Abstract
Objective: This study evaluated the use of novel peptides derived from platelet-derived growth factor (PDGF-BB) as potential wound healing stimulants. One of the compounds (named PDGF2) was subjected for further research after cytotoxicity and proliferation assays on human skin cells. Further investigation included evaluation of: migration and chemotaxis of skin cells, immunological and allergic safety, the transcriptional analyses of adipose-derived stem cells (ASCs) and dermal fibroblasts stimulated with PDGF2, and the use of dorsal skin wound injury model to evaluate the effect of wound healing in mice.
Approach: Colorimetric lactate dehydrogenase and tetrazolium assays were used to evaluate the cytotoxicity and the effect on proliferation. PDGF2 effect on migration and chemotaxis was also checked. Immunological safety and allergic potential were evaluated with a lymphocyte activation and basophil activation test. Transcriptional profiles of ASCs and primary fibroblasts were assessed after stimulation with PDGF2. Eight-week-old BALB/c female mice were used for dorsal skin wound injury model.
Results: PDGF2 showed low cytotoxicity, pro-proliferative effects on human skin cells, high immunological safety, and accelerated wound healing in mouse model. Furthermore, transcriptomic analysis of ASCs and fibroblasts revealed the activation of processes involved in wound healing and indicated its safety.
Innovation: A novel peptide derived from PDGF-BB was proved to be safe drug candidate in wound healing. We also present a multifaceted in vitro model for the initial screening of new compounds that may be potentially useful in wound healing stimulation.
Conclusion: The results show that peptide derived from PDGF-BB is a promising drug candidate for wound treatment.
Keywords: wound healing, peptides, PDGF, immunogenicity, cytotoxicity, transcriptomics

Michał Pikuła, MSc, MPharm, PhD

Sylwia Rodziewicz-Motowidło, PhD
Introduction
Wound healing is an intricate and dynamic process consisting of several overlapping stages, and three main phases can be distinguished: inflammation, proliferation, and remodelling.1 Many cell types (e.g., fibroblasts and keratinocytes), the extracellular matrix (ECM), growth factors, and cytokines are involved in the healing process.2,3
Platelet-derived growth factor (PDGF-BB) was the first growth factor taking part in wound healing to be described and purified.4 It is considered to be the most important and strongest stimulant, participating in almost all stages of wound healing.5 PDGF is released mostly by degranulating platelets and also by keratinocytes, fibroblasts, endothelial cells, and macrophages.1 It acts as a mitogen for various cell types, such as fibroblasts, keratinocytes, and endothelial cells.6 It causes chemotaxis of neutrophils, macrophages, fibroblasts, and smooth muscle cells to the wound site, which helps to initiate the inflammatory phase.7 In addition, it acts as a chemoattractant for bone marrow mesenchymal stem cells, which, when accumulated in the wound, can give rise to fibroblasts. PDGF also increases the proliferation of fibroblasts and the production of ECM components, such as fibronectin, collagen, proteoglycan, and hyaluronic acid.1,8,9
Several approaches to cutaneous wound healing/regenerative medicine, including negative pressure wound therapy,10 electrostimulation therapy,11 skin grafts,12 engineered skin substitutes,13 wound dressings,14 and stem cell therapy,15 have been studied. In particular, therapies based on stem cells offer considerable opportunities in treating chronic wounds because of the capacity of stem cells for self-renewal and differentiation into almost any cell type.16 However, current methods focused on effectively isolating and culturing these cells and safely delivering them require extensive time, funds, and human effort. Therefore, the search for novel pharmaceutical compounds that are beneficial in wound healing stimulation is crucial.
The utilization of peptides as compounds with regenerative potency has emerged over the past decade.17 They can mimic the functions of proteins but have lower production complexity and costs. In addition, peptides can target certain “flat pockets” that are considered undruggable in small-molecule therapy.18 Peptides are easily synthesized and biocompatible and have controlled sizes, functional groups, and activity, making them the molecules with the most potential for drug development. Based on their mechanism of action or origin, several classes, including peptides with antimicrobial/regenerative activity, compounds based on the sequence of collagen/elastin, derivatives of growth factors/hormones, proteins/peptides involved in immune and hematopoietic processes, and compounds of animal origin, can be distinguished.19–26 Some of these types of peptides have already been translated into the clinic; one example is MSI-78 (pexiganan; the peptide GIGKFLKKAKKFGKAFVKILKK), which has been used as an antimicrobial and wound healing compound.27
In this work, we designed and synthesized novel short peptides derived from the PDGF-BB sequence enclosing fragments of loop L1 or L3, which interact with the PDGFRβ receptor, and evaluated their potential as wound healing stimulants. For the initial screening, we checked the cytotoxicity of the peptides and observed a positive effect on the proliferation of human skin cells. The conducted research allowed us to select peptide named PDGF2 for further evaluation of its potential as a wound healing drug candidate in cellular and animal models. PDGF2 may have the potential to be used in wound healing stimulation and, in the future, after clinical evaluation, become an alternative to recombinant human PDGF-BB for the treatment of chronic wounds. These results also indicate that growth factors that naturally occur in the human body and participate in proper wound healing can be the basis for the design of new drugs for wound treatment. We also present a multifaceted in vitro model based on cell culture tests, animal models, immunogenicity and allergenicity assessments, and RNA sequencing (RNA-Seq) transcriptional analysis for the development and initial screening of new compounds that stimulate wound healing.
Clinical Problem Addressed
There is currently a significant need for the development of new methods of treatment of injuries requiring surgical interventions, such as thermal, chemical, or radiation burns of large area or chronic wounds resulting from civilization diseases such as diabetes or ischemia. Wound healing complications are an immense problem in modern medicine and are associated with various types of diseases, including diabetes, vascular problems, ischemia, cancer, and oncological treatment (radio- and chemotherapy).28,29 It is estimated that 1–2% of Europeans suffer from chronic wounds and that 2–3% of health care budgets are devoted to chronic wound treatment. Therefore, chronic wounds constitute a significant economic problem and create a high demand for novel drugs and methods that effectively stimulate wound healing.30,31
Recombinant human PDGF-BB in a hydrogel (becaplermin) is the only growth factor commercially available for the cutaneous wound healing. It was approved by the U.S. Food and Drug Administration (FDA) in 1997 for topical use in the treatment of lower limb ulcers in diabetic wounds.4,32 However, this product is not widely used because of its high costs and initial speculation about tumor growth acceleration due to the high concentration of PDGF-BB (100 μg/1 g of gel) used in the formulation.7,33 Nonetheless, after continued study, a statistically significant increased risk of death due to cancer was not observed.7,32,34 Therefore, the utilization of PDGF-BB as a template for novel small-molecule wound healing stimulators (e.g., peptides) is justified.
Materials and Methods
Synthesis of PDGF-derived peptides
Synthesis of PDGF1 and PDGF2
The synthesis of the peptides PDGF1 and PDGF2 was performed on a CEM Liberty Blue automated microwave peptide synthesizer using a TentaGel R RAM amide solid support (loading 0.18 mmol/g; Rapp Polymere) utilizing Fmoc/tBu chemistry. The coupling of the Fmoc/tBu standard protected amino acids was achieved using DIPCDI/oxyma pure reagents and the Fmoc deprotection cycles with 20% piperidine in DMF (v/v). Both cycles were carried out at 90°C according to protocols provided by CEM corporation. The dried peptidyl-resins with synthesized PDGF1 or PDGF2 were treated with a cleavage cocktail (92% trifluoroacetic acid (TFA), 4% triisopropylsilane (TIPSI), and 4% H2O) and precipitated with cold diethyl ether. Then, the precipitants were collected by centrifugation and washed three times with cold diethyl ether. Crude peptides were purified to at least 97% purity by reversed-phase high-performance liquid chromatography (RP-HPLC) on Jupiter Proteo column (21.2 × 250 mm, 4 μm, 90 Å; Phenomenex). Chromatographic separations were carried out in a linear gradient of 5 → 100% B over 180 min at a flow rate of 15 mL/min and with ultraviolet (UV) detection at λ = 223 nm and the following solvents: A = 0.1 M aqueous ammonium acetate (pH 4.75) and B = 30% in 0.1 M aqueous ammonium acetate (pH 4.75). The purity of peptides was determined by UPLC Shimadzu Nexera X2, and the sequence of compounds was confirmed with electrospray ionization ion trap time-of-flight liquid chromatography mass spectrometry (ESI-IT-TOF-LCMS). More details are listed in Supplementary Figs. S1 and S2 and Supplementary Table S1.
Synthesis of the cyclic peptide PDGF2-HTT
The synthesis of PDGF2-HTT (head-to-tail cyclization methodology) was performed on a CEM Liberty Blue automated microwave peptide synthesizer using a Cl-TCP(Cl) ProTide (CEM Corporation) acid solid support (loading 0.4 mmol/g) utilizing the Fmoc/tBu chemistry. The coupling of the Fmoc/tBu standard protected amino acids was achieved using DIPCI/Oxyma pure reagents and the Fmoc deprotection cycles with 20% piperidine in DMF (v/v). Both cycles were carried out at 90°C and addition of 0.1 M DIPEA into Oxyma pure reagent bottle according to the protocols provided by CEM corporation. The peptide was cleaved from the solid support with 1% TFA in dichloromethane (v/v) while leaving the side chain–protecting groups of all residues for 1 h. The peptide was precipitated with cold diethyl ether and washed with it three times. The crude product was cyclized by the formation of a peptide bond between the amino group at the N-terminus and the carboxyl group at the C-terminus with HATU/HOAt according to previously described protocol.35 After cyclization step, the peptide was purified by HPLC on a Jupiter Proteo C12 semi-preparative column (Phenomenex) with dimensions of 21.2 × 250 mm, 90 Å, and 4 μm. The chromatographic separation was carried out in a linear gradient of 5 → 100% B over 180 min with the following eluents: A = 0.1% TFA in H2O and B = 0.1% TFA and 80% isopropanol in H2O. The eluent flow rate was 14 mL/min, and UV detection at λ = 223 nm was performed. Pure fractions were collected and lyophilized. Cleavage of the remaining side chain–protecting groups in the peptide was carried out by treating the cyclic form with a mixture of TFA/TIPSI/H2O (94:3:3, v/v/v) for 2 h. Then, the peptide was treated and purified as described above. Purification was carried out with following solvents: A = 0.1% TFA in H2O and B = 0.1% TFA and 35% acetonitrile (ACN) in H2O. The exchange of the trifluoroacetic counterion with an acetate ion was performed on a Strata X-C 33U Polymeric Strong Cation column (Phenomenex) according to the protocol provided by the manufacturer. The purity of peptides was determined by UPLC Shimadzu Nexera X2, and the sequence of compounds was confirmed with ESI-IT-TOF-LCMS. More details are listed in Supplementary Fig. S3 and Supplementary Table S1.
