Abstract
Purpose
To determine the diagnostic parameters of breast ultrasound (US) in the setting of routine radiological surveillance after a diagnosis of breast cancer and evaluate costs of the inclusion of breast US as well as any survival benefit of US detected cases of recurrence in surveillance.
Methods
622 patients underwent breast cancer surgery and follow up at Austin Health from July 2009 to December 2015. Retrospective data analysis was performed to determine; diagnostic parameters, financial costs of US and survival outcomes of US detected cases of recurrence.
Results
Patients underwent 1–9 years of breast cancer surveillance, with a median of 4.24 years. 390 (62.7%) patients underwent additional breast US surveillance to mammography. 232 (38.3%) fit criteria for use of additional breast US. 199 abnormal imaging episodes occurred, leading to 16 screen detected-cases of locoregional recurrence. US alone generated 107 abnormal images and found 9 cancers. US had a sensitivity of 44.1%, specificity of 95.2% and positive predictive value of 11.7% in comparison to mammography; 20.6%, 97.4% and 9.9% respectively. US had a biopsy rate of 4.0% and lead to an incremental cancer detection rate of 0.38%. The cost of incremental cancer found was $31,463.72 AUD. Survival outcomes based on method of detection of recurrence were insignificant (p value = 0.71).
Conclusions
Breast US has a sensitivity of 44.1% and detected seven recurrences that were mammographically occult. Breast US has a similar PPV to mammography in surveillance. Breast US generated considerable biopsy rates and costs. Survival analysis was not able to detect any benefit of US detected cases of recurrence.
Keywords: Breast cancer, Breast ultrasound, Surveillance, Biopsy rate, Diagnostic accuracy, Recurrence
Abbreviations: ASCO, American Society of Clinical Oncology; AUD, Australian Dollar; BIRADS, Breast Imaging- Reporting and Data System; DBT, Digital Breast Tomosynthesis; EBC, Early Breast Cancer; ESMO, European Society for Medical Oncology; FNA, Fine Needle Aspiration; ICDR, Incidental Cancer Detection Ratio; LABC, Locally Advanced Breast Cancer; MMG, Mammogram; MRI, Magnetic Resonance Imaging; NCCN, National Comprehensive Cancer Network; NHMRC, National Health and Medical Research Medical Council; NICE, National Institute of Clinical Excellence; NPV, Negative Predictive Value; PPV, Positive Predictive Value; RANZCR, Royal Australian and New Zealand College of Radiologists; UK, United Kingdom; US, Ultrasound; USA, United States of America
Highlights
-
•
Breast ultrasound in asymptomatic surveillance after breast cancer surgery was found to have a sensitivity of 44.1%.
-
•
Breast ultrasound detected 7 recurrences in 390 patients who did not meet criteria for adjunct breast US and had normal mammography
-
•
Breast ultrasound generated 26 additional biopsies per 1000 US compared to mammography in surveillance, creating considerablecosts.
-
•
Cancer detection by breast US alone did not lead to statistically significant survival benefit over mammography.
Funding declaration
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
1. Introduction
Breast cancer is the most common cancer in Australia, accounting for 13% of all newly diagnosed cancers in 2018 [1]. With excellent survival outcomes, there is a growing number of patients entering surveillance. Routine surveillance after a diagnosis of breast cancer involves annual clinical review along with breast imaging. Australian Clinical Practice Guidelines recommend the use of annual mammography as primary method of radiological surveillance, and to include breast ultrasound (US) if indicated, in addition to a clinical examination schedule(2). The indication for US applies to women under the age of 35, women with dense breasts, or women with mammographically occult primary breast cancers(2).
Imaging surveillance after breast cancer is a valuable tool to detect recurrence in women in absence of clinical suspicion, leading to better outcomes associated with early detection of recurrence(3). There are a number of imaging modalities available for the detection of breast cancer recurrence, with mammography being the primary method selected by several international guidelines(2, 4–7). The Australian guidelines are the sole organisation to comment on breast ultrasound, with USA, UK and European guidelines not recommending breast US or omitting it entirely, these guidelines are summarised in Table 1.
