Abstract
Ectoenzyme and receptor BST-1/CD157 has been considered as a key molecule involved in the regulation of functional activity of cells in various tissues and organs. It is commonly accepted that CD157 catalyzes NAD+ hydrolysis and acts as a component of integrin adhesion receptor complex. Such properties are important for the regulatory role of CD157 in neuronal and glial cells: in addition to recently discovered role in the regulation of emotions, motor functions, and social behavior, CD157 might serve as an important component of innate immune reactions in the central nervous system. Activation of innate immune system in the brain occurs in response to infectious agents as well as in brain injury and neurodegeneration. As an example, in microglial cells, association of CD157 with CD11b/CD18 complex drives reactive gliosis and neuroinflammation evident in brain ischemia, chronic neurodegeneration, and aging. There are various non-substrate ligands of CD157 belonging to the family of extracellular matrix proteins (fibronectin, collagen I, finbrinogen, and laminin) whose activity is required for controlling cell adhesion and migration. Therefore, CD157 could control structural and functional integrity of the blood-brain barrier and barriergenesis. On the other hand, contribution of CD157 to the regulation of brain development is rather possible since in the embryonic brain, CD157 expression is very high, whereas in the adult brain, CD157 is expressed on neural stem cells and, presumably, is involved in the neurogenesis. Besides, CD157 could mediate astrocytes’ action on neural stem and progenitor cells within neurogenic niches. In this review we will summarize how CD157 may affect brain plasticity acting as a molecule at the crossroad of neurogenesis, cerebral angiogenesis, and immune regulation.
Keywords: CD157, immune system, brain plasticity, social brain, brain development
Introduction
NAD+ metabolism is recognized as an important factor contributing to numerous metabolic events and intercellular communications. Several NAD+-consuming enzymes have been discovered in recent 3 decades. Particularly, CD38 and CD157 represent the pair of ectoenzymes involved in the catalytic degradation of NAD+ leading to production of second messengers with pivotal biological effects (i.e., control of Ca2+ release from intracellular stores, regulation of cell movement, etc.) (1–3).
CD157 is well known as bone marrow stromal cell antigen-1 (BST-1) (4), that was first isolated from a bone marrow stromal cells (5), and the BST1 gene was identified by gene cloning as CD157 (6). CD157/BST-1 together with CD38 belong to the NADase/ADP-ribosyl cyclase family, catalyzing the conversion of NAD+ and NADP+ to cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (4, 7–15). CD157/BST-1 exhibits the same dualism of properties as CD38 like receptor and enzyme activity in leukocytes and ovarian cancer cells (16, 17), bone marrow stromal cells (18), myeloid cells (6, 19, 20), and netrophils and hematopoietic stem cells (14, 18, 21–23).
Interesting, the ADP-ribosyl cyclase activity of CD157 is not so much strong like that one of CD38 including brain. However, it is not clear whether CD157 has other enzyme activities, such as NAD glycohydrolase or NAD base exchange activities. The product of base exchange is nicotinic acid adenine dinucleotide phosphate (NAADP). NAADP also has Ca2+ mobilization activity from different Ca2+ pools. CD38 initiates calcium mobilization through two products: NAADP and cADPR, but CD157 uses only cADPR pathway (11, 24, 25). Perhaps, sibling rivalry between CD157 and CD38 has become more exiting (26).
From the point of the social brain the role of CD38 in oxytocin (OT) secretion into the brain has been established: CD38 mediates cADPR production, TRMP2 and ERK1/2 activation, Ca2+-mobilization, and OT release. Additionally, CD38 is involved in OT release by activating molecular cascades of OT autoregulation (27–30). In contrast, CD157 binds with the serotonin transporter and integrin β and invokes multiple circuits to control anxiety- and depression-like behaviors (24, 31, 32). CD157 plays a role in cADPR-induced OT release, which may not be same to that of CD38. The deficiency of CD157 leads to aberrant behaviors, such as increased anxiety.
Interestingly, despite the important role of CD157 in the immune system (33–35), the CD157/BST1 gene has been identified as a risk factor for neurodegeneration (36), particularly in Parkinson’s disease (PD) (37–48). A new role for CD157 was also found in stem cells when CD157 induces catalysis of cADPR in Paneth cells, which promote self-renewal of stem cells in the intestines in mice on a low-calorie diet (49) and CD157 is responsible for the proliferation of stem and progenitor cells in the lungs (50). The role of CD157 in the regulation of brain activity and plasticity is very intriguing and needs in further evaluation.
CD157 and Brain Immune Response
CD157 is widely expressed in the brain. CD157 immunoreactivity was detected in the cytoplasm or at the cell surface of many but not all nestin-positive cells in the ventricular and subventricular zones beside the third ventricle (1, 24). In the nervous system, CD157 may play a role in neuronal migration during neural stem cell (NSC) proliferation and neurogenesis. It has been shown that CD157 binds with members of the integrin family (24, 51).
It is also interesting that CD157 is highly expressed in immune cells that are very active in a case of local brain immune response and neuroinflammation. Indeed, microglia cells demonstrate co-expression of CD157 with CD11b and CD18 in experimental Parkinson’s disease in rats (52).
CD11b and CD18 are molecules involved in the regulation of (micro)glial activity being the beta 2-integrin receptor, or complement 3 receptor (Mac-1), or receptor for double-stranded RNA (i.e., of viral origin) (53–55).
CD157 may serve as a part of this complex (56) where its role is in the regulation of leukocyte functional activity. Since activity of Mac-1 as a receptor for extracellular double-stranded RNA (dsRNA), CD157 may be important for sensing dsRNA by glial cells in neuroinflammation as we have suggested before (57). In brain, expression of CD11b/CD18 receptor complex is evident in either astroglial or microglial cells. Stimulation of Mac-1 results in the activation of tall-like receptor-3 (TLR3) and triggers TLR3-independent oxidative inflammatory signaling. Moreover, coordinated activity of Mac-1 and the receptor for advanced glycation end products (RAGE) is involved in development of inflammation, i.e., in neurodegeneration (52, 58). CD157, CD11b, and CD18 co-expression has been detected in microglial cells in experimental Parkinson’s disease in rats (52). It is well-known that activation of TLR3 in blood brain barrier (BBB) cells leads to BBB breakdown, activation of innate immune response and inflammation in the brain affected by degeneration or ischemia (59). Also, TLR3-coupled cell signaling is involved in the disruption of BBB evident in immunologically induced chronic fatigue syndrome (60) when inflammation-induced changes in neuron-glial interactions result in the activation of local immune response and cytokines-driven BBB breakdown. Involvement of viral dsRNA in the pathogenesis of chronic fatigue syndrome that was confirmed in the fatigue model induced by dsRNA poly (I:C) (61), and critical role of CD11b/CD18 complex in the recognition of dsRNA in immune cells (55) provide novel insights on the activity of Mac-1 complex and associated molecules (including CD157). Thus, it is reasonable to speculate that physical and functional association of CD157 with CD11b/CD18 could affect TLR3-dependent and independent signaling, thereby resulting in development of local inflammation caused by the release of PAMPs and DAMPs to the extracellular space, or by the presence of viral dsRNA in the brain tissue.
