Abstract
Since the first identification of bombykol, sex pheromones of about 700 moth species have been elucidated. Additionally, field evaluations of synthetic pheromones and their related compounds have revealed the male attraction of another 1,300 species. These pheromones and attractants are listed on the web-sites, “Pheromone Database, Part I.” Pheromone components are classified according to their chemical structures into two major groups (Types I and II) and miscellaneous. Based on our previous review published in 2004, studies reported during the last two decades are highlighted here to provide information on the structure characteristics of newly identified pheromones, current techniques for structure determination, new enantioselective syntheses of methyl-branched pheromones, and the progress of biosynthetic research. Besides the moth sex pheromones, various pheromones and allomones from many arthropod species have been uncovered. These semiochemicals are being collected in the “Pheromone Database, Part II.” The chemical diversity provides a wonderland for natural product chemists.
Keywords: chemical ecology, Lepidoptera, structural diversity, diagnostic ions, SN2 reaction, epoxidase
Introduction
Lepidoptera, the second largest order in Insecta, contains more than 150,000 species that are classified into a large group of moths and a small group of butterflies. While female butterflies do not produce a long-range active chemical, female moths, even diurnal moths, usually secrete species-specific sex pheromones to attract males of the same species and mate with them. Bombykol, (10E,12Z)-hexadeca-10,12-dien-1-ol (1), is the first pheromone compound identified from females of the silk moth (Bombyx mori) about 60 years ago.1) After this pioneering identification, the chemical communication systems of numerous species in many insect groups have been extensively studied, and the chemical structures of various pheromones with a characteristic role such as mating, trail, and alarm have been determined. Among them, research on the lepidopteran sex pheromones has been extensively developed, and the female pheromones of 700 moth species from around the world have been characterized to date.2) The lepidopteran sex pheromone is one of the most interesting bioactive natural products investigated in a large number of species. Because the pheromones can effectively attract male moths, many synthetic pheromones are now used as lures to monitor insect population in farmlands and forests. Furthermore, mating disruption has been achieved by permeating a field with a synthetic pheromone to regulate a pest insect directly.3)
In addition to the identification from female moths, field evaluations of synthetic pheromones and their related compounds have revealed the male attraction of another 1,300 species. Structures of the known pheromones and attractants are listed on the web-sites, “The Pherobase”4) and “Pheromone Database, Part I”,5) which are maintained by El-Sayed and our research group, respectively. The components are classified according to their chemical structures into two major groups and miscellaneous: unsaturated C10–C18 fatty alcohols and their derivatives (Type I), C17–C25 polyenyl hydrocarbons and their epoxides (Type II), and others including methyl-branched compounds. In addition to creating this list, we have summarized the pheromone research published until 2002, at which time the pheromones of about 530 species had been identified.6) Since then, information on another 170 lepidopteran species has increased, and structure determination techniques have advanced by using gas chromatography combined with a Fourier transform infrared spectrophotometer (GC/FT-IR) and a mass spectrometer (GC/MS). A new strategy for the enantioselective syntheses has been achieved and the knowledge of pheromone biosynthesis has also deepened. This review deals with the chemical structural features of newly identified pheromones and current pheromone studies. Additionally, “Pheromone Database, Part II” is introduced here. It includes many kinds of pheromones and allomones uncovered from a huge number of arthropods, including insects, spiders, mites, and millipedes, other than moth pheromones. The vast number of studies on semiochemicals shows how strongly they have attracted researchers.
1. Classification of Lepidopteran Sex Pheromones
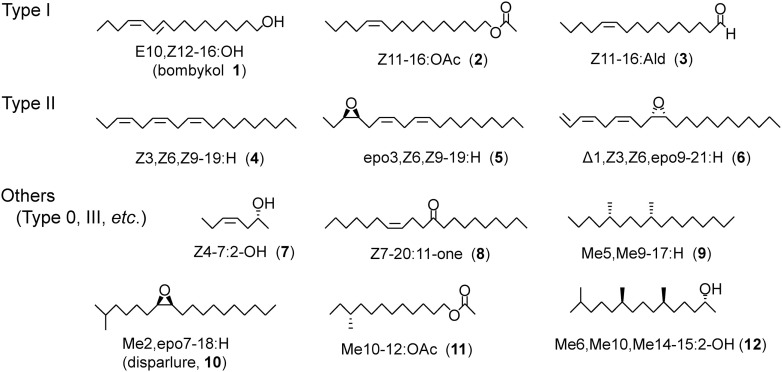
Taxonomically related moths are expected to produce pheromones with structural similarity, but some differences are necessary to establish their reproductive isolation. The chemical structures of sex pheromones are highly varied, reflecting the diversity of moth species. In addition to structural variation, the diversity of lepidopteran sex pheromones is generated by blending multiple components. Innumerable pheromone blends are based not only on combinations of different components but also on variations in the mixing ratio. Figure 1 shows some representative sex pheromones. The chemical formulae are symbolized as follows: (1) The numeral before the hyphen gives the position of the double bond (Z: Z configuration, E: E configuration, Δ=terminal double bond), triple bond (≡), cis-epoxy ring (epo), trans-epoxy ring (t-epo), or methyl branch (Me). (2) The numeral before the colon gives the carbon number of the straight chain. (3) The words after the colon give the functional groups as follows: alcohol (OH), acetate of alcohol (OAc), aldehyde (Ald), ketone (one), ester of carboxylic acid (Ate), and hydrocarbon (H).
Fig. 1. Formulas and abbreviations of representative lepidopteran sex pheromones, Type I, Type II, and other compounds.
Primary alcohols and their derivatives (mainly acetates and aldehydes) with a long straight chain (mainly a monoenyl or dienyl even-numbered chain of C10–C18) have been most commonly identified such as bombykol (1) and pheromone components of the diamond back moth (Plutella xylostella, 2 and 3).7) These chemicals, which are biosynthesized de novo via general saturated fatty acids and referred to as Type I compounds, comprise about 75% of known lepidopteran sex pheromones. The Type I pheromones have been widely recorded from almost all of the superfamilies of Ditrysia (Supplemental Fig. S1), whose females have two distinct genital openings, different from primitive species with one opening. Polyunsaturated hydrocarbons and their epoxy derivatives with a longer straight chain (C17–C25) comprise a second group, such as the pheromone components of the giant looper (Ascotis selenaria, 4 and 5)8) and the fall webworm moth (Hyphantria cunea, 6).9) These chemicals, referred to as Type II compounds, comprise about 15% of the known lepidopteran pheromones and have been identified only from some limited groups, such as Geometridae in Geometroidea and Erebidae in Noctuoidea.
The pheromones of the remaining 10% of species consist of components that belong to neither Type I nor Type II, such as a 2-hydroxy compound (7) of Eriocrania cicatricella10) and ketone (8) of the peach fruit moth (Carposina sasakii).11) Furthermore, several kinds of methyl-branched compounds are known: the hydrocarbon (9) of the mountain-ash bentwing (Leucoptera scitella),12,13) epoxide (disparlure, 10) of the gypsy moth (Lymantria dispar),14) acetate (11) of the smaller tea tortrix (Adoxophyes honmai),15) and secondary alcohol (12) of the rice moth (Corcyra cephalonica).16,17) Recently, Löfstedt and Millar proposed to classify the secondary alcohols with a short chain (C7 and C9) as Type 0 and methyl-branched compounds as Type III.18) The Type 0 secondary alcohols have been found from species only in the nonditrysian superfamilies Eriocranioidea and Nepticuloidea. Since heptan-2-ol and nonan-2-ol are sex pheromone components of some caddis flies in Trichoptera, the sister group of Lepidoptera,5) the short-chain pheromones of primitive species are categorized as Type 0, meaning an ancestral type of moth pheromones. On the other hand, Type III compounds with structural variations have been identified from species in some more distantly related superfamilies such as Yponomeutoidea and Noctuoidea.2,6)
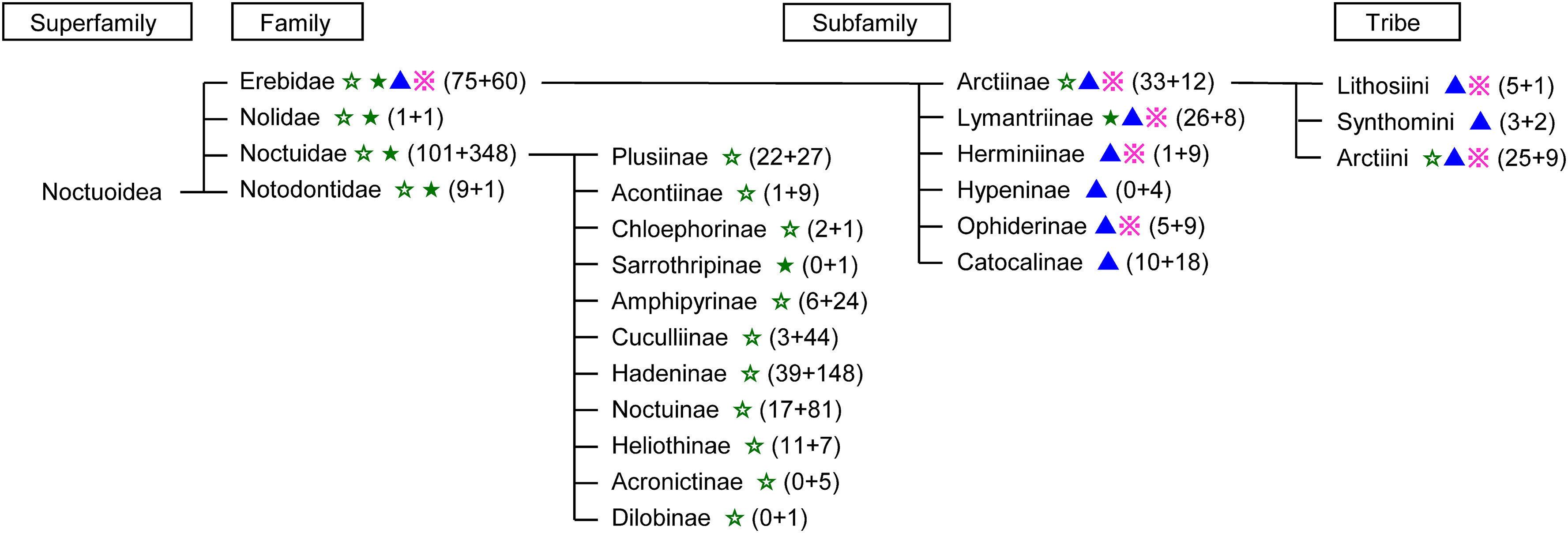
Superfamilies that include many species whose pheromones are already known are as follows: Noctuoidea (186 species), Tortricoidea (171 species), Pyraloidea (100 species), Geometroidea (54 species), Bombycoidea (34 species), Sesioidea (28 species), and Gelechioidea (25 species) (Supplemental Fig. S1).2) Noctuoidea is a highly evolved group with more than 70,000 described species, the largest number in a lepidopteran superfamily. Figure 2 shows the taxonomy of Noctuoidea, pheromone types of the constituent species, and frequency of pheromone studies. While this superfamily was classified into several families, such as Arctiidae, Lymantriidae, Noctuidae, Nolidae, and Notodontidae, the former two families have now been combined with some subfamilies in Noctuidae to make a new family, Erebidae.19) Namely, Arctiidae, including H. cunea, and Lymantriidae, including L. dispar, have been changed to Arctiinae and Lymantriinae, respectively. Subfamilies in Arctiidae have been changed to tribes in Arctiinae. This figure clearly indicates that species producing a Type II pheromone are gathered into one family, Erebidae. Previously, Noctuidae was broadly divided into two groups, Quadrifinae and Trifinae. Four subfamilies (Hermininae, Hypeninae, Ophiderinae, and Catocalinae) that were moved to Erebidae from previous Noctuidae belonged to Quadrifinae, and new Noctuidae includes the Trifinae species, which produce only Type I pheromones. In Noctuoidea, Type II and methyl-branched pheromones have been found only from Erebidae species. The types of pheromone structures are consistent with the new taxonomy.
