SUMMARY
Objective:
Quantitative, micrometer length scale assessment of human articular cartilage is essential to enable progress toward new functional tissue engineering approaches, including utilization of emerging 3D bioprinting technologies, and for improved computational modeling of the osteochondral unit. Thus the objective of this study was to characterize the structural organization, material properties, and chemical composition of human skeletally mature articular cartilage with respect to depth and defined morphological features: normal to the articulating surface, parallel to the split-line, and transverse to the split-line.
Method:
Three samples from the lateral femoral condyles of 4 healthy adult donors (55–61 years old) were evaluated via histology, second harmonic generation, microindentation, and Raman spectroscopy. All metrics were evaluated as a function of depth and direction relative to the split-line.
Results:
All donors presented with intact and healthy tissue. Collagen fiber orientation varied significantly between testing directions and with increasing depth from the articular surface. Both compressive and tensile modulus increased significantly with depth and differed across the middle and deep zones and depended on orthogonal direction relative to the split-line. Similarly, matrix components varied with both depth and direction, where chondroitin sulfate steadily increased with depth while collagen prevalence was highest in the surface layer.
Conclusions:
Microscale measurements of human articular cartilage demonstrate that properties are both depth-dependent and orthotropic and depend on the underlying tissue structure and composition. These findings improve upon existing knowledge establishing more accurate measurements, with greater degree of depth and spatial specificity, as inputs for tissue engineering and computational modeling.
Keywords: Articular cartilage, Anisotropy, SHG, Indentation, Raman
Introduction
Left untreated, cartilage injuries and defects progress to tissue degeneration, pain, and functional impairment culminating in osteoarthritis (OA) and, total joint arthroplasty in advanced OA1. While arthroscopic cartilage repair strategies exist, clinical outcomes are unsatisfactory2-5. Emerging technologies, including 3D bioprinting, hold exciting promise by enabling formation of heterogeneous, zonally-appropriate biomimetic constructs. However, such approaches require deriving design inputs from the quantitative assessment of articular cartilage zonal anisotropy, tissue composition, and architecture. The field of articular cartilage characterization is quite mature, yet we lack empirical data that describe how human articular cartilage tissue mechanics, structure, and composition vary both with depth and direction at micro-to millimeter length scales, i.e., scales that are relevant to cells and important for guiding novel biomaterial design.
Articular cartilage has a preferred surface organization, often described as split-line orientation6-9, characterized by the primary collagen fiber direction which coincides with the direction of maximal tensile stress10. Benninghoff first described the collagen arrangement in articular cartilage as arch-like structures that are organized by depth and orientation11. Early scanning electron microscopy images of cartilage revealed a complex 3D architecture and preferential collagen orientation10,12,13. Subsequent studies have determined that fractures within articular cartilage often occur along the split-lines, which are not only present on the surface of the tissue but also in the underlying intermediate zone14-16. Thus, researchers have suggested matching split-line direction when performing osteochondral transplants to optimize treatment success rates6. Furthermore, material characterization of bovine articular cartilage suggests the tissue might have orthotropic symmetry17,18, which has yet to be thoroughly investigated in human articular cartilage.
Consideration of the mechanical, compositional, and structural anisotropy collectively will improve our understanding of the tissue as a whole and aid in the design of tissue-engineered constructs. The microarchitecture of articular cartilage undoubtedly plays a role in the biomechanics of the tissue. Mechanical anisotropy is thought to be dominated by the collagen fiber structural organization17,19,20, and also may orient orthogonally to cartilage split-lines18. This bulk tissue-level anisotropy provides essential design criteria, but local directional and microscale properties are likely more integral than previously thought. The mechanobiological responses of the chondrocytes in articular cartilage drive the production of extracellular matrix (ECM) components such as proteoglycans which, in turn, impact tissue mechanical properties21. However, excessive loading can halt ECM synthesis and cause chondrocyte apoptosis and necrosis22,23. Furthermore, since chondrocytes are sensitive to small mechanobiological perturbations, understanding the gradation of articular cartilage properties both directionally and depth-wise, and at length scales that are relevant to cells within the cartilage matrix, would supply a repository of information that can be used to drive design of emerging regenerative approaches.
The objective of the current work was to characterize structural organization, material properties, and chemical composition of skeletally mature human articular cartilage normal to the articulating surface, parallel to the split-line, and transverse to the split-line throughout the full tissue depth at length scales relevant to cells. Here, we evaluated articular cartilage ECM structure using Second Harmonic Generation (SHG) imaging, mechanics with micro-indentation, and composition (i.e., tissue biochemistry) via Raman Spectroscopy through the full depth of the tissue. These results provide novel insight into cartilage anisotropy and enable novel correlations to be made to better inform tissue-engineered material design.
