Abstract
Background
To inform World Health Organization (WHO) global guidelines, we updated and expanded the evidence base to assess the comparative efficacy, tolerability, and safety of first-line antiretroviral therapy (ART) regimens.
Methods
We searched Embase, Medline and CENTRAL on 28 February 2020 to update the systematic literature review of clinical trials comparing recommended first-line ART that informed previous WHO guidelines. Outcomes included viral suppression, change in CD4 cell counts, mortality, serious and overall adverse events (AEs), discontinuation, discontinuations due to AEs (DAEs); and new outcomes: drug-resistance, neuropsychiatric AEs, early viral suppression, weight gain and birth outcomes. Comparative effects were assessed through network meta-analyses and certainty in the evidence was assessed using the GRADE framework.
Findings
We identified 156 publications pertaining to 68 trials for the primary population. Relative to efavirenz, dolutegravir had improved odds of viral suppression across all time points (odds ratio [OR]: 1·94; 95% credible interval [CrI]: 1·48–2·56 at 96 weeks); was protective of drug-resistance (OR: 0·13; 95%CrI: 0·04–0·48); and led to fewer discontinuations (OR: 0·58; 95%CrI: 0·48–0·70). Evidence supported dolutegravir use among TB-HIV co-infected persons and pregnant women. Adverse birth outcomes were observed in 33.2% of dolutegravir-managed pregnancies and 35.0% of efavirenz-managed pregnancies. Low-dose efavirenz had comparable efficacy and safety to standard-dose efavirenz, but led to fewer DAEs (OR: 0·70; 95%CrI: 0·50–0·92).
Interpretation
The evidence supports choosing dolutegravir in combination with lamivudine/emtricitabine and tenofovir disoproxil fumarate as the preferred first-line regimen and low-dose efavirenz-based regimens as an alternative. Dolutegravir can be considered to be effective, safe and tolerable.
Funding
WHO.
Keywords: HIV, Antiretroviral therapy, First-line, Network meta-analysis
Research in Context.
Evidence before this study
The 2016 WHO consolidated guidelines on the use of antiretrovirals for the treatment and prevention of HIV identified efavirenz with tenofovir disoproxil fumarate and lamivudine/emtricitabine as the preferred first-line regimen and both dolutegravir and low-dose efavirenz as alternative first-line anchor treatments. The 2018 WHO interim guidelines provided a recommendation for DTG based on an update to the review and analyses and cautioned against the potential safety signal of increased risk of neural tube defects with perinatal exposure to dolutegravir in women. For the purpose of this research in context, a search of PubMed for studies published in English through to 4 May 2020, using the search terms “HIV”, “first-line”, “treatment-naïve” and “meta-analysis” confirmed no newer studies exploring global evidence on the matter.
Added value of this study
This study is an update and expansion to the ongoing review and analyses evaluating the therapeutic landscape for first-line antiretroviral therapy regimens. Adding evidence pertaining to reduced drug-resistance, faster viral suppression, reduced neuropsychiatric adverse events, and non birth-defect pregnancy outcomes relative to efavirenz help better contextualize the potential increased risks with dolutegravir – namely neural tube defects and weight gains. Overall, this study establishes the high to moderate certainty of improved efficacy, safety and tolerability of dolutegravir relative to standard-dose efavirenz and adds evidence supporting the use of low-dose efavirenz as an alternative first-line anchor treatment.
Implications to all available evidence
Dolutegravir offers numerous advantages to help in the continued pursuit of the antiretroviral therapy scale-up. The improvements in efficacy and tolerability are likely to be amplified in settings with high drug-resistance to non-nucleoside reverse transcriptase inhibitors, which are becoming more common. Potential safety issues regarding neural tube defects and side-effects such as weight gain need to continue to be monitored.
Alt-text: Unlabelled box
1. Introduction
The 2016 World Health Organization (WHO) antiretroviral therapy (ART) consolidated guidelines on the use of antiretrovirals for treating and preventing HIV included a strong recommendation for the combination of efavirenz, tenofovir disoproxil and lamivudine (or emtricitabine) [EFV+TDF+3TC/FTC] as the preferred first-line ART regimen [1]. The supporting evidence synthesis suggested favourable efficacy, tolerability and safety of dolutegravir (DTG) relative to EFV and improved tolerability with low-dose efavirenz (EFV400) [2]. Nonetheless, DTG and EFV400 based regimens were recommended as alternative first-line regimens [1]. A lack of data among pregnant and breastfeeding women, TB-HIV co-infected persons and other sub-populations – as well as a higher price for DTG – did not allow for a change in recommendation that would align with the public health framework [3]. This framework aims to keep the guidance as simple as possible in order to facilitate task-shifting and other strategies to optimize the ART scale-up in low- and middle-income countries (LMICs) [4]. Improvements in potency, tolerability, simplicity and availability of first-line ART have not only resulted in increased life expectancy and quality of life for people living with HIV (PLWH) [5,6], but also in improved public health through reduced HIV transmissions among virally suppressed PLWH [7]. To this end, the ongoing ART scale-up in LMICs has played a critical role in the fight against HIV/AIDS [8] and the WHO consolidated guidelines are influential in this regard [9].
With numerous changes, including the availability of generic fixed-dose combinations of DTG+TDF+3TC/FTC, an updated evidence synthesis was conducted in 2018 [10]. Results confirmed those of the review supporting the 2016 guidelines and added that results extended to sub-populations. The primary support for the first-line use of DTG in pregnant women was the TSEPAMO surveillance study, conducted in Botswana. Simultaneous to the 2018 evidence synthesis, a separate analysis conducted by the TSEPAMO study group led to a signal of potential increased risk of neural tube defects (NTDs) among women with periconception exposure to DTG [11]. Although based on four cases, the signal was serious and the results were statistically significant. Among others, the WHO, Food and Drug Administration, European Medicines Agency and Center for Disease Control released notes of caution [12,13]. Risk benefit assessments helped weigh all of the evidence, and in late 2018 the WHO interim guidelines recommended DTG+TDF+3TC/FTC as the preferred first-line ART regimen with a note of caution for women of childbearing potential [14].
The purpose of this review was to update and expand the systematic literature review (SLR) and network meta-analysis (NMA) used to answer multiple PICO (population, intervention, comparator and outcome) questions pertaining to the choice of preferred first-line regimen. NMA is an expansion of traditional pairwise meta-analysis by which a connected network of treatments can be analysed simultaneously. A single analysis providing an overview over a whole disease lends itself naturally to informing clinical guidelines with respect to efficacy and safety [15]. By including all treatment options within a single analysis, treatments can be compared despite not having been compared in head-to-head trials. Furthermore, by combining direct and indirect estimates, these estimates can be more precise.
