Supplemental Digital Content is available in the text.
Keywords: brain diseases, coma, coronavirus disease 2019, critical illness, delirium, respiratory insufficiency
Abstract
Objectives:
To determine delirium occurrence rate, duration, and severity in patients admitted to the ICU with coronavirus disease 2019.
Design:
Retrospective data extraction study from March 1, 2020, to June 7, 2020. Delirium outcomes were assessed for up to the first 14 days in ICU.
Setting:
Two large, academic centers serving the state of Indiana.
Patients:
Consecutive patients admitted to the ICU with positive severe acute respiratory syndrome coronavirus 2 nasopharyngeal swab polymerase chain reaction test from March 1, 2020, to June 7, 2020, were included. Individuals younger than 18 years of age, without any delirium assessments, or without discharge disposition were excluded.
Measurements and Main Results:
Primary outcomes were delirium rates and duration, and the secondary outcome was delirium severity. Two-hundred sixty-eight consecutive patients were included in the analysis with a mean age of 58.4 years (sd, 15.6 yr), 40.3% were female, 44.4% African American, 20.7% Hispanic, and a median Acute Physiology and Chronic Health Evaluation II score of 18 (interquartile range, 13–25). Delirium without coma occurred in 29.1% of patients, delirium prior to coma in 27.9%, and delirium after coma in 23.1%. The first Confusion Assessment Method for the ICU assessment was positive for delirium in 61.9%. Hypoactive delirium was the most common subtype (87.4%). By day 14, the median number of delirium/coma-free were 5 days (interquartile range, 4–11 d), and median Confusion Assessment Method for the ICU-7 score was 6.5 (interquartile range, 5–7) indicating severe delirium. Benzodiazepines were ordered for 78.4% of patients in the cohort. Mechanical ventilation was associated with greater odds of developing delirium (odds ratio, 5.0; 95% CI, 1.1–22.2; p = 0.033) even after adjusting for sedative medications. There were no between-group differences in mortality.
Conclusions:
Delirium without coma occurred in 29.1% of patients admitted to the ICU. Delirium persisted for a median of 5 days and was severe. Mechanical ventilation was significantly associated with odds of delirium even after adjustment for sedatives. Clinical attention to manage delirium duration and severity, and deeper understanding of the virus’ neurologic effects is needed for patients with coronavirus disease 2019.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or novel coronavirus disease 2019 (COVID-19) has emerged as a global pandemic and is associated with rapid spread, severe respiratory failure, and significant morbidity and mortality (1, 2). As clinical experience with COVID-19 grows, neurologic manifestations of the disease are receiving increased attention. A recently published small case series from France reported delirium occurred in 26 of 40 patients (65%) admitted to the ICU with COVID-19 (3). However, the duration and severity of delirium in critically ill COVID-19 patients have not been well described.
Delirium is a serious neurologic syndrome independently associated with longer duration of mechanical ventilation, prolonged ICU and hospital stays, increased mortality, and institutionalization after discharge (4–8). Increasing levels of delirium severity and duration amplify these outcomes and are independently associated with worsening cognitive and functional outcomes post discharge (9–12). Prior to COVID-19, the prevalence of delirium in mechanically ventilated patients has been decreasing from a historically high rate of 80% to a range of 16.5–33% (13–19). In the setting of the current global health crisis, hospital resources have been stretched to their limits to meet the needs of a large number of critically ill patients. The unintended impact of limited resources on clinical practice has raised concerns that current ICU delirium rates have returned to the historically high levels (20–22). As of August 9, 2020, there are over 5 million confirmed COVID-19 cases in the United States and approximately 12% of COVID-19 patients required ICU level care (1, 2, 23). In this context, delirium is likely to pose a long-term public health challenge if rates in the United States are as high as recently reported in France.
Therefore, we conducted this study at two large academic health systems in the urban Midwest to measure incidence of delirium, delirium duration, and delirium severity and investigate risk factors associated with delirium in critically ill patients admitted with COVID-19.
MATERIALS AND METHODS
The observational, retrospective data extraction study was conducted at two large, urban, academic, level I trauma centers (Indiana University Health Methodist Hospital and Eskenazi Health) serving as major referral hospitals for the state of Indiana and affiliated with Indiana University School of Medicine (Indianapolis, Indiana). Methodist Hospital is an 802-bed quaternary care referral center with an average of 150 ICU admissions per month. Eskenazi Health is a 336-bed safety net hospital with an average of 120 ICU admissions per month. The study received ethical approval from the Institutional Review Board at Indiana University (April 17, 2020, categorized as exempt, number 2004316653). All consecutive patients admitted to the ICUs of Methodist Hospital and Eskenazi Health with a positive result by SARS-CoV-2 nasopharyngeal swab polymerase chain reaction test from March 1, 2020, to June 7, 2020, were included in the electronic health record data abstraction. Exclusion criteria were as follows: patients under the age of 18, admitted after June 7, 2020, patients with no delirium assessments recorded in the electronic medical record for the duration of the follow-up period, and patients remaining admitted at the end of the study follow-up period (i.e., August 8, 2020).
