Abstract
The sensitivity to volatile carbon dioxide (CO2) produced by humans and other animals is a critical component in the host preference behaviors of the malaria vector mosquito Anopheles coluzzii. The molecular receptors responsible for the ability to sense CO2 are encoded by three putative gustatory receptor (Gr) genes (Gr22,23,24) which are expressed in a distinctive array of sensory neurons housed in maxillary palp capitate peg sensilla of An. coluzzii. Despite the identification of these components and subsequent studies, there is a paucity of understanding regarding the respective roles of these three GRs in the mosquito’s CO2 transduction process. To address this, we have used CRISPR/Cas9-based gene editing techniques combined with in vivo electrophysiological recordings to directly examine the role of Gr22,23,24 in detecting CO2 in An. coluzzii. These studies reveal that both Gr23 and Gr24 are absolutely required to maintain in vivo CO2 sensitivity while, in contrast, Gr22 knock out mutants are still able to respond to CO2 stimuli albeit with significantly weaker sensitivity. Our data supports a model in which Gr22 plays a modulatory role to enhance the functionality of Gr23/24 complexes that are responsible for CO2 sensitivity of mosquitoes.
Keywords: Anopheles coluzzii, CO2, Gustatory receptor, CRISPR/Cas9, Malaria, Single sensillum recording
Graphical Abstract
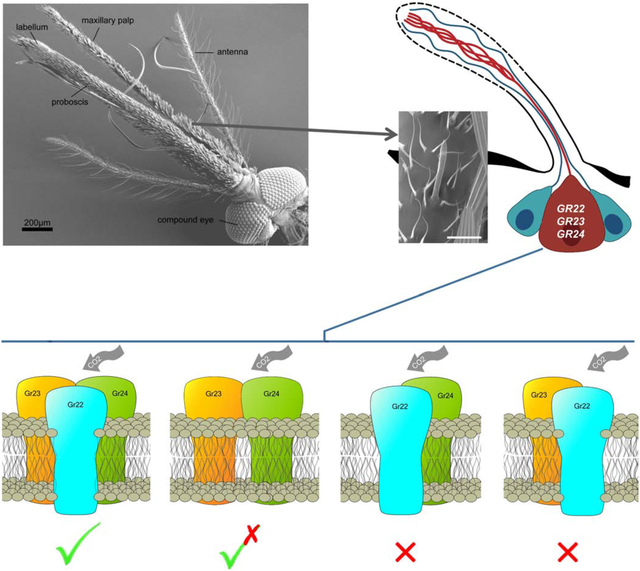
Introduction
The malaria parasite Plasmodium falciparum infected an estimated 228 million people worldwide in 2018, accounting for more than 400,000 deaths (WHO, 2019). Accordingly, controlling Anopheles mosquitoes, which are the sole vectors for transmitting human malaria, has long been a formidable concern to public health. Blood feeding is a requisite activity for the reproductive cycle in female Anopheles mosquitoes and in that context serves as the mode of transport for P. falciparium to transit between mosquito vectors and host animals (Aly et al., 2009; Beier, 1998; Whitten et al., 2006).
The mosquito host-seeking process relies heavily on a broad array of sensory cues released from the human body including heat, odors, and CO2 which in particular has proven to play a critical role in long-range host seeking by mosquitoes (Cooperband and Carde, 2006; Healey andand Copland, 1995; Lorenz et al., 2013). This is exemplified by the ability of mosquitoes to detect CO2 at distances of nearly 50 meters for An. arabiensis (Lorenz et al., 2013) and 150 centimeters for Culex mosquitoes (Cooperband and Carde, 2006). Numerous studies have shown that when CO2 is combined with other sensory cues such as heat or odors in creating mosquito traps, the trapping efficiency is considerably higher than when using the heat or odor alone (Geier and Boeckh, 1999; Kline et al., 2012; Spitzen et al., 2008). Indeed, CO2 is the most common component of odor baits found in commercial mosquito traps, which illustrates the mosquito’s remarkable attraction to it in the field (Hoel et al., 2007; Kline et al., 1990; Roiz et al., 2012).
