Toxoplasma gondii is known to infect a considerable number of mammalian and avian species and a substantial proportion of the world’s human population. The parasite has an impressive ability to disseminate within the host’s body and employs various tactics to overcome the highly regulatory blood-brain barrier and reside in the brain. In healthy individuals, T. gondii infection is largely tolerated without any obvious ill effects. However, primary infection in immunosuppressed patients can result in acute cerebral or systemic disease, and reactivation of latent tissue cysts can lead to a deadly outcome.
KEYWORDS: Toxoplasma gondii, toxoplasmic encephalitis, immunocompromised patients, pathophysiology, diagnosis, treatment, cerebral toxoplasmosis
SUMMARY
Toxoplasma gondii is known to infect a considerable number of mammalian and avian species and a substantial proportion of the world’s human population. The parasite has an impressive ability to disseminate within the host’s body and employs various tactics to overcome the highly regulatory blood-brain barrier and reside in the brain. In healthy individuals, T. gondii infection is largely tolerated without any obvious ill effects. However, primary infection in immunosuppressed patients can result in acute cerebral or systemic disease, and reactivation of latent tissue cysts can lead to a deadly outcome. It is imperative that treatment of life-threatening toxoplasmic encephalitis is timely and effective. Several therapeutic and prophylactic regimens have been used in clinical practice. Current approaches can control infection caused by the invasive and highly proliferative tachyzoites but cannot eliminate the dormant tissue cysts. Adverse events and other limitations are associated with the standard pyrimethamine-based therapy, and effective vaccines are unavailable. In this review, the epidemiology, economic impact, pathophysiology, diagnosis, and management of cerebral toxoplasmosis are discussed, and critical areas for future research are highlighted.
INTRODUCTION
The opportunistic organism Toxoplasma gondii has reached a global interest due to its public health and socioeconomic impacts. This apicomplexan parasite can infect a large number of domestic and wild animals and has infected a significant number of people throughout the world (1). People become infected primarily through consuming raw or improperly cooked meat (particularly lamb and pork) containing infectious tissue cysts or via ingestion of sporulated oocysts in vegetables, fruits, or water contaminated with feline feces (2). At the initial infection site in the intestine, T. gondii infects various immune cells and use them to migrate to and infiltrate the brain, where it employs various strategies to overcome the complex cellular structure of the blood-brain barrier (BBB). In the vast majority of individuals with competent immune responses, primary infection is asymptomatic or may produce a mild, flu-like illness, and the parasite eventually lies dormant within a tissue cyst. However, in less than 10% of infections, a mononucleosis-like syndrome with headache, malaise, fever, cervical lymphadenopathy, and fatigue may occur (3). Primary T. gondii infection can also cause ocular disease, and in pregnant women, can lead to fetal death or brain damage in congenitally infected children (4–6).
In addition to the three classical clinical forms of toxoplasmosis (ocular, congenital, and cerebral), association of latent T. gondii infection with a number of behavioral modifications and neuropsychiatric disorders has also been reported (7). Recurrence of toxoplasmosis from latency is a frequent cause of toxoplasmic encephalitis (TE) in people with immunosuppressive conditions such as advanced HIV infection, organ transplantation, and neoplastic disease, or in those receiving immunosuppressive therapies (e.g., rituximab). These patients are particularly vulnerable to recrudescence of latent infection, in which slowly dividing bradyzoites transform into rapidly replicating tachyzoites, which can result in fatal consequences (3). Symptoms of TE may include diffuse encephalopathy, headaches, confusion, weakness, numbness, incoordination, and seizures. Extracerebral manifestations such as respiratory and visual problems can also occur.
Here, we outline the epidemiology and pathobiology of T. gondii infection as it relates to the cerebral form of toxoplasmosis, and we highlight the mechanisms by which this parasite migrates to, invades, and colonizes the brain to causes neurological dysfunctions. We also discuss diagnostic approaches using molecular methods and neuroimaging techniques to confirm brain involvement in infected individuals and summarize current strategies for the treatment and control of TE, particularly in immunocompromised patients. Finally, we shed light on new targets for future research that may accelerate the discovery of improved methods for managing this condition.
PARASITE BIOLOGY
Life Cycle Forms and Routes of Transmission
The provenance of the multistage life cycle of Toxoplasma gondii has been well established. During its development, T. gondii progresses through 3 main distinctive forms, namely, oocyst (containing sporozoites), tachyzoite, and tissue cyst (containing bradyzoites). Oocysts are the final product of sexual reproduction and are formed exclusively in the intestine of infected felines. The obligate intracellular tachyzoite and the bradyzoite represent the two main stages in the parasite’s asexual reproductive cycle. Tachyzoites represent the rapidly dividing stage within host cells. Tachyzoites can disseminate to multiple and distant tissues within the host’s body and can elicit significant immune responses. Although chemotherapeutic drugs and host immune defenses can limit their growth, some tachyzoites have the ability to overcome these formidable challenges and transform into slowly replicating bradyzoites. In stark contrast to tachyzoites, bradyzoites divide slowly and remain dormant, protected within a stage-specific cyst that is mainly located in the brain and muscle (skeletal and cardiac), presumably due to less rapid parasite elimination caused by reduced cellular turnover in these tissues compared to that in other organs. The preference of T. gondii to infect neurons, and their particular location within neuronal processes, was demonstrated in vivo for the first time by using a T. gondii reporter strain that secretes Cre into host cells, which enabled the specific identification and direct visualization of infected neurons (8). Bradyzoites are contained within tissue cysts that can evade the host immune responses and facilitate the establishment of a long-term persistent infection without causing overt disease (9). A recent study identified the Myb-like transcription factor (BFD1) as a key regulator for bradyzoite differentiation (10). Infection in humans commonly occurs via the consumption of food or water contaminated with tissue cysts or oocysts (11). Congenital “vertical” transmission also occurs, in which T. gondii tachyzoites are passed from mother to fetus through the placenta (4). Other routes of transmission of T. gondii include organ transplantation and blood transfusion (4).
Reproductive Strategies
According to the established paradigm, the life cycle of T. gondii involves sexual and asexual reproductive phases. The sexual reproductive phase, known as “gametogony,” occurs within cats (and other felids), which serve as the exclusive “definitive” hosts for T. gondii. In the intestinal epithelium of the definitive host, T. gondii differentiates into male and female gametes that form zygotes, which leave the cat intestine and are excreted with feces as oocysts (12). This is the main source of genetic recombination, should the cat be coinfected with more than one T. gondii strains (13). Infected cats shed millions of oocytes for a nonrecurring period lasting up to 3 weeks (14). Within 2 to 3 days following excretion, depending on environmental conditions, oocysts undergo maturation/sporulation to become infectious. Once they become sporulated, oocysts can survive in the environment and maintain their viability for more than a year. The reason that the feline gut epithelium is the only tissue that can accommodate sexual reproduction of this parasite remains largely unknown. In recent years, interest in the development of methods for establishing in vitro culturing models of the cat intestine has increased, and it is hoped that these models may help in unraveling the mechanisms that allow this particular location in this particular host to support the sexual development of T. gondii (15–17). A recent study using feline intestinal organoids has shown that a critical factor in the exclusive occurrence of sexual reproduction and oocyst production in the feline gut is the intrinsic abundance of linoleic acid in cats compared to that in other mammalian (nonfelid) hosts (18). The cat gut is genetically deficient in the enzyme delta-6-desaturase which converts linoleic acid to arachidonic acid. Astonishingly, high levels of linoleic acid, together with inhibiting delta-6-desaturase that suppresses metabolic conversion of linoleic acid to arachidonic acid, have also triggered sexual reproduction and oocyst formation in mice (18). Temporal analysis of the transcriptome of cat intestine during the first 96 h post T. gondii infection revealed significant changes in transcripts associated with immune response and metabolic pathways (19). A recent study showed that sexual differentiation in T. gondii can be inhibited by the histone deacetylase HDAC3, in a process mediated by microrchidia (MORC) protein and the Apetala 2 transcription factor (20).
T. gondii shows a remarkable diversity in the range of vertebrate species that it uses as intermediate hosts. Most warm-blooded mammals, birds, and humans can serve as the intermediate host. Although humans are permissive to T. gondii infection, they are dead-end hosts unless they are eaten by a feline species. Mammals and birds serve as proper intermediate hosts and transmit tissue cysts to both the definitive feline host and to other intermediate hosts. Within humans and other intermediate hosts, T. gondii exists in either the more rapidly proliferating tachyzoite stage or the more dormant bradyzoite stage. Ingestion of bradyzoite-containing cysts in raw or poorly cooked meat or infectious oocysts that are excreted in felid fecal material are the major infection sources. Upon entry into the small intestine, bradyzoites within the cysts, or sporozoites within the sporulated oocysts, are released and invade the intestinal epithelial cells, where they differentiate into tachyzoites. T. gondii tachyzoites have a distinctive ability to migrate to distant body regions, including peripheral and immunoprivileged regions such as the eye, brain, and placenta. As they invade, tachyzoites produce a membrane-bound parasitophorous vacuole (PV). Within this protective shelter, T. gondii tachyzoites secrete many effector molecules, exploit host cell metabolic resources, and reproduce asexually in a process known as “endodyogeny,” whereby each parental tachyzoite divides to form two daughter tachyzoites. This reproductive process in the intermediate host continues until the accumulation of newly produced tachyzoites causes rupture of the infected cells. Tachyzoites then continue with further rounds of invasion and proliferation in new cells. Without therapeutic interventions or a strong immune response, these repeated cycles of intracellular replications will cause severe or even fatal pathologies. In the setting of effective immune response or treatment, T. gondii establishes a chronic infection in the infected host as bradyzoites inside cysts, surrounded by a wall formed by modification of the membrane that limits the bradyzoite-containing PV (21). Under certain conditions, T. gondii undergoes phenotypic transformation from bradyzoites (the hallmark of latent infection) to tachyzoites (associated with acute infection) (22), and this differentiation can lead to adverse, or even life-threatening, consequences in immunocompromised individuals (3, 23).
EPIDEMIOLOGY
Burden, Global Prevalence, and Risk Factors of Toxoplasmosis
As a single disease, toxoplasmosis exerts a significant impact on health care services, individual health care costs, and health insurance companies. Earlier estimates, published in 2012, suggest that toxoplasmosis accounted for nearly $3 billion of illness-related costs and approximately 11,000 quality-adjusted life years (QALYs) lost per year (24, 25). In the United States alone, the total annual incidence of toxoplasmosis was estimated to be 9,832; with ocular (n = 2,169) and cerebral (n = 1,399) toxoplasmosis being the most prevalent forms of disease (26). Another 11-year study (2000 to 2010) in the United States reported 789 toxoplasmosis-related deaths, predominantly in people from Black and Hispanic backgrounds, with cumulative productivity losses of approximately $815 million (27). The economic cost of foodborne toxoplasmosis in pork was estimated to be $1.9 billion in the United States (28). During 1998 to 2010, an annual average of 20,258 encephalitis-associated hospitalizations (attributed to T. gondii and other infectious and noninfectious causes) were reported in the United States (29). A 9-year Canadian study (2002 to 2011) reported an overall annual health care cost of Can$1,686,860 attributed to toxoplasmosis (30). In the Netherlands, the toxoplasmosis burden has been estimated to be ∼€44 million in health costs, with the loss of 1,900 disability-adjusted life years (DALYs) annually (31). In Denmark, foodborne congenital toxoplasmosis was estimated to cause the loss of ∼100 years of healthy life in 2017 (32). Estimates of the burden of toxoplasmosis in other countries are needed to support country-specific toxoplasmosis control planning.
T. gondii is a highly prevalent parasite in humans worldwide (1). The seroprevalence of T. gondii varies substantially between the geographic regions throughout the world. The parasite is particularly more prevalent in Western European, South American, and African countries (33). The prevalence of toxoplasmosis can be seen as a proxy reflecting the hygienic and dietary practices of human populations. An increased risk for T. gondii infection has been associated with ingesting raw or undercooked meat, particularly pork or lamb meat, or unwashed raw vegetables or fruits. Others factors that can predispose an individual to risk of infection include age, gender, race, educational level, socioeconomic status, cultural background, level of health literacy, lifestyle, living in rural areas, proximity to cats, contact with soil, scooping cat litter, pregnancy, number of births, frequent travel to areas where T. gondii is endemic, immigration, quality and source of drinking water, and T. gondii strain genotype/virulence (4, 34). Because a cure for the persistent cystic stage is not currently available, latent toxoplasmosis can be present for decades, leading to considerable individual and societal burdens. All of the aforementioned risk factors are, fortunately, readily modifiable, and reducing their impact could potentially reduce the prevalence of T. gondii infection. Health care systems and current health-management practices should take all the risk factors into consideration so that the burden of toxoplasmosis, particularly in certain vulnerable groups or populations, can be minimized.
The Particular Impact on Immunocompromised Populations
TE is often reported in immunosuppressed people, such as persons living with HIV (PLWH) (23) and patients who received a hematopoietic stem cell or a solid organ transplant (1, 35). Also, patients receiving high doses of immunosuppressive chemotherapy or antineoplastic treatment and patients with an underpinning condition such as cancer or connective tissue diseases, are at a greater risk of toxoplasmosis-associated deaths. In these vulnerable groups, TE imposes a tremendous individual and socioeconomic burden. For example, in the United States, from 1988 to 1997, toxoplasmosis accounted for more than 21,000 hospitalizations, with a mean estimated cost of $28,151 per person attributed to TE-associated hospitalization (36). In the United States, ∼3,000 toxoplasmosis-related hospitalizations were also reported in PLWH in 2008 (37). In Canada, HIV comorbidity with toxoplasmosis was detected in 40% of clinical cases between 2002 and 2011, which correlated with an increased number of hospitalizations and increased treatment cost per case and accounted for 53% of the total toxoplasmosis-related health care costs (30). In Tanzania, most deaths attributed to toxoplasmosis were highly associated with HIV/AIDS, and TE was responsible for 15.4% of toxoplasmosis deaths (38). In West Africa, TE accounted for 10% of deaths due to AIDS (39). Fortunately, the use of appropriate testing, combination antiretroviral therapy (cART), and antimicrobial drugs to prevent opportunistic infection by T. gondii and Pneumocystis jirovecii has significantly helped to reduce the incidence of reactivation of latent infection and toxoplasmosis-associated deaths in PLWH (37). Implementation of an early prophylaxis treatment using trimethoprim-sulfamethoxazole (TMP-SMX) starting on the day of engraftment in T. gondii-seropositive patients can significantly reduce the rate of parasite reactivation in stem cell recipients (40).
The findings of a comprehensive review of 72 studies revealed a higher T. gondii infection rate in immunocompromised patients versus that in the control group (35.9 versus 24.7%; P < 0.001), in PLWH with advanced HIV infection versus that in the control (42.1 versus 32.0%; P < 0.05), in cancer patients versus that in the control (26.0 versus 12.1%; P < 0.001), and in organ transplant recipients versus that in the control (42.1 versus 34.5%; P > 0.05) (41). Therefore, prevention and treatment of toxoplasmosis should aim to include both HIV and non-HIV immunocompromised populations. Emerging epidemiologic evidence based on meta-analysis of 74 studies regarding concurrent infections by T. gondii and HIV from 34 countries revealed a worldwide pooled seroprevalence of 35.8%. The prevalence in Asia and the Pacific was 25.1%, that in sub-Saharan Africa was 44.9%, that in South American and Caribbean countries was 49.1%, and that in North Africa and Middle East was 60.7%. As expected, populations in developing countries exhibited higher comorbidity (54.7%) compared to those in middle-income (34.2%) and high-income (26.3%) countries. The particularly high burden (87.1%) in sub-Saharan Africa was attributed to the lack of resources, poor dietary and sanitary conditions, poor health literacy, limited health care capacities, and limited access to safe water, all of which may have increased odds of infection (42). In these resource-limited settings, TE can represent a particularly high risk for PLWH patients, particularly those with less than 200 peripheral blood CD4+ T cells per microliter. A more recent systematic review analyzing 111 studies from 37 countries reported a pooled prevalence in PLWH of T. gondii of 44.22% by IgG, which was higher than the prevalences obtained based on IgM analysis (3.24%) and molecular methods (26.22%), highlighting the high T. gondii infection rate in PLWH (43). The same study also detected a correlation between T. gondii positivity and a number of variables, such as gender, consumption of raw meat, proximity to cats, and awareness of toxoplasmosis, suggesting that risk factors for toxoplasmosis are the same regardless of the individual’s immune status. Given that toxoplasmosis results in significant illness in immunosuppressed persons, these highly vulnerable groups should be advised by their health care providers to avoid all behaviors that can put them at risk of serious disease. A greater understanding of the levels of T. gondii infection in PLWH is also important in order to inform policies on allocation of resources and to guide early detection of seroconversion.
