Abstract
Simple Summary
As the only predatory group in the family Pentatomidae, Asopinae are a diverse group of specialized soft-bodied insect predators, which have the potential for use in controlling pests of orchards, forests, and field crops. However, the feeding behavior remains poorly known for Asopinae, especially how the mouthpart structures relate to various functions in feeding. The fine structure of the mouthparts, including distribution and abundance of receptor sensilla, was observed in three species of Asopinae using scanning electron microscopy and structural details are described for the first time. The morphology of the mouthparts is similar to those of other Heteroptera. The four-segmented labium and labrum in all studied species have fourteen types of sensilla. Unlike phytophagous pentatomids, two types of olfactory sensilla with nanopores (St1, Sb3) were observed in these three predatory insects, which probably function to locate prey by smell. In predatory stink bugs, each mandibular stylet tip has five irregular teeth and three long, pointed hooks; the apices of the right maxilla have small teeth and few short barbs along the edge of the food canal. The structure and function of the mouthparts are adapted for predatory feeding in the three studied species. The detailed structure of the predatory pentatomid’s mouthparts shown in this study can provide more data for the morphological differentiation of the mouthparts in the future.
Abstract
Mouthpart structures were observed in three species of Asopinae using scanning electron microscopy to investigate their morphological disparity. The examined species attack mainly slow-moving, soft-bodied insects, primarily larval forms of the Lepidoptera, and are the natural enemies of many pests. This is the first detailed description of their external mouthparts. The triangular and elongated labrum and four-segmented tube-like labium are longer in Picromerus species (Picromerus bidens (Linnaeus, 1758) and Picromerus lewisi Scott, 1874 than in Cazira bhoutanica Schouteden, 1907. The labrum of P. lewisi and C. bhoutanica appear to be equipped with olfactory sensilla basiconica Sb3, a special type of sensilla with nanopores. The labium surface in all studied species bears 14 types of sensilla (St1–St4, Sb1–7, Sst, Sca1–2). A new characteristic of sensilla trichodea is represented in sensillum St1; in both Picromerus species, it is classified as an olfactory sensillum with nanopores. The tripartite apex of the labium consists of two lateral lobes and a central membranous lobe having microtrichial extensions. Each lobe has one sensory field, including sensilla basiconica (Sb7), sensilla styloconica (Sst), and sensilla trichodea (St4). In the three studied predatory stink bugs, each mandibular stylet tip has five irregular teeth and three long, pointed hooks. The two opposing maxillae, which are held together by a tongue-and-groove system, form a food canal and a salivary canal. The apices of the right maxilla have small teeth and few short barbs along the edge of the food canal. In P. bidens and P. lewisi, there are 5 teeth, while in C. bhoutanica there are 2. Based on structural differences, we inferred that the hook-shaped mandibular teeth, right maxilla with small teeth, and few short barbs along edge of the food canal are more adapted for a predatory lifestyle. Predatory stink bugs use sharp recurved hooks and irregular teeth penetrating, tearing, or filing devices that aid in the mechanical disruption of host tissue. Stiff bristles in the food canal may indicate their possible adaptation to feeding on insect larvae. The evolution of mouthpart morphology and the putative functional significance of sensilla are discussed, providing insight into the sensory mechanism.
Keywords: predatory stink bugs, stylet, sensilla, fine morphology
1. Introduction
The mouthparts of insects display a wondrous diversity of form and function. As feeding structures, mouthparts play an important role in the evolution of insects. The structure of mouthparts varies according to the nature of their food. The structural differences are especially significant in the more derived orders and can be used as features for identification and classification [1,2,3]. During the feeding process, sensory organs on the surface of the mouthparts can identify and locate the host [4,5]. In Hemiptera, because the apex of the labium first comes into contact with the feeding site, the sensory structures on the labial tip are most concentrated and diverse. The types and quantity of sensilla are closely related to the feeding habits of the insect [6]. In addition to these sensory organs, the stylet fascicle is also very important for feeding. After the mandibular stylet is inserted into the host tissue, it is mainly used for fixation, providing support for the maxillary stylets, and helping penetrate deeper to feed [1]. The ridge on the outer surface of the proximal end of the mandibular stylet and the teeth on both sides contribute to the depth of puncture. During evolutionary diversification, the morphology of stylets became differentiated, with substantial differences emerging among different lineages [1,2,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Most prior studies of hemipteran mouthparts only focused on one aspect, the sensilla on the labium tip or the stylet fascicle, and detailed study of the mouthparts overall is often lacking. More comprehensive studies are needed in order to provide useful comparative data.
Heteroptera is one of the most abundant groups of hemimetabolous insects and includes many economically important taxa, such as agricultural pests, animal parasites, and natural enemies of pest insects. Pentatomidae is one of the most common large families in Heteroptera and members are found worldwide. While most members of Pentatomidae are phytophagous, most Asopinae are predatory, preying on Coleoptera and Lepidoptera larvae [23]. Some species of this group have been used in biocontrol of agricultural pests [23]. Several prior studies reported on the morphology of the mouthparts of Pentatomidae [1,8,24,25,26,27,28]. Most of these studies focused on the tip of the labium [26,27], stylets [1,8,12,13] and the general structure of mouthparts [24,25,28]. Among them, only four predatory pentatomids were studied: Perillus bioculatus (Fabricius) by Cobben [1] and Parveen et al. [27]; Podisus maculiventris (Say) by Cohen [8]; Eocanthecona furcellata (Wolff) by Rani [26] and Parveen et al. [27]; and Canthecona furcellata (Wolff) by Barsagade and Gathalkar [28]. More species and more detailed information on fine structure are needed to determine how much variability in mouthparts occurs among pentatomid groups with different feeding habits.
The subfamily Asopinae, commonly called predatory stinkbugs or soldier bugs, has more than 300 species in 63 genera worldwide [29] and most of the genera and species are distributed in the trans-Palearctic [30,31,32]. The main synapomorphy of this subfamily is the stout rostrum, which is an adaptation for predation on other insects [33]. Predatory stinkbugs inhabit forests and grasslands as well as agroecosystems such as orchards and vegetable fields. Many species appear to prefer shrubland and forests. As the only predatory group in the family Pentatomidae, Asopinae attack mainly slow-moving, soft-bodied insects, primarily larval forms of Lepidoptera, and are the natural enemy of pests (e.g., Colorado potato beetle and Mexican bean beetle) [23]. The predatory pentatomids, therefore, have potential for use in in controlling pests of orchards, forests, and field crops [34,35,36].
This study explores the sensory structures, both maxillary and mandibular, of three species of Asopinae, belonging to two genera, Picromerus bidens (Linnaeus, 1758), Picromerus lewisi Scott, 1874, and Cazira bhoutanica Schouteden, 1907, which are all predatory. P. bidens is distributed in forests and grasslands of China (Anhui, Hunan, Hebei, Heilongjiang, Jilin, Liaoning, Inner Mongolia), Europe, and northeastern North American [23,37,38]. It is a highly polyphagous bug that feeds mainly on leaf-feeding larvae of the Lepidoptera, Coleoptera, and Hymenoptera [23]. Prior studies have reported that this species has the potential to reduce the number of pests in various ecosystems [23,39,40,41,42,43,44,45,46,47,48,49]. P. lewisi is found in China, Japan, Korea, and Russia [37,50,51], and feeds on Lepidoptera, Diptera and other pests [52,53]. In Yanbian, Jilin, it may attack the larvae of tussah silkworms, and in Tonggu, Jiangxi, it is found to injure tea. C. bhoutanica is distributed in China (Jiangsu, Anhui, Zhejiang, Jiangxi, Fujian, Sichuan, Guizhou, Yunnan), India, Nepal, and Bhutan [50]. This bug mainly preys on cotton aphids. Prior research on these three stink bugs focused on taxonomy and bionomic characteristics. There are no detailed reports on their mouthparts.
For this study, we have three aims: (1) to provide the first detailed fine morphological characterization of the mouthparts of three Asopinae species using scanning electron microscopy (SEM); (2) to show how the mouthparts of these predatory pentatomids differ from those of phytophagous pentatomids (incorporating data from references); and (3) to determine what roles the mouthpart structures (labrum, labium, sensilla, mandibular, and maxillary stylets) play in their feeding process.
2. Material and Methods
2.1. Insect Collecting
The specimens (n = 7) of Asopinae used in this study were collected in China and Poland. P. bidens was collected by Yan Wang in Szczawnica, Poland (49°43′ N, 20°50′ E, elev. 674 m) in August 2019 and preserved in 95% ethanol. P. lewisi was collected by Chao Wen in Fanjing Mountain, Guizhou Province, China (27°913′ N, 108°694′ E, elev. 2572 m), in July 2016 and preserved in 95% ethanol. C. bhoutanica was collected by Wang Yan in Siming Mountain, Ningbo, Zhejiang Province, China (29°75′ N, 121°90′ E, elev. 823 m), in July 2015 and preserved in 95% ethanol.
2.2. Samples for SEM
The sampled specimens (n = 7) were dipped into 10% NaOH solution for 2 h and cleaned twice by using an ultrasonic cleaner (KQ118, Kunshan, China) for 15s each time. Dehydration involved serial baths of 80%, 90% and 100% ethanol each for 15 min. The specimens were air dried, coated with a film of gold (Q150T-S, Quorum, West Sussex, UK), and then imaged with a Nova Nano SEM-450 (FEI, Hillsboro, OR, USA) at 5 kV in the scanning microscopy laboratories of the Life Science Research Core Services of Northwest A & F University, Yangling, Shaanxi, China.
2.3. Image Processing and Morphometric Measurement
SEMs were observed and measured after being imported into Adobe Photoshop CC 2019 (Adobe Systems, San Jose, CA, USA). Statistical analyses were executed using SPSS 19.0 (SPSS, Chicago, IL, USA). Graphs were fitted using Microsoft Office Excel 2007.
2.4. Terminology
The sensilla were classified according to their external morphology, length, distribution, and position. The terminology of sensilla follows Altner and Prillinger [54] and Frazier [55] with less specialized nomenclature from Parveen et al. [27].
3. Results
3.1. Overall Morphology of the Mouthparts
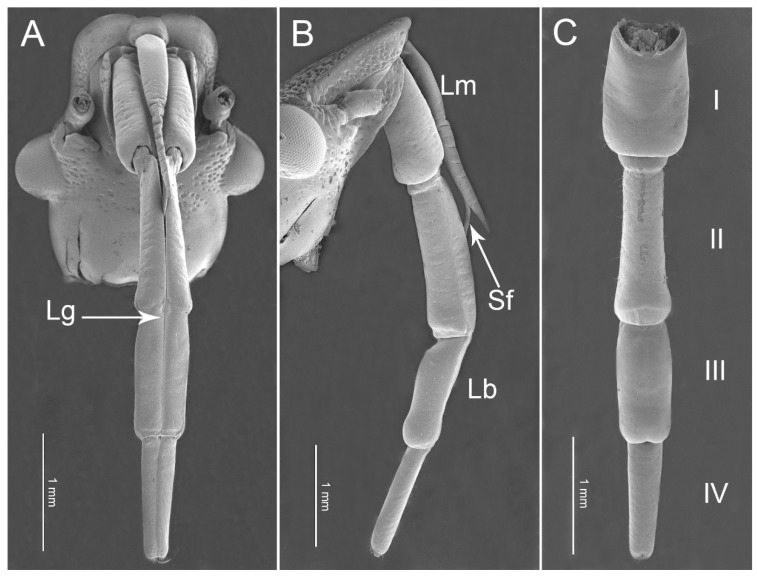
The mouthparts of P. bidens, P. lewisi, and C. bhoutanica are similar to those of other true bugs, consisting of a tapered labrum (Lm), a long four-segmented labium (Lb), and a pair of separated mandibular and interlocked maxillary stylets, which are enclosed in the groove of the labium (Figure 1A–C). When the insect is resting or not feeding, the mouthparts (rostrum) are pressed to the sternum, parallel to the body.