Mosquito CO2 detection is primarily mediated through the maxillary palp which is an important accessory olfactory head appendage (Grant et al., 1995; Lu et al., 2007; Syed and Leal, 2007). While there appears to be only a single type of olfactory sensillum on the maxillary palp, the capitate-peg (cp), recent studies suggest there may be a degree of functional heterogeneity among these seemingly uniform sensory structures (Ye et al., 2020). That said, there are three different neurons housed in the cp sensillum only one of which, the cpA neuron, is responsible for detecting CO2 and other cues; the two other neurons (cpB/C) respond to a range of odors associated with human emanations and other biological sources (Lu et al., 2007). Molecular studies in Anopheles gambiae revealed three gustatory receptor genes, Grs 22, 23, and 24, are expressed in the cpA neuron and play a key role in its firing in response to CO2 stimulation (Lu et al., 2007).
Phylogenetic analyses reveal the three CO2-sensitive Gr genes are highly conserved across insect taxa, extending across dipterans and broad evolutionary distances to include Coleoptera, Hemiptera, Hymenoptera and Lepidoptera (Benoit et al., 2016; McMeniman et al., 2014; Robertson and Kent, 2009; Terrapon et al., 2014). Paradoxically, while the genomes of most insect species contain homologs of all three CO2-sensitive Gr genes, the common fruit fly (Drosophila melanogaster) has only two CO2-sensitive Gr genes, Gr21a and Gr63a which are orthologous to Gr1/22 and Gr3/24 in Aedes and Anopheles mosquitoes, respectively (Kwon et al., 2007). Considering the evolutionary advancement of Drosophila in Hexapods this is likely to be the result of a selective gene-loss event for the third (Gr2/23) CO2 receptor gene (Misof et al., 2014). Nevertheless, while D. melanogaster only possess two CO2-sensitive GRs on the CO2-sensitive ab1C neuron, this complex is clearly sufficient for sensing environmental CO2 (Jones et al., 2007). Heterologous over-expression of Gr21a and Gr63a in the Drosophila ab3A neuron conferred CO2 sensitivity, further confirming these genes are sufficient in mediating CO2 responses in Drosophila (Kwon et al., 2007). These results raise questions as to the requirement and role of the third Gr gene in the CO2-receptor triad complex that is likely to be active in other insect species. These issues are especially salient for mosquitoes which rely heavily on CO2-responses for obtaining reproductively essential blood meals and largely forms the basis of their vectorial capacity insofar the transmission of malaria and a range of arboviral diseases.
To answer these questions, we have taken an in vivo approach in the Afrotropical malaria vector mosquito An. coluzzii using gene-editing techniques to knock out each CO2-sensitive Gr gene and directly characterize the cpA neuron’s response to CO2 in mutant mosquitoes. Comprehensive interrogation of the CO2-sensitive neurons of Gr22, 23, and 24 mutant mosquitoes reveals that Gr22 plays a modulatory role to enhance the essential and irreplaceable functionality of Gr23/24 complexes that together are responsible for CO2 sensitivity of An. coluzzii. Our in vivo studies align with similar models derived from insect and Xenopus heterologous expression studies of the three CO2-sensitive Grs from Aedes aegypti and Culex quinquefaciatus (Kumar et al., 2020; Xu et al., 2020), respectively, suggesting that mosquitoes utilize a similarly distinctive mechanism for CO2 reception.
Material and Methods
Mosquito maintenance
Anopheles coluzzii (SUA 2La/2La), previously known as Anopheles gambiae sensu stricto “M-form”(Coetzee et al., 2013), originated from Suakoko, Liberia, were reared as described (Fox et al., 2001; Qiu et al., 2004) and 5- to 7-day-old females that had not been blood fed were used for all experiments. All mosquito lines were reared at 27°C, 75% relative humidity under a 12:12 light-dark cycle and supplied with 10% sucrose water in the Vanderbilt University Insectary.
