Significance
A classic explanation for the prevalence of complex warfare in human societies is leadership by exploitative individuals who reap the benefits of conflict while avoiding the costs. Here, we extend the classic hawk−dove model to show that leadership of this kind can also explain the evolution of severe collective violence in certain animal societies. We test our model using long-term data from wild banded mongooses, and show that female leaders incite fights with rival groups to gain genetic benefits, while males bear the costs of fighting. The result is unusually severe levels of intergroup violence. Our findings suggest that the decoupling of leaders from the costs that they incite amplifies the destructive nature of intergroup conflict.
Keywords: social evolution, aggression, human evolution, animal behavior, collective violence
Abstract
Collective conflicts among humans are widespread, although often highly destructive. A classic explanation for the prevalence of such warfare in some human societies is leadership by self-serving individuals that reap the benefits of conflict while other members of society pay the costs. Here, we show that leadership of this kind can also explain the evolution of collective violence in certain animal societies. We first extend the classic hawk−dove model of the evolution of animal aggression to consider cases in which a subset of individuals within each group may initiate fights in which all group members become involved. We show that leadership of this kind, when combined with inequalities in the payoffs of fighting, can lead to the evolution of severe intergroup aggression, with negative consequences for population mean fitness. We test our model using long-term data from wild banded mongooses, a species characterized by frequent intergroup conflicts that have very different fitness consequences for male and female group members. The data show that aggressive encounters between groups are initiated by females, who gain fitness benefits from mating with extragroup males in the midst of battle, whereas the costs of fighting are borne chiefly by males. In line with the model predictions, the result is unusually severe levels of intergroup violence. Our findings suggest that the decoupling of leaders from the costs that they incite amplifies the destructive nature of intergroup conflict.
Humans are capable of astonishing feats of altruism and cooperation (1–3), but, at the same time, of violent and destructive conflicts (4–8). A key factor contributing to the latter may be that wars are often waged at the behest of leaders who do not share fully in the immediate risks of conflict, and stand to gain benefits in terms of resources and status that are not enjoyed by the majority of combatants (4, 9–11). Could such “warmongering” be a feature of animal conflicts too? Only recently have models of animal aggression begun to explore the impact of inequalities among combatants in collective conflict (12, 13), and the usual assumption of existing theory is that individuals who initiate intergroup conflicts also contribute most to group conflict effort and thereby confer fitness benefits on the rest of their group (a positive or “heroic” model of leadership) (14–17). Here, we explore the more sinister possibility that those who initiate conflict may actually harm their fellows in pursuit of their own interests by exposing them to the risks of conflict while contributing little to fighting themselves (a negative or “exploitative” model of leadership).
The Model
In the classic hawk−dove game (18), members of a population engage in pairwise antagonistic interactions, with each individual choosing whether to adopt aggressive (hawk) or peaceful (dove) tactics. We extend this model to consider pairwise encounters between groups of size n (assuming that each individual engages in an average of n group interactions, so that the number of encounters per individual remains the same as in the original game). In each encounter, individuals are grouped at random, and one member of each group (chosen at random) becomes the “leader,” while the rest become “followers.” A group may collectively adopt aggressive (hawk) or peaceful (dove) tactics, with the leader making this decision for the group via their disproportionate influence on collective behavior (19). Thus our model applies to cohesive groups in which there are costs to individuals that do not follow the tactic chosen by the leader, for example, because refuseniks are punished (20, 21) or forego the benefits of group membership (22, 23). In human societies, leaders can embroil other group members in conflict by wielding political influence, including peer pressure and the use of threats to coerce other group members to fight (10, 24–27). Societies in which warriors follow orders given by their leaders [i.e., exhibiting a high degree of “subordination” (9)] are common in the ethnographic record [27 out of 36 societies in one cross-cultural sample (9)]. In mobile animal groups, leaders are individuals that wield disproportionate influence over collective movement (19, 28), which may enable them to draw or coerce their followers into antagonistic encounters with other groups that benefit themselves but not necessarily the rest of the group. Total fitness payoffs to contending groups in our model are identical to the payoffs to individuals in the classic game (see Materials and Methods), but while, in the simplest case, all benefits and costs are divided equally among group members, we allow for unequal sharing [or “political bias” (26)]. Formally, we suppose that each follower’s share of any benefit obtained is reduced by a proportion dv compared to that of the leader, while the leader’s share of any costs incurred is reduced by a proportion dc compared to that of a follower.
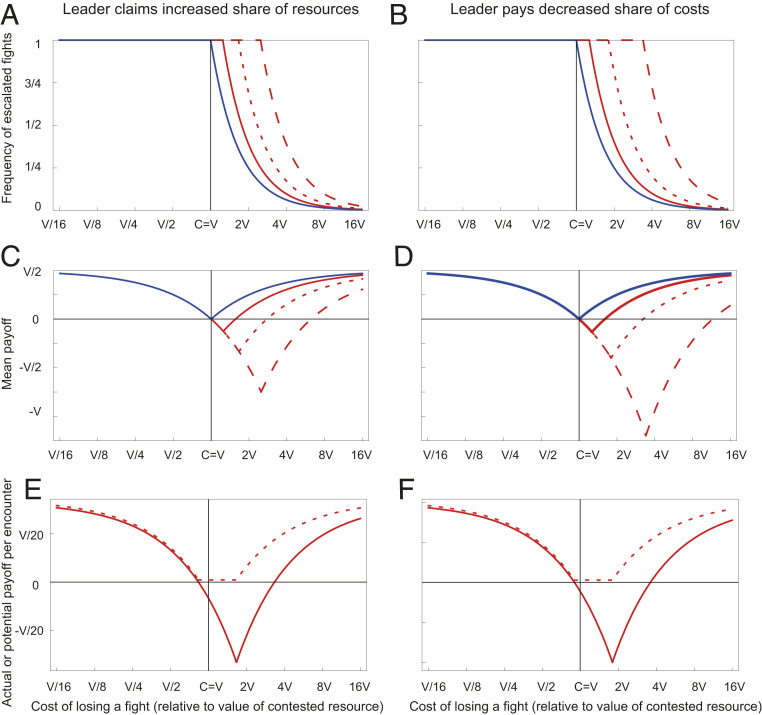
In the original (pairwise interaction) game, when the fitness cost of losing a fight (c) is no greater than the value of victory (v), the model yields an evolutionarily stable strategy (ESS) in which all individuals play hawk. Consequently, over this range, all encounters involve fighting, and the expected mean payoff decreases with the cost of losing, reaching a minimum of zero when c = v. As the cost of losing increases further, however, the evolutionarily stable probability of playing hawk starts to decrease, so that fights become rarer (Fig. 1). The expected mean payoff thus increases once again, despite the fact that each individual fight is more damaging to the loser. For no value of c does the mean payoff fall below zero. In the group conflict game, by contrast, the probability of playing hawk is equal to 1 for cL < vL and to vL/cL for cL > vL, where vL denotes the individual benefit gained by the leader of a victorious group and cL denotes the individual cost incurred by the leader of a defeated group. Thus, when leaders gain a disproportionately large share of the benefits of conflict (dv > 0; Fig. 1, A, C, and E) and/or pay a disproportionately small share of the costs (dc > 0; Fig. 1, B, D, and F), hawk continues to be played with probability 1, and the mean fitness payoff continues to decline, even as the total cost of losing a fight c rises above the total value of the benefit of winning v (Fig. 1 A and B). This leads to mean fitness payoffs at equilibrium that are negative for part of the parameter space of the model (Fig. 1 C and D). The greater the inequality in the division of costs and benefits within groups (i.e., the larger the values of dv and dc), the greater the potential negative impact of conflict. These conclusions, moreover, are unaffected by the introduction into the model of persistent groups (associating always with the same group mates in every encounter), and consistent leaders (where the same individual within a group always leads; see SI Appendix for details of the extended model).