Primary cell isolation
Human skin and subcutaneous adipose tissue were sampled from patients at the Plastic Surgery Clinic or Oncologic Surgery Clinic of the Medical University of Gdansk. The procedure was approved by the Independent Bioethics Commission for Research of the Medical University of Gdansk (NKBBN/387/2014). Primary epidermal cells were isolated with the protocol described previously by Langa et al.36 The isolation of adipose-derived stem cells (ASCs) was based on the standard protocol previously described by Mieczkowska et al.37 involving enzymatic digestion and erythrocyte lysis. ASC “stemness” was confirmed by differentiating the isolated ASCs into chondrocytes, osteocytes, and adipocytes as described previously (Supplementary Fig. S4). The isolation of fibroblasts was performed according to the protocol described by Kosikowska et al.,38 which utilizes collagenase enzymatic digestion, followed by culturing in Dulbecco's modified Eagle's medium (DMEM) with a high-glucose (HG) content.
Cell culture
In our study, five types of cells were used: immortalized human HaCaT keratinocytes (DKFZ, Heidelberg, Germany),39,40 a human dermal fibroblast cell line (46BR.1N), human primary fibroblasts, human primary keratinocytes, and ASCs. The 46BR.1N cell line was obtained from the European Collection of Cell Cultures (ECACC). This cell line was originally derived from the skin of an anonymous individual with hypogammaglobulinemia and was transformed with the pSV3neo plasmid.41 HaCaT and 46BR.1N cells and human primary fibroblasts were grown in DMEM (Sigma–Aldrich Co.) supplemented with 4,500 mg/L glucose, 584 mg/L l-glutamine, sodium pyruvate, and sodium bicarbonate. The medium also contained 10% fetal bovine serum (FBS) and was supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin (Sigma–Aldrich Co.). Human primary keratinocytes were grown in Keratinocyte Growth Medium (KGM) (Cat. No. CC-3103; Lonza-Clonetics, Basel, Switzerland) supplemented with epidermal growth factor, hydrocortisone, transferrin, epinephrine, insulin, and gentamycin (Cat. No. CC-4152; Lonza-Clonetics). ASCs were grown in DMEM (Sigma–Aldrich Co.) supplemented with 1,000 mg/L glucose, 584 mg/L l-glutamine, sodium pyruvate, and sodium bicarbonate. Cells were routinely cultured in a humidified atmosphere with 5% CO2 at 37°C in culture flasks (growth surface area: 25 cm2).
Lactate dehydrogenase assay
Cell death was quantified by measuring lactate dehydrogenase (LDH) activity in cell supernatants (Cat. No. MK401; Takara, Japan). Cells were seeded in 96-well plates at a density of 5,000 cells per well in DMEM supplemented with 10% FBS. After 24 h, the medium was changed to serum-free medium containing appropriate concentrations of PDGF2. After 48 h, the supernatants were collected for LDH content analysis. Cell death was normalized with respect to the level of cell death in a non-PDGF2-treated control (0%). Triton X-100 detergent (1%) was used as a positive control for maximum LDH release (maximum cytotoxicity).
Tetrazolium assay
HaCaT keratinocytes, 46BR.1N fibroblasts, and primary fibroblasts were seeded at a density of 5,000 cells per well in 96-well plates (BD) in DMEM HG medium supplemented with 10% FBS. After 24 h, the medium was exchanged for serum-free DMEM HG medium containing appropriate concentrations of PDGF2. All solutions used in the experiments were prepared with water under sterile conditions. The XTT cell proliferation assay was then performed according to the manufacturer's instructions (Roche Diagnostics). The cells were incubated with PDGF2 for 48 or 72 h, and then, XTT reagent was added. The plates were incubated at 37°C for 4 h in the presence of 5% CO2. The absorbance was then read using a standard plate reader at 490 nm. Cell proliferation was normalized with respect to the proliferation of a non-PDGF2-treated control (100%).
Migration and chemotaxis assays
The effect of PDGF2 on cell migration after 24 h was determined using Ibidi culture inserts with a defined cell-free gap suitable for wound healing and migration assays (Cat. No. 81176; Ibidi). Cells were seeded in the culture inserts at a density of 20,000 per well in DMEM supplemented with 10% FBS. After 24 h, the medium was exchanged for serum-free DMEM, and cell proliferation was blocked by adding mitomycin C (5 μg/mL) for 2 h. Next, the medium was changed, and the cells were stimulated with the appropriate concentration of PDGF2. After 24 h, the cells were fixed with 3.7% paraformaldehyde and stained with 0.05% crystal violet, and the effect was measured with a microscope. The effect of PDGF2 on cell chemotaxis was assessed with ThinCert cell culture inserts (8.0 μm; Greiner Bio-One, Germany). Cells were starved overnight in serum-free medium. Then, they were seeded on inserts placed in a 24-well plate at a density of 100,000 cells per well in serum-free medium. Culture medium with PDGF2 was added to the plate well. After 24 h, the cells were stained with 8.0 μM Calcein-AM (a cell viability dye; Sigma–Aldrich Co.), migratory cells were detached with trypsin-EDTA, and their fluorescence was read with a plate reader at an excitation wavelength of 485 nm and an emission wavelength of 520 nm.
Analysis of protein and cytokine levels in culture supernatants
For the analysis of proteins and cytokines in supernatants collected from cell cultures of primary fibroblasts or keratinocytes stimulated with the PDGF2 peptide at a 1.0 μg/mL concentration, we used Luminex® xMAP® technology. This technology is a powerful platform for multiplex detection of proteins in a single biological sample. For analysis of keratinocyte and fibroblast supernatants, we assessed the concentrations of 12 human angiogenesis and growth factor biomarkers: angiopoietin-2, BMP-9, EGF, endoglin, endothelin-1, FGF-1 (acidic FGF), FGF-2 (basic FGF), follistatin, G-CSF, HB-EGF, HGF, IL-8, leptin, PLGF, VEGF-A, VEGF-C, and VEGF-D with the Human Angiogenesis/Growth Factor Magnetic Bead Panel 1 (Merck Millipore, Germany). Supernatants were thawed on ice. The analysis was performed according to the manufacturer's instructions. Briefly, the supernatants were incubated with a mixture of color-coded beads precoated with analyte-specific capture antibodies. Next, a cocktail of biotinylated detection antibodies specific to the analyte of interest was added, followed by the addition of phycoerythrin (PE)-conjugated streptavidin, which bound to the detection antibodies. The prepared samples were read by the Luminex MAGPIX® Analyzer (Merck Millipore). Data were analyzed using xPONENT 4.2 software and are presented as the cytokine concentration in units of pg per 1.0 mL.
Basophil activation test
The commercially available Flow CAST® highsens test (Bühlmann Laboratories, Switzerland) was used to assess the activity of granulocytes. This test was performed with blood samples collected from healthy volunteers within 24 h, according to the protocol. Blood (100 μL) was incubated with PDGF2 at a final concentration of 1.0 μg/mL. Blood cells were then stained with fluorochrome-conjugated monoclonal antibodies (anti-CD63, anti-CD203c, and anti-CCR3) and incubated for 15 min at 37°C. After the incubation, erythrocytes were lysed, and the cells were washed and then subjected to flow cytometry analysis (BD FACSCanto II). Each sample was accompanied by its own negative and positive controls (anti-FcɛRI mAb and N-Formylmethionyl-leucyl-phenylalanine [fMLP]).
Immunological studies
Tests were conducted with human peripheral blood mononuclear cells (PBMCs) isolated from “buffy coats” using a Ficoll density gradient (Histopaque; Sigma–Aldrich Co.). Following two wash steps in phosphate-buffered saline (PBS), erythrocyte lysis, and cell counting (cell counter; Bio-Rad), the PBMCs were seeded in a 24-well culture plate at a density of 1 × 106 cells/1 mL of RPMI 1640 medium (antibiotics penicillin and streptomycin, 10% FBS) per well. The cells were then allowed to adapt to the culture conditions for the next 24 h. After cell adaptation, PDGF2 was added to the wells at a final concentration of 1.0 μg/mL, and the cells were incubated for the next 48 h under appropriate conditions (37°C and 5% CO2). Untreated cells constituted a negative control.
After the incubation, the PBMCs were collected, washed with PBS, counted, and prepared for flow cytometry analysis under the following conditions: 104 cells/100 μL were stained with fluorochrome-conjugated monoclonal antibodies (anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD56, anti-CD25, anti-CD69, anti-CD71, anti-HLA-DR, anti-CD11c, anti-CD80, and anti-CD83). Following 30 min of incubation at room temperature in the dark, the cells were analyzed using a flow cytometer (LSRFortessa; BD).
Cell stimulation for transcriptomic analysis
Cells (ASCs after the second passage and fibroblasts after the third passage) were seeded and cultured for 24 h in medium supplemented with 10% FBS, followed by a 24-h incubation in medium supplemented with 5% FBS and then a 48-h incubation in serum-free medium supplemented with the PDGF2 peptide (1 μg/mL). Cell cultured in the same condition but nontreated with PDGF2 were used as the parallel control. After the stimulation, the cells were trypsinized, collected, and centrifuged, and the pellet was snap frozen for transcriptomic analysis.
Human epidermal cells were seeded in a 25 cm2 t-flask in KGM supplemented with epidermal growth factor, hydrocortisone, transferrin, epinephrine, insulin, gentamycin, and 5% FBS. After 24 h, the medium was changed to serum-free KGM. After another 2–5 days, the medium was changed to keratinocyte basal medium, and the cells were stimulated with PDGF2. After 48 h of incubation, cell pellets were collected and snap frozen at −80°C until RNA isolation.
