Abstract
Vaspin, visceral-adipose-tissue-derived serine protease inhibitor, is involved in the development of obesity, insulin resistance, inflammation, and energy metabolism. Our previous study showed vaspin expression and its regulation in the ovary; however, the role of this adipokine in ovarian cells has never been studied. Here, we studied the in vitro effect of vaspin on various kinase-signaling pathways: mitogen-activated kinase (MAP3/1), serine/threonine kinase (AKT), signal transducer and activator of transcription 3 (STAT3) protein kinase AMP (PRKAA1), protein kinase A (PKA), and on expression of nuclear factor kappa B (NFKB2) as well as on steroid synthesis by porcine ovarian cells. By using western blot, we found that vaspin (1 ng/ml), in a time-dependent manner, increased phosphorylation of MAP3/1, AKT, STAT3, PRKAA1, and PKA, while it decreased the expression of NFKB2. We observed that vaspin, in a dose-dependent manner, increased the basal steroid hormone secretion (progesterone and estradiol), mRNA and protein expression of steroid enzymes using real-time PCR and western blot, respectively, and the mRNA of gonadotropins (FSHR, LHCGR) and steroids (PGR, ESR2) receptors. The stimulatory effect of vaspin on basal steroidogenesis was reversed when ovarian cells were cultured in the presence of a PKA pharmacological inhibitor (KT5720) and when GRP78 receptor was knocked down (siRNA). However, in the presence of insulin-like growth factor type 1 and gonadotropins, vaspin reduced steroidogenesis. Thus, vaspin, by activation of various signaling pathways and stimulation of basal steroid production via GRP78 receptor and PKA, could be a new regulator of porcine ovarian function.
Keywords: steroid hormones, kinases, ovary, pig, vaspin
Vaspin, by activation of various signaling pathways and stimulation of basal steroid production via GRP78 receptor and protein kinase A, could be a new regulator of porcine ovarian function.
Introduction
Vaspin was recently identified as a member of the serine protease inhibitor (serpin) family, from the visceral white adipose tissue of Otsuka Long-Evans Tokushima fatty (OLETF) rats, an animal model of obesity and related metabolic disorders such as type 2 diabetes [1]. In the OLETF rat, vaspin expression in visceral adipose tissue and vaspin serum concentration were highest when insulin resistance and obesity reached their peak and decreased with the exacerbation of diabetes and concomitant body weight loss [1]. Rat, mouse, and human vaspins are made up of 392, 394, and 395 amino acids, respectively; they exhibit approximately 40% homology with alpha-1-antitrypsin and are related to the serine-protease-inhibitor family. Vaspin is a 47-kDa protein that is constitutively synthesized by adipose tissue and many other tissues including gastric mucosa, liver, pancreas, cerebrospinal fluid, skin, hypothalamus, heart, small intestine, skeletal muscle and lung in humans, mice and bovines [2–4]. A higher vaspin level is observed in the serum of women than of men and is also correlated with obesity and impaired insulin sensitivity [5]. Vaspin is an adipokine involved in obesity development, insulin resistance and the pathogenesis of inflammatory reactions of the body. Recent meta-analysis covering six studies with 1826 participants found significantly higher levels of serum vaspin in obese subjects [6]. The receptor of vaspin is not yet identified; however, literature data show that in endothelial cells vaspin acts as a ligand for the cell-surface anion channel complex heat shock protein family A (Hsp70) member GRP78 receptor [7]. This receptor plays important roles in the regulation of several processes in the body like reproduction, cancer progression, malignancy, drug resistance, and neurologic disorders, by activation of different signaling pathways [8]. Moreover, recent published studies documented that vaspin activates various signaling pathways in different cells, which lead to the regulation of its physiology; for example, mitogen-activated kinase (MAP3/1) in human osteoblasts [9], serine/threonine kinase (AKT) in pancreatic beta cells [10], signal transducer and activator of transcription 3 (STAT3) in endothelial cells and isolated aorta from Sprague-Dawley rats [11], and protein kinase AMP-activated catalytic subunit alpha 1 (PRKAA1) in vascular endothelial cells [12], and it has an inhibitory effect on the nuclear factor kappa B subunit 2 (NFKB2) pathway in cytokine-induced inflammation in 3T3-L1 adipocytes [13]. Also, several studies have shown the key role of activation of the signaling pathways MAP3/1, PI3/AKT, STAT3, protein kinase A (PKA), and PRKAA1 on female fertility including ovarian functions like oocyte maturation, granulosa (Gc) cell proliferation, differentiation, and apoptosis as well as steroid synthesis [14–18].
In recent years, growing evidence has demonstrated that vaspin is actively involved in the female reproduction system. Indeed, vaspin is constitutively expressed in the hypothalamus [3], placenta [19], and ovary [20]. In porcine ovarian follicles, vaspin expression is detected in cumulus, Gc, and theca (Tc) cells as well as in oocytes [20]. We showed that a higher vaspin mRNA and protein expression in the porcine ovarian follicles and adipose tissue at 10–12 days of the estrous cycle in fat Meishan compared to lean Large White [20]. Moreover, ovarian vaspin expression is dependent on days of the estrous cycle and is regulated by gonadotropins, insulin, insulin-like growth factor type 1 (IGF1), and steroid hormones, interestingly through the activation of different kinases such as STAT3, MAP3/1, PI3/AKT, and PRKAA1, as well as NFKB2 [20]. Additionally, Tan et al. [21] showed a significantly higher expression of vaspin mRNA in white adipose tissue and serum of overweight women with polycystic ovary syndrome (PCOS) compared to a control group without PCOS, while metformin treatment decreases serum vaspin level in PCOS women [21].
Despite these advances, there are still questions regarding the role of vaspin in ovarian function, in addition to signaling pathways or steroid synthesis. Furthermore, it is well known that other adipokines regulate ovarian steroidogenesis; for example, resistin increases progesterone (P4), androstenedione (A4), and testosterone (T) secretion by stimulating the expression of steroidogenic enzymes like cytochrome P450 family 11 subfamily A member 1 (CYP11A1), hydroxy-delta-5-steroid dehydrogenase (HSD3B), and cytochrome P450 family 19 subfamily A member 1 (CYP19A1) [22], while apelin stimulates basal P4 and estradiol (E2) secretion by expression of HSD3B and CYP19A1 and activation of the PRKAA1 kinase pathway [16] in porcine ovary. An in vitro study showed that visfatin increases P4 and E2 secretion as well as steroidogenic acute regulatory protein (STAR) and HSD3B activity by the MAP3/1 kinase pathway in bovine Gc [23], while in hen Gc, an inhibitory effect on P4 secretion STAR and HSD3B but not CYP11A1 expression is observed [24]. Similarly, chemerin also decreases P4 and E2 secretion as well as CYP19A1 expression by the MAP3/1 kinase pathway in bovine Gc [25].
Based on these observations, we hypothesized that vaspin, like other described adipokines, may be an important factor in regulation of signaling pathways and steroid synthesis in porcine ovarian follicles. Thus, the first aim of this study was to investigate the direct effect of vaspin on the phosphorylation of several kinases including MAP3/1, AKT, STAT3, PRKAA1, and PKA as well as protein expression of NFKB2 in porcine ovarian cells. We focused on these pathways because the literature data confirm that other adipokines, by activation of kinase phosphorylation, regulate steroid synthesis, proliferation/apoptosis, and oocyte maturation in ovarian cells [16, 26–28]. The next goal was to examine the dose-dependent effect of vaspin on basal and IGF1-, follicle-stimulating hormone (FSH), and luteinizing hormone (LH)-induced secretion of steroid hormones (P4 and E2), expression of the steroid enzymes CYP11A1, HSD3B, CYP17A1, CYP19A1, and STAR as well gonadotropin (FSHR and LHCGR) and steroid receptors (PGR and ESR2). We also determined the involvement of GRP78 receptor and MAP3/1 and PKA kinases in the steroidogenic effect of vaspin on ovarian cells.
Materials and methods
Ethics statement
Porcine tissues will be obtained post mortem from a local abattoir, as a by-product, so the agreement of the ethical commission is not necessary. Sows will be euthanized in the slaughter according to European Legislation (EFSA, AHAW/04-027).
Reagents
Fetal bovine serum (FBS), TaqMan Gene Expression Cells-to-CT Kit (product no. AM1728), electrophoresis markers, Lipofectamine 3000 (product no. L3000001) and GRP78 siRNA were purchased from ThermoFisher Scientific (Waltham, MA, USA). Phosphate-buffered saline (PBS) was purchased from BioWest (Riverside, MO, USA). Medium M199 (product no. M2154), antibiotic–antimycotic solution, Tris, trypsin, FSH (product no. F4021), LH (product no. L5259), IGF1 (product no. I3769), human vaspin (product no. SRP4915), KT5720 (product no. K3761), Laemmli buffer (product no. 38733), Na-deoxycholate, Nonidet NP-40, sodium dodecyl sulfate (SDS), protease inhibitors (ethylene diamine tetraacetic acid-free), dithiothreitol (DTT), Tween 20, bromophenol blue, and 1 bromo-3-chloro-propane were obtained from Sigma-Aldrich (St. Louis, MO, USA). Pig vaspin was not available at the beginning of experiments; therefore, human vaspin was used (there is 90% homology of vaspin nucleotides between these species; sequences were analyzed against the NCBI, USA database using the BLAST program). PD98059 (product no. 1213) was obtained from Tocris (Bristol, GB). Bradford protein assay kit, 4–20% gels (product no. 456-1093) and membranes (product no. 1704156) were obtained from Bio-Rad (Hercules, CA, USA).