Fig. 1.
(A and B) Evolutionarily stable probabilities of escalated fighting and (C and D) mean payoffs per individual across all encounters, as a function of the total cost c of losing a fight (expressed relative to the value of victory v, and plotted on a log scale), in the original pairwise-interaction hawk−dove game (blue curves), and in the group-interaction game (red curves). A and C show results when leaders claim a disproportionately large share of the benefits of victory, that is, when dv > 0 (solid red curves, dv = 0.25; dotted red curves, dv = 0.5; dashed red curves dv = 0.75; dc = 0 in each case); B and D show results when leaders suffer a disproportionately small share of the cost of losing a fight, that is, when dc > 0 (solid red curves, dc = 0.25; dotted red curves, dc = 0.5; dashed red curves dc = 0.75; dv = 0 in each case). In all cases, n = 5. (E and F) Mean payoffs to followers per encounter when leaders control group behavior (solid curves), compared to the potential maximum payoffs obtainable if a follower were to seize control of the group and impose its own preferred behavior (dashed curves), as a function of the total cost c of losing a fight (expressed relative to the value of victory v, and plotted on a log scale). Exploitative leadership occurs where the solid and dashed curves diverge. Parameter values E, (dv = 0.5, dc = 0, n = 5); F, (dv = 0, dc = 0.5, n = 5).
The exploitative nature of leadership in the above model is highlighted in Fig. 1 E and F, which contrasts the mean payoff to followers per encounter when leaders control group behavior (solid curves) with the potential maximum payoff obtainable if a follower were to seize control of the group and impose its own preferred choice of behavior (dotted line). When the benefits of victory outweigh the costs of losing even for followers (i.e., when vF > cF), then all group members favor the hawk tactic, and the interests of followers coincide with those of leaders. But, over a large part of the model’s parameter space (when cF > vF), followers favor a lower frequency of hawk than do leaders and, due to this conflict, obtain a lower payoff under leader control than they could potentially do if they were able to seize control for themselves and impose their own preferred choice of group behavior.
Results and Discussion
We tested whether this model could help to explain patterns of intergroup aggression, using data from an obligately social mammal, the banded mongoose (Mungos mungo). This species lives in highly territorial, mixed-sex groups averaging ∼20 adults plus offspring (29). Group members forage and sleep together in an underground burrow, and cooperate to rear young and to defend against aerial and terrestrial predators (30). Group members never leave the group unless they are forcibly expelled as a same-sex group (31) or, more rarely, leave voluntarily as a same-sex group (32). Exploitative leadership is plausible in obligately social, group-foraging species because followers may have little option but to remain as part of the group, even when the self-serving decisions of their leaders place them at personal risk. In obligately social species, individuals that leave the group forego the survival and fitness benefits of group living (22), and may suffer reduced body condition (33) and elevated stress (34), and are at increased risk of predation (35) and/or attack by groups of conspecifics (36).
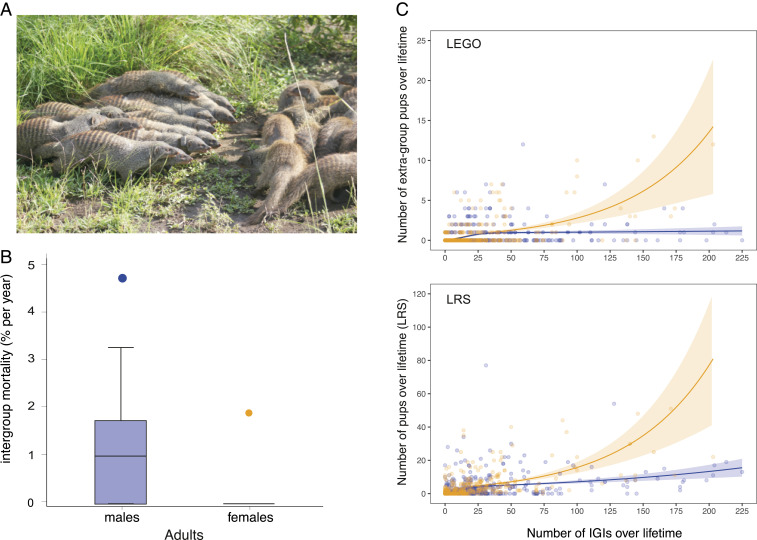
Reproduction in banded mongooses is synchronized within but not between groups (29). Multiple females in each group give birth together on the same day in an underground den, and offspring are raised cooperatively by the whole group. All adult female group members enter estrus within 7 d to 10 d of one another (the “group estrus” period), during which females are closely guarded by males from their own group (29). The adult sex ratio is significantly male biased [1.6 males:females (29)], and males compete intensely for access to females in their own group and in rival groups. Throughout group estrus, mate-guarding males follow females of their own group nose-to-tail all day long, and aggressively defend them from other males in their own group. Encounters between groups are frequent [0.6 to 3 per month (37)] and always aggressive (e.g., 95/95 interactions in ref. 38), usually escalating into chases and physical combat (see Materials and Methods). When members of rival groups detect each other, they stand up and emit a piercing call which brings the group running together into a tight formation. Bunched groups often advance toward one another in tight “battle lines” which may function to assess relative group resource holding potential; these face-offs erupt into individual fights and chases that involve biting and scratching (Fig. 2A and Movies S1–S3). In the midst of these encounters, individual mongooses may become separated from their group and attacked on all sides (Movie S1), leading to injury and sometimes death (37, 38).
Fig. 2.