Figure 1.
Scanning electron micrographs of the head of Picromerus lewisi. (A) Ventral view; (B) Lateral view; (C) Dorsal view showing four-segmented labium (I–IV); Lg, labial groove; Lm, labrum; Lb, labium; Sf, stylet fascicle.
3.1.1. Labrum
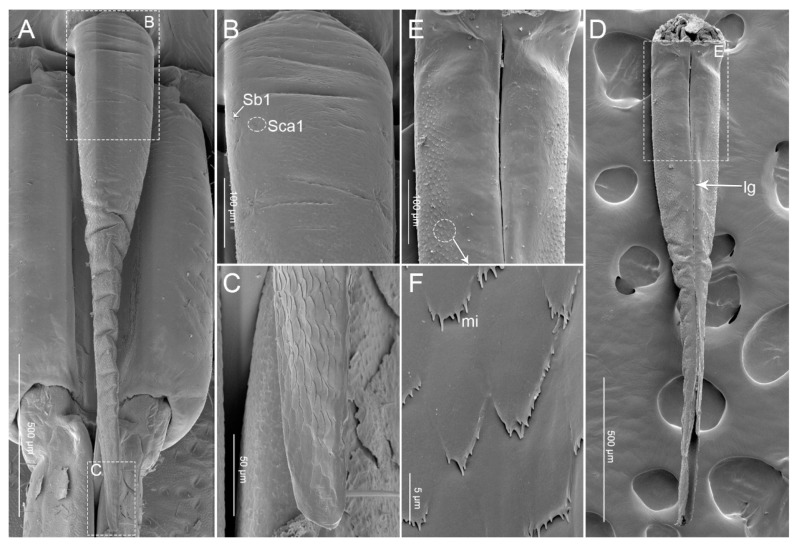
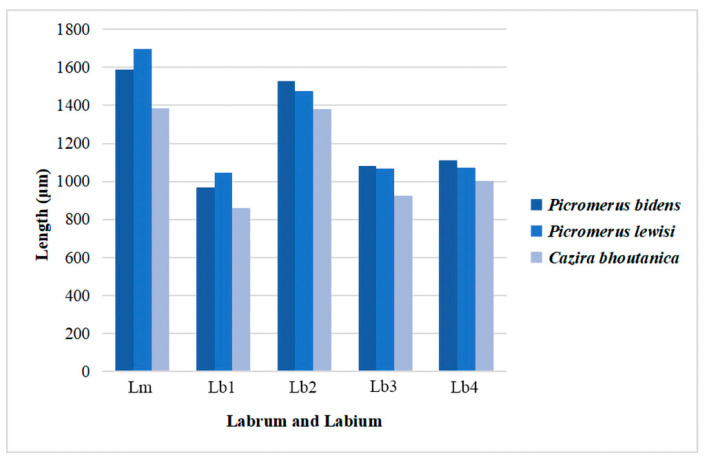
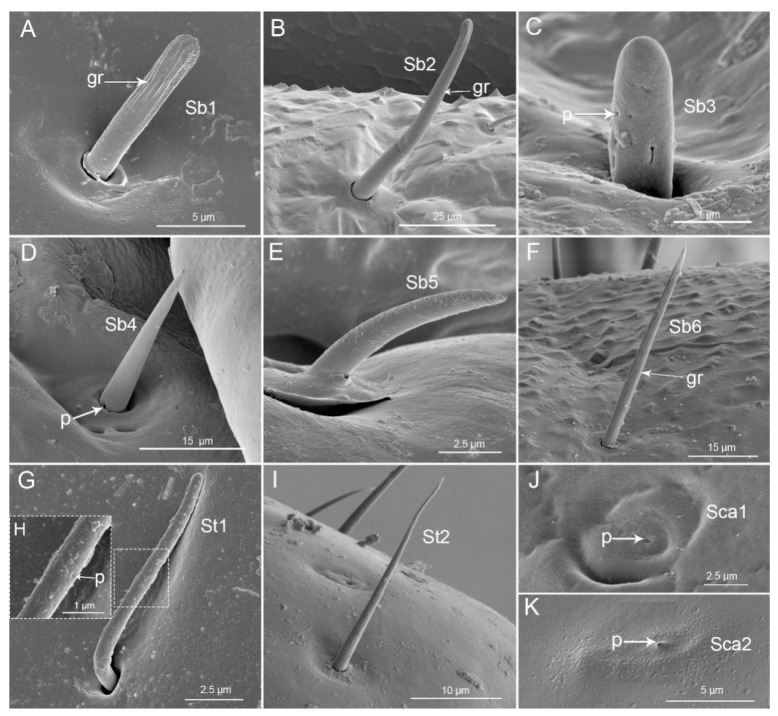
The labrum (Lm) is triangular, elongated, with the base connected to the anteclypeus (Figure 2A and Figure 3A–C). In P. bidens, the basal half is wide and relatively smooth, while the distal half is extremely narrow and wrinkled. In P. lewisi, the basal quarter is relatively smooth, the distal three quarters are strongly plicated. However, in C. bhoutanica, the basal quarter is relatively smooth while the distal four quarters are strongly plicated. The lengths of the labrum are 1586.8 µm in P. bidens, 1694.8 µm in P. lewisi, and 1385.9 µm in C. bhoutanica, respectively. In all three species, the labrum is longer than the first labial segment and completely covers the groove of the first labial segment (Table 1, Figure 3A–C and Figure 4). The ventral side is covered with some sensilla basiconica Sb1, Sb2, and Sb3 (Table 2, Figure 2B). Sensilla basiconica I (Sb1) are present in P. bidens, P. lewisi, and C. bhoutanica; sensilla basiconica II (Sb2) occur only in P. lewisi; and sensilla basiconica III (Sb3) are found in P. lewisi and C. bhoutanica. According to their external morphology (not porous and embedded in flexible sockets), Sb1 and Sb2 represent mechanosensilla (Table 3). The surfaces of sensilla basiconica Sb3 are slightly porous and several very small pores (diameters of these nanopores approximately 31.6 ± 2.0 nm (n = 5)) are present. Many scale-like projections cover the ventral surface of the labrum (Figure 2A–C). On the dorsal side of the labrum is the conspicuous labral groove (lg) together with a strongly sclerotized hypopharyngeal lobe forming a trough for the stylet bundle (Figure 2D,E). Clusters of microtrichia (mi) are arranged in irregular transverse rows on the dorsal area of the labrum (Figure 2D–F).
Figure 2.
SEM images of labrum of Picromerus bidens. (A) Ventral view; (B,C) Enlarged view of box in (A), showing sensilla basiconica (Sb1) and sensilla campaniformia Sca1; (D) Dorsal view; (E) Enlarged view of box in (D); (F) Clusters of microtrichia (mi); lg, labral groove.
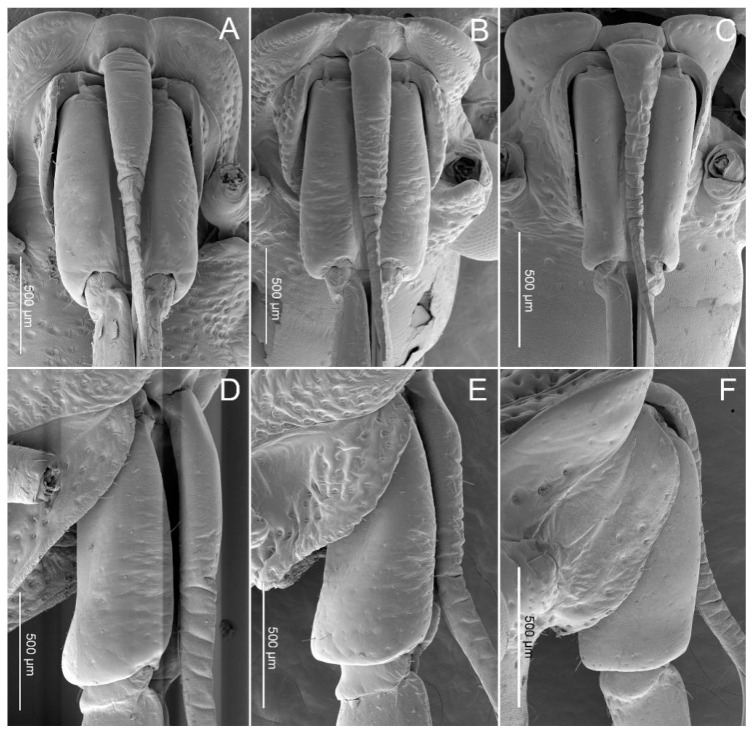
Figure 3.
SEM images of the labrum and first labial segment. (A) Ventral view of Picromerus bidens; (B) Ventral view of Picromerus lewisi; (C) Ventral view of Cazira bhoutanica; (D) Lateral view of Picromerus bidens; (E) Lateral view of Picromerus lewisi; (F) Lateral view of Cazira bhoutanica.
Table 1.
Comparison of the lengths of labial segments in studied species. Data are means ± SE values obtained from scanning electron microscopy.
| Species | Labrum | Labial Segments | ||||
|---|---|---|---|---|---|---|
| I (μm) | II (μm) | III (μm) | IV (μm) | Whole | ||
| Picromerus bidens (Linnaeus) | 1586.8 ± 10.6 | 967.3 ± 8.9 | 1527.7 ± 33.2 | 1080.5 ± 34.5 | 1113.4± 24.4 | 4687.8 ± 93.9 |
| Picromerus lewisi Scott | 1694.8 ± 55.9 | 1046.0 ± 12.1 | 1474.1 ± 74.2 | 1068.3 ± 9.2 | 1073.9 ± 17.8 | 4661.5 ± 89.2 |
| Cazira bhoutanica Schouteden | 1385.9 ± 2.9 | 862.1 ± 7.4 | 1379.1 ± 28.6 | 925.2 ± 7.4 | 1001.2 ± 12.5 | 4179.8 ± 43.1 |
Figure 4.
The length of labrum and each labial segment in different species. Lm, labrum; Lb1, 2, 3, and 4: the first, second, third, and fourth labial segments, respectively.
Table 2.
