Abstract
Background: It is now well established that microbes play a key and causative role in the pathogenesis of anastomotic leak. Yet, in patients, determining whether a cultured pathogen retrieved from an anastomotic leak site is a cause or a consequence of the complication remains a challenge. The aim of this study was to test a methodology to invoke causality between a retrieved microbe from a leak site and its role in anastomotic leak.
Methods: The commensal organism Bacillus subtilis was isolated from an esophagojejunostomy leak site in a 35-year-old patient with a CDH1 mutation after a prophylactic gastrectomy whose body mass index (BMI) was 35 kg/m2. The organism was screened for its ability to degrade collagen, shift human recombinant matrix metalloprotease-9 (MMP9) to its active form, and induce a clinical anastomotic leak when introduced to anastomotic tissues of mice fed their standard diet (SD) of chow or an obesogenic Western-type diet (WD).
Results: The Bacillus subtilis strain retrieved from the anastomotic leak site displayed a high degree of collagenolytic activity and was able to activate human MMP9 consistent with other pathogens expressing this characteristic “leak phenotype.” Exposure of the Bacillus subtilis to the anastomotic tissues of obese mice fed a WD led to dehiscence of the anastomosis, abscess formation with peritonitis, and mortality in 50% of mice (3/6). When anastomotic healing was evaluated by a validated anastomotic healing score (AHS), substantially worse healing was observed (i.e., higher AHS) in WD-fed mice exposed to Bacillus subtilis compared to SD-fed mice (analysis of variance [ANOVA], p = 0.0006).
Conclusions: Microbial strains obtained from patients' anastomotic leak sites can be evaluated for their pathogenic in the leak process by assessing their ability to produce collagenase, activate MMP9 and cause clinical leaks in mice fed a WD. These studies may aid in identifying those bacterial strains that play a causal role in patients with an anastomotic leak.
Keywords: anastomotic leak, Western diet, obesity, Bacillus subtilis
Anastomotic leak remains a real and present danger to patients despite improvement in operative technique, use of antibiotics, and the rapid availability of imaging. Although leak rates across high-volume centers seem to have decreased substantially over the last several decades, even among high-volume expert surgeons, leaks still occur and result in substantial disability. A series of reports has invoked a microbial pathogenesis for anastomotic healing suggesting that microbes can play a causative role in anastomotic leak [1–7]. In the aggregate, these studies suggest that bacteria that express the tissue-destroying enzyme collagenase can activate key tissues proteases resulting in impairment of the normal process of healing. In fact, across all the body's tissues, excess protease activity is the hallmark of abnormal healing.
We have recently shown that endogenous Enterococcus faecalis colonizes anastomotic tissues in rats undergoing colon surgery [8] but only causes leak when strains express the tissue destroying enzyme collagenase [9]. Exposure of the normally anaerobic environment of the colon to ambient oxygen when performing an anastomosis can lead to elimination of the health promoting Bacteroides species [10]. In addition, gastrointestinal surgery invariably involves the use of both oral and parenteral antibiotic agents, leaving mucosal tissues vulnerable to colonization by remaining pathogens. We have modeled colon anastomotic leak in the mouse and observed that when anastomotic tissues are exposed to collagenolytic bacteria such as Enterococcus faecalis [11,12], Serratia marcescens [13], or Pseudomonas aeruginosa [11,13] anastomotic disruption occurs that involves the activation of tissue proteases such as matrix metalloprotease-9 (MMP9) and plasminogen [9,11–13]. Of note is that these pathogens are commonly retrieved from sites of anastomotic leak [9,14–16]. Further modeling of anastomotic leak in our laboratory has determined that feeding mice a high-fat Western-type diet (WD) can enrich the colonic anastomotic mucosa with collagenolytic bacteria, further implicating their role in anastomotic leak pathogenesis. In the present report, we isolated a pathogen from an anastomotic leak site of an esophagojejunostomy after a total gastrectomy. The isolated organism, Bacillus subtilis, considered to be a non-pathogenic commensal organism, was tested for its ability to produce collagenase, activate MMP9, and cause anastomotic leak in our established mouse models. Results indicate that Bacillus subtilis is an organism that produces substantial collagenase, can activate MMP9, and can cause anastomotic leak in mice fed high-fat WD. Taken together these data indicate, for the first time that Bacillus subtilis may be considered a leak pathogen given its properties to express collagenase and activate tissue proteases consistent with other pathogens identified to cause anastomotic leak.
