Abstract
Background
Knee osteoarthritis (OA) is a major cause of disability in the elderly, however, there are few studies to estimate the global prevalence, incidence, and risk factors of knee OA.
Methods
For this study, we searched PUBMED, EMBASE and SCOPUS from inception to April 4, 2020, without language restriction. We identified eligible studies with information on the prevalence or incidence of knee OA in population-based observational studies and extracted data from published reports. We did random-effects meta-analysis to generate estimates. This study was registered with PROSPERO (CRD42020181035).
Findings
Out of 9570 records identified, 88 studies with 10,081,952 participants were eligible for this study. The pooled global prevalence of knee OA was 16⋅0% (95% CI, 14⋅3%-17⋅8%) in individuals aged 15 and over and was 22⋅9% (95% CI, 19⋅8%-26⋅1%) in individuals aged 40 and over. Correspondingly, there are around 654⋅1 (95% CI, 565⋅6–745⋅6) million individuals (40 years and older) with knee OA in 2020 worldwide. The pooled global incidence of knee OA was 203 per 10,000 person-years (95% CI, 106–331) in individuals aged 20 and over. Correspondingly, there are around annual 86⋅7 (95% CI, 45⋅3–141⋅3) million individuals (20 years and older) with incident knee OA in 2020 worldwide. The prevalence and incidence varied substantially between individual countries and increased with age. The ratios of prevalence and incidence in females and males were 1⋅69 (95% CI, 1⋅59–1⋅80, p<0⋅00) and 1⋅39 (95% CI, 1⋅24–1⋅56, p<0⋅00), respectively.
Interpretation
Our study provides the global prevalence (16⋅0% [95% CI, 14⋅3%-17⋅8%]) and incidence (203 per 10,000 person-years [95% CI, 106–331]) of knee OA. These findings can be used to better assess the global health burden of knee OA. Further prospective cohort studies are warranted to identify modifiable risk factors for providing effectively preventive strategies in the early stages of the disease.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81772384 and 81572174).
Research in context.
Evidence before this study
To our knowledge, only the Global Burden of Disease Study estimated the global prevalence and incidence of osteoarthritis (OA) from 2000 onwards. The latest estimate was in 2017.
Added value of this study
We included 88 studies for this systematic review and meta-analysis, of which 18 studies were published from 2017 to 2020. We found that the global prevalence of knee OA was 16⋅0% (95% CI, 14⋅3%−17⋅8%) in individuals aged 15 and over and was 22⋅9% (95% CI, 19⋅8%−26⋅1%) in individuals aged 40 and over. Correspondingly, there are around 654⋅1 (95% CI, 565⋅6–745⋅6) million individuals (40 years and older) with knee OA in 2020 worldwide. We found that the global incidence of knee OA was 203 per 10,000 person-years (95% CI, 106–331) in individuals aged 20 and over. Correspondingly, there are around annual 86⋅7 (95% CI, 45⋅3–141⋅3) million individuals (20 years and older) with incident knee OA in 2020 worldwide. The ratios of prevalence and incidence in females and males were 1⋅69 (95% CI, 1⋅59–1⋅80, p<0⋅00) and 1⋅39 (95% CI, 1⋅24–1⋅56, p<0⋅00), respectively. We also assessed risk factors, the results showed that the prevalence and incidence increased with age, peaked at the advanced age on prevalence and at 70–79 years old on incidence; increasing education attainment was negatively associated with the prevalence of knee OA.
Implications of all the available evidence
Global health-care providers, policy makers, and the general public should be aware of the high prevalence of knee OA and the modifiable risk factors such as obesity, injury, and education. More efforts should be made to explore the cause of the high prevalence and incidence of knee OA in women and elderly and provide effectively preventive strategies in the early stages of the disease.
Alt-text: Unlabelled box
1. Introduction
Knee osteoarthritis (OA) is a common progressive multifactorial joint disease and is characterized by chronic pain and functional disability [1]. Knee OA accounts for almost four fifths of the burden of OA worldwide and increases with obesity and age [2]. Up to now, knee OA is incurable except knee arthroplasty which is considered as an effective treatment at an advanced stage of the disease, however, which is responsible for substantial health costs [3]. Many researchers have shifted their focus to the prevention and treatment in the early stage of the disease [4]. Accordingly, it is essential to understand the prevalence, incidence, and modifiable risk factors of knee OA for providing effectively preventive strategies.
The World Health Organization, the International League Against Rheumatism, and global OA experts have made substantial efforts over the past few decades, many population-based epidemiological studies of knee OA have been conducted worldwide [5]. Exploring the differences of prevalence, incidence, and risk factors of knee OA in age, gender, region, and others can help us better understand the potential etiology of knee OA. However, there is a paucity of the epidemiologic data of knee OA in the global population. The Global Burden of Disease (GBD) study 2017 and recent several reviews assessed the epidemiological characteristics of knee OA, but they still used data published before 2000 [6,7]. Moreover, the main objective of the GBD study was to assess the burden of OA. Furthermore, the GBD study 2017 only included the symptomatic knee OA which was radiologically confirmed, which tended to collect relatively late cases, because there is discordance between symptomatic and radiographic changes. The early painful patients with knee OA may be not suffer from radiographic changes. On the contrary, the patients with severe radiographic changes may not have any symptoms [8]. The possible explanations are that structural changes on radiographs may not have a nociceptive innervation and the experience of pain is related to many factors such as tolerance and susceptibility. In general, the patient with both radiographic changes and symptoms is usually considered to be more severe. Accordingly, only either symptomatic or radiological knee OA may occur earlier than the radiologically confirmed symptomatic knee OA, and the former may provide more opportunities for disease prevention. Even Du H et al. found that almost a quarter of patients with the radiographic OA, but no symptoms, complained of disability [9]. Moreover, with the changes of demographic structure and lifestyle, the epidemiological data of knee OA are also changing. Wallace IJ et al. demonstrated that the prevalence of knee OA has doubled since the mid-20th century in the United States [10]. Consequently, investigating the contemporary epidemiology of knee OA is considerably important. However, it is time-consuming, expensive, and difficult to examine the prevalence or incidence of knee OA in large-scale population.
Therefore, we quantitatively synthesized the published epidemiological data of knee OA in individual country to estimate the global, regional prevalence of knee OA from 2000 to 2020, to estimate the global, regional incidence of knee OA from 1990 to 2020, and to identify risk factors of knee OA, aiming to produce more accurate and up-to-date evidence-based information for health-care providers and policy makers.
2. Methods
2.1. Search strategy and selection criteria
We performed this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline, the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline, and Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER Statement [11], [12], [13]. The protocol of this study was preregistered in the PROSPERO database (CRD42020181035).
The criteria for study inclusion are as follows: an original study reporting the prevalence or incidence of knee OA; a study examining radiographical (femorotibial), symptomatic, or self-report knee OA; a cross-sectional or longitudinal observational study; a population-based study; on prevalence, a study collecting data from 2000 onwards; on incidence, a study collecting data from 1990 onwards. In addition, several studies reporting register primary care data [14], [15], [16] and claims data [17] were included, because those data were a close representation of the national population and had been validated [18,19]. Studies are excluded: examined individuals only with radiographically patellofemoral OA; diagnosed knee OA by MRI and ultrasonography; a hospital-based study; used sample sizes less than 150.
Two researchers (CAY, LHZ) searched PUBMED, EMBASE, and SCOPUS from inception to April 4, 2020 using search terms defined according to PECOS (participants, exposure, control, outcome, study) principle (Appendix 1.1–3), without limitation in language. We also identified reference lists of relevant systematic reviews and included studies, but we did not search gray literature. To select eligible studies, we firstly used endnote software (version 19⋅0) to remove duplicates from different bibliographic databases. Then two investigators (CAY, LHZ) independently screened titles and abstracts of remaining literature search records and assessed all potential eligible articles against the inclusion and exclusion criteria. The two researchers (CAY, LHZ) and another professor (LHD) discussed together to resolve any discrepancy in the process of selecting studies.
2.2. Data analysis
Two investigators (CAY, LHZ) extracted data from published reports. We extracted the data related to prevalence and risk factors of knee OA (sample sizes, the number of cases of knee OA) and the data related to incidence of knee OA (sample sizes at risk, the number of cases of incident knee OA, follow-up time, person-years at risk). Where several studies used data from the same study cohort, we only analyzed one study that presented the most comprehensive and latest data.
The primary outcome of this study are the prevalence and incidence of knee OA. The prevalence of knee OA is defined as a proportion, namely, the number of cases of knee OA divided by the sample sizes. The incidence equals the number of cases of incident knee OA divided by the person-years at risk. Concomitantly, we also estimated the global number of individuals with knee OA (40 years and older) and with incident knee OA (20 years and older) in 2020 according to world population prospects obtained from the UN Population Division [20]. The secondary outcome are risk factors (age, gender, area of residence, cigarettes, and education level) of knee OA, and the relationships between risk factors and the prevalence and incidence of knee OA are expressed by risk ratios (RRs).
We assessed the quality of prevalence studies using the risk of bias tool developed by Hoy D et al., which includes 10 items and a summary assessment [21]. And we assessed the quality of incidence studies using risk of bias tool designed by ourselves according to previously validated quality assessment tools, which includes 6 items and a summary assessment. Two raters (CAY, LHZ) independently assessed each item and any discrepancy was resolved by consensus. Finally, we used Revman software (version 5⋅3) to visualize the results.
We analyzed heterogeneity using the I2 statistic. To explore the source of heterogeneity, we firstly conducted subgroup analysis according to the characteristics of studies, the classification are as follows: location (continent, country); definition (radiological, symptomatic, and self-report knee OA); sex (female, male); age. Then we performed sensitively analysis to explore the impact of individual study on the overall effects. Univariate meta-regression models were further used to assess the associations between the characteristics of studies and the pooled prevalence and incidence of knee OA. We qualitatively and quantitatively detected publication bias using funnel plots and the Egger linear regression test, respectively [22].
We used R software (version 4⋅0) and Stata software (version 15⋅1) to conduct the meta-analysis. Considering the anticipated high heterogeneity of the observational studies, we employed random-effects model to generate estimates. To stabilize variances, study data were transformed using the Freeman-Tukey double arcsine transformation [23]. All results were generated accompanying 95% confidence interval (CI). A 2-sided P value < 0⋅05 was considered statistically significant in all analyses.
