Abstract
Background
Falls amongst older people are common; however, around 40% of falls could be preventable. Medications are known to increase the risk of falls in older adults. The debate about reducing the number of prescribed medications remains controversial, and more evidence is needed to understand the relationship between polypharmacy and fall-related hospital admissions. We examined the effect of polypharmacy on hospitalization due to a fall, using a large nationally representative sample of older adults.
Methods
Data from the English Longitudinal Study of Ageing (ELSA) were used. We included 6220 participants aged 50+ with valid data collected between 2012 and 2018.The main outcome measure was hospital admission due to a fall. Polypharmacy -the number of long-term prescription drugs- was the main exposure coded as: no medications, 1–4 medications, 5–9 medications (polypharmacy) and 10+ medications (heightened polypharmacy). Competing-risk regression analysis was used (with death as a potential competing risk), adjusted for common confounders, including multi-morbidity and fall risk-increasing drugs.
Results
The prevalence of people admitted to hospital due to a fall increased according to the number of medications taken, from 1.5% of falls for people reporting no medications, to 4.7% of falls among those taking 1–4 medications, 7.9% of falls among those with polypharmacy and 14.8% among those reporting heightened polypharmacy. Fully adjusted SHRs for hospitalization due to a fall among people who reported taking 1–4 medications, polypharmacy and heightened polypharmacy were 1.79 (1.18; 2.71), 1.75 (1.04; 2.95), and 3.19 (1.61; 6.32) respectively, compared with people who were not taking medications.
Conclusions
The risk of hospitalization due to a fall increased with polypharmacy. It is suggested that prescriptions in older people should be revised on a regular basis, and that the number of medications prescribed be kept to a minimum, in order to reduce the risk of fall-related hospital admissions.
Keywords: Older people, Polypharmacy, Falls, Hospitalization
Background
Falls, defined as an unanticipated incident in which a person come to rest on the ground or a lower level, [1] are the most frequent type of accidents among older people [2]. One in three people over 65 years of age experience at least one fall each year, and injuries occur in approximately 20% of such cases [3]. Older people who have suffered a fall experience an increased risk of recurrence and of being hospitalized. Falls not only carry a human burden, but they can incur considerable medical care costs, with estimates being suggested between 0.85 and 1.5% of total healthcare expenditure in the UK [4].
It has been estimated that around 40% of falls in older people are preventable [5]. As a consequence, a large body of research has emerged to explore risk factors that might determine whether someone is at risk of experiencing a fall, especially a fall for which they might require treatment in hospital. Polypharmacy, defined as the chronic co-prescription of multiple medications, has been identified as one of the most significant factors associated with falls among older people [6, 7]. Several studies in ageing populations have reported that the risk of a fall increases with the use of four or more medications [8–16]. However, older adults using multiple medications might also have several long-term conditions, whose pharmacological treatment often requires the concomitant use of several medications. Therefore, the risk of falls might not be independent of these long-term conditions. Indeed, those with multimorbidity (defined as reporting three or more long-term conditions) who also take multiple medications have a higher risk of falls [17].
Recent studies also suggest that medications such as cardiovascular agents, central nervous system drugs, analgesics and endocrine drugs, increase the risk of falls [14, 15]. The possible underlying mechanisms for the increased risk of falls related to the use of these medications, called “fall risk-increasing drugs” (FRIDs), relate to the adverse effects (eg, dizziness, imbalance or mobility difficulties, reduced attention and vigilance). However, Seppala et al., in their systematic review, point out that adjustment for long-term conditions and “fall risk-increasing drugs” has rarely been carried out in studies of polypharmacy and falls [18]. Properly adjusting for both is imperative, since polypharmacy is often the consequence of long term conditions, [19] and the risk of polypharmacy on falls may not be independent of “fall risk-increasing drugs” [14].
