Abstract
Objective
The COVID-19 pandemic is a global public health crisis, with over 33 million cases and 999 000 deaths worldwide. Data are needed regarding the clinical course of hospitalised patients, particularly in the USA. We aimed to compare clinical characteristic of patients with COVID-19 who had in-hospital mortality with those who were discharged alive.
Design
Demographic, clinical and outcomes data for patients admitted to five Mount Sinai Health System hospitals with confirmed COVID-19 between 27 February and 2 April 2020 were identified through institutional electronic health records. We performed a retrospective comparative analysis of patients who had in-hospital mortality or were discharged alive.
Setting
All patients were admitted to the Mount Sinai Health System, a large quaternary care urban hospital system.
Participants
Participants over the age of 18 years were included.
Primary outcomes
We investigated in-hospital mortality during the study period.
Results
A total of 2199 patients with COVID-19 were hospitalised during the study period. As of 2 April, 1121 (51%) patients remained hospitalised, and 1078 (49%) completed their hospital course. Of the latter, the overall mortality was 29%, and 36% required intensive care. The median age was 65 years overall and 75 years in those who died. Pre-existing conditions were present in 65% of those who died and 46% of those discharged. In those who died, the admission median lymphocyte percentage was 11.7%, D-dimer was 2.4 μg/mL, C reactive protein was 162 mg/L and procalcitonin was 0.44 ng/mL. In those discharged, the admission median lymphocyte percentage was 16.6%, D-dimer was 0.93 μg/mL, C reactive protein was 79 mg/L and procalcitonin was 0.09 ng/mL.
Conclusions
In our cohort of hospitalised patients, requirement of intensive care and mortality were high. Patients who died typically had more pre-existing conditions and greater perturbations in inflammatory markers as compared with those who were discharged.
Keywords: infectious diseases, public health, internal medicine
Strengths and limitations of this study.
Our cohort includes greater racial diversity as compared with previously published work.
Largest hospitalised cohort of patients with COVID-19 in New York City.
Limited by lack of access to clinical notes.
Our study is limited by the short follow-up time period.
Our study is limited by high censoring rate.
Introduction
The COVID-19 pandemic, caused by SARS-CoV-2, has held the world at a standstill with its virulence. As of 28 September 2020, over 33 million people have been affected, and >999 000 patients have died worldwide.1 In addition to being highly contagious, the disease manifestations and clinical course are variable, spanning from asymptomatic status to severe acute respiratory distress syndrome with multiorgan failure and death,2 3 acute kidney injury,4 5 acute myocardial injury6 and coagulopathy.7–11
Reports from China and Italy provided early data on disease presentation and management12–14 but also revealed varying geographic disease expressions. Nearly one-third of the world’s cases are now in the USA, and with nearly one-third of those cases located in New York, it represents the current epicentre of the COVID-19 pandemic.15
As the number of cases continues to climb, hospitals are being stretched well-beyond capacity while facing challenges of insufficient personal protective equipment, ventilators and workforce. Thus, understanding the clinical course of hospitalised patients with COVID-19 is critical for providing optimal patient care and to inform resource management in other locations across the USA likely to experience similar case surges.16 Data specifically examining differences in admission laboratory data in patients who died as compared with those who were ultimately discharged are lacking.
The Mount Sinai Healthcare System (MSHS) is the largest academic health system in New York City and serves as an ideal platform to better understand the evolving landscape of COVID-19 across a diverse population. Here, we present the largest case series of patients hospitalised with laboratory confirmed COVID-19 to date in the USA.
Methods
Patient involvement
Patients and the public were not directly involved in the study design or implementation.
Study population
The MSHS serves a large, racially and ethnically diverse patient population. In this study, patient data came from five major hospitals: the Mount Sinai Hospital located in East Harlem, Manhattan; Mount Sinai Morningside located in Morningside Heights, Manhattan; Mount Sinai West located in Midtown and the West Side, Manhattan; Mount Sinai Brooklyn located in Midwood, Brooklyn and Mount Sinai Queens located in Astoria, Queens. We included patients who were at least 18 years of age, had a laboratory-confirmed COVID-19 infection and were admitted to any of the aforementioned five MSHS hospitals between 27 February and 00:00 hours 2 April 2020 (time of data freeze). A confirmed case of COVID-19 was defined by a positive reverse transcriptase polymerase chain reaction (RT-PCR) assay of a specimen collected via nasopharyngeal swab.
Detection of viral RNA
Nasopharyngeal swab specimens were taken from all patients and placed in 120–140 µL viral transport media (VTM). RNA was purified from specimens using either the Qiacube Connect (Qiagen), QIAamp Viral RNA mini kit (Qiagen), or the EZ1 DSP Virus kit (Qiagen). SARS-CoV-2 RNA was detected using the qualitative cobas SARS-CoV-2 kit using the cobas 6800 system. For detection, a two-target RT-PCR using (1) SARS-CoV-2-specific primers and (2) pan-sarbecovirus primers as included in the cobas master mix. All assays were performed in a CLIA-certified high complexity laboratory at the Mount Sinai Health System.