Exposures and Outcomes
The main exposure variables were patients’ demographics, comorbidities, laboratory results, and severity of illness at admission. The primary outcomes were rate of delirium and delirium/coma duration during the first 14 days of admission to the ICU. Delirium/coma duration was defined by the number of days the patient was alive and free from delirium or coma by day 14. Patients who were discharged from the ICU prior to 14 days did not have subsequent delirium or coma assessments performed outside the ICU. Coma was assessed using the Richmond Agitation-Sedation Scale (RASS) and delirium was identified through the Confusion Assessment Method for the ICU (CAM-ICU). Coma was defined as a RASS score of –4 or –5, making patients ineligible for a CAM-ICU screening, while patients with a RASS score of –3 or greater were eligible for a CAM-ICU assessment (24, 25). The CAM-ICU score was determined by examining the patient for 1) acute or fluctuating changes in mental status, 2) inattention, 3) altered level of consciousness, and 4) disorganized thinking. Patients were considered delirious if they displayed acute or fluctuating changes in mental status and inattention, plus altered level of consciousness, and/or disorganized thinking on the CAM-ICU. ICU nurses administered the RASS and CAM-ICU bid (around 07:00 to 08:00, and then between 19:00 and 20:00) to measure level of consciousness and delirium, respectively. These standardized and validated screening tools were implemented in our healthcare systems in 2011 and are normally obtained throughout the ICU stay. Hyperactive delirium was defined as a RASS score of +1 to +4 at the time of positive CAM-ICU, and hypoactive delirium was defined as a RASS score –3 to 0 with a positive CAM-ICU score. The secondary outcome of delirium severity was assessed using the CAM-ICU-7, which requires all components of the CAM-ICU to be assessed for each patient rather than a dichotomous CAM-ICU positive or negative result. The CAM-ICU-7 was implemented into the electronic medical record at Eskenazi Health in 2017 and is assessed bid in the subset of patients receiving care at this hospital site. CAM-ICU-7 scores range from 0 to 7, with 0–2 indicating no delirium, 3–5 mild to moderate delirium, and 6–7 as severe delirium (12).
Data Collection
Research assistants familiar with electronic medical systems at the hospitals (Cerner PowerChart, Epic Health Systems) retrospectively abstracted study data from the medical record, including CAM-ICU assessments performed by clinical nurses, and results were entered directly into an electronic Research Electronic Data Capture database. Data obtained from the medical record included patient demographics (age, gender, self-reported race), insurance status, comorbidities, vital signs, laboratory and imaging results (within 24 hr of ICU admission), level of consciousness (RASS), date/time, and results of delirium assessments for up to the first 14 days of ICU stay (overall CAM-ICU positive or negative, including CAM-ICU features as applicable: altered mental status, disorganized thinking, altered level of consciousness, disorganized thinking, and CAM-ICU-7 scores), SARS-CoV-2 test results, medication orders, and dates of admission and discharge from hospital and ICU. Date of death during the hospitalization was also recorded including level of care at time of death (ICU vs non-ICU). Comorbidities are presented as Charlson Comorbidity Index using diagnoses lists documented in the medical record. Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated using laboratory values, vital signs, and neurologic assessments from first 24 hours of ICU admission.
Statistical Analysis
Demographic and clinical characteristics were compared between patients who had delirium positive and those without delirium using two-sample t tests (normal data) and Wilcoxon rank-sum tests (skewed data) for continuous outcomes or Fisher exact test for categorical variables. Summary statistics, including median and interquartile range (IQR), were provided for patients with delirium. Logistic regression was used including demographic or clinical characteristics that were significantly different between patients with delirium and those without delirium as independent variables to identify factors associated with delirium.
RESULTS
A total of 301 consecutive patients with COVID-19 were admitted from March 1, 2020, to June 7, 2020, to the ICUs at the two hospital systems. We excluded 33 patients, 32 did not have any delirium assessments and one remained admitted at the end of the follow-up period (eFig. 1, Supplemental Digital Content, http://links.lww.com/CCX/A439). In total, 268 patients comprised the study cohort. Demographics and clinical characteristics for the cohort are presented in Table 1 and eTable 1 (Supplemental Digital Content, http://links.lww.com/CCX/A439). The mean age of the cohort was 58.4 years (sd, 15.6 yr), 40.3% were female, 44.4% African American, and 20.7% Hispanic, 27.3% used commercial insurance, and 23.2% Medicare. The median Charlson Comorbidity Index score was 1 (IQR, 0–2), with hypertension (60.1%), obesity (57.5%), tobacco use (25.7%), and chronic lung disease (20.9%) the most frequent comorbid conditions. The median APACHE II score was 18 (IQR, 13–25), and 80.2% of patients in the cohort underwent invasive mechanical ventilation. Cerebrovascular accident (ischemic or hemorrhagic) was identified in seven of 268 patients.