The debate about reducing the number of prescribed medications remains controversial. On the one hand, the prescription of several medications is largely justified by the complex clinical profile of older adults and it has been shown that interventions to reduce the number of concurrent medications have been unsuccessful [20–22]. On the other hand, studies have shown that medication withdrawal, especially FRIDs, has been effective in reducing the risk of falls [23]. Therefore, more evidence is needed to understand the relationship between polypharmacy and fall-related hospital admissions. Large nationally representative longitudinal studies of ageing, which collect a broad range of factors and have been linked to administrative health data, are best placed to provide insights into this relationship. Accordingly, the aim of this study is to examine, in a nationally representative sample of older adults, whether polypharmacy is a risk factor for hospitalization due to a fall. We will examine this independently of other risk factors, including long-term conditions and drugs known to increase the risk of falling (FRIDs).
Methods
Data
These data are from the English Longitudinal Study of Ageing (ELSA) [24] a nationally representative sample of individuals aged 50 and older living in private households in England, followed and re-interviewed every 2 years. The main objective of the study is to understand the complex dynamics of the ageing process, that is the relationships between economic and family circumstances, behaviour, social participation, biology, retirement, and health and well-being. The study began in 2002–2003 (first phase of data collection referred to as wave 1). Data collection comprises face-to-face interviews, self-completion questionnaires and nurse visits in participants’ homes every other wave. For the purpose of this study, we used data from wave 6 (2012–2013) as our baseline, when information on medication was first collected during the nurse visit. A total of 6220 individuals had valid data on medications and covariates of interest at baseline. All individuals included in the baseline sample (2012–2013) had their data linked to Hospital Episode Statistics, and to mortality even those who dropped out of the study after baseline.
Outcome measure
Hospitalization due to a fall was derived from the Hospital Episode Statistics data linked by NHS digital to ELSA participants’ NHS number, date of birth, gender and postcode. All participants were followed-up from the interview date up to March 2018. For each participant, a record of each hospitalization to secondary care is available, with admission date, episode duration, primary diagnosis and secondary diagnoses. Diagnoses are coded according to the international classification of disease 10th version (ICD-10). Falls correspond to the ICD-10 codes W00 to W19. The event “fall” is defined as the first episode where a primary diagnosis of fall was recorded.
Exposure: polypharmacy
At wave 6 (2012–2013) during the nurse visits to participants’ homes nurses recorded medications taken by each participant. These drugs, both generic and brand name, were allocated codes based on the British National Formulary. In the definition of polypharmacy, only long-term medications were considered. Long-term medications were either drugs for long-term diseases such as diabetes and hypertension, or drugs for long-term symptoms such as sedatives. Despite variation, which exists in the definition of polypharmacy, the number of medications in this sample was recoded according to the most commonly used cut-offs: No medications, 1–4 medications, 5–9 medications (polypharmacy) and 10 or more medications (heightened polypharmacy) [25].
Potential confounders
Socio-demographic variables included age (continuous, ranging from 54 to 101 at wave 6), sex (males vs females), cohabitation status (currently living or not with a partner whether married or not), and educational attainment (high-college and above, medium-A-levels, low-below O-levels). For cognitive function we used a memory score computed from a word-list learning test [26] in which a list of ten words was read out to study participants, who were then asked to recall as many words as possible immediately and after around a five-minute delay (total score ranged from 0 to 20 with higher scores indicating better cognitive function). Health behaviours included frequency of alcohol intake (in days) in the last 12 months ascertained from self-reported responses and coded as daily (5/7 days week) or less than daily (< 5 days a week); smoking status (non-smoker vs current smoker); body mass index (computed from objectively measured height and weight); physical activity (active vs sedentary). Physical activity was measured using responses to questions on leisure-time physical activity and aggregated to compute a five-level score from inactive to active, and used in the analysis as binary. We also used a self-reported measure of eyesight (poor vs good). Health conditions were ascertained from self-reported doctor diagnosis and included: coronary heart disease (CHD), stroke, diabetes, depression (defined as 4 or more depressive symptoms), respiratory illness, arthritis, cancer, dementia, Parkinson’s disease and Alzheimer’s disease. In addition we computed a variable for multimorbidity by recoding the number of long-term conditions reported into a dichotomous variable, with a cut-off of 3 or more [16]. FRIDs were also taken into account as a binary variable (2+ FRIDS versus none). FRIDs included cardiovascular agents, central nervous system drugs (not including antiparkinsonians), analgesics (non-steroidal anti-inflammatory drugs), thyroid drugs, and antihyperglycemic drugs [14]. Physical functioning was measured using number of limitations with mobility items (continuous) and the number of difficulties with activities of daily living (ADLs) and instrumental activities of daily living (IADLs) (binary, one or more versus no difficulties), and cognitive function. ADLs items were: dressing, walking across a room, bathing or showering, eating, getting out of bed, using the toilet; IADLs items were: using a map, preparing a hot meal, shopping for groceries, making phone calls, taking medications, doing work around the house, managing money.
