Significance
Many neurons release neuropeptides as well as classical neurotransmitters. Neuropeptides often regulate neural circuits controlling behaviors such as emotional behaviors. However, how neuropeptide release is regulated is not well-understood. We identified a Drosophila gene, Dstac, that is similar to a vertebrate gene that regulates voltage-dependent calcium channels in skeletal muscles and found that it is active in muscles and a subset of neurons, including motor neurons. Using live imaging, electrophysiology, and genetic manipulations, we found that Dstac localizes to motor synapses with muscles and regulates the voltage response of calcium channels and the release of neuropeptides. Since Stac proteins are found in neurons in the vertebrate CNS, they may also be regulators of neuropeptide release in the vertebrate brain.
Keywords: stac, neuropeptide, L-type voltage-gated calcium channel, Drosophila melanogaster
Abstract
Neuropeptides are important for regulating numerous neural functions and behaviors. Release of neuropeptides requires long-lasting, high levels of cytosolic Ca2+. However, the molecular regulation of neuropeptide release remains to be clarified. Recently, Stac3 was identified as a key regulator of L-type Ca2+ channels (CaChs) and excitation–contraction coupling in vertebrate skeletal muscles. There is a small family of stac genes in vertebrates with other members expressed by subsets of neurons in the central nervous system. The function of neural Stac proteins, however, is poorly understood. Drosophila melanogaster contain a single stac gene, Dstac, which is expressed by muscles and a subset of neurons, including neuropeptide-expressing motor neurons. Here, genetic manipulations, coupled with immunolabeling, Ca2+ imaging, electrophysiology, and behavioral analysis, revealed that Dstac regulates L-type CaChs (Dmca1D) in Drosophila motor neurons and this, in turn, controls the release of neuropeptides.
Neuropeptides are required for a myriad of brain functions, such as regulation of complex social behaviors, including emotional behavior (1). The activities of many neuropeptides within the brain are now well-established, but, despite the important role neuropeptides play, our understanding of the mechanisms by which neuropeptides are released by neurons and how release is regulated is not as advanced as that of classical neurotransmitters. A greater understanding of neuropeptide release could help untangle the mechanisms of complex behaviors as well as reveal therapeutic targets that could modulate aberrant behaviors.
Release of neuropeptides, which are packaged in dense core vesicles (DCVs) rather than in small synaptic vesicles that contain neurotransmitters, often involves activation of L-type Ca2+ channels (CaChs) and Ca2+-induced Ca2+ release (CICR) (2). Much of what is known about DCV exocytosis is from the study of nonneural cells. For example, in adrenal chromaffin cells, the exocytosis of DCVs containing catecholamines and/or peptide hormones involves CICR either via the ryanodine receptor (RyR) or inositol trisphosphate receptor (iP3R) (3, 4) initiated by an influx of Ca2+ through voltage-gated CaChs, such as L-type CaChs. In neurons, the release of neuropeptides is more complex. Neuropeptides can be released from dendrites, cell bodies, axons, and presynaptic terminals (5–7), and release can involve a kiss-and-run mechanism (8). DCVs are not tightly clustered as are small synaptic vesicles, and DCVs are generally not associated with presynaptic specializations for release of neurotransmitters, such as active zones (9). Furthermore, the DCVs within the central nervous system (CNS) are not as numerous nor as large as they are in chromaffin cells and neurohypophyseal terminals. In many neurons, the release of neuropeptides requires bursts of action potentials (10, 11). Based primarily upon pharmacological experiments, it appears that an influx of Ca2+ via L-type CaChs is necessary for release of neuropeptides from some neurons (8, 12–18), with some cases also involving Ca2+ release from internal stores while others not. This suggests the possibility that, in neurons, neuropeptide release may be initiated by an influx of Ca2+ via L-type CaCh.
Drosophila provide an ideal system to study neuropeptide release due to the vast array of molecular and genetic tools available for manipulating them. Numerous neuropeptides are expressed by the Drosophila nervous system, including proctolin by motor neurons (19, 20). Furthermore, larval motor neurons fire bursts of high frequency action potentials (21, 22) so motor boutons are likely to have the long-lasting increases in Ca2+ transients in motor boutons that are thought to be required for the release of neuropeptides. Motor boutons contain a network of endoplasmic reticulum (ER) (23), and there is a RyR-dependent release of Ca2+ from the ER presumably via CICR, which is required for sustained release of neuropeptides at the neuromuscular junction (NMJ) of third instar larvae (24). Elegant dynamic examination of DCVs in boutons with fluorescence recovery after photobleaching showed that they are immobile in the resting state, but they move randomly and release neuropeptides for several minutes following activity-dependent Ca2+ increases (25). Furthermore, simultaneous photobleaching and imaging of DCVs suggest that DCVs release neuropeptides via multiple rounds of kiss-and-run events (26). Thus, in Drosophila, neuropeptide release at the NMJ appears to involve CICR from the ER.
One way to regulate the release of neuropeptides is to control changes in cytoplasmic Ca2+ levels. In mammals, stac3 is a member of a small family of genes, along with stac1 and stac2, which are expressed by subsets of neurons (27–29). Stac3 was identified as a regulator of L-type CaChs and excitation–contraction (EC) coupling in zebrafish skeletal muscles (30, 31). Stac3 also regulates EC coupling in murine skeletal muscles (32) and is causal for the congenital Native American myopathy (30). EC coupling is the process that transduces changes in muscle membrane voltage to initiate release of Ca2+ from the sarcoplasmic reticulum (SR) and contraction. In vertebrate skeletal muscles, EC coupling is mediated by the L-type CaCh DHPR, which is in the transverse tubule membrane (t-tubules) and is the voltage sensor for EC coupling, and the RyR, which is the Ca2+ release channel in the SR (33–36). In zebrafish, Stac3 regulates EC coupling by colocalizing with DHPR and RyR, and by regulating DHPR stability and functionality, including the response to voltage of DHPRs, but not trafficking of DHPRs (31, 37).
The in vivo function of the stac1 and stac2 genes expressed by neurons is, however, unknown. Recently, a stac-like gene, Dstac, was identified in Drosophila (38). There is a single stac gene in Drosophila, and it is expressed both by muscles and a subset of neurons, including in the lateral ventral neurons (LNVs) that express the neuropeptide, pigment-dispersing factor (PDF), in the brain. Previously, genetic manipulation of PDF demonstrated the necessity of PDF for circadian rhythm (39). Interestingly, knocking down Dstac selectively in the PDF neurons disrupted circadian rhythm, suggesting the hypothesis that Dstac regulates the release of neuropeptides such as PDF. Since Stac3 regulates the L-type CaCh in vertebrate skeletal muscle, Dstac might control neuropeptide release via regulation of the single L-type CaCh in Drosophila neurons (Dmca1D) (40). We tested this hypothesis by examining the role of Dstac for neuropeptide release by the more accessible presynaptic boutons of motor neurons at the NMJs of third instar larvae.
Results
Motor Boutons Express Dstac, Dmca1D, and Proctolin.
The hypothesis that Dstac regulates Dmca1D and the release of neuropeptide by motor boutons at the NMJ predicts that Dstac, Dmca1D, and a neuropeptide are all expressed by motor boutons. We examined the expression of Dstac in the motor boutons by labeling them with anti-Dstac, anti-proctolin, and an antibody directed against Dmca1D that we generated (Materials and Methods). Labeling larvae selectively expressing membranous GFP in proctolin+ motor neurons (Proct:GAL4 > UAS:mCD8GFP) with anti-proctolin confirmed that motor boutons expressed proctolin (Fig. 1A). There are four types of boutons on larval muscles based upon their size (41). Type Is and III boutons showed strong labeling by anti-proctolin while type Ib showed weak labeling and type II no labeling (SI Appendix, Fig. S1). We detected reliable anti-proctolin labeling on muscles 1, 2, 3, 4, 6/7, 9, 12, 13, 15/16, and 19, which extends the result of an earlier study that found labeling reliably in 4, 12, and 13 (19). Labeling larvae with anti-Dstac and anti-Dmca1D showed that both type Ib and Is boutons expressed Dstac and Dmca1D (Fig. 1 B and C) in addition to proctolin. The specificity of anti-Dmca1D was demonstrated by labeling Dmca1D null embryos (Dmca1DX10) (42), which are larval lethals, with anti-Dmca1D and finding that there was no detectable labeling in mutant embryos while, in control embryos, the longitudinal tracts in the CNS were labeled (SI Appendix, Fig. S2D). The colocalization of Dstac, Dmca1D, and proctolin was confirmed using an independent approach. Examination of third instar larvae from a Dstac-gfp gene trap line (38, 43) that also were transgenic for ShakB:Gal4 > UAS:mCD8RFP, which is expressed by a subset of motor neurons in the ventral ganglia (44), revealed that GFP and RFP were colocalized in the ventral ganglia (SI Appendix, Fig. S2A). This is consistent with Dstac expression by at least some motor neurons in the ventral ganglia. Labeling ShakB:Gal4 > UAS:mCD8GFP larvae with anti-proctolin (20) and anti-GFP showed that the neuropeptide proctolin is expressed by these motor neurons as well (SI Appendix, Fig. S2B). Labeling Proct:Gal4 > UAS:mCD8GFP larvae with anti-Dstac (38) further suggested that Dstac is expressed by proctolin+ motor neurons including the RP2 neuron (SI Appendix, Fig. S2C). Thus, motor boutons at the NMJ express Dstac, Dmca1D, and the neuropeptide proctolin.