2.3. Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
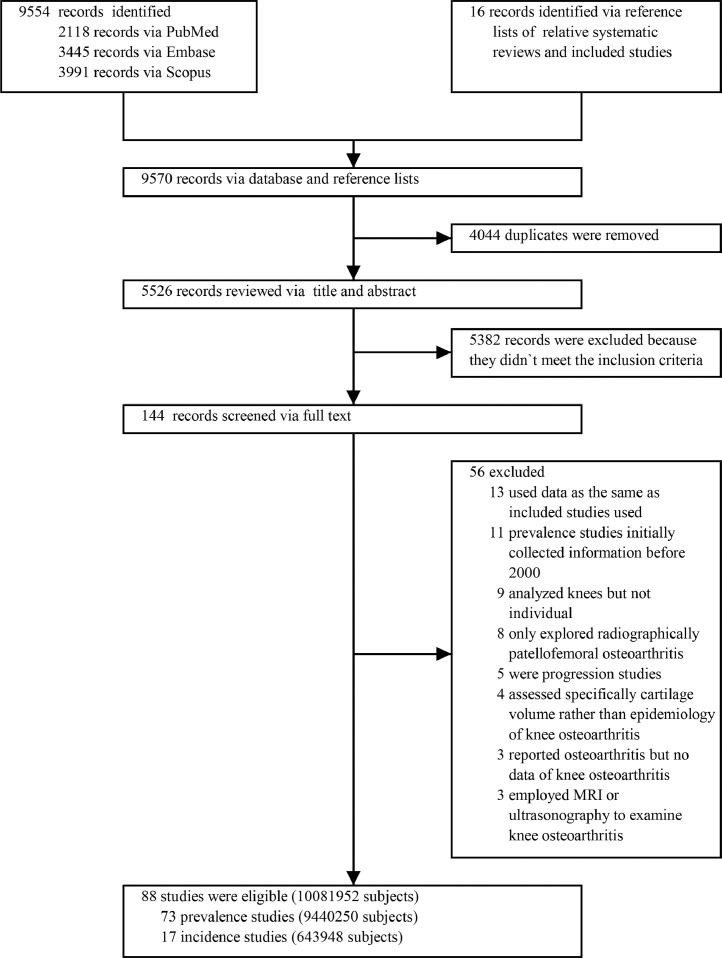
In total, 9570 citations were retrieved, of which 4044 were duplicated articles and 5438 were ineligible for inclusion criteria, of 88 remaining articles were included for this meta-analysis. The process of selecting studies was showed in Fig. 1.
Fig. 1.
PRISMA flow diagram.
We finally conducted the present meta-analysis based on 88 articles with 10,081,952 participants, of those, 73 prevalence studies were showed in Table 1, 17 incidence studies were showed in Table 2. Those studies were from six continents (47 in Asian (144,838 participants), 23 in Europe (9,042,232 participants), 11 in North America (236,263 participants), five in South America (15,670 participants), one in Oceania (847 participants), one in Africa (400 participants)) and 34 countries. 70 articles were cross-sectional studies, four articles were retrospective cohort studies. 14 articles were prospective cohort studies. On prevalence, 19 studies examined the radiographic knee OA, 56 studies examined the symptomatic knee OA, and 3 studies examined the self-report knee OA; on incidence, ten studies reported the radiological knee OA and seven studies reported the symptomatic knee OA. The number of females was more than that of males in most of the included studies. 16 studies were high risk of bias, 29 were moderate risk of bias, 43 were low risk of bias (Appendix 2.1–4).
Table 1.
The summary characteristics of included prevalence studies.
| Countries | NO. of Total | NO. of knee OA | Age (Mean (SD); Rang (years)) | BMI (kg/m2) | NO. of Female | Methods of Diagnosis | Time of Data Collection | Type of Study | Sampling Method | Type of Article | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang Y et al. 2001 [34] | China | 1,781 | 692 | ≥60 | 25⋅7 | 1,051 | KL≥2 | 2001 | Cross-sectional | Random sampling | Full text |
| Zhang Y et al. 2001 [34] | China | 1,781 | 199 | ≥60 | 25⋅7 | 1,051 | KL≥2 and Symptomatic (pain) | 2001 | Cross-sectional | Random sampling | Full text |
| Minh Hoa TT et al. 2003 [35] | Vietnam | 2,119 | 52 | 40 (17); 16–93 | NA | 1,138 | ACR | 2000 | Cross-sectional | No random | Full text |
| Minaur N 2004 [36] | Australia | 847 | 26 | 35 (13); 15–86 | NA | 446 | Doctor-diagnosed | 2002 | Cross-sectional | All subjects were interviewed | Full text |
| Senna ER et al. 2004 [37] | Brazil | 3,038 | 47 | 36 (16); 16–92 | NA | 1,929 | ACR | 2004 | Cross-sectional | Probabilistic sampling | Full text |
| Du H et al. 2005 [9] | China | 2,093 | 934 | 54; ≥40 | 22⋅9 | 1,099 | KL≥2 | 2001 | Cross-sectional | Random sampling | Full text |
| Du H et al. 2005 [9] | China | 2,093 | 150 | 54; ≥40 | 22⋅9 | 1,099 | KL≥2 and Symptomatic (pain) | 2001 | Cross-sectional | Random sampling | Full text |
| Haq SA et al. 2005 [38] | Bangladesh | 5,077 | 441 | >15 | NA | 2,517 | ACR | 2001 | Cross-sectional | Random sampling | Full text |
| Kaçar C et al. 2005 [39] | Turkey | 655 | 97 | 60 (8); >50 | NA | 306 | ACR | 2005 | Cross-sectional | Cluster sampling | Full text |
| Salaffi F et al. 2005 [40] | Italy | 2,155 | 116 | 58 (18); 18–91 | 25⋅1 | 1,151 | ACR | 2004 | Cross-sectional | Random sampling | Full text |
| Wang W et al. 2006 [41] | China | 1,450 | 176 | ≥40 | NA | 746 | KL≥2 | 2005 | Cross-sectional | Multiple stage cluster sampling | Abstract |
| Zeng QY et al. 2006 [42] | China | 2,188 | 244 | 35–64 | 23⋅9 | 1,139 | ACR | 2005 | Cross-sectional | House by house | Full text |
| Felson DT et al. 2007 [43] | USA | 1,128 | 104 | 62; 34–90 | 27⋅4 | NA | KL≥2 | 2005 | Cross-sectional | No random | Full text |
| Tangtrakulwanich B et al. 2007 [44] | Thailand | 506 | 234 | 59; >40 | 24⋅4 | 288 | KL≥2 | 2007 | Cross-sectional | Cluster stratified sampling | Full text |
| Davatchi F et al. 2008 [45] | Iran | 10,291 | 1,532 | >15 | NA | 5,413 | Doctor-diagnosed | 2004–2005 | Cross-sectional | Multistage sampling | Full text |
| Grotle M et al. 2008 [46] | Norway | 3,266 | 232 | 24–74 | NA | 1,786 | Self-report doctor-diagnosed | 2004 | Cross-sectional | No random | Full text |
| Quintana JM et al. 2008 [47] | Spain | 7,577 | 925 | 71 (7); 60–90 | NA | 4,264 | ACR | 2002–2003 | Cross-sectional | Stratified random sampling | Full text |
| Joshi VL et al. 2009 [48] | India | 8,145 | 451 | >16 | NA | NA | ACR | 2004–2008 | cross-sectional | House by house | Full text |
| Kang X et al. 2009 [49] | China | 1,025 | 155 | 58 (8); >50 | 22⋅5 | 520 | KL≥2 | 2005 | Cross-sectional | Compact segment sampling | Full text |
| Kang X et al. 2009 [49] | China | 1,026 | 109 | 58 (8); >50 | 22⋅5 | 520 | KL≥2 and Symptomatic (pain) | 2005 | Cross-sectional | Compact segment sampling | Full text |
| Yoshimura N et al. 2009 [50] | Japan | 3,040 | 1,660 | 70 (11); >40 | 23⋅0 | 1,979 | KL≥2 | 2005–2007 | Cross-sectional | Random sampling | Full text |
| Kim I et al. 2010 [51] | Korea | 504 | 188 | 70 (8); >50 | 24⋅6 | 274 | KL≥2 | 2007 | Cross-sectional | No random | Full text |
| Kim I et al. 2010 [51] | Korea | 504 | 121 | 70 (8); >50 | 24⋅6 | 274 | KL≥2 and Symptomatic (pain) | 2007 | Cross-sectional | No random | Full text |
| Salve H et al. 2010 [52] | India | 260 | 123 | >40 | NA | All female | ACR | 2009 | Cross-sectional | House by house | Full text |
| Cibere J et al. 2010 [53] | Canada | 255 | 98 | 40–79 | NA | 173 | K/L ≥ 2 | 2010 | Cross-sectional | Multistage random sampling | Full text |
| Guillemin F et al. 2011 [54] | France | 27,109 | 1,962 | 40–75 | NA | 18,111 | KL≥2 and Symptomatic (pain) | 2007–2009 | Cross-sectional | Random sampling | Full text |
| Horváth G et al. 2011 [55] | Hungary | 672 | 111 | 52; 20–67 | NA | 432 | K/L ≥ 2 | 2011 | Cross-sectional | No random | Full text |
| Namali H et al. 2011 [56] | Sri Lanka | 1,750 | 207 | ≥50 | NA | NA | ACR | 2006–2007 | Cross-sectional | Stratified cluster sampling | Abstract |
| Cakır N et al. 2012 [57] | Turkey | 4,952 | 265 | ≥40 | NA | 2,575 | ARA | 2012 | Cross-sectional | No random | Full text |
| Chaaya M et al. 2012 [58] | Lebanon | 3,530 | 106 | 38(17); 15–90 | NA | 1,730 | ACR | 2007 | Cross-sectional | Multistage cluster sampling | Full text |
| Ajit NE et al. 2012 [59] | India | 342 | 58 | 43 (17) | NA | NA | ACR | 2011–2012 | Cross-sectional | Stratified random sampling | Abstract |
| Jiang L et al. 2012 [60] | China | 1,196 | 193 | 63 (9); 40–84 | NA | 623 | KL≥2 and Symptomatic (pain) | 2005 | Cross-sectional | Stagestratified sampling | Full text |
| Sandoughi M et al. 2013 [61] | Iran | 2,100 | 321 | 33 (15); >15 | NA | 1,179 | ACR | 2008–2009 | Cross-sectional | Multistage cluster randomly sampling | Full text |
| Zhang J et al. 2013 [62] | China | 7,126 | 983 | 44 (17); 16–90 | NA | 3,517 | KL≥2 and Symptomatic (pain) | 2013 | Cross-sectional | Stratified multistage cluster sampling | Full text |
| Yeşil H et al. 2013 [63] | Turkey | 522 | 109 | 54 (9); ≥40 | 29⋅6 | 390 | ACR | 2013 | Cross-sectional | No random | Full text |
| Xiang Z et al. 2013 [64] | China | 1,499 | 499 | 62 (11); ≥40 | NA | 790 | ACR | 2010–2011 | Cross-sectional | Cluster stratified multistage random sampling | Full text |
| Turkiewicz A et al. 2013 [65] | Sweden | 7,737 | 1,965 | 70 (8); 56–84 | 27⋅0 | 947 | KL≥2 | 2007 | Cross-sectional | Random sampling | Abstract |
| Turkiewicz A et al. 2013 [65] | Sweden | 7,737 | 812 | 70 (8); 56–84 | 27⋅0 | 947 | KL≥2 and Symptomatic (pain) | 2007 | Cross-sectional | Random sampling | Abstract |
| Turkiewicz A et al. 2013 [65] | Sweden | 7,737 | 696 | 70 (8); 56–84 | 27⋅0 | 947 | ACR | 2007 | Cross-sectional | Random sampling | Abstract |
| Thomas E et al. 2014 [66] | England | 26,100 | 8,000 | 66 (11); ≥50 | NA | 14,172 | Symptomatic OA | 2004 | Cross-sectional | All subjects were interviewed | Full text |
| Visser AW et al. 2014 [67] | Netherlands | 5,284 | 991 | 57; 45–65 | 29⋅9 | 2,794 | ACR | 2008–2013 | Cross-sectional | No random | Full text |
| Singh AK et al. 2014 [68] | India | 496 | 204 | ≥60 | NA | 238 | ACR | 2009–2010 | Cross-sectional | House by house | Full text |
| Tehrani-Banihashemi A et al. 2014 [69] | Iran | 1,565 | 303 | 38 (19); >15 | NA | 862 | ACR | 2006 | Cross-sectional | Random sampling | Full text |
| Ho-Pham LT et al. 2014 [70] | Vietnam | 658 | 225 | 56; 40–98 | NA | 488 | KL≥2 | 2014 | Cross-sectional | Simple random sampling | Full text |
| Haouichat C et al. 2014 [71] | Algeria | 400 | 84 | 65 (9); ≥50 | 30⋅1 | All female | KL≥2 | 2014 | Cross-sectional | Stratified sampling | Abstract |
