Abstract
Objective:
Although behaviorally inhibited (BI) temperament predicts risk for anxiety, anxiety in BI may involve distinct neural responses to errors. The current study examines the relations between anxiety and neural correlates of error processing both in youths identified as BI in early childhood and in youths seeking treatment for an anxiety disorder.
Method:
All participants underwent functional magnetic resonance imaging using a flanker task to assess responses to errors. A study in healthy subjects assessed test–retest reliability to inform analyses in two other samples. For one sample, a cohort of BI youths (Low BI, n = 28; High BI, n = 27) was followed into adolescence. For the other, participants were recruited based on the presence or absence of an anxiety disorder. Using identical methods in medication-free subjects, analyses compared relations between anxiety and error processing across the two samples.
Results:
Error-processing exhibited acceptable reliability. Within a ventromedial–prefrontal–cortex (vmPFC) cluster, anxiety related to error processing only in youths whose early-life BI status was known. In the high BI group, anxiety related to reduced neural response to errors. No such associations manifested in treatment-seeking youths. Other analyses mapped relations between error-processing and anxiety in each sample on its own. However, only the vmPFC cluster statistically differentiated the neural correlates of anxiety in BI.
Conclusion:
BI temperament may define a unique pathway into anxiety involving perturbed neural responding to errors. Although BI is a risk factor for later anxiety, the neural and associated features of anxiety in BI youths may differ from those in treatment-seeking youths.
Keywords: pediatric anxiety, behavioral inhibition, neuroimaging, error processing
Behavioral inhibition (BI), an early-childhood temperament, predicts risk for anxiety.1-4 However, BI is only one pathway through which anxiety develops. Thus, similarities and differences exist between anxiety in children with BI and anxiety in children seeking treatment for anxiety disorders in which BI level is unknown. Youths with BI who develop high levels of anxiety and youths seeking treatment for an anxiety disorder share many clinical symptoms (eg, wariness toward novelty, avoidant behaviors). Moreover, functional magnetic resonance imaging (fMRI) research on threat processing demonstrates comparable correlates of anxiety in these two phenotypes.5,6 Other data, however, suggest that the correlates of anxiety differ in children with and without early-life BI. In particular, neurophysiological research on error processing differentiates the neural correlates of anxiety in children with and without a history of BI.7 Notably, an independent line of research also links anxiety in treatment-seeking youths to atypical error responses.8-10 Nevertheless, no study directly compares the relationship between anxiety and neural correlates of error processing in youths identified as having BI in early childhood and in youths seeking treatment for an anxiety disorder whose BI status is unknown. The current fMRI study reports such data to test the hypothesis that the relationship between anxiety and neural responding to errors differs in children characterized by levels of BI and children with a range of anxiety symptoms, including those seeking treatment for anxiety disorders.
Prior research on error processing describes more consistent findings in BI than in anxiety disorders. In individuals identified as BI versus non-BI in childhood, the most consistent finding is larger event-related potentials (ERP) in response to errors in regions underlying error monitoring, such as the anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (dlPFC). 8-10 Of note, other data also suggest that anxious, compared to nonanxious, youths express larger ERP responses to errors.11-14 However, fMRI studies find the opposite pattern, in which anxious compared to nonanxious youths manifest less engagement of error-related regions.8 Such reduced activation is also found in other forms of pediatric and adult psychopathology.15 Finally, studies in youths with anxiety also find that individual differences, particularly the age of the patient, moderate error-related neural correlates of anxiety.16 Of note, unlike these findings for brain function, most work on task performance finds no associations between anxiety and control-relevant behaviors.8-10 However, because fMRI studies of error processing in both pediatric anxiety and BI are limited, conclusions remain tentative.
Beyond the need for more data, methodological factors further limit attempts to compare relations between anxiety and error processing in youths with BI as opposed to treatment-seeking youths with anxiety disorders whose level of childhood BI is unknown. For instance, error-processing studies of BI and treatment-seeking youths use different methodology.8,9,11,12 The current report uses identical anxiety assessments, cognitive tasks, and imaging methods in two samples of medication-free youths, one assessed for BI and another with a wide range of anxiety symptoms, including individuals seeking treatment for one or more anxiety disorders. Moreover, the study directly compares findings in the two samples, using the same dimensional measure of anxiety. Finally, the modest reliability of fMRI paradigms complicates attempts to perform this comparison and reflects broader concerns about failures to replicate findings both within and across phenotypes.17,18 To address this issue, the current report also provides new data on reliability of the fMRI paradigm to bolster any anxiety-related findings.
Evidence for distinct relations between anxiety and error processing in BI and anxious youths would significantly influence developmental perspectives on anxiety. This would provide imaging-based evidence that BI represents only one pathway through which children develop anxiety symptoms. This pathway would involve some features, such as threat-processing biases, shared with treatment-seeking youths, as well as other features, possibly error-processing perturbation, unique to anxiety symptoms related to BI. We consider this possibility by collecting data in two cohorts—one characterized by level of BI in early childhood and followed longitudinally through adolescence (n = 54), and another recruited in adolescence based on the presence or absence of an anxiety disorder requiring treatment (n = 78). The main analysis, Analysis 1, compares the neural correlates of anxiety symptoms in the 132 adolescents spanning the cohorts. Using a dimensional measure of anxiety, we test the hypothesis that the relationship between anxiety and error processing differs among subjects recruited based on early-life levels of BI as compared to subjects recruited based on the presence or absence of an anxiety disorder with an unknown level of BI. Given prior work concerning these two phenotypes examined in isolation, we expected a direct comparison to yield different patterns in these groups.
