Abstract
Significance: In humans, imbalances in the reduction–oxidation (redox) status of cells are associated with many pathological states. In addition, many therapeutics and prophylactics used as interventions for diverse pathologies either directly modulate oxidant levels or otherwise influence endogenous cellular redox systems.
Recent Advances: The cellular machineries that maintain redox homeostasis or that function within antioxidant defense systems rely heavily on the regulated reactivities of sulfur atoms either within or derived from the amino acids cysteine and methionine. Recent advances have substantially advanced our understanding of the complex and essential chemistry of biological sulfur-containing molecules.
Critical Issues: The redox machineries that maintain cellular homeostasis under diverse stresses can consume large amounts of energy to generate reducing power and/or large amounts of sulfur-containing nutrients to replenish or sustain intracellular stores. By understanding the metabolic pathways underlying these responses, one can better predict how to protect cells from specific stresses.
Future Directions: Here, we summarize the current state of knowledge about the impacts of different stresses on cellular metabolism of sulfur-containing molecules. This analysis suggests that there remains more to be learned about how cells use sulfur chemistry to respond to stresses, which could in turn lead to advances in therapeutic interventions for some exposures or conditions.
Keywords: methionine cycle, trans-sulfuration, disulfide reductase systems, oxidative stress, drug metabolism
Introduction
The element sulfur (S) is found in two proteinogenic amino acids, L-cysteine (Cys) and L-methionine (Met), as well as in a diverse group of other biologically important organic and inorganic small molecules. The ability of S to donate and accept electrons, and to access hypervalent states through bonding in its empty d-orbital, allows it to form bonds with other S atoms as well as with carbon, nitrogen, some metals/metalloids, and up to four oxygen atoms. This property, for example, allows S residues to participate in structural, yet nonetheless reversible, disulfide bonds within or between some proteins. More importantly for this discussion, however, it underlies Biology's expansive utilization of S in molecules and situations in which transient bonding is used as a conduit for electron transfers; that is, reduction and oxidation (redox) reactions. Indeed, S is probably the atom most often used to directly alter the oxidation state of oxygen atoms within our cells!
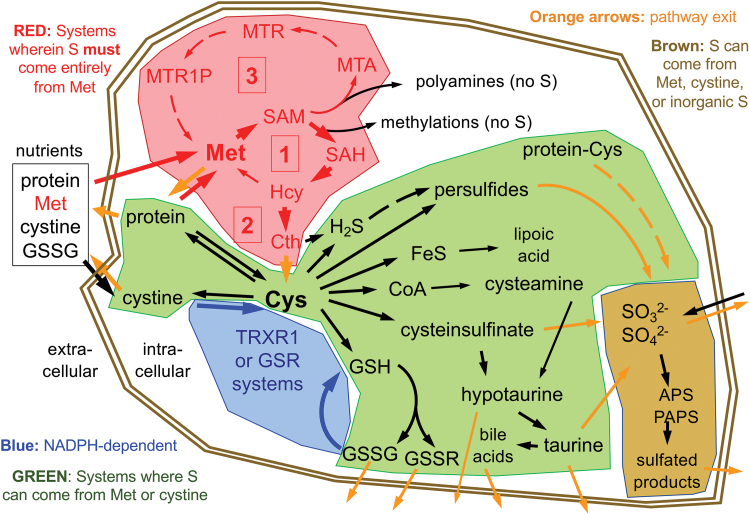
Within the active forms of Cys and Met used for protein synthesis and found participating in most cellular processes, the S is in a fully reduced state, with either the thiol-form in Cys or thioether-form in Met. To synthesize Met or Cys, cells need access to a source of S in its fully reduced state. Whereas many plants and microbes have sulfate reducing enzymes that allow them to use abundant sources of oxidized inorganic S to synthesize these S-amino acids, animals cannot reduce these oxidized forms (82). In animals, S-amino acids come entirely from dietary protein. Moreover, Met is essential in all metazoans, meaning that animals are incapable of synthesizing Met from other dietarily available S-containing nutrients, so a healthy human diet must provide an adequate source of Met (Fig. 1, box labeled [1]) (82, 118). Cys, on the contrary, can be synthesized from Met via the trans-sulfuration pathway (Fig. 1, box labeled [2]) and is only essential in humans in specific situations (discussed below). Whereas both Met and Cys are needed for protein synthesis, as compared with Met, Cys is more abundant in proteins, is a more broadly used precursor for the biosynthesis of other products in cells, and unlike for Met, many of these products are excreted from cells (Fig. 1). Thus, in addition to its incorporation into protein, Cys is the critical upstream S-containing precursor for the biosynthesis of glutathione (GSH), coenzyme A (CoA), hydrogen sulfide (H2S), hypotaurine, taurine, iron–sulfur (Fe–S) clusters, much of the sulfate in adenosine-5′-phosphosulfate and phosphoadenosine-5′-phosphosulfate (APS and PAPS, respectively), and several other S-containing molecules in cells (Fig. 1).
FIG. 1.
Basal mammalian S-metabolism. Major cellular pathways are shaded to distinguish those that are NADPH dependent (blue), those in which the S must come entirely from extracellular Met sources (red), those in which the sulfur can come from either Met in an NADPH-independent process or from cystine in an NADPH-dependent process (green), and those in which the S can come from Met, cystine, or inorganic sulfite or sulfate (brown). Red boxes denote the following: [1] the Met cycle, [2] trans-sulfuration, and [3] the polyamine synthesis (methionine salvage) pathway. Dashed arrows denote multistep processes. Abbreviations are listed in Abbreviations Used section. Different cell types will have different activities of the various pathways; arrow weights reflect predictions of approximate flux in liver hepatocytes under basal states. As discussed in the text, stresses will variously affect flux rates at many points in these pathways. Color images are available online.
Diverse stresses on living cells or systems will interact profoundly with cellular S-containing molecules and therefore with S-metabolism. Most evident among these, perhaps, is oxidative stress, but it also includes other electrophilic stresses, reductive stress, toxin/drug/metal exposures, other energetic metabolic imbalances, carcinogenic stress, and likely other cellular or systemic insults. Interestingly, cancers can often experience overlapping combinations of many or all of these stresses, in particular during therapeutic interventions. Although each of these stresses can impact structural S-based bonds, this article will focus on the transient S-based bonds underlying the redox reactions affected by representative stresses.
Basal Mammalian S Metabolism
Dietary sources and systemic distribution
The predominant sources of all S in humans and other animals come from dietary protein. Uniquely among nutritional sources of S, Met is both essential and can generally serve as the sole source of nutritional S, if needed. In most diets, cystine (the oxidized disulfide form of Cys usually found in foods) is also a source of S. As long as sufficient Met is available in the diet, dietary Cys is not required in adult humans; however, dietary Cys is important during postnatal growth and development, making Cys a “semiessential” amino acid (96). Inorganic sulfate or sulfite from dietary sources can be utilized by human cells in a limited set of reactions, and some S-containing trace nutrients such as lipoic acid can be obtained directly from nutritional sources. Like all nutrients, sources of nutritional S enter the circulation via the portal vein and transit directly to the liver. In liver hepatocytes, considerable metabolism of S-based nutrients occurs, which are detailed below. Unsurprisingly, liver plays the predominant role in intermediary metabolism of S-amino acids, in particular Cys, by excretion of S-containing molecules into the peripheral circulation.
Unlike the reducing conditions found within the cytosol of most if not all living cells, extracellular fluids in the body, including blood plasma and extravascular interstitial fluids, are moderately oxidizing and will promote spontaneous oxidation of most free thiols, such as that within Cys. Moreover, abundant sulfhydryl oxidases in plasma, such as Qsox, catalytically oxidize free Cys into its homodimeric disulfide form, cystine (126). As a result, cells do not have access to substantial extracellular sources of free Cys. Rather, Cys is typically obtained from assimilation of cystine followed by intracellular reduction by the cytosolic NADPH-dependent disulfide reductase systems to yield 2Cys (discussed below), or from other abundant thiol- or disulfide-containing molecules in plasma. These include reduced glutathione (glutathione-thiol or GSH), its oxidized homodimeric form (glutathione disulfide [GSSG]), or oxidized glutathione conjugates (GSSX). Of particular importance is glutathionylated serum albumin (19, 129). Most of the glutathione in plasma is thought to come from hepatocytes as GSSG or GSSX; however, a substantial portion likely comes from erythrocytes as GSH (40). Importantly, however, there are no known transporters that can allow GSH, GSSG, or other glutathionylated products to enter the cytosol of cells. Rather, γ-glutamyl transpeptidase (GGT) on the cell surface hydrolyzes the γ-glutamyl bond of the glutathione moieties releasing the glutamate and, in combination with ubiquitous dipeptidases, also liberating l-glycine (Gly) and either Cys (in the case of GSH) or cystine (in the case of disulfide forms), all of which can then enter the cell through specific amino acid transporters (1, 43, 44, 80, 124). In addition to being cleaved to its constitutive amino acids by dipeptidases, circulating Cys-Gly or the oxidized disulfide form, either as the product of GGT cleavage or of export by many of the splanchnic organs, is removed from circulation by the kidneys (39).
The S demands for synthesis of low abundance low turnover S-molecules, such as lipoic acid, CoA, FeS clusters, and polyamine synthesis (also called “methionine salvage”) pathway intermediates, represent a minor portion of total S metabolism and are not likely to impact S availability for the high-demand stress responses considered here. The different nutritional S sources, predominantly Met, cystine, and sulfite/sulfate, each enter cells with their sulfurs in different redox states, which determines how each can be utilized.
Sulfate/sulfite metabolism
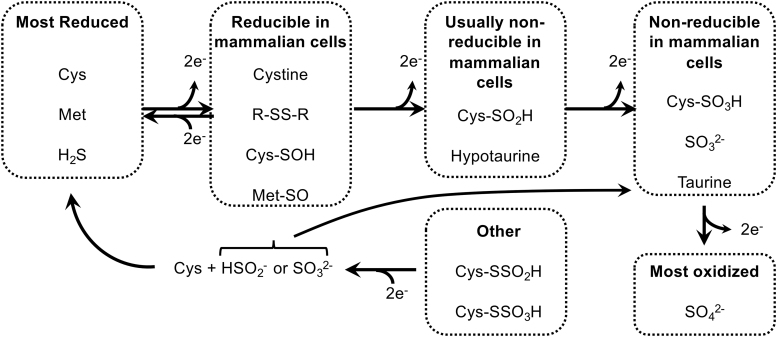
Inorganic sulfate is in the most oxidized state of the nutritional S-sources that mammalian cells can use; sulfite is one 2-electron state more reduced than sulfate (Fig. 2). Either sulfate or sulfite can be used to synthesize APS for production of PAPS (133). Subsequently, the oxidized S group from PAPS can be used in diverse reactions catalyzed by the sulfotransferase (SULT) family of enzymes. These enzymes function in the production of extracellular matrix molecules (e.g., heparin sulfate and chondroitin sulfate), sulfated steroids (e.g., estrone sulfate), bile acid sulfates, and sulfation-based phase-2 drug metabolism/detoxification reactions (22, 59). However, mammalian cells are unable to reduce inorganic sulfate or sulfite, nor are they able to reduce the oxidized S residues within any organosulfates. Therefore, dietary sulfite/sulfate cannot be used to generate S-amino acids. The inverse, however, is not true. Specific mechanisms, and likely also some spontaneous oxidation reactions, within our cells will allow S residues from more reduced species, for example within S-amino acids, to be oxidized to generate disulfides, sulfoxides, sulfenic acids, sulfinic acids, sulfonic acids, and inorganic sulfite or sulfate (101, 120). H2S may also act as a primary S source for generation of highly oxidized S species, although the specific mechanisms for formation of thiosulfate (S2O32−), S0, and sulfite from H2S are still debated (8, 53). As H2S is primarily derived from trans-sulfuration of Cys and Met, dietary S-amino acids can fully supply cellular oxidized sulfur needs for PAPS production and sulfation in the absence of nutritional inorganic sulfate/sulfite.
