Abstract
Simple Summary
In this manuscript we review the recent literature supporting a biological link between circadian clock disruption and thyroid cancer development and progression. After a brief description of the involvement of the circadian clock machinery in the cell cycle, stemness and cancer, we discuss the scientific evidence supporting the contribution of circadian clockwork dysfunction in thyroid tumorigenesis and the possible molecular mechanisms underlying this relationship. We also point out the potential clinical implications of this link highlighting its impact on thyroid cancer prevention, diagnosis and therapy.
Abstract
Thyroid cancer (TC) represents the most common malignancy of the endocrine system, with an increased incidence across continents attributable to both improvement of diagnostic procedures and environmental factors. Among the modifiable risk factors, insulin resistance might influence the development of TC. A relationship between circadian clock machinery disfunction and TC has recently been proposed. The circadian clock machinery comprises a set of rhythmically expressed genes responsible for circadian rhythms. Perturbation of this system contributes to the development of pathological states such as cancer. Several clock genes have been found deregulated upon thyroid nodule malignant transformation. The molecular mechanisms linking circadian clock disruption and TC are still unknown but could include insulin resistance. Circadian misalignment occurring during shift work, jet lag, high fat food intake, is associated with increased insulin resistance. This metabolic alteration, in turn, is associated with a well-known risk factor for TC i.e., hyperthyrotropinemia, which could also be induced by sleep disturbances. In this review, we describe the mechanisms controlling the circadian clock function and its involvement in the cell cycle, stemness and cancer. Moreover, we discuss the evidence supporting the link between circadian clockwork disruption and TC development/progression, highlighting its potential implications for TC prevention, diagnosis and therapy.
Keywords: thyroid cancer, clock genes, machinery, circadian misalignment, sleep disturbances, insulin resistance, occupational and environmental factors
1. Introduction
Thyroid cancer (TC) represents the most common endocrine malignancy, which has shown a strikingly increasing incidence over the past few decades [1,2]. Well-differentiated TC histotypes, comprising papillary TCs (PTCs, 85%) and follicular TCs (FTCs, 15%) account for the majority of TCs and are considered to be low risk tumors [3]. Poorly differentiated TCs (PDTCs) and anaplastic TCs (ATCs) are less common but more aggressive histotypes, often unresponsive to conventional treatments [3].
Epidemiological studies have suggested that environmental factors and lifestyle modifications can be responsible for the increased incidence of TC worldwide. Among the potential modifiable risk factors of TC, particular attention has been paid to insulin resistance and hyperinsulinemia [4]. These metabolic alterations have also been rapidly increasing worldwide due to lifestyle modifications, which may also include circadian clock disruption. At present, it is not completely clear how insulin resistance and related metabolic disorders may affect well-known molecular pathways involved in the pathogenesis of TC such as MAPK, PI3K/PTEN/AKT, TSH-R, and mTOR/p70S6K. To date, many etiopathogenetic features of TC still remain unknown.
Recently a relationship between the circadian clock machinery function and TC has been proposed [5,6,7,8,9,10]. During evolution, organisms have developed biological clocks to better adapt to various rhythmic events such as daily and seasonal fluctuations. Circadian rhythms are generated by a central clock located in the brain’s suprachiasmatic nuclei and by multiple peripheral cellular clocks [11]. A 24 h cell-autonomous circadian clock, virtually present in all cells of the body, regulates several physiological functions, including endocrine rhythms [12].
Disruption of the circadian timing system caused by circadian misalignment such as shift work, chronic jet lag, high fat intake, inappropriate eating times, and abnormal sleep patterns could be responsible of insulin resistance, diabetes mellitus type 2, obesity, metabolic syndrome, cardiovascular diseases and several types of cancers, including TC [13,14,15]. Conversely, proper coordination of circadian behavior and sleep homeostasis may improve several conditions including insulin resistance and overall metabolic fitness [16,17]. The molecular mechanisms linking circadian clock disruption and TC are still unknown but could be, at least in part, insulin resistance. Indeed, this metabolic alteration is associated with a well-known risk factor for TC i.e., hyperthyrotropinemia [18,19,20] which, in turn, has also been associated to sleep disturbances [21]. Alterations in the rhythmicity of thyroid stimulating hormone (TSH) secretion and hypothalamic-pituitary-thyroid (HPT) axis function as well as modifications in genes controlling the cell cycle, apoptosis, DNA damage, inflammation, and immune response are the main mechanisms proposed to mediate circadian-related thyroid disorders [22,23]. Furthermore, variants of various clock genes (PER2–3, CRYs, BMAL1, REV-ERBs and RORs) and strong changes in their expression profile have been found on thyroid nodule malignant transformation and have been proposed as potential biomarkers for thyroid nodule pre-operative diagnostics [5,7,8]. Although at present fine-needle aspiration biopsy (FNA) represents the gold standard for the preoperative diagnosis of TC, 20–30% of lesions are indeterminate based on cytological features [3,24]. However, FNA is unable to distinguish between follicular adenoma and follicular carcinoma [25,26,27,28,29]. Molecular testing of FNA samples is a new strategy that can help to rule in or rule out the diagnosis of TC, to reduce the use of diagnostic surgery and to better define the prognosis. Genomic studies of differentiated TC have demonstrated that the most recurrently altered genes are BRAFV600E, RAS and RET/PTC [30]. Over the last several years new molecular alterations (such as gene fusions, copy number variations, driver mutations, indels, abnormal gene expression, miRNAs) either entirely novel in this cancer or novel alterations of known drivers have been identified [30,31,32]. Some of these molecular markers sometimes coexist with BRAF or RAS mutation influencing fundamental aspects of TC phenotype and its biological behavior. For example, the combination of TERT mutation and a BRAF or RAS mutation within the same tumor is associated with low degree of differentiation, aggressive behavior and high risk of recurrence and mortality [33]. Yet, it has been demonstrated that BRAFV600E PCTs represent a spectrum of tumors consisting of at least four distinct molecular subtypes with different genomic, epigenomic and proteomic profiles, suggesting the presence of molecular diversity among PTCs [30,32]. At present, the available molecular tests of FNA samples (ThyroSeq version 3, Afirma Genomic Sequencing Classifier, ThyGeNEXT/ThyraMIR and ThyroPrint) allow one to detect a broad spectrum of molecular alterations [34,35]. Despite the efforts that have been made over the last decade, the diagnostic and prognostic performance of these molecular approaches and their applicability in the routine diagnostic laboratory for thyroid nodules, especially those with indeterminate cytology, are poor and require further validation [36]. Expanding the existing tests by incorporating further reliable preoperative markers predictive of malignancy for suspicious or indeterminate thyroid nodules and/or of disease progression, in combination with the already known molecular alterations and clinical examination, would enhance the diagnostic performance of molecular testing and could have great clinical importance.
In this review, we explore the relationship between disrupted circadian clock machinery and TC. We first describe the mechanisms controlling the circadian clock functions and its involvement in the cell cycle, stemness and cancer. The molecular mechanisms underlying thyroid tumorigenesis will then be summarized. Finally, the scientific evidence supporting the possible biological link between the disruption of circadian clockwork and TC development/progression and its potential role for the TC prevention and treatment will be discussed.
2. Circadian Clock
2.1. Regulation
All living organisms have developed an internal circadian system oscillating within a period of roughly 24 h in order to adapt to environmental cues. This system is composed of two components: a central master clock and peripheral clocks, all of which are developmentally regulated [11]. The central clock is located in the anterior hypothalamic suprachiasmatic nucleus (SCN), as suggested by the observation that SCN lesions disrupt circadian rhythm, while SCN transplantation restores it [37,38]. The SCN is composed of thousands of neurons, which contain a cell-autonomous circadian clock with a specific rhythm [39,40]. The SCN entrains to environmental light-dark cycles sending signals to the peripheral clocks of each tissue and of almost all of the cells in the body [41] to control rhythms in physiology, metabolism, behavior, immune, hormonal and neural functions [12].
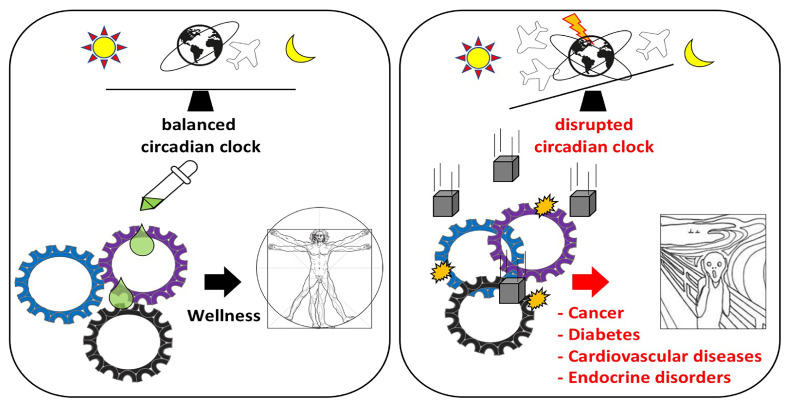
At the molecular level, the core pacemaker of each clock is regulated by a set of genes named “clock genes”, which control the cycling of mRNAs and proteins, called “clock-controlled genes (CCGs)”, through positive or negative transcriptional/post-transcriptional feedback loops [42]. Many CCGs are involved in important physiological and pathophysiological networks and signaling pathways regulating tissue and organ functions. Therefore, the disruption of the circadian clocks in the body could contribute to develop different pathological conditions. Indeed, shift workers who live in a chronic state of circadian misalignment, show an increased prevalence of many diseases including insulin resistance, cardiovascular disorders, gastrointestinal disturbances, depression, neurological alterations, and cancer [11,43] (Figure 1).
Figure 1.
Effect of circadian rhythm disruption on body health. Circadian alignment is associated with wellness and body health. Circadian clock malfunctioning induced by genetic factors (clock gene mutations) and/or environmental factors (inappropriate light exposure, sleep restriction, jetlag, shift work, irregular food intake) can lead to the development of several disorders including cancer, diabetes, cardiovascular disorders, endocrine diseases, inflammation, mental disorders, immune system alterations and reproductive disorders.
In mammals, the circadian clock molecular machinery includes several genes, among which are the following: CLOCK, BMAL1, NPAS2, Per1-2-3, CRY1-2, DEC1-2, REV-ERBα, RORα, CK1ε, CK1δ and TIM [40,41,44]. The central elements are represented by BMAL1, CLOCK and NPAS2. They form the positive control loop of the circadian clock. In the nucleus, BMAL1/CLOCK and BMAL1/NPAS2 heteromize and activate the transcription of other clock genes such as CRY, PER and DEC, which translocate to the cytoplasm. In turn, phosphorylated PERs/CRYs cytoplasmic heterodimers are transported back into the nucleus. PER and CRY proteins, present in the nucleus, inactivate BMAL1/CLOCK and BMAL1/NPAS2 complexes repressing their own transcription as well as the transcription of DEC1-2 and REV-ERBα-RORα, thereby closing a negative feedback loop [45,46,47]. DEC1 and DEC2, by binding to the regulatory DNA core enhancer sequences “CANNTG” of their promoter, directly inhibit their own transcription [48]. RORα and REV-ERBα constitute a supplementary loop, which acts through RORE elements present in the BMAL1 promoter to activate or inactivate BMAL1 transcription, respectively. A further modulation of the nucleocytoplasmic shuttling of all these core clock components is represented by the protein kinases CK1ε and CK1δ, which phosphorylate elements belonging to both positive and negative loops [49,50]. Post-translational and transcriptional modifications such as acetylation, methylation, SUMOylation and ubiquitination contribute to regulate the oscillating of the clockwork circuitry [51,52].
2.2. Circadian Clock and Cell Cycle
Recent evidence has highlighted a connection between the circadian clock and cell cycle machinery in healthy and pathological states. The physiological circadian-dependent regulation of cell cycle phases is suggested by the observation that cell cycle progression occurs at specific times of the day/night rhythm [53]. Furthermore, several proteins controlling G1/S and G2/M phases as well as checkpoints involved in DNA repair after damage are rhythmically expressed and regulated by CCGs [54,55]. For instance, P21 WAF1/CIP1, a negative regulator of G1/S phase progression, is alternatively activated or repressed by RORα and REV-ERBα, respectively [56]. These two proteins bind the same RORE element in the P21 promoter leading to the activation or inhibition of the CDK2/Cyclin E complex and, consequently, G1/S progression. The expression of another component of cell cycle machinery, CyclinD1, is indirectly regulated by PER1 and PER2 genes by inhibiting the transcription of c-MYC. In fact, PER1-2 ablation abolishes c-MYC repression, resulting in elevated cyclin D1 expression, G1/S progression and, therefore, cell proliferation [57]. In contrast, overexpression of PER2 induces cell cycle arrest [58]. PER2 is also involved in the regulation of p53 stability [59,60]. PER2 directly associates with p53 and with its negative regulator MDM-2. The formation of this trimeric complex in the nucleus impairs MDM2-mediated ubiquitination and degradation of p53, resulting in p53 stabilization. On the other hand, p53, acting as a direct competitor of the BMAL1/CLOCK binding to PER2 promoter, represses PER2 gene expression [61]. However, high levels of BMAL1/CLOCK or BMAL1/NPAS2 activate the expression of the tyrosine kinase WEE1, which inhibits CDK1/Cyclin B complex and represses G2/M transition. Conversely, CRYs repress WEE1, favoring cell proliferation [55]. PER1 and TIM, by acting as co-factors or adaptor proteins, lead to the activation of Ataxia Telangiectasia Mutated (ATM) or Ataxia Telangiectasia and Rad3-related protein (ATR) [18,54,62], which in turn activate Checkpoint kinase 1 (CHK1) and Checkpoint kinase 2 (CHK2). Phosphorylated CHK1 and CHK2 are responsible for cell cycle arrest and apoptosis by the inactivation of CDKs [63,64]. All these molecular interactions may represent a regulatory link between the cell cycle, p53-mediated cellular damage response and the circadian clock-regulated cellular pathways. The disruption of cell cycle regulation as a consequence of circadian clock rhythm perturbation could lead to uncontrolled cell division and, consequently, to the development of cancer.
