Abstract
Similar to the global scenario, pneumococcal diseases are a significant health concern in India. Pneumococcal diseases occur frequently among adults and are largely preventable through vaccines. Globally, several guidelines and recommendations are available for pneumococcal vaccination in adults. However, owing to wide variations in the disease burden, regulatory landscape, and health-care system in India, such global guidelines cannot be unconditionally implemented throughout the country. To address these gaps, the Indian Chest Society and National College of Chest Physicians of India jointly conducted an expert meeting in January 2019. The aim of the discussion was to lay down specific evidence-based recommendations on adult pneumococcal vaccination for the country, with a view to further ameliorate the disease burden in the country. This article presents an overview of the closed-door discussion by the expert members on clinical practice guidelines to be followed for adult pneumococcal vaccination in India.
KEY WORDS: Adult vaccination, consensus-based recommendations, elderly, guidelines, India, PCV13, pneumonia, PPSV23
EXECUTIVE SUMMARY
Pneumococcal diseases are a preventable set of diseases caused mainly by Streptococcus pneumoniae (Pneumococcus). The disease can be broadly classified into invasive and noninvasive forms. The incidence rates of pneumococcal disease in India in 2018 were found to be 31.3%, 22.7%, and 13.9% among adults aged ≥60 years, 44–60 years, and 18–44 years, respectively. Apart from the increased burden of disease in the country, antibiotic resistance to strains of S. pneumoniae is also increasing at an alarming rate, which is an obstacle in the effective treatment of the disease. Hence, pneumococcal vaccination has potential as a preventive strategy in the country.
PNEUMOCOCCAL VACCINATION
-
Is the use of pneumococcal vaccines for preventing pneumococcal disease in India justifiable?
- The use of pneumococcal vaccine is worthwhile in India, as it not only reduces the burden of pneumococcal disease but also leads to a positive impact on health-care economics by reducing overall health-care expenditure (3A).
-
What is the dose and route of administration for pneumococcal vaccines?
- Two inactivated pneumococcal vaccines are available and approved for use in the country for preventing pneumococcal disease, viz., conjugated 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23)
- Pneumococcal vaccines are administered as mentioned in Table 1.
-
What are the contraindications and precautions for pneumococcal vaccines?
- PCV13 and PPSV23 are contraindicated if the individual has hypersensitivity to any component of the vaccine
- Both PPSV23 and PCV13 should not be given during acute respiratory illness
- Caution should be exercised in individuals with altered immunocompetence (congenital or acquired splenic dysfunction, HIV [human immunodeficiency virus] infection, malignancy, hematopoietic stem cell transplant, and nephrotic syndrome), including those at higher risk for invasive pneumococcal disease, as such individuals may have reduced antibody responses to immunization with the pneumococcal vaccine
- Appropriate agents should be made available immediately if allergic reactions occur due to the vaccine
- In the case of pregnancy, although not contraindicated, there is no recommendation regarding the use of PCV13. Even the use of PPSV23 during pregnancy is not specifically recommended, due to the lack of adequate safety data[1]
- The use of pneumococcal vaccine in solid-organ transplant recipients is not contraindicated. However, adequate data are lacking for a proper recommendation[2]
- Pneumonia vaccine is not contraindicated in rheumatoid arthritis patients[3]
-
What is the coadministration schedule of pneumococcal vaccines with respect to other recommended vaccines?
-
In adults, PCV13 can be concomitantly administered with:
- Trivalent/quadrivalent inactivated influenza vaccine
- Tetanus–diphtheria vaccine
- Diphtheria–tetanus–acellular pertussis hepatitis B-inactivated polio-Haemophilus influenzae Type B.
- PCV13 and PPSV23 can be administered concomitantly with all other recommended vaccines:
- At the same clinical visit, using separate syringes and at different anatomical sites
- At any later time with no waiting period following the vaccination (except for the quadrivalent meningococcal conjugate vaccine [MCV4]-D).
-
-
Which among PCV13 and PPSV23 should be administered first and what is the immunization schedule of pneumococcal vaccines for the adult population at risk for pneumococcal disease in India?
Adults Aged 19–64 Years
- Pneumococcal vaccination is usually not recommended for healthy adults under the age of 65 years (UPP)
- In immunocompetent patients with chronic conditions such as chronic heart disease, chronic liver disease, poorly controlled diabetes mellitus, chronic lung disease, and in current smokers and those with alcohol abuse, a single dose of PCV13 followed by PPSV23 ≥8 weeks later is recommended (UPP) [Box 1]
- In adults with a history of invasive pneumococcal disease, those with cochlear implants, cerebrospinal fluid (CSF) leak, or impaired splenic function (anatomic asplenia or hyposplenism, sickle cell disease or other hemoglobinopathy or functional asplenia or hyposplenism), a single dose of PCV13 followed by PPSV23 ≥8 weeks later is recommended (2A) [Box 1]
- In all immunocompromised individuals, such as those with HIV infection, iatrogenic immunosuppression, chronic kidney disease, hematologic malignancy, other solid tumor malignancies with or without metastasis, hematopoietic stem cell transplantation and solid-organ transplantation, administration of a single dose of PCV13 followed by PPSV23 ≥ 8 weeks later is recommended (2A) [Box 1].
Adults aged 65 years or older
- Vaccination with PPSV23 in all adults above 65 years of age is recommended because of the overall higher incidence of invasive pneumococcal disease in this age group (2A)
- Vaccination with PCV13 first, followed by PPSV23, is usually recommended for individuals who have immunocompromising conditions, functional or anatomic asplenia, cochlear implant, CSF leak or history of invasive pneumococcal disease (2A). However, in this group of patients, the decision regarding giving PCV13 before PPSV23 can be discussed on a case-to-case basis between the physician and the patient
- For adults with chronic conditions such as chronic heart disease, chronic liver disease, poorly controlled diabetes mellitus, chronic lung disease, and in current smokers and those with alcohol abuse, the decision to administer a dose of PCV13 preceding PPSV23 should be taken jointly by the physician and the patient, on a case-to-case basis (UPP)
- Repeat vaccination with PPSV23 causes hyporesponsiveness, and hence revaccination of an individual with PPSV23 must be solely based on clinical judgment.
-
What are the recommendations for pneumococcal vaccination for travelers, Hajj pilgrims, and individuals attending mass gatherings?