FIG. 2.
Biological redox states and transitions of S. Most activities of S in mammalian cells transition between the most reduced state (far left) and a 2-electron oxidation of that state, fueled by the NADPH-dependent GSR and TRXR1 disulfide reductase systems. A further 2-electron oxidation yields sulfinic acids, which are not reducible in mammalian cells except in the special situation of sulfiredoxin-catalyzed repair. Further oxidation states cannot be reduced in mammalian cells, with the exception of persulfide-linked higher oxidation states. In the figure, “Cys” is used to refer to the free amino acid, Cys in the context of a protein or other Cys-containing molecule, or a Cys-derived S in the context of another metabolite. See text for more details. GSR, glutathione reductase; TRXR1, thioredoxin reductase-1.
Cys and cystine metabolism
The reduced thiol group of Cys spontaneously oxidizes in most extracellular environments. In blood plasma, a common stable product of this oxidation is the cysteine-mixed disulfide of albumin; however, the most common nutritional form of Cys is the disulfide bond-linked homodimer of Cys, cystine (129, 132). After uptake through amino acid transporters (e.g., SLC7A11, also called xCT), cellular utilization of nutritional cystine requires cytosolic reduction of the disulfide bond to generate two molecules of Cys. The only systems capable of catalyzing this reaction are the cytosolic disulfide reductase systems; that is, the GSH system or the cytosolic thioredoxin-1 (TRX1) system (Fig. 1) (6, 82). Reducing power for the reaction comes from one of the two cytosolic NADPH-dependent disulfide reductase enzymes, glutathione reductase (GSR) or thioredoxin reductase-1 (TRXR1). These enzymes are able to obtain 2 electrons of reducing power from NADPH through a flavin cofactor and use this to reduce their respective substrates, generating the reduced form of their substrates (two GSH or TRX1-dithiol) and oxidized NADP+ as products (7). The relative contributions of each system, and of the different redoxin effectors of each system, including TRX1, other thioredoxin-related proteins (TRPs), or various members of the glutaredoxin (GRX) family, remain largely undefined. Biochemical and genetic studies suggest, however, that there is considerable redundancy and promiscuity in the systems that catalyze this reaction (83, 93).
Cys constitutes 2%–3% of the amino acids within proteins of mammalian cells, and this results in a concentration of cytosol-accessible protein-thiols in the low-millimolar range (41, 47). The redox activity of the thiol-S makes Cys residues particularly common in enzyme active sites, regulatory sites, and in positions where they can participate in disulfide bond formation. In addition to its roles in proteins, however, Cys is a “metabolic hub” for biosynthesis of other S-containing small molecules in cells (Fig. 1) (117, 119). Most abundant among these is the tripeptide GSH (γ-L-glutamyl-L-cysteinylglycine), which is also present at low-millimolar concentrations in cells (80). Importantly, whereas the predominant redox roles of GSH involve considerable recycling between the reduced GSH and oxidized GSSG forms, other often major roles for GSH, including hepatic export of GSSG as a systemic source of amino acids for intermediary metabolism and many GSH-S-transferase (GST)-catalyzed conjugation activities involved in detoxification reactions, result in loss of GSH from the cell. Unlike Cys, a substantial fraction of the plasma glutathione is in the reduced GSH form, with GSSG and protein-glutathione–mixed disulfides accounting for the remainder (129). Despite both protein and GSH contributing fairly equivalently to the total pools of solvent-accessible thiols within cells, the rapid turnover (43, 81) and frequent excretion of GSH from cells (71), as compared with proteins, suggest that a considerably greater amount of Cys might be committed to GSH biosynthesis than to protein synthesis. Other metabolites either synthesized from Cys or obtaining their S from Cys include CoA, Fe–S clusters, H2S, hypotaurine, taurine, and sulfate. Whereas due to their cellular retention and/or low cellular concentrations, CoA and Fe–S clusters are not likely to consume substantial amounts of Cys, taurine and likely also H2S and sulfate can be abundantly excreted, and therefore might drive substantial consumption of cellular Cys (38, 130).
Met metabolism
The S in Met is in the same fully reduced state as that in Cys; however as a thioether, it is refractory to spontaneous oxidation as compared with the thiol-S found in Cys (83). For that reason, cells are able to obtain Met in its fully reduced form directly from nutritional sources. Met is the initiator amino acid for translation of nearly all proteins in mammalian cells and, even though the initiator Met is often post-translationally removed, due largely to the use of Met internally in proteins, it constitutes 1%–2% of the total steady-state amino acids in animal protein (41). Like Cys, Met also acts as a metabolic hub, although to a lesser extent (120). Specifically, Met enters the Met cycle, which provides the methyl source for most cellular methylation reactions. The Met cycle, which is the transit of Met to S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), l-homocysteine (Hcy), and finally remethylation to Met, feeds the polyamine biosynthesis pathway, the trans-sulfuration pathway, and two Hcy remethylation reactions: (i) the cobalamin-dependent methionine synthase (MS) reaction, which obtains methyl groups from the folate cycle; or (ii) the betaine-homocysteine methyltransferase reaction, which obtains methyl groups from betaine (Fig. 1) (32, 33). In the absence of trans-sulfuration activity, remethylation and the polyamine biosynthesis pathway each fully recycle the S back into Met, and therefore are not major consumers of Met. By contrast, the trans-sulfuration pathway represents an irreversible exit of S from the Met cycle and can consume substantial amounts of Met. Indeed, trans-sulfuration and utilization of Met for synthesis of excreted proteins are the only two substantial outlets of S from the Met cycle in healthy cells (Fig. 1), and severe genetically determined deficiencies in trans-sulfuration or remethylation can cause overaccumulation of toxic levels of Hcy (10, 68, 69, 105, 106, 142). The cellular stopgap for overaccumulation of intracellular Hcy is excretion of the Hcy into the circulation, wherein it remains toxic and can lead to hyperhomocysteinemia (20, 77).
As its name implies, trans-sulfuration effectively transfers the fully reduced Met-derived S from Hcy to Cys. In this two-step process, all other Met-derived atoms are discarded to other metabolic pathways as α-ketobutyrate and ammonia, and a new amino acid skeleton is obtained from L-serine (Ser). Thus, in addition to providing an “outlet” to prevent toxic overaccumulation of Met-cycle intermediates, this pathway provides cells with a route of de novo Cys biosynthesis. In hepatocytes and some other cell types, this pathway is exceptionally robust. In hepatocytes, ∼1/2 of the S in Cys comes from Met via this route (87). Importantly, liver is largely responsible for intermediary metabolism of Cys, and it provides Cys to the body by excretion of GSSG into the blood. In addition, red blood cells provide a source of circulating free GSH (40). Likely dependent on the respective nutritional availability of cystine versus Met, the S in the GSSG excreted from liver can likely be fully derived from either Met or cystine.
Besides trafficking S from the Met cycle to Cys, the trans-sulfuration pathway is the predominant source of cellular H2S production in mammals (61, 114). Since metazoans cannot synthesize Cys from H2S, this has been generally considered a one-way outlet of S from the pathway; however, more recent advances in understanding the relationships between H2S, protein persulfidation, and the enzymatic activities involved in catalyzing sulfide-based chemistry in mammalian cells hint at potentially far more complex and physiologically important fates for cellular H2S (see the S-Persulfidation in Mammalian Cells section and other papers in this issue).
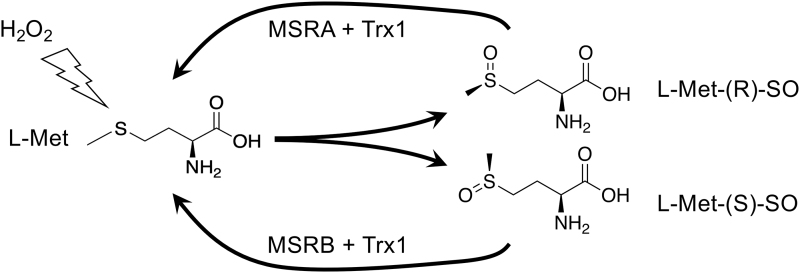
Although the thioether of Met is resistant to oxidation, exposure to oxidants can result in a 2-electron oxidation to Met-sulfoxide (Met-SO), which can adopt either of two diastereoisomeric forms: the R- or the S-form (Fig. 3). Although the R- and S-forms will occur randomly upon oxidation of free Met, the context of a Met within a protein or complex might influence which form will be adopted. Met-SO can be reduced back to Met by the TRX1-dependent Met-SO reductase (MSR) enzymes of which, interestingly, MSRA will only reduce the R-isomer and MSRB will only reduce the S-isomer (70, 123). MSRA and MSRB can be differentially expressed and differently subcellularly localized, and it has been proposed that different accumulation or repair of Met-SO diastereomers could serve regulatory or signaling functions in cells (92).
FIG. 3.
Oxidation and reduction of Met. Oxidation of Met will yield Met-SO in either R or S diastereomeric forms. On free Met, this will occur randomly; however in the context of a protein or under influence of an enzyme, preference for one or the other could occur. Reduction of the R form can only be catalyzed by MSRA, whereas the S isoform can only be reduced by MSRB. These two enzymes are differentially expressed. It has been proposed that diastereomer-specific oxidation and resolution of protein-Met-SO forms might participate in redox regulation (see text). Met-SO, Met-sulfoxide; MSR, Met-SO reductase.