2.3. Tumor Suppressor or Oncogene: The Janus Face of the Circadian Clock Machinery
Circadian clock function and cancer are interlinked. The synchronized circadian clock is an important tumor suppressor, while disruption of clock genes affects tumor development and cancer susceptibility [65,66,67,68]. Although several in vitro and in vivo studies support this observation, the molecular connections and the relationship between clockwork and cancer are still not well understood and remain controversial [69]. For instance, PER1 and PER2 behave as tumor suppressors in vivo [57]. Mice bearing the PER2 mutation and lacking circadian rhythm show increased incidence of malignant lymphomas and an increased rate of mortality after ionizing radiation relative to wild-type controls. This tumor promoting effect is likely due to decreased BMAL1 expression and consequent increased c-MYC expression [57,70]. However, other findings have shown that deficiency in PER genes (PER1 or PER2) has no effect on the rate of spontaneous and radiation-induced carcinogenesis [71].
Conversely, PER2 overexpression causes growth inhibition, apoptosis and cell cycle arrest in different cancer cell models [72,73,74]. Altered expression of PER1, PER2 and/or PER3 have been reported in colorectal, pancreatic, gastric, oral, breast, prostate, bladder, renal, and non-small cell lung cancers, as well as in glioma, hepatocellular carcinoma, head and neck squamous cell carcinoma and myeloid leukemia [75,76,77,78,79,80,81,82,83,84,85,86,87].
With respect to the other components of the core clock, CRY mutant mice lacking circadian rhythm [88] have a faster rate of implanted tumor growth, more susceptibility to ionizing radiation-induced cancer, and increased morbidity and mortality, likely due to defective cell cycle checkpoints and DNA repair ability [89,90]. However, the increased predisposition of arrhythmic CRY−/− mice to spontaneous and DNA damage-induced cancers has not been confirmed by other studies. Gauger et al. have showed that CRY double knockout (DKO) mice behave similarly to wild-type controls with respect to spontaneous and radiation-induced morbidity, mortality and cancer [70]. Similarly, fibroblasts derived from the CRY mutant mice have the same sensitivity to ionizing and UV radiations and the same cellular response to DNA damage, compared to wild-type control fibroblasts [70]. On the other hand, later studies demonstrated that CRY1−/−; CRY2−/− deficient mice in a p53−/− background showed an increased survival and protection from tumor development [91]. However, CRY mutation makes RAS-transformed p53 null cells, but not p53 wild type cells, more susceptible to apoptosis [92,93].
Unlike CRY DKO mice, loss of CRY2 alone induces increased tumor burden and enhanced susceptibility to transformation [94], supporting an unexpected function of CRY2 in contributing to circadian protection from tumor formation.
Furthermore, recently it has been demonstrated that CRY1 and CRY2 exert opposite roles in modulating transcription of several factors, such as c-MYC, in response to DNA damage [95]. The discrepancies observed among various studies may be attributable to several reasons: the real divergent roles of CRY1 and CRY2; the different genetic backgrounds of mice; the severity of the circadian clock disruption caused by CRY knockout; the establishment of homeostatic mechanisms; the cooperation between CRY2 deficiency and multiple oncogenes in the control of proliferation and transformation.
The other central component of clock machinery, BMAL1, has notoriously been considered a tumor suppressor gene. However, as seen for other circadian clock genes, there are different findings from different laboratories showing both pro- and anti-cancer effects of BMAL1 KO mutation. Some studies have demonstrated that downregulation of BMAL1 gene expression promotes cancer cell proliferation, invasion, and tumor growth and decreases apoptosis induced by DNA damage [96,97,98]. Conversely, BMAL1 overexpression has been seen to inhibit cell proliferation, invasiveness and to increase sensitivity to anticancer drugs [99,100,101]. In support of the anticancer effect of this clock gene, whole-body or organ specific KO of BMAL1 in mice has been associated with increased lung cancer and hepatocellular carcinoma [102,103]. In contrast to these data, BMAL1 KO has been found to suppress proliferation and anchorage-dependent and independent clonal growth of malignant pleural mesothelioma cells [104]. Similarly, BMAL1 KO decreases apoptosis of murine colon cancer cells and fibroblast cells in response to chemotherapeutic drugs [98]. However, a study by Puram et al. has shown that genetic deletion of BMAL1 results in suppression of leukemia formation [105]. The opposite and divergent effects of BMAL1 on carcinogenetic mechanisms have recently been confirmed in untransformed MCF10A and in invasive MDA-MB231 breast epithelial cell lines. In these cellular models, BMAL1 deletion by CRISPR technology induced apoptosis in response to genotoxic agents but at the same time increased the invasive potential of MDA-MB231 cells. Altogether these results suggest that BMAL1 may exert both protective and pro-tumor effects based on the different cellular contexts and on the activation of circadian dependent or independent functions of the BMAL1 gene in different organs [106].
Similar to BMAL1, studies on the role of the CLOCK gene in carcinogenesis have often been contradictory. A study by Lee et al. [89] found that CLOCK Δ19/ Δ19 mice had enhanced tumorigenesis under basal and irradiated conditions in contrast to other studies showing that CLOCK gene deletion in mice did not increase the incidence of cancer [54,71]. In support of the pro-tumor role of the CLOCK gene, other evidence found that CLOCK knocking-down decreased cancer proliferation, progression and invasion as well as expression of several cancer-associated genes [107,108]. These pro-tumor effects of the CLOCK gene are likely due to its transcriptional functions as well as to its intrinsic histone acetyltransferase (HAT) activity [109]. Through this HAT activity, CLOCK may play a pivotal role in chromatin remodeling and in modulating the activity and the transcription of proteins involved in cell cycle control and DNA damage response, thereby influencing cancer development [110]. For example, in breast cancer, CLOCK may modulate estrogen receptor-α mediated gene expression using its HAT activity [110].
Epidemiological evidence supports the possibility that disruption of the circadian clock periodicity may be implicated in increased cancer risk and in the progression of the disease [111,112]. As suggested by different independent studies and meta-analyses, night workers, shift workers or people often subjected to jet lag or to prolonged light exposure during the night, present an increased incidence of breast [113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128], prostate [129,130,131,132,133,134,135,136,137,138,139], colon [40,140,141] and endometrial epithelial cancers [142], as well as non-Hodgkin’s lymphoma [143]. Furthermore, cancer patients with altered circadian rhythm have poorer survival compared to those with normal circadian clock periodicity [144]. All these epidemiological studies strongly suggest that the lack of circadian rhythm homeostasis contributes to cancer risk, cancer development and progression. In light of these results and based on sufficient evidence from experimental animal models, the Agency for Research on Cancer has classified “shift work with circadian clock desynchrony” as a potential carcinogenic to humans (group 2A) [145,146].
Several plausible hypotheses have been proposed to explain the link between circadian clock disruption and cancer, among them: the suppression of nocturnal peak of melatonin after exposure to light at night; immune system alterations as a consequence of sleep deprivation; shift in the ratio between anti-tumor and pro-tumor cytokines, induction of inflammation response, modifications in the levels of appetite-regulating hormones, internal desynchronization and disturbances in the regulation of several clock genes controlling the cell cycle, apoptosis, DNA damage repair and cell proliferation. However, further studies are needed to better investigate the different day/night alternation systems, sleep patterns, chronotypes, measurement of biomarkers, presence of polymorphisms or other abnormalities in clock genes in order to discover new potential prognostic markers and novel therapeutic targets for specific cancers [66,139,147,148].
2.4. Circadian Clock and Stemness
A large body of evidence has shown that the circadian clock influences stem cell biology, lineage commitment, tissue regeneration and aging [149]. The core of the clock machinery, including CLOCK and BMAL1 genes, is common to different organs and tissues, while the resulting rhythmic and phased transcription of peripheral output clock genes controlled by the central core circuitry is highly tissue-specific. The functional integrity of both central and peripheral clocks and the tissue-specific gene expression programs meet the physiological needs of every organ, thereby ensuring tissue homeostasis and adaptation to the circadian rhythm of the environment. Perturbation of physiological circadian clock equilibrium has been implicated in several processes of tumorigenesis, even at early stages of its development [149,150,151,152].
In vitro and in vivo studies have demonstrated that regulation of circadian clock programs is different in pluripotent stem cells, adult stem cells and differentiating cells. Pluripotent embryonic stem cells (ES), although expressing most of the clock genes at low levels, lack a rhythmic clock system [150,153,154]. The diurnal oscillatory network starts to be gradually activated during the differentiation process [149,150]. Conversely, reversing differentiation through reprogramming processes decreases rhythmicity of the expression of clock-related genes [154]. It is still unknown whether clock factors expressed in ES exert a role in stem cell maintenance. BMAL1, CLOCK, and PER2 KO mice are not embryonically lethal [11,155,156] but they show premature aging and age-related diseases [155]. As suggested by Dierickx et al., it is plausible that the different level of clock factor expression at embryonic stages compared to differentiated cells might exert an unrelated clock function during embryonic development, which becomes important and prevalent at later stages in life [149]. Adult stem cells, unlike ES, possess a functional circadian clock [152], which guarantees stem cell proliferation and self-renewal, thereby facilitating tissue homeostasis, regeneration and a stress-associated response [157].
In fact, disruption of the clock components PERs, CLOCK and BMAL1 has been shown to affect regulation of hair follicle bulge stem cell cycling [158], cell-intrinsic keratinocyte differentiation or proliferation responses [159,160,161,162], epidermal wound repair [163], myocardial response to infarction [164], lung response to pro-inflammatory cues [165], hematopoietic system replenishment [166,167], intestinal stem cell renewal and intestinal epithelial regeneration especially after damage from gastrointestinal disease [168,169,170,171].
Dysregulation of the circadian network has also been implicated in cancer stem cell biology.
Targeting BMAL1/CLOCK machinery using small molecule agonists of CRY and REV-ERB, induced a synergistic anti-proliferative effect in glioma stem cells (GSCs) [172]. The oncogenic role for circadian clock activity in the cancer stem cell compartment has been confirmed by other observations. For instance, PER2 mRNA and protein expression was down-regulated in glioma stem cells (GSCs) compared to non-stem glioma cells, while PER2 overexpression induced GSC cell cycle arrest at the G0/G1 phase and suppression of proliferation, a stem cell-like phenotype and invasion capability by targeting the Wnt/β-catenin signaling pathway [173]. However, PER1/2 expression correlates with WHO grading of glioma, being downregulated in glioma tissue compared to normal brain tissue [85]. All these findings suggest that the PER2 gene exerts a potential role in regulating stemness, self-renewal, cell growth, cell cycle distribution, migration and invasion of GCS in glioma and are consistent with similar results obtained in colon cancer stem-like cells (CCSCs). In this cell subtype, PER overexpression inhibits self-renewal properties and chemo-resistance via downregulation of β-catenin and NOTCH signaling pathways [174]. Involvement of the core circadian clock genes in stemness has also been demonstrated in other CSC contexts such as myeloid leukemia stem cells [105], breast cancer stem cells [175] and in the initial steps of hepatocarcinogenesis [102]. Despite this evidence, some aspects and control mechanisms of stem/progenitor cell biology by clock machinery still remain unknown also because they may be influenced by the cellular context, tumor development and differentiation stages. However, on the basis of the data present in the literature to date, targeting one or more components of the circadian machinery could represent a new opportunity for the development of novel anti-cancer therapies.
3. Thyroid Tumorigenesis
Based on the cell of origin, TC can be divided into two main categories: follicular epithelial cell-derived carcinomas (>95%) and medullary TC (3–5%) arising from C cells. Tumor arising from follicular epithelial cells include papillary TC (PTC), follicular TC (FTC), Hurthle cell carcinoma (HCC), poorly differentiated TC (PDTC) and anaplastic TC (ATC). The last two tumor subtypes are very rare but more aggressive follicular-derived TCs compared to differentiated TCs [1]. Recently, integrated genomic, transcriptomic, proteomic and miRNA analysis has been developed to better examine the molecular mechanisms responsible of the different structural features and behaviors between the different TC subtypes. Thyroid tumorigenesis classically occurs through a multistep dedifferentiation process, which starts from well-differentiated TCs and proceeds through poorly differentiated to anaplastic carcinoma. According to this model of tumorigenesis, constitutional activation of the MAPK signaling pathway via RAS, BRAF mutations and/or RET/PTC rearrangements and Paired-box gene 8/Peroxisome Proliferator-Activated Receptor gamma (PAX8/PPARγ ) fusion transmit growth signals to normal thyrocytes, thereby playing a driver role in their malignant transformation. The most common molecular alteration includes the mutation in the BRAF gene, which appears activated in 35–60% of PTCs [176]. Rearrangements of RET gene (RET/PTC) (especially RET/PTC1 and RET/PTC3) are specific molecular alterations present in 5% to 30% of PTCs [176]. The follicular variant of PTCs usually harbor RAS mutations or PAX-8/PPAR-γ translocations [177,178,179]. However, several other molecular alterations including abnormal gene expression, point mutations, copy number changes, gene fusions in components of other survival-signaling cascades, such as TSH-R, PI3-K/Akt, mTOR, and the IGF pathways have been identified as potential contributors to TC development and progression [180,181,182,183,184,185]. For instance, roughly 40% of well differentiated TCs and more than 50% of highly aggressive TCs carry PTEN downregulation or gene silencing [186]. Point mutations or copy number alterations of PIK3CA and Protein Kinase B (PKB also known as AKT) are present in ~23% of ATCs sometimes coexisting with either RAS or BRAF mutations [185]. A proportion of TCs, showing an aggressive behavior, often overexpress components of the IGF system such as insulin receptor isoform A (IR-A), insulin-like growth factor-2 (IGF-2) and insulin-like growth factor-1 receptor (IGF-1R) [187]. Indeed, overexpression of IR-A and the activation of IR-A/IGF-2 loop is a feature of PDTCs, ATCs or stem-like TC cells [188,189] and it is associated to resistance to some targeted therapies [190,191]. The functional interactions between the IGF system and other molecules, such as the non-integrin collagen receptor discoidin domain receptor 1 (DDR1) and the receptor for the hepatocyte growth factor (HGF) MET, may amplify the biological response to insulin, insulin-like growth factors (IGFs), and HGF contributing to favor TC initiation, progression, de-differentiation and metastatic features [192,193,194,195,196,197,198,199,200]. Much evidence has suggested that overactivation of the IR/insulin axis, present in different metabolic disorders characterized by insulin resistance and hyperinsulinemia, plays a putative role in TC tumorigenesis being associated with TC increased risk and worse prognosis [4,201]. In addition, mutations in p53 family members, TERT promoter, ATM, RB1, MEN1, NF1, NF2, SWI/SNF, mismatch repair genes, and histone methyltransferase have been associated with tumor de-differentiation process and tumor progression [202,203,204,205,206,207,208,209,210].