TABLE 1.
Characteristics of Critically Ill Patients Admitted With Coronavirus Disease 2019 (n = 268)
| Demographic/Clinical Characteristic | Total (n = 268) | Delirium Positivea (n = 215) | Delirium Negativea (n = 53) | p |
|---|---|---|---|---|
| Age, mean (sd) | 58.4 (15.6) | 59.4 (15.0) | 54.3 (17.3) | 0.034 |
| Age, stratified, n (%) | 0.053 | |||
| 18–49 | 77 (28.7) | 57 (26.5) | 20 (37.7) | |
| 50–64 | 88 (32.8) | 68 (31.6) | 20 (37.7) | |
| 65+ | 103 (38.4) | 90 (41.9) | 13 (24.5) | |
| Sex, n (%) | ||||
| Female | 108 (40.3) | 90 (41.9) | 18 (34.0) | 0.349 |
| Race, n (%) | 0.499 | |||
| African American | 118 (44.4) | 93 (43.7) | 25 (47.2) | |
| Caucasian | 76 (28.6) | 59 (27.7) | 17 (32.1) | |
| Hispanic | 55 (20.7) | 45 (21.1) | 10 (18.9) | |
| Other | 17 (6.4) | 16 (7.5) | 1 (1.9) | |
| Insurance, n (%) | 0.176 | |||
| Medicare | 62 (23.2) | 50 (23.4) | 12 (22.6) | |
| Medicaid | 45 (16.9) | 40 (18.7) | 5 (9.4) | |
| Medicare and Medicaid | 34 (12.7) | 30 (14.0) | 4 (7.5) | |
| Commercial | 73 (27.3) | 54 (25.2) | 19 (35.8) | |
| Self-pay | 33 (12.4) | 23 (10.7) | 10 (18.9) | |
| Other | 20 (7.5) | 17 (7.9) | 3 (5.7) | |
| Comorbidities, n (%) | ||||
| Hypertension | 161 (60.1) | 133 (61.9) | 28 (52.8) | 0.273 |
| Diabetes | 111 (41.4) | 89 (41.4) | 22 (41.5) | 1.000 |
| Obesity, body mass index (calculated with height and weight at admission) > 30 | 142 (57.5) | 114 (57.3) | 28 (58.3) | 1.000 |
| Tobacco use | 69 (25.7) | 55 (25.6) | 14 (26.4) | 1.000 |
| Chronic obstructive pulmonary disease or asthma | 56 (20.9) | 45 (20.9) | 11 (20.8) | 1.000 |
| Chronic kidney diseaseb | 42 (15.7) | 37 (17.2) | 5 (9.4) | 0.207 |
| Chronic heart failure | 34 (12.7) | 27 (12.6) | 7 (13.2) | 1.000 |
| Cardiac artery disease | 30 (11.2) | 27 (12.6) | 3 (5.7) | 0.223 |
| Dementia | 9 (3.4) | 0 (0.0) | 9 (4.2) | 0.212 |
| Charlson Comorbidity Index | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 0.407 |
| Acute Physiology and Chronic Health Evaluation IIc | 18.0 (13.0–25.0) | 20.0 (15.0–26.0) | 11.0 (9.0–16.0) | < 0.001 |
| Laboratory values, clinical and respiratory characteristics, median (interquartile range) or n (%)d | ||||
| WBC count × 109/L | 9.0 (6.5–12.7) | 9.4 (6.5–13.3) | 7.5 (6.5–10.0) | 0.067 |
| Glasgow Coma Scale (0–15)c | 10.0 (6.0–15.0) | 9.0 (6.0–14.0) | 15.0 (14.0–15.0) | < 0.001 |
| Richmond Agitation-Sedation Scale (14 d)c,e | –1.5 (–2.5 to –0.3) | –2.0 (–2.8 to –1.0) | 0.0 (0.0–0.0) | < 0.001 |
| Pao2, mm Hg | 70.0 (56.0–89.0) | 69.5 (56.5–89.0) | 73.5 (53.0–85.0) | 0.959 |
| Pao2:Fio2 ratioc | 87.0 (64.0–127.8) | 81.8 (62.0–121.0) | 103.1 (91.7–185.7) | 0.001 |
| Invasive mechanical ventilationc | 215 (80.2) | 201 (93.5) | 14 (26.4) | < 0.001 |
| Presence of shock | 47 (17.5) | 45 (20.9) | 2 (3.8) | 0.002 |
aDelirium status determined by a positive Confusion Assessment Method for the ICU clinical assessment.
bChronic kidney disease includes end-stage renal disease.
cSeverity of illness, Glasgow Coma Scale, Pao2:Fio2, Richmond Agitation-Sedation Scale (RASS) scores, and percent mechanically ventilated were significantly different (p < 0.05) between groups (no delirium/delirium) in univariate analysis.
dLaboratory values, clinical, respiratory characteristics, and severity of illness measurements represent data from first 24 hr of ICU admission.
eRASS scores were calculated using scores for up to the first 14 d of ICU admission.
