Abstract
Background
The fecal microbiota, fecal bile acid concentrations, and abundance of Clostridium perfringens and Clostridium difficile are altered in acute and chronic gastrointestinal disease in adult dogs. However, less is known in young puppies.
Hypothesis/Objectives
To determine composition of the fecal microbiota, assess development of fecal bile acid profiles, and determine the abundance of Clostridial species in puppies, young adult dogs, and adult dogs.
Animals
Healthy puppies from a whelping kennel (n = 53) and healthy client‐owned dogs <1 year old (n = 20) were separated into 6 age groups, then compared to client‐owned dogs over 1 year of age (n = 13).
Methods
Prospective observational study. Naturally voided fecal samples were analyzed by quantitative polymerase chain reaction to measure bacterial abundances. Fecal bile acids were quantified using gas chromatography‐mass spectrometry.
Results
Puppies up to 5 to 6 weeks of age had increased Dysbiosis Index (median [min‐max]: 5.39 [1.32‐8.6], P < .001), increased abundance of C. difficile (4.1 [0.01‐4.85] log DNA, P < .001), decreased secondary bile acid concentrations (0.61 [0.28‐5.06] μg/mg, P = .006), and decreased abundance of C. hiranonis (0.84 [0.01‐6.71], P = .005) compared to adult dogs (−4.62 [−8.36 to −0.61], 0.01 [0.01‐0.01], 4.12 [0.32‐8.94], and 6.02 [5.06‐7.00], respectively). Secondary bile acid concentration positively correlated with C. hiranonis abundance (ρ = 0.77; P < .001).
Conclusions and Clinical Importance
The increase in secondary bile acids and simultaneous decrease of C. difficile and C. perfringens after 5 to 6 weeks of age warrants further investigation into regulatory impacts that secondary bile acids could have on clostridial species in dogs.
Keywords: canine, Clostridium difficile, Clostridium hiranonis, microbiota
Abbreviations
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- CPE
Clostridium perfringens enterotoxin gene
- DCA
deoxycholic acid
- DI
dysbiosis index
- GI
gastrointestinal
- LCA
lithocholic acid
- qPCR
quantitative polymerase chain reaction
- UDCA
ursodeoxycholic acid
1. INTRODUCTION
The gastrointestinal (GI) microbiome is critical to the health of young dogs as it plays several roles, including the maturation of the immune system, protection against enteropathogens, and utilization of nutrients. 1 , 2 While there are numerous studies pertaining to the microbiome in adult dogs, few describe the postnatal development of the GI microbiome. 3 , 4 , 5 The microbiome increases in species richness beginning on day 2 after birth, and a major shift occurs from predominantly Firmicutes on day 2 to a codominance of Bacteroidetes, Fusobacteria, and Firmicutes by day 21 of age in dogs. 3 Further characterization is merited, as development of the microbiome is important for the health of the developing puppy.
Alteration of the fecal microbiota is common in adult dogs with GI diseases. 6 , 7 , 8 , 9 , 10 , 11 The fecal dysbiosis index (DI) is a tool to evaluate the GI microbiota during disease and monitor response to treatment. 12 The DI is a rapid quantitative polymerase chain reaction (qPCR)‐based assay that measures total bacteria and 7 key bacterial taxa (Fusobacterium, Clostridium hiranonis, Faecalibacterium, Streptococcus, Turicibacter, Blautia, and Escherichia coli) which were selected for having the highest discriminatory power in identifying dysbiosis in adult dogs with chronic enteropathies. A DI below zero is indicative of a healthy microbiota in dogs. Importantly, no studies have examined if the same index is applicable in young dogs. Based on previous studies, 3 , 5 puppies have an increased abundance of E. coli and a decreased abundance of Faecalibacterium, which would be expected to lead to an apparently increased DI in young healthy dogs.
Additional bacterial groups used to evaluate GI diseases in adult dogs include Clostridium difficile, Clostridium perfringens, Salmonella spp., and Campylobacter jejuni. Although multiple studies show these bacterial groups and their respective toxins to be present in healthy adult dogs and dogs with GI disease, little is known about their presence in young healthy dogs. 13 , 14 , 15 , 16 , 17 , 18 , 19
Bile acids are of growing interest in study of GI disease in dogs. Bile acids aid in digestion and absorption of lipids in the GI tract, but also serve as signaling molecules. Primary bile acids are converted to secondary bile acids by bacteria with 7α‐dehydroxylation capabilities. Secondary bile acids inhibit growth and germination of C. difficile in humans, 20 , 21 and it is expected that the same relationship exists in dogs. Secondary bile acids also inhibited growth of isolates from dogs of E. coli and C. perfringens in vitro. 22 Conversely, Clostridium hiranonis has 7α‐dehydroxylation activity, 23 is part of the DI of dogs, 12 and is likely the main contributor to the secondary bile acid pool in dogs. 6 , 24 Therefore, the association between the development of bile acid profiles and the development of the microbiota, and the potential influence of bile acids on Clostridial species in growing puppies needs further examination.
The objective of our study was to determine at which age the fecal DI resembles that of adult dogs and to assess the development of fecal bile acid profiles and their correlation with abundances of C. hiranonis and C. difficile in puppies of different ages.
2. MATERIALS AND METHODS
2.1. Animal enrollment
Puppies equal to or less than 10 weeks of age were enrolled from the Guide Dogs for the Blind, Inc in San Rafael, California (n = 53), and freely passed fecal samples (total n = 58) were collected. For the purpose of our study, fecal samples were separated into 5 groups based on the age of the puppy rounded up to the nearest week (Table 1): 1 to 2 weeks (n = 14), 3 to 4 weeks (n = 14), 5 to 6 weeks (n = 13), 7 to 9 weeks (n = 16), and 10 to 16 weeks (n = 1). Five of the puppies were represented in 2 age groups. All of these puppies were Golden Retrievers, Labrador Retrievers, or Golden Labrador mixes. They were born in a whelping kennel, and all puppies were fed the same diet plan during their entire time in the kennels. Starting at 3 weeks of age (21 days), puppies were offered Purina Pro Plan Large Breed Puppy Chicken & Rice formula in a 1 : 1.2 kibble to water ratio 3 times daily. At 4 weeks and 4 days to 4 weeks and 5 days (32‐33 days) of age, puppies were transitioned to a 3 : 1 kibble to water ratio and intermittently separated from brood to encourage weaning. By 6 weeks of age, puppies were fully weaned and then moved to a puppy kennel. Biosecurity measures were present in each kennel to prevent outside pathogens from entering. At 8 to 10 weeks of age, the puppies were sent to homes across the Western United States to be raised and socialized by volunteers until returning for formal guide dog training. Feces were stored at −20°C at the whelping facility until the end of collection, upon which they were shipped on dry ice to the Gastrointestinal Laboratory at Texas A&M University. Upon arrival to the laboratory, samples were inventoried while remaining on dry ice over the course of 3 hours and then transferred to −80°C storage until further processing.
TABLE 1.