(A) Banded mongoose battle lines during an IGI. Image credit: Dave Seager (photographer). (B) Costs of intergroup aggression in male and female banded mongooses. Mortality rate of adult (>1 y) males and females resulting from intergroup aggression. Box, median and interquartile range; whisker, 90th percentile; points, outliers. *P = 0.018. N = 478 males, 335 females followed for 1,899 mongoose-years. (C) LRS and intergroup conflict. LEGO (Top) and LRS (Bottom) of males (blue) and females (orange) are plotted against the number of IGIs in which individuals were involved across their lifespan. Data are for 499 males and 367 female adults monitored from birth to death over 20 y.
The model predicts that damaging levels of intergroup aggression can evolve where a subset of group members can initiate conflicts that involve the whole group, and where the initiators of conflicts gain a disproportionate benefit or suffer lower costs from fighting than do others. To test this prediction in banded mongooses, we focused on sex differences in the payoffs of intergroup conflict, because, as we describe below, females and males may have different fitness interests in intergroup interactions (IGIs). We asked three questions: 1) Do males and females experience different costs and/or benefits of IGIs? 2) Does the sex that experiences the lowest costs and/or highest benefits of IGIs initiate the encounters? 3) Is the result unusually severe levels of intergroup violence (measured in terms of the mortality costs of IGIs)?
Fitness Costs and Benefits of IGIs
First, to evaluate sex differences in the costs of IGIs, we analyzed all of the adult deaths attributable to intergroup fighting over a 16-y period. We found that almost all mortality attributable to fighting occurred in males. Females, by contrast, almost never died in, or as a result of, fighting (generalized linear model [GLM], β ± SE = 1.63 ± 0.80, χ21 = 5.62, P = 0.018; Fig. 2B). This result is consistent with a previous study using staged encounters, which found that males are highly aggressive toward experimental intruders, while females remain nonaggressive (38).
To evaluate fitness benefits to males and females of engaging in IGIs, we examined how the lifetime number of extragroup offspring (LEGO), and lifetime reproductive success (LRS) varied with the number of IGIs experienced across the lifetime (Fig. 2C). This analysis used genetic pedigree data on 499 adult males and 367 adult females for which genetic data are available across the entire lifespan. Both LEGO and LRS increased more steeply with the number of IGIs in females compared to males (GLM, sex * number of IGIs interaction: LEGO β ± SE = −0.014 ± 0.005, χ22 = 8.77, P = 0.012; LRS β ± SE = −0.008 ± 0.003, χ22 = 7.43, P = 0.024). This result held when we included lifespan as an offset, suggesting that the rate of production of surviving offspring (both extragroup and all offspring) increased more steeply with number of IGIs in females than males (GLM, sex * number of IGIs interaction with offset: LEGO β ± SE = −0.014 ± 0.006, χ22 = 18.74, P < 0.001; LRS β ± SE = −0.009 ± 0.004, χ22 = 6.34, P = 0.042). The true fitness benefits of IGIs to females are likely to be even greater than this relationship suggests, because extragroup offspring are more heterozygous, heavier, and have higher survival compared to pups fathered within the group (39).
These analyses suggest that not only do females suffer lower mortality costs from IGIs than males, but they also gain greater fitness benefits. In these circumstances, our model predicts females, as a class, should prefer a higher rate of IGI than males (Fig. 1 E and F). If they can exert disproportionate control over group movement, females are predicted to lead their groups into intergroup encounters that are beneficial for themselves but not for the males in their group.
Initiators of IGIs
Because encounters between banded mongoose groups always involve aggression (38), individuals with disproportionate influence on group movement can initiate intergroup aggression by leading their group into detection range of a rival group. Several lines of evidence suggest that females lead their groups into IGIs to gain access to extragroup matings. First, reproductive females exert disproportionate influence on group leadership decisions compared to males (40), particularly during the “group estrus” period when mate-guarding males follow estrus females nose-to-tail all day long, for days at a time (41). Estrus females have been observed to lead their group deep into enemy territory, closely followed by mate-guarding males, directly inciting intergroup fights. In the ensuing chaos, females escape their mate guards and mate with males from the rival group (38). Second, IGIs are most common when females are in estrus, compared to when they are pregnant or in nonbreeding periods (37). Third, genetic analysis shows that the probability that females conceive to extragroup males is significantly higher when there is a high risk of inbreeding within their own group (39). Fourth, as our data show, females stand to gain substantial fitness benefits from IGIs through increased production of extragroup offspring (Fig. 2).
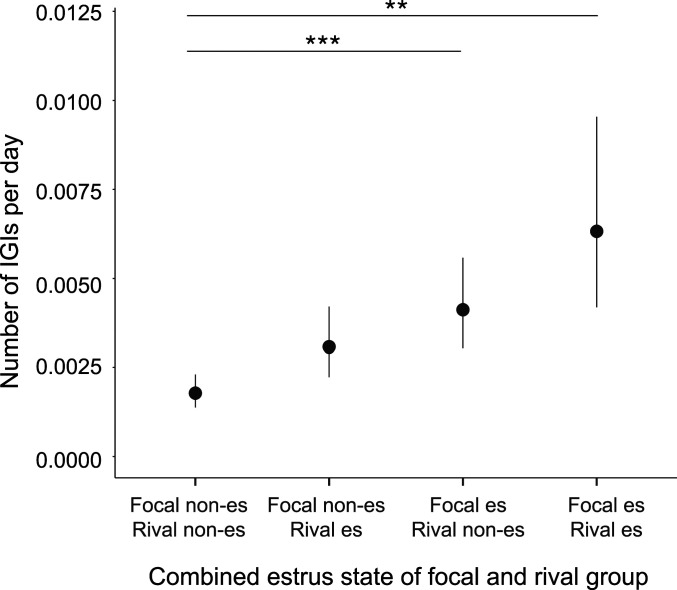
To test more explicitly whether females are the primary initiators of IGIs, we carried out a further analysis of our long-term data, examining how the IGI rate of focal groups varied with their own group estrus state (focal estrus [FE] vs. focal nonestrus [FNE]) and with the group estrus state of rival groups (rival estrus [RE] vs. rival nonestrus [RNE]). If females control the rate of IGIs, and seek matings with rival groups when it is in their interest to do so, we predict a higher interaction rate when those females are in group estrus (and therefore most likely to conceive), irrespective of the group estrus state of rival groups. By contrast, if males control the IGI rate, we predict lower encounter rates when females in their own group are in estrus, because males closely mate guard females in their own group throughout the group estrus period. Consistent with the hypothesis that females initiate IGIs, encounter rates increased when females were in group estrus, irrespective of the group estrus state of rival groups (generalized linear mixed model [GLMM]: χ23 = 17.86, P < 0.001; post hoc Tukey’s test: FERNE vs. FNERNE: β ± SE = 0.84 ± 0.22, z = 3.76, P < 0.001; FERE vs. FNERNE: β ± SE = 1.27 ± 0.35, z = 3.64, P = 0.001; all other pairwise comparisons P > 0.1; Fig. 3).