Whole-transcriptome RNA-Seq analysis of the PDGF2 stimulatory effect on primary ASCs and fibroblasts
RNA was isolated from cell pellets stored at −80°C using the miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol with the following two modifications: 1-bromo-3-chloropropane was used instead of chloroform, and the elution was conducted with 40 μL of water and repeated with a second elution of the entire volume of the original eluate. RNA quantity and quality (RNA Integrity Number [RIN]) were assessed using the Bioanalyzer 2100 Instrument and RNA 6000 Nano Kit (Agilent, Waldbronn, Germany). RNA transcripts isolated from three ASC and three fibroblasts replicates with an RIN above 7.0 were pooled in equal amounts (by mass) and were later used for massive parallel transcriptome sequencing. The reads were mapped to a reference human genome (hg19 edition) using TopHat software. Differential expression analyses comparing between the PDGF2-treated and control cells were performed with the Cufflinks package using the complete workflow for version 2.2.0 (and higher) as described by the authors (http://cole-trapnell-lab.github.io/cufflinks/manual). The results of the comparative analyses were used to build differential gene expression matrices that were further processed by Ingenuity Pathway Analysis (IPA).
Quantitative PCR gene expression analysis
Total RNA was extracted from epidermal cells with an RNeasy kit (74104; Qiagen) according to the manufacturer's protocol. RNA quality and quantity were evaluated with a NanoDrop 2000 spectrophotometer. cDNA synthesis was performed with Maxima Reverse Transcriptase (EP0741; Thermo Scientific) according to the manufacturer's protocol. Real-time PCR analyses were carried out on a LightCycler 96 with FastStart Essential DNA Green Master Mix (06402712001; Roche), and ACTB and TBP were used as reference genes. The analyzed transcripts and primer sequences are listed in Supplementary Table S2.
Excision wound model in mice
Eight-week-old BALB/c female mice were used for experiments. All experimental procedures were approved by the local ethics committee in Bydgoszcz (Approval No. 49/2016). Before experiments, mice were randomized and divided into groups comprising seven to eight individuals. The mice were anesthetized with inhaled 2–5% isoflurane. The skin on the back was shaved and disinfected. The skin was folded and raised cranially and caudally along the spine. Then, the mouse was placed in a lateral position, and the folded skin was pierced with a φ 6.0-mm biopsy punch, resulting in the formation of two excisional wounds in the dorsal skin. A total of 25 μL of peptide (0.2 mg/mL) suspended in hydrogel was applied to each wound. The wounds were covered with transparent Tegaderm film, and an adhesive plaster was wrapped around the mouse torso. During the first week of the experiment, the mice were treated with the peptide, and the dressing was replaced once a day for 5 days. During the second week of the experiment, the dressing was replaced every other day. In the beginning of the third week, the dressing was removed. The same protocol was applied to the control mice, which were treated with hydrogel alone. To measure the wound area, a ruler was placed next to the injury site, and the wound was photographed. Wound areas were quantitated using the ImageJ software.
Tissue isolation for histological analyses
Mice were sacrificed at days 4 and 21 postinjury. After the mice were euthanized, the skin from the back was dissected and stored in formalin. The skin samples were then embedded in paraffin, cut into 5 μm sections, and stained with either hematoxylin and eosin or Masson's trichrome. The stained sections were evaluated using a light microscope.
Statistical analysis
Statistical significance was determined with the Mann–Whitney U-test (p < 0.05) using STATISTICA software (StatSoft Polska, Krakow, Poland) and XLSTAT (Addinsoft). Graphs were prepared with GraphPad Prism 5 software.
Results
Design of PDGF-derived peptides
Functional and structural studies of human PDGF proteins allowed pinpointing loops L1, L2, and L3 as crucial regions in the binding and activation of PDGFRs.42–44 To date, only one X-ray crystal structure of the complex formed by the human PDGF-BB homodimer with two PDGFRβ receptor particles has been described (PDB: 3MJG).45 In the crystal structure, PDGF-BB interacts with its receptor by three interstrand loops (L1-residues 25–38, L2-residues 53–58, and L3-residues 78–81) and the C-terminal segment. Nonetheless, on the PDGF-BB side, the majority of the interface is contributed by the L1 and L3 loops of the protruding protomer, which contrarily, in complex-free state, are defined as flexible and solvent exposed.46 Previous mutagenesis and deletion mapping data of PDGF-BB:PDGFR interactions suggest that the L1 and L3 loops of PDGF-BB are crucial for its biological activity.47,48 To date, most efforts have focused on the search for small-molecule inhibitors (or intercalators) of the interactions of PDGFs with their receptors in the context of cancer, chronic inflammatory conditions, or atherosclerosis.49 Only a few articles have reported the utilization of peptide molecules based on the receptor-interacting loop of PDGF-BB. Authors have emphasized the role of the loop L3 of PDGF-BB as the template to synthesize new inhibitors or synthetic antigens for the native protein.50–52 Nonetheless, only Brennand et al. showed stimulatory effects of some analogues based on the interacting loop L3 of PDGF-BB on proliferation or DNA synthesis in cell cultures during the search for inhibitors.50 However, none of the articles evaluated the potency of the full covering sequence of the L1 or L3 loop as a stimulant of skin cell proliferation or migration in regard to wound healing.
Therefore, we decided to evaluate the potency of the loops L1 and L3 of the PDGF-BB protein by synthesizing peptides covering the interacting sequences based on the crystal structure complex (Table 1 and Supplementary Fig. S5) and determine the biological activity of these peptides as stimulants of skin regeneration. After preliminary testing of the peptides designated PDGF1 and PDGF2 (Table 1) in cell cultures, the latter was selected for further modification. PDGF2 was cyclized by the attachment of a polyethylene glycol linker (PEG2) to its N-terminus following the formation of a peptide bond with the C-terminus (PDGF2-HTT in Table 1). The PEG2 linker, which had an estimated length of ∼11 Å, was chosen as corresponding to the linear estimation of the distance between the N-terminus of Arg28 and the C-terminus of Leu37 of the PDGF-BB protein in the crystal structure complex.45 This modification was chosen because, in general, cyclic peptides exhibit reduced conformational freedom, which often results in higher receptor selectivity and binding affinity or improvement in their intercalation abilities when acting as inhibitors.53,54 Furthermore, cyclic peptides exhibit improved metabolic stability in the serum and elevated resistance to microbial degradation.55
Table 1.
Structures of the peptides under study
| Name | Sequence | PDGF-BB Loop Origin |
|---|---|---|
| PDGF1 | R73KIEIVRKKPIF84-NH2 | L3 |
| PDGF2 | R28LIDRTNANFL37-NH2 | L1 |
| PDGF2-HTT | Cyclo-[PEG2-R28LIDRTNANFL37] | L1 |
Amino acid residues were assigned according to the numeric convention in the crystal structure of PDGFRβ:PDGF-BB (PDB:3MJG).
PDGF, platelet-derived growth factor.
PDGF derivatives show different cytotoxicities in human cells
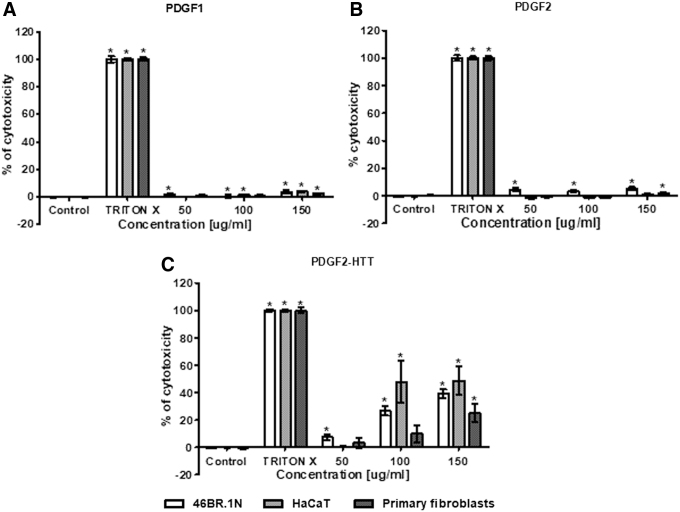
Taking into consideration the fact that peptides can be cytotoxic to human cells, we decided to evaluate the effect of PDGF-BB-derived peptides on human skin cells at concentrations of 50–150 μg/mL with an LDH test. This method measures LDH activity in culture supernatants. LDH is released into culture medium when the plasma membrane is damaged. In the LDH test, the PDGF1 and PDGF2 peptides did not induce cytotoxicity in human HaCaT keratinocytes, 46BR.1N fibroblasts, or primary fibroblasts isolated from patient skin samples (Fig. 1). However, PDGF2-HTT appeared to be cytotoxic to all tested cells. The highest cytotoxicity was observed in the HaCaT cells (50% cytotoxicity at concentrations of 100 and 150 μg/mL), and the 46BR.1N fibroblasts were slightly less susceptible. The lowest cytotoxicity was observed in the primary fibroblasts (∼30% cytotoxicity at a concentration of 150 μg/mL and no statistically significant effect at lower concentrations). Moreover, the PDGF2 peptide did not induce cytotoxicity in ASCs, and at the concentrations of 50 and 100 μg/mL, we observed lower LDH activity in the supernatants from PDGF2-treated ASCs than in control samples (Supplementary Fig. S6A).
Figure 1.
Cytotoxicity of PDGF1 (A), PDGF2 (B), and PDGF2-HTT (C) in human 46BR.1N fibroblasts, HaCaT keratinocytes, and human dermal primary fibroblasts. Graphs show the results of four independent experiments, which are presented as the mean ± SEM (*statistically significant difference, Mann–Whitney U-test, p < 0.05).