Impacts of Stress on S Metabolism
Oxidative stress and endogenous antioxidant systems
The predominant source of reactive oxygen species (ROS) in most cells under most conditions is the mitochondrial electron transport chain (ETC) (17). In addition, ROS can arise as collateral by-products of energetic metabolism (e.g., β-oxidation of fatty acids), anabolic metabolism, detoxification or anabolic activities of cytochrome p450 (CYP) enzymes, as primary products of NADPH-oxidases (NOX), or as a result of toxin-driven redox cycling by diverse NADPH-dependent enzymes (76, 79, 95, 113). Another major source of oxidative stress arises extracellularly, largely from either defensive or pathological production of ROS by activated inflammatory cells (111).
For the classical case of mitochondrially generated ROS, a small amount of incompletely reduced oxygen as superoxide radical (O2−•) is collaterally generated by the ETC even under normal conditions. This O2−• is rapidly dismutated to hydrogen peroxide (H2O2) by abundant superoxide dismutases (SODs). Conditions like ETC disruption by various toxins or ischemia/reperfusion injury can dramatically increase the production of O2−• by the ETC. The efficacy of SODs renders H2O2 the predominant species in both the mitochondria and the cytosol (109, 110, 112). Each compartment has its own similar systems for detoxifying H2O2; for simplicity, we will focus here on only the cytosolic systems.
Cells efficiently eliminate cytosolic H2O2 via highly active and abundant peroxidases: the peroxiredoxins (PRXs) and glutathione peroxidases (GPXs), each of which obtain reducing power from the disulfide reductase systems (35, 111, 136). These peroxidases utilize cysteine thiols either intermolecularly (e.g., in “1-Cys PRXs”), intramolecularly (in “2-Cys PRXs”), or within a Cys-L-selenocysteine (Cys-Sec) pair in their active sites (e.g., in most GPXs, in which the GPX-Sec residue collaborates intramolecularly with a Cys from GSH or a GRX) (91). After each catalytic cycle, the oxidized enzymes require 2 electrons of reducing power to return the peroxidase to its active state. This reducing power is most often acquired from other thiol-based reductants, such as TRX1 or GRX/GSH. As discussed above, the upstream source of this reducing power is NADPH, which fuels the disulfide reducing systems via GSR or TRXR1 (82).
Other stresses
Electrophilic stress
Whereas small inorganic nonmetallic oxidizing molecules are typically called “oxidants,” metallic or somewhat larger organic oxidizing molecules are more commonly referred to as “electrophiles.” Both groups will readily accept electrons from other molecules, and therefore qualify as oxidants and electrophiles. Moreover, both are primarily detoxified by S-based reductase systems in cells. However, based on their other molecular properties, each of them is generally targeted by different detoxification enzymes, and this activity can have very different impacts on the cells and on S metabolism pathways. For example, H2O2 is considered an oxidant, and is detoxified by reduction to water and molecular oxygen. In the case of detoxification of H2O2 by GPXs, the reducing power for this comes initially from cellular GSH pools (16, 35, 127). In contrast, organic electrophiles such as reactive quinones are more often detoxified by conjugation to GSH by GSTs, followed by excretion of the glutathionylated product out of the cell (49). Lipid-hydroperoxides (ROOH) are relatively large organic electrophiles, yet they are often detoxified by direct enzymatic reduction by GPX4, for which the reducing power comes from GSH (35). Heavy metals (e.g., Au) and metalloids (e.g., As), on the contrary, are often monoatomic oxidants, yet they are more likely to be primarily detoxified by glutathionylation (104). Although both GPX- and GST-driven detoxification reactions use GSH, each has distinct impacts on the GSH system and on supporting metabolic pathways. GSH-driven reduction by GPXs generates intracellular GSSG, which, as long as the intracellular disulfide reductase systems are intact, consumes NADPH and fully regenerates the 2GSH without loss of the component atoms of the GSH. This reaction, therefore, consumes cellular energy sources to maintain NADPH:NADP+ pool ratios, but does not result in loss of cellular S (35). GSH conjugation and export, by contrast, irreversibly removes S from the cell (14, 27), which must be replaced by acquisition of new S-amino acids from extracellular sources (83).
Reductive stress
The need for cells to maintain a balanced redox environment, not only avoiding excessive accumulation of oxidants but also avoiding an overly reducing environment, is often overlooked. Oxidative activities of cells play critical roles in all aspects of physiology, and if the intracellular milieu becomes excessively reducing, it might interfere with these activities (15, 143). Among these activities is redox signaling, wherein oxidants need to react with regulatory protein-thiols as a part of the signaling mechanisms that coordinate cellular activities. Indeed, it is easy to artificially overexpress antioxidant systems in cells, showing that it would be a small matter for cells to have increased antioxidant activities. However, evolution did not give us a more reducing cytosol. Why? Likely because any benefits from increased resistance to oxidative stress would be negated by the detriments of the resultant reductive stress.
Excessive accumulation of GSH can cause reductive stress (143). However, a diverse battery of metabolic and regulatory systems in cells generally prevent this. For example, the heterodimeric rate-limiting enzyme for GSH biosynthesis, γ-glutamyl-Cys ligase (GCL), is strictly regulated to keep GSH levels balanced. GCL, itself, is catalytically inhibited by GSH; this feedback prevents excessive GSH accumulation (36, 103). Moreover, both the catalytic (GCLC) and modulatory (GCLM) subunits of GCL are regulated by the stress-responsive nuclear factor erythroid 2-related factor 2 (NRF2) pathway (85), such that under conditions of oxidative or electrophilic stress, GSH biosynthesis capacity is increased to restore the redox balance (see Master-Regulation of S Metabolism in Stress section). Recent evidence shows that cells also use export of GSSG from subcellular compartments (90, 97) or from the cytosol (28, 86, 89) to eliminate excessive GSSG, thereby modulating both total levels and the redox status of glutathione pools.
Drug/toxin/xenobiotic stress
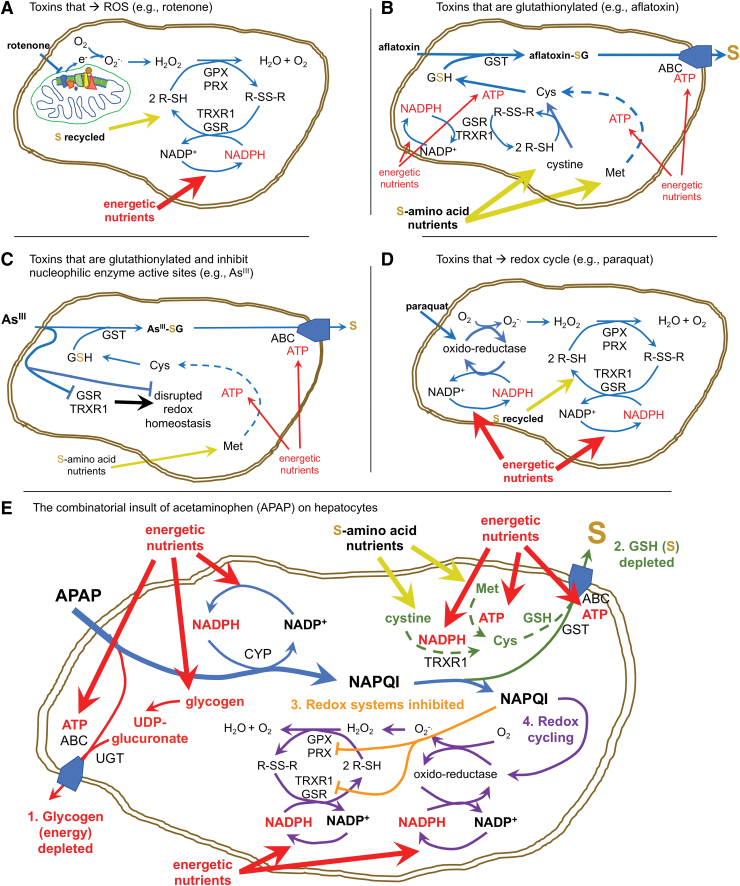
Many drugs, toxins, and other xenobiotics interact with the cellular disulfide reductase systems. However, in terms of understanding how this impacts the cells, much can be inferred by assessing whether the end-result of this activity consumes cellular NADPH, cellular S, or both (Fig. 4). Compounds that uncouple mitochondrial electron transport, such as rotenone, can induce production of O2−• → H2O2 (Fig. 4A) (84). Typically, this will be remediated by GPX and PRX responses, which consume recyclable disulfide reducing power (from GSH or TRX1, respectively), supported by GSR and TRXR1 using electrons obtained from NAPDH (35). Upstream of this, the NADPH-regenerating systems typically consume resources that must be diverted from energetic or anabolic processes (glucose, malate, or isocitrate) (21, 82). By contrast, drugs or toxins that are conjugated to S-containing molecules (e.g., glutathionylated by GSTs or sulfated by SULTs) and excreted, for example aflatoxin-B1, will irreversibly consume Cys/GSH and tax cellular S-amino acid stores, but this will not substantially consume NADPH (Fig. 4B) (24, 45). Some electrophilic drugs or toxins, for example, organoarsenites (As3+) or organomercurial compounds, are glutathionylated yet also covalently bind with high affinity to Cys or Sec residues of GSR, TRXR1, and other critical enzymes, thereby irreversibly disrupting activity of these enzymes (104). Importantly, most of these metals are cytotoxic at low doses, suggesting that consumption of cellular S-stores is not a major determinant of toxicity. Rather it is likely the dominant disruption of enzymatic activities in the cells is critical (Fig. 4C). Redox-cycling drugs or toxins, such as doxorubicin or paraquat, will interact with NADPH-dependent reductases in the presence of molecular oxygen to catalytically generate large amounts of O2−• (37). This redox cycling will consume cellular NADPH stores and, after the O2−• is dismutated to H2O2, additional NADPH will be consumed by GSR or TRXR1 to support peroxidase-driven reduction of the H2O2 (Fig. 4D).
FIG. 4.