Table 1.
Summary of International recommendations for breast cancer surveillance.
| Guideline | Mammography | Breast U/S | Population |
|---|---|---|---|
| NHMRC (AUS), 2001 [2] | Annually 6–12 months post treatment, for 5 years | Only if indicated | EBC |
| ASCO (USA), 2012 [4] | Annually 6–12 months post treatment, for 5 years | Not recommended | EBC and LABC |
| NICE (UK), 2011[5] | Annually 12 months post treatment for 5 years | Not recommended | EBC and LABC |
| NCCN (USA), 2018 [6] | Annually 3–12 months post treatment | Not mentioned | Stage I or II |
| ESMO (EU), 2013 [7] | Every 1–2 years 6–12 months post treatment | Not mentioned | EBC |
NHMRC, National Health and Medical Research Council; ASCO, American Society of Clinical Oncology; NICE, National Institute of Clinical Excellence; NCCN, National Comprehensive Cancer Network; ESMO, European Society for Medical Oncology; EBC, Early Breast Cancer; LABC, Locally Advanced Breast Cancer.
With advances in technology of breast US, Magnetic Resonance Imaging (MRI) and molecular imaging, there has been a shift in using these modalities in adjunct to mammography for the detection of recurrence. Breast US has shown promise in diagnosing mammographically occult breast cancers as its diagnostic performance is largely unaltered by increased breast density [8]. Breast density has become the subject of new legislation in the United States, where mandatory reporting of breast density and recommendation to discuss adjunct screening methods with primary care physicians has recently been introduced [9]. Most states within Australia and New Zealand do not recommend further discussion regarding breast screening in women with dense breasts. These legislations apply to an asymptomatic screening cohort, however given the similarity to patients in breast cancer surveillance, these laws provide insight towards the importance of breast density and its impact on standard mammography.
Presently at Austin Health, most breast cancer surveillance patients are receiving adjunct bilateral breast US regardless of the guidelines in addition to standard clinical examination and mammography. Increased imaging surveillance may seem beneficial for the patient cohort, there is concern for increased detection of benign or indeterminate breast lesions in patients who have already underwent primary diagnosis and treatment, and may have to undergo further imaging or biopsy of a lesion detected on US.
The aims of this study are to evaluate the use of US in breast cancer surveillance and to determine diagnostic parameters of US in this setting. Further analysis including financial aspects of US and survival impact of US detected recurrences will be performed. We hypothesise that breast US provides minor additional benefit to cancer detection despite its cost.
2. Materials & methods
2.1. Patient recruitment
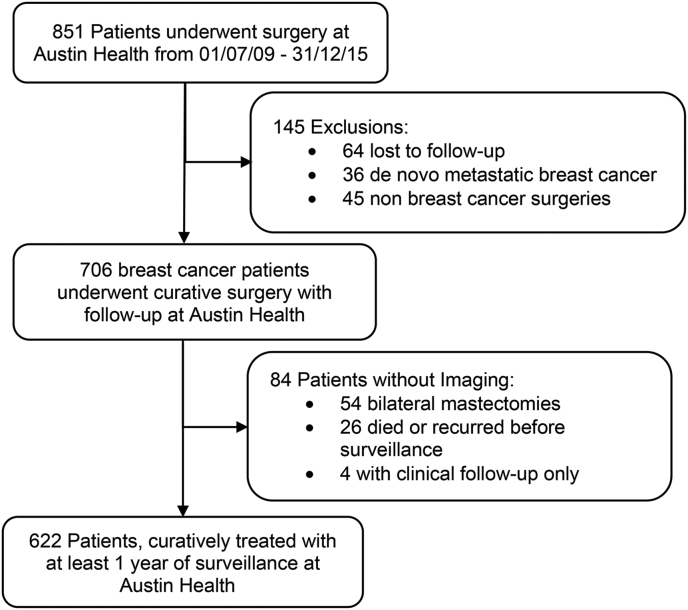
Patient recruitment is summarised in Fig. 1. 851 patients underwent breast cancer surgery at Austin Health from July 2009 to December 2015. 145 patients were excluded from the study. Patients who have had bilateral mastectomy and had axillary US surveillance were included, 54 bilateral mastectomy patients were excluded as they didn’t not receive axillary ultrasound surveillance.
