Abstract
Background
Many studies investigate the role of pharmacological treatments on disease course in Corona Virus Disease 2019 (COVID-19). Sex disparities in genetics, immunological responses, and hormonal mechanisms may underlie the substantially higher fatality rates reported in male COVID-19 patients. To optimise care for COVID-19 patients, prophylactic and therapeutic studies should include sex-specific design and analyses. Therefore, in this scoping review, we investigated whether studies on pharmacological treatment in COVID-19 were performed based on a priori sex-specific design or post-hoc sex-specific analyses.
Methods
We systematically searched PubMed, EMBASE, UpToDate, clinical trial.org, and MedRxiv for studies on pharmacological treatment for COVID-19 until June 6th, 2020. We included case series, randomized controlled trials, and observational studies in humans (≥18 years) investigating antiviral, antimalarial, and immune system modulating drugs. Data were collected on 1) the proportion of included females, 2) whether sex stratification was performed (a priori by design or post-hoc), and 3) whether effect modification by sex was investigated.
Findings
30 studies were eligible for inclusion, investigating remdesivir (n = 2), lopinavir/ritonavir (n = 5), favipiravir (n = 1), umifenovir (n = 1), hydroxychloroquine/chloroquine (n = 8), convalescent plasma (n = 6), interleukin-6 (IL-6) pathway inhibitors (n = 5), interleukin-1 (IL-1) pathway inhibitors (n = 1) and corticosteroids (n = 3). Only one study stratified its data based on sex in a post-hoc analysis, whereas none did a priori by design. None of the studies investigated effect modification by sex. A quarter of the studies included twice as many males as females.
Interpretation
Analyses assessing potential interference of sex with (side-)effects of pharmacological therapy for COVID-19 are rarely reported. Considering sex differences in case-fatality rates and genetic, immunological, and hormonal mechanisms, studies should include sex-specific analyses in their design to optimise COVID-19 care.
Funding
None
Keywords: Sex, Diversity, COVID-19, Clinical trials, Therapy
Research in context.
Evidence before this study
Given the impact of sex on diseases and therapy as revealed in the past, we systematically reviewed this potential knowledge gap by performing a scoping review on clinical trials investigating pharmacological therapies for COVID-19. Searching the literature in PubMed, EMBASE, UpToDate, clinical trial.org and Medrixv up to June 6th 2020, we included case series, randomized controlled trials and observational studies in humans, which investigated antiviral, antimalarial and immune system modulating drugs.
Added value of this study
This scoping review reports four main findings. First, none of thirty clinical trials on pharmacological treatment for COVID-19 included sex-stratified randomization in its design. Second, only one study stratified its results based on sex by post-hoc analysis. Third, none of the studies evaluated sex as a potential effect modifier. Finally, almost a quarter of the studies included two times more males than females.
Implications of all the available evidence
We call investigators who are developing and testing therapeutic and prophylactic approaches for COVID-19 to incorporate sex-stratified randomization into their study protocol. For already published studies, we recommend investigators to perform post-hoc sex-specific analyses, while considering potential power insufficiencies in the interpretation of the results. Furthermore, we recommend generating an open access database with data from completed clinical COVID-19 trials, to improve the power to detect sex-differences compared to the current trials.
Alt-text: Unlabelled box
1. Introduction
In early 2020, a novel β-coronavirus causing Severe Acute Respiratory Syndrome (SARS-CoV-2) rapidly spread around the world, resulting in a pandemic with global impact [1,2]. On June 9th, 2020, SARS-CoV-2, causing Corona Virus Disease 2019 (COVID-19), reached a worldwide case fatality rate of 5.7% [3,4]. Although patients are unique individuals, they are often categorised according to disease or condition and treated using a 'one-size-fits-all'-approach. However, the disease can unfold in individuals diversely and variably. This heterogeneity may have significant implications in therapy effectiveness. For COVID-19, heterogeneity in ethnicity, comorbidities and age might impact disease course (ranging from common flu-like symptoms to critical illness requiring intensive care admission) and complications (pulmonary embolisms, kidney failure or cardiac injury) [2].
Although males and females are affected by COVID-19 at comparable incidence, case fatality rates are higher for males (10.4%) compared to females (7.0%), resulting in a markedly male-to-female case fatality ratio of 1.5, according to the Global Health 50/50 data tracker [5]. Sex-stratified proportions differ from global numbers as not all countries report their data specified for males and females, including the United States of America. Age and comorbidities also strongly affect the fatality rates as mainly elderly, and patients with underlying cardiovascular diseases appear to be affected [6]. Despite these differences in fatality rates, a thorough analysis of underlying contributory factors is lacking [7], [8], [9]. Sex has been shown to contribute to disparities in widespread diseases in the past and may have an influence on vulnerability and differentials in incidence and case fatality between males and females [10].
There is growing evidence that sex differences play a role in the immunological, hormonal, and cardiovascular pathophysiological responses to SARS-CoV-2 [10,11]. Historically, in medical research on other cardiovascular and infectious diseases, women seem underrepresented in clinical and pharmacological trials, and data are rarely reported separately for males and females [10], [11], [12]. Consequently, unrevealed differences in disease presentation and progression between men and women may have been missed, and system-biological differences in (side)-effects of pharmacological therapy undetected. In COVID-19 patients, it is expected that these disparities also affect the severity of the virus infection, disease course, and (side)-effects of initiated therapy [11,12].
In this scoping review, we systematically reviewed completed clinical studies on pharmacological therapy for COVID-19 patients. For these studies, we reported male-to-female ratios of included patients and whether the studies were performed based on an a priori sex-specific design or post-hoc sex-specific analyses.