Fig. 2. Taxonomy and pheromone studies of insects in the superfamily of Noctuoidea. The numbers before and after + in parentheses in each group indicate the total number of species whose female sex pheromone and male attractant have been reported, respectively. Each mark after the group name indicates that some species within the group produces a pheromone component of Type I (☆ with a common functional group, ★ with a novel functional group), Type II (▲), or others (※). (This figure is a significant revision of Fig. 2 in Ref. 6 due to the change in the classification of Noctuoidea).
2. Current Identification of Sex Pheromones
2.1. Structures of new pheromones in Type I
Type I pheromones from 119 species have been studied since 2003.2) Most of them are composed of known pheromone compounds previously identified from other species, but 16 new compounds (13–28) have been reported from 14 species, as shown in Table 1-A. In the case of monoenyl components (13–18), it was shown that female moths could also construct 8-10 (dec-8-enyl), 6-12, 5-16, 13-16, and 6-18 structures. The components unsaturated at the 6-position are noteworthy because a 6-monoenyl compound had not been known for any length of carbon chain.6) As a result, known double-bond positions were increased to be 5, 7, and 8 for C10 monoenes; 3 and 5 to 11 for C12 monoenes; 3, 5, and 7 to 12 for C14 monoenes; 5 to 7, 9 to 11, and 13 for C16 monoenes; and 2, 6, 7, 11, and 13 for C18 monoenes (Supplemental Fig. S2). Double bonds of Type I pheromones are introduced into saturated fatty acyl intermediates by desaturases. The desaturase corresponding directly to each double-bond position of each carbon chain is not always necessary because an enzyme for chain shortening or elongation converts the original structure. For example, the 6-12 structure can be biosynthesized from a 10-16 acyl compound if β-oxidation happens twice. The variety of the double-bond positions, however, indicates that moths established many different desaturases over their long history.
Table 1. Type I and II lepidopteran female pheromones with a new chemical structure discovered since 2003.
| Identified compound | Insect | Publication year of 2000s [Reference] | ||
|---|---|---|---|---|
| Chem. class | Structure abbreviation | [Superfamily]a) Family (Subfamily) | Species | |
| (A) Type I | ||||
| Monoene | E8-10:OH (13) | [Zyg] Limacodidae | Monema flavescens | 13 [20] |
| E6-12:OAc (14) | [Gel] Gelechiidae | Anthistarcha binocularis | 20 [21] | |
| E6-12:OH (15) | [Gel] Gelechiidae | Anthistarcha binocularis | 20 [21] | |
| E5-16:OAc (16) | [Gel] Stathmopodidae | Stathmopoda auriferella | 13 [22] | |
| Z13-16:OAc (17) | [Pyr] Crambidae (Spilomelinae) | Herpetogramma submarginale | 15 [23] | |
| E6-18:Ald (18) | [Bom] Saturniidae | Actias luna | 16 [24] | |
| Diene | Z4,Z7-10:OAc (19) | [Gel] Batrachedridae | Batrachedra amydraula | 11 [25], 13 [26] |
| E3,Z5-14:OAc (20) | [Tor] Cochylidae | Phtheochroa cranaodes | 01 [27], 03 [28] | |
| Z3,E5-14:OAc (21) | [Cos] Cossidae | Holcocerus vicarius | 15 [29] | |
| E9,Z11-15:Ald (22) | [Bom] Sphingidae | Dolbina tancrei | 13 [30] | |
| Z9,Z11-15:Ald (23) | [Bom] Sphingidae | Dolbina tancrei | 13 [30] | |
| Z7,Z10-16:Ald (24) | [Cos] Cossidae | Chilecomadia valdiviana | 16 [31], 17 [32] | |
| E10,E14-16:Ald (25) | [Pyr] Crambidae (Spilomelinae) | Omphisa anastomosalis | 10 [33], 14 [34] | |
| E6,Z11-18:Ald (26) | [Bom] Saturniidae | Actias luna | 16 [24] | |
| Tetraene | E4,E6,Z11,Z13-16:Ald (27) | [Bom] Saturniidae | Callosamia promethea | 13 [35] |
| Dienyne | Z9,≡11,Δ13–14:Ald (28) | [Gel] Elachistidae | Stenoma catenifer | 08 [36], 09 [37] |
| (B) Type II | ||||
| Monoene | Z6,epo9-19:H (29) | [Geo] Geometridae (Ennominae) | Ennomos subsignaria | 10 [38] |
| Diene | Z6,Z9-22:H (30) | [Noc] Erebidae (Arctiinae) | Eilema japonica | 10 [39] |
| Z6,Z9-21:11-OH (31) | [Noc] Erebidae (Lymantriinae) | Orgyia detrita | 03 [40] | |
| Z6,Z9-21:11-one (32) | [Noc] Erebidae (Lymantriinae) | Orgyia leucostigma | 03 [41], 08 [42] | |
| Teia anartoides | 05 [43], 05 [44] | |||
| Triene | Z3,Z6,Z9-22:H (33) | [Noc] Erebidae (Arctiinae) | Eilema japonica | 10 [39] |
| E4,Z6,Z9-21:H (34) | [Noc] Erebidae (Arctiinae) | Arctia plantaginis | 17 [45] | |
| Z6,Z9,Z12-18:H (35) | [Geo] Geometridae (Geometrinae) | Hemithea tritonaria | 09 [46], 11 [47] | |
| Pamphlebia rubrolimbraria | ||||
| Z6,Z9,Z12-20:H (36) | [Geo] Geometridae (Geometrinae) | Maxates versicausa | 09 [46], 11 [47] | |
| Tetraene | Z2,E4,Z6,Z9-21:H (37) | [Noc] Erebidae (Arctiinae) | Arctia plantaginis | 17 [45] |
| Z3,Z6,Z9,Z12-20:H (38) | [Geo] Geometridae (Geometrinae) | Thalassodes immissaria | 09 [46] | |
| Z3,Z6,Z9,Z19-23:H (39) | [Tis] Tischeriidae | Tischeria ekebladella | 12 [48] | |
| Pentaene | Z3,Z6,Z9,Z12,Z15-23:H (40) | [Pyr] Pyralidae (Phycitinae) | Amyelois transitella | 05 [49], 10 [50], 10 [51] |
| (Pyralinae) | Pyralis farinalis | 10 [50] | ||
| (Epipaschiinae) | Orthaga achatina | 18 [52] | ||
| Z3,Z6,Z9,Z12,Z15-25:H (41) | [Pyr] Pyralidae (Phycitinae) | Amyelois transitella | 05 [49] | |
| Dioryctria abietivorella | 05 [53], 08 [54] | |||
| Dioryctria abietella | 12 [55] | |||
| Dioryctria mendacella | 17 [56] | |||
a) Superfamilies are abbreviated as follows; [Bom] Bombycoidea, [Cos] Cossoidea, [Gel] Gelechioidea, [Geo] Geometroidea, [Noc] Noctuoidea, [Pyr] Pyraloidea, [Tin] Tineoidea, [Tis] Tischerioidea, [Tor] Tortricoidea, and [Zyg] Zygaenoidea.
In the case of dienyl compounds (19–26), 4,7-10 (deca-4,7-dienyl), 3,5-14, 9,11-15, 7,10-16, 10,14-16, and 6,11-18 structures have been newly reported. The second and third structures of 20–23 have a 1,3-diene system. Among the previously identified dienes with a C12, C14, or C16 chain, the conjugated system is most frequently found, for example 8,10-, 9,11-, 10,12-, and 11,13-dines in C14 compounds (Supplemental Fig. S3). The double bonds of these C14 1,3-dienes locate at a terminal methyl side of the C14 chain. On the other hand, the newly identified 3,5-14 acetates (20 and 21) from the species of Cochylidae and Cossidae include them near a functional group. A structurally related 3,5-12 compound is a known pheromone component of another Cochylidae species. The 9,11-15 aldehydes (22 and 23) produced by Sphingidae species in Bombycoidea have the conjugated system in an unusual C15 chain. The double bonds are located at the ω4- and ω6-positions counted from the terminal methyl group. The positions are the same as those of 10,12-16 compounds such as bombykol (1).
The new 4,7-10 and 7,10-16 compounds (19 and 24) have a 1,4-diene system. The homoconjugated diene system is not common, and only 9,12-14, 9,12-18, and 11,14-18 compounds had been identified before 2003. The 10,14-16 and 6,11-18 compounds (25 and 26) have other uncommon 1,5-diene and 1,6-diene systems, respectively. Only 3,7-14 and 7,11-16 compounds with the 1,5-diene system and 3,8-14, 4,9-14, and 6,11-16 compounds with the 1,6-diene system had been previously identified. These 1,6-dienes, except for the 3,8-14 compound are pheromone components of some species in Saturniidae. Interestingly, the 4,6,11,13-16 structure of 27 has been determined to be the first tetraene system among the Type I compounds. The tetraene (27) is a pheromone component of Saturniidae, and its structure corresponds to those of the 6,11-16 and 4,6,11-16 compounds produced by another Saturniidae species. In addition to the 4,6,11-16 compound, trienyl compounds with a 9,11,13-14, 4,6,10-16, 10,12,14-16, or 9,12,15-18 structure had been previously known, but no new trienes have been identified since 2003.