Therefore, it is not surprising that ATP-mediated purinergic signaling via P2X7 receptors induces expression of CD157 in brain microvessel endothelial cells (BMECs) in mice (62), thereby providing a basis for the transmigration of activated peripheral immune cells through the blood-brain barrier into brain tissue. Taking into the consideration the well-established role of CD157 in the regulation of leukocytes movement across the endothelial layer (34), one could propose that integrin-mediated control of the blood-brain barrier integrity might be partly provided by CD157 expressed on brain microvessel endothelial cells. In addition to molecules involved into the control of transendothelial leukocytes migration (CD31, cadherins, JAMs etc.), CD157 expressed by neutrophils and endothelial cells was found to be involved into the regulation of cell adhesion during chemotaxis and transmigration at imflammatory loci (51). This could suggest new clues to the pathogenesis of immune response-associated blood-brain barrier breakdown and progression of neuroinflammation due to excessive migration of peripheral blood leukocytes into the brain tissue at the sites of compromised blood-brain barrier permeability. Moreover, it should be noted, that NAD+ metabolism is of great importance for maintaining metabolic activity of brain microvessel endothelial cells and BBB integrity (63); therefore, catalytic activity of CD157 expressed in endothelial cells might be required for the activation of BMECs seen in brain injury. Recent data on the role of NAD+ depletion in endothelial cells accompanied aging and degeneration further support such assumption (64); however, whether or not it relates to the activation of CD157 remains to be evaluated.
CD157 and Social Brain
Systemic and local inflammation often associates with behavioral and cognitive deficits (65). Recent data suggest that it might result in prominent alterations in social behavior (66). Moreover, aberrant immune response has been reported in various models of chronic neurodegeneration, autism spectrum disorder (ASD), and schizophrenia (66, 67). As an example, maternal immune activation caused by poly(I:C) drives development of autism-like phenotype in the offspring, demonstrating dependence on purinergic receptors (P2X7) expression in the brain (68). So, the question arises whether CD157 could contribute to the immunity-mediated control of social behavior.
Recently, it was found that the CD157/BST1 gene polymorphisms are associated with some neurological diseases, including ASD (69, 70), which provoked to work with animals with a deletion of the CD157/BST1 gene and to evaluate the possibility of using CD157−/− mice as models of ASD or autistic-like behavior with a social deficit in the absence of motor dysfunctions (especially in childhood and early adolescent).
Social interaction and communication is the most vulnerable behavioral trait in children with ASD (71, 72). Communicative skills are formed in the early period of life and require the use of language as the main tool for the two-way transmission of information. At the same time, mice are social animals with their own communication system with ultrasonic vocalization (USV) in various contexts (73–76). Mouse USV are a whole complex with various qualitative (form, frequency, duration, intensity of sounds, etc.) and quantitative (number of USV produced) (77, 78) characteristics; which, of course, is not a human language.
It was shown that deletion of the Cd157 gene in mice causes a deviation in the development of the production of ultrasonic vocalization during lactation (32). Young mice, removed from the nest and isolated from the mother, vocalize with a communicative orientation, which correlates with social contacts and research behavior of rodents (79). Neonatal USV can be a guide in understanding remote adult anxiety profiles (78, 80). However, the decrease in vocalization after PND 3, which we observed in CD157−/− mice, can be considered as a delayed violation of communication skills. This may be partially associated with the previously noted autistic (anxious and restless) similar behavior in CD157−/− mice (32).
The structure and organization of the language is actively studied in patients with ASD. Pragmatics is the most “socially motivated” and consistently devalued domain for ASD because it requires an understanding of the language and its correct interpretation during social interactions (72). The language of instruction is tied to mechanical and motor functions, since there is a connection between motor skills and speech function. Atypical motor movements were observed with various phenotypes of ASD (81). Poor vocabulary and its active use in patients with ASD seems to be associated with reduced motivation for communication (82). The registration of USV produced by mouse cubs during social isolation can be distinguished as an index of social motivation of cubs to stimulate parental care and as a marker of early communication deficits in ASD models in mice in anticipation of other, long-term, changes (83, 84). Such scientific studies demonstrate the potential positive effect of introducing oxytocin on increasing motivation for targeted behavior (85) and promoting fundamental psychophysiological functions in the implementation of social behavior, which in turn contributes to social activity (86). Administration of exogenous oxytocin can improve brain function in children with ASD (87). Taking into account all the data we obtained and previously published scientific studies, we assume that the introduction of oxytocin activates the potential flexibility of neuron functions, which does not directly stimulate indirect motivation and, possibly, helps to develop vocabulary and increase vocabulary.
Actually, this is the first study demonstrating the relationship of CD157 with early postnatal development and communicative abilities (which can be restored by introducing exogenous oxytocin). The CD157 gene can be a candidate gene and a risk factor for the development of states of anxiety and social avoidance (social fear). This data can make a great contribution to the study of molecular mechanisms that underlie disturbances in social interactions, in particular, communicative deficits in ASD. However, one study demonstrates that single-nucleotide polymorphisms (SNPs) are rs28532698 and rs4301112 in CD157not predictors of childhood ASD in the Chinese Han population (88), but CD157 sequence variation predicts scores on the Friendship questionnaire (89).
Results of experiments show that CD157−/− young adult male mice displayed anxiety-related behaviors for the novel environment; exhibited anxiety for non-social and/or social novel targets (31, 90). Weak sociability with novel target mice and social avoidance for target males were recover with oxytocin application (31, 32). A new oxytocin analog, lipo-oxytocin-1 (LOT-1), similar to OT, rescued anxiety-like behavior and social avoidance in CD157 knockout mice (91).
In addition, CD157−/− mice displayed depression-like behaviors and response well for antidepressant treatment (90). Significant differences in the activity of ADP-ribosyl cyclase in the hypothalamus and the pituitary gland between the two genotypes were not observed. Measurement of the oxytocin level in blood plasma showed that the concentration of oxytocin in CD157−/− mice was significantly lower than in mice of the control group (31) ( Figure 1 ). It was interesting to analyze how these behavioral impairments in CD157−/− mice could reflect impairment of the amygdala (31, 92, 93) and what is the role of CD157 in adult neurogenesis. However, there is no direct evidence that CD157 plays a role in neuronal migration during neurogenesis, although CD157 binds integrins in human monocytes and plays a role in neutrophil migration (31, 32).
