Highlights
-
•
Schizophrenia patients have intact reward anticipation and early stage reward outcome processing.
-
•
However, for patients early in their illness course, deficits were evident during late stage reward outcome processing.
-
•
Patients with schizophrenia who have an older neural brain age have worse depressive symptoms.
Keywords: First-episode schizophrenia, Chronic schizophrenia, Event-related potentials, Reward processing, Age, Depression
Abstract
Background
Reward processing abnormalities may underlie characteristic pleasure and motivational impairments in schizophrenia. Some neural measures of reward processing show age-related modulation, highlighting the importance of considering age effects on reward sensitivity. We compared event-related potentials (ERPs) reflecting reward anticipation (stimulus-preceding negativity, SPN) and evaluation (reward positivity, RewP; late positive potential, LPP) across individuals with schizophrenia (SZ) and healthy controls (HC), with an emphasis on examining the effects of chronological age, brain age (i.e., predicted age based on neurobiological measures), and illness phase.
Methods
Subjects underwent EEG while completing a slot-machine task for which rewards were not dependent on performance accuracy, speed, or response preparation. Slot-machine task EEG responses were compared between 54 SZ and 54 HC individuals, ages 19 to 65. Reward-related ERPs were analyzed with respect to chronological age, categorically-defined illness phase (early; ESZ versus chronic schizophrenia; CSZ), and were used to model brain age relative to chronological age.
Results
Illness phase-focused analyses indicated there were no group differences in average SPN or RewP amplitudes. However, a group × reward outcome interaction revealed that ESZ differed from HC in later outcome processing, reflected by greater LPP responses following loss versus reward (a reversal of the HC pattern). While brain age estimates did not differ among groups, depressive symptoms in SZ were associated with older brain age estimates while controlling for negative symptoms.
Conclusions
ESZ and CSZ did not differ from HC in reward anticipation or early outcome processing during a cognitively undemanding reward task, highlighting areas of preserved functioning. However, ESZ showed altered later reward outcome evaluation, pointing to selective reward deficits during the early illness phase of schizophrenia. Further, an association between ERP-derived brain age and depressive symptoms in SZ extends prior findings linking depression with reward-related ERP blunting. Taken together, both illness phase and age may impact reward processing among SZ, and brain aging may offer a promising, novel marker of reward dysfunction that warrants further study.
1. Introduction
Reward processing deficits are a core feature of schizophrenia (SZ) (Barch and Dowd, 2010, Morris, 2018, Strauss et al., 2014, Whitton et al., 2015) that may underlie characteristic motivational impairments (Barch and Dowd, 2010) and contribute to poor functional outcomes (Fervaha et al., 2014, Fervaha et al., 2015, Foussias et al., 2011). Many reward-focused studies i) implement paradigms that confound reward processing with other cognitive or behavioral demands known to be impaired in SZ (Strauss et al., 2014, Knutson et al., 2001), ii) do not focus on key demographic variables (like age) (Hill et al., 2018), or iii) do not consider clinical variables that may be associated with accelerated brain changes (like illness phase) (Palaniyappan, 2017).
Prior research demonstrates impairments in SZ when anticipating future reward enjoyment, but suggests that “in-the-moment” consummatory, pleasure may be relatively intact (based on experience sampling, behavioral performance, and functional MRI) (Kring and Barch, 2014, Cohen and Minor, 2010). With millisecond resolution, event-related potentials (ERPs) can capture temporally distinct reward processing phases. We examined three sequential reward-related ERP components that span the anticipatory and consummatory reward phases: the stimulus preceding negativity (SPN, reflecting reward anticipation), the reward positivity (RewP, reflecting early reward evaluation), and the late positive potential (LPP, reflecting later, and therefore higher-order, aspects of reward evaluation).
The SPN builds several hundred milliseconds prior to an anticipated stimulus (van Boxtel and Böcker, 2004, Brunia et al., 2011). In the context of a reward task, the SPN is sensitive to reward anticipation, i.e., larger (more negative) when expecting reward versus non-reward (Kotani et al., 2003, Ohgami et al., 2004). Although the SPN has not been a focus of many reward studies in SZ, aberrant SPN responses to certain versus uncertain reward-signaling cues have been demonstrated in SZ (Clayson et al., 2019).
The RewP is a positive medial frontal component occurring ~250–300 ms after reward feedback relative to non-reward or loss (Proudfit, 2015). Historically, research focused on the negativity following loss, typically referred to as the feedback-related negativity (FRN) (Hajcak et al., 2006, Yeung et al., 2005, Gehring and Willoughby, 2002), and calculated as a loss – win difference; although more recent work emphasizes win-related contributions to RewP variance (Holroyd et al., 2008, Holroyd et al., 2011, Baker et al., 2017) and promotes calculation of the difference score to reflect a positivity from rewards (i.e., by measuring a win – loss difference wave, rather than the originally measured loss – win) (Proudfit, 2015). Blunted RewP is thought to reflect reduced reward sensitivity and is evident in those with, or at-risk-for, major depressive disorder (MDD) (Proudfit, 2015). Studies using passive reward tasks reported intact RewP in SZ (Horan et al., 2012, Morris et al., 2011), though a study of probabilistic learning found attenuated RewP correlated with more severe negative symptoms (Morris et al., 2008), raising the possibility that early reward feedback processing (i.e., “consummatory”) deficits are most evident among people with SZ who have prominent negative symptoms.
The LPP is a slow, centro-parietal, ERP component typically measured starting ~600 ms following stimulus onset, reflecting sustained engagement with salient content (Hajcak et al., 2010, Cuthbert et al., 2000). The LPP follows the RewP in time, capturing later-stage outcome processing in reward tasks (Glazer et al., 2018), and may reflect more downstream processing of output from the earlier evaluation systems (Cunningham et al., 2005). The LPP has frequently been examined using emotion-picture viewing tasks (Hajcak et al., 2010; Horan et al., 2010, Horan et al., 2012, Horan et al., 2013), though more recent studies demonstrate the LPP’s role in reward processing in healthy controls (HC) (Angus et al., 2017, Donaldson et al., 2016, Glazer et al., 2019, Meadows et al., 2016, Pornpattananangkul and Nusslock, 2015), and motivated attention to potential rewards or punishments in people with SZ (Horan et al., 2016). Blunted LPP following rewards has also been associated with higher negative symptoms in a transdiagnostic sample that included schizophrenia-spectrum disorders (Bedwell et al., 2016).
Normal and pathological aging effects may impact reward processing neural signals. RewP magnitude shows a negative association with age among HC (Hill et al., 2018), and a depression-focused meta-analysis found that RewP blunting was most pronounced in younger depressed samples (Keren et al., 2018). Beyond reward processing, growing evidence supports hypotheses of accelerated brain aging in SZ (Nenadić et al., 2017, Schnack et al., 2016, Hajek et al., 2019, Shahab et al., 2019, Koutsouleris et al., 2014), particularly early in the illness course (Schnack et al., 2016, Shahab et al., 2019), which may be due to abnormal brain maturation (van Haren et al., 2008). Brain aging has been measured as the difference between chronological and predicted age based on neurobiological measures (referred to as “BrainAGE” gap). Most of the BrainAGE literature to date has used structural MRI data to estimate age, though this framework is expanding to other neurobiological measures, including a recent study using resting EEG (Al Zoubi et al., 2018). Incorporating neural age estimates may enhance sensitivity for detecting age-associated pathophysiological processes in psychiatric disorders (Franke and Gaser, 2019). Given age relationships for some reward ERPs and the importance of motivational and reward processes in normal neurodevelopment (Tau and Peterson, 2010), examining brain aging relationships in SZ with ERP reward-processing metrics is warranted.
SZ and depression are highly comorbid disorders (Siris, 2000, Buckley et al., 2009). The presence of reward processing deficits among individuals with SZ and depressive disorders has led researchers to characterize the neural processes linking common symptoms across these disorders, like anhedonia (Whitton et al., 2015). While there is a sizeable literature on the RewP and depressive symptoms and risk for depression (Proudfit, 2015, Proudfit et al., 2015), no studies have examined RewP in SZ as a function of depressive features (Foti et al., 2018). Accordingly, there is strong empirical impetus to evaluate relationships between RewP and depressive symptoms in SZ.