Fig. 1.
Patient selection process.
2.2. Data collection
Patient data was extracted from electronic medical records at Austin Health. Domains of data included were; age, date and method of diagnosis, tumour histology, neoadjuvant and adjuvant therapies, and breast density according to Breast Imaging Reporting and Data System (BIRADS). Imaging outcomes were recorded by the Royal Australian and New Zealand College of Radiologists (RANZCR) breast lesion classification system. Dates of last follow-up, recurrence, or death were obtained. The data cut-off date was arbitrarily set at the 1st of April 2019. Tumour characteristics were summarised using the TNM system for breast malignancies. Nodal stage was scored based on pathological findings (pN). Tumour size was scored based on the pathological findings or best radiological estimation if undergoing neoadjuvant chemotherapy. Breast density was scored by reporting radiologists, if there were any discrepancies between reports than the most frequent density reported was scored.
2.3. Imaging surveillance
During the study period, Siemens Mammomat Inspiration Digital Mammography unit was used, without tomosynthesis. The standard Australian mammography screening protocol was used, consisting of Medial Lateral Oblique and Craniocaudal projections for each breast, followed by further views if deemed necessary. Two ultrasound units were used; Philips iU22 ultrasound machine and General Electric LOCIQ E9 ultrasound system. The ultrasound examinations were performed by sonographers accredited by the Australian Sonographer Accreditation registry. Images were reviewed by accredited breast radiologists.
2.4. Data analysis
Recorded data was utilised to carry out simple statistical equations to determine sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) of breast US and mammography. Abnormal findings seen on both mammography and ultrasound were included for both US and mammography in data analysis. The PPV1 was calculated in this study based on the number of BIRADS 3/4/5 findings compared to biopsy proven cancers. Concurrent local and distant recurrences were counted as distant recurrences. All patients who developed an interval locoregional recurrence were classified as a false negative finding on the previous round of surveillance imaging. Economic analysis was determined using Medicare Benefits Scheme [10] item numbers only, additional costs not included. Survival analysis was carried out using GraphPad Prism (version 8.4.1 for Mac, GraphPad Software, La Jolla California USA, http://www.graphpad.com) and statistical significance of survival outcome differences was determined using the Logrank test.
3. Results
Seven hundred and six patients were suitable for retrospective analysis and 622 underwent at least one year of radiological surveillance. Baseline characteristics of the patient cohort can be found in Table 2. The median number of surveillance was 4.24 rounds (range: 1–9). Mammography and US used concurrently accounted for 579 patients primary surveillance. Three hundred and ninety (63.7%) patients did not meet a guideline recommendation for supplemental US.
Table 2.
Baseline characteristics of the patient cohort.