Materials and methods
Gross morphology
Samples were obtained from femoral condyles of two male and two female human donors (55–61 years old) with no clinical history of osteoarthritis from a tissue bank (Science Care Inc., Phoenix, AZ 85207). Specimens were fresh-frozen and subjected to two additional freeze–thaw cycles throughout the testing process. Prior to dissection, joints were wrapped in gauze with phosphate-buffered saline (PBS) and secured at the isocenter of a 7T Biospec magnetic resonance imaging (MRI) scanner (Bruker Medical GmbH) for imaging. Photographs were taken of cartilage surfaces to identify regions of potential arthritis, and synovial fluid was assessed for discoloration (e.g., blood). Three blind graders assessed gross morphology in the anterior and posterior regions of both condyles. A score of 1–4 was assigned for intact cartilage, cartilage with few surface lesions, cartilage with moderate fibrillation, and cartilage with full-thickness erosion exposing underlying bone, respectively. An intraclass correlation coefficient using a two-way mixed effects model, absolute agreement, and multiple raters/measurements was calculated to assess intra-grader reliability24.
Sample preparation
Three osteochondral plugs were harvested from each donor from the center of the lateral femoral condyle (Fig. 1); adjacent slices were used for histology. Split-line direction was determined by inserting a dye dipped pin into the center of each cartilage slice10. The samples were cut to approximately 5 mm cubes, with faces both perpendicular and parallel to the split-line direction (Fig. 1), and mounted using cyanoacrylate onto 20 mm2 Delrin blocks that were machined to support the tissue specimen, aid in polishing, and preserve sample orientation. During preparation, sample hydration was maintained with 1 × PBS. All testing was performed submerged in 1 × PBS containing 1% (v/v) Protease Inhibitors (PI) (Halt, Thermo Fisher 78438); this solution was changed every 3 days. The full depth of the samples was assessed and normalized to the total cartilage thickness.
Fig. 1.
Schematic of sample location and test setup show separate slices were harvested for histological analysis and all other characterization. Three separate cubes were harvested from each donor and split-line orientation was referenced when shaping the cubes so the three faces evaluated were normal (face 3), parallel (face 2), and perpendicular (face 1) to the split-line.
Histological analysis
Sections for histology were fixed in 4% paraformaldehyde and decalcified in 4% ethylenediaminetetraacetic acid (EDTA) for 10 days; then dehydrated, paraffin-embedded, and serially sectioned (10 μm). Slices were stained with Safranin-O/Fast Green to assess glycosaminoglycan (GAG) content. Histological appearance of samples was evaluated by three blind graders using a modified Mankin scoring system to assess structure, cellular abnormalities, matrix staining, and tidemark integrity25. Similar to gross morphology grading, an intraclass correlation coefficient was calculated.
Second harmonic generation (SHG)/Two photon fluorescence imaging
SHG was used to visualize prevalence, orientation, and crystallinity of type I and type II collagen26,27 in the uncalcified cartilage (Fig. 1). Collagen alignment was investigated using SHG and two photon fluorescence (BioRad Radiance 2100 confocal and Coherent Chameleon Ultra II laser tuned to 800 nm wavelength). A dichroic mirror was used to separate the output onto a non-descanned QUASAR Detection Unit with two detectors enabling the observation of SHG and two photon emissions separately. Imaging was performed without staining or contrast agents on faces 1 and 2 spanning the full sample depth. Collagen orientation was observed from the SHG signal using ImageJ (v 2.0.0 with java v 1.6.0_24), with the OrientationJ plugin (v 2.0.5)28 implementing the finite difference gradient method. The mean interface between articular cartilage and subchondral bone established the zero axis and all collagen fiber angle measurements were calculated as the smallest absolute value with 90° as perpendicular to the tidemark.
Uncalcified tissue microindentation
Microindentation was performed on a Bruker TI-950 Tri-boindenter system following SHG imaging. Indent arrays, five indents wide and spanning the full depth from the articular surface to the zone of calcified cartilage on faces 1 and 2. On face 3, 25 indents were placed at three depths: (1) on the articular cartilage gliding surface, (2) on a surface cut and polished 500 mm below the articular surface (middle zone), and (3) on a surface cut and polished 500 mm above the calcified cartilage (deep zone). Indents were performed using a conico-spherical probe (R = 250 μm), spaced at 250 × 250 μm, and at a minimum of 10× the contact radius from the tissue corner to avoid edge effects. All indents were performed in displacement control to a max indentation depth of 12.5 μm. Tests at multiple load rates (1, 4, and 8 μm/s) were performed using a trapezoidal load-hold-unload function with 60 s hold time, and 1 μm/s unload rate. The point of initial surface contact employed a method outlined by Guo29 using a 1 μm lift and a 20 μN preload.
The cartilage was modeled as a nonlinear biphasic material with a Poisson’s ratio near zero from Soltz and Ateshian30 and applied to indentation by Burris et al. 31,32. The “Hertz Burris Theory” (HBT) was used to obtain measures of tensile modulus (Et), equilibrium contact modulus (Ec), and permeability (k)33. These combined tests at multiple rates enabled determination of the fluid load fraction for each test and also used to determine material properties applicable to all loading rates31.
Raman spectroscopy imaging
Raman spectroscopy was performed with an upright InVia confocal microscope (Renishaw plc, Wotton-under-Edge, UK) on the same cartilage cubes while immersed in 1 × PBS+PI. Maps were created of cartilage sections with a 50 μm × 50 μm spacing on both faces 1 and 2, and subsequently at the three face 3 depths. The regions were coincident with the regions tested with microindentation. The sample was excited by a 785 nm laser (Innovative Photonic Solutions, I0785SR0090B-IS1) without polarization filters. An immersion 63 × microscope (Leica, NA = 0.9) provided an approximately 1 μm spot size on submerged samples. A 1200 l/mm grating and a charge-coupled device (CCD) camera yielded a spectral resolution of approximately 1 cm−1 for wavelengths from 620 to 1711 cm−1. The system was calibrated using both internal and external silicon standards, by setting the primary silicon peak to 520 cm−1.