Sample collection and in vitro culture of ovarian cells
Porcine ovaries were collected from sexually mature Polish Large White pigs (6–8 months old) at days 10–12 of the estrous cycle at a local abattoir under veterinarian control. Ovaries were transported to the laboratory in PBS with antibiotic–antimycotic solution within 30 min of collection, then medium antral ovarian follicles (4–6 mm) were isolated from ovaries after morphological examination [29].
Gc were isolated by the technique described by Stoklosowa et al. [30]. Briefly, Gc were scrubbed from the follicular wall with round-tipped ophthalmologic tweezers and rinsed several times with PBS. After isolation, Gc were exposed to DNAse I (500 U for 1 min), washed three times in M199, collected, and resuspended in M199 supplemented with 10% FBS. In the meantime, the Tc were prepared from the same follicles by placing the theca layers in a drop of saline under the dissecting microscope. Isolated theca interna tissue was then washed with PBS, cleaned, cut with scissors, and exposed to 0.25% trypsin in PBS for 10 min at 37 °C [30]. Isolated cells were separated by decantation, and the procedure was repeated three times. Finally, the cells were centrifuged and resuspended in M199/FBS. The viability of the cells was determined before seeding by the Trypan blue exclusion test, and viability was found to be 90% for Gc and 85% for Tc. For mono-culture experiments, Gc or Tc were inoculated at concentrations of 5 × 104 in 96-well tissue culture plates. Co-culture of Gc and Tc was subsequently prepared according to the technique described by Stoklosowa et al. [30], which compared Gc and Tc cultured singly and in co-culture combination and these authors documented that mixed cultures of Tc and Gc secreted the largest amount of estrogen and androgen vs Tc alone and vs Gc. Moreover, the co-culture model of both cell types in ovarian follicles is better than a monoculture of one cell type because all interactions (structural and functional) between Gc and Tc cells are preserved in vitro. This in vitro model was used in previous studies examining the role of ghrelin or other adipokines in ovarian steroid synthesis in pig [31–33]. After cell isolation, the viability of the cells was determined before seeding, using the Trypan blue exclusion test (92% for Gc and 84% for Tc). For co-culture experiments, Gc and Tc were inoculated at concentrations of 4 × 104 and 1 × 104 cells/well, respectively, in 96-well tissue culture plates; the ratio of the two types of cell was comparable to that observed in vivo (Gc: Tc = 4: 1) [34]. Cells were plated in 96-well culture plates in M199 medium with 10% FBS for 24 h. Next, the medium was changed to 5% FBS. In monoculture, we cannot added substrates for estrogens production because Stoklosowa et al. [30] clearly documented that Tc have the potential to secrete estrogen in the absence of additional androgen substrate. All cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2/95% O2.
Experimental procedure
Experiment 1. Effect of vaspin on phosphorylation of several kinases and on protein expression of NFKB2. Ovarian cells: Gc alone, Tc alone, and Gc plus Tc in coculture were incubated for 1, 5, 15, 30, 45, and 60 min with 1 ng/ml vaspin in M199 supplemented with 5% FBS. The dose of vaspin was chosen based on our preliminary experiments. After incubation, cells were washed in PBS and then boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of MAP3/1, AKT, STAT3, PRKAA1, PKA, and NFKB2 expression. For each type of culture (Gc, Tc or cocultures), ovarian cells were collected from two different animals (four ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 72 and 96, and all experiments were repeated five times. The total number of pigs was 30.
Experiment 2. Dose-dependent effect of vaspin on basal and IGF1-, FSH- and LH-induced steroid secretion, steroid enzyme mRNA and protein expression, and gonadotropin and steroid receptor mRNA expression. Coculture of ovarian cells was incubated for 24 h in M199 supplemented with 5% FBS as a control medium or with (i) vaspin at doses of 0.01, 0.1, 1, and 10 ng/ml alone; (ii) FSH or LH at 100 ng/ml, or IGF1 at 50 ng/ml alone; and (iii) vaspin at dose 1 ng/ml with combination with FSH or LH at 100 ng/ml, or IGF1 at 50 ng/ml. Doses of hormones were chosen based on a preliminary study and the literature [20]. After incubation, the medium was stored at −20 °C for P4 and E2 secretion, while cells were washed in PBS and then boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of STAR, CYP11A1, HSD3B, CYP17A1, and CYP19A1 protein expression or washed in PBS and frozen at −80 °C to determine the mRNA expression of steroid enzymes, STAR and receptors for FSHR, LHR, PGR, and ESR2. For each analysis, ovarian cells were collected from three different animals (six ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 36 and 48, and all experiments were repeated five times. The total number of pigs was 15.
Experiment 3. mRNA and protein expression of GRP78 in ovarian follicles and in vitro effect of vaspin on GRP78 level. To study GRP78 expression, antral ovarian follicles: small (2–4 mm; SFs, n = 6), medium (4–6 mm; MFs, n = 6), and large (8–12 mm; LFs, n = 6) were collected at days 4–6, 10–12, and 16–18 of estrus cycle, respectively [20]. Ovarian follicles were homogenized twice in ice-cold lysis buffer (50 mM TrisHCl [pH 7.5] containing 100 mM NaCl, 0.5% sodium deoxycholate, 0.5% NP-40, 0.5% SDS and protease inhibitors), and the lysates were cleared by centrifugation (15.000 g) at 4 °C for 30 min. Protein content was determined by a protein Bradford assay using bovine serum albumin (BSA) as a standard. All samples were stored at −20 °C for GRP78 protein expression analysis. To analyze GRP78 mRNA expression, ovarian follicles were immediately frozen in liquid nitrogen and then stored at −70 °C. For real-time PCR and western blot analysis, ovarian follicles were collected from six different animals in each stage of the estrous cycle; the total number of follicles was 36. The total number of pigs was 18.
Coculture of ovarian cells was incubated for 24 h in M199 supplemented with 5% FBS as a control medium or with 0.01, 0.1, 1, and 10 ng/ml vaspin. After incubation, cells were washed in PBS and boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of GRP78 protein expression. For each analysis, ovarian cells were collected from two different animals (four ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 24 and 32, and all experiments were repeated five times. The total number of pigs was 10.
Experiment 4. GRP78 receptor silencing. Ovarian cells were incubated for 24 h in M199 without FBS. Then, cells were cultured with GRP78 siRNA at doses 1, 2, and 4 nM diluted in Lipofectamine 3000 at a ratio of 1: 1 for 24 h. Doses of siRNA were chosen based on the literature [33]. After incubation, cells were washed in PBS and boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of GRP78 protein expression. For each analysis, ovarian cells were collected from two different animals (four ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 24 and 32, and all experiments were repeated five times. The total number of pigs was 10.
Experiment 5. Involvement of GRP78 receptor in the steroidogenic effect of vaspin in ovarian cells. Ovarian cells were incubated in M199 without FBS and treated with 2 nM GRP78 siRNA for 24 h, then 1 ng/ml vaspin was added for the next 24 h. After incubation, the medium was stored at −20 °C for steroid secretion, while the cells were washed in PBS and boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of HSD3B and CYP19A1 protein expression. For each analysis, ovarian cells were collected from two different animals (four ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 24 and 32, and all experiments were repeated five times. The total number of pigs was 10.
Experiment 6. Involvement of kinases MAP3/1 and PKA in the steroidogenic effect of vaspin in ovarian cells. After 24 h of incubation, the medium was changed to M199 with 5% FBS and the cells were pretreated for 1 h with the PKA inhibitor KT5720 at 50 ng/ml or the MAP3/1 inhibitor PD098059 at 50 μM. The concentrations of the inhibitors were chosen based on previous data [20]. Then, 1 ng/ml vaspin was added for 24 h. After incubation, the medium was stored at −20 °C for P4 and E2 secretion, while the cells were washed in PBS and boiled in Laemmli buffer for 4 min and finally stored at −20 °C for analysis of HSD3B and CYP19A1 protein expression. For each analysis, ovarian cells were collected from two different animals (four ovaries), and six to eight ovarian follicles were taken from each ovary; the total number of follicles per experiment varied between 24 and 32, and all experiments were repeated five times. The total number of pigs was 10.
RNA isolation and real-time PCR
For GRP78 mRNA expression analysis in ovarian follicles, total RNA was extracted from whole SFs, MFs, and LFs, without follicular fluid using Trizol reagent as per the manufacturer’s procedure (Sigma Aldrich, Saint-Quentin-Fallavier, France) as described previously [28]. Briefly, 1 μg of total RNA was reverse transcribed for 1 h at 37 °C in a final reaction volume of 20 μL, containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 200 μM of each deoxynucleotide triphosphate (Amersham, Piscataway, NJ, USA), 50 pmol of oligo(dT) 15, 5 U of ribonuclease inhibitor, and 15 U of MMLV reverse transcriptase. Afterwards, porcine cDNA was diluted 1:5. Real-time PCR was performed in a 20-μL final volume containing 10 μL of iQ SYBR Green supermix (Bio-Rad), 0.25 μL of each primer (10 μM), 4.5 μL of water, and 5.0 μL of template. The cDNA templates were amplified and detected using the MYIQ Cycler real-time PCR system (Bio-Rad) following the protocol previously described by Rak et al. [16]. The abundance of housekeeping gene PPIA (cyclophilin A) was examined and normalized. The descriptions of the different primers are as follows: GRP78 (forward “5′-ATCGAGTTGGCTTTCCGTGT-3′ and reverse 5′-CCAGTCAGTCAGTCAGCAGG-3′), and PPIA (forward “5′-GCATACAGGTCCTGGCATCT-3′ and reverse 5′-TGTCCACATGCAGCAATGGT-3′). The specificity of the amplified fragment sequence was assessed by Beckman Coulter Genomics. The efficiency was between 1.8 and 2.0.