Patients and Methods
Isolation of Bacillus subtilis from a patient with a clinical anastomotic leak
A 48-year-old female patient with a body mass index (BMI) of 35 and a known CDH-1 mutation underwent a prophylactic gastrectomy via the laparoscopic approach. A hand-sewn Roux-en-Y esophagojejunostomy was performed in standard fashion. Intra-operative endoscopy at the conclusion of the procedure revealed an air-tight anastomosis with no evidence of ischemia. A routine upper gastrointestinal (UGI) study on post-operative day three was normal and a diet was introduced. The night of post-operative day three the patient spiked fever of 39.2°C and became mildly tachycardic. A chest radiograph revealed free air, however, the patient did not appear to have sepsis (normotensive with adequate urine output) nor did she have any signs of peritonitis on physical examination. A decision was made to forego computed tomography (CT) imaging and perform repeat laparoscopy. Approximately 50 mL of turbid intra-peritoneal fluid was identified near the anastomosis and obtained for culture. Upper endoscopy was performed during the surgery and a 2-mm hole was observed without any grossly visible areas of ischemia. The area was lavaged and a drain was placed.
Screening of the intra-operative perianastomotic fluid sample for the presence of collagenase-producing (i.e., collagenolytic) bacteria
Initially, intra-operative fluid samples were sent to the clinical microbiology laboratory at the University of Chicago for bacterial isolation and antibiotic sensitivity assay. Results indicated normal mouth flora and the results were not speciated further. To characterize the microbial content of the leak fluid further, samples were inoculated onto skim milk plates to examine a zone of milk clearance as an indication of the presence of collagenolytic bacteria as our group has previously described [5]. Briefly, plates specified for gram-negative, gram-positive, Enterococcus, and Pseudomonas were created with incorporation of a skim milk overlay onto either Enterococcus, MacConkey gram-negative, Pseudomonas isolation agar, or by with Columbia colistin-nalidixic acid (CNA) agar with 5% sheep blood (gram-positive) [5]. Colonies surrounded by zones of skim milk clearance were collected and identified. Speciation of isolated collagenolytic colonies was then determined at the Mayo Clinic Hospital in Rochester, Minnesota, and identified Bacillus subtilis as the collagenolytic bacteria.
Quantitative collagenase assay
Confirmative quantitative analysis of isolated colonies for collagenolytic activity was performed as previously described using the EnzChek™ gelatinase/collagenase assay (ThermoFisher Scientific, Eugene, OR) [9]. Bacteria were grown overnight in TY media consisting of 10 g/L tryptone and 5 g/L yeast extract and then were inoculated into 96-well plates at 1:100 dilution with fresh TY media. Fluorescein-conjugated gelatin from pig skin (D12054, ThermoFisher Scientific) at a final concentration of 5 mcg/mL was introduced immediately before measuring using a multichannel pipette. Cell density (optical density [OD] = 600 nm) and collagenolytic activity (495/515 nm, excitation/emission) were measured immediately (t = 0) and serially for up to 24 hours. Start point values were subtracted, and collagenolytic activity was normalized to cell density.
MMP9 activation assay
Given the central role of MMP9 activation in the mechanism by which intestinal bacteria can cause anastomotic leak, assays were performed using recombinant human proenzyme MMP9 (r-MMP9) (Calbiochem, catalog #PF038). The r-MMP9 was diluted to a final concentration of 1 mcg/mL in the assay buffer (50 mM Tris-HCl [pH 7.5], 10 mM CaCl2, and 0.05% Triton X-100). Thirty microliters of Bacillus subtilis grown in tryptic soy broth (TSB) (OD 600 nm = 1.0) was incubated with 20 mcL of r-MMP9 (1 mcg/mL) for two hours. After co-incubation, the conditioned medium was centrifuged (6,000g, 20 min), and 5 mcL of the supernatant was mixed with an equal volume of 2 × sodium dodecyl sulfate loading buffers and subjected to zymography. Zymography for identification of zones of enzyme activity was performed using 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels containing 0.1% gelatin as previously described [9]. Enterococcus faecalis strain E2, previously shown to activate MMP9 [9], was used as a positive control.