MOLECULAR PATHOGENESIS
Marching to the Brain
To cause encephalitis, T. gondii must migrate to and enter the central nervous system (CNS) and establish a persistent infection in neural and other brain cells. Following ingestion of the infective stage, either oocysts or tissue cysts, the parasite develops into rapidly proliferating tachyzoites, which invade and proliferate within the intestinal epithelium. The tachyzoites then exit and infect dendritic cells (DCs) and other immune system cell types that are important in protecting against T. gondii infection (44). These patrolling immune cells are permissive to T. gondii infection and represent an important niche for the parasite’s replication. In addition to using immune cells as a replicative niche, T. gondii manipulates the functions of these cells to increase their metastatic behavior, which is crucial for the dissemination of T. gondii to distant organs, particularly the brain (45). The exact molecular mechanisms that promote the hypermigratory behavior of infected cells are not fully understood, but cellular migration seems to depend on chemokines and their receptors. For example, restructuring of the cytoskeleton, upregulation of the chemokine receptor CCR7, downregulation of CCR5, increase of the secretion of gamma-aminobutyric acid (GABA), induction of the GABA-A receptor, and activation of calcium channels and calcium signaling are all implicated in the migration of infected DCs (46–49). Additionally, the upregulation of tissue inhibitor of metalloproteinases-1 (TIMP-1), through CD63-integrin β1 (ITGB1)-focal adhesion kinase (FAK) signaling promoted the motility of infected DCs (50). An increased velocity of infected DCs and microglia was also mediated by the secretory T. gondii 14-3-3 protein (51). Moreover, the secreted kinase ROP17 promoted the mobility and dissemination of T. gondii-infected monocytes (52).
Crossing the BBB
T. gondii spreads to various tissues, such as the eye, heart, liver, lung, lymph nodes, and muscles; however, this parasite seems to persist in neurons (and muscle), probably due to reduced parasite elimination or cellular turnover in these tissues compared to other organs. The parasite’s entry to the brain from the blood through cerebral capillary endothelial cells occurs via paracellular transfer into the brain following BBB damage, invasion of cerebrovascular endothelial cells allowing transcellular transfer, and trafficking within infected immune cells into the brain in so-called “Trojan horse” attack (Fig. 1A) (53). The accumulation of cell-free T. gondii tachyzoites at the intercellular junctions prior to transmigration indicates that crossing of the BBB can occur via breaching intercellular tight junctions (TJs). T. gondii adversely affects the resistance of cerebrovascular endothelial cell monolayers and impairs the barrier’s function, thus facilitating paracellular migration (54). Although extracellular tachyzoites can overcome the BBB, invasion and replication within brain microvascular endothelium is an alternative pathway by which T. gondii enters the CNS (55). Secretion of toxofilin (56) and proteases (57) by T. gondii facilitates its traversal of the BBB and may underpin the cellular destruction observed along the BBB, when only focal sites are infected by the parasite. Crossing the BBB by T. gondii can also occur via exploitation of DCs and monocytes as vehicles for their protection and transport across the BBB. T. gondii preferentially enters the brain within parasitized CD11c− CD11b+ monocytes and, to a lesser extent, CD11c+ CD11b+/− DCs. The increased mobilization of T. gondii-infected phagocytes involves Gi protein-coupled signaling (57), which promotes diapedesis and extravasation of infected cells from cerebral capillaries into the brain. Migration of murine T. gondii-infected macrophages in Matrigel correlates with upregulation of matrix metalloproteinases (MMPs) such as MMP-9, MT1-MMP, and ADAM10 (58).
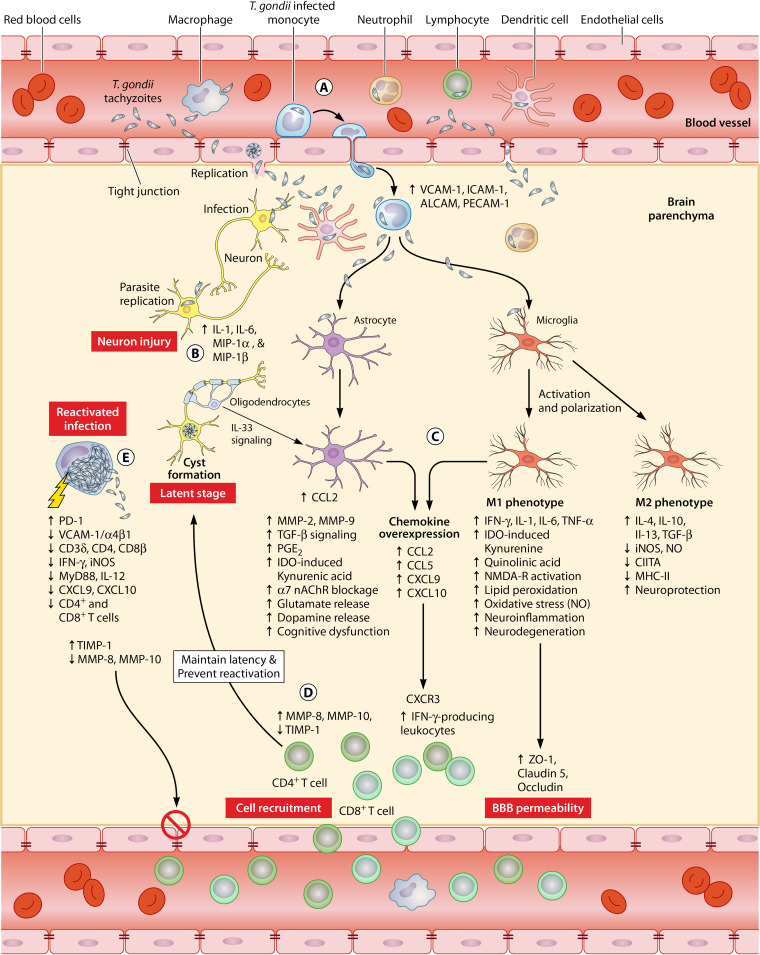
FIG 1.
Schematic illustration of T. gondii traversal across the blood-brain barrier (BBB) and the mechanisms that underlie the disruption of BBB permeability and brain dysfunction. (A) Different routes of T. gondii entry into the brain and associated alterations in the tight junction (TJ) proteins and adhesion molecules of the cerebrovascular endothelium. The extracellular tachyzoites can directly enter the brain by paracellular or transcellular route or via a “Trojan horse” mechanism in which tachyzoites cross the BBB within infected leukocytes. (B) Due to their unique metabolic and immunological attributes, neurons are often vulnerable to the parasite’s attack; the parasite replicates within neurons, causing neuronal injury with production of cytokines and chemokines and resulting in more impairment of the neurological function and disturbance of brain metabolism. Neurons also provide a permissive niche to the development of cysts, which persist in dormancy for a long time within the brain. (C) Following entry to the brain, the tachyzoites activate resident microglia and astrocytes and elicit immune responses to limit the parasite proliferation. The M1 phenotype of activated microglia produces proinflammatory cytokines, which exacerbate BBB dysfunction by altering the architecture of the TJ proteins ZO-1, claudin-5, and occludin. The effects of M1 microglia are counterbalanced by alternatively activated M2 microglia, which produce anti-inflammatory cytokines. Maintaining this immune-inflammatory equilibrium is key to the establishment of latent infection. Infection of astrocytes and microglia also leads to the disruption of neuroreceptors, such as the alpha-7 nicotinic acetylcholine receptor (α7 nAChR) and N-methyl-d-aspartate receptor (NMDAR), which may lead to cognitive dysfunction and neurodegeneration. Activated microglia and astrocytes secrete chemokines (e.g., CXCL9 and CXCL10) which function as ligands for CXCR3 to promote the influx of T cells and myeloid cells (granulocytes and monocytes) into the brain. (D) Matrix metalloproteinases (e.g., MMP-8 and MMP-10) and TIMP-1 also contribute to the regulation of the perivascular accumulation and influx of lymphocytes into the brain to prevent the reactivation of dormant cysts. (E) Reactivation from latency can occur due to various mechanisms, such as reduced expression of VCAM-1/α4β1 integrin, CD3δ, CD4, CD8β, interferon gamma (IFN-γ), and inducible nitric oxide synthase (iNOS). Reduced levels of CXCL9, CXCL10, MyD88, interleukin 12 (IL-12), MMP-8, or MMP-10 during reactivated toxoplasmosis decrease the influx of T cells into the brain.
The infiltration of infected cells into such an immune-privileged site as the brain is a highly orchestrated process involving interactions with cerebrovascular endothelial cells and is mediated by a number of adhesion molecules and chemokine receptors (59). Through activation of the chemokine receptor CXCR3, the chemokines CXCL9 and CXCL10 promote the chemotactic recruitment of T cells to the brain (60). Through their adhesive function, ICAM-1 and its counterpart VCAM-1 facilitate the binding of leukocytes to the cerebrovascular endothelium. The interleukin 1 (IL-1) signaling stimulates the expression of VCAM-1 and ICAM-1 during T. gondii infection in mice (61). When crossing the BBB, infected leukocytes use their LFA-1 integrin to adhere to ICAM-1. Infected leukocytes also use selectin and its glycoprotein ligand to cross the BBB endothelium. Using antibodies that block the ligand for VCAM-1 prevents cell entry, further supporting the important role that adhesion molecules and endothelial cell surface receptors have in the influx of myeloid cells, particularly infected monocytes, into the brain (62). Interestingly, T. gondii tachyzoites were found in the choroid plexus (CP) of PLWH (63). However, the role of the CP and blood-cerebrospinal fluid barrier in the parasite’s entry into the CNS is unknown. Collectively, these studies have provided significant insights into the tactics employed by T. gondii to cross the BBB in animal models or ex vivo studies. Although our current understanding of these mechanisms in humans remain largely speculative, it is logical to assume that T. gondii does not cross the BBB via one exclusive mechanism in humans.
What Mediates the Course of a Persistent Brain Infection?
The preference of T. gondii for neurons is apparent, perhaps because neurons (Fig. 1B), unlike other brain cell types, do not react to inflammatory cytokines and thus do not induce a strong antiparasitic immune response (64). Although neurons are the main host cell type preferred by T. gondii (8), other nonneuronal cell types also contribute to TE (65). Despite continuous immune surveillance, neuronal degeneration is rarely observed in immunocompetent hosts infected by T. gondii. This is attributed to the effective and balanced proinflammatory (T helper 1 [Th1] antiparasitic) and anti-inflammatory (neuroprotective) immune responses. Maintaining this balance is crucial for containing parasite proliferation by promoting persistent infection and preventing parasite reactivation, while limiting excessive immune pathology.
Homing of immune cells into the brain.
Immune cell migration and extravasation through blood vessels, and their homing into infection sites, are important mechanisms for establishing an effective immune response. The chemokines and metalloproteinases are key regulators of these events. During TE, the cross talk between chemokines and the cerebral immune response involve CD4+ and CD8+ T cells and is mainly mediated by interferon gamma (IFN-γ). Increased production of T-cell chemoattractants, particularly chemokines CXCL9, CXCL10, and CCL5, was detected in the brains of mice during latent infection and was dependent on IFN-γ (66). Infection of astrocytes or microglia by T. gondii induced the expression of chemokines CCL5, CCL2, CXCL9, and CXCL10 (Fig. 1C), which actively recruit IFN-γ-expressing T cells to control tachyzoite proliferation (59).
The chemokine receptor CCR2 (and its ligand CCL2) contribute to the migration of monocytes and neutrophils into the brain following infection. In CCR2-deficient (CCR2−/−) mice, leukocyte trafficking was decreased, and immune cells within the brain became less active, leading in turn to an increased parasite burden (67). Astrocytes express CCL21 and CXCL10 to promote brain infiltration of CD8+ T cells (68), and microglia-derived CXCL10 modulates the search behavior of CD8+ T cells in a way that enhances their ability to detect infection sites in the brain parenchyma (60). The damage signal protein IL-33, expressed by oligodendrocytes, induced the expression of monocyte chemoattractant CCL2 by astrocytes (Fig. 1B), which is required for the recruitment of monocyte-derived myeloid cells, and the expansion of focal myeloid cell-derived inducible nitric oxide synthase (iNOS), which is crucial for survival during chronic infection (69). T. gondii can also increase the production of the chemokines CCL3 and CCL4 in the brain of C57Bl/10 ScSn mice (70) and in cultured cerebellar neurons of mice (64). Infected neurons increase the production of the chemotactic macrophage inflammatory protein-1 alpha and beta (MIP-1α and MIP-1β), which have proinflammatory effects, leading to an influx of leucocytes at the site of inflammation (Fig. 1B).
The interplay of the TIMP-1 and MMPs also influences the infiltration of T cells and parasite control (Fig. 1D). Astrocytes infected with T. gondii produced MMP-2 and MMP-9, possibly to increase extravasation and infiltration of inflammatory cells to the infection sites (71). In a mouse model, MMP-8 increased infiltration of CD4+/CD8+ T cells, and MMP-10 increased infiltration of CD4+ T cells into the brain. TIMP-1 in astrocytes and in the infiltrating CD4+/CD8+ T cells decreased the brain parasite load without the development of adverse pathology. An increase in CD4+ T cells and a significant reduction in parasite load were detected in the brain of mice deficient in TIMP-1 compared to those in wild-type (WT) controls, without substantial brain injury or any reduction in the peripheral parasite burden (72). This suggests that production of TIMP-1 may be an attempt by astrocytes to block parasite elimination by restricting MMP-mediated recruitment of lymphocytes. This scenario seems to favor parasite survival and to restrict neuroinflammation and brain damage, which ultimately promotes persistent infection.
The roles of cytokines in containing infection.
Following brain invasion, T. gondii tachyzoites encounter strong cell-mediated (type 1) immune responses marked by the production of IFN-γ, interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α), which clear most of the invading tachyzoites (73). T. gondii infection induces the expression of Toll-like receptor 11 (TLR11) in astrocytes, neurons, and microglia in mice (74). TLR11 stimulation by the parasite’s profilin protein induces IL-12 in DCs (75). MyD88 knockout mice rapidly succumbed to acute infection, with a commensurate impairment in IL-12 response, suggesting that mice resistance to toxoplasmosis depends on MyD88-dependent signals regulated by TLRs (76). Many in vitro and in vivo studies have indicated the fundamental role that IFN-γ plays in protecting the host from severe T. gondii infection. IFN-γ controls parasite replication via various mechanisms, such as by stimulating the degradation of the PV via the production of immunity-related GTPases (IRGs) and interferon-inducible guanylate-binding proteins (GBPs), increasing expression of major histocompatibility complex (MHC) and induction of iNOS and the immunosuppressive enzyme indoleamine 2,3-dioxygenase (IDO) (77–80). Production of nitric oxide (NO) by iNOS inhibited T. gondii proliferation within macrophages in vitro (81) and in the mouse brain (82). The procurement of nutrients such as tryptophan from the host cells is essential for intracellular T. gondii survival. Hence, the breakdown of tryptophan into kynurenine due to IFN-γ-mediated IDO induction can result in inhibition of tachyzoite growth (83). Increased kynurenic acid concentrations during infection acts as an agonist of the immunosuppressive aryl hydrocarbon receptor (AhR), which results in controlling the pathological immune response associated with toxoplasmosis. Mice deficient in AhR (AhR−/−) succumbed more readily to illness but developed fewer brain cysts than WT mice infected by T. gondii (84). Additionally, the excessive production of kynurenic acid, which blocks the alpha-7 nicotinic acetylcholine receptor (α7 nAChR) and disrupts other neuroreceptors in infected astrocytes and microglia, can lead to abnormal neurotransmission (85) and was hypothesized to lead to cognitive dysfunction (Fig. 1C). In response, T. gondii secretes TgIST, an inhibitor of signal transducer and activator of transcription 1 (STAT1) transcriptional activity, which antagonizes IFN-γ-induced IDO1-mediated immunity against T. gondii in human cells (86). Mice deficient in the lymphotoxin-β receptor (LTβR), which has diverse antimicrobial immunoregulatory roles, had a higher parasite burden and increased mortality in acute T. gondii infection than immunocompetent control mice. The impaired resistance of LTβR−/− mice was attributed to the deregulation of interferons and interleukins, as well as a reduced upregulation of murine GBP, which is mediated by IFN-γ and is crucial for parasite elimination, further supporting the importance of IFN-γ in controlling T. gondii (87). Mice lacking mGBP7 failed to control parasite replication and succumbed to acute infection (88).
The contribution of astrocytes and microglia to the pathogenesis of TE is notable. Inhibition of T. gondii replication in astrocytes was attributed to an increased induction of IFN-γ–induced GTPase (89). Genetic deletion of STAT1 in astrocytes promoted the spread of T. gondii infection and increased susceptibility to TE (90). The orphan nuclear hormone receptor TLX, expressed in astrocytes and neural stem cells, can support resistance to T. gondii through enhancing STAT1 activity (91). Mice lacking cytokine receptor gp130 (a protein of the IL-6 signaling pathway) in astrocytes showed increased astrocyte apoptosis and inflammation during T. gondii infection. Although these mice were able to control parasite numbers, they developed excessive inflammation and succumbed more easily to rapid development of TE (92). Interestingly, microglia did not seem to be the only cells in charge of controlling T. gondii in the brain through T cell-derived IFN-γ mechanisms. Mice in which both monocyte-derived macrophages and microglia were made deficient in IFN-γ signaling succumbed to infection by 19 days postinfection. Conversely, mice in which microglia were only deficient in IFN-γ signaling were able to resist infection (93). Interestingly, a recent study using a microglia reporter mouse model showed that microglia can promote neuroinflammation via the release of alarmin IL-1α, which recruits more immune cells to control chronic brain infection (61).
The cysticidal role of CD8+ cytotoxic T lymphocytes.
