Abstract
There is growing recognition that the gut microbiome is an important regulator for neurological functions. This review provides a summary on the role of gut microbiota in various neurological disorders including neurotoxicity induced by environmental stressors such as drugs, environmental contaminants, and dietary factors. We propose that the gut microbiome remotely senses and regulates CNS signaling through the following mechanisms: 1) intestinal bacteria-mediated biotransformation of neurotoxicants that alters the neuro-reactivity of the parent compounds; 2) altered production of neuro-reactive microbial metabolites following exposure to certain environmental stressors; 3) bi-directional communication within the gut-brain axis to alter the intestinal barrier integrity; and 4) regulation of mucosal immune function. Distinct microbial metabolites may enter systemic circulation and epigenetically reprogram the expression of host genes in the CNS, regulating neuroinflammation, cell survival, or cell death. We will also review the current tools for the study of the gut-brain axis and provide some suggestions to move this field forward in the future.
I. Introduction
The gut-brain axis is increasingly recognized as an important target for the health of the central nervous system (CNS) (Carabotti et al., 2015; Sharon et al., 2016; Skonieczna-Zydecka et al., 2018; Zhu et al., 2017). As a multidirectional communication network, the key components of the gut-brain axis include the CNS, the autonomic nervous system (ANS), the enteric nervous system (ENS), as well as the hypothalamic pituitary adrenal axis (HPA) (Carabotti et al., 2015). The CNS is crucial for cognitive functions including memory, social, and emotional responses, and it communicates with ANS, ENS, and HPA to orchestrate signal transduction. The ENS regulates the production of intestinal hormones and mucus secretion. In addition, the ENS and enteric immune system interact to maintain gut integrity (Yoo and Mazmanian, 2017). The ENS can polarize macrophages and put them in close proximity to extrinsic and mucosal nerve fibers (Gabanyi et al., 2016). The bioavailability of catecholamines—a sympathetic neurotransmitters and immune modulator—can be modified by gut bacteria; catecholamines are glucuronidated for excretion, whereas some gut bacteria can de-glucuronidate catecholamines and possibly affect leukocytes in the gut (Asano et al., 2012; Yoo and Mazmanian, 2017). Gut permeability and integrity can also be regulated and monitored by the ENS through mechanoreceptors responsive to mucosal abrasions, intrinsic primary afferent neurons responsive to molecular and mechanical aberrations, and tension receptors responsive to stretch. Several compounds, including from bacteria such as short-chain fatty acids) activate receptors in neurons that regulate gut motility (Cherbut et al., 1998). The HPA modulates the release of cortisol from adrenal glands during stress response. In addition to host signaling, the gut microbiome, is important in the bidirectional communications through modulating gastrointestinal tract (GI) functions, remotely signaling to the brain and other metabolic organs, and is one of many regulatory targets of brain-to-gut signaling (Carabotti et al., 2015; Fu and Cui, 2017; Sharon et al., 2016; Skonieczna-Zydecka et al., 2018; Zhu et al., 2017).
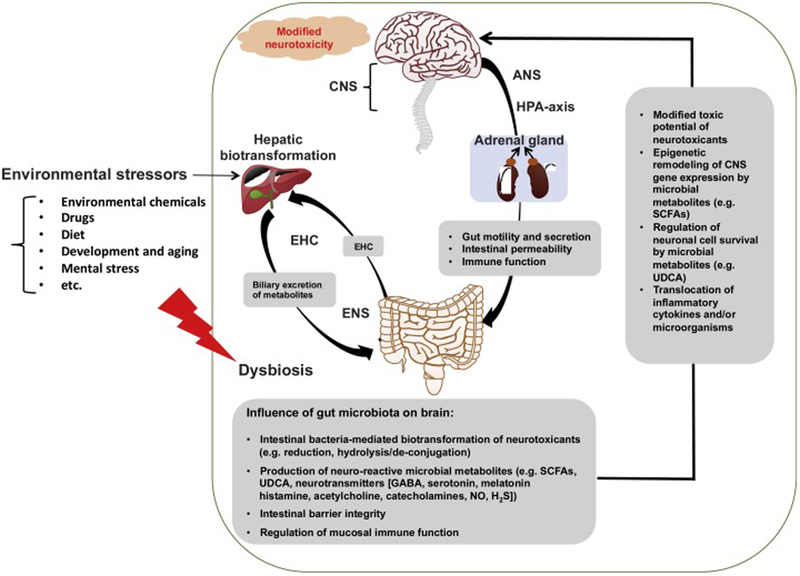
The human microbiome dwarfs the human genome with a 10:1 ratio of nucleated cells, and 100:1 ratio of genes (Sender et al., 2016). The microorganisms in the GI tract represent the majority of the human microbiome, including bacteria, fungi, parasites, and viruses (Zhu et al., 2017). This review focuses primarily on bacteria, as they are the best characterized members of the microbiome at this time. At the phylum level, the dominant intestinal bacteria are Firmicutes and Bacteroidetes in healthy human subjects, whereas other minor phyla include Proteobacteria, Actinomycetes, Verrucomicrobia, and Fusobacteria (Eckburg et al., 2005). As illustrated in Figure 1, following exposure to environmental stressors such as environmental contaminants, drugs, dietary factors, and other xenobiotics, the gut microbiome is thought to modify the toxicological outcomes through several mechanisms. 1) Gut microbiome can directly metabolize neurotoxicants primarily through reduction and hydrolysis/de-conjugation reactions (Claus et al., 2016; Lu et al., 2015); the gut microbiome may communicate with the liver through the enterohepatic circulation of primary and secondary metabolites, altering hepatic xenobiotic biotransformation and nutrient homeostasis, which are the major functions of the liver (Klaassen and Cui, 2015; Spanogiannopoulos et al., 2016; Swanson, 2015; Visschers et al., 2013). 2) Intestinal dysbiosis as a result of chemical exposure may lead to local inflammation and gut leakiness, subsequently increasing levels of pro-inflammatory cytokines in the systemic circulation, which may contribute to neuroinflammation (Fournier et al., 2018; Janakiraman and Krishnamoorthy, 2018; Lin et al., 2018; Rea et al., 2016; Sampson et al., 2016). 3) Gut microbiota may produce neuro-reactive microbial metabolites, including short-chain fatty acids (SCFAs), ursodeoxycholic acid (UDCA), as well as various neurotransmitters such as gamma-Aminobutyric acid (GABA), histamine, acetylcholine, serotonin, melatonin, gut lumen-derived bioreactive free catecholamines, nitric oxide, and hydrogen sulfide (Asano et al., 2012; Iyer et al., 2004; Schicho et al., 2006; Sobko et al., 2006). These microbial metabolites may enter the systemic circulation and reach the molecular targets in the brain to modulate various types of cognitive functions (Carabotti et al., 2015).
Figure 1.
An illustration of the gut-brain axis in environmental stressor induced neurotoxicity.
In this review, we will first discuss the role of the gut microbiome in various types of neurological diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD), autism spectrum disorder, attention-deficit hyperactivity disorder (ADHD), anorexia nervosa, depression, schizophrenia, bipolar disorders, as well as post-traumatic stress disorder (Table 1). We will then provide a comprehensive analysis of the literature regarding the interactions between various neurotoxicants and gut microbiome (Table 2). These neurotoxicants are selected based on research articles as well as the Casarett and Doull’s Toxicology Textbook (Klaassen CD 2013), including various drugs and environmental contaminants. While a substantial portion of the research body in this area still remains at the association level, several mechanistic studies have been performed taking advantage of novel research tools, including germ-free (GF) models, fecal/single microbial strain inoculations, anaerobic cultures, and microbial metabolite supplementations (Table 2). We will also discuss the challenges and opportunities characterizing the gut-brain axis in neurotoxicology.
Table 1.
Studies investigating neurological diseases in the gut-brain axis.
| Disease | Model | Bacteria influence | Source |
|---|---|---|---|
| ADHD | Human adolescents and adults | Association | (Aarts et al., 2017) |
| Human children | Association (biomarker) | (Jiang et al., 2018) | |
| Humans: juvenile males | Association (diversity; biomarker) | (Prehn-Kristensen et al., 2018) | |
| Humans: cesarean delivery or antibiotic use during first two years of life | No association | (Axelsson etal., 2019a) | |
| Alzheimer's disease | Mice | Association (diversity) | (Hoffman et al., 2017) |
| Artificial Aβ aggregation assays | Inverse association with SCFAs | (Ho et al., 2018) | |
| Anorexia nervosa | Human adult females | Association | (Borgo et al., 2017; Kleiman et al., 2015; Mack et al., 2016; Morita et al., 2015; Morkl et al., 2017) |
| Mice | Association | (Chen et al., 2016) | |
| Autism | Human children | No association | (Gondalia et al., 2012) |
| Human children | Association | (Coretti et al., 2018; De Angelis et al., 2013; Finegold et al., 2010; Hicks et al., 2018; Kang et al., 2013; Liu et al., 2019; Pulikkan et al., 2018; Qiao et al., 2018; Rose et al., 2018; Son et al., 2015; Wang, M. et al., 2019) | |
| Mice valproic acid-induced | Association | (de Theije et al., 2014) | |
| BTBR T+ltpr3tf/J mouse model of ASD | Association | (Coretti et al., 2017; Golubeva et al., 2017) | |
| Juvenile hamsters clindamycin and propionic acid induced | Mechanistic | (El-Ansary et al., 2018) | |
| Shank3-null mice | Mechanistic | (Tabouy et al., 2018) | |
| Rat valproic acid-induced | Association (biomarker) | (Liu et al., 2018) | |
| Autism and antibiotics | Human children in Manitoba, Canada or Denmark | No association | (Axelsson et al., 2019b; Hamad et al., 2018) |
| Autism and Vitamin A | Human children with autism | Association | (Liu, J. et al., 2017) |
| Behavior - generic germ-free | Germ-free mice | Association | (Arentsen et al., 2015; Lu et al., 2018; Neufeld et al., 2011; Sudo et al., 2004) |
| Germ-free mice | Mechanistic | (Diaz Heijtz et al., 2011) | |
| Germ-free, stress-sensitive rats | Association | (Crumeyrolle-Arias et al., 2014) | |
| Antibiotic-exposed mice | Association | (Desbonnet et al., 2015) | |
| Bipolar | Human adults | Association | (Evans et al., 2017; Painold et al., 2018; Vinberg et al., 2019) |
| Human adults | Mechanistic | (Painold et al., 2018) | |
| Depression | Mice | Mechanistic | (Sun, J. et al., 2018; Zheng et al., 2016)30521978 (Huang et al., 2019) |
| Rats | Association | (Tillmann et al., 2018; Yu et al., 2017) | |
| Rats | Mechanistic | (Abildgaard et al., 2017; Kelly et al., 2016) | |
| Human adults | Association | (Aizawa et al., 2016; Chen, J.J. et al., 2018; Chen, Z. et al., 2018; Jiang et al., 2015; Lurie et al., 2015; Naseribafrouei et al., 2014) | |
| Human adults | Mechanistic | (Kazemi et al., 2018; Miyaoka et al., 2018; Rudzki et al., 2019) | |
| Parkinson’s disease | Human adults | Association | (Hill-Burns et al., 2017; Unger et al., 2016) |
| Mice | Mechanistic | (Sampson et al., 2016) | |
| Postpartum depression | Adult women | Mechanistic | (Slykerman et al., 2017) |
| Pregnant women | Association | (Murphy et al., 2018) | |
| PTSD | Humans | Association | (Hemmings et al., 2017) |
| Male mice | Association | (Gautam et al., 2015; Gautam et al., 2018) | |
| Schizophrenia | Rats | Association | (Dunphy-Doherty et al., 2018; Pyndt Jorgensen et al., 2015) |
| Humans | Association | (Castro-Nallar et al., 2015; Flowers et al., 2019; Nagamine et al., 2018; Nguyen et al., 2019; Olde Loohuis et al., 2018; Shen et al., 2018) | |
| Humans | Mechanistic | (Okubo et al., 2019) | |
| Mice | Mechanistic | (Zheng et al., 2019) |
Table 2.
Studies investigating neurotoxicants and microbiome.
| Category | Toxicant | Model | Dose or exposure | End point and bacteria result | Source |
|---|---|---|---|---|---|
| Air pollution | PM2.5 | Mouse (8–12 weeks old) | 135.4 ± 6.4 mg/m3 8 hours for 5 days/week for 3 weeks | Increased genera, as well as alpha and beta diversity | (Mutlu et al., 2018) |
| PM10 | Mouse (6–8 weeks old) | 18 μg/g/day (oral gavage) | Altered short chain fatty acid concentrations and microbial composition | (Kish et al., 2013) | |
| Dietary and herbal medicine | Fructooligosaccharides | Rat (adults given 100 mg/kg/d d-galactose i.p. for inducing AD) | 100 mg/kg/d (oral gavage) | Maintains gut microbiota diversity while improving neurological endpoints | (Chen et al., 2017) |
| Ketogenic diet | Mouse (adult 6-Hz induced seizure model of refractory epilepsy) | 6:1 fat:protein ketogenic diet (ad libitum) | Increased hippocampal GABA/glutamate levels mechanistically by Akkermansia and Parabacteroides | (Olson et al., 2018) | |
| Drugs | Amiodarone | Rat (adult) | Probiotic: 1.5 × 109 CFU/dose once daily for 7 days Amiodarone: 50 mg/kg (single oral dose) |
E. coli Nissle 1917 increased the bioavailability whereas L. casei DN-114 001 delayed max plasma concentration | (Matuskova et al., 2017; Matuskova et al., 2014) |
| Chemotherapeutics | Bacteria (in vitro) | Variable—dose-response relatationship (screens to identify bacteria susceptible to chemotherapeutic s) | Lactic acid bacteria and bifidobacteria may be susceptible whereas other bacteria may affect efficacy | (Florez et al., 2016; Lehouritis et al., 2015) | |
| Cisplatin | Mouse (8–12 weeks) | Cisplatin: 10mg/kg Probiotic: healthy donor fecal pellet or 2×108 Ruminococcus gnavus | Probiotic ameliorated intestinal toxicity and systemic inflammation of cisplatin | (Perales-Puchalt et al., 2018) | |
| Disulfiram | Bacteria (in vitro) | Variable (disulfiram and metabolites) | Antimicrobial activity against gram-positive bacteria | (Frazier et al., 2019; Long, 2017; Sheppard et al., 2018) | |
| Doxorubicin | Mouse (8–10-week-old females) | 20 mg/kg by i.p. | Enteric injury (crypt depth, crypt number, and proliferative cell number) is dependent on presence of bacteria | (Rigby et al., 2016) | |
| Bacteria (in vitro) | 150 μg/mL | Deglycosylation of doxorubicin reduces toxicity | (Yan et al., 2018) | ||
| Mouse (7–10-week-old females) | 10 mg/kg by i.p. | Pre-treatment with pectin (1.5 mg per day orally) protected against ileitis independent of SCFAs | (Sahasrabudhe et al., 2018) | ||
| Lithium, valproate and aripiprazole | Rat (adult) | Variable | Increased microbial species richness and diversity; some antimicrobial activity | (Cussotto et al., 2018) | |
| Metronidazole | Rat (adult males) | 1 mg/ml in drinking water for 1 week | Increased in Bifidobacterium spp. and Enterobacteriaceae and increased mucosal thickness | (Pelissier et al., 2010) | |
| Mouse (8-week-old males) | 30 mg/kg by i.p. | Decreased Firmicutes and order Clostridiales; increased Proteobacteria, Turicibacterales and Enterobacteriales; PD-like effects abrogated by FMT | (Sun, M.F. et al., 2018) | ||
| MPTP | Mouse (male and/or female adults, WT and metabotropic flutamate receptors knockout) | 18 mg/kg 2 times per week for 5 weeks 10 mg/kg for 5 days, rest 2 days, 20 mg/kg for 5 days by i.p. |
Altered microbiome composition; Gender- and genotype-specific results; associations between microbiome diversity and sensorimotor performance | (Lai et al., 2018; Torres et al., 2018) | |
| Nano-encapsulated doxorubicin and paclitaxel | Mouse (8-week-old males) | 12.93 μmol/kg | Altered microbial activity indicated by levels of hippurate and indoxyl sulfate | (Song et al., 2015) | |
| Nitrofurantoin | Human (adults) | 100 mg twice per day | Decreased Clostridium sp. and increased Faecalibacterium sp. | (Stewardson et al.,2015; Vervoort et al., 2015) | |
| Metals | Arsenic | Mouse (8-week-old males and females) | 100 ppb sodium arsenite for 13 weeks Up to 250 ppb Antibiotic-treated for 3 days then exposed up to 1 ppm arsenic in drinking water for 2 weeks (females only) |
Altered gene abundances for genes involved in carbohydrate metabolism, pyruvate fermentation, short-chain fatty acid synthesis, and starch utilization Arsenic eroded bacterial biofilms adjacent to the mucosa 1 ppm arsenic did not alter gut microbiome |
(Chi et al., 2016; Chi et al., 2017a; Chi et al., 2019; Dheer et al., 2015; Lu et al., 2014) |
| Mouse (7–13-week-old males and females) | 25 and 100 ppm sodium arsenate | Antibiotic-treated and GF mice accumulate more arsenic than controls; human fecal transplants protect mice lacking the arsenic detoxification enzyme from arsenic-induced mortality, but may depend on Faecalibacterium spp. | (Coryell et al., 2018) | ||
| Earthworm (Lumbricus rubellus) | Devon Great Consols (DGC) mine site | Slight trend for an association between worm microbiome diversity and arsenic contamination | (Pass et al., 2015) | ||
| Human (in vitro; SHIME) | Soil arsenic concentrations (varies) 100μg/L As, 600 μg/L As, 600 μg/L As and 0.1 mg/L Fe, 600 μg/L As and 0.3 mg/L Fe, and 600 μg/L and As+3 mg/L Fe |
Bioaccessibility in the Arsenic was 1.8–2.8 times more bioaccessible in the colon than in the small intestinal phase Iron decreased bioaccessibiility of arsenic with increased arsenic methylation; |
(Yin et al., 2015; Yu et al., 2016) | ||
| Human (in vitro; Caco-2 cells) | Soil arsenic samples ranging from 15.5 to 3225.6 mg/kg | Gut microbiota can directly release soil arsenic; arsenic in colon is digested more quickly than in soil | (Yin et al., 2017) | ||
| Human | Children: high group (218.8 μg/L in drinking water) and low group (1.7 μg/L in drinking water) Infants at 6 weeks of age Adult men and women |
Children: high arsenic group had increased Gammaproteobacteria class, Enterobacteriales order, and Enterobacteriaceae family Infants: 8 genera were enriched with higher arsenic exposure, whereas 15 genera were decreased; changes were associated with males, but not females Time-weighted urinary arsenic associated with order Campylobacterales and the genera Anaerostipes and Faecalibacterium | (Dong et al., 2017)(Hoen et al.,2018)(Wu et al., 2019) | ||
| Zebrafish (larva) | 10, 50, and 100 ppb for 20 days | Increased the genera Acinetobacter, Sediminibacterium, and Janthinobacterium; decreased the genera Bdellovibrio and Pseudomonas | (Dahan et al., 2018) | ||
| Copper | Broiler chicken (Start from Day 0 as chicks) | 8 or 187.5 mg/kg of Cu from Cu Sul or 187.5 mg/kg of Cu from TBCC | No affect on performance by Cu source or concentration | (Pang et al., 2009) | |
| Holstein-Friesian calves (Dairy cows; 7 month old males) | 3g/100 L of copper supplementation as cupric sulphate in drinking water for 75 days | Increased microbial alpha diversity in rumen; altered bacteria S24–7, Planctomycetaceae p-1088-a5 gut group, and Azospira spp. | (Biscarini et al., 2018) | ||
| Gold | Mouse (7–8-week-old males) | Up to 25 μg gold/kg bodyweight for 8 7 different types of gold nanoparticles for 8 days during and after 5-day dextran sodium sulfate exposure | 5 nm/Citrate and Au-5 nm/ polyvinylpyrrolidone attenuated colonic and systemic inflammation; gold nanoparticles decreased alpha diversity; | (Zhu, S. et al., 2018) | |
| Lead | Mouse | 8 week old females: 10 ppm for 13 weeks; 32 ppm to dams in drinking water 2 weeks prior to breeding and up to 40 weeks of age for pups 6-week-old males and females: 0.01, 0.03, or 0.1 mg/L Pbfor15 weeks |
Majority of bacteria decreased following lead exposure; Cultivable aeobes decreased and anaerobes increased; at highest exposure, decreased Firmicutes and increased Bacteroidetes and Proteobacteria |
(Gao et al., 2017c; Wu et al., 2016; Xia, J. et al.,2018a) | |
| Zebrafish (adult males) | 10 and 30 μg/L for 7 days | 52 microbes altered by 30 ug/L PB group; altered metabolites in pathways for glucose and lipid metabolism, amino acid metabolism, nucleotide metabolism | (Xia, J. et al., 2018b) | ||
| Carp (Cyprinus carpio; 105 days old) | 1 mg/L | Pb decreased the expressions of pro-inflammatory cytokines; Lactobacillus reuteri P16 decreased mortality, improved the growth performance, and abrogated changes in gene expression | (Giri et al., 2018) | ||
| Magnesium | Mouse (8-week-old males) | Standard diet with 500 mg Mg/kg food and magnesium deficient diet with 50 mg Mg/kg food for 6 weeks | Increased depressive-like behavior with magnesium deficiency; the gut microbiome was altered by magnesium deficiency and was positively correlated with hippocampal interleukin-6 | (Winther et al., 2015) | |
| Manganese | Mouse (8-week-old males and females) | 100 ppm in drinking water for 13 weeks | Altered tmicrobial tryptophan and phenylalanine biosynthesis pathways; bacteria of male mice had altered GABA/putrescine metabolism | (Chi et al., 2017b) | |
| Mercury | Mummichog (Fundulus heteroclitus) | Total mercury in food: 0.08, 24.4, or 131 μg/g dry weight for 15 days | Microbial Hg resistance gene mercuric reductase 8 times higher in fish at Hg contamination site | (Lloyd et al., 2016) | |
| Methylmercury | Human (36–39 weeks pregnant) | Hair: 57 ng total Hg/g hair; Stool: 150(2.1–810) ng total mercury /g stool; Cord blood: 0.23 (0.061–0.73) μg MeHg/L cord blood |
17 genera were correlated with mercury concentration in stool or hair | (Rothenberg et al., 2016) | |
| Fathead minnow and mouse | Minnow: 0.02, 0.72, 0.87, or 5.50 μg/g, mercury in food twice daily for 30 days Mice: 0.02 μg/g, 0.43 μg/g, or 4.39 ± 0.57 μg/g mercury in food twice daily for 30 days |
Minnow gut microbiome adapted to detoxify MeHg; Mouse midbrain L-glutamine, O-phosphatidylcholine, dopamine, tagatose, hydroquinone, L-ascorbic acid, inosine 5-monophosphate, and uracil decreased |
(Bridges et al., 2018) | ||
| Bacteria (in vitro; fresh human fecal samples in anaerobic chamber) | 10 ng/g monomethylmerc ury and 1 ng/g mercury for 0, 12, 24, 36, and 48 hours | Monomethylmercury concentration decreased under a balanced or protein rich diet, but not a carbohydrate rich diet | (Guo, G. et al., 2018) | ||
| Arsenic and zinc | Mouse (4-week-old females) | Zinc-adequate diet with 0, 50, or 500 ppb arsenic or zinc-deficient diet with 0, 50, or 500 ppb arsenic And drinking water with 0, 50, or 500 ppb sodium arsenite for 6 weeks | No interaction between arsenic exposure and zinc restriction; plasma zinc concentration was positively correlated with the genera Shewanella, Rheinheimera, and Bifidobacterium | (Gaulke et al., 2018) | |
| Arsenic, cadmium, cobalt, chromium, and nickel | Rat (adults) | 15, 22, or 31 mg/kg/day sodium arsenite; 35, 54, or 85 mg/kg/day cadmium chloride; 44, 62, or 88 mg/kg/day sodium dichromate, 27, 47, or 82 mg/kg/day cobalt chloride, or 177, 232, or 300 mg/kg/day nickel chloride for 5 days | 47 genera were affected by at least one metal exposure; nickel uniquely altered 25 genera; bacteria with higher iron importing genes were increased by arsenic and nickel | (Richardson et al., 2018) | |
| Lead and cadmium | Mouse (6-week-old females) | For 8 weeks: Cadmium: 20 or 100 ppm Lead: 100 or 500 ppm | Decreased Lachnospiraceae and increased Lactobacillaceae and Erysipelotrichaceae | (Breton et al., 2013) | |
| Mercury and copper | Mouse (8-week-old females) | 5 mg/kg copper, 2 mg/kg mercury, or 2.5 mg/kg copper and 1 mg/kg mercury | Copper decreased Rikenella spp., Jeotgailcoccus spp., and Staphylococcus spp.; mercury decreased Rikenella spp, Jeotgailcoccus spp, and Staphylococcus spp | (Ruan et al., 2018) | |
| Mercury, lead, arsenic, and cadmium | Humans (children and pregnant women) | Lead (μg/L): 22.6 or 47.1 Mercury (nmol/L): 8.8 and 9.5 Arsenic (nmol/L): 3.0 and 6.5 Cadmium (nmol/L): 1.1 and 1.2) | Lactobacillus rhamnosus GR-1 protected against mercury and arsenic blood levels in pregnant women; Increased blood lead levels was associated with increased Succinivibrionaceae and Gammaproteobacteria | (Bisanz et al., 2014) | |
| Lead and PCBs | Human (males and females age 60–84) | Lead geometric mean of 2.17 μg/dL; many PCBs; geometric mean range of individual PCBs in blood of 7.86–66.99 ng/g lipid | Increased PCB-146 and lead concentrations result in lower Digit Symbol Coding Test of the Weschler Adult Intelligence Scale | (Przybyla et al., 2017) | |
| Noise | Noise | Mouse (3-month-old male senescence-accelerated mouse prone 8) | <40 (background), 88, or 98 decibels for 30 days | Cognitive impairment and Aβ accumulation; changes similar to aged mice; decreased gut microbiota diversity; fecal transplant of noised-exposed mice induced epithelial integrity impairment and Aβ accumulation to unexposed mice | (Cui et al., 2018) |
| Environmental chemicals -PBDEs | BDE-47 and BDE-99 | Mouse (9-week-old male CV and GF) | 100 μmol/kg for 4 days | Lack of gut microbiome altered PBDE metabolite profiles; increased Akkermansia muciniphila and Allobaculum spp.; BDE-99 increased unconjugated bile acids | (Li et al., 2018; Li et al., 2017) |
| DE-71 | Zebrafish (male and female adult) | 5.0 ng/L for 7 days | Decreased Mycoplasma spp., Ruminiclostridium spp., unclassified Firmicutes sensu stricto spp., and Fusobacterium spp.; disrupted intestinal neural signaling, epithelial barrier integrity in males | (Chen, L. et al., 2018a) | |
| Environmental chemicals -PCBs | PCB-126 | Mouse (8-week-old male Ldlr−/−) | 1 μmol/kg at weeks 2 and 4 of a 12 week study | Decreased S24.7, Clostridiales, Bifidobacterium spp., Ruminococcus spp., Oscillospira spp., and Lactobacillus spp. and increased Akkermansia spp.; positive associations between Bifidobacterium spp. and GLP-1, as well as Akkermansia spp. and fasting blood glucose | (Petriello et al., 2018) |
| Multiple | Mouse | Adult females: Varied up to 50 mg/kg 11–13 month old males with voluntary exercise 5 weeks prior: 150 μmol/kg |
Parasutterella, Ruminococcus, Prevotellaceae_UCG-001, Alloprevotella and Parabacteroides were decreased by PCBs; exercise attenuated PCB-induced changes in gut microbiome | (Chi et al., 2018; Choi et al., 2013) | |
| Pesticide -Carbamates | Aldicarb | Mouse (8-week-old males) | 2 ppm for 13 weeks in drinking water | Increased genes involved in virulence, adhesion, bacteriocins, antioxidant defense, protein degradation, DNA repair | (Gao et al., 2018b) |
| Many | Bacteria from rumen of Holstein dairy cows (in vitro) | Concentration varied; screened for bacteria that degrade carbamates through esterase activity | 26 isolates had esterase activity and degraded at least 1 polyurethane and pesticide carbamate | (Ufarte et al., 2017) | |
| Pesticide -Neonicotinoid | Imidacloprid | Fruit fly (Drosophila melanogaster, adults and larva) | 10, 50, and 100 μM in food | Increased Acetobacter spp. and Lactobacillus spp.; survival decreased with co-exposure to bacterial infection or heat stress | (Daisley et al., 2017) |
| Honey bee (Apis mellifera; adults) | 500 μg/liter imidacloprid suspended in sterilized sucrose syrup for 3 days | Little or no impact on the gut microbiome of adult worker bees | (Raymann et al., 2018) | ||
| Pesticide -Organochlorine | p, p’-DDE and β-HCH | Mouse (adult males) | p, p’-DDE: 1 mg/kg body weight/day β-HCH: 10mg/kg body weight/day |
Both chemicals decreased the genera Parabacteroides, Prevotella, Bacteroides, Clostridium XlVa and Clostridium IV and increased Barnesiella, Alloprevotella, Oscillibacter, Lactobacillus, Parasutterella and Akkermansia | (Liu, Q. etal., 2017) |
| Pesticide -Organophosp hate | Azinphos-methyl | Human | 0.021 to 6.192 ng azinphos-methyl/g blood serum | Decreased Streptococcus, Micrococcineae, Gemella, Haemophilus, Halomonas, Actinomycineae, and Granulicatella | (Stanaway et al., 2017) |
| Chlorpyrifos | Rat and human (in vitro; SHIME) | SHIME: 1 mg/day for 30 days Rat dams and pups: 1 mg/kg/day from gestation till 60 days of age |
SHIME: increased Enterococcus spp. and Bacteroides spp.; decreased Lactobacillus spp. and Bifidobacterium spp. Rats: Decreased Lactobacillus spp. and Bifidobacterium spp. |
(Joly et al., 2013) | |
| Rat | Adult males exposed to 0.3 or 3 mg/kg/day for 9 weeks Dams exposed orally from gestation to weaning and pups exposed thereafter at 1 or 3.5 mg/kg/day Dams exposed orally from gestation to weaning and pups exposed thereafter at 1 or 5 mg/kg/day |
Sutterella spp. consistently enriched regardless of diet (normal or high-fat) In ileum, increased Enterococcus spp., Clostridium spp., Staphylococcus spp., and Bacteroides spp.; Decreased Bifidobacterium spp. in ileum and colon Inulin supplementation in drinking water abrogated chlorpyrifos-induced metabolic disorders in adults exposed in utero |
(Fang et al., 2018; Joly Condette et al., 2015; Reygner et al., 2016b) | ||
| Mouse (10-week-old male mice) | 1 mg/kg/day for 30 days | Increase lipopolysaccharide and diamine oxidase in the serum; increased Lactobacillaceae and decreased Bacteroidaceae | (Zhao et al., 2016) | ||