Mouse model of anastomosis leak
Six-week-old BALB/c mice (Charles River Laboratories, Wilmington, MA) were used in all experiments. Mice were maintained in accordance with the University of Chicago IACUC Protocol 72491 with guidelines prepared by the University of Chicago Institutional Animal Care and Use Committee. To determine if the Bacillus subtilis species isolated from the patient was capable of causing a clinical anastomotic leak, the isolate was introduced into our standard mouse model for clinical anastomotic leak. Previous work with our mouse model indicated that mice fed a WD, similar to that consumed by patients, and exposure to antibiotic agents, similar to those used by patients, is a sensitive model to detect clinical anastomotic leak [1]. Therefore, mice were randomly assigned to two feeding regimens, a standard chow diet (SD) characterized by low fat and high fiber (n = 14) and a high-fat, low-fiber WD, BioServe S3282) (n = 12). The detailed compositions of the diets have been described recently [10]. Mice were allowed their defined diet and water ad libitum for six weeks, and the mice were weighed at the end of six weeks.
Colon surgery on mice was performed as previously described [13]. Prior to the surgery, mice received oral clindamycin (100 mg/kg, ∼50 mcL oral gavage of 50 mg/mL), and a subcutaneous injection of cefoxitin (40 mg/kg, ∼100 mcL of 10 mg/mL). Subsequently the mice were anesthetized with an intra-peritoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg) and underwent a standard hand-sewn distal colon anastomosis at the peritoneal reflection as previously described. The anastomosis integrity was verified by administration of 100-mcL enema of normal saline via a 22-gauge blunt, olive-tip needle, after which the abdomen was closed by a double layer of 5-0 Vicryl followed by a 5-0 nylon. Animals were resuscitated with 1 mL subcutaneous injection of 0.9% normal saline. Post-operative analgesia consisted of subcutaneous injections of buprenorphine (0.05 mg/kg Henry Schein) every eight to 12 hours for first 48 hours and one-time subcutaneous dose of meloxicam (1 mg/kg Henry Schein). All mice were operated on by the same surgeon. On post-operative day one, 100 mcL of a freshly prepared bacterial suspension with Bacillus subtilis (OD 600 nm = 1.0 in 10% glycerol) or a vehicle (10% glycerol) was administered via a rectal enema with a 22-gauge blunt olive-tip needle (n = 6 in WD group, n = 7 SD group). Mice were randomly assigned to each group and subsequently redistributed over different cages in order to prevent cage effects [17]. The mice were observed post-operatively for clinical signs of leak such as lethargy, decreased movement, and visible chills. Mice that exhibited signs of leak were euthanized and their anastomoses were evaluated visually by two individual researchers. The rest of mice were euthanized on post-operative day six by carbon dioxide asphyxiation with confirmatory cervical dislocation, upon which their anastomoses were visually inspected. The anastomosis was evaluated using the validated anastomotic healing score (AHS) as previously described [13]: 0 = normal healing; 1 = loose adhesion; 2 = dense adhesion; 3 = gross abscess formation; 4 = gross leak with peritoneal contamination. The AHS = 5 was additionally created to evaluate mice with gross leak (dehiscence) leading to peritoneal contamination and moribundity.
Statistics
Analysis of variance (ANOVA) and unpaired t-test were used for the significance analysis with GraphPad Prism (GraphPad Software, San Diego, CA). A p value <0.05 was estimated as a significant difference.