Fig. 1.
Dstac and Dmca1D are expressed by proctolin+ motor boutons. (A) Anti-proctolin and anti- horseradish peroxidase (anti-HRP) labeling of motor nerves in larvae selectively expressing membranous GFP in proctolin+ motor neurons (Proct:GAL4 > UAS:mCD8GFP) showed expression of mCD8GFP and proctolin at type Ib and type Is branches. The anti-HRP labels an unknown protein that is present on the plasma membrane of motor nerves and boutons. Shown are the type Is branch of the RP2 (arrow) and the type Ib branch of the MN4-Ib motor neuron (asterisk) on muscle 4 (45). Merge is Proct:GAL4 > UAS:mCD8GFP and anti-Proctolin. The images are a single focal plane. (Scale bar: 3 μm.) (B) Anti-Dstac and anti-HRP labels type 1b (asterisk) and 1s (arrow) boutons. Left Inset shows higher magnification view of type 1b boutons. Right Inset shows higher magnification view of type 1s boutons. Shown are type 1s boutons of RP2 and type 1b boutons of MN4-1b on muscle 4. (C) Anti-Dmca1D and anti-HRP labels type 1b (asterisk) and 1s (arrow) boutons. Left Inset shows higher magnification view of type 1b boutons. Right Inset shows higher magnification view of type 1s boutons. Shown are type 1s boutons of RP2 and type 1b boutons of MN4-1b on muscle 4. Some of the labeling by both anti-Dstac and anti-Dmca1D beyond the motor nerves may represent muscle labeling since body wall muscles also express Dstac and Dmca1D (38, 46). The images show a single focal plane. (Scale bar: 3 μm.) See also SI Appendix, Fig. S1.
Dstac, Dmca1D, and Proctolin Deficiency in Motor Neurons Decreases Locomotion.
The hypothesis that Dstac regulates neuropeptide release by motor boutons predicts that a defect in Dstac should lead to a decrease in the release of proctolin at the NMJ. This may result in decreased locomotion since proctolin is known to increase contractions when applied to Drosophila larval muscles (47). To examine this prediction, we assayed a Dstac p-element insertion line and generated a CRISPR-Cas9–mediated mutation targeting the SH3 domain of Dstac (Materials and Methods). The rationale for targeting the SH3 domain was based upon our earlier finding that the SH3 domain was critical for Stac3 function in vertebrate skeletal muscles. A missense mutation in the SH3 domain of the skeletal muscle gene STAC3 caused Native American myopathy that is characterized by muscle weakness (30), and the corresponding mutation in zebrafish resulted in dysregulation of L-type CaCh in skeletal muscle, disrupted EC coupling, and decreased locomotion (31).
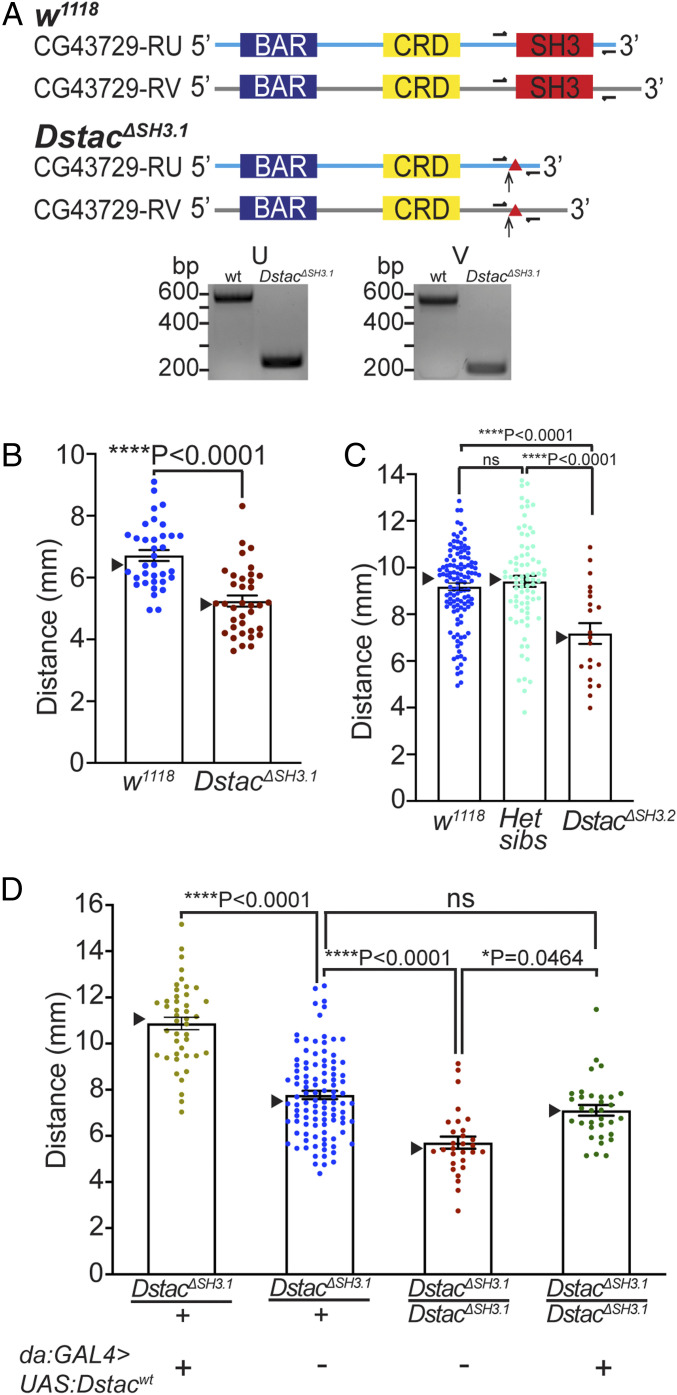
We examined two founders for the CRISPR-Cas9 mutation in which genomic DNA sequencing showed that the SH3 domain of Dstac was deleted (Materials and Methods and SI Appendix, Supplementary Text). We outcrossed founder 1 (DstacΔSH3.1) for five generations to clarify the mutant phenotype and found that third instar larvae of the outcrossed line exhibited decreased locomotion (Fig. 2B). Complementary DNA (cDNA) sequencing of the outcrossed DstacΔSH3.1 confirmed that the SH3 domain of Dstac transcripts was deleted, resulting in a frameshift and predicted premature stop codon (Fig. 2A and SI Appendix, Supplementary Text). Offsprings from DstacΔSH3.1 were viable with normal morphology (SI Appendix, Fig. S3 A–C), and mutant adults produced embryos in comparable numbers to that of wild type (wt) (SI Appendix, Fig. S3D), suggesting that the mutation does not lead to a generalized lethargic state. Founder 2 (DstacΔSH3.2) produced a few small larvae, which did not eclose and exhibited decreased locomotion. However, after outcrossing 10 times, the outcrossed DstacΔSH3.2 line became viable and eclosed, but larval locomotion was decreased (Fig. 2C), similar to DstacΔSH3.1. Thus, it appears that Dstac mutant larvae exhibit decreased locomotion.
Fig. 2.
Dstac mutants generated by CRISPR-Cas9 showed reduced locomotion. (A) Schematic of two Dstac protein variants (CG43729-RU and CG43729-RV) is shown. Arrows denote the location where early stop codons occurred due to the indels (triangles) created by CRISPR-Cas9. Half arrows denote primer sites used to sequence the mutations. All primers used are listed in SI Appendix, Table S1 and Supplementary Materials and Methods. The cDNA PCR fragments of DstacΔSH3.1 are smaller than wt for both RU and RV variants, suggesting the deletions of the SH3 domain in the Dstac cDNA. The deletions of SH3 were confirmed by sequencing the wt and DstacΔSH3.1 cDNA bands (SI Appendix, Supplementary Text). The translated mutant cDNA sequences showed that an early stop codon was produced in DstacΔSH3.1 (SI Appendix, Supplementary Text). The schematic is not to scale. (B) Offsprings of founder 1 after five generations of outcrossing (DstacΔSH3.1) showed decreased locomotion compared with wt (wt, n = 35; DstacΔSH3.1, n = 36; one-tailed, unpaired t test). In this histogram and all subsequent ones, SEMs are shown, and triangles denote the median. (C) Offsprings of founder 2 after 10 generations of outcrossing (DstacΔSH3.2, n = 21) showed decreased locomotion compared with wt (n = 120) and heterozygous siblings (n = 78) (one-way ANOVA, Tukey’s multiple comparisons test). ns denotes not significant. (D) Expression of Dstacwt by heterozygous DstacΔSH3.1 larvae (n = 43) increased locomotion compared with heterozygous controls (n = 104), and expression of Dstacwt by homozygous DstacΔSH3.1 (n = 33) rescued DstacΔSH3.1 (n = 29) locomotion (Kruskal–Wallis test, Dunn’s multiple comparisons test). The larvae were genotyped by PCR after the locomotion assay.