The anticyst activity of CD8+ cytotoxic T lymphocytes (CTLs) and the control of TE are mediated by mechanisms that utilize both the IFN-γ (the key effector of protective immunity against tachyzoites) and perforin-mediated immune responses (94). Control of brain cyst burden is mediated largely by CD8+ CTLs that produce perforin and M2 microglia expressing CXCR3, acidic mammalian chitinase (AMCase), and arginase-1 (95, 96). Although M2-like microglia populations act to inhibit neuroinflammation, AMCase-deficient mice can develop a high parasite burden in the brain, indicating a role for M2-like microglia in controlling the parasite cysts. Both perforin and granzyme B underpin the capacity of CD8+ CTLs for direct invasion and elimination of the existing T. gondii cysts (97). Adoptive transfer of perforin-competent CD8+ CTLs to immunodeficient mice significantly reduced cyst load and increased the level of CXCR3 and CXCR6, which promote the recruitment of microglia and macrophages to destroy bradyzoites within T cell-attacked cysts (98). The induction of the CD8+ CTL response is also mediated by rhoptry protein ROP7 and dense granule protein GRA6 (99, 100). The cysticidal capacity of CD8+ CTLs is related to the amino-terminal region of T. gondii GRA6 (GRA6Nt) (101). Markedly, one epitope in the C-terminal region of GRA6 was a strong inducer of IFN-γ-mediated protection against acute toxoplasmosis in mice (99). These results suggest that host immune responses can recognize different GRA6 regions to stimulate CD8+ T cell-based protection against both tachyzoites and tissue cysts. The cysticidal mechanism is initiated by CD8+ CTLs, but the removal of cysts is accomplished by microglia and macrophages, which invade the cysts and destroy the bradyzoites (95). iNOS-mediated protection conferred by CD8+ CTLs is important for inhibiting the development of toxoplasmic cysts, but it does not play a role in the mechanism required to eradicate the cysts (102).
Immune evasion strategies.
T. gondii is an exceptionally successful intracellular parasite. Despite being at constant risk of a sustained and efficient immune defensive patrol, it can linger inside the body of the host for a long time. Some tachyzoites can escape destruction from the immune response (or from drugs used to treat toxoplasmosis) and transform into bradyzoites inside quiescent cysts. Paradoxically, the Th1-polarized immune response essential for eradication of acute infection is the same response required to develop latent infection in the brain. T. gondii seems to subvert CD4+-mediated immune responses through the inhibition of the MHC II transactivator CIITA, which contributes to its long-term survival in the brain (103). T. gondii also produces cyclophilin‐18-mimicking chemokine, which, when bound to CCR5 on DCs, evokes a Th1 response (e.g., production of IL‐12), required for the establishment of persistent infections (104). The expansion and functional maturation of cerebral DCs contributes to the development of latent infection (105). T. gondii possesses secretory organelles that discharge a number of polymorphic effector proteins that seem spatially and temporally controlled, as different effectors are secreted at different stages of host cell invasion and colonization and effector secretion is targeted to specific locations to selectively modulate a specific function or a regulatory pathway within the host cell (106–108). These effectors are utilized by T. gondii to circumvent host immunity and to support the establishment of a latent infection (109). For example, GRA23 and GRA17 control the delivery of small metabolites to the PV and promotes the structural stability of the PV (110). More recently, GRA17 and GRA23 have been shown to mediate the growth, virulence, cyst burden, and immunogenicity of the type II PRU strain (111). GRA39 contributes to parasite virulence and tissue cyst burden (108). Additionally, ROP16 serves as a virulence factor that activates STAT3 and STAT6 and inhibits T-cell responses (112). ROP5 forms a complex with ROP17 and ROP18 to prevent the recruitment of IFN-γ–mediated IRGs, which are essential for PV destruction and parasite control (113). In addition to inhibiting STAT1 transcriptional activity (86), TgIST binds to the STAT1/STAT2 heterodimer, leading to the inhibition of type I IFN pathway (114).
Striking a balance between friend and foe.
The long-term presence of dormant cysts in the host tissues requires a well-coordinated immune arsenal that is sufficiently potent to combat the infection, yet moderate enough to counterbalance hyperinflammation. Mice deficient in 5-lipoxygenase (5-LO) (115) or suppressor of cytokine signaling 2 (SOCS-2) (116), succumbed to chronic infection and showed increased mortality due to excessive inflammation caused by elevated IL-12 and IFN-γ, regardless of the reduction in the brain cysts. Therefore, the effects of proinflammatory cytokines (e.g., IFN-γ) must be buffered by immunosuppressive effectors (IL-27, IL-10, and transforming growth factor β [TGF-β]) and receptors (e.g., PD-1, LAG3, and TIGIT), without which the continued secretion of inflammatory cytokines and homing of T cells into the brain can lead to an exaggerated inflammatory response and encephalitis (117, 118). Therefore, the relative balance between IL-10 and IFN-γ produced by T cells will dictate whether the immune response will eliminate the parasite with limited immunopathology or whether chronic infection will be established. Regulatory T cells (Tregs) are activated by IL-27, which is secreted by monocytes, to induce IL-10 and T-box transcription factor (T-bet) expression to attenuate the inflammatory responses (119). In the absence of IL-27, T. gondii increases the levels of IL-17 and granulocyte-macrophage colony-stimulating factor (GM-CSF) and results in neuroinflammation in mice (118). The reduction of IL-10 by blocking the IL-10 receptor in chronically infected mice led to significant tissue destruction due to the extensive inflammatory response in the brain. The anti-inflammatory role of IL-10 during toxoplasmosis is also evidenced by the fact that mice lacking IL-10 succumbed to fatal CNS inflammation (120, 121). Deletion of the transcription factor Bhlhe40, an IL-10 repressor, resulted in severe T. gondii infection in mice, which was attributed to reduced IFN-γ and increased IL-10 production (122).
Although IL-10, which is commonly produced by Th2 cells, has broad anti-inflammatory properties and suppresses Th1-cell proinflammatory responses (123) via its inhibitory effect on the function of macrophages and DCs (124), Th1 cells secreting IL-10 and IFN-γ were detected in animals infected by T. gondii (125, 126). Recent studies have identified a number of immune cells (for example, B cells, eosinophils, CD8+ CTLs, Tregs, and antigen-driven regulatory CD4+ T cells) that can produce IL-10 (127). Tregs expressing CXCR3 were also found to express T-bet and Foxp3 and to secrete IFN-γ and IL-10. It remains to be completely defined how IL-10 functions to limit tissue damage while at the same time promoting persistent infection. More research is required to reveal the extent of dependence between the mechanisms used by different types of cells to secrete IL-10 and to elucidate how and to what magnitude different sources of IL-10 execute the aforementioned distinct activities.
TGF-β signaling in astrocytes is of particular interest for its immunosuppressive role during brain injury (128). Infected astrocytes secrete prostaglandin E2 (PGE2) to activate microglia, which suppress NO production (129). TGF-β1 plays a role in the inhibition of iNOS and NO production by IFN-γ-activated microglia (130). TGF-β signaling in astrocytes controls neuroinflammation and neuronal injury via the inhibition of NF-κB signaling, which in turn inhibits CCL5 and the infiltration of T cells and macrophages (131). Thus, lack of a TGF-β signaling in astrocytes, while not necessarily affecting parasite burden, can increase inflammation and neuronal damage.
The role of Tregs in TE.
The role of Tregs in toxoplasmosis has yet to be fully understood. However, elucidating of the mechanisms that underpin the interaction of Tregs with CD11c-expressing DCs in the meninges and perivascular space is warranted (132). Reduction of the suppressive Tregs during acute toxoplasmosis is commensurate with increased IFN-γ in an IL-10-independent/IL-2-dependent manner (133). In TE, Tregs expressing Th1 response-related molecules, such as T-bet, CXCR3, and IFN-γ, were detected in the brain, where they probably limit neuroinflammation via IFN-γ-mediated increased IDO expression (134). Studies in knockout (KO) mice have shown that inducible costimulator (ICOS) signaling promotes inflammation and antibody production—both of which are important for parasite control—while helping to control inflammation by inhibiting effector T-cell proliferation and inducing Tregs in the brain and spleen (135) or by inducing IL-10 production in T cells (136).
What makes the murine model different from humans?
Our knowledge of the pathophysiology of toxoplasmosis has mostly been derived from studies in mice and must be interpreted with caution. Marked differences exist between murine models and humans and their mechanisms of innate recognition and cytokine production. For example, IFN-γ-mediated immune responses of IRGs and GBPs in mice does not seem to have an equivalent in humans (106). Furthermore, parasite recognition and the initiation of innate immunity in mice require stimulation of TLR11 and TLR12 by the parasite profilin for the production of cytokines by myeloid cells (75, 137). The role of TLR11/TLR12 in recognizing the parasite profilin in mice is absent in humans; instead, immune recognition triggered by phagocytosis of the live parasite leads to IL-12 being produced by myeloid cells (138). In contrast, immune cells in mice do not require direct contact with live tachyzoites (139). Human peripheral blood mononuclear cells can produce IL-12 and TNF-α after stimulation with T. gondii nucleic acids, suggesting the involvement of TLRs 7, 8, and 9, all of which function to recognize foreign nucleic acids (140). A recent study using human monocytes has shown that the recognition pathway for T. gondii relies on the detection of S100A11, a damage-related molecule secreted from adjacent infected cells (141). Taken together, these results indicate that recognition of the pathogen is not a prerequisite for the induction of an anti-T. gondii immune response, because immunity in humans and mice can also arise from sensing active infection. Additional differences include the production of large amounts of IL-12 from mouse DCs, whereas the human response to T. gondii mainly involves the expression of CCL2 (141). In mice, T. gondii induces the production of IL-12 by myeloid cCD2 cells (e.g., CD8α+), which are distinct from the IL-12-producing cDC1 subset (e.g., CD1c+) in humans (138). Differences in cytokine production also exist between human and murine subpopulations of monocytes in response to T. gondii stimulation (138). Despite the aforementioned differences, mice remain an important model for the investigation of T. gondii infection and for the development of remedial measures. Work toward developing animal models continues, as illustrated by a recent report using zebrafish as a model to study T. gondii replication, T. gondii strain-specific variations in host response, and interaction with immune cells (142).
Reactivation of Latent Infection
Dormant tissue cysts encapsulating slow-growing bradyzoites are fundamental for the long-term survival and persistence of T. gondii in the host’s brain, due to their ability to evade immune-mediated destruction. The following question arises: what advantage does T. gondii gain by undergoing phenotypic transformation from dormant bradyzoites protected within cysts to the more active tachyzoites, which are less able to counter host immune responses? Some studies provide direct evidence supporting a link between immunosuppression and reactivation of latent infection. Indeed, parasite cyst reactivation is a high-frequency occurrence in immunocompromised people, such as individuals who received organ transplantation and PLWH who have AIDS, in particular those who are not on cART and are not receiving prophylactic TMP-SMX (23, 143, 144). In these groups of patients, phenotypic switching provides an opportunity for uncontrolled proliferation of tachyzoites in excessive numbers, which can overwhelm the capacity of the host’s already-compromised immune system, resulting in life-threatening consequences (53). Therefore, durable cell-mediated immunity to T. gondii is essential for protecting the host from reactivation of any latent infection. It remains to be shown whether immune suppression triggers reactivation or if reactivation continues at a set rate and the immune system, which would normally control any tachyzoites that are released, is suppressed, allowing replication without control and leading to a clinical reactivation.
As stated above, IFN-γ has a crucial role in the control of latent infection (73, 145). Microglia are a further source of IFN-γ production and contribute to the protective immune response that maintains the CNS tolerance to T. gondii presence and prevents any recrudescence of latent cerebral infection (146). The production of IFN-γ in microglia is mediated by GRA6Nt, which likely serves as a warning signal of the parasite’s presence for neighboring uninfected microglia. This would be a preemptive strategy to limit any further growth of tachyzoites in the brain via activation of the protective immune responses and by increasing microglial production of IFN-γ (147). Astrocytic TGF-β signaling exerts anti-inflammatory and neuroprotective functions during T. gondii infection (131). Astrocyte dysfunction during HIV infection can therefore lead to loss of their protective roles and diminish their ability to counter infection (131). CXCL9 recruits and accumulates T cells around the parasite-infected brain areas to prevent any reactivation of latent cerebral infection (148). The influx of T cells into the brain is also mediated by the action of IFN-γ on the cerebrovascular endothelium; IFN-γ increases VCAM-1/α4β1 integrin interactions to suppress TE; this appear to serve as a host defense mechanism during the early reactivation of dormant cysts (149, 150).
Pharmacological agents, through their antiparasitic or immunomodulatory effects, can also modulate the reactivation of latent cysts and the development of TE. For example, termination of sulfadiazine treatment resulted in reactivation of latent infection in a murine model (151). The use of biological agents, such as anti-α4 integrin monoclonal antibodies used in the treatment of Crohn’s disease or multiple sclerosis, can interfere with the VCAM-1/α4β1 integrin interaction and brain homing of CD4+ and CD8+ cells, potentially increasing the odds of reactivation of latent toxoplasmosis (Fig. 1E). In line with this assumption, administration of anti-α4 integrin antibody inhibited key factors that play roles in controlling T. gondii growth, such as iNOS, T cells, and IFN-γ in the mouse brain (150). In addition to taking into account the effect of using immune-modulatory biologics on the reactivation of tissue cysts, the impact of secondary/opportunistic infections in chronically infected patients should be also considered. The interaction between T. gondii and HIV is just one example. While HIV-induced impairment in immune response triggers reactivation of dormant cysts, coinfection with T. gondii compromises host immune defenses to HIV-1 and herpes simplex virus 1 (HSV-1) by suppressing plasmacytoid dendritic cell (pDC) activation and inhibition of IFN-α production (152). During latent toxoplasmosis, exhaustion of CD8+ CTLs and induction of programmed cell death 1 (PD-1) rendered CD8+ CTLs liable to destruction via apoptosis, leading to reactivation of latent infection and host mortality (153, 154). Blocking of PD-1 and its ligand PD-L1 has a potential therapeutic value, as anti-PDL-1 treatment reinvigorated CD8+ CTL response, which prevented reactivation of tissue cysts and improved survival of chronically infected mice (153). Also, inhibition of the PD-1–PD-L1 pathway can decrease the expression of caspase 3 on polyfunctional and IFN-γ+/granzyme B− memory CD8+ CTLs in vitro (154). Additionally, blockade of the immune checkpoint inhibitor PD-1 significantly reduced brain cyst burden and increased brain infiltration of CD8+ CTLs, CD11b+ DCs, and CD3+ T cells in mice (155). All of the aforementioned studies indicate that development of strategies to prevent T. gondii reactivation is achievable by maintaining enhanced proinflammatory effectors without disproportionate reduction of the anti-inflammatory mediators.
CLINICAL SIGNS AND DIAGNOSIS
Infection of the CNS with T. gondii, or TE, can cause severe illness and death in immunocompromised individuals. The worldwide T. gondii seroprevalence in PLWH ranges from ∼26% in high-income countries to ∼55% in low-income countries (42). The risk factors for TE in PLWH include blood CD4+ T cell counts of <200/μl, lack of TMP-SMX prophylaxis, which may be given to specifically prevent TE or Pneumocystis jirovecii pneumonia (143, 156), and lack of cART used to control HIV infection (23, 144). Because the greatest current experience with TE is in PLWH, and clinical guidelines are available (157), we focus on this risk group. However, the principles used in the diagnosis, screening and prevention of TE in PLWH are applicable to other at-risk populations.
Clinical Features
TE most commonly presents as one or, more commonly, multiple brain abscesses with a predilection for the deep gray matter structures or the junction between cortical gray and white matter. However, any part of the brain can be affected. As such, neurological abnormalities can be similar to what might be seen in an individual with one or multiple brain lesions from any cause. More unique to TE is fever. Commonly described neurological abnormalities in PLWH with TE are subacute onset of headache, hemiparesis, cranial nerve palsies, ataxia, change in level of consciousness, or seizures (158, 159). Due to involvement of the basal ganglia, abnormal movements, including chorea, ballism, and rigidity may also be observed (158, 160). Uncommonly, patients may present with an encephalitic illness rather than with discrete brain abscesses. These individuals may present with fever; meningeal signs such as headache, stiff neck, and photophobia; and encephalopathy that is rapidly fatal (161, 162). Similar clinical manifestations and course have been reported in people with necrotizing ventriculitis due to T. gondii (163).
Diagnostic Tests
Serological diagnosis.
The Sabin-Feldman dye test, first reported in 1948, remains the “gold standard” for serological detection of anti-Toxoplasma IgG and IgM antibodies (164). However, this method requires using live tachyzoites, which is not feasible in most laboratories. An enzyme-linked immunosorbent assay (ELISA) is commonly used to detect specific IgG and IgM antibodies against T. gondii. IgG titers peak within 1 to 2 months postinfection and stay high for life, which is why they are a good marker of risk for TE in PLWH, which almost always occurs following reactivation of a preexisting latent infection. Because the proportion of PLWH with TE who have serum anti-T. gondii IgG antibodies can reach 100% (165, 166), identification of PLWH who are IgG seropositive identifies those at risk for the disease. However, empirical diagnosis based on IgG level can be misleading because elevated serum anti-T. gondii IgG titers are common in the general population, even in the absence of active illness (167). An avidity assay, which is a modification of the ELISA, incorporates a denaturing agent (e.g., urea) within serum dilutions to test for antibody avidity. The avidity values are lower during acute infection and increase over time, which can be used to estimate when seroconversion has occurred, and are useful in the evaluation of reactivated infection and in pregnant women who acquire infection during gestation (168). IgM antibodies can be detectable for over a year. Thus, the presence of IgM antibodies does not necessarily indicate a recent infection; however, the lack of IgM rules out recent infection. Perhaps unexpectedly, PLWH with TE do not mount an IgM response.
