Abstract
Thrombosis is a life-threatening pathological condition in which blood clots form in blood vessels, obstructing or interfering with blood flow. Thrombolytic agents (TAs) are enzymes that can catalyze the conversion of plasminogen to plasmin to dissolve blood clots. The plasmin formed by TA breaks down fibrin clot into soluble fibrin that finally dissolves thrombus. Several TAs have been developed to treat various thromboembolic diseases, such as pulmonary embolisms, acute myocardial infarction, deep vein thrombosis, and extensive coronary emboli. However, systemic TA administration can trigger non-specific activation that can increase the incidence of bleeding. Moreover, protein-based TAs are rapidly inactivated upon injection resulting in the need for large doses. To overcome these limitations, various types of nanocarriers have been introduced that enhance the pharmacokinetic effects by protecting the TA from the biological environment and targeting the release into coagulation. The nanocarrier showed increasing half-life, reducing side effects, and improving overall TA efficacy. In this review, we thoroughly review the recent advances in various types of TAs and nanocarriers. We describe various types of nanocarriers, including lipid-based, polymer-based, and metal-based nanoparticles, for the targeted delivery of TAs. It also provides insight into issues related to TA’s future development and successful clinical translation.
Keywords: thrombosis, thrombolytic agents, plasminogen activators, nanoparticles, drug delivery
Graphical Abstract

In this review paper, the significant role of thrombolytic agent-loaded nanocarriers in thrombolysis is described.
1. Introduction
Thrombosis is a life-threatening pathological condition in which unwanted blood clots occlude blood vessels. It is an acute disorder caused by an accumulation of platelets within a blood vessel and is a hallmark of many cardiovascular diseases [1]. Among other symptoms, thrombosis frequently results in obstructive cardiovascular disorders, which cause ischemic injury to blood vessels and surrounding tissue [2]. Thoracic outlet syndrome, deep vein thrombosis (DVT), pulmonary embolism (PE), acute myocardial infarction (AMI), and stroke are life-threatening thrombus-related diseases especially in developed countries [3]. There are two types of thrombosis: arterial thrombosis, a common characteristic of advanced atherosclerosis, and venous thrombosis, encompassing DVT (with or without PE), renal vein thrombosis, portal vein thrombosis, cerebral venous sinus thrombosis, and hepatic vein thrombosis (Budd-Chiari syndrome). Arterial thrombosis occurs when the atherosclerotic plaque ruptures from a vessel wall and provokes the formation of a thrombus or embolus. Venous thrombosis, on the other hand, is a blood clot originating in a vein and is known to form in places where blood flow slows or changes. Blood clots from both arterial and venous thrombosis can partially or completely occlude blood vessels, causing damage and dysfunction of the organs supplied by the affected blood vessels [4].
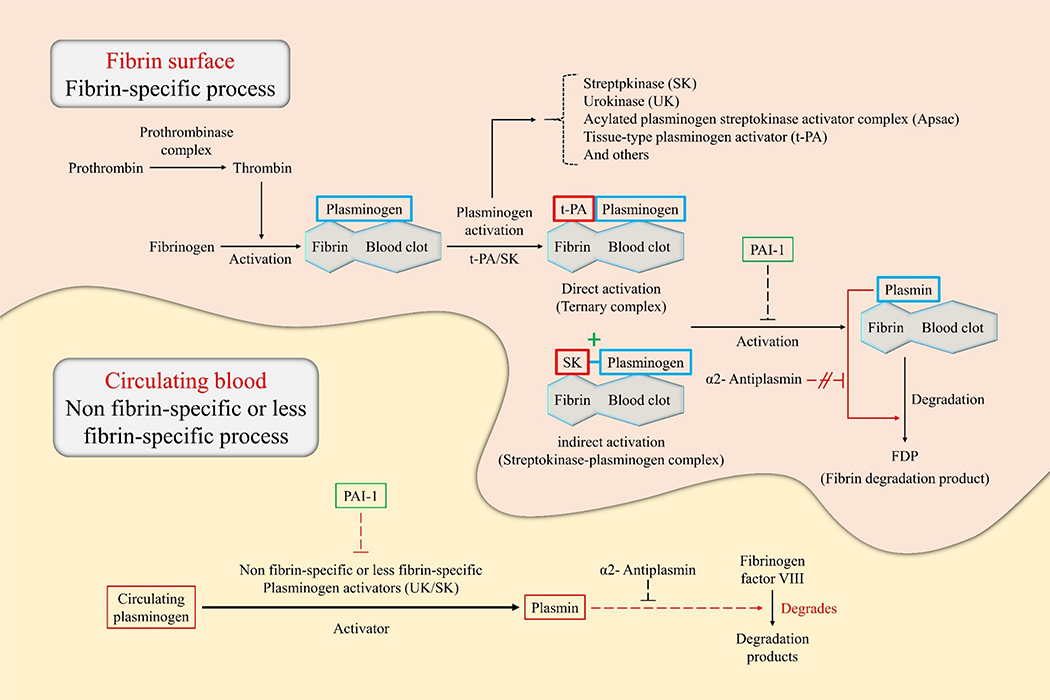
When the vessel wall is damaged, the thrombogenic cascade is immediately activated to reduce bleeding. Vasoconstriction occurs as one of the first steps followed by an injury to prevent blood loss from the affected area. Following that, the formation of a fibrin-platelet composite is the primary component of the typical blood clot [5]. In these coagulation cascades, thrombin is the serine protease enzyme generated to convert fibrinogen into fibrin, which forms the thrombus [6]. There are two exosites in thrombin that are essential for proper function; the first one binds thrombomodulin and fibrinogen while the second one interacts with heparin, factor VII (FVII), glycoprotein Ib-IX, and factor V (FV), a platelet glycoprotein [7]. Thrombin is also a multifunctional enzyme that contributes to the anticoagulant, procoagulant, mitogenic, and inflammatory responses. Each of these assists in the maintenance of hemostatic balance [6]. Activation of fibrinolytic systems (Figure 1) through the administration of thrombolytic agents (TA) causes the pharmacological dissolution of the blood clot, termed thrombolysis. In the thrombolysis reaction, the enzyme plasmin is activated and cleaves the fibrin mesh, which constitutes coagulation. To complement the primary thrombolytic mechanism, TA development research has primarily focused on the inhibition of thrombus formation, as well as thrombolysis [8].
Figure 1.
Illustration of the principles of thrombolysis in a fibrin surface and circulating blood environment. This figure describes the catalytic principle of the conversion of plasminogen to plasmin according to the binding method of the plasminogen activator (e.g., tissue-type plasminogen activator [t-PA], urokinase [UK], and streptokinase [SK]). Plasminogen specifically binds to the surface of the fibrin blood clot. In direct activation, t-PA preferentially attaches to plasminogen, resulting in the formation of a ternary complex. On the other hand, in indirect activation, SK cannot directly bind to the plasminogen but induce conformational changes of the plasminogen to form a streptokinase-plasminogen complex. Subsequently, these complexes form plasmin through cleavage of the fibrin-associated plasminogen. Plasmin formed by direct/indirect activation breaks down fibrin into fibrin breakdown products (FDP), which eventually dissolves blood clots. The thrombolytic process in circulating blood is triggered by non-fibrin-specific or less fibrin-specific plasminogen activators. Plasminogen activators such as the UK and SK induce plasmin production by cleavage of circulating plasminogen. Subsequently, plasmin degrades fibrinogen factor VIII instead of fibrinogen. Plasmin activator inhibitor-1 acts on plasminogen, blocking cleavage into plasmin, causing blood clot formation. α2-antiplasmin acts only on circulating blood, can inhibit thrombolysis by interfering with plasmin binding sites with fibrinogen factor VIII.
However, the current clinical application of TAs still has several limitations such as low targeting capability, short half-life, and increased risk of uncontrolled bleeding. Systemic delivery and non-specific activation of TA increase the likelihood of hemorrhage and limit the use of certain therapeutic drugs. Moreover, protein-based TAs are rapidly inactivated upon injection resulting in the need for large doses [9]. Accordingly, research has focused on surmounting these challenges through selective delivery systems capable of targeting the site of vascular occlusion [10]. Targeting drug delivery to the clotting site is an appealing approach as it could reduce side effects and minimize the impact of short molecular half-life. An efficient methodology for delivering TAs is a drug delivery system (DDS) which utilizes carrier materials to maintain drug stability in transit to the site of action [11]. These nanocarrier-based DDS technologies can deliver optimized concentrations of medication to the target tissue, minimizing systemic exposure and overdose, facilitating a reduction in administered dosage and accompanying side effects [12].
Here, we thoroughly review the recent advances in various types of TAs and nanocarriers for targeted delivery. We also provide insights on barriers and discussions on expanding aid for developing on TAs and minimally invasive therapeutics.
2. Types of TAs
Plasminogen activators (PAs) are proenzymes, precursors to a class of proteolytic enzymes, that are broadly applied as TAs to remedy blockages caused by thrombi. PAs have great specificity for plasminogen and convert plasminogen into plasmin [13]. Then, the newly formed plasmin degrades insoluble fibrin clots into soluble fibrin molecules [14]. Plasmin also enzymatically degrades any peptide or protein with accessible arginine (Arg)-lysine (Lys) sequences, which include fibrin, fibrinogen, and albumin, blood coagulation factors V, VIII, IX, and X[15]. Due to these thrombolytic potentials, PAs are used to treat cerebrovascular and cardiovascular obstructions that lead to stroke and heart attack [16].
PAs can be classified as either direct or indirect. Indirect PAs include streptokinase (SK), staphylokinase (SAK), and vampire bat PA (bat-PA) [17]. SK and SAK are produced by bacteria and do not show proteolytic activity. These proteins indirectly facilitate plasmin formation by generating a 1:1 stoichiometric compound with plasminogen [18]. Direct PAs include tissue PA (t-PA), urokinase (UK), prourokinase (ProUK), acylated plasminogen–streptokinase activator complex (APSAC), alteplase (rt-PA), reteplase (r-PA), tenecteplase (TNK-t-PA), monteplase, and lanoteplase (n-PA) [17]. The most prevalent direct PA is t-PA, a single-chain glycoprotein synthesized in endothelial cells, which is activated by fibrin to boost the conversion of plasminogen to plasmin [18–19].
2.1. Streptokinase (SK)
The first TA to reach the commercial market was SK. As the first drug of its kind, it was a significant advance that contributed to a 50% decrease in mortality related to lethal disorders [20]. SK was first isolated in 1933 and used as a therapeutic agent in the mid-1940s. In 1947, Dr. Charles Dotter prescribed SK to treat peripheral arterial occlusive disease [18]. It currently remains as one of the most inexpensive and broadly prescribed fibrinolytic agents to treat thromboembolic diseases by preventing thrombus formation [21].
SK is a 47 kDa single-chain polypeptide composed of 414 amino acids. Various strains of β-hemolytic streptococci produce SK as an extracellular protein. It forms a complex after binding to plasminogen, and induces the substrate’s conversion to plasmin [18]. After intravenous delivery into the physiological environment, SK has a short half-life of only 20 to 30 minutes as it is quickly bound by antibodies and removed from circulation by the reticuloendothelial system [22].
2.2. Urokinase (UK)
The fibrinolytic activity of urine was discovered by Dr. J. Pilling and Dr. R. G. Macfarlane in 1947 [23]. In 1951, Dr. J. R. B. Williams identified that the activity was due to the presence of a PA now known as UK [24]. UK is a serine protease that is found in human urine and can also be isolated from human parenchyma in kidney tissue culture [18, 25]. The PA consists of two polypeptide chains and has a molecular weight between 32 and 54 kDa. The low-molecular-weight protein was isolated from cultured kidney cells; whereas, the high-molecular-weight molecule was isolated from urine. UK is a direct PA that has a half-life of approximately 15 minutes in the blood. Additionally, a glycosylated, recombinant form of UK has also been harvested from murine hybridoma cells; this recombinant protein features a higher molecular weight and a shorter half-life [18, 25].