Fig. 3.
Intergroup encounters and female reproductive state. The number of IGIs observed per day when a focal and rival group were in each of four possible combined group estrus (es) states. Points show means from the GLMM ± SE; ***P < 0.001; **P = 0.001; asterisks refer to post hoc Tukey’s all-pairwise comparison of means across all four categories.
These findings, together with our observations (37, 38) and genetic data (39), suggest that IGIs are driven by estrus females who lead their group in search of outbred matings, and use the cover of battle to escape reproductive control by their mate guards. Once females have initiated a conflict by leading their group into detection range of another, males on the opposing side attempt to drive off mate guards to gain access to guarded females, and mate-guarding males have little option but to fight rival males to defend access to their estrus females. In the chaos and confusion, females can escape their mate guards and mate with males from the rival group (Movie S2).
Mortality due to Intergroup Conflict in Other Species
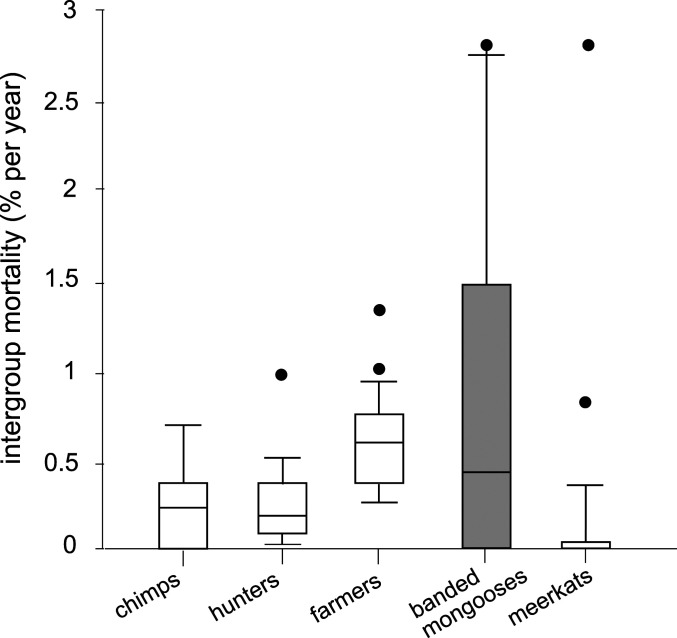
Finally, to assess whether the severity of intergroup aggression is unusually high in banded mongooses, we compared rates of adult death attributable to intergroup fighting with other mammals for which comparable data exist. One way to quantify the severity of conflict is to calculate the proportion of deaths of known cause that are attributable to intergroup aggression. In the vast majority of social mammals, lethal intergroup conflict is absent or extremely rare (36, 42–44). Frequent, lethal coalitionary violence, accounting for 10% more of adult deaths, is probably limited to 10 or fewer mammalian species (45). In banded mongooses, IGIs account for 10% of total adult mortality where the cause of death is known; for juveniles, this figure is 20% (39). In wolves (Canis lupus), relations between packs are highly aggressive, and, in some populations, intraspecific (presumed intergroup) killing accounts for 43% (46) to 71% (47) of adult deaths of known cause. In one well-studied lion population, a large majority of adult deaths are a consequence, directly or indirectly, of intergroup aggression (48). In chimpanzees, rates of intergroup (or intercommunity) killing vary widely across populations (49, 50), but accounted for 17% of adult deaths in one long-term study (51). In humans, the percentage of adult mortality that is attributable to war averaged 14% for 14 Late Pleistocene and Early Holocene hunter-gatherers [mean across 14 sites, range 0 to 30% (52)], and 18% in 14 contemporary small-scale human societies [mean across 14 societies, range 0 to 33% (52, 53)].
For three species (in addition to banded mongooses), sufficient information was available on the number of deaths and periods of exposure to calculate annual total adult mortality attributable to intergroup violence: humans, represented by small-scale human societies (subsistence hunter-gatherers [N = 12 societies] and farmers [n = 21 societies (5)]), chimpanzees (Pan troglodytes) (54), and another intensely studied social mongoose, the meerkat (Suricata suricatta) (55), using data that we extracted from the long-term database. Banded mongooses exhibit very high levels of annual mortality from intergroup conflict (“intergroup mortality”) compared to meerkats, showing that such high mortality is not a peculiarity of social mongooses. In meerkats, breeding females typically have access to an unrelated in-group male, and so have little or no incentive to pursue matings with rival groups. Only around 3% of meerkat pups are fathered by extragroup males (56), compared to 18% of pups in banded mongooses (57). Dominant female meerkats exert disproportionate influence over some aspects of group movement (e.g., selection of sleeping burrows), but use this control to avoid rather than pursue interaction with other groups (58). The mean intergroup mortality rate in banded mongooses (0.4% per annum) is greater than that observed in chimpanzees, and comparable to the rate of death observed in small-scale human societies (Fig. 4). These data, and the data on proportional causes of death, confirm that intergroup conflict in banded mongooses is extremely damaging, involving mortality costs that are comparable to those seen in the most warlike mammals.
Fig. 4.
Comparable mortality costs of intergroup aggression. Adult mortality rate from intergroup aggression in chimpanzees P. troglodytes (N = 5 populations studied for >5 y), small-scale human societies (N = 12 hunter-gatherers and N = 20 subsistence farmer societies), meerkats (N = 24 groups studied for >5 y), and banded mongooses (N = 10 groups studied for >5 y). Box, median and IQR; whisker, 90th percentile; points, outliers. Human and nonhuman primate data are redrawn from ref. 54.
Conclusions
In sum, our results suggest that banded mongooses experience high levels of mortality from intergroup conflict because encounters are initiated by females to gain genetic benefits, while males bear the cost of collective aggression. Estrus females incite fights among rival groups as a means to acquire a fitness benefit that they could not otherwise obtain, and mate-guarding males are forced into conflicts that it is not in their interests to seek out. The result is an unusually high level of intergroup violence and mortality. These findings do not fit a heroic model of leadership, in which leaders contribute most to aggression and bear greater costs, but rather an exploitative model, in which the initiators of conflict expose others to greater risks while contributing little to fighting themselves. As our model shows, this type of inequality can favor the evolution of increased aggression in collective animal conflicts, even to a point at which mean per capita payoffs are negative (such that groups would be better off if the potential benefits that they fight to obtain never existed in the first place). Since unequal division of costs and benefits is also a feature of complex warfare in humans (59), it is possible that similar processes could contribute to the destructive nature of human warfare. Consequently, while there are many ecological and social factors that may have shaped patterns of human intergroup violence [including kinship, subsistence strategy, military technology, and systems of political organization (11, 60, 61)], our findings highlight the value of exploring when and how leaders may become decoupled from the costs that they incite.
