Abstract
The chemical identity of RNA molecules beyond the four standard ribonucleosides has fascinated scientists since pseudouridine was characterized as the “fifth” ribonucleotide in 1951. Since then, the ever‐increasing number and complexity of modified ribonucleosides have been found in viruses and throughout all three domains of life. Such modifications can be as simple as methylations, hydroxylations, or thiolations, complex as ring closures, glycosylations, acylations, or aminoacylations, or unusual as the incorporation of selenium. While initially found in transfer and ribosomal RNAs, modifications also exist in messenger RNAs and noncoding RNAs. Modifications have profound cellular outcomes at various levels, such as altering RNA structure or being essential for cell survival or organism viability. The aberrant presence or absence of RNA modifications can lead to human disease, ranging from cancer to various metabolic and developmental illnesses such as Hoyeraal–Hreidarsson syndrome, Bowen–Conradi syndrome, or Williams–Beuren syndrome. In this review article, we summarize the characterization of all 143 currently known modified ribonucleosides by describing their taxonomic distributions, the enzymes that generate the modifications, and any implications in cellular processes, RNA structure, and disease. We also highlight areas of active research, such as specific RNAs that contain a particular type of modification as well as methodologies used to identify novel RNA modifications.
This article is categorized under:
RNA Processing > RNA Editing and Modification
Keywords: mRNA, ncRNA, RNA modification, rRNA, tRNA
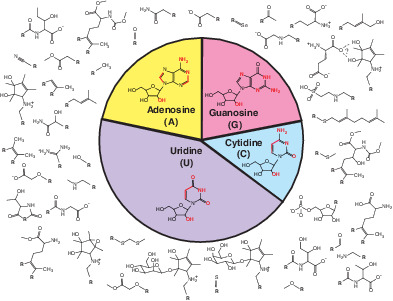
Adenosine, guanosine, cytidine, and uridine can be modified at various positions (red) with a myriad of functional groups (outside circle).
1. INTRODUCTION
Adenosine (A), guanosine (G), cytidine (C), and uridine (U) comprise the four ribonucleosides typically found in ribonucleic acid (RNA) molecules. These four ribonucleosides were divided into two categories based on the shape and chemical properties of the nucleobases: pyrimidines and purines. Pyrimidines, composed of C and U (represented as Y), feature a six‐membered heterocyclic configuration, similar to pyridine. Purines, composed of A and G (represented as R), possess a heterocyclic configuration of a pyrimidine fused to an imidazole ring. The pyrimidines and purines can be added to ribose molecules, forming ribonucleosides (Figure 1). From these pyrimidines and purines, several derivatives such as thiamin pyrophosphate (TPP), flavin mononucleotide (FMN), and cyclic di‐guanosine monophosphate (c‐di‐GMP) are thought to belong to the RNA world, a world in which genes, genomes, and enzymes were composed solely of RNA (Crick, 1968; Joyce & Szostak, 2018; Nelson & Breaker, 2017; Orgel, 1968; Robertson & Joyce, 2012; Woese, 1967). However, pyrimidines and purines have also been discovered to be the precursors for numerous modifications (Cohn & Volkin, 1951; Dunn, 1960; Hall, 1963; Holley, 1965; Iwanami & Brown, 1968; D. A. Smith & Visser, 1965; reviewed in Boccaletto et al., 2018; Cantara et al., 2011). The discovery and characterization of RNA modifications underscore their biological significance and embody exciting strides throughout RNA biology (Boccaletto et al., 2018; Jung & Goldman, 2018; Shi, Wei, & He, 2019; Thapar et al., 2019).
FIGURE 1.
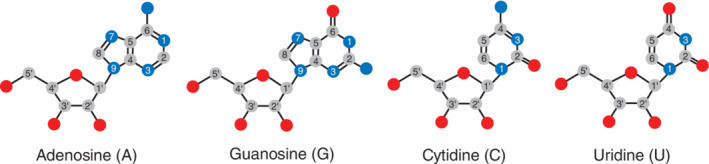
Ribonucleoside numbering of adenine, guanine, cytosine, uracil bases and numbering of the ribose sugar for adenosine (A), guanosine (G), cytidine (C), and uridine (U). Carbon atoms are in gray, oxygen atoms are in red, and nitrogen atoms are in blue. Single lines between atoms depict single bonds, double lines between atoms depict double bonds, and hydrogens are not depicted for simplicity
Ribonucleoside modifications encompass a myriad of chemical moieties, which are added to adenosine, guanosine, cytidine, or uridine. From simple methylations or hydrations of double bonds to ring closures of the nitrogenous base or the addition of large groups such as amino acids or monosaccharides, almost all modifications are catalyzed by an enzyme. Some enzymes form multimers with auxiliary proteins (e.g., helper methyltransferases), expanding the range of applicable substrates or allowing for the integration of cellular activities and enzymatic processes (Guy & Phizicky, 2014). However, a few modifications arise from nonenzymatic processes or oxidative damage (Figure 2, Table 1). A further distinction pertains to RNA editing, which was originally described as a process of adding polyuridine residues within the coding region of select RNAs (Grosjean & Benne, 1998). RNA editing has since been expanded to include RNA base excisions or additions (e.g., deletion of uridine residues in pre‐mRNAs or addition of G to select tRNAs) (Benne, 1994; Jackman & Phizicky, 2006) as well as RNA base conversions (e.g., A‐to‐I or C‐to‐U editing), although further distinctions have remained somewhat incoherent (Grosjean & Benne, 1998). While there are 111 modifications that can be found within transfer RNAs (tRNAs), 33 modifications can be found in ribosomal RNAs (rRNAs), 17 in messenger RNAs (mRNAs), and 11 in long noncoding RNAs (lncRNAs) and other noncoding RNAs (ncRNAs) (Figures 3 and 4, Table 2) (Boccaletto et al., 2018; Cantara et al., 2011; Lorenz et al., 2017).
FIGURE 2.
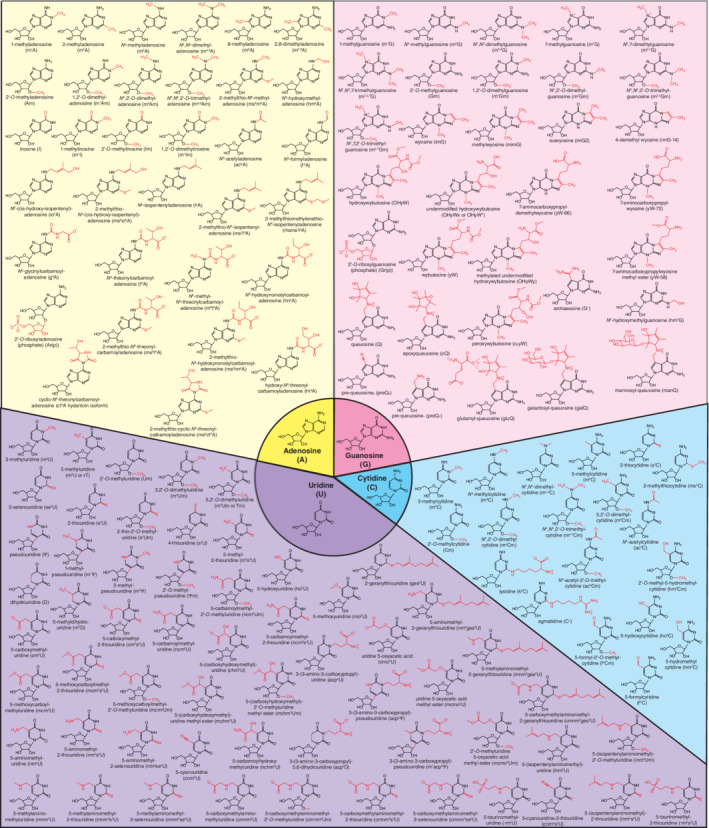
Chemical structures of all currently known RNA modifications. Adenosine‐derived (yellow), guanosine‐derived (pink), uridine‐derived (purple), and cytidine‐derived (cyan) modifications are classified based on the parent ribonucleoside. Red moieties indicate which portion of the modified ribonucleoside is different from the parent ribonucleoside, whose structures are shown in the central circle. A poster‐size image is available as Figure S1
TABLE 1.
All 143 presently known modifications and their abbreviations
| Count | RNA modification | Symbol | New symbol | Original NT |
|---|---|---|---|---|
| Adenosine | A | A | A | |
| 1 | N 6‐methyladenosine or 6‐methyladenosine | m6A | 6A | A |
| 2 | N 1‐methyladenosine or 1‐methyladenosine | m1A | 01A | A |
| 3 | N 2‐methyladenosine or 2‐methyladenosine | m2A | 2A | A |
| 4 | 2′‐O‐methyladenosine | Am | 0A | A |
| 5 | 2‐Methylthio‐N 6‐methyladenosine | ms2m6A | 621A | A |
| 6 | N 6‐isopentenyladenosine | i6A | 61A | A |
| 7 | N 6‐(cis‐hydroxyisopentenyl)‐adenosine | io6A | 60A | A |
| 8 | 2‐Methylthio‐N 6‐isopentenyladenosine | ms2i6A | 2161A | A |
| 9 | 2‐Methylthio‐N 6‐(cis‐hydroxyisopentenyl)‐adenosine | ms2io6A | 2160A | A |
| 10 | N 6‐glycinylcarbamoyladenosine | g6A | 65A | A |
| 11 | N 6‐threonylcarbamoyladenosine | t6A | 62A | A |
| 12 | 2‐Methylthio‐N 6‐threonylcarbamoyl‐adenosine | ms2t6A | 2162A | A |
| 13 | N 6‐methyl‐N 6‐threonylcarbamoyladenosine | m6t6A | 662A | A |
| 14 | N 6‐hydroxynorvalylcarbamoyladenosine | hn6A | 63A | A |
| 15 | 2‐Methylthio‐N 6‐hydroxynorvalylcarbamoyladenosine | ms2hn6A | 2163A | A |
| 16 | 2′‐O‐ribosyladenosine (phosphate) | Ar(p) | 00A | A |
| 17 | Inosine | I | 9A | A |
| 18 | N 1‐inosine or 1‐methylinosine | m1I | 19A | A |
| 19 | 1,2′‐O‐dimethylinosine | m1Im | 019A | A |
| 20 | N 6,N 6‐dimethyladenosine | m6 2A or m6,6A | 66A | A |
| 21 | 2′‐O‐methylinosine | Im | 09A | A |
| 22 | N 6,2′‐O‐dimethyladenosine | m6Am | 06A | A |
| 23 | N 6,N 6,2′‐O‐trimethyladenosine | m6 2Am or m6,6Am | 066A | A |
| 24 | N 1,2′‐O‐dimethyladenosine | m1Am | 01A | A |
| 25 | N 6‐acetyladenosine | ac6A | 64A | A |
| 26 | 8‐Methyladenosine | m8A | 8A | A |
| 27 | N 6‐formyladenosine | f6A | 67A | A |
| 28 | Cyclic N 6‐threonylcarbamoyladenosine | ct6A | 69A | A |
| 29 | N 6‐hydroxymethyladenosine | hm6A | 68A | A |
| 30 | 2,8‐Dimethyladenosine | m2,8A | 28A | A |
| 31 | Cyclic 2‐methylthio‐N 6‐threonylcarbamoyladenosine | ms2ct6A | 2164A | A |
| 32 | N 6‐hydroxythreonylcarbamoyladenosine | ht6A | 2165A | A |
| 33 | 2‐Methylthiomethylenethio‐N 6‐isopentenyl‐adenosine | msms2i6A | None | A |
| Cytidine | C | C | C | |
| 34 | 3‐Methylcytidine | m3C | 3C | C |
| 35 | 5‐Methylcytidine | m5C | 5C | C |
| 36 | 2′‐O‐methylcytidine | Cm | 0C | C |
| 37 | 2‐Thiocytidine | s2C | 2C | C |
| 38 | N 4‐acetylcytidine | ac4C | 42C | C |
| 39 | 5‐Formylcytidine | f5C | 71C | C |
| 40 | 5,2′‐O‐dimethylcytidine | m5Cm | 05C | C |
| 41 | Lysidine | k2C | 21C | C |
| 42 | N 4‐methylcytidine | m4C | 4C | C |
| 43 | 4,2′‐O‐dimethylcytidine | m4Cm | 04C | C |
| 44 | 5‐Hydroxymethylcytidine | hm5C | 51C | C |
| 45 | 5‐Formyl‐2′‐O‐methylcytidine | f5Cm | 071C | C |
| 46 | N 4,N 4,2′‐O‐trimethylcytidine | m4 2Cm or m4,4Cm | 044C | C |
| 47 | Agmatidine | C+ | 20C | C |
| 48 | 5‐Hydroxycytidine | ho5C | 50C | C |
| 49 | N 4‐acetyl‐2′‐O‐methylcytidine | ac4Cm | 042C | C |
| 50 | N 4,N 4‐dimethylcytidine | m4,4C | 44C | C |
| 51 | 2‐Methylthiocytidine | ms2C | None | C |
| 52 | 2′‐O‐methyl‐5‐hydroxymethylcytidine | hm5Cm | None | C |
| Guanosine | G | G | G | |
| 53 | 1‐Methylguanosine | m1G | 1G | G |
| 54 | N 2‐methylguanosine | m2G | 2G | G |
| 55 | 7‐Methylguanosine | m7G | 7G | G |
| 56 | 2′‐O‐methylguanosine | Gm | 0G | G |
| 57 | N 2,N 2‐dimethylguanosine | m2,2G | 22G | G |
| 58 | N 2‐2′‐O‐dimethylguanosine | m2Gm | 02G | G |
| 59 | N 2,N 2,2′‐O‐trimethylguanosine | m2,2Gm | 022G | G |
| 60 | 2′‐O‐ribosylguanosine (phosphate) | Gr(p) | 00G | G |
| 61 | Wybutosine | yW | 3483G | G |
| 62 | Peroxywybutosine | o2yW | 34832G | G |
| 63 | Hydroxywybutosine | OHyW | 34830G | G |
| 64 | Undermodified hydroxywybutosine | OHyWx or OHyW* | 3470G | G |
| 65 | Wyosine | imG | 34G | G |
| 66 | Methylwyosine | mimG | 342G | G |
| 67 | Queuosine | Q | 10G | G |
| 68 | Epoxyqueuosine | oQ | 102G | G |
| 69 | Galactosyl‐queuosine | galQ | 104G | G |
| 70 | Mannosyl‐queuosine | manQ | 106G | G |
| 71 | Glutamyl‐queuosine | gluQ | 105G | G |
| 72 | Pre‐queuosine0 | preQ0 | 100G | G |
| 73 | Pre‐queuosine1 | preQ1 | 101G | G |
| 74 | Archaeosine | G+ | 103G | G |
| 75 | N 2 ,7‐dimethylguanosine | m2,7G | 27G | G |
| 76 | N 2,2‐7‐trimethylguanosine | m2,2,7G | 227G | G |
| 77 | 1,2′‐O‐dimethylguanosine | m1Gm | 01G | G |
| 78 | 4‐Demethylwyosine | imG‐14 | 4G | G |
| 79 | Isowyosine | imG2 | 42G | G |
| 80 | N 2,2′‐O‐7‐trimethylguanosine | m2,7Gm | 027G | G |
| 81 | 7‐Aminocarboxypropylwyosine methyl ester | yW‐58 | 348G | G |
| 82 | 7‐Aminocarboxypropyl‐demethylwyosine | yW‐86 | 47G | G |
| 83 | 7‐Aminocarboxypropylwyosine | yW‐72 | 347G | G |
| 84 | Methylated undermodified hydroxywybutosine | OHyWy | 3480G | G |
| 85 | 2‐Hydroxymethylguanosine | hm2G | None | G |
| Uridine | U | U | U | |
| 86 | Pseudouridine | ψ | 9U | U |
| 87 | Dihydrouridine | D | 8U | U |
| 88 | 5‐Methyluridine, ribosylthymine, or ribothymidine | m5U or rT | 5U | U |
| 89 | 2′‐O‐methyluridine | Um | 0U | U |
| 90 | 5,2′‐O‐dimethyluridine | m5Um or Tm | 05U | U |
| 91 | 1‐Methylpseudouridine | m1ψ | 19U | U |
| 92 | 2′‐O‐methylpseudouridine | ψm | 09U | U |
| 93 | 2‐Thiouridine | s2U | 2U | U |
| 94 | 4‐Thiouridine | s4U | 74U | U |
| 95 | 2‐Thio‐2′‐O‐methyluridine | s2Um | 02U | U |
| 96 | 3‐(3‐Amino‐3‐carboxypropyl)uridine | acp3U | 30U | U |
| 97 | 5‐Hydroxyuridine | ho5U | 50U | U |
| 98 | 5‐Methoxyuridine | mo5U | 501U | U |
| 99 | Uridine 5‐oxyacetic acid | cmo5U | 502U | U |
| 100 | Uridine 5‐oxyacetic acid methyl ester | mcmo5U | 503U | U |
| 101 | 5‐Carboxyhydroxymethyluridine | chm5U | 520U | U |
| 102 | 5‐Carboxyhydroxymethyluridine methyl ester | mchm5U | 522U | U |
| 103 | 5‐Methoxycarbonylmethyluridine | mcm5U | 521U | U |
| 104 | 5‐Methoxycarbonylmethyl‐2′‐O‐methyluridine | mcm5Um | 0521U | U |
| 105 | 5‐Aminomethyl‐2‐thiouridine | nm5s2U | 2,510U | U |
| 106 | 5‐Methylaminomethyluridine | mnm5U | 511U | U |
| 107 | 5‐Methylaminomethyl‐2‐thiouridine | mnm5s2U | 2,511U | U |
| 108 | 5‐Methylaminomethyl‐2‐selenouridine | mnm5se2U | 20,511U | U |
| 109 | 5‐Carbamoylmethyluridine | ncm5U | 53U | U |
| 110 | 5‐Carbamoylmethyl‐2′‐O‐methyluridine | ncm5Um | 053U | U |
| 111 | 5‐Carboxymethylaminomethyluridine | cmnm5U | 51U | U |
| 112 | 5‐Carboxymethylaminomethyl‐2′‐O‐methyluridine | cmnm5Um | 051U | U |
| 113 | 5‐Carboxymethylaminomethyl‐2‐thiouridine | cmnm5s2U | 251U | U |
| 114 | 3‐Methyluridine | m3U | 3U | U |
| 115 | 1‐Methyl‐3(3‐amino‐3‐carboxypropyl) pseudouridine | m1acp3ψ | 1309U | U |
| 116 | 5‐Carboxymethyluridine | cm5U | 52U | U |
| 117 | 3,2′‐O‐dimethyluridine | m3Um | 03U | U |
| 118 | 5‐Methyldihydrouridine | m5D | 58U | U |
| 119 | 3‐Methylpseudouridine | m3ψ | 39U | U |
| 120 | 5‐Taurinomethyluridine | τm5U | 54U | U |
| 121 | 5‐Taurinomethyl‐2‐thiouridine | τm5s2U | 254U | U |
| 122 | 5‐(Isopentenylaminomethyl)uridine | inm5U | 583U | U |
| 123 | 5‐(Isopentenylaminomethyl)‐2‐thiouridine | inm5s2U | 2583U | U |
| 124 | 5‐(Isopentenylaminomethyl)‐2′‐O‐methyluridine | inm5Um | 0583U | U |
| 125 | 5‐Cyanomethyluridine | cnm5U | 55U | U |
| 126 | 5‐(Carboxyhydroxymethyl)‐2′‐O‐methyluridine methyl ester | mchm5Um | 0522U | U |
| 127 | 5‐Carboxymethylaminomethyl‐2‐selenouridine | cmnm5se2U | 2051U | U |
| 128 | 5‐Carboxymethylaminomethyl‐2‐geranylthiouridine | cmnm5ges2U | 2151U | U |
| 129 | 5‐Methylaminomethyl‐2‐geranylthiouridine | mnm5ges2U | 21511U | U |
| 130 | 5‐Aminomethyl‐2‐geranylthiouridine | nm5ges2U | 21510U | U |
| 131 | 5‐Methoxycarbonylmethyl‐2‐thiouridine | mcm5s2U | 2521U | U |
| 132 | 5‐Carbamoylmethyl‐2‐thiouridine | ncm5s2U | 253U | U |
| 133 | 3(3‐Amino‐3‐carboxypropyl)‐5,6‐dihydrouridine | acp3D | 308U | U |
| 134 | 5‐Aminomethyl‐2‐selenouridine | nm5se2U | 20510U | U |
| 135 | 5‐Carbamoylhydroxymethyluridine | nchm5U | 531U | U |
| 136 | 5‐Carboxymethyl‐2‐thiouridine | cm5s2U | 2540U | U |
| 137 | 5‐Methyl‐2‐thiouridine | m5s2U | 25U | U |
| 138 | 2‐Geranylthiouridine | ges2U | 21U | U |
| 139 | 2‐Selenouridine | se2U | 20U | U |
| 140 | 5‐Aminomethyluridine | nm5U | 510U | U |
| 141 | 2′‐O‐methyluridine 5‐oxyacetic acid methyl ester | mcmo5Um | 0503U | U |
| 142 | 3‐(3‐Amino‐3‐carboxypropyl)pseudouridine | acp3ψ | 309U | U |
| 143 | 5‐Cyanomethyl‐2‐thiouridine | cnm5s2U | None | U |
Note: The column labeled “RNA modification” lists the modification name. The column labeled “Symbol” lists the traditionally named abbreviations or symbols for the modification. Nonalphanumeric characters seen in abbreviations, such as plus signs, commas, parentheses, asterisks, and other such characters, significantly complicate regular expression scripting in Linux‐based environments. To mitigate this problem, the column labeled “New symbol” lists a new set of symbols for these modifications that is easier for regular expressions to use (Boccaletto et al., 2018; Jonkhout et al., 2017). The column labeled “Original NT” indicates which nucleotide (A, C, G, or U) is the ultimate originating nucleotide for the modification.
FIGURE 3.
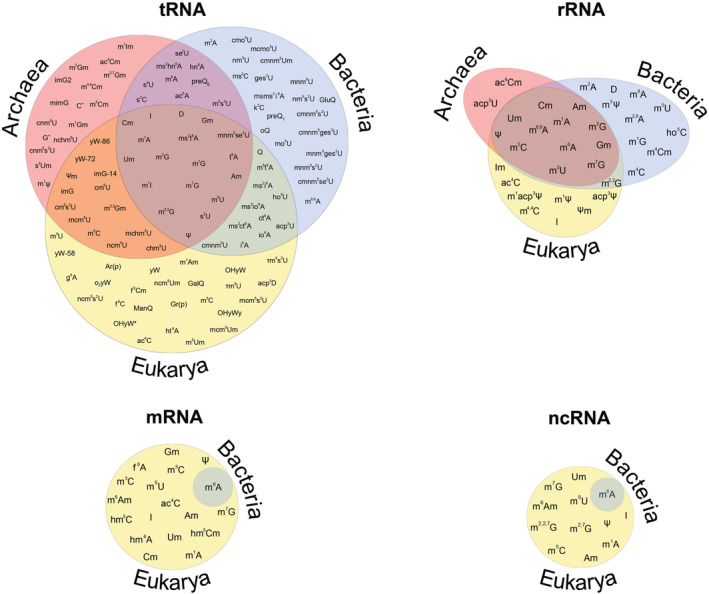
Euler diagrams showing the currently known phylogenetic distribution of ribonucleoside modifications in tRNA, rRNA, mRNA, and ncRNA classes. Archaeal modifications are in pink, bacterial modifications are in blue, and eukaryotic modifications are in yellow
FIGURE 4.
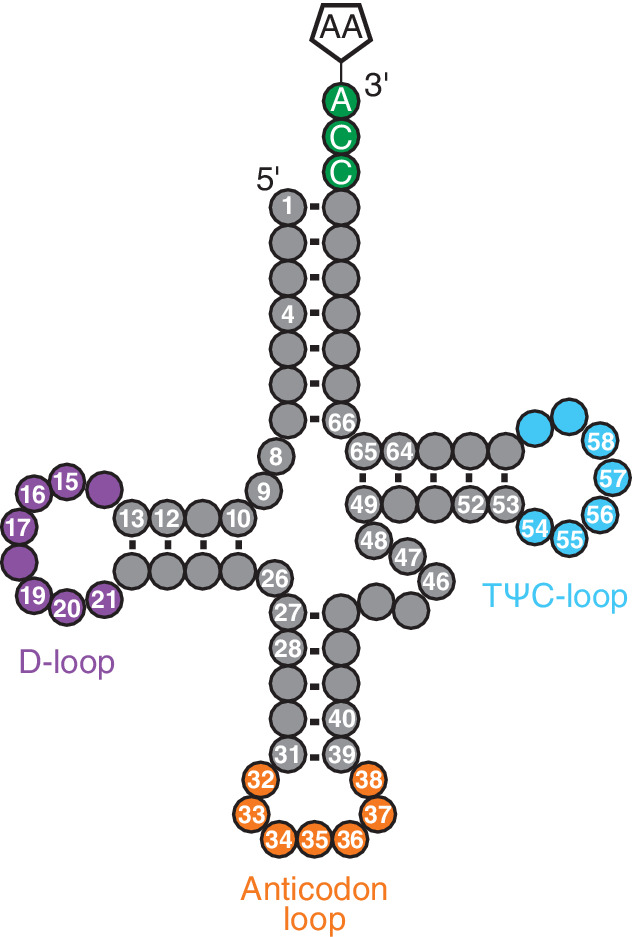
tRNA‐specific modifications are clustered around specific nucleotides in tRNAs. A schematic of the secondary structure of tRNA is depicted as a cloverleaf structure, having a D‐loop (purple), an anticodon loop (orange), a TΨC‐loop (blue), and the 3′‐CCA sequence (green) that is aminoacylated when charged with an amino acid (AA). Numbers refer to specific nucleotide positions where modifications have been mapped (Lorenz, Lünse, & Mörl, 2017; Phillips & de Crécy‐Lagard, 2011; Phizicky & Hopper, 2010; Powell, Nicholls, & Minczuk, 2015). The modifications cataloged in Table 2 occur at numbered positions in Figure 4
TABLE 2.
Modifications at known nucleotide positions in tRNAs
| tRNA position | Modification(s) |
|---|---|
| 1 | Ψ |
| 4 | Am, Cm |
| 8 | s4U |
| 9 | m1A, m1G, s4U |
| 10 | m2G, m2,2G, m2Gm, m2,2Gm |
| 12 | ac4C |
| 13 | Ψ |
| 15 | G+, preQ0 |
| 16 | D |
| 17 | D, Gm |
| 19 | D |
| 20 | D, acp3U |
| 21 | Ψ |
| 26 | m2G, m2,2G, m2Gm, m2,2Gm |
| 27 | Ψ |
| 28 | Ψ |
| 31 | Ψ |
| 32 | Ψ, m3C, Cm, s2C, s2U, ms2C |
| 33 | s2U |
| 34 | Ψ, cnm5U, ho5U, mo5U, cmo5U, mcmo5U, mcmo5Um, m3U, I, f5C, m5C, m3C, preQ0, preQ1, Q, oQ, galQ, manQ, gluQ, Cm, s2U, s2Um, nm5s2U, mnm5s2U, cmnm5s2U, C+, k2C, ac4C, Gm, mcm5U, mcm5Um, ncm5U, ncm5Um, τm5U, τm5s2U, cmnm5U, cmnm5s2U, mnm5se2U, cmnm5ges2U, mnm5ges2U, cmnm5se2U, mnm5se2U, mnm5U, nm5U, nchm5U, se2U, ges2U, chm5U, mchm5U, mcm5s2U, ncm5s2U, cm5s2U |
| 35 | Ψ |
| 36 | Ψ |
| 37 | i6A, io6A, ms2i6A, ms2io6A, t6A, g6A, ht6A, m6t6A, ct6A, ms2t6A, ms2ct6A, I, m1A, m1I, m2A, m1G, imG‐14, imG, imG2, mimG, yW‐86, yW‐72, yW‐58, yW, OHyW*, OHyWy, o2yW, OHyW, m6A, s2C |
| 38 | Ψ |
| 39 | Ψ, Um |
| 40 | m5C |
| 46 | m7G |
| 47 | acp3U |
| 48 | m5C |
| 49 | m5C |
| 52 | Ψ |
| 53 | Ψ |
| 54 | Ψ,m1Ψ, m5U, m5s2U |
| 55 | Ψ |
| 56 | Cm |
| 57 | m1A, m1I, I |
| 58 | m1A, m1I |
| 64 | Ar(p), Gr(p) |
| 65 | Ψ |
| 66 | Ψ |
| Unknown location | msms2i6A, ac6A, hn6A, ms2hn6A, m6,6A, acp3D, m3Ψ, m4,4Cm, m6Am, m6,6Am, m1Am, Im, m1Im, m1Gm, m2,7Gm, m4Cm, m5Cm, f5Cm, ac4Cm, mchm5Um, mcmo5Um, inm5Um |
Note: Specific positions within tRNAs, labeled “tRNA position,” indicate which nucleotide is modified (related to Figure 4). The column labeled “Modification(s)” indicates which modification(s) can be found at a given nucleotide position. All modifications are abbreviated as indicated in “Symbol” column of Table 1.
In this review article, we summarize all currently known ribonucleoside modifications. Each section highlights one or more modifications that are structurally or functionally related to each other or belong to established biosynthetic pathways. Each section begins with the full chemical name of the ribonucleoside modification(s) and the corresponding IUPAC abbreviation(s), domain‐of‐life assignments, and RNA classes. Domain assignments refer to the domain‐of‐life of the host organisms that harbor a modification, using the abbreviations “A” for archaeal organisms, “B” for bacterial organisms, and “E” for eukaryotic organisms. Within the text, usage of the term “prokaryote” encompasses both archaeal and bacterial species (Woese & Fox, 1977). RNA modifications in different RNA classes are denoted as follows: a black cloverleaf ( ) for modifications in tRNAs, a red square (
) for modifications in tRNAs, a red square ( ) for modifications in rRNAs, a green dot (
) for modifications in rRNAs, a green dot ( ) for modifications in mRNAs, and a blue triangle (
) for modifications in mRNAs, and a blue triangle ( ) for modifications in ncRNAs. Instances where two or more modifications are grouped in a section but are found in different domains of life will be noted in parentheses. Next, we provide a description of which enzymes or pathways are involved in the synthesis of the modification. Also, we provide any additional data or discoveries associated with the modification, including impact on RNA structure, functional significance, disease relevance, or any other notable findings. We initially describe the pseudouridine (Ψ), inosine (I), and dihydrouridine (D) modifications for historical purposes, as these were among the first modifications to be found in RNA. We then characterize all adenosine‐, guanosine‐, cytidine‐, and uridine‐based modifications followed by ribose modifications. We conclude the review by summarizing the most recent technological advances in finding novel modifications, while also describing conflicting experimental evidence for the existence of select modifications.
) for modifications in ncRNAs. Instances where two or more modifications are grouped in a section but are found in different domains of life will be noted in parentheses. Next, we provide a description of which enzymes or pathways are involved in the synthesis of the modification. Also, we provide any additional data or discoveries associated with the modification, including impact on RNA structure, functional significance, disease relevance, or any other notable findings. We initially describe the pseudouridine (Ψ), inosine (I), and dihydrouridine (D) modifications for historical purposes, as these were among the first modifications to be found in RNA. We then characterize all adenosine‐, guanosine‐, cytidine‐, and uridine‐based modifications followed by ribose modifications. We conclude the review by summarizing the most recent technological advances in finding novel modifications, while also describing conflicting experimental evidence for the existence of select modifications.
2. ABBREVIATIONS USED IN THIS WORK
Throughout this work, we use abbreviations that are commonplace or from publications that we reference. In addition to the ribonucleoside abbreviations that we catalog in Table 1, the following abbreviations appear in the text.
| ABH1: | alpha‐ketoglutarate‐dependent dioxygenase 1 or AlkB Homolog 1 |
| ACP: | 3‐amino‐3‐carboxypropyl |
| ADAR: | adenosine deaminase acting on RNA |
| ADAT: | adenosine deaminase acting on tRNA |
| AHK3: | histidine kinase 3 |
| AHK4: | histidine kinase 4 |
| AID: | activation‐induced cytidine deaminase |
| ALKB: | alpha‐ketoglutarate‐dependent dioxygenase |
| ALKBH1: | alkylated DNA repair protein (AlkB) homolog 1 |
| ALKBH3: | alkylated DNA repair protein (AlkB) homolog 3 |
| ALKBH5: | alkylated DNA repair protein (AlkB) homolog 5 |
| ALKBH8: | alkylated DNA repair protein (AlkB) homolog 8 |
| AML: | acute myeloid leukemia |
| ArcS/arcTGT: | archaeosine synthase |
| AroD: | 3‐dehydroquinate dehydratase |
| Atm1: | mitochondrial iron–sulfur clusters transporter |
| BMT5/6: | 25S rRNA (uridine(2634)‐N 3)‐methyltransferases 5 and 6 |
| Bud23: | rRNA methyltransferase and ribosome maturation factor |
| c‐di‐GMP: | cyclic di‐guanosine monophosphate |
| CDK5RAP1: | cyclin‐dependent kinase 5 regulatory subunit associated protein 1 |
| CDKAL1: | threonylcarbamoyladenosine tRNA methythiotransferase or cyclin‐dependent kinase (CDK) 5‐regulatory subunit associated protein 1 like 1 |
| cDNA: | complementary DNA |
| CDS: | coding sequence |
| Cfr: | chloramphenicol‐florfenicol resistance |
| CMC: | N‐cyclohexyl‐N′‐(2‐morpholinoethyl)‐carbodiimide |
| CMCT: | N‐cyclohexyl‐N′‐(2‐morpholinoethyl)‐carbodiimide metho‐p‐toluenesulphonate |
| c‐myc: | cellular avian myelocytomatosis viral oncogene homolog |
| CmoABM: | carboxy‐S‐adenosyl‐L‐methionine synthase A, B, or M |
| COG1738: | conserved protein domain 1738 |
| CRE1: | cytokinin response element 1 |
| CsdAE: | cysteine desulfurase A or E |
| Ctu1‐2: | cytoplasmic tRNA 2‐thiolation proteins 1 or 2 |
| DDX6: | DEAD‐box helicase 6 |
| Dim1: | dimethyladenosine transferase 1 |
| DKC1: | dyskerin pseudouridine synthase 1 |
| DNMT3A: | DNA methyltransferase 3 alpha or DNA (cytosine‐5‐)‐methyltransferase 3 alpha |
| dpCoA: | 3′‐dephospho‐coenzyme A |
| DROSHA: | double‐stranded RNA‐specific endoribonuclease |
| DTWD1‐2: | DTW (aspartate‐threonine‐tryptophan) domain‐containing proteins 1 or 2 |
| Dus: | dihydrouridine synthase |
| Dus1‐4p: | dihydrouridine synthase proteins 1–4 |
| DusABC: | dihydrouridine synthase A, B, or C |
| eEF1A: | eukaryotic elongation factor 1A |
| EF‐Tu: | elongation factor thermo unstable |
| EGFR: | epidermal growth factor receptor |
| eIF3H: | eukaryotic translation initiation factor 3 subunit H |
| eL41: | eukaryotic ribosomal protein L41 |
| Elp1‐6: | Elongator proteins 1–6 |
| Emg1: | N 1‐specific pseudouridine methyltransferase |
| FICC‐seq: | fluorouracil‐induced‐catalytic‐crosslinking‐sequencing |
| FMN: | flavin mononucleotide |
| FTO: | fat mass and obesity‐associated related |
| Fur: | ferric uptake regulator |
| GAT‐QueC: | glutamine amidotransferase class‐II‐7‐cyano‐7‐deazaguanine synthase fusion protein |
| GCD: | tRNA (adenine‐N 1‐)‐methyltransferase |
| Gcd10p/Gcd14p: | tRNA (adenine‐N 1‐)‐methyltransferases 10 and 14 proteins or tRNA (adenine(58)‐N(1))‐methyltransferase noncatalytic subunit Trm6 and Trm61 |
| GidA: | uridine 5‐carboxymethylaminomethyl modification enzyme |
| GluQRS: | glutamyl‐Q tRNA(Asp) synthetase |
| GRIA2: | glutamate ionotropic receptor alpha‐amino 3‐hydroxy‐5‐methyl‐4‐isoxazole propionate (AMPA) type subunit 2 |
| GTPBP3: | guanosine triphosphate binding protein 3 |
| H/ACA: | H‐ and ACA‐box‐containing small nucleolar RNA |
| HER2+: | human epidermal growth factor receptor 2 positive |
| hDus2p: | human dihydrouridine synthase protein 2 |
| hnRNP: | heterogeneous nuclear ribonucleoprotein |
| hTRMT10A or B: | human tRNA methyltransferase 10 A or B |
| IGF2BP: | insulin‐like growth factor 2 mRNA‐binding protein |
| IPP: | isopentenyl pyrophosphate |
| IscS: | iron–sulfur cluster S or cysteine desulfurase |
| IscU: | iron–sulfur cluster assembly enzyme |
| Isu1/2: | iron–sulfur cluster assembly proteins 1 and 2 |
| KEOPS: | kinase, putative endopeptidase and other proteins of small size |
| KsgA: | rRNA adenine dimethyltransferase or ribosomal RNA small subunit methyltransferase A |
| L7Ae: | 50S ribosomal protein |
| LC–MS: | liquid chromatography–mass spectrometry |
| MALAT1: | metastasis‐associated lung adenocarcinoma transcript 1 |
| MAT2A: | methionine adenosyltransferase 2A |
| MELAS: | mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke‐like episodes |
| MERRF: | myoclonus epilepsy associated with ragged‐red fibers |
| METTL: | methyltransferase‐like protein |
| METTL2: | methyltransferase‐like protein 2 |
| METTL3/14: | methyltransferase‐like proteins 3 and 14 |
| METTL5: | methyltransferase‐like protein 5 |
| METTL6: | methyltransferase‐like protein 6 |
| METTL8: | methyltransferase‐like protein 8 |
| METTL15: | methyltransferase‐like protein 15 |
| METTL16: | methyltransferase‐like protein 16 |
| MiaA: | tRNA delta(2)‐isopentenylpyrophosphate transferase A or tRNA dimethylallyltransferase A |
| MiaB: | tRNA delta(2)‐isopentenylpyrophosphate transferase B or tRNA dimethylallyltransferase B |
| MiaE: | tRNA delta(2)‐isopentenylpyrophosphate transferase E or tRNA dimethylallyltransferase E |
| miR/miRNA: | microRNA |
| MK2: | MAPK activated protein kinase 2 or mitogen‐activated protein kinase‐activated protein kinase 2 or MAPKAPK2 |
| MLASA: | mitochondrial myopathy and sideroblastic anemia |
| MnmACEGH: | tRNA‐specific 2‐thiouridylase A, C, E, G, or H |
| MnmC(m): | tRNA‐specific 2‐thiouridylase C, methyltransferase domain |
| MnmC(o): | tRNA‐specific 2‐thiouridylase C, oxidoreductase domain |
| Moco: | molybdenum cofactor |
| Mod5: | tRNA isopentenyltransferase 1 |
| MtaB: | threonylcarbamoyladenosine tRNA methylthiotransferase or TcdA |
| mTOR: | mammalian target of rapamycin |
| MTO1: | mitochondrial tRNA translation optimization 1 |
| MTU1: | mitochondrial tRNA‐specific 2‐thiouridylase 1 or TRMU |
| NAD+: | nicotinamide adenine dinucleotide (oxidized form) |
| NADH: | nicotinamide adenine dinucleotide (reduced form) |
| NAP57: | nucleolar and coiled‐body phosphoprotein 57 or dyskerin |
| NAT10: | N‐acetyltransferase 10 |
| Ncs6: | cytoplasmic tRNA 2‐thiolation protein 1 |
| Nep1/Emg1: | N 1‐specific pseudouridine methyltransferase |
| NFS1: | mitochondrial cysteine desulfurase |
| NifZ: | nitrogen fixation protein |
| Nm: | 2′‐O‐methyl mark |
| NML: | nucleomethylin |
| NOP56: | nucleolar protein 56 |
| NOP58: | nucleolar protein 58 |
| NRF2: | nuclear factor erythroid 2 like 2 |
| NSUN: | nucleolar protein 22/Sun domain RNA methyltransferase |
| Nt: | Nucleotide |
| Opa1: | optic atrophy 1 |
| P body: | processing body |
| PAR: | poly(ADP‐ribose) |
| PAR‐CLIP: | photoactivatable ribonucleoside‐enhanced crosslinking and immunoprecipitation |
| PCIF1: | phosphorylated C‐terminal domain‐interacting factor 1 |
| PKR: | interferon‐inducible double‐stranded RNA‐activated protein kinase or eIF‐2A protein kinase 2 |
| pri‐miRNA: | primary microRNA transcripts |
| PRC2: | polycomb responsive complex 2 |
| PRPP: | phosphoribosylpyrophosphate |
| PrrC: | anticodon nuclease |
| PRRC2A: | proline‐rich coiled‐coil 2A |
| PTC: | peptidyl transferase center |
| Pus: | pseudouridine synthase |
| Pus1p: | pseudouridine synthase 1 protein |
| Pus10: | pseudouridine synthase 10 |
| QueA: | S‐adenosyl‐l‐methionine‐dependent tRNA ribosyltransferase‐isomerase |
| QueCDE: | 7‐cyano‐7‐deazaguanine synthase, 6‐carboxy‐5,6,7,8‐tetrahydropterine synthase, and 7‐carboxy‐7‐deazaguanine synthase |
| QueF: | NADPH‐dependent 7‐cyano‐7‐deazaguanine reductase |
| QueF‐like: | similar to NADPH‐dependent 7‐cyano‐7‐deazaguanine reductase |
| QueG: | epoxyqueuosine reductase |
| QueH: | epoxyqueuosine reductase |
| QueT: | queuosine precursor transporter |
| RaSEA: | radical SAM enzyme for archaeosine formation |
| Rit1: | ribosylation of initiator tRNA 1 |
| RlhA: | 23S rRNA 5‐hydroxycytidine C2501 synthase |
| RlmA: | ribosomal RNA large subunit methyltransferase A or 23S rRNA (guanine(745)‐N(1))‐methyltransferase |
| RlmC: | ribosomal RNA large subunit methyltransferase C or 23S rRNA (uracil(747)‐C(5))‐methyltransferase |
| RlmD: | ribosomal RNA large subunit methyltransferase D or 23S rRNA (uracil(1939)‐C(5))‐methyltransferase |
| RlmF: | ribosomal RNA large subunit methyltransferase F |
| RlmFO: | ribosomal RNA large subunit methyltransferase FO or methylenetetrahydrofolate‐tRNA‐(uracil‐5‐)‐methyltransferase |
| RlmG: | ribosomal RNA large subunit methyltransferase G |
| RlmH: | ribosomal RNA large subunit methyltransferase H |
| RlmJ: | ribosomal RNA large subunit methyltransferase J |
| RlmL: | ribosomal RNA large subunit methyltransferase L |
| RlmN: | 23S rRNA m2A2503 methyltransferase/tRNA or dual‐specificity RNA methyltransferase |
| RluA: | ribosomal large subunit pseudouridine synthase A or dual‐specificity RNA pseudouridine synthase |
| RluD: | ribosomal large subunit pseudouridine synthase D |
| RNMT: | RNA guanine‐7 methyltransferase |
| RRF: | ribosome release factor |
| RsmA: | ribosomal RNA small subunit methyltransferase A |
| RsmC: | ribosomal RNA small subunit methyltransferase C |
| RsmD: | ribosomal RNA small subunit methyltransferase D |
| RsmE: | ribosomal RNA small subunit methyltransferase E |
| RsmH: | ribosomal RNA small subunit methyltransferase H |
| RsmI: | ribosomal RNA small subunit methyltransferase I |
| RsuA: | ribosomal small subunit pseudouridine synthase A |
| SAH: | S‐adensoyl‐L‐homocysteine |
| SAM: | S‐adenosyl‐L‐methionine |
| SCARLET: | site‐specific cleavage and radioactive labeling followed by ligation‐assisted extraction and thin‐layer chromatography |
| SCM‐SAH: | S‐carboxy‐S‐adenosyl‐L‐homocysteine |
| SELEX: | systematic evolution of ligands by exponential enrichment |
| SelU: | tRNA 2‐selenouridine synthase |
| snoRNA: | small nucleolar RNA |
| snoRNP: | small nucleolar ribonucleo protein |
| SNP: | single nucleotide polymorphism |
| snR35: | small nucleolar RNA 35 |
| snRNA: | small nuclear RNA |
| snRNP: | small nuclear ribonucleoprotein |
| SNU13: | small nuclear ribonucleoprotein 13 or 15.5K |
| SOS: | inducible DNA repair system |
| SRA: | steroid receptor RNA activator |
| T: | thymidine |
| Tad1p: | tRNA‐specific adenosine deaminase 1 protein |
| Tad2p: | tRNA‐specific adenosine deaminase 2 protein |
| Tad3p: | tRNA‐specific adenosine deaminase 3 protein |
| TadA: | tRNA‐specific adenosine deaminase |
| Tan1: | tRNA acetyltransferase 1 |
| TapT: | tRNA aminocarboxypropyltransferase |
| Taw1‐3: | SAM‐dependent tRNA 4‐demethylwyosine synthases 1–3 |
| Taw22: | SAM‐dependent tRNA 4‐demethylwyosine synthase 22 |
| TAZ: | tafazzin |
| TCD1/2: | threonylcarbamoyladenosine dehydratase 1 or 2 |
| TcdA: | threonylcarbamoyladenosine dehydratase A |
| Tcs1/2: | threonylcarbamoyladenylate synthase 1 or 2 |
| TermY: | tRNA m1Ψ54‐methyltransferase |
| TET: | ten‐eleven translocation methylcytosine dioxygenase |
| TET1: | ten‐eleven translocation methylcytosine dioxygenase 1 |
| Tgs1: | trimethylguanosine synthase 1 |
| TGT: | queuine tRNA‐ribosyltransferase |
| ThiI: | tRNA sulfurtransferase |
| TiaS: | tRNAIle‐agmatidine synthetase |
| TilS: | tRNAIle‐lysidine synthetase |
| Tit1: | tRNA isopentenyltransferase 1 |
| tmRNA: | transfer‐messenger RNA |
| TOR: | target of rapamycin |
| TP53: | tumor protein P53 |
| TPA: | 12‐O‐tetradecanoylphorbol‐13‐acetate |
| TPP: | thiamin pyrophosphate |
| TrhOP: | metallophos domain‐containing protein O or P |
| TRIT1: | tRNA isopentenyltransferase 1 |
| Trm‐G10: | tRNA (guanine‐10‐N 2)‐dimethyltransferase |
| Trm1: | tRNA methyltransferase 1 |
| Trm1p: | tRNA methyltransferase 1 protein |
| TRM4B: | tRNA cytosine‐C5‐methyltransferase |
| Trm5: | tRNA methyltransferase 5 |
| Trm6‐Trm61: | tRNA methyltransferases 6 and 61 |
| Trm8p/Trm82p: | tRNA (guanine‐N 7‐)‐methyltransferase |
| Trm9/Trm112: | tRNA (carboxymethyluridine(34)‐5‐O)‐methyltransferase protein |
| Trm10: | tRNA (guanine(9)‐N 1)‐methyltransferase |
| Trm11p/112p: | tRNA (guanine(10)‐N 2)‐methyltransferase |
| Trm140: | tRNA(Thr) (cytosine(32)‐N 3)‐methyltransferase |
| TrmA: | tRNA/tmRNA (uracil‐C5)‐methyltransferase |
| TrmD: | tRNA (guanine‐N 1‐)‐methyltransferase |
| TrmFO: | methylenetetrahydrofolate‐tRNA‐(uracil‐5‐)‐methyltransferase |
| TrmI: | tRNA m1A58/m7G46 methyltransferase |
| TrmM: | tRNA (adenosine(37)‐N 6)‐methyltransferase or YfiC or TrmN6 |
| TrmO: | tRNA methyltransferase O or tRNA (adenine(37)‐N 6‐methyltransferase |
| TrmU: | tRNA 5‐methylaminomethyl‐2‐thiouridylate methyltransferase |
| TRMT: | tRNA methyltransferase |
| TRMT1: | tRNA methyltransferase 1 or tRNA (guanine(26)‐N(2))‐dimethyltransferase |
| TRMT10: | tRNA methyltransferase 10 |
| TRMT2A: | tRNA methyltransferase 2A or tRNA (uracil‐5‐)‐methyltransferase |
| TRMT6: | tRNA methyltransferase 6 or tRNA (adenine(58)‐N(1))‐methyltransferase |
| TRMT12: | tRNA methyltransferase 12 homolog or tRNA wybutosine‐synthesizing protein 2 homolog |
| TRMT61A: | tRNA methyltransferase 61A or tRNA (adenine(58)‐N(1))‐methyltransferase |
| TRMT61B: | tRNA methyltransferase 61B or tRNA (adenine(58)‐N(1))‐methyltransferase |
| TRMT112: | tRNA methyltransferase 112 |
| TruA: | tRNA pseudouridine synthase A or Pus1 |
| TruB: | tRNA pseudouridine synthase B or Pus4 |
| TruD: | tRNA pseudouridine synthase D or Pus7 |
| TtcA: | tRNA‐cytidine‐32 2‐sulfurtransferase |
| TsaBCDE: | tRNA adenosine(37) threonylcarbamoyltransferase complex, dimerization subunit type 1; threonylcarbamoyladenylate synthase; tRNA adenosine(37) threonylcarbamoyltransferase complex, transferase subunit; tRNA adenosine(37) threonylcarbamoyltransferase complex, ATPase subunit type 1 |
| TsaC2: | threonylcarbamoyladenylate synthase |
| Tsr3: | acp transferase ribosome maturation factor |
| TuaA: | tRNA U47 acp transferase A or YfiP |
| Tum1: | thiosulfate sulfurtransferase |
| Tus: | sulfur carrier protein or Moa |
| TYW1‐5: | SAM‐dependent tRNA 4‐demethylwyosine synthases 1–5 |
| UTR: | untranslated region |
| WBSCR22: | Willams–Beuren syndrome chromosomal region 22 protein |
| WTAP: | Wilms Tumor 1‐Associating Protein |
| X‐DC: | X‐linked dyskeratosis congenita |
| YbbB: | tRNA 2‐selenouridine/geranyl‐2‐thiouridine synthase |
| YbeA: | m3Ψ synthase or RlmH |
| YhhQ: | queuosine precursor transporter |
| YrvO: | cysteine desulfurase |
| YTH: | YT521‐B protein domain |
| YTHDF1‐3: | YTH N 6‐methyladenosine RNA binding proteins 1–3 |
| ZCCHC4: | zinc finger CCHC‐type containing 4 |
3. DISCOVERY OF MODIFIED RIBONUCLEOSIDES: AN HISTORICAL RETROSPECTIVE ON PSEUDOURIDINE, INOSINE, AND DIHYDROURIDINE
Nucleoside modification (IUPAC abbreviation): pseudouridine (Ψ); inosine (I); dihydrouridine (D).