Animal enrollment and data (if available) for dogs included in the study
| Age group | Sample number | Source | Sex (female/male) |
|---|---|---|---|
| 1‐2 weeks | 14 | Guide dogs | 6/8 |
| 3‐4 weeks | 14 | Guide dogs | 8/5 |
| 5‐6 weeks | 13 | Guide dogs | 8/5 |
| 7‐9 weeks | 17 | Guide dogs (16) and Client‐owned dogs (1) | 9/8 |
| 10‐16 weeks | 11 | Guide dogs (1) and client‐owned dogs (10) | 7/4 |
| 20‐48 weeks | 11 | Client‐owned dogs | 6/5 |
| >52 weeks | 13 | Client‐owned dogs | 7/6 |
Note: Seven dogs were represented in 2 age groups.
Since further sampling was not able to be completed on the puppies once they left the whelping facility, 33 additional healthy dogs aged 8 weeks and older were enrolled in College Station, Texas. Freely passed fecal samples (total n = 35) were collected and separated into 4 age groups based on the age of the dog at the time of sampling: 7 to 9 weeks (n = 1), 10 to 16 weeks (n = 10), 20 to 48 weeks (n = 11), and >52 weeks (n = 13). Two dogs were represented in 2 age groups. These dogs were client‐owned, lived indoors, and were fed a variety of diets. Recruitment was conducted at puppy training classes, dog parks, and through social media at the veterinary school. Owners verbally consented to the use of their pet's feces in research and filled out a questionnaire to provide information about their dog (Supporting Information: Questionnaire 1). Inclusion criteria for all dogs included the absence of clinical signs and no antibiotic usage in the previous 6 months. Feces utilized in the study were stored at −80°C until processing.
Institutional animal care and use committee approval was not necessary for our study because all fecal samples used were naturally voided.
2.2. DNA isolation and qPCR
DNA was extracted from approximately 100 mg feces using a commercially available kit (PowerSoil DNA Isolation Kit, MOBIO Laboratories, Inc, Carlsbad, California) following manufacturer's instructions. Then, the DNA was normalized for concentration on a 96‐well plate utilizing DNA concentration and purity obtained on a Nanodrop 1000 (Thermo Scientific, Rockford, Illinois).
The abundances of selected bacterial groups were assessed by qPCR assays using published oligonucleotides described in Table 2. All samples were analyzed in duplicate on a commercially available qPCR thermal cycler (CFX96TM, Bio‐Rad Laboratories, California), and data expressed as LogSQ, or log target DNA (fg) per 10 ng starting quantity of total DNA.
TABLE 2.
Primers and cycling conditions used in qPCR assays
| Target | Primer sequences (5′ ‐ 3′) | Initial denaturing, # cycles | Denaturing | Annealing | Extension | Reference |
|---|---|---|---|---|---|---|
| Universal |
F‐CCTACGGGAGGCAGCAGT R‐ATTACCGCGGCTGCTGG |
98°C, 2 min, 35 | 98°C, 5 s | 59°C, 5 s | 25 | |
| Faecalibacterium spp. |
F‐GAAGGCGGCCTACTGGGCAC R‐GTGCAGGCGAGTTGCAGCCT |
98°C, 2 min, 40 | 98°C, 5 s | 60°C, 5 s | 26 | |
| Turicibacter spp. |
F‐CAGACGGGGACAACGATTGGA R‐TACGCATCGTCGCCTTGGTA |
98°C, 2 min, 40 | 98°C, 3 s | 57°C, 3 s | 27 | |
| Streptococcus spp. |
F‐TTATTTGAAAGGGGCAATTGCT R‐GTGAACTTTCCACTCTCACAC |
95°C, 2 min, 40 | 95°C, 5 s | 54°C, 10 s | 28 | |
| Escherichia coli |
F‐GTTAATACCTTTGCTCATTGA R‐ACCAGGGTATCTAATCCTGTT |
98°C, 2 min, 40 | 98°C, 3 s | 55°C, 3 s | 29 | |
| Blautia spp. |
F‐TCTGATGTGAAAGGCTGGGGCTTA R‐GGCTTAGCCACCCGACACCTA |
98°C, 2 min, 40 | 98°C, 4 s | 56°C, 4 s | 27 | |
| Fusobacterium spp. |
F‐KGGGCTCAACMCMGTATTGCGT R‐TCGCGTTAGCTTGGGCGCTG |
98°C, 2 min, 40 | 98°C, 4 s | 50.5°C, 4 s | 27 | |
| Clostridium hiranonis |
F‐AGTAAGCTCCTGATACTGTCT R‐AGGGAAAGAGGAGATTAGTCC |
95°C, 3 min, 40 | 95°C, 30 s | 59°C, 5 s | 30 | |
| C perfringens NetF toxin |
F‐AACAATATGTACAGGTATAACT R‐TTGATAGGTATAATATGGTTCT |
98°C, 2 min, 40 | 98°C, 30 s | 55°C, 30 s | 16 | |
| C perfringens 16S rRNA |
F‐CGCATAACGTTGAAAGATGG R‐CCTTGGTAGGCCGTTACCC P‐TCATCATTCAACCAAAGGAGCAATCC |
94°C, 10 min, 45 | 94°C, 10 s | 58°C, 20 s | 70°C, 10 s | 31 |
| C difficile 16S rRNA |
F‐TTGAGCGATTTACTTCGGTAAAGA R‐TGTACTGGCTCACCTTTGATATTCA P‐CCACGCGTTACTCACCCGTCCG |
95°C, 2 min, 40 | 95°C, 5 s | 61°C, 10 s | 32 | |
| C perfringens enterotoxin |
F‐AACTATAGGAGAACAAAATACAATAG R‐TGCATAAACCTTATAATATACATATTC P‐TCTGTATCTACAACTGCTGGTCCA |
95°C, 2 min, 40 | 95°C, 5 s | 55°C, 10 s | 33 | |
| Salmonella spp. |
F‐GCAATTACAGGAACAGACGCT R‐CCTGACGCCCGTAAGAGA P‐TAAAACTTCGCCATACCAGCCAGACA |
95, 2 min, 40 | 95, 5 s | 60, 10 s | 34 | |
| Campylobacter jejuni |
F‐TTAATGACGCGGTAAAAGTAACTATGG R‐TGCTTGGAGCACCAAAGCT P‐CCAAGAGGACGCAATGT |
95, 2 min, 40 | 95, 5 s | 52.5, 20 s | 35 |
Abbreviations: F, forward primer; P, probe; qPCR, quantitative polymerase chain reaction; R, reverse primer.
The qPCR assays for total bacteria, Faecalibacterium spp., Turicibacter spp., Streptococcus spp., Escherichia coli, Blautia spp., Fusobacterium spp., Clostridium hiranonis, and C. perfringens NetF toxin gene were performed using previously published cycling and annealing conditions described in Table 2. 16 , 25 , 26 , 27 , 28 , 29 , 30 Briefly, SYBR Green based reaction mixtures (total 10 μL) contained 5 μL of SsoFast EvaGreen Supermix (Bio‐Rad Laboratories), 2.2 μL of PCR water, 0.4 μL of each primer (final concentration: 400 nM), and 2 μL of normalized DNA (final concentration: 5 ng/μL). A melt curve analysis, with increments of 0.5°C from 65°C to 95°C for 5 seconds each, was performed after the amplification cycles.