Statistical analysis
To examine the association between polypharmacy and hospitalization due to a fall we employed competing-risk regression analysis with subdistribution hazard ratios (SHR) and related 95% Confidence Intervals, using a version of the Fine and Gray method [27]. This method allows a competing risk – an event that might occur during the follow-up instead of the event of interest – to be taken into account in the analysis. In this case, death is a potential competing risk when examining incidence rates of admission to hospital due to a fall; therefore, it is important to take this into account, rather than treating those who had died as censored. Mortality status was ascertained from linked register data, up to the end of March 2018. By the end of this follow-up period (six years) 295 admissions to hospital due to a fall were recorded and 594 deaths occurred.
In a sensitivity analysis, we explored whether fall hospitalization was associated with the use of polypharmacy also among people in the 0–1 FRID category, as previous studies suggested that the risk of polypharmacy of falls might not be associated with fall risk, independently of FRIDs [9, 14].
Results
The baseline characteristics of the sample in 2012–2013 according to polypharmacy status are reported in Table 1. The prevalence of people admitted to hospital due to a fall increased steadily according to polypharmacy status. This ranged from 1.5% in people reporting no medications, to 4.7% of falls among people reporting 1–4 medications, 7.9% of falls occurred among people with polypharmacy (5–9 medications) and 14.8% among those reporting heightened polypharmacy (10 + medications). Respondents reporting polypharmacy and heightened polypharmacy were also older and reported poorer health outcomes at baseline than those not taking medications.
Table 1.
Baseline characteristics of participants according to polypharmacy, England 2012–2013
| Polypharmacy | |||||
|---|---|---|---|---|---|
| No medications | 1–4 medications | 5–9 medications (polypharmacy) |
10+ medications (heightened polypharmacy) |
P Value | |
| Number of Respondents | 1720 | 3051 | 1290 | 159 | |
| Age, years: mean (s.e.) | 64.07 (0.23) | 66.04 (0.24) | 71.00 (0.39) | 71.02 (0.81) | < 0.001 |
| Women, % (n) | 47.3 (909) | 54.5 (1729) | 52.3 (675) | 50.2 (85) | < 0.001 |
| Hospitalization due to falls by 2018, % (n) | 1.5 (33) | 4.7 (153) | 7.9 (99) | 14.8 (25) | < 0.001 |