- The working group for the prevention of pneumococcal disease in Hajj pilgrims has recommended administration of PCV13 4 weeks before travel to Hajj, while PPSV23 can be administered after return from the Hajj pilgrimage[4]
- The data regarding other mass gatherings such as Kumbh Mela are lacking; however, we recommend extrapolating the data from Hajj pilgrims to vaccinate individuals attending large mass gatherings, as mentioned in the above statement.
-
Why should PCV13 be administered before PPSV23?
- PCV13 is recommended first, as it amplifies the antipneumococcal response to subsequent administration of PPSV23 for many common vaccine serotypes. In line with the evidence, there is unanimous agreement on the benefits of PCV13 over a polysaccharide vaccine, as the former has a T-cell-dependent response and causes induction of immunological memory and long-lasting immunity (2A)
-
What is the pneumococcal vaccination recommendation if an individual has received PPSV23 first?
- For individuals who have already received PPSV23 initially, and where PCV13 is indicated, a single dose of PCV13 should be administered ≥1 year following the receipt of PPSV23 (UPP)
- Further revaccination with PPSV23 should be done as per the usual regime, maintaining a gap of 5–7 years between the two doses of PPSV23
- Repeat vaccination with PPSV23 causes hyporesponsiveness and hence revaccination of an individual with PPSV23 must be solely based on clinical judgment
-
What is the recommendation on coadministration of the two pneumococcal vaccines?X
- The experts recommended that a gap of at least 8 weeks be maintained between the two vaccines and that they should not be given simultaneously (UPP)
Table 1.
Dose and route of administration for pneumococcal vaccines
| Pneumococcal vaccine | Route and dose |
|---|---|
| PCV13 | Each 0.5-milliliter (mL) suspension of intramuscular injection is supplied in a single-dose prefilled syringe |
| In adults ≥18 years, the vaccine should be administered as a single dose | |
| The preferred sites of administration are the anterolateral aspect of the thigh in infants or the deltoid muscle of the upper arm in toddlers, children, and adults | |
| The vaccine should not be injected in the gluteal area or areas where there may be a major nerve trunk and/or blood vessel | |
| PPSV23 | A single 0.5-mL dose of PPSV23 is administered intramuscularly or subcutaneously only |
PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine
Box 1.
Indian guidelines on pneumococcal vaccination with PCV13 and PPSV23
| The Indian guidelines are different from the ACIP guidelines, in terms of recommendation regarding vaccination with PCV13 vaccine. The ACIP guidelines are based on the fact that in the United States, PCV13 is being administered to all children since the year 2010, and therefore, there is herd immunity, which prevents adults from being affected by the 13 serotypes covered in PCV13 |
| In India, pneumococcal vaccination in the pediatric immunization schedule has only been incorporated recently. Thus, the concept of herd immunity is not applicable. Hence, in the current guidelines, for individuals aged 19-64 years and with health conditions as stated above, we recommend vaccination with PCV13 followed by PPSV23 |
ACIP: Advisory Committee on Immunization Practices
INTRODUCTION
Pneumonia and influenza are key clinical conditions encountered by clinicians and pulmonologists in the country. Several evidence-based global guidelines and recommendations from numerous bodies are available for pneumococcal and influenza vaccination for the diverse adult population. However, due to differences in the disease burden, regulatory landscape, health-care infrastructure, and functioning in India, these global guidelines cannot be implemented unconditionally in the region. Hence, in the absence of country-specific structured clinical recommendations on adult respiratory vaccination, this document is a first-of-its-kind multidisciplinary effort to lay down specific evidence-based recommendations on adult pneumococcal and influenza vaccination for the country. It further aims (1) to guide practicing clinicians and health-care professionals in the country from various specialties to make informed decisions about adult respiratory vaccination and (2) to facilitate consistent adult respiratory vaccinations in the region and further help reduce the disease burden in the country.
METHODOLOGY
The pioneering attempt to develop easily implementable clinical recommendations for the prevention of pneumonia and influenza with vaccination in India was undertaken as a joint exercise by the Indian Chest Society and National College of Chest Physicians of India by a panel of experts at a closed-door meeting held in Kolkata on 13 January 2019.
There were 24 expert members with four moderators and four rapporteurs. The expert panel members included an oncologist, pulmonologists, a nephrologist, a diabetologist, and an infectious disease specialist. These experts were from various parts of India. The experts critically analyzed existing literature, including randomized clinical trials, systematic reviews, and meta-analyses, as well as key global and Indian guidelines and recommendations on pneumococcal and influenza vaccine. Evidence to frame specific questions was obtained through a literature search of MEDLINE (via PubMed) and Cochrane-indexed databases on pneumococcal and influenza vaccination published between January 1995 and December 2018. The keywords for the literature search were as follows: definitions, epidemiology, India, pneumonia, prevention, mortality, vaccine, impact, elderly, serotype, safety, immunogenicity, comorbid conditions, community-acquired pneumonia, mass gatherings, antibiotics, guidelines, and recommendations. These questions were formulated following discussions on several factors unique to the Indian context.
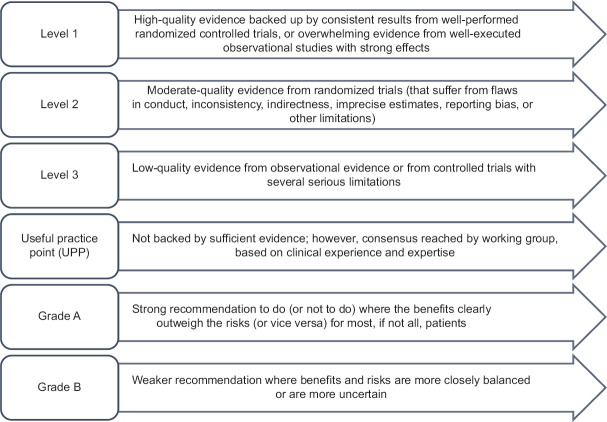
Literature review and discussions for each disease were coordinated by group chairs and were well documented by rapporteurs. Discussions regarding grading of evidence and recommendations were held independently in four parallel-group sessions and, thereafter, in a joint session including all the experts. A consensus-based approach was used to arrive at the final decision on clinical recommendations in the joint session. The modified Grading of Recommendations, Assessment, Development and Evaluation (GRADE) system was utilized for categorizing the level of evidence as 1, 2, 3, or usual practice point (UPP) [Figure 1].[5]
Figure 1.