2.3. Staphylokinase (SAK)
Although SK is the most notable microbe-based PA, it is not the only one. SAK is an alternative derived from Staphylococcus aureus bacteria with a half-life of approximately 6.3 minutes [25–26]. SAK binds to plasminogen, forming an inactive complex, and requires a PA to activate it [26c]. S. aureus produces a recombinant protein consisting of a single 136-amino acid polypeptide chain weighing approximately 16.5 kDa [18, 27]. Early studies of SAK in the 1960’s demonstrated the drug’s pros and cons as they proved the protein’s efficacy as a TA while also demonstrating its ability to cause severe side effects due to impurities resulting from poor purification. Major advances in bacterial cloning and recombinant gene expression in the 1980s facilitated the production of pure SAK to be evaluated as a TA in humans [28]. These studies demonstrated one of the unique, attractive properties of SAK-specificity to fibrin. α2-antiplasmin rapidly neutralizes SAK when fibrin is not included in the reaction; however, in the presence of fibrin, SAK does not easily neutralize at the surface of the clot when fibrin is present [28].
2.4. Prourokinase (proUK)
A novel fibrinolytic agent currently undergoing clinical trials is proUK - a 414 amino acid, 49 kDa, single polypeptide chain [25]. Eight clinical trials have been conducted in China and the Netherlands while three of them are currently ongoing [29]. In the blood, proUK has a half-life of 7 to 8 minutes. In the physiological environment, plasmin partially converts proUK to an active 276 amino acid, low-molecular-weight, two-chain UK. Also, the unconverted proUK fraction directly activates plasminogen [18]. Currently, proUK is manufactured through recombinant processes in glycosylated mammalian cells or non-glycosylated E. coli [25].
2.5. Anisoylated plasminogen-streptokinase activator complex (APSAC)
The impact of acute myocardial infarction warranted the development of APSAC, which is suitable for intravenous bolus injection for treatment. It is composed of the acylated active site of human plasminogen in complex with bacteria-derived SK [26a, 30]. APSAC has a molecular weight of 131 kDa and half-life observed as 70 to 120 minutes, which is greater than that of SK [31]. Acylation of the active site in human plasminogen inhibits the formation of plasmin, yet it does not impact the ability of APSAC’s Lys-binding residue to adhere to fibrin. Upon injection, the catalytic center of APSAC is immediately deacylated to activate the thrombolytic capacity of APSAC. A certain amount of plasminogen-SK complex is attached to fibrin during circulation. Circulating α2-antiplasmin protects the plasmin from neutralization, while the plasmin is now available to degrade fibrin in the thrombi. However, the circulating deacylated complex had previously formed free plasmin, which is neutralized until it exceeds the neutralizing capacity of the α2-antiplasmin [29].
2.6. Alteplase (rt-PA)
t-PA is a serine protease composed of 527 amino acids yielding a molecular weight of 68 kDa and a half-life of 4 to 6 minutes. t-PA is one of the two PAs found in the organs and blood of mammals that can convert plasminogen into active plasmin. The other endogenous enzyme is UK which differs in molecular structure [32]. rt-PA is manufactured using recombinant CHO cells transfected with cDNA that is transcribed and translated to express t-PA [25, 33]. It was the first recombinant t-PA to cleave arginine-valine interactions to activate plasminogen into plasmin in the presence of fibrin [19, 31b]. However, t-PA inhibits this transformation in the absence of fibrin [19]. rt-PA is widely used for controlling thrombosis, especially acute ischemic strokes which must be treated before three hours from onset [12a].
2.7. Reteplase (r-PA)
r-PA is a second-generation rt-PA consisting of 355 amino acids (amino acids 1 to 3 and 176 to 527 are from native t-PA) and weighs 39 kDa [25, 31b]. r-PA is a mutant form of t-PA in which two functional protein domains are deleted, leading to extended half-life in plasma, reduced fibrin specificity, and improved blood clot penetration [39]. The extended half-life is approximately 14 minutes [18]. The analogous function of the protein is due to its inclusion of kringle domain 2, the portion responsible for stimulating protease domains to degrade fibrin, instead of the kringle domain 1 and epidermal growth factor (EGF) found in t-PA [19]. While t-PA forms a complex with plasminogen to facilitate conversion to plasmin, the protein domain’s deletion in r-PA removes this requirement, facilitating an increase in thrombolytic activity. In some patients, the increased enzymatic activity can lead to complications as rapid thrombolysis impairs the formation of blood clots to stop bleeding [19].
2.8. Tenecteplase (TNK-t-PA)
Tenecteplase (TNK-t-PA) is a modified t-PA designed to protect the fibrinolytic activity of wild type t-PA. T, N, and K refer to the three amino acids (threonine (Thr), asparagine (Asn), and lysine (Lys) respectively) that vary from the native sequence of t-PA. The functional protein kringle domain 1 in TNK-t-PA contains a threonine as the 103rd residue, instead of an Asn, while the 117th residue, glutamine (Gln), is exchanged for Asn. Additional changes are made to the catalytic protease domain; the Lys-histidine (His)-Arg-Arg (296–299) sequence in the active site is substituted for 4 alanines (Ala) [17, 25]. These modifications to TNK-t-PA expand the molecule, leading to greater half-life (t-PA:TNm K-t-PA, 3.5:22 minutes) [34]. Despite the modifications, the altered form of human t-PA still has functional protease activity which attaches to fibrin and catalyzes the formation of plasmin [18].
2.9. Monteplase
Monteplase is the bioengineered modified form of rt-PA, in which a single amino acid is exchanged in the EGF protein domain (84th residue cysteine (Cys) to serine (Ser)), and has a molecular weight of 68 kDa [35]. The single substitution results in a drastic increase in half-life to more than 20 minutes compared to the 4-minute half-life of natural rt-PA [36]. Monteplase has been used in clinical trials in combination with coronary angioplasty and has shown higher patency rates than angioplasty alone [37].
2.10. Lanoteplase (n-PA)
n-PA is in the third generation of engineered PAs. It is modified by mutations in which the glycosylated position in the kringle domain 1 has been altered (117th residue Asn to Gln), and the EGF protein domain and finger domain of t-PA have been removed [38]. Like t-PA, n-PA converts inactive plasminogen to plasmin mediating fibrinolysis. The half-life of n-PA is 30 to 45 minutes [18].
2.11. Vampire bat plasminogen activator (bat-PA)
Desmodus salivary plasminogen activators (DSPAs) are a family of 4 PAs found in vampire bat saliva. One of the DSPAs, bat-PA (alpha-1), is structurally and functionally similar to human t-PA with 477 amino acids and a molecular weight of 43 kDa [25, 39]. It contains an EGF, finger, and kringle domain 1, but differs in its lack of kringle domain 2 as well as the plasmin cleavage region which is required for transformation into a double chain PA [40]. The absent plasmin cleavage sequence makes bat-PA a unique PA that demonstrates enzymatic activity as a single-chain molecule. The bat-PA has a half-life of 2.8 hours, a 5 to 9-fold increase compared to that of rt-PA [18].
2.12. Heparin
Heparin is the most broadly adopted indirect anticoagulant used to prevent DVT and coronary syndromes. It can be distinguished into two main types, low molecular weight heparins (LMWHs) and unfractionated heparin (UFH) [4, 7]. The molecular weight of UFH is approximately 15 kDa on average. UFH has varied half-lives which depend on the dosage. For example, a half-life of 25 U/kg is approximately 30 minutes; whereas, higher doses like 100 and 400 U/kg should have half-lives of 60 minutes and 150 minutes, respectively [41]. The primary LMWHs range in molecular weight from 4.5 to 6.5 kDa, with half-lives from 4.5 to 3.4 hours, respectively. They are replacing UFHs for use in therapeutic anticoagulation as a treatment for venous thromboembolism and DVT, but their frequency of use depends on the clot location. As a TA, heparin shows minimal side effects and drug interaction. However, its required parenteral administration route, intravenous infusion, or subcutaneous injection, limits its adoption in the clinic [42]. Heparin is also involved in several physiological and pathological pathways such as immune cell migration [43], smooth muscle cell proliferation [44], inflammation [45], and tumor cell metastasis [46].
3. Strategies for site-specific delivery of TAs
The development of drug delivery methods is a broad field of research due to the diverse needs of each therapeutic. Significant effort has been directed to research novel targeted delivery systems to surmount the limitations of traditional systemic delivery including non-specific bio-distribution, inappropriate dosage, and minimized therapeutic efficacy. Controlled-release and targeted delivery systems aim to solve these problems using spatial and temporal regulation of drug administration [42, 47]. The research progress of nanocarriers shows promise in overcoming hurdles toward targeted molecule delivery for developing more effective drug therapies [47–48].
The delivery of nanocarriers can be tuned to treat a wide array of diseases. One study attempted to enhance TA action by delivering rt-PA with a fucoidan-functionalized NP system [49]. Juenet et al., used fucoidan functionalized polysaccharide-poly(isobutylcyanoacrylate) NPs (Fuco-NPs) loaded with rt-PA to localize accumulation on thrombi. The Fuco-NPs were synthesized using a redox radical emulsion procedure to produce a hydrodynamic diameter of 136 ± 4 nm through. These NPs were combined with dextran modified-amino groups. Subsequently, Fuco-NPs were successfully loaded with rt-PA which was released in both venous and arterial conditions (Figure 2). The fibrinolytic activity of rt-PA and the interactions between rt-PA and P-selectin were observed under physiological flow conditions. Fuco-NPs were observed to specifically accumulate at the site of thrombosis. Furthermore, thrombolytic efficacy and efficiency were improved in an acute venous thrombosis mouse model compared to rt-PA delivered alone [49].
Figure 2.
Fucoidan functionalized core-shell polymeric NPs targeted to P-selectin to improve the specific aggregation of loaded recombinant tissue PA at the thrombus Redrawn from Ref [49].
Beyond targeting platelets within a thrombus by P-selectin, fucoidan is also able to target platelets through binding with dilysin, a protein that contains two Lys groups and a C18H37 chain [50]. This bonding is covalent, whereas the interaction with rt-PA is non-covalent in vivo. This structure has been used as a vector for delivering rt-PA into the thrombus, which makes it suitable for improving fibrinolytic efficiency while minimizing deleterious impacts. Ghebouli et al reported that rt-PA combined with dilysin fucoidan (DLF) exhibited increased fibrinolytic activity in vitro compared to free rt-PA [51]. Also, in C57BL/6 mouse models, occluded vessels (epileptic vessels, carotid arteries, vena cava) were more effectively re-perfused by rt-PA-DLF than using free rt-PA. These results have enabled the development of TAs that facilitate the delivery of rt-PA using fucoidan through studies that target P-selectin in NP form or platelet in DLF form.
A heparin-based targeting system for the delivery of t-PA was developed based on concurrent administration of a t-PA prodrug and heparin [52]. The activity of t-PA was reduced by conjugation with LMWH and camouflaging with multiple human serum albumin (HSA) using a protamine-albumin conjugate (Figure 3). This camouflaging facilitated the site-specific delivery of t-PA. The in vitro activity of camouflaged t-PA was tested using a human blood clot model and showed that activity was minimally reduced (by approximately 3%) compared to native t-PA. The prodrug (“camouflaged”) version of t-PA showed no enzymatic activity. However, full activity was recovered upon the addition of heparin. The camouflaged protein was stable for 30 minutes in human blood and was activated in vitro by 0.4 U/mL of heparin. Rat models of jugular vein thrombosis demonstrated that the activity of heparin-induced camouflaged t-PA was equal to that of t-PA in the presence of heparin, which indicated that the heparin-induced camouflaged t-PA is as effective as t-PA in the presence of heparin. This and other similar heparin-based targeting systems are promising as thrombolytic activity can be locally induced by site-specific delivery of heparin. These systems can improve therapeutic efficacy and reduce the risk of bleeding in patients, especially those with MI and other cardiovascular diseases [52].
Figure 3.
a) Formation of camouflaged tissue PA composed of tissue-PA-low molecular-weight heparin (t-PA-LMWH) and HSA protamine-targeting peptide. b) The linkage of albumin with tissue plasminogen activator serves as a steric barrier to blood plasma protein. c) Binding of peptide-GP IIb/IIIa (expressed on the activated platelets surface) to the thrombus leads to complex deposition on the activated platelet surface. d) Administering heparin after the complex has accumulated at the thrombus region. e) Provoked release of t-PA and fibrinolysis. Redrawn from Ref [52].