Updates to this NMA include new information on the NTD risk as well as the emerging issue of potential weight gain on a DTG-based ART regimen [16,17]. There are numerous ways in which this review and analysis expand upon the previous work. First, the addition of the high quality double-blind NAMSAL and ADVANCE trials in 2019 were predicted to be important in tackling this question [16,17]. Second, several outcomes, such as drug resistance, body weight, neuropsychiatric adverse events, and birth outcomes were added to the review and analyses. These additional outcomes help provide a more comprehensive evaluation of the efficacy and safety of first-line ART regimen being considered for use.
2. Methods
As described above, this is an evolving SLR that is an update and expansion to previously published work [2,10]. The research questions being assessed were: whether DTG can serve as the anchor treatment to the preferred first-line ART and whether EFV400 can be an alternative to EFV in first-line ART. A comprehensive systematic search of the literature was conducted on 28 February 2020 using the following databases: Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (EMBASE), and Cochrane Central Register of Controlled Trials (CENTRAL). Further manual searches of the Conference on Retroviruses and Opportunistic Infections (CROI), and the International AIDS Society and HIV Glasgow conferences were conducted. Clinical trial registries (US National Institutes of Health Clinical Trial Registry [http://www.clinicaltrials.gov] and the EU Clinical Trials Register [https://www.clinicaltrialsregister.eu]) and reference lists of identified publications were also searched. See the Web Appendix for search strategies.
2.1. Systematic literature review
For the primary population, eligible studies were randomized clinical trials (RCTs) among treatment-naïve adults and adolescents (aged 10 years or more) with HIV. The eligible study design was expanded to include comparative observational studies for sub-populations and non-comparative studies for NTDs. Sub-populations of interest were: pregnant and breastfeeding women, TB-HIV co-infected persons, pre-treatment drug-resistant PLWH, and women of childbearing age. Eligible treatment arms included EFV, EFV400, doravirine (DOR), nevirapine (NVP), rilpivirine (RPV), DTG, raltegravir (RAL), cobicistat-boosted elvitegravir (EVG/c), bictegravir (BIC), and ritonavir-boosted atazanavir (ATV/r), ritonavir or cobicistat-boosted darunavir (DRV/r-c), and lopinavir (LPV/r); each in combination with a two-NRTI (nucleoside reverse transcriptase inhibitor) backbone and each using the FDA approved dosage. Older treatments (indinavir, fosamprenavir, unboosted atazanavir, saquinavir, nelfinavir, and triple NRTIs) that were previously found to be poorly connected to the network were no longer eligible.
The primary outcomes included those previously considered: viral suppression and change in CD4 at 48, 96, and 144 weeks, mortality, discontinuations, discontinuations due to adverse events (DAEs), and treatment-related and -emergent adverse events (AEs) and serious AEs (SAEs). These were supplemented by viral suppression at 4, 12, and 24 weeks (to assess the speed of suppression), HIV drug-resistance, neuropsychiatric adverse events, weight gains and birth outcomes. See Web Appendix for full PICOS criteria.
Two investigators worked independently through abstract and full-text screening and data extraction. The validity of individual RCTs was assessed using the Cochrane Collaboration Risk of Bias instrument [18]. The validity of non-randomized studies were evaluated using the Tool to Assess the Risk of Bias in Cohort Studies, developed by the Clinical Advances through Research and Information Translation (CLARITY) group at McMaster University.
2.2. Statistical analysis
For all outcomes, we conducted NMA using Bayesian hierarchical models. We used a logistic regression model with the logit link-function and a binomial likelihood for binary outcomes (reporting treatment effects in terms of odds ratios [ORs]) and linear regression models with an identity-link and normal likelihood for continuous outcomes (reporting treatment effects in terms of mean differences). Where possible, fixed or random-effects models were applied. Model selection was conducted using the deviance information criterion according to NICE conventions [19]. Model fit was also assessed using leverage plots, and any identified outliers were further investigated. Finally, the consistency condition was evaluated using both node-splitting [20] and the Bucher test [21].
Given that the research questions concerned third agent antivirals, we defined the nodes in terms of specific antivirals rather than specific ART regimens. Defining nodes according to a single ARV simplified the network and interpretation of results. To account for differences in backbone therapies, primary analyses categorized backbone regimens as TDF+3TC/FTC (the reference category), TAF+3TC/FTC, abacavir+3TC/FTC, zidovudine+3TC/FTC, and as other and used arm-specific meta-regression to adjust estimates according to differences in backbones. A sensitivity analysis consisted of reducing the evidence base to trials that did not differ with respect to backbones.
Adjusted analyses were conducted through meta-regression adjustments to evaluate whether differences in baseline CD4, baseline log-transformed viral load, and the proportion of males impacted relative efficacy and safety estimates. Second, for viral suppression, we conducted analyses restricted to persons with high viremia (>100,000 copies/ml at baseline) given the apparent impact observed in the NAMSAL trial [22]. Further details on methods are provided in the Web Appendix.
2.3. GRADE
We employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for rating overall quality of evidence [23], [24], [25]. First, the GRADE system was applied to the direct evidence, which was examined in pairwise meta-analyses. We then updated the score according to changes obtained through NMA evidence. The certainty of evidence for each main outcome can be determined after considering direction and measure of effect, risk of bias and sample size, and categorized as either high, moderate, low, or very low [26].