Cerebrospinal fluid (CSF) analysis.
Mild mononuclear pleocytosis and elevated protein can be detected in the CSF of patients with TE. Diagnostic tests for detection of toxoplasmosis-specific IgG and IgM antibodies in the CSF do not have proven utility beyond an empirical treatment trial. The detection of T. gondii DNA in CSF using PCR is specific, but not sensitive, for diagnosis of TE (169, 170), meaning that a positive test confirms the diagnosis but a negative test does not rule it out. Diagnostic accuracy decreases if CSF is examined after more than a week of TE therapy (170). In a PLWH with a focal brain lesion with mass effect who is not receiving anti-Toxoplasma prophylaxis, detection of T. gondii DNA in CSF increases the likelihood of TE from 87% to 96% (171). It is important to remember that lumbar puncture for collection of CSF may not be safe in TE due to mass effect, which increases the risk of brain herniation after lumbar puncture.
Histopathology.
CNS toxoplasmosis in PLWH can lead to granulomatous reactions with gliosis and microglial nodules and necrotizing encephalitis (172). The detection of tachyzoites either alone or together with tissue cysts is diagnostic. Immunohistochemistry can improve the detection and localization of the parasite (173).
Neuroimaging.
Brain imaging is particularly useful for the diagnosis and management of patients with TE. Brain examination using computed tomography (CT) scan revealed hypodense and contrast-enhancing focal brain lesions with mass effect, primarily in basal ganglia, thalami, and cortico-medullary junction in 70–80% of PLWH with TE. Less often, TE in PLWH presents with one lesion or with no lesions on brain CT. Rarely, ventriculitis can be seen on brain CT scan of PLWH with TE (163, 174, 175). Magnetic resonance imaging (MRI) is the clinical imaging standard used in PLWH with suspected TE (176, 177). Given the better sensitivity of MRI compared to CT, often patients with one lesion or no lesions on CT scan may have multiple lesions detectable by MRI (Fig. 2). Concentric and eccentric “target sign” enhancement on contrast enhanced MR images has been described (178, 179). Although TE can occasionally cause a single brain lesion on MR images, an alternative diagnosis such as primary CNS lymphoma should be considered in these individuals (180). Both TE and primary CNS lymphoma can cause contrast-enhancing lesions with mass effect and thus cannot be easily differentiated based on neuroradiologic criteria. However, the presence of hyperattenuation on nonenhanced brain CT scan and subependymal location are more specific for lymphoma (181). Based on the retention of thallium-201, single-photon emission computed tomography ([201Tl]-SPECT) imaging can reliably differentiate CNS lymphoma (uptake) from TE (no uptake). Indeed, SPECT demonstrated a 92% sensitivity and 85% specificity in distinguishing CNS lymphoma from other focal brain lesions in PLWH (182). The use of cART may decrease the specificity of SPECT in distinguishing between the two diagnoses (183).
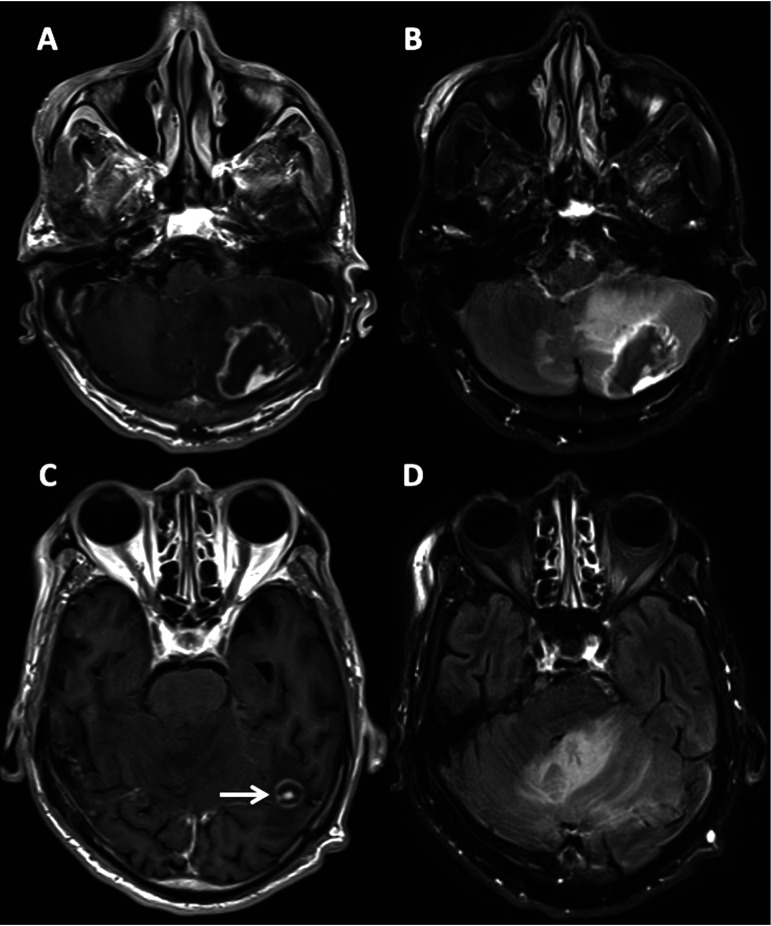
FIG 2.
Representative magnetic resonance images from a 68-year-old man living with HIV with toxoplasmic encephalitis. (A and C) T1 FLAIR post contrast. (B and D) Corresponding FLAIR. Note contrast enhancement of both lesions (A and C, bright white rim around the lesion), the “target sign” of the left temporal lobe lesion (white arrow), and significant mass effect (dark [low] signal on panels A and C; white [high] signal on panels B and D).
How Clinicians Use Laboratory Tests in Their Diagnostic Algorithms
In PLWH, the differential diagnosis for those with brain mass lesions with contrast enhancement and mass effect most commonly includes TE, primary CNS lymphoma, and tuberculoma or tuberculous abscess (Table 1). Individuals who are seropositive for anti-Toxoplasma IgG, have blood CD4+ T cell counts of <200/μl, and are not receiving prophylaxis for TE have a high likelihood of TE (23, 184–186), and the approach to these individuals is an empirical treatment trial. As noted above, a positive CSF PCR for T. gondii further increases the likelihood of TE to more than 90% (171), but lumbar puncture for collection of CSF is often not safe in patients with mass lesions. Clinical and radiographic improvement after 10 to 14 days of empirical treatment for TE is used to establish the diagnosis. Although brain biopsy is the gold standard for diagnosis (187), it is reserved for patients with low probability of TE, for example, those who are seronegative, and for individuals who fail to respond to a treatment trial (188). Accurate identification of TE can be challenging in individuals who do not have HIV, including recipients of hematopoietic stem cell or solid organ transplants, and those who received immunomodulatory therapies, in whom a wide variety of bacterial and fungal pathogens are possible. An example of this challenge is an individual with multiple sclerosis (MS) who developed TE during treatment with fingolimod (189). Brain lesions resembled tumefactive MS and prompted intensification of MS treatment. Serological testing was useful in establishing the correct diagnosis of TE.
TABLE 1.
Diagnostic pearls in the management of toxoplasmic encephalitis
| Summary of recommendations regarding testing for toxoplasmic encephalitis |
|---|
|
|
|
|
|
|
|
MANAGEMENT
Pharmacotherapy
Parasite dormancy exhibited by the cystic stage remains the main stumbling block to achieve effective eradication of toxoplasmosis because no drugs can clear tissue cysts of T. gondii (190). Current treatments can only manage acute and reactivated infections; both are caused by tachyzoites. If treatment is delayed, mortality can reach a very high level in immunocompromised individuals. The activity of pyrimethamine (PYR) is potentiated when combined with sulfadiazine (SDZ) or clindamycin (CLD) for TE in PLWH (191, 192) and, in combination with folinic acid, is the recommended initial treatment (193). High dose TMP-SMX is an alternative when the preferred regimen is not available (194, 195). Owing to the potential allergy or toxicity that can occur with the use of these drugs, alternative regimens have been used in PLWH, including the macrolide antibiotic azithromycin or the antimalarial agent atovaquone. Unfortunately, limited access to first-line drugs for acute TE may oblige physicians to choose an alternative therapy. A lower-priced PYR oral suspension successfully controlled TE in a PLWH (196), providing a new solution to tackle the challenge of affordability of the PYR-based regimen. While the aim of the study is laudable, it highlights concerns beyond issues of efficacy and safety, such as rising drug prices, industry profits, and patient access to crucial drugs, especially that of uninsured patients. More information about the treatment of toxoplasmosis in immunocompromised patients can be found in other reviews (195, 197).
Prevention
Over the past few decades, knowledge of T. gondii biology, epidemiology, and ecology has expanded exponentially and has provided the underpinning of the measures currently used to control T. gondii infection. Preventative measures focus on limiting the contact with known routes of transmission and reducing exposure to the parasite’s infective stages. As mentioned above, humans acquire T. gondii by consuming food or water contaminated with oocysts shed in cat feces or by ingesting the parasite cysts in raw or undercooked meat. Also, acquisition of infection can occur via ingestion of raw shellfish (198). Therefore, PLWH who are seronegative should only eat meat that has been thoroughly cooked, avoid eating raw shellfish, thoroughly clean their hands following touching raw meat, avoid gardening or handling soil without gloves, and eat fruits and vegetables that are properly washed. If PLWH have a cat, daily changing of the litter box should be delegated to another person who is not immunocompromised and is nonpregnant. Wearing disposable gloves and washing hands thoroughly with antiseptic should be a common practice for individuals who manage the litter box. When possible, cats should stay indoors and not be fed raw or undercooked meat, and seronegative PLWH should not adopt or handle stray cats. The above recommendations for prevention of T. gondii infection are also applicable to individuals in other specific at-risk groups. Standardized guidelines recommend that all PLWH be tested for serological evidence of previous T. gondii infection soon after HIV diagnosis (157). PLWH who are also seropositive for T. gondii with peripheral blood CD4+ T cell counts of <100/μl should receive primary prophylaxis to prevent TE (157). Individuals treated with cART who have more than 200 CD4+ T cells/μl for>3 months can safely discontinue primary prophylaxis. PLWH who have been successfully treated for TE and are receiving cART can discontinue maintenance treatment when they achieve more than 200 CD4+ T cells/μl for >6 months (157). It is worth noting that despite these preventive measures, no intervention is capable of completely preventing T. gondii infection.
QUALITY OF LIFE
Link to Psychiatric Illness and Cognitive Function
Interest in exploring the connection between infection and behavioral alterations and brain illnesses in humans was sparked following the discovery that T. gondii can induce neurologic changes in its intermediate murine hosts in order to make them easier prey for the definitive feline host (199). Despite a considerable amount of data, evidence surrounding the impact of T. gondii on neurological functions, particularly in regard to behavioral modifications and neurodegenerative disease, remains conflicting (200). There is serological evidence, albeit indirect and preliminary, pointing to an association between T. gondii infection and psychiatric abnormalities such as schizophrenia and bipolar disorder (201–203). However, randomized clinical trials of toxoplasmosis treatment have not shown a benefit in individuals with schizophrenia (204). Also, correlations between serological evidence of T. gondii infection and poorer neurocognitive function has been reported in some, but not all, studies, including in PLWH (205, 206). Cognitive impairment in individuals with schizophrenia who are also T. gondii seropositive was attributed to immune-inflammation mediated by alterations of kynurenine metabolism (207, 208). T. gondii can also alter other pathways, such as dopaminergic and GABAergic pathways, which have also been implicated in the neurobiology of schizophrenia (209). To what extent T. gondii-related alterations of the aforementioned pathways lead to psychiatric illness and cognitive impairment remains to be clarified.
Prognosis of TE in PLWH
More than half of PLWH who survive TE have residual neurological abnormalities (210). In addition, they are faced with the possibility of HIV progression and cognitive impairment. A prospective study of 205 PLWH and TE showed that initiation of cART within 2 months of TE diagnosis reduced HIV disease progression compared to that in those who started cART after 2 months (23). These results are consistent with studies that have shown improved survival of PLWH who develop TE in the cART era (210). PLWH with prior TE may be at greater risk of subsequent cognitive impairment and dementia compared to those with other CNS or non-CNS opportunistic infections (211, 212).
PROGRESS, CHALLENGES, AND FUTURE PROSPECTS
New Pharmacological Targets
As mentioned above, PYR and SDZ are the drugs of choice. This drug combination synergistically inhibits dihydropteroate synthase and dihydrofolate reductase, both of which play key roles in folate biosynthesis, which is essential for the growth and proliferation of T. gondii (213). Other treatment regimens involve atovaquone, which exerts its effect by inhibiting the mitochondrial cytochrome bc1, leading to interruption of cellular respiration and suppression of the parasite growth (214). Clindamycin exerts its antimicrobial activity via inhibiting protein synthesis in the apicoplast, a vestigial plastid organelle presents in T. gondii and other protozoa in the phylum Apicomplexa (215). Intolerance, poor absorption (191), variations in the susceptibility of T. gondii strains to PYR (216), increased pricing (196), adverse dermatologic and hematologic reactions associated with the use of PYR-based therapy (194), and inability to kill bradyzoites (190) are major challenges that limit the effectiveness of the current first-line interventions. For these reasons, there have been significant interests in the discovery of new formulations to treat TE. An earlier study showed that atovaquone nanosuspensions have improved bioavailability and high therapeutic efficacy against reactivated toxoplasmosis in mice (151). Another study demonstrated that treatment of mice with sodium dodecyl sulfate-coated atovaquone nanosuspensions reduced parasite load and inflammatory reactions in the brain (217).
The last few decades have witnessed significant progress toward exploring novel and better therapeutic agents for TE. Parasite-targeted therapeutics has been the most common approach used to discover new generations of anti-T. gondii drugs. This approach involves in vitro high-throughput screening of libraries of compounds for identification of those novel molecules with a potent toxoplasmicidal activity at nanomolar concentrations against the tachyzoite and/or bradyzoite stage. The clinical benefit of this approach is limited owing to the need to understand the compound’s toxicity and potential side effects, mode of action, and pharmacokinetics. Addressing these issues can be costly and time-consuming. An alternative strategy to overcome these hurdles is to test compounds approved to treat other indications (218). Drug repurposing has the added advantage of discovering new leads with novel scaffolds (different from the scaffold of PYR) and exhibit novel mechanism of actions. Screening molecules in the open access Malaria Box (i.e., Medicines for Malaria Venture) has revealed new anti-T. gondii drug candidates that seem to hold a promising therapeutic potential (219, 220). Encouraging results have been reported in other repurposing screens of existing drugs, in which several compounds were found to have new anti-T. gondii indications (218, 221).
Tackling the Treatment Impasse of Latent Infection
Elimination of persistent T. gondii cysts is a prerequisite for the success of any prophylactic program that aims to eliminate the risk of TE in vulnerable populations. As noted above, researchers have tested the efficacies of repurposed approved drugs used for other indications. For elimination of persistent T. gondii cysts, the antimalarial endochin-like quinolones (inhibitors of the apicomplexan cytochrome bc1) (222), the antileishmanial and antineoplastic miltefosine (inhibitor of phosphatidylcholine biosynthesis and the PI3K/Akt/PKB pathway) (223, 224), and guanabenz (inhibitor of translational control and protein synthesis) significantly decreased the brain cyst burden in latently infected mice (225). Additional compounds, such as the histone deacetylase inhibitor FR235222 (226), tanshinone IIA (inhibitor of inflammation and cancer cell cycle), and the antihistaminic hydroxyzine (227) have shown efficacy against T. gondii cysts. More validation studies are needed to select the compounds with the most potent cysticidal activity from these and many other approved drugs (195).
Vaccines
Vaccination may be used to prevent T. gondii infection or to clear latent infection. The long-term goal to control TE can be achieved by preventing its development in at-risk individuals. Therefore, there is a widely acknowledged need for human vaccines to reinforce the existing arsenal of anti-T. gondii therapeutics. Given the obvious importance of the cysts in TE, there is a need to have more in-depth understanding of the evolutionary pressure and the molecular pathways that mediate cyst development and the associated immune responses. This is especially important in order to provide well-informed perspectives for a rational development of safe and effective vaccine capable of inducting potent immunity against T. gondii cysts. Given the crucial role of CD8+ CTLs in controlling the cysts during chronic infection, GRA6 molecules released from bradyzoites within cysts can be a candidate immunogen for stimulating of CD8+ CTL response (101). Hence, in designing a therapeutic vaccine, inclusion of full-length GRA6 protein or GRA6Nt epitope(s) to induce CD8+ T cell responses can be a good approach to boost immune responses against the persistent cysts. Using an adjuvant to induce IgG2a and switch the cytokine balance toward Th1 immune responses can potentiate the immunogenicity of a vaccine. An alternative preemptive vaccination strategy may involve induction of GRA6Nt-primed CD8+ CTLs in healthy individuals so that when immunized individuals acquire infection, the elicited immune response can thwart the development of new cysts, protecting the vaccinated individuals from developing latent infection. There is evidence that the microenvironment of the cystic stage is more dynamic than what was previously thought because bradyzoites’ replication seems to continue within the cyst, although more slowly than in tachyzoites (228).