Here we use a slot-machine task in which rewards were not dependent on decision-making, response speed, or performance accuracy (Morris et al., 2011), to isolate reward processes from other preparatory and/or executive demands (Dowd and Barch, 2012). Each trial depends on the pseudorandom population of three reels with fruit symbols. The reels sequentially populate, left to right, enabling parsing of anticipatory from early and late-consummatory signals. Because neurodevelopmental changes are central to SZ pathogenesis and healthy reward processing (Tau and Peterson, 2010, Rapoport et al., 2005), we compared individuals with SZ and HC across a wide age range, with the goal of examining contributions of i) age through analyses of chronological age and BrainAGE (derived from reward ERPs), and ii) categorically-defined illness phase through recruitment of early illness (ESZ) and chronic (CSZ) patient groups (Hill et al., 2018, Palaniyappan, 2017).
We expected blunting of anticipatory signals (SPN) based on current models of reward deficits in SZ (Kring and Barch, 2014), and more pronounced group differences during later reward evaluation (LPP) given theories that downstream processes following initial feedback may be integral to reward dysfunction in SZ (Strauss et al., 2014). Based on prior electrophysiology studies, we did not expect group differences in early reward evaluation indexed by the RewP (Horan et al., 2012, Morris et al., 2011), but did expect reduced reward-related ERP amplitudes (SPN, RewP, LPP) to correlate with greater negative symptoms (Morris et al., 2008, Bedwell et al., 2016). We also directly compared reward-anticipation with reward-outcome ERPs across the groups, as a prior report found that anticipatory signals (SPN) correlated with later outcome processes (LPP) in HC (Pornpattananangkul and Nusslock, 2015). Finally, we hypothesized depressive features would relate to RewP amplitudes, based on a substantial literature supporting blunted RewP as a marker of depression vulnerability (Proudfit, 2015, Keren et al., 2018), and because depressive symptoms frequently occur in SZ (Siris, 2000).
2. Methods and materials
2.1. Subjects
Fifty-four individuals with SZ (76% men; age range = 19.07–64.70 years) and 54 HC (78% men; age range = 19.25–64.41 years) were recruited via community advertisements; results from the HC sample are described in a previous study (Fryer et al., 2020). SZ subjects were either early illness (ESZ) within 5 years of onset (Fryer et al., 2016, Fryer et al., 2013, Hay et al., 2015) (n = 26; mean illness duration = 2.90 ± 1.47 years), or chronic (CSZ; n = 28; mean illness duration = 23.55 ± 15.35 years). ESZ and CSZ were comparable in gender representation, handedness, chlorpromazine equivalents (CPZeq) (Woods, 2003), haloperidol equivalents (HPeq) (Andreasen et al., 2010), as well as concomitant anti-depressant, mood stabilizer, and benzodiazepine treatments; and, as expected based on subgroup assignment, ESZ and CSZ differed in age and illness duration (Table 1).
Table 1.
Sample Demographics.
| HC (n = 54) | ESZ (n = 26) | CSZ (n = 28) | F/t | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 33.72 (14.42) | 24.47 (4.01) | 44.05 (14.73) | 15.77*** |
| Age range, years | 19.25 – 64.41 | 19.07 – 37.59 | 22.60 – 64.70 | – |
| Gender (% male) | 77.78 | 73.08 | 78.57 | 0.14 |
| Handedness (% right) | 87.04 | 92.31 | 82.14 | 0.43 |
| Illness Duration, years | – | 2.90 (1.47) | 23.55 (15.35) | −6.71*** |
| aCPZeq, mg | – | 301.65 (257.85) | 461.58 (366.41) | −1.53 |
| bHPeq, mg | – | 7.06 (6.77) | 6.21 (3.45) | 0.44 |
| Antidepressants (% group) | – | 38.46 | 28.57 | 0.76 |
| Mood stabilizers (% group) | – | 26.92 | 14.29 | 1.15 |
| Benzodiazepines (% group) | – | 0.00 | 7.14 | −1.39 |
| Clinical Symptoms | ||||
| CAINS Motivation & Pleasure | – | 16.64 (7.44) | 14.48 (8.00) | 1.02 |
| CAINS Expression | – | 4.35 (3.63) | 3.63 (4.43) | 0.64 |
| PANSS Total Negative | – | 15.35 (4.45) | 15.73 (6.75) | −0.24 |
| PANSS Total Positive | – | 16.27 (6.39) | 16.67 (4.97) | −0.25 |
| PANSS Depression | – | 8.77 (3.67) | 7.79 (3.60) | 0.33 |
*p < .05.
***p < .001.
Abbreviations: HC, healthy controls; ESZ, early illness schizophrenia subjects; CSZ, chronic illness schizophrenia subjects; CPZeq, chlorpromazine equivalents; HPeq, haloperidol equivalents; CAINS, Clinical Assessment Interview for Negative Symptoms; PANSS, Positive and Negative Syndrome Scale.
aThree subjects were taking first-generation antipsychotics, and 34 subjects were taking second-generation antipsychotics.
bCPZeq and HPeq were correlated at 0.48.
We used the Structured Clinical Interview for DSM-IV (SCID-IV-TR) (First et al., 2002) to confirm a schizophrenia or schizoaffective diagnosis for SZ subjects and to exclude HC if they met criteria for a past or current Axis I disorder. HC were also excluded for having a first-degree relative with a schizophrenia-spectrum disorder. Urine toxicology tested for common drugs of abuse (e.g., opiates, cocaine, amphetamines) and potential subjects with a positive test were excluded. English fluency was required for participation. Additional exclusion criteria for SZ and HC subjects were history of head injury, neurological illness, or other major medical illness that impacts the central nervous system. Study procedures were approved by the Institutional Review Board at the University of California, San Francisco. All subjects provided written informed consent.
3. Task description
We developed a 288-trial slot-machine reward task adapted from prior studies (Habib and Dixon, 2010, Clark et al., 2009, Donkers et al., 2005), and shown to elicit expected SPN, RewP, and LPP condition effects in the same HC sample studied here (Fryer et al., 2020). Subjects initiated each trial via a button press, after which reels 1–3 (R1, R2, R3) populated automatically from left to right with single, sequential fruit symbols. After R3 populated, feedback indicated a win or loss. The reward anticipation phase spanned the population of R1 and R2 (culminating just prior to R3; 0–3666 ms); the reward evaluation phase began with the population of the R3 symbol (3666–6115 ms).
Trial types were wins, near misses, and total misses. Wins occurred when all three reels populated identical fruit symbols (AAA). Near misses occurred when R1 and R2 populated matching symbols but the R3 symbol was incongruent (AAB). Wins and near misses were congruent on R1 and R2, inducing similar reward anticipation just prior to R3. Total misses occurred when R2 did not match R1 (ABC), indicating a loss at R2 and eliminating further reward anticipation prior to R3 (with R3 providing no additional information about the trial’s outcome). To reflect real-world slot-machine outcomes, subjects encountered more frequent total misses (n = 144, P = .50) than wins (n = 72, P = .25) and near misses (n = 72, P = .25).
Wins yielded a $1.25 payout, while near and total misses yielded $0 payouts. Subjects were instructed that they would receive monetary compensation reflective of their slot-machine winnings, in addition to routine compensation for participation. Supplemental Materials contain additional task details.
3.1. Clinical symptom ratings
Negative symptoms were evaluated using the Clinical Interview for Negative Symptoms (CAINS) (Horan et al., 2011) and the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). The CAINS is a newer negative symptom measure, with strong psychometric properties (Kring et al., 2013), that was designed to parse deficits in motivation and pleasure for social, vocational, and recreational activities (MAP; 9 items) from deficits in verbal and non-verbal expression (EXP; 4 items). From the PANSS, we computed a Total Negative symptom score (items 8–14). We also used the PANSS to compute a Depression composite score based on a previously validated five-factor model (Lindenmayer et al., 1995) shown to correlate with depression in SZ (Kim et al., 2006, Kontaxakis et al., 2000); summing the anxiety (G2), guilt (G3), and depression (G6) items yielded the Depression composite score (Wallwork et al., 2012).
3.2. EEG acquisition and preprocessing
EEG data were recorded from a 64-channel electrode cap using the BioSemi ActiveTwo system (www.biosemi.com). Data were digitized at 1024 Hz and a 0.1 Hz high-pass filter was applied using ERPlab (Lopez-Calderon and Luck, 2014). Reference electrodes were placed on the mastoids. Electrodes were placed above and below the right eye, and at the outer canthus of each eye, to record vertical and horizontal electrooculograms (VEOG, HEOG, respectively).