2.4. Role of the funding source
The study sponsor, the WHO HIV department, helped devise the research question, but otherwise had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The first author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. Adults and adolescents
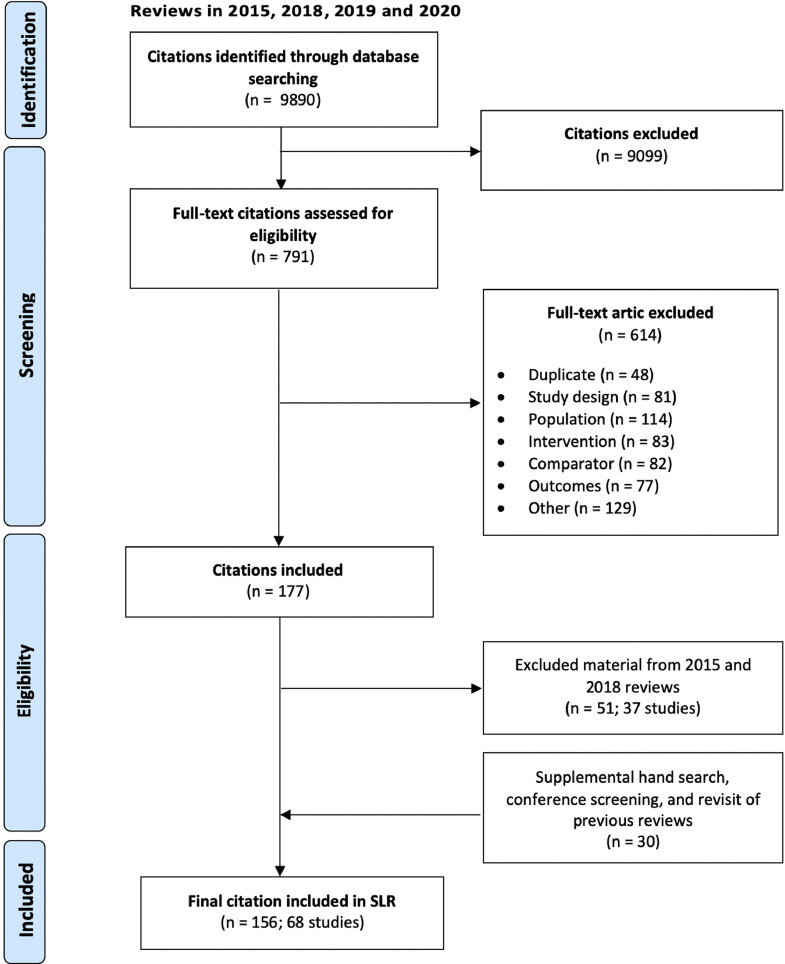
Fig. 1 presents the study selection flow diagram cumulated over all reviews. After reviewing the eligibility of studies included in previous review iterations, the final evidence set consisted of 156 publications describing 68 studies (Fig. 2; full list in the Web Appendix). The primary new studies directly addressing the research questions were the ADVANCE and NAMSAL trials [16,17]. Other new trials compared DRV/r and RAL [27], EFV to ATV/r [28], and two others differed only in backbone [29], [30], [31], [32], [33]. Further additional publications pertained to follow-up data on the more recently added trials: DRIVE-AHEAD, DRIVE-FORWARD, GS-US-380–1489, and GS-US-380–1490 [34], [35], [36], [37], [38], [39], [40], [41], [42], [43].
Fig. 1.
Flow diagram for study selection of trial publications of clinical trials comparing antiretroviral therapy on adults and adolescents living with HIV.
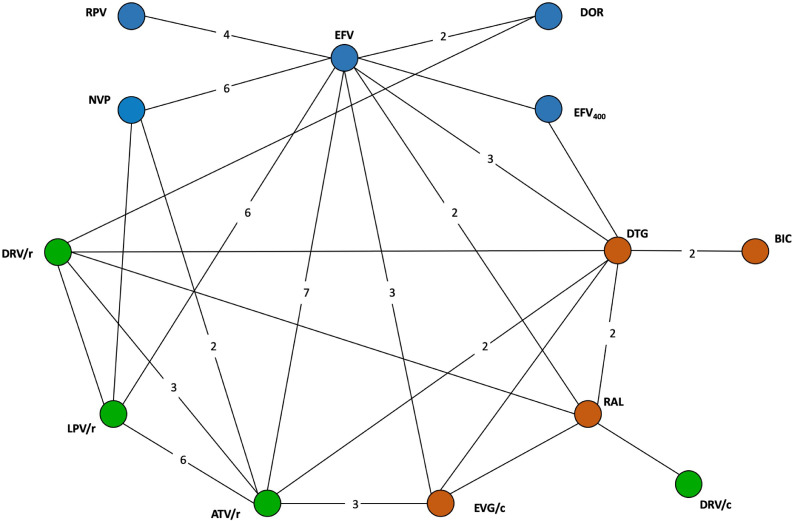
Fig. 2.
Network of evidence for adults and adolescents.
Legend: Circles (nodes) in the diagrams represent individual treatments, lines between circles represent availability of head-to-head evidence between two treatments, and the numbers on the lines are the number of RCTs informing each head-to-head comparison. Blue: NNRTIs; Green: Protease inhibitors; Orange: Integrase inhibitors. ATV/r: ritonavir-boosted atazanavir; BIC: bictegravir; DRV/r: ritonavir-boosted darunavir; DTG: dolutegravir; EFV: efavirenz; EVG/c: Elvitegravir/cobicistat; LPV/r: ritonavir-boosted lopinavir; NVP: nevirapine; RAL: raltegravir; RPV: rilpivirine.
The five key trials informing the comparisons of interest were: SINGLE [44], [45], [46], SPRING-1 [47,48], ADVANCE [17], ENCORE-1 [49], [50], [51], and NAMSAL [16]. All were multinational, double-blind, placebo-controlled randomized trials. SPRING-1 was a Phase-II trial, while the others were Phase-III trials. The risk of bias assessment suggested trials were of high quality with the most common concern being lack of blinding in some trials. Trial and patient characteristics and risk of bias assessment are provided in the Web Appendix.
Viral suppression was the most frequently reported outcome. Most trials used the FDA Snapshot algorithm whereby discontinuations are considered failures. The FDA Snapshot was used throughout and in trials not reporting such results, the missing=failure values were used instead. The only median time to suppression provided was from the SINGLE trial: 28 days with DTG and 84 days with EFV (p<0·0001) [46]. Fig. 3 presents the modelled response from networks at 4, 12, 24, and 48 weeks. These estimates suggest a more rapid progress to viral suppression using DTG followed by other integrase inhibitors relative to EFV and EFV400.
Fig. 3.
Comparisons of treatments with respect to viral suppression over time relative to treatment initiation.
ATV/r: ritonavir-boosted atazanavir; BIC: bictegravir; DRV/r: ritonavir-boosted darunavir; DTG: dolutegravir; EFV: efavirenz; EVG/c: Elvitegravir/cobicistat; LPV/r: ritonavir-boosted lopinavir; NVP: nevirapine; RAL: raltegravir; RPV: rilpivirine.
