Abstract
Wild jujube “Ziziphus lotus (L.) Desf.” belongs to the Rhamnaceae family and is a traditionally herbaceous medicinal plant. It is very common in arid and semi-arid regions and is currently used for its antidiabetic, sedative, analgesic, anti-inflammatory and hypoglycemic activities. The aim of the present work was to characterize the physico-chemical properties and the phytochemical profile of wild jujube sample collected from the Guercif region, in order to determine the polyphenolic compounds and the antioxidant ability Analyses were carried out directly after the harvest for the determination of pH, refractive index, total soluble solid (°Brix), dry matter, sugar/acidity, total sugars, reducing sugars, as well as lipid and protein content. Results showed that the investigated fruit is acidic (pH 4.9 ± 0.23) and rich in sugars (80.2 g/100 g ± 3.81). The GC-MS analysis of the fruit revealed a number of volatile compounds, as many as 97, belonging to different chemical classes. The HPLC-DAD-ESI/MS analysis showed the presence of a total of 20 polyphenolic compounds in both EtOAc and MeOH-water extracts. Among them, p-Hydroxybenzoic acid was the most abundant in the EtOAc extract (185.68 µg/100 mg ± 0.5) whereas Quercetin 3-O-rhamnoside-7-O-glucoside was found in higher amounts in the MeOH-water extract (25.40 µg/100 mg ± 0.5). These components have medical interest, notably for human nutrition, as well as health benefits and therapeutic effects. Therefore, Moroccan jujube “Zizyphus lotus (L.)” fruit may have potential industrial applications for food formulations.
Keywords: Rhamnaceae, phenolic compounds, flavonoids, tannins, antioxidant activity, liquid chromatography
1. Introduction
Moroccan wild jujube (Ziziphus lotus (L.) Desf), widely called “Sedra” or “Nbag”, is found in several arid and semi-arid regions such as Chaouia, Haouz, Zear, Rhamna, the Middle Atlas Gharb, Errachidia, Souss, the coastal region of Safi in Sidi Ifni, Khenifra, eastern Morocco Sahara, and in the region of Oujda [1].
Jujube fruits are spherical drupes with a size of a pupil, and are eaten at full maturity in October. Their taste evokes candied apple and their texture is similar to dates. They are marketed for human consumption as a fermented drink by mixing crushed fruits with water, and as flour after drying it [2].
This species is known worldwide for many different medical uses e.g. its antipyretic and antiviral properties [3,4]. In antiquity, Z. lotus was used for its emollient properties; the mixture of dried leaves and fruits was applied topically in the treatment of boils and throat and bronchopulmonary irritations [5]. In previous studies, it has been also reported that Z. lotus root bark has anti-inflammatory, analgesic, and antidiabetic activities [6,7,8].
These fruits are famous for their high biologically active material contents such as polyphenols, exhibiting antioxidant, antimicrobial, and immunomodulatory properties [9]. Some studies carried out on butanol extracts of Zizyphus spina-christi leaves showed that they are rich in saponins and improve the glucose-induced insulin release in type II diabetic rats [10]. Moreover, Z. lotus aqueous and organic extracts are characterized by the presence of flavonoids and tannins [11]. Particularly, cyclopeptide alkaloids, termed lotusines, and dammarane saponins have been isolated from this shrub, along with polyunsaturated fatty acids (oleic acid and linoleic acid), carbohydrates, and fibers [12,13,14,15,16,17,18,19].
A comparative study of two Zizyphus species, namely spina christi and lotus from Morocco, highlighted the presence of essential nutrients and phytochemical compounds in the fruits, pulps, seeds, and almonds. Flavonoid and anthocyanin contents were found to be highest in methanolic extract of the seeds and almonds of Z. lotus [16]. An earlier study reported the concentrations of different vitamins (vitamin A, C, and E) and fatty acids in root, stem, leaves, fruit pulp, and seed of Z. lotus L. The results achieved showed higher vitamin A and C contents in the fruit pulp and a richer source of linoleic acid (18:2n−6) than that in other parts of the plant [20].
To the best of our knowledge, there is a lack of information about the physico-chemical properties, as well as the phytochemical composition of such a shrub growing wild in Morocco.; therefore, the present article will serve as an addition to the data that exists about Moroccan wild jujube. This work aims to evaluate the physico-chemical and bioactive properties of Z. lotus (L.) Desf fruit in order to potentially exploit them for industrial applications and incorporate them into food formulations to improve human health.
2. Results and Discussion
Nutritional quality of fruits is usually first characterized by physicochemical parameters, which can indicate a general estimate of the overall composition of their nutrient content. The most discriminating criteria are related to their sweet and sour taste but also their firmness. These are important factors in the sensory quality determination and the food products acceptability by consumers. In addition, the organoleptic quality is mainly determined by these chemical indicators. The phytochemical screening is required to detect the majority of compounds possessing essential biological activity.
2.1. Variation of Physicochemical Parameters
Jujube fruits are almost unknown to the majority of the Moroccan population, and there is lack of knowledge about their nutritional quality. Therefore, their physicochemical parameters have not been previously reported. The results achieved for refractive index, acidity, total soluble solid (°Brix), sugar/acidity, dry matter, ash, total sugars, and reducing sugars are shown in Table 1.
Table 1.
Different physico-chemical parameters of Z. Lotus samples. The results are expressed as mean ± standard deviation.
| Parameter | Crude Extract | Solvent Fractions | |
|---|---|---|---|
| EtOAc | MeOH-H2O | ||
| pH | 4.9 ± 0.23 | - | - |
| Acidity | 1.5 ± 0.06 | - | - |
| RI | 1.3 ± 0.02 | 2.8 ± 0.00 | 2.7 ± 0.02 |
| TSS | 6.5 ± 0.92 | 60.8 ± 0.20 | 16.7 ± 0.48 |
| S/A | 4.2 ± 0.40 | - | - |
| DM (%) | 87.1 ± 0.25 | - | - |
| Ash (%) | 3.2 ± 0.54 | - | - |
| TS (%) | 80.2 ± 3.81 | 6.2 ± 0.75 | 76.5 ± 1.21 |
| RS (%) | 9.6 ± 0.39 | - | - |
| Lipids (mg/g) | 2.3 ± 0.09 | - | - |
| Proteins (mg/g) | 0.9 ± 0.02 | 0.9 ± 0.00 | 0.00 |
| Vitamin C (mg/g) | 34.5 ± 0.30 | 12.7 ± 0.51 | 33.6 ± 0.45 |
RI: refractive index; TSS: total soluble solid (°Brix); DM: dry matter; S/A: sugar/acidity; TS: total sugars; RS: reducing sugars.
