Abstract
Turnover of the mitochondrial pool due to coordinated processes of mitochondrial biogenesis and mitophagy is an important process in maintaining mitochondrial stability. An important role in this process is played by the Nrf2/ARE signaling pathway, which is involved in the regulation of the expression of genes responsible for oxidative stress protection, regulation of mitochondrial biogenesis, and mitophagy. The p62 protein is a multifunctional cytoplasmic protein that functions as a selective mitophagy receptor for the degradation of ubiquitinated substrates. There is evidence that p62 can positively regulate Nrf2 by binding to its negative regulator, Keap1. However, there is also strong evidence that Nrf2 up-regulates p62 expression. Thereby, a regulatory loop is formed between two important signaling pathways, which may be an important target for drugs aimed at treating neurodegeneration. Constitutive activation of p62 in parallel with Nrf2 would most likely result in the activation of mTORC1-mediated signaling pathways that are associated with the development of malignant neoplasms. The purpose of this review is to describe the p62-Nrf2-p62 regulatory loop and to evaluate its role in the regulation of mitophagy under various physiological conditions.
Keywords: mitochondria, Nrf2, p62, mitophagy, regulatory loop, neurodegenerative disease
1. Introduction
Mitochondrial dysfunctions play a key role in a wide range of neurodegenerative diseases, especially Alzheimer’s and Parkinson’s diseases as the most common forms of neurodegenerative diseases [1]. Worldwide, over 46 million people over 65 years old are living with some kind of dementia. Many of them would develop Alzheimer’s disease, eventually [2], a plague of developed countries with a huge negative social-economic impact. Parkinson’s disease is the second most common neurodegenerative disease that affects approximately ten million people worldwide [3]. One of the common biochemical features of these neurodegenerative diseases is neuronal mitochondrial dysfunctions. The latter are very diverse, including overproduction of reactive oxygen species (ROS) [4], oxidative mtDNA damage [5], impaired oxidative phosphorylation as a result of dysfunction of the respiratory complexes [6], ATPase damage [7], a loss of inner membrane integrity [8], a dysfunction of metabolites transport systems [9], and dysfunction of Ca2+ metabolism [10]. Each of these dysfunctions can trigger a chain of events that can cause neuronal cell death.
There are few ways of overcoming mitochondrial dysfunctions pharmacologically. The most effective methods are antioxidant protection [11] and manipulating/modulating the turnover of the mitochondrial pool in the cell [12]. The latter includes mitochondrial biogenesis (the formation of new mitochondria), mitophagy (elimination of damaged mitochondria), and fission/fusion processes (mitochondrial dynamics) [13]. Maintaining a dynamic balance between these processes is important for the normal functioning of the structural components of the brain and for the preventive therapy of neurodegenerative diseases [14].
In recent years, the Nrf2 (Nuclear factor erythroid 2-related factor 2) protein has emerged as one of the most promising targets for the therapy of neurodegenerative diseases. It is a transcription factor that regulates the expression of a large number of antioxidant and detoxifying enzymes [15]. However, in the last decade, data have begun to emerge that show its effects on mitochondrial biogenesis and mitophagy [16]. It became clear that Nrf2′s role in maintaining mitochondrial homeostasis is not limited to just antioxidant protection but also extends to the turnover of the mitochondrial pool.
To note, Nrf2 expression and function are also regulated by the same factors that regulate mitochondrial biogenesis. We have previously considered the possibility of the existence of a regulatory loop between Nrf2 and PGC1α in the context of regulation of mitochondrial biogenesis during aging and neurodegeneration [17]. This review is focused on the regulatory loop between Nrf2 and p62, which can potentially be an important target for drugs to cure neurodegenerative diseases.
2. Nrf2/ARE Signal Pathway
The transcription factor NF-E2 p45-related factor 2 (Nrf2; gene name NFE2L2) regulates the expression of a wide variety of genes that encode proteins with cytoprotective properties, such as antioxidant enzymes, xenobiotic detoxification protein, and anti-inflammatory enzymes, as well as metabolic enzymes and regulators involved in maintaining redox homeostasis [18]. Nrf2 is translocated into the nucleus and binds to the antioxidant response element (ARE) of the promotor in the absence of negative regulators [18]. The regulatory cis-activating element of ARE in the promoter regions of genes is the nucleotide sequence 5′-A (G) TGAC (T) nnnGCA (G) -3′ [19]. There are several variants of the Nrf2 interaction with ARE sequences in the cell nucleus. The most canonical way of activation is the interaction of Nrf2 with basic-leucine zipper (bZip) transcription factors (most often small musculoaponeurotic fibrosarcoma (MAFs)) and CREB-binding protein (CBP) coactivator, which has histone acetyltransferase activity, which leads to changes in the chromatin structure. This allows increasing expression of target genes [20].
Nrf2 is a short-lived protein (about 15 min). In the absence of activating factors, it undergoes ubiquitination and proteasomal degradation. Three ubiquitin ligase systems are known that provide for the degradation of Nrf2. The first to be described was Kelch-like ECH-associated protein 1 (Keap1). Keap1 functions as an adapter protein that mediates the interaction of Nrf2 with the E3 ubiquitin ligase complex Cullin 3 (Cul3), and with RING-box protein 1 (Rbx1), which is required for the interaction of Nrf2 with the ubiquitin ligase system [21]. The second negative regulator of Nrf2 is Glycogen synthase kinase 3 beta (GSK3β), which is able to phosphorylate protein at serine and threonine amino acids. That makes Nrf2 to be recognized by SCF/β-TrCP (SCF is an abbreviation formed from the first letters of the subunits of the complex: Skp1, Cul1, F-box; β-TrCP—β -transducin repeat containing protein). The complex formed by SCF/β-TrCP binds to Cullin 1 (Cul1), which leads to the formation of an ubiquitin ligase complex and subsequent Keap1-independent degradation of Nrf2 [22]. Recently, a third way of negative regulation of Nrf2 was described by the E3 ubiquitin ligase HRD1 [23].
Nrf2 contains seven NRF2-ECH (Neh) domains (Figure 1). The Neh1 domain is required for the formation of a heterodimer with small MAFs and mediates interaction with the ARE sequence of targeted genes [24]. Keap1 binds to the N-terminal Neh2 domain. Thus, Neh2 can be considered the domain responsible for the cytoplasmic localization of Nrf2 [25]. The C-terminal Neh3 domain, as well as the tandem Neh4 and Neh5, provide the transactivating effect of Nrf2 by binding to histone acetyltransferases [26]. In addition, Neh4 and Neh5 mediate interaction with HRD1 [23]. Nrf2 is phosphorylated by GSK3β at the Neh6 domain [27]. The Neh7 domain is responsible for binding to the retinoid X receptor α (RXRα), which can also act as a negative regulator of Nrf2 [28].
