Abstract
AARS1 deficiency belongs to the group of disorders affecting aminoacyl-tRNA synthetases. To date, AARS1 deficiency has only been linked to neurologic disorders. We report a 6-year-old girl with microcephaly and developmental delay who presented with repeated episodes of acute liver failure. Whole-exome sequencing revealed compound heterozygosity for two missense variants within the AARS1 gene, p.[Leu298Gln];[Arg751Gly]), whose functional relevance was demonstrated by decreased enzymatic activity in fibroblasts. This is the first report that shows that AARS1 variants may be associated with recurrent acute liver failure.
Keywords: Recurrent acute liver failure, Protein biosynthesis, Aminoacylation, AARS1, Metabolic disease
Abbreviations: aaRS, aminoacyl-tRNA synthetase; AARS1, alanyl-(aminoacyl)-tRNA synthetase-1; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; PICU, pediatric intensive care unit
1. Introduction
In cytoplasm and mitochondria of essentially all cells, aminoacyl-tRNA synthetases (aaRSs) contribute to the first step of protein synthesis charging tRNAs with their cognate amino acids. With few exceptions, cytosolic and mitochondrial aaRSs are encoded by different gene sets. Patients with different aaRS deficiencies may present with variable clinical symptoms, frequently early-onset neurodegenerative disorders and epileptic encephalopathies; only few have been associated with a variable degree of liver involvement [1]. Here, we report for the first time that functionally relevant AARS1 variants are associated with recurrent acute liver failure.
2. Case report
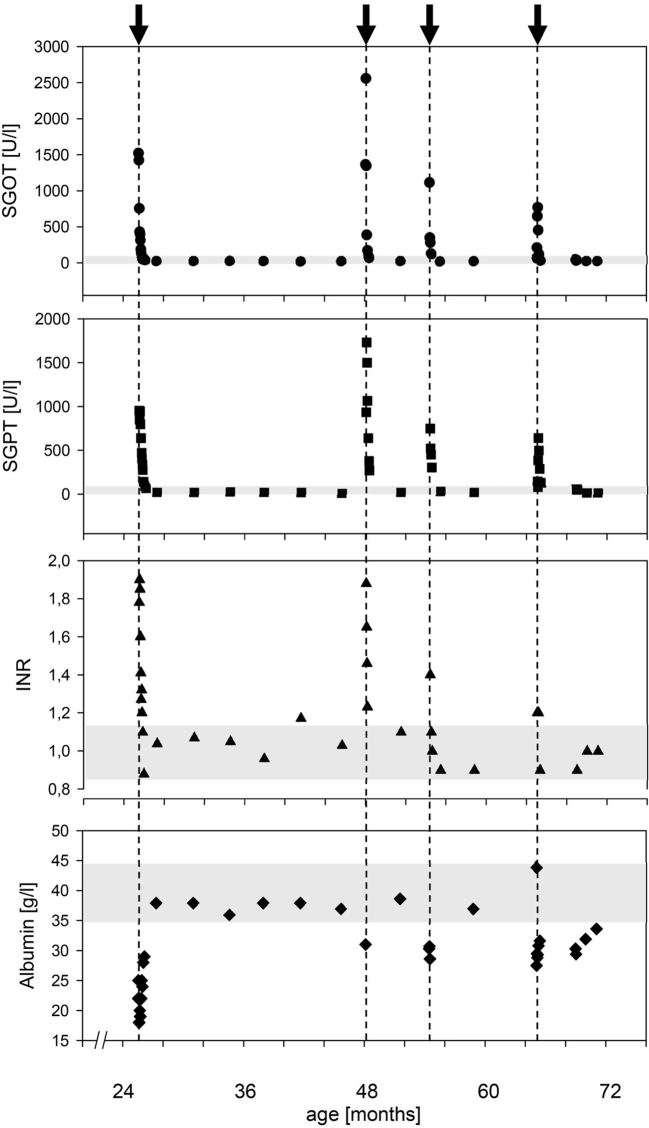
The 6-year-old girl was born at term after uneventful gestation. Family history was unremarkable without signs of neurological or liver disease. She presented with hyperexcitability in the neonatal period, and strabism, muscular hypotonia, and developmental delay subsequently. She learned to sit at 14 months and started to walk unaided recently; she follows simple instructions and only speaks few words. Already small at birth (2725 g, −2.0z, 47 cm, −2.4z, head circumference 32 cm, −2.5z), she soon crossed percentiles; at 24 months her weight was −3.3z, length − 2.9z, and head circumference − 6.1z. Extensive routine blood tests including metabolic work-up showed no specific pathological results; particularly liver function tests and plasma lactate concentration were normal. At 24 months, she developed a first episode of acute liver failure shortly after a norovirus infection: she presented with a generalized seizure and hypoglycemia, markedly elevated liver enzymes (SGOT 1500 U/l, SGPT 950 U/l), INR 1.9, albumin 18 g/l, and bilirubin 2.0 mg/dl. Ultrasound showed ascites and pleural effusions, the liver was enlarged with parenchymal irregularity and diminished portal flow velocity. She was admitted to the PICU for supportive treatment (iv glucose, acetylcysteine, antibiotics). Liver biopsy after stabilization showed structurally normal liver tissue with swollen hepatocytes, macro- and microvesicular steatosis, and small areas of necrosis. The mtDNA/nDNA-ratio was normal (0.63; normal >0.15). She fully recovered with normalization of all liver function tests within two weeks (Fig. 1). At 3.5 years, she presented with a second episode of liver disease following an upper airway infection. Again, liver enzymes were markedly increased (SGOT 2500 U/I, SGPT 1700 U/I), INR was 1.9, and she fully recovered within 2 weeks. Except for intermittent mild lactic acidosis, laboratory results were normal. Repeated cMRI studies between age 24 and 46 months showed T2w hyperintensities within the occipital semioval center and a lactate peak on spectroscopy.