2. Methods
This scoping review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline [13]. Four independent investigators (E.J., V.S., L.J., and C.G.) systematically searched PubMed and EMBASE. They reviewed original research articles investigating antiviral, antimalarial, and immune-modulating pharmacological treatments for COVID-19, that were thought to be potentially effective to treat symptoms of COVID-19 at time of study inclusion and/or mentioned in the "Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19″ [14] (for keywords used in the literature search, see Supplementary file). Randomized controlled trials (RCTs), observational studies, and case series (defined as ≥5 reported patients) were eligible for inclusion. Besides, studies had to investigate a single, specific therapy in COVID-19 patients aged ≥18 years. To be eligible for inclusion, RCTs and observational studies had to investigate both a treatment and control group, in which the latter was allowed to vary between standard care, a specific pharmacological treatment or no pharmacological treatment. We excluded in vitro studies, animal studies, trials investigating different doses or settings of one similar drug in different groups, and trials not reporting the proportion of male and female patients. The search was limited to English and Dutch studies published between December 2019 and June 6th, 2020.
The initial study selection, based on title and abstract, and the subsequent selection, based on the full-text, were performed independently by four investigators (E.J., V.S., L.J., and C.G.). All disagreements were resolved by discussion and mutual agreement. Furthermore, reviews, UpToDate, clinicaltrial.org, and MedRxiv, were examined for additional eligible studies. After checking the preselected publications and cross-checking the reference lists, the relevant data were extracted from the final selection of studies, as described in the section below.
2.1. Data extraction
A pre-defined data-extraction sheet, including characteristics and outcomes of interest, was used. Data were extracted on the description of sex, sex stratification (predesigned a priori or by post-hoc analyses), and effect modification by sex. The potential effect of treatments was beyond the scope of this review. Extractions were performed independently by two investigators (E.J. and V.S.). Discrepancies were resolved by dialogue (E.J. and V.S.) and discussion with a third investigator (C.G.). The proportion of female inclusions was reported for the total population (pooling case series, observational studies, and RCT's) and separately for treatment and control groups (pooling only observational studies and RCT's). Pharmacological treatment modalities were clustered in the following groups: antiviral, antimalarial, and immune system modulating drugs. We reported male-to-female ratios of included patients for each study and each pharmacological treatment under investigation. To calculate treatment-stratified male-to-female ratios, we used the absolute inclusion numbers and reported a minimum and maximum range of the individual study ratios. A ratio of >1 indicates a higher proportion of included men compared to women.
2.2. Role of the funding source
There were no funders or sponsors that had any contribution to the study design, data collection, analysis, preparation or decision to publish this manuscript.
3. Results
3.1. General
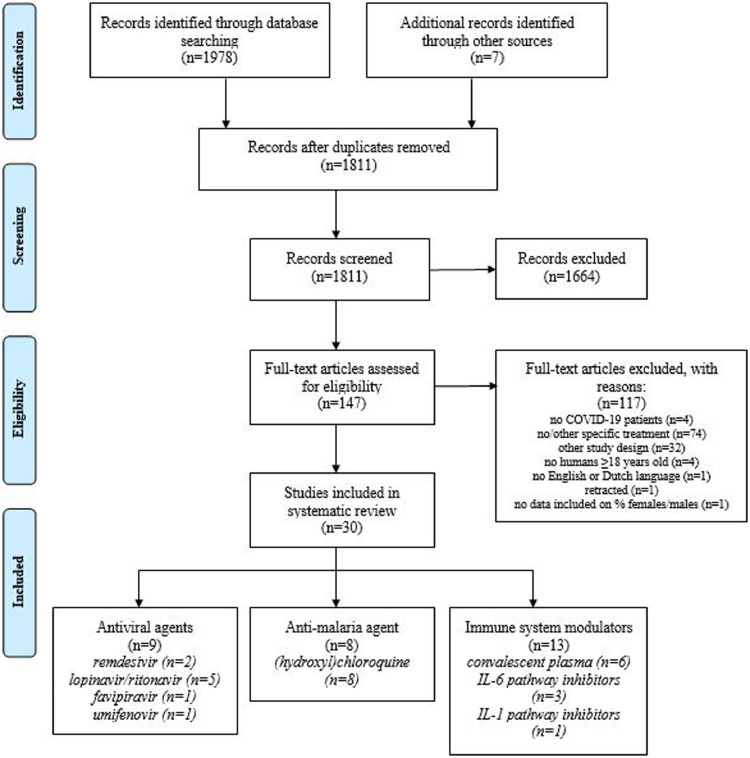
Our database search resulted in 1978 studies and by cross-checking reviews, clinicaltrial.org [15], UpToDate [16], and MedRxiv [17] we added seven additional studies that were in preprint stage by the time we performed our search. After removing duplicates, we started our first selection with 1811 studies (see Fig. 1 ). After screening articles based on title and abstract, we excluded 1664 studies, resulting in 147 studies eligible for the second screening based on full-text assessment. Based on the second screening, we additionally excluded 117 studies, amongst which the study of Mehra et al. [18] as it was retracted and the study of Klopfenstein et al. [19] as it did not report the number of included females and males. As a result, we included 30 eligible unique research articles on pharmacologic therapies for COVID-19 in this scoping review (Fig. 1).
Fig. 1.
Flowchart of study selection and inclusion after systematic literature search.
