Abstract
Motivation
Co-expression of two genes across different conditions is indicative of their involvement in the same biological process. However, when using RNA-Seq datasets with many experimental conditions from diverse sources, only a subset of the experimental conditions is expected to be relevant for finding genes related to a particular Gene Ontology (GO) term. Therefore, we hypothesize that when the purpose is to find similarly functioning genes, the co-expression of genes should not be determined on all samples but only on those samples informative for the GO term of interest.
Results
To address this, we developed Metric Learning for Co-expression (MLC), a fast algorithm that assigns a GO-term-specific weight to each expression sample. The goal is to obtain a weighted co-expression measure that is more suitable than the unweighted Pearson correlation for applying Guilt-By-Association-based function predictions. More specifically, if two genes are annotated with a given GO term, MLC tries to maximize their weighted co-expression and, in addition, if one of them is not annotated with that term, the weighted co-expression is minimized. Our experiments on publicly available Arabidopsis thaliana RNA-Seq data demonstrate that MLC outperforms standard Pearson correlation in term-centric performance. Moreover, our method is particularly good at more specific terms, which are the most interesting. Finally, by observing the sample weights for a particular GO term, one can identify which experiments are important for learning that term and potentially identify novel conditions that are relevant, as demonstrated by experiments in both A. thaliana and Pseudomonas Aeruginosa.
Availability and implementation
MLC is available as a Python package at www.github.com/stamakro/MLC.
Supplementary information
Supplementary data are available at Bioinformatics online.
1 Introduction
Knowing which biological processes and pathways are affected by each gene would be a useful tool for plant biologists and breeders. With this information, they can more easily identify genes that are likely to affect the phenomenon or trait they are studying and prioritize genes for experimental testing. The Biological Process Ontology (BPO) of the Gene Ontology (GO) (Ashburner et al., 2000) provides us with a set of terms that describe biological processes at different levels of granularity and can be used to annotate genes from all species in a systematic way. However, the use of computational methods to accurately predict BPO annotations, also known as Automatic Function Prediction (AFP), remains challenging, as demonstrated in the Critical Assessment of Functional Annotation (CAFA) challenges (Jiang et al., 2016a).
Most AFP methods use the Guilt-By-Association (GBA) principle. They define a similarity or dissimilarity measure between genes and use it as a proxy for functional similarity. Then, they assign GO annotations to genes of unknown function based on the functions of the genes most similar to them. The choice of similarity measure is always motivated by biology. For instance, sequence similarity points towards a conserved structure which in turn implies similar function. Alternatively, co-expression across different conditions may hint at involvement in the same pathways. Recent results from the third CAFA challenge hint at the great potential of gene expression data to find which genes are involved in a specific biological process (Zhou et al., 2019). Combining multiple similarity measures in order to better approximate functional similarity is also possible, as done for instance in (Cozzetto et al., 2013; Lan et al., 2013; Zhang et al., 2017).
Genes that are involved in the same biological processes are expected to show similar expression patterns, as they respond similarly to perturbations related to these processes. Discovering BPO annotations for all unannotated genes requires data from a wide range of different experimental conditions. For example, we need samples from different tissues, different time points across development, from wild-type or mutant plants etc. Thanks to world-wide sequencing efforts, more and more RNA-Seq data are becoming available to public databases, such as ArrayExpress (Parkinson et al., 2007) and GEO (Clough and Barrett, 2016).
The Pearson Correlation Co-efficient (PCC) is the most widely used measure of gene co-expression similarity and has been largely successful, especially for microarray-derived expression data. For instance, for MS-kNN (Lan et al., 2013), one of the top-performing methods in CAFA2 (Jiang et al., 2016a), the PCC was calculated on samples from 392 human microarray datasets to quantify co-expression similarity between genes, outperforming sequence similarity for AFP in BPO (Lan et al., 2013).
PCC might, however, not be the optimal co-expression measure due to the diversity of biological processes and heterogeneity of public expression datasets. This means that only a subset of all available experimental conditions is likely to be truly informative about a specific GO term. For example, let us assume that we are looking for genes involved in plant immune response. Using the PCC across all possible conditions, we implicitly expect that all such genes are expressed similarly not only during immune response, but across all conditions and tissues. However, differential co-expression analysis has shown that several Arabidopsis thaliana immune genes, such as FLS2, ADR1 and JAR1, change co-expressed partners before and after infection with Pseudomonas syringae (Jiang et al., 2016b). A gene that is co-expressed with immune genes during (only) infection is still a good candidate gene for immune response, even if it has different expression patterns to the immune genes in other tissues or developmental stages. Including many unrelated expression samples, essentially adds noise to the correlations. According to this reasoning, we should be able to improve the performance of co-expression-based gene function prediction by calculating co-expression only over the samples that are relevant for each term.
This insight that the PCC might be suboptimal is not new. For example, Jaskowiak et al. showed that k-means clustering of gene expression data heavily relies on the choice of similarity measure (PCC, Spearman correlation, Euclidean distance etc.) and that the most suitable measure varies across different datasets (Jaskowiak et al., 2012). As another example, Hu et al. showed that using an inappropriate distance metric can really harm the performance of the k-Nearest Neighbors (k-NN) classifier in biomedical datasets (Hu et al., 2016).
Adapting a distance measure is a subfield within machine learning that is called metric learning: learning a distance function from a dataset of examples that can most effectively be utilized to perform a task, e.g. discriminating between two classes. It is most explored in combination with the k-NN classifier (Bellet et al., 2013). In the context of AFP, Ray and Misra developed a metric learning method called Genetic Algorithm for Assigning Weights to Gene Expressions using Functional Annotations (GAAWGEFA) that learns a weighted PCC on microarray data using a genetic algorithm to find the optimal values for the weights (Ray and Misra, 2019). They showed that their weighted correlation increases the protein-centric precision compared to PCC in a yeast dataset. Metric learning has also been applied to AFP combined with multiple-instance learning (Xu et al., 2017). In that work, each protein is viewed as a ‘bag of domains’ and metric learning is used to learn a distance function between proteins (based on their domains) that is representative of functional similarity.