Data collection
The dataset was obtained from different sources and aggregated by the New York Covid Informatics Taskforce (NYCIT) (further description of NYCIT is provided in online supplemental material 1). We obtained demographics, diagnosis codes (International Classification of Diseases-9/10-Clinical Modification (ICD-9/10-CM)) codes and procedures, as well as vital signs and laboratory measurements during hospitalisation. Demographics included age, sex and language, as well as race and ethnicity in the electronic health records (EHRs). Racial groups included white, black or African-American, Asian, Pacific Islander, other and unknown. Ethnic groups included non-Hispanic/Latino, Hispanic/Latino or unknown. All vital signs and laboratory values were obtained as part of indicated clinical care.
bmjopen-2020-040736supp001.pdf (54.1KB, pdf)
Definitions of pre-existing conditions
We defined a pre-existing condition as the presence of diagnosis codes associated with specific diseases. Diagnoses and corresponding ICD codes are provided in online supplemental table 1.
bmjopen-2020-040736supp002.pdf (328.1KB, pdf)
Definitions of outcomes
We assessed in-hospital mortality and admission to intensive care.
Statistical analysis
Results are reported as medians and IQRs or means and SD, as appropriate. Categorical variables were summarised as counts and percentages. Statistical significance was evaluated using a Wilcoxon test for continuous variables and a Fisher’s exact test for categorical variables. We visualised length of stay (LOS) using a cumulative incidence function with competing risks for mutually exclusive events of in-hospital mortality or discharge. Patients who were still hospitalised at the time of data freeze were regarded as having a censored LOS. We assumed censored observations from patients with ongoing hospitalisation will not exceed the longest LOS in our dataset when calculating the restricted mean LOS. No imputation was made for missing data. Analysis was performed with R.17
Data availability
Please contact authors for information on data availability.
Results
A consort diagram of included patients and outcomes is depicted in online supplemental figure 1.
bmjopen-2020-040736supp003.pdf (105.8KB, pdf)
Demographic and clinical characteristics
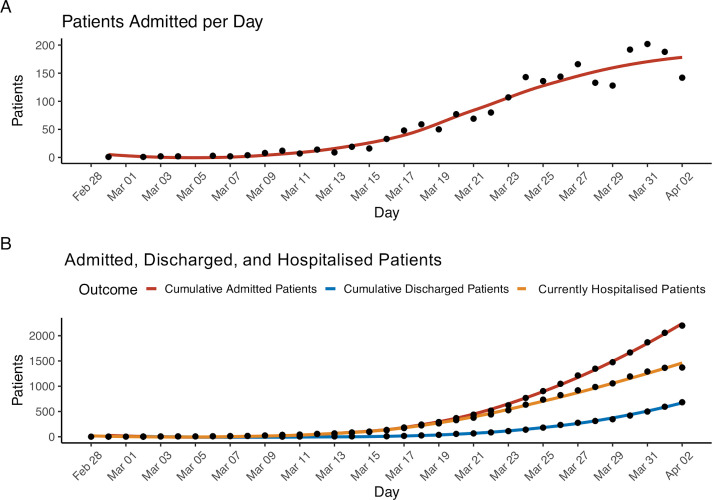
From February 27 to 2 April 2020, 2199 patients with COVID-19 were hospitalised at one of five MSHS New York City hospitals. At the time of writing this report, 1121 (51%) patients remained hospitalised and 1078 (49%) completed their hospital course, with 768 discharges and 310 deaths. Figure 1 details the number of patients admitted to the hospital per day and the total number of patients admitted cumulatively over time. During the study period, the trend of hospital admissions per day consistently increased.
Figure 1.
Hospital admissions of patients with COVID-19 within our cohort. Panel A: the number of total patients (n=2199) admitted each day to one of the hospitals for the duration of the study period. Panel B: the number of patients cumulatively admitted, cumulatively discharged or still hospitalised by day.
Patient demographics, pre-existing conditions as well as vital signs and laboratory values at the time of admission are displayed in table 1. Median age was 65 years with only 3% of patients <30 years and 36% over 70 years. The proportion of men was higher (59%) than women (41%) and 25% had their race identified as white, 25% as African-American and 3% as Asian. One-quarter of the population has their ethnicity identified as Hispanic/Latino. More than half of the population had at least one pre-existing condition. Specifically, 37% presented with a history of hypertension, 27% with diabetes mellitus, 16% with coronary artery disease, 10% with heart failure and 9% with chronic kidney disease.
Table 1.