Materials and Methods
All research procedures received prior approval from Uganda Wildlife Authority and Uganda National Council for Science and Technology, and adhered to the Guidelines for the Treatment of Animals in Behavioural Research and Teaching, published by the Association for the Study of Animal Behaviour. All research was approved by the Ethical Review Committee of the University of Exeter.
The Model.
In the classic hawk−dove game (18), members of a population contest engage in pairwise agonistic interactions, with each individual choosing whether to adopt aggressive (hawk) or peaceful (dove) tactics. An individual that plays hawk always defeats one that plays dove, the former obtaining a payoff v and the latter a payoff of zero; two doves resolve their encounter peacefully, or each have equal probabilities of winning, so that each obtains an expected payoff of v/2; lastly, two hawks become embroiled in an escalated conflict that each is equally likely to win, the winner obtaining a payoff of v while the loser suffers a cost c, so that each individual obtains an expected payoff of (v – c)/2.
In the group conflict game, each encounter involves two randomly assembled groups of size n (≥2), in each of which a single (randomly selected) individual acts as leader, choosing whether its group will collectively adopt hawk or dove tactics. We assume that there is one such encounter per pair of individuals in the population (as in the original, pairwise interaction game), so that each individual engages in an average of n group contests. The total payoffs to contending groups are identical to the payoffs to individuals in the pairwise game. In the simplest case, any benefits obtained by a group are divided equally among its members, as are any costs incurred through escalated conflict. Allowing for inequality, however, we suppose that each follower’s share of any benefits gained is reduced by a proportion dv compared to that of the leader, while the leader’s share of any costs incurred is reduced by a proportion dc compared to that of a follower. The leader’s share vL of the collective payoff of victory is thus equal to v/(n – (n – 1) dv), while each follower’s share vF is equal to v (1 – dv)/(n – (n – 1) dv). Similarly, the leader’s share cL of costs incurred by a losing group is equal to c (1 – dc)/(n – dc), while each follower’s share cF is equal to c/(n – dc).
Consider a population of individuals that (if selected as leader) play hawk with probability p. The expected payoff in this population to a rare mutant type that plays hawk with probability pm, denoted w(pm, p), is given by
where the first term in square brackets on the right-hand side represents the payoff from the fraction (1/n) of group encounters in which the focal individual is chosen as leader, and the second term represents the payoff from the fraction ((n – 1)/n)) of group encounters in which the focal individual is a follower.
We seek an evolutionarily stable probability of playing Hawk p*, which satisfies
and if
then
This yields the unique solution
which leads to mean payoffs per encounter to leaders and followers of and given by
and a mean overall payoff of (over the n encounters in which an individual can, on average, expect to participate) given by
The evolutionarily stable probability p* with which leaders play hawk differs, over a large part of the model’s parameter range, from that which would be favored by followers (if the latter were able to enforce their own preferences on their group). To illustrate this evolutionary conflict, we can calculate the maximum payoff obtainable (in a population in which leaders adopt the ESS) by a “rebellious” follower able to enforce its own optimal choice of action on its group (shown in Fig. 2). This payoff, denoted , is given by
When , a rebellious follower would do best always to play hawk, just as its leader does under these circumstances, so that (i.e., there is, in this case, no conflict between leaders and followers, and nothing for the latter to gain even if they could seize control of their group from the former). By contrast, when cF > vF, a rebellious follower would do best always to play dove, while its leader (following the ESS derived above) plays hawk with some nonzero probability, so that (i.e., there is, in this case, a conflict between leaders and followers, such that the latter do stand to gain by seizing control from the former). In this second case, we refer to the ESS outcome discussed in The Model as an instance of exploitative leadership, since a leader, by enforcing a positive probability of playing hawk, prevents its followers from obtaining their maximum potential payoff.
Persistent Groups and Consistent Leaders.
In the above analysis, we have adopted a classical game theoretical approach similar to that of the original hawk−dove model, assuming a well-mixed population in which groups are repeatedly formed at random in each encounter (so that a given individual will be associated with different group mates in every interaction). In SI Appendix, we consider, instead, a population in which individuals form persistent groups (associating always with the same group mates in every encounter), and also allow for the possibility of consistent leaders (so that the same individual within a group always leads). As we show there, the introduction of persistent groups does not alter the results at all, while the addition of consistent leaders has only a very small quantitative impact that does not affect the qualitative predictions of the model.
Study System.
We collected data from a population of banded mongooses living on the Mweya Peninsula, Queen Elizabeth National Park, Uganda (0°12′S, 29°54′E) between January 2000 and March 2019. For details of the climate and habitat, see ref. 29. Our study population typically consists of 10 to 12 social groups occupying distinct territories (62). Groups sleep together at night in underground dens, moving dens every 2 d to 5 d, and spend the day foraging as a group for insects and small vertebrates. Reproduction occurs year-round, with each group producing an average of four communal litters per year. Around 85% of individuals are born and die in the same group (62); among individuals that reach adulthood, ∼73% die without leaving their natal group. When dispersal does occur, it is almost always the result of violent eviction events targeted at young adults, particularly females (31).
Groups were visited every 1 d to 3 d to record data on group composition, life history, and reproductive behavior. Incidences of IGIs were recorded ad libitum during group visits. IGIs are conspicuous and aggressive, and usually escalate into physical contact (38). Following Thompson et al. (37), we defined an IGI as any occasion that two groups sighted each other and responded by vocalizing, chasing and/or fighting. We use this definition because interactions often involve much chasing in and out of bushes, and it is sometimes difficult to determine whether physical contact has occurred (37). Our dataset comprised 597 IGIs among 28 groups over a 19-y period.
Because groups are highly cohesive and mate-guarding males follow females very closely throughout the group estrus period, banded mongoose females are able to lead other group members into intergroup encounters that embroil the whole group (38). Upon detecting a rival group, individuals emit specific calls (“war cries”) which alert their own group and bring them rapidly together into a bunched formation, before advancing upon the rival group, snarling, growling, feinting, and emitting high-pitched squeals (Fig. 2 and Movies S1–S3). Each IGI can involve multiple rounds of physical encounter and last for up to an hour, ending when one or both groups retreat. Deaths and serious injury occur when individual mongooses become separated from their group mates and are attacked on all sides (Movie S1). Most IGIs have a clear winner and loser: Losing groups are displaced while winners hold their ground or advance further into the rival’s territory. Group size is an important factor contributing to group success in IGIs: in 74% of 314 IGIs between 2000 and 2019, the larger group won the encounter and succeeded in driving off the rival group. Further description of behavior during IGIs is given in ref. 38.
