Abstract
Chagas disease, caused by the parasite Trypanosoma cruzi, affects millions of people in South America. The current treatments are limited, have severe side effects, and are only partially effective. Drug repositioning, defined as finding new indications for already approved drugs, has the potential to provide new therapeutic options for Chagas. In this work, we conducted a structure-based drug repositioning approach with over 130,000 3D protein structures to identify drugs that bind therapeutic Chagas targets and thus represent potential new Chagas treatments. The screening yielded over 500 molecules as hits, out of which 38 drugs were prioritized following a rigorous filtering process. About half of the latter were already known to have trypanocidal activity, while the others are novel to Chagas disease. Three of the new drug candidates—ciprofloxacin, naproxen, and folic acid—showed a growth inhibitory activity in the micromolar range when tested ex vivo on T. cruzi trypomastigotes, validating the prediction. We show that our drug repositioning approach is able to pinpoint relevant drug candidates at a fraction of the time and cost of a conventional screening. Furthermore, our results demonstrate the power and potential of structure-based drug repositioning in the context of neglected tropical diseases where the pharmaceutical industry has little financial interest in the development of new drugs.
Keywords: Chagas, Trypanosoma cruzi, drug repositioning, structural screening, non-covalent interactions
1. Introduction
Chagas disease, also known as American trypanosomiasis, is a life-threatening infection caused by the protozoan parasite Trypanosoma cruzi [1]. According to the World Health Organization (WHO), about 7,000,000 people worldwide are afflicted with Chagas disease. Most cases of Chagas disease occur in South America, mostly in rural areas, with the infection being endemic in 21 countries [2]. The parasite is often transmitted via Triatomine insects. The infection occurs when the insect bites and subsequently defecates in the bite, allowing T. cruzi to enter the bloodstream. Moreover, T. cruzi can be congenitally transmitted from a pregnant mother to her baby, via blood transfusion, organ transplantation, or even due to laboratory accidents [1].
The acute phase of the disease occurs during the first few months after infection. T. cruzi propagates in the bloodstream, which produces mild symptoms, such as a skin lesion at the infection site, headache, fever, and muscle aches [3]. During the chronic phase of infection, the parasite lodges itself mainly in digestive and cardiac tissues. During this phase, about 30% of patients suffer from cardiac issues and 10% suffer from digestive or neurological issues, which can be fatal [2]. Fatality is frequently caused by Chronic Chagas Cardiomyopathy, which is the weakening of the heart muscles due to the parasite invasion.
Currently, there are only two drugs on the market for Chagas disease: Benznidazole works via inducing reductive stress, whereas Nifurtimox causes the generation of free radicals. Both drugs cause the parasite to be vanquished within 60–90 days. However, they are only effective in the predominantly asymptotic acute phase of the disease [4]. Once the disease reaches the chronic stage, there is not much that can be done. Furthermore, both drugs produce severe side effects in over 40% of patients and are contraindicated for use in pregnancy, reducing their applicability. Nifurtimox has severe side effects related to the nervous system, including depression, anorexia, neuropathy, insomnia, headache, and vomiting. On the other hand, Benznidazole has severe toxicities related to skin hypersensitivity, such as dermatitis and severe symptoms like depression of bone marrow, thrombocytopenic purpura, and agranulocytosis [5]. Due to the unspecific mechanism of action, the severe side effects, and the limited efficacy of the current chemotherapeutic options, there is a need for improved drugs with targeted action and less severe side effects.
The cost for pharmaceutical companies to research, develop, test, and bring a new drug to market is about $2.6 billion and takes about 10–15 years [6]. Drug repositioning, sometimes referred to as drug repurposing, is the utilization of already approved or experimental drugs for a novel indication [7]. The risks and development costs are reduced as there is already a wealth of knowledge available for approved and experimental drugs, such as safety, adsorption, distribution, metabolism, excretion, and other biological data, as well as clinical data in some cases [7]. In fact, about 60% of all drugs, both approved and experimental, have been tested for more than one disease [8]. The need for more effective and less toxic drugs coupled with the low commercial interest of pharmaceutical companies makes Chagas disease the perfect case for drug repurposing.
Several studies have reported repositioning candidates for Chagas with promising trypanocidal effects. Examples are Amiodarone, which is actually used as a Class III anti-arrhythmic agent [9]; Trimetrexate, an antifolate drug used against Pneumocystis carinii infection in patients with Acquired Immune Deficiency Syndrome (AIDS) [10]; and, most relevant, Posaconazole and Ravuconazole, which entered phase II clinical trials. Unfortunately, the latter showed poor results compared to Benznidazole [11,12]. Still, combination therapy could lead to better results [13]. Nowadays, with the exponential growth of structural data, it is possible to exploit drug repositioning at a structural level and to screen vast amounts of drug–target interactions to predict polypharmacological potential and repositioning opportunities [14]. For instance, Haupt et al. explored shared binding sites between Chagas targets and other proteins to identify novel drugs for the treatment of Chagas disease. Using their approach known as Target Hopping, they predicted that the antiviral Foscarnet would inhibit the target Farnesyl Pyrophosphate Synthase (FPPS) in T. cruzi [15]. In a more recent study, a virtual screening approach combining classical docking with protein–ligand interaction profiling identified drug repositioning candidates against T. cruzi infection. Nilotinib, Glipizide, Glyburide, and Gliquidone were predicted to bind to the Chagas target Dihydrofolate Reductase-Thymidylate Synthase (TcDHFR-TS) with high affinity. They were tested on T. cruzi epimastigotes, where they showed a growth inhibitory activity in the micromolar range, making them potential lead compounds in the development of new treatments for Chagas disease [16].
Over time, multiple T. cruzi enzymes have been highlighted as crucial therapeutic targets [15,17,18]. With the aim to identify novel repositioning candidates for Chagas disease, we explored the wide space of FDA approved drugs using a novel hybrid structure/knowledge-based repositioning method presented in this work.
2. Results
2.1. Identification of Relevant Chagas Targets and Their Structural Data
In the case of Chagas disease, a “good” target is a protein that is found in T. cruzi and not in humans and is important to parasite survival, or, alternatively, a T. cruzi protein that is different enough from its human homolog to reduce off target effects. It is important to consider that the current chemotherapeutic options for Chagas—nifurtimox and benznidazole—do not act through a primary target but kill the parasite by generating free radicals and inducing reductive stress, respectively [4]. To find a drug with a more focused mechanism of action, a list of drugable targets first had to be established.
As the starting point of this work, a set of 20 Chagas targets was assessed. Structural data was available for 16 targets (see Table 1). The targets were evaluated based on their dependability as a Chagas target. The seven targets listed above the bold line in Table 1 have been thoroughly researched and there is a high confidence that their modulation will produce the desired effect in T. cruzi, while the nine targets below the line have at least been researched as T. cruzi targets.
Table 1.
T. cruzi targets used as input for the computational screening. The targets above the bold line have been thoroughly researched and there is a high confidence that modulating these targets will produce the desired effect in T. cruzi. The targets below the line have been at least researched for being involved in T. cruzi’s survival. For each target, the target name, the specific binding site (in case of a multi-binding site target), the enzyme class, the PDB ID of the available structures, and the binding ligand are indicated. An independent screening was conducted for each target binding site. The last column on the right indicates the number of hit complexes predicted for each screening.
| Target | Binding Site | Enzyme Class | PDB ID | Ligand Name | Ligand Type | Nr. Hits | |
|---|---|---|---|---|---|---|---|
| 1 | Bifunctional DHFR-TS [19] | DHFR site | Oxidoreductase | 3IRN | Cycloguanil | Inhibitor | 43 |
| 3CLB | Trimetrexate | Inhibitor | |||||
| 3CL9 | Methotrexate | Inhibitor | |||||
| 3HBB | Trimetrexate | Inhibitor | |||||
| 3IRM | Cycloguanil | Inhibitor | |||||
| TS site | Transferase | 3CL9 | DUMP | substrate | 7 | ||
| 2 | Cruzipain [20] | unspecified | Transferase | 2OZ2 | K11777 | Inhibitor | 15 |
| 3LXS | WRR483 | Inhibitor | |||||
| 3 | FPPS [21] | allylic site | Transferase | 3IBA | Zoledronate | Inhibitor | 20 |
| 1YHL | Risedronate | Inhibitor | |||||
| 1YHM | Alendronate | Inhibitor | |||||
| 3ICK | Minodronate | Inhibitor | |||||
| homoallylic site | Transferase | 1YHM | Isopentyl Pyrophosphate | Substrate | 8 | ||
| 3ICK | Isopentyl Pyrophosphate | Substrate | |||||
| 3IBA | Isopentyl Pyrophosphate | Substrate | |||||
| 4 | GAPDH [22] | active site | Oxidoreductase | 1QXS | 1,3-bisphospho-d-glyceric acid | Product | 18 |
| 1K3T | Chalepin | Inhibitor | |||||
| covalent site | Oxidoreductase | 3IDS | Iodoacetamide | Inhibitor | 24 | ||
| 5 | Lanosterol Demethylase [23] | unspecified | Oxidoreductase | 5AJR | VT-1161 | Inhibitor | 69 |
| 3ZG3 | UDD | Inhibitor | |||||
| 3ZG2 | UDO | Inhibitor | |||||
| 3KSW | VNF | Inhibitor | |||||
| 2WX2 | Fluconazole | Inhibitor | |||||
| 3KHM | Fluconazole | Inhibitor | |||||
| 2WUZ | Fluconazole | Inhibitor | |||||
| 3K1O | Posaconazole | Inhibitor | |||||
| 6 | trans-sialidase [24] | acceptor site | Hydrolase | 1MS9 | Beta-lactose | Substrate | 20 |
| 1MS0 | Beta-lactose | Substrate | |||||
| sialic acid site | Hydrolase | 1MS0 | DANA | Inhibitor | 12 | ||
| 1S0J | Methylumbelliferyl Sialic Acid | Substrate | |||||
| 1S0I | Lactose Sialic Acid | Substrate | |||||
| 7 | Trypanothione Reductase [25] | unspecified | Oxidoreductase | 1GXF | Quinacrine Mustard | Inhibitor | 13 |
| 1BZL | Trypanothione | Substrate | |||||
| 8 | B Cell Mitogen [24] | unspecified | Isomerase | 1W61 | Pyrrole-2-Carboxylic acid | Substrate | 10 |
| 9 | Dihydroorate Dehydrogenase [26] | unspecified | Oxidoreductase | 2E6A | Orotate | Product | 16 |
| 2E6D | Fumarate | Substrate | |||||
| 2E68 | Dihydroorotate | Substrate | |||||
| 2DJL | Succinate | Product | |||||
| 2E6F | Oxonate | Inhibitor | |||||
| 10 | Glucose 6-phosphate dehydrogenase [18] | unspecified | Oxidoreductase | 6D24 | Beta-Glucose-6 Phosphate | Substrate | 2 |
| 5AQ1 | Beta-Glucose-6 Phosphate | Substrate | |||||
| 11 | HGPRT [27] | PRPP site | Transferase | 1TC2 | PRPP | Substrate | 0 |
| purine site | Transferase | 1TC2 | 7HPP | Inhibitor | 42 | ||
| 1P19 | Inosinic Acid | Product | |||||
| 1TC1 | Formycin B | Product | |||||
| 12 | Old Yellow Enzyme [28] | unspecified | Oxidoreductase | 3ATZ | Hydroxybenzaldehyde | Substrate | 34 |
| 13 | Pteridine Reductase [29] | unspecified | Oxidoreductase | 1MXF | Methotrexate | Inhibitor | 26 |
| 1MXH | Dihydrofolic Acid | Substrate | |||||
| 14 | Spermidine Synthase [30] | dcSAM site | Transferase | 5B1S | dcSAM | Substrate | 6 |
| 4YUW | dcSAM | Substrate | |||||
| putrescine site | Transferase | 5B1S | 2-(2-fluorophenyl)ethanamine | Inhibitor | 30 | ||
| 4YUW | trans-4-methylcyclohexanamine | Inhibitor | |||||
| 15 | Squalene Synthase [17] | unspecified | Transferase | 3WSB | SQ109 | Inhibitor | 97 |
| 3WCA | Farnesyl Thiopyrophosphate | Substrate | |||||
| 3WCB | BPH1237 | Inhibitor | |||||
| 3WCC | E5700 | Inhibitor | |||||
| 3WCE | ER119884 | Inhibitor | |||||
| 3WCG | BPH1344 | Inhibitor | |||||
| 16 | UDP-galactapyranose mutase [31] | unspecified | Isomerase | 4DSH | UDP | Substrate | 11 |
| 4DSG | UDP | Substrate |
As for any other structural approach, the availability of 3D structural data of proteins and ligands is a must. Currently, the Protein Data Bank (PDB) holds more than 169,000 structures, with a current annual growth of nearly 12,000 entries [32]. It is estimated to cover the vast majority of the known drug targets (about 92%) [33], and most of the structures (more than 60%) are in complex with biologically relevant ligands [34]. Most of the Chagas targets have at least one structure with a biologically relevant ligand available in the PDB (see Table 1), with the exception of cyclic nucleotide specific phosphodiesterase, Dihydrolipyl dehydrogenase, Ribose-5-phosphate dehydrogenase, and Triosephosphate Isomerase. Interestingly, only five structures of dihydroorate contained relevant ligands, although there were 58 structures available. The other complex structures involved cofactors, molecules from the buffer solution, or molecular fragments.