Characteristics of hospitalised patients with COVID-19 at baseline (n=2199)
| Characteristics of admission | N with characteristic available | |
| Admission source, n (%) | ||
| Emergency department | 1558 (71) | 2199 |
| Other | 641 (29) | |
| Race, n (%) | ||
| White | 554 (25.2) | 2199 |
| Black or African-American | 543 (24.7) | |
| Asian | 74 (3.4) | |
| Pacific Islander | 25 (1.1) | |
| Other | 912 (41.5) | |
| Unknown | 91 (4.1) | |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 576 (26.2) | 2199 |
| Non-Hispanic/Latino | 1305 (59.4) | |
| Unknown | 318 (14.5) | |
| Age, median (IQR) | 65 (54–76) | 2199 |
| Age groups, n (%) | ||
| 18–30 | 73 (3.3) | 2199 |
| 31–40 | 179 (8.1) | |
| 41–50 | 225 (10.2) | |
| 51–60 | 395 (18.0) | |
| 61–70 | 527 (24.0) | |
| 71–80 | 444 (20.2) | |
| 81–90 | 274 (12.5) | |
| 91 or older | 82 (3.7) | |
| Sex, n (%) | ||
| Male | 1293 (58.8) | 2199 |
| Female | 906 (41.2) | |
| Body mass index in kg/m2, median (IQR) | 28 (6) | 1316 |
| Medical history, n (%) | ||
| Atrial fibrillation | 156 (7.1) | 2199 |
| Asthma | 180 (8.2) | |
| Coronary artery disease | 343 (15.6) | |
| Cancer | 151 (6.9) | |
| Chronic kidney disease | 207 (9.4) | |
| Chronic obstructive pulmonary disease | 113 (5.1) | |
| Diabetes mellitus | 583 (26.5) | |
| Heart failure | 217 (9.9) | |
| Hypertension | 812 (37) | |
| Stroke | 153 (7) | |
| Vital signs at hospital admission | ||
| Heart rate in beats per min, median (IQR) | 95 (83–108) | 2181 |
| Number of patients >100 beats per min, n (%) | 857 (39) | |
| Temperature in °F, median (IQR) | 99 (98.2–100.4) | 2180 |
| Number of patients >100.4°F, n (%) | 1926 (88) | |
| Respiratory rate in breaths/min, median (IQR) | 20 (18–21) | |
| Systolic blood pressure in mm Hg, median (IQR) | 130 (116–145) | 2176 |
| Diastolic blood pressure in mm Hg, median (IQR) | 74 (65–82) | |
| Oxygen saturation, median (IQR) | 95 (92–97) | 2176 |
| Admission laboratory parameters, median (IQR) | ||
| White blood cell count in K/μL, median (IQR) | 7 (5.2–9.9) | 2029 |
| Number of patients >10, n (%) | 498 (25) | 2029 |
| Number of patients <4, n (%) | 224 (11) | 2029 |
| Lymphocyte count in K/μL, median (IQR) | 0.9 (0.7–1.3) | 1888 |
| Lymphocyte percentage, median (IQR) | 13.8 (8.5–20.5) | 1972 |
| Haemoglobin g/dL, median (IQR) | 131 (116–144) | 2031 |
| Platelet count in K/μL, median (IQR) | 195 (151–253) | 2028 |
| Prothrombin time in s, median (IQR) | 13.9 (13.2–15) | 937 |
| Activated partial thromboplastin time in s, median (IQR) | 32 (29–36.1) | 926 |
| Serum sodium in mEq/L, median (IQR) | 137 (134–140) | 2015 |
| Serum potassium in mEq/L, median (IQR) | 4.2 (3.9–4.6) | 2006 |
| Serum creatinine in mg/dL, median (IQR) | 1 (0.8–1.5) | 2018 |
| Aspartate aminotransferase in U/L, median (IQR) | 42 (30–70.5) | 1815 |
| Alanine aminotransferase in U/L, median (IQR) | 32 (19–56) | 1025 |
| Serum albumin in g/dL, median (IQR) | 3.2 (2.8–3.6) | 1848 |
| Venous lactate in mmol/L, median (IQR) | 1.5 (1.1–2) | 775 |
| Number of patients >1.5, n (%) | 392 (51) | 775 |
| Inflammatory markers | ||
| C reactive protein in mg/L, median (IQR) | 110 (57.8–196) | 1354 |
| Ferritin in ng/L, median (IQR) | 714 (324–1730) | 1324 |
| Procalcitonin in ng/mL, median (IQR) | 0.18 (0.08–0.57) | 1241 |
| Number of patients >0.49, n (%) | 344 (28) | 1241 |
| Number of patients <0.15, n (%) | 573 (46) | 1241 |
| Lactate dehydrogenase in U/L, median (IQR) | 416 (314–545) | 1290 |
| Creatine kinase in U/L, median (IQR) | 199 (97–565) | 502 |
| D-dimer in μg/mL, median (IQR) | 1.31 (0.74–2.44) | 1079 |
| Number of patients >2.0, n (%) | 352 (33) | 1079 |
| Required mechanical ventilation, n (%) | 446 (20) | 2199 |
Baseline values are defined as first values on hospitalisation. All continuous characteristics are in median (IQR) unless specified otherwise and all categorical characteristics are in number (percentage). The percentage is calculated with the number of patients who had the characteristic available as the denominator. For further clarity, the number in which that characteristic was available for is provided separately in adjacent column.
Laboratory results and vital signs at presentation
Overall, 1558 (71%) patients were admitted through the emergency department. On hospital admission, 39% of all patients were tachycardic and 88% of all patients were febrile (table 1). The median white blood cell count was 7 K/μL and lymphocyte percentage was 13.8. The median serum creatinine was 1 mg/dL. Select inflammatory markers performed in subsets of patients in accordance with clinical indication were markedly elevated on admission (table 1). Specifically, the median C reactive protein (CRP) was 110 mg/L, lactate dehydrogenase (LDH) was 416 U/L and ferritin was 714 ng/L. Over one-quarter of patients (28%) had a procalcitonin level above 0.49 ng/mL, and nearly half of patients (46%) had a procalcitonin level <0.15 ng/mL. The median D-dimer was 1.31 μg/mL; one-third of patients (33%) had a D-dimer >2 μg/mL.
The frequencies of otherwise non-routine laboratory assessments ordered on day of admission increased over time and are shown in online supplemental figure 2. In contrast, haemoglobin, a routinely measured clinical lab value, was ordered at admission in the majority of patients without variation over the study period.
bmjopen-2020-040736supp004.pdf (69.7KB, pdf)
Clinical outcomes
Due to the unknown future clinical course of those patients hospitalised at the time of data freeze, below we present clinical characteristics of only those patients who had completed their hospital course. A total of 1078 COVID-19 confirmed hospitalised patients completed their hospital course (died or discharged alive) by the date of data freeze on 2 April 2020. Of these, 768 (71%) were discharged and 310 (29%) died in the hospital. Estimates for mortality and need for intensive care unit (ICU) admissions over time are displayed in figure 2.