TaqMan real-time PCR
TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA, USA) were used to quantify mRNA expression of STAR, steroid enzymes, and receptors for steroid and gonadotropin as previously described [16]. Total RNA isolation and cDNA synthesis were performed following the manufacturer’s protocol for the TaqManGene Expression Cells-to-CT Kit; RNA and cDNA quantities were determined using spectrophotometry to obtain the optical density at 260 and 280 nm. Amplifications were performed using the StepOnePlus system (Applied Biosystems, Carlsbad, CA, USA) following the manufacturer’s instructions, and the TaqMan-specific primers: STAR (assay ID: Ss03381250_u1), CYP11A1 (assay ID: Ss03384849_u1), HSD3B (assay ID: Ss03391752_m1), CYP19A1 (assay ID: Ss03384876_u1), FSHR (assay ID: Ss03384581_u1), LHCGR (assay ID: Ss03384991_u1), PGR (assay ID: Ss03374440_m1) or ESR2 (assay ID: Ss03391479_m1), and a TaqMan Gene Expression Master Mix; PCR was performed using a final volume of 20 μL, including 50 ng/reaction of cDNA. Relative gene expression was normalized against the reference gene GAPDH (assay ID: Ss03375629_u1) and converted to relative expression (RQ) using the 2−ΔΔCq method.
GRP78 knockdown
The GRP78 silencing experiment was designed according to the rules described by Park et al. [35]. Ovarian cells were transfected with either GRP78 or control siRNA (2 nM) using Lipofectamine 3000 according to the manufacturer’s instructions. The sequences of siRNA used against GRP78 were #909: CCU UCU CAC CAU UGA UAA UTT (sense), AUU AUC AAU GGU GAG AAG GTT (antisense); #693: GGG AAA GAA GGU UAC UCA UTT (sense), AUG AGU AAC CUU UCC CTT (antisense); and #1570: GCC UCU GAU AAU CAG CCA ATT (sense), UUG GCU GAU UAU CAG AGG CTT (antisense). The sequences of porcine negative control siRNA used were: UUC UCC GAA CGU GUC ACG UTT (sense) and ACG UGA CAC GUU CG AGA ATT (antisense).
Protein extraction and western blot
Western blotting and quantification were performed as previously described [22]. For each sample, 30 μg of protein was reconstituted directly in the appropriate amount of sample buffer and separated in Mini-Protean TGX System Precast Protein Gels, then transferred to Trans-Blot Turbo Mini PVDF Transfer. The membranes were washed and blocked in 0.02 M Tris-buffered saline containing 5% BSA and 0.1% Tween 20, and then incubated overnight at 4 °C with the respective antibody (Table 1). Next, the membranes were washed with Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated for 1 h with a horseradish-peroxidase-conjugated secondary antibody diluted at 1: 1000. An anti-actin antibody was used as the loading control. Signals were detected by chemiluminescence using WesternBright Quantum HRP substrate (product no. K-12043 D20, Advansta Inc., Menlo Park, USA) and visualized using a Chemidoc XRS + System (Bio-Rad, Hercules, USA). All visible bands were quantified using a densitometer and ImageJ software (US National Institutes of Health, Bethesda, MD, USA).
Table 1.
Antibodies used in western blot experiments.
| Antibody | Host species | Dilution | Vendor |
|---|---|---|---|
| pMAP3/1 | Rabbit | 1:1000 | Cell Signaling Technology product no. #9101S |
| MAP3/1 | Rabbit | 1:1000 | Cell Signaling Technology product no. #9102S |
| pAKT | Rabbit | 1:1000 | Cell Signaling Technology product no. #9271S |
| AKT | Rabbit | 1:1000 | Cell Signaling Technology product no. #9272S |
| pSTAT3 | Rabbit | 1:1000 | Cell Signaling Technology product no. #9131S |
| STAT3 | Rabbit | 1:2000 | Cell Signaling Technology product no. #4904S |
| pPRKAA1 | Rabbit | 1:1000 | Cell Signaling Technology product no. #2535 L |
| PRKAA1 | Rabbit | 1:1000 | Cell Signaling Technology product no. #2532 L |
| pPKA | Rabbit | 1:1000 | Abcam product no. ab5815 |
| PKA | Rabbit | 1:500 | Abcam product no. ab187515 |
| NFKB2 | Mouse | 1:200 | Santa Cruz Biotechnology product no. sc-7386 |
| STAR | Rabbit | 1:500 | Abcam product no. ab233427 |
| CYP11A1 | Goat | 1:200 | Santa Cruz Biotechnology product no. sc-18040 |
| HSD3B | Mouse | 1:1000 | Abcam product no. ab75710 |
| CYP17A1 | Goat | 1:200 | Santa Cruz Biotechnology product no. sc-46084 |
| CYP19A1 | Rabbit | 1:200 | ThermoFisher Scientific product no. PA1-21398 |
| GRP78 | Rabbit | 1:700 | ThermoFisher Scientific product no. PA5-19503 |
| ACTIN | Mouse | 1:5000 | Sigma-Aldrich product no. A5316 |
| VINCULIN | Mouse | 1:1000 | Sigma-Aldrich product no. V9264 |
| ANTI-MOUSE | Horse | 1:1000 | Cell Signaling Technology product no. #7076 |
| ANTI-RABBIT | Goat | 1:1000 | Cell Signaling Technology product no. #7074 |
ELISA assay
Commercially available P4 (product no. EIA-1561) and E2 (product no. EIA-2693) ELISA kits were used to quantify steroid secretion in the culture medium. The sensitivity of assays was 0.045 ng/ml for P4 and 9.714 pg/ml for E2. The inter- and intra-experimental coefficients of variation were, respectively, <9.96% and <6.99% for P4 and <9.39% and <6.81% for E2. Samples were run in triplicate within the same assay.
Statistical analysis
Statistical data are presented as the means ± standard error of the mean (SEM) of five independent experiments. Distribution of normality was checked by Shapiro–Wilk test. Statistical analysis was carried out using one-way or two-way ANOVA, followed by Tukey’s test (PRISM software version 5; GraphPad, La Jolla, CA, USA). Statistical significance is indicated by different letters (P < 0.05) or by *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Time-dependent effect of vaspin on signaling pathways in porcine ovarian cells
Vaspin is known to activate various signaling pathways in different cells [9]. Therefore, we investigated the effect of 1 ng/ml vaspin on the phosphorylation of MAP3/1, AKT, STAT3, PRKAA1, and PKA as well as on NFKB2 expression over different exposure periods in monoculture of Gc or Tc alone and in coculture of Gc plus Tc. The dose of vaspin was chosen based on our previous research about the vaspin concentration in porcine serum and follicular fluid, which varies around 1 ng/ml [20]. We observed that vaspin at 1 ng/ml had a significant effect on kinase phosphorylation and NFKB2 protein expression depending on the incubation time and model of ovarian cells culture. As shown in Figure 1, vaspin significantly increased MAP3/1, AKT, STAT3, PRKAA1, and PKA, while decreased NFKB2 protein expression both in coculture model of Gc plus Tc and monoculture of Gc (P < 0.05, Figure 1). In Tc monoculture, we noted that vaspin increased MAP3/1, AKT, PRKAA1, and PKA phosphorylation while decreased NFKB2 protein expression (P < 0.05, Figure 1).
Figure 1.

Time-dependent effect of vaspin on signaling pathways of MAP3/1, AKT, STAT3, PRKAA1, PKA, and expression of NFKB2 in coculture of Gc plus Tc and monoculture of Gc or Tc. Representative blots from five independent experiments are shown (A). The protein levels were shown as the ratio of phosphorylated protein to total protein for kinases, and the protein level in relation to actin for NFKB2 (B). Western blotting experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and vaspin treatments is indicated by different letters (P < 0.05) from all five experiments.
Dose-dependent effect of vaspin on basal and IGF1-, FSH-, and LH-induced P4 secretion and on expression of STAR, CYP11A1, HSD3B, and CYP17A1
We have previously documented that some adipokines like resistin, adiponectin, and apelin regulate steroid production in porcine ovarian cells [16, 27, 36]. Therefore, we investigated the direct action of vaspin on steroid secretion and steroid enzyme expression. Cells were incubated with various concentrations of vaspin (0.01, 0.1, 1, and 10 ng/ml) for 24 h alone or in combination with IGF1 (50 ng/ml), or FSH (100 ng/ml) or LH (100 ng/ml). We observed that vaspin at doses of 0.1 and 1 ng/ml significantly increased P4 secretion to 3.809 and 3.696 ng/ml, respectively, vs 2.506 ng/ml in controls (Figure 2A). Moreover, we noted that vaspin at 0.1 and 1 ng/ml significantly increased HSD3B mRNA expression, 1.5- and 2.4-fold vs the control, respectively (Figure 2B), and increased HSD3B protein expression at 0.1 and 1 ng/ml but decreased it at a dose of 10 ng/ml (Figure 2C). As expected, treatment with either IGF1 or both gonadotropins alone increased P4 and HSD3B expression in porcine ovarian cells (Figure 2E–G). In the presence of IGF1 and gonadotropin, 1 ng/ml vaspin decreased IGF1- (1.809 vs 3.730 ng/ml in IGF1 alone), FSH- (1.766 vs 3.890 ng/ml in FSH alone), and LH-induced P4 secretion (1.835 vs 3.156 ng/ml in LH alone) (Figure 2E). Similar results were observed for the effect on HSD3B. Vaspin decreased significantly IGF1- (1.6 vs 2.8 in IGF1 alone), FSH- (2.0 vs 2.6 in FSH alone), and LH-induced HSD3B mRNA expression (4.6 vs 11.7 in LH alone) (Figure 2F) as well as HSD3B protein expression (Figure 2G). Finally, vaspin in all doses significantly increased protein expression of CYP17A1 (Figure 2D). In the presence of IGF1 and gonadotropin, vaspin decreased IGF1- and LH-induced CYP17A1 protein expression (Figure 2H).
