Abstract
Unlike widely perceived, resveratrol (RSV) decreased the average lifespan and extended only the replicative lifespan in yeast. Similarly, although not widely discussed, RSV is also known to evoke neurite degeneration, kidney toxicity, atherosclerosis, premature senescence, and genotoxicity through yet unknown mechanisms. Nevertheless, in vivo animal models of diseases and human clinical trials demonstrate inconsistent protective and beneficial effects. Therefore, the mechanism of action of RSV that elicits beneficial effects remains an enigma. In a previously published work, we demonstrated structural similarities between RSV and tyrosine amino acid. RSV acts as a tyrosine antagonist and competes with it to bind to human tyrosyl-tRNA synthetase (TyrRS). Interestingly, although both isomers of RSV bind to TyrRS, only the cis-isomer evokes a unique structural change at the active site to promote its interaction with poly-ADP-ribose polymerase 1 (PARP1), a major determinant of cellular NAD+-dependent stress response. However, retention of trans-RSV in the active site of TyrRS mimics its tyrosine-bound conformation that inhibits the auto-poly-ADP-ribos(PAR)ylation of PARP1. Therefore, we proposed that cis-RSV-induced TyrRS-regulated auto-PARylation of PARP1 would contribute, at least in part, to the reported health benefits of RSV through the induction of protective stress response. This observation suggested that trans-RSV would inhibit TyrRS/PARP1-mediated protective stress response and would instead elicit an opposite effect compared to cis-RSV. Interestingly, most recent studies also confirmed the conversion of trans-RSV and its metabolites to cis-RSV in the physiological context. Therefore, the finding that cis-RSV and trans-RSV induce two distinct conformations of TyrRS with opposite effects on the auto-PARylation of PARP1 provides a potential molecular basis for the observed dichotomic effects of RSV under different experimental paradigms. However, the fact that natural RSV exists as a diastereomeric mixture of its cis and trans isomers and cis-RSV is also a physiologically relevant isoform has not yet gained much scientific attention.
Keywords: Resveratrol (RSV), Aminoacyl-tRNA synthetases (aaRSs), Tyrosyl-tRNA synthetase (YARS, TyrRS), Nicotinamide adenine nucleotide (NAD+), Poly-ADP-ribose polymerase (PARP), Sirtuins (SIRT), AMP-activated protein kinase (AMPK), Nicotinamide (NAM)
Introduction
Natural resveratrol (RSV or 3,5,4′-trihydroxystilbene) is an important constituent of the ayurvedic medicine “Drakshasava,” a well-known Indian herbal preparation from grapes prescribed as a cardiotonic [1]. (Draksha is the Sanskrit word for grape. “Asava” means “distillate,” “juice,” or “extract.” Thus, “Drakshasava” means “extract from grapes.”) Similarly, RSV is also considered responsible for the fundamental principle behind the “French Paradox” [2, 3]. It is produced by many different plant species, especially grapevines, pines, and legumes and therefore is abundant in peanuts, soybeans, blueberries, and pomegranates [4]. RSV is produced in plants as a protective agent in response to stressful conditions such as injury or attack by bacterial/fungal pathogens or UV exposure and believed to activate the innate defense mechanism of plants against fungal/microbial pathogens [5]. Interestingly, Botrytis cinerea infection in grapes leads to the exclusive synthesis of RSV in the leaf epidermis and grape skins [5–7]. Since grape skins are fermented during red wine production, they contain higher amounts of RSV than white wines. RSV was first isolated in 1939 by Takaoka from Veratrum grandiflorum Loes (the root of the white hellebore) [8]. Hence, it is speculated that the name “resveratrol”: was created based on its chemical structure and the source of the plant used for its isolation (a resorcinol derivative or polyphenol in resin from a Veratrum species). Despite being a protective molecule in plants, the ingestion of RSV could also evoke similar protective stress responses in animals and was widely expected to overcome stressful/harmful conditions [9], including auto-immune disorders [10]. Unfortunately, despite being one of the widely studied small molecules, the in vivo animal models of diseases and human clinical studies using RSV brought out inconclusive results [11, 12], indicating our understanding of the mechanism of action of natural RSV is not yet complete.
The enigma of the dichotomic and pro-aging effects of RSV
Natural RSV captured widespread scientific and public interest due to its reported anti-cancer [13] and anti-aging effects [14], supported by further longevity demonstrations in other organisms [15–18]. However, unlike widely perceived, RSV decreased the normal (chronological) lifespan in yeast [19–21], and it extended only the replicative lifespan which is calculated based on the number of daughter cells an individual yeast mother cell produces before dying [14]. Moreover, the replicative lifespan effect of RSV in yeast was immediately questioned as it was not reproducible [22] indicating that the mechanism of action of the anti- and pro-aging effects of RSV is not yet understood. Similarly, higher doses of RSV decreased the normal lifespan in mice as well (mice died within 3–4 months) [23] and resulted in kidney toxicity in rats [24]. Consistent with the pro-aging effects of RSV [19–21, 23], it is known to evoke toxic effects such as induction of neurite degeneration [25], atherosclerosis [26], premature senescence [27–32], genotoxicity [33–38], and inhibition of hippocampal neurogenesis [39]. These observations suggest that the mechanism of action of RSV that promoted longevity in other organisms [15–18] is not yet completely understood. Consistently, research works using RSV showed dichotomic effects resulting in inconsistent therapeutic outcomes. For example, while RSV shows protective effects against experimental models of multiple sclerosis (MS) and autoimmune encephalomyelitis (EAE) in some studies [40–42], it exacerbated the progression of MS and EAE in another study [43]. Similarly, RSV protects against peripheral neuropathy in some studies [44, 45] while it exacerbates the disease condition in another study [46]. Likewise, while RSV is known to protect against anxiety and depression [47], it is also shown to exacerbate anxiety and depression [48]. Interestingly, acute RSV treatment enhanced cocaine-induced dopamine neurotransmission and behavioral responses suggesting that RSV promotes drug addiction [49, 50]. However, RSV is also shown to protect against drug addiction through an unknown mechanism [51, 52]. Consistent with the dichotomic effects discussed above, RSV protects against Wallerian degeneration [53], but it also abolishes neuroprotection mediated through Wallerian-slow degeneration mutants [25]. Similarly, RSV acts as an antagonist on aryl hydrocarbon receptor (AhR) [54] and estrogen receptor alpha (ERα) [55], but it also acts as an agonist of AhR [40] and ERα [56, 57]. The dichotomic effect of RSV is also observed in the induction of autophagy. While RSV stimulates autophagy [17, 27, 58], it is also known to inhibit autophagy [59, 60]. Interestingly, although RSV inhibits nuclear factor kappa B (NF-κB) in some studies [61, 62], RSV instead activates NF-κB in other studies [63–66]. Moreover, although RSV exacerbates atherosclerosis [26], it is also shown to prevent atherosclerosis [67, 68]. Consistently, RSV also exacerbated inflammation state and superoxide production and diminishing aortic distensibility in aged mice [69]. Likewise, RSV protects against oxidative stress in some studies [70–72] while it exacerbates oxidative stress in other studies [73, 74]. Consistently, RSV exhibits both pro-oxidant and antioxidant effects [75, 76]. Interestingly, the pro-oxidant activity of RSV inhibits hydrogen peroxide (H2O2)-induced apoptosis [77] and evokes cardiac protection [78] and anti-tumor effects [38]. Although RSV induces premature senescence [27–32], RSV is also known to inhibit senescence through an unknown mechanism [79]. RSV is widely believed to enhance mitochondrial function [80], despite being a potent inhibitor of mitochondria [81–85]. RSV inhibits oxidative phosphorylation (OXPHOS) activity at two sites: mitochondrial complex I and complex III of the electron transport chain [82, 83]. Consistently, RSV increases the mitochondrial H2O2 production [84] and decreases ATP production [83, 85]. Therefore, RSV induces mitochondria-driven apoptosis [86] through the elevation in intracellular Ca2+, resulting in the collapse of the membrane potential with mitochondrial permeability transition pore (mPTP) opening and cytochrome c release into the cytosol [87]. Although RSV is known to evoke anti-tumor effects [13, 88–91], it is also known to exacerbate cancer proliferation [56, 92, 93] and shows dichotomic effects in angiogenic response as well [94, 95]. Similarly, despite being reported to evoke anti-obesity effects [96–98], intriguingly, higher doses of RSV rather had a weight-promoting effect when mice were fed with a high-fat diet [89]. Although RSV is shown to evoke anti-diabetic effects [98] and restore insulin sensitivity [99] through inhibition of gluconeogenesis [100], and by facilitating cellular glucose uptake [101–103] through GLUT4 translocation [102, 104, 105], enigmatically, RSV is also known to inhibit cellular glucose uptake [106–108], potentially through the inhibition of class I phosphoinositide 3-kinase (PI3K) [109] and glucose transporter 1 (GLUT1) [110] or through the downregulation of GLUT4 translocation [108, 111]. Furthermore, RSV inhibits coronaviral replication [112, 113] but facilitates herpes simplex virus (HSV) [114], hepatitis B virus (HBV) [115], and hepatitis C virus [116] replication. Therefore, studies spanning over the last two decades show that treatment with RSV can evoke dichotomic effects resulting in either unfavorable or beneficial outcomes.
RSV elicits favorable and unexpected outcomes in human clinical trials
Consistent with the dichotomic effects of RSV, as discussed above, human clinical trials using RSV also ended up in favorable and unexpected outcomes [11, 12]. For example, despite numerous animal models showing neuroprotective effects [80, 98, 117–120], intriguingly, a previous human Alzheimer’s disease (AD) clinical trial using high-dose RSV (2000 mg/day) resulted in the upregulation of amyloid-beta (Aβ) levels with exacerbation of the brain volume loss [121]. However, another recent human AD clinical trial using low-dose RSV (5 mg/day) showed beneficial effects [122] and human clinical trial using low-dose RSV (75 mg twice daily) enhanced cognitive benefits in postmenopausal women [123, 124]. Consistently, RSV improved memory performance and increased the functional connectivity of the hippocampus in older human adults [125], but worsened episodic memory in a human clinical trial for schizophrenia (SZ) [126]. Although extensive data show RSV protects against nephropathy [70, 127, 128], renal oxidative DNA damage [129], and kidney diseases [130], a phase 2 clinical trial of RSV targeting multiple myeloma was terminated due to patients developing kidney failure [131]. Similarly, despite known to improve several cardiovascular health parameters [98] and suggested to evoke an anabolic function in exercise-induced adaptations of older persons to reverse sarcopenia [132], RSV supplementation in humans also resulted in the reduction of high-density lipoprotein (HDL) cholesterol concentrations [133] and blunted exercise-mediated effects [134, 135] and impaired the improvements in markers of oxidative stress and inflammation in skeletal muscle [135]. Therefore, for unknown reasons, studies in humans have shown that RSV may reduce training-induced adaptations [95, 134, 135] and lead to bicytopenia in patients treated for non-alcoholic fatty liver disease (NAFLD) [136] and caused an elevation of biomarkers of cardiovascular disease (CVD) risk in overweight older adults [137]. Although some studies did not show any improvement in the glucose intolerance in older adults [138] and type 2 diabetic patients [139, 140], however, RSV has a positive impact on blood pressure [141, 142], improves insulin sensitivity [143], and reduces blood glucose levels [144] in type 2 diabetic patients and patients with non-alcoholic fatty liver disease [145], in the treatment of pre-eclampsia [146] and obese people [97]. Consistently, most recent meta analysis also indicates that RSV improves cardiometabolic health by decreasing some risk factors (HOMA-IR, LDL-C, and T-Chol) associated with cardiovascular disease (CVD) [147, 148] and significantly reduced total cholesterol and increased gamma-glutamyl transferase (GGT) concentrations in patients with metabolic syndrome (MetS) and related disorders [149]. In conclusion, along with substantial protective/beneficial effects of low-dose RSV demonstrated in various studies, exacerbation of the disease conditions especially by higher doses of RSV is also an accompanying theme in human clinical trials. However, the molecular basis for these dichotomic effects of RSV largely remains unexplained, and these opposing effects observed upon RSV treatment remain an enigma [150].
Low-dose beneficial effects versus high-dose detrimental effects of RSV
Beyond the unexpected outcomes in multiple models of diseases, it is apparent that low doses of RSV evoke health-promoting effects and detrimental effects are generally observed only upon treatment with high doses of RSV as observed in the cases of human AD clinical trails [121, 122]. Therefore, RSV often displays a biphasic dose-response [151] with features consistent with the hormetic dose-response, a phenomenon that is characterized by low-dose stimulation and a high-dose inhibition [152]. RSV inhibits DNA type II topoisomerase [153], and histone deacetylases (HDACs) [154], enzymes that are critical for the maintenance of chromosomal stability [155], and neuronal survival [156, 157], resulting in genotoxic effects. Consistently, treatment with high-dose RSV induces DNA double-strand breaks (DSBs) [153, 158, 159] along with downregulation of DNA repair proteins [160], while low-dose RSV rather upregulates DNA repair proteins [161, 162]. Furthermore, a lower dose of RSV significantly reduced chromosomal instability (CIN) as well as defective spindle assembly checkpoint (SAC), while higher doses of RSV significantly induced them [36]. Therefore, RSV reduces cell growth and induces apoptosis in healthy cells in a dose-dependent manner triggering biphasic effects over low to high concentrations [163, 164]. For example, low-dose RSV enhances self-renewal of stem cells through inhibition of senescence whereas higher dose RSV instead inhibits self-renewal and induces senescence [165–168] through cell cycle arrest at S/G2 phase [36, 169, 170]. Hence, at a lower concentration, RSV can have a positive impact on the proliferation, survival of neuronal progenitor cells (NPCs), and rat hippocampal neurogenesis [167], which is also consistent with its angiogenic effects at a lower dose [94]. Consistently, low-dose RSV protected against amyotrophic lateral sclerosis (ALS) [171] and Huntington’s disease (HD) [172], but a higher dose of RSV rather failed to improve motor deficits associated with the HD phenotype in a transgenic mouse model [173]. Intriguingly, pre-treatment with low-dose RSV, however, failed to reduce amyloid beta-mediated elevation of H2O2 production [174]. Although RSV was identified as an anti-inflammatory compound [175, 176], RSV modulates the inflammatory response in an ERα-dependent manner [57]. At medium concentrations (10–25 μM), RSV acts as a superagonist of estradiol [177], while at lower concentrations, RSV relatively inhibited the estradiol-driven transcription through a yet unknown mechanism [177]. Similarly, RSV binds directly to mitochondrial complex I and induces oxidative stress in aged mice in a dose-dependent manner [73]. At low doses, RSV stimulated complex I and F0/F1 ATPase activities, whereas, at high doses, it inhibited them [73, 178]. Therefore, low concentrations of RSV trigger and high concentrations inhibit respiratory chain complexes [179]. Similarly, low doses of RSV promoted in vitro muscle regeneration and attenuated the impact of reactive oxygen species (ROS), while high doses exacerbated the reduction in plasticity and metabolism induced by oxidative stress [180]. Intriguingly, lower doses of RSV generate ROS, and higher doses of RSV act as an anti-oxidant in erythrocytes [181] and pro-oxidant properties of low-dose RSV inhibit caspase activation and DNA fragmentation induced by oxidative stress [182]. However, in indomethacin-induced gastric ulcers, high-dose RSV instead exacerbated ulcerative damage in mice [183]. Similarly, a low-dose RSV administration partly improved renal function in mice with kidney damage caused by a unilateral ureteral obstruction (UUO) while high dose of RSV lost its anti-fibrotic effect and exacerbated kidney fibrosis [184]. Likewise, RSV induced a dose-dependent pro-oxidant effect in hepatic stellate cells (HSC) with the highest dose of RSV inducing oxidation-related damage and drastically reducing cell viability [185, 186]. Similarly, high-dose RSV upregulated genes involved in gluconeogenesis [100] and inhibited insulin signaling [108, 187, 188], but low-dose RSV rather downregulated gluconeogenesis [100] and improved insulin sensitivity [99, 103, 143, 145, 189, 190].
RSV attains high micromolar levels in human tissues and plasma after oral ingestion
Although approximately 75% of RSV is absorbed after oral consumption [191], it is rapidly metabolized by the liver, intestinal tract, and gut microbiota into the sulfated and glucuronidated forms such as RSV-3’-O-β-d-glucuronide (RSV3G), RSV-4′-O-d-glucuronide (RSV4G), and RSV-3-O-sulfate (RSV3S). Interestingly, RSV is more stable in human plasma compared to rat plasma [192], and RSV crosses the blood-brain barrier (BBB) and accumulates in the brain tissue as well [117]. Therefore, the concentrations of these RSV metabolites far exceed the concentrations of their parent compound (free RSV) in human serum [27, 193] and ocular tissues [194]. For example, human oral ingestion of low dose (5 mg) RSV daily can achieve the plasma concentrations of 0.12–0.6 μM RSV [89], and human clinical trials receiving 5–1000 mg daily dose resulted in average peak concentrations of blood levels between 0.6 and 137 μM [89, 194]. Furthermore, sustained intake of 1 g of RSV as a food supplement resulted in a concentration of 50–640 μM RSV in human colonic tissues [27]. Moreover, sulfate metabolites of RSV contribute to the in vivo activity of these metabolites by regenerating free RSV in colorectal cell lines, supporting the hypothesis that RSV metabolites potentially serve as a reservoir for the parent compound [27, 195]. These paradigm shifting studies have not only challenged the classical notion that low bioavailability of RSV is the reason for the lack of therapeutic effects in human clinical trials but have also provided a potential molecular basis for the unexpected effects of high-dose RSV in human clinical trials such as adverse gastrointestinal effects [196, 197]. The recent results from clinical trials and in vivo studies also support the hormetic response of RSV with lower doses retarding age-related cardiac dysfunction [198], preventing cancer [89], slowing down AD symptoms [122], and improving cardiovascular and cerebrovascular functions [23, 196] more potently than higher doses [89].
trans-RSV is a direct activator of SIRT1 at higher micromolar concentrations
Although there are many known targets for trans-RSV [199], the most well-studied target is SIRT1 [14]. Later studies also indicated a direct link between trans-RSV and 5′ adenosine monophosphate-activated protein kinase (AMPK) and SIRT1 by showing RSV’s inhibitory effect on several phosphodiesterases (PDE) that increased cyclic AMP (cAMP) levels to enhance the intracellular Ca2+ to activate Ca2+/calmodulin-dependent protein kinase kinase β (CaMKKβ), which phosphorylates AMPK, finally leading to SIRT1 activation [200, 201]. Although, the attempts to modulate the activity and specificity of RSV through different targets or signaling cascades failed to reproduce the complete spectrum of its activity, [202, 203] highlighting some technical problems associated with the “Fluor-de-Lys” substrate (FdL) assay used to determine RSV-mediated SIRT1 activation [20, 22, 204, 205], subsequent works finally concluded that trans-RSV is indeed a direct activator of SIRT1 [206–208] with a potency of RSV against SIRT1 in the FdL assay (EC50 ~ 30–100 μM) [14, 207] whereas the Km value of SIRT1 for NAD+ was found to be 94 ± 5 μM [203]. Finally, patients who received 500 mg/day RSV demonstrated the activation of SIRT1 in peripheral blood mononuclear cell (PBMC), suggesting that oral administration of higher doses of RSV achieve sufficient cellular concentrations to activate SIRT1 (≥ 30 μM) in humans as well [209]. This finding is also consistent with other human clinical trials that used 5–1000 mg/day RSV resulted in average peak concentrations of blood levels between 0.6 and 137 μM [89, 194].