PDGF-derived peptides stimulate the proliferation of human skin cells
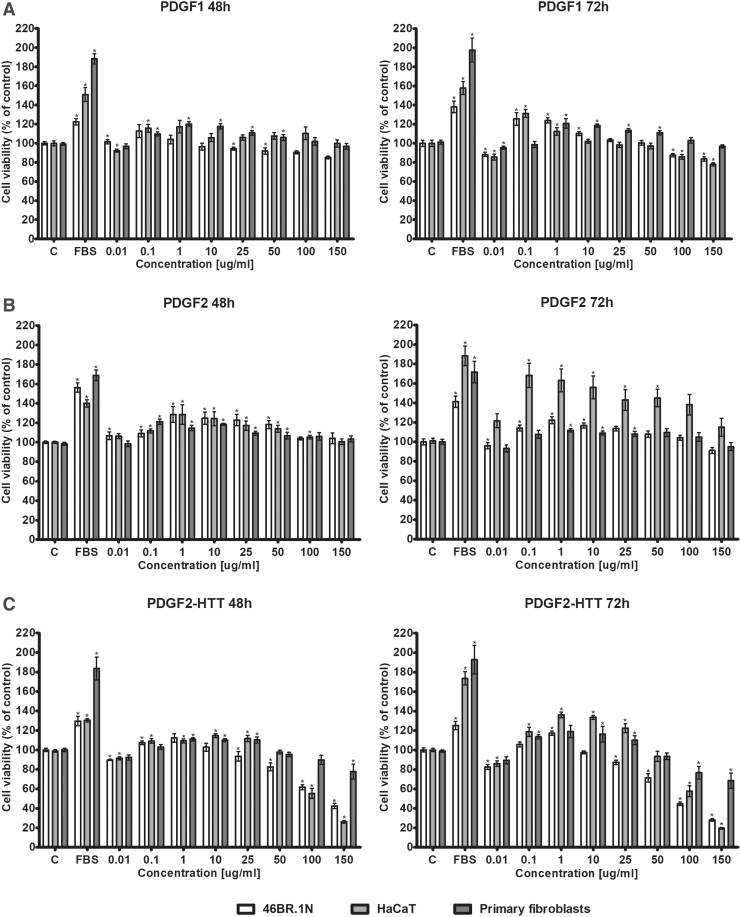
The proliferation of skin cells plays a crucial role in wound healing. Therefore, we decided to check the effect of the tested peptides on the proliferation of HaCaT keratinocytes, 46BR.1N fibroblasts, and human primary fibroblasts. The results obtained from an XTT test (Fig. 2) showed that PDGF1 stimulated proliferation in all examined cells. The strongest effects were observed in the 46BR.1N fibroblasts and HaCaT keratinocytes at a concentration of 0.1 μg/mL after 72 h of incubation. PDGF1 slightly inhibited proliferation in the HaCaT keratinocytes at the two highest concentrations, but it did not inhibit proliferation in the primary fibroblasts.
Figure 2.
Effects of PDGF1 (A), PDGF2 (B), and PDGF2-HTT (C) on the proliferation of human 46BR.1N fibroblasts, HaCaT keratinocytes, and human dermal primary fibroblasts. Graphs show the results of four independent experiments, which are presented as the mean ± SEM (*statistically significant difference, Mann–Whitney U-test, p < 0.05).
PDGF2 stimulated proliferation in all tested cells. However, the strongest effect was obtained in the HaCaT keratinocytes after a 72-h stimulation. For all tested cell types, the pro-proliferative effect was observed with concentrations up to 50 μg/mL. Moreover, PDGF2 did not inhibit proliferation at any of the tested concentrations.
PDGF2-HTT, the heterocyclic derivative of PDGF2, showed a pro-proliferative effect on all tested cell lines. The strongest effect was observed in the HaCaT keratinocytes at concentrations of 0.1–25 μg/mL after a 72-h incubation. However, this effect was weaker than that obtained with PDGF2. In addition, PDGF2-HTT significantly inhibited the proliferation of the 46BR.1N fibroblasts and HaCaT keratinocytes at the two highest concentrations. A slight decrease in 46BR.1N cell proliferation was also observed at the 25 and 50 μg/mL concentrations. The primary fibroblasts showed the lowest sensitivity to the inhibitory effects of PDGF2-HTT.
The results obtained from the XTT and LDH tests allowed us to choose PDGF2 for further examination. PDGF2 was considered the most promising candidate due to its strong pro-proliferative effect and low cytotoxicity.
An additional analysis of PDGF2 activity in human ASCs showed that after 72 h of incubation, PDGF2 slightly stimulated ASCs proliferation at concentrations of 0.1–50 μg/mL (Supplementary Fig. S6B).
PDGF2 has a slight effect on the migration and chemotaxis of skin cells and does not cause changes in the secretion of cytokines and growth factors
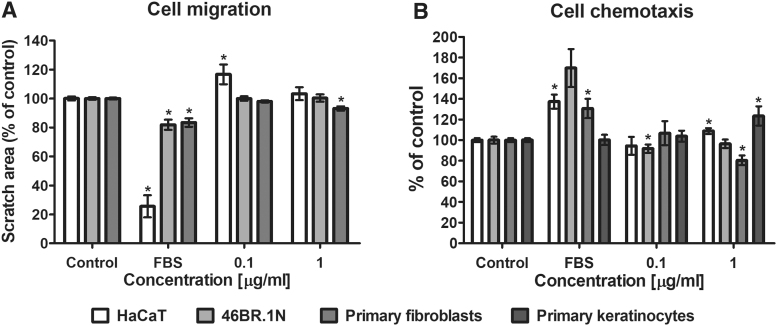
The effect of PDGF2 on cell migration was evaluated in HaCaT keratinocytes, 46BR.1N fibroblasts, and primary dermal fibroblasts isolated from skin samples. The cells were stimulated with PDGF2 at concentrations of 0.1 and 1.0 μg/mL for 24 h. The obtained results showed a slight pro-migratory effect on the human primary fibroblasts (reduction in the scratch surface area by 5–20% relative to the control scratch surface area) at the concentration of 1.0 μg/mL. However, PDGF2 did not stimulate the migration of the 46BR.1N fibroblasts and caused a small reduction in the migration of the HaCaT keratinocytes at the concentration of 0.1 μg/mL (Fig. 3A). In addition, PDGF2 showed a slight chemotactic effect on the human primary and HaCaT keratinocytes at the concentration of 1.0 μg/mL (Fig. 3B). Additionally, to a small extent, PDGF2 reduced the chemotaxis of the 46BR.1N cells and primary fibroblasts at the 0.1 and 1.0 μg/mL concentrations, respectively (Fig. 3B).
Figure 3.
Effects of PDGF2 on the migration (A) and chemotaxis (B) of skin cells. Graphs show the results of at least three experiments, which are presented as the mean ± SEM. (*statistically significant difference, Mann–Whitney U-test, p < 0.05).
The assessment of levels of selected cytokines and growth factors involved in wound healing in postculture medium after the stimulation of primary fibroblasts and keratinocytes with the PDGF2 peptide (1.0 μg/mL) was made by Luminex xMAP technology. The analysis of obtained results did not reveal statistically significant changes in the concentrations of the examined growth factors and cytokines (data not shown).
PDGF2 is immunologically safe
Peptides, similar to other biological drugs, can cause allergic responses and induce an immune response, so it is crucial to check their allergic potential. Several tests are useful for the preclinical evaluation of the immunogenicity of peptide-based drugs.25 In our research, we used a lymphocyte activation assay and basophil activation test (BAT) test.
The results obtained from the BAT assay showed no basophil activation in the presence of PDGF2 (1.0 μg/mL), as the PDGF2-treated samples showed values comparable to the negative control samples (Fig. 4A).
Figure 4.
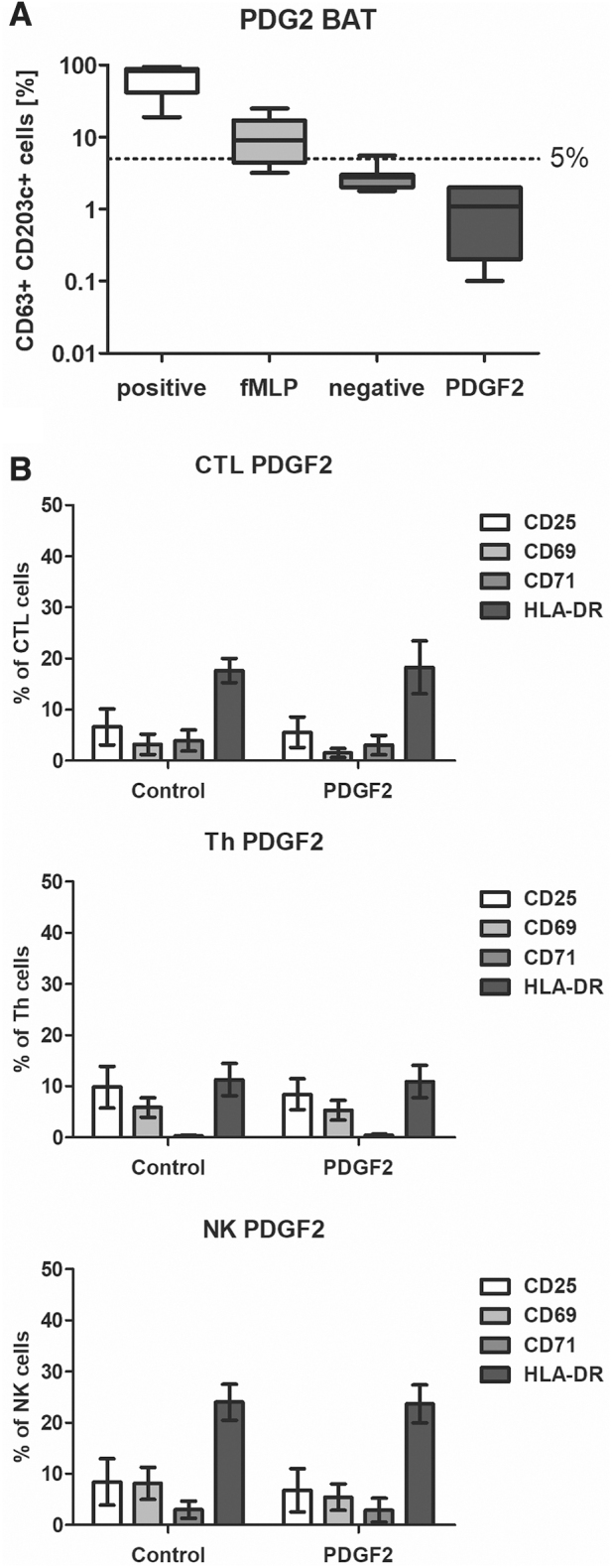
Analysis of PDGF2 immunological safety. (A) The BAT was used to evaluate the allergic potential of PDGF2. In vitro activation of basophils in the presence of activating antibodies (first positive control), fMLP (second positive control), a negative control vehicle (water), or PDGF2 was monitored. (B) An analysis was performed via flow cytometry to evaluate the activation levels of selected immune cell subpopulations. Graphs present the expression levels of activation markers on CTLs, Th cells, and NK cells after an incubation with PDGF2 (1 μg/mL). BAT, basophil activation test; CTLs, cytotoxic T lymphocytes; fMLP, N-Formylmethionyl-leucyl-phenylalanine; Th, T helper; NK, natural killer.