The qPCR assay for the C. perfringens 16S rRNA gene was performed as previously described. 13 , 31 Briefly, TaqMan based reaction mixtures (total 10 μL) contained 5 μL of SsoAdvanced Universal Probes Supermix, 2.2 μL of PCR water, 0.3 μL of each primer (final concentration: 300 nM), 0.2 μL of the probe (final concentration: 200 nM), and 2 μL of normalized DNA (final concentration: 5 ng/μL). The qPCR cycling and annealing conditions are described in Table 2.
The qPCR assays for the Clostridium difficile 16S rRNA gene, Clostridium perfringens enterotoxin gene (CPE), Salmonella spp., and Campylobacter jejuni were performed as previously described. 13 , 32 , 33 , 34 , 35 Briefly, TaqMan based reaction mixtures (total 10 μL) contained 5 μL of SsoAdvanced Universal Probes Supermix (Bio‐Rad Laboratories), 2.35 μL of PCR water, 0.25 μL of each forward and reverse primer (final concentration: 250 nM), 0.15 μL of the probe (final concentration: 150 nM), and 2 μL of normalized DNA (final concentration: 5 ng/μL). The qPCR cycling and annealing conditions are described in Table 2.
2.3. Dysbiosis index
The fecal DI was calculated from the abundance of key bacterial taxa as previously described. 12 Briefly, the abundances of total bacteria, Faecalibacterium spp., Turicibacter spp., Streptococcus spp., Escherichia coli, Blautia spp., Fusobacterium spp., and Clostridium hiranonis are combined using a mathematical algorithm and reported as the DI. A DI below zero is indicative of a normal adult microbiota in dogs.
2.4. Clostridium spp. toxins
Fecal samples that tested positive for C. difficile 16S rRNA through qPCR were subjected to further screening with the C. Diff Quik Chek Complete test (TechLab, Inc, Blackburg, Virginia) for the detection of C. difficile glutamate dehydrogenase antigen and toxins A and B when leftover feces were available.
Fecal samples that tested positive for the C. perfringens enterotoxin gene through qPCR were subjected to further screening with an ELISA based C. perfringens Enterotoxin Test (TechLab Inc) for the detection of enterotoxin produced by C. perfringens when leftover feces were available.
2.5. Quantification of fecal bile acids
The following unconjugated bile acids were quantified by an in‐house targeted gas chromatography‐mass spectrometry assay utilizing previously described methods 6 : cholic acid (CA), chenodeoxycholic acid (CDCA), deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA).
2.6. Statistical analysis
Statistical analysis was performed using statistical software packages (JMP Pro version 14, SAS Institute, Inc, Cary, North Carolina; and Prism version 8, GraphPad Software, Inc, La Jolla, California). Some data showed a nonparametric distribution based on the Shapiro‐Wilk normality test results. Therefore, nonparametric testing was used for further analysis. Kruskal‐Wallis tests were used to assess differences in measured variables across the age groups. Dunn's multiple comparisons post‐tests were used to identify differences between each of the 6 age groups <1 year of age and healthy adult dogs >1 year of age. Spearman's correlation analysis was performed between Clostridial species abundances and total secondary bile acid concentrations. Significance was set at P < .05.
3. RESULTS
Statistical test results for all variables are listed in Table S1.
3.1. Dysbiosis index
The DI was significantly higher in puppies that were 1 to 2 weeks, 3 to 4 weeks, and 5 to 6 weeks of age (P < .001) when compared to adult dogs (dogs >52 weeks old; Figure 1). Dogs that were 1 to 2 weeks, 3 to 4 weeks, and 5 to 6 weeks old also showed a decreased abundance of Faecalibacterium when compared to adult dogs (P < .001, P < .001, and P = .02, respectively; Figure 2). Abundance of Turicibacter was decreased in puppies 1 to 2 and 3 to 4 weeks of age (P < .001) compared to adult dogs (Figure 2). Abundance of Streptococcus was increased in puppies that were 3 to 4, 5 to 6, and 7 to 9 weeks of age (P = .03, P < .001, and P = .007, respectively), and the abundance of E. coli was significantly increased in puppies that were 1 to 2, 3 to 4, 5 to 6, and 7 to 9 weeks old (P = .005, P < .001, P = .02, and P = .03, respectively) compared to adult dogs (Figure 2). Abundances of Blautia and Fusobacterium were significantly decreased in puppies that were 1 to 2 weeks of age (P < .001 and P = .003, respectively) when compared to adult dogs (Figure 2). Abundance of C. hiranonis was significantly decreased in puppies that were 1 to 2, 3 to 4, and 5 to 6 weeks old (P < .001, P < .001, and P = .005, respectively) when compared to adult dogs (Figure 3). Abundance of total bacteria was increased in puppies that were 3 to 4 weeks and 5 to 6 weeks old (P = .05 and P = .02, respectively) when compared to adult dogs (Figure S1).
FIGURE 1.
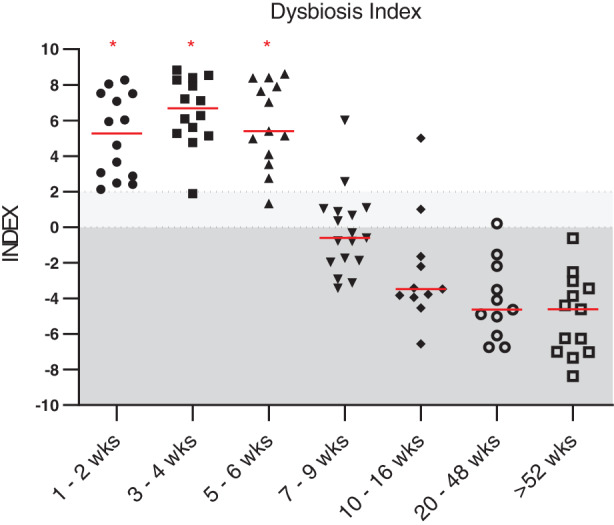
Fecal dysbiosis index of dogs age 1 to 2 weeks (n = 14), 3 to 4 weeks (n = 14), 5 to 6 weeks (n = 13), 7 to 9 weeks (n = 17), 10 to 16 weeks (n = 11), 20 to 48 weeks (n = 11), and adult dogs (ie, dogs >52 weeks of age; n = 13). Dysbiosis index was significantly increased in dogs age 1 to 2 weeks, 3 to 4 weeks, and 5 to 6 weeks (P < .001 for all) when compared to adult dogs. Dysbiosis index was not significantly different from adult dogs at 7 to 9 weeks of age (P = .05), 10 to 16 weeks of age (P > .99), and 20 to 48 weeks of age (P > .99). Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (P < .05). Dark shaded area (values <0) represents reference interval, and light shaded area (0‐2) represents an equivocal range. wks, weeks
FIGURE 2.