Several approaches to toxoplasmosis vaccine development have been explored, such as live attenuated strains, nanoparticle-based, exosome-based, and carbohydrate-based vaccination (229). Despite significant progress in vaccine discovery, including many promising proof-of-concept vaccination trials in mice, none of the tested vaccines has been advanced to clinical trials in humans. Future work toward a commercial vaccine requires detailed validation studies in order to optimize the potency and sustainability of the protective immunity, and practicality of administration. When researchers reported in the year 2014 the first application of the gene-editing tool CRISPR/CAS9 in T. gondii research (230), they kicked off a new era for the identification of genes with immunogenic potentials. Several genes have since been tested in vaccination studies in mice, which have helped to identify correlates of protection and candidates for generating attenuated strains of the tachyzoite stage (231, 232). On the downside, challenges remain as to prioritization of the most promising candidates from among the many fitness-conferring genes, the relevance of these candidates for the bradyzoite stage, and the difficulty of regulatory approval of live vaccines for human use (229, 233). Therefore, we must remain realistic about how soon we expect a human vaccine. T. gondii is a eukaryotic protozoan with a large ∼69.35-Mb genome, and it has >8,300 protein-coding genes (234). Also, this parasite has a complex life cycle with several antigenically distinct developmental stages that elicit different immune responses. Thus, developing a vaccine targeting several developmental stages will not be straightforward. Although the path to an effective vaccine is full of hurdles, we should remain hopeful that vaccines may, in the near future, become available for the control of toxoplasmosis. Finally, while a series of landmark studies has provided important contributions to current understanding of TE, many challenges concerning the pathogenesis and management of TE remain unsolved (Table 2). Overcoming these challenges is critical to successful development and realization of efficient therapeutic interventions.
TABLE 2.
Key questions to consider for future research
| Topic | Question or research need |
|---|---|
| Pathogenesis | What drives the remarkable diversity of T. gondii in regard to its intermediate host range and clinical pathogenicity? |
| How does the mechanism of crossing the blood-brain barrier (BBB) differ among T. gondii strains? | |
| To what extent do alterations in the actin cytoskeleton of cerebrovascular endothelial cells contribute to BBB disruption during T. gondii infection? | |
| What parasite gene products or effectors (e.g., soluble factors and extracellular exosomes and their contents) induce changes in the organization or expression of tight junction (TJ) proteins in endothelial cells? | |
| To what extent does T. gondii interaction with nonendothelial cellular components of the BBB (e.g., astrocytes, microglia, and pericytes) contribute to the pathogenesis of cerebral toxoplasmosis? | |
| Immunity | In which ways can trafficking and influx of CD4+ and CD8+ T cells into the brain be modulated by matrix metalloproteinase (MMP) agonists or tissue inhibitor of metalloproteinase (TIMP) antagonists? |
| How do cytokines, MMPs, and chemokines orchestrate T-cell migration to and recruitment into the brain to combat toxoplasmosis? | |
| How much overlap exists in the role of interleukin 10 (IL-10) originating from different sources in maintaining brain immune homeostasis during T. gondii infection? | |
| How does IL-10 limit T. gondii growth while at the same time attenuating excessive immune responses? | |
| Latent infection | What is the evolutionary advantage to the T. gondii parasite of infecting the CNS? |
| Which molecular mechanisms regulate T. gondii transitioning from dormant bradyzoites to actively proliferating tachyzoites, and vice versa? | |
| What are the factors that make neurons the most preferable host cell type, and what effects may the neural cell microenvironment have on the latency and reactivation of infection? | |
| Treatment | What can be done to improve the efficacy, safety, and tolerability profiles of existing anti-T. gondii therapeutics? |
| Comparative studies evaluating effectiveness of different treatment strategies for toxoplasmosis are needed, especially for those populations of patients at highest risk of TE. | |
| Can host-targeted therapy provide new adjunctive treatments for toxoplasmosis and reduce the development of drug resistance? | |
| Vaccination | Can vaccine cocktails incorporating multiple antigens induce more effective anti-T. gondii immune responses than a single-antigen vaccine? What mechanisms might be involved? |
| What are other potent immunogenic antigens are involved in T. gondii immunopathogenesis and can be targeted for vaccine development? | |
| Would genetically manipulated parasites be accepted by regulatory authorities as a potential live attenuated vaccine? |
ACKNOWLEDGMENTS
We thank Kevin Webb and Paul Goodwin from the University of Nottingham for helpful comments and valuable suggestions on the manuscript.
Biographies

Hany M. Elsheikha, Ph.D., is an Associate Professor in the Global Health Department, School of Veterinary Medicine and Science, University of Nottingham (SVMS-UoN). Dr. Elsheikha is also a European Veterinary Parasitology College diplomate and a European Scientific Counsel Companion Animal Parasites member. He earned his Ph.D. from Michigan State University for research performed on the molecular evolution of the causative agent of equine protozoal myeloencephalitis. In 2005, he was awarded the prestigious American Society for Microbiology (ASM)/National Center for Infectious Diseases (NCID) Postdoctoral Fellowship. Over the last 13 years, he has been spearheading the development and delivery of parasitology teaching at SVMS-UoN. Also, he has established a multidisciplinary research program focused on decoding the interkingdom chemical communication between host cells and neuropathogenic protozoan parasites, with a special interest in Toxoplasma gondii. He has published more than 170 peer-reviewed papers and many other articles in professional magazines and science communication journals. Dr. Elsheikha has also published 6 textbooks in veterinary parasitology for students, residents, and veterinary professionals.

Christina M. Marra, M.D., completed residency training in neurology and fellowship training in infectious diseases at the University of Washington, in Seattle, Washington, USA. She is Professor and Vice Chair for Academic Affairs in Neurology, and she has an adjunct appointment in Medicine (Infectious Diseases), at the University of Washington. Dr. Marra directs a U.S. National Institutes of Health-funded clinical and translational research program on factors that influence the clinical course of syphilis, with particular focus on HIV and on neurosyphilis. She received the American Sexually Transmitted Diseases Association Achievement Award in 2014 for work in this area. She also participates in multicenter clinical research on the neurological consequences of HIV, and she provides general neurological care in inpatient and outpatient settings, including a multispecialty HIV clinic.

Xing-Quan Zhu obtained a Bachelor of Veterinary Science (B.V.Sc.) degree from the Sichuan Institute of Animal Sciences and Veterinary Medicine, a Master of Veterinary Science (M.V.Sc.) degree from the Graduate School of the Chinese Academy of Agricultural Sciences, China, and a Ph.D. in parasitology from the Department of Veterinary Science, The University of Melbourne, Australia. He acquired postdoctoral training in molecular parasitology at the Department of Veterinary Science, The University of Melbourne. He is currently Distinguished Professor of Parasitology in the College of Veterinary Medicine, Shanxi Agricultural University, Shanxi Province, China. His research interests focus on the genetics, genomics, transcriptomics, proteomics, metabolomics, and microRNAs (miRNAs) of parasites; parasite-host interactions; molecular vaccines; and the epidemiology and control strategies of parasitic infections. He serves as a Subject Editor for the journal Parasites and Vectors and as a Section Editor for the journal Parasitology Research. He also serves on the editorial boards for Trends in Parasitology, Veterinary Parasitology, Experimental Parasitology, and Journal of Helminthology. Professor Zhu has published more than 300 articles in well-regarded international journals, including The Lancet Infectious Diseases, Clinical Microbiology Reviews, Nature Communications, Biotechnology Advances, PNAS, Trends in Parasitology, Emerging Infectious Diseases, The Journal of Infectious Diseases, and The Journal of Immunology.
REFERENCES
- 1.Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubey JP, Lindsay DS, Speer CA. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 11:267–299. doi: 10.1128/CMR.11.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 4.Elsheikha HM. 2008. Congenital toxoplasmosis: priorities for further health promotion action. Public Health 122:335–353. doi: 10.1016/j.puhe.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Maldonado YA, Read JS, Committee on Infectious Diseases . 2017. Diagnosis, treatment, and prevention of congenital toxoplasmosis in the United States. Pediatrics 139:e20163860. doi: 10.1542/peds.2016-3860. [DOI] [PubMed] [Google Scholar]
- 6.El Bissati K, Levigne P, Lykins J, Adlaoui EB, Barkat A, Berraho A, Laboudi M, El Mansouri B, Ibrahimi A, Rhajaoui M, Quinn F, Murugesan M, Seghrouchni F, Gomez-Marin JE, Peyron F, McLeod R. 2018. Global initiative for congenital toxoplasmosis: an observational and international comparative clinical analysis. Emerg Microbes Infect 7:165. doi: 10.1038/s41426-018-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyebji S, Seizova S, Hannan AJ, Tonkin CJ. 2019. Toxoplasmosis: a pathway to neuropsychiatric disorders. Neurosci Biobehav Rev 96:72–92. doi: 10.1016/j.neubiorev.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Cabral CM, Tuladhar S, Dietrich HK, Nguyen E, MacDonald WR, Trivedi T, Devineni A, Koshy AA. 2016. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog 12:e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cerutti A, Blanchard N, Besteiro S. 2020. The bradyzoite: a key developmental stage for the persistence and pathogenesis of toxoplasmosis. Pathogens 9:234. doi: 10.3390/pathogens9030234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldman BS, Schwarz D, Wadsworth MH 2nd, Saeij JP, Shalek AK, Lourido S. 2020. Identification of a master regulator of differentiation in Toxoplasma. Cell 180:359–372.e16. doi: 10.1016/j.cell.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones JL, Dubey JP. 2012. Foodborne toxoplasmosis. Clin Infect Dis 55:845–851. doi: 10.1093/cid/cis508. [DOI] [PubMed] [Google Scholar]
- 12.Dubey JP, Miller NL, Frenkel JK. 1970. The Toxoplasma gondii oocyst from cat feces. J Exp Med 132:636–662. doi: 10.1084/jem.132.4.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrmann DC, Barwald A, Maksimov A, Pantchev N, Vrhovec MG, Conraths FJ, Schares G. 2012. Toxoplasma gondii sexual cross in a single naturally infected feline host: generation of highly mouse-virulent and avirulent clones, genotypically different from clonal types I, II and III. Vet Res 43:39. doi: 10.1186/1297-9716-43-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey JP. 2009. History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol 39:877–882. doi: 10.1016/j.ijpara.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Luu L, Johnston LJ, Derricott H, Armstrong SD, Randle N, Hartley CS, Duckworth CA, Campbell BJ, Wastling JM, Coombes JL. 2019. An open-format enteroid culture system for interrogation of interactions between Toxoplasma gondii and the intestinal epithelium. Front Cell Infect Microbiol 9:300. doi: 10.3389/fcimb.2019.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Muno RM, Moura MA, de Carvalho LC, Seabra SH, Barbosa HS. 2014. Spontaneous cystogenesis of Toxoplasma gondii in feline epithelial cells in vitro. Folia Parasitol (Praha) 61:113–119. doi: 10.14411/fp.2014.017. [DOI] [PubMed] [Google Scholar]
- 17.Moura M. d A, Amendoeira MRR, Barbosa HS. 2009. Primary culture of intestinal epithelial cells as a potential model for Toxoplasma gondii enteric cycle studies. Mem Inst Oswaldo Cruz 104:862–864. doi: 10.1590/s0074-02762009000600007. [DOI] [PubMed] [Google Scholar]
- 18.Martorelli Di Genova B, Wilson SK, Dubey JP, Knoll LJ. 2019. Intestinal delta-6-desaturase activity determines host range for Toxoplasma sexual reproduction. PLoS Biol 17:e3000364. doi: 10.1371/journal.pbio.3000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang M, Zhang FK, Elsheikha HM, Zhang NZ, He JJ, Luo JX, Zhu XQ. 2018. Transcriptomic insights into the early host-pathogen interaction of cat intestine with Toxoplasma gondii. Parasit Vectors 11:592. doi: 10.1186/s13071-018-3179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhat DC, Swale C, Dard C, Cannella D, Ortet P, Barakat M, Sindikubwabo F, Belmudes L, De Bock PJ, Couté Y, Bougdour A, Hakimi MA. 2020. A MORC-driven transcriptional switch controls Toxoplasma developmental trajectories and sexual commitment. Nat Microbiol 5:570–583. doi: 10.1038/s41564-020-0674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss LM, Kim K. 2000. The development and biology of bradyzoites of Toxoplasma gondii. Front Biosci 5:D391–405. doi: 10.2741/weiss. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons RE, McLeod R, Roberts CW. 2002. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol 18:198–201. doi: 10.1016/S1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 23.Antinori A, Larussa D, Cingolani A, Lorenzini P, Bossolasco S, Finazzi MG, Bongiovanni M, Guaraldi G, Grisetti S, Vigo B, Gigli B, Mariano A, Dalle Nogare ER, De Marco M, Moretti F, Corsi P, Abrescia N, Rellecati P, Castagna A, Mussini C, Ammassari A, Cinque P, d’Arminio Monforte A, Italian Registry Investigative NeuroAIDS . 2004. Prevalence, associated factors, and prognostic determinants of AIDS-related toxoplasmic encephalitis in the era of advanced highly active antiretroviral therapy. Clin Infect Dis 39:1681–1691. doi: 10.1086/424877. [DOI] [PubMed] [Google Scholar]
- 24.Batz MB, Hoffmann S, Morris JG Jr. 2012. Ranking the disease burden of 14 pathogens in food sources in the United States using attribution data from outbreak investigations and expert elicitation. J Food Prot 75:1278–1291. doi: 10.4315/0362-028X.JFP-11-418. [DOI] [PubMed] [Google Scholar]
- 25.Hoffmann S, Batz MB, Morris JG Jr. 2012. Annual cost of illness and quality-adjusted life year losses in the United States due to 14 foodborne pathogens. J Food Prot 75:1292–1302. doi: 10.4315/0362-028X.JFP-11-417. [DOI] [PubMed] [Google Scholar]
- 26.Belk K, Connolly MP, Schlesinger L, Ben-Harari RR. 2018. Patient and treatment pathways for toxoplasmosis in the United States: data analysis of the Vizient Health Systems Data from 2011 to 2017. Pathog Glob Health 112:428–437. doi: 10.1080/20477724.2018.1552644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cummings PL, Kuo T, Javanbakht M, Sorvillo F. 2014. Trends, productivity losses, and associated medical conditions among toxoplasmosis deaths in the United States, 2000–2010. Am J Trop Med Hyg 91:959–964. doi: 10.4269/ajtmh.14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scharff RL. 2020. Food attribution and economic cost estimates for meat- and poultry-related illnesses. J Food Prot 83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- 29.Vora NM, Holman RC, Mehal JM, Steiner CA, Blanton J, Sejvar J. 2014. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology 82:443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 30.Schurer JM, Rafferty E, Schwandt M, Zeng W, Farag M, Jenkins EJ. 2016. Toxoplasmosis and toxocariasis: an assessment of humanimmunodeficiency virus comorbidity and health-care costs in Canada. Am J Trop Med Hyg 95:168–174. doi: 10.4269/ajtmh.15-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangen MJ, Friesema IHM, Haagsma JA, Van Pelt W. 2017. Disease burden of food-related pathogens in the Netherlands, 2016. RIVM, Bilthoven, The Netherlands. [DOI] [PubMed] [Google Scholar]
- 32.Monteiro Pires S, Jakobsen LS, Ellis-Iversen J, Pessoa J, Ethelberg S. 2020. Burden of disease estimates of seven pathogens commonly transmitted through foods in Denmark, 2017. Foodborne Pathog Dis 17:322–339. doi: 10.1089/fpd.2019.2705. [DOI] [PubMed] [Google Scholar]
- 33.Mose JM, Kagira JM, Kamau DM, Maina NW, Ngotho M, Karanja SM. 2020. A review on the present advances on studies of toxoplasmosis in eastern Africa. Biomed Res Int 2020:7135268. doi: 10.1155/2020/7135268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robert-Gangneux F, Dardé ML. 2012. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25:264–296. doi: 10.1128/CMR.05013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gajurel K, Dhakal R, Montoya JG. 2015. Toxoplasma prophylaxis in haematopoietic cell transplant recipients: a review of the literature and recommendations. Curr Opin Infect Dis 28:283–292. doi: 10.1097/QCO.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 36.Khetsuriani N, Holman RC, Anderson LJ. 2002. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clin Infect Dis 35:175–182. doi: 10.1086/341301. [DOI] [PubMed] [Google Scholar]