Ubiquitous expression of wt Dstac (da:Gal4 > UAS:Dstacwt) (48) in DstacΔSH3.1 mutant larvae rescued normal locomotion compared with mutants, confirming that the locomotion defect was due to the DstacΔSH3.1 mutation (Fig. 2D). Furthermore, ubiquitous expression of wt Dstac in DstacΔSH3.1/Dstacwt larvae increased locomotion compared with heterozygous controls. These findings in DstacΔSH3.1 larvae were corroborated by analysis of a line with a single p-element inserted into the 3′ untranslated region (UTR) of the Dstac locus (DstacLA00216) (SI Appendix, Fig. S4A). These larvae also exhibited decreased locomotion, and precise excision of the p-element rescued the reduced locomotion (SI Appendix, Fig. S4B). Furthermore, locomotion by larvae that were heterozygous for both DstacΔSH3.1 and DstacLA00216 (DstacΔSH3.1/DstacLA00216) was also reduced (SI Appendix, Fig. S4C). Thus, Dstac is required for normal larval locomotion.
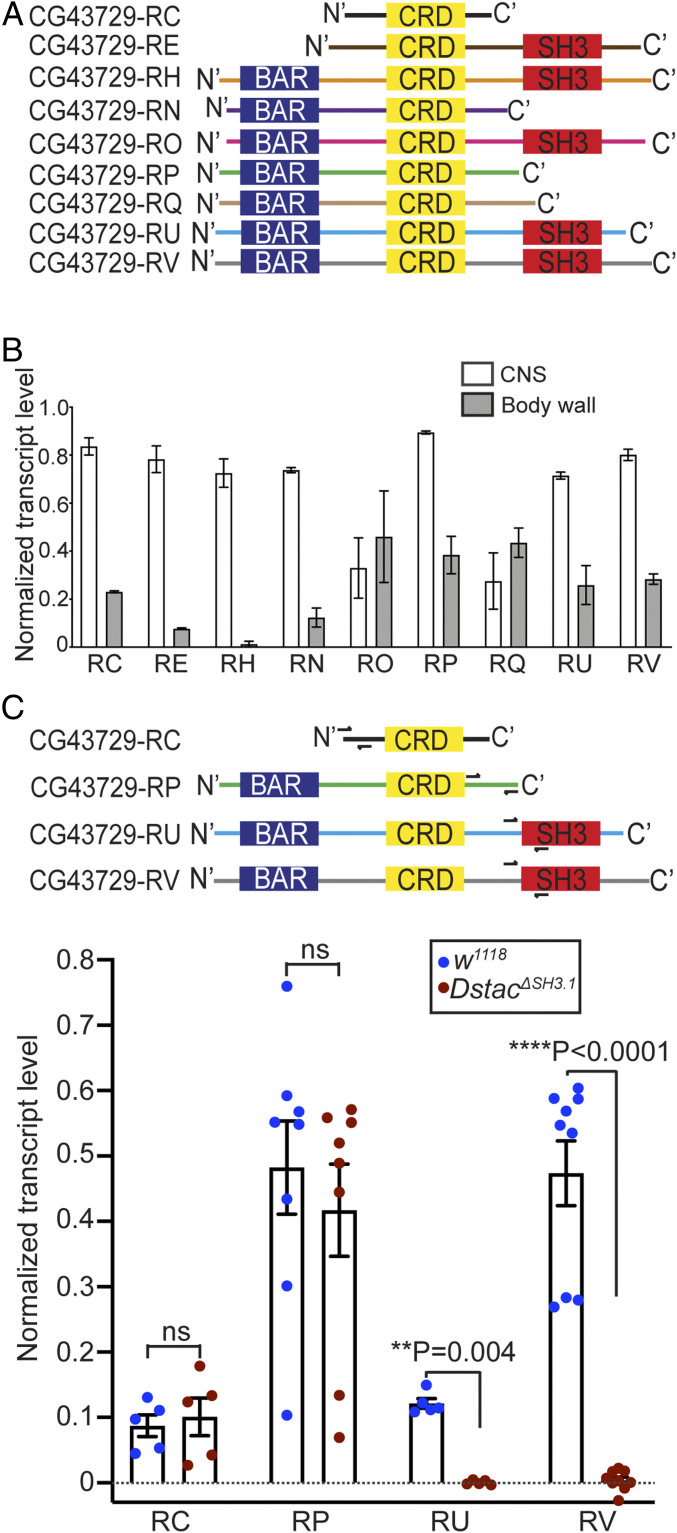
Dstac is alternatively spliced and expressed both by muscles and neurons (38), with some splice variants containing different combinations of the BAR, CRD, and SH3 domains (Fig. 3A). RT-PCR from dissected CNS or body wall, which represents mostly muscle, showed that splice variants were expressed at varying levels in the CNS and body wall of wt larvae (Fig. 3B). RT-PCR of DstacΔSH3.1 larvae of four of the splice variants showed that transcripts RU and RV that contain the SH3 domain in wt larvae, which were expressed primarily in the CNS, were missing the SH3 domain while transcripts RC and RP without the SH3 domain appeared unaffected in DstacΔSH3.1 mutants (Figs. 2A and 3C). Thus, it appears there is a deficiency in transcripts containing the SH3 domain that is correlated with decreased locomotion in DstacΔSH3.1 larvae.
Fig. 3.
Dstac isoforms that contain the SH3 domain are affected in DstacΔSH3.1. (A) Schematic of domain composition of nine Dstac protein variants. Not all variants contain an SH3 domain. (B) Quantification of Dstac transcript levels in wt third instar larval CNS and body wall assayed by RT-PCR. Error bars represent SEM. RT-PCR primers for Dstac (CG43729) transcripts and GAPDH used for normalization can be found in SI Appendix, Table S1 and Supplementary Materials and Methods. (C) Two isoforms without the SH3 domain (CG43729-RC and CG43729-RP) and two isoforms that contain the SH3 domain (CG43729-RU and CG43729-RV) were PCR amplified from wt and DstacΔSH3.1 cDNA. Half arrows denote the primer sites. The levels of isoforms that don’t have SH3 domain (RC and RP) were comparable between wt and DstacΔSH3.1 (Mann–Whitney U test), but the isoforms that normally contained the SH3 domain (RU and RV) were diminished in DstacΔSH3.1 (Mann–Whitney U test) (RC: wt n = 5, DstacΔSH3.1 n = 5; RP: wt n = 8, DstacΔSH3.1 n = 8; RU: wt n = 5, DstacΔSH3.1 n = 5; RV: wt n = 9, DstacΔSH3.1 n = 9; each dot represents one cDNA gel band).
Since Dstac is expressed both by body wall muscles and a subset of neurons (38), the decreased locomotion of the mutants could be due to disruption of muscle and/or neural function. In fact, muscle-specific expression of wt Dstac in DstacΔSH3.1 larvae did not rescue the mutant phenotype, consistent with the idea that a deficiency of Dstac in neurons could result in decreased locomotion (SI Appendix, Fig. S5A). One caveat to this interpretation is that the expression of wt Dstac in muscles may have been insufficient for rescue of muscle function. Thus, the hypothesis that Dstac is required by neurons for normal locomotion was tested directly by assaying locomotion in larvae that were deficient in Dstac in neurons. In fact, pan-neural knockdown of Dstac (elav:Gal4 > UAS: DstacRNAi) using an RNA interference (RNAi) line that was previously shown to knockdown Dstac (38) decreased locomotion compared to controls (SI Appendix, Fig. S5B). A neuron-specific rescue of the Dstac mutant locomotion phenotype might also establish a neuronal requirement of Dstac for locomotion. However, muscle-specific knockdown of Dstac also resulted in a locomotion phenotype that was similar to that of the DstacΔSH3.1 mutants, thus making a neuron-specific rescue in mutants not useful. However, the finding that pan-neuronal knockdown of Dstac resulted in reduced locomotion suggests that Dstac is required by neurons for normal locomotion by larvae.
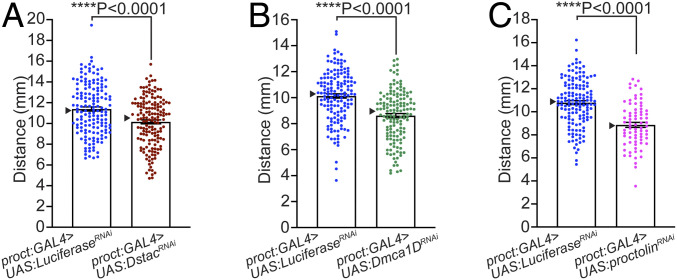
Since larval motor neurons express Dstac, Dmca1D, and proctolin, we tested whether knocking them down specifically in motor neurons could lead to a locomotion phenotype. Indeed, in larvae with Dstac knocked down selectively in motor neurons (Proct:Gal4 > UAS:DstacRNAi and ShakB:Gal4 > UAS:DstacRNAi), locomotion was decreased compared to controls (Fig. 4A and SI Appendix, Fig. S5C). In these larvae (Proct:Gal4 > UAS:DstacRNAi), anti-Dstac labeling showed that Dstac expression was reduced in motor boutons (SI Appendix, Fig. S5D), confirming that Dstac was indeed knocked down. Furthermore, in larvae with Dmca1D knocked down in motor neurons (Proct:Gal4 > UAS:Dmca1DRNAi and ShakB:Gal4 > UAS:Dmca1DRNAi) locomotion was also decreased (Fig. 4B and SI Appendix, Fig. S5E). In these larvae (Proct:Gal4 > UAS:Dmca1DRNAi), anti-Dmca1D labeling showed a small decrease in Dmca1D levels in motor neuron boutons (SI Appendix, Fig. S5F). The Dmca1D knockdown phenotype was corroborated by a partial loss-of-function mutation of Dmca1D (Dmca1DAR66) (42) that also exhibited decreased locomotion (SI Appendix, Fig. S5G). Finally, knocking down proctolin in motor neurons (Proct:Gal4 > UAS:ProctRNAi) also decreased larval locomotion (Fig. 4C). Thus, deficiency in Dstac, Dmca1D, and proctolin in normally proctolin+ motor neurons decreased larval locomotion as hypothesized by the proposed regulation of Dmca1D by Dstac and subsequent release of proctolin at the NMJ.