Results
Bacillus subtilis isolated from the perianastomotic fluid expresses the collagenolytic phenotype
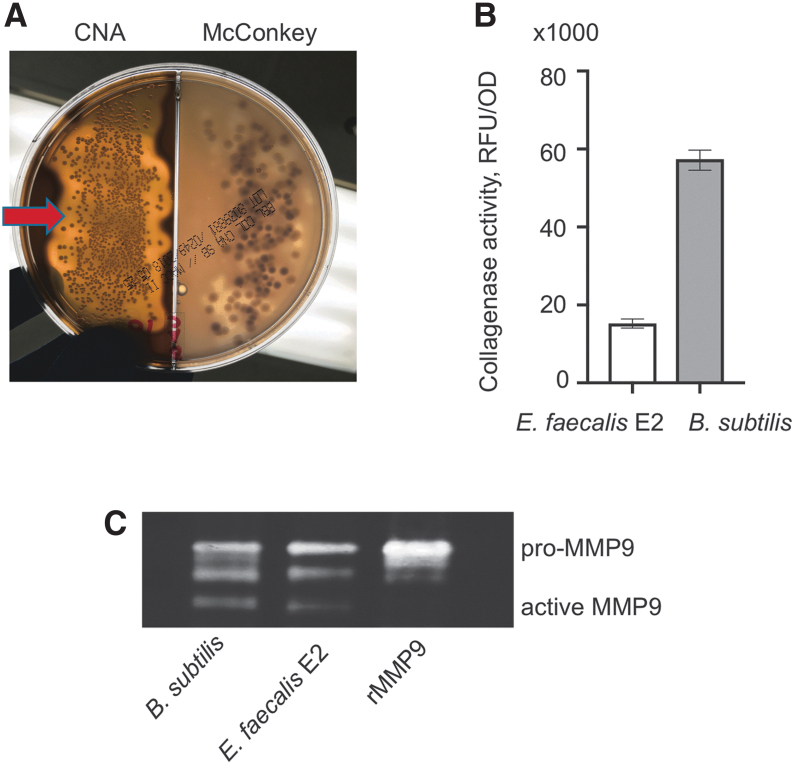
Collagenolytic colonies were identified on gram-positive CNA plates covered with skim milk. Massive colonization of gram-positive colonies with adjusted clearance zone was observed on the left part of the test plate containing CNA agar covered with skim milk (Fig. 1A). As mentioned, the colonies were identified as facultative anaerobe Bacillus subtilis. Quantitative collagenase assay revealed a high degree of collagenase activity as seen by the degradation of fluorescein-labeled gelatin (Fig. 1B). Bacillus subtilis collagenase activity was three-fold higher that of the other gram-positive collagenolytic strain of Enterococcus faecalis E2 isolated previously from anastomotic tissue in a rat model of anastomotic leak [9]. Matrix metalloprotease-9 activity assay demonstrated that Bacillus subtilis was able to cleave MMP9 to its active form similar to that of our reference Enterococcus faecalis E2 strain (Fig. 1C) [9].
FIG. 1.
Bacillus subtilis isolated from IP of the patient with anastomotic leak is characterized by a leaking phenotype. (A) Identification of collagenolytic bacteria on skim milk plate. Red arrow depicts a massive clearance zone surrounded by bacterial colonies on CNA side of the plate. (B) Confirmation of collagenolytic activity of the isolate identified as Bacillus subtilis using fluorescein-labeled gelatin. Enterococcus faecalis strain E2 included as a control. (C) Zymography image with pro- and active recombinant MMP9 bands demonstrating the ability of Bacillus subtilis to activate the host extracellular matrix metalloprotease. IP = intraperitoneal fluid; CNA = colistin-nalidixic acid; RFU = relative fluorescence units; OD = optical density; rMMP9 = recombinant human proenzyme matrix metalloprotease-9; MMP9 = matrix metalloprotease-9; SD = standard diet; WD = Western-type diet. Color image is available online.