| Human (in vitro; SHIME) | 0.35 and 1 mg/mL working solution | inulin co-treatment partially reversed dysbiosis and inhibited pro-inflammatory signaling when effluent was applied to Caco-2/TC7 intestinal cells; Increased in Enterobacteriaceae, Bacteroides spp. and Clostridia | (Requile et al., 2018; Reygner et al., 2016a) | ||
| Diamondback moth larva (Plutella xylostella) | Cabbage leaves dipped in 50 g/L solution for 10 minutes | Larva resistant to insecticides had increased levels of Lactobacillales order; Isolated from P. xylostella, Enterococcus sp. increased, Serratia sp. decreased, and Enterobacter sp. had not effect on insecticide resistance | (Xia, X. et al., 2018; Xia et al., 2013) | ||
| Fruit fly (Drosophila melangaster) | 10 μM in food with or without Lactobacillus rhamnosus GG | L. rhamnosus GG prevented chlorpyrifos toxicity; abutbiotic-treated and GF flies live longer than CV flies; gut-derived Lactobacillus plantarum metabolizes chlorpyrifos to the oxon | (Daisley et al., 2018; Trinder et al., 2016) | ||
| Zebrafish (adult males) | 30, 100 and 300 μg/L for 21 days | Altered 25 microbial genera and increased malondialdehyde and decreased glutathione in the gut | (Wang, X. et al., 2019) | ||
| Diazinon | Mouse (8-week-old males and females) | 4 mg/L in drinking water for 13 weeks | Altered expression of 677 microbial genes; disrupted quorum sensing, and enriched motility, sporulation, and stress response genes; sex-specific changes in altered bacteria | (Gao et al., 2017a; Gao et al., 2017b) | |
| Fenitrothion | Mosquito (Anopheles albimanus) | Captured mosquitos were exposed to bottles containing 50 μg fenitothion and categorized as susceptible or resistant | Resistant mosquitos had a lower bacterial diversity but an enrichment for OP-degrading bacteria and enzymes | (Dada et al., 2018) | |
| Malathion | Mouse (8-week-old males) | 2 mg/L in drinking water (~0.6 mg/kg/day) for 13 weeks | Enrichment of genes encoding virulence, mobility, and cell wall; altered quorum sensing | (Gao et al., 2018a) | |
| Monocrotophos and other OPs | Mouse (8-week-old females) | 28 μg/kg/day in drinking water for 30 days | Mice given fecal microbiota of monocrotophos-exposed mice had significant blood glucose intolerance | (Velmurugan et al., 2017) | |
| Pesticide -Pyrethrin | Permethrin | Rat (90-day-old males and females) | 34 mg/ 4 mL/kg per day from 6 to 21 days of age | Decreased Provatella family, increased Bacteroides-Prevotella-Porphyromonas spp. and Bifidobacterium spp. | (Nasuti et al., 2016) |
| Mouse (adult males) | 200 mg/kg permethrin and 2 mg/kg pyridostigmine bromide for 3 days in 2 week | Butyrate exposure before treatment improves proinflammatory phenotype mediated by TLR4 | (Seth et al., 2018) | ||
| Pesticide -rotenoids | Rotenone | Rat (8-week-old) Mouse | 2.75 mg/kg 5 days a week for 4 weeks | Increased Bifidobacterium spp. in colon; changes in microbiota were consistent with PD patients | (Johnson et al., 2018) |
| Mouse | 15–17 week old males (after training and restrain stress): 10 mg/kg/day for 6 weeks 7-week-old males: 10 mg/kg/day for 4 weeks 8–9 week-old males: 30 mg/kg/day for 4 weeks |
Increased relative abundance of fecal Akkermansia Decreased Bifidobacterium spp. Gastrointestinal dysfunction and microbiome dysbiosis occurred before motor dysfunction; increased Lactobacillus spp. and decreased Desulfovibrio spp. associated with gastrointestinal dysfunction and motor dysfunction |
(Dodiya et al., 2018; Perez-Pardo et al., 2018; Yang et al., 2017) | ||
| Pesticide-Herbicides | Atrazine | Zebrafish (4 months) | 0.42 ± 0.02 μg/L for 7 days | Altered gut microbiota composition and function, but did not differ overall from control; decreased body weight and gonadosomatic index of females; induced intestinal inflammation in males | (Chen, L. et al., 2018b) |
| Tree frog tadpoles and adults (Osteopilus spetentionalis) | 178.2 ± 7.8 μg/L for 6 days | No effect on gut bacteria of tadpoles or adults | (Knutie et al., 2018) | ||
| Glyphosate | Bacteria (in vitro isolated from poultry) | 5.0, 2.40, 1.20, 0.60, 0.30, 0.15 and 0.075 mg/ml | Pathogenic bacteria are resistant to glyphosate whereas beneficial bacteria are susceptible | (Shehata et al., 2013) | |
| Enterococcus spp. (in vitro from cattle and horses) | Serial dilutions from 0.001 to 10 mg/mL | All tested Enterococcus spp. inhibit Clostridium botulinum; higher concentrations of glyphosate inhibited E. faecalis growth but not C. botulinum | (Kruger et al., 2013) | ||
| Rat | 1.75 mg/kg bw/day from gestational day 6 to postnatal day 125 0.1 ppb, 400 ppm and 5000 ppm in drinking water in adults for 673 days 2.5 mg/kg/day in adults for 2 weeks |
Increased Prevotella spp. and decreased Lactobacillus spp. Increased Bacteroidetes family S24–7 and a decreased Lactobacillaceae across all doses No observable short term effects; aromatic amino acids alleviate the antimicrobial effect of glyphosate |
(Lozano et al., 2018; Mao et al., 2018; Nielsen et al., 2018) | ||
| Cultural bacteria from green turtles (Chelonia mydas) | 0.00022, 0.00044, 0.056,0.1125, 1.8, and 3.6 g/L of glyphosate from Rodeo® for 24 hours | Reduced growth and decreased survival at ceoncentrations greater than 0.00022 | (Kittle et al., 2018) | ||
| Mouse (4-week-old males) | 250 or 500 mg/kg/day for 1 day, 6 weeks, or 12 weeks | Increase of anxiety and depression-like behaviors associated with decreased Firmicutes, Bacteroidetes Corynebacterium spp. and Lactobacillus spp. | (Aitbali et al., 2018) | ||
| Honey bee (Apis mellifera; larva and adults) | Larva: 0.8, 4, and 20 mg/L Adults: 5 and 10 mg/L |
development rate, but higher doses decreased survival; decrease in beta diversity of 20 mg/L group; increased Acidobacteria and Gemmatimonadaceae Adult: sensitivity was dependent on microbiome containing an insensitive 5-enolpyruvylshikimate-3-phosphate synthase gene; increased for opportunistic pathogen Serratia marcescens | (Dai et al., 2018; Motta et al., 2018) | ||
| Pesticide -Fungicide | Copper sulfate | Piglets (28-days-old) | Up to 175 mg/kg food for 2 weeks | May increase villi and crypt depth in duodenum; decreased Enterobacteriaceae and Streptococci spp. | (Hojberg et al., 2005) (Di Giancamillo et al., 2018) |
| Pesticide -Mixture | Boscalid, captan, chlorpyrifos, thiofanate, thiacloprid, and ziram | Mouse (16-week-old males and females) | Ziram <0.01 mg/kg food, chlorpyrifos 47 μg/kg food, thiacloprid 56 μg/kg food, boscalid 240 μg/kg food, thiofanate 205 μg/kg food, captan 165 μg/kg food for 52 weeks | In females, increased microbial associated metabolites 3-indoxyl sulphate and phenyl derivatives phenylacetylglycine and p-cresol glucuronide | (Lukowicz et al., 2018) |
| Coumaphos, tau-fluvalinate, and chlorothalonil | Honey bee (Apis mellifera) | Colonies treated with tau-fluvalinate and coumaphos given strips with ~10% of the active ingredient; Colonies treated with chlorothalonil at 10 μg/L in 30% sucrose; all treatments for 6 weeks | Pesiticde-dependent changes in microbiome and fungal communities; chlorothalonil increased genes for oxidative phosphorylation and decreased sugar and peptidase metabolism | (Kakumanu et al., 2016) | |
| Lambda-cyhalothrin, deltamethrin, chlorpyrifos ethyl, spinosad and lufenuron | Bacteria from insecticide-resistant fall armyworm (Spodoptera fruqiperda) | In vitro culturing with insecticide doses of 10, 20, 40, 80, and 160 ug/mL | 16 microbial strains were isolated and shown to be resistant against at least 1 insecticide | (Almeida et al., 2017) | |
| Plant and Animal Toxins | Bamboo (cyanide) | Giant panda (Ailuropoda melanoleuca) and red panda (Ailurus fulgens) | N/A | Metagenome enriched with cyanide degrading enzymes; high abundance of Pseudomonas | (Zhu, L. et al., 2018) |
| Domoic acid | Mollusks | N/A | Blue mussels (Mytilus edulis) and soft-shell clams (Mya arenaria) carry bacteria that can degrade domoic acid | (Stewart et al., 1998) | |
| Nicotine | Mouse (8-week-old males and females) | 60 mg/L for 13 weeks in drinking water | Microbiome of male mice enriched for oxidative stress response, DNA repair genes, and acetate synthesis | (Chi et al., 2017c) | |
| SCFA | propionic acid (PPA) | Human (in vitro; lymphoblasoid cell lines from male children with autism) | 0.1, 0.5 and 1 mM | Mitochondrial function increased with PPA; however high PPA and long exposure duration increased proton leaking | (Frye et al., 2016) |
| Solvents | Ethanol | Mouse (6–8-week-old females) | 5% for 10 days and 5 g/kg via oral gavage 9 hours prior to euthanizing | Neuroinflammation and increased SI cytokines were abbrogated in antibiotic-treated mice | (Lowe et al., 2018) |
| Mouse (8–10-week-old males) | 5% for 6 weeks and/or Lactobacillus rhamnosus GG 1×109 cfu daily | Increased Alcaligenes sp. and Corynebacterium sp.; Lactobacillus rhamnosus GG prevented hepatic injury | (Bull-Otterson et al., 2013) | ||
| Human (adults) | Group mean 177–188 g/day | Dorea spp. and Blautia spp. were increased in alcohol dependent subjects and correlated with intestinal permeability | (Leclercq et al., 2014) | ||
| Mouse (7-week-old females) | Up to 20% for 8 weeks | Altered genera in Lachnospiraceae family and decreased Alistipes spp.; decreased Clostridium spp. with saccharin co-consumption; decreased Adlercreutzia spp. was positively correlated with alcohol preference | (Labrecque et al., 2015; Wang et al.,2018;Xu et al., 2018) | ||
| Rat (adults) | 20% for 13 weeks (voluntary consumption) | Decreased microbiome diversity; decreased Lactobacillus spp. | (Kosnicki et al., 2018) | ||
| Formaldehyde | Mouse (6 weeks old) | 1 or 3 ng/mL intragastrically | Increased abundance of 13 genera and decreased 4 genera | (Guo, J. etal., 2018) | |
| Trichloroethylene | Mouse (gestational day 0 to postnatal day 154 or 259; females) | 0.05 or 500 μg/ml | High dose, 259 day exposure, decreased Bacteroides spp. and Lactobaccilus spp. and increased Bifidobactrium spp. and Enterobacteriaceae | (Khare et al., 2019) |
II. The gut-brain axis and neurological diseases
II-1. neurodevelopmental disorders.
II-1.1. ADHD.
Attention-deficit hyperactivity disorder (ADHD) is a common condition characterized by inattention, hyperactivity, and/or impulsivity that affects 5% of children and 2.5% of adults (Faraone et al., 2015). Factors that increase the risk of ADHD include age, sex (4:1 male to female) (Faraone and Glatt, 2010), socioeconomic status (low family income) (Larsson et al., 2014), genetic variants, as well as environmental factors such as parental behavior (Harold et al., 2013; Stevens et al., 2008), prenatal metrics, and some environmental contaminants including organophosphate pesticides, polychlorinated biphenyls (PCBs), and lead (Banerjee et al., 2007; Scassellati et al., 2012). Recent studies demonstrate that the composition and predicted functions of gut microbiome are altered in ADHD patients. In one study, a decrease in parent-reported ADHD symptoms was associated with an increase in the abundance of Faecalibacterium spp. with no alteration in the richness of the gut microbiota (Jiang et al., 2018). A second study of male juveniles found that relative to healthy controls, subjects with ADHD had lower species richness as well as an increase in Bacteroidaceae (Prehn-Kristensen et al., 2018). Young adult subjects with ADHD had increased Bifidobacterium spp. associated with a predicted increase in cyclohexadienyl dehydratase l, which is important for the generation of the dopamine and noradrenaline precursor phenylalanine (Aarts et al., 2017). Dysregulation of dopamine and noradrenaline is implicated in ADHD etiology; however, it is unclear whether bacteria can independently induce ADHD-like behavior.
II-1.2. Autism spectrum disorder.
Autism spectrum disorder (ASD) is a severe neurodevelopmental disorder characterized by persistent social and communicative deficits as well as repetitive and restrictive patterns of behaviors, interests, and activities. It is increasingly recognized that both environmental and genetic factors play important roles in the etiology of ASD (Hallmayer et al., 2011), including the gut microbiome and ASD in children (Coretti et al., 2018; De Angelis et al., 2013; Finegold et al., 2010; Hicks et al., 2018; Kang et al., 2013; Liu et al., 2019; Pulikkan et al., 2018; Qiao et al., 2018; Rose et al., 2018; Son et al., 2015; Wang, M. et al., 2019). Interestingly, there is a high prevalence of gastrointestinal disorders in children with ASD. Gastrointestinal-related symptoms in ASD were associated with a less diverse gut microbiome and decreased abundances of the genera Prevotella, Coprococcus, and an unclassified taxon in Veillonellaceae, which are carbohydrate-degrading and fermenting bacteria, suggesting that carbohydrate metabolism or SCFAs may be involved in ASD (Kang et al., 2013). In ASD patients, a positive correlation was identified between high levels of the SCFA butyrate and Faecalibacterium prausnitzii (Coretti et al., 2018). Male mice perinatally exposed to valproic acid to induce ASD-like behaviors also had increased caecum butyrate (de Theije et al., 2014). ASD children were found to have higher fecal abundances of Caloramator, Sarcina and Clostridium genera along with a decrease in Bifidobacterium, which is known to produce SCFAs (De Angelis et al., 2013). A similar study comparing children with ASD and neurotypical healthy controls found no differences in fecal microbiome diversity, but did identify a significant interaction between ASD and the Cyanobacteria/Chloroplast genus (Son et al., 2015). A comparison of Indian children with ASD relative to healthy controls identified ASD as a significant factor to explain the differences in gut phylotypes; Lactobacillus spp. was significantly associated with ASD using a meta-analysis of the Indian children cohort with a US cohort (Pulikkan et al., 2018). Behavioral tests and hazard models from studies investigating antibiotics or vitamin A and ASD do not support a causal relationship (Axelsson et al., 2019b; Hamad et al., 2018; Liu, J. et al., 2017). Other studies have found decreased alpha diversity and altered composition of the oral microbiome (Hicks et al., 2018; Qiao et al., 2018).
In 12 month old BTBR T + tf/J (BTBR) inbred mice, an established model of ASD for social interaction and behavior, 16 taxa were altered in a sex-specific manner relative to sex-matched C57BL/6 mice (Coretti et al., 2017). For female BTBR mice, this included an increase in Akkermansia spp. and a decrease in Oscillospira spp., whereas in male BTBR mice, there was an increase in Lactobacillus spp. and a decrease in Desulfovibrio spp. (Coretti et al., 2017). It was later shown that compared to C57BL/6J mice, adult male BTBR mice have decreased Bifidobacterium spp. and Blautia spp. associated with dysregulated bile acid and tryptophan metabolism related to ASD behavior (Golubeva et al., 2017). Male Syrian hamsters exposed to a neurotoxic dose of propionic acid exhibit ASD-like behaviors and glutamate excitotoxicity in the brain. A probiotic mixture of Bifidobacterium breve, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus rhamnosus, and Streptococcus thermophiles abrogated the ASD effects of high-dose propionic acid (El-Ansary et al., 2018). Deletion of the gene SHANK3 is associated with dysregulated neurodevelopment and autistic like behaviors (Durand et al., 2007). In Shank3 knockout mice, there was a decrease in the levels of Lactobacillus reuteri that correlated with decreased expression of gamma-aminobutyric acid (GABA) receptor subunits in the brain; supplementation of L. reuteri attenuated unsocial behavior in male Shank3 knockout mice and increased GABA receptor expression (Tabouy et al., 2018).
In summary, both ADHD and ASD patients have higher levels of Faecalibacterium, whereas Bifidobacterium is higher in ADHD but lower in ASD. Conflicting observations in the richness of gut microbiome were made in both diseases.
II-2. Neurodegenerative diseases
II-2.1. Alzheimer’s disease.
AD is a progressive degenerative neurological disorder that is among the most common causes of dementia worldwide. An estimated 5.5 million Americans are living with AD (Hebert et al., 2013). These include 5.3 million patients above 65 years of age (10% of all Americans older than 65) and 200,000 individuals below 65 (early-onset AD). More than 90% of AD cases are idiosyncratic and late-onset (after age 65).
Growing evidence supports a critical role of environmental factors interacting with genes of susceptibility to influence the initiation and progression of AD. There is an immense impetus to understand the interactions between host genes and the functional microbiome that may lead to the pathogenesis of AD (Bhattacharjee and Lukiw, 2013). In humans, gut microbiome from AD patients has decreased microbial diversity and is compositionally distinct from age- and gender-matched healthy controls, evidenced by a decrease in Firmicutes and Bifidobacterium spp., but an increase in Bacteroidetes, and such dysbiosis correlated with cerebrospinal fluid biomarkers of AD (Vogt et al., 2017). In the APPS1 transgenic AD mouse model (human transgenes for both amyloid beta precursor protein [APP] and presenilin 1 [PSEN1]), GF mice had a drastic reduction of cerebral amyloid β (Aβ) pathology, a hallmark for clinical AD, whereas colonizing these GF mice with microbiota from the conventional mice increased cerebral Aβ pathology (Harach et al., 2017). Similarly, life-long combinatorial antibiotics treatment of AD mice also reduced Aβ pathology (Minter et al., 2016). Interestingly, probiotic supplementation reduced cognitive decline, accumulation of Aβ aggregates, and plasma inflammatory cytokines (Bonfili et al., 2017). Acute perinatal antibiotic-treatment resulted in long-term alterations of gut microbiota, reduction in brain Aβ deposition, and inflammatory signaling in serum and brain of aged APPS1 mice, indicating that there is a critical time window early in life to target the microbiome and modulate late-onset of AD (Minter et al., 2017).
Microbial metabolites may modulate the gut-brain axis during the pathogenesis of AD. Fecal SCFAs were decreased in AD mice (Zhang et al., 2017). In an AD mouse model, administration of butyrate, a major microbial-derived SCFA, led to epigenetic reprogramming (by inhibiting histone deacetylation [HDAC]) and improved memory, even at an advanced stage of disease progression (Govindarajan et al., 2011). Inflammation is a critical contributor to the pathogenesis of AD (Akiyama, 1994; Ardura-Fabregat et al., 2017; Eikelenboom et al., 1994), and SCFAs also have anti-inflammatory effects through HDAC inhibition (Vinolo et al., 2011). These studies suggest that SCFAs are neuroprotective. In addition to SCFAs, the microbial-derived ursodeoxycholic acid (UDCA) has been shown to be neuroprotective in various in vitro and in vivo AD models by reducing apoptosis and promoting cell survival (Ramalho et al., 2008). UDCA treatment can reduce inflammation and increase intestinal Akkermansia muciniphila, which is a normal gut bacterial species responsible for SCFA production (Van den Bossche et al., 2017).
In summary, gut microbiome may contribute to the pathogenesis of AD through dysbiosis-induced pro-inflammatory signaling, whereas beneficial microbial metabolites and/or bacteria that produce these metabolites may serve as novel therapeutic modalities for AD.
II-2.2. Parkinson’s disease.
Parkinson’s disease (PD) affects an estimated 1 million people in the US and is the second most common neurodegenerative disease; however, less than 10% of cases are considered hereditary (Nalls et al., 2014). Symptoms of PD include motor deficits (tremors), muscle rigidity, bradykinesia, and impaired gait. PD is one of a group of neurodegenerative diseases called synucleinopathies characterized by an aggregation of α-synuclein (αSyn). Interestingly, there is a rostrocaudal gradient of αSyn in the ENS with higher expression in the upper gastrointestinal tract, and gastrointestinal symptoms often occur before motor symptoms in PD (Cersosimo et al., 2013). In a landmark study, mice overexpressing αSyn were protected from aggregation by antibiotic treatment, and exposing αSyn-overexpressing mice to microbiota of PD patients increased physical impairments compared to colonization by healthy donors (Sampson et al., 2016). In an effort to identify microbial metabolites that may antagonize PD development, Sampson et al. (2016) demonstrated that oral exposure to high concentrations of SCFAs (propionate, acetate, and butyrate) was sufficient to produce the motor deficits similar to PD in mice (Sampson et al., 2016). A human study, however, found decreased SCFAs in PD patients compared to healthy controls (Unger et al., 2016). Gut dybsiosis in PD patients included increased Proteus spp., Bilophila spp., and Roseburia spp. along with decreased Lachnospiraceae, Rikenellaceae, Peptostreptococcaceae, and Butyricicoccus spp. (Sampson et al., 2016). A cross-sectional study comparing PD patients to healthy controls found increased Ruminococcaceae to be associated with disease duration (Hill-Burns et al., 2017).
In summary, for AD and PD, fecal SFCAs were lower, suggesting that this class of microbial metabolites may be beneficial to prevent the disease onset. In mouse models, SCFA supplementation improved memory likely due to the HDAC inhibitor and anti-inflammation properties; however, conflicting results were observed in PD mouse models, because oral exposure to high concentrations of SCFAs actually produced motor deficit similar to PD.
II-3. Psychiatric disorders.
II-3.1. Depression.
Major depressive disorder (MDD) is a common, life-disrupting mental health disorder that is a leading cause of disability worldwide (Moussavi et al., 2007). Diagnosed by at least two weeks of low mood, MDD is accompanied by low self-esteem, loss of interest, and low energy. Environmental factors are associated with MDD, and several human studies have examined the association between gut microbiome and patients with MDD (Aizawa et al., 2016; Chen, Z. et al., 2018; Jiang et al., 2015; Lurie et al., 2015; Naseribafrouei et al., 2014). Of note, Oscilibacter spp. and Alistipes spp. showed a high association with MDD (Naseribafrouei et al., 2014). A second study also found increased Alistipes spp. and Enterobacteriaceae, as well as decreased Faecalibacterium spp. (Jiang et al., 2015). Bifidobacterium spp. and Lactobacillus spp. were decreased in another MDD cohort (Aizawa et al., 2016), and increased Actinobacteria and decreased Bacteroidetes were identified in female MDD patients (Chen, Z. et al., 2018). In the chronic variable stress rat model for depression, the genera Candidatus Arthromitus and Oscillibacter were increased and Marvinbryantia, Corynebacterium, Psychrobacter, Christensenella, Lactobacillus, Peptostreptococcaceae incertae sedis, Anaerovorax, Clostridiales incertae sedis and Coprococcus were decreased in depressed rats compared to control rats (Yu et al., 2017). The Flinders sensitive rat line—a strain used for depression studies—had decreased sample richness (alpha diversity) compared to controls with increased Proteobacteria and decreased Elusimicrobia and Saccharibacteria (Tillmann et al., 2018). Interestingly, transplantation of fecal microbiota from human MDD patients to microbiota-depleted rats or mice induced depression-like behaviors, such as anhedonia and anxiety (Kelly et al., 2016; Zheng et al., 2016). The transplantation of fecal microbiota from depressed patients to GF mice suggested that the gut microbiota could disrupt CAMP Responsive Element Binding Protein 1 (CREB) signaling by down-regulating calcium voltage-gated channel subunit alpha1 E (CACNA1E) and disrupting axonogenesis of the olfactory bulb (Huang et al., 2019). Clinical trials of patients with MDD and one of three probiotics improved cognitive performance. Treatment with Lactobacillus plantarum 299v decreased the concentration of kynurenine, suggesting that microbial tryptophan metabolism may be important for MDD’s etiology (Kazemi et al., 2018; Rudzki et al., 2019). In a male C57BL/6 mouse model for chronic unpredictable mild stress-induced (CUMS) depression, Clostridium butyricum treatment improved depressive behaviors, possibly because C. butyricum upregulated glucagon-like peptide-1 (GLP-1) in the intestine, activated GLP-1R in the brain and increased cerebral serotonin (Sun, J. et al., 2018). Overall, gut dysbiosis may contribute to MDD and supplementation of probiotics may alleviate the disorder.
II-3.2. Bipolar affective disorder and schizophrenia.
Bipolar affective disorder (BD) is a spectrum of psychiatric syndromes through a cyclical pattern of excitement and depressive behavior (Akiskal et al., 2000; Dacquino et al., 2015). BD is a major cause of global disability and premature mortality (Painold et al., 2018). Several human studies have investigated the association between the gut microbiome and BD. A relatively small cross-sectional comparison of fecal microbiome revealed a negative correlation between duration of BD and sample richness (Painold et al., 2018), and decreased microbiome richness was also observed in a monozygotic twin study comparing the risk of developing or having BD (Vinberg et al., 2019). The abundance of Faecalibacterium spp. was decreased in a small cohort of BD patients (Painold et al., 2018), and this was replicated in a larger cohort of BD patients that also showed a positive correlation between Faecalibacterium spp. abundance and better self-reported outcomes (Flowers et al., 2017). The use of atypical antipsychotics as a treatment for BD increased Lachnospiraceae and decreased Akkermansia spp. and Sutterela spp. (Flowers et al., 2017). Of clinical interest, patients diagnosed with BD and given the probiotic OMNi-BiOTiC® Stress Repair (Bifidobacterium bifidum W23, B. lactis W51, B. lactis W52, Lactobacillus acidophilus W22, L. casei W56, L. paracasei W20, L. plantarum W62, L. salivarius W24, Lactococcus lactis W19) had significant improvement in attention and psychomotor processing speed after taking the probiotic for 1 to 3 months (Painold et al., 2018).
Schizophrenia is a debilitating chronic mental health disorder characterized by delusional thoughts, disorganized behavior, and decreased participation in daily activities. Schizophrenia has increased mortality (Saha et al., 2007) and creates a heavy financial burden (Knapp et al., 2004). Although the etiology of schizophrenia is not well understood, risk factors include genetic inheritance (Schizophrenia Psychiatric Genome-Wide Association Study, 2011) and environmental interactions (Severance et al., 2015; van Os et al., 2010). Both microbial richness index (Chao) and diversity index (Shannon) were lower in patients with schizophrenia compared to healthy controls (Zheng et al., 2019). GF mice given human microbiome fecal transplants of people with schizophrenia exhibited schizophrenic-like behaviors and displayed similar glutamatergic hypofunction as other mouse schizophrenic models, which have decreased glutamate and increased glutamine and GABA (Zheng et al., 2019). A metagenomics analysis of the oropharyngeal microbiome comparing 16 schizophrenia patients with 16 controls demonstrated increased Lactobacillus spp. and Bifidobacterium spp., as well as an increase in the fungal phylum Ascomycota (Castro-Nallar et al., 2015). Of note, a single-arm study treating schizophrenia patients with the probiotic Bifidobacterium breve A-1 had improved anxiety and depression scores, and they reported fewer negative symptoms (Okubo et al., 2019). Bifidobacterium spp. was increased in the fecal microbiota of older schizophrenic subjects given 4G-β-D-galactosylsucrose as a prebiotic treatment for underweight patients (Nagamine et al., 2018).