An analysis of the impact on immune cell activity was performed with human PBMCs. The cells were incubated with PDGF2 (1.0 μg/mL) for 48 h. The effects of the stimulation were evaluated by flow cytometry. We assessed the activation level of T cells (CD3/CD4/CD8) and natural killer (NK) cells (CD16/CD56) through the evaluation of the expression of the activation markers CD69, CD71, CD25, and HLA-DR. The influence of the examined compound on dendritic cells (CD11c, HLA-DR) was also investigated by assessing the expression of CD80 and CD83.
The obtained results (Fig. 4B) showed no immunogenic properties for PDGF2. The analysis of the expression of activation markers (CD25, CD69, CD71, and HLA-DR) in individual lymphocyte subpopulations (cytotoxic T lymphocytes [CTLs], T helper [Th] cells, and NK cells) did not show differences between the negative control cells and the peptide-stimulated cells. In addition, dendritic cells were not activated in the presence of PDGF2 (Supplementary Fig. S7) (inactive dendritic cell phenotype: CD11c+, CD80−, and CD83−; active phenotype: CD11+, CD80+, and CD83+).
Therefore, based on the conducted tests, the immunological safety of PDGF2 was confirmed.
Transcriptional responses of ASCs, fibroblasts, and keratinocytes to PDGF2 stimulation
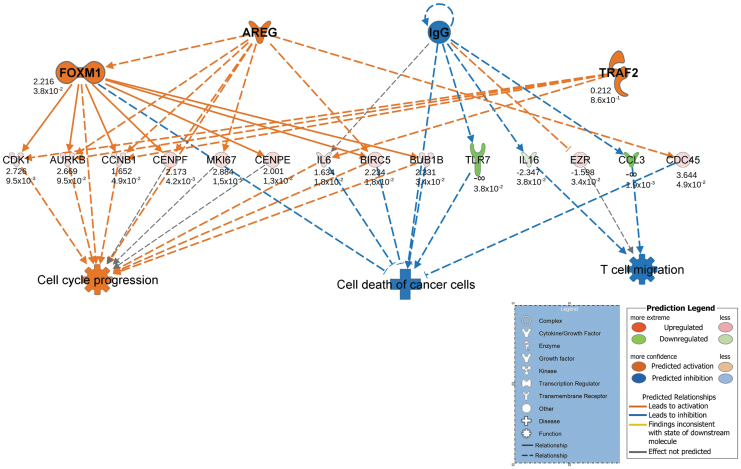
Examinations of ASC and fibroblast transcriptomic profiles in response to PDGF2 were performed using RNA-Seq analysis followed by bioinformatic analyses with the IPA software. The IPA software enables the analysis of transcript changes in experimental data sets and assigns these changes to shifts in upstream regulators and downstream effects. The analyses were limited to human tissues and primary cell lines. The regulatory network for genes with significant expression changes (p-score <0.05) and with the highest consistency score (6.414) in the ASCs revealed distinct activation of cell cycle progression and inhibition of T lymphocyte migration (Fig. 5). Additionally, the disease and function mode implemented in the IPA showed the activation of effects related to cell cycle progression and cell viability and the concurrent inhibition of phenomena associated with the inflammatory response in the ASCs cultured with PDGF2 (Supplementary Table S3). An analysis of fibroblasts stimulated with PDGF2 using the same settings generated no regulatory networks and showed a rather weak response to PDGF2 stimulation. However, several effects related to cell and stem cell migration and movement and l-tyrosine phosphorylation adhesion were found to be activated in the fibroblasts stimulated with PDGF2. The latter is connected with crucial signaling pathways that control cell proliferation, migration, adhesion, and differentiation. Apoptotic pathways were inhibited at the same time (Supplementary Table S4). Additionally, IPA toxicity (TOX) analysis of both cell types was performed to assess the biofunctions and toxic effects of PDGF2 stimulation. This analysis revealed no toxic effects of PDGF2 at the transcriptomic level: none of the phenomena connected with hyperproliferation, inflammation, apoptosis, or oncogenesis was activated in either the ASCs (Supplementary Table S5) or the fibroblasts (Supplementary Table S6).
Figure 5.
IPA regulatory network with the highest consistency score (6.414) in ASCs isolated from three patients (after RNA pooling) and stimulated with the PDGF2 peptide (1 μg/mL) compared with parallel controls untreated with PDGF2. The consistency score is a measurement used to describe the relationship among the observed expression profile and upstream and downstream regulatory effects. The values below the molecules correspond to log2-fold changes in expression and p-value. Negative and positive values indicate a decrease and an increase in expression, respectively. In the bottom panel, the arrow shows the activation or inhibition of a downstream effect. ASCs, adipose-derived stem cells; IPA, Ingenuity Pathway Analysis.
The transcriptional responses to PDGF2 stimulation in keratinocyte cultures expanded from epidermal cells collected from patients were evaluated using quantitative PCR (qPCR) analyses for the CDKN1A, KIT, MYC, POU5F1, TGFβ3, and TP53 transcripts. The gene expression levels displayed no consistent changes among the keratinocyte cultures isolated from different patients (Supplementary Fig. S8). However, some individual responses were noted. For one of the cell lines, the transcript levels of CDKN1A, KIT, MYC, POU5F1, and TGFB3 dropped significantly after stimulation with PDGF2.
PDGF2 topically delivered in P407 hydrogel stimulates dorsal skin wound healing in mice
To evaluate the effectiveness of the PDGF2 peptide in stimulating wound healing, we established a dorsal skin excisional injury mouse model and applied the peptide topically in 18% P407 hydrogel (0.2 mg/mL) (Fig. 6). Notably, the regions corresponding to the PDGF2 peptide sequence were identical between the mouse orthologue of human PDGFB and human PDGFB (Supplementary Fig. S9). Four days after wounding, we observed the formation of an epithelial membrane in the wound area.
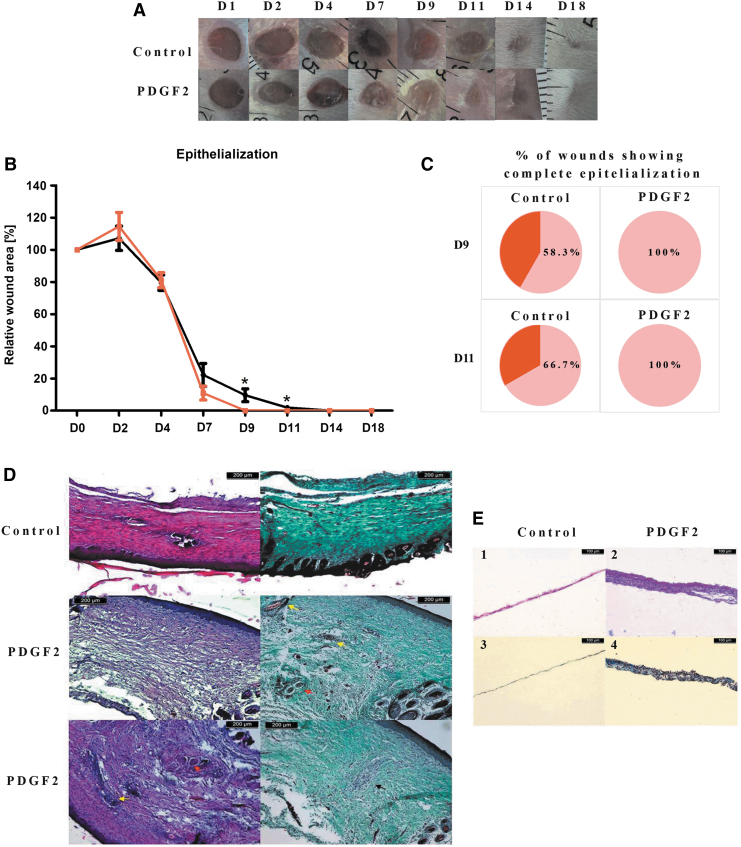
Figure 6.
Representative images of dorsal skin wounds in mice (A), wound epithelialization (B), and the percentage of wounds showing complete epithelialization (C) in mice treated with PDGF2. Histological analysis of wound healing: (D) images of skin samples at day 21 after injury and stained with hematoxylin and eosin (on the left) or Masson's trichrome (on the right). Yellow arrows indicate hair follicles, and red arrows indicate glands. Black arrows show areas of cells with a distinct morphology. (E). Images of epithelial membrane samples at day 4 postinjury stained with hematoxylin and eosin (1–2) or Masson's trichrome (3–4).
The analysis of wound epithelialization showed that PDGF2 accelerated this process (Fig. 6A, B). Changes were visible beginning on day 7. In the PDGF2-treated group, all wounds showed complete epithelialization on day 9, whereas in the control group, complete epithelialization was observed in 58.3% and 66.7% of wounds on days 9 and 11, respectively. No adverse effects of PDGF2 on the mice were observed during the experiments.
Histological analysis
Histological samples collected 3 weeks after injury showed remarkable differences between the PDGF2-treated and control animals (Fig. 6D). The control group displayed extensive scar tissue with highly marked collagen. Sporadic formation of developing hair follicles was observed within the scar. The outlets of the hair follicles could be seen along the edges of the scar. In the samples from the mice receiving PDGF2, scar tissue could also be observed; however, it was much less extensive than the scar tissue in the control samples. The collagen structure was looser and less regular, especially in the deeper scar regions, in the PDGF2-treated mice, and we observed much more frequent occurrences of hair follicles.