Considering the moisture content of the harvested fruits, a value of 12.9% was attained. This value does not fall within the range (58.34–76.5%) reported for other species of jujube (Zizyphus jujuba Mill.) present in China [21]. The moisture content is considered as a critical parameter to evaluate the quality of jujube fruits and can be considerably affected by genotype and culture conditions [22]. In general, the water content can be influenced by the age of the plant, the period of the vegetative cycle, and even genetic factors [20]. This variation may also be due to the different soil and climatic conditions and to the geographic distribution [23]. Other parameters studied by Chen et al. revealed the following values: ash content (0.8–1.1), total sugars (TS) (27.19–31.7), acidity (1.98–3.12), and sugar/acidity (S/A) (8.8–14.75) [21]. Studies on the same genus have found the percentage of dry matter values between these intervals: 7.88–77.93, 18.99–74.08, and 2.26–3.01, respectively, for % reducing sugars (RS), water content, and ash content. The same collected fruits in India revealed the following values, namely 1.4–6.2, 81.6–83, and 5.83, respectively, for RS, water content, and ash content [24]. The jujube fruits have a high total soluble solid (TSS) value, which is due to their high sugar content. This agrees with the results found by Zia-Ul-Haq et al. [25]. However, our values were different from those found by Gao et al. [26], probably due to the different extraction conditions. High quantities of TS were recovered from freeze-dried berries of jujube (80.2% ± 3.81). Cultivars of the Chinese jujube (Zizyphus jujuba cv) have shown values varying between 69.2% and 85.3% [27]. The average value of TS in the fractions was 6.23% ± 0.75 and 76.5% ± 1.21, respectively in EtOAc and MeOH-H2O fractions. The difference noted can be explained by the different polarity (p < 0.05) and the number of extraction cycles. There was a large gap between the values found in this study and those reported in China and India. This indicates that Moroccan jujubes, particularly from the Guercif region, have the sweetest character among the other studied jujubes. The soluble sugars of the Chinese jujube in five cultivars were identified as fructose, glucose, rhamnose, sorbitol, and sucrose [27]. Fructose and glucose were identified as the main sugars while sorbitol was present in small amounts. Other studies have also shown that glucose and fructose are the main present sugars [28]. The content of sucrose was found to be lower than the content of fructose and glucose. In fact, sucrose is synthesized in the leaves and is hydrolyzed to glucose and fructose with the invertase enzyme once translocated to the flesh of the fruit, which is known to occur during the ripening of the fruits [27]. TS content variations can be attributed to several factors such as the age of the plant, the burn load, the stage of ripening, and the fruit physiological state during analysis. Other factors such as the length of time in the sun, the climate, and the availability of water can also affect sugar content [21,29,30,31,32]. Indeed, a high concentration of sugars prevents bacterial proliferation in jams and jellies, and this contributes to the transformation of the studied fruit into several food products, in particular, jams, compotes, marmalades, and juice [24]. The average refractive index (RI) values were 2.8 ± 0.00 (EtOAc) and 2.7 ± 0.02 (MeOH-H2O). RI is influenced by the polarity of the solvents employed, as demonstrated by the ANOVA test (p < 0.05) [33]. Regarding protein content, a very low content was determined: 0.9 mg/g. The total concentrations of vitamin C were 34.5 mg/g ± 0.30, 12.7 mg/g ± 0.51, and 33.6 mg/g ± 0.45, respectively, for fresh fruits, EtOAc fraction, and MeOH-H2O fraction. The ANOVA test demonstrated a significant effect (p < 0.05) of solvent polarity on vitamin C content of jujubes.
2.2. Phytochemical Screening
The phytochemical screening of the wild jujube investigated in this work revealed the presence of different families of molecules, and results are presented in Table 2.
Table 2.
Phytochemicals detected in Z. lotus extracts.
| Compounds Group/Solvent of Extraction | Crude Extract | EtOAc | MeOH-H2O | |
|---|---|---|---|---|
| Alkaloids | - | ± | ± | |
| Polyphenols | Flavonoids | C++ | B | A+ |
| Tannins | + | - | ++ | |
| Anthocyanins | + | - | ± | |
| Catechic tannins | + | - | + | |
| Gallic tannins | + | - | + | |
| Coumarins | + | - | - | |
| Steroids | Soponosides | + | - | + |
| Unsaturated Sterols/Terpenes | - | + | - | |
| Sterols and Steroids | ++ | - | ++ | |
| Sugars | Deoxysugars | + | - | - |
| Glycosides | - | + | ± | |
| Mucilages | + | - | + | |
A: Flavone; B: Isoflavone; C: Flavonones; ++: Abundant; +: Presence of metabolite; -: Absence of metabolite; ±: trace.
Results showed the presence of flavonoids, tannins, anthocyanins, coumarins, saponosides, sterols, deoxysugars, and mucilages. These results are in agreement with previous studies carried on the same species [34,35] and in a similar species, Ziziphus mauritiana [36]. Concerning polyphenols, the best solvent extraction was MeOH-H2O. For anthocyanins, only traces were detected in the MeOH-H2O fraction whereas they were totally absent in the EtOAc one. These results are in agreement with previous findings reported by Tiwari et al. [37]. Total tannins were the most abundant in the MeOH-H2O, as demonstrated by other studies [38,39,40]. Sterols and steroids were present in higher amounts in the MeOH-H2O fraction, in agreement with data obtained by Tiwari et al. [37]. Unsaturated sterols and terpenes were present in the EtOAc extract [39]. Saponosides were detected in the crude and MeOH-H2O extracts [37,39]; the same results were attained for mucilages [41].
Such a phytochemical prospecting of the studied fruits could be a good starting point for determining the presence of various classes of secondary metabolites [42,43,44,45,46,47].
Table 3 reports the quantification of total polyphenols (TPP), total flavonoids (TFv), total anthocyanins (TA), and total tannins (TT) content in Z. lotus solvent fractions. TPP was expressed as mg/g gallic acid equivalents (GAE), whereas flavonoid content was expressed in terms of mg/g quercetin equivalents (QE).
Table 3.
Total polyphenols (TPP), total flavonoids (TFv), total anthocyanins (TA), and total tannins (TT) content in Z. lotus solvent fractions.
| Extract | Vit. C | TPP | TFv | TA | TT | IC50 |
|---|---|---|---|---|---|---|
| EtOAc | 12.7 ± 1.01 | 3.0 ± 0.10 | 2.0 ± 0.10 | 0.1 ± 0.00 | 5.2 ± 0.10 | 1.5 ± 0.00 |
| MeOH-H20 | 33.6 ± 2.50 | 4.8 ± 1.05 | 5.7 ± 0.05 | 0.1 ± 0.00 | 11.1 ± 0.50 | 1.3 ± 0.00 |
Polyphenols: For each fraction, the average value of polyphenols was 3.0 ± 0.10 mg GAE/g dry matter (DM) and 4.8 ± 1.05 mg GAE/g DM, respectively, for EtOAc and MeOH. The statistical analysis of variance test revealed that there was a significant difference between the levels of polyphenols in the fractions according to the extraction solvent used (p < 0.05) [48]. In many published reports, the most suitable solvent for polyphenols extraction was represented by ethyl acetate [49,50]; in the present work, the MeOH-H2O mixture yielded the highest content of polyphenols, in agreement with other works [51,52]. As a consequence, the recovery of polyphenols from plant materials was indeed influenced by the solubility of the polyphenolic compounds in the extraction solvent.
Flavonoids: The average value of TFv was 2.0 ± 0.10 mg QE/g DM and 5.7 ± 0.05 mg QE/g DM, respectively, for jujube fractions of EtOAc and MeOH-H2O. The difference between the two results was significant (p < 0.05). A study conducted on the phytochemical composition of sea buckthorn exposed a level of flavonoids ranging from 2.18 to 6.6 mg QE/g DM [53]. A similar result was found by Vinatoru et al. [54] who extracted flavonoids from carrot powder using ultrasound extraction. In another work carried out in Brazil, a research team found that acetonitrile could recover an optimal amount of Macela’s flavonoids “Achyrolcine satureioides” [55].
Anthocyanins: The mean value of TA for each fraction was equal to 0.1 ± 0.00 for both EtOAc and MeOH-H2O fractions. The statistical analysis of variance test (ANOVA) showed that there was a significant difference depending on the diversity between all the anthocyanin contents (p < 0.05). In Mexico, pure methanol showed the greatest capacity to extract TA from the skin of Renealmia alpinia fruit compared to acetonitrile [56]. Similar results were reported by Ju and Howard [57] where MeOH 60% showed a greater capacity than ethanol 60% and water for the extraction of TA and phenolic compounds from grape skin. In addition, Khonkarn et al. [58] pointed out that MeOH was the solvent with the highest yield of anthocyanins from coconut, rambutan, and mangosteen barks. The difference between the levels of anthocyanins in each solvent can be explained by the stability of the anthocyanins, which can react with the solvent present in the mother solution, as reported by Benabdeljalil et al. [59]. It is also important to point out that, on a theoretical level, anthocyanins increase with the ripening of the fruits [60].