RSV is also an indirect inhibitor of SIRT1
Intriguingly, in mammalian cells, RSV is also known to inhibit SIRT1 [14], and this inhibitory effect of RSV on SIRT1 is required for its longevity effects in Caenorhabditis elegans [15]. Moreover, RSV decreases yeat chronological lifespan in a Sir2-dependent manner [19]. This apparent contradictory observation was termed as the “dichotomy” of RSV’s action, and the mechanism remained unknown. Furthermore, later studies also demonstrated that RSV inhibits SIRT1 to mediate part of its biochemical and functional outcomes. For example, while SIRT1 activates vascular endothelial growth factor (VEGF) expression [210, 211], treatment with RSV rather downregulates it [212, 213]. Likewise, activation of SIRT1 inhibits muscle differentiation and mitochondrial biogenesis [214–217], whereas RSV rather potentiates muscle differentiation [218, 219] and acts as an exercise mimetic [220]. Activation of SIRT1 sensitizes neurons to oxidative stress [221, 222] and inhibits neurogenesis [223] while RSV protects neurons against oxidative stress [224, 225] and enhances neurogenesis [226, 227]. Similarly, activation of SIRT1 promotes mitochondrial fission [228], and treatment with RSV rather enhances mitochondrial fusion [229]. Similarly, although brain-specific activation of SIRT1 drives anxiety and exploratory drive [48, 230], RSV rather protects against autistic features [231–234]. Interestingly, SIRT1 activates monoamine oxidase A (MAO-A) in the brain [230], but RSV inhibits it [235, 236]. Furthermore, RSV is known to exert metabolic benefits by increasing metabolic rate, insulin sensitivity, mitochondrial biogenesis, and physical endurance, and reduce fat accumulation in mice [80, 98, 201]. Although lower doses of RSV (≥ 25 μM) fail to inhibit PDEs [207], RSV is also shown to upregulate the cellular levels of cyclic AMP (cAMP) though phosphodiesterases (PDEs) [201]. However, increasing cAMP levels via the Epac pathway retards the clearance of autophagy substrates and inhibits α-synuclein clearance, and enhances polyglutamine aggregation in the Parkinson’s disease (PD) mouse model [237]. Intriguingly, RSV rescues mutant polyglutamine cytotoxicity [118] and alleviates motor and cognitive deficits in the A53T α-synuclein mouse model of PD [238], whereas SIRTuin inhibition also rescues polyglutamine cytotoxicity [239], and PD [240] and motor deficits after peripheral nerve injury [241]. Similarly, SIRT1 inhibits p53 [242], a known anti-tumor protein, and RSV is known to exert anti-cancer effects through p53 activation [13, 243]. Although SIRT1 is essential for coronavirus replication and survival [113], RSV instead inhibits coronavirus replication [112, 113], and the acetylation of p53 is a critical mediator of antiviral response [244]. Interestingly, RSV treatment is known to activate p300 acetyltransferase to protect against rat spinal cord affected by sciatic nerve injury [245] and is also known to activate AMPK independent of SIRT1 in neurons [246] through yet unknown mechanisms. However, RSV activates AMPK in a poly-ADP-ribose polymerase 1 (PARP1)-dependent manner [71], which is also a potent inhibitor of SIRT1 [247]. Although the mechanism of RSV-mediated inhibition of SIRT1 [14, 15] and activations of p53 in human PBMC [209] and AMPK in neurons [246] remain an enigma, the observations mentioned above suggest that the inhibitory effects of RSV on SIRT1 [14, 15] through PARP1 activation [71] may also contribute to the observed physiological functions of RSV.
cis-RSV is present in the commercial wines
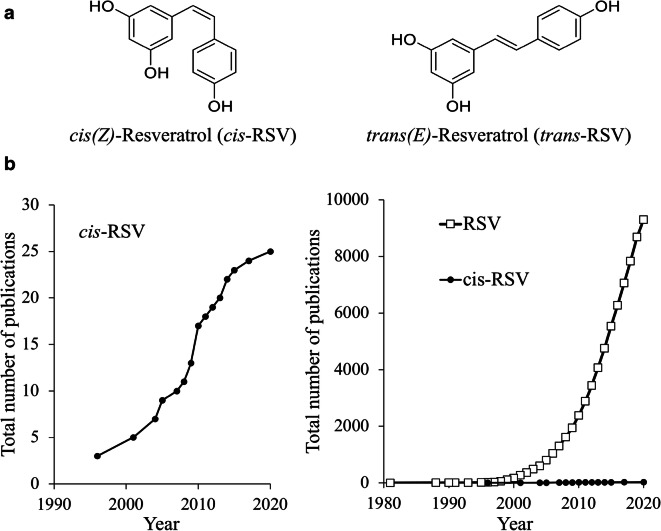
RSV exists as a mixture of its diastereomeric cis (Z) and trans (E) isomers in wines [248–250] (Fig. 1a), and both isomers are stable when protected from light for at least 6 weeks at 4 °C and are not prone to oxidation within at least 48 h of exposure to air [248]. Although cis-RSV has not been detected in fresh grapes [5–7], it is nevertheless a significant component of commercial wines from every wine-producing region of the world as well [248, 250]. This observation suggests that cis-RSV is produced during fermentation through an unknown mechanism. Wines that are high in trans-RSV tend to be also high in cis-RSV, and their concentrations may be subject to the same variables such as cultivar, climate, soil composition, and drainage characteristics, fungal pressure, and wine-making techniques [248]. In general, the absorbance of cis-RSV is lower than that of trans-RSV [250], and the accurate quantitation of cis forms showed that the concentration of cis-RSV could sometimes be the predominant form in specific grape varietals such as Pinot noir [248, 250].
Fig. 1.
a Natural resveratrol (RSV or 3,5,4′-trihydroxystilbene) exists as two diastereomeric forms (cis (Z)- and trans(E)-RSV). b Number of Pubmed publications on RSV. Graphical representation of the total number of publications obtained by searching in Pubmed using the term “resveratrol” and “cis-resveratrol” in the titles of the publications as of 15th September, 2020
cis-RSV exerts anti-inflammatory and anti-oxidant effects
Although cis-RSV is not well-explored, the limited amount of scientific literature shed light on its biological effects. cis-RSV inhibits both canonical and non-canonical inflammasome activation in macrophages resulting in the downregulation of caspases 1 and 4 and reduction in the secretion of the pro-inflammatory cytokine, interleukin-1β (IL-1β) [251]. Interestingly, the reduction of IL-1β secretion was more pronounced with cis-RSV pre-treatment than trans-RSV [251]. cis-RSV also scavenges intracellular reactive oxygen species (ROS) and downregulates mRNA and protein levels of NOS-2 and COX-2, resulting in the attenuation of the pro-inflammatory responses [252]. cis-RSV modulates the pro-inflammatory transcription factor, NF-κB, reducing the expression of chemokines such as monocyte chemoattractant protein-1 (MCP-1) and regulated on activation normal T cell expressed and secreted (RANTES), pro-inflammatory cytokines that attract monocyte–granulocyte cells such as M-CSF (colony-stimulating factor 1), GM-CSF (colony-stimulating factor 2) and G-CSF (colony-stimulating factor 3), the cytokine tumor growth factor-beta (TGF-β), and the extracellular ligand IL-1α [253]. The methylated forms of both isomers have anti-tumorogenic properties, but the cis-RSV-derived methylated forms have a higher anti-tumor effect than the trans derivatives [254]. In contrast, for the unmodified isomers, trans-RSV has greater anti-cancer activity [255]. RSV has vasorelaxant properties, and in this aspect, both isomers showed similar effects on the reduction of intracellular calcium levels when pre-treated in vascular myocytes, although co-treatment with angiotensin II, cis-RSV showed more potent effects [256, 257]. However, when tested in mice model against angiotensin II (AngII)-mediated vascular inflammation, only the trans-isomer was found to be effective, although it is not clear whether such a result is caused by a difference in potency or is due to different mechanisms [258]. Interestingly, both cis-RSV and trans-RSV suppress the platelet aggregation induced by pro-aggregatory stimuli such as collagen, ADP, and thrombin [259, 260]. Although one study showed the potency of the cis-RSV was lower than that of the trans-RSV [260], an earlier study showed that cis-RSV is more potent than trans-RSV to inhibity the platelet aggregation [259]. Despite early indications of the physiological effects, cis-RSV did not gain much scientific attention in terms of the number of publications (Fig. 1b) majorly due to the lack of commercial availability of cis-RSV in early days [261].
cis-RSV is metabolized faster than its trans counterparts in humans
Although glucuronidation of RSV is done preferentially by different UDP-glucuronosyltransferase (UGT) isoforms resulting in the formation of two glucuronides (RSV 3-O- and 4*-O-glucuronides (RSV3G and RSV4G)), interestingly, this enzymatic action can be selective with different reaction rates and occurs at a faster rate with the cis-RSV [262, 263]. For example, trans-RSV is glucoronidated by bilirubin conjugating UGT1A1 and cis-RSV is glucoronidated by the phenol conjugating UGT1A6 [262, 264]. However, both cis- and trans-RSV isomers are actively glucuronidated by enzymes like UGT1A9 and 1A10 [262, 264] and the biological significance of faster glucuronidation of cis-RSV remains to be evaluated. Moreover, the gastrointestinal (GI) tract contributes significantly to the first pass metabolism of these naturally occurring polyphenols [263] and cis-RSV sulfates and glucuronides (cis-RSV3G, cis-RSV4G, and cis-RSV3S) are found in higher concentrations than their trans counterparts in urine of subjects given a 250-ml single dose of red wine [265].
trans-RSV and its metabolites convert to cis-RSV in the physiological context
Interestingly, trans-RSV is known to be converted to cis-RSV in the physiological context [78, 195], and both trans- and cis-RSV metabolites with a preference for cis-isomer are detected in the profiles of culture media and lysates of cells exposed to trans-RSV [195]. Importantly, in endothelial cells, when the RSV metabolites (RSV3G, RSV4G, and RSV3S) were converted back to their parent form, i.e., free RSV, they were preferentially converted to cis-RSV through a yet unknown mechanism [195]. Similarly, another recent study also showed that trans-RSV is converted to cis-RSV in the physiological context to evoke cardioprotective functions [78]. This trans to cis-RSV conversion exploits a novel thiol-dependent mechanism to activate protein kinase 1α (PKG1α) that mediates the beneficial actions of RSV. However, the thiol oxidation-mediated activity of RSV is restricted to the pro-oxidative environment of tissues [78]. In light of the new findings that trans-RSV converts to cis-RSV and RSV reaches high micromolar concentrations in target tissues [27, 89, 194], and RSV metabolites potentially serve as a reservoir for the parent compound [27, 195] and cis-RSV is glucuronidated faster than trans-RSV [262–264, 266] and cis-RSV sulfates and glucuronides are found in higher concentrations than their trans counterparts in humans [265], it is critical to explore whether a combination of cis- and trans-RSV would elicit synergistic or antagonistic effects in future studies.
“Moonlighting” functions of aminoacyl-tRNA synthetases (aaRSs)
Aminoacyl-tRNA synthetases (aaRSs) are ancient proteins that activate L-amino acids for protein synthesis (translational function) [267]. However, during evolution, aaRSs also progressively accrued “moonlighting” functions activated under conditions of diminished protein synthesis, such as cellular stress, which is well reviewed in many recent publications [268]. These functions beyond protein synthesis (non-translational “moonlighting” functions) enable aaRSs to play critical roles in various cellular functions, including metabolic homeostasis and modulation of signal transduction pathways [268]. However, the molecular mechanisms by which aaRSs “switch ON” their “moonlighting” functions and “switch OFF” their role in protein synthesis are not very well understood. Intriguingly, knocking down aaRSs enhances longevity in C. elegans [269] but decreases it in Drosophila [270], indicating that the non-canonical functions of aaRSs are probably significant only in higher eukaryotic organisms [271]. Each aaRS has a unique active site that precisely differentiates closely related amino acids. Interestingly, the depletion of tryptophan from the active site of tryptophanyl-tRNA synthetase (TrpRS) activates its “moonlighting” nuclear function poly-ADP-ribose-polymerase 1 (PARP1)-dependent activation of p53 [272]. Therefore, potential binding of natural amino acid analogs (metabolites, neurotransmitters, and bioactive compounds) to the active sites of aaRSs could “switch ON” their “moonlighting” functions by temporarily transforming them to catalytic nulls [273].
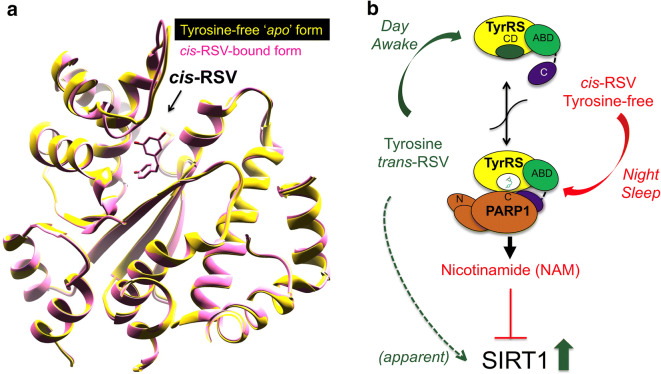
cis-RSV evokes a tyrosine-free (apo) conformation in human tyrosyl-tRNA synthetase (TyrRS)
We astutely observed structural similarities between RSV and tyrosine (RSV harbors a tyrosine-like phenolic ring), and interestingly, human serum levels of L-tyrosine remain elevated under conditions that drive various metabolic dysfunctions [274–282], including cancer [283, 284]. Therefore, we tested if RSV would behave as an “active site-directed inhibitor” of TyrRS and consistently found that RSV is a direct inhibitor of TyrRS catalytic activity with an inhibition constant (Ki) value of 22 μM [285]. Unlike other known biological targets of RSV (F0/F1 ATPase, ERα, SIRT3, COX-1/2) that bind to its trans-isomer [57, 286–288], intriguingly, we found that despite using trans-RSV, we obtained the crystal structure of TyrRS bound with only the cis-isomer of RSV. Therefore, TyrRS is the first and so far, the only biological target that binds to the cis-RSV. While the phenolic ring of RSV and tyrosine have the same disposition in the respective co-crystals, accommodation of the cis conformation of the dihydroxy ring of RSV forces a local structural change near the linker to the C-terminal domain of TyrRS. Based on the structural information, we proposed that RSV-triggered conformational switch in the active site of TyrRS might drive the predominant trans-RSV into a cis conformation in the physiological context. Consistently, downregulation of the cellular protein levels using siRNA against the mRNA of TyrRS mitigated the signaling effects of low-dose trans-RSV and overexpression of TyrRS was sufficient to mimic at least in part, the signaling events evoked by low dose (≥ 10 μM) trans-RSV. Therefore, we concluded that while high-dose RSV (≥ 25 μM) inhibits tyrosine-AMP formation [285] and activates SIRT1 [14, 207], at lower concentrations of RSV (≤ 15 μM), TyrRS protein would instead facilitate the conversion of trans-RSV to cis-RSV in the physiological context, providing an underappreciated contribution of TyrRS in the biological effects of natural RSV. Moreover, a structural comparison between cis-RSV bound TyrRS, and its the tyrosine-free (apo) form demonstrated that cis-RSV mimics a tyrosine-free “apo” state of TyrRS (Fig. 2a) and the physiological significance of this observation remains to be explored in the future. Because mutations in human TyrRS are known to cause neuropathy [289] and multi-system diseases [290–292] in a protein synthesis function-independent manner [293], our findings suggested that RSV would be a potent modulator of the emerging “moonlighting functions” of TyrRS [46, 294–296]. In this context, it is interesting to note that inflammation drives the matrix metalloproteinase (MMP)-mediated cleavage of TyrRS [297] and RSV is a known inhibitor of MMP-2 and MMP-9 [298, 299]. Therefore, it is apparent that RSV would contribute to the systemic protein levels of TyrRS and vice versa, TyrRS would contribute to the distinct physiological outcomes of RSV, if any that are mediated through the cis and trans isomers of RSV.
Fig. 2.
a RSV mimics/induces a tyrosine-free (apo) conformation in TyrRS. Ribbon illustration of structural comparison between the superimposed stuctures of TyrRS. Structures of TyrRS with cis-RSV bound at the active site and a tyrosine-free (apo) form does not show any significant conformational differences. b cis- and trans-RSV have opposite effects on TyrRS-regulated PARP1 activation. Cartoon illustration of the mechanism of isomer-specific opposite effects of cis- and trans-RSV on TyrRS-regulated PARP1 activation. cis-RSV facilitates TyrRS-regulated PARP1 activation, which results in the production of nicotinamide (NAM). Similarly, circadian downregulation of tyrosine in the night would also facilitate TyrRS-regulated PARP1 activation. In contrast, trans-RSV and tyrosine inhibit TyrRS-regulated PARP1 activation and the production of NAM, which is a well-known inhibitor of SIRT1. Therefore, treatment with trans-RSV or the circadian upregulation of tyrosine in the day time would also result in an apparent activation of SIRT1 due to the absence of NAM production by TyrRS-regulated PARP1 activation
cis- and trans-RSV have opposite effects on TyrRS-regulated PARP1 activation
Although RNA does not activate PARP1 [300], broken DNA ends are the best-known activators of PARP1 [301]. Intriguingly, the presence of PARP1 on the broken DNA impairs efficient DNA repair [302, 303], suggesting that eviction of PARP1 from the DNA through auto-poly-ADP-ribos(PAR)ylation is required for efficient DNA repair [304, 305]. Consistently, NAD+ supplementation that enhances auto-PARylation of PARP1 facilitates PARP1-dependent DNA repair [306]. Interestingly, our work demonstrated that nuclear localization of TyrRS after either serum starvation, heat shock, or endoplasmic reticulum (ER) stress stimulates the auto-PARylation of PARP1 suggesting that eukaryotic TyrRS activates PARP1 in a DNA-independent manner [285] to facilitate TyrRS-mediated DNA repair [295, 296]. Because our previous work [272] provided the structural basis of amino acids mediated inhibition of the “moonlighting” functions of aaRSs, we hypothesized that RSV that binds to TyrRS would modulate its PARP1-activating “moonlighting” function as well. Importantly, RSV was previously shown to activate PARP1-dependent protection against oxidative stress and mitochondrial dysfunctions [71]. As expected, we found that binding of cis-RSV induced conformational switch promoted the interaction of TyrRS with PARP1 and stimulated the generation of nicotinamide (NAM) and ADP-ribose (ADPR)- two potent inhibitors of SIRT1 [285]. Most significantly, RSV potentiated TyrRS-mediated auto-PARylation of PARP1 in vitro at nanomolar (nM) levels with a half-maximal effect (EC50) at roughly 10 nM, indicating that TyrRS/PARP1 complex is the biological target of RSV rather than TyrRS by itself. TyrRS-cis-RSV-PARP1-driven NAD+ signaling thus upregulated the expression and activation of a battery of genes, proteins, and signaling cascades that elicit a protective stress response. Most importantly, we observed significant upregulation of the acetylome of proteins, including p53, upon treatment with RSV in vitro and in vivo [285]. This observation was also consistent with RSV-mediated stimulation of the acetylation of nuclear proteins that drives autophagy [58] and CR stimulated upregulation of acetylome, including p53 [307]. However, TyrRS-regulated auto-PARylation of PARP1 was lost in the presence of broken DNA [285], suggesting that factors that induce DNA damage would abolish TyrRS/PARP1-mediated protective stress response.