Domain assignments: A, B, E.
RNA classes:  ,
,  ,
,  (Ψ and I),
(Ψ and I),  (Ψ and I).
(Ψ and I).
tRNAs play a central role in translation. These adaptor molecules serve as links between the informational level of codons in mRNA and the functional level of amino acids incorporated into a growing polypeptide chain (Lorenz et al., 2017). From the initial total sequence determination of tRNAAla, the specific locations of seven different modified nucleosides, including Ψ, inosine, and D, were described by Holley (1965). Since then, tRNAs have been recognized as the RNA class containing the highest percentage of post‐transcriptional modifications (Figure 4, Table 2), which are mostly found in the tRNA loops (D‐ and TΨC‐loops) and the tRNA anticodon loop. Currently, 111 distinct modifications have been identified in tRNAs from all three domains of life (Figure 3, Table 2). One such modification, and the first RNA modification to be discovered, is Ψ.
In 1951, Ψ was discovered as the first post‐transcriptional modification in RNA and today is recognized as the most abundant RNA modification (Cohn & Volkin, 1951; reviewed in Spenkuch, Motorin, & Helm, 2014). Ψ is an isomer of uridine, where the nitrogen–carbon glycosidic bond is replaced by a carbon–carbon bond and an imine group is projected into the major groove (Figure 2). Ψ and U have identical molecular weights, making discerning Ψ and U problematic using size‐based methods (L. Johnson & Söll, 1970). However, the use of CMCT and reverse transcription or the use of SCARLET are two methods frequently used to find Ψ in RNAs (Adachi, DeZoysa, & Yu, 2019; X. Li et al., 2015; N. Liu et al., 2013; Penzo, Guerrieri, Zacchini, Treré, & Montanaro, 2017). Ψ‐seq uses CMCT to modify Ψ into N 3‐CMC‐Ψ, which can terminate reverse transcription when preparing RNAs for RNA‐seq (Carlile et al., 2014; Schwartz et al., 2014; B. S. Zhao & He, 2015). Because N 3‐CMC‐Ψ blocks reverse transcription, the Ψ nucleotide is identified as one nucleotide downstream of the 3′ end of the fragment (Ofengand, Del Campo, & Kaya, 2001).
Pseudouridine synthases isomerize the U incorporated within RNA transcripts rather than individual ribonucleosides (L. Johnson & Söll, 1970). The synthases known to date are divided into five families (RluA, RsuA, TruA, TruB, and TruD) in bacteria. In archaeal and eukaryotic organisms, Pus family enzymes write pseudouridine marks (Hamma & Ferré‐D'Amaré, 2006). The Pus enzymes have a conserved enzymatic core and use various protein domains to recognize different RNA targets (Hamma & Ferré‐D'Amaré, 2006). However, the pseudouridine synthase NAP57, dyskerin, and other homologs of pseudouridine synthase, coupled with other core proteins, use H/ACA snoRNAs as guides to position a uridine at the active site of the machinery which introduces Ψ marks in rRNA and snRNAs (Ganot, Bortolin, & Kiss, 1997; reviewed in Y. T. Yu & Meier, 2014). Ψ is found nearly ubiquitously throughout various classes of RNAs (lncRNAs, mRNAs, miRNAs, mitochondrial tRNAs, rRNAs, small Cajal‐body specific RNAs, snRNA, snoRNAs, and tRNAs) and in all three domains of life (reviewed in Penzo et al., 2017). However, it is unknown if any enzymes exist that revert Ψ back to U or completely remove Ψ from RNAs (reviewed in Eyler et al., 2019).
Nearly all tRNAs are populated with some abundance of Ψ, although some exceptions exist, such as the initiator tRNA in Saccharomyces cerevisiae (Boyer et al., 1970). Ψ55 is found in nearly all tRNAs within the TΨC stem‐loop while other sites, such as the anticodon stem‐loop and D‐arm, also possess Ψ (Charette & Gray, 2000) (Figure 4, Table 2). U55 is modified to Ψ55 by tRNA Ψ55 synthase, an enzyme from the TruB family (Nurse, Wrzesinski, Bakin, Lane, & Ofengand, 1995). Ψ55 is proposed to stabilize the tertiary structure of tRNA, particularly under extreme thermal stress (Kinghorn, O'Byrne, Booth, & Stansfield, 2002). Within rRNA, Ψ can be found in both the small and large subunits of the ribosome in prokaryotes and eukaryotes as well as in chloroplasts and mitochondria (Charette & Gray, 2000). Ψ can be found in locations crucial for peptide bond formation, such as the PTC region (Ofengand, 2002). The inclusion of Ψ in various regions of tRNA and rRNA provides structural stabilization to enable the formation of highly ordered structures. snRNAs have Ψ within both the major (U1, U2, U4, U5, and U6) and minor (U12, U4atac, and U6atac) spliceosomal complexes (A. T. Yu, Ge, & Yu, 2011). Ψ in spliceosomal RNAs plays a crucial role in the assembly and function of snRNPs or the spliceosome via intermolecular RNA–RNA and RNA–protein interactions (A. T. Yu et al., 2011).
Ψ can be found in mRNA (5′ UTRs, coding sequences, and 3′ UTRs) (Carlile et al., 2014). Additionally, pseudouridylation of mRNA was found to have a role in translation. Ψ‐containing mRNA impede Escherichia coli translation elongation by preventing the canonical interactions between the CCA‐end of a tRNA and the A site of the PTC in ribosome (Eyler et al., 2019). Further, Ψ promoted aberrant amino acid substitutions, yielding multiple peptide products from one luciferase transcript in E. coli and human cells (Eyler et al., 2019). While many of these substitutions were anticipated not to have deleterious effects on luciferase, the authors also hypothesized that these substitutions could be harmful to host organisms under stress conditions (Eyler et al., 2019). As pseudouridylation helps stabilize RNA structures, Ψ is crucial in forming the active structure of the SRA lncRNA, which promotes transcription of several genes (X. Zhao et al., 2007; Y. Zhao, Dunker, Yu, & Karijolich, 2018). Moreover, mutating the pseudouridylation site to adenosine in mice causes SRA to become hyperpseudouridylated and act as a dominant negative suppressor of gene transcription, notably c‐myc (X. Zhao et al., 2007; Y. Zhao, Dunker, et al., 2018).
Ψ has relevance in human disease, but its roles are not clearly defined in most diseases. Urine samples contain Ψ and could be used as a potential biomarker for cancer due to elevated levels in some cancer patients (Penzo et al., 2017; Seidel, Brunner, Seidel, Fritz, & Herbarth, 2006). Correlations between defects in Ψ formation and X‐DC and Hoyeraal–Hreidarsson syndrome have been characterized (Penzo et al., 2017). Both diseases involve a mutation in the dkc1 gene, leading to a decrease of Ψ in mRNA and many epidermal complications such as unusual skin pigmentation and white patches lining the oral cavity (leukoplakia) (Penzo et al., 2017). However, some individuals with either disease have been found to have normal levels of Ψ in mRNA, while telomerase impairment and shortened telomeres result in the diseases (Penzo et al., 2017). Separately, abrogation of Pus1p‐mediated pseudouridylation of SRA has also been linked to MLASA in humans (Bykhovskaya, Casas, Mengesha, Inbal, & Fischel‐Ghodsian, 2004; X. Zhao et al., 2007). Further studies are required to determine if pseudouridylation has a direct role in these diseases.
As early as 1957, inosine was discovered as a biosynthetic precursor to purine synthesis in every domain of life (Brown, Hoopes, White, & Sarisky, 2011; Graupner, Xu, & White, 2002; Hartman & Buchanan, 1958; Levin & Magasanik, 1961; Marolewski, Smith, & Benkovic, 1994; E. Meyer, Leonard, Bhat, Stubbe, & Smith, 1992; Nygaard & Saxild, 2000; Ownby, Xu, & White, 2005; L Warren, Flaks, & Buchanan, 1957). Inosine, which occurs in RNA and DNA, is the product of hydrolytic deamination at the C6 position of adenosine (Alseth, Dalhus, & Bjørås, 2014). In DNA, inosine formation can be caused by nonenzymatic processes, such as spontaneous hydrolysis, endogenous or environmental factors, or exposure to nitrosative compounds (Alseth et al., 2014). While these nonenzymatic processes can also occur in RNA, deaminase enzymes can also introduce inosine into RNAs (Alseth et al., 2014). In DNA, inosine preferentially base pairs with cytidine, where A‐to‐I deamination results in miscoding and is considered a premutagenic event (Alseth et al., 2014; Nordmann, Makris, & Reznikoff, 1988). In RNA, inosine can base pair to A, C, or U, allowing inosine to function as a Wobble base in the anticodon loop of tRNA or within other RNAs, such as mRNAs (reviewed in Wright, Force, & Znosko, 2018). Inosine was found in the Wobble position (I34) of tRNAAla (Holley, 1965) and in several tRNAs (Figure 4) (reviewed in Rubio et al., 2017; Torres et al., 2014). I34 was later determined to be essential in bacteria and eukaryotes, but not in archaea (reviewed in Rubio et al., 2017; Torres et al., 2014).
In contrast to DNA, enzymatically derived inosine modifications found in double‐stranded RNA (dsRNA) occur via ADARs (Bass et al., 1997; Bass & Weintraub, 1988; Rebagliati & Melton, 1987) or ADATs (Bass et al., 1997; Danan‐Gotthold & Levanon, 2017). Deamination of A‐to‐I has been reported in tRNAs (reviewed in Torres et al., 2014), recently for the first time in the small subunit of mitochondrial rRNA from the eukaryote Diplonema papillatum (Moreira, Valach, Aoulad‐Aissa, Otto, & Burger, 2016), mRNAs (Paul & Bass, 1998), ncRNAs (Kawahara et al., 2007; Luciano, Mirsky, Vendetti, & Maas, 2004; Y. Yang, Zhou, & Jin, 2013), and viral RNAs (Samuel, 2011). Translational and splicing machinery recognize inosine as G, resulting in the altered informational content of the RNA. When A‐to‐I editing occurs in the coding region of an mRNA, the corresponding codon is altered, which can change the sequence of an encoded protein. Though recoding is rare, A‐to‐I editing events predominantly occur in the noncoding intronic or untranslated terminal regions of mRNAs and in ncRNAs (Athanasiadis, Rich, & Maas, 2004; D. D. Y. Kim et al., 2004; E. Y. Levanon et al., 2005; Y. Yang et al., 2013). For example, A‐to‐I editing events have been observed at repeated Alu‐rich regions in RNAs from multiple classes (Levanon et al., 2004). Additionally, with several A‐to‐I editing events in the primary transcript of mouse miRNA‐142 (pri‐miR‐142), both pri‐miR‐142 and the mature miR‐142 levels are repressed (W. Yang et al., 2006). Consequently, in adar1 or adar2 null mice, both pri‐miR‐142 and mature miR‐142 are increased (W. Yang et al., 2006). Interestingly, ADAR‐catalyzed A‐to‐I conversion of DNA bases in DNA/RNA hybrids has been recently described, which may expand the spectrum of biological functions of ADARs (Y. Zheng, Lorenzo, & Beal, 2017).
While A‐to‐I editing is widespread across Metazoa, the number of edits can vary considerably across species (Porath, Knisbacher, Eisenberg, & Levanon, 2017). Three classes of ADAR proteins are involved in the regulation of pre‐mRNA A‐to‐I editing in vertebrates. ADAR1 and ADAR2 are expressed in most tissues, while ADAR3 is expressed only in the central nervous system (C. X. Chen et al., 2000; Oakes, Anderson, Cohen‐Gadol, & Hundley, 2017). Importantly, while ADAR1 and ADAR2 catalyze A‐to‐I editing (Zinshteyn & Nishikura, 2009), ADAR3 competitively interferes with the activity of RNA‐bound ADAR1 and ADAR2, suggesting ADAR3 has a regulatory role (C. X. Chen et al., 2000). ADAT, a class of deaminases that are specific to tRNAs, are related to ADAR but are inactive on known pre‐mRNA substrates and extended dsRNAs (Maas, Gerber, & Rich, 1999; Torres et al., 2014). ADAT1, or its homolog Tad1p, catalyzes the A‐to‐I conversion at position 37 in the anticodon loop of eukaryotic tRNAAla (Figure 4, Table 2) (A. Gerber, Grosjean, Melcher, & Keller, 1998), while the ADAT2/ADAT3 heterodimer or its homolog heterodimer Tad2p/Tad3p catalyzes the Wobble position A‐to‐I conversion of eukaryotic tRNAs (A. P. Gerber & Keller, 1999). The formation of I34 in bacterial tRNAArg is catalyzed by the TadA enzyme (A. A. H. Su & Randau, 2011; Wolf, Gerber, & Keller, 2002). Inosine at position 57 in the TΨC loop of tRNA has been reported for only archaea, where it occurs as N 1‐methylinosine (m1I57) (Figure 4) (Yamaizumi et al., 1982). Here, the first step of A‐to‐I conversion involves methylation of A57 by the SAM‐dependent methyltransferase TrmI; this methylated adenosine is then deaminated to m1I57 by an unknown enzyme (Grosjean, Constantinesco, Foiret, & Benachenhou, 1995; Roovers et al., 2004; reviewed in Torres et al., 2014).
The effects of A‐to‐I editing have been observed primarily in brain tissue or neurons and can exhibit different phenotypes in these organs or cellular environments. A‐to‐I editing can affect brain tissue or neurons through dampening signaling through G‐protein receptors, enhancing the activity of voltage‐gated ion channels, promoting proper brain and neuron function, or proper splicing of glutamate‐gated cation selective channels, among other observed functions (Burns et al., 1997; Hoopengardner, Bhalla, Staber, & Reenan, 2003; Levanon et al., 2005; Seeburg, Higuchi, & Sprengel, 1998; Sommer, Köhler, Sprengel, & Seeburg, 1991). ADAR activity plays an essential role in nervous system function by restoring or establishing neuronal growth (Benowitz, Goldberg, & Irwin, 2002). Further, mutations in ADARs have been shown to cause neural and behavioral defects in Drosophila melanogaster and Caenorhabditis elegans (Goldstein et al., 2017). Disrupted ADAR activity is associated with brain cancers, prostate cancer, hepatocellular carcinoma, and chronic myeloid leukemia (Fritzell, Xu, Lagergren, & Öhman, 2018; L.‐D. Xu & Öhman, 2018). ADAR2 is essential for editing some mature miRNAs in glioblastoma cells and ADAR2 can also reduce the expression of numerous oncogenic miRNAs (Tomaselli et al., 2015). Astrocytoma and glioblastoma in humans have been correlated with an overexpression of ADAR3, in which ADAR3 likely competes with ADAR2 for a binding site in the gria2 mRNA (Oakes et al., 2017). This competition prevents RNA editing and promotes cell migration as well as tumor invasion in glioblastoma (Oakes et al., 2017). A‐to‐I editing involvement in cancer, as well as neuronal behavior studies, remains an area of active research and could potentially lead to drug targets for cancer or behavioral treatments.
Another modification that was among the first found in total RNA was D in 1952 from beef spleen, though D had been synthesized previously (Funk, Merritt, & Ehrlich, 1952; Weidel & Roithner, 1896). D is a product of the post‐transcriptional enzymatic reduction of the C5–C6 double bond in uridine to a single bond (Figures 1 and 2). Present in all three domains of life, D is a common modification in tRNA, where the D‐loop was aptly named because it has several D modifications (Figure 4) (Sprinzl, Horn, Brown, Ioudovitch, & Steinberg, 1998). However, D also resides in the variable loop (V‐loop) of tRNAs and in the E. coli 23S rRNA (Bishop, Xu, Johnson, Schimmel, & de Crécy‐Lagard, 2002; Kowalak, Bruenger, & McCloskey, 1995; Xing, Hiley, Hughes, & Phizicky, 2004). D typically destabilizes stacking interactions with other nucleosides and D destabilizes the C3′‐endo sugar conformation of its ribose, which is a hallmark for the formation of the A‐RNA double helix (J. Dalluge, Hashizume, Sopchik, McCloskey, & Davis, 1996). Instead, D prefers a C2′‐endo conformation, resulting in increased flexibility and dynamic motion where RNA tertiary interactions need to be accommodated (J. Dalluge et al., 1996). In psychrophiles, the increased usage of D is thought to be helpful in maintaining conformational flexibility of RNA under instances where thermal‐based motion is limited or compromised (J. J. Dalluge et al., 1997). Finally, D is an important factor in ensuring the recognition of tRNA by specific aminoacyl‐tRNA synthetases and the fidelity of translation (Hendrickson, 2001).
Though D is an abundant modification in all three domains of life, Dus enzymes that catalyze the formation of D belong to a broad family of FMN‐dependent enzymes (Bishop et al., 2002). Three classes of Dus enzymes are found in E. coli (DusA, DusB, and DusC), four classes are found in S. cerevisiae (Dus1p, Dus2p, Dus3p, and Dus4p), and one class is archaeal (Dus) (Bishop et al., 2002; Kasprzak, Czerwoniec, & Bujnicki, 2012). Thus far, one homolog has been discovered in humans (hDus2p), though homologs for Dus1, Dus3, and Dus4 may also exist in humans (Kasprzak et al., 2012; Whelan et al., 2015). By conducting a structural analysis of bacterial DusABC, it was determined that DusB is the ancestral Dus enzyme (Bou‐Nader et al., 2018). Additional structural analyses of DusABC proteins revealed that each Dus enzyme has distinct substrate specificity on tRNAs (Bou‐Nader et al., 2018). Further analysis of the otherwise evolutionarily conserved double‐stranded RNA‐binding domain of hDus2p revealed a novel N‐terminal extension in the hDus2p enzyme, which confers the ability to recognize specifically tRNAs, instead of nonspecifically recognizing double‐stranded RNAs (Bou‐Nader et al., 2019).
Because elevated levels of dihydrouridine were correlated with Novikoff hepatoma and in Ehrlich ascites previously (Kuchino & Borek, 1978), subsequent research sought to determine involvement of hDus2p in cancer (T. Kato et al., 2005). In lung cancer, there is an increased amount of hDus2p, leading to greater amounts of D, which in turn increases conformational flexibility of tRNAs and enhances translational efficiencies (T. Kato et al., 2005). Concordantly, overexpression of hDus2p was correlated with a poor prognosis in lung cancer patients (T. Kato et al., 2005). Separately, hDus2p interacts with PKR, inhibiting its kinase activity and interferon‐induced antiviral innate immunity (Mittelstadt et al., 2007). Overexpression of hDus2p also inhibits stress‐induced apoptosis in sarcoma cells (Mittelstadt et al., 2007). The discovery of additional Dus homologs in humans may provide further clues in its involvement in human health. Moreover, the natural occurrence of D in psychrophilic organisms may provide useful applications or insights into synthetic biological applications at colder temperatures. The discovery and characterization of the modifications Ψ, I, and D served as a basis for the subsequent discovery and characterization of modifications in RNA from all three domains of life.
4. ADENOSINE‐DERIVED MODIFICATIONS
4.1. The roles of m6A in biology and its metabolic breakdown products of hm6A and f6A
Nucleoside modification (IUPAC abbreviation): N 6‐methyladenosine (m6A); N 6‐hydroxymethyladenosine (hm6A); N 6‐formyladenosine (f6A).
Domain assignments: A (m6A), B (m6A), E.
RNA classes:  (m6A),
(m6A),  (m6A),
(m6A),  ,
,  (m6A).
(m6A).
The biological function of N 6‐methyladenosine (m6A) represents one of the most prolific areas of recent research in modified ribonucleosides. Although m6A was originally discovered in 1974 in mRNA (Perry & Kelley, 1974), it was 25 years later when the first enzyme that could synthesize m6A (known as an m6A writer) was discovered. For eukaryotic mRNAs, this writer was identified as METTL3, which exists in a complex with the catalytically inactive METTL14 and WTAP (Bokar, Shambaugh, Polayes, Matera, & Rottman, 1997; Ping et al., 2014; Shi et al., 2019). METTL3/14 methylate RNAs at a consensus nucleotide sequence: 5′‐RRACH‐3′ (R = purine, H = A, C, or U nucleotides) (Dominissini et al., 2012; K. D. Meyer et al., 2012). Subsequent m6A methyltransferases were discovered in humans and E. coli acting on different RNA classes. One of these methyltransferases is human METTL16, which modifies the U6 snRNA, the MAT2A mRNA, and likely other RNAs (Koh, Goh, & Goh, 2019; Pendleton et al., 2017; Warda et al., 2017). Recently, the heterodimer complex METTL5 and Trmt12 was characterized as an m6A methyltransferase that modifies A1832 in the human 18S rRNA (van Tran et al., 2019) and ZCCHC4 was recently characterized as an m6A methyltransferase that modifies A4220 in the human 28S rRNA (Pinto et al., 2020; van Tran et al., 2019). RlmF and RlmJ place two distinct m6A marks (A1618 and A2030, respectively) on the E. coli 23S rRNA (Golovina et al., 2012; Sergiev, Serebryakova, Bogdanov, & Dontsova, 2008). Although m6A is present in bacterial mRNAs, the methyltransferase(s) that confer m6A marks remains elusive. The consensus sites for E. coli and Pseudomonas aeruginosa, respectively, are 5′‐UGCCAG‐3′ or 5′‐GGYCAG‐3′, which are distinct from the 5′‐RRACH‐3′ sequence recognized by METTL3/14 in humans (Deng et al., 2015). Moreover, m6A modifications have been detected in one tRNAVal species in E. coli (Saneyoshi, Harada, & Nishimura, 1969) and its writer was determined to be TrmM (Golovina et al., 2009). Further, an m6A modification at A1500 has been detected in the small ribosomal subunit of the crenarchaeote Sulfolobus solfataricus and Haloferax volcanii (Kowalak, Bruenger, Crain, & McCloskey, 2000; Noon, Bruenger, & McCloskey, 1998), although it is currently unknown which enzymes confer these modifications.
For some RNAs from bacterial and eukaryotic organisms, there are validated demethylases that can remove m6A marks from RNAs (known as m6A erasers) (Fedeles, Singh, Delaney, Li, & Essigmann, 2015). One such demethylase is the ALKBH5 protein, which specifically removes m6A marks from mRNAs (G. Zheng et al., 2013). Another demethylase is the FTO protein, which demethylates m6A, m6Am, and m1A marks in mRNA, snRNA, and tRNA, respectively (Shi et al., 2019; Wei et al., 2018; X. Zhang, Wei, et al., 2019). Instead of a direct demethylation reaction, human and mouse FTO can demethylate m6A marks by oxidizing them to yield hm6A, which is further oxidized to yield f6A (Fu et al., 2013). The half‐lives of these modifications, whose biological roles remain unexplored, is ~3 hr in vitro, and then both modifications nonenzymatically decompose back to adenosine (Fu et al., 2013). The reversibility of m6A marks in mRNAs remains controversial, for a small percent of m6A marks vary during the life of an mRNA (Darnell, Ke, & Darnell, 2018; Zaccara, Ries, & Jaffrey, 2019; B. S. Zhao, Nachtergaele, Roundtree, & He, 2018). Further, the role of m6A in splicing has been questioned because mouse embryonic stem cells without Mettl3 continue to grow and properly splice mRNAs (Darnell et al., 2018). However, there is agreement that nascent mRNAs are cotranscriptionally modified with m6A marks and that m6A‐containing mRNAs are more expeditiously turned over than non‐m6A mRNAs (Darnell et al., 2018; B. S. Zhao, Nachtergaele, et al., 2018). Regardless, the removal of m6A is a controlled process, though it is unclear what significance hm6A and f6A have in biology.
In addition to m6A writers and erasers, several proteins have been characterized whose binding activity depends on the presence or absence of an m6A mark (known as m6A readers). For example, m6A marks may weaken secondary structure to create new protein‐binding sites, such as hnRNPC binding to a U‐rich loop in an m6A‐disrupted hairpin (Alarcón, Goodarzi, et al., 2015; N. Liu et al., 2015, 2017; Shi et al., 2019; B. Wu et al., 2018). These findings led to the model of an m6A switch, whereby the absence of an m6A mark may result in a stable hairpin that obscures protein binding sequences (N. Liu et al., 2015, 2017; X. Wang et al., 2014). Another possible m6A switch regulates miRNA‐binding site accessibility in MALAT1. Here, the absence of an m6A mark in MALAT1 may prevent the formation of a long‐range pseudoknot and, concordantly, expose two miRNA‐binding sites that could sequester miRNAs away from their oncogenic mRNA targets (McCown, Wang, Jaeger, & Brown, 2019). Proteins containing the YTH domain, such as YTHDF2, can recognize and bind to m6A marks on mRNAs and ncRNAs in humans. These binding events destabilize the structure of their RNA targets and then lead these YTHDF2‐bound RNAs to P bodies for degradation (H. Du et al., 2016; Shi et al., 2017, 2019; X. Wang et al., 2014, 2015). IGF2BP1–3 proteins increase the mRNA half‐life of mRNAs with m6A marks, while PRRC2A also increases mRNA half‐life of an m6A‐containing transcript that is necessary for myelination (H. Huang et al., 2018; Shi et al., 2019; R. Wu et al., 2019). The binding of the splicing‐factor hnRNPA2B1 enables m6A‐dependent nuclear processing, especially for pri‐miRNAs (Alarcón, Goodarzi, et al., 2015). METTL3 and its corresponding m6A marks are sufficient to permit proper pri‐miRNA processing by DROSHA in a non‐cell‐type specific fashion in human cells (Alarcón, Lee, Goodarzi, Halberg, Tavazoie, 2015). hm6A and f6A are not recognized by the YTHDF2 protein, suggesting that they could either bind to different RNA‐binding proteins or serve as markers for newly synthesized RNAs, among other possibilities (Fu et al., 2013). Recently, it was discovered that YTHDF1–3 facilitates phase separation of m6A‐marked mRNAs and influences the transport of these mRNAs into different phase‐separated compartments: P bodies for nonstress conditions, or stress granules for heat shock or arsenite stress (Ries et al., 2019).
The involvement of m6A in human health is a highly active area of research that cannot be adequately addressed in this review. Several recent reviews capture the trends on m6A in disease (X.‐Y. Chen, Zhang, & Zhu, 2019; K. Du, Zhang, Lee, & Sun, 2019; Lan et al., 2019; Tong, Flavell, & Li, 2018). Nonetheless, we highlight some of the more striking discoveries involving m6A in human disease. In select cancer cells, an abundance of METTL3 was found to promote A549 lung adenocarcinoma cancer cell growth, survival, and invasion phenotypes, while the methylation activity of METTL3 was found to regulate the protein expression of several oncogenes, including EGFR, TAZ, MK2, and DNMT3A (S. Lin, Choe, Du, Triboulet, & Gregory, 2016). METTL3 enhances the translation of these oncogenes and others by physically interacting with eIF3H, which increases the formation of polysomes on the EGFR, TAZ, MK2, and DNMT3A mRNAs (Choe et al., 2018). Small molecule ligands that bind to the METTL3–METTL14–WTAP complex activate this complex and may aid in cell survival after UV exposure (Selberg et al., 2019; Xiang et al., 2017). YTHDF2 is often overexpressed in AML and is required for AML initiation and propagation (J. Paris et al., 2019). YTHDF2 was found to be dispensable in noncancerous hematopoietic stem cell function, yet essential for leukemic stem cell function. Concordantly, YTHDF2 represents an attractive therapeutic target for AML, which also permits hematopoietic stem cell expansion after treatment (J. Paris et al., 2019). Small molecule inhibitors targeting FTO have eradicated AML cells in vitro and in mouse xenograft models of AML (Y. Huang et al., 2019). In neurology, m6A marks were shown to shorten the radial glial cell cycle, while also extending cortical neurogenesis beyond birth in mouse brains (Yoon et al., 2017). Moreover, m6A marks are significant in memory formation in mice, as a knockout of the ythdf1 gene in mice showed learning and memory deficits (Shi et al., 2018). In cardiology, m6A marks have been shown to be crucial in proper heart development and in heart function; for an siRNA knockdown of METTL3 and a cardiomyocyte‐specific knockout mouse line of mettl3 resulted in the depletion of m6A, which also resulted in increased rates of heart failure in mice and possibly in humans (Dorn et al., 2019). Much research has been conducted on the significance of the presence or absence of m6A marks to human health. However, further research is needed to determine precisely how m6A, hm6A, and f6A affect human health instead of correlative observations based on their global alterations in knocking down or overexpressing enzymes that write or erase these modifications. Also, research into the significance of hm6A and f6A remains almost nonexistent and may further elucidate the temporal presence of m6A in RNAs.
4.2. The methyl groups of m1A and its derivative m1I disrupt hydrogen bonding along the Watson–Crick face of their host ribonucleotides
Nucleoside modification (IUPAC abbreviation): N 1‐methyladenosine (m1A); N 1‐inosine (m1I).
Domain assignments: A, B, E.
RNA classes:  ,
,  (m1A),
(m1A),  (m1A),
(m1A),  (m1A).
(m1A).
m1A is a prevalent, dynamic base modification found in tRNAs, rRNAs, mRNAs, and ncRNAs (Bar‐Yaacov et al., 2016, 2017; Dominissini et al., 2016; Khoddami et al., 2019; Kirpekar, Hansen, Rasmussen, Poehlsgaard, & Vester, 2005; Koscinski, Feder, & Bujnicki, 2007; X. Li et al., 2016; Maden, 1990; McCown et al., 2019; Oerum, Dégut, Barraud, & Tisné, 2017; Roovers et al., 2004; Safra et al., 2017; Schwartz, 2018; Sharma, Watzinger, Kötter, & Entian, 2013; Wachino et al., 2007). m1A disrupts U‐A Watson–Crick base pairing and introduces a positive charge at physiological pH (Dominissini et al., 2016; Parsyan et al., 2011; Roundtree, Evans, Pan, & He, 2017; Zur & Tuller, 2013). m1A marks have been found at several positions in tRNAs in all three domains of life (Figure 4, Table 2) (Oerum et al., 2017; Roovers et al., 2008; Sprinzl et al., 1998). The two most well‐studied m1A modifications in tRNA are m1A9 and m1A58, which have been related to proper tRNA folding and structural stability (reviewed in Oerum et al., 2017). The m1A9 modification has been found in archaeal and mitochondrial mammalian tRNAs (reviewed in Oerum et al., 2017). In human mitochondrial tRNALys, tRNALeu(UUR), and tRNAAsp, the m1A9 modifications disrupt Watson–Crick interactions that would otherwise render these tRNAs as hairpins. Instead, these tRNAs possess the functional L‐shape structure seen in other tRNAs (Figure 4, Table 2) (Helm et al., 1998; Helm, Giegé, & Florentz, 1999; Helm & Attardi, 2004; Oerum et al., 2017; Voigts‐Hoffmann et al., 2007). Recently, the enzyme hTRMT10B was indicated as the tRNAAsp‐specific m1A9 methyltransferase in humans (Howell, Jora, Jepson, Limbach, & Jackman, 2019). The m1A58 modification in tRNAs can be found in all three domains of life, though it is found in both cytosolic and mitochondrial tRNAs of eukaryotes (reviewed in Oerum et al., 2017). For the tRNA‐specific m1A58 modification, prokaryotes use the enzyme TrmI to methylate A58, while the enzyme complex Trm6–Trm61 (previously known as Gcd10p/Gcd14p) methylates this adenosine in S. cerevisiae (Droogmans et al., 2003; Oerum et al., 2017; Roovers et al., 2004). Among prokaryotic organisms such as Thermus thermophilus, m1A58, Gm18, and m5s2U54 have been linked to the structural thermostability of tRNA as these modifications increase the melting temperature of tRNA by 10°C when compared with unmodified tRNAs (Droogmans et al., 2003; Oerum et al., 2017; Yokoyama, Watanabe, & Miyazawa, 1987). In S. cerevisiae, m1A58 has been shown to be important for the maturation of the initiator tRNAMet (Anderson et al., 1998). In humans, m1A58 situated in tRNALys3 appears to be essential for the fidelity and efficiency of plus‐strand DNA transfer during HIV‐1 reverse transcription (Auxilien, Keith, Le Grice, & Darlix, 1999). m1A marks have also been discovered in rRNA in archaeal and aminoglycoside‐resistant bacteria. The m1A1408 modification in 16S rRNA disrupts the binding of aminoglycosides to rRNA in E. coli (Kirpekar et al., 2005; Koscinski et al., 2007; Wachino et al., 2007). Like prokaryotes, m1A modifications can be found in eukaryotic rRNA, such as A645 in S. cerevisiae 25S rRNA and A1322 in human 28S rRNA. This modification functions in the biogenesis of the human 60S ribosome through methylation of A1322, as catalyzed by NML (Peifer et al., 2013; Sharma et al., 2018; Waku et al., 2016). In the mitochondrial 16S rRNA, the enzyme TRMT61B confers this methyl mark (Bar‐Yaacov et al., 2016, 2017).
For m1A modifications in human mRNAs, TRMT6/TRMT61A has been shown to confer this mark (Schwartz, 2018). For m1A erasers, FTO and ALKBH3 have been shown to demethylate m1A marks in human tRNAs and mRNAs (Wei et al., 2018; Woo & Chambers, 2019). The presence of m1A in mRNAs increases the efficiency of translation initiation, early elongation, and enhances translation rates (Dominissini et al., 2016; Roundtree et al., 2017). m1A has been determined to be involved in cancer, as either drastic upregulation or downregulation of m1A levels and m1A writer proteins are found in patients with poor prognoses in several cancer types, including gastrointestinal cancers, hepatocellular carcinoma, pancreatic adenocarcinoma, and colorectal adenocarcinoma (Y. Zhao et al., 2019).
In archaeal tRNAs, m1A marks are present at positions 57 and 58, where m1A57 is an obligatory precursor in the formation of m1I (Droogmans et al., 2003; Grosjean et al., 1995; Roovers et al., 2004; Yamaizumi et al., 1982). Adenosine methylation occurs first via the TrmI enzyme to form the m1A marks; m1A57 is further deaminated by an unknown enzyme to form m1I (Grosjean et al., 1995, 1996; Roovers et al., 2004). m1I has also been found in eukaryotic cytosolic tRNAAla at position m1I37 (Grosjean et al., 1995, 1996; Holley, 1965). The synthesis of m1I in eukaryotes differs from archaea, however. In S. cerevisiae and animal cells, the homodimeric Tad1p and ADAT1 enzymes, respectively, catalyze the conversion of A37 to I37 (A. Gerber et al., 1998; Maas et al., 1999). I37 is then methylated to m1I by Trm5 in a SAM‐dependent reaction (Björk et al., 2001). In the autoimmune disease polymyositis, antibodies were detected and could target m1I‐containing tRNAs and stem‐loops (Grosjean et al., 1996). Though m1A‐related health research is being conducted, further m1I‐related research may also yield interesting discoveries in human health that are currently unknown.
4.3. i6A and the related ribonucleosides io6A, ms2i6A, ms2io6A, and msms2i6A have roles in tRNA formation, translation, cytokinin formation, and prion development
Nucleoside modification (IUPAC abbreviation): N 6‐isopentenyladenosine (i6A); N 6‐(cis‐hydroxyisopentenyl)‐adenosine (io6A); 2‐methylthio‐N 6‐isopentenyladenosine (ms2i6A); 2‐methylthio‐N 6‐(cis‐hydroxyisopentenyl)‐adenosine (ms2io6A); 2‐methylthiomethylenethio‐N 6‐isopentenyl‐adenosine (msms2i6A).
Domain assignments: B, E (not msms2i6A).
RNA classes:  .
.
i6A appears at position 37 in many tRNA molecules, corresponding to the anticodon loop (nt 37 in Figure 4) (U. Schweizer, Bohleber, & Fradejas‐Villar, 2017). In bacteria and eukaryotes, the synthesis of i6A within a tRNA is accomplished by conjugating an isopentenyl pyrophosphate (IPP) molecule to A37 via the bacterial miaA enzyme, the S. cerevisiae mod5 enzyme, the human TRIT1 enzyme, or organism‐relevant homologs (Figure 5a) (U. Schweizer et al., 2017). In select plants, i6A can also exist as an unincorporated ribonucleoside excised from a recycled tRNA molecule or synthesized as a ribonucleotide for use as a hormone (Hwang & Sakakibara, 2006; Riefler, Novak, Strnad, & Schmülling, 2006). i6A can be hydroxylated by the bacterial MiaE enzyme and its eukaryotic homologs to yield io6A (U. Schweizer et al., 2017).
FIGURE 5.
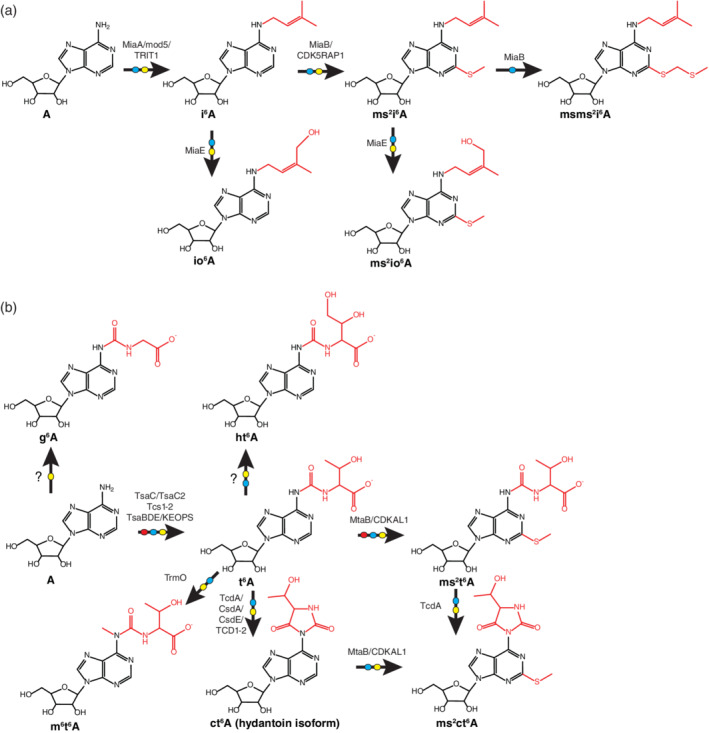
Schemes showing the production of i6A and t6A derivatives of adenosine. (a) Modifications that occur in the production of i6A, io6A, ms2i6A, ms2io6A, and msms2i6A. (b) Modifications that occur in the production of g6A, t6A, ht6A, ms2t6A, m6t6A, ct6A, and ms2ct6A. In both panels, arrows denote enzymatic reactions and enzyme(s) that participate in each reaction are abbreviated if known (see abbreviations list) or denoted by a question mark (?) if unknown. Red moieties represent the modification(s) added to the adenosine molecule. Arrow tails containing red, blue, or yellow ellipses denote reactions that occur in archaea, bacteria, or eukaryotes, respectively
Further modifications of i6A are established by bacterial MiaB and mammalian CDK5RAP1 enzymes, which perform a thiomethylation of i6A to form ms2i6A (U. Schweizer et al., 2017). ms2i6A can be further modified by the miaE enzyme, which hydroxylates the ms2i6A modification to form ms2io6A. While ms2io6A is chemically distinct from ms2i6A, the two appear to be functionally interchangeable in stabilizing weak base pairing between the mRNA codon and the tRNA anticodon (Persson, Esberg, Ólafsson, & Björk, 1994). The sulfur in ms2i6A and ms2io6A comes from cysteine and is delivered by the cysteine desulfurase IscS, itself a hub of seven different pathways in which sulfur can be utilized in the biogenesis of ms2i6A/ms2io6A, s2U, s4U, s2C, thiamin, Moco, and additional Fe‐S proteins (Shigi, 2014). msms2i6A, predominantly found in E. coli tRNASer and tRNATyr, was determined to be synthesized from the MiaB enzyme in a sequential thioacetylation reaction using ms2i6A as a precursor in vitro (Dal Magro et al., 2018). However, it is unknown whether msms2i6A is found in other organisms and its function remains unknown.
At position 37 in the anticodon loop, the modified ribonucleosides i6A, ms2i6A, m1G, inosine, m1A, m1I, m2A, and yW and its derivatives disrupt potential base pairing with a neighboring uridine residue (U33) that is close to A37 in the tertiary structure of several tRNA species (U. Schweizer et al., 2017). These modifications also strengthen anticodon–codon pairings of adjacent tRNA nucleotides to their cognate mRNA targets (U. Schweizer et al., 2017). i6A and ms2i6A have significant roles in proper codon recognition in tRNA molecules, particularly in the stationary phase sigma factor in E. coli (U. Schweizer et al., 2017). Moreover, i6A formation on tRNATrp was shown to optimize trp operon attenuation in bacteria (Eisenberg, Yarus, & Soll, 1979). In bacteria, ms2i6A limits P‐site slippage in the ribosome, while also increasing the synthesis of an iron chelator (Buck & Griffiths, 1982; reviewed in Pollo‐Oliveira & De Crécy‐Lagard, 2019; Urbonavicius, Qian, Durand, Hagervall, & Björk, 2001). On elucidating its role in iron chelation, it was discovered that MiaB is dependent upon an iron–sulfur cluster (Pierrel, Björk, Fontecave, & Atta, 2002). Further, the translation of the major iron homeostasis regulator in E. coli, Fur, is coupled to translation of a leader peptide that is properly decoded by a tRNASer‐UGA which is modified at A37 to contain an ms2i6A mark (Pollo‐Oliveira & De Crécy‐Lagard, 2019; Veĉerek, Moll, & Bläsi, 2007). In S. cerevisiae, a reduction in the efficiency of the tRNATyr(UAA) suppressor was attributed to lack of i6A (Laten, Gorman, & Bock, 1978; U. Schweizer et al., 2017). Further, S. cerevisiae strains that were homozygous for a deletion of mod5 fail to sporulate (Laten et al., 1978). The mod5 protein in S. cerevisiae can also form prions, which were shown to regulate the sterol biosynthesis pathway and ultimately led to an acquired resistance against several azole‐derived antifungal agents (G. Suzuki, Shimazu, & Tanaka, 2012). Prion formation of mod5 also resulted in a decrease of i6A production (G. Suzuki et al., 2012). In Schizosaccharomyces pombe, the mod5 homolog tit1 renders resistance to rapamycin, an inhibitor of mTOR (U. Schweizer et al., 2017).
Within other eukaryotes, mod5 or its homologs can modify cytosolic and mitochondrial tRNAs. Both S. cerevisiae and S. pombe retain a preference for using i6A in mitochondrial tRNAs. However, the preferential modification in mammals shifts to using the ms2i6A modification in mitochondrial tRNAs; ms2i6A may also be absent in cytosolic tRNAs. Finally, in mammals, selenoprotein expression is dependent upon proper formation of i6A and mcm5U in tRNA[Ser]Sec (U. Schweizer et al., 2017; Sierant et al., 2018).
Within plants, i6A functions as a cytokinin, a plant hormone with regulatory roles in plant growth and proper plant development (Riefler et al., 2006). The i6A receptors CRE1/AHK4 and AHK3 are expressed significantly in root tissue and aerial portions of Arabidopsis, respectively (Riefler et al., 2006). CRE1/AHK4 has roles in phosphate starvation responses and sulfate acquisition, in addition to the formation of significant defects in the formation and development of plant vascular tissues (Riefler et al., 2006). Further, these receptors have roles in regulating germination rates, light needed for germination, and sensitivity to far‐red light (Riefler et al., 2006). Within humans, the role of i6A as a cytokinin has been investigated as a potential antitumor treatment for the last 50 years (Dassano et al., 2014). Though the initial investigation into i6A in clinical trials failed, the development of i6A analogs has yielded compounds that inhibit growth of the MCF7 breast cancer cell line and activate the NRF2‐mediated oxidative stress response, which is triggered typically by hydrogen peroxide or TPA (Dassano et al., 2014). What roles i6A or its derivatives may have as an anticancer drug remain unknown but are quite exciting. Further, while IPP is used to generate i6A, it remains unknown if any additional IPP derivatives are used in organisms to modify adenosine molecules. Specifically, as a geranyl moiety can be used in modifying uridine molecules (Sierant et al., 2016), it would be fascinating to see if geranyl pyrophosphate or another lipid molecule can similarly be incorporated into an adenosine molecule.
4.4. The biological roles of t6A and its derivatives or relatives: ct6A, m6t6A, ms2t6A, ms2ct6A, ht6A, ac6A, g6A, hn6A, and ms2hn6A
Nucleoside modification (IUPAC abbreviation): N 6‐threonylcarbamoyladenosine (t6A); cyclic N 6‐threonylcarbamoyladenosine (ct6A); N 6‐methyl‐N 6‐threonylcarbamoyladenosine (m6t6A); 2‐methylthio‐N 6‐threonylcarbamoyladenosine (ms2t6A); cyclic 2‐methylthio‐N 6‐threonylcarbamoyladenosine (ms2ct6A); N 6‐hydroxythreonylcarbamoyladenosine (ht6A); N 6‐acetyladenosine (ac6A); N 6‐glycinylcarbamoyladenosine (g6A); N 6‐hydroxynorvalylcarbamoyladenosine (hn6A); 2‐methylthio‐N 6‐hydroxynorvalylcarbamoyladenosine (ms2hn6A).
Domain assignments: A (t6A, ac6A, hn6A, ms2t6A, and ms2hn6A), B (not g6A, ht6A), E (not ac6A, hn6A, and ms2hn6A).
RNA classes:  .