Different toxic stresses have different impacts on S metabolism. (A) Toxins like rotenone disrupt mitochondrial respiration, leading to ROS production that will be addressed primarily by GPX and PRX enzymes. The pathways used generally recycle their S-metabolites and therefore will not deplete cellular S amino acids, but rather will consume energetic nutrients to maintain NADPH pools. (B) Toxins like aflatoxin B1 that are glutathionylated and exported will predominantly consume S amino acids to maintain GSH pools. Compared with (A), these will have lower consumption of NADPH and energetic resources, though some NADPH and ATP will be used to reduce cystine, synthesize GSH, and export the glutathionylated toxin through ABC exporters. (C) Electrophilic toxins such as arsenite (As3+) will be glutathionylated, but will also disrupt nucleophilic active site Cys or Sec residues, thereby dominantly inhibiting redox homeostasis systems. Typically, arsenite is a potent low-dose exposure, so GSH depletion is less important than is the disruption of redox enzymes. (D) Electrophilic compounds that redox cycle, like paraquat, can induce catalytic production of superoxide radical → H2O2. Whereas this alone will not deplete S amino acid pools, it can consume large amounts of NADPH and can generate considerable ROS/oxidative damage. (E) At high doses, APAP can exhibit toxicity at many levels by many mechanisms, as summarized in the text. In the online version of this panel, red denotes energetic impacts; green denotes impacts on S amino acid metabolism; orange denotes inhibition of nucleophilic active sites in redox enzymes; and purple indicates possible redox cycling. To summarize, APAP that enters hepatocytes is first conjugated to sulfate (not shown) or glycogen-derived glucuronic acid and then exported by ABC transporters. This can rapidly deplete all glycogen reserves in the hepatocytes [1]. Once glycogen reserves are depleted, excess APAP is reduced by CYP enzymes, consuming NADPH and generating the electrophilic quinone NAPQI. NAPQI is then conjugated to GSH by GST enzymes and exported through ABC transporters, thereby depleting GSH and consuming S amino acids [2]. Once GSH pools are exhausted, accumulating NAPQI dominantly inhibits nucleophilic active sites in redox enzymes [3] and likely redox cycles in some NADPH-dependent oxidoreductases [4]. ABC, ATP-binding cassette; APAP, acetaminophen; CYP, cytochrome p450; GPX, glutathione peroxidase; GSH glutathione; GST, GSH-S-transferase; NAPQI, N-acetylbenzoquinoneimine; PRX, peroxiredoxin. Color images are available online.
As a culminative example, some drugs, such as acetaminophen (paracetamol), can have even broader impacts on these metabolic systems (Fig. 4E) (99). Upon entering liver hepatocytes, much of the acetaminophen is conjugated to sulfate by SULTs or to glycogen-derived sugars by UDP-glucuronyltransferase and exported by multidrug resistance (MDR) or ATP-binding cassette (ABC) organic ion transporters, thereby removing oxidized sulfur and depleting energetic resources (glycogen) (56). At higher doses, these systems become exhausted and excess acetaminophen is chemically reduced by CYP enzymes, thereby consuming NADPH. The product of this reaction, N-acetylbenzoquinoneimine (NAPQI), is a highly electrophilic quinone that is aggressively glutathionylated by GSTs. Subsequently, the conjugated product is excreted by ABC/MDR exporters, thereby irreversibly consuming cellular GSH reserves and depleting S-amino acids. At still higher doses, GSH pools can become exhausted, allowing free NAPQI to accumulate (34, 58). In these conditions, NAPQI also inhibits the active site residue in TRXR1 (56) and likely with nucleophilic active sites in other redox enzymes. This interferes with the cells' ability to regenerate disulfide reducing power and it might induce redox cycling, which would consume more NADPH and catalytically generate O2−• → H2O2. Subsequently, reduction of the H2O2 by peroxidases will require disulfide reducing power and therefore more NADPH, although these activities might already be compromised by inactivation of TRXR1 and depletion of GSH. In severe overdose situations, hepatic glycogen, GSH, S-amino acids, and NADPH all become severely depleted; TRXR1 becomes unable to reduce TRX1-disulfide, peroxidases cannot be supported, and O2−• production increases. This “perfect storm” can result in necrotic hepatocyte death and acute liver failure. Indeed, this is the leading cause of acute liver failure in the United States and Europe (12). However, even under such severe overdose conditions, hepatocytes that have not yet died can often be rescued by administration of N-acetyl-L-cysteine (NAC), and in the clinic NAC treatment saves the lives of many acetaminophen-overdose patients (99). Whereas NAC is often referred to as an “antioxidant” or a “ROS scavenger,” it likely does not serve as a direct antioxidant because the reaction of the NAC thiol with H2O2 is too slow to be biologically relevant (68 M−1s−1), in particular under severe oxidative challenge (138). Although much remains to be resolved about the complex roles of NAC in cells (30), in the case of acetaminophen overdose, one likely role of NAC is to provide the hepatocytes with an alternate source of Cys that can be assembled into GSH to support continued glutathionylation and export of the NAPQI, and possibly also to allow continued trafficking reducing power from GSR to GPXs (2, 42, 56). In addition, recent studies have shown that NAC can act as a potent Cys-persulfidating agent, which might protect protein thiols from overoxidation, not only in the context of GSH depletion but more universally under diverse oxidative stress conditions as well (discussed below) (30).
Metabolic stresses
The interplay between the disulfide reductase systems and metabolism is finely tuned by a complex network of feedback mechanisms that are still being resolved. As suggested above, the relative availability of Cys versus Met sources in the diet will influence flux through S-amino acid metabolic pathways (54, 78). However, most mammalian cell types have only modest trans-sulfuration activity, and likely rely on having a ready supply of extracellular cystine and intracellular disulfide reducing power to support their intracellular Cys demands. Intermediary metabolism by liver hepatocytes provides a constant supply of extracellular cystine to the whole body. Thus, the hepatocytes maintain circulating levels of plasma GSSG/GSSX, and erythrocytes provide circulating GSH (see the Dietary sources and systemic distribution section). Within the hepatocytes, GSH is synthesized using Cys, which can be acquired from either dietary cystine and reduce to Cys by the NADPH-dependent disulfide reductase systems, or from dietary Met via their robust NADPH-independent trans-sulfuration pathway (83, 87, 101, 118).
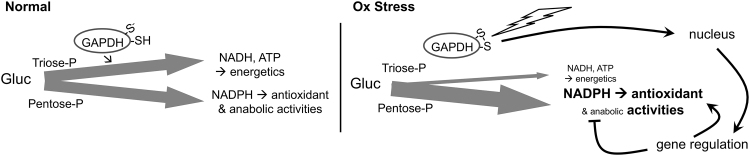
β-oxidation of fatty acids, which becomes elevated with high-fat diets, and glycolysis, which is elevated in many cancers, are each associated with increased production of ROS, increased oxidative damage, and increased demands on the GSH and TRX1 systems (4, 48, 55, 57, 76, 115). During oxidative stress, GSR and TRXR1 likely compete with other NADPH-dependent enzymatic systems to obtain the reducing power needed to maintain redox homeostasis. Favoring GSR and TRXR1 under these conditions, the active site Cys of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is highly redox reactive and functions as an oxidant-sensitive switch (94). Relatively subtle increases in H2O2 will preferentially oxidize this Cys, which inactivates the glycolytic activities of GAPDH and converts the protein into a nuclear-localized transcription factor (Fig. 5). With the disruption of glycolysis, more glucose is shunted to the pentose phosphate pathway, resulting in increased production of NADPH. Moreover, as a transcription factor, oxidized GAPDH realigns expression of diverse genes, resulting in a global metabolic rewiring by which NADPH-consuming anabolic activities are attenuated and redox homeostasis activities are elevated (52).
FIG. 5.
Redox sensing and metabolic reprioritization by GAPDH. GAPDH, a critical enzyme on the energy generating triosephosphate pathway, has a highly reactive Cys-thiolate ion in the active site (S−). In normal conditions, GAPDH is active and flux of glucose through the triose-P pathway for energetics versus through the pentose-P pathway for generating NADPH is balanced. During oxidative stress, H2O2 oxidizes the active site thiolate, which inactivates glycolytic activity of GAPDH, lowering glycolytic flux and making more glucose available for the pentose-P pathway; and converts GAPDH into a nuclear-localized transcription factor that further favors antioxidant activities of NADPH arising from the pentose-P pathway. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Carcinogenic stress
Transformation to a cancer phenotype and the characteristics of the tumor microenvironment are associated with diverse stresses on the cancer cells that can influence, or be influenced by, S metabolism activities. Most of these are redundant with stresses already mentioned above, but in cancer these can often occur in combination, leading to greater deviations from normal cell physiology and larger impacts on S metabolism. Metabolic realignments in transformed cells, such as the preferential switch to glycolysis, are often associated with increased levels of ROS in these cells, which, in turn, is expected to increase their reliance on the disulfide reductase-based antioxidant systems (63, 128). Conditions of hypoxia, nutrient restriction, or inflammation within the tumor microenvironment can further increase metabolic and oxidative stresses. The replicative activity of cancer cells is associated with proliferative stress, and therapeutic measures taken to combat the cancer can be associated with electrophilic stress, oxidative stress, metabolic stress, and drug/xenobiotic stress. Whereas different cancers might exhibit diverse combinations of these stresses, in some cases potentially targetable liabilities in S amino acid metabolism might be found. For example, many cancers upregulate the glutamate/cystine antiporter, xCT, to acquire cystine, which might be a vulnerability that can be targeted therapeutically (see the S-Overoxidation in Mammalian Cells section). By contrast, however, some other cancers are critically dependent on glutamine anaplerosis during glucose starvation and overexpression of xCT or cystine supplementation can drive glutamate exchange and potentially starve the tumor (66, 67, 88, 128).
S-Overoxidation in Mammalian Cells
In addition to oxidant-driven disulfide formation, which is reversible by the NADPH-driven disulfide reductase systems, biological “overoxidation” reactions can occur (Fig. 2). In these, Cys-thiols within proteins or small molecules can react with successive oxidants to first yield a sulfenic acid (Cys-SOH), then a sulfinic acid (Cys-SO2H), and finally a sulfonic acid (Cys-SO3H) (83). Cys-sulfenic acids are highly unstable species that usually rapidly react with another thiol to yield H2O and a disulfide. However, when concentrations of an oxidant are high, a second oxidation of the sulfenic acid to the sulfinic acid form can occur (46). Historically, it had been thought that neither the sulfinic- nor the sulfonic form could be reduced back to a Cys-thiol in mammalian cells. However, the discovery of the enzyme sulfiredoxin (Srx) revealed that some Cys-sulfinic acids can be reduced back to the thiol. Srx uses both ATP and reduced dithiols from TRX1 in a slow 4-electron reaction to repair an overoxidized Cys-sulfininc acid within the active site of 2-Cys PRXs back to the Cys-thiol form (13, 18, 139). This discovery led to the proposed “floodgate model” in redox signaling, wherein a burst of H2O2 could generate a Cys-sulfinic acid on PRX in a rapid two-step process, which would transiently inactivate the PRX peroxidase activity and allow H2O2 to accumulate locally and oxidize Cys residues on other proteins as a signaling molecule (140). Srx will then slowly repair the PRX-sulfinic acid, thereby restoring peroxidase activity and shutting off the signaling event (102). Recently, an unexpected diversity of other potential protein-sulfinic acid targets that can be repaired by Srx, at least in vitro, were reported, including oxidatively damaged PTPN12, DJ-1, and others (5). A mechanism to reduce Cys-sulfonic acid in mammalian cells has not been demonstrated, supporting the current understanding that this is, indeed, an irreversible modification. The fate of Cys-sulfonic acid in damaged proteins is also poorly understood; but it is presumed that proteins with Cys-sulfonic acids, being irreversibly damaged, are degraded by the proteasome. This will release Cys-sulfonic acid, which can be converted to taurine or further oxidized to liberate sulfate (116, 120). There is also evidence of some chaperones stably carrying Cys-sulfonic acid modifications, which might be critical to their specialized activities (72).