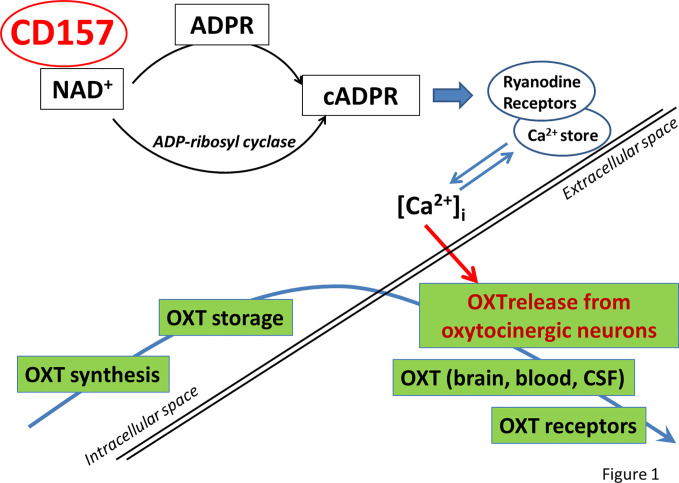
Figure 1.
Model illustrating the potential role of CD157 in oxytocin release. CD157 catalyzes NAD+ hydrolysis and synthesis of cADPR, then cADPR acts at Ryanodine receptors expressed in intracellular Ca2+ stores to initiate [Ca2+]irelease to cytosole. As a result, OXT is secreted from the cell into the blood and brain tissue. ADPR, ADP-ribose; cADPR, cyclic ADP-ribose; OXT, oxytocin.
In sum, CD157 directly or indirectly affects the central axonal release of OXT, which proves the observed change in the oxytocin system (plasma concentration) and the compensatory effect of oxytocin on the behavior of CD157−/− mice. Targeted modulation of CD157 expression in the brain could be considered as an approach to restore behavioral deficits seen in brain disorders associated with impaired social behavior and stress tolerance.
CD157 and Neuroplasticity
Subgranular zone (SGZ) of hippocampus and subventricular zone (SVZ) are the main areas of the brain where neurogenesis occurs in adulthood due to presence of neurogenic niches with the optimal microenvironment required for maintenance of populations of NSCs, proliferation of neural progenitor cells (NPCs), further differentiation and migration of cells of neuronal and glial lineages (94, 95).
Deletion of the Cd157 gene is associated with behavioral characteristic of a number of neurodevelopment and neurodegenerative diseases. Violations of socialization, social recognition, and anxiety are associated with learning and memory processes, while autism is associated with impaired synaptogenesis. Moreover, deletion of the Cd157 gene causes a decrease in the proliferation of neuronal progenitor cells in the SGZ, which can be seen from the significant decrease in the expression of the Nestin marker in the SGZ of the dentate gyrus of CD157−/− mice compared with wild-type C57BL/6 mice (1.43 ± 2.36% and 8.0 ± 2.41%, respectively, P = 0.0039). A tendency toward a decrease in the expression of the marker of neuroblasts (MAP2) in the SGZ of CD157−/− mice (12.64 ± 4.44%) compared with the control group (25.12 ± 5.77%, P = 0.0410). However, no differences were found in the expression of the marker for immature neurons (doublecortin, DCX) in the experimental and control groups, which indicates that CD157 does not control the number of immature neurons.
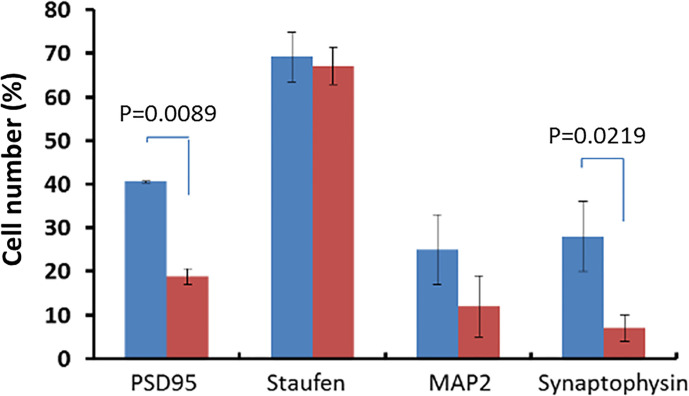
There was statistically significant decrease of the expression of the postsynaptic density marker PSD95 in the SGZ of the dentate gyrus in CD157−/− mice (18.79 ± 1.88%) was revealed compared with the control (40.51 ± 0.13%, P = 0.0089, Figure 2 ). We found no difference in Staufen expression (an RNA-binding protein involved in the localization and transport of dendritic mRNA; a marker of neuronal RNA granules) in the dentate gyrus of the hippocampus of CD157−/− mice and wild-type C57BL/6 mice (67.07 ± 4.28% and 69.19 ± 5.67%, respectively, P = 0.7834) ( Figure 2 ). But we detected a tendency to a decrease in the expression of MAP2 (associated with protein microtubules) in the same brain area in CD157−/− mice compared to the control group ( Figure 2 ). Deletion of the Cd157 gene is statistically significantly associated with decreased expression of Synaptophysin in the studied brain area ( Figure 2 ). These results demonstrate that absence of CD157 expression suppresses synaptogenesis in the hippocampus.
Figure 2.
Deficiency of synaptogenesis under deletion of the Cd157 gene. Expression of synaptogenesis markers (PSD95, Staufen, MAP2, Synaptophysin) in the subgranular zone of the dentate gyrus of the hippocampus in CD157−/− mice (red marker) and in the control group of mice (CD157+/+ mice, blue marker). The number of positive cells is presented as a percentage of the total number of cells in the field of view (five visual fields were evaluated). Two-way ANOVA (genotype effect, F (1.32) = 36.26, P = 0.0007) followed by Bonferroni’s post hoc test.
Olfactory bulbs in rodents serve as platform for the integration of newly-formed neurons in neuronal circuits throughout the life being in close functional connection with the SVZ (96). We found significant increase in the expression of CD157 by microglial cells during neurodegeneration in olfactory bulbs (4.12 ± 1.52%) compared with the control group of sham-operated mice (0.72 ± 0.38%, P = 0.027). Also, a statistically significant increase of CD157 expression was recorded in astrocytes expressing GFAP in neurodegeneration group(5.74 ± 1.45%) compared with the sham-operated control (1.03 ± 0.39%, P = 0.05). In S100β+ astrocytes, the expression of CD157 demonstrated tendency to increase (P = 0.086, Table 1 ). Thus, progression of neurodegeneration is accompanied by an increase in the expression of CD157 in microglia cells in rodent olfactory bulbs, thereby suggesting a role of activated microglial cells in the control of newly-formed neurons integration into pre-existing circuits. In microglial cell, similar to immune cells, CD157 is responsible for cytoskeletal rearrangement associated with cell activation and migration and can form a functional complex with the CD11b/CD18, thus contributing to cell adhesion (97). Meta-analysis suggests that the rs11931532 and rs4698412 in CD157, but not rs11724635 might be risk factors for Parkinson’s disease in Asian populations (98).