Data were entered into a modified version of the Fully Automated Statistical Threshold for EEG artifact Rejection (FASTER) pre-processing pipeline (Nolan et al., 2010). This entailed: i) identifying outlier channels and interpolating their values in the continuous data, ii) removing outlier epochs from each subject’s trial set, iii) applying spatial independent components analysis (ICA) to the remaining trials, iv) identifying outlier components from spatial ICA using the ADJUST procedure (Mognon et al., 2011), and v) removing outlier channels (see Supplemental Materials).
3.3. ERP measurement
Epochs were time-locked to R3 onset and baseline corrected using the −100 to 0 ms preceding R3 for RewP and LPP, and −100 to 0 ms preceding R2 (−1300 to −1200 ms preceding R3) for SPN. A trimmed means approach excluded the top and bottom 10% at each time point before averaging to obtain a more robust estimate (Leonowicz et al., 2005).
SPN (reward anticipation): measured as the average voltage from −100 to 0 ms prior to the R3 outcome (Brunia et al., 2011) from representative electrode Cz (Donkers et al., 2005, Donkers and van Boxtel, 2005). We computed separate ERP averages for possible win trials (AA; collapsed across trials eventually revealed as wins (AAA) or near misses (AAB), as these are equivalent at R2) and total miss trials (AB; trials revealed as a loss at R2). For the SPN analyses, we removed two SZ subjects with average SPN values on total miss (AB) trials more than 3 SD above the mean.
RewP (early reward evaluation): measured as the average voltage from 228 to 344 ms post R3 onset; this time-window was chosen based on an average of measurements from a meta-analysis of 54 RewP studies (Sambrook and Goslin, 2015). Statistical analyses were conducted based on representative electrode FCz (Holroyd et al., 2008, Donkers et al., 2005, Donkers and van Boxtel, 2005, Cockburn and Holroyd, 2018). The RewP was computed as a difference score of wins minus near misses (AAA – AAB) (Umemoto and Holroyd, 2017); this isolates a pure valence effect as the stimulus probabilities are equated across these two conditions. We note that because the RewP is derived from a difference score, we cannot disentangle the contributions of wins from near misses.
LPP (late reward evaluation): measured as the average voltage from 600 to 800 ms after R3 (Hajcak and Olvet, 2008) from representative electrode Pz (Glazer et al., 2018). We computed separate ERP averages for wins (AAA) and near misses (AAB). For the LPP analyses, we removed four SZ subjects with average LPP values on win (AAA) or near miss (AAB) trials ± 3 SD from the mean.
3.4. Data analysis
Age-adjusted ERP z-scores: We calculated age-adjusted z-scores for each ERP component to account for expected age differences between the clinical groups, ongoing neuromaturation processes expected in younger subjects, and normal aging processes expected in older subjects. This procedure removes normal aging effects while preserving variance associated with pathological age effects in SZ, similar to our prior ERP (Mathalon et al., 2019, Perez et al., 2014, Mathews et al., 2016) and MRI studies (Mathalon et al., 2003, Pfefferbaum et al., 1992).
To calculate age-adjusted z-scores, we first produced a HC age-adjusted regression model that regressed each ERP component onto age. We then extracted the intercept (), slope (), and root mean squared error (RMSE) from each model to compute the age-adjusted z-scores for all subjects, for the respective ERP component. Age-adjusted z-scores were produced using the HC age-regression model:
where the predicted value was calculated for each subject as follows:
Accordingly, a given subject’s score reflects the deviation of their ERP component amplitude, in standard deviations units, from that expected for a HC of the same age. Correlations between original and age-adjusted ERP scores, within the HC and SZ groups, are presented in Table S1.
Between-group ERP effects: We compared age-adjusted ERP measures across the three groups. For SPN, we used a mixed-effects ANOVA that included SPN z-scores as the outcome variable, Condition (AA, AB), Group (HC, ESZ, CSZ), and Group × Condition as fixed effects, and Subject as a random effect. We built an equivalent mixed-effects model for the LPP, but with Condition as AAA – ABC and AAB – ABC difference scores. For the RewP, we used a one-way ANOVA with RewP difference z-scores as the outcome variable and Group as a fixed effect. Follow-up tests were adjusted using Benjamini and Hochberg’s false discovery rate (FDR) algorithm (Benjamini and Hochberg, 1995).
Relationships between anticipatory and consummatory ERP components: Using three regression models, we tested if reward-anticipation was related to reward-outcome responses between the HC and SZ groups. Each model included reward outcome ERPs (RewP, LPP AAA – ABC, LPP AAB – ABC) as the outcome variable, and Reward Anticipation (SPN AA – AB), Group (HC, SZ), and a Group × Reward Anticipation interaction term as the predictor variables.
ERP and negative symptom correlations: We correlated each ERP component of interest (SPN AA – AB, RewP, LPP AAA – ABC, and LPP AAB – ABC) with negative symptoms (CAINS MAP, CAINS EXP, and PANSS Total Negative), yielding 12 correlations total; we adjusted for multiple comparisons using the FDR algorithm. Correlations were computed across all SZ as we were interested in dimensional associations regardless of illness phase.
RewP and depressive symptom correlation: We correlated RewP difference scores with the PANSS Depression composite score.
BrainAGE analyses: To derive a reward-related BrainAGE model, we included the following predictors: i) to isolate reward anticipation, we included the difference between SPN possible wins and total misses (AA – AB); ii) to measure reward outcome processing, we included the RewP difference wave (wins AAA – near misses AAB) and the difference between LPP wins and near misses (AAA – AAB); iii) lastly, to account for general motivational salience following an outcome, we collapsed across wins and near misses by including the average of LPP wins and near misses (AAA + AAB)/2. We constructed the BrainAGE model from unadjusted HC ERP data, including the four conditions described above. Results from this model are described in Supplemental Materials.
We used the HC regression weights from the BrainAGE model to predict BrainAGEs for all subjects, and then calculated the BrainAGE gap as BrainAGE minus chronological age (Schnack et al., 2016). (One subject with a predicted BrainAGE more than 3 SD above the mean was removed from subsequent analyses.)
Lastly, we tested whether negative and depressive symptoms were related to the BrainAGE gap, based on evidence that accelerated aging is related to symptom levels in SZ (Koutsouleris et al., 2014); we regressed BrainAGE gap onto CAINS MAP, CAINS EXP, and PANSS Depression scores for all SZ (we did not include PANSS Total Negative given high collinearity with the CAINS; 59% of PANSS Total Negative variance was explained by the CAINS MAP and EXP scores).
Effects of antipsychotic medication dosage and anti-depressant treatment: We evaluated pairwise correlations between CPZeq and HPeq with all ERP, symptom, and BrainAGE metrics. We also tested whether ERP, symptom, or BrainAGE metrics were related to concomitant anti-depressant treatment via t-tests.
4. Results
4.1. Reward-related ERP effects
Grand average ERP waveforms are presented in Fig. 1 and RewP difference waveforms in Fig. 2 (Supplemental Materials). As reported in (Fryer et al., 2020), HC showed the expected condition effects for SPN, i.e., more negative for anticipated wins than total misses (AA < AB), and the expected condition effects for RewP and LPP, i.e., more positive for wins than near misses (AAA > AAB).
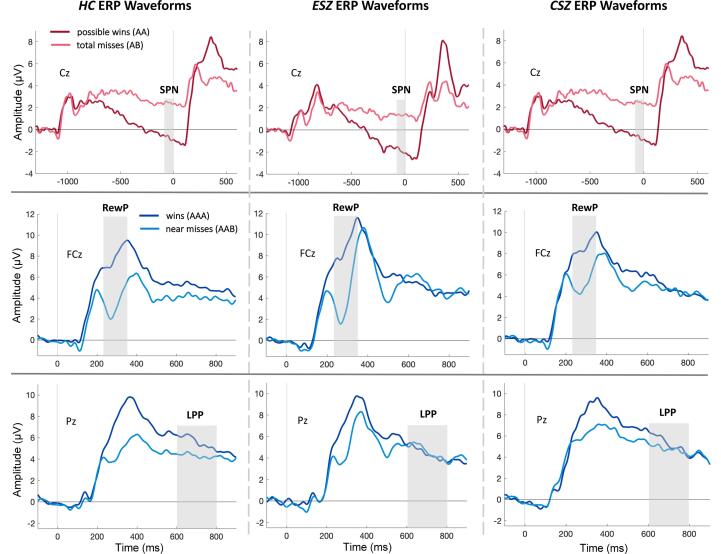
Fig. 1.