Importantly, severe oxidative stress is not the only means of inducing Cys-overoxidation in cells. The enzyme Cys-dioxygenase (CDO) catalyzes the irreversible conversion of free Cys + O2 → Cys-sulfinic acid. Deamination converts this to hypotaurine. Hypotaurine can further oxidize to the sulfonic acid form taurine, and then further oxidize to release inorganic sulfate (117). These regulated overoxidations play several roles in physiology. First, taurine is an important osmolyte in blood plasma that is secreted predominantly by the liver and, to a lesser extent, the kidney (130, 131). Taurine is also conjugated onto bile acids within hepatocytes in many animal species, which likely plays important roles in digestive processes (131). Overoxidation of Cys in liver provides an amino acid-derived source of sulfate that can be assembled into PAPS for important anabolic and defensive SULT reactions (see Dietary Sources and Systemic Distribution section). Finally, it has more recently been recognized that Cys overoxidation might provide an important “outlet” that prevents excessive accumulation of Cys in cells, thereby rectifying conditions of potential reductive stress (see Other Stresses section). Indeed, some cancer cells are critically addicted to CDO activity for modulating intracellular Cys levels, which represents a potentially targetable liability to these cancers (64, 65).
S-Persulfidation in Mammalian Cells
Another important Cys modification in cells is Cys-persulfidation (Cys-SSH) (11, 51). Protein-Cys-persulfides are readily reduced by cellular redoxins (25, 26, 29, 135), yielding a Cys-thiol and H2S. This modification is therefore a likely regulator of enzyme activity and signaling pathways, although the extent of this use of protein-persulfidation remains largely undefined (60). Liberation of H2S as a result of regulation of specific individual proteins is unlikely to be substantial; however, some studies suggest that protein persulfides might be more abundant than expected, in which case their redoxin-catalyzed conversion to thiols could generate large amounts of H2S in cells or subcellular compartments. For example, the mitochondrial Cys-tRNA synthetase, CARS2, has Cys-persulfidation activity, and this could lead to extensive cotranslational insertion of Cys-persulfide into diverse proteins (3). It has also been proposed that cells might generate a reserve of certain proteins with persulfide modifications on critical Cys residues that, although perhaps inactive in their normal roles, could be activated to thiols by redoxins after oxidative stress-mediated overoxidation to support stress recovery (discussed further below) (25). The reader is directed to other papers in this issue for updates on mechanisms and activities of protein persulfidation.
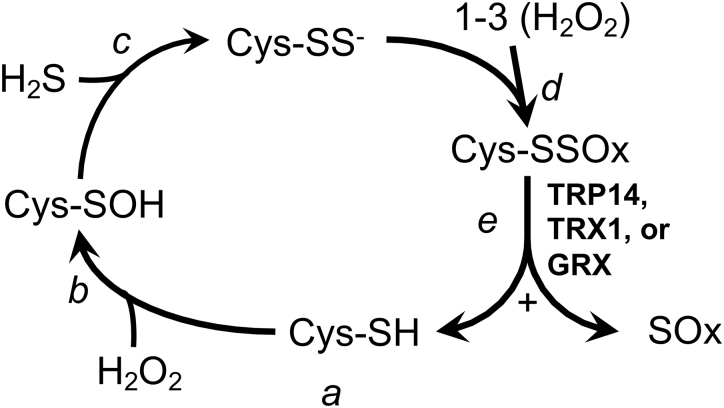
The contributions of H2S in generating Cys-persulfides on proteins or small molecules remain unclear. The S in H2S is in the same reduced state as thiols and cannot react with a thiol to generate a persulfide unless an oxidant is added. However, if H2S is present coincident with a 2-electron thiol-oxidation, for example by H2O2, the unstable Cys-sulfenic acid intermediate could be resolved with the H2S to form a Cys-persulfide rather than with another thiol to form a disulfide (Fig. 6, steps a–c). Due to the combined nucleophilicity of the two S residues in a Cys-persulfide, the terminal S more readily ionizes as compared with the Cys-thiol (pKa ∼4.5 as compared with pKa ∼8.2, respectively), so at physiological pH the Cys-persulfide will be predominantly in the highly reactive perthiolate-anion state (Cys-SS−) (25). Whereas the reaction of most Cys-thiols and H2O2 at physiological pH is slow (137, 138), a Cys-perthiolate anion is likely to be dramatically more reactive with H2O2, forming Cys-persulfenylate (Cys-SSO−) and possibly Cys-persulfinylate (-SSO2−) and -persulfonylate (-SSO3−) anions upon subsequent reactions with additional equivalents of H2O2 (Fig. 6, step d) (11, 51). Cys-SSO3− can also be generated by the reduction of a disulfide with SO32− (9, 75). Interestingly, unlike Cys overoxidation, which yields typically irreversible sulfinic and sulfonic acids, the persulfide forms have a disulfide bond linking the overoxidized terminal S to the Cys. Recent work shows that this disulfide bond remains a reducible substrate for TRX1, thioredoxin-related protein of 14 kDa (TRP14), and GRXs (Fig. 6, step e) (25). The redoxin-catalyzed reduction of an overoxidized Cys-persulfide, whether generated on proteins cotranslationally by CARS2 or on any molecule by thiol oxidation in the presence of H2S, yields, in each case, the native Cys-thiol-containing molecule and liberates the inorganic overoxidized S species (25, 31). Although the overoxidized inorganic S have been irretrievably lost from the pool of metabolites that could contribute S to Met or Cys in the future, in doing so it could have removed up to four molecules of H2O2 from the cell. Such nonrecycling use of S amino acids to reduce H2O2 might seem unfavorable under normal conditions, when the disulfide reductase-fueled GPXs and PRXs are able to remove H2O2 without loss of S. However, under conditions wherein these systems are disrupted or overwhelmed (28, 82, 98, 104), “sacrificial” production of H2S to support a persulfide-based overoxidation mechanism to eliminate H2O2, as depicted in Figure 6, could provide a stopgap measure for limiting oxidative damage.
FIG. 6.
Hypothetical persulfidation-based thiol protection. a, In the unstressed state, Cys residues in proteins or metabolites will generally be in the reduced thiol form. b, Exposure to H2O2 yields the unstable sulfenic acid. c, Whereas this will typically react with another thiol to yield a disulfide, in the presence of H2S, a persulfide can form, which will spontaneously ionize to the reactive perthiolate anion at physiological pH. d, The reactive perthiolate anion can react with H2O2, yielding persulfenylate anion. This might further react with H2O2, yielding persulfinylate and persulfonylate anions (in combination, these overoxidized forms designated “-SSOx”). e, Reduction of the disulfide bond in any of the -SSOx species by TRX1, TRP14, or GRX will regenerate the active thiol on the original substrate and liberate an oxidized inorganic S product, designated “SOx.” GRX, glutaredoxin; H2O2, hydrogen peroxide; H2S, hydrogen sulfide; TRP14, thioredoxin-related protein of 14 kDa; TRX1, thioredoxin-1.
Regulation of S Metabolism in Stressed Cells
Regulation of the Met cycle and trans-sulfuration
A previous overview of regulatory circuits in the Met cycle and trans-sulfuration pathway summarizes how these pathways are differently regulated to support intermediary metabolism in hepatocytes versus cell-autonomous activities in other cell types (78). Here, we will emphasize how stresses might impact these pathways.
The first step in the Met cycle generates SAM, which is the methyl donor for most methyltransferase enzymes in cells, and which must be demethylated by one of these methyltransferases to continue through the Met cycle (Fig. 1). SAM is synthesized from Met + ATP by methionine adenosyltransferase-II (MATII, encoded by the MAT2A gene) in nonliver cells or by MATI and MATIII (homodimeric and homotetrameric forms, respectively, both encoded by the MAT1A gene) in hepatocytes. MATI and MATII are feedback inhibited by SAM, thereby adjusting Met-cycle flux to match cellular needs. Conversely, MATIII is activated by SAM, which generates feed-forward activation in support of intermediary metabolism in hepatocytes (78). SAM also activates trans-sulfuration and inhibits methylene-tetrahydrofolate reductase, the latter of which provides new methyl groups to MS for conversion of Hcy → Met. These activities effectively shunt excess Hcy toward Cys rather than back to Met. Importantly, however, there are situations, for example, Cys deficiency, wherein flux from SAM → Hcy → Cys can become restricted by the need to demethylate SAM. Evidence suggests that conversion of glycine to sarcosine by the enzyme glycine N-methyltransferase, which accounts for ∼3% of liver protein by mass, is used by hepatocytes to promote conversion of SAM → Hcy by removing excess methyl groups, preventing accumulation of SAM, thereby allowing unrestricted transit through the Met cycle (50, 54). In yeast and mammalian cells, phospholipid biosynthesis and, in more extreme situations, histone methylation, have also been shown to provide a “methyl-sink” for SAM-dependent methyltransferase activity, again ensuring rapid flux through the cycle in support of Cys biosynthesis, when needed (141).
The signals that determine Cys- versus H2S production by the trans-sulfuration pathway remain incompletely resolved. The balance between Cys versus H2S production by cystathionine-β-synthase (CBS) is influenced by the Ser:Cys ratio; when Ser is limiting, H2S production increases (74). In addition, work from the Banerjee laboratory has demonstrated a CO-dependent switch in the trans-sulfuration products (62). Exposure to CO inhibits the first enzyme in the trans-sulfuration pathway, CBS, leading to an accumulation of Hcy and depletion of cystathionine (Cth). In response, the second enzyme in the pathway, cystathionine-γ-lyase (CSE), which normally converts Cth to Cys, switches substrate utilization from Cth to Cys or Hcy, resulting in the production of H2S. Preference can be returned to Cys production with the addition of Ser, the amino acid skeleton donor for Cys biosynthesis (62).
Other major metabolic regulation of S metabolism
Some critical S-metabolites are normally kept within narrow limits by either substrate- or product-feedback regulation. As discussed already, GSH biosynthesis is repressed by the pathway product, GSH (108), a mechanism that both prevents overaccumulation of GSH leading to reductive stress and allows rapid recovery of GSH levels after depletion. Cys levels, by contrast, are feedback regulated by the reactant, Cys. Thus, CDO is activated by Cys, leading to shunting of excess Cys into overoxidized forms that can be excreted (23, 121).