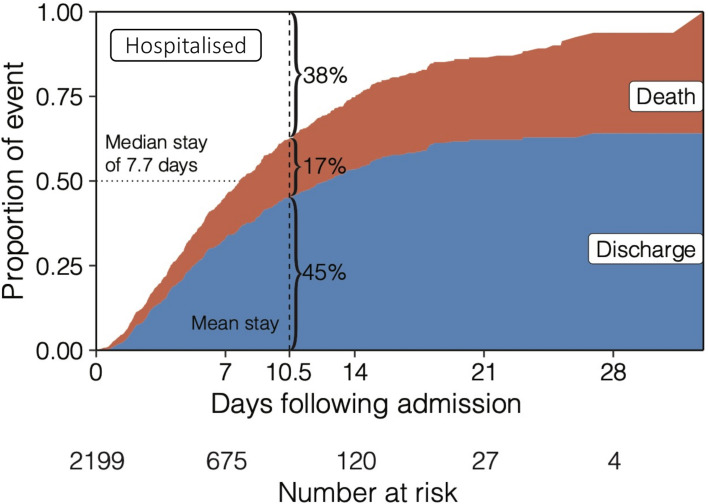
Figure 2.
Cumulative incidence function displays the probability of mutually exclusive events of discharge (blue) or death (red) by a given day of hospitalisation, accounting for the changing number of patients at risk including censoring. The remaining portion (white) shows patients that are still hospitalised, where the median length of stay is 7.7 days.
The median LOS was 7.7 days, accounting for censoring of patients with ongoing hospitalisation. By the mean LOS (10.5 days), 45% of patients had been discharged, 17% had died and 38% were still hospitalised (figure 2). Demographics and admission laboratory measurements for patients who completed their hospital course are displayed in table 2, stratified by mortality. The median age was significantly greater in those who died as compared with those who were discharged (75 years vs 59 years; p<0.001). Pre-existing conditions were present in 64% of those who died and 46% of those discharged. We observed a significantly greater prevalence of diabetes (34% vs 20%; p=0.004), chronic obstructive pulmonary disease (9% vs 4%; p=0.002), heart failure (21% vs 7%; p<0.001), stroke (10% vs 5%; p=0.004) and hypertension (45% vs 30%; p<0.001) in those who died as compared with those who were discharged. Prevalence of other comorbidities are provided in table 2.
Table 2.
Characteristics of hospitalised patients with COVID-19 by patients who had in-hospital mortality versus those who were discharged alive (n=1078)
| Patients with in-hospital mortality (n=310) | N with characteristic available | Patients who were discharged alive (n=768) | N with characteristic available | P value | |
| Required mechanical ventilation, n (%) | 165 (53) | 310 | 14 (1.8) | 768 | <0.001 |
| Time to ICU (hours), median (IQR) | 11.0 (4.9–44.7) | 121 | 12.6 (3.1–11.1) | 264 | <0.001 |
| Race, n (%) | <0.001 | ||||
| White | 98 (31.6) | 310 | 216 (28.1) | 768 | |
| Black or African-American | 71 (22.9) | 185 (24.1) | |||
| Asian | 17 (5.5) | 22 (2.9) | |||
| Pacific Islander | 1 (0.3) | 7 (0.9) | |||
| Other | 111 (35.8) | 310 (40.4) | |||
| Unknown | 12 (3.9) | 28 (3.6) | |||
| Ethnicity, n (%) | 0.005 | ||||
| Hispanic/Latino | 58 (18.7) | 310 | 208 (27.1) | 768 | |
| Non-Hispanic/Latino | 198 (63.9) | 464 (60.4) | |||
| Unknown | 54 (17.4) | 96 (12.5) | |||
| Age, median (IQR) | 75 (64–85) | 59 (45–72) | <0.001 | ||
| Age groups, n (%) | |||||
| 18–30 | 1 (0.32) | 310 | 53 (6.9) | 768 | |
| 31–40 | 2 (0.65) | 108 (14.1) | |||
| 41–50 | 17 (5.5) | 100 (13.0) | |||
| 51–60 | 37 (11.9) | 143 (18.6) | |||
| 61–70 | 66 (21.3) | 157 (20.4) | |||
| 71–80 | 83 (26.8) | 130 (16.9) | |||
| 80–90 | 75 (24.2) | 62 (8.1) | |||
| >90 | 29 (9.4) | 15 (2.0) | |||
| Sex, n (%) | 0.16 | ||||
| Male | 191 (61.6) | 310 | 436 (56.8) | 768 | |
| Female | 119 (38.4) | 332 (43.2) | |||
| Body mass index in kg/m2, median (IQR) | 32 (27–34) | 241 | 28 (25–32) | 627 | <0.001 |
| Previous medical history, n (%) | |||||
| Atrial fibrillation | 43 (13.9) | 768 | 42 (5.5) | 768 | 0.91 |
| Asthma | 23 (7.4) | 61 (7.9) | 0.87 | ||
| Coronary artery disease | 83 (26.8) | 84 (10.9) | <0.001 | ||
| Cancer | 24 (7.7) | 40 (5.2) | 0.147 | ||
| Chronic kidney disease | 41 (13.2) | 54 (7) | 0.002 | ||
| Chronic obstructive pulmonary disease | 28 (9) | 31 (4) | 0.002 | ||
| Diabetes mellitus | 105 (33.9) | 151 (19.7) | <0.001 | ||
| Heart failure | 64 (20.6) | 53 (6.9) | <0.001 | ||
| Hypertension | 140 (45.2) | 233 (30.3) | <0.001 | ||
| Stroke | 32 (10.3) | 40 (5.2) | 0.004 | ||
| Admission laboratory parameters | |||||
| White blood cell count in K/μL, median (IQR) | 8.6 (5.9–12) | 281 | 6.2 (4.7–8.2) | 728 | <0.001 |