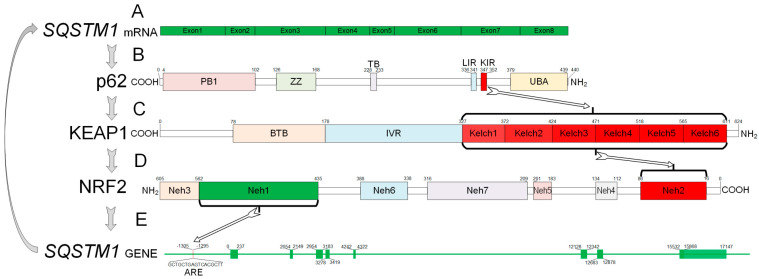
Figure 1.
Scheme of p62-Nrf2 loop with protein and gene structure. KIR of p62 interacts with the Kelch repeat of KEAP1 protein and inhibits its activity. Kelch repeat interacts with the Neh2 domain of the Nrf2 protein and inactivates it. Free Nrf2 can interact with the ARE region of the SQSTM1 gene and increase its expression. A. mRNA structure of SQSTM1 of humans. mRNA contains 1352 protein-coding bp and includes 8 exons. B. Domain structure of p62 protein of human (440 aa). Phox and Bem1p (PB1)—4–102 aa; ZZ-type zinc finger domain (ZZ)—126–168 aa; TRAF6 binding domain (TB)—228–233 aa; LC3 interacting region (LIR)—336–341 aa; KEAP1 interacting region (KIR)—347–352 aa; ubiquitin-associated domain (UBA)—379–439 aa. C. Domain structure of KEAP1 protein of human (624 aa). BTB domain (BTB)—78–178 aa; intervening region (IVR)—179–327 aa; Kelch repeat (Kelch1–Kelch6)—327–611 aa. D. Domain structure of KEAP1 protein of human (605 aa). Neh domains. Neh2—16–86 aa; Neh4—112–134 aa; Neh5—183–201 aa; Neh7—209–316 aa; Neh6—338–388 aa; Neh1—435–562 aa; Neh3—563–605 aa. E. SQSTM1 gene structure of humans. Gene contains 8 exons. 1st exon (0–237 bp); 2nd exon (2054–2149 bp); 3rd exon (2954–3183 bp); 4th exon (3278–3419 bp); 5th exon (4249–4322 bp); 6th exon (12128–12342 bp); 7th exon (12683–12878 bp); 8th exon (15532–17147 bp). SQSTM1 gene contains ARE region at (−1306)–(−1295) bp.
Nrf2 inductors are well studied and well described in published studies. These are natural, synthetic, and endogenous quinones, diphenols, phenylenediamines, isothiocyanates, heavy metal ions (Cd, Co, Cu, Au, Hg, Pb), carotenoids, ROS, and chemical compounds that promote their production [18,21,29].
3. p62 (Sequestosome 1)
p62 (Sequestosome 1; gene name SQSTM1) is a multifunctional cytoplasmic protein that is an important regulatory molecule that functions as a selective autophagy receptor for the degradation of ubiquitinated substrates [30]. Autophagy is an important process that can be divided into two types. Non-selective autophagy occurs in a cell suffering nutritional deficiencies. In this situation, selective autophagy serves to selectively remove organelles in order to regulate their number. Mitophagy is a particular manifestation of selective mitochondrial autophagy [31].
Mitophagy depends on PTEN-induced kinase 1 (PINK1). PINK1 contains a mitochondrial targeting sequence (MTS). In the absence of mitochondrial damage, penetrates into mitochondria through the outer membrane (through the TOM complex) and partially through the inner mitochondrial membrane (through the TIM complex). On the inner membrane, PINK1 undergoes partial cleavage to presenilins-associated rhomboid-like protein (PARL). This form of PINK1 is cleaved by proteases in the mitochondrial matrix [32].
In the damaged mitochondria, the inner membrane is depolarized, which affects TIM-mediated protein import. As a result, PINK1 protein does not enter the mitochondrial matrix, where it is usually degraded. Therefore, the PINK1 protein accumulates on the outer mitochondrial membrane. It leads to the activation of PARKIN, which is the cytosolic E3-ubiquitin ligase. PARKIN can ubiquitinate a number of proteins on the outer mitochondrial membrane, which triggers mitophagy. PARKIN promotes K63-linked polyubiquitination of the mitochondrial substrate and recruits ubiquitin- and LC3-binding protein p62 into mitochondria [33]. The p62 acts as an adapter molecule that directly interacts with ubiquitinated molecules on the autophagosome. Ablating p62 completely blocks the clearance of damaged mitochondria [34]. Thus, activation of the PINK1/PARKIN/p62 axis plays an important role in the selective elimination of damaged mitochondria, which is essential for maintaining their quality control. It should be noted, that some recent data suggest that p62-mediated ubiquitination and mitophagy can also be carried out in the PINK1/PARKIN-independent pathway [35].
The p62 protein is made of multiple domains that provide a wide range of functions (Figure 1). The PB1 domain (Phox and Bem1p) is responsible for interacting with the autophagy receptor NBR1 and with a number of protein kinases (ERK, MEKK3, MEK5, and aPKCs). This domain is also responsible for protein di- and multimerization. The ZZ-type zinc finger domain is responsible for binding to RIP1 (receptor-interacting serine threonine kinase 1). The TB domain (TNF receptor-associated factor 6 (TRAF6) binding domain) contains the E3 binding site of the ubiquitin-protein ligase TRAF6. The LIR domain (C-terminal LC3- interacting region) and UBA domain (ubiquitin-associated domain) link the autophagic machinery to ubiquitinated protein substrates. Finally, KIR (Keap-interacting region) binds Keap1 and induce Nrf2 nuclear translocation [36].
4. p62-Nrf2 Regulatory Loop
The ability of p62 to activate Nrf2 was first described in 2010 by at least three research groups. The first was done by Komatsu et al. (2010), who demonstrated that p62 interacts with the Nrf2-binding site in Keap1, and that p62 accumulation results in an activation of Nrf2 [37]. At about the same time, Lau et al. (2010) presented the data that there is a direct interaction between p62 and Keap1. Accumulation of p62 sequesters Keap1 into aggregates, resulting in an inhibition of Keap1-dependent ubiquitination and subsequent degradation of Nrf2 [38]. At the same time, by means of immunopurification and mass spectrometry, an interaction between Keap1 and p62 was shown [39]. In the same year, the Komatsu group demonstrated that a simultaneous knockout of p62 and Nrf2 resulted in autophagy suppression, which also indirectly indicates the relationship between p62 and Nrf2 and the fact that the accumulation of p62-Keap1 aggregates leads to constitutive activation of Nrf2 [40]. However, it was shown that these aggregates are observed in more than 25% of human hepatocellular carcinomas. It is noteworthy that it is an increase in p62 expression, rather than Keap1 mutants, that causes malignant growth [41].