.
t6A and subsets of its derivatives (Figure 5b) are essential modified bases found in all three domains of life at position 37 of tRNAs (Figure 4, Table 2), comparable with i6A and its derivatives above (Kang et al., 2017). Several tRNAs that read codons starting with a U residue are universally modified to contain t6A or its derivatives, while various tRNAs that read codons that start with an A residue are modified to contain i6A or its derivatives (U. Schweizer et al., 2017). To create t6A, all three domains of life undergo a two‐step process to covalently attach threonine to the N6 position of A37 in tRNA molecules. In bacteria, the TsaC or the TsaC2 protein adds a threonylcarbamoyl group onto an ATP molecule, whereas archaea and eukaryotes use Tcs1 or Tcs2 (reviewed in Thiaville, Iwata‐Reuyl, & de Crécy‐Lagard, 2014). Once the resulting threonylcarbamoyl‐AMP molecule is formed, bacteria use the TsaBDE proteins to transfer the threonylcarbamoyl moiety into A37 to form t6A37, while archaea and eukaryotes use the KEOPS macromolecular complex to form t6A37 (reviewed in Thiaville et al., 2014; Wan et al., 2013). Instead of incorporation with threonine into A37 in tRNAs, a glycine residue can occasionally be placed on the tRNA instead, generating g6A through an unknown enzymatic process (M. P. Schweizer, McGrath, & Baczynskyj, 1970; Wan et al., 2013). t6A can be cyclized into ct6A by the E. coli enzymes TcdA, CsdA, and CsdE or the S. cerevisiae homologs TCD1‐TCD2 (Miyauchi, Kimura, & Suzuki, 2013). t6A can also be further methylated or hydroxylated, forming m6t6A or ht6A via the enzyme TrmO and by an unknown enzyme, respectively (Figure 5b) (Kang et al., 2017; Nagao et al., 2017). In all three domains of life, a thiomethyl group from SAM can also be added onto t6A by the bacterial MtaB enzyme or the eukaryotic enzyme CDKAL1, forming ms2t6A (Arragain et al., 2010). ms2t6A can be further cyclized into ms2ct6A by the enzyme TcdA (Kang et al., 2017). However, ms2ct6A can be generated directly from ct6A by CDKAL1, as the necessary 2‐methylthiolation and cyclization events are independent of each other (Kang et al., 2017). Further, while the cyclic modifications ct6A and ms2ct6A can be artificially synthesized in an oxazolone isoform, the hydantoin isoforms are the biologically relevant forms (Figure 5b) (Kang et al., 2017). ct6A, m6t6A, ms2t6A, ms2ct6A, ht6A, and g6A do not appear to be found in archaeal species (Boccaletto et al., 2018; Kang et al., 2017).
While archaeal tRNAs contain t6A and some related derivatives, ac6A has been hypothesized to be a structural analog of t6A and related molecules within archaeal tRNA (Sauerwald, Sitaramaiah, McCloskey, Söll, & Crain, 2005). ac6A is the most abundant modified t6A‐like derivative in tRNA molecules from Methanopyrus kandleri (Sauerwald et al., 2005). Further, the t6A derivative hn6A and its relative ms2hn6A were also present in the characterization studies of ac6A (Reddy et al., 1992; Sauerwald et al., 2005). As was seen with ac6A, both hn6A and ms2hn6A were found in thermophilic archaeal species (Sauerwald et al., 2005). However, their roles and precise nucleotide positions within archaea tRNA remain unknown.
As with i6A, t6A and its derivatives are essential in decoding and recognizing codons. However, each t6A derivative has unique specificity. t6A and ct6A decode ANN (N represents any nucleotide) codons, m6t6A decodes ACY codons, ms2t6A and ms2ct6A decode AAR codons, and ht6A decodes the AAA codon in sea urchins and reads as asparagine instead of lysine (Kang et al., 2017; Miyauchi et al., 2013; Nagao et al., 2017). t6A is necessary for proper and efficient aminoacylation of tRNAs, efficient translocation, proper scanning of initiation codons, and proper read‐through of stop codons (Kang et al., 2017). While t6A is essential in E. coli and many bacteria, it is not essential to Deinococcus radiodurans, Thermus thermophilus, Synechocystis PCC6803, and Streptococcus mutans (Thiaville et al., 2015). While dispensable in D. radiodurans, t6A‐deficient strains of D. radiodurans were more sensitive to the DNA crosslink inducing drug mitomycin C and had a marked increase in protein expression of stress response genes related to protein homeostasis (Onodera, Satoh, Ohta, & Narumi, 2013; Thiaville et al., 2015). In E. coli and S. mutans, it was determined that t6A was a positive determinant for the PrrC toxins in both organisms (Bacusmo et al., 2017). In S. cerevisiae, t6A37 is not essential, though lack of t6A results in substantial growth defects. These defects include an increase in sensitivity to select stresses (i.e., heat, ethanol, and salt), an increase in sensitivity to TOR pathway inhibitors, and an increase in rates of translation at non‐AUG start codons upstream of the actual translation start site (Thiaville et al., 2016). In D. melanogaster, a homozygous mutation of one component of the KEOPS complex results in larvae that are significantly smaller than wild type and which do not pupate, ultimately dying in larval stages (Rojas‐Benitez, Thiaville, Valérie De Crécy‐Lagard, & Glavic, 2015). In humans, mutations in CDKAL1 revealed a risk association with type 2 diabetes (Kang et al., 2017). Further, mutations in several KEOPS genes result in Galloway–Mowat syndrome, a disease characterized by microcephaly and nephrotic syndrome (Arrondel et al., 2019; Braun et al., 2017; Edvardson et al., 2017). Further, t6A deficiency in human mitochondria due to TsaC knockout results in a MERRF‐like phenotype (H. Lin et al., 2018). What additional roles or modifications may be generated from t6A remain an unclear, though some comparisons of t6A and its derivatives may be made with k2C and C+, as k2C and C+ are composed of cytidine residues that have been attached to a lysine or an arginine, respectively (Phillips & de Crécy‐Lagard, 2011; Soma et al., 2003). This strategy of attaching an amino acid to the nitrogenous base of a ribonucleotide may be a relic from the RNA world (Ikeuchi et al., 2010; Soma et al., 2003) and may be used as a strategy for finding additional unknown modifications throughout biology.
4.5. m6,6A plays an important structural role in rRNA and can be found in tRNAs
Nucleoside modification (IUPAC abbreviation): N 6,N 6‐dimethyladenosine (m6,6A or m6 2A).
Domain assignments: A, B, E.
RNA classes:  ,
,  .
.
In its capacity as a structurally important modification, m6,6A can be found in the final helix (helix 45) at A1518 and A1519 of the small ribosome subunit of E. coli and T. thermophilus. m6,6A1518 and m6,6A1519 are formed by RsmA (previously KsgA) in prokaryotes. In eukaryotes, the corresponding enzyme is Dim1 (Chan et al., 2011; Demirci et al., 2010; Seistrup et al., 2017). RsmA/Dim1 catalyze both dimethylation events at m6,6A1518 and m6,6A1519, which are necessary for assembling the minimal, functional ribosome (Grosjean et al., 2014; Seistrup et al., 2017). Owing to the steric interference of the dimethyl groups and the removal of hydrogen bond donors at m6,6A1518 and m6,6A1519, the normally structured tetraloop of helix 45 is weakened and results in decreased errors in translation and decreased non‐AUG translation initiation (Demirci et al., 2010). However, inactivation of RsmA can occur in some organisms, resulting in impaired organismal growth and the removal of a late‐stage checkpoint in 30S ribosome biosynthesis. In mammalian cells, a buildup of unprocessed small ribosomal subunits that occurs with Dim1 inactivation is lethal to the cells (Seistrup et al., 2017). However, there are a few archaeal organisms that do not have RsmA/Dim1 and have fully functional ribosomes, though RsmA/Dim1 is normally found in archaeal species (Seistrup et al., 2017). While m6,6A modifications were initially found in the ribosome, m6,6A has also been found in tRNA from the bacteria Mycobacterium bovis, though its location within tRNA and its function remain presently unsolved (Chan et al., 2011). Moreover, while the loss of a hydrogen bond donor in m6,6A is exploited in the ribosome for proper function, it remains to be seen if the loss of the hydrogen bond donor has similar effects in other RNAs, such as mRNAs and other ncRNAs.
4.6. The roles of m2A, m8A, and m2,8A in translational fidelity and antibiotic resistance in bacteria
Nucleoside modification (IUPAC abbreviation): 2‐methyladenosine (m2A); 8‐methyladenosine (m8A); 2,8‐dimethyladenosine (m2,8A).
Domain assignments: B.
RNA classes:  (m2A),
(m2A),  .
.
The adenosine modifications m2A and m8A are conferred by RlmN and Cfr, respectively. In some RNAs, m2A and m8A can be further methylated at either position 8 or position 2 by Cfr or RlmN, respectively. Regardless of which singly methylated adenosine precursor is methylated, this additional methylation results in the formation of m2,8A. The methylation order of m2,8A is currently not known to be of biological significance (Yan & Fujimori, 2011). RlmN confers an m2A mark on tRNA species in E. coli at position 37 (Benítez‐Páez, Villarroya, & Armengod, 2012). m2A37 is reported to occur in at least six E. coli tRNAs (tRNAArg ICG, tRNAAsp QUC, tRNAGln cmnm5sUUG, tRNAGln CUG, tRNAGlu mnm5s2UUC, and tRNAHis QUG) (Schwalm, Grove, Booker, & Boal, 2016). Also, m2A, m8A, and m2,8A can be found, separately, at A2503 in the PTC of the 23S rRNA (Yan & Fujimori, 2011). RlmN and Cfr can utilize protein‐free 23S rRNA as a substrate, but the enzymes cannot utilize the fully assembled large ribosomal subunit. This observation suggests that methylation events occur during formation of the ribosome instead of after its full assembly (Yan et al., 2010). The presence of a methyl group on C2 of A2503 improves translational fidelity (Schwalm et al., 2016). The presence of a methyl group on A2503 confers resistance to more than five classes of ribosomal‐targeting antibiotics (Long, Poehlsgaard, Kehrenberg, Schwarz, & Vester, 2006). As A2503 is located at the PTC of the ribosome, m2A may influence contacts between the exit tunnel and the nascent peptide chain and further strengthens the structure of the PTC (Polikanov, Melnikov, Söll, & Steitz, 2015; Toh, Xiong, Bae, & Mankin, 2008). Also, it is not specifically indicated if the dimethylation seen in m2,8A exerts both translational fidelity and antibiotic resistance effects. It is known that antibiotic resistance from C8 methylation is independent of methylation of C2 (Giessing et al., 2009). While there has not been further experimentation that has been published about these methylation marks, the methylases that confer these marks are potentially interesting antibiotic targets of interest that may be of use in future drug discovery.
5. GUANOSINE‐DERIVED MODIFICATIONS
5.1. Alterations of the base‐pairing capabilities of RNAs by m2G and m2,2G may be crucial in proper translation, while m2G can undergo hydroxymethylation to form hm2G
Nucleoside modification (IUPAC abbreviation): N 2‐methylguanosine (m2G); N 2, N 2‐dimethylguanosine (m2,2G or m2 2G); N 2‐hydroxymethylguanosine (hm2G).
Domain assignments: A (not hm2G), B (not hm2G), E.
RNA classes:  (not hm2G),
(not hm2G),  .
.
m2G and m2,2G are two common modifications. m2G marks can be frequently found in archaeal and eukaryotic tRNAs but more rarely found in bacterial tRNAs at positions 10 or 26 (Table 2). However, the role of m2G within tRNA remains elusive (Purushothaman, Bujnicki, Grosjean, & Lapeyre, 2005; Roovers et al., 2012). While the methyl mark on m2G is found on the Watson–Crick face of guanosine, m2G base pairing to C in an RNA duplex occurs with the same energetics as G base pairing to C (Rife, Cheng, Moore, & Strobel, 1998). However, m2G‐U Wobble base pairs are slightly more energetically stabilizing than G‐U Wobble base pairs and do not affect the energetics of GNRA tetraloops (Rife et al., 1998). One methyltransferase complex that is known to form m2G10 marks in eukaryotes is the Trm11p/Trm112p complex (Purushothaman et al., 2005). The methyltransferase Trm‐G10 was characterized in Pyrococcus furiosus to confer m2G marks, while homologs of the trm‐G10 gene were determined to be nearly ubiquitous in archaea and eukaryotes (Armengaud et al., 2004). Homologs of the Trm‐G10 methyltransferase could also confer m2,2G marks, as seen in Pyrococcus abyssi (Gabant et al., 2006). A separate methyltransferase, Trm1, was found to confer m2G marks in Aquifex aeolicus, though Trm1 also conducts a second methylation event to confer m2,2G marks (Awai et al., 2009). In S. cerevisiae and other eukaryotes, the enzyme Trm1p or its homologs writes an m2,2G mark at G26 on numerous tRNAs (Dewe, Fuller, Lentini, Kellner, & Fu, 2017; Hopper, Furukawa, Pham, & Martin, 1982; K. Zhang et al., 2020). m2,2G prevents a potential G26‐A44 base pair in tRNAs that could form with unmodified guanosine (Figure 4, Table 2) (Pallan, Kreutz, Bosio, Micura, & Egli, 2008). The dimethyl modification at G26 permits base pairing with any nucleotide except cytidine, thereby allowing base pairing between the m2,2G‐D pair (D representing every nucleotide other than cytidine, not the modification dihydrouridine). m2,2G also prohibits the misfolding of eukaryotic tRNAs containing this modification (Figure 4) (Pallan et al., 2008).
The E. coli methyltransferases RlmG, RlmL, RsmC, and RsmD and their bacterial homologs are responsible for forming m2G in rRNA, where m2G occurs at five different positions in E. coli rRNA (Pallan et al., 2008; Sergiev, Bogdanov, & Dontsova, 2007). m2G marks in rRNA are hypothesized to be involved in codon–anticodon recognition, formation of the binding pocket for A‐site‐bound tRNA, translocation, overall ribosome structure formation, and formation of the PTC within the large ribosomal subunit (Polikanov et al., 2015; Sergiev et al., 2007). In E. coli, Clostridium acetobutylicum, and T. thermophilus 16S rRNA, the m2,2G966 mark contacts the Wobble base pair and enhances translation initiation (Burakovsky et al., 2012; Emmerechts, Barbé, Herdewijn, Anné, & Rozenski, 2007; Polikanov et al., 2015; Woese, Gutell, Gupta, & Noller, 1983).
Recently, a total RNA extraction from HEK293T cells resulted in the discovery of hm2G (You et al., 2019). While it is unclear which RNAs contain hm2G, which enzymes synthesize hm2G, and what additional organisms may have hm2G, it was discovered that hm2G levels are statistically higher in thyroid carcinoma samples compared to tumor‐adjacent noncancerous thyroid tissue (You et al., 2019). This finding suggests that hm2G may be useful as a cancer biomarker, but also that cancer‐related processes may drive hm2G expression. In humans, RNA Pol III mutations associated with hypomyelinating leukodystrophy result in an increase in the presence of m2,2G in tRNAs, yet also a decrease in overall tRNA transcription that can be mimicked by addition of rapamycin (Arimbasseri et al., 2015). Further, a missense variant of TRMT1, the human homolog of Trm1p, resulted in a deficiency of m2,2G marks in tRNAs and may be linked to intellectual disability, developmental delay, and epilepsy in some patients (K. Zhang et al., 2020). Additional studies in this area of human health and in discovering the functionalities of m2G, m2,2G, and hm2G in other organisms may yield additional functionalities of these modifications and their cognate enzymes.
5.2. The many roles of m7G: from capping mRNAs and presence in mRNAs, to their presence in rRNAs and tRNAs, to forming the precursor molecule for m2,7G and m2,2,7G within spliceosomal snRNAs
Nucleoside modification (IUPAC abbreviation): 7‐methylguanosine (m7G); N 2,7‐dimethylguanosine (m2,7G); N 2,2‐7‐trimethylguanosine (m2,2,7G).
Domain assignments: A, B (m7G), E.
RNA classes:  (m7G),
(m7G),  (m7G),
(m7G),  (m7G),
(m7G),  .
.
5′‐capping of mRNAs is usually accomplished by the addition of an m7G to the 5′‐most nucleotide of a nascent mRNA transcript. The eukaryotic m7G‐containing cap is used in the recruitment of pre‐mRNA splicing and initiation factors to further mature mRNA transcripts. The eukaryotic m7G‐containing cap is added co‐transcriptionally to the 5′ end of newly transcribed mRNA in an unusual inverted 5′‐5′ triphosphate linkage with a GTP molecule that is subsequently methylated by RNMT or its homologs (reviewed in Galloway & Cowling, 2019; Topisirovic, Svitkin, Sonenberg, & Shatkin, 2011). Once the m7G cap is added, the next first nucleotide in the nascent transcript is methylated to contain an Nm mark (Akichika et al., 2019; Boulias et al., 2019; Sendinc et al., 2019). The cap also allows for further maturation of mRNAs by polyadenylation and eventually nuclear export. Once successfully spliced and formed as mRNA, cap‐dependent initiation of protein synthesis of mRNAs occurs. Further, this cap is used to protect mRNAs from 5′‐to‐3′ exoribonuclease activity and degradation of mRNAs, while also looping mRNAs throughout translation (Ramanathan, Robb, & Chan, 2016). While m7G is found in the majority of 5′ caps in eukaryotic mRNAs, caps exist that are formed from chemical groups other than m7G. These include NAD+, NADH, and dpCoA (Bird et al., 2016; Cahová, Winz, Höfer, Nübel, & Jäschke, 2015; Y. G. Chen, Kowtoniuk, Agarwal, Shen, & Liu, 2009; Julius & Yuzenkova, 2017; Kowtoniuk, Shen, Heemstra, Agarwal, & Liu, 2009).
Until 2019, m7G in mRNAs was thought to be found exclusively within the 5′ cap of mRNAs. However, m7G marks have since been identified in the CDS, 3′ UTR, and more rarely in the 5′ UTR of human mRNAs (L.‐S. Zhang, Liu, et al., 2019). Additionally, m7G marks have been found in human ncRNAs (McCown et al., 2019; L.‐S. Zhang, Liu, et al., 2019). At least 90 internal m7G methylation sites have been identified in human mRNA, with high degrees of conservation of the m7G marks found across murine and human cell lines (L.‐S. Zhang, Liu, et al., 2019). While m7G marks occur at several motifs within mRNA (AGm7GA, GGm7GAA, AUCGm7GA, and UUm7GAU), the enzyme or enzymes that confer these marks remain uncharacterized (L.‐S. Zhang, Liu, et al., 2019).
In bacteria and S. cerevisiae, m7G marks in tRNAs are conferred by Trm8p/Trm82p. The m7G mark at m7G46 extends tRNA half‐life (Alexandrov et al., 2006). In archaeal tRNAs, m7G is present but the precise nucleotide location and tRNA identity are unknown (Edmonds et al., 1991; Tomikawa, 2018). Within pre‐rRNA transcripts in eukaryotes, m7G marks are catalyzed by Bud23 (S. cerevisiae) (Létoquart et al., 2014) or WBSCR22 (human) (Zorbas et al., 2015). In humans, m7G marks are required for proper nuclear export of premature 18S ribosome complexes (Sloan et al., 2017), while rRNA is modified in the cytosol in S. cerevisiae (Létoquart et al., 2014).
Further hypermethylation of the guanosine in the 5′ m7G cap can occur, as is the case in eukaryotic spliceosomal snRNAs, some snoRNAs, and telomerase RNA (Monecke, Dickmanns, & Ficner, 2009). Sequential methylation of the m7G cap via Tgs1 gives rise to m2,7G, then m2,2,7G (Monecke et al., 2009). The trimethylated guanosine residue marks the maturation of spliceosomal RNAs, which has further roles in enhancing the nuclear receptor signal transduction cascade, in enhancing transcriptional regulation, and in mitigating spinal muscular atrophy (reviewed in Monecke et al., 2009). While it remains unknown whether these modifications affect the structural stability of mRNAs, m7G and its derivatives have a few roles in human health. Overexpression of RNMT, which stimulates mRNA cap formation, is sufficient to induce tumor formation in human mammary epithelial cells (reviewed in Galloway & Cowling, 2019). Additional discoveries in characterizing writers of m7G may yield further insights into the significance of the m7G marks in mRNAs and in human health.
5.3. Biosynthetic pathways of Q and G+ and their function in tRNAs
Nucleoside modification (IUPAC abbreviation): pre‐queuosine0 (preQ0); pre‐queuosine1 (preQ1); epoxyqueuosine (oQ); queuosine (Q); galactosyl‐queuosine (GalQ); mannosyl‐queuosine (ManQ); glutamyl‐queuosine (GluQ); archaeosine (G+).
Domain assignments: A (preQ0 and G+), B (not G+, ManQ, and GalQ), E (not preQ0, preQ1, oQ, G+, and GluQ).
RNA classes:  .
.
Q and G+ represent two modified ribonucleosides that are derived from a split in a central biosynthetic pathway in archaea and bacteria, following the processing of guanosine into preQ0 via the QueCDE enzyme complex (Figure 6) (Iwata‐Reuyl, 2008; Reader, Metzgar, Schimmel, & de Crécy‐Lagard, 2004). In bacterial species, the nitrile group on the preQ0 molecule is reduced into an amine to form preQ1 by the QueF enzyme (Van Lanen et al., 2005). The resulting preQ1 molecule is incorporated into a tRNA molecule at position 34 by TGT synthase (Iwata‐Reuyl, 2008; Reader et al., 2004). Once incorporated, the preQ1 moiety on tRNA is further modified to contain a cyclopentane ring with two hydroxyl groups and an epoxy ring into oQ34 by the enzyme QueA. The epoxide is removed from oQ34 by the enzyme QueG to form Q34 (Miles, McCarty, Molnar, & Bandarian, 2011; Zallot, Ross, et al., 2017). Separately, Q34 was also determined to be the product of a reaction catalyzed by a nonhomologous enzyme, QueH (Miles et al., 2011; Zallot, Ross, et al., 2017). Once Q34 is formed, Q34 can be conjugated to a mannose (ManQ) or galactose (GalQ) via a Man/Gal‐Q‐transferase in eukaryotes, respectively (Okada & Nishimura, 1977). Q34 can also be further modified to contain a glutamine (GluQ) moiety via the bacterial GluQRS enzyme (Campanacci et al., 2004; Ehrenhofer‐Murray, 2017; Salazar, Ambrogelly, Crain, McCloskey, & Soll, 2004). In some archaeal species, preQ0 is incorporated into a tRNA molecule at G15 and subsequently modified to become G+15 by the enzymatic complex consisting of the ArcS/arcTGT enzyme and the RaSEA enzyme (Mohammad et al., 2017; Phillips et al., 2010; Yokogawa et al., 2019). However, numerous Crenarchaeota species lack ArcS and could instead form G+ with either the GAT‐QueC or QueF‐like protein families (Phillips et al., 2012).
FIGURE 6.
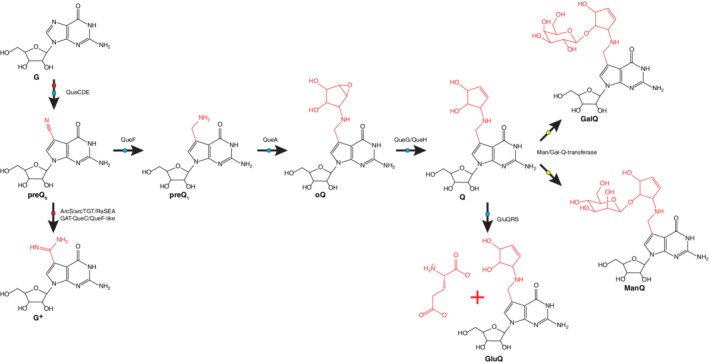
Scheme for queuosine derivatives. Pathway depicts the modifications that occur during the production of queuosine derivatives preQ0, preQ1, oQ, Q, GalQ, ManQ, GluQ, and G+. Red color indicates the modification added to the guanosine molecule. Enzymes that catalyze each reaction are adjacent to arrow. The plus sign by GluQ indicates that glutamate is incorporated onto one of the hydroxyl groups from Q, though it is unknown at present which of the two hydroxyl groups receives the glutamate. Arrow tails containing red, blue, or yellow ellipses denote reactions that occur in archaea, bacteria, or eukaryotes, respectively
While most bacteria and eukaryotes contain Q or its derivatives, Q biosynthesis genes are not found in many bacterial species and are absent in eukaryotes. In bacteria that cannot synthesize Q and in eukaryotes, Q or its corresponding nucleobase queuine are imported or salvaged. One class of bacterial transport proteins, known as COG1738, QueT, or YhhQ, is involved in transporting preQ0 or preQ1 (reviewed in McCown, Liang, Weinberg, & Breaker, 2014; Zallot, Yuan, & De Crécy‐Lagard, 2017). Three additional transport proteins in Clostridioides difficile were also characterized in transporting preQ1, queuine, and Q (Yuan et al., 2019). Owing to the incredible metabolic demand to synthesize Q, at least two salvage pathways exist to salvage or scavenge Q from the gut microbiota. The first bacterial pathway involves homologs of the YhhQ and TGT enzymes that transport or utilize queuine as a substrate (Yuan et al., 2019). The second bacterial pathway utilizes a queuosine hydrolase to generate queuine from queuosine; the cyano group in queuine is then converted into an aminomethyl group, forming preQ1 via a queuine lyase (Yuan et al., 2019). However, it is presently unknown how queuine or other Q‐related molecules are transported into eukaryotes. Regardless of how Q or related molecules enter into or are synthesized by organisms, the presence of Q34 in tRNAs improves their ability to read degenerate codons (Meier, Suter, Grosjean, Keith, & Kubli, 1985). The absence of enzymes that incorporate Q into tRNA or enzymes that synthesize preQ1 have different deleterious phenotypes in a wide array of bacterial and eukaryotic organisms (Durand, Dagberg, Uhlin, & Bjork, 2000; Rakovich et al., 2011). Further, in S. pombe and in other eukaryotes, Q‐containing tRNAAsp further enhances rates of m5C38 formation, while enhancing the translation speed for C‐ending codons and reducing the translation speed for U‐ending codons (Müller et al., 2019; Tuorto et al., 2018). Separately, G+ is used in the D‐loops of archaeal tRNAs to stabilize the overall tRNA structure (Iwata‐Reuyl, 2008; Lorenz et al., 2017).
Several of the Q‐derived modifications have effects on the health or functionality of organisms that use Q. preQ1 and Q biosynthesis genes are required for effective nitrogen‐fixing symbiosis between Sinorhizobium meliloti and the barrel clover plant (Marchetti et al., 2013). Q deficiency halts tyrosine production in germ‐free mice (Rakovich et al., 2011). In several human and murine cancer cell lines, lack of Q modifications in tRNAs has been associated with cell proliferation, anaerobic metabolism, and malignancy (Elliott, Katze, & Trewyn, 1984; Pathak, Jaiswal, & Vinayak, 2008). The presence of Q was shown to directly affect m5C levels in S. pombe tRNAAsp (Ehrenhofer‐Murray, 2017). Further, a queuosine‐deficient diet results in endoplasmic reticulum stress and an unfolded protein response in HeLa cells and in germ‐free mice (Tuorto et al., 2018). Additionally, Q‐containing tRNAs in HeLa cells were recently demonstrated to protect tRNA fragmentation from oxidative stress and angiogenin (Xiaoyun Wang et al., 2018). As tRNA fragments generate functional small RNA molecules similar to miRNAs, this protective mechanism may be helpful to cellular survival or a viable drug target in oncology studies (Xiaoyun Wang et al., 2018). GluQ has been linked to effective stress response in Shigella flexneri (Caballero et al., 2012). While previous experimental work has determined the biosynthetic enzymes involved in Q and G+ biosynthesis, the roles of ManQ and GalQ remain unknown. However, further appreciation of Q as a micronutrient (Fergus, Barnes, Alqasem, & Kelly, 2015) may further elucidate the roles of these modified Qs and other molecules in this pathway.
5.4. m1G has effects in neurodegenerative diseases and glucose metabolism
Nucleoside modification (IUPAC abbreviation): 1‐methylguanosine (m1G).
Domain assignments: A, B, E.
RNA classes:  ,
,  .
.
m1G occurs in several paradigms. m1G acts as a precursor to yW and imG, as discussed below. At G9 in tRNAs from all domains of life, the enzyme Trm10 or homologous enzymes confer the m1G mark (Howell et al., 2019; Jackman, Montange, Malik, & Phizicky, 2003). Separately, m1G can also be found at G37 in tRNAs. While m1G37 occurs in archaeal and eukaryotic tRNAs as a precursor for yW and imG, TrmD can also confer an m1G37 mark in bacteria, though this mark is not further modified (Goto‐Ito, Ito, & Yokoyama, 2017). Despite this research on m1G marks in tRNAs, it is unclear what effects m1G has on tRNA structure. In the E. coli 23S ribosome, an m1G mark was found at G745, which supports proper polysome formation on mRNAs and sensitizes the E. coli ribosome to the ribosome‐binding drug viomycin (Gustafsson & Persson, 1998; Hsuchen & Dubin, 1980; Ishiguro, Arai, & Suzuki, 2019). The enzyme that confers the m1G745 mark was determined to be RlmA (Gustafsson & Persson, 1998). There are biologically relevant phenotypes that are associated with m1G. In S. cerevisiae, deletion strains of Trm10 are hypersensitive to 5‐fluorouracil (Krishnamohan & Jackman, 2017). In humans, two mutations in hTRMT10A were determined to be causative in neurodegenerative defects and in glucose metabolism disorders (Krishnamohan & Jackman, 2017). Further effects of m1G in a biological context may further elucidate previously unknown mechanisms of neuron formation and may be of significance in central metabolism.
5.5. The yW and imG biosynthesis pathways start with m1G biosynthesis
Nucleoside modification (IUPAC abbreviation): 4‐demethylguanosine (imG‐14); wyosine (imG); isowyosine (imG2); methylwyosine (mimG); 7‐aminocarboxypropyl‐demethylwyosine (yW‐86); 7‐aminocarboxypropylwyosine (yW‐72); 7‐aminocarboxypropylwyosine methyl ester (yW‐58); wybutosine (yW); undermodified hydroxywybutosine (OHyW*); methylated undermodified hydroxywybutosine (OHyWy); peroxywybutosine (o2yW); hydroxywybutosine (OHyW).
Domain assignments: A (not yW‐58, yW, OHyW*, OHyWy, o2yW, and OHyW), E (not imG2 and mimG).
RNA classes:  .
.
yW and related analogs are essential in the stabilization of codon–anticodon pairing in tRNAPhe within eukaryotic and archaeal species (Noma, Kirino, Ikeuchi, & Suzuki, 2006). Synthesis of yW and imG represents a complicated biosynthesis pathway within many archaeal and eukaryotic organisms (Figure 7). The biosynthesis pathway begins with the formation of m1G at G37 in tRNAPhe, as catalyzed by the TRM5 protein (Urbonavičius, Meškys, & Grosjean, 2014). The subsequent reactions are catalyzed by TYW1‐4 proteins in eukaryotes and Taw1‐3 proteins in Euryarchaeota (Noma et al., 2006; Urbonavičius et al., 2014). Further, the modifications imG‐14, yW‐86, yW‐72, and yW‐58 are abbreviated based on how many fewer Daltons of mass are observed with these modifications than imG or yW (Noma et al., 2006). For example, imG‐14 has 14 Da less mass than imG.
FIGURE 7.
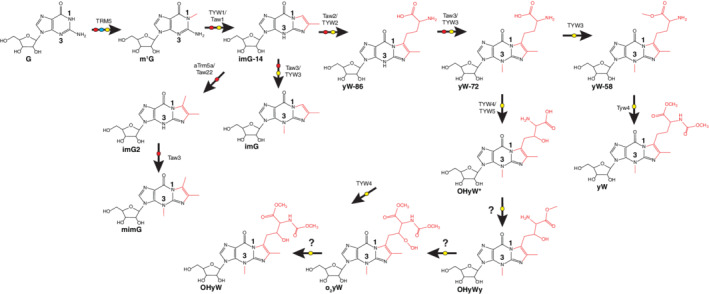
Scheme for wybutosine derivatives. Chemical modifications (red) that occur in the production of wybutosine derived from guanosine (G) include: m1G, imG‐14, imG, imG2, mimG, yW‐86, yW‐72, yW‐58, yW, OHyW*, OHyWy, o2yW, and OHyW. Enzymes that catalyze each reaction are adjacent to arrow, otherwise a question mark (?) indicates enzyme is unknown. Arrow tails containing red, blue, or yellow ellipses denote reactions that occur in archaea, bacteria, or eukaryotes, respectively. The bolded numbers 1 and 3 are added to indicate position N1 and N3, respectively, in guanosine
Using TYW1/Taw1 enzymes, a ring closure occurs on the nucleobase of m1G, forming imG‐14 (Figure 7). From here, there are no fewer than three outcomes of imG‐14, due to the genetic differences in archaeal and eukaryotic species. For eukaryotes (e.g., such as S. cerevisiae) and archaea of the phylum Euryarchaeota, imG‐14 is methylated by TYW3/Taw3 at N3 into imG or attached to an α‐amino‐α‐carboxypropyl group from SAM by TYW2/Taw2 to form yW‐86. yW‐86 can then be methylated at N3 into yW‐72. Euryarchaeota do not appear to conduct any further reactions on yW‐72. All eukaryotes can carboxymethyate yW‐72 to form yW‐58 with the TYW3 enzyme. yW‐58 can then be carboxymethylated again by the enzyme TYW4 to form yW. However, in higher order eukaryotes, yW‐72 can also be hydroxylated into OHyW* by the TYW4 and TYW5 enzymes. OHyW* is then further carboxymethylated into OHyW by TYW4 (M. Kato et al., 2011; Noma et al., 2010). While the synthesis reactions involving OHyWy and o2yW have not been determined, these molecules may be precursors of OHyW. Separately, Crenarchaeota conduct a completely different set of enzymatic reactions on imG‐14. Instead, imG‐14 is methylated at a carbon placed next to position N1 by the enzymes aTrm5a/Taw22 to form imG2. Taw3 can subsequently methylate imG2 at position N3 to form mimG (Urbonavičius et al., 2014). Some of these enzymes or the overall yW biosynthetic process may be of significance to biology, as overexpression of the TYW2 gene was found in breast cancer cell lines. However, yW‐containing modifications were not significantly increased in tRNA modifications (Rodriguez et al., 2012). This possibly means that TYW2 has additional functions in breast cancer that are presently unknown (Rodriguez et al., 2012). Lack of yW modifications in tRNAPhe may cause an activation of the replication of HIV RNA and human T‐cell lymphotrophic virus 1 due to enhancing codon frameshifting in cells (Landgraf, McCarthy, & Booker, 2016; Y. Suzuki, Noma, Suzuki, Ishitani, & Nureki, 2009). There have been instances where a lack of yW modifications has been correlated with rat and mouse tumors and Ehrlich ascites in mice, and represent a point of interest in future oncology research (reviewed in Landgraf et al., 2016). At present, none of the yW‐related modifications themselves are found to be directly culpable in any human diseases, though they may have additional functions that are presently unknown. Moreover, given the complexity of the yW synthesis pathway, it is also possible that additional yW‐derived modifications exist which may reveal yW derivatives in bacteria or may add to further complexity of yW biosynthesis.
6. CYTIDINE‐DERIVED MODIFICATIONS
6.1. The dual lives of m5C, hm5C, and f5C as DNA‐ and RNA‐based modifications
Nucleoside modification (IUPAC abbreviation): 5‐methylcytidine (m5C); 5‐hydroxymethylcytidine (hm5C); 5‐formylcytidine (f5C).
Domain assignments: A (m5C), B (m5C), E.
RNA classes:  (m5C and f5C),
(m5C and f5C),  (m5C),
(m5C),  (m5C and hm5C),
(m5C and hm5C),  (m5C).
(m5C).
While the DNA forms of m5C, hm5C, and f5C have significant roles in epigenetics (Hashimoto, Zhang, Vertino, & Cheng, 2015), the RNA forms of these nucleosides have distinct roles (Cui et al., 2017; Delatte et al., 2016; Nakano et al., 2016; Trixl & Lusser, 2019; R. Wang et al., 2016). m5C can be found in archaeal and eukaryotic tRNAs (Dunn, 1960; Helm, 2006; Motorin & Helm, 2010), rRNAs from all three domains of life (Edelheit, Schwartz, Mumbach, Wurtzel, & Sorek, 2013; Squires & Preiss, 2010), archaeal and eukaryotic mRNAs (D. T. Dubin & Taylor, 1975; Edelheit et al., 2013), human lncRNAs (Amort et al., 2013; McCown et al., 2019), human vault RNAs (Hussain et al., 2013; Squires et al., 2012), and in the HIV‐1 viral genome (Courtney et al., 2019). The TRM4B protein was determined to have m5C RNA methyltransferase activity in S. pombe and Arabidopsis (Cui et al., 2017). NSUN1‐6 and other close homologs have also been attributed to the genesis of m5C in numerous eukaryotes, including humans (Heissenberger et al., 2019; Van Haute, Powell, & Minczuk, 2017).
In tRNAs, it has been proposed that the formation of m5C48 contributes to the L‐shape of tRNAs in vivo (Väre, Eruysal, Narendran, Sarachan, & Agris, 2017). m5C2278 in the 25S rRNA of S. cerevisiae is one modification that serves as an anchor for the ribosomal protein eL41. Once secured by m5C2278, eL41 forms a bridge between the large and small ribosomal subunits (Sharma & Lafontaine, 2015). Loss of m5C2278 in S. cerevisiae results in oxidative stress‐induced refolding of rRNA (Schosserer et al., 2015). Recently, it was discovered that NSUN5 confers an m5C mark on C3782 in human 28S rRNA, while the mouse homolog of NSUN5 confers a related m5C mark on C3438 in mouse 28S rRNA (Heissenberger et al., 2019). It appears that the m5C3782 and m5C3438 marks in human and mice 28S rRNA are analogous to m5C2278 in S. cerevisiae ribosomes. Likewise, m5C3782 in human 28S rRNA is necessary for anchoring the eL41 protein, which then forms a bridge between the large and small ribosomal subunits (Heissenberger et al., 2019). While the precise number of m5C sites in mRNAs is currently under debate, its structural roles are less well‐understood (reviewed in Trixl & Lusser, 2019). In Arabidopsis, m5C is present in mRNAs that are associated with low translational activity during the early stages of plant development, while mutated TRM4B proteins result in defects in root development and a decreased abundance of m5C (Cui et al., 2017). In lncRNAs, m5C in the A‐region of Xist interferes with binding to PRC2 via an unknown mechanism (Amort et al., 2013). m5C in vault RNAs is necessary for the maturation of smaller miRNA‐like RNAs, but it is unclear if m5C confers additional functionalities to vault RNAs (Hussain et al., 2013).
hm5C and f5C are oxidized from m5C in humans by TET or ABH1 enzymes (Van Haute et al., 2017; R. Wang et al., 2016). Though it is unknown which enzymes generate hm5C, this modification is found in archaea and bacteria (Huber et al., 2015). hm5C‐containing mRNAs have increased rates of translation in vitro, while also promoting brain development in D. melanogaster (Delatte et al., 2016). However, the recent discovery of hm5Cm has suggested that the antibodies used in identifying hm5C marks may not be able to distinguish between hm5C or hm5Cm, as the anti‐hm5C antibody was not validated for being incapable of binding to hm5Cm (Huber, van Delft, Tanpure, Miska, & Balasubramanian, 2017). f5C is necessary for deciphering the tRNAMet that aids in translating the AUA and AUG codons in mitochondrial peptide synthesis (Van Haute et al., 2017).
Lack of appropriate methylation of C at target sites and/or oxidation of m5C marks results in mitochondrial‐associated diseases, Leber's hereditary optic neuropathy, myopathy, and hypotonia (reviewed in Van Haute et al., 2017). These diseases occur concordantly with knockdown of either nsun3 or abh1 genes (Haag et al., 2016; Van Haute et al., 2017). A knockout of nsun5 results in a decrease in mouse weight, lean mass, and overall protein synthesis, while a depletion of NSUN5 in cancer cells results in a reduction of cell proliferation and size due to aberrant ribosome formation (Heissenberger et al., 2019). Further, a heterozygous microdeletion within chromosome 7 that includes nsun5 results in Williams–Beuren syndrome, characterized by cardiac defects, lack of weight gain, and mental deficits (Heissenberger et al., 2019). Deletion or mutation of nsun2 in T cells results in a loss of m5C marks on HIV‐1 genomic RNA that consequently inhibits HIV‐1 mRNA translation, alternative splicing of viral RNAs, and viral replication (Courtney et al., 2019). Further research into the roles of m5C in mRNAs, the roles of hm5C and f5C, and the impact of the enzymes that generate these marks may glean further insights into areas of significant impact on human and organismal biology.
6.2. m3C marks are formed by four methyltransferases
Nucleoside modification (IUPAC abbreviation): 3‐methylcytidine (m3C).
Domain assignments: E.
RNA classes:  ,
,  .
.
m3C was first discovered in S. cerevisiae tRNA (Hall, 1963; Iwanami & Brown, 1968; Maden & Salim, 1974). Subsequently, m3C was found to be synthesized by at least four enzymes, with each enzyme having specificity for a particular RNA class (Noma et al., 2011; L. Xu et al., 2017). METTL2 and METTL6 methylate C32 on tRNAThr(UGU), tRNAArg(CCU), and several tRNASer eukaryotic isoacceptors (Noma et al., 2011; L. Xu et al., 2017). METTL6 interacts with seryl‐tRNA synthetase in an RNA‐dependent fashion, which may mean that METTL6 is involved in the methylation of serine tRNA isoacceptors (L. Xu et al., 2017). However, only METTL8 can form m3C marks on mRNA in murine and human cell lines (L. Xu et al., 2017). ALKBH1 was subsequently identified as a demethylase for m3C marks on mRNAs (Ma, Ding, Ye, Yuan, & Feng, 2019). Further, knockouts of mettl2, 6, and 8 did not demonstrate any observable developmental defects in mice (L. Xu et al., 2017). However, it is possible that deficiencies in METTL8 increase ribosome stalling in colon cancer cells (L. Xu et al., 2017). METTL2 and METTL6 account for only 20–40% of m3C in tRNAs from HeLa and HCT116 cells and mouse liver extracts, indicating that there are likely more than two enzymes that methylate cytidine residues in tRNA (L. Xu et al., 2017). As METTL8 accounts for ~100% of m3C marks in mRNAs from HeLa and HCT116 cells, it is very likely that METTL8 may be the major or the only methyltransferase that confers m3C marks in mRNAs (L. Xu et al., 2017). Additionally, m3C levels in mRNAs were lower in hepatocellular carcinoma biopsied tissues compared to noncancerous tissues, indicating m3C is a potential liver cancer diagnostic marker (Ma et al., 2019). Moreover, the same study noted that m3C levels in cancerous and noncancerous tissues were significantly higher than m3C levels in cultured cancerous and noncancerous human cell lines that they had examined (Ma et al., 2019), which may indicate biological roles involving m3C may be missed in only examining human cell lines.
Above, A‐to‐I editing was discussed as one type of RNA editing. Another type of RNA editing is C‐to‐U editing, which can use m3C as an intermediate modification. In Trypanosoma brucei, the enzyme TRM140 methylates C32 into m3C in tRNAThr(AGU) as part of a pathway to convert C32 to U32 (Rubio et al., 2017). Co‐expression of TRM140 and ADAT2/3 prevents wholesale deamination of the trypanosomal genome, while also providing a key insight into the functionality of AID in antibody class diversification (Rubio et al., 2017). While C‐to‐U editing has been observed in several eukaryotes and archaea, it is unclear whether the trypanosomal process involving m3C is present in these organisms (Rubio et al., 2017). While the structural roles of m3C in RNA remain unstudied, additional studies in how m3C levels can be used as a cancer diagnostic and whether m3C is involved in C‐to‐U editing in other organisms remains two areas of research that may elucidate more functions and unknown biology involved with m3C.
6.3. s2C allows for anticodon flexibility and enhanced codon binding in bacterial and archaeal tRNAs and can be methylated to form ms2C
Nucleoside modification (IUPAC abbreviation): 2‐thiocytidine (s2C); 2‐methylthiocytidine (ms2C).
Domain assignments: A (not ms2C), B.
RNA classes:  .
.
s2C is present in archaeal and bacterial tRNAs, specifically tRNASer(GCU) and three tRNAArg molecules (Jager, Leipuviene, Pollard, Qian, & Bjork, 2004; Shigi, 2014). The sulfur for s2C is derived from cysteine via the IscS enzyme, which serves as a central sulfur hub. IscS then moves the cysteine‐derived sulfur to the IscU enzyme and then the TtcA enzyme. The thiolated TtcA incorporates sulfur into position C32 on tRNA, generating s2C32 (Shigi, 2014). While there were no observed growth deficits found in mutagenized strains of E. coli or Salmonella enterica that lacked TtcA, s2C modifications were determined to have a role in increasing the selection rate by ~30% of the ternary complex of aminoacylated‐tRNA/EF‐Tu/GTP within the A‐site of the ribosome (Jager et al., 2004). Further, s2C in tRNA interferes with codon recognition in the A‐site of the ribosome and decreases the translation rate of some mRNAs (Jager et al., 2004). Lack of s2C corresponds to an increase in frameshifting rates during translation (Jager et al., 2004). Additional studies have determined that s2C modifications allow for flexibility in the anticodon stem and loop structures, which is necessary for select tRNAs to properly decode mRNAs (Cantara et al., 2012).
ms2C was recently discovered in E. coli and P. aeruginosa tRNAs that had been previously determined to have s2C marks (Reichle, Petrov, Weber, Jung, & Kellner, 2019). While it does not appear that there are any known methyltransferases that confer the ms2C mark, it appears that the modification is the result of methylating agents or damage that occurs on tRNAs (Reichle et al., 2019). At present, it does not appear that all s2C marks have the capability of being methylated. Moreover, AlkB seems to be at least one enzyme that can revert the ms2C mark back to s2C (Reichle et al., 2019). However, there is a separate AlkB‐independent process that removes the 2‐methylthio group and rethiolates C to form s2C, though it is unclear how this process occurs (Reichle et al., 2019). ms2C marks may reduce the structural flexibility in tRNAs that would otherwise have the s2C mark and the presence of ms2C marks in tRNA reduces translational efficiency (Reichle et al., 2019). With the presence of ms2C and the extensive modifications featuring s2U below, it is curious to see if a similar myriad of modifications can also be found with thiolated cytidine nucleotides.
6.4. m4C, m4Cm, ac4C, and ho5C are within rRNA and m4,4C is present in viral‐infected human cells
Nucleoside modification (IUPAC abbreviation): N 4‐methylcytidine (m4C); 4,2′‐O‐dimethylcytidine (m4Cm); N 4,N 4,2′‐O‐trimethylcytidine (m4,4Cm); N 4‐acetylcytidine (ac4C); 5‐hydroxycytidine (ho5C); N 4,N 4‐dimethylcytidine (m4,4C).
Domain assignments: A (m4,4Cm), B (m4C, m4Cm, and ho5C), E (ac4C, m4,4C, m4,4Cm).
RNA classes:  (ac4C, m4,4Cm),
(ac4C, m4,4Cm),  (not m4,4Cm),
(not m4,4Cm),  (ac4C).
(ac4C).
Extensive research on the DNA form of N 4‐methylcytidine (m4C) has shown its involvement in the epigenetic regulation of genes (W. Chen, Yang, Feng, Ding, & Lin, 2017). However, current knowledge of the RNA form of m4C appears to be limited to its presence within bacterial rRNA and its writer, the RsmH enzyme (Kimura & Suzuki, 2010). The initial discovery of m4C was considered a possible side effect of treating RNA samples with acid, with m4Cm possibly being the native modified ribonucleoside (Limbach, Crain, & McCloskey, 1994). However, subsequent analysis indicated that the RsmH protein is a methyltransferase that methylates the N4 position of C1402 in the E. coli 16S rRNA. The enzyme RsmI encodes for a methyltransferase that confers the 2′‐O‐methyl mark to make m4Cm at C1402 (Kimura & Suzuki, 2010). Subsequently, both the m4C1402 and m4Cm1402 modifications were determined to prevent non‐AUG initiation in E. coli by decreasing read‐through effects of the UGA stop codon (Jiang, Seo, & Chow, 2016; Kimura & Suzuki, 2010). m4Cm1402 in the 30S small ribosomal subunit stabilizes helix 44 and may assist in interactions between the ribosome, P‐site tRNA docking, and mRNA interactions (Jiang et al., 2016). Determining how widespread m4C and m4Cm marks are in bacteria may be helpful in determining if the RsmH or RsmI proteins can be a viable option for antibiotic targeting. Separately, m4,4Cm has been identified in the tRNAs of several archaeal species (Noon et al., 2003).
Previously, ac4C exists within several tRNAs and the 18S rRNA of eukaryotes and both marks are formed by the enzyme Tan1 (Johansson & Byström, 2004; Parthasarathy, Ginell, De, & Chheda, 1978). Deletion of tan1 in S. cerevisiae results in a decrease in A‐site occupancy of the ribosome (Chou, Donnard, Gustafsson, Garber, & Rando, 2017). However, P‐site occupancy rates increase (Chou et al., 2017). It was hypothesized that ac4C could have roles in positioning peptidyl‐tRNA molecules within the ribosome, enhancing the translocation of codons of mRNA from the P‐site to the E‐site of the ribosome, or in delaying the progression of mRNA when translocating from the A‐site to the P‐site (Chou et al., 2017). This hypothesis was confirmed in an RNA‐seq‐based analysis of the transcriptome‐wide presence of ac4C marks in HeLa cells (Arango et al., 2018). The enzyme NAT10 was determined to catalyze the formation of ac4C in a variety of mRNAs, where the presence of these marks in mRNAs was shown to promote translation and mRNA stability (Arango et al., 2018). It remains to be seen if the ac4C mRNA modifications exist in other eukaryotes or if this modification is seen in bacteria or archaea. Also, it is possible that NAT10 may present a viable anticancer therapeutic target and could be pursued in further research.