The total number of 30 included studies consisted of seven case series, eight RCTs, and fifteen observational studies. As shown in table 1 , included studies investigated remdesivir (n = 2), lopinavir/ritonavir (n = 5), favipiravir (n = 1), umifenovir (n = 1), hydroxychloroquine/chloroquine (n = 8), convalescent plasma (n = 6), interleukin-6 (IL-6) pathway inhibitors (n = 3), interleukin-1 (IL-1) pathway inhibitors (n = 1) and corticosteroids (n = 3). The five included case series reported individual patient data, including sex. Of all observational studies and RCTs, only one study by Beigel et al. [20] performed post-hoc sex-specific analysis (Table 2 ). As a result, none of the 30 included studies incorporated sex-stratified randomization a priori into their design, and 29 of 30 studies (97%) did not stratify their results based on sex post-hoc (Table 1 and 2). None of the included studies investigated effect modification by sex using interaction-terms between therapy and sex in their analyses (Table 1 and 2). Study characteristics are comprehensively described in Supplementary Table 1.
Table 1.
Total and therapy-stratified male-to-female ratios of included studies.
| Studies (n) | Sex-specific analysis (n) | Patients (n) | Female (%) | Male-to-female ratio (range) | |
|---|---|---|---|---|---|
| I. TOTAL | 30 | 1 | 6156 | 41 | 1•4 (0•7–7•0) |
| treatment group | 23 | . | 3292 | 40 | 1•5 (0•8–5•0) |
| control group | 23 | . | 2757 | 43 | 1•3 (0•5–7•0) |
| II. THERAPY GROUPS | |||||
| II.I ANTIVIRAL AGENTS | |||||
| Viral entry and replication inhibitors | |||||
| Remdesivir | 2 | 1 | 1299 | 37 | 1•7 (1•5–1•8) |
| treatment group | 2 | . | 699 | 36 | 1•7 (1•3–1•9) |
| control group | 2 | . | 600 | 36 | 1•8 (1•7–1•9) |
| Lopinavir/ritonavir | 5 | 0 | 460 | 47 | 1•1 (0•7–1•5) |
| treatment group | 5 | . | 247 | 46 | 1•2 (0•8–1•6) |
| control group | 5 | . | 196 | 47 | 1•1 (0•5–1•4) |
| Favipiravir | 1 | 0 | 236 | 53 | 0•9 (-) |
| treatment group | 1 | . | 116 | 49 | 1•0 (-) |
| control group | 1 | . | 120 | 58 | 0•7 (-) |
| Umifenovir | 1 | 0 | 81 | 44 | 1•3 (-) |
| treatment group | 1 | . | 45 | 38 | 1•6 (-) |
| control group | 1 | . | 36 | 53 | 0•9 (-) |
| II.II ANTIMALARIA AGENT | |||||
| (hydroxy)chloroquine | 8 | 0 | 3325 | 41 | 1•4 (0•7–2•7) |
| treatment group | 8 | . | 1814 | 40 | 1•5 (0•8–3•4) |
| control group | 8 | . | 1511 | 43 | 1•3 (0•6–2•1) |
| II.III IMMUNE MODULATORS | |||||
| Convalescent plasma | 6 | 0 | 75 | 39 | 1•6 (1•0–7•0) |
| case series | 5 | . | 54 | 44 | 1•3 (0•8–7•0) |
| observational study | 1 | . | 21 | 24 | 3•2 (-) |
| treatment group | 1 | . | 6 | 17 | 5•0 (-) |
| control group | 1 | . | 15 | 27 | 2•8 (-) |
| IL-6 pathway inhibitors | 3 | 0 | 147 | 27 | 2•7 (2•3–6•0) |
| treatment group | 1 | . | 42 | 21 | 3•7 (-) |
| control group | 1 | . | 69 | 36 | 1•8 (-) |
| IL-1 pathway inhibitors | 1 | 0 | 45 | 16 | 5•4 (-) |
| treatment group | 1 | . | 29 | 17 | 4•8 (-) |
| control group | 1 | . | 16 | 13 | 7•0 (-) |
| Corticosteroids | 3 | 0 | 488 | 47 | 1•1 (1•0–1•8) |
| treatment group | 3 | . | 294 | 46 | 1•2 (1•1–2•7) |
| control group | 3 | . | 194 | 49 | 1•0 (0•9–1•5) |
Data are presented as numbers and percentages.
Table 2.
Study-specific male-to-female ratios of included studies.