Jain et al. (2010) discovered ARE sequences in the promoter region of the gene encoding p62 and verified that Nrf2 binds to this cis-element in vivo and in vitro [42] (Figure 1). It has been shown that PMI (P62-mediated mitophagy inducer) and sulforaphane (Nrf2 inducer) are able to activate p62 expression via the Nrf2/ARE signaling pathway [43]. Recently, Liao et al. (2019) proved the existence of a positive feedback loop that is activated by cisplatin, which induces oxidative stress in an acute kidney injury model. The p62 knockdown significantly decreased Nrf2 protein expression, which was accompanied by an increase in oxidative stress. In turn, Nrf2 knockdown significantly reduced the cisplatin-induced expression of p62, and this caused a disruption in autophagosome formation [44].
However, there are some data suggesting that p62 may not activate Nrf2, but suppress its activity. There is a splicing variant of p62 that is lacking the last half of the KIR domain, which interacts with Keap1. As a result, there is ubiquitination of Nrf2 that is leading to its degradation by the 26S proteasome. This suppresses the expression of Nrf2 target genes [45]. However, the role of this p62 splicing variant in the regulation of mitophagy and its consequences for the pathogenesis of neurodegenerative diseases requires further studies.
5. Physiological Role of p62-Nrf2 Regulatory Loop
Both separately and together, p62 and Nrf2 signaling pathways are firmly associated with cell survival [46]. On the one hand, p62 and Nrf2 signaling pathways can protect a tissue from degeneration, which is especially important considering neurodegeneration. On the other hand, activation of these signaling pathways can lead to the development of oncological processes. A review by Katsuragi et al. (2016) discussed in detail the role of p62 and Nrf2 signaling pathways in the pathogenesis of hepatocellular carcinoma [47]. p62 and Nrf2 include activation phosphatidylinositol 3-kinase (PI3K)-Akt pathway and mammalian target of rapamycin complex 1 (mTORC1). Nrf2 positively regulates MTOR expression [48]. p62 interacts with molecules, which interact with mTOR and forms mTORC1. The p62 activation is a crucial step for mTOR activation [49]. The mTORC1 plays a dual role in the regulation of cellular processes. It is definitely necessary for long-term potentiation via regulation of protein synthesis [50]. On the other hand, mTOR promotes cell growth signaling, and its mutations were identified in several types of human cancer [51].
However, the p62-Keap1-Nrf2 axis promotes malignancy of hepatocellular carcinoma through enhancing UDP-glucuronate and glutathione production, which can promote hepatocellular carcinoma growth [52]. There are several reasons why p62 and Nrf2, which are thus important for mitophagy, may cause hepatocellular carcinoma. The reasons are mutations in the NFE2L2 and KEAP1 genes [53], chronic inflammation [54], which cause constitutive Nrf2 activation, and stable overexpression of p62.
In either case, the p62-Nrf2 regulatory loop is attractive for pharmacological intervention because it appears to be a good target for developing compounds that are aimed to suppress neurodegenerative processes. E.g., an accumulation of misfolded peptides (α-synuclein in Parkinson’s disease, a-beta and tau fibrils in Alzheimer’s disease, et cetera). In so far as ubiquitin plays a critical role in the elimination of misfolded and aggregated protein molecules, p62 is a good target for modulating proteasomal pathways [55]. A decrease in expression or inactivation of p62 gene in mice had caused some of the symptoms associated with Alzheimer’s disease (loss of working memory) and resulted in hyperphosphorylated tau, neurofibrillary tangles, and neurodegeneration [56]. Similar results were observed for Alzheimer’s disease rat model by injecting β-amyloid protein into the hippocampus, where p62 expression was reduced [57]. An increase in p62 expression resulted in a decrease in Aβ level and improved cognitive ability in APP/PS1 mice (a mouse model of Alzheimer’s disease) [58]. These results indicate that an increase in p62 expression may be a target to reduce the Aβ level and cognitive impairment.
At present, there are no compounds that modulate p62 expression; these have to be developed. However, a large number of Nrf2 activators have been described (Table 1). At the moment, only dimethyl fumarate is approved by the Food and Drug Administration (FDA) for the treatment of neurodegenerative disease (multiple sclerosis) [59]. In addition to dimethyl fumarate, Nrf2 activators such as curcumin, resveratrol, sulforaphane, masatinib, methylene blue, omaveloxolone, tideglusib, Dl-3-n-butylphthalide ide, ALKS-8700, benfotiamine, and ketogenic diet undergoing clinical trials for treating various neurodegenerative disease such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Friedreich’s ataxia, multiple sclerosis, amyotrophic lateral sclerosis, cataract, schizophrenia, bipolar disorder, mild cognitive impairment, depression, autism, obstructive sleep apnea, etc. [59]. A number of compounds of Nrf2 activators have been studied in animal and cellular models of Alzheimer’s and Parkinson’s disease. Among the most promising compounds (besides compounds undergoing clinical trials) for the treatment of Parkinson’s disease are carnosic acid, monomethyl fumarate, salidroside, β-ecdysterone, pinostrobin, berberine, vildagliptin, glaucocalyxin B, fasudil, protocatechuic acid, chrysin, hypoestoxide, α-Asarone [16,60]. Among the most promising compounds (besides compounds undergoing clinical trials) for the treatment of Alzheimer’s disease are carnosic acid, gypenoside XVII, eriodictyol, hesperidin, puerarin, orientin, antroquinonol, sodium hydrosulfide, vanillic acid, methysticin, 3H-1,2-dithiole-3-thione, mini-GAGR, allicin, triterpenoid CDDO-methylamide (CDDO-MA) [60,61]. Other triterpenoids such as CDDO-ethyl amide (CDDO-EA) and CDDO-trifluoroethyl amide (CDDO-TFEA) improve the behavioral phenotype in a model of Huntington’s disease [62] (Table 1). Unfortunately, none of these compounds are in clinical trials as of now. The major reason for that is—in our understanding—is that the signaling mechanisms underlying the interaction of mitochondria with neuronal metabolism are insufficiently studied, as well as the mechanisms that actually control the survival of neurons in their native location (human brain).