Delirium in Critically Ill Patients With COVID-19
Delirium without coma occurred in 29.1% (78/268), delirium prior to coma in 27.9% (75/268), and delirium after coma in 23.1% (62/268) of the cohort. In patients with delirium, 61.9% (133/215) were positive on the first CAM-ICU assessment. As shown in Table 1, patients with delirium had higher median APACHE II severity of illness scores (20, IQR 15–26 vs 11.0, IQR 9–16; p < 0.001) and were more likely to be mechanically ventilated (93.5% vs 26.4%; p < 0.001) than patients without delirium. There were lower Pao2:Fio2 ratios (81.8, IQR 62–121 vs 103.1, IQR 91.7–185.7; p = 0.001) and lower Glasgow Coma Scale scores (9.0, IQR 6.0–14.0 vs 15.0, IQR 14–15.0; p < 0.001) in patients with delirium during the first 24 hours of ICU admission compared with those without delirium. Decreased level of consciousness was found in patients with delirium (median RASS score: –2.0, IQR–2.8 to –1.0 vs 0, IQR 0–0) compared with those without delirium (Table 1).
Delirium Duration, Subtypes of Delirium, and Delirium Severity
As shown in Table 2, patients in the cohort had median 5 (IQR, 2–9) delirium/coma-free days by day 14 with median delirium duration of 5 days (IQR, 2–8 d). Patients had a median RASS of –2 (IQR, –3 to 0) at the time of ICU admission indicating light sedation. Figure 1 shows the daily rates of patient’s delirium, coma, or delirium/coma-free status for up to 14 days of ICU admission. In our study, hypoactive delirium occurred in 87.4% of patients on the first CAM-ICU assessment, and the median duration of hypoactive delirium was 4 days (IQR, 2–6 d). Details of the subtypes of delirium for the entire cohort are shown in Table 2 and Figure 2. In the subset of patients with delirium severity assessments (n = 134), the median CAM-ICU-7 score was 6.5 (IQR, 5–7) representing severe delirium.
TABLE 2.
Clinical Outcomes in Critically Ill Patients Admitted With Coronavirus Disease 2019 Who Developed Delirium
| Outcome Measures | Patients With Delirium (n = 215) |
|---|---|
| Duration of delirium and coma, median (IQR)a | |
| Delirium/coma-free days by day 14 | 5 (2–9) |
| Duration of delirium, median (IQR) | 5 (2–8) |
| Duration of coma, median (IQR) | 1 (0–5) |
| Subtypes of delirium, n (%) | |
| Hypoactive delirium at first ICU assessmentb | 188 (87.4) |
| Hyperactive delirium at first ICU assessmentc | 27 (12.6) |
| Duration of subtypes of delirium, median days (IQR) | |
| Hypoactive delirium duration days | 4 (2–6) |
| Hyperactive delirium duration days | 0 (0–1) |
| Delirium severity, median (IQR)d | |
| Confusion Assessment Method for the ICU-7 score | 6.5 (5–7) |
IQR = interquartile range.
aDelirium was defined as a positive Confusion Assessment Method for the ICU (CAM-ICU) assessment in the patient medical record for up to 14 d during their ICU coronavirus disease 2019 stay. Coma was defined by Richmond Agitation-Sedation Scale (RASS) score of –4 or –5. Delirium/coma-free days: defined as number of days patient was alive and free of delirium or coma by day 14. Delirium was defined by CAM-ICU positive on either morning or afternoon assessment for up to 14 d while admitted to the ICU. Duration of coma was defined as number of days patient had coma by RASS score on either morning or afternoon assessment for up to 14 d of ICU stay.
bHypoactive delirium was defined by RASS of –1 to –3 with positive CAM-ICU.
cHyperactive delirium was defined by a RASS score of +1 to +3 with positive CAM-ICU.
dDelirium severity was measured using the CAM-ICU-7 in 134 patients (0–7, 0–2: no delirium; 3–5: mild to moderate delirium; and 6–7: severe delirium).
Missing data: There were 2,861 possible days of delirium follow-up, and 2,257 d had complete assessments. The remaining 604 d had incomplete assessment data as follows: 1) 157 d with no CAM or RASS (n = 98 patients) and 2) 447 d where RASS was other than –4 or –5, but CAM-ICU assessment was missing (n = 144). All patients had at least one RASS assessment and at least one CAM-ICU assessment.