Preprocessing of Raman spectra was performed as outlined by Bonifacio et al.34 and Bergholt et al.35. Cosmic rays were identified and removed, linear baseline subtracted, and intensity normalized. A univariate method where peak intensities were summed at multiple target wavelengths per constituent was used to reduce noise. The intensities observed at 1578, 1488 and 782 cm−1 were summed for deoxyribonucleic acid (DNA), those at 1380, 1342 and 1068 cm−1 were summed for chondroitin sulfate (ChS); peaks at 1271,1246, 920, 857 and 816 cm−1 were summed to represent collagen; and peaks at 1555, 1127 and 1004 cm−1 were summed for non-collagenous proteins (NCP). The ratio of ChS, a sulfated GAG, and collagen was calculated. Similar to all other evaluations, locations were converted to a depth that was normalized to the tidemark.
Statistical analysis
Outliers were removed using the Huber method36 (multiplier of 4) for SHG, micro-mechanical testing, and Raman spectroscopy measures. The total number of observations taken for each sample, both before and after outliers were removed, can be seen for each modality in supplemental material (Table S1). A linear mixed-model approach used random repeated variables of donor and sample and fixed variables of face and depth. Depth was nested in tissue testing direction, testing direction in sample, and sample in donor. The mixed-model accounted for the dependence between samples from the same subject. If significant effects were identified, post-hoc assessments utilized a Tukey’s HSD test with α = 0.05 to evaluate pairwise comparisons. A multivariate correlation analysis evaluated for correlations between structure, mechanics, and chemistry, where R > 0.5 was reported as significant. When assessing anisotropy, a one-sample Student’s t-test was implemented with the previously described mixed-model to compare differences between groups. All statistical evaluations were performed using JMP Pro (v 14.1.10).
Results
Gross morphology indicates healthy donor tissue
MR imaging of the intact human knees revealed one joint with a patellar fracture and narrow joint space. Otherwise, normal joint space was observed [Fig. 2(A)]. All analyzed tissues were visually healthy upon dissection with a mean gross morphology grade of 1.5 ± 0.7 [Fig. 2(B) and (C)]. The intraclass correlation coefficient measuring interrater reliability was 0.7. Records of dissection observations and gross morphological scoring are presented in Table I. Average cartilage thickness across all samples was 2.7 ± 0.5 mm, subsequently on face 3 after polishing 500 μm below the surface tests were run at a mean % of total thickness of 32 ± 19 % and 500 μm above the calcified cartilage represented 78 ± 5% of the total thickness.
Fig. 2.
Representative images showing donors had healthy articular cartilage A) MRI, B) gross dissection, C) Mankin scoring for each donor with inset representative histology slice (representative images from donor C170243).
Table I.
Results from MRI, synovial fluid evaluations, and articular cartilage gross morphological grading (mean ± SD) show all samples were relatively healthy
| Sample | Intake MRI | Synovial Fluid Observations | Medial |
Lateral |
||
|---|---|---|---|---|---|---|
| Anterior | Posterior | Anterior | Posterior | |||
| C161473 | Weaker cartilage signal | Yellow | 1.8 ± 0.4 | 1.8 ± 0.4 | 2.3 ± 0.8 | 1.3 ± 0.4 |
| C170123 | Broken patella | Red | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.4 |
| C170125 | Healthy | Low Volume | 1.5 ± 0.9 | 2.0 ± 0.7 | 2.0 ± 0.0 | 2.3 ± 0.4 |
| C170243 | Weaker cartilage signal | Light Pink | 1.5 ± 0.9 | 1.0 ± 0.0 | 2.3 ± 0.4 | 1.0 ± 0.0 |
Histological analysis shows only minor reduction of GAGs in surface layer
Histological assessment yielded a mean Mankin score of 2.1 ± 1.8, suggesting all donors had intact and predominantly healthy tissue. The intraclass correlation coefficient between graders was 0.9. All samples had slight reduction of Safranin-O that was often confined to the superficial layer [Fig. 2(C) inset].