According to the classical multistep carcinogenesis model, accumulating multiple alterations of some of the above-mentioned molecular components are responsible for TC heterogeneity and the transition from well differentiated normal thyrocytes to well differentiated TC subtypes and finally, to most undifferentiated ATCs. Recently, an alternative model named “fetal/stem cell carcinogenesis hypothesis” has been proposed [211]. According to this new model, mutations or epigenetic alterations of normal thyroid adult stem cells or their committed progenitors present within the thyroid gland, induce their malignant transformation toward specific TC stem cells (TCSC), which, in turn, become the potential origin of distinct TC histotypes and the cells responsible for tumor progression, therapeutic resistance and recurrence [212]. Therefore, this last model regards the thyroid carcinogenesis process as an abnormal development of fetal-like thyroid cells, instead of de-differentiation of normal thyrocytes [213]. Preclinical data have shown that several pathways regulating self-renewal, proliferation and differentiation abilities are deregulated in TCSC. Alterations in the insulin/IGF system components, including increased expression of IR-A, IGF-1R, and IGF-2, and as a consequence, over-activation of the IR-A/IGF-2 autocrine loop have been found in TC stem/progenitor cells derived from PDTCs [214]. These results suggest that the IGF system may also be involved in follicular thyroid precursor regulation and biology. Other well-studied molecular alterations present in TCSCs include RET/PTC and Pax8/PPAR-γ rearrangements as well as deregulation in the MAPK pathway or Wnt/β-catenin, NOTCH, Hedgehog, JAK/STAT3 and NFkB pathways [214,215,216,217]. Furthermore, TCSCs obtained from undifferentiated thyroid carcinoma show constitutive activation of AKT, MET, and β-catenin and loss of E-cadherin, TWIST and SNAIL. MET or AKT targeting repressed the migration and metastatic behavior of thyroid stem cells as well as the expression of TWIST and SNAIL. These data suggest a role for AKT, MET, β-catenin and the IGF system in mediating an aggressive metastatic phenotype of cancer stem cells that is consistent with that shown by PDTCs [214,218].
Recent evidence suggests that also non-coding RNAs, both microRNAs (miRNAs) and long-non coding RNAs (lncRNAs), may play a role in thyroid carcinogenesis due to their ability to modulate target genes involved in several pathological pathways and biological processes such as differentiation, proliferation, apoptosis, and stemness [219,220,221]. Sheng et al. have identified miRNA-148a and its target INO80 as crucial regulators of the proliferative and tumor-forming capacity of ATC-CSCs [222]. In another study, the antisense-mediated downregulation of miR-21 has been seen to enhance differentiation and apoptosis and to reduce cancer stemness features and cell cycle progression of ATC cells [223]. However, lncRNA-H19 was found highly expressed in cancer stem cells from PTCs where its depletion significantly reversed E2-induced sphere formation capability and stem-like properties [224]. Similarly, LIN00311 was found upregulated in PTC tissues and cells, where it promoted cancer stem-like properties by targeting miR-330-5p/TLR4 pathway [225].
Although many aspects of thyroid cancer initiation and progression still remain unclear, the discovery of TCSCs and signals regulating their biology may provide new insight into the pathologic mechanism of thyroid tumorigenesis and may open new perspectives in terms of prevention, diagnosis and therapy. Indeed, targeting TCSCs and/or the signaling pathways and/or the factors involved in their self-renewal, proliferation and differentiation abilities may contribute to overcome the resistance to anti-cancer therapies and achieve long-lasting remission.
4. Circadian Clock and Thyroid Tumorigenesis
A large body of evidence has suggested that different components and functions of the endocrine system, including the hypothalamic-pituitary-thyroid axis, the rhythmicity of TSH and of thyroid hormones secretion, are driven not only by behavior-associated factors, but also by an intrinsic timekeeping machinery, including the central hypothalamic clock as well as peripheral clocks [226,227]. The connection between circadian clock and thyroid function is reciprocal. The circadian and ultradian TSH rhythm, the daily rhythmicity of circulating thyroid hormones T4, Free T4 and T3 are influenced by sleep-wake homeostasis [21,23,228,229,230].
In turn, thyroid hormone deficiency or excess may affect the expression of core clock genes and metabolic clock-controlled genes in several peripheral tissues [231,232,233,234]. Similarly to most cells of the body, a rhythm-generating circuitry composed of a number of clock genes and several autoregulatory feedback loops has also been revealed in cultured human primary thyrocytes derived from healthy thyroid tissue [8]. In support of the existence of a thyroid clock, circadian oscillations for core clock genes have been demonstrated in rats as well as in in vitro synchronized human primary thyrocytes, which present a circadian period length of about 27 h [8,228]. Different studies, although sometimes with conflicting results, have demonstrated a possible relationship between circadian clockwork and thyroid tumorigenesis [5,6,7,8,9,10].
Insulin resistance could represent a plausible biological link for this association. Indeed, insulin resistance has been implicated in TC development and progression and it is often increased upon circadian clock disruption. Furthermore, this metabolic alteration is associated with an elevation of serum TSH level, which is, in turn, a well-known risk factor for TC [19,20] and is also increased upon sleep-wake cycle disturbances [21,235,236]. However, to our knowledge, clinical studies conducted to better understand this relationship are not available to date. Furthermore, the studies regarding the association between sleep disorders and risk of TC do not help, because they have often been contradictory. Indeed, two cohort studies conducted in flight attendants and flight crews did not support this association [237,238]. Conversely, a large prospective study has indicated that postmenopausal women affected by sleep disorders showed a significantly increased risk of TC (HR = 1.44), which was surprisingly limited to non-obese subjects (HR = 1.71) and was not seen in obese women (HR = 0.94) [239]. These contradictory results suggest that additional clinical studies with sufficient sample size and strong statistical power are urgently needed to apply and validate these findings on a larger population. Several in vitro studies have tried to answer some questions in order to confirm the connection between circadian clocks and TC transformation and to better characterize the possible biological mechanisms underlying this association.
SNPs or deregulation of several clock genes including PER1-2-3, CRYs, REV-ERBα−β and RORα−β−γ have recently been found associated with a higher risk of TC [14,240]. Increased expression levels of the circadian clock factor Differentially Expressed in Chondrocyte 1 (DEC1), has been implicated in TC promotion by the induction of several cell-cycle-related genes [241]. Up-regulation of BMAL1 and downregulation of CRY2 have been observed in tissue samples from FTC and PTC nodule tissues compared to benign tissues, which show functional circadian oscillators. Endogenous transcript analysis of primary thyrocytes established from PDTCs revealed a robust disruption of circadian gene expression [8]. In particular, PER1 transcripts showed ablated circadian amplitude, whereas BMAL1, PER2/3 and REV-ERBα displayed a strong phase shift compared to thyrocytes established from benign nodules. Similar results were obtained using a long-term continuous circadian bioluminescence oscillation monitoring by transducing BMAL1–luciferase lentivectors into healthy, benign nodules and PDTC-PTC-derived thyrocytes. These last types of cells showed a robust shifted or even anti-phasic pattern of BMAL1-luc reporter oscillatory expression compared to healthy and benign tissue counterparts [8]. These results suggest that the circadian clock machinery is altered upon thyroid malignant transformation. Similar findings were recently confirmed by a Nanostring approach in PTC, FTC, and PDTC tissue samples, which showed significant alterations in core clock genes (BMAL1 and CRY2) and in other genes related to the cell-cycle and apoptosis [5,7]. In particular, PER2 core clock transcript level was found downregulated in oncocytic FTCs and in PDTCs; CRY2 was significantly downregulated in PTCs and PDTCs, while BMAL1 was upregulated in PTCs compared to normal thyroid and benign nodules [5,7]. Based on these alterations in gene expression, a correlation coefficient for the diagnosis of FTCs has been proposed [7]. Furthermore, distinct molecular profiles of key components of clock machinery, cell cycle, apoptosis and Wnt signaling were observed for oncocytic and non-oncocytic FTCs and PDTCs. The more aggressive oncocytic subgroups showed higher numbers of altered genes compared to their non-oncocytic counterparts, revealing that alteration levels of several transcripts might correlate to tumor progression [7]. In line with these data, another study reported an altered expression of REV-ERBα and RORα genes in PTCs especially in those positive for BRAF-mutation [242].
Overall these results suggest that circadian clock characteristics are altered upon thyroid nodule malignant transformation/progression and that changes in clock gene expression profiles may be potentially employed in clinics as potential biomarkers for FTCs and disease progression (Figure 2). Despite the fact that this attempt might represent a great potential for the preoperative diagnosis of TC, further preclinical and epidemiological studies are needed for a rigorous confirmation.
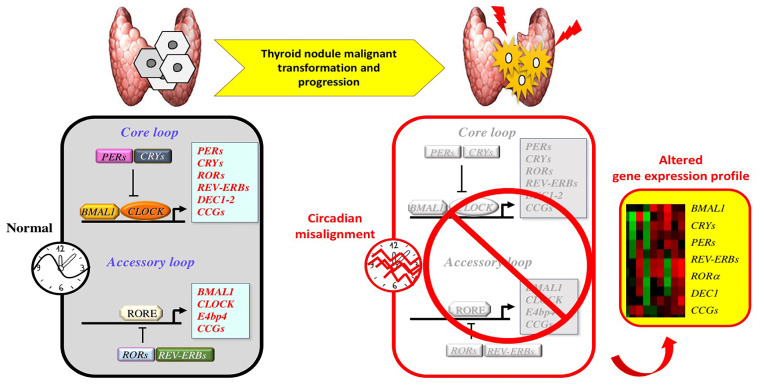
Figure 2.
Molecular alterations in circadian clock gene machinery during thyroid nodule malignant transformation and progression. The circadian transcriptional/translation machinery physiologically acts through a core loop in which CLOCK/BMAL1 activate transcription by binding E-boxes in the promoters of target genes (PERs, CRYs, REV-ERBs, RORs, DECs, WEE1, c-MYC and other clock-controlled genes (CCGs)). In the same loop, the negative PERs and CRYs proteins multimerize and inhibit CLOCK/BMAL1 activity. Clock machinery is also regulated by an accessory loop, consisting of antagonizing transcription factors such as REV-ERBs (α−β) and RORs (α−β−γ), which regulate CLOCK/BMAL1 gene expression and CLOCK/BMAL1-mediated CCGs transcription through ROR-elements (RORE). During thyroid tumorigenesis, circadian misalignment is associated with an altered expression of several clock genes and other CCGs controlling cellular and metabolic functions. These molecular alterations may contribute to thyroid nodule malignant transformation and progression.
The name and functions of the main genes and corresponding proteins involved in circadian clock machinery regulation and thyroid tumorigenesis are listed in Table 1.
Table 1.
List describing in alphabetic order name, major putative functions/signaling pathways of genes and corresponding products involved in circadian clock machinery regulation and thyroid tumorigenesis.
| Gene Name | Protein Name | Function/Signaling Pathway |
|---|---|---|
| AKT or PKB (Protein kinase B) | Protein kinase B (PKB) | Survival, proliferation, apoptosis resistance. PI3K/AKT signaling pathway |
| ATM (ataxia telangiectasia) | ATM | DNA damage response, cell cycle, apoptosis, mitochondrial homeostasis |
| ATR (ataxia telangiectasia and Rad-3 related protein) | ATR | DNA damage response, cell cycle. PI3K/AKT signaling pathway |
| BMAL1 (aryl hydrocarbon receptor nuclear translocator like) | BMAL1 | Circadian clock, exercise-induced circadian regulation, melatonin metabolism and effects, bone metabolism, energetic metabolism, cell stress |
| BRAF (B-Raf proto-oncogene, serine/threonine kinase) | BRAF | Oncogene. Proliferation, differentiation. MAPK/ERK signaling pathway |
| c-MYC | C-MYC | Proto-oncogene, transcription factor. Cell growth, apoptosis, differentiation, stem cell self-renewal. |
| CK1 (casein kinase 1) | CK1α-β-γ-δ-ε casein kinase 1α-β-γ-δ-ε) | Tumor suppressor. Circadian clock, metabolism, DNA damage, cellular stress, cell cycle, cytoskeleton associated functions. Developmental pathways |
| CLOCK (clock circadian regulator) | CLOCK | Circadian clock, exercise-induced circadian regulation, melatonin metabolism and effects. |
| CRYs (cryptochrome circadian regulators) | CRY1-2 | Tumor suppressor. Circadian clock. |
| DEC1-2 (differentially expressed in chondrocytes 1-2) | DEC1-2 | Tumor suppressor. Circadian clock. |
| INO80 | Chormatin-remodeling ATPase INO80 | Cell cycle, cell division, DNA damage, DNA recombination, DNA repair, mitosis, chromatin remodeling. |
| MEN1 | Menin | Transcriptional regulator. Telomerase repressor. Cell proliferation, DNA repair. TGFB1 and NFkB signaling |
| MET | Proto-oncogene c-Met | Proliferation, scattering, morphogenesis, survival, differentiation, angiogenesis. RAS/ERK, PI3K/AKT, PLC-γ/PKC signaling |
| NF1 (neurofibromatosis Type 1 Protein) | Neurofibromin 1 | Tumor suppressor. Cell growth and division. Ras inhibition. Circadian clock. |
| NPAS2 (neuronal PAS domain protein 2) | NPAS2 | Tumor suppressor. DNA damage response. Negative regulator of cell death. Circadian clock. Central nervous system development. Metabolism. |
| P53 | P53 | Tumor suppressor. Response to DNA damage. Cell cycle arrest. Apoptosis. Aging. Gene expression |
| PI3KCA (phosphatidylinositol-4,5-bisphosphate 3-kinase 110 kDa catalytic subunit alpha) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha isoform | Catalytic activity (performs the action of PI3K). Proliferation, survival, migration. PI3K/AKT/mTOR signaling pathway. |
| PER1-2-3 (Period circadian regulator 1-2-3) | PER1-2-3 | Tumor suppressor. Circadian clock. Exercise-induced circadian regulation. Melatonin metabolism and effects. Chromatin DNA binding |
| PTEN (phosphatase and tensin homolog) | PTEN | Tumor suppressor. AKT/PKB signaling pathway |
| RAS | RAS | Oncogene. Cell growth, differentiation, survival, Cell adhesion, apoptosis, migration. MAPK/ERK and PI3K/AKT/mTOR pathway |
| RB1 (RB transcriptional corepressor 1) | Retinoblastoma associated protein RB1 | Tumor suppressor. Cell cycle. Chromatin remodeling. Cell differentiation, cell growth |
| REV-ERB or NR1D1 (nuclear receptor subfamily 1 group D member 1) | NR1D1 | Tumor suppressor. Circadian clock. Mitochondrial biogenesis. Nuclear Receptor transcription pathway |
| RET (RET proto-oncogene) | RET | Proto-oncogene. Protein tyrosine kinase activity. MAPK signaling. |
| RORα (RAR related orphan receptor A) | Nuclear receptor ROR-alpha | Tumor suppressor. Circadian clock. Metabolism. Transcription factor activity. |
| SWI/SNF (SMARCC1) | SWI/SNF complex subunit SMARCC1 | Chromatin remodeling. Transcription regulator |
| TERT (telomerase reverse transcriptase) | Telomerase reverse transcriptase | Chromosome replication. Telomerase activity. Transcription regulator |
| TIM (timeless circadian regulator) | Protein timeless homolog, hTIM | Tumor suppressor. Circadian clock. DNA replication, replication fork stability |
| WEE1 | WEE1-like protein kinase | Cell division, cell cycle, microtubule cytoskeleton organization |
5. Conclusions
The connection between circadian clock machinery dysfunction and TC has different clinical implications in terms of TC prevention, diagnosis and therapy.