Mortality Rates from Intergroup Conflict.
Incidences where adult individuals (older than 12 mo) died as a result of intergroup fighting (either during intergroup fighting or as a direct result of injury) were analyzed to calculate the rate of mortality from intergroup conflict (n = 19; 17 males and 2 females) between January 2000 and December 2015. We focus on adult mortality since these are the data available for comparison with human societies. We restricted our analyses to include only those groups for which we had more than 5 y of detailed life history data (10 groups), the minimum number of data years in Wrangham et al.’s (54) analyses of intergroup mortality in chimpanzees. Following Wrangham et al. (54), we assembled data on group composition in these 10 study groups across the study period to calculate exposure to mortality from intergroup conflict. For each adult mongoose, we calculated the number of years that it was alive during the study period to generate individual exposure in mongoose-years (n = 813 adults; 478 males, and 335 females). We then summed these individual exposures to calculate total adult exposure for each study pack (1,899 mongoose-years), and also separately summed individual exposure for males (1,171 mongoose-years) and females (728 mongoose-years). The total adult mortality rate (% per year) from intergroup conflict in each study pack was calculated as (D/E)*100, where D = number of adult deaths from intergroup fighting, and E = total adult exposure in mongoose years. Mortality rates for adult males and adult females were calculated similarly. Note that, since we do not observe all IGIs, there may be additional deaths due to fighting that we do not see, so the total adult intergroup mortality rate is likely to be an underestimate.
Sex Differences in Intergroup Mortality Rate.
To analyze sex differences in the costs of collective conflict, we fitted the number of deaths observed as a result of intergroup fighting as the response variable in a generalized linear model with a negative binomial error structure (to account for overdispersion) and a log-link function. Analysis was carried out using R version 3.6.0 (63) and the “MASS” package (64). We included sex as the main term of interest, and log(mongoose-years) as an offset term as an additional fixed effect to account for differences in exposure. To test the effect of sex on the mortality rate from intergroup conflict, we compared the likelihood ratio of the model with and without this fixed effect (65). We fitted the model to data from 10 social groups.
Sex Differences in Fitness Benefits from IGIs.
To analyze sex differences in the fitness benefits obtained through IGIs, we analyzed how the LEGO and LRS varied with the number of IGIs experienced across the lifetime. For all individuals in our population that were adults (> 1 y old) after January 2000 and for whom we had lifetime data, LEGO and LRS was calculated using genetic pedigree data with assignment probabilities for maternity and paternity of 90% [for assignments made using a full panel of 43 microsatellites (66)] and 95% [for assignments made using a subset of 35 microsatellites (67)]. We fitted either LEGO or LRS as the response variable in a generalized linear model with a zero-inflated negative binomial error structure (to account for zero inflation and overdispersion) and a log-link function using the “glmmTMB” package (68). In each model, we included, as fixed effects, the number of IGIs experienced as an adult across the lifetime, sex, and the interaction between these variables. To test the effect of the interaction between the number of IGIs and sex on LEGO and LRS, we compared the likelihood ratio of each model with and without this fixed effect (65). We then repeated the analyses of LEGO and LRS to account for differences in lifespan by including log(lifespan) as an offset term as an additional fixed effect. We fitted each model to data from 866 individuals (499 males and 367 females).
Females as the Primary Initiators of Conflict in Banded Mongooses.
For every IGI observed between groups in our study population between January 2000 and March 2019, we assigned each group in the interacting pair as either the focal or the rival group. In cases where one group in the interacting pair was being observed at the time the IGI took place, this group was assigned as the focal and the other group as the rival. In cases where both interacting groups were being observed, or when it was not known which of the two groups was being observed, the focal and rival were assigned randomly. For each IGI where we knew the group estrus state of the focal and rival group (n = 539 IGIs), we assigned whether the encounter occurred when focal and rival females in the interacting pair were simultaneously in one of four estrus states: both focal and rival females in group estrus; focal females in group estrus but rival females not in group estrus; focal females not in group estrus but rival females in group estrus; both focal and rival females not in group estrus. We summed the number of IGIs occurring between each unique focal−rival pair when in each of these four estrus states, and we also calculated the total number of days that females in the pair were simultaneously in each of these four group estrus states.
Using the “lme4” package (65), we fitted the number of IGIs between each focal−rival pair as the response variable in a generalized linear mixed model with a Poisson error structure and a log-link function. We included the group estrus state of rival and focal females as the main term of interest, and the log(number of days in each estrus state) as an offset term as an additional fixed effect to account for differences in the opportunity of focal−rival pairs to interact during each group estrus state. We fitted the focal group identity, and the rival group identity as random effects, as well as an observation level random effect to correct overdispersion of our response variable (69). To test the effect of group estrus state on the number of IGIs between each focal−rival pair, we compared the likelihood ratio of the model with and without this fixed effect (65). To determine differences in the number of IGIs observed in each group estrus state, we conducted a post hoc multiple comparison of means using the “glht” function with Tukey’s all-pairwise comparisons in the “multcomp” package (70). We fitted the model to data on 19 unique groups comprising 61 unique focal−rival pairs.
Intergroup Mortality Rates in Other Species.
To assess the intensity of lethal intergroup conflict in banded mongooses, we compared our data to that from groups of wild chimpanzees (P. troglodytes), independent subsistence human societies (hunter-gatherers and farmers), and meerkats (S. suricatta). Annual intergroup mortality data from chimpanzees and humans were taken from Wrangham et al. (54). Intergroup mortality rates for meerkats were calculated using data from the Kalahari Meerkat Project, a long-term behavioral study of a population of wild meerkats in the Kalahari, South Africa (55). Individual deaths attributable to intergroup conflict between 1997 and 2016 were recorded and mortality rates for adults calculated in the same way as for banded mongooses (n = 2,440 adults; 1,386 males and 1,054 females; individual exposures: 1,168 male meerkat-years and 964 female meerkat-years). In meerkat groups, the dominant female is typically unrelated to her within-group mate, and therefore has no incentive to lead the group on dangerous forays into neighboring territories in pursuit of outbreeding opportunities (55). Meerkat females exert disproportionate control over movement between sleeping burrows (58), but use this leadership ability to avoid rather than pursue contact with rival groups. During foraging trips, control of group movement appears to be shared among group members irrespective of dominance or sex (71). Thus females appear to have no incentive to engage in exploitative leadership in meerkats, and show no inclination to incite IGIs. Finally, while deaths from intergroup fights are extremely rare in meerkats, they are equally likely for adult males and females.