| Author (reference number) | Design | Sex-stratified randomization (a priori) | Sex-specific analyses (post hoc) | Patients (n) | Female (%) | Male-to-female ratio | |
|---|---|---|---|---|---|---|---|
| REMDESIVIR | |||||||
| Beigel et al. (19) | RCT | No | Yes | 1063 | 36 | 1•8 | |
| Wang et al. (20) | RCT | No | No | 236 | 41 | 1•5 | |
| LOPINAVIR/RITONAVIR | |||||||
| Cao et al. (21) | RCT | No | No | 199 | 40 | 1•5 | |
| Li et al. (22)* | RCT | No | No | 86 | 54 | 0•9 | |
| Yan et al. (23) | OBS | – | No | 120 | 55 | 0•8 | |
| Cheng et al. (24) | CASE | – | No | 5 | 60 | 0•7 | |
| Zhu et al. (25) | OBS | – | No | 50 | 48 | 1•1 | |
| FAVIPIRAVIR | |||||||
| Chen et al. (26)* | RCT | No | No | 236 | 53 | 0•9 | |
| UMIFENOVIR | |||||||
| Lian et al. (27) | OBS | – | No | 81 | 44 | 1•3 | |
| (HYDROXY)CHLOROQUINE | |||||||
| Huang et al. (28) | RCT | No | No | 22 | 41 | 1•2 | |
| Tang et al. (29) | RCT | No | No | 150 | 45 | 1•2 | |
| Gautret et al. (30) | OBS | – | No | 36 | 58 | 0•7 | |
| Chen et al. (31) | RCT | No | No | 62 | 53 | 0•9 | |
| Geleris et al. (32) | OBS | – | No | 1376 | 43 | 1•3 | |
| Yu et al. (33) | OBS | – | No | 550 | 38 | 1•7 | |
| Rosenberg et al. (34) | OBS | – | No | 956 | 41 | 1•5 | |
| Mahévas et al. (35) | OBS | – | No | 173 | 27 | 2•7 | |
| CONVALESCENT PLASMA | |||||||
| Shen et al. (36) | CASE | – | No | 5 | 40 | 1•5 | |
| Duan et al. (37) | CASE | – | No | 10 | 40 | 1•5 | |
| Zeng et al. (38) | OBS | – | No | 21 | 24 | 3•2 | |
| Ye et al. (39) | CASE | – | No | 6 | 50 | 1•0 | |
| Adeli et al. (40) | CASE | – | No | 8 | 13 | 7•0 | |
| Salazar et al. (41) | CASE | – | No | 25 | 56 | 0•8 | |
| IL-6 PATHWAY INHIBITORS | |||||||
| Luo et al. (42) | CASE | – | No | 15 | 20 | 4•0 | |
| Gritti et al. (43)* | OBS | – | No | 21 | 14 | 6•0 | |
| Quartuccio et al. (44) | OBS | – | No | 111 | 31 | 2•3 | |
| IL-1 PATHWAY INHIBITORS | |||||||
| Cavalli et al. (45) | OBS | – | No | 45 | 16 | 5•4 | |
| CORTICOSTEROIDS | |||||||
| Fadel et al. (46) | OBS | – | No | 213 | 49 | 1•0 | |
| Zha et al. (47) | OBS | – | No | 31 | 36 | 1•8 | |
| Lu et al. (48) | OBS | – | No | 244 | 48 | 1•1 | |
RCT = randomized controlled trial, OBS = observational study, CASE = case series.*In preprint and not peer reviewed at time of study inclusion.
Table 1 and 2 present the treatment- and study-specific inclusion rates of females and males with the corresponding male-to-female ratio. A total of 6156 patients were reported in the 30 included studies, of which 2531 (41%) were female. In the treatment group, 3292 patients were included, of which 1325 (40%) women. In the control group, 2757 patients were included, of which 1181 (43%) women. The average overall male-to-female ratio was 1•4 (range 0•7–7•0), indicating an overall inclusion of more males than females (Table 1). In the 23 studies reporting treatment and control groups, the male-to-female ratio in the treatment group was 1•5 (range 0•8–5•0) and in the control group 1•3 (range 0•5–7•0). A quarter of the studies included twice as many males as females (i.e. male-to-female ratio >2).
3.2. Antiviral agents (viral entry and replication inhibitors)
3.2.1. Remdesivir (nucleoside analogue)
Two RCTs investigated remdesivir in COVID-19 patients [20,21], and none of them predesigned a priori sex-stratified randomization (Table 2). Of both studies, only Beigel et al. [20] stratified their results based on sex in a post-hoc analysis (Table 1). They reported a Recovery Rate Ratio (RRR) in favour of remdesivir, showing a 1•31 (95% CI 1•07–1•59) RRR for men and 1•38 (95% CI 1•05–1•81) for women (not shown). Although the authors did not investigate the interaction between therapy and sex to test for effect modification by sex statistically, the largely overlapping 95% confidence intervals around the estimated RRRs suggest no difference in treatment effect of remdesivir between males and females. Wang et al. did not stratify their outcomes based on sex and did not perform interaction analyses [21]. A total of 1299 patients were included in both studies, of which 475 (37%) were female (Table 1). In the treatment groups, 699 patients were included, of which 255 (36%) were female. In the control groups, 600 patients were included, of which 217 (36%) were female. Pregnancy was an exclusion criterion in both studies.
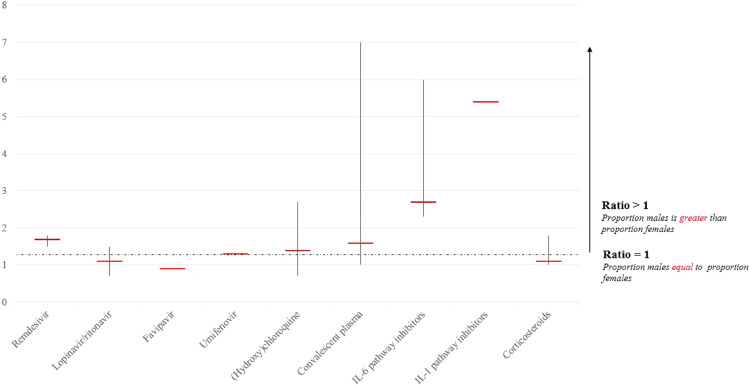
The male-to-female ratio in both studies on remdesivir was 1•7 (range 1•5–1•8) (Table 1, Fig. 2 ). The ratio in the treatment group was 1•7 (range 1•3–1•9) and in the control group 1•8 (range 1•7–1•9).
Fig. 2.
Total male-to-female ratio of included patients stratified per pharmacological treatment under investigation, including the range of study-specific male-to-female ratios.
3.2.2. Lopinavir/ritonavir (boosted protease inhibitor)
Lopinavir/ritonavir was reported in five studies for their effectivity and side-effects in the treatment for COVID-19, including one case series, two RCTs, and two observational studies [22], [23], [24], [25], [26] (Table 2). Five studies compared the use of lopinavir/ritonavir with a control group, consisting of standard care or treatment with umifenovir. None of these studies stratified their randomization for sex a priori by design (i.e. for RCTs), nor stratified their results for sex in a post-hoc analysis, nor performed interaction analyses to investigate effect modification by sex (Table 1). A total of 460 patients were included in the studies, of which 218 (47%) were female (Table 1). The treatment groups included 247 patients, of which 113 (46%) were female. The control groups included a total of 196 patients, of which 95 (48%) were female. The average male-to-female ratio in studies on lopinavir/ritonavir was 1•1 (range 0•7–1•5) (Table 1, Fig. 2). In the treatment groups, the average male-to-female ratio was 1•2 (range 0•8–1•6) and in the control groups 1•1 (range 0•5–1•4). Li et al. excluded pregnant women; the other four studies did not report any data on pregnancy.