2.2. In Silico Screening
We analyzed drug interaction pattern similarities between binding sites of the query complexes and over 130,000 protein structures. The identified targets comprised 22 binding sites in complex with several relevant ligands (Table 1). Each binding site accounted for an independent screening. The screening output was a list of hit complexes with high interaction pattern similarity ranked by p-value and aggregated by ligand. In total, there were 523 hits across the 22 screenings, ranging from 0 to 97 hits per screening with a mean of 23. There was a modest positive correlation between the number of query structures and the number of hits, as indicated by a Pearson correlation coefficient of 0.6. Several compounds yielded a hit in multiple screenings, while others were unique to only one screening. Most of the frequent hitters were not relevant to Chagas disease, such as glutathione, citric acid, and different amino acids. On the other hand, some screenings produced hit numbers well above the average, such as the screenings of squalene synthase with 96 hits and lanosterol demethylase with 69 hits.
To get a first impression of the significance of the screening predictions, we checked the Binding DB [35] for binding evidence. Affinity data were available for only three drug target pairs. Of these three, the binding of Risedronate to the target FPPS was also predicted by our screening. In contrast, there was binding evidence for Etravirine and the target Cruzipain as well as Chlorpromazine and Trypanthione reductase, but these drug target pairs were not a hit in the screenings.
Following several filtering steps (see Methods section), the primary list of 512 compounds was narrowed down to 38 high-priority predictions (see Table 2). These compounds were selected as our top repositioning candidates for Chagas disease.
Table 2.
Computational screening hits predicted to bind Chagas targets. Top 38 hits from the screening sorted by novelty with regard to trypanocidal activity, where ? indicates unknown activity, + indicates indirect evidence of activity, and ++ indicates direct evidence of activity. Within each of the previous classifications, the hits are sorted by screening p-value and the original indication is shown. The ✔ represents positive visual inspection, meaning high interaction pattern similarity between query and hit.
| Drug | Predicted Target | p-Value | Current Indication | Visual Inspection |
Trypan. Activity |
Reference | |
|---|---|---|---|---|---|---|---|
| 1 | Glutathione | Dihydroorate | 3.80 × 10−6 | Antioxidant | ✔ | ? | |
| 2 | Naproxen | FPPS Homoallylic | 6.46 × 10−6 | Anti-inflammatory | ✔ | ? | |
| 3 | Amphetamine | Spermidine Synthase Putrescine | 1.14 × 10−5 | Attention deficit/Hyperactivity | ✔ | ? | |
| 4 | Folic acid | DHFR | 2.66 × 10−5 | Megaloblastic Anemia | ✔ | ? | |
| 5 | Sapropterin | Pteridine Reductase | 2.66 × 10−5 | Phenylketonuria | ✔ | ? | |
| 6 | Clioquinol | Trypanothione Reductase | 2.66 × 10−5 | antifungal | ✔ | ? | |
| 7 | Celecoxib | DHFR | 3.04 × 10−5 | Anti-inflammatory | ✔ | ? | |
| 8 | Leucovorin | DHFR | 4.18 × 10−5 | Toxicity of Pyrimethamine | ✔ | ? | |
| 9 | Theophylline | HGPRT purine | 7.22 × 10−5 | Asthma | ✔ | ? | |
| 10 | Fosfomycin | FPPS allylic | 1.52 × 10−4 | Antibiotic | ✔ | ? | |
| 11 | Ticagrelor | Pteridine Reductase | 1.90 × 10−4 | Platelet aggregation inhibitor | ✔ | ? | |
| 12 | Fludarabine | HGPT purine | 4.18 × 10−4 | Cancer | ✔ | ? | |
| 13 | Varenicline | Spermidine Synthase Putrescine | 4.45 × 10−4 | Nicotine Addiction | ✔ | ? | |
| 14 | Progesterone | Old Yellow | 3.80 × 10−6 | Hormone | ✔ | + | Schuster et al. [36] |
| 15 | Pemetrexed | Pteridine Reductase | 3.80 × 10−6 | Cancer | ✔ | + | Sienkiewicz et al. [37] |
| 16 | Ciprofloxacin | Trans-sialidase sailic | 9.51 × 10−5 | Antibiotic | ✔ | + | Hiltensperger et al. [38] |
| 17 | Aunomycin | Trypanothione Reductase | 1.29 × 10−4 | Cancer | ✔ | + | Andrews et al. [39] |
| 18 | Pentoxifylline | Trypanothione Reductase | 2.24 × 10−4 | Muscle Pain Reliever | ✔ | + | Villa-Pereira et al. [40] |
| 19 | Pyrimethamine | DHFR | 3.80 × 10−6 | Toxoplasmosis | ✔ | ++ | Gilbert et al. [19] |
| 20 | Trimethoprim | DHFR | 3.80 × 10−6 | Antibiotic | ✔ | ++ | Gilbert et al. [19] |
| 21 | Risedronate | FPPS Allylic | 3.80 × 10−6 | Osteoporosis | ✔ | ++ | Huang et al. [41] |
| 22 | Triamterene | Pteridine Reductase | 3.80 × 10−6 | Diuretic | ✔ | ++ | Planer et al. [13] |
| 23 | Tioguanine | Pteridine Reductase | 7.61 × 10−6 | Cancer | ✔ | ++ | fernandes et al. [42] |
| 24 | Nicotinamide | Spermidine Synthase Putrescine | 7.61 × 10−6 | Pellagra | ✔ | ++ | Soares et al. [43] |
| 25 | Zidovudine | Squalene Synthase | 1.14 × 10−5 | HIV | ✔ | ++ | Nakajima-Shimada et al. [44] |
| 26 | Zanamivir | Trans-sialidase sailic | 1.14 × 10−5 | Antiviral | ✔ | ++ | Kashif et al. [45] |
| 27 | Rimantadine | DHFR | 1.54 × 10−5 | Anti-viral | ✔ | ++ | Kelly et al. [46] |
| 28 | Quinine | DHFR | 1.90 × 10−5 | Malaria | ✔ | ++ | Ceole et al. [47] |
| 29 | Benzoic acid | Galactopyranose Mutase | 1.90 × 10−5 | Antifungal | ++ | Neres et al. [48] | |
| 30 | Imatinib | Cruzipain | 6.84 × 10−5 | Cancer | ✔ | ++ | Simoes-Silva et al. [49] |
| 31 | Isotretinoin | DHFR | 7.22 × 10−5 | Acne | ✔ | ++ | Reigada et al. [50] |
| 32 | Foscarnet | FPPS allylic | 7.61 × 10−5 | Antiviral | ✔ | ++ | Haupt et al. [15] |
| 33 | Paclitaxel | Trans-sailidase Acceptor | 8.75 × 10−5 | Cancer | ++ | Baum et al. [51] | |
| 34 | Citalopram | Squalene Synthase | 9.13 × 10−5 | Antidepressant | ✔ | ++ | Jones et al. [52] |
| 35 | Thioridazine | Squalene Synthase | 1.48 × 10−4 | Antipsychotic | ✔ | ++ | Lo Presti et al. [53] |
| 36 | Memantine | Squalene Synthase | 2.51 × 10−4 | Alzheimer’s | ✔ | ++ | Damasceno et al. [54] |
| 37 | Saquinavir | Spermidine Synthase dcSAM | 2.70 × 10−4 | HIV | ✔ | ++ | Sangenito et al. [55] |
| 38 | Minocycline | Spermidine Synthase Putrescine | 3.65 × 10−4 | Antibiotic | ++ | Planer et al. [13] |
2.3. Characterization of the Hit Candidates
The 38 hits cover a diverse set of primary indications (see Table 2), such as osteoporosis, cancer, asthma, antifungal, antibiotics, and antivirals, among others. Surprisingly, Pyrimethamine and Quinine are the only two drugs with a parasitic effect (Toxoplasmosis and Malaria, respectively) as primary indication. Independently of their primary indication, the hit candidates were classified according to their novelty for Chagas. According to literature evidence, 20 drugs have a direct evidence of Chagas activity(++), five drugs have rather an indirect link to Chagas(+), and the other 13 have no previous evidence(?), meaning they are fully novel to Chagas disease.
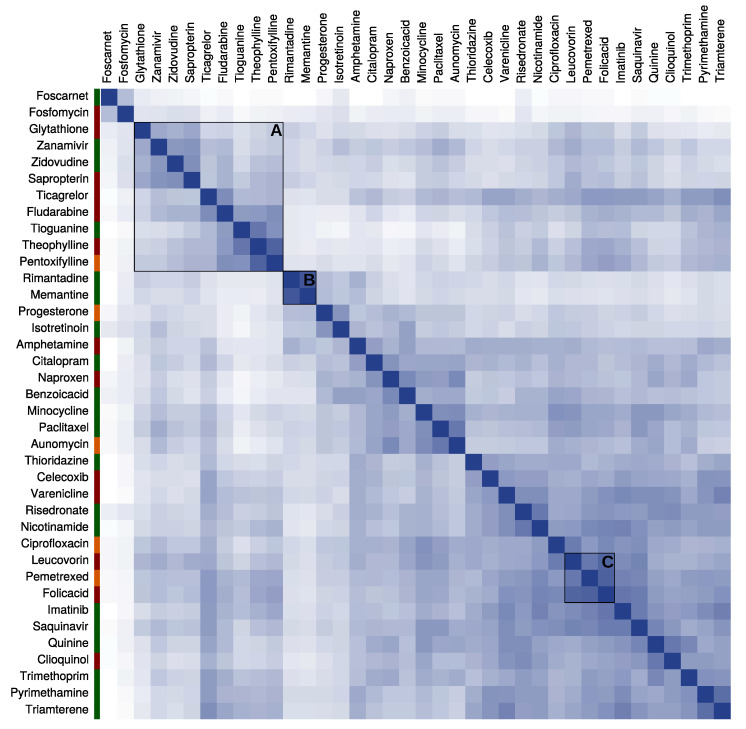
Moreover, the chemical space of the selected hit drugs was characterized to determine the scaffold diversity that the screening achieved. As shown in Figure 1, overall there is very little chemical similarity between the top hits, with only 1.5% of the similarity scores above the threshold of 0.7. In general, most hits have unique chemical profiles and therefore remain without cluster. Nonetheless, a few clusters can be spot in the similarity chart and are worth further exploration. For instance, there is a small cluster (cluster B) with the highest chemical similarity score (0.87) grouping the adamantane derivative rimantadine and memantine. Cluster C groups compounds derivative of folate, e.g., folic acid, pemetrexed, and leucovorin, with a mean similarity of 0.77 ± 0.29. In cluster A, there is a big group of nucleic acids and their analogs with a relatively high mean similarity of 0.62 ± 0.21. Although they all share similar core scaffolds, the rest of their chemical structure is very distinct from one another. Finally, the chart shows no clear relation between the chemical structure of the hits and the novelty of their trypanocidal activity (color tags along the left side of the chart). The hits cover a vast chemical space, regardless of whether they have a direct (green), indirect (orange), or no (red) trypanocidal evidence.