Fig. 4.
Knockdown of Dstac, Dmca1D, and proctolin selectively in proctolin+ motor neurons reduced larval locomotion. (A) DstacRNAi larvae in which Dstac was knocked down in proctolin neurons (proct:GAL4 > UAS:DstacRNAi) showed decreased locomotion compared with control LuciferaseRNAi larvae (proct:GAL4 > UAS:LuciferaseRNAi) (control n = 156, DstacRNAi n = 156; one-tailed Mann–Whitney U test). (B) Dmca1DRNAi in which Dmca1D was knocked down in proctolin neurons (proct:GAL4 > UAS:Dmca1DRNAi) showed decreased locomotion compared with control LuciferaseRNAi larvae (control n = 150, Dmca1DRNAi n = 143; one-tailed, unpaired t test). (C) proctolinRNAi larvae in which proctolin was knocked down in proctolin neurons (proct:GAL4 > UAS:proctolinRNAi) showed decreased locomotion compared with control LuciferaseRNAi larvae (control n = 144, proctolinRNAi n = 75; one-tailed, unpaired t test). See also SI Appendix, Fig. S4.
Dstac and Dmca1D Deficiency Decreases Ca2+ Transients in Motor Boutons.
In zebrafish, Stac3 regulates DHPR, the skeletal muscle L-type CaCh (31). If, by analogy to Stac3, Dstac regulates Dmca1D in motor boutons, then there might be abnormal Ca2+ transients in the boutons of DstacΔSH3.1 larvae. First, we examined whether Ca2+ transients in boutons were dependent on Dmca1D by assaying Ca2+ transients in Proct:Gal4 > UAS:GCaMP6f larvae. Ca2+ imaging of motor boutons was performed in completely intact larvae placed in a microfluidics chamber designed to physically restrain larvae (49). Under these conditions, large Ca2+ transient increases in free Ca2+ within type Is boutons of RP2 were observed (Fig. 5 A and B and Movie S1). These presumably represent endogenous locomotor activity. The peak and area under the curve of Ca2+ transients recorded from Dmca1D knockdown larvae (Proct:Gal4 > UAS: Dmca1DRNAi) were decreased compared with controls (Fig. 5 C and D). However, the frequency of transients was comparable between Dmca1D knockdown and control larvae (Fig. 5E). Thus, it appears that, during normal, physiological activation of motor neurons, there are Ca2+ transients that are dependent on Dmca1D CaChs in the motor boutons.
Fig. 5.
Deficiencies in Dmca1D and Dstac decrease Ca2+ transients in motor boutons. (A) GCaMP6f expressed selectively in proctolin+ motor boutons of a wt control larva (Proct:GAL4 > UAS:GCaMP6f;UAS:mCD8tdTomato;UAS:LuciferaseRNAi). Asterisk denotes type Is motor branch on muscle 4. (Scale bar: 3 μm.) (B) Example of Ca2+ transients from a type Is bouton on muscle 4 in a wt control larva (Top) and in a Dmca1D KD (proct:GAL4 > UAS: Dmca1DRNAi) larva (Bottom). See Movie S1. (C and D) The peaks and area under the peaks of Ca2+ transients (ΔF/F) in the boutons of Dmca1D KD larvae (Proct:GAL4 > UAS:GCaMP6f;UAS:Dmca1DRNAi) were smaller compared with wt control. Peaks (C), one-tailed, unpaired t test. Area under peaks (D), one-tailed Mann–Whitney U test. (E) The number of Ca2+ transients over 5 min in Dmca1D KD and wt control boutons were comparable (Mann–Whitney U test). Data in C–E were from 24 boutons in 19 wt control larvae and 21 boutons in 16 Dmca1D KD larvae. (F) Example of Ca2+ transients from a type Is bouton on muscle 4 in a wt control larva (Top) and in a DstacΔSH3.1 larva (Bottom). (G and H) The peaks and area under the peaks of Ca2+ transients in the boutons of DstacΔSH3.1 larvae (DstacΔSH3.1;Proct:GAL4 > UAS:GCaMP6f) are smaller compared with wt control (one-tailed Mann–Whitney U test). (I) The number of Ca2+ transients over 5 min in DstacΔSH3.1 and wt boutons are comparable (Mann–Whitney U test). Data in G–I were from 25 boutons in 25 wt control larvae and 18 boutons in 18 DstacΔSH3.1 larvae. Each data point represents the averaged peaks or area under peaks of Ca2+ transients per bouton.
Ca2+ transients were also assayed in the boutons of DstacΔSH3.1 (Fig. 5F), and the peak and area under the curve of Ca2+ transients were also decreased (Fig. 5 G and H), but not the frequency (Fig. 5I). The decrease in Ca2+ transients in the boutons of DstacΔSH3.1 larvae is consistent with the hypothesized regulation of Dmca1D by Dstac and subsequent release of neuropeptide.
Dstac Regulates Currents Passed by Dmca1D Channels.
In zebrafish, Stac3 regulates the voltage response of the L-type CaCh in skeletal muscle (31). To see if Dstac also regulates the Dmca1D channels, we performed voltage-clamp analysis of L-type currents in the RP2 motor neurons that express Dstac, Dmca1D, and proctolin. Dmca1D channels are the major voltage-dependent Ca2+ currents recorded from larval motor neuron cell bodies (50) and are also found in their dendrites and axons (51). RP2 neurons were identified by position and expression of markers in Proct:Gal4 > UAS:Dilp2-GFP; UAS:mCD8tdTomato larvae. L-type currents were isolated by pharmacologically blocking Na+ and K+ currents with 1 μM tetrodotoxin, 50 mM tetraethylammonium, and 1.5 mM 4-aminopyridine added to the extracellular saline containing 1.8 mM CaCl2 and 144 mM CsCl in the electrode (see SI Appendix, Supplementary Materials and Methods for details). Extracellular CaCl2 was replaced with 1.8 mM BaCl2 for recording Ba2+ currents. First, we recorded currents in the Ca2+ and Ba2+ solutions by stepping to −10 mV from a holding potential of −70 mV. When Ca2+ was present, the voltage step produced transient inward currents (mean time to peak: 5.7 ± 0.2 ms) that declined along an exponential time course (τ: 21.3 ± 2.5 ms) to a steady state value of 15.6% ± 4.7% of the peak (n = 6). In contrast, when Ba2+ replaced Ca2+, the peak inward currents were larger, and the extent of the time-dependent decline was much smaller (Fig. 6A). These findings are similar to those found earlier (50, 51). The addition of Cd2+ (500 μM) blocked much of the Ba2+ current in control larvae, confirming earlier results (50). Furthermore, both the currents generated in Ca2+ and Ba2+ solutions were decreased in DstacΔSH3.1 larvae (Fig. 6A). A second set of voltage clamp experiments in Ca2+ solution using 100-mS steps to −90 mV to +50 mV from a holding potential of −70 mV was performed to generate current/voltage (I/V) curves. This showed that peak current occurred at +10 mV in both control and DstacΔSH3.1 larvae and that the currents were significantly decreased in DstacΔSH3.1 compared to controls at −10 mV, 0 mV, +10 mV, and +20 mV (Fig. 6 C–E and SI Appendix, Fig. S6A). Furthermore, the time to peak current following the voltage steps to 0 mV, +10 mV, and +20 mV was greater in mutants compared with wt controls (SI Appendix, Fig. S6B). The sustained current measured at the end of the voltage step was also decreased in DstacΔSH3.1 larvae compared with wt controls (SI Appendix, Fig. S7A). The ratio of peak currents between DstacΔSH3.1 and control and the ratio of sustained currents between DstacΔSH3.1 and control, however, were comparable (SI Appendix, Fig. S7B), which is consistent with no differential effect of the mutation on the magnitude of the peak and sustained currents. Thus, Dstac regulates the voltage-dependent currents of L-type CaChs in motor neurons. Although this result only applies to currents in the cell bodies, the electrophysiological findings in conjunction with the decrease in Ca2+ transients in motor boutons of DstacΔSH3.1 larvae are consistent with the regulation of Dmca1D CaChs by Dstac in proctolin+ motor boutons.
Fig. 6.