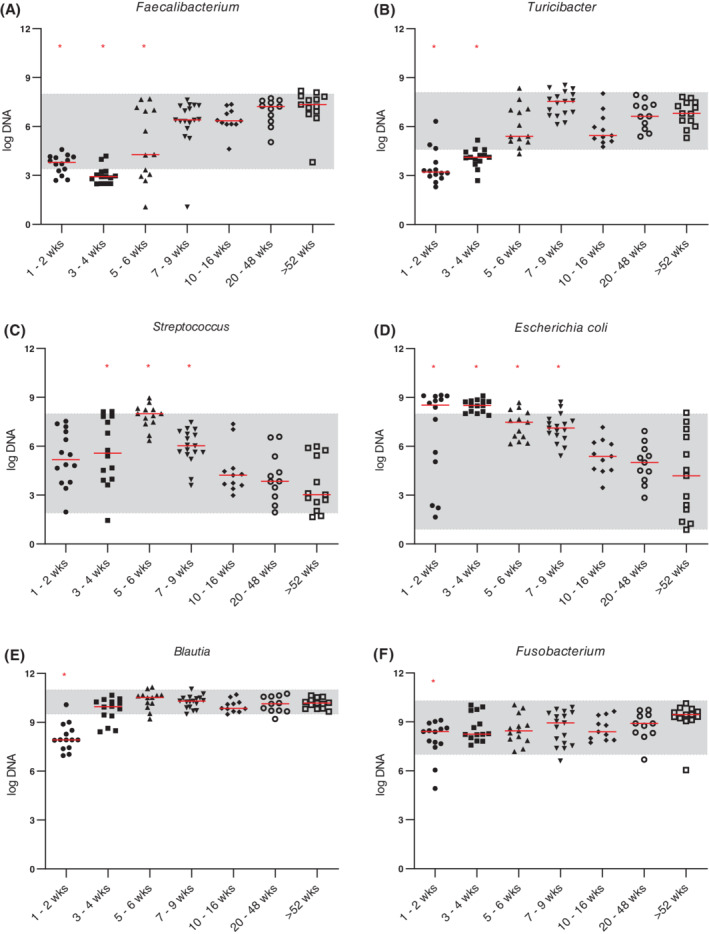
Abundances (log DNA) of bacterial groups included in the dysbiosis index for dogs age 1 to 2 weeks (n = 14), 3 to 4 weeks (n = 14), 5 to 6 weeks (n = 13), 7 to 9 weeks (n = 17), 10 to 16 weeks (n = 11), 20 to 48 weeks (n = 11), and adult dogs (ie, dogs >52 weeks of age; n = 13). A, Abundance of Faecalibacterium was significantly decreased in dogs age 1 to 2 weeks (P < .001), 3 to 4 weeks (P < .001), and 5 to 6 weeks (P = .02) when compared to adult dogs. B, Abundance of Turicibacter was significantly decreased in dogs age 1 to 2 weeks and 3 to 4 weeks (both P < .001) compared to adult dogs. C, Abundance of Streptococcus was significantly increased in dogs age 3 to 4 weeks (P = .03), 5 to 6 weeks (P < .001), and 7 to 9 weeks (P = .007) compared to adult dogs. D, Abundance of Escherichia coli was significantly increased in dogs age 1 to 2 weeks (P = .005), 3 to 4 weeks (P < .001), 5 to 6 weeks (P = .02), and 7 to 9 weeks (P = .03) compared to adult dogs. E, Abundance of Blautia and, F, Fusobacterium was significantly decreased in dogs 1 to 2 weeks of age (P < .001 and P = .003, respectively) compared to adult dogs. Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (P < .05). Shaded areas correspond to reference intervals. wks, weeks
FIGURE 3.
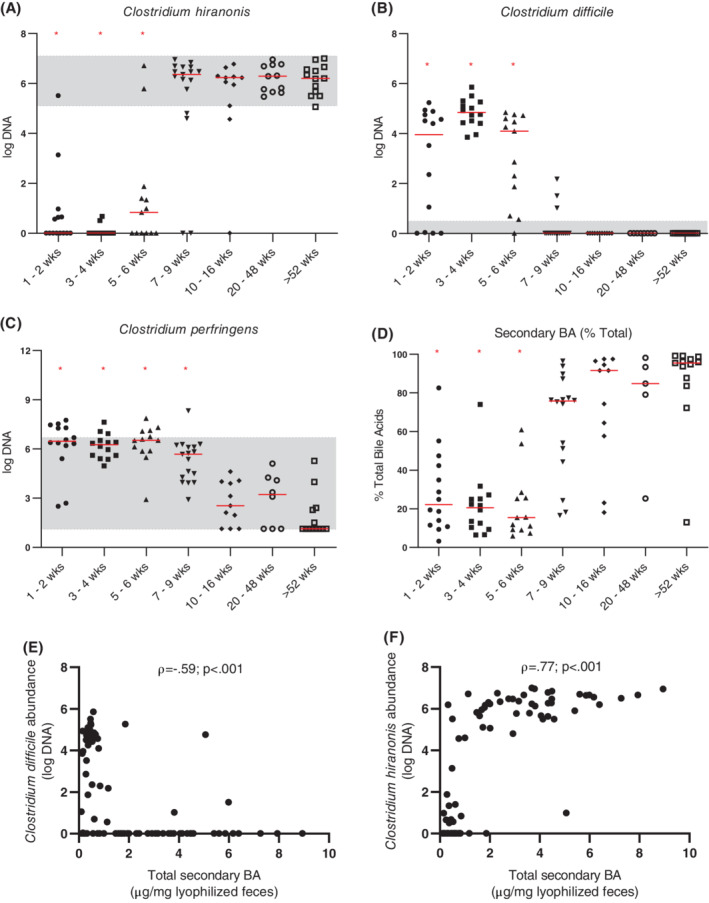
Abundances (log DNA) of Clostridial spp. (A, B, C), percentage of secondary bile acids as a percent of total measured bile acids (D), and Spearman's correlations of total secondary bile acids, Clostridium difficile (E), and Clostridium hiranonis (F). Graphs (A‐D) depict dogs age 1 to 2 weeks (n = 14 for all), 3 to 4 weeks (n = 14 for all), 5 to 6 weeks (n = 13 for all), 7 to 9 weeks (n = 17 for A‐C, n = 16 for D), 10 to 16 weeks (n = 11 for all), 20 to 48 weeks (n = 11 for A, n = 8 for B and C, n = 5 for D), and adult dogs (ie, dogs >52 weeks of age; n = 13 for all). Abundance of Clostridium hiranonis (A) was significantly decreased in dogs age 1 to 2 weeks (P < .001), 3 to 4 weeks (P < .001), and 5 to 6 weeks (P = .005) when compared to adult dogs. Abundance of Clostridium difficile (B) was significantly increased in dogs age 1 to 2 weeks, 3 to 4 weeks, and 5 to 6 weeks (P < .001 for all) compared to adult dogs. Abundance of Clostridium perfringens (C) was significantly increased in dogs age 1 to 2 weeks (P < .001), 3 to 4 weeks (P < .001), 5 to 6 weeks (P < .001), and 7 to 9 weeks (P = .002) compared to adult dogs. Percentage of secondary bile acids was significantly decreased in dogs age 1 to 2 weeks, 3 to 4 weeks, and 5 to 6 weeks (P < .001 for all) compared to adult dogs. Total secondary bile acid concentration was negatively correlated with C. difficile abundance (E; ρ = −0.59) and positively correlated with C. hiranonis abundance (F; ρ = 0.77; P < .001 and n = 86 for both). Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (P < .05). Shaded areas correspond to reference intervals. ρ, Spearman's rho; BA, bile acid; wks, weeks
3.2. Clostridium spp., Salmonella, and Campylobacter jejuni
None of the fecal samples from any of the dogs evaluated contained the NetF toxin gene. The abundance of C. perfringens was significantly increased in puppies that were 1 to 2, 3 to 4, 5 to 6, and 7 to 9 weeks old (P < .001, P < .001, P < .001, and P = .002, respectively) when compared to the healthy adult dogs (Figure 3). The abundance of C. difficile was significantly increased in puppies that were 1 to 2, 3 to 4, and 5 to 6 weeks of age (P < .001) when compared to adult dogs (Figure 3). Out of 87 samples total, 40 tested positive for C. difficile by qPCR. Of those 40 samples, the C. Diff Quik Chek Complete test was performed on 30 samples for which leftover sample material was available. All 30 samples were toxin negative, and 27 out of 30 samples were antigen positive. Additionally, 8 out of 87 samples were positive for CPE by qPCR. Of those 8 samples, 6 samples were tested for C. perfringens enterotoxin by ELISA, and none of the 6 samples tested positive. The abundance of CPE was decreased in puppies that were 1 to 2 or 5 to 6 weeks of age (P = .01 and P = .02, respectively) when compared to the adult dogs (Figure S2). Clostridium difficile abundance was negatively correlated with the abundance of C. hiranonis (ρ = −0.7; P < .001; Figure 3).