Finally, for completeness, the study reports two additional secondary analyses in each cohort on its own. Analysis 2 focuses specifically on BI to further map the relations between anxiety symptoms and error processing in children with varying levels of BI.12-14 This is particularly important, as the majority of prior research uses electroencephalography, rather than fMRI. Analysis 3 examines the neural correlates of error processing in youths stratified based on the presence or absence of an anxiety disorder. Here, the study tests the hypothesis that error processing differs as a function of both anxiety and age, given prior evidence on age moderation in anxiety.16 This analysis is important because it seeks to replicate patterns from other studies and thereby to evaluate the robustness of study methodology. Together, the studies test the hypothesis that the relationship between anxiety and error processing differs among children recruited based on their early-life temperament as compared to the presence or absence of an anxiety disorder.
METHOD
Procedure
All procedures were approved by the National Institute of Mental Health (NIMH) and University of Maryland Institutional Review Boards. Parents and participants provided written consent/assent. All participants were paid for their participation. All participants underwent a semi-structured clinical interview with a trained clinical psychologist19 to ascertain current or past DSM-5 disorders. Participants recruited through NIMH who met DSM-5 criteria for an anxiety disorder also received treatment for their time and participation. All participants and their parents completed the Screen for Child Anxiety Related Emotional Disorders (SCARED)20 to assess current anxiety severity.
Two cohorts were enrolled in the current study. For the first cohort, 71 participants, 12-15 years of age (mean = 13.02, SD = 0.66) were enrolled from a larger longitudinal study on early life temperament (BI cohort). During the recruitment process, 11 potential participants were excluded for current selective serotonin reuptake inhibitor use and 19 for fMRI contraindications (ie, braces and other metal in the body). At the time of the scan, eight participants met DSM-5 criteria for a current anxiety disorder (for full demographics, see Table S1, available online). Following participation, 15 participants were excluded based on task accuracy (n = 9 above 90%; n = 4 below 70%; and unusable fMRI data [n = 2]).
In this cohort, BI was assessed via maternal report and laboratory observations at ages 2 and 3 years.21 Scores from observations and maternal report were standardized and averaged to generate a BI composite, with higher scores representing greater levels of BI (range = −1.64 to 1.46; mean = 0.04; SD = 0.69).22 Although the positive correlation between BI at ages 2 to 3 years and anxiety at age 13 years was not significant in the scanned subsample (r = 0.16, p = .24), the correlation was similar in magnitude to the significant correlation in the larger sample.23 Our recruitment strategy (ie, exclusion of individuals taking selective serotonin reuptake inhibitors or with contraindications) likely lowered the significance of the relation between BI and anxiety in the current sample.
The second cohort of participants included 118 youths, 8 to 18 years of age, who were enrolled based on the presence of absence or an anxiety disorder. Because these participants were recruited in adolescence, no comparable measures of early childhood BI were available for this cohort. Of these 118 youths, 40 were excluded based on task accuracy (n = 19 above 90%; n = 13 below 70%), and unusable fMRI data (n = 8). Of the 78 remaining participants, 39 (13 male and 26 female) met DSM-5 criteria for an anxiety disorder (anxiety group) and 39 (16 male and 23 female) did not (healthy group). Within this cohort, the anxious and healthy groups did not differ in age [t(76) = −0.82, p = .42], sex [χ2(1) = 0.49, p = .48], or IQ [t(76) = 1.34, p = .18]. Full recruitment details and demographics are provided in Supplement 1, available online.
Task
Participants completed a modified flanker task24 at NIMH while undergoing functional magnetic resonance imaging (fMRI) (Figure 1). Participants completed four 6-minute runs of the task. Each run was divided into three blocks to provide rest and feedback regarding task performance. At the end of each block, participants were given one of three feedback messages based on their task performance: “be more accurate” for accuracy under 75%, “respond faster” for accuracy above 90%, and “good job” for accuracy between 75% and 90%. Feedback was used to maintain accuracy within a 75% to 90% range and to maximize errors.25 For accurate modeling of brain responses, we first removed participants with poor accuracy (<70%). Next, to accurately estimate neural response to errors for imaging analyses, we removed all participants who did not have 20 errors of commission on incongruent trials (ie, >90% accuracy).26,27 Full details on participant exclusions are provided below. For behavioral variables and analyses, see Supplement 1, available online.
FIGURE 1. Flanker Task.
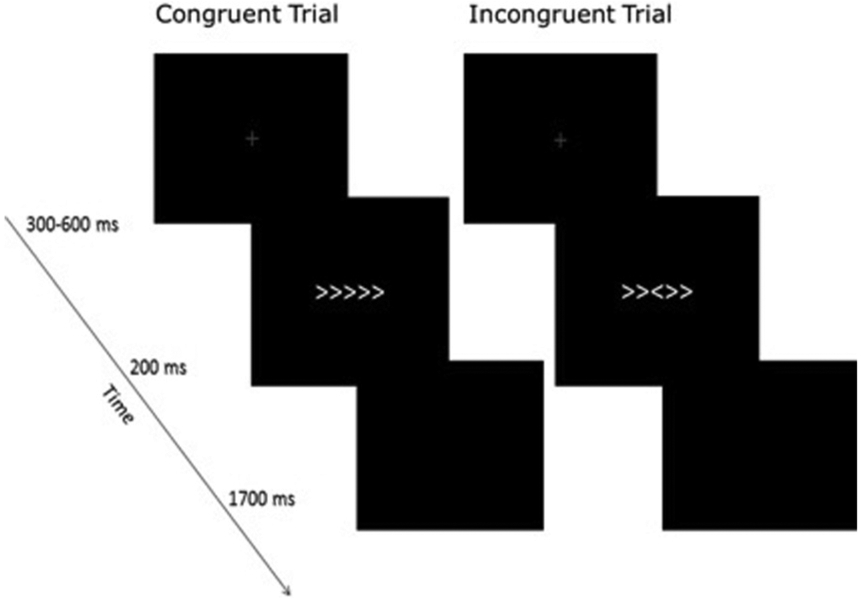
Note: In the task, participants were instructed to press a button indicating which direction a center arrow, flanked by two arrows on either side, was facing. In 50% of the experimental trials, the flanking arrows were facing the same direction as the center arrow (congruent trials), whereas in the other 50%, they were facing the opposite direction (incongruent trials). Congruent and incongruent trials were randomized across task runs. The task also included null trials, during which no stimuli was presented. Each trial began with the presentation of a fixation cross in the center of the screen. The fixation remained on the screen for a randomized amount of time, ranging from 300 to 600 milliseconds. Next, the flanker stimuli appeared on the screen (200 milliseconds) followed by a blank screen (1,700 milliseconds). Participants’ responses were recorded during the 1,700-ms response window. All task stimuli were white and presented on a black background.