| Deaths [by March 2018], % (n) | 3.1 (63) | 8.1 (245) | 19.3 (240) | 30.8 (46) | < 0.001 |
| Living alone, % (n) | 23.5 (430) | 28.8 (917) | 36.1 (469) | 38.7 (66) | < 0.001 |
| Highest level of education, % (n) | |||||
| Degree, | 22.6 (404) | 15.7 (533) | 11.5 (148) | 11.5 (21) | < 0.001 |
| Intermediate | 61.5 (1064) | 58.7 (1805) | 46.8 (722) | 46.8 (81) | |
| No qualification | 15.9 (252) | 25.6 (713) | 41.7 (420) | 41.7 (57) | |
| Poor vision, % (n) | 7.8 (123) | 11.1 (314) | 21.2 (255) | 29.8 (48) | < 0.001 |
| Diagnoses and Health conditions, % (n) | |||||
| CHD | 0.4 (10) | 4.9 (158) | 28.5 (355) | 53.3 (82) | < 0.001 |
| Diabetes | 0.7 (15) | 8.2 (235) | 30.9 (383) | 47.3 (77) | < 0.001 |
| Depression | 3.9 (66) | 8.1 (224) | 11.4 (115) | 18.9 (29) | < 0.001 |
| Asthma or lung disease | 4.7 (74) | 14.6 (437) | 25.2 (310) | 53.4 (80) | < 0.001 |
| Stroke | 0.3 (6) | 2.9 (92) | 11.2 (142) | 17.0 (23) | < 0.001 |
| Cancer | 3.2 (59) | 5.4 (171) | 7.8 (108) | 7.3 (13) | < 0.001 |
| Arthritis | 20.0 (411) | 39.1 (1268) | 58.8 (753) | 72.0 (117) | < 0.001 |
| Parkinson’s disease | 0.0 (1) | 0.6 (20) | 1.0 (14) | 2.6 (4) | < 0.001 |
| Alzheimer’s disease | 0.0 (0) | 0.1 (3) | 0.4 (4) | 0 (0) | 0.055 |
| Dementia | 0.1 (3) | 0.7 (18) | 1.9 (20) | 2.1 (2) | 0.001 |
| Has 3+ long-term conditions, % (n) | 0.6 (12) | 3.2 (100) | 22.0 (255) | 53.5 (84) | < 0.001 |
| Takes 2+ FRIDsa, % (n) | 0 | 30.1 (924) | 85.6 (1100) | 95.3 (151) | < 0.001 |
| Difficulty in ADL or IADL, % (n) | 8.6 (149) | 22.1 (649) | 49.6 (582) | 68.9 (109) | < 0.001 |
| Limitations in mobility, mean (s.e.) | 0.69 (0.04) | 1.70 (0.05) | 3.58 (0.10) | 5.57 (0.26) | < 0.001 |
| Hardly ever engage in physical activity, % (n) | 5.3 (83) | 14.1 (388) | 34.8 (381) | 55.4 (83) | < 0.001 |
| BMI value: mean (s.e.) | 27.19 (0.14) | 28.55 (0.11) | 30.37 (0.19) | 31.57 (0.55) | < 0.001 |
| Current smoker, % (n) | 15.0 (205) | 12.4 (302) | 13.4 (151) | 21.7 (32) | 0.021 |
| Almost daily alcohol consumption, % (n) | 19.7 (382) | 20.5 (673) | 15.9 (227) | 13.7 (22) | 0.007 |
| Memory index: mean (s.e.) | 11.80 (0.09) | 10.92 (0.07) | 9.51 (0.11) | 8.40 (0.32) | < 0.001 |
Source: ELSA, Wave 6. Weighted data. a FRID Fall-risk increasing drugs
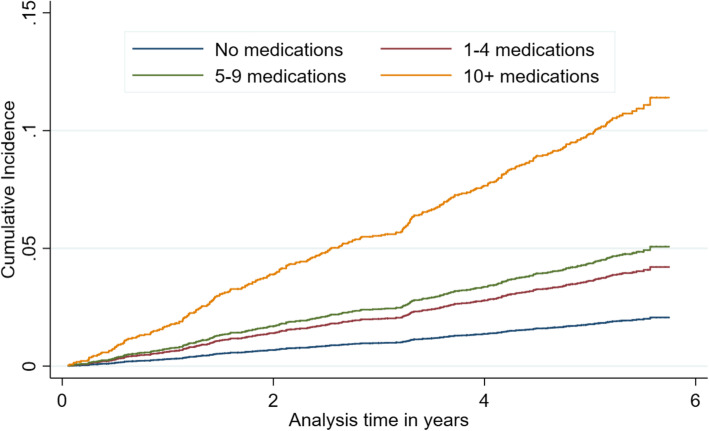
The unadjusted cumulative incidence function shows a dose-response association in the risk of hospitalization due to a fall and polypharmacy; in particular, the cumulative incidence curve for 10+ medications increased steeply with time (Fig. 1).