Fig. 1.
Time course of liver function tests (SGOT (AST), SGPT (ALT), INR, and albumin) in the index patient with 4 episodes with impaired liver function triggered by fever and/or infection. Note that in between all episodes liver function tests returned to normal values (indicated by shaded areas).
Trio whole-exome sequencing revealed compound heterozygosity for two functionally relevant missense variants within the AARS1 gene (NM_001605.2, NP_001596.2; c.[893 T > A];[2251A > G], p.[Leu298Gln];[Arg751Gly]). The functional significance of these variants was suggested by the high level of conservation at this position across species, results of pathogenicity prediction programs, and their absence in various databases (for details see Supplementary Fig. S1b). Functional effects were confirmed by measuring aminoacylation activity in patient fibroblasts [2], showing reduced cytosolic AARS activity of 37% when compared to healthy controls (Fig. S1a).
At 4 years, the patient developed a milder third and fourth episode, again following febrile infections. No other febrile infections were documented, and no other episodes of impaired liver function occurred.
3. Discussion
Our report of a patient compound heterozygous for relevant AARS1 variants is the first of impaired cytosolic AARS activity and recurrent acute liver failure. Before, biallelic AARS1 variants with a comparable effect on measured enzyme activity have been observed in congenital and progressive microcephaly, central hypomyelination, and early infantile epileptic encephalopathy (EIEE29) [3,4]. Severe liver disease has not yet been reported in this context; it has only been associated with pathogenic variants in other genes coding for cytosolic aaRS, i.e., FARS1 (FARSA/FARSB) [5,6], IARS1 [7], LARS1 [8], MARS1 [9,10], QARS1 [1], and YARS1 [[11], [12], [13]], and has been associated by very variable additional symptoms (Supplementary Table 1).
The distinct pathogenic mechanisms of aaRS deficiencies and explanations for the tissue specific affection are still elusive. aaRS deficiencies may directly impair protein biosynthesis (in liver e.g., albumin). This may be the result of a quantitative effect, due to an impaired editing mechanism, however, this may also cause qualitative effects with altered protein sequences, misfolding and an intracellular accumulation of unstable proteins. Impaired synthesis might also affect cellular proteins that are imported into mitochondria resulting in an energetic problem which possibly explains the multisystem disorder and trigger mechanism (fever, infections) with higher energy demands in our patient.
The clinical presentation mimicking mitochondrial disease may lead to an approach with high glucose supply during catabolic crises, as implemented in our patient. Others have suggested protein supplementation to maximize possible residual function of an affected aaRS [1]. Interestingly, a recent publication has revealed reduced aminoacylation activity with increasing temperatures in samples from patients with bi-allelic LARS1 variants [8]. This might be an explanation for the development of acute liver disease and encephalopathic crises in these patients when exposed to febrile infections. Since this observation might be relevant to other cytosolic aaRS deficiencies, early and sufficient antipyretic treatment should be accomplished. Most likely, however, pathogenesis is more complex since several studies describe a growing spectrum of cytosolic aaRS functions exceeding aminoacylation of tRNAs [14].