Tannins: The average values obtained for tannin concentrations of EtOAc and MeOH-H2O fractions were, respectively, equal to 5.2 ± 0.01 µg catechin equivalents (CE)/g DM and 11.1 ± 0.50 µg EC/g DM. The MeOH-H2O solvent mixture turned out to be the best solvent for extracting the maximum level of tannins. Mokhtarpour et al. reported that using 50% aqueous MeOH, revealed high tannin levels compared to that of other treatments [61]. Ghasemi et al. [62] evaluated pistachio shells and attained maximum tannin content in the MeOH fraction. In addition, in a Moroccan study on Acacia mollissima, the best yield of tannins was observed for the MeOH-H2O fraction [63].
2.3. Antioxidant Activity
The mean IC50 of each solvent fraction studied showed that Z. lotus has the highest antioxidant power in the MeOH-H2O fraction (smallest IC50). Analysis by the ANOVA test showed a very significant difference between the results of the two fractions (p < 0.01). These values were lower than the ones by Najjaa et al. [64] but were higher than the ones reported by Ghazghazi et al. for methanol extracts [65].
Methanol has a great reduction capability and powerful free radical scavenging activity [66]. In this regard, in some medicinal plants, it has been found that the DPPH radical scavenging effects of methanolic extracts are greater than that of aqueous extracts. The same authors reported that the exhibited antioxidant activity by methanol extracts is due to the presence of phenolic substances such as rosmarinic acid from Salvia officinalis and Origanum vulgare [67]. Numerous in vitro studies have confirmed the ability of Z. lotus to scavenge free radicals and prevent cell damage [65,68]. In addition, it has been shown that Z. lotus do present antioxidant compounds belonging to different classes such as phenolic acids, flavonoids, and saponins. These components prevent oxidative stress by reducing reactive oxygen species (ROS), and a regular intake of natural antioxidants can lower the risk of various diseases by reducing oxidative stress [64].
2.4. GC-MS Analyses
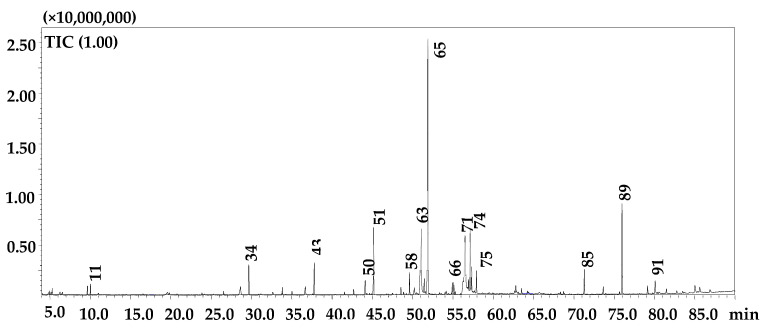
The attained results of the GC-MS analysis of the n-hexane fraction showed the presence of lipids, alkanes, alcohols, sterols, and terpenoids. The studied fruits revealed a high number (N = 97) of these compounds as reported in Figure 1 and Table 4 with a % of similarity ranging from 83 to 97%.
Figure 1.
GC-MS profile of the n-hexane fraction of Z. lotus. Main peaks are labeled. Peak assignment as in Table 4.
Table 4.
List of compounds identified in Z. lotus by GC-MS.
| Peak | Compound | LRI (lib) | LRI (exp) | Similarity (%) | Library |
|---|---|---|---|---|---|
| 1 | Isobutyric acid | 752 | 740 | 83 | FFNSC 4.0 |
| 2 | 3-Hexanone | 782 | 781 | 93 | FFNSC 4.0 |
| 3 | Butyl methyl ketone | 786 | 787 | 98 | FFNSC 4.0 |
| 4 | 3-Hexanol | 795 | 798 | 91 | FFNSC 4.0 |
| 5 | 2-Hexanol | 802 | 801 | 92 | FFNSC 4.0 |
| 6 | Isovaleric acid | 842 | 838 | 97 | FFNSC 4.0 |
| 7 | 2-methylbutanoic acid | 881 | 849 | 94 | FFNSC 4.0 |
| 8 | n-Hexanol | 867 | 867 | 88 | FFNSC 4.0 |
| 9 | n-Pentanoic acid | 911 | 876 | 96 | FFNSC 4.0 |
| 10 | n-Heptanal | 906 | 903 | 90 | FFNSC 4.0 |
| 11 | (E)-Hept-2-enal | 956 | 957 | 93 | FFNSC 4.0 |
| 12 | n-Hexanoic acid | 997 | 980 | 96 | FFNSC 4.0 |
| 13 | 2-pentyl Furan | 991 | 992 | 86 | FFNSC 4.0 |
| 14 | n-Octanal | 1006 | 1004 | 91 | FFNSC 4.0 |
| 15 | Limonene | 1030 | 1030 | 93 | FFNSC 4.0 |
| 16 | Oct-3-en-2-one | 1036 | 1039 | 90 | FFNSC 4.0 |
| 17 | (E)-Oct-2-enal | 1058 | 1059 | 93 | FFNSC 4.0 |
| 18 | n-Nonanal | 1107 | 1105 | 96 | FFNSC 4.0 |
| 19 | methyl-Octanoate | 1125 | 1124 | 93 | FFNSC 4.0 |
| 20 | Benzenecarboxylic acid | 1213 | 1171 | 97 | FFNSC 4.0 |
| 21 | n-Octanoic acid | 1192 | 1176 | 96 | FFNSC 4.0 |
| 22 | ethyl-Octanoate | 1202 | 1196 | 95 | FFNSC 4.0 |
| 23 | n-Decanal | 1208 | 1207 | 91 | FFNSC 4.0 |
| 24 | methyl-Nonanoate | 1224 | 1224 | 88 | FFNSC 4.0 |
| 25 | (Z)-Dec-2-enal | 1250 | 1250 | 89 | FFNSC 4.0 |
| 26 | (E)-Dec-2-enal | 1265 | 1264 | 97 | FFNSC 4.0 |
| 27 | n-Nonanoic acid | 1289 | 1270 | 94 | FFNSC 4.0 |
| 28 | ethyl-Nonanoate | 1297 | 1295 | 93 | FFNSC 4.0 |
| 29 | Carvacrol | 1300 | 1302 | 92 | FFNSC 4.0 |
| 30 | n-Undecanal | 1309 | 1309 | 91 | FFNSC 4.0 |
| 31 | (E,E)-2,4-Decadienal | 1322 | 1321 | 89 | FFNSC 4.0 |
| 32 | methyl-Decanoate | 1327 | 1324 | 96 | FFNSC 4.0 |
| 33 | n-Decanoic acid | 1398 | 1372 | 97 | FFNSC 4.0 |
| 34 | ethyl-Decanoate | 1399 | 1395 | 97 | FFNSC 4.0 |
| 35 | methyl-Undecanoate | 1423 | 1424 | 95 | FFNSC 4.0 |