Fig. 1.
Estimates of the cumulative incidence curves of risk of hospitalization following a fall according to polypharmacy, England 2012–2018
In Table 2 we report the subdistribution hazard ratios (SHR) for the association between polypharmacy and risk of hospitalization due to a fall estimated using competing risk analysis. The age- and sex- adjusted SHRs for hospitalization due to a fall among people who reported taking 1–4 medications, polypharmacy and heightened polypharmacy were 2.06 (95%CI:1.38;3.07), 2.49 (95%CI:1.62;3.82), and 5.79 (95%CI:3.33;10.1) respectively, compared with people who were not taking medications. After adjustment for all covariates, the association between polypharmacy and hospitalization due to a fall, reduced to 1.79 (95%CI:1.18; 2.71) among people who reported taking 1–4 medications, reduced to 1.75 (95%CI: 1.04; 2.95) among those reporting polypharmacy and was 3.19 (95%CI: 1.61; 6.32) for heightened polypharmacy.
Table 2.
Subdistribution hazard ratios (95 CIs) for the association between the number polypharmacy and hospitalization following a fall (N = 6220), England 2012–2018
| Age and gender adjusted | Fully adjusteda | |||
|---|---|---|---|---|
| Polypharmacy | SHR (95%CI) | P Value | SHR (95%CI) | P Value |
| No medications | 1 (Ref) | 1 (Ref) | ||
| 1–4 medications | 2.06 (1.38; 3.07) | < 0.0001 | 1.79 (1.18; 2.71) | < 0.01 |
|
5–9 medications (polypharmacy) |
2.49 (1.62; 3.82) | < 0.0001 | 1.75 (1.04; 2.95) | < 0.05 |
|
10+ medications (heightened polypharmacy) |
5.79 (3.33; 10.1) | < 0.0001 | 3.19 (1.61; 6.32) | < 0.001 |
Hospitalization following a fall N = 295, competing event deaths N = 594. aAdjusted for age, gender, living alone, education, poor vision, all diagnoses and health conditions, 3+ long-term conditions, 2+ FRIDs, any functional impairment, health behaviours, tests of cognitive function
In further sensitivity analysis, we investigated whether the association between polypharmacy and admissions to hospital due to a fall remained when FRIDs were included in the model. We ran the analyses again among those who were taking 0–1 FRIDs. We found that the association between polypharmacy and hospitalization due to a fall reduced in magnitude, but was still statistically significant by polypharmacy status, including the group of 1–4 medications (SHR 1.65 95%1.1;2.5 p = 0.025 compared to not taking medications).
Discussion
Using a large nationally representative sample of older people in England, our study showed a strong association between polypharmacy status and the risk of hospitalization due to a fall. We found that the risk was highest among people reporting polypharmacy and heightened polypharmacy compared to those who reported taking no medications. We also observed a slightly elevated risk among older adults who reported the concurrent use of 1–4 medications compared with those who reported taking none. These associations were not explained by common risk factors for falls, neither by multi-morbidities nor by FRIDs.
In agreement with results from a population-based case control study of people 65 years and older living in Stockholm, [12] we found that the use of one or more medications led to an increased risk of hospitalization due to a fall. In a previous investigation using data from ELSA [8] it was found that polypharmacy and heightened polypharmacy significantly increased the risk of falls among people aged 60 and over, however, the study only used a short follow-up period (2 years) and a self-reported measure of falls. Our analysis has improved the results of previous studies by using an objective measure of falls and by studying a younger cohort at the first assessment (aged 50 years old and over) [8, 9, 11–14]. Furthermore, we showed that this increased risk of falls among people taking medications was independent of long-term conditions and FRIDs.