In 2014, Absar et al. designed an albumin-camouflaged/thrombin-induced DDS [53]. HSA facilitated the site-specific delivery of t-PA, similar to the heparin-based targeting system, by camouflaging the protein with a thrombin-cleavable peptide. A homing peptide specific to glycoprotein (GP) IIb/IIIa on activated platelets was conjugated to the surface of albumin (Figure 4). This system was expected to suppress the enzymatic activity of t-PA while supporting the thrombolytic activity of t-PA near thrombi. 75% of t-PA activity was suppressed by HSA shielding until exposed to 25 nM thrombin which restored approximately 90% of its native enzymatic activity. An in vitro experiment using fibrin-agar plates verified the release of t-PA upon thrombin administration from the albumin-camouflaged composition. Fluorescence microscopy provided evidence of camouflaged t-PA’s affinity for activated platelets; specifically, the affinity of the homing peptide to GPIIb/IIIa. Furthermore, an in vivo rat thrombosis model was used to show the similar thrombolytic activity of HSA-camouflaged and natural t-PA. Additionally, HSA-modified t-PA reduced circulating fibrinogen degradation in the rat model by a factor of 2 compared to its unmodified counterpart. These results suggest that HSA can effectively suppress t-PA activity in circulation until the protein is reactivated by thrombin present in thrombi [53].
Figure 4.
Schematic illustration of an albumin-camouflaged/thrombin-provoked system for targeted delivery of t-PA. A) Formation of camouflaged t-PA. Albumins linked with thrombin-cleavable peptides mask the activity of the t-PA. B) Complex accumulated on the surface of a thrombus through a targeting peptide. This leads to the targeted release of camouflaged t-PA. Redrawn from Ref. [53].
4. Types of nanocarriers for TA delivery
A variety of carriers have been considered for controlling the release of therapeutic agents which include liposomes, dendrimers, polymeric carriers, and magnetic NPs. Here we summarize the diverse methods used to deliver TAs.
4.1. Liposomes
The application of lipid-based nanocarriers to TA is important because they enable conjugation of site-specific ligands, facile preparation, low immunogenicity, and versatility of form and formulation [54]. This artificial microscopic phospholipid vesicle protects the TA from the external environment, preventing the breakdown and transformation. Lipid-based carriers contribute to the prevention of undesirable side reactions due to uncontrolled or excessive systemic release. These features make liposomes versatile nanocarriers for delivering PAs [54b].
Liposomes were initially investigated for diagnostic and therapeutic purposes related to vascular disease caused by thrombus. Attempts to use liposome as t-PA carriers were first reported in 1995 [55]. These studies aimed to increase the duration of t-PA activity and improve thrombolytic efficiency. Heeremans et al. demonstrated that liposomes loaded with t-PA have greater anti-thrombolytic activity in comparison to t-PA alone [56]. In addition to the conventional liposome-based PA delivery, recent research continues to extend the half-life by polymer coating the liposomes [57]. Kim et al. prepared conventional liposomes using sodium cholesterol-3-sulfate, egg phosphatidylcholine, and cholesterol using a lipid film technique. Also, distearolyphosphatidyl ethanolamine-N-poly(ethylene glycol) 2000 (DSPE-PEG 2000), which exhibits hydrophilic self-assembly properties, was added to provide a steric barrier to improve the stability of the liposomes. A series of modifications to the liposome formation process and PEGylation did not affect the fibrinolytic action of native t-PA. Compared to t-PA in solution, both conventional liposomes and PEGylated liposomes showed extended t-PA half-life by 16 and 21-fold, respectively [57]. Further examples are given in Table 1.
Table 1.
Lipid based nanocarriers for delivery of Tas.
| Nanocarrier | TA | Modification of nanocarrier with | Target | In vivo/ In vitro | Consequence | Ref. |
|---|---|---|---|---|---|---|
| Liposome | t-PA | pegylated and non-pegylated peptide sequence of fibrinogen γ-chain (CQQHHLGGAKQAGDV) | GPIIb/IIIa | in vitro | Increased half-life (from 7 min to 103 and 141 min) and thrombolytic activity of t-PA | [107] |
| Liposome | SK | — | activated platelets | in vitro and in vivo | Decreased time of dissolving the clot | [108] |
| Liposome | SK | RGD peptide | Platelets | in vitro | Improved thrombolytic activity and prolonged circulation time | [109] |
| ELIP1 | t-PA | — | Fibrin | in vitro | Enhanced clot treatment with t-PA–ELIP | [110] |
| Liposome | SK | polyethyleneglycol (PEG) | — | in vivo | Enhanced thrombolytic activity | [111] |
| ELIP | rt-PA | 120-kHz ultrasound | — | In vitro | Promoted stable activity | [112] |
| Liposome | r-Sak2 | — | — | in vivo | Prolonged r-Sak plasma half-life, enhanced therapeutic effect, reduced toxicity | [113] |
| Liposome | UK | DDmAb (D-dimer monoclonal antibody) | Thrombus | in vivo | Successful treatment of Acute PE3 | [114] |
| Liposome | UK | RGDS (H-Arg-Gly-Asp-Ser-OH) | Thrombus | in vivo | Good thrombolytic activity | [115] |
| ELIP | t-PA | Color Doppler ultrasound | — | in vivo | Effective thrombolytic activity | [116] |
| Liposome | t-PA | — | anti-actin | in vivo | Damage to vascular membrane ameliorated after thrombolytic treatment | [117] |
| Liposome | SK | — | — | In vitro | Enhanced subconjunctival hemorrhage | [22] |
| ELIP | t-PA | 20 MHz ultrasound | — | In vitro | A potential for specific clot lysis and targeted thrombolysis | [118] |
| Liposome | SK | — | — | In vitro | Improved fibrinolytic treatment | [119] |
| Liposome | SK | — | activated platelets | In vitro | Enhanced therapeutic efficiency | [120] |
| TSL, LTSL, TTSL4 | Staphylokinase (SAK), UK, t-PA | — | — | In vitro | SAK-LTSL demonstrated significant thrombolysis | [121] |
| Microbubble | UK | low-frequency ultrasound | — | in vitro | Decreased required in vitro dose of UK for thrombolysis | [122] |
| Microbubble | rt-PA | 1.0 MHz ultrasound | Fibrin | in vitro | Fibrin degradation and clot lysis | [64b] |
| liposomal bubbles | rt-PA | low-frequency (27 kHz) ultrasound | thrombus | in vitro and in vivo | Enhanced ultrasonic thrombus disruption | [123] |
| ELIP | rt-PA | perfluorocarbon gas | — | in vitro | Improved encapsulation efficacy of both perfluorocarbon microbubbles and rt-PA within ELIP | [124] |
Echogenic liposomes
Recombinant staphylokinase
pulmonary thromboembolism
TSL: temperature-sensitive liposome, LTSL: low temperature-sensitive liposome, TTSL: traditional temperature-sensitive liposome
The surface of liposomes can be functionalized to conjugate moieties like fibrin-linking t-PA or anti-fibrin antibodies to target thrombi and deliver TAs [58]. Zhang et al. prepared cyclic arginyl-glycyl-aspartic acid (cRGD)-functionalized liposomes for encapsulating UK to overcome the limitations of low efficacy, adverse side effects, and short half-life (Figure 5) [58]. According to the flow cytometry analysis, cRGD-functionalized liposomes could continuously release the UK in response to activated platelets. An in vitro study demonstrated that 60% of the UK was released from the functionalized liposomes in approximately five hours. Additional in vitro thrombolysis examination confirmed that cRGD-UK-liposomes had greater thrombolysis potential than UK alone, particularly due to the increase in functionalized drug release. Further, in vivo thrombolysis experiments in a mesenteric thrombosis mouse model showed that the use of cRGD-functionalized liposomes reduced the required UK dose by 75% to obtain equal thrombolytic activity as UK alone. UK-loaded cRGD-functionalized liposomes show promise for better thrombolysis treatment than the free UK due to the dramatically increased thrombolytic efficacy (approximately 4-fold) [58].
Figure 5.
A) A schematic representation of targeted thrombolytic therapy via cyclic RGD functionalized liposomes loaded with UK. B) Flow cytometry analysis demonstrating that cRGD-liposomes only slightly interact with fresh platelets, C) while they interact well with activated platelets. D) The thrombolysis consequences of the cyclic RGD liposomes loaded with UK in the mesenteric vessels of a mouse. The images were taken after treatment with 100 U/g of cyclic RGD-UK-liposomes at 0 min, 5 min, 10 min, 20 min, 30 min, 40min to demonstrate the change in thrombus size over time. Redrawn from Ref. [58].
In 2017, platelet microparticle (PMP)-inspired nanovesicles (PMINs) were developed to encapsulate, transport, and deliver TAs in circulation [59]. These particles actively targeted thrombi through PMP molecular mechanisms and facilitated the release of the drug through a thrombus-related enzymatic trigger. SK was encapsulated in PMINs and targeted to the thrombus by two types of peptide ligands on the activated platelets, P-selectin and integrin GPIIb-IIIa. Secreted phospholipase-A2 (sPLA2-group II) from activated platelets and glycerophospholipids destabilized the designed vesicles by cleaving the SN-2 ester bonds which induced release of SK [60]. PMIN succeeded in localized, targeted delivery without causing systemic bleeding. This experiment demonstrates the great promise for safe and site-specific targeted therapies to vascular diseases using lipid-based nanocarriers (Figure 6) [59].
Figure 6.
A) A schema illustrating surface entities of PMP (platelet-derived microparticles). B) Schematic illustration of PMIN (platelet microparticle-inspired nanovesicles), B1) cryo-TEM image of platelet microparticle-inspired nanovesicles. C) Representation of the PMIN mechanism of targeting thrombi, C1) PMINs actively connect to thrombi through P-selectin and GPIIb-IIIa on activated platelets, C2) PMIN-bound clot is targeted by secreted phospholipase-A2 produced from activated platelets and leukocytes in the thrombus, C3) Degraded PMINs release SK. D) In vivo examination set-up. A FeCl3 (ferric chloride)- induced mouse model of carotid artery thrombosis was utilized to assess the linking of PMINs, E) Representative image of thrombosed carotid as observed by (E1) intravital microscopy and (E2) ex vivo immunofluorescence. Redrawn from Ref. [59].
To further improve the targeting capability and drug release profiles of liposomes, they have been applied in tandem with ultrasound. Ultrasound-assisted thrombolysis improved drug delivery and imaging quality using contrast agents such as echogenic liposomes (ELIPs) and micro-bubbles. Once an ELIP is exposed to ultrasound, the lipid shell breaks down and releases the therapeutic agent, facilitating externally controlled release [54c]. Besides, micro-bubbles encapsulated in liposomes near the core or between the phospholipid bilayers of the particles can be released upon treatment with ultrasound making it easier to identify liposomes for imaging.
Shaw et al. evaluated the effect of pulsed 120-kHz ultrasound on the thrombolytic efficiency of t-PA and a t-PA-integrated ELIP (t-ELIP) in an in vitro model of a human blood clot [61]. The thrombolytic efficiency of t-ELIP was comparable to that of free tPA. Treatment with 120-kHz ultrasound enhanced the efficacy of thrombolytic therapy for both t-ELIP and t-PA, which suggests that liposomes filled with t-PA may be beneficial in thrombolytic therapies [61]. A separate study conducted by Smith, et al., quantified the ultrasound-assisted therapeutic agent release from ELIPs loaded with rt-PA [82]. ELIPs were exposed to 600 kPa low pressure 6.9-MHz B-mode ultrasound to investigate the echogenicity of the particles under four experimental circumstances. These conditions were as follows: 1) flow, to check diffusion of gas from T-ELIPs; 2) color Doppler 6.0-MHz greater than 0.8 MPa, for gas leaking out of the liposome; 3) color Doppler 6.0-MHz greater than 2.6 MPa, for fast fragmentation; and 4) in the presence of Triton X-100, to serve as a positive control to chemically dissolve the T-ELIPs. The release of rt-PA was evaluated spectrophotometrically for each circumstance. rt-PA-loaded ELIPs had echogenicity in the 5 mL/min flow model for 30 minutes. The TA remained connected to the liposome while exposed to low pressure B-mode ultrasound for 60 minutes but was released while exposed to Triton X-100 or color Doppler pulses. rt-PA released from the liposomes had a similar enzymatic activity to free rt-PA. The rt-PA-loaded ELIPs were robustly activated during continuous 600 kPa-low pressure, 6.9-MHz imaging. Additionally, fragmentation of T-ELIP with color Doppler ultrasound (6.0-MHz) greater than the 1.59 MPa fast fragmentation threshold led to the release of a therapeutic concentration of rt-PA [62].
