Abstract
Anaerobic digestion was one of the first bioenergy strategies developed, yet the interactions of the microbial community that is responsible for the production of methane are still poorly understood. For example, it has only recently been recognized that the bacteria that oxidize organic waste components can forge electrical connections with methane-producing microbes through biologically produced, protein-based, conductive circuits. This direct interspecies electron transfer (DIET) is faster than interspecies electron exchange via diffusive electron carriers, such as H2. DIET is also more resilient to perturbations such as increases in organic load inputs or toxic compounds. However, with current digester practices DIET rarely predominates. Improvements in anaerobic digestion associated with the addition of electrically conductive materials have been attributed to increased DIET, but experimental verification has been lacking. This deficiency may soon be overcome with improved understanding of the diversity of microbes capable of DIET, which is leading to molecular tools for determining the extent of DIET. Here we review the microbiology of DIET, suggest molecular strategies for monitoring DIET in anaerobic digesters, and propose approaches for re-engineering digester design and practices to encourage DIET.
Subject Areas: Chemistry, Energy Resources, Biotechnology, Energy Materials
Graphical Abstract
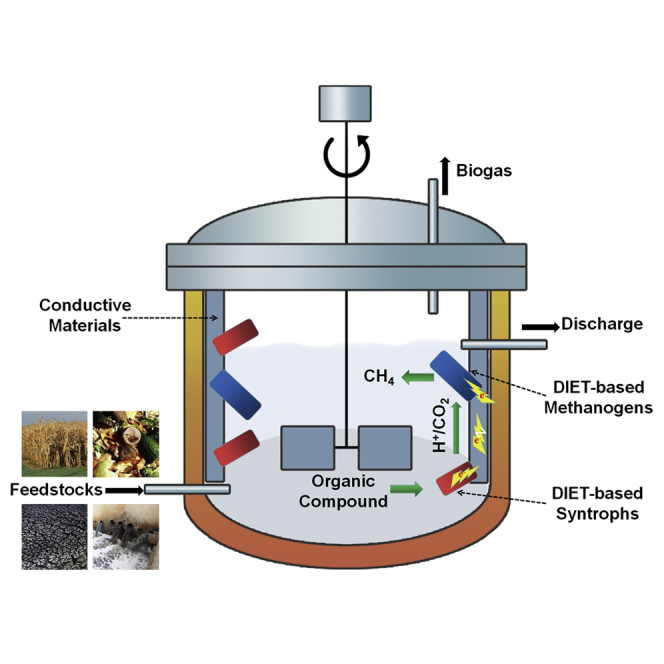
Chemistry; Energy Resources; Biotechnology; Energy Materials
Introduction
Anaerobic digestion (the conversion of organic wastes to methane) is one of the few economically successful large-scale bioenergy strategies (Appels et al., 2011; Holm-Nielsen et al., 2009; Mao et al., 2015). The three major factors limiting broader adoption of anaerobic digestion are slow rates of waste conversion to methane, low conversion efficiencies (50%–70% of theoretical methane yield for conventional digesters), and susceptibility to system disruption by toxins or system overloads (Chen et al., 2008; De Clercq et al., 2016; Holm-Nielsen et al., 2009). Anaerobic digestion has been practiced for hundreds of years (Meynell, 1982). Thus, the possibility of dramatically changing the performance of anaerobic digestion after all this time might have been considered slim.
However, simple strategies for accelerating, stabilizing, and increasing the efficiency of anaerobic digestion has recently been documented for a broad diversity of organic wastes. As detailed below, a diversity of inexpensive electrically conductive materials promotes methane production from many organic substrates. The most likely explanation for conductive materials enhancing anaerobic digestion process is that they facilitate direct interspecies electron transfer (DIET) between the bacteria contributing to the degradation of organics and the methane-producing archaea. Digester process designs that favor DIET have also been discovered. The possibility of rewiring the electrical connections between microbes offers substantial new opportunities for re-engineering and optimizing anaerobic digestion. The purpose of this review is to summarize the evidence that conductive materials improve anaerobic digestion, to discuss how this is related to the microbiology of DIET, and to emphasize urgent research and engineering needs that could lead to further improvements in the function of DIET-based anaerobic digesters.
Evidence for Conductive Materials Promoting Anaerobic Digestion
Over 80 studies have demonstrated that electrically conductive materials such as iron minerals, granular activated carbon, and biochar enhance anaerobic digestion (Tables 1 and S1 in Supplemental Information). In most instances, the evaluation of the impact of the conductive material was based solely upon comparisons with controls with no additions. However, in some instances controls of non-conductive materials were also included and the results demonstrated that the improvements in methane production could be attributed to the conductivity of the material (Dang et al., 2016, 2017; Guo et al., 2018; Liu et al., 2020; Mei et al., 2018; Wang et al., 2020a, 2020b; Zhang et al., 2017b; Zhao et al., 2017a, 2017c; Zhao and Zhang, 2019; Zhu et al., 2019).
Table 1.
Studies Reporting Stimulating Anaerobic Digestion to Methane with Conductive Materials
More detailed expanded table is available as Table S1.
Abbreviations: AnSBR, anaerobic sequencing batch reactor; CSTR, continuous stirred tank reactor; UASB, up-flow anaerobic sludge blanket; EGSB, expanded granular sludge blanket.
Designates control studies with non-conductive materials were conducted.
Conductive materials have enhanced methane production under a diversity of conditions. For example, methane production is often sensitive to low pH. Conductive materials can improve methane production in digesters under acidic conditions and also hasten the resumption of methane production once acidity is neutralized (Dang et al., 2016; Lei et al., 2019; Ren et al., 2020; Wang et al., 2017, 2018c, 2019b; Zhao et al., 2017c). Conductive materials also lessen the usual negative impacts of high ammonium concentrations on anaerobic digestion (Baek et al., 2016; Cheng et al., 2020; Lü et al., 2016; Shen et al., 2016; Wang et al., 2020b; Yan et al., 2020; Zhuang et al., 2018). High rates of organic loading can inhibit methane production through the accumulation of organic acids and ammonia. Conductive materials also help protect anaerobic digestion against these metabolic imbalances (Chen et al., 2020; Dang et al., 2016; Lei et al., 2016; Wang et al., 2019a, 2019b, 2020a; Yang et al., 2020; Zhang et al., 2018a, 2020c; Zhao et al., 2015).
Direct Interspecies Electron Transfer as the Likely Target for Conductive Materials
In order to most effectively take utmost advantage of the possibility of enhancing anaerobic digestion with conductive particles it is important to understand how conductive particles might influence methane-producing microbial communities. The elucidation of these stimulatory mechanisms can be evaluated within the existing paradigms for microbial community function.
Interspecies Hydrogen Transfer
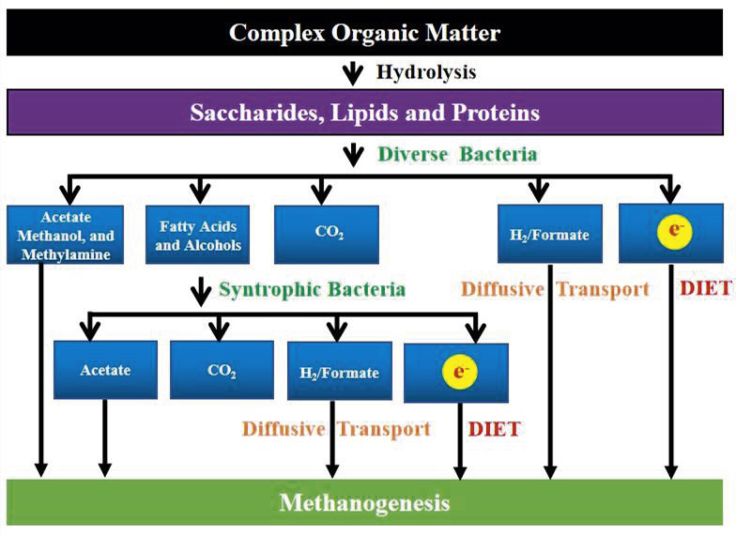
Overall, anaerobic digestion is the production of methane and carbon dioxide from multi-carbon substrates (Figure 1). Polymeric organic compounds must be hydrolyzed to simpler monomers. Methanogens are only able to directly metabolize mono-methyl compounds and acetate, and thus most of these organic monomers must be further metabolized down to acetate prior to methanogenesis. This typically requires further oxidation. Oxidation requires removal and disposal of electrons. The best known strategy for electron disposal in methanogenic communities is the reduction of protons to produce H2. A functional equivalent is the reduction of carbon dioxide to produce formate. Some methanogens can oxidize H2 and/or formate and use the electrons to reduce carbon dioxide to methane. Subsequent discussion will focus on H2 with the understanding that formate may substitute for H2.
Figure 1.
Schematic Diagram of Methane Formation in Anaerobic Digestion
Bacterial production of H2 from organic substrates and H2 consumption by methanogens, known as interspecies hydrogen transfer, has been the guiding principal for interspecies electron transfer methanogenic systems for over 50 years (Bryant et al., 1967; Kato and Watanabe, 2010; Sieber et al., 2012; Stams and Plugge, 2009). Much of the research focus on interspecies hydrogen transfer has been on the metabolism of non-fermentable compounds such as short- and long-chain fatty acids, alcohols, and aromatic compounds because metabolism of these compounds with the production of H2 is only thermodynamically feasible when H2-consuming methanogens maintain pace with the H2-producing bacteria and maintain H2 at very low levels. However, a substantial amount of H2 may also be produced from the fermentation of sugars and amino acids, which is less sensitive to high H2 concentrations.
Systems that depend on interspecies hydrogen transfer are susceptible to disruption. Trafficking in H2 is a relatively slow way to transfer electrons. It relies on diffusion. If H2 consumption fails to keep up with production, the system breaks down as non-fermentable intermediates, some of which are toxic to methanogens buildup. No engineering strategies to speed up interspecies hydrogen transfer are readily apparent. It is not apparent how the stimulation of methane production by conductive particles could be attributed to an impact on interspecies hydrogen transfer.
What if there was an alternative? Prior to 2010 data from studies on methanogenic communities were interpreted through the lens of interspecies hydrogen transfer, but there were results that suggested something else was going on. Most notably, a study of H2 turnover in anaerobic sludge found that H2 turnover rates could only account for 4.7% of the methane produced from carbon dioxide reduction to methane (Conrad et al., 1985). No studies have provided evidence for a greater contribution of H2 to interspecies electron transfer. As previously discussed (Walker et al., 2020), the most likely interpretation of these results is that H2 is not an important electron donor for methane production.
Direct Interspecies Electron Transfer
Direct interspecies electron transfer (DIET) is an alternative to interspecies hydrogen/formate transfer. In DIET, electron-donating partners pass electrons to electron-accepting partners through electrical contacts rather than producing intermediary soluble electron carriers, like H2 and formate, that carry electrons via diffusive transport (Figure 2). As previously reviewed in detail (Lovley, 2017c), conductive non-biological materials can often replace or enhance the electron transfer via the biologically produced proteins that are common conduits for DIET.