| Edwards M et al. 2014 [72] | Europe countries | 2,904 | 587 | 74 (5); 65–85 | 27⋅3 | 1,507 | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | Germany | 405 | 45 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | Italy | 467 | 135 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | Netherlands | 558 | 102 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | Spain | 535 | 129 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | Sweden | 506 | 101 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Edwards M et al. 2014 [72] | England | 433 | 69 | 74 (5); 65–85 | 27⋅3 | NA | ACR | 2010–2011 | Cross-sectional | Random sampling | Full text |
| Ara R et al. 2014 [73] | Bangladesh | 4,850 | 401 | 56 (13); ≥15 | NA | 3,502 | ACR | 2011 | Cross-sectional | House by house and census | Abstract |
| Moghimi N et al. 2015 [74] | Iran | 5,830 | 1,096 | >15 | NA | 3,236 | Doctor-diagnosed | 2011–2012 | Cross-sectional | Clusters randomly sampling | Full text |
| Granados Y et al. 2015 [75] | Venezuela | 3,973 | 218 | 44 (18); >18 | NA | 2,367 | ACR | 2011 | Cross-sectional | House by house | Full text |
| Zeng SY et al. 2015 [76] | China | 4,056 | 421 | 48; ≥16 | NA | 2,108 | ACR | 2012 | Cross-sectional | Random sampling | Full text |
| Plotnikoff R et al. 2015 [77] | Canada | 4,733 | 497 | ≥18 | NA | NA | Self-report doctor-diagnosed OA | 2015 | Cross-sectional | Random sampling | Abstract |
| Cho HJ et al. 2015 [78] | Korea | 696 | 265 | 72 (5); 65–91 | 24⋅0 | 398 | KL≥2 | 2005–2006 | Cross-sectional | Simple random sampling | Full text |
| Tang X et al. 2016 [79] | China | 17,128 | 1,387 | 60; ≥45 | NA | 8,761 | Doctor-diagnosed OA | 2011–2012 | Cross-sectional | Multistage probability sampling | Full text |
| Guevara-Pacheco S et al. 2016 [80] | Ecuador | 4,877 | 361 | 43 (18); >18 | NA | 2,916 | ACR | 2014 | Cross-sectional | Mixed multistage and random sampling | Full text |
| Pal CP et al. 2016 [81] | India | 4,909 | 1,412 | ≥45 | NA | NA | KL≥2 | 2016 | Cross-sectional | Simple random sampling | Full text |
| Branco JC et al. 2016 [82] | Portugal | 7,911 | 981 | >18 | NA | 4,153 | ACR | 2011–2013 | Cross-sectional | Stratified multistage random sampling | Full text |
| Liu Y et al. 2016 [83] | China | 3,428 | 568 | 55 (10); ≥40–74 | NA | 1,767 | KL≥2 and Symptomatic (pain) | 2010 | Cross-sectional | Proportionately stratified random sampling | Full text |
| Deshpande BR et al. 2016 [84] | USA | 198,600 | 13,750 | ≥25 | NA | 103,140 | Doctor-diagnosed OA | 2007–2008 | Cross-sectional | NA | Full text |
| Al Saleh J et al. 2016 [85] | United Arab Emirates | 3,984 | 1,028 | 42 (16); 18–85 | 28⋅8 | 3,084 | ACR | 2009 | Cross-sectional | Random sampling | Full text |
| Murphy LB et al. 2016 [86] | USA | 1,246 | 192 | ≥51 | NA | 723 | KL≥2 | 2003 | Longitudinal | Stratified simple random sampling | Full text |
| Murphy LB et al. 2016 [86] | USA | 1,342 | 155 | ≥51 | NA | 778 | KL≥2 and Symptomatic (pain) | 2003 | Longitudinal | Stratified simple random sampling | Full text |
| Kolahi S et al. 2017 [87] | Iran | 952 | 143 | 50 (9); 35–70 | NA | 516 | ACR | 2014–2015 | Cross-sectional | Random sampling | Full text |
| Ahmed S et al. 2017 [88] | Bangladesh | 1,843 | 135 | ≥18 | NA | NA | Doctor-diagnosed OA | 2017 | Cross-sectional | Clusters sampling | Abstract |
| Prashansanie Hettihewa A et al. 2018 [89] | Sri Lanka | 666 | 134 | 63 (9); ≥50 | NA | All female | ACR | 2013 | Cross-sectional | Multistage cluster sampling | Full text |
| Venkatachalam J et al. 2018 [90] | India | 1,986 | 538 | >18 | NA | 1,260 | ACR | 2014 | Cross-sectional | Random sampling | Full text |
| Vega-Hinojosa O et al. 2018 [91] | Peru | 1,095 | 17 | 39; ≥18 | NA | 614 | ARA | 2010 | Cross-sectional | Probabilistic, stratified random sampling | Full text |
| Seoane-Mato D et al. 2018 [92] | Spain | 4,753 | 661 | ≥20 | NA | NA | ACR | 2016 | Cross-sectional | Stratified cluster sampling | Abstract |
| Kumar P et al. 2018 [93] | India | 10,171 | 766 | ≥0 | NA | NA | Doctor-diagnosed OA | 2004–2007 | Cross-sectional | Cluster sampling | Full text |
| Gavali M et al. 2018 [94] | India | 647 | 84 | 45; 35–57 | NA | 386 | Doctor-diagnosed OA | 2017–2018 | Cross-sectional | House by house | Abstract |
| Ananto M et al. 2018 [95] | Indonesia | 2,067 | 296 | ≥15 | NA | NA | Doctor-diagnosed OA | 2018 | Cross-sectional | Multistage random sampling | Abstract |
| Leung YY et al. 2018 [96] | Singapore | 3,364 | 370 | ≥18 | NA | 1,822 | Symptomatic OA | 2013–2014 | Cross-sectional | A nationally representative sample | Full text |
| Macías-Hernández SI et al. 2018 [97] | Mexico | 204 | 52 | 57 (11); ≥40 | NA | 124 | KL≥2 | 2018 | Cross-sectional | No random | Full text |
| Macías-Hernández SI et al. 2018 [97] | Mexico | 204 | 36 | 57 (11); ≥40 | NA | 124 | KL≥2 and Symptomatic (pain) | 2018 | Cross-sectional | No random | Full text |
| Postler A et al. 2018 [17] | Germany | 2,700,000 | 329,216 | 75; >60 | NA | 1,884,600 | ICD-10 codes | 2014 | Cross-sectional | De-identified claims data | Full text |
| Badley EM et al. 2018 [98] | Canada | 30,097 | 4,460 | 45–85 | NA | NA | Self-report doctor-diagnosed OA | 2018 | Cross-sectional | No random | Abstract |
| Rodriguez-Veiga D et al. 2019 [99] | Spain | 707 | 207 | 62; ≥40 | NA | 398 | Doctor-diagnosed OA | 2019 | Cross-sectional | Random sampling | Abstract |
| Guevara SV et al. 2019 [100] | Ecuador | 2,687 | 177 | 44 (20); ≥18 | NA | 1,690 | ACR | 2016–2018 | Cross-sectional | Random and mixed (stratified and conglomerate) | Full text |
| Hvidberg MF et al. 2019 [14] | Denmark | 4,555,439 | 177,662 | ≥16 | NA | 2,314,163 | ICD-10 codes | 2013 | Longitudinal | NA | Full text |
| Hong JW et al. 2020 [101] | Korea | 12,287 | 4,313 | 63; ≥50 | NA | 7,056 | KL≥2 | 2010–2013 | Cross-sectional | Proportional-allocation systematic sampling with multistage stratification | Full text |
| Sasaki E et al. 2020 [102] | Japan | 1,104 | 282 | 54; ≥20 | 22⋅9 | 661 | KL≥2 | 2005 | Cross-sectional | No random | Full text |
| Swain S et al. 2020 [15] | England | 1,690,618 | 49,028 | ≥20 | NA | NA | ICD-10 codes | 2017 | Longitudinal | NA | Full text |
NO=numero. OA=osteoarthiritis. SD=standard deviation. BMI=body mass index. KL=the Kellgren and Lawrence. NA=not applicate. ACR=the American College of Rheumatology classification criteria. ARA=the clinical criteria of the American Rheumatism Association. ICD=International Classification of Disease.
Table 2.
The summary characteristics of included incidence studies.
| Countries | NO. of knee OA | Person- years | Time of Follow- up (years) | Age at baseline, Mean; Range (years) | NO. of Female | Methods of Diagnosis | Time of Data Collection | |
|---|---|---|---|---|---|---|---|---|
| Oliveria SA et al. 1995 [103] | USA | 461 | 196,990 | 3⋅0 | 20–89 | NA | KL≥2 + symptoms | 1988-(1991,1992) |
| Felson DT et al. 1995 [104] | USA | 93 | 4,844 | 8⋅1 | 71; (63–91) | 381 | K/L grade >2 | (1983–1985)-(1992–1993) |
| Slemenda C et al. 1998 [105] | USA | 27 | 728 | 2⋅6 | 71; (≥65) | 113 | K/L grade >2 | NA |
| Gelber AC et al. 1999 [106] | USA | 62 | 42,480 | 36⋅0 | 23; (21–25) | All men | Self-report | NA |
| Murphy LB et al. 2016 [86] | USA | 193 | 6,853 | 5⋅5 | ≥45 | 723 | K/L grade >2 | (1991–1997)-(1999–2003) |
| Cooper C et al. 2000 [107] | England | 45 | 1,234 | 5⋅1 | 70; (≥55) | NA | K/L grade >2 | (1990–1991)-(1995–1996) |
| Duncan R et al. 2011 [108] | England | 55 | 759 | 3⋅0 | 65 (57–73) | NA | K/L grade >2 | (2002–2003)-(2005–2006) |
| Driban JB et al. 2020 [109] | England | 93 | 3,575 | 15⋅0 | 45–64 | All female | K/L grade >2 | (1988–1989)−2004 |
| Swain S et al. 2020 [15] | England | 3,432 | 1,492,174 | 1⋅0 | ≥20 | NA | CDI-10 | 2007 |
| Prieto-Alhambra D et al. 2014 [16] | Spain | 96,222 | 3,266,826 | 4⋅5 | ≥40 | NA | Doctor-diagnosed | 2006–2010 |
| Grotle M et al. 2008 [110] | Norway | 114 | 16,750 | 10⋅0 | 42; (24–66) | NA | Self-report | 1994–2004 |
| Mork PJ et al. 2012 [111] | Norway | 351 | 329,527 | 11⋅0 | 44; ≥20 | 15,191 | Self-report | (1984–1986)-(1995–1997) |
| Reijman M et al. 2007 [112] | Netherlands | 76 | 9,055 | 6⋅6 | 66; (≥55) | 792 | K/L grade >2 | (1990–1993)-(1996–1999) |
| Nishimura A et al. 2011 [113] | Japan | 57 | 1,044 | 4⋅0 | 71; (65–89) | 165 | K/L grade >2 | (1997,1999,2001,2003)−2007 |
| Muraki S 2012 [114] | Japan | 107 | 3,623 | 3⋅3 | 69; (23–95) | 631 | K/L grade >2 | (2005–2007)-(2008–2010) |
| Sasaki E et al. 2015 [115] | Japan | 129 | 1,615 | 5⋅0 | 55; (25–85) | NA | K/L grade >2 | 2008–2013 |
| Toivanen AT et al. 2010 [116] | Finland | 94 | 18,106 | 22⋅0 | 42; (30–72) | 454 | Doctor-diagnosed | (1978,1980)-(2000,2001) |
NO.=numero. OA=osteoarthiritis. KL=the Kellgren and Lawrence. NA=not applicate. ICD=International Classification of Disease.