Skin sampled on day 4 revealed that the membranes in the wound area were significantly thicker in the mice receiving the PDGF2 peptide. The membranes were composed of several cell layers, while in the control skin samples, a single layer of cells was present. This finding suggested that PDGF2 increased early epithelization of the wound (Fig. 6E).
Discussion
Nonhealing wounds constitute an increasingly significant medical issue. Conventional methods of therapy often do not produce effective clinical outcomes. In this study, we presented an original unpublished peptide designated PDGF2, which may be a promising candidate drug for stimulating wound healing. We also proposed a method of applying the peptide in the form of a hydrogel. In addition, we demonstrated a multidimensional strategy for developing and testing new candidate drugs for wound treatment.
The experiments were carried out with the use of immortalized human HaCaT keratinocyte and 46BR.1N fibroblast cell lines as well as human primary fibroblasts, keratinocytes, and ASCs. Immortalized cell lines are a reliable research model, ensuring high reproducibility.56–58 In our study, we also decided to use dermal fibroblasts and keratinocytes isolated from clinical samples. We also carried out tests with ASCs, which perform an active role in wound healing, and recently, there have been trials studying the application of ASCs in the therapy of chronic wounds.59,60 Under appropriate conditions, ASCs can also differentiate into fibroblasts, keratinocytes, chondrocytes, and osteocytes.60–63
The treatment of chronic wounds is based on the application of a drug on the open lesion, which involves direct interactions with local cells. Therefore, the evaluation of toxicity of the potential dermal drugs is crucial.64,65 In our study, we performed preliminary tests of three novel peptides designed on the basis of the PDGF-BB:PDGFRβ complex structure. Only one of the three evaluated peptides, PDGF2-HTT, showed significant cytotoxicity in skin cells and proliferation inhibition. Interestingly, the weakest effect was observed in primary fibroblasts, and additionally, there were differences in the responses of the cells from different donors. A number of peptides have been reported to be cytotoxic to skin cells.65 Therefore, it is worth noting that the lack of cytotoxicity of PDGF2 is its great asset and shows that PDGF2 can be used without the risk of damaging skin cells.
Next, we checked the effect of our designed peptides on the proliferation of human keratinocytes and fibroblasts. The proliferation of these cells is crucial for proper wound healing, including the formation of the epidermis and production of the ECM.1 Our PDGF-BB-derived peptides stimulated the proliferation of these cells mainly at concentrations of 0.1–50 μg/mL. The strongest effect was observed after a 72-h stimulation of HaCaT keratinocytes with PDGF2. Primary fibroblasts showed the weakest response to the tested compounds. This outcome may be because primary cell lines are not homogeneous and interindividual differences can appear. The cyclization of PDGF2 resulted in a decrease in its activity in HaCaT cells. Additionally, PDGF2-HTT at concentrations of 100 and 150 μg/mL significantly inhibited HaCaT and 46BR.1N cell proliferation. The weakest effect was observed with primary fibroblasts. The obtained data correlate with the results of Brennand et al.,50 who demonstrated that the cyclization of a PDGFB peptide derivative led to the inhibition of DNA synthesis in fibroblasts.
We also should refer to the influence of PDGF-BB peptide derivatives on skin cells in regard to the activity of the protein itself. Interestingly, Park et al.66 reported that PDGF-BB does not stimulate proliferation of HaCaT keratinocytes. The authors also showed that PDGF-BB stimulates proliferation in primary fibroblasts at a concentration of 5 ng/mL. In addition, this effect was significantly greater than that obtained by using 10% bovine serum. Agren et al.67 reported similar data for primary fibroblasts isolated from various sources (healthy skin, acute wounds, and chronic wounds). In the abovementioned publication, stimulation was observed at concentrations of 1–10 ng/mL. However, it should be noted that in the case of the compounds tested in this work, cells were stimulated with only a small fragment of protein, and therefore, the biological effect may be different.
Based on the results of cytotoxicity and proliferation tests, we chose PDGF2 for further, more complex analyses. The migration and chemotaxis of skin cells are crucial for proper wound healing. While it is known that PDGF-BB stimulates the migration of primary fibroblasts at concentrations of 1–30 ng/mL,68 we did not observe significant stimulation of these processes in cells treated with PDGF2. Only a small increase in the chemotaxis of HaCaT cells and only a small pro-migratory effect on primary fibroblasts were observed. However, it should be noted that cells undergoing division (proliferation) usually have limited migratory/chemotactic properties; hence, the effect observed in this work may have a biological justification.
The risk of immunogenicity of peptide drugs is a constantly increasing issue. Excessive activation of the immune system can cause side effects and limit the effectiveness of a therapy.69–71 Furthermore, it can hinder the healing of chronic wounds, for example, diabetic wounds in which the wound healing process often arrests in the inflammatory phase.29 Our results indicate that PDGF2 does not activate the immune system (no activation of CTLs, Th cells, or NK cells), thus indicating a low risk of immunogenic reactions. It is worth noting that in this work, an additional in vitro method of assessing allergenic potential, namely the BAT, was applied. This test allowed us to examine the possibility of causing an allergic reaction without having to apply the compound to a patient.25 This test in our work showed low allergenic potential of the PDGF2 peptide, which confirmed the high safety profile of this peptide.
Transcriptomic analysis provides a great deal of data regarding the activity of tested compounds, which makes it a powerful tool for assessing potential drugs. The process of discovering and developing new drugs is a challenge in part because of the complexity of humans. RNA-Seq is a high-throughput technology that allows the simultaneous measurement of the expression of thousands of genes and provides insight into functional pathways and regulation in biological processes.72 It can provide a large amount of valuable data about the potential mechanism of action of a tested compound, its biological activity, and possible side effects. Understanding drug side effects seems to be particularly important since the withdrawal of a potential drug during clinical trials generates enormous costs. Therefore, increasing emphasis is being placed on identifying the effects of a drug before it enters this costly and time-consuming stage.73 In our work, we performed RNA-Seq analysis of ASCs and primary fibroblasts stimulated with PDGF2, which revealed neither toxic effects nor activation of oncogenesis pathways, thus indicating the safety of PDGF2 (Supplementary Tables S5 and S6). In addition, in PDGF2-stimulated cells, we observed the activation of wound healing–related pathways, such as cell and stem cell migration, proliferation, and cell cycle progression pathways, with concurrent slight anti-inflammatory activity (Supplementary Tables S2 and S3). These results showed a strong potential for PDGF2 as a wound healing stimulant.
A model of excisional skin injury in mice showed that the application of PDGF2 with a P407 poloxamer slightly stimulated wound healing. The effects of PDGF2 were especially evident on days 9 and 11 and manifested as increased epithelialization. Accelerated wound closure seems to be crucial because it can limit the possibility of bacterial infection.74 Moreover, on the fourth day after wounding, the epidermis of the mice receiving PDGF2 was much thicker than that of the controls. However, it is worth remembering that wound healing in healthy mice is fast and effective, and it is difficult to accelerate this process. Therefore, further studies utilizing a model of delayed wound healing, for example, diabetic wound healing, are required.
Also, the model of skin wound in mice has limited relevance to human skin wound healing due to anatomical differences and significant contraction observed in rodents but not in humans.75 Despite the limitations, animal experiments are necessary to assess the pro-regenerative potential of the tested compounds in preclinical studies. What is more, it has been demonstrated that contraction and re-epithelialization contribute to full-thickness excisional skin wound closure in mice to a comparable extent.76
In summary, we proved that a newly synthesized peptide designated PDGF2 stimulated human skin cells; showed high immunological safety, low cytotoxicity, and significant pro-proliferative effects on human skin cells; and accelerated dorsal skin wound healing in an excisional mouse model. Therefore, this peptide is a promising drug candidate for stimulating chronic wound healing in humans, and after clinical evaluation, it may become a safe alternative to becaplermin, the recombinant form of human PDGF-BB.33 However, further research is necessary to dissect the mechanism of action of PDGF2 and its influence on other processes, such as angiogenesis, extracellular production, and skin cell migration. In addition, we showed that a peptide derived from a human protein growth factor could be a starting point for the development of novel wound healing drugs. We also presented a multifaceted in vitro model for the initial selection and evaluation of the regenerative potential of novel molecules.
Innovation
The results of the study demonstrate that short peptide named PDGF2, derived from PDGF-BB, may be potentially used as an alternative for recombinant growth factors for treating chronic wounds. Our results also indicate that endogenous growth factors participating in wound healing may be used as a starting point to design pro-regenerative small-molecule peptide. In our work, we also present a multifaceted in vitro model based on cell culture tests, animal model, immunological tests, and transcriptomic analysis for the screening and development of new compounds that can improve wound healing.
Key Findings
New peptide compound derived from PDGF-BB protein, in concentrations of 0.1–50 μg/mL, shows strong pro-proliferation effect on human HaCaT keratinocytes after 72 h at stimulation.
The new compound is not cytotoxic and does not activate the immune system (no activation of CTLs, Th cells, or NK cells), thus indicating a low risk of immunogenic reactions confirmed also with low allergenic potential in the BAT.
The compound delivered topically in P407 hydrogel caused acceleration of wound healing (evident on days 9 and 11 from injury) in dorsal skin wound injury model in mice.
RNA-Seq analysis of dermal skin cells and ASCs stimulated with the new compound did not reveal the activation of oncogenesis pathways and any toxic effects.
A multifaceted in vitro model, based on cell culture tests, animal models, immunogenicity and allergenicity assessments, and transcriptomic analysis, for the development and initial screening of new compounds that stimulate wound healing is presented.