Dmca1D currents in RP2 motor neuron cell bodies were largely decreased in DstacΔSH3.1. (A) Voltage-clamp recording in response to a single voltage step of a wt control RP2 (Left) and a DstacΔSH3.1 RP2 (Right). The RP2 motor neuron cell bodies were held at −70 mV and stepped to −10 mV for 200 ms in four different solutions (basal: 0 Ca2+ solution plus TTX; Ca2+: normal Ca2+ solution; Ba2+: Ba2+ solution; Cd2+: Ba2+ solution plus Cd2+). See SI Appendix, Supplementary Materials and Methods for details. The wt control RP2 cell bodies showed an inward Ca2+ current that inactivated and a larger Ba2+ current that barely inactivated whereas DstacΔSH3.1 RP2 cell bodies showed significantly smaller Ca2+ and Ba2+ currents. Addition of Cd 2+ to the Ba2+ solution eliminated the Ba2+ current. (B) The peak Ba2+ current densities from DstacΔSH3.1 RP2 cell bodies were reduced compared with control (control n = 7 cells from seven larvae, DstacΔSH3.1 n = 6 cells from six larvae, one-tailed Mann–Whitney U test). The RP2 motor neuron cell bodies were held at −70 mV and stepped to −10 mV for 200 ms. (C) Full I/V curves from control and DstacΔSH3.1 RP2 cell bodies were created by holding the membrane voltage (Vm) at −70 mV and stepping from −90 mV to +50 mV with 10-mV increments for 100 ms. Ca2+ current peaked at +10 mV in both control (n = 8) and DstacΔSH3.1 (n = 11) larvae, and the currents were significantly decreased in DstacΔSH3.1 compared to controls at −10 mV, 0 mV, +10 mV, and +20 mV (one-tailed Mann–Whitney U test; P value: 0.0034, 0.0004, 0.0003, and 0.0018, respectively). (D) Voltage-clamp Ca2+ currents at +10 mV of a control and DstacΔSH3.1 RP2 cell body. (E) The peak Ca2+ current density from the voltage steps of DstacΔSH3.1 RP2 cell bodies were reduced significantly compared with control. (Control n = 8 cells from eight larvae, DstacΔSH3.1 n = 11 cells from 11 larvae, one-tailed Mann–Whitney U test).
Dstac and Dmca1D Deficiency Decreases Release of Neuropeptide by Motor Boutons.
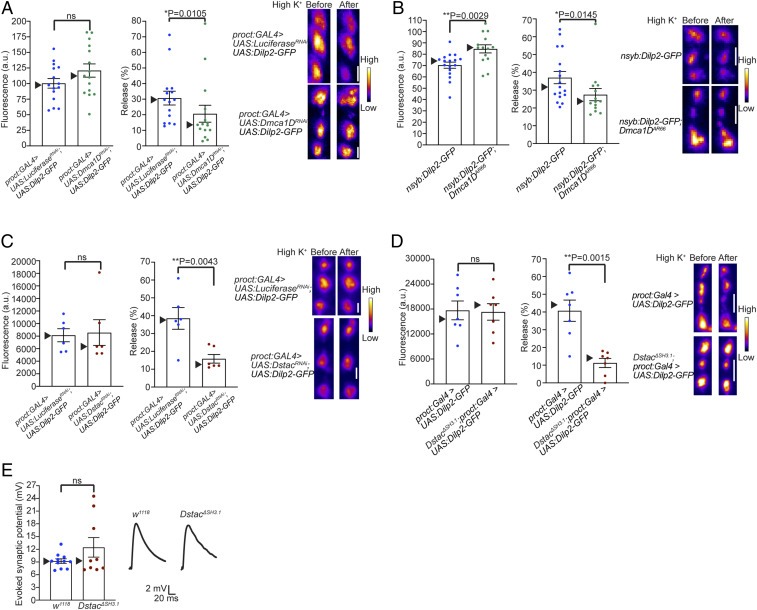
The final prediction of the hypothesis is that Dstac and Dmca1D regulate the release of neuropeptides by motor boutons. This prediction was examined by assaying type Is boutons of the RP2 motor neuron in larvae expressing the Drosophila neuropeptide Dilp2-GFP, following activation of motor boutons with high K+ (HL3 containing 70 mM KCl) (52). In this assay, release of Dilp2-GFP causes a decrease in fluorescence within the boutons. Expression of Dilp2-GFP by type Is motor boutons on muscle 4 of Dmca1D knockdown larvae (Proct:Gal4 > UAS:Dilp2-GFP; UAS:Dmca1DRNAi) and control (Proct:Gal4 > UAS:Dilp2-GFP; UAS:LuciferaseRNAi) was comparable (Fig. 7 A, Left), and the release of Dilp2-GFP was decreased in boutons of Dmca1D knockdown larvae compared with control (Fig. 7 A, Middle and Right). Expression of Dilp2-GFP by motor boutons of Dmca1DAR66 was higher than that by control (Fig. 7 B, Left), and the release of Dilp2-GFP was decreased in Dmca1DAR66 compared with control (Fig. 7 B, Middle and Right). Thus, deficiencies in Dmca1D resulted in decreased neuropeptide release by motor boutons.
Fig. 7.
Release of neuropeptides is diminished in Dstac and Dmca1D deficient motor boutons. (A) Expression of Dilp2-GFP (fluorescence in arbitrary units [a.u.]) by type Is motor boutons on muscle 4 of Dmca1D KD (proct:GAL4 > UAS:Dmca1DRNAi; n = 15) and control (proct:GAL4 > UAS:LuciferaseRNAi; n = 15) was comparable (Left; unpaired t test). Release of Dilp2-GFP was decreased in Dmca1D KD compared with control (Middle; one-tailed Mann–Whitney U test). Examples of Dilp2-GFP expression before and after exposure to high potassium of control (Upper Right) and Dmca1D KD (Lower Right). The boutons are shown in pseudocolor. (Scale bar: 2 μm.) (B) Expression of Dilp2-GFP by motor boutons of Dmca1DAR66 (nysb:Dilp2-GFP;Dmca1DAR66; n = 14) was higher than that by control (nysb:Dilp2-GFP; n = 17) (Left; unpaired t test). Release of Dilp2-GFP was decreased in Dmca1DAR66 compared with control (Middle; one-tailed Mann–Whitney U test). Examples of Dilp2-GFP expression before and after exposure to high KCl of control (Upper Right) and Dmca1DAR66 (Lower Right). (Scale bar: 2 μm.) (C) Expression of Dilp2-GFP by motor boutons of Dstac KD (proct:GAL4 > UAS:DstacRNAi; n = 6) and control (n = 6) was comparable (Left; Mann–Whitney U test). Release of Dilp2-GFP was decreased in Dstac KD compared with control (Middle; one-tailed Mann–Whitney U test). Examples of Dilp2-GFP expression before and after exposure to high KCl of control (Upper Right) and Dstac KD (Lower Right). (Scale bar: 2 μm.) (D) Expression of Dilp2-GFP by motor boutons of DstacΔSH3.1 (DstacΔSH3.1;proct:GAL4 > UAS:Dilp2-GFP; n = 7) and control (proct:GAL4 > UAS:Dilp2-GFP; n = 7) was comparable (Left; Mann–Whitney U test). Release of Dilp2-GFP was decreased in DstacΔSH3.1 compared with control (Middle; one-tailed Mann–Whitney U test). Examples of Dilp2-GFP expression before and after exposure to high KCl of control (Upper Right) and DstacΔSH3.1 (Lower Right). (Scale bar: 2 μm.) (E) Nerve evoked synaptic potentials of muscle 4 were comparable between wt (n = 11) and DstacΔSH3.1 (n = 9) (unpaired t test).
Expression of Dilp2-GFP by motor boutons of Dstac knockdown larvae (Proct:Gal4 > UAS:Dilp2-GFP; UAS: DstacRNAi) and control (Proct:Gal4 > UAS:Dilp2-GFP; UAS: LuciferaseRNAi) was comparable (Fig. 7 C, Left), and the release of Dilp2-GFP was decreased in boutons of Dstac knockdown larvae compared with control (Fig. 7 C, Middle and Right). Expression of Dilp2-GFP by motor boutons of DstacΔSH3.1 and control was comparable (Fig. 7 D, Left), and the release of Dilp2-GFP was decreased in DstacΔSH3.1 compared with control (Fig. 7 D, Middle and Right). Therefore, deficiencies in both Dmca1D and Dstac resulted in decreased neuropeptide release by motor boutons. Thus, Dmca1D and Dstac regulate the release of neuropeptides by motor boutons.
Interestingly, application of proctolin, the endogenous neuropeptide released by motor boutons (19), enhances body wall muscle contraction without affecting the membrane potential of muscles (47). The excitatory neurotransmitter released by motor neuron boutons is glutamate so the evoked synaptic potential at the NMJ is due to the action of glutamate (53). We examined whether Dstac might also regulate release of glutamate by recording the synaptic potential following stimulation of the motor nerve in mutant larvae. Evoked synaptic potentials in DstacΔSH3.1 larvae were comparable to that in wt control (Fig. 7E), suggesting that the release of glutamate was not affected in DstacΔSH3.1 larvae. This finding is consistent with the possibility that Dstac selectively regulates neuropeptide release, but not glutamate release at the NMJ.