Table 1.
Nrf2 activators undergoing clinical trials and research in the cells and animal models of neurodegenerative diseases.
| Compound | Disease | Research Type | Reference |
|---|---|---|---|
| Curcumin | Schizophrenia | Clinical trial. Phase I | NCT02104752 * |
| Alzheimer’s disease | Clinical trial. Phase I/II | NCT00164749 | |
| Schizophrenia | Clinical trial. Phase IV | NCT02298985 | |
| Mild cognitive impairment | Clinical trial. Phase II | NCT01811381 | |
| Major depression | Clinical trial. Phase IV | NCT01750359 | |
| Parkinson’s disease | Rat model. Rotenone-injured | [63] | |
| Resveratrol | Alzheimer’s disease | Clinical trial. Phase II | NCT01504854 |
| Clinical trial. Phase III | NCT00743743 | ||
| Huntington’s disease | Clinical trial. Phase III | NCT02336633 | |
| Friedreich’s ataxia | Clinical trial. Phase II | NCT03933163 | |
| Sulforaphane | Schizophrenia | Clinical trial. Phase II | NCT02880462 |
| Clinical trial. Phase II | NCT02810964 | ||
| Clinical trial. Phase II | NCT01716858 | ||
| Autism | Clinical trial. Phase II | NCT01474993 | |
| Clinical trial. Phase II | NCT02909959 | ||
| Clinical trial. Phase II | NCT02677051 | ||
| Clinical trial. Phase III | NCT02654743 | ||
| Clinical trial. Phase I/II | NCT02561481 | ||
| Alzheimer’s disease | Clinical trial. Recruiting | NCT04213391 | |
| Parkinson’s disease | Cell models. Mice models. 6-OHDA treated. |
[64] | |
| Dimethyl fumarate | Multiple sclerosis | Clinical trial. FDA Approved in 2013 |
NCT02047097, NDA 204063 ** |
| Obstructive sleep apnea | Clinical trial. Phase II | NCT02438137 | |
| Alzheimer’s disease | Mice models. P301L mice | [65] | |
| Parkinson’s disease | Mice models. MPTP treated |
[66] | |
| Huntington’s disease | Mice models. R6/2 and YAC128 mice |
[67] | |
| Masatinib | Multiple sclerosis | Clinical trial. Phase III | NCT01433497 |
| Alzheimer’s disease | Clinical trial. Phase II/III | NCT01872598 | |
| Amyotrophic lateral sclerosis | Clinical trial. Phase II | NCT02588677 | |
| Methylene blue | Alzheimer’s disease | Clinical trial. Phase II | NCT02380573 |
| Bipolar disorder | Clinical trial. Phase III | NCT00214877 | |
| Parkinson’s disease | Mice models. MPTP treated |
[68] | |
| Omaveloxolone | Friedreich’s ataxia | Clinical trial. Phase II | NCT02255435 |
| Cataract | Clinical trial. Phase II | NCT02128113 | |
| Tideglusib | Autism | Clinical trial. Phase II | NCT02586935 |
| Alzheimer’s disease | Clinical trial. Phase II | NCT01350362 | |
| Ketogenic diet | Alzheimer’s disease | Clinical trial. Phase II | NCT04466735 |
| Clinical trial. Not Applicable | NCT03690193 | ||
| Dl-3-n-butylphthalide ide | Alzheimer’s disease | Clinical trial. Not Applicable | NCT02711683 |
| ALKS-8700 | Multiple sclerosis | Clinical trial. Phase III | NCT02634307 |
| Benfotiamine | Alzheimer’s disease | Clinical trial. Phase II | NCT02292238 |
| Carnosic acid | Alzheimer’s disease | Mice models.(hAPP)-J20 and 3×Tg-AD mice | [69] |
| Parkinson’s disease | Cell models. Paraquat treated SH-SY5Y cells | [70] | |
| ITH12674 | Brain ischemia | Culture of rat cortical neurons | [71] |
| Monomethyl fumarate | Parkinson’s disease | Mice models. MPTP treated | [66] |
| Salidroside | Parkinson’s disease | Cell models. MPP+/MPTP treated | [72] |
| β-Ecdysterone | Parkinson’s disease | Cell models. MPP+ treated | [73] |
| Pinostrobin | Parkinson’s disease | Cell models. MPTP treated SH-SY5Y cells | [74] |
| Berberine | Parkinson’s disease | Zebrafish models. 6-OHDA treated | [75] |
| Vildagliptin | Parkinson’s disease | Rat models. Rotenone treated | [76] |
| Glaucocalyxin B | Parkinson’s disease | Rat models. Lipopolysaccharide-injected | [77] |
| Fasudil | Parkinson’s disease | Mice models. MPTP treated | [78] |
| Protocatechuic acid | Parkinson’s disease | Cell models. 6-OHDA treated PC12 cells | [79] |
| Chrysin | Parkinson’s disease | Cell models. 6-OHDA treated PC12 cells | [79] |
| Hypoestoxide | Parkinson’s disease | Mice models. mThy1-α-syn transgenic mice |
[80] |
| α-Asarone | Parkinson’s disease | Mice models. MPTP treated | [81] |
| Gypenoside XVII | Alzheimer’s disease | Cell models. Aβ treated | [82] |
| Eriodictyol | Alzheimer’s disease | Cell models. Aβ treated | [83] |
| Hesperidin | Alzheimer’s disease | Mice models. APP/PS1 mice | [84] |
| Puerarin | Alzheimer’s disease | Mice models. APP/PS1 mice | [85] |
| Orientin | Alzheimer’s disease | Mice models. Aβ injected | [86] |
| Antroquinonol | Alzheimer’s disease | Mice models. Aβ injected | [87] |
| Sodium hydrosulfide | Alzheimer’s disease | Mice models. APP/PS1 mice | [88] |
| Vanillic acid | Alzheimer’s disease | Mice models. Aβ injected | [89] |
| Methysticin | Alzheimer’s disease | Mice models. APP/PS1 mice | [90] |
| 3H-1,2-dithiole-3-thione | Alzheimer’s disease | Mice models. Tg2576 mice | [91] |
| Mini-GAGR | Alzheimer’s disease | Mice models. 3xTg-AD mice | [92] |
| Allicin | Alzheimer’s disease | Rat models. Tunicamycin-injected | [93] |
| CDDO-MA | Alzheimer’s disease | Mice models. Tg19959 mice | [94] |
| CDDO-EA | Huntington’s disease | Mice models. N171-82Q mice | [62] |
| CDDO-TFEA | Huntington’s disease | Mice models. N171-82Q mice | [62] |
* NCT—national clinical trial; ** NDA—new drug approval.