Recently, studies on liposomes that are responsive to various stimuli have emerged. One example is the thermosensitive magnetic liposome (TMLs) developed by Hsu et al. through the use of DSPE-PEG 2000, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, Fe3O4 magnetic NPs, and cholesterol [63]. TMLs enable self-targeted rt-PA delivery to the clotting site and temperature-triggered controlled drug release via an altering magnetic field. This approach allows for more sophisticated tuning parameters to optimize drug delivery by factoring in response to multiple simultaneous stimuli.
4.2. Micro-bubbles (MB)
Micro-bubbles (MBs) are small gas-filled microspheres (1–8 μm in diameter) that can be used as delivery carriers for ultrasound-assisted thrombolysis [64]. The vibration of these MBs causes clot surface erosion, increasing TAs penetration into the clot. As the acoustic power increases, the MB explodes, increasing the dissolution and permeation of TAs [54c]. In 2018, Zhang, Bohua, and colleagues coated MBs with 50 nm magnetic NPs (MNPs) called magnetic-MBs (MNP-MBs) as an ultrasound thrombosis strategy. A rotating magnetic field was applied to vibrate and place the MNP-MBs at the target clotting area. The MNP-MB was then acoustically activated using an intravascular ultrasound transducer. In vitro intravascular sonothrombolysis results showed that vibrating MNP-MBs produced vortex-like micro-streaming in the targeted coagulation region. In addition, MNP-MB not only enhance the ultrasonic cavitation, but also showed a greater thrombolytic rate (1.5 ± 0.06%/min) than solitary MB sonothrombolysis method (0.7 ± 0.15%/min) [65]. In 2010, Hau et al. designed an ultrasonic MB carrying lyophilized t-PA and an RGDS tetrapeptide (Arg-Gly-Asp-Ser) that could target thrombi and be activated by ultrasound [66]. Their results demonstrated that MBs were appropriate for intravenous administration. ELISA was used to detect the envelope rate of t-PA (81.12 ± 2.44%) and flow cytometry was used for the detection of RGDS conjugation (94.49 ± 6.19%). An experiment testing fibrinolytic activity using an agarose-fibrin plate demonstrated that t-PA encapsulated in MBs had a comparable fibrinolytic activity to the unencapsulated molecule. The in vitro thrombolytic effect of MBs was tested on a healthy human blood clot. The TA-loaded MBs with ultrasound exposure showed enhanced thrombolytic activity at a low dose. This study shows that t-PA-loaded MBs made by lyophilization can be applied to targeted thrombolytic therapy [66]. In another study by Hau and colleagues, t-PA-loaded MBs with RGDS were prepared by lyophilization and used to evaluate the in vivo thrombolytic effect in 40 rabbits with thrombi in the bilateral femoral arteries [67]. The results showed that targeted t-PA-loaded MBs allowed a reduction in the required dose while enhancing thrombolytic efficiency and reducing the risk of hemorrhage upon exposure to ultrasound.
In a recent study, coaxial electrohydrodynamic atomization (CEHDA) was used to prepare t-PA-loaded MBs from two kinds of shell substances, bovine serum albumin (BSA) and phospholipids [68]. The t-PA was labeled with fluorescein isothiocyanate (FITC) for imaging. t-PA was successfully loaded in the shell for both lipid and BSA-MBs. The average diameter of t-PA-BSA MBs was 41 μm, while 36% of t-PA-lipid MBs varied from 6 to 9 μm and 41% varied from 3 to 6 μm under optimal manufacturing conditions. Sulfur hexafluoride was used as the core gas to enhance t-PA-lipid MB stability. t-PA microbubbles are another promising tool for the treatment of thrombosis (Figure 7) [68]. A similar study employed CEHDA for the fabrication of t-PA-loaded MBs to treat ischemic stroke. The performance of the MB and therapeutic agent release were evaluated under exposure to 2 MHz ultrasound. The maximum concentration of t-PA was 109.89 μg t-PA/ml MB, and the t-PA retained about 80% of its performance and activity [69].
Figure 7.
Schematic illustration of the preparation and effect of t-PA loaded micro-bubble in thrombolysis. Reproduced from Ref. [68] with permission from Elsevier.
4.3. Polymer-based nanocarriers
Biodegradable and biocompatible polymer-based nanocarriers such as poly(lactide-co-glycolide) (PLGA), poly(lactic acid) (PLA), chitosan (CS), and their copolymers modified with poly(ethylene glycol) (PEG) have been employed in the advancement of DDSs. PLGA is the most common polymer for delivery of t-PA. Natural polymers like gelatin and CS are also advantageous alternatives used with the molecule. Furthermore, PEGylated or surface-modified NPs (with hydrophilic shells) are used to increase residence time in the blood and facilitate the loading and transport of a variety of different therapeutic agents [54c, 70].
4.3.1. PLGA
PLGA NPs are appropriate nanocarriers for macromolecules such as proteins and peptides. The FDA has approved PLGA for the delivery of t-PA and has been broadly utilized due to its biodegradability and biocompatibility [71]. Zamanlu and co-workers developed t-PA-loaded biocompatible and biodegradable polymeric NPs with extended thrombolytic activity and circulating time, and also increased the t-PA self-targeting capacity. The PEG was bound to PLGA via carbodiimide/N-hydroxysuccinimide chemistry. PEG-PLGA NPs loaded with t-PA was prepared through a single emulsion solvent diffusion/evaporation method. The morphology and physicochemical characteristics of the NPs and release profile were investigated in in vitro models. The prepared NPs exhibited smooth sphere with a size between 250–280 nm and the drug loading efficiencey was 80–100%. Functional release evaluation demonstrated a decrease in NP’s thrombolytic activity during the experimental period. Thrombolytic activity results indicated that the t-PA-loaded PEG-PLGA NPs showed an improved thrombolytic activity compared to pure t-PA [72]. In 2001, Park et al., reported that loading t-PA into a porous PLGA semi-interpenetrating polymer network (semi-IPN) hydrogel enabled efficient thrombolytic treatment [73]. The semi-IPN hydrogel was prepared through a polymerization process using free-radicals, and crosslinking of PEG in the PLGA network. The hydrogel pore size was 10–20 μm in diameter and did not considerably change the encapsulated t-PA activity. The activity of plasmin was also evaluated using the chromogenic substrate S-2251 to examine tissue plasminogen release from the hydrogel; this test showed that this system may be beneficial for local delivery of t-PA at the thrombus [73]. Korin and colleagues developed t-PA-coated PLGA NPs micro-aggregates which were intravenously administered to mice [74]. In general, blood flow near the thrombus is accelerated due to the small open cross-section, which leads to high shear stresses. Shear-activated NPs that exposed to high shear stress around these thrombi, leading to rapid coagulation lysis by locally releasing the therapeutic agent. This targeting strategy maximizes drug efficacy while decreasing side effects and reducing the required dose, making it a potential approach to treat life-threatening illnesses that stem from acute vascular occlusion. Clot analysis required a dose of shear-activated t-PA NPs that was approximately 100 times lower than the necessary dose for free t-PA [74].
A microparticulate (MP) system for LMWH and enoxaparin sodium (ENX) delivery was developed especially for DVT treatment [75]. A double emulsification/solvent evaporation technique was used to prepare MPs from PLGA to be loaded with ENX. MPs were spherical with a smooth surface and mean size of 2.0 ± 0.9 μm. The MP system improved the encapsulation efficiency of less than 30%, characteristic of hydrophilic macromolecules, to 50.2%. ENX release from MPs in vitro showed pseudo-zero-order kinetics suggesting that the primary mechanism for release of the therapeutic agent is diffusion [75]. Wang and colleagues encapsulated t-PA in PLGA NPs coated with CS or GRGD (Gly-Arg-Gly-Asp) grafted onto CS via pressure-driven permeation [76]. The permeation patterns of free t-PA in solution were different compared to encapsulated t-PA. Clot dissolution by PLGA/CS-GRGD and PLGA/CS-NPs resulted in a rough clot surface on which thrombolysis extended to a depth of 100 μm. By comparison, free t-PA in solution dissolved 12 μm of the clot and yielded only a slightly rough surface. The clot lysis pattern from t-PA encapsulated in PLGA coated with CS-GRGD or CS distinctly varied from that of free t-PA in solution in an in vitro model of thrombolysis [76]. Chung et al., also reported that the PLGA NPs coated with CS expedited thrombolysis and changed the pattern of clot dissolution and permeation through the thrombus compared to t-PA alone [77].
Fe3O4-based PLGA NPs were designed for detecting and targeting thrombi through monitoring with MRI [78]. cRGD was integrated into CS to synthesize a CS-cRGD film as the molecules formed an amide bond mediated by carbodiimide. Fe3O4-based PLGA nanocarriers for rt-PA (Fe3O4–PLGA-rt-PA/CS-cRGD) were prepared using a double emulsion/solvent evaporation technique (water in oil in water, W/O/W). Fe3O4-based PLGA nanocarriers provided high Fe3O4 loading capacity as well as high rt-PA encapsulation efficiency and thrombolytic activity. Compared to free rt-PA and nanocarriers including Fe3O4-PLGA-rt-PA/CS, Fe3O4-PLGA-rt-PA, and Fe3O4-PLGA, accumulation of Fe3O4–PLGA-rt-PA/CS-cRGD NPs on the edge of thrombi was observed, thus leading to a monumental increase in thrombolysis both in vivo and in vitro. These results suggest that Fe3O4–PLGA-rt-PA/CS-cRGD NPs act as a dual-functioning instrument in early thrombus detection and dynamic observation of thrombolytic activity using MRI [78].
4.3.2. Chitosan (CS)
CS is a cationic, water-soluble polysaccharide that has been employed in biomedical processes due to its biodegradability and permeability [79]. Shamsi et al., compared the methods of generating SK-loaded CS NP by the microfluidic chip-based methods with bulky mixing methods. Dynamic light scattering and scanning electron microscopy analysis were used to evaluate the physicochemical features of the specimens. The CS NP generated using the microfluidic chip had a uniform spherical shape with an average of 67± 13 nm, with a narrow multi-dispersed than that fabricated by the bulk mixing technique. The CS NPs prepared by bulky mixing strategy had a disordered and irregular shape with a wide distribution at particle size (452 ± 300 nm). As a result of the in vitro SK release experiment for 48 hours, the SK release of CS NP based on microfluidic chips showed a controlled release without a stable plateau regime compared to CS NP by bulky mixing strategy. The drug release kinetics showed that the main release mechanism of the microfluidic chip-CS NP was Fickian diffusion. In vivo animal study showed that SK action in plasma showed greater amidolytic activity by microfluidic chip-based CS NP, compared to those NPs fabricated by bulk mixing as well as SK alone [80].