Firstly, circadian misalignment could represent a putative risk factor suspected to play a potential role in the changing epidemiology of TC. The increased incidence of TC is largely dependent on modifiable risk factors, such as environmental carcinogens, diet habits, insulin resistance, therapies and lifestyle modifications [243], which may include circadian misalignment. Sleep disturbances and disruption in circadian synchronization are spreading worldwide as a consequence of occupational and personal pressure [11,239].
Chronic disruption of the clockwork has long-term consequences on health becoming a risk factor for insulin resistance, type 2 diabetes mellitus, obesity, atherosclerosis, cardiovascular diseases and cancers including endocrine-dependent tumors [11,67,68,147,244,245].
A reciprocal connection between circadian clock and thyroid disorders has been described in both in vitro and in vivo studies. Chronic sleep deprivation has been associated with disruption of rhythmic TSH secretion, which, in turn, is linked to an increased incidence of human TC [19,20]. Furthermore, disruption of circadian rhythm has been linked to alterations in gene-related apoptosis, DNA damage, cell cycle, and stemness, and thereby to carcinogenesis [11,55,67,68,149,246]. However, some oncogenes such as RAS, which is implicated in thyroid tumorigenesis, induce dysregulation of circadian clocks in human cancer cell lines [6,247]. In light of this evidence, it is biologically plausible that circadian clock alterations could represent a potential risk factor of developing TC. However, so far, no epidemiologic study has been directly addressed in this relationship.
Another concept to be highlighted is that clock gene expression profile could be helpful to improve the pre-operative diagnostics of thyroid nodules, especially those cytologically indeterminate or with a follicular pattern. Alterations in the expression profiles of clock genes (i.e. BMAL1, CRYs, REV-ERBα and PERs) and of cell cycle key components have recently been observed in both PTCs and FTCs when compared to benign nodules or healthy tissue [5,7,8]. Based on these distinct molecular expression profiles a predictive score correlation coefficient with high sensitivity and specificity has been proposed to distinguish between FTCs and benign follicular lesions [7]. The potential use of clock gene expression profiling as predictive markers of TC provides new insights into the molecular mechanisms underlying the pathophysiology of malignant thyroid nodules giving important perspectives in scientific and clinical fields. However, there is an urgent need to launch large prospective studies to confirm this preclinical evidence.
Last but not least, the synchronization of circadian rhythm and/or targeting clock gene alterations starting from TC progenitor cells may represent new adjunct therapeutic strategies to improve the clinical management of TCs especially those developed in insulin resistant patients with circadian clock disruption. Hyperinsulinemia, present in insulin resistant conditions, may worsen the prognosis of TC likely by potentiating IR-A/IGF2-dependent mitogenic functions. Dysfunction of circadian timing leads to an increased risk of insulin resistance-related metabolic disorders [11,245,248,249,250]. Conversely, pharmacological treatments enhancing circadian rhythm or chrono-pharmacology exert beneficial effects on metabolic fitness [16,17]. Based on these observations, it is reasonable to expect that improving insulin resistance through synchronization of circadian rhythm or chronotherapy in conjunction with a healthy diet, physical activity and conventional anti-cancer therapies, could exert beneficial effects on prevention and treatment of TCs developed in insulin resistant patients with disrupted circadian rhythms. However, to date, studies aimed at evaluating the efficacy of all these therapeutic options as an add-on therapy for patients with TCs in the context of insulin resistance and circadian misalignment are lacking.
Acknowledgments
We wish to thank the Scientific Bureau of the University of Catania for language support.
Abbreviations
| ATC | Anaplastic Thyroid Cancer |
| ATM | Ataxia Telangiectasia Mutated |
| ATR | Ataxia Telangiectasia and Rad3-related protein |
| CCGs | Clock-Controlled Genes |
| CCSC | Colon Cancer Stem-like Cells |
| DBP | D-box-binding protein |
| DDR1 | Discoidin Domain Receptor 1 |
| DEC1 | Differentially Expressed in Chondrocyte 1 |
| DKO | Double knockout |
| ES | Embryonic stem cells |
| FNA | Fine-Needle Aspiration |
| FTC | Follicular Thyroid Cancer |
| GSC | Glioma Stem Cells |
| HAT | Histone acetyltransferase |
| HCC | Hurthle Cell Carcinoma |
| HGF | Hepatocyte Growth Factor |
| HPT | Hypothalamic-Pituitary-Thyroid |
| IGF-1R | Insulin-like growth factor-1 receptor |
| IGF-2 | Insulin-like growth factor-2 |
| IGF | Insulin-like growth factor |
| KO | Knockout |
| lncRNAs | Long-non coding RNAs |
| miRNAs | MicroRNAs |
| PAX8/PPAR | Paired-box gene 8/Peroxisome Proliferator-Activated Receptor gamma |
| PDTC | Poorly Differentiated Thyroid Cancer |
| PKB | Protein Kinase B |
| PTC | Papillary Thyroid Cancer |
| SCN | Suprachiasmatic Nucleus |
| TC | Thyroid Cancer |
| TCSC | Thyroid Cancer Stem Cell |
| TSH | Thyroid-Stimulating Hormone |
Author Contributions
Conceptualization, design and original draft preparation, R.M. and S.P.; critical reading and editing C.L., F.F., V.C.F. and V.R.; preparation of references with a bibliography software package T.R.; supervision R.M., S.P. and C.L.; preparation of figures, A.F. and M.C.P. All authors performed the literature search. All authors were involved in manuscript writing and approved the submitted version of the manuscript.
Funding
This study received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016;388:2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaguarnera R., Vella V., Nicolosi M.L., Belfiore A. Insulin Resistance: Any Role in the Changing Epidemiology of Thyroid Cancer? Front. Endocrinol. 2017;8:314. doi: 10.3389/fendo.2017.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chitikova Z., Pusztaszeri M., Makhlouf A.M., Berczy M., Delucinge-Vivier C., Triponez F., Meyer P., Philippe J., Dibner C. Identification of new biomarkers for human papillary thyroid carcinoma employing NanoString analysis. Oncotarget. 2015;6:10978–10993. doi: 10.18632/oncotarget.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegami K., Refetoff S., Van Cauter E., Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019;15:590–600. doi: 10.1038/s41574-019-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makhlouf A.M., Chitikova Z., Pusztaszeri M., Berczy M., Delucinge-Vivier C., Triponez F., Meyer P., Philippe J., Dibner C. Identification of CHEK1, SLC26A4, c-KIT, TPO and TG as new biomarkers for human follicular thyroid carcinoma. Oncotarget. 2016;7:45776–45788. doi: 10.18632/oncotarget.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannic T., Meyer P., Triponez F., Pusztaszeri M., Le Martelot G., Mariani O., Schmitter D., Sage D., Philippe J., Dibner C. Circadian clock characteristics are altered in human thyroid malignant nodules. J. Clin. Endocrinol. Metab. 2013;98:4446–4456. doi: 10.1210/jc.2013-2568. [DOI] [PubMed] [Google Scholar]
- 9.Philippe J., Dibner C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J. Biol. Rhythms. 2015;30:76–83. doi: 10.1177/0748730414557634. [DOI] [PubMed] [Google Scholar]
- 10.Angelousi A., Kassi E., Ansari-Nasiri N., Randeva H., Kaltsas G., Chrousos G. Clock genes and cancer development in particular in endocrine tissues. Endocr. Relat. Cancer. 2019;26:R305–R317. doi: 10.1530/ERC-19-0094. [DOI] [PubMed] [Google Scholar]
- 11.Turek F.W. Circadian clocks: Not your grandfather’s clock. Science. 2016;354:992–993. doi: 10.1126/science.aal2613. [DOI] [PubMed] [Google Scholar]
- 12.Mohawk J.A., Green C.B., Takahashi J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buxton O.M., Cain S.W., O’Connor S.P., Porter J.H., Duffy J.F., Wang W., Czeisler C.A., Shea S.A. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci. Transl. Med. 2012;4:129ra143. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kettner N.M., Katchy C.A., Fu L. Circadian gene variants in cancer. Ann. Med. 2014;46:208–220. doi: 10.3109/07853890.2014.914808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheer F.A., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He B., Nohara K., Park N., Park Y.S., Guillory B., Zhao Z., Garcia J.M., Koike N., Lee C.C., Takahashi J.S., et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016;23:610–621. doi: 10.1016/j.cmet.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winter C., Silvestre-Roig C., Ortega-Gomez A., Lemnitzer P., Poelman H., Schumski A., Winter J., Drechsler M., de Jong R., Immler R., et al. Chrono-pharmacological Targeting of the CCL2-CCR2 Axis Ameliorates Atherosclerosis. Cell Metab. 2018;28:175–182 e175. doi: 10.1016/j.cmet.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Boelaert K., Horacek J., Holder R.L., Watkinson J.C., Sheppard M.C., Franklyn J.A. Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. J. Clin. Endocrinol. Metab. 2006;91:4295–4301. doi: 10.1210/jc.2006-0527. [DOI] [PubMed] [Google Scholar]
- 19.Haymart M.R., Repplinger D.J., Leverson G.E., Elson D.F., Sippel R.S., Jaume J.C., Chen H. Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J. Clin. Endocrinol. Metab. 2008;93:809–814. doi: 10.1210/jc.2007-2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Polyzos S.A., Kita M., Efstathiadou Z., Poulakos P., Slavakis A., Sofianou D., Flaris N., Leontsini M., Kourtis A., Avramidis A. Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. J. Cancer Res. Clin. Oncol. 2008;134:953–960. doi: 10.1007/s00432-008-0373-7. [DOI] [PubMed] [Google Scholar]
- 21.Moon S.H., Lee B.J., Kim S.J., Kim H.C. Relationship between thyroid stimulating hormone and night shift work. Ann. Occup. Environ. Med. 2016;28:53. doi: 10.1186/s40557-016-0141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellastella A., Pisano G., Iorio S., Pasquali D., Orio F., Venditto T., Sinisi A.A. Endocrine secretions under abnormal light-dark cycles and in the blind. Horm. Res. 1998;49:153–157. doi: 10.1159/000023163. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 24.Cibas E.S., Ali S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid. 2009;19:1159–1165. doi: 10.1089/thy.2009.0274. [DOI] [PubMed] [Google Scholar]
- 25.Baloch Z.W., Fleisher S., LiVolsi V.A., Gupta P.K. Diagnosis of “follicular neoplasm”: A gray zone in thyroid fine-needle aspiration cytology. Diagn. Cytopathol. 2002;26:41–44. doi: 10.1002/dc.10043. [DOI] [PubMed] [Google Scholar]
- 26.Walsh P.S., Wilde J.I., Tom E.Y., Reynolds J.D., Chen D.C., Chudova D.I., Pagan M., Pankratz D.G., Wong M., Veitch J., et al. Analytical performance verification of a molecular diagnostic for cytology-indeterminate thyroid nodules. J. Clin. Endocrinol. Metab. 2012;97:E2297–E2306. doi: 10.1210/jc.2012-1923. [DOI] [PubMed] [Google Scholar]
- 27.Yang G.C., Liebeskind D., Messina A.V. Should cytopathologists stop reporting follicular neoplasms on fine-needle aspiration of the thyroid? Cancer. 2003;99:69–74. doi: 10.1002/cncr.10957. [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Schnadig V., Logrono R., Wasserman P.G. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer. 2007;111:306–315. doi: 10.1002/cncr.22955. [DOI] [PubMed] [Google Scholar]
- 29.Yassa L., Cibas E.S., Benson C.B., Frates M.C., Doubilet P.M., Gawande A.A., Moore F.D., Jr., Kim B.W., Nose V., Marqusee E., et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. doi: 10.1002/cncr.23116. [DOI] [PubMed] [Google Scholar]
- 30.Cancer Genome Atlas Research N. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eszlinger M., Lau L., Ghaznavi S., Symonds C., Chandarana S.P., Khalil M., Paschke R. Molecular profiling of thyroid nodule fine-needle aspiration cytology. Nat. Rev. Endocrinol. 2017;13:415–424. doi: 10.1038/nrendo.2017.24. [DOI] [PubMed] [Google Scholar]
- 32.Song Y.S., Park Y.J. Genomic Characterization of Differentiated Thyroid Carcinoma. Endocrinol. Metab. 2019;34:1–10. doi: 10.3803/EnM.2019.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing M., Liu R., Liu X., Murugan A.K., Zhu G., Zeiger M.A., Pai S., Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 2014;32:2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grani G., Sponziello M., Pecce V., Ramundo V., Durante C. Contemporary Thyroid Nodule Evaluation and Management. J. Clin. Endocrinol. Metab. 2020;105 doi: 10.1210/clinem/dgaa322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikiforova M.N., Mercurio S., Wald A.I., Barbi de Moura M., Callenberg K., Santana-Santos L., Gooding W.E., Yip L., Ferris R.L., Nikiforov Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. 2018;124:1682–1690. doi: 10.1002/cncr.31245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon R., Radmacher M.D., Dobbin K., McShane L.M. Pitfalls in the use of DNA microarray data for diagnostic and prognostic classification. J. Natl. Cancer Inst. 2003;95:14–18. doi: 10.1093/jnci/95.1.14. [DOI] [PubMed] [Google Scholar]