Figure 2.
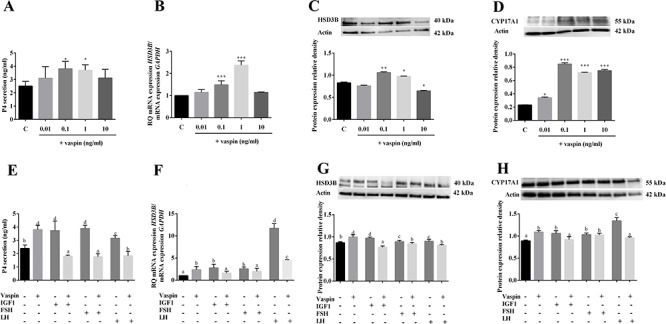
Dose-dependent effect of vaspin added alone (A–D) or in combination with IGF1, or FSH or LH (E–H) on P4 secretion (A, E) and HSD3B mRNA (B, F) and protein (C, G) as well CYP17A1 protein (D, H) expression. ELISA, real-time PCR, and western blotting experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001; or by different letters (P < 0.05), C: control.
To complete the results of vaspin effect on P4 secretion and HSD3B expression, we focused on early step of steroidogenesis by analysis action of vaspin on STAR and CYP11A1 expression (Figure 3). CYP11A1 initiates steroidogenesis by converting cholesterol to pregnenolone on the inner mitochondrial membrane (IMM). Acute steroidogenic responses are regulated by cholesterol delivery from outer mitochondrial membrane to inner, triggered by the STAR. We observed that vaspin significantly increased both STAR and CYP11A1 expression: at 1 ng/ml increased STAR mRNA and at all investigated doses STAR protein expression and CYP11A1 mRNA as well protein level (Figure 3). However, vaspin decreased significantly IGF1-, FSH-, and LH-induced STAR and CYP11A1 mRNA expression as well as CYP11A1 protein expression (Figure 3E, G, H), while only in culture with FSH decreased STAR protein expression (Figure 3F).
Figure 3.
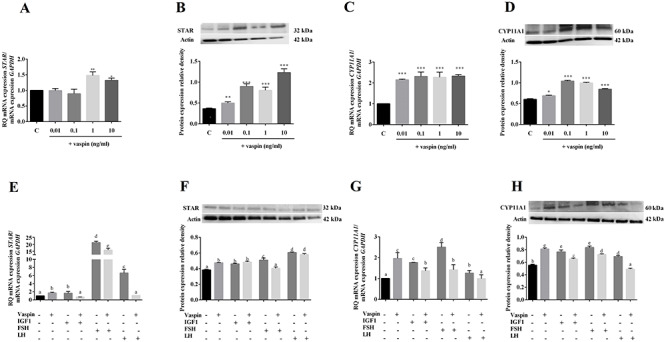
Dose-dependent effect of vaspin added alone (A–D) or in combination with IGF1, or FSH or LH (E–H) on STAR and CYP1A1 mRNA and expression. Real-time PCR experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001; or by different letters (P < 0.05), C: control.
Dose-dependent effect of vaspin on basal and IGF1-, FSH-, and LH-induced E2 secretion and on mRNA and protein expression of CYP19A1
We also investigated whether vaspin could alter E2 secretion and CYP19A1 expression in porcine ovarian cells in basal (Figure 4A–C) or in combination with IGF1, or FSH or LH (Figure 4D–F). We found that vaspin at all doses investigated (0.01, 0.1, 1, and 10 ng/ml) significantly increased E2 secretion to 25.094, 25.224, 23.507, and 23.494 pg/ml, respectively, vs 15.417 pg/ml in the control (Figure 4A). Furthermore, we observed that vaspin at 1 and 10 ng/ml significantly increased CYP19A1 mRNA expression (4.0- and 2.4-fold vs control, respectively), while at the 0.01 ng/ml dose, we observed an inhibitory effect (0.1-fold vs control) (Figure 4B). Western blot analysis showed that protein expression of CYP19A1 was significantly higher for treatments with 0.1 and 1 ng/ml of vaspin, while it decreased at the dose of 0.01 ng/ml vaspin (Figure 4C). As expected, we noted that in ovarian cells treated with IGF1 and both gonadotropins, E2 secretion and CYP19A1 were significantly higher compared to control cells (Figure 4D–F). However, we noted that 1 ng/ml vaspin decreased IGF1- (24.544 vs 28.041 pg/ml), FSH- (23.960 vs 26.951 pg/ml) and LH-induced E2 secretion (22.681 vs 25.654 pg/ml) (Figure 4D) as well as CYP19A1 mRNA expression: 1 ng/ml vaspin with IGF1 (1.3 vs 1.7), FSH (0.7 vs 1.3) and LH (1.2 vs 1.5) (Figure 4E); and CYP19A1 protein expression (Figure 4F).
Figure 4.

Dose-dependent effect of vaspin added alone (A–C) or in combination with IGF1, or FSH or LH (D-F) on E2 secretion (A, D) and CYP19A1 mRNA (B, E), and protein (C, F) expression. ELISA, real-time PCR, and western blotting experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001; or by different letters (P < 0.05), C: control.
Dose-dependent effect of vaspin on gonadotropin and steroidogenic receptor mRNA expression
To confirm the direct role of vaspin on steroid synthesis, we also studied the mRNA expression of both gonadotropin (FSHR and LHCGR) and steroid (PGR and ESR2) receptors (Figure 5). The results of the present study showed that vaspin increases mRNA expression of receptors in a dose-dependent manner: at 1 and 10 ng/ml for FSHR (5.1- and 3.8-fold vs control, respectively), at 0.01 and 0.1 ng/ml for LHCGR (1.2- and 1.2-fold vs control, respectively), at 0.01, 0.1, 1 and 10 ng/ml for PGR (1.7-, 6.0-, 5.2- and 1.7-fold vs control, respectively), and at 0.1 ng/ml for ESR2 mRNA expression (1.3-fold vs control, respectively). However, we observed that vaspin at the 10 ng/ml dose decreased ESR2 mRNA expression (0.7-fold vs control, respectively) (Figure 5).
Figure 5.

Dose-dependent effect of vaspin on mRNA expression levels of FSHR (A), LHCGR (B), PGR (C), and ESR2 (D). Real-time PCR experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by *P < 0.05, **P < 0.01, and ***P < 0.001; C: control.
Expression of GRP78 in the ovarian follicles and dose-dependent effect of vaspin on GRP78 receptor expression and GRP78 receptor silencing
To investigate whether the effect of vaspin on steroidogenesis depends on GRP78 receptor, first we studied level of GRP78 in follicles at different stages of estrous cycle, next, the direct effect of vaspin on GRP78 protein expression in ovarian cells and then we used siRNA technology to specifically knock down the expression of GRP78 in porcine ovarian cells. We observed that both mRNA and protein level of GRP78 does not change in ovarian follicles during estrous cycle. As observed in Figure 6C, vaspin at all doses investigated (0.01, 0.1, 1 and 10 ng/ml) significantly decreased GRP78 protein expression after 24 h of incubation. To validate siRNA silences method, we noted that 2 and 4 nM of GRP78 siRNA significantly decreased GRP78 expression, 0.8- and 0.7-fold vs control, respectively (Figure 6D). Additionally, we observed no difference between cells treated with negative control siRNA and untreated cells (data not shown).
Figure 6.
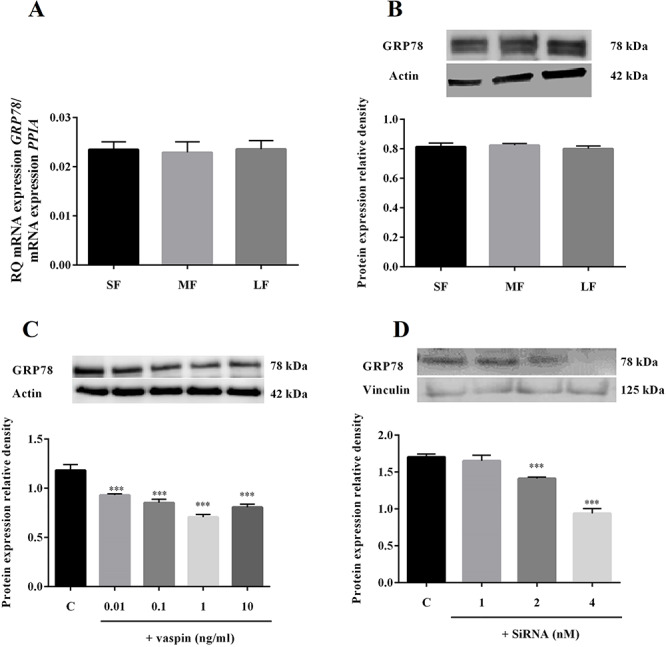
mRNA (A) and protein (B) expression of GRP78 in ovarian follicles: small (SF), medium (MF), and large (LF) and in vitro effect of vaspin on GRP78 level (C) as well GRP78 receptor silencing (D). Real-time PCR experiments were performed independently and repeated five times (n = 5). Representative blots from five independent experiments are shown. Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by ***P < 0.001; C: control.