Table 1.
The number of CD157+ microglia and astrocytes (%) in olfactory bulbs in control animals and with neurodegeneration.
| Groups | Cell type | ||
|---|---|---|---|
| Microglia (MAC-1, CD18/CD11b) | Astrocyte (GFAP+) | Astrocytes(S100β+) | |
| Neurodegeneration (β-amyloid injection) | 4.12 ± 1.52 | 5.74 ± 1.45 | 2.45 ± 1.48 |
| Control (sham-operated) | 0.72 ± 0.38 | 1.03 ± 0.39 | 0.46 ± 0.08 |
| Р | 0.027 | 0.05 | 0.086 |
Animals, n = 5 in every group; slices, n = 5 from every brain; field of view, n = 5 from every slice.
In sum, it is clear that CD157 is involved in the formation of immature neurons and in the proliferation of neuronal cells. In this case CD157 does not affect the number of immature neurons. However, the absence of CD157 negatively affects the processes of synaptogenesis, whereas progression of neurodegeneration is accompanied by CD157 overexpression in brain cells.
Functional association of CD157 and CD200 on stem cells (99) may provide novel role of CD157 in the regulation of stem cells development in neurogenic niches established in a close vicinity to the sites of hyperpermeable BBB (100). Recently, CD157 was confirmed as a marker of tissue-resident vascular endothelial stem cells (VESCs) in large arteries and veins of numerous mouse organs (101), thereby supporting new idea on its role in the immune-controlled regulation of angiogenesis and vascular remodeling (99). It was proposed that CD200+CD157+ endothelial cells (ECs) are self-renewing stem cells contributing to angiogenesis and vasculogenesis by supplying terminally differentiating ECs through a stage of CD200+CD157− endothelial progenitors (102). Moreover, therapeutic application of CD200+CD157+ progenitors aimed to restore angiogenic potential in the affected tissues could be proposed (102). The same approach might be applied for the establishment of new BBB in vitro models, particularly those reflecting aberrant barrier integrity evident in neuroinflammation and neurodgeneration (103). In sum, CD157-immunopositive endothelial progenitor cells may display great regenerative potential in tissues, including brain (101); however, nothing is known about this cell pool in the context of brain microvessel development in embryonic or adult stages, and it requires further assessment.
Conclusion
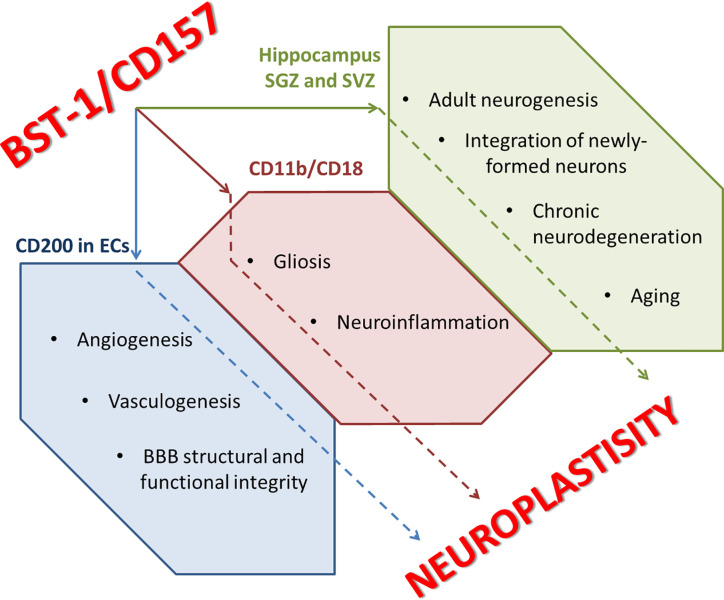
CD157 is defined as a neuro-entero-immunological regulator. The Cd157 gene can be a candidate gene and a risk factor for the development of states of anxiety and social avoidance (social fear), and the CD157−/− mice is a relevant model for the study of mental disorders and brain plasticity, including those characteristic of humans. In addition to recently discovered role in the regulation of emotions, motor functions and social behavior, CD157 might serve as an important component of innate immune reactions in the central nervous system. Involvement of CD157 to the regulation of brain development is rather possible since in the embryonic brain where CD157 expression is very high, whereas in the adult brain, CD157 is expressed on NSCs and surrounding glial cells, being, probably, involved in the regulation of adult neurogenesis and integration of newly-formed neurons into pre-existing circuits. Functional association of CD157 with CD11b/CD18 complex may drive reactive gliosis and neuroinflammation evident in brain ischemia, chronic neurodegeneration, and aging. Coupling of CD157 to CD200 in endothelial cells may affect angiogenesis and vasculogenesis, whereas expression of CD157 on mature brain endothelial cells may contribute to controlling BBB structural and functional integrity ( Figure 3 ). In sum, CD157 should be recognized as a target molecule for the therapy of brain disorders associated with immune dysfunction, aberrant neuroplasticity, and neuroinflammation.
Figure 3.
CD157 and neuroplastisity. ECs, endothelial cells; SGZ, subgranular zone of hippocampus; SVZ, subventricular zone of hippocampus.
Author Contributions
AS and OL conceived of the presented idea. YK, NM, YP, and AM worked on the table and figures. All authors contributed to the article and approved the submitted version.