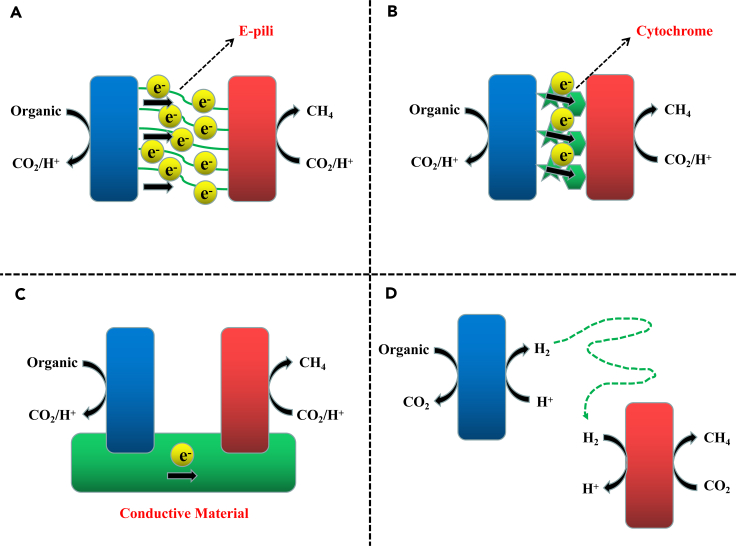
Figure 2.
Schematic Diagram of Interspecies Electron Transfer between Electron-Donating Bacteria (Blue) and Electron-Accepting Methanogens (Red)
DIET mechanisms: cell-to-cell electron transfer through electrically conductive pili (e-pili) (A), cell-to-cell electron transfer through cytochromes associated with outer cell surfaces (B), and cell-to-cell electron transfer through a conductive material (C). In contrast, cell-to-cell electron transfer via intermediary soluble electron carriers, like H2, is a diffusive transport process (D).
The general phenomena of DIET (Lovley, 2011; Summers et al., 2010) and the possibility of DIET in anaerobic digesters (Morita et al., 2011; Rotaru et al., 2014a) are relatively new concepts, but the potential importance of DIET is being increasingly recognized as evidenced by increased rapid increase in publications on this topic (Figure S1). The likely connection between the addition of conductive materials, which could potentially supplement biological electrical connections, DIET, and enhanced anaerobic digestion is readily apparent, and general practice has been to assume that better methane production means improved DIET. However, the evidence has often been circumstantial and indirect. For example, the metabolism of non-fermentable substrates such as propionate are less susceptible to inhibition by high H2 concentrations in the presence of conductive materials, suggesting that interspecies electron transfer is proceeding via DIET rather than interspecies hydrogen transfer (Cruz et al., 2014; Guo et al., 2020; Xu et al., 2016; Zhang et al., 2020c; Zhao et al., 2017c). In some instances the enhancement of methane production following the addition of conductive materials is associated with the enrichment of microbial populations for which pure culture representatives are known to participate in DIET (Guo et al., 2020; Lei et al., 2016; Lei et al., 2019; Lin et al., 2017; Ma et al., 2020a; Mei et al., 2018; Sun et al., 2020; Tian et al., 2017; Wang et al., 2018a, 2018b, 2019b; Xing et al., 2020; Xu et al., 2016; Yang et al., 2017; Zhang et al., 2018b, 2020a, 2020c; Zhao et al., 2016a, 2017c). However, in very few instances has the in situ physiological state of the microbial community been investigated in sufficient detail to verify the predominance of DIET (Rotaru et al., 2014a), and in those studies the impact of conductive materials was not investigated.
Therefore, in order to move beyond the empirical approach of assuming that enhanced methane production when conductive materials are added reflects an engineered shift toward more DIET, it will be necessary document how, why, and how much conductive materials actually improve DIET in different treatment systems. Only then with the information it will be available to knowledgably design even better anaerobic digestion strategies. Such an analysis will require a basic understanding of the microbiology of DIET and how its importance can be diagnosed in anaerobic digesters.
Microbiology of DIET
The study of DIET is still in its infancy with a rather limited understanding of which microorganisms can participate in DIET and the nature of the electrical connection between the microorganisms. Some insights into general mechanistic aspects of DIET are available from the few model microbes available in pure culture that have been studied in detail. However, it must be recognized that, as detailed below, different electrical contacts for DIET have independently evolved many times and thus the specific proteins that serve as electrical contacts in the currently available model microbes may not be found in other microbes that participate in DIET.
Bacterial Models for DIET
The electrical connections for DIET have been most extensively studied in Geobacter species. Geobacter are abundant and metabolically active in a diversity of methanogenic environments, including some anaerobic digesters (Morita et al., 2011; Rotaru et al., 2014a; Shrestha et al., 2014) and rice paddy soils (Holmes et al., 2017; Kato et al., 2012a; Li et al., 2015a; Rotaru et al., 2018). The first defined co-culture participating in DIET comprised G. metallireducens as the electron-donating partner and G. sulfurreducens as the electron-accepting partner in a medium with ethanol as the electron donor and fumarate as the electron acceptor (Summers et al., 2010). Much of the focus was on the electrical connections expressed by G. sulfurreducens because it is the most intensively studied Geobacter species and readily genetically manipulated for functional studies (Lovley et al., 2011). It is important to recognize that as an electron-accepting partner G. sulfurreducens differs significantly from methanogens in likely outer surface electrical contacts. The outer-surface electrical contacts involved in DIET in G. metallireducens does serve as a likely model for one approach to expressing electrical contacts in bacteria that serve as the electron-donating partner for DIET in methanogenic systems.
Electrically conductive pili (e-pili) are important electrical contacts for DIET in both G. metallireducens and G. sulfurreducens. The evolution of e-pili in Geobacter and closely related species as well as the features that confer conductivity has been previously reviewed in detail (Lovley, 2017a; Lovley and Holmes, 2020; Lovley and Walker, 2019) and will not be repeated here. Initial studies demonstrated that mutant strains of G. metallireducens and G. sulfurreducens that could not express their e-pili could not participate in DIET (Rotaru et al., 2014a, 2014b; Summers et al., 2010). Studies with Geobacter strains that expressed pili, but with greatly reduced conductivity, demonstrated that DIET was possible if the electron-accepting partner (G. sulfurreducens) lacked e-pili, but DIET with a strain of G. metallireducens that expressed poorly conductive pili was not possible (Ueki et al., 2018). Geobacter e-pili enable electron transfer over micrometer distances, and networks of interconnected e-pili confer conductivity to thick (up to ca. 100 μm) biofilms and large (millimeter diameters) cell aggregates (Malvankar et al., 2011, 2012; Morita et al., 2011; Summers et al., 2010).
e-Pili have independently evolved multiple times in bacteria (Walker et al., 2018, 2020). Of particular interest to anaerobic digestion is the finding that Syntrophus aciditrophicus expresses e-pili (Walker et al., 2020). S. aciditrophicus has been intensively studied as a model organism for the metabolism of non-fermentable substrates via interspecies H2 (or formate) electron transfer in methanogenic environments (Sieber et al., 2012, 2014). However, S. aciditrophicus expresses e-pili with a conductance comparable with G. sulfurreducens and can grow via DIET (Walker et al., 2020). Many of the other microbes also known to grow via interspecies hydrogen transfer have genes expected to yield e-pili, suggesting that the capacity for DIET is widespread among microbes that metabolize non-fermentable substrates in anaerobic digesters and other methanogenic environments (Walker et al., 2020).
Another important component in DIET for G. metallireducens and G. sulfurreducens is multi-heme c-type cytochromes that are exposed on the outer surface of the cell. Strains in which some of these cytochrome genes have been deleted are unable to participate in DIET (Rotaru et al., 2014a; Summers et al., 2010). Only one of these c-type cytochromes, the OmcS cytochrome of G. sulfurreducens, has been studied in detail (Filman et al., 2019; Leang et al., 2010; Mehta et al., 2005; Qian et al., 2011). As previously reviewed in detail (Lovley and Walker, 2019), OmcS may be associated with the outer surface of the cell, attached to e-pili, and may even form its own conductive filaments. More studies on the role of outer surface c-type cytochromes in DIET are needed, especially in microbes like G. metallireducens that can serve as an electron-donating partner. Furthermore, the ability of S. aciditrophicus, which lacks outer-surface cytochromes, to participate in DIET demonstrates that c-type cytochromes are not an absolute requirement for microbes to function as the electron-donating partner for DIET (Walker et al., 2020).
Methanogens Participating in DIET
The electrical contacts on methanogens are more enigmatic. Methanothrix harundinaceae, the first methanogen found to directly accept electrons in pure culture (Rotaru et al., 2014a), lacks c-type cytochromes. Several Methanosarcina species, including M. barkeri, M. horonobensis, M. mazeii, M. subterranean, and M. vacuolate, have also been shown to also participate in DIET (Rotaru et al., 2014b; Yee and Rotaru, 2020), but none of these species are known to display c-type cytochromes on their outer surface.
The archaella of archaea are homologous to the type IV pili that have evolved into e-pili in bacteria, and the one methanogen archaellum that has been examined appears to be more conductive than G. sulfurreducens e-pili (Walker et al., 2019). However, this archaellum was from Methanospirillum hungatei, which could not be grown via DIET with G. metallireducens as the electron-donating partner (Rotaru et al., 2014a). As noted above, a strain of G. sulfurreducens that expressed poorly conductive pili could serve as the electron-accepting partner in co-cultures in which the electron-donating partner (G. metallireducens) expressed e-pili (Ueki et al., 2018). By analogy it seems likely that methanogens could also function as the electron-accepting partner in DIET without electrically conductive archaella. Energy conservation in methanogens with DIET as the sole potential energy source has been demonstrated (Wang et al., 2016), and mechanisms to describe methane production and energy conservation from the reduction of carbon dioxide to methane with electrons received via DIET have been described (Holmes et al., 2018). However, how those electrons enter the cell remains a mystery. Additional information on the mechanisms for extracellular electron exchange in methanogens could aid in further tailoring conductive materials to further enhance anaerobic digestion and provide additional molecular targets for diagnosing the prevalence of DIET.
The Role of Conductive Materials in Defined Culture Systems
Carbon Materials
The potential role of electrically conductive carbon materials and the conductive mineral magnetite in DIET has been studied with defined co-cultures, and the function of these materials have been evaluated with mutant strains in which genes for key extracellular electron transfer constituents were deleted. For example, when granular activated carbon (GAC) was added to medium in which growth of G. metallireducens and G. sulfurreducens was only possible via DIET, there was little or no lag in metabolism and growth (Liu et al., 2012). This is in contrast to the long lag periods (30–35 days) before DIET was established in the absence of GAC. Glass beads did not promote DIET. A similar stimulation of DIET was observed with carbon cloth (Chen et al., 2014a) and biochar (Chen et al., 2014b), two other conductive carbon materials. Poorly conductive cotton cloth did not promote DIET, and conductive carbon cloth did not have a stimulatory effect on a co-culture in which Desulfovibrio vulgaris (a microbe that can participate in interspecies hydrogen transfer, but not DIET) was substituted for G. metallireducens as the electron-donating partner (Chen et al., 2014a). GAC also greatly accelerated the initiation of DIET in defined co-cultures of G. metallireducens and M. barkeri (Rotaru et al., 2014b) and in samples from a laboratory digester in which Geobacter and Methanothrix species were involved in DIET (Liu et al., 2012).
Conductive carbon materials with particle sizes larger than cells appear to function as an electrical grid connecting electricity generation (the electron-donating partners) with electricity consumers (the electron-accepting partners). Both partners attach to the conductive carbon material rather than making cell-to-cell electrical connections (Figure 3). The simplicity of the partners attaching anywhere on a conductive carbon material versus two or more species locating each other and establishing direct contact may be one of the factors leading to much faster establishment of DIET with conductive carbon materials.
Figure 3.
Scanning Electron Micrograph of a Syntrophic Co-culture of G. metallireducens (Rods) and M. barkeri (Spheres) with Biochar
Image reprinted with permission from Chen et al. (2014b).