Distribution and morphometric data of sensilla in studied species. Data are means ± SE values obtained from scanning electron microscopy. N = sensilla number; Lm, Labrum; St1–4, sensilla trichodea I–IV; Sb1–7, sensilla basiconica I–VII; Sst, Sensilla styloconica; Sca1–2, sensilla campaniformia I–II; Lb, labium; Lb1, 2, 3, 4, the first, second, third, fourth segment of labium; SF, sensory field on the labial tip.
| Sensilla | Picromerus bidens (Linnaeus) | Picromerus lewisi Scott | Cazira bhoutanica Schouteden | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distri- bution |
Length (μm) |
Basal Diameter (μm) |
N | Distri- bution |
Length (μm) |
Basal Diameter (μm) |
N | Distri- bution |
Length (μm) |
Basal Diameter (μm) |
N | |
| St1 | Lb1-4 | 22.9 ± 1.2 | 0.83 ± 0 | 10 | Lb1-4 | 16.4 ± 1.5 | 0.8 ± 0 | 10 | ||||
| St2 | Lb4 | 17.3 ± 1.8 | 1.6 ± 0.1 | 8 | Lb4 | 20.7 ± 1.1 | 1.2 ± 0.1 | 10 | Lb4 | 22.5 ± 2.0 | 1.7 ± 0.1 | 7 |
| St3 | Lb4 | 63.5 ± 1.9 | 2.1 ± 0.1 | 5 | Lb4 | 66 ± 4.9 | 2.3 ± 0.1 | 6 | Lb4 | 97.6 ± 9.8 | 2.5 ± 0.1 | 6 |
| St4 | SF | 15.6 ± 0.8 | 1.7 ± 0.1 | 10 | SF | 15.5 ± 1.1 | 1.6 ± 0.1 | 10 | SF | 4.2 ± 0.3 | 1.2 ± 0.1 | 6 |
| Sb1 | Lm, Lb1-4 | 9.4 ± 0.6 | 1.7 ± 0.1 | 10 | Lm, Lb1 | 12 ± 1.8 | 1.9 ± 0.1 | 10 | Lm, Lb1 | 9.2 ± 0.7 | 1.5 ± 0.1 | 9 |
| Sb2 | Lb1 | 67.8 ± 9.5 | 3.8 ± 0.2 | 5 | Lm, Lb2 | 38 ± 5.9 | 3.7 ± 0.6 | 7 | Lb1, 2 | 56.2 ± 4.6 | 4.3 ± 0.2 | 4 |
| Sb3 | Lm | 1.7 ± 0.6 | 0.7 ± 0 | 3 | Lm, Lb1-3 | 1.1 ± 0.1 | 0.8 ± 0.1 | 3 | ||||
| Sb4 | Lb2, Lb4 | 23.3 ± 0.7 | 4.2 ± 0.1 | 5 | Lb2, Lb4 | 18.3 ± 0.6 | 3.6 ± 0.1 | 4 | Lb2, Lb4 | 11.2 ± 1.3 | 3.5 ± 0.2 | 4 |
| Sb5 | Lb1, Lb3 | 14.9 ± 0.4 | 1.8 ± 0.1 | 3 | Lb1,2,4 | 8.7 ± 0.7 | 1.4 ± 0.1 | 4 | Lb2 | 10.9 ± 1.0 | 1.8 ± 0.1 | 5 |
| Sb6 | Lb1-4 | 18 ± 1.4 | 1.5 ± 0.1 | 8 | Lb3,4 | 40.3 ± 0.4 | 3.5 ± 0.4 | 3 | Lb2-4 | 75.3 ± 3.9 | 4.1 ± 0.1 | 3 |
| Sb7 | SF | 4.7 ± 0.4 | 2.6 ± 0.1 | 4 | SF | 4.4 ± 0.8 | 1.4 ± 0.1 | 4 | SF | 4.9 ± 0.2 | 2.5 ± 0.1 | 4 |
| Sst | SF | 22.8 ± 0.9 | 7.3 ± 0.2 | 18 | SF | 22.8 ± 0.5 | 7.5 ± 0.3 | 10 | SF | 11.6 ± 0.8 | 5.1 ± 0.2 | 9 |
| Sca1 | Lm | 7.1 ± 0.5 | 4 | Lm, Lb2 | 6.7 ± 0.2 | 3 | Lm, Lb2, 4 | 9.1 ± 0.9 | 3 | |||
| Sca2 | Lb4 | 5.5 ± 0.3 | 12 | Lb4 | 5.8 ± 0.7 | 10 | Lb4 | 7.4 ± 0.4 | 4 | |||
Table 3.
Terminology and definition of sensilla used in this paper including terms from previous studies [27,54,55], Wp, wall pore; Tp, tip pore.
| Type | Shape | Socket | Surface | Pore | Category | Function |
|---|---|---|---|---|---|---|
| St1 | Hair in pit | Inflexible | Smooth | Wp (porous) diameter 0.043 (μm) = 43 nm | Chemoreceptive sensilla | Olfactory |
| St2 | Hair | Flexible | Smooth | No | Mechanoreceptive sensilla | Tactile |
| St3 | Hair | Flexible | Smooth | No | Mechanoreceptive sensilla | Tactile |
| St4 | Hair | Inflexible | Smooth | No | Mechanoreceptive sensilla | Tactile |
| Sb1 | Peg | Flexible | Grooved | No | Mechanoreceptive sensilla | Tactile |
| Sb2 | Peg | Flexible | Grooved | No | Mechanoreceptive sensilla | Tactile |
| Sb3 | Peg in pit | Inflexible | Smooth | Diameter 0.043 (μm) = 43 nm | Chemoreceptive sensilla | Olfactory |
| Sb4 | Peg | Flexible | Smooth | Wp (molting pore) | Proprioceptive sensilla | Perceive the degree of flexion of the joint |
| Sb5 | Peg | Flexible | Smooth | No | Mechanoreceptive sensilla | Tactile |
| Sb6 | Peg, hair | Flexible | Grooved | No | Mechanoreceptive sensilla | Tactile |
| Sb7 | Peg in pit | Inflexible | Smooth | TP | Chemoreceptive sensilla | Gustatory |
| Sst | Peg | Cone sitting on a style(high socket) | Smooth | TP | Chemoreceptive sensilla | Gustatory |
| Sca1 | Oval plate | Inflexible | Smooth | Central pore (molting pore) | Mechanoreceptive sensilla | Responding to strains in the exoskeletons |
| Sca2 | Domelike structures | Inflexible | Smooth | Central pore (molting pore) | Mechanoreceptive sensilla | Responding to strains in the exoskeletons |
3.1.2. Labium
The labium (Lb) (Figure 1A–C) is elongated to form a protective sheath within which the stylet bundle is enclosed. In the studied species, the first (I), second (II), third (III), and fourth segments (IV) have a similar shape but differ in length (Table 1, Figure 4). Based on the surface sculpture and pore system, socket form, shaft shape, and length, fourteen subtypes of sensilla were observed on the surfaces of the labium (Table 2 and Table 3).
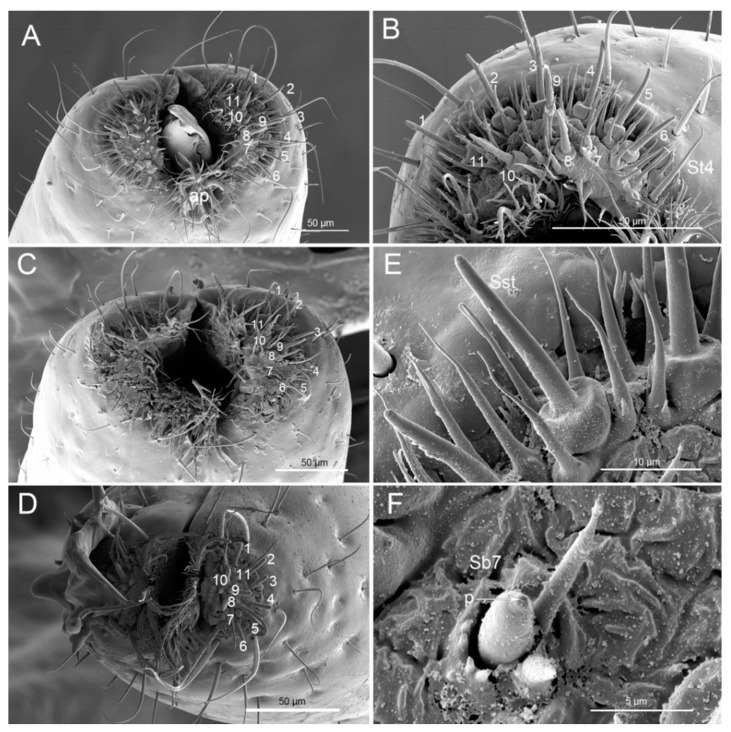
In these three species, the first (basal) segment (I) is short and thick, surrounded and partially covered by the small low bucculae, but the other segments are clearly exposed (Figure 3A–F). The first segment is the shortest segment of the labium in these species (Figure 4). On the ventral surface, the labial groove (Lg) is very wide and open enough to accommodate the labrum (Figure 3A–C). The distal ventro-lateral part of the segment is slightly widened (Figure 3D–F). Sensilla found on this segment are as follows: Sensilla trichodea I (St1) are hair-like, with a curved and round apex and are inserted in a pit (Figure 5G) in P. bidens and P. lewisi. The surfaces of these sensilla are covered by nanopores (diameters of approximately 0.0587µm = 58.7 ± 4.3 nm (n = 8)) but visible only at very high magnification. These sensilla are positioned solitarily on the surface of the first segment. Sensilla basiconica I (Sb1) that are short, straight, robust, and have a grooved surface and flexible socket (Figure 5A) are present in all the studied species. Sensilla basiconica II (Sb2) are longer with a curved, grooved surface and a flexible socket (Figure 5B). This type of sensilla occurs in P. bidens and C. bhoutanica. Sensilla basiconica III (Sb3) are similar to sensilla coeloconica, being short cones that arise from inflexible sockets (diameter of these nanopores on Sb3 is 31.6 ± 2.0 nm (n = 5)) (Figure 5C). This type of sensillum is present in C. bhoutanica. Sensilla basiconica V (Sb5) are conical, curved, and robust with a smooth surface (Figure 5E). This type of sensillum is found in P. bidens and P. lewisi. Sensilla basiconica VI (Sb6) are very long, straight, and almost perpendicular to the surface of the labium (Table 2, Figure 5F). The bases of these sensilla have a flexible socket, the surface with a vertical groove, and the tip is narrow (Figure 5F). This type of sensilla is only found in P. bidens.
Figure 5.
SEM images of sensilla of three species. (A) Sensilla basiconica I (Sb1); (B) Sensilla basiconica II (Sb2); (C) Sensilla basiconica III (Sb3); (D) Sensilla basiconica IV (Sb4); (E) Sensilla basiconica V (Sb5); (F) Sensilla basiconica VI (Sb6); (G) Sensilla trichodea I (St1); (H) Enlarged view of sensilla trichodea I (St1); (I) Sensilla trichodea II (St2); (J) Sensilla campaniformia I (Sca1); (K) Sensilla campaniformia II (Sca2); gr, groove; p, pore.
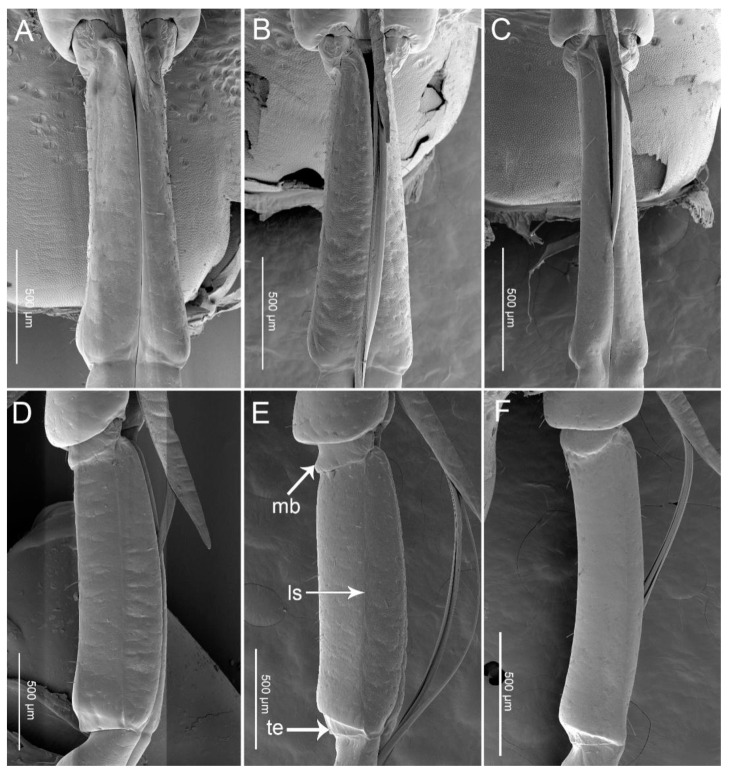
The second segment (II) is the longest segment of the labium (Figure 4). In the ventral view, the second segment gradually widens from base to apex (Figure 6A–C); in the lateral view, however, it is of uniform width throughout most of its length (Figure 6D–F). The base of this segment forms a distinct articulation with the first segment, consisting of a band-like dorsal plate and a ventro-lateral band of membrane (mb) (Figure 6D–F). A longitudinal suture (ls) extends along each side of the second segment (Figure 6D–F). The distal part of the second labial segment has a tapered edge (te) with a lateral membrane (Figure 6D–F). There are three pairs of sensilla basiconica (Sb4) at the base of the second segment of all three species. They are of medium length, straight, with a smooth surface, and have a basal wall pore (molting pore), a rounded tip, and a flexible socket (Table 2, Figure 5D). Aside from sensilla basiconica IV (Sb4), there are six types of sensilla (St1, Sb1, Sb2, Sb3, Sb5, Sca1) on the second segment that slightly differ in these three species (Table 2). Sensilla trichodea I (St1) have the same characters as on the first segment and are also visible in P. bidens and P. lewisi. Sb1 are only found in P. bidens and Sb3 in C. bhoutanica (the same as the Sb3 on the labrum). Sensilla basiconica II (Sb2), sensilla basiconica V (Sb5), and sensilla campaniformia I (Sca1) are distributed in P. lewisi and C. bhoutanica. Sca1 are cupola-shaped structures with a slightly convex central part and with a central pore (molting pore) (Figure 5I).