Funding
The study is supported by the grant НШ-2547.2020.7 of the President of Russian Federation given to Russian Leading Research Teams.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Pehar M, Harlan BA, Killoy KM, Vargas MR. Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration. Antioxid Redox Signal (2018) 28:1652–68. 10.1089/ars.2017.7145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Salmina AB, Morgun AV, Kuvacheva NV, Lopatina OL, Komleva YK, Malinovskaya NA, et al. Establishment of neurogenic microenvironment in the neurovascular unit: the connexin 43 story. Rev Neurosci (2014) 25(1):97–111. 10.1515/revneuro-2013-0044 [DOI] [PubMed] [Google Scholar]
- 3. Salmina AB, Inzhutova AI, Morgun AV, Okuneva OS, Malinovskaia NA, Lopatina OL, et al. [NAD+-converting enzymes in neuronal and glial cells: CD38 as a novel target for neuroprotection]. Vestn Akad Med Nauk SSSR (2012) 10:29–37. 10.15690/vramn.v67i10.413 [DOI] [PubMed] [Google Scholar]
- 4. Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, et al. CD38 and CD157: A long journey from activation markers to multifunctional molecules: CD38 and CD157. Cytometry B Clin Cytometry (2013) 84B:207–17. 10.1002/cyto.b.21092 [DOI] [PubMed] [Google Scholar]
- 5. Kaisho T, Ishikawa J, Oritani K, Inazawa J, Tomizawa H, Muraoka O, et al. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc Natl Acad Sci (1994) 91:5325–9. 10.1073/pnas.91.12.5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itoh M, Ishihara K, Tomizawa H, Tanaka H, Kobune Y, Ishikawa J, et al. Molecular Cloning of Murine BST-1 Having Homology with CD38 and Aplysia ADP-Ribosyl Cyclase. Biochem Biophys Res Commun (1994) 203:1309–17. 10.1006/bbrc.1994.2325 [DOI] [PubMed] [Google Scholar]
- 7. Ferrero E, Saccucci F, Malavasi F. The human CD38 gene: polymorphism, CpG island, and linkage to the CD157 (BST-1) gene. Immunogenetics (1999) 49:597–604. 10.1007/s002510050654 [DOI] [PubMed] [Google Scholar]
- 8. Guse AH. Second messenger function and the structure-activity relationship of cyclic adenosine diphosphoribose (cADPR). FEBS J (2005) 272:4590–7. 10.1111/j.1742-4658.2005.04863.x [DOI] [PubMed] [Google Scholar]
- 9. Hirata Y, Kimura N, Sato K, Ohsugi Y, Takasawa S, Okamoto H, et al. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett (1994) 356:244–8. 10.1016/0014-5793(94)01279-2 [DOI] [PubMed] [Google Scholar]
- 10. Itoh M, Ishihara K, Hiroi T, Lee BO, Maeda H, Iijima H, et al. Deletion of bone marrow stromal cell antigen-1 (CD157) gene impaired systemic thymus independent-2 antigen-induced IgG3 and mucosal TD antigen-elicited IgA responses. J Immunol (1998) 161:3974–83. [PubMed] [Google Scholar]
- 11. Lee HC. Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling. Sci China Life Sci (2011) 54:699–711. 10.1007/s11427-011-4197-3 [DOI] [PubMed] [Google Scholar]
- 12. Lee HC. Structure and Enzymatic Functions of Human CD38. Mol Med (2006) 12:317–23. 10.2119/2006-00086.Lee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee HC. Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu Rev Pharmacol Toxicol (2001) 41:317–45. 10.1146/annurev.pharmtox.41.1.317 [DOI] [PubMed] [Google Scholar]
- 14. Malavasi F, Deaglio S, Ferrero E, Funaro A, Sancho J, Ausiello CM, et al. CD38 and CD157 as Receptors of the Immune System: A Bridge Between Innate and Adaptive Immunity. Mol Med (2006) 12:334–41. 10.2119/2006-00094.Malavasi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamamoto-Katayama S, Ariyoshi M, Ishihara K, Hirano T, Jingami H, Morikawa K. Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities. J Mol Biol (2002) 316:711–23. 10.1006/jmbi.2001.5386 [DOI] [PubMed] [Google Scholar]
- 16. Morone S, Augeri S, Cuccioloni M, Mozzicafreddo M, Angeletti M, Lo Buono N, et al. Binding of CD157 Protein to Fibronectin Regulates Cell Adhesion and Spreading. J Biol Chem (2014) 289:15588–601. 10.1074/jbc.M113.535070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morone S, Lo-Buono N, Parrotta R, Giacomino A, Nacci G, Brusco A, et al. Overexpression of CD157 Contributes to Epithelial Ovarian Cancer Progression by Promoting Mesenchymal Differentiation. PloS One (2012) 7:e43649. 10.1371/journal.pone.0043649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta K, Malavasi F. eds. Human CD38 and related molecules. Basel: Karger; (2000). [DOI] [PubMed] [Google Scholar]
- 19. Muraoka O, Tanaka H, Itoh M, Ishihara K, Hirano T. Genomic structure of human BST-1. Immunol Lett (1996) 54:1–4. 10.1016/S0165-2478(96)02633-8 [DOI] [PubMed] [Google Scholar]
- 20. Okuyama Y, Ishihara K, Kimura N, Hirata Y, Sato K, Itoh M, et al. Human BST-1 Expressed on Myeloid Cells Functions as a Receptor Molecule. Biochem Biophys Res Commun (1996) 228:838–45. 10.1006/bbrc.1996.1741 [DOI] [PubMed] [Google Scholar]
- 21. Funaro A, Ortolan E, Ferranti B, Gargiulo L, Notaro R, Luzzatto L, et al. CD157 is an important mediator of neutrophil adhesion and migration. Blood (2004) 104:4269–78. 10.1182/blood-2004-06-2129 [DOI] [PubMed] [Google Scholar]
- 22. Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: A therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol (2013) 48:397–408. 10.3109/10409238.2013.789479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Podestà M, Benvenuto F, Pitto A, Figari O, Bacigalupo A, Bruzzone S, et al. Concentrative Uptake of Cyclic ADP-ribose Generated by BST-1 + Stroma Stimulates Proliferation of Human Hematopoietic Progenitors. J Biol Chem (2005) 280:5343–9. 10.1074/jbc.M408085200 [DOI] [PubMed] [Google Scholar]
- 24. Higashida H, Liang M, Yoshihara T, Akther S, Fakhrul A, Stanislav C, et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci (2017) 18:1–12. 10.1186/s12868-017-0350-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim U-H. Multiple Enzymatic Activities of CD38 for Ca2 + Signaling Messengers. Messenger (2014) 3:6–14. 10.1166/msr.2014.1030 [DOI] [Google Scholar]
- 26. Ferrero E, Lo Buono N, Morone S, Parrotta R, Mancini C, Brusco A, et al. Human canonical CD157/Bst1 is an alternatively spliced isoform masking a previously unidentified primate-specific exon included in a novel transcript. Sci Rep (2017) 7(1):15923. 10.1038/s41598-017-16184-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Higashida H. Somato-axodendritic release of oxytocin into the brain due to calcium amplification is essential for social memory. J Physiol Sci (2016) 66:275–82. 10.1007/s12576-015-0425-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Higashida H, Yokoyama S, Kikuchi M, Munesue T. CD38 and its role in oxytocin secretion and social behavior. Horm Behav (2012) 61:351–8. 10.1016/j.yhbeh.2011.12.011 [DOI] [PubMed] [Google Scholar]