A 16S rDNA gene sequencing study of the fecal microbiome found increased relative abundance of the genera Succinivibrio, Megasphaera, Collinsella, Clostridium, Klebsiella and Methanobrevibacter in schizophrenia patients compared to healthy controls, whereas the abundance of Blautia, Coprococcus, Roseburia was decreased (Shen et al., 2018). A second group found increased Anaerococcus whereas Haemophilus, Sutterella, and Clostridium were decreased; there was also an association between worsening depressive symptoms and the abundance of Bacteroides (Nguyen et al., 2019). Interestingly, metatranscriptomics of whole blood from healthy individuals compared to schizophrenia patients found increased microbial sample richness; increased diversity was inversely associated with CD8+ CD28− CD45RA−, indicating an association between the microbiome, immunity, and schizophrenia (Olde Loohuis et al., 2018). A schizophrenia model in rats using social isolation had increased Actinobacteria and decreased Clostridia compared to controls and also noted associations between gut microbiota and hippocampal interleukin (IL)-6 and IL-10 (Dunphy-Doherty et al., 2018).
In summary, among the 3 types of psychiatric disorders, decreased gut microbiome richness appeared to be a common feature. Faecalibacterium was decreased in both depression and BD; whereas Lactobacillus supplementation was beneficial to improve both depression and BD. Depression and schizophrenia appeared to have opposite microbial patterns: for example, depression has lower Bifidobacterium and Lactobacillus, whereas schizophrenia has higher levels of these bacteria.
II-4. Other neurological disorders associated with stress
II-4.1. Postpartum depression.
Postpartum depression is a clinically diagnosed form of MDD with onset during the peripartum period; several risk factors for postpartum depression include history of depression, pregnancy and birth complications, and psychoneuroimmune dysregulation (Association, 2013; Corwin et al., 2015; Dunn et al., 2015; Osborne and Monk, 2013). As many as 19.2% of women have a major depressive episode within the first three months postpartum (Gavin et al., 2005). Remarkably, antibiotic exposure was identified as an independent risk factor up to 2 months postpartum (Murphy et al., 2018). A cohort of 380 women in New Zealand were given the probiotic Lactobacillus rhamnosus HN001 or a placebo from 14–16 weeks gestation through 6 months postpartum; women given the probiotic reported lower depression and anxiety scores during the postpartum period (Slykerman et al., 2017). Dysbiosis may increase the risk of postpartum depression, whereas probiotic supplementation may decrease the risk of depressive episodes.
II-4.2. Post-traumatic stress disorder.
Post-traumatic stress disorder (PTSD) is a commonly occurring condition manifesting after exposure to trauma, such as war, sexual assault, and other distressing experiences, and has a high rate of psychiatric comorbidity (Kessler, 2000). PTSD can often occur for many years and is frequently associated with exposure to multiple traumas (Hemmings et al., 2017). In a mouse model of PTSD, in which adult male C57BL/6J mice were exposed to extreme aggression by SJL albino male mice, the microbial-influenced metabolites 3-phenylpropionate, hippurate, and phenylpropionylglycine were increased in PTSD mice (Gautam et al., 2015), indicating an altered gut microbiome. A follow-up study using the same PTSD aggressive mouse model showed an immediate and inconsistent dysregulation of microbiome composition, specifically for Akkermansia spp, Anaeroplasma spp., Lactobacillus spp., and Oscillospira spp. (Gautam et al., 2018). In a preliminary human study investigating the fecal microbiome in South African PTSD-affected individuals, there was no difference in the diversity of the microbiota, but there was a decrease in the total abundance of the phyla Actinobacteria, Lentisphaerae, and Verrucomicrobia associated with PTSD (Hemmings et al., 2017).
II-4.3. Anorexia nervosa.
Anorexia nervosa is an eating disorder characterized by severe weight loss (lack of appropriate weight gain or maintenance) often caused by the limitation of calories, fear of gaining weight, and denial of being underweight (Miller and Golden, 2010). Anorexia nervosa is more likely to affect females than males (Herpertz-Dahlmann, 2009). The fecal microbiota of 16 female patients showed increased sample richness at the time of admission compared to discharge (Kleiman et al., 2015). Relative to controls, samples from anorexic patients during admission were higher in the genera Anaerostipes and Faecalibacterium, but an undefined genus in the family Coribacteriales was lower (Kleiman et al., 2015). Another study identified decreased Clostridium coccoides, Clostridium leptum, and Bacteroides fragilis, as well as decreased SCFAs acetic acid and propionic acid, in the stool of female patients with anorexia nervosa compared to healthy controls (Morita et al., 2015). In another cohort of anorexia nervosa patients, propionate and butyrate were decreased, corresponding to increased Enterobacteriaceae and Methanobrevibacter smithii and decreased Roseburia spp., Ruminococcus spp., and Clostridium spp. (Borgo et al., 2017). Anorexia nervosa patients who gained weight had increased sample richness; the genera in Clostridium XI and Bacteroidetes were unique to anorexia nervosa patients before weight gain (Mack et al., 2016). In BALB/c mice, a chronic caloric restriction study was conducted to determine the effect on gut microbiota (Chen et al., 2016). Briefly, caloric intake was limited to prevent weight gain starting at 28 days of age and restored to ad libitum feeding after day 97 until day 120 when the tissues were collected. Interestingly, the changes in gut microbiome persisted even after lifting the caloric restriction. Collectively, it is unclear if the compositional changes mechanistically contribute to anorexia nervosa.
III. Gut microbiome mechanistically contributes to behavior changes – lessons learned from germ free (GF) research models.
Studies have established the association between intestinal dysbiosis and neurological disorders of various etiologies (Alam et al., 2017; Bourassa et al., 2016; Galland, 2014; Heiss and Olofsson, 2019; Kelly et al., 2017; McKay et al., 2017; Moos et al., 2016; Tremlett et al., 2017; Zhu et al., 2017), but the role of the gut microbiome in modulating various behavior changes requires mechanistic investigations using laboratory models such as GF rats and mice. GF rats had exacerbated neuroendocrine and behavior responses to acute stress, coinciding with alterations of the dopaminerigic turnover rate in the upper structures of the brain known to regulate stress response and anxiety-like behavior (Crumeyrolle-Arias et al., 2014). The GF rats also had higher serum corticosterone levels and elevated mRNA of corticotropin releasing factor but reduced mRNA of glucocorticoid receptor in hippocampus (Crumeyrolle-Arias et al., 2014). Similarly, GF mice had impaired cognitive behaviors in response to novel objects, corresponding to decreased expression of genes involved in brain-derived neurotrophic factor (BDNF) signaling in amygdala, which is a key region for the social brain network (Arentsen et al., 2015). In response to restraint stress, plasma levels of adrenocorticotropic hormone (ATCH) and corticosterone were also substantially higher in GF mice (Sudo et al., 2004). Conversely, under basal conditions (i.e. without an environmental stimulus), GF mice exhibited anxiolytic behavior accompanied by decreased mRNA expression of the N-methyl-D-aspartate receptor subunit NR2B in the central amygdala, as well as increased mRNA of BDNF but decreased mRNA of serotonin receptor 1A in the dentate granule layer of the hippocampus (Neufeld et al., 2011). There are also many morphological differences between specific pathogen-free (SPF) and GF mice. For example, GF mice had delayed brain maturation and organization, evidenced by lower volumes and fractional anisotropy in major gray and white matter areas, as well as lower levels of myelination in total brain and major white and grey matter structures at either 4 or 12-weeks of age, which demonstrate delayed brain maturation and organization (Lu et al., 2018). This coincided with lower mobility and higher anxiety of GF mice in an open field test, which is a photo beam tracking method to monitor the movement of animals with two sets of infrared beams, at 4-weeks of age (Lu et al., 2018). At 12-weeks of age, GF mice also had reduced spatial and learning memory in the Morris water maize test, which measures spatial learning for subjects that use distal cues to navigate from start locations around the perimeter of an open swimming field to locate a submerged escape platforms. At this age, GF mice also exhibited reduced contextual memory in contextual fear conditioning test, which measures persistent freezing behavior using a foot shock context arena and a conditioned stimulus, in order to quantify hippocampus-dependent learning and memory test (Lu et al., 2018). Last but not least, in a three-chamber social test, which assesses cognition in the form of interest in a never-before-met intruder (i.e. social novelty), GF mice had reduced social novelty at 12-week of age. Similar behavioral changes were also observed in antibiotic-treated SPF mice (Desbonnet et al., 2015).
While the absence of gut microbiota leads to marked behavior changes, microbial colonization of GF mice reprogrammed the postnatal development of the HPA system. For example, the heightened HPA stress response in GF mice was attenuated by inoculation with Bifidobacterium infantis, but was enhanced by the enteropathogen Escherichia coli (Sudo et al., 2004). There appears to be a critical time window for microbiome inoculation to affect behavior in GF mice as fecal transplants from SPF mouse donor to 6 week old but not 8 week old GF mice reduced elevated HPA response in GF mice (Sudo et al., 2004). Early-life inoculation using SPF feces reduced the expression of postsynaptic density protein 95 (PSD-95) and synaptophysin in the striatum, which are involved in the neuronal circuits for motor control and anxiety behavior (Diaz Heijtz et al., 2011). Together these studies demonstrate that the presence of the commensal gut microbiota has dual functions in modulating both cognitive functions under basal conditions as well as stress response.
IV. Microbiome and neurotoxicants
IV-1. Air pollution.
Recent epidemiological studies have established a positive correlation between exposure to ubiquitous traffic-related air pollution and the exacerbations of various neurological disorders such as Alzheimer’s disease and Parkinson’s disease (Babadjouni et al., 2017; Calderon-Garciduenas et al., 2016). Large populations living in highly polluted metropolitan regions are at higher risk for robust central nervous system pathology (Babadjouni et al., 2017). The primary cause of urban air pollution is through vehicular emissions. Exposure to fine particulate matter (PM2.5) and ozone above US EPA standards, have been linked to both Alzheimer’s and Parkinson’s diseases (Calderon-Garciduenas et al., 2016; Palacios, 2017; Shin et al., 2018). Air pollution positively associates with the hallmark clinical characteristics of neuroinflammation and CNS diseases, including increased expression of pro-inflammatory markers in brain, diffused amyloid plaques, neuronal cell loss, and impaired cognition (Block and Calderon-Garciduenas, 2009). It has been suggested that inhaled particulate matter can directly translocate to the brain and cause neuroinflammation, which in turn contributes to neuro-degeneration (Calderon-Garciduenas et al., 2016; Oberdorster et al., 2005). In addition, the novel contribution of the gut-brain axis in several CNS disorders suggests that gut-derived pro-inflammatory signaling accompanied with dys-regulated microbial or host-derived lipid profiles may contribute to neurotoxicity (Russo et al., 2018; Valles and Francino, 2018). In human subjects, exposure to freeway air pollution correlated with decreased Bacteroidaceae and increased Coriobacteriaceae as well as increased fasting glucose levels (Alderete et al., 2018). In mice, inhalation of PM air pollution altered the composition of the gut microbiome and was suggested to play a role in PM-induced GI inflammation (Beamish et al., 2011; Kish et al., 2013; Mutlu et al., 2018). Because intestinal bacteria contribute to both local and systemic inflammation, the latter may lead to neuroinflammation (Grigg and Sonnenberg, 2017; Reinoso Webb et al., 2016). Investigation of the interplay among air pollution, gut microbiome, and inflammatory signaling is an intriguing direction in research on mechanisms of neurotoxicity.
IV-2. Antibiotics and drugs.
The important role of gut microbiome in the biotransformation of various therapeutic drugs has been extensively reviewed (Bisanz et al., 2018; Carmody and Turnbaugh, 2014; Haiser and Turnbaugh, 2013; Spanogiannopoulos et al., 2016). Briefly, gut microbiome can either utilize their own enzymes to metabolize drugs into reactive or inactive metabolites or can secrete endogenous microbial metabolites into circulation to interact with host receptors in various organs. We will focus on studies on gut microbiome and the drug categories that have neurotoxic side effects as summarized in Table 2. These drugs are selected based on Chapter 16 of the Casarett & Doull’s Toxicology Textbook (Klaassen, 2013) as well as literature search regarding the involvement of the gut microbiome in the metabolism and/or toxicities of these chemicals.
Microbiome and compounds associated with neuropathies.
The anti-cancer drug doxorubicin is known to produce progressive ataxia in laboratory animal models through degeneration of dorsal root ganglion cells and axonal degeneration (Graham and Lantos, 1997; Spencer and Schaumburg, 2000). In the human gut microbiome, doxorubicin can be inactivated by Raoultella planticola via reductive deglycosylation (Yan et al., 2018), thus targeting this bacteria is promising to reduce its side effects including neurotoxicity. Intestinal bacteria contribute to doxorubicin-induced intestinal damage (Rigby et al., 2016), whereas the dietary fiber pectin, which is a substrate of microbial fermentation, can reduce doxorubicin-induced intestinal inflammation through direct interaction with host Toll-like receptors 1 and 2 (Sahasrabudhe et al., 2018). In addition, gut microbiota can affect the efficacy of doxorubicin and many other anticancer drugs (Florez et al., 2016; Lehouritis et al., 2015), whereas doxorubicin can alter the gut microbial activity (Song et al., 2015). It may be interesting to investigate whether the interactions between doxorubicin and gut microbiota may modify the CNS toxicity.
MPTP (1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an industrial chemical and contaminant of illicit narcotics that selectively destroys dopaminergic neurons of the nigrostriatal pathway, leading to Parkinsonian syndromes (Calne et al., 1985; Kopin, 1987). In mice, sub-chronic MPTP exposure affected the gut microbiome diversity, which was associated with sensorimotor performance and fear learning (Torres et al., 2018). Importantly, dysbiosis and GI dysfunction precedes motor dysfunction (Lai et al., 2018), whereas fecal microbiota transplantation from MPTP-exposed mice alone can induce motor impairment and striatal neurotransmitter decreased in normal mice (Sun, M.F. et al., 2018). Therefore, microbiota may mechanistically contribute to the pathogenesis of Parkinson’s disease.
Microbiome and compounds associated with axonopathies.
The antibiotic and antiprotozoal drug metronidazole is a drug that can produce peripheral neuropathy (Goolsby et al., 2018), leading to axonal degeneration, lesions of cerebellar nuclei, mostly affecting myelinated fibers (Graham and Lantos, 1997; Spencer and Schaumburg, 2000). Studies showed that metronidazole also altered gut microbiota in the colon of healthy rats, evidenced by increased Bifidobacteria spp. (especially Bifidobacterium pseudolongum) and Enterobacteria, and this associated with increased mucus layer thickness (Pelissier et al., 2010). While this effect has been suggested to benefit the treatment of inflammatory bowel diseases, potential systemic effects following changes in the microbiota, especially on the CNS, need further investigation.
The antibiotic nitrofurantoin produces peripheral neuropathy via axonal degeneration (Spencer and Schaumburg, 2000). In human subjects with urinary tract infections, nitrofurantoin treatment correlated with a reduced relative abundance of the genus Clostridium and an increased relative abundance of the genus Facalibacterium (Stewardson et al., 2015), whereas another clinical study showed that nitrofurantoin treatment resulted in a temporary increase in Bifidobacterium (Vervoort et al., 2015). Overall, it is generally considered that this drug only has a mild effect on the gut microbiota community, although the potential changes in microbial functions locally and in distal organs need additional investigations.
The anticancer drug cisplatin produces peripheral neuropathy evidenced by axonal degeneration and microtubule accumulation in early stages (Graham and Lantos, 1997; Spencer and Schaumburg, 2000). In mice, cisplatin treatment disrupts the gut microbiome as well as the mucosal integrity, and this can be partially corrected by Ruminococcus gnavus, which is one of the bacteria that is depleted by cisplatin, or by fecal gavage (Perales-Puchalt et al., 2018).
Microbiome and compounds associated with myelinopathies.
Amiodarone is an anti-arrhythmic drug that is known to produce myelinopathy (Graham and Lantos, 1997; Spencer and Schaumburg, 2000) as well as peripheral neuropathy following long-term high-dose therapy (Fraser et al., 1985). In rats, oral administration of Lactobacillus casei DN-114 001 slowed the absorption of amiodarone without altering the pharmacokinetics of its main metabolite (Matuskova et al., 2017); however, oral administration of Escherichia coli Nissel 1917 (EcN) increased the oral bioavailability of the parent compound as well as the P450-mediated metabolism (Matuskova et al., 2014). These studies at the single-strain resolution have suggested that targeting distinct microbiota can alter the pharmacokinetics and potentially the toxicity of amiodarone.
Disulfiram, which treats chronic alcoholism by inhibiting aldehyde dehydrogenase, produces peripheral neuropathy and swelling in distal axons (Graham and Lantos, 1997). Interestingly, disulfiram was repurposed as an antibiotic for multi-drug resistant Staphylococcus aureus infections, and it can increase the vancomycin susceptibility of three clinical vancomycin-resistant S. aureus strains (Long, 2017). The disulfiram metabolite diethyldithiocarbamate also has antibacterial activity towards Bacillus anthracis (Frazier et al., 2019). In addition, disulfiram-based disulfide derivatives have been shown to exhibit antibacterial activity against gram-positive Staphylococcus, Streptococcus, Enterococcus, Bacillus, and Listeria spp. (Sheppard et al., 2018). It remains unknown whether the antibacterial effect of disulfiram can affect the CNS.
Psychotropic drugs.
Several psychotropic drugs, including fluoxetine and escitalopram, have antimicrobial effects in vitro (Cussotto et al., 2018). Alternatively, lithium, valproate, and aripiprazole increased the richness and diversity of gut microbiome in human gut. In addition, escitalopram, venlafaxine, fluoxetine and aripiprazole have also been shown to increase the permeability in the ileum. Because it is known that microbiota plays an important role in regulating the gut-brain interactions, it has been suggested that the intestinal effect of the psychotropics may contribute to the mechanism of action and side effects of these medications (Cussotto et al., 2018).
IV-4. Metals and metalloids
Arsenic.
Arsenic is a carcinogenic metalloid that is widely distributed in nature in its trivalent (arsenic trioxide and sodium arsenite) and pentoxide forms (sodium arsenate, arsenic pentoxide, and arsenic acid). Other organoarsenicals are also common. Occupational exposure to arsenic compounds comes in the form of pesticides, herbicides, and other agricultural products. Environmental arsenic is in drinking water with sources in the United States less than 5 μg/L (Environmental Protection Agency maximum contaminant limit is 10 μg/L), however it is estimated that many people in Bangladesh drink water in excess of 50 μg/L (Klaassen, 2013). Acute exposure to arsenic has many symptoms, including fever, anorexia, hepatomegaly, melanosis, cardiac arrhythmia, and terminal cardiac failure, as well as a delay in sensory loss in the peripheral nervous system (Wallerian degeneration of axons) (Klaassen, 2013). Chronic arsenic exposure can cause diffuse or spotted hyperpigmentation or hypopigmentation, as well as liver injury. Interestingly, peripheral neuropathy also occurs with the development of numbness or “pins and needles” in the hands and feet, caused by dying-back axonopathy with demyelination.
From environmental observations, there was a non-significant trend in microbiome composition dependent on arsenic contamination observed in soil and earthworm microbiome samples around an arsenic mine, with about 47 taxa (about 7% of the abundance) driving the differences (Pass et al., 2015). Zebrafish exposed to varying low concentrations of arsenic (10, 50, and 100 ppb for 20 days) found 43 amplicon sequence variants that increased in abundance and 43 that decreased at the genus level (Dahan et al., 2018).
The Simulator of the Human Microbial Intestinal Ecosystem (SHIME) system is a dynamic series of six compartments and pumps that are used to mimic the intestines by controlling the pH, relative volume of fluid, and residence time, as well as the inoculation of the human microbiome (Joly et al., 2013; Requile et al., 2018). Regarding arsenic toxification and detoxification via methylation by gut microbiome, a study using the human SHIME model showed that arsenite (As[II]) is more readily metabolized than arsenate (As[V]), 66.5–92% to 22.1–38.2% respectively (Yin et al., 2015), and gut microbiota can also release arsenic from the soil in solid phase for absorption (Yin et al., 2017). Another SHIME model demonstrated that co-administration of drinking water with ferric iron and arsenic increased arsenic methylation and decreased absorption in the colon. Effluents from iron-exposed SHIME colons decreased toxicity in the human hepatoma cell line HepG2 cells (Yu et al., 2016). Bacteroides spp., Clostridium spp., Alistipes spp., and Bilophila spp. had resistance to and ability to methylate arsenic (Yu et al., 2016).
Several mouse studies have looked at the effect of arsenic exposure on the microbiome using 16S rDNA gene sequencing data. One of the first arsenic microbiome studies exposed C57BL/6 mice to 10 ppm arsenic in drinking water for 4 weeks and showed clear microbiome compositional differences between control and exposed mice with 2 microbial classes increased and 7 classes decreased (Lu et al., 2014). Another study showed that microbiome compositional and functional changes could be sex-specific (Chi et al., 2016), and a follow-up study exposing mice to 100 ppb arsenic for 13 weeks showed altered abundance of microbial genes involved in carbohydrate metabolism, especially pyruvate fermentation, short-chain fatty acid synthesis, and starch utilization, as well as stress response genes (Chi et al., 2017a). However, mice exposed to arsenite (10 or 250 ppb) did not have altered expression of arsenate reductase (arsA) or the arsenite exporter (arsB), thus bacteria with genes protective against arsenic may not be the bacteria that were increased during arsenic exposure (Dheer et al., 2015).
Importantly, Coryell et al. (2018) showed that GF mice and antibiotic-treated conventional mice exposed to arsenate (25 and 100 ppm) excreted less arsenic in stool and accumulated more arsenic in organics relative to control mice. GF arsenite methyltransferase (As3mt)-knockout mice are hypersensitive to arsenic-induced mortality but are protected following human fecal microbiome transplants, likely in part due to protective properties of Faecalibacterium prausnitzii (Coryell et al., 2018). Similarly, antibiotic-treated mice exposed to arsenite (III; 250 ppb and 1 ppm) had increased total urinary arsenic, but decreased fecal arsenic than conventionally raised mice, indicating that a depleted microbiome could absorb more arsenic (Chi et al., 2019). This evidence suggests that gut microbiota may limit the arsenic absorption, but no conclusions are definitive between arsenic-induced microbiome neurotoxicity. Additionally, many of the studies used exposures greater than the described human exposures (50 ppb or 50 μg/L in the population from Bangladesh); therefore, translational conclusions regarding arsenic exposure and microbiome in humans should remain cautious.
Copper.
Copper is a trace element found in all tissues and is required for a plethora of molecular events including cellular respiration, peptide amidation, neurotransmitter biosynthesis, pigment formation, and connective tissue strength. Copper is present throughout the brain with many enzymes in the CNS being copper-dependent for proper function. Both a deficiency and an excess of copper due to genetics or environmental factors can lead to several neurological conditions, including aceruloplasminemia, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, Menkes disease, occipital horn syndrome, Parkinson’s disease, prion disease, and Wilson disease (Desai and Kaler, 2008). Copper ions are naturally antimicrobial, but very few papers have investigated the antimicrobial properties of copper on the gut microbiome. Culturing of chicken ileal microbiome exposed to copper increased Lactobacillus spp. while decreasing Escherichia coli, but the effect of copper on the in vivo microbiome was negligible (Pang et al., 2009). Supplementation of copper to dairy calves for 75 days increased the richness of the rumen microbiome and decreased the lipopolysaccharide biosynthetic pathway (Biscarini et al., 2018). It appears that copper supplementation, not necessarily excess copper, may alter the microbiome composition, but it is unclear if this may play a role in copper neurotoxicity.
Gold.
Elemental gold is considered nontoxic and is used as an aesthetic enhancement in food and drinks. However, gold in its ionic form can have neurotoxic effects. For example, used as an anti-inflammatory treatment for rheumatoid arthritis, gold sodium thiomalate decreases the number of unmyelinated axons, suggesting gold could slow signal response to the peripheral nervous system (Levine et al., 1986; Salzer and Zalc, 2016). A study investigating gold nanoparticle protection against colitis also identified a gut dysbiosis in mice following 8 days of exposure with decreased species richness as well as decreased Lactobacillus spp. and other SCFA-producing bacteria (Kase et al., 1987). Further research is needed to understand the effect of ionic gold on the gut microbiome and neurotoxicity.
Lead.
Lead is a non-essential, heavy metal toxicant that can cause severe neurotoxicity with the EPA dictating the action level at 15 ppb. Adults can be exposed to lead through contamination of drinking water via lead pipes or by its natural occurrence in the environment, as well as in occupational settings such as smelting and soldering. Children can also be exposed through mouthing or consuming objects other than food, such as dust and lead contaminated paint. Children more readily absorb lead compared to adults with limited protection by the developing blood-brain barrier (Perlstein and Attala, 1966). Shown in Figure 2A, lead concentrations in mussels on the coastal regions of the US are highest on the East coast between Washington and Boston with large areas of exposure around the Lake Erie region in northern Ohio, Chicago, and along the West coast near San Francisco and Seattle (see Methods for GIS data analysis). Concentrations of lead in bivalves ranged from 0.172 to 73.6 μg/dry g. Large acute exposures result in severe cerebral edema, likely due to damaged endothelial cells, with children more likely to develop lead encephalopathy than adults (Johnston and Goldstein, 1998). Although not well understood, chronic lead exposure results in peripheral neuropathy and can also include gastritis, abdominal pain, anemia, and deposits of lead in gums and long bones.
Figure 2.
Collection sites and concentrations of BDE-47, BDE-99, PCB-95, PCB-153, lead, and manganese in bivalves collected from the coastal United States. (A) Green circles indicate BDE-47 sampling sites while purple circles correspond to BDE-99 sampling sites in bivalves with size corresponding to concentration (ng/dry g). (B) Green circles indicate PCB-95 sampling sites while purple circles correspond to PCB-153 sampling sites in bivalves with size corresponding to concentration (ng/dry g). (C) Green circles indicate lead sampling sites while purple circles correspond to manganese sampling sites in bivalves with size corresponding to concentration (μg/dry g).
Animal studies showed that lead exposure (acute, chronic, and perinatal) alters the composition of the gut microbiome, which may contribute to metabolic disorders. For instance, perinatal lead exposure in drinking water (32 ppm; 2 weeks before mating to postnatal day 21) increased the adult body weight of male mouse offspring, but not female offspring, and this was highly associated with microbiome compositional change, suggesting that lead-exposed gut microbiome may play a role in sex-specific differences (Wu et al., 2016). Adult female C57BL/6J mice exposed to lead (10 ppm) for 13 weeks decreased the abundance of the genera Blautia, Coprococcus, two species of Ruminococcus, and 1 genus in each of the families Clostridiales and Lachnospiraceae, as well as increased abundance of a genus in the Clostridiaceae family (Gao et al., 2017c). Lead exposure also reduced microbial metabolites in the vitamin E and bile acid pathways as well as altering the gene abundance for nitrogen metabolism and energy metabolism (Gao et al., 2017c). Dysregulated energy metabolism and altered microbiome composition were also found in 5-week-old mice exposed up to 0.1 mg/L (0.1 ppm) of lead for 15 weeks; a dose-dependent decrease in the phylum Bacteroides and an increase in Firmicutes was observed (Xia, J. et al., 2018a). Adult male zebrafish exposed to 0.03 mg/L (0.03 ppm) lead for 7 days had an increase in Firmicutes, but a decrease in Proteobacteria; in total 30 genera were altered by acute lead exposure (Lai et al., 2018). Many of the microbiome-lead exposure studies identified changes in gut microbiome, however the exposures are greater than the EPA action limit of 15 ppb and no studies related the changes in microbiome to neurotoxic effects in the host. More studies are needed to identify if gut microbiome is a contributing factor to lead-induced neurotoxicity.
Magnesium.
Magnesium is an important cofactor that helps regulate diverse chemical reactions in the body, including cardiovascular, alimentary, endocrine, and osteoarticular systems, as well as brain biochemistry by influencing neurotransmission pathways associated with depression (Rajizadeh et al., 2017; Serefko et al., 2016). Mice exposed to a magnesium-deficient diet for 6 weeks had increased depressive-like behaviors and a gut microbiome composition correlated with magnesium deficiency (Winther et al., 2015). However, it is unclear if the compositional changes in gut microbiome contribute to the observed depressive-like behaviors.
Manganese.
Manganese is an essential trace element needed for many enzymatic reactions, such as arginase and glutamine synthatase. Excessive consumption of manganese can lead to Parkinson’s disease-like symptoms, including dysfunction of the basal ganglia (Dobson et al., 2004). In the United States, the highest concentration of manganese in bivalves is in northern Ohio and northwestern New York around the Great Lakes region (Lake Erie and Lake Ontario; Figure 2A). Adult mice exposed to neurotoxic concentrations of manganese for 13 weeks induced a gut dysbiosis for 12 genera in female mice and 16 genera in male mice. Furthermore, there was an increase in microbial tryptophan synthesis pathways in females, but in males, there was a general decrease in GABA/Putrescine metabolism (Chi et al., 2017b). Again, it is not known if the changes in gut microbiome contribute to manganese toxicity.