Another mechanism by which conductive carbon materials may facilitate DIET is by reducing the need for microbes to express an extensive array of extracellular electrical connects. Although mutant strains that lacked e-pili or key outer-surface c-type cytochromes were inhibited in DIET, these same strains readily participated in DIET in the presence of electrically conductive carbon materials (Chen et al., 2014a; Liu et al., 2012; Rotaru et al., 2014b). The reduced requirement for biological extracellular electrical contacts in the presence of conductive carbon materials further illustrates that greater simplicity of different species establishing electrical connections when the cells have the option to plug into a large conductive surface that can be shared with substantial numbers of electron-donating and electron-accepting cells.
Furthermore, eliminating the need to produce abundant e-pili in order to increase the odds of making contact with an electron-accepting partner may yield a substantial energetic advantage. The high conductivity of e-pili is associated with a greater abundance of aromatic amino acids than found in poorly conductive pili (Lovley, 2017a; Lovley and Holmes, 2020; Lovley and Walker, 2019). Biosynthesis of aromatic amino acids requires substantial metabolic energy, a cost to the cell that can be avoided when cells can attach to conductive carbon materials. The combined effects of simplifying the establishment of interspecies electrical connections and reducing the metabolic costs to make those connections are likely contributors to enhanced DIET in the presence of conductive carbon materials.
Magnetite
Nanoparticles of the conductive iron mineral magnetite stimulated methane production in enrichment cultures initiated with rice paddy soils with an accompanying enrichment of Geobacter and Methanosarcina species, suggesting an “electric syntrophy” in which magnetite served as a conduit for interspecies electron transfer (Kato et al., 2012a). Magnetite greatly enhanced DIET in defined co-cultures of G. sulfurreducens and Thiobacillus denitrificans (Kato et al., 2012b), G. metallireducens and G. sulfurreducens (Liu et al., 2015), and G. metallireducens and M. barkeri (Wang et al., 2018d).
The available evidence suggests that the mechanism for magnetite stimulation is different from that for conductive carbon materials (Liu et al., 2015; Ueki et al., 2018). In addition to composition, a major difference between magnetite and conductive carbon materials is size. The conductive carbon materials examined in defined co-cultures are much larger than cells, whereas the typical size of magnetite (20–50 nm) is much smaller. At best, each magnetite crystal contacts a small proportion of an individual cell’s surface and two cells would need to be within nanometers of each other to be simultaneously in contact with the same magnetite crystal. This contrasts with conductive carbon materials facilitate long range (hundreds of micrometers or more) electron exchange between DIET partners. It is conceivable that multiple magnetite particles could agglomerate to form larger particles or chains, but such formations have not been observed in the transmission electron micrographs of co-cultures in which DIET was promoted with magnetite (Kato et al., 2012b; Liu et al., 2015; Wang et al., 2018d). Some added magnetite adsorbs to cell surfaces, but in co-cultures of G. metallireducens as the electron-donating partner and either G. sulfurreducens or M. barkeri as the electron-accepting partner, much of the magnetite is specifically associated with pili (Liu et al., 2015; Wang et al., 2018d).
The association of magnetite with pili is notable because, in contrast to conductive carbon materials (Chen et al., 2014a; Rotaru et al., 2014b; Ueki et al., 2018), the addition of magnetite cannot restore the capacity for DIET when Geobacter cannot express e-pili or expresses poorly conductive pili (Liu et al., 2015; Ueki et al., 2018). This result is consistent with the concept that magnetite crystals are too small to enable long-range electron transport. Magnetite additions did enable DIET in a G. sulfurreducens strain deficient in OmcS that did not grow via DIET without magnetite (Liu et al., 2015). This result suggests that magnetite might replace a proposed function of OmcS, which is facilitating electron transport between e-pili and the external environment. The addition of magnetite to DIET-based co-cultures with wild-type G. sulfurreducens repressed expression of the OmcS gene (Liu et al., 2015), further suggesting that magnetite could substitute for OmcS and that the cell sensed the reduced need for OmcS.
The size of magnetite, the lack of evidence for long magnetite chains in DIET-based co-cultures, and the ability of magnetite to rescue OmcS deficient, but not e-pili deficient, mutants suggest that the electron transfer capabilities of magnetite enable it to substitute for outer-surface c-type cytochromes in DIET. The differences between conductive carbon materials and magnetite in their mechanisms for stimulating DIET are significant, and thus strategies to promote anaerobic digestion with conductive carbon materials may not be effective with magnetite and vice versa.
Potential Reasons for DIET Stabilizing and Accelerating Anaerobic Digestion
Owing to the limited understanding of the routes for electron transfer during DIET, the reasons why promoting DIET can accelerate and stabilize anaerobic digestion are still somewhat a matter of speculation. The potential energy yield from the overall reaction of the conversion of organic substrates to methane is the same with DIET and when H2/formate serve as an intermediary electron carrier. Increased energy conservation may be available to DIET partners because electron transfer via DIET is more direct with fewer electron transfer steps incurring energy losses (Lovley, 2011). However, the key comparative studies on energy conservation and cells yields in support of this speculation have yet to be conducted (Lovley, 2017c). Furthermore, high efficiency in energy conservation may not be the prime consideration (Lovley, 2011). Energetic considerations must also include the energy that DIET partners need to invest to establish their extracellular electrical connections and the relative reduction in this cost when conductive materials are added (Lovley, 2017b).
Once interspecies electrical connections are established, electron transfer via DIET should be faster than diffusive electron exchange via soluble electron shuttles. DIET is also less sensitive to the accumulation of H2 (Cruz et al., 2014; Guo et al., 2020; Xu et al., 2016; Zhang et al., 2020c; Zhao et al., 2017c). Thus, DIET is expected to be especially beneficial when rates of organic loading are high and more rapid metabolism of fermentable substrates releases H2 at elevated levels. As more data become available it may be possible to construct mathematical models that can quantify the cost/benefits of DIET versus interspecies H2 transfer under different environmental conditions, as well as the benefits of conductive material amendments, providing a valuable tool for anaerobic digester design. However, there are not yet enough data on the fundamental parameters necessary to build reliable/predictive models (Lovley, 2017c).
Diagnosing Success in Promoting DIET
The rational design of strategies to improve anaerobic digestion by promoting DIET will require methods for quantifying the degree to which DIET has been stimulated. An underlying assumption in many studies appears to be that, prior to the addition of conductive materials, interspecies hydrogen transfer predominates in most anaerobic digester samples. However, as discussed previously, in general, there is no supporting evidence for this assumption, which is a remnant of a time prior to the discovery of DIET. In some instances, such as the treatment of relatively simple brewery wastes in UASB digesters, DIET is prevalent prior to the addition of conductive materials, as evidenced with detailed analysis of the composition and gene expression of the microbial community (Morita et al., 2011; Rotaru et al., 2014a; Shrestha et al., 2014). The prevalence of DIET in other types of digesters is not well understood. The degree to which methanogenesis is stimulated with conductive materials is not a reliable indication of the extent of DIET prior to amendment because, even when DIET appears to be the predominant route for interspecies electron exchange, the addition of conductive materials can further accelerate methanogenesis (Liu et al., 2012).
In the current early stages of DIET studies stimulation of methane production following addition of electrically conductive carbon materials or magnetite is typically attributed to promotion of DIET based on extrapolation from the results with defined co-cultures in which DIET can be verified in greater detail. In some instances, enhanced methane production is associated with an increased enrichment of microbes closely related to microbes that are available in pure culture that are known to participate in DIET, such as Geobacter, Syntrophus, Methnothrix, and Methanoarcina species (Baek et al., 2016; Barua and Dhar, 2017; Guo et al., 2020; Kato et al., 2012a; Lei et al., 2019; Lin et al., 2020; Ma et al., 2020a; Mei et al., 2018; Shen et al., 2020; Tian et al., 2017; Wang et al., 2018a, 2018b, 2019b; Xing et al., 2020; Xu et al., 2015, 2016; Yan et al., 2018; Yang et al., 2017; Zhang et al., 2018b, 2020c; Zhao et al., 2016a, 2017c; Zhuang et al., 2015b). However, in many other studies the addition of conductive materials to samples from anaerobic digesters or other methanogenic environments has led to the enrichment of other bacteria and methanogens (Baek et al., 2015; Barua et al., 2018; Fu et al., 2018; Guo et al., 2018, 2020; Hu et al., 2017; Im et al., 2019; Jing et al., 2017; Lei et al., 2016, 2018; Lin et al., 2018; Luo et al., 2015; Ma et al., 2020b; Mostafa et al., 2020; Ren et al., 2020; Rotaru et al., 2018; Sun et al., 2020; Tan et al., 2015; Usman et al., 2019; Wang et al., 2019a, 2020a; Xia et al., 2019; Xu et al., 2018; Yamada et al., 2015; Yang et al., 2017; Yan et al., 2018; Yang et al., 2015, 2016, 2020; Yin et al., 2017a, 2017b, 2018; Zhang et al., 2018b, 2020a; Zhang and Lu, 2016; Zhuang et al., 2015a) (Table S2). Known characteristics of some of the enriched microbes further support the possibility that they are involved in DIET. For example, Syntrophus, Smithella, Syntrophobacter, Syntrophorhabdus, Syntrophomonas, Desulfatibacillum, Syntrophaceticus, Flexistipes, Desulfurivibrio, and Calditerrivibrio (Walker et al., 2018, 2020) have genes likely to yield e-pili, and several of these microbes were enriched in studies in which conductive materials were added to methanogenic samples (Baek et al., 2016; Capson-Tojo et al., 2018; Fu et al., 2018; Guo et al., 2020; Guo et al., 2020, 2020; Lei et al., 2016; Lim et al., 2020; Luo et al., 2015; Ma et al., 2020a, 2020b; Mostafa et al., 2020; Wang et al., 2019b, 2020a; Xia et al., 2019; Xing et al., 2020; Xu et al., 2018; Zhang et al., 2018b, 2020c; Zhang and Lu, 2016) (Table S2). However, as previously discussed in detail (Lovley, 2017c), the mechanisms for electron exchange via DIET may be diverse, and more studies are required to further identify the diversity of bacteria capable of functioning as electron-donating partners for DIET.
In a similar manner, the diversity of methanogens capable of DIET probably extends well beyond the Methanothrix and Methanosarcina species, which have been shown to participate in DIET in defined co-culture studies (Rotaru et al., 2014a, 2014b; Yee and Rotaru, 2020). A broad range of methanogenic genera, including Methanobacterium, Methanospirillum, Methanocella, Methanoregula, Methanolinea, Methanosphaerula, Methanomassiliicoccus, Methanothermobacter, and Methanoculleus species, were enriched with the addition of electrically conductive materials and inferred to be capable of accepting electrons via DIET (Capson-Tojo et al., 2018; Chowdhury et al., 2019; Fu et al., 2018; Guo et al., 2018, 2020; Im et al., 2019; Jing et al., 2017; Lei et al., 2016; Lei et al., 2016; Lin et al., 2017, 2018; Ma et al., 2020b; Tian et al., 2017; Xia et al., 2019; Yang et al., 2016; Zhang and Lu, 2016; Zhang et al., 2018b; Zhuang et al., 2015a) (Table S2). The inability of Methanobacterium formicum and Methanospirillium hungateti to grow in defined co-culture with G. metallireducens as the electron-donating partner argues against some of these methanogens being capable of DIET (Rotaru et al., 2014a). However, it must be considered that the available pure cultures of these methanogens were enriched, cultured, and maintained for years with H2 or formate as the electron donor and may have lost their ability to participate in DIET. For example, enrichment conditions that favored DIET yielded a Methanobacterium species capable of DIET with G. metallireducens as the electron-donating partner. Therefore, it is not possible to classify microbes in terms of their capacity to participate in DIET based solely on the currently available analysis of a limited number of laboratory isolates.