Grand average waveforms. Top: Stimulus preceding negativity (SPN) grand average waveforms at electrode Cz; win (AA) trials shown in red and total miss (AB) trials in pink. Middle: Reward positivity (RewP) grand average waveforms at electrode FCz; win (AAA) trials shown in dark blue and near miss (AAB) trials in light blue. Bottom: Late positive potential (LPP) grand average waveforms at electrode Pz; win (AAA) trials shown in dark blue and near miss (AAB) trials in light blue. Time at −1200 ms corresponds to Reel-2 outcome (top row); time at 0 ms corresponds to Reel-3 outcome. Grey bars represent the ERP measurement window. Abbreviations: HC, healthy controls; ESZ, early illness schizophrenia subjects; CSZ, chronic illness schizophrenia subjects. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
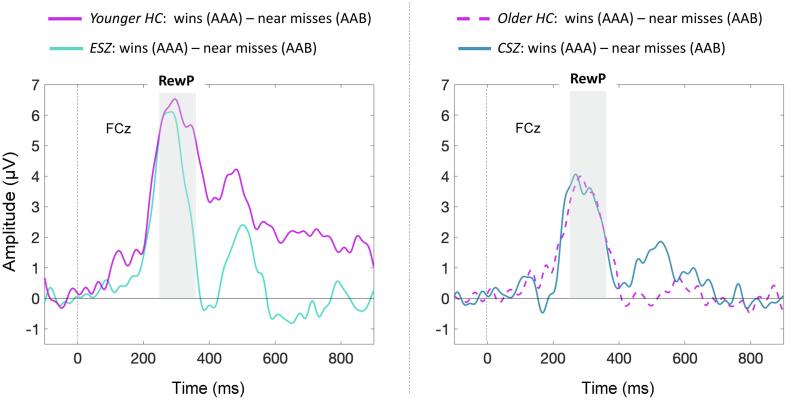
Fig. 2.
RewP difference waveforms. Difference ERP waveforms (wins AAA – near misses AAB) at electrode FCz. Time at 0 ms corresponds to Reel-3 outcome. Grey bar depicts reward positivity (RewP) measurement window. HC were divided using a median split, given a significant decline in RewP amplitude with age (Supplemental Materials). Abbreviations: HC, healthy controls; ESZ, early illness schizophrenia subjects; CSZ, chronic illness schizophrenia subjects.
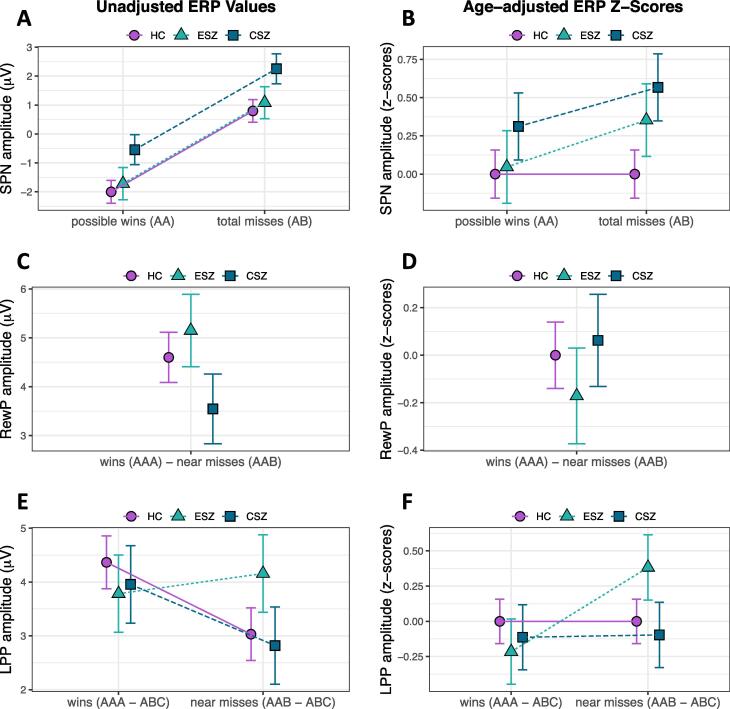
SPN (reward anticipation): There was no Group × Condition interaction for SPN z-scores (F2,103 = 0.55, p = .58), nor a main effect of Group (F2,103 = 2.07, p = .13; Fig. 3B). All groups showed the expected AA < AB condition effect in the unadjusted data (Fig. 3A).
Fig. 3.
Group ERP effects. Results depicting group differences for the three ERPs; group means ± standard error. (A) Stimulus preceding negativity (SPN) Condition effect for possible wins versus total misses regardless of Group (HC, healthy controls; ESZ, early illness schizophrenia subjects; CSZ, chronic illness schizophrenia subjects). (B) No Group × Condition interaction for age-adjusted SPN z-scores. (C, D) No Group differences for Reward Positivity (RewP) unadjusted or age-adjusted z-scores. (E, F) Significant Group × Condition interaction for late positive potential (LPP); whereby the ESZ group showed a heightened LPP response to near misses as compared to wins (F) when adjusting for HC age-related variance. For SPN analyses, we removed two SZ subjects with an average SPN value on AB trials more than 3 SD above the mean; for the LPP analyses, we removed four SZ subjects with average LPP win (AAA – ABC) or near miss (AAB – ABC) values ± 3 SD from the mean. Note for age-adjusted data: data were adjusted to account for normal aging effects using a z-scoring procedure based on a HC age regression model; as a result, HC z-scores have mean = 0 and SD = 1, and the patient groupmeans reflect the degree and direction of abnormality, in standard units, from the HC-derived norms.
RewP (early reward evaluation): There was no main effect of Group for RewP difference z-scores (F2,105 = 0.38, p = .68; Fig. 3D, age-adjusted and Fig. 3C, unadjusted).
LPP (late reward evaluation): We observed a significant Group × Condition interaction for LPP z-scores (F2,101 = 3.76, p = .03): ESZ exhibited abnormally large LPP following near misses and abnormally small LPP following wins (AAA – ABC < AAB – ABC; t101 = −3.18, p = .002, padj = 0.02; Fig. 3F; Table S2), compared to the HC pattern of greater LPP responses to wins versus near misses (Fig. 3E) (Fryer et al., 2020), and the CSZ group, which showed no age-adjusted LPP condition difference.
The pattern of group effects for SPN, RewP, and LPP did not differ when using a HC age-matched grouping strategy in place of age-adjusted z-scores (see Supplemental Materials for full set of analyses).
4.2. Anticipatory and consummatory ERP relationships
There was a significant Group × Reward Anticipation interaction for RewP difference scores (F1,102 = 6.56, p = .01; Fig. S1A), whereby SZ (r50 = −0.53, p < .001) showed a negative relationship between SPN and RewP that was not observed among HC (r52 = −0.01, p = .92). There was also a trend-level Group × Reward Anticipation interaction for LPP wins (F1,99 = 3.69, p = .06; Fig. S1B); here, both HC and SZ had a negative correlation between SPN and LPP wins (HC: r52 = −0.31, p = .02, SZ: r47 = −0.59, p < .001). For LPP near misses and SPN, there was a significant negative common slope across the groups (β = −0.53, p < .001; Fig. S1C), but no interaction (p > .10).
4.3. ERP clinical symptom correlations
Across all SZ, negative symptoms were not correlated with any ERP measures (all p > .10). Depressive symptoms were unrelated to RewP difference scores (r52 = −0.12, p = .38).
4.4. BrainAGE predictions for HC and SZ
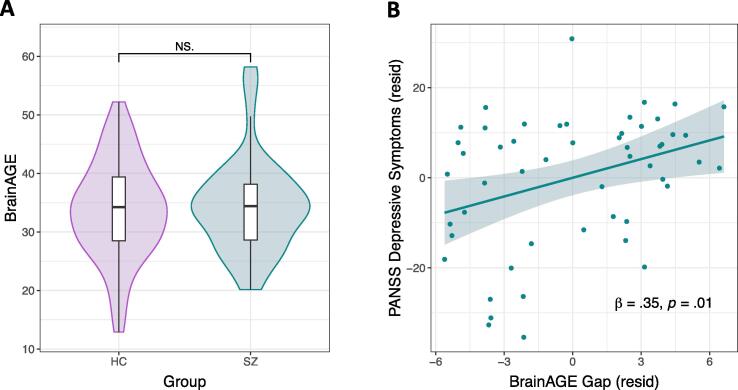
Table S3 shows the HC model used to produce BrainAGEs for all subjects, which indicated that Age was negatively associated with RewP, positively associated with LPP, and had a marginal relationship with SPN, when controlling for all other measures. A t-test indicated that BrainAGE predictions, on average, were similar for HC and SZ (t105 = −0.46, p = .65; Fig. 4A), and both groups showed a positive relationship between chronological age and BrainAGE (HC: r52 = 0.58, p < .001; SZ: r51 = 0.30, p = .03).