Figure 6.
SEM images of the second labial segment. (A) Ventral view of Picromerus bidens; (B) Ventral view of Picromerus lewisi; (C) Ventral view of Cazira bhoutanica; (D) Lateral view of Picromerus bidens; (E) Lateral view of Picromerus lewisi; (F) Lateral view of Cazira bhoutanica; mb, membrane; ls, longitudinal suture; te, tapered edge.
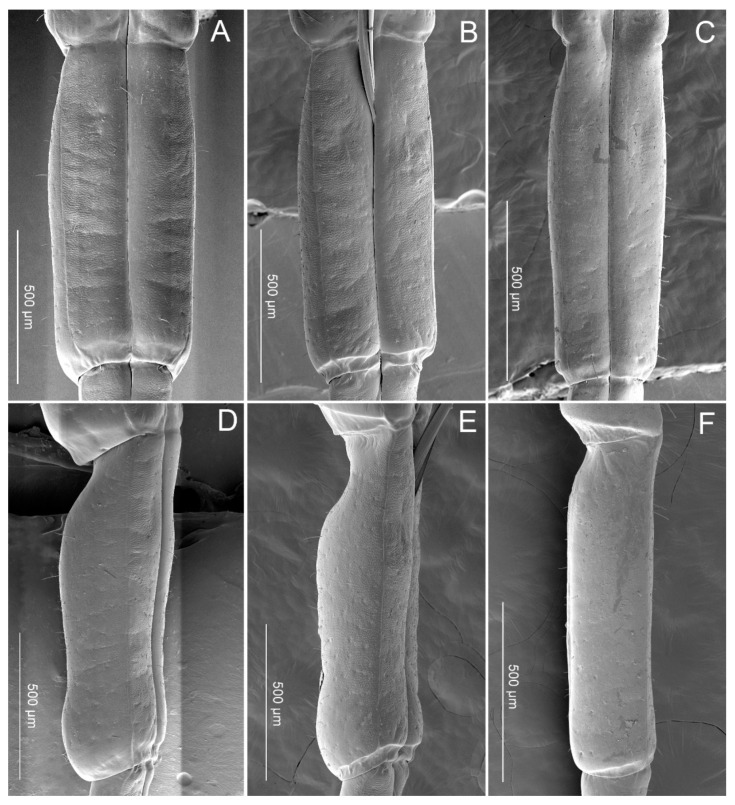
The third segment (III) is shorter than the second segment and is nearly uniform in width through most of its length (Figure 7A–C). In lateral view, the base is constricted (Figure 7D–F). A longitudinal suture (ls) also extends through each side of this segment. There are fewer sensilla distributed on this segment. St1 are present in small quantities in P. bidens and P. lewisi. Sb3 are only found in C. bhoutanica, Sb5 in P. bidens and Sb6 in P. lewisi.
Figure 7.
SEM images of the third labial segment. (A) Ventral view of Picromerus bidens; (B) Ventral view of Picromerus lewisi; (C) Ventral view of Cazira bhoutanica; (D) Lateral view of Picromerus bidens; (E) Lateral view of Picromerus lewisi; (F) Lateral view of Cazira bhoutanica.
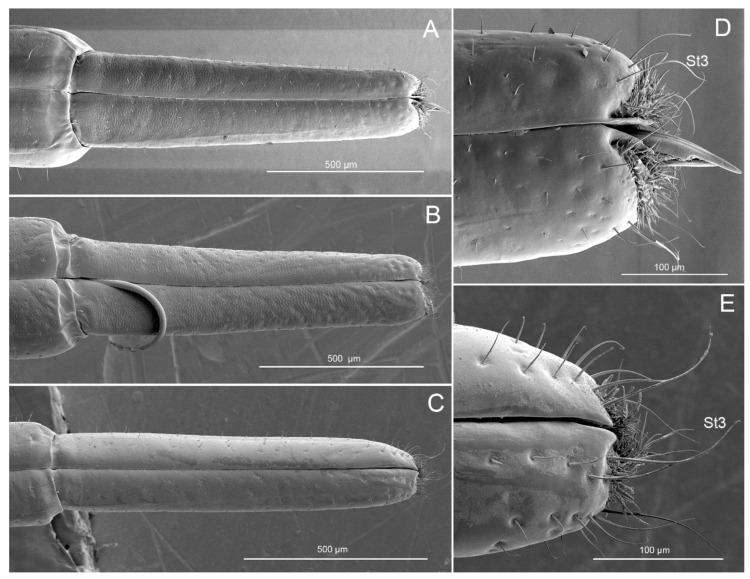
The fourth, or distal labial, segment is short (Figure 4) and conical in shape (Figure 8A–C). A longitudinal suture (ls) is also found on each side of this segment. A large number of sensilla are present on the surface of this segment. One pair of Sb4 are present on each side of the junction of the third and fourth segments of the species. St1 are visible in P. bidens and P. lewisi. St2, St3, Sb4, Sb6, and Sca2 are found in these species. Sb1 are only distributed in P. bidens, Sb5 in P. lewisi, and Sca1 in C. bhoutanica. St2 arranged roughly on the apical 1/6 are smooth and slender, slightly curved in the apical half, and inserted in a flexible socket (Figure 5H). St3, arranged on the labial subapex, are long and slender, slightly curved, and smooth with a flexible socket (Figure 8D,E). Several Sca2 are located on the antero-lateral surface near the apical 1/5. Each is a sunken circular plate with a central pore (Figure 5J).
Figure 8.
SEM images of ventral view of the fourth labial segment. (A) Ventral view of Picromerus bidens; (B) Ventral view of Picromerus lewisi; (C) Ventral view of Cazira bhoutanica; (D) Apex of the fourth labial segment of Picromerus bidens; (E) Apex of the fourth labial segment of Cazira bhoutanica, St3, sensilla trichodea III.
The tripartile labial tip is divided into two lateral lobes and a central membranous lobe bearing microtrichial extensions (Figure 9A–F). Each lobe has one sensory field, including sensilla basiconica (Sb7) (nos. 7, 11), sensilla styloconica (Sst) (nos. 1–6, 8–10), and sensilla trichodea (St4). Nine Sst are present at the center of each lobe (Figure 9B). Sst are robust, straight, with a smooth surface, and are inserted on a raised platform (Figure 9E). Two Sb7 have an ovoid body, a socket surrounding the base of the shaft, and a partial hood formed by fused cuticular processes (Figure 9F). A mass of St4 are present at each lobe (Figure 9B). St4 are hair-like with a curved, pointed apex and smooth surface. Spines and comb-like structures are laterally situated within the stylet groove of the last labial segment, probably serving to clean the mandibles during and after feeding.
Figure 9.
SEM images of the tip of the labium. (A) Vertical view of the tip of Picromerus bidens; (B) Right side of the tip of Picromerus bidens showing sensilla basiconica VII (Sb7) (nos. 7 and 11), sensilla styloconica (nos. 1–6, 8–10) and sensilla trichodea IV (St4); (C) Vertical view of the tip of Picromerus lewisi; (D) Vertical view of the tip of Cazira bhoutanica; (E) Sensillum styloconicum (Sst); (F) Sensillum basiconicum (Sb7); p, pore.
According to their morphology, putative functions of the aforementioned labial tip sensilla are presented in Table 3.
3.2. Stylet Fascicle
The stylet bundle consists of two separate mandibular stylets (Md) and two interlocked maxillary stylets (Mx). Usually, the mandibular stylets used for tearing are shorter than the absorbed maxillary stylets.
3.2.1. Mandibles
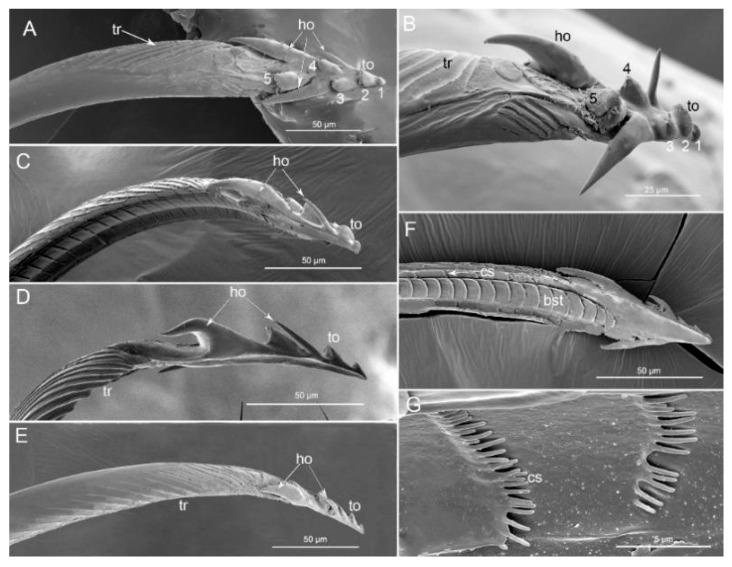
The mandibular stylets (Md) are attached to and surround the two interlocking maxillary stylets (Mx). The apical region, external, lateral, and internal parts of the mandibular (Md) stylets were observed in all three predatory stinkbugs (P. bidens, P. lewisi, and C. bhoutanica). The apices of the mandibles have a distinct curvature and bear rows of transverse ridges (tr) over much of their outer surfaces. Their apices are irregular with teeth (to) and long, pointed hooks (ho) (Figure 10A–E). In the three species of Asopinae (P. bidens, P. lewisi, and C. bhoutanica) two irregular teeth (to) are placed anteriorly. Three big irregular teeth (to) and three long, pointed hooks (ho) are placed dorso-laterally (Figure 10A,B). The mandibles have some longitudinal grooves over much of their inner surfaces that differ between the left and right in all studied species. The first row consists of small cuticular spines (cs) (Figure 10F,G). The second row possesses big squamous textures (bst) (Figure 10F). The inner surfaces of the ends of the mandibles are smooth (Figure 10F).
Figure 10.