Involvement of GRP78 receptor and kinases MAP3/1 and PKA in the steroidogenic effect of vaspin in ovarian cells
We next investigated the role of GRP78 receptor and kinases MAP3/1 and PKA in the steroidogenic properties of vaspin in ovarian cells. Secretion of P4 and E2 as well as protein expression of HSD3B and CYP19A1 were studied after 24 h of cell incubation with GRP78 siRNA (2 nM) or the pharmacological inhibitors KT5720 (50 ng/ml) or PD098059 (50 μM), which are known to block the PKA and MAP3/1 signaling pathways, respectively. As shown in Figure 7, we observed that siRNA and KT5720 added with vaspin reduced secretion of steroids P4 (2.668 and 2.739 ng/ml, respectively) and E2 (15.216 and 15.856 pg/ml, respectively) and protein expression of both steroidogenic enzymes to the control level. Thus, these data suggest that both GRP78 and PKA are involved in vaspin-induced steroid synthesis in porcine ovarian follicles. Additionally, we noted that in ovarian cell culture treated with PD98059 and vaspin, steroid secretion of P4 and E2 as well as protein expression of HSD3B and CYP19A1 were significantly higher than control levels, similar to those in culture with vaspin alone. We also observed that both siRNA and inhibitors KT5720 and PD98059 added alone had no effect on steroid secretion or steroid enzyme expression (Figure 7A–D).
Figure 7.

Involvement of GRP78 receptor and kinases MAP3/1 and PKA in vaspin action on P4 (A) and E2 (C) secretion as well as HSD3B (B) and CYP19A1 (D) protein expression. Representative blots from five independent experiments are shown. ELISA and Western blotting experiments were performed independently and repeated five times (n = 5). Data are plotted as the mean ± SEM. Significance between control and treatments is indicated by ***P < 0.001; or by different letters (P < 0.05), C: control.
Discussion
In the present study, we report for the first time the role of vaspin in the ovary in phosphorylation of various signaling kinase pathways and its steroidogenic function in porcine ovarian follicle cells. Vaspin at 1 ng/ml significantly increased phosphorylation of kinases MAP3/1, AKT, STAT3, PRKAA1, and PKA in a time-dependent manner, while it decreased protein expression of NFKB2 in coculture model of Gc plus Tc and monoculture of Gc or Tc, except STAT3 activation in Tc. The dose of vaspin (1 ng/ml) was chosen based on our previous paper, where we indicated that the vaspin level in the follicular fluid and blood serum of Large White pigs varies around 1 ng/ml [20]. Furthermore, vaspin increases P4 and E2 secretion by stimulating steroid enzyme expression and STAR protein in a dose-dependent manner in the basal state, while it decreases in response to IGF1 or gonadotropins in coculture model of ovarian cells. Additionally, the results of our study document that vaspin also stimulates gonadotropin (FSHR and LHCGR) and steroid (PGR and ESR2) receptor gene expression. The stimulatory effect of vaspin on ovarian steroidogenesis is reversed by addition of a PKA pharmacological inhibitor (KT5720) or by silencing GRP78 receptor.
The results of our data clearly document that vaspin, by activating phosphorylation of various kinases, could be an important hormone-regulating ovarian physiological functions like other adipokines including resistin [27], apelin [16, 37], RARRES2 [28], and NAMPT [23]. To our knowledge, phosphorylation of kinases is necessary for proper ovarian physiology including steroid hormone secretion, proliferation, oocyte maturation, angiogenesis, cell migration, and apoptosis [15, 16, 38]. In epithelial ovarian cancer cells, gonadotropins induce both ovarian cancer cell migration and proliferation by activating the MAP3/1 signaling pathway [15]. Moreover, FSH regulates rats’ Gc differentiation by activating PKA [38]. In porcine ovarian follicles, phosphorylation of PRKAA1 is involved in the action of apelin in P4 production, while MAP3/1 and AKT mediate the proliferative effect of this adipokine [16]. Modulation of MAP3/1 and AKT kinase phosphorylation by ghrelin is the pathway for bovine oocyte maturation [39]. Also, in bovine Gc cells, interleukin 6 (IL-6) can promote FSH-induced vascular endothelial growth factor (VEGF) expression by JAK/Stat-3 activation (JAK) [40]. Interesting, in our results, we observed that both type of ovarian cells Gc or Tc, similar as in coculture, response to vaspin on signaling molecules, expect STAT3 activation in Tc. It is a well-known fact that signaling pathways participate in cross-talks, which should be considered here. We can suggest that no effect on STAT3 activation may result that some other kinases can regulate the activity and subcellular localization of STAT3 in Tc or increased Gc remains detectable in coculture in the absence of a change in Tc. Stimulatory effect of vaspin on kinases phosphorylation in coculture can be the result of the interaction of both types of cells e.g. pMAPK3/1 activation or only one type of ovarian cells e.g. pSTAT3.
The results in the present study document that vaspin decreases significantly protein expression of NFKB2 in ovarian follicle cells both in coculture model and monoculture of Gc or Tc, regardless of the cell models used. It is well known that NFKB2 is involved in ovarian cell physiology. For example, NFKB2 regulates the mechanism of luteal angiogenesis during formation of the corpus luteum in mammals [41], ovarian cell apoptosis [42] and steroid production [43], and inflammation [44] in Gc. Inhibition of NFKB2 in rat luteal cells decreases endothelial growth factor, which is an important factor connected to regulation of angiogenesis in the ovary [41] and which also has a negative effect on mouse Gc steroidogenesis induced by Di-n-butyl phthalate, one of the most common phthalate esters [43]. Furthermore, ovulation is a critical inflammation-like event that is central to ovarian physiology, which acts by activation of the NFKB2 signaling pathway [44]. The present results show that vaspin decreases NFKB2 expression significantly in ovarian cells, which suggests that this adipokine could be an anti-inflammatory factor in ovarian physiology. Additionally, in rat chondrocytes, vaspin prevents leptin-induced inflammation by inhibiting the activation of NFKB2 [45], and in cultured rat vascular smooth muscle cells, it also inhibits the phosphorylation of NFKB2 induced by tumor necrosis factor alpha [46].
In our experiments, we stimulated porcine ovarian cells with vaspin at different doses in the range 0.01–10 ng/ml, concentrations close to those observed in porcine follicular fluid and plasma [20]. The DNA sequence of porcine vaspin is highly homologous to those of humans, mice and rats, suggesting also a greater sequence identity for the amino acid sequences. Thus, we used recombinant human vaspin to determine effect of vaspin, e.g. steroidogenesis, in porcine ovarian cells. During steroid synthesis, cholesterol is first transported into the mitochondrial inner membrane, facilitated by STAR, and is then converted to the important P4 under action of the mitochondrial enzyme CYP11A1 and endoplasmic reticulum enzyme HSD3B. P4 is further enzymatically processed into estrogen through activation of different enzymes including CYP19A1. In our study, we showed that vaspin increases basal secretion of steroids P4 and E2 by stimulating STAR protein as well steroid enzyme expression of CYP11A1, HSD3B, CYP17A1, and CYP19A1. The stimulatory effect of vaspin on steroid production is dose dependent: at doses 1 and 10 ng/ml, vaspin increased STAR mRNA expression, while at all investigated doses, STAR protein expression as well as CYP11A1 expression were increased. Moreover, vaspin at 0.1 and 1 ng/ml significantly increased P4 secretion and gene expression of HSD3B, while at 10 ng/ml, only protein enzyme expression decreased. Our previous study documented that the vaspin concentration in plasma and follicular fluid is around 1 ng/ml in lean Large White pigs, while it increases to around 5 ng/ml in fat Meishan pigs [20]. The physiological level of vaspin in women’s serum varies around 1 ng/ml [47]. The results of our data show that vaspin significantly increased E2 secretion at all doses used, by stimulating CYP19A1 mRNA and protein expression; however, we observed that vaspin at 0.01 ng/ml decreased mRNA and protein expression of CYP19A1. One hypothesis to explain these results is that vaspin could modulate the activity of steroid enzymes. However, this needs to be investigated. Literature data document differences between enzyme activity of steroids and their expression; for example, in rat testis, ciprofibrate decreases HSD3B activity without affecting either HSD3B protein or mRNA expression [48]. In the present results, we observed opposite effects of vaspin on mRNA and protein levels of steroidogenic enzymes, suggesting translational regulations of these enzymes. We report for the first time that in porcine ovarian cells, vaspin has a stimulatory effect on gonadotropin (FSHR and LHCGR) and steroid (PGR and ESR2) hormone receptors, confirming role of vaspin in ovarian steroidogenesis as well as steroid hormone action on ovarian cells. Gonadotropins and steroids like P4 and E2 can precisely regulate female fertility depending on the development of ovarian follicles and final ovulation [49, 50]. The interaction between gonadotropins and the receptors FSHR or LHCGR activates multiple signaling pathways, leading to steroidogenesis that modulates the differentiation and proliferation of ovarian cells. It is also well known that other adipokines have an impact on steroidogenesis in different species; for example, leptin decreases E2 secretion and increases P4 secretion in preovulatory porcine ovarian follicles [26], while apelin increases basal steroid secretion [16]. Moreover, in bovine Gc, visfatin increases the release of P4 and E2 and this is associated with stimulation of the protein level of STAR and HSD3B [23].