Electrophysiology
Single sensillum recordings (SSR) were carried out as previously described (Liu et al., 2013) with minor modifications. Mated female mosquitoes (4–10 days after eclosion) were mounted on a microscope slide (76 × 26 mm) (Ghaninia et al., 2007). Maxillary palps were fixed using double-sided tape to a cover slip resting on a small bead of dental wax to facilitate manipulation and the cover slip was placed at approximately 30 degrees to the mosquito head. Once mounted, the specimen was placed under an Olympus BX51WI microscope and antennae were viewed at high magnification (1000×). Two tungsten microelectrodes were sharpened in 10% KNO2 at 10 V. The grounded reference electrode was inserted into the compound eye of the mosquito using a WPI micromanipulator and the recording electrode was connected to the pre-amplifier (Syntech universal AC/DC 10x, Syntech, Hilversum, The Netherlands) and inserted into the shaft of the olfactory sensillum to complete the electrical circuit to extracellularly record OSN potentials (Den Otter et al., 1980). Controlled manipulation of the recording electrode was performed using a Burleigh micromanipulator (Model PCS6000). The preamplifier was connected to an analog-to-digital signal converter (IDAC-4, Syntech, Hilversum, The Netherlands), which in turn was connected to a PC-computer for signal recording and visualization.
Customized CO2 tanks at different concentrations (0.001%, 0.005%, 0.01%, 0.05%, 0.1%. 0.5%, 1%) were purchased from Airgas Inc. (Nashville, TN). Compounds with highest purity, typically ≧99% (Sigma-Aldrich) were diluted in dimethyl disulfide (DMSO) to make 1% v/v (for liquids) or m/v (for solids) solutions. For each compound, a 10μL portion was dispersed onto a filter paper (3 × 10mm) which was then inserted into a Pasteur pipette to create the stimulus cartridge. A sample containing the solvent alone served as the control. The airflow across the antennae was maintained at a constant 20 mL/s throughout the experiment. Purified and humidified air was delivered to the preparation through a glass tube (10-mm inner diameter) perforated by a small hole 10cm away from the end of the tube into which the tip of the Pasteur pipette could be inserted. The stimulus was delivered to the sensilla by inserting the tip of the stimulus cartridge into this hole and diverting a portion of the air stream (0.5 L/min) to flow through the stimulus cartridge for 500ms using a Syntech stimulus controller CS-55 (Syntech, Hilversum, The Netherlands). The distance between the end of the glass tube and the antennae was ⩽1cm. The CO2 stimulus was pulsed through a separate delivery system that delivered controlled pulses using a PSM 8000 microinjector (WPI, Sarasota, FL., variable 5 mL s−1) into the same humidified airstream, from CO2 tanks of different concentrations.
Signals were recorded for 10s starting 1 second before stimulation, and the action potentials were counted off-line over a 500ms period before and after stimulation. Spike rates observed during the 500ms stimulation were subtracted from the spontaneous activities observed in the preceding 500ms and counts recorded in units of spikes/s.
CRISPR-Cas9 gene editing
The CRISPR gene targeting vector was a generous gift from the lab of Dr. Andrea Crisanti of Imperial College London, UK (Hammond et al., 2016). The single guide RNA (sgRNA) sequences for each CO2 receptor gene were designed for high efficiency using the Chopchop online tool (http://chopchop.cbu.uib.no/), commercially synthesized (Integrated DNA Technologies, Coralville, IA) and subcloned into the CRISPR vector via Golden Gate cloning (New England Biolabs, Ipswich, MA). The homology templates for Gr23 and Gr24 were constructed based on a pHD-DsRed vector (a gift from Kate O’Connor-Giles; Addgene plasmid #51434; http://n2t.net/addgene:51434; RRID:Addgene 51434). Here, the 2kb homology arms extending in either direction from the double-stranded break (DSB) sites were PCR amplified and sequentially inserted into the AarI/ SapI restriction sites on the vector. The DsRed sequence was replaced by the ECFP fluorescence marker sequence to construct a pHD-ECFP vector for the knockout of Gr22.