6. Conclusions
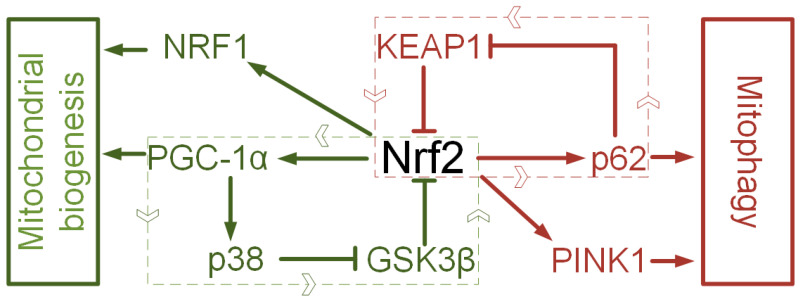
The value of Nrf2 activators is limited not only to their ability to reduce oxidative stress, which has been repeatedly discussed [95,96,97]. Oxidative stress is not only a cause of mitochondrial dysfunction but a consequence of a disruption of mitochondrial quality control [98]. An imbalance occurs when there is a violation of the coordination of mitochondrial biogenesis and mitophagy [99]. When biogenesis is suppressed, and mitophagy is activated, an energy deficit occurs. When mitophagy is suppressed, and mitochondrial biogenesis is activated, a large number of damaged mitochondria can accumulate in the cell, which will produce a lot of ROS, but at the same time not fully meet the energy requirements due to the non-functional respiratory chain [99]. Nrf2 is capable of forming regulatory loops that are involved in the regulation of mitochondrial biogenesis. There is evidence that Nrf2 increases the expression of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (PGC-1α) (master regulator mitochondrial biogenesis) and nuclear respiratory factor (NRF1), which are directly involved in the regulation of mtDNA transcription. PGC-1α, in turn, deactivates GSK3β via p38 [17]. Another loop involves interaction with p62, which forms a loop with Nrf2 by inactivating Keap1. However, the regulation of mitophagy by Nrf2 is not limited to this. There is evidence that Nrf2 regulates the expression of Pink1, which plays a key role in mitophagy induction (Figure 2). It is important that Nrf2 activation does not lead to an imbalance in the direction of mitophagy or mitochondrial biogenesis but rather to maintain a dynamic balance, which is essential for mitochondrial stability.
Figure 2.
Two regulatory loops for mitochondria turnover. Nrf2—PGC1α—p38—GSK3β—Nrf2 loop and Nrf2—NRF1 interaction regulate of mitochondrial biogenesis. Nrf2—p62—KEAP1—Nrf2 loop and Nrf2—PINK1 interaction regulate of mitochondrial biogenesis.
Author Contributions
Conceptualization, A.P.G., V.N.P. and A.A.S; writing—original draft preparation, A.P.G. and I.S.S.; supervision, N.N.S.; funding acquisition, V.N.P. and A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation within the framework of the State assignment to universities in the field of scientific activity on 2020–2022 (project FZGU-2020-0044) to V.N.P, the Russian Foundation for Basic Research (project 17-29-06036 ofi_m) to V.N.P, and AG014930/AG/NIA NIH HHS/United States to A.A.S.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yan M.H., Wang X., Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic. Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luczynski P., Laule C., Hsiung G.-Y.R., Wayne Moore G.R., Tremlett H. Coexistence of Multiple Sclerosis and Alzheimer’s disease: A review. Mult. Scler. Relat. Disord. 2019;27:232–238. doi: 10.1016/j.msard.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 3. [(accessed on 20 October 2020)]; Available online: https://www.parkinson.org/Understanding-Parkinsons/Statistics.
- 4.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018;592:692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 5.Nissanka N., Moraes C.T. Mitochondrial DNA damage and reactive oxygen species in neurodegenerative disease. FEBS Lett. 2018;592:728–742. doi: 10.1002/1873-3468.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAvoy K., Kawamata H. Glial mitochondrial function and dysfunction in health and neurodegeneration. Mol. Cell Neurosci. 2019;101:103417. doi: 10.1016/j.mcn.2019.103417. [DOI] [PubMed] [Google Scholar]
- 7.Payne B.A.I., Chinnery P.F. Mitochondrial dysfunction in aging: Much progress but many unresolved questions. Biochim. Biophys. Acta. 2015;1847:1347–1353. doi: 10.1016/j.bbabio.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merkwirth C., Martinelli P., Korwitz A., Morbin M., Brönneke H.S., Jordan S.D., Rugarli E.I., Langer T. Loss of prohibitin membrane scaffolds impairs mitochondrial architecture and leads to tau hyperphosphorylation and neurodegeneration. PLoS Genet. 2012;8:e1003021. doi: 10.1371/journal.pgen.1003021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoshan-Barmatz V., Nahon-Crystal E., Shteinfer-Kuzmine A., Gupta R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol. Res. 2018;131:87–101. doi: 10.1016/j.phrs.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Silachev D.N., Plotnikov E.Y., Zorova L.D., Pevzner I.B., Sumbatyan N.V., Korshunova G.A., Gulyaev M.V., Pirogov Y.A., Skulachev V.P., Zorov D.B. Neuroprotective Effects of Mitochondria-Targeted Plastoquinone and Thymoquinone in a Rat Model of Brain Ischemia/Reperfusion Injury. Molecules. 2015;20:14487–14503. doi: 10.3390/molecules200814487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markaki M., Tavernarakis N. Mitochondrial turnover and homeostasis in ageing and neurodegeneration. FEBS Lett. 2020 doi: 10.1002/1873-3468.13802. [DOI] [PubMed] [Google Scholar]
- 13.Fu W., Liu Y., Yin H. Mitochondrial Dynamics: Biogenesis, Fission, Fusion, and Mitophagy in the Regulation of Stem Cell Behaviors. Stem Cells Int. 2019;2019:9757201. doi: 10.1155/2019/9757201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertholet A.M., Delerue T., Millet A.M., Moulis M.F., David C., Daloyau M., Arnauné-Pelloquin L., Davezac N., Mils V., Miquel M.C., et al. Mitochondrial fusion/fission dynamics in neurodegeneration and neuronal plasticity. Neurobiol. Dis. 2016;90:3–19. doi: 10.1016/j.nbd.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Silva-Islas C.A., Maldonado P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018;134:92–99. doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Gureev A.P., Popov V.N. Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 2019;44:2273–2279. doi: 10.1007/s11064-018-02711-2. [DOI] [PubMed] [Google Scholar]
- 17.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasserman W.W., Fahl W.E. Comprehensive analysis of proteins which interact with the antioxidant responsive element: Correlation of ARE-BP-1 with the chemoprotective induction response. Arch. Biochem. Biophys. 1997;344:387–396. doi: 10.1006/abbi.1997.0215. [DOI] [PubMed] [Google Scholar]