Here, we use metric learning to identify the most informative conditions for a given GO term. Similar to GAAWGEFA, our goal is to assign a weight to every RNA-Seq sample. GAAWGEFA learns one weighted PCC for all GO terms (Ray and Misra, 2019). On the contrary, our approach, Metric Learning for Co-expression (MLC), optimizes the weights per term. Our philosophy (graphically shown in Fig. 1) is that weights should be chosen in such a way that a pair of genes annotated with the same term should have maximally similar expression profiles, i.e. comply with our assumption that these genes should be co-expressed. On the other hand, when one gene is annotated with the term and the other not, we expect that such a pair should not have high co-expression. In other words, we would like to select weights that minimize the co-expression for these pairs. For pairs of genes both not annotated with the term, we cannot say anything about the co-expression since they might be annotated with another term, and thus be co-expressed too (albeit for other conditions/samples). Consequently, the co-expression of these pairs should be ignored when optimizing weights for the GO term of consideration. A high weight for a sample will put emphasis on that sample when calculating the co-expression over all samples, whereas a low weight for a sample will reduce the influence of that sample. When a weight becomes zero the sample is even ignored. To enforce selecting informative samples, we additionally apply an L1 sparsity constraint on the weights, which will set a weight to zero when a sample is uninformative (Tibshirani, 1996). In contrast to GAAWGEFA, where they have used a genetic algorithm to find the weights, we are able to pose the weight optimization in an elegant mathematical formulation that can be minimized efficiently using standard methods. To reduce the computational burden even further, we use the weighted inner product as a similarity function instead of the weighted PCC. We evaluate our algorithm on public RNA-Seq data from A. thaliana and on the microarray data from Pseudomonas aeruginosa that were used for the CAFA-π challenge, which included experimental validation of gene functions. (Zhou et al., 2019).
Fig. 1.
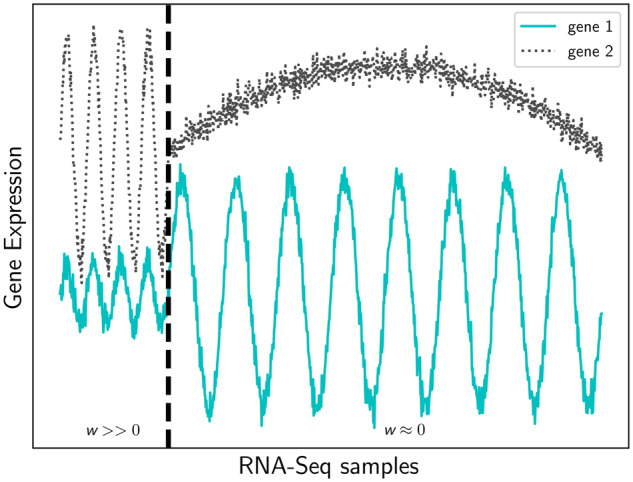
Illustrative example of the expression of two hypothetical genes (y-axis, solid and dashed lines) involved in the same biological process over a large set of samples (x-axis). The total Pearson correlation between the genes is 0.09. MLC sets large weight values (wm) for the samples left of the vertical dashed line (where the unweighted correlation is 0.92) and small or zero weights for the samples on the right (unweighted correlation = 0.002)
2 Materials and methods
2.1 Data and preprocessing
We used the API of the European Bioinformatics Institute (Petryszak et al., 2017) to download all A. thaliana RNA-seq studies available at ArrayExpress (Parkinson et al., 2007). All samples had been processed using the same pipeline and expression was measured using raw read counts. We restricted our dataset to samples that used the latest version of the A. thaliana genome (TAIR10) and had fewer than 10% unmapped reads. After removing duplicate experiments, we had 4215 samples from 298 different studies (batches) for 32 833 genes, 26 925 of which were protein-coding. We used a preprocessing pipeline similar to the one used to construct the ATTED-II RNA-Seq co-expression network (Obayashi et al., 2018). We first removed samples with fewer than 10 000 000 mapped reads. Then, we removed lowly expressed genes (genes with maximum expression over the remaining samples less than 100). To diminish the zero-inflation of the dataset, we also removed all genes that were not expressed in at least half of the samples (median expression smaller than 1). Finally, we log-transformed the expression counts using a pseudocount of 0.125. Supplementary Material S1 and Supplementary Figure S1 describe the results when excluding the filtering of low-coverage samples.
We then mapped the TAIR gene ID’s to UniProt ID’s. BPO annotations were downloaded from GOA (https://www.ebi.ac.uk/GOA) in September 2016 and annotations with the IEA evidence code were removed. A total of 2978 samples and 6013 genes with BPO annotations remained after these filtering steps. We applied ComBat (Johnson et al., 2007) to remove unwanted variation stemming from the fact that the different samples come from different studies (batch effects). ComBat uses a Bayesian method to standardize the mean and the variance of each gene in each study (batch). In order to be able to estimate within-batch variances, we removed all studies that had only one sample, leaving us with 2959 samples (Supplementary Material S2).
For the experiments on P. aeruginosa we used a pre-processed microarray expression compendium from (Tan et al., 2017). This dataset contains the expression of 5549 genes measured over 1051 samples. The gene annotations were downloaded from the Supplementary Material of the CAFA-π paper (Zhou et al., 2019).
2.2 Notation
We use to denote the expression of gene i across all f = 2959 samples. xim is the expression of gene i at sample m. is the mean of gene i across all samples. Given N genes and a GO term l, we denote as the vector with the class labels of the genes, with iff gene i is annotated with l. The sample weights are represented by a vector with f non-negative elements .
2.3 Weighted and unweighted measures of Co-Expression
The most widely-used measure of co-expression between two gene expression vectors is the Pearson Correlation Coefficient (PCC), which is defined as follows:
| (1) |
Note that the numerator is the covariance between xi and xj and the denominator is the product of the SDs of the two vectors.
A related, but simpler measure is the inner product similarity (S), which, on the contrary, is sensitive to the mean expression of both genes:
| (2) |
If two vectors xi, xj both have zero mean and unit L2-norm, then their PCC is equal to their inner product. This equality does not hold anymore if we weigh each vector element (sample) differently. However, since the two metrics are related, we chose to use the weighted inner product similarity instead of the weighted PCC as our expression similarity function in order to simplify the problem. We center and scale our data so that the (unweighted) mean of every gene is zero across all conditions and its (unweighted) L2-norm is equal to one:
| (3) |
Then, we define our similarity function as the weighted inner product of the two scaled expression vectors ():
| (4) |
Where is a diagonal matrix containing the sample weights.
2.4 MLC
The rationale for learning the weights is to maximize the performance of the k-NN classifier. For this purpose, we want the expression similarity between two genes that are both annotated with a given GO term l to be higher (on average) than the similarity between a gene that is annotated with l and a gene that is not. We group each gene pair into one of the following three categories:
Both genes are annotated with l (we call these ‘positive–positive pairs’ or ‘p–p’).
Exactly one of the two genes is annotated with l (‘positive–negative pairs’ or ‘p–n’).
Neither gene is annotated with l (‘negative–negative pairs’ or ‘n–n’).