Magnetic Imaging Acquisition
Neuroimaging was completed on a 3T GE Scanner using a 32-channel head coil. Each functional imaging run consisted of 170 whole-brain (forty-two 3-mm axial slices) T*2-weighted echoplanar images (TR = 2,000 milliseconds, TE = 25, flip angle = 60°, 24 field of view, 96 × 96 matrix). A structural magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence was acquired for coregistration. A structural scan was collected in the sagittal direction (TI/TE = 425/min full, 1-mm slices, flip angle = 7°, 256 × 256 matrix).
Imaging Analyses
All imaging analyses were conducted using AFNI 18.0.27.28 Preprocessing (afni.proc.py) included despiking, slice time correction, coregistration, spatial smoothing with a 5-mm full-width half maximum smoothing kernel, and warping to standard space. Acquisitions with greater than 1 mm of movement were censored. All included participants had more than 80% of acquisitions in each run following censoring. At the individual subject level, we applied a general linear model with 6 regressors time-locked to stimulus onset reflecting trial type (incongruent, congruent) and error condition (correct, commission, omission).
To examine error-specific processing, whole-brain group-level multivariate linear models (AFNI’s 3dMVM) contrasted errors of commission errors and correct responses (ie, Error Condition) only within incongruent trials, including motion and number of error trials as covariates (see Table S2, available online, for full trial information). Between-subject variables for each study are described below. Masked output maps included gray matter voxels where more than 90% of participants had signal. Significance for all output maps was determined based on 10,000 Monte Carlo simulations in AFNI’s 3dClustsim program. Each output map’s spatial autocorrelation function (two-sided thresholding) was used to maximize accuracy.29 Separate estimations were used for each group map because they contained different individual-subject maps (reported below).
Data Analysis Plan
Analysis 1.
To examine the impact of anxiety severity across phenotypes, we used a dimensional, rather than a categorical, approach. In this analysis, SCARED Total scores (SCARED–Total, averaged across parent and child reports) were used as a continuous measure of anxiety severity. Participants were categorized into three groups based on phenotype: Low BI, High BI, and participants recruited based on the presence or absence of an anxiety disorder (NIMH-recruited cohort). BI groups were determined based on a median-split of BI scores (Low BI: n = 28, mean = −0.45, SD = 0.50; High BI: n = 27, mean = 0.56, SD = 0.42). Across the NIMH-recruited cohort, there was a wide range of anxiety severity (SCARED range 0–51); therefore, to use this group dimensionally, we collapsed across diagnostic groups. We will refer to this combined group as the Distributed Anxiety (dAnx) group. This dimensional approach was taken to avoid collinearity between anxiety severity and diagnosis group, particularly in the NIMH-recruited cohort.
The three groups (Low BI, High BI, dAnx) did not differ in age (F2,132 = 0.15, p = .86), sex [χ2(2) = 0.80, p = .67], or IQ (F2,132 = 1.67, p = .19). However, the groups did differ on SCARED–Total (F2,132 = 13.08, p <.001). More specifically the dAnx had higher self-reported anxiety than the Low BI [t(104) = 4.08, p < .001] and High BI [t(103) = 3.47, p < .001] groups. Full demographics for these groups are reported in Table 1.
TABLE 1.