Liu et al., fabricated new NPs via the formation of a polyelectrolyte complex between CS and heparin [81]. The MW and concentration of CS, heparin, and BSA as well as the environmental pH influenced the NP yield, size, and encapsulation efficiency. When the pH value of the CS solution and the CS MW were moderately high and both the heparin and CS concentrations increased at the optimum concentration ratio, more NPs were formed, resulting in higher BSA encapsulation [81]. UK is one of the widely used venous thrombosis treatments in China, but there are some limitations due to the risk of hemorrhagic complications. NP conjugation with the UK not only reduces side effects, but can also increase thrombolytic efficacy simultaneously. Jin and co-workers studied the combination of UK-NPs and CDT (catheter direct thrombolysis) for the novel thrombolytic therapy. NP was prepared by self-assembly of CS and tripolyphosphate. The prepared NP was administered into the rabbit thrombosis model, and the ratio of the residual thrombus cross-sectional area to the vascular cross-sectional area was evaluated. The encapsulation efficacy of UK-loaded NP was 94.8±2.1%, the drug loading efficacy was 14.5±1.3%, and the particle size was 236 nm. Compared to the UK administration, UK-loaded NP significantly increased the thrombolytic efficiency, and further improved upon co-treatment with CDT. Intravenous administration of UK-loaded NPs led to an increase of fibrinogen. Consequently, water-soluble UK-load CS NPs exhibited great encapsulation efficacy while maintaining the UK activity as well as sustained release and superior thrombolytic performance over the sole UK treatment [82].
In 2013, Trapani and colleagues evaluated the application of glycol chitosan (GCS) and CS holding the S100 surfactant lipoid for systemic LMWH delivery through pulmonary administration (Figure 8) [83]. An ionotropic gelation method was used to prepare NPs in neutral and acidic conditions to control zeta potential and NP size based on the pH and polysaccharide used (GCS or CS). The encapsulation efficiency of LMWH varied between 43% and 100% for NPs created in neutral and acidic circumstances, respectively. In vivo experiments demonstrated that NPs fabricated in acidic environments did not lead to prolonged blood coagulation in mice. Conversely, lipoid S100-LMWH GCS NPs formed in a neutral environment demonstrated pharmacological efficacy [83]. Recently, a combination of thermo-responsive poloxamer with LMWH/CS nano-complexes was designed to prolong the release of LMWH. Incorporation of LMWH/CS nanocomplexes in the gel elongated heparin release over 4 days [84].
Figure 8.
A schematic illustration of chitosan/glycol chitosan-based nanocarriers. Redrawn from Ref. [83].
Jin, et al., coated CS NPs with UK for thrombolytic treatment [82]. NPs fabricated through self-assembly of tripolyphosphate and CS were introduced into a white rabbit model of thrombosis. The size of the UK-coated NPs was 236 nm and the encapsulation and drug-loading efficiencies were 94.8 ± 2.1% and 14.5 ± 1.3%, respectively. Water-soluble UK-loaded CS NPs preserved UK activity, continuously released UK, and augmented thrombolytic activity compared to free UK [84]. Another study investigated nanoparticulate platforms based on N-trimethyl chitosan (TMC) and CS for improvement of LMWH oral bioavailability [85]. TMC was synthesized via CS methylation; NMR and IR spectra of TMC verified the presence of the trimethyl groups. The results showed that TMC-NPs were more readily absorbed resulting in increased oral bio-accessibility of LMWH in comparison to CS-NPs and free LMWH. This study provides evidence of the advantages of TMC and CS NP-based therapies in the treatment of vascular diseases such as PE and DVT [85].
A combination of heparin/CS nanocomplexes and functional ingredients (hydroxypropylmethylcellulose (HPMC) and two poloxamers, P188 and P407) has been used to fabricate thermo-reversible hydrogels [86]. An increase in gelation temperature, reduction of viscosity, and hastening of gel dissolution occurred when P188 was added to the gel formulation of P407. Contrary to this result, HPMC-containing formulations showed relatively long gel dissolution (18-day-long) with complete heparin release from the gel (9 days). The integration of CS/heparin nanocomplexes into the gel substantially increased the sustained release of heparin. These results suggest that modulating concentrations of poloxamer compounds, adding HPMC, and utilizing nanocomplexes of heparin/CS can lead to the optimal formulation of thermo-reversible NPs for sustained heparin release [86].
4.3.3. Dendrimers
Dendrimers are branched polymers that can be easily controlled and rigidly monitored due to their predictable synthesis [87]. Polyamidoamine (PAMAM), poly(etherhydroxylamine) (PEHAM), poly(esteramine) (PEA), poly(propylene imine) (PPI), poly-L-lysine, polyglycerol, and melamine are all derivatives of dendrimers reported for use as therapeutic agent carriers [88].
Among these derivatives, PAMAM is the most widely investigated dendritic derivative for biomedical processes [47]. Bai et al. developed dendrimer-LMWH complexes that effectively prevent DVT after pulmonary administration [89]. Azure A and FTIR spectroscopy analysis were used to evaluate the dendrimer-ENX interaction and verify interactions between sulfate and carboxylic groups of ENX and amino groups of positively charged dendrimers. Cationic dendrimers increased ENX bioavailability by 40% while anionic dendrimers had no impact. Formulations including 0.5% G3 or 1% G2 PAMAM coupled with ENX were efficient in the prevention of DVT after subcutaneous delivery in a rat model. Cationic dendrimers were effective carriers for LMWH in pulmonary delivery as they decreased the negative charge density on the therapeutic agent’s surface [89]. In another study, researchers focused on lengthening the half-life of LMWH and boosting pulmonary absorption using PEGylated dendrimeric micelles [90]. G3 PAMAM dendrimers conjugated to the methyl ester of PEG-2000 formed PEGylated PAMAM. The encapsulation efficiency of LMWH in PEGylated dendrimer micelles was approximately 40%. Encapsulation of LMWH increased the particle size of PEG-dendrimer micelles, while the LMWH trapped in the PEG-dendrimer induced a significant increase in pulmonary uptake and bioavailability. The half-life of the system was 11.9 hours, 2.4-fold higher than that of LMWH. Additionally, the dendrimer-encapsulated LMWH was effective in decreasing thrombus weight in a rodent model when subcutaneously administered. The PAMAM dendrimer has the potential to be utilized as a carrier for LMWH delivered to the lungs to prevent and control DVT [90].
4.4. Mesoporous silica NPs (MSNs)
Due to its unique properties, such as large surface area and volume of pores, mesoporous silica NPs (MSNs) are considered as promising nanocarriers for the drug delivery and protection. Huang et al., [91] developed the magnetic-MSNs-polyglutamic acid peptide dendrimer-arginine-glycine-aspartic peptide (M-MSNs-G3-RGD) nanocomposite to deliver and protect the new generation of thrombolytic drug, nattokinase (NK). The entire core of the NP was prepared using M-MSNs and then G3 was linked. Subsequently, RGD was introduced to the margin of the NP. By these strategies, M-MSNs-G3-RGD improves the thrombolytic activities by 1) reducing the binding of receptor GP IIb/IIIa and fibrinogen, and 2) preventing platelet aggregation [91].
In another study [92], large pore size M-MSN (6 nm) was examined for effective targeted thrombolysis. M-MSN was prepared with a pore size of 6 nm, and the thrombolytic effect was evaluated in vitro using a dynamic flow system and a fibrin agarose plate assay (FAPA). M-MSN with 6 nm mesopore showed 30 times increased UK loading capacity than the particles without MSN or control sized mesopore (3.7nm) M-MSN. Also, FAPA analysis showed that UK/M-MSNs exhibited faster thrombolysis than non-MSN magnetic sphere or control sized M-MSN up to 3 days. Besides, dynamic flow system revealed that M-MSN with 6 nm-sized mesopore increased thrombolysis efficacy 3.5 folds than the native UK in 30 minutes. All together, these results indicated that larger pore-sized MSN can improve thrombolytic effects. [92].
4.5. Gold NPs (Au NPs)
Au NPs are widely used in DDSs development due to their unique material properties [93]. Without the need to change the structure of the drug, hydrophobic drugs can be loaded onto the surface of Au NPs through non-covalent interactions [137]. In addition, drug can be released through the break down of covalent conjugation, and its release can be controlled by intrinsic/extrinsic stimuli [94].
Wang et al., [95] introduced a controlled UK-PA release system using gold NPs-mesoporous silica core-shell nanospheres (Au-MSNs) and photothermal thrombolysis. As a result, local hyperthermia in combination with the use of Au-MSNs was effective in increasing the thrombolysis. Thus, the results indicate that the Au-MSN system has two potential advantages in thrombolysis: reducing the dose of the drug through local hyperthermia and controlling drug release, thereby reducing the risk of side effects. Through thermal thrombolysis, the Au-MSN provides a more secure protocol for thrombolytic agent delivery through high efficiency and drug secretion [95].
In another study, Zhou et al., [96] delivered the UK to the deep vein thrombosis of the lower extremities using mesoporous silica/gold shell-core nanostructures (Au@MSNs). The eutectic mixture of fatty acids (stearic acid and lauric acid) was applied to the Au@MSNs pores for hyperthermia-triggered UK release. Under the near-infrared irradiation, the UK was rapidly released with the photothermal effect of gold particles. In addition to the rapid drug release, thrombolytic effect was further enhanced by local hyperthermia [96].
4.6. Magnetic nanocarriers
Magnetic NP (MNP) has been used as a technique for targeted DDS based on magnetic fields. The mechanism of action of MNP-based DDS is that when the MNP is exposed to an external magnetic field, it releases the therapeutic agent locally, ultimately increasing the concentration of the local therapeutic agent. [97]. Prilepskii and colleagues developed a unique nanocomposite composed of heparin-mediated UK cross-linked MNP (MNPs@uPA) to effectively deliver TA to the the site of thrombus formation. MNPs@uPA showed that heparin inside the nanocomposite did not interfere the activity of UK. Magnetic-control resulted in a significant increase in thrombolytic efficacy of the MNPs@uPA. MNPs@uPA showed effective dissolution of blood clot in vitro and in vivo. There was no sign of bleeding or side effects in rabbits and rats by the therapeutic dose of single bolus intravenous administration of MNPs@uPA. Based on these results, MNPs@uPA can be a promising candidate for non-invasive nanomedicine for thrombolytic agents due to its biocompatibility, inexpensive and easy manufacturing characteristics. [98].
Pernal et al., used MNPs to target the delivery of t-PA at a human-scale distance (1/8 inch) [99]. The MNP cluster was controlled by a rotating permanent magnet and delivered to the vascular channels through a surface walking method. MNPs with or without t-PA were evaluated to assess the effect of fibrinolysis in trays and wells as well as their speed in vascular channels. By using a rotating permanent magnet, the MNP cluster was able to move a human-scale distance through a branched or straight vascular channel filled with blood-like fluid. In addition, MNP showed improved t-PA delivery and induced fibrinolysis in both dynamic and static studies. In particular, 85% fibrinolysis was observed in dynamic MNP-t-PA analysis. These results indicate that MNP can travel a human-scale distance upon magnetic guidance, which is a promising option for t-PA delivery to treat stroke and various thrombolytic situations [99].
Li and colleagues proposed to improve the thrombolytic effect by covalently binding UK and water-soluble MNPs [100]. In the first step, the hydrophobic NPs of oleic acid (OA)-coated Fe3O4 were synthesized, and then the surface of these NPs were modified with amphipathic co-polymer poly(maleic anhydride-alt-1-octadecylene)(PMAO) to fabricate water-soluble PMAO-OA-Fe3O4 NP. PMAO-OA-Fe3O4 NPs showed improved water solubility without aggregation close to the neutral pH and also exhibited excellent magnetic separation properties. UK was covalently bound to the PMAO-OA- Fe3O4 NPs by anhydride dehydration of N-hydroxysuccinimide (NHS) and N-Ethyl-N′-(3-dimethylaminopropyl) carbodiimide (EDC). Drug release analysis results showed that an alternating magnetic field could be used as an accelerator for drug release. In addition, NP could be used as a switch for drug release. Moreover, the thrombolytic efficiency was four times higher than that of the UK. These results showed that binding of the magnetic field may be a promising strategy to improve the thrombolytic effect of the magnetic drug carrier conjugate [100].