Unique pheromone components including a triple bond had been previously known, namely a C16 11-monoynyl compound from Crambidae species and C16 13-en-11-ynyl compounds from Notodontidae species.6) Additionally, a C14 9,13-dien-11-ynyl aldehyde (28) has been identified from an Elachistidae species. Even though these compounds are produced by the species with taxonomically low relation, the triple bond is located at the same 11-position. Interestingly, a corresponding 9,11,13-trienyl compound had already been determined as a pheromone component of another Elachistidae species in the same genus.2)
2.2. Structures of new pheromones in Type II
Type II pheromone compounds are 6,9-dienes, 3,6,9-trienes, and their derivatives, which are expected to be biosynthesized from dietary linoleic and linolenic acids. Double bonds at the 3-, 6-, and 9-positions have a Z configuration (Supplemental Table S1-A).6) Type II pheromones from 36 species have been studied since 2003, and 13 new compounds (29–41) have been identified from 18 species as shown in Table 1-B. Most of the known Type II pheromones consist of the compounds with an odd-number chain, mainly C19 and C21, such as a new epoxymonoene Z6,epo9-19:H (29), and no compounds with a C22 chain had been identified before that.2) As a result of the discovery of Z6,Z9-22:H (30) and Z3,Z6,Z9-22:H (33) from an Arctiinae species in Erebidae, the 6,9-dienes with a C19–C22 chain and the 3,6,9-trienes with a C17–C23 chain have become recognized as natural pheromone components. Z6,Z9-21:11-OH (31) and Z6,Z9-21:11-one (32) are new pheromone components found from three Lymantriinae species. One of them also secretes corresponding common Type II compounds, Z6,Z9-21:H and Z6,epo9-21:H. Although the biosynthesis of the new compounds from linoleic acid has not been experimentally proven, the dienyl structure suggests their production by enzymatic oxidation at the allylic 11-position of the 6,9-diene.
A 4,6,9-triene with a C19 chain is a known pheromone component of a Geometridae species.6) Recently, its homolog and a 2,4,6,9-tetraene with a C21 chain (34 and 37) were identified from an Arctiinae species in Erebidae. Additionally, 1,3,6,9- and 3,6,9,11-tetraenes are known Type II components produced by Arctiinae and Geometridae species (Supplemental Table S1-B).6) These compounds include an extra double bond at an allylic position of original homoconjugated 6,9-dienes and 3,6,9-trienes to create the conjugated system that causes a long retention time in GC analysis with a polar column. As a new pheromone component with another structural modification, two 6,9,12-trienes (35 and 36) and one 3,6,9,12-tetraene (38) were identified from Geometrinae species in Geometridae. In this family, many pheromone studies had been conducted with the species in the subfamilies Ennominae and Larentiinae, but not in Geometrinae, which has a lot of species. It was very possible to that new pheromone components could be found from the Geometrinae species, and in fact, new structures further unsaturated at the 12-position were determined, indicating the diversity of Type II pheromones. Furthermore, a 3,6,9,19-tetraene (39) has been identified from a Tischeriidae species in nonditrysian superfamily Tischerioidea. This is the first pheromone study in this primitive moth group, and the females surprisingly produce a unique Type II compound further unsaturated at the 19-position.
Besides the evolved species in Erebidae and Geometridae, the secretion of Z3,Z6,Z9-23:H by one Oecophoridae species in Gelechioidea and six Crambidae species in Pyraloidea has been reported (Supplemental Table S1-A).2) These Crambidae females also secrete one or two Type I compounds as a main pheromone component. The Type I component(s) of the hybrid pheromones are species specific but the Type II component is the universal C23 triene. Different hybrid pheromones have been identified from six species of Pyralidae, another family in Pyraloidea. Their Type II components are new pentaenes, Z3,Z6,Z9,Z12,Z15-23:H (40) and Z3,Z6,Z9,Z12,Z15-25:H (41). Since the volatility of the C25 pentaene is very low, a synthetic lure for male attraction contains it as a major component. In some of these species using the hybrid pheromone, the unsaturated hydrocarbons were found by reexamination of their pheromone extracts because of the weak attraction activity of the synthetic lures baited only with the Type I components. This kind of hybrid pheromone has not been found in any other insect groups. Nor has a hybrid with a Type I and an epoxy compound been reported.2)
New epoxy derivatives have not been reported, except for Z6,epo9-19:H (29), since 2003. As a C17–C23 chain compound, there are 14 epoxymonoenes and 21 epoxydienes derived from 6,9-dienes and 3,6,9-trienes, respectively. Among them, only four epoxymonoenes and nine epoxydienes have been identified, suggesting insufficient studies of the Type II pheromones (Supplemental Table S1-A).6) Geometridae and Erebidae include a large number of species, and their reproductive isolation is compensated by the diversity of pheromone structures. Many new epoxy compounds are expected to be found by further studies with these species.
2.3. Structures of new pheromones in other groups
Table 2 shows new methyl-branched compounds (42–50) and unbranched compounds (51–60) that have been characterized in analytical studies on 14 species since 2003. In the case of methyl-branched hydrocarbons, 14 compounds had been previously recorded from several species in Lyonetiidae, Geometridae, and Erebidae, and two compounds (42 and 43) were added. While pheromone components are usually secreted from a pheromone gland, as a close-range courtship component, Me11-23:H (42) was identified from female body scales of a Gelechiidae species in Gelechioidea, which had been known to produce two Type I pheromone compounds. This is the first identification of the branched pheromone component from a Gelechioidea species. From a Lymantriinae species in Erebidae, Me2,Z7,E9-18:H (43) was newly identified. This compound is structurally related to Me2,Z7-18:H, a known pheromone component of some other species in the same subfamily. The 14 previously recorded hydrocarbons have a monomethyl or dimethyl structure. The methyl branches are variously located at a position from 2 to 14, with the exception of the 4-, 6-, 8-, and 12-positions, in a saturated and monounsaturated C15–C21 main chain, such as Me5,Me9-17:H (9). Different from them, 42 and 43 have a C23 chain and a conjugated diene system, respectively. Furthermore, Me2,epo7,Δ17-18:H (44) was identified as a new minor pheromone component of the gypsy moth, a well-known Lymantriinae species that secrets disparlure (10).
Table 2. Other lepidopteran female pheromones with a new chemical structure discovered since 2003.
| Identified compound | Insect | Publication year of 2000s [Reference] | |||
|---|---|---|---|---|---|
| Chem. class | Structure abbreviation | Configuration | [Superfamily]a) Family (Subfamily) | Species | |
| Methyl-branched (Type III) | |||||
| Hydrocarbon | Me11-23:H (42) | R + S | [Gel] Gelechiidae | Anarsia lineatella | 05 [57] |
| Me2,Z7,E9-18:H (43) | achiral | [Noc] Erebidae (Lymantriinae) | Lymantria bantaizana | 05 [58] | |
| Epoxide | Me2,epo7,Δ17-18:H (44) | 7R,8S | [Noc] Erebidae (Lymantriinae) | Lymantria dispar | 05 [59] |
| Ester | Me3,Me13-15:Ateb) (45) | 3R,13R,1′S | [Tin] Psychidae | Clania variegata | 06 [60], 10 [61] |
| Me10,Me14-15:OisoBuc) (46) | R | [Noc] Erebidae (Lymantriinae) | Artaxa subflava | 07 [62] | |
| 2° OH | Me5-17:7-OH (47) | 5R,7R | [Noc] Erebidae (Arctiinae) | Miltochrista calamina | 11 [63], 14 [64] |
| Ketone | Me6,Me10,Me14-15:2-one (48) | unknown | [Pyr] Pyralidae (Galleriinae) | Aphomia sociella | 12 [65] |
| Me6-18:2-one (49) | S | [Noc] Erebidae (Arctiinae) | Lyclene dharma | 07 [66], 09 [67], 10 [68] | |
| Me14-18:2-one (50) | S | [Noc] Erebidae (Arctiinae) | Lyclene dharma | 07 [66], 09 [67], 10 [68] | |
| Un-branched | |||||
| Hydrocarbon | Z7-23:H (51) | achiral | [Cop] Carposinidae | Coscinoptycha improbana | 06 [69] |
| Ester | isopropyl Z5-10:Ate (52) | achiral | [Tin] Psychidae | Whittleia retiella | 20 [70] |
| sec-butyl Z5-10:Ate (53) | S | [Tin] Psychidae | Whittleia retiella | 20 [70] | |
| butyl E7,9-10:Ate (54) | achiral | [Zyg] Limacodidae | Darna pallivitta | 07 [71] | |
| sec-butyl Z7-12:Ate (55) | R | [Zyg] Zygaenidae | Illiberis rotundata | 09 [72] | |
| 2° OH deriv. | Z12-17:2-OAc (56) | S | [Tin] Tineidae | Kermania pistaciella | 06 [73] |
| 17:7-OPr (57) | S | [Noc] Erebidae (Arctiinae) | Barsine expressa | 13 [74] | |
| 17:8-OPr (58) | S | [Noc] Erebidae (Arctiinae) | Barsine expressa | 13 [74] | |
| Ketone | Z7-18:11-one (=Z11-18:8-one) (59) | achiral | [Cop] Carposinidae | Coscinoptycha improbana | 06 [69] |
| Z7-23:11-one (60) | achiral | [Cop] Carposinidae | Coscinoptycha improbana | 06 [69] | |
a) Superfamilies are abbreviated as follows; [Cop] Copromorphoidea, [Gel] Gelechioidea, [Noc] Noctuoidea, [Pyr] Pyraloidea, [Tin] Tineoidea, and [Zyg] Zygaenoidea. b) 1-Ethyl-2-methylpropyl ester. c) This compound was identified from Arna pseudoconspersa in 1994,75) but not cited in a previous review.
The ester of a novel acid with a Me3,Me13-15 structure (45) is a pheromone component of a bagworm moth. A pheromone gland of female moths is usually placed at a terminal abdominal segment, but a vermiform apterous female in Psychidae has it in the dorsal mesothorax. The female stays within a bag and releases pheromone-impregnated scales from the bag to attract a winged male of the conspecies. The identification of this methyl-branched ester demonstrates an example of further structural diversity of the lepidopteran pheromones, because all other esters of a long-chain acid, including newly identified compounds (52–55), have no branches in the even-numbered chain.6) In addition to two known esters of saturated acids, new isopropyl and sec-butyl esters of Z5-10 acid (52 and 53) have been identified from another Psychidae species. Besides these Psychidae pheromones, seven esters of unsaturated acids had been previously recorded from several species in Zygaenoidea, and a butyl ester of 7,9-10 acid (54) and sec-butyl ester of 7-12 acid (55) were added. The butyl alcohol and 7-12 acid are new moieties included in the ester pheromones with a long-chain acid. These unbranched esters might be considered extraordinary Type I compounds. Normal Type I compounds are biosynthesized via the step of converting an acyl intermediate to an alcohol by a fatty acyl reductase. The unsaturated acid moieties in the ester pheromones are expected to bind to a certain alcohol without being reduced after being produced in the same manner as the normal Type I pheromones.