Figure 1.
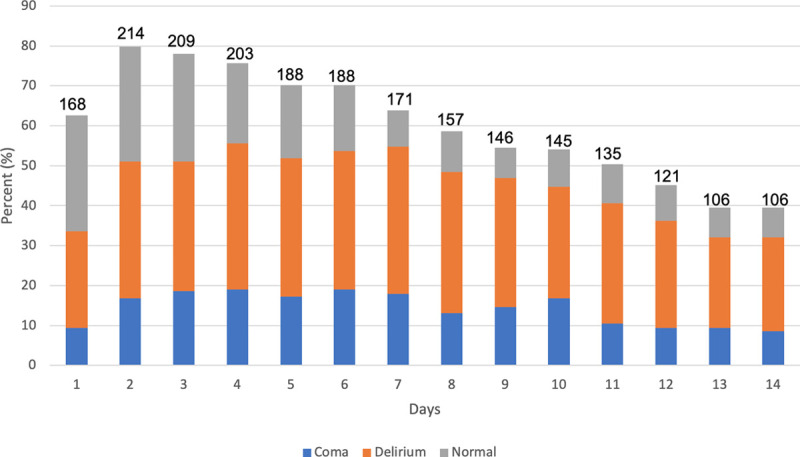
Daily rates of delirium, coma, or without delirium/coma status as assessed up to first 14 d of ICU stay (n = 268). Number above each bar column indicates number of patients assessed per day. Daily percentages do not equal 100% due to incomplete assessments, death, or discharge from ICU. Delirium was defined as a positive Confusion Assessment Method for the ICU (CAM-ICU) assessment on either morning or afternoon assessment. Coma was defined by Richmond Agitation-Sedation Scale (RASS) score of –4 or –5. Without delirium or coma was defined by RASS greater than –4 and a negative CAM-ICU on either morning or afternoon assessment.
Figure 2.
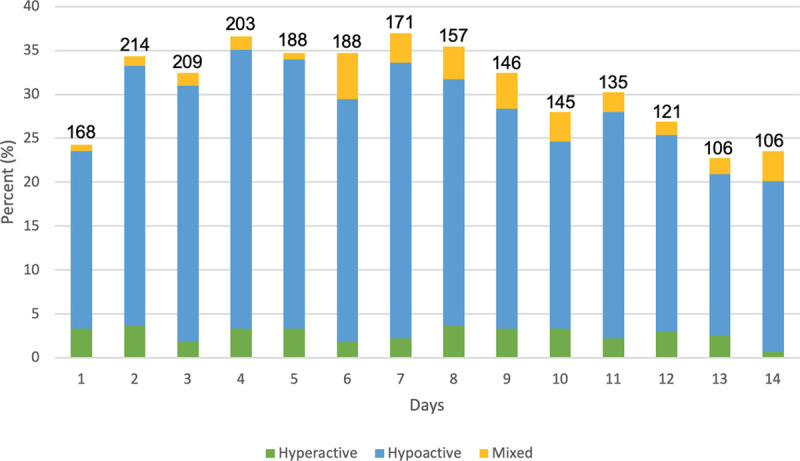
Subtypes of delirium in critically ill patients with coronavirus disease 2019 (n = 268). Number above each bar column indicates number of patients assessed for delirium per day. Daily percentages do not equal 100% due to incomplete assessments, death, discharge from ICU, or screening negative for delirium. Number of patients screening positive for delirium per day: 65 (day 1), 92 (day 2), 87 (day 3), 98 (day 4), 93 (day 5), 93 (day 6), 99 (day 7), 95 (day 8), 87 (day 9), 75 (day 10), 81 (day 11), 72 (day 12), 61 (day 13), and 63 (day 14). Confusion Assessment Method for the ICU (CAM-ICU) and Richmond Agitation-Sedation Scale (RASS) assessments were performed up to bid while patient was admitted to the ICU. Hypoactive delirium was defined by RASS of –1 to –3 with positive CAM-ICU and hyperactive delirium was defined by a RASS score of +1 to +3 with positive CAM-ICU. Mixed delirium was defined as patients with both hyperactive and hypoactive delirium assessment on a given ICU day.