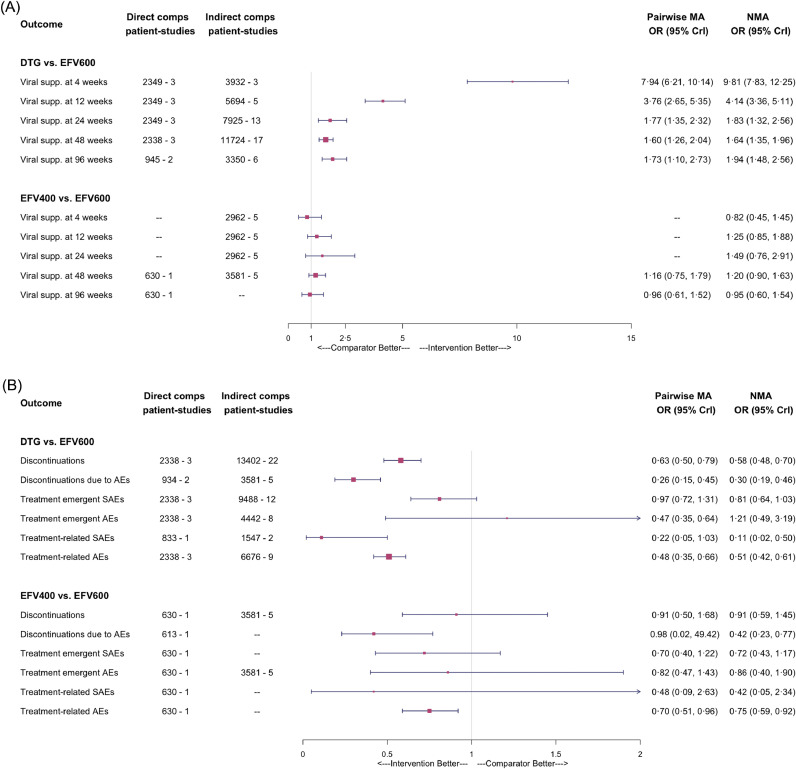
Fig. 4a presents all of the estimated relative treatment effects for viral suppression. Overall, we found high certainty evidence of durable viral suppression with DTG relative to EFV and EFV400 and moderate certainty that there is probably non-inferior viral suppression with EFV400 relative to EFV. DTG had significantly higher odds of leading to suppression at all timepoints relative to EFV (OR: 1·64; 95% credible interval [CrI]: 1·35–1·96 at 48 weeks and OR: 1·94; 95% CrI: 1·48–2·56 at 96 weeks). In contrast, the OR between the two doses of EFV were not statistically significant, with EFV400 emerging marginally better (OR: 1·20; 95% CrI: 0·90–1·63 at 48 weeks). Results of the analysis restricted to persons with baseline viremia >100,000 copies/mL were similar to the full evidence base at 48 weeks, but DTG and EFV400 appeared comparable at 96 weeks (OR: 1·06; 95% CrI: 0·55–2·09). Importantly, adjustments for differences in backbones in the subgroup analysis were only feasible at 48 weeks.
Fig. 4.
Forest plot of odds ratios obtained through network meta-analyses comparing dolutegravir to standard and low-dose efavirenz: for (A) measures of efficacy and (B) measures of tolerability and safety
DTG: dolutegravir; EFV600: standard-dose efavirenz; EFV400: low-dose efavirenz; AE: adverse events; SAE: serious adverse events; OR: odds ratio; CrI: credible interval; MA: meta-analysis; NMA:network meta-analysis.
Similarly, there was high certainty that DTG was more effective than EFV in increasing CD4 cell counts at each time point, with the increase in CD4 cell counts ranging from 41 cells/ml to 57 cells/ml. EFV400 was differentiable from standard-dose EFV at 48 and 96 weeks, with the effect-size being roughly half of that observed with DTG.
No DTG patient developed integrase inhibitor resistance (9 trials; 2639 patients) and the odds of developing resistance to the anchor treatments were lower than for EFV (OR: 0·13; 95% CrI: 0·04–0·47). Although there were no DTG events, there were sufficient events in other treatments in the network to establish a high certainty of the protectiveness of DTG with respect to selection of drug-resistance (Web Appendix).
Tolerability is an area of highest certainty for both DTG and EFV400 relative to EFV. Both demonstrated a large treatment effect for DAEs, with ORs of 0·30 and 0·42, respectively (Fig. 4b). DTG also had lower odds (OR: 0·58; 95% CrI: 0·48–0·70) of overall discontinuations relative to EFV, while the EFV400 was comparable to EFV.
For overall safety, all treatments were comparable with respect to treatment-emergent AEs and SAEs, but DTG and EFV400 were safer relative to EFV with respect to treatment-related AEs and SAEs. DTG appeared to be associated with fewer neuropsychiatric adverse events relative to EFV. Of note, the odds ratio of sleep disorders was 1·40 (95%CrI: 0·96–2·04) suggesting comparing DTG and EFV in the pairwise analysis but 0·58 (95%CrI: 0·05- 4·74) when combining direct and indirect evidence; suggesting low certainty of its benefit or harm in this regard.
More recently, the potential issue of weight gain has emerged. Unfortunately, the evidence base is sparse. At 48 weeks DTG led to an additional +1·63 Kg (95% CrI: 0·56–2·70) relative to EFV, and increased to +3·56 Kg (95% CrI: 0·75–6·40) at 96 weeks in the ADVANCE trial among patients with TDF-based backbones. Weight gains were larger in patients using TAF-based backbones.
Full results and comparisons to the full therapeutic landscape are presented in the Web Appendix.
3.2. TB co-infected PLWH
Overall, 9 studies described by 14 publications were identified, with 7 studies included in the network, and 2 studies pertaining to EFV400 only [52,53]. In this review, the evidence for HIV-TB co-infected persons treated with DTG is based primarily on the findings of the INSPIRING trial, which compared twice-daily DTG 50 mg to once-daily EFV 600 mg.
The ORs of viral suppression for DTG relative to EFV was 6·52 (95%CrI: 2·44–17·40) at 4 weeks, 2·98 (95%CrI: 1·27–6·99) at 12 weeks, 0·53 (95%CrI: 0·15–1·52) at 24 weeks, and 0·68 (95%CrI: 0·25–1·71) at 48 weeks. This moderate certainty of a faster suppression became non-differentiable at 24 weeks and beyond. The difference at 48 weeks appears to be driven by discontinuations rather than viral failure. With moderate certainty, treatment with DTG was associated with higher increases in CD4 cell count compared to all other treatments.
Treatments appeared to be comparable with respect to tolerability, but DTG appeared to be safer (overall AEs OR: 0·29; 95% CrI: 0·08–0·89). Weight gains were not measured in the INSPIRNG trial. Neuropsychiatric adverse events were only reported in the INSPIRING trial and DTG and EFV were comparable.