The microinjection protocol was carried out as described (Pondeville et al., 2014; Ye et al., 2020). Briefly, newly laid (approximately 1hr-old) embryos of wild-type An. coluzzii were immediately collected and aligned on a filter paper moistened with 25mM sodium chloride solution. All the embryos were fixed on a coverslip with double-sided tape and a drop of halocarbon oil 27 was applied to cover the embryos. The coverslip was further fixed on a slide under a Zeiss Axiovert 35 microscope with a 40X objective. The microinjection was performed using an Eppendorf FemtoJet 5247 (Eppendorf, Enfield, CT) and quartz needles prepared using a customized protocol (Sutter Instrument, Novato, CA). The gene targeting vector and the homology template were diluted to 300ng/μL each and co-injected to maximum capacity/embryo. Injected embryos were subsequently placed in deionized water with artificial sea salt (0.3g/L) and thereafter reared under normal VU insectary conditions.
The first generation (G0) of injected adults were separated based on gender and crossed to 5X wild-type gender-counterparts. Their offspring (F1) were manually screened for DsRed/ECFP-derived red/cyan eye fluorescence using an Olympus BX60 Compound Fluorescent Microscope (Olympus, PA). Red/cyan-eyed F1 males were individually backcrossed to 5-fold wild-type females to establish a stable mutant line. DNA extraction was performed using Qiagen Gel Extraction protocols (Qiagen, Germantown, MD) and genomic DNA templates for PCR analyses of all individuals were performed (after mating) to validate the fluorescence marker insertion using primers that cover DSB sites (Table S1). PCR products were sequenced to confirm the accuracy of the genomic insertion. Heterozygous mutant lines were thereafter backcrossed to wild-type An. coluzzii for at least 3 generations before putative homozygous individuals were manually screened for DsRed/ECFP-derived red/cyan eye fluorescence intensity. Putative homozygous mutant individuals were mated to each other before being sacrificed for genomic DNA extraction and PCR analyses (as above) to confirm their homozygosity.
Results
Generation of mosquito mutant lines
In order to generate loss of function mutant mosquito lines for each of the Gr22, 23,24 CO2 receptors, guide RNAs (gRNA) were designed to target early exon coding regions to generate truncated and nonfunctional proteins (Figure 1A, B) using the Chopchop online tool (Labun et al., 2019). At the same time, visible markers were inserted into the DSB site of each Gr gene target to drive the expression of red eye color in Gr23 and Gr24 mutants, and cyan eye color in Gr22 mutants to facilitate selection (Fig.1C).
Figure 1.

Generation of three Gr mutants using CRISPR/Cas9 gene editing. (A) Schematic diagram showing the gRNA target site and marker gene (ECFP for Gr22, DsRed for Gr23 and Gr24; 3xP3 is the promoter sequence) inserted into the exon of each Gr gene. (B) Truncation of amino acid sequences after insertion of marker gene in the open reading frame of Gr genes. Left: model for secondary structure of normal Gr protein; Right: model for secondary structure of truncated Gr protein. (C) Florescence marker presenting in the compound eye of mutant mosquitoes with a specific Gr gene knockout. (D) Genotyping of the mutant mosquito lines using a pair of primers which amplify a PCR product across the DSB site.
Heterozygous mutant mosquito lines were thereafter back crossed to wild-type mosquitoes for at least three generations before homozygous Gr gene mutant lines were established by self-crossing. Each mutant line was molecularly confirmed as homozygotic insertions into each respective Gr gene by PCR with a pair of primers to amplify genomic DNA sequences covering the DSB sites of each Gr gene, which also served to confirm the specific insertion of eye color markers (Figure 1D). It is noteworthy that while Gr22−/− and Gr24−/− mosquitoes appear fully capable of successful breeding leading to the stable generation of laboratory colonies, Gr23−/− mosquitoes have thus far failed to successfully self-reproduce even after nine generations of backcrosses with wild-type partners.