Participant Demographics
| dAnx |
Low BI |
High BI |
Test |
||
|---|---|---|---|---|---|
| (n = 78) | (n = 28) | (n = 27) | Statistic | Contrasts | |
| Demographics | |||||
| Age, y | |||||
| Mean | 13.22 | 12.96 | 13.09 | 0.15 | — |
| SD | 2.92 | 0.55 | 0.77 | ||
| Sex | |||||
| Female (%) | 49 (62.8) | 15 (53.6) | 17 (63.0) | χ2(2) = 0.81 | — |
| Male (%) | 29 (37.2) | 13 (46.4) | 10 (37.0) | ||
| BI score (z scored) | |||||
| Mean | — | −0.45 | 0.56 | 8.06*** | High > Low |
| SD | — | 0.50 | 0.42 | ||
| SCARED–Child | |||||
| Mean | 21.04 | 10.79 | 13.16 | 8.03*** | dAnx > Low |
| SD | 14.64 | 8.93 | 10.84 | dAnx >High | |
| SCARED–Parent | |||||
| Mean | 22.00 | 9.36 | 9.94 | 11.74*** | dAnx > Low |
| SD | 17.47 | 7.84 | 7.97 | dAnx >High | |
| SCARED–Average | |||||
| Mean | 21.26 | 10.07 | 11.55 | 13.08*** | dAnx > Low |
| SD | 13.78 | 7.52 | 7.78 | dAnx >High | |
| Additional Demographics | |||||
| IQ | |||||
| Mean | 113.03 | 116.64 | 117.89 | 1.67 | — |
| SD | 13.25 | 13.88 | 13.33 | ||
| Race | |||||
| White (%) | 43 (55.1) | 20 (71.4) | 18 (66.7) | χ2(8) = 7.57 | — |
| African American (%) | 19 (24.5) | 3 (10.7) | 3 (11.1) | ||
| Asian (%) | 5 (6.4) | 1 (3.6) | 2 (7.4) | ||
| Multiple race (%) | 10 (12.8) | 2 (7.1) | 3 (11.1) | ||
| Unknown (%) | 1 (1.2) | 2 (7.1) | 1 (3.7) | ||
| Combined Family Income | |||||
| <$24,999 (%) | 4 (5.1) | 0 | 0 | χ2(10) = 21.98** | |
| $25,000–$59,999 (%) | 9 (11.5) | 2 (7.1) | 2 (7.4) | ||
| $60,000–$89,999 (%) | 8 (10.3) | 4 (14.2) | 2 (7.4) | ||
| $90,000–$179,999 (%) | 35 (44.9) | 7 (25) | 7 (25.9) | ||
| >$180,000 (%) | 19 (24.4) | 7 (25) | 8 (29.6) | ||
| Unknown/missing | 3 (3.8) | 8 (28.6) | 8 (29.6) | ||
| Highest Level of Education (Household) | |||||
| <High school (%) | 4 (5.1) | 0 | 0 | χ2(12) = 14.86 | |
| High school (%) | 1 (1.3) | 1 (3.5) | 3 (11.1) | ||
| Partial college (%) | 9 (11.5) | 0 | 3 (11.1) | ||
| 4-Year college (%) | 16 (20.5) | 9 (32.1) | 5 (18.5) | ||
| Graduate/professional (%) | 47 (60.3) | 17 (60.7) | 15 (55.6) | ||
| Other/unknown (%) | 0 | 1 (3.5) | 1 (3.7) | ||
| Anxiety Diagnoses | |||||
| Generalized anxiety | |||||
| n (%) | 36 (92.3) | 3 (10.7) | 1 (3.7) | ||
| Social anxiety | |||||
| n (%) | 24 (61.5) | 1 (1.7) | 1 (3.7) | ||
| Separation anxiety | |||||
| n (%) | 18 (46.2) | — | — | ||
| Specific phobia | |||||
| n (%) | 21 (53.8) | 1 (1.7) | 1 (3.7) | ||
| Panic disorder | |||||
| n (%) | 1 (2.6) | — | — |
Note: Distributed anxiety (dAnx) cohort ranged from 8 to 18 years of age. The behavioral inhibition (BI) cohort ranged from 12 to 15 years of age. SCARED = Screen for Child Anxiety Related Emotional Disorders.
p < .01
p < .001.
One whole-brain group-level mixed-effects model (AFNI’s 3dMVM) was performed that contrasted errors of commission and correct responses (ie, Error Condition) only within incongruent trials, with anxiety severity (SCARED–Total) as a between-subjects continuous variable, and Group (Low BI, High BI, dAnx) as a between-subjects categorical variable. Age, number of error trials, and motion parameters were included as covariates. All continuous variables were automatically mean-centered (across groups) in 3dMVM. With a voxelwise probability threshold of p < .005 and familywise error rate of α =0.05, the cluster contiguity threshold was calculated at 1,078 mm3.
Analysis 2.
For this secondary analysis, a whole-brain analysis was run to map relations among BI in childhood, anxiety symptoms in adolescence, and neural correlates of error monitoring within the BI cohort (excluding all participants in the dAnx group). The whole-brain group-level mixed-effects model (AFNI’s 3dMVM) contrasted errors of commission and correct responses (ie, Error Condition) only within incongruent trials, with BI and anxiety levels treated as fully continuous between-subjects variables, and with age, motion, and number of error trials as covariates. With a voxelwise probability threshold of p < .005 and familywise error rate of α = 0.5, the cluster contiguity threshold was calculated at 1,078 mm3.
Analysis 3.
Finally, to map relations among anxiety and age with neural correlates of error processing in youths with and without current anxiety disorders (excluding the BI cohort), an additional whole-brain analysis was run. Similarly, this group-level mixed-effects model (AFNI’s 3dMVM) contrasted errors of commission and correct responses (ie, Error Condition) only within incongruent trials; however, Group and Age were included as between-subjects variables. Motion and number of error trials were entered as covariates. With a voxelwise probability threshold of p < .005 and familywise error rate of α = 0.05, the cluster contiguity threshold was calculated at 1,078 mm3.
Reliability Analysis
Given concern regarding the replicability of fMRI results,30 we also conducted an independent reliability study in 44 healthy individuals aged 8 to 40 years (mean = 20.60, SD = 8.73). All participants completed an identical flanker task twice on separate occasions (days between scans: mean = 54.37, SD = 11.97). Intraclass correlation coefficient (ICC) analyses were performed in AFNI’s LME program31 on two contrasts. First, one analysis considered the contrast of errors on incongruent trials > correct responses on incongruent trials, where subject and visit were entered as random variables in the model. Second, another analysis considered the contrast of correct incongruent > correct congruent trials. For both contrasts, ICCs were modeled with a 2-way mixed model with absolute agreement [ie, ICC (2,1)]. The initial ICC threshold value was set to 0.39, corresponding to a p < .005 threshold with 43 degrees of freedom.32 Reliability and cognitive control analyses are reported in Supplement 1 (available online) along with full methods and results (Tables S3-S7, available online).
Finally, we present the reliability data for the error-related contrast to highlight proportions of overlapping voxels between significant clusters in the reliability maps and in group maps from analyses 1 through 3. Both percent overlap and ICC value at the peak voxel of each cluster appear in tables for each study.