3.2.3. Favipiravir (viral RNA polymerase inhibitor)
Chen et al. [27] performed a RCT to investigate the effect of favipiravir in COVID-19 patients compared to umifenovir (Table 2). This study neither stratified its randomization for sex a priori, nor stratified its results for sex in a post-hoc analysis, nor performed interaction analyses to examine effect modification by sex (Table 1). A total of 236 patients were included, of which 126 (53%) were female (Table 1). In the treatment group, 116 patients were included, of which 57 (49%) were female. In the control group, 120 patients were included, of which 69 (58%) were female. The total male-to-female ratio in this study was 0•9 (Table 1, Fig. 2). In the treatment group, the male-to-female ratio was 1•0, and in the control group 0•7.
3.2.4. Umifenovir (viral entry inhibitor)
Lian et al. performed an observational study on umifenovir and did not stratify its results for sex, nor performed interaction analyses to examine effect modification by sex [28] (Table 2). A total of 81 patients were included, of which 36 (44%) were female (Table 1). In the treatment group, 45 patients were included, of which 17 (38%) were female. In the control group (receiving standard care), 36 patients were included, of which 19 (53%) were female. The total male-to-female ratio in this study was 1•3 (Table 1, Fig. 2). In the treatment group, and 1•6 in the control group.
4. Anti-malaria agent
4.1. (Hydroxy)chloroquine
(Hydroxy)chloroquine was investigated in eight studies; three RCTs and five observational studies [29], [30], [31], [32], [33], [34], [35], [36] (Table 2). None of these studies stratified their randomization for sex a priori, nor stratified their results for sex in a post-hoc analysis, nor performed interaction analyses to investigate effect modification by sex (Table 1). A total of 3325 patients were included in these studies, of which 1369 (41%) were female (Table 1). In the treatment groups, 1814 patients were included, of which 733 (40%) were female . In the control groups, 1511 patients were included, of which 654 (43%) were female. The male-to-female ratio for included patients in studies on (hydroxy)chloroquine was 1•4 (range 0•7–2•7) (Table 1, Fig. 2). The male-to-female ratio in the treatment groups was 1•5 (range 0•8–3•4) and in the control groups 1•3 (range 0•6–2•1). Five studies reported pregnancy as an exclusion criterion (Supplementary Table 1).
5. Immune system modulators
5.1. Convalescent plasma
Six studies investigated convalescent plasma in COVID-19 patients [37], [38], [39], [40], [41] (Table 2). Five studies were case series, whereas Zeng et al. [39] performed an observational study comparing the efficacy of the patients who received plasma collected from recovered COVID-19 individuals with a control group who did not receive convalescent plasma. None of these studies stratified their results for sex, nor performed interaction analyses to investigate effect modification by sex (Table 1). The five case series described in total 54 cases, of which 24 (44%) were women (Table 1). Zeng et al. included 21 patients, of which five (24%) were female. Their study population consisted of six patients in the treatment group, of which only one (17%) was female and 15 patients in the control group, of which only four (27%) were female. The total male-to-female ratio for convalescent plasma studies was 1•6 (range 0•8–7•0) (Table 1, Fig. 2). The male-to-female ratio in the treatment group of the study of Zeng et al. was 5•0 and in the control group 2•8. Moreover, only one study reported that 56% of plasma was donated by men [42].
5.2. IL-6 pathway inhibitors and IL-1 pathway inhibitors
Tocilizumab was investigated in one observational study and one case series, whereas siltuximab was investigated in one observational study [43], [44], [45] (Table 2). None of these studies stratified their results for sex, nor performed interaction analyses to investigate effect modification by sex (Table 1). These three studies included 147 patients, of whom 40 were female (27%) (Table 1). Quartuccio et al. was the only study reporting a control group consisting of 69 patients, of which 25 (36%) were female (Table 1). The male-to-female ratio in the total group was 2•7 (range 2•3–6•0) (Table 1, Fig. 2).
Cavalli et al. observationally investigated anakinra in both low and high dosages in patients with COVID-19 [46] (Table 2). Given the design of this review, to evaluate male-to-female ratios, we used the high-dose group as the treatment group and the standard as the control group. Cavalli et al. study did neither stratify its results for sex, nor performed interaction analyses to examine effect modification by sex (Table 1). A total of 45 patients were included in their study, of which 7 (16%) were female, resulting in a male-to-female ratio of 5•4 (Table 1, Fig. 2). A total of 29 patients received high-dose anakinra, of which five (17%) were female, resulting in a male-to-female ratio of 4•8 in the treatment group. The control group included 16 patients, of which 2 (13%) were female, resulting in a male-to-female ratio of 7•0 in the control group.
5.3. Corticosteroids
Three observational studies examined the effect of methylprednisolone (n = 2) and the combination with dexamethasone (n = 1) on COVID-19 outcomes [47], [48], [49] (Table 2). None of these studies stratified their results for sex, nor performed interaction analyses to investigate effect modification by sex (Table 1). A total of 488 patients were included in these studies, of which 231 (47%) were female (Table 1). In the treatment groups, 294 patients were included, of which 135 (46%) were female. In the control groups, a total of 194 patients were included, of which 96 (49%) were female. The average male-to-female ratio was 1•1 (range 1•0–1•8) (Table 1, Fig. 2). In the treatment group, the male-to-female ratio was 1•2 (range 1•1–2•7) and in the control group 1•0 (range 0•9–1•5).