Classification of level of evidence and grading of clinical recommendations
The strength of recommendation was graded as A or B, based on the level of evidence. Grade A should be interpreted as “recommended,” while Grade B as “suggested.” All aspects related to practicality, implementation, costs, and clinical feasibility in the region at various health-care levels were duly considered while formulating the clinical recommendations.[6]
EPIDEMIOLOGY
01. What is the burden of pneumococcal disease in India?
Pneumococcal diseases are a group of preventable diseases caused by the pathogen Streptococcus pneumoniae (Pneumococcus) and are preventable.[7,8,9] They are broadly categorized into invasive and noninvasive forms. The invasive form leads to bacteremia, sepsis, and meningitis. The noninvasive form, or the less severe variant, causes sinusitis, acute otitis media, and community-acquired pneumonia (CAP). The noninvasive form of pneumonia can change into the severe invasive variant when accompanied by bacteremia.[7,10,11] According to the 2016 Global Burden of Disease estimates, pneumonia along with bronchiolitis (low respiratory tract infection) was the leading cause of mortality and morbidity globally.[12] Furthermore, according to the 2008 World Health Organization (WHO) data, Asia had the highest burden of pneumonia, with the Indian subcontinent contributing to a major share of the disease burden.[13]
Various studies evaluating the bacteriological profiles of invasive pneumococcal bacteria from across various parts of India revealed the predominance of S. pneumoniae in the country. Meningitis and pneumonia were the most common clinical conditions found in the adult population and were associated with a high fatality rate despite treatment in hospital settings.[14,15,16,17,18,19]
A recent laboratory-based surveillance study on invasive pneumococcal disease (IPD) in the Indian adult population revealed that S. pneumoniae-positive cultures were characterized by serotype prevalence and antimicrobial resistance patterns. The study is consistent with findings from previous studies in the country, which showed pneumonia and meningitis to be the most common clinical conditions, accounting for 39% and 24.3%, respectively, of the total of IPD cases.[20]
Prevention of pneumonia through vaccination
02. What are the various vaccines available for preventing pneumococcal disease in India?
At present, two inactive vaccines are recommended and clinically used in India: PPSV23 and PCV13. The characteristics of both vaccines are summarized in Table 2.[4,21,22,23,24]
Table 2.
Detailed characteristics and serotype coverage of pneumococcal vaccines used in India
| Characteristic | PPSV23 | PCV13 |
|---|---|---|
| Type of vaccine | Unconjugated capsular polysaccharide antigens | Capsular polysaccharides conjugated with a protein carrier |
| Serotype coverage | 1, 2*, 3, 4, 5, 6B, 7F, 8*, 9N*, 9V, 10A*, 11A*, 12F*, 14, 15B*, 17F*, 18C, 19A, 19F, 20*, 22F*, 23F, and 33F* | 1, 3, 4, 5, 6A**, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F |
| Serotypes not covered | 9A, 9L, 12B, 18, 38, 10, 15, 22, 6A, 15A, 21, 35B, 9, 35F, 16F, 23A, 34, 37, 7, 11, 15, 23, 6C | 8, 9A, 12F, 9N, 9L, 12B, 20, 18, 22F, 33F, 38, 10, 15, 22, 11A, 15A, 21, 35B, 9, 35F, 10A, 16F, 23A, 34, 37, 7, 11, 23, 6C |
| Mechanism of action | B-cell activation | T-cell-dependent immune response |
| T-cell-independent immune response | Combination of polysaccharide-protein antigen conjugate is captured by B cells and other APC | |
| Weak and short-lasting IgM immune response | ||
| Response declines in 3-5 years | Larger duration and boosting effect at revaccination |
*Strains unique to PPSV23, **Strain unique to PCV13. APC: Antigen-presenting cell, Ig: Immunoglobulin, PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine
03. What is the dose and route of administration for PPSV23 and PCV13?
Pneumococcal vaccines have a pivotal role in preventing pneumococcal disease; the dosing and administration of these vaccines are discussed in Table 3.[25,26,27,28]
Table 3.
Administration and dosage details for pneumococcal vaccines
| Pneumococcal vaccine | Route | Dose |
|---|---|---|
| PCV13[23,24,25] | PCV13 is administered by intramuscular injection | Each 0.5-mL suspension of intramuscular injection is supplied in a single-dose prefilled syringe |
| The preferred sites of administration are the anterolateral aspect of the thigh in infants or the deltoid muscle of the upper arm in toddlers, children, and adults | In children aged 6 weeks through 5 years, a four-dose immunization series consisting of a 0.5-mL injection is administered at 2, 4, 6, and 12-15 months of age | |
| The vaccine should not be injected in the gluteal area or areas where there may be a major nerve trunk and/or blood vessel | Children aged six through 17 years of age should be administered a single dose. Similarly, in adults 18 years and older, the vaccine should be administered as a single dose | |
| PPSV23[25,26] | PPSV23 is given either by intramuscular or subcutaneous injection only | A single 0.5-mL dose of PPSV23 is administered intramuscularly or subcutaneously only |
PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine
Recommendation
The experts recommended the dose and route of administration of the two pneumococcal vaccines in keeping with the approved prescribing information [Table 3].[25,26,27,28]
04. Is the use of pneumococcal vaccines for preventing pneumococcal disease in India justifiable?
Pneumococcal vaccination offers several benefits, ranging from reduction of disease burden and mortality to a positive economic impact. A population-based study conducted over a 2-year period revealed CAP patients previously vaccinated with PPSV23 to have a 40% lower rate of mortality or intensive care unit admission compared with nonvaccinated patients.[29] Pneumococcal vaccination exerts a potentially beneficial economic impact, as it leads to reduced usage of antibiotics and decreased overall health-care expenditure.[30] In a retrospective study conducted among an elderly population with chronic obstructive pulmonary disease (COPD), elderly vaccinated patients were found to have reduced hospitalization, mortality, and direct medical care costs compared to unvaccinated patients. Pneumococcal vaccines led to a 43% reduction in hospitalization due to pneumonia.[31]
Furthermore, PCV13 also has the potential to slow the rate of antibiotic resistance. The vaccine exerts this effect by preventing the disease in the first place, slowing the spread of resistant pneumococcal serotypes (19A) and thereby eliminating the need for antibiotics.[22]
A reduction in the rate of antibiotic-resistant infection among older adults was also observed in a laboratory-based study. The incidence rate of pneumococcal diseases caused by penicillin-resistant strains reduced to 8.4 from 16.4 cases per 100,000. Furthermore, the rates of resistant pneumococcal disease caused by vaccine serotypes reduced by 87%.[32]
PCV13 immunization was also found to be relatively more cost-effective for immunocompromised individuals versus previously recommended vaccinations.[33] This benefit is especially noteworthy for developing nations such as India.