Like m4C, ho5C has a DNA counterpart and is studied in the context of C‐to‐T transition mutations in DNA (Zahn, Averill, Wallace, & Doublié, 2011). The enzyme that synthesizes the RNA form of ho5C is RlhA and AroD, which generates ho5C from chorismate and iron–sulfur clusters (Kimura, Sakai, Ishiguro, & Suzuki, 2017; Popova & Williamson, 2014). The role of ho5C in biology was obscured until recently. ho5C was identified at position 2501 in the 23S rRNA of the E. coli ribosome and at an equivalent position within the 23S ribosome within Deinococcus radiodurans (Havelund et al., 2011). As ho5C2501 localizes to the PTC and forms a base triple that interacts with P‐site tRNA, it is quite likely that the structural function of ho5C2501 plays significant roles in translation (Kimura et al., 2017). As methods to extract ho5C from nascent RNA would often obscure the modification, the presence of this modification in other RNA classes should be re‐examined (Havelund et al., 2011). The function of ho5C in the ribosome appears to have some connection with environmental iron, as the ho5C levels were found to be directly proportional to the amount of environmental iron (Kimura et al., 2017). Given the sensitivity of ho5C to purification methods, careful re‐examination of RNAs in other species could be conducted to determine if this modification is seen in other domains of life.
Though presently unknown how the m4,4C modification is generated, it is detected in the human liver cell line Huh7 after exposure to one of the following RNA viruses: Zika virus, Dengue virus, Hepatitis C virus, or poliovirus (McIntyre et al., 2018). It is unclear whether this modification is due to host methyltransferase(s) or if the viral genomes themselves have a factor that could methylate m4C or demethylate the 2′‐O‐methyl group in cellular m4,4Cm. Of note, arsenite exposure to Huh7 cells caused the appearance of m4,4C in total RNA samples, suggesting m4,4C formation could occur via a general stress response mechanism in humans (McIntyre et al., 2018). The enzymes RsmH, METTL15, and the DEAD‐box RNA helicase DDX6 may also have roles in the formation of m4,4C, based on homology searches and siRNA depletion of the DDX6 mRNA having a deleterious effect on m4,4C levels (McIntyre et al., 2018). Further analysis may reveal additional properties of m4,4C that have gone previously undetected in human cells.
6.5. k2C and C+ are modified cytidines that use amino acid residues to switch codon recognition in tRNAIle
Nucleoside modification (IUPAC abbreviation): lysidine (k2C); agmatidine (C+).
Domain assignments: A (C+), B (k2C).
RNA classes:  .
.
Codons in mRNA for isoleucine represent a challenge for proper decoding due to the Wobble effect. For codons whose sequence is AUN, three of the possible codons (AUU, AUC, and AUA) encode for isoleucine incorporation into nascent peptide chains while the remaining codon (AUG) encodes for methionine incorporation into nascent peptides (Agris, Vendeix, & Graham, 2007; Crick, 1966; Köhrer et al., 2014). If a tRNA has a guanosine residue in the first position of the anticodon, this guanosine residue can read codons that end in uridine or cytidine (AUU and AUC for isoleucine). However, if the first anticodon residue is uridine, this residue can read codons ending in adenosine or guanosine (AUA for isoleucine and AUG for methionine). Eukaryotes solve this problem of amino acid misincorporation by using either IAU (inosine‐adenosine‐uridine) or ΨAΨ (pseudouridine‐adenosine‐pseudouridine) anticodon sequences in tRNAs (Crick, 1966; Senger, Auxilien, Englisch, Cramer, & Fasiolo, 1997).
However, prokaryotes employ a different approach to overcoming amino acid misincorporation. Almost all bacteria utilize k2C in place of inosine for the first anticodon sequence (k2CAU instead of IAU) (Harada & Nishimura, 1974; Nakanishi et al., 2005; Senger et al., 1997; Soma et al., 2003). However, archaea make use of C+ in this location (C+AU instead of IAU) (Ikeuchi et al., 2010; Mandal et al., 2010). In the case of k2C, a lysine residue is added onto the C34 residues of tRNAIle 2 at what would be the C2‐oxo position by the TilS enzyme. In the case of C+, a decarboxylated arginine residue is placed at the same position on a cytidine residue by the TiaS enzyme (Ikeuchi et al., 2010; Köhrer et al., 2014; Mandal et al., 2010). While TilS and TiaS are essential for their host organisms, there are exceptions. These organisms do not have tRNA molecules with a CAU sequence in their anticodon loops; instead, they have UAU sequences (Köhrer et al., 2014). However, a strain of Bacillus subtilis was mutated to remove its copy of the tilS gene. The resulting viable strain of B. subtilis had a low level of misreading AUG codons and possessed a strong binding preference in tRNAIle for AUA codons (Köhrer et al., 2014). Additional roles that k2C and C+ may have in biology remain unclear. However, it has been speculated that k2C, C+, and other RNA–amino acid conjugates may be remnants from the RNA world (Soma et al., 2003) and may have additional aspects of their biology and chemistry that remains unknown. Further, different amino acids may also be conjugated to nucleotides in ways similar to k2C and C+ that are currently unknown.
7. URIDINE‐DERIVED MODIFICATIONS
7.1. cnm5U modifications in tRNAIle avoid mistranslation at the Wobble position
Nucleoside modification (IUPAC abbreviation): 5‐cyanomethyluridine (cnm5U).
Domain assignments: A.
RNA classes:  .
.
As discussed in the previous section, many prokaryotes can use a modified cytidine residue in the anticodon loop of tRNAIle to appropriately translate AUU, AUA, and AUC codons as isoleucine, while AUG codons are translated as methionine (Mandal et al., 2014). However, mistranslation occurred when the first nucleotide in the anticodon loop of tRNAIle was mutated to a uridine residue (i.e., CAU to UAU) (Mandal et al., 2014). Surprisingly, this mutation allowed for retention of wild‐type binding of tRNAIle to AUA codons to be preserved. However, the mutant tRNAIle binds to AUU (isoleucine) and AUG (methionine) codons, which poses a problem in translation. Eventually, this mutagenized uridine in UAU was determined to be modified into cnm5U through unknown mechanisms (Mandal et al., 2014). While this modification appears to be in haloarchaea, in the euryarchaeote Methanococcus maripaludis, and in the euryarchaeote Methanocaldococcus jannaschii, it is absent in other archaea, E. coli, and S. cerevisiae (Mandal et al., 2014; N. Yu, Jora, et al., 2019). How widely distributed this modification is in archaea, which enzyme or enzymes are involved in its synthesis, and its broader roles in biology remain currently unknown.
7.2. m5U/rT marks are essential for proper tRNA formation
Nucleoside modification: 5‐methyluridine (m5U) or ribothymidine (rT).
Domain assignments: A, B, E.
RNA classes:  ,
,  ,
,  ,
,  .
.
m5U, also referred to as rT, is a common modification in the tRNAs of bacteria and eukaryotes, but limited to the order Thermococcales within Euryarchaeota and the phylum Nanoarchaeota in archaea (Auxilien et al., 2011). In E. coli tRNAs, TrmA catalyzes the formation of m5U. A mammalian homolog of TrmA is TRMT2A (Chang et al., 2019). m5U is normally found in the TΨC‐loop of tRNAs at position 54 as part of the G‐m5U‐Ψ‐C motif, where m5U54 aids in the stabilization of tRNA tertiary structure (Figure 4, Table 2) (Auxilien et al., 2011; Björk & Neidhardt, 1975; Hur, Stroud, & Finer‐Moore, 2006; Urbonavicius, Skouloubris, Myllykallio, & Grosjean, 2005). One enzyme that catalyzes the formation of m5U54 in tRNA is TrmFO, while another is TrmA (Lartigue et al., 2014; Urbonavicius et al., 2005). Further, a TrmFO homolog in Mycoplasma capricolum subsp. capricolum, renamed to be RlmFO, was also discovered to confer m5U1939 marks in the large ribosomal subunit (Lartigue et al., 2014). However, there are other enzymes that form m5U marks in rRNA. In the large ribosomal subunit in E. coli, RlmC catalyzes m5U747 while RlmD catalyzes m5U1939 (Auxilien et al., 2011; Madsen, Mengel‐Jørgensen, Kirpekar, & Douthwaite, 2003). These m5U methyltransferases are fairly universal in bacteria and eukaryotes, but are unique to Thermococcales and Nanoarchaeota. It is possible that the common ancestor of the two archaeal groups may have received an rlmD‐like gene of bacterial origin via horizontal gene transfer (Auxilien et al., 2011). m5U can also be found on the mitochondrial small rRNA in hamster and human at m5U429 and the 23S rRNA large subunit in E. coli at m5U747 and m5U1939 (Baer & Dubin, 1981; D. Dubin, 1974; Madsen et al., 2003; Rebelo‐Guiomar, Powell, Van Haute, & Minczuk, 2019). TrmA catalyzes the formation of m5U marks on E. coli ribosomes (Ranaei‐Siadat et al., 2013). However, the roles of m5U in structural features of rRNA remain unclear. Also, two recent high‐throughput sequencing projects examined m5U in RNAs from human cells (Carter et al., 2019; Cheng et al., 2020). While the vast majority of m5U marks were detected in tRNAs, several mRNAs and lncRNAs also contained m5U marks (Carter et al., 2019; Cheng et al., 2020). Importantly, several RNAs were bound to TRMT2A, but did not contain methylated uridines, suggesting that TRMT2A has additional biological roles that remain currently unknown (Carter et al., 2019). Moreover, in human cells, the methyl group donor for m5U in mRNAs is SAM (Cheng et al., 2020). In tmRNA, an m5U mark is positioned within the tRNA‐like domain at the location that would correspond to the TΨC‐loop in tRNA (Ranaei‐Siadat et al., 2013).
The protein TRMT2A has also been studied for its effects in cancer. Ectopic expression of TRMT2A has been shown to have an inhibitory effect on cell growth and TRMT2A was subsequently determined to be a cell cycle regulator (Chang et al., 2019). While TRMT2A is found in nuclei of HeLa cells (Chang et al., 2019), the cytosolic localization of TRMT2A in HER2+ breast cancer patients represents a high degree of likelihood that a recurrence of breast cancer would occur (Hicks et al., 2010). Further research into the functionality of m5U and whether its overabundance can be useful in cancer diagnostics remains an intriguing avenue of research.
7.3. m1Ψ marks are essential for proper tRNA formation in archaea and can be found in rRNA
Nucleoside modification (IUPAC abbreviation): N 1‐methylpseudouridine (m1Ψ).
Domain assignments: A, E.
RNA classes:  ,
,  .
.
m1Ψ and m5U modifications have been found in multiple archaeal tRNAs in the TΨC‐loop at position 54 (Gupta, 1984; Pang et al., 1982). m1Ψ54 is formed in two steps. First, Ψ54 is generated by Pus10 (Gurha & Gupta, 2008). Subsequently, Ψ54 is methylated by TermY (Chatterjee et al., 2012). Notably, the residue identity at position 55 affects the methylation of Ψ54. The formation of Ψ55 by Pus10 enables the generation of m1Ψ54, while a cytidine at nucleotide 55 decreases methylation efficiency (Chatterjee et al., 2012; Gurha & Gupta, 2008). However, a purine at nucleotide 55 abrogates the formation of m1Ψ54 from Ψ54 (Chatterjee et al., 2012). m1Ψ54/m5U54 and the adjacent Ψ55 in tRNAs likely have roles in the formation of a stable tertiary structure of host tRNAs. Ψ55 strengthens a tertiary interaction with the conserved G18 and facilitates intra‐loop stacking with a conserved purine at position 57 and with m1Ψ54/m5U54, which pairs via a reverse‐Hoogsteen interaction with the conserved A58 (Chatterjee et al., 2012; Romby et al., 1987).
m1Ψ forms via a two‐step mechanism in archaeal (16S) and eukaryotic (18S) rRNAs (Brand, Klootwijk, Planta, & Maden, 1978; B. Meyer et al., 2016; Wurm et al., 2010). In the first step, an snR35 H/ACA snoRNP guides the conversion of U54 into Ψ54. Next, the eukaryotic enzyme Nep1/Emg1 and archaeal homologs of Nep1 methylate Ψ54 to form m1Ψ54 (B. Meyer et al., 2016; Wurm et al., 2010). In eukaryotes, m1Ψ is also a precursor of m1acp3Ψ (Brand et al., 1978). For example, methylation of Ψ1191 takes place shortly after transcription, whereas the installation of the N 3‐acp group via the enzyme Tsr3 possibly occurs before the final cleavage of pre‐rRNA into mature 18S rRNA in the cytoplasm (Brand et al., 1978; B. Meyer et al., 2016). Of note, a mutation in the nep1 gene causes the developmental disorder called Bowen–Conradi syndrome (Armistead et al., 2009; Bowen & Conradi, 1976; B. Meyer et al., 2011). With a homozygous mutation of nep1 in mice, reduced cell proliferation in brain and other tissues was seen in addition to severe defects in overall growth and in neural tube formation (Armistead et al., 2015). While Bowen–Conradi syndrome is characterized as a ribosomopathy (Armistead et al., 2015), it is unclear how m1Ψ marks play roles in the structure of ribosomes or in this syndrome and in other ribosomopathies. Bowen–Conradi syndrome or m1Ψ could provide further insights into ribosomopathies in general or perhaps also in additional cellular processes with further research.
7.4. acp3U and related marks acp3D, acp3Ψ, and m1acp3Ψ are found in either tRNA or rRNA
Nucleoside modification (IUPAC abbreviation): 3(3‐amino‐3‐carboxypropyl) uridine (acp3U); 3(3‐amino‐3‐carboxypropyl)‐5,6‐dihydrouridine (acp3D); 3(3‐amino‐3‐carboxypropyl) pseudouridine (acp3Ψ); 1‐methyl‐3(3‐amino‐3‐carboxypropyl) pseudouridine (m1acp3Ψ).
Domain assignments: A (acp3U), B (acp3U), E.
RNA classes:  (acp3U, acp3D),
(acp3U, acp3D),  (acp3U, acp3Ψ, m1acp3Ψ).
(acp3U, acp3Ψ, m1acp3Ψ).
acp3U was first detected in E. coli tRNAPhe and was found to be in a signature sequence (D[dihydrouridine]‐acp3U‐A) in the D‐loop of prokaryotic and eukaryotic tRNAs (Nishimura, Taya, Kuchino, & Oashi, 1974; Stuart et al., 1996). The enzyme in E. coli that synthesizes acp3U on tRNA was recently determined to be YfiP, which was renamed as TuaA or TapT (B. Meyer et al., 2020; Takakura, Ishiguro, Akichika, Miyauchi, & Suzuki, 2019). The human homologs of TapT, DTWD1 and DTWD2, confer acp3U marks at U20 in human tRNA (Takakura et al., 2019). Moreover, the effects of acp3U were determined to promote regional conformational shifting in the D‐loop (acp3U20) and the variable loop (acp3U47) of tRNAs containing acp3U (Stuart et al., 1996). The presence of this modification in the tRNAs of several additional eukaryotic species, including D. melanogaster, wheat germ, lupine seed, rat, cow (B. Meyer et al., 2020; Takakura et al., 2019), and human, suggests that this modification is conserved in eukaryotes (Chheda et al., 1988). Outside of eukaryotes, there has been an acp3U modification observed in the rRNA at U966 of the 16S rRNA of Haloferax volcanii and it is conferred by the archaeal homolog of the SAM‐dependent enzyme Tsr3 (Kowalak etal., 2000; B. Meyer et al., 2020). The archaeal homologs of Tsr3 were found in the archaeal species Vulcanisaeta distributa and Sulfolobus solfataricus, suggesting that the acp3U mark may be widely distributed in archaea (B. Meyer et al., 2020). Of interest to human health, a decreased abundance of acp3U was noted in cancer patient urine, similar to the decreased abundance of Q derivatives in cancer cell lines (Chheda et al., 1988; Elliott et al., 1984; Pathak et al., 2008). This decrease of acp3U in urine may be used as a biomarker for cancer diagnostics. Further, a double knockout of DTWD1/2 in HeLa cells exhibited a severe growth phenotype that did not reveal a loss of tRNA stability, suggesting that DTWD1 and DTWD2 have redundant roles in normal cellular growth in human cells (Takakura et al., 2019).
acp3D has been found only in T. brucei tRNALys(UUU), although the synthesis, function, and distribution of acp3D remain to be elucidated (Krog et al., 2011). Separately, a chemically related molecule, m1acp3Ψ, was discovered. At U1191 on helix 31 of eukaryotic 18S rRNA, the parent uridine molecule is converted into Ψ by the snRNA35 H/ACA snoRNP (Samarsky, Balakin, & Fournier, 1995; Sloan et al., 2017). Next, Nep1/Emg1 methylates this pseudouridine into m1Ψ (B. Meyer et al., 2011, 2016). Finally, Tsr3 confers the acp group from SAM onto m1Ψ, giving rise to m1acp3Ψ (B. Meyer et al., 2016). If Nep1 is missing or deficient, the Ψ can still have an acp group attached, yielding acp3Ψ (B. Meyer et al., 2011). Neither acp3Ψ nor m1acp3Ψ has been detected in tRNA. Given the conservation of Tsr3 in archaea and throughout eukaryotes, it is quite likely that archaea also have some form of acp3Ψ or m1acp3Ψ, though this proposed distribution of acp3Ψ or m1acp3Ψ remains speculative. Several phenotypes emerged with knockouts of tsr3 in S. cerevisiae and knockdowns of tsr3 in HCT116 human cells. In S. cerevisiae, the Δtsr3 mutant resulted in hypersensitivity to the protein synthesis inhibitor paromomycin (B. Meyer et al., 2016). The depletion of Tsr3 in human cells caused a buildup of 20S pre‐rRNA, suggesting that Tsr3 is crucial in 18S rRNA processing (B. Meyer et al., 2016). Further, as mentioned above, specific tsr3 mutations result in the formation of Bowen–Conradi syndrome (Wurm et al., 2010). However, what additional functions acp‐derived nucleotides may have in overall biology remain unclear.
7.5. m3U and m3Ψ marks may affect rRNA and tRNA functionality
Nucleoside modification (IUPAC abbreviation): 3‐methyluridine (m3U); 3‐methylpseudouridine (m3Ψ).
Domain assignments: A (m3U), B, E (m3U).
RNA classes:  (m3U),
(m3U),  .
.
While m3U and m3Ψ are isomers of each other, their functionalities in RNAs are strikingly different. In T. brucei tRNAThr, m3C is deaminated into m3U by Trm140 and ADAT2/3 during C‐to‐U editing (Rubio et al., 2017). The association of Trm140 to ADAT2/3 prevents massive genome‐ and transcriptome‐wide deamination by the ADAT2/3 enzyme (Rubio et al., 2017). Both m3U and m3Ψ have been identified in the rRNA of bacteria. However, only m3U has been found in archaeal and eukaryotic rRNAs. First discovered in S. cerevisiae cells (Hall, 1963), m3U modifications have since been found in 25S rRNA of S. cerevisiae, 23S rRNA of S. solfataricus, and 16S rRNA of E. coli, among others (J. D. Johnson & Horowitz, 1971; Noon et al., 1998). Though originally found in 18S rRNA of HeLa cells, later studies did not find evidence for the existence of m3U there (Iwanami & Brown, 1968; Klagsbrun, 1973). Several enzymes that catalyze this m3U modification in rRNAs have been identified. In S. cerevisiae, U2634 and U2843 of 28S rRNA are methylated by the SAM‐dependent methyltransferases BMT5 and BMT6, respectively (Sharma et al., 2014). In E. coli, the only known m3U site (U1498 in 16S rRNA) is methylated by the site‐specific SAM‐dependent methyltransferase RsmE (Basturea, Rudd, & Deutscher, 2006). Interestingly, the human obesity‐linked FTO protein is capable of demethylating m3U sites in vitro (Jia et al., 2008).
The sole m3Ψ mark discovered in E. coli occurs at the highly conserved Ψ1915 position in hairpin 69 of the 23S rRNA (Kowalak et al., 1996; Ofengand, Del Campo, et al., 2001; Ofengand, Malhotra, et al., 2001). To make the m3Ψ mark, the uridine is first pseudouridylated by the RluD enzyme before being methylated by the SAM‐dependent methyltransferase RlmH, also known as YbeA (Ero, Peil, Liiv, & Remme, 2008; Purta, Kaminska, Kasprzak, Bujnicki, & Douthwaite, 2008; Raychaudhuri, Conrad, Hall, & Ofengand, 1998). This methylation of Ψ1915 stabilizes hairpin 69 relative to the pseudouridine alone (Chui, Desaulniers, Scaringe, & Chow, 2002). The m3Ψ1915 mark in 23S rRNA is conserved in other bacteria, including the 23S analogs in D. radiodurans, B. subtilis, and the chloroplast of Zea mays (Del Campo et al., 2005). Pseudouridylation, and possibly methylation, of this site may be essential for proper E. coli growth and development since knockouts of rluD showed growth rates reduced by ~50% when compared with wild‐type E. coli. However, knockouts of rlmH also showed slightly slower growth rates when compared with wild‐type E. coli (Purta et al., 2008; Raychaudhuri et al., 1998). m3Ψ1915 may have a further important role in large ribosomal subunit structure and function. The loop of hairpin 69, which contains m3Ψ1915 in E. coli, interacts with anticodon arm of A‐site tRNA (Yusupov et al., 2001). Additionally, m3Ψ1915 has been shown to directly contact residues of the RRF protein, which disassembles the post‐translational termination complex of mRNA, tRNA, and rRNA (Agrawal et al., 2004). Additional biological roles of m3U and m3Ψ remain unclear, though further discovery of these marks in previously unknown RNAs may elucidate novel functions. Further, the role of m3U in C‐to‐U editing may also be more prevalent in eukaryotic organisms or in bacteria and archaea, which may elicit unexpected functions of m3U.
7.6. ho5U and its derivatives mo5U, cmo5U, and mcmo5U affect base‐pairing specificity in tRNAs that contain these modifications
Nucleoside modification (IUPAC abbreviation): 5‐hydroxyuridine (ho5U); 5‐methoxyuridine (mo5U); uridine 5‐oxyacetic acid (cmo5U); uridine 5‐oxyacetic acid methyl ester (mcmo5U).
Domain assignments: B, E (ho5U).
RNA classes:  .
.
Under nonenzymatic processes, ho5U is commonly found to spontaneously occur due to oxidative damage to cytosines in DNA or RNA. However, ho5U can be enzymatically synthesized at U34 of tRNAs via the TrhOP enzyme complex (Sakai, Kimura, & Suzuki, 2019). Homologs of TrhO were found in bacterial and eukaryotic organisms, while TrhP was exclusive to bacterial species (Sakai et al., 2019). Regardless of origin, ho5U remains capable of base pairing with guanosine by adopting a normal Watson–Crick base pair arrangement instead of a Wobble base pair arrangement (Sakai et al., 2019). Knocking out the TrhOP enzymes introduces a temperature‐sensitive phenotype in E. coli and decreases the efficiency of decoding codons ending in G, specifically the GCG and UCG codons (Sakai et al., 2019).
ho5U is often utilized as an intermediate for mo5U, cmo5U, and mcmo5U in bacteria (Sakai et al., 2019). The enzyme CmoB carboxymethylates ho5U34 in tRNA into cmo5U34 using a highly unusual SCM‐SAH molecule, which is generated when the enzyme CmoA uses a carboxyl group from prephenate to carboxylate SAH (J. Kim et al., 2013). The cmo5U34 modification can be further methylated by the SAM‐dependent enzyme CmoM into mcmo5U (Sakai, Miyauchi, Kimura, & Suzuki, 2016). The mcmo5U mark in one tRNA, tRNAPro3, is dynamic, as its abundance is positively correlated with the number of E. coli and perhaps in other organisms (Sakai et al., 2016). The presence of cmo5U and mcmo5U appears to be exclusive to bacteria, possibly due to the requirement of prephenate, one of the intermediates in the chorismate–shikimate pathway (Sakai et al., 2016, 2019). In a prior observation, ho5U can also slow the rate of RNA and protein synthesis in bacteria, viruses, and tumor cells if introduced in media as a mononucleotide (D. A. Smith & Visser, 1965). Additional research is underway for finding previously unknown ways in which ho5U participates in cellular processes, especially with its role in oxidative damage and whether ho5U and its derivatives have any roles in RNA structure (Sakai et al., 2019). Perhaps these ho5U‐related processes could become a new drug target or be useful diagnostics for diseases related to oxidative damage.
7.7. cmnm5U, cm5U, and their derivatives decorate tRNA molecules
Nucleoside modification (IUPAC abbreviation): 5‐carboxymethylaminomethyluridine (cmnm5U); 5‐methylaminomethyluridine (mnm5U); 5‐aminomethyluridine (nm5U); 5‐carboxymethyluridine (cm5U); 5‐methoxycarbanylmethyluridine (mcm5U); 5‐carboxyhydroxymethyluridine (chm5U); 5‐carbamoylmethyluridine (ncm5U)5‐carboxyhydroxymethyluridine methyl ester (mchm5U); 5‐carbamoylhydroxymethyluridine (nchm5U).
Domain assignments: A (not cmnm5U, mnm5U and nm5U), B (cmnm5U, mnm5U, and nm5U), E (not mnm5U, nm5U, and nchm5U).
RNA classes:  .
.
Uridines in the anticodon loop (positions 34–37) of select tRNA molecules can be modified with methyl (m) or carboxymethyl (cm) derivatives (Figure 8) (C. Chen, Huang, Anderson, & Byström, 2011). U5 in bacterial tRNA is modified by the bacterial enzymatic complex MnmE and MnmG/GidA to contain either a carboxymethylaminomethyl (cmnm) or a aminomethyl (nm) group resulting in the formation of either cmnm5U or nm5U, respectively (Ayadi, Galvanin, Pichot, Marchand, & Motorin, 2019; Mihara et al., 2002; Moukadiri et al., 2009; Moukadiri, Villarroya, Benítez‐Páez, & Armengod, 2018; Sierant et al., 2018). The formation of cmnm5U makes use of a 5‐formyluridine intermediate (J. Kim & Almo, 2013). An additional interconversion can occur from cmnm5U into mnm5U. Here, the carboxymethyl group is cleaved off cmnm5U via the oxidoreductase domain of the MnmC(o) enzyme, yielding nm5U (J. Kim & Almo, 2013; Moukadiri et al., 2018). nm5U, either newly formed or originating from cmnm5U, can then be methylated by the methyltransferase domain of the MnmC(m) enzyme to yield mnm5U (J. Kim & Almo, 2013; Moukadiri et al., 2018). In an unrelated reaction mechanism in S. cerevisiae, MTO1 adds a glycine residue to U34, resulting in the formation of cmnm5U (Fakruddin et al., 2018; Umeda et al., 2005). However, it is unknown if cmnm5U can also interconvert into mnm5U or nm5U in eukaryotes using enzymatic processes similar to bacteria.
FIGURE 8.
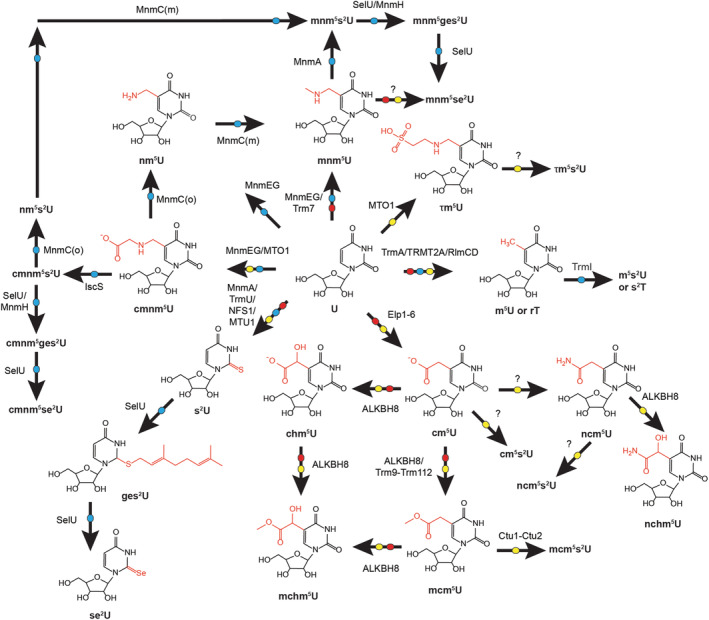
Scheme for cmnm5U, cm5U, τm5U, s2U, se2U, and related derivatives. Shown in red are chemical modifications that occur in the production of 5‐methyluridine or thiolated uridine derivatives: m5U or rT, cmnm5U, mnm5U, nm5U, cm5U, mcm5U, chm5U, mchm5U, nchm5U, ncm5U, τm5U, s2U, m5s2U or s2T, cmnm5s2U, mnm5s2U, nm5s2U, τm5s2U, mcm5s2U, ncm5s2U, cm5s2U, se2U, ges2U, cmnm5ges2U, mnm5ges2U, cmnm5se2U, and mnm5se2U. Modifications containing a 2‐thio group, a 2‐geranyl group, or a 2‐seleno group are listed with only abbreviations, while the structures for s2U, ges2U, and se2U are shown as exemplars for each of these modification types. Enzymes that catalyze each reaction are adjacent to arrow, otherwise a question mark (?) indicates enzyme is unknown. Arrow tails containing red, blue, or yellow ellipses denote reactions that occur in archaea, bacteria, or eukaryotes, respectively
The Elongator complex (Elp1‐6), which is highly conserved in eukaryotes and supports mRNA transcription elongation by RNA Pol II, carboxymethylates uridine residues and forms cm5U (Figure 8) (Karlsborn et al., 2014). Archaeal homologs of the third subunit of the Elongator complex, Elp3, have also been shown to carboxymethylate uridine residues in tRNAs (Selvadurai, Wang, Seimetz, & Huang, 2014). Once cm5U forms, it can undergo one of several synthetic pathways. cm5U can be methylated with a methyl group from SAM by the enzyme ALKBH8 to form mcm5U (Songe‐Møller et al., 2010). In archaea and in eukaryotes, methylation of cm5U to mcm5U is catalyzed by the Trm9–Trm112 complex (van Tran et al., 2018). cm5U can also be hydroxylated by the ALKBH8 enzyme to form chm5U (van den Born et al., 2011). Both chm5U and mcm5U can be independently methylated or carboxyhydroxylated, respectively, by ALKBH8 to form mchm5U (Karlsborn et al., 2014; van den Born et al., 2011). Finally, cm5U can be modified by an unknown enzyme to contain a carbamoylmethyl group to form ncm5U and hydroxylated to form nchm5U with the eukaryotic ALKBH8 enzyme (C. Chen et al., 2011; van den Born et al., 2011). Altogether, these modifications have roles in proper decoding of codons and specificity of tRNA‐binding events to mRNA (C. Chen et al., 2011). However, many of the implications of these modifications in biology remain unknown.
7.8. τm5U is important to mitochondrial tRNAs
Nucleoside modification (IUPAC abbreviation): 5‐taurinomethyluridine (τm5U).
Domain assignments: E.
RNA classes:  .
.
τm5U was discovered in mammalian tRNALys (Fakruddin et al., 2018). The enzyme MTO1 adds the taurine modification in mitochondrial tRNAs and can be found in position U34 of the anticodon loop within tRNALys (Figures 4 and 8) (Fakruddin et al., 2018). An siRNA knockdown of a mitochondrial homolog of MTO1 resulted in the absence of τm5U and reduced translational activity of UUG codon (Umeda et al., 2005). The lack of τm5U results in a deficiency of mitochondrial complex I that is seen in patients with MELAS and MERRF (Umeda et al., 2005). MELAS and MERRF result from mitochondrial distress, as indicated by the severely impaired translation and respiration events within the mitochondria (Fakruddin et al., 2018). Additionally, trafficking of mitochondrial proteins encoded by nuclear DNA was negatively affected by the resulting abnormal mitochondrial shape (Fakruddin et al., 2018). Inner membrane proteins of the mitochondria, such as Opa1, are unable to localize to the inner membrane; therefore, they aggregate in the cytosol, inducing a cytotoxic unfolded protein response (Fakruddin et al., 2018). This response has been studied as a drug target, where a taurine‐conjugated bile acid molecule was discovered to possibly supply the missing taurine in mouse livers and could alleviate this unfolded protein response (Fakruddin et al., 2018). However, as mitochondrial proteins are still mistranslated, further research is necessary to elucidate a cure for these diseases (Fakruddin et al., 2018).
7.9. Sulfur‐containing modifications, such as s2U and its derivatives and s4U, are essential for tRNA structural stability as well as translational fidelity and efficiency
Nucleoside modification (IUPAC abbreviation): 2‐thiouridine (s2U); 5‐methyl‐2‐thiouridine (m5s2U); 5‐carboxymethylaminomethyl‐2‐thiouridine (cmnm5s2U); 5‐methylaminomethyl‐2‐thiouridine (mnm5s2U); 5‐aminomethyl‐2‐thiouridine (nm5s2U); 5‐taurinomethyl‐2‐thiouridine (τm5s2U); 5‐methoxycarbonylmethyl‐2‐thiouridine (mcm5s2U); 5‐carbamoylmethyl‐2‐thiouridine (ncm5s2U); 5‐carboxymethyl‐2‐thiouridine (cm5s2U); 5‐cyanomethyl‐2‐thiouridine (cnm5s2U); 4‐thiouridine (s4U).
Domain assignments: A (s2U, m5s2U, cm5s2U, cnm5s2U, s4U), B (not cm5s2U, mcm5s2U, ncm5s2U, τm5s2U, cnm5s2U), E (not m5s2U, mnm5s2U, nm5s2U, cmnm5s2U, cnm5s2U, s4U).
RNA classes:  .
.
The formation of s2U involves an array of enzymatic processes, ultimately resulting in a modification that is found in nine other uridine modifications (Figure 8, Table 1). The modified nucleoside derivatives featuring an xs2U (where x denotes one of several possible modifications listed above) are covered more in‐depth in sections below. However, the common feature for all these modifications is the substitution of a sulfur at O2 of uracil. To add this sulfur, a cysteine is dethiolated by either IscS or YrvO in E. coli or B. subtilis, respectively (Black & Dos Santos, 2015; Lauhon, 2002; Mihara et al., 2002; Shigi, 2014), or by homologs of NFS1/Ncs6/Ctu1 in archaea and eukaryotes (Y. Liu, Long, Wang, Söll, & Whitman, 2014). E. coli continues shuttling the sulfur molecule through TusABCDE proteins before transferring the sulfur to the MnmA protein, which thiolates the uridine at nt 34 in tRNA (Black & Dos Santos, 2015). B. subtilis does not have the TusABCDE operon; therefore, YrvO directly passes the sulfur molecule to MnmA, which then thiolates tRNAs (Black & Dos Santos, 2015). In archaea and possibly eukaryotes, NFS1/Ncs6/Ctu1 first adenylates U34 in select tRNAs prior to thiolation from NFS1/Ncs6/Ctu1 (Y. Liu et al., 2014). While it is not known precisely which type of sulfur modification is directly replaced on uridine residues in eukaryotic tRNAs, mitochondrial NFS1, Isu1/2, and Atm1 in S. cerevisiae and Tum1, a persulfide mediator and activator of NFS1, have roles in forming and transporting s2U marks (Nakai et al., 2004; Noma, Sakaguchi, & Suzuki, 2009; Pandey et al., 2018). Moreover, the MTU1 enzyme is a mitochondrial‐specific 2‐thiouridylase that is a homolog of the MnmA enzyme (Black & Dos Santos, 2015; Noma et al., 2009). Humans use a homolog of MTU1, also known as TrmU, to thiolate uridine residues in mitochondrial tRNAs (Black & Dos Santos, 2015; Umeda et al., 2005).
The functions of the 2‐thio group in numerous uridine nucleotides depend on where the modified ribonucleotide occurs within the tertiary structure of tRNA. For example, s2U in the acceptor stems (TΨC‐loop and D‐loop) is important for ensuring tRNA structural stability and recognition by specific enzymes (reviewed in C. Zheng, Black, & Dos Santos, 2017). Sulfur‐containing modifications within the anticodon stem‐loop (nts 32, 33, 34, 37) are usually important for translation. s2U33 can have roles in negatively regulating C34‐to‐U34 editing and in subsequent translation (reviewed in Čavužić & Liu, 2017; Z. Paris, Fleming, & Alfonzo, 2012). The s2U33 mark has been found in T. brucei and Leishmania spp. mitochondrial tRNATrp‐CCA (Crain et al., 2002; Z. Paris et al., 2012). s2U and its derivatives that are present at nt 34 within tRNAGlu, tRNAGln, and tRNALys ensure specificity or flexibility in the discrimination of multiple codons (reviewed in Čavužić & Liu, 2017; Lim & Curran, 2001; C. Zheng, Black, et al., 2017). Early studies indicated that the presence of s2U34 maintains a Watson–Crick base pair with A and restricts Wobble pairing with guanosine, though s2U34 can still pair with both A and G. However, additional modifications to position C5 of s2U34 can affect the preference for U–A pairing and favored U–G interactions (Agris et al., 1992; Egert, Lindner, Hillen, & Boehm, 1980; Krüger & Sørensen, 1998). The 2‐thio group of m5s2U34 and its derivatives enhances translation specificity, contributing to the recognition of tRNAs by aminoacyl‐tRNA synthetases (Madore et al., 1999; Rodriguez‐Hernandez et al., 2013; Sylvers, Rogers, Shimizu, Ohtsuka, & Söll, 1993; Tamura, Himeno, Asahara, Hasegawa, & Shimizu, 1992). These modifications ensure preferential base pairing with purines in the codons of mRNAs and prevent misreading caused by interactions with pyrimidines (reviewed in Shigi, 2014; C. Zheng, Black, et al., 2017). s2U and its derivatives are involved in codon recognition within the ribosome and prevent frameshifting during translation (Rodriguez‐Hernandez et al., 2013; Shigi, 2014; C. Zheng, Black, et al., 2017). For example, m5s2U (also known as s2T) at nt 54 plays an important role in protein expression in thermophilic organisms such as the bacteria T. thermophilus or the archaea P. furiosus (Kowalak, Dalluge, McCloskey, & Stetter, 1994; Shigi, 2014; Shigi et al., 2006; Shigi, Sakaguchi, Asai, Suzuki, & Watanabe, 2008; Yokoyama et al., 1987). The rigid structure of this modification increases tRNA thermostability through stabilization of the D‐ and TΨC‐loops (reviewed in Shigi, 2014).
Thiolation events can be found in other modified uridine residues on tRNAs. cmnm5U and mnm5U can be thiolated at C2 via the bacterial enzymes IscS and MnmA to form cmnm5s2U or mnm5s2U (Mihara et al., 2002; Sierant et al., 2018). cmnm5s2U can also be converted into mnm5s2U or nm5s2U via the bacterial MnmC enzyme (Bujnicki et al., 2004). Though it is unclear which thiolase forms cm5s2U from cm5U, mcm5U can be thiolated to form mcm5s2U using the eukaryotic Ctu1–Ctu2 enzymatic complex (Dewez et al., 2008; Lentini, Ramos, & Fu, 2018). Also, once ncm5U is formed, it can be thiolated through an unknown thiolase to form ncm5s2U (C. Chen et al., 2011). Through an unknown mechanism, τm5U can be thiolated in human mitochondrial tRNA to form τm5s2U (Fakruddin et al., 2018). τm5s2U‐deficient human mitochondrial tRNAs were also found in patients who had MERRF and cannot translate the codons AAA or AAG (Umeda et al., 2005). Through an unknown mechanism, cnm5U can be thiolated in tRNALeu from euryarchaeote M. jannaschii to form cnm5s2U (N. Yu, Jora, et al., 2019). While cnm5s2U has been found only in M. jannaschii to date, M. jannaschii and Haloferax volcanii represent the only two archaeal species to have had their tRNAs analyzed for the presence of modified ribonucleosides. This dearth of knowledge of archaeal tRNA modifications and their distributions within archaea may lead to further discoveries of previously unknown modified ribonucleosides. Moreover, given the large number of s2U derivatives, it would be interesting to see if there are additional thiolated cytidine derivatives beyond s2C and ms2C.
Like s2U modifying enzymes, there are enzymes specific to the Gram‐negative and Gram‐positive bacteria synthetic reactions of s4U. In the Gram‐negatives E. coli and S. enterica, IscS removes a sulfur atom from cysteine while ThiI thiolates U8 in tRNA (Rajakovich, Tomlinson, & Dos Santos, 2012). However, in Gram‐positive B. subtilis and the other Gram‐positives in Firmicutes, NifZ instead removes the sulfur from cysteine and directly thiolates the uridine residue in tRNA with a ThiI homolog (Rajakovich et al., 2012). The archaeal homologs of ThiI also incorporate s4U in tRNA. Eukaryotes do not appear to have s4U, though homologs of ThiI have been found in some eukaryotic genomes (reviewed in Čavužić & Liu, 2017; Kotera et al., 2010). s4U can be located at nt 8 in tRNA, which is at the intersection of the tRNA acceptor‐ and D‐arms (Figure 4, Table 2). Upon exposure to near‐UV light, s4U crosslinks to C13 in tRNA (reviewed in Palenchar, Buck, Cheng, Larson, & Mueller, 2000). The s4U‐C13 crosslink prevents proper tRNA aminoacylation, leading to uncharged tRNA accumulation and translational pausing (Čavužić & Liu, 2017; Favre, Yaniv, & Michels, 1969). These effects then activate the SOS response, which mitigates damage to DNA due to UV‐light exposure (Björk & Hagervall, 2014; Čavužić & Liu, 2017; Thomas & Favre, 1980). The thiolation of s4U8 of tRNAs has also been shown to positively maintain tRNA homeostasis in Vibrio cholerae (Kimura & Waldor, 2019). In thiI deletion strains of V. cholerae, several tRNAs were decreased, as it was determined that the lack of s4U8 designates these hypomodified tRNAs for degradation by the RNA degradosome (Kimura & Waldor, 2019). While s4U is used in PAR‐CLIP at high concentrations that are in excess of 100 μM, this high concentration has been shown to interfere with proper 47S rRNA processing in U2OS and 2fTGH human cell cultures; therefore, caution is warranted when using s4U in PAR‐CLIP or other related experiments (Burger et al., 2013). Further roles of sulfur‐containing ribonucleotides and the enzymes involved may have further unknown roles in structural formation and biological relevance to thermophilic organisms.
7.10. Selenium‐modified uridines and their precursors are thought to aid in translational accuracy and efficiency
Nucleoside modification (IUPAC abbreviation): 2‐selenouridine (se2U); 2‐geranylthiouridine (ges2U); 5‐carboxymethylaminomethyl‐2‐geranylthiouridine (cmnm5ges2U); 5‐methylaminomethyl‐2‐geranylthiouridine (mnm5ges2U); 5‐carboxymethylaminomethyl‐2‐selenouridine (cmnm5se2U); 5‐methylaminomethyl‐2‐selenouridine (mnm5se2U).
Domain assignments: A (se2U and mnm5se2U), B, E (mnm5se2U).
RNA classes:  .
.
While there are several sulfur‐modified uridine residues currently known, selenium‐containing ribonucleosides can also be found in tRNAs, specifically in the anticodon loop at position 34 (Figures 4 and 8, Table 1). In bacteria, converting s2U to se2U is a two‐step process catalyzed by SelU (Sierant et al., 2018). In the first step, the sulfur of s2U is geranylated to yield ges2U. By itself, ges2U is a stable modification that is thought to shift codon bias and frameshifting rates during translation, which is possibly due to a preference of ges2U to base pair with guanosine and not adenosine (Sierant et al., 2016). Once ges2U is formed on tRNA, the second step results in a selenation by replacing the geranyl‐sulfate with a selenide group, thus forming se2U (Sierant et al., 2016). In archaea, a YbbB‐like enzyme forms se2U, though it is unknown if this requires a geranylated intermediate (D. Su, Ojo, Söll, & Hohn, 2012). Separately, once cmnm5s2U and mnm5s2U are thiolated in bacteria, these modified uridines can be geranylated by SelU or by MnmH, yielding cmnm5ges2U or mnm5ges2U (Jäger, Chen, & Björk, 2016; Sierant et al., 2016, 2018). These modifications can be subsequently selenated by the SelU enzyme to yield cmnm5se2U or mnm5se2U (Dumelin, Chen, Leconte, Chen, & Liu, 2012; Sierant et al., 2018). mnm5se2U is the most abundant selenonucleoside, found in all three domains of life, and commonly occurs in the Wobble position of the anticodon of three tRNA acceptors (tRNAGlu, tRNAGln, and tRNALys) (D. Su et al., 2012). Se2 of mnm5se2U residues discriminates against the formation of G–U Wobble pairs. Instead, the selenium promotes the formation of A–U base pairs in RNA in vitro, suggesting selenium modifications may be important in translational fidelity (Huiyan Sun et al., 2012). While it is presently unknown if the bacterial selenation process is present in archaea or eukaryotes, the presence of mnm5se2U in archaea and eukaryotes suggests that a similar process may be possibly occurring.
Further, GTPBP3 and the taurine‐adding enzyme MTO1 have also been linked to selenation reactions in mitochondrial tRNAs (Bykhovskaya, Mengesha, et al., 2004; Kopajtich et al., 2014; Xinjian Wang, Yan, & Guan, 2010; Yim, Moukadiri, Björk, & Armengod, 2006). Deficiencies in mitochondrial GTPBP3 have been linked to hypertrophic cardiomyopathy, lactic acidosis, encephalopathy, and deafness through deficiencies in cmnm‐containing uridines (Bykhovskaya, Mengesha, et al., 2004; Kopajtich et al., 2014). While MTO1 deficiency can also be linked to these diseases, MTO1 deficiency is typically linked to deficiencies in taurine‐containing uridine modifications (Fakruddin et al., 2018; Jonkhout et al., 2017; X. Wang et al., 2010). Additional discoveries of geranyl derivatives and selenium‐containing RNAs will undoubtedly continue to provide new insights into biology and in further elucidating the differences between sulfur‐ and selenium‐containing RNAs.
8. MODIFICATIONS TO THE RIBOSE MOIETY
8.1. Ribosyl‐RNA modifications are found in select RNAs
Nucleoside modification (IUPAC abbreviation): 2′‐O‐ribosyladenosine (Ar(p)); 2′‐O‐ribosylguanosine (Gr(p)).
Domain assignments: E.
RNA classes:  .
.
Ar(p) and Gr(p) represent disaccharide‐modified ribonucleosides, whereby the 2′‐hydroxyl of either adenosine or guanosine forms an α‐(1,2)‐glycosidic linkage with an additional 5′‐phosphorylated ribose molecule. Though first discovered at position 64 in eukaryotic tRNAMet that initiates translation, these disaccharide‐modified ribonucleosides have been found in poly(ADP‐ribose) (PAR), in select enzymes as prosthetic groups, and in assorted naturally derived compounds or secondary metabolites (Desgrès, Keith, Kuo, & Gehrke, 1989; Efimtseva, Kulikova, & Mikhaĭlov, 2009; Glasser, Desgres, Heitzler, Gehrke, & Keith, 1991; Juarez‐Salinas, Mendoza‐Alvarez, Levi, Jacobson, & Jacobson, 1983; Keith, Glasser, Desgrés, Kuo, & Gehrke, 1990; Q. Liu, van der Marel, & Filippov, 2019; Simsek & RajBhandary, 1972). Ar(p) and Gr(p) are synthesized by the enzyme Rit1 through addition of a PRPP group directly onto A64 or G64 in tRNAMet, which potentially distorts the RNA phosphodiester backbone of the initiator tRNAMet and prevents the initiator tRNAMet from binding to eEF1A (Åström & Byström, 1994; Kiesewetter, Ott, & Sprinzl, 1990; Kolitz & Lorsch, 2010). PAR is synthesized from NAD+ via eukaryotic PAR polymerases (Berger, Ramírez‐Hernández, & Ziegler, 2004; Pleschke, Kleczkowska, Strohm, & Althaus, 2000; Rongvaux, Andris, Van Gool, & Leo, 2003; Schreiber, Dantzer, Ame, & de Murcia, 2006). One of the primary functions of PAR is aiding in the DNA‐strand repair process, as it is added to DNA‐strand breaks and serves as a signaling molecule to initiate DNA repair processes (Schreiber et al., 2006). Moreover, PAR can have roles in acute and chronic inflammation, while also contributing to the progression of degenerative diseases (Schreiber et al., 2006). Further discoveries pertaining to PAR, Ar(p), and Gr(p) will perhaps expand the roles of these modified nucleosides throughout eukaryotic biology.