Factors Associated With Delirium
Patients with delirium had greater mechanical ventilation days (median 9.1 d, IQR 4.6–14.0 vs 0, IQR 0–0.2; p < 0.001) and ICU days (median 14.4, IQR 9.2–19.5 vs 4.0, IQR 2.2–6.8; p < 0.001) compared with patients without delirium (Table 3). We did not find a significant difference in hospital mortality between patients with delirium compared with those without (23.3% vs 15.1%; p = 0.264), as shown in Table 3. Sedative medication orders for the study cohort are shown in Table 4. Patients with delirium had greater frequency of orders for benzodiazepines (86.5% vs 45.3%; p < 0.001), opioids (94.4% vs 50.9%; p < 0.001), propofol (83.3% vs 24.5%; p < 0.001), and dexmedetomidine (34.9% vs 5.7%; p < 0.001) compared with patients without delirium. Frequency of antipsychotics and other sedatives used during the pandemic are shown in Table 4. Daily rates of patient’s discharge from ICU and death over the first 14 days are shown in eFigure 2 (Supplemental Digital Content, http://links.lww.com/CCX/A439). In the logistic regression model consisting of age, receipt of mechanical ventilation, APACHE II scores, Glasgow Coma Scale RASS, and sedative medications, only mechanical ventilation was significantly associated with greater odds of developing delirium (odds ratio, 5.0; 95% CI, 1.1–22.2; p = 0.033).
TABLE 3.
Clinical Outcomes of Patients With Coronavirus Disease 2019 in the ICU
| Clinical Outcomes | Total (n = 268) | Delirium Positivea (n = 215) | Delirium Negativea (n = 53) | p |
|---|---|---|---|---|
| Median (interquartile range), d | ||||
| Mechanical ventilation | 8.0 (0.2–13.0) | 9.1 (4.6–14.0) | 0 (0–0.2) | < 0.001 |
| ICU LOS | 12.0 (6.0–18.7) | 14.4 (9.2–19.5) | 4.0 (2.2–6.8) | < 0.001 |
| Hospital LOS | 17 (11–25) | 19 (13–27) | 9 (6–13) | < 0.001 |
| Mortality, n (%) | ||||
| In-hospital | 58 (21.6) | 50 (23.3) | 8 (15.1) | 0.264 |
LOS = length of stay.
aDelirium status determined by a positive Confusion Assessment Method for the ICU clinical assessment. Univariate testing was completed to investigate statistical significance.
TABLE 4.
Medication Orders of Patients With Coronavirus Disease 2019 in the ICU
| Medication | Total (n = 268), n (%) | Delirium Positivea (n = 215), n (%) | Delirium Negativea (n = 53), n (%) | p |
|---|---|---|---|---|
| Benzodiazepines | 210 (78.4) | 186 (86.5) | 24 (45.3) | < 0.001 |
| Opioids | 230 (85.8) | 203 (94.4) | 27 (50.9) | < 0.001 |
| Propofol | 192 (71.6) | 179 (83.3) | 13 (24.5) | < 0.001 |
| Dexmedetomidine | 78 (29.1) | 75 (34.9) | 3 (5.7) | < 0.001 |
| Phenobarbital | 51 (19.0) | 51 (23.7) | 0 (0.0) | < 0.001 |
| Ketamine | 86 (32.1) | 83 (38.6) | 3 (5.7) | < 0.001 |
| Haloperidol | 42 (15.7) | 41 (19.1) | 1 (1.9) | 0.001 |
| Quetiapine | 67 (25.0) | 61 (28.4) | 6 (11.3) | 0.012 |
| Olanzapine | 19 (7.1) | 19 (8.8) | 0 (0.0) | 0.017 |
aDelirium status determined by a positive Confusion Assessment Method for the ICU clinical assessment. Univariate testing was completed to investigate statistical significance.
DISCUSSION
In this retrospective study of COVID-19 patients admitted to the ICU at two large hospitals, nearly 29% experienced delirium without coma, nearly 28% developed delirium before coma, while approximately 23% had delirium after coma. Delirium occurred early in the ICU course (within the first 2 d) and persisted for median length of 5 days. In addition, patients with COVID-19 experienced severe delirium, and invasive mechanical ventilation was associated with a marked increase in odds of delirium. While mortality rates did not statistically differ by delirium status likely due to the small sample size of patients without delirium, we found mortality to be 8% higher in patients with delirium. To the best of our knowledge, our study is the first to describe delirium rates, duration, and severity in critically ill patients with COVID-19 using standardized delirium assessment tools. Due to the increased risk of mortality and morbidity following delirium, including the development of long-term cognitive impairment and post intensive care syndrome, this study has important implications for clinical practice, the recovery of patients with COVID-19 admitted to intensive care, public health decision making, and even future research priorities (26, 27).