Our goal is to find the weight values wm that maximize the separability between ‘p–p’ and ‘p–n’ pairs. Let denote the mean and variance of the weighted similarity value Sw of all ‘p–p’ gene pairs and, similarly, for all ‘p–n’ gene pairs. Let also and denote the number of gene pairs in each category. We use Welch’s two-sample t-statistic with unequal variances to quantify the notion of separability:
| (5) |
Note that μ and are functions of w, but this dependence is not shown explicitly in Equation (5) to keep the notation simple. Maximizing is equivalent to minimizing . In order to enable sample selection, we also added an L1 regularization term that forces the weights of uninformative samples to zero (Tibshirani, 1996). Our optimization problem then becomes:
| (6) |
Parameter α controls the trade-off between the actual cost and the regularization. The minimization of Equation (6) is done with the Broyden-Fletcher-Goldfarb-Shanno method (Byrd et al., 1995).
2.4.1 Global MLC
To investigate the effect of creating GO-term specific predictors, we also implemented a version of MLC that is applicable to all terms simultaneously. To this purpose, we redefined ‘p–p’ gene pairs as pairs of two genes which share at least one GO term and ‘p–n’ pairs as pairs of two genes that share no GO annotations. All the ensuing steps remain the same as for the term-specific MLC. We call this method ‘Global MLC’ (MLCG).
2.5 Experimental set-up
We compared MLC to the unweighted PCC baseline. To investigate the effect of the use of a term-specific classifier, we created term-specific classifiers from the PCC by tuning the classifier parameter k individually per GO term and not globally over all terms. We called this approach PCC(k). We also compared to GAAWGEFA which, like MLC, learns a weighted co-expression measure (Ray and Misra, 2019). GAAWGEFA is not GO-term-specific and optimizes the mean protein-centric precision using a genetic algorithm, so we also constructed a non-term-specific version of MLC (MLCG) to compare against. Another way to measure co-expression is the Mutual Rank (MR) (Obayashi et al., 2018) which is used in the ATTED-II database. Although MR neither selects samples nor weighs samples differently, it has been shown to outperform the PCC for function prediction (Obayashi et al., 2018), so we included it in the comparison as a stronger baseline. Input to MR are typically the PCC values of all gene pairs, although it can be applied to any co-expression measure. More details on the definition and implementation of each of these methods are given in Supplementary Material S3.
We evaluated the methods in three ways: (1) A cross-validation experiment using all A. thaliana genes with at least one GO annotation (reported as ‘CV results’), (2) a time-course experiment using the preliminary test set from CAFA3 containing 6077 training and 90 test genes also from A. thaliana (reported as ‘CAFA3 results’) and (3) the dataset of CAFA-π from bacterium P. aeruginosa, where the goal was to make term-specific predictions for biofilm formation and motility (Zhou et al., 2019). For the cross validation experiment, we used nested cross-validation (Varma and Simon, 2006), using the inner loop to optimize the parameters and the outer loop to evaluate performance on previously unseen genes (Supplementary Fig. S2, Supplementary Material S4). As in this work we are dealing with the problem of identifying which genes should be annotated with a specific GO term, we focus on term-centric evaluation using the mean ROCAUC. Details about the three evaluation modes are provided in Supplementary Material S4 and details about the used term-centric evaluation metrics as well as protein-centric metrics that we also used are given in Supplementary Material S5.
3 Results
3.1 All methods outperform the PCC
We compared our metric learning approach (MLC), as well as MR and GAAWGEFA to the standard, unweighted PCC using 3-fold cross-validation. PCC achieved a mean term-centric ROCAUC of 0.69, while the performance of both MR and MLC with the weighted inner product was 0.72 (Table 1). The performance of GAAWGEFA was 0.71. MLCG, the non-term-specific version of MLC, also achieved a mean ROCAUC of 0.72. Although all methods perform fairly similarly according to protein-centric measures (Supplementary Tables S1–2, Supplementary Material S6), PCC performs significantly worse than the other methods on term-centric ROCAUC [False Discovery Rate (FDR) < 0.036, Table S3–6, Supplementary Material S7, effect size 4%]. This shows that the PCC is indeed a suboptimal co-expression measure. When randomly permuting the GO annotations of the genes, both PCC and MLC had a mean ROCAUC of 0.5, i.e. equal to random guessing, implying that MLC does not artificially generate information in the absence of real structure (Supplementary Material S8).
Table 1.
Mean term-centric ROCAUC achieved by the methods under comparison using 3-fold cross-validation (CV, second and third column) and when testing on the CAFA3 dataset (CAFA3, fourth and fifth column)
| Method | ROCAUC (CV) | Weighted ROC AUC (CV) | ROC AUC (CAFA3) | Weighted ROCAUC (CAFA3) |
|---|---|---|---|---|
| PCC | 0.69 ± 0.003 | 0.69 ± 0.003 | 0.68 [0.63, 0.72] | 0.68 [0.63, 0.73] |
| PCC(k) | 0.69 ± 0.003 | 0.69 ± 0.003 | 0.68 [0.63, 0.71] | 0.68 [0.63, 0.72] |
| PCC + MR | 0.72 ± 0.002 | 0.72 ± 0.002 | 0.69 [0.65, 0.73] | 0.69 [0.65, 0.73] |
| GAAWGEFA | 0.71 ± 0.002 | 0.71 ± 0.002 | 0.69 [0.65, 0.73] | 0.70 [0.65, 0.74] |
| MLC () | 0.72 ± 0.003 | 0.73 ± 0.003 | 0.69 [0.65, 0.73] | 0.69 [0.65, 0.73] |
| MLCG | 0.72 ± 0.003 | 0.72 ± 0.003 | 0.71 [0.67, 0.75] | 0.72 [0.67, 0.76] |
| MLC-MR Hybrid | 0.73 ± 0.005 | 0.73 ± 0.005 | 0.69 [0.65, 0.73] | 0.69 [0.66, 0.73] |
Notes: For the cross-validation, we report the average performance over the three folds as well as the corresponding standard error. For the CAFA3 results we report the performance on the test set as well as the 95% confidence intervals from doing 1000 bootstrapped tests. The top performance of every column is shown in bold.
3.2 MLC is the best at predicting specific GO terms
Although MR, GAAWGEFA and MLC perform equally on average, one is typically not interested in predicting GO terms that are ‘near’ the ontology root, as most of them describe too general biological processes (Clark and Radivojac, 2013). Therefore, we compared the performances of these methods as a function of term specificity. As measures of specificity, we used the maximum path length to the ontology root and the Resnik Information Content (IC) (Resnik, 1995). One way to take term specificity into account is to calculate the weighted term-centric ROCAUC, where each term is weighted by its IC when calculating the average. As shown in Table 1, MLC achieves the highest weighted ROCAUC. The difference is statistically significant for all methods except for MR (Supplementary Table S4, Supplementary Material S7), although the effect size is small (1.5%).