Mercury and methylmercury.
Elemental mercury (Hg) is a heavy, silvery liquid metal that is used in products such as thermometers, barometers, float valves, and fluorescent lamps. Exposure to elemental mercury may cause emotional disturbances, tremors, and fatigue, but there is limited evidence of toxicity in humans (Klaassen, 2013). The National Institute for Occupational Safety and Health (NIOSH) limits occupational exposures to 0.05 mg/m3 (about 6 ppb) over a 10-hour workday and the EPA limits mercury to 2 ppb in drinking water. In the context of the microbiome, one study examined the development of Hg and antibiotic resistance in bacteria of the estuarine fish mummichog (Fundulus heteroclitus) upon exposure to Hg in the diet for 15 days. The microbiome of fish exposed to mercury developed resistance to at least 3 antibiotics compared to control fish; furthermore, there was an 8-fold increase in the mercury resistance gene mercuric reductase at a mercury contamination site (Lloyd et al., 2016). This indicates that mercury exposure induced the co-selection of mercury and antibiotic resistance. An epidemiological study of pregnant women and children found that consumption of probiotic yogurt was protective against increased blood levels of mercury and arsenic in pregnant women but not children (Bisanz et al., 2014). Interestingly, a combinational exposure of copper and mercury in female Kunming mice decreased the abundance of the genera Sporosarcina, Jeotgailcoccus, and Staphylococcus, but increased the genus Anaeroplasma (Ruan et al., 2018). It is unclear how the shift would affect neurotoxicity, but this demonstrates the need to consider mixtures when assessing risk.
Methylmercury (organic mercury) is highly toxic, causing neuronal degeneration, ataxia, paresthesia, and psychomotor retardation in adults (Klaassen, 2013). In children, exposure to methylmercury can cause developmental disabilities, retardation, and cognitive deficits, which is attributed to an immature blood-brain barrier and higher disposition of mercury in the developing brain. In an epidemiological study, methylmercury concentration in the stool or total mercury in hair of pregnant women (36–39 weeks gestation) was correlated with 17 bacterial genera, but there was no detection of the mercury methylation gene or the methylmercury detoxification genes (Rothenberg et al., 2016). The inability to identify known mercury biotransformation genes in the gut microbiome of pregnant women could be attributed to small sample size (n=6) or there could be exciting new alternative pathways for microbial mercury metabolism. One group cultured the microbiome of two human subjects to investigate methylmercury metabolism rate in relation to nutrients; there was a dependency of diet on demethylation of methylmercury by an increase in protein content, but the responsible genes and mechanism could not be identified (Guo, G. et al., 2018). Changes in gut microbiome following dietary exposure to methylmercury is further supported by a study in fathead minnows which showed significant compositional differences between methylmercury exposed and unexposed groups with 18 genera altered by exposure (Bridges et al., 2018). They found an increase in bacteria that generally have genes for xenobiotic metabolism and metal removal, including Deltaproteobacteria FAC87, Xanthomonadaceae, Comamonadaceae, Cloacibacterium spp., and Pirellula spp. (Bridges et al., 2018). Overall, more studies at human relative exposures are needed to conclude the effect of mercury and microbiome on host neurotoxicity.
IV-5. Persistent environmental toxicants.
PCBs.
Polychlorinated biphenyls (PCBs) were formerly used in industrial and consumer projects before they were banned in the United States in 1979. Due to their bio-accumulative nature, these persistent environmental contaminants are still of significant public health concern and are listed on the 2015 Agency for Toxic Substances and Disease Registry Substance Priority List (http://www.atsdr.cdc.gov/SPL/index.html). PCBs are frequently detected in fatty food (Gorchev and Jelinek, 1985; Newsome et al., 1998), air in public schools (Herrick, 2010; Herrick et al., 2016), industrial paint pigments (Anezaki and Nakano, 2014; Hu and Hornbuckle, 2010), as well as human samples including blood, adipose, milk, and placenta (Nakagawa et al., 1987; Suzuki et al., 2005; Wang et al., 2010). In humans, epidemiological studies showed a positive link between developmental PCB exposure and neurological deficits in infants and children (Korrick and Sagiv, 2008; Schantz, 1996; Winneke, 2011). Symptoms associated with PCB neurotoxicity include decreased motor and cognitive skills in newborns, greater incidence of behavioral problems and lower IQ scores in children, and lower cognitive functioning in older adults (Przybyla et al., 2017; Ribas-Fito et al., 2001; Schantz, 1996). Developmental neurotoxicity following PCB exposure has also been observed in laboratory animals including mouse and rat pups, as well as chicken embryos (Kania-Korwel et al., 2017; Roelens et al., 2005; Yang et al., 2009) and in neuronal cell cultures (Lyng and Seegal, 2008; Lyng et al., 2007). Suggested molecular mechanisms of PCB neurotoxicity include binding to aryl hydrocarbon receptor (for dioxin-like PCB congeners), decreasing dopamine content, GABAergic neuronal dysfunction, interference with calcium signaling, as well as altering the dendritic growth and plasticity by promoting the activity of ryanodine receptors (Lyng et al., 2007; Tilson and Kodavanti, 1998; Yang et al., 2009).
As shown in Figure 2B, Concentrations of PCB-95 in bivalves ranged from 0.12 to 153.88 ng/dry g while concentrations of PCB-153 ranged from 0.04 to 472.5 ng/dry g. Similar to PBDEs, concentrations of both PCB congeners tended to increase with increasing proximity to coastal cities with large populations, with overall higher concentrations of PCB-153 measured. One exception to this trend appears south of Miami, Florida where PCB-95 is present in higher concentrations than PCB-153.
Coinciding with PCB-associated neurodevelopmental disorders, there is strong evidence that intestinal dysbiosis also plays a role in the etiology of these diseases (Borre et al., 2014; Warner, 2019). In adult mice, oral exposure to an environmentally relevant PCB mixture (PCB-153, PCB-138, and PCB-180) was shown to decrease the levels of Proteobacteria correlating with the activity level of the mice, whereas exercise attenuated PCB-induced changes in gut microbiome (Choi et al., 2013). Dioxin-like PCB-126 increased both intestinal and systemic inflammation correlating with gut dysbiosis (Petriello et al., 2018). Gut dysbiosis may lead to altered microbial metabolites as well as increased pro-inflammatory cytokines in circulation. Oral exposure to PCBs increased pro-inflammatory mediators in brain and other metabolic organs (Chi et al., 2018; Sipka et al., 2008). Because systemic inflammation has been linked to neuroinflammation (Bendorius et al., 2018), it is possible that PCB-induced gut dysbiosis contributes to PCB-induced neurotoxicity partially through inflammation. In addition, there is a positive association between circulating glucagon-like peptide-1 (GLP-1) and Bifidobacterium spp. following PCB-126 exposure (Petriello et al., 2018) with GLP-1 protecting against neurotoxicity of various etiologies (Athauda and Foltynie, 2016; Chang et al., 2018; Khalilnezhad and Taskiran, 2018).
PBDEs.
Polybrominated diphenyl ethers (PBDEs) are a class of persistent environmental contaminants that were previously used as flame retardants in various consumer products. Although the commercial use of PBDEs has been recently banned in the United States, PBDEs are still ubiquitously found in the environment due to their resistance to degradation, bio-accumulation along the food chain, as well as the recycling of PBDE-containing products worldwide (Schecter et al., 2010; Schecter et al., 2003). PBDE levels in human specimens, such as blood, breast milk, and adipose tissue, have increased exponentially over the last 30 years, and are especially high in North America as compared to European and Asian countries (Costa et al., 2014; Costa and Giordano, 2007). Infants and toddlers are particularly vulnerable to PBDE-induced adverse effects due to exposure to PBDE-contaminated breast milk and dust (Costa et al., 2014). In contrast to many studies, a 2017 study in middle-aged and older Californian women showed a modest average annual percent increase in serum concentrations of 3 major PBDE congeners (BDE-47, BDE-100, and BDE-153) from 2011 to 2015 (Hurley et al., 2017). The Geographic Information System (GIS)-based distribution of 2 breast milk-enriched PBDE congeners (BDE-47 and BDE-99) in coastal areas of the United States is shown in Figure 2C. Concentrations of BDE-47 in bivalves ranged from 0.3 to 68.4 ng/dry g and concentrations of BDE-99 ranged from 0.2 to 38.4 ng/dry g. The concentrations of both congeners tended to increase with proximity to a major coastal city, and overall higher concentrations of BDE-47 measured. Higher brominated congeners are thought to debrominate into lower forms through photolytic degradation, which possibly explains this overall trend (Lagalante et al., 2011). BDE-47 was present in notably higher concentrations than BDE-99 near Houston, Texas, Biloxi, Mississippi, Mobile, Alabama, and Tampa, Florida.
PBDEs are present in 97% of adults in the United States as measured by the National Health and Nutrition Examination Survey (NHANES), with BDE-47 and BDE-99 present in the highest serum concentrations (Sjodin et al., 2008). Pregnant Mexican immigrant women living in California were found to have increasing levels of total PBDEs with each year of residence as measured by the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) cohort, with BDE-47 the most common congener found (Castorina et al., 2011). In fact, BDE-47 can reach higher levels in Americans than total PBDE levels in humans in other countries (Fromme et al., 2009). For 223 Mexican women living in California as measured through the CHAMACOS cohort, increasing total PBDE (including BDE-47 and BDE-99) serum concentrations were associated with lower chance of pregnancy (Harley et al., 2010).
PBDEs have been linked to developmental neurotoxicity. A 2018 review and meta-analysis of animal evidence showed that exposure to BDE-47, BDE-99, and BDE-209 affects learning (Dorman et al., 2018). In humans, large prospective cohorts showed that in utero exposure to PBDEs is associated with impaired executive function and poor control of attention in children and that prenatal and postnatal PBDE exposure adversely impact externalizing behavior (Vuong et al., 2018). However, the exact mechanisms of PBDE-induced neurotoxicity in humans is unknown. In mice and rats, perinatal PBDE exposure at environmentally relevant concentrations produced persistent changes in spontaneous motor activity, including hyperactivity and decreased habituation, as well as disruptions in learning and memory (Costa and Giordano, 2007). Proposed mechanisms of PBDE developmental neurotoxicity include reduction of circulating thyroid hormones, induction of apoptotic cell death of neurons and astrocytes, oxidative stress-induced damage, interference with calcium signaling and neurotransmitters, as well as biotransformation of PBDEs (Costa et al., 2014; Costa and Giordano, 2007; Giordano et al., 2008). In zebrafish, developmental neurotoxicity of DE-71 (a commercial PBDE mixture) decreased expression of genes involved in central nervous system development and decreased locomotion activity (Chen et al., 2012).
Investigation of the involvement of gut microbiome in PBDE-induced neurotoxicity is still at an early stage. We hypothesize that PBDE neurotoxicity may be regulated through the gut-liver axis. This hypothesis is based on 1) the evidence that PBDEs are primarily oxidized by hepatic cytochrome P450s (CYPs) into hydroxylated metabolites, which are considered more toxic than the parent compound (Dingemans et al., 2008), and 2) the observations that the hepatic P450 levels are profoundly modified by lack of gut microbiome in mice (Kuno et al., 2016; Selwyn et al., 2015; Toda et al., 2009). In primary adult neural stem/progenitor cells isolated from subventricular zone of mice, the hydroxylated BDE-47 metabolite 6-OH-BDE-47, but not its parent compound, inhibited adult neurogenesis demonstrated by decreased survival, proliferation, and neuronal differentiation associated with inhibition of ERK5 signaling (Li et al., 2013). We have reported that in BDE-47 orally exposed GF mice, there was an increase in 5-OH-BDE-47 but lower levels of 4 other BDE-47 hydroxylated metabolites in liver; whereas in BDE-99 orally exposed GF mice, there was a decrease in 4 minor BDE-99 hydroxylated metabolites in liver (Liu, Q. et al., 2017). Interestingly, the lack of gut microbiome potentiated PBDE-mediated up-regulation of Cyp1a2 and Cyp3a11 at both mRNA and protein levels in mouse liver (Liu, Q. et al., 2017). We also showed that in large intestinal content of adult male mice, PBDEs induced a dysbiosis, including an increase in Akkermansia muciniphila and Erysipelotrichaceae Allobaculum spp., positively associated with increased unconjugated bile acids in serum as well as increased DNA abundance in microbial 7α-dehydroxylation enzymes involved in secondary bile acid synthesis (Li et al., 2018). Although evidence in humans is limited, it has been shown in mice that deoxycholic acid (a microbial-derived secondary bile acid) may contribute to a neurological decline (McMillin and DeMorrow, 2016; McMillin et al., 2016). Microbial metabolic functions in the intestine have also been shown to be altered by DE-71 in zebrafish, associated with disruption of neural signaling, epithelial barrier integrity, inflammatory response, oxidative stress, and compromised detoxification potential in males (Vuong et al., 2018). Similar to the studies on heavy metal exposure, a mechanism linking microbiome and PBDE-induced neurotoxicity remains elusive.
Organochlorines.
Organochlorines are a class of pesticides that include dichlorodiphenyltrichloroethane (DDT) and hexachlorocyclohexane (HCH), which were banned in the 1970s and 1980s due to their reproductive (Salihovic et al., 2016), neurobehavioral (Saeedi Saravi and Dehpour, 2016), and immunological (Mrema et al., 2013) toxicities, as well as carcinogenicity (Freund et al., 2014). In addition, organochlorines are environmentally persistent (Jayaraj et al., 2016), and are still detected in Atlantic bluefin tuna (Thunnus thynnus), an indicator organism for the oceanic environment and therefore a potential risk to human health if consumed chronically (Maisano et al., 2016). Chronic exposure to the major metabolites of DDT, dichlorodiphenyldichloroethylene (p,p’-DDE) and β-hexachlorocyclohexane (β-HCH), in mice altered the composition of the gut microbiota and increased the presence of bacteria with microbial bile salt hydrolase, which is required for the production of secondary bile acids (Liu, Q. et al., 2017). However, it is unclear how the metabolites modified the gut microbiota and how this may contribute to organochlorine neurotoxicity.
IV-6. Pesticides
Carbamates.
Carbamates are derived from carbamic acid that kill insects by reversible inhibition of acetylcholinesterase (AChE). The inhibition of AChE prevents the degradation of the neurotransmitter acetylcholine causing over-stimulation of a nerve or muscle, resulting in exhaustion and tetany (Fukuto, 1990). Carbamate pesticide has increased along with organophosphorus compounds and some compounds can have toxicities for mammals and aquatic organisms.
In the rumen microbiome of cows, Ufarté et al. (2017) identified a bacterium named clone 44I12 that could metabolize the carbamate fenobucarb to a less toxic compound, but not two other carbamates tested, fenoxycarb and prosulfocarb (Ufarte et al., 2017). The carbamate-specific metabolism by microbes is similar to a study that isolated bacteria which could metabolize fenobucarb from rice paddy soils, but not other carbamates (Kim et al., 2014). However, neither of these studies associated the microbiome to neurological health. Consumption of aldicarb in drinking water of C57BL/6 mice for 13 weeks resulted in a differential abundance of 17 genera and also significantly disrupted the brain metabolism marked by a reduction in glucose and malic acid, whereas 3-hydroxybutyric acid was increased (Gao et al., 2018b). Although toxicity was not shown to be dependent on a gut dysbiosis, this study demonstrated a potential association between aldicarb, neurotoxicity, and gut microbiome.
Neonicotinoids.
Neonicotinoids are a popular and growing class of insecticide thought to selectively and irreversibly target the nicotinic acetylcholine receptors in the CNS of insects, making them generally safe for humans and animals (Han et al., 2018). Because of this selectivity, neonicotinoids also increase the mortality of pollinators, such as honey bees (Apis mellifera) (Ihara and Matsuda, 2018). For example, imidacloprid, the first neonicotinoid, suppresses immune function and increases the risk of pathogen-infection in honey bees (Di Prisco et al., 2013). Using Drosophila melanogaster (fruit fly) as a model for honey bees, it was shown that sublethal exposure to imidacloprid induced a gut dysbiosis and decreased survival following Serratia marcescens infection (Daisley et al., 2017). However, a study examining this effect in honey bees showed that decreased survival due to imidacloprid exposure and metabolism of the compound is not gut microbiome-dependent (Raymann et al., 2018).
Organophosphates.
Organophosphates (OPs) are esters of phosphoric acid that covalently bind to AChE, causing over-stimulation of neurons (Ruark et al., 2013). More recently, some OPs are recognized to have off-target toxicities at concentrations below the inhibition of AChE. For example, some OPs can inhibit or activate serine hydrolases and inhibit cannabinoid receptor 1 (CNR1) (Casida and Quistad, 2004). Diazinon exposure can also alter signaling of the neurotransmitter serotonin, production of which is influenced by the microbial metabolites short-chain fatty acids (SCFAs) and tryptophan (Slotkin et al., 2008; Timofeeva et al., 2008; Waclawikova and El Aidy, 2018). Gao et al. (2017b) demonstrated that C57BL/6 mice exposed to diazinon in drinking water for 13 weeks altered gut microbiome composition (19 genera), function, and metabolic profiles in a sex-specific manner (16S rDNA sequencing and whole genome shotgun sequencing) (Gao et al., 2017b), including decreased tryptophan synthase, which is required for the biosynthesis of tryptophan in bacteria. In a follow-up study, metatranscriptomic analysis revealed an increase in the RNA of quorum sensing genes as well as bacterial motility and sporulation, which could explain the detection of pathogenic bacteria (Gao et al., 2017a). Similarly, C57BL/6 male mice exposed to malathion for 13 weeks exhibited increased DNA expression of quorum sensing, motility, and pathogenicity genes, indicating that exposure to some OPs may increase the gut microbiota susceptibility to colonization by pathogenic bacteria (Gao et al., 2018a). One study compared the gut microbiome of the malaria vector-containing mosquito Anopheles albimanus and demonstrated that mosquitos resistant to the OP fenitrothion had a relatively lower diversity than mosquitos who were not resistant, possibly due to fenitrothion-exposure selecting for a few bacteria which utilize fenitrothion as an energy source, leading to their overabundance (Dada et al., 2018). Interestingly, BALB/c mice exposed to monocrotophos for 180 days had increased production of the microbial SCFA acetate and show that increased acetate induced gluconeogenesis and glucose intolerance (Velmurugan et al., 2017). Fecal microbiome transplants of monocrotophos-exposed mice to un-exposed mice showed that glucose intolerance was microbiome-dependent. In humans, an examination of the oral microbiome of farmworkers by season demonstrated that microbiome perturbations may act persistently as the dysbiosis persisted in winter after the potential for exposure during the spring and summer (Stanaway et al., 2017). Therefore, while the majority of these chemicals have not been investigated for gut microbiome-dependent neurotoxicity, it has been noted that gut dysbiosis causes neurotoxicity via multiple mechanisms (Galland, 2014), thus exposure to chemicals such as carbamates could potentially further these effects.
Chlorpyrifos is the most well studied OP insecticide in relation to changes in the microbiomes of humans and animal models, with some evidence for neurotoxic effects. Most notably, adult male Wistar rats exposed to chlorpyrifos for 9 weeks increased bacteria associated with neurotoxicity such as Candidatus Arthromitus, in addition to inducing metabolic disorders (obesity and diabetes) (Fang et al., 2018). Rats exposed perinatally to chlorpyrifos had altered gut microbiome profiles as adults and were associated with impaired epithelium protection (Joly Condette et al., 2015) and induced metabolic disorders, but these conditions were partially abrogated by inulin supplementation (Reygner et al., 2016b). The microbial metabolites SCFAs and bile acids were altered in the urine of mice exposed to chlorpyrifos for 30 days (Zhao et al., 2016). The metabolome profiles correlated with changes in gut microbiome. Increased lipopolysaccharide, intestinal inflammation, and intestinal permeability indicates that the perturbations of the mouse microbiome by chlorpyrifos significantly affect gut health and the microbial metabolites that can act as signaling to the host (Zhao et al., 2016). Zebrafish exposed to chlorpyrifos also had altered gut microbiome composition (25 taxa) as well as metabolic changes in glucose and lipid metabolism, TCA cycle, and amino acid metabolism (Wang, X. et al., 2019). In the SHIME model, chlorpyrifos decreased the colony forming units (cfu) of Bifidobacterium spp. and Lactobacillus spp. after 15 to 30 days of exposure (Requile et al., 2018). Decreased Bifidobacterium spp. and Lactobacillus spp. was reflected in two other SHIME studies, as well as increased Entereoccocus spp. (Joly et al., 2013; Reygner et al., 2016a).
Two other environmentally relevant animal models, which have been used to explore gut microbiome-chlorpyrifos interactions, are the diamondback moth (Plutella xylosterlla) and fruit fly (Drosophila melanogaster). The diamondback moth is an economically deleterious pest of cruciferous crops, and it is suspected that the development of pesticide-resistance is partly due to the microbiome of this insect. In vitro experiments from bacteria isolated from the gut microbiome of the diamondback moth revealed that Enterococcus spp., vitamin C, and acetylsalicylic acid enhanced resistance to chlorpyrifos; however, the bacteria do not detoxify the insecticide (Xia, X. et al., 2018). An earlier sequencing study found that an insecticide resistant line of diamondback moth larva had increased Lactobacillales, Pseudomonadales, and Xanthomondales (Xia et al., 2013). Contradictorily, GF and antibiotic treated fruit flies exposed to chlorpyrifos lived significantly longer than conventionally raised flies, and this was due to metabolism of chlorpyrifos to the stronger AChE inhibitor chlorpyrifos oxon by Lactobacillus plantarum (Daisley et al., 2018). This effect was mitigated by supplementation of the human probiotic Lactobacillus rhamnosus GG, which binds, but does not metabolize chlorpyrifos as demonstrated in a previous study along with L. rhamnosus GR-1 (Trinder et al., 2016). Studies in the diamondback moth and fruit fly demonstrate that certain bacteria species can increase or decrease the toxicity of chlorpyrifos.
Overall, microbiome may contribute to neurotoxicity and other toxicological endpoints due to organophosphate exposure, but more evidence (behavior and biochemical data) is needed to ascertain a direct mechanism of OP exposure to gut dysbiosis to neurotoxicity.
Pyrethrins.
Pyrethroids are derived from the chrysanthemum flower (originally known as pyrethrum) and are found in many household insecticides and repellents. Pyrethroids prevent the closure of voltage-gated sodium channels, making them axonic excitotoxins that prevent repolarization that leads to paralysis of an affected organism. Pyrethroids are broad spectrum pesticides, affecting insects indiscriminately, including bees, but are poorly absorbed by humans and often are used to treat clothing and prevent mosquito-borne diseases.
Permethrin has been linked to the depressive- and anxiety-like behavior of Gulf War Illness (GWI), as well as reduced hippocampal volume, neural stem cell activity, and neurogenesis (Parihar et al., 2013). Co-exposure of permethrin and another GWI compound pyridostigmine bromide in a mouse model decreased Lactobacillus spp. and Bifidobacterium spp. and supplementation of the microbial SCFA butyrate restored gut homeostasis (Seth et al., 2018). Chronic exposure to permethrin in rats decreased abundance of the beneficial bacteria Bifidobacterium spp. and Lactobacillus paracasei in the feces (Nasuti et al., 2016). Overall, pyrethrins, and specifically permethrin, have an effect on the microbiome, but more research is needed to identify if changes in the gut microbiome are associated with the neuroinflammation and behavioral changes associated with GWI.
Rotenoids.
Rotenoids are naturally occurring substances, particularly in the flowering plant subfamily Faboideae as well as Nyctaginaceae. Many rotenoids such as rotenone have broad spectrum insecticidal properties. A hydrophobic compound, rotenone is an inhibitor of the mitochondrial complex I that easily crosses the blood-brain barrier causing toxicity to the central nervous system. Specifically, rotenone selectively induces apoptosis in serotonergic and dopaminergic neurons (Bisbal and Sanchez, 2019; Ren and Feng, 2007; Ren et al., 2005). Interestingly, rotenone exposure in Lewis rats for 7 days induces the pathology of Parkinson’s disease and is therefore used in animal models for Parkinson’s disease (Betarbet et al., 2000; Bisbal and Sanchez, 2019). In mice orally exposed to rotenone for 28 days, were shown to have 14 bacteria taxa at the family level dysregulated with a marked decrease in Bifidobacterium spp. (30099890). Furthermore, dopaminergic cell loss was inversely correlated with increased Rikenellaceae, Erysipelotrichaceae, Ruminococcaceae and S24–7, as well as a positive correlation with Bifidobacteriaceae. A second study in mice that used unpredictable restraint stress for 12 weeks and 6 weeks of rotenone exposure found that rotenone exposure furthered the restraint stress-associated increase in the relative abundance of the mucin-degrading bacteria Akkermansia muciniphila (Dodiya et al., 2018). This result is interesting because an increase in A. muciniphila has been noted in patients with Parkinson’s disease, possibly due to the bacterium’s ability to activate TLR2 as explored in a review by Radisavljevic et al (Radisavljevic et al., 2018). In a 4-week longitudinal rotenone exposure study in mice, gastrointestinal dysfunction started at 3 weeks along with significant inflammation; rotenone also decreased the microbial richness at 3 weeks compared to baseline with decreased in Bacteroidetes and increased Firmicutes (Yang et al., 2017). However, in adult male rats exposed to rotenone for 4 weeks, there was a tendency for Lactobacillus spp. and Bifidobacterium spp. to increase despite matching intestinal inflammatory properties (Johnson et al., 2018). It should be noted that some species of Lactobacillus produce D-lactic acid, which can cause neurological effects (Yilmaz et al., 2018). Overall, it is unclear if gut microbiome altered by rotenone may contribute to Parkinson’s disease or if rotenone neuronal toxicity induces a gut dysbiosis; further studies are necessary to identify mechanisms of toxicity and disease pathology.
Herbicides and fungicides.
In general, herbicides and fungicides are designed to prevent and kill unwanted plants, molds, and fungi; however, their chemical properties can have unintended neurotoxicity and hormonal disruption in humans and animals. For example, atrazine, a triazine herbicide used in corn and sugarcane fields as well as grass turfs (lawns and golf courses), demasculinizes male gonads during developmental exposure (Hayes et al., 2011). This occurs across amphibians, reptiles, and mammals and causes an increase in the size of testicular tubules, loss of Sertoli cells, and a marked loss of germ cells. One experiment in Cuban tree frogs (Osteopilus septentionalis) found that atrazine did not affect the amphibian chytrid fungus Batrachochytrium dendrobatidis in a gut microbiome-dependent manner, however the intensity of the fungus was negatively associated with the microbial phylum Fusobacteria (Knutie et al., 2018). In zebrafish, atrazine decreased serotonin and increased inflammation in males, and affected the abundance of many genera of bacteria (Chen, L. et al., 2018b).
Glyphosate is a broad-spectrum herbicide that is the leading product for weed management by acting as a competitive inhibitor of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), preventing the biosynthesis of aromatic amino acids and other metabolites in plants and some microorganisms that have the shikimate pathway (Motta et al., 2018). However, recent evidence suggests that glyphosate has off target effects on non-shikimate organisms. For example, chronic exposure of rat hippocampal cells to 1% Roundup® (commercial product with 0.38% glyphosate) showed decreased 3H-glutamate uptake with increased glutamine uptake, indicating glutamatergic excitotoxicity (Cattani et al., 2014). Glyphosate alters the behavior of honey bees, and, interestingly, the genomes of the bee gut microbiome contain EPSPS (Motta et al., 2018). Young worker bees fed glyphosate for 5 days significantly decreased the abundance of the dominant gut microbiota species and increased their susceptibility to the opportunistic pathogen Serratia marcescens (Motta et al., 2018). The gut microbiome of honey bee larva fed a high concentration of glyphosate (20mg/L) had 9 taxa that were decreased relative to controls or low dose glyphosate exposures (Dai et al., 2018). Cultured gut bacteria from the gastrointestinal tracts of green turtles (Chelonia mydas) exposed to six different glyphosate concentrations, as well as a deionized water control, showed a decreased in four bacteria genera: Pantoea, Proteus, Shigella, and Staphylococcus (Kittle et al., 2018). Interestingly, exposing the gut bacteria of poultry in vitro to glyphosate demonstrated that pathogenic bacteria such as pathogenic bacteria as Salmonella Entritidis, Salmonella Gallinarum, Salmonella Typhimurium, Clostridium perfringens, and Clostridium botulinum are highly resistant to glyphosate, whereas beneficial bacteria Enterococcus faecalis, Enterococcus faecium, Bacillus badius, Bifidobacterium adolescentis, and Lactobacillus spp. were susceptible to glyphosate (Shehata et al., 2013), indicating that glyphosate could increase the risk for pathogenic diseases. Indeed, glyphosate exposure reduces the most prevalent Enterococcus spp. in German cattle, which can produce bacteriocins against Clostridium botulinum (Kruger et al., 2013).