- 29. Jin D, Liu H-X, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature (2007) 446:41–5. 10.1038/nature05526 [DOI] [PubMed] [Google Scholar]
- 30. Tao R, Sun H-Y, Lau C-P, Tse H-F, Lee H-C, Li G-R. Cyclic ADP ribose is a novel regulator of intracellular Ca2+ oscillations in human bone marrow mesenchymal stem cells. J Cell Mol Med (2011) 15:2684–96. 10.1111/j.1582-4934.2011.01263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lopatina O, Yoshihara T, Nishimura T, Zhong J, Akther S, Fakhrul AAKM, et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson’s disease. Front Behav Neurosci (2014) 8:133. 10.3389/fnbeh.2014.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopatina OL, Furuhara K, Ishihara K, Salmina AB, Higashida H. Communication Impairment in Ultrasonic Vocal Repertoire during the Suckling Period of Cd157 Knockout Mice: Transient Improvement by Oxytocin. Front Neurosci (2017) 11:266. 10.3389/fnins.2017.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lo Buono N. CD157 at the intersection between leukocyte trafficking and epithelial ovarian cancer invasion. Front Biosci (Landmark Ed) (2014) 19:366. 10.2741/4213 [DOI] [PubMed] [Google Scholar]
- 34. Lo Buono N, Parrotta R, Morone S, Bovino P, Nacci G, Ortolan E, et al. The CD157-Integrin Partnership Controls Transendothelial Migration and Adhesion of Human Monocytes. J Biol Chem (2011) 286:18681–91. 10.1074/jbc.M111.227876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shimaoka Y, Attrep JF, Hirano T, Ishihara K, Suzuki R, Toyosaki T, et al. Nurse-like cells from bone marrow and synovium of patients with rheumatoid arthritis promote survival and enhance function of human B cells. J Clin Invest (1998) 102:606–18. 10.1172/JCI3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chung SJ, Jung Y, Hong M, Kim MJ, You S, Kim YJ, et al. Alzheimer’s disease and Parkinson’s disease genome-wide association study top hits and risk of Parkinson’s disease in Korean population. Neurobiol Aging (2013) 34:2695.e1–2695.e7. 10.1016/j.neurobiolaging.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 37. Chang X-L, Mao X-Y, Li H-H, Zhang J-H, Li N-N, Burgunder J-M, et al. Association of GWAS loci with PD in China. Am J Med Genet B Neuropsychiatr Genet (2011) 156:334–9. 10.1002/ajmg.b.31167 [DOI] [PubMed] [Google Scholar]
- 38. Chen M-L, Lin C-H, Lee M-J, Wu R-M. BST1 rs11724635 interacts with environmental factors to increase the risk of Parkinson’s disease in a Taiwanese population. Parkinsonism Relat Disord (2014) 20:280–3. 10.1016/j.parkreldis.2013.11.009 [DOI] [PubMed] [Google Scholar]
- 39. Liu J, Xiao Q, Wang Y, Xu Z-M, Wang Y, Yang Q, et al. Analysis of genome-wide association study-linked loci in Parkinson’s disease of Mainland China: GWAS-Linked PD Loci in Mainland China. Mov Disord (2013) 28:1892–5. 10.1002/mds.25599 [DOI] [PubMed] [Google Scholar]
- 40. Miyake Y, Tanaka K, Fukushima W, Kiyohara C, Sasaki S, Tsuboi Y, et al. Lack of association between BST1 polymorphisms and sporadic Parkinson’s disease in a Japanese population. J Neurol Sci (2012) 323:162–6. 10.1016/j.jns.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 41. Saad M, Lesage S, Saint-Pierre A, Corvol J-C, Zelenika D, Lambert J-C, et al. Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson’s disease in the European population. Hum Mol Genet (2011) 20:615–27. 10.1093/hmg/ddq497 [DOI] [PubMed] [Google Scholar]
- 42. Satake W, Nakabayashi Y, Mizuta I, Hirota Y, Ito C, Kubo M, et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat Genet (2009) 41:1303–7. 10.1038/ng.485 [DOI] [PubMed] [Google Scholar]
- 43. Sharma M, Ioannidis JPA, Aasly JO, Annesi G, Brice A, Van Broeckhoven C, et al. Large-scale replication and heterogeneity in Parkinson disease genetic loci. Neurology (2012) 79:659–67. 10.1212/WNL.0b013e318264e353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Simón-Sánchez J, van Hilten JJ, van de Warrenburg B, Post B, Berendse HW, Arepalli S, et al. Genome-wide association study confirms extant PD risk loci among the Dutch. Eur J Hum Genet (2011) 19:655–61. 10.1038/ejhg.2010.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tan E-K, Kwok H-K, Tan LC, Zhao W-T, Prakash KM, Au W-L, et al. Analysis of GWAS-linked loci in Parkinson disease reaffirms PARK16 as a susceptibility locus. Neurology (2010) 75:508–12. 10.1212/WNL.0b013e3181eccfcd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang C, Cai Y, Zheng Z, Tang B-S, Xu Y, Wang T, et al. Penetrance of LRRK2 G2385R and R1628P is modified by common PD-associated genetic variants. Parkinsonism Relat Disord (2012) 18:958–63. 10.1016/j.parkreldis.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 47. Zhu L, Luo X, Zhou Y, Li F, Yang Y, Ren Y, et al. Lack of association between three single nucleotide polymorphisms in the PARK9, PARK15, and BST1 genes and Parkinson’s disease in the northern Han Chinese population. Chin Med J (2012) 125:588–92. 10.1016/S1353-8020(11)70766-0 [DOI] [PubMed] [Google Scholar]
- 48. Zimprich A. Genetics of Parkinson’s disease and essential tremor. Curr Opin Neurol (2011) 24:318–23. 10.1097/WCO.0b013e3283484b87 [DOI] [PubMed] [Google Scholar]
- 49. Yilmaz ÖH, Katajisto P, Lamming DW, Gültekin Y, Bauer-Rowe KE, Sengupta S, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature (2012) 486:490–5. 10.1038/nature11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu N, Li Z, Su Y. The association between oxytocin receptor gene polymorphism (OXTR) and trait empathy. J Affect Disord (2012) 138:468–72. 10.1016/j.jad.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 51. Ortolan E, Augeri S, Fissolo G, Musso I, Funaro A. CD157: From immunoregulatory protein to potential therapeutic target. Immunol Lett (2019) 205:59–64. 10.1016/j.imlet.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 52. Malinovskaya NA, Salmina AB, Prokopenko SV, Morgun AV, Kuvacheva NV, Yu AP, et al. The coexpression of CD157/CD11b/CD18 in an experimental model of Parkinson’s disease. Neurochem J (2015) 9:279–83. 10.1134/S181971241504011X [DOI] [Google Scholar]