Because cis-RSV and tyrosine evoke two distinct conformations in TyrRS, treatment with a higher affinity tyrosine-adenylate analog (Tyr-SA, (5’-O-[N-(9 L-tyrosyl) sulfamoyl] adenosine)) resulted in the inhibition of cis-RSV/TyrRS-regulated auto-PARylation of PARP1 both in vitro and in vivo in mice; however, we were intrigued by the observation that despite using the commonly available trans-RSV, we obtained only the cis-isomer of RSV bound to TyrRS. To better understand it, we performed in silico modeling using the trans-RSV in the active of TyrRS. This modeling showed that the binding of trans-RSV to TyrRS did not induce any conformational change and is identical to its tyrosine-bound form [285]. This data indicated that unlike cis-RSV, retension of trans-RSV in the active site of TyrRS by higher concentrations of RSV (≥ 25 μM) would prevent the interaction of TyrRS with PARP1, leading to inhibition of PARP1 and an apparent activation of SIRT1 (due to the absence of PARP1 activation) (Fig. 2b). Thus, our previous work for the first time suggested that the “cis” and the “trans” isomers of RSV and lower (≤ 15 μM) and higher (≥ 25 μM) doses of RSV would have opposite effects on TyrRS-regulated PARP1 activation and associated NAD+ signaling [285] and indicated a potential molecular basis to resolve the “dichotomy” of RSV.
PARP1 maintains genomic stability and inflammation inhibits PARP1-mediated DNA repair
PARP1 senses and responds to DNA damage [305, 308–310], oxidative, and environmental stresses by transcriptionally activating cytoprotective and DNA repair pathways [303, 311]. To protect cells from damage, PARP1 metabolizes NAD+ to nicotinamide and ADP-ribose resulting in the activation of cellular cytoprotective pathways [312–319] along with rapid nuclear ATP synthesis that sustains the transcriptional upregulation of stress response genes [318, 320]. PARP1 modulates the function of the CCCTC-binding factor (CTCF) [321, 322] and feeding behavior [323] in a circadian transcription-dependent manner [311, 324]. Consistently, dopamine activates the PARP1/CTCF-regulated transcriptional network to trigger morphological remodeling in astrocytes [325]. PARP1 is not only a potent modulator of SIRTuin activity [326] but also protects against genotoxic stress [315, 327, 328], optimizes efficient DNA repair [303], and facilitates long-term memory formation [329–332] and neuronal survival under stress [333–335]. PARP1 plays a critical role in induced pluripotent stem cells (iPSCs) generation [336] and neuronal differentiation [337]. Interestingly, PARP1 knockout mice exhibited decreased neurogenesis with a concomitant increase in gliosis [338], exacerbates diet-induced obesity [339], induces schizophrenia-like symptoms such as anxiety, depression, social interaction deficits, and cognitive impairments in mice [340]. Interestingly, treatment with nerve growth factor (NGF) [341, 342] and NAD+ supplementation [343] activate PARP1 and protect neuron-like PC-12 cells from H2O2-mediated cell death [344, 345], despite the accumulation of poly-ADP-ribose (PAR) [343]. Furthermore, activation of PARP1 is required for nuclear proteasome function [346, 347] that prevents the accumulation of misfolded protein aggregates, a hallmark of neurodegenerative diseases. Basal PARP1 activation is higher in the CNS of young mice [348], and it is downregulated in AD [349]. PARylation protects against coronavirus [350] and modulates glucose metabolism [351], and consistently, PARylation is downregulated in an age-dependent manner [352, 353]. Interestingly, naked mole-rat (NMR) has higher basal PARylation levels than the mouse [354] and is resistant to Alzheimer’s disease (AD) [355]. Most significantly, inhibition of PARP1 leads to the induction of DNA damage and cytotoxicity [356, 357] and mitochondrial dysfunction [358] through the upregulation of aerobic glycolysis [359–361], which are implicated in the etiology of various metabolic disorders and cancer. Moreover, inflammation inhibits PARP1-dependent DNA repair [359, 362, 363] and depletion of PARP1 not only triggers sustained induction of interferon-stimulated genes (ISGs) [364] and senescence [365] but also exacerbates autoimmune diseases [366–368] and spontaneous cancer formation through accelerated aging [369]. In this context, it is interesting to note that inflammation also drives the cleavage of TyrRS [297], indicating a potential role of full-length TyrRS in PARP1-mediated DNA repair [359, 362, 363]. Consistently, emerging works suggest that inhibition of PARP1 induces DNA damage-dependent pro-inflammatory response [362, 363, 370–372] and results in cancer metastasis [373] and dampens the anti-cancer immune response through the induction of programmed death-ligand 1 (PD-L1) [374]. These observations suggested that activation of PARP1 not only enhances DNA repair but also triggers an anti-inflammatory signaling cascade [362–365] to maintain genomic stability. Consistent with the inhibitory role of PARP1 on DNA repair [302, 303], recently, PARP “trapping” has gained much attention in the anti-cancer treatment regimen [375]. Intriguingly, PARP1 inhibition protects neurons from toxic effects [376–382], suggesting that PARP-dependent DNA repair and survival are context dependent.
Human serum L-tyrosine level is circadian regulated and RSV has circadian effects
Physiologically, human serum L-tyrosine level is modulated in a circadian manner with a peak of serum tyrosine in the morning to noon (at light) and a drop in the night (at dark) [383, 384]. Therefore, during deep sleep, humans have low serum tyrosine levels [385]. Likewise, there is a daily rhythm in the content and utilization of tyrosine in the whole mouse [386]. Interestingly, tyrosine transaminase that modulates the serum tyrosine levels [387] is regulated by vagal cholinergic nerves, which in turn, is regulated by the central nervous system (CNS) [388, 389]. Consistent with RSV being a modulator of L-tyrosine-mediated signaling [285] and tyrosine kinases [390], RSV also restores circadian rhythm in mice [391] and upregulates circadian gene expression in fibroblasts [392, 393]. Because rhythmic histone acetylation regulates circadian gene transcription [394], these studies are also consistent with RSV’s role as a modulator of histone acetyltransferases (HDACs) [154, 207]. Intriguingly, when administered during the activity phase (at dark) in rat, RSV behaved as a potent antioxidant in the heart, the liver, and the kidney [395], but, when administered during the rest phase (at light), RSV instead exerted pro-oxidant effects in the organs for unknown reasons [395]. Furthermore, RSV supplementation significantly increased the proportion of active-wake time, occurring mainly during the resting phase of the sleep-wake cycle (+ 163%) of adult mouse lemurs. The increase in active-wake time with RSV supplementation was accompanied by a significant reduction of both paradoxical sleep (− 95%) and slow-wave sleep (− 38%). Therefore, RSV can act as a potent modulator of sleep-wake rhythms [396, 397]. Furthermore, the nocturnal administration of RSV sharply decreased tumor frequency up to 40% and lowered tumor incidence [398]. However, daytime administration of RSV in the same N-methyl-N-nitrosourea (NMU) rat model was significantly less effective with no change in tumor incidence [398]. These observations are consistent with the recent finding that different circadian cycles in nocturnal rodents versus diurnal humans may contribute to the failure in human translational studies [399]. Therefore, an important consideration should be given to the timing of RSV administration (day vs. night) in the future clinical trials to make the best out of the circadian effects of L-tyrosine and RSV.
Calorie restriction (CR) lowers serum L-tyrosine, a biomarker for metabolic dysfunctions and aging
The most recent human metabolomic analysis demonstrated that serum L-tyrosine level is upregulated during aging [400, 401], and its downregulation is an indicator of CR in humans [402, 403]. However, the potential mechanisms for an association between reduced serum levels of L-tyrosine and improvement in metabolic dysfunctions during CR remain unclear. Nevertheless, recent studies have confirmed the long-standing observation that elevated L-tyrosine level is a biomarker for various metabolic dysfunctions including the development of type 2 diabetes, obesity [274–282], cancer [283, 284], and memory dysfunction in human Alzheimer’s disease (AD) [404, 405]. Moreover, serum L-tyrosine level has also emerged as a novel marker that links diabetes and cardiovascular disease (CVD) susceptibility [406]. Interestingly, L-tyrosine influences developmental decisions and longevity in Caenorhabditis elegans [407, 408] and potentiates the detrimental effects of oxidative stress either by decreasing glutathione and stimulating lipid and protein oxidation in rat cerebral cortex [409] or by increasing the thiobarbituric acid reactive species levels in the hippocampus and the carbonyl levels in the cerebellum, hippocampus, and striatum [410]. Moreover, chronic administration of L-tyrosine increased DNA damage frequency and damage index in the hippocampus, striatum, cerebral cortex, and blood [411, 412]. Recent studies demonstrated that chronic administration of L-tyrosine inhibited the activity of complex I, II–III, and IV in the striatum, which can be prevented by antioxidant treatment [413, 414]. Consistently, oral supplementation of L-tyrosine impairs glucose uptake and insulin secretion in rats [415], and the capacity to detoxify excess L-tyrosine is an essential life trait for the blood-sucking arthropods [416, 417]. Quite interestingly, L-tyrosine was negatively correlated with hypothalamic transcriptional levels of Drd5, a dopamine receptor expressed in the limbic regions of the brain [418] that not only activates memory formation [419] but also evokes anti-tumor effects through autophagy induction [420]. Moreover, unlike branched-chain amino acids (BCAA) that upregulate BDNF levels [421], the acute administration of L-tyrosine instead decreased BDNF levels in the hippocampus and striatum of rats [422]. Consistently, the administration of L-tyrosine exacerbates the cognitive decline in aged people [423]. Most significantly, a tyrosine-restricted diet stimulates human immunocompetence [424], and CR significantly downregulates tyrosine biosynthesis and serum tyrosine levels in mice [418] as well as in humans [402, 403]. Intriguingly, similar to RSV-mediated antiatherogenic effects modulated through the focal adhesion kinase (FAK) [425] and anti-tumor effects [426, 427], a tyrosine-free diet is also known to evoke anti-tumor effects against melanoma [428–430] in a FAK-dependent manner [431].
cis- and trans-RSV would exert opposite effects in the physiological contexts
Although a significant bottleneck in tapping the therapeutic potential of RSV is the lack of a proven physiologically relevant mechanism of action, our previous work addressed some fundamental aspects of this issue and laid a foundation to bridge the gap between the observed in vitro and in vivo effects of RSV. Moreover, our discovery of TyrRS being a biologically significant and physiologically relevant target of RSV that facilitates the conversion of trans-RSV to cis-RSV suggests that the cis-isomer of RSV is also a major isomer that evokes protective stress response. Other recent studies have also confirmed the TyrRS-PARP1 signaling in mediating the protective effects of RSV [120, 229, 296, 432–434]. However, there are still critical gaps in our knowledge. The biological significance of the two distinct conformations induced by cis-and trans-RSV in TyrRS (Fig. 2) [285] has not been explored extensively. Moreover, the biological significance of cis-RSV has not gained scientific attention in terms of the number of publications (Fig. 1b) despite showing significant physiological effects, as mentioned above. In light of the new findings that RSV attains high micromolar levels in human tissues and plasma after oral ingestion [27, 89, 193, 194] and trans-RSV converts to cis-RSV in the physiological context [78, 195], and cis-RSV has anti-inflammatory [251–253] and anti-platelet [259] and anti-cancer activities [88] and cis-RSV sulfates and glucuronides are found in higher concentrations in humans [265], future studies should determine if cis- and trans-RSV would evoke distinct physiological outcomes in various experimental paradigms.
Acknowledgments
The authors acknowledge funding from NIH (2P20GM109091-06) and NSF (Award Number: 1755670) and American Cancer Society (ACS)-Institutional Research Grant (IRG).
Funding
This study received funding from NIH (2P20GM109091-06) and NSF (Award Number: 1755670) and American Cancer Society (ACS)-Institutional Research Grant (IRG).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paul B, Masih I, Deopujari J, Charpentier C. Occurrence of resveratrol and pterostilbene in age-old Drakshasava, an ayurvedic medicine from India. J Ethnopharmacol. 1999;68(1–3):71–76. doi: 10.1016/S0378-8741(99)00044-6. [DOI] [PubMed] [Google Scholar]
- 2.Wu JM, Wang ZR, Hsieh TC, Bruder JL, Zou JG, Huang YZ. Mechanism of cardioprotection by resveratrol, a phenolic antioxidant present in red wine (review) Int J Mol Med. 2001;8(1):3–17. doi: 10.3892/ijmm.8.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339(8808):1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 4.Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 5.Langcake P, Pryce RJ. Production of resveratrol by Vitis-Vinifera and other members of Vitaceae as a response to infection or injury. Physiol Plant Pathol. 1976;9(1):77–86. doi: 10.1016/0048-4059(76)90077-1. [DOI] [Google Scholar]
- 6.Jeandet P, Bessis R, Gautheron B. The production of resveratrol (3,5,4′-Trihydroxystilbene) by grape berries in different developmental stages. Am J Enol Vitic. 1991;42(1):41–46. [Google Scholar]
- 7.Jeandet P, Bessis R, Sbaghi M, Meunier P. Production of the phytoalexin resveratrol by grapes as a response to Botrytis attack under natural conditions. J Phytopathol. 1995;143(3):135–139. doi: 10.1111/j.1439-0434.1995.tb00246.x. [DOI] [Google Scholar]
- 8.Takaoka M. Resveratrol, a new phenolic compound, from Veratrum grandiflorum. J Chem Soc Jpn. 1939;60:1090–1100. [Google Scholar]
- 9.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira ALB, Monteiro VVS, Navegantes-Lima KC, Reis JF, Gomes RS, Rodrigues DVS, et al. Resveratrol role in autoimmune disease-a mini-review. Nutrients. 2017;9(12). 10.3390/nu9121306. [DOI] [PMC free article] [PubMed]
- 11.Kjaer TN, Ornstrup MJ, Poulsen MM, Stodkilde-Jorgensen H, Jessen N, Jorgensen JOL, et al. No beneficial effects of resveratrol on the metabolic syndrome: a randomized placebo-controlled clinical trial. J Clin Endocrinol Metab. 2017;102(5):1642–1651. doi: 10.1210/jc.2016-2160. [DOI] [PubMed] [Google Scholar]
- 12.Vang O, Ahmad N, Baile CA, Baur JA, Brown K, Csiszar A, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One. 2011;6(6):e19881. doi: 10.1371/journal.pone.0019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425(6954):191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C-elegans life span. Dev Cell. 2005;9(5):605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:e10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Orlandi I, Stamerra G, Strippoli M, Vai M. During yeast chronological aging resveratrol supplementation results in a short-lived phenotype Sir2-dependent. Redox Biol. 2017;12:745–754. doi: 10.1016/j.redox.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramos-Gomez M, Olivares-Marin IK, Canizal-Garcia M, Gonzalez-Hernandez JC, Nava GM, Madrigal-Perez LA. Resveratrol induces mitochondrial dysfunction and decreases chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. J Bioenerg Biomembr. 2017;49(3):241–251. doi: 10.1007/s10863-017-9709-9. [DOI] [PubMed] [Google Scholar]
- 21.Orozco H, Matallana E, Aranda A. Two-carbon metabolites, polyphenols and vitamins influence yeast chronological life span in winemaking conditions. Microb Cell Factories. 2012;11:104. doi: 10.1186/1475-2859-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280(17):17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 23.Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82(2):614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki K, Koike T. Resveratrol abolishes resistance to axonal degeneration in slow Wallerian degeneration (WldS) mice: activation of SIRT2, an NAD-dependent tubulin deacetylase. Biochem Biophys Res Commun. 2007;359(3):665–671. doi: 10.1016/j.bbrc.2007.05.164. [DOI] [PubMed] [Google Scholar]
- 26.Wilson T, Knight TJ, Beitz DC, Lewis DS, Engen RL. Resveratrol promotes atherosclerosis in hypercholesterolemic rabbits. Life Sci. 1996;59(1):PL15–PL21. doi: 10.1016/0024-3205(96)00260-3. [DOI] [PubMed] [Google Scholar]
- 27.Patel KR, Andreadi C, Britton RG, Horner-Glister E, Karmokar A, Sale S, et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med. 2013;5(205):205ra133. doi: 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Wang B, Zang W, Wang X, Liu Z, Li W, et al. Resveratrol inhibits the growth of gastric cancer by inducing G1 phase arrest and senescence in a Sirt1-dependent manner. PLoS One. 2013;8(11):e70627. doi: 10.1371/journal.pone.0070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Z, Xu MS, Barnett TL, Xu CW. Resveratrol induces cellular senescence with attenuated mono-ubiquitination of histone H2B in glioma cells. Biochem Biophys Res Commun. 2011;407(2):271–276. doi: 10.1016/j.bbrc.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Faragher RG, Burton DG, Majecha P, Fong NS, Davis T, Sheerin A, et al. Resveratrol, but not dihydroresveratrol, induces premature senescence in primary human fibroblasts. Age (Dordr) 2011;33(4):555–564. doi: 10.1007/s11357-010-9201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Hou D, Guo H, Zhou H, Zhang S, Xu X, et al. Resveratrol sequentially induces replication and oxidative stresses to drive p53-CXCR2 mediated cellular senescence in cancer cells. Sci Rep. 2017;7(1):208. doi: 10.1038/s41598-017-00315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eren MK, Kilincli A, Eren O. Resveratrol induced premature senescence is associated with DNA damage mediated SIRT1 and SIRT2 down-regulation. PLoS One. 2015;10(4):e0124837. doi: 10.1371/journal.pone.0124837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmitt E, Lehmann L, Metzler M, Stopper H. Hormonal and genotoxic activity of resveratrol. Toxicol Lett. 2002;136(2):133–142. doi: 10.1016/s0378-4274(02)00290-4. [DOI] [PubMed] [Google Scholar]
- 34.Fukuhara K, Nagakawa M, Nakanishi I, Ohkubo K, Imai K, Urano S, et al. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II) Bioorg Med Chem. 2006;14(5):1437–1443. doi: 10.1016/j.bmc.2005.09.070. [DOI] [PubMed] [Google Scholar]
- 35.Fukuhara K, Miyata N. Resveratrol as a new type of DNA-cleaving agent. Bioorg Med Chem Lett. 1998;8(22):3187–3192. doi: 10.1016/S0960-894x(98)00585-X. [DOI] [PubMed] [Google Scholar]