Exposure of the mouse colon anastomosis to Bacillus subtilis results in a clinical leak
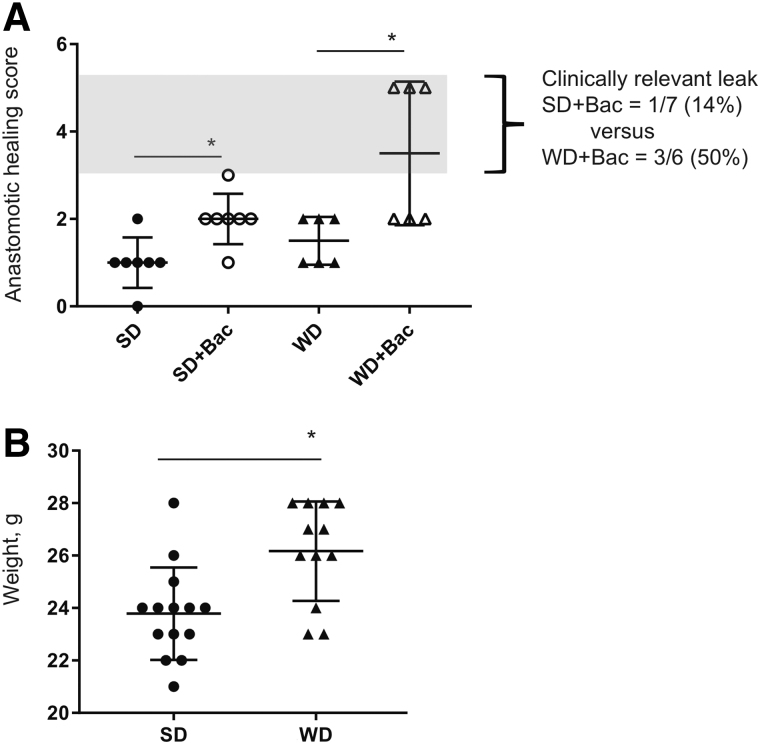
In mice fed standard chow, exposure of anastomotic tissues to Bacillus subtilis via enema on post-operative day one [12,13]. Results indicated that only one of seven mice developed a clinical leak as judged by an AHS = 3, meaning the presence of an abscess. However, dense adhesions indicating abnormal healing with an AHS = 2 were observed in the majority of mice (Fig. 2A). To render the model more sensitive to the Bacillus subtilis strain and to shift the animals microbiome to reflect that of an obese patient (such as the patient from whom the Bacillus subtilis strain was isolated), reiterative studies were performed in mice fed a high-fat/low-fiber WD in which they gained substantial weight (p = 0.0029, unpaired t-test; Fig. 2B). In this model a substantial incidence of clinical leak was observed with an AHS = 5 in 50% of mice (3/6; Fig. 2A). Visible dehiscence in these mice was accompanied by substantial perianastomotic adhesion, abscess formation, and spilled luminal contents and led to mortality. The percent leak rate was 14% in SD plus Bacillus subtilis versus 50% in WD plus Bacillus subtilis (Fig. 2A) whereas gross leak was identified only in WD and Bacillus subtilis-injected mice (Fig. 2A).
FIG. 2.
Enema injection of Bacillus subtilis at the anastomotic site of Western diet (WD)-fed mice leads to gross leak and moribundity in 50% of mice. (A) The anastomotic healing score (AHS). Enema injection of Bacillus subtilis leads to substantial increase of AHS in both standard diet (SD)-fed mice (p = 0.0071, n = 7 per group) and Western diet (WD)-fed mice (p = 0.0179, n = 6 per group). Group difference significance = 0.0006, analysis of variance (ANOVA). (B) Weight of mice after 6 weeks feeding on SD vs. WD. p = 0.0029, Unpaired t-test.
Discussion
To our knowledge, this is the first report to indicate the recovery of a species of Bacillus subtilis from an anastomotic leak site. The fact that this organism is considered to be a commensal of the upper gut flora and in general not a pathogen is intriguing given its high collagenolytic potential as indicated by the results of this report. Remarkably, the clinical laboratory did not speciate the organisms from the clinical sample that were submitted as they demonstrated normal mouth flora. Yet, screening the clinical sample for the appearance of collagenolytic colonies on selective media allowed us to capture the Bacillus subtilis species and identify it as a collagenase producing organism. Although Bacillus subtilis and other Bacillus species are known to produce gelatinase (i.e., collagenase) [18], relevant to the current report, it is noteworthy that Bacillus subtilis was able to persist under the environmental conditions of surgery and the use of prophylactic antibiotics. Although some have considered Bacillus subtilis to be associated with clinical diarrhea, it is also considered to be a probiotic organism in other circumstances [19,20].