| Number of patients >10 K/µL, n (%) | 105 (37) | 281 | 101 (14) | 728 | <0.001 |
| Number of patients <4 K/µL, n (%) | 17 (6) | 281 | 106 (15) | 728 | <0.001 |
| Haemoglobin in g/dL, median (IQR) | 12.6 (10.9–14.3) | 282 | 13.2 (11.9–14.4) | 728 | <0.001 |
| Platelet count in K/µL, median (IQR) | 185 (144–239) | 282 | 193 (153–247) | 726 | 0.070 |
| Lymphocyte count K/μL, median (IQR) | 0.9 (0.6–1.3) | 248 | 1 (0.8–1.4) | 689 | <0.001 |
| Lymphocyte percentage, median (IQR) | 11.7 (6.6–18.6) | 270 | 16.6 (11.7–24.2) | 710 | <0.001 |
| Prothrombin time in s, median (IQR) | 14.2 (13.6–16.3) | 142 | 13.5 (13–14.4) | 304 | <0.001 |
| Activated partial thromboplastin time in s, median (IQR) | 32.9 (30.2–37.6) | 140 | 31.4 (28.7–34.8) | 302 | 0.001 |
| Serum sodium in mEq/L, median (IQR) | 138 (134–141) | 284 | 137 (135–140) | 708 | 0.059 |
| Serum potassium in mEq/L, median (IQR) | 4.4 (4–5) | 288 | 4.1 (3.8–4.5) | 706 | <0.001 |
| Serum creatinine in mg/dL, median (IQR) | 1.3 (0.9–2.2) | 288 | 0.9 (0.7–1.2) | 710 | <0.001 |
| Aspartate aminotransferase in U/L, median (IQR) | 58.5 (34–102) | 246 | 36 (26–54) | 628 | <0.001 |
| Alanine aminotransferase in U/L, median (IQR) | 32 (19–58) | 119 | 30 (19–52) | 358 | 0.47 |
| Serum albumin in g/dL, median (IQR) | 3 (2.7–3.4) | 255 | 3.4 (3–3.7) | 635 | <0.001 |
| Venous lactate in mmol/L, median (IQR) | 1.8 (1.3–2.6) | 108 | 1.3 (1.1–1.8) | 236 | <0.001 |
| Number of patients >1.5 mmol/L, n (%) | 74 (69) | 108 | 88 (37) | 236 | <0.001 |
| Inflammatory markers | |||||
| C reactive protein in mg/L, median (IQR) | 162 (75.7–266) | 141 | 79.3 (37.5–144) | 422 | <0.001 |
| Ferritin in ng/L, median (IQR) | 798 (397–2020) | 146 | 509 (235–987) | 410 | <0.001 |
| Procalcitonin -in ng/mL, median (IQR) | 0.44 (0.16–1.45) | 138 | 0.09 (0.05–0.22) | 392 | <0.001 |
| Number of patients >0.49 ng/mL, n (%) | 67 (49) | 138 | 46 (12) | 392 | <0.001 |
| Number of patients <0.15 ng/mL, n (%) | 34 (25) | 138 | 263 (67) | 392 | <0.001 |
| Lactate dehydrogenase in U/L, median (IQR) | 517 (371–756) | 134 | 347 (272–448) | 419 | <0.001 |
| Creatine kinase in U/L, median (IQR) | 472 (139–940) | 74 | 154 (84–319) | 126 | <0.001 |
| D-dimer in μg/mL, median (IQR) | 2.41 (1.18–3.79) | 117 | 0.93 (0.58–1.61) | 282 | <0.001 |
| Number of patients >2.0 µg/mL, n (%) | 66 (56) | 117 | 59 (21) | 282 | <0.001 |
Continuous and categorical variables were compared using a Wilcoxon test and Fisher’s exact test, respectively.
Baseline values are defined as first values on hospitalisation. All continuous characteristics are in median (IQR) unless specified otherwise and all categorical characteristics are in number (percentage). The percentage is calculated with the number of patients who had the characteristic available as the denominator. For further clarity, the number in which that characteristic was available for is provided separately in adjacent column.
ICU, intensive care unit.
We present key laboratory markers at the time of hospital admission in subsets of patients for whom they were measured. Patients who died had a significantly lower median lymphocyte percentage (11.7% vs 16.6%; p<0.001) and greater aspartate aminotransferase (AST) (58.5 U/L vs 36 U/L; p<0.001), CRP (162 mg/L vs 79 mg/L; p<0.001), ferritin (798 ng/L vs 509 ng/L; p<0.001), LDH (517 U/L vs 347 U/L; p<0.001) and procalcitonin (0.44 ng/mL vs 0.09 ng/mL; p<0.001) as compared with those who were discharged (table 2). We also observed a significant elevation in D-dimer (56% >2.0 μg/mL vs 21% >2.0 μg/mL; p<0.001) in those who died as compared with those who were discharged.