Both Gr23 and Gr24 are obligatory for CO2 detection in the cpA neuron
In light of the absence of a Gr23 ortholog in D. melanogaster, we initially determined the functional relevance of Gr23 in An. coluzzii by assessing neuronal activity in the CO2 sensitive maxillary palp cp sensillum (Figure 2A). Here, the cpA neuron in wild-type females display pre-stimulus background activity as well as robust responses to 1% CO2 along with a characteristic post-CO2 stimulation latency together with low threshold sensitivity to 1-Octen-3-ol (10−7 v/v) in cpB/C neurons (Figure 2A). As expected, heterozygous Gr23+/− mutants displayed near wild-type responses across several parameters including background activity, CO2 sensitivity, and post-response latency (Figure 2B). In contrast, homozygous Gr23−/− mutant maxillary palp cpA neurons display no background activity and are completely insensitive to stimulation with 1% CO2 even though their cpB/C neurons display wild-type levels of background firing and low threshold sensitivity to 1-Octen-3-ol (Figure 2C, Supplemental Figure S1). These data suggest that, unlike Drosophila which lacks a Gr23 ortholog, in An. coluzzii, this gene encodes an essential component for detection of CO2 in the cpA neuron and that complexes made up of solely Gr22/Gr24 are insufficient for robust detection of CO2 in Anopheline mosquitoes.
Figure 2.

Neuronal responses of wild-type, Gr23 heterozygous and homozygous mutant mosquitoes when challenged with CO2. (A) Representative recordings from capitate peg sensilla in the maxillary palp of wild-type, Gr23 heterozygous and homozygous mutant mosquitoes; Top to bottom: background activity, response to 1% CO2 and response to 10−7 (v/v in paraffin oil) 1-Octen-3-ol. (B) Statistical analysis of background activity (unstimulated spike frequency), firing frequency of cpA neuron in response to CO2 challenge, and latency of cpA neuron after CO2 challenge. Nonparametric Mann-Whitney test was applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant differences.
Gr24 homologs are found in the genomes of various insect species and moreover are highly conserved across most, if not all, mosquitoes. Previous studies in Ae. aegypti revealed the essential role of Gr3 (the Anopheles Gr24 ortholog) in cpA sensitivity to CO2 (McMeniman et al., 2014) and, not surprisingly, this phenotype is also seen in our electrophysiological characterization of An. coluzzii Gr24 mutant females. Here, the cpA neuron in heterozygous An. coluzzii Gr24+/− mutants display wild-type background activity as well as robust responses to 1% CO2 stimulation (Figure 3A, B) while cpA neurons of homozygous Gr24−/− mutants manifest no background activity and are unresponsive when stimulated with 1% CO2 (Figure 3A, B). As was the case for Gr23, these data confirm that Gr24/Gr3 is similarly absolutely required for maintaining cpA function and CO2 sensitivity in mosquitoes.
Figure 3.

Neuronal responses of wild-type, Gr24 heterozygous and homozygous mutant mosquitoes when challenged with CO2. (A) Representative recordings from capitate peg sensilla in the maxillary palp of wild-type, Gr24 heterozygous and homozygous mutant mosquitoes; Top to bottom: background activity, response to 1% CO2 and response to 10−7 (v/v in paraffin oil) 1-Octen-3-ol. (B) Statistical analysis of background activity, firing frequency of cpA neuron in response to CO2 challenge, and latency of cpA neuron after CO2 challenge. Nonparametric Mann-Whitney test was applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant difference.