SEM images of mandibular stylets of three species. (A) External view of stylet in Picromerus bidens showing transverse ridges (tr), five irregular teeth (to) and three long and pointed hooks (ho); (B) External view of stylet in Picromerus bidens; (C) Lateral view of stylet in Picromerus bidens; (D) Lateral view of stylet in Picromerus lewisi; (E) Lateral view of stylet in Cazira bhoutanica; (F) Interior side of stylet of Cazira bhoutanica showing small cuticular spines (cs) and big squamous textures (bst); (G) Small cuticular spines (cs); 1–5, irregular teeth.
3.2.2. Maxillae
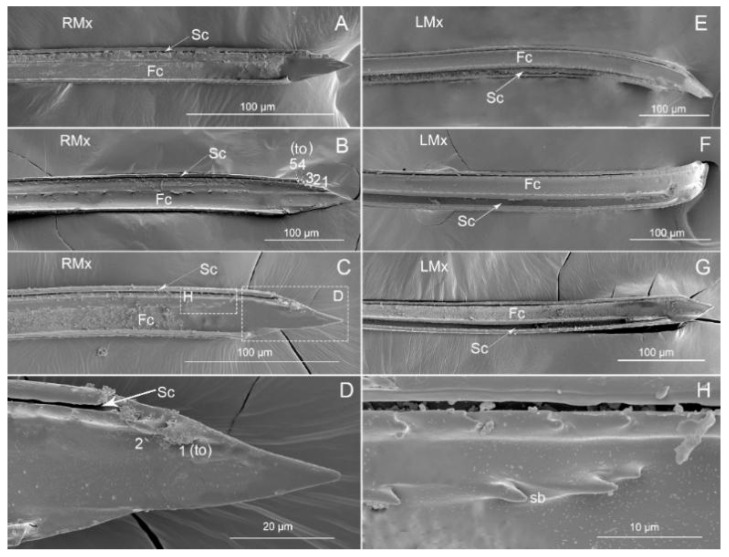
In these three species, maxillary stylets are equipped with a series of ridges and grooves internally and the left and right maxillary stylets are asymmetric. The two opposing maxillae, which are held together by a system of tongue-and-grooves, form a food canal and a salivary canal. The apex of the right maxilla (RMx) is straight with several ventral teeth (to) and ventral rows that have short barbs (sb) in the food canal (Figure 11A–D,H). In P. bidens and P. lewisi, the number of teeth (to) is 5, and there are 2 in C. bhoutanica. The apex of the left maxilla (LMx) is tapered and has a distinct curvature (Figure 11E–G). In our observation, the curvature P. bidens and P. lewisi is greater than that of C. bhoutanica.
Figure 11.
SEM images of maxillary stylets of three species. (A) Picromerus bidens; (B) Picromerus lewisi; (C) Cazira bhoutanica; (D) Enlarged view of box in (C); (E) Picromerus bidens; (F) Picromerus lewisi; (G) Cazira bhoutanica; (H) Enlarged view of box in (C); Fc, food canal; Sc, salivary canal; LMx, left maxillary stylet; RMx, right maxillary stylet; to, tooth; sb, short barbs; The number represents the number of teeth.
4. Discussion
This study presents detailed observations of the mouthpart structures in three species of Asopinae (Heteroptera: Pentatomidae). This reveals some interesting morphological features and allows for morphological comparison as well as a better understanding of the feeding mechanism and the sensory system of these predatory stinkbugs when compared to phytophagous heteropterans (Table 4). Because the Asopinae are likely derived from plant-feeding ancestors [56], differences in the mouthparts of this group may reflect a shift from phytophagy to predation [57].
Table 4.
Main features of mouthparts of stinkbugs (Pentatomidae).
| Feeding Behavior | Species | Labrum | Labium | Number of Sensilla Types | The Number of Sensilla of Labium Tip | Apical Plate | Internal Side of Maxillary Stylet | Internal Morphology of Mandibular Stylet | Distal Mandibular Stylet; Serration | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Predatory | Canthecona furcellata (Wolff) | triangular sclerite | elongated | 3 types | unreported | unreported | dentations | unreported | serrated | Barsagade & Gathalkar [28] |
| Predatory | Eocanthecona furcellata (Wolff) | unreported | robust and stout | 6 types | 3 types | unreported | unreported | unreported | unreported | Rani [26]; Parveen et al. [27] |
| Predatory | Perillus bioculatus Fabricius | unreported | robust and stout | 6 types | 3 types | membranous lobe microtrichial extensions | unreported | squamous texture | sharp recurved hooks | Cobben [1]; Parveen et al. [27] |
| Predatory | Podisus maculiventris (Say) | unreported | unreported | unreported | unreported | present | unreported | unreported | teeth | Cohen [8] |
| Predatory | Picromerus bidens (Linnaeus) | elongated, triangular | robust and stout | 14 types | 3 types | membranous lobe microtrichial extensions | short barbs | squamous textures and cuticular spines | five teeth and three hooks | This study |
| Predatory | Picromerus lewisi Scott | elongated, triangular | robust and stout | 14 types | 3 types | membranous lobe microtrichial extensions | short barbs | squamous textures and cuticular spines | five teeth and three hooks | This study |
| Predatory | Cazira bhoutanica Schouteden | elongated, triangular | robust and stout | 14 types | 3 types | membranous lobe microtrichial extensions | short barbs | squamous textures and cuticular spines | five teeth and three hooks | This study |
| Phytophagous | Graphosoma lineatum (Linnaeus) | unreported | unreported | unreported | unreported | unreported | unreported | small, widely spaced notches arranged in longitudinal strips | complex ribbed texture, the apices are knotted with irregular prominences | Cobben [1] |
| Phytophagous | Chrysocoris purpurea (Westwood) | unreported | unreported | unreported | 2 types | present | unreported | unreported | knotted with irregular prominences |
Rani & Madhavendra [25] |
| Phytophagous | Cyclopelta siccifolia Westwood | unreported | unreported | unreported | 2 types | present | unreported | unreported | knotted with irregular prominences |
Rani & Madhavendra [25] |
| Phytophagous | Dichelops melacanthus (Dallas) | unreported | unreported | unreported | unreported | unreported | unreported | squamous texture | dentition | Depieri & Panizzi [12] |
| Phytophagous | Dolycoris indicus (Stål) | unreported | slender and long | 6 types | 2 types | unreported | unreported | unreported | unreported | Parveen et al. [27] |
| Phytophagous | Euschistus heros Fabricius | unreported | unreported | unreported | unreported | unreported | unreported | squamous texture | dentition | Depieri et al. [13]; Depieri & Panizzi [12] |
| Phytophagous | Nezara viridula (Linnaeus) | unreported | unreported | unreported | 2 types | present | grooves | squamous texture | knotted with irregular prominences | Rani and Madhavendra [24]; Depieri & Panizzi [12] |
| Phytophagous | Piezodorus guildinii (Westwood) | unreported | unreported | unreported | unreported | unreported | unreported | squamous texture | dentition | Depieri & Panizzi [12] |
| Phytophagous | Piezodorus hybneri (Gmelin) | unreported | slender and long | 6 types | 2 types | unreported | unreported | unreported | unreported | Parveen et al. [27] |
| Phytophagous | Plautia crossota (Dallas) | unreported | slender and long | 6 types | 2 types | unreported | unreported | unreported | unreported | Parveen et al. [27] |
4.1. The Mouthparts of Predatory Specialist
Diagnostic characteristics of the Asopinae include, among others, the following modifications of the head associated with their feeding habits: rostrum strongly incrassate, the insertion of labium very close to base of labrum, and the posterior margins of buccula merged [33,58]. Because the labrum is frequently omitted in most studies as an element of the mouthparts, we included it in this study for structural and functional analysis.
The lengths of the labrums vary among the three species of predatory stinkbugs we observed (1586.8 µm in P. bidens, 1694.8 µm in P. lewisi and 1385.9 µm in C. bhoutanica), but they are consistently longer than the first segment of the labium (Table 1 and Figure 3). This is consistent with two other predatory stinkbugs, Canthecona furcellata (Wolff) [28] and Apateticus cynicus (Say) [59], in which a narrow elongate labrum rests over the proximal portion of the labial groove and extends distally through the first and part way into the second labial segment. The median part of the ventral surface of the labrum is slightly concave, forming a sort of trough holding the stylet bundle [59]. Based on our images of Asopinae species (Figure 3) and other information [59] it can be assumed that the long labrum may be important for predatory stinkbugs to hold the proximal part of the stylets together during feeding, preventing excessive bending in this part of the stylet bundle. The short membrane at the anterior basal portion of the labrum allows freedom of forward movement of the labrum from its normal concealed position beneath the head and controls the path of the stylet bundle. Comparison of similar-sized phytophagous pentatomid species (Chinavia hilaris (Say) vs. Euschistus servus (Say), and Oebalus pugnax (Fabricius) vs. Piezodorus guildinii Westwood) showed differences in lengths of the labial segments (Table 3, [60]) and their influence on depth of penetration of the stylets in host tissues, as well as the role of the labrum in feeding. However, the role of labrum size has not been demonstrated. Esquivel [60] described how, during probing, the labrum remains against the ventral surface of the head and the stylet bundle remains within segments 2, 3, and 4. However, when assuming a feeding position, the labrum swings away from the body and the stylet bundle emerges from the length of segment 2 [60]. We found more general information in prior publications on pentatomids on the total length of the labium (rostrum) than on the labrum. Nevertheless, a comparison of Asopinae with different phytophagous heteropteran bugs, e.g., Pyrrhocoris sibiricus (Kuschakevich) [16], Cheilocapsus nigrescens (Liu and Wang) [18], Stephanitis nashi (Esaki and Takeya) [19] and Macrocheraia grandis (Gray), Physopelta quadriguttata Bergroth, Physopelta cincticollis (Stål), and Physopelta gutta (Burmeister) [20] showed that the labrum is shorter than the first labial segment in these species. More data on the labrum length of phytophagous pentatomids is needed to facilitate further comparison.
The labium of these three predatory stinkbugs (P. bidens, P. lewisi, and C. bhoutanica) is four-segmented as in most other heteropteran bugs [16,18,19,20,28]. In these species, the base of the second segment forms a distinct articulation (band-like dorsal plate and ventro-lateral band of the membrane) with the first segment (Figure 6D–F). Such features have not been reported in previous studies of phytophagous bugs [16,18,19,20].
The lengths of the labial segments of the species studied here (Table 1) are different from another species of Asopinae, Eocanthecona japanicola (Esaki & Ishihara) (I—1120 µm, II—1430 µm, III—1220 µm, IV—1140 µm, cumulative length 4910 µm) [61]. Phytophagous Pentatomidae have similar relative proportions of individual labial segments to those found in Asopinae, but the cumulative length of the labial segments is significantly longer in phytophages (Table 3, [60]). In both groups, the first segment is shorter than the second and the third and fourth have a similar length but are shorter than the second. In asopines, the first segment of the labium (rostrum) is markedly thickened and free (not concealed between bucculae) [62], which enables the rostrum to swing forward fully, making it easier for the predator to feed on active prey [23]. This is probably an essential difference of the rostrum structure between asopines and other pentatomids. In the two groups, feeding stages differ slightly in the relative positions of labial segments. In phytophage feeding, the angle between the first and second segment is more acute [63] in Asopinae. This causes a shortening of the overall distance from the apex of the head to the distal end of the labium. This results in the stylet bundle exiting the distal end of the rostrum, thereby causing stylet penetration into the host [63]. In the Asopinae, the labium assumes a more prognathous position during feeding. The first and second segments also bend, but not as strongly, shortening the length of the labium to allow deep penetration of the stylets. The second segment (Figure 4) probably controls the activity of the short third and fourth segments.