Our previous study reported that vaspin expression in porcine ovarian cells is higher in cells treated with gonadotropins and IGF1, thus in the present study, we investigated the interaction of vaspin with these hormones in steroid secretion. Moreover, we chose these hormones because they play important role in the initiation and maintenance of follicular growth, as well as in the selection of the dominant follicle and its maturation to preovulatory status [51]. The developing responsiveness of follicles to stimulation by gonadotropins may result from changes in the production and alterations in follicular sensitivity to intraovarian paracrine and/or autocrine factors, such as IGF1 and steroid hormones. In mammals, IGF1 is well known to play a key role on the development of antral follicles and steroid synthesis [52]. As we expected, we noted that in ovarian cells treated with IGF1 and both gonadotropins, steroid hormone secretion and enzyme expression were significantly higher [27]. However, the results of our study document that vaspin reverses the stimulatory effect of IGF1, FSH and LH on secretion of both P4 and E2 as well as on STAR, CYP11A1, HSD3B, CYP17A1, and CYP19A1 expression. These results are in agreement with other previous studies that showed that resistin and apelin decrease IGF1- and FSH-induced steroid secretion and HSD3B and CYP19A1 expression in porcine ovarian cells [16, 27]. Similarly, in human Gc, chemerin inhibits IGF1-induced production of both P4 and E2 [25], while adiponectin in bovine Gc decreases insulin-induced steroid secretion [36]. Further investigations are required to understand molecular mechanisms involved in vaspin interaction with different hormones such as gonadotropin or IGF1 on steroid synthesis in the ovarian cells.
In the next experiments, we analyzed the participation of GRP78 and kinases MAP3/1 and PKA in the steroidogenic effect of vaspin in ovarian cells. Natsuka et al. [7, 53] described that vaspin is a ligand for cell-surface GRP78; it prevents metabolic dysfunctions caused by endoplasmic reticulum stress, promotes proliferation, and inhibits apoptosis in rat endothelial cells. However, GRP78 plays an important role in the female reproduction system, such as in embryo implantation [54]. In the present study, we showed that GRP78 expression does not change in the ovarian follicles at different stages of the estrous cycle. Expression of GRP78 is lower in the endometrium of women with PCOS compared to controls [55], while in the placenta of women with preeclampsia, it is higher [56]. Our data show that vaspin significantly decreases GRP78 protein expression in a dose-dependent manner. We can explain the observed effect by increasing doses of vaspin, which we used in this experiment, leading to downregulation of the GRP78 receptor in the cell membrane, hence we observed lower protein expression of GRP78 in ovarian follicle cells. It is well know that downregulation of receptors can occur when receptors have been chronically exposed to an excessive amount of a ligand, resulting from ligand-induced desensitization or internalization of that receptor. For example, elevated serum levels of insulin led to downregulation of the associated receptors, when insulin binds to its receptors on the surface of a cell, the hormone receptor complex undergoes endocytosis and is subsequently attacked by intracellular lysosomal enzymes [57]. The internalization of the insulin molecules provides a pathway for degradation of the hormone as well as for regulation of the number of sites that are available for binding on the cell surface [58]. At high plasma concentrations, the number of surface receptors for insulin is gradually reduced by the accelerated rate of receptor internalization and degradation brought about by increased hormonal [58]. Additionally, our results are in agreement with previous studies that reported that higher doses of leptin in serum decrease mRNA expression of leptin receptor (LEPR) in mouse liver [59], or glucocorticoids leading to progressive diminution in the number of cellular glucocorticoid receptors in mouse pituitary tumor cells [60]. Furthermore, E2 treatment dose dependently decreases the gene expression of its own receptor ER, especially its subtype ER-α mRNA, in the mammary gland [61]. Moreover, some studies reported that steroids T and E2 decrease GRP78 receptor expression in endometrial cells [55, 62].
In our study, the stimulatory effect of vaspin on steroid hormone levels and protein expression of enzymes HSD3B and CYP19A1 was negated, as they returned to control levels in culture, when we studied GRP78 gene knockdown using siRNA or when we used the pharmacological inhibitor of kinase PKA (KT5720), not MAP3/1 (PD98059). These results suggest that vaspin stimulates porcine ovarian cell steroidogenesis through GRP78 receptor and activation of PKA. Some studies confirm our result, documenting that GRP78 positively regulates estrogen receptor in gastric tumor cells [63], while estrogen induces GRP78 expression in endometrial cancer [64]. Also, steroid production in the placenta BeWo cell line [65] or in the bovine corpus luteum [66] is mediated by activation of PKA. Moreover, FSH enhances the development of medium-sized follicles in goats via increased the production of IGF1 and steroids, possibly through the PKA pathway [67]. Interestingly, in our study, we observed that the kinase MAP3/1 is not involved in vaspin’s action in steroid production. Similarly, in human ovarian cells, insulin induces P4 secretion without activation of the MAP3/1 kinase pathway [68]. However, the opposite effect was observed by Roche et al. [37] and Maillard et al. [36], who showed that other adipokines: apelin and adiponectin increase steroidogenesis by the MAP3/1 pathway in human and bovine ovarian cells, so, from the difference in MAP3/1 activation, we can explain the various adipokines used or species-specific action. Our last paper [69] documented that vaspin have mitogenic effect in porcine Gc via activation of GRP78 receptor and kinases MAP3/1/ERK1/2, STAT3, and AKT, indicating that vaspin as a new regulator of ovarian growth, development, or folliculogenesis.
Presented novel data focused of understanding molecular mechanism of vaspin on activation of various signaling pathways as well ovarian steroid secretion in in vitro experiments of ovarian cells. To our knowledge, the lack data documented role of vaspin in female fertility in in vivo experiments. Further studies on vaspin-null transgenic animals are necessary to better understand the role of vaspin on female reproduction infertility like in the case of other adipokines including adiponectin or resistin [70]. Indeed, overexpression of adiponectin leads to increased insulin sensitivity and infertility or subfertility [71] and a recent study demonstrated that disruption of adiponectin can cause subfertility in female mice [72]. Furthermore, female adiponectin-null mice displayed reduced retrieval of oocytes, a disrupted estrous cycle, an elevated number of atretic follicles, and impaired late folliculogenesis. Also, their serum has lower levels of P4 at diestrus that can be explained by a lower expression of CYP11A1 and reduction in E2 and FSH at proestrus [72]. Recent published studies clearly documented that vaspin could be a potential novel biomarker for the prediction and early diagnosis of reproductive pathology like PCOS [21] and pregnancy [73, 74].
In conclusion, we have demonstrated for the first time a role of vaspin in porcine ovarian function by investigating activation of signaling pathways and steroid production. We observed that vaspin, in a time-dependent manner, stimulates phosphorylation of MAP3/1, AKT, STAT3, PRKAA1, and PKA kinase pathways, while it inhibits NFKB2 protein expression. Furthermore, on our coculture model, we noted a stimulatory, dose-dependent effect of vaspin on basal steroid hormone secretion, steroidogenic enzyme expression, and gonadotropin/steroid receptor expression. However, we observed that vaspin reverses the stimulatory effect on steroidogenesis in ovarian cell culture induced by IGF1 and gonadotropins. The stimulatory effect of vaspin on steroidogenesis was reversed when we used GRP78 siRNA and a pharmacological inhibitor of PKA. Thus, vaspin, by activating various signaling pathways and stimulating basal steroid production via GRP78 receptor and PKA, could be a new regulator of porcine ovarian function (Figure 8).
Figure 8.

Proposed intracellular mechanism of GRP78 and protein kinase A (PKA) signaling pathway activation by vaspin on basal and IGF-1 and gonadotropin-induced steroid synthesis in ovarian cells.
Acknowledgements
This paper was prepared with Polish–French cooperation and an international project between supervisors Agnieszka Rak and Joelle Dupont. The authors want to thank Alix Barbe and Christelle Rame from the French laboratory for their technical support including ovarian cell culture and gene silencing, and Monika Sroka from the Polish laboratory for her preparation of ELISA tests. The present publication contains parts of Patrycja Kurowska’s PhD dissertation.
Conflict of interest
The authors declare no conflict of interest regarding the publication of this article.
Conference Presentation: Presented in part at the 52nd Annual Meeting of Society for the Study of Reproduction, 18–21 July, San Jose, California.
Footnotes
† Grant Support: National Science Center, Poland (HARMONIA grant no. 2016/22/M/NZ9/00316).