Salmonella and C. jejuni assays were only able to be performed on adult dog samples and samples from dogs 9 weeks of age and younger due to limited amount of feces (n = 70 total). Two samples from puppies that were 5 to 6 weeks of age and 2 samples from puppies that were 7 to 9 weeks of age were positive for Salmonella, but all samples were negative for C. jejuni.
3.3. Fecal unconjugated bile acids
Due to limited amount of feces, fecal bile acid concentrations were not able to be obtained for 1 dog in the 7 to 9 week age group and 6 dogs in the 20 to 48 week age group. Graphs of concentrations of individual bile acids are provided in Figure S3. Fecal concentrations of CA were significantly increased in puppies that were 3 to 4 or 5 to 6 weeks of age (P = .003 and P < .001, respectively) when compared to the adult dogs. Fecal concentrations of CDCA were significantly increased in puppies that were 3 to 4, 5 to 6, and 7 to 9 weeks of age (P = .02, P < .001, and P < .001, respectively) when compared to the adult dogs. Fecal concentrations of LCA and DCA were significantly decreased in puppies that were 1 to 2 weeks (P < .001), 3 to 4 weeks (P < .001), or 5 to 6 weeks old (P = .05 and P = .001, respectively) when compared to the adult dogs. Fecal concentrations of UDCA were significantly increased in puppies that were 3 to 4, 5 to 6, or 7 to 9 weeks of age (P = .003, P < .001, and P < .001, respectively) when compared to the adult dogs. The total concentration of fecal primary bile acids (sum of CA and CDCA) was significantly increased in puppies that were 3 to 4, 5 to 6, or 7 to 9 weeks of age (P = .003, P < .001, and P = .02, respectively) when compared to adult dogs (Figure 4). Total concentration of fecal secondary bile acids (sum of DCA, LCA, and UDCA) was significantly decreased in puppies that were 1 to 2, 3 to 4, or 5 to 6 weeks of age (P < .001, P < .001, and P = .006, respectively) age groups when compared to adult dogs (Figure 4). The total concentration of fecal bile acids was decreased in puppies that were 1 to 2 weeks of age (P < .001) when compared to adult dogs (Figure 4). Total secondary bile acid concentration was negatively correlated with C. difficile abundance (ρ = −0.59; P < .001) and positively correlated with C. hiranonis abundance (ρ = 0.77; P < .001; Figure 3) across all tested samples.
FIGURE 4.
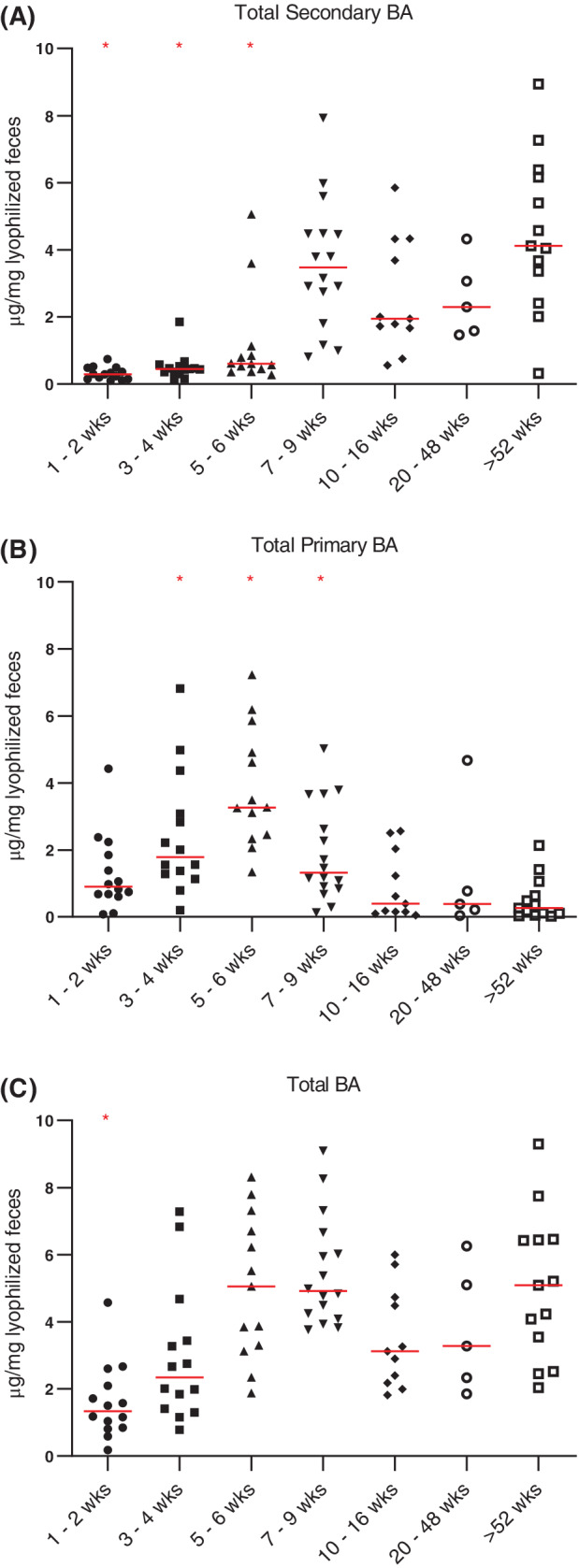
Concentration of fecal bile acids (μg/mg lyophilized feces) for dogs age 1 to 2 weeks (n = 14), 3 to 4 weeks (n = 14), 5 to 6 weeks (n = 13), 7 to 9 weeks (n = 16), 10 to 16 weeks (n = 11), 20 to 48 weeks (n = 5), and adult dogs (ie, dogs >52 weeks of age; n = 13). Total secondary BA represents the sum of concentrations of deoxycholic acid, lithocholic acid, and ursodeoxycholic acid. Total primary BA represents the sum of cholic acid and chenodeoxycholic acid concentrations. Total BA represents the sum of total primary and total secondary bile acid concentrations. Concentration of secondary bile acids (A) was significantly decreased in dogs age 1 to 2 weeks (P < .001), 3 to 4 weeks (P < .001), and 5 to 6 weeks (P = .006) when compared to adult dogs. Concentration of primary bile acids (B) was significantly increased in dogs age 3 to 4 weeks (P = .003), 5 to 6 weeks (P < .001), and 7 to 9 weeks (P = .02) compared to adult dogs. Concentration of total bile acids (C) was significantly decreased in dogs age 1 to 2 weeks compared to adult dogs (P < .001). Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (P < .05). BA, bile acid; wks, weeks
4. DISCUSSION
Results of our study suggest that the fecal microbiome changes significantly with age in young dogs. The DI was significantly higher in puppies that were 1 to 2, 3 to 4, or 5 to 6 weeks of age (P < .001) when compared to the adult dogs. Several individual bacterial groups were also altered in puppies less than 7 to 9 weeks of age (Figures 1, 2, 3). These results are consistent with previous studies that show puppies under 6 weeks of age to have different fecal and GI microbial communities from adult dogs. 3 , 4 , 5 The increased DI in young dogs appeared to be driven by an increased abundance of E. coli and a decreased abundance of anaerobic bacterial groups (ie, Faecalibacterium, Turicibacter, and C. hiranonis). As the abundance of E. coli decreased and that of C. hiranonis, Faecalibacterium, Blautia, and Turicibacter increased, the microbiome of puppies started to become similar to that of adult dogs and the DI was negative in older puppies. These results suggest that the DI might need a different cutoff value for differentiation of normobiosis and dysbiosis in puppies that are 6 weeks of age or younger.