- 37.Moore R.Y., Eichler V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- 38.Ralph M.R., Foster R.G., Davis F.C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 39.Herzog E.D., Takahashi J.S., Block G.D. Clock controls circadian period in isolated suprachiasmatic nucleus neurons. Nat. Neurosci. 1998;1:708–713. doi: 10.1038/3708. [DOI] [PubMed] [Google Scholar]
- 40.Shearman L.P., Sriram S., Weaver D.R., Maywood E.S., Chaves I., Zheng B., Kume K., Lee C.C., van der Horst G.T., Hastings M.H., et al. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- 41.Harmer S.L., Panda S., Kay S.A. Molecular bases of circadian rhythms. Annu. Rev. Cell Dev. Biol. 2001;17:215–253. doi: 10.1146/annurev.cellbio.17.1.215. [DOI] [PubMed] [Google Scholar]
- 42.Darlington T.K., Wager-Smith K., Ceriani M.F., Staknis D., Gekakis N., Steeves T.D., Weitz C.J., Takahashi J.S., Kay S.A. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- 43.Wickwire E.M., Geiger-Brown J., Scharf S.M., Drake C.L. Shift Work and Shift Work Sleep Disorder: Clinical and Organizational Perspectives. Chest. 2017;151:1156–1172. doi: 10.1016/j.chest.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hastings M.H. Circadian clockwork: Two loops are better than one. Nat. Rev. Neurosci. 2000;1:143–146. doi: 10.1038/35039080. [DOI] [PubMed] [Google Scholar]
- 45.King D.P., Takahashi J.S. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 2000;23:713–742. doi: 10.1146/annurev.neuro.23.1.713. [DOI] [PubMed] [Google Scholar]
- 46.Lowrey P.L., Takahashi J.S. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and posttranslational regulation. Annu. Rev. Genet. 2000;34:533–562. doi: 10.1146/annurev.genet.34.1.533. [DOI] [PubMed] [Google Scholar]
- 47.Reppert S.M., Weaver D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 48.Sato F., Kawamoto T., Fujimoto K., Noshiro M., Honda K.K., Honma S., Honma K., Kato Y. Functional analysis of the basic helix-loop-helix transcription factor DEC1 in circadian regulation. Interaction with BMAL1. Eur. J. Biochem. 2004;271:4409–4419. doi: 10.1111/j.1432-1033.2004.04379.x. [DOI] [PubMed] [Google Scholar]
- 49.Eide E.J., Kang H., Crapo S., Gallego M., Virshup D.M. Casein kinase I in the mammalian circadian clock. Methods Enzymol. 2005;393:408–418. doi: 10.1016/S0076-6879(05)93019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee C., Weaver D.R., Reppert S.M. Direct association between mouse PERIOD and CKIepsilon is critical for a functioning circadian clock. Mol. Cell. Biol. 2004;24:584–594. doi: 10.1128/MCB.24.2.584-594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardone L., Hirayama J., Giordano F., Tamaru T., Palvimo J., Sassone-Corsi P. Circadian clock control by SUMOylation of BMAL1. Science. 2005;309:1390–1394. doi: 10.1126/science.1110689. [DOI] [PubMed] [Google Scholar]
- 52.Yagita K., Tamanini F., Yasuda M., Hoeijmakers J.H., van der Horst G.T., Okamura H. Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBO J. 2002;21:1301–1314. doi: 10.1093/emboj/21.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjarnason G.A., Jordan R. Circadian variation of cell proliferation and cell cycle protein expression in man: Clinical implications. Prog. Cell Cycle Res. 2000;4:193–206. doi: 10.1007/978-1-4615-4253-7_17. [DOI] [PubMed] [Google Scholar]
- 54.Kondratov R.V., Antoch M.P. Circadian proteins in the regulation of cell cycle and genotoxic stress responses. Trends Cell Biol. 2007;17:311–317. doi: 10.1016/j.tcb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Matsuo T., Yamaguchi S., Mitsui S., Emi A., Shimoda F., Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 56.Grechez-Cassiau A., Rayet B., Guillaumond F., Teboul M., Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J. Biol. Chem. 2008;283:4535–4542. doi: 10.1074/jbc.M705576200. [DOI] [PubMed] [Google Scholar]
- 57.Fu L., Pelicano H., Liu J., Huang P., Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/S0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 58.Hua H., Wang Y., Wan C., Liu Y., Zhu B., Yang C., Wang X., Wang Z., Cornelissen-Guillaume G., Halberg F. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97:589–596. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gotoh T., Vila-Caballer M., Santos C.S., Liu J., Yang J., Finkielstein C.V. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol. Biol. Cell. 2014;25:3081–3093. doi: 10.1091/mbc.e14-05-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou X., Kim D.W., Gotoh T., Liu J., Kim J.K., Finkielstein C.V. A Systems Biology Approach Identifies Hidden Regulatory Connections Between the Circadian and Cell-Cycle Checkpoints. Front. Physiol. 2020;11:327. doi: 10.3389/fphys.2020.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miki T., Matsumoto T., Zhao Z., Lee C.C. p53 regulates Period2 expression and the circadian clock. Nat. Commun. 2013;4:2444. doi: 10.1038/ncomms3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unsal-Kacmaz K., Mullen T.E., Kaufmann W.K., Sancar A. Coupling of human circadian and cell cycles by the timeless protein. Mol. Cell. Biol. 2005;25:3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corellou F., Bisgrove S.R., Kropf D.L., Meijer L., Kloareg B., Bouget F.Y. A S/M DNA replication checkpoint prevents nuclear and cytoplasmic events of cell division including centrosomal axis alignment and inhibits activation of cyclin-dependent kinase-like proteins in fucoid zygotes. Development. 2000;127:1651–1660. doi: 10.1242/dev.127.8.1651. [DOI] [PubMed] [Google Scholar]
- 64.Takai H., Tominaga K., Motoyama N., Minamishima Y.A., Nagahama H., Tsukiyama T., Ikeda K., Nakayama K., Nakanishi M., Nakayama K. Aberrant cell cycle checkpoint function and early embryonic death in Chk1(-/-) mice. Genes Dev. 2000;14:1439–1447. [PMC free article] [PubMed] [Google Scholar]
- 65.Fu L., Kettner N.M. The circadian clock in cancer development and therapy. Prog. Mol. Biol. Transl. Sci. 2013;119:221–282. doi: 10.1016/B978-0-12-396971-2.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haus E.L., Smolensky M.H. Shift work and cancer risk: Potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med. Rev. 2013;17:273–284. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Kelleher F.C., Rao A., Maguire A. Circadian molecular clocks and cancer. Cancer Lett. 2014;342:9–18. doi: 10.1016/j.canlet.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 68.Lahti T., Merikanto I., Partonen T. Circadian clock disruptions and the risk of cancer. Ann. Med. 2012;44:847–853. doi: 10.3109/07853890.2012.727018. [DOI] [PubMed] [Google Scholar]
- 69.West A.C., Bechtold D.A. The cost of circadian desynchrony: Evidence, insights and open questions. Bioessays. 2015;37:777–788. doi: 10.1002/bies.201400173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gauger M.A., Sancar A. Cryptochrome, circadian cycle, cell cycle checkpoints, and cancer. Cancer Res. 2005;65:6828–6834. doi: 10.1158/0008-5472.CAN-05-1119. [DOI] [PubMed] [Google Scholar]
- 71.Antoch M.P., Toshkov I., Kuropatwinski K.K., Jackson M. Deficiency in PER proteins has no effect on the rate of spontaneous and radiation-induced carcinogenesis. Cell Cycle. 2013;12:3673–3680. doi: 10.4161/cc.26614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gery S., Gombart A.F., Yi W.S., Koeffler C., Hofmann W.K., Koeffler H.P. Transcription profiling of C/EBP targets identifies Per2 as a gene implicated in myeloid leukemia. Blood. 2005;106:2827–2836. doi: 10.1182/blood-2005-01-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gery S., Komatsu N., Baldjyan L., Yu A., Koo D., Koeffler H.P. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol. Cell. 2006;22:375–382. doi: 10.1016/j.molcel.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 74.Xiang S., Coffelt S.B., Mao L., Yuan L., Cheng Q., Hill S.M. Period-2: A tumor suppressor gene in breast cancer. J. Circadian Rhythms. 2008;6:4. doi: 10.1186/1740-3391-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao Q., Gery S., Dashti A., Yin D., Zhou Y., Gu J., Koeffler H.P. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen S.T., Choo K.B., Hou M.F., Yeh K.T., Kuo S.J., Chang J.G. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 77.Gery S., Komatsu N., Kawamata N., Miller C.W., Desmond J., Virk R.K., Marchevsky A., McKenna R., Taguchi H., Koeffler H.P. Epigenetic silencing of the candidate tumor suppressor gene Per1 in non-small cell lung cancer. Clin. Cancer Res. 2007;13:1399–1404. doi: 10.1158/1078-0432.CCR-06-1730. [DOI] [PubMed] [Google Scholar]
- 78.Hsu C.M., Lin S.F., Lu C.T., Lin P.M., Yang M.Y. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33:149–155. doi: 10.1007/s13277-011-0258-2. [DOI] [PubMed] [Google Scholar]
- 79.Jung-Hynes B., Huang W., Reiter R.J., Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y.M., Chang J.H., Yeh K.T., Yang M.Y., Liu T.C., Lin S.F., Su W.W., Chang J.G. Disturbance of circadian gene expression in hepatocellular carcinoma. Mol. Carcinog. 2008;47:925–933. doi: 10.1002/mc.20446. [DOI] [PubMed] [Google Scholar]
- 81.Litlekalsoy J., Rostad K., Kalland K.H., Hostmark J.G., Laerum O.D. Expression of circadian clock genes and proteins in urothelial cancer is related to cancer-associated genes. BMC Cancer. 2016;16:549. doi: 10.1186/s12885-016-2580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mazzoccoli G., Piepoli A., Carella M., Panza A., Pazienza V., Benegiamo G., Palumbo O., Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed. Pharmacother. 2012;66:175–179. doi: 10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 83.Mostafaie N., Kallay E., Sauerzapf E., Bonner E., Kriwanek S., Cross H.S., Huber K.R., Krugluger W. Correlated downregulation of estrogen receptor beta and the circadian clock gene Per1 in human colorectal cancer. Mol. Carcinog. 2009;48:642–647. doi: 10.1002/mc.20510. [DOI] [PubMed] [Google Scholar]
- 84.Pogue-Geile K.L., Lyons-Weiler J., Whitcomb D.C. Molecular overlap of fly circadian rhythms and human pancreatic cancer. Cancer Lett. 2006;243:55–57. doi: 10.1016/j.canlet.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 85.Xia H.C., Niu Z.F., Ma H., Cao S.Z., Hao S.C., Liu Z.T., Wang F. Deregulated expression of the Per1 and Per2 in human gliomas. Can. J. Neurol. Sci. 2010;37:365–370. doi: 10.1017/S031716710001026X. [DOI] [PubMed] [Google Scholar]
- 86.Xiong H., Yang Y., Yang K., Zhao D., Tang H., Ran X. Loss of the clock gene PER2 is associated with cancer development and altered expression of important tumor-related genes in oral cancer. Int. J. Oncol. 2018;52:279–287. doi: 10.3892/ijo.2017.4180. [DOI] [PubMed] [Google Scholar]
- 87.Yang M.Y., Chang J.G., Lin P.M., Tang K.P., Chen Y.H., Lin H.Y., Liu T.C., Hsiao H.H., Liu Y.C., Lin S.F. Downregulation of circadian clock genes in chronic myeloid leukemia: Alternative methylation pattern of hPER3. Cancer Sci. 2006;97:1298–1307. doi: 10.1111/j.1349-7006.2006.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Okamura H., Miyake S., Sumi Y., Yamaguchi S., Yasui A., Muijtjens M., Hoeijmakers J.H., van der Horst G.T. Photic induction of mPer1 and mPer2 in cry-deficient mice lacking a biological clock. Science. 1999;286:2531–2534. doi: 10.1126/science.286.5449.2531. [DOI] [PubMed] [Google Scholar]
- 89.Lee S., Donehower L.A., Herron A.J., Moore D.D., Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS ONE. 2010;5:e10995. doi: 10.1371/journal.pone.0010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vitaterna M.H., Selby C.P., Todo T., Niwa H., Thompson C., Fruechte E.M., Hitomi K., Thresher R.J., Ishikawa T., Miyazaki J., et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad Sci. USA. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ozturk N., Lee J.H., Gaddameedhi S., Sancar A. Loss of cryptochrome reduces cancer risk in p53 mutant mice. Proc. Natl. Acad Sci. USA. 2009;106:2841–2846. doi: 10.1073/pnas.0813028106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee J.H., Sancar A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc. Natl. Acad Sci. USA. 2011;108:12036–12041. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee J.H., Sancar A. Circadian clock disruption improves the efficacy of chemotherapy through p73-mediated apoptosis. Proc. Natl. Acad Sci. USA. 2011;108:10668–10672. doi: 10.1073/pnas.1106284108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huber A.L., Papp S.J., Chan A.B., Henriksson E., Jordan S.D., Kriebs A., Nguyen M., Wallace M., Li Z., Metallo C.M., et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Mol. Cell. 2016;64:774–789. doi: 10.1016/j.molcel.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Papp S.J., Huber A.L., Jordan S.D., Kriebs A., Nguyen M., Moresco J., Yates J.R., Lamia K.A. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. eLife. 2015;4 doi: 10.7554/eLife.04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jung C.H., Kim E.M., Park J.K., Hwang S.G., Moon S.K., Kim W.J., Um H.D. Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol. Rep. 2013;29:2109–2113. doi: 10.3892/or.2013.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J., Li S., Li X., Li B., Li Y., Xia K., Yang Y., Aman S., Wang M., Wu H. Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase9 expression. Cancer Cell Int. 2019;19:182. doi: 10.1186/s12935-019-0902-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng Z.L., Wu M.W., Sun J., Sun Y.L., Cai Y.C., Huang Y.J., Xian L.J. Effects of the biological clock gene Bmal1 on tumour growth and anti-cancer drug activity. J. Biochem. 2010;148:319–326. doi: 10.1093/jb/mvq069. [DOI] [PubMed] [Google Scholar]