| N = 622 | ||
|---|---|---|
| Age at Diagnosis | Mean | Range |
| 59.7 | 24.7–94.8 | |
| Mode of Detection | n | % |
| Symptomatic | 377 | 60.6 |
| Screen | 245 | 39.4 |
| Tumour size (T Stage) | ||
| 1 | 273 | 43.9 |
| 2 | 271 | 43.5 |
| 3 | 75 | 12.1 |
| 4 | 3 | 0.5 |
| Nodal stage | ||
| Negative | 424 | 68.1 |
| 1 | 113 | 18.2 |
| 2 | 49 | 7.9 |
| 3 | 21 | 3.4 |
| Unknown | 15 | 2.4 |
| Immunohistochemistry | ||
| ER1 | ||
| Negative | 107 | 17.2 |
| <50 | 502 | 80.7 |
| > or = 50 | 13 | 2.1 |
| PR2 | ||
| Negative | 167 | 26.9 |
| <50 | 135 | 21.7 |
| > or = 50 | 320 | 51.4 |
| HER23 | ||
| Amplified | 99 | 15.9 |
| Non-Amplified | 523 | 84.1 |
| Ki67% | ||
| <14% | 63 | 10.1 |
| > or = 14% | 127 | 20.4 |
| Unknown | 432 | 69.5 |
| Grade (BRE4) | ||
| 1 | 87 | 14.0 |
| 2 | 264 | 42.4 |
| 3 | 271 | 43.6 |
| Surgery Type | ||
| Breast Conserving Surgery | 340 | 54.6 |
| Mastectomy | 282 | 45.4 |
| Other Therapies | ||
| Chemotherapy | 291 | 46.8 |
| Endocrine Therapy | 501 | 80.5 |
| HER2 Therapy | 83 | 13.3 |
| Radiotherapy | 437 | 70.2 |
| Neoadjuvant Therapy | ||
| Chemotherapy | 36 | 5.8 |
| Endocrine Therapy | 8 | 1.3 |
| Radiotherapy | 6 | 1.0 |
| HER2 Therapy | 4 | 0.6 |
1 ER; Oestrogen Receptor, 2 PR; Progesterone Receptor, 3 HER2; Human Epidermal Growth Factor Receptor 2, 4BRE; Bloom-Richardson-Elston Grading System.
From all imaging episodes, 199 abnormal images were generated. Of these abnormal images, 168 (84.4%) were reported as RANZCR category 3 lesions, 61 by mammography, 107 by US. Twenty-five images (12.6%) were classified as category 4 lesions 18 by US and 7 by mammography and 6 images (3.0%) were considered category 5, 3 by US and 3 by mammography. One hundred and eight percutaneous biopsies were performed, 78 (72.2%) of which were triggered by US alone. From 70 mammographic abnormal findings, 33 (47.1%) lead to biopsy, in US, 129 abnormal images were found and 95 (73.6%) of abnormal findings lead to biopsy.
The diagnostic parameters of both ultrasound and mammography can be found in Table 3.
Table 3.
Diagnostic parameters of US and mammography.
| Sensitivity | Specificity | PPV1 | PPV3 | NPV | Biopsy Rate | ICDR | |
|---|---|---|---|---|---|---|---|
| Mammography | 20.59 | 44.12 | 9.86 | 23.33 | 98.91 | 1.34 | |
| US | 97.45 | 95.21 | 11.72 | 19.23 | 99.16 | 3.97 | 0.38 |
PPV1; Positive predictive value (abnormal imaging), PPV3 (biopsy) NPV; negative predictive value, ICDR; incremental cancer detection rate.
There were 85 episodes of recurrence in the cohort, 34 locoregional and 41 distant. The median time from diagnosis of primary breast cancer to recurrence was 39.9 months (range: 1.4–100). In the local recurrence cohort, 16 were detected due to radiological surveillance and 18 were interval cancers. Nine (56.3%) episodes of recurrence were detected using US alone, six (37.5%) were detected on both modalities, and one (6.2%) was occult on ultrasound. Of the nine US alone detected cases, only two fit criteria for inclusion of US in their surveillance. Further analysis of the 18 cases of locoregional recurrence can be found within Table 4.
Table 4.
Characteristics of locoregional recurrence cases.