Several studies have used rodent models to evaluate glyphosate-induced perturbations to the gut microbiome as well as possible neurotoxic associations. Mice exposed chronically to 250 or 500 mg/kg/day of glyphosate had increased anxiety and depression like behaviors as well as decreased Corynebacterium spp. and Lactobacillus spp., indicating that key microbes could increase neurobehavioral alterations (Aitbali et al., 2018). Exposing female Sprague-Dawley rats to three doses of Roundup® increased the family S24–7 and decreased Lactobacillaceae; culturable bacteria in the rat microbiome showed varying sensitivities to glyphosate, with one highly resistant Escherichia coli strain lacking EPSPS (Lozano et al., 2018). Another study examining the rat pups from in utero and developmental exposure to postnatal day 125 of glyphosate in drinking water showed an increase in Prevotella spp., but a decrease in Lactobacillus spp. at postnatal day 31 (Mao et al., 2018). However, another study found that a diet sufficient in aromatic amino acids would prevent the antimicrobial effects of glyphosate on the rat microbiome (Nielsen et al., 2018). Overall, some bacteria that decrease from glyphosate exposure, such as Lactobacillus spp., may increase the neurotoxicity of glyphosate, but further studies are warranted.
IV-7. Plant and animal toxins.
In addition to the manmade neurotoxicants, microbiome may contribute to the detoxification/toxification of the plant and animal derived toxins. For example, the neurotoxin domoic acid is produced by the diatom Pseudo-nitzschia multiseries and is responsible for the amnesic shellfish poisoning. Domoic acid exerts its neurotoxicity through activating the excitatory glutamate receptors as a structural analog to glutamate (Bates et al., 2018; Chegini and Metcalfe, 2005; Mills et al., 2016; Smith and Swoboda, 2019). Autochthonous bacteria have been suggested to contribute to the biodegradation and disposal of domoic acid, and blue mussels and soft-shell clams have been demonstrated as the unique sources of these domoic acid-utilizing bacteria (Stewart et al., 1998).
Nicotine, which is the primary active substance in tobacco, interacts with the nicotinic receptors in CNS for excitatory signaling. Studies using mouse models have shown that nicotine exposure affects the gut microbiome in a sex-dependent manner, in that there is a male gut microbiome-specific modulation of microbial pathways involved in carbohydrate metabolism, oxidative stress, and DNA repair. In addition, fecal metabolomics studies showed that multiple neurotransmitters including glutamate, GABA, and glycine, as well as the neuro-active metabolites leucine and uric acid, were altered by nicotine in both sexes (Chi et al., 2017c). This indicates that gut microbiome may at least partly contribute to nicotine’s CNS effect.
Cyanide can induce tremors through neuronal calcium signaling (Johnson et al., 1986), and is enriched in secondary metabolites in bamboo. Interestingly, the bamboo-eating pandas are deficient in rhodanese, which is one of the essential cyanide-detoxifying enzymes as compared to other herbivores. Correspondingly, the gut microbiota of pandas have high proportions of Pseudomonas bacteria and enriched putative genes coding for rhodanese-like enzymes involved in cyanide degradation. This diet-driven symbiotic relationship is advantageous for the host survival during evolution (Zhu, L. et al., 2018).
IV-8. Solvents.
Solvents refer to a class of organic chemicals of varying lipophilicity and volatility classified by molecular structure and functional groups that general lack a charge and are of small molecular size. Toxicity of solvents is determined by the number of carbon atoms, saturation (number of bonds between carbon atoms), configuration of the carbon atoms, halogenation, and the presence of functional groups (Klaassen, 2013).
One of the greatest solvent exposures for most humans is ethanol through intoxicating beverages as well as from its use in household products, pharmaceuticals, industry, and gasoline additive. The metabolism of ethanol by the liver is well characterized by the formation of acetaldehyde by CYP2E1 or alcohol dehydrogenase followed by acetate by aldehyde dehydrogenase, and it is well known that chronic alcohol ingestion can induce alcoholic liver disease. Mice fed the liquid Liber-DeCarli diet with alcohol (5% v/v) for 6 weeks to induce alcoholic liver disease increased the fecal pH and increased fecal Corynebacterium spp. as well as the alkaline tolerant Alcaligenes spp. compared to mice not exposed to alcohol (Bull-Otterson et al., 2013). Supplementation with the probiotic bacterium Lactobacillus rhamnosus GG prevented the ethanol induced pathogenic changes. A combination of ethanol and the artificial sweetener saccharin increased Eubacteria in pregnant mice, whereas it decreased in non-pregnant mice; in pregnant mice, ethanol and saccharin increased the abundance of Clostridium spp. compared to ethanol and weight (Labrecque et al., 2015). Another study found that alcohol dependent mice had increased Bifidobacterium spp., and metabolomics analysis revealed changes in bacteria relevant metabolism, including secondary bile acids and serotonin (Wang et al., 2018). Ethanol exposure for 3 weeks in mice induced anxiety and depression-like behaviors also increased Adlercreutzia spp., Allobaculum spp. and Turicibacter spp. and decreased Helicobacter spp. (Xu et al., 2018). Interestingly, decreased Adlercreutzia spp. was positively correlated with alcohol preference and negatively correlated with anxiety-like behavior and the decreased expression of brain-derived neurotrophic factor (BDNF) and α1 subunit of γ-aminobutyric acid A receptor (Gabra1) in prefrontal cortex.
Alcohol dependent human subjects admitted to a gastroenterology ward for a 3-week detoxification and rehabilitation program had increased gut permeability by the second day of alcohol withdrawal. Alcohol dependent subjects with high gut permeability had decreased abundance of the genera Ruminococcus, Faecalibacterium, Subdoligranulum, Oscillibacter, and Anaerofilum as well as an increase in Dorea (Leclercq et al., 2014). Interestingly, increased gut permeability was negatively correlated with the reduced total number of bacteria and microbial metabolites from tryptophan metabolism, which is used for generating neurotransmission molecules such as serotonin, were low or not present in high gut permeability subjects. Mice fed a liquid diet with alcohol were protected from alcohol-induced neuroinflammation, increased small intestine and brain cytokine expression by antibiotic treatment, and antibiotics abrogated microglia activation and morphological changes in the cortex and hippocampus, demonstrating a direct connection between the gut microbiome and the CNS effects of alcohol (Lowe et al., 2018).
Associations between gut microbiome and solvents have also been investigated for formaldehyde and trichloroethylene (TCE). Formaldehyde is an irritating, gaseous solvent that is an environmental toxic hazard found in paint, cloth, cigarette smoke and exhaust gas. Formaldehyde has many detrimental effects on various tissues including skin, eye, gonads, the gastrointestinal system and the respiratory tract, as well as the nervous system, which can cause neurobehavioral impairment and seizures (Kilburn, 1994; Tang et al., 2011). Blab-C mice exposed to formaldehyde had increased abundance of the genera Prevotella, Dorea, Desulfovibrio, Adlercreutzia, Anaeroplasma, Coprococcus, Candidatus Arthromitus, Delftia, Lactococcus, and Serratia and decreased abundance of Bacteroides (Guo, J. et al., 2018). TCE is a clear sweet-smelling industrial solvent with a variety of uses, including dry cleaning, film cleaning, and degreaser. TCE is a CNS depressant by inhibiting GABAA and glycine receptors (Beckstead et al., 2000; Krasowski and Harrison, 2000). Mice exposed to TCE perinatally and up to postnatal day 259 decreased the abundance of the genera Bacteroides and Lactobacillus and increased the abundance of Bifidobactrium (Khare et al., 2019). Overall, more research is needed to understand the contribution of the microbiome to neurotoxicity by solvents.
V. Closing remarks: microbiome as a contributor to neurotoxicity
Taken together, our review provides a comprehensive literature update regarding the role of gut microbiome in various neurological disorders, especially during chemical-induced neurotoxicity in both human subjects and animal models. Although the research in neurotioxicants and gut-brain axis is still in its early phase, we hypothesize that gut microbiome may contribute to the pathogenesis and/or resolution of neurotoxicity by 1) direct biotransformation of the xenobiotics into neuro-reactive or inactive metabolites; 2) alteration of endogenous microbial neuro-reactive metabolites, some of which may have epigenetic reprogramming potential in regulating the transcription of host genes involved in cognitive functions in brain; 3) modulating neuroinflammation by modulating intestinal barrier integrity and the systemic availability of gut-derived pro-inflammatory cytokines; and 4) regulation of mucosal immune function (Figure 1). Among various neurological disorders and chemical-induced neurotoxicity, distinct neuroactive microbial metabolites appear to be common targets, this includes decreased SCFAs and/or decreased abundance of SCFA-producing bacteria in AD mouse models (Zhang et al., 2017), anorexia nervosa patients (Borgo et al., 2017; Morita et al., 2015), PD patients (Unger et al., 2016), ASD patients (De Angelis et al., 2013; Kang et al., 2013), as well as ionic gold (Kase et al., 1987) and permethrin/pyridostigmine bromide co-exposed mice (Seth et al., 2018). Conversely, SCFA supplementation has been shown to improve AD (Govindarajan et al., 2011; Vinolo et al., 2011), and restore permethrin-induced gut dysbiosis, stalls microbiome-induced GI inflammation in Gulf War illness mouse model (Seth et al., 2018). This is likely contributed by the HDAC inhibitor and anti-inflammation properties of SCFAs (Bourassa et al., 2016; Matt et al., 2018; Vinolo et al., 2011). Tryptpohan metabolism is another important common target in neurotoxicity, as it is dysregulated in ASD (Golubeva et al., 2017), MDD (Kazemi et al., 2018; Rudzki et al., 2019), alcohol addition (Leclercq et al., 2014), as well as manganese- and organophosphate-induced neurotoxicity (Chi et al., 2017b; Slotkin et al., 2008; Timofeeva et al., 2008; Waclawikova and El Aidy, 2018). These common targets should be especially focused on as they may represent common signaling pathways that can be harnessed therapeutically to mitigate neurotoxicity.
In the endeavor to move forward the research on gut-brain axis and neurotoxicity, we propose that it will be important to consider the following aspects:
1). Beyond associations: more mechanistic investigations are needed in vitro and at the single microbial species resolution to establish the causality.
A combination of research tools including anaerobic culture of bacteria, GF mice, fecal transplant/single strain inoculations, metabolomics, as well as biochemical and behavioral assessment of CNS functions, will likely lead to a more thorough understanding of the exact molecular mechanisms of gut microbiota-mediated regulation of CNS functions. For example, Dr. Elaine Hsiao’s group demonstrated how the ketogenic diet exerts its anti-seizure effects in mice through modulating the gut microbiota by initially using a multi-omics approach of 16S rDNA sequencing and metabolomics followed by identification of key bacteria that mitigate seizures (Olson et al., 2018). The ketogenic diet promoted the enrichment of the genera Akkermansia and Parabacteroides in the intestine and correlated with reduction in systemic gamma-glutamylated amino acids and increased hippocampal GABA/glutamate levels. The dependency of gut microbiota in ketogenic diet mediated anti-seizure effect was confirmed using antibiotic-exposed mice and GF mice, inoculation of specific bacteria (Akkermansia and Parabacteroides), and fecal microbiome transfer (Olson et al., 2018). Overall, this study demonstrated which bacteria modified by ketogenic diet mechanistically contributed to seizure protection in mice.
The SHIME system, or a similar microbial culturing method, is another tool that could be used to study the specific effects of chemical exposures on bacteria and understand how the chemicals may alter gut microbiome composition in humans. Because the system uses a dynamic series of six compartments that can mimic the intestines (Joly et al., 2013; Requile et al., 2018), some bacteria that have not be cultured by traditional methods may colonize with the system. Furthermore, the bacteria cultured before and after exposure could be used to colonize GF mice to elucidate a microbiome dependent effect in a paradigm such that exposure causes a gut dysbiosis which leads to neurotoxicity. The SHIME system was used to characterize the effect of exposures on the microbiome, including arsenic (Yin et al., 2017; Yin et al., 2015; Yu et al., 2016) and chlorpyrifos (Joly et al., 2013; Requile et al., 2018; Reygner et al., 2016a).
In general, an obstacle to study the role of intestinal bacteria using single strain anaerobic cultures is that a substantial number of the intestinal bacteria are not culturable in vitro (Lagier et al., 2015; Lagkouvardos et al., 2017). This is partly because the artificial media may lack key growth factors that are provided by other symbiotic bacteria in the gut (D’Onofrio et al., 2010; Fenn et al., 2017). Co-cultures of multiple microbial species may be the solution to this problem, and this method has been shown to be successful in characterizing GABA-modulating bacteria in the human gut (Strandwitz et al., 2019). Because microbes live in a symbiotic environment in the gut, metabolites produced by one microbe may serve as important substrates for the function of another microbe. Conversely, antagonistic microbes can compete with each other for the same nutrient niche and may inhibit a particular signaling pathway under physiological or pathophysiological conditions (Das et al., 2018). Therefore, in the effort to determine the mechanistic roles of microbes at single species resolution, it is important to perform co-culture experiments to investigate the microbe-microbe interactions, and identify key microbial metabolites in the context of microbial networks that contribute to the pathogenesis of neurological diseases. In addition, it is important to include host enterocytes in the co-culturing system, so as to determine the interactions between distinct microbes and host receptors (Sadaghian Sadabad et al., 2015).
In addition, steady-state pre-rRNA analysis, which quantifies the ribosomal RNA precursors instead of mature 16S rDNA, has been shown to be a more promising culture-independent tool to assess the active growth status of the bacteria. Pre-rRNA is rapidly replenished when growth-limited bacteria encounter a more favorable growth environment, and such changes can occur only in viable bacteria but not in dead cells or with free nucleic acids. Pre-rRNAs also appear to be a more sensitive biomarker to environmental stress as compared to the mature rDNA signals (Cangelosi and Brabant, 1997; Cangelosi and Meschke, 2014; Mackow and Chang, 1985; Oerther et al., 2000; Srivastava and Schlessinger, 1990). Lastly, taking the advantage of the CRISPR-Cas9 gene-editing system, one could engineer individual intestinal bacteria to investigate the necessity of the microbial genes at single species resolution (Mimee et al., 2015).
2). Beyond 16S rDNA survey: investigating microbial genes and neuroactive microbial metabolites to further decipher the bacterial functions.
As reviewed in Tables 1 and 2, a substantial amount of research in gut microbiome and neurological disorders has utilized 16S rDNA gene sequencing, which has provided important and valuable information regarding what taxa are differentially regulated during the progression of diseases. However, 16S rDNA gene sequencing often does not resolve taxonomy past the genus level and provides only moderately accurate predictions of the functional changes. Deep whole-metagenome shotgun sequencing, as well as a more cost-effective approach, namely shallow shotgun metagenomic sequencing, are highly recommended alternatives for high resolution taxonomic and functional microbiome analysis (Hillmann et al., 2018). In addition, we believe that more research using metatranscriptomics (Bashiardes et al., 2016) and metaproteomics (Mills et al., 2019) are needed to further characterize the gut microbiome functions beyond DNA level. Computational tools for functional predictions of the microbiome, such as FishTaco and AGORA (Magnusdottir et al., 2017; Manor and Borenstein, 2017), followed by wet lab validations using metabolomics approach (Daliri et al., 2017), will also provide further mechanistic insights into further understanding the gut-brain axis in neurotoxicity.
Specifically, building on the known neuroactive microbial metabolites that contribute to the pathogenesis and/or mitigation of neurotoxicity, it is important to determine the regulation of these microbial genes that are responsible for the production of these metabolites. As summarized in this review, SCFAs are commonly reduced in AD mouse models (Zhang et al., 2017), anorexia nervosa patients (Borgo et al., 2017; Morita et al., 2015), PD patients (Unger et al., 2016); whereas decreased SCFA-producing bacteria were also observed in intestines of ASD patients (De Angelis et al., 2013; Kang et al., 2013), ionic gold exposed mice (Kase et al., 1987), permethrin and pyridostigmine bromide co-exposed mice (Seth et al., 2018). Dysregulation in microbial and/or host tryptophan metabolism was found associated linking to ASD behavior (Golubeva et al., 2017); MDD (Kazemi et al., 2018; Rudzki et al., 2019), alcohol addition (Leclercq et al., 2014), as well as manganese- and organophosphate-induced neurotoxicities (Chi et al., 2017b; Slotkin et al., 2008; Timofeeva et al., 2008; Waclawikova and El Aidy, 2018). It is especially important to focus on the neuroactive metabolites that are commonly regulated in neurological disorders of various etiology, such as SCFAs and tryptophan microbial metabolites.
3). Beyond intestinal bacteria: characterizing the involvement of other microorganisms in neurotoxicity
Although most of the current endeavors in exploring the gut microbiome have focused on intestinal bacteria, other microorganisms are important for neurodegenerative diseases and may also provide alternative therapeutic options. These other microorganisms that could be essential to the normal function of the host or contribute to pathogencity of diseases include fungi, porotozoa, and viruses (Barko et al., 2018). Fungi have been detected in different brain regions in Alzheimer’s disease patients (Alonso et al., 2018; Pisa et al., 2015), as well as in patients with amyotrophic lateral sclerosis (Alonso et al., 2017). One hypothesis is that these fungi are of gut origin and may be a result of gut leakage. Regarding viruses, it is not known what viruses in the gut microbiome may be beneficial to host health, however bacteriophage therapy may be method to alter the gut microbiome. The gut microbiome of gnotobiotic mice colonized with known human gut bacteria was modulated by lytic phages that shifted the gut microbiome composition and the gut metabolome, suggesting that bacteriophage could be used to modulate the microbiome (Hsu et al., 2019). Indeed, intravenous treatment with engineered phages was used to treat a cystic fibrosis patient with a drug-resistant strain of Mycobacterium abscessus (Dedrick et al., 2019). More research is needed in this field to further characterize the sources, types, and mechanistic involvement of these microorganisms during various types of neurological diseases. The bioavailability of gut-derived microorganisms and microbial products should also be assessed when considering the presence of the blood-brain barrier.
4). Beyond early-life exposure: epigenetic reprogramming via microbial metabolites.
Early-life exposure to environmental chemicals has been shown to be an important contributing factor for developmental origins of human diseases. Along those lines, early-life induced dysbiosis may also predispose the host organism to delayed onset of adverse health outcomes including neurodegenerative diseases. There is a critical time window early in life to target the microbiome and modulate late-onset of Alzheimer’s disease, because acute antibiotic-treatment in perinatal period resulted in long-term alterations of gut microbiota (especially Lachnospiraceae and S24–7) and reduction in brain Aβ deposition, as well as reduced inflammatory signaling in serum and brain of aged AD mice (Minter et al., 2017). Neuroactive microbial metabolites, such as SCFAs which have anti-inflammatory HDAC inhibitor properties, may be beneficial against the pathogenesis of AD (Govindarajan et al., 2011; Vinolo et al., 2011). Therefore, linking gut microbiome and metabolome to host epigenome and inflammasome is an exciting approach to investigate the remote-sensing mechanisms between the gut and brain.
Table 3.
List of neurotoxicants and type of toxicity.
| Toxicant | Neurotoxicity | Sources |
|---|---|---|
| Amiodarone | Myelinopathy | (Graham and Lantos, 1997) |
| Peripheral neuropathy | (Fraser et al., 1985) | |
| Antibiotics (unspecified) | Postpartum depression | (Murphy et al., 2018) |
| Arsenic | Wallerian degeneration of axons | (Klaassen, 2013) |
| Atrazine | Decreased serotonin | (Chen, L. et al., 2018b) |
| Carbamates | Inhibition of hippocampal neurogenesis, memory dysfunctions, impaired myelination | (Seth et al., 2019) |
| Cisplatin | Peripheral neuropathy | (Graham and Lantos, 1997) |
| Copper imbalance | Aceruloplasminemia, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, Menkes disease, occipital horn syndrome, Parkinson’s disease, prion disease, and Wilson disease | (Desai and Kaler, 2008) |
| Disulfiram | Peripheral neuropathy | (Graham and Lantos, 1997) |
| Doxorubicin | Progressive ataxia | (Graham and Lantos, 1997; Spencer and Schaumburg, 2000) |
| Ethanol | Worsened short-term memory, impaired neuronal signaling | (Huf et al., 2019) |
| Glial dysfunction, neuroinflammation | (Gomez et al., 2018) | |
| Formaldehyde | Neurobehavioral impairment and seizures | (Tang et al., 2011) |
| Gold sodium thiomalate | Decreased unmyelinated axons | (Levine et al., 1986) |
| Lead | ADHD | (Banerjee et al., 2007; Scassellati et al., 2012) |
| Cerebral edema, lead encephalopathy, peripheral neuropathy | (Johnston and Goldstein, 1998) | |
| Magnesium deficiency | Depression | (Winther et al., 2015) |
| Manganese | Parkinson’s disease | (Calderon-Garciduenas et al., 2016; Dobson et al., 2004) |
| Methylmercury | Neuronal degeneration, ataxia, paresthesia, psychomotor retardation, developmental disabilities, and cognitive deficits | (Klaassen, 2013) |
| Metronidazole | Peripheral neuropathy | (Goolsby et al., 2018) |
| MPTP(1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine) | Parkinson’s disease | (Kopin, 1987) |
| Nitrofurantoin | Peripheral neuropathy | (Spencer and Schaumburg, 2000) |
| Organochlorines | Blocks chloride channels of the GABA-A receptor, seizures, weak association with Parkinson’s disease | (Costa, 2015) |
| Organophosphates | ADHD | (Banerjee et al., 2007; Scassellati et al., 2012) |
| Over-stimulation of neurons | (Ruark et al., 2013) | |
| Altered serotonin signaling | Slotkin et al., 2008 | |
| Ozone | Alzheimer’s disease | (Calderon-Garciduenas et al., 2016) |
| PM2.5 | Alzheimer’s disease | (Calderon-Garciduenas et al., 2016) |
| Parkinson’s disease | (Shin et al., 2018) | |
| Polybrominated diphenyl ethers (PBDEs) | Developmental neurotoxicity | (Dorman et al., 2018) |
| Inhibited adult neurogenesis | (Li et al., 2013) | |
| Polychlorinated biphenyls (PCBs) | ADHD | (Banerjee et al., 2007; Scassellati et al., 2012) |
| Neurological deficits (differences in neuromotor development, decrements in cognition and behavioral deficits) | (Korrick and Sagiv, 2008) | |
| Lower cognitive functioning | (Przybyla et al., 2017) | |
| Pyrethroids | Gulf War Illness (GWI), hippocampal volume, neural stem cell activity, and neurogenesis | (Parihar et al., 2013) |
| Rotenoids | Apoptosis in serotonergic and dopaminergic neurons | (Bisbal and Sanchez, 2019) |
| Parkinson’s disease | (Betarbet et al., 2000) | |
| Trichloroethylene (TCE) | CNS depressant by inhibiting GABAA and glycine receptors | (Beckstead et al., 2000; Krasowski and Harrison, 2000) |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, Smeekens SP, Netea MG, Buitelaar JK, Franke B, van Hijum S, Arias Vasquez A, 2017. Gut microbiome in ADHD and its relation to neural reward anticipation. PLoS One 12(9), e0183509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abildgaard A, Elfving B, Hokland M, Wegener G, Lund S, 2017. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 79, 40–48. [DOI] [PubMed] [Google Scholar]
- Aitbali Y, Ba-M’hamed S, Elhidar N, Nafis A, Soraa N, Bennis M, 2018. Glyphosate based-herbicide exposure affects gut microbiota, anxiety and depression-like behaviors in mice. Neurotoxicol Teratol 67, 44–49. [DOI] [PubMed] [Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S, Ota M, Koga N, Hattori K, Kunugi H, 2016. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 202, 254–257. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Bourgeois ML, Angst J, Post R, Moller H, Hirschfeld R, 2000. Re-evaluating the prevalence of and diagnostic composition within the broad clinical spectrum of bipolar disorders. J Affect Disord 59 Suppl 1, S5–S30. [DOI] [PubMed] [Google Scholar]
- Akiyama H, 1994. Inflammatory response in Alzheimer’s disease. Tohoku J Exp Med 174(3), 295–303. [DOI] [PubMed] [Google Scholar]
- Alam R, Abdolmaleky HM, Zhou JR, 2017. Microbiome, inflammation, epigenetic alterations, and mental diseases. Am J Med Genet B Neuropsychiatr Genet 174(6), 651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete TL, Jones RB, Chen Z, Kim JS, Habre R, Lurmann F, Gilliland FD, Goran MI, 2018. Exposure to traffic-related air pollution and the composition of the gut microbiota in overweight and obese adolescents. Environ Res 161, 472–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida LG, Moraes LA, Trigo JR, Omoto C, Consoli FL, 2017. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS One 12(3), e0174754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R, Pisa D, Fernandez-Fernandez AM, Carrasco L, 2018. Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer’s Disease. Front Aging Neurosci 10, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso R, Pisa D, Fernandez-Fernandez AM, Rabano A, Carrasco L, 2017. Fungal infection in neural tissue of patients with amyotrophic lateral sclerosis. Neurobiol Dis 108, 249–260. [DOI] [PubMed] [Google Scholar]
- Anezaki K, Nakano T, 2014. Concentration levels and congener profiles of polychlorinated biphenyls, pentachlorobenzene, and hexachlorobenzene in commercial pigments. Environ Sci Pollut Res Int 21(2), 998–1009. [DOI] [PubMed] [Google Scholar]
- Ardura-Fabregat A, Boddeke E, Boza-Serrano A, Brioschi S, Castro-Gomez S, Ceyzeriat K, Dansokho C, Dierkes T, Gelders G, Heneka MT, Hoeijmakers L, Hoffmann A, Iaccarino L, Jahnert S, Kuhbandner K, Landreth G, Lonnemann N, Loschmann PA, McManus RM, Paulus A, Reemst K, Sanchez-Caro JM, Tiberi A, Van der Perren A, Vautheny A, Venegas C, Webers A, Weydt P, Wijasa TS, Xiang X, Yang Y, 2017. Targeting Neuroinflammation to Treat Alzheimer’s Disease. CNS Drugs 31(12), 1057–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentsen T, Raith H, Qian Y, Forssberg H, Diaz Heijtz R, 2015. Host microbiota modulates development of social preference in mice. Microb Ecol Health Dis 26, 29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, Koga Y, Sudo N, 2012. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol 303(11), G1288–1295. [DOI] [PubMed] [Google Scholar]
- Association AP, 2013. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Publishing, Arlington. [Google Scholar]
- Athauda D, Foltynie T, 2016. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today 21(5), 802–818. [DOI] [PubMed] [Google Scholar]
- Axelsson PB, Clausen TD, Petersen AH, Hageman I, Pinborg A, Kessing LV, Bergholt T, Rasmussen SC, Keiding N, Lokkegaard ECL, 2019a. Investigating the effects of cesarean delivery and antibiotic use in early childhood on risk of later attention deficit hyperactivity disorder. J Child Psychol Psychiatry 60(2), 151–159. [DOI] [PubMed] [Google Scholar]
- Axelsson PB, Clausen TD, Petersen AH, Hageman I, Pinborg A, Kessing LV, Bergholt T, Rasmussen SC, Keiding N, Lokkegaard ECL, 2019b. Relation Between Infant Microbiota and Autism?: Results from a National Cohort Sibling Design Study. Epidemiology 30(1), 52–60. [DOI] [PubMed] [Google Scholar]
- Babadjouni RM, Hodis DM, Radwanski R, Durazo R, Patel A, Liu Q, Mack WJ, 2017. Clinical effects of air pollution on the central nervous system; a review. J Clin Neurosci 43, 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee TD, Middleton F, Faraone SV, 2007. Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 96(9), 1269–1274. [DOI] [PubMed] [Google Scholar]