RESULTS
fMRI Analysis 1
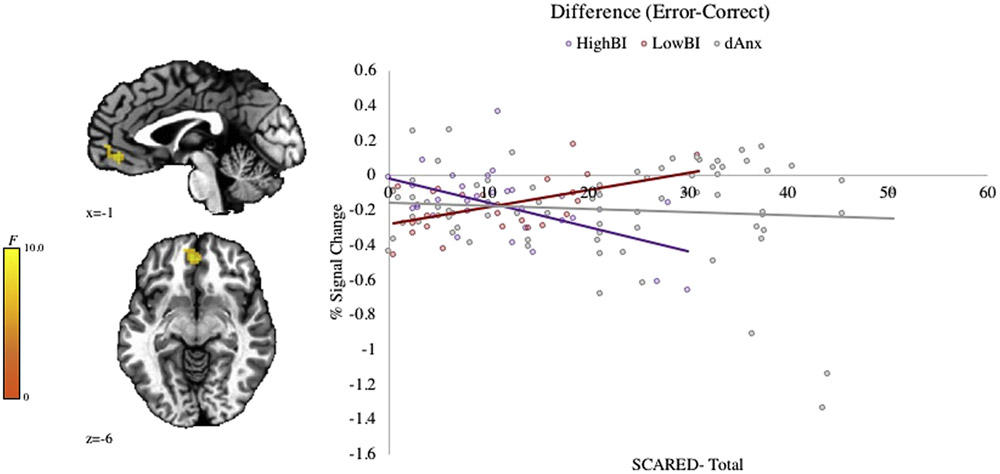
The full interaction (Group-by-Anxiety-by-Error Condition) revealed a significant cluster in the ventromedial prefrontal cortex (vmPFC) (Figure 2). This region overlapped with the reliability map. Follow-up tests demonstrate a positive correlation with error-related signal in the vmPFC and anxiety severity (SCARED–Total) in the Low BI group (r = 0.61, p = .003), whereas the opposite pattern is seen among individuals in the High BI group (r = −0.62, p = .001). Furthermore, the relationship between anxiety and error-related response in the vmPFC was not significant in the dAnx sample (r = −0.02, p = .88). For full imaging results, see Table S8, available online.
FIGURE 2. Neural Response to Error: The Impact of Anxiety Across Anxious Phenotypes.
Note: Results of a whole-brain analysis (p < .005, k < 1,078 mm3) reveal a significant Group (Low BI, High BI, dAnx) × Anxiety (SCARED) × Error interaction in the ventromedial prefrontal cortex (vmPFC; −4, 44, −6; k = 1,469 mm3). BI = behavioral inhibition; dAnx = distributed anxiety; SCARED = Screen for Child Anxiety Related Emotional Disorders.
fMRI Analysis 2
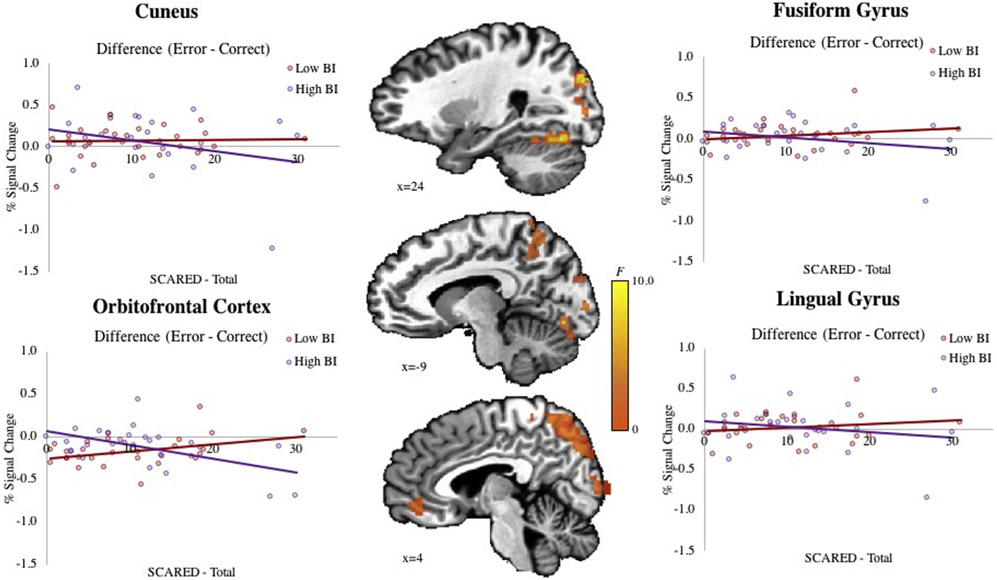
Significant BI-by-Anxiety-by-Error Condition interactions emerged in the cuneus, fusiform gyrus, lingual gyrus, orbitofrontal gyrus, and middle occipital gyrus (Figure 3 and Table S9, available online). All regions overlapped with the reliability map. To decompose interactions, signal from each region of interest was examined in post hoc tests using fully continuous variables. However, to facilitate interpretation, results are presented using the same median-split on BI scores presented in Analysis 1. All regions exhibited patterns similar to those seen in Analysis 1. In the Low BI group, anxiety scores positively correlated with activation during error-versus-correct trials, whereas in the High BI group, anxiety severity negatively correlated with such response patterns.
FIGURE 3. Neural Response to Error in Behaviorally Inhibited Children.