Figure 1.
Chemical space of the hit candidates. The heatmap shows the pairwise similarity of the chemical structures of the hits. The similarity scores range from 0 (low) to 1 (high) with a color scheme from white to blue, respectively. The color tags on the right indicate the novelty of the drug with regard to the trypanocidal activity: direct evidence (green), indirect evidence (orange), and no previous evidence (red). Some clusters of drugs with a relatively high chemical similarity are marked: (A) nucleic acid analogs, (B) adamantane analogs, and (C) folate analogs.
2.4. Inhibitory Effect of Selected Drugs in T. cruzi Epimastigotes and Trypomastigotes
From the list of high priority drug repositioning candidates, eight were selected for further experimental validation. The selection was based on the following criteria; novelty of Chagas activity (+ or ?), a reasonable interaction pattern similarity between query and hit (positive visual inspection), and availability at a low price.
The trypanocidal activity of the eight candidates was assessed in vitro for the epimastigote stage and ex vivo for the trypomastigote stage using the T. cruzi strains Ninoa and INC-5 (see Table 3). Of the compounds tested, ciprofloxacin, folic acid, and naproxen showed the greatest inhibitory activity against T. cruzi trypomastigotes, with IC50 values of 25.7 µM, 28.1 µM, and 58.5 µM for the Ninoa strain, and 21.3 µM, 21.5 µM, and 38.3 µM for the INC-5 strain, respectively. Moreover, the active drugs demonstrated relatively low cytotoxicity with CC50 (the concentration of the drug that causes the death of 50% of viable cells in the host) values of 7.7 × 1023 µM for ciprofloxacin, 2.5 × 1018 µM for naproxen, and 9.1 × 1017 µM for folic acid. Based on these results, the selectivity indices (CC50/IC50) were 2.9 × 1022, 4.2 × 1016, and 3.2 × 1016, respectively. While ciprofloxacin, folic acid, and naproxen had IC50 values in the same range as the standard Chagas disease medications nifurtimox and benznidazole, they exhibited lower cytotoxicity and higher selectivity in comparison. Yet, none of the drug repositioning candidates showed trypanocidal activity in the lower micromolar range against T. cruzi epimastigotes (see Table 3).
Table 3.
Inhibition of proliferation of Ninoa and INC-5 strains of T. cruzi, cytotoxicity and selectivity index of tested FDA-approved drugs compared to the known treatments with nifurtimox and benznidazole (positive controls).
| T.cruzi blood trypomastigote | Cytotoxicity | Selectivity Index | T.cruzi epimastigote | Cytotoxicity | Selectivity Index | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | IC50 (µM) | CC50 (µM) | CC50/IC50 | IC50 (µM) | CC50 (µM) | CC50/IC50 | ||||
| Ninoa | INC-5 | Ninoa | INC-5 | Ninoa | INC-5 | Ninoa | INC-5 | |||
| Ciprofloxacin | 25.7 ± 0.04 | 21.3 ± 0.09 | 7.7 × 1023 ± 0.31 | 3.0 × 1022 | 3.6 × 1022 | > 400 ± 0.17 | > 400 ± 0.17 | 7.7 × 1023 ± 0.31 | < 1.9 × 1021 | < 1.9 × 1021 |
| Folic Acid | 28.1 ± 0.01 | 21.5 ± 0.03 | 9.1 × 1017 ± 0.31 | 3.2 × 1016 | 4.2 × 1016 | > 400 ± 0.03 | > 400 ± 0.01 | 9.1 × 1017 ± 0.14 | < 2.3 × 1015 | < 2.3 × 1015 |
| Naproxen | 58.5 ± 0.07 | 38.3 ± 0.06 | 2.5 × 1018 ± 0.16 | 4.3 × 1016 | 6.5 × 1016 | > 400 ± 0.10 | > 400 ± 0.04 | 2.5 × 1018 ± 0.08 | < 6.3 × 1015 | < 6.3 × 1015 |
| Celecoxib | > 100 ± 0.06 | > 100 ± 0.15 | 1.2 × 1016 ± 0.19 | < 1.2 × 1014 | < 1.2 × 1014 | > 400 ± 0.13 | > 400 ± 0.02 | 1.2 × 1016 ± 0.32 | < 3.1 × 1015 | < 3.1 × 1015 |
| Glutathione | > 100 ± 0.08 | > 100 ± 0.05 | 1.7 × 1018 ± 0.07 | < 1.7 × 1014 | < 1.7 × 1014 | > 400 ± 0.10 | > 400 ± 0.17 | 1.7 × 1018 ± 0.42 | < 4.3 × 1015 | < 4.3 × 1015 |
| Leucovirin | > 100 ± 0.18 | > 100 ± 0.05 | 7.9 × 1023 ± 0.23 | < 7.9 × 1021 | < 7.9 × 1021 | > 400 ± 0.12 | > 400 ± 0.08 | 7.9 × 1023 ± 0.17 | < 2.0 × 1021 | < 2.0 × 1021 |
| Pentoxyfiline | > 100 ± 0.25 | > 100 ± 0.12 | 7.9 × 1017 ± 0.05 | < 7.9 × 1015 | < 7.9 × 1015 | > 400 ± 0.14 | > 400 ± 0.16 | 7.9 × 1017 ± 0.02 | < 2.0 × 1015 | < 2.0 × 1015 |
| Theophyline | > 100 ± 0.18 | > 100 ± 0.15 | 8.3 × 1035 ± 0.14 | < 8.3 × 1033 | < 8.3 × 1033 | > 400 ± 0.21 | > 400 ± 0.03 | 8.3 × 1035 ± 0.08 | < 2.1 × 1033 | < 2.1 × 1033 |
| Nifurtimox | 167.1 ± 0.03 | 115.2 ± 0.17 | 164.2 ± 0.25 | 0.10 | 1.42 | 7.09 ± 0.12 | 6.47 ± 0.42 | 164.2 ± 0.08 | 23.2 | 25.4 |
| Benznidazole | 156.0 ± 0.11 | 130.6 ± 0.08 | 133.9 ± 0.06 | 0.85 | 1.02 | 30.3 ± 0.03 | 19.9 ± 0.23 | 133.9 ± 0.71 | 4.42 | 6.74 |
2.5. In Vivo Trypanocidal Activity of the Drug Repositioning Candidates
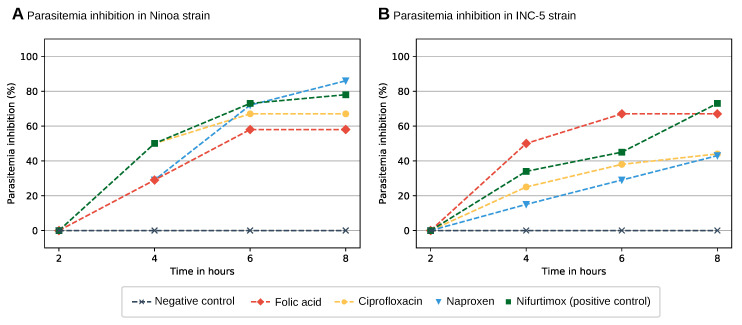
As ciprofloxacin, naproxen, and folic acid showed a growth inhibitory activity in the micromolar range against T. cruzi, they were tested for parasitemia inhibition in vivo. Mice were infected with blood trypomastigotes and at day 13 post-infection, a single dose (at 100 mg/kg body weight) of the drugs ciprofloxacin, nifurtimox, or folic acid was orally administered. Parasitemia was monitored at 2, 4, 6, and 8 h after administration (see Figure 2).
Figure 2.
Parasitemia inhibition (%) of T. cruzi NINOA (A) and INC-5 (B) strains by the tested FDA-approved drugs during 8 h after administration. The plot shows parasitemia inhibition by the drugs ciprofloxacin (yellow line), naproxen (blue line), and folic acid (red line) at 2, 4, 6, and 8 h after administration. Drugs at a single dose of 100 mg/kg body weight were orally administered at day 13 post-infection when the infected mice reached an average parasitemia of 5 × 106 parasitemia/mL. Infected mice treated with nifurtimox (green line) and infected non-treated mice (gray line) were used as positive and negative controls, respectively.
All drugs tested demonstrated inhibition of parasitemia. Eight hours after administration to the mice infected with the Ninoa strain of T. cruzi, folic acid, ciprofloxacin, and naproxen showed parasitemia inhibition of 57.2%, 66.7%, and 85.8%, respectively, while the established Chagas treatment nifurtimox exhibited parasitemia inhibition of 77.8%. Interestingly, the trypanocidal activity of naproxen was significanclty higher than that shown by nifurtimox (p ≤ 0.05). In mice infected with the T. cruzi strain INC-5, folic acid, ciprofloxacin, and naproxen showed parasitemia inhibition of 66.7%, 43.8%, and 42.9%, respectively, eight hours after administration. In comparison, nifurtimox exhibited an inhibition of 72.3%. It is worth mentioning that folic acid showed significantly higher activity than nifurtimox against INC-5 strain at 4 and 6 h after drug administration (p≤ 0.05), although it was maintained at 8 h, it was lower than the reference drug. Overall, the drug repositioning candidates achieved parasitemia inhibition of T. cruzi trypomastigotes in vivo.
2.6. Characterization of the Validated Drugs
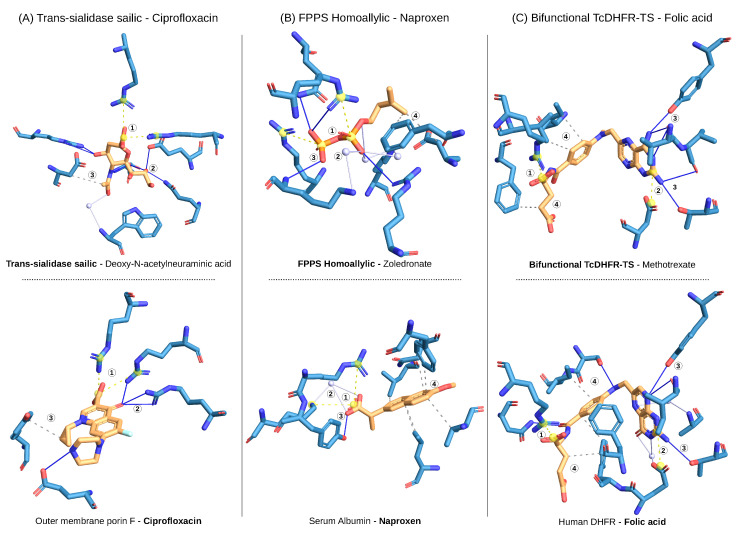
Ciprofloxacin, naproxen, and folic acid were identified as drug repositioning candidates for Chagas disease based on the tanimoto similarity score of the interaction fingerprints between the query and the hit complexes of the screening. In other terms, the screening identified a high non-covalent interaction pattern similarity between the drugs in complex with their original targets and the therapeutic Chagas targets in complex with their original binders. Figure 3A shows the query complex of the Chagas target trans-sialidase (sialic acid site) and its original binder deoxy-N-acetylneuraminic acid. Among the interactions that define the binding mode of the query, there is a clear set of interactions (a pattern) that are also present in the hit complex of ciprofloxacin and its original target outer membrane porin F. The pattern includes a pair of salt bridges (1), three hydrogen bonds (2), and one hydrophobic interaction within the same distance and angle.