Supplementary Material
ACKNOWLEDGMENTS AND FUNDING SOURCES
We thank Prof. Marek Grzybiak, Prof. Adam Kosiński (Department of Clinical Anatomy, Medical University of Gdansk, Poland), Prof. Janusz Moryś (Chair of Anatomy, Medical University of Gdansk, Poland), and Prof. Piotr Trzonkowski (Department of Medical Immunology, Medical University of Gdansk, Poland) for providing access to needed equipment. We would also like to thank Maciej Zielinski, PhD, and the personnel of the Laboratory of Immunology and Clinical Transplantology from the University Clinical Centre in Gdansk for performing the BAT assays, Michael R. Crowley, PhD, and David K. Crossman, PhD (Heflin Center for Genomic Sciences, University of Alabama at Birmingham, Birmingham, AL), for their constant support and advice regarding the NGS analyses, and Prof. Marta Miączyńska, PhD, and Kamil Jastrzębski, PhD, from the International Institute of Molecular and Cell Biology in Warsaw for substantive support. This work was supported by funds from the National Centre for Research and Development, Poland, Grant No. STRATEGMED1/235077/9/NCBR/2014.
Abbreviations and Acronyms
- ACN
acetonitrile
- ASCs
adipose-derived stem cells
- BAT
basophil activation test
- ECM
extracellular matrix
- HG
high glucose
- IL-8
interleukin 8
- LDH
lactate dehydrogenase
- NK
natural killer
- PDGFRβ
beta-type platelet-derived growth factor receptor
- qPCR
quantitative PCR
- TFA
trifluoroacetic acid
- Th
T helper
- TIPSI
triisopropylsilane
- TOX
toxicity
- VEGF
vascular endothelial growth factor
- XTT
sodium 3′-[1- (phenylaminocarbonyl)- 3,4- tetrazolium]-bis (4-methoxy6-nitro) benzene sulfonic acid hydrate
Authors' Contributions
M.D.—designed and performed cell culture experiments, isolated cells from skin samples, conducted Luminex analyses, performed statistical analyses, analyzed and interpreted results, and wrote the article. P.K.—designed and synthesized peptides, participated in the study concept design, participated in manuscript writing, and revised the article. A.W.—designed and conducted immunological experiments, performed statistical analyses, and interpreted results. Pi.S. and P.So.—designed and performed qPCR analyses and animal experiments and collected tissue samples. A.M. and N.F.—designed and performed RNA-Seq transcriptomic analyses and interpreted the obtained results. M.Dz. and J.S.—participated in peptide purity confirmation and preparation for biological experiments. E.N.—performed histological staining. P.L.—isolated primary keratinocytes and stimulated cells for qPCR analyses. A.S.—isolated ASCs and stimulated them for transcriptomic analyses. M.C.—participated in histological analyses and revised the article. J.Z. and K.K.—provided clinical material for experiments. F.K.—participated in the peptide design. A.C., Ł.J., P.M., and P.Sk.—participated in the study concept design. A.P.—provided financial support, participated in the study design, and supervised RNA-Seq transcriptomic analyses. P.Sa.—provided financial support, participated in the study design, supervised animal and qPCR analyses, performed statistical analyses and multiple sequence alignment, and revised the article. S.R.-M.—provided financial support, created the study concept, designed experiments, supervised peptide design and synthesis, and revised the article. M.P.—provided financial support, created the study concept, designed experiments, supervised cell culture and immunological analyses and histological examinations, and revised the article.
Author Disclosure and Ghostwriting
Patent applications (EP18000305.5, P.425038) to protect new peptide derivatives of PDGF have been filed (S.R.-M., M.P., M.D. P.K., Pi.S., A.W., J.S., M.D., F.K., P.So., A.M., N.F., Piotr Madanecki, A.P., A.C., P.M., P.Sk., Ł.J., P.Sa).
The authors declare that there are no other conflicts of interest regarding the publication of this article. No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article. All authors approved the final article.
About the Authors
Milena Deptuła, Eng., MSc, PhD, is specialist at the Laboratory of Tissue Engineering and Regenerative Medicine, Department of Embryology, Medical University of Gdansk, Poland. She has an expertise in cell isolation, culture, and biology. Her research is focused on tissue engineering and regenerative medicine. Przemysław Karpowicz, MSc, PhD, is currently an Assistant Professor in the Department of Organic Chemistry, Faculty of Chemistry, University of Gdansk. His research is focused on the design and synthesis of novel compounds with regenerative potential. Sylwia Rodziewicz-Motowidło, MSc, PhD, Dsc, is an Associate Professor and the Head of Biomedical Chemistry Department, Faculty of Chemistry, University of Gdansk. She is a specialist in the field of research on the SAR studies of biologically active peptides using advanced physicochemical methods. Michał Pikuła, MSc, MPharm, PhD, is an Associate Professor at the Laboratory of Tissue Engineering and Regenerative Medicine, Department of Embryology, Medical University of Gdansk. He is a specialist in experimental immunology, tissue engineering, and regenerative medicine. All authors are a part of multicenter consortium REGENNOVA conducting research in the grant entitled “Novel Technologies for Pharmacological Stimulation of Regeneration.” The consortium brings together specialists in such fields as: chemistry, surgery, biochemistry, or animal surgery.
Supplementary Material
References
- 1. Pikuła M, Langa P, Kosikowska P, Trzonkowski P. Stem cells and growth factors in wound healing. Postepy Hig Med Dosw 2015;69:874–885 [DOI] [PubMed] [Google Scholar]
- 2. Olczyk P, Mencner Ł, Komosinska-Vassev K. The role of the extracellular matrix components in cutaneous wound healing. Biomed Res Int 2014;2014:747584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pikuła M, Trzonkowski P. Biology of epidermal stem cells: impact on medicine. Postepy Hig Med Dosw 2009;63:449–456 [PubMed] [Google Scholar]
- 4. Bowen-Pope DF, Raines EW. History of discovery: platelet-derived growth factor. Arterioscler Thromb Vasc Biol 2011;31:2397–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 6. Cheng B, Liu HW, Fu XB, Sun TZ, Sheng ZY. Recombinant human platelet-derived growth factor enhanced dermal wound healing by a pathway involving ERK and c-fos in diabetic rats. J Dermatol Sci 2007;45:193–201 [DOI] [PubMed] [Google Scholar]
- 7. Barrientos S, Brem H, Stojadinovic O, Tomic-Canic M. Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014;22:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. PERSPECTIVE ARTICLE: growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 9. Shah P, Keppler L, Rutkowski J. A review of platelet derived growth factor playing pivotal role in bone regeneration. J Oral Implantol 2014;40:330–340 [DOI] [PubMed] [Google Scholar]
- 10. Capobianco CM, Zgonis T. An overview of negative pressure wound therapy for the lower extremity. Clin Podiatr Med Surg 2009;26:619–631 [DOI] [PubMed] [Google Scholar]
- 11. Hunckler J, de Mel A. A current affair: electrotherapy in wound healing. J Multidiscip Healthc 2017;10:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun BK, Siprashvili Z, Khavari PA. Advances in skin grafting and treatment of cutaneous wounds. Science 2014;346:941–945 [DOI] [PubMed] [Google Scholar]
- 13. Yu JR, Navarro J, Coburn JC, et al. Current and future perspectives on skin tissue engineering: key features of biomedical research, translational assessment, and clinical application. Adv Healthc Mater 2019;8:e1801471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aljghami ME, Saboor S, Amini-Nik S. Emerging innovative wound dressings. Ann Biomed Eng 2019;47:659–675 [DOI] [PubMed] [Google Scholar]
- 15. Kanji S, Das H. Advances of stem cell therapeutics in cutaneous wound healing and regeneration. Mediators Inflamm 2017;2017:5217967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch T, Rothoeft T, Teig N, et al. Regeneration of the entire human epidermis by transgenic stem cells. Nature 2017;551:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Du QS, Xie NZ, Huang RB. Recent development of peptide drugs and advance on theory and methodology of peptide inhibitor design. Med Chem 2015;11:235–247 [DOI] [PubMed] [Google Scholar]
- 18. Tsomaia N. Peptide therapeutics: targeting the undruggable space. Eur J Med Chem 2015;94:459–470 [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Duan Z, Tang J, Lv Q, Rong M, Lai R. A short peptide from frog skin accelerates diabetic wound healing. FEBS J 2014;281:4633–4643 [DOI] [PubMed] [Google Scholar]
- 20. Di Grazia A, Cappiello F, Imanishi A, et al. The frog skin-derived antimicrobial peptide esculentin-1a(1–21)NH2 promotes the migration of human haCaT keratinocytes in an EGF receptor-dependent manner: a novel promoter of human skin wound healing? PLoS One 2015;10:e0128663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung Kim D, Lee YW, Park MK, et al. Efficacy of the designer antimicrobial peptide SHAP1 in wound healing and wound infection. Amino Acids 2014;46:2333–2343 [DOI] [PubMed] [Google Scholar]
- 22. Pickart L, Vasquez-Soltero JM, Margolina A. GHK peptide as a natural modulator of multiple cellular pathways in skin regeneration. Biomed Res Int 2015;2015:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Kilsdonk JWJ, Jansen PAM, Van Den Bogaard EH, et al. The effects of human beta-defensins on skin cells in vitro. Dermatology 2017;233:155–163 [DOI] [PubMed] [Google Scholar]
- 24. Banerjee P, Suguna L, Shanthi C. Wound healing activity of a collagen-derived cryptic peptide. Amino Acids 2015;47:317–328 [DOI] [PubMed] [Google Scholar]