In our study, we evaluated the fecal abundance of 4 bacterial species that are listed on commercially available fecal enteropathogen panels (ie, C. difficile, C. perfringens, Salmonella, and Campylobacter jejuni) in addition to evaluating fecal samples for C. difficile and C. perfringens toxins and toxin‐encoding genes.
Clostridium perfringens is an anaerobic, spore‐forming bacterium that is found in fecal samples of both healthy dogs and dogs with diarrhea. 13 , 14 , 36 There have been no studies in puppies that investigate whether C. perfringens colonizes at an early age. Our results suggest that C. perfringens does colonize at an early age and that its abundance decreased over time. This could be because it is an opportunistic bacterium and its decrease could be associated with an increased competition for space. It is also possible that the increase in secondary bile acids over time is associated with a decrease in C. perfringens (see discussion below). 22
Clostridium perfringens enterotoxin (CPE) is a virulence factor that is found in both healthy dogs and dogs with diarrhea. 13 , 14 , 36 One study suggested that CPE plays no role in acute hemorrhagic diarrhea syndrome. 14 While its presence in adult dogs has been established, it was unknown whether this toxin is expressed in puppies. Our results suggest that CPE is present with a 5% prevalence in dogs under 1 year of age. Further studies should be performed looking at this enterotoxin gene presence in young dogs with GI symptoms to determine if it may be a contributing factor in the pathogenesis of diarrhea.
NetF toxin is a novel toxin associated with C. perfringens. As it is commonly associated with acute hemorrhagic diarrhea syndrome in dogs 16 , we did not expect to find it in samples of healthy dogs. This expectation was confirmed by our data.
Salmonella and Campylobacter jejuni are well‐known zoonoses that have been isolated in both healthy dogs and dogs with diarrhea. The prevalence of Salmonella in our healthy cohort (0.6%) fell within the range previously reported for healthy dogs (0%‐3.6%) 37 , and there were no significant differences between puppies of any age and adult dogs. Campylobacter jejuni was not detected in any of the 70 fecal samples evaluated, regardless of age. Previous studies show a prevalence of C. jejuni of 0.8% to 14.7% in dogs, and suggest that it is more frequently isolated from dogs under 1 year of age. 35 , 36 , 38 , 39 , 40 , 41 However, this is the first study, to our knowledge, that has looked at dogs under 10 weeks of age for C. jejuni. Further studies with a larger number of dogs in this age group could give a more accurate estimation of population prevalence and association with disease.
Clostridium difficile is a human pathogen that causes GI disease. While dogs are carriers for this bacterium, their carriage is not clearly associated with GI disease. 15 Additionally, while there is an association with increasing age and an increase in risk for fecal shedding 18 , puppies have yet to be included in such studies. Our results suggest that C. difficile is initially abundant and decreases after 5 to 6 weeks of age. Similarly, in human infants, colonization by C. difficile is more common than in adults, but rarely causes disease. 42 In our cohort of dogs, 40 out of 87 fecal samples tested positive for C. difficile by qPCR. Of those 40 samples, we were able to measure antigen and toxin A/B in 30 samples. Three of the 30 samples were antigen negative. This discordance is likely because of the relatively low quantity of C. difficile 16S rRNA detected in those 3 samples. None of the 30 samples were positive for C. difficile toxin A or B, similar to what has been observed in adult healthy dogs. 14 Toxigenic strains of C. difficile have been identified in adult dogs with GI diseases 17 ; however, the prevalence of toxigenic strains in dogs under 6 months of age was unknown. Our study suggests that nontoxigenic C. difficile is common in healthy puppies under 6 weeks of age.
A decreased abundance of C. difficile in puppies 7 to 9 weeks of age and older closely mirrored an increased abundance of C. hiranonis (Figure 3). One potential explanation of this observation could be the relationship that both of these Clostridial species have with bile acids. Clostridium hiranonis plays a role in the conversion of primary to secondary bile acids 23 , and its presence was strongly correlated with concentration of secondary bile acids (Figure 3). Additionally, the growth and spore germination of C. difficile is inhibited by secondary bile acids, 20 , 21 and 1 recent study shows that this inhibition is dose‐dependent. 43 Fecal concentrations of secondary bile acids in puppies up to 5 to 6 weeks of age in our study were significantly lower than those in adult dogs (Figure 4). The concurrent increase in the concentration of secondary bile acids in the feces and decrease of C. difficile abundance supports the hypothesis that such a relationship exists in dogs.
Furthermore, bile acids influence the growth of other bacterial groups as well. Secondary bile acids inhibit the growth of isolates from dogs of E. coli and C. perfringens, in vitro. 22 Additionally, a deoxycholic acid supplemented diet reduces invasion of C. perfringens into the ileum in chickens with C. perfringens‐induced necrotic enteritis. 44 The results of our study suggest that such an inhibitory relationship may exist in dogs, albeit with a slight delay, as E. coli and C. perfringens abundances remained significantly increased in puppies that were 7 to 9 weeks of age.