3.3. Pregnant and breast-feeding women
Overall, 54 references reporting on 15 studies were identified through the SLR (Web Appendix). The previous review (2018) focused on four studies: DolPHIN-1, TSEPAMO, EPPICC/PANNA, and IMPAACT 1026s [54,55]. In this review, DOLPHIN-2, ADVANCE and NAMSAL were added [16,17,56]. The TSEPAMO study is a large cohort study of pregnant women initiating DTG+TDF+3TC/FTC (n = 1729) or EFV+TDF+3TC/FTC (n = 4593) across 8 government hospitals in Botswana. Due to its size relative to other studies, most information on pregnancy outcomes comes primarily from Tsepamo. In all studies combined, the proportion of pregnancies with any adverse birth outcome was similar with 33.2% of DTG-exposed pregnancies and 35.0% of EFV-exposed pregnancies.
Fig. 5 presents the NMA-modelled proportions for pregnancy outcomes. In all but one outcome, the negative outcome estimate was lower or comparable for DTG relative to EFV, suggesting that DTG initiated during pregnancy is a relatively safe option. The only exception was severe adverse events among mothers, which was based on 26 and 18 events in the DTG and EFV arms, respectively, in the DOLPHIN-2 trial.
Fig. 5.
Modelled risk of birth and maternal outcomes among women using dolutegravir and efavirenz
DTG: dolutegravir; EFV: efavirenz; SGA: small for gestational age
Proportion of patients with virologic response with the following definitions: - Plasma HIV RNA <50 copies/ml at week 24 - Rate of strategy discontinuation and treatment changes - Proportion of death - Proportion of patients loss to follow-up24 weeks; Proportion of patients with virologic response with the following definitions: o Plasma HIV RNA <50 copies/ml o Plasma HIV RNA <400 copies/ml24 and 48 weeks; Evolution in HIV RNA and HIV DNA (total and 2 LTR circular) from baseline to week 4848 weeks; Rate of viral resistance mutations in the plasma at the time of virologic failure and in comparison with HIV-RNA mutations at W0At the time of virologic failure; Evolution of CD4 cell counts from baseline to week 4848 weeks; Frequency, type and time to a new AIDS-defining event or deathThrough out the trial; Frequency, type, time to grade 3 or 4 adverse eventThrough out the trial; Rate of success of TB treatment48 weeks; Anti-TB resistance rate48 weeks; Evolution of raltegravir and efavirenz trough concentrationThrough out the trial.
For NTDs, the estimate combining the updated Tsepamo results through March 201,957 with the Botswana MoH/CDC study[58] results is a risk difference between DTG and EFV of 0·29% (95% CI: 0·10–0·68%). We excluded other data from the meta-analysis given the substantial heterogeneity between countries with respect to folic acid supplements and lower background rates of NTDs (See Web-Appendix).
3.4. Pre-treatment drug-resistance
There was no network of evidence for this subpopulation. ENCORE-1 and NAMSAL were the only trials to report on this subpopulation and comparing EFV, EFV400, and/or DTG; however, there were too few data from these comparisons to draw meaningful inferences (Web-Appendix) [22,50]. There were no data on PLWH with pre-treatment drug-resistance using DTG.
4. Discussion
The purpose of this study was to determine the comparative efficacy, safety, and tolerability of DTG and EFV400 relative to EFV, each with a TDF+3TC/FTC backbone, as a first-line ART regimen. These comprehensive SLR and NMAs found improved efficacy and tolerability of DTG relative to EFV, now reinforced by its robustness among sub-populations. The concern over the higher risk of NTD among peri‑conception exposures to DTG still exists; however, as more data has become available since the previous guideline revisions, the estimated effect has been considerably reduced. This updated data on risk, together with a comprehensive modeling of the risks and benefits of giving DTG to women of childbearing potential supports DTG+TDF+3TC/FTC as the preferred first-line regimen across all populations [59,60]. Similarly, this study demonstrated that EFV400 is comparable to EFV in terms of efficacy and safety but is more tolerable.
Despite high quality evidence of improved efficacy and tolerability of DTG relative to EFV in the 2016 [2], the uncertainty around its use in sub-populations (pregnant women and TB-HIV co-infection), the unavailability of a fixed-dose combination with TDF+3TC/FTC, and the prohibitive price prevented its recommendation as the preferred first-line regimen [1]. All of these factors have now been addressed. In 2018, with the availability of a generic fixed-dose combination with TDF+3TC, referred to as TLD [61], and studies among sub-populations having begun to report results, the SLR and NMA informed the change in recommendations to make DTG+TDF+3TC/FTC the preferred first-line ART regimen in the interim guideline [14]. These updated results found high certainty of faster viral suppression, protectiveness with respect to drug-resistance and improved safety, in addition to the already established high certainty with respect to viral suppression, discontinuations and DAEs. The high barrier to drug-resistance is important given the growing proportion of PLWH with PDR to NNRTIs [62]. Despite this compelling support for DTG as the preferred first-line treatment, two new concerns did emerge: elevated risk of NTDs and potential elevated weight gains.
An unplanned interim analysis of the Tsepamo study in Spring 2018 provided a signal of potentially elevated risk of NTDs in women with periconception exposure to DTG. The updated TSEPAMO analysis using data up to March 2019 did show a large reduction in the estimated risk of NTDs with DTG compared to EFV [57], from close to 1% increase in risk to 0.29%. Since the SLR and analyses were conducted for this study, new data from Botswana were presented at the AIDS2020 conference showing a 0.09% (95% CI: −0.03% to 0.30%) difference in prevalence between DTG and all other exposures at conception (i.e., a decrease in difference that is no longer statistically different) [63]. At the same conference, another meta-analysis supported our findings of no difference in vertical transmissions and marginal evidence of reduced adverse pregnancy outcomes between DTG and EFV [64].
Concerns over weight gain primarily arise from the ADVANCE and NAMSAL trials[16,17]; however, they are also supported by cohort studies [65,66]. Given the chronic nature of HIV and the aging HIV population, weight gain is an important safety concern. At the moment, there is only moderate certainty of important weight gains with DTG. This degree of certainty is attributable to lack of historical data to support findings from newer trials, a need for a better understanding of factors that influence weight gain, and a need to better understand the nature of the weight gain (e.g., a return to normal weight vs. a movement into high BMI). Factors that may influence weight gain include race, sex, and backbone combinations. The combination of DTG and TAF appears to lead to double the relative weight gain than DTG combined with TDF, possibly explained by the weight loss induced by TDF in some patients. ART regimens lead to weight gains and it is important to better understand what constitutes a safe average weight gain. Moreover, it is unclear whether these weight gains are sustained over longer periods or whether they plateau over time. It will be necessary to monitor weight gains as they can lead to notable co-morbidities and adversely impact pregnancy outcomes.