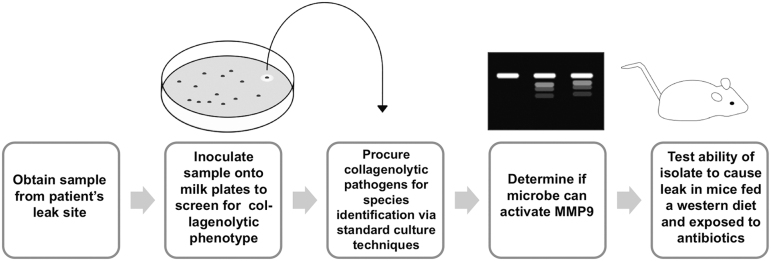
Verifying whether a bacterial isolate recovered from an anastomotic leak site is a cause or a consequence of an anastomotic leak remains a major challenge. Although we have previously proven in animals (rats, mice) that collagenolytic bacteria with the capacity to cleave MMP9 can play a key and causative role in anastomotic leak, that this occur in humans remains to be demonstrated. Here we attempted to provide a mechanism to test this hypothesis by first examining the phenotype of the isolated pathogen, in this case Bacillus subtilis, its ability to produce collagen, and cleave MMP9 (Fig. 3). Next, we introduced the organism into a well-characterized mouse model of anastomotic leak to demonstrate its in vivo capacity to cause a clinically relevant leak (i.e., abscess) (Fig. 3). Although neither of these assays is sufficient to prove causality between the Bacillus subtilis species herein reported and the occurrence of the anastomotic leak in this patient, the results indicate an indirect method to infer its causality to the occurrence of a clinical leak. This information can then potentially be used to generate a library of leak pathogens that may provide key information for antibiotic prophylaxis, particularly in high-risk patients. Admittedly, the patient's leak in the case report described here could have been caused by a technical issue with the anastomosis involving the suture technique, ischemia, or tension, although there was no evidence for this either during the operation or at the second exploration.
FIG. 3.
Proposed workflow to determine the potential bacteria with leaking phenotype in the patients.
The use of mice fed a WD in the present report is based on recent work from our laboratory indicating this diet renders mice highly sensitive to the development of a clinical anastomotic leak (i.e., abscess formation, peritonitis) [1]. Previous work has indicated that feeding of a WD, such as the one used here, has a dramatic effect on the gut microbiome rendering it particularly vulnerable to colonization by exogenously introduced pathogenic species [10]. Colonization resistance or the competitive exclusions of exogenous pathogens is diminished when mice are fed a WD similar to that used here and is postulated to be a result of a loss of probiotic species such as Bacteroidetes. In addition, feeding of a WD to mice results in a near four-order magnitude increase in the population of collagenolytic bacteria in the colon [1]. We assume that against this background microbiome, the addition of another collagenolytic bacteria such as Bacillus subtilis may disrupt the healing process further resulting in uncontrolled collagen degradation through MMP9 activation as we have previously shown in mice [9].
This study has several limitations. First, the number of test animals used was small, because we tried to limit their use to obtain a signal that Bacillus subtilis can cause anastomotic leak, a process that can cause abscess formation and peritonitis. By having one surgeon perform the anastomosis and by assigning mice randomly to the treatment groups we sought to limit technical issues. Second, by not analyzing the entire gut microbiome in mice at the site of the anastomosis, we limited our understanding of how Bacillus subtilis strains introduced into the anastomotic site alter the overall microbial ecology. Future studies will need to incorporate a more in-depth analysis of the microbiome at anastomotic tissues sites when an exogenous organism is introduced.
In summary, here we describe a method in an attempt to ascribe a causal relation between a microbe retrieved from an anastomotic leak site and the occurrence of a clinical anastomotic leak by using an established mouse model of anastomotic leak into which the test organism is introduced. This approach may be useful in screening patients who may be harboring highly pathogenic species that can complicate anastomotic healing. Further work is needed to verify the accuracy of this approach to reduce clinical anastomotic leak in high-risk patients via this screening method.
Authors' Contributions
J.v.P., J.L., O.Z., and J.A. all made substantial contributions to the concept and design of the work and to the drafting and corrections of the manuscript. All authors approve of the final version to be published.