Abed et al., developed a magnetic iron oxide NP for loading SK as a modified-TA [101]. In vitro thrombolytic activity analysis showed that SK-loaded NPs exhibited improved thrombolytic activity in blood clots compared to pure SK [101]. In a recent study by Chen et al., a dual targeting method was used to deliver rt-PA to reduce the dose of rt-PA for the thrombolytic therapy [102]. For the preparation of rt-PA/peptide conjugated PLGA MNPs, fibrin avid peptide and rt-PA were co-immobilized to PLGA-MNP (PMNP). First, the PMMP surface was modified avidin to interact with biotin. Subsequently, rt-PA was bound to biotin-PEG-maleimide through click chemistry between the thiol group of rt-PA and maleimide. PMNP-avidin was then attached to biotin-PEG-rt-PA. In an in vitro application of rt-PA-PMNP, fibrin binding and magnetic guidance resulted in a greater thrombolytic rate than in rt-PA alone. In addition, the 20% dose of rt-PA-PMNP showed thrombolytic efficacy similar to that of pure rt-PA in a rat embolic model [102].
Kempe et al. synthesized MNPs that were 10–30 nm in size to perform in-stent thrombosis treatment [103]. Tetraethyl orthosilicate was used for salinization of the particles in the presence of PEG and/or triethylene glycol. Tresyl chloride or N-hydroxysulfosuccinimide were employed to activate the surface of the MNPs for covalent immobilization of t-PA. The MNPs were intravascularly delivered through a stented brachial artery without short-term side-effects. The initial results demonstrated that MNP-t-PA conjugates can be applied to in-stent thrombolysis in coronary arteries [103].
rt-PA can also be delivered via covalent bonds with MNP [104]. An external magnet was used to target the NPs in vivo. Polyacrylic acid-covered magnetite NPs (PPA-MNP) were synthesized (particle diameter of 246 nm), and rt-PA was immobilized to its surface via the formation of carbodiimide-mediated amide linkages. PPA–MNP–rt-PA was injected intra-arterially and recovered 82% of iliac blood flow within 75 minutes. Additionally, covalent immobilization of rt-PA to PAA-MNP improved rt-PA stability and facilitated NP accumulation around the target site while guided with a magnet [104a]. Yang et al. designed low-toxicity magnetic nanocarriers (MNC) consisting of a Fe3O4 core and a poly [aniline-co-N-(1-one-butyric acid) aniline] shell (14.8 nm mean diameter) for targeted delivery of rt-PA [104b]. MNC-rt-PA improved thrombolysis greater than free rt-PA in vitro. It also reduced lysis time from 39.2 ± 3.2 minutes to 10.8 ± 4.2 minutes. Moreover, at 20% of the rt-PA dosage, magnet-induced MNC-rt-PA restored blood flow within 15–25 minutes in an embolism model with no hematologic toxicity. Subsequently, this magnet-based targeting system expedited thrombolysis and is another promising treatment for thromboembolic disorders [104b].
Chen and colleagues modified MNPs with carboxymethyl dextran (CMD) [105]. Coating the surface of NPs with CMD provided carboxyl groups (-COOH) for conjugation to rt-PA. CMD-covered MNPs (CMD-MNPs) were made with low iron and high CMD content providing an abundance of surface carboxyl groups while minimizing saturation magnetization and hydrodynamic diameter. Conjugation of 0.25 mg of rt-PA with 5 mg of CMD-MNPs yielded the optimum loading conditions in which the immobilized rt-PA maintained its full thrombolytic function [105].
Polymer/inorganic material-covered MNPs were used for targeted delivery of t-PA [106]. The conjugation of 0.5 mg of t-PA to 5 mg of CS-MNP preserved 95% of t-PA’s thrombolytic activity. CS-MNP-t-PA reduced clot lysis time by 53% compared to free t-PA alone and by 58% compared to use in the absence of a magnetic field [106b]. Silica-covered MNPs (SiO2-MNPs) were utilized as nanocarriers for t-PA as well. The core and shell of the nanocarrier were composed of superparamagnetic iron oxide and SiO2, respectively [106a]. Researchers used 3-aminopropyltrimethoxysilane to functionalize the SiO2 nanocarrier surface which provided numerous amine groups for t-PA conjugation. Conjugating 0.5 mg/mL t-PA with 5 mg silica-covered MNP preserved 94% of t-PA activity. Magnetic guidance enhanced SiO2-MNP-t-PA penetration into clots, demonstrating the therapeutic potential of these NPs [106a]. Further examples of a wide variety of different nanocarriers for the delivery of TAs are summarized in Table 2.
Table 2.
Range of nanocarriers for TAs’ delivery.
| Nanocarrier | Formulation of nanocarrier | Modification | TA | In vivo/ In vitro | Outcome | Ref. |
|---|---|---|---|---|---|---|
| PLGA | PEG, PLGA | LMWH | In vivo | Biocompatible and efficient carriers for highly hydrophilic drugs for pulmonary delivery | [125] | |
| PLGA | PLGA | polyethyleneimine (PEI),stearyl amine and Span 60 | LMWH | In vivo/ In vitro | Delivered via dry-powder inhaler as an alternative to several parenteral LMWH administrations | [126] |
| Gelatin NPs | Ethylenediamine cationized gelatins, PEG-gelatin | t-PA | In vivo/ In vitro | Prolonged half-life of t-PA by approximately 3 times in the blood circulation, enhanced the biological activity of t-PA | [11] | |
| Gelatin NPs | zinc acetate, magnesium acetate or calcium acetate | t-PA | In vivo | Prolonged half-life of t-PA | [127] | |
| Chitosan NPs | SK | In vivo/ In vitro | Increased entrapment capacity and half-life of SK, potential in thrombolytic diseases treatment | [80] | ||
| Gold NPs (AuNPs) | Glycol, chitosan | t-PA | In vivo/ In vitro | Targeted delivery of t-PA for thrombolysis | [128] | |
| Hydrogels | PEG-SH and Mal1-LMWH | PEG-SH | LMWH | In vivo | Anticoagulants released controllably | [129] |
| MNPs | dextran | UK | In vivo | 5-fold enhancement of thrombolytic efficiency, prolonged half-life | [130] | |
| Magnetic Mesoporous Silica NPs (M-MSNs) | M-MSNs, UK | UK | In vitro | Greatly improved lysis efficacy | [92] | |
| Metallic NPs | CuNP, SiO2 | t-PA, SK | In vivo | Decreased lysis time of blood clot | [131] | |
| Nanorods | Fe3O4, magnetic guidance | t-PA | in vitro and in vivo | Enhanced thrombolysis and decrease the hemorrahge risk | [132] | |
| Perfluorocarbon NPs | SK | In vitro | Rapid and specific fibrinolysis | [133] | ||
| Porous magnetic Fe3O4-microrods | External magnet | t-PA | In vivo | Overcome restrictions of thrombolytic treatment | [134] | |
| MNP | Fe3O4 | UK | In vitro | High rate of thrombolysis with low-dosage of UK in use | [135] | |
| MNP | Polyacrylic acid (PAA) | rt-PA | In vivo | Effective and feasible treatment | [136] |
Maleimide-modified
5. Conclusion
A wide range of nanocarriers has been studied to optimize the delivery of TAs, enhance thrombolytic efficacy, improve circulation half-life, and overcome problems associated with conventional thrombolytic therapies. The combination of TAs and nanocarriers is clinically useful as their integration can protect TAs from inactivation and degradation in the bio-environment. Additionally, linkage leads to a reduction in the required dose of TAs and an increase in thrombolytic efficiency all while facilitating targeted delivery of agents to clot sites. Targeting techniques ranging from the use of targeting moieties, such as antibodies or peptides, to the incorporation of the drug into inducible, site-specific nanocarriers have been used to augment the clinical efficacy of TAs. These targeting methods show promise to promote thrombolysis and prevent systemic bleeding and other side effects by localizing TAs to the surface of the clot. Modification of TA-loaded nanocarriers with various moieties can target blood clots and decrease drug off-target effects. Furthermore, employing suitable NPs and choosing optimal administration times can decrease the leakage of TA-loaded nanocarriers. Further development of these technologies in addition to in vitro and in vivo experiments will yield the next generation of efficient thrombolytic therapies.
Supplementary Material
Acknowledgments
S. Hassanpour and H-J Kim contributed equally to this work. The authors acknowledge funding from the National Institutes of Health (HL140951, HL137193). The authors are grateful for financial support from the Immunology Research Center, Tabriz University of Medical Sciences.
Contributor Information
Soodabeh Hassanpour, Department of Analytical Chemistry, Faculty of Science, Palacky University Olomouc, 17. Listopadu 12, 77146 Olomouc, Czech Republic.
Han-Jun Kim, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Arezoo Saadati, Pharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Peyton Tebon, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Chengbin Xue, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Floor W. van den Dolder, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA Division Heart and Lungs, Department of Cardiothoracic Surgery, University Medical Center Utrecht, 3508 GA, The Netherlands; Regenerative Medicine Center Utrecht, University Medical Center Utrecht, 3584 CT, Utrecht, The Netherlands.
Jai Thakor, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Behzad Baradaran, Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Jafar Mosafer, Research Center of Advanced Technologies in Medicine, Torbat Heydariyeh University of Medical Sciences, Torbat Heydariyeh, Iran.
Amir Baghbanzadeh, Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Natan Roberto de Barros, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Mahmoud Hashemzaei, Department of Pharmacodynamics and Toxicology, School of Pharmacy, Zabol University of Medical Sciences, Zabol, Iran.
Kangju Lee, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Junmin Lee, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Shiming Zhang, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Wujin Sun, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Hyun-Jong Cho, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA; College of Pharmacy, Kangwon National University, Chuncheon, Gangwon 24341, Republic of Korea.
Samad Ahadian, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA.
Nureddin Ashammakhi, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA; Jonsson Comprehensive Cancer Center, Department of Radiology and Department of Chemical and Biomolecular Engineering, University of California-Los Angeles, Los Angeles, CA 90095, USA.
Mehmet R. Dokmeci, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA Jonsson Comprehensive Cancer Center, Department of Radiology and Department of Chemical and Biomolecular Engineering, University of California-Los Angeles, Los Angeles, CA 90095, USA.
Ahad Mokhtarzadeh, Immunology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ali Khademhosseini, Department of Bioengineering, Center for Minimally Invasive Therapeutics (C-MIT) and California NanoSystems Institute University of California-Los Angeles, Los Angeles, CA 90095, USA; Jonsson Comprehensive Cancer Center, Department of Radiology and Department of Chemical and Biomolecular Engineering, University of California-Los Angeles, Los Angeles, CA 90095, USA; Department of Chemical and Biomolecular Engineering, Henry Samueli School of Engineering and Applied Sciences, University of California - Los Angeles, Los Angeles, CA 90095, USA.