- 25. Deptuła M, Wardowska A, Dzierżyńska M, Rodziewicz-Motowidło S, Pikuła M. Antibacterial peptides in dermatology-strategies for evaluation of allergic potential. Molecules 2018;23:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Demidova-Rice TN, Wolf L, Deckenback J, Hamblin MR, Herman IM. Human platelet-rich plasma- and extracellular matrix-derived peptides promote impaired cutaneous wound healing in vivo. PLoS One 2012;7:e32146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomes A, Teixeira C, Ferraz R, et al. Wound-healing peptides for treatment of chronic diabetic foot ulcers and other infected skin injuries. Molecules 2017;22:1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deptuła M, Zieliński J, Wardowska A, Pikuła M. Wound healing complications in oncological patients: perspectives for cellular therapy. Adv Dermatol Allergol 2019;36:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trostrup H, Bjarnsholt T, Kirketerp-Moller K, Hoiby N, Moser C. What is new in the understanding of non healing wounds epidemiology, pathophysiology, and therapies. Ulcers 2013;2013:1–8 [Google Scholar]
- 30. Schreml S, Szeimies R-M, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. J Am Acad Dermatol 2010;63:866–881 [DOI] [PubMed] [Google Scholar]
- 31. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 2015;4:560–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dreifke MB, Jayasuriya AA, Jayasuriya AC. Current wound healing procedures and potential care. Mater Sci Eng C Mater Biol Appl 2015;48:651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Das S, Majid M, Baker AB. Syndecan-4 enhances PDGF-BB activity in diabetic wound healing. Acta Biomater 2016;42:56–65 [DOI] [PubMed] [Google Scholar]
- 34. Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014;6:265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malesevic M, Strijowski U, Bächle D, Sewald N. An improved method for the solution cyclization of peptides under pseudo-high dilution conditions. J Biotechnol 2004;112:73–77 [DOI] [PubMed] [Google Scholar]
- 36. Langa P, Wardowska A, Zieliński J, et al. Transcriptional profile of in vitro expanded human epidermal progenitor cells for the treatment of non-healing wounds. J Dermatol Sci 2018;89:272–281 [DOI] [PubMed] [Google Scholar]
- 37. Mieczkowska A, Schumacher A, Filipowicz N, et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci Rep 2018;8:11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kosikowska P, Pikula M, Langa P, Trzonkowski P, Obuchowski M, Lesner A. Synthesis and evaluation of biological activity of antimicrobial pro-proliferative peptide conjugates. PLoS One 2015;10:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988;106:761–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boukamp P, Popp S, Altmeyer S, et al. Sustained nontumorigenic phenotype correlates with a largely stable chromosome content during long-term culture of the human keratinocyte line HaCat. Genes Chromosom Cancer 1997;19:201–214 [DOI] [PubMed] [Google Scholar]
- 41. Dȩbowski D, Łukajtis R, Łȩgowska A, et al. Inhibitory and antimicrobial activities of OGTI and HV-BBI peptides, fragments and analogs derived from amphibian skin. Peptides 2012;35:276–284 [DOI] [PubMed] [Google Scholar]
- 42. Bonner JC, Osornio-Vargas AR. Differential binding and regulation of platelet-derived growth factor A and B chain isoforms by alpha 2-macroglobulin. J Biol Chem 1995;270:16236–16242 [DOI] [PubMed] [Google Scholar]
- 43. Mahadevan D, Yu JC, Saldanha JW, et al. Structural role of extracellular domain 1 of alpha-platelet-derived growth factor (PDGF) receptor for PDGF-AA and PDGF-BB binding. J Biol Chem 1995;270:27595–27600 [DOI] [PubMed] [Google Scholar]
- 44. Andersson M, Ostman A, Kreysing J, Bäckström G, Van de Poll MHC. Involvement of loop 2 of platelet-derived growth factor-AA and -BB in receptor binding. Growth Factors 1995;12:159–164 [DOI] [PubMed] [Google Scholar]
- 45. Hye-Ryong Shim A, Liu H, Focia PJ, Chen X, Lin PC, He X. Structures of a platelet-derived growth factor/propeptide complex and a platelet-derived growth factor/receptor complex. Proc Natl Acad Sci U S A 2010;107:11307–11312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oefner C, D'Arcy A, Winkler FK, Eggimann B, Hosang M. Crystal structure of human platelet-derived growth factor BB. EMBO J 1992;11:3921–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jaumann M, Tatje D, Hoppe J. Identification of individual amino acids in platelet-derived growth factor that contribute to the specificity towards the β-type receptor. FEBS Lett 1992;302:265–268 [DOI] [PubMed] [Google Scholar]
- 48. Clements JM, Bawden LJ, Bloxidge RE, et al. Two PDGF-B chain residues, arginine 27 and isoleucine 30, mediate receptor binding and activation. EMBO J 1991;10:4113–4120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heldin CH, Lennartsson J, Westermark B. Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J Intern Med 2018;283:16–44 [DOI] [PubMed] [Google Scholar]
- 50. Brennand DM, Dennehy U, Ellis V, et al. Identification of a cyclic peptide inhibitor of platelet-derived growth factor-BB receptor-binding and mitogen-induced DNA synthesis in human fibroblasts. FEBS Lett 1997;413:70–74 [DOI] [PubMed] [Google Scholar]
- 51. Brennand DM, Scully MF, Kakkar VV, Patel G. A cyclic peptide analogue of loop III of PDGF-BB causes apoptosis in human fibroblasts. FEBS Lett 1997;419:166–170 [DOI] [PubMed] [Google Scholar]
- 52. Patel G, Husman W, Jehanli AM, et al. A cyclic peptide analogue of the loop III region of platelet-derived growth factor-BB is a synthetic antigen for the native protein. J Pept Res 1999;53:68–74 [DOI] [PubMed] [Google Scholar]
- 53. Merzouk A, Salari H, Arab L, et al. C-terminal cyclization of an SDF-1 small peptide analogue dramatically increases receptor affinity and activation of the CXCR4 receptor. J Med Chem 2002;45:2024–2031 [DOI] [PubMed] [Google Scholar]
- 54. Yap BK, Leung EWW, Yagi H, et al. A potent cyclic peptide targeting SPSB2 protein as a potential anti-infective agent. J Med Chem 2014;57:7006–7015 [DOI] [PubMed] [Google Scholar]
- 55. Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SAJ, Vogel HJ. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS One 2010;5:e12648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kukowska M, Pikuła M, Kukowska-Kaszuba M, Schumacher A, Dzierzbicka K, Trzonkowski P. Synthetic lipopeptides as potential topical therapeutics in wound and skin care: in vitro studies of permeation and skin cells behaviour. RSC Adv 2016;6:115120–115131 [Google Scholar]
- 57. Zbytek B, Pikula M, Slominski RM, et al. Corticotropin-releasing hormone triggers differentiation in HaCaT keratinocytes. Br J Dermatol 2005;152:474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pikuła M, Żebrowska ME, Pobłocka-Olech L, Krauze-Baranowska M, Sznitowska M, Trzonkowski P. Effect of enoxaparin and onion extract on human skin fibroblast cell line—therapeutic implications for the treatment of keloids. Pharm Biol 2014;52:262–267 [DOI] [PubMed] [Google Scholar]
- 59. Lau K, Paus R, Tiede S, Day P, Bayat A. Exploring the role of stem cells in cutaneous wound healing. Exp Dermatol 2009;18:921–933 [DOI] [PubMed] [Google Scholar]
- 60. Pikuła M, Marek-Trzonkowska N, Wardowska A, Renkielska A, Trzonkowski P. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther 2013;13:1357–1370 [DOI] [PubMed] [Google Scholar]
- 61. Auxenfans C, Lequeux C, Perrusel E, Mojallal A, Kinikoglu B, Damour O. Adipose-derived stem cells (ASCs) as a source of endothelial cells in the reconstruction of endothelialized skin equivalents. J Tissue Eng Regen Med 2012;6:512–518 [DOI] [PubMed] [Google Scholar]
- 62. Du Y, Roh DS, Funderburgh ML, et al. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol Vis 2010;16:2680–2689 [PMC free article] [PubMed] [Google Scholar]
- 63. Schumacher A, Cichorek M, Pikuła M. Adipose-derived stem cells for tissue engineering and therapy of non-healing wounds. Postepy Hig Med Dosw 2018;72:806–821 [Google Scholar]
- 64. Barańska-Rybak W, Pikula M, Dawgul M, Kamysz W, Trzonkowski P, Roszkiewicz J. Safety profile of antimicrobial peptides: camel, citropin, protegrin, temporin a and lipopeptide on HaCaT keratinocytes. Acta Pol Pharm 2013;70:795–801 [PubMed] [Google Scholar]
- 65. Pikuła M, Smużyńska M, Krzystyniak A, et al. Cystatin C peptidomimetic derivative with antimicrobial properties as a potential compound against wound infections. Bioorg Med Chem 2017;25:1431–1439 [DOI] [PubMed] [Google Scholar]
- 66. Park SA, Raghunathan VK, Shah NM, et al. PDGF-BB does not accelerate healing in diabetic mice with splinted skin wounds. PLoS One 2014;9:e104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Agren MS, Steenfos HH, Dabelsteen S, Hansen JB, Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Invest Dermatol 1999;112:463–469 [DOI] [PubMed] [Google Scholar]
- 68. De Donatis A, Comito G, Buricchi F, et al. Proliferation versus migration in platelet-derived growth factor signaling. J Biol Chem 2008;283:19948–19956 [DOI] [PubMed] [Google Scholar]
- 69. Corominas M, Gastaminza G, Lobera T. Hypersensitivity reactions to biological drugs. J Investig Allergol Clin Immunol 2014;24:212–225 [PubMed] [Google Scholar]
- 70. Wadhwa M, Bird C, Dilger P, Gaines-Das R, Thorpe R. Strategies for detection, measurement and characterization of unwanted antibodies induced by therapeutic biologicals. J Immunol Methods 2003;278:1–17 [DOI] [PubMed] [Google Scholar]
- 71. Shankar G, Shores E, Wagner C, Mire-Sluis A. Scientific and regulatory considerations on the immunogenicity of biologics. Trends Biotechnol 2006;24:274–280 [DOI] [PubMed] [Google Scholar]
- 72. Khatoon Z, Figler B, Zhang H, Cheng F. Introduction to RNA-Seq and its applications to drug discovery and development. Drug Dev Res 2014;75:324–330 [DOI] [PubMed] [Google Scholar]
- 73. Fischer HP, Heyse S. From targets to leads: the importance of advanced data analysis for decision support in drug discovery. Curr Opin Drug Discov Devel 2005;8:334–346 [PubMed] [Google Scholar]
- 74. Krishnaswamy VR, Mintz D, Sagi I. Matrix metalloproteinases: the sculptors of chronic cutaneous wounds. Biochim Biophys Acta Mol Cell Res 2017;1864:2220–2227 [DOI] [PubMed] [Google Scholar]
- 75. Zomer HD, Trentin AG. Skin wound healing in humans and mice: challenges in translational research. J Dermatol Sci 2018;90:3–12 [DOI] [PubMed] [Google Scholar]
- 76. Chen L, Mirza R, Kwon Y, DiPietro LA, Koh TJ. The murine excisional wound model: contraction revisited. Wound Repair Regen 2015;23:874–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.