- Barko PC, McMichael MA, Swanson KS, Williams DA, 2018. The Gastrointestinal Microbiome: A Review. J Vet Intern Med 32(1), 9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiardes S, Zilberman-Schapira G, Elinav E, 2016. Use of Metatranscriptomics in Microbiome Research. Bioinform Biol Insights 10, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SS, Hubbard KA, Lundholm N, Montresor M, Leaw CP, 2018. Pseudo-nitzschia, Nitzschia, and domoic acid: New research since 2011. Harmful Algae 79, 3–43. [DOI] [PubMed] [Google Scholar]
- Beamish LA, Osornio-Vargas AR, Wine E, 2011. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis 5(4), 279–286. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI 2nd, Gong DH, Mihic SJ, 2000. Glycine and gamma-aminobutyric acid(A) receptor function is enhanced by inhaled drugs of abuse. Mol Pharmacol 57(6), 1199–1205. [PubMed] [Google Scholar]
- Bendorius M, Po C, Muller S, Jeltsch-David H, 2018. From Systemic Inflammation to Neuroinflammation: The Case of Neurolupus. Int J Mol Sci 19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT, 2000. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12), 1301–1306. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S, Lukiw WJ, 2013. Alzheimer’s disease and the microbiome. Front Cell Neurosci 7, 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz JE, Enos MK, Mwanga JR, Changalucha J, Burton JP, Gloor GB, Reid G, 2014. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. MBio 5(5), e01580–01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz JE, Spanogiannopoulos P, Pieper LM, Bustion AE, Turnbaugh PJ, 2018. How to Determine the Role of the Microbiome in Drug Disposition. Drug Metab Dispos 46(11), 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisbal M, Sanchez M, 2019. Neurotoxicity of the pesticide rotenone on neuronal polarization: a mechanistic approach. Neural Regen Res 14(5), 762–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscarini F, Palazzo F, Castellani F, Masetti G, Grotta L, Cichelli A, Martino G, 2018. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS One 13(11), e0205670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L, 2009. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci 32(9), 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfili L, Cecarini V, Berardi S, Scarpona S, Suchodolski JS, Nasuti C, Fiorini D, Boarelli MC, Rossi G, Eleuteri AM, 2017. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep 7(1), 2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgo F, Riva A, Benetti A, Casiraghi MC, Bertelli S, Garbossa S, Anselmetti S, Scarone S, Pontiroli AE, Morace G, Borghi E, 2017. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS One 12(6), e0179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF, 2014. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 20(9), 509–518. [DOI] [PubMed] [Google Scholar]
- Bourassa MW, Alim I, Bultman SJ, Ratan RR, 2016. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci Lett 625, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton J, Massart S, Vandamme P, De Brandt E, Pot B, Foligne B, 2013. Ecotoxicology inside the gut: impact of heavy metals on the mouse microbiome. BMC Pharmacol Toxicol 14, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges KN, Zhang Y, Curran TE, Magnuson JT, Venables BJ, Durrer KE, Allen MS, Roberts AP, 2018. Alterations to the Intestinal Microbiome and Metabolome of Pimephales promelas and Mus musculus Following Exposure to Dietary Methylmercury. Environ Sci Technol 52(15), 8774–8784. [DOI] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S, 2013. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One 8(1), e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Avila-Ramirez J, Calderon-Garciduenas A, Gonzalez-Heredia T, Acuna-Ayala H, Chao CK, Thompson C, Ruiz-Ramos R, Cortes-Gonzalez V, Martinez-Martinez L, Garcia-Perez MA, Reis J, Mukherjee PS, Torres-Jardon R, Lachmann I, 2016. Cerebrospinal Fluid Biomarkers in Highly Exposed PM2.5 Urbanites: The Risk of Alzheimer’s and Parkinson’s Diseases in Young Mexico City Residents. J Alzheimers Dis 54(2), 597–613. [DOI] [PubMed] [Google Scholar]
- Calne DB, Langston JW, Martin WR, Stoessl AJ, Ruth TJ, Adam MJ, Pate BD, Schulzer M, 1985. Positron emission tomography after MPTP: observations relating to the cause of Parkinson’s disease. Nature 317(6034), 246–248. [DOI] [PubMed] [Google Scholar]
- Cangelosi GA, Brabant WH, 1997. Depletion of pre-16S rRNA in starved Escherichia coli cells. J Bacteriol 179(14), 4457–4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangelosi GA, Meschke JS, 2014. Dead or alive: molecular assessment of microbial viability. Appl Environ Microbiol 80(19), 5884–5891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C, 2015. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28(2), 203–209. [PMC free article] [PubMed] [Google Scholar]
- Carmody RN, Turnbaugh PJ, 2014. Host-microbial interactions in the metabolism of therapeutic and diet-derived xenobiotics. J Clin Invest 124(10), 4173–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casida JE, Quistad GB, 2004. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol 17(8), 983–998. [DOI] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Sjodin A, Fenster L, Jones RS, Harley KG, Eisen EA, Eskenazi B, 2011. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ Sci Technol 45(15), 6553–6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Nallar E, Bendall ML, Perez-Losada M, Sabuncyan S, Severance EG, Dickerson FB, Schroeder JR, Yolken RH, Crandall KA, 2015. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. PeerJ 3, e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattani D, de Liz Oliveira Cavalli VL, Heinz Rieg CE, Domingues JT, Dal-Cim T, Tasca CI, Mena Barreto Silva FR, Zamoner A, 2014. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: involvement of glutamate excitotoxicity. Toxicology 320, 34–45. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, Raina GB, Pecci C, Pellene A, Calandra CR, Gutierrez C, Micheli FE, Benarroch EE, 2013. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol 260(5), 1332–1338. [DOI] [PubMed] [Google Scholar]
- Chang CC, Lin TC, Ho HL, Kuo CY, Li HH, Korolenko TA, Chen WJ, Lai TJ, Ho YJ, Lin CL, 2018. GLP-1 Analogue Liraglutide Attenuates Mutant Huntingtin-Induced Neurotoxicity by Restoration of Neuronal Insulin Signaling. Int J Mol Sci 19(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chegini S, Metcalfe DD, 2005. Contemporary issues in food allergy: seafood toxin-induced disease in the differential diagnosis of allergic reactions. Allergy Asthma Proc 26(3), 183–190. [PubMed] [Google Scholar]
- Chen D, Yang X, Yang J, Lai G, Yong T, Tang X, Shuai O, Zhou G, Xie Y, Wu Q, 2017. Prebiotic Effect of Fructooligosaccharides from Morinda officinalis on Alzheimer’s Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front Aging Neurosci 9, 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Toyomasu Y, Hayashi Y, Linden DR, Szurszewski JH, Nelson H, Farrugia G, Kashyap PC, Chia N, Ordog T, 2016. Altered gut microbiota in female mice with persistent low body weights following removal of post-weaning chronic dietary restriction. Genome Med 8(1), 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, Xie P, 2018. Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat 14, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Hu C, Lok-Shun Lai N, Zhang W, Hua J, Lam PKS, Lam JCW, Zhou B, 2018a. Acute exposure to PBDEs at an environmentally realistic concentration causes abrupt changes in the gut microbiota and host health of zebrafish. Environ Pollut 240, 17–26. [DOI] [PubMed] [Google Scholar]
- Chen L, Yu K, Huang C, Yu L, Zhu B, Lam PK, Lam JC, Zhou B, 2012. Prenatal transfer of polybrominated diphenyl ethers (PBDEs) results in developmental neurotoxicity in zebrafish larvae. Environ Sci Technol 46(17), 9727–9734. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang W, Hua J, Hu C, Lok-Shun Lai N, Qian PY, Lam PKS, Lam JCW, Zhou B, 2018b. Dysregulation of Intestinal Health by Environmental Pollutants: Involvement of the Estrogen Receptor and Aryl Hydrocarbon Receptor. Environ Sci Technol 52(4), 2323–2330. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, Hu Z, Wang H, Zhong X, Zeng L, Chen K, Li P, Xie P, 2018. Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 29(5), 417–425. [DOI] [PubMed] [Google Scholar]
- Cherbut C, Ferrier L, Roze C, Anini Y, Blottiere H, Lecannu G, Galmiche JP, 1998. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol 275(6), G1415–1422. [DOI] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Ru H, Tu P, Lu K, 2016. Sex-Specific Effects of Arsenic Exposure on the Trajectory and Function of the Gut Microbiome. Chem Res Toxicol 29(6), 949–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Bian X, Gao B, Tu P, Ru H, Lu K, 2017a. The Effects of an Environmentally Relevant Level of Arsenic on the Gut Microbiome and Its Functional Metagenome. Toxicol Sci 160(2), 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Gao B, Bian X, Tu P, Ru H, Lu K, 2017b. Manganese-induced sex-specific gut microbiome perturbations in C57BL/6 mice. Toxicol Appl Pharmacol 331, 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L, Mahbub R, Gao B, Bian X, Tu P, Ru H, Lu K, 2017c. Nicotine Alters the Gut Microbiome and Metabolites of Gut-Brain Interactions in a Sex-Specific Manner. Chem Res Toxicol 30(12), 2110–2119. [DOI] [PubMed] [Google Scholar]
- Chi L, Xue J, Tu P, Lai Y, Ru H, Lu K, 2019. Gut microbiome disruption altered the biotransformation and liver toxicity of arsenic in mice. Arch Toxicol 93(1), 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Lin Y, Zhu H, Huang Q, Ye G, Dong S, 2018. PCBs-high-fat diet interactions as mediators of gut microbiota dysbiosis and abdominal fat accumulation in female mice. Environ Pollut 239, 332–341. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M, 2013. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect 121(6), 725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus SP, Guillou H, Ellero-Simatos S, 2016. The gut microbiota: a major player in the toxicity of environmental pollutants? NPJ Biofilms Microbiomes 2, 16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coretti L, Cristiano C, Florio E, Scala G, Lama A, Keller S, Cuomo M, Russo R, Pero R, Paciello O, Mattace Raso G, Meli R, Cocozza S, Calignano A, Chiariotti L, Lembo F, 2017. Sex-related alterations of gut microbiota composition in the BTBR mouse model of autism spectrum disorder. Sci Rep 7, 45356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coretti L, Paparo L, Riccio MP, Amato F, Cuomo M, Natale A, Borrelli L, Corrado G, Comegna M, Buommino E, Castaldo G, Bravaccio C, Chiariotti L, Berni Canani R, Lembo F, 2018. Gut Microbiota Features in Young Children With Autism Spectrum Disorders. Front Microbiol 9, 3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K, Paul S, Lowe N, Weber M, McCarthy DO, 2015. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun 49, 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell M, McAlpine M, Pinkham NV, McDermott TR, Walk ST, 2018. The gut microbiome is required for full protection against acute arsenic toxicity in mouse models. Nat Commun 9(1), 5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, 2015. The neurotoxicity of organochlorine and pyrethroid pesticides. Handb Clin Neurol 131, 135–148. [DOI] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C, 2014. A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity. Toxicol Lett 230(2), 282–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Giordano G, 2007. Developmental neurotoxicity of polybrominated diphenyl ether (PBDE) flame retardants. Neurotoxicology 28(6), 1047–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Dauge V, Naudon L, Rabot S, 2014. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology 42, 207–217. [DOI] [PubMed] [Google Scholar]
- Cui B, Su D, Li W, She X, Zhang M, Wang R, Zhai Q, 2018. Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer’s disease. J Neuroinflammation 15(1), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussotto S, Strain CR, Fouhy F, Strain RG, Peterson VL, Clarke G, Stanton C, Dinan TG, Cryan JF, 2018. Differential effects of psychotropic drugs on microbiome composition and gastrointestinal function. Psychopharmacology (Berl). [DOI] [PubMed] [Google Scholar]
- D’Onofrio A, Crawford JM, Stewart EJ, Witt K, Gavrish E, Epstein S, Clardy J, Lewis K, 2010. Siderophores from neighboring organisms promote the growth of uncultured bacteria. Chem Biol 17(3), 254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacquino C, De Rossi P, Spalletta G, 2015. Schizophrenia and bipolar disorder: The road from similarities and clinical heterogeneity to neurobiological types. Clin Chim Acta 449, 49–59. [DOI] [PubMed] [Google Scholar]
- Dada N, Sheth M, Liebman K, Pinto J, Lenhart A, 2018. Whole metagenome sequencing reveals links between mosquito microbiota and insecticide resistance in malaria vectors. Sci Rep 8(1), 2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan D, Jude BA, Lamendella R, Keesing F, Perron GG, 2018. Exposure to Arsenic Alters the Microbiome of Larval Zebrafish. Front Microbiol 9, 1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai P, Yan Z, Ma S, Yang Y, Wang Q, Hou C, Wu Y, Liu Y, Diao Q, 2018. The Herbicide Glyphosate Negatively Affects Midgut Bacterial Communities and Survival of Honey Bee during Larvae Reared in Vitro. J Agric Food Chem 66(29), 7786–7793. [DOI] [PubMed] [Google Scholar]
- Daisley BA, Trinder M, McDowell TW, Collins SL, Sumarah MW, Reid G, 2018. Microbiota-Mediated Modulation of Organophosphate Insecticide Toxicity by Species-Dependent Interactions with Lactobacilli in a Drosophila melanogaster Insect Model. Appl Environ Microbiol 84(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisley BA, Trinder M, McDowell TW, Welle H, Dube JS, Ali SN, Leong HS, Sumarah MW, Reid G, 2017. Neonicotinoid-induced pathogen susceptibility is mitigated by Lactobacillus plantarum immune stimulation in a Drosophila melanogaster model. Sci Rep 7(1), 2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daliri EB, Wei S, Oh DH, Lee BH, 2017. The human microbiome and metabolomics: Current concepts and applications. Crit Rev Food Sci Nutr 57(16), 3565–3576. [DOI] [PubMed] [Google Scholar]
- Das P, Ji B, Kovatcheva-Datchary P, Backhed F, Nielsen J, 2018. In vitro co-cultures of human gut bacterial species as predicted from co-occurrence network analysis. PLoS One 13(3), e0195161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M, Piccolo M, Vannini L, Siragusa S, De Giacomo A, Serrazzanetti DI, Cristofori F, Guerzoni ME, Gobbetti M, Francavilla R, 2013. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8(10), e76993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Theije CG, Wopereis H, Ramadan M, van Eijndthoven T, Lambert J, Knol J, Garssen J, Kraneveld AD, Oozeer R, 2014. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav Immun 37, 197–206. [DOI] [PubMed] [Google Scholar]
- Dedrick RM, Guerrero-Bustamante CA, Garlena RA, Russell DA, Ford K, Harris K, Gilmour KC, Soothill J, Jacobs-Sera D, Schooley RT, Hatfull GF, Spencer H, 2019. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 25(5), 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai V, Kaler SG, 2008. Role of copper in human neurological disorders. Am J Clin Nutr 88(3), 855S–858S. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, Cotter PD, Dinan TG, Cryan JF, 2015. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav Immun 48, 165–173. [DOI] [PubMed] [Google Scholar]
- Dheer R, Patterson J, Dudash M, Stachler EN, Bibby KJ, Stolz DB, Shiva S, Wang Z, Hazen SL, Barchowsky A, Stolz JF, 2015. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol Appl Pharmacol 289(3), 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giancamillo A, Rossi R, Martino PA, Aidos L, Maghin F, Domeneghini C, Corino C, 2018. Copper sulphate forms in piglet diets: Microbiota, intestinal morphology and enteric nervous system glial cells. Anim Sci J 89(3), 616–624. [DOI] [PubMed] [Google Scholar]
- Di Prisco G, Cavaliere V, Annoscia D, Varricchio P, Caprio E, Nazzi F, Gargiulo G, Pennacchio F, 2013. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc Natl Acad Sci U S A 110(46), 18466–18471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Heijtz R, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, Hibberd ML, Forssberg H, Pettersson S, 2011. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci U S A 108(7), 3047–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingemans MM, de Groot A, van Kleef RG, Bergman A, van den Berg M, Vijverberg HP, Westerink RH, 2008. Hydroxylation increases the neurotoxic potential of BDE-47 to affect exocytosis and calcium homeostasis in PC12 cells. Environ Health Perspect 116(5), 637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson AW, Erikson KM, Aschner M, 2004. Manganese neurotoxicity. Ann N Y Acad Sci 1012, 115–128. [DOI] [PubMed] [Google Scholar]
- Dodiya HB, Forsyth CB, Voigt RM, Engen PA, Patel J, Shaikh M, Green SJ, Naqib A, Roy A, Kordower JH, Pahan K, Shannon KM, Keshavarzian A, 2018. Chronic stress-induced gut dysfunction exacerbates Parkinson’s disease phenotype and pathology in a rotenone-induced mouse model of Parkinson’s disease. Neurobiol Dis. [DOI] [PubMed] [Google Scholar]
- Dong X, Shulzhenko N, Lemaitre J, Greer RL, Peremyslova K, Quamruzzaman Q, Rahman M, Hasan OS, Joya SA, Golam M, Christiani DC, Morgun A, Kile ML, 2017. Arsenic exposure and intestinal microbiota in children from Sirajdikhan, Bangladesh. PLoS One 12(12), e0188487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman DC, Chiu W, Hales BF, Hauser R, Johnson KJ, Mantus E, Martel S, Robinson KA, Rooney AA, Rudel R, Sathyanarayana S, Schantz SL, Waters KM, 2018. Polybrominated diphenyl ether (PBDE) neurotoxicity: a systematic review and meta-analysis of animal evidence. J Toxicol Environ Health B Crit Rev 21(4), 269–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AB, Paul S, Ware LZ, Corwin EJ, 2015. Perineal Injury During Childbirth Increases Risk of Postpartum Depressive Symptoms and Inflammatory Markers. J Midwifery Womens Health 60(4), 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy-Doherty F, O’Mahony SM, Peterson VL, O’Sullivan O, Crispie F, Cotter PD, Wigmore P, King MV, Cryan JF, Fone KCF, 2018. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav Immun 68, 261–273. [DOI] [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, Nygren G, Rastam M, Gillberg IC, Anckarsater H, Sponheim E, Goubran-Botros H, Delorme R, Chabane N, Mouren-Simeoni MC, de Mas P, Bieth E, Roge B, Heron D, Burglen L, Gillberg C, Leboyer M, Bourgeron T, 2007. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39(1), 25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA, 2005. Diversity of the human intestinal microbial flora. Science 308(5728), 1635–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Zhan SS, van Gool WA, Allsop D, 1994. Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol Sci 15(12), 447–450. [DOI] [PubMed] [Google Scholar]
- El-Ansary A, Bacha AB, Bjorklund G, Al-Orf N, Bhat RS, Moubayed N, Abed K, 2018. Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab Brain Dis 33(4), 1155–1164. [DOI] [PubMed] [Google Scholar]
- Evans SJ, Bassis CM, Hein R, Assari S, Flowers SA, Kelly MB, Young VB, Ellingrod VE, McInnis MG, 2017. The gut microbiome composition associates with bipolar disorder and illness severity. J Psychiatr Res 87, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang B, Li JW, Zhang M, Ren FZ, Pang GF, 2018. Chronic chlorpyrifos exposure elicits diet-specific effects on metabolism and the gut microbiome in rats. Food Chem Toxicol 111, 144–152. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, Rohde LA, Sonuga-Barke EJ, Tannock R, Franke B, 2015. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers 1, 15020. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Glatt SJ, 2010. A comparison of the efficacy of medications for adult attention-deficit/hyperactivity disorder using meta-analysis of effect sizes. J Clin Psychiatry 71(6), 754–763. [DOI] [PubMed] [Google Scholar]
- Fenn K, Strandwitz P, Stewart EJ, Dimise E, Rubin S, Gurubacharya S, Clardy J, Lewis K, 2017. Quinones are growth factors for the human gut microbiota. Microbiome 5(1), 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, Liu M, Molitoris DR, Green JA 3rd, 2010. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16(4), 444–453. [DOI] [PubMed] [Google Scholar]
- Florez AB, Sierra M, Ruas-Madiedo P, Mayo B, 2016. Susceptibility of lactic acid bacteria, bifidobacteria and other bacteria of intestinal origin to chemotherapeutic agents. Int J Antimicrob Agents 48(5), 547–550. [DOI] [PubMed] [Google Scholar]
- Flowers SA, Baxter NT, Ward KM, Kraal AZ, McInnis MG, Schmidt TM, Ellingrod VL, 2019. Effects of Atypical Antipsychotic Treatment and Resistant Starch Supplementation on Gut Microbiome Composition in a Cohort of Patients with Bipolar Disorder or Schizophrenia. Pharmacotherapy 39(2), 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers SA, Evans SJ, Ward KM, McInnis MG, Ellingrod VL, 2017. Interaction Between Atypical Antipsychotics and the Gut Microbiome in a Bipolar Disease Cohort. Pharmacotherapy 37(3), 261–267. [DOI] [PubMed] [Google Scholar]
- Fournier CN, Houser M, Tansey MG, Glass JD, Hertzberg VS, 2018. The gut microbiome and neuroinflammation in amyotrophic lateral sclerosis? Emerging clinical evidence. Neurobiol Dis. [DOI] [PubMed] [Google Scholar]
- Fraser AG, McQueen IN, Watt AH, Stephens MR, 1985. Peripheral neuropathy during longterm high-dose amiodarone therapy. J Neurol Neurosurg Psychiatry 48(6), 576–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier KR, Moore JA, Long TE, 2019. Antibacterial activity of disulfiram and its metabolites. J Appl Microbiol 126(1), 79–86. [DOI] [PubMed] [Google Scholar]
- Freund R, Kelsberg G, Safranek S, 2014. Clinical Inquiry: do oral contraceptives put women with a family history of breast cancer at increased risk? J Fam Pract 63(9), 540, 549. [PubMed] [Google Scholar]
- Fromme H, Korner W, Shahin N, Wanner A, Albrecht M, Boehmer S, Parlar H, Mayer R, Liebl B, Bolte G, 2009. Human exposure to polybrominated diphenyl ethers (PBDE), as evidenced by data from a duplicate diet study, indoor air, house dust, and biomonitoring in Germany. Environ Int 35(8), 1125–1135. [DOI] [PubMed] [Google Scholar]
- Frye RE, Rose S, Chacko J, Wynne R, Bennuri SC, Slattery JC, Tippett M, Delhey L, Melnyk S, Kahler SG, MacFabe DF, 2016. Modulation of mitochondrial function by the microbiome metabolite propionic acid in autism and control cell lines. Transl Psychiatry 6(10), e927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZD, Cui JY, 2017. Remote Sensing between Liver and Intestine: Importance of Microbial Metabolites. Curr Pharmacol Rep 3(3), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuto TR, 1990. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect 87, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D, 2016. Neuro-immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 164(3), 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland L, 2014. The gut microbiome and the brain. J Med Food 17(12), 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bian X, Chi L, Tu P, Ru H, Lu K, 2017a. Editor’s Highlight: OrganophosphateDiazinon Altered Quorum Sensing, Cell Motility, Stress Response, and Carbohydrate Metabolism of Gut Microbiome. Toxicol Sci 157(2), 354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Bian X, Mahbub R, Lu K, 2017b. Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions. Environ Health Perspect 125(2), 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Chi L, Mahbub R, Bian X, Tu P, Ru H, Lu K, 2017c. Multi-Omics Reveals that Lead Exposure Disturbs Gut Microbiome Development, Key Metabolites, and Metabolic Pathways. Chem Res Toxicol 30(4), 996–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Chi L, Tu P, Bian X, Thomas J, Ru H, Lu K, 2018a. The organophosphate malathion disturbs gut microbiome development and the quorum-Sensing system. Toxicol Lett 283, 52–57. [DOI] [PubMed] [Google Scholar]
- Gao B, Chi L, Tu P, Gao N, Lu K, 2018b. The carbamate aldicarb altered the gut microbiome, metabolome and lipidome of C57BL/6J mice. Chem Res Toxicol. [DOI] [PubMed] [Google Scholar]
- Gaulke CA, Rolshoven J, Wong CP, Hudson LG, Ho E, Sharpton TJ, 2018. Marginal Zinc Deficiency and Environmentally Relevant Concentrations of Arsenic Elicit Combined Effects on the Gut Microbiome. mSphere 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, D’Arpa P, Donohue DE, Muhie S, Chakraborty N, Luke BT, Grapov D, Carroll EE, Meyerhoff JL, Hammamieh R, Jett M, 2015. Acute and chronic plasma metabolomic and liver transcriptomic stress effects in a mouse model with features of post-traumatic stress disorder. PLoS One 10(1), e0117092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A, Kumar R, Chakraborty N, Muhie S, Hoke A, Hammamieh R, Jett M, 2018. Altered fecal microbiota composition in all male aggressor-exposed rodent model simulating features of post-traumatic stress disorder. J Neurosci Res 96(7), 1311–1323. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T, 2005. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 106(5 Pt 1), 1071–1083. [DOI] [PubMed] [Google Scholar]
- Giordano G, Kavanagh TJ, Costa LG, 2008. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol 232(2), 161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri SS, Yun S, Jun JW, Kim HJ, Kim SG, Kang JW, Kim SW, Han SJ, Sukumaran V, Park SC, 2018. Therapeutic Effect of Intestinal Autochthonous Lactobacillus reuteri P16 Against Waterborne Lead Toxicity in Cyprinus carpio. Front Immunol 9, 1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golubeva AV, Joyce SA, Moloney G, Burokas A, Sherwin E, Arboleya S, Flynn I, Khochanskiy D, Moya-Perez A, Peterson V, Rea K, Murphy K, Makarova O, Buravkov S, Hyland NP, Stanton C, Clarke G, Gahan CGM, Dinan TG, Cryan JF, 2017. Microbiota-related Changes in Bile Acid & Tryptophan Metabolism are Associated with Gastrointestinal Dysfunction in a Mouse Model of Autism. EBioMedicine 24, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez GI, Falcon RV, Maturana CJ, Labra VC, Salgado N, Rojas CA, Oyarzun JE, Cerpa W, Quintanilla RA, Orellana JA, 2018. Heavy Alcohol Exposure Activates Astroglial Hemichannels and Pannexons in the Hippocampus of Adolescent Rats: Effects on Neuroinflammation and Astrocyte Arborization. Front Cell Neurosci 12, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondalia SV, Palombo EA, Knowles SR, Cox SB, Meyer D, Austin DW, 2012. Molecular characterisation of gastrointestinal microbiota of children with autism (with and without gastrointestinal dysfunction) and their neurotypical siblings. Autism Res 5(6), 419–427. [DOI] [PubMed] [Google Scholar]
- Goolsby TA, Jakeman B, Gaynes RP, 2018. Clinical relevance of metronidazole and peripheral neuropathy: a systematic review of the literature. Int J Antimicrob Agents 51(3), 319–325. [DOI] [PubMed] [Google Scholar]
- Gorchev HG, Jelinek CF, 1985. A review of the dietary intakes of chemical contaminants. Bull World Health Organ 63(5), 945–962. [PMC free article] [PubMed] [Google Scholar]
- Govindarajan N, Agis-Balboa RC, Walter J, Sananbenesi F, Fischer A, 2011. Sodium butyrate improves memory function in an Alzheimer’s disease mouse model when administered at an advanced stage of disease progression. J Alzheimers Dis 26(1), 187–197. [DOI] [PubMed] [Google Scholar]
- Graham, Lantos PL, 1997. Greenfield’s neuropathology. Arnold, London. [Google Scholar]
- Grigg JB, Sonnenberg GF, 2017. Host-Microbiota Interactions Shape Local and Systemic Inflammatory Diseases. J Immunol 198(2), 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Yumvihoze E, Poulain AJ, Man Chan H, 2018. Monomethylmercury degradation by the human gut microbiota is stimulated by protein amendments. J Toxicol Sci 43(12), 717–725. [DOI] [PubMed] [Google Scholar]
- Guo J, Zhao Y, Jiang X, Li R, Xie H, Ge L, Xie B, Yang X, Zhang L, 2018. Exposure to Formaldehyde Perturbs the Mouse Gut Microbiome. Genes (Basel) 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiser HJ, Turnbaugh PJ, 2013. Developing a metagenomic view of xenobiotic metabolism. Pharmacol Res 69(1), 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, Miller J, Fedele A, Collins J, Smith K, Lotspeich L, Croen LA, Ozonoff S, Lajonchere C, Grether JK, Risch N, 2011. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11), 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF, 2018. Early childhood antibiotics use and autism spectrum disorders: a population-based cohort study. Int J Epidemiol 47(5), 1497–1506. [DOI] [PubMed] [Google Scholar]
- Han W, Tian Y, Shen X, 2018. Human exposure to neonicotinoid insecticides and the evaluation of their potential toxicity: An overview. Chemosphere 192, 59–65. [DOI] [PubMed] [Google Scholar]