- 37.Jones JL, Roberts JM. 2012. Toxoplasmosis hospitalizations in the United States, 2008, and trends, 1993–2008. Clin Infect Dis 54:e58–61–e61. doi: 10.1093/cid/cir990. [DOI] [PubMed] [Google Scholar]
- 38.Mboera LEG, Kishamawe C, Kimario E, Rumisha SF. 2019. Mortality patterns of toxoplasmosis and its comorbidities in Tanzania: a 10-year retrospective hospital-based survey. Front Public Health 7:25. doi: 10.3389/fpubh.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewden C, Drabo YJ, Zannou DM, Maiga MY, Minta DK, Sow PS, Akakpo J, Dabis F, Eholié SP, IeDEA West Africa Collaboration . 2014. Disease patterns and causes of death of hospitalized HIV-positive adults in West Africa: a multicountry survey in the antiretroviral treatment era. J Int AIDS Soc 17:18797. doi: 10.7448/IAS.17.1.18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paccoud O, Guitard J, Labopin M, Surgers L, Malard F, Battipaglia G, Duléry R, Hennequin C, Mohty M, Brissot E. 2020. Features of Toxoplasma gondii reactivation after allogeneic hematopoietic stem-cell transplantation in a high seroprevalence setting. Bone Marrow Transplant 55:93–99. doi: 10.1038/s41409-019-0641-y. [DOI] [PubMed] [Google Scholar]
- 41.Wang ZD, Liu HH, Ma ZX, Ma HY, Li ZY, Yang ZB, Zhu XQ, Xu B, Wei F, Liu Q. 2017. Toxoplasma gondii infection in immunocompromised patients: a systematic review and meta-analysis. Front Microbiol 8:389. doi: 10.3389/fmicb.2017.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang ZD, Wang SC, Liu HH, Ma HY, Li ZY, Wei F, Zhu XQ, Liu Q. 2017. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: a systematic review and meta-analysis. Lancet HIV 4:e177–e188. doi: 10.1016/S2352-3018(17)30005-X. [DOI] [PubMed] [Google Scholar]
- 43.Safarpour H, Cevik M, Zarean M, Barac A, Hatam-Nahavandi K, Rahimi MT, Bannazadeh Baghi H, Koshki TJ, Pagheh AS, Shahrivar F, Ebrahimi M, Ahmadpour E. 2020. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. AIDS 34:469–474. doi: 10.1097/QAD.0000000000002424. [DOI] [PubMed] [Google Scholar]
- 44.Cohen SB, Denkers EY. 2015. The gut mucosal immune response to Toxoplasma gondii. Parasite Immunol 37:108–117. doi: 10.1111/pim.12164. [DOI] [PubMed] [Google Scholar]
- 45.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. 2006. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol 8:1611–1623. doi: 10.1111/j.1462-5822.2006.00735.x. [DOI] [PubMed] [Google Scholar]
- 46.Fuks JM, Arrighi RB, Weidner JM, Kumar Mendu S, Jin Z, Wallin RP, Rethi B, Birnir B, Barragan A. 2012. GABAergic signaling is linked to a hypermigratory phenotype in dendritic cells infected by Toxoplasma gondii. PLoS Pathog 8:e1003051. doi: 10.1371/journal.ppat.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weidner JM, Kanatani S, Hernández-Castañeda MA, Fuks JM, Rethi B, Wallin RP, Barragan A. 2013. Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell Microbiol 15:1735–1752. doi: 10.1111/cmi.12145. [DOI] [PubMed] [Google Scholar]
- 48.Kanatani S, Fuks JM, Olafsson EB, Westermark L, Chambers B, Varas-Godoy M, Uhlén P, Barragan A. 2017. Voltage-dependent calcium channel signaling mediates GABAA receptor-induced migratory activation of dendritic cells infected by Toxoplasma gondii. PLoS Pathog 13:e1006739. doi: 10.1371/journal.ppat.1006739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhandage AK, Barragan A. 2019. Calling in the caValry—Toxoplasma gondii hijacks GABAergic signaling and voltage-dependent calcium channel signaling for Trojan horse-mediated dissemination. Front Cell Infect Microbiol 9:61. doi: 10.3389/fcimb.2019.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ólafsson EB, Ross EC, Varas-Godoy M, Barragan A. 2019. TIMP-1 promotes hypermigration of Toxoplasma-infected primary dendritic cells via CD63-ITGB1-FAK signaling. J Cell Sci 132:jcs225193. doi: 10.1242/jcs.225193. [DOI] [PubMed] [Google Scholar]
- 51.Weidner JM, Kanatani S, Uchtenhagen H, Varas-Godoy M, Schulte T, Engelberg K, Gubbels MJ, Sun HS, Harrison RE, Achour A, Barragan A. 2016. Migratory activation of parasitized dendritic cells by the protozoan Toxoplasma gondii 14–3-3 protein. Cell Microbiol 18:1537–1550. doi: 10.1111/cmi.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drewry LL, Jones NG, Wang Q, Onken MD, Miller MJ, Sibley LD. 2019. The secreted kinase ROP17 promotes Toxoplasma gondii dissemination by hijacking monocyte tissue migration. Nat Microbiol 4:1951–1963. doi: 10.1038/s41564-019-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elsheikha HM, Khan NA. 2010. Protozoa traversal of the blood-brain barrier to invade the central nervous system. FEMS Microbiol Rev 34:532–553. doi: 10.1111/j.1574-6976.2010.00215.x. [DOI] [PubMed] [Google Scholar]
- 54.Harun MSR, Taylor M, Zhu XQ, Elsheikha HM. 2020. Transcriptome profiling of Toxoplasma gondii-infected human cerebromicrovascular endothelial cell response to treatment with monensin. Microorganisms 8:842. doi: 10.3390/microorganisms8060842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konradt C, Ueno N, Christian DA, Delong JH, Pritchard GH, Herz J, Bzik DJ, Koshy AA, McGavern DB, Lodoen MB, Hunter CA. 2016. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol 1:16001. doi: 10.1038/nmicrobiol.2016.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delorme-Walker V, Abrivard M, Lagal V, Anderson K, Perazzi A, Gonzalez V, Page C, Chauvet J, Ochoa W, Volkmann N, Hanein D, Tardieux I. 2012. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci 125:4333–4342. doi: 10.1242/jcs.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masocha W, Kristensson K. 2012. Passage of parasites across the blood-brain barrier. Virulence 3:202–212. doi: 10.4161/viru.19178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seipel D, Oliveira BC, Resende TL, Schuindt SH, Pimentel PM, Kanashiro MM, Arnholdt AC. 2010. Toxoplasma gondii infection positively modulates the macrophages migratory molecular complex by increasing matrix metalloproteinases, CD44 and alpha v beta 3 integrin. Vet Parasitol 169:312–319. doi: 10.1016/j.vetpar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 59.Landrith TA, Harris TH, Wilson EH. 2015. Characteristics and critical function of CD8+ T cells in the Toxoplasma-infected brain. Semin Immunopathol 37:261–270. doi: 10.1007/s00281-015-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harris TH, Banigan EJ, Christian DA, Konradt C, Tait Wojno ED, Norose K, Wilson EH, John B, Weninger W, Luster AD, Liu AJ, Hunter CA. 2012. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature 486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batista SJ, Still KM, Johanson D, Thompson JA, O’Brien CA, Lukens JR, Harris TH. 2020. Gasdermin-D-dependent IL-1α release from microglia promotes protective immunity during chronic Toxoplasma gondii infection. Nat Commun 11:3687. doi: 10.1038/s41467-020-17491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. 2009. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity 30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Falangola MF, Petito CK. 1993. Choroid plexus infection in cerebral toxoplasmosis in AIDS patients. Neurology 43:2035–2040. doi: 10.1212/wnl.43.10.2035. [DOI] [PubMed] [Google Scholar]
- 64.Schlüter D, Deckert M, Hof H, Frei K. 2001. Toxoplasma gondii infection of neurons induces neuronal cytokine and chemokine production, but gamma interferon- and tumor necrosis factor-stimulated neurons fail to inhibit the invasion and growth of T. gondii. Infect Immun 69:7889–7893. doi: 10.1128/IAI.69.12.7889-7893.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lüder CG, Giraldo-Velásquez M, Sendtner M, Gross U. 1999. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol 93:23–32. doi: 10.1006/expr.1999.4421. [DOI] [PubMed] [Google Scholar]
- 66.Wen X, Kudo T, Payne L, Wang X, Rodgers L, Suzuki Y. 2010. Predominant interferon-gamma-mediated expression of CXCL9, CXCL10, and CCL5 proteins in the brain during chronic infection with Toxoplasma gondii in BALB/c mice resistant to development of toxoplasmic encephalitis. J Interferon Cytokine Res 30:653–660. doi: 10.1089/jir.2009.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benevides L, Milanezi CM, Yamauchi LM, Benjamim CF, Silva JS, Silva NM. 2008. CCR2 receptor is essential to activate microbicidal mechanisms to control Toxoplasma gondii infection in the central nervous system. Am J Pathol 173:741–751. doi: 10.2353/ajpath.2008.080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ploix CC, Noor S, Crane J, Masek K, Carter W, Lo DD, Wilson EH, Carson MJ. 2011. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav Immun 25:883–896. doi: 10.1016/j.bbi.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Still KM, Thompson J, Batista S, Hayes N, Harris TH. 2018. The damage signal IL-33 facilitates focal immune responses to Toxoplasma gondii in the brain. J Immunol 200:43.4. [Google Scholar]
- 70.Hunter CA, Roberts CW, Murray M, Alexander J. 1992. Detection of cytokine mRNA in the brains of mice with toxoplasmic encephalitis. Parasite Immunol 14:405–413. doi: 10.1111/j.1365-3024.1992.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 71.Lu CY, Lai SC. 2013. Matrix metalloproteinase-2 and -9 lead to fibronectin degradation in astroglia infected with Toxoplasma gondii. Acta Trop 125:320–329. doi: 10.1016/j.actatropica.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Clark RT, Nance JP, Noor S, Wilson EH. 2011. T-cell production of matrix metalloproteinases and inhibition of parasite clearance by TIMP-1 during chronic Toxoplasma infection in the brain. ASN Neuro 3:e00049. doi: 10.1042/AN20100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol 149:175–180. [PubMed] [Google Scholar]
- 74.Atmaca HT, Kul O, Karakuş E, Terzi OS, Canpolat S, Anteplioğlu T. 2014. Astrocytes, microglia/macrophages, and neurons expressing Toll-like receptor 11 contribute to innate immunity against encephalitic Toxoplasma gondii infection. Neuroscience 269:184–191. doi: 10.1016/j.neuroscience.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 75.Yarovinsky F, Zhang D, Andersen JF, Bannenberg GL, Serhan CN, Hayden MS, Hieny S, Sutterwala FS, Flavell RA, Ghosh S, Sher A. 2005. TLR11 activation of dendritic cells by a protozoan profilin-like protein. Science 308:1626–1629. doi: 10.1126/science.1109893. [DOI] [PubMed] [Google Scholar]
- 76.Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol 168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- 77.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. 2005. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog 1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto M, Okuyama M, Ma JS, Kimura T, Kamiyama N, Saiga H, Ohshima J, Sasai M, Kayama H, Okamoto T, Huang DC, Soldati-Favre D, Horie K, Takeda J, Takeda K. 2012. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 37:302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 79.Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klümpers V, Lahme S, Wischmann E, Mausberg AK, Beer-Hammer S, Pfeffer K. 2013. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci U S A 110:294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sturge CR, Yarovinsky F. 2014. Complex immune cell interplay in the gamma interferon response during Toxoplasma gondii infection. Infect Immun 82:3090–3097. doi: 10.1128/IAI.01722-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adams LB, Hibbs JB Jr, Taintor RR, Krahenbuhl JL. 1990. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J Immunol 144:2725–2729. [PubMed] [Google Scholar]
- 82.Scharton-Kersten TM, Yap G, Magram J, Sher A. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med 185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bando H, Lee Y, Sakaguchi N, Pradipta A, Ma JS, Tanaka S, Cai Y, Liu J, Shen J, Nishikawa Y, Sasai M, Yamamoto M. 2018. Inducible nitric oxide synthase is a key host factor for Toxoplasma GRA15-dependent disruption of the gamma interferon-induced antiparasitic human response. mBio 9:e01738-18. doi: 10.1128/mBio.01738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez Y, Rosado J. d D, Vega L, Elizondo G, Estrada-Muñiz E, Saavedra R, Juárez I, Rodríguez-Sosa M. 2010. The unexpected role for the aryl hydrocarbon receptor on susceptibility to experimental toxoplasmosis. J Biomed Biotechnol 2010:505694. doi: 10.1155/2010/505694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwarcz R, Hunter CA. 2007. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull 33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bando H, Sakaguchi N, Lee Y, Pradipta A, Ma JS, Tanaka S, Lai DH, Liu J, Lun ZR, Nishikawa Y, Sasai M, Yamamoto M. 2018. Toxoplasma effector TgIST targets host ido1 to antagonize the IFN-γ-induced anti-parasitic response in human cells. Front Immunol 9:2073. doi: 10.3389/fimmu.2018.02073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wichert A, Ursula S, Degrandi D, Pfeffer K. 2019. Lymphotoxin β receptor: a crucial role in Toxoplasma gondii infection. J Immunol 202:190.87. [Google Scholar]
- 88.Steffens N, Beuter-Gunia C, Kravets E, Reich A, Legewie L, Pfeffer K, Degrandi D. 2020. Essential role of mGBP7 for survival of Toxoplasma gondii infection. mBio 11:e02993-19. doi: 10.1128/mBio.02993-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halonen SK, Taylor GA, Weiss LM. 2001. Gamma interferon-induced inhibition of Toxoplasma gondii in astrocytes is mediated by IGTP. Infect Immun 69:5573–5576. doi: 10.1128/iai.69.9.5573-5576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hidano S, Randall LM, Dawson L, Dietrich HK, Konradt C, Klover PJ, John B, Harris TH, Fang Q, Turek B, Kobayashi T, Hennighausen L, Beiting DP, Koshy AA, Hunter CA. 2016. STAT1 Signaling in astrocytes is essential for control of infection in the central nervous system. mBio 7:e01881-16. doi: 10.1128/mBio.01881-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beiting DP, Hidano S, Baggs JE, Geskes JM, Fang Q, Wherry EJ, Hunter CA, Roos DS, Cherry S. 2015. The orphan nuclear receptor TLX is an enhancer of STAT1-mediated transcription and immunity to Toxoplasma gondii. PLoS Biol 13:e1002200. doi: 10.1371/journal.pbio.1002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drögemüller K, Helmuth U, Brunn A, Sakowicz-Burkiewicz M, Gutmann DH, Mueller W, Deckert M, Schlüter D. 2008. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol 181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- 93.Thompson J, Nikolas H, Gevaria A, Harris T. 2017. The role of microglia and monocyte-derived macrophages in Toxoplasma gondii infection of the brain. J Immunol 198:68.16. [Google Scholar]
- 94.Suzuki Y, Sa Q, Gehman M, Ochiai E. 2011. Interferon-gamma- and perforin-mediated immune responses for resistance against Toxoplasma gondii in the brain. Expert Rev Mol Med 13:e31. doi: 10.1017/S1462399411002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, Michie SA, Xu B, Kudo T, Perkins S. 2010. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol 176:1607–1613. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nance JP, Vannella KM, Worth D, David C, Carter D, Noor S, Hubeau C, Fitz L, Lane TE, Wynn TA, Wilson EH. 2012. Chitinase dependent control of protozoan cyst burden in the brain. PLoS Pathog 8:e1002990. doi: 10.1371/journal.ppat.1002990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tiwari A, Hannah R, Lutshumba J, Ochiai E, Weiss LM, Suzuki Y. 2019. Penetration of CD8+ Cytotoxic T cells into large target, tissue cysts of Toxoplasma gondii, leads to its elimination. Am J Pathol 189:1594–1607. doi: 10.1016/j.ajpath.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lutshumba J, Ochiai E, Sa Q, Anand N, Suzuki Y. 2020. Selective upregulation of transcripts for six molecules related to T cell costimulation and phagocyte recruitment and activation among 734 immunity-related genes in the brain during perforin-dependent, CD8+ T cell-mediated elimination of Toxoplasma gondii cysts. mSystems 5:e00189-20. doi: 10.1128/mSystems.00189-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. 2008. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol 9:937–944. doi: 10.1038/ni.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanecka A, Yoshida N, Dougan SK, Jackson J, Shastri N, Ploegh H, Blanchard N, Frickel EM. 2016. Transnuclear CD8 T cells specific for the immunodominant epitope Gra6 lower acute-phase Toxoplasma gondii burden. Immunology 149:270–279. doi: 10.1111/imm.12643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sa Q, Ochiai E, Tiwari A, Mullins J, Shastri N, Mercier C, Cesbron-Delauw MF, Suzuki Y. 2017. Determination of a key antigen for immunological intervention to target the latent stage of Toxoplasma gondii. J Immunol 198:4425–4434. doi: 10.4049/jimmunol.1700062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sa Q, Tiwari A, Ochiai E, Mullins J, Suzuki Y. 2018. Inducible nitric oxide synthase in innate immune cells is important for restricting cyst formation of Toxoplasma gondii in the brain but not required for the protective immune process to remove the cysts. Microbes Infect 20:261–266. doi: 10.1016/j.micinf.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lüder CG, Lang C, Giraldo-Velasquez M, Algner M, Gerdes J, Gross U. 2003. Toxoplasma gondii inhibits MHC class II expression in neural antigen-presenting cells by down-regulating the class II transactivator CIITA. J Neuroimmunol 134:12–24. doi: 10.1016/S0165-5728(02)00320-X. [DOI] [PubMed] [Google Scholar]