Note: Results of a whole-brain analysis (p < .005, k < 1,078 mm3) show significant BI × Anxiety × Error interactions in the cuneus (24, −84, 29; k = 13,703 mm3), fusiform gyrus (24, −69, −16; k = 7,484 mm3), lingual gyrus −9, −76, −9; k = 3,922 mm3), orbitofrontal cortex (−4, 44, −9; k = 1,484 mm3), and middle occipital gyrus (29, −79, 11; k = 1,078 mm3). BI = behavioral inhibition.
fMRI Analysis 3
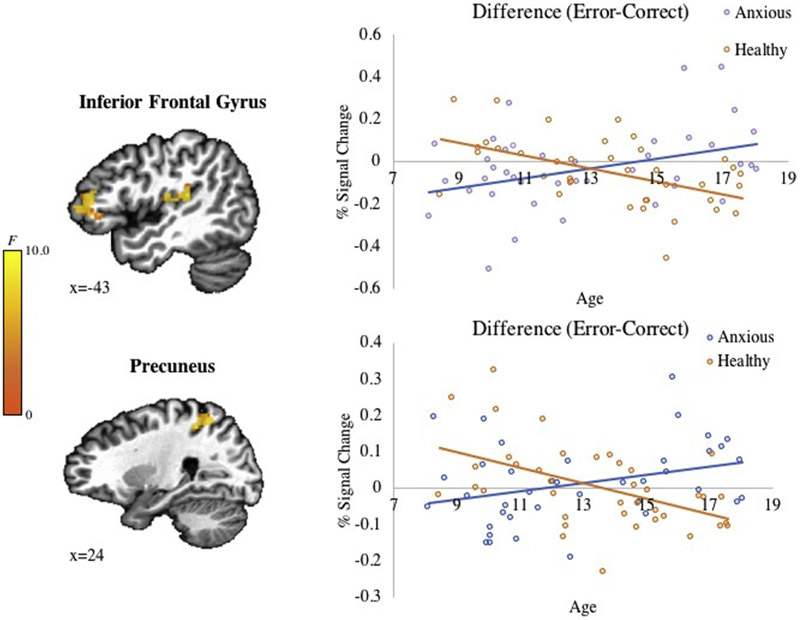
Significant findings emerged in the inferior frontal gyrus (IFG), precuneus, and superior temporal gyrus (STG)/insula for the full interaction (Group-by-Age-by-Error Condition) (Figure 4). All regions showed overlapping voxels with the reliability map. Follow-up tests showed that these three-way interactions reflected negative correlations with age in healthy participants on error-versus-correct trials in the IFG (r = −0.52, p = .001) and precuneus (r = −0.50, p = .001). The STG/Insula showed the same pattern but did not reach significance (r = −0.31, p = .06). In contrast, clinically anxious participants exhibited significant (IFG: r = 0.41, p = .01; STG/insula: r = 0.51, p = .001) or nonsignificant (precuneus r = 0.30, p = .07) positive correlations with age. Full results are provided in Table S10, available online.
FIGURE 4. Neural Response to Error in Pediatric Anxiety.
Note: Results of a whole-brain analysis (p < .005, k < 1,078 mm3) demonstrate significant Group × Age × Error interactions in the inferior frontal gyrus (−46, 41, 4; k = 1,297 mm3), precuneus (24, −44, 46; k = 1,297 mm3), and superior temporal gyrus (−44, −31, 14; k = 1,141 mm3).
DISCUSSION
The main result from the current study emerges from Analysis 1, in two pediatric cohorts. Specifically, anxiety severity relates to error-related patterns of activation in the vmPFC among individuals characterized based on their levels of early-childhood BI. However, anxiety severity is not associated with such patterns in adolescents recruited based on their current level of anxiety severity (dAnx). Secondary analyses in the BI cohort reveal anxiety-related patterns in other brain regions resembling the pattern in vmPFC. Secondary analyses in the dAnx cohort show age to moderate the relationship between anxiety and neural response to errors in various brain regions.
Analysis 1 showed that, in youths with histories of low or high BI levels, current anxiety severity predicts unique patterns of vmPFC engagement following errors. These patterns do not relate to anxiety in youths recruited based on the presence or absence of an anxiety disorder requiring treatment. Such findings suggest that patterns of aberrant error processing may relate to anxiety symptoms in particular developmental contexts. Moreover, when combined with other research, this finding suggests that pediatric anxiety involves multiple components with varying developmental profiles. Some such components, such as patterns of error processing, may manifest uniquely when anxiety arises in the context of early-life BI. However, other components may manifest similarly when anxiety is diagnosed in adolescence, with or without a history of BI. For example, anxiety predicts similar patterns of aberrant threat processing in children with a history of BI and in youths recruited when they seek treatment.5,6 Thus, as compared to other pathways into anxiety, an anxiety pathway in behaviorally inhibited children may contain both shared and phenotype-specific substrates that differentiate this BI-related pathway from other anxiety pathways. This suggests that heterogeneity in anxiety emerges from multiple pathophysiological processes that show unique associations with BI temperament.
Additional insights might arise from considering specific error-processing patterns in the current study. Participants characterized as High BI showed a negative correlation between anxiety severity and activation in the vmPFC during errors, such that subjects who are high in both BI and anxiety exhibit particularly low activation after an error, compared to subjects high in BI but low in anxiety. The opposite pattern was found in the Low BI group, with higher activation relating to higher levels of anxiety. A recent meta-analysis33 implicates the vmPFC in the integration of affective signals from subcortical-to-cortical regions. From this perspective, anxiety in children with BI relates to perturbations in the ability of vmPFC to incorporate subcortical error-related signals. These findings were further extended in Analysis 2, which eliminated the dAnx group. This secondary analysis revealed several posterior brain regions, including the cuneus, fusiform, and occipital gyrus, as well as the orbitofrontal cortex, where patterns resembled those found for vmPFC in Analysis 1. Of note, beyond the vmPFC, none of these regions emerged in the direct comparisons between BI and the dAnx group. Nevertheless, the presence of comparable patterns may implicate these regions along with the vmPFC in a pathway into anxiety symptoms arising specifically in youths with a history of BI.