Collagen orientation differs parallel and perpendicular to split-line direction
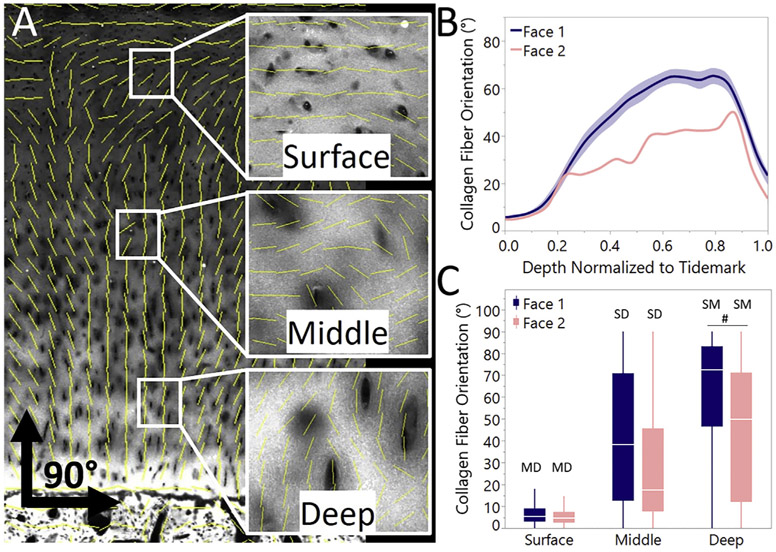
Collagen fiber orientation was found to vary significantly between testing directions and with increasing depth from the articular surface [Fig. 3(C)]. Similar to previously described37, three zones were identified from the full depth data: a surface (i.e., superficial) zone from 0 to 20% of the full cartilage thickness, middle zone from 20 to 60%, and deep zone from 60 to 90%. The deepest 10% was excluded to avoid irregularities associated with the nonuniform border of the mineralized-unmineralized cartilage interface.
Fig. 3.
Collagen fiber orientation varied with depth and showed directional dependence relative to the split-line. A) SHG and OrientationJ overlay with insets highlighting fiber orientation analysis across zones B) continuous fit from face 1 and face 2 across the full depth of the articular cartilage (shaded regions represent confidence of fit) showing differences in collagen fiber orientation with depth between faces and C) binned zones with 0–20% representing surface, 20–60% middle, 60–90% deep zones (box plot with data outliers removed for visualization). (# denotes significant difference within zone between faces; S, M, and D denot significant difference with the surface, middle, and deep zone respectively within face).
Both depth and testing direction were significant predictors (F < 0.001) of collagen fiber orientation. The surface, middle, and deep zones were compared statistically, and within each face, collagen alignment was statistically different across all zones [Fig. 3(C)]. Similarly, significant differences were seen between faces in the middle and deep zones suggesting organization in the deeper zones is orientation dependent. The mean surface orientation was 7.9 ± 8.8° while the mean deep zone orientation was 54.3 ± 28.6°.
Microindentation suggests depth dependent orthotropic material properties
The tensile modulus, regardless of testing direction, exceeded compressive modulus (mean Et/Ec = 2.33 ± 1.7). Results from indentation testing were normalized to cartilage depth and binned to represent each zone as previously described. Depth and testing direction were determined to be significant predictors of both compressive and tensile modulus (F < 0.001 for all). Significant differences with respect to depth were identified for both Ec and Et [Fig. 4(A) and (B)]. In all cases, mean modulus values increased with depth. Regardless of testing direction or face, Ec was significantly different across the surface, middle, and deep zones [Fig. 4(A)], whereas only deep zone Et was significantly different across faces. A less consistent pattern was seen when comparing modulus values between faces within each zone. Post-hoc analysis suggested Ec on face 3 was significantly different from both face 1 and 2 in the deep zone and different from only face 2 in the middle zone. Et differences across testing directions were significantly different only in the deep zone where face 1 had a higher modulus value than faces 2 or 3 [Fig. 4(B)]. Permeability decreased with depth across all faces with mean values of 3.1e-16 m4/N•s, 1.5e-16 m4/N•s, and 6.1e-17 m4/N•s for the surface, middle, and deep zones, respectively. Face 3 had a much higher permeability in the surface zone compared to the two other testing directions. Permeability results for all depths and faces can be seen in supplemental material (Fig. S1).
Fig. 4.
Modulus is dependent on both depth and testing direction, and anisotropy indicates cartilage as a zonally orthotropic material. A) compressive modulus across all three zones with directional dependence in the middle and deep zones, B) directionally and depth dependent tensile modulus values in the deep zone, C) compressive modulus anisotropy in the surface and middle zone, and D) tensile modulus anisotropy in the middle and deep zones (box plot with data outliers removed for visualization). (# denotes significant difference within zone between faces; S, M, and D denote significant difference with the surface, middle, and deep zone respectively within face; % denotes significant difference from the isotropic value of 1).
Significant anisotropy (p < 0.05) was observed when evaluating both the tensile and compressive moduli. Regardless of metric, the modulus on face 3 was significantly lower than that of the other two faces except when evaluating the deep zone compressive modulus [Fig. 4(C) and (D)]. Within the surface and middle zones, all three ratios of Ec suggested anisotropy [Fig. 5(C)] and Ec on face 1 was universally higher than the modulus on the two other faces. The ratio of Et on face 3 to 1 was found to be anisotropic in both the middle and deep zones while Et on face 3 to 2 was anisotropic only in the middle zone [Fig. 5(D)]. These results suggest articular cartilage is not transversely isotropic, as it is often characterized, but rather orthotropic.
Fig. 5.
Representative heat maps of Raman spectroscopy results highlighting depth-dependent distributions of DNA, collagen, ChS, and NCP.
Chemical composition depth and directionally-dependent
Raman spectra intensities were normalized, and all values presented represent relative contributions rather than actual concentrations. Qualitatively, the major components exhibited some depth-dependent spatial variations (Fig. 5). For all measures, there was a high degree of variance between samples, but generally, collagen and DNA were maintained throughout tissue depth while ChS and NCP increased with depth [Fig. 6(A)].