Another ester with a dimethyl structure in a long-chain alcohol moiety has been identified from Lymantriinae species. Two species in this evolved group produce an isobutylate of a primary alcohol with a Me10,Me14-15 structure (46) as a pheromone component. Thus, the Lymantriinae pheromones are composed of a variety of compounds, such as Type I and II compounds, unsaturated ketones, branched hydrocarbons, their epoxy derivatives, and esters of a branched primary alcohol. On the other hand, a known secondary alcohol with a Me6,Me10,Me14-15 structure (12) and the corresponding new 2-ketone (48) were identified from a Galleriinae species in Pyralidae. In a laboratory bioassay, the mixture initiated male courtship behavior associated with ultrasonic production but not long-distance attraction. Different from many other Phycitiinae species in Pyralidae, Type I compounds with attraction activity have not been found from this Galleriinae species.
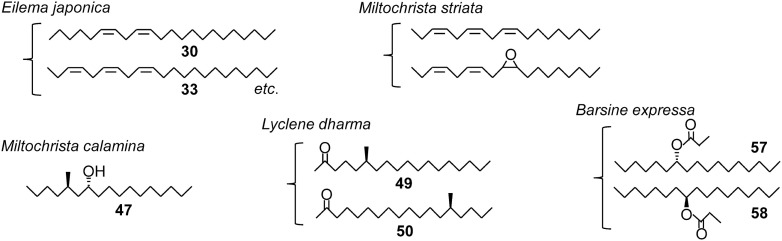
Two methyl-branched 2-ketones (49 and 50) were found from a lichen moth, a Lithosiini species in Arctiinae. In this subfamily, pheromone researches had been conducted mainly with the species in a different Arctiini tribe, not in Lithosiini, which has a lot of species, until the mid-2000s. While Lithosiini species whose larvae feed on lichen are not research subjects in applied entomology, experiments that expect to find new compounds from this insect group yielded further fruitful results, discovering new methyl-branched secondary alcohol (47) and propyl esters of unbranched secondary alcohols (57 and 58). These compounds have a hydroxyl or propyloxy group at an unusual 7- or 8-position. Figure 3 shows structures of the pheromones identified from five Lithosiini species. Two species utilize Type II compounds, but each of the other three species produces different compounds with a simple but characteristic structure. These unique pheromone compounds of the three species have little commonality with respect to the presence or absence of a methyl branch, the position of a methyl branch present, and the type and position of functional groups. Studies with a limited number of species designate three new chemical groups of lepidopteran pheromones. It will be interesting to see whether further studies will find new pheromone components, such as methyl-branched derivatives of 57 and 58, that connect discontinuity structures in these compounds.
Fig. 3. Sex pheromones identified from female lichen moths.
Pheromone studies of Tineidae species are limited, but many male attractants have been reported.4) While all of them are Type I compounds, an acetate of a C17 secondary alcohol (56) was newly identified. In Ditrysia, Tineidae is the family most closely taxonomically related to nonditrysian Eriocranioidea, whose female moths produce Type 0 compound such as Z4-7:2-OH (7). The acetate (56) has a long chain, as usual with Type I pheromones, but the acetoxy group is located at the 2-position as the hydroxyl group of the Type 0 pheromones. Information about pheromones of Carposinidae species is also limited.4) However, in addition to two known unsaturated ketones, Z7-20:11-one (8) and its analog with a C19 chain, C18 and C23 analogs (59 and 60), and a monoenyl hydrocarbon (51) were newly identified from a species in this family. All species in the related families use only Type I compounds for their mating communication, but their production by Carposinidae females and attraction of the males have not been reported.2) Some unsaturated 11-ketones have been identified from Lymantriinae, which is taxonomically far from Carposinidae. The pheromones of Lymantriinae species have a C21 chain and their double bonds are located at the 6-, 8-, or 9-position, not at the 7-position.6)
3. Current Techniques for Structure Determination and Synthesis
3.1. GC/FT-IR analysis
The sex pheromone, a volatile secreted by a female moth, is usually stored in the pheromone gland and can be easily extracted with hexane. While the pheromone content is generally very low, the extract is effectively analyzed using gas chromatography combined with an electro-antennogram detector (GC-EAD) and a mass spectrometer (GC/MS) with high sensitivity. The GC-EAD analysis indicates the number of pheromone candidates that stimulate a male antenna. The mass spectra of active components measured by GC/MS suggest the outline of their chemical structures. In addition to microchemical reactions of the pheromone extract followed by GC/MS analysis, a comparison with the chemical data of authentic synthetic compounds helps to reveal the precise structure of the natural pheromone. Although an IR spectrum gives information about the functional group and double-bond configuration, IR analysis had rarely been utilized for pheromone identifications, primarily due to the difficulty of isolating each pheromone component and the low sensitivity of the spectrometer. Recently, a new type of GC/Fourier transform infrared spectrophotometer (GC/FT-IR), equipped with a zinc selenide disk cooled to around −40°C, was developed. The disk is turned slowly, and compounds eluting from a capillary column are fixed on the disk in discrete positions. The resulting IR spectra, which are similar to the spectra recorded by the usual KBr or liquid-film methods, are measured continuously. Furthermore, the increased analytical sensitivity facilitated the application of GC/FT-IR to pheromone studies of lepidopteran species, particularly to determine the double-bond configurations of Type I and II compounds.
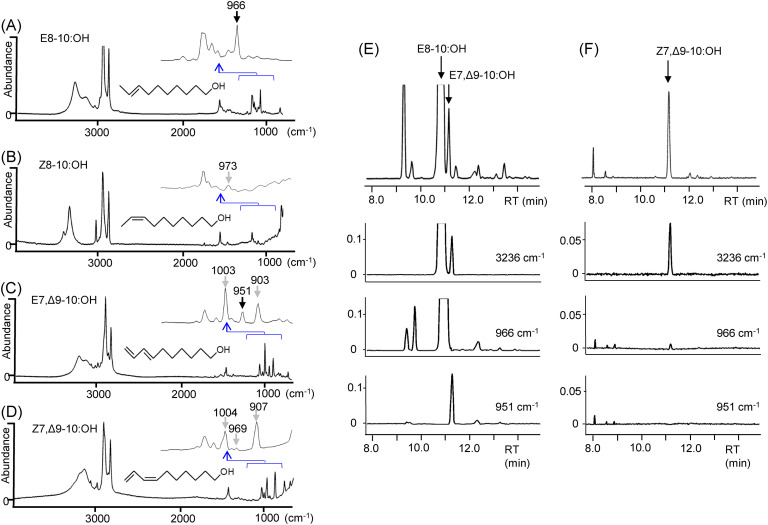
GC-EAD and GC/MS analysis indicated that females of the nettle moth, Monema flavescens (Limacodidae), produced dec-8-en-1-ol (13) and deca-7,9-dien-1-ol at a ratio of approximately 9 : 1.20) Based on these results, the structure determination was completed via GC/FT-IR analysis with the crude extract. The IR spectra of the monoenyl and dienyl alcohols showed characteristic absorptions at 966 and 951 cm−1, respectively (Fig. 4-A, -C), indicating an E configuration for both components. Their synthetic (Z)-isomers did not show these absorptions (Fig. 4-, -D), and band chromatograms of the extract confirmed this configuration (Fig. 4-E). The chromatogram at 3236 cm−1 reveals the two alcohol components in the extract, and those at 966 and 951 cm−1 clarify their E configuration. The absorption at 951 cm−1 is weak, but the band chromatogram indicates that the absorption is derived from the minor alcohol component, not any impurities. This chromatogram at 951 cm−1 is particularly useful for distinguishing the geometric isomers of terminal conjugated dienes, which are difficult to resolve even on highly polar capillary columns. On the other hand, a Z configuration of the same 7,9-dienyl alcohol secreted by another nettle moth, Parasa lepida, was confirmed through the absence of this absorption (Fig. 4-F).20)
Fig. 4. Infrared spectra obtained by GC/FT-IR of the pheromone components of Monema flavescens, (A) and (C), and synthetic geometric isomers, (B) and (D), and band chromatograms of pheromone extracts; M. flavescens (E) and Parasa lepida (F).