Figure 3.
Non-covalent interaction patterns accounting for the repositioning predictions. The structure-based drug repositioning approach predicted that ciprofloxacin binds trans-sialidase (sialic acid site), naproxen binds FPPS (homoallylic site) binder, and folic acid binds TcDHFR. The repositioning is based on the similarity of the non-covalent interactions defining the binding mode of inhibitors (orange) to their targets (blue) between query (top) and hit (bottom) complexes. (A) The binding mode of ciprofloxacin to porin F (PDB ID: 4kra) is similar to the one of deoxy-N-acetylneuraminic acid to trans-sialidase (sialic acid site) (PDB ID: 1ms0). Both have in common (1) a double salt bridge (yellow dashed lines), (2) a triple set of hydrogen bonds (blue lines), and (3) a hydrophobic interaction (gray dashed lines). In the same way, (B) the binding mode of naproxen to serum albumin (PDB ID: 4ot2) is similar to the one of zoledronate to FPPS (PDB ID: 3iba) as they have (1) two salt bridges, (2) one water bridge (lightblue lines and sphere), and (3) one hydrogen bond in common. (C) The binding mode of folic acid to human DHFR (PDB ID: 1drf) is similar to the one of methotrexate to TcDHFR (PDB ID: 3cl9) with (1,2) two salt bridges, (3) a set of hydrogen bonds, and (4) a set of hydrophobic interactions.
The same applies to the repositioning of naproxen (see Figure 3B), where there is a common interaction pattern between FPPS (homoallylic site) with its binder zoledronate (query) and naproxen with its original target serum albumin (hit). In the case of folic acid, there is an explicit interaction pattern that is present both in the complex of TcDHFR with its binder methotrexate (query) and in the complex of folic acid with human DHFR (hit) (see Figure 3C). However, it should be noted that the protein targets are homologs and the residues that define the binding pocket are very similar.
3. Discussion
3.1. The Diversity of Chagas Targets and the Promiscuous Hits
Over the years, several T. cruzi enzymes have been identified as therapeutic targets (Table 1) and they cover different enzyme classes like hydrolases, isomerases, transferases, and oxidoreductases, with the majority of them being either transferases or oxidoreductases. Consistent with this tendency, the PDB mainly contains hydrolases, transferases, and oxidoreductases, which currently make up 38.9%, 30.4%, and 15.7% of the enzyme structures, respectively [32].
The binding of a molecule to two or more different targets is known as drug promiscuity [56,57]. In drug development, compound promiscuity is often regarded as an unfavorable property because drugs binding multiple targets are expected to cause side effects. However, promiscuity can also positively affect the efficacy of a drug, i.e., a drug binding several targets linked to the same disease will have an increased impact compared to a single-target modulator. Before prioritization of the screening results, several hit drugs were predicted to bind multiple targets. More surprisingly, some multi-target hits, such as amino acids, glycerol, glutathione, citric acid, and bile salt, did not even discriminate on enzyme class. There is a significant correlation between compound promiscuity and binding site similarity [58]. According to Gao et al., the number of ways a drug can interact with its target is limited to 1000 distinct interface types [59]. Therefore, the promiscuous binding of the hit drugs to several targets is not an exception but rather a frequent observation. The multi-target hits could also be directly related to the coincidence of targets among the same pathways (Supplementary Table S5). For instance, FPPS, lanosterol demethylase, and squalene synthase are all involved in ergosterol biosynthesis; DHFR and pteridine reductase are involved in folate metabolism; trypanothione reductase and spermidine synthase are involved in trypanothione metabolism; and finally, GADPH, G6PDH, and triosephosphate isomerase are involved in glycolysis.
Another reason for the promiscuous predictions might be unspecific and simple interaction patterns. For instance, it is known that hydrogen bonds and hydrophobic contacts occur more frequently than other interaction types and therefore lead to less specific predictions [60]. Many of the multi-target hits are very important substances for metabolism and other cellular functions [61,62]. As these ligands are involved in multiple pathways and bind several different enzymes, it makes sense that simple interactions are preferred.
3.2. Predicted Drugs with a Known Activity in Chagas Disease
More than half of the drugs predicted by the drug repositioning screening had a previous evidence of trypanocidal activity (Table 2). They serve as a good validation of the approach and demonstrate its ability to retrieve drugs with a known link to the disease. Nonetheless, the quality of evidence varies from case to case.
Risedronate, for example, is an osteoporosis medication that acts by inhibiting poly-isoprenoid biosynthesis. It has been shown to bind T. cruzi FPPS [41] and its trypanocidal activity against T. cruzi has been confirmed [63,64].
Pyrimethamine, on the other hand, has a defined trypanocidal activity with a proposed mechanism of action via the target predicted by the screening but in a different organism. It is an antiparasitic used to treat malaria by inhibiting the target DHFR, which is an important enzyme in folate metabolism and the production of the nucleic acid thymidine. The disruption of thymidine biosynthesis consequently interferes with DNA biosynthesis [19]. DHFR is also a well-known Chagas target and essential for the survival of T. cruzi. Several studies have already explored pyrimethamine in the context of trypanosoma diseases, African sleeping sickness (caused by Trypanosoma brucei), and Chagas disease. In fact, pyrimethamine has been shown to be a potent inhibitor of T. cruzi DHFR with a Ki of [19] and has been reported to inhibit T. cruzi growth in vitro with an EC50 of 3800 [13].
Another example is Zidovudine, an antiretroviral used in the treatment of HIV [65], which has an anti-trypanosomal activity known from phenotypic studies. Yet, the target and mechanism of action remain unknown. Nakajima et al. reported a growth inhibition of T. cruzi by zidovudine with an between 200 and 300 . However, they were unable to elucidate the mechanism of the trypanocidal activity. While T. cruzi has genes for reverse transcriptase, which are activated in several stages of its life cycle, zidovudine does not inhibit T. cruzi reverse transcriptase as it was initially thought [44]. This suggests that the activity of zidovudine relies on the inhibition of another enzyme. The prediction made in our screening proposes for the first time a mechanism of action for the trypanocidal activity of zidovudine. It was predicted to bind Squalene synthase (SQS) (see Table 2), which is relevant to T. cruzi survival due to its role in Ergosterol synthesis. Ergosterol is an essential membrane sterol in many trypanosomatid parasites that plays the same structural role as cholesterol in humans [17].
At a lower level, there are drugs with a weak evidence of indirect trypanocidal activity. For instance, Progesterone is a female steroidal hormone important in pregnancy with various medical applications, e.g., birth control, fertility treatments, and hormone replacement therapy [66]. It has not been shown to kill T. cruzi in vitro or in vivo, but there is indirect evidence of its potential effectiveness. It has been reported that female infected mice with high endogenous progesterone levels also show low parasite loads [36].
3.3. Predicted Drugs with a Novel Activity to Chagas
Three predicted drugs with no previous evidence of activity in Chagas disease were validated as inhibitors of T. cruzi proliferation: ciprofloxacin (a “normal” antibiotic), naproxen (an over the counter pain killer), and folic acid (a vitamin).
Ciprofloxacin is a broad-spectrum antibiotic of the semisynthetic fluoroquinolone class. Fluoroquinolones have two main bacterial targets: the type II topoisomerases DNA gyrase and DNA topoisomerase IV [67]. Type II topoisomerases introduce a transient double-strand break into DNA segments, pass an intact double strand through this break, and re-ligate the break to relax topological stress or generate supercoils [68]. Fluoroquinolones inhibit the ligase activity of bacterial type II topoisomerases, thereby blocking bacterial growth [69]. Several studies have reported the relevance of fluoroquinolones as parasitic inhibitors and suggested inhibition of DNA topoisomerases as mechanism of action [38,70,71,72]. In our screening, ciprofloxacin was predicted to bind trans-sialidase (see Figure 3), which catalyses the transfer of sialic acid from host glycoconjugates to acceptor molecules on the parasite surface and is fundamental for T. cruzi survival [73]. The prediction suggests for the first time that the antiparasitic effect of quinolones is caused by trans-sialidase inhibition. Moreover, Nenortas et al. have reported that ciprofloxacin is active against T. brucei in vitro with an of 52 [70]. We have now shown that ciprofloxacin is also a potent inhibitor of T. cruzi trypomastigotes with an of 21 (see Table 3) and blocks T. cruzi growth in vivo. Generally, ciprofloxacin is considered a relatively safe, well tolerated, and widely used drug in clinical practice. As for most other drugs on the market, several side effects have been reported that among others cause gastrointestinal, central nervous system, skin, cardiovascular, lymphatic, or nutritional issues. However, most of them are reversible and occur at a mild or moderate intensity (in about 94% of cases) [74]. The widespread use of ciprofloxacin has caused a remarkable increase in bacterial resistance to the drug, making it a less effective antibiotic [75]. For this reason, its repositioning to a parasitic indication would be sustainable and profitable
Naproxen is a bicyclic propionic acid derivative that has analgesic, anti-inflammatory, and antipyretic effects and is classified as non-steroidal anti-inflammatory drug (NSAID). Like most NSAIDs, naproxen acts via the inhibition of Cyclooxygenase (COX) isoforms I and II, which are involved in the synthesis of prostaglandins, prostacyclin, and thromboxane from arachidonic acid [76,77]. Our results show that naproxen is a potent inhibitor of T. cruzi trypomastigote (see Table 3). Furthermore, our screening predicted that naproxen binds T. cruzi farnesyl pyrophosphate synthase (TcFPPS) (see Figure 3). TcFPPS synthesises farnesyl pyrophosphate (FPP). Farnesylation is a post-translational modification required for membrane localization of many proteins, such as small GTPases. Therefore, the inhibition on TcFPPS disrupts various cellular functions [41]. This makes TcFPPS a well-known Chagas target and could explain the trypanocidal activity of naproxen. Although naproxen effectively inhibits T. cruzi proliferation, several aspects should be considered prior to its repositioning. The safety of NSAIDs with regards to cardiovascular events has been evaluated in a large number of retrospective and prospective clinical studies, all of which have reported cardiotoxicity [78]. Moreover, Cossentini et al. demonstrated that the gastric mucosal damage caused by the intake of Aspirin, a commonly used NSAID, facilitates T. cruzi infection by the oral route [79]. While this has not been tested particularly for naproxen, the side effects in the gastrointestinal tract and kidneys are a well-known disadvantage of NSAIDs [78]. Additionally, a comparison of the structures of trypanosomal and human FPPS revealed that their active site residues are highly conserved, which makes the design of parasite-specific drugs difficult [41].
Folic acid is the manufactured version of folate. As the human body is not able to produce folate on its own, folic acid is used as a dietary supplement and food fortification. In our screening, folic acid was predicted to bind TcDHFR. The repositioning has been proposed between the same protein among different species (from human to T. cruzi DHFR), meaning that the targets are expected to have similar binding sites and thus form similar non-covalent interactions with their ligands. Although the repositioning itself is not very surprising, we demonstrated the trypanocidal activity of folic acid in this study for the first time. However, there is no clear explanation for the trypanocidal activity of folic acid since it is a known substrate of the predicted target. Several studies have discussed the positive effect of folic acid intake in different diseases and conditions. Folate plays an essential role in the human body as a major coenzyme in one-carbon metabolism, including DNA synthesis (dTMP) and methylation [80]. Moreover, folate deficiency has been associated with a risk for several diseases, involving, among others [81], cancer [82,83,84], Alzheimer [85], hypertension [86], and some pregnancy and birth complications, such as megaloblastic anemia [87] or neural tube defects (NTDs) [88,89]. Moreover, it has been shown that the cell-mediated immunity is highly affected by folate deficiency [90]. In fact, the blastogenic response of T lymphocytes to certain mitogens is decreased in folate-deficient humans and animals, and the thymus is preferentially altered. In light of this, it might be that the trypanocidal effect of Folic acid is not due to Chagas target inhibition, but rather to a beneficial effect of the compound as a supplementary diet. In that sense, the intake of Folic acid might help to improve the immune system of the infected host, indirectly affecting the proliferation of T. cruzi. However, further studies are necessary to test this hypothesis.