Falls are common among older adults, and as the proportion of elderly people in the population continues to increase, falls in this group are predicted to pose a serious burden on healthcare expenditure. Strategies to prevent falls include the identification of potential modifiable risk factors, such as multiple medications [6]. Our results contribute to current discussions in the UK about reducing the number of prescribed medications in older age. The National Institute for Health and Care Excellence published new guidelines for the management of multimorbidity among individuals taking 10 or more prescribed medications. However, we have shown that the risk of hospitalization due to a fall is also high in patients taking 1–4 and 5–9 medications, and these people might be excluded from the target group for medication reviews [28]. Period reviews of prescriptions among older patients should be in place to assure that the number of medications consumed is minimalized, especially amongst frail people who might be at higher risk of falling.
Strengths and limitations
This study examined the association between polypharmacy and hospitalization due to a fall in a nationally representative sample of non-institutionalised individuals in England. The use of medication data collected by a nurse, and hospital administrative data, is less susceptible to recall bias. Moreover, we considered the number of long-term conditions from which participants suffered and were able to adjust for these in our analyses. Lastly, we used a competing risk analysis strategy to consider mortality as a competing event.
The main limitation of our study is that the information on medication was collected for the first time in 2012/2013; it would have been preferable to have multiple time points of medication records to establish whether the duration of polypharmacy had an impact on the risk of being admitted to hospital due to a fall. We were able to investigate the number of medications prescribed, but unfortunately, we were not able to explore the nature of these medications, since the specific drug name was often not available. We were also not able to test common drug-drug interactions directly, [29] such as with antihypertensive, diuretics and selective serotonin reuptake inhibitors (SSRIs) [30]. Potentially serious drug-drug interactions have been reported in drugs recommended by clinical guidelines for different long-term conditions, such as cardiovascular diseases, type 2 diabetes, depression and dementia [29, 31]. Severe drug-drug interactions could happen between SSRIs and serotonin–norepinephrine reuptake inhibitors or between beta-blockers and certain antiarrhythmic agents; we cannot entirely exclude that these interactions occurred in our sample [29, 31]. Future studies should examine specific drug-drug interactions in detail [31].
An additional issue is that the assessment of polypharmacy was based on the long-term medications that were being taken by participants at the time of the nurse visits. Although excluded medications were primarily painkillers, a small proportion of antihistamines, both sedating and non-sedating types, were also excluded. Furthermore, we do not have information about whether the medication prescribed changed over the follow-up period. People might also have taken other medications acutely that might have provoked problems with balance and increased risk of falls.
It is also possible that the risk of falls may be increased by strong doses of FRIDs, but we did not have information on medication dosage. Finally, although we used a wide range of confounders, some residual confounding might exist. For example, we could not adjust for objective measures of physical functioning since those were collected only among those aged 60 and over.
Conclusions
In conclusion, we found that the risk of hospitalization due to a fall increased with polypharmacy status. The increased risk was apparent among those reporting polypharmacy and heightened polypharmacy, but also among those reporting the concurrent use of 1–4 medications. It is advisable that drug prescriptions in older people be revised on a regular basis, and that the number of medications should be kept to the minimum possible as it might reduce the risk of fall-related hospital admissions.
Acknowledgements
Not applicable.
Abbreviations
- ADLs
Activities of Daily Living
- CHD
Coronary Heart Disease
- ELSA
English Longitudinal Study of Ageing
- FRIDs
Fall Risk-Increasing Drugs
- IADLs
Instrumental Activities of Daily Living
- ICD-10
International Classification of Disease
- SHR
Subdistribution Hazard Ratios
Authors’ contributions
All authors contributed to the study concept and design. PZ wrote the first and successive drafts of the paper. YTH prepared the polypharmacy data. CL and JA prepared the HES data. PZ carried out all the statistical analysis with help from GDG. All authors contributed to the interpretation of results and approved the final version of the paper.
Funding
The English Longitudinal Study of Ageing is supported by the National Institute on Aging (grant numbers: 2RO1AG7644 and 2RO1AG017644-01A1) and a consortium of the UK government departments coordinated by the National Institute for Health Research. The funding bodies had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Availability of data and materials
The ELSA datasets are available in the UK Data Service, [https://beta.ukdataservice.ac.uk/datacatalogue/series/series?id=200011].