Supplementary Material
Acknowledgments
We are grateful to Uganda Wildlife Authority and Uganda National Council for Science and Technology for permission to carry out our research, and the Wardens of Queen Elizabeth National Park for logistical support. We are grateful to Tim Clutton-Brock for granting us access to long-term data from the Kalahari Meerkat Project, and Christopher Duncan for assistance with the meerkat data. We thank Tim Clutton-Brock, Darren Croft, Tom Currie, Patrick Green, Jenny York, and three anonymous referees for comments on the manuscript, and Sarah Leclaire, David Mech, and Craig Packer for additional information on their study species. For field data collection, we thank the Banded Mongoose Research Project field team: Francis Mwanguhya, Solomon Kyabulima, Kenneth Mwesige, Robert Businge, and Solomon Ahabyona. We are grateful to Harry Marshall and Emma Vitikainen for curation and maintenance of the long-term data, and Tim Clutton-Brock, Jason Gilchrist, Sarah Hodge, Matthew Bell, Corsin Müller, Neil Jordan, Bonnie Metherell, Roman Furrer, David Jansen, Jenni Sanderson, and Beth Preston for contributions to the project. For generating the genetic pedigree we thank Hazel Nichols, Jenni Sanderson, David Wells, and Joe Hoffman. The research was funded by a grant from the Natural Environment Research Council of the United Kingdom to M.A.C., F.J.T., and R.A.J. (Grant NE/S000046/1) and a European Research Council (ERC) grant to M.A.C. (Grant 309249). D.C. was supported by ERC Grants 294494 and 725185.
Footnotes
The authors declare no competing interest.
See online for related content such as Commentaries.
This article is a PNAS Direct Submission. R.W.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003745117/-/DCSupplemental.
Data Availability.
Behavioral and life history history data have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.13102586).
References
- 1.Silk J. B., House B. R., Evolutionary foundations of human prosocial sentiments. Proc. Natl. Acad. Sci. U.S.A. 108 (suppl. 2), 10910–10917 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkart J. M., et al. , The evolutionary origin of human hyper-cooperation. Nat. Commun. 5, 4747 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Tomasello M., Vaish A., Origins of human cooperation and morality. Annu. Rev. Psychol. 64, 231–255 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Waltz K. N., Man, the State, and War: A Theoretical Analysis (Columbia University Press, 2001). [Google Scholar]
- 5.Keeley L. H., War Before Civilization (Oxford University Press, 1997). [Google Scholar]
- 6.Gat A., War in Human Civilization (Oxford University Press, 2008). [Google Scholar]
- 7.Allen M. W., Bettinger R. L., Codding B. F., Jones T. L., Schwitalla A. W., Resource scarcity drives lethal aggression among prehistoric hunter-gatherers in central California. Proc. Natl. Acad. Sci. U.S.A. 113, 12120–12125 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirazón Lahr M., et al. , Inter-group violence among early Holocene hunter-gatherers of West Turkana, Kenya. Nature 529, 394–398 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Otterbein K. F., The Evolution of War: A Cross-Cultural Study (HRAF Press, 1970). [Google Scholar]
- 10.Fearon J. D., Rationalist explanations for war. Int. Organ. 49, 379–414 (1995). [Google Scholar]
- 11.Jackson M. O., Morelli M., “The reasons for wars: An updated survey” in The Handbook on the Political Economy of War, Coyne C. J., Mathers R. L., Eds. (Edward Elgar, 2011), pp. 34−57. [Google Scholar]
- 12.Gavrilets S., Fortunato L., A solution to the collective action problem in between-group conflict with within-group inequality. Nat. Commun. 5, 3526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rusch H., Gavrilets S., The logic of animal intergroup conflict: A review. J. Econ. Behav. Organ. 178, 1014–1030 (2020). [Google Scholar]
- 14.Lehmann L., Feldman M. W., War and the evolution of belligerence and bravery. Proc. Biol. Sci. 275, 2877–2885 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glowacki L., Wrangham R., Warfare and reproductive success in a tribal population. Proc. Natl. Acad. Sci. U.S.A. 112, 348–353 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Micheletti A. J., Ruxton G. D., Gardner A., Intrafamily and intragenomic conflicts in human warfare. Proc. Biol. Sci. 284, 20162699 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doğan G., Glowacki L., Rusch H., Spoils division rules shape aggression between natural groups. Nat. Hum. Behav. 2, 322–326 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Maynard Smith J., Evolution and the Theory of Games (Cambridge University Press, 1982). [Google Scholar]
- 19.Smith J. E., et al. , Leadership in mammalian societies: Emergence, distribution, power, and payoff. Trends Ecol. Evol. 31, 54–66 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Arseneau-Robar T. J. M., et al. , Female monkeys use both the carrot and the stick to promote male participation in intergroup fights. Proc. Roy. Soc. B Biol. Sci. 283, 20161817 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathew S., Boyd R., Punishment sustains large-scale cooperation in prestate warfare. Proc. Natl. Acad. Sci. U.S.A. 108, 11375–11380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kingma S. A., Santema P., Taborsky M., Komdeur J., Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476–484 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Sääksvuori L., Intergroup conflict, ostracism, and the evolution of cooperation under free migration. Behav. Ecol. Sociobiol. 68, 1311–1319 (2014). [Google Scholar]
- 24.Turney-High H. H., Primitive War: Its Practices and Concepts (University of South Carolina Press, 1991). [Google Scholar]
- 25.Wrangham R. W., Wilson M. L., Collective violence: Comparisons between youths and chimpanzees. Ann. N. Y. Acad. Sci. 1036, 233–256 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Jackson M. O., Morelli M., Political bias and war. Am. Econ. Rev. 97, 1353–1373 (2007). [Google Scholar]
- 27.Glowacki L., Wrangham R. W., The role of rewards in motivating participation in simple warfare. Hum. Nat. 24, 444–460 (2013). [DOI] [PubMed] [Google Scholar]
- 28.King A. J., Johnson D. D., Van Vugt M., The origins and evolution of leadership. Curr. Biol. 19, R911–R916 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Cant M. A., Vitikainen E., Nichols H. J., “Demography and social evolution of banded mongooses” in Advances in the Study of Behavior, Brockmann H. J., et al., Eds. (Elsevier, 2013), vol. 45, pp. 407–445. [Google Scholar]
- 30.Rood J. P., Banded mongoose rescues pack member from eagle. Anim. Behav. 31, 1261–1262 (1983). [Google Scholar]
- 31.Thompson F. J., et al. , Reproductive competition triggers mass eviction in cooperative banded mongooses. Proc. Biol. Sci. 283, 20152607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cant M. A., Otali E., Mwanguhya F., Eviction and dispersal in co‐operatively breeding banded mongooses (Mungos mungo). J. Zool. 254, 155–162 (2001). [Google Scholar]