Master regulation of S metabolism in stress
The NRF2/kelch-like ECH-associated protein 1 (KEAP1) pathway plays the role of master regulator of many genes involved in stress responses of S-metabolism. This pathway is post-translationally induced in response to oxidative or electrophilic exposures. The reader is directed to an outstanding recent review for more information on the mechanisms of NRF2 regulation (122); here, we will consider the impacts of this on S-metabolism pathways. Upon induction, the transcription factor NRF2 regulates a suite of ∼102 genes that coordinately defend cells against oxidants and electrophiles, including genes encoding critical enzymes on several S-metabolism pathways and genes that more peripherally impact these pathways. In the latter case, activation of NRF2 induces expression of enzymes that generate NADPH (e.g., malic enzyme and pentose phosphate pathway enzymes), and represses expression of anabolic genes that might compete with GSR and TRXR1 for NADPH (e.g., fatty acid biosynthesis pathways). In the former case, NRF2 activates expression of genes for glutathione biosynthesis enzymes (e.g., GCLC and GCLM) (73, 85, 107, 125). Another interesting target of the NRF2 pathway is heme oxygenase 1 (HO-1), which is strongly activated by NRF2. As discussed above, in the presence of free heme, induction of HO-1 will generate CO, which will both impede Cys synthesis and therefore GSH biosynthesis, and favor H2S production by the trans-sulfuration pathway (62). Although disruption of Cys/GSH biosynthesis seems at first counterproductive in face of a stress response, it is possible that the special situation of heme exposure, including release of iron leading to possible Fenton chemistry-catalyzed generation of extremely damaging radicals (134), is less hazardous if Cys/GSH biosynthesis is transiently repressed. Alternatively, it is possible that H2S, itself, is better than Cys/GSH at remediating the hazards associated with heme exposure. Future studies will hopefully resolve the rationale for how CO, H2S, Cys, and GSH are coordinated by Nfr2 and HO-1 as a stress response.
Acknowledgments
We thank our colleagues and collaborators for fruitful discussions on these topics.
Abbreviations Used
- ABC
ATP-binding cassette family exporter
- APAP
acetaminophen (paracetamol)
- APS
adenosine-5′-phosphosulfate
- CBS
cystathionine-β-synthase
- CDO
Cys-dioxygenase
- CoA
coenzyme A
- CSE
cystathionine-γ-lyase
- Cth
cystathionine
- CYP
cytochrome p450
- Cys
l-cysteine
- Cys-Sec
Cys-L-selenocysteine
- ETC
electron transport chain
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCL
γ-glutamyl-Cys ligase
- GCLC
GCL catalytic subunit
- GCLM
GCL modulatory subunit
- GGT
γ-glutamyl transpeptidase
- Glu
l-glutamate
- Gly
l-glycine
- GPX
glutathione peroxidase
- GRX
glutaredoxin
- GSH
glutathione (γ-L-glutamyl-L-cysteinylglycine)
- GSR
glutathione reductase
- GSSG
glutathione disulfide
- GST
GSH-S-transferase
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- Hcy
l-homocysteine
- HO-1
heme oxygenase 1
- KEAP1
kelch-like ECH-associated protein 1
- MAT
methionine adenosyltransferase
- MDR
multidrug resistance organic ion transporters
- Met
l-methionine
- Met-SO
Met sulfoxide
- MS
methionine synthase
- MSR
Met-SO reductase
- NAC
N-acetyl-L-cysteine
- NAPQI
N-acetylbenzoquinoneimine
- NOX
NADPH-oxidase
- NRF2
nuclear factor erythroid 2-related factor 2
- PAPS
phosphoadenosine-5′-phosphosulfate
- PRX
peroxiredoxin
- ROS
reactive oxygen species
- S
sulfur
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- Sec
l-selenocysteine
- Ser
l-serine
- SOD
superoxide dismutase
- Srx
sulfiredoxin
- SULT
sulfotransferase
- TRP14
thioredoxin-related protein of 14 kDa
- TRPs
thioredoxin-related proteins
- TRX1
thioredoxin-1
- TRXR1
thioredoxin reductase-1
Funding Information
Our research is supported by funding to E.E.S. from the National Institute of Health (DK123738, OD026444, AG055022, and CA215784), the Montana Agricultural Experiment Station (MONB00443), and the Montana State University Department of Microbiology & Immunology.
References
- 1. Adams JD Jr., Lauterburg BH, and Mitchell JR.. Plasma glutathione and glutathione disulfide in the rat: regulation and response to oxidative stress. J Pharmacol Exp Ther 227: 749–754, 1983 [PubMed] [Google Scholar]
- 2. Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, and Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337: 110–116, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akaike T, Ida T, Wei FY, Nishida M, Kumagai Y, Alam MM, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, Nishimura A, Morita M, Tomizawa K, Nishimura A, Watanabe S, Inaba K, Shima H, Tanuma N, Jung M, Fujii S, Watanabe Y, Ohmuraya M, Nagy P, Feelisch M, Fukuto JM, and Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 8: 1177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akram M, Ali Shah SM, Munir N, Daniyal M, Tahir IM, Mahmood Z, Irshad M, Akhlaq M, Sultana S, and Zainab R. Hexose monophosphate shunt, the role of its metabolites and associated disorders: a review. J Cell Physiol 2019 [Epub ahead of print]; Doi: 10.1002/jcp.28228 [DOI] [PubMed]
- 5. Akter S, Fu L, Jung Y, Conte ML, Lawson JR, Lowther WT, Sun R, Liu K, Yang J, and Carroll KS. Chemical proteomics reveals new targets of cysteine sulfinic acid reductase. Nat Chem Biol 14: 995–1004, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arner ES and Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem 267: 6102–6109, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Arnér ESJ. Focus on mammalian thioredoxin reductases—important selenoproteins with versatile functions. Biochim Biophys Acta 1790: 495–526, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Augustyn KD, Jackson MR, and Jorns MS. Use of tissue metabolite analysis and enzyme kinetics to discriminate between alternate pathways for hydrogen sulfide metabolism. Biochemistry 56: 986–996, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailey JL and Cole RD. Studies on the reaction of sulfite with proteins. J Biol Chem 234: 1733–1739, 1959 [PubMed] [Google Scholar]
- 10. Baumgartner MR and Fowler B. Vitamin B12 disorders. In: Physician's Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases, edited by Blau N, Duran M, Gibson KM, and Dionisi Vici C. Berlin: Springer, 2014, pp. 205–218 [Google Scholar]
- 11. Benchoam D, Cuevasanta E, Moller MN, and Alvarez B. Hydrogen sulfide and persulfides oxidation by biologically relevant oxidizing species. Antioxidants (Basel) 8: 48, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bernal W, Auzinger G, Dhawan A, and Wendon J. Acute liver failure. Lancet 376: 190–201, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Biteau B, Labarre J, and Toledano MB. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425: 980–984, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Bousova I and Skalova L. Inhibition and induction of glutathione S-transferases by flavonoids: possible pharmacological and toxicological consequences. Drug Metab Rev 44: 267–286, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Brewer AC, Mustafi SB, Murray TV, Rajasekaran NS, and Benjamin IJ. Reductive stress linked to small HSPs, G6PD, and Nrf2 pathways in heart disease. Antioxid Redox Signal 18: 1114–1127, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brigelius-Flohe R and Maiorino M. Glutathione peroxidases. Biochim Biophys Acta 1830: 3289–3303, 2013 [DOI] [PubMed] [Google Scholar]
- 17. Cadenas S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim Biophys Acta Bioenerg 1859: 940–950, 2018 [DOI] [PubMed] [Google Scholar]
- 18. Chang TS, Jeong W, Woo HA, Lee SM, Park S, and Rhee SG. Characterization of mammalian sulfiredoxin and its reactivation of hyperoxidized peroxiredoxin through reduction of cysteine sulfinic acid in the active site to cysteine. J Biol Chem 279: 50994–51001, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Chawla RK, Lewis FW, Kutner MH, Bate DM, Roy RG, and Rudman D. Plasma cysteine, cystine, and glutathione in cirrhosis. Gastroenterology 87: 770–776, 1984 [PubMed] [Google Scholar]
- 20. Chen NC, Yang F, Capecci LM, Gu Z, Schafer AI, Durante W, Yang XF, and Wang H. Regulation of homocysteine metabolism and methylation in human and mouse tissues. FASEB J 24: 2804–2817, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cordeiro AT. NADPH producing enzymes as promising drug targets for Chagas disease. Curr Med Chem 26: 6564–6571, 2019 [DOI] [PubMed] [Google Scholar]
- 22. Coughtrie MWH. Function and organization of the human cytosolic sulfotransferase (SULT) family. Chem Biol Interact 259: 2–7, 2016 [DOI] [PubMed] [Google Scholar]
- 23. Cresenzi CL, Lee JI, and Stipanuk MH. Cysteine is the metabolic signal responsible for dietary regulation of hepatic cysteine dioxygenase and glutamate cysteine ligase in intact rats. J Nutr 133: 2697–2702, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Dohnal V, Wu Q, and Kuca K. Metabolism of aflatoxins: key enzymes and interindividual as well as interspecies differences. Arch Toxicol 88: 1635–1644, 2014 [DOI] [PubMed] [Google Scholar]
- 25. Doka E, Ida T, Dagnell M, Abiko Y, Luong NC, Balog N, Takata T, Espinosa B, Nishimura A, Cheng Q, Funato Y, Miki H, Fukuto JM, Prigge JR, Schmidt EE, Arner ESJ, Kumagai Y, Akaike T, and Nagy P. Control of protein function through oxidation and reduction of persulfidated states. Sci Adv 6: eaax8358, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Doka E, Pader I, Biro A, Johansson K, Cheng Q, Ballago K, Prigge JR, Pastor-Flores D, Dick TP, Schmidt EE, Arner ES, and Nagy P. A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci Adv 2: e1500968, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dourado DF, Fernandes PA, and Ramos MJ. Mammalian cytosolic glutathione transferases. Curr Protein Pept Sci 9: 325–337, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Eriksson S, Prigge JR, Talago EA, Arner ES, and Schmidt EE. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nat Commun 6: 6479, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Espinosa B and Arner ESJ. Thioredoxin-related protein of 14 kDa as a modulator of redox signalling pathways. Br J Pharmacol 176: 544–553, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ezerina D, Takano Y, Hanaoka K, Urano Y, and Dick TP. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular H2S and sulfane sulfur production. Cell Chem Biol 25: 447–459.e4, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filipovic MR, Zivanovic J, Alvarez B, and Banerjee R. Chemical biology of H2S signaling through persulfidation. Chem Rev 118: 1253–1337, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finkelstein JD. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin Chem Lab Med 45: 1694–1699, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Finkelstein JD and Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem 259: 9508–9513, 1984 [PubMed] [Google Scholar]