- 36.Guo XH, Ni J, Dai XQ, Zhou T, Yang GF, Xue JL, et al. Biphasic regulation of spindle assembly checkpoint by low and high concentrations of resveratrol leads to the opposite effect on chromosomal instability. Mutat Res-Gen Tox En. 2018;825:19–30. doi: 10.1016/j.mrgentox.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Azmi AS, Bhat SH, Hadi SM. Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: implications for anticancer properties. FEBS Lett. 2005;579(14):3131–3135. doi: 10.1016/j.febslet.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 38.Azmi AS, Bhat SH, Hanif S, Hadi SM. Plant polyphenols mobilize endogenous copper in human peripheral lymphocytes leading to oxidative DNA breakage: a putative mechanism for anticancer properties. FEBS Lett. 2006;580(2):533–538. doi: 10.1016/j.febslet.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 39.Park HR, Kong KH, Yu BP, Mattson MP, Lee J. Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. J Biol Chem. 2012;287(51):42588–42600. doi: 10.1074/jbc.M112.406413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol. 2007;72(6):1508–1521. doi: 10.1124/mol.107.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imler TJ, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17(+)IL-10(+) T cells, CD4(-) IFN-gamma(+) cells, and decreased macrophage IL-6 expression. Int Immunopharmacol. 2009;9(1):134–143. doi: 10.1016/j.intimp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Fonseca-Kelly Z, Nassrallah M, Uribe J, Khan RS, Dine K, Dutt M, et al. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol. 2012;3:84. doi: 10.3389/fneur.2012.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato F, Martinez NE, Shahid M, Rose JW, Carlson NG, Tsunoda I. Resveratrol exacerbates both autoimmune and viral models of multiple sclerosis. Am J Pathol. 2013;183(5):1390–1396. doi: 10.1016/j.ajpath.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassereau J, Chevrollier A, Codron P, Goizet C, Gueguen N, Verny C, et al. Oxidative stress contributes differentially to the pathophysiology of Charcot-Marie-Tooth disease type 2K. Exp Neurol. 2020;323:113069. doi: 10.1016/j.expneurol.2019.113069. [DOI] [PubMed] [Google Scholar]
- 45.Pan PT, Lin HY, Chuang CW, Wang PK, Wan HC, Lee MC, et al. Resveratrol alleviates nuclear factor-kappaB-mediated neuroinflammation in vasculitic peripheral neuropathy induced by ischaemia-reperfusion via suppressing endoplasmic reticulum stress. Clin Exp Pharmacol Physiol. 2019;46(8):770–779. doi: 10.1111/1440-1681.13105. [DOI] [PubMed] [Google Scholar]
- 46.Bervoets S, Wei N, Erfurth ML, Yusein-Myashkova S, Ermanoska B, Mateiu L, et al. Transcriptional dysregulation by a nucleus-localized aminoacyl-tRNA synthetase associated with Charcot-Marie-Tooth neuropathy. Nat Commun. 2019;10(1):5045. doi: 10.1038/s41467-019-12909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finnell JE, Lombard CM, Melson MN, Singh NP, Nagarkatti M, Nagarkatti P, et al. The protective effects of resveratrol on social stress-induced cytokine release and depressive-like behavior. Brain Behav Immun. 2017;59:147–157. doi: 10.1016/j.bbi.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim HD, Hesterman J, Call T, Magazu S, Keeley E, Armenta K, et al. SIRT1 mediates depression-like behaviors in the nucleus accumbens. J Neurosci. 2016;36(32):8441–8452. doi: 10.1523/JNEUROSCI.0212-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shuto T, Kuroiwa M, Koga Y, Kawahara Y, Sotogaku N, Toyomasu K, et al. Acute effects of resveratrol to enhance cocaine-induced dopamine neurotransmission in the striatum. Neurosci Lett. 2013;542:107–112. doi: 10.1016/j.neulet.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 50.Renthal W, Kumar A, Xiao G, Wilkinson M, Covington HE, 3rd, Maze I, et al. Genome-wide analysis of chromatin regulation by cocaine reveals a role for sirtuins. Neuron. 2009;62(3):335–348. doi: 10.1016/j.neuron.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Yu L, Zhao L, Zeng F, Liu QS. Resveratrol modulates cocaine-induced inhibitory synaptic plasticity in VTA dopamine neurons by inhibiting phosphodiesterases (PDEs) Sci Rep. 2017;7(1):15657. doi: 10.1038/s41598-017-16034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu P, Zhu W, Zhu C, Jin L, Guan Y, Guan X. Resveratrol fails to affect cocaine conditioned place preference behavior, but alleviates anxiety-like behaviors in cocaine withdrawn rats. Psychopharmacology. 2016;233(7):1279–1287. doi: 10.1007/s00213-016-4210-4. [DOI] [PubMed] [Google Scholar]
- 53.Calliari A, Bobba N, Escande C, Chini EN. Resveratrol delays Wallerian degeneration in a NAD(+) and DBC1 dependent manner. Exp Neurol. 2014;251:91–100. doi: 10.1016/j.expneurol.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 54.Casper RF, Quesne M, Rogers IM, Shirota T, Jolivet A, Milgrom E, et al. Resveratrol has antagonist activity on the aryl hydrocarbon receptor: implications for prevention of dioxin toxicity. Mol Pharmacol. 1999;56(4):784–790. [PubMed] [Google Scholar]
- 55.Bowers JL, Tyulmenkov VV, Jernigan SC, Klinge CM. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141(10):3657–3667. doi: 10.1210/en.141.10.3657. [DOI] [PubMed] [Google Scholar]
- 56.Gehm BD, McAndrews JM, Chien PY, Jameson JL. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. P Natl Acad Sci USA. 1997;94(25):14138–14143. doi: 10.1073/pnas.94.25.14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nwachukwu JC, Srinivasan S, Bruno NE, Parent AA, Hughes TS, Pollock JA, et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. Elife. 2014;3:e02057. doi: 10.7554/eLife.02057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morselli E, Marino G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol. 2011;192(4):615–629. doi: 10.1083/jcb.201008167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Armour SM, Baur JA, Hsieh SN, Land-Bracha A, Thomas SM, Sinclair DA. Inhibition of mammalian S6 kinase by resveratrol suppresses autophagy. Aging (Albany NY) 2009;1(6):515–528. doi: 10.18632/aging.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin CJ, Lee CC, Shih YL, Lin TY, Wang SH, Lin YF, et al. Resveratrol enhances the therapeutic effect of temozolomide against malignant glioma in vitro and in vivo by inhibiting autophagy. Free Radic Biol Med. 2012;52(2):377–391. doi: 10.1016/j.freeradbiomed.2011.10.487. [DOI] [PubMed] [Google Scholar]
- 61.Manna SK, Mukhopadhyay A, Aggarwal BB. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: potential role of reactive oxygen intermediates and lipid peroxidation. J Immunol. 2000;164(12):6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 62.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, et al. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakajima S, Ishimaru K, Kobayashi A, Yu G, Nakamura Y, Oh-Oka K, et al. Resveratrol inhibits IL-33-mediated mast cell activation by targeting the MK2/3-PI3K/Akt axis. Sci Rep. 2019;9(1):18423. doi: 10.1038/s41598-019-54878-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uchida Y, Yamazaki H, Watanabe S, Hayakawa K, Meng Y, Hiramatsu N, et al. Enhancement of NF-kappaB activity by resveratrol in cytokine-exposed mesangial cells. Clin Exp Immunol. 2005;142(1):76–83. doi: 10.1111/j.1365-2249.2005.02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jhou JP, Chen SJ, Huang HY, Lin WW, Huang DY, Tzeng SJ. Upregulation of FcgammaRIIB by resveratrol via NF-kappaB activation reduces B-cell numbers and ameliorates lupus. Exp Mol Med. 2017;49(9):e381. doi: 10.1038/emm.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palomer X, Capdevila-Busquets E, Alvarez-Guardia D, Barroso E, Pallas M, Camins A, et al. Resveratrol induces nuclear factor-kappaB activity in human cardiac cells. Int J Cardiol. 2013;167(6):2507–2516. doi: 10.1016/j.ijcard.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 67.Zhou L, Long J, Sun Y, Chen W, Qiu R, Yuan D. Resveratrol ameliorates atherosclerosis induced by high-fat diet and LPS in ApoE(-/-) mice and inhibits the activation of CD4(+) T cells. Nutr Metab (Lond) 2020;17:41. doi: 10.1186/s12986-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berbee JFP, Wong MC, Wang YN, van der Hoorn JWA, Khedoe PPSJ, van Klinken JB, et al. Resveratrol protects against atherosclerosis, but does not add to the antiatherogenic effect of atorvastatin, in APOE*3-Leiden.CETP mice. J Nutr Biochem. 2013;24(8):1423–1430. doi: 10.1016/j.jnutbio.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 69.Baron S, Bedarida T, Cottart CH, Vibert F, Vessieres E, Ayer A, et al. Dual effects of resveratrol on arterial damage induced by insulin resistance in aged mice. J Gerontol A Biol Sci Med Sci. 2014;69(3):260–269. doi: 10.1093/gerona/glt081. [DOI] [PubMed] [Google Scholar]
- 70.Kitada M, Kume S, Imaizumi N, Koya D. Resveratrol improves oxidative stress and protects against diabetic nephropathy through normalization of Mn-SOD dysfunction in AMPK/SIRT1-independent pathway. Diabetes. 2011;60(2):634–643. doi: 10.2337/db10-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3 beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76(4):884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 72.Jackson JR, Ryan MJ, Alway SE. Long-term supplementation with resveratrol alleviates oxidative stress but does not attenuate sarcopenia in aged mice. J Gerontol a-Biol. 2011;66(7):751–764. doi: 10.1093/gerona/glr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gueguen N, Desquiret-Dumas V, Leman G, Chupin S, Baron S, Nivet-Antoine V, et al. Resveratrol directly binds to mitochondrial complex I and increases oxidative stress in brain mitochondria of aged mice. PLoS One. 2015;10(12):e0144290. doi: 10.1371/journal.pone.0144290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miki H, Uehara N, Kimura A, Sasaki T, Yuri T, Yoshizawa K, et al. Resveratrol induces apoptosis via ROS-triggered autophagy in human colon cancer cells. Int J Oncol. 2012;40(4):1020–1028. doi: 10.3892/ijo.2012.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de la Lastral CA, Villegas I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem Soc T. 2007;35:1156–1160. doi: 10.1042/Bst0351156. [DOI] [PubMed] [Google Scholar]
- 76.Plauth A, Geikowski A, Cichon S, Wowro SJ, Liedgens L, Rousseau M, et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic Biol Med. 2016;99:608–622. doi: 10.1016/j.freeradbiomed.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Ahmad KA, Clement MV, Pervaiz S. Pro-oxidant activity of low doses of resveratrol inhibits hydrogen peroxide-induced apoptosis. Ann N Y Acad Sci. 2003;1010:365–373. doi: 10.1196/annals.1299.067. [DOI] [PubMed] [Google Scholar]
- 78.Prysyazhna O, Wolhuter K, Switzer C, Santos C, Yang XP, Lynham S, et al. Blood pressure-lowering by the antioxidant resveratrol is counterintuitively mediated by oxidation of cGMP-dependent protein kinase. Circulation. 2019;140(2):126–137. doi: 10.1161/Circulationaha.118.037398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demidenko ZN, Blagosklonny MV. At concentrations that inhibit mTOR, resveratrol suppresses cellular senescence. Cell Cycle. 2009;8(12):1901–1904. doi: 10.4161/cc.8.12.8810. [DOI] [PubMed] [Google Scholar]
- 80.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 81.Olivares-Marin IK, Gonzlez-Hernndez JC, Madrigal-Perez LA. Resveratrol cytotoxicity is energy-dependent. J Food Biochem. 2019;43(9):e13008. doi: 10.1111/jfbc.13008. [DOI] [PubMed] [Google Scholar]
- 82.Zini R, Morin C, Bertelli A, Bertelli AA, Tillement JP. Effects of resveratrol on the rat brain respiratory chain. Drugs Exp Clin Res. 1999;25(2–3):87–97. [PubMed] [Google Scholar]
- 83.Zheng J, Ramirez VD. Inhibition of mitochondrial proton F0F1-ATPase/ATP synthase by polyphenolic phytochemicals. Br J Pharmacol. 2000;130(5):1115–1123. doi: 10.1038/sj.bjp.0703397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moreira AC, Silva AM, Santos MS, Sardao VA. Resveratrol affects differently rat liver and brain mitochondrial bioenergetics and oxidative stress in vitro: investigation of the role of gender. Food Chem Toxicol. 2013;53:18–26. doi: 10.1016/j.fct.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 85.Gledhill JR, Montgomery MG, Leslie AG, Walker JE. Mechanism of inhibition of bovine F1-ATPase by resveratrol and related polyphenols. Proc Natl Acad Sci U S A. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sareen D, Darjatmoko SR, Albert DM, Polans AS. Mitochondria, calcium, and calpain are key mediators of resveratrol-induced apoptosis in breast cancer. Mol Pharmacol. 2007;72(6):1466–1475. doi: 10.1124/mol.107.039040. [DOI] [PubMed] [Google Scholar]
- 87.Ma X, Tian X, Huang X, Yan F, Qiao D. Resveratrol-induced mitochondrial dysfunction and apoptosis are associated with Ca2+ and mCICR-mediated MPT activation in HepG2 cells. Mol Cell Biochem. 2007;302(1–2):99–109. doi: 10.1007/s11010-007-9431-8. [DOI] [PubMed] [Google Scholar]
- 88.Jayatilake GS, Jayasuriya H, Lee ES, Koonchanok NM, Geahlen RL, Ashendel CL, et al. Kinase inhibitors from Polygonum cuspidatum. J Nat Prod. 1993;56(10):1805–10. doi: 10.1021/np50100a021. [DOI] [PubMed] [Google Scholar]
- 89.Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A, et al. Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci Transl Med. 2015;7(298):298ra117. doi: 10.1126/scitranslmed.aaa7619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang H, Lin H, Zhang X, Li J. Resveratrol reverses temozolomide resistance by downregulation of MGMT in T98G glioblastoma cells by the NF-kappaB-dependent pathway. Oncol Rep. 2012;27(6):2050–2056. doi: 10.3892/or.2012.1715. [DOI] [PubMed] [Google Scholar]
- 91.Yuan Y, Xue X, Guo RB, Sun XL, Hu G. Resveratrol enhances the antitumor effects of temozolomide in glioblastoma via ROS-dependent AMPK-TSC-mTOR signaling pathway. CNS Neurosci Ther. 2012;18(7):536–546. doi: 10.1111/j.1755-5949.2012.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andreani C, Bartolacci C, Wijnant K, Crinelli R, Bianchi M, Magnani M, et al. Resveratrol fuels HER2 and ER alpha-positive breast cancer behaving as proteasome inhibitor. Aging-Us. 2017;9(2):508–523. doi: 10.18632/aging.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klink JC, Tewari AK, Masko EM, Antonelli J, Febbo PG, Cohen P, et al. Resveratrol worsens survival in SCID mice with prostate cancer xenografts in a cell-line specific manner, through paradoxical effects on oncogenic pathways. Prostate. 2013;73(7):754–762. doi: 10.1002/pros.22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H, Zhou HB, Zou YX, Liu QA, Guo CH, Gao GM, et al. Resveratrol modulates angiogenesis through the GSK3 beta/beta-catenin/TCF-dependent pathway in human endothelial cells. Biochem Pharmacol. 2010;80(9):1386–1395. doi: 10.1016/j.bcp.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 95.Gliemann L, Olesen J, Bienso RS, Schmidt JF, Akerstrom T, Nyberg M, et al. Resveratrol modulates the angiogenic response to exercise training in skeletal muscles of aged men. Am J Physiol-Heart C. 2014;307(8):H1111–H11H9. doi: 10.1152/ajpheart.00168.2014. [DOI] [PubMed] [Google Scholar]
- 96.Jimoh A, Tanko Y, Ahmed A, Mohammed A, Ayo JO. Resveratrol prevents high-fat diet-induced obesity and oxidative stress in rabbits. Pathophysiology. 2018;25(4):359–364. doi: 10.1016/j.pathophys.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622. doi: 10.1016/j.cmet.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Rodriguez A, Santamaria B, Mas-Gutierrez JA, Rada P, Fernandez-Millan E, Pardo V, et al. Resveratrol treatment restores peripheral insulin sensitivity in diabetic mice in a sirt1-independent manner. Mol Nutr Food Res. 2015;59(8):1431–1442. doi: 10.1002/mnfr.201400933. [DOI] [PubMed] [Google Scholar]
- 100.Zhao H, Shu L, Huang W, Song G, Ma H. Resveratrol affects hepatic gluconeogenesis via histone deacetylase 4. Diabetes Metab Syndr Obes. 2019;12:401–411. doi: 10.2147/DMSO.S198830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su HC, Hung LM, Chen JK. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2006;290(6):E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 102.Deng JY, Hsieh PS, Huang JP, Lu LS, Hung LM. Activation of estrogen receptor is crucial for resveratrol-stimulating muscular glucose uptake via both insulin-dependent and -independent pathways. Diabetes. 2008;57(7):1814–1823. doi: 10.2337/db07-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Patel MI, Gupta A, Dey CS. Potentiation of neuronal insulin signaling and glucose uptake by resveratrol: the involvement of AMPK. Pharmacol Rep. 2011;63(5):1162–1168. doi: 10.1016/s1734-1140(11)70635-1. [DOI] [PubMed] [Google Scholar]
- 104.Tan Z, Zhou LJ, Mu PW, Liu SP, Chen SJ, Fu XD, et al. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats. J Nutr Biochem. 2012;23(12):1716–1724. doi: 10.1016/j.jnutbio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 105.Kang BB, Chiang BH. Amelioration of insulin resistance using the additive effect of ferulic acid and resveratrol on vesicle trafficking for skeletal muscle glucose metabolism. Phytother Res. 2020;34(4):808–816. doi: 10.1002/ptr.6561. [DOI] [PubMed] [Google Scholar]
- 106.Park JB. Inhibition of glucose and dehydroascorbic acid uptakes by resveratrol in human transformed myelocytic cells. J Nat Prod. 2001;64(3):381–384. doi: 10.1021/np000411t. [DOI] [PubMed] [Google Scholar]
- 107.Varshney P, Dey CS. Resveratrol regulates neuronal glucose uptake and insulin sensitivity via P21-activated kinase 2 (PAK2) Biochem Biophys Res Commun. 2017;485(2):372–378. doi: 10.1016/j.bbrc.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 108.Lee H, Kim JW. High-dose resveratrol inhibits insulin signaling pathway in 3T3-L1 adipocytes. J Lifestyle Med. 2013;3(1):41–47. [PMC free article] [PubMed] [Google Scholar]
- 109.Frojdo S, Cozzone D, Vidal H, Pirola L. Resveratrol is a class IA phosphoinositide 3-kinase inhibitor. Biochem J. 2007;406:511–518. doi: 10.1042/Bj20070236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Salas M, Obando P, Ojeda L, Ojeda P, Perez A, Vargas-Uribe M, et al. Resolution of the direct interaction with and inhibition of the human GLUT1 hexose transporter by resveratrol from its effect on glucose accumulation. Am J Phys Cell Phys. 2013;305(1):C90–C99. doi: 10.1152/ajpcell.00387.2012. [DOI] [PubMed] [Google Scholar]
- 111.Breen DM, Sanli T, Giacca A, Tsiani E. Stimulation of muscle cell glucose uptake by resveratrol through sirtuins and AMPK. Biochem Bioph Res Co. 2008;374(1):117–122. doi: 10.1016/j.bbrc.2008.06.104. [DOI] [PubMed] [Google Scholar]
- 112.Lin SC, Ho CT, Chuo WH, Li S, Wang TT, Lin CC. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17(1):144. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weston S, Matthews KL, Lent R, Vlk A, Haupt R, Kingsbury T, et al. A yeast suppressor screen used to identify mammalian SIRT1 as a proviral factor for Middle East Respiratory Syndrome coronavirus replication. J Virol. 2019;93(16):e00197–e00119. doi: 10.1128/JVI.00197-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ding L, Jiang P, Xu X, Lu W, Yang C, Zhou P, et al. Resveratrol promotes HSV-2 replication by increasing histone acetylation and activating NF-kappaB. Biochem Pharmacol. 2020;171:113691. doi: 10.1016/j.bcp.2019.113691. [DOI] [PubMed] [Google Scholar]