Recommendation
The use of pneumococcal vaccine is worthwhile in India, as it not only reduces the burden of pneumococcal disease but also leads to a positive impact on health-care economics by reducing overall health-care expenditure.
05. What are the contraindications and precautions for pneumococcal vaccines?
Although pneumococcal vaccines are indispensable elements in the preventive strategy for pneumococcal diseases, certain contraindications and precautions govern their administration [Table 4].[24,25,27,34,35,36,37,38,39]
Table 4.
Contraindications and precautions for pneumococcal vaccines
| Contraindication(s) | Precaution(s) |
|---|---|
| PCV13[24,25,34,35,36,37,38,39] | |
| Hypersensitivity to any component of the vaccine, including diphtheria toxoid | Appropriate agents should be made available immediately to manage allergic reactions that occur with the vaccine |
| Individuals with altered immunocompetence, including those at higher risk for IPD*, may have reduced antibody responses to immunization with the pneumococcal vaccine | |
| PPSV2327 | |
| The vaccine should not be administered to individuals with a history of anaphylactic/anaphylactoid or severe allergic reaction to any component of PPSV23 | Defer vaccination with PPSV23 in individuals with moderate or severe acute illness |
| Use caution and appropriate care for individuals with severely compromised cardiovascular and/or pulmonary function in whom a systemic reaction would pose a significant risk, individuals with altered immunocompetence and chronic cerebrospinal fluid leakage | |
| This vaccine does not replace penicillin (other antibiotics) prophylaxis against pneumococcal infection. Hence, in patients who require penicillin (or other antibiotics) prophylaxis against pneumococcal infection, such prophylaxis should not be discontinued after vaccination with PPSV23 | |
*Individuals with congenital or acquired splenic dysfunction, HIV infection, malignancy, hematopoietic stem cell transplant, nephrotic syndrome. IPD: Invasive pneumococcal disease, PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine, HIV: Human immunodeficiency virus
Recommendations
PCV13 and PPSV23 are contraindicated if an individual has hypersensitivity to any component of the vaccine
Both PPSV23 and PCV13 should not be given during acute respiratory illness. Caution should be exercised in individuals with altered immunocompetence, CSF leakage, severely compromised cardiovascular and/or pulmonary function receiving the pneumococcal vaccine, since in such cases, the pneumococcal vaccines may not be effective in preventing pneumococcal infection
Appropriate agents should be made available immediately if allergic reactions occur due to the vaccines.
06. What is the coadministration schedule of pneumococcal vaccines with respect to other recommended vaccines?
Evidence indicates that coadministration or concomitant administration of PCV13 with other vaccines (trivalent inactivated influenza vaccine [MF59-aTIV], tetanus–diphtheria vaccine [Td], diphtheria–tetanus–acellular pertussis hepatitis B-inactivated polio-Haemophilus influenzae type B [DTaP-HBV-IPV/Hib] vaccine) in the adult as well as pediatric populations is associated with functional opsonophagocytic activity (OPA) responses for all serotypes, induces sufficient immunity without significant interference, and has a good safety profile.[40,41,42,43,44,45,46,47] All other recommended vaccines can be coadministered with PPSV23 and PCV13 at the same visit using different syringes or at any later date with no waiting period following the vaccination, except the quadrivalent meningococcal conjugate vaccine (MCV4)-D.
For the pediatric population (aged ≥2 years) at high risk for invasive meningococcal disease with functional or anatomic asplenia, two doses of MCV4-D are administered 2 months apart and ≥4 weeks after completion of the PCV13 vaccine series.[48,49,50] Furthermore, PPSV23 and PCV13 are inactivated vaccines.
Recommendations
-
Experts recommended that in adults, PCV13 can be concomitantly administered with:
- Trivalent/quadrivalent inactivated influenza vaccine
- Tetanus–diphtheria vaccine
- Diphtheria–tetanus–acellular pertussis hepatitis B-inactivated polio-Haemophilus influenzae type B.
-
PCV13 and PPSV23 can be administered concomitantly with all other recommended vaccines:
- At the same clinical visit, using separate syringes and at different anatomical sites
- At any later time with no waiting period following the vaccination (except for the quadrivalent meningococcal conjugate vaccine [MCV4]-D).
PPSV23 and PCV13 should not be administered simultaneously at the same visit, and a time gap of at least 8 weeks should be maintained between the administration of the two vaccines.
07. What is the immunogenicity and safety profile of PCV13 and PPSV23?
PPSV23 has been available for clinical use for more than three decades now and has an established, protective effect against all-cause pneumonia in healthy adults. Evidence generated over the years has shown that PPSV23's effectiveness ranges from 50% to 80% against invasive pneumococcal forms among adults with comorbid conditions. However, the vaccine has not shown benefit in reducing the risk of CAP associated with seasonal influenza in the adult population.[51] The major limitations associated with the vaccine are hyporesponsiveness on repeated administration; uncertain effectiveness for prevention against nonbacteremic pneumococcal pneumonia; low immunological response in adults who are immunocompromised and/or with underlying comorbid conditions; and a decrease in clinical protection with age in the adult population.[52,53,54,55,56]
As PPSV23 has a T-cell-independent immune response, it does not provide long-lasting immunity to adult patients and, further, has no anamnestic effect upon revaccination. Immune response to the vaccine is also found to be low in immunocompromised adults and adults with various underlying comorbid conditions.[53] A meta-analysis by Huss et al. found little evidence in favor of the effectiveness of PPSV23 against pneumonia in elderly patients or adults with chronic illness.[54] In view of such studies, in general, PCV13 is usually preferred over PPSV23 in the older adult population. The immunogenicity and safety profiles of PPSV23 and PCV13 are summarized in Table 5.[55,56,57,58,59,60,61,62]
Table 5.