- 33.Kingma S. A., Komdeur J., Hammers M., Richardson D. S., The cost of prospecting for dispersal opportunities in a social bird. Biol. Lett. 12, 20160316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young A. J., Monfort S. L., Stress and the costs of extra-territorial movement in a social carnivore. Biol. Lett. 5, 439–441 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasa O. A. E., The costs and effectiveness of vigilance behaviour in the dwarf mongoose: Implications for fitness and optimal group size. Ethol. Ecol. Evol. 1, 265–282 (1989). [Google Scholar]
- 36.Boydston E. E., Morelli T. L., Holekamp K. E., Sex differences in territorial behavior exhibited by the spotted hyena (Hyaenidae, Crocuta crocuta). Ethology 107, 369–385 (2001). [Google Scholar]
- 37.Thompson F. J., Marshall H. H., Vitikainen E. I., Cant M. A., Causes and consequences of intergroup conflict in cooperative banded mongooses. Anim. Behav. 126, 31–40 (2017). [Google Scholar]
- 38.Cant M. A., Otali E., Mwanguhya F., Fighting and mating between groups in a cooperatively breeding mammal, the banded mongoose. Ethology 108, 541–555 (2002). [Google Scholar]
- 39.Nichols H. J., Cant M. A., Sanderson J. L., Adjustment of costly extra-group paternity according to inbreeding risk in a cooperative mammal. Behav. Ecol. 26, 1486–1494 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furrer R. D., Kunc H. P., Manser M. B., Variable initiators of group departure in a cooperative breeder: The influence of sex, age, state and foraging success. Anim. Behav. 84, 205–212 (2012). [Google Scholar]
- 41.Cant M. A., Social control of reproduction in banded mongooses. Anim. Behav. 59, 147–158 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Lazaro-Perea C., Intergroup interactions in wild common marmosets, Callithrix jacchus: Territorial defence and assessment of neighbours. Anim. Behav. 62, 11–21 (2001). [Google Scholar]
- 43.Rosenbaum S., Vecellio V., Stoinski T., Observations of severe and lethal coalitionary attacks in wild mountain gorillas. Sci. Rep. 6, 37018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan N. R., et al. , Dynamics of direct inter-pack encounters in endangered African wild dogs. Behav. Ecol. Sociobiol. 71, 115 (2017). [Google Scholar]
- 45.Wrangham R. W., Evolution of coalitionary killing. Am. J. Phys. Anthropol. 110, 1–30 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Mech L. D., Productivity, mortality, and population trends of wolves in northeastern Minnesota. J. Mammal. 58, 559–574 (1977). [Google Scholar]
- 47.Mech L. D., The Wolves of Denali (University of Minnesota Press, 2003). [Google Scholar]
- 48.Pusey A. E., Packer C., The evolution of sex-biased dispersal in lions. Behaviour 101, 275–310 (1987). [Google Scholar]
- 49.Boesch C., et al. , Intergroup conflicts among chimpanzees in Taï National Park: Lethal violence and the female perspective. Am. J. Primatol. 70, 519–532 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Wilson M. L., et al. , Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature 513, 414–417 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Williams J. M., et al. , Causes of death in the Kasekela chimpanzees of Gombe National Park, Tanzania. Am. J. Primatol. 70, 766–777 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Bowles S., Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science 324, 1293–1298 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Gurven M., Kaplan H., Longevity among hunter‐gatherers: A cross‐cultural examination. Popul. Dev. Rev. 33, 321–365 (2007). [Google Scholar]
- 54.Wrangham R. W., Wilson M. L., Muller M. N., Comparative rates of violence in chimpanzees and humans. Primates 47, 14–26 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Clutton-Brock T., Manser M., “Meerkats: Cooperative breeding in the Kalahari” in Cooperative Breeding in Vertebrates, Koenig W. D., Dickinson J. L., Eds. (Cambridge University Press, 2016), pp. 294–317. [Google Scholar]
- 56.Leclaire S., Nielsen J. F., Sharp S. P., Clutton-Brock T. H., Mating strategies in dominant meerkats: Evidence for extra-pair paternity in relation to genetic relatedness between pair mates. J. Evol. Biol. 26, 1499–1507 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Nichols H. J., Cant M. A., Hoffman J. I., Sanderson J. L., Evidence for frequent incest in a cooperatively breeding mammal. Biol. Lett. 10, 20140898 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strandburg-Peshkin A., Clutton-Brock T., Manser M. B., Burrow usage patterns and decision-making in meerkat groups. Behav. Ecol. 31, 292–302 (2019). [Google Scholar]
- 59.Wrangham R. W., Glowacki L., Intergroup aggression in chimpanzees and war in nomadic hunter-gatherers: Evaluating the chimpanzee model. Hum. Nat. 23, 5–29 (2012). [DOI] [PubMed] [Google Scholar]
- 60.Turchin P., Currie T. E., Turner E. A., Gavrilets S., War, space, and the evolution of Old World complex societies. Proc. Natl. Acad. Sci. U.S.A. 110, 16384–16389 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glowacki L., Wilson M. L., Wrangham R. W., The evolutionary anthropology of war. J. Econ. Behav. Organ. 178, 963–982 (2020). [Google Scholar]
- 62.Cant M. A., Nichols H. J., Thompson F. J., Vitikainen E., Banded Mongooses: Demography, Life History, and Social Behavior, Koenig W. D., Dickinson J. L., Eds. (Cambridge University Press, Cambridge, United Kingdom, 2016), pp. 318–337. [Google Scholar]
- 63.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2013). [Google Scholar]
- 64.Venables W., Ripley B., Modern Applied Statistics (Springer, New York, NY, ed. 4, 2002). [Google Scholar]
- 65.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015). [Google Scholar]
- 66.Sanderson J. L., Wang J., Vitikainen E. I. K., Cant M. A., Nichols H. J., Banded mongooses avoid inbreeding when mating with members of the same natal group. Mol. Ecol. 24, 3738–3751 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells D. A., Cant M. A., Nichols H. J., Hoffman J. I., A high-quality pedigree and genetic markers both reveal inbreeding depression for quality but not survival in a cooperative mammal. Mol. Ecol. 27, 2271–2288 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Brooks M. E., et al. , glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017). [Google Scholar]
- 69.Harrison X. A., Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2, e616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hothorn T., Bretz F., Westfall P., Simultaneous inference in general parametric models. Biom. J. 50, 346–363 (2008). [DOI] [PubMed] [Google Scholar]
- 71.Gall G. E., Strandburg-Peshkin A., Clutton-Brock T., Manser M. B., As dusk falls: Collective decisions about the return to sleeping sites in meerkats. Anim. Behav. 132, 91–99 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Behavioral and life history history data have been deposited in Figshare (https://doi.org/10.6084/m9.figshare.13102586).