| 36 | n-Undecanoic acid | 1473 | 1466 | 95 | FFNSC 4.0 |
| 37 | ethyl-Undecanoate | 1498 | 1494 | 96 | FFNSC 4.0 |
| 38 | ethyl 9-oxononanoate | - | 1505 | - | W11N17 |
| 39 | methyl-Dodecanoate | 1527 | 1524 | 96 | FFNSC 4.0 |
| 40 | isobutyl-Decanoate | 1545 | 1545 | 92 | FFNSC 4.0 |
| 41 | n-Dodecanoic acid | 1581 | 1566 | 96 | FFNSC 4.0 |
| 42 | butyl-Decanoate | 1585 | 1586 | 88 | FFNSC 4.0 |
| 43 | ethyl-Dodecanoate | 1598 | 1594 | 97 | FFNSC 4.0 |
| 44 | n-Tetradecanal | 1614 | 1614 | 91 | FFNSC 4.0 |
| 45 | n-Tridecanoic acid | 1668 | 1663 | 93 | FFNSC 4.0 |
| 46 | Apiole | 1683 | 1679 | 92 | FFNSC 4.0 |
| 47 | Ethyl tridecanoate | - | 1694 | - | W11N17 |
| 48 | Tridecyl methyl ketone | 1697 | 1698 | 92 | FFNSC 4.0 |
| 49 | methyl-Tetradecanoate | 1727 | 1725 | 97 | FFNSC 4.0 |
| 50 | n-Tetradecanoic acid | 1773 | 1765 | 90 | FFNSC 4.0 |
| 51 | ethyl-Tetradecanoate | 1794 | 1794 | 98 | FFNSC 4.0 |
| 52 | Hexadecanal | - | 1818 | - | W11N17 |
| 53 | methyl pentadecanoate | - | 1825 | - | W11N17 |
| 54 | Neophytadiene | 1836 | 1837 | 93 | FFNSC 4.0 |
| 55 | Phytone | 1841 | 1842 | 94 | FFNSC 4.0 |
| 56 | Pentadecylic acid | 1869 | 1862 | 96 | FFNSC 4.0 |
| 57 | ethyl-Pentadecanoate | 1893 | 1893 | 94 | FFNSC 4.0 |
| 58 | methyl (Z)-9-hexadecenoate | - | 1904 | - | W11N17 |
| 59 | methyl (Z)-11-hexadecenoate | - | 1913 | - | W11N17 |
| 60 | methyl-Hexadecanoate | 1925 | 1926 | 96 | FFNSC 4.0 |
| 61 | 9-Hexadecenoic acid | - | 1944 | - | W11N17 |
| 62 | (Z)-11-Hexadecenoic acid | - | 1953 | - | W11N17 |
| 63 | n-Hexadecanoic acid | 1977 | 1971 | 95 | FFNSC 4.0 |
| 64 | Ethyl 9-hexadecenoate | - | 1982 | - | W11N17 |
| 65 | ethyl-Palmitate | 1993 | 1996 | 97 | FFNSC 4.0 |
| 66 | propyl hexadecanoate | - | 2090 | - | W11N17 |
| 67 | ethyl heptadecanoate | - | 2094 | - | W11N17 |
| 68 | methyl-Oleate | 2098 | 2100 | 93 | FFNSC 4.0 |
| 69 | methyl-Octadecanoate | 2127 | 2127 | 93 | FFNSC 4.0 |
| 70 | Linoleic acid | 2144 | 2139 | 95 | FFNSC 4.0 |
| 71 | Oleic acid | 2147 | 2142 | 90 | FFNSC 4.0 |
| 72 | (Z)-Vaccenic acid | - | 2150 | - | W11N17 |
| 73 | ethyl-Linoleate | 2164 | 2161 | 93 | FFNSC 4.0 |
| 74 | ethyl-Oleate | 2166 | 2168 | 87 | FFNSC 4.0 |
| 75 | ethyl-Stearate | 2198 | 2194 | 96 | FFNSC 4.0 |
| 76 | (Z)-9-Octadecenamide | - | 2363 | - | W11N17 |
| 77 | hexyl hexadecanoate | - | 2380 | - | W11N17 |
| 78 | ethyl-Eicosanoate | 2394 | 2395 | 90 | FFNSC 4.0 |
| 79 | n-Tetracosane | 2400 | 2400 | 87 | FFNSC 4.0 |
| 80 | n-Pentacosane | 2500 | 2500 | 90 | FFNSC 4.0 |
| 81 | benzyl hexadecanoate | - | 2581 | - | W11N17 |
| 82 | ethyl-Docosanoate | 2595 | 2595 | 87 | FFNSC 4.0 |
| 83 | n-Hexacosane | 2600 | 2600 | 90 | FFNSC 4.0 |
| 84 | ethyl docosanoate | - | 2581 | - | W11N17 |
| 85 | n-Heptacosane | 2700 | 2700 | 95 | FFNSC 4.0 |
| 86 | ethyl-Tetracosanoate | 2796 | 2796 | 88 | FFNSC 4.0 |
| 87 | n-Octacosane | 2800 | 2800 | 94 | FFNSC 4.0 |
| 88 | Squalene | 2810 | 2814 | 87 | FFNSC 4.0 |
| 89 | n-Nonacosane | 2900 | 2902 | 92 | FFNSC 4.0 |
| 90 | n-Triacontane | 3000 | 3000 | 85 | FFNSC 4.0 |
| 91 | Octacosanal | - | 3045 | - | W11N17 |
| 92 | 10-Nonacosanone | - | 3088 | - | W11N17 |
| 93 | n-Hentriacontane | 3100 | 3100 | 92 | FFNSC 4.0 |
| 94 | Octacosanol | - | 3111 | - | W11N17 |
| 95 | Vitamin E | - | 3131 | - | W11N17 |
| 96 | Triacontanal | - | 3250 | - | W11N17 |
| 97 | γ-Sitosterol | - | 3323 | - | W11N17 |
2.5. HPLC-DAD-ESI/MS Analyses
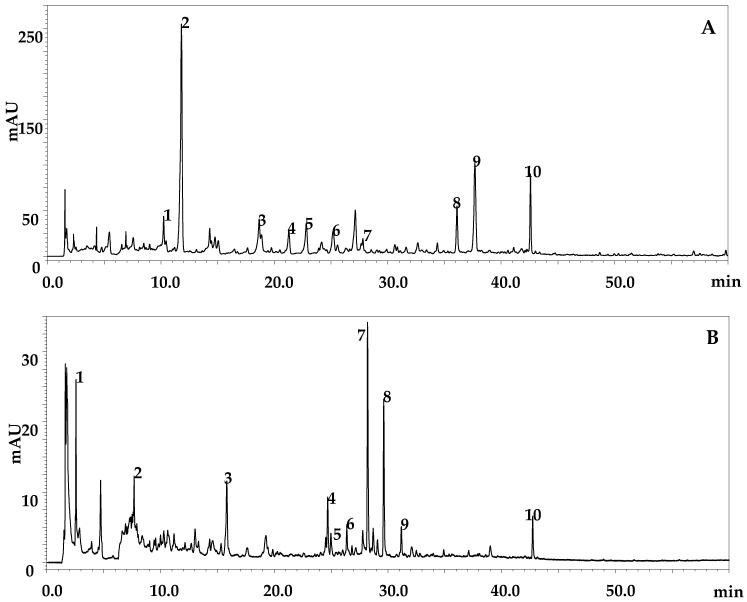
Figure 2 and Table 5 and Table 6 report the polyphenolic compounds identified in Z. lotus fruit extracts. A total of 20 different polyphenolic compounds were detected according to DAD, MS, and literature data. For the EtOAc extract, the phenolic compounds belonged to phenolic acid and derivatives and flavonols; in the MeOH-H2O extract, in addition to those compounds found in the EtOAc extract, organic acids and flavan-3-ols were detected. Considering phenolic acid and derivatives, the highest value was found for the EtOAc extract viz. 199.43 ± 0.8 vs. 2.42 ± 0.02 for the MeOH-H2O extract. Hydroxycinnamic acids were detected only in the EtOAc extract (84.69 ± 0.5); flavonols were more abundant in the MeOH-H2O extracts (31.99 ± 0.05 vs. 14.45 ± 0.01) (Table 7). These results are consistent with those of other studies carried out on the same species [20,35]. The difference in compounds detection can be due to their solubility in the extraction solvent, the degree of polymerization of the phenols, and the interaction of the phenols with other constituents of the plant [51,69].
Figure 2.