3.4. Drugs Known to Bind Chagas Targets but not Retrieved by the Screening
Two confirmed binders in BindingDB [35] were not predicted by the screening: etravirine, which is an HIV reverse transcriptase inhibitor known to bind cruzipain, and chlorpromazine, an antipsychotic, promiscuous drug that is known to bind the Chagas target trypanothione reductase [91]. A simple explanation for the missing prediction is that a ligand can bind a protein with multiple different binding modes [92], and a PDB structure is only a snapshot of one of these binding modes. For both targets, there were only two query structures available, so it is possible that not all binding modes were captured and therefore the binding mode required to predict the binding of etravirine to cruzipain and chlorpromazine to trypanothione reductase was missing.
3.5. Drugs Effective against Trypomastigotes but not against Epimastigotes
During the life cycle of T. cruzi, the parasite experiences multiple changes in its morphology, metabolism, and gene expression, going from its epimastigote replicative stage in the insect to its pathogenic metacyclic trypomastigote form [93]. After penetration into the host cell, T. cruzi differentiates into the amastigote form and initiates the intracellular binary division. Finally, the amastigotes transform into trypomastigotes, which break open the host cell and enter the bloodstream. The trypomastigotes spread through the bloodstream to penetrate cells of different organs, where the cycle process is repeated [94,95].
The validated drugs in this study were potently effective against the trypomastigote but not the epimastigote form. This behavior can be explained by the morphological differences between the two stages of T. cruzi and has already been observed in previous studies [96,97,98]
Over time, it has been assumed that replicating epimastigotes present in the insect gut are not infectious to the mammalian host since only the epimastigote stage is susceptible to the innate immune system of mammals and can be killed by the complement system [99]. However, recent studies have remarked the relevance of treatments that also kill T. cruzi epimastigotes [94,100,101].
3.6. A Promising Approach for Neglected Diseases
Neglected tropical diseases (NTDs) are a group of bacterial, parasitic, viral, and fungal infections. As they are prevalent in many tropical and sub-tropical developing countries, where poverty is flagrant [102], they are diseases that have not only health but also socio-economic impact. However, they do not receive the attention they require and are generally less studied than other diseases.
Our structure-based drug repositioning approach has proved to be a viable option for the exploration of novel Chagas treatments. Despite being considered a neglected disease, there was sufficient data to conduct a screen for over 75% of targets and return positive results at a very high hit rate, which translates into low cost and time. This is a promising option for all rare and neglected diseases for which reliable treatment has not yet been found.
Structure-based approaches rely on the availability and quality of structural data. Notwithstanding the great amount of structural data available in the PDB, there is an inherent bias towards easily tractable and therapeutically relevant proteins, which is clearly not the case for neglected diseases. Nonetheless, huge efforts are being put into practice to overcome these limitations, e.g., the revolutionary cryogenic electron microscopy technique, which is a more sensitive and widely available approach to solving protein structures [103], or novel modeling techniques that benefit from recent breakthroughs in artificial intelligence [14]. It is expected that such techniques will lead to an immense growth in structural data in the next years and lead to an expansion of promising structural analyses for drug discovery.
4. Materials and Methods
4.1. Identification and Collection of Chagas Targets and Their Structural Data
Therapeutic Chagas targets were collected from literature evidence. Most of the targets were taken from a preliminary work by Haupt et al. [15] with additional targets added from other literature sources [17,18].
Selection and characterization of the Chagas targets. Each target was mapped to the corresponding PDB structures using the UniProt (Universal Protein Resource) accession number. Only targets with a structure available in the PDB were considered for the study. The targets were further characterized and classified according to their Protein family and Enzyme classification categories, primarily using two databases: Pfam (version 31.0) [104] and ENZYME [105]. A protein family is a collection of proteins that have related regions or domains. Knowing about the protein families of the targets provides valuable information about the function of the protein. Furthermore, Enzyme classification was used to analyze whether there are differences between the different enzyme groups in terms of the availability of structural data or the amount of hit compounds.
Classification and selection of the query ligands. From the structural point of view, it is important to select high-quality ligands that specifically interact with the target. For this reason, only structures in complex with a ligand were considered. Based on literature and data given in the PDB, the ligands were annotated and divided into 6 different categories: inhibitors, products, substrates, cofactors, fragments, and substances from the buffer or solution used in the crystallization process. In this way, only structures with high quality ligands that specifically interact with their targets (e.g, inhibitors, products, or substrates) were selected for the screening, while cofactors, buffers, and solvents were excluded (Supplementary Tables S1 and S2).
Sub-classification of binding sites. The targets were further classified by binding site in order to easily elucidate the predicted binding mechanism of resulting hit compounds. In particular, some of the targets had two different binding/active sites, as shown in Table 1, which was determined via literature review. Additionally, some structures had to be prepared manually with special parameters to detect interaction patterns in alternate atom locations or to split covalently attached cofactors from the ligands (Supplementary Table S3).
4.2. Computational Screening to Identify Novel Chagas Target Binders
The targets in Table 1 were the input for the in silico screening. One independent screening was conducted per target binding site. First, the interaction profiles were obtained from the Protein Ligand Interaction Profiler (PLIP) [106], a tool that detects the non-covalent interactions describing the binding mode of a drug to a target. Second, the interaction profiles were encoded into interaction fingerprints, which are binary vectors. Each bin on the vector represents a feature defined by the combination of two non-covalent interactions within an angle and distance range. This value is set to 1 if the feature is present in the binding or to 0 if it is not, as previously described in Adasme et al. [107]. Finally, each fingerprint was screened against the full PDB to identify other complexes (fingerprints) with a similar binding mode. The results were aggregated by ligands and ranked by p-value per screen. That is, if there were multiple complexes of the same drug with different proteins, they were all grouped together and regarded as one hit, with the lowest p-value being chosen to represent the group. In addition, the results were filtered to contain only FDA approved drugs or drugs approved elsewhere in the world.
4.3. Hit Candidate Prioritization
Following several filtering steps, the primary list of 512 compounds was narrowed to 38 of the top predictions for Chagas disease. First, a p-value cut-off of 5 × 10−4 was introduced. Subsequently, biologically irrelevant drugs (sugars, amino acids, nucleic acids, fatty acids, citric acids, collagen, glycerol, and others) and compounds with poor drug properties for Chagas (sedatives, disinfectants, hand wash, and others) were excluded, which further decreased the number of hits to 297. Redundant hits were removed while keeping the hit with the lowest p-value, reducing the hit count to 164. Each of these hits was evaluated according to three criteria: (1) Visual inspection of binding mode similarity, meaning an interaction pattern similarity with a score greater than 0.75 in case of simple interactions (less than two interaction types or less than five patterns) or greater than 0.6 similarity in case of more complex interactions. (2) Binding affinity values for query protein and hit drug in Binding DB with , , or less than or equal to 5 M. (3) Literature evidence of trypanocidal activity. Direct evidence was defined as an in vitro or in vivo experimental assay in which the drug showed growth inhibition or killing of the parasite. Indirect evidence was defined as the potential of a drug to work against T. cruzi (i.e., activity against related organisms, combination therapy studies with known Chagas drugs, etc.) while there is no literature evidence of an assay that directly studies the effect of the drug on T. cruzi. If a hit fulfilled at least 1 of the 3 criteria, it was included in the final selection.
4.4. Chemical Space of the Hit Candidates.
The chemical similarity of the high priority hits was calculated to assess the novel scaffolds identified in the screen. A chemical similarity score matrix was obtained via PubChem service, where the 2D chemical structure of the compounds is compared pairwise and a similarity score is calculated. The similarity matrix was used as input to generate a clustered heatmap with complete linkage in Rstudio v1.0.153 using the packages heatmap.2 and hclust.
4.5. Experimental Validation
The high-priority hit drugs were subjected to experimental validation in vitro, ex vivo, and in vivo, together with a cytotoxicity assay. Ninoa and INC-5 strains of T. cruzi were used in this study. The Ninoa strain was obtained in 1986 by xenodiagnoses from an acute case of Chagas’ disease in Oaxaca, Mexico, whereas the INC-5 strain was obtained in 1997 from a chronic case of Chagas’ disease in a 58-year-old woman in Guanajuato, Mexico.
CD1 strain mice, 6–8 weeks old, were used to maintain Ninoa and INC-5 strains of T. cruzi. Animals were given by the University of Science and Arts of Chiapas. To maintain the strains, animals were intraperitoneally inoculated every 14 days with 1 × 105 blood trypomastigote, obtained from parasite-infected mice. Parasitemia was monitored every three days by the Pizzi method [108]. For this, 5 µL of blood from the caudal vein of infected mice were deposited on a slide and evenly distributed with an 18 × 18 mm coverslip. Parasite counting was performed by observing 15 microscopic fields using a 40 × objective lens. For the in vivo short term evaluation of the trypanocidal activity of the FDA-approved drugs, six groups of six CD1 mice each with homogeneous weight and parasitemia were used.
In vitro evaluation of different FDA-approved drugs against Trypanosoma cruzi epimastigotes. The epimastigote stage of Ninoa and INC5 strains of T. cruzi were used to evaluated the in vitro trypanocidal activity of different FDA-approved drugs. Both strains were maintained in liver infusion tryptose (LIT) medium, supplemented with 10% fetal bovine serum (FBS) and 0.1% penicillin–streptomycin. They were preserved by transferring 1 × 106 parasites/mL into a new culture medium every week. The activity of eight FDA-approved drugs as well as nifurtimox and benznidazole—two FDA-approved drugs for Chagas’s disease treatment—were evaluated against T. cruzi strains. All compounds were initially prepared at 10 mg/mL, using dimethyl sulfoxide (DMSO) as diluent. Serial dilutions were performed using complemented LIT medium until concentrations of 100 to 0.46 µg/mL of each drug were obtained. T. cruzi epimastigotes (1 × 106/well) were cultured in 96-well microliter plates, and incubated for 48 h at 28 °C with the drugs at different concentrations in a final volume of 200 µL. DMSO was included as negative control and nifurtimox and benznidazole as positive controls. After the incubation period, 20 µL of 2.5 mM resazurin solution were added to each well and incubated for 3 h. All assays were carried out in triplicate. The IC50 value were determined by Probit analysis [109,110].
Ex vivo evaluation of different FDA-approved drugs against Trypanosoma cruzi blood trypomastigotes. The ex vivo evaluation of the trypanocidal activity of the FDA-approved drugs was carried out according to the methodology described above. Blood from parasite infected animals was obtained from intracardiac puncture and diluted with phosphate-buffered saline (PBS), pH 7.2, until a concentration of 2 × 106 blood trypomastigotes/mL was obtained. From this, 195 µL of blood/well was placed in a 96-well plate and incubated at 4 °C for 24 h with 5 µL of each drug tested at different concentrations (5 µg/mL to 250 mg/mL). DMSO was included as negative control and nifurtimox and benznidazole as positive controls. After incubation, live parasites were counted in a Neubauer chamber and the IC50 was calculated. Three independent experiments by triplicate were performed.
In vivo short-term evaluation of the trypanocidal activity of the FDA-approved drugs. Six groups of six CD1 female mice were infected with blood trypomastigotes of Ninoa and INC-5 strains following the methodology previously described in Romanha et al. [111] and in Díaz-Chiguer et al. [112]. At day 13 post-infection, when the infected mice reached an average of 5 × 106 parasites/mL, a single dose of the FDA-approved drugs at 100 mg/kg body weight was orally administered. Parasitemia was monitored following the Pizzi method at 2, 4, 6, and 8 h post-treatment with a Neubauer chamber using mineral oil as a vehicle. Nifurtimox, vehicle control, and infected non-treated mice were used as controls [108,113]. Statistical significance between the trypanocidal activity of FDA-approved drugs and nifurtimox was analyzed by Student’s t test (Start Plus). Mice examination was performed as stated in the Norma Official Mexicana (NOM-062-Z00-1999), published on August 2009.