References
- 1. Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA 2005; 102:10610–10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Q, Chen R, Moriya J, Yamakawa J, Sumino H, Kanda T, Takahashi T. A novel adipocytokine, visceral adipose tissue-derived serine protease inhibitor (vaspin), and obesity. J Int Med Res 2008; 36:625–629. [DOI] [PubMed] [Google Scholar]
- 3. Klöting N, Kovacs P, Kern M, Heiker JT, Fasshauer M, Schön MR, Stumvoll M, Beck-Sickinger AG, Blüher M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011; 54:1819–1823. [DOI] [PubMed] [Google Scholar]
- 4. Lai X, Zhang C, Wang J, Wang C, Lan X, Zhang C, Lei C, Chen H. mRNA expression pattern and association study with growth traits of bovine vaspin gene. Mol Biol Rep 2013; 40:4499–4505. [DOI] [PubMed] [Google Scholar]
- 5. Youn BS, Klöting N, Kratzsch J, Lee N, Park JW, Song ES, Ruschke K, Oberbach A, Fasshauer M, Stumvoll M, Blüher M. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes 2008; 57:372–377. [DOI] [PubMed] [Google Scholar]
- 6. Feng R, Li Y, Wang C, Luo C, Liu L, Chuo F, Li Q, Sun C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract 2014; 106:88–94. [DOI] [PubMed] [Google Scholar]
- 7. Nakatsuka A, Wada J, Iseda I, Teshigawara S, Higashio K, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Hida K, Eguchi J et al. Visceral adipose tissue-derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell-surface GRP78/voltage-dependent anion channel complex. Circ Res 2013; 112:771–780. [DOI] [PubMed] [Google Scholar]
- 8. Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 2009; 11:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu X, Jiang Y, Shan PF, Shen J, Liang QH, Cui RR, Liu Y, Liu GY, Wu SS, Lu Q, Xie H, Liu YS et al. Vaspin attenuates the apoptosis of human osteoblasts through ERK signaling pathway. Amino Acids 2013; 44:961–968. [DOI] [PubMed] [Google Scholar]
- 10. Liu S, Li X, Wu Y, Duan R, Zhang J, Du F, Zhang Q, Li Y, Li N. Effects of vaspin on pancreatic β cell secretion via PI3K/AKT and NF-κB signaling pathways. PLoS One 2017; 12:e0189722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jung CH, Lee WJ, Hwang JY, Lee MJ, Seol SM, Kim YM, Lee YL, Kim HS, Kim MS, Park JY. Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. PLoS One 2012; 7:e52346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung CH, Lee MJ, Kang YM, Lee YL, Yoon HK, Kang SW, Lee WJ, Park JY. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Diabetol 2014; 13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zieger K, Weiner J, Krause K, Schwarz M, Kohn M, Stumvoll M, Blüher M, Heiker JT. Vaspin suppresses cytokine-induced inflammation in 3T3-L1 adipocytes via inhibition of NFKB2 pathway. Mol Cell Endocrinol 2018; 460:181–188. [DOI] [PubMed] [Google Scholar]
- 14. Makarevich A, Sirotkin A, Chrenek P, Bulla J, Hetenyi L. The role of IGF-I, cAMP/protein kinase A and MAP-kinase in the control of steroid secretion, cyclic nucleotide production, granulosa cell proliferation and preimplantation embryo development in rabbits. J Steroid Biochem Mol Biol 2000; 73:123–133. [DOI] [PubMed] [Google Scholar]
- 15. Mertens-Walker I, Bolitho C, Baxter RC, Marsh DJ. Gonadotropin-induced ovarian cancer cell migration and proliferation require extracellular signal-regulated kinase 1/2 activation regulated by calcium and protein kinase C{delta}. Endocr Relat Cancer 2010; 17:335–349. [DOI] [PubMed] [Google Scholar]
- 16. Rak A, Drwal E, Rame C, Knapczyk-Stwora K, Słomczyńska M, Dupont J, Gregoraszczuk EL. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017; 96:126–135. [DOI] [PubMed] [Google Scholar]
- 17. Yuan J, Zhang F, Niu R. Multiple regulation pathways and pivotal biological functions of STAT3 in cancer. Sci Rep 2015; 5:17663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romorini L, Garate X, Neiman G, Luzzani C, Furmento VA, Guberman AS, Sevlever GE, Scassa ME, Miriuka SG. AKT/GSK3β signaling pathway is critically involved in human pluripotent stem cell survival. Sci Rep 2016; 6:35660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caminos JE, Bravo SB, Garcés MF, González CR, Cepeda LA, González AC, Nogueiras R, Gallego R, García-Caballero T, Cordido F, López M, Diéguez C. Vaspin and amylin are expressed in human and rat placenta and regulated by nutritional status. Histol Histopathol 2009; 24:979–990. [DOI] [PubMed] [Google Scholar]
- 20. Kurowska P, Mlyczyńska E, Barbe A, Staub C, Gregoraszczuk E, Dupont J, Rak A. Vaspin in the pig ovarian follicles: expression and regulation by different hormones. Reproduction 2019; 158:137–148. [DOI] [PubMed] [Google Scholar]
- 21. Tan BK, Heutling D, Chen J, Farhatullah S, Adya R, Keay SD, Kennedy CR, Lehnert H, Randeva HS. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008; 57:1501–1507. [DOI] [PubMed] [Google Scholar]
- 22. Rak-Mardyła A, Durak M, Gregoraszczuk EL. Effects of resistin on porcine ovarian follicle steroidogenesis in prepubertal animals: an in vitro study. Reprod Biol Endocrinol 2013; 11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reverchon M, Rame C, Bunel A, Chen W, Froment P, Dupont J. VISFATIN (NAMPT) improves in vitro IGF1-induced steroidogenesis and IGF1 receptor signaling through SIRT1 in bovine granulosa cells. Biol Reprod 2016; 94:54. [DOI] [PubMed] [Google Scholar]
- 24. Diot M, Reverchon M, Ramé C, Baumard Y, Dupont J. Expression and effect of NAMPT (visfatin) on progesterone secretion in hen granulosa cells. Reproduction 2015; 150:53–63. [DOI] [PubMed] [Google Scholar]
- 25. Reverchon M, Cornuau M, Ramé C, Guerif F, Royère D, Dupont J. Chemerin inhibits IGF1-induced progesterone and estradiol secretion in human granulosa cells. Hum Reprod 2012; 27:1790–1800. [DOI] [PubMed] [Google Scholar]
- 26. Gregoraszczuk EL, Rak A, Wójtowicz A, Ptak A, Wojciechowicz T, Nowak KW. Gh and IGF-I increase leptin receptor expression in prepubertal pig ovaries: the role of leptin in steroid secretion and cell apoptosis. Acta Vet Hung 2006; 54:413–426. [DOI] [PubMed] [Google Scholar]
- 27. Rak A, Drwal E, Karpeta A, Gregoraszczuk EL. Regulatory role of gonadotropins and local factors produced by ovarian follicles on in vitro resistin expression and action on porcine follicular steroidogenesis. Biol Reprod 2015; 92:142. [DOI] [PubMed] [Google Scholar]
- 28. Reverchon M, Bertoldo MJ, Ramé C, Froment P, Dupont J. CHEMERIN (RARRES2) decreases in vitro granulosa cell steroidogenesis and blocks oocyte meiotic progression in bovine species. Biol Reprod 2014; 90:102. [DOI] [PubMed] [Google Scholar]
- 29. Akins EL, Morrissette MC. Gross ovarian changes during estrous cycle of swine. Am J Vet Res 1968; 29:1953–1957. [PubMed] [Google Scholar]
- 30. Stoklosowa S, Gregoraszczuk E, Channing CP. Estrogen and progesterone secretion by isolated cultured porcine thecal and granulosa cells. Biol Reprod 1982; 26:943–952. [DOI] [PubMed] [Google Scholar]
- 31. Rak-Mardyla A, Gregoraszczuk EL. ERK 1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin action on cell proliferation and apoptosis in the porcine ovarian follicular cells. J Physiol Pharmacol 2010; 61:451–458. [PubMed] [Google Scholar]
- 32. Rak A, Gregoraszczuk EL. Modulatory effect of ghrelin in prepubertal porcine ovarian follicles. J Physiol Pharmacol 2008; 59:781–793. [PubMed] [Google Scholar]
- 33. Rak A, Drwal E, Wróbel A, Gregoraszczuk EŁ. Resistin is a survival factor for porcine ovarian follicular cells. Reproduction 2015; 150:343–355. [DOI] [PubMed] [Google Scholar]
- 34. Stoklosowa S. Tissue culture of gonadal cells. Acta Biol Acad Sci Hung 1982; 33:367–379. [PubMed] [Google Scholar]
- 35. Park KW, Eun Kim G, Morales R, Moda F, Moreno-Gonzalez I, Concha-Marambio L, Lee AS, Hetz C, Soto C. The endoplasmic reticulum chaperone GRP78/BiP modulates prion propagation in vitro and in vivo. Sci Rep 2017; 7:44723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maillard V, Uzbekova S, Guignot F, Perreau C, Ramé C, Coyral-Castel S, Dupont J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod Biol Endocrinol 2010; 8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roche J, Ramé C, Reverchon M, Mellouk N, Cornuau M, Guerif F, Froment P, Dupont J. Apelin (APLN) and apelin receptor (APLNR) in human ovary: expression, signaling, and regulation of steroidogenesis in primary human luteinized granulosa cells. Biol Reprod 2016; 95:104. [DOI] [PubMed] [Google Scholar]