Fig. 6.
Samples exhibited varying distributions of Raman spectroscopy results for DNA, ChS, collagen, and NCP with depth and direction relative to the split-line. A) Mean results as a function of depth (confidence interval shaded), B) ratio of ChS to collagen by zone (box plot with data outliers removed for visualization). (# denotes significant difference within zone between faces; S, M, and D denote significant difference with the surface, middle, and deep zonerespectively within face).
As with previous measures, Raman testing results were normalized to cartilage depth and binned to represent the surface, middle, and deep zones. Mean results for all faces and zones with statistical analysis can be found in the supplemental material (Fig. S2). A prevalent collagen signal was observed regardless of depth, and the ratio of ChS/collagen significantly increased with depth and differed across all faces [Fig. 6(B)]. Depth and orientation were predictors (F < 0.001) for all five metrics: DNA, collagen, ChS, NCP, and ChS/Col ratio, and subsequently significant zonal and directional differences were frequently observed (Fig. S2).
Structure function correlations highly dependent on direction relative to split-line
Multivariate correlation analysis was performed using the mean within sample value for each measure with correlations across metrics and across modalities (nanoindentation, SHG, and Raman). Without accounting for depth or testing direction, correlations within modality, between Ec and Et, as well as correlations across Raman measures, were observed, but no across–modality correlations were identified as significant. However, when depth and orientation were accounted for, significant correlations across metrics and modalities were numerous (Table II). The most numerous across–metrics correlations occurred with mechanical properties and composition for face 3, with the most frequent correlations in the surface zone. Collagen fiber orientation correlated with Ec only in the face 1 surface zone but Ec was correlated with permeability in the deep zones of both faces 1 and 2. No across–metric correlations were seen between chemistry and structural organization (SHG).
Table II.
Correlations were found between metrics both across and within modalities suggesting a relationship between mechanics, structure, and composition. However, these correlations were most notable when evaluating face 3 (normal to the split-line direction). All correlations were positive unless denoted by a “↓” signifying a negative correlation (ABS = collagen fiber orientation)
| Face 1 |
Face 2 |
Face 3 |
||||
|---|---|---|---|---|---|---|
| Across | Within | Across | Within | Across | Within | |
| Surface | ABS:Ec ↓ | DNA:ChS | k: Ec ↓ | Ec:Col | Ec:Et | |
| Ec:Col | DNA:Col | DNA:ChS | Ec:ChS | DNA:ChS | ||
| DNA:NCP | DNA:Col | Ec:NCP | DNA:Col | |||
| ChS:Col | DNA:NCP | Et:Col ↓ | DNA:NCP | |||
| ChS:NCP | ChS:Col | Et:ChS ↓ | ChS:Col | |||
| Col:NCP | ChS:NCP | Et:NCP ↓ | ChS:NCP | |||
| Col:NCP | Et:DNA ↓ | Col:NCP | ||||
| Middle | k:Ec | Ec:Et | Ec:ChS | DNA:Col | ||
| k:Ec | DNA:ChS | Ec:NCP | ||||
| Ec:Et | DNA:Col | |||||
| DNA:ChS | DNA:NCP | |||||
| ChS:Col | ChS:Col | |||||
| ChS:NCP | ChS:NCP | |||||
| Col:NCP | ||||||
| Deep | k:Ec ↓ | k:Ec ↓ | Ec:Col ↓ | k:Ec ↓ | ||
| Ec:Et | DNA:ChS | Ec:NCP | k:Et ↓ | |||
| ChS:NCP | DNA:Col | Et:ChS | Ec:Et | |||
| ChS:Col | DNA:NCP | DNA:ChS | ||||
| ChS:Col | ChS:NCP | |||||
| ChS:NCP | Col:NCP | |||||
| Col:NCP | ||||||
Discussion
With increased resolution and the ability to accurately recapitulate microscale compositional and structural features38-41, 3D printing and other manufacturing technologies are no longer limiting factors when developing new treatment modalities or engineered replacements for articular cartilage. We are instead limited by our current understanding of orientation-specific tissue composition and material property variations at small length scales. The results of this study provide design criteria that are, to date, absent in the literature but needed to understand cartilage tissue mechanics at length scales relevant to cells. Further, these data are critical for the design of engineered materials and therapeutic strategies to repair and regenerate damaged articular cartilage. Thus, this work is an important extension of prior efforts to reveal the microscale zonal and directional variations across the structural, mechanical, and chemical composition of human articular cartilage. Previous work has described variation in collagen arrangement throughout articular cartilage thickness as well as zonal mechanical anisotropy42. However, few have combined these efforts and looked at the composition, structural organization, and mechanical properties within the same tissue samples across multiple orientations. This work quantifies these measures and demonstrates human articular cartilage has orthotropic symmetry. Further we describe both tissue composition and behavior are a function of distance from the articular surface as well as orientation with respect to the tissue’s natural split-line.