In addition to the relationship bile acids have with the microbiota, they can also have independent effects on the host. A disruption in the bile acid pool that leads to secretory diarrhea, also known as bile acid diarrhea, is implicated in 25% to 33% of human patients with chronic functional diarrhea. 45 This could be a contributing factor to diarrhea in some dogs with GI disease, as previous studies show an increase in fecal primary bile acids. 6 , 10 Additionally, 18% of dogs with chronic diarrhea have higher than normal values of serum 7α‐hydroxy‐4‐cholesten‐3‐1, a marker of bile acid malabsorption. 46 Dogs with chronic enteropathy also have decreased ileal expression of the apical sodium‐dependent bile acid transporter. 10 Interestingly, we found that the fecal bile acid profiles of healthy puppies 5 to 6 weeks of age and younger resembled that of adult dogs with GI disease, with increased primary bile acid concentrations and decreased secondary bile acid concentrations. It remains unclear if this observation is due to the maturing microbial community, physical changes in puppies, or a change in the diet with weaning. Further studies should examine Clostridial species and fecal bile acids in puppies with diarrhea to see if there is an altered pattern of maturation.
Limitations of our study include a small number of samples from puppies that were 10 weeks to 1 year of age and a lack of follow‐up for the vast majority of samples. The investigators were not able to follow the dogs over time, but instead sampled dogs from different age groups. Seven dogs were sampled twice and represented in 2 age groups in the study. Dogs were enrolled at 2 different locations because the first location did not have dogs that were 10 weeks to 1 year of age. The variability in environments could also be considered a limitation, as dogs cohoused in kennels have a different environment from those in a single pet household. However, most observed changes occurred already within the first 5 to 6 weeks of life, when all dogs were still living in the same environment. Fecal samples were not tested for Giardia or Cryptosporidium beforehand, as these dogs did not display clinical symptoms of GI disease. Future studies should consider following a greater number of puppies, with and without diarrhea, throughout their development in a standardized setting.
5. CONCLUSION
Similar to the relationship seen in humans, it is likely that secondary bile acids play a role in regulating growth of C. difficile in dogs. Our study suggests a relationship between C. difficile, C. hiranonis, and fecal bile acid concentrations in dogs, and highlights the use of metabolites in conjunction with microbial communities to elucidate function in the GI tract.
CONFLICT OF INTEREST DECLARATION
The following authors are either currently or within the last 3 years employed by the Gastrointestinal Laboratory at Texas A&M University that performs diagnostic testing on a fee‐for‐service basis: Amanda B. Blake, Annalis Cigarroa, Hannah L. Klein, Mohammad R. Khattab, Jonathan A. Lidbury, Joerg M. Steiner, and Jan S. Suchodolski.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Questionnaire 1 A questionnaire collected at the time of sampling to gain health history, dietary information, and signalment of the patient. PDF format.
Supplemental Table 1 A table listing statistical test results for all variables. Xlsx format.
Supplemental Figure 1 Abundance (log DNA) of total bacteria. Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).
Supplemental Figure 2 Abundance (log DNA) of Clostridium perfringens enterotoxin gene. Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).
Supplemental Figure 3 Concentration of fecal bile acids (μg/mg lyophilized feces). Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).
ACKNOWLEDGMENTS
Funding provided by U.S. Department of Health and Human Services, National Institutes of Health (NIH), NIH Office of the Director, award number T35OD010991. A. Cigarroa was supported by the Veterinary Student Research Training Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Some authors are employed by the Gastrointestinal Laboratory ‐ Texas A&M University that offers the Dysbiosis index and tests for Clostridial species on a fee for service basis.
Blake AB, Cigarroa A, Klein HL, et al. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J Vet Intern Med. 2020;34:2345–2356. 10.1111/jvim.15928
Funding information NIH Office of the Director, Grant/Award Number: T35OD010991
REFERENCES
- 1. Suchodolski JS. Companion animals symposium: microbes and gastrointestinal health of dogs and cats. J Anim Sci. 2011;89:1520‐1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blake AB, Suchodolski JS. Importance of gut microbiota in the health and disease of dogs and cats. Anim Front. 2016;6:37‐42. [Google Scholar]
- 3. Guard BC, Mila H, Steiner JM, Mariani C, Suchodolski JS, Chastant‐Maillard S. Characterization of the fecal microbiome during neonatal and early pediatric development in puppies. PLoS One. 2017;12:e0175718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burton EN, O'Connor E, Ericsson AC, et al. Evaluation of fecal microbiota transfer as treatment for postweaning diarrhea in research‐colony puppies. J Am Assoc Lab Anim Sci. 2016;55:582‐587. [PMC free article] [PubMed] [Google Scholar]
- 5. Buddington RK. Postnatal changes in bacterial populations in the gastrointestinal tract of dogs. Am J Vet Res. 2003;64:646‐651. [DOI] [PubMed] [Google Scholar]
- 6. Guard BC, Honneffer JB, Jergens AE, et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid‐responsive chronic inflammatory enteropathy. J Vet Intern Med. 2019;33:1295‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guard BC, Barr JW, Reddivari L, et al. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS One. 2015;10:e0127259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Isaiah A, Parambeth JC, Steiner JM, et al. The fecal microbiome of dogs with exocrine pancreatic insufficiency. Anaerobe. 2017;45:50–58. [DOI] [PubMed] [Google Scholar]
- 9. Minamoto Y, Minamoto T, Isaiah A, et al. Fecal short‐chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J Vet Intern Med. 2019;33:1608‐1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giaretta PR, Rech RR, Guard BC, et al. Comparison of intestinal expression of the apical sodium‐dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J Vet Intern Med. 2018;32:1918‐1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Markel M, Berghoff N, Unterer S, et al. Characterization of fecal dysbiosis in dogs with chronic enteropathies and acute hemorrhagic diarrhea. J Vet Intern Med. 2012;26:765‐766. [Google Scholar]
- 12. AlShawaqfeh MK, Wajid B, Minamoto Y, et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol Ecol. 2017;93:fix136. [DOI] [PubMed] [Google Scholar]
- 13. Minamoto Y, Dhanani N, Markel ME, Steiner JM, Suchodolski JS. Prevalence of Clostridium perfringens, Clostridium perfringens enterotoxin and dysbiosis in fecal samples of dogs with diarrhea. Vet Microbiol. 2014;174:463‐473. [DOI] [PubMed] [Google Scholar]
- 14. Busch K, Suchodolski JS, Kuhner KA, et al. Clostridium perfringens enterotoxin and Clostridium difficile toxin A/B do not play a role in acute haemorrhagic diarrhoea syndrome in dogs. Vet Rec. 2015;176:253. [DOI] [PubMed] [Google Scholar]