GC/FT-IR analysis was also applied to the structure determination of new Type II compounds. A pheromone extract of the wood tiger moth, Arctia plantaginis (Erebidae: Arctiinae), included four pheromone components, A–D, with an unsaturated C21 chain at a ratio of 30 : 3 : 5 : 1.45) Comparing their mass spectra with those of synthetic standards, Z3,Z6,Z9-21:H and Δ1,Z3,Z6,Z9-21:H were assigned for Comps. A and C, respectively. Furthermore, Comps. B and D were estimated to be new pheromone compounds with 4,6,9-trienyl and 2,4,6,9-tetraenyl structures, respectively. As a next step, the extract was analyzed using GC/FT-IR. The Z configuration of three double bonds in Comps. A and C was confirmed by the absorption at around 3010 cm−1 of C–H stretching at a 1,2-disubstituted double bond and the absence of absorption at around 970 cm−1 of the C–H bending with an E configuration. Additionally, Comp. C showed absorptions at 1000 and 899 cm−1, caused by C–H bending of geminal hydrogen atoms at the terminal double bond. On the other hand, Comp. B showed two absorptions at 983 and 951 cm−1, which are characteristic for conjugated dienes with an E,Z configuration and revealed an E configuration at the 4-position. Similar absorption at 993 and 941 cm−1 of Comp. D suggests that it includes an E,Z configuration. A conjugated diene with a Z,Z configuration is characterized by a C-H stretching absorption at around 3040 cm−1, but Comp. D showed absorption at 3021 cm−1, indicating the absence of a conjugated dienyl system with a Z,Z configuration. Therefore, Z and E configurations were assigned for the 2- and 4-positions of Comp. D, respectively. Their Z double bonds at the 6- and 9-positions were indicated by the fact that there were no absorptions around 970 cm−1; thus, Comps. B and D are identified as E4,Z6,Z9-21:H (34) and Z2,E4,Z6,Z9-21:H (37), respectively.45)
The sensitivity of the GC/FT-IR is comparable to that of GC/MS, and IR spectra offer some important information for structure determination that is difficult to obtain with mass spectra. In the future, it is expected that GC/FT-IR instruments will become widespread and be routinely used in many analytical studies on trace natural products, especially insect pheromones.76)
3.2. Synthesis and GC/MS analysis of Type II epoxy pheromone candidates
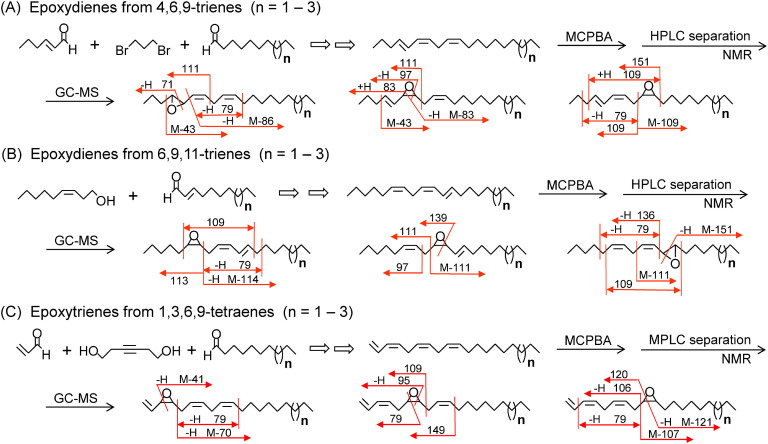
Monoepoxides derived from Z6,Z9-dienes and Z3,Z6,Z9-trienes are key components of Type II pheromones. Each epoxide shows characteristic fragment ions in the mass spectrum, and thus positional isomers with an epoxy ring at a different position are easily differentiated by some diagnostic ions.77) Additionally, the 4,5-epoxide of E4,Z6,Z9-19:H (t-epo4,Z6,Z9-19:H), the 11,12-expoxide of Z6,Z9,E11-21:H (Z6,Z9,t-epo11-21:H), and the 9,10-epoxide of Δ1,Z3,Z6,Z9-21:H (Δ1,Z3,Z6,epo9-21:H,6) have been identified from Geometridae and Erebidae species.2) Considering the diversity of these insect groups, it seems quite possible that some species utilize their positional isomers as a pheromone component. Therefore, to provide a useful tool for studying the structure of a new component in the future, monoepoxy compounds including one more double bond than the basic Type II epoxy compounds were systematically synthesized, and their mass spectra were analyzed (Fig. 5).
Fig. 5. Preparation of epoxy derivatives from E4,Z6,Z9-trienes (A), Z6,Z9,E11-trienes (B), and Δ1,Z3,Z6,Z9-tetraenes (C), and their diagnostic fragment ions for the GC/MS analysis. See mass spectra of Suppl. Fig. S4 for the epoxydienes from E4,Z6,Z9-21:H, Supplemental Fig. S5 for the epoxydienes from Z6,Z9,E11-21:H, and Supplemental Fig. S6 for the epoxytrienes from Δ1,Z3,Z6,Z9-21:H.
As the first step, polyunsaturated hydrocarbons with a C19–C21 chain were prepared using a Wittig reaction.78) E4,Z6,Z9-trienes were obtained via the coupling reaction between a bis(ylide) prepared from 1,3-dibromopropane and two aldehydes, (E)-hex-2-enal and a C10–C12 alkanal, in one pot. Synthesis of the Z6,Z9,E11-trienes was started from (Z)-non-3-en-1-ol. After iodination of the alcohol, an ylide prepared from the iodide was coupled with a C10–C12 (E)-alk-2-enal synthesized from a C8–C10 alkanal to yield the objective trienes. Synthesis of the Δ1,Z3,Z6,Z9-tetraenes was started from hex-3-yne-1,6-diol, which was converted into (Z)-1,6-diiodohex-3-ene by hydrogenation and iodination. The coupling reaction between a bis(ylide) prepared from the diiodide and two aldehydes, acrolein and a C10–C12 alkanal, yielded the objective tetraenes. Treatment of each polyene with a peracid produced a mixture of monoepoxides as follows: a 3 : 6 : 2 mixture of 4,5-epoxide, 6,7-epoxide, and 9,10-epoxide from the E4,Z6,Z9-triene,79) a 1 : 6 : 2 mixture of 6,7-epoxide, 9,10-epoxide, and 11,12-epoxide from the Z6,Z9,E11-triene,80) and a 1 : 2 : 2 mixture of 3,4-epoxide, 6,7-epoxide, and 9,10-epoxide from the Δ1,Z3,Z6,Z9-tetraene.81) This peracid oxidation of the tetraene did not yield 1,2-epoxide. Before GC/MS analysis, each epoxide was isolated by HPLC or MPLC and its structure was confirmed by NMR.
Comparing the mass spectra of three compounds with the same unsaturated epoxy structure in a C19–C21 chain, characteristic fragment ions reflecting the structure were understood as shown in Fig. 5.79–81) A molecular ion (M+) was detectable in all epoxides (relative intensity: 1–8%), and positional isomers were also separable by GC. When a female moth secrets a compound in these groups, the new pheromone components can be easily identified by examining these diagnostic ions. These monoepoxides derived from the polyenes showed an abundant ion at m/z 79, [H(CH=CH)3]+, except for E4,epo6,Z9 and 6Z,epo9,E11 compounds which exhibit a base peak at m/z 83 and 69, respectively. The m/z 79 ion is a base peak of E4,Z6,epo9, epo6,Z9,E11, Δ1,epo3,Z6,Z9, and Δ1,Z3,epo6,Z9 compounds, while t-epo4,Z6,Z9, Z6,Z9,t-epo11, and Δ1,Z3,Z6,epo9 compounds show a base peak at m/z 71, M-151 (or 57), and 106, respectively. Diagnostic ions of the epoxytrienes derived from the Δ1,Z3,Z6,Z9-tetraenes are compatible with those of the epoxydienes derived from Z3,Z6,Z9-trienes. Namely, the following ions are characteristic in the mass spectra of the epoxydienes: ions at m/z M-72 of 3,4-epoxides; ions at m/z 97 and 111 of 6,7-epoxides; and ions at m/z 108, 122, M-123, and M-109 of 9,10-epoxides.77)
3.3. Enantioselective synthesis of methyl-branched pheromones
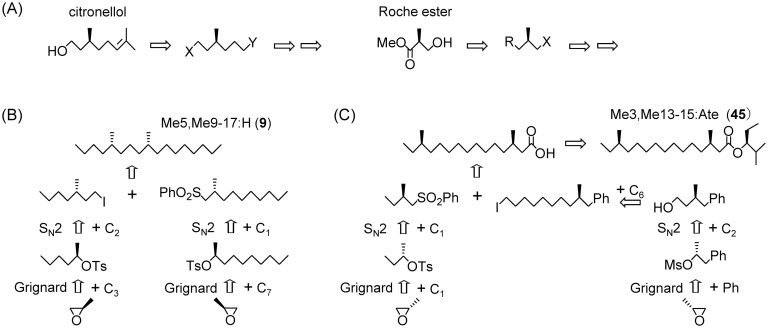
Not only lepidopteran insects but also a vast number of arthropod species use methyl-branched compounds for their chemical communication. Almost all of the branched compounds are chiral because a tertiary carbon is a stereogenic center, except for that at the 2- or ω2-position. Since optically active synthetic standards are necessary in order to clarify the absolute configuration of natural components, many studies on enantioselective synthesis have been carried out.82) One of the most important methods is the utilization of citronellol and a Roche ester as starting materials (Fig. 6-A). Citronellol is converted into a chiral methyl-branched synthon with two different functional groups at the ends of the C6 chain, and the Roche ester is another compact bifunctional synthon. Their both enantiomers are commercially available. In addition, both enantiomers of propylene oxide with a high enantiomeric excess (ee), also commercially available, have recently been utilized as chiral sources for some branched pheromones (Fig. 6-B, -C).
Fig. 6. Notable chiral synthons for enantioselective syntheses of methyl-branched pheromones (A) and new synthetic approaches using chiral propylene oxide, (B) and (C).
The coupling reaction between the oxide and a Grignard reagent formed a chiral 2-hydroxy compound, and its tosylate was converted into two kinds of methyl-branched building blocks with an inverse configuration by an SN2 reaction with appropriate alkylating reagents, such as the anion of methyl phenyl sulfone (PhSO2CH2−) and the enolate of dimethyl malonate [CH−(CO2Me)2]. The former reagent produced the chiral block branched at the 2-position, and the latter produced another chiral block branched at the 3-positon after decarboxylation. Stereospecific conversion has rarely been used in pheromone syntheses because of the risk of racemization. However, enantioselective HPLC analysis verified the high enantiomeric purity (>99% ee) of these blocks, indicating perfect inversion. Compounds with a 1,5-dimethyl structure were obtained by coupling these two blocks with a methyl branch at the 2- or 3-position. Namely, four stereoisomers of Me5,M9-17:H (9) were synthesized with the chiral 2- and 3-methyl blocks including a C7 and C3 alkyl chain of the Grignard reagents, respectively (Fig. 6-B).83) In the same manner, four stereoisomers of Δ1,Me10,Me14-18:H, a pheromone of the apple leafminer, were synthesized with other two blocks including a C3 alkyl and C8 alkenyl chain of the Grignard reagents.84) In order to determine the absolute configuration of Me6,Me10,Me13-14:2-one, a new pheromone component of a stink bug (Heteroptera), four stereoisomers were also synthesized by coupling two chiral blocks prepared with different Grignard reagents.85)
Enantioselective synthesis using the SN2 reaction was also applied to the sex pheromone of the bagworm moth, Me3,Me13-15:Ate (45), containing two methyl branches at distant positions of the acid moiety (Fig. 6-C).86) The 2-methyl block was prepared from propylene oxide via successive reactions with MeMgBr, tosyl chloride, and PhSO2CH2−. The 3-methyl block was prepared differently, via successive reactions with PhMgBr, mesyl chloride, CH−(CO2Me)2, and LiCl for decarboxylation. The phenyl group is a key structural element because it can be converted into a carboxyl group by oxidation with RuO4 with moderate yield. This building block is a useful bifunctional chiral synthon including a methyl branch, and it is possible to elongate the carbon chain. The block with a long chain was coupled with the 2-methyl block, and the produced acid moiety was esterified with (S)-2-methylpentan-3-ol synthesized from (S)-valine to yield the ester pheromone (45). The advantage of starting with a chiral propylene oxide is that any type of building block can be prepared by modifying the Grignard reagent at the first reaction and the nucleophile of the SN2 reaction.