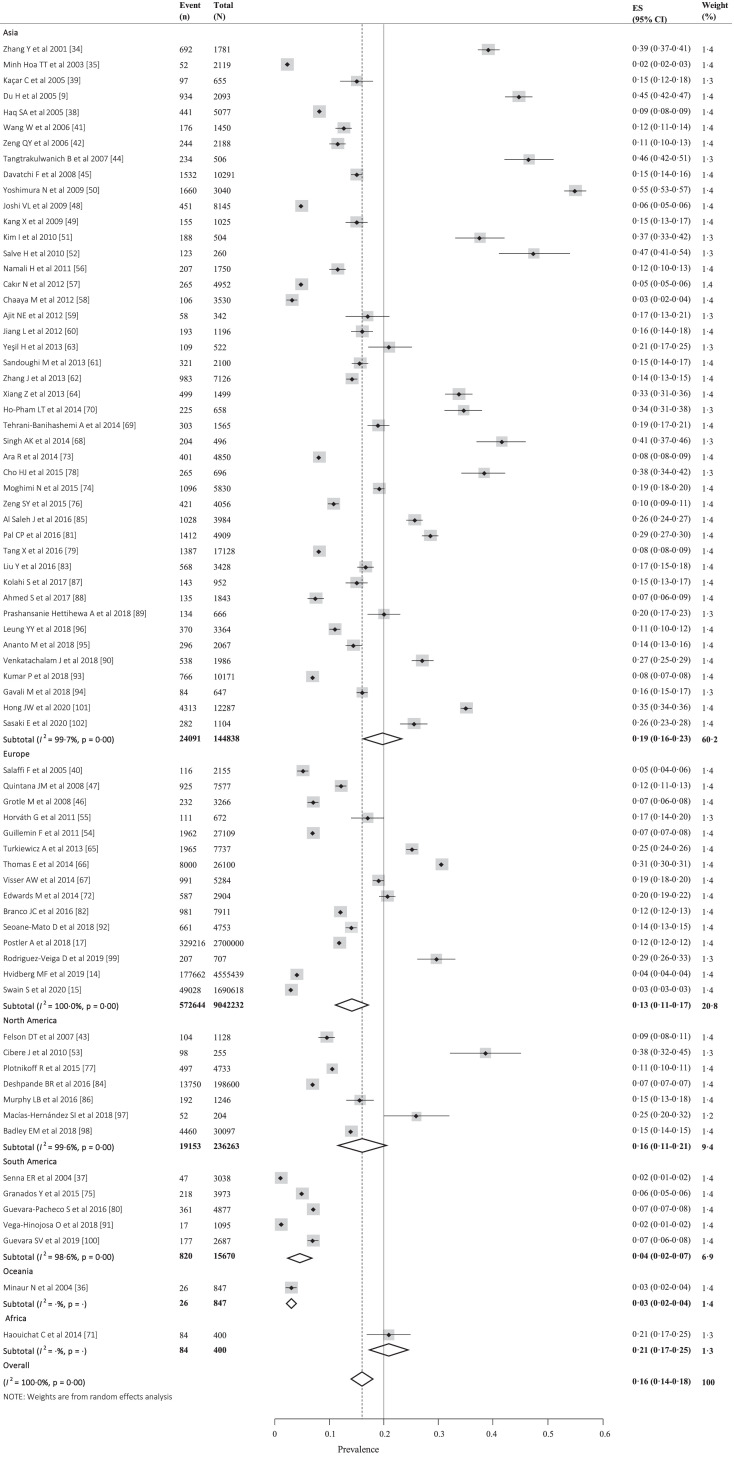
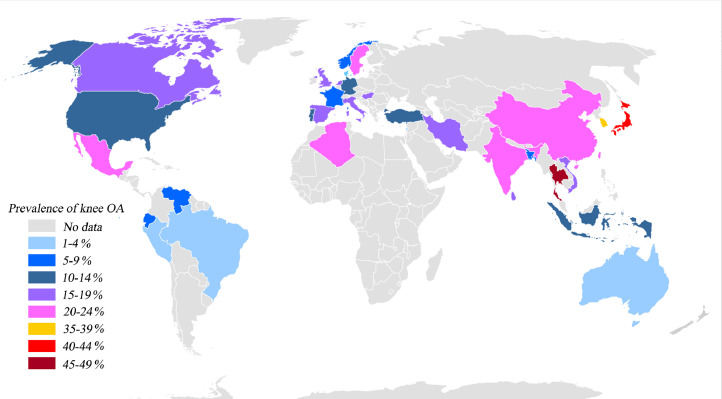
We estimated the global prevalence of knee OA based on 9,440,250 participants from 34 countries, most of participants were ≥15 years old (mean 57⋅2 (standard deviation (SD) 6⋅5)). The pooled global prevalence of knee OA was 16⋅0% (95% CI, 14⋅3%−17⋅8%) (Fig. 2) in individuals aged 15 and over and was 22⋅9% (95% CI, 19⋅8%−26⋅1%) (Appendix 3) in individuals aged 40 and over. Correspondingly, there are around 654⋅1 (95% CI, 565⋅6–745⋅6) million individuals (40 years and older) with knee OA in 2020 worldwide. The prevalence was 19⋅2% (95% CI, 15⋅7%−23⋅0%) in Asian, 13⋅4% (95% CI, 10⋅1%−17⋅0%) in Europe, 15⋅8% (95% CI, 11⋅2%−20⋅9%) in North America, 4⋅1% (95% CI, 2⋅1%−6⋅9%) in South America, 3⋅1% (95% CI, 2⋅0%−4⋅4%) in Oceania, 21⋅0% (95% CI, 17⋅1%−25⋅1%) in Africa (Fig. 2). The prevalence in individual country ranged from 1⋅6% (95% CI, 1⋅1%−2⋅0%) to 46⋅3% (95% CI, 41⋅9%−50⋅6%) (Fig. 3 and Appendix 4). The radiological knee OA (28⋅7% [95% CI, 23⋅6%−34⋅1%]) (Appendix 5) was more common than the symptomatic (12⋅5% [95% CI, 10⋅8%−14⋅3%]) (Appendix 6) and self-report (10⋅6% [95% CI, 6⋅5%−15⋅6%]) (Appendix 7) knee OA. The prevalence was higher in women (21⋅7% [95% CI, 19⋅0%−24⋅5%]) (Appendix 8) than in men (11⋅9% [95% CI, 10⋅2%−13⋅8%]) (Appendix 9).
Fig. 2.
The forest plot of the global prevalence of knee osteoarthritis.
Fig. 3.
Global distribution of the prevalence of knee osteoarthritis (OA).
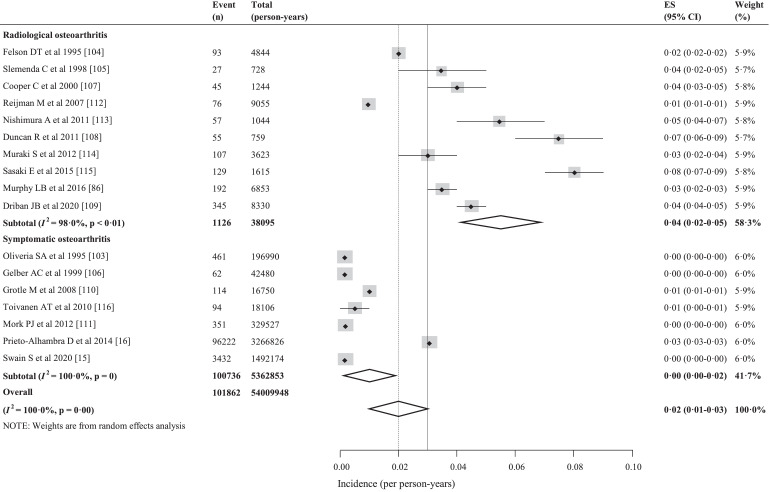
We estimated the global incidence of knee OA based on 643,948 participants from 7 countries, most of participants were ≥20 (mean 46⋅0 (SD 5⋅7)) years old at baseline. The global incidence of knee OA was 203 per 10,000 person-years (95% CI, 106–331) in individuals aged 20 and over (Fig. 4). Correspondingly, there are around annual 86⋅7 (95% CI, 45⋅3–141⋅3) million individuals (20 years and older) with incident knee OA in 2020 worldwide. The incidence was 130 per 10,000 person-years (95% CI, 59–228) in the United States, 315 per 10,000 person-years (95% CI, 42–824) in the United Kingdom, 525 per 10,000 person-years (95% CI, 245–902) in the Japan, 52 per 10,000 person-years (95% CI, 42–63) in the Finland, 84 per 10,000 person-years (95% CI, 66–106) in the Netherlands, 33 per 10,000 person-years (95% CI, 1–113) in the Norway, 295 per 10,000 person-years (95% CI, 293–296) in the Spain (Appendix 10). The incidence was higher in the radiological knee OA (373 per 10,000 person-years [95% CI, 248–522]) than in the symptomatic knee OA (50 per 10,000 person-years [95% CI, 1–175]) (Fig. 4).
Fig. 4.
The forest plot of the global incidence of knee osteoarthritis.
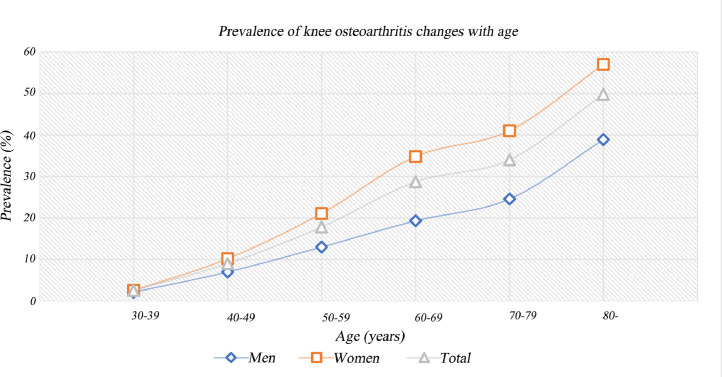
We assessed that the risk factors were age, gender, area of residence, cigarettes, and education level. We found that the risk of prevalence and incidence increased with age, peaked at the advanced age on prevalence (Fig. 5 and Appendix 11) and at 70–79 years old on incidence (Appendix 12 and 13). The risk of prevalence and incidence in females 1⋅69 (95% CI, 1⋅59–1⋅80, p<0⋅00) and 1⋅39 (95% CI, 1⋅24–1⋅56, p<0⋅00) times as much as males, respectively (Appendix 14 and 15). The risk of prevalence was not significantly different in rural and urban (RRs 0⋅97, 95% CI, 0⋅74–1⋅28, p<0⋅00) (Appendix 16). The risk of prevalence was higher in junior and senior high school than in college degree and above (RRs 2⋅01, 95% CI, 1⋅28–3⋅16, p<0⋅00) (Appendix 17) and was higher in elementary school and below than in junior and senior high school (RRs 2⋅14, 95% CI, 1⋅70–2⋅69, p<0⋅00) (Appendix 18). The risk of prevalence was not significantly different in smoking and no-smoking in cross-sectional studies (RRs 0⋅81, 95% CI, 0⋅44–1⋅51, p<0⋅51) (Appendix 19), but the risk of incidence was lower in smoking than in no-smoking in prospective cohort studies (RRs 0⋅60, 95% CI, 0⋅44–0⋅82, p<0⋅00)) (Appendix 20).