Studies with defined co-cultures have focused on the limited subset of non-fermentable substrates such as short-chain fatty acids, alcohols, and aromatic compounds (Rotaru et al., 2014a, 2014b; Summers et al., 2010; Walker et al., 2020; Wang et al., 2016). However, a broad range of organic compounds are metabolized faster when DIET in methanogenic mixed-species systems is promoted with conductive materials in Table S2. These include glycerol (Im et al., 2019), oleic acid (Mostafa et al., 2020), glucose (Guo et al., 2018, 2020; Luo et al., 2015; Ren et al., 2020; Tian et al., 2017; Xu et al., 2016; Yang et al., 2017; Zhang et al., 2020b), sucrose (Hu et al., 2017; Wang et al., 2018a, 2019a, 2019b), phenol (Yan et al., 2018), tryptone (Yin et al., 2017a, 2017b, 2018), complex mixtures of fructose and polyethylene glycol (Mei et al., 2018), hydrocarbons (Usman et al., 2019; Yang et al., 2020), and N-heterocyclic compounds (Wang et al., 2018b), dairy waste (Baek et al., 2016), whey (Baek et al., 2015), leachate (Lei et al., 2016, 2018, 2019), dog food (Dang et al., 2016, 2017), kitchen waste (Capson-Tojo et al., 2018; Lim et al., 2020; Zhang et al., 2018a), waste activated sludge (Li et al., 2018; Sun et al., 2020; Wang et al., 2020d; Yang et al., 2017), corn straw (Zhu et al., 2019), and cyanobacterial biomass (Tan et al., 2015). The possibility that these substrates directly serve as substrates for DIET will require more in-depth microbiological investigations.
As more is learned about the scope of DIET in the microbial world it should be possible to estimate the importance of DIET in anaerobic digesters with metatranscriptomic analysis that identifies the levels of expression for key genes diagnostic of DIET. The promise of this approach is evident from transcriptomic analysis of DIET in defined co-cultures (Holmes et al., 2018; Rotaru et al., 2014a; Shrestha et al., 2013), rice paddy soils (Holmes et al., 2017), and a UASB reactor (Rotaru et al., 2014a). Ideally, advanced analytical techniques capable of directly quantifying in situ fluxes of interspecies electron transfer as well as H2 and formate turnover will be developed so that the relative importance of DIET and interspecies hydrogen (formate) transfer can be determined.
Engineering a Better DIET-Based Digester
Alternative Strategies to Favor DIET
The inclusion of conductive materials is likely to be just one component of the best strategies for enhancing anaerobic digestion. The prevalence of DIET in UASB digesters treating ethanol (Morita et al., 2011; Rotaru et al., 2014a; Shrestha et al., 2014) even without the addition of conductive materials suggests that strategies that retain biomass within the digester may provide time for biological interspecies electrical connections to be established and justify the biological energy expenditure to synthesize these additional proteins. Also, the fact that ethanol is a good substrate for establishing DIET-based communities should be considered in digester start-up. For example, UASB digesters initially developed with ethanol feed to establish DIET-based communities formed highly conductive aggregates and metabolized subsequent propionate and butyrate inputs faster than digesters that were only fed with propionate or butyrate (Zhao et al., 2016b).
It is uneconomical to continuously feed digesters to promote DIET. An alternative strategy is to pretreat complex organic wastes to favor ethanol production. This approach has been evaluated with dairy wastes (Zhao et al., 2017a), kitchen wastes (Zhao et al., 2020), and corn straw (Zhu et al., 2019). For example, UASB digesters fed with dairy wastes pretreated with an ethanol-producing fermentation (ethanol content 15%–20%) could tolerate higher organic loading rates (53–79.5 kgCOD [chemical oxygen demand]/m3/day) versus control digesters (13.25–39.75 kgCOD/m3/day) and methane conversion efficiency was increased to more than 85% (Zhao et al., 2017a). Other studies also noted the increased energy yield derived from an ethanol fermentation pretreatment (Yu et al., 2018; Calicioglu and Brennan, 2018; Dererie et al., 2011; Wu et al., 2015), but the possibility of DIET enhancement was not considered.
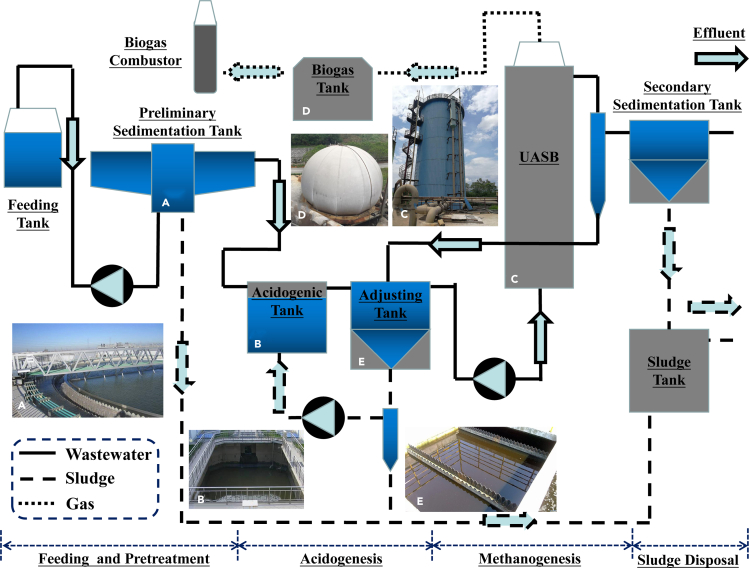
Ethanol fermentation pretreatment is optimal at pH 4.0–4.5 (Ren et al., 1997), too low for effective methanogenesis. However, in a full-scale anaerobic digestion system, the excessive acidity can be alleviated by mixing with a fraction of the effluent of the anaerobic digester (neutral reflux liquid) with the acidic ethanol-rich feed (Zhao and Zhang, 2019). This relatively simple, low-cost design (Figure 4) does not require any changes to internal structure of the anaerobic digester.
Figure 4.
Flow Diagram of a Full-Scale Anaerobic Digestion System Treating Bagasse Wastes
Image reprinted with permission from Zhao and Zhang (2019).
Economically Providing Conductive Surfaces in Full-Scale Digesters
Although laboratory studies have routinely mixed conductive materials into small-scale batch systems (Figure 5), this approach is not scalable economically. In a study evaluating this approach, granular activated carbon (15 g/L, 0.037–0.149 mm) was mixed with kitchen wastes that were then fed to a semi-continuous-flow pilot-scale digester for methane production, increasing the methane production rate by 40% (Zhang et al., 2018a). However, the daily loss of granular activated carbon in the digester discharge (0.345 kg) needed to be replenished each day (Zhang et al., 2018a). The costs of maintaining the granular activated carbon levels (0.97–2.07 Yuan/gVSS [volatile suspended solid]/day) account for 38.3%–81.8% of the income expected from the methane produced (approximately 2.53 Yuan/gVSS/day). Furthermore, additional costs would be associated with the labor and equipment for the daily additions of granular activated carbon. DIET has been successfully stimulated in laboratory studies with the addition of carbon nanotubes and graphene oxides (Li et al., 2015b; Lin et al., 2017, 2018; Mostafa et al., 2020; Shen et al., 2020; Tian et al., 2017; Yang et al., 2017) but cost more than granular activated carbon. Biochar, which costs ca. 15%–35% as much as granular activated carbon, would help the economics. However, there is no apparent method for removing either biochar or granular activated carbon from the digester discharge, and thus their use will increase the final solid volume of waste residues. Therefore, application of carbon-based conductive materials with the particle sizes that have typically been employed in laboratory studies seems unlikely to be an economically feasible strategy for improving full-scale anaerobic digestion.
Figure 5.
Image of a Semi-Continuous-Flow Pilot-Scale Anaerobic Digester Treating Kitchen Wastes with Granular Activated Carbon
Image reprinted with permission from Zhang et al. (2018a).
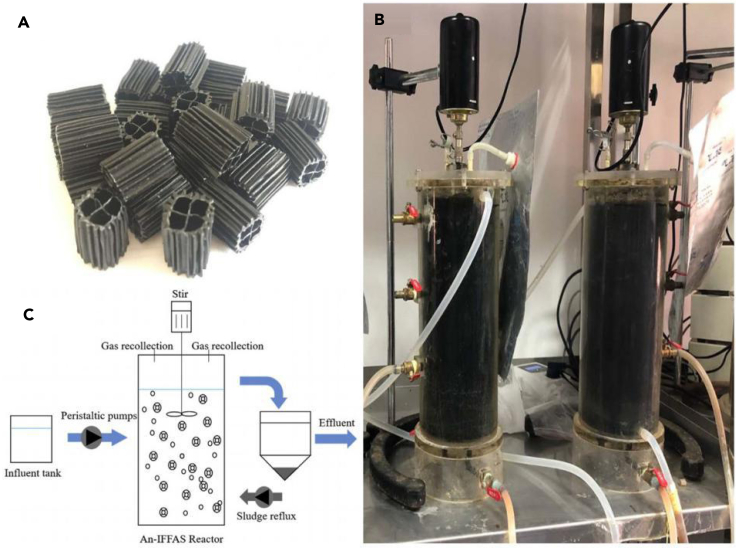
More promising are approaches that retain conductive materials within digesters, such as increasing their size. The addition of larger particles of granular activated carbon provided greater resilience to high rates of organic loading in UASB digesters than smaller particles (Xu et al., 2015) despite the fact that larger particles are expected to have less surface area per volume than smaller particles. Graphite powder mixed with high-density polyethylene formed yielded conductive carriers, suspended by hydraulic mixing and external reflux (Figure 6), that increased methane production rates from brewery wastes by 7.8%–23.3% over controls with non-conductive high-density polyethylene suspended carriers (Liu et al., 2020).
Figure 6.
Study Reporting a Laboratory-Scale Moving-Bed Anaerobic Digester with Graphite-Based Suspended Carriers
Images of graphite-based suspended carriers (A), a laboratory-scale moving-bed anaerobic digester (B), and schematic diagram of the moving-bed anaerobic digestion process (C). Image (C) reprinted with permission from Liu et al. (2020).
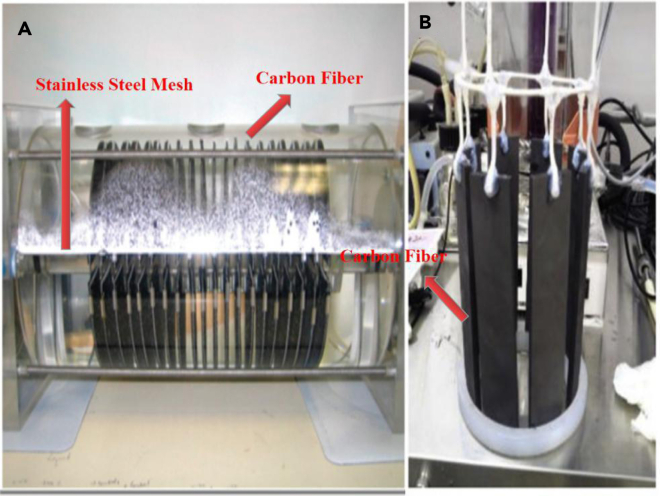
Carbon cloth, felt, or fiber is amendable to incorporation in anaerobic digesters. Carbon fiber has been employed as a packed bed for biomass retention in continuous-flow anaerobic digesters (Sasaki et al., 2007; Tatara et al., 2005, 2008; Zhang et al., 2012) and bioelectrochemical reactors (Cheng et al., 2011; Sasaki et al., 2013), but could have the added benefit of providing a conduit for DIET. High mechanical strength and corrosion-resistant materials such as stainless steel mesh can serve as the physical support to fix the carbon fiber sheets to the top or bottom of the digester (Figure 7).