- 115.Shi YX, Li YJ, Huang CJ, Ying LX, Xue JH, Wu HC, et al. Resveratrol enhances HBV replication through activating Sirt1-PGC-1 alpha-PPAR alpha pathway. Sci Rep-Uk. 2016;6:24744. doi: 10.1038/srep24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nakamura M, Saito H, Ikeda M, Hokari R, Kato N, Hibi T, et al. An antioxidant resveratrol significantly enhanced replication of hepatitis C virus. World J Gastroenterol. 2010;16(2):184–192. doi: 10.3748/wjg.v16.i2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958(2):439–447. doi: 10.1016/S0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 118.Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, et al. Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet. 2005;37(4):349–350. doi: 10.1038/ng1534. [DOI] [PubMed] [Google Scholar]
- 119.Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci Rep. 2015;5:8075. doi: 10.1038/srep08075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng HY, Mi MT. Resveratrol attenuates a beta(25-35) caused neurotoxicity by inducing autophagy through the TyrRS-PARP1-SIRT1 signaling pathway. Neurochem Res. 2016;41(9):2367–2379. doi: 10.1007/s11064-016-1950-9. [DOI] [PubMed] [Google Scholar]
- 121.Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, et al. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu CW, Grossman H, Neugroschl J, Parker S, Burden A, Luo X, et al. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer's disease: a pilot study. Alzheimers Dement (N Y) 2018;4:609–616. doi: 10.1016/j.trci.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thaung Zaw JJ, Howe PR, Wong RH. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: a 24-month randomised, double-blind, placebo-controlled, crossover study. Clin Nutr. 2020. 10.1016/j.clnu.2020.08.025. [DOI] [PubMed]
- 124.Thaung Zaw JJ, Howe PRC, Wong RHX. Sustained cerebrovascular and cognitive benefits of resveratrol in postmenopausal women. Nutrients. 2020;12(3). 10.3390/nu12030828. [DOI] [PMC free article] [PubMed]
- 125.Witte AV, Kerti L, Margulies DS, Floel A. Effects of resveratrol on memory performance, hippocampal functional connectivity, and glucose metabolism in healthy older adults. J Neurosci. 2014;34(23):7862–7870. doi: 10.1523/JNEUROSCI.0385-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zortea K, Franco VC, Guimaraes P, Belmonte-de-Abreu PS. Resveratrol supplementation did not improve cognition in patients with schizophrenia: results from a randomized clinical trial. Front Psychiatry. 2016;7:159. doi: 10.3389/fpsyt.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Morales AI, Rodriguez-Barbero A, Vicente-Sanchez C, Mayoral P, Lopez-Novoa JM, Perez-Barriocanal F. Resveratrol inhibits gentamicin-induced mesangial cell contraction. Life Sci. 2006;78(20):2373–2377. doi: 10.1016/j.lfs.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 128.Morales AI, Buitrago JM, Santiago JM, Fernandez-Tagarro M, Lopez-Novoa JM, Perez-Barriocanal F. Protective effect of trans-resveratrol on gentamicin-induced nephrotoxicity. Antioxid Redox Signal. 2002;4(6):893–898. doi: 10.1089/152308602762197434. [DOI] [PubMed] [Google Scholar]
- 129.Cadenas S, Barja G. Resveratrol, melatonin, vitamin E, and PBN protect against renal oxidative DNA damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med. 1999;26(11–12):1531–1537. doi: 10.1016/s0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 130.Den Hartogh DJ, Tsiani E. Health benefits of resveratrol in kidney disease: evidence from in vitro and in vivo studies. Nutrients. 2019;11(7). 10.3390/nu11071624. [DOI] [PMC free article] [PubMed]
- 131.Popat R, Plesner T, Davies F, Cook G, Cook M, Elliott P, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160(5):714–717. doi: 10.1111/bjh.12154. [DOI] [PubMed] [Google Scholar]
- 132.Alway SE, McCrory JL, Kearcher K, Vickers A, Frear B, Gilleland DL, et al. Resveratrol enhances exercise-induced cellular and functional adaptations of skeletal muscle in older men and women. J Gerontol A Biol Sci Med Sci. 2017;72(12):1595–1606. doi: 10.1093/gerona/glx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, et al. Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors--results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55. doi: 10.1016/j.ijcard.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 134.Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, et al. Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591(20):5047–5059. doi: 10.1113/jphysiol.2013.258061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olesen J, Gliemann L, Bienso R, Schmidt J, Hellsten Y, Pilegaard H. Exercise training, but not resveratrol, improves metabolic and inflammatory status in skeletal muscle of aged men. J Physiol. 2014;592(8):1873–1886. doi: 10.1113/jphysiol.2013.270256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heeboll S, Kreuzfeldt M, Hamilton-Dutoit S, Kjaer Poulsen M, Stodkilde-Jorgensen H, Moller HJ, et al. Placebo-controlled, randomised clinical trial: high-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand J Gastroenterol. 2016;51(4):456–464. doi: 10.3109/00365521.2015.1107620. [DOI] [PubMed] [Google Scholar]
- 137.Mankowski RT, You L, Buford TW, Leeuwenburgh C, Manini TM, Schneider S, et al. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults - a pilot study. Exp Gerontol. 2020;131:110821. doi: 10.1016/j.exger.2019.110821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pollack RM, Barzilai N, Anghel V, Kulkarni AS, Golden A, O'Broin P, et al. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J Gerontol a-Biol. 2017;72(12):1703–1709. doi: 10.1093/gerona/glx041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bo S, Ponzo V, Ciccone G, Evangelista A, Saba F, Goitre I, et al. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol Res. 2016;111:896–905. doi: 10.1016/j.phrs.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 140.Jeyaraman MM, Al-Yousif NSH, Singh Mann A, Dolinsky VW, Rabbani R, Zarychanski R, et al. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;1:CD011919. doi: 10.1002/14651858.CD011919.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fogacci F, Fogacci S, Cicero A. Resveratrol for high blood pressure: a total failure or the need to identify the right patient? High Blood Press Cardiovasc Prev. 2019;26(5):421–423. doi: 10.1007/s40292-019-00333-5. [DOI] [PubMed] [Google Scholar]
- 142.Akbari M, Tamtaji OR, Lankarani KB, Tabrizi R, Dadgostar E, Kolahdooz F, et al. The effects of resveratrol supplementation on endothelial function and blood pressures among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. High Blood Press Car. 2019;26(4):305–319. doi: 10.1007/s40292-019-00324-6. [DOI] [PubMed] [Google Scholar]
- 143.Brasnyo P, Molnar GA, Mohas M, Marko L, Laczy B, Cseh J, et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br J Nutr. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 144.Khodabandehloo H, Seyyedebrahimi S, Esfahani EN, Razi F, Meshkani R. Resveratrol supplementation decreases blood glucose without changing the circulating CD14(+) CD16(+) monocytes and inflammatory cytokines in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Nutr Res. 2018;54:40–51. doi: 10.1016/j.nutres.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 145.Chen S, Zhao X, Ran L, Wan J, Wang X, Qin Y, et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: a randomized controlled trial. Dig Liver Dis. 2015;47(3):226–232. doi: 10.1016/j.dld.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 146.Sridharan K, Sequeira RP. Drugs for treating severe hypertension in pregnancy: a network meta-analysis and trial sequential analysis of randomized clinical trials. Br J Clin Pharmacol. 2018;84(9):1906–1916. doi: 10.1111/bcp.13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sergi C, Chiu B, Feulefack J, Shen F, Chiu B. Usefulness of resveratrol supplementation in decreasing cardiometabolic risk factors comparing subjects with metabolic syndrome and healthy subjects with or without obesity: meta-analysis using multinational, randomised, controlled trials. Arch Med Sci Atheroscler Dis. 2020;5:e98–e111. doi: 10.5114/amsad.2020.95884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci. 2019;20(4). 10.3390/ijms20040904. [DOI] [PMC free article] [PubMed]
- 149.Akbari M, Tamtaji OR, Lankarani KB, Tabrizi R, Dadgostar E, Haghighat N, et al. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2020;19(1):25. doi: 10.1186/s12944-020-1198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pezzuto JM. Resveratrol: twenty years of growth, development and controversy. Biomol Ther (Seoul) 2019;27(1):1–14. doi: 10.4062/biomolther.2018.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Calabrese EJ, Mattson MP, Calabrese V. Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol. 2010;29(12):980–1015. doi: 10.1177/0960327110383625. [DOI] [PubMed] [Google Scholar]
- 152.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 153.Leone S, Cornetta T, Basso E, Cozzi R. Resveratrol induces DNA double-strand breaks through human topoisomerase II interaction. Cancer Lett. 2010;295(2):167–172. doi: 10.1016/j.canlet.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 154.Venturelli S, Berger A, Bocker A, Busch C, Weiland T, Noor S, et al. Resveratrol as a Pan-HDAC inhibitor alters the acetylation status of jistone proteins in human-derived hepatoblastoma cells. PLoS One. 2013;8(8):e73097. doi: 10.1371/journal.pone.0073097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miller KM, Tjeertes JV, Coates J, Legube G, Polo SE, Britton S, et al. Human HDAC1 and HDAC2 function in the DNA-damage response to promote DNA nonhomologous end-joining. Nat Struct Mol Biol. 2010;17(9):1144–1151. doi: 10.1038/nsmb.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pao PC, Patnaik D, Watson LA, Gao F, Pan L, Wang J, et al. HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer's disease. Nat Commun. 2020;11(1):2484. doi: 10.1038/s41467-020-16361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu CC, Jin LW, Wang IF, Wei WY, Ho PC, Liu YC, et al. HDAC1 dysregulation induces aberrant cell cycle and DNA damage in progress of TDP-43 proteinopathies. EMBO Mol Med. 2020;12(6):e10622. doi: 10.15252/emmm.201910622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gatz SA, Keimling M, Baumann C, Dork T, Debatin KM, Fulda S, et al. Resveratrol modulates DNA double-strand break repair pathways in an ATM/ATR-p53- and -Nbs1-dependent manner. Carcinogenesis. 2008;29(3):519–527. doi: 10.1093/carcin/bgm283. [DOI] [PubMed] [Google Scholar]
- 159.Tyagi A, Gu M, Takahata T, Frederick B, Agarwal C, Siriwardana S, et al. Resveratrol selectively induces DNA damage, independent of Smad4 expression, in its efficacy against human head and neck squamous cell carcinoma. Clin Cancer Res. 2011;17(16):5402–5411. doi: 10.1158/1078-0432.CCR-11-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leon-Galicia I, Diaz-Chavez J, Garcia-Villa E, Uribe-Figueroa L, Hidalgo-Miranda A, Herrera LA, et al. Resveratrol induces downregulation of DNA repair genes in MCF-7 human breast cancer cells. Eur J Cancer Prev. 2013;22(1):11–20. doi: 10.1097/CEJ.0b013e328353edcb. [DOI] [PubMed] [Google Scholar]
- 161.Papoutsis AJ, Lamore SD, Wondrak GT, Selmin OI, Romagnolo DF. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J Nutr. 2010;140(9):1607–1614. doi: 10.3945/jn.110.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fustier P, Le Corre L, Chalabi N, Vissac-Sabatier C, Communal Y, Bignon YJ, et al. Resveratrol increases BRCA1 and BRCA2 mRNA expression in breast tumour cell lines. Br J Cancer. 2003;89(1):168–172. doi: 10.1038/sj.bjc.6600983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ferry-Dumazet H, Garnier O, Mamani-Matsuda M, Vercauteren J, Belloc F, Billiard C, et al. Resveratrol inhibits the growth and induces the apoptosis of both normal and leukemic hematopoietic cells. Carcinogenesis. 2002;23(8):1327–1333. doi: 10.1093/carcin/23.8.1327. [DOI] [PubMed] [Google Scholar]
- 164.Fujimoto A, Sakanashi Y, Matsui H, Oyama T, Nishimura Y, Masuda T, et al. Cytometric analysis of cytotoxicity of polyphenols and related phenolics to rat thymocytes: potent cytotoxicity of resveratrol to normal cells. Basic Clin Pharmacol Toxicol. 2009;104(6):455–462. doi: 10.1111/j.1742-7843.2009.00386.x. [DOI] [PubMed] [Google Scholar]
- 165.Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, et al. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One. 2012;7(5):e37162. doi: 10.1371/journal.pone.0037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang X, Ma S, Meng N, Yao N, Zhang K, Li Q, et al. Resveratrol exerts dosage-dependent effects on the self-renewal and neural differentiation of hUC-MSCs. Mol Cell. 2016;39(5):418–425. doi: 10.14348/molcells.2016.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kumar V, Pandey A, Jahan S, Shukla RK, Kumar D, Srivastava A, et al. Differential responses of trans-resveratrol on proliferation of neural progenitor cells and aged rat hippocampal neurogenesis. Sci Rep. 2016;6:28142. doi: 10.1038/srep28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kornienko JS, Smirnova IS, Pugovkina NA, Ivanova JS, Shilina MA, Grinchuk TM, et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci Rep. 2019;9(1):1296. doi: 10.1038/s41598-018-37972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Della Ragione F, Cucciolla V, Borriello A, Della Pietra V, Racioppi L, Soldati G, et al. Resveratrol arrests the cell division cycle at S/G2 phase transition. Biochem Bioph Res Co. 1998;250(1):53–58. doi: 10.1006/bbrc.1998.9263. [DOI] [PubMed] [Google Scholar]
- 170.Sui XX, Zhang CJ, Zhou JN, Cao SX, Xu C, Tang F, et al. Resveratrol inhibits extranodal NK/T cell lymphoma through activation of DNA damage response pathway. J Exp Clin Cancer Res. 2017;36:133. doi: 10.1186/s13046-017-0601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mancuso R, del Valle J, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Nunez O, et al. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics. 2014;11(2):419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Naia L, Rosenstock TR, Oliveira AM, Oliveira-Sousa SI, Caldeira GL, Carmo C, et al. Comparative mitochondrial-based protective effects of resveratrol and nicotinamide in Huntington's disease models. Mol Neurobiol. 2017;54(7):5385–5399. doi: 10.1007/s12035-016-0048-3. [DOI] [PubMed] [Google Scholar]
- 173.Ho DJ, Calingasan NY, Wille E, Dumont M, Beal MF. Resveratrol protects against peripheral deficits in a mouse model of Huntington's disease. Exp Neurol. 2010;225(1):74–84. doi: 10.1016/j.expneurol.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 174.Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, et al. Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer's disease neurons. J Alzheimers Dis. 2010;20(Suppl 2):S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Srinivasan S, Nwachukwu JC, Parent AA, Cavett V, Nowak J, Hughes TS, et al. Ligand-binding dynamics rewire cellular signaling via estrogen receptor-alpha. Nat Chem Biol. 2013;9(5):326–332. doi: 10.1038/nchembio.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yanez M, Jhanji M, Murphy K, Gower RM, Sajish M, Jabbarzadeh E. Nicotinamide augments the anti-inflammatory properties of resveratrol through PARP1 activation. Sci Rep-Uk. 2019;9:10219. doi: 10.1038/s41598-019-46678-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Basly JP, Marre-Fournier F, Le Bail JC, Habrioux G, Chulia AJ. Estrogenic/antiestrogenic and scavenging properties of (E)- and (Z)-resveratrol. Life Sci. 2000;66(9):769–777. doi: 10.1016/s0024-3205(99)00650-5. [DOI] [PubMed] [Google Scholar]
- 178.Kipp JL, Ramirez VD. Effect of estradiol, diethylstilbestrol, and resveratrol on F0F1-ATPase activity from mitochondrial preparations of rat heart, liver, and brain. Endocrine. 2001;15(2):165–175. doi: 10.1385/ENDO:15:2:165. [DOI] [PubMed] [Google Scholar]
- 179.Madreiter-Sokolowski CT, Sokolowski AA, Graier WF. Dosis Facit Sanitatem-concentration-dependent effects of resveratrol on mitochondria. Nutrients. 2017;9(10). 10.3390/nu9101117. [DOI] [PMC free article] [PubMed]
- 180.Bosutti A, Degens H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci Rep-Uk. 2015;5:8093. doi: 10.1038/srep08093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pignitter M, Schueller K, Burkon A, Knorr V, Esefelder L, Doberer D, et al. Concentration-dependent effects of resveratrol and metabolites on the redox status of human erythrocytes in single-dose studies. J Nutr Biochem. 2016;27:164–170. doi: 10.1016/j.jnutbio.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 182.Ahmad KA, Clement MV, Hanif IM, Pervaiz S. Resveratrol inhibits drug-induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 2004;64(4):1452–1459. doi: 10.1158/0008-5472.can-03-2414. [DOI] [PubMed] [Google Scholar]
- 183.Guha P, Dey A, Chatterjee A, Chattopadhyay S, Bandyopadhyay SK. Pro-ulcer effects of resveratrol in mice with indomethacin-induced gastric ulcers are reversed by L-arginine. Br J Pharmacol. 2010;159(3):726–734. doi: 10.1111/j.1476-5381.2009.00572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu S, Zhao M, Zhou Y, Wang C, Yuan Y, Li L, et al. Resveratrol exerts dose-dependent anti-fibrotic or pro-fibrotic effects in kidneys: a potential risk to individuals with impaired kidney function. Phytomedicine. 2019;57:223–235. doi: 10.1016/j.phymed.2018.12.024. [DOI] [PubMed] [Google Scholar]
- 185.Martins LA, Coelho BP, Behr G, Pettenuzzo LF, Souza IC, Moreira JC, et al. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem Biophys. 2014;68(2):247–257. doi: 10.1007/s12013-013-9703-8. [DOI] [PubMed] [Google Scholar]
- 186.Souza IC, Martins LA, Coelho BP, Grivicich I, Guaragna RM, Gottfried C, et al. Resveratrol inhibits cell growth by inducing cell cycle arrest in activated hepatic stellate cells. Mol Cell Biochem. 2008;315(1–2):1–7. doi: 10.1007/s11010-008-9781-x. [DOI] [PubMed] [Google Scholar]
- 187.Zhang JD. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/Bj20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li S, Bouzar C, Cottet-Rousselle C, Zagotta I, Lamarche F, Wabitsch M, et al. Resveratrol inhibits lipogenesis of 3T3-L1 and SGBS cells by inhibition of insulin signaling and mitochondrial mass increase. Biochim Biophys Acta. 2016;1857(6):643–652. doi: 10.1016/j.bbabio.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 189.Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, et al. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2012;61(3):424–433. doi: 10.1016/j.metabol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 190.Jimenez-Gomez Y, Mattison JA, Pearson KJ, Martin-Montalvo A, Palacios HH, Sossong AM, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18(4):533–545. doi: 10.1016/j.cmet.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chedea VS, Vicas SI, Sticozzi C, Pessina F, Frosini M, Maioli E, et al. Resveratrol: from diet to topical usage. Food Funct. 2017;8(11):3879–3892. doi: 10.1039/c7fo01086a. [DOI] [PubMed] [Google Scholar]