Although no similar studies use fMRI to evaluate error processing in BI, the current findings do differ from findings in electrophysiological research. This research consistently finds larger neural response to errors in BI youths with high relative to low levels of anxiety.11,14 The current findings, for fMRI, reveal the opposite patterns: namely, larger neural response to errors in non-BI youths with high relative to low levels of anxiety. Such discrepancies may be due to methodological differences between fMRI and electroencephalography. That is, not only do the temporal and spatial properties of these two modalities differ, but they are also thought to assess different sources of neural activity.34 However, taken together, both the current and past findings relate aberrant error processing to variations in early-childhood temperament and adolescent anxiety.
Findings from Analyses 1 and 2 suggest that youths identified as BI demonstrate a unique neural profile following an error. Importantly, such findings differ from findings in youths with anxiety symptoms seen in other contexts, including those seeking treatment for anxiety disorders. These findings, like other research on information processing in anxiety, may inform phenotype-specific treatments. For instance, work on threat-processing biases finds similar neural correlates in BI youths with high anxiety and youths seeking treatment for anxiety disorders. In this case, similar forms of treatments targeting such threat-processing biases might be appropriate for both groups of anxious individuals.35,36 However, the emergence of phenotype-specific patterns during error processing suggests that these groups may respond differently to treatments aimed at reducing error perseverance. Thus, different therapeutic decisions may be warranted based on the presence or absence of early childhood BI.
Analysis 3 uses only the dAnx group after eliminating the cohort recruited based on early life BI history. A few features speak to the importance of findings from this analysis. For example, prior studies have found age to moderate the relationship between anxiety and error processing.16 The presence of findings resembling results in past research suggests that study methodology was sensitive to some anxiety-related associations. Moreover, the pattern in these findings was also notable. Specifically, Analysis 3 revealed older youths with anxiety to show increased IFG, precuneus, and STG/insula activation during errors, as compared to their nonanxious counterparts. For younger children, the opposite pattern emerged, whereby youths with anxiety showed decreased response to errors compared to healthy controls. Similar-appearing age-related differences in brain function in youths with anxiety have been documented in prior work. For instance, prior ERP research shows greater neural response to errors in older, but not younger, anxious youths.16
The present study has limitations. First, the direct comparison between BI and pediatric anxiety are limited by sample-specific restrictions. Most evident is our inability to quantify childhood BI in our treatment-seeking youths. Research identifies individuals as BI using many metrics such as observation, parent-report, and/or retrospective self-report. However, our studies use a stringent measure that includes both observational and parent-reported data acquired prospectively over the first 3 years of life, thereby focusing on early-identified BI. Because our measure of BI is quantified from infancy through early childhood, this information could not be obtained from the NIMH-recruited cohort, who were enrolled based on the presence or absence of anxiety requiring treatment. Conversely, it was not possible to study a large sample of medication-free, treatment-seeking youths in the BI cohort. In this cohort, anxiety disorders emerged gradually over time and were treated as they intensified, at time points distributed throughout childhood and adolescence. This makes some behaviorally inhibited children ineligible for our studies.
Furthermore, the relation between BI and anxiety symptoms in the sample of BI participants in the current analysis is weaker than that of the larger cohort12 and similar cohorts.3 It is possible that our exclusions, the relatively young age of the sample, or biased attrition resulted in a relatively weak correlation. Prior work suggests that enhanced error response increases risk for developing an anxiety disorder at later ages among youths with BI.12-14,37 Therefore, it remains unclear whether we would find similar phenotype-specific patterns in a BI sample with higher, clinical levels of anxiety. In addition, our BI sample had a restricted age range compared to our clinically anxious sample (although the ages were not significantly different). It is possible that during specific developmental periods, these two phenotypes show similar patterns of response during error processing. Moreover, cross-cohort differences (eg, age range, anxiety severity) may make the current results vulnerable to confound. For instance, the level of anxiety in subjects used for Analysis 2 was elevated but subclinical. Exploring patterns of brain function in a larger longitudinal sample, recruited based on both BI and anxiety, would strengthen our ability to look at BI at more clinically relevant levels and would allow us to examine age effects in the BI sample.
Finally, similar to other studies of cognitive control in youths with anxiety, we failed to find clear anxiety-related differences in task behavior (eg, number of errors8-10). Although this limits our ability to discuss group differences in the ability to engage control behaviors, the current article’s focus on error processing is not affected by the lack of behavioral differences. Despite these limitations, the current investigation’s demonstration of different neural patterns between these phenotypes provides novel insights into the heterogeneity of anxiety pathophysiology. However, more fMRI research in BI youths is needed to tease apart relations among childhood BI, anxiety in adolescence, and neural correlates of error processing.
The current article also provides a separate analysis of the test–retest reliability of neural responses to errors. Neuroimaging experts view test–retest analyses as a critical step toward reproducible findings.30 However, application of these analyses to other imaging findings is relatively uncharted territory. The present reliability sample includes only healthy individuals because of ethical considerations of withholding treatment (ie, medication and/or cognitive-behavioral therapy) from treatment-seeking patients. Although this design is not ideal for comparing anxiety-related differences, it has been successful in other domains38 and may be similarly helpful in the current instance.
The current findings highlight distinct neural correlates between the phenotypes of pediatric anxiety with and without childhood BI. Unlike research on threat processing, finding similar neural responses across these two phenotypes, the current study demonstrates that these two populations appear to display different response patterns during error processing. This set of findings suggests that anxiety is a heterogeneous construct, whereby some subcomponents (eg, threat processing) are shared across different phenotypes of anxiety, whereas others (eg, error processing) are not.