Fig. 4.
BrainAGE estimates and relationships with depressive symptoms. (A) Comparison of BrainAGE estimates (derived from reward ERPs) across groups (HC, healthy controls; SZ, schizophrenia subjects). (B) Relationship between depressive symptoms and BrainAGE gap scores in SZ; variables are residualized to account for the other predictors in Model 1 (Table 2). We removed one SZ subject with a BrainAGE estimate more than 3 SD above the mean from these analyses. Abbreviations: PANSS, Positive and Negative Syndrome Scale; resid, residuals.
4.5. BrainAGE gap correlates with SZ depressive symptoms
Depressive symptoms were related to the BrainAGE gap among SZ when controlling for negative symptoms (βdepressive_symptoms = 0.35, p = .01; Fig. 4B; Model 1, Table 2), while negative symptoms were unrelated to the BrainAGE gap; the bivariate correlation between the BrainAGE gap and depressive symptoms was also significant (r51 = 0.34, p = .01). Thus, the greater the estimated BrainAGE relative to an individual’s chronological age, the worse the depressive symptoms. Because depressive symptom ratings were higher for SZ with a schizoaffective diagnosis versus those with a schizophrenia diagnosis (t52 = 2.53, p = .01), and higher for SZ receiving concomitant antidepressant treatment (t52 = 2.53, p = .01), we used hierarchical regression to test whether our BrainAGE-symptom effect was better explained by diagnosis type (schizophrenia versus schizoaffective) or anti-depressant treatment (concomitant anti-depressant treatment versus not). Diagnosis and antidepressant treatment did not predict the BrainAGE gap, account for the depressive symptom association, or improve the model (R2-change = 0.49, p = .61; Model 2, Table 2).
Table 2.
Depressive symptoms correlate with BrainAGE gap in SZ.
| β (SE) | t-stat | Adj-R2 | |
|---|---|---|---|
| Model 1 | |||
| CAINS Motivation & Pleasure | 0.05 (0.14) | 0.36 | 0.07 |
| CAINS Expression | 0.08 (0.14) | 0.58 | |
| PANSS Depression | 0.35 (0.14) | 2.57* | |
| Model 2 | |||
| CAINS Motivation & Pleasure | 0.02 (0.14) | 0.15 | 0.05 |
| CAINS Expression | 0.05 (0.15) | 0.34 | |
| PANSS Depression | 0.41 (0.15) | 2.68* | |
| Schizophrenia/Schizoaffective Diagnosis | -0.09 (0.33) | −0.29 | |
| Anti-depressant Treatment | -0.30 (0.32) | -0.93 | |
*p < .05.
Model 1: F3,48 = 2.35, p = .08
Model 2: F5,46 = 1.57, p = .19
Abbreviations: CAINS, Clinical Assessment Interview for Negative Symptoms; PANSS, Positive and Negative Syndrome Scale.
4.6. Effects of antipsychotic medication dosage and antidepressant treatment
CPZeq and HPeq were unrelated to any ERP, symptom, or BrainAGE metrics (all p > .10). Concomitant antidepressant treatment was unrelated to any ERP, negative symptom, or BrainAGE metrics (all p > .10).
5. Discussion
We compared ERP components reflecting reward anticipation and evaluation in SZ versus HC, with a focus on investigating effects of chronological age, BrainAGE, and illness phase. ESZ exhibited aberrant late-stage reward evaluation signals relative to HC, indicated by heightened LPP responses following near misses versus wins (a reversal of the HC pattern). In comparison, reward anticipation and early reward outcome signals were intact in SZ, across early and chronic illness phases. Lastly, when we derived estimates of neural age from HC reward ERPs, these brain age estimates did not differ between HC and SZ groups, but “older” neural age in SZ correlated with worse depressive symptoms, even after controlling for negative symptoms, schizoaffective diagnosis, and antidepressant treatment. Together, our findings reveal novel age- and illness-phase indicators of altered reward processing in SZ.
Contrary to our hypotheses, SZ subjects did not differ from HC in their SPN amplitudes, and SPN amplitudes were unrelated to negative symptoms. This suggests that the SPN is relatively preserved in SZ during basic incentive processing. It is possible that anticipatory reward differences may emerge for individuals with SZ under more complex conditions, such as evaluating reward cues of varying certainty (Clayson et al., 2019). This follows from interpretations that certain reward deficits stem from interactions with higher-order cognitive processes like attention or working memory (Morris et al., 2011, Collins et al., 2014, Gold et al., 2013, Gold et al., 2012), and from evidence that avolition and anhedonia are linked to learning and other higher-order processes (Dowd et al., 2016). Effects of negative symptoms on reward anticipation have also been observed in tasks where cues indicate the need to prepare a response to either win, or avoid losing, different amounts of money, like in the monetary incentive delay task (Juckel et al., 2006, Juckel et al., 2006). Taken together, passive reward anticipation functions (Diekhof et al., 2012) may be relatively intact in SZ, whereas deficits in performance-based reward anticipation may have implications for negative symptoms. Future research studying both passive and operant reward tasks, in the same sample, will help to further elucidate reward deficits in SZ.
RewP responses for reward versus non-reward outcomes were comparable for SZ and HC subjects during a simple reward task, replicating earlier case-control studies that found intact RewP among people with SZ (Horan et al., 2012, Morris et al., 2011). This finding also fits with broader evidence for intact immediate consummatory responses in SZ (Kring and Barch, 2014). However, when comparing slopes of SPN reward anticipatory signals with early reward outcome processing measured by RewP, we found a relationship in SZ that was not observed in HC: that is, more reward anticipation (i.e., more negative SPN) was associated with a larger immediate response to reward (i.e., more positive RewP) in SZ. This could suggest that individuals with SZ have a unique temporal coupling between these reward signals that differs from HC; although it is unclear whether this is a disease-driven correlation or a typical relationship that was not present in our HC. In comparison, both HC and SZ subjects showed a significant SPN and LPP relationship, consistent with a prior report from a HC sample during a rewarded time estimation task (Pornpattananangkul and Nusslock, 2015).
Illness phase differences emerged during later reward outcome processing. More specifically, ESZ had larger LPP responses following near misses than wins, a reversal of the HC pattern (Fryer et al., 2020). This alteration was specific to ESZ, as the CSZ group showed no age-adjusted LPP differences. One potential explanation for the ESZ findings could be differences in attention allocation during reward processing. The LPP is modulated by attention and can be reduced if attention is directed away from emotionally-arousing content (Hajcak et al., 2013). Greater LPP in ESZ following near miss events could reflect more focus on negative outcomes or insufficient focus to positive outcomes, or both. Alternatively, HC and CSZ could be suppressing their LPP to near misses by paying less attention to unrewarding outcomes. HC and CSZ might also be enhancing their LPP to wins by paying more attention to rewarding outcomes, or by enhancing their anticipation of the next trial; i.e., this could reflect an adaptive strategy among HC and CSZ that is absent in ESZ. This fits with interpretations that the LPP indicates reward-related attentional deployment; for instance, one study found that LPP amplitudes were increased if subjects directed their attention towards cues indicating monetary earnings (Langeslag and van Strien, 2013). Evidence for normalization of the LPP response in CSZ warrants further study using longitudinal approaches, to understand how these downstream reward processes change over the illness course.
Finally, when collapsing across illness phase, accelerated brain aging correlated with higher depressive symptoms, when controlling for negative symptoms and schizoaffective diagnosis; i.e., a higher BrainAGE relative to one’s chronological age was associated with worse depressive symptoms in SZ. Blunted reward responsiveness, including RewP, has been associated with MDD diagnosis, self-reported depressive symptoms, and anhedonia (Foti et al., 2014, Liu et al., 2014, Proudfit et al., 2015), but the relationship between depressive features and reward processing has been less investigated in individuals with SZ. Notably this finding was not explained by negative symptom severity, antidepressant medication, or diagnosis (schizophrenia vs. schizoaffective), indicating some specificity of the relationship between depressive symptom expression and reward-related brain age. Our data correspond with findings that blunted reward-related fMRI activation following reward receipt is associated with worse depressive symptoms in SZ (Simon et al., 2010). However, in our study the relationship was specific to those SZ individuals who showed “older” reward-related brain functioning than expected based on chronological age, suggesting that the relationship between reward responsiveness and depression liability in SZ may depend on aspects of aging. This finding also underscores that brain-predicted aging measures can be used to capture meaningful variation in illness attributes within SZ (Cole et al., 2019).