- 192.Robinson K, Mock C, Liang D. Pre-formulation studies of resveratrol. Drug Dev Ind Pharm. 2015;41(9):1464–1469. doi: 10.3109/03639045.2014.958753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sergides C, Chirila M, Silvestro L, Pitta D, Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp Ther Med. 2016;11(1):164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang S, Wang Z, Yang S, Yin T, Zhang Y, Qin Y, et al. Tissue distribution of trans-resveratrol and its metabolites after oral administration in human eyes. J Ophthalmol. 2017;2017:4052094. doi: 10.1155/2017/4052094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fernandez-Castillejo S, Macia A, Motilva MJ, Catalan U, Sola R. Endothelial cells deconjugate resveratrol metabolites to free resveratrol: a possible role in tissue factor modulation. Mol Nutr Food Res. 2019;63(3):e1800715. doi: 10.1002/mnfr.201800715. [DOI] [PubMed] [Google Scholar]
- 196.Shukla Y, Singh R. Resveratrol and cellular mechanisms of cancer prevention. Ann N Y Acad Sci. 2011;1215:1–8. doi: 10.1111/j.1749-6632.2010.05870.x. [DOI] [PubMed] [Google Scholar]
- 197.Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, et al. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One. 2008;3(6):e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pirola L, Frojdo S. Resveratrol: one molecule, many targets. IUBMB Life. 2008;60(5):323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 200.Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148(3):421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Um JH, Park SJ, Kang H, Yang S, Foretz M, McBurney MW, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285(11):8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74(6):619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 205.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280(17):17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 206.Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339(6124):1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sinclair DA, Guarente L. Small-molecule allosteric activators of sirtuins. Annu Rev Pharmacol Toxicol. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bo S, Togliatto G, Gambino R, Ponzo V, Lombardo G, Rosato R, et al. Impact of sirtuin-1 expression on H3K56 acetylation and oxidative stress: a double-blind randomized controlled trial with resveratrol supplementation. Acta Diabetol. 2018;55(4):331–340. doi: 10.1007/s00592-017-1097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21(20):2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Das A, Huang GX, Bonkowski MS, Longchamp A, Li C, Schultz MB, et al. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang M, Li W, Yu L, Wu S. The suppressive effect of resveratrol on HIF-1alpha and VEGF expression after warm ischemia and reperfusion in rat liver. PLoS One. 2014;9(10):e109589. doi: 10.1371/journal.pone.0109589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yu HB, Zhang HF, Zhang XA, Li DY, Xue HZ, Pan CE, et al. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa-B mediated mechanism. Hepato-Gastroenterol. 2010;57(102–03):1241–1246. [PubMed] [Google Scholar]
- 214.Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, et al. Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell. 2008;14(5):661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, et al. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12(1):51–62. doi: 10.1016/S1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 216.Gurd BJ, Yoshida Y, Lally J, Holloway GP, Bonen A. The deacetylase enzyme SIRT1 is not associated with oxidative capacity in rat heart and skeletal muscle and its overexpression reduces mitochondrial biogenesis. J Physiol-London. 2009;587(8):1817–1828. doi: 10.1113/jphysiol.2008.168096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Boutant M, Kulkarni SS, Joffraud M, Raymond F, Metairon S, Descombes P, et al. SIRT1 gain of function does not mimic or enhance the adaptations to intermittent fasting. Cell Rep. 2016;14(9):2068–2075. doi: 10.1016/j.celrep.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 218.Montesano A, Luzi L, Senesi P, Mazzocchi N, Terruzzi I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J Transl Med. 2013;11:310. doi: 10.1186/1479-5876-11-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kaminski J, Lancon A, Aires V, Limagne E, Tili E, Michaille JJ, et al. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem Pharmacol. 2012;84(10):1251–1259. doi: 10.1016/j.bcp.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 220.Momken I, Stevens L, Bergouignan A, Desplanches D, Rudwill F, Chery I, et al. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25(10):3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 221.Ng F, Tang BL. When is Sirt1 activity bad for dying neurons? Front Cell Neurosci. 2013;7:186. doi: 10.3389/fncel.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Li Y, Xu W, McBurney MW, Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8(1):38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Prozorovski T, Schulze-Topphoff U, Glumm R, Baumgart J, Schroter F, Ninnemann O, et al. Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol. 2008;10(4):385–394. doi: 10.1038/ncb1700. [DOI] [PubMed] [Google Scholar]
- 224.Wang R, Liu YY, Liu XY, Jia SW, Zhao J, Cui D, et al. Resveratrol protects neurons and the myocardium by reducing oxidative stress and ameliorating mitochondria damage in a cerebral ischemia rat model. Cell Physiol Biochem. 2014;34(3):854–864. doi: 10.1159/000366304. [DOI] [PubMed] [Google Scholar]
- 225.Renaud J, Bournival J, Zottig X, Martinoli MG. Resveratrol protects DAergic PC12 cells from high glucose-induced oxidative stress and apoptosis: effect on p53 and GRP75 localization. Neurotox Res. 2014;25(1):110–123. doi: 10.1007/s12640-013-9439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Torres-Perez M, Tellez-Ballesteros RI, Ortiz-Lopez L, Ichwan M, Vega-Rivera NM, Castro-Garcia M, et al. Resveratrol enhances neuroplastic changes, including hippocampal neurogenesis, and memory in Balb/C mice at six months of age. PLoS One. 2015;10(12):e0145687. doi: 10.1371/journal.pone.0145687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Liu L, Zhang Q, Cai Y, Sun D, He X, Wang L, et al. Resveratrol counteracts lipopolysaccharide-induced depressive-like behaviors via enhanced hippocampal neurogenesis. Oncotarget. 2016;7(35):56045–56059. doi: 10.18632/oncotarget.11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Iwata R, Casimir P, Vanderhaeghen P. Mitochondrial dynamics in postmitotic cells regulate neurogenesis. Science. 2020;369(6505):858–862. doi: 10.1126/science.aba9760. [DOI] [PubMed] [Google Scholar]
- 229.Yang JN, Zhou X, Zeng XL, Hu O, Yi L, Mi MT. Resveratrol attenuates oxidative injury in human umbilical vein endothelial cells through regulating mitochondrial fusion via TyrRS-PARP1 pathway. Nutr Metab. 2019;16. 9 10.1186/s12986-019-0338-7. [DOI] [PMC free article] [PubMed]
- 230.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147(7):1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bhandari R, Kuhad A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem Int. 2017;103:8–23. doi: 10.1016/j.neuint.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 232.Ahmad SF, Ansari MA, Nadeem A, Alzahrani MZ, Bakheet SA, Attia SM. Resveratrol improves Neuroimmune dysregulation through the inhibition of neuronal toll-like receptors and COX-2 signaling in BTBR T+ Itpr3(tf)/J mice. NeuroMolecular Med. 2018;20(1):133–146. doi: 10.1007/s12017-018-8483-0. [DOI] [PubMed] [Google Scholar]
- 233.Ahmad SF, Ansari MA, Nadeem A, Bakheet SA, Alzahrani MZ, Alshammari MA, et al. Resveratrol attenuates pro-inflammatory cytokines and activation of JAK1-STAT3 in BTBR T(+) Itpr3(tf)/J autistic mice. Eur J Pharmacol. 2018;829:70–78. doi: 10.1016/j.ejphar.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 234.Bakheet SA, Alzahrani MZ, Ansari MA, Nadeem A, Zoheir KMA, Attia SM, et al. Resveratrol ameliorates dysregulation of Th1, Th2, Th17, and T regulatory cell-related transcription factor signaling in a BTBR T + tf/J mouse model of autism. Mol Neurobiol. 2017;54(7):5201–5212. doi: 10.1007/s12035-016-0066-1. [DOI] [PubMed] [Google Scholar]
- 235.Zhou CX, Kong LD, Ye WC, Cheng CH, Tan RX. Inhibition of xanthine and monoamine oxidases by stilbenoids from Veratrum taliense. Planta Med. 2001;67(2):158–161. doi: 10.1055/s-2001-11500. [DOI] [PubMed] [Google Scholar]
- 236.Yanez M, Fraiz N, Cano E, Orallo F. Inhibitory effects of cis- and trans-resveratrol on noradrenaline and 5-hydroxytryptamine uptake and on monoamine oxidase activity. Biochem Biophys Res Commun. 2006;344(2):688–695. doi: 10.1016/j.bbrc.2006.03.190. [DOI] [PubMed] [Google Scholar]
- 237.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4(5):295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang LF, Yu XL, Ji M, Liu SY, Wu XL, Wang YJ, et al. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T alpha-synuclein mouse model of Parkinson's disease. Food Funct. 2018;9(12):6414–6426. doi: 10.1039/c8fo00964c. [DOI] [PubMed] [Google Scholar]
- 239.Smith MR, Syed A, Lukacsovich T, Purcell J, Barbaro BA, Worthge SA, et al. A potent and selective Sirtuin 1 inhibitor alleviates pathology in multiple animal and cell models of Huntington's disease. Hum Mol Genet. 2014;23(11):2995–3007. doi: 10.1093/hmg/ddu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007;317(5837):516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 241.Romeo-Guitart D, Leiva-Rodriguez T, Casas C. SIRT2 inhibition improves functional motor recovery after peripheral nerve injury. Neurotherapeutics. 2020. 10.1007/s13311-020-00860-3. [DOI] [PMC free article] [PubMed]
- 242.Vaziri H, Dessain SK, Eagon EN, Imai SI, Frye RA, Pandita TK, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107(2):149–159. doi: 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- 243.Huang CS, Ma WY, Goranson A, Dong ZG. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis. 1999;20(2):237–242. doi: 10.1093/carcin/20.2.237. [DOI] [PubMed] [Google Scholar]
- 244.Munoz-Fontela C, Gonzalez D, Marcos-Villar L, Campagna M, Gallego P, Gonzalez-Santamaria J, et al. Acetylation is indispensable for p53 antiviral activity. Cell Cycle. 2011;10(21):3701–3705. doi: 10.4161/cc.10.21.17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ding Z, Cao J, Shen Y, Zou Y, Yang X, Zhou W, et al. Resveratrol promotes nerve regeneration via activation of p300 acetyltransferase-mediated VEGF signaling in a rat model of sciatic nerve crush injury. Front Neurosci. 2018;12:341. doi: 10.3389/fnins.2018.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Guarente L. Cell metabolism The resurgence of NAD(+) Science. 2016;352(6292):1396–1397. doi: 10.1126/science.aag1718. [DOI] [PubMed] [Google Scholar]
- 248.Goldberg DM, Karumanchiri A, Ng E, Yan J, Diamandis EP, Soleas GJ. Direct gas-chromatographic mass-spectrometric method to assay Cis-resveratrol in wines - preliminary survey of its concentration in commercial wines. J Agric Food Chem. 1995;43(5):1245–1250. doi: 10.1021/jf00053a023. [DOI] [Google Scholar]
- 249.Cvejic JM, Djekic SV, Petrovic AV, Atanackovic MT, Jovic SM, Brceski ID, et al. Determination of trans- and cis-resveratrol in Serbian commercial wines. J Chromatogr Sci. 2010;48(3):229–234. doi: 10.1093/chromsci/48.3.229. [DOI] [PubMed] [Google Scholar]
- 250.RomeroPerez AI, LamuelaRaventos RM, Waterhouse AL, delaTorreBoronat MC. Levels of cis- and trans-resveratrol and their glucosides in white and rose Vitis vinifera wines from Spain. J Agric Food Chem. 1996;44(8):2124–2128. doi: 10.1021/jf9507654. [DOI] [Google Scholar]
- 251.Huang TT, Lai HC, Chen YB, Chen LG, Wu YH, Ko YF, et al. cis-Resveratrol produces anti-inflammatory effects by inhibiting canonical and non-canonical inflammasomes in macrophages. Innate Immun. 2014;20(7):735–750. doi: 10.1177/1753425913507096. [DOI] [PubMed] [Google Scholar]
- 252.Leiro J, Alvarez E, Arranz JA, Laguna R, Uriarte E, Orallo F. Effects of cis-resveratrol on inflammatory murine macrophages: antioxidant activity and down-regulation of inflammatory genes. J Leukoc Biol. 2004;75(6):1156–1165. doi: 10.1189/jlb.1103561. [DOI] [PubMed] [Google Scholar]
- 253.Leiro J, Arranz JA, Fraiz N, Sanmartin ML, Quezada E, Orallo F. Effect of cis-resveratrol on genes involved in nuclear factor kappa B signaling. Int Immunopharmacol. 2005;5(2):393–406. doi: 10.1016/j.intimp.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 254.Morris VL, Toseef T, Nazumudeen FB, Rivoira C, Spatafora C, Tringali C, et al. Anti-tumor properties of cis-resveratrol methylated analogs in metastatic mouse melanoma cells. Mol Cell Biochem. 2015;402(1–2):83–91. doi: 10.1007/s11010-014-2316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Pettit GR, Grealish MP, Jung MK, Hamel E, Pettit RK, Chapuis JC, et al. Antineoplastic agents. 465. Structural modification of resveratrol: Sodium resverastatin phosphate. J Med Chem. 2002;45(12):2534–2542. doi: 10.1021/jm010119y. [DOI] [PubMed] [Google Scholar]
- 256.Campos-Toimil M, Elies J, Orallo F. Trans- and cis-resveratrol increase cytoplasmic calcium levels in A7r5 vascular smooth muscle cells. Mol Nutr Food Res. 2005;49(5):396–404. doi: 10.1002/mnfr.200400108. [DOI] [PubMed] [Google Scholar]
- 257.Campos-Toimil M, Elies J, Alvarez E, Verde I, Orallo F. Effects of trans- and cis-resveratrol on Ca2+ handling in A7r5 vascular myocytes. Eur J Pharmacol. 2007;577(1–3):91–99. doi: 10.1016/j.ejphar.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 258.Rius C, Abu-Taha M, Hermenegildo C, Piqueras L, Cerda-Nicolas JM, Issekutz AC, et al. Trans- but not Cis-resveratrol impairs angiotensin-II-mediated vascular inflammation through inhibition of NF-kappa B activation and peroxisome proliferator-activated receptor-gamma upregulation. J Immunol. 2010;185(6):3718–3727. doi: 10.4049/jimmunol.1001043. [DOI] [PubMed] [Google Scholar]
- 259.Bertelli AA, Giovannini L, Bernini W, Migliori M, Fregoni M, Bavaresco L, et al. Antiplatelet activity of cis-resveratrol. Drugs Exp Clin Res. 1996;22(2):61–63. [PubMed] [Google Scholar]
- 260.Kim H, Oh SJ, Liu Y, Lee MY. A comparative study of the anti-platelet effects of cis- and trans-resveratrol. Biomol Ther. 2011;19(2):201–205. doi: 10.4062/biomolther.2011.19.2.201. [DOI] [Google Scholar]
- 261.Orallo F. Comparative studies of the antioxidant effects of cis- and trans-resveratrol. Curr Med Chem. 2006;13(1):87–98. doi: 10.2174/092986706775197962. [DOI] [PubMed] [Google Scholar]
- 262.Aumont V, Krisa S, Battaglia E, Netter P, Richard T, Merillon JM, et al. Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch Biochem Biophys. 2001;393(2):281–289. doi: 10.1006/abbi.2001.2496. [DOI] [PubMed] [Google Scholar]
- 263.Sabolovic N, Humbert AC, Radominska-Pandya A, Magdalou J. Resveratrol is efficiently glucuronidated by UDP-glucuronosyltransferases in the human gastrointestinal tract and in Caco-2 cells. Biopharm Drug Dispos. 2006;27(4):181–189. doi: 10.1002/bdd.498. [DOI] [PubMed] [Google Scholar]
- 264.Iwuchukwu OF. Nagar S. Cis-resveratrol glucuronidation kinetics in human and recombinant UGT1A sources. Xenobiotica. 2010;40(2):102–108. doi: 10.3109/00498250903406754. [DOI] [PubMed] [Google Scholar]
- 265.Urpi-Sarda M, Zamora-Ros R, Lamuela-Raventos R, Cherubini A, Jauregui O, de la Torre R, et al. HPLC-tandem mass spectrometric method to characterize resveratrol metabolism in humans. Clin Chem. 2007;53(2):292–299. doi: 10.1373/clinchem.2006.071936. [DOI] [PubMed] [Google Scholar]
- 266.Sabolovic N, Heurtaux T, Humbert AC, Krisa S, Magdalou J. cis- and trans-resveratrol are glucuronidated in rat brain, olfactory mucosa and cultured astrocytes. Pharmacology. 2007;80(2–3):185–192. doi: 10.1159/000104149. [DOI] [PubMed] [Google Scholar]
- 267.Ibba M, Soll D. Aminoacyl-tRNA synthesis. Annu Rev Biochem. 2000;69:617–650. doi: 10.1146/annurev.biochem.69.1.617. [DOI] [PubMed] [Google Scholar]
- 268.Guo M, Schimmel P. Essential nontranslational functions of tRNA synthetases. Nat Chem Biol. 2013;9(3):145–53. doi: 10.1038/nchembio.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Anderson LL, Mao XR, Scott BA, Crowder CM. Survival from hypoxia in C-elegans by inactivation of aminoacyl-tRNA Synthetases. Science. 2009;323(5914):630–633. doi: 10.1126/science.1166175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Suh YS, Yeom E, Nam JW, Min KJ, Lee J, Yu K. Methionyl-tRNA synthetase regulates lifespan in Drosophila. Mol Cell. 2020;43(3):304–311. doi: 10.14348/molcells.2019.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Guo M, Yang XL, Schimmel P. New functions of aminoacyl-tRNA synthetases beyond translation. Nat Rev Mol Cell Biol. 2010;11(9):668–674. doi: 10.1038/nrm2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Sajish M, Zhou Q, Kishi S, Valdez DM, Jr, Kapoor M, Guo M, et al. Trp-tRNA synthetase bridges DNA-PKcs to PARP-1 to link IFN-gamma and p53 signaling. Nat Chem Biol. 2012;8(6):547–554. doi: 10.1038/nchembio.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lo WS, Gardiner E, Xu Z, Lau CF, Wang F, Zhou JJ, et al. Human tRNA synthetase catalytic nulls with diverse functions. Science. 2014;345(6194):328–332. doi: 10.1126/science.1252943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tillin T, Hughes AD, Wang Q, Wurtz P, Ala-Korpela M, Sattar N, et al. Diabetes risk and amino acid profiles: cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall and Brent REvisited) study. Diabetologia. 2015;58(5):968–979. doi: 10.1007/s00125-015-3517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Hellmuth C, Kirchberg FF, Lass N, Harder U, Peissner W, Koletzko B et al. Tyrosine is associated with insulin resistance in longitudinal metabolomic profiling of obese children. J Diabetes Res. 2016. 2108909 10.1155/2016/2108909. [DOI] [PMC free article] [PubMed]