Fig. 5.
The trend of the prevalence of knee osteoarthritis changes with age.
Sensitivity analysis showed that there was no significant change in the estimates of prevalence and incidence after omitting any one of the included studies (Appendix 21 and 22). The univariate meta-regression results could explain some heterogeneity caused by publication year, type of study, sampling method, type of knee OA, mean BMI, but couldn`t explain all heterogeneity (Appendix 23 and 24). The funnel plots showed visually asymmetrical distribution of published studies on prevalence and incidence of knee OA (Appendix 25 and 26). But Egger tests indicated that there was no statistically significant publication bias on prevalence (Egger test: bias=11⋅98; 95% CI −2⋅33 to −26⋅28; p = 0⋅099) and incidence (Egger test: bias= −17⋅35; 95% CI −40⋅45 to 5⋅74; p = 0⋅130).
4. Discussion
The present systematic review and meta-analysis is, to our knowledge, the first study pooling the latest data and the largest sample sizes to estimate the epidemiologic characteristics of knee OA at the beginning of the 21st century. Our main findings were that the global prevalence of knee OA was 16⋅0% (95% CI, 14⋅3%−17⋅8%) in individuals aged 15 and over and was 22⋅9% (95% CI, 19⋅8%−26⋅1%) in individuals aged 40 and over; the incidence was 203 per 10,000 person-years (95% CI, 106–331) in individuals aged 20 and over; the prevalence and incidence increased with age, peaked at the advanced age on prevalence and at 70–79 years old on incidence; and the ratios of prevalence and incidence in females and males were 1⋅69 (95% CI, 1⋅59–1⋅80, p<0⋅00) and 1⋅39 (95% CI, 1⋅24–1⋅56, p<0⋅00), respectively.
The prevalence varied with the characteristics of studies (e.g., age, sex, BMI, etc.). In general, the global prevalence of knee OA was high from 2000 to 2020, which may bring a huge burden to global health care system. At continent-level, the prevalence was higher in Asian (19⋅2% [95% CI, 15⋅7%−23⋅0%]) than in Europe (13⋅4% [95% CI, 10⋅1%−17⋅0%]) and North America (15⋅8% [95% CI, 11⋅2%−20⋅9%]). The prevalence was lowest in South America (4⋅1% [95% CI, 2⋅1%−6⋅9%]). There was not adequate data to draw a conclusion in Oceania and Africa. At country-level, the prevalence ranged from 1⋅6% (95% CI, 1⋅1%−2⋅0%) to 46⋅3% (95% CI, 41⋅9%−50⋅6%). Thailand (46⋅3% [95% CI, 41⋅9%−50⋅6%]), Japan (39⋅6% [95% CI, 14⋅1%−68⋅7%]), Korea (36⋅1% [95% CI, 34⋅1%−38⋅1%]) and Indian (21⋅0% [95% CI, 11⋅0%−34⋅%]) had higher prevalence. Given Asian were relatively thinner than others, however, they had higher prevalence in our study. We analyzed that the number of studies examining radiographic knee OA was more in Asian than in other continents in the meta-analysis, which was the main reason why knee OA was more prevalent in Asian. Other possible reasons were that Asian were used to bending knee and squatting and genetic or environmental factors. In conclusion, we failed to determine accurately which country or continent had a higher prevalence of knee OA based on the existing evidence in our study, because there was no comparability of baseline data between studies. We found that the radiographic knee OA (28⋅7% [95% CI, 23⋅6%−34⋅1%]) was more prevalent than the symptomatic knee OA (12⋅4% [95%CI, 10⋅8%−14⋅2%]), which was consistent with a previous systematic review conducted by Pereira D et al. and they concluded that the prevalence of OA depended on definition used [24]. Accordingly, according to the results of our study, we speculated that the radiographic knee OA may be detected earlier, which can provide more opportunities for prevention of knee OA. We found the positive correlation between the prevalence of knee OA and increased age, similarly, the same result could be seen in GBD 2017 Study [25] and other studies [26,27]. However, the prevalence peaked at around 50 years of age for knee OA in GBD 2014 Study, the potential explanation may be that the GBD 2014 Study used inadequate data. Considering the older people easier encountered with knee OA and according to the current consensus, tailored care should be given to them [3]. We pooled a large sample (3,686,544 participants) to explore the ratio of prevalence and incidence of knee OA in women and men, to our knowledge, which was the strongest evidence so far [28]. Consequently, a more accurate estimate can be obtained. The results showed that prevalence and incidence in females 1⋅69 (95% CI, 1⋅59–1⋅80, p<0⋅00) and 1⋅39 (95% CI, 1⋅24–1⋅56, p<0⋅00) times as much as males, respectively, which was corresponded to established consensus [1,29]. More efforts should be made to explore the cause of the high prevalence of knee OA in women.
There were very rare studies examining the incidence of knee OA, most of the published studies were in Europe and North America. In Asian, only Japan has the published data of incidence of knee OA. The data of incidence was unavailable in South America, Oceania, and Africa. Considering most of whole world population in Asian, Oceania, and Africa, their data may be highly significant to ascertain the etiology and reduce global burden. And in consideration of the economic situation of those areas, initiating new program like Community Oriented for Control of the Rheumatic Diseases (COPCORD) may be a cost-effective strategy for understanding the epidemiological characteristics of knee OA. The data of the United Kingdom and the United States was more comprehensive and the pooled results showed that the incidence was 315 per 10,000 person-years (95% CI, 42–824) in the United Kingdom and 130 per 10,000 person-years (95% CI, 59–228) in the United States. We found that the incidence of knee OA increased with age and peaked at 70–79 years, which was consistent with the latest result of the study in the United Kingdom [15]. This may be an interesting topic, the prospective studies are warranted to validate it in future.
It is well known that knee OA is a multiple factors disease, which include modifiable (obesity, knee injury) and unmodifiable (age, sex) factors [30]. In our meta-analysis, the pooled estimates showed that the prevalence of knee OA was negatively associated with education level, which was in agreement with several previous studies [31,32]. This may be because of the fact that individuals with lower education level frequently involved heavy physical activities or accessed to few the knowledge of prevention for knee OA. We found that there was no statistically significant difference in prevalence between rural and urban (RRs 0⋅97, 95% CI, 0⋅74–1⋅28, p = 0⋅84), which was contradictory to some previous studies included in the meta-analysis. We speculated that the sample size of the individual study may be not enough. However, above estimates should be interpreted with caution because articles included in this meta-analysis were cross-sectional studies, which didn`t allow to draw a causal conclusion. Our pooled estimates showed that the incidence was lower in smokers than in no-smokers in cohort study, which was inconsistent with a previous systematic review and meta-analysis conducted by Hui M et al. [33]. They argued that the relationship between smoking and knee OA may be confounded by body mass index (BMI). Because BMI was a significant risk factor for the occurrence and development of knee OA and smokers were usually thinner than no-smokers. Another possible reason was that smokers often suffered from multiple comorbidities such as lung cancer, cardiovascular disease, so the no-smokers may have a longer life span and they were more likely to develop knee OA. Further prospective studies are needed to verify the dose-response relationship between the incidence of knee OA and cigarettes. Nevertheless, researchers have realized that knee OA is amenable to prevention and treatment at early stages [30]. Increasing education attainment, weight control, injury prevention, improving muscle function, and others may play an important role for early prevention in future.
There are some limitations in our study. Firstly, there is statistically significant heterogeneity in our meta-analysis. However, it is anticipated that the observational studies in epidemiology usually have substantial heterogeneity given established associations between the prevalence and incidence of knee OA and age, sex, BMI, type of knee OA, genetic factors and so on. Secondly, due to included studies had few data directly examining the difference between prevalence of knee OA and ethnicity, we were unable to differentiate it. Thirdly, we couldn`t assess the prevalence of knee OA for individual country accurately owing to the limitations of the number of original studies. Fourthly, despite we assessed the relationships between risk factors (area of residence, cigarettes, and education level) and the prevalence and incidence of knee OA, those evidences were not strong enough because of the limited number of included studies. Fifthly, considering the preference of the journal and individual, the potential publication bias can hardly be excluded.
In conclusion, this study provides the global prevalence (16⋅0% [95% CI, 14⋅3%−17⋅8%]) and incidence (203 per 10,000 person-years [95% CI, 106–331]) of knee OA. These findings can be used to better estimate the global health burden of knee OA. The knee OA is continuing to be prevalent in global adults and over, especially in older and women. Further prospective cohort studies are warranted to identify modifiable risk factors for providing effectively preventive strategies in the early stages of the disease.
Declaration of Competing Interests
We declare that we have no conflicts of interest.
Acknowledgments
Contributors
LHD, CAY, and LHZ conducted the study protocol. CAY and LHZ searched the databases, selected the literature and extracted the data. CAY and LHZ did the statistical analyses. CAY draw figures, CAY and LHZ prepared the first draft under the guidance of LHD. All authors interpreted the results, reviewed drafts of the Article, and approved the final version. CAY and LHZ contributed equally to this work.
Acknowledgments
We would like to thank the National Natural Science Foundation Council of China.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81772384 and 81572174).
Data sharing statement
The study protocol is available with this publication. No additional unpublished data are available.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2020.100587.
Appendix. Supplementary materials
Appendix 1.1. Search strategy in PUBMED. Appendix 1.2. Search strategy in EMBASE. Appendix 1.3. Search strategy in SCOPUS.
Appendix 2.1. Risk of bias summary of the prevalence studies
Appendix 2.2. Risk of bias graph of the prevalence studies
Appendix 2.1\elsamp #x2013;2. We used the risk of bias tool for prevalence studies developed by Hoy D et\elsamp #x00A0;al. (2012), which comprises 10 items plus a summary assessment. Items 1 to 4 assess the external validity of the study (domains are selection and nonresponse bias), and items 5 to 10 assess the internal validity (items 5 to 9 assess the domain of measurement bias, and item 10 assesses bias related to the analysis). The summary assessment evaluates the overall risk of study bias and is based on the rater`s subjective judgment given responses to the preceding 10 items. Response options for individual items were either low or high risk of bias; Response options for the summary assessment were low, moderate, or high risk of bias.