6. Discussion
Our scoping review reports four main findings. First, none of thirty clinical trials on pharmacological treatment for COVID-19 included sex-stratified randomization in its design. Second, only one study stratified its results based on sex by post-hoc analysis. Third, none of the studies evaluated sex as a potential effect modifier. Finally, almost a quarter of the studies included two times more males than females.
The main findings of this study should be interpreted in the context of a large body of evidence showing sex differences in COVID-19 survival. COVID-19 appears to affect men more severely than women [10]. Although this may partly explain the higher male-to-female ratio in the included studies, it does not justify including more than two times more males than females, when evaluating treatment for a novel disease with varying risks worldwide [10]. For example, amongst women in the U.K., women of African ancestry were more severely affected. This stresses the necessity for a more personalised approach [50]. Also, comorbidities, such as chronic lung disease, hypertension, and cardiovascular disease are associated with more severe COVID-19 infection [10]. Worldwide, these comorbidities are more prevalent amongst men than women, except for older age groups, where a reversed prevalence has been observed [51]. Although underlying comorbidities may account for differences in male-to-female outcomes, the possible biological contributions of sex in COVID-19 remain unknown.
To cause infection, SARS-Cov-2 binds to the angiotensin-converting enzyme (ACE) 2 receptor and the cellular serine protease TMPRSS2 for priming to enter cells [52]. Sex hormones affect not only ACE2 but also the Renin-Angiotensin-Aldosterone System (RAAS) components that modulate ACE2 [53], [54], [55], [56], [57]. Moreover, TMPRSS2 is sevenfold more frequently expressed in prostate epithelium than in other human tissue, and its transcription is regulated by androgenic ligands and an androgen receptor binding element in the promoter [58]. However, its function is not understood yet, allowing speculation on a potential sex-specific role of TMPRSS2 in the worse prognosis in males compared to females [59]. Not only the initial cell-entering mechanisms are subject to biological interference, but also immune responses to viruses can vary with changes in sex hormone concentrations. These fluctuations in sex hormone concentrations are naturally observed during the menstrual cycle, following contraception, after menopause, hormone replacement therapy (HRT), and pregnancy [60]. Sex steroids, particularly testosterone (T), oestradiol (E2), and progesterone (P4), influence the functioning of immune cells by altering cell signalling pathways resulting in differential production of cytokines and chemokines [10,61]. Therefore, a 'one-size-fits-all'-approach to immunotherapies is not expected to be effective, and sex may contribute to treatment success in clinical settings. In addition to the potential sex-specific biological mechanisms driving pharmacodynamic heterogeneity of potential treatment effects for women with COVID-19, sex-related differences in absorption, distribution, metabolism, and excretion of drugs are usually explained by differences in weight, body surface area and water/fat distribution. This often results in higher drug exposure if a fixed dose is given irrespective of body weight or composition, a mechanism which may contribute to more side effects in women.
A large body of evidence shows that responses regarding the pharmacokinetics of antiviral drugs differ between men and women and that women encounter adverse drug reactions to antiviral treatment more often than men, which also applies to lopinavir/ritonavir [62]. For the same dose administered, higher plasma concentrations of ritonavir have been reported in females. On the other hand, an atazanavir plus ritonavir regimen was associated with a higher risk of virologic failure in women than in men [63]. Unfortunately, only one of nine studies on remdesivir, lopinavir/ritonavir, favipiravir or umifenovir performed any post-hoc sex-specific analyses. None of them investigated sex as a potential effect modifier, and none of the RCTs stratified its randomization for sex. Moreover, studies on these antiviral drugs included overall more male than female patients.
Chloroquine and hydroxychloroquine are known to trigger life-threatening polymorphic ventricular tachycardia (torsades de pointes) by prolonging the heart rate-corrected QT (QTc) interval [32, 64]. Previous reports indicate that women are more prone to develop drug-induced torsades de pointes than men, with 65–75% of drug-induced torsades de pointes occurring in women [65]. Substantial sex differences in the electrocardiographic pattern of ventricular repolarisation are observed, with a longer QTc interval at baseline in women [55,66]. Protective effects of testosterone have been suggested to account for the shorter QTc interval and the reduced incidence of drug-induced torsades de pointes in men. However, underlying mechanisms are not fully understood.
Besides antiviral and antimalarial medication, the immune-modulating therapies IL-6 inhibitors, IL-1 inhibitors, convalescent plasma, and corticosteroids were evaluated. IL-6 and IL-1 inhibitors are monoclonal antibodies against IL‐6 and IL-1. The latter are cytokines that play an essential role in inflammatory reaction and immune response. Three studies investigated IL-6 pathway inhibitors, of which none performed sex-specific analyses. Almost three times more male than female patients were included in these studies.
Convalescent plasma is plasma from COVID-19 recovered donors, being transfused to treat severe COVID-19 patients [37]. The majority of studies on convalescent plasma were case series, which all reported the sex of their included cases. One observational study on convalescent plasma did not perform sex-specific analyses. This study included five times more male patients than female patients in the treatment group and almost three times more male than female patients in the control group.
For proof of principle, a study in mice revealed that the transfer of serum from female immune mice was significantly better at protecting naïve mice (both males and females) against influenza than the transfer of immune serum from males [67]. It is unknown whether these findings also account for in vivo studies, which stresses the importance of sex-specific randomization and analyses, not only for the patient but also for the donor.