- 277.Hellmuth C, Kirchberg FF, Brandt S, Moss A, Walter V, Rothenbacher D, et al. An individual participant data meta-analysis on metabolomics profiles for obesity and insulin resistance in European children. Sci Rep. 2019;9(1):5053. doi: 10.1038/s41598-019-41449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Moran-Ramos S, Ocampo-Medina E, Gutierrez-Aguilar R, Macias-Kauffer L, Villamil-Ramirez H, Lopez-Contreras BE, et al. An amino acid signature associated with obesity predicts 2-year risk of hypertriglyceridemia in school-age children. Sci Rep. 2017;7(1):5607. doi: 10.1038/s41598-017-05765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Li J, Cao YF, Sun XY, Han L, Li SN, Gu WQ, et al. Plasma tyrosine and its interaction with low high-density lipoprotein cholesterol and the risk of type 2 diabetes mellitus in Chinese. J Diabetes Investig. 2019;10(2):491–498. doi: 10.1111/jdi.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and Lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. 1969;281(15):811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- 282.Kawanaka M, Nishino K, Oka T, Urata N, Nakamura J, Suehiro M, et al. Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepat Med. 2015;7:29–35. doi: 10.2147/HMER.S79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Leichtle AB, Nuoffer JM, Ceglarek U, Kase J, Conrad T, Witzigmann H, et al. Serum amino acid profiles and their alterations in colorectal cancer. Metabolomics. 2012;8(4):643–653. doi: 10.1007/s11306-011-0357-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20(10):1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sajish M, Schimmel P. A human tRNA synthetase is a potent PARP1-activating effector target for resveratrol. Nature. 2015;519(7543):370–373. doi: 10.1038/nature14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Gledhill JR, Montgomery MG, Leslie AGW, Walker JE. Mechanism of inhibition of bovine F-1-ATPase by resveratrol and related polyphenols. P Natl Acad Sci USA. 2007;104(34):13632–13637. doi: 10.1073/pnas.0706290104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nguyen GT, Gertz M, Steegborn C. Crystal structures of Sirt3 complexes with 4′-bromo-resveratrol reveal binding sites and inhibition mechanism. Chem Biol. 2013;20(11):1375–1385. doi: 10.1016/j.chembiol.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 288.Calamini B, Ratia K, Malkowski MG, Cuendet M, Pezzuto JM, Santarsiero BD, et al. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem J. 2010;429(2):273–282. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Jordanova A, Irobi J, Thomas FP, Van Dijck P, Meerschaert K, Dewil M, et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet. 2006;38(2):197–202. doi: 10.1038/ng1727. [DOI] [PubMed] [Google Scholar]
- 290.Nowaczyk MJ, Huang L, Tarnopolsky M, Schwartzentruber J, Majewski J, Bulman DE, et al. A novel multisystem disease associated with recessive mutations in the tyrosyl-tRNA synthetase (YARS) gene. Am J Med Genet A. 2017;173(1):126–134. doi: 10.1002/ajmg.a.37973. [DOI] [PubMed] [Google Scholar]
- 291.Williams KB, Brigatti KW, Puffenberger EG, Gonzaga-Jauregui C, Griffin LB, Martinez ED, et al. Homozygosity for a mutation affecting the catalytic domain of tyrosyl-tRNA synthetase (YARS) causes multisystem disease. Hum Mol Genet. 2019;28(4):525–538. doi: 10.1093/hmg/ddy344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Tracewska-Siemiatkowska A, Haer-Wigman L, Bosch DGM, Nickerson D, Bamshad MJ, University of Washington Center for Mendelian G, et al. An expanded multi-organ disease phenotype associated with mutations in YARS. Genes (Basel). 2017;8(12). 10.3390/genes8120381. [DOI] [PMC free article] [PubMed]
- 293.Blocquel D, Li S, Wei N, Daub H, Sajish M, Erfurth ML, et al. Alternative stable conformation capable of protein misinteraction links tRNA synthetase to peripheral neuropathy. Nucleic Acids Res. 2017;45(13):8091–8104. doi: 10.1093/nar/gkx455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Fu G, Xu T, Shi Y, Wei N, Yang XL. tRNA-controlled nuclear import of a human tRNA synthetase. J Biol Chem. 2012;287(12):9330–9334. doi: 10.1074/jbc.C111.325902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Wei N, Shi Y, Truong LN, Fisch KM, Xu T, Gardiner E, et al. Oxidative stress diverts tRNA synthetase to nucleus for protection against DNA damage. Mol Cell. 2014;56(2):323–332. doi: 10.1016/j.molcel.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cao X, Li C, Xiao S, Tang Y, Huang J, Zhao S, et al. Acetylation promotes TyrRS nuclear translocation to prevent oxidative damage. Proc Natl Acad Sci U S A. 2017;114(4):687–692. doi: 10.1073/pnas.1608488114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Jobin PG, Solis N, Machado Y, Bell PA, Rai SK, Kwon NH, et al. Moonlighting matrix metalloproteinase substrates: enhancement of proinflammatory functions of extracellular tyrosyl-tRNA synthetase upon cleavage. J Biol Chem. 2020;295(8):2186–2202. doi: 10.1074/jbc.RA119.010486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen J, Bai Q, Zhao Z, Sui H, Xie X. Resveratrol improves delayed r-tPA treatment outcome by reducing MMPs. Acta Neurol Scand. 2016;134(1):54–60. doi: 10.1111/ane.12511. [DOI] [PubMed] [Google Scholar]
- 299.Pandey AK, Bhattacharya P, Shukla SC, Paul S, Patnaik R. Resveratrol inhibits matrix metalloproteinases to attenuate neuronal damage in cerebral ischemia: a molecular docking study exploring possible neuroprotection. Neural Regen Res. 2015;10(4):568–575. doi: 10.4103/1673-5374.155429. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 300.Nakamoto MY, Rudolph J, Wuttke DS, Luger K. Nonspecific binding of RNA to PARP1 and PARP2 does not Lead to catalytic activation. Biochemistry-Us. 2019;58(51):5107–5111. doi: 10.1021/acs.biochem.9b00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18(10):610–621. doi: 10.1038/nrm.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Strom CE, Johansson F, Uhlen M, Szigyarto CA, Erixon K, Helleday T. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 2011;39(8):3166–3175. doi: 10.1093/nar/gkq1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Caron MC, Sharma AK, O'Sullivan J, Myler LR, Ferreira MT, Rodrigue A, et al. Poly(ADP-ribose) polymerase-1 antagonizes DNA resection at double-strand breaks. Nat Commun. 2019;10:2954. doi: 10.1038/s41467-019-10741-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Satoh MS, Lindahl T. Role of poly(ADP-ribose) formation in DNA repair. Nature. 1992;356(6367):356–358. doi: 10.1038/356356a0. [DOI] [PubMed] [Google Scholar]
- 305.Bilokapic S, Suskiewicz MJ, Ahel I, Halic M. Bridging of DNA breaks activates PARP2-HPF1 to modify chromatin. Nature. 2020. 10.1038/s41586-020-2725-7. [DOI] [PMC free article] [PubMed]
- 306.Wilk A, Hayat F, Cunningham R, Li J, Garavaglia S, Zamani L, et al. Extracellular NAD(+) enhances PARP-dependent DNA repair capacity independently of CD73 activity. Sci Rep. 2020;10(1):651. doi: 10.1038/s41598-020-57506-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Roberts MN, Wallace MA, Tomilov AA, Zhou Z, Marcotte GR, Tran D, et al. A ketogenic diet extends longevity and Healthspan in adult mice. Cell Metab. 2017;26(3):539–46 e5. doi: 10.1016/j.cmet.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Suskiewicz MJ, Zobel F, Ogden TEH, Fontana P, Ariza A, Yang JC, et al. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature. 2020;579(7800):598–602. doi: 10.1038/s41586-020-2013-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Zandarashvili L, Langelier MF, Velagapudi UK, Hancock MA, Steffen JD, Billur R, et al. Structural basis for allosteric PARP-1 retention on DNA breaks. Science. 2020;368(6486). 10.1126/science.aax6367. [DOI] [PMC free article] [PubMed]
- 310.Langelier MF, Planck JL, Roy S, Pascal JM. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science. 2012;336(6082):728–732. doi: 10.1126/science.1216338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Gibson BA, Zhang Y, Jiang H, Hussey KM, Shrimp JH, Lin H, et al. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science. 2016;353(6294):45–50. doi: 10.1126/science.aaf7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.de Murcia JM, Niedergang C, Trucco C, Ricoul M, Dutrillaux B, Mark M, et al. Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc Natl Acad Sci U S A. 1997;94(14):7303–7307. doi: 10.1073/pnas.94.14.7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Ishizuka S, Martin K, Booth C, Potten CS, de Murcia G, Burkle A, et al. Poly(ADP-ribose) polymerase-1 is a survival factor for radiation-exposed intestinal epithelial stem cells in vivo. Nucleic Acids Res. 2003;31(21):6198–6205. doi: 10.1093/nar/gkg840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Robu M, Shah RG, Purohit NK, Zhou P, Naegeli H, Shah GM. Poly(ADP-ribose) polymerase 1 escorts XPC to UV-induced DNA lesions during nucleotide excision repair. Proc Natl Acad Sci U S A. 2017;114(33):E6847–E6E56. doi: 10.1073/pnas.1706981114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Schuhwerk H, Bruhn C, Siniuk K, Min W, Erener S, Grigaravicius P, et al. Kinetics of poly(ADP-ribosyl)ation, but not PARP1 itself, determines the cell fate in response to DNA damage in vitro and in vivo. Nucleic Acids Res. 2017;45(19):11174–11192. doi: 10.1093/nar/gkx717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Chen JK, Lin WL, Chen Z, Liu HW. PARP-1-dependent recruitment of cold-inducible RNA-binding protein promotes double-strand break repair and genome stability. Proc Natl Acad Sci U S A. 2018;115(8):E1759–E1E68. doi: 10.1073/pnas.1713912115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Ronson GE, Piberger AL, Higgs MR, Olsen AL, Stewart GS, McHugh PJ, et al. PARP1 and PARP2 stabilise replication forks at base excision repair intermediates through Fbh1-dependent Rad51 regulation. Nat Commun. 2018;9:746. doi: 10.1038/s41467-018-03159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wright RHG, Lioutas A, Le Dily F, Soronellas D, Pohl A, Bonet J, et al. ADP-ribose-derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science. 2016;352(6290):1221–1225. doi: 10.1126/science.aad9335. [DOI] [PubMed] [Google Scholar]
- 319.Navarro J, Gozalbo-Lopez B, Mendez AC, Dantzer F, Schreiber V, Martinez C, et al. PARP-1/PARP-2 double deficiency in mouse T cells results in faulty immune responses and T lymphomas. Sci Rep. 2017;7:41962. doi: 10.1038/srep41962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Mashimo M, Kato J, Moss J. ADP-ribosyl-acceptor hydrolase 3 regulates poly (ADP-ribose) degradation and cell death during oxidative stress. P Natl Acad Sci USA. 2013;110(47):18964–18969. doi: 10.1073/pnas.1312783110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yu W, Ginjala V, Pant V, Chernukhin I, Whitehead J, Docquier F, et al. Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet. 2004;36(10):1105–10. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]
- 322.Zhao HL, Sifakis EG, Sumida N, Millan-Arino L, Scholz BA, Svensson JP, et al. PARP1-and CTCF-mediated interactions between active and repressed chromatin at the lamina promote oscillating transcription. Mol Cell. 2015;59(6):984–997. doi: 10.1016/j.molcel.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 323.Asher G, Reinke H, Altmeyer M, Gutierrez-Arcelus M, Hottiger MO, Schibler U. Poly(ADP-ribose) polymerase 1 participates in the phase entrainment of circadian clocks to feeding. Cell. 2010;142(6):943–953. doi: 10.1016/j.cell.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 324.Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119(6):803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 325.Galloway A, Adeluyi A, O'Donovan B, Fisher ML, Rao CN, Critchfield P, et al. Dopamine triggers CTCF-dependent morphological and genomic remodeling of astrocytes. J Neurosci. 2018. 10.1523/JNEUROSCI.3349-17.2018. [DOI] [PMC free article] [PubMed]
- 326.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Maya-Mendoza A, Moudry P, Merchut-Maya JM, Lee M, Strauss R, Bartek J. High speed of fork progression induces DNA replication stress and genomic instability. Nature. 2018;559(7713):279–284. doi: 10.1038/s41586-018-0261-5. [DOI] [PubMed] [Google Scholar]
- 328.Fischer JMF, Zubel T, Jander K, Fix J, Trussina I, Gebhard D, et al. PARP1 protects from benzo[a]pyrene diol epoxide-induced replication stress and mutagenicity. Arch Toxicol. 2018;92(3):1323–1340. doi: 10.1007/s00204-017-2115-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Cohen-Armon M, Visochek L, Katzoff A, Levitan D, Susswein AJ, Klein R, et al. Long-term memory requires polyADP-ribosylation. Science. 2004;304(5678):1820–1822. doi: 10.1126/science.1096775. [DOI] [PubMed] [Google Scholar]
- 330.Fontan-Lozano A, Suarez-Pereira I, Horrillo A, del-Pozo-Martin Y, Hmadcha A, Carrion AM. Histone H1 poly[ADP]-ribosylation regulates the chromatin alterations required for learning consolidation. J Neurosci. 2010;30(40):13305–13313. doi: 10.1523/JNEUROSCI.3010-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Inaba H, Tsukagoshi A, Kida S. PARP-1 activity is required for the reconsolidation and extinction of contextual fear memory. Mol Brain. 2015;8:63. doi: 10.1186/s13041-015-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Goldberg S, Visochek L, Giladi E, Gozes I, Cohen-Armon M. PolyADP-ribosylation is required for long-term memory formation in mammals. J Neurochem. 2009;111(1):72–79. doi: 10.1111/j.1471-4159.2009.06296.x. [DOI] [PubMed] [Google Scholar]
- 333.Midorikawa R, Takei Y, Hirokawa N. KIF4 motor regulates activity-dependent neuronal survival by suppressing PARP-1 enzymatic activity. Cell. 2006;125(2):371–383. doi: 10.1016/j.cell.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 334.Diaz-Hernandez JI, Moncada S, Bolanos JP, Almeida A. Poly(ADP-ribose) polymerase-1 protects neurons against apoptosis induced by oxidative stress. Cell Death Differ. 2007;14(6):1211–1221. doi: 10.1038/sj.cdd.4402117. [DOI] [PubMed] [Google Scholar]
- 335.Naumann M, Pal A, Goswami A, Lojewski X, Japtok J, Vehlow A, et al. Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat Commun. 2018;9(1):335. doi: 10.1038/s41467-017-02299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Doege CA, Inoue K, Yamashita T, Rhee DB, Travis S, Fujita R, et al. Early-stage epigenetic modification during somatic cell reprogramming by Parp1 and Tet2. Nature. 2012;488(7413):652–655. doi: 10.1038/nature11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Ju BG, Solum D, Song EJ, Lee KJ, Rose DW, Glass CK, et al. Activating the PARP-1 sensor component of the groucho/ TLE1 corepressor complex mediates a CaMKinase IIdelta-dependent neurogenic gene activation pathway. Cell. 2004;119(6):815–829. doi: 10.1016/j.cell.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 338.Plane JM, Grossenbacher SK, Deng W. PARP-1 deletion promotes subventricular zone neural stem cells toward a glial fate. J Neurosci Res. 2012;90(8):1489–1506. doi: 10.1002/jnr.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Devalaraja-Narashimha K, Padanilam BJ. PARP1 deficiency exacerbates diet-induced obesity in mice. J Endocrinol. 2010;205(3):243–252. doi: 10.1677/JOE-09-0402. [DOI] [PubMed] [Google Scholar]
- 340.Hong S, Yi JH, Lee S, Park CH, Ryu JH, Shin KS, et al. Defective neurogenesis and schizophrenia-like behavior in PARP-1-deficient mice. Cell Death Dis. 2019;10(12):943. doi: 10.1038/s41419-019-2174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wang SH, Liao XM, Liu D, Hu J, Yin YY, Wang JZ, et al. NGF promotes long-term memory formation by activating poly(ADP-ribose)polymerase-1. Neuropharmacology. 2012;63(6):1085–1092. doi: 10.1016/j.neuropharm.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 342.Cohen-Armon M, Visochek L, Rozensal D, Kalal A, Geistrikh I, Klein R, et al. DNA-independent PARP-1 activation by phosphorylated ERK2 increases Elk1 activity: a link to histone acetylation. Mol Cell. 2007;25(2):297–308. doi: 10.1016/j.molcel.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 343.Alano CC, Garnier P, Ying WH, Higashi Y, Kauppinen TM, Swanson RA. NAD(+) depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30(8):2967–2978. doi: 10.1523/Jneurosci.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Kamata H, Tanaka C, Yagisawa H, Hirata H. Nerve growth factor and forskolin prevent H2O2-induced apoptosis in PC12 cells by glutathione independent mechanism. Neurosci Lett. 1996;212(3):179–182. doi: 10.1016/0304-3940(96)12806-8. [DOI] [PubMed] [Google Scholar]
- 345.Satoh T, Sakai N, Enokido Y, Uchiyama Y, Hatanaka H. Free radical-independent protection by nerve growth factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered apoptosis. J Biochem. 1996;120(3):540–546. doi: 10.1093/oxfordjournals.jbchem.a021447. [DOI] [PubMed] [Google Scholar]
- 346.Ullrich O, Reinheckel T, Sitte N, Hass R, Grune T, Davies KJ. Poly-ADP ribose polymerase activates nuclear proteasome to degrade oxidatively damaged histones. Proc Natl Acad Sci U S A. 1999;96(11):6223–6228. doi: 10.1073/pnas.96.11.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Catalgol B, Wendt B, Grimm S, Breusing N, Ozer NK, Grune T. Chromatin repair after oxidative stress: role of PARP-mediated proteasome activation. Free Radic Biol Med. 2010;48(5):673–680. doi: 10.1016/j.freeradbiomed.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 348.Pieper AA, Blackshaw S, Clements EE, Brat DJ, Krug DK, White AJ, et al. Poly(ADP-ribosyl)ation basally activated by DNA strand breaks reflects glutamate-nitric oxide neurotransmission. Proc Natl Acad Sci U S A. 2000;97(4):1845–1850. doi: 10.1073/pnas.97.4.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Zeng JY, Libien J, Shaik F, Wolk J, Hernandez AI. Nucleolar PARP-1 Expression is decreased in Alzheimer's disease: consequences for epigenetic regulation of rDNA and cognition. Neural Plast. 2016; 8987928 10.1155/2016/8987928. [DOI] [PMC free article] [PubMed]
- 350.Grunewald ME, Chen YT, Kuny C, Maejima T, Lease R, Ferraris D, et al. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog. 2019;15(5):e1007756. doi: 10.1371/journal.ppat.1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Hopp AK, Gruter P, Hottiger MO. Regulation of glucose metabolism by NAD(+) and ADP-ribosylation. Cells. 2019;8(8). 10.3390/cells8080890. [DOI] [PMC free article] [PubMed]
- 352.Grube K, Burkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc Natl Acad Sci U S A. 1992;89(24):11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Muiras ML, Muller M, Schachter F, Burkle A. Increased poly(ADP-ribose) polymerase activity in lymphoblastoid cell lines from centenarians. J Mol Med (Berl) 1998;76(5):346–354. doi: 10.1007/s001090050226. [DOI] [PubMed] [Google Scholar]
- 354.Evdokimov A, Kutuzov M, Petruseva I, Lukjanchikova N, Kashina E, Kolova E, et al. Naked mole rat cells display more efficient excision repair than mouse cells. Aging-Us. 2018;10(6):1454–1473. doi: 10.18632/aging.101482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A, et al. Amyloid beta and the longest-lived rodent: the naked mole-rat as a model for natural protection from Alzheimer's disease. Neurobiol Aging. 2013;34(10):2352–2360. doi: 10.1016/j.neurobiolaging.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Ito S, Murphy CG, Doubrovina E, Jasin M, Moynahan ME. PARP inhibitors in clinical use induce genomic instability in normal human cells. PLoS One. 2016;11(7):e0159341. doi: 10.1371/journal.pone.0159341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hopkins TA, Ainsworth WB, Ellis PA, Donawho CK, DiGiammarino EL, Panchal SC, et al. PARP1 trapping by PARP inhibitors drives cytotoxicity in both cancer cells and healthy bone marrow. Mol Cancer Res. 2019;17(2):409–419. doi: 10.1158/1541-7786.Mcr-18-0138. [DOI] [PubMed] [Google Scholar]