Gr22 is required for comprehensive CO2 detection in the cpA neuron
Recent heterologous expression-based studies have reported somewhat conflicting results on the modulatory role of Gr22 orthologs in two mosquito species where they are both known as Gr1 (Kumar et al., 2020; Xu et al., 2020). Xenopus oocyte-based studies suggest that in Cx. quinquefasciatus, Gr1 serves as an inhibitor to fine-tune responses to CO2 (Xu et al., 2020). In Drosophila empty neuron-based studies, Ae. aegypti Gr1 acts as both an activator of CO2 detection and an inhibitor of sensitivity to pyridine and potentially other non-CO2 odors (Kumar et al., 2020). Cognizant of the need to examine these questions in An. coluzzii as well as the potential limitations of studies using in vitro/heterologous expression systems, we elected to work in vivo to directly examine the role of Gr22 in CO2 sensitivity of peripheral neurons. As expected, maxillary palp cpSSRs of heterozygous Gr22+/− mutants showed no significant differences when compared to wild-type preparations insofar as their background activity or in their responses to CO2 or 1-Octen-3-ol (Figure 4). However, in contrast to its near absolute silence in Gr23−/− and Gr24−/− mutants, the cpA neuron in Gr22−/− mutants displays modest, albeit significantly reduced background activity, sensitivity to CO2 and post-stimulus latency when compared to both wild-type and heterozygous mosquitoes (Figure 4). Exploring this further, Gr22−/− homozygous mutants displayed consistently deficient CO2 responses compared to both wild-type and Gr22+/− heterozygous mosquitoes at serial CO2 dilutions covering 3 orders of magnitude (Supplemental Figure S2A). Importantly, these assays revealed no significant difference in the CO2-evoked responses between wild-type and Gr22+/− heterozygotes. Additionally, when exploring the temporal dynamics of CO2-evoked cpA neuron firing it is clear that Gr22−/− mutants respond more phasically resulting in a firing pattern with a kurtosis value of 5.685 while wild-type female maxillary palp cpA neurons respond to CO2 more tonically giving rise to a firing pattern with a kurtosis value of 0.016 (Supplemental Figure S2B).