In previous reviews, the evidence on EFV400 compared to standard-dose EFV came entirely from the ENCORE1 trial [49]. For this review, the indirect comparison through DTG became possible with the addition of the NAMSAL trial [22]. The comparable efficacy and safety and improved tolerability suggest that EFV400 may represent a better choice of alternative first-line regimens. As opposed to the 2015 review, there were no studies providing support for the use of EFV400 in both TB-HIV co-infected PLWH and in pregnant women. An area of potential concern for EFV400 is its use in highly drug-resistant populations; however, there are few data among highly drug-resistant patients and conclusions from ENCORE-1 and NAMSAL were discordant. Interestingly, the addition of the NAMSAL trial had minimal impact on the conclusions drawn in 2016, providing reassurance of the NMA methods used to draw these inferences.
This study has numerous strengths and limitations. In addition to strengths previously discussed [2], the expansion of outcomes allowed for a more complete picture of the benefits of DTG (e.g., drug-resistance) and areas of potential concern (e.g., NTDs). With respect to limitations, first, there were too few data to draw definite conclusions regarding the effect of DTG on NTDs. Progress was made and while there remains evidence of an elevated risk, the possibility of no elevated risk is still reasonable given the downward trend in risk observed with additional data. Second, more data on weight are needed and given that it affects people differently, it is critical that more sophisticated analyses with individual PLWH be conducted to help better assess risk for individuals. Third, evidence was at times sparse. Despite a large network, the comparisons with EFV400 were primarily driven by two trials only: ENCORE-1 and NAMSAL. Although a trial was available comparing DTG to EFV among TB-HIV co-infected PLWH, the trial was small and only 24-week results were available, leading to low-quality evidence. Finally, some significant outcomes (mortality, drug-resistance, etc.) were limited by a very low number of events. Although the low counts influenced the precision or precluded the conduct of evidence synthesis for these outcomes, it can also be viewed as evidence of improved efficacy and safety of these treatments.
In conclusion, the evidence supports the choice of a DTG in combination with TDF+3TC/FTC as the preferred first-line regimen and EFV400 regimens as an alternative. DTG can be considered to be an effective, safe and tolerable anchor treatment. Across a variety of outcomes, evidence strongly suggests that it is superior to efavirenz. Moreover, generic fixed-dose combinations of DTG-based regimens are now available and there is greater certainty of its use in critical sub-populations resulting in greater confidence in the choice of this regimen as the preferred ARV treatment for a public health approach.
Author contributions
Steve Kanters had full access to all of the data in the study. Steve Kanters takes responsibility for the integrity of the data, the accuracy of the data analysis, and the final decision to submit for publication.
Study concept and design: Kanters, Vitoria, Rangaraj, Doherty, Penazzato, Mofenson and Bansback
Acquisition, analysis, or interpretation of data: Kanters, Zoratti, Mofenson, and Vitoria
Drafting of the manuscript: Kanters, Rangaraj, Vitoria, and Bansback
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Kanters, Thorlund, Bansback
Obtained funding: Kanters
Administrative, technical, or material support: Kanters, Vitoria, Thorlund and Bansback
Study supervision: Doherty, Vitoria, Penazzato, and Bansback
Declaration of Competing Interest
Dr. Karim reports grants from Michael Smith Foundation for Health Research, grants from Natural Sciences and Engineering Research Council, grants from BC SUPPORT Unit, grants from Canadian Institutes of Health Research, personal fees from Biogen Inc., outside the submitted work. Dr. Alexandra reports grants from Unrestriceted Educational Grant (for the Unit) by MSD and Gilead Sciences, grants from Financial support by Gilead Sciences, AbbVie, MSD, ViiV Healthcare and Janssen Cilag for the day hospital, outside the submitted work. All other authors have nothing to declare.
Acknowledgments
Acknowledgments
The authors would like to thank the WHO guideline development group for their support and critical feedback:
https://www.who.int/hiv/mediacentre/news/Bio_ARV_GDG-2019-a.pdf . The authors would also like to thank Monica Hughes, Evan Popoff, and Jamie I Forrest for their role in the systematic literature review over the lifetime of this dynamic systematic literature review.
Data sharing
All non-confidential data are available upon request.
Funding
WHO HIV department.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100573.
Appendix. Supplementary materials
References
- 1.World Health Organization . 2016. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection recommendations for a public health approach - Second edition. Geneva, Switzerland. [PubMed] [Google Scholar]
- 2.Kanters S., Vitoria M., Doherty M. Comparative efficacy and safety of first-line antiretroviral therapy for the treatment of HIV infection: a systematic review and network meta-analysis. Lancet HIV. 2016;3(11) doi: 10.1016/S2352-3018(16)30091-1. e510–e20. [DOI] [PubMed] [Google Scholar]
- 3.Vitoria M., Ford N., Clayden P., Pozniak A.L., Hill A.M. When could new antiretrovirals be recommended for national treatment programmes in low-income and middle-income countries: results of a WHO Think Tank. Curr Opin HIV AIDS. 2017;12(4):414–422. doi: 10.1097/COH.0000000000000380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilks C.F., Crowley S., Ekpini R. The WHO public-health approach to antiretroviral treatment against HIV in resource-limited settings. Lancet. 2006;368(9534):505–510. doi: 10.1016/S0140-6736(06)69158-7. [DOI] [PubMed] [Google Scholar]
- 5.Mills E.J., Bakanda C., Birungi J. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 6.Nsanzimana S., Remera E., Kanters S. Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet Glob Health. 2015;3(3):e169–e177. doi: 10.1016/S2214-109X(14)70364-X. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M.S., Chen Y.Q., McCauley M. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Sadr W.M., Holmes C.B., Mugyenyi P. Scale-up of HIV treatment through PEPFAR: a historic public health achievement. J Acquir Immune Defic Syndr. 2012;60(Suppl 3):S96–104. doi: 10.1097/QAI.0b013e31825eb27b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. March 2014 supplement to the 2013 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2014; [PubMed]
- 10.Kanters S., Jansen J., Zoratti M., Forrest J., Humphries B., Campbell J. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. World Health Organization; Geneva, Switzerland: 2018. WEB ANNEX B. Systematic literature review and network meta-analysis assessing first-line ART treatments. [Google Scholar]
- 11.Zash R., Holmes L., Makhema J. Surveillance for neural tube defects following antiretroviral exposure from conception, the Tsepamo study (Botswana). Proceedings of the 22nd international AIDS conference 23-27 July 2018; Amsterdam Netherlands; 2018. [Google Scholar]
- 12.Food and Drug Administration. FDA drug safety communication: FDA to evaluate potential risk of neural tube birth defects with HIV medicine dolutegravir (Juluca, Tivicay, Triumeq). 2018.