Our study findings represent a departure from our own center’s recently reported rates of ICU delirium (22.7%) and rates of mechanical ventilation (36%) during the influenza pandemic occurring in 2009–2010 (eTable 2, Supplemental Digital Content, http://links.lww.com/CCX/A439). Reductions in the prevalence of ICU delirium from a historical high of 80% to rates of 16.5–33% have been reported over the past 2 years (13, 16, 17, 19). These reductions were likely linked to the implementation of the Society of Critical Care Medicine’s Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in the ICU guidelines, multidisciplinary bundled protocols for delirium prevention (ABCDEF), avoidance of deliriogenic agents, increasing use of noninvasive ventilation strategies and heated high-flow nasal cannula devices leading to reduced rates of invasive ventilation, and increasing clinician awareness regarding the harms of delirium (17, 28–30). The COVID-19 crisis has seriously challenged these care improvements as preestablished multidisciplinary care models of ICU care become disrupted and health systems are overwhelmed with critically ill patients requiring invasive mechanical ventilation. Our study also emphasizes the increased use of benzodiazepines, opioids, and other sedative medications in the management of critically ill patients with COVID-19 at our center. While the high rates of hypoactive delirium found in our study cohort may be associated with sedative exposure or severity of illness, direct neurotoxicity of COVID-19 has also been proposed, including infiltration of the CNS leading to delirium (31–33). A recent meta-analysis on neuropsychiatric symptoms associated with severe coronavirus infections concluded that signs of delirium (confusion 27.9%, 95% CI, 20.5–36.0; impaired concentration or attention 38.2%, 95% CI, 29.0–47.9; and altered consciousness 20.7%, 95% CI, 12.6–30.3) were common in SARS and Middle East respiratory syndrome (34). Further, as recent studies have reported neurologic symptoms such as anosmia in COVID-19 and SARS-CoV-2 has been identified in cerebrospinal fluid as well as brain tissue, the neurotoxic impact of COVID-19 is increasingly plausible (31–33, 35). Our study design does not permit exclusion of viral neurotoxicity as the cause of delirium or coma, and therefore, pathways for encephalopathy due to COVID-19 require additional study.
While effective pharmacological therapies for treatment of COVID-19 as well as delirium are not yet available, our study sheds light on an alarming burden of delirium and coma in patients admitted to the ICU and the need for continued efforts on delirium prevention. Following and implementing evidence-based ICU practices (such as the ABCDEF bundle) to minimize delirium occurrence and severity under the pandemic conditions will likely remain an ongoing challenge (30). The continued use of screening tools for delirium and delirium severity can also provide bedside clinicians with dynamic assessments to measure the impact of interventions in real-time (9, 12). As resources shrink in the face of the pandemic and the healthcare response disrupts, it is imperative to continue to follow and implement time-tested evidence-based practices. Finally, delirium in critically ill patients has been associated with long-term cognitive decline (10, 36). If other studies confirm higher rates of delirium in COVID-19 ICU patients, longitudinal follow-up will be crucial to understand the full impact of COVID-19 and understand the pathophysiology of COVID-19 related delirium.
Our study does have important limitations. This analysis is limited by its reliance on data from the medical record including clinician-administered delirium assessments obtained when RASS of –3 to greater than +1 was present, leading to potentially false positive results. Data on the RASS score was extracted in concordance with the delirium assessment; therefore, we were not able to provide a more global view of sedation depth, and patients may not have received consistent sedative interruption to provide more reliable delirium assessment. The limitation of clinician-administered delirium assessments has been minimized by the rigorous implementation and continued education on the CAM-ICU and CAM-ICU-7 at the participating institutions. While we included medication orders to estimate sedative exposure, medication doses, frequency, and administration details were not available for the analysis. Additionally, adherence to the ABCDEF bundle at the patient level, as well as risk factors for delirium such as baseline cognitive and functional status, were not available. Our analysis is also limited to delirium and coma assessments performed in the first 14 days of ICU stay, and therefore, we are unable to describe the trajectory of delirium and coma for the duration of the hospitalization in this report. Together, these limitations preclude discrimination between sedative-associated delirium and delirium secondary to neurotoxicity as a consequence of COVID-19 infection. Strengths of the study include incorporation of delirium severity data, a racially and socioeconomically diverse cohort of patients and protocolized delirium assessments conducted by bedside clinicians at two high volume and high acuity centers.
CONCLUSIONS
In our cohort of critically ill patients with COVID-19, delirium without coma occurred in 29.1%, delirium prior to coma in 27.9%, and delirium after coma in 23.1%. Delirium persisted for approximately 5 days and occurred at high severity. Invasive mechanical ventilation is significantly associated with delirium even after adjustment for sedatives. Given these findings, continued attention to prevent and manage delirium, and a deeper understanding of the virus’ neurotoxic effects are critical.
ACKNOWLEDGMENTS
We thank our patients, caregivers, healthcare team members, Christiana Purnell, and “Team Vitality,” without whom this work would not be possible.
Supplementary Material
Footnotes
Drs. Khan and Lindroth shared primary authorship.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccxjournal).