Appendix 2.3. Risk of bias summary of the incidence studies
Appendix 2.4. Risk of bias graph of the incidence studies
Appendix 2.3\elsamp #x2013;4. we assessed the quality of the incidence studies using risk of bias tool designed by ourselves according to previous validated quality assessment tools, which includes 6 items and a summary assessment: (1) Was the target population clearly defined? (2) Was random sampling method used? (3) Were the samples at risk representative of the target population? (4) Were non-responders clearly described? (5) Were data collection methods standardized? (6) Was validated criteria used to assess the presence of disease? (7) Summary item on the overall risk of study bias. The summary assessment evaluates the overall risk of study bias and is based on the rater`s subjective judgment given responses to the preceding 6 items. Response options for individual items were either low (answer YES) or high (answer NO) risk of bias; Response options for the summary assessment were low, moderate, or high risk of bias.
Appendix 3. The forest plot of the global prevalence of knee osteoarthritis in individuals aged 40 and over
Appendix 4. The forest plot of the prevalence of knee osteoarthritis in a single country
Appendix 5. The forest plot of the prevalence of the radiological knee osteoarthritis
Appendix 6. The forest plot of the prevalence of the symptomatic knee osteoarthritis
Appendix 7. The forest plot of the prevalence of the self-report knee osteoarthritis
Appendix 8. The forest plot of the prevalence of knee osteoarthritis in women
Appendix 9. The forest plot of the prevalence of knee osteoarthritis in men
Appendix 10. The forest plot of the incidence of knee osteoarthritis in a single country
Appendix 11. The relationship between age and the prevalence of knee osteoarthritis: to accurately assess the relationship between age in different genders and the prevalence of knee osteoarthritis, we only extracted data from the study which not only reported the prevalence in women but also in men at each age group (such as 30\elsamp #x2013;39, 40\elsamp #x2013;49, \elsamp #x2026;).
Appendix 12. The relationship between age and the incidence of knee osteoarthritis: because original study didn`t report the incidence in different genders for each age group (such as 40\elsamp #x2013;49, 50\elsamp #x2013;59, \elsamp #x2026;), so we only assessed the relationship between age in all genders and the incidence of knee osteoarthritis.
Appendix 13. The trend of the incidence of knee osteoarthritis changes with age
Appendix 14. The forest plot of the ratio of the prevalence of knee osteoarthritis in women and in men
Appendix 15. The forest plot of the ratio of the incidence of knee osteoarthritis in women and in men
Appendix 16. The forest plot of the ratio of the prevalence of knee osteoarthritis in rural and urban
Appendix 17. The forest plot of the ratio of the prevalence of knee osteoarthritis in Junior and Senior high school and College and above
Appendix 18. The forest plot of the ratio of the prevalence of knee osteoarthritis in Elementary school and below and Junior and Senior high school
Appendix 19. The forest plot of the ratio of the prevalence of knee osteoarthritis in smoking and no-smoking in cross-sectional studies
Appendix 20. The forest plot of the ratio of the incidence of knee osteoarthritis in smoking and no-smoking in prospective cohort studies
Appendix 21. Sensitivity analyses for the estimates of the global prevalence of knee osteoarthritis
Appendix 22. Sensitivity analyses for the estimates of the global incidence of knee osteoarthritis
Appendix 23. The univariate meta-regression results on the prevalence of knee osteoarthritis.
Appendix 24. The univariate meta-regression results on the incidence of knee osteoarthritis.
Appendix 25. The funnel plots for published studies on prevalence of knee osteoarthritis
Appendix 26. The funnel plots for published studies on prevalence of knee osteoarthritis
References
- 1.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2.Vos T., Allen C., Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannuru R.R., Osani M.C., Vaysbrot E.E. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Glyn-Jones S., Palmer A.J., Agricola R. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 5.Darmawan J., Muirden K.D. WHO-ILAR COPCORD perspectives past, present, and future. J Rheumatol. 2003;30(11):2312–2314. [PubMed] [Google Scholar]
- 6.James S.L., Abate D., Abate K.H. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel-Pelletier J., Barr A.J., Cicuttini F.M. Osteoarthritis. Nat Rev Dis Primers. 2016;2 doi: 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 8.Litwic A., Edwards M.H., Dennison E.M., Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du H., Chen S.L., Bao C.D. Prevalence and risk factors of knee osteoarthritis in Huang-Pu District, Shanghai, China. Rheumatol Int. 2005;25(8):585–590. doi: 10.1007/s00296-004-0492-7. [DOI] [PubMed] [Google Scholar]
- 10.Wallace I.J., Worthington S., Felson D.T. Knee osteoarthritis has doubled in prevalence since the mid-20th century. PNAS. 2017;114(35):9332–9336. doi: 10.1073/pnas.1703856114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. w64. [DOI] [PubMed] [Google Scholar]
- 12.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 13.Stevens G.A., Alkema L., Black R.E. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388(10062):e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 14.Hvidberg M.F., Johnsen S.P., Davidsen M., Ehlers L. Pharmacoeconomics - open. 2019. A nationwide study of prevalence rates and characteristics of 199 chronic conditions in Denmark. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swain S., Sarmanova A., Mallen C. Trends in incidence and prevalence of osteoarthritis in the United Kingdom: findings from the Clinical Practice Research Datalink (CPRD) Osteoarthritis Cartilage. 2020 doi: 10.1016/j.joca.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Prieto-Alhambra D., Judge A., Javaid M.K., Cooper C., Diez-Perez A., Arden N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73(9):1659–1664. doi: 10.1136/annrheumdis-2013-203355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Postler A., Ramos A.L., Goronzy J. Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: an analysis based on health insurance claims data. Clin Interv Aging. 2018;13:2339–2349. doi: 10.2147/CIA.S174741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieto-Alhambra D., Nogues X., Javaid M.K. An increased rate of falling leads to a rise in fracture risk in postmenopausal women with self-reported osteoarthritis: a prospective multinational cohort study (GLOW) Ann Rheum Dis. 2013;72(6):911–917. doi: 10.1136/annrheumdis-2012-201451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan N.F., Harrison S.E., Rose P.W. Validity of diagnostic coding within the general practice research database: a systematic review. Br J General Practice. 2010;60(572):e128–e136. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Department of Economic and Social Affairs, Population Division. World Population Prospects, the 2019 revision. United Nations, 2019. https://esa.un.org/unpd/wpp/ (accessed May 29, 2020).
- 21.Hoy D., Brooks P., Woolf A. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 23.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 24.Pereira D., Peleteiro B., Araújo J., Branco J., Santos R.A., Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage. 2011;19(11):1270–1285. doi: 10.1016/j.joca.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Safiri S., Kolahi A.A., Smith E. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the global burden of disease study 2017. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2019-216515. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson D.T., Lawrence R.C., Dieppe P.A. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 28.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30(2):160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos E.M., Arden N.K. Strategies for the prevention of knee osteoarthritis. Nature reviews Rheumatology. 2016;12(2):92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 31.Hannan M.T., Anderson J.J., Pincus T., Felson D.T. Educational attainment and osteoarthritis: differential associations with radiographic changes and symptom reporting. J Clin Epidemiol. 1992;45(2):139–147. doi: 10.1016/0895-4356(92)90006-9. [DOI] [PubMed] [Google Scholar]
- 32.Callahan L.F., Shreffler J., Siaton B.C. Limited educational attainment and radiographic and symptomatic knee osteoarthritis: a cross-sectional analysis using data from the Johnston County (North Carolina) Osteoarthritis Project. Arthritis Res Ther. 2010;12(2):R46. doi: 10.1186/ar2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hui M., Doherty M., Zhang W. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann Rheum Dis. 2011;70(7):1231–1237. doi: 10.1136/ard.2010.142323. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y., Xu L., Nevitt M.C. Comparison of the prevalence of knee osteoarthritis between the elderly Chinese population in Beijing and whites in the United States: the Beijing osteoarthritis study. Arthritis Rheum. 2001;44(9):2065–2071. doi: 10.1002/1529-0131(200109)44:9<2065::AID-ART356>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Minh Hoa T.T., Darmawan J., Chen S.L., Van Hung N., Thi Nhi C., Ngoc An T. Prevalence of the rheumatic diseases in urban Vietnam: a WHO-ILAR COPCORD study. J Rheumatol. 2003;30(10):2252–2256. [PubMed] [Google Scholar]
- 36.Minaur N., Sawyers S., Parker J., Darmawan J. Rheumatic disease in an Australian Aboriginal community in North Queensland, Australia. A WHO-ILAR COPCORD survey. J Rheumatol. 2004;31(5):965–972. [PubMed] [Google Scholar]
- 37.Senna E.R., De Barros A.L., Silva E.O. Prevalence of rheumatic diseases in Brazil: a study using the COPCORD approach. J Rheumatol. 2004;31(3):594–597. [PubMed] [Google Scholar]
- 38.Haq S.A., Darmawan J., Islam M.N. Prevalence of rheumatic diseases and associated outcomes in rural and urban communities in Bangladesh: a COPCORD study. J Rheumatol. 2005;32(2):348–353. [PubMed] [Google Scholar]
- 39.Kaçar C., Gilgil E., Urhan S. The prevalence of symptomatic knee and distal interphalangeal joint osteoarthritis in the urban population of Antalya, Turkey. Rheumatol Int. 2005;25(3):201–204. doi: 10.1007/s00296-003-0415-z. [DOI] [PubMed] [Google Scholar]
- 40.Salaffi F., De Angelis R., Grassi W. Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. I. The mapping study. Clin Exp Rheumatol. 2005;23(6):819–828. [PubMed] [Google Scholar]
- 41.Wang W., Wang K.Z., Dang X.Q. Relevant factors for knee osteoarthritis in middle aged and old population. Chin J Clin Rehab. 2006;10(44):15. 8. [Google Scholar]
- 42.Zeng Q.Y., Zang C.H., Li X.F., Dong H.Y., Zhang A.L., Lin L. Associated risk factors of knee osteoarthritis: a population survey in Taiyuan, China. Chin Med J. 2006;119(18):1522–1527. [PubMed] [Google Scholar]
- 43.Felson D.T., Niu J., Clancy M., Sack B., Aliabadi P., Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum. 2007;57(1):6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 44.Tangtrakulwanich B., Chongsuvivatwong V., Geater A.F. Habitual floor activities increase risk of knee osteoarthritis. Clin Orthop Relat Res. 2007;454:147–154. doi: 10.1097/01.blo.0000238808.72164.1d. [DOI] [PubMed] [Google Scholar]