Discussion
Pharmacological experiments suggest that the release of neuropeptides by neurons involves Ca2+ influx via L-type CaChs (8, 12–16, 18, 54) and CICR from internal Ca2+ stores (55). In Drosophila, the release of neuropeptides at the NMJ also appears to involve CICR (24), but it was unknown whether Ca2+ influx via the L-type CaCh Dmca1D triggers CICR. The results from this study established that Dmca1D is required for the normal release of neuropeptides at the NMJ and suggested a mechanism by which Dstac regulates Dmca1D and the release of neuropeptides at the Drosophila NMJ. First, Dstac, Dmca1D, and proctolin are all expressed by motor boutons. Second, Dstac mutations and Dmca1D knockdowns decrease Ca2+ transients in motor boutons. Third, Dstac mutations decrease currents through Dmca1D channels in motor neurons. Fourth, mutations and knockdowns of Dstac and Dmca1D decrease the release of neuropeptides by motor boutons. Fifth, mutations and knockdowns of Dstac, Dmca1D, and proctolin decrease locomotion. Thus, Dstac regulates voltage-dependent influx of Ca2+ through Dmca1D channels, which leads to an increase in cytosolic Ca2+, perhaps involving CICR, and the release of neuropeptides at the NMJ. This, in turn, controls the intensity of muscle contractions and thus locomotion by Drosophila larvae (Fig. 8).
Fig. 8.
Model for the role of Dstac in neuropeptide release from RP2 motor neuron boutons. In wt (Left) Dstac regulates Ca2+ influx (circles) through Dmca1D channels (thick dashed arrow) to initiate CICR from the RyR Ca2+ release channel in the ER into the cytosol (thick arrow) and subsequent release of proctolin neuropeptide (hexagon) from motor boutons. In Dstac∆SH3.1 (Right) Ca2+ influx through Dmca1D channels is decreased (thin dashed arrow) resulting in less CICR (thin arrow), and thus reduced release of proctolin neuropeptide.
Our experiments demonstrated that normal levels of Dilp2-GFP release by motor boutons require Dstac. The endogenous neuropeptide expressed by these boutons is proctolin (ref. 19 and this study) so the presumption is that proctolin release by the boutons also requires Dstac. Previous studies showed that proctolin increases muscle contractions in a variety of arthropods (56–58), including Drosophila larvae, without affecting the membrane voltage of muscles (47). Thus, a decrease in proctolin predicts a decrease in locomotion by larvae. In fact, we found that larvae in which proctolin was knocked down selectively in motor neurons exhibited decreased locomotion. Furthermore, Dstac mutant larvae and larvae with Dstac selectively knocked down in motor neurons also showed decreased locomotion. These findings are consistent with the requirement of Dstac for the release of proctolin by motor boutons.
A deficiency in Dstac reduces the release of neuropeptides but not the synaptic potential due to the release of glutamate at the larval NMJ. Since Dstac regulates Dmca1D, normal synaptic function at the NMJ when Dstac is deficient suggests that Dmca1D is dispensable for synaptic release of glutamate. This is consistent with previous evidence that cacophony (CaV2) is the most important CaCh for producing fast excitatory postsynaptic potentials (EPSPs) mediated by the release of glutamate (59, 60).
Knockdown (KD) of Dmca1D specifically in motor neurons decreased the influx of Ca2+ into motor boutons at the NMJ, and both the Dmca1D KD and Dmca1DAR66 mutation decreased the release of neuropeptide. This is consistent with the requirement for the influx of Ca2+ through the Dmca1D channels in the boutons for normal neuropeptide release. However, the decrease in Dmca1D in the dendrites, cell bodies, and axons leading to the boutons of motor neurons may also contribute to decreased release of neuropeptide. Motor neuron-specific knockdown of Dmca1D also leads to a decrease in the synaptic response of motor neurons to the central pattern generator and a decrease in duration and maximal firing rate of action potential bursts initiated by synaptic input to motor neurons (51). These appear to be due to the decrease in Dmca1D currents in both the dendrites and axons of motor neurons. Therefore, normal activation of Dmca1D channels in boutons in intact larvae is likely to require Dmca1D channels in dendrites and axons of motor neurons. Thus, Dmca1D in dendrites, axons, and boutons is likely required for normal Ca2+ influx into boutons and the release of neuropeptides.
We previously showed that Dstac and Dmca1D were expressed and that Dstac was required in the LNV neurons in the brain for normal circadian rhythm of locomotor behavior in Drosophila (38). These neurons express the neuropeptide PDF (61, 62) that is critical for circadian rhythm (39). Thus, it is possible that Dstac may regulate Dmca1D and the release of PDF by these neurons and that the release of PDF by LNV neurons is essential for normal circadian rhythm. There are ∼50 neuropeptide and peptide hormone genes in the Drosophila genome (63, 64). Whether Dstac regulates the release of other neuropeptides remains to be investigated, but, given the wide range of time scales, cellular actions, and sites of release of different neuropeptides and peptide hormones, one might imagine several different mechanisms for the regulation of their release, including those not involving Dstac. In fact, the finding that Dstac mutant larvae are normal in size and morphology and eclose properly is consistent with the possibility that Dstac does not regulate the release of peptide hormones such as Dilp2 or eclosion hormone.
Dstac is also expressed by body wall muscles in Drosophila (38). Of note in vertebrates, stac3 is expressed selectively by skeletal muscles and required for EC coupling (30–32). In vertebrate skeletal muscles, EC coupling involves the direct interaction of the L-type CaCh, DHPR, which is the voltage detector for EC coupling in the t-tubules, with the ryanodine receptor, which is the Ca2+ release channel in the SR (36, 65, 66). Stac3 regulates EC coupling in skeletal muscles by regulating the stability and properties of the L-type CaCh in skeletal muscles (31). The muscle expression of Dstac suggests that Dstac might regulate EC coupling in larval body wall muscles of Drosophila just as Stac3 in vertebrate skeletal muscles. In fact, selective knockdown of either Dmca1D or Dstac in muscles decreased activation-mediated Ca2+ transients in muscles, suggesting that Dstac regulates EC coupling in Drosophila larval muscles (67). In some invertebrate muscles, including those of Drosophila, EC coupling is thought to involve CICR (68–72). Thus, Dstac might regulate EC coupling by controlling the stability and voltage dependency of Dmca1D in Drosophila muscles.
There are few known regulators of neuropeptide release and DCV exocytosis. Most recently the RAB3/RIM pathway was found to be required for fusion of DCVs at the presynaptic terminals of mammalian hippocampal neurons (73). Earlier, the cytosolic calcium-activated protein for secretion (CAPS) was identified to be necessary for normal Ca2+-dependent release of norepinephrine by permeabilized PC12 cells (74). Interestingly, CAPS appears to be specific for release from DCVs since antibody block with anti-CAPS decreases DCV exocytosis but not synaptic vesicle exocytosis in semiintact, rat brain synaptosomes (75). In vivo mutations in CAPS result in defective locomotion, feeding, egg laying, and failure to recover from the dauer stage in nematodes (76, 77) and an apparent failure to exocytose DCVs by motor boutons in larval Drosophila (78). In flies, unlike in mammalian neurons, CAPS appears also to regulate release of glutamate from synaptic vesicles, as well as DCV exocytosis.
In our experiments, deficiencies of Dmca1D and Dstac decreased neuropeptide release by motor boutons but did not eliminate release. This could be due to incomplete inhibition of Dmca1D and Dstac by the knockdowns and mutations of these genes. Alternatively, it is possible that other CaChs in boutons might contribute to the increase in cytosolic Ca2+ necessary for normal release of neuropeptides. cacophony/Dmca1A (CaV2), the CaCh required for release of neurotransmitter by boutons (60), might participate in neuropeptide release independently of Dstac. Other CaChs found in motor neurons, such as the T-type CaCh, DmαG/CaαIT (79, 80), and TRPV1 (81), could also participate in neuropeptide release. In this regard, Stac1, which is expressed by vertebrate neurons, is sufficient for the surface localization of CaV3.2, a mammalian T-type CaCh, in cultured cells and coimmunoprecipitates with CaV3.2 (82). Finally, how Dstac regulates Dmca1D channels is unknown. It is possible that regulation of Dmca1D by Dstac may be a direct one since Stac3 directly binds the cytoplasmic loop between repeats II and III of CaV1.1 and CaV1.2 (83). Future analysis of neuropeptide release may elucidate answers to these issues.
Materials and Methods
Full materials and methods are available in SI Appendix.
Drosophila melanogaster Strains.
All experiments used age- and size-matched larvae. Both male and female third instar larvae were used. All experiments were conducted at room temperature (21 to 23.5 °C). The fly stocks used in this study are specified in SI Appendix.
Generation of Dstac Mutant Strains by CRISPR-Mediated Homology-Directed Repair.
The Dstac mutant strains were generated by CRISPR-mediated homology-directed repair (84). Details of the methods for generation of the mutants are described in SI Appendix.
P-Element Excision.
The P{Mae-UAS.6.11} insertion of DstacLA00216 ([1] w[*]; P{y[+t7.7]=Mae UAS.6.11}CG43729[LA00216]) contains a yellow+ gene and was inserted in the 3′ UTR of the Dstac gene (85). See SI Appendix for detailed methods and SI Appendix, Table S1 for primer sequences.