- 13.World Health Organization. Statement on DTG. Geneva Switzerland2018.
- 14.World Health Organization . Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization; Geneva, Switzerland: 2018. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines. [Google Scholar]
- 15.Kanters S., Ford N., Druyts E., Thorlund K., Mills E.J., Bansback N. Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94(10):782–784. doi: 10.2471/BLT.16.174326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouanfack C., Mpoudi-Etame M., Omgba Bassega P. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. doi: 10.1056/NEJMoa1904340. [DOI] [PubMed] [Google Scholar]
- 17.Venter W.D.F., Moorhouse M., Sokhela S. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803–815. doi: 10.1056/NEJMoa1902824. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J., Altman D., Gotzsche P. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias S., Welton N., Sutton A., Ades A. Technical support document 2: a generalized linear modelling framework for pairwise and network meta-analysis of randomized controlled trials. 2011; [PubMed]
- 20.Dias S., Welton N.J., Caldwell D.M., Ades A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 21.Bucher H.C., Guyatt Gh Fau - Griffith L.E., Griffith Le Fau - Walter S.D., Walter S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol(0895-4356 (Print)): [DOI] [PubMed]
- 22.Cournil A., Kouanfack C., Eymard-Duvernay S. Dolutegravir-versus an efavirenz 400mg-based regimen for the initial treatment of HIV-infected patients in Cameroon: 48-week efficacy results of the NAMSAL ANRS 12313 trial. J Int AIDS Soc. 2018;21(Supplement 8):16. [Google Scholar]
- 23.Guyatt G., Oxman A., Kunz R. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol. 2011;64(12):1303–1310. doi: 10.1016/j.jclinepi.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Guyatt G., Oxman A., Sultan S. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G., Oxman A., Vist G. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias) J Clin Epidemiol. 2011;64(4):407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G., Oxman A., Vist G. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mussini C., Roncaglia E., Sighinolfi L. A prospective randomised trial on abacavir/lamivudine plus darunavir/r or raltegravir in patients with CD4 + <200 cells/uL (PRADAR study) J Int AIDS Soc. 2018;21(Supplement 8):41–42. [Google Scholar]
- 28.Perez-Valero I., Bailon L., Gonzalez A. Brain volumes changes after ABC/3TC + EFV or TDF/FTC + ATV/R as first-line ART. Top Antivir Med. 2018;26(Supplement 1):171s. [Google Scholar]
- 29.Gallant J., Orkin C., Molina J. Week-48 results of AMBER: phase 3, randomised, double-blind trial in antiretroviral treatment (ART)-naive HIV-1-infected adults to evaluate the efficacy and safety of the once-daily, single-tablet regimen (STR) of darunavir/ cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) versus darunavir/cobicistat (DRV/c) plus emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) HIV Med. 2018;19(Supplement 2):S32. [Google Scholar]
- 30.Orkin C., Eron J., Rockstroh J. Efficacy and safety of the once-daily, darunavir/cobicistat/ emtricitabine/tenofovir alafenamide (D/C/F/TAF) singletablet regimen (STR) in ART-naive, HIV-1-infected adults: AMBER Week 96 results. J Int AIDS Soc. 2018;21(Supplement 8):8–10. [Google Scholar]
- 31.Rashbaum B., McDonald C., Mussini C. Age, gender, and race analyses of D/C/F/TAF in HIV-1 treatment naive patients. Top Antivir Med. 2018;26(Supplement 1):203s. [Google Scholar]
- 32.Venter F., Hill A., Kambugu A. Efficacy and safety of tenofovir disoproxil fumarate versus low-dose stavudine over 96 weeks: a randomised, noninferiority trial. J Int AIDS Soc. 2018;21(Supplement 8):44. doi: 10.1097/QAI.0000000000001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venter F., Kambugu A., Chersich M. Efficacy and Safety of Tenofovir Disoproxil Fumarate Versus Low-Dose Stavudine Over 96 Weeks: a Multicountry Randomized, Noninferiority Trial. J Acquir Immune Defic Syndr. 2019;80(2):224–233. doi: 10.1097/QAI.0000000000001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acosta R., White K., Garner W. HIV-1 subtype (B or non-B) had no impact on the efficacy of B/F/TAF or resistance development in five phase 3 traetment-naive or switch studies. Proceedings of the 22nd international AIDS conference; Amsterdam, Netherlands; 2018. [Google Scholar]
- 35.Gupta S., Mills A., Brinson C. 96 week efficacy and safety of B/F/TAF in treatment-naive adults and adults ≥50 years. Proceedings of the 26th conference on retroviruses and opportunistic infections (CROI); Seattle, Washington; 2019. [Google Scholar]
- 36.Molina J., Squires K., Sax P. Doravirine (DOR) versus ritonavir-boosted darunavir (DRV+r): 96-week results of the randomized, doubleblind, phase 3 DRIVE-FORWARD Noninferiority trial. Proceedings of the 22nd international AIDS conference; Amsterdam, Netherlands; 2018. [Google Scholar]
- 37.Molina J., Squires K., Sax P.E. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV. 2018;5(5) doi: 10.1016/S2352-3018(18)30021-3. e211–e20. [DOI] [PubMed] [Google Scholar]
- 38.Orkin C., Molina J., Lombaard J. Integrated efficacy analysis of doravirine in HIV-1 infection treatment-naive adults. Proceedings of the 26th conference on retroviruses and opportunistic infections (CROI); Seattle, Washington; 2019. [Google Scholar]
- 39.Orkin C., Molina J., Lombaard J. Once-daily doravirine in HIV-1-infected, antiretroviral-naive adults: an integrated efficacy analysis. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz424. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Orkin C., Squires K., Molina J. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis. 2019;68(4):535–544. doi: 10.1093/cid/ciy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podzamczer D., Stellbrink H., Orkin C. B/F/TAF versus ABC/DTG/3TC or DTG + F/TAF in treatment-naïve adults with high baseline viral load or low baseline CD4 count in 2 Phase 3 randomized, controlled clinical trials. Proceedings of the 22nd international AIDS conference; Amsterdam, Netherlands; 2018. [Google Scholar]