The switch from a plant-feeding ancestor to a predaceous true bug likely occurred in the most recent common ancestor of all Heteroptera and the predatory life-style was retained in most lineages, although secondary transitions to phytophagy occurred, especially in the Pentatomomorpha and Cimicomorpha, with subsequent reversals to predation in some taxa [64]. The main functional elements of the mouthparts are mandibular and maxillary stylets, and their adaptive characters reflect trophic differences [1,65].
Different numbers and shapes of teeth on the mandibles in many heteropteran taxa have been reported by several authors [1,2,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,24,66,67]. Generally, Faucheux [2] commented that the number of mandibular teeth in heteropterans ranges from 4 to 40, with the number of teeth in phytophagous bugs significantly lower and less variable among different groups. The tips of mandibular stylets with teeth and hooks and their numbers and shapes are very similar among the studied species. In P. bidens, P. lewisi, and C. bhoutanica, two irregular teeth are placed anteriorly, three big teeth and three very pointed, long and recurved hooks are placed dorso-laterally (Figure 10). Cobben [1] suggested the predatory stink bug Perillus bioculatus Fabricius (Asopinae) uses sharp recurved hooks and irregular teeth as a penetrating, tearing or filing device that aids in the mechanical disruption of host tissues.
The abovementioned characters of the mandible in species studied so far correspond to Cobben’s [1] description of the mandibular tips in Perillus bioculatus Fabricius (Asopinae), which also have an inward curvature similar to those of typical phytophagous bugs. The mandibular stylet behavior of Perillus feeding on lepidopteran larvae is similar to that of seed-feeding bugs [1]; however, some special elements of the mandible structures are different. We documented in previous studies that mandibular structures of such predators differ from those of seed feeding bugs (three central blunt teeth and two pairs of lateral teeth in Pyrrhocoris sibiricus [16], and 1–2 teeth on the anterior and 1–2 dorso-lateral side in Largidae [20]). This very similar pattern of the mandible serration to seed-feeding bugs is present in phytophagous stink bugs. Depieri and Panizzi [12] reported that species Dichelops melacanthus (Dallas), Euschistus heros Fabricius, Nezara viridula (Linnaeus), and Piezodorus guildinii (Westwood) have four central blunt teeth and three pairs of small lateral teeth. This study of Asopinae species shows the consistent presence of pointed and long hooks on the mandible, which are absent in seed and stink bugs.
The basic plan of the maxillary stylets constitutes a powerful morphological adaptation revealing external features adapted for tissue penetration and internally includes a delivery channel for saliva and food equipped with small teeth-like/brush-like structures. Generally, the maxillary tips of heteropteran species are sharp, but this varies between taxa [1,2,7,8,14,15,16,17,18,19,20,21,22,66,67] depending on feeding habits. A common feature in most heteropterans the asymmetric tips of the left and right maxillary stylets (curvature on the right and straight on the left) and the distal end of each maxilla being smooth [1,8,15], a feature also visible in asopine species. This analysis of the maxillary stylets showed some differences among three Asopinae species (P. bidens, P. lewisi, and C. bhoutanica). In P. bidens and P. lewisi, the number of inner teeth is 5, with 2 in C. bhoutanica. In these species, the right maxilla is straight with ventral rows that have short barbs in the food canal. Images of the maxillae of related predaceous heteropterans [1,2,8,14,15,17,21,66,67] indicate that the distal parts of the maxillary stylets have different types of barbs consisting of rows of very well-developed stiff bristles in the food canal. A system of barbs in the food canal of predaceous heteropterans is needed to filter larger solid food so that they do not obstruct the digestive tract [1,8]. However, our observations of Asopinae show very few stiff bristles in the canal compared to other predatory insects [1,15,21].
4.2. Sensilla Types
Usually, the labium surface of heteropteran insects bears different sensilla with functions that vary between mechanoreceptive, chemoreceptive (gustatory and olfactory), proprioceptive, and thermo-hygrosensitive [1,15,16,18,19,20,24,26,27,28,68,69]. Here, we focus on comparing three predatory stinkbugs with other asopine species as well as with phytophagous pentatomid bugs.
The morphological characteristics of sensilla in Asopinae have been little studied. Basic information the labial tip sensilla and sensilla located around the distal part of last labial segment described in Canthecona furcellata [28] and in Eocanthecona furcellata [26]. More data on the labial sensilla were presented for Perillus bioculatus and Eocanthecona furcellata [27]. A full analysis of sensilla on each labial segment in other species indicates that up to fourteen different morphological types of sensilla may be present. In general, the basic shapes of labial sensilla are morphologically similar to sensilla reported for other heteropteran insects, including asopine species [26,27,28]. Nevertheless, in P. bidens, P. lewisi, and C. bhoutanica a new characteristic of sensilla was also distinguished. Our observations of the sensilla (St1) on the labial surface show that the number, types and distribution are similar between the two Picromerus species (P. bidens and P. lewisi) but lacking in C. bhoutanica. In the latter species the sensilla basiconica Sb3 are analogous to St1 because both have a similar system of pores. The short sensilla trichodea (St1) and sensilla basiconica Sb3 with several cuticular (nano) pores (in approximate diameters of St1 = 58.7 ± 4.3 nm, Sb3 = 31.6 ± 2.0 nm) were observed on the surfaces of segments 1–4; however, these sensilla are rarely arranged on the labium. Nanopores are small (1–100 nm diameter) holes or channels formed in biological membranes [70]. The permeation of ions and small molecules through nanopores is common in biological systems [70]. We suggest that these sensilla St1 and Sb3 might perform some kind olfactory function, because they have a special channel for uptake of nanoparticles. The ability to smell airborne odorants contributes to the insect’s ability to search for food and mates and detect environmental cues. In insects, the cuticle covering sensilla has small pores with diameters between 50 and 200 nanometers [70]. These nanopores are believed to function as filters that allow odorant molecules to enter but prevent the entry of larger airborne particles and help the insects avoid liquid loss [70]. Cuticular nanopores were described in the fruit fly’s olfactory sensilla [70]. The presence of pores on the wall of the sensillum is characteristic of olfactory sensilla [5,54,55]. These types of sensilla have not been reported in other predatory stink bugs [26,27,28]. However, a recent study on Haematoloecha nigrorufa (Reduviidae: Ectrichodiinae) [21] that employed very high magnification did provide the first evidence of the presence of St1 with pores in a predaceous heteropteran. As such types of sensilla are similar to antennal sensilla and are reported to have an olfactory function [5,71,72], it seems possible that the hemipteran labium and probably the labrum assist the antenna in this function.
The sensilla trichodea II (St2) are smooth, slender, and inserted in a flexible socket in the subapical region of the labium in all three species (P. bidens, P. lewisi and C. bhoutanica) and appear to be identical to those of other heteropterans also in Asopinae [16,18,19,20,26,27,28]. Their basic morphological appearance corresponds with the features of mechanosensilla also reported in other insects [54,55].
Observations of sensilla basiconica (Sb1, Sb2, Sb5, Sb6) on the labium in P. bidens, P. lewisi, and C. bhoutanica show obvious similarities. Only slight differences in the size and distribution of these sensilla were observed (Table 2); however, this may be attributed to the individual characteristics of the studied species. These sensilla basiconica are robust and have a flexible socket. This structure indicates a mechanical function, and they appear to be identical to those of the asopines studied by Rani [26] and Parveen et al. [27]. The sensilla campaniformia (Sca1, Sca2) distributed in various places on the mouthparts in Asopinae, in phytophagous pentatomids as well as in other heteropterans [16,18,19,20,27,28,73,74] represent the same form and function. These are mechanosensilla with a function of proprioception, responding to strains in the exoskeleton [75,76]. Similar functions are performed by sensilla basiconica (Sb4) described in this study. These are present on the junction between the first and second labial segment, and between the third and fourth segment. Such sensilla are also present in other heteropterans [16,18,19,20]. Proprioceptive sensilla perceive the degree of flexion of the integument [55,77].
The comparison of the labial tip sensilla of the studied species with other Asopinae species (Eocanthecona furcellata by Rani [26] and Parveen et al. [27], Perillus bioculatus by Parveen et al. [27] and Canthecona furcellata by Barsagade and Gathalkar [28] reveals a few differences in distribution of various sensilla types. This study of three species revealed nine pairs of sensilla styloconica (Sst) (nos. 1–6, 8–10), similar to gustatory sensilla corresponding to sensilla basiconica type A described in Eocanthecona furcellata by Rani [26], to sensilla styloconica (SStc), and peg sensilla (SP1) in Perillus bioculatus and Eocanthecona furcellata reported by Parveen et al. [27], and to sensilla basiconica (SB-I) in Canthecona furcellata reported by Barsagade and Gathalkar [28]. Two pairs of sensilla basiconica VII (Sb7) (nos. 7, 11 are the same type of gustatory sensilla) are short and have a big terminal pore; these pegs are located among sensilla styloconica. Sb7 are similar to sensilla basiconica type B and C [26] and to peg sensilla (SP2) in all five species studied by Parveen et al. [27], and to sensilla basiconica (SB-II, SB-III) in Canthecona furcellata. According to Rani [26] the type C and SB-III distinguished by Barsagade and Gathalkar [28] are only slightly different from each other because they are surrounded by hair-like structures. Rani [26] suggested that because sensilla basiconica type B and C have wall pores, they may have an olfactory function. Barsagade and Gathalkar [28] did not attribute specific functions to the sensilla reported. In this study, the system pores of the sensilla basiconica (Sb7) are not visible, although a large magnification was used in SEM observations. We agree with Parveen et al. [27] that sensilla (Sb7) are chemosensilla with gustatory function (uniporous sensilla). Although typical olfactory sensilla are not found on the labial tip in the Asopinae species, the reception of olfactory stimuli probably takes place through uniporous sensilla (gustatory). The uniporous sensilla present on the labial tip, e.g., near the rostral groove opening, might perceive the volatile chemicals emanating from prey. According to Zacharuk [5], all types of uniporous sensilla that respond to gustatory stimuli can also respond to strong odors. Thus, olfactory stimuli can be conducted even in the absence of typical multiporous olfactory sensilla. Sensilla styloconica are restricted to the predatory bugs, i.e., E. furcellata and P. bioculatus [27] and are located more marginally on the sensory fields. In phytophagous stinkbugs (Dolycoris indicus (Stål), Piezodorus hybneri (Gmelin) and Plautia crossota (Dallas)) uniporous peg sensilla (SP1) are more numerous on the sensory fields.
Sensilla trichodea (St3, St4) on the apical and subapical region of the labium in Asopinae species probably represent mechano-chemosensilla. Their appearance and location are similar to those reported as having this dual function in other insects [54,76,78]. The counterpart mechano-chemosensilla (St3, St4) are sensilla trichodea embedded in a flexible socket with cuticular walls smooth and a terminal pore, that are observed in phytophagous as well as predatory stinkbugs as three subtypes—sensilla trichodea ST1, ST2 and ST3. Sensilla trichodea were more abundant near the labial tip in such a way that they make first contact with the substratum during feeding [27,28]. A similar three subtypes of sensilla trichodea A, B and C were arranged around the labial tip as well as the sensorial area of the labial apex in E. furcellata and C. furcellata [26,28].
In all mentioned Asopinae species, there are special structures near the rostral groove referred to as cuticular projections (types i, ii, iii) by Parveen et al. [27] or multilobed sensillae (MLS) by Barsagade and Gathalkar [28] and Rani [26]. In our studied species, these structures are visible Figure 9. They apparently lack sensillar structures, although short and smooth peg sensilla with thermo-hygroreceptive function may be hidden among them. This type of sensillum is common in heteropteran species [16,18,19,20,24,27,69] and other insects [79].