6. Limitations
Though we found no relationships with chlorpromazine equivalents, medication status could still influence results. The ERP components we assessed were unrelated to negative symptoms, and we might have observed the hypothesized negative symptom relationships had we oversampled individuals meeting deficit syndrome criteria (Carpenter et al., 1988); it is also possible that these null results are due to symptom measure imperfections (Mathalon and Ford, 2012), and/or failures to parse primary versus secondary negative symptoms (the latter which are caused by positive symptoms, treatment side effects, depression, or substance use). Within-group sample sizes diminish our statistical power to detect relationships with smaller effect sizes than those observed; accordingly, replication in larger samples is warranted. Future studies are also needed to consider more inclusive BrainAGE models (e.g., adding multiple neurobiological modalities and extending the age range) that might improve prediction of normal and pathological reward processing trajectories, as we focused exclusively on ERP-derived reward-related metrics. We measured depressive symptoms using a scale developed for SZ, and did not assess HC, preventing dimensional analysis. A final caveat is that, while age and illness phase both modulated reward-related brain functioning, these features are inextricable in cross-sectional samples, in that younger patients tend to be earlier in their illness course. Thus, longitudinal studies are needed to clarify the nature of the age and illness phase reward relationships suggested by our data.
7. Conclusions
Our data highlight the impact of age, illness phase, and depressive features on reward-related brain functioning in SZ. Using a cognitively undemanding reward task, we identified areas of preserved functioning and illness-phase specific deficits: more specifically, reward anticipation (SPN) and early reward evaluation (RewP) were intact in ESZ and CSZ, while aberrant late-stage reward evaluation (LPP) emerged as a selective deficit among ESZ. Lastly, accelerated brain aging correlated with higher depressive symptoms across SZ, extending prior findings linking depressive features and blunted RewP to the schizophrenia spectrum.
CRediT authorship contribution statement
Samantha V. Abram: Methodology, Formal analysis, Visualization, Writing - original draft. Brian J. Roach: Methodology, Software, Formal analysis, Data curation, Writing - review & editing. Clay B. Holroyd: Conceptualization, Writing - review & editing. Martin P. Paulus: Conceptualization, Writing - review & editing. Judith M. Ford: Conceptualization, Methodology, Writing - review & editing. Daniel H. Mathalon: Conceptualization, Methodology, Supervision, Writing - review & editing. Susanna L. Fryer: Funding acquisition, Conceptualization, Methodology, Investigation, Supervision, Project administration, Writing - review & editing.
Acknowledgments
Acknowledgments
Research supported by VA CX001028 to Dr. Fryer. Dr. Abram is supported by the Department of Veteran Affairs Sierra Pacific Mental Illness Research, Education, and Clinical Center (MIRECC). Dr. Ford is supported by a VA Senior Research Career Scientist award.
Disclosures
Dr. Mathalon is a consultant for Boehringer Ingelheim, Greenwich Biosciences, and Cadent Therapeutics. All authors declare that there are no conflicts of interest for the current study. Drs. Abram, Fryer, Ford, and Mathalon are U.S. Government employees. The content is solely the responsibility of the authors and does not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102492.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Whitton A.E., Treadway M.T., Pizzagalli D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry. 2015;28(1):7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Dowd E.C. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G.P., Waltz J.A., Gold J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr. Bull. 2014;40(Suppl 2):S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fervaha G., Foussias G., Agid O., Remington G. Motivational and neurocognitive deficits are central to the prediction of longitudinal functional outcome in schizophrenia. Acta Psychiatr. Scand. 2014;130(4):290–299. doi: 10.1111/acps.12289. [DOI] [PubMed] [Google Scholar]
- Foussias G., Mann S., Zakzanis K.K., van Reekum R., Agid O., Remington G. Prediction of longitudinal functional outcomes in schizophrenia: The impact of baseline motivational deficits. Schizophr. Res. 2011;132(1):24–27. doi: 10.1016/j.schres.2011.06.026. [DOI] [PubMed] [Google Scholar]
- Fervaha G., Foussias G., Agid O., Remington G. Motivational deficits in early schizophrenia: prevalent, persistent, and key determinants of functional outcome. Schizophr. Res. 2015;166(1-3):9–16. doi: 10.1016/j.schres.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J. Neurosci. 2001;21(16):1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K.E., Ait Oumeziane B., Novak K.D., Rollock D., Foti D. Variation in reward- and error-related neural measures attributable to age, gender, race, and ethnicity. Int. J. Psychophysiol. 2018;132:353–364. doi: 10.1016/j.ijpsycho.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L. Progressive cortical reorganisation: A framework for investigating structural changes in schizophrenia. Neurosci. Biobehav. Rev. 2017;79:1–13. doi: 10.1016/j.neubiorev.2017.04.028. [DOI] [PubMed] [Google Scholar]
- Kring A.M., Barch D.M. The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. Eur. Neuropsychopharmacol. 2014;24(5):725–736. doi: 10.1016/j.euroneuro.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A.S., Minor K.S. Emotional experience in patients with schizophrenia revisited: Meta-analysis of laboratory studies. Schizophr. Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boxtel G.J.M., Böcker K.B.E. Cortical measures of anticipation. J. Psychophysiol. 2004;18:61–76. doi: 10.1027/0269-8803.18.23.61. [DOI] [Google Scholar]
- Brunia C.H.M., Hackley S.A., van Boxtel G.J.M., Kotani Y., Ohgami Y. Waiting to perceive: reward or punishment? Clin. Neurophysiol. 2011;122(5):858–868. doi: 10.1016/j.clinph.2010.12.039. [DOI] [PubMed] [Google Scholar]
- Kotani Y., Kishida S., Hiraku S., Suda K., Ishii M., Aihara Y. Effects of information and reward on stimulus-preceding negativity prior to feedback stimuli. Psychophysiology. 2003;40(5):818–826. doi: 10.1111/1469-8986.00082. [DOI] [PubMed] [Google Scholar]
- Ohgami Y., Kotani Y., Hiraku S., Aihara Y., Ishii M. Effects of reward and stimulus modality on stimulus-preceding negativity. Psychophysiology. 2004;41(5):729–738. doi: 10.1111/j.1469-8986.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- Clayson P.E., Wynn J.K., Infantolino Z.P., Hajcak G., Green M.F., Horan W.P. Reward processing in certain versus uncertain contexts in schizophrenia: An event-related potential (ERP) study. J. Abnorm. Psychol. 2019;128(8):867–880. doi: 10.1037/abn0000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit G.H. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015;52(4):449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Moser J.S., Holroyd C.B., Simons R.F. The feedback-related negativity reflects the binary evaluation of good versus bad outcomes. Biol. Psychol. 2006;71(2):148–154. doi: 10.1016/j.biopsycho.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Yeung N., Holroyd C.B., Cohen J.D. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cereb. Cortex. 2005;15(5):535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]
- Gehring W.J., Willoughby A.R. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295(5563):2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Pakzad-Vaezi K.L., Krigolson O.E. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Krigolson O.E., Lee S. Reward positivity elicited by predictive cues. NeuroReport. 2011;22(5):249–252. doi: 10.1097/WNR.0b013e328345441d. [DOI] [PubMed] [Google Scholar]
- Baker T.E., Lesperance P., Tucholka A., Potvin S., Larcher K., Zhang Y.u. Reversing the atypical valuation of drug and nondrug rewards in smokers using multimodal neuroimaging. Biol. Psychiatry. 2017;82(11):819–827. doi: 10.1016/j.biopsych.2017.01.015. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Foti D., Hajcak G., Wynn J.K., Green M.F. Impaired neural response to internal but not external feedback in schizophrenia. Psychol. Med. 2012;42(8):1637–1647. doi: 10.1017/S0033291711002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S.E., Holroyd C.B., Mann-Wrobel M.C., Gold J.M. Dissociation of response and feedback negativity in schizophrenia: Electrophysiological and computational evidence for a deficit in the representation of value. Front. Hum. Neurosci. 2011;5(123):1–16. doi: 10.3389/fnhum.2011.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R.W. Goal-Directed Deficits in Schizophrenia. In: Morris R.W., Bornstein A., Shenhaw A., editors. Goal-Directed Decision Making: Computations and Neural Circuits. Academic Press; 2018. pp. 387–406. [Google Scholar]
