Abstract
Sox proteins are a family of lineage-associated transcription factors. They regulate expression of genes involved in control of self-renewal and multipotency in both developmental and adult stem cells. Overexpression of Sox proteins is frequently observed in many different human cancers. Despite their importance as therapeutic targets, Sox proteins are difficult to ‘drug’ using structure-based design. However, Sox protein localisation, activity and interaction partners are regulated by a plethora of post-translational modifications (PTMs), such as: phosphorylation, acetylation, sumoylation, methylation, and ubiquitylation. Here we review the various reported post-translational modifications of Sox proteins and their potential functional importance in guiding cell fate processes. The enzymes that regulate these PTMs could be useful targets for anti-cancer drug discovery.
Keywords: SOX, Post-translational modification, Stem cell, Cancer, Transcription
1. Introduction
Within many tissues, undifferentiated multipotent stem and progenitor cells sustain the turnover of new cells during tissue homeostasis and repair. Developmental transcription factors (TFs) and their associated core transcriptional apparatus are key determinants of cell type identity. These orchestrate the balance between self-renewal and differentiation. In many types of cancer, these developmental TFs (e.g. HOX, FOX, SOX and bHLH proteins) become deregulated and drive “stemness”, enforcing an immature cellular phenotype and limiting possibilities for terminal cell differentiation [1]. Excellent reviews have discussed the diverse SOX protein functions across stem cells, development and cancer [2,3]. Here we focus our discussions on the function of SOX post-translational modifications (PTMs) and whether these could be critical targets for new anti-cancer drug discovery.
Developmental TFs often operate in a combinatorial manner, as ‘co-operatives’ and are often termed master regulatory transcription factors [4]. In many cancers changes in gene expression which underpin tumour growth, are driven by key oncogenic signalling TFs, such as: STAT, SMAD, TCF, ETS [5,6]; these are the transcriptional effectors of LIF, TGFbeta, Wnt and ERK pathways, respectively. Convergence of these oncogenic, or signalling TFs, with master developmental TFs likely occurs by their shared binding/co-localisation at key enhancers or superenhancers, which drives robust expression of cell type specific target genes [5]. This is why high-grade cancers typically have histological resemblance to the corresponding foetal tissue or regenerating tissue from the cell of origin. They exploit the molecular apparatus used by stem cells. Targeting cancer stem-cell-like identity could therefore be achieved by targeting the activity of these core TFs.
2. ‘Drugging’ transcription factors
There are ∼1600 TFs encoding genes in the human genome [7]. TFs form protein complexes that activate or repress transcription at specific target genes, by binding to regulatory sites in the genome. TFs typically have a modular design, with core DNA binding domains separate from effector domains (e.g. activation, repression or chromatin-interacting). Many also harbour non-structured domains, such as stretches of alanine, proline or glycine residues, and these confer an important functional role in supporting the formation of biophysically separated sub-nuclear domains, termed transcriptional condensates.
Disrupting these TFs, or their complexes, using small molecules has proven difficult due them targeting protein-protein interactions, with large, flat surface areas which do not offer druggable binding pockets. They have therefore been viewed as difficult to targets to ‘drug’ using classic structure-based design methods that have been so successful for targets with catalytic sites. Alternative strategies are therefore needed.
PTM is a dynamic, reversible process adapted to rapidly transmit signalling information. PTM dynamics are frequently centred on enzymes that catalyse the addition, removal and “reading” of these sites. In the last 10–20 years there has been great interest in targeting cancer signalling pathways, which often involve complex networks and cascades of post-translation modifications (PTMs) [8]. Genetic disruption to these core cancer pathways (e.g. receptor tyrosine kinase signalling) is frequently observed across many human cancers. Therefore, the tools for studying and identifying new small molecule inhibitors are well established. Perhaps the most widely explored PTM is phosphorylation, which is catalysed by kinases and reversed by phosphatases [9].
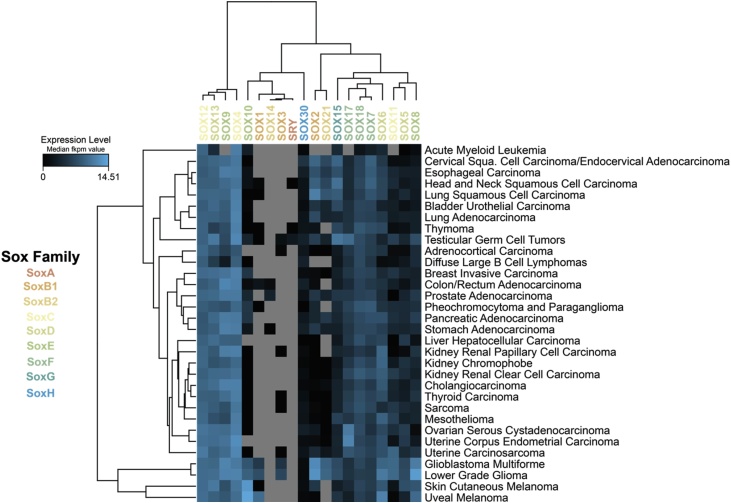
PTMs can be broadly separated into 2 categories: chemical modifications (e.g. phosphorylation/methylation/acetylation) and small protein modifications (e.g. ubiquitylation/SUMOylation). Searching for post-translational modifications (PTMs) which might be critical for TF activity, might offer a wealth of new targets, as the associated enzymes could be amenable to inhibition. However, there remain extensive gaps in our knowledge. Below, we will focus discussion on how these modifications and associated interactions provide a novel target for cancer therapies. How do these PTMs arise? What is their key function? Can they be modulated using small molecules? SOX proteins are attracting particular attention, as their overexpression is a recurrent feature of the majority of human cancers (Fig. 1).
Fig. 1.
Sox proteins are highly expressed in different cancers. Heatmap showing the median fkpm value of Sox family expression from RNA-seq of The Cancer Genome Atlas [10].
3. SOX proteins
The male sex determination gene SRY was the founding member of the SOX family (from which the family takes its name: Sex determining region Y (SRY)-related high-mobility group HMG-box) [11,12]. SOX proteins bind to the DNA sequence motif ATTGTT (or related sequences) through the three alpha helices within the HMG domains that form a L-shaped domain that interacts with the DNA minor groove [13,14]. This interaction is thought to trigger a conformational change, bending the DNA, and this may contribute to Sox function [15]. However, it is also the case that Sox factors have affinity to ‘pre-bent’ DNA that is present within nucleosomes – wrapped around the histone octamer – explaining their activity as pioneer factors (see pioneer factor discussion below).
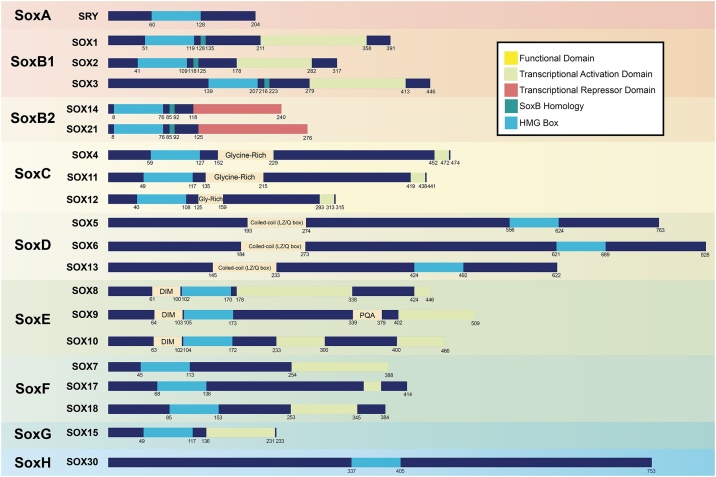
The SOX transcription factor family comprises of over 20 members of which there are 11 subgroups categorised on the basis of their HMG box and associated domains (Fig. 2). These are classified into groups termed A-H, depending on the similarity of the HMG domains [3,16]. Sry, is the only member of the SoxA group [17]. The SoxB family is subdivided into two subgroups with similar HMG domains: SoxB1 and B2. SoxB1 members (Sox1, Sox2 and Sox3) have a transcriptional activation domain in their C-terminus whereas SoxB2 (Sox14, Sox21) have transcriptional repression domain in the C-terminus [18,19]. The SoxG protein, Sox15, is related to SoxB; however its molecular functions remain unclear [20]. SoxC/E/F are analogous to SoxB1 with N-terminal HMG domains and transcriptional activation domains; however, there are significant differences in protein sequences beyond these domains.