- 358.Lapucci A, Pittelli M, Rapizzi E, Felici R, Moroni F, Chiarugi A. Poly(ADP-ribose) polymerase-1 is a nuclear epigenetic regulator of mitochondrial DNA repair and transcription. Mol Pharmacol. 2011;79(6):932–940. doi: 10.1124/mol.110.070110. [DOI] [PubMed] [Google Scholar]
- 359.Regdon Z, Robaszkiewicz A, Kovacs K, Rygielska Z, Hegedus C, Bodoor K, et al. LPS protects macrophages from AIF-independent parthanatos by downregulation of PARP1 expression, induction of SOD2 expression, and a metabolic shift to aerobic glycolysis. Free Radic Biol Med. 2019;131:184–196. doi: 10.1016/j.freeradbiomed.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 360.Fouquerel E, Goellner EM, Yu Z, Gagne JP, Barbi de Moura M, Feinstein T, et al. ARTD1/PARP1 negatively regulates glycolysis by inhibiting hexokinase 1 independent of NAD+ depletion. Cell Rep. 2014;8(6):1819–1831. doi: 10.1016/j.celrep.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Andrabi SA, Umanah GKE, Chang C, Stevens DA, Karuppagounder SS, Gagne JP, et al. Poly(ADP-ribose) polymerase-dependent energy depletion occurs through inhibition of glycolysis. P Natl Acad Sci USA. 2014;111(28):10209–10214. doi: 10.1073/pnas.1405158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Liu H, Zhang H, Wu X, Ma D, Wu J, Wang L, et al. Nuclear cGAS suppresses DNA repair and promotes tumorigenesis. Nature. 2018;563(7729):131–136. doi: 10.1038/s41586-018-0629-6. [DOI] [PubMed] [Google Scholar]
- 363.Malireddi RKS, Ippagunta S, Lamkanfi M, Kanneganti TD. Cutting edge: proteolytic inactivation of poly(ADP-ribose) polymerase 1 by the Nlrp3 and Nlrc4 inflammasomes. J Immunol. 2010;185(6):3127–3130. doi: 10.4049/jimmunol.1001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Ghosh R, Roy S, Franco S. PARP1 depletion induces RIG-I-dependent signaling in human cancer cells. PLoS One. 2018;13(3):e0194611. doi: 10.1371/journal.pone.0194611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Nassour J, Martien B, Martin N, Deruy E, Tomellini E, Malaquin N, et al. Defective DNA single-strand break repair is responsible for senescence and neoplastic escape of epithelial cells. Nat Commun. 2016;7:10399. doi: 10.1038/ncomms10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Kiss B, Szanto M, Hegedus C, Antal D, Szodenyi A, Marton J, et al. Poly(ADP-ribose) polymerase-1 depletion enhances the severity of inflammation in an imiquimod-induced model of psoriasis. Exp Dermatol. 2020;29(1):79–85. doi: 10.1111/exd.14061. [DOI] [PubMed] [Google Scholar]
- 367.Tepper S, Mortusewicz O, Czlonka E, Bello A, Schmidt A, Jeschke J, et al. Restriction of AID activity and somatic hypermutation by PARP-1. Nucleic Acids Res. 2019;47(14):7418–7429. doi: 10.1093/nar/gkz466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Selvaraj V, Soundarapandian MM, Chechneva O, Williams AJ, Sidorov MK, Soulika AM, et al. PARP-1 deficiency increases the severity of disease in a mouse model of multiple sclerosis. J Biol Chem. 2009;284(38):26070–26084. doi: 10.1074/jbc.M109.013474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Piskunova TS, Yurova MN, Ovsyannikov AI, Semenchenko AV, Zabezhinski MA, Popovich IG, et al. Deficiency in poly(ADP-ribose) polymerase-1 (PARP-1) accelerates aging and spontaneous carcinogenesis in mice. Curr Gerontol Geriatr Res. 2008;754190. 10.1155/2008/754190. [DOI] [PMC free article] [PubMed]
- 370.Pantelidou C, Sonzogni O, De Oliveria TM, Mehta AK, Kothari A, Wang D, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019. 10.1158/2159-8290.CD-18-1218. [DOI] [PMC free article] [PubMed]
- 371.Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, et al. PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian Cancer. Cell Rep. 2018;25(11):2972–2980. doi: 10.1016/j.celrep.2018.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Parkes EE, Walker SM, Taggart LE, McCabe N, Knight LA, Wilkinson R et al. Activation of STING-dependent innate immune signaling by S-phase-specific DNA damage in breast cancer. J Natl Cancer Inst. 2017;109(1). 10.1093/jnci/djw199. [DOI] [PMC free article] [PubMed]
- 373.Zuo H, Yang D, Yang Q, Tang H, Fu YX, Wan Y. Differential regulation of breast cancer bone metastasis by PARP1 and PARP2. Nat Commun. 2020;11(1):1578. doi: 10.1038/s41467-020-15429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Ding L, Chen X, Xu X, Qian Y, Liang G, Yao F, et al. PARP1 suppresses the transcription of PD-L1 by poly(ADP-Ribosyl)ating STAT3. Cancer Immunol Res. 2019;7(1):136–149. doi: 10.1158/2326-6066.CIR-18-0071. [DOI] [PubMed] [Google Scholar]
- 375.Pommier Y, O'Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8(362):362ps17. doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 376.Hoch NC, Hanzlikova H, Rulten SL, Tetreault M, Komulainen E, Ju LM, et al. XRCC1 mutation is associated with PARP1 hyperactivation and cerebellar ataxia. Nature. 2017;541(7635):87. doi: 10.1038/nature20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med. 1997;3(10):1089–1095. doi: 10.1038/nm1097-1089. [DOI] [PubMed] [Google Scholar]
- 378.Lee Y, Karuppagounder SS, Shin JH, Lee YI, Ko HS, Swing D, et al. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. Nat Neurosci. 2013;16(10):1392–1400. doi: 10.1038/nn.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297(5579):259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 380.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 381.Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal CM, Smulson ME, et al. Poly(ADP-ribose) polymerase activation mediates 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism. Proc Natl Acad Sci U S A. 1999;96(10):5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, et al. NMDA but not non-NMDA excitotoxicity is mediated by poly(ADP-ribose) polymerase. J Neurosci. 2000;20(21):8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wurtman RJ, Chou C, Rose CM. Daily rhythm in tyrosine concentration in human plasma: persistence on low-protein diets. Science. 1967;158(3801):660–662. doi: 10.1126/science.158.3801.660. [DOI] [PubMed] [Google Scholar]
- 384.Wurtman RJ, Rose CM, Chou C, Larin FF. Daily rhythms in the concentrations of various amino acids in human plasma. N Engl J Med. 1968;279(4):171–175. doi: 10.1056/NEJM196807252790401. [DOI] [PubMed] [Google Scholar]
- 385.Zir LM, Parker DC, Smith RA, Rossman LG. The relationship of human growth hormone and plasma tyrosine during sleep. J Clin Endocrinol Metab. 1972;34(1):1–6. doi: 10.1210/jcem-34-1-1. [DOI] [PubMed] [Google Scholar]
- 386.Rose CM, Wurtman RJ. Daily rhythms in content and utilization of tyrosine in the whole mouse. Nature. 1970;226(5244):454–455. doi: 10.1038/226454a0. [DOI] [PubMed] [Google Scholar]
- 387.Wurtman RJ, Shoemaker WJ, Larin F. Mechanism of the daily rhythm in hepatic tyrosine transaminase activity: role of dietary tryptophan. Proc Natl Acad Sci U S A. 1968;59(3):800–807. doi: 10.1073/pnas.59.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Black IB. Induction of hepatic tyrosine aminotransferase mediated by a cholinergic agent. Nature. 1970;225(5233):648. doi: 10.1038/225648a0. [DOI] [PubMed] [Google Scholar]
- 389.Black IB, Reis DJ. Central neural regulation by adrenergic nerves of the daily rhythm in hepatic tyrosine transaminase activity. J Physiol. 1971;219(2):267–280. doi: 10.1113/jphysiol.1971.sp009661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Palmieri L, Mameli M, Ronca G. Effect of resveratrol and some other natural compounds on tyrosine kinase activity and on cytolysis. Drugs Exp Clin Res. 1999;25(2–3):79–85. [PubMed] [Google Scholar]
- 391.Sun L, Wang Y, Song Y, Cheng XR, Xia S, Rahman MR, et al. Resveratrol restores the circadian rhythmic disorder of lipid metabolism induced by high-fat diet in mice. Biochem Biophys Res Commun. 2015;458(1):86–91. doi: 10.1016/j.bbrc.2015.01.072. [DOI] [PubMed] [Google Scholar]
- 392.Oike H, Kobori M. Resveratrol regulates circadian clock genes in Rat-1 fibroblast cells. Biosci Biotechnol Biochem. 2008;72(11):3038–3040. doi: 10.1271/bbb.80426. [DOI] [PubMed] [Google Scholar]
- 393.Okada Y, Okada M. Quercetin, caffeic acid and resveratrol regulate circadian clock genes and aging-related genes in young and old human lung fibroblast cells. Mol Biol Rep. 2020;47(2):1021–1032. doi: 10.1007/s11033-019-05194-8. [DOI] [PubMed] [Google Scholar]
- 394.Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421(6919):177–182. doi: 10.1038/nature01314. [DOI] [PubMed] [Google Scholar]
- 395.Gadacha W, Ben-Attia M, Bonnefont-Rousselot D, Aouani E, Ghanem-Boughanmi N, Touitou Y. Resveratrol opposite effects on rat tissue lipoperoxidation: pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009;14(4):154–158. doi: 10.1179/135100009x466131. [DOI] [PubMed] [Google Scholar]
- 396.Pifferi F, Rahman A, Languille S, Auffret A, Babiloni C, Blin O, et al. Effects of dietary resveratrol on the sleep-wake cycle in the non-human primate gray mouse lemur (Microcebus murinus) Chronobiol Int. 2012;29(3):261–270. doi: 10.3109/07420528.2011.654019. [DOI] [PubMed] [Google Scholar]
- 397.Pifferi F, Dal-Pan A, Menaker M, Aujard F. Resveratrol dietary supplementation shortens the free-running circadian period and decreases body temperature in a prosimian primate. J Biol Rhythm. 2011;26(3):271–275. doi: 10.1177/0748730411401788. [DOI] [PubMed] [Google Scholar]
- 398.Kiskova T, Demeckova V, Jendzelovska Z, Kiktava M, Venglovska K, Bohmdorfer M, et al. Nocturnal resveratrol administration inhibits chemically induced breast cancer formation in rats. J Physiol Pharmacol. 2017;68(6):867–875. [PubMed] [Google Scholar]
- 399.Esposito E, Li W, Mandeville ET, Park JH, Sencan I, Guo S, et al. Potential circadian effects on translational failure for neuroprotection. Nature. 2020;582(7812):395–398. doi: 10.1038/s41586-020-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Darst BF, Koscik RL, Hogan KJ, Johnson SC, Engelman CD. Longitudinal plasma metabolomics of aging and sex. Aging (Albany NY) 2019;11(4):1262–1282. doi: 10.18632/aging.101837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Chak CM, Lacruz ME, Adam J, Brandmaier S, Covic M, Huang JL, et al. Ageing investigation using two-time-point metabolomics data from KORA and CARLA studies. Metabolites. 2019;9(3):44. doi: 10.3390/metabo9030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Zou H, Wang D, Ren H, Cai K, Chen P, Fang C et al. Effect of caloric restriction on BMI, gut microbiota, and blood amino acid levels in non-obese adults. Nutrients. 2020;12(3). 10.3390/nu12030631. [DOI] [PMC free article] [PubMed]
- 403.Zheng Y, Ceglarek U, Huang T, Li L, Rood J, Ryan DH, et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am J Clin Nutr. 2016;103(2):505–511. doi: 10.3945/ajcn.115.117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Storga D, Vrecko K, Birkmayer JGD, Reibnegger G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci Lett. 1996;203(1):29–32. doi: 10.1016/0304-3940(95)12256-7. [DOI] [PubMed] [Google Scholar]
- 405.Huo Z, Yu L, Yang J, Zhu Y, Bennett DA, Zhao J. Brain and blood metabolome for Alzheimer's dementia: findings from a targeted metabolomics analysis. Neurobiol Aging. 2020;86:123–133. doi: 10.1016/j.neurobiolaging.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Magnusson M, Lewis GD, Ericson U, Orho-Melander M, Hedblad B, Engstrom G, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Ferguson AA, Roy S, Kormanik KN, Kim Y, Dumas KJ, Ritov VB, et al. TATN-1 mutations reveal a novel role for tyrosine as a metabolic signal that influences developmental decisions and longevity in Caenorhabditis elegans. PLoS Genet. 2013;9(12):e1004020. doi: 10.1371/journal.pgen.1004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Ipson BR, Green RA, Wilson JT, Watson JN, Faull KF, Fisher AL. Tyrosine aminotransferase is involved in the oxidative stress response by metabolizing meta-tyrosine in Caenorhabditis elegans. J Biol Chem. 2019;294(24):9536–9554. doi: 10.1074/jbc.RA118.004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Sgaravatti AM, Magnusson AS, de Oliveira AS, Rosa AP, Mescka CP, Zanin FR, et al. Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis. 2009;24(3):415–425. doi: 10.1007/s11011-009-9153-6. [DOI] [PubMed] [Google Scholar]
- 410.Macedo LG, Carvalho-Silva M, Ferreira GK, Vieira JS, Olegario N, Goncalves RC, et al. Effect of acute administration of L-tyrosine on oxidative stress parameters in brain of young rats. Neurochem Res. 2013;38(12):2625–2630. doi: 10.1007/s11064-013-1180-3. [DOI] [PubMed] [Google Scholar]
- 411.De Pra SD, Ferreira GK, Carvalho-Silva M, Vieira JS, Scaini G, Leffa DD, et al. L-tyrosine induces DNA damage in brain and blood of rats. Neurochem Res. 2014;39(1):202–207. doi: 10.1007/s11064-013-1207-9. [DOI] [PubMed] [Google Scholar]
- 412.Streck EL, De Pra SDT, Ferro PR, Carvalho-Silva M, Gomes LM, Agostini JF, et al. Role of antioxidant treatment on DNA and lipid damage in the brain of rats subjected to a chemically induced chronic model of tyrosinemia type II. Mol Cell Biochem. 2017;435(1–2):207–214. doi: 10.1007/s11010-017-3070-5. [DOI] [PubMed] [Google Scholar]
- 413.Teodorak BP, Scaini G, Carvalho-Silva M, Gomes LM, Teixeira LJ, Rebelo J, et al. Antioxidants reverse the changes in energy metabolism of rat brain after chronic administration of L.-tyrosine. Metab Brain Dis. 2017;32(2):557–564. doi: 10.1007/s11011-016-9936-5. [DOI] [PubMed] [Google Scholar]
- 414.Ferreira GK, Carvalho-Silva M, Gomes LM, Scaini G, Teixeira LJ, Mota IT, et al. The characterization of neuroenergetic effects of chronic L-tyrosine administration in young rats: evidence for striatal susceptibility. Metab Brain Dis. 2015;30(1):215–221. doi: 10.1007/s11011-014-9615-3. [DOI] [PubMed] [Google Scholar]
- 415.Korner J, Cline GW, Slifstein M, Barba P, Rayat GR, Febres G, et al. A role for foregut tyrosine metabolism in glucose tolerance. Mol Metab. 2019;23:37–50. doi: 10.1016/j.molmet.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Sterkel M, Perdomo HD, Guizzo MG, Barletta ABF, Nunes RD, Dias FA, et al. Tyrosine detoxification is an essential trait in the life history of blood-feeding arthropods. Curr Biol. 2016;26(16):2188–2193. doi: 10.1016/j.cub.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 417.Sterkel M, Oliveira PL. Developmental roles of tyrosine metabolism enzymes in the blood-sucking insect Rhodnius prolixus. Proc Biol Sci. 2017;284(1854). 10.1098/rspb.2016.2607. [DOI] [PMC free article] [PubMed]
- 418.Green CL, Soltow QA, Mitchell SE, Derous D, Wang Y, Chen L, et al. The effects of graded levels of calorie restriction: XIII. Global Metabolomics Screen Reveals Graded Changes in Circulating Amino Acids, Vitamins, and Bile Acids in the Plasma of C57BL/6 Mice. J Gerontol A Biol Sci Med Sci. 2019;74(1):16–26. doi: 10.1093/gerona/gly058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Shetty MS, Gopinadhan S, Sajikumar S. Dopamine D1/D5 receptor signaling regulates synaptic cooperation and competition in hippocampal CA1 pyramidal neurons via sustained ERK1/2 activation. Hippocampus. 2016;26(2):137–150. doi: 10.1002/hipo.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Leng ZG, Lin SJ, Wu ZR, Guo YH, Cai L, Shang HB, et al. Activation of DRD5 (dopamine receptor D5) inhibits tumor growth by autophagic cell death. Autophagy. 2017;13(8):1404–1419. doi: 10.1080/15548627.2017.1328347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Scaini G, Morais MO, Furlanetto CB, Kist LW, Pereira TC, Schuck PF, et al. Acute administration of branched-chain amino acids increases the pro-BDNF/total-BDNF ratio in the rat brain. Neurochem Res. 2015;40(5):885–893. doi: 10.1007/s11064-015-1541-1. [DOI] [PubMed] [Google Scholar]
- 422.Ferreira GK, Scaini G, Jeremias IC, Carvalho-Silva M, Goncalves CL, Pereira TC, et al. An evaluation of the effects of acute and chronic L-tyrosine administration on BDNF levels and BDNF mRNA expression in the rat brain. Mol Neurobiol. 2014;49(2):734–740. doi: 10.1007/s12035-013-8552-1. [DOI] [PubMed] [Google Scholar]
- 423.van de Rest O, Bloemendaal M, de Heus R, Aarts E. Dose-dependent effects of oral tyrosine administration on plasma tyrosine levels and cognition in aging. Nutrients. 2017;9(12). 10.3390/nu9121279. [DOI] [PMC free article] [PubMed]
- 424.Norris JR, Meadows GG, Massey LK, Starkey JR, Sylvester DM, Liu SY. Tyrosine- and phenylalanine-restricted formula diet augments immunocompetence in healthy humans. Am J Clin Nutr. 1990;51(2):188–196. doi: 10.1093/ajcn/51.2.188. [DOI] [PubMed] [Google Scholar]
- 425.Seo Y, Park J, Choi W, Ju Son D, Sung Kim Y, Kim MK, et al. Antiatherogenic effect of resveratrol attributed to decreased expression of ICAM-1 (intercellular adhesion molecule-1) Arterioscler Thromb Vasc Biol. 2019;39(4):675–684. doi: 10.1161/ATVBAHA.118.312201. [DOI] [PubMed] [Google Scholar]
- 426.Zhao HL, Han LM, Jian Y, Ma YT, Yan WY, Chen XW, et al. Resveratrol induces apoptosis in human melanoma cell through negatively regulating Erk/PKM2/Bcl-2 axis. Oncotargets Ther. 2018;11:8995–9006. doi: 10.2147/Ott.S186247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Gong CH, Xia HL. Resveratrol suppresses melanoma growth by promoting autophagy through inhibiting the PI3K/AKT/mTOR signaling pathway. Exp Ther Med. 2020;19(3):1878–1886. doi: 10.3892/etm.2019.8359. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 428.Meadows GG, Pierson HF, Abdallah RM, Desai PR. Dietary influence of tyrosine and phenylalanine on the response of B16 melanoma to carbidopa-levodopa methyl ester chemotherapy. Cancer Res. 1982;42(8):3056–3063. [PubMed] [Google Scholar]
- 429.Lorincz AB, Kuttner RE, Brandt MB. Tumor response to phenylalanine-tyrosine-limited diets. J Am Diet Assoc. 1969;54(3):198–205. [PubMed] [Google Scholar]
- 430.Demopoulos HB. Effects of reducing the phenylalanine-tyrosine intake of patients with advanced malignant melanoma. Cancer. 1966;19(5):657–664. doi: 10.1002/1097-0142(196605)19:5<657::aid-cncr2820190509>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 431.Fu YM, Yu ZX, Pelayo BA, Ferrans VJ, Meadows GG. Focal adhesion kinase-dependent apoptosis of melanoma induced by tyrosine and phenylalanine deficiency. Cancer Res. 1999;59(3):758–765. [PubMed] [Google Scholar]
- 432.Gao P, Li N, Ji K, Wang Y, Xu C, Liu Y, et al. Resveratrol targets TyrRS acetylation to protect against radiation-induced damage. FASEB J. 2019:fj201802474RR. 10.1096/fj.201802474RR. [DOI] [PubMed]
- 433.Huang SZ, Wang X, Lin GF, Cheng J, Chen XL, Sun WN, et al. Discovery of human TyrRS inhibitors by structure-based virtual screening, structural optimization, and bioassays. RSC Adv. 2019;9(16):9323–9330. doi: 10.1039/c9ra00458k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Chatterjee S, Olsen S, Blanch EW, Wang F. Resveratrol's hidden hand: a route to the optical detection of biomolecular binding. J Phys Chem B. 2018;122(11):2841–2850. doi: 10.1021/acs.jpcb.7b10278. [DOI] [PubMed] [Google Scholar]