8.2. 2′‐O‐Methyl RNA modifications exist in otherwise unmodified and additionally modified ribonucleosides
Nucleoside modification (IUPAC abbreviation): 2′‐O‐methyladenosine (Am); N 6,2′‐O‐dimethyladenosine (m6Am); N 6,N 6,2′‐O‐trimethyladenosine (m6,6Am); N 1,2′‐O‐dimethyladenosine (m1Am); 2′‐O‐methylinosine (Im); 1,2′‐O‐dimethylinosine (m1Im); 2′‐O‐methylguanosine (Gm); 1,2′‐O‐dimethylguanosine (m1Gm); N 2,2′‐O‐dimethylguanosine (m2Gm); N 2,N 2,2′‐O‐trimethylguanosine (m2,2Gm); N 2,2′‐O‐7‐trimethylguanosine (m2,7Gm); 2′‐O‐methylcytidine (Cm); 4,2′‐O‐dimethylcytidine (m4Cm); 5,2′‐O‐dimethylcytidine (m5Cm); 5‐formyl‐2′‐O‐methylcytidine (f5Cm); N 4,N 4,2′‐O‐trimethylcytidine (m4,4Cm); N 4‐acetyl‐2′‐O‐methylcytidine (ac4Cm); 2′‐O‐methyl‐5‐hydroxymethylcytidine (hm5Cm); 2′‐O‐methyluridine (Um); 3,2′‐O‐dimethyluridine (m3Um); 5,2′‐O‐dimethyluridine (m5Um); 2′‐O‐methylpseudouridine (Ψm); 2‐thio‐2′‐O‐methyluridine (s2Um); 5‐methoxycarbonylmethyl‐2′‐O‐methyluridine (mcm5Um); 5‐carboxymethylaminomethyl‐2′‐O‐methyluridine (cmnm5Um); 5‐carbamoylmethyl‐2′‐O‐methyluridine (ncm5Um); 5‐(isopentenylaminomethyl)‐2′‐O‐methyluridine (inm5Um); 5‐(carboxyhydroxymethyl)‐2′‐O‐methyluridine methyl ester (mchm5Um); 2′‐O‐methyluridine 5‐oxyacetic acid methyl ester (mcmo5Um).
Domain assignments: A, B, E; see the following reviews for prokaryotic assignments (Ayadi et al., 2019; Boccaletto et al., 2018) and RMBase for eukaryotic assignments (Xuan et al., 2018).
RNA classes:  ,
,  ,
,  ,
,  ; see for RNA classes (Ayadi et al., 2019; Boccaletto et al., 2018; Xuan et al., 2018).
; see for RNA classes (Ayadi et al., 2019; Boccaletto et al., 2018; Xuan et al., 2018).
While there are at least 28 2′‐O‐methyl modifications (Nm) to ribonucleosides, their origins are thought to be quite similar (Table 1). Assorted archaeal and eukaryotic snoRNAs, many of which are Box C/D snoRNAs, aid in assembling the snoRNPs NOP56, NOP58, fibrillarin, and 15.5K/Snu13/L7Ae. This protein complex then coordinates 2′‐O‐methylation events on pre‐rRNA transcripts and other RNAs (reviewed in Sloan et al., 2017; Yip, Shigematsu, Taylor, & Baserga, 2016; Zhu, Pirnie, & Carmichael, 2017). Moreover, snoRNAs are also used by archaeal species to conduct 2′‐O‐methylation events on tRNA. Separately, bacteria also conduct 2′‐O‐methylations on ribosomes, although these methylations are made by site‐specific enzymes (Marchand, Blanloeil‐Oillo, Helm, & Motorin, 2016). There are also methyltransferases that are involved in the formation of 2′‐O‐methylation marks, as in the case of m6Am by PCIF1 (Akichika et al., 2019; Boulias et al., 2019; Sendinc et al., 2019).
While the existence of 2′‐O‐methyl modifications has been documented since 1960s, much of the function of several 2′‐O‐methyl modifications still remains unknown, though studies of 2′‐O‐methylation have garnered useful insights that may be helpful in elucidating future discoveries involving 2′‐O‐methylated nucleotides (reviewed in Ayadi, Galvanin, Pichot, Marchand, & Motorin, 2019; Baskin & Dekker, 1967). The presence of 2′‐O‐methyl marks results in degradation resistance upon exposure to alkaline solutions, the disruption of protein‐binding activities, and disrupts RNA–DNA duplex formation to limiting off‐target effects of siRNAs (Ayadi et al., 2019; Iribe et al., 2017). In eukaryotes, 2′‐O‐methyl modifications are essential for splicing reactions by the major spliceosome (Y.‐T. Yu, Shu, & Steitz, 1998). ac4Cm and m2,2Gm have been shown to have additive effects in stabilizing tRNA structure and conformation at elevated temperatures in archaeal hyperthermophiles, a trend seen with Nm marks overall in 16S rRNA in T. thermophilus and possibly other thermophiles (Guymon, Pomerantz, Crain, & McCloskey, 2006; Kawai et al., 1991). In humans, self‐ and non‐self‐identification of mRNAs is dependent upon the presence or absence, respectively, of 2′‐O‐methyl marks and elicit an interferon‐induced response during a human coronavirus infection and potentially for other viral infections (Abbas et al., 2017). Moreover, knockdown of fibrillarin resulted in a corresponding decrease in ribosome 2′‐O‐methylation and a corresponding increase in translational infidelity (Erales et al., 2017). Colorectal cancer, AML, and cancers that originate due to mutation or deletion of tp53 may form due to a knockdown or a knockout of fibrillarin (Ayadi et al., 2019; Erales et al., 2017; Sharma, Marchand, Motorin, & Lafontaine, 2017; Zhou et al., 2017). While these modifications have been known for over 50 years, further advances in research on 2′‐O‐methyl marks may elucidate previously unknown biological functions.
9. CONSIDERATIONS OF MODIFIED RIBONUCLEOSIDES AND EVIDENCE FOR THE EXISTENCE OF ADDITIONAL MODIFIED RIBONUCLEOSIDES
9.1. Caution must be exercised for identification of modified ribonucleosides
Historically, several modifications or studies on modifications have demonstrated that caution must be used when interpreting data on RNA modifications, as noted by these nine examples: (a) Initial efforts to map A‐to‐I modifications across the human transcriptome (M. Li et al., 2011) were found to be filled with false‐positive results (Kleinman & Majewski, 2012; W. Lin, Piskol, Tan, & Li, 2012; Pickrell, Gilad, & Pritchard, 2012; Piskol, Peng, Wang, & Li, 2013; Schrider, Gout, & Hahn, 2011). Subsequent research endeavors have been conducted to report more accurately instances of A‐to‐I modified RNAs (Bahn et al., 2012; Kleinman, Adoue, & Majewski, 2012; Peng et al., 2012; Ramaswami et al., 2012; Ramaswami & Li, 2014). (b) Likewise, failures in correcting for duplications, misannotations, mismapping, SNPs, sequencing errors, and incorporation of nontemplate nucleotides from reverse transcriptase in postsequencing analysis proved detrimental to studies on m1A modifications and led to some confusion over which dataset(s) or conditions were accurate (Schwartz, 2018). (c) Prior work indicated that when ms2t6A was exposed to Tris, an in vivo artifact formed that was a higher ms2t6A‐related compound in mass spectrometry analyses. This artifact was later determined to be ms2ct6A (Kang et al., 2017; Krog et al., 2011). (d) Early studies on m6A involved use of an anti‐m6A antibody that could also detect m6Am in RNA (Linder et al., 2015). m6Am marks were localized to being immediately adjacent to the m7G cap, although m6Am marks found in mRNAs and ncRNAs were misannotated to be m6A marks. These misannotated m6Am marks were localized to alternative transcription start sites in mRNA isoforms (Akichika et al., 2019; Boulias et al., 2019; Linder et al., 2015; Sendinc et al., 2019; Hanxiao Sun, Zhang, Li, Bai, & Yi, 2019). (e) Peroxywybutosine, o2yW, has only been isolated once in tRNAPhe from rat liver extracts despite a separate lab attempting to replicate the o2yW isolation, leaving its biological relevance in question (Kasai, Yamaizumi, Kuchino, & Nishimura, 1979). (f) While 5‐methyldihydrouridine (m5D) was initially identified in the chromosomal RNA of rat ascites tumor cells, a later paper found no evidence of the modification in mouse ascites (Jacobson & Bonner, 1968; Tolstoshev & Wells, 1973). (g) ms2m6A is another modification with conflicting evidence of existence. There is no manuscript that describes the existence of the modification and it is only referred to as a preliminary modification in The RNA Modification Database. However, it is listed as being present in the majority of tRNAs, in addition to m6A (Cantara et al., 2011; Kierzek & Kierzek, 2003). (h) Separately, in the production of mnm5se2U and cmnm5se2U, it has been speculated that formation of 5‐aminomethyl‐2‐geranylthiouridine (nm5ges2U) and 5‐aminomethyl‐2‐selenouridine (nm5se2U) can occur due to the possibility of decarboxylation of cmnm5ges2U and cmnm5se2U. The conversion from cmnm5ges2U and cmnm5se2U into nm5ges2U and nm5se2U is consistent with prior decarboxylation events with other precursors in this synthesis cascade (C. Zheng, Black, et al., 2017). (i) Historically, in vitro analysis of the effects of modifications on RNAs has been conducted through direct incorporation of the desired modification via synthetic means (reviewed in Dellafiore, Montserrat, & Iribarren, 2016; A. J. Meyer et al., 2015). However, through adjustments of SELEX protocols (reviewed in Dellafiore et al., 2016) or by guided gain‐of‐function mutations to T7 RNA polymerase (A. J. Meyer et al., 2015), some modified ribonucleotides (e.g., Nm and other 2′‐containing modifications) can be incorporated. Though all ribonucleoside modifications contain the potential for newly discovered and exciting biology, they must be carefully vetted.
9.2. Modified ribonucleosides have therapeutic applications
Several strategies involving the use of modified ribonucleosides or their chemical analogs have been exploited in the treatment of human disease. Examples include ribavirin, acyclovir, valacyclovir, and other ribonucleoside analogs in the treatment of viral infections or the use of cytarabine in the treatment of cancer (Seley‐Radtke & Yates, 2018). Another example is the use of modified ribonucleobases when transfecting RNAs to increase their stability, to increase their lifetime in complex biological systems that include RNases, or to increase rates of translation (Andries et al., 2015; Karikó, Buckstein, Ni, & Weissman, 2005; Orellana et al., 2017). One type of modification strategy incorporates m1Ψ into transfected mRNAs to increase RNA stability and translation rates, consequently enhancing protein expression (Andries et al., 2015). m1Ψ and other modifications such as m6A, m5U, s2U, and Ψ reduce immunogenicity of mRNA (Karikó et al., 2005). While a combination of Ψ and m5U modifications in transfected RNAs has been successfully used for therapeutic applications in preclinical research, further studies show that m1Ψ introduced into mRNA alone or combined with m5U have increased rates of mRNA stabilization than mRNAs carrying only Ψ (Andries et al., 2015; Kormann et al., 2011; Miki et al., 2015; Luigi Warren et al., 2010; Wroblewska et al., 2015; Zangi et al., 2013). Incorporation of m1Ψ or m1Ψ/m5C into mRNA results in enhanced translation, lower cytotoxicity, and diminished activation of the intracellular innate immune response in comparison with Ψ or Ψ/m5C (Andries et al., 2015). Recent strategies of chemically modifying RNAs have been applied to antisense oligonucleotides or to miRNA derivatives to enhance their therapeutic potential and have even resulted in a cure for diseases such as spinal muscular atrophy or transthyretin‐mediated amyloidosis (Adams et al., 2018; Bennett, Krainer, & Cleveland, 2019; Orellana et al., 2017). However, as with all therapeutics, caution must be exercised in the design of RNA‐based therapeutics, as immune responses to RNA and normal RNases present in humans can hamper or negate these synthetic RNAs (A.‐M. Yu, Jian, Yu, &Tu, 2019).
9.3. Previously undiscovered ribonucleosides may exist
2′‐O‐ribosyluridine may exist, as a part of this previously unknown ribonucleoside was detected in the small molecule Hellecaucaside A, which is found in the hellebore plant Helleborus caucasicus or Lenten Rose (Kulikova, Muradova, Drenichev, & Mikhailov, 2015; Sylla et al., 2014). 5‐Carboxycytidine (ca5C) may exist, as the DNA form of this nucleoside exists and the ribonucleoside form of ca5C can be made under in vitro conditions using the TET1 protein to catalyze the conversion of f5C to ca5C (Basanta‐Sanchez et al., 2017). However, despite searches for ca5C, it cannot be found in vivo at present (Nakano et al., 2016). While 5‐isopentenylaminomethyluridine (inm5U) and 5‐isopentenylaminomethyl‐2‐thiouridine (inm5s2U) have been thought to exist in the tRNA of Thermodesulfobacterium commune, these two modifications may be a source of interest, due to the modifications using the 2‐thiouridine precursor (Cantara et al., 2011; Limbach et al., 1994). Separately, N 6‐hydroperoxymethyladenosine (oxm6A) was discovered in an in vitro chemical reaction when m6A was exposed to bicarbonate‐activated peroxide (J. Wu et al., 2015). Some or all of the new technologies mentioned above could possibly be applied in finding these modifications and others, thereby elucidating previously unknown aspects of biology that may also have medical significance.
10. METHODS OF DETECTING MODIFIED RIBONUCLEOSIDES
10.1. Mass spectrometry‐based methods of detecting modified ribonucleosides
Ongoing improvements of well‐established technologies and the development of new technology, research tools, and protocols may yield newfound modifications. For example, using mass spectrometry to analyze RNAs has been prevalent for over 20 years, as this method is able to directly distinguish all known modifications with the exception of Ψ‐containing modifications (Wetzel & Limbach, 2016). However, new technological breakthroughs to mass spectrometry may further elucidate novel modifications in a high‐throughput fashion or at the attomolar level that is consistent with small amounts of cellular RNA from individual cells (Basanta‐Sanchez, Temple, Ansari, D'Amico, & Agris, 2016; Jora et al., 2019). For example, conducting LC–MS on E. coli tRNAs resulted in the discovery of msms2i6A and potentially 36 other modifications that could have previously gone undetected (Dal Magro et al., 2018). Additional LC–MS analysis could also yield any of the 143 known modifications in other organisms that were not known to contain them.
In addition to finding RNA modifications in total RNA, sequence‐specific detection of RNA modifications can be performed with LC–MS (Addepalli, Venus, Thakur, & Limbach, 2017; Björkbom et al., 2015; Jora et al., 2019). Historically and in current research, finding sequence‐specific locations of RNA modifications relies upon the purification of a specific RNA and subsequent partial enzymatic or chemical degradation of RNA into sequencing ladders (Addepalli et al., 2017; Björkbom et al., 2015; N. Zhang, Shi, et al., 2019). Recently, multiple RNases were used to generate unique, overlapping digestion products, which has bypassed the need for the RNA to be highly purified (Thakur, Estevez, Lobue, Limbach, & Addepalli, 2020). Because LC–MS can be limited by the RNase cleavage site, novel RNases that have different substrate specificities can be helpful in sequence‐specific detection of modifications (Addepalli et al., 2017). Novel techniques that adapt mass‐retention time ladders or hydrophobic end‐labeling for LC–MS have been used to address some of the problems in identifying sequence‐specific RNA modifications (Björkbom et al., 2015; N. Zhang, Shi, et al., 2019). Reacting CMCT with mRNAs pooled from human cells increases sensitivity of mass spectrometry experiments to m5U, as the introduction of a positively charged quaternary amine group found in CMCT onto uridine allowed for improved ionization efficiency in mass spectrometry experiments (Cheng et al., 2020). This use of CMCT was recently successful in determining m5U content in mRNAs from HEK293T, HeLa, HL‐7702, and HepG2 human cell lines (Cheng et al., 2020).
Mass spectrometry‐based approaches of detecting RNA modifications may also have clinical relevance or may be helpful in elucidating previously unknown aspects of biology. Quantitative measurements of RNA modifications have shown reproducible and predictable changes in tRNA modification states under different cellular stresses (Chan et al., 2010). For example, exposure of S. cerevisiae to hydrogen peroxide resulted in an increase of m5C in tRNALeu(UUG), which enhances translation of oxidative stress response genes (reviewed in Huber, Leonardi, Dedon, & Begley, 2019). Analysis of Plasmodium falciparum m6A levels in mRNA by mass spectrometry showed that overall m6A levels increase slightly and proportionally to the amount of time postinfection of human red blood cells (Baumgarten et al., 2019). However, individual m6A peaks within mRNA can vary drastically in the entire amount of time that P. falciparum are inside red blood cells (Baumgarten et al., 2019). Collectively, with further improvements to both mass spectrometers and reagents, more RNA modifications, their locations in the transcriptome, and their roles in biology and in disease will doubtlessly be discovered.
10.2. RNA sequencing‐based methods can detect modified ribonucleosides
Direct sequencing of RNAs poses an exciting opportunity for being able to detect known and unknown modifications in RNA transcripts. Both Oxford Nanopore Technologies and Pacific Biosciences possess sequencing methods that allow for quantification of RNA modifications, such as m6A marks, and can likely be expanded to find previously unknown modifications (H. Liu, Begik, et al., 2019; Parker et al., 2020). These sequencing methods directly sequence the RNA transcript, rather than cDNA, which allows for modified nucleotides to be detected. Sequencing methodologies to directly sequence tRNAs have begun to elucidate the content and extent of tRNA modifications, especially in disease contexts (Goodarzi et al., 2016). Presently, the noise in high‐throughput sequencing unfortunately leads to false‐positive RNA modifications. All proposed modifications should be validated using at least one biochemical assay, such as mass spectrometry or SCARLET (Grozhik & Jaffrey, 2018; N. Liu et al., 2013).
The use of antibodies and high‐throughput sequencing has represented a recent boon in finding RNA modifications, as several have already been highlighted in this review (Arango et al., 2018; Dai et al., 2017; Dominissini et al., 2012, 2016; K. D. Meyer et al., 2012). Using antibodies greatly aids in reducing the noise of data derived from either mass spectrometry or through downstream high‐throughput sequencing methods, as the RNA pool is enriched with the modified RNA (Grozhik & Jaffrey, 2018). Recent progress to use activated nucleotides of modified ribonucleotides or engineering mutants of RNA‐modifying enzymes, especially enzymes that form an enzyme–substrate covalent intermediate, offer an intriguing way to pull out RNA targets (Khoddami & Cairns, 2013; T. S. Smith, Zoltek, & Simon, 2019; Tomkuvienė, Mickutė, Vilkaitis, & Klimašauskas, 2019). Further, use of AlkB to demethylate total RNA from human cells, coupled with comparative methylation analysis, was used to identify methylated small RNAs that were derived from tRNAs (Cozen et al., 2015). High‐throughput sequencing will continue to be used in determining the location of the modified RNA and may aid in determining the substrates of specific RNA‐modifying proteins. The use of new technology, or improvements on classic techniques, continues to unveil previously unknown modifications.
11. CONCLUSIONS
Since the discovery and characterization of pseudouridine, numerous modifications from all domains of life and all four parent nucleotides have been discovered. Much research has been conducted on modifications in eukaryotes, specifically in humans. For example, the m6A modification is intimately involved in oncology, cardiology, and several other fields in human biology. However, there are several instances where the presence of modifications in prokaryotes or in nonhuman organisms remains unexplored. There are RNA classes that are not currently known to contain any modifications (e.g., archaeal mRNAs and ncRNAs in Figure 3). Moreover, there are domain‐specific modifications that could be found in other domains of life (e.g., cnm5U in archaea only). While tRNAs and rRNAs remain a well‐spring of RNA modifications, the use of antibodies in probing for modifications and additional technological advances may find modifications not previously known to exist in RNA classes. Moreover, while modifications such as methylations or hydroxylations occur, more novel and complicated modifications may exist that have not been considered. With the advances of LC–MS and other technologies, perhaps additional glyco‐ and lipid‐ribonucleotide conjugates may be found. While threonine, glycine, lysine, arginine, and taurine can be found attached to ribonucleotides, it is possible that other amino acids could be coupled to pyrimidines or purines. The additional possibilities of thiolation or selenation events elsewhere in modifications may also yield new discoveries. As with all of the modifications, their impact on health is only beginning to be understood.
With the increasing progress of finding modifications and identifying their chemical composition, many outstanding questions remain. What is the limit to the number of chemical modifications that exist? Can the abundance of each modification be fit to a power‐law plot that may aid in finding previously unknown modifications, similar to how a power‐law plot was used to predict riboswitch classes and abundances (McCown, Corbino, Stav, Sherlock, & Breaker, 2017)? In which RNAs will these modifications appear? Will there be any additional discoveries of previously known ribonucleosides in domains in which they are not currently known to exist (Figure 3)? What is the chemical identity and three‐dimensional structure of these modifications? Will they establish direct links to the primordial RNA world (Heuberger, Pal, Del Frate, Topkar, & Szostak, 2015; Larsen, Fahrenbach, Sheng, Pian, & Szostak, 2015; Nelson & Breaker, 2017)? Will they be variations of known modifications, or will these modifications sample chemical space in novel ways? Will these modifications have a direct role in catalysis? Will these modifications underlie disease states or elucidate truly unknown biology? All these exciting questions face this ever‐growing field of RNA biology and leave much to be anticipated.
ACKNOWLEDGEMENTS
This work was supported by the NIH under Grants R00GM111430 and R35GM133696 to J.A.B., the Clare Boothe Luce Program of the Henry Luce Foundation, and startup funds from the University of Notre Dame. C.N.K. is a fellow of the Chemistry‐Biochemistry‐Biology Interface (CBBI) Training Program at the University of Notre Dame, supported by NIH Grant T32GM075762. M.C.W. is a College of Science Summer Undergraduate Research Fellow at the University of Notre Dame with funds provided from the Sean O'Keefe Cancer Fund. N.A.S. is a College of Science Summer Undergraduate Research Fellow at the University of Notre Dame with funds provided from the Glynn Family Honors Program.
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
AUTHOR CONTRIBUTIONS
Phillip J. McCown: Conceptualization; data curation; formal analysis; writing‐original draft; writing‐review and editing. Agnieszka Ruszkowska: Data curation; formal analysis; investigation; writing‐review and editing. Charlotte N. Kunkler: Data curation; formal analysis; investigation; writing‐review and editing. Kurtis Breger: Data curation; formal analysis; investigation; writing‐review and editing. Jacob P. Hulewicz: Data curation; formal analysis; investigation; writing‐review and editing. Matthew C. Wang: Data curation; formal analysis; investigation; writing‐review and editing. Noah A. Springer: Data curation; formal analysis; investigation; writing‐review and editing. Jessica A. Brown: Conceptualization; funding acquisition; investigation; supervision; writing‐review and editing.
RELATED WIREs ARTICLE
Transfer RNA modifications: Nature's combinatorial chemistry playground
Supporting information
Figure S1. Supporting information.
McCown PJ, Ruszkowska A, Kunkler CN, et al. Naturally occurring modified ribonucleosides. WIREs RNA. 2020;11:e1595. 10.1002/wrna.1595
Funding information Glynn Family Honors Program; Sean O'Keefe Cancer Fund; University of Notre Dame; Henry Luce Foundation; NIH, Grant/Award Number: R00GM111430, R35GM133696, T32GM075762
REFERENCES
- Abbas, Y. M. , Laudenbach, B. T. , Martínez‐Montero, S. , Cencic, R. , Habjan, M. , Pichlmair, A. , … Nagar, B. (2017). Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′‐O‐methylations. Proceedings of the National Academy of Sciences of the United States of America, 114(11), E2106–E2115. 10.1073/pnas.1612444114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi, H. , DeZoysa, M. D. , & Yu, Y.‐T. (2019). Detection and quantification of pseudouridine in RNA. Methods in molecular biology (Clifton, N.J.), 1870, 219–235. 10.1007/978-1-4939-8808-2_17 [DOI] [PubMed] [Google Scholar]
- Adams, D. , Gonzalez‐Duarte, A. , O'Riordan, W. D. , Yang, C.‐C. , Ueda, M. , Kristen, A. V. , … Suhr, O. B. (2018). Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. New England Journal of Medicine, 379(1), 11–21. 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]
- Addepalli, B. , Venus, S. , Thakur, P. , & Limbach, P. A. (2017). Novel ribonuclease activity of cusativin from Cucumis sativus for mapping nucleoside modifications in RNA. Analytical and Bioanalytical Chemistry, 409(24), 5645–5654. 10.1007/s00216-017-0500-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal, R. K. , Sharma, M. R. , Kiel, M. C. , Hirokawa, G. , Booth, T. M. , Spahn, C. M. T. , … Frank, J. (2004). Visualization of ribosome‐recycling factor on the Escherichia coli 70S ribosome: Functional implications. Proceedings of the National Academy of Sciences of the United States of America, 101(24), 8900–8905. 10.1073/pnas.0401904101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agris, P. F. , Sierzputowska‐Gracz, H. , Smith, W. , Malkiewicz, A. , Sochacka, E. , & Nawrot, B. (1992). Thiolation of uridine carbon‐2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. Journal of the American Chemical Society, 114(7), 2652–2656. 10.1021/ja00033a044 [DOI] [Google Scholar]
- Agris, P. F. , Vendeix, F. A. P. , & Graham, W. D. (2007). tRNA's wobble decoding of the genome: 40 years of modification. Journal of Molecular Biology, 366(1), 1–13. 10.1016/j.jmb.2006.11.046 [DOI] [PubMed] [Google Scholar]
- Akichika, S. , Hirano, S. , Shichino, Y. , Suzuki, T. , Nishimasu, H. , Ishitani, R. , … Suzuki, T. (2019). Cap‐specific terminal N 6‐methylation of RNA by an RNA polymerase II‐associated methyltransferase. Science, 363(6423), eaav0080. 10.1126/science.aav0080 [DOI] [PubMed] [Google Scholar]
- Alarcón, C. R. , Goodarzi, H. , Lee, H. , Liu, X. , Tavazoie, S. , & Tavazoie, S. F. (2015). HNRNPA2B1 is a mediator of m6A‐dependent nuclear RNA processing events. Cell, 162(6), 1299–1308. 10.1016/J.CELL.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón, C. R. , Lee, H. , Goodarzi, H. , Halberg, N. , & Tavazoie, S. F. (2015). N 6‐methyladenosine marks primary microRNAs for processing. Nature, 519(7544), 482–485. 10.1038/nature14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov, A. , Chernyakov, I. , Gu, W. , Hiley, S. L. , Hughes, T. R. , Grayhack, E. J. , & Phizicky, E. M. (2006). Rapid tRNA decay can result from lack of nonessential modifications. Molecular Cell, 21(1), 87–96. 10.1016/J.MOLCEL.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Alseth, I. , Dalhus, B. , & Bjørås, M. (2014). Inosine in DNA and RNA. Current Opinion in Genetics & Development, 26, 116–123. 10.1016/j.gde.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Amort, T. , Soulière, M. F. , Wille, A. , Jia, X.‐Y. , Fiegl, H. , Wörle, H. , … Lusser, A. (2013). Long non‐coding RNAs as targets for cytosine methylation. RNA Biology, 10(6), 1002–1008. 10.4161/rna.24454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J. , Phan, L. , Cuesta, R. , Carlson, B. A. , Pak, M. , Asano, K. , … Hinnebusch, A. G. (1998). The essential Gcd10p‐Gcd14p nuclear complex is required for 1‐methyladenosine modification and maturation of initiator methionyl‐tRNA. Genes & Development, 12(23), 3650–3662. 10.1101/gad.12.23.3650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries, O. , Mc Cafferty, S. , De Smedt, S. C. , Weiss, R. , Sanders, N. N. , & Kitada, T. (2015). N 1‐methylpseudouridine‐incorporated mRNA outperforms pseudouridine‐incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. Journal of Controlled Release: Official Journal of the Controlled Release Society, 217, 337–344. 10.1016/j.jconrel.2015.08.051 [DOI] [PubMed] [Google Scholar]
- Arango, D. , Sturgill, D. , Alhusaini, N. , Dillman, A. A. , Sweet, T. J. , Hanson, G. , … Oberdoerffer, S. (2018). Acetylation of cytidine in mRNA promotes translation efficiency. Cell, 175(7), 1872–1886.e24. 10.1016/j.cell.2018.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimbasseri, A. G. , Blewett, N. H. , Iben, J. R. , Lamichhane, T. N. , Cherkasova, V. , Hafner, M. , & Maraia, R. J. (2015). RNA polymerase III output is functionally linked to tRNA dimethyl‐G26 modification. PLoS Genetics, 11(12), e1005671. 10.1371/journal.pgen.1005671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengaud, J. , Urbonavicius, J. , Fernandez, B. , Chaussinand, G. , Bujnicki, J. M. , & Grosjean, H. (2004). N 2‐methylation of guanosine at position 10 in tRNA is catalyzed by a THUMP domain‐containing, S‐adenosylmethionine‐dependent methyltransferase, conserved in Archaea and Eukaryota. The Journal of Biological Chemistry, 279(35), 37142–37152. 10.1074/jbc.M403845200 [DOI] [PubMed] [Google Scholar]
- Armistead, J. , Khatkar, S. , Meyer, B. , Mark, B. L. , Patel, N. , Coghlan, G. , … Triggs‐Raine, B. (2009). Mutation of a gene essential for ribosome biogenesis, EMG1, causes Bowen‐Conradi syndrome. American Journal of Human Genetics, 84(6), 728–739. 10.1016/j.ajhg.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead, J. , Patel, N. , Wu, X. , Hemming, R. , Chowdhury, B. , Basra, G. S. , … Triggs‐Raine, B. (2015). Growth arrest in the ribosomopathy, Bowen‐Conradi syndrome, is due to dramatically reduced cell proliferation and a defect in mitotic progression. Biochimica et Biophysica Acta (BBA): Molecular Basis of Disease, 1852(5), 1029–1037. 10.1016/j.bbadis.2015.02.007 [DOI] [PubMed] [Google Scholar]
- Arragain, S. , Handelman, S. K. , Forouhar, F. , Wei, F.‐Y. , Tomizawa, K. , Hunt, J. F. , … Atta, M. (2010). Identification of eukaryotic and prokaryotic methylthiotransferase for biosynthesis of 2‐methylthio‐N 6‐threonylcarbamoyladenosine in tRNA. Journal of Biological Chemistry, 285(37), 28425–28433. 10.1074/jbc.M110.106831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondel, C. , Missoury, S. , Snoek, R. , Patat, J. , Menara, G. , Collinet, B. , … Mollet, G. (2019). Defects in t6A tRNA modification due to GON7 and YRDC mutations lead to Galloway‐Mowat syndrome. Nature Communications, 10(1), 3967. 10.1038/s41467-019-11951-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åström, S. U. , & Byström, A. S. (1994). Rit1, a tRNA backbone‐modifying enzyme that mediates initiator and elongator tRNA discrimination. Cell, 79(3), 535–546. 10.1016/0092-8674(94)90262-3 [DOI] [PubMed] [Google Scholar]
- Athanasiadis, A. , Rich, A. , & Maas, S. (2004). Widespread A‐to‐I RNA editing of Alu‐containing mRNAs in the human transcriptome. PLoS Biology, 2(12), e391. 10.1371/journal.pbio.0020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auxilien, S. , Keith, G. , Le Grice, S. F. , & Darlix, J. L. (1999). Role of post‐transcriptional modifications of primer tRNALys,3 in the fidelity and efficacy of plus strand DNA transfer during HIV‐1 reverse transcription. The Journal of Biological Chemistry, 274(7), 4412–4420. 10.1074/jbc.274.7.4412 [DOI] [PubMed] [Google Scholar]
- Auxilien, S. , Rasmussen, A. , Rose, S. , Brochier‐Armanet, C. , Husson, C. , Fourmy, D. , … Douthwaite, S. (2011). Specificity shifts in the rRNA and tRNA nucleotide targets of archaeal and bacterial m5U methyltransferases. RNA, 17(1), 45–53. 10.1261/rna.2323411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awai, T. , Kimura, S. , Tomikawa, C. , Ochi, A. , Ihsanawati Bessho, Y. , Yokoyama, S. , … Hori, H. (2009). Aquifex aeolicus tRNA (N 2,N 2‐guanine)‐dimethyltransferase (Trm1) catalyzes transfer of methyl groups not only to guanine 26 but also to guanine 27 in tRNA. Journal of Biological Chemistry, 284(31), 20467–20478. 10.1074/JBC.M109.020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayadi, L. , Galvanin, A. , Pichot, F. , Marchand, V. , & Motorin, Y. (2019). RNA ribose methylation (2′‐O‐methylation): Occurrence, biosynthesis and biological functions. Biochimica et Biophysica Acta (BBA): Gene Regulatory Mechanisms, 1862(3), 253–269. 10.1016/J.BBAGRM.2018.11.009 [DOI] [PubMed] [Google Scholar]
- Bacusmo, J. M. , Orsini, S. S. , Hu, J. , Demott, M. , Thiaville, P. C. , Elfarash, A. , … De Crécy‐Lagard, V. (2017). The t6A modification acts as a positive determinant for the anticodon nuclease PrrC, and is distinctively nonessential in Streptococcus mutans . RNA Biology, 15(4–5), 508–517. 10.1080/15476286.2017.1353861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer, R. J. , & Dubin, D. T. (1981). Methylated regions of hamster mitochondrial ribosomal RNA: Structural and functional correlates. Nucleic Acids Research, 9(2), 323–337. 10.1093/nar/9.2.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn, J. H. , Lee, J.‐H. , Li, G. , Greer, C. , Peng, G. , & Xiao, X. (2012). Accurate identification of A‐to‐I RNA editing in human by transcriptome sequencing. Genome Research, 22(1), 142–150. 10.1101/gr.124107.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Yaacov, D. , Frumkin, I. , Yashiro, Y. , Chujo, T. , Ishigami, Y. , Chemla, Y. , … Mishmar, D. (2016). Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biology, 14(9), e1002557. 10.1371/journal.pbio.1002557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar‐Yaacov, D. , Frumkin, I. , Yashiro, Y. , Chujo, T. , Ishigami, Y. , Chemla, Y. , … Mishmar, D. (2017). Correction: Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biology, 15(1), e1002594. 10.1371/journal.pbio.1002594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta‐Sanchez, M. , Temple, S. , Ansari, S. A. , D'Amico, A. , & Agris, P. F. (2016). Attomole quantification and global profile of RNA modifications: Epitranscriptome of human neural stem cells. Nucleic Acids Research, 44(3), e26. 10.1093/nar/gkv971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanta‐Sanchez, M. , Wang, R. , Liu, Z. , Ye, X. , Li, M. , Shi, X. , … Sheng, J. (2017). TET1‐mediated oxidation of 5‐formylcytosine (5fC) to 5‐carboxycytosine (5caC) in RNA. ChemBioChem: A European Journal of Chemical Biology, 18(1), 72–76. 10.1002/cbic.201600328 [DOI] [PubMed] [Google Scholar]
- Baskin, F. , & Dekker, C. A. (1967). A rapid and specific assay for sugar methylation in ribonucleic acid. The Journal of Biological Chemistry, 242(22), 5447–5449 http://www.ncbi.nlm.nih.gov/pubmed/4863753 [PubMed] [Google Scholar]
- Bass, B. L. , Nishikura, K. , Keller, W. , Seeburg, P. H. , Emeson, R. B. , O'Connell, M. A. , … Herbert, A. (1997). A standardized nomenclature for adenosine deaminases that act on RNA. RNA, 3(9), 947–949 http://www.ncbi.nlm.nih.gov/pubmed/9292492 [PMC free article] [PubMed] [Google Scholar]
- Bass, B. L. , & Weintraub, H. (1988). An unwinding activity that covalently modifies its double‐stranded RNA substrate. Cell, 55(6), 1089–1098. 10.1016/0092-8674(88)90253-X [DOI] [PubMed] [Google Scholar]
- Basturea, G. N. , Rudd, K. E. , & Deutscher, M. P. (2006). Identification and characterization of RsmE, the founding member of a new RNA base methyltransferase family. RNA, 12(3), 426–434. 10.1261/rna.2283106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten, S. , Bryant, J. M. , Sinha, A. , Reyser, T. , Preiser, P. R. , Dedon, P. C. , & Scherf, A. (2019). Transcriptome‐wide dynamics of extensive m6A mRNA methylation during Plasmodium falciparum blood‐stage development. Nature Microbiology, 4(12), 2246–2259. 10.1038/s41564-019-0521-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez‐Páez, A. , Villarroya, M. , & Armengod, M.‐E. (2012). The Escherichia coli RlmN methyltransferase is a dual‐specificity enzyme that modifies both rRNA and tRNA and controls translational accuracy. RNA, 18(10), 1783–1795. 10.1261/rna.033266.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benne, R. (1994). RNA editing in trypanosomes. European Journal of Biochemistry, 221(1), 9–23. 10.1111/j.1432-1033.1994.tb18710.x [DOI] [PubMed] [Google Scholar]
- Bennett, C. F. , Krainer, A. R. , & Cleveland, D. W. (2019). Antisense oligonucleotide therapies for neurodegenerative diseases. Annual Review of Neuroscience, 42(1), 385–406. 10.1146/annurev-neuro-070918-050501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz, L. I. , Goldberg, D. E. , & Irwin, N. (2002). Inosine stimulates axon growth in vitro and in the adult CNS. Progress in Brain Research, 137, 389–399. 10.1016/s0079-6123(02)37030-4 [DOI] [PubMed] [Google Scholar]
- Berger, F. , Ramírez‐Hernández, M. H. , & Ziegler, M. (2004). The new life of a centenarian: Signalling functions of NAD(P). Trends in Biochemical Sciences, 29(3), 111–118. 10.1016/j.tibs.2004.01.007 [DOI] [PubMed] [Google Scholar]
- Bird, J. G. , Zhang, Y. , Tian, Y. , Panova, N. , Barvík, I. , Greene, L. , … Nickels, B. E. (2016). The mechanism of RNA 5′ capping with NAD+, NADH and desphospho‐CoA. Nature, 535(7612), 444–447. 10.1038/nature18622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop, A. C. , Xu, J. , Johnson, R. C. , Schimmel, P. , & de Crécy‐Lagard, V. (2002). Identification of the tRNA‐dihydrouridine synthase family. The Journal of Biological Chemistry, 277(28), 25090–25095. 10.1074/jbc.M203208200 [DOI] [PubMed] [Google Scholar]
- Björk, G. R. , Jacobsson, K. , Nilsson, K. , Johansson, M. J. , Byström, A. S. , & Persson, O. P. (2001). A primordial tRNA modification required for the evolution of life? The EMBO Journal, 20(1–2), 231–239. 10.1093/emboj/20.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G. R. , & Neidhardt, F. C. (1975). Physiological and biochemical studies on the function of 5‐methyluridine in the transfer ribonucleic acid of Escherichia coli . Journal of Bacteriology, 124(1), 99–111 http://www.ncbi.nlm.nih.gov/pubmed/1100618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G. R. , & Hagervall, T. G. (2014). Transfer RNA modification: Presence, synthesis, and function. EcoSal Plus, 6(1). 10.1128/ecosalplus.ESP-0007-2013, [DOI] [PubMed] [Google Scholar]
- Björkbom, A. , Lelyveld, V. S. , Zhang, S. , Zhang, W. , Tam, C. P. , Blain, J. C. , & Szostak, J. W. (2015). Bidirectional direct sequencing of noncanonical RNA by two‐dimensional analysis of mass chromatograms. Journal of the American Chemical Society, 137(45), 14430–14438. 10.1021/jacs.5b09438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, K. A. , & Dos Santos, P. C. (2015). Abbreviated pathway for biosynthesis of 2‐thiouridine in Bacillus subtilis . Journal of Bacteriology, 197(11), 1952–1962. 10.1128/JB.02625-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccaletto, P. , Machnicka, M. A. , Purta, E. , Piątkowski, P. , Bagiński, B. , Wirecki, T. K. , … Bujnicki, J. M. (2018). MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Research, 46(D1), D303–D307. 10.1093/nar/gkx1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokar, J. A. , Shambaugh, M. E. , Polayes, D. , Matera, A. G. , & Rottman, F. M. (1997). Purification and cDNA cloning of the AdoMet‐binding subunit of the human mRNA (N 6‐adenosine)‐methyltransferase. RNA, 3(11), 1233–1247 http://www.ncbi.nlm.nih.gov/pubmed/9409616 [PMC free article] [PubMed] [Google Scholar]
- Boulias, K. , Toczydłowska‐Socha, D. , Hawley, B. R. , Liberman, N. , Takashima, K. , Zaccara, S. , … Greer, E. L. (2019). Identification of the m6Am methyltransferase PCIF1 reveals the location and functions of m6Am in the transcriptome. Molecular Cell, 75(3), 631–643.e8. 10.1016/J.MOLCEL.2019.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou‐Nader, C. , Barraud, P. , Pecqueur, L. , Pérez, J. , Velours, C. , Shepard, W. , … Hamdane, D. (2019). Molecular basis for transfer RNA recognition by the double‐stranded RNA‐binding domain of human dihydrouridine synthase 2. Nucleic Acids Research, 47(6), 3117–3126. 10.1093/nar/gky1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou‐Nader, C. , Montémont, H. , Guérineau, V. , Jean‐Jean, O. , Brégeon, D. , & Hamdane, D. (2018). Unveiling structural and functional divergences of bacterial tRNA dihydrouridine synthases: Perspectives on the evolution scenario. Nucleic Acids Research, 46(3), 1386–1394. 10.1093/nar/gkx1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen, P. , & Conradi, G. J. (1976). Syndrome of skeletal and genitourinary anomalies with unusual facies and failure to thrive in Hutterite sibs. Birth Defects Original Article Series, 12(6), 101–108 http://www.ncbi.nlm.nih.gov/pubmed/974244 [PubMed] [Google Scholar]
- Boyer, P. D. , Krebs, E. G. , Sigman, D. S. , Tamanoi, F. , Dalbey, R. E. , Hackney, D. D. , … Luan, S. (1970). The enzymes. Academic Press; https://www.sciencedirect.com/bookseries/the-enzymes/vol/14 [Google Scholar]
- Brand, R. C. , Klootwijk, J. , Planta, R. J. , & Maden, B. E. (1978). Biosynthesis of a hypermodified nucleotide in Saccharomyces carlsbergensis 17S and HeLa‐cell 18S ribosomal ribonucleic acid. The Biochemical Journal, 169(1), 71–77. 10.1042/bj1690071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun, D. A. , Rao, J. , Mollet, G. , Schapiro, D. , Daugeron, M. C. , Tan, W. , … Hildebrandt, F. (2017). Mutations in KEOPS‐complex genes cause nephritic syndrome with primary microcephaly. Nature Genetics, 49(10), 1529–1538. 10.1038/ng.3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, A. M. , Hoopes, S. L. , White, R. H. , & Sarisky, C. A. (2011). Purine biosynthesis in archaea: Variations on a theme. Biology Direct, 6(1), 63. 10.1186/1745-6150-6-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, M. , & Griffiths, E. (1982). Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli . Nucleic Acids Research, 10(8), 2609–2624. 10.1093/nar/10.8.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujnicki, J. M. , Oudjama, Y. , Roovers, M. , Owczarek, S. , Caillet, J. , & Droogmans, L. (2004). Identification of a bifunctional enzyme MnmC involved in the biosynthesis of a hypermodified uridine in the wobble position of tRNA. RNA, 10(8), 1236–1242. 10.1261/rna.7470904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakovsky, D. E. , Prokhorova, I. V. , Sergiev, P. V. , Milón, P. , Sergeeva, O. V. , Bogdanov, A. A. , … Dontsova, O. A. (2012). Impact of methylations of m2G966/m5C967 in 16S rRNA on bacterial fitness and translation initiation. Nucleic Acids Research, 40(16), 7885–7895. 10.1093/nar/gks508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger, K. , Mühl, B. , Kellner, M. , Rohrmoser, M. , Gruber‐Eber, A. , Windhager, L. , … Eick, D. (2013). 4‐Thiouridine inhibits rRNA synthesis and causes a nucleolar stress response. RNA Biology, 10(10), 1623–1630. 10.4161/rna.26214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns, C. M. , Chu, H. , Rueter, S. M. , Hutchinson, L. K. , Canton, H. , Sanders‐Bush, E. , & Emeson, R. B. (1997). Regulation of serotonin‐2C receptor G‐protein coupling by RNA editing. Nature, 387(6630), 303–308. 10.1038/387303a0 [DOI] [PubMed] [Google Scholar]
- Bykhovskaya, Y. , Casas, K. , Mengesha, E. , Inbal, A. , & Fischel‐Ghodsian, N. (2004). Missense mutation in pseudouridine synthase 1 (PUS1) causes mitochondrial myopathy and sideroblastic anemia (MLASA). American Journal of Human Genetics, 74(6), 1303–1308. 10.1086/421530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaya, Y. , Mengesha, E. , Wang, D. , Yang, H. , Estivill, X. , Shohat, M. , & Fischel‐Ghodsian, N. (2004). Phenotype of non‐syndromic deafness associated with the mitochondrial A1555G mutation is modulated by mitochondrial RNA modifying enzymes MTO1 and GTPBP3. Molecular Genetics and Metabolism, 83(3), 199–206. 10.1016/J.YMGME.2004.07.009 [DOI] [PubMed] [Google Scholar]
- Caballero, V. C. , Toledo, V. P. , Maturana, C. , Fisher, C. R. , Payne, S. M. , & Salazar, J. (2012). Expression of Shigella flexneri gluQ‐rs gene is linked to dksA and controlled by a transcriptional terminator. BMC Microbiology, 12(1), 226. 10.1186/1471-2180-12-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahová, H. , Winz, M. L. , Höfer, K. , Nübel, G. , & Jäschke, A. (2015). NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature, 519(7543), 374–377. 10.1038/nature14020 [DOI] [PubMed] [Google Scholar]
- Campanacci, V. , Dubois, D. Y. , Becker, H. D. , Kern, D. , Spinelli, S. , Valencia, C. , … Cambillau, C. (2004). The Escherichia coli YadB gene product reveals a novel aminoacyl‐tRNA synthetase like activity. Journal of Molecular Biology, 337(2), 273–283. 10.1016/j.jmb.2004.01.027 [DOI] [PubMed] [Google Scholar]
- Cantara, W. A. , Bilbille, Y. , Kim, J. , Kaiser, R. , Leszczyńska, G. , Malkiewicz, A. , & Agris, P. F. (2012). Modifications modulate anticodon loop dynamics and codon recognition of E. coli tRNAArg1,2 . Journal of Molecular Biology, 416(4), 579–597. 10.1016/j.jmb.2011.12.054 [DOI] [PubMed] [Google Scholar]