Fig. 2.
A schematic of the domain structure of SOX family transcription factors. Sox groups A-H are grouped based on their DNA-binding high mobility group (HMG) domain. The domain architecture of SOX proteins varies between each group and harbour additional motifs, such as Transcriptional Activation/Repressor domains (SOX domains reviewed in: Ref. [3]). SOX30 is uniquely expressed in humans and is related to SOXD family proteins through the remanence of a coiled-coiled domain. Proteins are drawn to scale.
Notably, SoxE family (Sox8, Sox9 and Sox10) contain a N-terminal dimerisation domain which plays a role in remodelling chromatin [[21], [22], [23]]. SoxD have C-terminal HMG domains and N-terminal coiled-coil domains which mediate SoxD family dimerization and thereby preferential binding to pairs of DNA recognition sites [24]. These factors are also less stringent in DNA binding motifs resulting in putative SoxD binding sites at most promoters/regulatory regions [25]. Loosely related to SoxD is the SoxG protein, Sox30, which is the most divergent member, these Sox proteins contain the remnants of a coiled coil domain and a C-terminal HMG domain [26]. PTMs may therefore have specific effects on each SOX member based on their distinct protein domain architectures. For this review modified residues will be stated in the species variant (mm: mus musculus; hs: homo sapien) in which the study was conducted.
4. Consequences of SOX post-translation modifications
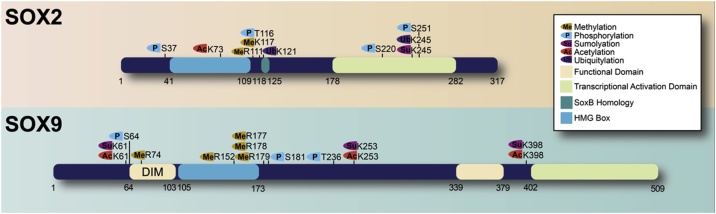
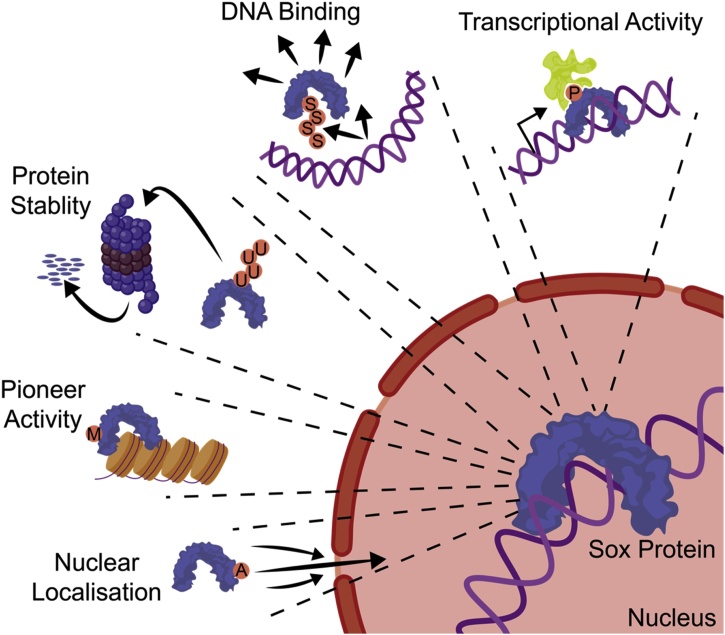
Sox protein PTMs have been reported across the spectrum of common modifications, including: phosphorylation, acetylation, methylation, SUMOylation, and ubiquitylation (Fig. 3). These modifications represent druggable targets through targeting the enzymes that catalyse either their addition or removal. Many known PTMs have an annotated function; however, numerous proteomic mass spectrometry screens have revealed novel and functionally uncharacterised PTMs on Sox factors [[27], [28], [29], [30], [31], [32], [33], [34]]. An increasingly long list of new PTMs has therefore emerged, and we do not know the identity or role of the associated enzyme. These provide many new avenues to investigate and will likely be corrupted in cancer. There are several strategies that have been uncovered in which SOX activity can be modulated (Fig. 4), and these are discussed below.
Fig. 3.
Posttranslational modifications of SOX2 and SOX9. PTMs of SOX2 and SOX9 are well-explored and highlight clusters of methylation, phosphorylation, acetylation, sumolyation and ubiquitination. Post-Translational modifications identified through low throughput approaches are annotated. Proteins are drawn to scale.
Fig. 4.
Schematic diagram illustrating how various post translational modifications of Sox factors modulate their function such as transcriptional actitivity, DNA binding, protein stability, pioneer activity and nuclear localisation.
5. PTMS that modulate DNA binding properties
Critical to Sox protein function is its ability to bind sequence-specific sites in the genome. Either disrupting or promoting DNA interactions may therefore influence Sox activity and shift key targets, either oncogene or tumour suppressor. All 20 Sox factors interact with DNA through a highly positive binding surface in the HMG box, which exhibits strong evolutionary conservation, and has been studied through numerous NMR and X-ray crystallography studies [[35], [36], [37], [38]]. Sox protein modifications that modulate DNA binding therefore represent a tractable target to disrupt/promote DNA interactions. However, it is notable those reported PTMs of Sox proteins which influence DNA binding lie outside of the HMG box, which suggests that it may not be directly accessible to modification (Fig. 3; Table 1) [39].
Table 1.
Table of Sox protein post translational modifications.
| SOX Protein | Modification | Species identified in | Human Residue | Catalytic enzyme | PTM Effect | Citation |
|---|---|---|---|---|---|---|
| SOX2 | Acetylation | Mouse | K35 | Unknown | Unknown | [91] |
| SOX2 | Phosphorylation | Mouse/Human | S37 | CDK | Enhances transcriptional activity | [43,98] |
| SOX2 | Acetylation | Mouse | K58 | Unknown | Unknown | [91] |
| SOX2 | Acetylation | Mouse | K65 | Unknown | Unknown | [91] |
| SOX2 | Acetylation | Mouse/Human | K73 | p300/CBP | Promotes nuclear export | [91] |
| SOX2 | Acetylation | Mouse | K87 | Unknown | Unknown | [91] |
| SOX2 | Acetylation | Mouse | K95 | Unknown | Unknown | [91] |
| SOX2 | Acetylation | Mouse | K103 | Unknown | Unknown | [91] |
| SOX2 | Acetylation | Mouse | K109 | Unknown | Unknown | [91] |
| SOX2 | Methylation | Mouse | R111 | CARM1 | Enhances transcriptional activity | [30] |
| SOX2 | Acetylation | Mouse | K115 | Unknown | Unknown | [91] |
| SOX2 | Methylation | Mouse | R117 | SETD7 | Promotes degradation | [75] |
| SOX2 | Phosphorylation | Human | T116 | PKCι | Required for promotor occupancy/Enhances transcriptional activity/Promotes stablisation | [40,42] |
| SOX2 | Acetylation | Mouse | K121 | Unknown | Unknown | [91] |
| SOX2 | Ubiquitination | Mouse | K121 | Unknown | Promotes Degradation | [86] |
| SOX2 | SUMOlylation | Mouse | K245 | PIAS, UBC9 | Impairs DNA binding/Impairs Protein interactions/Reduces transcriptional activity | [41,56] |
| SOX2 | Phosphorylation | Mouse/Human | S251 | CDK1, MAPK1/3, Aurora Kinase A | Promotes SOX2 associated transcriptional programs | [43,79,98] |
| SOX4 | Acetylation | Human | K95 | KAT5 | Chromatin Remodelling | [73] |
| SOX6 | Phosphorylation | Mouse/Human | T119 | CDK5 | Promotes degradation | [87] |
| SOX6 | SUMOylation | Human | K404 | UBC9 | Represses SOX6 transcriptional activity | [57] |
| SOX6 | SUMOylation | Human | K417 | UBC9 | [57] | |
| SOX9 | Acetylation | Human | K61 | KAT5 | Enhances transcriptional activity | [65] |
| SOX9 | SUMOylation | Chicken | K61 | Unknown | [46] | |
| SOX9 | Phosphorylation | Mouse/Human/Chicken | S64 | Upstream: BMP, TGF Kinase: PKA, | Enhances transcriptional activation | [[44], [45], [46], [47],63,64] |
| SOX9 | Methylation | Mouse | R74 | CARM1/PRMT4 | Possibly enhances transcriptional activity | [55] |
| SOX9 | Methylation | Mouse | R152 | CARM1/PRMT4 | [55] | |
| SOX9 | Methylation | Mouse | R177 | CARM1/PRMT4 | [55] | |
| SOX9 | Methylation | Mouse | R178 | CARM1/PRMT4 | [55] | |
| SOX9 | Methylation | Mouse | R179 | CARM1/PRMT4 | [55] | |
| SOX9 | Phosphorylation | Mouse/Human/Chicken | S181 | Upstream: BMP, TGF, Rho kinase Kinase: PKA, | Enhances transcriptional activity | [[44], [45], [46], [47],[60], [61], [62], [63]] |
| SOX9 | Phosphorylation | Mouse/Human | S211 | PKA, p38, SMAD | [45,64] | |
| SOX9 | Phosphorylation | Human | T236 | Upstream: PI3K/AKT/mTOR Kinase: GSK3 | Promotes degradation | [88] |