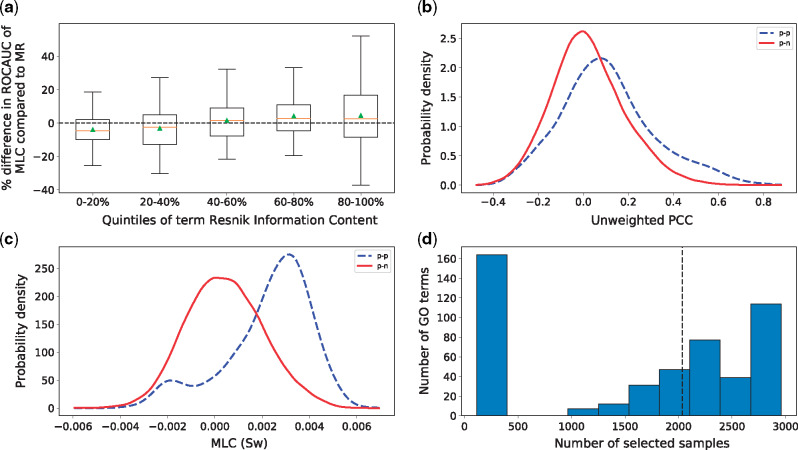
Furthermore, we grouped the GO terms into quintiles (quantiles at 0, 20%, 40%, 60% and 80%) and plotted the distribution of the percent differences in performance of MLC from MR for each quintile (Fig. 2a). We observed that for the first two quintiles (i.e. the 40% most frequent terms), MLC performs worse than MR, while for the 60% most specific terms, both the mean and the median performance of MLC is better. Further analysis showed that for the very general terms, MLC makes a lot more type I errors (false positives) than for the more specific ones (Supplementary Fig. S3, Supplementary Material S9) and that makes it underperform with respect to MR. The Spearman correlation between percent difference and Resnik IC was 0.26. The same pattern is evident when comparing MLC to all other methods (PCC, GAAWGEFA and MLCG), as well as when replacing Resnik IC with the path length to the ontology root (Supplementary Tables S7 and 8, Supplementary Figs. S4 and 5, Supplementary Material S10). From that we can conclude that term-specific MLC is the preferred method for finding genes belonging to rarer terms.
Fig. 2.
(a) Percent increase in ROCAUC of MLC (Sw) with respect to MR as a function of Resnik IC. For each set of terms in each quintile of IC, the corresponding box includes the two middle quartiles of the percent increase for these terms. An orange line denotes the median and a green triangle the mean. The error bars extend to 1.5 times the range of the two middle quartiles. Note, that the 0–20% quintile corresponds to the 20% least specific terms and the 80–100% quintile to the 20% most specific ones. (b–c) Distributions of co-expressions for genes annotated with term GO: 1903047. In dashed blue lines, the co-expression values between a test and a training gene that both are annotated with that term. In solid red lines, the co-expression between test genes annotated with that GO term and training genes that are not. Co-expression is measured as the PCC (b) and the Sw trained by MLC (c). The x-axis shows the co-expression values and the y-axis the probability density estimated with Gaussian kernels. Note that the PCC and Sw have different ranges due to the weight optimization. (d) Histogram of the number of samples that were selected for each GO term. The x-axis corresponds to the number of selected samples and the y-axis to how many GO-term-specific similarity functions selected that many samples. The dashed line denotes the median number of non-zero weights
3.3 MLC tunes the weights to find ‘p–p’ pairs
The goal of MLC is to choose the weights so that for test genes that have a particular GO annotation, the learned similarities are higher to training genes that have the same annotation than to genes that do not. As an example, Figure 2b and c show the distribution of co-expression similarities between the test genes annotated with term GO: 1903047 (mitotic cell cycle process) and all training genes for the PCC and MLC similarities, respectively. It is clear that for MLC, the test genes are a lot more similar to training genes with the same annotation. Note, however, that, for this term, a significant portion of the similarities are negative (small blue peak in Fig. 2b). This means that some positive genes are anti-correlated to the rest. For these cases MLC will make Type II errors (false negatives).
Figure 2d shows the distribution of the number of selected samples for each GO term. For about 33% of all GO terms, MLC selected less than 9% of the available samples (252 or fewer), setting all other weights to zero. The median number of selected samples was 2035 out of 2959 or about 69% of all samples. Randomly selecting samples did not improve the mean performance of the PCC (Supplementary Material S11), implying that the correct samples have to be selected for each term. Moreover, for about 23% of the terms, MLC kept all the samples and weighted them more or less equally (maximum SD of weights = 0.006), in which case MLC was almost equivalent to the unweighted inner product. As expected, for those terms MLC had on average similar performance to the baseline PCC. We also found that individually tuning k per GO term for the PCC gave on average the same term-centric ROCAUC as the baseline PCC [PCC (k), Table 1], so the performance improvement is not caused by simply choosing the optimal k value for each GO term. Finally, we observed a small negative correlation between term IC and the number of samples selected (Spearman ρ = –0.09, P-value = 0.057). This means that MLC has a slight tendency to select fewer samples for more specific terms, but this result is not statistically significant.
3.4 The weights learned by MLC help at identifying relevant experimental conditions
The weights learned by GAAWGEFA are roughly uniformly distributed between 0 and 1 (Kolmogorov–Smirnov test statistic = 0.011, P-value = 0.847) and are not correlated to any of the term-specific weight profiles of MLC, which tend to have an exponential-like distribution (Supplementary Figs. S6 and 7, Supplementary Material S12), as many samples get a weight of zero. Furthermore, samples from the same study (batch) tend to be either selected or not selected together by MLC (Supplementary Table S9, Supplementary Material S13).
We used simulated data to validate the sample selection of MLC. We created an artificial dataset with 7000 genes, 3000 samples and three GO terms, where the genes with a particular GO term are correlated over a predefined set of samples. The sets of informative samples are of equal length and do not overlap (Supplementary Fig. S8, Supplementary Material S14). We varied the number of informative samples from 10 to 500 and compared MLC to the PCC calculated over all samples and the PCC calculated only over the informative ones [which can be seen as the optimal, ground-truth (GT) performance]. As seen in Figure 3, when up to 200 samples drive the similarity, MLC performs considerably better than the PCC and only slightly worse than the GT PCC (GT). When the number of informative samples increases, both MLC and PCC increase performance, eventually both converging to GT. Yet, MLC converges much faster, thus achieving GT PCC performance with fewer informative samples. We also found that in all cases, and for all numbers of relevant samples except 500, the MLC weights of the GT samples were on average significantly larger than those in the rest of the samples (two-sample t-test, FDR ¡ 0.01, Supplementary Fig. S9a, Supplementary Material S14). Moreover, there was a significant enrichment of the informative samples in the samples selected by MLC (Fisher’s exact test, FDR ¡ 0.01, Supplementary Fig. S9b, Supplementary Material S14). When the number of informative samples increased to 500, MLC selected all the samples with rather similar weights (Fig. S9) and performed consistently equal to both PCC and GT.