Cytotoxicity assay. Mouse macrophage cell line J774.2 was cultivated in RPMI medium supplemented with 10% FBS, 100 U µg/mL penicillin, and 100 mg/mL streptomycin, at 37 °C and in an atmosphere of CO2 at 5%. The culture medium was replaced at intervals of 2–3 days, according to cell confluence. In order to evaluate the cytotoxicity of the FDA-approved drugs, 50,000 cells/well were placed in a 96-well plate and allowed to adhere for 24 h at 37 °C. Afterwards, FDA-approved drugs were added at 0.8 to 100 µg/mL in a final volume of 200 µL and plates were incubated for 48 h at 37 °C with CO2 at 5%. DMSO at 0.1% (the maximum concentration used) was included as negative control, and nifurtimox and benznidazole were used as positive controls. The metabolic activity of the cells was determined following the resazurin method. The percentage of cell viability was calculated and the half maximal cytotoxicity concentration (CC50) was determined by Probit analysis. Three independent tests were carried out in triplicates. The selectivity index (SI) was calculated with the formula: CC50/IC50.
5. Conclusions
Chagas disease affects millions of people in South America. As current medications are ineffective and have severe side effects, drug repositioning is a promising approach to find new therapies. The approach presented in this study has demonstrated the potential of structure-based drug repositioning to produce a high rate of true positive hits, which translates into low cost and time. Half of the predicted drugs were already known to have trypanocidal activity, which serves as a good validation of the approach. In addition, the screening proposed a Chagas target for the drugs with no clear mechanism of action, the explanation being based on the similarity of the non-covalent interactions. On the other hand, the screening also predicted drugs with no prior evidence of an association with Chagas disease and with novel chemical scaffolds, indicating the potential of the approach to reveal promising drug repositioning candidates. We present three candidate Chagas drugs: ciprofloxacin, naproxen, and folic acid, which demonstrated inhibitory activity against T. cruzi trypomastigotes ex vivo and in vivo. Importantly, these candidates are inexpensive, easily available, widely used, and have few side effects. This makes them perfect candidates for further validation in models and in humans, and could eventually lead to a more efficient and better tolerated treatment of Chagas disease. Overall, our approach represents a promising option for all rare and neglected diseases for which no reliable treatment has yet been found.
Acknowledgments
The authors would like to express their thanks to Alex Mestiashvili for his technical support.
Abbreviations
| AIDS | Acquired Immune Deficiency Syndrome |
| COX | Cyclooxigenase |
| dTMP | Deoxythymidine monophosphate |
| FDA | Food and Drug Administration |
| FPPS | Farnesyl Pyrophosphate Synthase |
| GDAPH | Glyceraldehyde-3-phosphate dehydrogenase |
| G6PDH | Glucose-6-phosphate dehydrogenase |
| NSAID | Non-steroidal anti-inflammatory drug |
| NTD | Neglected tropical diseases |
| PDB | Protein Data Bank |
| PLIP | Protein Ligand Interactions Profiler |
| SQS | Squalene synthase |
| TcDHFR | T. cruzi Dihydrofolate reductase |
| TS | Thymidilate synthase |
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/22/8809/s1.
Author Contributions
Conceptualization, M.F.A., S.N.B., S.S., and M.S.; methodology, M.F.A., S.N.B., L.A., S.S., and V.J.H.; validation, A.M.-R., B.N.-T., V.C.-C., L.Y.-M., J.A.D.F.-V., and G.R.; formal analysis and investigation, M.F.A., S.N.B., and L.A.; data curation, M.F.A. and L.A.; writing—original draft preparation, M.F.A.; writing—review and editing, M.F.A., S.N.B., L.Y.-M., B.N.-T., A.M.-R., G.R., and M.S.; visualization, M.F.A.; supervision, M.S. and G.R. All authors have read and agreed to the published version of the manuscript.
Funding
Secretaría de Investigación y Posgrado del Instituto Politécnico Nacional, Grant 20200491.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chatelain E. Chagas disease research and development: Is there light at the end of the tunnel? Comput. Struct. Biotechnol. J. 2017;15:98–103. doi: 10.1016/j.csbj.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Chagas Disease (American Trypanosomiasis) [(accessed on 3 November 2020)]; Available online: http://www.who.int/mediacentre/factsheets/fs340/en/
- 3.Coura J.R., Castro S.L.D. A critical review on chagas disease chemotherapy. Memórias de Instituto Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/S0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez J., Davies C., Simonazzi A., Real J.P., Palma S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Trop. 2016;156:1–16. doi: 10.1016/j.actatropica.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 5.Castro J.A., deMecca M.M., Bartel L.C. Toxic side effects of drugs used to treat chagas’ cisease (American Trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 6.DiMasi J.A., Grabowski H.G., Hansen R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016;47:20–33. doi: 10.1016/j.jhealeco.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Li J., Zheng S., Chen B., Butte A.J., Swamidass S.J., Lu Z. A survey of current trends in computational drug repositioning. Brief. Bioinform. 2015;17:2–12. doi: 10.1093/bib/bbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker N.C., Ekins S., Williams A.J., Tropsha A. A bibliometric review of drug repurposing. Drug Discov. Today. 2018;23:661–672. doi: 10.1016/j.drudis.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oldfield E. Targeting isoprenoid biosynthesis for drug discovery: Bench to bedside. Acc. Chem. Res. 2010;43:1216–1226. doi: 10.1021/ar100026v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senkovich O., Bhatia V., Garg N., Chattopadhyay D. Lipophilic antifolate trimetrexate is a potent inhibitor of trypanosoma cruzi: Prospect for chemotherapy of Chagas’ disease. Antimicrob. Agents Chemother. 2005;49:3234–3238. doi: 10.1128/AAC.49.8.3234-3238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morillo C.A., Waskin H., Sosa-Estani S., del Carmen Bangher M., Cuneo C., Milesi R., Mallagray M., Apt W., Beloscar J., Gascon J., et al. Benznidazole and posaconazole in eliminating parasites in asymptomatic T. Cruzi carriers: The STOP-CHAGAS trial. J. Am. Coll. Cardiol. 2017;69:939–947. doi: 10.1016/j.jacc.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Torrico F., Gascon J., Ortiz L., Alonso-Vega C., Pinazo M.J., Schijman A., Almeida I.C., Alves F., Strub-Wourgaft N., Ribeiro I., et al. Treatment of adult chronic indeterminate Chagas disease with benznidazole and three E1224 dosing regimens: A proof-of-concept, randomised, placebo-controlled trial. Lancet Infect. Dis. 2018;18:419–430. doi: 10.1016/S1473-3099(17)30538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planer J.D., Hulverson M.A., Arif J.A., Ranade R.M., Don R., Buckner F.S. Synergy testing of FDA-approved drugs identifies potent drug combinations against Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2014;8:e2977. doi: 10.1371/journal.pntd.0002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adasme M.F., Parisi D., Sveshnikova A., Schroeder M. Structure-based drug repositioning: Potential and limits. Semin. Cancer Biol. 2020 doi: 10.1016/j.semcancer.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Haupt V.J., Uvalle J.E.A., Salentin S., Daminelli S., Leonhardt F., Konc J., Schroeder M. Computational drug repositioning by target hopping: A use case in Chagas disease. Curr. Pharm. Des. 2016;22:3124–3134. doi: 10.2174/1381612822666160224143008. [DOI] [PubMed] [Google Scholar]
- 16.Juárez-Saldivar A., Schroeder M., Salentin S., Haupt V.J., Saavedra E., Vázquez C., Reyes-Espinosa F., Herrera-Mayorga V., Villalobos-Rocha J.C., García-Pérez C.A., et al. Computational drug repositioning for chagas disease using protein-ligand interaction profiling. Int. J. Mol. Sci. 2020;21:4270. doi: 10.3390/ijms21124270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang N., Li Q., Ko T.P., Chan H.C., Li J., Zheng Y., Huang C.H., Ren F., Chen C.C., Zhu Z., et al. Squalene synthase as a target for Chagas disease therapeutics. PLoS Pathog. 2014;10:e1004114. doi: 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igoillo-Esteve M., Cazzulo J.J. The glucose-6-phosphate dehydrogenase from Trypanosoma cruzi: Its role in the defense of the parasite against oxidative stress. Mol. Biochem. Parasitol. 2006;149:170–181. doi: 10.1016/j.molbiopara.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert I.H. Inhibitors of dihydrofolate reductase in leishmania and trypanosomes. Biochim. Biophys. Acta Mol. Basis Dis. 2002;1587:249–257. doi: 10.1016/S0925-4439(02)00088-1. [DOI] [PubMed] [Google Scholar]
- 20.Stoka V., Nycander M., Lenarčič B., Labriola C., Cazzulo J.J., Björk I., Turk V. Inhibition of cruzipain, the major cysteine proteinase of the protozoan parasite, Trypanosoma cruzi, by proteinase inhibitors of the cystatin superfamily. FEBS Lett. 1995;370:101–104. doi: 10.1016/0014-5793(95)00798-E. [DOI] [PubMed] [Google Scholar]
- 21.Gabelli S.B., McLellan J.S., Montalvetti A., Oldfield E., Docampo R., Amzel L.M. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: Implications for drug design. Proteins. 2005;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- 22.Guido R., Balliano T., Andricopulo A., Oliva G. Kinetic and crystallographic studies on glyceraldehyde-3-Phosphate dehydrogenase from trypanosoma cruzi in complex with iodoacetate. Lett. Drug Des. Discov. 2009;6:210–214. doi: 10.2174/157018009787847774. [DOI] [Google Scholar]
- 23.Hargrove T.Y., Wawrzak Z., Alexander P.W., Chaplin J.H., Keenan M., Charman S.A., Perez C.J., Waterman M.R., Chatelain E., Lepesheva G.I. Complexes of trypanosoma cruzi sterol 14α-demethylase (CYP51) with two pyridine-based drug candidates for Chagas disease. J. Biol. Chem. 2013;288:31602–31615. doi: 10.1074/jbc.M113.497990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buschiazzo A., Goytia M., Schaeffer F., Degrave W., Shepard W., Goire C.G., Chamond N., Cosson A., Berneman A., Coatnoan N., et al. Crystal structure, catalytic mechanism, and mitogenic properties of Trypanosoma cruzi proline racemase. Proc. Natl. Acad. Sci. USA. 2006;103:1705–1710. doi: 10.1073/pnas.0509010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan M.O. Trypanothione reductase: A viable chemotherapeutic target for antitrypanosomal and antileishmanial drug design. Drug Target Insights. 2007;2:129–146. doi: 10.1177/117739280700200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inaoka D.K., Sakamoto K., Shimizu H., Shiba T., Kurisu G., Nara T., Aoki T., Kita K., Harada S. Structures of trypanosoma cruzi dihydroorotate dehydrogenase complexed with substrates and products: Atomic resolution insights into mechanisms of dihydroorotate oxidation and fumarate reduction. Biochemistry. 2008;47:10881–10891. doi: 10.1021/bi800413r. [DOI] [PubMed] [Google Scholar]