- 99.Jiang W., Zhao S., Jiang X., Zhang E., Hu G., Hu B., Zheng P., Xiao J., Lu Z., Lu Y., et al. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016;371:314–325. doi: 10.1016/j.canlet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 100.Tang Q., Cheng B., Xie M., Chen Y., Zhao J., Zhou X., Chen L. Circadian Clock Gene Bmal1 Inhibits Tumorigenesis and Increases Paclitaxel Sensitivity in Tongue Squamous Cell Carcinoma. Cancer Res. 2017;77:532–544. doi: 10.1158/0008-5472.CAN-16-1322. [DOI] [PubMed] [Google Scholar]
- 101.Zeng Z.L., Luo H.Y., Yang J., Wu W.J., Chen D.L., Huang P., Xu R.H. Overexpression of the circadian clock gene Bmal1 increases sensitivity to oxaliplatin in colorectal cancer. Clin. Cancer Res. 2014;20:1042–1052. doi: 10.1158/1078-0432.CCR-13-0171. [DOI] [PubMed] [Google Scholar]
- 102.Kettner N.M., Voicu H., Finegold M.J., Coarfa C., Sreekumar A., Putluri N., Katchy C.A., Lee C., Moore D.D., Fu L. Circadian Homeostasis of Liver Metabolism Suppresses Hepatocarcinogenesis. Cancer Cell. 2016;30:909–924. doi: 10.1016/j.ccell.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Papagiannakopoulos T., Bauer M.R., Davidson S.M., Heimann M., Subbaraj L., Bhutkar A., Bartlebaugh J., Vander Heiden M.G., Jacks T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016;24:324–331. doi: 10.1016/j.cmet.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Elshazley M., Sato M., Hase T., Yamashita R., Yoshida K., Toyokuni S., Ishiguro F., Osada H., Sekido Y., Yokoi K., et al. The circadian clock gene BMAL1 is a novel therapeutic target for malignant pleural mesothelioma. Int. J. Cancer. 2012;131:2820–2831. doi: 10.1002/ijc.27598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Puram R.V., Kowalczyk M.S., de Boer C.G., Schneider R.K., Miller P.G., McConkey M., Tothova Z., Tejero H., Heckl D., Jaras M., et al. Core Circadian Clock Genes Regulate Leukemia Stem Cells in AML. Cell. 2016;165:303–316. doi: 10.1016/j.cell.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Korkmaz T., Aygenli F., Emisoglu H., Ozcelik G., Canturk A., Yilmaz S., Ozturk N. Opposite Carcinogenic Effects of Circadian Clock Gene BMAL1. Sci. Rep. 2018;8:16023. doi: 10.1038/s41598-018-34433-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hoffman A.E., Yi C.H., Zheng T., Stevens R.G., Leaderer D., Zhang Y., Holford T.R., Hansen J., Paulson J., Zhu Y. CLOCK in breast tumorigenesis: Genetic, epigenetic, and transcriptional profiling analyses. Cancer Res. 2010;70:1459–1468. doi: 10.1158/0008-5472.CAN-09-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xiao L., Chang A.K., Zang M.X., Bi H., Li S., Wang M., Xing X., Wu H. Induction of the CLOCK gene by E2-ERalpha signaling promotes the proliferation of breast cancer cells. PLoS ONE. 2014;9:e95878. doi: 10.1371/journal.pone.0095878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Doi M., Hirayama J., Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 110.Sahar S., Sassone-Corsi P. Circadian clock and breast cancer: A molecular link. Cell Cycle. 2007;6:1329–1331. doi: 10.4161/cc.6.11.4295. [DOI] [PubMed] [Google Scholar]
- 111.Mormont M.C., Hecquet B., Bogdan A., Benavides M., Touitou Y., Levi F. Non-invasive estimation of the circadian rhythm in serum cortisol in patients with ovarian or colorectal cancer. Int. J. Cancer. 1998;78:421–424. doi: 10.1002/(SICI)1097-0215(19981109)78:4<421::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 112.Mormont M.C., Levi F. Circadian-system alterations during cancer processes: A review. Int. J. Cancer. 1997;70:241–247. doi: 10.1002/(SICI)1097-0215(19970117)70:2<241::AID-IJC16>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 113.Davis S., Mirick D.K., Stevens R.G. Night shift work, light at night, and risk of breast cancer. J. Natl. Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 114.Fritschi L., Erren T.C., Glass D.C., Girschik J., Thomson A.K., Saunders C., Boyle T., El-Zaemey S., Rogers P., Peters S., et al. The association between different night shiftwork factors and breast cancer: A case-control study. Br. J. Cancer. 2013;109:2472–2480. doi: 10.1038/bjc.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ijaz S., Verbeek J., Seidler A., Lindbohm M.L., Ojajarvi A., Orsini N., Costa G., Neuvonen K. Night-shift work and breast cancer--a systematic review and meta-analysis. Scand. J. Work Environ. Health. 2013;39:431–447. doi: 10.5271/sjweh.3371. [DOI] [PubMed] [Google Scholar]
- 116.James P., Bertrand K.A., Hart J.E., Schernhammer E.S., Tamimi R.M., Laden F. Outdoor Light at Night and Breast Cancer Incidence in the Nurses’ Health Study II. Environ. Health Perspect. 2017;125:087010. doi: 10.1289/EHP935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia Y., Lu Y., Wu K., Lin Q., Shen W., Zhu M., Huang S., Chen J. Does night work increase the risk of breast cancer? A systematic review and meta-analysis of epidemiological studies. Cancer Epidemiol. 2013;37:197–206. doi: 10.1016/j.canep.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 118.Kamdar B.B., Tergas A.I., Mateen F.J., Bhayani N.H., Oh J. Night-shift work and risk of breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013;138:291–301. doi: 10.1007/s10549-013-2433-1. [DOI] [PubMed] [Google Scholar]
- 119.Kojo K., Pukkala E., Auvinen A. Breast cancer risk among Finnish cabin attendants: A nested case-control study. Occup. Environ. Med. 2005;62:488–493. doi: 10.1136/oem.2004.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lin X., Chen W., Wei F., Ying M., Wei W., Xie X. Night-shift work increases morbidity of breast cancer and all-cause mortality: A meta-analysis of 16 prospective cohort studies. Sleep Med. 2015;16:1381–1387. doi: 10.1016/j.sleep.2015.02.543. [DOI] [PubMed] [Google Scholar]
- 121.Megdal S.P., Kroenke C.H., Laden F., Pukkala E., Schernhammer E.S. Night work and breast cancer risk: A systematic review and meta-analysis. Eur. J. Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 122.Menegaux F., Truong T., Anger A., Cordina-Duverger E., Lamkarkach F., Arveux P., Kerbrat P., Fevotte J., Guenel P. Night work and breast cancer: A population-based case-control study in France (the CECILE study) Int. J. Cancer. 2013;132:924–931. doi: 10.1002/ijc.27669. [DOI] [PubMed] [Google Scholar]
- 123.Pukkala E., Auvinen A., Wahlberg G. Incidence of cancer among Finnish airline cabin attendants, 1967–1992. BMJ. 1995;311:649–652. doi: 10.1136/bmj.311.7006.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schernhammer E.S., Kroenke C.H., Laden F., Hankinson S.E. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 125.Schernhammer E.S., Laden F., Speizer F.E., Willett W.C., Hunter D.J., Kawachi I., Colditz G.A. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J. Natl. Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 126.Thompson C.L., Li L. Association of sleep duration and breast cancer OncotypeDX recurrence score. Breast Cancer Res. Treat. 2012;134:1291–1295. doi: 10.1007/s10549-012-2144-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Travis R.C., Balkwill A., Fensom G.K., Appleby P.N., Reeves G.K., Wang X.S., Roddam A.W., Gathani T., Peto R., Green J., et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-analysis of Published Studies. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw169. [DOI] [Google Scholar]
- 128.Wang F., Yeung K.L., Chan W.C., Kwok C.C., Leung S.L., Wu C., Chan E.Y., Yu I.T., Yang X.R., Tse L.A. A meta-analysis on dose-response relationship between night shift work and the risk of breast cancer. Ann. Oncol. 2013;24:2724–2732. doi: 10.1093/annonc/mdt283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chu L.W., Zhu Y., Yu K., Zheng T., Yu H., Zhang Y., Sesterhenn I., Chokkalingam A.P., Danforth K.N., Shen M.C., et al. Variants in circadian genes and prostate cancer risk: A population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–348. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 130.Conlon M., Lightfoot N., Kreiger N. Rotating shift work and risk of prostate cancer. Epidemiology. 2007;18:182–183. doi: 10.1097/01.ede.0000249519.33978.31. [DOI] [PubMed] [Google Scholar]
- 131.Flynn-Evans E.E., Mucci L., Stevens R.G., Lockley S.W. Shiftwork and prostate-specific antigen in the National Health and Nutrition Examination Survey. J. Natl. Cancer Inst. 2013;105:1292–1297. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kubo T., Oyama I., Nakamura T., Kunimoto M., Kadowaki K., Otomo H., Fujino Y., Fujimoto N., Matsumoto T., Matsuda S. Industry-based retrospective cohort study of the risk of prostate cancer among rotating-shift workers. Int. J. Urol. 2011;18:206–211. doi: 10.1111/j.1442-2042.2010.02714.x. [DOI] [PubMed] [Google Scholar]
- 133.Markt S.C., Grotta A., Nyren O., Adami H.O., Mucci L.A., Valdimarsdottir U.A., Stattin P., Bellocco R., Lagerros Y.T. Insufficient Sleep and Risk of Prostate Cancer in a Large Swedish Cohort. Sleep. 2015;38:1405–1410. doi: 10.5665/sleep.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Papantoniou K., Castano-Vinyals G., Espinosa A., Aragones N., Perez-Gomez B., Burgos J., Gomez-Acebo I., Llorca J., Peiro R., Jimenez-Moleon J., et al. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int. J. Cancer. 2015;137:1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 135.Parent M.E., El-Zein M., Rousseau M.C., Pintos J., Siemiatycki J. Night work and the risk of cancer among men. Am. J. Epidemiol. 2012;176:751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 136.Rao D., Yu H., Bai Y., Zheng X., Xie L. Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. Onco Targets Ther. 2015;8:2817–2826. doi: 10.2147/OTT.S89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sigurdardottir L.G., Valdimarsdottir U.A., Fall K., Rider J.R., Lockley S.W., Schernhammer E., Mucci L.A. Circadian disruption, sleep loss, and prostate cancer risk: A systematic review of epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 2012;21:1002–1011. doi: 10.1158/1055-9965.EPI-12-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wendeu-Foyet M.G., Koudou Y., Cenee S., Tretarre B., Rebillard X., Cancel-Tassin G., Cussenot O., Boland A., Bacq D., Deleuze J.F., et al. Circadian genes and risk of prostate cancer: Findings from the EPICAP study. Int. J. Cancer. 2019;145:1745–1753. doi: 10.1002/ijc.32149. [DOI] [PubMed] [Google Scholar]
- 139.Wendeu-Foyet M.G., Menegaux F. Circadian Disruption and Prostate Cancer Risk: An Updated Review of Epidemiological Evidences. Cancer Epidemiol. Biomarkers Prev. 2017;26:985–991. doi: 10.1158/1055-9965.EPI-16-1030. [DOI] [PubMed] [Google Scholar]
- 140.Jiao L., Duan Z., Sangi-Haghpeykar H., Hale L., White D.L., El-Serag H.B. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br. J. Cancer. 2013;108:213–221. doi: 10.1038/bjc.2012.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Thompson C.L., Larkin E.K., Patel S., Berger N.A., Redline S., Li L. Short duration of sleep increases risk of colorectal adenoma. Cancer. 2011;117:841–847. doi: 10.1002/cncr.25507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Viswanathan A.N., Hankinson S.E., Schernhammer E.S. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 143.Lahti T.A., Partonen T., Kyyronen P., Kauppinen T., Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int. J. Cancer. 2008;123:2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 144.Mormont M.C., Waterhouse J., Bleuzen P., Giacchetti S., Jami A., Bogdan A., Lellouch J., Misset J.L., Touitou Y., Levi F. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin. Cancer Res. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 145.Baan R., Grosse Y., Straif K., Secretan B., El Ghissassi F., Bouvard V., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., et al. A review of human carcinogens—Part F: Chemical agents and related occupations. Lancet Oncol. 2009;10:1143–1144. doi: 10.1016/S1470-2045(09)70358-4. [DOI] [PubMed] [Google Scholar]
- 146.Straif K., Baan R., Grosse Y., Secretan B., El Ghissassi F., Bouvard V., Altieri A., Benbrahim-Tallaa L., Cogliano V., Group WHOIAFRoCM Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 147.Costa G., Haus E., Stevens R. Shift work and cancer-considerations on rationale, mechanisms, and epidemiology. Scand. J. Work Environ. Health. 2010;36:163–179. doi: 10.5271/sjweh.2899. [DOI] [PubMed] [Google Scholar]