- 20.Nam L.B., Keum Y.S. Binding partners of NRF2: Functions and regulatory mechanisms. Arch. Biochem. Biophys. 2019;678:108184. doi: 10.1016/j.abb.2019.108184. [DOI] [PubMed] [Google Scholar]
- 21.Tkachev V.O., Menshchikova E.B., Zenkov N.K. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc.) 2011;76:407–422. doi: 10.1134/S0006297911040031. [DOI] [PubMed] [Google Scholar]
- 22.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu T., Zhao F., Gao B., Tan C., Yagishita N., Nakajima T., Wong P.K., Chapman E., Fang D., Zhang D.D. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta K., Ohigashi M., Naganawa A., Ikeda H., Sakai M., Nishikawa J., Imagawa M., Osada S., Nishihara T. Histone acetyltransferase MOZ acts as a co-activator of Nrf2-MafK and induces tumour marker gene expression during hepatocarcinogenesis. Biochem. J. 2007;402:559–566. doi: 10.1042/BJ20061194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J.D., et al. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 29.Erlank H., Elmann A., Kohen R., Kanner J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011;51:2319–2327. doi: 10.1016/j.freeradbiomed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 30.Lamark T., Svenning S., Johansen T. Regulation of selective autophagy: The p62/SQSTM1 paradigm. Essays Biochem. 2017;61:609–624. doi: 10.1042/EBC20170035. [DOI] [PubMed] [Google Scholar]
- 31.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell. Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S.M., Lazarou M., Wang C., Kane L.A., Narendra D.P., Youle R.J. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J. Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narendra D., Kane L.A., Hauser D.N., Fearnley I.M., Youle R.J. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 35.Yamada T., Dawson T.M., Yanagawa T., Iijima M., Sesaki H. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy. Autophagy. 2019;15:2012–2018. doi: 10.1080/15548627.2019.1643185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippai M., Lőw P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Res. Int. 2014;2014:832704. doi: 10.1155/2014/832704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 38.Lau A., Wang X.J., Zhao F., Villeneuve N.F., Wu T., Jiang T., Sun Z., White E., Zhang D.D. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: Direct interaction between Keap1 and p62. Mol. Cell. Biol. 2010;30:3275–3285. doi: 10.1128/MCB.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Copple I.M., Lister A., Obeng A.D., Kitteringham N.R., Jenkins R.E., Layfield R., Foster B.J., Goldring C.E., Park B.K. Physical and functional interaction of sequestosome 1 with Keap1 regulates the Keap1-Nrf2 cell defense pathway. J. Biol. Chem. 2010;285:16782–16788. doi: 10.1074/jbc.M109.096545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley B.E., Kaiser S.E., Shaler T.A., Ng A.C., Hara T., Hipp M.S., Lage K., Xavier R.J., Ryu K.Y., Taguchi K., et al. Ubiquitin accumulation in autophagy-deficient mice is dependent on the Nrf2-mediated stress response pathway: A potential role for protein aggregation in autophagic substrate selection. J. Cell Biol. 2010;191:537–552. doi: 10.1083/jcb.201005012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inami Y., Waguri S., Sakamoto A., Kouno T., Nakada K., Hino O., Watanabe S., Ando J., Iwadate M., Yamamoto M., et al. Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J. Cell Biol. 2011;193:275–284. doi: 10.1083/jcb.201102031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain A., Lamark T., Sjøttem E., Larsen K.B., Awuh J.A., Øvervatn A., McMahon M., Hayes J.D., Johansen T. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. J. Biol. Chem. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.East D.A., Fagiani F., Crosby J., Georgakopoulos N.D., Bertrand H., Schaap M., Fowkes A., Wells G., Campanella M. PMI: A ΔΨm independent pharmacological regulator of mitophagy. Chem. Biol. 2014;21:1585–1596. doi: 10.1016/j.chembiol.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao W., Wang Z., Fu Z., Ma H., Jiang M., Xu A., Zhang W. p62/SQSTM1 protects against cisplatin-induced oxidative stress in kidneys by mediating the cross talk between autophagy and the Keap1-Nrf2 signalling pathway. Free Radic. Res. 2019;53:800–814. doi: 10.1080/10715762.2019.1635251. [DOI] [PubMed] [Google Scholar]
- 45.Kageyama S., Saito T., Obata M., Koide R.H., Ichimura Y., Komatsu M. Negative Regulation of the Keap1-Nrf2 Pathway by a p62/Sqstm1 Splicing Variant. Mol. Cell. Biol. 2018;38:e00642-17. doi: 10.1128/MCB.00642-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchal J.A., Lopez G.J., Peran M., Comino A., Delgado J.R., García-García J.A., Conde V., Aranda F.M., Rivas C., Esteban M., et al. The impact of PKR activation: From neurodegeneration to cancer. FASEB J. 2014;28:1965–1974. doi: 10.1096/fj.13-248294. [DOI] [PubMed] [Google Scholar]
- 47.Katsuragi Y., Ichimura Y., Komatsu M. Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr. Opin. Toxicol. 2016;1:54–61. doi: 10.1016/j.cotox.2016.09.005. [DOI] [Google Scholar]
- 48.Shibata T., Saito S., Kokubu A., Suzuki T., Yamamoto M., Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 49.Duran A., Amanchy R., Linares J.F., Joshi J., Abu-Baker S., Porollo A., Hansen M., Moscat J., Diaz-Meco M.T. p62 is a key regulator of nutrient sensing in the mTORC1 pathway. Mol. Cell. 2011;44:134–146. doi: 10.1016/j.molcel.2011.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Switon K., Kotulska K., Janusz-Kaminska A., Zmorzynska J., Jaworski J. Molecular neurobiology of mTOR. Neuroscience. 2017;341:112–153. doi: 10.1016/j.neuroscience.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 51.Murugan A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019;59:92–111. doi: 10.1016/j.semcancer.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Saito T., Ichimura Y., Taguchi K., Suzuki T., Mizushima T., Takagi K., Hirose Y., Nagahashi M., Iso T., Fukutomi T., et al. p62/Sqstm1 promotes malignancy of HCV-positive hepatocellular carcinoma through Nrf2-dependent metabolic reprogramming. Nat. Commun. 