| SOX9 | Acetylation | Human | K253 | KAT5 | Enhances transcriptional activity | [65] |
| SOX9 | SUMOylation | Chicken | K253 | Unknown | [46] | |
| SOX9 | SUMOylation | Chicken | K398 | Unknown | [46] | |
| SOX9 | Acetylation | Human | K398 | KAT5 | [65] | |
| SOX10 | Phosphorylation | Mouse/Human | S24 | Predicted: CDK, MAPK | Represses transcriptional activity/Promotes degradation | [48] |
| SOX10 | Phosphorylation | Mouse/Human | S45 | Predicted: CDK, MAPK | Slight increase in activity | [48] |
| SOX10 | SUMOylation | Human | K55 | Ubc9 | Represses transcriptional activity | [58] |
| SOX10 | Phosphorylation | Human | S224 | Unknown | Unknown | [48] |
| SOX10 | Phosphorylation | Human | S232 | Unknown | Unknown | [48] |
| SOX10 | Phosphorylation | Human | T240 | Predicted: CDK, MAPK | Represses transcriptional activity | [48] |
| SOX10 | Phosphorylation | Human | T244 | [48] | ||
| SOX10 | SUMOylation | Human | K246 | Unknown | Unknown | [58] |
| SOX10 | SUMOylation | Human | K357 | Ubc9 | Represses transcriptional activity | [58] |
| SOX11 | Phosphorylation | Mouse | S30 | Unknown | Promotes nuclear localisation | [92] |
| SOX11 | Phosphorylation | Mouse | S133 | PKA | Fine-tunes transcriptional activity | [99] |
| SOX18 | Phosphorylation | Mouse | S278/S281 | Predicted: PKA, CK2 | Enhances transcriptional activity | [50] |
| SRY | Phosphorylation | Human | S31-33 | PKA | Promotes DNA binding | [90] |
| SRY | Acetylation | Human | K136 | p300 | Promotes nuclear import | [90] |
Protein phosphorylation adds a phosphate group to either a tyrosine, threonine or serine. The addition of negative charge to a protein can result in a distinct change in its biophysical properties. In Sertoli cells SRY is phosphorylated by PKA, at the N-terminal of its HMG box which positively regulates DNA-binding and results in inhibition of basal promotor activity located downstream of an SRY DNA-binding site concatemer [35]. This could be due to phosphorylation altering protein conformation of SRY which promotes binding to DNA. In lung squamous cell carcinoma, Sox2 is phosphorylated by PKCι at Thr116 (hs; mm Thr118) and this is necessary for occupancy at the HHAT promoter leading to promotion of the stem cell-like phenotype [40]. Sox2 Thr116 (hs; mm Thr118) resides between the HMG domain and a consensus nuclear localisation sequence in Sox2 indicating that phosphorylation may modulate HMG-DNA interaction or nuclear localisation [40]. As there were no apparent localisation defects, these data suggest that modulating DNA interactions around the HMG box increase the ability of Sox proteins to bind to DNA, which could possibly be due to the introduction of a conformational change which increases affinity to DNA and/or chromatin.
SUMOylation (small ubiquitin-like modifier) is an important PTM that often alters the activity of many different transcription factors when it is covalently attached to a lysine residue. This is analogous to ubiquitination, with a series of enzymatic reactions leading to attachment of these proteins to a target protein. SUMOylation is most commonly reported to play a role in repressing transcriptional activity. Sox2 is SUMOylated, and this can influence its transcriptional activity. An example is Lys247 of Sox2 (mm; hs Lys245) which under normal conditions binds to the Fgf4 enhancer but following conjugation of SUMO1 at Lys247 (mm; hs Lys245) there is reduced binding [41]. Sox2 SUMOylation therefore can negatively regulate transcription at key target genes through impairing of DNA binding (Fig. 4).
6. Perturbing SOX family transcriptional activity
A related mode of action for PTMs is to influence SOX protein-protein interactions. Protein partner changes can dramatically switch activation, repression or transcriptional specificity of targets (e.g. motif binding). Phosphorylation often acts as an anchor for protein-protein interactions (e.g. dimerisation) and therefore can stimulate recruitment of transcriptional coactivators/repressors (Fig. 4). Sox2 is phosphorylated at Thr118 (mm; hs Thr116) by Akt and this has been reported to enhance the transcriptional activation of Sox2 in mouse embryonic stem cells (ESCs) and also enhancing the activity of Sox2 in reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells [42]. Sox2 is also phosphorylated by Cyclin-dependent kinase (CDK) at Ser37 (hs; mm Ser39) and Ser251 (hs; mm Ser253) with proposed functional roles in reprogramming to pluripotency [43]. Ser37 (hs; mm Ser39) and Thr118 (mm; hs Thr116) are flanking the HMG box which could confer conformational changes and/or promote interaction with transcriptional co-regulators.
Another example of phosphorylation modulating transcriptional activity is the phosphorylation of Sox9 at Ser64 (hs/mm) and Ser181 (hs/mm) by PKA in response to BMP/TGF signalling, which results in its transcriptional activation, in turn driving Sox9 dependent changes like delamination and osteochondrogenic differentiation [[44], [45], [46], [47]]. These amino acids lie near its dimerisation domain (Ser64) and HMG box (Ser181) which indicates that phosphorylation may modulate dimerisation of Sox9 and/or its interaction with DNA. Phosphorylation may therefore either alter the different dimers that Sox9 can make by recruiting different SoxE members (heterodimers vs. homodimers), preventing dimerisation, or modulating DNA interactions. In melanoma, Sox10 which is phosphorylated at Ser24 (hs/mm) and Thr240 (hs/mm) at sites which are predicted to be controlled by MAPK/CDK phosphorylation [48]. These sites are also highly conserved amongst SoxE family members suggesting a broad method of targeting these proteins in various different cancers [48]. Loss of these sites triggers an small increase in Sox10 transcriptional activity [48]. In all, SoxE family members have a wide range of phosphorylation sites that regulate their transcriptional activity by either increasing activity or changing activity. SoxE family members have been shown to be important in both maintenance of neural crest progenitors and ensuring their proper differentiation [49]. It could therefore be speculated that phosphorylation at the residues identified above could act as a TF switch that influences cell differentiation state. This could be exploited in a cancer therapeutic approach to influence the fate of cancer stem cells, such as driving terminal differentiation. Alterations to phosphatases might also influence SOX activity. Okadaic acid can broadly inhibiting serine/threonine phosphatases and results in an increase in Sox18 transcriptional activity. Sox18 transcriptional activity is reduced when prospective sites are mutated [50]. These sites are suggested to be downstream of either PKA or CK2 [50].