In conclusion, this clinical observation expands the spectrum of AARS1 deficiency and adds to the causes of (recurrent) acute liver failure in children. Extending the knowledge about aaRS deficiencies and recognition of clinical similarities will lead to further understanding of underlying pathophysiological processes and improve treatment options.
Funding/support
This study was supported by the German network for mitochondrial disorders (mitoNET) sponsored by the German Federal Ministry of Education and Research (BMBF).
Declaration of Competing Interest
All authors have no conflicts of interest to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2020.100681.
Appendix A. Supplementary data
Supplementary material
References
- 1.Fuchs S.A., Schene I.F., Kok G. Aminoacyl-tRNA synthetase deficiencies in search of common themes. Genet. Med. 2019;21(2):319–330. doi: 10.1038/s41436-018-0048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Knaap M.S., Bugiani M., Mendes M.I. Biallelic variants in LARS2 and KARS cause deafness and (ovario)leukodystrophy. Neurology. 2019;92(11):e1225–e1237. doi: 10.1212/WNL.0000000000007098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons C., Griffin L.B., Helman G. Loss-of-function alanyl-tRNA synthetase mutations cause an autosomal-recessive early-onset epileptic encephalopathy with persistent myelination defect. Am. J. Hum. Genet. 2015;96(4):675–681. doi: 10.1016/j.ajhg.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakayama T., Wu J., Galvin-Parton P. Deficient activity of alanyl-tRNA synthetase underlies an autosomal recessive syndrome of progressive microcephaly, hypomyelination, and epileptic encephalopathy. Hum. Mutat. 2017;38(10):1348–1354. doi: 10.1002/humu.23250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krenke K., Szczałuba K., Bielecka T. FARSA mutations mimic phenylalanyl-tRNA synthetase deficiency caused by FARSB defects. Clin. Genet. 2019 Nov;96(5):468–472. doi: 10.1111/cge.13614. [DOI] [PubMed] [Google Scholar]
- 6.Xu Z., Lo W.S., Beck D.B. Bi-allelic mutations in Phe-tRNA synthetase associated with a multi-system pulmonary disease support non-translational function. Am. J. Hum. Genet. 2018;103(1):100–114. doi: 10.1016/j.ajhg.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopajtich R., Murayama K., Janecke A.R. Biallelic IARS mutations cause growth retardation with prenatal onset, intellectual disability, muscular hypotonia, and infantile hepatopathy. Am. J. Hum. Genet. 2016;99(2):414–422. doi: 10.1016/j.ajhg.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz D., Smith D.E.C., Crushell E. Genotypic diversity and phenotypic spectrum of infantile liver failure syndrome type 1 due to variants in LARS1. Genet. Med. 2020;22(11):1863–1873. doi: 10.1038/s41436-020-0904-4. [DOI] [PubMed] [Google Scholar]
- 9.van Meel E., Wegner D.J., Cliften P. Rare recessive loss-of-function methionyl-tRNA synthetase mutations presenting as a multi-organ phenotype. BMC Med. Genet. 2013;14:106. doi: 10.1186/1471-2350-14-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun Y., Hu G., Luo J. Mutations in methionyl-tRNA synthetase gene in a Chinese family with interstitial lung and liver disease, postnatal growth failure and anemia. J. Hum. Genet. 2017;62(6):647–651. doi: 10.1038/jhg.2017.10. [DOI] [PubMed] [Google Scholar]
- 11.Nowaczyk M.J., Huang L., Tarnopolsky M. A novel multisystem disease associated with recessive mutations in the tyrosyl-tRNA synthetase (YARS) gene. Am. J. Med. Genet. A. 2017;173(1):126–134. doi: 10.1002/ajmg.a.37973. [DOI] [PubMed] [Google Scholar]
- 12.Tracewska-Siemiątkowska A., Haer-Wigman L., Bosch D.G.M. An expanded multi-organ disease phenotype associated with mutations in YARS. Genes (Basel) 2017;8(12):381. doi: 10.3390/genes8120381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams K.B., Brigatti K.W., Puffenberger E.G. Homozygosity for a mutation affecting the catalytic domain of tyrosyl-tRNA synthetase (YARS) causes multisystem disease. Hum. Mol. Genet. 2019;28(4):525–538. doi: 10.1093/hmg/ddy344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yakobov N., Debard S., Fischer F. Cytosolic aminoacyl-tRNA synthetases: unanticipated relocations for unexpected functions. Biochim. Biophys. Acta Gene Regul. Mech. 2018;1861(4):387–400. doi: 10.1016/j.bbagrm.2017.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material