- Morris S.E., Heerey E.A., Gold J.M., Holroyd C.B. Learning-related changes in brain activity following errors and performance feedback in schizophrenia. Schizophr. Res. 2008;99(1-3):274–285. doi: 10.1016/j.schres.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Olvet D.M. Event-related potentials, emotion, and emotion regulation: An integrative review. Dev. Neuropsychol. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Cuthbert B.N., Schupp H.T., Bradley M.M., Birbaumer N., Lang P.J. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biol. Psychol. 2000;52(2):95–111. doi: 10.1016/S0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Glazer J.E., Kelley N.J., Pornpattananangkul N., Mittal V.A., Nusslock R. Beyond the FRN: Broadening the time-course of EEG and ERP components implicated in reward processing. Int. J. Psychophysiol. 2018;132:184–202. doi: 10.1016/j.ijpsycho.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Cunningham W.A., Espinet S.D., DeYoung C.G., Zelazo P.D. Attitudes to the right- and left: Frontal ERP asymmetries associated with stimulus valence and processing goals. NeuroImage. 2005;28(4):827–834. doi: 10.1016/j.neuroimage.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Wynn J.K., Kring A.M., Simons R.F., Green M.F. Electrophysiological correlates of emotional responding in schizophrenia. J. Abnorm. Psychol. 2010;119(1):18–30. doi: 10.1037/a0017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Foti D., Hajcak G., Wynn J.K., Green M.F. Intact motivated attention in schizophrenia: Evidence from event-related potentials. Schizophr. Res. 2012;135(1-3):95–99. doi: 10.1016/j.schres.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Hajcak G., Wynn J.K., Green M.F. Impaired emotion regulation in schizophrenia: Evidence from event-related potentials. Psychol. Med. 2013;43(11):2377–2391. doi: 10.1017/S0033291713000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K.R., Ait Oumeziane B., Hélie S., Foti D. The temporal dynamics of reversal learning: P3 amplitude predicts valence-specific behavioral adjustment. Physiol. Behav. 2016;161:24–32. doi: 10.1016/j.physbeh.2016.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows C.C., Gable P.A., Lohse K.R., Miller M.W. The effects of reward magnitude on reward processing: An averaged and single trial event-related potential study. Biol. Psychol. 2016;118:154–160. doi: 10.1016/j.biopsycho.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Pornpattananangkul N., Nusslock R. Motivated to win: Relationship between anticipatory and outcome reward-related neural activity. Brain Cogn. 2015;100:21–40. doi: 10.1016/j.bandc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer J.E., Kelley N.J., Pornpattananangkul N., Nusslock R. Hypomania and depression associated with distinct neural activity for immediate and future rewards. Psychophysiology. 2019;56:e13301. doi: 10.1111/psyp.13301. [DOI] [PubMed] [Google Scholar]
- Angus D.J., Latham A.J., Harmon-Jones E., Deliano M., Balleine B., Braddon-Mitchell D. Electrocortical components of anticipation and consumption in a monetary incentive delay task. Psychophysiology. 2017;54(11):1686–1705. doi: 10.1111/psyp.12913. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Wynn J.K., Hajcak G., Altshuler L., Green M.F. Distinct patterns of dysfunctional appetitive and aversive motivation in bipolar disorder versus schizophrenia: An event-related potential study. J. Abnorm. Psychol. 2016;125(4):576–587. doi: 10.1037/abn0000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell J.S., Potts G.F., Gooding D.C., Trachik B.J., Chan C.C., Spencer C.C. Transdiagnostic psychiatric symptoms and event-related potentials following rewarding and aversive outcomes. PLoS One. 2016;11:1–16. doi: 10.1371/journal.pone.0157084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H., O’Callaghan G., Vidal-Ribas P., Buzzell G.A., Brotman M.A., Leibenluft E. Reward processing in depression: A conceptual and meta-analytic review across fMRI and EEG studies. Am. J. Psychiatry. 2018;175(11):1111–1120. doi: 10.1176/appi.ajp.2018.17101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadić I., Dietzek M., Langbein K., Sauer H., Gaser C. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Res. Neuroimaging. 2017;266:86–89. doi: 10.1016/j.pscychresns.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Sambrook T.D., Goslin J. A neural reward prediction error revealed by a meta-analysis of ERPs using great grand averages. Psychol. Bull. 2015;141(1):213–235. doi: 10.1037/bul0000006. [DOI] [PubMed] [Google Scholar]
- Schnack H.G., van Haren N.E.M., Nieuwenhuis M., Hulshoff Pol H.E., Cahn W., Kahn R.S. Accelerated brain aging in schizophrenia: A longitudinal pattern recognition study. Am. J. Psychiatry. 2016;173(6):607–616. doi: 10.1176/appi.ajp.2015.15070922. [DOI] [PubMed] [Google Scholar]
- Hajcak G., Olvet D.M. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8(2):250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajek T., Franke K., Kolenic M., Capkova J., Matejka M., Propper L. Brain age in early stages of bipolar disorders or schizophrenia. Schizophr. Bull. 2019;45:190–198. doi: 10.1093/schbul/sbx172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahab S., Mulsant B.H., Levesque M.L., Calarco N., Nazeri A., Wheeler A.L. Brain structure, cognition, and brain age in schizophrenia, bipolar disorder, and healthy controls. Neuropsychopharmacol. 2019;44(5):898–906. doi: 10.1038/s41386-018-0298-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsouleris N., Davatzikos C., Borgwardt S., Gaser C., Bottlender R., Frodl T. Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophr. Bull. 2014;40(5):1140–1153. doi: 10.1093/schbul/sbt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren N.E.M., Pol H.E.H., Schnack H.G., Cahn W., Brans R., Carati I. Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol. Psychiatry. 2008;63(1):106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Tau G.Z., Peterson B.S. Normal development of brain circuits. Neuropsychopharmacol. 2010;35(1):147–168. doi: 10.1038/npp.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siris S.G. Depression in schizophrenia: Perspective in the era of “atypical” antipsychotic agents. Am. J. Psychology. 2000;157(9):1379–1389. doi: 10.1176/appi.ajp.157.9.1379. [DOI] [PubMed] [Google Scholar]
- Buckley P.F., Miller B.J., Lehrer D.S., Castle D.J. Psychiatric comorbidities and schizophrenia. Schizophr. Bull. 2009;35(2):383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfit G.H., Bress J.N., Foti D., Kujawa A., Klein D.N. Depression and event-related potentials: Emotional disengagement and reward insensitivity. Curr. Opin. Psychol. 2015;4:110–113. doi: 10.1016/j.copsyc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Novak K.D., Hill K.E., Ait Oumeziane B. Neurophysiological assessment of anhedonia in depression and schizophrenia. In: Sangha S., Foti D., editors. Neurobiology of Abnormal Emotion and Motivated Behaviors: Integrating Animal and Human Research. Elsevier Academic Press; 2018. pp. 242–256. [Google Scholar]
- Dowd E.C., Barch D.M. Pavlovian reward prediction and receipt in schizophrenia: Relationship to anhedonia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport J.L., Addington A.M., Frangou S., Psych M.R.C. The neurodevelopmental model of schizophrenia: Update 2005. Mol. Psychiatry. 2005;10(5):434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Fryer S.L., Roach B., Holroyd C., Paulus M., Sargent K., Boos A. Electrophysiological investigation of reward anticipation and outcome evaluation during slot machine play. BioRxiv. 2020 doi: 10.1016/j.neuroimage.2021.117874. [DOI] [PubMed] [Google Scholar]