Methylation and acetylation result in the addition of a hydrophobic moiety to a protein, in the form of either a methyl group or acetyl group [51,52]. These PTMs have been extensively studied in the context of histone tail modifications. However, many proteins can be modified in this way [53]. Methylation has indeed been reported for Sox2 in ESCs, and is regulated by the coactivator-associated arginine methyltransferase 1 (CARM1). CARM1 mediates Sox2 transactivation by methylating Sox2 at Arg113 (hs; mm Arg115) resulting in enhanced oligomerization – possibly through the formation of dimers or larger protein complexes [54]. Interestingly, the chromatin-bound pool of Sox2 has a reduced methylation compared to unbound, suggesting that methylation may be important in loading and retaining Sox2 to transcriptional sites. Arg113 (hs; mm Arg115) flanks the HMG-domain of SOX2 which suggests that methylation may perturb DNA binding or that a region near the HMG is important in oligomerisation. CARM1 has also been shown to methylate Sox9 at multiple arginine residues near/on its HMG box domain in chondrocytes. This modification disrupts Sox9 interaction with beta-catenin which increases Cyclin D1 expression and therefore drives cell cycle progression [55]. It is unclear if this is due to Sox9 repressing beta-catenin transcriptional activity through its interaction, or if the methylation drives Sox9 transcriptional activity.
In ESCs, SOX2 is SUMOylated by the SUMO-E3 ligase, Pias3, which results in the impairment of the interaction with Oct4 and downstream disruption of key transcriptional targets, such as Nanog [56]. Furthermore, Sox6 sumoylation of either Lys404 (hs/mm) and Lys417 (hs/mm) perturbs its transcriptional activity [57]. Disruption of the SUMO modification site or its conjugating enzyme, Ubc9, result in an increase in transcriptional activity in both Sox2 and Sox6 [56,57]. Ubc9 has also been found to interact with Sox10 to drive its sumoylation at Lys55/357 (hs/mm) which leads to repression of its transcriptional activity at 2 of its target genes, GJB1 and MITF (by altering how it interacts with its cofactors EGR2 and PAX3) [58]. Furthermore, SUMOylation of SOX10 is antagonised by predicted MAPK sites at Thr240 and/or Thr244 (hs/mm) however Lys55 (hs/mm) SUMOlyation is required for SOX10 transcriptional activity [59]. Sox9 is SUMOylated at Lys61 (hs/mm), Lys253 (hs/mm), Lys398 (hs; mm Lys396) during chick development, within the neural crest progenitors during delamination. These Sox9 sites are required for its interaction with the zinc-finger-type transcription factor, Snail2, suggesting that SUMOylation is driving transcriptional activity of this complex [46]. This SUMOylation of Sox9 is mediated by Sox9 phosphorylation (Ser181/Ser211 (hs/mm) [[44], [45], [46], [47],[60], [61], [62], [63], [64]]. Thus, while modification with SUMO most commonly results in a suppression of transcriptional activity of Sox factors, it can also act to activate transcription as seen from the above papers (Fig. 4).
With regard to acetylation, Sox9 interacts with and is acetylated by KAT5 (Tip60) at Lys61 (hs/mm), Lys253 (hs/mm), Lys398 (hs; mm Lys396). These are also SUMOylation sites, indicating that there is a potential switch between acetylation and SUMOylation [65]. This modification increases Sox9 transcriptional activity. It would therefore be interesting to examine how these two modifications interact and how acetylation affects Snail2 interaction and how SUMOylation effects KAT5 interaction.
Altogether the above studies indicate that targeting the upstream enzymes that modulate (add/remove) PTMs on specific Sox protein transcriptional activity would have dramatic effects on Sox protein activity and function – although in complex and difficult to predict ways.
7. Disrupting pioneer activity
Eukaryotic genomes are organised into distinct chromatin territories – either compacted or more open and accessible – that stabilise the existing patterns of gene expression, helping to preserve cell type identity and limit inappropriate cell fate conversions. It is therefore critical to understand how transcription factors such as SOX members interact with distinct forms of chromatin. It has generally been viewed that transcription factors would bind most strongly to naked DNA, and become more restricted in their binding once the DNA becomes wrapped around histones, forming the nucleosome. However, it is become increasingly clear that certain transcription factors can strongly interact with nucleosome bound DNA. For example, SOX2 binds nucleosomes more strongly than naked DNA [66]. Transcription factors with these properties can therefore access closed chromatin and target silent genes for subsequent reactivation; they have been termed ‘pioneer’ factors [67]. This might be a key biochemical feature underpinning their importance as master regulators of cell type and as reprogramming factors [16,68]. Related to this, recent studies have suggested that binding to nucleosomes may impart the ability of pioneer factors to bind mitotic chromatin, acting as ‘book-marking’ proteins [69]. This mitotic bookmarking ability has been found to be more prevalent than previously thought and has been reported for Sox2 in ES cells [70]. The mitotic bookmarking function play a crucial role for maintaining cell identity through the cell cycle stages (reviewed in: Ref. [71]), which has direct implications on cycling and quiescent cancer cells. The role of PTM of Sox factors in regulating their pioneer activities is currently unknown yet could provide a key regulatory step.
Although not a Sox factor, HMG-14, which shares features with Sox proteins, is acetylated by p300 which weakens its interaction with nucleosomes [72]. We would speculate that large modifications like SUMOylation/ubiquitylation would disrupt the intricate chromatin-transcription factor interactions; however, phosphorylation, methylation, and acetylation would be harder to predict. Mechanistic insights have been made by studies of how Sox4 orchestrates chromatin remodelling during myoblast differentiation. Sox4, a SoxC member, interacts with and is acetylated by the acetyltransferase, KAT5, at Lys95 (hs/mm). This facilitates SOX4 recruitment to DNA and through the KAT5 chromodomain aids with chromatin remodelling [73]. This highlights that even small PTMs can modulate Sox family pioneer activity (Fig. 4).
8. Degrading SOX family members
Sox protein levels are tightly controlled to maintain cellular homeostasis. Sox levels are modulated throughout the cell cycle, and transcriptional regulation is in part achieved by varying steady-state protein levels. Proteins can be downregulated through extinguishing transcription, but also by post-translational control via protein degradation pathways. The most studied protein degradation is the ubiquitination system, although other PTMs can modulate this, such as phosphorylation [74]. Regulation of protein degradation pathways is therefore a clear route to perturbing the promiscuous activity of Sox factors in cancer (Fig. 4).
PTMs are not mutually exclusive, and an example of how they cooperate or antagonise one another, is the interplay between methylation, ubiquitination and phosphorylation on Sox2. In mouse ESCs, Sox2 protein expression is regulated by a novel methylation/phosphorylation switch. Setd7 efficiently monomethylates Sox2 at Lys119 (ms; hs Lys117). This methylation controls Sox2 protein stability by recruiting the ubiquitin ligase WWP2, whose catalytic HECT domain binds to the methylated lysine which in turn ubiquitylates Sox2 which results in its degradation [75]. In contrast, Akt1 phosphorylates SOX2 at Thr118 (mm; hs Thr116) which antagonises Lys119 (mm; hs Thr117) methylation and prevents subsequent degradation [75]. Additionally, SOX2 phosphorylation at Thr116 (hs; ms Thr118) by AKT antagonises degradation by the ubiquitin ligase UBR5 by preventing ubiquitination at Lys115 (hs; mm Lys117) [76]. It has been suggested that SOX phosphor-Thr118 (mm; hs Thr116) lies downstream of IGF-1 in a novel RIT/AKT/SOX2 axis [77]. Phosphorylation of SOX2 Ser251 (hs; mm Ser253) by ERK1/2 is also reported to promote autophagic degradation in nasopharyngeal carcinoma stem cells [78]. Mitotic SOX2 Ser251 (hs; mm Ser253) phosphorylation is also reported to be catalysed by Aurora A which maintains that a stem cell-like state [79]. These data demonstrate the importance of Sox factor protein stability in regulating cell fate.