Hospital Episode Statistics data and mortality data will be made available in due course.
Ethics approval and consent to participate
The ELSA study was conducted in accordance with the Declaration of Helsinki and ethical approval and experimental protocols were granted by NHS Research Ethics Committees under the National Research and Ethics Service.
Participants provided informed written consent to the investigation.
Consent for publication
Not applicable.
Competing interests
The author(s) declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lamb SE, Jorstad-Stein EC, Hauer K, Becker C. Prevention of falls network E, outcomes consensus G. development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53(9):1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 2.Gale CR, Cooper C, Aihie SA. Prevalence and risk factors for falls in older men and women: the English longitudinal study of ageing. Age Ageing. 2016;45(6):789–794. doi: 10.1093/ageing/afw129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lord S, Sherrington C, Menz H. Falls in older people. Risk factors and strategies for prevention. Cambridge: University Press; 2001. [Google Scholar]
- 4.Heinrich A, de la Rosa S, Schneider BA. The role of stimulus complexity, spectral overlap, and pitch for gap-detection thresholds in young and old listeners. J Acoust Soc Am. 2014;136(4):1797–1807. doi: 10.1121/1.4894788. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME, Speechley M. Prevention of falls among the elderly. N Engl J Med. 1989;320(16):1055–1059. doi: 10.1056/NEJM198904203201606. [DOI] [PubMed] [Google Scholar]
- 6.Zia A, Kamaruzzaman SB, Tan MP. Polypharmacy and falls in older people: balancing evidence-based medicine against falls risk. Postgrad Med. 2015;127(3):330–337. doi: 10.1080/00325481.2014.996112. [DOI] [PubMed] [Google Scholar]
- 7.Burt J, Elmore N, Campbell SM, Rodgers S, Avery AJ, Payne RA. Developing a measure of polypharmacy appropriateness in primary care: systematic review and expert consensus study. BMC Med. 2018;16(1):91. doi: 10.1186/s12916-018-1078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhalwani NN, Fahami R, Sathanapally H, Seidu S, Davies MJ, Khunti K. Association between polypharmacy and falls in older adults: a longitudinal study from England. BMJ Open. 2017;7(10):e016358. doi: 10.1136/bmjopen-2017-016358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziere G, Dieleman JP, Hofman A, Pols HA, van der Cammen TJ, Stricker BH. Polypharmacy and falls in the middle age and elderly population. Br J Clin Pharmacol. 2006;61(2):218–223. doi: 10.1111/j.1365-2125.2005.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong H, Heuberger R, Logomarsino J, Hewlings S. Associations between alcohol use, polypharmacy and falls in older adults. Nurs Older People. 2016;28(1):30–36. doi: 10.7748/nop.28.1.30.s22. [DOI] [PubMed] [Google Scholar]
- 11.Baranzini F, Diurni M, Ceccon F, Poloni N, Cazzamalli S, Costantini C, et al. Fall-related injuries in a nursing home setting: is polypharmacy a risk factor? BMC Health Serv Res. 2009;9:228. doi: 10.1186/1472-6963-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Helgadottir B, Laflamme L, Monarrez-Espino J, Moller J. Medication and fall injury in the elderly population; do individual demographics, health status and lifestyle matter? BMC Geriatr. 2014;14:92. doi: 10.1186/1471-2318-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kojima T, Akishita M, Nakamura T, Nomura K, Ogawa S, Iijima K, et al. Polypharmacy as a risk for fall occurrence in geriatric outpatients. Geriatr Gerontol Int. 2012;12(3):425–430. doi: 10.1111/j.1447-0594.2011.00783.x. [DOI] [PubMed] [Google Scholar]
- 14.Zia A, Kamaruzzaman SB, Tan MP. The consumption of two or more fall risk-increasing drugs rather than polypharmacy is associated with falls. Geriatr Gerontol Int. 2017;17(3):463–470. doi: 10.1111/ggi.12741. [DOI] [PubMed] [Google Scholar]