Figure 4.
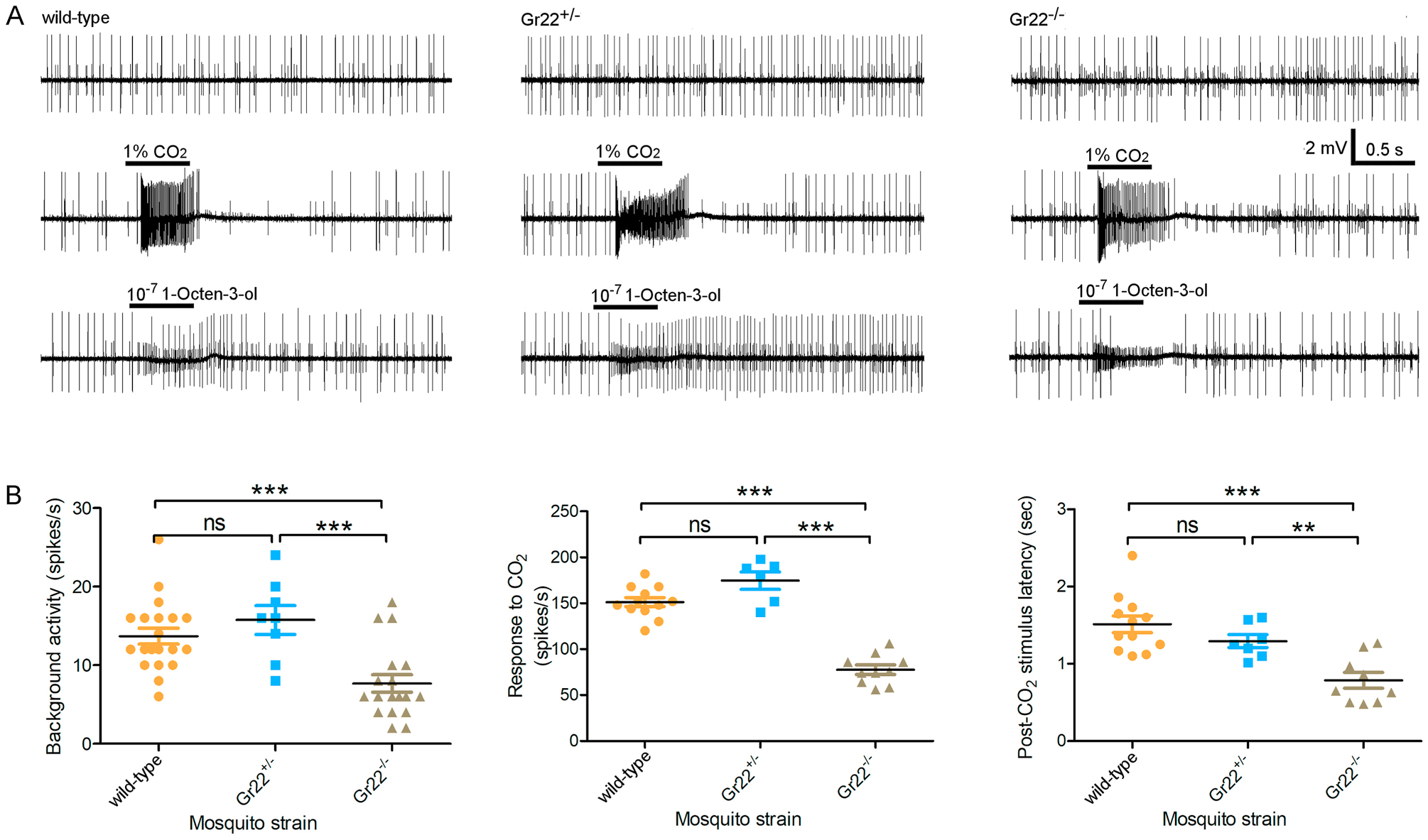
Neuronal response of wild-type, Gr22 heterozygous and homozygous mutant mosquitoes when challenged with CO2. (A) Representative recording from capitate peg sensilla in the maxillary palp of wild-type, Gr22 heterozygous and homozygous mutant mosquitoes; Top to bottom: background activity, response to 1% CO2 and response to 10−7 (v/v in paraffin oil) 1-Octen-3-ol. (B) Statistical analysis of background activity, firing frequency of cpA neuron in response to CO2 challenge, and latency of cpA neuron after CO2 challenge. Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant difference.
Gr22 is required for complete detection of CO2-mimic compounds in the cpA neuron
While in An. coluzzii and other mosquitoes it is likely the maxillary palp cpA neuron’s primary role is to sense host-derived CO2, the cpA neuron also responds to a range of non-CO2 odorants (Lu et al., 2007; Coutinho-Abreu et al., 2019). Insofar as such compounds represent potential CO2-augments and/or mimics that could be useful to further examine the mechanistic roles of Grs 22,23, and 24 as well as opportunities for optimizing odor-baited mosquito traps, we screened the An. coluzzii cpA neuron for responses to an in-lab chemical library of 325 compounds (Figure 5A). To begin with, stimulation with these non-CO2 compounds revealed three classes of temporally distinct cpA neuron response patterns: 1) CO2-like responses were elicited by compounds which not only evoked excitatory cpA responses but also gave rise to an obvious post-stimulus latency, these were identified as potential CO2 “mimics”; 2) Non-CO2-like excitatory responses without a post-stimulus latency; 3) Suppressive responses that significantly inhibited cpA neuron firing (Figure 5B, Supplemental Figure S3). Of the 50 compounds which elicited significant cpA excitatory responses, 15 including pyridine, cyclohexanone, and 2-methyl-2-thiazole display CO2-like mimic responses (Figure 5C, blue bars).
Figure 5.