Immunogenicity and safety evidence summarization for pneumococcal vaccines
| Publication (year) | Study design and analysis | Key observations |
|---|---|---|
| McLaughlin et al. (2018)[55] | Real-world observational analysis for PCV13 in CAP patients aged ≥65 years | Effectiveness of PCV13 proved against vaccine-type CAP in older adults |
| Prato et al. (2018)[56] | Prospective, 2-year cohort study for PCV13 in CAP patients aged ≥65 years | PCV13 effectiveness: 42.3% against VT-CAP in the age group with higher vaccine uptake |
| PCV13 was promisingly effective against all confirmed CAP forms | ||
| Solanki et al. (2017)[57] | Open-label, single-arm study for PCV13 in individuals aged 50-65 years (post hoc comparator studies enrolled PPSV23-naïve adults aged 50-64 years) | PCV13 showed a robust immune response in the Indian population |
| Safe and well-tolerated in Indian adults aged 50-65 year | ||
| Bonten et al. (2015)[58] | Randomized controlled trial PCV13 versus placebo (3 years) in adults aged >65 years | PCV13 showed a reduction of |
| 45.6% in VT-pneumococcal CAP | ||
| 45% in VT-nonbacteremic pneumococcal pneumonia | ||
| 75% in VT-IPD | ||
| Serious AE and mortality profiles were comparable between two groups | ||
| Greenberg et al. (2014)[59] | Population: Vaccine-naïve adults aged 60-64 years | Initial PCV13 elevated antipneumococcal response to subsequent PPSV23 administration for various common serotypes |
| Repeat vaccination at 12 months following initial vaccination (3 arms) | Initial PPSV23 decreased the OPA response to subsequent PCV13 for all serotypes. | |
| PCV13 → PCV13 | ||
| PCV13 → PPSV23 | ||
| PPSV23 → PPSV23 | ||
| Jackson et al. (a) (2013)[60] | Population: Elderly patients aged >70 years; vaccinated with PPSV23 at least 5 years earlier | Group A with PPSV23 administered first had significantly lower OPA titers for the majority of common serotypes (diminished immune response) PCV13 should be administered first |
| Sequential vaccination separated by 1 year (study duration: 13 months) | ||
| PPSV23 → PCV13 | ||
| PCV13 → PCV13 | ||
| Jackson et al. (b) (2013)[61] | Single dose of PCV13 versus PPSV23 in adults aged 60-64 years, vaccine-naïve (study duration: 12 months) | PCV13 had significantly higher 1-month postvaccination OPA titers in 8 of 12 common serotypes versus PPSV23 |
| Immune response to PCV13 was higher in adults aged 50-59 years versus adults aged 60-64 years | ||
| Jackson et al. (c) (2013)[62] | Extension study (5 years) in vaccine-naïve adults aged 60-64 years | Repeat PCV13 had OPA titers significantly higher for 7 of 13 serotypes versus after the initial vaccination. (Immune memory and recall of antipneumococcal responses) |
| Repeat vaccination 4 years following initial vaccination | Repeat PPSV23 had OPA titers significantly lower for 9 of 13 serotypes versus after the initial vaccination | |
| PCV13 → PCV13 | ||
| PCV13 → PPSV23 | ||
| PPSV23 → PPSV23 |
AE: Adverse event, CAP: Community-acquired pneumonia, OPA: Opsonophagocytic activity, PPSV23: 23-valent pneumococcal polysaccharide vaccine, PCV13: 13-valent pneumococcal conjugate vaccine, VT: Vaccine type
08. Which among PCV13 and PPSV23 should be administered first and what is the immunization schedule of pneumococcal vaccines for the adult population at risk for pneumococcal disease in India?
A single dose of PCV13 should be given first, followed by a single dose of PPSV23. In older adults (>65 years), a single dose of PCV13 is administered first, followed by a single dose of PPSV23 a year later. According to established global guidelines, adults aged ≥65 years with immunocompromising conditions, functional or anatomic asplenia, CSF leaks, or cochlear implants are recommended a single dose of PCV13 first, followed by a single dose of PPSV23 at ≥8 weeks.[63,64,65,66] As enumerated in the immunogenicity profile of the pneumococcal vaccine Table 5,[55,56,57,58,59,60,61,62] evidence shows that the initial single dose of PCV13 amplifies the antipneumococcal response to subsequent administration of PPSV23 for many common vaccine serotypes.
Contrary to this, the administration of PPSV23 before PCV13 results in a diminished response to subsequent administration of PCV13 for all serotypes. These findings have helped in providing a reasonable rationale for the recommendation for administering PCV13 first followed by PPSV23.[67,68]
Recommendations
Adults aged 19–64 years
Pneumococcal vaccination is usually not recommended for healthy adults under the age of 65 years
In immunocompetent patients with chronic conditions such as chronic heart disease, chronic liver disease, poorly controlled diabetes mellitus, chronic lung disease, and in current smokers and those with alcohol abuse, a single dose of PCV13 followed by PPSV23 ≥8 weeks later is recommended
In adults with a history of invasive pneumococcal disease, those with cochlear implants, CSF leak, or impaired splenic function (anatomic asplenia or hyposplenism, sickle cell disease or other hemoglobinopathy or functional asplenia or hyposplenism), a single dose of PCV13 followed by PPSV23 ≥8 weeks later is recommended
In all immunocompromised individuals, such as those with HIV infection, iatrogenic immunosuppression, chronic kidney disease, hematologic malignancy, other solid tumor malignancies with or without metastasis, hematopoietic stem cell transplantation, and solid-organ transplantation, administering a single dose of PCV13 followed by PPSV23 ≥8 weeks later is recommended [Box 1].
Adults aged 65 years or older
Vaccination with PPSV23 in all adults above 65 years of age is recommended because of the overall higher incidence of invasive pneumococcal disease in this age group
Vaccination with PCV13 first, followed by PPSV23, is usually recommended for individuals who have immunocompromising conditions, functional or anatomic asplenia, cochlear implant, CSF leak, or history of invasive pneumococcal disease. In this group of patients, the decision regarding giving PCV13 before PPSV23 can be discussed on a case-to-case basis between the physician and the patient
For adults with chronic conditions such as chronic heart disease, chronic liver disease, poorly controlled diabetes mellitus, chronic lung disease, and in current smokers and those with alcohol abuse, the decision to administer a dose of PCV13 preceding PPSV23 should be taken jointly by the physician and the patient, on a case-to-case basis
The experts acknowledged that repeated vaccination with PPSV23 causes hyporesponsiveness and hence revaccination with PPSV23 must be based only on clinical judgment.