The role of protein ubiquitination in regulating Sox factor stability is also exemplified in the stability of SOX2 during neural stem cell differentiation. During neural differentiation, the decrease in SOX2 protein expression is directly correlated to the expression of the deubiqutinating enzyme, OTUD7B and inversely to the reciprocal ubiquitin ligase complex, CUL4ADET1-COP1 [80]. Sox2 directly interacts with the substrate adaptor, COP1 which results in its polyubiquitination on multiple Lysine residues on Sox2, whereas OTUD7B deubiquinates Sox2 [80]. This modification is distinct from the WWP2 turnover of Sox2 which indicates that there may be differential regulation of Sox proteins between cell types. The CUL4A complexes are critical in regulating cell cycle machinery and indicates that Sox2 may have a novel role in the cell cycle [81]. Ubiquitin can form a polymer chain at any of its seven lysine residues which regulate, K11 linkages only accounts for a small percentage the total ubiquitination events in normal mammalian cells [82]. K11 is associated with proteasomal degradation of cell cycle components through the anaphase-promoting complex (APC) [83,84]. In addition, APC has been found to regulate neuronal morphogenesis and differentiation [85]. The generation of K11 linked chains are almost specifically driven by the priming E2, Ube2s, which has been identified as critical for maintaining pluripotency [86]. Interestingly, Sox2 is K11 ubiquitinated at Lys123 (mm; hs Lys121), thus marking it for degradation and contributes to Sox2-controlled differentiation to neuroectoderm [86]. This suggests that Sox2 is degraded at specific cell cycle stages to allow differentiation and could be manipulated in order to enforce differentiation of cancer stem cells.
Phosphorylation of proteins at specific residues can alter interactions with the degradation machinery: a phospho-degron. Sox6 is expressed in differentiating neurons with its expression gradually decreases through development. Sox6 is phosphorylated a phospho-degron at Thr119 (hs/mm) by Cdk5 which regulates Sox6 steady state protein levels in order to coordinate the proper differentiation into a post-mitotic state neuron [87]. Another example of a phospho-degron is on Sox9. Sox9 is phosphorylated by GSK3 kinase at Thr236 (hs/mm), FBW7 recognises a conserved degron surrounding this site which is degraded by the SCFFBW7α complex [88]. This was shown to be negatively regulated by a PI3K/AKT/mTOR signalling axis. As shown in Fig. 1, Sox9 is highly expressed in medulloblastoma; in cases where FBW7 is dysregulated, Sox9 protein is elevated [88]. Inhibition of PI3K/AKT/mTOR signalling destabilises Sox9 which renders medulloblastoma cells sensitive to cytostatic treatment [88]. Interestingly, this residue and motif is conserved amongst all SoxE family members which suggest that there may be a unifying approach to targeting cancers where SoxE family members are overexpressed (Fig. 1). SOX10, which is highly expressed in melanoma tumours, was identified to be phosphorylated at Ser24 (hs/mm). This site is predicted to be a MAPK/CDK substrate motif and mutation of this resulted in an increase in stability of SOX10 suggesting the presence of a phosphodegron [48].
These findings further the understanding of Sox10 protein levels and provides possibly novel targets for more effective combinatorial therapeutics. Modulation of the stability of Sox proteins may provide a way to compensate against the effects of oncogenic or SOX2 genomic amplifications observed in cancers. Therefore, targeting the pathways upstream with activators or agonists may provide a unique way to decrease Sox expression.
9. Mediating SOX nuclear localisation
Within the Sox HMG domain there is a conserved region which is a predicted to be a nuclear localisation signal (Reviewed in: Ref. [89]). Disruption of Sox factor localisation (less nuclear) has been shown upon perturbation of the acetylation of SRY by p300 at Lys136 (hs; ms not conserved) where this residue increases its interaction with importin-beta [90]. This is reversed by HDAC3. These data suggest that SRY acetylation may play an important regulatory role. Acetylation by p300 can also promote the export of other Sox factors. SOX2 is acetylated at Lys75 (mm. hs Lys73), within its HMG box, which disrupts it nuclear localisation and promotes nuclear export [91]. Disruption of this acetylation promotes SOX2 nuclear localisation and maintains its target gene expression. Therefore, p300 represents an interesting target for regulating the localisation of Sox proteins that are overexpressed in cancer (Fig. 4).
Sox11 is transiently active during neurogenesis which ensures precise execution of the neurogenic program. Sox11 phosphorylation at Ser30 (hs/mm), a residue N-terminal of the HMG box, promotes nuclear localisation [92]. The upstream kinases for this PTM remain unclear and could represent an interesting and druggable target to regulate Sox11 localisation.
10. Future perspectives
This review has summarised the numerous roles of PTMs that influence Sox proteins. There is a wealth of possibilities to influence SOX activity via modulation of PTMs. SOX PTMs that are at the core of controlling “stemness” can act as molecular switches for various cancer types. It is therefore important to understand how to target these using either small molecules, gene therapies or immunotherapies. The therapeutic exploitation of PTMs other than phosphorylation is still in its infancy. Phosphorylation, which is the most well studied PTM, led to 38 small molecules approved for clinical applications [93]. The targeting of other PTMs remains more elusive, with acetylation being of the next most frequently targeted (e.g. HDAC inhibitors).
In addition to finding novel sites on Sox factors for the pre-existing array of PTMs, there is the possibility that unknown PTMs may be having a novel function in regulating Sox activity. There is much to explore. For example, the recent discovery of non-canonical phosphorylation events which are highly liable or the recently identified PTMs like CoAlation [94,95]. However, a challenge will be to identify PTMs and associated enzymatic activities that are cell type/lineage specific to prevent off target tissue toxicities. Furthermore, it remains unclear how PTMs will effect changes to the biophysical properties of Sox factors which are required to initiative transcription. A biophysical mechanism may explain the robust and high levels of expression of many developmental master regulatory TFs: namely, the aggregation of these high valency proteins into complexes and formation of phase-separated nuclear microcompartments, or condensates, that provide robust and high levels of expression [96]. Indeed, it has been identified that phosphorylation can prevent phase-separated protein condensation of core transcriptional machinery [97].
11. Concluding remarks
Although many genetic or RNAi loss of function studies have identified critical roles for Sox proteins in cancer, unfortunately less is known about the biochemistry of Sox proteins and how they could be modulated using small molecules. PTMs have been identified which regulate almost every aspect of Sox function, but the functional roles, cross-talk between these modifications needs further investigation and is not easily dissected. With improved understanding of the mechanisms by which PTMs are added/removed opportunities will open up for new strategies to tackle many human cancers. Such targeted can potentially disrupt tumour growth without the significant tissue toxicities that are a feature of current cytotoxic or anti-mitotic treatments.
Acknowledgements
SMP is a Cancer Research UK Senior Research Fellow (A19778) and is supported by The Brain Tumour Charity Quest for Cures Collaborative Team Award (GN-000358). SP and AS are supported by the BBSRC/EPSRC/MRC funded, UK Mammalian Synthetic Biology Research Centre (BB/M018040/1). AS is supported by an MRC career development award (MR/N024028/1), and by a Cancer research UK grant award (C65925/A26986).