Fig. 3.
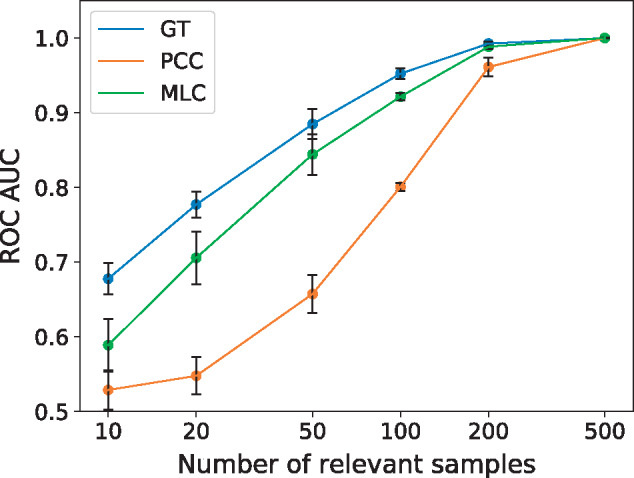
ROCAUC (y axis) on simulated data as a function of the number of relevant samples (x axis in log scale) for MLC (green), PCC (orange) and the PCC using only the GT samples (blue). The error bars denote the SD over five repetitions
Continuing on the example from before, for term GO: 1903047 (mitotic cell cycle process) MLC gives the highest weight to a sample of a plant grown in the absence of phosphorus, which has been shown to restrict the cell division rate (Kavanová et al., 2006). Among the samples with highest weights are also many samples from experiments studying seed germination, a process closely linked to cell cycle (Vázquez-Ramos and de la Paz Sánchez, 2003). Finally, two IBM1 mutant samples were selected with very high weights. The IBM1 gene codes for histone demethylation protein and has GO annotations that include flower, root and pollen development. The complete weight profile is shown in Supplementary Figure S10 (Supplementary Material S15).
As another example, we looked at ‘regulation of flower development’ (GO: 0009909). For this term, MLC selected 164 samples and achieved ROC AUC of 0.74 while the PCC score was 0.55. The top scoring sample came from an AS1 mutant plant. AS1 is a well-known transcription factor, key in many developmental processes including flowering (Xu et al., 2008). Several wild-type samples were selected, many of them from meristems, tissues which contain undifferentiated cells and drive tissue differentiation. In the top-10 samples, we also found a CORYNE mutant [CORYNE is involved in the signaling of cell differentiation in flowers (Muller et al., 2008)] and an AGO1 mutant, a gene crucial for miRNA-based mRNA splicing (Vaucheret et al., 2004). Finally, 3 out of the top-10 samples were from knock-outs of well-known DNA methylation genes. Chen et al. were—to our knowledge—the first to extensively study the interplay between epigenetics, transcription factors and miRNAs in flower development, which is otherwise largely not understood (Chen et al., 2018). MLC was able to highlight that all these three regulatory mechanisms are at the same time informative for understanding flower development, without using the data from that work or any Chip-Seq or miRNA data.
Together these examples highlight that MLC has the ability to identify novel experimental conditions for a specific GO term. Moreover, the weights learned by MLC are also consistent with the ontology structure and the existing annotations, as shown in Supplementary Figure S11 in Supplementary Material S16.
3.5 Using all samples obscures co-expression
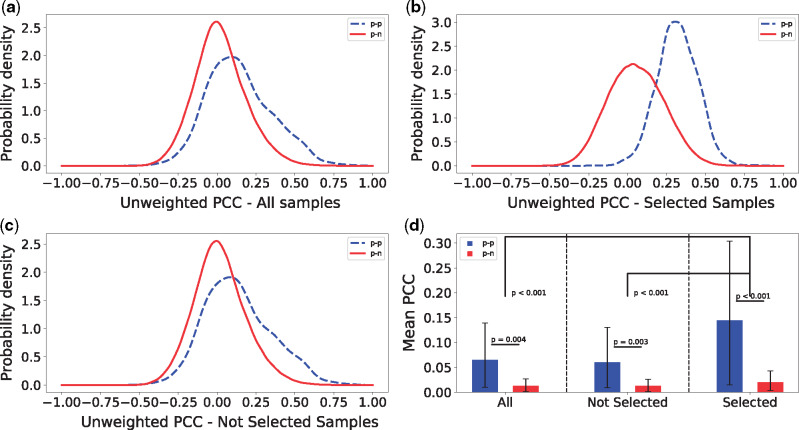
Next, we investigated the terms for which MLC performed sample selection, i.e. assigning a non-zero weight to at most 9% of the samples. We looked at the PCC values for ‘p–p’ and ‘p–n’ gene pairs. Figure 4a shows an example of the distributions of the PCC values for ‘p–p’ and ‘p–n’ pairs for term ‘GO: 1903047’ (mitotic cell cycle process). Next, we calculated the PCC for all ‘p–p’ and ‘p–n’ pairs, but only considering the samples that were selected by MLC (i.e. had a non-zero weight assigned), as well as only considering the samples that were not selected by MLC (i.e. were assigned a weight of zero), shown in Figure 4b and c, respectively. One can notice that the two distributions (‘p–p’ and ‘p–n’ PCC values) differ more when taking the MLC selected samples in consideration (compare Fig. 4a with Fig. 4b). When only considering the samples that were not selected, the two distributions differ in a similar way to when all samples are being considered (compare Fig. 4a with Fig. 4c).
Fig. 4.