Enantioselective synthesis with high flexibility is useful to supply not only targeted pheromones but also their analogues, which could possibly be produced by taxonomically related species. Furthermore, the structure and activity relationships of a pheromone can be understood with a series of the analogues. A simple synthetic route to the pheromone of a lichen moth, Me5-17:7-OH (47), starting from (S)-propylene oxide was developed using the SN2 reaction and the Jacobsen hydrolytic kinetic resolution of an epoxide intermediate as key steps. Six analogues with the same configuration as (5R,7R)-47 but with a different alkyl chain(s) connected to the stereogenic centers were prepared. Their field tests revealed that males distinguished the configurations of methyl and hydroxyl groups but were less able to perceive differences in the lengths of the two alkyl chains in the pheromone.64)
4. Pheromone Biosynthesis
Type I compounds are biosynthesized in a pheromone gland via following steps: (1) de novo synthesis of a saturated fatty acid skeleton using acetyl-CoA and malonyl-CoA, (2) desaturation to an unsaturated fatty acyl intermediate, (3) chain shortening or elongation (if necessary), (4) reduction of the acyl moiety into a hydroxyl group, and (5) acetylation or oxidation of the alcohol (if necessary).18) This biosynthetic pathway has been investigated in many species and understood well on the enzymatic level. In particular, desaturases are noticeable enzymes because the variety of unsaturated chain skeletons is due to their reaction site specificity and substrate selectivity. After cloning and functional expression of a cDNA encoding the Δ11-desaturase of the cabbage looper moth,87) many enzymes have been identified from more than 20 species; i.e., Δ5-, Δ6-, Δ8-, Δ9-, Δ10-, Δ11-, and Δ14-desaturases have been characterized. They conserve three histidine-rich motifs implicated in iron-binding and four protein transmembrane domains.88) Their phylogenetic analysis shows groupings that reflect the desaturating positions.18) Further structural analysis is required to show the mechanism by which each desaturase performs desaturation at a particular position.
On the other hand, polyunsaturated hydrocarbons of Type II pheromones are biosynthesized from dietary linoleic and linolenic acids via steps such as chain elongation and decarboxylation in oenocytes, where cuticle hydrocarbons are produced. The lipophilic polyenes are transported to a pheromone gland through hemolymph after association with lipophorin and released outside the gland or converted into epoxyalkenyl components. Experiments with several synthetic polyenes with a different chain length showed low substrate selectivity of epoxidases in the gland. The formation of species-specific epoxyalkenyl pheromones results from the rigid formation of polyunsaturated precursors and their epoxidation at a fixed position.89) The 1,3,6,9-tetraene with a C21 chain, a precursor of the 9,10-epoxy pheromone of H. cunea (6), was found in the hemolymph of the female moth,90) suggesting that not only 6,9-dienes and 3,6,9-trienes but also further-desaturated hydrocarbons were prepared in the oenocytes. Namely, desaturases for modified Type II pheromones act outside the pheromone gland, which plays a role in the epoxidation and release of pheromone components.
Whereas biosynthetic studies on Type II pheromones have been conducted with a limited number of species, three cytochrome P450s have recently been identified as epoxidation enzymes; i.e., a 3,4-epoxidase belonged to a CYP340 family from A. selenaria91) and 9.10-epoxidases belonged to a CYP341 family from H. cunea92) and the mulberry tiger moth (Lemyra imparilis).93) The identification of a 6,7-epoxidase is desired. The sex pheromone of H. cunea is composed of Z3,Z6,epo9-21:H, Δ1,Z3,Z6,epo9-21:H (6), Z6,Z9-18:Ald, and Z3,Z6,Z9-18:Ald. The structures of the two unsaturated aldehydes demonstrate their derivatization from linoleic and linolenic acids, origin materials of the epoxy components. Conversion of the corresponding alcohols into the aldehydes in the pheromone gland has been experimentally confirmed, indicating another role of the pheromone gland in which an alcohol dehydrogenase acts.90) The Type I and II pheromones are differentiated by the presence or absence of a terminal functional group. However, considering the biosynthetic aspect, these aldehyde components of the H. cunea pheromone can be assigned to Type II compounds.
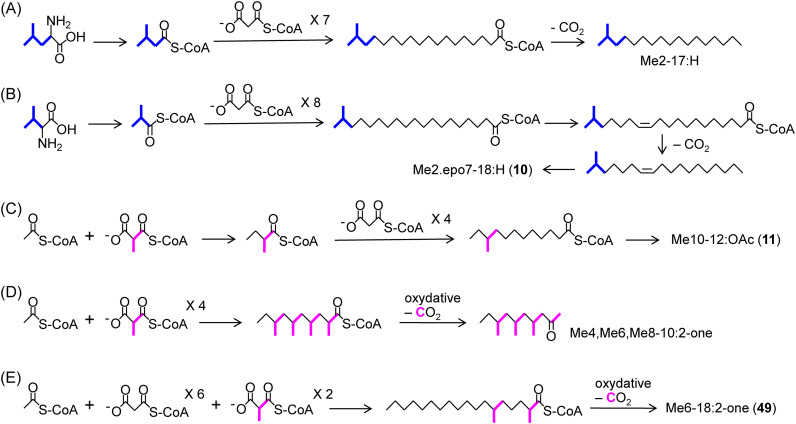
In the case of methyl-branched pheromones, only two studies have been reported. Experiments with three Homomelina species in Arctiinae have shown the biosynthesis of Me2-17:H starting from leucine, which is first converted to isovaleryl-CoA. The C4 chain is extended by a fatty acid synthase into a C18 chain that incorporates the C2 unit from malonyl-CoA seven times, and then decarboxylation produces the C17 chain pheromone (Fig. 7-A).94) Another study with L. dispar has revealed the biosynthesis of Me2,epo7-18:H (10) starting from valine, which is converted to isobutyryl-CoA. The C3 chain is extended to C19, and Me2,Z7-18:H, a precursor of 10, is formed by desaturation and decarboxylation (Fig. 7-B).95) These methyl-branched hydrocarbons are produced in oenocytes. On the other hand, most of the compounds with a methyl-branch(es) located other than at the 2- or ω2-position are expected to be propanogenins formed by the incorporation of C3 unit(s) derived from methylmalonyl-CoA in the biosynthesis catalyzed by fatty acid synthases or polyketide synthases.82) For example, the skeleton of Me10-12:OAc (11), a minor pheromone component of A. honmai, can be constructed with one acetyl-CoA, one methylmalonyl-CoA, and four malonyl-CoAs (Fig. 7-C). This biosynthesis may proceed in a pheromone gland like other Type I components of this species; meanwhile, methyl-branched hydrocarbons, such as Me5,Me9-17:H (9), are expected to be biosynthesized in oenocytes.
Fig. 7. Biosynthetic pathways for methyl-branched pheromones of Arctiinae moths (A), the gypsy moth (B), the smaller tea tortrix (C), the storage mite (D), and the lichen moth (E). The pathways of (C) and (E) are putative.
Among the new compounds found from lichen moths, methyl-branched 2-ketones (49 and 50) are interesting, because several insect and mite species utilize compounds in the same group as a chemical communication cue, e.g., Me3,Me11-29:2-one of the German cockroach (Blattella germanica) and Me4,Me6,Me8-10:2-one of the storage mite (Chortoglyphus arcuatus). The former sex pheromone is biosynthesized from the corresponding hydrocarbon by selective oxidation at the 2-position.96) The latter aggregation pheromone is produced by oxidative decarboxylation of 2,4,6,8-tetramethyldecyl-CoA, which includes four C3 units derived from methylmalonyl-CoA (Fig. 7-D).97) This pathway of the mite not through a hydrocarbon is reasonable because of the similarity of two terminal parts in its short chain. The structures of 49 and 50 with a C18 main chain also negate the possibility of their biosynthesis via branched hydrocarbons. If 6-methyl (=13-methyl) and 14-methyl (=5-methyl) hydrocarbons are precursors of the 2-ketones of the lichen moth, it would be difficult to make a mixture of only two 2-ketones. Experimental demonstration of whether the monomethyl compounds are biosynthesized via the incorporation of two C3 units and oxidative decarboxylation in the pheromone gland is a challenge for the future (Fig. 7-E).
5. Database of Arthropod Semiochemicals
The chemical structures of semiochemicals such as pheromones and allomones acting intra- or interspecies have been elucidated from a huge number of arthropods, including not only insects but also some species of spiders, mites, and millipedes. Based on reviews by Francke and Schulz98) and our group,82) a new database (“Pheromone database, Part II”) is being created to summarize the research results accumulated over time by many chemists and entomologists around the world.5) It contains 806 compounds, whose identification and synthesis have been reported by more than 1,300 publications. These compounds are broadly divided into three groups: terpenes, methyl-branched nonterpene compounds, and others. Furthermore, they are subdivided as follows: acyclic comp. (123), small-ring comp. (54), large-ring comp. (14), fused-ring comp. (68), and heterocyclic comp. (16) for the terpenes (total 275); hydrocarbons (49), primary alcohols and derivatives (28), secondary alcohols and esters (31), ketones (32), acids and derivatives (34), and ring comp. (27) for methyl-branched nonterpene compounds (total 201); acyclic comp. (185), aromatic comp. (79), and ring comp. (66) for others (total 330). Figures in parentheses show number of compounds included. Different from Part I, the data in Part II were collected with an emphasis on chemical structures. The compounds are listed in ascending order of the total carbon numbers and the main chain length or ring size, which are described for each compound in the database. Therefore, it is easy to retrieve a specific compound and its structurally related compounds.
Arthropods most widely utilize terpenes with various chemical structures as semiochemicals. In addition to the differences in carbon skeletons, modification with different functional groups increases their variety. Methyl-branched nonterpene compounds are relatively simple in structure, but most of them contain chiral centers. Since determination of their absolute configuration is an important point, the database distinguishes between publications by the presence or absence of stereochemical analysis. The number of acyclic compounds listed in other groups is also large, even though they do not contain moth Type I and II pheromones, which are separately organized as “Part I.” Chain skeletons of the other compounds differ significantly from C4 to C37. The structures of moth pheromones are characteristic, and insects of other groups rarely secrete the same compounds identified from moths. The database shows that there are a few exceptions, such as Z9-16:Ald and Z9-18:Ald. These aldehydes are trail pheromones of an ant99) and sex pheromones of some moths in Crambidae and Noctuidae.4) The structural diversity of semiochemicals that induce a specific behavior to sustain the life and reproduction of species in arthropods provides a wonderland for natural product chemists. The database will help us to understand the chemical diversity of the semiochemicals and provide tips for future research.
Electronic supplementary materials
The online version of this article contains supplementary material (Supplemental Figures S1-S6 and Table S1), which is available at https://www.jstage.jst.go.jp/browse/jpestics/.