Figure 7.
Study Reporting Laboratory-Scale Bioelectrochemical Reactors with Carbon Fiber
Images of carbon fiber sheets horizontally (A) or vertically (B) physically supported by stainless steel mesh. Image (A) reprinted with permission from Cheng et al. (2011). Image (B) reprinted with permission from Sasaki et al. (2013).
Carbon cloth is less expensive than carbon felt and carbon fiber and enhanced the stability of continuous-flow laboratory-scale methanogenic digesters treating the non-fermentable substrates such as ethanol (Zhao et al., 2015) and butanol (Zhao et al., 2017c), leachate (Lei et al., 2016), and dog food (Dang et al., 2016). Carbon cloth promoted methane production from dog food better than carbon felt with the same geometry (Dang et al., 2016). The cost of the one-time addition of carbon cloth was only 0.84–1.27 Yuan/L (working volume of digester), which corresponds to the 200- to 300-day income derived from the biogas production under the organic loading rate of 8.5 kgCOD/m3/day (Dang et al., 2016). Therefore, from the perspective of a long-term economic and technical feasibility, carbon cloth may be the best of carbon-based conductive materials that can be used and designed as an electron conduit to permanently promote DIET in a full-scale anaerobic digester.
Although magnetite has shown promise in promoting methane production in continuous-flow systems (Baek et al., 2016; Lei et al., 2018; Wang et al., 2018b, 2019b), there are also issues with retaining magnetite for long-term operations. There is no apparent method for recycling magnetite from the digester discharge, and in some instances, an equal amount of magnetite was periodically supplemented into the digester to compensate for the loss during the sludge withdrawal period (Wang et al., 2018b; Yin et al., 2017a, 2018). A potential strategy to avoid magnetite loss is to modify magnetite loaded with a porous and biocompatible carrier (Song et al., 2019). Another source of magnetite loss can be microbial reduction of the Fe(III) in magnetite, which destroys the crystalline structure (Wang et al., 2019b). In one study the diameter of large particles of aggregated magnetite derived from industrial waste residues declined over 100 days of digester operation from 10–15 mm to 8–13 mm (Zhao et al., 2017b), whereas in another study (with similarly sized magnetite, 10–15 mm) no magnetite was detected after 56 days (Peng et al., 2018). Therefore, magnetite does not appear to be a good solution for long-term improvement of methane production in full-scale anaerobic digesters.
Outlook
The finding that promoting DIET improves methane yields and increases resilience against instabilities has suggested that re-engineering anaerobic digesters to favor DIET will enhance the effectiveness of this important bioenergy strategy. To date, investigations on the impact of amendments of conductive materials on DIET have primarily been empirical. The improvements in methane production observed are generally attributed to a shift in electron flow toward more DIET, but the evidence has been circumstantial at best. In the absence of suitable technologies for directly tracking the flow of electrons in methanogenic microbial communities, molecular analyses such as metatranscriptomics are likely to be the best approach for documenting the extent of DIET. The potential for diagnosing DIET with metatranscriptomics has already been demonstrated in anaerobic digesters in which a limited diversity of microbes was metabolizing one simple primary substrate (Rotaru et al., 2014a). It will be more challenging to diagnose DIET with metatranscriptomics for systems anaerobically digesting more complex wastes because the diversity of microorganisms and metabolic pathways is much greater. Development of the paradigms for DIET diagnosis in such systems will require basic research to better understand the diversity of bacteria and methanogens that have the potential to participate in DIET and the mechanisms by which these microbes participate in extracellular electron exchange. The insights derived from such research is also likely to lead to new concepts for the design of new materials to enhance DIET.
Improved engineering of anaerobic digestion, based on existing information, should proceed in parallel with the suggested microbiological studies with a focus on technologies that are sustainable and economically feasible for long-term operation. A holistic approach that considers physical/chemical modifications to favor DIET-based communities as well as the deployment of conductive materials is likely to be most effective. For example, co-digestion of wastes with a high saccharide content likely to yield ethanol with other types of wastes could provide an ethanol “spiked” fuel likely to promote DIET-based communities. Digester designs should consider that conductive materials are only likely to be economical if they are a permanent fixture within the digester.
This is an exciting time in anaerobic digestion. Modern tools of microbiology and engineering show promise for advancing the understanding of the function of this early bioenergy technology and improving its effectiveness to enhance the sustainable conversion of wastes to energy.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors acknowledged Mr. Jifu Liu of Dalian University of Technology and Dr. Jingxin Zhang of National University of Singapore for providing the images of their experimental equipment. Z.Z., Y.L., and Y.Z. acknowledge the financial support from National Scientific Foundation of China (51808097), State Key Research and Development Plan of China (2018YFC1900901), and Liaoning Scientifific Technological Major Project (2019JH1/10300001).
Author Contributions
Conceptualization, Z.Z., Y.Z., and D.R.L.; Literature, Z.Z. and Y.L.; Figure and Table, Z.Z. and Y.L.; Writing - Original Draft, Z.Z. and D.R.L.; Review and Editing, Z.Z., Y.Z., and D.R.L.
Declaration of Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101794.
Supplemental Information
References
- Appels L., Lauwers J., Degrève J., Helsen L., Lievens B., Willems K., Van Impe J., Dewil R. Anaerobic digestion in global bio-energy production: potential and research challenges. Renew. Sustain. Energ. Rev. 2011;15:4295–4301. [Google Scholar]
- Baek G., Kim J., Cho K., Bae H., Lee C. The biostimulation of anaerobic digestion with (semi)conductive ferric oxides: their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015;99:10355–10366. doi: 10.1007/s00253-015-6900-y. [DOI] [PubMed] [Google Scholar]
- Baek G., Kim J., Lee C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent-Enhancement in process performance and stability. Bioresour. Technol. 2016;222:344–354. doi: 10.1016/j.biortech.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Barua S., Zakaria B.S., Dhar B.R. Enhanced methanogenic co-degradation of propionate and butyrate by anaerobic microbiome enriched on conductive carbon fibers. Bioresour. Technol. 2018;266:259–266. doi: 10.1016/j.biortech.2018.06.053. [DOI] [PubMed] [Google Scholar]
- Barua S., Dhar B.R. Advances towards understanding and engineering direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017;244:698–707. doi: 10.1016/j.biortech.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Bryant M.P., Wolin E.A., Wolin M.J., Wolfe R.S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch. Mikrobiol. 1967;59:20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Calicioglu O., Brennan R.A. Sequential ethanol fermentation and anaerobic digestion increases bioenergy yields from duckweed. Bioresour. Technol. 2018;257:344–348. doi: 10.1016/j.biortech.2018.02.053. [DOI] [PubMed] [Google Scholar]
- Chen Q., Liu C., Liu X., Sun D., Li P., Qiu B., Dang Y., Karpinski N.A., Smith J.A., Holmes D.E. Magnetite enhances anaerobic digestion of high salinity organic wastewater. Environ. Res. 2020;189:109884. doi: 10.1016/j.envres.2020.109884. [DOI] [PubMed] [Google Scholar]
- Chen S., Rotaru A., Liu F., Philips J., Woodard T.L., Nevin K.P., Lovley D.R. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour. Technol. 2014;173:82–86. doi: 10.1016/j.biortech.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Chen S., Rotaru A., Shrestha P.M., Malvankar N.S., Liu F., Fan W., Nevin K.P., Lovley D.R. Promoting interspecies electron transfer with biochar. Sci. Rep. 2014;4:5019. doi: 10.1038/srep05019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capson-Tojo G., Moscoviz R., Ruiz D., Santa-Catalina G., Trably E., Rouez M., Crest M., Steyer J., Bernet N., Delgenès J., Escudié R. Addition of granular activated carbon and trace elements to favor volatile fatty acid consumption during anaerobic digestion of food waste. Bioresour. Technol. 2018;260:157–168. doi: 10.1016/j.biortech.2018.03.097. [DOI] [PubMed] [Google Scholar]
- Chen Y., Cheng J.J., Creamer K.S. Inhibition of anaerobic digestion process: a review. Bioresour. Technol. 2008;99:4044–4064. doi: 10.1016/j.biortech.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Cheng K.Y., Ho G., Cord-Ruwisch R. Novel methanogenic rotatable bioelectrochemical system operated with polarity inversion. Environ. Sci. Technol. 2011;45:796–802. doi: 10.1021/es102482j. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Xu C., Huang W., Jiang M., Yan J., Fan G., Zhang J., Chen K., Xiao B., Song G. Improving anaerobic digestion of piggery wastewater by alleviating stress of ammonia using biochar derived from rice straw. Environ. Technol. Inno. 2020;19:100948. [Google Scholar]
- Chowdhury B., Lin L., Dhar B.R., Islam M.N., McCartney D., Kumar A. Enhanced biomethane recovery from fat, oil, and grease through co-digestion with food waste and addition of conductive materials. Chemosphere. 2019;236:124362. doi: 10.1016/j.chemosphere.2019.124362. [DOI] [PubMed] [Google Scholar]
- Conrad R., Phelps T.J., Zeikus J.G. Gas metabolism evidence in support of the juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl. Environ. Microbiol. 1985;50:595–601. doi: 10.1128/aem.50.3.595-601.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz V.C., Rossetti S., Fazi S., Paiano P., Majone M., Aulenta F. Magnetite particles triggering a faster and more robust syntrophic pathway of methanogenic propionate degradation. Environ. Sci. Technol. 2014;48:7536–7543. doi: 10.1021/es5016789. [DOI] [PubMed] [Google Scholar]
- Dang Y., Holmes D.E., Zhao Z., Woodard T.L., Zhang Y., Sun D., Wang L., Nevin K.P., Lovley D.R. Enhancing anaerobic digestion of complex organic waste with carbon-based conductive materials. Bioresour. Technol. 2016;220:516–522. doi: 10.1016/j.biortech.2016.08.114. [DOI] [PubMed] [Google Scholar]
- Dang Y., Sun D., Woodard T.L., Wang L., Nevin K.P., Holmes D.E. Stimulation of the anaerobic digestion of the dry organic fraction of municipal solid waste (OFMSW) with carbon-based conductive materials. Bioresour. Technol. 2017;238:30–38. doi: 10.1016/j.biortech.2017.04.021. [DOI] [PubMed] [Google Scholar]