| US |
MMG |
Interval |
|
|---|---|---|---|
| Total (number) | 9 | 7 | 18 |
| Breast Density | |||
| <50% | 77.8% | 56.2% | 55.6% |
| >50% | 22.2% | 42.8% | 33.4% |
| Tumour Size | |||
| <2 cm | 55.6% | 57.1% | 27.8% |
| >2 cm | 11.1% | 42.9% | 27.8% |
| Nodal only recurrence | 33.3% | 0% | 44.4% |
| Nodal Stage | |||
| Positive | 44.4% | 14.3% | 55.6% |
| Negative | 55.6% | 85.7% | 44.4% |
| Tumour Phenotype | |||
| ER/PR +/HER2- | 77.8% | 57.1% | 44.4% |
| ER/PR -/HER2+ | 0% | 0% | 5.6% |
| Triple Positive Breast Cancer | 0% | 0% | 11.1% |
| Triple Negative Breast Cancer | 22.2% | 42.9% | 38.9% |
| Primary Treatment | |||
| Surgery | 88.9% | 100% | 61.1% |
| Endocrine/Chemo/Radio Therapy | 11.1% | 0% | 39.9% |
| Overall Survival | 55.6% | 57.1% | 50.0% |
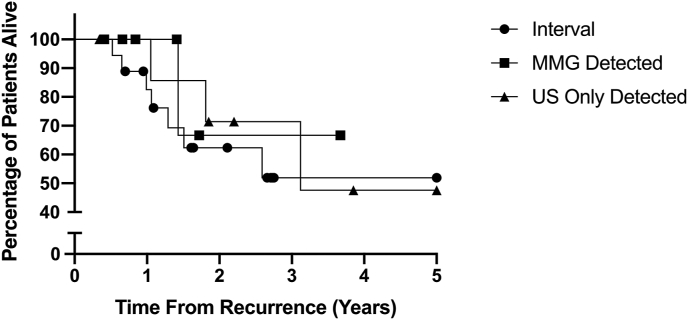
There was no significant difference in overall survival outcomes based on the method of detection of recurrence (Interval vs US alone vs MMG detected) with a p value of 0.71 as demonstrated in Fig. 2.
Fig. 2.
Kaplan-Meier Survival Curve of Survival after locoregional recurrence detection.
Economic analysis of US was performed. All prices are in Australian dollars (AUD). The total cost of additional US in this cohort was $283,173.50, the cost per incremental cancer found by US was $31,463.72, and additional US cost $468.06 per patient in the cohort.
4. Discussion
In our single centre cohort, the 63.7% of patients who underwent both mammography and US did not meet the Australian guidelines criteria for the inclusion of US in surveillance, leading to a considerable number of adjuvant tests and highlights the non-adherence to local guidelines. Explanations for this non-adherence may include surgeon personal preference of utilising adjuvant ultrasound regardless of breast density, patient anxiety of omitting a supplementary test that they may have previously had, or as a consequence of the use of breast US in the diagnostic setting of breast cancer. These factors become evident in subsequent years of US surveillance as data indicates that US is continually used despite documented breast density on previous radiology reports of less than 50%.
The diagnostic parameters of breast US were reviewed in this study to add to the base of evidence regarding its value in surveillance. The sensitivity of breast US at 44.12% reported is lesser than most literature available, with studies indicating a range between 45% and 90.5% [[11], [12], [13], [14], [15], [16]]. In our study, this lesser sensitivity is likely derived from interval cancers being treated as a false negative. This is true for both mammography and US, as it is important to note that mammography similarly had a low sensitivity of 20.59%. The decreased sensitivity in both mammography and US found in this study suggests under performance of these modalities in breast cancer surveillance. There is contention in regarding the classification of interval cancers as false negatives, as previous studies have indicated only a percentage of interval cancers are true false negatives [17,18].
In comparison of BIRADS and RANZCR reporting, category 3 lesions differ greatly, as BIRADS suggests a malignancy potential of <2%, probably benign in comparison to an “indeterminate” finding [19]., In Australian practice, a RANZCR category 3 lesion usually prompts percutaneous biopsy, as opposed to BIRADS category 3 where shorter interval follow up is recommended [19]. A recent review of the literature available for category 3 lesions indicates the nuance of interpreting category 3 lesions with the clinical picture; including age, personal history of breast cancer among other indicators to guide decision for biopsy as opposed to reimaging [20]. Given in a surveillance setting all patients had a personal history of breast cancer, this elevates suspicion of recurrence and therefore may explain why biopsy was commonly chosen than not.