- 148.Fritschi L., Glass D.C., Heyworth J.S., Aronson K., Girschik J., Boyle T., Grundy A., Erren T.C. Hypotheses for mechanisms linking shiftwork and cancer. Med. Hypotheses. 2011;77:430–436. doi: 10.1016/j.mehy.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 149.Dierickx P., Van Laake L.W., Geijsen N. Circadian clocks: From stem cells to tissue homeostasis and regeneration. EMBO Rep. 2018;19:18–28. doi: 10.15252/embr.201745130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dierickx P., Vermunt M.W., Muraro M.J., Creyghton M.P., Doevendans P.A., van Oudenaarden A., Geijsen N., Van Laake L.W. Circadian networks in human embryonic stem cell-derived cardiomyocytes. EMBO Rep. 2017;18:1199–1212. doi: 10.15252/embr.201743897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Paatela E., Munson D., Kikyo N. Circadian Regulation in Tissue Regeneration. Int. J. Mol. Sci. 2019;20:2263. doi: 10.3390/ijms20092263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weger M., Diotel N., Dorsemans A.C., Dickmeis T., Weger B.D. Stem cells and the circadian clock. Dev. Biol. 2017;431:111–123. doi: 10.1016/j.ydbio.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 153.Umemura Y., Koike N., Ohashi M., Tsuchiya Y., Meng Q.J., Minami Y., Hara M., Hisatomi M., Yagita K. Involvement of posttranscriptional regulation of Clock in the emergence of circadian clock oscillation during mouse development. Proc. Natl. Acad Sci. USA. 2017;114:E7479–E7488. doi: 10.1073/pnas.1703170114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yagita K., Horie K., Koinuma S., Nakamura W., Yamanaka I., Urasaki A., Shigeyoshi Y., Kawakami K., Shimada S., Takeda J., et al. Development of the circadian oscillator during differentiation of mouse embryonic stem cells in vitro. Proc. Natl. Acad Sci. USA. 2010;107:3846–3851. doi: 10.1073/pnas.0913256107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kondratov R.V., Kondratova A.A., Gorbacheva V.Y., Vykhovanets O.V., Antoch M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zheng B., Larkin D.W., Albrecht U., Sun Z.S., Sage M., Eichele G., Lee C.C., Bradley A. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 157.Brown S.A. Circadian clock-mediated control of stem cell division and differentiation: Beyond night and day. Development. 2014;141:3105–3111. doi: 10.1242/dev.104851. [DOI] [PubMed] [Google Scholar]
- 158.Plikus M.V., Chuong C.M. Complex hair cycle domain patterns and regenerative hair waves in living rodents. J. Investig. Dermatol. 2008;128:1071–1080. doi: 10.1038/sj.jid.5701180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gaddameedhi S., Selby C.P., Kaufmann W.K., Smart R.C., Sancar A. Control of skin cancer by the circadian rhythm. Proc. Natl. Acad Sci. USA. 2011;108:18790–18795. doi: 10.1073/pnas.1115249108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Janich P., Toufighi K., Solanas G., Luis N.M., Minkwitz S., Serrano L., Lehner B., Benitah S.A. Human epidermal stem cell function is regulated by circadian oscillations. Cell Stem Cell. 2013;13:745–753. doi: 10.1016/j.stem.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 161.Lin K.K., Kumar V., Geyfman M., Chudova D., Ihler A.T., Smyth P., Paus R., Takahashi J.S., Andersen B. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 2009;5:e1000573. doi: 10.1371/journal.pgen.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Plikus M.V., Van Spyk E.N., Pham K., Geyfman M., Kumar V., Takahashi J.S., Andersen B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythms. 2015;30:163–182. doi: 10.1177/0748730414563537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoyle N.P., Seinkmane E., Putker M., Feeney K.A., Krogager T.P., Chesham J.E., Bray L.K., Thomas J.M., Dunn K., Blaikley J., et al. Circadian actin dynamics drive rhythmic fibroblast mobilization during wound healing. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Durgan D.J., Young M.E. The cardiomyocyte circadian clock: Emerging roles in health and disease. Circ. Res. 2010;106:647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sundar I.K., Yao H., Sellix M.T., Rahman I. Circadian molecular clock in lung pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L1056–L1075. doi: 10.1152/ajplung.00152.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tsinkalovsky O., Filipski E., Rosenlund B., Sothern R.B., Eiken H.G., Wu M.W., Claustrat B., Bayer J., Levi F., Laerum O.D. Circadian expression of clock genes in purified hematopoietic stem cells is developmentally regulated in mouse bone marrow. Exp. Hematol. 2006;34:1249–1261. doi: 10.1016/j.exphem.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 167.Tsinkalovsky O., Rosenlund B., Laerum O.D., Eiken H.G. Clock gene expression in purified mouse hematopoietic stem cells. Exp. Hematol. 2005;33:100–107. doi: 10.1016/j.exphem.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 168.Karpowicz P., Zhang Y., Hogenesch J.B., Emery P., Perrimon N. The circadian clock gates the intestinal stem cell regenerative state. Cell Rep. 2013;3:996–1004. doi: 10.1016/j.celrep.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Konturek P.C., Brzozowski T., Konturek S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. 2011;62:591–599. [PubMed] [Google Scholar]
- 170.Pagel R., Bar F., Schroder T., Sunderhauf A., Kunstner A., Ibrahim S.M., Autenrieth S.E., Kalies K., Konig P., Tsang A.H., et al. Circadian rhythm disruption impairs tissue homeostasis and exacerbates chronic inflammation in the intestine. FASEB J. 2017;31:4707–4719. doi: 10.1096/fj.201700141RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stokes K., Cooke A., Chang H., Weaver D.R., Breault D.T., Karpowicz P. The Circadian Clock Gene BMAL1 Coordinates Intestinal Regeneration. Cell. Mol. Gastroenterol. Hepatol. 2017;4:95–114. doi: 10.1016/j.jcmgh.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dong Z., Zhang G., Qu M., Gimple R.C., Wu Q., Qiu Z., Prager B.C., Wang X., Kim L.J.Y., Morton A.R., et al. Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov. 2019;9:1556–1573. doi: 10.1158/2159-8290.CD-19-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma D., Hou L., Xia H., Li H., Fan H., Jia X., Niu Z. PER2 inhibits proliferation and stemness of glioma stem cells via the Wnt/betacatenin signaling pathway. Oncol. Rep. 2020;44:533–542. doi: 10.3892/or.2020.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhang F., Sun H., Zhang S., Yang X., Zhang G., Su T. Overexpression of PER3 Inhibits Self-Renewal Capability and Chemoresistance of Colorectal Cancer Stem-Like Cells via Inhibition of Notch and beta-Catenin Signaling. Oncol. Res. 2017;25:709–719. doi: 10.3727/096504016X14772331883976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Matsunaga N., Ogino T., Hara Y., Tanaka T., Koyanagi S., Ohdo S. Optimized Dosing Schedule Based on Circadian Dynamics of Mouse Breast Cancer Stem Cells Improves the Antitumor Effects of Aldehyde Dehydrogenase Inhibitor. Cancer Res. 2018;78:3698–3708. doi: 10.1158/0008-5472.CAN-17-4034. [DOI] [PubMed] [Google Scholar]
- 176.Nikiforov Y.E., Nikiforova M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011;7:569–580. doi: 10.1038/nrendo.2011.142. [DOI] [PubMed] [Google Scholar]
- 177.DeLellis R.A. Pathology and genetics of thyroid carcinoma. J. Surg. Oncol. 2006;94:662–669. doi: 10.1002/jso.20700. [DOI] [PubMed] [Google Scholar]
- 178.Jin S., Borkhuu O., Bao W., Yang Y.T. Signaling Pathways in Thyroid Cancer and Their Therapeutic Implications. J. Clin. Med. Res. 2016;8:284–296. doi: 10.14740/jocmr2480w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nikiforov Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008;21(Suppl. 2):S37–S43. doi: 10.1038/modpathol.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.De Felice M., Postiglione M.P., Di Lauro R. Minireview: Thyrotropin receptor signaling in development and differentiation of the thyroid gland: Insights from mouse models and human diseases. Endocrinology. 2004;145:4062–4067. doi: 10.1210/en.2004-0501. [DOI] [PubMed] [Google Scholar]
- 181.Franco A.T., Malaguarnera R., Refetoff S., Liao X.H., Lundsmith E., Kimura S., Pritchard C., Marais R., Davies T.F., Weinstein L.S., et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc. Natl. Acad Sci. USA. 2011;108:1615–1620. doi: 10.1073/pnas.1015557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kimura T., Van Keymeulen A., Golstein J., Fusco A., Dumont J.E., Roger P.P. Regulation of thyroid cell proliferation by TSH and other factors: A critical evaluation of in vitro models. Endocr. Rev. 2001;22:631–656. doi: 10.1210/edrv.22.5.0444. [DOI] [PubMed] [Google Scholar]
- 183.Malaguarnera R., Chen K.Y., Kim T.Y., Dominguez J.M., Voza F., Ouyang B., Vundavalli S.K., Knauf J.A., Fagin J.A. Switch in signaling control of mTORC1 activity after oncoprotein expression in thyroid cancer cell lines. J. Clin. Endocrinol. Metab. 2014;99:E1976–E1987. doi: 10.1210/jc.2013-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nozhat Z., Hedayati M. PI3K/AKT Pathway and Its Mediators in Thyroid Carcinomas. Mol. Diagn. Ther. 2016;20:13–26. doi: 10.1007/s40291-015-0175-y. [DOI] [PubMed] [Google Scholar]
- 185.Ricarte-Filho J.C., Ryder M., Chitale D.A., Rivera M., Heguy A., Ladanyi M., Janakiraman M., Solit D., Knauf J.A., Tuttle R.M., et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gimm O., Attie-Bitach T., Lees J.A., Vekemans M., Eng C. Expression of the PTEN tumour suppressor protein during human development. Hum. Mol. Genet. 2000;9:1633–1639. doi: 10.1093/hmg/9.11.1633. [DOI] [PubMed] [Google Scholar]
- 187.Malaguarnera R., Morcavallo A., Belfiore A. The insulin and igf-I pathway in endocrine glands carcinogenesis. J. Oncol. 2012;2012:635614. doi: 10.1155/2012/635614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Malaguarnera R., Belfiore A. The insulin receptor: A new target for cancer therapy. Front. Endocrinol. 2011;2:93. doi: 10.3389/fendo.2011.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vella V., Pandini G., Sciacca L., Mineo R., Vigneri R., Pezzino V., Belfiore A. A novel autocrine loop involving IGF-II and the insulin receptor isoform-A stimulates growth of thyroid cancer. J. Clin. Endocrinol. Metab. 2002;87:245–254. doi: 10.1210/jcem.87.1.8142. [DOI] [PubMed] [Google Scholar]
- 190.Malaguarnera R., Belfiore A. The emerging role of insulin and insulin-like growth factor signaling in cancer stem cells. Front. Endocrinol. 2014;5:10. doi: 10.3389/fendo.2014.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malaguarnera R., Morcavallo A., Giuliano S., Belfiore A. Thyroid cancer development and progression: Emerging role of cancer stem cells. Minerva Endocrinol. 2012;37:103–115. [PubMed] [Google Scholar]
- 192.Belfiore A., Gangemi P., Costantino A., Russo G., Santonocito G.M., Ippolito O., Di Renzo M.F., Comoglio P., Fiumara A., Vigneri R. Negative/low expression of the Met/hepatocyte growth factor receptor identifies papillary thyroid carcinomas with high risk of distant metastases. J. Clin. Endocrinol. Metab. 1997;82:2322–2328. doi: 10.1210/jc.82.7.2322. [DOI] [PubMed] [Google Scholar]
- 193.Di Renzo M.F., Olivero M., Ferro S., Prat M., Bongarzone I., Pilotti S., Belfiore A., Costantino A., Vigneri R., Pierotti M.A., et al. Overexpression of the c-MET/HGF receptor gene in human thyroid carcinomas. Oncogene. 1992;7:2549–2553. [PubMed] [Google Scholar]
- 194.Mineo R., Costantino A., Frasca F., Sciacca L., Russo S., Vigneri R., Belfiore A. Activation of the hepatocyte growth factor (HGF)-Met system in papillary thyroid cancer: Biological effects of HGF in thyroid cancer cells depend on Met expression levels. Endocrinology. 2004;145:4355–4365. doi: 10.1210/en.2003-1762. [DOI] [PubMed] [Google Scholar]
- 195.Ruco L., Scarpino S. The Pathogenetic Role of the HGF/c-Met System in Papillary Carcinoma of the Thyroid. Biomedicines. 2014;2:263–274. doi: 10.3390/biomedicines2040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Malaguarnera R., Nicolosi M.L., Sacco A., Morcavallo A., Vella V., Voci C., Spatuzza M., Xu S.Q., Iozzo R.V., Vigneri R., et al. Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses. Oncotarget. 2015;6:16084–16105. doi: 10.18632/oncotarget.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malaguarnera R., Vella V., Pellegriti G., Belfiore A. Editorial: Clinical and Molecular Epidemiology of Thyroid Cancer of Follicular Origin. Front. Endocrinol. 2018;9:67. doi: 10.3389/fendo.2018.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vella V., Malaguarnera R. The Emerging Role of Insulin Receptor Isoforms in Thyroid Cancer: Clinical Implications and New Perspectives. Int. J. Mol. Sci. 2018;19:3814. doi: 10.3390/ijms19123814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vella V., Malaguarnera R., Nicolosi M.L., Morrione A., Belfiore A. Insulin/IGF signaling and discoidin domain receptors: An emerging functional connection. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:118522. doi: 10.1016/j.bbamcr.2019.118522. [DOI] [PubMed] [Google Scholar]