- 53. Choucair-Jaafar N, Laporte V, Levy R, Poindron P, Lombard Y, Gies J-P. Complement receptor 3 (CD11b/CD18) is implicated in the elimination of β-amyloid peptides: CR3 and β-amyloid peptides phagocytosis. Fundam Clin Pharmacol (2011) 25:115–22. 10.1111/j.1472-8206.2010.00811.x [DOI] [PubMed] [Google Scholar]
- 54. Roy A, Fung YK, Liu X, Pahan K. Up-regulation of Microglial CD11b Expression by Nitric Oxide. J Biol Chem (2006) 281:14971–80. 10.1074/jbc.M600236200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhou H, Liao J, Aloor J, Nie H, Wilson BC, Fessler MB, et al. CD11b/CD18 (Mac-1) Is a Novel Surface Receptor for Extracellular Double-Stranded RNA To Mediate Cellular Inflammatory Responses. J Immunol (2013) 190:115–25. 10.4049/jimmunol.1202136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lavagno L, Ferrero E, Ortolan E, Malavasi F, Funaro A. CD157 is part of a supramolecular complex with CD11b/CD18 on the human neutrophil cell surface. J Biol Regul Homeost Agents (2007) 21:5–11. [PubMed] [Google Scholar]
- 57. Salmina AB, Komleva YK, Lopatina OL, Kuvacheva NV, Gorina YV, Panina YA, et al. Astroglial control of neuroinflammation: TLR3-mediated dsRNA-sensing pathways are in the focus. Rev Neurosci (2015) 26(2):143–59. 10.1515/revneuro-2014-0052 [DOI] [PubMed] [Google Scholar]
- 58. Zhang D, Hu X, Qian L, Chen S-H, Zhou H, Wilson B, et al. Microglial MAC1 receptor and PI3K are essential in mediating β-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroinflammation (2011) 8:3. 10.1186/1742-2094-8-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNα/β and IL-1β responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immun (2010) 24:996–1007. 10.1016/j.bbi.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Noda M, Ifuku M, MdS H, Katafuchi T. Glial Activation and Expression of the Serotonin Transporter in Chronic Fatigue Syndrome. Front Psychiatry (2018) 9:589. 10.3389/fpsyt.2018.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ifuku M, Hossain SM, Noda M, Katafuchi T. Induction of interleukin-1β by activated microglia is a prerequisite for immunologically induced fatigue. Eur J Neurosci (2014) 40:3253–63. 10.1111/ejn.12668 [DOI] [PubMed] [Google Scholar]
- 62. Maeda T, Inagaki M, Fujita Y, Kimoto T, Tanabe-Fujimura C, Zou K, et al. ATP increases the migration of microglia across the brain endothelial cell monolayer. Biosci Rep (2016) 36(2):e00318. 10.1042/BSR20160054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malinovskaya NA, Komleva YK, Salmin VV, Morgun AV, Shuvaev AN, Panina YA, et al. Endothelial Progenitor Cells Physiology and Metabolic Plasticity in Brain Angiogenesis and Blood-Brain Barrier Modeling. Front Physiol (2016) 7:599. 10.3389/fphys.2016.00599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ungvari Z, Tarantini S, Kiss T, Wren JD, Giles CB, Griffin CT, et al. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat Rev Cardiol (2018) 15:555–65. 10.1038/s41569-018-0030-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cunningham C, Campion S, Lunnon K, Murray CL, Woods JFC, Deacon RMJ, et al. Systemic Inflammation Induces Acute Behavioral and Cognitive Changes and Accelerates Neurodegenerative Disease. Biol Psychiatry (2009) 65:304–12. 10.1016/j.biopsych.2008.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Haida O, Al Sagheer T, Balbous A, Francheteau M, Matas E, Soria F, et al. Sex-dependent behavioral deficits and neuropathology in a maternal immune activation model of autism. Trans Psychiatry (2019) 9(124):1–12. 10.1038/s41398-019-0457-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haddad FL, Patel SV, Schmid S. Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci Biobehav Rev (2020) 113:546–67. 10.1016/j.neubiorev.2020.04.012 [DOI] [PubMed] [Google Scholar]
- 68. Horváth G, Otrokocsi L, Bekő K, Baranyi M, Kittel Á, Antonio Fritz-Ruenes P, et al. P2X7 receptors drive poly(I:C) induced autism-like behavior in mice. J Neurosci (2019) 39(13):2542–61. 10.1523/JNEUROSCI.1895-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ceroni F, Sagar A, Simpson NH, Gawthrope AJT, Newbury DF, Pinto D, et al. A Deletion Involving CD 38 and BST 1 Results in a Fusion Transcript in a Patient With Autism and Asthma: CD38/BST1 deletion with autism and asthma. Autism Res (2014) 7:254–63. 10.1002/aur.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yokoyama S, Al Mahmuda N, Munesue T, Hayashi K, Yagi K, Yamagishi M, et al. Association Study between the CD157/BST1 Gene and Autism Spectrum Disorders in a Japanese Population. Brain Sci (2015) 5:188–200. 10.3390/brainsci5020188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. DiStefano C, Shih W, Kaiser A, Landa R, Kasari C. Communication growth in minimally verbal children with ASD: The importance of interaction: Communication in minimally verbal children. Autism Res (2016) 9:1093–102. 10.1002/aur.1594 [DOI] [PubMed] [Google Scholar]
- 72. Eigsti I-M, de Marchena AB, Schuh JM, Kelley E. Language acquisition in autism spectrum disorders: A developmental review. Res Autism Spectr Disord (2011) 5:681–91. 10.1016/j.rasd.2010.09.001 [DOI] [Google Scholar]
- 73. Gaub S, Fisher SE, Ehret G. Ultrasonic vocalizations of adult male Foxp2 -mutant mice: behavioral contexts of arousal and emotion: Ultrasonic vocalizations of Foxp2 mutant mice. Genes Brain Behav (2016) 15:243–59. 10.1111/gbb.12274 [DOI] [PubMed] [Google Scholar]
- 74. Kim H, Son J, Yoo H, Kim H, Oh J, Han D, et al. Effects of the Female Estrous Cycle on the Sexual Behaviors and Ultrasonic Vocalizations of Male C57BL/6 and Autistic BTBR T+ tf/J Mice. Exp Neurobiol (2016) 25:156–62. 10.5607/en.2016.25.4.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Matsumoto YK, Okanoya K. Phase-Specific Vocalizations of Male Mice at the Initial Encounter during the Courtship Sequence. PloS One (2016) 11:e0147102. 10.1371/journal.pone.0147102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wöhr M, Schwarting RKW. Ultrasonic Communication in Rats: Can Playback of 50-kHz Calls Induce Approach Behavior? PloS One (2007) 2:e1365. 10.1371/journal.pone.0001365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lahvis GP, Alleva E, Scattoni ML. Translating mouse vocalizations: prosody and frequency modulation1. Genes Brain Behav (2011) 10:4–16. 10.1111/j.1601-183X.2010.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual Repertoire of Vocalizations in the BTBR T+tf/J Mouse Model of Autism. PloS One (2008) 3:e3067. 10.1371/journal.pone.0003067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: A tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev (2009) 33:508–15. 10.1016/j.neubiorev.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dichter GS, Brunelli SA, Hofer MA. Elevated plus-maze behavior in adult offspring of selectively bred rats. Physiol Behav (1996) 60:299–304. 10.1016/0031-9384(95)02222-8 [DOI] [PubMed] [Google Scholar]