Funding Information
This work was partially funded by NIH grant R01-GM062344-18 (JCA).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Gaines S, van Praagh JB, Williamson AJ, et al. Western diet promotes intestinal colonization by collagenolytic microbes and promotes tumor formation following colorectal surgery. Gastroenterology 2020;158:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaines S, Shao C, Hyman N, Alverdy JC. Gut microbiome influences on anastomotic leak and recurrence rates following colorectal cancer surgery. Br J Surg 2018;105:e131–e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol 2017;14:43–54 [DOI] [PubMed] [Google Scholar]
- 4. Guyton KL, Hyman NH, Alverdy JC. Prevention of perioperative anastomotic healing complications: Anastomotic stricture and anastomotic leak. Adv Surg 2016;50:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guyton KL, Levine ZC, Lowry AC, et al. Identification of collagenolytic bacteria in human samples: Screening methods and clinical implications for resolving and preventing anastomotic leaks and wound complications. Dis Colon Rectum 2019;62:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shogan BD, An GC, Schardey HM, et al. Proceedings of the first international summit on intestinal anastomotic leak, Chicago, Illinois, October 4–5, 2012. Surg Infect 2014;15:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schardey HM, Kamps T, Rau HG, et al. Bacteria: A major pathogenic factor for anastomotic insufficiency. Antimicrob Agents Chemother 1994;38:2564–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shogan BD, Smith DP, Christley S, et al. Intestinal anastomotic injury alters spatially defined microbiome composition and function. Microbiome 2014;2:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shogan BD, Belogortseva N, Luong PM, et al. Collagen degradation and MMP9 activation by Enterococcus faecalis contribute to intestinal anastomotic leak. Sci Transl Med 2015;7:286ra268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hyoju SK, Zaborin A, Keskey R, et al. Mice fed an obesogenic Western diet, administered antibiotics, and subjected to a sterile surgical procedure develop lethal septicemia with multidrug-resistant pathobionts. mBio 2019;10:e00903–00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacobson RA, Wienholts K, Williamson AJ, et al. Enterococcus faecalis exploits the human fibrinolytic system to drive excess collagenolysis: Implications in gut healing and identification of druggable targets. Am J Physiol Gastrointest Liver Physiol 2020;318:G1–G9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wiegerinck M, Hyoju SK, Mao J, et al. Novel de novo synthesized phosphate carrier compound ABA-PEG20k-Pi20 suppresses collagenase production in Enterococcus faecalis and prevents colonic anastomotic leak in an experimental model. Br J Surg 2018;105:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hyoju SK, Klabbers RE, Aaron M, et al. Oral polyphosphate suppresses bacterial collagenase production and prevents anastomotic leak due to Serratia marcescens and Pseudomonas aeruginosa. Ann Surg 2018;267:1112–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belmouhand M, Krohn PS, Svendsen LB, et al. The occurrence of Enterococcus faecium and faecalis is significantly associated with anastomotic leakage after pancreaticoduodenectomy. Scand J Surg 2018;107:107–113 [DOI] [PubMed] [Google Scholar]
- 15. Ohigashi S, Sudo K, Kobayashi D, et al. Significant changes in the intestinal environment after surgery in patients with colorectal cancer. J Gastrointest Surg 2013;17:1657–1664 [DOI] [PubMed] [Google Scholar]
- 16. Wirth U, Rogers S, Haubensak K, et al. Local antibiotic decontamination to prevent anastomotic leakage short-term outcome in rectal cancer surgery. Int J Colorectal Dis 2018;33:53–60 [DOI] [PubMed] [Google Scholar]
- 17. Alexander AD, Orcutt RP, Henry JC, et al. Quantitative PCR assays for mouse enteric flora reveal strain-dependent differences in composition that are influenced by the microenvironment. Mamm Genome 2006;17:1093–1104 [DOI] [PubMed] [Google Scholar]
- 18. Evans DG, Wardlaw AC. Gelatinase and collagenase production by certain species of Bacillus. J Gen Microbiol 1953;8:481–487 [DOI] [PubMed] [Google Scholar]
- 19. Elshaghabee FMF, Rokana N, Gulhane RD, et al. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front Microbiol 2017;8:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Starosila D, Rybalko S, Varbanetz L, et al. Anti-influenza activity of a Bacillus subtilis probiotic strain. Antimicrob Agents Chemother. 2017;61 61:e00539–17 [DOI] [PMC free article] [PubMed] [Google Scholar]