09. What are the recommendations for pneumococcal vaccination for travelers, Hajj pilgrims, and individuals attending mass gatherings?
The working group for the prevention of pneumococcal disease in Hajj pilgrims has recommended administration of PCV13 4 weeks before travel to Hajj, while PPSV23 can be administered after return from the Hajj pilgrimage.[4]
The data regarding other mass gatherings such as Kumbh Mela are lacking; however, we recommend extrapolating the data from Hajj pilgrims to vaccinate individuals attending large mass gatherings, as mentioned in the above statement.
10. Why should PCV13 be administered before PPSV23?
PCV13 should always be administered first.[69,70,71,72,73] Administration of PPSV23 8 weeks after PCV13 provides protection against an additional 11 pneumococcal serotypes covered by PPSV23 that are not covered by PCV13.[69] However, evidence reveals that repeated administration of PPSV23 causes hyporesponsiveness: The response to revaccination will not reach the levels achieved with primary vaccination.[71,72,73]
Although both vaccines elicit a B-cell-mediated immune response, only PCV13 has a T-cell-dependent immune response, which is essential for the maturation of the B-cell response and development of immunological memory.[70,71,72,73,74]
Recommendations
The initial single dose of PCV13 is recommended first, as it amplifies the antipneumococcal response to subsequent administration of PPSV23 for many common vaccine serotypes
Furthermore, in line with the evidence, unanimous agreement was obtained on the benefits of PCV13 over a polysaccharide vaccine, as the former is associated with a T-cell-dependent response and causes induction of immunological memory and long-lasting immunity.
11. What is the pneumococcal vaccination recommendation if an individual has received PPSV23 first?
PCV13 should still be administered to individuals who have already received PPSV23.[67] The Advisory Committee on Immunization Practices (ACIP) recommends that all adults aged ≥ 65 years who have already received PPSV23 receive a dose of PCV13 ≥ 1 year after receiving PPSV23, wherever indicated.[67] Furthermore, only PCV13 produces a T-cell-dependent response, which is essential for the development of immunological memory and therefore, primes the immune system for natural exposure.[69,75,76]
Recommendation
If individuals (aged ≥65 years) have already received PPSV23 initially, and wherever it is indicated to give PCV13, a single dose of PCV13 should be administered ≥1 year after the receipt of PPSV23.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank BioQuest Solutions for their contribution in research, and data analysis, and for providing writing assistance.
REFERENCES
- 1.Centers for Disease Control and Prevention. Guidelines for Vaccinating Pregnant Women. [[Last accessed on 2019 Dec 11]]. Available from: https://wwwcdcgov/vaccines/pregnancy/hcp-toolkit/guidelineshtml .
- 2.Dendle C, Stuart RL, Mulley WR, Holdsworth SR. Pneumococcal vaccination in adult solid organ transplant recipients: A review of current evidence. Vaccine. 2018;36:6253–61. doi: 10.1016/j.vaccine.2018.08.069. [DOI] [PubMed] [Google Scholar]
- 3.Meroni PL, Zavaglia D, Girmenia C. Vaccinations in adults with rheumatoid arthritis in an era of new disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2018;36:317–28. [PubMed] [Google Scholar]
- 4.Mathai D, Shamsuzzaman AK, Feroz AA, Virani AR, Hasan A, Ravi Kumar KL, et al. Consensus recommendation for India and Bangladesh for the use of pneumococcal vaccine in mass gatherings with special reference to Hajj Pilgrims. J Glob Infect Dis. 2016;8:129–38. doi: 10.4103/0974-777X.193749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt GH, Rennie D, Meade MO, Cook DJ. Users' Guide to the Medical Literature: A Manual for Evidence-Based Clinical Practice. 2nd ed. New York: McGraw Hill; 2008. [Google Scholar]
- 7.Ludwig E, Bonanni P, Rohde G, Sayiner A, Torres A. The remaining challenges of pneumococcal disease in adults. Eur Respir Rev. 2012;21:57–65. doi: 10.1183/09059180.00008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pneumococcal disease Immunization Vaccines and Biologicals. WHO. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwwhoint/immunization/diseases/pneumococcal/en/
- 9.Randle E, Ninis N, Inwald D. Invasive pneumococcal disease. Arch Dis Child Educ Pract Ed. 2011;96:183–90. doi: 10.1136/adc.2010.191718. [DOI] [PubMed] [Google Scholar]
- 10.Drijkoningen JJ, Rohde GG. Pneumococcal infection in adults: Burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 11.Dockrell DH, Whyte MKB, Mitchell TJ. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest. 2012;142:482–91. doi: 10.1378/chest.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Burden of Disease (GBD) Group. GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–10. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghoshal AG. Burden of pneumonia in the community. JAPI. 2016;64:8–11. [Google Scholar]
- 14.Muley VA, Ghadage DP, Yadav GE, Bhore AV. Study of invasive pneumococcal infection in adults with reference to penicillin resistance. J Lab Physicians. 2017;9:31–5. doi: 10.4103/0974-2727.187918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomas K, Mukkai Kesavan L, Veeraraghavan B, Jasmine S, Jude J, Shubankar M, et al. Invasive pneumococcal disease associated with high case fatality in India. J Clin Epidemiol. 2013;66:36–43. doi: 10.1016/j.jclinepi.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Shah BA, Singh G, Naik MA, Dhobi GN. Bacteriological and clinical profile of Community acquired pneumonia in hospitalized patients. Lung India. 2010;27:54–7. doi: 10.4103/0970-2113.63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberoi A, Aggarwal A. Bacteriological profile, serology and antibiotic sensitivity pattern of micro-organisms from community acquired pneumonia. JK Sci. 2006;8:79–82. [Google Scholar]
- 18.Bansal S, Kashyap S, Pal LS, Goel A. Clinical and bacteriological profile of community acquired pneumonia in Shimla, Himachal Pradesh. Indian J Chest Dis Allied Sci. 2004;46:17–22. [PubMed] [Google Scholar]
- 19.Para RA, Fomda BA, Jan RA, Shah S, Koul PA. Microbial etiology in hospitalized North Indian adults with community-acquired pneumonia. Lung India. 2018;35:108–15. doi: 10.4103/lungindia.lungindia_288_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayaraman R, Varghese R, Kumar JL, Neeravi A, Shanmugasundaram D, Ralph R, et al. Invasive pneumococcal disease in Indian adults: 11 years' experience. J Microbiol Immunol Infect. 2019;52:736–42. doi: 10.1016/j.jmii.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Hayward S, Thompson LA, McEachern A. Is 13-valent pneumococcal conjugate vaccine (PCV13) Combined With 23-valent pneumococcal polysaccharide vaccine (PPSV23) superior to PPSV23 alone for reducing incidence or severity of pneumonia in older adults.A Clin-IQ? J Patient Cent Res Rev. 2016;3:111–5. doi: 10.17294/2330-0698.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cafiero-Fonseca ET, Stawasz A, Johnson ST, Sato R, Bloom DE. The full benefits of adult pneumococcal vaccination: A systematic review. PLoS One. 2017;12:e0186903. doi: 10.1371/journal.pone.0186903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Pneumococcal Vaccines. [[Last accessed on 2019 Dec 09]]. Available from: http://wwwwhoint/vaccine_safety/initiative/tools/Pneumococcal_Vaccine_rates_information_sheetpdf .