References
- [1].O’reilly R, The pharmacologic basis of therapeutics. 6th ed. New York, NY: Macmillan; 1980, 1347. [Google Scholar]
- [2].Fuster V, Badimon L, Badimon JJ, Chesebro JH, New England Journal of Medicine 1992, 326, 242. [DOI] [PubMed] [Google Scholar]
- [3].Maldonado-Peña J, Rivera K, Ortega C, Betancourt M, Lugo JE, Camargo E, International journal of cardiology 2016, 219, 282. [DOI] [PubMed] [Google Scholar]
- [4].Elbayoumi TA, Torchilin VP, Expert opinion on drug delivery 2008, 5, 1185. [DOI] [PubMed] [Google Scholar]
- [5].Julien RM, Drugs and the Body, WH Freeman, 1988. [Google Scholar]
- [6].Esmon C, Journal of Thrombosis and haemostasis 2003, 1, 1343. [DOI] [PubMed] [Google Scholar]
- [7].Ilinskaya AN, Dobrovolskaia MA, in HANDBOOK OF IMMUNOLOGICAL PROPERTIES OF ENGINEERED NANOMATERIALS: Volume 2: Haematocompatibility of Engineered Nanomaterials, World Scientific; 2016, p. 261. [Google Scholar]
- [8].Collen D, Lijnen HR, Thromb Haemost 2005, 93, 627. [DOI] [PubMed] [Google Scholar]
- [9].Eppler S, Senn T, Gilkerson E, Modi NB, Biopharmaceutics & drug disposition 1998, 19, 31. [DOI] [PubMed] [Google Scholar]
- [10].Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, The Lancet 1998, 352, 1245. [DOI] [PubMed] [Google Scholar]
- [11].Uesugi Y, Kawata H, Jo J.-i., Saito Y, Tabata Y, Journal of Controlled Release 2010, 147, 269. [DOI] [PubMed] [Google Scholar]
- [12].a) Martins S, Sarmento B, Ferreira DC, Souto EB, International journal of nanomedicine 2007, 2, 595; [PMC free article] [PubMed] [Google Scholar]; b) Pan Y, Ren X, Wang S, Li X, Luo X, Yin Z, Biomacromolecules 2017, 18, 865. [DOI] [PubMed] [Google Scholar]
- [13].Collen D, Stump D, Gold H, Annual review of medicine 1988, 39, 405. [DOI] [PubMed] [Google Scholar]
- [14].Rouf SA, Moo-Young M, Chisti Y, Biotechnology advances 1996, 14, 239. [DOI] [PubMed] [Google Scholar]
- [15].Bell WR, Drugs 1997, 54, 11. [DOI] [PubMed] [Google Scholar]
- [16].Stamatoyannopoulos G, Nienhuis AW, Leder P, Majerus P, 1987. [Google Scholar]
- [17].Ross AM, Clinical cardiology 1999, 22, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Baruah DB, Dash RN, Chaudhari M, Kadam S, Vascular pharmacology 2006, 44, 1. [DOI] [PubMed] [Google Scholar]
- [19].Rho JP, Louie SG, Handbook of pharmaceutical biotechnology, CRC Press, 2003. [Google Scholar]
- [20].Bryan J, Stroke 2018, 13, 57. [Google Scholar]
- [21].a) Courval M, Palisaitis DA, Diodati JG, Lesperance B, Pharand C, Thrombosis research 2003, 111, 243; [DOI] [PubMed] [Google Scholar]; b) Perler B, Journal of Endovascular Therapy 2005, 12, 224. [DOI] [PubMed] [Google Scholar]
- [22].Baek S-H, Park SJ, Jin S-E, Kim J-K, Kim C-K, Hwang J-M, European Journal of Pharmaceutics and Biopharmaceutics 2009, 72, 546. [DOI] [PubMed] [Google Scholar]
- [23].MacFarlane R, Pilling J, Nature 1947, 159, 779. [DOI] [PubMed] [Google Scholar]
- [24].Williams J, British journal of experimental pathology 1951, 32, 530. [PMC free article] [PubMed] [Google Scholar]
- [25].Ouriel K, Reviews in cardiovascular medicine 2002, 3, S17. [PubMed] [Google Scholar]
- [26].a) Banerjee A, Chisti Y, Banerjee U, Biotechnology advances 2004, 22, 287; [DOI] [PubMed] [Google Scholar]; b) Collen D, Van de Werf F, Circulation 1993, 87, 1850; [DOI] [PubMed] [Google Scholar]; c) Llevadot J, Giugliano RP, current interventional cardiology reports 2000, 2, 250. [PubMed] [Google Scholar]
- [27].Lyden PD, Thrombolytic therapy for stroke, Springer Science & Business Media, 2001. [Google Scholar]
- [28].Stockx L, Lacroix H, Suy R, Vanderschueren S, Circulation 1997, 95, 463. [DOI] [PubMed] [Google Scholar]
- [29].ClinicalTrials.gov, Prourokinase, https://clinicaltrials.gov/ct2/results?cond=&term=prourokinase&cntry=&state=&city=&dist=, accessed: November 25, 2019. [Google Scholar]
- [30].Monk JP, Heel RC, Drugs 1987, 34, 25. [DOI] [PubMed] [Google Scholar]
- [31].a) Iqbal O, Tobu M, DEMÝR M, Fareed J, Aziz S, Messmore H, Turkish Journal of Hematology 2002, 19, 151; [PubMed] [Google Scholar]; b) Feied C, Handler J, 2005. [Google Scholar]
- [32].Loscalzo J, Braunwald E, New England Journal of Medicine 1988, 319, 925. [DOI] [PubMed] [Google Scholar]
- [33].Kaneshiro K, Medical Reference Services Quarterly 2004, 23, 101. [DOI] [PubMed] [Google Scholar]
- [34].Tanswell P, Modi N, Combs D, Danays T, Clinical pharmacokinetics 2002, 41, 1229. [DOI] [PubMed] [Google Scholar]
- [35].Verstraete M, The American journal of medicine 2000, 109, 52. [DOI] [PubMed] [Google Scholar]
- [36].Deitcher SR, Jaff MR, Rev Cardiovasc Med 2002, 3, S25. [PubMed] [Google Scholar]
- [37].Inoue T, Yaguchi I, Takayanagi K, Hayashi T, Morooka S, Eguchi Y, American heart journal 2002, 144, H1. [DOI] [PubMed] [Google Scholar]
- [38].Llevadot J, Giugliano RP, Expert opinion on investigational drugs 2000, 9, 2689. [DOI] [PubMed] [Google Scholar]
- [39].Barrett AJ, Woessner JF, Rawlings ND, Handbook of proteolytic enzymes, Elsevier, 2012. [Google Scholar]
- [40].Gardell S, Duong LT, Diehl RE, York JD, Hare TR, Register RB, Jacobs JW, Dixon R, Friedman PA, Journal of Biological Chemistry 1989, 264, 17947. [PubMed] [Google Scholar]
- [41].Merli GJ, Groce JB, Pharmacy and Therapeutics 2010, 35, 95. [PMC free article] [PubMed] [Google Scholar]
- [42].Das Kurmi B, Tekchandani P, Paliwal R, Rai Paliwal S, Current pharmaceutical design 2015, 21, 4509. [DOI] [PubMed] [Google Scholar]
- [43].Lider O, Mekori YA, Miller T, Bar‐Tana R, Vlodavsky I, Baharav E, Cohen IR, Naparstek Y, European journal of immunology 1990, 20, 493. [DOI] [PubMed] [Google Scholar]
- [44].Gilotti AC, Nimlamool W, Pugh R, Slee JB, Barthol TC, Miller EA, Lowe‐Krentz LJ, Journal of cellular physiology 2014, 229, 2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tyrrell DJ, Horne AP, Holme KR, Preuss J, Page CP, Advances in pharmacology (San Diego, Calif.) 1999, 46, 151. [DOI] [PubMed] [Google Scholar]
- [46].Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A, Proceedings of the National Academy of Sciences 2001, 98, 3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Rahisuddin PSN, Akrema RA, Abid M, Recent Trends in Nanomaterials: Synthesis and Properties 2017, 83, 49. [Google Scholar]
- [48].Korin N, Therapeutic delivery 2015, 6, 895. [DOI] [PubMed] [Google Scholar]
- [49].Juenet M, Aid-Launais R, Li B, Berger A, Aerts J, Ollivier V, Nicoletti A, Letourneur D, Chauvierre C, Biomaterials 2018, 156, 204. [DOI] [PubMed] [Google Scholar]
- [50].Fischer P, Göritz D, Langmuir 1996, 12, 6315. [Google Scholar]
- [51].Ghebouli R, Loyau S, Maire M, Saboural P, Collet J-P, Jandrot-Perrus M, Letourneur D, Chaubet F, Michel J-B, Thrombosis and haemostasis 2018, 118, 042. [DOI] [PubMed] [Google Scholar]
- [52].Absar S, Choi S, Yang VC, Kwon YM, Journal of controlled release 2012, 157, 46. [DOI] [PubMed] [Google Scholar]
- [53].Absar S, Kwon YM, Ahsan F, Journal of Controlled Release 2014, 177, 42. [DOI] [PubMed] [Google Scholar]
- [54].a) Lewandowska-Łańcucka J, Mystek K, Gilarska A, Kamiński K, Romek M, Sulikowski B, Nowakowska M, Colloids and Surfaces B: Biointerfaces 2016, 143, 359; [DOI] [PubMed] [Google Scholar]; b) Vaidya B, Agrawal G, Vyas SP, International journal of pharmaceutics 2012, 424, 1; [DOI] [PubMed] [Google Scholar]; c) Liu S, Feng X, Jin R, Li G, Expert opinion on drug delivery 2018, 15, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Heeremans J, Gerrttsen H, Meusen S, Mijnheer F, Gangaram Panday R, Prevost R, Kluft C, Crommelin D, Journal of Drug targeting 1995, 3, 301. [DOI] [PubMed] [Google Scholar]
- [56].Heeremans J, Prevost R, Bekkers M, Los P, EMEIS L, Kluft C, Crommelin D, Liposomes in thrombolytic therapy 1995, 102. [PubMed] [Google Scholar]
- [57].Kim J-Y, Kim J-K, Park J-S, Byun Y, Kim C-K, Biomaterials 2009, 30, 5751. [DOI] [PubMed] [Google Scholar]
- [58].Zhang N, Li C, Zhou D, Ding C, Jin Y, Tian Q, Meng X, Pu K, Zhu Y, Acta biomaterialia 2018. [DOI] [PubMed] [Google Scholar]
- [59].Pawlowski CL, Li W, Sun M, Ravichandran K, Hickman D, Kos C, Kaur G, Gupta AS, Biomaterials 2017, 128, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhu G, Mock JN, Aljuffali I, Cummings BS, Arnold RD, Journal of pharmaceutical sciences 2011, 100, 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Shaw GJ, Meunier JM, Huang S-L, Lindsell CJ, McPherson DD, Holland CK, Thrombosis research 2009, 124, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Smith DA, Vaidya SS, Kopechek JA, Huang S-L, Klegerman ME, McPherson DD, Holland CK, Ultrasound in Medicine and Biology 2010, 36, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Hsu H-L, Chen J-P, Journal of Magnetism and Magnetic Materials 2017, 427, 188. [Google Scholar]
- [64].a) Hernot S, Klibanov AL, Advanced drug delivery reviews 2008, 60, 1153; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Petit B, Yan F, Bussat P, Bohren Y, Gaud E, Fontana P, Tranquart F, Allémann E, Journal of Drug Delivery Science and Technology 2015, 25, 29; [Google Scholar]; c) Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Stroke 2006, 37, 425. [DOI] [PubMed] [Google Scholar]
- [65].Zhang B, Jiang X, Wu H, presented at 2018 IEEE 13th Nanotechnology Materials and Devices Conference (NMDC) 2018. [Google Scholar]
- [66].Hua X, Liu P, Gao Y-H, Tan K-B, Zhou L-N, Liu Z, Li X, Zhou S-W, Gao Y-J, Journal of thrombosis and thrombolysis 2010, 30, 29. [DOI] [PubMed] [Google Scholar]
- [67].Hua X, Zhou L, Liu P, He Y, Tan K, Chen Q, Gao Y, Gao Y, Journal of thrombosis and thrombolysis 2014, 38, 57. [DOI] [PubMed] [Google Scholar]
- [68].Yan W-C, Ong XJ, Pun KT, Tan DY, Sharma VK, Tong YW, Wang C-H, Chemical Engineering Journal 2017, 307, 168. [Google Scholar]
- [69].Yan W-C, Chua QW, Ong XJ, Sharma VK, Tong YW, Wang C-H, Journal of colloid and interface science 2017, 501, 282. [DOI] [PubMed] [Google Scholar]
- [70].a) Mosafer J, Abnous K, Tafaghodi M, Mokhtarzadeh A, Ramezani M, European Journal of Pharmaceutics and Biopharmaceutics 2017, 113, 60; [DOI] [PubMed] [Google Scholar]; b) Zamanlu M, Farhoudi M, Eskandani M, Mahmoudi J, Barar J, Rafi M, Omidi Y, Journal of drug targeting 2018, 26, 95. [DOI] [PubMed] [Google Scholar]