- Cantara, W. A. , Crain, P. F. , Rozenski, J. , McCloskey, J. A. , Harris, K. A. , Zhang, X. , … Agris, P. F. (2011). The RNA modification database, RNAMDB: 2011 update. Nucleic Acids Research, 39(Database), D195–D201. 10.1093/nar/gkq1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile, T. M. , Rojas‐Duran, M. F. , Zinshteyn, B. , Shin, H. , Bartoli, K. M. , & Gilbert, W. V. (2014). Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature, 515(7525), 143–146. 10.1038/nature13802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter, J.‐M. , Emmett, W. , Mozos, I. R. , Kotter, A. , Helm, M. , Ule, J. , & Hussain, S. (2019). FICC‐Seq: A method for enzyme‐specified profiling of methyl‐5‐uridine in cellular RNA. Nucleic Acids Research, 47(19), e113. 10.1093/nar/gkz658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čavužić, M. , & Liu, Y. (2017). Biosynthesis of sulfur‐containing tRNA modifications: A comparison of bacterial, archaeal, and eukaryotic pathways. Biomolecules, 7(1), 27. 10.3390/biom7010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C. T. Y. , Chionh, Y. H. , Ho, C.‐H. , Lim, K. S. , Babu, I. R. , Ang, E. , … Dedon, P. C. (2011). Identification of N 6,N 6‐dimethyladenosine in transfer RNA from Mycobacterium bovis Bacille Calmette‐Guérin. Molecules, 16(6), 5168–5181. 10.3390/molecules16065168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, C. T. Y. , Dyavaiah, M. , DeMott, M. S. , Taghizadeh, K. , Dedon, P. C. , & Begley, T. J. (2010). A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genetics, 6(12), e1001247. 10.1371/journal.pgen.1001247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y.‐H. , Nishimura, S. , Oishi, H. , Kelly, V. P. , Kuno, A. , & Takahashi, S. (2019). TRMT2A is a novel cell cycle regulator that suppresses cell proliferation. Biochemical and Biophysical Research Communications, 508(2), 410–415. 10.1016/j.bbrc.2018.11.104 [DOI] [PubMed] [Google Scholar]
- Charette, M. , & Gray, M. W. (2000). Pseudouridine in RNA: What, where, how, and why. IUBMB Life (International Union of Biochemistry and Molecular Biology: Life), 49(5), 341–351. 10.1080/152165400410182 [DOI] [PubMed] [Google Scholar]
- Chatterjee, K. , Blaby, I. K. , Thiaville, P. C. , Majumder, M. , Grosjean, H. , Yuan, Y. A. , … de Crécy‐Lagard, V. (2012). The archaeal COG1901/DUF358 SPOUT‐methyltransferase members, together with pseudouridine synthase Pus10, catalyze the formation of 1‐methylpseudouridine at position 54 of tRNA. RNA, 18(3), 421–433. 10.1261/rna.030841.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. , Huang, B. , Anderson, J. T. , & Byström, A. S. (2011). Unexpected accumulation of ncm5U and ncm5s2U in a trm9 mutant suggests an additional step in the synthesis of mcm5U and mcm5s2U. PLoS One, 6(6), e20783. 10.1371/journal.pone.0020783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. X. , Cho, D. S. , Wang, Q. , Lai, F. , Carter, K. C. , & Nishikura, K. (2000). A third member of the RNA‐specific adenosine deaminase gene family, ADAR3, contains both single‐ and double‐stranded RNA binding domains. RNA, 6(5), 755–767. 10.1017/s1355838200000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. , Yang, H. , Feng, P. , Ding, H. , & Lin, H. (2017). iDNA4mC: Identifying DNA N 4‐methylcytosine sites based on nucleotide chemical properties. Bioinformatics, 33(22), 3518–3523. 10.1093/bioinformatics/btx479 [DOI] [PubMed] [Google Scholar]
- Chen, X.‐Y. , Zhang, J. , & Zhu, J.‐S. (2019). The role of m6A RNA methylation in human cancer. Molecular Cancer, 18(1), 103. 10.1186/s12943-019-1033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. G. , Kowtoniuk, W. E. , Agarwal, I. , Shen, Y. , & Liu, D. R. (2009). LC/MS analysis of cellular RNA reveals NAD‐linked RNA. Nature Chemical Biology, 5(12), 879–881. 10.1038/nchembio.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Q.‐Y. , Xiong, J. , Ma, C.‐J. , Dai, Y. , Ding, J.‐H. , Liu, F.‐L. , … Feng, Y.‐Q. (2020). Chemical tagging for sensitive determination of uridine modifications in RNA. Chemical Science, 11(7), 1878–1891. 10.1039/c9sc05094a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda, G. B. , Tworek, H. A. , Bhargava, A. K. , Rachlin, E. , Dutta, S. P. , & Patrzyc, H. B. (1988). Isolation and characterization of 3‐(3‐amino‐3‐carboxypropyl)uridine from human urine. Nucleosides and Nucleotides, 7(4), 417–429. 10.1080/07328318808075388 [DOI] [Google Scholar]
- Choe, J. , Lin, S. , Zhang, W. , Liu, Q. , Wang, L. , Ramirez‐Moya, J. , … Gregory, R. I. (2018). mRNA circularization by METTL3–eIF3h enhances translation and promotes oncogenesis. Nature, 561(7724), 556–560. 10.1038/s41586-018-0538-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, H.‐J. , Donnard, E. , Gustafsson, H. T. , Garber, M. , & Rando, O. J. (2017). Transcriptome‐wide analysis of roles for tRNA modifications in translational regulation. Molecular Cell, 68(5), 978–992.e4. 10.1016/j.molcel.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chui, H. M.‐P. , Desaulniers, J.‐P. , Scaringe, S. A. , & Chow, C. S. (2002). Synthesis of helix 69 of Escherichia coli 23S rRNA containing its natural modified nucleosides, m3Ψ and Ψ. The Journal of Organic Chemistry, 67(25), 8847–8854. 10.1021/jo026364m [DOI] [PubMed] [Google Scholar]
- Cohn, W. E. , & Volkin, E. (1951). Nucleoside‐5′‐phosphates from ribonucleic acid. Nature, 167(4247), 483–484. 10.1038/167483a0 [DOI] [Google Scholar]
- Courtney, D. G. , Tsai, K. , Bogerd, H. P. , Kennedy, E. M. , Law, B. A. , Emery, A. , … Cullen, B. R. (2019). Epitranscriptomic addition of m5C to HIV‐1 transcripts regulates viral gene expression. Cell Host & Microbe, 26(2), 217–227.e6. 10.1016/j.chom.2019.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozen, A. E. , Quartley, E. , Holmes, A. D. , Hrabeta‐Robinson, E. , Phizicky, E. M. , & Lowe, T. M. (2015). ARM‐seq: AlkB‐facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nature Methods, 12(9), 879–884. 10.1038/nmeth.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain, P. F. , Aflonzo, J. D. , Rozenski, J. , Kapushoc, S. T. , McCloskey, J. A. , & Simpson, L. (2002). Modification of the universally unmodified uridine‐33 in a mitochondria‐imported edited tRNA and the role of the anticodon arm structure on editing efficiency. RNA, 8(6), S1355838202022045. 10.1017/S1355838202022045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick, F. H. C. (1966). Codon–anticodon pairing: The wobble hypothesis. Journal of Molecular Biology, 19(2), 548–555. 10.1016/S0022-2836(66)80022-0 [DOI] [PubMed] [Google Scholar]
- Crick, F. H. C. (1968). The origin of the genetic code. Journal of Molecular Biology, 38(3), 367–379. 10.1016/0022-2836(68)90392-6 [DOI] [PubMed] [Google Scholar]
- Cui, X. , Liang, Z. , Shen, L. , Zhang, Q. , Bao, S. , Geng, Y. , … Yu, H. (2017). 5‐Methylcytosine RNA methylation in Arabidopsis thaliana . Molecular Plant, 10(11), 1387–1399. 10.1016/j.molp.2017.09.013 [DOI] [PubMed] [Google Scholar]
- Dai, Q. , Moshitch‐Moshkovitz, S. , Han, D. , Kol, N. , Amariglio, N. , Rechavi, G. , … He, C. (2017). Nm‐seq maps 2′‐O‐methylation sites in human mRNA with base precision. Nature Methods, 14(7), 695–698. 10.1038/nmeth.4294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Magro, C. , Keller, P. , Kotter, A. , Werner, S. , Duarte, V. , Marchand, V. , … Helm, M. (2018). A vastly increased chemical variety of RNA modifications containing a thioacetal structure. Angewandte Chemie International Edition, 57(26), 7893–7897. 10.1002/anie.201713188 [DOI] [PubMed] [Google Scholar]
- Dalluge, J. , Hashizume, T. , Sopchik, A. E. , McCloskey, J. A. , & Davis, D. R. (1996). Conformational flexibility in RNA: The role of dihydrouridine. Nucleic Acids Research, 24(6), 1073–1079. 10.1093/nar/24.6.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalluge, J. J. , Hamamoto, T. , Horikoshi, K. , Morita, R. Y. , Stetter, K. O. , & McCloskey, J. A. (1997). Posttranscriptional modification of tRNA in psychrophilic bacteria. Journal of Bacteriology, 179(6), 1918–1923. 10.1128/jb.179.6.1918-1923.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danan‐Gotthold, M. , & Levanon, E. Y. (2017). Promoting RNA editing by ADAR attraction. Genome Biology, 18(1), 196. 10.1186/s13059-017-1343-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell, R. B. , Ke, S. , & Darnell, J. E. (2018). Pre‐mRNA processing includes N 6 methylation of adenosine residues that are retained in mRNA exons and the fallacy of “RNA epigenetics”. RNA, 24(3), 262–267. 10.1261/rna.065219.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassano, A. , Mancuso, M. , Giardullo, P. , De Cecco, L. , Ciuffreda, P. , Santaniello, E. , … Colombo, F. (2014). N 6‐isopentenyladenosine and analogs activate the NRF2‐mediated antioxidant response. Redox Biology, 2, 580–589. 10.1016/J.REDOX.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo, M. , Recinos, C. , Yanez, G. , Pomerantz, S. C. , Guymon, R. , Crain, P. F. , … Ofengand, J. (2005). Number, position, and significance of the pseudouridines in the large subunit ribosomal RNA of Haloarcula marismortui and Deinococcus radiodurans . RNA, 11(2), 210–219. 10.1261/rna.7209905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte, B. , Wang, F. , Ngoc, L. V. , Collignon, E. , Bonvin, E. , Deplus, R. , … Fuks, F. (2016). RNA biochemistry. Transcriptome‐wide distribution and function of RNA hydroxymethylcytosine. Science, 351(6270), 282–285. 10.1126/science.aac5253 [DOI] [PubMed] [Google Scholar]
- Dellafiore, M. A. , Montserrat, J. M. , & Iribarren, A. M. (2016). Modified nucleoside triphosphates for in‐vitro selection techniques. Frontiers in Chemistry, 4, 18. 10.3389/fchem.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci, H. , Murphy, F. , Belardinelli, R. , Kelley, A. C. , Ramakrishnan, V. , Gregory, S. T. , … Jogl, G. (2010). Modification of 16S ribosomal RNA by the KsgA methyltransferase restructures the 30S subunit to optimize ribosome function. RNA, 16(12), 2319–2324. 10.1261/rna.2357210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, X. , Chen, K. , Luo, G.‐Z. , Weng, X. , Ji, Q. , Zhou, T. , & He, C. (2015). Widespread occurrence of N 6‐methyladenosine in bacterial mRNA. Nucleic Acids Research, 43(13), 6557–6567. 10.1093/NAR/GKV596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrès, J. , Keith, G. , Kuo, K. C. , & Gehrke, C. W. (1989). Presence of phosphorylated O‐ribosyl‐adenosine in T‐psi‐stem of yeast methionine initiator tRNA. Nucleic Acids Research, 17(3), 865–882. 10.1093/nar/17.3.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewe, J. M. , Fuller, B. L. , Lentini, J. M. , Kellner, S. M. , & Fu, D. (2017). TRMT1‐catalyzed tRNA modifications are required for redox homeostasis to ensure proper cellular proliferation and oxidative stress survival. Molecular and Cellular Biology, 37(21). 10.1128/MCB.00214-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewez, M. , Bauer, F. , Dieu, M. , Raes, M. , Vandenhaute, J. , & Hermand, D. (2008). The conserved Wobble uridine tRNA thiolase Ctu1‐Ctu2 is required to maintain genome integrity. Proceedings of the National Academy of Sciences of the United States of America, 105(14), 5459–5464. 10.1073/pnas.0709404105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominissini, D. , Moshitch‐Moshkovitz, S. , Schwartz, S. , Salmon‐Divon, M. , Ungar, L. , Osenberg, S. , … Rechavi, G. (2012). Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature, 485(7397), 201–206. 10.1038/nature11112 [DOI] [PubMed] [Google Scholar]
- Dominissini, D. , Nachtergaele, S. , Moshitch‐Moshkovitz, S. , Peer, E. , Kol, N. , Ben‐Haim, M. S. , … He, C. (2016). The dynamic N 1‐methyladenosine methylome in eukaryotic messenger RNA. Nature, 530(7591), 441–446. 10.1038/nature16998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn, L. E. , Lasman, L. , Chen, J. , Xu, X. , Hund, T. J. , Medvedovic, M. , … Accornero, F. (2019). The N 6‐methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation, 139(4), 533–545. 10.1161/CIRCULATIONAHA.118.036146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans, L. , Roovers, M. , Bujnicki, J. M. , Tricot, C. , Hartsch, T. , Stalon, V. , & Grosjean, H. (2003). Cloning and characterization of tRNA (m1A58) methyltransferase (TrmI) from Thermus thermophilus HB27, a protein required for cell growth at extreme temperatures. Nucleic Acids Research, 31(8), 2148–2156. 10.1093/nar/gkg314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, H. , Zhao, Y. , He, J. , Zhang, Y. , Xi, H. , Liu, M. , … Wu, L. (2016). YTHDF2 destabilizes m6A‐containing RNA through direct recruitment of the CCR4‐NOT deadenylase complex. Nature Communications, 7(1), 12626. 10.1038/ncomms12626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, K. , Zhang, L. , Lee, T. , & Sun, T. (2019). m6A RNA methylation controls neural development and is involved in human diseases. Molecular Neurobiology, 56(3), 1596–1606. 10.1007/s12035-018-1138-1 [DOI] [PubMed] [Google Scholar]
- Dubin, D. (1974). Methylated nucleotide content of mitochondrial ribosomal RNA from hamster cells. Journal of Molecular Biology, 84(2), 257–273. 10.1016/0022-2836(74)90584-1 [DOI] [PubMed] [Google Scholar]
- Dubin, D. T. , & Taylor, R. H. (1975). The methylation state of poly A‐containing messenger RNA from cultured hamster cells. Nucleic Acids Research, 2(10), 1653–1668. 10.1093/nar/2.10.1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumelin, C. E. , Chen, Y. , Leconte, A. M. , Chen, Y. G. , & Liu, D. R. (2012). Discovery and biological characterization of geranylated RNA in bacteria. Nature Chemical Biology, 8(11), 913–919. 10.1038/nchembio.1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, D. B. (1960). The isolation of 5‐methylcytidine from RNA. Biochimica et Biophysica Acta, 38, 176–178. 10.1016/0006-3002(60)91219-1 [DOI] [PubMed] [Google Scholar]
- Durand, J. M. B. , Dagberg, B. , Uhlin, B. E. , & Bjork, G. R. (2000). Transfer RNA modification, temperature and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: The expression of the virF gene. Molecular Microbiology, 35(4), 924–935. 10.1046/j.1365-2958.2000.01767.x [DOI] [PubMed] [Google Scholar]
- Edelheit, S. , Schwartz, S. , Mumbach, M. R. , Wurtzel, O. , & Sorek, R. (2013). Transcriptome‐wide mapping of 5‐methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs. PLoS Genetics, 9(6), e1003602. 10.1371/journal.pgen.1003602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds, C. G. , Crain, P. F. , Gupta, R. , Hashizume, T. , Hocart, C. H. , Kowalak, J. A. , … McCloskey, J. A. (1991). Posttranscriptional modification of tRNA in thermophilic archaea (Archaebacteria). Journal of Bacteriology, 173(10), 3138–3148. 10.1128/jb.173.10.3138-3148.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvardson, S. , Prunetti, L. , Arraf, A. , Haas, D. , Bacusmo, J. M. , Hu, J. F. , … Elpeleg, O. (2017). tRNA N 6‐adenosine threonylcarbamoyltransferase defect due to KAE1/TCS3 (OSGEP) mutation manifest by neurodegeneration and renal tubulopathy. European Journal of Human Genetics, 25(5), 545–551. 10.1038/ejhg.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimtseva, E. V. , Kulikova, I. V. , & Mikhaĭlov, S. N. (2009). [Disaccharide nucleosides, an important group of natural compounds]. Molekuliarnaia Biologiia, 43(2), 327–338 http://www.ncbi.nlm.nih.gov/pubmed/19425500 [PubMed] [Google Scholar]
- Egert, E. , Lindner, H. J. , Hillen, W. , & Boehm, M. C. (1980). Influence of substituents at the 5 position on the structure of uridine. Journal of the American Chemical Society, 102(11), 3707–3713. 10.1021/ja00531a007 [DOI] [Google Scholar]
- Ehrenhofer‐Murray, A. E. (2017). Cross‐talk between Dnmt2‐dependent tRNA methylation and queuosine modification. Biomolecules, 7(1). 10.3390/biom7010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg, S. P. , Yarus, M. , & Soll, L. (1979). The effect of an Escherichia coli regulatory mutation on transfer RNA structure. Journal of Molecular Biology, 135(1), 111–126. 10.1016/0022-2836(79)90343-7 [DOI] [PubMed] [Google Scholar]
- Elliott, M. S. , Katze, J. R. , & Trewyn, R. W. (1984). Relationship between a tumor promoter‐induced decrease in queuine modification of transfer RNA in normal human cells and the expression of an altered cell phenotype. Cancer Research, 44(8), 3215–3219 http://www.ncbi.nlm.nih.gov/pubmed/6589040 [PubMed] [Google Scholar]
- Emmerechts, G. , Barbé, S. , Herdewijn, P. , Anné, J. , & Rozenski, J. (2007). Post‐transcriptional modification mapping in the Clostridium acetobutylicum 16S rRNA by mass spectrometry and reverse transcriptase assays. Nucleic Acids Research, 35(10), 3494–3503. 10.1093/nar/gkm248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erales, J. , Marchand, V. , Panthu, B. , Gillot, S. , Belin, S. , Ghayad, S. E. , … Diaz, J.‐J. (2017). Evidence for rRNA 2′‐O‐methylation plasticity: Control of intrinsic translational capabilities of human ribosomes. Proceedings of the National Academy of Sciences of the United States of America, 114(49), 12934–12939. 10.1073/pnas.1707674114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ero, R. , Peil, L. , Liiv, A. , & Remme, J. (2008). Identification of pseudouridine methyltransferase in Escherichia coli . RNA, 14(10), 2223–2233. 10.1261/rna.1186608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler, D. E. , Franco, M. K. , Batool, Z. , Wu, M. Z. , Dubuke, M. L. , Dobosz‐Bartoszek, M. , … Koutmou, K. S. (2019). Pseudouridinylation of mRNA coding sequences alters translation. Proceedings of the National Academy of Sciences of the United States of America., 116, 23068–23074. 10.1073/pnas.1821754116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakruddin, M. , Wei, F.‐Y. , Suzuki, T. , Asano, K. , Kaieda, T. , Omori, A. , … Tomizawa, K. (2018). Defective mitochondrial tRNA taurine modification activates global proteostress and leads to mitochondrial disease. Cell Reports, 22(2), 482–496. 10.1016/j.celrep.2017.12.051 [DOI] [PubMed] [Google Scholar]
- Favre, A. , Yaniv, M. , & Michels, A. M. (1969). The photochemistry of 4‐thiouridine in Escherichia coli t‐RNAVal1 . Biochemical and Biophysical Research Communications, 37(2), 266–271. 10.1016/0006-291X(69)90729-3 [DOI] [PubMed] [Google Scholar]
- Fedeles, B. I. , Singh, V. , Delaney, J. C. , Li, D. , & Essigmann, J. M. (2015). The AlkB family of Fe(II)/α‐ketoglutarate‐dependent dioxygenases: Repairing nucleic acid alkylation damage and beyond. Journal of Biological Chemistry, 290(34), 20734–20742. 10.1074/jbc.R115.656462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergus, C. , Barnes, D. , Alqasem, M. A. , & Kelly, V. P. (2015). The queuine micronutrient: Charting a course from microbe to man. Nutrients, 7(4), 2897–2929. 10.3390/nu7042897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzell, K. , Xu, L.‐D. , Lagergren, J. , & Öhman, M. (2018). ADARs and editing: The role of A‐to‐I RNA modification in cancer progression. Seminars in Cell & Developmental Biology, 79, 123–130. 10.1016/j.semcdb.2017.11.018 [DOI] [PubMed] [Google Scholar]
- Fu, Y. , Jia, G. , Pang, X. , Wang, R. N. , Wang, X. , Li, C. J. , … He, C. (2013). FTO‐mediated formation of N 6‐hydroxymethyladenosine and N 6‐formyladenosine in mammalian RNA. Nature Communications, 4(1), 1798. 10.1038/ncomms2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk, C. , Merritt, A. J. , & Ehrlich, A. (1952). The isolation of hydrouracil from beef spleen. Archives of Biochemistry and Biophysics, 35(2), 468–469. 10.1016/S0003-9861(52)80029-3 [DOI] [PubMed] [Google Scholar]
- Gabant, G. , Auxilien, S. , Tuszynska, I. , Locard, M. , Gajda, M. J. , Chaussinand, G. , … Armengaud, J. (2006). THUMP from archaeal tRNA:m2 2G10 methyltransferase, a genuine autonomously folding domain. Nucleic Acids Research, 34(9), 2483–2494. 10.1093/nar/gkl145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway, A. , & Cowling, V. H. (2019). mRNA cap regulation in mammalian cell function and fate. Biochimica et Biophysica Acta: Gene Regulatory Mechanisms, 1862(3), 270–279. 10.1016/j.bbagrm.2018.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot, P. , Bortolin, M. L. , & Kiss, T. (1997). Site‐specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell, 89(5), 799–809. 10.1016/s0092-8674(00)80263-9 [DOI] [PubMed] [Google Scholar]
- Gerber, A. , Grosjean, H. , Melcher, T. , & Keller, W. (1998). Tad1p, a yeast tRNA‐specific adenosine deaminase, is related to the mammalian pre‐mRNA editing enzymes ADAR1 and ADAR2. The EMBO Journal, 17(16), 4780–4789. 10.1093/emboj/17.16.4780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, A. P. , & Keller, W. (1999). An adenosine deaminase that generates inosine at the Wobble position of tRNAs. Science, 286(5442), 1146–1149. 10.1126/science.286.5442.1146 [DOI] [PubMed] [Google Scholar]
- Giessing, A. M. B. , Jensen, S. S. , Rasmussen, A. , Hansen, L. H. , Gondela, A. , Long, K. , … Kirpekar, F. (2009). Identification of 8‐methyladenosine as the modification catalyzed by the radical SAM methyltransferase Cfr that confers antibiotic resistance in bacteria. RNA, 15(2), 327–336. 10.1261/rna.1371409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser, A. L. , Desgres, J. , Heitzler, J. , Gehrke, C. W. , & Keith, G. (1991). O‐ribosyl‐phosphate purine as a constant modified nucleotide located at position 64 in cytoplasmic initiator tRNAsMet of yeasts. Nucleic Acids Research, 19(19), 5199–5203. 10.1093/nar/19.19.5199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein, B. , Agranat‐Tamir, L. , Light, D. , Ben‐Naim Zgayer, O. , Fishman, A. , & Lamm, A. T. (2017). A‐to‐I RNA editing promotes developmental stage‐specific gene and lncRNA expression. Genome Research, 27(3), 462–470. 10.1101/gr.211169.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina, A. Y. , Dzama, M. M. , Osterman, I. A. , Sergiev, P. V. , Serebryakova, M. V. , Bogdanov, A. A. , & Dontsova, O. A. (2012). The last rRNA methyltransferase of E. coli revealed: The yhiR gene encodes adenine‐N 6 methyltransferase specific for modification of A2030 of 23S ribosomal RNA. RNA, 18(9), 1725–1734. 10.1261/rna.034207.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina, A. Y. , Sergiev, P. V. , Golovin, A. V. , Serebryakova, M. V. , Demina, I. , Govorun, V. M. , & Dontsova, O. A. (2009). The yfiC gene of E. coli encodes an adenine‐N 6 methyltransferase that specifically modifies A37 of tRNA1 Val(cmo5UAC). RNA, 15(6), 1134–1141. 10.1261/rna.1494409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi, H. , Nguyen, H. C. B. , Zhang, S. , Dill, B. D. , Molina, H. , & Tavazoie, S. F. (2016). Modulated expression of specific tRNAs drives gene expression and cancer progression. Cell, 165(6), 1416–1427. 10.1016/J.CELL.2016.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto‐Ito, S. , Ito, T. , & Yokoyama, S. (2017). Trm5 and TrmD: Two enzymes from distinct origins catalyze the identical tRNA modification, m1G37. Biomolecules, 7(1), 32. 10.3390/biom7010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupner, M. , Xu, H. , & White, R. H. (2002). New class of IMP cyclohydrolases in Methanococcus jannaschii . Journal of Bacteriology, 184(5), 1471–1473. 10.1128/jb.184.5.1471-1473.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, H. , Auxilien, S. , Constantinesco, F. , Simon, C. , Corda, Y. , Becker, H. F. , … Fourrey, J. L. (1996). Enzymatic conversion of adenosine to inosine and to N 1‐methylinosine in transfer RNAs: A review. Biochimie, 78(6), 488–501. 10.1016/0300-9084(96)84755-9 [DOI] [PubMed] [Google Scholar]
- Grosjean, H. , Constantinesco, F. , Foiret, D. , & Benachenhou, N. (1995). A novel enzymatic pathway leading to 1‐methylinosine modification in Haloferax volcanii tRNA. Nucleic Acids Research, 23(21), 4312–4319. 10.1093/nar/23.21.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, H. , & Benne, R. (1998). In Grosjean H. & Benne R. (Eds.), Modification and editing of RNA. ASM Press. [Google Scholar]
- Grosjean, H. , Breton, M. , Sirand‐Pugnet, P. , Tardy, F. , Thiaucourt, F. , Citti, C. , … Blanchard, A. (2014). Predicting the minimal translation apparatus: Lessons from the reductive evolution of Mollicutes. PLoS Genetics, 10(5), e1004363. 10.1371/journal.pgen.1004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozhik, A. V. , & Jaffrey, S. R. (2018). Distinguishing RNA modifications from noise in epitranscriptome maps. Nature Chemical Biology, 14(3), 215–225. 10.1038/nchembio.2546 [DOI] [PubMed] [Google Scholar]
- Gupta, R. (1984). Halobacterium volcanii tRNAs. Identification of 41 tRNAs covering all amino acids, and the sequences of 33 class I tRNAs. The Journal of Biological Chemistry, 259(15), 9461–9471 http://www.ncbi.nlm.nih.gov/pubmed/6746655 [PubMed] [Google Scholar]
- Gurha, P. , & Gupta, R. (2008). Archaeal Pus10 proteins can produce both pseudouridine 54 and 55 in tRNA. RNA, 14(12), 2521–2527. 10.1261/rna.1276508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson, C. , & Persson, B. C. (1998). Identification of the rrmA gene encoding the 23S rRNA m1G745 methyltransferase in Escherichia coli and characterization of an m1G745‐deficient mutant. Journal of Bacteriology, 180(2), 359–365 http://www.ncbi.nlm.nih.gov/pubmed/9440525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy, M. P. , & Phizicky, E. M. (2014). Two‐subunit enzymes involved in eukaryotic post‐transcriptional tRNA modification. RNA Biology, 11(12), 1608–1618. 10.1080/15476286.2015.1008360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guymon, R. , Pomerantz, S. C. , Crain, P. F. , & McCloskey, J. A. (2006). Influence of phylogeny on posttranscriptional modification of rRNA in thermophilic prokaryotes: The complete modification map of 16S rRNA of Thermus thermophilus . Biochemistry, 45(15), 4888–4899. 10.1021/bi052579p [DOI] [PubMed] [Google Scholar]
- Haag, S. , Sloan, K. E. , Ranjan, N. , Warda, A. S. , Kretschmer, J. , Blessing, C. , … Bohnsack, M. T. (2016). NSUN3 and ABH1 modify the wobble position of mt‐tRNAMet to expand codon recognition in mitochondrial translation. The EMBO Journal, 35(19), 2104–2119. 10.15252/embj.201694885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, R. H. (1963). Isolation of 3‐methyluridine and 3‐methylcytidine from soluble ribonucleic acid. Biochemical and Biophysical Research Communications, 12(5), 361–364. 10.1016/0006-291X(63)90105-0 [DOI] [PubMed] [Google Scholar]
- Hamma, T. , & Ferré‐D'Amaré, A. R. (2006). Pseudouridine synthases. Chemistry & Biology, 13(11), 1125–1135. 10.1016/J.CHEMBIOL.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Harada, F. , & Nishimura, S. (1974). Purification and characterization of AUA specific isoleucine transfer ribonucleic acid from Escherichia coli B. Biochemistry, 13(2), 300–307. 10.1021/bi00699a011 [DOI] [PubMed] [Google Scholar]
- Hartman, S. C. , & Buchanan, J. M. (1958). Biosynthesis of the purines. XXI. 5‐Phosphoribosylpyrophosphate amidotransferase. The Journal of Biological Chemistry, 233(2), 451–455 http://www.ncbi.nlm.nih.gov/pubmed/13563519 [PubMed] [Google Scholar]
- Hashimoto, H. , Zhang, X. , Vertino, P. M. , & Cheng, X. (2015). The mechanisms of generation, recognition, and erasure of DNA 5‐methylcytosine and thymine oxidations. The Journal of Biological Chemistry, 290(34), 20723–20733. 10.1074/jbc.R115.656884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelund, J. F. , Giessing, A. M. B. , Hansen, T. , Rasmussen, A. , Scott, L. G. , & Kirpekar, F. (2011). Identification of 5‐hydroxycytidine at position 2501 concludes characterization of modified nucleotides in E. coli 23S rRNA. Journal of Molecular Biology, 411(3), 529–536. 10.1016/j.jmb.2011.06.036 [DOI] [PubMed] [Google Scholar]
- Heissenberger, C. , Liendl, L. , Nagelreiter, F. , Gonskikh, Y. , Yang, G. , Stelzer, E. M. , … Schosserer, M. (2019). Loss of the ribosomal RNA methyltransferase NSUN5 impairs global protein synthesis and normal growth. Nucleic Acids Research, 47, 11807–11825. 10.1093/nar/gkz1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm, M. (2006). Post‐transcriptional nucleotide modification and alternative folding of RNA. Nucleic Acids Research, 34(2), 721–733. 10.1093/nar/gkj471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm, M. , Brule, H. , Degoul, F. , Cepanec, C. , Leroux, J.‐P. , Giege, R. , & Florentz, C. (1998). The presence of modified nucleotides is required for cloverleaf folding of a human mitochondrial tRNA. Nucleic Acids Research, 26(7), 1636–1643. 10.1093/nar/26.7.1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm, M. , Giegé, R. , & Florentz, C. (1999). A Watson–Crick base‐pair‐disrupting methyl group (m1A9) is sufficient for cloverleaf folding of human mitochondrial tRNALys . Biochemistry, 38(40), 13338–13346. 10.1021/bi991061g [DOI] [PubMed] [Google Scholar]
- Helm, M. , & Attardi, G. (2004). Nuclear control of cloverleaf structure of human mitochondrial tRNALys . Journal of Molecular Biology, 337(3), 545–560. 10.1016/j.jmb.2004.01.036 [DOI] [PubMed] [Google Scholar]
- Hendrickson, T. L. (2001). Recognizing the D‐loop of transfer RNAs. Proceedings of the National Academy of Sciences of the United States of America, 98(24), 13473–13475. 10.1073/pnas.251549298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger, B. D. , Pal, A. , Del Frate, F. , Topkar, V. V. , & Szostak, J. W. (2015). Replacing uridine with 2‐thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. Journal of the American Chemical Society, 137(7), 2769–2775. 10.1021/jacs.5b00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks, D. G. , Janarthanan, B. R. , Vardarajan, R. , Kulkarni, S. A. , Khoury, T. , Dim, D. , … Ross, D. T. (2010). The expression of TRMT2A, a novel cell cycle regulated protein, identifies a subset of breast cancer patients with HER2 over‐expression that are at an increased risk of recurrence. BMC Cancer, 10, 108. 10.1186/1471-2407-10-108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley, R. W. (1965). Structure of an alanine transfer ribonucleic acid. JAMA, 194(8), 868–871 http://www.ncbi.nlm.nih.gov/pubmed/5898068 [PubMed] [Google Scholar]
- Hoopengardner, B. , Bhalla, T. , Staber, C. , & Reenan, R. (2003). Nervous system targets of RNA editing identified by comparative genomics. Science, 301(5634), 832–836. 10.1126/science.1086763 [DOI] [PubMed] [Google Scholar]
- Hopper, A. K. , Furukawa, A. H. , Pham, H. D. , & Martin, N. C. (1982). Defects in modification of cytoplasmic and mitochondrial transfer RNAs are caused by single nuclear mutations. Cell, 28(3), 543–550. 10.1016/0092-8674(82)90209-4 [DOI] [PubMed] [Google Scholar]
- Howell, N. W. , Jora, M. , Jepson, B. F. , Limbach, P. A. , & Jackman, J. E. (2019). Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA, 25(10), 1366–1376. 10.1261/rna.072090.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchen, C. C. , & Dubin, D. T. (1980). Methylation patterns of mycoplasma transfer and ribosomal ribonucleic acid. Journal of Bacteriology, 144(3), 991–998 http://www.ncbi.nlm.nih.gov/pubmed/6160144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, H. , Weng, H. , Sun, W. , Qin, X. , Shi, H. , Wu, H. , … Chen, J. (2018). Recognition of RNA N 6‐methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nature Cell Biology, 20(3), 285–295. 10.1038/s41556-018-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y. , Su, R. , Sheng, Y. , Dong, L. , Dong, Z. , Xu, H. , … Yang, C.‐G. (2019). Small‐molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell, 35(4), 677–691.e10. 10.1016/j.ccell.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S. M. , Leonardi, A. , Dedon, P. C. , & Begley, T. J. (2019). The versatile roles of the tRNA epitranscriptome during cellular responses to toxic exposures and environmental stress. Toxics, 7(1), 17. 10.3390/toxics7010017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S. M. , Van Delft, P. , Mendil, L. , Bachman, M. , Smollett, K. , Werner, F. , … Balasubramanian, S. (2015). Formation and abundance of 5‐hydroxymethylcytosine in RNA. Chembiochem, 16(5), 752–755. 10.1002/cbic.201500013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, S. M. , van Delft, P. , Tanpure, A. , Miska, E. A. , & Balasubramanian, S. (2017). 2′‐O‐Methyl‐5‐hydroxymethylcytidine: A second oxidative derivative of 5‐methylcytidine in RNA. Journal of the American Chemical Society, 139(5), 1766–1769. 10.1021/jacs.6b12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur, S. , Stroud, R. M. , & Finer‐Moore, J. (2006). Substrate recognition by RNA 5‐methyluridine methyltransferases and pseudouridine synthases: A structural perspective. The Journal of Biological Chemistry, 281(51), 38969–38973. 10.1074/jbc.R600034200 [DOI] [PubMed] [Google Scholar]
- Hussain, S. , Sajini, A. A. , Blanco, S. , Dietmann, S. , Lombard, P. , Sugimoto, Y. , … Frye, M. (2013). NSUN2‐mediated cytosine‐5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Reports, 4(2), 255–261. 10.1016/j.celrep.2013.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, I. , & Sakakibara, H. (2006). Cytokinin biosynthesis and perception. Physiologia Plantarum, 126(4), 528–538. 10.1111/j.1399-3054.2006.00665.x [DOI] [Google Scholar]
- Ikeuchi, Y. , Kimura, S. , Numata, T. , Nakamura, D. , Yokogawa, T. , Ogata, T. , … Suzuki, T. (2010). Agmatine‐conjugated cytidine in a tRNA anticodon is essential for AUA decoding in archaea. Nature Chemical Biology, 6(4), 277–282. 10.1038/nchembio.323 [DOI] [PubMed] [Google Scholar]
- Iribe, H. , Miyamoto, K. , Takahashi, T. , Kobayashi, Y. , Leo, J. , Aida, M. , & Ui‐Tei, K. (2017). Chemical modification of the siRNA seed region suppresses off‐target effects by steric hindrance to base‐pairing with targets. ACS Omega, 2(5), 2055–2064. 10.1021/acsomega.7b00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, K. , Arai, T. , & Suzuki, T. (2019). Depletion of S‐adenosylmethionine impacts on ribosome biogenesis through hypomodification of a single rRNA methylation. Nucleic Acids Research, 47(8), 4226–4239. 10.1093/nar/gkz111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanami, Y. , & Brown, G. M. (1968). Methylated bases of ribosomal ribonucleic acid from HeLa cells. Archives of Biochemistry and Biophysics, 126(1), 8–15. 10.1016/0003-9861(68)90553-5 [DOI] [PubMed] [Google Scholar]
- Iwata‐Reuyl, D. (2008). An embarrassment of riches: The enzymology of RNA modification. Current Opinion in Chemical Biology, 12(2), 126–133. 10.1016/j.cbpa.2008.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman, J. E. , & Phizicky, E. M. (2006). tRNAHis guanylyltransferase adds G‐1 to the 5′ end of tRNAHis by recognition of the anticodon, one of several features unexpectedly shared with tRNA synthetases. RNA, 12(6), 1007–1014. 10.1261/rna.54706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman, J. E. , Montange, R. K. , Malik, H. S. , & Phizicky, E. M. (2003). Identification of the yeast gene encoding the tRNA m1G methyltransferase responsible for modification at position 9. RNA, 9(5), 574–585. 10.1261/rna.5070303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson, R. A. , & Bonner, J. (1968). The occurrence of dihydro ribothymidine in chromosomal RNA. Biochemical and Biophysical Research Communications, 33(5), 716–720. 10.1016/0006-291X(68)90217-9 [DOI] [PubMed] [Google Scholar]
- Jäger, G. , Chen, P. , & Björk, G. R. (2016). Transfer RNA bound to MnmH protein is enriched with geranylated tRNA – A possible intermediate in its selenation? PLoS One, 11(4), e0153488. 10.1371/journal.pone.0153488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager, G. , Leipuviene, R. , Pollard, M. G. , Qian, Q. , & Bjork, G. R. (2004). The conserved Cys‐X1‐X2‐Cys motif present in the TtcA protein is required for the thiolation of cytidine in position 32 of tRNA from Salmonella enterica serovar Typhimurium. Journal of Bacteriology, 186(3), 750–757. 10.1128/JB.186.3.750-757.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, G. , Yang, C.‐G. , Yang, S. , Jian, X. , Yi, C. , Zhou, Z. , & He, C. (2008). Oxidative demethylation of 3‐methylthymine and 3‐methyluracil in single‐stranded DNA and RNA by mouse and human FTO. FEBS Letters, 582(23–24), 3313–3319. 10.1016/j.febslet.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Seo, H. , & Chow, C. S. (2016). Post‐transcriptional modifications modulate rRNA structure and ligand interactions. Accounts of Chemical Research, 49(5), 893–901. 10.1021/acs.accounts.6b00014 [DOI] [PubMed] [Google Scholar]
- Johansson, M. J. O. , & Byström, A. S. (2004). The Saccharomyces cerevisiae TAN1 gene is required for N 4‐acetylcytidine formation in tRNA. RNA, 10(4), 712–719. 10.1261/rna.5198204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, J. D. , & Horowitz, J. (1971). Characterization of ribosomes and RNAs from Mycoplasma hominis . Biochimica et Biophysica Acta (BBA): Nucleic Acids and Protein Synthesis, 247(2), 262–279. 10.1016/0005-2787(71)90675-7 [DOI] [PubMed] [Google Scholar]
- Johnson, L. , & Söll, D. (1970). In vitro biosynthesis of pseudouridine at the polynucleotide level by an enzyme extract from Escherichia coli . Proceedings of the National Academy of Sciences of the United States of America, 67(2), 943–950. 10.1073/pnas.67.2.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkhout, N. , Tran, J. , Smith, M. A. , Schonrock, N. , Mattick, J. S. , & Novoa, E. M. (2017). The RNA modification landscape in human disease. RNA, 23(12), 1754–1769. 10.1261/rna.063503.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jora, M. , Lobue, P. A. , Ross, R. L. , Williams, B. , & Addepalli, B. (2019). Detection of ribonucleoside modifications by liquid chromatography coupled with mass spectrometry. Biochimica et Biophysica Acta (BBA): Gene Regulatory Mechanisms, 1862(3), 280–290. 10.1016/j.bbagrm.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce, G. F. , & Szostak, J. W. (2018). Protocells and RNA self‐replication. Cold Spring Harbor Perspectives in Biology, 10(9), a034801. 10.1101/cshperspect.a034801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez‐Salinas, H. , Mendoza‐Alvarez, H. , Levi, V. , Jacobson, M. K. , & Jacobson, E. L. (1983). Simultaneous determination of linear and branched residues in poly(ADP‐ribose). Analytical Biochemistry, 131(2), 410–418. 10.1016/0003-2697(83)90192-6 [DOI] [PubMed] [Google Scholar]
- Julius, C. , & Yuzenkova, Y. (2017). Bacterial RNA polymerase caps RNA with various cofactors and cell wall precursors. Nucleic Acids Research, 45(14), 8282–8290. 10.1093/nar/gkx452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, Y. , & Goldman, D. (2018). Role of RNA modifications in brain and behavior. Genes, Brain, and Behavior, 17(3), e12444. 10.1111/GBB.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, B. , Miyauchi, K. , Matuszewski, M. , D'Almeida, G. S. , Rubio, M. A. T. , Alfonzo, J. D. , … Suzuki, T. (2017). Identification of 2‐methylthio cyclic N 6‐threonylcarbamoyladenosine (ms2ct6A) as a novel RNA modification at position 37 of tRNAs. Nucleic Acids Research, 45(4), 2124–2136. 10.1093/nar/gkw1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikó, K. , Buckstein, M. , Ni, H. , & Weissman, D. (2005). Suppression of RNA recognition by Toll‐like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity, 23(2), 165–175. 10.1016/j.immuni.2005.06.008 [DOI] [PubMed] [Google Scholar]
- Karlsborn, T. , Tükenmez, H. , Mahmud, A. K. M. F. , Xu, F. , Xu, H. , & Byström, A. S. (2014). Elongator, a conserved complex required for wobble uridine modifications in eukaryotes. RNA Biology, 11(12), 1519–1528. 10.4161/15476286.2014.992276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, H. , Yamaizumi, Z. , Kuchino, Y. , & Nishimura, S. (1979). Isolation of hydroxy‐Y base from rat liver tRNAPhe . Nucleic Acids Research, 6(3), 993–999. 10.1093/nar/6.3.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasprzak, J. M. , Czerwoniec, A. , & Bujnicki, J. M. (2012). Molecular evolution of dihydrouridine synthases. BMC Bioinformatics, 13(1), 153. 10.1186/1471-2105-13-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, M. , Araiso, Y. , Noma, A. , Nagao, A. , Suzuki, T. , Ishitani, R. , & Nureki, O. (2011). Crystal structure of a novel JmjC‐domain‐containing protein, TYW5, involved in tRNA modification. Nucleic Acids Research, 39(4), 1576–1585. 10.1093/nar/gkq919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, T. , Daigo, Y. , Hayama, S. , Ishikawa, N. , Yamabuki, T. , Ito, T. , … Nakamura, Y. (2005). A novel human tRNA‐dihydrouridine synthase involved in pulmonary carcinogenesis. Cancer Research, 65(13), 5638–5646. 10.1158/0008-5472.CAN-05-0600 [DOI] [PubMed] [Google Scholar]
- Kawahara, Y. , Zinshteyn, B. , Sethupathy, P. , Iizasa, H. , Hatzigeorgiou, A. G. , & Nishikura, K. (2007). Redirection of silencing targets by adenosine‐to‐inosine editing of miRNAs. Science, 315(5815), 1137–1140. 10.1126/science.1138050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai, G. , Ue, H. , Yasuda, M. , Sakamoto, K. , Hashizume, T. , McCloskey, J. A. , … Yokoyama, S. (1991). Relation between functions and conformational characteristics of modified nucleosides found in tRNAs. Nucleic Acids Symposium Series, 25, 49–50 http://www.ncbi.nlm.nih.gov/pubmed/1726811 [PubMed] [Google Scholar]
- Keith, G. , Glasser, A. L. , Desgrés, J. , Kuo, K. C. , & Gehrke, C. W. (1990). Identification and structural characterization of O‐β‐ribosyl‐(1′→2′)‐adenosine‐5′‐phosphate in yeast methionine initiator tRNA. Nucleic Acids Research, 18(20), 5989–5993. 10.1093/nar/18.20.5989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami, V. , & Cairns, B. R. (2013). Identification of direct targets and modified bases of RNA cytosine methyltransferases. Nature Biotechnology, 31(5), 458–464. 10.1038/nbt.2566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoddami, V. , Yerra, A. , Mosbruger, T. L. , Fleming, A. M. , Burrows, C. J. , & Cairns, B. R. (2019). Transcriptome‐wide profiling of multiple RNA modifications simultaneously at single‐base resolution. Proceedings of the National Academy of Sciences of the United States of America, 116(14), 6784–6789. 10.1073/pnas.1817334116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierzek, E. , & Kierzek, R. (2003). The thermodynamic stability of RNA duplexes and hairpins containing N 6‐alkyladenosines and 2‐methylthio‐N 6‐alkyladenosines. Nucleic Acids Research, 31(15), 4472–4480. 10.1093/nar/gkg633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiesewetter, S. , Ott, G. , & Sprinzl, M. (1990). The role of modified purine 64 in initiator/elongator discrimination of tRNAiMet from yeast and wheat germ. Nucleic Acids Research, 18(16), 4677–4681. 10.1093/nar/18.16.4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D. D. Y. , Kim, T. T. Y. , Walsh, T. , Kobayashi, Y. , Matise, T. C. , Buyske, S. , & Gabriel, A. (2004). Widespread RNA editing of embedded Alu elements in the human transcriptome. Genome Research, 14(9), 1719–1725. 10.1101/gr.2855504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , & Almo, S. C. (2013). Structural basis for hypermodification of the wobble uridine in tRNA by bifunctional enzyme MnmC. BMC Structural Biology, 13(1), 5. 10.1186/1472-6807-13-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Xiao, H. , Bonanno, J. B. , Kalyanaraman, C. , Brown, S. , Tang, X. , … Almo, S. C. (2013). Structure‐guided discovery of the metabolite carboxy‐SAM that modulates tRNA function. Nature, 498(7452), 123–126. 10.1038/nature12180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, S. , Sakai, Y. , Ishiguro, K. , & Suzuki, T. (2017). Biogenesis and iron‐dependency of ribosomal RNA hydroxylation. Nucleic Acids Research, 45(22), 12974–12986. 10.1093/nar/gkx969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, S. , & Suzuki, T. (2010). Fine‐tuning of the ribosomal decoding center by conserved methyl‐modifications in the Escherichia coli 16S rRNA. Nucleic Acids Research, 38(4), 1341–1352. 10.1093/nar/gkp1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, S. , & Waldor, M. K. (2019). The RNA degradosome promotes tRNA quality control through clearance of hypomodified tRNA. Proceedings of the National Academy of Sciences of the United States of America, 116(4), 1394–1403. 10.1073/pnas.1814130116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn, S. M. , O'Byrne, C. P. , Booth, I. R. , & Stansfield, I. (2002). Physiological analysis of the role of truB in Escherichia coli: A role for tRNA modification in extreme temperature resistance. Microbiology, 148(Pt 11), 3511–3520. 10.1099/00221287-148-11-3511 [DOI] [PubMed] [Google Scholar]
- Kirpekar, F. , Hansen, L. H. , Rasmussen, A. , Poehlsgaard, J. , & Vester, B. (2005). The archaeon Haloarcula marismortui has few modifications in the central parts of its 23S ribosomal RNA. Journal of Molecular Biology, 348(3), 563–573. 10.1016/j.jmb.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Klagsbrun, M. (1973). An evolutionary study of the methylation of transfer and ribosomal ribonucleic acid in prokaryote and eukaryote organisms. The Journal of Biological Chemistry, 248(7), 2612–2620 http://www.ncbi.nlm.nih.gov/pubmed/4633356 [PubMed] [Google Scholar]