Overall, more awareness in scientific research is needed to understand the role of sex in causing differential outcomes and effects related to COVID-19 between women and men. Although outside the scope of this review, the distinction and interplay between sex (system-biological) and gender (socio-economic) and their effect on differences in outcome remain subject for elaboration and investigation in future studies.
There are several limitations to mention. First, three of the included studies were not peer-reviewed, which increases the risk of bias amongst these studies. However, we believe that this potential bias does not impact our conclusions, as our outcome was based on sex stratification and randomization rather than therapy outcome. Second, we did not use a risk of bias assessment tool as analysing outcome concerning drugs was outside the scope of this review. Finally, the higher survival rate and more favourable disease course of COVID-19 in women compared to men may have influenced the numbers of women included in the studies, as often disease severity increased the chance of inclusion. However, this should not influence sex-stratified randomization in its design or post hoc, or evaluating sex as a potential effect modifier. Strengths of the study are; first, the extensive systematic search aimed to include all relevant studies which resulted in a numeric analysis of the lack of sex consideration in COVID-19. Second, we provided numeric evidence that strengthens arguments and debates on the extent of sex-neglect in clinical trials. Finally, the results show literature-based, novel and easily applicable recommendations to improve future COVID-19 research in an early stage, as described below.
Concluding, in currently available clinical studies on pharmacological therapies for COVID-19 patients, none of the RCTs incorporated sex-stratified randomization, nor investigated sex as a potential effect modifier. Only one out of 30 studies stratified for sex in their analyses post-hoc. Moreover, almost a quarter of the included studies reported twice as many males compared to females. To allow for more personalised patient care, we call investigators to incorporate sex-stratification into their study protocol when developing and testing therapeutic and prophylactic approaches for COVID-19. Although we are aware that these designs are expensive, the trial costs can be reduced by specifying the power to detect a clinically relevant interaction effect between sex and treatment a priori, recruiting a pre-specified number of women and men and ceasing enrolment of the particular sex when the sample size target is reached. For already published studies, we recommend investigators to perform post-hoc sex-specific analyses, while considering potential power insufficiencies in the interpretation of the results [68,69]. Furthermore, we recommend generating an open-access database with data from completed clinical COVID-19 trials in order to improve the power to detect sex-differences compared to current trials.
Funding
None.
Author's contribution
EJ and VS: performed the search, study selection, analysed the data, wrote the initial draft of the paper, revised the paper and finalized the manuscript
LJ and CG: study selection, wrote the paper
CG, BvB, IvdH: initiated the project, developed the idea and coordinated the writing process
JT, JS, DB, TD, SvK, AH, JS, CS, DM, BS, GM, AvtH, MS, WvM: wrote the paper and critically reviewed the content.
Data sharing statement
No individual patient data are included in this study. Search strategy and results of included papers are presented within the manuscript and are available at the corresponding author upon request.
Declaration of Competing Interest
Dr. Marx reports grants and personal fees from Bbraun Melsungen AG, grants and personal fees from Sphingotec AG, grants and personal fees from 4TEEN4 Pharmaceuticals GmbH, outside the submitted work. In addition, Dr. Marx has a patent Modulation ofTLR4 signalling European Patent 2855519/US Patent: US9,745,369 B2 issued, and a patent Combination of C1-Inh and Lung surfactant for the treatment of respiratory disorders PatentÐo.: US 7053,176 B1 PCT/EP99/06845 issued. All other authors declare no interest. Furthermore, there are no known conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that all have approved the order of authors listed in the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100652.
Appendix. Supplementary materials
References
- 1.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Coronavirus disease 2019 (COVID-19): situation Report – 141. 2020.
- 4.WHO. WHO director general's opening remarks at the media briefing on COVID-19. 2020.
- 5.Sex, gender and Covid-19. Global health 50/50. 2020.
- 6.Leung C. Clinical features of deaths in the novel coronavirus epidemic in China. Rev Med Virol. 2020;30(3):e2103. doi: 10.1002/rmv.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conti P., Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34(2) doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 8.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenham C., Smith J., Morgan R., Gender Group C-W. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395(10227):846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020;11(1):29. doi: 10.1186/s13293-020-00304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bischof E., Wolfe J., Klein S.L. Clinical trials for COVID-19 should include sex as a variable. J Clin Invest. 2020 doi: 10.1172/JCI139306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bischof E., Oertelt-Prigione S., Morgan R., Klein S.L. The S, gender in covid clinical trials working group S, towards precision medicine: inclusion of sex and gender aspects in COVID-19 clinical studies-acting now before it is too late-a joint call for action. Int J Environ Res Public Health. 2020;17(10) doi: 10.3390/ijerph17103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinicaltrial.org. 2020.
- 16.UptoDate. 2020.
- 17.Medrxiv. 2020.