Patients requiring ICU admission
Of the 1078 patients who completed their hospital course, 385 (36%) required intensive care during their hospital stay. For these patients, vital signs and laboratory values immediately before transfer to intensive care are displayed in table 3, stratified by mortality outcome. Immediately before their ICU admission, patients who died were more likely to be tachycardiac (38% vs 19%; p<0.001) and hypotensive (22% vs 4%; p<0.001) as compared with those who were ultimately discharged. We also observed that patients who died had a lower median lymphocyte percentage (9.6% vs 16.6%) and greater serum creatinine (1.5 mg/dL vs 0.6 mg/dL; p<0.001), AST (62 U/L vs 35 U/L; p<0.001), CRP (220 mg/L vs 76 mg/L; p<0.001), ferritin (920 ng/L vs 503 ng/L; p<0.001), procalcitonin >0.49 mg/mL (59% vs 10%; p<0.001), LDH (513 U/L vs 333 U/L; p<0.001), CK (659 U/L vs 146 U/L; p<0.001) and D-dimer >2 μg/mL (63% vs 22%; p<0.001) as compared with those who were discharged (table 3).
Table 3.
Selected characteristics for hospitalised patients with COVID-19 before transfer to intensive care stratified by in-hospital mortality (n=385)
| Patients with in-hospital mortality who required intensive care (n=121) | N with characteristic available | Patients who were discharged alive but required intensive care (n=264) | N with characteristic available | P value | |
| Required mechanical ventilation, n (%) | 95 (79) | 121 | 11 (4) | 264 | <0.001 |
| Vital signs, n (%) | |||||
| Number of patients with heart rate >100 beats per min, n (%) | 45 (38) | 118 | 51 (19) | 264 | <0.001 |
| Number of patients with temperature >100.4°F, n (%) | 107 (91) | 228 (86) | 0.370 | ||
| Systolic blood pressure <100 mm Hg, n (%) | 27 (22) | 10 (4) | <0.001 | ||
| Oxygen saturation, median (IQR) | 92 (88–95) | 95 (92–97) | <0.001 | ||
| Admission laboratory parameters, median (IQR) | |||||
| White blood cell count in K/μL, median (IQR) | 10.1 (6.3–14.7) | 111 | 5.6 (4.3–7.7) | 258 | <0.001 |
| Number of patients >10 K/µL, n (%) | 58 (52) | 111 | 28 (11) | 258 | <0.001 |
| Number of patients <4 K/µL, n (%) | 6 (5) | 111 | 54 (21) | 258 | <0.001 |
| Haemoglobin in g/dL, median (IQR) | 12.1 (10.4–13.8) | 111 | 13 (11.5–14.2) | 258 | <0.001 |
| Platelet count in K/μL, median (IQR) | 185 (146–230) | 111 | 191(153-248) | 258 | 0.308 |
| Lymphocyte count in K/μL, median (IQR) | 0.8 (0.6–1.25) | 91 | 1 (0.75–1.4) | 239 | 0.003 |
| Lymphocyte percentage, median (IQR) | 9.6 (5.5–17.2) | 103 | 16.6 (12–26.4) | 251 | <0.001 |
| Prothrombin time in s, median (IQR) | 14.9 (13.8–16.7) | 63 | 13.7 (13.2–14.7) | 97 | <0.001 |
| Activated partial thromboplastin time in s, median (IQR) | 34 (30.6–38.2) | 62 | 31.4 (29.4–35) | 96 | 0.027 |
| Serum sodium in mEq/L, median (IQR) | 137 (135–140) | 114 | 137 (135–140) | 237 | 0.449 |
| Serum potassium in mEq/L, median (IQR) | 4.3 (4–4.9) | 112 | 4.1 (3.8–4.4) | 237 | <0.001 |
| Serum creatinine in mg/dL, median (IQR) | 1.5 (0.94–2.4) | 114 | 0.8 (0.7–1.1) | 261 | <0.001 |
| Aspartate aminotransferase in U/L, median (IQR) | 62 (36.2–105) | 106 | 35 (26–54.5) | 236 | <0.001 |
| Alanine aminotransferase in U/L, median (IQR) | 28 (16–54) | 56 | 28 (19–52) | 215 | 0.514 |
| Serum albumin in g/dL, median (IQR) | 2.7 (2.3–3.2) | 107 | 3.3 (2.9–3.6) | 236 | <0.001 |
| Venous lactate in mmol/L, median (IQR) | 1.55 (1.02–2.15) | 34 | 1.3 (1.1–1.7) | 49 | 0.157 |
| Number of patients >1.5 mmol/L, n (%) | 19 (56) | 34 | 20 (41) | 49 | 0.157 |
| Inflammatory markers | |||||
| C reactive protein in mg/L, median (IQR) | 220 (113–297) | 80 | 75.6 (36.2–134) | 182 | <0.001 |
| Ferritin in ng/L, median (IQR) | 920 (470–2220) | 76 | 503 (266–1020) | 187 | <0.001 |
| Procalcitonin in ng/mL, median (IQR) | 1.02 (0.20–4.5) | 58 | 0.08 (0.05–0.22) | 163 | <0.001 |
| Number of patients >0.49 ng/mL, n (%) | 34 (59) | 58 | 17 (10) | 163 | <0.001 |
| Number of patients <0.15 ng/mL, n (%) | 11 (19) | 58 | 106 (65) | 163 | <0.001 |
| Lactate dehydrogenase in U/L, median (IQR) | 513 (399–729) | 67 | 333 (274–422) | 188 | <0.001 |
| Creatine kinase in U/L, median (IQR) | 659 (305–1450) | 42 | 146 (60–268) | 73 | <0.001 |
| D-dimer in μg/mL, median (IQR) | 2.7 (1.6–5.8) | 57 | 0.83 (0.5–1.6) | 127 | <0.001 |
| Number of patients >2.0 µg/mL, n (%) | 36 (63) | 57 | 28 (22) | 127 | <0.001 |
Continuous and categorical variables were compared using a Wilcoxon test and Fisher’s exact test, respectively.