- Kleinman, C. L. , Adoue, V. , & Majewski, J. (2012). RNA editing of protein sequences: A rare event in human transcriptomes. RNA, 18(9), 1586–1596. 10.1261/rna.033233.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman, C. L. , & Majewski, J. (2012). Comment on “Widespread RNA and DNA sequence differences in the human transcriptome”. Science, 335(6074), 1302–1302. 10.1126/science.1209658 [DOI] [PubMed] [Google Scholar]
- Koh, C. W. Q. , Goh, Y. T. , & Goh, W. S. S. (2019). Atlas of quantitative single‐base‐resolution N 6‐methyl‐adenine methylomes. Nature Communications, 10(1), 1–15. 10.1038/s41467-019-13561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhrer, C. , Mandal, D. , Gaston, K. W. , Grosjean, H. , Limbach, P. A. , & RajBhandary, U. L. (2014). Life without tRNAIle‐lysidine synthetase: Translation of the isoleucine codon AUA in Bacillus subtilis lacking the canonical tRNA2 Ile . Nucleic Acids Research, 42(3), 1904–1915. 10.1093/nar/gkt1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolitz, S. E. , & Lorsch, J. R. (2010). Eukaryotic initiator tRNA: Finely tuned and ready for action. FEBS Letters, 584(2), 396–404. 10.1016/j.febslet.2009.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopajtich, R. , Nicholls, T. J. , Rorbach, J. , Metodiev, M. D. , Freisinger, P. , Mandel, H. , … Prokisch, H. (2014). Mutations in GTPBP3 cause a mitochondrial translation defect associated with hypertrophic cardiomyopathy, lactic acidosis, and encephalopathy. American Journal of Human Genetics, 95(6), 708–720. 10.1016/j.ajhg.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormann, M. S. D. , Hasenpusch, G. , Aneja, M. K. , Nica, G. , Flemmer, A. W. , Herber‐Jonat, S. , … Rudolph, C. (2011). Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nature Biotechnology, 29(2), 154–157. 10.1038/nbt.1733 [DOI] [PubMed] [Google Scholar]
- Koscinski, L. , Feder, M. , & Bujnicki, J. M. (2007). Identification of a missing sequence and functionally important residues of 16S rRNA:m1A1408 methyltransferase KamB that causes bacterial resistance to aminoglycoside antibiotics. Cell Cycle, 6(10), 1268–1271. 10.4161/cc.6.10.4231 [DOI] [PubMed] [Google Scholar]
- Kotera, M. , Bayashi, T. , Hattori, M. , Tokimatsu, T. , Goto, S. , Mihara, H. , & Kanehisa, M. (2010). Comprehensive genomic analysis of sulfur‐relay pathway genes. Genome Informatics. International Conference on Genome Informatics, 24, 104–115. 10.1142/9781848166585_0009 [DOI] [PubMed] [Google Scholar]
- Kowalak, J. A. , Bruenger, E. , Crain, P. F. , & McCloskey, J. A. (2000). Identities and phylogenetic comparisons of posttranscriptional modifications in 16S ribosomal RNA from Haloferax volcanii . Journal of Biological Chemistry, 275(32), 24484–24489. 10.1074/jbc.M002153200 [DOI] [PubMed] [Google Scholar]
- Kowalak, J. A. , Bruenger, E. , Hashizume, T. , Peltier, J. M. , Ofengand, J. , & McCloskey, J. A. (1996). Structural characterization of U*‐1915 in domain IV from Escherichia coli 23S ribosomal RNA as 3‐methylpseudouridine. Nucleic Acids Research, 24(4), 688–693. 10.1093/nar/24.4.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalak, J. A. , Bruenger, E. , & McCloskey, J. A. (1995). Posttranscriptional modification of the central loop of domain V in Escherichia coli 23S ribosomal RNA. The Journal of Biological Chemistry, 270(30), 17758–17764. 10.1074/jbc.270.30.17758 [DOI] [PubMed] [Google Scholar]
- Kowalak, J. A. , Dalluge, J. J. , McCloskey, J. A. , & Stetter, K. O. (1994). The role of posttranscriptional modification in stabilization of transfer RNA from hyperthermophiles. Biochemistry, 33(25), 7869–7876. 10.1021/bi00191a014 [DOI] [PubMed] [Google Scholar]
- Kowtoniuk, W. E. , Shen, Y. , Heemstra, J. M. , Agarwal, I. , & Liu, D. R. (2009). A chemical screen for biological small molecule‐RNA conjugates reveals CoA‐linked RNA. Proceedings of the National Academy of Sciences of the United States of America, 106(19), 7768–7773. 10.1073/pnas.0900528106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamohan, A. , & Jackman, J. E. (2017). Mechanistic features of the atypical tRNA m1G9 SPOUT methyltransferase, Trm10. Nucleic Acids Research, 45(15), 9019–9029. 10.1093/nar/gkx620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krog, J. S. , Español, Y. , Giessing, A. M. B. , Dziergowska, A. , Malkiewicz, A. , de Pouplana, L. R. , & Kirpekar, F. (2011). 3‐(3‐Amino‐3‐carboxypropyl)‐5,6‐dihydrouridine is one of two novel post‐transcriptional modifications in tRNALys(UUU) from Trypanosoma brucei . FEBS Journal, 278(24), 4782–4796. 10.1111/j.1742-4658.2011.08379.x [DOI] [PubMed] [Google Scholar]
- Krüger, M. K. , & Sørensen, M. A. (1998). Aminoacylation of hypomodified tRNAGlu in vivo . Journal of Molecular Biology, 284(3), 609–620. 10.1006/jmbi.1998.2197 [DOI] [PubMed] [Google Scholar]
- Kuchino, Y. , & Borek, E. (1978). Tumour‐specific phenylalanine tRNA contains two supernumerary methylated bases. Nature, 271(5641), 126–129. 10.1038/271126a0 [DOI] [PubMed] [Google Scholar]
- Kulikova, I. V. , Muradova, D. A. , Drenichev, M. S. , & Mikhailov, S. N. (2015). Stereoselective synthesis of 2′‐O‐α‐d‐ribofuranosyluridine, a structural fragment of Hellecaucaside A. Chemistry of Natural Compounds, 51(2), 256–260. 10.1007/s10600-015-1256-1 [DOI] [Google Scholar]
- Lan, Q. , Liu, P. Y. , Haase, J. , Bell, J. L. , Hüttelmaier, S. , & Liu, T. (2019). The critical role of RNA m6A methylation in cancer. Cancer Research, 79(7), 1285–1292. 10.1158/0008-5472.CAN-18-2965 [DOI] [PubMed] [Google Scholar]
- Landgraf, B. J. , McCarthy, E. L. , & Booker, S. J. (2016). Radical S‐adenosylmethionine enzymes in human health and disease. Annual Review of Biochemistry, 85(1), 485–514. 10.1146/annurev-biochem-060713-035504 [DOI] [PubMed] [Google Scholar]
- Larsen, A. T. , Fahrenbach, A. C. , Sheng, J. , Pian, J. , & Szostak, J. W. (2015). Thermodynamic insights into 2‐thiouridine‐enhanced RNA hybridization. Nucleic Acids Research, 43(16), 7675–7687. 10.1093/nar/gkv761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartigue, C. , Lebaudy, A. , Blanchard, A. , El Yacoubi, B. , Rose, S. , Grosjean, H. , & Douthwaite, S. (2014). The flavoprotein Mcap0476 (RlmFO) catalyzes m5U1939 modification in Mycoplasma capricolum 23S rRNA. Nucleic Acids Research, 42(12), 8073–8082. 10.1093/nar/gku518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laten, H. , Gorman, J. , & Bock, R. M. (1978). Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae . Nucleic Acids Research, 5(11), 4329–4342. 10.1093/nar/5.11.4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauhon, C. T. (2002). Requirement for IscS in biosynthesis of all thionucleosides in Escherichia coli . Journal of Bacteriology, 184(24), 6820–6829. 10.1128/JB.184.24.6820-6829.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini, J. M. , Ramos, J. , & Fu, D. (2018). Monitoring the 5‐methoxycarbonylmethyl‐2‐thiouridine (mcm5s2U) modification in eukaryotic tRNAs via the γ‐toxin endonuclease. RNA, 24(5), 749–758. 10.1261/rna.065581.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létoquart, J. , Huvelle, E. , Wacheul, L. , Bourgeois, G. , Zorbas, C. , Graille, M. , … Lafontaine, D. L. J. (2014). Structural and functional studies of Bud23‐Trm112 reveal 18S rRNA N 7‐G1575 methylation occurs on late 40S precursor ribosomes. Proceedings of the National Academy of Sciences of the United States of America, 111(51), E5518–E5526. 10.1073/pnas.1413089111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon, E. Y. , Hallegger, M. , Kinar, Y. , Shemesh, R. , Djinovic‐Carugo, K. , Rechavi, G. , … Eisenberg, E. (2005). Evolutionarily conserved human targets of adenosine to inosine RNA editing. Nucleic Acids Research, 33(4), 1162–1168. 10.1093/nar/gki239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levanon, E. Y. , Eisenberg, E. , Yelin, R. , Nemzer, S. , Hallegger, M. , Shemesh, R. , … Jantsch, M. F. (2004). Systematic identification of abundant A‐to‐I editing sites in the human transcriptome. Nature Biotechnology, 22(8), 1001–1005. 10.1038/nbt996 [DOI] [PubMed] [Google Scholar]
- Levin, A. P. , & Magasanik, B. (1961). The effect of purines on the formation of two enzymes involved in purine biosynthesis. The Journal of Biological Chemistry, 236, 184–188 http://www.ncbi.nlm.nih.gov/pubmed/13761395 [PubMed] [Google Scholar]
- Li, M. , Wang, I. X. , Li, Y. , Bruzel, A. , Richards, A. L. , Toung, J. M. , & Cheung, V. G. (2011). Widespread RNA and DNA sequence differences in the human transcriptome. Science, 333(6038), 53–58. 10.1126/science.1207018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Xiong, X. , Wang, K. , Wang, L. , Shu, X. , Ma, S. , & Yi, C. (2016). Transcriptome‐wide mapping reveals reversible and dynamic N 1‐methyladenosine methylome. Nature Chemical Biology, 12(5), 311–316. 10.1038/nchembio.2040 [DOI] [PubMed] [Google Scholar]
- Li, X. , Zhu, P. , Ma, S. , Song, J. , Bai, J. , Sun, F. , & Yi, C. (2015). Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nature Chemical Biology, 11(8), 592–597. 10.1038/nchembio.1836 [DOI] [PubMed] [Google Scholar]
- Lim, V. I. , & Curran, J. F. (2001). Analysis of codon:anticodon interactions within the ribosome provides new insights into codon reading and the genetic code structure. RNA, 7(7), 942–957. 10.1017/s135583820100214x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbach, P. A. , Crain, P. F. , & McCloskey, J. A. (1994). Summary: The modified nucleosides of RNA. Nucleic Acids Research, 22(12), 2183–2196. 10.1093/nar/22.12.2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H. , Miyauchi, K. , Harada, T. , Okita, R. , Takeshita, E. , Komaki, H. , … Suzuki, T. (2018). CO2‐sensitive tRNA modification associated with human mitochondrial disease. Nature Communications, 9(1), 1875. 10.1038/s41467-018-04250-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S. , Choe, J. , Du, P. , Triboulet, R. , & Gregory, R. I. (2016). The m6A methyltransferase METTL3 promotes translation in human cancer cells. Molecular Cell, 62(3), 335–345. 10.1016/J.MOLCEL.2016.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, W. , Piskol, R. , Tan, M. H. , & Li, J. B. (2012). Comment on “Widespread RNA and DNA sequence differences in the human transcriptome”. Science, 335(6074), 1302–1302. 10.1126/science.1210624 [DOI] [PubMed] [Google Scholar]
- Linder, B. , Grozhik, A. V. , Olarerin‐George, A. O. , Meydan, C. , Mason, C. E. , & Jaffrey, S. R. (2015). Single‐nucleotide‐resolution mapping of m6A and m6Am throughout the transcriptome. Nature Methods, 12(8), 767–772. 10.1038/nmeth.3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H. , Begik, O. , Lucas, M. C. , Mason, C. E. , Schwartz, S. , Mattick, J. S. , … Novoa, E. M. (2019). Accurate detection of m6A RNA modifications in native RNA sequences. Nature Communications, 10, 4079. 10.1101/525741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Parisien, M. , Dai, Q. , Zheng, G. , He, C. , & Pan, T. (2013). Probing N 6‐methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA, 19(12), 1848–1856. 10.1261/rna.041178.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Dai, Q. , Zheng, G. , He, C. , Parisien, M. , & Pan, T. (2015). N 6‐methyladenosine‐dependent RNA structural switches regulate RNA‐protein interactions. Nature, 518(7540), 560–564. 10.1038/nature14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, N. , Zhou, K. I. , Parisien, M. , Dai, Q. , Diatchenko, L. , & Pan, T. (2017). N 6‐methyladenosine alters RNA structure to regulate binding of a low‐complexity protein. Nucleic Acids Research, 45(10), 6051–6063. 10.1093/nar/gkx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , van der Marel, G. A. , & Filippov, D. V. (2019). Chemical ADP‐ribosylation: Mono‐ADPr‐peptides and oligo‐ADP‐ribose. Organic & Biomolecular Chemistry, 17(22), 5460–5474. 10.1039/C9OB00501C [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Long, F. , Wang, L. , Söll, D. , & Whitman, W. B. (2014). The putative tRNA 2‐thiouridine synthetase Ncs6 is an essential sulfur carrier in Methanococcus maripaludis . FEBS Letters, 588(6), 873–877. 10.1016/j.febslet.2014.01.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, K. S. , Poehlsgaard, J. , Kehrenberg, C. , Schwarz, S. , & Vester, B. (2006). The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrobial Agents and Chemotherapy, 50(7), 2500–2505. 10.1128/AAC.00131-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, C. , Lünse, C. E. , & Mörl, M. (2017). tRNA modifications: Impact on structure and thermal adaptation. Biomolecules, 7(2), 35. 10.3390/biom7020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano, D. J. , Mirsky, H. , Vendetti, N. J. , & Maas, S. (2004). RNA editing of a miRNA precursor. RNA, 10(8), 1174–1177. 10.1261/rna.7350304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, C.‐J. , Ding, J.‐H. , Ye, T.‐T. , Yuan, B.‐F. , & Feng, Y.‐Q. (2019). AlkB homologue 1 demethylates N 3‐methylcytidine in mRNA of mammals. ACS Chemical Biology, 14(7), 1418–1425. 10.1021/acschembio.8b01001 [DOI] [PubMed] [Google Scholar]
- Maas, S. , Gerber, A. P. , & Rich, A. (1999). Identification and characterization of a human tRNA‐specific adenosine deaminase related to the ADAR family of pre‐mRNA editing enzymes. Proceedings of the National Academy of Sciences of the United States of America, 96(16), 8895–8900. 10.1073/pnas.96.16.8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden, B. E. (1990). The numerous modified nucleotides in eukaryotic ribosomal RNA. Progress in Nucleic Acid Research and Molecular Biology, 39, 241–303 http://www.ncbi.nlm.nih.gov/pubmed/2247610 [DOI] [PubMed] [Google Scholar]
- Maden, B. E. , & Salim, M. (1974). The methylated nucleotide sequences in HeLa cell ribosomal RNA and its precursors. Journal of Molecular Biology, 88(1), 133–152. 10.1016/0022-2836(74)90299-x [DOI] [PubMed] [Google Scholar]
- Madore, E. , Florentz, C. , Giegé, R. , Sekine, S. , Yokoyama, S. , & Lapointe, J. (1999). Effect of modified nucleotides on Escherichia coli tRNAGlu structure and on its aminoacylation by glutamyl‐tRNA synthetase. Predominant and distinct roles of the mnm5 and s2 modifications of U34. European Journal of Biochemistry, 266(3), 1128–1135. 10.1046/j.1432-1327.1999.00965.x [DOI] [PubMed] [Google Scholar]
- Madsen, C. T. , Mengel‐Jørgensen, J. , Kirpekar, F. , & Douthwaite, S. (2003). Identifying the methyltransferases for m5U747 and m5U1939 in 23S rRNA using MALDI mass spectrometry. Nucleic Acids Research, 31(16), 4738–4746. 10.1093/nar/gkg657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal, D. , Köhrer, C. , Su, D. , Babu, I. R. , Chan, C. T. Y. , Liu, Y. , … Rajbhandary, U. L. (2014). Identification and codon reading properties of 5‐cyanomethyl uridine, a new modified nucleoside found in the anticodon wobble position of mutant haloarchaeal isoleucine tRNAs. RNA, 20(2), 177–188. 10.1261/rna.042358.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal, D. , Köhrer, C. , Su, D. , Russell, S. P. , Krivos, K. , Castleberry, C. M. , … RajBhandary, U. L. (2010). Agmatidine, a modified cytidine in the anticodon of archaeal tRNAIle, base pairs with adenosine but not with guanosine. Proceedings of the National Academy of Sciences of the United States of America, 107(7), 2872–2877. 10.1073/pnas.0914869107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand, V. , Blanloeil‐Oillo, F. , Helm, M. , & Motorin, Y. (2016). Illumina‐based RiboMethSeq approach for mapping of 2′‐O‐Me residues in RNA. Nucleic Acids Research, 44(16), e135. 10.1093/nar/gkw547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti, M. , Capela, D. , Poincloux, R. , Benmeradi, N. , Auriac, M.‐C. , Le Ru, A. , … Masson‐Boivin, C. (2013). Queuosine biosynthesis is required for Sinorhizobium meliloti‐induced cytoskeletal modifications on HeLa cells and symbiosis with Medicago truncatula . PLoS One, 8(2), e56043. 10.1371/journal.pone.0056043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolewski, A. , Smith, J. M. , & Benkovic, S. J. (1994). Cloning and characterization of a new purine biosynthetic enzyme: A non‐folate glycinamide ribonucleotide transformylase from E. coli . Biochemistry, 33(9), 2531–2537. 10.1021/bi00175a023 [DOI] [PubMed] [Google Scholar]
- McCown, P. J. , Corbino, K. A. , Stav, S. , Sherlock, M. E. , & Breaker, R. R. (2017). Riboswitch diversity and distribution. RNA, 23(7), 995–1011. 10.1261/rna.061234.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown, P. J. , Liang, J. J. , Weinberg, Z. , & Breaker, R. R. (2014). Structural, functional, and taxonomic diversity of three preQ1 riboswitch classes. Chemistry and Biology, 21(7), 880–889. 10.1016/j.chembiol.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCown, P. J. , Wang, M. C. , Jaeger, L. , & Brown, J. A. (2019). Secondary structural model of human MALAT1 reveals multiple structure–function relationships. International Journal of Molecular Sciences, 20(22), 5610. 10.3390/ijms20225610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre, W. , Netzband, R. , Bonenfant, G. , Biegel, J. M. , Miller, C. , Fuchs, G. , … Pager, C. T. (2018). Positive‐sense RNA viruses reveal the complexity and dynamics of the cellular and viral epitranscriptomes during infection. Nucleic Acids Research, 46(11), 5776–5791. 10.1093/nar/gky029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier, F. , Suter, B. , Grosjean, H. , Keith, G. , & Kubli, E. (1985). Queuosine modification of the wobble base in tRNAHis influences “in vivo” decoding properties. The EMBO Journal, 4(3), 823–827 http://www.ncbi.nlm.nih.gov/pubmed/2988936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, A. J. , Garry, D. J. , Hall, B. , Byrom, M. M. , McDonald, H. G. , Yang, X. , … Ellington, A. D. (2015). Transcription yield of fully 2′‐modified RNA can be increased by the addition of thermostabilizing mutations to T7 RNA polymerase mutants. Nucleic Acids Research, 43(15), 7480–7488. 10.1093/nar/gkv734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Immer, C. , Kaiser, S. , Sharma, S. , Yang, J. , Watzinger, P. , … Wöhnert, J. (2020). Identification of the 3‐amino‐3‐carboxypropyl (acp) transferase enzyme responsible for acp3U formation at position 47 in Escherichia coli tRNAs. Nucleic Acids Research, 48(3), 1435–1450. 10.1093/nar/gkz1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Wurm, J. P. , Kötter, P. , Leisegang, M. S. , Schilling, V. , Buchhaupt, M. , … Entian, K.‐D. (2011). The Bowen–Conradi syndrome protein Nep1 (Emg1) has a dual role in eukaryotic ribosome biogenesis, as an essential assembly factor and in the methylation of Ψ1191 in yeast 18S rRNA. Nucleic Acids Research, 39(4), 1526–1537. 10.1093/nar/gkq931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, B. , Wurm, J. P. , Sharma, S. , Immer, C. , Pogoryelov, D. , Kötter, P. , … Entian, K.‐D. (2016). Ribosome biogenesis factor Tsr3 is the aminocarboxypropyl transferase responsible for 18S rRNA hypermodification in yeast and humans. Nucleic Acids Research, 44(9), 4304–4316. 10.1093/nar/gkw244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, E. , Leonard, N. J. , Bhat, B. , Stubbe, J. , & Smith, J. M. (1992). Purification and characterization of the purE, purK, and purC gene products: Identification of a previously unrecognized energy requirement in the purine biosynthetic pathway. Biochemistry, 31(21), 5022–5032. 10.1021/bi00136a016 [DOI] [PubMed] [Google Scholar]
- Meyer, K. D. , Saletore, Y. , Zumbo, P. , Elemento, O. , Mason, C. E. , & Jaffrey, S. R. (2012). Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell, 149(7), 1635–1646. 10.1016/J.CELL.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara, H. , Kato, S. , Lacourciere, G. M. , Stadtman, T. C. , Kennedy, R. A. J. D. , Kurihara, T. , … Esaki, N. (2002). The iscS gene is essential for the biosynthesis of 2‐selenouridine in tRNA and the selenocysteine‐containing formate dehydrogenase H. Proceedings of the National Academy of Sciences of the United States of America, 99(10), 6679–6683. 10.1073/pnas.102176099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, K. , Endo, K. , Takahashi, S. , Funakoshi, S. , Takei, I. , Katayama, S. , … Yoshida, Y. (2015). Efficient detection and purification of cell populations using synthetic microRNA switches. Cell Stem Cell, 16(6), 699–711. 10.1016/j.stem.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Miles, Z. D. , McCarty, R. M. , Molnar, G. , & Bandarian, V. (2011). Discovery of epoxyqueuosine (oQ) reductase reveals parallels between halorespiration and tRNA modification. Proceedings of the National Academy of Sciences of the United States of America, 108(18), 7368–7372. 10.1073/PNAS.1018636108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelstadt, M. , Frump, A. , Khuu, T. , Fowlkes, V. , Handy, I. , Patel, C. V. , & Patel, R. C. (2007). Interaction of human tRNA‐dihydrouridine synthase‐2 with interferon‐induced protein kinase PKR. Nucleic Acids Research, 36(3), 998–1008. 10.1093/nar/gkm1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi, K. , Kimura, S. , & Suzuki, T. (2013). A cyclic form of N 6‐threonylcarbamoyladenosine as a widely distributed tRNA hypermodification. Nature Chemical Biology, 9(2), 105–111. 10.1038/nchembio.1137 [DOI] [PubMed] [Google Scholar]
- Mohammad, A. , Bon Ramos, A. , Lee, B. , Cohen, S. , Kiani, M. , Iwata‐Reuyl, D. , … Swairjo, M. (2017). Protection of the queuosine biosynthesis enzyme QueF from irreversible oxidation by a conserved intramolecular disulfide. Biomolecules, 7(4), 30. 10.3390/biom7010030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke, T. , Dickmanns, A. , & Ficner, R. (2009). Structural basis for m7G‐cap hypermethylation of small nuclear, small nucleolar and telomerase RNA by the dimethyltransferase TGS1. Nucleic Acids Research, 37(12), 3865–3877. 10.1093/nar/gkp249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira, S. , Valach, M. , Aoulad‐Aissa, M. , Otto, C. , & Burger, G. (2016). Novel modes of RNA editing in mitochondria. Nucleic Acids Research, 44(10), 4907–4919. 10.1093/nar/gkw188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin, Y. , & Helm, M. (2010). tRNA stabilization by modified nucleotides. Biochemistry, 49(24), 4934–4944. 10.1021/bi100408z [DOI] [PubMed] [Google Scholar]
- Moukadiri, I. , Prado, S. , Piera, J. , Velázquez‐campoy, A. , Björk, G. R. , & Armengod, M. E. (2009). Evolutionarily conserved proteins MnmE and GidA catalyze the formation of two methyluridine derivatives at tRNA wobble positions. Nucleic Acids Research, 37(21), 7177–7193. 10.1093/nar/gkp762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukadiri, I. , Villarroya, M. , Benítez‐Páez, A. , & Armengod, M. E. (2018). Bacillus subtilis exhibits MnmC‐like tRNA modification activities. RNA Biology, 15(9), 1167–1173. 10.1080/15476286.2018.1517012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller, M. , Legrand, C. , Tuorto, F. , Kelly, V. P. , Atlasi, Y. , Lyko, F. , & Ehrenhofer‐Murray, A. E. (2019). Queuine links translational control in eukaryotes to a micronutrient from bacteria. Nucleic Acids Research, 47(7), 3711–3727. 10.1093/nar/gkz063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao, A. , Ohara, M. , Miyauchi, K. , Yokobori, S.‐I. , Yamagishi, A. , Watanabe, K. , & Suzuki, T. (2017). Hydroxylation of a conserved tRNA modification establishes non‐universal genetic code in echinoderm mitochondria. Nature Structural & Molecular Biology, 24(9), 778–782. 10.1038/nsmb.3449 [DOI] [PubMed] [Google Scholar]
- Nakai, Y. , Umeda, N. , Suzuki, T. , Nakai, M. , Hayashi, H. , Watanabe, K. , & Kagamiyama, H. (2004). Yeast Nfs1p is involved in thio‐modification of both mitochondrial and cytoplasmic tRNAs. Journal of Biological Chemistry, 279(13), 12363–12368. 10.1074/jbc.M312448200 [DOI] [PubMed] [Google Scholar]
- Nakanishi, K. , Fukai, S. , Ikeuchi, Y. , Soma, A. , Sekine, Y. , Suzuki, T. , & Nureki, O. (2005). Structural basis for lysidine formation by ATP pyrophosphatase accompanied by a lysine‐specific loop and a tRNA‐recognition domain. Proceedings of the National Academy of Sciences of the United States of America, 102(21), 7487–7492. 10.1073/pnas.0501003102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, S. , Suzuki, T. , Kawarada, L. , Iwata, H. , Asano, K. , & Suzuki, T. (2016). NSUN3 methylase initiates 5‐formylcytidine biogenesis in human mitochondrial tRNAMet . Nature Chemical Biology, 12(7), 546–551. 10.1038/nchembio.2099 [DOI] [PubMed] [Google Scholar]
- Nelson, J. W. , & Breaker, R. R. (2017). The lost language of the RNA world. Science Signaling, 10(483), eaam8812. 10.1126/scisignal.aam8812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, S. , Taya, Y. , Kuchino, Y. , & Oashi, Z. (1974). Enzymatic synthesis of 3‐(3‐amino‐3‐carboxypropyl)uridine in Escherichia coli phenylalanine transfer RNA: Transfer of the 3‐amino‐acid‐3‐carboxypropyl group from S‐adenosylmethionine. Biochemical and Biophysical Research Communications, 57(3), 702–708. 10.1016/0006-291x(74)90603-2 [DOI] [PubMed] [Google Scholar]
- Noma, A. , Ishitani, R. , Kato, M. , Nagao, A. , Nureki, O. , & Suzuki, T. (2010). Expanding role of the jumonji C domain as an RNA hydroxylase. Journal of Biological Chemistry, 285(45), 34503–34507. 10.1074/jbc.M110.156398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, A. , Kirino, Y. , Ikeuchi, Y. , & Suzuki, T. (2006). Biosynthesis of wybutosine, a hyper‐modified nucleoside in eukaryotic phenylalanine tRNA. The EMBO Journal, 25(10), 2142–2154. 10.1038/sj.emboj.7601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, A. , Sakaguchi, Y. , & Suzuki, T. (2009). Mechanistic characterization of the sulfur‐relay system for eukaryotic 2‐thiouridine biogenesis at tRNA wobble positions. Nucleic Acids Research, 37(4), 1335–1352. 10.1093/nar/gkn1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma, A. , Yi, S. , Katoh, T. , Takai, Y. , Suzuki, T. , & Suzuki, T. (2011). Actin‐binding protein ABP140 is a methyltransferase for 3‐methylcytidine at position 32 of tRNAs in Saccharomyces cerevisiae . RNA, 17(6), 1111–1119. 10.1261/RNA.2653411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon, K. R. , Bruenger, E. , & McCloskey, J. A. (1998). Posttranscriptional modifications in 16S and 23S rRNAs of the archaeal hyperthermophile Sulfolobus solfataricus . Journal of Bacteriology, 180(11), 2883–2888 http://www.ncbi.nlm.nih.gov/pubmed/9603876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon, K. R. , Guymon, R. , Crain, P. F. , McCloskey, J. A. , Thomm, M. , Lim, J. , & Cavicchioli, R. (2003). Influence of temperature on tRNA modification in archaea: Methanococcoides burtonii (optimum growth temperature [Topt], 23°C) and Stetteria hydrogenophila (Topt, 95°C). Journal of Bacteriology, 185(18), 5483–5490. 10.1128/JB.185.18.5483-5490.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann, P. L. , Makris, J. C. , & Reznikoff, W. S. (1988). Inosine induced mutations. MGG Molecular & General Genetics, 214(1), 62–67. 10.1007/BF00340180 [DOI] [PubMed] [Google Scholar]
- Nurse, K. , Wrzesinski, J. , Bakin, A. , Lane, B. G. , & Ofengand, J. (1995). Purification, cloning, and properties of the tRNA Ψ55 synthase from Escherichia coli . RNA, 1(1), 102–112 http://www.ncbi.nlm.nih.gov/pubmed/7489483 [PMC free article] [PubMed] [Google Scholar]
- Nygaard, P. , & Saxild, H. H. (2000). The yexA gene product is required for phosphoribosylformylglycinamidine synthetase activity in Bacillus subtilis . Microbiology, 146(4), 807–814. 10.1099/00221287-146-4-807 [DOI] [PubMed] [Google Scholar]
- Oakes, E. , Anderson, A. , Cohen‐Gadol, A. , & Hundley, H. A. (2017). Adenosine deaminase that acts on RNA 3 (ADAR3) binding to glutamate receptor subunit B pre‐mRNA inhibits RNA editing in glioblastoma. The Journal of Biological Chemistry, 292(10), 4326–4335. 10.1074/jbc.M117.779868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerum, S. , Dégut, C. , Barraud, P. , & Tisné, C. (2017). m1A post‐transcriptional modification in tRNAs. Biomolecules, 7(4), 20. 10.3390/biom7010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofengand, J. , Del Campo, M. , & Kaya, Y. (2001). Mapping pseudouridines in RNA molecules. Methods, 25(3), 365–373. 10.1006/meth.2001.1249 [DOI] [PubMed] [Google Scholar]
- Ofengand, J. , Malhotra, A. , Remme, J. , Gutgsell, N. S. , Del Campo, M. , Jean‐Charles, S. , … Kaya, Y. (2001). Pseudouridines and pseudouridine synthases of the ribosome. Cold Spring Harbor Symposia on Quantitative Biology, 66, 147–159 http://www.ncbi.nlm.nih.gov/pubmed/12762017 [DOI] [PubMed] [Google Scholar]
- Ofengand, J. (2002). Ribosomal RNA pseudouridines and pseudouridine synthases. FEBS Letters, 514(1), 17–25. 10.1016/s0014-5793(02)02305-0 [DOI] [PubMed] [Google Scholar]
- Okada, N. , & Nishimura, S. (1977). Enzymatic synthesis of Q* nucleoside containing mannose in the anticodon of tRNA: Isolation of a novel mannosyltransferase from a cell‐free extract of rat liver. Nucleic Acids Research, 4(8), 2931–2937. 10.1093/nar/4.8.2931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera, T. , Satoh, K. , Ohta, T. , & Narumi, I. (2013). Deinococcus radiodurans YgjD and YeaZ are involved in the repair of DNA cross‐links. Extremophiles, 17(1), 171–179. 10.1007/s00792-012-0506-4 [DOI] [PubMed] [Google Scholar]
- Orellana, E. A. , Tenneti, S. , Rangasamy, L. , Lyle, L. T. , Low, P. S. , & Kasinski, A. L. (2017). FolamiRs: Ligand‐targeted, vehicle‐free delivery of microRNAs for the treatment of cancer. Science Translational Medicine, 9(401), eaam9327. 10.1126/scitranslmed.aam9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel, L. E. (1968). Evolution of the genetic apparatus. Journal of Molecular Biology, 38(3), 381–393. 10.1016/0022-2836(68)90393-8 [DOI] [PubMed] [Google Scholar]
- Ownby, K. , Xu, H. , & White, R. H. (2005). A Methanocaldococcus jannaschii archaeal signature gene encodes for a 5‐formaminoimidazole‐4‐carboxamide‐1‐β‐D‐ribofuranosyl 5′‐monophosphate synthetase: A new enzyme in purine biosynthesis. Journal of Biological Chemistry, 280(12), 10881–10887. 10.1074/jbc.M413937200 [DOI] [PubMed] [Google Scholar]
- Palenchar, P. M. , Buck, C. J. , Cheng, H. , Larson, T. J. , & Mueller, E. G. (2000). Evidence that ThiI, an enzyme shared between thiamin and 4‐thiouridine biosynthesis, may be a sulfurtransferase that proceeds through a persulfide intermediate. Journal of Biological Chemistry, 275(12), 8283–8286 http://www.jbc.org/ [DOI] [PubMed] [Google Scholar]
- Pallan, P. S. , Kreutz, C. , Bosio, S. , Micura, R. , & Egli, M. (2008). Effects of N 2,N 2‐dimethylguanosine on RNA structure and stability: Crystal structure of an RNA duplex with tandem m2,2G:A pairs. RNA, 14(10), 2125–2135. 10.1261/rna.1078508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, A. , Pain, J. , Dziuba, N. , Pandey, A. K. , Dancis, A. , Lindahl, P. A. , & Pain, D. (2018). Mitochondria export sulfur species required for cytosolic tRNA thiolation. Cell Chemical Biology, 25(6), 738–748.e3. 10.1016/j.chembiol.2018.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang, H. , Ihara, M. , Kuchino, Y. , Nishimura, S. , Gupta, R. , Woese, C. R. , & McCloskey, J. A. (1982). Structure of a modified nucleoside in archaebacterial tRNA which replaces ribosylthymine. 1‐Methylpseudouridine. The Journal of Biological Chemistry, 257(7), 3589–3592 http://www.ncbi.nlm.nih.gov/pubmed/7061499 [PubMed] [Google Scholar]
- Paris, J. , Morgan, M. , Campos, J. , Spencer, G. J. , Shmakova, A. , Ivanova, I. , … Kranc, K. R. (2019). Targeting the RNA m6A reader YTHDF2 selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell, 25(1), 137–148.e6. 10.1016/j.stem.2019.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris, Z. , Fleming, I. M. C. , & Alfonzo, J. D. (2012). Determinants of tRNA editing and modification: Avoiding conundrums, affecting function. Seminars in Cell & Developmental Biology, 23(3), 269–274. 10.1016/j.semcdb.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, M. T. , Knop, K. , Sherwood, A. V. , Schurch, N. J. , Mackinnon, K. , Gould, P. D. , … Simpson, G. G. (2020). Nanopore direct RNA sequencing maps the complexity of Arabidopsis mRNA processing and m6A modification. eLife, 9. 10.7554/eLife.49658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsyan, A. , Svitkin, Y. , Shahbazian, D. , Gkogkas, C. , Lasko, P. , Merrick, W. C. , & Sonenberg, N. (2011). mRNA helicases: The tacticians of translational control. Nature Reviews. Molecular Cell Biology, 12(4), 235–245. 10.1038/nrm3083 [DOI] [PubMed] [Google Scholar]
- Parthasarathy, R. , Ginell, S. L. , De, N. C. , & Chheda, G. B. (1978). Conformation of N 4‐acetylcytidine, a modified nucleoside of tRNA, and stereochemistry of codon‐anticodon interaction. Biochemical and Biophysical Research Communications, 83(2), 657–663. 10.1016/0006-291X(78)91040-9 [DOI] [PubMed] [Google Scholar]
- Pathak, C. , Jaiswal, Y. K. , & Vinayak, M. (2008). Queuine promotes antioxidant defence system by activating cellular antioxidant enzyme activities in cancer. Bioscience Reports, 28(2), 73–81. 10.1042/BSR20070011 [DOI] [PubMed] [Google Scholar]
- Paul, M. S. , & Bass, B. L. (1998). Inosine exists in mRNA at tissue‐specific levels and is most abundant in brain mRNA. The EMBO Journal, 17(4), 1120–1127. 10.1093/emboj/17.4.1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer, C. , Sharma, S. , Watzinger, P. , Lamberth, S. , Kötter, P. , & Entian, K.‐D. (2013). Yeast Rrp8p, a novel methyltransferase responsible for m1A 645 base modification of 25S rRNA. Nucleic Acids Research, 41(2), 1151–1163. 10.1093/nar/gks1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton, K. E. , Chen, B. , Liu, K. , Hunter, O. V. , Xie, Y. , Tu, B. P. , & Conrad, N. K. (2017). The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell, 169(5), 824–835.e14. 10.1016/j.cell.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Z. , Cheng, Y. , Tan, B. C.‐M. , Kang, L. , Tian, Z. , Zhu, Y. , … Wang, J. (2012). Comprehensive analysis of RNA‐Seq data reveals extensive RNA editing in a human transcriptome. Nature Biotechnology, 30(3), 253–260. 10.1038/nbt.2122 [DOI] [PubMed] [Google Scholar]
- Penzo, M. , Guerrieri, A. N. , Zacchini, F. , Treré, D. , & Montanaro, L. (2017). RNA pseudouridylation in physiology and medicine: For better and for worse. Genes, 8(11), 301. 10.3390/genes8110301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, R. P. , & Kelley, D. E. (1974). Existence of methylated messenger RNA in mouse L cells. Cell, 1(1), 37–42. 10.1016/0092-8674(74)90153-6 [DOI] [Google Scholar]
- Persson, B. C. , Esberg, B. , Ólafsson, Ó. , & Björk, G. R. (1994). Synthesis and function of isopentenyl adenosine derivatives in tRNA. Biochimie, 76(12), 1152–1160. 10.1016/0300-9084(94)90044-2 [DOI] [PubMed] [Google Scholar]
- Phillips, G. , Chikwana, V. M. , Maxwell, A. , El‐Yacoubi, B. , Swairjo, M. A. , Iwata‐Reuyl, D. , & de Crécy‐Lagard, V. (2010). Discovery and characterization of an amidinotransferase involved in the modification of archaeal tRNA. Journal of Biological Chemistry, 285(17), 12706–12713. 10.1074/jbc.M110.102236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, G. , & de Crécy‐Lagard, V. (2011). Biosynthesis and function of tRNA modifications in archaea. Current Opinion in Microbiology, 14(3), 335–341. 10.1016/J.MIB.2011.03.001 [DOI] [PubMed] [Google Scholar]
- Phillips, G. , Swairjo, M. A. , Gaston, K. W. , Bailly, M. , Limbach, P. A. , Iwata‐Reuyl, D. , & De Crécy‐Lagard, V. (2012). Diversity of archaeosine synthesis in crenarchaeota. ACS Chemical Biology, 7(2), 300–305. 10.1021/cb200361w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phizicky, E. M. , & Hopper, A. K. (2010). tRNA biology charges to the front. Genes & Development, 24(17), 1832–1860. 10.1101/gad.1956510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell, J. K. , Gilad, Y. , & Pritchard, J. K. (2012). Comment on “Widespread RNA and DNA sequence differences in the human transcriptome”. Science, 335(6074), 1302–1302. 10.1126/science.1210484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrel, F. , Björk, G. R. , Fontecave, M. , & Atta, M. (2002). Enzymatic modification of tRNAs. MiaB is an iron‐sulfur protein. Journal of Biological Chemistry, 277(16), 13367–13370. 10.1074/jbc.C100609200 [DOI] [PubMed] [Google Scholar]
- Ping, X.‐L. , Sun, B.‐F. , Wang, L. , Xiao, W. , Yang, X. , Wang, W.‐J. , … Yang, Y.‐G. (2014). Mammalian WTAP is a regulatory subunit of the RNA N 6‐methyladenosine methyltransferase. Cell Research, 24(2), 177–189. 10.1038/cr.2014.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, R. , Vågbø, C. B. , Jakobsson, M. E. , Kim, Y. , Baltissen, M. P. , O'Donohue, M.‐F. , … Falnes, P. Ø. (2020). The human methyltransferase ZCCHC4 catalyses N6‐methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Research, 48(2), 830–846. 10.1093/nar/gkz1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskol, R. , Peng, Z. , Wang, J. , & Li, J. B. (2013). Lack of evidence for existence of noncanonical RNA editing. Nature Biotechnology, 31(1), 19–20. 10.1038/nbt.2472 [DOI] [PubMed] [Google Scholar]
- Pleschke, J. M. , Kleczkowska, H. E. , Strohm, M. , & Althaus, F. R. (2000). Poly(ADP‐ribose) binds to specific domains in DNA damage checkpoint proteins. Journal of Biological Chemistry, 275(52), 40974–40980. 10.1074/jbc.M006520200 [DOI] [PubMed] [Google Scholar]
- Polikanov, Y. S. , Melnikov, S. V. , Söll, D. , & Steitz, T. A. (2015). Structural insights into the role of rRNA modifications in protein synthesis and ribosome assembly. Nature Structural & Molecular Biology, 22(4), 342–344. 10.1038/nsmb.2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollo‐Oliveira, L. , & De Crécy‐Lagard, V. (2019). Can protein expression be regulated by modulation of tRNA modification profiles? Biochemistry, 58(5), 355–362. 10.1021/acs.biochem.8b01035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova, A. M. , & Williamson, J. R. (2014). Quantitative analysis of rRNA modifications using stable isotope labeling and mass spectrometry. Journal of the American Chemical Society, 136(5), 2058–2069. 10.1021/ja412084b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath, H. T. , Knisbacher, B. A. , Eisenberg, E. , & Levanon, E. Y. (2017). Massive A‐to‐I RNA editing is common across the Metazoa and correlates with dsRNA abundance. Genome Biology, 18(1), 185. 10.1186/s13059-017-1315-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell, C. A. , Nicholls, T. J. , & Minczuk, M. (2015). Nuclear‐encoded factors involved in post‐transcriptional processing and modification of mitochondrial tRNAs in human disease. Frontiers in Genetics, 6, 79. 10.3389/fgene.2015.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purta, E. , Kaminska, K. H. , Kasprzak, J. M. , Bujnicki, J. M. , & Douthwaite, S. (2008). YbeA is the m3Ψ methyltransferase RlmH that targets nucleotide 1915 in 23S rRNA. RNA, 14(10), 2234–2244. 10.1261/rna.1198108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman, S. K. , Bujnicki, J. M. , Grosjean, H. , & Lapeyre, B. (2005). Trm11p and Trm112p are both required for the formation of 2‐methylguanosine at position 10 in yeast tRNA. Molecular and Cellular Biology, 25(11), 4359–4370. 10.1128/MCB.25.11.4359-4370.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakovich, L. J. , Tomlinson, J. , & Dos Santos, P. C. (2012). Functional analysis of Bacillus subtilis genes involved in the biosynthesis of 4‐thiouridine in tRNA. Journal of Bacteriology, 194(18), 4933–4940. 10.1128/JB.00842-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakovich, T. , Boland, C. , Bernstein, I. , Chikwana, V. M. , Iwata‐Reuyl, D. , & Kelly, V. P. (2011). Queuosine deficiency in eukaryotes compromises tyrosine production through increased tetrahydrobiopterin oxidation. Journal of Biological Chemistry, 286(22), 19354–19363. 10.1074/jbc.M111.219576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan, A. , Robb, G. B. , & Chan, S.‐H. (2016). mRNA capping: Biological functions and applications. Nucleic Acids Research, 44(16), 7511–7526. 10.1093/nar/gkw551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami, G. , & Li, J. B. (2014). RADAR: A rigorously annotated database of A‐to‐I RNA editing. Nucleic Acids Research, 42(Database), D109–D113. 10.1093/nar/gkt996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami, G. , Lin, W. , Piskol, R. , Tan, M. H. , Davis, C. , & Li, J. B. (2012). Accurate identification of human Alu and non‐Alu RNA editing sites. Nature Methods, 9(6), 579–581. 10.1038/nmeth.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranaei‐Siadat, E. , Fabret, C. , Seijo, B. , Dardel, F. , Grosjean, H. , & Nonin‐Lecomte, S. (2013). RNA‐methyltransferase TrmA is a dual‐specific enzyme responsible for C5‐methylation of uridine in both tmRNA and tRNA. RNA Biology, 10(4), 572–578. 10.4161/rna.24327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri, S. , Conrad, J. , Hall, B. G. , & Ofengand, J. (1998). A pseudouridine synthase required for the formation of two universally conserved pseudouridines in ribosomal RNA is essential for normal growth of Escherichia coli . RNA, 4(11), 1407–1417. 10.1017/s1355838298981146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader, J. S. , Metzgar, D. , Schimmel, P. , & de Crécy‐Lagard, V. (2004). Identification of four genes necessary for biosynthesis of the modified nucleoside queuosine. The Journal of Biological Chemistry, 279(8), 6280–6285. 10.1074/jbc.M310858200 [DOI] [PubMed] [Google Scholar]
- Rebagliati, M. R. , & Melton, D. A. (1987). Antisense RNA injections in fertilized frog eggs reveal an RNA duplex unwinding activity. Cell, 48(4), 599–605. 10.1016/0092-8674(87)90238-8 [DOI] [PubMed] [Google Scholar]
- Rebelo‐Guiomar, P. , Powell, C. A. , Van Haute, L. , & Minczuk, M. (2019). The mammalian mitochondrial epitranscriptome. Biochimica et Biophysica Acta (BBA): Gene Regulatory Mechanisms, 1862(3), 429–446. 10.1016/J.BBAGRM.2018.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, D. M. , Crain, P. F. , Edmonds, C. G. , Gupta, R. , Hashizume, T. , Stetter, K. O. , … McCloskey, J. A. (1992). Structure determination of two new amino acid‐containing derivatives of adenosine from tRNA of thermophilic bacteria and archaea. Nucleic Acids Research, 20(21), 5607–5615. 10.1093/nar/20.21.5607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichle, V. F. , Petrov, D. P. , Weber, V. , Jung, K. , & Kellner, S. (2019). NAIL‐MS reveals the repair of 2‐methylthiocytidine by AlkB in E. coli . Nature Communications, 10(1). 10.1038/s41467-019-13565-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler, M. , Novak, O. , Strnad, M. , & Schmülling, T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. The Plant Cell, 18(1), 40–54. 10.1105/tpc.105.037796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries, R. J. , Zaccara, S. , Klein, P. , Olarerin‐George, A. , Namkoong, S. , Pickering, B. F. , … Jaffrey, S. R. (2019). m6A enhances the phase separation potential of mRNA. Nature, 571(7765), 424–428. 10.1038/s41586-019-1374-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rife, J. P. , Cheng, C. S. , Moore, P. B. , & Strobel, S. A. (1998). N 2‐Methylguanosine is iso‐energetic with guanosine in RNA duplexes and GNRA tetraloops. Nucleic Acids Research, 26(16), 3640–3644. 10.1093/nar/26.16.3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson, M. P. , & Joyce, G. F. (2012). The origins of the RNA world. Cold Spring Harbor Perspectives in Biology, 4(5), a003608. 10.1101/cshperspect.a003608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, V. , Vasudevan, S. , Noma, A. , Carlson, B. A. , Green, J. E. , Suzuki, T. , & Chandrasekharappa, S. C. (2012). Structure‐function analysis of human TYW2 enzyme required for the biosynthesis of a highly modified wybutosine (yW) base in phenylalanine‐tRNA. PLoS One, 7(6), e39297. 10.1371/journal.pone.0039297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez‐Hernandez, A. , Spears, J. L. , Gaston, K. W. , Limbach, P. A. , Gamper, H. , Hou, Y.‐M. , … Perona, J. J. (2013). Structural and mechanistic basis for enhanced translational efficiency by 2‐thiouridine at the tRNA anticodon wobble position. Journal of Molecular Biology, 425(20), 3888–3906. 10.1016/j.jmb.2013.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas‐Benitez, D. , Thiaville, P. C. , Valérie De Crécy‐Lagard, X. , & Glavic, A. (2015). The levels of a universally conserved tRNA modification regulate cell growth. Journal of Biological Chemistry, 290, 18699–18707. 10.1074/jbc.M115.665406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romby, P. , Carbon, P. , Westhof, E. , Ehresmann, C. , Ebel, J. P. , Ehresmann, B. , & Giegé, R. (1987). Importance of conserved residues for the conformation of the T‐loop in tRNAs. Journal of Biomolecular Structure & Dynamics, 5(3), 669–687. 10.1080/07391102.1987.10506419 [DOI] [PubMed] [Google Scholar]
- Rongvaux, A. , Andris, F. , Van Gool, F. , & Leo, O. (2003). Reconstructing eukaryotic NAD metabolism. BioEssays, 25(7), 683–690. 10.1002/bies.10297 [DOI] [PubMed] [Google Scholar]