- 34. Fisher ES and Curry SC. Evaluation and treatment of acetaminophen toxicity. Adv Pharmacol 85: 263–272, 2019 [DOI] [PubMed] [Google Scholar]
- 35. Flohe L, Toppo S, Cozza G, and Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid Redox Signal 15: 763–780, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, and Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 30: 86–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fussell KC, Udasin RG, Gray JP, Mishin V, Smith PJ, Heck DE, and Laskin JD. Redox cycling and increased oxygen utilization contribute to diquat-induced oxidative stress and cytotoxicity in Chinese hamster ovary cells overexpressing NADPH-cytochrome P450 reductase. Free Radic Biol Med 50: 874–882, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garcia RA and Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122: 1693–1701, 1992 [DOI] [PubMed] [Google Scholar]
- 39. Garibotto G, Sofia A, Saffioti S, Russo R, Deferrari G, Rossi D, Verzola D, Gandolfo MT, and Sala MR. Interorgan exchange of aminothiols in humans. Am J Physiol Endocrinol Metab 284: E757–E763, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Giustarini D, Milzani A, Dalle-Donne I, and Rossi R. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol Dis 40: 174–179, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Gorissen SHM and Witard OC. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc Nutr Soc 77: 20–31, 2018 [DOI] [PubMed] [Google Scholar]
- 42. Green JL, Heard KJ, Reynolds KM, and Albert D. Oral and intravenous acetylcysteine for treatment of acetaminophen toxicity: a Systematic review and meta-analysis. West J Emerg Med 14: 218–226, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Griffith OW and Meister A. Glutathione: interorgan translocation, turnover, and metabolism. Proc Natl Acad Sci U S A 76: 5606–5610, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Griffith OW and Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A 76: 268–272, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gross-Steinmeyer K and Eaton DL. Dietary modulation of the biotransformation and genotoxicity of aflatoxin B(1). Toxicology 299: 69–79, 2012 [DOI] [PubMed] [Google Scholar]
- 46. Gupta V and Carroll KS. Sulfenic acid chemistry, detection and cellular lifetime. Biochim Biophys Acta 1840: 847–875, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hansen RE, Roth D, and Winther JR. Quantifying the global cellular thiol-disulfide status. Proc Natl Acad Sci U S A 106: 422–427, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, Elia A, Berger T, Cescon DW, Adeoye A, Brustle A, Molyneux SD, Mason JM, Li WY, Yamamoto K, Wakeham A, Berman HK, Khokha R, Done SJ, Kavanagh TJ, Lam CW, and Mak TW. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 27: 211–222, 2015 [DOI] [PubMed] [Google Scholar]
- 49. Hayes JD, Flanagan JU, and Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88, 2005 [DOI] [PubMed] [Google Scholar]
- 50. Heady JE and Kerr SJ. Purification and characterization of glycine N-methyltransferase. J Biol Chem 248: 69–72, 1973 [PubMed] [Google Scholar]
- 51. Heppner DE, Hristova M, Ida T, Mijuskovic A, Dustin CM, Bogdandi V, Fukuto JM, Dick TP, Nagy P, Li J, Akaike T, and van der Vliet A. Cysteine perthiosulfenic acid (Cys-SSOH): a novel intermediate in thiol-based redox signaling? Redox Biol 14: 379–385, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hildebrandt T, Knuesting J, Berndt C, Morgan B, and Scheibe R. Cytosolic thiol switches regulating basic cellular functions: GAPDH as an information hub? Biol Chem 396: 523–537, 2015 [DOI] [PubMed] [Google Scholar]
- 53. Hildebrandt TM and Grieshaber MK. Three enzymatic activities catalyze the oxidation of sulfide to thiosulfate in mammalian and invertebrate mitochondria. FEBS J 275: 3352–3361, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Hughey CC, Trefts E, Bracy DP, James FD, Donahue EP, and Wasserman DH. Glycine N-methyltransferase deletion in mice diverts carbon flux from gluconeogenesis to pathways that utilize excess methionine cycle intermediates. J Biol Chem 293: 11944–11954, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Indo HP, Hawkins CL, Nakanishi I, Matsumoto KI, Matsui H, Suenaga S, Davies MJ, St Clair DK, Ozawa T, and Majima HJ. Role of mitochondrial reactive oxygen species in the activation of cellular signals, molecules, and function. Handb Exp Pharmacol 240: 439–456, 2017 [DOI] [PubMed] [Google Scholar]
- 56. Iverson SV, Eriksson S, Xu J, Prigge JR, Talago EA, Meade TA, Meade ES, Capecchi MR, Arner ES, and Schmidt EE. A Txnrd1-dependent metabolic switch alters hepatic lipogenesis, glycogen storage, and detoxification. Free Radic Biol Med 63: 369–380, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, and Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci 65: 166–176, 2002 [DOI] [PubMed] [Google Scholar]
- 58. James LP, Mayeux PR, and Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos 31: 1499–1506, 2003 [DOI] [PubMed] [Google Scholar]
- 59. James MO. Enzyme kinetics of conjugating enzymes: PAPS sulfotransferase. Methods Mol Biol 1113: 187–201, 2014 [DOI] [PubMed] [Google Scholar]
- 60. Kabil O and Banerjee R. Enzymology of H2S biogenesis, decay and signaling. Antioxid Redox Signal 20: 770–782, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kabil O, Vitvitsky V, Xie P, and Banerjee R. The quantitative significance of the transsulfuration enzymes for H2S production in murine tissues. Antioxid Redox Signal 15: 363–372, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kabil O, Yadav V, and Banerjee R. Heme-dependent metabolite switching regulates H2S synthesis in response to endoplasmic reticulum (ER) stress. J Biol Chem 291: 16418–16423, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kang SW, Lee S, and Lee EK. ROS and energy metabolism in cancer cells: alliance for fast growth. Arch Pharm Res 38: 338–345, 2015 [DOI] [PubMed] [Google Scholar]
- 64. Kang YP, Torrente L, Falzone A, Elkins CM, Liu M, Asara JM, Dibble CC, and DeNicola GM. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. Elife 8: e45572, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kang YP, Torrentte L, Liu M, Asara JM, Dibble Cc, and DeNicola GM. Cysteine dioxygenase 1 is a metabolic liability for non-small cell lung cancer. BioRxiv 8: e45572, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koppula P, Zhang Y, Shi J, Li W, and Gan B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J Biol Chem 292: 14240–14249, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Koppula P, Zhang Y, Zhuang L, and Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 38: 12, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kozich V, Ditroi T, Sokolova J, Krizkova M, Krijt J, Jesina P, and Nagy P. Metabolism of sulfur compounds in homocystinurias. Br J Pharmacol 176: 594–606, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kozich V, Morris AAM, and Blom HJ. Disorders of sulfur amino acid metabolism. In: Inborn Metabolic Diseases: Diagnosis and Treatment, edited by Saudubray J-M, Baumgartner MR, and Walter J. Berlin: Springer, 2016, pp. 309–320 [Google Scholar]
- 70. Lee BC, Dikiy A, Kim HY, and Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta 1790: 1471–1477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee TK, Li L, and Ballatori N. Hepatic glutathione and glutathione S-conjugate transport mechanisms. Yale J Biol Med 70: 287–300, 1997 [PMC free article] [PubMed] [Google Scholar]
- 72. Lim JC, Choi HI, Park YS, Nam HW, Woo HA, Kwon KS, Kim YS, Rhee SG, Kim K, and Chae HZ. Irreversible oxidation of the active-site cysteine of peroxiredoxin to cysteine sulfonic acid for enhanced molecular chaperone activity. J Biol Chem 283: 28873–28880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lu SC. Glutathione synthesis. Biochim Biophys Acta 1830: 3143–3153, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Majtan T, Krijt J, Sokolova J, Krizkova M, Ralat MA, Kent J, Gregory JF, 3rd, Kozich V, and Kraus JP. Biogenesis of hydrogen sulfide and thioethers by cystathionine beta-synthase. Antioxid Redox Signal 28: 311–323, 2018 [DOI] [PubMed] [Google Scholar]
- 75. Mannervik B, Persson G, and Eriksson S. Enzymatic catalysis of the reversible sulfitolysis of glutathione disulfide and the biological reduction of thiosulfate esters. Arch Biochem Biophys 163: 283–289, 1974 [DOI] [PubMed] [Google Scholar]
- 76. Mansouri A, Gattolliat CH, and Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155: 629–647, 2018 [DOI] [PubMed] [Google Scholar]
- 77. Martinez Y, Li X, Liu G, Bin P, Yan W, Mas D, Valdivie M, Hu CA, Ren W, and Yin Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids 49: 2091–2098, 2017 [DOI] [PubMed] [Google Scholar]
- 78. Martinov MV, Vitvitsky VM, Banerjee R, and Ataullakhanov FI. The logic of the hepatic methionine metabolic cycle. Biochim Biophys Acta 1804: 89–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mazat JP, Devin A, and Ransac S. Modelling mitochondrial ROS production by the respiratory chain. Cell Mol Life Sci 77: 455–465, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Meister A, Griffith OW, Novogrodsky A, and Tate SS. New aspects of glutathione metabolism and translocation in mammals. Ciba Found Symp 135–161, 1979 [DOI] [PubMed] [Google Scholar]
- 81. Meredith MJ and Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J Biol Chem 257: 3747–3753, 1982 [PubMed] [Google Scholar]
- 82. Miller CG, Holmgren A, Arner ESJ, and Schmidt EE. NADPH-dependent and -independent disulfide reductase systems. Free Radic Biol Med 127: 248–261, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Miller CG and Schmidt EE. Disulfide reductase systems in liver. Br J Pharmacol 176: 532–543, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mills EL, Kelly B, Logan A, Costa ASH, Varma M, Bryant CE, Tourlomousis P, Dabritz JHM, Gottlieb E, Latorre I, Corr SC, McManus G, Ryan D, Jacobs HT, Szibor M, Xavier RJ, Braun T, Frezza C, Murphy MP, and O'Neill LA. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 167: 457–470.e13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mitsuishi Y, Motohashi H, and Yamamoto M. The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2: 200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morgan B, Ezerina D, Amoako TN, Riemer J, Seedorf M, and Dick TP. Multiple glutathione disulfide removal pathways mediate cytosolic redox homeostasis. Nat Chem Biol 9: 119–125, 2013 [DOI] [PubMed] [Google Scholar]
- 87. Mosharov E, Cranford MR, and Banerjee R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39: 13005–13011, 2000 [DOI] [PubMed] [Google Scholar]
- 88. Muir A, Danai LV, Gui DY, Waingarten CY, Lewis CA, and Vander Heiden MG. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife 6: e27713, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Muller A, Schneider JF, Degrossoli A, Lupilova N, Dick TP, and Leichert LI. Fluorescence spectroscopy of roGFP2-based redox probes responding to various physiologically relevant oxidant species in vitro. Data Brief 11: 617–627, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Muller A, Schneider JF, Degrossoli A, Lupilova N, Dick TP, and Leichert LI. Systematic in vitro assessment of responses of roGFP2-based probes to physiologically relevant oxidant species. Free Radic Biol Med 106: 329–338, 2017 [DOI] [PubMed] [Google Scholar]