2016;7:12030. doi: 10.1038/ncomms12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menegon S., Columbano A., Giordano S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016;22:578–593. doi: 10.1016/j.molmed.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Ahmed S.M., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 55.Ma S., Attarwala I.Y., Xie X.Q. SQSTM1/p62: A Potential Target for Neurodegenerative Disease. ACS Chem. Neurosci. 2019;10:2094–2114. doi: 10.1021/acschemneuro.8b00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramesh Babu J., Lamar Seibenhener M., Peng J., Strom A.L., Kemppainen R., Cox N., Zhu H., Wooten M.C., Diaz-Meco M.T., Moscat J., et al. Genetic inactivation of p62 leads to accumulation of hyperphosphorylated tau and neurodegeneration. J. Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- 57.Zheng X., Wang W., Liu R., Huang H., Zhang R., Sun L. Effect of p62 on tau hyperphosphorylation in a rat model of Alzheimer’s disease. Neural Regen. Res. 2012;7:1304–1311. doi: 10.3969/j.issn.1673-5374.2012.17.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caccamo A., Ferreira E., Branca C., Oddo S. p62 improves AD-like pathology by increasing autophagy. Mol. Psychiatry. 2017;22:865–873. doi: 10.1038/mp.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell Longev. 2019;2019:9372182. doi: 10.1155/2019/9372182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fão L., Mota S.I., Rego A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2019;54:100942. doi: 10.1016/j.arr.2019.100942. [DOI] [PubMed] [Google Scholar]
- 61.Bahn G., Jo D.G. Therapeutic Approaches to Alzheimer’s Disease Through Modulation of NRF2. Neuromol. Med. 2019;21:1–11. doi: 10.1007/s12017-018-08523-5. [DOI] [PubMed] [Google Scholar]
- 62.Stack C., Ho D., Wille E., Calingasan N.Y., Williams C., Liby K., Sporn M., Dumont M., Beal M.F. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic. Biol. Med. 2010;49:147–158. doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui Q., Li X., Zhu H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol. Med. Rep. 2016;13:1381–1388. doi: 10.3892/mmr.2015.4657. [DOI] [PubMed] [Google Scholar]
- 64.Morroni F., Sita G., Djemil A., D’Amico M., Pruccoli L., Cantelli-Forti G., Hrelia P., Tarozzi A. Comparison of Adaptive Neuroprotective Mechanisms of Sulforaphane and its Interconversion Product Erucin in in Vitro and in Vivo Models of Parkinson’s Disease. J. Agric. Food Chem. 2018;66:856–865. doi: 10.1021/acs.jafc.7b04641. [DOI] [PubMed] [Google Scholar]
- 65.Cuadrado A., Kügler S., Lastres-Becker I. Pharmacological targeting of GSK-3 and NRF2 provides neuroprotection in a preclinical model of tauopathy. Redox Biol. 2018;14:522–534. doi: 10.1016/j.redox.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahuja M., Ammal Kaidery N., Yang L., Calingasan N., Smirnova N., Gaisin A., Gaisina I.N., Gazaryan I., Hushpulian D.M., Kaddour-Djebbar I., et al. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J. Neurosci. 2016;36:6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ellrichmann G., Petrasch-Parwez E., Lee D.H., Reick C., Arning L., Saft C., Gold R., Linker R.A. Efficacy of fumaric acid esters in the R6/2 and YAC128 models of Huntington’s disease. PLoS ONE. 2011;6:e16172. doi: 10.1371/journal.pone.0016172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biju K.C., Evans R.C., Shrestha K., Carlisle D., Gelfond J., Clark R.A. Methylene Blue Ameliorates Olfactory Dysfunction and Motor Deficits in a Chronic MPTP/Probenecid Mouse Model of Parkinson’s Disease. Neuroscience. 2018;380:111–122. doi: 10.1016/j.neuroscience.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 69.Lipton S.A., Rezaie T., Nutter A., Lopez K.M., Parker J., Kosaka K., Satoh T., McKercher S.R., Masliah E., Nakanishi N. Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer’s disease models. Cell Death Dis. 2016;7:e2499. doi: 10.1038/cddis.2016.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Oliveira M.R., Ferreira G.C., Schuck P.F. Protective effect of carnosic acid against paraquat-induced redox impairment and mitochondrial dysfunction in SH-SY5Y cells: Role for PI3K/Akt/Nrf2 pathway. Toxicol. Vitr. 2016;32:41–54. doi: 10.1016/j.tiv.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 71.Egea J., Buendia I., Parada E., Navarro E., Rada P., Cuadrado A., López M.G., García A.G., León R. Melatonin-sulforaphane hybrid ITH12674 induces neuroprotection in oxidative stress conditions by a ‘drug-prodrug’ mechanism of action. Br. J. Pharmacol. 2015;172:1807–1821. doi: 10.1111/bph.13025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li R., Wang S., Li T., Wu L., Fang Y., Feng Y., Zhang L., Chen J., Wang X. Salidroside Protects Dopaminergic Neurons by Preserving Complex I Activity via DJ-1/Nrf2-Mediated Antioxidant Pathway. Parkinson’s Dis. 2019;2019:6073496. doi: 10.1155/2019/6073496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou Y., Wang R., Guo H., Dong M. Phytoestrogen β-Ecdysterone Protects PC12 Cells Against MPP+-Induced Neurotoxicity In Vitro: Involvement of PI3K-Nrf2-Regulated Pathway. Toxicol. Sci. 2015;147:28–38. doi: 10.1093/toxsci/kfv111. [DOI] [PubMed] [Google Scholar]
- 74.Li C., Tang B., Feng Y., Tang F., Pui-Man Hoi M., Su Z., Ming-Yuen Lee S. Pinostrobin Exerts Neuroprotective Actions in Neurotoxin-Induced Parkinson’s Disease Models through Nrf2 Induction. J. Agric. Food Chem. 2018;66:8307–8318. doi: 10.1021/acs.jafc.8b02607. [DOI] [PubMed] [Google Scholar]
- 75.Zhang C., Li C., Chen S., Li Z., Jia X., Wang K., Bao J., Liang Y., Wang X., Chen M., et al. Berberine protects against 6-OHDA-induced neurotoxicity in PC12 cells and zebrafish through hormetic mechanisms involving PI3K/AKT/Bcl-2 and Nrf2/HO-1 pathways. Redox Biol. 2017;11:1–11. doi: 10.1016/j.redox.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdelsalam R.M., Safar M.M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: Role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015;133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 77.Xu W., Zheng D., Liu Y., Li J., Yang L., Shang X. Glaucocalyxin B Alleviates Lipopolysaccharide-Induced Parkinson’s Disease by Inhibiting TLR/NF-κB and Activating Nrf2/HO-1 Pathway. Cell Physiol. Biochem. 2017;44:2091–2104. doi: 10.1159/000485947. [DOI] [PubMed] [Google Scholar]