References
- 1.Kreso A., Dick J.E. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14:275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar A., Hochedlinger K. The Sox family of transcription factors: versatile regulators of stem and progenitor cell fate. Cell Stem Cell. 2013;12:15–30. doi: 10.1016/j.stem.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamachi Y., Kondoh H. Sox proteins: regulators of cell fate specification and differentiation. Development. 2013;140:4129–4144. doi: 10.1242/dev.091793. [DOI] [PubMed] [Google Scholar]
- 4.Spitz F., Furlong E.E.M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 5.Hnisz D., Schuijers J., Lin C.Y., Weintraub A.S., Abraham B.J., Lee T.I., Bradner J.E., Young R.A. Convergence of developmental and oncogenic signaling pathways at transcriptional super-enhancers. Mol. Cell. 2015;58:362–370. doi: 10.1016/j.molcel.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradner J.E., Hnisz D., Young R.A. Transcriptional addiction in Cancer. Cell. 2017;168:629–643. doi: 10.1016/j.cell.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert S.A., Jolma A., Campitelli L.F., Das P.K., Yin Y., Albu M., Chen X., Taipale J., Hughes T.R., Weirauch M.T. The human transcription factors. Cell. 2018;172:650–665. doi: 10.1016/j.cell.2018.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Guerra S., Cichowski K. Targeting cancer at the intersection of signaling and epigenetics. Annu. Rev. Cancer Biol. 2018;3 doi: 10.1146/annurev-cancerbio-030617-050400. annurev-cancerbio-030617-050400. [DOI] [Google Scholar]
- 9.Cohen P. Protein kinases—the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002;1:309–315. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 10.Hoadley K.A., Yau C., Hinoue T., Wolf D.M., Lazar A.J., Drill E. Cell-of-origin patterns dominate the molecular classification of 10,000 tumors from 33 types of cancer. Cell. 2018;173 doi: 10.1016/J.CELL.2018.03.022. 291-304.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair A.H., Berta P., Palmer M.S., Hawkins J.R., Griffiths B.L., Smith M.J., Foster J.W., Frischauf A.M., Lovell-Badge R., Goodfellow P.N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- 12.Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 13.Badis G., Berger M.F., Philippakis A.A., Talukder S., Gehrke A.R., Jaeger S.A., Chan E.T., Metzler G., Vedenko A., Chen X., Kuznetsov H., Wang C.F., Coburn D., Newburger D.E., Morris Q., Hughes T.R., Bulyk M.L. Diversity and complexity in DNA recognition by transcription factors. Science (80-.) 2009;324:1720–1723. doi: 10.1126/science.1162327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reményi A., Lins K., Nissen L.J., Reinbold R., Schöler H.R., Wilmanns M. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 2003;17:2048–2059. doi: 10.1101/gad.269303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaffidi P., Bianchi M.E. Spatially precise DNA bending is an essential activity of the Sox2 transcription factor. J. Biol. Chem. 2001;276:47296–47302. doi: 10.1074/jbc.M107619200. [DOI] [PubMed] [Google Scholar]
- 16.Bowles J., Schepers G., Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 2000;227:239–255. doi: 10.1006/dbio.2000.9883. [DOI] [PubMed] [Google Scholar]
- 17.Guth S.I.E., Wegner M. Having it both ways: sox protein function between conservation and innovation. Cell. Mol. Life Sci. 2008;65:3000–3018. doi: 10.1007/s00018-008-8138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu S., Kenne L., Pedersén M. α-1,4-Glucan lyase, a new class of starch/glycogen degrading enzyme. I. Efficient purification and characterization from red seaweeds. BBA Gen. Subj. 1993;1156:313–320. doi: 10.1016/0304-4165(93)90049-E. [DOI] [PubMed] [Google Scholar]
- 19.Uchikawa M., Kamachi Y., Kondoh H. Two distinct subgroups of Group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech. Dev. 1999;84:103–120. doi: 10.1016/S0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 20.Ito M. Function and molecular evolution of mammalian Sox15, a singleton in the SoxG group of transcription factors. Int. J. Biochem. Cell Biol. 2010;42:449–452. doi: 10.1016/j.biocel.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Coustry F., do Oh C., Hattori T., Maity S.N., de Crombrugghe B., Yasuda H. The dimerization domain of SOX9 is required for transcription activation of a chondrocyte-specific chromatin DNA template. Nucleic Acids Res. 2010;38:6018–6028. doi: 10.1093/nar/gkq417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y.H., Jankowski A., Cheah K.S.E., Prabhakar S., Jauch R. SOXE transcription factors form selective dimers on non-compact DNA motifs through multifaceted interactions between dimerization and high-mobility group domains. Sci. Rep. 2015;5:10398. doi: 10.1038/srep10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peirano R.I. The glial transcription factor Sox10 binds to DNA both as monomer and dimer with different functional consequences. Nucleic Acids Res. 2000;28:3047–3055. doi: 10.1093/nar/28.16.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefebvre V., Li P., De Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefebvre V. The SoxD transcription factors - Sox5, Sox6, and Sox13 - are key cell fate modulators. Int. J. Biochem. Cell Biol. 2010;42:429–432. doi: 10.1016/j.biocel.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osaki E., Nishina Y., Inazawa J., Copeland N.G., Gilbert D.J., Jenkins N.A., Ohsugi M., Tezuka T., Yoshida M., Semba K. Identification of a novel Sry-related gene and its germ cell-specific expression. Nucleic Acids Res. 1999;27:2503–2510. doi: 10.1093/nar/27.12.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim W., Bennett E.J., Huttlin E.L., Guo A., Li J., Possemato A., Sowa M.E., Rad R., Rush J., Comb M.J., Harper J.W., Gygi S.P. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol. Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeNardo B.D., Holloway M.P., Ji Q., Nguyen K.T., Cheng Y., Valentine M.B., Salomon A., Altura R.A. Quantitative phosphoproteomic analysis identifies activation of the RET and IGF-1R/IR signaling pathways in neuroblastoma. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanivan S., Gnad F., Wickström S.A., Geiger T., Macek B., Cox J., Fässler R., Mann M. Solid tumor proteome and phosphoproteome analysis by high resolution mass spectrometry. J. Proteome Res. 2008;7:5314–5326. doi: 10.1021/pr800599n. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen M.L., Jensen L.J., Larsen S.C., Mund A., Mullari M., Lyon D., Daniel J.A., Sylvestersen K.B., Madsen M.V. Proteome-wide analysis of arginine monomethylation reveals widespread occurrence in human cells. Sci. Signal. 2016;9 doi: 10.1126/scisignal.aaf7329. rs9–rs9. [DOI] [PubMed] [Google Scholar]
- 31.Old W.M., Shabb J.B., Houel S., Wang H., Couts K.L., yu Yen C., Litman E.S., Croy C.H., Meyer-Arendt K., Miranda J.G., Brown R.A., Witze E.S., Schweppe R.E., Resing K.A., Ahn N.G. Functional proteomics identifies targets of phosphorylation by B-Raf signaling in melanoma. Mol. Cell. 2009;34:115–131. doi: 10.1016/j.molcel.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mertins P., Mani D.R., Ruggles K.V., Gillette M.A., Clauser K.R., Wang P., Wang X., Qiao J.W., Cao S., Petralia F., Kawaler E., Mundt F., Krug K., Tu Z., Lei J.T., Gatza M.L., Wilkerson M., Perou C.M., Yellapantula V., Huang K.L., Lin C., McLellan M.D., Yan P., Davies S.R., Townsend R.R., Skates S.J., Wang J., Zhang B., Kinsinger C.R., Mesri M., Rodriguez H., Ding L., Paulovich A.G., Fenyö D., Ellis M.J., Carr S.A. Proteogenomics connects somatic mutations to signalling in breast cancer. Nature. 2016;534:55–62. doi: 10.1038/nature18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiromizu T., Adachi J., Watanabe S., Murakami T., Kuga T., Muraoka S., Tomonaga T. Identification of missing proteins in the neXtProt database and unregistered phosphopeptides in the phosphositeplus database as part of the chromosome-centric human proteome project. J. Proteome Res. 2013;12:2414–2421. doi: 10.1021/pr300825v. [DOI] [PubMed] [Google Scholar]