- 24.Song JY, Nahm MH, Moseley MA. Clinical implications of pneumococcal serotypes: Invasive disease potential, clinical presentations, and antibiotic resistance. J Korean Med Sci. 2013;28:4–15. doi: 10.3346/jkms.2013.28.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevenar 13® Prescribing Information. 2017. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwfdagov/media/107657/download .
- 26.Prevenar 13® European Summary of Product Characteristics. 2014. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwemaeuropaeu/en/documents/product-information/prevenar-13-epar-product-information_enpdf .
- 27.Swaminathan S, Mathai D. Protocols for pneumococcal vaccination understanding the term. JAPI. 2016;64:52–62. [Google Scholar]
- 28.Pneumovax23. Prescribing Information. 2015. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwmerckcom/product/usa/pi_circulars/p/pneumovax_23/pneum ovax_pipdf .
- 29.Johnstone J, Marrie TJ, Eurich DT, Majumdar SR. Effect of pneumococcal vaccination in hospitalized adults with community-acquired pneumonia. Arch Intern Med. 2007;167:1938–43. doi: 10.1001/archinte.167.18.1938. [DOI] [PubMed] [Google Scholar]
- 30.Bärnighausen T, Bloom DE, Cafiero-Fonseca ET, O'Brien JC. Valuing vaccination. Proc Natl Acad Sci USA. 2014;111:12313–9. doi: 10.1073/pnas.1400475111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Arch Intern Med. 1999;159:2437–42. doi: 10.1001/archinte.159.20.2437. [DOI] [PubMed] [Google Scholar]
- 32.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumonia. N Engl J Med. 2006;354:1455–63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 33.Smith KJ, Nowalk MP, Raymund M, Zimmerman RK. Cost-effectiveness of pneumococcal conjugate vaccination in immunocompromised adults. Vaccine. 2013;31:3950–6. doi: 10.1016/j.vaccine.2013.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention. Pink Book. 2014. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwcdcgov/vaccines/pubs/pinkbook/downloads/pneumopdf .
- 35.Immunization Action Coalition. Standing Orders for Administering Pneumococcal Vaccines (PCV13 and PPSV23) to Adults. 2018. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwimmunizeorg/catgd/p3075pdf .
- 36.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices Contraindications and Precautions. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwcdcgov/vaccines/hcp/acip-recs/general-recs/contraindicationshtml#ref-49 .
- 37.Centers for Disease Control and Prevention PCV 13 VIS. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwcdcgov/vaccines/hcp/vis/vis-statements/pcv13html .
- 38.Nilsson L, Brockow K, Alm J, Cardona V, Caubet JC, Gomes E, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28:628–40. doi: 10.1111/pai.12762. [DOI] [PubMed] [Google Scholar]
- 39.McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868–78. doi: 10.1016/j.jaci.2015.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song JY, Cheong HJ, Noh JY, Choi MJ, Yoon JG, Lee SN, et al. Immunogenicity and safety of a tetanus diphtheria vaccine and a 13-valent pneumococcal conjugate vaccine after concomitant vaccination in≥50-year-old adults. BMC Infect Dis. 2018;18:628. doi: 10.1186/s12879-018-3479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song JY, Cheong HJ, Hyun HJ, Seo YB, Lee J, Wie SH, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine and an MF59-adjuvanted influenza vaccine after concomitant vaccination in P60-year-old adults. Vaccine. 2017;35:313–20. doi: 10.1016/j.vaccine.2016.11.047. [DOI] [PubMed] [Google Scholar]
- 42.Esposito S, Tansey S, Thompson A, Razmpour A, Liang J, Jones TR, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010;17:1017–26. doi: 10.1128/CVI.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frenck RW, Jr, Gurtman A, Rubino J, Smith W, van Cleeff M, Jayawardene D, et al. Randomized, controlled trial of a 13-valent pneumococcal conjugate vaccine administered concomitantly with an influenza vaccine in healthy adults. Clin Vaccine Immunol. 2012;19:1296–303. doi: 10.1128/CVI.00176-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwarz TF, Flamaing J, Rümke HC, Penzes J, Juergens C, Wenz A, et al. A randomized, double-blind trial to evaluate immunogenicity and safety of 13-valent pneumococcal conjugate vaccine given concomitantly with trivalent influenza vaccine in adults aged≥65 years. Vaccine. 2011;29:5195–2. doi: 10.1016/j.vaccine.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 45.DeStefano F, Goodman RA, Noble GR, McClary GD, Smith SJ, Broome CV, et al. Simultaneous administration of influenza and pneumococcal vaccines. JAMA. 1982;247:2551–4. [Google Scholar]
- 46.Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM VSD Rapid Cycle Analysis Influenza Working Group. Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine. 2012;30:2024–31. doi: 10.1016/j.vaccine.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 47.Leroy Z, Broder K, Menschik D, Shimabukuro T, Martin D. Febrile seizures after 2010-2011 influenza vaccine in young children, United States: A vaccine safety signal from the vaccine adverse event reporting system. Vaccine. 2012;30:2020–3. doi: 10.1016/j.vaccine.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. Timing and Spacing of Immunobiologics. 2018. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwcdcgov/vaccines/hcp/acip-recs/general-recs/timinghtml .