Supplementary Material
Acknowledgments
Funding for this manuscript was provided by the National Institute on Mental Health (NIMH) Intramural Research Program (Principal Investigator [PI]: Daniel S. Pine) and MH093349 (PI: Nathan A. Fox) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) P01HD064653 (PI: Nathan A. Fox).
Footnotes
Disclosure: Dr. Buzzell has served as PI on a grant from the Bill and Melinda Gates Foundation ($80,000 Optimizing Prediction of Infant Brain Health Outcomes Through Advanced EEG Signal Processing and Network Analysis). Drs. Smith, White, Leibenluft, Haller, Fox, Pine, and Mss. McGlade and Heckelman have reported no biomedical financial interests or potential conflicts of interest.
Contributor Information
Ashley R. Smith, National Institute of Mental Health, Bethesda, Maryland..
Lauren K. White, Lifespan Brain Institute, Children’s Hospital of Philadelphia, Pennsylvania..
Ellen Leibenluft, National Institute of Mental Health, Bethesda, Maryland..
Anastasia L. McGlade, University of California, Los Angeles..
Adina C. Heckelman, Mailman School of Public Health, Columbia University, New York..
Simone P. Haller, National Institute of Mental Health, Bethesda, Maryland..
George A. Buzzell, University of Maryland, College Park..
Nathan A. Fox, University of Maryland, College Park..
Daniel S. Pine, National Institute of Mental Health, Bethesda, Maryland..
REFERENCES
- 1.Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J Am Acad Child Adolesc Psychiatry. 2012;51:1066–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buzzell GA, Toller-Renfree SV, Barker TV, et al. A neurobiological mechanism linking behaviorally inhibited temperament and later adolescent social anxiety. J Am Acad Child Adolesc Psychiatry. 2017;56:1097–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronis-Tuscano A, Degnan KA, Pine DS, et al. Stable, early behavioral inhibition predicts the development of social anxiety disorder in adolescence. J Am Acad Child Adolesc Psychiatry. 2009;49:928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J Am Acad Child Adolesc Psychiatry. 1999; 38:1008–1015. [DOI] [PubMed] [Google Scholar]
- 5.Hardee JE, Benson BE, Bar-Haim Y, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013;74:273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.White LK, Sequeira S, Britton JC, et al. Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry. 2017;174:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40:207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald KD, Liu Y, Stern ER, et al. Reduced error-related activation of dorsolateral prefrontal cortex across pediatric anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2013;52:1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladouceur CD, Tan PZ, Sharma V, et al. Error-related brain activity in pediatric anxiety disorders remains elevated following individual therapy: a randomized clinical trial. J Child Psychol Psychiatry. 2018;59:1152–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. J Child Psychol Psychiatry. 2006;47:1073–1082. [DOI] [PubMed] [Google Scholar]
- 11.Meyer A, Hajcak G, Torpey-Newman D, et al. Early temperamental fearfulness and the developmental trajectory of error-related brain activity. Dev Psychobiol. 2018;60: 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Henderson HA, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J Am Acad Child Adolesc Psychiatry. 2014;53:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamm C, Walker OL, Degnan KA, et al. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev Sci. 2014;17:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol Psychiatry. 2009;65:445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McTeague LM, Huemer J, Carreon DM, Jiang Y, Eickhoff SB, Etkin A. Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry. 2017;174:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer A, Weinberg A, Klein DN, Hajcak G. The development of the error-related negativity (ERN) and its relationship with anxiety: evidence from 8 to 13 year-olds. Dev Cogn Neurosci. 2012;2:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moran TP. Anxiety and working memory capacity: a meta-analysis and narrative review. Psychol Bull. 2016;142:831–864. [DOI] [PubMed] [Google Scholar]
- 18.Herting MM, Gautam P, Chen Z, Mezher A, Vetter NC. Test-retest reliability of longitudinal task-based fMRI: implications for developmental studies. Develop Cogn Neuro. 2018;33:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADSPL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 20.Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545–553. [DOI] [PubMed] [Google Scholar]
- 21.Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001; 72:1–21. [DOI] [PubMed] [Google Scholar]
- 22.White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. J Abnorm Child Psychol. 2011;39:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahat A, Walker OL, Lamm C, Degnan KA, Henderson HA, Fox NA. Cognitive conflict links behavioral inhibition and social problem solving during social exclusion in childhood. Infant Child Dev. 2014;23:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 25.Gehring W, Liu Y, Orr J, Carp J. The error-related negativity (ERN/Ne) In: Luck SK, Kappenman E, eds. Oxford Handbook of Event-Related Potential Components. New York, NY: Oxford University Press; 2012:231–291. [Google Scholar]
- 26.Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. [DOI] [PubMed] [Google Scholar]
- 27.Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psych Rev. 2004;111:931–959. [DOI] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 29.Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7:152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poldrack RA, Farah MJ. Progress and challenges in probing the human brain. Nature. 2015;526:371–379. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013;73:176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. [DOI] [PubMed] [Google Scholar]
- 33.de la Vega A, Chang LJ, Banich MT, Wager TD, Yarkoni T. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J Neurosci. 2016; 15(36):6553–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He B, Liu Z. Multimodal functional neuroimaging: integrating functional MRI and EEG/MEG. IEEE Rev Biomed Eng. 2008;1:23–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abend R, Swetlitz C, White LK, et al. Levels of early-childhood behavioral inhibition predict distinct neurodevelopmental pathways to pediatric anxiety. Psychol Med. 2019. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White LK, Sequeira S, Britton JC, et al. Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry. 2017;174:775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer A, Hajcak G, Torpey-Newman DC, Kujawa A, Klein DN. Enhanced error-related brain activity in children predicts the onset of anxiety disorders between the ages of 6 and 9. J Abnorm Psychol. 2015;124:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White LK, Britton JC, Sequeira S, et al. Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage. 2016;136:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.