- 91. Nagy P, Karton A, Betz A, Peskin AV, Pace P, O'Reilly RJ, Hampton MB, Radom L, and Winterbourn CC. Model for the exceptional reactivity of peroxiredoxins 2 and 3 with hydrogen peroxide: a kinetic and computational study. J Biol Chem 286: 18048–18055, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Novoselov SV, Kim HY, Hua D, Lee BC, Astle CM, Harrison DE, Friguet B, Moustafa ME, Carlson BA, Hatfield DL, and Gladyshev VN. Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice. Antioxid Redox Signal 12: 829–838, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pader I, Sengupta R, Cebula M, Xu J, Lundberg JO, Holmgren A, Johansson K, and Arner ES. Thioredoxin-related protein of 14 kDa is an efficient L-cystine reductase and S-denitrosylase. Proc Natl Acad Sci U S A 111: 6964–6969, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Peralta D, Bronowska AK, Morgan B, Doka E, Van Laer K, Nagy P, Grater F, and Dick TP. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol 11: 156–163, 2015 [DOI] [PubMed] [Google Scholar]
- 95. Peter Guengerich F and Avadhani NG.. Roles of cytochrome P450 in metabolism of ethanol and carcinogens. Adv Exp Med Biol 1032: 15–35, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pohlandt F. Cystine: a semi-essential amino acid in the newborn infant. Acta Paediatr Scand 63: 801–804, 1974 [DOI] [PubMed] [Google Scholar]
- 97. Ponsero AJ, Igbaria A, Darch MA, Miled S, Outten CE, Winther JR, Palais G, D'Autreaux B, Delaunay-Moisan A, and Toledano MB. Endoplasmic reticulum transport of glutathione by Sec61 is regulated by Ero1 and Bip. Mol Cell 67: 962–973.e5, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Prigge JR, Coppo L, Martin SS, Ogata F, Miller CG, Bruschwein MD, Orlicky DJ, Shearn CT, Kundert JA, Lytchier J, Herr AE, Mattsson A, Taylor MP, Gustafsson TN, Arner ESJ, Holmgren A, and Schmidt EE. Hepatocyte hyperproliferation upon liver-specific co-disruption of thioredoxin-1, thioredoxin reductase-1, and glutathione reductase. Cell Rep 19: 2771–2781, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ramachandran A and Jaeschke H. Acetaminophen hepatotoxicity. Semin Liver Dis 39: 221–234, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. This reference has been deleted
- 101. Rao AM, Drake MR, and Stipanuk MH. Role of the transsulfuration pathway and of gamma-cystathionase activity in the formation of cysteine and sulfate from methionine in rat hepatocytes. J Nutr 120: 837–845, 1990 [DOI] [PubMed] [Google Scholar]
- 102. Rhee SG, Chae HZ, and Kim K. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic Biol Med 38: 1543–1552, 2005 [DOI] [PubMed] [Google Scholar]
- 103. Richman PG and Meister A. Regulation of gamma-glutamyl-cysteine synthetase by nonallosteric feedback inhibition by glutathione. J Biol Chem 250: 1422–1426, 1975 [PubMed] [Google Scholar]
- 104. Roggenbeck BA, Leslie EM, Walk ST, and Schmidt EE. Redox metabolism of ingested arsenic: integrated activities of microbiome and host on toxicological outcomes. Curr Opin Toxicol 13: 90–98, 2019 [Google Scholar]
- 105. Saudubray JM and Garcia-Cazorla A. An overview of inborn errors of metabolism affecting the brain: from neurodevelopment to neurodegenerative disorders. Dialogues Clin Neurosci 20: 301–325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Scaglia F and Blau N. Disorders of folate metabolism and transport. In: Physician's Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Diseases, edited by Blau N, Duran M, Gibson KM, and Dionisi Vici C. Berlin: Springer, 2014, pp. 167–178 [Google Scholar]
- 107. Schmidt EE. Interplay between cytosolic disulfide reductase systems and the Nrf2/Keap1 pathway. Biochem Soc Trans 43: 632–638, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seelig GF, Simondsen RP, and Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem 259: 9345–9347, 1984 [PubMed] [Google Scholar]
- 109. Siegrist J and Sies H. Disturbed redox homeostasis in oxidative distress: a molecular link from chronic psychosocial work stress to coronary heart disease? Circ Res 121: 103–105, 2017 [DOI] [PubMed] [Google Scholar]
- 110. Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol 82: 291–295, 1997 [DOI] [PubMed] [Google Scholar]
- 111. Sies H. Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289: 8735–8741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol 4: 180–183, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sies H, Berndt C, and Jones DP. Oxidative stress. Annu Rev Biochem 86: 715–748, 2017 [DOI] [PubMed] [Google Scholar]
- 114. Singh S, Padovani D, Leslie RA, Chiku T, and Banerjee R. Relative contributions of cystathionine beta-synthase and gamma-cystathionase to H2S biogenesis via alternative trans-sulfuration reactions. J Biol Chem 284: 22457–22466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Gruning NM, Kruger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, and Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc 90: 927–963, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Stipanuk MH. Metabolism of sulfur-containing amino acids. Annu Rev Nutr 6: 179–209, 1986 [DOI] [PubMed] [Google Scholar]
- 117. Stipanuk MH. Role of the liver in regulation of body cysteine and taurine levels: a brief review. Neurochem Res 29: 105–110, 2004 [DOI] [PubMed] [Google Scholar]
- 118. Stipanuk MH. Sulfur amino acid metabolism: pathways for production and removal of homocysteine and cysteine. Annu Rev Nutr 24: 539–577, 2004 [DOI] [PubMed] [Google Scholar]
- 119. Stipanuk MH, Dominy JE Jr., Lee JI, and Coloso RM.. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136: 1652S–1659S, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Stipanuk MH and Ueki I. Dealing with methionine/homocysteine sulfur: cysteine metabolism to taurine and inorganic sulfur. J Inherit Metab Dis 34: 17–32, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stipanuk MH, Ueki I, Dominy JE Jr., Simmons CR, and Hirschberger LL.. Cysteine dioxygenase: a robust system for regulation of cellular cysteine levels. Amino Acids 37: 55–63, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Suzuki T and Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem 292: 16817–16824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tarrago L and Gladyshev VN. Recharging oxidative protein repair: catalysis by methionine sulfoxide reductases towards their amino acid, protein, and model substrates. Biochemistry (Mosc) 77: 1097–1107, 2012 [DOI] [PubMed] [Google Scholar]
- 124. Tate SS, Grau EM, and Meister A. Conversion of glutathione to glutathione disulfide by cell membrane-bound oxidase activity. Proc Natl Acad Sci U S A 76: 2715–2719, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, and Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 88(Pt B): 108–146, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Thorpe C, Hoober KL, Raje S, Glynn NM, Burnside J, Turi GK, and Coppock DL. Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch Biochem Biophys 405: 1–12, 2002 [DOI] [PubMed] [Google Scholar]
- 127. Toppo S, Flohe L, Ursini F, Vanin S, and Maiorino M. Catalytic mechanisms and specificities of glutathione peroxidases: variations of a basic scheme. Biochim Biophys Acta 1790: 1486–1500, 2009 [DOI] [PubMed] [Google Scholar]
- 128. Torresano L, Nuevo-Tapioles C, Santacatterina F, and Cuezva JM. Metabolic reprogramming and disease progression in cancer patients. Biochim Biophys Acta Mol Basis Dis 1866: 165721, 2020 [DOI] [PubMed] [Google Scholar]
- 129. Turell L, Radi R, and Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radic Biol Med 65: 244–253, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ueki I, Roman HB, Hirschberger LL, Junior C, and Stipanuk MH. Extrahepatic tissues compensate for loss of hepatic taurine synthesis in mice with liver-specific knockout of cysteine dioxygenase. Am J Physiol Endocrinol Metab 302: E1292–E1299, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ueki I, Roman HB, Valli A, Fieselmann K, Lam J, Peters R, Hirschberger LL, and Stipanuk MH. Knockout of the murine cysteine dioxygenase gene results in severe impairment in ability to synthesize taurine and an increased catabolism of cysteine to hydrogen sulfide. Am J Physiol Endocrinol Metab 301: E668–E684, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Ueland PM, Mansoor MA, Guttormsen AB, Muller F, Aukrust P, Refsum H, and Svardal AM. Reduced, oxidized and protein-bound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status—a possible element of the extracellular antioxidant defense system. J Nutr 126: 1281S–1284S, 1996 [DOI] [PubMed] [Google Scholar]
- 133. Venkatachalam KV. Human 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase: biochemistry, molecular biology and genetic deficiency. IUBMB Life 55: 1–11, 2003 [DOI] [PubMed] [Google Scholar]
- 134. Wardman P and Candeias LP. Fenton chemistry: an introduction. Radiat Res 145: 523–531, 1996 [PubMed] [Google Scholar]
- 135. Wedmann R, Onderka C, Wei S, Szijarto IA, Miljkovic JL, Mitrovic A, Lange M, Savitsky S, Yadav PK, Torregrossa R, Harrer EG, Harrer T, Ishii I, Gollasch M, Wood ME, Galardon E, Xian M, Whiteman M, Banerjee R, and Filipovic MR. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem Sci 7: 3414–3426, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Winterbourn CC. The biological chemistry of hydrogen peroxide. Methods Enzymol 528: 3–25, 2013 [DOI] [PubMed] [Google Scholar]
- 137. Winterbourn CC and Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45: 549–561, 2008 [DOI] [PubMed] [Google Scholar]
- 138. Winterbourn CC and Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med 27: 322–328, 1999 [DOI] [PubMed] [Google Scholar]
- 139. Woo HA, Chae HZ, Hwang SC, Yang KS, Kang SW, Kim K, and Rhee SG. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 300: 653–656, 2003 [DOI] [PubMed] [Google Scholar]
- 140. Wood ZA, Poole LB, and Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 300: 650–653, 2003 [DOI] [PubMed] [Google Scholar]
- 141. Ye C, Sutter BM, Wang Y, Kuang Z, and Tu BP. A metabolic function for phospholipid and histone methylation. Mol Cell 66: 180–193.e8, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zavadakova P, Fowler B, Suormala T, Novotna Z, Mueller P, Hennermann JB, Zeman J, Vilaseca MA, Vilarinho L, Gutsche S, Wilichowski E, Horneff G, and Kozich V. cblE type of homocystinuria due to methionine synthase reductase deficiency: functional correction by minigene expression. Hum Mutat 25: 239–247, 2005 [DOI] [PubMed] [Google Scholar]
- 143. Zhang H, Limphong P, Pieper J, Liu Q, Rodesch CK, Christians E, and Benjamin IJ. Glutathione-dependent reductive stress triggers mitochondrial oxidation and cytotoxicity. FASEB J 26: 1442–1451, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]