Polyphenolic profiles of Z. lotus extracts obtained by HPLC-PDA-ESI/MS analysis at λ = 280 nm: (A) EtOAc, (B) MeOH-H2O.
Table 5.
Polyphenolic compounds detected in Z. lotus (EtOAc extract) by HPLC-DAD-ESI/MS.
| Peak | Tentative Identification | tR (min) | Identification Type | λMAX (nm) | m/z | Fragments |
|---|---|---|---|---|---|---|
| Phenolic acid and derivatives | ||||||
| 1 | synapic acid | 10.23 | DAD/MS | 309 | 223 | 193, 161 |
| 2 | p-Hydroxybenzoic acid | 11.80 | DAD/MS | 254 | 137 | - |
| 4 | p-coumaric acid | 21.27 | DAD/MS | 308 | 163 | - |
| 5 | p-Coumaroyl glucose | 22.81 | DAD/MS | 293 | 325 | 163 |
| 6 | benzoic acid | 25.17 | DAD/MS | 273 | 121 | - |
| 9 | cinnamic acid derivative | 37.68 | DAD/MS | 277 | 650 | 616, 147 |
| Flavonol | ||||||
| 7 | Rutin | 27.80 | DAD/MS | 255–353 | 609 | - |
| Not identified | ||||||
| 3 | Unknown | 18.65 | - | 266 | 281 | 265+ |
| 10 | Unknown | 42.58 | - | 294–381 | 698 | - |
| 8 | Unknown | 36.10 | - | 264 | 263 | - |
Table 6.
Polyphenolic compounds detected in Z. lotus (MeOH-H2O extract) by HPLC-DAD-ESI/MS.
| Peak | Tentative Identification | tR (min) | Identification Type |
λMAX (nm) | m/z | Fragments |
|---|---|---|---|---|---|---|
| Organic acid | ||||||
| 1 | Malic acid derivative | 2.51 | DAD/MS | - | 503 | 191,133 |
| Phenolic acid and derivatives | ||||||
| 3 | Galloyl shikimic acid | 15.3 | DAD/MS | 252–286 | 325 | |
| Flavan-3-ols | ||||||
| 2 | (-)-Catechin 3-O-gallate | 7.35 | DAD/MS | 258 | 441 | - |
| Flavonols | ||||||
| 4 | Quercetin rhamnosyl-rhamnosyl-glucoside | 24.98 | DAD/MS | 253–357 | 755 | 303+ |
| 5 | Quercetin di-glucoside | 25.25 | DAD/MS | 254–357 | 625 | 303+ |
| 7 | Quercetin rhamnoside-glucoside | 28.53 | DAD/MS | 286 | 609 | 303+ |
| 8 | Eriodictyol derivative | 29.80 | DAD/MS | 285 | 597 | 287 |
| Non-identified | ||||||
| 6 | Unknown | 26.51 | DAD/MS | 351 | 613 | - |
| 9 | Unknown | 31.31 | DAD/MS | 255–352 | 141 | - |
| 10 | Unknown | 43.02 | DAD/MS | 277–373 | 698 | - |
Table 7.
Semi-quantification of polyphenols detected in Z. lotus fruits in µg/100 mg (w/w).
| Compound | EtOAc | MeOH-Water | Standard Used |
|---|---|---|---|
| Phenolic acid and derivatives | |||
| p-Hydroxybenzoic acid | 185.7 ± 0.50 | - | Gallic acid |
| benzoic acid | 13.7 ± 0.50 | - | Gallic acid |
| galloyl shikimic acid | - | 2.4 ± 0.02 | Gallic acid |
| Total of Hydroxybenzoic acids | 199.4 ± 0.80 | 2.4 ± 0.02 | |
| sinapic acid | 60.0 ± 0.10 | - | Cinnamic acid |
| p-coumaric acid | 3.7 ± 0.04 | - | Cinnamic acid |
| p-coumaroyl glucose | 6.5 ± 0.01 | - | Cinnamic acid |
| cinnamic acid derivative | 14.5 ± 0.50 | - | Cinnamic acid |
| Total of hydroxycinnamic acid | 84.7 ± 0.50 | - | |
| Flavonols | |||
| Rutin | 14.4 ± 0.01 | - | Rutin |
| Quercetin rhamnosyl-rhamnosyl-glucoside | - | 4.1 ± 0.02 | Rutin |
| Quercetin di-glucoside | - | 2.5 ± 0.05 | Rutin |
| Quercetin rhamnoside-glucoside | - | 25.4 ± 0.03 | Rutin |
| Total flavonols | 14.4 ± 0.01 | 32.0 ± 0.05 |
3. Materials and Methods
3.1. Samples
Wild jujube fruits (Z. lotus) were collected for 4 months (May-June-July and August), all the harvest areas were between the longitude 3°38′13007 and the latitude 34°23′45746 of the Guercif region. The fruits were harvested at their physiological maturity in the early morning, transported in well-closed boxes, and stored at −10 °C in the Materials and Resources Valorization Laboratory, Faculty of Sciences and Technology of Tangier.
3.2. Chemical Reagents and Solvents
2,20-Diphenyl-1-picrylhydrazyl (DPPH), 2,20-azobis (2-amidinopropane), gallic acid dihydrochloride (AAPH), L-ascorbic acid, trichloroacetic acid (TCA), 1,1,3,3-tetraethoxypropane (TEP), thiobarbituric acid (TBA), and butylated hydroxytoluene (BHT) were purchased from Sigma (St. Lois, MO, USA). Folin-Ciocalteu phenol reagent was obtained from Fluka. Standards (gallic acid, cinnamic acid, rutin) were obtained from Merck Life Science (Merck KGaA, Darmstadt, Germany). LC-MS grade methanol, acetonitrile, acetic acid, acetone, and water were purchased from Merck Life Science (Merck KGaA, Darmstadt, Germany). All other chemicals were of analytical grade and obtained from Sigma (St. Louis, MO, USA).
3.3. Extraction Method
Five grams of lyophilized powder was defatted three times in 50 mL of n-hexane, dried, and homogenized with 50 mL of two solvents with increased polarity viz. EtOAc/water or MeOH/water (80:20 v/v) [70]. Each fraction was extracted by sonication in an ultrasound bath (130 kHz) for 45 min. The temperature was controlled by using a thermometer and gel ice box. After centrifugation for 5 min, the supernatant was filtered through a paper filter, dried, reconstituted with MeOH/water 80:20, v/v, and then filtered through a 0.45 μm Acrodisc nylon membrane (Merck Life Science, Merck KGaA, Darmstadt, Germany) prior to LC-PDA-MS analysis.
3.4. Physico-Chemical Analyses
The AOAC international standard methods 16 were used to determine the physico-chemical characteristics: ashes and DM content. The pH measurement was performed using a digital pH meter. Titratable acidity (TAc) was measured by the titrimetric method. RI and %TSS were determined by a digital refractometer.
3.5. Phytochemical Screening
Phytochemical screening was performed according to the method of Trease, E. and Evans, W.C. (1987). The tests were based on visual observation of the color change or the formation of a precipitate after addition of specific reagents [71].
3.6. Determination of the Polyphenolic Content
3.6.1. Quantification of TPP, TFv, and TT in Z. lotus extracts
TPP content was estimated using the Folin–Ciocalteu method [72] with a few modifications. Gallic acid was used as the standard (10/25/50/100/200 ppm). TPP content was recorded at 755 nm and was expressed as mg of GAE/g of DM. Flavonoids were quantified according to the method of Zhishen et al. [29], using AlCl3 10%, NaOH 4%, and NaNO2 5%. The absorbance was determined at 510 nm. A curve of catechin was also carried out. TFv content was expressed as mg QE/g DM. TT content was determined by the vanillin method of Julkunen–Tiitto and expressed as mg CE/g DW [30].