- 200.Vella V., Nicolosi M.L., Cantafio P., Massimino M., Lappano R., Vigneri P., Ciuni R., Gangemi P., Morrione A., Malaguarnera R., et al. DDR1 regulates thyroid cancer cell differentiation via IGF-2/IR-A autocrine signaling loop. Endocr. Relat. Cancer. 2019;26:197–214. doi: 10.1530/ERC-18-0310. [DOI] [PubMed] [Google Scholar]
- 201.Belfiore A., Malaguarnera R., Vella V., Lawrence M.C., Sciacca L., Frasca F., Morrione A., Vigneri R. Insulin Receptor Isoforms in Physiology and Disease: An Updated View. Endocr. Rev. 2017;38:379–431. doi: 10.1210/er.2017-00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Frasca F., Vella V., Aloisi A., Mandarino A., Mazzon E., Vigneri R., Vigneri P. p73 tumor-suppressor activity is impaired in human thyroid cancer. Cancer Res. 2003;63:5829–5837. [PubMed] [Google Scholar]
- 203.Kunstman J.W., Juhlin C.C., Goh G., Brown T.C., Stenman A., Healy J.M., Rubinstein J.C., Choi M., Kiss N., Nelson-Williams C., et al. Characterization of the mutational landscape of anaplastic thyroid cancer via whole-exome sequencing. Hum. Mol. Genet. 2015;24:2318–2329. doi: 10.1093/hmg/ddu749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Landa I., Ibrahimpasic T., Boucai L., Sinha R., Knauf J.A., Shah R.H., Dogan S., Ricarte-Filho J.C., Krishnamoorthy G.P., Xu B., et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J. Clin. Investig. 2016;126:1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Malaguarnera R., Mandarino A., Mazzon E., Vella V., Gangemi P., Vancheri C., Vigneri P., Aloisi A., Vigneri R., Frasca F. The p53-homologue p63 may promote thyroid cancer progression. Endocr. Relat. Cancer. 2005;12:953–971. doi: 10.1677/erc.1.00968. [DOI] [PubMed] [Google Scholar]
- 206.Malaguarnera R., Vella V., Pandini G., Sanfilippo M., Pezzino V., Vigneri R., Frasca F. TAp73 alpha increases p53 tumor suppressor activity in thyroid cancer cells via the inhibition of Mdm2-mediated degradation. Mol. Cancer Res. 2008;6:64–77. doi: 10.1158/1541-7786.MCR-07-0005. [DOI] [PubMed] [Google Scholar]
- 207.Malaguarnera R., Vella V., Vigneri R., Frasca F. p53 family proteins in thyroid cancer. Endocr. Relat. Cancer. 2007;14:43–60. doi: 10.1677/erc.1.01223. [DOI] [PubMed] [Google Scholar]
- 208.Pozdeyev N., Gay L.M., Sokol E.S., Hartmaier R., Deaver K.E., Davis S., French J.D., Borre P.V., LaBarbera D.V., Tan A.C., et al. Genetic Analysis of 779 Advanced Differentiated and Anaplastic Thyroid Cancers. Clin. Cancer Res. 2018;24:3059–3068. doi: 10.1158/1078-0432.CCR-18-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sykorova V., Dvorakova S., Vcelak J., Vaclavikova E., Halkova T., Kodetova D., Lastuvka P., Betka J., Vlcek P., Reboun M., et al. Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Res. 2015;35:2029–2036. [PubMed] [Google Scholar]
- 210.Xu B., Ghossein R. Genomic Landscape of poorly Differentiated and Anaplastic Thyroid Carcinoma. Endocr. Pathol. 2016;27:205–212. doi: 10.1007/s12022-016-9445-4. [DOI] [PubMed] [Google Scholar]
- 211.Nagayama Y., Shimamura M., Mitsutake N. Cancer Stem Cells in the Thyroid. Front. Endocrinol. 2016;7:20. doi: 10.3389/fendo.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zane M., Scavo E., Catalano V., Bonanno M., Todaro M., De Maria R., Stassi G. Normal vs. cancer thyroid stem cells: The road to transformation. Oncogene. 2016;35:805–815. doi: 10.1038/onc.2015.138. [DOI] [PubMed] [Google Scholar]
- 213.Takano T. Fetal cell carcinogenesis of the thyroid: A hypothesis for better understanding of gene expression profile and genomic alternation in thyroid carcinoma. Endocr. J. 2004;51:509–515. doi: 10.1507/endocrj.51.509. [DOI] [PubMed] [Google Scholar]
- 214.Malaguarnera R., Frasca F., Garozzo A., Giani F., Pandini G., Vella V., Vigneri R., Belfiore A. Insulin receptor isoforms and insulin-like growth factor receptor in human follicular cell precursors from papillary thyroid cancer and normal thyroid. J. Clin. Endocrinol. Metab. 2011;96:766–774. doi: 10.1210/jc.2010-1255. [DOI] [PubMed] [Google Scholar]
- 215.Kimura E.T., Nikiforova M.N., Zhu Z., Knauf J.A., Nikiforov Y.E., Fagin J.A. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 216.Lowry W.E., Richter L. Signaling in adult stem cells. Front. Biosci. 2007;12:3911–3927. doi: 10.2741/2360. [DOI] [PubMed] [Google Scholar]
- 217.Shiraiwa K., Matsuse M., Nakazawa Y., Ogi T., Suzuki K., Saenko V., Xu S., Umezawa K., Yamashita S., Tsukamoto K., et al. JAK/STAT3 and NF-kappaB Signaling Pathways Regulate Cancer Stem-Cell Properties in Anaplastic Thyroid Cancer Cells. Thyroid. 2019;29:674–682. doi: 10.1089/thy.2018.0212. [DOI] [PubMed] [Google Scholar]
- 218.Todaro M., Iovino F., Eterno V., Cammareri P., Gambara G., Espina V., Gulotta G., Dieli F., Giordano S., De Maria R., et al. Tumorigenic and metastatic activity of human thyroid cancer stem cells. Cancer Res. 2010;70:8874–8885. doi: 10.1158/0008-5472.CAN-10-1994. [DOI] [PubMed] [Google Scholar]
- 219.Forte S., La Rosa C., Pecce V., Rosignolo F., Memeo L. The role of microRNAs in thyroid carcinomas. Anticancer Res. 2015;35:2037–2047. [PubMed] [Google Scholar]
- 220.He H., Olesnanik K., Nagy R., Liyanarachchi S., Prasad M.L., Stratakis C.A., Kloos R.T., de la Chapelle A. Allelic variation in gene expression in thyroid tissue. Thyroid. 2005;15:660–667. doi: 10.1089/thy.2005.15.660. [DOI] [PubMed] [Google Scholar]
- 221.Pallante P., Visone R., Ferracin M., Ferraro A., Berlingieri M.T., Troncone G., Chiappetta G., Liu C.G., Santoro M., Negrini M., et al. MicroRNA deregulation in human thyroid papillary carcinomas. Endocr. Relat. Cancer. 2006;13:497–508. doi: 10.1677/erc.1.01209. [DOI] [PubMed] [Google Scholar]
- 222.Sheng W., Chen Y., Gong Y., Dong T., Zhang B., Gao W. miR-148a inhibits self-renewal of thyroid cancer stem cells via repressing INO80 expression. Oncol. Rep. 2016;36:3387–3396. doi: 10.3892/or.2016.5203. [DOI] [PubMed] [Google Scholar]
- 223.Haghpanah V., Fallah P., Tavakoli R., Naderi M., Samimi H., Soleimani M., Larijani B. Antisense-miR-21 enhances differentiation/apoptosis and reduces cancer stemness state on anaplastic thyroid cancer. Tumour Biol. 2016;37:1299–1308. doi: 10.1007/s13277-015-3923-z. [DOI] [PubMed] [Google Scholar]
- 224.Li M., Chai H.F., Peng F., Meng Y.T., Zhang L.Z., Zhang L., Zou H., Liang Q.L., Li M.M., Mao K.G., et al. Estrogen receptor beta upregulated by lncRNA-H19 to promote cancer stem-like properties in papillary thyroid carcinoma. Cell Death Dis. 2018;9:1120. doi: 10.1038/s41419-018-1077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gao Y., Wang P.X., Cheng J.W., Sun Y.F., Hu B., Guo W., Zhou K.Q., Yin Y., Li Y.C., Wang J., et al. Chemotherapeutic perfusion of portal vein after tumor thrombectomy and hepatectomy benefits patients with advanced hepatocellular carcinoma: A propensity score-matched survival analysis. Cancer Med. 2019;8:6933–6944. doi: 10.1002/cam4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gamble K.L., Berry R., Frank S.J., Young M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014;10:466–475. doi: 10.1038/nrendo.2014.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weeke J., Gundersen H.J. Circadian and 30 minutes variations in serum TSH and thyroid hormones in normal subjects. Acta Endocrinol. 1978;89:659–672. doi: 10.1530/acta.0.0890659. [DOI] [PubMed] [Google Scholar]
- 228.Fahrenkrug J., Georg B., Hannibal J., Jorgensen H.L. Hypophysectomy abolishes rhythms in rat thyroid hormones but not in the thyroid clock. J. Endocrinol. 2017;233:209–216. doi: 10.1530/JOE-17-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gronfier C., Brandenberger G. Ultradian rhythms in pituitary and adrenal hormones: Their relations to sleep. Sleep Med. Rev. 1998;2:17–29. doi: 10.1016/S1087-0792(98)90051-X. [DOI] [PubMed] [Google Scholar]
- 230.Magrini A., Pietroiusti A., Coppeta L., Babbucci A., Barnaba E., Papadia C., Iannaccone U., Boscolo P., Bergamaschi E., Bergamaschi A. Shift work and autoimmune thyroid disorders. Int. J. Immunopathol. Pharmacol. 2006;19:31–36. [PubMed] [Google Scholar]
- 231.Amir S., Robinson B. Thyroidectomy alters the daily pattern of expression of the clock protein, PER2, in the oval nucleus of the bed nucleus of the stria terminalis and central nucleus of the amygdala in rats. Neurosci. Lett. 2006;407:254–257. doi: 10.1016/j.neulet.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 232.Bargi-Souza P., Peliciari-Garcia R.A., Nunes M.T. Disruption of the Pituitary Circadian Clock Induced by Hypothyroidism and Hyperthyroidism: Consequences on Daily Pituitary Hormone Expression Profiles. Thyroid. 2019;29:502–512. doi: 10.1089/thy.2018.0578. [DOI] [PubMed] [Google Scholar]
- 233.Peliciari-Garcia R.A., Bargi-Souza P., Young M.E., Nunes M.T. Repercussions of hypo and hyperthyroidism on the heart circadian clock. Chronobiol. Int. 2018;35:147–159. doi: 10.1080/07420528.2017.1388253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Peliciari-Garcia R.A., Previde R.M., Nunes M.T., Young M.E. Interrelationship between 3,5,3 -triiodothyronine and the circadian clock in the rodent heart. Chronobiol. Int. 2016;33:1444–1454. doi: 10.1080/07420528.2016.1229673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hirschfeld U., Moreno-Reyes R., Akseki E., L’Hermite-Baleriaux M., Leproult R., Copinschi G., Van Cauter E. Progressive elevation of plasma thyrotropin during adaptation to simulated jet lag: Effects of treatment with bright light or zolpidem. J. Clin. Endocrinol. Metab. 1996;81:3270–3277. doi: 10.1210/jcem.81.9.8784082. [DOI] [PubMed] [Google Scholar]
- 236.Ledda C., Cina D., Matera S., Mucci N., Bracci M., Rapisarda V. High HOMA-IR Index in Healthcare Shift Workers. Medicina. 2019;55:186. doi: 10.3390/medicina55050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Liu G.S., Cook A., Richardson M., Vail D., Holsinger F.C., Oakley-Girvan I. Thyroid cancer risk in airline cockpit and cabin crew: A meta-analysis. Cancers Head Neck. 2018;3:7. doi: 10.1186/s41199-018-0034-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Pinkerton L.E., Hein M.J., Anderson J.L., Christianson A., Little M.P., Sigurdson A.J., Schubauer-Berigan M.K. Melanoma, thyroid cancer, and gynecologic cancers in a cohort of female flight attendants. Am. J. Ind. Med. 2018;61:572–581. doi: 10.1002/ajim.22854. [DOI] [PubMed] [Google Scholar]
- 239.Luo J., Sands M., Wactawski-Wende J., Song Y., Margolis K.L. Sleep disturbance and incidence of thyroid cancer in postmenopausal women the Women’s Health Initiative. Am. J. Epidemiol. 2013;177:42–49. doi: 10.1093/aje/kws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Morales-Santana S., Morell S., Leon J., Carazo-Gallego A., Jimenez-Lopez J.C., Morell M. An Overview of the Polymorphisms of Circadian Genes Associated with Endocrine Cancer. Front. Endocrinol. 2019;10:104. doi: 10.3389/fendo.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Gallo C., Fragliasso V., Donati B., Torricelli F., Tameni A., Piana S., Ciarrocchi A. The bHLH transcription factor DEC1 promotes thyroid cancer aggressiveness by the interplay with NOTCH1. Cell Death Dis. 2018;9:871. doi: 10.1038/s41419-018-0933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mond M., Alexiadis M., Eriksson N., Davis M.J., Muscat G.E., Fuller P.J., Gilfillan C. Nuclear receptor expression in human differentiated thyroid tumors. Thyroid. 2014;24:1000–1011. doi: 10.1089/thy.2013.0509. [DOI] [PubMed] [Google Scholar]
- 243.Leenhardt L., Grosclaude P. [Epidemiology of thyroid carcinoma over the world] Ann. Endocrinol. 2011;72:136–148. doi: 10.1016/j.ando.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 244.Copinschi G., Spiegel K., Leproult R., Van Cauter E. Pathophysiology of human circadian rhythms. Novartis Found. Symp. 2000;227:143–157; discussion 157–162. doi: 10.1002/0470870796.ch9. [DOI] [PubMed] [Google Scholar]
- 245.Maury E., Ramsey K.M., Bass J. Circadian rhythms and metabolic syndrome: From experimental genetics to human disease. Circ. Res. 2010;106:447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Ledda C., Rapisarda V. Occupational and Environmental Carcinogenesis. Cancers. 2020;12:2547. doi: 10.3390/cancers12092547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Relogio A., Thomas P., Medina-Perez P., Reischl S., Bervoets S., Gloc E., Riemer P., Mang-Fatehi S., Maier B., Schafer R., et al. Ras-mediated deregulation of the circadian clock in cancer. PLoS Genet. 2014;10:e1004338. doi: 10.1371/journal.pgen.1004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kettner N.M., Mayo S.A., Hua J., Lee C., Moore D.D., Fu L. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab. 2015;22:448–459. doi: 10.1016/j.cmet.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pan X., Bradfield C.A., Hussain M.M. Global and hepatocyte-specific ablation of Bmal1 induces hyperlipidaemia and enhances atherosclerosis. Nat. Commun. 2016;7:13011. doi: 10.1038/ncomms13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Shi S.Q., Ansari T.S., McGuinness O.P., Wasserman D.H., Johnson C.H. Circadian disruption leads to insulin resistance and obesity. Curr. Biol. 2013;23:372–381. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]