What is less evident from our data is the trend for biopsy after an ultrasound finding as opposed to mammography. From an abnormal US, 72.9% led to biopsy, whereas in mammographic findings, 33.3% received a biopsy. This subsequently lead to a decreased PPV3 in US. In a large multicentre trial of US in primary screening for breast cancer, US was similarly found to lead to a large number of subsequent biopsy, and a significant number of which derived from category 3 lesions [21]. Surgeon and patient preference may influence the decision to undergo surgical biopsy, however it is also noted that radiological reporting suggesting percutaneous biopsy was common.
The ICDR rate of US in our cohort is 0.38%. This is amongst lowest reported in the range of detection rates in the literature, which ranges from 0.28 to 0.8% [11,12,[14], [15], [16],[22], [23], [24], [25], [26]]. The lower ICDR in this cohort owes to the design of the study and methods of US screening. It could be argued that the ICDR in this study is artificially low, as most existing literature utilises US in areas that are mammographically inaccessible such as visualising the supraclavicular fossa and chest wall after mastectomy. As a result of this, cases of recurrence within locoregional lymph node stations are inherently “mammographically occult” and increase the ICDR. US of the chest wall was utilised sporadically in our study in patients with bilateral mastectomy. US in this study was confined to the breast and axilla in most cases, and therefore would not detect lymphadenopathy in other regional lymph node stations. In comparison to the ACRIN trial, where US was used as a primary screening method in the asymptomatic population that were not in routine follow up after a diagnosis of breast cancer, US had an ICDR of 0.3–0.4% [21]. It would be expected that in a cohort of women with personal history of breast cancer, US would have an increased ICDR due to the inherent increased risk of this cohort.
The detection of each incremental cancer in our study appears significant at $31,463. Only one other study reviewing economic costs of supplemental US was found, which was $10,000 AUD less per cancer, however still significant [27]. Whilst our study derived an impressive cost of US per cancer found, it is important to consider the opportunity cost of these cancers not being found. Breast cancers detected later may lead to increased costs to the healthcare system of adjuvant therapy for advanced cancers. Furthermore, it is important to consider the ramifications of early cancer detection on the patient level. Early detection of recurrence is invaluable to individual patients who benefit from avoiding progression of disease.
Our study yielded nine US only episodes of recurrence, of these 7 patients did not meet criteria for US inclusion in surveillance. Further analysis of these seven patients would be required to determine whether there are any specific cancer phenotypes or patient factors that need inclusion in the guidelines to prevent these episodes of recurrence from being missed. This statistic may suggest that the current Australian clinical guidelines do not reflect all groups at increased risk of mammographically occult recurrence.
The survival benefit of these nine US only detected cancers remains unknown. The demonstration of survival outcomes indicates that there is no method of detection that aligns with a better survival benefit. There is literature to suggest that sonographic detection of recurrence has survival benefit over clinical examination detection(24), and this likely reflects the extent of tumour growth before it is clinically detectable. Furthermore, it is suggested that cancers may be detected on US earlier than possible with mammography and lead to better survival outcomes [3,14,24]. The exact reason for this is unclear in the literature. Survival outcomes and method of detection do not occur in isolation, and tumour characteristics and patient comorbidity are vital factors for patient survival after diagnosis of recurrence. It remains unknown whether survival outcomes would have been altered if these mammographically occult cancers were eventually detected by mammography at a later surveillance round.
4.1. Limitations and scope for further research
A number of limitations arose due to the retrospective nature of this study. Primarily, a retrospective observational study meant that selection bias could not be accounted for. Specifically to our study, the co-reporting of mammography and US may have affected the final reporting of imaging abnormalities. Further unblinded review of imaging of interval cancers could be performed to identify true false negatives as opposed to true interval cancers. The survival benefits of earlier US detected cancers could not be evaluated. The small locoregional recurrence rate lead to statistical insignificance of survival benefits. Finally, as this was a single centre study, the recommendations that may arise regarding the use of supplemental US may not be applicable globally. Larger prospective studies would be required to determine the survival benefits of US detected cancers. Another large aspect of costs of breast US is the psychological impacts to patients. A qualitative study of patient acceptability of additional breast US could give valuable information regarding patient perspectives.