(a–c) Distributions of Pearson correlations for pairs of training genes that are both annotated with term GO: 1903047 (‘p–p’, blue dashed), and for pairs of training genes of which only one is annotated with that term (‘p–n’, red solid). The correlations are calculated using all samples (a), the samples that were selected by MLC (b) and the samples that were not selected (c). (d) The means of the distributions of (a–c), over all GO terms where less than 10% of the samples where used to calculate the co-expression (more than 90% zero weights). Values for ‘p–p’ pairs are colored blue and for ‘p–n’ pairs red. The error bars show the 95% confidence intervals for the means, calculated with 1000 bootstraps. Above the bars the bootstrap P-values are shown for the pairwise comparisons
For every GO term for which MLC performs sample selection, we calculated the mean PCC of all ‘p–p’ and ‘p–n’ pairs under the three sample sets (all samples, not selected samples and selected samples). Figure 4d shows the average of these values of all GO terms. We also performed 1000 bootstraps, sampling GO terms with replacement to obtain 95% confidence intervals for these averages. We observed that the difference in mean co-expression between ‘p–p’ and ‘p–n’ in the samples not selected by MLC is similar to the difference in all the samples. Although these differences are statistically significant, they are also significantly smaller than the difference in the MLC-selected samples (bootstrap P-value ¡0.001) (Fig. 4d). This means that although the whole dataset does contain a few samples that are informative for these GO terms, calculating the co-expression over a larger set of samples can corrupt the ‘real’ co-expression signal, increasing the difficulty of discovering new genes that play a role in these processes.
3.6 Combining MR and MLC
The performances of MLC and MR are positively correlated (Spearman ρ = 0.13, P-value =0.003). We also applied MR on the co-expression similarities obtained with MLC, as MR is in principle not restricted to using only the PCC. We found a small improvement compared to standalone MLC, with a mean ROC AUC of 0.73. Also, the performances of MLC and MLC + MR were highly correlated (Spearman ρ = 0.97, P-value ≪ 10–20).
We tried another approach to combine MLC and MR depending on the performance of the methods. If the training ROCAUC of MR was larger than 0.8 for a GO term, we used the predictions of MR for that term, otherwise we used the predictions of MLC. This combined classifier had an incrementally larger term-centric ROCAUC (0.73, Table 1–Hybrid), though statistically significant (P-value = 0.008, two-sample t-test). The threshold of 0.8 training ROCAUC was chosen arbitrarily and was not tuned to maximize performance. This naïve hybrid classifier shows that there is potential to improve performance by combining MLC and MR in more sophisticated ways.
3.7 CAFA results
Moreover, we benchmarked MLC on 90 temporary A. thaliana targets from the CAFA3 competition. The results are similar, both in absolute numbers and the ranking of the methods (Table 1), with both MR and MLC outperforming the PCC on average. However, due to the small size of the dataset, the confidence intervals are much wider, so no significant conclusions can be drawn.
Lastly, we compared MLC and MR to PCC in the term-centric challenge CAFA-π for P. aeruginosa, where PCC on microarray data was the top-performing method (Zhou et al., 2019). The goal of the challenge was to predict proteins involved in motility and biofilm formation and the ground truth was obtained using genome-wide assays. Note that these two terms are relatively frequent (about 14% and 12% of the tested genes were annotated with biofilm and motility, respectively). Comparing them to our experiments in Arabidopsis (Fig. 2a), they would fall in the left-most, least informative bin, where MLC is expected to be on average slightly worse than MR and about similar to PCC. The results show that PCC, MR and MLC achieve similar ROCAUC (around 0.6 for both biofilm and motility, which is also very similar to the performance reported in Zhou et al., 2019) (Supplementary Table S10, Supplementary Material S17). However, MLC selected samples from relevant conditions (Supplementary Material S17), enabling interpretability of the predictions. We also examined the genes for which MLC was most confident for their involvement in either biofilm formation or motility, but they were not confirmed by the assay (i.e. false positives) and found evidence in the literature that indeed these genes are likely part of these processes under certain biological conditions. For example, two genes in the top-10 for biofilm formation were annotated with the biofilm pathway in KEGG (more detailed discussion and interpretation can be found in Supplementary Material S17). Interestingly, these genes received low scores by PCC and MR (see e.g. Supplementary Fig. S12, Supplementary Material S17).
4 Discussion
4.1 MLC
We introduced MLC, a metric learning method for building automatic function predictors from a large collection of expression data. MLC calculates gene co-expression by assigning GO-term-specific weights to each sample. The weights aim at maximizing the co-expression similarity between genes that are annotated with that GO term. In general, training GO-term specific classifiers (also known as the ‘Binary Relevance’ approach in the machine learning literature) has the disadvantage that individual classifiers fail to see the ‘bigger picture’ and cannot exploit the correlations between terms imposed by the ontological structure. Several works on multi-label classification have shown that Binary Relevance performs worse than models that incorporate label correlations (Li and Zhang, 2014; Suzuki et al., 2001; Tanaka et al., 2015). Despite this, we showed that the weight profiles learned by MLC do correlate with real biological knowledge, such as semantic similarity in the ontology graph and gene annotation similarity, meaning that our method is powerful enough to capture at least some of the label similarities even though it was not exposed to them. Due to the use of the L1 regularization, MLC can also select informative samples by setting the weights of non-informative samples to zero. Moreover, we showed that the samples that are selected come from biological conditions relevant to the GO term in question.
Our method is designed to work well with a GBA approach like the k-NN classifier. This classifier assigns a GO term to a test gene if a large enough fraction of its top co-expressed training genes are annotated with that term. To achieve this, MLC tries to maximize the difference between the average co-expression between gene pairs that are both annotated with the GO term of interest (‘p–p’ pairs) and the average co-expression between gene pairs only one of which is annotated with the term (‘p–n’ pairs). During the training phase, our model ignores gene pairs where neither gene has the term of interest (‘n–n’ pairs). Such pairs could either include two genes that have common GO annotations, but different from the GO term of interest or two genes with completely different annotations. For the first case, one might be tempted to think that the co-expression of such pairs should be high. However, if their common function is different from the term of interest, it is likely that they are correlated for another set of samples than the one related to the GO term of interest and, consequently, are thus uninformative for that GO term. For the second type of ‘n–n’ pairs, the ones that share no annotations whatsoever, it might make sense to want their co-expression to be 0, as they are expected to be dissimilar over any set of samples. However, we decided to ignore these pairs as they do not add any term-specific information, so it is not clear how they will affect the identification of samples specifically relevant for a specific term. This might be problematic as for a negative test gene (i.e. a gene that should not be annotated with the GO term of interest) we cannot exclude that it can be as highly co-expressed to positive as to negative genes, because we did not tune the co-expression values for ‘n–n’ pairs. For very frequent terms with a lot of positive training genes this leads to a lot of false positive predictions, which might explain the poor performance of MLC for frequent terms.