- 104.Aliberti J, Valenzuela JG, Carruthers VB, Hieny S, Andersen J, Charest H, Reis e Sousa C, Fairlamb A, Ribeiro JM, Sher A. 2003. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol 4:485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 105.Fischer HG, Bonifas U, Reichmann G. 2000. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol 164:4826–4834. doi: 10.4049/jimmunol.164.9.4826. [DOI] [PubMed] [Google Scholar]
- 106.Hakimi MA, Olias P, Sibley LD. 2017. Toxoplasma effectors targeting host signaling and transcription. Clin Microbiol Rev 30:615–645. doi: 10.1128/CMR.00005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Boothroyd JC, Dubremetz JF. 2008. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol 6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 108.Nadipuram SM, Kim EW, Vashisht AA, Lin AH, Bell HN, Coppens I, Wohlschlegel JA, Bradley PJ. 2016. In vivo biotinylation of the Toxoplasma parasitophorous vacuole reveals novel dense granule proteins important for parasite growth and pathogenesis. mBio 7:e00808-16. doi: 10.1128/mBio.00808-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clough B, Frickel EM. 2017. The Toxoplasma parasitophorous vacuole: an evolving host-parasite frontier. Trends Parasitol 33:473–488. doi: 10.1016/j.pt.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 110.Gold DA, Kaplan AD, Lis A, Bett GC, Rosowski EE, Cirelli KM, Bougdour A, Sidik SM, Beck JR, Lourido S, Egea PF, Bradley PJ, Hakimi MA, Rasmusson RL, Saeij JP. 2015. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 17:642–652. doi: 10.1016/j.chom.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li TT, Wang JL, Liang QL, Sun LX, Zhang HS, Zhang ZW, Zhu XQ, Elsheikha HM. 2020. Effect of deletion of gra17 and gra23 genes on the growth, virulence, and immunogenicity of type II Toxoplasma gondii. Parasitol Res 119:2907–2916. doi: 10.1007/s00436-020-06815-z. [DOI] [PubMed] [Google Scholar]
- 112.Chen L, Christian DA, Kochanowsky JA, Phan AT, Clark JT, Wang S, Berry C, Oh J, Chen X, Roos DS, Beiting DP, Koshy AA, Hunter CA. 2020. The Toxoplasma gondii virulence factor ROP16 acts in cis and trans, and suppresses T cell responses. J Exp Med 217:e20181757. doi: 10.1084/jem.20181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD. 2014. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Matta SK, Olias P, Huang Z, Wang Q, Park E, Yokoyama WM, Sibley LD. 2019. Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc Natl Acad Sci U S A 116:17480–17491. doi: 10.1073/pnas.1904637116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aliberti J, Serhan C, Sher A. 2002. Parasite-induced lipoxin A4 is an endogenous regulator of IL-12 production and immunopathology in Toxoplasma gondii infection. J Exp Med 196:1253–1262. doi: 10.1084/jem.20021183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, Aliberti J. 2006. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med 12:330–334. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 117.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. 2007. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol 7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 119.Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS, Harris TH, Grainger J, Wojno ED, Wagage S, Roos DS, Scott P, Turka LA, Cherry S, Reiner SL, Cua D, Belkaid Y, Elloso MM, Hunter CA. 2012. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gazzinelli RT, Wysocka M, Hieny S, Scharton-Kersten T, Cheever A, Kuhn R, Muller W, Trinchieri G, Sher A. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent on CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma and TNF-alpha. J Immunol 157:798–805. [PubMed] [Google Scholar]
- 121.Wilson EH, Wille-Reece U, Dzierszinski F, Hunter CA. 2005. A critical role for IL-10 in limiting inflammation during toxoplasmic encephalitis. J Neuroimmunol 165:63–74. doi: 10.1016/j.jneuroim.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 122.Yu F, Sharma S, Jankovic D, Gurram RK, Su P, Hu G, Li R, Rieder S, Zhao K, Sun B, Zhu J. 2018. The transcription factor Bhlhe40 is a switch of inflammatory versus antiinflammatory Th1 cell fate determination. J Exp Med 215:1813–1821. doi: 10.1084/jem.20170155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. 2001. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 124.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. 1991. IL-10 inhibits cytokine production by activated macrophages. J Immunol 147:3815–3822. [PubMed] [Google Scholar]
- 125.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. 2002. In the absence of IL-12, CD4+ T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10−/− setting. Immunity 16:429–439. doi: 10.1016/S1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 126.Shaw MH, Freeman GJ, Scott MF, Fox BA, Bzik DJ, Belkaid Y, Yap GS. 2006. Tyk2 negatively regulates adaptive Th1 immunity by mediating IL-10 signaling and promoting IFN-gamma-dependent IL-10 reactivation. J Immunol 176:7263–7271. doi: 10.4049/jimmunol.176.12.7263. [DOI] [PubMed] [Google Scholar]
- 127.O'Garra A, Vieira P. 2007. T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- 128.Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. 2010. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rozenfeld C, Martinez R, Figueiredo RT, Bozza MT, Lima FR, Pires AL, Silva PM, Bonomo A, Lannes-Vieira J, De Souza W, Moura-Neto V. 2003. Soluble factors released by Toxoplasma gondii-infected astrocytes down-modulate nitric oxide production by gamma interferon-activated microglia and prevent neuronal degeneration. Infect Immun 71:2047–2057. doi: 10.1128/iai.71.4.2047-2057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rozenfeld C, Martinez R, Seabra S, Sant'anna C, Goncalves JG, Bozza M, Moura-Neto V, De Souza W. 2005. Toxoplasma gondii prevents neuron degeneration by interferon-gamma-activated microglia in a mechanism involving inhibition of inducible nitric oxide synthase and transforming growth factor-beta1 production by infected microglia. Am J Pathol 167:1021–1031. doi: 10.1016/S0002-9440(10)61191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, Egusquiza R, Wai KM, Koshy AA, Buckwalter MS. 2014. Astrocytic TGF-β signaling limits inflammation and reduces neuronal damage during central nervous system Toxoplasma infection. J Immunol 193:139–149. doi: 10.4049/jimmunol.1303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O'Brien CA, Overall C, Konradt C, O'Hara Hall AC, Hayes NW, Wagage S, John B, Christian DA, Hunter CA, Harris TH. 2017. CD11c-expressing cells affect regulatory T cell behavior in the meninges during central nervous system infection. J Immunol 198:4054–4061. doi: 10.4049/jimmunol.1601581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tenorio EP, Fernandez J, Castellanos C, Olguin JE, Saavedra R. 2011. CD4+ Foxp3+ regulatory T cells mediate Toxoplasma gondii-induced T-cell suppression through an IL-2-related mechanism but independently of IL-10. Eur J Immunol 41:3529–3541. doi: 10.1002/eji.201141507. [DOI] [PubMed] [Google Scholar]
- 134.Harris T. 2014. Regulatory T cells in CNS Toxoplasma gondii infection. J Neuroimmunology 275:77–78. doi: 10.1016/j.jneuroim.2014.08.205. [DOI] [Google Scholar]
- 135.O”Brien CA, Harris TH. 2020. ICOS-deficient and ICOS YF mutant mice fail to control Toxoplasma gondii infection of the brain. PLoS One 15:e0228251. doi: 10.1371/journal.pone.0228251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. 2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med 204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. 2013. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sher A, Tosh K, Jankovic D. 2017. Innate recognition of Toxoplasma gondii in humans involves a mechanism distinct from that utilized by rodents. Cell Mol Immunol 14:36–42. doi: 10.1038/cmi.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christian DA, Koshy AA, Reuter MA, Betts MR, Boothroyd JC, Hunter CA. 2014. Use of transgenic parasites and host reporters to dissect events that promote interleukin-12 production during toxoplasmosis. Infect Immun 82:4056–4067. doi: 10.1128/IAI.01643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andrade WA, Souza M. d C, Ramos-Martinez E, Nagpal K, Dutra MS, Melo MB, Bartholomeu DC, Ghosh S, Golenbock DT, Gazzinelli RT. 2013. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe 13:42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Safronova A, Araujo A, Camanzo ET, Moon TJ, Elliott MR, Beiting DP, Yarovinsky F. 2019. Alarmin S100A11 initiates a chemokine response to the human pathogen Toxoplasma gondii. Nat Immunol 20:64–72. doi: 10.1038/s41590-018-0250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yoshida N, Domart MC, Peddie CJ, Yakimovich A, Mazon-Moya MJ, Hawkins TA, Collinson L, Mercer J, Frickel EM, Mostowy S. 2020. The zebrafish as a novel model for the in vivo study of Toxoplasma gondii replication and interaction with macrophages. Dis Model Mech 13:dmm043091. doi: 10.1242/dmm.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carr A, Tindall B, Brew BJ, Marriott DJ, Harkness JL, Penny R, Cooper DA. 1992. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med 117:106–111. doi: 10.7326/0003-4819-117-2-106. [DOI] [PubMed] [Google Scholar]
- 144.Abgrall S, Rabaud C, Costagliola D, Clinical Epidemiology Group of the French Hospital Database on HIV . 2001. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clin Infect Dis 33:1747–1755. doi: 10.1086/322622. [DOI] [PubMed] [Google Scholar]
- 145.Wang X, Claflin J, Kang H, Suzuki Y. 2005. Importance of CD8+Vbeta8+ T cells in IFN-gamma-mediated prevention of toxoplasmic encephalitis in genetically resistant BALB/c mice. J Interferon Cytokine Res 25:338–344. doi: 10.1089/jir.2005.25.338. [DOI] [PubMed] [Google Scholar]
- 146.Sa Q, Ochiai E, Tiwari A, Perkins S, Mullins J, Gehman M, Huckle W, Eyestone WH, Saunders TL, Shelton BJ, Suzuki Y. 2015. Cutting Edge: IFN-γ produced by brain-resident cells is crucial to control cerebral infection with Toxoplasma gondii. J Immunol 195:796–800. doi: 10.4049/jimmunol.1500814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sa Q, Mercier C, Cesbron-Delauw M-F, Suzuki Y. 2020. The amino-terminal region of dense granule protein 6 of Toxoplasma gondii stimulates IFN-γ production by microglia. Microbes Infect 22:375–378. S1286-4579(20)30001–0. doi: 10.1016/j.micinf.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ochiai E, Sa Q, Brogli M, Kudo T, Wang X, Dubey JP, Suzuki Y. 2015. CXCL9 is important for recruiting immune T cells into the brain and inducing an accumulation of the T cells to the areas of tachyzoite proliferation to prevent reactivation of chronic cerebral infection with Toxoplasma gondii. Am J Pathol 185:314–324. doi: 10.1016/j.ajpath.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang X, Michie SA, Xu B, Suzuki Y. 2007. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J Interferon Cytokine Res 27:329–338. doi: 10.1089/jir.2006.0154. [DOI] [PubMed] [Google Scholar]
- 150.Sa Q, Ochiai E, Sengoku T, Wilson ME, Brogli M, Crutcher S, Michie SA, Xu B, Payne L, Wang X, Suzuki Y. 2014. VCAM-1/α4β1 integrin interaction is crucial for prompt recruitment of immune T cells into the brain during the early stage of reactivation of chronic infection with Toxoplasma gondii to prevent toxoplasmic encephalitis. Infect Immun 82:2826–2839. doi: 10.1128/IAI.01494-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Scholer N, Krause K, Kayser O, Muller RH, Borner K, Hahn H, Liesenfeld O. 2001. Atovaquone nanosuspensions show excellent therapeutic effect in a new murine model of reactivated toxoplasmosis. Antimicrob Agents Chemother 45:1771–1779. doi: 10.1128/AAC.45.6.1771-1779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pierog PL, Zhao Y, Singh S, Dai J, Yap GS, Fitzgerald-Bocarsly P. 2018. Toxoplasma gondii inactivates human plasmacytoid dendritic cells by functional mimicry of IL-10. J Immunol 200:186–195. doi: 10.4049/jimmunol.1701045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhadra R, Gigley JP, Weiss LM, Khan IA. 2011. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A 108:9196–9201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bhadra R, Gigley JP, Khan IA. 2012. PD-1-mediated attrition of polyfunctional memory CD8+ T cells in chronic toxoplasma infection. J Infect Dis 206:125–134. doi: 10.1093/infdis/jis304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xiao J, Li Y, Yolken RH, Viscidi RP. 2018. PD-1 immune checkpoint blockade promotes brain leukocyte infiltration and diminishes cyst burden in a mouse model of Toxoplasma infection. J Neuroimmunol 319:55–62. doi: 10.1016/j.jneuroim.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 156.Oksenhendler E, Charreau I, Tournerie C, Azihary M, Carbon C, Aboulker JP. 1994. Toxoplasma gondii infection in advanced HIV infection. AIDS 8:483–488. doi: 10.1097/00002030-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 157.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H, Centers for Disease Control and Prevention, National Institutes of Health, HIV Medicine Association of the Infectious Diseases Society of America . 2009. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 58:1–207, quiz CE1–4. [PubMed] [Google Scholar]
- 158.Navia BA, Petito CK, Gold JW, Cho ES, Jordan BD, Price RW. 1986. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol 19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 159.Porter SB, Sande MA. 1992. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med 327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 160.Nath A, Hobson DE, Russell A. 1993. Movement disorders with cerebral toxoplasmosis and AIDS. Mov Disord 8:107–112. doi: 10.1002/mds.870080119. [DOI] [PubMed] [Google Scholar]
- 161.Arendt G, Hefter H, Figge C, Neuen-Jakob E, Nelles HW, Elsing C, Freund HJ. 1991. Two cases of cerebral toxoplasmosis in AIDS patients mimicking HIV-related dementia. J Neurol 238:439–442. doi: 10.1007/BF00314650. [DOI] [PubMed] [Google Scholar]
- 162.Gray F, Gherardi R, Wingate E, Wingate J, Fenelon G, Gaston A, Sobel A, Poirier J. 1989. Diffuse “encephalitic” cerebral toxoplasmosis in AIDS: report of four cases. J Neurol 236:273–277. doi: 10.1007/BF00314455. [DOI] [PubMed] [Google Scholar]
- 163.Mauhin W, Demoule A, Leclercq D, Gasnault J, Paris L, Katlama C, Epelboin L. 2016. Toxoplasmic ventriculitis. Med Mal Infect 46:100–103. doi: 10.1016/j.medmal.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 164.Reiter-Owona I, Petersen E, Joynson D, Aspock H, Darde ML, Disko R, Dreazen O, Dumon H, Grillo R, Gross U, Hayde M, Holliman R, Ho-Yen DO, Janitschke K, Jenum PA, Naser K, Olszewski M, Thulliez P, Seitz HM. 1999. The past and present role of the Sabin-Feldman dye test in the serodiagnosis of toxoplasmosis. Bull World Health Organ 77:929–935. [PMC free article] [PubMed] [Google Scholar]
- 165.Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. 1984. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA 252:913–917. doi: 10.1001/jama.1984.03350070031018. [DOI] [PubMed] [Google Scholar]
- 166.Wong B, Gold JW, Brown AE, Lange M, Fried R, Grieco M, Mildvan D, Giron J, Tapper ML, Lerner CW. 1984. Central-nervous-system toxoplasmosis in homosexual men and parenteral drug abusers. Ann Intern Med 100:36–42. doi: 10.7326/0003-4819-100-1-36. [DOI] [PubMed] [Google Scholar]
- 167.Berger JR. 2003. Mass lesions of the brain in AIDS: the dilemmas of distinguishing toxoplasmosis from primary CNS lymphoma. AJNR Am J Neuroradiol 24:554–555. [PMC free article] [PubMed] [Google Scholar]
- 168.Lappalainen M, Koskela P, Koskiniemi M, Ammala P, Hiilesmaa V, Teramo K, Raivio KO, Remington JS, Hedman K. 1993. Toxoplasmosis acquired during pregnancy: improved serodiagnosis based on avidity of IgG. J Infect Dis 167:691–697. doi: 10.1093/infdis/167.3.691. [DOI] [PubMed] [Google Scholar]
- 169.Alfonso Y, Fraga J, Jimenez N, Fonseca C, Dorta-Contreras AJ, Cox R, Capo V, Bandera F, Pomier O, Ginorio D. 2009. Detection of Toxoplasma gondii in cerebrospinal fluid from AIDS patients by nested PCR and rapid identification of type I allele at B1 gene by RFLP analysis. Exp Parasitol 122:203–207. doi: 10.1016/j.exppara.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 170.Anselmo LM, Vilar FC, Lima JE, Yamamoto AY, Bollela VR, Takayanagui OM. 2014. Usefulness and limitations of polymerase chain reaction in the etiologic diagnosis of neurotoxoplasmosis in immunocompromised patients. J Neurol Sci 346:231–234. doi: 10.1016/j.jns.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 171.Antinori A, Ammassari A, De Luca A, Cingolani A, Murri R, Scoppettuolo G, Fortini M, Tartaglione T, Larocca LM, Zannoni G, Cattani P, Grillo R, Roselli R, Iacoangeli M, Scerrati M, Ortona L. 1997. Diagnosis of AIDS-related focal brain lesions: a decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology 48:687–694. doi: 10.1212/wnl.48.3.687. [DOI] [PubMed] [Google Scholar]