- 27.Eakin A.E., Guerra A., Focia P.J., Torres-Martinez J., Craig S.P. Hypoxanthine phosphoribosyltransferase from Trypanosoma cruzi as a target for structure-based inhibitor design: Crystallization and inhibition studies with purine analogs. Antimicrob. Agents Chemother. 1997;41:1686–1692. doi: 10.1128/AAC.41.8.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murakami M.T., Rodrigues N.C., Gava L.M., Honorato R.V., Canduri F., Barbosa L.R., Oliva G., Borges J.C. Structural studies of the Trypanosoma cruzi Old Yellow Enzyme: Insights into enzyme dynamics and specificity. Biophys. Chem. 2013;184:44–53. doi: 10.1016/j.bpc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Schormann N., Pal B., Senkovich O., Carson M., Howard A., Smith C., DeLucas L., Chattopadhyay D. Crystal structure of Trypanosoma cruzi pteridine reductase 2 in complex with a substrate and an inhibitor. J. Struct. Biol. 2005;152:64–75. doi: 10.1016/j.jsb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Amano Y., Namatame I., Tateishi Y., Honboh K., Tanabe E., Niimi T., Sakashita H. Structural insights into the novel inhibition mechanism ofTrypanosoma cruzispermidine synthase. Acta Crystallogr. D. 2015;71:1879–1889. doi: 10.1107/S1399004715013048. [DOI] [PubMed] [Google Scholar]
- 31.Oppenheimer M., Valenciano A.L., Kizjakina K., Qi J., Sobrado P. Chemical mechanism of UDP-galactopyranose mutase from Trypanosoma cruzi: A potential drug target against Chagas’ disease. PLoS ONE. 2012;7:e32918. doi: 10.1371/journal.pone.0032918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman H.M. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overington J.P., Al-Lazikani B., Hopkins A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 34.Yang J., Roy A., Zhang Y. BioLiP: A semi-manually curated database for biologically relevant ligand–protein interactions. Nucleic Acids Res. 2012;41:D1096–D1103. doi: 10.1093/nar/gks966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilson M.K., Liu T., Baitaluk M., Nicola G., Hwang L., Chong J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2015;44:D1045–D1053. doi: 10.1093/nar/gkv1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuster J.P., Schaub G.A. Trypanosoma cruzi: The development of estrus cycle and parasitemia in female mice maintained with or without male pheromones. Parasitol. Res. 2001;87:985–993. doi: 10.1007/s004360100472. [DOI] [PubMed] [Google Scholar]
- 37.Sienkiewicz N., Jarosławski S., Wyllie S., Fairlamb A.H. Chemical and genetic validation of dihydrofolate reductase–thymidylate synthase as a drug target in African trypanosomes. Mol. Microbiol. 2008;69:520–533. doi: 10.1111/j.1365-2958.2008.06305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiltensperger G., Jones N.G., Niedermeier S., Stich A., Kaiser M., Jung J., Puhl S., Damme A., Braunschweig H., Meinel L., et al. Synthesis and structure–activity relationships of new quinolone-type molecules against trypanosoma brucei. J. Med. Chem. 2012;55:2538–2548. doi: 10.1021/jm101439s. [DOI] [PubMed] [Google Scholar]
- 39.Andrews K.T., Fisher G., Skinner-Adams T.S. Drug repurposing and human parasitic protozoan diseases. Int. J. Parasitol. 2014;4:95–111. doi: 10.1016/j.ijpddr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vilar-Pereira G., Resende Pereira I., de Souza Ruivo L.A., Cruz Moreira O., da Silva A.A., Britto C., Lannes-Vieira J. Combination chemotherapy with suboptimal doses of benznidazole and pentoxifylline sustains partial reversion of experimental Chagas’ heart disease. Antimicrob. Agents Chemother. 2016;60:4297–4309. doi: 10.1128/AAC.02123-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C.H., Gabelli S.B., Oldfield E., Amzel L.M. Binding of nitrogen-containing bisphosphonates 651 (N-BPs) to the Trypanosoma cruzi farnesyl diphosphate synthase homodimer. Proteins. 2010;78:888–899. doi: 10.1002/prot.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes J.F., Castellani O., Pa G. Nucleotide and polynucleotide synthesis in trypanosoma crud. II. In vitro effect of tioguanine and of the aminonucleoside of atylomycin. Exp. Parasitol. 1959;8:480–485. doi: 10.1016/0014-4894(59)90035-9. [DOI] [PubMed] [Google Scholar]
- 43.Soares M.B., Silva C.V., Bastos T.M., Guimarães E.T., Figueira C.P., Smirlis D., Azevedo W.F. Anti-Trypanosoma cruzi activity of nicotinamide. Acta Trop. 2012;122:224–229. doi: 10.1016/j.actatropica.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima-Shimada J., Hirota Y., Aoki T. Inhibition of Trypanosoma cruzi growth in mammalian cells by purine and pyrimidine analogs. Antimicrob. Agents Chemother. 1996;40:2455–2458. doi: 10.1128/AAC.40.11.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kashif M., Moreno-Herrera A., Lara-Ramirez E.E., Ramírez-Moreno E., Bocanegra-García V., Ashfaq M., Rivera G. Recent developments in trans-sialidase inhibitors of Trypanosoma cruzi. J. Drug Target. 2017;25:485–498. doi: 10.1080/1061186X.2017.1289539. [DOI] [PubMed] [Google Scholar]
- 46.Kelly J.M., Miles M.A., Skinner A.C. The anti-influenza virus drug rimantadine has trypanocidal activity. Antimicrob. Agents Chemother. 1999;43:985–987. doi: 10.1128/AAC.43.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ceole L.F., Gandhi H., Villamizar L.H., Soares M.J., O’Sullivan T.P. Synthesis of novel quinine analogs and evaluation of their effects on Trypanosoma cruzi. Future Med. Chem. 2018;10:391–408. doi: 10.4155/fmc-2017-0184. [DOI] [PubMed] [Google Scholar]
- 48.Neres J., Bonnet P., Edwards P.N., Kotian P.L., Buschiazzo A., Alzari P.M., Bryce R.A., Douglas K.T. Benzoic acid and pyridine derivatives as inhibitors of Trypanosoma cruzi trans-sialidase. Bioorg. Med. Chem. 2007;15:2106–2119. doi: 10.1016/j.bmc.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Simões-Silva M.R., Araújo J.S.D., Peres R.B., Silva P.B.D., Batista M.M., Azevedo L.D.D., Bastos M.M., Bahia M.T., Boechat N., Soeiro M.N.C. Repurposing strategies for Chagas disease therapy: The effect of imatinib and derivatives against Trypanosoma cruzi. Parasitology. 2019;146:1006–1012. doi: 10.1017/S0031182019000234. [DOI] [PubMed] [Google Scholar]
- 50.Reigada C., Valera-Vera E.A., Sayé M., Errasti A.E., Avila C.C., Miranda M.R., Pereira C.A. Trypanocidal effect of isotretinoin through the inhibition of polyamine and amino acid transporters in Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2017;11:e0005472. doi: 10.1371/journal.pntd.0005472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baum S.G., Wittner M., Nadler J.P., Horwitz S.B., Dennis J.E., Schiff P.B., Tanowitz H.B. Taxol, a microtubule stabilizing agent, blocks the replication of Trypanosoma cruzi. Proc. Natl. Acad. Sci. USA. 1981;78:4571–4575. doi: 10.1073/pnas.78.7.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones D.C., Ariza A., Chow W.H.A., Oza S.L., Fairlamb A.H. Comparative structural, kinetic and inhibitor studies of Trypanosoma brucei trypanothione reductase with T. cruzi. Mol. Biochem. Parasitol. 2010;169:12–19. doi: 10.1016/j.molbiopara.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lo Presti M.S., Bazán P.C., Strauss M., Báez A.L., Rivarola H.W., Paglini-Oliva P.A. Trypanothione reductase inhibitors: Overview of the action of thioridazine in different stages of Chagas disease. Acta Tropica. 2015;145:79–87. doi: 10.1016/j.actatropica.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Damasceno F.S., Barisón M.J., Pral E.M.F., Paes L.S., Silber A.M. Memantine, an antagonist of the NMDA glutamate receptor, affects cell proliferation, differentiation and the intracellular cycle and induces apoptosis in Trypanosoma cruzi. PLoS Negl. Trop. Dis. 2014;8:e2717. doi: 10.1371/journal.pntd.0002717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sangenito L.S., Menna-Barreto R.F.S., d’Avila Levy C.M., Santos A.L.S., Branquinha M.H. Decoding the anti-Trypanosoma cruzi action of HIV peptidase inhibitors using epimastigotes as a model. PLoS ONE. 2014;9:e113957. doi: 10.1371/journal.pone.0113957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins A.L., Mason J.S., Overington J.P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 2006;16:127–136. doi: 10.1016/j.sbi.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y., Gupta-Ostermann D., Bajorath J. Exploring compound promiscuity patterns and multi-target activity spaces. Comput. Struct. Biotechnol. J. 2014;9:e201401003. doi: 10.5936/csbj.201401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haupt V.J., Daminelli S., Schroeder M. Drug promiscuity in PDB: Protein binding site similarity is key. PLoS ONE. 2013;8:e65894. doi: 10.1371/annotation/0852cc69-8cea-4966-bb8a-ae0b348d1bd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao M., Skolnick J. Structural space of protein-protein interfaces is degenerate, close to complete, and highly connected. Proc. Natl. Acad. Sci. USA. 2010;107:22517–22522. doi: 10.1073/pnas.1012820107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salentin S., Adasme M.F., Heinrich J.C., Haupt V.J., Daminelli S., Zhang Y., Schroeder M. From malaria to cancer: Computational drug repositioning of amodiaquine using PLIP interaction patterns. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-11924-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.RODWELL A.W., ABBOT A. The function of glycerol, cholesterol and long-chain fatty acids in the nutrition of mycoplasma mycoides. J. Gen. Microbiol. 1961;25:201–214. doi: 10.1099/00221287-25-2-201. [DOI] [PubMed] [Google Scholar]
- 62.Chen X., Lv Q., Liu Y., Deng W. Effect of food additive citric acid on the growth of human esophageal Carcinoma Cell Line EC109. Cell J. (Yakhteh) 2017;18:493. doi: 10.22074/cellj.2016.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Montalvetti A., Bailey B.N., Martin M.B., Severin G.W., Oldfield E., Docampo R. Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. J. Biol. Chem. 2001;276:33930–33937. doi: 10.1074/jbc.M103950200. [DOI] [PubMed] [Google Scholar]
- 64.Garzoni L.R., Caldera A., de Nazareth L., Meirelles M., Castro S.L., Docampo R., Meints G.A., Oldfield E., Urbina J.A. Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. Int. J. Antimicrob. Agents. 2004;23:273–285. doi: 10.1016/j.ijantimicag.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 65.Furman P.A., Barry D.W. Spectrum of antiviral activity and mechanism of action of zidovudine. An overview. Am. J. Med. 1988;85:176–181. [PubMed] [Google Scholar]
- 66.Sitruk-Ware R., El-Etr M. Progesterone and related progestins: Potential new health benefits. Climacteric. 2013;16:69–78. doi: 10.3109/13697137.2013.802556. [DOI] [PubMed] [Google Scholar]
- 67.Khodursky A.B., Zechiedrich E.L., Cozzarelli N.R. Topoisomerase IV is a target of quinolones in Escherichia coli. Proc. Natl. Acad. Sci. USA. 1995;92:11801–11805. doi: 10.1073/pnas.92.25.11801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bush N.G., Maxwell A., Evans-Roberts K. DNA Topoisomerases. EcoSal Plus. 2015;6 doi: 10.1128/ecosalplus.ESP-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heisig P. Type II topoisomerases–inhibitors, repair mechanisms and mutations. Mutagenesis. 2009;24:465–469. doi: 10.1093/mutage/gep035. [DOI] [PubMed] [Google Scholar]