- [71].a) Makadia HK, Siegel SJ, Polymers 2011, 3, 1377; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lü J-M, Wang X, Marin-Muller C, Wang H, Lin PH, Yao Q, Chen C, Expert review of molecular diagnostics 2009, 9, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zamanlu M, Eskandani M, Barar J, Jaymand M, Pakchin PS, Farhoudi M, Journal of Drug Delivery Science and Technology 2019, 53, 101165. [Google Scholar]
- [73].Park Y-J, Liang J, Yang Z, Yang VC, Journal of controlled release 2001, 75, 37. [DOI] [PubMed] [Google Scholar]
- [74].Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatta D, Science 2012, 1217815. [DOI] [PubMed] [Google Scholar]
- [75].Oliveira SS, Oliveira FS, Gaitani CM, Marchetti JM, Journal of pharmaceutical sciences 2011, 100, 1783. [DOI] [PubMed] [Google Scholar]
- [76].Wang SS, Chou NK, Chung TW, Journal of Biomedical Materials Research Part A 2009, 91, 753. [DOI] [PubMed] [Google Scholar]
- [77].Chung T-W, Wang S-S, Tsai W-J, Biomaterials 2008, 29, 228. [DOI] [PubMed] [Google Scholar]
- [78].Zhou J, Guo D, Zhang Y, Wu W, Ran H, Wang Z, ACS applied materials & interfaces 2014, 6, 5566. [DOI] [PubMed] [Google Scholar]
- [79].a) Cheung RCF, Ng TB, Wong JH, Chan WY, Marine drugs 2015, 13, 5156; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dash M, Chiellini F, Ottenbrite RM, Chiellini E, Progress in polymer science 2011, 36, 981. [Google Scholar]
- [80].Shamsi M, Zahedi P, Journal of pharmaceutical sciences 2017, 106, 3623. [DOI] [PubMed] [Google Scholar]
- [81].Liu Z, Jiao Y, Liu F, Zhang Z, Journal of Biomedical Materials Research Part A 2007, 83, 806. [DOI] [PubMed] [Google Scholar]
- [82].Jin H.-j., Zhang H, Sun M.-l., Zhang B.-g., Zhang J.-w., Journal of thrombosis and thrombolysis 2013, 36, 458. [DOI] [PubMed] [Google Scholar]
- [83].Trapani A, Di Gioia S, Ditaranto N, Cioffi N, Goycoolea FM, Carbone A, Garcia-Fuentes M, Conese M, Alonso MJ, International journal of pharmaceutics 2013, 447, 115. [DOI] [PubMed] [Google Scholar]
- [84].Matanović MR, Grabnar I, Gosenca M, Grabnar PA, International journal of pharmaceutics 2015, 488, 127. [DOI] [PubMed] [Google Scholar]
- [85].Paliwal R, Paliwal SR, Agrawal GP, Vyas SP, International journal of pharmaceutics 2012, 422, 179. [DOI] [PubMed] [Google Scholar]
- [86].Radivojša M, Grabnar I, Grabnar PA, European Journal of Pharmaceutical Sciences 2013, 50, 93. [DOI] [PubMed] [Google Scholar]
- [87].a) Palmerston Mendes L, Pan J, Torchilin VP, Molecules 2017, 22, 1401; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barrett T, Ravizzini G, Choyke PL, Kobayashi H, IEEE engineering in medicine and biology magazine: the quarterly magazine of the Engineering in Medicine & Biology Society 2009, 28, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Madaan K, Kumar S, Poonia N, Lather V, Pandita D, Journal of pharmacy & bioallied sciences 2014, 6, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bai S, Thomas C, Ahsan F, Journal of pharmaceutical sciences 2007, 96, 2090. [DOI] [PubMed] [Google Scholar]
- [90].Bai S, Ahsan F, Pharmaceutical research 2009, 26, 539. [DOI] [PubMed] [Google Scholar]
- [91].Huang M, Zhang SF, Lü S, Qi T, Yan J, Gao C, Liu M, Li T, Ji Y, Journal of Biomedical Materials Research Part A 2019, 107, 1824. [DOI] [PubMed] [Google Scholar]
- [92].Wang M, Zhang J, Yuan Z, Yang W, Wu Q, Gu H, Journal of biomedical nanotechnology 2012, 8, 624. [DOI] [PubMed] [Google Scholar]
- [93].a) Gibson JD, Khanal BP, Zubarev ER, Journal of the American Chemical Society 2007, 129, 11653; [DOI] [PubMed] [Google Scholar]; b) Paciotti GF, Kingston DG, Tamarkin L, Drug development research 2006, 67, 47. [Google Scholar]
- [94].Han G, You CC, Kim B. j., Turingan RS, Forbes NS, Martin CT, Rotello VM, Angewandte Chemie International Edition 2006, 45, 3165. [DOI] [PubMed] [Google Scholar]
- [95].Wang X, Wei C, Liu M, Yang T, Zhou W, Liu Y, Hong K, Wang S, Xin H, Ding X, Advanced Functional Materials 2017, 27, 1701824. [Google Scholar]
- [96].Xu J, Zhou Y, Nie H, Xiong Z, OuYang H, Huang L, Fang H, Jiang H, Huang F, Yang Y, Journal of Materials Chemistry B 2020. [DOI] [PubMed] [Google Scholar]
- [97].Huang J, Li Y, Orza A, Lu Q, Guo P, Wang L, Yang L, Mao H, Advanced functional materials 2016, 26, 3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Prilepskii AY, Fakhardo AF, Drozdov AS, Vinogradov VV, Dudanov IP, Shtil AA, Bel’tyukov PP, Shibeko AM, Koltsova EM, Nechipurenko DY, ACS applied materials & interfaces 2018, 10, 36764. [DOI] [PubMed] [Google Scholar]
- [99].Pernal SP, Willis AJ, Sabo ME, Moore LM, Olson ST, Morris SC, Creighton FM, Engelhard HH, International Journal of Nanomedicine 2020, 15, 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li Q, Liu X, Lu Z, Yang W, Lei Z, Chang M, Applied Sciences 2019, 9, 4862. [Google Scholar]
- [101].Abed HH, Alwasiti EA, Tawfeeq AT. [Google Scholar]
- [102].Chen H-A, Ma Y-H, Hsu T-Y, Chen J-P, International Journal of Molecular Sciences 2020, 21, 2690. [Google Scholar]
- [103].Kempe H, Kempe M, Biomaterials 2010, 31, 9499. [DOI] [PubMed] [Google Scholar]
- [104].a) Ma Y-H, Wu S-Y, Wu T, Chang Y-J, Hua M-Y, Chen J-P, Biomaterials 2009, 30, 3343; [DOI] [PubMed] [Google Scholar]; b) Yang H-W, Hua M-Y, Lin K-J, Wey S-P, Tsai R-Y, Wu S-Y, Lu Y-C, Liu H-L, Wu T, Ma Y-H, International journal of nanomedicine 2012, 7, 5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chen J-P, Yang P-C, Ma Y-H, Lu Y-J, Journal of nanoscience and nanotechnology 2011, 11, 11089. [DOI] [PubMed] [Google Scholar]
- [106].a) Chen J-P, Yang P-C, Ma Y-H, Tu S-J, Lu Y-J, International journal of nanomedicine 2012, 7, 5137; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen J-P, Yang P-C, Ma Y-H, Wu T, Carbohydrate polymers 2011, 84, 364. [Google Scholar]
- [107].Absar S, Nahar K, Kwon YM, Ahsan F, Pharmaceutical research 2013, 30, 1663. [DOI] [PubMed] [Google Scholar]
- [108].Vaidya B, Nayak MK, Dash D, Agrawal GP, Vyas SP, Drug delivery 2016, 23, 791. [DOI] [PubMed] [Google Scholar]
- [109].Vaidya B, Agrawal G, Vyas SP, European Journal of Pharmaceutical Sciences 2011, 44, 589. [DOI] [PubMed] [Google Scholar]
- [110].Tiukinhoy-Laing SD, Buchanan K, Parikh D, Huang S, Macdonald RC, Mcpherson DD, Klegerman ME, Journal of drug targeting 2007, 15, 109. [DOI] [PubMed] [Google Scholar]
- [111].Jin S-E, Kim I-S, Kim C-K, Archives of pharmacal research 2015, 38, 1822. [DOI] [PubMed] [Google Scholar]
- [112].Bader KB, Bouchoux G, Peng T, Klegerman ME, McPherson DD, Holland CK, Journal of thrombosis and thrombolysis 2015, 40, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yang L, Shi C, Chang Y, Li H, Xiao P, Yang W, Zeng Q, Journal of Drug Delivery Science and Technology 2008, 18, 245. [Google Scholar]
- [114].Lü C, Yang H, Wang J, Dong X, Zhonghua xin xue guan bing za zhi 2009, 37, 1035. [PubMed] [Google Scholar]
- [115].Wang X, Li S, Zhang X, Hou X, Yao xue xue bao= Acta pharmaceutica Sinica 2003, 38, 231. [PubMed] [Google Scholar]
- [116].Laing ST, Moody M, Smulevitz B, Kim H, Kee P, Huang S, Holland CK, McPherson DD, Arteriosclerosis, thrombosis, and vascular biology 2011, 31, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Asahi M, Rammohan R, Sumii T, Wang X, Pauw RJ, Weissig V, Torchilin VP, Lo EH, Journal of Cerebral Blood Flow & Metabolism 2003, 23, 895. [DOI] [PubMed] [Google Scholar]
- [118].Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD, Thrombosis research 2007, 119, 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Leach KJ, Edgar A, Patterson E, Miao Y, Johnson AE, Thrombosis and haemostasis 2003, 89, 64. [PubMed] [Google Scholar]
- [120].Vaidya B, Nayak MK, Dash D, Agrawal G, Vyas SP, International journal of pharmaceutics 2011, 403, 254. [DOI] [PubMed] [Google Scholar]
- [121].Saxena V, Gacchina Johnson C, Negussie AH, Sharma KV, Dreher MR, Wood BJ, International Journal of Hyperthermia 2015, 31, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ren S-T, Zhang H, Wang Y-W, Jing B-B, Li Y-X, Liao Y-R, Kang X-N, Zang W-J, Wang B, Ultrasound in Medicine and Biology 2011, 37, 1828. [DOI] [PubMed] [Google Scholar]
- [123].Hagisawa K, Nishioka T, Suzuki R, Maruyama K, Takase B, Ishihara M, Kurita A, Yoshimoto N, Nishida Y, Iida K, Journal of Thrombosis and Haemostasis 2013, 11, 1565. [DOI] [PubMed] [Google Scholar]
- [124].Kandadai MA, Mukherjee P, Shekhar H, Shaw GJ, Papautsky I, Holland CK, Biomedical microdevices 2016, 18, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Patel B, Gupta V, Ahsan F, Journal of controlled release 2012, 162, 310. [DOI] [PubMed] [Google Scholar]
- [126].Rawat A, Majumder QH, Ahsan F, Journal of Controlled Release 2008, 128, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Uesugi Y, Kawata H, Saito Y, Tabata Y, Journal of drug targeting 2012, 20, 224. [DOI] [PubMed] [Google Scholar]
- [128].Kim J-Y, Ryu JH, Schellingerhout D, Sun I-C, Lee S-K, Jeon S, Kim J, Kwon IC, Nahrendorf M, Ahn C-H, Theranostics 2015, 5, 1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Baldwin AD, Robinson KG, Militar JL, Derby CD, Kiick KL, Akins RE, Journal of Biomedical Materials Research Part A 2012, 100, 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bi F, Zhang J, Su Y, Tang Y-C, Liu J-N, Biomaterials 2009, 30, 5125. [DOI] [PubMed] [Google Scholar]
- [131].Tadayon A, Jamshidi R, Esmaeili A, International journal of pharmaceutics 2016, 501, 300. [DOI] [PubMed] [Google Scholar]
- [132].Hu J, Huang W, Huang S, ZhuGe Q, Jin K, Zhao Y, Nano Research 2016, 9, 2652. [Google Scholar]
- [133].Marsh JN, Senpan A, Hu G, Scott MJ, Gaffney PJ, Wickline SA, Lanza GM, 2007. [DOI] [PubMed] [Google Scholar]
- [134].Hu J, Huang S, Zhu L, Huang W, Zhao Y, Jin K, ZhuGe Q, ACS applied materials & interfaces 2018, 10, 32988. [DOI] [PubMed] [Google Scholar]
- [135].Li Q, Liu X, Chang M, Lu Z, Materials 2018, 11, 2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Huang L, Wang J, Huang S, Siaw-Debrah F, Nyanzu M, Zhuge Q, Biochemical and biophysical research communications 2019, 516, 565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.