Generation of UAS:Dstacwt.
For making UAS:Dstacwt transgenic flies, a Dstac cDNA (CG43729-RE) that contains an SH3 domain and a CRD domain was amplified from the cDNA of the whole third instar larvae of the w1118 strain using primer 34 and 35 (SI Appendix, Table S1) and the Platinum taq high fidelity DNA polymerase (cat. no. 11304011; Thermo Fisher). The amplified Dstac cDNA was cloned and tagged with an eGFP into a modified pJFRC14 vector (Addgene plasmid no. 26223; http://www.addgene.org/26223/; RRID: Addgene_26223). The plasmid was injected into the strain carrying the attP2 sites (RRID: BDSC_8622) for integration of the transgene via the Phic31 integrase. The transgenic flies were isolated and balanced by a commercial vendor (BestGene).
Antibody Production.
Anti-Dmca1D was generated against the 94 amino acids at the N terminus of the Dmca1D. The specificity of anti-Dmca1D was confirmed by labeling of the CNS in wt but not in Dmca1D-null Drosophila embryos (SI Appendix, Fig. S2D). Details are described in SI Appendix.
Immunostaining.
Third instar larvae were filleted in HL3 solution and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS). For immunostaining of Dmca1DX10 embryos, stage 12 to 17 embryos were collected, and the chorion and vitelline membrane was removed manually by needles, followed by fixation. Immunolabeling followed the procedure described previously (38). Details are described in SI Appendix.
Motility Assay.
Details of the methods for generation of the mutants are described in SI Appendix.
In Vivo Ca2+ Imaging.
For Ca2+ imaging of motor boutons, whole intact larvae selectively expressing GCaMP6f and UAS:mCD8tdTomato in proctolin+ motor neurons (Proct:GAL4 > UAS:GCaMP6f; UAS:mCD8tdTomato) were placed into a microfluidics device (49), and GCaMP fluorescence was observed on a Leica SP8 confocal microscope equipped with an 8,000-Hz resonance scanner and a 63× objective.
Electrophysiology.
Voltage-clamp of RP2 motor neuron cell bodies followed the published protocols (50, 51). Details of the methods for voltage clamping and for recording evoked synaptic potentials in larval muscles are specified in SI Appendix.
Dilp2-GFP Release Assay.
Previously published procedures for assaying the release of Dilp2-GFP from motor boutons were followed (25). Proctolin:GAL4 > UAS:Dilp2-GFP was expressed in Dstac KD, DstacΔSH3.1, and Dmca1D KD larvae. nsyb:Dilp2-GFP was expressed in Dmca1DAR66 larvae. Previously published procedures for quantification of the release of Dilp2-GFP from motor boutons were followed (25).
Supplementary Material
Acknowledgments
We thank Dick Nassel (Stockholm University) for sharing anti-proctolin; Christopher Vecsey (Skidmore College) and Carsten Duch (Johannes Gutenberg University of Mainz) for protocols for desheathing the larval CNS; and William Yau, Naveen Jasti, and Bethany Folk-Middlebrook (University of Michigan) for technical assistance. This project was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR063056 (to J.Y.K.), National Institute of Neurological Disorders and Stroke (NINDS) Grant RO1 NS069844 (to C.A.C.), and NINDS Grant RO1 NS032385 (to E.S.L.). I.-U.H. was supported by a Rackham International Student Fellowship, Rackham Barbour Scholarship, Rackham Predoctoral Fellowship, Rackham Research Grant, and Rackham One-Term Fellowship from the University of Michigan; J.W.L. by a Rackham Merit Fellowship from the University of Michigan and National Institute of General Medical Sciences Grant T32 GM007315; and M.C.L., A.M.O., L.E.R. and J.E.V. by Summer Research Fellowships from the University of Michigan.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2009224117/-/DCSupplemental.
Data and Materials Availability.
All study data are included in the article, SI Appendix, and Movie S1.
References
- 1.Donaldson Z. R., Young L. J., Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904 (2008). [DOI] [PubMed] [Google Scholar]
- 2.García A. G., García-De-Diego A. M., Gandía L., Borges R., García-Sancho J., Calcium signaling and exocytosis in adrenal chromaffin cells. Physiol. Rev. 86, 1093–1131 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Alonso M. T., et al. , Ca2+-induced Ca2+ release in chromaffin cells seen from inside the ER with targeted aequorin. J. Cell Biol. 144, 241–254 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue M., et al. , Homogeneous Ca2+ stores in rat adrenal chromaffin cells. Cell Calcium 33, 19–26 (2003). [DOI] [PubMed] [Google Scholar]
- 5.Morris J. F., Pow D. V., Widespread release of peptides in the central nervous system: Quantitation of tannic acid-captured exocytoses. Anat. Rec. 231, 437–445 (1991). [DOI] [PubMed] [Google Scholar]
- 6.Ludwig M., Leng G., Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Sobota J. A., Mohler W. A., Cowan A. E., Eipper B. A., Mains R. E., Dynamics of peptidergic secretory granule transport are regulated by neuronal stimulation. BMC Neurosci. 11, 32 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia X., Lessmann V., Martin T. F., Imaging of evoked dense-core-vesicle exocytosis in hippocampal neurons reveals long latencies and kiss-and-run fusion events. J. Cell Sci. 122, 75–82 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Pol A. N., Neuropeptide transmission in brain circuits. Neuron 76, 98–115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bondy C. A., Gainer H., Russell J. T., Effects of stimulus frequency and potassium channel blockade on the secretion of vasopressin and oxytocin from the neurohypophysis. Neuroendocrinology 46, 258–267 (1987). [DOI] [PubMed] [Google Scholar]
- 11.Muschol M., Salzberg B. M., Dependence of transient and residual calcium dynamics on action-potential patterning during neuropeptide secretion. J. Neurosci. 20, 6773–6780 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perney T. M., Hirning L. D., Leeman S. E., Miller R. J., Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc. Natl. Acad. Sci. U.S.A. 83, 6656–6659 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cazalis M., Dayanithi G., Nordmann J. J., Hormone release from isolated nerve endings of the rat neurohypophysis. J. Physiol. 390, 55–70 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rane S. G., Holz G. G. IV, Dunlap K., Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 409, 361–366 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lemos J. R., Nowycky M. C., Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron 2, 1419–1426 (1989). [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Treistman S., Wilson A., Nordmann J., Lemos J., Ca2+ channels and peptide release from neurosecretory terminals. Physiology (Bethesda) 8, 64–68 (1993). [Google Scholar]
- 17.Simmons M. L., Terman G. W., Gibbs S. M., Chavkin C., L-type calcium channels mediate dynorphin neuropeptide release from dendrites but not axons of hippocampal granule cells. Neuron 14, 1265–1272 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Kolarow R., Brigadski T., Lessmann V., Postsynaptic secretion of BDNF and NT-3 from hippocampal neurons depends on calcium calmodulin kinase II signaling and proceeds via delayed fusion pore opening. J. Neurosci. 27, 10350–10364 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson M. S., Halpern M. E., Keshishian H., Identification of the neuropeptide transmitter proctolin in Drosophila larvae: Characterization of muscle fiber-specific neuromuscular endings. J. Neurosci. 8, 242–255 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor C. A., et al. , Identification of a proctolin preprohormone gene (proct) of Drosophila melanogaster: Expression and predicted prohormone processing. J. Neurobiol. 58, 379–391 (2004). [DOI] [PubMed] [Google Scholar]
- 21.Cattaert D., Birman S., Blockade of the central generator of locomotor rhythm by noncompetitive NMDA receptor antagonists in Drosophila larvae. J. Neurobiol. 48, 58–73 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Barclay J. W., Atwood H. L., Robertson R. M., Impairment of central pattern generation in Drosophila cysteine string protein mutants. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 71–78 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Summerville J. B., et al. , The effects of ER morphology on synaptic structure and function in Drosophila melanogaster. J. Cell Sci. 129, 1635–1648 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakiryanova D., et al. , Presynaptic ryanodine receptor-activated calmodulin kinase II increases vesicle mobility and potentiates neuropeptide release. J. Neurosci. 27, 7799–7806 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shakiryanova D., Tully A., Hewes R. S., Deitcher D. L., Levitan E. S., Activity-dependent liberation of synaptic neuropeptide vesicles. Nat. Neurosci. 8, 173–178 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Wong M. Y., Cavolo S. L., Levitan E. S., Synaptic neuropeptide release by dynamin-dependent partial release from circulating vesicles. Mol. Biol. Cell 26, 2466–2474 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H., et al. , Stac, a novel neuron-specific protein with cysteine-rich and SH3 domains. Biochem. Biophys. Res. Commun. 229, 902–909 (1996). [DOI] [PubMed] [Google Scholar]
- 28.Lein E. S., et al. , Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Legha W., et al. , stac1 and stac2 genes define discrete and distinct subsets of dorsal root ganglia neurons. Gene Expr. Patterns 10, 368–375 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Horstick E. J., et al. , Stac3 is a component of the excitation-contraction coupling machinery and mutated in Native American myopathy. Nat. Commun. 4, 1952 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linsley J. W., et al. , Congenital myopathy results from misregulation of a muscle Ca2+ channel by mutant Stac3. Proc. Natl. Acad. Sci. U.S.A. 114, E228–E236 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson B. R., et al. , Skeletal muscle-specific T-tubule protein STAC3 mediates voltage-induced Ca2+ release and contractility. Proc. Natl. Acad. Sci. U.S.A. 110, 11881–11886 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider M. F., Chandler W. K., Voltage dependent charge movement of skeletal muscle: A possible step in excitation-contraction coupling. Nature 242, 244–246 (1973). [DOI] [PubMed] [Google Scholar]