- 18.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Klopfenstein T., Zayet S., Lohse A., Balblanc J.C., Badie J., Royer P.Y. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y. An exploratory randomized controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020 2020.03.19.20038984. [Google Scholar]
- 24.Yan D., Liu X-y, Zhu Y-n, Huang L., Dan B-t, Zhang G-j. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in patients with SARS-CoV-2 infection. medRxiv. 2020 doi: 10.1183/13993003.00799-2020. 2020.03.22.20040832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C.-.Y., Lee Y.-.L., Chen C.-.P., Lin Y.-.C., Liu C.-.E., Liao C.-.H. Lopinavir/ritonavir did not shorten the duration of SARS CoV-2 shedding in patients with mild pneumonia in Taiwan. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–ee3. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ce al. Favipiravir versus Arbidol for COVID-19: a Randomized Clinical Trial. MedRxiv. 2020 [Google Scholar]
- 28.Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect: Off Publ Eur Soc Clin Microbiol Infect Dis. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang M., Tang T., Pang P., Li M., Ma R., Lu J. Treating COVID-19 with Chloroquine. J Mol Cell Biol. 2020;12(4):322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang W., Cao Z., Han M., Wang Z., Chen J., Sun W. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. Bmj. 2020;369:m1849. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C.Y., Wang F.L., Lin C.C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila) 2006;44(2):173–175. doi: 10.1080/15563650500514558. [DOI] [PubMed] [Google Scholar]
- 33.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G. Observational Study of Hydroxychloroquine in Hospitalized Patients with Covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu B., Li C., Chen P., Zhou N., Wang L., Li J. Low dose of hydroxychloroquine reduces fatality of critically ill patients with COVID-19. Sci China Life Sci. 2020:1–7. doi: 10.1007/s11427-020-1732-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients With COVID-19 in New York State. JAMA. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahévas M., Tran V.T., Roumier M., Chabrol A., Paule R., Guillaud C. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. Bmj. 2020;369:m1844. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117(17):9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng Q.L., Yu Z.J., Gou J.J., Li G.M., Ma S.H., Zhang G.F. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020 doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adeli S.H., Asghari A., Tabarraii R., Shajari R., Afshari S., Kalhor N. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol Arch Internal Med. 2020;130(5):455–458. doi: 10.20452/pamw.15340. [DOI] [PubMed] [Google Scholar]
- 42.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A. Treatment of coronavirus disease 2019 (COVID-19) patients with convalescent plasma. Am J Pathol. 2020 doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gritti G., Raimondi F., Ripamonti D., Riva I., Landi F., Alborghetti L. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. MedRxiv. 2020 [Google Scholar]
- 45.Quartuccio L., Sonaglia A., McGonagle D., Fabris M., Peghin M., Pecori D. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020 doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fadel R., Morrison A., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P. Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. medRxiv. 2020 doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zha L., Li S., Pan L., Tefsen B., Li Y., French N. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID‐19) Med J Aust. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):1–4. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS-CoV-2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. doi: 10.1136/bmj.m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2) doi: 10.1016/j.cell.2020.02.052. 271-80 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer M., Baessler A., Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc Res. 2002;53(3):672–677. doi: 10.1016/s0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- 54.Chappell M.C., Marshall A.C., Alzayadneh E.M., Shaltout H.A., Diz D.I. Update on the Angiotensin converting enzyme 2-Angiotensin (1-7)-MAS receptor axis: fetal programing, sex differences, and intracellular pathways. Front Endocrinol (Lausanne) 2014;4:201. doi: 10.3389/fendo.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regitz-Zagrosek V. Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov. 2006;5(5):425–438. doi: 10.1038/nrd2032. [DOI] [PubMed] [Google Scholar]
- 56.Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler Thromb Vasc Biol. 2012;32(6):1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J., Ji H., Zheng W., Wu X., Zhu J.J., Arnold A.P. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1(1):6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lucas J.M., Heinlein C., Kim T., Hernandez S.A., Malik M.S., True L.D. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 2014;4(11):1310–1325. doi: 10.1158/2159-8290.CD-13-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Donaldson S.H., Hirsh A., Li D.C., Holloway G., Chao J., Boucher R.C. Regulation of the epithelial sodium channel by serine proteases in human airways. J Biol Chem. 2002;277(10):8338–8345. doi: 10.1074/jbc.M105044200. [DOI] [PubMed] [Google Scholar]
- 60.Klein S.L. Sex differences in prophylaxis and therapeutic treatments for viral diseases. Handb Exp Pharmacol. 2012;214:499–522. doi: 10.1007/978-3-642-30726-3_22. [DOI] [PubMed] [Google Scholar]
- 61.Arnold A.P., Chen X. What does the "four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shiau S., Kuhn L., Strehlau R., Martens L., McIlleron H., Meredith S. Sex differences in responses to antiretroviral treatment in South African HIV-infected children on ritonavir-boosted lopinavir- and nevirapine-based treatment. BMC Pediatr. 2014;14:39. doi: 10.1186/1471-2431-14-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith K.Y., Tierney C., Mollan K., Venuto C.S., Budhathoki C., Ma Q. Outcomes by sex following treatment initiation with atazanavir plus ritonavir or efavirenz with abacavir/lamivudine or tenofovir/emtricitabine. Clin Infect Dis. 2014;58(4):555–563. doi: 10.1093/cid/cit747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the qtc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abi-Gerges N., Philp K., Pollard C., Wakefield I., Hammond T.G., Valentin J.P. Sex differences in ventricular repolarization: from cardiac electrophysiology to Torsades de Pointes. Fundam Clin Pharmacol. 2004;18(2):139–151. doi: 10.1111/j.1472-8206.2004.00230.x. [DOI] [PubMed] [Google Scholar]
- 66.Group E.U.C.C.S., Regitz-Zagrosek V., Oertelt-Prigione S., Prescott E., Franconi F., Gerdts E. Gender in cardiovascular diseases: impact on clinical manifestations, management, and outcomes. Eur Heart J. 2016;37(1):24–34. doi: 10.1093/eurheartj/ehv598. [DOI] [PubMed] [Google Scholar]
- 67.Fink A.L., Engle K., Ursin R.L., Tang W.Y., Klein S.L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A. 2018;115(49):12477–12482. doi: 10.1073/pnas.1805268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ioannidis J.P. Why most discovered true associations are inflated. Epidemiology (Cambridge, Mass) 2008;19(5):640–648. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 69.Peto R. Current misconception 3: that subgroup-specific trial mortality results often provide a good basis for individualising patient care. Br J Cancer. 2011;104(7):1057–1058. doi: 10.1038/bjc.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.