- 38. Puri P, Little-Ihrig L, Chandran U, Law NC, Hunzicker-Dunn M, Zeleznik AJ. Protein kinase A: a master kinase of granulosa cell differentiation. Sci Rep 2016; 6:28132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chouzouris TM, Dovolou E, Krania F, Pappas IS, Dafopoulos K, Messinis IE, Anifandis G, Amiridis GS. Effects of ghrelin on activation of AKT1 and ERK1/2 pathways during in vitro maturation of bovine oocytes. Zygote 2017; 25:183–189. [DOI] [PubMed] [Google Scholar]
- 40. Yang M, Wang L, Wang X, Wang X, Yang Z, Li J. IL-6 promotes FSH-induced VEGF expression through JAK/STAT3 signaling pathway in bovine granulosa cells. Cell Physiol Biochem 2017; 44:293–302. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Z, Huang Y, Zhang J, Liu Z, Lin Q, Wang Z. Activation of NF-κB signaling pathway during HCG-induced VEGF expression in luteal cells. Cell Biol Int 2019; 43:344–349. [DOI] [PubMed] [Google Scholar]
- 42. Peng H, Zhao XH, Bi TT, Yuan XY, Guo JB, Peng S-Q. PM (2.5) obtained from urban areas in Beijing induces apoptosis by activating nuclear factor-kappa B. Mil Med Res 2017; 4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang C, Gong P, Ye Y, Zhang L, Chen M, Hu Y, Gu A, Chen S, Wang Y. NF-κB-vimentin is involved in steroidogenesis stimulated by mono-butyl phthalate in primary cultured ovarian granulosa cells. Toxicol In Vitro 2017; 45:25–30. [DOI] [PubMed] [Google Scholar]
- 44. Ou HL, Sun D, Peng YC, Wu YL. Novel effects of the cyclooxygenase-2-selective inhibitor NS-398 on IL-1β-induced cyclooxygenase-2 and IL-8 expression in human ovarian granulosa cells. Innate Immun 2016; 22:452–465. [DOI] [PubMed] [Google Scholar]
- 45. Bao JP, Xu LH, Ran JS, Xiong Y, Wu LD. Vaspin prevents leptin induced inflammation and catabolism by inhibiting the activation of nuclear factor κB in rat chondrocytes. Mol Med Rep 2017; 16:2925–2930. [DOI] [PubMed] [Google Scholar]
- 46. Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF-α-induced intracellular adhesion molecule-1 via inhibiting reactive oxygen species-dependent NF-κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol Res 2011; 64:493–500. [DOI] [PubMed] [Google Scholar]
- 47. Auguet T, Quintero Y, Riesco D, Morancho B, Terra X, Crescenti A, Broch M, Aguilar C, Olona M, Porras JA, Hernandez M, Sabench F et al. New adipokines vaspin and omentin. Circulating levels and gene expression in adipose tissue from morbidly obese women. BMC Med Genet 2011; 12:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hierlihy AM, Cooke GM, Curran IH, Mehta R, Karamanos L, Price CA. Effects of ciprofibrate on testicular and adrenal steroidogenic enzymes in the rat. Reprod Toxicol 2006; 22:37–43. [DOI] [PubMed] [Google Scholar]
- 49. Field SL, Dasgupta T, Cummings M, Orsi NM. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol Reprod Dev 2014; 81:284–314. [DOI] [PubMed] [Google Scholar]
- 50. Xu H, Deng K, Luo Q, Chen J, Zhang X, Wang X, Diao H, Zhang C. High serum FSH is associated with brown oocyte formation and a lower pregnancy rate in human IVF practice. Cell Physiol Biochem 2016; 39:677–684. [DOI] [PubMed] [Google Scholar]
- 51. Yoshimura Y, Koyama N, Karube M, Oda T, Akiba M, Yoshinaga A, Shiokawa S, Jinno M, Nakamura Y. Gonadotropin stimulates ovarian renin-angiotensin system in the rabbit. J Clin Invest 1994; 93:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silva JR, Figueiredo JR, Hurk R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009; 71:1193–1208. [DOI] [PubMed] [Google Scholar]
- 53. Nakatsuka A, Wada J, Iseda I, Teshigawara S, Higashio K, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Hida K, Eguchi J et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012; 61:2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lin P, Jin Y, Lan X, Yang Y, Chen F, Wang N, Li X, Sun Y, Wang A. GRP78 expression and regulation in the mouse uterus during embryo implantation. J Mol Histol 2014; 45:259–268. [DOI] [PubMed] [Google Scholar]
- 55. Rosas C, Oróstica L, Poblete C, Carvajal R, Gabler F, Romero C, Lavandero S, Vega M. Hyperandrogenism decreases GRP78 protein level and glucose uptake in human endometrial stromal cells. Reprod Sci 2016; 23:761–770. [DOI] [PubMed] [Google Scholar]
- 56. Fu J, Zhao L, Wang L, Zhu X. Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan J Obstet Gynecol 2015; 54:19–23. [DOI] [PubMed] [Google Scholar]
- 57. Zaliauskiene L, Kang S, Brouillette CG, Lebowitz J, Arani RB, Collawn JF. Down-regulation of cell surface receptors is modulated by polar residues within the transmembrane domain. Mol Biol Cell. 2000; 11:2643–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Carpentier J-L. Insulin receptor internalization: molecular mechanisms and physiopathological implications. Diabetologia 1994; 37:117–124. [DOI] [PubMed] [Google Scholar]
- 59. Hegyi K, Fülöp KA, Kovács KJ, Falus A, Tóth S. High leptin level is accompanied with decreased long leptin receptor transcript in histamine deficient transgenic mice. Immunol Lett 2004; 92:193–197. [DOI] [PubMed] [Google Scholar]
- 60. Svec F, Rudis M. Glucocorticoids regulate the glucocorticoid receptor in the AtT-20 cell. J Biol Chem 1981; 256:5984–5987. [PubMed] [Google Scholar]
- 61. Hatsumi T, Yamamuro Y. Downregulation of estrogen receptor gene expression by exogenous 17beta-estradiol in the mammary glands of lactating mice. Exp Biol Med. 2006; 231:311–316. [DOI] [PubMed] [Google Scholar]
- 62. Choi JY, Jo MW, Lee EY, Lee DY, Choi DS. Ovarian steroid dependence of endoplasmic reticulum stress involvement in endometrial cell apoptosis during the human endometrial cycle. Reproduction 2018; 155:493–503. [DOI] [PubMed] [Google Scholar]
- 63. Fu Z, Wang X, Zhou H, Li Y, Chen Y, Wang Z, Liu L. GRP78 positively regulates estrogen-stimulated cell growth mediated by ER-α36 in gastric cancer cells. Mol Med Rep 2017; 16:8329–8334. [DOI] [PubMed] [Google Scholar]
- 64. Luvsandagva B, Nakamura K, Kitahara Y, Aoki H, Murata T, Ikeda S, Minegishi T. GRP78 induced by estrogen plays a role in the chemosensitivity of endometrial cancer. Gynecol Oncol 2012; 126:132–139. [DOI] [PubMed] [Google Scholar]
- 65. Manna PR, Jo Y, Stocco DM. Regulation of Leydig cell steroidogenesis by extracellular signal-regulated kinase 1/2: role of protein kinase A and protein kinase C signaling. J Endocrinol 2007; 193:53–63. [DOI] [PubMed] [Google Scholar]
- 66. Jiang YF, Tsui KH, Wang PH, Lin CW, Wang JY, Hsu MC, Chen YC, Chiu CH. Hypoxia regulates cell proliferation and steroidogenesis through protein kinase A signaling in bovine corpus luteum. Anim Reprod Sci 2011; 129:152–161. [DOI] [PubMed] [Google Scholar]
- 67. Yu Y, Li W, Han Z, Luo M, Chang Z, Tan J. The effect of follicle-stimulating hormone on follicular development, granulosa cell apoptosis and steroidogenesis and its mediation by insulin-like growth factor-I in the goat ovary. Theriogenology 2003; 60:1691–1704. [DOI] [PubMed] [Google Scholar]
- 68. Seto-Young D, Avtanski D, Varadinova M, Park A, Suwandhi P, Leiser A, Parikh G, Poretsky L. Differential roles of MAPK-ERK1/2 and MAPK-p38 in insulin or insulin-like growth factor-I (IGF-I) signaling pathways for progesterone production in human ovarian cells. Horm Metab Res 2011; 43:386–390. [DOI] [PubMed] [Google Scholar]
- 69. Kurowska P, Mlyczyńska E, Dawid M, Opydo-Chanek M, Dupont J, Rak A. In vitro effects of vaspin on porcine granulosa cell proliferation, cell cycle progression, and apoptosis by activation of GRP78 receptor and several kinase signaling pathways including MAP3/1, AKT, and STAT3. Int J Mol Sci 2019; 20:5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rak A, Mellouk N, Froment P, Dupont J. Adiponectin and resistin: potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction. 2017; 153:215–226. [DOI] [PubMed] [Google Scholar]
- 71. Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004; 145:367–383. [DOI] [PubMed] [Google Scholar]
- 72. Cheng L, Shi H, Jin Y, Li X, Pan J, Lai Y, Lin Y, Jin Y, Roy G, Zhao A, Li F. Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology. 2016; 157:4875–4887. [DOI] [PubMed] [Google Scholar]
- 73. Mm WQ, Fan J, Khor S, Song M, Hong W, Dai X. Serum vaspin levels and vaspin mRNA expression in subcutaneous adipose tissue in women with gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol. 2014; 182:98–101. [DOI] [PubMed] [Google Scholar]
- 74. Mierzyński R, Poniedziałek-Czajkowska E, Dłuski D, Patro-Małysza J, Kimber-Trojnar Ż, Majsterek M, Leszczyńska-Gorzelak B. Nesfatin-1 and vaspin as potential novel biomarkers for the prediction and early diagnosis of gestational diabetes mellitus. Int J Mol Sci 2019; 20:159. [DOI] [PMC free article] [PubMed] [Google Scholar]