- 81. Barbeau EB, Lewis JD, Doyon J, Benali H, Zeffiro TA, Mottron L. A greater involvement of posterior brain areas in interhemispheric transfer in autism: fMRI, DWI and behavioral evidences. NeuroImage: Clin (2015) 8:267–80. 10.1016/j.nicl.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Arunachalam S, Luyster RJ. The integrity of lexical acquisition mechanisms in autism spectrum disorders: A research review: lexical acquisition in ASD. Autism Res (2016) 9:810–28. 10.1002/aur.1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Hamilton SM, Spencer CM, Harrison WR, Yuva-Paylor LA, Graham DF, Daza RAM, et al. Multiple autism-like behaviors in a novel transgenic mouse model. Behav Brain Res (2011) 218:29–41. 10.1016/j.bbr.2010.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Homberg JR, Kyzar EJ, Nguyen M, Norton WH, Pittman J, Poudel MK, et al. Understanding autism and other neurodevelopmental disorders through experimental translational neurobehavioral models. Neurosci Biobehav Rev (2016) 65:292–312. 10.1016/j.neubiorev.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 85. Nawijn L, van Zuiden M, Koch SBJ, Frijling JL, Veltman DJ, Olff M. Intranasal oxytocin enhances neural processing of monetary reward and loss in post-traumatic stress disorder and traumatized controls. Psychoneuroendocrinology (2016) 66:228–37. 10.1016/j.psyneuen.2016.01.020 [DOI] [PubMed] [Google Scholar]
- 86. Kemp AH, Quintana DS, Kuhnert R-L, Griffiths K, Hickie IB, Guastella AJ. Oxytocin Increases Heart Rate Variability in Humans at Rest: Implications for Social Approach-Related Motivation and Capacity for Social Engagement. PloS One (2012) 7:e44014. 10.1371/journal.pone.0044014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci (2013) 110:20953–8. 10.1073/pnas.1312857110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mo W, Liu J, Zhang Z, Yu H, Yang A, Qu F, et al. Hu F. A study of single nucleotide polymorphisms in CD157, AIM2 and JARID2 genes in Han Chinese children with autism spectrum disorder. Nord J Psychiatry (2018) 72:179–83. 10.1080/08039488.2017.1410570 [DOI] [PubMed] [Google Scholar]
- 89. Chong A, Malavasi F, Israel S, Khor CC, Yap VB, Monakhov M, et al. ADP ribosyl-cyclases (CD38 / CD157), social skills and friendship. Psychoneuroendocrinology (2017) 78:185–92. 10.1016/j.psyneuen.2017.01.011 [DOI] [PubMed] [Google Scholar]
- 90. Kasai S, Yoshihara T, Lopatina O, Ishihara K, Higashida H. Selegiline Ameliorates Depression-Like Behavior in Mice Lacking the CD157/BST1 Gene, a Risk Factor for Parkinson’s Disease. Front Behav Neurosci (2017) 11:75. 10.3389/fnbeh.2017.00075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mizuno A, Cherepanov S, Kikuchi Y, Fakhrul A, Akther S, Deguchi K, et al. Lipo-oxytocin-1, a Novel Oxytocin Analog Conjugated with Two Palmitoyl Groups, Has Long-Lasting Effects on Anxiety-Related Behavior and Social Avoidance in CD157 Knockout Mice. Brain Sci (2015) 5:3–13. 10.3390/brainsci5010003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain (2002) 125:2431–45. 10.1093/brain/awf251 [DOI] [PubMed] [Google Scholar]
- 93. Surdhar I, Gee M, Bouchard T, Coupland N, Malykhin N, Camicioli R. Intact limbic-prefrontal connections and reduced amygdala volumes in Parkinson’s disease with mild depressive symptoms. Parkinsonism Relat Disord (2012) 18:809–13. 10.1016/j.parkreldis.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 94. Komleva Y, Kuvacheva NV, Malinocskaya NA, Gorina Y, Lopatina OL, Teplyashina EA, et al. Regenerative potential of the brain: Composition and forming of regulatory microenvironment in neurogenic niches. Hum Physiol (2016) 42:865–73. 10.1134/S0362119716080077 [DOI] [Google Scholar]
- 95. Pozhilenkova EA, Lopatina OL, Komleva YK, Salmin VV, Salmina AB. Blood-brain barrier-supported neurogenesis in healthy and diseased brain. Rev Neurosci (2017) 28(4):397–415. 10.1515/revneuro-2016-0071 [DOI] [PubMed] [Google Scholar]
- 96. Menini A. ed. The neurobiology of olfaction. Boca Raton, FL: CRC Press/Taylor & Francis; (2010). [PubMed] [Google Scholar]
- 97. Salmina AB, Komleva YK, Lopatina OL, Gorina YV, Malinovskaya NA, Pozhilenkova EA, et al. CD38 and CD157 Expression: Glial Control of Neurodegeneration and Neuroinflammation. Messenger (2014) 3:78–85. 10.1166/msr.2014.1037 [DOI] [Google Scholar]
- 98. Li J, Luo J, Liu L, Fu H, Tang L. The association between CD157/BST1 polymorphisms and the susceptibility of Parkinson’s disease: a meta-analysis. Neuropsychiatr Dis Treat (2019) 15:1089–102. 10.2147/NDT.S190935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Katoh M, Katoh M. CD157 and CD200 at the crossroads of endothelial remodeling and immune regulation. Stem Cell Invest (2019) 6:10–0. 10.21037/sci.2019.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lin R, Cai J, Nathan C, Wei X, Schleidt S, Rosenwasser R, et al. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol Dis (2015) 74:229–39. 10.1016/j.nbd.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 101. Wakabayashi T, Naito H, Suehiro J, Lin Y, Kawaji H, Iba T, et al. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell (2018) 22:384–97. 10.1016/j.stem.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 102. Takakura N. Discovery of a Vascular Endothelial Stem Cell (VESC) Population Required for Vascular Regeneration and Tissue Maintenance. Circ J (2018) 83:12–7. 10.1253/circj.CJ-18-1180 [DOI] [PubMed] [Google Scholar]
- 103. Osipova ED, Komleva YK, Morgun AV, Lopatina OL, Panina YA, Olovyannikova R, et al. Designing in vitro Blood-Brain Barrier Models Reproducing Alterations in Brain Aging. Front Aging Neurosci (2018) 10:234. 10.3389/fnagi.2018.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]