- 15.Richardson K, Bennett K, Kenny RA. Polypharmacy including falls risk-increasing medications and subsequent falls in community-dwelling middle-aged and older adults. Age Ageing. 2015;44(1):90–96. doi: 10.1093/ageing/afu141. [DOI] [PubMed] [Google Scholar]
- 16.Morin L, Calderon Larranaga A, Welmer AK, Rizzuto D, Wastesson JW, Johnell K. Polypharmacy and injurious falls in older adults: a nationwide nested case-control study. Clin Epidemiol. 2019;11:483–493. doi: 10.2147/CLEP.S201614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damian J, Pastor-Barriuso R, Valderrama-Gama E, de Pedro-Cuesta J. Factors associated with falls among older adults living in institutions. BMC Geriatr. 2013;13:6. doi: 10.1186/1471-2318-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seppala LJ, van de Glind EMM, Daams JG, Ploegmakers KJ, de Vries M, Wermelink A, et al. Fall-Risk-Increasing Drugs: A Systematic Review and Meta-analysis: III. Others. J Am Med Dir Assoc. 2018;19(4):372 e1–372 e8. doi: 10.1016/j.jamda.2017.12.099. [DOI] [PubMed] [Google Scholar]
- 19.Morin L, Johnell K, Laroche ML, Fastbom J, Wastesson JW. The epidemiology of polypharmacy in older adults: register-based prospective cohort study. Clin Epidemiol. 2018;10:289–298. doi: 10.2147/CLEP.S153458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salisbury C, Man MS, Bower P, Guthrie B, Chaplin K, Gaunt DM, et al. Management of multimorbidity using a patient-centred care model: a pragmatic cluster-randomised trial of the 3D approach. Lancet. 2018;392(10141):41–50. doi: 10.1016/S0140-6736(18)31308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace E, Salisbury C, Guthrie B, Lewis C, Fahey T, Smith SM. Managing patients with multimorbidity in primary care. BMJ. 2015;350:h176. doi: 10.1136/bmj.h176. [DOI] [PubMed] [Google Scholar]
- 22.Wise J. Polypharmacy: a necessary evil. BMJ. 2013;347:f7033. doi: 10.1136/bmj.f7033. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steptoe A, Breeze E, Banks J, Nazroo J. Cohort profile: the English longitudinal study of ageing. Int J Epidemiol. 2013;42(6):1640–1648. doi: 10.1093/ije/dys168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17(1):230. doi: 10.1186/s12877-017-0621-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaninotto P, Batty GD, Allerhand M, Deary IJ. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English longitudinal study of ageing. J Epidemiol Community Health. 2018;72(8):685–694. doi: 10.1136/jech-2017-210116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fine JP, Grey RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 28.Farmer C, Fenu E, O'Flynn N, Guthrie B. Clinical assessment and management of multimorbidity: summary of NICE guidance. BMJ. 2016;354:i4843. doi: 10.1136/bmj.i4843. [DOI] [PubMed] [Google Scholar]
- 29.Dumbreck S, Flynn A, Nairn M, Wilson M, Treweek S, Mercer SW, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350:h949. doi: 10.1136/bmj.h949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersson ML, Bottiger Y, Kockum H, Eiermann B. High prevalence of drug-drug interactions in primary health care is caused by prescriptions from other healthcare units. Basic Clin Pharmacol Toxicol. 2018;122(5):512–516. doi: 10.1111/bcpt.12939. [DOI] [PubMed] [Google Scholar]
- 31.Strandell J, Caster O, Hopstadius J, Edwards IR, Noren GN. The development and evaluation of triage algorithms for early discovery of adverse drug interactions. Drug Saf. 2013;36(5):371–388. doi: 10.1007/s40264-013-0053-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ELSA datasets are available in the UK Data Service, [https://beta.ukdataservice.ac.uk/datacatalogue/series/series?id=200011].
Hospital Episode Statistics data and mortality data will be made available in due course.