- 172.Farkash AE, Maccabee PJ, Sher JH, Landesman SH, Hotson G. 1986. CNS toxoplasmosis in acquired immune deficiency syndrome: a clinical-pathological-radiological review of 12 cases. J Neurol Neurosurg Psychiatry 49:744–748. doi: 10.1136/jnnp.49.7.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Conley FK, Jenkins KA, Remington JS. 1981. Toxoplasma gondii infection of the central nervous system. Use of the peroxidase-antiperoxidase method to demonstrate toxoplasma in formalin fixed, paraffin embedded tissue sections. Hum Pathol 12:690–698. doi: 10.1016/S0046-8177(81)80170-0. [DOI] [PubMed] [Google Scholar]
- 174.Cohen W, Koslow M. 1985. An unusual CT presentation of cerebral toxoplasmosis. J Comput Assist Tomogr 9:384–386. doi: 10.1097/00004728-198503000-00033. [DOI] [PubMed] [Google Scholar]
- 175.Sell M, Sander B, Klingebiel R. 2005. Ventriculitis and hydrocephalus as the primary manifestation of cerebral toxoplasmosis associated with AIDS. J Neurol 252:234–236. doi: 10.1007/s00415-005-0620-7. [DOI] [PubMed] [Google Scholar]
- 176.Levy RM, Mills CM, Posin JP, Moore SG, Rosenblum ML, Bredesen DE. 1990. The efficacy and clinical impact of brain imaging in neurologically symptomatic AIDS patients: a prospective CT/MRI study. J Acquir Immune Defic Syndr (1988) 3:461–471. [PubMed] [Google Scholar]
- 177.Ciricillo SF, Rosenblum ML. 1990. Use of CT and MR imaging to distinguish intracranial lesions and to define the need for biopsy in AIDS patients. J Neurosurg 73:720–724. doi: 10.3171/jns.1990.73.5.0720. [DOI] [PubMed] [Google Scholar]
- 178.Kumar GG, Mahadevan A, Guruprasad AS, Kovoor JM, Satishchandra P, Nath A, Ranga U, Shankar SK. 2010. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging 31:1469–1472. doi: 10.1002/jmri.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mahadevan A, Ramalingaiah AH, Parthasarathy S, Nath A, Ranga U, Krishna SS. 2013. Neuropathological correlate of the “concentric target sign” in MRI of HIV-associated cerebral toxoplasmosis. J Magn Reson Imaging 38:488–495. doi: 10.1002/jmri.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ciricillo SF, Rosenblum ML. 1991. Imaging of solitary lesions in AIDS. J Neurosurg 74:1029. doi: 10.3171/jns.1991.74.6.1029. [DOI] [PubMed] [Google Scholar]
- 181.Dina TS. 1991. Primary central nervous system lymphoma versus toxoplasmosis in AIDS. Radiology 179:823–828. doi: 10.1148/radiology.179.3.2027999. [DOI] [PubMed] [Google Scholar]
- 182.Yang M, Sun J, Bai HX, Tao Y, Tang X, States LJ, Zhang Z, Zhou J, Farwell MD, Zhang P, Xiao B, Yang L. 2017. Diagnostic accuracy of SPECT, PET, and MRS for primary central nervous system lymphoma in HIV patients: a systematic review and meta-analysis. Medicine (Baltimore) 96:e6676. doi: 10.1097/MD.0000000000006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Giancola ML, Rizzi EB, Schiavo R, Lorenzini P, Schinina V, Alba L, Del Grosso B, Gigli B, Rosati S, Mango L, Bibbolino C, Antinori A. 2004. Reduced value of thallium-201 single-photon emission computed tomography in the management of HIV-related focal brain lesions in the era of highly active antiretroviral therapy. AIDS Res Hum Retroviruses 20:584–588. doi: 10.1089/0889222041217446. [DOI] [PubMed] [Google Scholar]
- 184.Kiderlen TR, Liesenfeld O, Schurmann D, Schneider T. 2011. Toxoplasmic encephalitis in AIDS-patients before and after the introduction of highly active antiretroviral therapy (HAART). Eur J Clin Microbiol Infect Dis 30:1521–1525. doi: 10.1007/s10096-011-1254-6. [DOI] [PubMed] [Google Scholar]
- 185.Marra CM, Krone MR, Koutsky LA, Holmes KK. 1998. Diagnostic accuracy of HIV-associated central nervous system toxoplasmosis. Int J STD AIDS 9:761–764. doi: 10.1258/0956462981921378. [DOI] [PubMed] [Google Scholar]
- 186.Raffi F, Aboulker JP, Michelet C, Reliquet V, Pelloux H, Huart A, Poizot-Martin I, Morlat P, Dupas B, Mussini JM, Leport C. 1997. A prospective study of criteria for the diagnosis of toxoplasmic encephalitis in 186 AIDS patients: the BIOTOXO Study Group. AIDS 11:177–184. doi: 10.1097/00002030-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 187.Bannoura S, El Hajj R, Khalifeh I, El Hajj H. 2018. Acute disseminated encephalomyelitis and reactivation of cerebral toxoplasmosis in a child: case report. IDCases 13:e00434. doi: 10.1016/j.idcr.2018.e00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Davaro RE, Thirumalai A. 2007. Life-threatening complications of HIV infection. J Intensive Care Med 22:73–81. doi: 10.1177/0885066606297964. [DOI] [PubMed] [Google Scholar]
- 189.Enriquez-Marulanda A, Valderrama-Chaparro J, Parrado L, Diego Vélez J, Maria Granados A, Luis Orozco J, Quiñones J. 2017. Cerebral toxoplasmosis in an MS patient receiving fingolimod. Mult Scler Relat Disord 18:106–108. doi: 10.1016/j.msard.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 190.Derouin F. 2001. Anti-toxoplasmosis drugs. Curr Opin Invest Drugs 2:1368–1374. [PubMed] [Google Scholar]
- 191.Dannemann B, McCutchan JA, Israelski D, Antoniskis D, Leport C, Luft B, Nussbaum J, Clumeck N, Morlat P, Chiu J, Vilde J-L, Orellana M, Feigel D, Bartok A, Heseltine P, Leedom J, Remington J. 1992. Treatment of toxoplasmic encephalitis in patients with AIDS: a randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. Ann Intern Med 116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 192.Katlama C, De Wit S, O’Doherty E, Van Glabeke M, Clumeck N. 1996. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clin Infect Dis 22:268–275. doi: 10.1093/clinids/22.2.268. [DOI] [PubMed] [Google Scholar]
- 193.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. 2020. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. https://clinicalinfo.hiv.gov/sites/default/files/inline-files/adult_oi.pdf.
- 194.Ben-Harari RR, Goodwin E, Casoy J. 2017. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: a systematic review. Drugs R D 17:523–544. doi: 10.1007/s40268-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Konstantinovic N, Guegan H, Stajner T, Belaz S, Robert-Gangneux F. 2019. Treatment of toxoplasmosis: current options and future perspectives. Food Waterborne Parasitol 15:e00036. doi: 10.1016/j.fawpar.2019.e00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hodgson HA, Sim T, Gonzalez H, Aziz M, Rhee Y, Lewis PO, Jhobalia N, Shields B, Wang SK. 2018. Successful treatment of cerebral toxoplasmosis using pyrimethamine oral solution compounded from inexpensive bulk powder. Open Forum Infect Dis 5:ofy055. doi: 10.1093/ofid/ofy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dunay IR, Gajurel K, Dhakal R, Liesenfeld O, Montoya JG. 2018. Treatment of toxoplasmosis: historical perspective, animal models, and current clinical practice. Clin Microbiol Rev 31:e00057-17. doi: 10.1128/CMR.00057-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. 2009. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 49:878–884. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- 199.Berdoy M, Webster JP, Macdonald DW. 2000. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci 267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Johnson HJ, Koshy AA. 2020. Latent toxoplasmosis effects on rodents and humans: how much is real and how much is media hype? mBio 11:e02164-19. doi: 10.1128/mBio.02164-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Torrey EF, Bartko JJ, Lun ZR, Yolken RH. 2007. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull 33:729–736. doi: 10.1093/schbul/sbl050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Brown AS, Derkits EJ. 2010. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry 167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hamdani N, Daban-Huard C, Lajnef M, Richard JR, Delavest M, Godin O, Le Guen E, Vederine FE, Lépine JP, Jamain S, Houenou J, Le Corvoisier P, Aoki M, Moins-Teisserenc H, Charron D, Krishnamoorthy R, Yolken R, Dickerson F, Tamouza R, Leboyer M. 2013. Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. J Affect Disord 148:444–448. doi: 10.1016/j.jad.2012.11.034. [DOI] [PubMed] [Google Scholar]
- 204.Chorlton SD. 2017. Toxoplasma gondii and schizophrenia: a review of published RCTs. Parasitol Res 116:1793–1799. doi: 10.1007/s00436-017-5478-y. [DOI] [PubMed] [Google Scholar]
- 205.Pearce BD, Kruszon-Moran D, Jones JL. 2014. The association of Toxoplasma gondii infection with neurocognitive deficits in a population-based analysis. Soc Psychiatry Psychiatr Epidemiol 49:1001–1010. doi: 10.1007/s00127-014-0820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Bharti AR, McCutchan A, Deutsch R, Smith DM, Ellis RJ, Cherner M, Woods SP, Heaton RK, Grant I, Letendre SL. 2016. Latent Toxoplasma infection and higher Toxoplasma gondii immunoglobulin G levels are associated with worse neurocognitive functioning in HIV-infected adults. Clin Infect Dis 63:1655–1660. doi: 10.1093/cid/ciw655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kindler J, Lim CK, Weickert CS, Boerrigter D, Galletly C, Liu D, Jacobs KR, Balzan R, Bruggemann J, O’Donnell M, Lenroot R, Guillemin GJ, Weickert TW. 2020. Dysregulation of kynurenine metabolism is related to proinflammatory cytokines, attention, and prefrontal cortex volume in schizophrenia. Mol Psychiatry 25:2860–2872. doi: 10.1038/s41380-019-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Pearce BD, Gandhi ZH, Massa N, Goldsmith DR, Hankus A, Alrohaibani A, Goel N, Cuthbert B, Fargotstein M, Barr DB, Panuwet P, Miller AH, Duncan E. 2019. ACNP 58th Annual Meeting: poster session II. T198. Toxoplasma gondii effects on the relationship of kynurenine pathway metabolites to cognition in schizophrenia versus control subjects. Neuropsychopharmacology 44:230–384. doi: 10.1038/s41386-019-0546-x. [DOI] [Google Scholar]
- 209.Elsheikha HM, Zhu XQ. 2016. Toxoplasma gondii infection and schizophrenia: an inter-kingdom communication perspective. Curr Opin Infect Dis 29:311–318. doi: 10.1097/QCO.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 210.Martin-Iguacel R, Ahlström MG, Touma M, Engsig FN, Stærke NB, Stærkind M, Obel N, Rasmussen LD. 2017. Incidence, presentation and outcome of toxoplasmosis in HIV infected in the combination antiretroviral therapy era. J Infect 75:263–273. doi: 10.1016/j.jinf.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 211.Arendt G, von Giesen HJ, Hefter H, Neuen-Jacob E, Roick H, Jablonowski H. 1999. Long-term course and outcome in AIDS patients with cerebral toxoplasmosis. Acta Neurol Scand 100:178–184. doi: 10.1111/j.1600-0404.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 212.Levine AJ, Hinkin CH, Ando K, Santangelo G, Martinez M, Valdes-Sueiras M, Saxton EH, Mathisen G, Commins DL, Moe A, Farthing C, Singer EJ. 2008. An exploratory study of long-term neurocognitive outcomes following recovery from opportunistic brain infections in HIV+ adults. J Clin Exp Neuropsychol 30:836–843. doi: 10.1080/13803390701819036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Derouin F, Chastang C. 1989. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob Agents Chemother 33:1753–1759. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.McFadden DC, Tomavo S, Berry EA, Boothroyd JC. 2000. Characterization of cytochrome b from Toxoplasma gondii and Qo domain mutations as a mechanism of atovaquone-resistance. Mol Biochem Parasitol 108:1–12. doi: 10.1016/S0166-6851(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 215.Camps M, Arrizabalaga G, Boothroyd J. 2002. An rRNA mutation identifies the apicoplast as the target for clindamycin in Toxoplasma gondii. Mol Microbiol 43:1309–1318. doi: 10.1046/j.1365-2958.2002.02825.x. [DOI] [PubMed] [Google Scholar]
- 216.Meneceur P, Bouldouyre MA, Aubert D, Villena I, Menotti J, Sauvage V, Garin JF, Derouin F. 2008. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother 52:1269–1277. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Shubar HM, Lachenmaier S, Heimesaat MM, Lohman U, Mauludin R, Mueller RH, Fitzner R, Borner K, Liesenfeld O. 2011. SDS-coated atovaquone nanosuspensions show improved therapeutic efficacy against experimental acquired and reactivated toxoplasmosis by improving passage of gastrointestinal and blood-brain barriers. J Drug Target 19:114–124. doi: 10.3109/10611861003733995. [DOI] [PubMed] [Google Scholar]
- 218.Dittmar AJ, Drozda AA, Blader IJ. 2016. Drug repurposing screening identifies novel compounds that effectively inhibit Toxoplasma gondii growth. mSphere 1:e00042-15. doi: 10.1128/mSphere.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Boyom FF, Fokou PV, Tchokouaha LR, Spangenberg T, Mfopa AN, Kouipou RM, Mbouna CJ, Donfack VF, Zollo PH. 2014. Repurposing the open access malaria box to discover potent inhibitors of Toxoplasma gondii and Entamoeba histolytica. Antimicrob Agents Chemother 58:5848–5854. doi: 10.1128/AAC.02541-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Spalenka J, Escotte-Binet S, Bakiri A, Hubert J, Renault JH, Velard F, Duchateau S, Aubert D, Huguenin A, Villena I. 2017. Discovery of new inhibitors of Toxoplasma gondii via the pathogen box. Antimicrob Agents Chemother 62:e01640-17. doi: 10.1128/AAC.01640-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Adeyemi OS, Sugi T, Han Y, Kato K. 2018. Screening of chemical compound libraries identified new anti-Toxoplasma gondii agents. Parasitol Res 117:355–363. doi: 10.1007/s00436-017-5698-1. [DOI] [PubMed] [Google Scholar]
- 222.Doggett JS, Nilsen A, Forquer I, Wegmann KW, Jones-Brando L, Yolken RH, Bordón C, Charman SA, Katneni K, Schultz T, Burrows JN, Hinrichs DJ, Meunier B, Carruthers VB, Riscoe MK. 2012. Endochin-like quinolones are highly efficacious against acute and latent experimental toxoplasmosis. Proc Natl Acad Sci U S A 109:15936–15941. doi: 10.1073/pnas.1208069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Eissa MM, Barakat AM, Amer EI, Younis LK. 2015. Could miltefosine be used as a therapy for toxoplasmosis? Exp Parasitol 157:12–22. doi: 10.1016/j.exppara.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 224.Dorlo TP, Balasegaram M, Beijnen JH, de Vries PJ. 2012. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J Antimicrob Chemother 67:2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- 225.Benmerzouga I, Checkley LA, Ferdig MT, Arrizabalaga G, Wek RC, Sullivan WJ Jr. 2015. Guanabenz repurposed as an antiparasitic with activity against acute and latent toxoplasmosis. Antimicrob Agents Chemother 59:6939–6945. doi: 10.1128/AAC.01683-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Maubon D, Bougdour A, Wong YS, Brenier-Pinchart MP, Curt A, Hakimi MA, Pelloux H. 2010. Activity of the histone deacetylase inhibitor FR235222 on Toxoplasma gondii: inhibition of stage conversion of the parasite cyst form and study of new derivative compounds. Antimicrob Agents Chemother 54:4843–4850. doi: 10.1128/AAC.00462-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Murata Y, Sugi T, Weiss LM, Kato K. 2017. Identification of compounds that suppress Toxoplasma gondii tachyzoites and bradyzoites. PLoS One 12:e0178203. doi: 10.1371/journal.pone.0178203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Watts E, Zhao Y, Dhara A, Eller B, Patwardhan A, Sinai AP. 2015. Novel approaches reveal that Toxoplasma gondii bradyzoites within tissue cysts are dynamic and replicating entities in vivo. mBio 6:e01155–15–e01115. doi: 10.1128/mBio.01155-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang JL, Zhang NZ, Li TT, He JJ, Elsheikha HM, Zhu XQ. 2019. Advances in the development of anti-Toxoplasma gondii vaccines: challenges, opportunities, and perspectives. Trends Parasitol 35:239–253. doi: 10.1016/j.pt.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 230.Sidik SM, Hackett CG, Tran F, Westwood NJ, Lourido S. 2014. Efficient genome engineering of Toxoplasma gondii using CRISPR/Cas9. PLoS One 9:e100450. doi: 10.1371/journal.pone.0100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Liang QL, Sun LX, Elsheikha HM, Cao XZ, Nie LB, Li TT, Li TS, Zhu XQ, Wang JL. 2020. RHΔgra17 Δnpt1 strain of Toxoplasma gondii elicits protective immunity against acute, chronic and congenital toxoplasmosis in mice. Microorganisms 8:352. doi: 10.3390/microorganisms8030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang JL, Liang QL, Li TT, He JJ, Bai MJ, Cao XZ, Elsheikha HM, Zhu XQ. 2020. Toxoplasma gondii tkl1 deletion mutant is a promising vaccine against acute, chronic, and congenital toxoplasmosis in mice. J Immunol 204:1562–1570. doi: 10.4049/jimmunol.1900410. [DOI] [PubMed] [Google Scholar]
- 233.Innes EA, Hamilton C, Garcia JL, Chryssafidis A, Smith D. 2019. A One Health approach to vaccines against Toxoplasma gondii. Food Waterborne Parasitol 15:e00053. doi: 10.1016/j.fawpar.2019.e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lau YL, Lee WC, Gudimella R, Zhang G, Ching XT, Razali R, Aziz F, Anwar A, Fong MY. 2016. Deciphering the draft genome of Toxoplasma gondii RH strain. PLoS One 11:e0157901. doi: 10.1371/journal.pone.0157901. [DOI] [PMC free article] [PubMed] [Google Scholar]