5. Conclusion
This single centre study highlighted the fact that US was being added to standard mammography surveillance in a large proportion, regardless of the clinical guidelines. Breast US detected mammographically occult cases of recurrence, but generated numerous percutaneous biopsy. US outside of clinical guidelines and further investigations added to surveillance generated costs, and no observable survival benefit. In a relatively small cohort of patients, incremental cancers found earlier may have had individual benefits. Given indeterminate lesions found on US drove biopsy rates, further clinical judgement of benefit of further investigation should be applied in this setting.
Compliance with ethical standards
Ethical approval: Ethics approval was granted by Austin Health HREC. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: No identifiable patient data has been included in this manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interest.
References
- 1.Cancer Australia . 2018. Breast cancer statistics.https://breast-cancer.canceraustralia.gov.au/statistics [Google Scholar]
- 2.Cancer Australia . Commonwealth of Australia; 2010. Recommendations for follow-up of women with early breast cancer.https://canceraustralia.gov.au/system/tdf/publications/fueg-follow-up-of-women-with-early-breast-cancer_504af0340ef02.pdf?file=1&type=node&id=2967 [Google Scholar]
- 3.Schneble E.J., Graham L.J., Shupe M.P., Flynt F.L., Banks K.P., Kirkpatrick A.D. Current approaches and challenges in early detection of breast cancer recurrence. J Canc. 2014;5(4):281–290. doi: 10.7150/jca.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khatcheressian J.L., Hurley P., Bantug E., Esserman L.J., Grunfeld E., Halberg F. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2013;31(7):961–965. doi: 10.1200/JCO.2012.45.9859. [DOI] [PubMed] [Google Scholar]
- 5.National Collaborating Centre for C . National Collaborating Centre for Cancer (UK) National Collaborating Centre for Cancer.; Cardiff (UK): 2009. National Institute for Health and clinical excellence: guidance. Early and locally advanced breast cancer: diagnosis and treatment. [Google Scholar]
- 6.National Comprehensive Cancer Network. Breast Cancer: National Comprehensive Cancer Network; [Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 7.Senkus E., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rutgers E. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol : official journal of the European Society for Medical Oncology. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 8.Berg W.A., Blume J.D., Cormack J.B., Mendelson E.B., Lehrer D., Bohm-Velez M. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. Jama. 2008;299(18):2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingman W.V., Richards B., Street J.M. Breast density notification: an Australian perspective. J Clin Med. 2020;9(3):681. doi: 10.3390/jcm9030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Australian Government Department of Health . 2020. Medicare benefits Scheme.http://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Home [ Available from: [Google Scholar]
- 11.Kim H.J., Kwak J.Y., Choi J.W., Bae J.H., Shin K.M., Lee H.J. Impact of US surveillance on detection of clinically occult locoregional recurrence after mastectomy for breast cancer. Ann Surg Oncol. 2010;17(10):2670–2676. doi: 10.1245/s10434-010-1087-z. [DOI] [PubMed] [Google Scholar]
- 12.Moon H.J., Kim M.J., Kim E.K., Park B.W., Youk J.H., Kwak J.Y. US surveillance of regional lymph node recurrence after breast cancer surgery. Radiology. 2009;252(3):673–681. doi: 10.1148/radiol.2523081977. [DOI] [PubMed] [Google Scholar]
- 13.Suh Y.J., Kim M.J., Kim E.K., Moon H.J., Kim S.I., Park B.W. Value of ultrasound for postoperative surveillance of asian patients with history of breast cancer surgery: a single-center study. Ann Surg Oncol. 2013;20(11):3461–3468. doi: 10.1245/s10434-013-3020-8. [DOI] [PubMed] [Google Scholar]