5. Conclusions
The mouthpart structures of three Asopinae species were investigated as examples of predatory stink bugs. Our results represent the first detailed reports of P. bidens, P. lewisi, and C. bhoutanica mouthpart structures. These species exhibit some similar structures compared to other stink bugs: similar labium shape but different lengths and similar basic types of sensilla on the labial surface.
The sensilla on the labium surface (especially several types of sensilla basiconica Sb1, Sb2, Sb4, Sb6) in these three species are not significantly different in terms of their presence and location. In general, the labial tip sensilla in Asopinae reflects the usual pentatomorphan pattern. However, in these predators the sensilla styloconica are predominant along with additional short and long cuticular projections that are numerous around these sensilla. The two types of olfactory sensilla with nanopores (St1, Sb3) probably function to locate prey by smell.
In Asopinae, adaptation to the predatory lifestyle contributed to the formation of several hook-shaped mandibular teeth, clearly different from homologous structures in phytophagous stinkbugs. Externally, however, the structures of the maxillary stylets do not show significant changes reflecting a shift from phytophagy to predation; rather the phytophagous characters are preserved. The presence of distinct, stiff bristles in the food canal may indicate a possible adaptation to feeding on insect larvae.
Acknowledgments
We thank John Richard Schrock (Emporia State University, Emporia, KS, USA) and Chris Dietrich (Illinois Natural History Survey, Champaign, IL, USA) for comments on an earlier draft of this paper. We thank the Life Science Research Core Services of Northwest A&F University for providing the scanning electron microscope. We also thank the PROM programme 2018/2019 International scholarship exchange of doctoral students and academic staff. Project was co-financed by the European Union under the European Social Fund, the Knowledge Education Development Operational Program-POWER, and the University of Silesia in Katowice, Bankowa 12, 40-007 Katowice, Poland.
Author Contributions
Data curation, W.D.; funding acquisition, W.D.; investigation, Y.W.; project administration, W.D.; resources, W.D.; writing—original draft, Y.W., J.B. and W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (No. 31772514).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cobben R.H. Part II Mouthpart-Structures and Feeding Strategies. Mededelingen Landbouwhogeschool; Wageningen, The Netherlands: 1978. Evolutionary Trends in Heteroptera; pp. 1–407. [Google Scholar]
- 2.Faucheux M.M. Relations entre l’ultrastructure des stylets manibulaires et maxillaires et la prise de nourriture chez les insects Hemipteres. CR Acad. Sci. Paris (Ser. D) 1975;281:41–44. [Google Scholar]
- 3.Brożek J. Phylogenetic signals from Nepomorpha (Insecta: Hemiptera: Heteroptera) mouthparts: Stylets bundle, sense organs, and labial segments. Sci. World J. 2014;2014:237854. doi: 10.1155/2014/237854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis C.T. Structure and function in some external receptors. Symp. R. Entomol. Soc. Lond. 1970;5:59–76. [Google Scholar]
- 5.Zacharuk R.Y. Ultrastructure and function of insect chemosensilla. Annu. Rev. Entomol. 1980;25:27–47. doi: 10.1146/annurev.en.25.010180.000331. [DOI] [Google Scholar]
- 6.Romani R., Salerno G., Frati F., Conti E., Isidoro N., Bin F. Oviposition behaviour in Lygus rugulipennis: A morpho-functional study. Entomol. Exp. Appl. 2005;115:17–25. doi: 10.1111/j.1570-7458.2005.00268.x. [DOI] [Google Scholar]
- 7.Rathore Y.K. Studies on the mouthparts and feeding mechanism in Dysdercus cingulatus Fabr. (Pyrrhocoridae: Heteroptera) Indian J. Entomol. 1961;23:163–185. [Google Scholar]
- 8.Cohen A.C. Feeding adaptations of some predaceous Hemiptera. Ann. Entomol. Soc. Am. 1990;83:1215–1223. doi: 10.1093/aesa/83.6.1215. [DOI] [Google Scholar]
- 9.Boyd D.W. Digestive enzymes and stylet morphology of Deraeocoris nigritulus (Uhler) (Hemiptera: Miridae) reflect adaptations for predatory habits. Ann. Entomol. Soc. Am. 2003;96:667–671. doi: 10.1603/0013-8746(2003)096[0667:DEASMO]2.0.CO;2. [DOI] [Google Scholar]
- 10.Roitberg B.D., Gillespie D.R., Quiring D.M.J., Alma C.R., Jenner W.H., Perry J., Peterson J.H., Salomon M., Van Laerhoven S. The cost of being an omnivore: Mandible wear from plant feeding in a true bug. Naturwissenschaften. 2005;92:431–434. doi: 10.1007/s00114-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 11.Bérenger J., Pluot-Sigwalt D. Notes sur Micrauchenus lineola (Fabricius 1787), espècetermitophile et termitophage (Heteroptera: Reduviidae: Harpactorinae, Apiomerini) Ann. Soc. Entomol. Fr. 2009;45:129–133. doi: 10.1080/00379271.2009.10697596. [DOI] [Google Scholar]
- 12.Depieri R.A., Panizzi A.R. Rostrum length, mandible serration, and food and salivary canal areas of selected species of stink bugs (Heteroptera, Pentatomidae) Rev. Bras. Entomol. 2010;54:584–587. doi: 10.1590/S0085-56262010000400008. [DOI] [Google Scholar]
- 13.Depieri R.A., Siqueia F., Panizzi A.R. Aging and food source effects on mandibular stylets teeth wear of Phytophagous Stink Bug (Heteroptera: Pentatomidae) Neotrop. Entomol. 2010;39:952–956. doi: 10.1590/S1519-566X2010000600017. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M., Sahayaraj K. Gross morphology and histology of head and salivary apparatus of the predatory bug, Rhynocoris marginatus. J. Insect Sci. 2012;12:19. doi: 10.1673/031.012.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brożek J. A comparison of external and internal maxilla and mandible morphology of water bugs (Hemiptera: Heteroptera: Nepomorpha) Zootaxa. 2013;3635:340–378. doi: 10.11646/zootaxa.3635.4.2. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Dai W. Fine structure of mouthparts and feeding performance of Pyrrhocoris sibiricus Kuschakevich with remarks on the specialization of sensilla and stylets for seed feeding. PLoS ONE. 2017;12:e0177209. doi: 10.1371/journal.pone.0177209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stubbins F.L., Mitchell P.L., Turnbull M.W., Reay-Jones F.P.F., Greene J.K. Mouthpart morphology and feeding behavior of the invasive kudzu bug, Megacopta cribraria (Hemiptera: Plataspidae) Invertebr. Biol. 2017;136:309–320. doi: 10.1111/ivb.12184. [DOI] [Google Scholar]
- 18.Wang Y., Li L.F., Dai W. Fine morphology of the mouthparts in Cheilocapsus nigrescens (Hemiptera: Heteroptera: Miridae) reflects adaptation for phytophagous habits. Insects. 2019;10:143. doi: 10.3390/insects10050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Brożek J., Dai W. Sensory armature and stylets of the mouthparts of Stephanitis nashi (Hemiptera: Cimicomorpha: Tingidae), their morphology and function. Micron. 2020;132:102840. doi: 10.1016/j.micron.2020.102840. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Brożek J., Dai W. Morphological disparity of the mouthparts in polyphagous species of Largidae (Heteroptera: Pentatomomorpha: Pyrrhocoroidea) reveals feeding specialization. Insects. 2020;11:145. doi: 10.3390/insects11030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Zhang J.R., Wang W.S., Brożek J., Dai W. Unique fine morphology of mouthparts in Haematoloecha nigrorufa (Stål) (Hemiptera: Reduviidae) adapted to millipede feeding. Insects. 2020;11:386. doi: 10.3390/insects11060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Dai W. How does the intricate mouthpart apparatus coordinate for feeding in the hemimetabolous insect pest Erthesina fullo? Insects. 2020;11:503. doi: 10.3390/insects11080503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Clercq P. Predaceous stinkbugs (Pentatomidae: Asopinae) In: Schaefer C.W., Panizzi A.R., editors. Heteroptera of Economic Importance. CRC Press; Boca Raton, FL, USA: 2000. pp. 737–789. [Google Scholar]
- 24.Rani P.U., Madhavendra S.S. Morphology and distribution of antennal sense organs and diversity of mouthpart structures in Odontopus nigricornis (Stål) and Nezara viridula L. (Hemiptera) Int. J. Insect Morphol. Embryol. 1995;24:119–132. doi: 10.1016/0020-7322(94)00020-Q. [DOI] [Google Scholar]
- 25.Rani P.U., Madhavendra S.S. External morphology of antennal and rostral sensillae in four hemipteran insects and their possible role in host plant selection. Int. J. Trop. Insect Sci. 2005;25:198–207. doi: 10.1079/IJT200577. [DOI] [Google Scholar]
- 26.Rani P.U. Sensillary morphology on the rostral apex and their possible role in prey location behaviour of the carnivorous stinkbug, Eocanthecona furcellata (Wolff) (Heteroptera: Pentatomidae) Acta. Zool. 2009;90:246–253. doi: 10.1111/j.1463-6395.2008.00346.x. [DOI] [Google Scholar]
- 27.Parveen S., Ahmad A., Brożek J., Ramamurthy V.V. Morphological diversity of the labial sensilla of phytophagous and predatory Pentatomidae (Hemiptera: Heteroptera), with reference to their possible functions. Zootaxa. 2015;4039:246–253. doi: 10.11646/zootaxa.4039.2.9. [DOI] [PubMed] [Google Scholar]
- 28.Barsagade D.D., Gathalkar G.B. First predation record of Canthecona furcellata (Wolff.) (Hemiptera: Pentatomidae) on spinning stage silkworm Antheraea mylitta (Drury) Entomol. Res. 2016;46:236–245. doi: 10.1111/1748-5967.12169. [DOI] [Google Scholar]
- 29.Rider D.A., Schwertner C.F., Vilímová J., Rédei D., Kment P., Thomas D.B. Higher systematics of the Pentatomoidea. In: McPherson J.E., editor. Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management. CRC Press; Boca Raton, FL, USA: 2018. pp. 25–201. [Google Scholar]
- 30.Schouteden H. Genera Insectorum. Heteroptera. Fam. Pentatomidae. Subfam. Asopinae (Amyoteinae) Fasc. 1906;52:1–82. [Google Scholar]
- 31.Thomas D.B. Taxonomic synopsis of the Old World asopine genera (Heteroptera: Pentatomidae) Insecta Mundi. 1994;8:145–212. [Google Scholar]
- 32.Rider D. Pentatomidae. In: Aukema B., Rieger C., editors. Catalogue of the Heteroptera of the Palaearctic region, 5. Netherlands Entomological Society; Amsterdam, The Netherlands: 2006. pp. 233–402. [Google Scholar]