Regardless of testing orientation relative to the split-line, this study demonstrates that with increasing depth there is increased collagen alignment perpendicular to the articulating surface [Fig. 3(B) and 3(C)]. Our findings are in agreement with previous descriptions of collagen fibers forming arch-like Benninghoff structures11,43. It has been hypothesized that this collagen fiber alignment and organization is critical to the unique physiological functions of articular cartilage10,11. We observed significant differences between testing directions parallel to the split-line, having a more gradual change in collagen orientation with depth, than in directions perpendicular to the split-line. The gradual transition of collagen orientation was more evident in the middle zone and could thus play a larger role in the formation of split-line direction than previously thought.
Mechanically, the superficial zone had the lowest indentation moduli values and highest permeability. In contrast, the deep zone had the highest moduli and lowest permeability. These findings are in agreement with research performed on bulk samples for compressive modulus32,44, tensile modulus20,30,31, and permeability45. Zonal variations observed herein agree with previous work evaluating long term compressive modulus46 and local zonal mechanical properties (i.e., from microindentation)33. Moduli values observed in human cartilage were 1.2 × – 1.7 × greater (Ec and Et, respectively) when indentation was performed perpendicular to the predominant collagen fiber direction (faces 1 and 2 vs face 3). Thus, the human cartilage tested is orthotropic with zonal variation in directional stiffness. These findings are in qualitative agreement with both observations from AFM47, which evaluates small volumes of tissue, as well as from macroscopic testing of cartilage17,30. Statistically, the split-line direction had little effect on surface properties but influenced compressive modulus in the middle and deep zones and tensile modulus in the deep zone. Collagen orientation also differed between split-line directions (i.e., face 1 vs 2) only in the middle and deep zones. Thus, the differences in collagen fiber alignment are strongly associated with modulus values in the three orthogonal directions.
This work supports and extends prior findings that the chemical composition of cartilage varies with depth34,35,48 by revealing significant differences in biochemical composition with respect to orientation. In agreement with others49, collagen was observed at high levels throughout the tissue depth, but ChS was found to increase with increasing distance from the articular surface. Orientation differences could point to various ECM components, not just collagen, having structural organization. The high degree of correlation across Raman spectroscopy measurements suggests that, in relatively healthy samples, the various major contributors to the articular cartilage composition exist in universal ratios across samples. Previous work by Saarakkala et al. concluded that both collagen and proteoglycan content decrease with depth and with advancing osteoarthritis, particularly in the surface and middle zones50. The role of orientation and the split-line were not described, yet we demonstrate that these two variables are important and, if evaluated in the context of degeneration, may be critical to illuminate how OA progresses. Moreover, these factors may be critical to interrogate how composition and structure changes ultimately affect articular cartilage biomechanics and function.
One of the strengths of this work lies in the novel correlations made when controlling for orientation and depth. While previous studies have evaluated two or more characteristics, correlations were challenging due to the high variability of results. Even within this study, no correlations were identified between mechanical outcomes and collagen orientation or biochemical composition prior to accounting for orientation and depth. However, when we account for these factors, strong correlations emerged with moduli and chemical composition and with permeability and collagen orientation. Most notably, mechanics and chemical composition were highly correlated on face 3 and in the surface zone, and ChS content and compressive modulus were only correlated in the surface and middle zones of face 3. Increased GAG content is often cited as a contributor to overall increased compressive moduli values, but our results suggest a much more complex mechanism that merits further investigation. Thus, accounting for both depth and orientation are particularly important for drawing correlations across testing modalities and interrogating the interplay between composition, structure, and mechanics.
There are several limitations to the current study. Our samples experienced multiple freeze–thaw cycles which could have affected the measured properties. This study was limited to four human donors with three samples taken per donor. While statistical models accounted for inter-relations of samples, additional sample populations would strengthen findings and make this work more translational. Lastly, the large number of observations in the Raman spectroscopy analysis and high degree of variance with only four donors limited the statistical effect size of the Raman measurements which could result in an increased chance of type I errors and overestimation of significance across groups.
Here, we describe healthy human articular cartilage as an orthotropic tissue where micrometer–length scale mechanical properties, tissue-level organization and biochemistry are zonally-dependent. Moreover, we show for the first time that these measures vary with orientation and, critically, in directions along the predominant line of failure – the split-line. These location and orientation-specific relationships between micrometer-scale measures of properties, structural elements (i.e., collagen), and biochemical constituents are needed to recapitulate articular cartilage and may emerge as critical design inputs for 3D bioprinting and scaffold design for osteochondral tissue engineering. Our results provide novel human data, that is currently lacking in the literature, for use in computational and finite element models of the osteochondral unit. While we demonstrate these measurements in healthy human cartilage, this work also establishes a multi-modal assessment approach that can be used to evaluate early cartilage damage or injured/diseased tissues following treatment or regeneration. Finally, this work highlights the importance of accounting for split-line orientation when evaluating material properties, composition, and structural organization of articular cartilage, and in osteochondral grafts, where matching split-line orientations between grafted and native cartilage may maximize integration.
Supplementary Material
Acknowledgments
The authors acknowledge Elizabeth Chlipala, Alicia Ortega, and Jennifer Coulombe for their assistance in histology, experiments, and tissue preparation.
Role of the funding source
This work was supported National Institutes of Health grants R01 AR063712 and R01 AR069060. The funding sources were not involved in the design, writing, or publication of the current study.
Footnotes
Competing interest statement
The authors have no competing interests.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.joca.2020.06.007.