- 14.Yoon J.H., Kim M.J., Kim E.K., Moon H.J. Imaging surveillance of patients with breast cancer after primary treatment: current recommendations. Korean J Radiol. 2015;16(2):219–228. doi: 10.3348/kjr.2015.16.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J.H., Kim E.K., Oh J.Y., Kwon H.C., Kim S.H., Kim D.C. US screening for detection of nonpalpable locoregional recurrence after mastectomy. Eur J Radiol. 2013;82(3):485–489. doi: 10.1016/j.ejrad.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Shin J.H., Han B.K., Choe Y.H., Nam S.J., Park W., Im Y.H. Ultrasonographic detection of occult cancer in patients after surgical therapy for breast cancer. J Ultrasound Med : official journal of the American Institute of Ultrasound in Medicine. 2005;24(5):643–649. doi: 10.7863/jum.2005.24.5.643. [DOI] [PubMed] [Google Scholar]
- 17.Simpson W., Neilson F., Young J.R. The identification of false negatives in a population of interval cancers: a method for audit of screening mammography. Breast. 1995;4(3):183–188. [Google Scholar]
- 18.Amos A.F., Kavanagh A.M., Cawson J. Radiological review of interval cancers in an Australian mammographic screening programme. J Med Screen. 2000;7(4):184–189. doi: 10.1136/jms.7.4.184. [DOI] [PubMed] [Google Scholar]
- 19.Royal Australian and New Zealand College of Radiologists . 2018. Breast imaging comparison and lesion classification lists.https://www.ranzcr.com/search/breast-imaging-grading-comparison-and-lesion-classification [Google Scholar]
- 20.Lee K.A., Talati N., Oudsema R., Steinberger S., Margolies L.R. BI-RADS 3: current and future use of probably benign. Curr Radiol Rep. 2018;6(2):5. doi: 10.1007/s40134-018-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr R.G., Zhang Z., Cormack J.B., Mendelson E.B., Berg W.A. Probably benign lesions at screening breast US in a population with elevated risk: prevalence and rate of malignancy in the ACRIN 6666 trial. Radiology. 2013;269(3):701–712. doi: 10.1148/radiol.13122829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Australia . 2001. Breast imaging - a guide for practice: cancer Australia. [Available from: https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/breast-imaging-guide-practice. [Google Scholar]
- 23.Park W.J., Kim E.K., Moon H.J., Kim M.J., Kim S.I., Park B.W. Breast ultrasonography for detection of metachronous ipsilateral breast tumor recurrence. Acta radiologica (Stockholm, Sweden. 1987;57(10):1171–1177. doi: 10.1177/0284185115618549. [DOI] [PubMed] [Google Scholar]
- 24.Tsai W.C., Wei H.K., Hung C.F., Kwang-Jane Lin C., Hung-Chun Cheng S., Chen C.M. Better overall survival for breast cancer patients by adding breast ultrasound to follow-up examinations for early detection of locoregional recurrence-A survival impact study. Ultrasound Med Biol. 2016;42(9):2058–2064. doi: 10.1016/j.ultrasmedbio.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 25.Wojcinski S., Farrokh A., Hille U., Hirschauer E., Schmidt W., Hillemanns P. Optimizing breast cancer follow-up: diagnostic value and costs of additional routine breast ultrasound. Ultrasound Med Biol. 2011;37(2):198–206. doi: 10.1016/j.ultrasmedbio.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 26.You J.K., Song M.K., Kim M.J., Kim E.K., Moon H.J., Youk J.H. Can biannual ultrasound surveillance detect smaller second cancers or detect cancers earlier in patients with breast cancer history? Ultrasound Med Biol. 2018;44(7):1355–1363. doi: 10.1016/j.ultrasmedbio.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Corsetti V., Houssami N., Ferrari A., Ghirardi M., Bellarosa S., Angelini O. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Canc. 2008;44(4):539–544. doi: 10.1016/j.ejca.2008.01.009. [DOI] [PubMed] [Google Scholar]