The similarity function that we used as a basis for MLC is the weighted inner product (Sw). We chose this measure because its unweighted version is identical to the unweighted PCC for centered and scaled data, but it has a simpler form which eases the computational burden. The weighted versions of the inner product and PCC are no longer identical, as the data are no longer scaled after weighing the samples. This has as side-effect that the similarity functions that MLC learns are not necessarily in the range [–1, 1], like the PCC. In most cases, their range is much narrower as can be seen in Figure 2b for GO: 1903047. Also, because of the range differences, it is not trivial to compare the similarity of two genes across different GO terms. For the purpose of classification with the k-NN classifier, however, the range of the metric is insignificant (only the relevant rankings are important to find the proper neighborhood).
Our model is more general and not restricted to only the inner product, though. The main idea is to maximize the difference between the similarity of p–p and p–n pairs. This is done by maximizing the t-statistic between the two distributions of similarities. This means that MLC can also be applied to any measure of similarity such as the weighted PCC, weighted Spearman correlation, Euclidean distance etc. Regardless of the chosen metric, the two classes (‘p–p’ and ‘p–n’) do not meet the assumptions for applying Student’s t-test, as the similarity values are neither normally distributed nor independent. This is not an issue, though, because we do not use the t-statistic to compute a P-value (exploit that the t-statistic is distributed according the Student’s t-distribution under these assumptions), but only to quantify the class separability (Theodoridis and Koutroumbas, 2008). Equivalently, we could have used any other measure of class separability, for instance the Fisher Discriminant Ratio (Fisher, 1936) or the Davies-Bouldin index (Davies and Bouldin, 1979).
4.2 Comparison to related methods
Our work validates the observation that PCC is not the optimal co-expression measure for AFP. The MR attempts to obtain more robust and noise-free co-expression values by converting the PCC values into ranks and averaging the reciprocal rankings of two genes (Obayashi et al., 2018). MLC takes a fundamentally different approach, operating on the sample level rather than the correlation level. First and foremost, as we mentioned above, it removes samples that do not help at discriminating between genes that do or do not perform a certain function. With that MLC gives insight into which samples are important for a given GO term, which subsequently can be used to investigate the expression patterns of the GO term related genes across these samples. Weighing samples differently can also be viewed as a way of denoising. For example, it can compensate for the issue that an expression change of one unit has a different meaning in different samples due to technical variations, such as differences in sequencing depth or sample preparations. Our results have shown that MLC is more beneficial than the MR approach for the more specific—and arguably more useful—GO terms.
A similar method to MLC is GAAWGEFA, which learns a weight for each sample in a dataset and then applies a weighted Pearson correlation. There are two fundamental differences between the two methods. Firstly, GAAWGEFA aims at good protein-centric performance, i.e. it tries to do well on average for all genes and therefore learns only one set of sample weights. On the other hand, MLC aims at maximizing the performance for each GO term individually. Secondly, GAAWGEFA learns the weights using a genetic algorithm. For MLC, we used the inner product, which allowed us to have a simple optimization problem that can be solved very efficiently. Even though MLC has to be run for each term separately, it is still 67% faster than GAAWGEFA and, unlike GAAWGEFA, runs for different GO terms can be carried out in parallel to achieve even greater speed-up. Next to those differences, MLC makes more accurate predictions for rarer terms and provides interpretability of the predictions by examining the term-specific sample weight distributions.
Furthermore, in the context of selecting expression samples a related technique is biclustering. Biclustering is an umbrella term for a diverse set of algorithms that simultaneously select subsets of genes and samples, so that the genes in the same subset (bicluster) have similar expression to each other within the samples of that bicluster. It is typically expected that each bicluster reflects a biological process and that makes the rationale of MLC appear similar to a biclustering approach. Although both approaches make use of sample selection and aim at discovering genes involved in the same biological processes, they are fundamentally different in the sense that MLC is supervised and biclustering unsupervised. Biclustering does not make use of GO annotations, but only of the expression matrix. Often, observing enrichment of certain GO terms or KEGG pathways in the genes of biclusters is one of the ways to validate a biclustering result (Santamaría et al., 2007). On the other hand, MLC starts with a set of genes whose GO annotations are known (or at least partly known) and tries to use the expression matrix in order to identify which of the remaining genes participate in a particular biological process by defining a co-expression measure specific to that process.
4.3 Possible extensions
MLC learns the sample weights automatically from the available data and does not rely on information about the samples’ biological condition or tissue. As curation efforts increase and the amount of well-annotated data in public databases grows larger with time, in the future it might be useful to extend MLC to incorporate such knowledge. A possible way to do that would be a group LASSO approach (Yuan and Lin, 2006). Group LASSO uses predefined groups of samples and forces the weights of all samples in a group to be equal. Each such group could contain technical and biological replicates, samples from the same tissue or samples from similar knockout experiments and perturbations.
A disadvantage of MLC is the fact that it does not account for the possibility that genes that show exactly opposite expression patterns (i.e. genes with large negative correlation) might also be involved in the same biological process. In fact, negative correlations are penalized, as our model explicitly tries to force the signed similarities of p–p pairs to be larger than those of the p–n pairs. In Figure 2b, we see that large negative PCC values are scarce within our dataset, implying that we might not suffer a lot from ignoring negative similarities, at least when considering the PCC as a method. The effect might be larger for our MLC approach though, which selects a subset of the samples, as in this smaller set negative correlations might be more frequent.
To handle this short-coming, one could directly use the absolute value of the weighted co-expression in the model. Doing this, one is faced with an additional challenge, namely that the absolute value is not differentiable at 0. This can be overcome by approximating the absolute value with a smooth function, such as , where ϵ is a small positive number (Ramírez et al., 2013), but this makes the co-expression function non-linear and the calculation of its derivative with respect to w more costly. More importantly, it makes the optimization problem more difficult, as it adds an extra non-linearity to an already non-convex objective function, meaning that it might be harder to find a good solution for the weights in this problem.
One could also think of alternative formulations of the objective function that would accommodate absolute co-expression values more easily. For instance, it would be possible to minimize the squared difference between the weighted absolute correlations and a target value (e.g. 0 for p–n pairs and 1 for p–p pairs). Another possibility would be to use a triplet loss, which has been successfully used in image retrieval (Husain et al., 2019). In the triplet loss, we look at sets of three genes at a time instead of two: two positive genes (p1, p2) and one negative (n1). Then, we maximize the difference , where Sw is in this case the absolute weighted similarity.
Finally, in this work, we applied MLC on finding candidate genes for GO terms from the BPO. However, it can be useful for any gene annotation problem that can be solved with expression data, such as finding members of KEGG pathways or genes that are likely to influence a given phenotypic trait. As MLC is computationally efficient, it can easily be applied to a large number of different terms/phenotypes, offering state-of-the-art performance with the added benefit of allowing users to understand which parts of the dataset influence the predictions.
Supplementary Material
Acknowledgements
The authors would like to thank the anonymous reviewers for their valuable suggestions.