References
- 1) A. Butenandt, R. Beckmann, D. Stamm and E. Hecker: Z. Naturforsch. B 14b, 283–284 (1959). [Google Scholar]
- 2) https://lepipheromone.sakura.ne.jp/lepi_phero_list_eng.html
- 3) R. T. Cardé: “Ecological Theory and Integrated Pest Management,” ed. by M. Kogan, Cambridge University Press, New York, pp. 122–169, 2007.
- 4) https://www.pherobase.com/
- 5) https://lepipheromone.sakura.ne.jp/pdb_top_eng.html
- 6) T. Ando, S. Inomata and M. Yamamoto: Top. Curr. Chem. 239, 51–96 (2004). [DOI] [PubMed] [Google Scholar]
- 7) Y. Tamaki, K. Kawasaki, H. Yamada, T. Koshihara, N. Osaki, T. Ando, S. Yoshida and H. Kakinohana: Appl. Entomol. Zool. 12, 208–210 (1977). [Google Scholar]
- 8) K. Witjaksono, K. Ohtani, M. Yamamoto, T. Miyamoto and T. Ando: J. Chem. Ecol. 25, 1633–1642 (1999). [Google Scholar]
- 9) M. Tóth, H. R. Buser, A. Peňa, H. Arn, K. Mori, T. Takeuchi, L. N. Nikolaeva and B. G. Kovalev: Tetrahedron Lett. 30, 3405–3408 (1989). [Google Scholar]
- 10) J.-W. Zhu, M. V. Kozlov, P. Philipp, W. Francke and C. Löfstedt: J. Chem. Ecol. 21, 29–43 (1995). [DOI] [PubMed] [Google Scholar]
- 11) Y. Tamaki, K. Honma and K. Kawasaki: Appl. Entomol. Zool. 12, 60–68 (1977). [Google Scholar]
- 12) W. Francke, S. Franke, M. Toth, G. Szöcs, P. Guerin and H. Arn: Naturwissenschaften 74, 143–144 (1987). [Google Scholar]
- 13) M. Tóth, G. Helmchen, U. Leikauf, G. Y. Sziráki and G. Szöcs: J. Chem. Ecol. 15, 1535–1543 (1989). [DOI] [PubMed] [Google Scholar]
- 14) B. A. Bierl, M. Beroza and C. W. Collier: Science 170, 87–89 (1970). [DOI] [PubMed] [Google Scholar]
- 15) Y. Tamaki, H. Sugie, M. Osakabe and P. Sonnet: Appl. Entomol. Zool. 18, 292–294 (1983). [Google Scholar]
- 16) D. R. Hall, A. Cork, R. Lester, B. F. Nesbitt and P. Zagatti: J. Chem. Ecol. 13, 1575–1589 (1987). [DOI] [PubMed] [Google Scholar]
- 17) K. Mori, H. Harada, P. Zagatti, A. Cork and D. R. Hall: Liebigs Ann. Chem. 1991, 259–267 (1991). [Google Scholar]
- 18) C. Löfstedt and J. G. Millar: “Pheromone Communication in Moths,” eds. by J. D. Allison and R. T. Cardé, University of California Press, CA, pp. 43–78, 2016.
- 19) R. Zahiri, I. J. Kitching, J. D. Lafontaine, M. Mutanen, L. Kaia, J. D. Holloway and N. Wahlberg: Zool. Scr. 40, 158–173 (2010). [Google Scholar]
- 20) H. Shibasaki, M. Yamamoto, Q. Yan, H. Naka, T. Suzuki and T. Ando: J. Chem. Ecol. 39, 350–357 (2013). [DOI] [PubMed] [Google Scholar]
- 21) A. M. L. Soares, P. H. B. França, M. F. Triana, J. M. D. Santos, N. S. Dias-Pini, H. F. Goulart, J. X. Araújo-Júnior and A. E. G. Santana: Pest Manag. Sci. 76, 1435–1442 (2020). [DOI] [PubMed] [Google Scholar]
- 22) C. Y. Yang, K. S. Choi and M. R. Cho: J. Chem. Ecol. 39, 555–558 (2013). [DOI] [PubMed] [Google Scholar]
- 23) Q. Yan, K. Kuriyama, K. Nishikawa, S. Tominaga, H. Tatsuta, T. Ando and H. Naka: J. Chem. Ecol. 41, 441–445 (2015). [DOI] [PubMed] [Google Scholar]
- 24) J. G. Millar, K. F. Haynes, A. T. Dossey, J. S. McElfresh and J. D. Allison: J. Chem. Ecol. 42, 869–876 (2016). [DOI] [PubMed] [Google Scholar]
- 25) A. Levi-Zada, D. Fefer, L. Anshelevitch, A. Litovsky, M. Bengtsson, G. Gindin and V. Soroker: Tetrahedron Lett. 52, 4550–4553 (2011). [Google Scholar]
- 26) A. Levi-Zada, A. Sadowsky, S. Dobrinin, M. David, T. Ticuchinski, D. Fefer, A. Greenberg and D. Blumberg: Chemoecology 23, 13–20 (2013). [Google Scholar]
- 27) M. D. A. Coracini, M. Bengtsson, A. Reckziegel, J. Löfqvist, W. Francke, E. F. Vilela, A. E. Eiras, A. Kovaleski and P. Witzgall: J. Econ. Entomol. 94, 911–914 (2001). [DOI] [PubMed] [Google Scholar]
- 28) M. D. A. Coracini, M. Bengtsson, A. Reckziegel, A. E. Eiras, E. F. Vilela, P. Anderson, W. Francke, J. Löfqvist and P. Witzgall: J. Appl. Entomol. 127, 427–434 (2003). [Google Scholar]
- 29) M.-H. Yang, H.-X. Liu, J.-L. Liu, X.-Y. Jing, J.-T. Zhang, L.-H. Fan and S.-F. Wang: Entomol. Exp. Appl. 154, 199–205 (2015). [Google Scholar]
- 30) T. Uehara, H. Naka, S. Matsuyama, L. V. Vang, T. Ando and H. Honda: J. Chem. Ecol. 39, 1441–1447 (2013). [DOI] [PubMed] [Google Scholar]
- 31) H. Herrera, W. Barros-Parada, M. F. Flores, W. Francke, E. Fuentes-Contreras, M. Rodriguez, F. Santis, P. H. G. Zarbin and J. Bergmann: J. Chem. Ecol. 42, 908–918 (2016). [DOI] [PubMed] [Google Scholar]
- 32) S. L. Lapointe, W. Barros-Parada, E. Fuentes-Contreras, H. Herrera, T. Kinsho, Y. Miyake, R. P. Niedz and J. Bergmann: J. Chem. Ecol. 43, 1046–1655 (2017). [DOI] [PubMed] [Google Scholar]
- 33) S. Wakamura, S. Ohno, N. Arakaki, T. Kohama, D. Haraguchi and H. Yasui: Appl. Entomol. Zool. 45, 635–640 (2010). [Google Scholar]
- 34) Q. Yan, L. V. Vang, C. N. Q. Khanh, H. Naka and T. Ando: J. Chem. Ecol. 40, 590–598 (2014). [DOI] [PubMed] [Google Scholar]
- 35) R. Gago, J. D. Allison, J. S. McElfresh, K. F. Haynes, J. McKenney, A. Guerrero and J. G. Millar: J. Chem. Ecol. 39, 1263–1272 (2013). [DOI] [PubMed] [Google Scholar]
- 36) J. G. Millar, M. Hoddle, J. S. McElfresh, Y. Zou and C. Hoddle: Tetrahedron Lett. 49, 4820–4823 (2008). [Google Scholar]
- 37) M. S. Hoddle, J. G. Millar, C. D. Hoddle, Y. Zou and J. S. McElfresh: J. Econ. Entomol. 102, 1460–1467 (2009). [DOI] [PubMed] [Google Scholar]
- 38) K. Ryall, P. J. Silk, J. Wu, P. Mayo, M. A. Lemay and D. MaGee: Naturwissenschaften 97, 717–724 (2010). [DOI] [PubMed] [Google Scholar]
- 39) T. Fujii, R. Nakano, Y. Takubo, S. Qian, R. Yamakawa, T. Ando and Y. Ishikawa: J. Insect Physiol. 56, 1986–1991 (2010). [DOI] [PubMed] [Google Scholar]
- 40) R. Gries, G. Khaskin, E. Khaskin, J. L. Foltz, P. W. Schaefer and G. Gries: J. Chem. Ecol. 29, 2201–2212 (2003). [DOI] [PubMed] [Google Scholar]
- 41) G. G. Grant, K. N. Slessor, W. Liu and M. M. Abou-Zaid: J. Chem. Ecol. 29, 589–601 (2003). [DOI] [PubMed] [Google Scholar]
- 42) G. G. Grant, M. D. Coppens, L. K. Hartling, D. O’Shea, D. Winter, J. Gordon, J. Rudderham and W. Liu: Entomol. Exp. Appl. 126, 174–178 (2008). [Google Scholar]
- 43) A. M. El-Sayed, A. R. Gibb, D. M. Suckling, B. Bunn, S. Fielder, D. Comeskey, L. A. Manning, S. P. Foster, B. D. Morris, T. Ando and K. Mori: J. Chem. Ecol. 31, 621–646 (2005). [DOI] [PubMed] [Google Scholar]
- 44) R. Gries, G. Khaskin, J. Cleawater, D. Hasman, P. W. Schaefer, E. Khaskin, O. Miroshnychenko, G. Hosking and G. Gries: J. Chem. Ecol. 31, 603–620 (2005). [DOI] [PubMed] [Google Scholar]
- 45) Y. Muraki, R. Yamakawa, M. Yamamoto, H. Naka, A. Honma, J. Mappes, K. Suisto and T. Ando: Am. J. Chem. Anal. 8, 645–656 (2017). [Google Scholar]
- 46) R. Yamakawa, N. D. Do, Y. Adachi, M. Kinjo and T. Ando: Tetrahedron Lett. 50, 4738–4740 (2009). [Google Scholar]
- 47) R. Yamakawa, N. D. Do, K. Kinjo, Y. Terashima and T. Ando: J. Chem. Ecol. 37, 105–113 (2011). [DOI] [PubMed] [Google Scholar]
- 48) B. P. Molnár, A. Tröger, T. B. Toshova, M. Subchev, E. J. van Nieukerken, J. C. Koster, G. Szöcs, M. Tóth and W. Francke: J. Chem. Ecol. 38, 1298–1305 (2012). [DOI] [PubMed] [Google Scholar]
- 49) W. S. Leal, A. L. Parra-Pedrazzoli, K.-E. Kaissling, T. I. Morgan, F. G. Zalom, D. J. Pesak, E. A. Dundulis, C. S. Burks and B. S. Higbee: Naturwissenschaften 92, 139–146 (2005). [DOI] [PubMed] [Google Scholar]