- 49.Centers for Disease Control and Prevention (CDC) Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391–2. [PubMed] [Google Scholar]
- 50.MenACWY-CRM (Menveo®) & MenACWY-D (Menactra™) Meningococcal Conjugate Vaccines Factsheet. 2014. [[Last accessed on 2019 Dec 09]]. Available from: http://publichealthlacountygov/acd/docs/MeningococcalVaccinespdf .
- 51.23-valent pneumococcal polysaccharide vaccine WHO position paper. Wkly Epidemiol Rec. 2008;83:373–84. [PubMed] [Google Scholar]
- 52.Sings HL. Pneumococcal conjugate vaccine use in adults-Addressing an unmet medical need for non-bacteremic pneumococcal pneumonia. Vaccine. 2017;35:5406–17. doi: 10.1016/j.vaccine.2017.05.075. [DOI] [PubMed] [Google Scholar]
- 53.Swaminathan S, Balajee G. Pneumococcal vaccines – A real world perspective cost-effectiveness of pneumococcal vaccination. JAPI. 2015;63:25–8. [PubMed] [Google Scholar]
- 54.Huss A, Scott P, Stuck AE, Trotter C, Egger M. Efficacy of pneumococcal vaccination in adults: A meta-analysis. CMAJ. 2009;180:48–58. doi: 10.1503/cmaj.080734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McLaughlin JM, Jiang Q, Isturiz RE, Sings HL, Swerdlow DL, Gessner BD, et al. Effectiveness of 13-valent pneumococcal conjugate vaccine against hospitalization for community-acquired pneumonia in older US adults: A test-negative design. Clin Infect Dis. 2018;67:1498–506. doi: 10.1093/cid/ciy312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prato R, Fortunato F, Cappelli MG, Chironna M, Martinelli D. Effectiveness of the 13-valent pneumococcal conjugate vaccine against adult pneumonia in Italy: A case-control study in a 2-year prospective cohort. BMJ Open. 2018;8:e019034. doi: 10.1136/bmjopen-2017-019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solanki BB, Juergens C, Chopada MB, Supe P, Sundaraiyer V, Le Dren-Narayanin N, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine in adults 50 to 65 years of age in India: An open-label trial. Hum Vaccin Immunother. 2017;13:2065–71. doi: 10.1080/21645515.2017.1331796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 59.Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, et al. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naïve adults 60-64 years of age. Vaccine. 2014;32:2364–74. doi: 10.1016/j.vaccine.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 60.Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013;31:3585–93. doi: 10.1016/j.vaccine.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2013;31:3577–84. doi: 10.1016/j.vaccine.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 62.Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013;31:3594–602. doi: 10.1016/j.vaccine.2013.04.084. [DOI] [PubMed] [Google Scholar]
- 63.Kobayashi M, Bennett NM, Gierke R, Almendares O, Moore MR, Whitney CG, et al. Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2015;64:944–7. doi: 10.15585/mmwr.mm6434a4. [DOI] [PubMed] [Google Scholar]
- 64.Centers for Disease Control and Prevention. Adults: Protect Yourself with Pneumococcal Vaccines. 2018. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwcdcgov/features/adult-pneumococcal/indexhtml .
- 65.Immunization Action Coalition. Ask the Experts: Pneumococcal Vaccines (PCV13 and PPSV23) 2018. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwimmunizeorg/askexperts/experts_pneumococcal_vaccinesasp .
- 66.Chilson E, Atkinson B, Hall-Murray C, Snow V, Schmoele-Thoma B, Isturiz RE, Scott DA. Sequential administration of PCV13 followed by PPSV23 results in a more robust immune response in PPSV23-naïve adults aged 60–64 years. OFID. 2017;4(Suppl 1):S470. [Google Scholar]
- 67.The Immunization Advisory Centre. 2017. [[Last accessed on 2019 Dec 09]]. Available from: https://wwwimmuneorgnz/sites/default/files/resources/Written%20Resources/VaccinePneumococcal20170807V01Finalpdf .
- 68.Pallotta A, Rehm SJ. Navigating pneumococcal vaccination in adults. Cleve Clin J Med. 2016;83:427–33. doi: 10.3949/ccjm.83a.15044. [DOI] [PubMed] [Google Scholar]
- 69.Papadatou I, Spoulou V. Pneumococcal vaccination in high-risk individuals: Are we doing it right? Clin Vaccine Immunol. 2016;23:388–95. doi: 10.1128/CVI.00721-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Papadatou I, Piperi C, Alexandraki K, Kattamis A, Theodoridou M, Spoulou V. Antigen-specific B-cell response to 13-valent pneumococcal conjugate vaccine in asplenic individuals with β-thalassemia previously immunized with 23-valent pneumococcal polysaccharide vaccine. Clin Infect Dis. 2014;59:862–5. doi: 10.1093/cid/ciu409. [DOI] [PubMed] [Google Scholar]
- 71.Orthopoulos GV, Theodoridou MC, Ladis VA, Tsousis DK, Spoulou VI. The effect of 23-valent pneumococcal polysaccharide vaccine on immunological priming induced by 7-valent conjugate vaccine in asplenic subjects with beta-thalassemia. Vaccine. 2009;27:350–4. doi: 10.1016/j.vaccine.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 72.Bajaj S. RSSDI clinical practice recommendations for the management of type 2 diabetes mellitus 2017. Int J Diabetes Dev Ctries. 2018;38:1–15. doi: 10.1007/s13410-018-0604-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–16. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mackay IR, Rosen FS. Vaccines and vaccination. N Engl J Med. 2001;345:1042–53. doi: 10.1056/NEJMra011223. [DOI] [PubMed] [Google Scholar]
- 75.Dhar R. Review of guidelines for the use of vaccines to prevent community-acquired pneumonia in Indian adults. JAPI. 2016;64:45–51. [Google Scholar]
- 76.Indian Society of Nephrology Vaccination Work Group Guidelines for vaccination in chronic kidney disease. Indian J Nephrol. 2016;26(Suppl 1):S15–S18. [Google Scholar]