- De Clercq D., Wen Z., Fan F., Caicedo L. Biomethane production potential from restaurant food waste in megacities and project level-bottlenecks: a case study in Beijing. Renew. Sustain. Energ. Rev. 2016;59:1676–1685. [Google Scholar]
- Dererie D.Y., Trobro S., Momeni M.H., Hansson H., Blomqvist J., Passoth V., Schnürer A., Sandgren M., Ståhlberg J. Improved bio-energy yields via sequential ethanol fermentation and biogas digestion of steam exploded oat straw. Bioresour. Technol. 2011;102:4449–4455. doi: 10.1016/j.biortech.2010.12.096. [DOI] [PubMed] [Google Scholar]
- Fagbohungbe M.O., Herbert B.M.J., Hurst L., Li H., Usmani S.Q., Semple K.T. Impact of biochar on the anaerobic digestion of citrus peel waste. Bioresour. Technol. 2016;216:142–149. doi: 10.1016/j.biortech.2016.04.106. [DOI] [PubMed] [Google Scholar]
- Filman D.J., Marino S.F., Ward J.E., Yang L., Mester Z., Bullitt E., Lovley D.R., Strauss M. Cryo-EM reveals the structural basis of long-range electron transport in a cytochrome-based bacterial nanowire. Commun. Biol. 2019;2:219. doi: 10.1038/s42003-019-0448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L., Song T., Zhang W., Zhang J., Lu Y. Stimulatory effect of magnetite nanoparticles on a highly enriched butyrate-oxidizing consortium. Front. Microbiol. 2018;9:1480. doi: 10.3389/fmicb.2018.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B., Zhang Y., Yu N., Liu Y. Impacts of conductive materials on microbial community during syntrophic propionate oxidization for biomethane recovery. Water Environ. Res. 2020 doi: 10.1002/wer.1357. [DOI] [PubMed] [Google Scholar]
- Guo Z., Gao L., Wang L., Liu W., Wang A. Enhanced methane recovery and exoelectrogen-methanogen evolution from low-strength wastewater in an up-flow biofilm reactor with conductive granular graphite fillers. Front. Environ. Sci. Eng. 2018;12:1–10. [Google Scholar]
- Holmes D.E., Rotaru A.E., Ueki T., Shrestha P.M., Ferry J.G., Lovley D.R. Electron and proton flux for carbon dioxide reduction in Methanosarcina barkeri during direct interspecies electron transfer. Front. Microbiol. 2018;9:3109. doi: 10.3389/fmicb.2018.03109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D.E., Shrestha P.M., Walker D., Dang Y., Nevin K.P., Woodard T.L., Lovley D.R. Metatranscriptomic evidence for direct interspecies electron transfer between geobacter and Methanothrix species in methanogenic rice paddy soils. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.00223-17. e00223–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm-Nielsen J.B., Al Seadi T., Oleskowicz-Popiel P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009;100:5478–5484. doi: 10.1016/j.biortech.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Hu Q., Sun D., Ma Y., Qiu B., Guo Z. Conductive polyaniline nanorods enhanced methane production from anaerobic wastewater treatment. Polymer. 2017;120:236–243. doi: 10.1016/j.jcis.2020.07.075. [DOI] [PubMed] [Google Scholar]
- Im S., Yun Y., Song Y., Kim D. Enhanced anaerobic digestion of glycerol by promoting DIET reaction. Biochem. Eng. J. 2019;142:18–26. [Google Scholar]
- Jing Y., Wan J., Angelidaki I., Zhang S., Luo G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017;108:212–221. doi: 10.1016/j.watres.2016.10.077. [DOI] [PubMed] [Google Scholar]
- Kato S., Hashimoto K., Watanabe K. Methanogenesis facilitated by electric syntrophy via (semi)conductive iron-oxide minerals. Environ. Microbiol. 2012;14:1646–1654. doi: 10.1111/j.1462-2920.2011.02611.x. [DOI] [PubMed] [Google Scholar]
- Kato S., Hashimoto K., Watanabe K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. U S A. 2012;109:10042–10046. doi: 10.1073/pnas.1117592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Watanabe K. Ecological and evolutionary interactions in syntrophic methanogenic consortia. Microbe Environ. 2010;25:145–151. doi: 10.1264/jsme2.me10122. [DOI] [PubMed] [Google Scholar]
- Leang C., Qian X., Mester T., Lovley D.R. Alignment of the c-type cytochrome OmcS along pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010;76:4080–4084. doi: 10.1128/AEM.00023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Sun D., Dang Y., Chen H., Zhao Z., Zhang Y., Holmes D.E. Stimulation of methanogenesis in anaerobic digesters treating leachate from a municipal solid waste incineration plant with carbon cloth. Bioresour. Technol. 2016;222:270–276. doi: 10.1016/j.biortech.2016.10.007. [DOI] [PubMed] [Google Scholar]
- Lei Y., Sun D., Dang Y., Feng X., Huo D., Liu C., Zheng K., Holmes D.E. Metagenomic analysis reveals that activated carbon aids anaerobic digestion of raw incineration leachate by promoting direct interspecies electron transfer. Water Res. 2019;161:570–580. doi: 10.1016/j.watres.2019.06.038. [DOI] [PubMed] [Google Scholar]
- Lei Y., Wei L., Liu T., Xiao Y., Dang Y., Sun D., Holmes D.E. Magnetite enhances anaerobic digestion and methanogenesis of fresh leachate from a municipal solid waste incineration plant. Chem. Eng. J. 2018;348:992–999. [Google Scholar]
- Li H., Chang J., Liu P., Fu L., Ding D., Lu Y. Direct interspecies electron transfer accelerates syntrophic oxidation of butyrate in paddy soil enrichments. Environ. Microbiol. 2015;17:1533–1547. doi: 10.1111/1462-2920.12576. [DOI] [PubMed] [Google Scholar]
- Li L., Tong Z., Fang C., Chu J., Yu H. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res. 2015;70:1–8. doi: 10.1016/j.watres.2014.11.042. [DOI] [PubMed] [Google Scholar]
- Li Q., Xu M., Wang G., Chen R., Qiao W., Wang X. Biochar assisted thermophilic co-digestion of food waste and waste activated sludge under high feedstock to seed sludge ratio in batch experiment. Bioresour. Technol. 2018;249:1009–1016. doi: 10.1016/j.biortech.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Lim E.Y., Tian H., Chen Y., Ni K., Zhang J., Tong Y.W. Methanogenic pathway and microbial succession during start-up and stabilization of thermophilic food waste anaerobic digestion with biochar. Bioresour. Technol. 2020;314:123751. doi: 10.1016/j.biortech.2020.123751. [DOI] [PubMed] [Google Scholar]
- Lin L., Chowdhury B., Zakaria B.S., Dhar B.R. Temperature-dependent (20–55 °C) electrocatalytic characteristics during ethanol/propionate degradation by methanogenic communities grown on conductive carbon fibers. Chem. Eng. J. 2020;391:123566. [Google Scholar]
- Lin R., Cheng J., Ding L., Murphy J.D. Improved efficiency of anaerobic digestion through direct interspecies electron transfer at mesophilic and thermophilic temperature ranges. Chem. Eng. J. 2018;350:681–691. [Google Scholar]
- Lin R., Cheng J., Zhang J., Zhou J., Cen K., Murphy J.D. Boosting biomethane yield and production rate with graphene: the potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017;239:345–352. doi: 10.1016/j.biortech.2017.05.017. [DOI] [PubMed] [Google Scholar]
- Liu F., Rotaru A.E., Shrestha P.M., Malvankar N.S., Nevin K.P., Lovley D.R. Promoting direct interspecies electron transfer with activated carbon. Energ. Environ. Sci. 2012;5:8982–8989. [Google Scholar]
- Liu F., Rotaru A.E., Shrestha P.M., Malvankar N.S., Nevin K.P., Lovley D.R. Magnetite compensates for the lack of a pilin-associated c-type cytochrome in extracellular electron exchange. Environ. Microbiol. 2015;17:648–655. doi: 10.1111/1462-2920.12485. [DOI] [PubMed] [Google Scholar]
- Liu J., Liu T., Chen S., Yu H., Zhang Y., Quan X. Enhancing anaerobic digestion in anaerobic integrated floating fixed-film activated sludge (An-IFFAS) system using novel electron mediator suspended biofilm carriers. Water Res. 2020;175:115697. doi: 10.1016/j.watres.2020.115697. [DOI] [PubMed] [Google Scholar]
- Lovley D.R. Electrically conductive pili: biological function and potential applications in electronics. Curr. Opin. Electrochem. 2017;4:190–198. [Google Scholar]
- Lovley D.R. Happy together: microbial communities that hook up to swap electrons. ISME J. 2017;11:327–336. doi: 10.1038/ismej.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D.R. Syntrophy goes electric: direct interspecies electron transfer. Annu. Rev. Microbiol. 2017;71:643–664. doi: 10.1146/annurev-micro-030117-020420. [DOI] [PubMed] [Google Scholar]
- Lovley D.R. Reach out and touch someone: potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev. Environ. Sci. Bio/technol. 2011;10:101–105. [Google Scholar]
- Lovley D.R., Ueki T., Zhang T., Malvankar N.S., Shrestha P.M., Flanagan K.A., Aklujkar M., Butler J.E., Giloteaux L., Rotaru A.E. Geobacter: the microbe electric's physiology, ecology, and practical applications. Adv. Microb. Physiol. 2011;59:1–100. doi: 10.1016/B978-0-12-387661-4.00004-5. [DOI] [PubMed] [Google Scholar]
- Lovley D.R., Holmes D.E. Protein nanowires: the electrification of the microbial world and maybe our own. J. Bacteriol. 2020;202 doi: 10.1128/JB.00331-20. e00331–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D.R., Walker D. Geobacter protein nanowires. Front. Microbiol. 2019;10:2078. doi: 10.3389/fmicb.2019.02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü F., Luo C., Shao L., He P. Biochar alleviates combined stress of ammonium and acids by firstly enriching Methanosaeta and then Methanosarcina. Water Res. 2016;90:34–43. doi: 10.1016/j.watres.2015.12.029. [DOI] [PubMed] [Google Scholar]
- Luo C., Lu F., Shao L., He P. Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res. 2015;68:710–718. doi: 10.1016/j.watres.2014.10.052. [DOI] [PubMed] [Google Scholar]
- Ma J., Wei H., Su Y., Gu W., Wang B., Xie B. Powdered activated carbon facilitates methane productivity of anaerobic co-digestion via acidification alleviating: microbial and metabolic insights. Bioresour. Technol. 2020;313:123706. doi: 10.1016/j.biortech.2020.123706. [DOI] [PubMed] [Google Scholar]
- Ma W., Li H., Zhang W., Shen C., Wang L., Li Y., Li Q., Wang Y. TiO2 nanoparticles accelerate methanogenesis in mangrove wetlands sediment. Sci. Total Environ. 2020;713:136602. doi: 10.1016/j.scitotenv.2020.136602. [DOI] [PubMed] [Google Scholar]
- Malvankar N.S., Tuominen M.T., Lovley D.R. Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energ. Environ. Sci. 2012;5:579–5797. [Google Scholar]
- Malvankar N.S., Vargas M., Nevin K.P., Franks A.E., Leang C., Kim B.C., Inoue K., Mester T., Covalla S.F., Johnson J.P. Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol. 2011;6:573–579. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- Mao C., Feng Y., Wang X., Ren G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energ. Rev. 2015;45:540–555. [Google Scholar]
- Mehta T., Coppi M.V., Childers S.E., Lovley D.R., Subsurface B.R.S. Outer membrane c-type cytochromes required for Fe(III) and Mn(IV) oxide reduction in geobacter sulfurreducens. Appl. Environ. Microbiol. 2005;71:8634–8641. doi: 10.1128/AEM.71.12.8634-8641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei R., Nobu M.K., Narihiro T., Yu J., Sathyagal A., Willman E., Liu W. Novel Geobacter species and diverse methanogens contribute to enhanced methane production in media-added methanogenic reactors. Water Res. 2018;147:403–412. doi: 10.1016/j.watres.2018.10.026. [DOI] [PubMed] [Google Scholar]