- 42.Stellbrink H., Arribas J., Stephens J. Phase III randomized, controlled clinical trial of bictegravir coformulated with FTC/TAF in a fixed-dose combination (B/F/TAF) versus dolutegravir (DTG) + F/TAF in treatmentnaive HIV-1 positive adults: week 96. J Int AIDS Soc. 2018;21(Supplement 8):8. [Google Scholar]
- 43.White K., Kulkarni R., Willkom M. Pooled week 48 efficacy and baseline resistance: B/F/TAF in treatment-naive patients. Top Antivir Med. 2018;26(Supplement 1) 224s–5s. [Google Scholar]
- 44.Walmsley S., Baumgarten A., Berenguer J. Brief report: dolutegravir plus abacavir/lamivudine for the treatment of HIV-1 infection in antiretroviral therapy-naive patients: week 96 and week 144 results from the SINGLE randomized clinical trial.[Erratum appears in J Acquir Immune Defic Syndr. 2016 Jan 1;71(1):e33] J Acquir Immune Defic Syndr. 2015;70(5):515–519. doi: 10.1097/QAI.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walmsley S., Berenguer J., Khuong-Josses M.A. Dolutegravir regimen statistically superior to efavirenz/tenofovir/emtricitabine: 96-week results from the SINGLE study (ING114467). Proceedings of the 21st CROI conference; Boston, USA; 2014. [Google Scholar]
- 46.Walmsley S.L., Antela A., Clumeck N. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. doi: 10.1056/NEJMoa1215541. [DOI] [PubMed] [Google Scholar]
- 47.Stellbrink H., Reynes J., Lazzarin A. Dolutegravir in antiretroviral-naive adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013;27(11):1771–1778. doi: 10.1097/QAD.0b013e3283612419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen C., Wohl D., Arribas J. STAR study: single tablet regimen emtricitabine/rilpivirine/ tenofovir DF is non-inferior to efavirenz/emtricitabine/ tenofovir DF in ART-naive adults. J Int AIDS Soc. 2012;15:24. [Google Scholar]
- 49.Amin J., Becker S., Belloso W. Efficacy of 400mg efavirenz versus standard 600mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial. Lancet. 2014;383(9927):1474–1482. doi: 10.1016/S0140-6736(13)62187-X. [DOI] [PubMed] [Google Scholar]
- 50.Encore Study Group. Carey D., Puls R. Efficacy and safety of efavirenz 400mg daily versus 600mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study.[Erratum appears in Lancet Infect Dis. 2015 Jul;15(7):761; PMID: 26122437] Lancet Infect Dis. 2015;15(7):793–802. doi: 10.1016/S1473-3099(15)70060-5. [DOI] [PubMed] [Google Scholar]
- 51.Puls R., Srasuebkul P., Petoumenos K. Efavirenz versus boosted atazanavir or zidovudine and abacavir in antiretroviral treatment-naive, HIV-infected subjects: week 48 data from the Altair study. Clin Infect Dis. 2010;51(7):855–864. doi: 10.1086/656363. [DOI] [PubMed] [Google Scholar]
- 52.Cerrone M., Wang X., Neary M. Pharmacokinetics of efavirenz 400mg once daily coadministered with isoniazid and rifampicin in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2019;68(3):446–452. doi: 10.1093/cid/ciy491. [DOI] [PubMed] [Google Scholar]
- 53.Kaboggoza J., Wang X., Neary M. A lower dose of efavirenz can be coadministered with rifampicin and isoniazid in tuberculosis patients. Open Forum Infect Dis. 2019;6(2):ofz035. doi: 10.1093/ofid/ofz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulligan N., Best B., Wang J. Dolutegravir pharmacokinetics in pregnant and postpartum women living with HIV. AIDS. 2018;32(6):729–737. doi: 10.1097/QAD.0000000000001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorne G., Favarato H., Peters A. 24th pregnancy and neonatal outcomes following prenatal exposure to dolutegravir. Proceedings of the conference on retroviruses and opportunistic infections (CROI); Seattle WA; 2017. [Google Scholar]
- 56.Kintu K., Malaba T., Nakibuka J. RCT of dolutegravir vs efavirenz-based therapy initiated in late pregnancy: DOLPHIN-2. Proceedings of the 26th conference on retroviruses and opportunistic infections (CROI); Seattle WA; 2019. [Google Scholar]
- 57.Zash R., Holmes L., Diseko M. Neural-Tube Defects and Antiretroviral Treatment Regimens in Botswana. N Engl J Med. 2019;381(9):827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raesima M.M., Ogbuabo C.M., Thomas V. Dolutegravir use at conception - additional surveillance data from Botswana. N Engl J Med. 2019;381(9):885–887. doi: 10.1056/NEJMc1908155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dugdale C.M., Ciaranello A.L., Bekker L.G. Risks and benefits of dolutegravir- and efavirenz-based strategies for South African women with HIV of child-bearing potential: a modeling study. Ann Intern Med. 2019;170(9):614–625. doi: 10.7326/M18-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips A.N., Bansi-Matharu L., Venter F. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: modelling to inform treatment guidelines. Lancet HIV. 2020;7(3):e193–e200. doi: 10.1016/S2352-3018(19)30400-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.UNAIDS. New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low-and middle-income countries at reduced price. 2017.
- 62.Hamers R.L., Rinke de Wit T.F., Holmes C.B. HIV drug resistance in low-income and middle-income countries. Lancet HIV. 2018;5(10) doi: 10.1016/S2352-3018(18)30173-5. e588-e96. [DOI] [PubMed] [Google Scholar]
- 63.Zash R, Holmes L, Diseko M, editors. Proceedings of the Botswana. international AIDS conference. 2020. Update on neural tube defects with antiretroviral exposure in the Tsepamo study. 2020; Virtual. [Google Scholar]
- 64.Asif S., Baxevanidi E., Hill A. Proceedings of the international AIDS conference; virtual. 2020. Assessing the safety and efficacy of dolutegravir in HIV-positive pregnant women in Sub-Saharan Africa: a meta-analysis. [Google Scholar]
- 65.Bourgi K., Jenkins C.A., Rebeiro P.F. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484. doi: 10.1002/jia2.25484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Taramasso L., Bonfanti P., Ricci E. Factors associated with weight gain in people treated with dolutegravir. Open Forum Infect Dis. 2020;7(6):ofaa195. doi: 10.1093/ofid/ofaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.