3.6.2. Quantification of Total Anthocyanin Content in Z. Lotus Extracts
TA content was estimated based on the differential pH (pH = 1 and pH = 4.5) by the method of Giusti and Wrolstad [31] with some modifications. Measurement was conducted at 510–700 nm in the UV-Vis spectrophotometer. The absorbance was calculated by the following formula:
| A = [(A510 − A700) to pH 1.0] − [(A510 − A700) at pH 4.5] |
The total anthocyanin content was calculated by the molecular weight of pelargonidine-3-glucoside using the following equation:
With M: molar mass of the pelargonidine-3-glucoside [g/mol], F: dilution factor, V: Volume of the extract (l), d: width of the Bowl (cm) and Q: quantity of homogenized fruit (g).
3.7. Determination of Antioxidant Activity
Free radical scavenging method DPPH(α, α-diphenyl-β-picrylhydrazyl) was carried out following the method described by Braca et al. [32] with minor modifications. Briefly, the fruit extracts were prepared from 25 µL of a methanolic solution and each of the pure compounds were added to 2 mL of DPPH (6.25 × 10−5 M). After gentle mixing and incubation for 30 min at room temperature in the dark, allowing for reactions to take place, the absorbance values of the resulting solutions were measured at 517 nm using a blank containing the same concentration of DPPH radicals. Inhibitions of DPPH radical in percent (I%) were calculated as follow:
| I% = [(Absblank − Abssample)/Absblank] × 100 |
where Ablank is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compounds. The sample concentration provided 50% inhibition (half-maximal inhibitory concentration, IC50) was calculated by plotting inhibition percentages against concentrations of the sample.
3.8. GC-MS
GC-MS analyses of volatile compounds was performed on an SLB-5ms column (30 m in length × 0.25 mm in diameter × 0.25 μm in thickness of film, Merck Life Science, Merck KGaA, Darmstadt, Germany). GC-MS detection involved an electron ionization system that utilized high energy electrons (70 eV). Pure helium gas (99.9%) was used as the carrier gas with a flow rate of 1 mL/min, and an injection volume of 0.7 μL was employed (a split ratio of 5:1). The initial temperature was set at 50 °C and increased up to 350 °C with an increase rate of 3 °C/min and holding time of about 5 min. Relative quantity of the chemical compounds present in each sample was expressed as percentage based on peak area produced in the chromatogram.
3.9. HPLC-DAD/ESI-MS
LC analyses were performed on a Shimadzu liquid chromatography system (Kyoto, Japan), consisting of a CBM-20A controller, two LC-30AD dual-plunger parallel-flow pumps, a DGU-20A5R degasser, a CTO-20AC column oven, a SIL-30AC autosampler, an SPD-M30A photo diode array detector, and an LCMS-8050 triple quadrupole mass spectrometer, through an ESI source (Shimadzu, Kyoto, Japan).
Chromatographic separations were carried out on 150 × 4.6 mm; 2.7 µm Ascentis Express RP C18 columns (Merck Life Science, Merck KGaA, Darmstadt, Germany). The mobile phase was composed of two solvents: water/acetic acid (99.85/0.15 v/v, solvent A) and acetonitrile/acetic acid (99.85/0.15 v/v, solvent B). The flow rate was set at 1 mL/min under gradient elution: 0–5 min, 5% B, 5–15 min, 10% B, 15–30 min, 20% B, 30–60 min, 50% B, 60 min, 100% B. DAD detection was applied in the range of λ = 200–400 nm and monitored at λ = 280 nm (sampling frequency: 40.0 Hz, time constant: 0.08 s). MS conditions were as follows: scan range and the scan speed were set at m/z 100–800 and 2500 u sec−1, respectively, event time: 0.3 sec, nebulizing gas (N2) flow rate: 1.5 L min−1, drying gas (N2) flow rate: 15 L min−1, interface temperature: 350 °C, heat block temperature: 300 °C, DL (desolvation line) temperature: 300 °C, DL voltage: 1 V, interface voltage: −4.5 kV. Calibration curves (R2 ≥ 0.997) of eleven polyphenolic standards used for the quantification in sample extracts were obtained using concentration (mg/mL) and according to the area of peaks at wavelengths of 270 nm, 277 nm, and 355 nm.
Compound identification was carried out by using complementary information coming from DAD, ESI-MS, and literature data.
3.10. Statistical Analysis
All analyses were performed in triplicate and data were reported as mean values and standard deviation (SD). The differences among treatments were detected by analysis of variance ANOVA (p < 0.05).
4. Conclusions
The present study shows that Z. lotus (L.) fruits possess interesting bioactive compounds as highlighted from the phytochemical profile. Results showed that the investigated fruits are acidic, rich in sugars, with a large array of volatile compounds belonging to different chemical classes. In addition, a total of 20 polyphenolic compounds were detected in both EtOAc and MeOH-water extracts. Such results demonstrate that Z. lotus (L.) is a potential source of bioactive compounds and can be potentially used for industrial applications in food formulations.
Acknowledgments
The authors thank Merck Life Science and Shimadzu Corporations for their continuous support.
Author Contributions
Conceptualization, H.E.C. and F.C.; Methodology, H.E.C. and F.C.; Investigation, H.E.C.; H.E.B.; G.S.; A.E.C.; B.R.; Y.O.E.M.; F.A.; and A.F.L.; Writing–Original Draft Preparation, H.E.C.; Writing–Review and Editing, F.C. and P.D.; Supervision, F.C. and J.B.; Project Administration: L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are not available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rsaissi N., Bouhache M., Bencharki B. Importance and agro-economical impact of wild jujube (Ziziphus lotus) in Chaouia region. Revue. Maroc. Prot. Des. Plantes. 2012;3:13–27. [Google Scholar]
- 2.Chevalier A. Les Jujubiers ou Ziziphus de l’Ancien monde et l’utilisation de leurs fruits. J. D’agric. Tradit. Bot. Appliquée. 1947;301–302:470–483. doi: 10.3406/jatba.1947.6125. [DOI] [Google Scholar]
- 3.Hammiche V., Maiza K. Traditional medicine in central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006;105:358–367. doi: 10.1016/j.jep.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Niamat R., Khan M.A., Khan K.Y., Mushtaq A., Barkat A., Paras M., Mazhar M., Hussain A. Element content of some ethnomedicinal Ziziphus Linn. species using atomic absorption spectroscopy technique. J. Appl. Pharm. Sci. 2012;2:96–100. [Google Scholar]
- 5.Glombitza K.W., Mahran G.H., Mirhom Y.W., Michel K.G., Motawi T.K. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats. Planta Med. 1994;60:244–247. doi: 10.1055/s-2006-959468. [DOI] [PubMed] [Google Scholar]
- 6.Mathon C.-C. Baumann Hellmut.—Le bouquet d’Athéna: Les plantes dans la mythologie et l’art grecs. Trad. de l’allemand par Roger Barbier, éd. allem. originale, 1982; éd. fr., La Maison rustique-Flammarion, 1984. J. D’agric. Tradit. Bot. Appliquée. 1984;31:129. [Google Scholar]
- 7.Borgi W., Ghedira K., Chouchane N. Anti-inflammatory and analgesic activities of Zizyphus lotus L. root barks. Fitoterapia. 2007;78:16–19. doi: 10.1016/j.fitote.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Borgi W., Recio M.C., Rios J.L., Chouchane N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. S. Afr. J. Bot. 2008;74:320–324. doi: 10.1016/j.sajb.2008.01.009. [DOI] [Google Scholar]