- Meynell P.J. Prism Press; 1982. Methane: Planning a Digester. [Google Scholar]
- Morita M., Malvankar N.S., Franks A.E., Summers Z.M., Giloteaux L., Rotaru A.E., Rotaru C., Lovley D.R. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. mBIO. 2011;2:e111–e159. doi: 10.1128/mBio.00159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa A., Im S., Song Y.C., Kang S., Kim D.H. Enhanced anaerobic digestion of long chain fatty acid by adding magnetite and carbon nanotubes. Microorganisms. 2020;8:333. doi: 10.3390/microorganisms8030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumme J., Srocke F., Heeg K., Werner M. Use of biochars in anaerobic digestion. Bioresour. Technol. 2014;164:189–197. doi: 10.1016/j.biortech.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Park J.H., Park J.H., Je S.H., Sul W.J., Jin K.H., Park H.D. Metagenomic insight into methanogenic reactors promoting direct interspecies electron transfer via granular activated carbon. Bioresour. Technol. 2018;259:414–422. doi: 10.1016/j.biortech.2018.03.050. [DOI] [PubMed] [Google Scholar]
- Peng H., Zhang Y., Tan D., Zhao Z., Zhao H., Quan X. Roles of magnetite and granular activated carbon in improvement of anaerobic sludge digestion. Bioresour. Technol. 2018;249:666–672. doi: 10.1016/j.biortech.2017.10.047. [DOI] [PubMed] [Google Scholar]
- Qian X., Mester T., Morgado L., Arakawa T., Sharma M.L., Inoue K., Joseph C., Salgueiro C.A., Maroney M.J., Lovley D.R. Biochemical characterization of purified OmcS, a c-type cytochrome required for insoluble Fe(III) reduction in Geobacter sulfurreducens. BBA-Bioenergetics. 2011;1807:404–412. doi: 10.1016/j.bbabio.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Ren N., Wang B., Huang J.C. Ethanol-type fermentation from carbohydrate in high rate acidogenic reactor. Biotechnol. Bioeng. 1997;54:428–433. doi: 10.1002/(SICI)1097-0290(19970605)54:5<428::AID-BIT3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ren S., Usman M., Tsang D., O-Thong S., Angelidaki I., Zhu X., Zhang S., Luo G. Hydrochar-Facilitated anaerobic digestion: evidence for direct interspecies electron transfer mediated through surface oxygen-containing functional groups. Environ. Sci. Technol. 2020;54:5755–5766. doi: 10.1021/acs.est.0c00112. [DOI] [PubMed] [Google Scholar]
- Rotaru A.E., Calabrese F., Stryhanyuk H., Musat F., Shrestha P.M., Weber H.S., Snoeyenbos-West O., Hall P., Richnow H.H., Musat N. Conductive particles enable syntrophic acetate oxidation between geobacter and Methanosarcina from coastal sediments. mBIO. 2018;9 doi: 10.1128/mBio.00226-18. e00226–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru A.E., Shrestha P.M., Liu F., Shrestha M., Shrestha D., Embree M., Zengler K., Wardman C., Nevin K.P., Lovley D.R. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energ. Environ. Sci. 2014;7:408–415. [Google Scholar]
- Rotaru A.E., Shrestha P.M., Liu F., Markovaite B., Chen S., Nevin K.P., Lovley D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014;80:4599–4605. doi: 10.1128/AEM.00895-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki D., Sasaki K., Watanabe A., Morita M., Matsumoto N., Igarashi Y., Ohmura N. Operation of a cylindrical bioelectrochemical reactor containing carbon fiber fabric for efficient methane fermentation from thickened sewage sludge. Bioresour. Technol. 2013;129:366–373. doi: 10.1016/j.biortech.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Sasaki K., Haruta S., Ueno Y., Ishii M., Igarashi Y. Microbial population in the biomass adhering to supporting material in a packed-bed reactor degrading organic solid waste. Appl. Microbiol. Biotechnol. 2007;75:941–952. doi: 10.1007/s00253-007-0888-x. [DOI] [PubMed] [Google Scholar]
- Shen N., Liang Z., Chen Y., Song H., Wan J. Enhancement of syntrophic acetate oxidation pathway via single walled carbon nanotubes addition under high acetate concentration and thermophilic condition. Bioresour Technol. 2020;306:123182. doi: 10.1016/j.biortech.2020.123182. [DOI] [PubMed] [Google Scholar]
- Shen Y., Linville J.L., Ignacio-de Leon P.A.A., Schoene R.P., Urgun-Demirtas M. Towards a sustainable paradigm of waste-to-energy process: enhanced anaerobic digestion of sludge with woody biochar. J. Clean. Prod. 2016;135:1054–1064. [Google Scholar]
- Shrestha P.M., Malvankar N.S., Werner J.J., Franks A.E., Elena-Rotaru A., Shrestha M., Liu F., Nevin K.P., Angenent L.T., Lovley D.R. Correlation between microbial community and granule conductivity in anaerobic bioreactors for brewery wastewater treatment. Bioresour. Technol. 2014;174:306–310. doi: 10.1016/j.biortech.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Shrestha P.M., Rotaru A.E., Summers Z.M., Shrestha M., Liu F., Lovley D.R. Transcriptomic and genetic analysis of direct interspecies electron transfer. Appl. Environ. Microbiol. 2013;79:2397–2404. doi: 10.1128/AEM.03837-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber J.R., Le H.M., McInerney M.J. The importance of hydrogen and formate transfer for syntrophic fatty, aromatic and alicyclic metabolism. Environ. Microbiol. 2014;16:177–188. doi: 10.1111/1462-2920.12269. [DOI] [PubMed] [Google Scholar]
- Sieber J.R., McInerney M.J., Gunsalus R.P. Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu. Rev. Microbiol. 2012;66:429–452. doi: 10.1146/annurev-micro-090110-102844. [DOI] [PubMed] [Google Scholar]
- Song X., Liu J., Jiang Q., Zhang P., Shao Y., He W., Feng Y. Enhanced electron transfer and methane production from low-strength wastewater using a new granular activated carbon modified with nano-Fe3O4. Chem. Eng. J. 2019;374:1344–1352. [Google Scholar]
- Stams A.J., Plugge C.M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 2009;7:568–577. doi: 10.1038/nrmicro2166. [DOI] [PubMed] [Google Scholar]
- Summers Z.M., Fogarty H.E., Leang C., Franks A.E., Malvankar N.S., Lovley D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science. 2010;330:1413–1415. doi: 10.1126/science.1196526. [DOI] [PubMed] [Google Scholar]
- Sun W., Fu S., Zhu R., Wang Z., Zou H., Zheng Y. Improved anaerobic digestion efficiency of high-solid sewage sludge by enhanced direct interspecies electron transfer with activated carbon mediator. Bioresour. Technol. 2020;313:123648. doi: 10.1016/j.biortech.2020.123648. [DOI] [PubMed] [Google Scholar]
- Tan J., Wang J., Xue J., Liu S., Peng S., Ma D., Chen T., Yue Z. Methane production and microbial community analysis in the goethite facilitated anaerobic reactors using algal biomass. Fuel. 2015;145:196–201. [Google Scholar]
- Tatara M., Makiuchi T., Ueno Y., Goto M., Sode K. Methanogenesis from acetate and propionate by thermophilic down-flow anaerobic packed-bed reactor. Bioresour. Technol. 2008;99:4786–4795. doi: 10.1016/j.biortech.2007.09.069. [DOI] [PubMed] [Google Scholar]
- Tatara M., Tatara M., Yamazawa A., Yamazawa A., Ueno Y., Ueno Y., Fukui H., Fukui H., Goto M., Goto M. High-rate thermophilic methane fermentation on short-chain fatty acids in a down-flow anaerobic packed-bed reactor. Bioproc. Biosyst. Eng. 2005;27:105–113. doi: 10.1007/s00449-004-0387-8. [DOI] [PubMed] [Google Scholar]
- Tian T., Qiao S., Li X., Zhang M., Zhou J. Nano-graphene induced positive effects on methanogenesis in anaerobic digestion. Bioresour. Technol. 2017;224:41–47. doi: 10.1016/j.biortech.2016.10.058. [DOI] [PubMed] [Google Scholar]
- Ueki T., Nevin K.P., Rotaru A.E., Wang L.Y., Ward J.E., Woodard T.L., Lovley D.R. Geobacter strains expressing poorly conductive pili reveal constraints on direct interspecies electron transfer mechanisms. mBio. 2018;9 doi: 10.1128/mBio.01273-18. e01273–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usman M., Hao S., Chen H., Ren S., Tsang D.C.W., O-Thong S., Luo G., Zhang S. Molecular and microbial insights towards understanding the anaerobic digestion of the wastewater from hydrothermal liquefaction of sewage sludge facilitated by granular activated carbon (GAC) Environ. Int. 2019;133:105257. doi: 10.1016/j.envint.2019.105257. [DOI] [PubMed] [Google Scholar]
- Walker D., Martz E., Holmes D.E., Zhou Z., Nonnenmann S.S., Lovley D.R. The archaellum of Methanospirillum hungatei is electrically conductive. mBIO. 2019;10 doi: 10.1128/mBio.00579-19. e00579–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D., Nevin K.P., Holmes D.E., Rotaru A.E., Ward J.E., Woodard T.L., Zhu J., Ueki T., Nonnenmann S.S., McInerney M.J. Syntrophus conductive pili demonstrate that common hydrogen-donating syntrophs can have a direct electron transfer option. ISME J. 2020;14:837–846. doi: 10.1038/s41396-019-0575-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.J., Adhikari R.Y., Holmes D.E., Ward J.E., Woodard T.L., Nevin K.P., Lovley D.R. Electrically conductive pili from pilin genes of phylogenetically diverse microorganisms. ISME J. 2018;12:48–58. doi: 10.1038/ismej.2017.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Ai J., Shen F., Yang G., Zhang Y., Deng S., Zhang J., Zeng Y., Song C. Improving anaerobic digestion of easy-acidification substrates by promoting buffering capacity using biochar derived from vermicompost. Bioresour. Technol. 2017;227:286–296. doi: 10.1016/j.biortech.2016.12.060. [DOI] [PubMed] [Google Scholar]
- Wang L.Y., Nevin K.P., Woodard T.L., Mu B.Z., Lovley D.R. Expanding the diet for DIET: electron donors supporting direct interspecies electron transfer (DIET) in defined Co-cultures. Front. Microbiol. 2016;7:236. doi: 10.3389/fmicb.2016.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu Y., Gao X., Chen H., Xu X., Zhu L. Role of biochar in the granulation of anaerobic sludge and improvement of electron transfer characteristics. Bioresour. Technol. 2018;268:28–35. doi: 10.1016/j.biortech.2018.07.116. [DOI] [PubMed] [Google Scholar]
- Wang D., Han Y., Han H., Li K., Xu C., Zhuang H. New insights into enhanced anaerobic degradation of Fischer-Tropsch wastewater with the assistance of magnetite. Bioresour. Technol. 2018;257:147–156. doi: 10.1016/j.biortech.2018.02.084. [DOI] [PubMed] [Google Scholar]
- Wang G., Li Q., Gao X., Wang X.C. Synergetic promotion of syntrophic methane production from anaerobic digestion of complex organic wastes by biochar: performance and associated mechanisms. Bioresour. Technol. 2018;250:812–820. doi: 10.1016/j.biortech.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Wang O., Zheng S., Wang B., Wang W., Liu F. Necessity of electrically conductive pili for methanogenesis with magnetite stimulation. Peer J. 2018;6:e4541. doi: 10.7717/peerj.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Qiao W., Chen H., Xu X., Zhu L. A short-term stimulation of ethanol enhances the effect of magnetite on anaerobic digestion. Appl. Microbiol. Biotechnol. 2019;103:1511–1522. doi: 10.1007/s00253-018-9531-2. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang C., Jin L., Lu D., Chen H., Zhu W., Xu X., Zhu L. Response of syntrophic aggregates to the magnetite loss in continuous anaerobic bioreactor. Water Res. 2019;164:114925. doi: 10.1016/j.watres.2019.114925. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang C., Liu J., Han Z., Xu Q., Xu X., Zhu L. Role of magnetite in methanogenic degradation of different substances. Bioresour. Technol. 2020;314:123720. doi: 10.1016/j.biortech.2020.123720. [DOI] [PubMed] [Google Scholar]