- Roovers, M. , Oudjama, Y. , Fislage, M. , Bujnicki, J. M. , Versees, W. , & Droogmans, L. (2012). The open reading frame TTC1157 of Thermus thermophilus HB27 encodes the methyltransferase forming N 2‐methylguanosine at position 6 in tRNA. RNA, 18(4), 815–824. 10.1261/rna.030411.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers, M. , Kaminska, K. H. , Tkaczuk, K. L. , Gigot, D. , Droogmans, L. , & Bujnicki, J. M. (2008). The YqfN protein of Bacillus subtilis is the tRNA: m1A22 methyltransferase (TrmK). Nucleic Acids Research, 36(10), 3252–3262. 10.1093/nar/gkn169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roovers, M. , Wouters, J. , Bujnicki, J. M. , Tricot, C. , Stalon, V. , Grosjean, H. , & Droogmans, L. (2004). A primordial RNA modification enzyme: The case of tRNA (m1A) methyltransferase. Nucleic Acids Research, 32(2), 465–476. 10.1093/nar/gkh191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roundtree, I. A. , Evans, M. E. , Pan, T. , & He, C. (2017). Dynamic RNA modifications in gene expression regulation. Cell, 169(7), 1187–1200. 10.1016/j.cell.2017.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio, M. A. T. , Gaston, K. W. , McKenney, K. M. , Fleming, I. M. C. , Paris, Z. , Limbach, P. A. , & Alfonzo, J. D. (2017). Editing and methylation at a single site by functionally interdependent activities. Nature, 542(7642), 494–497. 10.1038/nature21396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra, M. , Sas‐Chen, A. , Nir, R. , Winkler, R. , Nachshon, A. , Bar‐Yaacov, D. , … Schwartz, S. (2017). The m1A landscape on cytosolic and mitochondrial mRNA at single‐base resolution. Nature, 551(7679), 251–255. 10.1038/nature24456 [DOI] [PubMed] [Google Scholar]
- Sakai, Y. , Kimura, S. , & Suzuki, T. (2019). Dual pathways of tRNA hydroxylation ensure efficient translation by expanding decoding capability. Nature Communications, 10(1), 2858. 10.1038/s41467-019-10750-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai, Y. , Miyauchi, K. , Kimura, S. , & Suzuki, T. (2016). Biogenesis and growth phase‐dependent alteration of 5‐methoxycarbonylmethoxyuridine in tRNA anticodons. Nucleic Acids Research, 44(2), 509–523. 10.1093/nar/gkv1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar, J. C. , Ambrogelly, A. , Crain, P. F. , McCloskey, J. A. , & Soll, D. (2004). A truncated aminoacyl‐tRNA synthetase modifies RNA. Proceedings of the National Academy of Sciences of the United States of America, 101(20), 7536–7541. 10.1073/pnas.0401982101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarsky, D. A. , Balakin, A. G. , & Fournier, M. J. (1995). Characterization of three new snRNAs from Saccharomyces cerevisiae: snR34, snR35 and snR36. Nucleic Acids Research, 23(13), 2548–2554. 10.1093/nar/23.13.2548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, C. E. (2011). Adenosine deaminases acting on RNA (ADARs) are both antiviral and proviral. Virology, 411(2), 180–193. 10.1016/j.virol.2010.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi, M. , Harada, F. , & Nishimura, S. (1969). Isolation and characterization of N 6‐methyladenosine from Escherichia coli valine transfer RNA. Biochimica et Biophysica Acta (BBA): Nucleic Acids and Protein Synthesis, 190(2), 264–273. 10.1016/0005-2787(69)90078-1 [DOI] [PubMed] [Google Scholar]
- Sauerwald, A. , Sitaramaiah, D. , McCloskey, J. A. , Söll, D. , & Crain, P. F. (2005). N 6‐Acetyladenosine: A new modified nucleoside from Methanopyrus kandleri tRNA. FEBS Letters, 579(13), 2807–2810. 10.1016/j.febslet.2005.04.014 [DOI] [PubMed] [Google Scholar]
- Schosserer, M. , Minois, N. , Angerer, T. B. , Amring, M. , Dellago, H. , Harreither, E. , … Grillari, J. (2015). Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nature Communications, 6(1), 6158. 10.1038/ncomms7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, V. , Dantzer, F. , Ame, J.‐C. , & de Murcia, G. (2006). Poly(ADP‐ribose): Novel functions for an old molecule. Nature Reviews Molecular Cell Biology, 7(7), 517–528. 10.1038/nrm1963 [DOI] [PubMed] [Google Scholar]
- Schrider, D. R. , Gout, J.‐F. , & Hahn, M. W. (2011). Very few RNA and DNA sequence differences in the human transcriptome. PLoS One, 6(10), e25842. 10.1371/journal.pone.0025842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalm, E. L. , Grove, T. L. , Booker, S. J. , & Boal, A. K. (2016). Crystallographic capture of a radical S‐adenosylmethionine enzyme in the act of modifying tRNA. Science, 352(6283), 309–312. 10.1126/science.aad5367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, S. (2018). m1A within cytoplasmic mRNAs at single nucleotide resolution: A reconciled transcriptome‐wide map. RNA, 24(11), 1427–1436. 10.1261/rna.067348.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, S. , Bernstein, D. A. , Mumbach, M. R. , Jovanovic, M. , Herbst, R. H. , León‐Ricardo, B. X. , … Regev, A. (2014). Transcriptome‐wide mapping reveals widespread dynamic‐regulated pseudouridylation of ncRNA and mRNA. Cell, 159(1), 148–162. 10.1016/j.cell.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, M. P. , McGrath, K. , & Baczynskyj, L. (1970). The isolation and characterization of N‐[9‐(β‐d‐ribofuranosyl)‐purin‐6‐ylcarbamoyl] glycine from yeast transfer RNA. Biochemical and Biophysical Research Communications, 40(5), 1046–1052. 10.1016/0006-291X(70)90899-5 [DOI] [PubMed] [Google Scholar]
- Schweizer, U. , Bohleber, S. , & Fradejas‐Villar, N. (2017). The modified base isopentenyladenosine and its derivatives in tRNA. RNA Biology, 14(9), 1197–1208. 10.1080/15476286.2017.1294309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg, P. H. , Higuchi, M. , & Sprengel, R. (1998). RNA editing of brain glutamate receptor channels: Mechanism and physiology. Brain Research Reviews, 26(2–3), 217–229. 10.1016/S0165-0173(97)00062-3 [DOI] [PubMed] [Google Scholar]
- Seidel, A. , Brunner, S. , Seidel, P. , Fritz, G. I. , & Herbarth, O. (2006). Modified nucleosides: An accurate tumour marker for clinical diagnosis of cancer, early detection and therapy control. British Journal of Cancer, 94(11), 1726–1733. 10.1038/sj.bjc.6603164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seistrup, K. H. , Rose, S. , Birkedal, U. , Nielsen, H. , Huber, H. , & Douthwaite, S. (2017). Bypassing rRNA methylation by RsmA/Dim1 during ribosome maturation in the hyperthermophilic archaeon Nanoarchaeum equitans . Nucleic Acids Research, 45(4), 2007–2015. 10.1093/nar/gkw839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selberg, S. , Blokhina, D. , Aatonen, M. , Koivisto, P. , Siltanen, A. , Mervaala, E. , … Karelson, M. (2019). Discovery of small molecules that activate RNA methylation through cooperative binding to the METTL3‐14‐WTAP complex active site. Cell Reports, 26(13), 3762–3771.e5. 10.1016/j.celrep.2019.02.100 [DOI] [PubMed] [Google Scholar]
- Seley‐Radtke, K. L. , & Yates, M. K. (2018). The evolution of nucleoside analogue antivirals: A review for chemists and non‐chemists. Part 1: Early structural modifications to the nucleoside scaffold. Antiviral Research, 154, 66–86. 10.1016/j.antiviral.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvadurai, K. , Wang, P. , Seimetz, J. , & Huang, R. H. (2014). Archaeal Elp3 catalyzes tRNA wobble uridine modification at C5 via a radical mechanism. Nature Chemical Biology, 10(10), 810–812. 10.1038/nchembio.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendinc, E. , Valle‐Garcia, D. , Dhall, A. , Chen, H. , Henriques, T. , Navarrete‐Perea, J. , … Shi, Y. (2019). PCIF1 catalyzes m6Am mRNA methylation to regulate gene expression. Molecular Cell, 75(3), 620–630.e9. 10.1016/J.MOLCEL.2019.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger, B. , Auxilien, S. , Englisch, U. , Cramer, F. , & Fasiolo, F. (1997). The modified wobble base inosine in yeast tRNAIle is a positive determinant for aminoacylation by isoleucyl‐tRNA synthetase. Biochemistry, 36(27), 8269–8275. 10.1021/bi970206l [DOI] [PubMed] [Google Scholar]
- Sergiev, P. V. , Bogdanov, A. A. , & Dontsova, O. A. (2007). Ribosomal RNA guanine‐(N2)‐methyltransferases and their targets. Nucleic Acids Research, 35(7), 2295–2301. 10.1093/nar/gkm104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergiev, P. V. , Serebryakova, M. V. , Bogdanov, A. A. , & Dontsova, O. A. (2008). The ybiN gene of Escherichia coli encodes adenine‐N 6 methyltransferase specific for modification of A1618 of 23S ribosomal RNA, a methylated residue located close to the ribosomal exit tunnel. Journal of Molecular Biology, 375(1), 291–300. 10.1016/J.JMB.2007.10.051 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , Hartmann, J. D. , Watzinger, P. , Klepper, A. , Peifer, C. , Kötter, P. , … Entian, K. D. (2018). A single N 1‐methyladenosine on the large ribosomal subunit rRNA impacts locally its structure and the translation of key metabolic enzymes. Scientific Reports, 8(1), 11904. 10.1038/s41598-018-30383-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , & Lafontaine, D. L. J. (2015). ‘View from a bridge’: A new perspective on eukaryotic rRNA base modification. Trends in Biochemical Sciences, 40(10), 560–575. 10.1016/j.tibs.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Sharma, S. , Marchand, V. , Motorin, Y. , & Lafontaine, D. L. J. (2017). Identification of sites of 2′‐O‐methylation vulnerability in human ribosomal RNAs by systematic mapping. Scientific Reports, 7(1), 11490. 10.1038/s41598-017-09734-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Watzinger, P. , Kötter, P. , & Entian, K.‐D. (2013). Identification of a novel methyltransferase, Bmt2, responsible for the N 1‐methyl‐adenosine base modification of 25S rRNA in Saccharomyces cerevisiae . Nucleic Acids Research, 41(10), 5428–5443. 10.1093/nar/gkt195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, S. , Yang, J. , Düttmann, S. , Watzinger, P. , Kötter, P. , & Entian, K.‐D. (2014). Identification of novel methyltransferases, Bmt5 and Bmt6, responsible for the m3U methylations of 25S rRNA in Saccharomyces cerevisiae . Nucleic Acids Research, 42(5), 3246–3260. 10.1093/nar/gkt1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , Wang, X. , Lu, Z. , Zhao, B. S. , Ma, H. , Hsu, P. J. , … He, C. (2017). YTHDF3 facilitates translation and decay of N 6‐methyladenosine‐modified RNA. Cell Research, 27(3), 315–328. 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , Wei, J. , & He, C. (2019). Where, when, and how: Context‐dependent functions of RNA methylation writers, readers, and erasers. Molecular Cell, 74(4), 640–650. 10.1016/j.molcel.2019.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, H. , Zhang, X. , Weng, Y.‐L. , Lu, Z. , Liu, Y. , Lu, Z. , … Zhou, T. (2018). m6A facilitates hippocampus‐dependent learning and memory through YTHDF1. Nature, 563(7730), 249–253. 10.1038/s41586-018-0666-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigi, N. (2014). Biosynthesis and functions of sulfur modifications in tRNA. Frontiers in Genetics, 5, 67. 10.3389/fgene.2014.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigi, N. , Sakaguchi, Y. , Asai, S. , Suzuki, T. , & Watanabe, K. (2008). Common thiolation mechanism in the biosynthesis of tRNA thiouridine and sulphur‐containing cofactors. The EMBO Journal, 27(24), 3267–3278. 10.1038/emboj.2008.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigi, N. , Suzuki, T. , Terada, T. , Shirouzu, M. , Yokoyama, S. , & Watanabe, K. (2006). Temperature‐dependent biosynthesis of 2‐thioribothymidine of Thermus thermophilus tRNA. Journal of Biological Chemistry, 281(4), 2104–2113. 10.1074/jbc.M510771200 [DOI] [PubMed] [Google Scholar]
- Sierant, M. , Leszczynska, G. , Sadowska, K. , Dziergowska, A. , Rozanski, M. , Sochacka, E. , & Nawrot, B. (2016). S‐Geranyl‐2‐thiouridine Wobble nucleosides of bacterial tRNAs: Chemical and enzymatic synthesis of S‐geranylated‐RNAs and their physicochemical characterization. Nucleic Acids Research, 44(22), 10986–10998. 10.1093/nar/gkw727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierant, M. , Leszczynska, G. , Sadowska, K. , Komar, P. , Radzikowska‐Cieciura, E. , Sochacka, E. , & Nawrot, B. (2018). Escherichia coli tRNA 2‐selenouridine synthase (SelU) converts S2U‐RNA to Se2U‐RNA via S‐geranylated‐intermediate. FEBS Letters, 592(13), 2248–2258. 10.1002/1873-3468.13124 [DOI] [PubMed] [Google Scholar]
- Simsek, M. , & RajBhandary, U. L. (1972). The primary structure of yeast initiator transfer ribonucleic acid. Biochemical and Biophysical Research Communications, 49(2), 508–515. 10.1016/0006-291x(72)90440-8 [DOI] [PubMed] [Google Scholar]
- Sloan, K. E. , Warda, A. S. , Sharma, S. , Entian, K.‐D. , Lafontaine, D. L. J. , & Bohnsack, M. T. (2017). Tuning the ribosome: The influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biology, 14(9), 1138–1152. 10.1080/15476286.2016.1259781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. A. , & Visser, D. W. (1965). Studies on 5‐hydroxyuridine. The Journal of Biological Chemistry, 240, 446–453 http://www.ncbi.nlm.nih.gov/pubmed/14253450 [PubMed] [Google Scholar]
- Smith, T. S. , Zoltek, M. A. , & Simon, M. D. (2019). Reengineering a tRNA methyltransferase to covalently capture new RNA substrates. Journal of the American Chemical Society, 141, 17460–17465. 10.1021/jacs.9b08529 [DOI] [PubMed] [Google Scholar]
- Soma, A. , Ikeuchi, Y. , Kanemasa, S. , Kobayashi, K. , Ogasawara, N. , Ote, T. , … Suzuki, T. (2003). An RNA‐modifying enzyme that governs both the codon and amino acid specificities of isoleucine tRNA. Molecular Cell, 12(3), 689–698 http://www.ncbi.nlm.nih.gov/pubmed/14527414 [DOI] [PubMed] [Google Scholar]
- Sommer, B. , Köhler, M. , Sprengel, R. , & Seeburg, P. H. (1991). RNA editing in brain controls a determinant of ion flow in glutamate‐gated channels. Cell, 67(1), 11–19. 10.1016/0092-8674(91)90568-j [DOI] [PubMed] [Google Scholar]
- Songe‐Møller, L. , van den Born, E. , Leihne, V. , Vågbø, C. B. , Kristoffersen, T. , Krokan, H. E. , … Klungland, A. (2010). Mammalian ALKBH8 possesses tRNA methyltransferase activity required for the biogenesis of multiple Wobble uridine modifications implicated in translational decoding. Molecular and Cellular Biology, 30(7), 1814–1827. 10.1128/MCB.01602-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spenkuch, F. , Motorin, Y. , & Helm, M. (2014). Pseudouridine: Still mysterious, but never a fake (uridine)! RNA Biology, 11(12), 1540–1554. 10.4161/15476286.2014.992278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl, M. , Horn, C. , Brown, M. , Ioudovitch, A. , & Steinberg, S. (1998). Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Research, 26(1), 148–153. 10.1093/nar/26.1.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires, J. E. , Patel, H. R. , Nousch, M. , Sibbritt, T. , Humphreys, D. T. , Parker, B. J. , … Preiss, T. (2012). Widespread occurrence of 5‐methylcytosine in human coding and non‐coding RNA. Nucleic Acids Research, 40(11), 5023–5033. 10.1093/nar/gks144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires, J. E. , & Preiss, T. (2010). Function and detection of 5‐methylcytosine in eukaryotic RNA. Epigenomics, 2(5), 709–715. 10.2217/epi.10.47 [DOI] [PubMed] [Google Scholar]
- Stuart, J. W. , Basti, M. M. , Smith, W. S. , Forrest, B. , Guenther, R. , Sierzputowska‐Gracz, H. , … Agris, P. F. (1996). Structure of the trinucleotide D‐acp3 U‐A with coordinated Mg2+ demonstrates that modified nucleosides contribute to regional conformations of RNA. Nucleosides and Nucleotides, 15(5), 1009–1028. 10.1080/07328319608002031 [DOI] [Google Scholar]
- Su, A. A. H. , & Randau, L. (2011). A‐to‐I and C‐to‐U editing within transfer RNAs. Biochemistry (Moscow), 76(8), 932–937. 10.1134/S0006297911080098 [DOI] [PubMed] [Google Scholar]
- Su, D. , Ojo, T. T. , Söll, D. , & Hohn, M. J. (2012). Selenomodification of tRNA in archaea requires a bipartite rhodanese enzyme. FEBS Letters, 586(6), 717–721. 10.1016/j.febslet.2012.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Zhang, M. , Li, K. , Bai, D. , & Yi, C. (2019). Cap‐specific, terminal N 6‐methylation by a mammalian m6Am methyltransferase. Cell Research, 29(1), 80–82. 10.1038/s41422-018-0117-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, H. , Sheng, J. , Hassan, A. E. A. , Jiang, S. , Gan, J. , & Huang, Z. (2012). Novel RNA base pair with higher specificity using single selenium atom. Nucleic Acids Research, 40(11), 5171–5179. 10.1093/nar/gks010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, G. , Shimazu, N. , & Tanaka, M. (2012). A yeast prion, Mod5, promotes acquired drug resistance and cell survival under environmental stress. Science, 336(6079), 355–359. 10.1126/science.1219491 [DOI] [PubMed] [Google Scholar]
- Suzuki, Y. , Noma, A. , Suzuki, T. , Ishitani, R. , & Nureki, O. (2009). Structural basis of tRNA modification with CO2 fixation and methylation by wybutosine synthesizing enzyme TYW4. Nucleic Acids Research, 37(9), 2910–2925. 10.1093/nar/gkp158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla, B. , Gauthier, C. , Legault, J. , Fleury, P.‐Y. , Lavoie, S. , Mshvildadze, V. , … Pichette, A. (2014). Isolation of a new disaccharide nucleoside from Helleborus caucasicus: Structure elucidation and total synthesis of hellecaucaside A and its β‐anomer. Carbohydrate Research, 398, 80–89. 10.1016/j.carres.2014.06.027 [DOI] [PubMed] [Google Scholar]
- Sylvers, L. A. , Rogers, K. C. , Shimizu, M. , Ohtsuka, E. , & Söll, D. (1993). A 2‐thiouridine derivative in tRNAGlu is a positive determinant for aminoacylation by Escherichia coli glutamyl‐tRNA synthetase. Biochemistry, 32(15), 3836–3841. 10.1021/bi00066a002 [DOI] [PubMed] [Google Scholar]
- Takakura, M. , Ishiguro, K. , Akichika, S. , Miyauchi, K. , & Suzuki, T. (2019). Biogenesis and functions of aminocarboxypropyluridine in tRNA. Nature Communications, 10(1), 5542. 10.1038/s41467-019-13525-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura, K. , Himeno, H. , Asahara, H. , Hasegawa, T. , & Shimizu, M. (1992). In vitro study of E.coli tRNA Arg and tRNA Lys identity elements. Nucleic Acids Research, 20(9), 2335–2339. 10.1093/nar/20.9.2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur, P. , Estevez, M. , Lobue, P. A. , Limbach, P. A. , & Addepalli, B. (2020). Improved RNA modification mapping of cellular non‐coding RNAs using C‐ and U‐specific RNases. The Analyst, 145(3). 10.1039/c9an02111f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar, R. , Bacolla, A. , Oyeniran, C. , Brickner, J. R. , Chinnam, N. B. , Mosammaparast, N. , & Tainer, J. A. (2019). RNA modifications: Reversal mechanisms and cancer. Biochemistry, 58(5), 312–329. 10.1021/acs.biochem.8b00949 [DOI] [PubMed] [Google Scholar]
- Thiaville, P. C. , El Yacoubi, B. , Köhrer, C. , Thiaville, J. J. , Deutsch, C. , Iwata‐Reuyl, D. , … de Crécy‐Lagard, V. (2015). Essentiality of threonylcarbamoyladenosine (t6A), a universal tRNA modification, in bacteria. Molecular Microbiology, 98(6), 1199–1221. 10.1111/mmi.13209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiaville, P. C. , Iwata‐Reuyl, D. , & de Crécy‐Lagard, V. (2014). Diversity of the biosynthesis pathway for threonylcarbamoyladenosine (t6A), a universal modification of tRNA. RNA Biology, 11(12), 1529–1539. 10.4161/15476286.2014.992277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiaville, P. C. , Legendre, R. , Rojas‐Benítez, D. , Baudin‐Baillieu, A. , Hatin, I. , Chalancon, G. , … De Crécy‐Lagard, V. (2016). Global translational impacts of the loss of the tRNA modification t6A in yeast. Microbial Cell, 3(1), 29–45. 10.15698/mic2016.01.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G. , & Favre, A. (1980). 4‐thiouridine triggers both growth delay induced by near‐ultraviolet light and photoprotection. European Journal of Biochemistry, 113(1), 67–74. 10.1111/j.1432-1033.1980.tb06140.x [DOI] [PubMed] [Google Scholar]
- Toh, S.‐M. , Xiong, L. , Bae, T. , & Mankin, A. S. (2008). The methyltransferase YfgB/RlmN is responsible for modification of adenosine 2503 in 23S rRNA. RNA, 14(1), 98–106. 10.1261/rna.814408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolstoshev, P. , & Wells, J. R. E. (1973). The absence of saturated pyrimidine bases in chromatin‐associated RNA from avian reticulocytes and mouse ascites cells. Biochemical and Biophysical Research Communications, 51(1), 223–231. 10.1016/0006-291X(73)90532-9 [DOI] [PubMed] [Google Scholar]
- Tomaselli, S. , Galeano, F. , Alon, S. , Raho, S. , Galardi, S. , Polito, A. A. , … Gallo, A. (2015). Modulation of microRNA editing, expression and processing by ADAR2 deaminase in glioblastoma. Genome Biology, 16(1), 5. 10.1186/s13059-014-0575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomikawa, C. (2018). 7‐Methylguanosine modifications in transfer RNA (tRNA). International Journal of Molecular Sciences, 19(12). 10.3390/ijms19124080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkuvienė, M. , Mickutė, M. , Vilkaitis, G. , & Klimašauskas, S. (2019). Repurposing enzymatic transferase reactions for targeted labeling and analysis of DNA and RNA. Current Opinion in Biotechnology, 55, 114–123. 10.1016/j.copbio.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, J. , Flavell, R. A. , & Li, H.‐B. (2018). RNA m6A modification and its function in diseases. Frontiers of Medicine, 12(4), 481–489. 10.1007/s11684-018-0654-8 [DOI] [PubMed] [Google Scholar]
- Topisirovic, I. , Svitkin, Y. V. , Sonenberg, N. , & Shatkin, A. J. (2011). Cap and cap‐binding proteins in the control of gene expression. WIREs: RNA, 2(2), 277–298. 10.1002/wrna.52 [DOI] [PubMed] [Google Scholar]
- Torres, A. G. , Piñeyro, D. , Filonava, L. , Stracker, T. H. , Batlle, E. , & Ribas de Pouplana, L. (2014). A‐to‐I editing on tRNAs: Biochemical, biological and evolutionary implications. FEBS Letters, 588(23), 4279–4286. 10.1016/j.febslet.2014.09.025 [DOI] [PubMed] [Google Scholar]
- Trixl, L. , & Lusser, A. (2019). The dynamic RNA modification 5‐methylcytosine and its emerging role as an epitranscriptomic mark. WIREs: RNA, 10(1), e1510. 10.1002/wrna.1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuorto, F. , Legrand, C. , Cirzi, C. , Federico, G. , Liebers, R. , Müller, M. , … Lyko, F. (2018). Queuosine‐modified tRNAs confer nutritional control of protein translation. The EMBO Journal, 37(18). 10.15252/embj.201899777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda, N. , Suzuki, T. , Yukawa, M. , Ohya, Y. , Shindo, H. , Watanabe, K. , & Suzuki, T. (2005). Mitochondria‐specific RNA‐modifying enzymes responsible for the biosynthesis of the wobble base in mitochondrial tRNAs. Implications for the molecular pathogenesis of human mitochondrial diseases. The Journal of Biological Chemistry, 280(2), 1613–1624. 10.1074/jbc.M409306200 [DOI] [PubMed] [Google Scholar]
- Urbonavičius, J. , Meškys, R. , & Grosjean, H. (2014). Biosynthesis of wyosine derivatives in tRNAPhe of archaea: Role of a remarkable bifunctional tRNAPhe:m1G/imG2 methyltransferase. RNA, 20(6), 747–753. 10.1261/rna.043315.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonavicius, J. , Qian, Q. , Durand, J. M. , Hagervall, T. G. , & Björk, G. R. (2001). Improvement of reading frame maintenance is a common function for several tRNA modifications. The EMBO Journal, 20(17), 4863–4873. 10.1093/emboj/20.17.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbonavicius, J. , Skouloubris, S. , Myllykallio, H. , & Grosjean, H. (2005). Identification of a novel gene encoding a flavin‐dependent tRNA:m5U methyltransferase in bacteria – Evolutionary implications. Nucleic Acids Research, 33(13), 3955–3964. 10.1093/nar/gki703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Born, E. , Vågbø, C. B. , Songe‐Møller, L. , Leihne, V. , Lien, G. F. , Leszczynska, G. , … Falnes, P. Ø. (2011). ALKBH8‐mediated formation of a novel diastereomeric pair of wobble nucleosides in mammalian tRNA. Nature Communications, 2(1), 172. 10.1038/ncomms1173 [DOI] [PubMed] [Google Scholar]
- Van Haute, L. , Powell, C. A. , & Minczuk, M. (2017). Dealing with an unconventional genetic code in mitochondria: The biogenesis and pathogenic defects of the 5‐formylcytosine modification in mitochondrial tRNAMet. Biomolecules, 7(1), 24. 10.3390/biom7010024 [DOI] [Google Scholar]
- Van Lanen, S. G. , Reader, J. S. , Swairjo, M. A. , de Crecy‐Lagard, V. , Lee, B. , & Iwata‐Reuyl, D. (2005). From cyclohydrolase to oxidoreductase: Discovery of nitrile reductase activity in a common fold. Proceedings of the National Academy of Sciences of the United States of America, 102(12), 4264–4269. 10.1073/pnas.0408056102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tran, N. , Ernst, F. G. M. , Hawley, B. R. , Zorbas, C. , Ulryck, N. , Hackert, P. , … Lafontaine, D. L. J. (2019). The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Research, 47, 7719–7733. 10.1093/nar/gkz619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tran, N. , Muller, L. , Ross, R. L. , Lestini, R. , Létoquart, J. , Ulryck, N. , … Graille, M. (2018). Evolutionary insights into Trm112‐methyltransferase holoenzymes involved in translation between archaea and eukaryotes. Nucleic Acids Research, 46(16), 8483–8499. 10.1093/nar/gky638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väre, V. , Eruysal, E. , Narendran, A. , Sarachan, K. , & Agris, P. (2017). Chemical and conformational diversity of modified nucleosides affects tRNA structure and function. Biomolecules, 7(4), 29. 10.3390/biom7010029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veĉerek, B. , Moll, I. , & Bläsi, U. (2007). Control of Fur synthesis by the non‐coding RNA RyhB and iron‐responsive decoding. EMBO Journal, 26(4), 965–975. 10.1038/sj.emboj.7601553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigts‐Hoffmann, F. , Hengesbach, M. , Kobitski, A. Y. , van Aerschot, A. , Herdewijn, P. , Nienhaus, G. U. , & Helm, M. (2007). A methyl group controls conformational equilibrium in human mitochondrial tRNALys . Journal of the American Chemical Society, 129(44), 13382–13383. 10.1021/ja075520+ [DOI] [PubMed] [Google Scholar]
- Wachino, J. , Shibayama, K. , Kurokawa, H. , Kimura, K. , Yamane, K. , Suzuki, S. , … Arakawa, Y. (2007). Novel plasmid‐mediated 16S rRNA m1A1408 methyltransferase, NpmA, found in a clinically isolated Escherichia coli strain resistant to structurally diverse aminoglycosides. Antimicrobial Agents and Chemotherapy, 51(12), 4401–4409. 10.1128/AAC.00926-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waku, T. , Nakajima, Y. , Yokoyama, W. , Nomura, N. , Kako, K. , Kobayashi, A. , … Fukamizu, A. (2016). NML‐mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53‐dependent manner. Journal of Cell Science, 129(12), 2382–2393. 10.1242/jcs.183723 [DOI] [PubMed] [Google Scholar]
- Wan, L. C. K. , Mao, D. Y. L. , Neculai, D. , Strecker, J. , Chiovitti, D. , Kurinov, I. , … Sicheri, F. (2013). Reconstitution and characterization of eukaryotic N 6‐threonylcarbamoylation of tRNA using a minimal enzyme system. Nucleic Acids Research, 41(12), 6332–6346. 10.1093/nar/gkt322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. , Luo, Z. , He, K. , Delaney, M. O. , Chen, D. , & Sheng, J. (2016). Base pairing and structural insights into the 5‐formylcytosine in RNA duplex. Nucleic Acids Research, 44(10), 4968–4977. 10.1093/nar/gkw235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Lu, Z. , Gomez, A. , Hon, G. C. , Yue, Y. , Han, D. , … He, C. (2014). N 6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature, 505(7481), 117–120. 10.1038/nature12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Zhao, B. S. , Roundtree, I. A. , Lu, Z. , Han, D. , Ma, H. , … He, C. (2015). N 6‐Methyladenosine modulates messenger RNA translation efficiency. Cell, 161(6), 1388–1399. 10.1016/J.CELL.2015.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Matuszek, Z. , Huang, Y. , Parisien, M. , Dai, Q. , Clark, W. , … Pan, T. (2018). Queuosine modification protects cognate tRNAs against ribonuclease cleavage. RNA, 24(10), 1305–1313. 10.1261/rna.067033.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Yan, Q. , & Guan, M.‐X. (2010). Combination of the loss of cmnm5U34 with the lack of s2U34 modifications of tRNALys, tRNAGlu, and tRNAGln altered mitochondrial biogenesis and respiration. Journal of Molecular Biology, 395(5), 1038–1048. 10.1016/j.jmb.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda, A. S. , Kretschmer, J. , Hackert, P. , Lenz, C. , Urlaub, H. , Höbartner, C. , … Bohnsack, M. T. (2017). Human METTL16 is a N 6‐methyladenosine (m6A) methyltransferase that targets pre‐mRNAs and various non‐coding RNAs. EMBO Reports, 18(11), 2004–2014. 10.15252/embr.201744940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, L. , Flaks, J. G. , & Buchanan, J. M. (1957). Biosynthesis of the purines. XX. Integration of enzymatic transformylation reactions. The Journal of Biological Chemistry, 229(2), 627–640 http://www.ncbi.nlm.nih.gov/pubmed/13502327 [PubMed] [Google Scholar]
- Warren, L. , Manos, P. D. , Ahfeldt, T. , Loh, Y.‐H. , Li, H. , Lau, F. , … Rossi, D. J. (2010). Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell, 7(5), 618–630. 10.1016/j.stem.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, J. , Liu, F. , Lu, Z. , Fei, Q. , Ai, Y. , He, P. C. , … He, C. (2018). Differential m6A, m6Am, and m1A demethylation mediated by FTO in the cell nucleus and cytoplasm. Molecular Cell, 71(6), 973–985.e5. 10.1016/J.MOLCEL.2018.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidel, H. , & Roithner, E. (1896). Über den Abbau einiger Säureamide. Monatshefte für Chemie, 17(1), 172–190. 10.1007/BF01519206 [DOI] [Google Scholar]
- Wetzel, C. , & Limbach, P. A. (2016). Mass spectrometry of modified RNAs: Recent developments. The Analyst, 141(1), 16–23. 10.1039/c5an01797a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, F. , Jenkins, H. T. , Griffiths, S. C. , Byrne, R. T. , Dodson, E. J. , & Antson, A. A. (2015). From bacterial to human dihydrouridine synthase: Automated structure determination. Acta Crystallographica Section D: Biological Crystallography, 71, 1564–1571. 10.1107/S1399004715009220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese, C. R. (1967). The genetic code: The molecular basis for genetic expression (p. 200). Harper and Row. Retrieved from http://www.sidalc.net/cgi-bin/wxis.exe/?IsisScript=bac.xis&method=post&formato=2&cantidad=1&expresion=mfn=035868
- Woese, C. R. , & Fox, G. E. (1977). Phylogenetic structure of the prokaryotic domain: The primary kingdoms. Proceedings of the National Academy of Sciences of the United States of America, 74(11), 5088–5090. 10.1073/pnas.74.11.5088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese, C. R. , Gutell, R. , Gupta, R. , & Noller, H. F. (1983). Detailed analysis of the higher‐order structure of 16S‐like ribosomal ribonucleic acids. Microbiological Reviews, 47(4), 621–669. 10.1128/mmbr.47.4.621-669.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf, J. , Gerber, A. P. , & Keller, W. (2002). tadA, an essential tRNA‐specific adenosine deaminase from Escherichia coli . The EMBO Journal, 21(14), 3841–3851. 10.1093/emboj/cdf362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, H.‐H. , & Chambers, S. K. (2019). Human ALKBH3‐induced m1A demethylation increases the CSF‐1 mRNA stability in breast and ovarian cancer cells. Biochimica et Biophysica Acta (BBA): Gene Regulatory Mechanisms, 1862(1), 35–46. 10.1016/j.bbagrm.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Wright, D. J. , Force, C. R. , & Znosko, B. M. (2018). Stability of RNA duplexes containing inosine·cytosine pairs. Nucleic Acids Research, 46(22), 12099–12108. 10.1093/nar/gky907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wroblewska, L. , Kitada, T. , Endo, K. , Siciliano, V. , Stillo, B. , Saito, H. , & Weiss, R. (2015). Mammalian synthetic circuits with RNA binding proteins for RNA‐only delivery. Nature Biotechnology, 33(8), 839–841. 10.1038/nbt.3301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, B. , Su, S. , Patil, D. P. , Liu, H. , Gan, J. , Jaffrey, S. R. , & Ma, J. (2018). Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nature Communications, 9(1), 420. 10.1038/s41467-017-02770-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J. , Xiao, H. , Wang, T. , Hong, T. , Fu, B. , Bai, D. , … Zhou, X. (2015). N 6‐Hydroperoxymethyladenosine: A new intermediate of chemical oxidation of N 6‐methyladenosine mediated by bicarbonate‐activated hydrogen peroxide. Chemical Science, 6(5), 3013–3017. 10.1039/C5SC00484E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. , Li, A. , Sun, B. , Sun, J.‐G. , Zhang, J. , Zhang, T. , … Yuan, Z. (2019). A novel m6A reader Prrc2a controls oligodendroglial specification and myelination. Cell Research, 29(1), 23–41. 10.1038/s41422-018-0113-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurm, J. P. , Meyer, B. , Bahr, U. , Held, M. , Frolow, O. , Kötter, P. , … Wöhnert, J. (2010). The ribosome assembly factor Nep1 responsible for Bowen‐Conradi syndrome is a pseudouridine‐N 1‐specific methyltransferase. Nucleic Acids Research, 38(7), 2387–2398. 10.1093/nar/gkp1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Y. , Laurent, B. , Hsu, C.‐H. , Nachtergaele, S. , Lu, Z. , Sheng, W. , … Shi, Y. (2017). RNA m6A methylation regulates the ultraviolet‐induced DNA damage response. Nature, 543(7646), 573–576. 10.1038/nature21671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing, F. , Hiley, S. L. , Hughes, T. R. , & Phizicky, E. M. (2004). The specificities of four yeast dihydrouridine synthases for cytoplasmic tRNAs. Journal of Biological Chemistry, 279(17), 17850–17860. 10.1074/jbc.M401221200 [DOI] [PubMed] [Google Scholar]
- Xu, L. , Liu, X. , Sheng, N. , Oo, K. S. , Liang, J. , Chionh, Y. H. , … Fu, X.‐Y. (2017). Three distinct 3‐methylcytidine (m3C) methyltransferases modify tRNA and mRNA in mice and humans. The Journal of Biological Chemistry, 292(35), 14695–14703. 10.1074/jbc.M117.798298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, L.‐D. , & Öhman, M. (2018). ADAR1 editing and its role in cancer. Genes, 10(1), 12. 10.3390/genes10010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan, J. J. , Sun, W. J. , Lin, P. H. , Zhou, K. R. , Liu, S. , Zheng, L. L. , … Yang, J. H. (2018). RMBase v2.0: Deciphering the map of RNA modifications from epitranscriptome sequencing data. Nucleic Acids Research, 46(D1), D327–D334. 10.1093/nar/gkx934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi, Z. , Ihara, M. , Kuchino, Y. , Gupta, R. , Woese, C. R. , & Nishimura, S. (1982). Archaebacterial tRNA contains 1‐methylinosine at residue 57 in TΨC‐loop. Nucleic Acids Symposium Series, 11, 209–213 http://www.ncbi.nlm.nih.gov/pubmed/7183961 [PubMed] [Google Scholar]
- Yan, F. , & Fujimori, D. G. (2011). RNA methylation by radical SAM enzymes RlmN and Cfr proceeds via methylene transfer and hydride shift. Proceedings of the National Academy of Sciences of the United States of America, 108(10), 3930–3934. 10.1073/pnas.1017781108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, F. , LaMarre, J. M. , Röhrich, R. , Wiesner, J. , Jomaa, H. , Mankin, A. S. , & Fujimori, D. G. (2010). RlmN and Cfr are radical SAM enzymes involved in methylation of ribosomal RNA. Journal of the American Chemical Society, 132(11), 3953–3964. 10.1021/ja910850y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, W. , Chendrimada, T. P. , Wang, Q. , Higuchi, M. , Seeburg, P. H. , Shiekhattar, R. , & Nishikura, K. (2006). Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nature Structural & Molecular Biology, 13(1), 13–21. 10.1038/nsmb1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Zhou, X. , & Jin, Y. (2013). ADAR‐mediated RNA editing in non‐coding RNA sequences. Science China Life Sciences, 56(10), 944–952. 10.1007/s11427-013-4546-5 [DOI] [PubMed] [Google Scholar]
- Yim, L. , Moukadiri, I. , Björk, G. R. , & Armengod, M.‐E. (2006). Further insights into the tRNA modification process controlled by proteins MnmE and GidA of Escherichia coli . Nucleic Acids Research, 34(20), 5892–5905. 10.1093/NAR/GKL752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip, W. S. V. , Shigematsu, H. , Taylor, D. W. , & Baserga, S. J. (2016). Box C/D sRNA stem ends act as stabilizing anchors for box C/D di‐sRNPs. Nucleic Acids Research, 44(18), 8976–8989. 10.1093/nar/gkw576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokogawa, T. , Nomura, Y. , Yasuda, A. , Ogino, H. , Hiura, K. , Nakada, S. , … Ohno, S. (2019). Identification of a radical SAM enzyme involved in the synthesis of archaeosine. Nature Chemical Biology, 15(12), 1148–1155. 10.1038/s41589-019-0390-7 [DOI] [PubMed] [Google Scholar]
- Yokoyama, S. , Watanabe, K. , & Miyazawa, T. (1987). Dynamic structures and functions of transfer ribonucleic acids from extreme thermophiles. Advances in Biophysics, 23, 115–147 http://www.ncbi.nlm.nih.gov/pubmed/2449804 [DOI] [PubMed] [Google Scholar]
- Yoon, K.‐J. , Ringeling, F. R. , Vissers, C. , Jacob, F. , Pokrass, M. , Jimenez‐Cyrus, D. , … Song, H. (2017). Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell, 171(4), 877–889.e17. 10.1016/j.cell.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You, X.‐J. , Liu, T. , Ma, C.‐J. , Qi, C.‐B. , Tong, Y. , Zhao, X. , … Feng, Y.‐Q. (2019). Determination of RNA hydroxylmethylation in mammals by mass spectrometry analysis. Analytical Chemistry, 91(16), 10477–10483. 10.1021/acs.analchem.9b01318 [DOI] [PubMed] [Google Scholar]
- Yu, A.‐M. , Jian, C. , Yu, A. H. , & Tu, M.‐J. (2019). RNA therapy: Are we using the right molecules? Pharmacology & Therapeutics, 196, 91–104. 10.1016/J.PHARMTHERA.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, A. T. , Ge, J. , & Yu, Y.‐T. (2011). Pseudouridines in spliceosomal snRNAs. Protein & Cell, 2(9), 712–725. 10.1007/s13238-011-1087-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, N. , Jora, M. , Solivio, B. , Thakur, P. , Acevedo‐Rocha, C. G. , Randau, L. , … Limbach, P. A. (2019). tRNA modification profiles and codon‐decoding strategies in Methanocaldococcus jannaschii . Journal of Bacteriology, 201(9), e00690–e00618. 10.1128/JB.00690-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. T. , & Meier, U. T. (2014). RNA‐guided isomerization of uridine to pseudouridine – Pseudouridylation. RNA Biology, 11(12), 1483–1494. 10.4161/15476286.2014.972855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y.‐T. , Shu, M. D. , & Steitz, J. A. (1998). Modifications of U2 snRNA are required for snRNP assembly and pre‐mRNA splicing. The EMBO Journal, 17(19), 5783–5795. 10.1093/emboj/17.19.5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, Y. , Zallot, R. , Grove, T. L. , Payan, D. J. , Martin‐Verstraete, I. , Šepić, S. , … de Crécy‐Lagard, V. (2019). Discovery of novel bacterial queuine salvage enzymes and pathways in human pathogens. Proceedings of the National Academy of Sciences of the United States of America, 116(38), 19126–19135. 10.1073/pnas.1909604116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov, M. M. , Yusupova, G. Z. , Baucom, A. , Lieberman, K. , Earnest, T. N. , Cate, J. H. , & Noller, H. F. (2001). Crystal structure of the ribosome at 5.5 Å resolution. Science, 292(5518), 883–896. 10.1126/science.1060089 [DOI] [PubMed] [Google Scholar]
- Zaccara, S. , Ries, R. J. , & Jaffrey, S. R. (2019). Reading, writing and erasing mRNA methylation. Nature Reviews Molecular Cell Biology, 20(10), 608–624. 10.1038/s41580-019-0168-5 [DOI] [PubMed] [Google Scholar]
- Zahn, K. E. , Averill, A. , Wallace, S. S. , & Doublié, S. (2011). The miscoding potential of 5‐hydroxycytosine arises due to template instability in the replicative polymerase active site. Biochemistry, 50(47), 10350–10358. 10.1021/bi201219s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallot, R. , Ross, R. , Chen, W. H. , Bruner, S. D. , Limbach, P. A. , & De Crécy‐Lagard, V. (2017). Identification of a novel epoxyqueuosine reductase family by comparative genomics. ACS Chemical Biology, 12(3), 844–851. 10.1021/acschembio.6b01100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallot, R. , Yuan, Y. , & De Crécy‐Lagard, V. (2017). The Escherichia coli COG1738 member YhhQ is involved in 7‐cyanodeazaguanine (preQ0) transport. Biomolecules, 7(1). 10.3390/biom7010012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi, L. , Lui, K. O. , von Gise, A. , Ma, Q. , Ebina, W. , Ptaszek, L. M. , … Chien, K. R. (2013). Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nature Biotechnology, 31(10), 898–907. 10.1038/nbt.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Lentini, J. M. , Prevost, C. T. , Hashem, M. O. , Alkuraya, F. S. , & Fu, D. (2020). An intellectual disability‐associated missense variant in TRMT1 impairs tRNA modification and reconstitution of enzymatic activity. Human Mutation, 41(3), 600–607. 10.1002/humu.23976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.‐S. , Liu, C. , Ma, H. , Dai, Q. , Sun, H.‐L. , Luo, G. , … He, C. (2019). Transcriptome‐wide mapping of internal N 7‐methylguanosine methylome in mammalian mRNA. Molecular Cell, 74, 1304–1316.e8. 10.1016/j.molcel.2019.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N. , Shi, S. , Jia, T. Z. , Ziegler, A. , Yoo, B. , Yuan, X. , … Zhang, S. (2019). A general LC‐MS‐based RNA sequencing method for direct analysis of multiple‐base modifications in RNA mixtures. Nucleic Acids Research, 47(20), e125–e125. 10.1093/nar/gkz731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Wei, L.‐H. , Wang, Y. , Xiao, Y. , Liu, J. , Zhang, W. , … Jia, G. (2019). Structural insights into FTO's catalytic mechanism for the demethylation of multiple RNA substrates. Proceedings of the National Academy of Sciences of the United States of America, 116(8), 2919–2924. 10.1073/pnas.1820574116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B. S. , & He, C. (2015). Pseudouridine in a new era of RNA modifications. Cell Research, 25(2), 153–154. 10.1038/cr.2014.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, B. S. , Nachtergaele, S. , Roundtree, I. A. , & He, C. (2018). Our views of dynamic N 6‐methyladenosine RNA methylation. RNA, 24(3), 268–272. 10.1261/rna.064295.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Patton, J. R. , Ghosh, S. K. , Fischel‐Ghodsian, N. , Shen, L. , & Spanjaard, R. A. (2007). Pus3p‐ and Pus1p‐dependent pseudouridylation of steroid receptor RNA activator controls a functional switch that regulates nuclear receptor signaling. Molecular Endocrinology, 21(3), 686–699. 10.1210/me.2006-0414 [DOI] [PubMed] [Google Scholar]
- Zhao, Y. , Dunker, W. , Yu, Y.‐T. , & Karijolich, J. (2018). The role of noncoding RNA pseudouridylation in nuclear gene expression events. Frontiers in Bioengineering and Biotechnology, 6, 8. 10.3389/fbioe.2018.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhao, Q. , Kaboli, P. J. , Shen, J. , Li, M. , Wu, X. , … Xiao, Z. (2019). m1A regulated genes modulate PI3K/AKT/mTOR and ErbB pathways in gastrointestinal cancer. Translational Oncology, 12(10), 1323–1333. 10.1016/j.tranon.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, C. , Black, K. A. , & Dos Santos, P. C. (2017). Diverse mechanisms of sulfur decoration in bacterial tRNA and their cellular functions. Biomolecules, 7(4), 33. 10.3390/biom7010033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, G. , Dahl, J. A. , Niu, Y. , Fedorcsak, P. , Huang, C.‐M. , Li, C. J. , … He, C. (2013). ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Molecular Cell, 49(1), 18–29. 10.1016/J.MOLCEL.2012.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y. , Lorenzo, C. , & Beal, P. A. (2017). DNA editing in DNA/RNA hybrids by adenosine deaminases that act on RNA. Nucleic Acids Research, 45(6), 3369–3377. 10.1093/nar/gkx050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Liu, Y. , Rohde, C. , Pauli, C. , Gerloff, D. , Köhn, M. , … Müller‐Tidow, C. (2017). AML1‐ETO requires enhanced C/D box snoRNA/RNP formation to induce self‐renewal and leukaemia. Nature Cell Biology, 19(7), 844–855. 10.1038/ncb3563 [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Pirnie, S. P. , & Carmichael, G. G. (2017). High‐throughput and site‐specific identification of 2′‐O‐methylation sites using ribose oxidation sequencing (RibOxi‐seq). RNA, 23(8), 1303–1314. 10.1261/rna.061549.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinshteyn, B. , & Nishikura, K. (2009). Adenosine‐to‐inosine RNA editing. Wiley Interdisciplinary Reviews: Systems Biology and Medicine, 1(2), 202–209. 10.1002/wsbm.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorbas, C. , Nicolas, E. , Wacheul, L. , Huvelle, E. , Heurgué‐Hamard, V. , & Lafontaine, D. L. J. (2015). The human 18S rRNA base methyltransferases DIMT1L and WBSCR22‐TRMT112 but not rRNA modification are required for ribosome biogenesis. Molecular Biology of the Cell, 26(11), 2080–2095. 10.1091/mbc.E15-02-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zur, H. , & Tuller, T. (2013). New universal rules of eukaryotic translation initiation fidelity. PLoS Computational Biology, 9(7), e1003136. 10.1371/journal.pcbi.1003136 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Supporting information.