- 34.Schweppe D.K., Rigas J.R., Gerber S.A. Quantitative phosphoproteomic profiling of human non-small cell lung cancer tumors. J. Proteomics. 2013;91:286–296. doi: 10.1016/j.jprot.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harley V.R., Jackson D.I., Hextall P.J., Hawkins J.R., Berkovitz G.D., Sockanathan S., Lovell-Badge R., Goodfellow P.N. DNA binding activity of recombinant SRY from normal males and XY females. Science (80-.) 1992;255:453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- 36.Palasingam P., Jauch R., Ng C.K.L., Kolatkar P.R. The structure of Sox17 bound to DNA reveals a conserved bending topology but selective protein interaction platforms. J. Mol. Biol. 2009;388:619–630. doi: 10.1016/j.jmb.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 37.Klaus M., Prokoph N., Girbig M., Wang X., Huang Y.H., Srivastava Y., Hou L., Narasimhan K., Kolatkar P.R., Francois M., Jauch R. Structure and decoy-mediated inhibition of the SOX18/Prox1-DNA interaction. Nucleic Acids Res. 2016;44:3922–3935. doi: 10.1093/nar/gkw130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jauch R., Ng C.K.L., Narasimhan K., Kolatkar P.R. The crystal structure of the Sox4 HMG domain–DNA complex suggests a mechanism for positional interdependence in DNA recognition. Biochem. J. 2012;443:39–47. doi: 10.1042/bj20111768. [DOI] [PubMed] [Google Scholar]
- 39.Hou L., Srivastava Y., Jauch R. Molecular basis for the genome engagement by Sox proteins. Semin. Cell Dev. Biol. 2017;63:2–12. doi: 10.1016/j.semcdb.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Justilien V., Walsh M.P., Ali S.A., Thompson E.A., Murray N.R., Fields A.P. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate hedgehog signaling in lung squamous cell carcinoma. Cancer Cell. 2014;25:139–151. doi: 10.1016/j.ccr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekita Y., Watanabe S., Kawasuji M., Tsuruzoe S., Niwa H., Saitoh H., Uchimura Y., Ishihara K., Nakao M., Baba H., Aoto T., Yuasa Y. Inhibition of DNA binding of Sox2 by the SUMO conjugation. Biochem. Biophys. Res. Commun. 2006;351:920–926. doi: 10.1016/j.bbrc.2006.10.130. [DOI] [PubMed] [Google Scholar]
- 42.Jeong C.H., Cho Y.Y., Kim M.O., Kim S.H., Cho E.J., Lee S.Y., Jeon Y.J., Lee K.Y., Yao K., Keum Y.S., Bode A.M., Dong Z. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–2150. doi: 10.1002/stem.540. [DOI] [PubMed] [Google Scholar]
- 43.Ouyang J., Yu W., Liu J., Zhang N., Florens L., Chen J., Liu H., Washburn M., Pei D., Xie T. Cyclin-dependent kinase-mediated Sox2 phosphorylation enhances the ability of Sox2 to establish the pluripotent state. J. Biol. Chem. 2015;290:22782–22794. doi: 10.1074/jbc.M115.658195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W., Zhou X., Lefebvre V., de Crombrugghe B. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase a enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 2000;20:7838. doi: 10.1128/mcb.20.20.7838-7838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coricor G., Serra R. TGF-β regulates phosphorylation and stabilization of Sox9 protein in chondrocytes through p38 and Smad dependent mechanisms. Sci. Rep. 2016;6:38616. doi: 10.1038/srep38616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu J.A.J., Wu M.-H., Yan C.H., Chau B.K.H., So H., Ng A., Chan A., Cheah K.S.E., Briscoe J., Cheung M. Phosphorylation of Sox9 is required for neural crest delamination and is regulated downstream of BMP and canonical Wnt signaling. Proc. Natl. Acad. Sci. 2013;110:2882–2887. doi: 10.1073/pnas.1211747110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar D., Lassar A.B. The transcriptional activity of Sox9 in chondrocytes is regulated by RhoA signaling and actin polymerization. Mol. Cell. Biol. 2009;29:4262–4273. doi: 10.1128/MCB.01779-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cronin J.C., Loftus S.K., Baxter L.L., Swatkoski S., Gucek M., Pavan W.J. Identification and functional analysis of SOX10 phosphorylation sites in melanoma. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung M. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 50.Hosking B.M., Wyeth J.R., Pennisi D.J., Wang S.C.M., Koopman P., Muscat G.E.O. Cloning and functional analysis of the Sry-related HMG box gene, Sox18. Gene. 2001;262:239–247. doi: 10.1016/S0378-1119(00)00525-4. [DOI] [PubMed] [Google Scholar]
- 51.McBride A.E., Silver P.A. State of the Arg: protein methylation at arginine comes of age. Cell. 2001;106:5–8. doi: 10.1016/S0092-8674(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 52.Glozak M.A., Sengupta N., Zhang X., Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 53.Hansen B.K., Gupta R., Baldus L., Lyon D., Narita T., Lammers M., Choudhary C., Weinert B.T. Analysis of human acetylation stoichiometry defines mechanistic constraints on protein regulation. Nat. Commun. 2019;10:1055. doi: 10.1038/s41467-019-09024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.yong Zhao H., jun Zhang Y., Dai H., Zhang Y., fei Shen Y. CARM1 mediates modulation of Sox2. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito T., Yadav N., Lee J., Furumatsu T., Yamashita S., Yoshida K., Taniguchi N., Hashimoto M., Tsuchiya M., Ozaki T., Lotz M., Bedford M.T., Asahara H. Arginine methyltransferase CARM1/PRMT4 regulates endochondral ossification. BMC Dev. Biol. 2009;9:47. doi: 10.1186/1471-213X-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu Y., Guo Z., Wu H., Wang X., Yang L., Shi X., Du J., Tang B., Li W., Yang L., Zhang Y. SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fernández-Lloris R., Osses N., Jaffray E., Shen L.N., Vaughan O.A., Girwood D., Bartrons R., Rosa J.L., Hay R.T., Ventura F. Repression of SOX6 transcriptional activity by SUMO modification. FEBS Lett. 2006;580:1215–1221. doi: 10.1016/j.febslet.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Girard M., Goossens M. Sumoylation of the SOX10 transcription factor regulates its transcriptional activity. FEBS Lett. 2006;580:1635–1641. doi: 10.1016/j.febslet.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Han S., Ren Y., He W., Liu H., Zhi Z., Zhu X., Yang T., Rong Y., Ma B., Purwin T.J., Ouyang Z., Li C., Wang X., Wang X., Yang H., Zheng Y., Aplin A.E., Liu J., Shao Y. ERK-mediated phosphorylation regulates SOX10 sumoylation and targets expression in mutant BRAF melanoma. Nat. Commun. 2018;9:28. doi: 10.1038/s41467-017-02354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang W., Chung U.-i., Kronenberg H.M., de Crombrugghe B. The chondrogenic transcription factor Sox9 is a target of signaling by the parathyroid hormone-related peptide in the growth plate of endochondral bones. Proc. Natl. Acad. Sci. 2001;98:160–165. doi: 10.1073/pnas.98.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haudenschild D.R., Chen J., Pang N., Lotz M.K., D’Lima D.D. Rho kinase-dependent activation of SOX9 in chondrocytes. Arthritis Rheum. 2010;62:191–200. doi: 10.1002/art.25051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Handorf A.M., Li W.J. Fibroblast growth Factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis. PLoS One. 2011;6 doi: 10.1371/journal.pone.0022887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Malki S., Nef S., Notarnicola C., Thevenet L., Gasca S., Méjean C., Berta P., Poulat F., Boizet-Bonhoure B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L., Li G., Zhou G.Q. SOX9 directly binds CREB as a novel synergism with the PKA pathway in BMP-2-induced osteochondrogenic differentiation. J. Bone Miner. Res. 2009;24:826–836. doi: 10.1359/jbmr.081236. [DOI] [PubMed] [Google Scholar]
- 65.Hattori T., Coustry F., Stephens S., Eberspaecher H., Takigawa M., Yasuda H., de Crombrugghe B. Transcriptional regulation of chondrogenesis by coactivator Tip60 via chromatin association with Sox9 and Sox5. Nucleic Acids Res. 2008;36:3011–3024. doi: 10.1093/nar/gkn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soufi A., Garcia M.F., Jaroszewicz A., Osman N., Pellegrini M., Zaret K.S. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zaret K.S., Carroll J.S. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soufi A., Donahue G., Zaret K.S. Facilitators and impediments of the pluripotency reprogramming factors’ initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caravaca J.M., Donahue G., Becker J.S., He X., Vinson C., Zaret K.S. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 2013;27:251–260. doi: 10.1101/gad.206458.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deluz C., Friman E.T., Strebinger D., Benke A., Raccaud M., Callegari A., Leleu M., Manley S., Suter D.M. A role for mitotic bookmarking of SOX2 in pluripotency and differentiation. Genes Dev. 2016;30:2538–2550. doi: 10.1101/gad.289256.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soufi A., Dalton S. Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development. 2016;143:4301–4311. doi: 10.1242/dev.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bergel M., Herrera J.E., Thatcher B.J., Prymakowska-Bosak M., Vassilev A., Nakatani Y., Martin B., Bustin M. Acetylation of novel sites in the nucleosomal binding domain of chromosomal protein HMG-14 by p300 alters its interaction with nucleosomes. J. Biol. Chem. 2000;275:11514–11520. doi: 10.1074/jbc.275.15.11514. [DOI] [PubMed] [Google Scholar]