- Franke K., Gaser C. Ten years of brainAGE as a neuroimaging biomarker of brain aging: what insights have we gained? Front. Neurol. 2019;10(789):1–26. doi: 10.3389/fneur.2019.00789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Roach B.J., Wiley K., Loewy R.L., Ford J.M., Mathalon D.H. Reduced amplitude of low-frequency brain oscillations in the psychosis risk syndrome and early illness schizophrenia. Neuropsychopharmacology. 2016;41(9):2388–2398. doi: 10.1038/npp.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer S.L., Woods S.W, Kiehl K.A., Calhoun V.D., Pearlson G.D., Roach B.J. Deficient suppression of default mode regions during working memory in individuals with early psychosis and at clinical high-risk for psychosis. Front. Psychiatry. 2013;4:92. doi: 10.3389/fpsyt.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R.A., Roach B.J., Srihari V.H., Woods S.W., Ford J.M., Mathalon D.H. Equivalent mismatch negativity deficits across deviant types in early illness schizophrenia-spectrum patients. Biol. Psychol. 2015;105:130–137. doi: 10.1016/j.biopsycho.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry. 2003;64(6):663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- Al Zoubi O., Wong C.K., Kuplicki R.T., Yeh H., Mayeli A., Refai H. Predicting age from brain EEG signals-a machine learning approach. Front. Aging Neurosci. 2018;10:1–12. doi: 10.3389/fnagi.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.-C. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib R., Dixon M.R. Neurobehavioral evidence for the “near-miss” effect in pathological gamblers. J. Exp. Anal. Behav. 2010;93:313–328. doi: 10.1901/jeab.2010.93-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L., Lawrence A.J., Astley-Jones F., Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61(3):481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donkers F.C.L., Nieuwenhuis S., van Boxtel G.J.M. Mediofrontal negativities in the absence of responding. Cognitive Brain Res. 2005;25(3):777–787. doi: 10.1016/j.cogbrainres.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Kring A.M., Gur R.E., Reise S.P., Blanchard J.J. Development and psychometric validation of the Clinical Assessment Interview for Negative Symptoms (CAINS) Schizophr. Res. 2011;132(2-3):140–145. doi: 10.1016/j.schres.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Wüstenberg T., Villringer A., Knutson B. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for Schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kring A.M., Gur R.E., Blanchard J.J., Horan W.P., Reise S.P. The clinical assessment interview for negative symptoms (CAINS): Final development and validation. Am. J. Psychiatry. 2013;170(2):165–172. doi: 10.1176/appi.ajp.2012.12010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmayer J.-P., Bernstein-Hyman R., Grochowski S., Bark N. Psychopathology of schizophrenia: Initial validation of a 5-factor model. Psychopathology. 1995;28:22–31. doi: 10.1159/000284896. [DOI] [PubMed] [Google Scholar]
- Kim S.-W., Kim S.-J., Yoon B.-H., Kim J.-M., Shin I.-S., Hwang M.Y. Diagnostic validity of assessment scales for depression in patients with schizophrenia. Psychiatry Res. 2006;144(1):57–63. doi: 10.1016/j.psychres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Kontaxakis V.P., Havaki-Kontaxaki B.J., Stamouli S.S., Margariti M.M., Collias C.T., Christodoulou G.N. Comparison of four scales measuring depression in schizophrenic inpatients. Eur. Psychiatry. 2000;15(4):274–277. doi: 10.1016/S0924-9338(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Wallwork R.S., Fortgang R., Hashimoto R., Weinberger D.R., Dickinson D. Searching for a consensus five-factor model of the Positive and Negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012;137(1-3):246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J., Luck S.J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: Fully automated statistical thresholding for EEG artifact rejection. J. Neurosci. Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Mognon A., Jovicich J., Bruzzone L., Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology. 2011;48(2):229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Leonowicz Z., Karvanen J., Shishkin S.L. Trimmed estimators for robust averaging of event-related potentials. J. Neurosci. Methods. 2005;142(1):17–26. doi: 10.1016/j.jneumeth.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Donkers F.C.L., van Boxtel G.J.M. Mediofrontal negativities to averted gains and losses in the slot-machine task: A further investigation. J. Psychophysiol. 2005;19(4):256–262. [Google Scholar]
- Cockburn J., Holroyd C.B. Feedback information and the reward positivity. Int. J. Psychophysiol. 2018;132:243–251. doi: 10.1016/j.ijpsycho.2017.11.017. [DOI] [PubMed] [Google Scholar]
- Umemoto A., Holroyd C.B. Neural mechanisms of reward processing associated with depression-related personality traits. Clin. Neurophysiol. 2017;128(7):1184–1196. doi: 10.1016/j.clinph.2017.03.049. [DOI] [PubMed] [Google Scholar]
- Mathalon D.H., Roach B.J., Ferri J.M., Loewy R.L., Stuart B.K., Perez V.B. Deficient auditory predictive coding during vocalization in the psychosis risk syndrome and in early illness schizophrenia: The final expanded sample. Psychol. Med. 2019;49(11):1897–1904. doi: 10.1017/S0033291718002659. [DOI] [PubMed] [Google Scholar]
- Perez V.B., Woods S.W., Roach B.J., Ford J.M., McGlashan T.H., Srihari V.H. Automatic auditory processing deficits in schizophrenia and clinical high-risk patients: Forecasting psychosis risk with mismatch negativity. Biol. Psychiatry. 2014;75(6):459–469. doi: 10.1016/j.biopsych.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews C.A., Perez V.B., Roach B.J., Fekri S., Vigil O., Kupferman E. Error-related brain activity dissociates hoarding disorder from obsessive-compulsive disorder. Psychol. Med. 2016;46(2):367–379. doi: 10.1017/S0033291715001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Ford J.M. Neurobiology of schizophrenia: Search for the elusive correlation with symptoms. Front. Hum. Neurosci. 2012;6:136. doi: 10.3389/fnhum.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon D.H., Pfefferbaum A., Lim K.O., Rosenbloom M.J., Sullivan E.V. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch. Gen. Psychiatry. 2003;60(3):245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A., Lim K.O., Zipursky R.B., Mathalon D.H., Rosenbloom M.J., Lane B. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: A quantitative MRI study. Alcoholism Clin. Exp. Res. 1992;16(6):1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Collins A.G.E., Brown J.K., Gold J.M., Waltz J.A., Frank M.J. Working memory contributions to reinforcement learning impairments in schizophrenia. J. Neurosci. 2014;34(41):13747–13756. doi: 10.1523/JNEUROSCI.0989-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Strauss G.P., Waltz J.A., Robinson B.M., Brown J.K., Frank M.J. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol. Psychiatry. 2013;74(2):130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J.M., Waltz J.A., Matveeva T.M., Kasanova Z., Strauss G.P., Herbener E.S. Negative symptoms and the failure to represent the expected reward value of actions: Behavioral and computational modeling evidence. Arch. Gen. Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E.C., Frank M.J., Collins A., Gold J.M., Barch D.M. Probabilistic reinforcement learning in patients with schizophrenia: Relationships to anhedonia and avolition. Biol. Psychiatry. 2016;1(5):460–473. doi: 10.1016/j.bpsc.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Filonov D., Wüstenberg T., Villringer A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Diekhof E.K., Kaps L., Falkai P., Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude – an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50(7):1252–1266. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Hajcak G., MacNamara A., Foti D., Ferri J., Keil A. The dynamic allocation of attention to emotion: Simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biol. Psychol. 2013;92(3):447–455. doi: 10.1016/j.biopsycho.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Langeslag S.J.E., van Strien J.W. Up-regulation of emotional responses to reward-predicting stimuli: An ERP study. Biol. Psychol. 2013;94(1):228–233. doi: 10.1016/j.biopsycho.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Liu W.-hua, Wang L.-zhi, Shang H.-rui, Shen Y., Li Z., Cheung E.F.C., Chan R.C.K. The influence of anhedonia on feedback negativity in major depressive disorder. Neuropsychologia. 2014;53:213–220. doi: 10.1016/j.neuropsychologia.2013.11.023. [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient. Edition. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- Foti D., Carlson J.M., Sauder C.L., Proudfit G.H. Reward dysfunction in major depression: Multimodal neuroimaging evidence for refining the melancholic phenotype. NeuroImage. 2014;101:50–58. doi: 10.1016/j.neuroimage.2014.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J.J., Biller A., Walther S., Roesch-Ely D., Stippich C., Weisbrod M. Neural correlates of reward processing in schizophrenia — relationship to apathy and depression. Schizophr. Res. 2010;118(1–3):154–161. doi: 10.1016/j.schres.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Cole J.H., Marioni R.E., Harris S.E., Deary I.J. Brain age and other bodily ‘ages’: Implications for neuropsychiatry. Mol. Psychiatry. 2019;24(2):266–281. doi: 10.1038/s41380-018-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter W.T., Heinrichs D.W., Wagman A.M.I. Deficit and nondeficit forms of schizophrenia: The concept. Am. J. Psychiatry. 1988;145(5):578–583. doi: 10.1176/ajp.145.5.578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.