- 9.Abdel-Zaher A.O., Salim S.Y., Assaf M.H., Abdel-Hady R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol. 2005;101:129–138. doi: 10.1016/j.jep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Hirsinger F. New Annual Oil Crops, in Oils Crops of the World. McGraw-Hill; New York, NY, USA: 1989. pp. 518–532. [Google Scholar]
- 11.Abdoul-Azize S., Bendahmane M., Hichami A., Dramane G., Simonin A.M., Benammar C., Sadou H., Akpona S., El Boustani E.S., Khan N.A. Effects of Zizyphus lotus L. (Desf.) polyphenols on Jurkat cell signaling and proliferation. Int. Immunopharmacol. 2013;15:364–371. doi: 10.1016/j.intimp.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Le Crouéour G., Thépenier P., Richard B., Petermann C., Ghédira K., Zèches-Hanrot M. Lotusine G: A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia. 2002;73:63–68. doi: 10.1016/S0367-326X(01)00363-X. [DOI] [PubMed] [Google Scholar]
- 13.Ghedira K., Chemli R., Richard B., Nuzillard J.-M., Zeches M., Le Men-Olivier L. Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry. 1993;32:1591–1594. doi: 10.1016/0031-9422(93)85186-U. [DOI] [Google Scholar]
- 14.Ghedira K., Chemli R., Caron C., Nuzilard J.-M., Zeches M., Le Men-Olivier L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry. 1995;38:767–772. doi: 10.1016/0031-9422(94)00669-K. [DOI] [Google Scholar]
- 15.El Maaiden E., El Kharrassi Y., Lamaoui M., Allai L., Essamadi A.K., Nasser B., Moustaid K. Variation in minerals, polyphenolics and antioxidant activity of pulp, seed and almond of different Ziziphus species grown in Morocco. Braz. J. Food Technol. 2020 doi: 10.1590/1981-6723.20619. [DOI] [Google Scholar]
- 16.El Maaiden E., El Kharrassi Y., Moustaid K., Essamadi A.K., Nasser B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. J. Food Meas. Charact. 2019;13:121–130. doi: 10.1007/s11694-018-9925-y. [DOI] [Google Scholar]
- 17.Renault J.-H., Ghedira K., Thepenier P., Lavaud C., Zeches-Hanrot M., Le Men-Olivier L. Dammarane saponins from Zizyphus lotus. Phytochemistry. 1997;44:1321–1327. doi: 10.1016/S0031-9422(96)00721-2. [DOI] [PubMed] [Google Scholar]
- 18.El Hachimi F., El Antari A., Boujnah M., Bendrisse A. Comparison of oils seed and fatty acid content of various Moroccan populations of jujube, grenadier and prickly pear. J. Mat. Env. Sci. 2015;6:1488–1502. [Google Scholar]
- 19.Abdeddaim M., Lombarkia O., Bacha A., Fahloul D., Abdeddaim D., Farhat R., Saadoudi M., Noui Y., Lekbir A. Biochemical Characterization and nutritional properties Zizyphus lotus L. fruits in Aures region, Northerastern of Algeria. Ann. Food Sci. Technol. 2014;15:75–81. [Google Scholar]
- 20.Benammar C., Hichami A., Yessoufou A., Simonin A.-M., Belarbi M., Allali H., Khan N.A. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010;20:54. doi: 10.1186/1472-6882-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen K., Fan D., Fu B., Zhou J., Li H. Comparison of physical and chemical composition of three chinese jujube (Ziziphus jujuba Mill.) cultivars cultivated in four districts of Xinjiang region in China. Food Sci. Technol. 2019;39:912–921. doi: 10.1590/fst.11118. [DOI] [Google Scholar]
- 22.Maraghni M., Gorai M., Neffati M. The Influence of Water-Deficit Stress on Growth, Water Relations and Solute Accumulation in WildJujube (Ziziphus lotus) J. Ornam. Hortic. 2011;1:63–72. [Google Scholar]
- 23.Karumi Y., Onyeyili P.A., Ogugduaja V.O. Identification des principles actifs de l’extrait de feuilles de M. balsamia (Baume de la pomme) J. Med. Sci. 2004;4:179–182. [Google Scholar]
- 24.Sheng J.P., Shen L. Postharvest Biology and Technology of Tropical and Subtropical Fruits. Elsevier; Amsterdam, The Netherlands: 2011. Chinese jujube (Ziziphus jujuba Mill.) and Indian jujube (Ziziphus mauritiana Lam.) pp. 299–326. [Google Scholar]
- 25.Zia-Ul-Haq M., Riaz M., De Feo V., Jaafar H., Moga M., Rubus Fruticosus L. Constituents, Biological Activities and Health Related Uses. Molecules. 2014;19:10998–11029. doi: 10.3390/molecules190810998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Q.-H., Wu P.-T., Liu J.-R., Wu C.-S., Parry J.W., Wang M. Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci. Hortic. 2011;130:67–72. doi: 10.1016/j.scienta.2011.06.005. [DOI] [Google Scholar]
- 27.Li J.-W., Fan L.-P., Ding S.-D., Ding X.-L. Nutritional composition of five cultivars of chinese jujube. Food Chem. 2007;103:454–460. doi: 10.1016/j.foodchem.2006.08.016. [DOI] [Google Scholar]
- 28.Sakamura F., Suga T. Changes in chemical components of ripening oleaster fruits. Phytochemistry. 1987;26:2481–2484. doi: 10.1016/S0031-9422(00)83858-3. [DOI] [Google Scholar]
- 29.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 30.Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985;33:213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- 31.Giusti M.M., Wrolstad R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001 doi: 10.1002/0471142913.faf0102s00. [DOI] [Google Scholar]
- 32.Braca A., Sortino C., Politi M., Morelli I., Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002;79:379–381. doi: 10.1016/S0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 33.Irakli M., Chatzopoulou P., Ekateriniadou L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018;124:382–388. doi: 10.1016/j.indcrop.2018.07.070. [DOI] [Google Scholar]
- 34.Chouaibi M., Mahfoudhi N., Rezig L., Donsì F., Ferrari G., Hamdi S. Nutritional composition of Zizyphus lotus L. seeds. J. Sci. Food Agric. 2012;92:1171–1177. doi: 10.1002/jsfa.4659. [DOI] [PubMed] [Google Scholar]
- 35.Rached W., Barros L., Ziani B.E.C., Bennaceur M., Calhelha R.C., Heleno S.A., Alves M.J., Abderrazak M., Ferreira I.C.F.R. HPLC-DAD-ESI-MS/MS screening of phytochemical compounds and the bioactive properties of different plant parts of Zizyphus lotus (L.) Desf. Food Funct. 2019;10:5898–5909. doi: 10.1039/C9FO01423C. [DOI] [PubMed] [Google Scholar]
- 36.Diallo D., Sanogo R., Yasambou H., Traoré A., Coulibaly K., Maïga A. Étude des constituants des feuilles de Ziziphus mauritiana Lam. (Rhamnaceae), utilisées traditionnellement dans le traitement du diabète au Mali. Comptes Rendus Chim. 2004;7:1073–1080. doi: 10.1016/j.crci.2003.12.035. [DOI] [Google Scholar]
- 37.Tiwari B.K., Patras A., Brunton N., Cullen P.J., O’Donnell C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010;17:598–604. doi: 10.1016/j.ultsonch.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Haminiuk C.W.I., Maciel G.M., Plata-Oviedo M.S.V., Peralta R.M. Phenolic compounds in fruits—An overview: Phenolic compounds in fruits. Int. J. Food Sci. Technol. 2012;47:2023–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
- 39.Chu W., Cheung S.C.M., Lau R.A.W., Benzie I.F.F. In: Bilberry (Vaccinium myrtillus L.) in Herbal Medicine: Biomolecular and Clinical Aspects. 2nd ed. Benzie I.F.F., Wachtel-Galor S., editors. CRC Press; Boca Raton, FL, USA: 2011. [Google Scholar]