Dr. Lindroth is supported by National Heart, Lung, Blood Institute (NHLBI) T32 5T32HL091816-07. Drs. Perkins, Gao, and B.A. Khan are supported through National Institute on Aging (NIA) R01 AG 055391, R01 AG 052493, and NHLBI R01 HL131730. Dr. Perkins is also supported by NIA grants 1K23AG062555-01 and R01AG056325. Dr. Machado is supported by 1R01HL111656, 1R01HL127342, and 1R01HL133951. Dr. Wang is supported by K23AG062555-01. Dr. Marcantonio is supported by grants R01AG044518 and K24AG035075 from the NIA. Dr. Boustani received funding from NIA R01AG034205 and disclosed that he has ownership equity in two for profit companies, Preferred Population Health Management and RestUp. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work was performed at Indiana University Health Methodist Hospital and Eskenazi Health.
REFERENCES
- 1.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020; 382:2372–2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020; 382:2268–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisani MA, Kong SY, Kasl SV, et al. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009; 180:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014; 42:369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayhurst CJ, Pandharipande PP, Hughes CG. Intensive care unit delirium: A review of diagnosis, prevention, and treatment. Anesthesiology. 2016; 125:1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014; 383:911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017; 377:1456–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindroth H, Khan BA, Carpenter JS, et al. Delirium severity trajectories and outcomes in ICU patients. Defining a dynamic symptom phenotype. Ann Am Thorac Soc. 2020; 17:1094–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard TD, Thompson JL, Pandharipande PP, et al. Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: A prospective cohort study. Lancet Respir Med. 2018; 6:213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasunilashorn SM, Marcantonio ER, Gou Y, et al. Quantifying the severity of a delirium episode throughout hospitalization: The combined importance of intensity and duration. J Gen Intern Med. 2016; 31:1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan BA, Perkins AJ, Gao S, et al. The confusion assessment method for the ICU-7 delirium severity scale: A novel delirium severity instrument for use in the ICU. Crit Care Med. 2017; 45:851–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan SH, Lindroth H, Hendrie K, et al. Time trends of delirium rates in the intensive care unit. Heart Lung. 2020; 49:572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowan SL, Preller J, Goudie RJB. Evaluation of the E-PRE-DELIRIC prediction model for ICU delirium: A retrospective validation in a UK general ICU. Crit Care. 2020; 24:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rood PJT, van de Schoor F, van Tertholen K, et al. Differences in 90-day mortality of delirium subtypes in the intensive care unit: A retrospective cohort study. J Crit Care. 2019; 53:120–124 [DOI] [PubMed] [Google Scholar]
- 16.Rosa RG, Falavigna M, da Silva DB, et al. ; ICU Visits Study Group Investigators and the Brazilian Research in Intensive Care Network (BRICNet). Effect of flexible family visitation on delirium among patients in the intensive care unit: The ICU visits randomized clinical trial. JAMA. 2019; 322:216–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo-Novoa CM, Figueroa-Ramos MI, Puntillo KA. Occurrence and practices for pain, agitation, and delirium in intensive care unit patients. P R Health Sci J. 2019; 38:156–162 [PMC free article] [PubMed] [Google Scholar]
- 18.Chaiwat O, Chanidnuan M, Pancharoen W, et al. Postoperative delirium in critically ill surgical patients: Incidence, risk factors, and predictive scores. BMC Anesthesiol. 2019; 19:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. ; REDUCE Study Investigators. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018; 319:680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phua J, Weng L, Ling L, et al. ; Asian Critical Care Clinical Trials Group. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir Med. 2020; 8:506–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(SCCM) SoCCM. Tiered Staffing Strategy for Pandemic. 2020, online, SCCM; Available at: https://sccm.org/Blog/March-2020/United-States-Resource-Availability-for-COVID-19. Accessed May 2020 [Google Scholar]
- 22.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020; 46:854–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA. 2020; 323:1545–1546 [DOI] [PubMed] [Google Scholar]
- 24.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: Validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002; 166:1338–1344 [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: Validation of the confusion assessment method for the intensive care unit (CAM-ICU). Crit Care Med. 2001; 29:1370–1379 [DOI] [PubMed] [Google Scholar]
- 26.Kotfis K, Williams Roberson S, Wilson JE, et al. COVID-19: ICU delirium management during SARS-CoV-2 pandemic. Crit Care. 2020; 24:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serrano-Castro PJ, Estivill-Torrús G, Cabezudo-García P, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: A delayed pandemic? Neurologia. 2020; 35:245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46:e825–e873 [DOI] [PubMed] [Google Scholar]
- 29.Hsieh SJ, Otusanya O, Gershengorn HB, et al. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. 2019; 47:885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: Results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019; 47:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020; 92:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baig AM, Khaleeq A, Ali U, et al. Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020; 11:995–998 [DOI] [PubMed] [Google Scholar]
- 33.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020; 173:268–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers JP, Chesney E, Oliver D, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020; 7:611–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020; 87:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med. 2013; 369:1306–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