- 33.Gapud V.P. A generic revision of the subfamily Asopinae, with consideration of its phylogenetic position in the family Pentatomidae and superfamily Pentatomoidea (Hemiptera-Heteroptera). Parts. I and II. Philipp. Entomol. 1991;8:865–961. [Google Scholar]
- 34.Khloptseva R.I. The use of entomophages in biological pest control in the USSR. Biocontrol News Inf. 1991;12:243–246. [Google Scholar]
- 35.Hough-Goldstein J., Whalen J. Inundative release of predatory stink bugs for control of Colorado potato beetle. Biol. Control. 1993;3:343–347. doi: 10.1006/bcon.1993.1045. [DOI] [Google Scholar]
- 36.Zanuncio J.C., Alves J.B., Zanuncio T.V., Garcia J.F. Hemipterous predators of eucalypt defoliator caterpillars. For. Ecol. Manag. 1994;65:65–73. doi: 10.1016/0378-1127(94)90258-5. [DOI] [Google Scholar]
- 37.Zhao Q., Liu G., Bu W.J. A review of the Chinese species of the genus Picromerus Amyot and Serville, with description of a new species (Hemiptera: Heteroptera: Pentatomidae: Asopinae) Zootaxa. 2013;3613:146–164. doi: 10.11646/zootaxa.3613.2.3. [DOI] [PubMed] [Google Scholar]
- 38.Roca-Cusachs M., Kim J., Park J., Jung S. Taxonomic review of the predatory stink bugs of the Korean Peninsula (Heteroptera: Pentatomidae: Asopinae), with a key to the Korean species and a discussion of their usefulness as biological control agents. J. Asia-Pacific Entomol. 2020;23:113–123. doi: 10.1016/j.aspen.2019.09.006. [DOI] [Google Scholar]
- 39.Gäbler H. Picromerus bidens L. als Feind der Lophyruslarven. Tharandter Forstl. Jahrb. 1937;88:51–58. [Google Scholar]
- 40.Gäbler H. Die Bedeutung einiger Wanzenarten als Feinde der Nonne. Z. Angew. Entomol. 1938;25:277–290. doi: 10.1111/j.1439-0418.1939.tb01197.x. [DOI] [Google Scholar]
- 41.Engel H. Populationsdynamik des Kieferspanners in verschiedenen Biotopen. Verh. VII Intern. Kongr. Entomol. 1939;3:1941–1949. [Google Scholar]
- 42.Clausen C.P. Entomophagous Insects. McGraw-Hill; New York, NY, USA: 1940. p. 688. [Google Scholar]
- 43.Forsslund K.H. Något om röda tallstekelns (Diprion sertifer Geoffr.) skadegörelse. Medd. Skogsförsöksanst. 1946;34:365–390. [Google Scholar]
- 44.Mayné R., Breny R. Picromerus bidens L.: Morphologie. Biologie. Détermination de sa valeur d’utilisation dans la lute biologique contre le doryphore de la pomme de terre—La valeur économique antidoryphorique des Asopines indigènes belges. Parasitica. 1948;4:189–224. [Google Scholar]
- 45.Pschorn-Walcher H., Zinnert K.D. Investigations on the ecology and natural control of the larch sawfly (Pristiphora erichsonii Htg, Hym: Tenthredinidae) in central Europe. Part II: Natural enemies: Their biology and ecology, and their role as mortality factors in P. erichsonii. Tech. Bull. Commonw. Inst. Biol. Control. 1971;14:1–50. [Google Scholar]
- 46.Mallach N. Zur Kenntnis der kleinen Kiefern-Buschhornblattwespe, Diprion (Microdiprion) pallipes (Fall.) (Hym.: Diprionidae). Teil 3. Populationsökologie. Z. Angew. Entomol. 1974;75:337–380. doi: 10.1111/j.1439-0418.1974.tb01861.x. [DOI] [Google Scholar]
- 47.Asanova R.B. New and rare species of bugs for the fauna of northern Kazakhstan (Heteroptera) Trudy Inst. Zool. Akad. Nauk Kazakhskoi SSR. 1980;39:49–54. (In Russian) [Google Scholar]
- 48.Javahery M. Biology and ecology of Picromerus bidens (Hemiptera: Pentatomidae) in southeastern Canada. Entomol. News. 1986;97:87–98. [Google Scholar]
- 49.Larivière M.-C., Larochelle A. Picromerus bidens (Heteroptera: Pentatomidae) in North America, with a world review of its distribution and bionomics. Entomol. News. 1989;100:133–146. [Google Scholar]
- 50.Lin Y.J., Long J., Zhang S.M., Lin Z. A checklist of species of Asopinae (Hemiptera: Pentatomoidea) from China. Jiangxi Plant Protection. 2000;23:36–39. (In Chinese) [Google Scholar]
- 51.Rider D.A., Zheng L.Y. Checklist and nomenclatural notes on the Chinese Pentatomidae (Heteroptera). III. Asopinae. Entomotaxonomia. 2002;24:107–115. [Google Scholar]
- 52.Tang Y.T., Guo Y., He G.W., Liu C.X., Chen H.Y., Zhang L.S., Wang M.Q. Functional Responses of Picromerus lewisi Scott (Hemiptera: Pentatomidae) attacking Mythimna separata (Walker) (Lepidoptera: Noctuidae) Chin. J. Biol. Control. 2018;34:825–830. (In Chinese) [Google Scholar]
- 53.Tang Y.T., Wang M.Q., Chen H.Y., Wang Y., Zhang H.M., Chen F.S., Zhao X.Q., Zhang L.S. Predation and behavior of Picromerus lewisi Scott to Spodoptera frugiperda (J. E. Smith) Chin. J. Biol. Con. 2019;35:698–703. [Google Scholar]
- 54.Altner H., Prillinger L. Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. Int. Rev. Cytol. 1980;67:69–139. [Google Scholar]
- 55.Frazier J.L. Nervous system: Sensory system. In: Blum M.S., editor. Fundamentals of Insect Physiology. John Wiley & Sons; New York, NY, USA: 1985. pp. 287–356. [Google Scholar]
- 56.Walker A.A., Weirauch C., Fry B.G., King G.F. Venoms of Heteropteran Insects: A treasure trove of diverse pharmacological toolkits. Toxins. 2016;8:43. doi: 10.3390/toxins8020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grazia J., Panizzi A.R., Greve C., Schwertner C.F., Campos L.A., Garbelotto T., Fernandes J.A.M. Stink Bugs (Pentatomidae) In: Panizzi A.R., Grazia J., editors. True Bugs (Heteroptera) of the Neotropics. Springer Science + Business Media; Dordrecht, The Netherlands: 2015. pp. 681–756. Chapter 22. [Google Scholar]
- 58.Gross G.F. In: Plant-feeding and Other Bugs (Hemiptera) of South Australia. Heteroptera, Part I. James A.B., editor. Handbooks Committee of the South Australian Government; Adelaide, Australia: 1975. [Google Scholar]
- 59.Stone P.C. Master’s Thesis. University of Massachusetts Amherst; Amherst, MA, USA: 1936. A Study of the External Morphology of a Predacious Stink-bug: Apateticus cynicus (Say); Family, Pentatomidae; Order, Hemiptera-Heteroptera. [Google Scholar]
- 60.Esquivel J.F. Stink bug rostrum length vs. stylet penetration potential. Èntomol. Exp. Appl. 2019;167:323–329. doi: 10.1111/eea.12782. [DOI] [Google Scholar]
- 61.Lee H., Kim J.G., Jung S.H. A New record of Genus Eocanthecona Bergroth (Hemiptera: Heteroptera: Pentatomidae: Asopinae) from the Korean Peninsula. Korean J. Appl. Entomol. 2015;54:257–261. doi: 10.5656/KSAE.2015.05.0.009. [DOI] [Google Scholar]
- 62.Schuh R.T., Slater J.A. True Bugs of the World (Hemiptera: Heteroptera—Classification and Natural History. Cornell University Press; Ithaca, NY, USA: 1995. [Google Scholar]
- 63.Esquivel J.F. Estimating potential stylet penetration of southern green stink bug—A mathematical modeling approach. Èntomol. Exp. Appl. 2011;140:163–170. doi: 10.1111/j.1570-7458.2011.01148.x. [DOI] [Google Scholar]
- 64.Weiraucha C., Schuhb R.T., Cassisc G., Wheeler W.C. Revisiting habitat and lifestyle transitions in Heteroptera (Insecta: Hemiptera): Insights from a combined morphological and molecular phylogeny. Cladistics. 2018;35:67–105. doi: 10.1111/cla.12233. [DOI] [PubMed] [Google Scholar]
- 65.McGavin G.C. Bugs of the World. Blandford; London, UK: 1993. Food and Feeding; pp. 139–158. Chapter 6. [Google Scholar]
- 66.Swart C.C., Felgenhauer B.E. Structure and function of the mouthparts and salivary gland complex of the giant waterbug, Belostoma lutarium (Stål) (Hemiptera: Belostomatidae) Ann. Entomol. Soc. Am. 2003;96:870–882. doi: 10.1603/0013-8746(2003)096[0870:SAFOTM]2.0.CO;2. [DOI] [Google Scholar]
- 67.Sahayaraj K., Kanna A.V., Kumar S.M. Gross morphology of feeding canal, salivary apparatus and digestive enzymes of salivary gland of Catamirus brevipennis (Serville ) (Hemiptera: Reduviidae) J. Entomol. Res. Soc. 2010;12:37–50. [Google Scholar]
- 68.Brożek J., Zettel H. A comparison of the external morphology and functions of labial tip sensilla in semiaquatic bugs (Hemiptera: Heteroptera: Gerromorpha) Eur. J. Entomol. 2014;111:275–297. doi: 10.14411/eje.2014.033. [DOI] [Google Scholar]
- 69.Taszakowski A., Nowińska A., Brożek J. Morphological study of the labial sensilla in Nabidae (Hemiptera: Heteroptera: Cimicomorpha) Zoomorphology. 2019;138:483–492. doi: 10.1007/s00435-019-00455-3. [DOI] [Google Scholar]
- 70.Ando T., Sekine S., Inagaki S., Misaki K., Badel L., Moriya H., Sami M.M., Itakura Y., Chihara T., Kazama H., et al. Nanopore formation in the cuticle of an insect olfactory sensillum. Curr. Biol. 2019;29:1512–1520. doi: 10.1016/j.cub.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 71.Slifer E.H. The structure of arthropod chemoreceptors. Annu. Rev. Entomol. 1970;15:121–141. doi: 10.1146/annurev.en.15.010170.001005. [DOI] [Google Scholar]
- 72.Steinhrccht R.A. Chemo-, hygro-, and thermoreceptors. In: Berciter-Hahn J., Matoltsy A.G., Richards K.S., editors. Biology of the Integument, Invertebrates. Volume 1. Springer; Berlin/Heidelberg, Germany: 1984. pp. 523–553. [Google Scholar]
- 73.Catalá S. Sensilla associated with the rostrum of eight species of Triatominae. J. Morphol. 1996;228:195–201. doi: 10.1002/(SICI)1097-4687(199605)228:2<195::AID-JMOR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 74.Brożek J., Chłond D. Morphology, arrangement and classification of sensilla on the apical segment of labium in Peiratinae (Hemiptera: Heteroptera: Reduviidae) Zootaxa. 2010;2476:39–52. doi: 10.11646/zootaxa.2476.1.5. [DOI] [Google Scholar]
- 75.McIver S.B. Structure of cuticular mechanoreceptors of arthropods. Ann. Rev. Entomol. 1975;20:381–397. doi: 10.1146/annurev.en.20.010175.002121. [DOI] [PubMed] [Google Scholar]
- 76.Chapman R.F. Communication. In: Chapman R.F., editor. The Insects: Structure and Function. 4th ed. Cambridge University Press; Cambridge, UK: 1998. pp. 610–652. [Google Scholar]
- 77.Gullan P.J., Cranston P.S., editors. The Insects: An Outline of Entomology. 5th ed. Wiley Blackwell; Oxford, UK: 2014. Sensory Systems and Behavior; pp. 85–112. [Google Scholar]
- 78.Backus E.A. Anatomical and sensory mechanisms of leafhopper and planthopper feeding behavior. In: Nault L.R., Rodriguez J.G., editors. The Leafhoppers and Planthoppers. John Wiley & Sons; New York, NY, USA: 1985. pp. 163–194. [Google Scholar]
- 79.Chapman R.F. Chemosensory regulation of feeding. In: Chapman R.F., de Boer G., editors. Regulatory Mechanisms in Insects Feeding. Springer Science + Business Media; Dordrecht, The Netherlands: 1995. pp. 101–136. [Google Scholar]