- 70.Nenortas E., Burri C., Shapiro T.A. Antitrypanosomal activity of fluoroquinolones. Antimicrob. Agents Chemother. 1999;43:2066–2068. doi: 10.1128/AAC.43.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cavalcanti D.P., Fragoso S.P., Goldenberg S., de Souza W., Motta M.C.M. The effect of topoisomerase II inhibitors on the kinetoplast ultrastructure. Parasitol. Res. 2004;94:439–448. doi: 10.1007/s00436-004-1223-4. [DOI] [PubMed] [Google Scholar]
- 72.Kulikowicz T., Shapiro T.A. Distinct genes encode type II topoisomerases for the nucleus and mitochondrion in the protozoan parasite trypanosoma brucei. J. Biol. Chem. 2005;281:3048–3056. doi: 10.1074/jbc.M505977200. [DOI] [PubMed] [Google Scholar]
- 73.Nardy A.F.F.R., de Lima C.G.F., Pérez A.R., Morrot A. Role of Trypanosoma cruzi Trans-sialidase on the Escape from Host Immune Surveillance. Front. Microbiol. 2016;7:348. doi: 10.3389/fmicb.2016.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schacht P., Arcieri G., Hullmann R. Safety of oral ciprofloxacin. Am. J. Med. 1989;87:S98–S102. doi: 10.1016/0002-9343(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 75.Conley Z.C., Bodine T.J., Chou A., Zechiedrich L. Wicked: The untold story of ciprofloxacin. PLoS Pathog. 2018;14:e1006805. doi: 10.1371/journal.ppat.1006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angiolillo D.J., Weisman S.M. Clinical pharmacology and cardiovascular safety of naproxen. Am. J. Cardiovasc. Drugs. 2016;17:97–107. doi: 10.1007/s40256-016-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wojcieszyńska D., Guzik U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020;104:1849–1857. doi: 10.1007/s00253-019-10343-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Varga Z., rafay ali Sabzwari S., Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: An under-recognized public health issue. Cureus. 2017;9:e1144. doi: 10.7759/cureus.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cossentini L.A., Silva R.V.D., Yamada-Ogatta S.F., Yamauchi L.M., Araújo E.J.D.A., Pinge-Filho P. Aspirin treatment exacerbates oral infections by Trypanosoma cruzi. Exp. Parasitol. 2016;164:64–70. doi: 10.1016/j.exppara.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Choi J.H., Yates Z., Veysey M., Heo Y.R., Lucock M. Contemporary issues surrounding folic acid fortification initiatives. Prev. Nutr. Food Sci. 2014;19:247–260. doi: 10.3746/pnf.2014.19.4.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y., Cantley L.C. Toward a better understanding of folate metabolism in health and disease. J. Exp. Med. 2018;216:253–266. doi: 10.1084/jem.20181965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang S. A Prospective Study of Folate Intake and the Risk of Breast Cancer. JAMA. 1999;281:1632. doi: 10.1001/jama.281.17.1632. [DOI] [PubMed] [Google Scholar]
- 83.Giovannucci E. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann. Intern. Med. 1998;129:517. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 84.Kim J., Kim D.H., Lee B.H., Kang S.H., Lee H.J., Lim S.Y., Suh Y.K., Ahn Y.O. Folate intake and the risk of colorectal cancer in a Korean population. Eur. J. Clin. Nutr. 2009;63:1057–1064. doi: 10.1038/ejcn.2009.37. [DOI] [PubMed] [Google Scholar]
- 85.D’Anci K.E., Rosenberg I.H. Folate and brain function in the elderly. Curr. Opin. Clin. Nutr. Metab. Care. 2004;7:659–664. doi: 10.1097/00075197-200411000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Xun P., Liu K., Loria C.M., Bujnowski D., Shikany J.M., Schreiner P.J., Sidney S., He K. Folate intake and incidence of hypertension among American young adults: A 20-y follow-up study. Am. J. Clin. Nutr. 2012;95:1023–1030. doi: 10.3945/ajcn.111.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hibbard B.M. The role of folic acid in pregnancy with particular reference to anaemia, abruption and abortion. BJOG. 1964;71:529–542. doi: 10.1111/j.1471-0528.1964.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 88.Kirke P.N., Molloy A.M., Daly L.E., Burke H., Weir D.G., Scott J.M. Maternal plasma folate and vitamin B12 are independent risk factors for neural tube defects. QJM. 1993;86:703–708. doi: 10.1093/oxfordjournals.qjmed.a068749. [DOI] [PubMed] [Google Scholar]
- 89.Molloy A.M., Mills J.L., Kirke P.N., Ramsbottom D., McPartlin J.M., Burke H., Conley M., Whitehead A.S., Weir D.G., Scott J.M. Low blood folates in NTD pregnancies are only partly explained by thermolabile 5,10-methylenetetrahydrofolate reductase: Low folate status alone may be the critical factor. Am. J. Med. Genet. 1998;78:155–159. doi: 10.1002/(SICI)1096-8628(19980630)78:2<155::AID-AJMG11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 90.Dhur A., Galan P., Hercberg S. Folate status and the immune system. Prog Food Nutr. Sci. 1991;15:43–60. [PubMed] [Google Scholar]
- 91.Faerman C.H., Savvides S.N., Strickland C., Breidenbach M.A., Ponasik J.A., Ganem B., Ripoll D., Krauth-Siegel R.L., Karplus P.A. Charge is the major discriminating factor for glutathione reductase versus trypanothione reductase inhibitors. Bioorg. Med. Chem. 1996;4:1247–1253. doi: 10.1016/0968-0896(96)00120-4. [DOI] [PubMed] [Google Scholar]
- 92.Ma B., Shatsky M., Wolfson H.J., Nussinov R. Multiple diverse ligands binding at a single protein site: A matter of pre-existing populations. Protein Sci. 2009;11:184–197. doi: 10.1110/ps.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonçalves C.S., Ávila A.R., de Souza W., Motta M.C.M., Cavalcanti D.P. Revisiting the Trypanosoma cruzi metacyclogenesis: Morphological and ultrastructural analyses during cell differentiation. Parasites Vectors. 2018;11:1–14. doi: 10.1186/s13071-018-2664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teixeira A.R., Gomes C., Lozzi S.P., Hecht M.M., Rosa A.d.E.C., Monteiro P.S., Bussacos A.C., Nitz N., McManus C. Environment, interactions between Trypanosoma cruzi and its host, and health. Cad Saude Publica. 2009;25(Suppl. S1):32–44. doi: 10.1590/S0102-311X2009001300004. [DOI] [PubMed] [Google Scholar]
- 95.Lourenço A.M., Faccini C.C., de Jesus Costa C.A., Mendes G.B., Filho A.A.F. Evaluation of in vitro anti-Trypanosoma cruzi activity of medications benznidazole, amiodarone hydrochloride, and their combination. Rev. Soc. Bras. Med. Trop. 2018;51:52–56. doi: 10.1590/0037-8682-0285-2017. [DOI] [PubMed] [Google Scholar]
- 96.Chacón-Vargas K., Nogueda-Torres B., Sánchez-Torres L., Suarez-Contreras E., Villalobos-Rocha J., Torres-Martinez Y., Lara-Ramirez E., Fiorani G., Krauth-Siegel R., Bolognesi M., et al. Trypanocidal activity of quinoxaline 1, 4 Di-N-oxide derivatives as Trypanothione reductase inhibitors. Molecules. 2017;22:220. doi: 10.3390/molecules22020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kashif M., Moreno-Herrera A., Villalobos-Rocha J., Nogueda-Torres B., Pérez-Villanueva J., Rodríguez-Villar K., Medina-Franco J., de Andrade P., Carvalho I., Rivera G. Benzoic acid derivatives with Trypanocidal activity: Enzymatic analysis and molecular docking studies toward Trans-Sialidase. Molecules. 2017;22:1863. doi: 10.3390/molecules22111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kashif M., Chacón-Vargas K.F., López-Cedillo J.C., Nogueda-Torres B., Paz-González A.D., Ramírez-Moreno E., Agusti R., Uhrig M.L., Reyes-Arellano A., Peralta-Cruz J., et al. Synthesis, molecular docking and biological evaluation of novel phthaloyl derivatives of 3-amino-3-aryl propionic acids as inhibitors of Trypanosoma cruzi trans-sialidase. Eur. J. Med. Chem. 2018;156:252–268. doi: 10.1016/j.ejmech.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Nogueira N., Cohn Z. Trypanosoma cruzi: Mechanism of entry and intracellular fate in mammalian cells. J. Exp. Med. 1976;143:1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Romano P.S., Cueto J.A., Casassa A.F., Vanrell M.C., Gottlieb R.A., Colombo M.I. Molecular and cellular mechanisms involved in the Trypanosoma cruzi/host cell interplay. IUBMB Life. 2012;64:387–396. doi: 10.1002/iub.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessler R.L., Contreras V.T., Marliére N.P., Guarneri A.A., Silva L.H.V., Mazzarotto G.A.C.A., Batista M., Soccol V.T., Krieger M.A., Probst C.M. Recently differentiated epimastigotes from Trypanosoma cruzi are infective to the mammalian host. Mol. Microbiol. 2017;104:712–736. doi: 10.1111/mmi.13653. [DOI] [PubMed] [Google Scholar]
- 102.Mitra A.K., Mawsonand A.R. Neglected tropical diseases: Epidemiology and global burden. Trop. Med. Infect. Dis. 2017;2:36. doi: 10.3390/tropicalmed2030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Callaway E. ‘It opens up a whole new universe’: Revolutionary microscopy technique sees individual atoms for first time. Nature. 2020;582:156–157. doi: 10.1038/d41586-020-01658-1. [DOI] [PubMed] [Google Scholar]
- 104.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bairoch A. The ENZYME database in 2000. Nucleic Acids Res. 2000;28:304–305. doi: 10.1093/nar/28.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salentin S., Schreiber S., Haupt V.J., Adasme M.F., Schroeder M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015;43:W443–W447. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Adasme M.F., Parisi D., Van Belle K., Salentin S., Haupt V.J., Jennings G.S., Heinrich J.C., Herman J., Sprangers B., Louat T., et al. Structure-based drug repositioning explains ibrutinib as VEGFR2 inhibitor. PLoS ONE. 2020;15:1–26. doi: 10.1371/journal.pone.0233089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brener Z. Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Rev. Inst. Med. Trop. Sao Paulo. 1962;4:389–396. [PubMed] [Google Scholar]
- 109.Moreno-Rodríguez A., Salazar-Schettino P.M., Bautista J.L., Hernández-Luis F., Torrens H., Guevara-Gómez Y., Pina-Canseco S., Torres M.B., Cabrera-Bravo M., Martinez C.M., et al. In vitro antiparasitic activity of new thiosemicarbazones in strains of Trypanosoma cruzi. Eur. J. Med. Chem. 2014;87:23–29. doi: 10.1016/j.ejmech.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 110.Espinoza B., Rico T., Sosa S., Oaxaca E., Vizcaino-Castillo A., Caballero M.L., Martínez I. MexicanTrypanosoma cruzi(TCI) strains with different degrees of virulence induce diverse humoral and cellular immune responses in a murine experimental infection model. J. Biomed. Biotechnol. 2010;2010:1–10. doi: 10.1155/2010/890672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romanha A.J., de Castro S.L., de Nazaré Correia Soeiro M., Lannes-Vieira J., Ribeiro I., Talvani A., Bourdin B., Blum B., Olivieri B., Zani C., et al. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Memórias do Instituto Oswaldo Cruz. 2010;105:233–238. doi: 10.1590/S0074-02762010000200022. [DOI] [PubMed] [Google Scholar]
- 112.Díaz-Chiguer D.L., Hernández-Luis F., Nogueda-Torres B., Castillo R., Reynoso-Ducoing O., Hernández-Campos A., Ambrosio J.R. JVG9, a benzimidazole derivative, alters the surface and cytoskeleton of Trypanosoma cruzi bloodstream trypomastigotes. Memórias do Instituto Oswaldo Cruz. 2014;109:757–760. doi: 10.1590/0074-0276140096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Filardi L.S., Brener Z. A rapid method for testing in vivo the susceptibility of different strains of Trypanosoma cruzi to active chemotherapeutic agents. Memórias do Instituto Oswaldo Cruz. 1984;79:221–225. doi: 10.1590/S0074-02761984000200008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.