- Harach T, Marungruang N, Duthilleul N, Cheatham V, Mc Coy KD, Frisoni G, Neher JJ, Fak F, Jucker M, Lasser T, Bolmont T, 2017. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Scientific reports 7, 41802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B, 2010. PBDE concentrations in women’s serum and fecundability. Environ Health Perspect 118(5), 699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Leve LD, Barrett D, Elam K, Neiderhiser JM, Natsuaki MN, Shaw DS, Reiss D, Thapar A, 2013. Biological and rearing mother influences on child ADHD symptoms: revisiting the developmental interface between nature and nurture. J Child Psychol Psychiatry 54(10), 1038–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes TB, Anderson LL, Beasley VR, de Solla SR, Iguchi T, Ingraham H, Kestemont P, Kniewald J, Kniewald Z, Langlois VS, Luque EH, McCoy KA, Munoz-de-Toro M, Oka T, Oliveira CA, Orton F, Ruby S, Suzawa M, Tavera-Mendoza LE, Trudeau VL, Victor-Costa AB, Willingham E, 2011. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. J Steroid Biochem Mol Biol 127(1–2), 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA, 2013. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80(19), 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss CN, Olofsson LE, 2019. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J Neuroendocrinol, e12684. [DOI] [PubMed] [Google Scholar]
- Hemmings SMJ, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, Bohr AD, Stamper CE, Hyde ER, Morton JT, Marotz CA, Siebler PH, Braspenning M, Van Criekinge W, Hoisington AJ, Brenner LA, Postolache TT, McQueen MB, Krauter KS, Knight R, Seedat S, Lowry CA, 2017. The Microbiome in Posttraumatic Stress Disorder and Trauma-Exposed Controls: An Exploratory Study. Psychosom Med 79(8), 936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz-Dahlmann B, 2009. Adolescent eating disorders: definitions, symptomatology, epidemiology and comorbidity. Child Adolesc Psychiatr Clin N Am 18(1), 31–47. [DOI] [PubMed] [Google Scholar]
- Herrick RF, 2010. PCBs in school-persistent chemicals, persistent problems. New Solut 20(1), 115–126. [DOI] [PubMed] [Google Scholar]
- Herrick RF, Stewart JH, Allen JG, 2016. Review of PCBs in US schools: a brief history, an estimate of the number of impacted schools, and an approach for evaluating indoor air samples. Environ Sci Pollut Res Int 23(3), 1975–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks SD, Uhlig R, Afshari P, Williams J, Chroneos M, Tierney-Aves C, Wagner K, Middleton FA, 2018. Oral microbiome activity in children with autism spectrum disorder. Autism Res 11(9), 1286–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Burns EM, Debelius JW, Morton JT, Wissemann WT, Lewis MR, Wallen ZD, Peddada SD, Factor SA, Molho E, Zabetian CP, Knight R, Payami H, 2017. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov Disord 32(5), 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmann B, Al-Ghalith GA, Shields-Cutler RR, Zhu Q, Gohl DM, Beckman KB, Knight R, Knights D, 2018. Evaluating the Information Content of Shallow Shotgun Metagenomics. mSystems 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Ono K, Tsuji M, Mazzola P, Singh R, Pasinetti GM, 2018. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev Neurother 18(1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen AG, Madan JC, Li Z, Coker M, Lundgren SN, Morrison HG, Palys T, Jackson BP, Sogin ML, Cottingham KL, Karagas MR, 2018. Sex-specific associations of infants’ gut microbiome with arsenic exposure in a US population. Sci Rep 8(1), 12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman JD, Parikh I, Green SJ, Chlipala G, Mohney RP, Keaton M, Bauer B, Hartz AMS, Lin AL, 2017. Age Drives Distortion of Brain Metabolic, Vascular and Cognitive Functions, and the Gut Microbiome. Front Aging Neurosci 9, 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojberg O, Canibe N, Poulsen HD, Hedemann MS, Jensen BB, 2005. Influence of dietary zinc oxide and copper sulfate on the gastrointestinal ecosystem in newly weaned piglets. Appl Environ Microbiol 71(5), 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu BB, Gibson TE, Yeliseyev V, Liu Q, Lyon L, Bry L, Silver PA, Gerber GK, 2019. Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 25(6), 803–814 e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC, 2010. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol 44(8), 2822–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Yang X, Zeng B, Zeng L, Gong X, Zhou C, Xia J, Lian B, Qin Y, Yang L, Liu L, Xie P, 2019. Proteomic analysis of olfactory bulb suggests CACNA1E as a promoter of CREB signaling in microbiota-induced depression. J Proteomics 194, 132–147. [DOI] [PubMed] [Google Scholar]
- Huf F, Bandiera S, Muller CB, Gea L, Carvalho FB, Rahmeier FL, Reiter KC, Tortorelli LS, Gomez R, da Cruz Fernandes M, 2019. Comparative study on the effects of cigarette smoke exposure, ethanol consumption and association: Behavioral parameters, apoptosis, glial fibrillary acid protein and S100beta immunoreactivity in different regions of the rat hippocampus. Alcohol 77, 101–112. [DOI] [PubMed] [Google Scholar]
- Hurley S, Goldberg D, Nelson DO, Guo W, Wang Y, Baek HG, Park JS, Petreas M, Bernstein L, Anton-Culver H, Reynolds P, 2017. Temporal Evaluation of Polybrominated Diphenyl Ether (PBDE) Serum Levels in Middle-Aged and Older California Women, 2011–2015. Environ Sci Technol 51(8), 4697–4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M, Matsuda K, 2018. Neonicotinoids: molecular mechanisms of action, insights into resistance and impact on pollinators. Curr Opin Insect Sci 30, 86–92. [DOI] [PubMed] [Google Scholar]
- Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV, 2004. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet 20(7), 292–299. [DOI] [PubMed] [Google Scholar]
- Janakiraman M, Krishnamoorthy G, 2018. Emerging Role of Diet and Microbiota Interactions in Neuroinflammation. Front Immunol 9, 2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraj R, Megha P, Sreedev P, 2016. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9(3–4), 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B, 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48, 186–194. [DOI] [PubMed] [Google Scholar]
- Jiang HY, Zhou YY, Zhou GL, Li YC, Yuan J, Li XH, Ruan B, 2018. Gut microbiota profiles in treatment-naive children with attention deficit hyperactivity disorder. Behav Brain Res 347, 408–413. [DOI] [PubMed] [Google Scholar]
- Johnson JD, Meisenheimer TL, Isom GE, 1986. Cyanide-induced neurotoxicity: role of neuronal calcium. Toxicol Appl Pharmacol 84(3), 464–469. [DOI] [PubMed] [Google Scholar]
- Johnson ME, Stringer A, Bobrovskaya L, 2018. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 65, 174–185. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Goldstein GW, 1998. Selective vulnerability of the developing brain to lead. Curr Opin Neurol 11(6), 689–693. [DOI] [PubMed] [Google Scholar]
- Joly C, Gay-Queheillard J, Leke A, Chardon K, Delanaud S, Bach V, Khorsi-Cauet H, 2013. Impact of chronic exposure to low doses of chlorpyrifos on the intestinal microbiota in the Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and in the rat. Environ Sci Pollut Res Int 20(5), 2726–2734. [DOI] [PubMed] [Google Scholar]
- Joly Condette C, Bach V, Mayeur C, Gay-Queheillard J, Khorsi-Cauet H, 2015. Chlorpyrifos Exposure During Perinatal Period Affects Intestinal Microbiota Associated With Delay of Maturation of Digestive Tract in Rats. J Pediatr Gastroenterol Nutr 61(1), 30–40. [DOI] [PubMed] [Google Scholar]
- Kakumanu ML, Reeves AM, Anderson TD, Rodrigues RR, Williams MA, 2016. Honey Bee Gut Microbiome Is Altered by In-Hive Pesticide Exposures. Front Microbiol 7, 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R, 2013. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8(7), e68322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania-Korwel I, Lukasiewicz T, Barnhart CD, Stamou M, Chung H, Kelly KM, Bandiera S, Lein PJ, Lehmler HJ, 2017. Editor’s Highlight: Congener-Specific Disposition of Chiral Polychlorinated Biphenyls in Lactating Mice and Their Offspring: Implications for PCB Developmental Neurotoxicity. Toxicol Sci 158(1), 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase H, Kaneko M, Yamada K, 1987. K-13, a novel inhibitor of angiotensin I converting enzyme produced by Micromonospora halophytica subsp. exilisia. I. Fermentation, isolation and biological properties. J Antibiot (Tokyo) 40(4), 450–454. [DOI] [PubMed] [Google Scholar]
- Kazemi A, Noorbala AA, Azam K, Eskandari MH, Djafarian K, 2018. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin Nutr. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, C OB, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG, 2016. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82, 109–118. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Minuto C, Cryan JF, Clarke G, Dinan TG, 2017. Cross Talk: The Microbiota and Neurodevelopmental Disorders. Front Neurosci 11, 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, 2000. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry 61 Suppl 5, 4–12; discussion 13–14. [PubMed] [Google Scholar]
- Khalilnezhad A, Taskiran D, 2018. Protective effects of glucagon-like peptide-1 (GLP-1) analogue exenatide against glucose and fructose-induced neurotoxicity. Int J Neurosci, 1–24. [DOI] [PubMed] [Google Scholar]
- Khare S, Gokulan K, Williams K, Bai S, Gilbert KM, Blossom SJ, 2019. Irreversible effects of trichloroethylene on the gut microbial community and gut-associated immune responses in autoimmune-prone mice. J Appl Toxicol 39(2), 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilburn KH, 1994. Neurobehavioral impairment and seizures from formaldehyde. Arch Environ Health 49(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Kim I, Kim DU, Kim NH, Ka JO, 2014. Isolation and characterization of fenobucarb-degrading bacteria from rice paddy soils. Biodegradation 25(3), 383–394. [DOI] [PubMed] [Google Scholar]
- Kish L, Hotte N, Kaplan GG, Vincent R, Tso R, Ganzle M, Rioux KP, Thiesen A, Barkema HW, Wine E, Madsen KL, 2013. Environmental particulate matter induces murine intestinal inflammatory responses and alters the gut microbiome. PLoS One 8(4), e62220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittle RP, McDermid KJ, Muehlstein L, Balazs GH, 2018. Effects of glyphosate herbicide on the gastrointestinal microflora of Hawaiian green turtles (Chelonia mydas) Linnaeus. Mar Pollut Bull 127, 170–174. [DOI] [PubMed] [Google Scholar]
- Klaassen C, 2013. Casarett and Doull’s Toxicology: The Basic Science of Poisons, Eighth Edition, in: Klaassen C (Ed.) 8th ed. McGraw-Hill Education. [Google Scholar]
- Klaassen CD, Cui JY, 2015. Review: Mechanisms of How the Intestinal Microbiota Alters the Effects of Drugs and Bile Acids. Drug Metab Dispos 43(10), 1505–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman SC, Watson HJ, Bulik-Sullivan EC, Huh EY, Tarantino LM, Bulik CM, Carroll IM, 2015. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom Med 77(9), 969–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M, Mangalore R, Simon J, 2004. The global costs of schizophrenia. Schizophr Bull 30(2), 279–293. [DOI] [PubMed] [Google Scholar]
- Knutie SA, Gabor CR, Kohl KD, Rohr JR, 2018. Do host-associated gut microbiota mediate the effect of an herbicide on disease risk in frogs? J Anim Ecol 87(2), 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ, 1987. MPTP: an industrial chemical and contaminant of illicit narcotics stimulates a new era in research on Parkinson’s disease. Environ Health Perspect 75, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrick SA, Sagiv SK, 2008. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr 20(2), 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosnicki KL, Penprase JC, Cintora P, Torres PJ, Harris GL, Brasser SM, Kelley ST, 2018. Effects of moderate, voluntary ethanol consumption on the rat and human gut microbiome. Addict Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski MD, Harrison NL, 2000. The actions of ether, alcohol and alkane general anaesthetics on GABAA and glycine receptors and the effects of TM2 and TM3 mutations. Br J Pharmacol 129(4), 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger M, Shehata AA, Schrodl W, Rodloff A, 2013. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe 20, 74–78. [DOI] [PubMed] [Google Scholar]
- Kuno T, Hirayama-Kurogi M, Ito S, Ohtsuki S, 2016. Effect of Intestinal Flora on Protein Expression of Drug-Metabolizing Enzymes and Transporters in the Liver and Kidney of Germ-Free and Antibiotics-Treated Mice. Mol Pharm 13(8), 2691–2701. [DOI] [PubMed] [Google Scholar]
- Labrecque MT, Malone D, Caldwell KE, Allan AM, 2015. Impact of Ethanol and Saccharin on Fecal Microbiome in Pregnant and Non-Pregnant Mice. J Pregnancy Child Health 2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagalante AF, Shedden CS, Greenbacker PW, 2011. Levels of polybrominated diphenyl ethers (PBDEs) in dust from personal automobiles in conjunction with studies on the photochemical degradation of decabromodiphenyl ether (BDE-209). Environ Int 37(5), 899–906. [DOI] [PubMed] [Google Scholar]
- Lagier JC, Hugon P, Khelaifia S, Fournier PE, La Scola B, Raoult D, 2015. The rebirth of culture in microbiology through the example of culturomics to study human gut microbiota. Clin Microbiol Rev 28(1), 237–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagkouvardos I, Overmann J, Clavel T, 2017. Cultured microbes represent a substantial fraction of the human and mouse gut microbiota. Gut Microbes 8(5), 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai F, Jiang R, Xie W, Liu X, Tang Y, Xiao H, Gao J, Jia Y, Bai Q, 2018. Intestinal Pathology and Gut Microbiota Alterations in a Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Mouse Model of Parkinson’s Disease. Neurochem Res 43(10), 1986–1999. [DOI] [PubMed] [Google Scholar]
- Larsson H, Sariaslan A, Langstrom N, D’Onofrio B, Lichtenstein P, 2014. Family income in early childhood and subsequent attention deficit/hyperactivity disorder: a quasi-experimental study. J Child Psychol Psychiatry 55(5), 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P, Windey K, Tremaroli V, Backhed F, Verbeke K, de Timary P, Delzenne NM, 2014. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A 111(42), E4485–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehouritis P, Cummins J, Stanton M, Murphy CT, McCarthy FO, Reid G, Urbaniak C, Byrne WL, Tangney M, 2015. Local bacteria affect the efficacy of chemotherapeutic drugs. Sci Rep 5, 14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JD, Goldstine J, Mayes M, Moskowitz MA, Basbaum AI, 1986. The neurotoxic effect of gold sodium thiomalate on the peripheral nerves of the rat. Insights into the antiinflammatory actions of gold therapy. Arthritis Rheum 29(7), 897–901. [DOI] [PubMed] [Google Scholar]
- Li CY, Dempsey JL, Wang D, Lee S, Weigel KM, Fei Q, Bhatt DK, Prasad B, Raftery D, Gu H, Cui JY, 2018. PBDEs Altered Gut Microbiome and Bile Acid Homeostasis in Male C57BL/6 Mice. Drug Metab Dispos 46(8), 1226–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Lee S, Cade S, Kuo LJ, Schultz IR, Bhatt DK, Prasad B, Bammler TK, Cui JY, 2017. Novel Interactions between Gut Microbiome and Host Drug-Processing Genes Modify the Hepatic Metabolism of the Environmental Chemicals Polybrominated Diphenyl Ethers. Drug Metab Dispos 45(11), 1197–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang W, Pan YW, Xu L, Xia Z, 2013. A hydroxylated metabolite of flame-retardant PBDE-47 decreases the survival, proliferation, and neuronal differentiation of primary cultured adult neural stem cells and interferes with signaling of ERK5 MAP kinase and neurotrophin 3. Toxicol Sci 134(1), 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Zheng LJ, Zhang LJ, 2018. Neuroinflammation, Gut Microbiome, and Alzheimer’s Disease. Mol Neurobiol 55(11), 8243–8250. [DOI] [PubMed] [Google Scholar]
- Liu F, Horton-Sparks K, Hull V, Li RW, Martinez-Cerdeno V, 2018. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol Autism 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu X, Xiong XQ, Yang T, Cui T, Hou NL, Lai X, Liu S, Guo M, Liang XH, Cheng Q, Chen J, Li TY, 2017. Effect of vitamin A supplementation on gut microbiota in children with autism spectrum disorders - a pilot study. BMC Microbiol 17(1), 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Shao W, Zhang C, Xu C, Wang Q, Liu H, Sun H, Jiang Z, Gu A, 2017. Organochloride pesticides modulated gut microbiota and influenced bile acid metabolism in mice. Environ Pollut 226, 268–276. [DOI] [PubMed] [Google Scholar]
- Liu S, Li E, Sun Z, Fu D, Duan G, Jiang M, Yu Y, Mei L, Yang P, Tang Y, Zheng P, 2019. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci Rep 9(1), 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd NA, Janssen SE, Reinfelder JR, Barkay T, 2016. Co-selection of Mercury and Multiple Antibiotic Resistances in Bacteria Exposed to Mercury in the Fundulus heteroclitus Gut Microbiome. Curr Microbiol 73(6), 834–842. [DOI] [PubMed] [Google Scholar]
- Long TE, 2017. Repurposing Thiram and Disulfiram as Antibacterial Agents for Multidrug-Resistant Staphylococcus aureus Infections. Antimicrob Agents Chemother 61(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe PP, Gyongyosi B, Satishchandran A, Iracheta-Vellve A, Cho Y, Ambade A, Szabo G, 2018. Reduced gut microbiome protects from alcohol-induced neuroinflammation and alters intestinal and brain inflammasome expression. J Neuroinflammation 15(1), 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano VL, Defarge N, Rocque LM, Mesnage R, Hennequin D, Cassier R, de Vendomois JS, Panoff JM, Seralini GE, Amiel C, 2018. Sex-dependent impact of Roundup on the rat gut microbiome. Toxicol Rep 5, 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Synowiec S, Lu L, Yu Y, Bretherick T, Takada S, Yarnykh V, Caplan J, Caplan M, Claud EC, Drobyshevsky A, 2018. Microbiota influence the development of the brain and behaviors in C57BL/6J mice. PLoS One 13(8), e0201829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Abo RP, Schlieper KA, Graffam ME, Levine S, Wishnok JS, Swenberg JA, Tannenbaum SR, Fox JG, 2014. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect 122(3), 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Mahbub R, Fox JG, 2015. Xenobiotics: Interaction with the Intestinal Microflora. ILAR J 56(2), 218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowicz C, Ellero-Simatos S, Regnier M, Polizzi A, Lasserre F, Montagner A, Lippi Y, Jamin EL, Martin JF, Naylies C, Canlet C, Debrauwer L, Bertrand-Michel J, Al Saati T, Theodorou V, Loiseau N, Mselli-Lakhal L, Guillou H, Gamet-Payrastre L, 2018. Metabolic Effects of a Chronic Dietary Exposure to a Low-Dose Pesticide Cocktail in Mice: Sexual Dimorphism and Role of the Constitutive Androstane Receptor. Environ Health Perspect 126(6), 067007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie I, Yang YX, Haynes K, Mamtani R, Boursi B, 2015. Antibiotic exposure and the risk for depression, anxiety, or psychosis: a nested case-control study. J Clin Psychiatry 76(11), 1522–1528. [DOI] [PubMed] [Google Scholar]
- Lyng GD, Seegal RF, 2008. Polychlorinated biphenyl-induced oxidative stress in organotypic co-cultures: experimental dopamine depletion prevents reductions in GABA. Neurotoxicology 29(2), 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng GD, Snyder-Keller A, Seegal RF, 2007. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci 97(1), 128–139. [DOI] [PubMed] [Google Scholar]
- Mack I, Cuntz U, Gramer C, Niedermaier S, Pohl C, Schwiertz A, Zimmermann K, Zipfel S, Enck P, Penders J, 2016. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci Rep 6, 26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow ER, Chang FN, 1985. Processing of precursor ribosomal RNA and the presence of a modified ribosome assembly scheme in Escherichia coli relaxed strain. FEBS Lett 182(2), 407–412. [DOI] [PubMed] [Google Scholar]
- Magnusdottir S, Heinken A, Kutt L, Ravcheev DA, Bauer E, Noronha A, Greenhalgh K, Jager C, Baginska J, Wilmes P, Fleming RM, Thiele I, 2017. Generation of genome-scale metabolic reconstructions for 773 members of the human gut microbiota. Nat Biotechnol 35(1), 81–89. [DOI] [PubMed] [Google Scholar]
- Maisano M, Cappello T, Oliva S, Natalotto A, Giannetto A, Parrino V, Battaglia P, Romeo T, Salvo A, Spano N, Mauceri A, 2016. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, T. thynnus, from the Mediterranean Sea. Mar Environ Res 121, 40–48. [DOI] [PubMed] [Google Scholar]
- Manor O, Borenstein E, 2017. Systematic Characterization and Analysis of the Taxonomic Drivers of Functional Shifts in the Human Microbiome. Cell Host Microbe 21(2), 254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q, Manservisi F, Panzacchi S, Mandrioli D, Menghetti I, Vornoli A, Bua L, Falcioni L, Lesseur C, Chen J, Belpoggi F, Hu J, 2018. The Ramazzini Institute 13-week pilot study on glyphosate and Roundup administered at human-equivalent dose to Sprague Dawley rats: effects on the microbiome. Environ Health 17(1), 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matt SM, Allen JM, Lawson MA, Mailing LJ, Woods JA, Johnson RW, 2018. Butyrate and Dietary Soluble Fiber Improve Neuroinflammation Associated With Aging in Mice. Front Immunol 9, 1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuskova Z, Anzenbacher P, Vecera R, Siller M, Tlaskalova-Hogenova H, Strojil J, Anzenbacherova E, 2017. Effect of Lactobacillus casei on the Pharmacokinetics of Amiodarone in Male Wistar Rats. Eur J Drug Metab Pharmacokinet 42(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Matuskova Z, Anzenbacherova E, Vecera R, Tlaskalova-Hogenova H, Kolar M, Anzenbacher P, 2014. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PLoS One 9(2), e87150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay KA, Kowalec K, Brinkman F, Finlay BB, Horwitz M, Manges AR, Osborne L, Tremlett H, 2017. From bugs to brains: The microbiome in neurological health. Mult Scler Relat Disord 12, 1–3. [DOI] [PubMed] [Google Scholar]
- McMillin M, DeMorrow S, 2016. Effects of bile acids on neurological function and disease. FASEB J 30(11), 3658–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillin M, Frampton G, Quinn M, Ashfaq S, de los Santos M 3rd, Grant S, DeMorrow S, 2016. Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure. Am J Pathol 186(2), 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Golden NH, 2010. An introduction to eating disorders: clinical presentation, epidemiology, and prognosis. Nutr Clin Pract 25(2), 110–115. [DOI] [PubMed] [Google Scholar]
- Mills BD, Pearce HL, Khan O, Jarrett BR, Fair DA, Lahvis GP, 2016. Prenatal domoic acid exposure disrupts mouse pro-social behavior and functional connectivity MRI. Behav Brain Res 308, 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RH, Vazquez-Baeza Y, Zhu Q, Jiang L, Gaffney J, Humphrey G, Smarr L, Knight R, Gonzalez DJ, 2019. Evaluating Metagenomic Prediction of the Metaproteome in a 4.5-Year Study of a Patient with Crohn’s Disease. mSystems 4(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimee M, Tucker AC, Voigt CA, Lu TK, 2015. Programming a Human Commensal Bacterium, Bacteroides thetaiotaomicron, to Sense and Respond to Stimuli in the Murine Gut Microbiota. Cell Syst 1(1), 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Hinterleitner R, Meisel M, Zhang C, Leone V, Zhang X, Oyler-Castrillo P, Zhang X, Musch MW, Shen X, Jabri B, Chang EB, Tanzi RE, Sisodia SS, 2017. Antibiotic-induced perturbations in microbial diversity during post-natal development alters amyloid pathology in an aged APPSWE/PS1DeltaE9 murine model of Alzheimer’s disease. Sci Rep 7(1), 10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minter MR, Zhang C, Leone V, Ringus DL, Zhang X, Oyler-Castrillo P, Musch MW, Liao F, Ward JF, Holtzman DM, Chang EB, Tanzi RE, Sisodia SS, 2016. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci Rep 6, 30028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Kanayama M, Wake R, Hashioka S, Hayashida M, Nagahama M, Okazaki S, Yamashita S, Miura S, Miki H, Matsuda H, Koike M, Izuhara M, Araki T, Tsuchie K, Azis IA, Arauchi R, Abdullah RA, Oh-Nishi A, Horiguchi J, 2018. Clostridium butyricum MIYAIRI 588 as Adjunctive Therapy for Treatment-Resistant Major Depressive Disorder: A Prospective Open-Label Trial. Clin Neuropharmacol 41(5), 151–155. [DOI] [PubMed] [Google Scholar]
- Moos WH, Faller DV, Harpp DN, Kanara I, Pernokas J, Powers WR, Steliou K, 2016. Microbiota and Neurological Disorders: A Gut Feeling. Biores Open Access 5(1), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita C, Tsuji H, Hata T, Gondo M, Takakura S, Kawai K, Yoshihara K, Ogata K, Nomoto K, Miyazaki K, Sudo N, 2015. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS One 10(12), e0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkl S, Lackner S, Muller W, Gorkiewicz G, Kashofer K, Oberascher A, Painold A, Holl A, Holzer P, Meinitzer A, Mangge H, Holasek S, 2017. Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. Int J Eat Disord 50(12), 1421–1431. [DOI] [PubMed] [Google Scholar]
- Motta EVS, Raymann K, Moran NA, 2018. Glyphosate perturbs the gut microbiota of honey bees. Proc Natl Acad Sci U S A 115(41), 10305–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B, 2007. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 370(9590), 851–858. [DOI] [PubMed] [Google Scholar]
- Mrema EJ, Rubino FM, Brambilla G, Moretto A, Tsatsakis AM, Colosio C, 2013. Persistent organochlorinated pesticides and mechanisms of their toxicity. Toxicology 307, 74–88. [DOI] [PubMed] [Google Scholar]
- Murphy JR, Paul S, Dunlop AL, Corwin EJ, 2018. Maternal peripartum antibiotic exposure and the risk of postpartum depression. Res Nurs Health. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Comba IY, Cho T, Engen PA, Yazici C, Soberanes S, Hamanaka RB, Nigdelioglu R, Meliton AY, Ghio AJ, Budinger GRS, Mutlu GM, 2018. Inhalational exposure to particulate matter air pollution alters the composition of the gut microbiome. Environ Pollut 240, 817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine T, Ido Y, Nakamura M, Okamura T, 2018. 4(G)-beta-D-galactosylsucrose as a prebiotics may improve underweight in inpatients with schizophrenia. Biosci Microbiota Food Health 37(2), 45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa R, Iida T, Takenaka S, Fukamachi K, Mori A, Takahashi K, Asahi M, 1987. [PCBs in human blood and adipose tissue: levels and similarities on gas chromatograms]. Fukuoka Igaku Zasshi 78(5), 309–313. [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson’s Disease Genomics, C., Parkinson’s Study Group Parkinson’s Research: The Organized, G.I., andMe, GenePd, NeuroGenetics Research, C., Hussman Institute of Human, G., Ashkenazi Jewish Dataset, I., Cohorts for, H., Aging Research in Genetic, E., North American Brain Expression, C., United Kingdom Brain Expression, C., Greek Parkinson’s Disease, C., Alzheimer Genetic Analysis, G., Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T., Singleton AB, 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46(9), 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linlokken A, Wilson R, Rudi K, 2014. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26(8), 1155–1162. [DOI] [PubMed] [Google Scholar]
- Nasuti C, Coman MM, Olek RA, Fiorini D, Verdenelli MC, Cecchini C, Silvi S, Fedeli D, Gabbianelli R, 2016. Changes on fecal microbiota in rats exposed to permethrin during postnatal development. Environ Sci Pollut Res Int 23(11), 10930–10937. [DOI] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, Foster JA, 2011. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil 23(3), 255–264, e119. [DOI] [PubMed] [Google Scholar]
- Newsome WH, Davies DJ, Sun WF, 1998. Residues of polychlorinated biphenyls (PCB) in fatty foods of the Canadian diet. Food Addit Contam 15(1), 19–29. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kosciolek T, Maldonado Y, Daly RE, Martin AS, McDonald D, Knight R, Jeste DV, 2019. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophr Res 204, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen LN, Roager HM, Casas ME, Frandsen HL, Gosewinkel U, Bester K, Licht TR, Hendriksen NB, Bahl MI, 2018. Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environ Pollut 233, 364–376. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Oberdorster E, Oberdorster J, 2005. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113(7), 823–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerther DB, Pernthaler J, Schramm A, Amann R, Raskin L, 2000. Monitoring precursor 16S rRNAs of Acinetobacter spp. in activated sludge wastewater treatment systems. Appl Environ Microbiol 66(5), 2154–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo R, Koga M, Katsumata N, Odamaki T, Matsuyama S, Oka M, Narita H, Hashimoto N, Kusumi I, Xiao J, Matsuoka YJ, 2019. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J Affect Disord 245, 377–385. [DOI] [PubMed] [Google Scholar]
- Olde Loohuis LM, Mangul S, Ori APS, Jospin G, Koslicki D, Yang HT, Wu T, Boks MP, Lomen-Hoerth C, Wiedau-Pazos M, Cantor RM, de Vos WM, Kahn RS, Eskin E, Ophoff RA, 2018. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Transl Psychiatry 8(1), 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, Hsiao EY, 2018. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 173(7), 1728–1741 e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LM, Monk C, 2013. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: Theory and literature review. Psychoneuroendocrinology 38(10), 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painold A, Morkl S, Kashofer K, Halwachs B, Dalkner N, Bengesser S, Birner A, Fellendorf F, Platzer M, Queissner R, Schutze G, Schwarz MJ, Moll N, Holzer P, Holl AK, Kapfhammer HP, Gorkiewicz G, Reininghaus EZ, 2018. A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, 2017. Air pollution and Parkinson’s disease - evidence and future directions. Rev Environ Health 32(4), 303–313. [DOI] [PubMed] [Google Scholar]