All values are closest values in 24 hours prior to ICU admission. All continuous characteristics are in median (IQR) unless specified otherwise and all categorical characteristics are in number (percentage). The percentage is calculated with the number of patients who had the characteristic available as the denominator. For further clarity, the number in which that characteristic was available for is provided separately in adjacent column.
ICU, intensive care unit.
Outcomes by race
Including all individuals (n=2199), we then investigated survival probability stratified self-reported race and ethnicity by fitting a Cox proportional hazards model adjusted for age and sex (see online supplemental figure 3). As compared with white individuals, we did not find a significant association of survival with self-reported black (HR=0.88; p=0.37), or other race (HR=1.12; p=0.45). We also did not find a significant association of survival with Hispanic ethnicity (HR=1.10; p=0.50).
bmjopen-2020-040736supp005.pdf (648.3KB, pdf)
Discussion
The COVID-19 pandemic represents the greatest public health emergency in the modern world. Limited data, especially in the USA, exists to guide clinical care, resource management and risk stratification in hospitalised patients. Our study is of the case series of patients reported with confirmed COVID-19 in the USA. Previous reports were either from other countries, examined smaller cohorts, or were focused on critically ill patients.13 14 18–23 The present report provides a broad perspective on patients admitted with COVID-19 in both general medicine ward and intensive care settings. Our study presents key clinical differences from laboratory data available at time of admission and thus would aid clinical management decision-making early in the hospital course. Additionally, our health system serves a unique population representative of the ethnic and socioeconomic diversity seen in both New York City and across the USA.
We highlight several key findings. Among the 1078 patients who completed their hospital course (discharge or in-hospital death), the overall mortality rate was 29% and 31% in patients who received ICU care. The overall case fatality rate likely represents an overestimation of the true disease mortality rate since patients who remained hospitalised at the date of data freeze were not included in this calculation. The mortality rate in intensive care is lower than previously described13 23–25 and may be reflective of early care escalation.
We observed that patients who died had a significantly higher median age with significantly more pre-existing conditions than those who were discharged. Although 25% of patients were febrile on admission, this may be an underestimation due to possible antipyretic use and/or selection bias. A substantial proportion of patients with COVID-19 displayed abnormal laboratory measurements at the time of admission. These included lymphopoenia and elevated inflammatory markers such as D-dimer, CRP, LDH and ferritin. These trends persisted among those who died and/or received intensive care, both on admission and at the time of ICU transfer. If formal epidemiological analyses confirm these observations, early laboratory evaluation may be crucial in identifying patients suspected for COVID-19 prior to RT-PCR test result. It may also aid clinicians in identifying patients at high risk of decompensation, ICU admission and potentially even death. Early identification of high-risk patients could enable timely patient triage and improved resource allocation. Additional work is needed to develop real-time, accurate predictive models for risk stratification in COVID-19, particularly to elucidate the clinical utility of specific laboratory measurements.
Han et al26 found a significant association of serum LDH and CRP with COVID-19 severity in patients from China.26 As the number of cases rose quickly in New York City, MSHS hospitals served as early adopters, creating a COVID-19 order set in our EHR to streamline objective data gathering, facilitate more cohesive workflow among team members and minimise ancillary staff exposure by completing all necessary admission labs at one time. This laboratory order set included serum D-dimer, CRP, procalcitonin, ferritin and LDH. In turn, we observed an increase in these orders from the first day of admission over the study period (see online supplemental figure 2). Given the abnormalities observed in patients who died, these laboratory measurements may be prognostic markers of disease severity or subsequent clinical course, although this requires further investigation. If confirmed, other health systems expecting impending case surges may consider similar workflows to promote improved healthcare delivery to affected patients.
We found a significantly higher procalcitonin level in individuals who died as compared with those who were discharged alive. Moreover, of the 2199 individuals in the study, 28% had a procalcitonin >0.49. In the context of COVID-19, an elevated procalcitonin may signify a superimposed bacterial infection, but may also be a marker of acute respiratory distress syndrome or a result of upregulated cytokine production secondary to respiratory failure. Additionally, we found that individuals who died in the ICU had higher procalcitonin levels, supporting previous work demonstrating that patients with more severe COVID-19 infections have higher procalcitonin levels.22 27
The clinical characteristics of our cohort were largely similar to other large cohorts of patients with COVID-19from China2 22 and Italy. Specifically, patients in our dataset were elderly and had a male predominance, similar to previous reports. There was also a high prevalence of comorbid conditions, including hypertension and diabetes mellitus. Similar to an early report from Wuhanz, patients largely had a normal procalcitonin at admission, but individual requiring ICU care had a higher procalcitonin level. However, as compared studies from Wuhan, China22 and Genoa, Italy,14 and New York City,28 our cohort had a significantly lower prevalence of lymphopoenia at admission.