References
- 1.Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med 2010;38(2):231–7, 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 2.Rönn K, Reischl N, Gautier E, Jacobi M. Current surgical treatment of knee osteoarthritis. Arthritis 2011;2011(7–8):454873, 10.1155/2011/454873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med 2017;5(5), 10.1177/2325967117704634. 2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camp CL, Stuart MJ, Krych AJ. Current concepts of articular cartilage restoration techniques in the knee. Sport Health 2014;6(3):265–73, 10.1177/1941738113508917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Familiari F, Cinque ME, Chahla J, Godin JA, Olesen ML, Moatshe G, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med 2018;46(14):3541–9, 10.1177/0363546517732531. [DOI] [PubMed] [Google Scholar]
- 6.Below S, Arnoczky SP, Dodds J, Kooima C, Walter N. The split-line pattern of the distal femur: a consideration in the orientation of autologous cartilage grafts. Arthroscopy 2002;18(6):613–7, 10.1053/jars.2002.29877. [DOI] [PubMed] [Google Scholar]
- 7.Bae WC, Wong VW, Hwang J, Antonacci JM, Nugent-Derfus GE, Blewis ME, et al. Wear-lines and split-lines of human patellar cartilage: relation to tensile biomechanical properties. Osteoarthritis Cartilage 2008;16(7):841–5, 10.1016/j.joca.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.BÖttcher P, Zeissler M, Maierl J, Grevel V, Oechtering G. Mapping of split-line pattern and cartilage thickness of selected donor and recipient sites for autologous osteochondral transplantation in the canine stifle joint. Vet Surg 2009;38(6):696–704, 10.1111/j.1532-950X.2009.00527.x. [DOI] [PubMed] [Google Scholar]
- 9.Leo BM, Turner MA, Diduch DR. Split-line pattern and histologic analysis of a human osteochondral plug graft. Arthroscopy 2004;20 Suppl 2(6):39–45, 10.1016/j.arthro.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 10.Bullough P, Goodfellow J. The significance of the fine structure of articular cartilage. J Bone Joint Surg Br 1968;50(4):852–7, 10.1302/0301-620X.50B4.852. [DOI] [PubMed] [Google Scholar]
- 11.Benninghoff A Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. Z Zellforsch Mikrosk Anat 1925;2(5):783–862. [Google Scholar]
- 12.Minns RJ, Steven FS. The collagen fibril organization in human articular cartilage. J Anat 1977;123(Pt 2):437–57, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1234543/. [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke IC. Articular cartilage: a review and scanning electron microscope study. II. The territorial fibrillar architecture. J Anat 1974;118(Pt 2):261–80, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1231507/. [PMC free article] [PubMed] [Google Scholar]
- 14.Clark JM. The organization of collagen in cryofractured rabbit articular cartilage: a scanning electron microscopic study. J Orthop Res 1985;3(1):17–29, 10.1002/jor.1100030102. [DOI] [PubMed] [Google Scholar]
- 15.Jeffery AK, Blunn GW, Archer CW, Bentley G. Three-dimensional collagen architecture in bovine articular cartilage. J Bone Joint Surg - Ser B 1991;73(5):795–801, 10.1302/0301-620x.73b5.1894669. [DOI] [PubMed] [Google Scholar]
- 16.Santos S, Emery N, Neu CP, Pierce DM. Propagation of micro-cracks in collagen networks of cartilage under mechanical loads. Osteoarthritis Cartilage 2019;27(9):1392–402, 10.1016/j.joca.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Chahine NO, Wang CC, Hung CT, Ateshian GA. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech 2004;37(8):1251–61, 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang CCB, Chahine NO, Hung CT, Ateshian GA. Optical determination of anisotropic material properties of bovine articular cartilage in compression. J Biomech 2003;36(3): 339–53, 10.1016/s0021-9290(02)00417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu Rev Biomed Eng 2002;4(1):175–209, 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 20.Huang CY, Stankiewicz A, Ateshian GA, Mow VC. Anisotropy, inhomogeneity, and tension-compression nonlinearity of human glenohumeral cartilage in finite deformation. J Biomech 2005;38(4):799–809, 10.1016/j.jbiomech.2004.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Adams J, Leddy HA, McNulty AL, O'Conor CJ, Guilak F. The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep 2014;16(10):451, 10.1007/s11926-014-0451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng 2008;36(5):780–92, 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 23.Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage 1994;2(2):91–101, 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 24.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 2016;15(2):155–63, 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mainil-Varlet P, Schiavinato A, Ganster MM. Efficacy evaluation of a new hyaluronan derivative HYADD® 4-G to maintain cartilage integrity in a rabbit model of osteoarthritis. Cartilage 2013;4(1):28–41, 10.1177/1947603512455193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh AT, Hammer-Wilson MJ, Van Sickle DC, Benton HP, Zoumi A, Tromberg BJ, et al. Nonlinear optical microscopy of articular cartilage. Osteoarthritis Cartilage 2005;13(4):345–52, 10.1016/j.joca.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Williams RM, Zipfel WR, Webb WW. Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 2005;88(2):1377–86, 10.1529/biophysj.104.047308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezakhaniha R, Agianniotis A, Schrauwen JTC, Griffa A, Sage D, Bouten CVC, et al. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech Model Mechanobiol 2012;11(3–4):461–73, 10.1007/s10237-011-0325-z. [DOI] [PubMed] [Google Scholar]