- 40.Nair S.K.P., Ganesan K., Sinaga M., Letha N., Gani B. Preliminary phytochemical screening of different solvent extracts of leaves of Echeveria elegans rose, an endangered mexican succulent herb. J. Glob. Biosci. 2016;5:3429–3432. [Google Scholar]
- 41.Mishra B., Kar D.M., Maharana L., Mishra G.P. Physicochemical and phytochemical investigation of different fractions from hydroalcoholic extract of Tectona grandis (Linn) barks. Der Pharm. Lett. 2016;8:80–85. [Google Scholar]
- 42.Ribéreau-Gayon J., Peynaud E. Les Composés Phénoliques des Végétaux, Traité D’oenologie. Dunod; Paris, France: 1968. [Google Scholar]
- 43.Macheix J.J., Fleuriet A. Phenolics in fruit products: Progress and prospects. In: Scalbert A., editor. Polyphenolic Phenomena. INRA; Paris, France: 1993. [Google Scholar]
- 44.Dib M.E.A., Allali H., Bendiabdellah A., Meliani N., Tabti B. Antimicrobial activity and phytochemical screening of Arbutus unedo L. J. Saudi Chem. Soc. 2013;17:381–385. doi: 10.1016/j.jscs.2011.05.001. [DOI] [Google Scholar]
- 45.Hadi S.M., Asad S.F., Singh S., Ahmad A. Putative. Mechanism for Anticancer and Apoptosis-Inducing Properties of Plant-Derived Polyphenolic Compounds. IUBMB Life. 2000;50:167–171. doi: 10.1080/152165400300001471. [DOI] [PubMed] [Google Scholar]
- 46.Bate-Smith E.C. The phenolic constituents of plants and their taxonomic significance. I. Dicotyledons. J. Linn. Soc. Lond. Bot. 1962;58:95–173. doi: 10.1111/j.1095-8339.1962.tb00890.x. [DOI] [Google Scholar]
- 47.Di Carlo G., Mascolo N., Izzo A.A., Capasso F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–353. doi: 10.1016/S0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- 48.Alhakmani F., Khan S.A., Ahmad A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac. J. Trop. Biomed. 2014;4:S656–S660. doi: 10.12980/APJTB.4.2014APJTB-2014-0273. [DOI] [Google Scholar]
- 49.Khadhri A., Neffati M., Aschi-Smiti S., Falé P., Lino A.R.L., Serralheiro M.L. Machado Araùjo ME. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. Lwt-Food Sci. Technol. 2010;43:331–336. doi: 10.1016/j.lwt.2009.08.004. [DOI] [Google Scholar]
- 50.Hsouna A.B., Saoudi M., Trigui M., Jamoussi K., Boudawara T., Jaoua S., ElFeki A. Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damage and renal failure in rats. Food Chem. Toxicol. 2011;49:3183–3191. doi: 10.1016/j.fct.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 51.Choi C.W., Kim S.C., Hwang S.S., Choi B.K., Ahn H.J., Lee M.Y., Park S.H., Kim S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant. Sci. 2002;163:1161–1168. doi: 10.1016/S0168-9452(02)00332-1. [DOI] [Google Scholar]
- 52.Nićiforović N., Mihailović V., Mašković P., Solujić S., Stojković A., Muratspahić D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010;48:3125–3130. doi: 10.1016/j.fct.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Alothman M., Bhat R., Karim A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- 54.Vinatoru M., Toma M., Radu O., Filip P.I., Lazurca D., Mason T.J. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason. Sonochem. 1997;4:135–139. doi: 10.1016/S1350-4177(97)83207-5. [DOI] [PubMed] [Google Scholar]
- 55.Goltz C., Ávila S., Barbieri J.B., Igarashi-Mafra L., Mafra M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop. Prod. 2018;115:227–234. doi: 10.1016/j.indcrop.2018.02.013. [DOI] [Google Scholar]
- 56.Vega Arroy J.D., Ruiz-Espinosa H., Luna-Guevara J.J., Luna-Guevara M.L., Hernández-Carranza P., Ávila-Sosa R., Ochoa-Velasco C.E. Effect of Solvents and Extraction Methods on Total Anthocyanins, Phenolic Compounds and Antioxidant Capacity of Renealmia alpinia (Rottb.) Maas Peel. Czech J. Food Sci. 2017;35:456–465. doi: 10.17221/316/2016-CJFS. [DOI] [Google Scholar]
- 57.Ju Z.Y., Howard L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003;51:5207–5213. doi: 10.1021/jf0302106. [DOI] [PubMed] [Google Scholar]
- 58.Khonkarn R., Okonogi S., Ampasavate C., Anuchapreeda S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem. Toxicol. 2010;48:2122–2129. doi: 10.1016/j.fct.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Benabdeljalil C., Cheynier V., Fulcrand H., Hafiki A., Mosaddak M., Moutounet M. Mise en évidence de nouveaux pigments formés par réaction des anthocyanes avec des métabolites de levure. Sci. Aliment. 2000;20:203–220. doi: 10.3166/sda.20.203-220. [DOI] [Google Scholar]
- 60.Mensor L.L., Mnezes F.S., Leitão G.G., Reis A.S., dos Santos T.C., Coube C.S., Leitão S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 61.Mokhtarpour A., Naserian A.A., Valizadeh R., Danesh Mesgaran M., Pourmollae F. Extraction of Phenolic Compounds and Tannins from Pistachio By-products. Annu. Res. Rev. Biol. 2014;4:1330–1338. doi: 10.9734/ARRB/2014/7793. [DOI] [Google Scholar]
- 62.Ghasemi S., Naserian A.A., Valizadeh R., Vakil A.R., Behgar M., Tahmasebi A.M., Ghovvati S. Partial and total substitution of alfalfa hay by pistachio byproduct modulated the counts of selected cellulolytic ruminal bacteria attached to alfalfa hay in sheep. Livest. Sci. 2012;150:342–348. doi: 10.1016/j.livsci.2012.09.024. [DOI] [Google Scholar]
- 63.Naima R., Oumam M., Hannache H., Sesbou A., Charrier B., Pizzi A., Charrier–El Bouhtoury F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crop. Prod. 2015;70:245–252. doi: 10.1016/j.indcrop.2015.03.016. [DOI] [Google Scholar]
- 64.Najjaa H., Ben Arfa A., Elfalleh W., Zouari N., Neffati M. Jujube (Zizyphus lotus L.): Benefits and its effects on functional and sensory properties of sponge cake. PLoS ONE. 2020;15:e0227996. doi: 10.1371/journal.pone.0227996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ghazghazi H., Aouadhi C., Riahi L., Maaroufi A., Hasnaoui B. Fatty acids composition of Tunisian Ziziphus lotus L. (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat. Prod. Res. 2014;28:1106–1110. doi: 10.1080/14786419.2014.913244. [DOI] [PubMed] [Google Scholar]
- 66.Barros L., Carvalho A.M., Morais J.S., Ferreira I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010;120:247–254. doi: 10.1016/j.foodchem.2009.10.016. [DOI] [Google Scholar]
- 67.Kintzios S., Papageorgiou K., Yiakoumettis I., Baričevič D., Kušar A. Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. J. Pharm. Biomed. Anal. 2010;53:773–776. doi: 10.1016/j.jpba.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Hammi K.M., Jdey A., Abdelly C., Majdoub H., Ksouri R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015;184:80–89. doi: 10.1016/j.foodchem.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 69.El Cadi H., El Cadi A., Kounnoun A., Oulad El Majdoub Y., Lovillo M.P., Brigui J., Dugo P., Mondello L., Cacciola F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arab. J. Chem. 2020;13:6299–6311. doi: 10.1016/j.arabjc.2020.05.022. [DOI] [Google Scholar]
- 70.Naczk M., Shahidi F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 71.Trease E., Evans W.C. Pharmacognosie. Billiaire Tindall; London, UK: 1987. [Google Scholar]
- 72.Singleton V., Rossi J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]