- 45.Davatchi F., Jamshidi A.R., Banihashemi A.T. WHO-ILAR COPCORD study (Stage 1, Urban Study) in Iran. J Rheumatol. 2008;35(7):1384. [PubMed] [Google Scholar]
- 46.Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Prevalence and burden of osteoarthritis: results from a population survey in Norway. J Rheumatol. 2008;35(4):677–684. [PubMed] [Google Scholar]
- 47.Quintana J.M., Arostegui I., Escobar A., Azkarate J., Goenaga J.I., Lafuente I. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med. 2008;168(14):1576–1584. doi: 10.1001/archinte.168.14.1576. [DOI] [PubMed] [Google Scholar]
- 48.Joshi V.L., Chopra A. Is there an urban-rural divide? Population surveys of rheumatic musculoskeletal disorders in the Pune Region of India using the COPCORD Bhigwan model. J Rheumatol. 2009;36(3):614–622. doi: 10.3899/jrheum.080675. [DOI] [PubMed] [Google Scholar]
- 49.Kang X., Fransen M., Zhang Y. The high prevalence of knee osteoarthritis in a rural Chinese population: the Wuchuan osteoarthritis study. Arthritis Rheum. 2009;61(5):641–647. doi: 10.1002/art.24464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimura N., Muraki S., Oka H. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metab. 2009;27(5):620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 51.Kim I., Kim H.A., Seo Y.I., Song Y.W., Jeong J.Y., Kim D.H. The prevalence of knee osteoarthritis in elderly community residents in Korea. J Korean Med Sci. 2010;25(2):293–298. doi: 10.3346/jkms.2010.25.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salve H., Gupta V., Palanivel C., Yadav K., Singh B. Prevalence of knee osteoarthritis amongst perimenopausal women in an urban resettlement colony in South Delhi. Indian J Public Health. 2010;54(3):155–157. doi: 10.4103/0019-557X.75739. [DOI] [PubMed] [Google Scholar]
- 53.Cibere J., Zhang H., Thorne A. Association of clinical findings with pre-radiographic and radiographic knee osteoarthritis in a population-based study. Arthritis Care Res (Hoboken) 2010;62(12):1691–1698. doi: 10.1002/acr.20314. [DOI] [PubMed] [Google Scholar]
- 54.Guillemin F., Rat A.C., Mazieres B. Prevalence of symptomatic hip and knee osteoarthritis: a two-phase population-based survey1. Osteoarthritis Cartilage. 2011;19(11):1314–1322. doi: 10.1016/j.joca.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Horváth G., Koroknai G., Ács B., Than P., Bellyei Á., Illés T. Prevalence of radiographic primary hip and knee osteoarthritis in a representative Central European population. Int Orthop. 2011;35(7):971–975. doi: 10.1007/s00264-010-1069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Namali H., Fonseka P., Gunathilake N. Prevalence of knee osteoarthritis among community dwelling older adults in Colombo district. Intern Med J. 2011;41:24. [Google Scholar]
- 57.Cakır N., Pamuk Ö N., Derviş E. The prevalences of some rheumatic diseases in western Turkey: havsa study. Rheumatol Int. 2012;32(4):895–908. doi: 10.1007/s00296-010-1699-4. [DOI] [PubMed] [Google Scholar]
- 58.Chaaya M., Slim Z.N., Habib R.R. High burden of rheumatic diseases in Lebanon: a COPCORD study. Int J Rheum Dis. 2012;15(2):136–143. doi: 10.1111/j.1756-185X.2011.01682.x. [DOI] [PubMed] [Google Scholar]
- 59.Ajit N.E., Nandish B., Fernandes R. Prevalence of knee osteoarthritis in rural areas of Bangalore urban district. Ind J Rheumatol. 2012;7:S16. [Google Scholar]
- 60.Jiang L., Rong J., Zhang Q. Prevalence and associated factors of knee osteoarthritis in a community-based population in Heilongjiang, Northeast China. Rheumatol Int. 2012;32(5):1189–1195. doi: 10.1007/s00296-010-1773-y. [DOI] [PubMed] [Google Scholar]
- 61.Sandoughi M., Zakeri Z., Tehrani Banihashemi A. Prevalence of musculoskeletal disorders in southeastern Iran: a WHO-ILAR COPCORD study (stage 1, urban study) Int J Rheum Dis. 2013;16(5):509–517. doi: 10.1111/1756-185X.12110. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J., Song L., Liu G. Risk factors for and prevalence of knee osteoarthritis in the rural areas of Shanxi Province, North China: a COPCORD study. Rheumatol Int. 2013;33(11):2783–2788. doi: 10.1007/s00296-013-2809-x. [DOI] [PubMed] [Google Scholar]
- 63.YefiL H., Hepgüler S., Öztürk C., Çapaci K., YesiL M. Prevalence of symptomatic knee, hand and hip osteoarthritis among individuals 40 years or older: a study conducted in izmir city. Acta Orthop Traumatol Turc. 2013;47(4):231–235. doi: 10.3944/aott.2013.2731. [DOI] [PubMed] [Google Scholar]
- 64.Xiang Z.Y., Mao J.C., Qu H.R. Epidemiological study on risk factors of knee osteoarthritis in Shanggang community in Pudong New District. J Shanghai Jiaotong Univ (Med Sci) 2013;33(3):318–322. [Google Scholar]
- 65.Turkiewicz A., De Verdier M.G., Engström G., Lohmander S., Englund M. Twenty-first century prevalence of frequent knee pain, radiographic, symptomatic and clinical knee osteoarthritis according to American college of rheumatology criteria in Southern Sweden. Arthritis Rheum. 2013;65:S39. [Google Scholar]
- 66.Thomas E., Peat G., Croft P. Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology (Oxford) 2014;53(2):338–345. doi: 10.1093/rheumatology/ket346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Visser A.W., de Mutsert R., Loef M. The role of fat mass and skeletal muscle mass in knee osteoarthritis is different for men and women: the NEO study. Osteoarthritis Cartilage. 2014;22(2):197–202. doi: 10.1016/j.joca.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Singh A.K., Kalaivani M., Krishnan A., Aggarwal P.K., Gupta S.K. Prevalence of osteoarthritis of knee among elderly persons in urban slums using American college of rheumatology (ACR) criteria. J Clin Diagnostic Res. 2014;8(9):Jc09–Jc11. doi: 10.7860/JCDR/2014/7763.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tehrani-Banihashemi A., Davatchi F., Jamshidi A.R., Faezi T., Paragomi P., Barghamdi M. Prevalence of osteoarthritis in rural areas of Iran: a WHO-ILAR COPCORD study. Int J Rheum Dis. 2014;17(4):384–388. doi: 10.1111/1756-185X.12312. [DOI] [PubMed] [Google Scholar]
- 70.Ho-Pham L.T., Lai T.Q., Mai L.D., Doan M.C., Pham H.N., Nguyen T.V. Prevalence of radiographic osteoarthritis of the knee and its relationship to self-reported pain. PLoS ONE. 2014;9(4):e94563. doi: 10.1371/journal.pone.0094563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haouichat C., Aiche M.F., Lekhal F.Z., Melal S., Djoudi H., Bouzid F.Z. Prevalence of knee and digital osteoarthritis in women in Douera City (Algiers): a population-based study. Osteoporos Int. 2014;25:S202. [Google Scholar]
- 72.Edwards M.H., van der Pas S., Denkinger M.D. Relationships between physical performance and knee and hip osteoarthritis: findings from the European Project on Osteoarthritis (EPOSA) Age Ageing. 2014;43(6):806–813. doi: 10.1093/ageing/afu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ara R., Haq S., Hoy D., Islam N., Alam Z., Rahman M. Estimation of the prevalence of knee osteoarthritis and its impacts on quality of life in rural Bangladesh. Intern Med J. 2014;44:17. [Google Scholar]
- 74.Moghimi N., Davatchi F., Rahimi E. WHO-ILAR COPCORD study (stage 1, urban study) in Sanandaj, Iran. Clin Rheumatol. 2015;34(3):535–543. doi: 10.1007/s10067-013-2430-0. [DOI] [PubMed] [Google Scholar]
- 75.Granados Y., Cedeño L., Rosillo C. Prevalence of musculoskeletal disorders and rheumatic diseases in an urban community in Monagas State, Venezuela: a COPCORD study. Clin Rheumatol. 2015;34(5):871–877. doi: 10.1007/s10067-014-2689-9. [DOI] [PubMed] [Google Scholar]
- 76.Zeng S.Y., Gong Y., Zhang Y.P. Changes in the prevalence of rheumatic diseases in Shantou, China, in the past three decades: a COPCORD study. PLoS ONE. 2015;10(9) doi: 10.1371/journal.pone.0138492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plotnikoff R., Karunamuni N., Lytvyak E. Osteoarthritis prevalence and modifiable factors: a population study chronic disease epidemiology. BMC Public Health. 2015;15(1) doi: 10.1186/s12889-015-2529-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cho H.J., Morey V., Kang J.Y., Kim K.W., Kim T.K. Prevalence and risk factors of spine, shoulder, hand, hip, and knee osteoarthritis in community-dwelling Koreans older than age 65 years. Clin Orthop Relat Res. 2015;473(10):3307–3314. doi: 10.1007/s11999-015-4450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang X., Wang S., Zhan S. The prevalence of symptomatic knee osteoarthritis in China: results from the China health and retirement longitudinal study. Arthrit Rheumatol (Hoboken, NJ) 2016;68(3):648–653. doi: 10.1002/art.39465. [DOI] [PubMed] [Google Scholar]
- 80.Guevara-Pacheco S., Feicán-Alvarado A., Sanín L.H. Prevalence of musculoskeletal disorders and rheumatic diseases in Cuenca, Ecuador: a WHO-ILAR COPCORD study. Rheumatol Int. 2016;36(9):1195–1204. doi: 10.1007/s00296-016-3446-y. [DOI] [PubMed] [Google Scholar]
- 81.Pal C.P., Singh P., Chaturvedi S., Pruthi K.K., Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian J Orthop. 2016;50(5):518–522. doi: 10.4103/0019-5413.189608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Branco J.C., Rodrigues A.M., Gouveia N. Prevalence of rheumatic and musculoskeletal diseases and their impact on health-related quality of life, physical function and mental health in Portugal: results from EpiReumaPt- a national health survey. RMD Open. 2016;2(1) doi: 10.1136/rmdopen-2015-000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu Y., Zhang H., Liang N. Prevalence and associated factors of knee osteoarthritis in a rural Chinese adult population: an epidemiological survey. BMC Public Health. 2016;16:94. doi: 10.1186/s12889-016-2782-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deshpande B.R., Katz J.N., Solomon D.H. Number of persons with symptomatic knee osteoarthritis in the us: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res (Hoboken) 2016;68(12):1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al Saleh J., Sayed M.E., Monsef N., Darwish E. The prevalence and the determinants of musculoskeletal diseases in emiratis attending primary health care clinics in Dubai. Oman Med J. 2016;31(2):117–123. doi: 10.5001/omj.2016.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy L.B., Moss S., Do B.T. Annual incidence of knee symptoms and four knee osteoarthritis outcomes in the johnston county osteoarthritis project. Arthritis Care Res (Hoboken) 2016;68(1):55–65. doi: 10.1002/acr.22641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kolahi S., Khabbazi A., Malek Mahdavi A. Prevalence of musculoskeletal disorders in azar cohort population in northwest of Iran. Rheumatol Int. 2017;37(4):495–502. doi: 10.1007/s00296-017-3661-1. [DOI] [PubMed] [Google Scholar]
- 88.Ahmed S., Haq S.A., Al-qadir A.Z., Rahman M.M., Paul S. Survey on prevalence of rheumatic disorders in Bangladeshi adults. Ann Rheum Dis. 2017;76:1044–1045. [Google Scholar]
- 89.Prashansanie Hettihewa A., Gunawardena N.S., Atukorala I., Hassan F., Lekamge I.N., Hunter D.J. Prevalence of knee osteoarthritis in a suburban, Srilankan, adult female population: a population-based study. Int J Rheum Dis. 2018;21(2):394–401. doi: 10.1111/1756-185X.13225. [DOI] [PubMed] [Google Scholar]
- 90.Venkatachalam J., Natesan M., Eswaran M., Johnson A.K.S., Bharath V., Singh Z. Prevalence of osteoarthritis of knee joint among adult population in a rural area of Kanchipuram District, Tamil Nadu. Indian J Public Health. 2018;62(2):117–122. doi: 10.4103/ijph.IJPH_344_16. [DOI] [PubMed] [Google Scholar]