- 50) L. P. S. Kuenen, J. S. McElfresh and J. G. Millar: J. Econ. Entomol. 103, 314–330 (2010). [DOI] [PubMed] [Google Scholar]
- 51) H. Kanno, L. P. S. Kuenen, K. A. Klingler, J. G. Millar and R. T. Cardé: J. Chem. Ecol. 36, 584–591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52) Q. Yan, H.-D. Li, Y. Chen, Z.-F. Ye, X.-Y. You, J. Zhou, L.-F. Mu, S.-J. Liu, X.-B. Kong, S. A. Khuhro and S.-L. Dong: J. Chem. Ecol. 44, 886–893 (2018). [DOI] [PubMed] [Google Scholar]
- 53) J. G. Millar, G. G. Grant, J. S. McElfresh, W. Strong, C. Rudolph, J. D. Stein and J. A. Moreira: J. Chem. Ecol. 31, 1229–1234 (2005). [DOI] [PubMed] [Google Scholar]
- 54) W. B. Strong, J. G. Millar, G. G. Grant, J. A. Moreira, J. M. Chong and C. Rudolph: Entomol. Exp. Appl. 126, 67–77 (2008). [Google Scholar]
- 55) C. Löfstedt, G. P. Svensson, E. V. Jirle, O. Rosenberg, A. Roques and J. G. Millar: J. Appl. Entomol. 136, 70–78 (2012). [Google Scholar]
- 56) D. R. Hall, D. Farman, J. C. Domínguez and J. A. Pajares: J. Chem. Ecol. 43, 433–442 (2017). [DOI] [PubMed] [Google Scholar]
- 57) K. K. Schlamp, R. Gries, G. Khaskin, K. Brown, E. Khaskin, G. J. R. Judd and G. Gries: J. Chem. Ecol. 31, 2897–2911 (2005). [DOI] [PubMed] [Google Scholar]
- 58) R. Gries, G. Khaskin, T. Gotoh, P. W. Schaefer and G. Gries: J. Chem. Ecol. 31, 879–891 (2005). [DOI] [PubMed] [Google Scholar]
- 59) R. Gries, G. Khaskin, P. W. Schaefer, R. Hahn, T. Gotoh and G. Gries: J. Chem. Ecol. 31, 49–62 (2005). [DOI] [PubMed] [Google Scholar]
- 60) R. Gries, G. Khaskin, Z.-X. Tan, B.-G. Zhao, G. G. S. King, A. Miroshnychenko, G.-Q. Lin, M. Rhainds and G. Gries: J. Chem. Ecol. 32, 1673–1685 (2006). [DOI] [PubMed] [Google Scholar]
- 61) K. Mori, T. Tashiro, B. Zhao, D. M. Suckling and A. M. El-Sayed: Tetrahedron 66, 2642–2653 (2010). [Google Scholar]
- 62) S. Wakamura, T. Yasuda, Y. Hirai, H. Tanaka, T. Doki, Y. Nasu, M. Shibao, A. Yunotani and K. Kadono: Appl. Entomol. Zool. 42, 375–382 (2007). [Google Scholar]
- 63) R. Yamakawa, R. Kiyota, T. Taguri and T. Ando: Tetrahedron Lett. 52, 5808–5811 (2011). [Google Scholar]
- 64) Y. Muraki, T. Taguri, R. Yamakawa and T. Ando: J. Chem. Ecol. 40, 250–258 (2014). [DOI] [PubMed] [Google Scholar]
- 65) J. Kindl, P. Jiroš, B. Kalinová, P. Žáček and I. Valterová: J. Chem. Ecol. 38, 400–407 (2012). [DOI] [PubMed] [Google Scholar]
- 66) M. Yamamoto, T. Kamata, N. D. Do, Y. Adachi, M. Kinjo and T. Ando: Biosci. Biotechnol. Biochem. 71, 2860–2863 (2007). [DOI] [PubMed] [Google Scholar]
- 67) N. D. Do, M. Kinjo, T. Taguri, Y. Adachi, R. Yamakawa and T. Ando: Biosci. Biotechnol. Biochem. 73, 1618–1622 (2009). [DOI] [PubMed] [Google Scholar]
- 68) Y. Adachi, N. D. Do, M. Kinjo, S. Makisako, R. Yamakawa, K. Mori and T. Ando: J. Chem. Ecol. 36, 814–823 (2010). [DOI] [PubMed] [Google Scholar]
- 69) A. R. Gibb, D. M. Suckling, B. D. Morris, T. E. Dawson, B. Bunn, D. Comeskey and J. J. Dymock: J. Chem. Ecol. 32, 221–237 (2006). [DOI] [PubMed] [Google Scholar]
- 70) R. Rahmani, D. Carrasco, G. P. Svensson, H. Roweck, N. Ryrholm, M. C. Larsson and E. Hedenstroem: J. Chem. Ecol. 46, 115–127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71) M. S. Siderhurst, E. B. Jang, A. H. Hara and P. Conant: Entomol. Exp. Appl. 125, 63–69 (2007). [Google Scholar]
- 72) M. Subchev, T. Toshova, C. Koshio, S. Franke, A. Tröger, R. Twele, W. Francke, J. A. Pickett, L. J. Wadhams and C. M. Woodcock: Chemoecology 19, 47–54 (2009). [Google Scholar]
- 73) R. Gries, G. Khaskin, H. Daroogheh, C. Mart, S. Karadag, M. Kubilay Er, R. Britton and G. Gries: J. Chem. Ecol. 32, 2667–2677 (2006). [DOI] [PubMed] [Google Scholar]
- 74) T. Fujii, R. Yamakawa, Y. Terashima, S. Imura, K. Ishigaki, M. Kinjo and T. Ando: J. Chem. Ecol. 39, 28–36 (2013). [DOI] [PubMed] [Google Scholar]
- 75) S. Wakamura, T. Yasuda, A. Ichikawa, T. Fukumoto and F. Mochizuki: Appl. Entomol. Zool. 29, 403–411 (1994). [Google Scholar]
- 76) C. Schlawis and S. Schulz: Nat. Prod. Rep. 37, in press (2020). https://doi.org/10.1039/D0NP00013B [DOI] [PubMed] [Google Scholar]
- 77) T. Ando and R. Yamakawa: Trends Analyt. Chem. 30, 990–1002 (2011). [Google Scholar]
- 78) M. Yamamoto, R. Yamakawa, T. Oga, Y. Takei, M. Kinjo and T. Ando: J. Chem. Ecol. 34, 1057–1064 (2008). [DOI] [PubMed] [Google Scholar]
- 79) Y. Sakamoto and T. Ando: unpublished data.
- 80) M. Yamamoto, R. Maruyama, Y. Murakami, Y. Sakamoto, R. Yamakawa and T. Ando: Anal. Bioanal. Chem. 405, 7405–7414 (2013). [DOI] [PubMed] [Google Scholar]
- 81) R. Yamakawa, Y. Takubo, H. Shibasaki, Y. Murakami, M. Yamamoto and T. Ando: J. Chem. Ecol. 38, 1042–1049 (2012). [DOI] [PubMed] [Google Scholar]
- 82) T. Ando and R. Yamakawa: Nat. Prod. Rep. 32, 1007–1041 (2015). [DOI] [PubMed] [Google Scholar]
- 83) T. Taguri, M. Yamamoto, T. Fujii, Y. Muraki and T. Ando: Eur. J. Org. Chem. 2013, 6924–6933 (2013). [Google Scholar]
- 84) T. Taguri, K. Yaginuma, M. Yamamoto, T. Fujii and T. Ando: Biosci. Biotechnol. Biochem. 78, 761–765 (2014). [DOI] [PubMed] [Google Scholar]
- 85) Y. Muraki, T. Taguri, M. Yamamoto, P. H. G. Zarbin and T. Ando: Eur. J. Org. Chem. 2013, 2209–2215 (2013). [Google Scholar]
- 86) T. Taguri, M. Yamamoto, T. Fujii, Y. Muraki and T. Ando: Eur. J. Org. Chem. 2013, 6924–6933 (2013). [Google Scholar]
- 87) D. C. Knipple, C.-L. Rosenfield, S. J. Miller, W. Liu, J. Tang, P. W. K. Ma and W. L. Roelofs: Proc. Natl. Acad. Sci. U.S.A. 95, 15287–15292 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88) D. C. Knipple, C.-L. Rosenfield, R. Nielsen, K. M. You and S. E. Jeong: Genetics 162, 1737–1752 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89) T. Ando, T. Kawai and K. Matsuoka: J. Pestic. Sci. 33, 17–20 (2008). [Google Scholar]
- 90) R. Kiyota, M. Arakawa, R. Yamakawa, A. Yasmin and T. Ando: Insect Biochem. Mol. Biol. 41, 362–369 (2011). [DOI] [PubMed] [Google Scholar]
- 91) Y. Rong, T. Fujii, S. Katsuma, M. Yamamoto, T. Ando and Y. Ishikawa: Insect Biochem. Mol. Biol. 54, 122–128 (2014). [DOI] [PubMed] [Google Scholar]
- 92) Y. Rong, T. Fujii, H. Naka, M. Yamamoto and Y. Ishikawa: Insect Biochem. Mol. Biol. 107, 46–52 (2019). [DOI] [PubMed] [Google Scholar]
- 93) Y. Rong, T. Fujii and Y. Ishikawa: Insect Biochem. Mol. Biol. 108, 9–15 (2019). [DOI] [PubMed] [Google Scholar]
- 94) R. E. Charlton and W. L. Roelofs: Arch. Insect Biochem. Physiol. 18, 81–97 (1991). [Google Scholar]
- 95) R. A. Jurenka, M. Subchev, J.-L. Abad, M.-Y. Choi and G. Fabrias: Proc. Natl. Acad. Sci. U.S.A. 100, 809–814 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96) J. Chase, R. A. Jurenka, C. Schal, P. P. Halarnkar and G. J. Blomquist: Insect Biochem. 20, 149–156 (1990). [Google Scholar]
- 97) S. Schulz, J. Fuhlendorff, J. L. M. Steidle, J. Collatz and J. T. Franz: ChemBioChem 5, 1500–1507 (2004). [DOI] [PubMed] [Google Scholar]
- 98) W. Francke and S. Schulz: “Comprehensive Natural Products II, Chemistry and Biology,” eds. by L. Mander and H.-W. Lui, Elsevier, Oxford, pp. 153–223, 2010.
- 99) A. B. Attygalle, A. Mutti, W. Rohe, U. Maschwitz, W. Garbe and H. J. Bestmann: Naturwissenschaften 85, 275–277 (1998). [Google Scholar]