Funding
This work was supported by Keygene N.V., a crop innovation company in the Netherlands.
Conflict of Interest: none declared.
References
- Ashburner M. et al. (2000) Gene Ontology: tool for the unification of biology. Nat. Genet., 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet A. et al. (2013) A survey on metric learning for feature vectors and structured data. arxiv, 1306.6709.
- Byrd R.H. et al. (1995) A limited memory algorithm for bound constrained optimization. SIAM J. Sci. Comput., 16, 1190–1208. [Google Scholar]
- Chen D. et al. (2018) Architecture of gene regulatory networks controlling flower development in Arabidopsis thaliana. Nat. Commun., 9, 4534.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W.T., Radivojac P. (2013) Information-theoretic evaluation of predicted ontological annotations. Bioinformatics, 29, i53–i61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough E., Barrett T. (2016) The gene expression omnibus database. Methods Mol. Biol., 1418, 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzetto D. et al. (2013) Protein function prediction by massive integration of evolutionary analyses and multiple data sources. BMC Bioinformatics, 14, S1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D.L., Bouldin D.W. (1979) A cluster separation measure. IEEE Trans. Pattern Anal. Machine Intel., PAMI-1, 224–227. [PubMed] [Google Scholar]
- Fisher R.A. (1936) The use of multiple measurements in taxonomic problems. Ann. Eugenics, 7, 179–188. [Google Scholar]
- Hu L.-Y. et al. (2016) The distance function effect on k-nearest neighbor classification for medical datasets. Springerplus, 5, 1304.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S.S. et al. (2019) ACTNET: end-to-end learning of feature activations and multi-stream aggregation for effective instance image retrieval. CoRR, abs/1907.05794. [Google Scholar]
- Jaskowiak P. et al. (2012) Evaluating correlation coefficients for clustering gene expression profiles of cancer In: de Souto,M.C.Kann,M.G. (eds.) Advances in Bioinformatics and Computational Biology. BSB 2012. Lecture Notes in Computer Science, Vol. 7409. Springer, Berlin, Heidelberg, pp. 120–131. [Google Scholar]
- Jiang Y. et al. (2016a) An expanded evaluation of protein function prediction methods shows an improvement in accuracy. Genome Biol., 17, 184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z. et al. (2016b) Differential coexpression analysis reveals extensive rewiring of arabidopsis gene coexpression in response to pseudomonas syringae infection. Sci. Rep., 6. doi: 10.1038/srep35064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.E. et al. (2007) Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics, 8, 118–127. [DOI] [PubMed] [Google Scholar]
- Kavanová M. et al. (2006) Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Physiol., 141, 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan L. et al. (2013) MS-kNN: protein function prediction by integrating multiple data sources. BMC Bioinformatics, 14 (Suppl. 3), S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-K., Zhang M.-L. (2014) Enhancing binary relevance for multi-label learning with controlled label correlations exploitation In: Pham D.-N., Park S.-B. (eds.) PRICAI 2014: Trends in Artificial Intelligence. Springer International Publishing, Cham, pp. 91–103. [Google Scholar]
- Muller R. et al. (2008) The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell, 20, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obayashi T. et al. (2018) ATTED-II in 2018: a plant coexpression database based on investigation of the statistical property of the mutual rank index. Plant Cell Physiol., 59, e3.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson H. et al. (2007) ArrayExpress–A public database of microarray experiments and gene expression profiles. Nucleic Acids Res., 35, D747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petryszak R. et al. (2017) The RNASeq-er API–a gateway to systematically updated analysis of public RNA-Seq data. Bioinformatics (March), 33, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez C. et al. (2013) is the most computationally efficient smooth approximation to |x|: a proof. J. Uncertain Syst., 8, 205–10. [Google Scholar]
- Ray S.S., Misra S. (2019) Genetic algorithm for assigning weights to gene expressions using functional annotations. Comput. Biol. Med., 104, 149.. [DOI] [PubMed] [Google Scholar]
- Resnik P. (1995) Using information content to evaluate semantic similarity in a taxonomy. In: Mellish,C.S. (ed.), Proceedings of the 14th International Joint Conference on Artificial Intelligence - Volume 1 (IJCAI'95), Morgan Kaufmann Publishers Inc., San Francisco, CA, pp. 448–453.
- Santamaría R. et al. (2007) Methods to bicluster validation and comparison in microarray data In: Yin H.et al. , (eds.) Intelligent Data Engineering and Automated Learning–IDEAL 2007. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 780–789. [Google Scholar]
- Suzuki E. et al. (2001) Bloomy decision tree for multi-objective classification In: De Raedt L., Siebes A. (eds.) Principles of Data Mining and Knowledge Discovery. Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 436–447. [Google Scholar]
- Tan J. et al. (2017) Unsupervised extraction of stable expression signatures from public compendia with an ensemble of neural networks. Cell Syst., 5, 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E.A. et al. (2015) A multi-label approach using binary relevance and decision trees applied to functional genomics. J. Biomed. Inform., 54, 85–95. [DOI] [PubMed] [Google Scholar]
- Theodoridis S., Koutroumbas K. (2008) Pattern Recognition. Academic Press, Orlando, FL. [Google Scholar]
- Tibshirani R. (1996) Regression selection and shrinkage via the Lasso. J. Royal Stat. Soc. B 58, 267–88. [Google Scholar]
- Varma S., Simon R. (2006) Bias in error estimation when using cross-validation for model selection. BMC Bioinformatics, 7, 91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaucheret H. et al. (2004) The action of argonaute1 in the mirna pathway and its regulation by the mirna pathway are crucial for plant development. Genes Dev., 18, 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez-Ramos J.M., de la Paz Sánchez M. (2003) The cell cycle and seed germination. Seed Sci. Res., 13, 113–130. [Google Scholar]
- Xu B. et al. (2008) Arabidopsis genes AS1, AS2, and JAG negatively regulate boundary-specifying genes to promote sepal and petal development. Plant Physiol., 146, 566–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. et al. (2017) Multi-instance metric transfer learning for genome-wide protein function prediction. Sci. Rep., 7. doi: 10.1038/srep41831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M., Lin Y. (2006) Model selection and estimation in regression with grouped variables. J. R. Stat. Soc. Ser. B Stat. Methodol., 68, 49–67. [Google Scholar]
- Zhang L. et al. (2017) Hierarchical multi-label classification using fully associative ensemble learning. Pattern Recogn., 70, 89–103. [Google Scholar]
- Zhou N. et al. (2019) The cafa challenge reports improved protein function prediction and new functional annotations for hundreds of genes through experimental screens. bioRxiv. doi: 10.1101/653105. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.