- 73.Jang S.M., Kim J.W., Kim C.H., An J.H., Johnson A., Song P.I., Rhee S., Choi K.H. KAT5-mediated SOX4 acetylation orchestrates chromatin remodeling during myoblast differentiation. Cell Death Dis. 2015;6 doi: 10.1038/cddis.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Filtz T.M., Vogel W.K., Leid M. Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 2014;35:76–85. doi: 10.1016/j.tips.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fang L., Zhang L., Wei W., Jin X., Wang P., Tong Y., Li J., Du J.X., Wong J. A methylation-phosphorylation switch determines Sox2 stability and function in ESC maintenance or differentiation. Mol. Cell. 2014;55:537–551. doi: 10.1016/j.molcel.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 76.Wang Z., Kang L., Zhang H., Huang Y., Fang L., Li M., Brown P.J., Arrowsmith C.H., Li J., Wong J. AKT drives SOX2 overexpression and cancer cell stemness in esophageal cancer by protecting SOX2 from UBR5-mediated degradation. Oncogene. 2019;38:5250–5264. doi: 10.1038/s41388-019-0790-x. [DOI] [PubMed] [Google Scholar]
- 77.Mir S., Cai W., Carlson S.W., Saatman K.E., Andres D.A. IGF-1 mediated Neurogenesis Involves a Novel RIT1/Akt/Sox2 Cascade. Sci. Rep. 2017;7:3283. doi: 10.1038/s41598-017-03641-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ji J., Yu Y., Li Z.L., Chen M.Y., Deng R., Huang X., Wang G.F., Zhang M.X., Yang Q., Ravichandran S., Feng G.K., Xu X.L., Yang C.L., Qiu M.Z., Jiao L., Yang D., Zhu X.F. XIAP limits autophagic degradation of Sox2 and is a therapeutic target in nasopharyngeal carcinoma stem cells. Theranostics. 2018;8:1494–1510. doi: 10.7150/thno.21717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qi D., Wang Q., Yu M., Lan R., Li S., Lu F. Mitotic phosphorylation of SOX2 mediated by Aurora kinase A is critical for the stem-cell like cell maintenance in PA-1 cells. Cell Cycle. 2016;15:2009–2018. doi: 10.1080/15384101.2016.1192729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cui C.P., Zhang Y., Wang C., Yuan F., Li H., Yao Y., Chen Y., Li C., Wei W., Liu C.H., He F., Liu Y., Zhang L. Dynamic ubiquitylation of Sox2 regulates proteostasis and governs neural progenitor cell differentiation. Nat. Commun. 2018;9:4648. doi: 10.1038/s41467-018-07025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sharma P., Nag A. CUL4A ubiquitin ligase: a promising drug target for cancer and other human diseases. Open Biol. 2014;4 doi: 10.1098/rsob.130217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng J., Schwartz D., Elias J.E., Thoreen C.C., Cheng D., Marsischky G., Roelofs J., Finley D., Gygi S.P. A proteomics approach to understanding protein ubiquitination. Nat. Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 83.Matsumoto M.L., Wickliffe K.E., Dong K.C., Yu C., Bosanac I., Bustos D., Phu L., Kirkpatrick D.S., Hymowitz S.G., Rape M., Kelley R.F., Dixit V.M. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol. Cell. 2010;39:477–484. doi: 10.1016/j.molcel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Meyer H.J., Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157:910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puram S.V., Bonni A. Novel functions for the anaphase-promoting complex in neurobiology. Semin. Cell Dev. Biol. 2011;22:586–594. doi: 10.1016/j.semcdb.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang J., Zhang Y., Hou J., Qian X., Zhang H., Zhang Z., Li M., Wang R., Liao K., Wang Y., Li Z., Zhong D., Wan P., Dong L., Liu F., Wang X., Wan Y., Xiao W., Zhang W.W. Ube2s regulates Sox2 stability and mouse ES cell maintenance. Cell Death Differ. 2016;23:393–404. doi: 10.1038/cdd.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rudrabhatla P., Utreras E., Jaffe H., Kulkarni A.B. Regulation of Sox6 by cyclin dependent kinase 5 in brain. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suryo Rahmanto A., Savov V., Brunner A., Bolin S., Weishaupt H., Malyukova A., Rosén G., Čančer M., Hutter S., Sundström A., Kawauchi D., Jones D.T., Spruck C., Taylor M.D., Cho Y., Pfister S.M., Kool M., Korshunov A., Swartling F.J., Sangfelt O. FBW7 suppression leads to SOX9 stabilization and increased malignancy in medulloblastoma. EMBO J. 2016;35:2192–2212. doi: 10.15252/embj.201693889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malki S., Boizet-Bonhoure B., Poulat F. Shuttling of SOX proteins. Int. J. Biochem. Cell Biol. 2010;42:411–416. doi: 10.1016/j.biocel.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Thevenet L., Méjean C., Moniot B., Bonneaud N., Galéotti N., Aldrian-Herrada G., Poulat F., Berta P., Benkirane M., Boizet-Bonhoure B. Regulation of human SRY subcellular distribution by its acetylation/deacetylation. EMBO J. 2004;23:3336–3345. doi: 10.1038/sj.emboj.7600352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baltus G.A., Kowalski M.P., Zhai H., Tutter A.V., Quinn D., Wall D., Kadam S. Acetylation of Sox2 induces its nuclear export in embryonic stem cells. Stem Cells. 2009;27:2175–2184. doi: 10.1002/stem.168. [DOI] [PubMed] [Google Scholar]
- 92.Balta E.-A., Wittmann M.-T., Jung M., Sock E., Haeberle B.M., Heim B., von Zweydorf F., Heppt J., von Wittgenstein J., Gloeckner C.J., Lie D.C. Phosphorylation modulates the subcellular localization of SOX11. Front. Mol. Neurosci. 2018;11:211. doi: 10.3389/fnmol.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferguson F.M., Gray N.S. Kinase inhibitors: the road ahead. Nat. Rev. Drug Discov. 2018;17:353–377. doi: 10.1038/nrd.2018.21. [DOI] [PubMed] [Google Scholar]
- 94.Gout I. Coenzyme A, protein CoAlation and redox regulation in mammalian cells. Biochem. Soc. Trans. 2018;46:721–728. doi: 10.1042/bst20170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hardman G., Perkins S., Brownridge P.J., Clarke C.J., Byrne D.P., Campbell A.E., Kalyuzhnyy A., Myall A., Eyers P.A., Jones A.R., Eyers C.E. Strong anion exchange‐mediated phosphoproteomics reveals extensive human non-canonical phosphorylation. EMBO J. 2019 doi: 10.15252/embj.2018100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sabari B.R., Dall’Agnese A., Boija A., Klein I.A., Coffey E.L., Shrinivas K., Abraham B.J., Hannett N.M., Zamudio A.V., Manteiga J.C., Li C.H., Guo Y.E., Day D.S., Schuijers J., Vasile E., Malik S., Hnisz D., Lee T.I., Cisse I.I., Roeder R.G., Sharp P.A., Chakraborty A.K., Young R.A. Coactivator condensation at super-enhancers links phase separation and gene control. Science (80-.) 2018;361 doi: 10.1126/science.aap9195. eaar3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guo Y.E., Manteiga J.C., Henninger J.E., Sabari B.R., Dall’Agnese A., Hannett N.M., Spille J.-H., Afeyan L.K., Zamudio A.V., Shrinivas K., Abraham B.J., Boija A., Decker T.-M., Rimel J.K., Fant C.B., Lee T.I., Cisse I.I., Sharp P.A., Taatjes D.J., Young R.A. Pol II phosphorylation regulates a switch between transcriptional and splicing condensates. Nature. 2019;572:543–548. doi: 10.1038/s41586-019-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim S., Bhinge A., Bragado Alonso S., Aksoy I., Aprea J., Cheok C.F., Calegari F., Stanton L.W., Kaldis P. Cyclin-dependent kinase-dependent phosphorylation of Sox2 at serine 39 regulates neurogenesis. Mol. Cell. Biol. 2017;37 doi: 10.1128/mcb.00201-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balta E.-A., Schäffner I., Wittmann M.-T., Sock E., von Zweydorf F., von Wittgenstein J., Steib K., Heim B., Kremmer E., Häberle B.M., Ueffing M., Lie D.C., Gloeckner C.J. Phosphorylation of the neurogenic transcription factor SOX11 on serine 133 modulates neuronal morphogenesis. Sci. Rep. 2018;8:16196. doi: 10.1038/s41598-018-34480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]