- 91.Vega-Hinojosa O., Cardiel M.H., Ochoa-Miranda P. Prevalence of musculoskeletal manifestations and related disabilities in a Peruvian urban population living at high altitude. COPCORD Study. Stage I. Reumatol Clin. 2018;14(5):278–284. doi: 10.1016/j.reuma.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 92.Seoane-Mato D., Sánchez-Piedra C., Díaz-González F., Bustabad S. Prevalence of rheumatic diseases in adult population in Spain. Episer 2016 study. Ann Rheum Dis. 2018;77:535–536. [Google Scholar]
- 93.Kumar P., Alok R., Das S.K., Srivastava R., Agarwal G.G. Distribution of rheumatological diseases in rural and urban areas: an adapted COPCORD Stage I Phase III survey of Lucknow district in north India. Int J Rheum Dis. 2018;21(11):1894–1899. doi: 10.1111/1756-185X.13382. [DOI] [PubMed] [Google Scholar]
- 94.Gavali M., Devarasetti P.K., Rajasekhar L. Prevalence of musculoskeletal pain in an urban slum: community-oriented program for control of rheumatic disorders study from Hyderabad. Ind J Rheumatol. 2018;13(6):S205–S2S6. [Google Scholar]
- 95.Ananto M., Rahman P.A., Al Rasyid H., Wahono C.S., Handono K., Kalim H. Risk factors for knee osteoarthritis in Malang population. Int J Rheum Dis. 2018;21:26. [Google Scholar]
- 96.Leung Y.Y., Ma S., Noviani M. Validation of screening questionnaires for evaluation of knee osteoarthritis prevalence in the general population of Singapore. Int J Rheum Dis. 2018;21(3):629–638. doi: 10.1111/1756-185X.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Macías-Hernández S.I., Zepeda-Borbón E.R., Lara-Vázquez B.I. Prevalence of clinical and radiological osteoarthritis in knee, hip, and hand in an urban adult population of Mexico city. Reumatol Clin. 2018 doi: 10.1016/j.reuma.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 98.Badley E.M., Yip C., Perruccio A.V. Symptoms compatible with osteoarthritis and self-reported osteoarthritis in the population: findings from the Canadian longitudinal study on aging. Arthr Rheumatol. 2018;70:1289–1290. [Google Scholar]
- 99.Rodriguez-Veiga D., González-Martín C., Pertega-Díaz S., Seoane-Pillado T., Barreiro-Quintás M., Balboa-Barreiro V. Prevalence of knee osteoarthritis in a random population sample in people aged 40 and over. Gac Med Mex. 2019;155(1):39–45. doi: 10.24875/GMM.18004527. [DOI] [PubMed] [Google Scholar]
- 100.Guevara S.V., Feicán E.A., Peláez I. Prevalence of rheumatic diseases and quality of life in the saraguro indigenous people, ecuador: a cross-sectional community-based study. J Clin Rheumatol. 2019;26(7S Suppl 2):S139–S147. doi: 10.1097/RHU.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 101.Hong J.W., Noh J.H., Kim D.J. The prevalence of and demographic factors associated with radiographic knee osteoarthritis in Korean adults aged ≥ 50 years: the 2010-2013 Korea national health and nutrition examination survey. PLoS ONE. 2020;15(3) doi: 10.1371/journal.pone.0230613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sasaki E., Ota S., Chiba D. Early knee osteoarthritis prevalence is highest among middle-aged adult females with obesity based on new set of diagnostic criteria from a large sample cohort study in the Japanese general population. Knee Surgery, Sports Traumatol, Arthroscopy. 2020;28(3):984–994. doi: 10.1007/s00167-019-05614-z. [DOI] [PubMed] [Google Scholar]
- 103.Oliveria S.A., Felson D.T., Reed J.I., Cirillo P.A., Walker A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 104.Felson D.T., Zhang Y., Hannan M.T. The incidence and natural history of knee osteoarthritis in the elderly. The framingham osteoarthritis study. Arthritis Rheum. 1995;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 105.Slemenda C., Heilman D.K., Brandt K.D. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41(11):1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 106.Gelber A.C., Hochberg M.C., Mead L.A., Wang N.Y., Wigley F.M., Klag M.J. Body mass index in young men and the risk of subsequent knee and hip osteoarthritis. Am J Med. 1999;107(6):542–548. doi: 10.1016/s0002-9343(99)00292-2. [DOI] [PubMed] [Google Scholar]
- 107.Cooper C., Snow S., McAlindon T.E. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43(5):995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 108.Duncan R., Peat G., Thomas E., Hay E.M., Croft P. Incidence, progression and sequence of development of radiographic knee osteoarthritis in a symptomatic population. Ann Rheum Dis. 2011;70(11):1944–1948. doi: 10.1136/ard.2011.151050. [DOI] [PubMed] [Google Scholar]
- 109.Driban J.B., Bannuru R.R., Eaton C.B. The incidence and characteristics of accelerated knee osteoarthritis among women: the Chingford cohort. BMC Musculoskelet Disord. 2020;21(1):60. doi: 10.1186/s12891-020-3073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grotle M., Hagen K.B., Natvig B., Dahl F.A., Kvien T.K. Obesity and osteoarthritis in knee, hip and/or hand: an epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet Disord. 2008;9:132. doi: 10.1186/1471-2474-9-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mork P.J., Holtermann A., Nilsen T.I. Effect of body mass index and physical exercise on risk of knee and hip osteoarthritis: longitudinal data from the Norwegian HUNT Study. J Epidemiol Community Health. 2012;66(8):678–683. doi: 10.1136/jech-2011-200834. [DOI] [PubMed] [Google Scholar]
- 112.Reijman M., Pols H.A., Bergink A.P. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nishimura A., Hasegawa M., Kato K., Yamada T., Uchida A., Sudo A. Risk factors for the incidence and progression of radiographic osteoarthritis of the knee among Japanese. Int Orthop. 2011;35(6):839–843. doi: 10.1007/s00264-010-1073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Muraki S., Akune T., Oka H. Incidence and risk factors for radiographic knee osteoarthritis and knee pain in Japanese men and women: a longitudinal population-based cohort study. Arthritis Rheum. 2012;64(5):1447–1456. doi: 10.1002/art.33508. [DOI] [PubMed] [Google Scholar]
- 115.Sasaki E., Tsuda E., Yamamoto Y. Serum hyaluronic acid concentration predicts the progression of joint space narrowing in normal knees and established knee osteoarthritis - a five-year prospective cohort study. Arthritis Res Ther. 2015;17:283. doi: 10.1186/s13075-015-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Toivanen A.T., Heliövaara M., Impivaara O. Obesity, physically demanding work and traumatic knee injury are major risk factors for knee osteoarthritis–a population-based study with a follow-up of 22 years. Rheumatology (Oxford) 2010;49(2):308–314. doi: 10.1093/rheumatology/kep388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1.1. Search strategy in PUBMED. Appendix 1.2. Search strategy in EMBASE. Appendix 1.3. Search strategy in SCOPUS.
Appendix 2.1. Risk of bias summary of the prevalence studies
Appendix 2.2. Risk of bias graph of the prevalence studies
Appendix 2.1\elsamp #x2013;2. We used the risk of bias tool for prevalence studies developed by Hoy D et\elsamp #x00A0;al. (2012), which comprises 10 items plus a summary assessment. Items 1 to 4 assess the external validity of the study (domains are selection and nonresponse bias), and items 5 to 10 assess the internal validity (items 5 to 9 assess the domain of measurement bias, and item 10 assesses bias related to the analysis). The summary assessment evaluates the overall risk of study bias and is based on the rater`s subjective judgment given responses to the preceding 10 items. Response options for individual items were either low or high risk of bias; Response options for the summary assessment were low, moderate, or high risk of bias.
Appendix 2.3. Risk of bias summary of the incidence studies
Appendix 2.4. Risk of bias graph of the incidence studies
Appendix 2.3\elsamp #x2013;4. we assessed the quality of the incidence studies using risk of bias tool designed by ourselves according to previous validated quality assessment tools, which includes 6 items and a summary assessment: (1) Was the target population clearly defined? (2) Was random sampling method used? (3) Were the samples at risk representative of the target population? (4) Were non-responders clearly described? (5) Were data collection methods standardized? (6) Was validated criteria used to assess the presence of disease? (7) Summary item on the overall risk of study bias. The summary assessment evaluates the overall risk of study bias and is based on the rater`s subjective judgment given responses to the preceding 6 items. Response options for individual items were either low (answer YES) or high (answer NO) risk of bias; Response options for the summary assessment were low, moderate, or high risk of bias.
Appendix 3. The forest plot of the global prevalence of knee osteoarthritis in individuals aged 40 and over
Appendix 4. The forest plot of the prevalence of knee osteoarthritis in a single country
Appendix 5. The forest plot of the prevalence of the radiological knee osteoarthritis
Appendix 6. The forest plot of the prevalence of the symptomatic knee osteoarthritis
Appendix 7. The forest plot of the prevalence of the self-report knee osteoarthritis
Appendix 8. The forest plot of the prevalence of knee osteoarthritis in women
Appendix 9. The forest plot of the prevalence of knee osteoarthritis in men
Appendix 10. The forest plot of the incidence of knee osteoarthritis in a single country
Appendix 11. The relationship between age and the prevalence of knee osteoarthritis: to accurately assess the relationship between age in different genders and the prevalence of knee osteoarthritis, we only extracted data from the study which not only reported the prevalence in women but also in men at each age group (such as 30\elsamp #x2013;39, 40\elsamp #x2013;49, \elsamp #x2026;).
Appendix 12. The relationship between age and the incidence of knee osteoarthritis: because original study didn`t report the incidence in different genders for each age group (such as 40\elsamp #x2013;49, 50\elsamp #x2013;59, \elsamp #x2026;), so we only assessed the relationship between age in all genders and the incidence of knee osteoarthritis.
Appendix 13. The trend of the incidence of knee osteoarthritis changes with age
Appendix 14. The forest plot of the ratio of the prevalence of knee osteoarthritis in women and in men
Appendix 15. The forest plot of the ratio of the incidence of knee osteoarthritis in women and in men
Appendix 16. The forest plot of the ratio of the prevalence of knee osteoarthritis in rural and urban
Appendix 17. The forest plot of the ratio of the prevalence of knee osteoarthritis in Junior and Senior high school and College and above
Appendix 18. The forest plot of the ratio of the prevalence of knee osteoarthritis in Elementary school and below and Junior and Senior high school
Appendix 19. The forest plot of the ratio of the prevalence of knee osteoarthritis in smoking and no-smoking in cross-sectional studies
Appendix 20. The forest plot of the ratio of the incidence of knee osteoarthritis in smoking and no-smoking in prospective cohort studies
Appendix 21. Sensitivity analyses for the estimates of the global prevalence of knee osteoarthritis
Appendix 22. Sensitivity analyses for the estimates of the global incidence of knee osteoarthritis
Appendix 23. The univariate meta-regression results on the prevalence of knee osteoarthritis.
Appendix 24. The univariate meta-regression results on the incidence of knee osteoarthritis.
Appendix 25. The funnel plots for published studies on prevalence of knee osteoarthritis
Appendix 26. The funnel plots for published studies on prevalence of knee osteoarthritis