- Pang Y, Patterson JA, Applegate TJ, 2009. The influence of copper concentration and source on ileal microbiota. Poult Sci 88(3), 586–592. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacology 38(12), 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass DA, Morgan AJ, Read DS, Field D, Weightman AJ, Kille P, 2015. The effect of anthropogenic arsenic contamination on the earthworm microbiome. Environ Microbiol 17(6), 1884–1896. [DOI] [PubMed] [Google Scholar]
- Pelissier MA, Vasquez N, Balamurugan R, Pereira E, Dossou-Yovo F, Suau A, Pochart P, Magne F, 2010. Metronidazole effects on microbiota and mucus layer thickness in the rat gut. FEMS Microbiol Ecol 73(3), 601–610. [DOI] [PubMed] [Google Scholar]
- Perales-Puchalt A, Perez-Sanz J, Payne KK, Svoronos N, Allegrezza MJ, Chaurio RA, Anadon C, Calmette J, Biswas S, Mine JA, Costich TL, Nickels L, Wickramasinghe J, Rutkowski MR, Conejo-Garcia JR, 2018. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J Leukoc Biol 103(5), 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Pardo P, Dodiya HB, Engen PA, Naqib A, Forsyth CB, Green SJ, Garssen J, Keshavarzian A, Kraneveld AD, 2018. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes 9(5), 799–814. [DOI] [PubMed] [Google Scholar]
- Perlstein M, Attala R, 1966. Neurologic Sequelae of Plumbism in Children. Clinical Pediatrics 5(5), 292–298. [Google Scholar]
- Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, Hennig B, 2018. Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis. Environ Pollut 242(Pt A), 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa D, Alonso R, Rabano A, Rodal I, Carrasco L, 2015. Different Brain Regions are Infected with Fungi in Alzheimer’s Disease. Sci Rep 5, 15015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A, 2018. Reduced microbiome alpha diversity in young patients with ADHD. PLoS One 13(7), e0200728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla J, Houseman EA, Smit E, Kile ML, 2017. A path analysis of multiple neurotoxic chemicals and cognitive functioning in older US adults (NHANES 1999–2002). Environ Health 16(1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulikkan J, Maji A, Dhakan DB, Saxena R, Mohan B, Anto MM, Agarwal N, Grace T, Sharma VK, 2018. Gut Microbial Dysbiosis in Indian Children with Autism Spectrum Disorders. Microb Ecol 76(4), 1102–1114. [DOI] [PubMed] [Google Scholar]
- Pyndt Jorgensen B, Krych L, Pedersen TB, Plath N, Redrobe JP, Hansen AK, Nielsen DS, Pedersen CS, Larsen C, Sorensen DB, 2015. Investigating the long-term effect of subchronic phencyclidine-treatment on novel object recognition and the association between the gut microbiota and behavior in the animal model of schizophrenia. Physiol Behav 141, 32–39. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Wu M, Feng Y, Zhou Z, Chen L, Chen F, 2018. Alterations of oral microbiota distinguish children with autism spectrum disorders from healthy controls. Sci Rep 8(1), 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisavljevic N, Cirstea M, Brett Finlay B, 2018. Bottoms up: the role of gut microbiota in brain health. Environ Microbiol. [DOI] [PubMed] [Google Scholar]
- Rajizadeh A, Mozaffari-Khosravi H, Yassini-Ardakani M, Dehghani A, 2017. Effect of magnesium supplementation on depression status in depressed patients with magnesium deficiency: A randomized, double-blind, placebo-controlled trial. Nutrition 35, 56–60. [DOI] [PubMed] [Google Scholar]
- Ramalho RM, Viana RJ, Low WC, Steer CJ, Rodrigues CM, 2008. Bile acids and apoptosis modulation: an emerging role in experimental Alzheimer’s disease. Trends Mol Med 14(2), 54–62. [DOI] [PubMed] [Google Scholar]
- Raymann K, Motta EVS, Girard C, Riddington IM, Dinser JA, Moran NA, 2018. Imidacloprid Decreases Honey Bee Survival Rates but Does Not Affect the Gut Microbiome. Appl Environ Microbiol 84(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Dinan TG, Cryan JF, 2016. The microbiome: A key regulator of stress and neuroinflammation. Neurobiol Stress 4, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinoso Webb C, Koboziev I, Furr KL, Grisham MB, 2016. Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology 23(2), 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Feng J, 2007. Rotenone selectively kills serotonergic neurons through a microtubule-dependent mechanism. J Neurochem 103(1), 303–311. [DOI] [PubMed] [Google Scholar]
- Ren Y, Liu W, Jiang H, Jiang Q, Feng J, 2005. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J Biol Chem 280(40), 34105–34112. [DOI] [PubMed] [Google Scholar]
- Requile M, Gonzalez Alvarez DO, Delanaud S, Rhazi L, Bach V, Depeint F, Khorsi-Cauet H, 2018. Use of a combination of in vitro models to investigate the impact of chlorpyrifos and inulin on the intestinal microbiota and the permeability of the intestinal mucosa. Environ Sci Pollut Res Int 25(23), 22529–22540. [DOI] [PubMed] [Google Scholar]
- Reygner J, Joly Condette C, Bruneau A, Delanaud S, Rhazi L, Depeint F, Abdennebi-Najar L, Bach V, Mayeur C, Khorsi-Cauet H, 2016a. Changes in Composition and Function of Human Intestinal Microbiota Exposed to Chlorpyrifos in Oil as Assessed by the SHIME((R)) Model. Int J Environ Res Public Health 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reygner J, Lichtenberger L, Elmhiri G, Dou S, Bahi-Jaber N, Rhazi L, Depeint F, Bach V, Khorsi-Cauet H, Abdennebi-Najar L, 2016b. Inulin Supplementation Lowered the Metabolic Defects of Prolonged Exposure to Chlorpyrifos from Gestation to Young Adult Stage in Offspring Rats. PLoS One 11(10), e0164614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas-Fito N, Sala M, Kogevinas M, Sunyer J, 2001. Polychlorinated biphenyls (PCBs) and neurological development in children: a systematic review. J Epidemiol Community Health 55(8), 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JB, Dancy BCR, Horton CL, Lee YS, Madejczyk MS, Xu ZZ, Ackermann G, Humphrey G, Palacios G, Knight R, Lewis JA, 2018. Exposure to toxic metals triggers unique responses from the rat gut microbiota. Sci Rep 8(1), 6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby RJ, Carr J, Orgel K, King SL, Lund PK, Dekaney CM, 2016. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 7(5), 414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelens SA, Beck V, Aerts G, Clerens S, Vanden Bergh G, Arckens L, Darras VM, van der Geyten S, 2005. Neurotoxicity of polychlorinated biphenyls (PCBs) by disturbance of thyroid hormone-regulated genes. Ann N Y Acad Sci 1040, 454–456. [DOI] [PubMed] [Google Scholar]
- Rose DR, Yang H, Serena G, Sturgeon C, Ma B, Careaga M, Hughes HK, Angkustsiri K, Rose M, Hertz-Picciotto I, Van de Water J, Hansen RL, Ravel J, Fasano A, Ashwood P, 2018. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun 70, 354–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SE, Keiser S, Ajami NJ, Wong MC, Gesell J, Petrosino JF, Johs A, 2016. The role of gut microbiota in fetal methylmercury exposure: Insights from a pilot study. Toxicol Lett 242, 60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y, Wu C, Guo X, Xu Z, Xing C, Cao H, Zhang C, Hu G, Liu P, 2018. High Doses of Copper and Mercury Changed Cecal Microbiota in Female Mice. Biol Trace Elem Res. [DOI] [PubMed] [Google Scholar]
- Ruark CD, Hack CE, Robinson PJ, Anderson PE, Gearhart JM, 2013. Quantitative structure-activity relationships for organophosphates binding to acetylcholinesterase. Arch Toxicol 87(2), 281–289. [DOI] [PubMed] [Google Scholar]
- Rudzki L, Ostrowska L, Pawlak D, Malus A, Pawlak K, Waszkiewicz N, Szulc A, 2019. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. [DOI] [PubMed] [Google Scholar]
- Russo R, Cristiano C, Avagliano C, De Caro C, La Rana G, Raso GM, Canani RB, Meli R, Calignano A, 2018. Gut-brain Axis: Role of Lipids in the Regulation of Inflammation, Pain and CNS Diseases. Curr Med Chem 25(32), 3930–3952. [DOI] [PubMed] [Google Scholar]
- Sadaghian Sadabad M, von Martels JZ, Khan MT, Blokzijl T, Paglia G, Dijkstra G, Harmsen HJ, Faber KN, 2015. A simple coculture system shows mutualism between anaerobic faecalibacteria and epithelial Caco-2 cells. Sci Rep 5, 17906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi Saravi SS, Dehpour AR, 2016. Potential role of organochlorine pesticides in the pathogenesis of neurodevelopmental, neurodegenerative, and neurobehavioral disorders: A review. Life Sci 145, 255–264. [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, McGrath J, 2007. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 64(10), 1123–1131. [DOI] [PubMed] [Google Scholar]
- Sahasrabudhe NM, Beukema M, Tian L, Troost B, Scholte J, Bruininx E, Bruggeman G, van den Berg M, Scheurink A, Schols HA, Faas MM, de Vos P, 2018. Dietary Fiber Pectin Directly Blocks Toll-Like Receptor 2–1 and Prevents Doxorubicin-Induced Ileitis. Front Immunol 9, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihovic S, Ganna A, Fall T, Broeckling CD, Prenni JE, van Bavel B, Lind PM, Ingelsson E, Lind L, 2016. The metabolic fingerprint of p,p’-DDE and HCB exposure in humans. Environ Int 88, 60–66. [DOI] [PubMed] [Google Scholar]
- Salzer JL, Zalc B, 2016. Myelination. Curr Biol 26(20), R971–R975. [DOI] [PubMed] [Google Scholar]
- Sampson TR, Debelius JW, Thron T, Janssen S, Shastri GG, Ilhan ZE, Challis C, Schretter CE, Rocha S, Gradinaru V, Chesselet MF, Keshavarzian A, Shannon KM, Krajmalnik-Brown R, Wittung-Stafshede P, Knight R, Mazmanian SK, 2016. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167(6), 1469–1480 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M, 2012. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry 51(10), 1003–1019 e1020. [DOI] [PubMed] [Google Scholar]
- Schantz SL, 1996. Developmental neurotoxicity of PCBs in humans: what do we know and where do we go from here? Neurotoxicol Teratol 18(3), 217–227; discussion 229–276. [DOI] [PubMed] [Google Scholar]
- Schecter A, Haffner D, Colacino J, Patel K, Papke O, Opel M, Birnbaum L, 2010. Polybrominated diphenyl ethers (PBDEs) and hexabromocyclodecane (HBCD) in composite U.S. food samples. Environ Health Perspect 118(3), 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Pavuk M, Papke O, Ryan JJ, Birnbaum L, Rosen R, 2003. Polybrominated diphenyl ethers (PBDEs) in U.S. mothers’ milk. Environ Health Perspect 111(14), 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M, 2006. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology 131(5), 1542–1552. [DOI] [PubMed] [Google Scholar]
- Schizophrenia Psychiatric Genome-Wide Association Study, C., 2011. Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43(10), 969–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn FP, Cui JY, Klaassen CD, 2015. RNA-Seq Quantification of Hepatic Drug Processing Genes in Germ-Free Mice. Drug Metab Dispos 43(10), 1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R, 2016. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol 14(8), e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serefko A, Szopa A, Poleszak E, 2016. Magnesium and depression. Magnes Res 29(3), 112–119. [DOI] [PubMed] [Google Scholar]
- Seth B, Yadav A, Tandon A, Shankar J, Chaturvedi RK, 2019. Carbofuran hampers oligodendrocytes development leading to impaired myelination in the hippocampus of rat brain. Neurotoxicology 70, 161–179. [DOI] [PubMed] [Google Scholar]
- Seth RK, Kimono D, Alhasson F, Sarkar S, Albadrani M, Lasley SK, Horner R, Janulewicz P, Nagarkatti M, Nagarkatti P, Sullivan K, Chatterjee S, 2018. Increased butyrate priming in the gut stalls microbiome associated-gastrointestinal inflammation and hepatic metabolic reprogramming in a mouse model of Gulf War Illness. Toxicol Appl Pharmacol 350, 64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance EG, Prandovszky E, Castiglione J, Yolken RH, 2015. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep 17(5), 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, Mazmanian SK, 2016. The Central Nervous System and the Gut Microbiome. Cell 167(4), 915–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata AA, Schrodl W, Aldin AA, Hafez HM, Kruger M, 2013. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol 66(4), 350–358. [DOI] [PubMed] [Google Scholar]
- Shen Y, Xu J, Li Z, Huang Y, Yuan Y, Wang J, Zhang M, Hu S, Liang Y, 2018. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- Sheppard JG, Frazier KR, Saralkar P, Hossain MF, Geldenhuys WJ, Long TE, 2018. Disulfiram-based disulfides as narrow-spectrum antibacterial agents. Bioorg Med Chem Lett 28(8), 1298–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, Copes R, Tu K, Goldberg MS, Villeneuve PJ, Martin RV, Murray BJ, Wilton AS, Kopp A, Chen H, 2018. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: a population-based cohort study. Int J Epidemiol 47(6), 2038–2048. [DOI] [PubMed] [Google Scholar]
- Sipka S, Eum SY, Son KW, Xu S, Gavalas VG, Hennig B, Toborek M, 2008. ORAL ADMINISTRATION OF PCBs INDUCES PROINFLAMMATORY AND PROMETASTATIC RESPONSES. Environ Toxicol Pharmacol 25(2), 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG Jr., 2008. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ Sci Technol 42(4), 1377–1384. [DOI] [PubMed] [Google Scholar]
- Skonieczna-Zydecka K, Marlicz W, Misera A, Koulaouzidis A, Loniewski I, 2018. Microbiome-The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J Clin Med 7(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Ryde IT, Levin ED, Seidler FJ, 2008. Developmental neurotoxicity of low dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull 75(5), 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, Kang J, Rowden J, Stone P, Crane J, Stanley T, Abels P, Purdie G, Maude R, Mitchell EA, Probiotic in Pregnancy Study, G., 2017. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-blind Placebo-controlled Trial. EBioMedicine 24, 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME, Swoboda HD, 2019. Shellfish Toxicity, StatPearls. Treasure Island (FL). [PubMed] [Google Scholar]
- Sobko T, Huang L, Midtvedt T, Norin E, Gustafsson LE, Norman M, Jansson EA, Lundberg JO, 2006. Generation of NO by probiotic bacteria in the gastrointestinal tract. Free Radic Biol Med 41(6), 985–991. [DOI] [PubMed] [Google Scholar]
- Son JS, Zheng LJ, Rowehl LM, Tian X, Zhang Y, Zhu W, Litcher-Kelly L, Gadow KD, Gathungu G, Robertson CE, Ir D, Frank DN, Li E, 2015. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLoS One 10(10), e0137725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhao R, Hu Y, Hao F, Li N, Nie G, Tang H, Wang Y, 2015. Assessment of the Biological Effects of a Multifunctional Nano-Drug-Carrier and Its Encapsulated Drugs. J Proteome Res 14(12), 5193–5201. [DOI] [PubMed] [Google Scholar]
- Spanogiannopoulos P, Bess EN, Carmody RN, Turnbaugh PJ, 2016. The microbial pharmacists within us: a metagenomic view of xenobiotic metabolism. Nat Rev Microbiol 14(5), 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer P, Schaumburg H, 2000. Experimental and Clinical Neurotoxicology, 2nd ed. Oxford University Press, New York. [Google Scholar]
- Srivastava AK, Schlessinger D, 1990. Mechanism and regulation of bacterial ribosomal RNA processing. Annu Rev Microbiol 44, 105–129. [DOI] [PubMed] [Google Scholar]
- Stanaway IB, Wallace JC, Shojaie A, Griffith WC, Hong S, Wilder CS, Green FH, Tsai J, Knight M, Workman T, Vigoren EM, McLean JS, Thompson B, Faustman EM, 2017. Human Oral Buccal Microbiomes Are Associated with Farmworker Status and Azinphos-Methyl Agricultural Pesticide Exposure. Appl Environ Microbiol 83(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SE, Sonuga-Barke EJ, Kreppner JM, Beckett C, Castle J, Colvert E, Groothues C, Hawkins A, Rutter M, 2008. Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. J Abnorm Child Psychol 36(3), 385–398. [DOI] [PubMed] [Google Scholar]
- Stewardson AJ, Gaia N, Francois P, Malhotra-Kumar S, Delemont C, Martinez de Tejada B, Schrenzel J, Harbarth S, Lazarevic V, Saturn WP, Groups WPS, 2015. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin Microbiol Infect 21(4), 344 e341–311. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Marks LJ, Gilgan MW, Pfeiffer E, Zwicker BM, 1998. Microbial utilization of the neurotoxin domoic acid: blue mussels (Mytilus edulis) and soft shell clams (Mya arenaria) as sources of the microorganisms. Can J Microbiol 44(5), 456–464. [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova D, Liu JK, Sharma A, Levering J, McDonald D, Dietrich D, Ramadhar TR, Lekbua A, Mroue N, Liston C, Stewart EJ, Dubin MJ, Zengler K, Knight R, Gilbert JA, Clardy J, Lewis K, 2019. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 4(3), 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, Kubo C, Koga Y, 2004. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol 558(Pt 1), 263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Wang F, Hu X, Yang C, Xu H, Yao Y, Liu J, 2018. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J Agric Food Chem 66(31), 8415–8421. [DOI] [PubMed] [Google Scholar]
- Sun MF, Zhu YL, Zhou ZL, Jia XB, Xu YD, Yang Q, Cui C, Shen YQ, 2018. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-alpha signaling pathway. Brain Behav Immun 70, 48–60. [DOI] [PubMed] [Google Scholar]
- Suzuki G, Nakano M, Nakano S, 2005. Distribution of PCDDs/PCDFs and Co-PCBs in human maternal blood, cord blood, placenta, milk, and adipose tissue: dioxins showing high toxic equivalency factor accumulate in the placenta. Biosci Biotechnol Biochem 69(10), 1836–1847. [DOI] [PubMed] [Google Scholar]
- Swanson HI, 2015. Drug Metabolism by the Host and Gut Microbiota: A Partnership or Rivalry? Drug Metab Dispos 43(10), 1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabouy L, Getselter D, Ziv O, Karpuj M, Tabouy T, Lukic I, Maayouf R, Werbner N, Ben-Amram H, Nuriel-Ohayon M, Koren O, Elliott E, 2018. Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav Immun 73, 310–319. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Ren YK, Chen RQ, Zhuang YY, Fang HR, Xu JH, Wang CY, Hu B, 2011. Formaldehyde induces neurotoxicity to PC12 cells involving inhibition of paraoxonase-1 expression and activity. Clin Exp Pharmacol Physiol 38(4), 208–214. [DOI] [PubMed] [Google Scholar]
- Tillmann S, Abildgaard A, Winther G, Wegener G, 2018. Altered fecal microbiota composition in the Flinders sensitive line rat model of depression. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilson HA, Kodavanti PR, 1998. The neurotoxicity of polychlorinated biphenyls. Neurotoxicology 19(4–5), 517–525. [PubMed] [Google Scholar]
- Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED, 2008. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol 30(1), 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Ohi K, Kudo T, Yoshida T, Ikarashi N, Ito K, Sugiyama K, 2009. Ciprofloxacin suppresses Cyp3a in mouse liver by reducing lithocholic acid-producing intestinal flora. Drug Metab Pharmacokinet 24(3), 201–208. [DOI] [PubMed] [Google Scholar]
- Torres ERS, Akinyeke T, Stagaman K, Duvoisin RM, Meshul CK, Sharpton TJ, Raber J, 2018. Effects of Sub-Chronic MPTP Exposure on Behavioral and Cognitive Performance and the Microbiome of Wild-Type and mGlu8 Knockout Female and Male Mice. Front Behav Neurosci 12, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremlett H, Bauer KC, Appel-Cresswell S, Finlay BB, Waubant E, 2017. The gut microbiome in human neurological disease: A review. Ann Neurol 81(3), 369–382. [DOI] [PubMed] [Google Scholar]
- Trinder M, McDowell TW, Daisley BA, Ali SN, Leong HS, Sumarah MW, Reid G, 2016. Probiotic Lactobacillus rhamnosus Reduces Organophosphate Pesticide Absorption and Toxicity to Drosophila melanogaster. Appl Environ Microbiol 82(20), 6204–6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufarte L, Laville E, Duquesne S, Morgavi D, Robe P, Klopp C, Rizzo A, Pizzut-Serin S, Potocki-Veronese G, 2017. Discovery of carbamate degrading enzymes by functional metagenomics. PLoS One 12(12), e0189201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Burmann J, Fassbender K, Schwiertz A, Schafer KH, 2016. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32, 66–72. [DOI] [PubMed] [Google Scholar]
- Valles Y, Francino MP, 2018. Air Pollution, Early Life Microbiome, and Development. Curr Environ Health Rep 5(4), 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet T, Vilchez-Vargas R, Vital M, Pieper DH, Vanden Bussche J, Vanhaecke L, Van de Wiele T, De Vos M, Laukens D, 2017. Ursodeoxycholic Acid and Its Taurine- or Glycine-Conjugated Species Reduce Colitogenic Dysbiosis and Equally Suppress Experimental Colitis in Mice. Appl Environ Microbiol 83(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, Rutten BP, 2010. The environment and schizophrenia. Nature 468(7321), 203–212. [DOI] [PubMed] [Google Scholar]
- Velmurugan G, Ramprasath T, Swaminathan K, Mithieux G, Rajendhran J, Dhivakar M, Parthasarathy A, Babu DD, Thumburaj LJ, Freddy AJ, Dinakaran V, Puhari SS, Rekha B, Christy YJ, Anusha S, Divya G, Suganya K, Meganathan B, Kalyanaraman N, Vasudevan V, Kamaraj R, Karthik M, Jeyakumar B, Abhishek A, Paul E, Pushpanathan M, Rajmohan RK, Velayutham K, Lyon AR, Ramasamy S, 2017. Gut microbial degradation of organophosphate insecticides-induces glucose intolerance via gluconeogenesis. Genome Biol 18(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort J, Xavier BB, Stewardson A, Coenen S, Godycki-Cwirko M, Adriaenssens N, Kowalczyk A, Lammens C, Harbarth S, Goossens H, Malhotra-Kumar S, 2015. Metagenomic analysis of the impact of nitrofurantoin treatment on the human faecal microbiota. J Antimicrob Chemother 70(7), 1989–1992. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Ottesen NM, Meluken I, Sorensen N, Pedersen O, Kessing LV, Miskowiak KW, 2019. Remitted affective disorders and high familial risk of affective disorders associate with aberrant intestinal microbiota. Acta Psychiatr Scand 139(2), 174–184. [DOI] [PubMed] [Google Scholar]
- Vinolo MA, Rodrigues HG, Nachbar RT, Curi R, 2011. Regulation of inflammation by short chain fatty acids. Nutrients 3(10), 858–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visschers RG, Luyer MD, Schaap FG, Olde Damink SW, Soeters PB, 2013. The gut-liver axis. Curr Opin Clin Nutr Metab Care 16(5), 576–581. [DOI] [PubMed] [Google Scholar]
- Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE, 2017. Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7(1), 13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM, Yolton K, Dietrich KN, Braun JM, Lanphear BP, Chen A, 2018. Exposure to polybrominated diphenyl ethers (PBDEs) and child behavior: Current findings and future directions. Horm Behav 101, 94–104. [DOI] [PubMed] [Google Scholar]
- Waclawikova B, El Aidy S, 2018. Role of Microbiota and Tryptophan Metabolites in the Remote Effect of Intestinal Inflammation on Brain and Depression. Pharmaceuticals (Basel) 11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liu Q, Guo L, Zeng H, Ding C, Zhang W, Xu D, Wang X, Qiu J, Dong Q, Fan Z, Zhang Q, Pan J, 2018. Gut Microbiota and Relevant Metabolites Analysis in Alcohol Dependent Mice. Front Microbiol 9, 1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhou J, He F, Cai C, Wang H, Wang Y, Lin Y, Rong H, Cheng G, Xu R, Zhou W, 2019. Alteration of gut microbiota-associated epitopes in children with autism spectrum disorders. Brain Behav Immun 75, 192–199. [DOI] [PubMed] [Google Scholar]
- Wang N, Kong D, Cai D, Shi L, Cao Y, Pang G, Yu R, 2010. Levels of polychlorinated biphenyls in human adipose tissue samples from southeast China. Environ Sci Technol 44(11), 4334–4340. [DOI] [PubMed] [Google Scholar]
- Wang X, Shen M, Zhou J, Jin Y, 2019. Chlorpyrifos disturbs hepatic metabolism associated with oxidative stress and gut microbiota dysbiosis in adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol 216, 19–28. [DOI] [PubMed] [Google Scholar]
- Warner BB, 2019. The contribution of the gut microbiome to neurodevelopment and neuropsychiatric disorders. Pediatr Res 85(2), 216–224. [DOI] [PubMed] [Google Scholar]
- Winneke G, 2011. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci 308(1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- Winther G, Pyndt Jorgensen BM, Elfving B, Nielsen DS, Kihl P, Lund S, Sorensen DB, Wegener G, 2015. Dietary magnesium deficiency alters gut microbiota and leads to depressive-like behaviour. Acta Neuropsychiatr 27(3), 168–176. [DOI] [PubMed] [Google Scholar]
- Wu F, Yang L, Islam MT, Jasmine F, Kibriya MG, Nahar J, Barmon B, Parvez F, Sarwar G, Ahmed A, Eunus M, Islam T, Slavkovich V, Hu J, Li H, Graziano JH, Pei Z, Ahsan H, Chen Y, 2019. The role of gut microbiome and its interaction with arsenic exposure in carotid intima-media thickness in a Bangladesh population. Environ Int 123, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wen XW, Faulk C, Boehnke K, Zhang H, Dolinoy DC, Xi C, 2016. Perinatal Lead Exposure Alters Gut Microbiota Composition and Results in Sex-specific Bodyweight Increases in Adult Mice. Toxicol Sci 151(2), 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Jin C, Pan Z, Sun L, Fu Z, Jin Y, 2018a. Chronic exposure to low concentrations of lead induces metabolic disorder and dysbiosis of the gut microbiota in mice. Sci Total Environ 631–632, 439–448. [DOI] [PubMed] [Google Scholar]
- Xia J, Lu L, Jin C, Wang S, Zhou J, Ni Y, Fu Z, Jin Y, 2018b. Effects of short term lead exposure on gut microbiota and hepatic metabolism in adult zebrafish. Comp Biochem Physiol C Toxicol Pharmacol 209, 1–8. [DOI] [PubMed] [Google Scholar]
- Xia X, Sun B, Gurr GM, Vasseur L, Xue M, You M, 2018. Gut Microbiota Mediate Insecticide Resistance in the Diamondback Moth, Plutella xylostella (L.). Front Microbiol 9, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Zheng D, Zhong H, Qin B, Gurr GM, Vasseur L, Lin H, Bai J, He W, You M, 2013. DNA sequencing reveals the midgut microbiota of diamondback moth, Plutella xylostella (L.) and a possible relationship with insecticide resistance. PLoS One 8(7), e68852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Wang C, Dong X, Hu T, Wang L, Zhao W, Zhu S, Li G, Hu Y, Gao Q, Wan J, Liu Z, Sun J, 2018. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors. [DOI] [PubMed] [Google Scholar]
- Yan A, Culp E, Perry J, Lau JT, MacNeil LT, Surette MG, Wright GD, 2018. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect Dis 4(1), 68–76. [DOI] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ, 2009. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect 117(3), 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Qian Y, Xu S, Song Y, Xiao Q, 2017. Longitudinal Analysis of Fecal Microbiome and Pathologic Processes in a Rotenone Induced Mice Model of Parkinson’s Disease. Front Aging Neurosci 9, 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz B, Schibli S, Macpherson AJ, Sokollik C, 2018. D-lactic Acidosis: Successful Suppression of D-lactate-Producing Lactobacillus by Probiotics. Pediatrics 142(3). [DOI] [PubMed] [Google Scholar]
- Yin N, Cai X, Du H, Zhang Z, Li Z, Chen X, Sun G, Cui Y, 2017. In vitro study of soil arsenic release by human gut microbiota and its intestinal absorption by Caco-2 cells. Chemosphere 168, 358–364. [DOI] [PubMed] [Google Scholar]
- Yin N, Zhang Z, Cai X, Du H, Sun G, Cui Y, 2015. In Vitro Method To Assess Soil Arsenic Metabolism by Human Gut Microbiota: Arsenic Speciation and Distribution. Environ Sci Technol 49(17), 10675–10681. [DOI] [PubMed] [Google Scholar]
- Yoo BB, Mazmanian SK, 2017. The Enteric Network: Interactions between the Immune and Nervous Systems of the Gut. Immunity 46(6), 910–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu B, Zhang XX, Liu S, Yu J, Cheng S, Ren HQ, Ye L, 2016. Arsenic Metabolism and Toxicity Influenced by Ferric Iron in Simulated Gastrointestinal Tract and the Roles of Gut Microbiota. Environ Sci Technol 50(13), 7189–7197. [DOI] [PubMed] [Google Scholar]
- Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z, 2017. Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 138, 231–239. [DOI] [PubMed] [Google Scholar]
- Zhang L, Wang Y, Xiayu X, Shi C, Chen W, Song N, Fu X, Zhou R, Xu YF, Huang L, Zhu H, Han Y, Qin C, 2017. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J Alzheimers Dis 60(4), 1241–1257. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhang Y, Wang G, Han R, Xie X, 2016. Effects of chlorpyrifos on the gut microbiome and urine metabolome in mouse (Mus musculus). Chemosphere 153, 287–293. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Liu M, Chen J, Pan J, Han Y, Liu Y, Cheng K, Zhou C, Wang H, Zhou X, Gui S, Perry SW, Wong ML, Licinio J, Wei H, Xie P, 2019. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv 5(2), eaau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P, 2016. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21(6), 786–796. [DOI] [PubMed] [Google Scholar]
- Zhu L, Yang Z, Yao R, Xu L, Chen H, Gu X, Wu T, Yang X, 2018. Potential Mechanism of Detoxification of Cyanide Compounds by Gut Microbiomes of Bamboo-Eating Pandas. mSphere 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Jiang X, Boudreau MD, Feng G, Miao Y, Dong S, Wu H, Zeng M, Yin JJ, 2018. Orally administered gold nanoparticles protect against colitis by attenuating Toll-like receptor 4- and reactive oxygen/nitrogen species-mediated inflammatory responses but could induce gut dysbiosis in mice. J Nanobiotechnology 16(1), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Han Y, Du J, Liu R, Jin K, Yi W, 2017. Microbiota-gut-brain axis and the central nervous system. Oncotarget 8(32), 53829–53838. [DOI] [PMC free article] [PubMed] [Google Scholar]