- 78.Zhao Y.F., Zhang Q., Xi J.Y., Li Y.H., Ma C.G., Xiao B.G. Multitarget intervention of Fasudil in the neuroprotection of dopaminergic neurons in MPTP-mouse model of Parkinson’s disease. J. Neurol. Sci. 2015;353:28–37. doi: 10.1016/j.jns.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z., Li G., Szeto S., Chong C.M., Quan Q., Huang C., Cui W., Guo B., Wang Y., Han Y., et al. Examining the neuroprotective effects of protocatechuic acid and chrysin on in vitro and in vivo models of Parkinson disease. Free Radic. Biol. Med. 2015;84:331–343. doi: 10.1016/j.freeradbiomed.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 80.Kim C., Ojo-Amaize E., Spencer B., Rockenstein E., Mante M., Desplats P., Wrasidlo W., Adame A., Nchekwube E., Oyemade O., et al. Hypoestoxide reduces neuroinflammation and α-synuclein accumulation in a mouse model of Parkinson’s disease. J. Neuroinflamm. 2015;12:236. doi: 10.1186/s12974-015-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim B.W., Koppula S., Kumar H., Park J.Y., Kim I.W., More S.V., Kim I.S., Han S.D., Kim S.K., Yoon S.H., et al. α-Asarone attenuates microglia-mediated neuroinflammation by inhibiting NF kappa B activation and mitigates MPTP-induced behavioral deficits in a mouse model of Parkinson’s disease. Neuropharmacology. 2015;97:46–57. doi: 10.1016/j.neuropharm.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 82.Meng X., Wang M., Sun G., Ye J., Zhou Y., Dong X., Wang T., Lu S., Sun X. Attenuation of Aβ25-35-induced parallel autophagic and apoptotic cell death by gypenoside XVII through the estrogen receptor-dependent activation of Nrf2/ARE pathways. Toxicol. Appl. Pharmacol. 2014;279:63–75. doi: 10.1016/j.taap.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 83.Jing X., Shi H., Zhu X., Wei X., Ren M., Han M., Ren D., Lou H. Eriodictyol Attenuates β-Amyloid 25-35 Peptide-Induced Oxidative Cell Death in Primary Cultured Neurons by Activation of Nrf2. Neurochem. Res. 2015;40:1463–1471. doi: 10.1007/s11064-015-1616-z. [DOI] [PubMed] [Google Scholar]
- 84.Hong Y., An Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-κB signaling. Arch. Pharm. Res. 2018;41:655–663. doi: 10.1007/s12272-015-0662-z. [DOI] [PubMed] [Google Scholar]
- 85.Yu W., An S., Shao T., Xu H., Chen H., Ning J., Zhou Y., Chai X. Active compounds of herbs ameliorate impaired cognition in APP/PS1 mouse model of Alzheimer’s disease. Aging. 2019;11:11186–11201. doi: 10.18632/aging.102522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu L., Wang S., Chen X., Yang H., Li X., Xu Y., Zhu X. Orientin alleviates cognitive deficits and oxidative stress in Aβ1-42-induced mouse model of Alzheimer’s disease. Life Sci. 2015;121:104–109. doi: 10.1016/j.lfs.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 87.Chang W.H., Chen M.C., Cheng I.H. Antroquinonol Lowers Brain Amyloid-β Levels and Improves Spatial Learning and Memory in a Transgenic Mouse Model of Alzheimer’s Disease. Sci. Rep. 2015;5:15067. doi: 10.1038/srep15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y., Deng Y., Liu H., Yin C., Li X., Gong Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: A novel mechanism mediated by the activation of Nrf2. Pharmacol. Biochem. Behav. 2016;150–151:207–216. doi: 10.1016/j.pbb.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 89.Amin F.U., Shah S.A., Kim M.O. Vanillic acid attenuates Aβ1-42-induced oxidative stress and cognitive impairment in mice. Sci. Rep. 2017;7:40753. doi: 10.1038/srep40753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fragoulis A., Siegl S., Fendt M., Jansen S., Soppa U., Brandenburg L.O., Pufe T., Weis J., Wruck C.J. Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer’s disease. Redox Biol. 2017;12:843–853. doi: 10.1016/j.redox.2017.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cui Y., Ma S., Zhang C., Li D., Yang B., Lv P., Xing Q., Huang T., Yang G.L., Cao W., et al. Pharmacological activation of the Nrf2 pathway by 3H-1, 2-dithiole-3-thione is neuroprotective in a mouse model of Alzheimer disease. Behav. Brain Res. 2018;336:219–226. doi: 10.1016/j.bbr.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Murphy K., Llewellyn K., Wakser S., Pontasch J., Samanich N., Flemer M., Hensley K., Kim D.S., Park J. Mini-GAGR, an intranasally applied polysaccharide, activates the neuronal Nrf2-mediated antioxidant defense system. J. Biol. Chem. 2018;293:18242–18269. doi: 10.1074/jbc.RA117.001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y.F., Li X.H., Yuan Z.P., Li C.Y., Tian R.B., Jia W., Xiao Z.P. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur. J. Pharmacol. 2015;762:239–246. doi: 10.1016/j.ejphar.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Dumont M., Wille E., Calingasan N.Y., Tampellini D., Williams C., Gouras G.K., Liby K., Sporn M., Nathan C., Flint Beal M., et al. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer’s disease. J. Neurochem. 2009;109:502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Vries H.E., Witte M., Hondius D., Rozemuller A.J., Drukarch B., Hoozemans J., van Horssen J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008;45:1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Cederbaum A.I. Nrf2 and antioxidant defense against CYP2E1 toxicity. Sub-Cell. Biochem. 2013;67:105–130. doi: 10.1007/978-94-007-5881-0_2. [DOI] [PubMed] [Google Scholar]
- 97.Hu L., Zhang Y., Miao W., Cheng T. Reactive Oxygen Species and Nrf2: Functional and Transcriptional Regulators of Hematopoiesis. Oxid. Med. Cell. Longev. 2019;2019:5153268. doi: 10.1155/2019/5153268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kotiadis V.N., Duchen M.R., Osellame L.D. Mitochondrial quality control and communications with the nucleus are important in maintaining mitochondrial function and cell health. Biochim. Biophys. Acta. 2014;1840:1254–1265. doi: 10.1016/j.bbagen.2013.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Palikaras K., Lionaki E., Tavernarakis N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015;22:1399–1401. doi: 10.1038/cdd.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]