- Wang C., Wang C., Liu J., Xu Q., Han Z., Xu X., Zhu L. Tolerance of aceticlastic methanogenesis enhanced by magnetite under the condition of ammonia stress. ACS Sustain. Chem. Eng. 2020;8:1417–1426. [Google Scholar]
- Wang G., Gao X., Li Q., Zhao H., Liu Y., Wang X.C., Chen R. Redox-based electron exchange capacity of biowaste-derived biochar accelerates syntrophic phenol oxidation for methanogenesis via direct interspecies electron transfer. J. Hazard. Mater. 2020;390:121726. doi: 10.1016/j.jhazmat.2019.121726. [DOI] [PubMed] [Google Scholar]
- Wang P., Peng H., Adhikari S., Higgins B., Roy P., Dai W., Shi X. Enhancement of biogas production from wastewater sludge via anaerobic digestion assisted with biochar amendment. Bioresour. Technol. 2020;309:123368. doi: 10.1016/j.biortech.2020.123368. [DOI] [PubMed] [Google Scholar]
- Wu C., Wang Q., Yu M., Zhang X., Song N., Chang Q., Gao M., Sonomoto K. Effect of ethanol pre-fermentation and inoculum-to-substrate ratio on methane yield from food waste and distillers’ grains. Appl. Energ. 2015;155:846–853. [Google Scholar]
- Xia X., Zhang J., Song T., Lu Y. Stimulation of Smithella-dominating propionate oxidation in a sediment enrichment by magnetite and carbon nanotubes. Environ. Microbiol. Rep. 2019;11:236–248. doi: 10.1111/1758-2229.12737. [DOI] [PubMed] [Google Scholar]
- Xing L., Wang Z., Gu M., Yin Q., Wu G. Coupled effects of ferroferric oxide supplement and ethanol co-metabolism on the methanogenic oxidation of propionate. Sci. Total Environ. 2020;723:137992. doi: 10.1016/j.scitotenv.2020.137992. [DOI] [PubMed] [Google Scholar]
- Xu H., Wang C., Yan K., Wu J., Zuo J., Wang K. Anaerobic granule-based biofilms formation reduces propionate accumulation under high H2 partial pressure using conductive carbon felt particles. Bioresour. Technol. 2016;216:677–683. doi: 10.1016/j.biortech.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Xu S., Han R., Zhang Y., He C., Liu H. Differentiated stimulating effects of activated carbon on methanogenic degradation of acetate, propionate and butyrate. Waste Manage. 2018;76:394–403. doi: 10.1016/j.wasman.2018.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., He C., Luo L., Lü F., He P., Cui L. Comparing activated carbon of different particle sizes on enhancing methane generation in upflow anaerobic digester. Bioresour. Technol. 2015;196:606–612. doi: 10.1016/j.biortech.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Yamada C., Kato S., Ueno Y., Ishii M., Igarashi Y. Conductive iron oxides accelerate thermophilic methanogenesis from acetate and propionate. J. Biosci. Bioeng. 2015;119:678–682. doi: 10.1016/j.jbiosc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Yan W., Mukherjee M., Zhou Y. Direct interspecies electron transfer (DIET) can be suppressed under ammonia-stressed condition – reevaluate the role of conductive materials. Water Res. 2020;183:116094. doi: 10.1016/j.watres.2020.116094. [DOI] [PubMed] [Google Scholar]
- Yan W., Sun F., Liu J., Zhou Y. Enhanced anaerobic phenol degradation by conductive materials via EPS and microbial community alteration. Chem. Eng. J. 2018;352:1–9. [Google Scholar]
- Yang L., Si B., Zhang Y., Watson J., Stablein M., Chen J., Zhang Y., Zhou X., Chu H. Continuous treatment of hydrothermal liquefaction wastewater in an anaerobic biofilm reactor: potential role of granular activated carbon. J. Clean. Prod. 2020;276:122836. [Google Scholar]
- Yang Y., Zhang Y., Li Z., Zhao Z., Quan X., Zhao Z. Adding granular activated carbon into anaerobic sludge digestion to promote methane production and sludge decomposition. J. Clean. Prod. 2017;149:1101–1108. [Google Scholar]
- Yang Z., Guo R., Shi X., Wang C., Wang L., Dai M. Magnetite nanoparticles enable a rapid conversion of volatile fatty acids to methane. RSC Adv. 2016;6:25662–25668. [Google Scholar]
- Yang Z., Xu X., Guo R., Fan X., Zhao X. Accelerated methanogenesis from effluents of hydrogen-producing stage in anaerobic digestion by mixed cultures enriched with acetate and nano-sized magnetite particles. Bioresour. Technol. 2015;190:132–139. doi: 10.1016/j.biortech.2015.04.057. [DOI] [PubMed] [Google Scholar]
- Yee M.O., Rotaru A.E. Extracellular electron uptake in Methanosarcinales is independent of multiheme c-type cytochromes. Sci. Rep. 2020;10:372. doi: 10.1038/s41598-019-57206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Q., He K., Liu A., Wu G. Enhanced system performance by dosing ferroferric oxide during the anaerobic treatment of tryptone-based high-strength wastewater. Appl. Microbiol. Biotechnol. 2017;101:3929–3939. doi: 10.1007/s00253-017-8194-8. [DOI] [PubMed] [Google Scholar]
- Yin Q., Miao J., Li B., Wu G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeter. Biodegr. 2017;119:104–110. [Google Scholar]
- Yin Q., Yang S., Wang Z., Xing L., Wu G. Clarifying electron transfer and metagenomic analysis of microbial community in the methane production process with the addition of ferroferric oxide. Chem. Eng. J. 2018;333:216–225. [Google Scholar]
- Yu M., Wu C., Wang Q., Sun X., Ren Y., Li Y. Ethanol prefermentation of food waste in sequencing batch methane fermentation for improved buffering capacity and microbial community analysis. Bioresour. Technol. 2018;248:187–193. doi: 10.1016/j.biortech.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Zhang D., Zhu W., Tang C., Suo Y., Gao L., Yuan X., Wang X., Cui Z. Bioreactor performance and methanogenic population dynamics in a low-temperature (5–18°C) anaerobic fixed-bed reactor. Bioresour. Technol. 2012;104:136–143. doi: 10.1016/j.biortech.2011.10.086. [DOI] [PubMed] [Google Scholar]
- Zhang J., Mao L., Zhang L., Loh K.C., Dai Y., Tong Y.W. Metagenomic insight into the microbial networks and metabolic mechanism in anaerobic digesters for food waste by incorporating activated carbon. Sci. Rep. 2017;7:11293. doi: 10.1038/s41598-017-11826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lu Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016;7:1316. doi: 10.3389/fmicb.2016.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Chang J., Lin C., Pan Y., Cui K., Zhang X., Liang P., Huang X. Enhancement of methanogenesis via direct interspecies electron transfer between Geobacteraceae and Methanosaetaceae conducted by granular activated carbon. Bioresour. Technol. 2017;245:132–137. doi: 10.1016/j.biortech.2017.08.111. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang J., Loh K. Activated carbon enhanced anaerobic digestion of food waste – laboratory-scale and Pilot-scale operation. Waste Manage. 2018;75:270–279. doi: 10.1016/j.wasman.2018.02.020. [DOI] [PubMed] [Google Scholar]
- Zhang W., Zhang J., Lu Y. Stimulation of carbon nanomaterials on syntrophic oxidation of butyrate in sediment enrichments and a defined coculture. Sci. Rep. 2018;8:12185. doi: 10.1038/s41598-018-30745-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F., Qian D.K., Wang X.B., Dai K., Wang T., Zhang W., Zeng R.J. Stimulation of methane production from benzoate with addition of carbon materials. Sci. Total Environ. 2020;723:138080. doi: 10.1016/j.scitotenv.2020.138080. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Guo B., Zhang L., Liu Y. Key syntrophic partnerships identified in a granular activated carbon amended UASB treating municipal sewage under low temperature conditions. Bioresour. Technol. 2020;312:123556. doi: 10.1016/j.biortech.2020.123556. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang L., Guo B., Zhou Y., Gao M., Sharaf A., Liu Y. Granular activated carbon stimulated microbial physiological changes for enhanced anaerobic digestion of municipal sewage. Chem. Eng. J. 2020;400:125838. [Google Scholar]
- Zhao Z., Li Y., Quan X., Zhang Y. New application of ethanol-type fermentation: stimulating methanogenic communities with ethanol to perform direct interspecies electron transfer. ACS Sustain. Chem. Eng. 2017;5:9441–9453. [Google Scholar]
- Zhao Z., Li Y., Quan X., Zhang Y. Towards engineering application: potential mechanism for enhancing anaerobic digestion of complex organic waste with different types of conductive materials. Water Res. 2017;115:266–277. doi: 10.1016/j.watres.2017.02.067. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Wang J., Li Y., Zhu T., Yu Q., Wang T., Liang S., Zhang Y. Why do DIETers like drinking: metagenomic analysis for methane and energy metabolism during anaerobic digestion with ethanol. Water Res. 2020;171:115425. doi: 10.1016/j.watres.2019.115425. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang Y., Holmes D.E., Dang Y., Woodard T.L., Nevin K.P., Lovley D.R. Potential enhancement of direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate with biochar in up-flow anaerobic sludge blanket reactors. Bioresour. Technol. 2016;209:148–156. doi: 10.1016/j.biortech.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang Y., Yu Q., Dang Y., Li Y., Quan X. Communities stimulated with ethanol to perform direct interspecies electron transfer for syntrophic metabolism of propionate and butyrate. Water Res. 2016;102:475–484. doi: 10.1016/j.watres.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang Y., Li Y., Dang Y., Zhu T., Quan X. Potentially shifting from interspecies hydrogen transfer to direct interspecies electron transfer for syntrophic metabolism to resist acidic impact with conductive carbon cloth. Chem. Eng. J. 2017;313:10–18. [Google Scholar]
- Zhao Z., Zhang Y., Woodard T.L., Nevin K.P., Lovley D.R. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015;191:140–145. doi: 10.1016/j.biortech.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Zhang Y. Application of ethanol-type fermentation in establishment of direct interspecies electron transfer: a practical engineering case study. Renew. Energ. 2019;136:846–855. [Google Scholar]
- Zhu Y., Zhao Z., Zhang Y. Using straw as a bio-ethanol source to promote anaerobic digestion of waste activated sludge. Bioresour. Technol. 2019;286:121388. doi: 10.1016/j.biortech.2019.121388. [DOI] [PubMed] [Google Scholar]
- Zhuang L., Ma J., Yu Z., Wang Y., Tang J. Magnetite accelerates syntrophic acetate oxidation in methanogenic systems with high ammonia concentrations. Microb. Biotechnol. 2018;11:710–720. doi: 10.1111/1751-7915.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang L., Tang J., Wang Y., Hu M., Zhou S. Conductive iron oxide minerals accelerate syntrophic cooperation in methanogenic benzoate degradation. J. Hazard. Mater. 2015;293:37–45. doi: 10.1016/j.jhazmat.2015.03.039. [DOI] [PubMed] [Google Scholar]
- Zhuang L., Xu J., Tang J., Zhou S. Effect of ferrihydrite biomineralization on methanogenesis in an anaerobic incubation from paddy soil. J. Geophys. Res. 2015;120:876–886. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.