- 34.Rios E., Brum G., Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle. Nature 325, 717–720 (1987). [DOI] [PubMed] [Google Scholar]
- 35.Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C., Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J. Cell Biol. 107, 2587–2600 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paolini C., Fessenden J. D., Pessah I. N., Franzini-Armstrong C., Evidence for conformational coupling between two calcium channels. Proc. Natl. Acad. Sci. U.S.A. 101, 12748–12752 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Linsley J. W., Hsu I. U., Wang W., Kuwada J. Y., Transport of the alpha subunit of the voltage gated L-type calcium channel through the sarcoplasmic reticulum occurs prior to localization to triads and requires the beta subunit but not Stac3 in skeletal muscles. Traffic 18, 622–632 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu I. U., Linsley J. W., Varineau J. E., Shafer O. T., Kuwada J. Y., Dstac is required for normal circadian activity rhythms in Drosophila. Chronobiol. Int. 35, 1016–1026 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shafer O. T., Taghert P. H., RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: The anatomical basis of a neuropeptide’s circadian functions. PLoS One 4, e8298 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng W., et al. , Cloning and characterization of a calcium channel alpha 1 subunit from Drosophila melanogaster with similarity to the rat brain type D isoform. J. Neurosci. 15, 1132–1143 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menon K. P., Carrillo R. A., Zinn K., Development and plasticity of the Drosophila larval neuromuscular junction. Wiley Interdiscip. Rev. Dev. Biol. 2, 647–670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eberl D. F., et al. , Genetic and developmental characterization of Dmca1D, a calcium channel α1 subunit gene in Drosophila melanogaster. Genetics 148, 1159–1169 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venken K. J., et al. , MiMIC: A highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8, 737–743 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takizawa E., Komatsu A., Tsujimura H., Identification of common excitatory motoneurons in Drosophila melanogaster larvae. Zool. Sci. 24, 504–513 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Hoang B., Chiba A., Single-cell analysis of Drosophila larval neuromuscular synapses. Dev. Biol. 229, 55–70 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Ren D., Xu H., Eberl D. F., Chopra M., Hall L. M., A mutation affecting dihydropyridine-sensitive current levels and activation kinetics in Drosophila muscle and mammalian heart calcium channels. J. Neurosci. 18, 2335–2341 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ormerod K. G., et al. , Characterizing the physiological and behavioral roles of proctolin in Drosophila melanogaster. J. Neurophysiol. 115, 568–580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wodarz A., Hinz U., Engelbert M., Knust E., Expression of crumbs confers apical character on plasma membrane domains of ectodermal epithelia of Drosophila. Cell 82, 67–76 (1995). [DOI] [PubMed] [Google Scholar]
- 49.Ghannad-Rezaie M., Wang X., Mishra B., Collins C., Chronis N., Microfluidic chips for in vivo imaging of cellular responses to neural injury in Drosophila larvae. PLoS One 7, e29869 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Worrell J. W., Levine R. B., Characterization of voltage-dependent Ca2+ currents in identified Drosophila motoneurons in situ. J. Neurophysiol. 100, 868–878 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kadas D., et al. , Dendritic and axonal L-type calcium channels cooperate to enhance motoneuron firing output during Drosophila larval locomotion. J. Neurosci. 37, 10971–10982 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong M. Y., et al. , Neuropeptide delivery to synapses by long-range vesicle circulation and sporadic capture. Cell 148, 1029–1038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo J., Liu Y., Nässel D. R., Transcriptional reorganization of Drosophila motor neurons and their muscular junctions toward a neuroendocrine phenotype by the bHLH protein dimmed. Front. Mol. Neurosci. 10, 260 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sasaki N., Dayanithi G., Shibuya I., Ca2+ clearance mechanisms in neurohypophysial terminals of the rat. Cell Calcium 37, 45–56 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Ludwig M., et al. , Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418, 85–89 (2002). [DOI] [PubMed] [Google Scholar]
- 56.O’Shea M., Bishop C. A., Neuropeptide proctolin associated with an identified skeletal motoneuron. J. Neurosci. 2, 1242–1251 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams M. E., O’Shea M., Peptide cotransmitter at a neuromuscular junction. Science 221, 286–289 (1983). [DOI] [PubMed] [Google Scholar]
- 58.Bishop C. A., Wine J. J., Nagy F., O’Shea M. R., Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J. Neurosci. 7, 1769–1779 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee J., Ueda A., Wu C. F., Distinct roles of Drosophila cacophony and Dmca1D Ca(2+) channels in synaptic homeostasis: Genetic interactions with slowpoke Ca(2+) -activated BK channels in presynaptic excitability and postsynaptic response. Dev. Neurobiol. 74, 1–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawasaki F., Felling R., Ordway R. W., A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J. Neurosci. 20, 4885–4889 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helfrich-Förster C., Development of pigment-dispersing hormone-immunoreactive neurons in the nervous system of Drosophila melanogaster. J. Comp. Neurol. 380, 335–354 (1997). [DOI] [PubMed] [Google Scholar]
- 62.Renn S. C., Park J. H., Rosbash M., Hall J. C., Taghert P. H., A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99, 791–802 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Hewes R. S., Taghert P. H., Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 11, 1126–1142 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nässel D. R., Zandawala M., Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Prog. Neurobiol. 179, 101607 (2019). [DOI] [PubMed] [Google Scholar]
- 65.Nakai J., et al. , Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380, 72–75 (1996). [DOI] [PubMed] [Google Scholar]
- 66.Grabner M., Dirksen R. T., Suda N., Beam K. G., The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the Bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 274, 21913–21919 (1999). [DOI] [PubMed] [Google Scholar]
- 67.Hsu I. U. et al., Dstac regulates excitation-contraction coupling in Drosophila body wall muscles. Front. Physiol. 11, 10.3389/fphys.2020.573723 (2020) Published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Györke S., Palade P., Calcium-induced calcium release in crayfish skeletal muscle. J. Physiol. 457, 195–210 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maryon E. B., Saari B., Anderson P., Muscle-specific functions of ryanodine receptor channels in Caenorhabditis elegans. J. Cell Sci. 111, 2885–2895 (1998). [DOI] [PubMed] [Google Scholar]
- 70.Sullivan K. M., Scott K., Zuker C. S., Rubin G. M., The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 97, 5942–5947 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Takekura H., Franzini-Armstrong C., The structure of Ca(2+) release units in arthropod body muscle indicates an indirect mechanism for excitation-contraction coupling. Biophys. J. 83, 2742–2753 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collet C., Excitation-contraction coupling in skeletal muscle fibers from adult domestic honeybee. Pflugers Arch. 458, 601–612 (2009). [DOI] [PubMed] [Google Scholar]
- 73.Persoon C. M., et al. , The RAB3-RIM pathway is essential for the release of neuromodulators. Neuron 104, 1065–1080.e12 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walent J. H., Porter B. W., Martin T. F., A novel 145 kd brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell 70, 765–775 (1992). [DOI] [PubMed] [Google Scholar]
- 75.Tandon A., et al. , Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron 21, 147–154 (1998). [DOI] [PubMed] [Google Scholar]
- 76.Brenner S., The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Avery L., Bargmann C. I., Horvitz H. R., The Caenorhabditis elegans unc-31 gene affects multiple nervous system-controlled functions. Genetics 134, 455–464 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renden R., et al. , Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron 31, 421–437 (2001). [DOI] [PubMed] [Google Scholar]
- 79.Ryglewski S., Lance K., Levine R. B., Duch C., Ca(v)2 channels mediate low and high voltage-activated calcium currents in Drosophila motoneurons. J. Physiol. 590, 809–825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeong K., et al. , Ca-α1T, a fly T-type Ca2+ channel, negatively modulates sleep. Sci. Rep. 5, 17893 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wong C. O., et al. , A TRPV channel in Drosophila motor neurons regulates presynaptic resting Ca2+ levels, synapse growth, and synaptic transmission. Neuron 84, 764–777 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rzhepetskyy Y., et al. , A Cav3.2/Stac1 molecular complex controls T-type channel expression at the plasma membrane. Channels (Austin) 10, 346–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong King Yuen S. M., Campiglio M., Tung C.-C., Flucher B. E., Van Petegem F., Structural insights into binding of STAC proteins to voltage-gated calcium channels. Proc. Natl. Acad. Sci. U.S.A. 114, E9520–E9528 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gratz S. J., et al. , Highly specific and efficient CRISPR/Cas9-catalyzed homology-directed repair in Drosophila. Genetics 196, 961–971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellen H. J., et al. , The BDGP gene disruption project: Single transposon insertions associated with 40% of Drosophila genes. Genetics 167, 761–781 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article, SI Appendix, and Movie S1.