- 15. Borriello SP, Honour P, Turner T, Barclay F. Household pets as a potential reservoir for Clostridium difficile infection. J Clin Pathol. 1983;36:84‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mehdizadeh Gohari I, Parreira VR, Nowell VJ, et al. A novel pore‐forming toxin in type A Clostridium perfringens is associated with both fatal canine hemorrhagic gastroenteritis and fatal foal necrotizing enterocolitis. PLoS One. 2015;10:e0122684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Orden C, Blanco JL, Álvarez‐Pérez S, et al. Isolation of Clostridium difficile from dogs with digestive disorders, including stable metronidazole‐resistant strains. Anaerobe. 2017;43:78‐81. [DOI] [PubMed] [Google Scholar]
- 18. Struble AL, Tang YJ, Kass PH, Gumerlock PH, Madewell BR, Silva J Jr. Fecal shedding of Clostridium difficile in dogs: a period prevalence survey in a veterinary medical teaching hospital. J Vet Diagn Invest. 1994;6:342‐347. [DOI] [PubMed] [Google Scholar]
- 19. Botha WJ, Schoeman JP, Marks SL, et al. Prevalence of Salmonella in juvenile dogs affected with parvoviral enteritis. J S Afr Vet Assoc. 2018;89:e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sorg JA, Sonenshein AL. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol. 2010;192:4983‐4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winston JA, Theriot CM. Impact of microbial derived secondary bile acids on colonization resistance against Clostridium difficile in the gastrointestinal tract. Anaerobe. 2016;41:44‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Martins R, Sullivan MC, et al. Diet‐induced remission in chronic enteropathy is associated with altered microbial community structure and synthesis of secondary bile acids. Microbiome. 2019;7:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitahara M, Takamine F, Imamura T, Benno Y. Clostridium hiranonis sp. nov., a human intestinal bacterium with bile acid 7alpha‐dehydroxylating activity. Int J Syst Evol Microbiol. 2001;51:39‐44. [DOI] [PubMed] [Google Scholar]
- 24. Chaitman J, Ziese A‐L, Pilla R, et al. Fecal microbial and metabolic profiles in dogs with acute diarrhea receiving either fecal microbiota transplantation or oral metronidazole. Front Vet Sci. 2020;7:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lubbs DC, Vester BM, Fastinger ND, Swanson KS. Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract. J Anim Physiol Anim Nutr. 2009;93:113‐121. [DOI] [PubMed] [Google Scholar]
- 26. Garcia‐Mazcorro JF, Suchodolski JS, Jones KR, et al. Effect of the proton pump inhibitor omeprazole on the gastrointestinal bacterial microbiota of healthy dogs. FEMS Microbiol Ecol. 2012;80:624‐636. [DOI] [PubMed] [Google Scholar]
- 27. Suchodolski JS, Markel ME, Garcia‐Mazcorro JF, et al. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One. 2012;7:e51907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Furet JP, Quenee P, Tailliez P. Molecular quantification of lactic acid bacteria in fermented milk products using real‐time quantitative PCR. Int J Food Microbiol. 2004;97:197‐207. [DOI] [PubMed] [Google Scholar]
- 29. Malinen E, Kassinen A, Rinttila T, et al. Comparison of real‐time PCR with SYBR Green I or 5′‐nuclease assays and dot‐blot hybridization with rDNA‐targeted oligonucleotide probes in quantification of selected faecal bacteria. Microbiology. 2003;149:269‐277. [DOI] [PubMed] [Google Scholar]
- 30. Kitahara M, Sakamoto M, Benno Y. PCR detection method of Clostridium scindens and C. hiranonis in human fecal samples. Microbiol Immunol. 2001;45:263‐266. [DOI] [PubMed] [Google Scholar]
- 31. Wise MG, Siragusa GR. Quantitative detection of Clostridium perfringens in the broiler fowl gastrointestinal tract by real‐time PCR. Appl Environ Microbiol. 2005;71:3911‐3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast‐fed and formula‐fed infants by real‐time PCR. FEMS Microbiol Lett. 2005;243:141‐147. [DOI] [PubMed] [Google Scholar]
- 33. Gurjar AA, Hegde NV, Love BC, Jayarao BM. Real‐time multiplex PCR assay for rapid detection and toxintyping of Clostridium perfringens toxin producing strains in feces of dairy cattle. Mol Cell Probes. 2008;22:90‐95. [DOI] [PubMed] [Google Scholar]
- 34. Kurowski PB, Traub‐Dargatz JL, Morley PS, Gentry‐Weeks CR. Detection of Salmonella spp. in fecal specimens by use of real‐time polymerase chain reaction assay. Am J Vet Res. 2002;63:1265‐1268. [DOI] [PubMed] [Google Scholar]
- 35. Leahy AM, Cummings KJ, Rodriguez‐Rivera LD, Hamer SA, Lawhon SD. Faecal Campylobacter shedding among dogs in animal shelters across Texas. Zoonoses Public Health. 2017;64:623‐627. [DOI] [PubMed] [Google Scholar]
- 36. Hackett T, Lappin MR. Prevalence of enteric pathogens in dogs of north‐central Colorado. J Am Anim Hosp Assoc. 2003;39:52‐56. [DOI] [PubMed] [Google Scholar]
- 37. Marks SL, Rankin SC, Byrne BA, Weese JS. Enteropathogenic bacteria in dogs and cats: diagnosis, epidemiology, treatment, and control. J Vet Intern Med. 2011;25:1195‐1208. [DOI] [PubMed] [Google Scholar]
- 38. Parsons BN, Porter CJ, Ryvar R, et al. Prevalence of Campylobacter spp. in a cross‐sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Vet J. 2010;184:66‐70. [DOI] [PubMed] [Google Scholar]
- 39. Hald B, Pedersen K, Waino M, Jorgensen JC, Madsen M. Longitudinal study of the excretion patterns of thermophilic Campylobacter spp. in young pet dogs in Denmark. J Clin Microbiol. 2004;42:2003‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Procter TD, Pearl DL, Finley RL, et al. A cross‐sectional study examining Campylobacter and other zoonotic enteric pathogens in dogs that frequent dog parks in three cities in south‐western Ontario and risk factors for shedding of Campylobacter spp. Zoonoses Public Health. 2014;61:208‐218. [DOI] [PubMed] [Google Scholar]
- 41. Holmberg M, Rosendal T, Engvall EO, et al. Prevalence of thermophilic Campylobacter species in Swedish dogs and characterization of C. jejuni isolates. Acta Vet Scand. 2015;57:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Adlerberth I, Huang H, Lindberg E, et al. Toxin‐producing Clostridium difficile strains as long‐term gut colonizers in healthy infants. J Clin Microbiol. 2014;52:173‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thanissery R, Winston JA, Theriot CM. Inhibition of spore germination, growth, and toxin activity of clinically relevant C. difficile strains by gut microbiota derived secondary bile acids. Anaerobe. 2017;45:86‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang H, Latorre JD, Bansal M, et al. Microbial metabolite deoxycholic acid controls Clostridium perfringens‐induced chicken necrotic enteritis through attenuating inflammatory cyclooxygenase signaling. Sci Rep. 2019;9:14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Camilleri M. Advances in understanding of bile acid diarrhea. Expert Rev Gastroenterol Hepatol. 2014;8:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kent AC, Cross G, Taylor DR, et al. Measurement of serum 7alpha‐hydroxy‐4‐cholesten‐3‐one as a marker of bile acid malabsorption in dogs with chronic diarrhoea: a pilot study. Vet Rec Open. 2016;3:e000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Questionnaire 1 A questionnaire collected at the time of sampling to gain health history, dietary information, and signalment of the patient. PDF format.
Supplemental Table 1 A table listing statistical test results for all variables. Xlsx format.
Supplemental Figure 1 Abundance (log DNA) of total bacteria. Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).
Supplemental Figure 2 Abundance (log DNA) of Clostridium perfringens enterotoxin gene. Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).
Supplemental Figure 3 Concentration of fecal bile acids (μg/mg lyophilized feces). Red lines indicate medians and red asterisks indicate significance in comparison to adult dogs (ie, dogs >52 weeks of age; P < .05).