- 29.Guo S, Akhremitchev BB. Packing density and structural heterogeneity of insulin amyloid fibrils measured by AFM nanoindentation. Biomacromolecules 2006;7(5):1630–6, 10.1021/bm0600724. [DOI] [PubMed] [Google Scholar]
- 30.Soltz MA, Ateshian GA. A conewise linear elasticity mixture model for the analysis of tension-compression nonlinearity in articular cartilage. J Biomech Eng 2000;122(6):576, 10.1115/1.1324669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonnevie ED, Baro VJ, Wang L, Burris DL. Fluid load support during localized indentation of cartilage with a spherical probe. J Biomech 2012;45(6):1036–41, 10.1016/j.jbiomech.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore AC, Zimmerman BK, Chen X, Lu XL, Burris DL. Experimental characterization of biphasic materials using rate-controlled Hertzian indentation. Tribol Int 2015;89(302):2–8, 10.1016/j.triboint.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahlquist JA, DelRio FW, Randolph MA, Aziz AH, Heveran CM, Bryant SJ, et al. Indentation mapping revealed poroelastic, but not viscoelastic, properties spanning native zonal articular cartilage. Acta Biomater 2017;64:41–9, 10.1016/j.actbio.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonifacio A, Beleites C, Vittur F, Marsich E, Semeraro S, Paoletti S, et al. Chemical imaging of articular cartilage sections with Raman mapping, employing uni- and multi-variate methods for data analysis. Analyst 2010;135(12):3193–204, 10.1039/c0an00459f. [DOI] [PubMed] [Google Scholar]
- 35.Bergholt MS, St-Pierre J-P, Offeddu GS, Parmar PA, Albro MB, Puetzer JL, et al. Raman spectroscopy reveals new insights into the zonal organization of native and tissue-engineered articular cartilage. ACS Cent Sci 2016;2(12):885–95, 10.1021/acscentsci.6b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huber PJ. Robust estimation of a location parameter. Ann Math Stat 1964;35(1):73–101, 10.1214/aoms/1177703732. [DOI] [Google Scholar]
- 37.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sport Health 2009;1(6):461–8, 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akizuki S, Mow VC, Müller F, Pita JC, Howell DS, Manicourt DH. Tensile properties of human knee joint cartilage: I. Influence of ionic conditions, weight bearing, and fibrillation on the tensile modulus. J Orthop Res 1986;4(4):379–92, 10.1002/jor.1100040401. [DOI] [PubMed] [Google Scholar]
- 39.Robinson DL, Kersh ME, Walsh NC, Ackland DC, de Steiger RN, Pandy MG. Mechanical properties of normal and osteoarthritic human articular cartilage. J Mech Behav Biomed Mater 2016;61:96–109, 10.1016/j.jmbbm.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Aisenbrey EA, Tomaschke A, Kleinjan E, Muralidharan A, Pascual-Garrido C, McLeod RR, et al. A stereolithography-based 3D printed hybrid scaffold for in situ cartilage defect repair. Macromol Biosci 2018;18(2):1–8, 10.1002/mabi.201700267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uzcategui AC, Muralidharan A, Ferguson VL, Bryant SJ, McLeod RR. Understanding and improving mechanical properties in 3D printed parts using a dual-cure acrylate-based resin for stereolithography. Adv Eng Mater 2018;20(12):1–10, 10.1002/adem.201800876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jurvelin JS, Buschmann MD, Hunziker EB. Mechanical anisotropy of human knee articular cartilage in compression. Trans Orthop Res Soc 2003;217:215–9. [DOI] [PubMed] [Google Scholar]
- 43.Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 1979;11(4):447–55, https://link.springer.com/content/pdf/10.1007/BF01002772.pdf. [DOI] [PubMed] [Google Scholar]
- 44.Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage 2004;12(1):65–73, 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Mow VC, Holmes MH, Lai WM. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech 1984;17(5):377–94, 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 46.Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res 1997;15(4):499–506, 10.1002/jor.1100150404. [DOI] [PubMed] [Google Scholar]
- 47.McLeod MA, Wilusz RE, Guilak F. Depth-dependent anisotropy of the micromechanical properties of the extracellular and pericellular matrices of articular cartilage evaluated via atomic force microscopy. J Biomech 2013;46(3):586–92, 10.1016/j.jbiomech.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albro MB, Bergholt MS, St-Pierre J-P, Vinals Guitart A, Zlotnick HM, Evita EG, et al. Raman spectroscopic imaging for quantification of depth-dependent and local heterogeneities in native and engineered cartilage. NPJ Regen Med 2018;3(1):3, 10.1038/s41536-018-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Müller C, Khabut A, Dudhia J, Reinholt FP, Aspberg A, Heinegård D, et al. Quantitative proteomics at different depths in human articular cartilage reveals unique patterns of protein distribution. Matrix Biol 2014;40:34–45, 10.1016/j.matbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Saarakkala S, Julkunen P, Kiviranta P, Mäkitalo J, Jurvelin JS, Korhonen RK. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage 2010;18(1):73–81, 10.1016/j.joca.2009.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.