Our study should be considered in light of several limitations. Since COVID-19 testing is frequently repeated in hospitalised patients and initial testing may result in false negatives, we are unable to determine whether patients developed their infection during or before hospital admission. Furthermore, COVID-19 has a variable incubation period of approximately 8–15 days,29 and patients may present to the hospital several days after initial infection or the onset of symptoms. Thus, we are unable to determine patients’ disease duration. Additionally, we separated discharged patients from those who died, but some patients may have expired after discharge. This could affect our listed case mortality rate. Our study is also confined by the inherent limitations (eg, biases) of EHR data. Although using structured EHR data allows for rapid integration of multiple data streams and real-time analysis, data present only in clinical note text, such as symptoms on presentation are missed. Additionally, symptoms present before the time of admission were not included. We chose not to perform comprehensive manual chart review to prioritise timely dissemination of our observations. Another limitation of our dataset is the large proportion of individuals that were censored. These individuals remained in the hospital at the time of data analysis and thus had unknown outcomes. Future work will analyse these patients’ hospital course in greater detail with complete outcomes data.
As the COVID-19 pandemic spreads from the current epicentre in New York City to other areas, our report provides meaningful clinical insights that may better inform care for diverse populations. Future work will aim to predict COVID-19 patient outcomes using a variety of approaches, thereby reducing healthcare system burden and permitting improved care delivery.
Supplementary Material
Footnotes
Twitter: @ParanjpeIshan, @RFreeman_RN, @BenGlicksberg
IP, AJR, JKDF and AL contributed equally.
Contributors: IP, AJR, JKDF, AL, MS, AV, KWJ, SS, AK, RO'H, SM, UN, SKJ, AK, PT, JJ and AF developed and performed the analysis. PG, MAL, JF, JAA, EB, CRH and BM critically appraised the manuscript and helped acquire data. IP, AL, AJR, JKDpF, RM, MD, EG, DM, LMH, RF, MS, PK, VF, EPB, JN, EJN, CC-C, DC and DLR supervised the project. IP, AL, AJR, JKDF, GNN, BSG, AWC and AJ drafted the manuscript.
Funding: This work was Supported by U54 TR001433-05, National Center for Advancing Translational Sciences, National Institutes of Health.
Competing interests: GNN reports grants, personal fees and non-financial support from Renalytix AI, non-financial support from Pensieve Health, personal fees from AstraZeneca, BioVie, GLG Consulting, from outside the submitted work. AL reports personal fees from Zoll, outside the submitted work. ZAF reports grants from Daiichi Sankyo, grants from Amgen, Bristol Myers Squibb and Siemens Healthineers, personal fees from Alexion, GlaxoSmithKline, Trained Therapeutix Discovery, outside the submitted work. In addition, ZAF has patents licensed to Trained Therapeutix Discovery. The other authors have nothing to disclose.
Patient consent for publication: Not required.
Ethics approval: The Mount Sinai Institutional Review Board approved this research under a broad regulatory protocol allowing for analysis of limited patient-level data.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available. Please contact authors for information on data availability.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Coronavirus resource center COVID-19 Map, 2020. Available: https://coronavirus.jhu.edu/map.html
- 2.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32. 10.1038/s41591-020-0968-3 [DOI] [PubMed] [Google Scholar]
- 4.Fisher M, Neugarten J, Bellin E, et al. Aki in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 2020;31:2145–57. 10.1681/ASN.2020040509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan L, Chaudhary K, Saha A, et al. Aki in hospitalized patients with COVID-19. J Am Soc Nephrol 2020:ASN.2020050615. 10.1681/ASN.2020050615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–46. 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paranjpe I, Fuster V, Lala A, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol 2020;76:122–4. 10.1016/j.jacc.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID‐19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020;77:198–209. 10.1111/his.14134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 2020;382:e38. 10.1056/NEJMc2007575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin S, Huang M, Li D, et al. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis 2020;395:1–4. 10.1007/s11239-020-02105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a new York City health system. JAMA 2020;324:799–801. 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–81. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vena A, Giacobbe DR, Di Biagio A, et al. Clinical characteristics, management and in-hospital mortality of patients with coronavirus disease 2019 in Genoa, Italy. Clin Microbiol Infect 2020. 10.1016/j.cmi.2020.07.049. [Epub ahead of print: 15 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Center for Disease Control and Prevention States reporting cases of COVID-19 to CDC, 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html
- 16.Lipsitch M, Swerdlow DL, Finelli L. Defining the Epidemiology of Covid-19 - Studies Needed. N Engl J Med 2020;382:1194–6. 10.1056/NEJMp2002125 [DOI] [PubMed] [Google Scholar]
- 17.R Core Team R: a language and environment for statistical computing. R found STAT comput Vienna, Austria; R foundation for statistical computing, 2017. Available: https://www.r-project.org/about.html
- 18.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a long-term care facility in King County, Washington. N Engl J Med 2020;382:2005–11. 10.1056/NEJMoa2005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng O-T, Marimuthu K, Chia P-Y, et al. SARS-CoV-2 infection among travelers returning from Wuhan, China. N Engl J Med 2020;382:1476–8. 10.1056/NEJMc2003100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis 2020;20:669–77. 10.1016/S1473-3099(20)30243-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically Ill patients in the seattle region - case series. N Engl J Med 2020;382:2012–22. 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC C. Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. China CDC Weekly 2020. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 2020;323:1612. 10.1001/jama.2020.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han Y, Zhang H, Mu S. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. medRxiv. 10.1101/2020.03.24.20040162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta 2020;505:190–1. 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020;382:2372–4. 10.1056/NEJMc2010419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 2020;172:577–82. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040736supp001.pdf (54.1KB, pdf)
bmjopen-2020-040736supp002.pdf (328.1KB, pdf)
bmjopen-2020-040736supp003.pdf (105.8KB, pdf)
bmjopen-2020-040736supp004.pdf (69.7KB, pdf)
bmjopen-2020-040736supp005.pdf (648.3KB, pdf)
Data Availability Statement
Please contact authors for information on data availability.