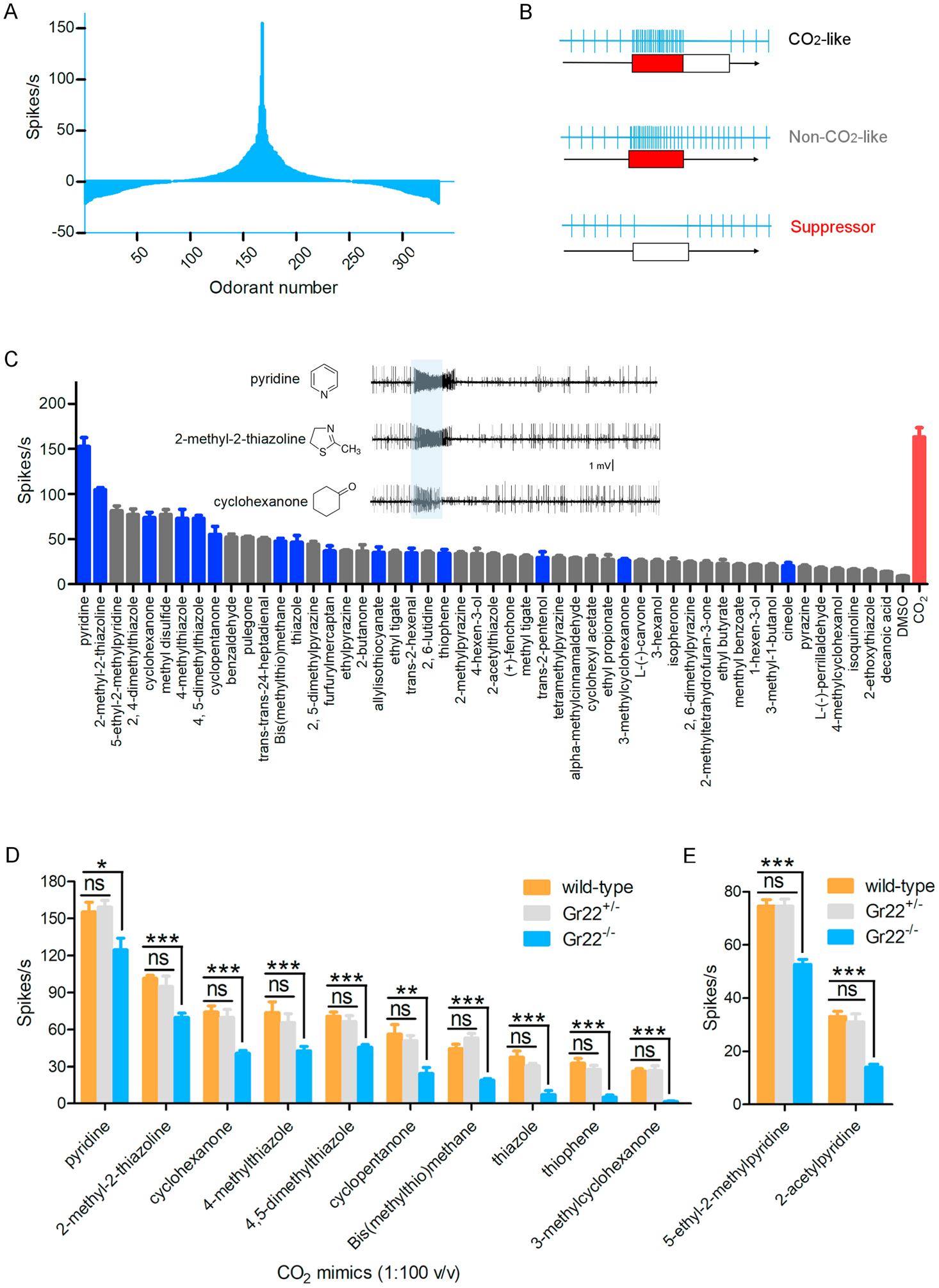
Neuronal responses of wild-type and Gr22−/− mosquitoes to CO2-mimic compounds. (A) Screening the potential CO2-mimic compounds (10−2 v/v or m/v in DMSO) on the cpA neuron with our laboratory chemical library (325 compounds) and identifying the most potent compounds in activating the cpA neuron. (B) Classification of response pattern of cpA neuron to different compounds compared with CO2-evoked response. (C) Compounds eliciting significant excitatory response on the cpA neuron compared to the paraffin oil control. CO2-like responses are labeled with blue color. Non-CO2-like response are shown with gray color. The response to 1% CO2 was placed on the right end of the figure with a red bar. Top three most potent CO2-mimcs which elicit the strongest responses in the neuron are placed in the left corner of the figure. Representative firing signal trace of those three compounds (10−2 v/v) is shown. (D) Lower sensitivity of the cpA neuron of Gr22 homozygous mutant mosquitoes in response to ten CO2 mimics compared to that observed in wild-type and Gr22 heterozygous mutant mosquito. (E) Lower sensitivity of the cpA neuron of Gr22−/− homozygous and Gr22+/− heterozygous mutant mosquitoes in response to two pyridine-derived compounds compared to wild-type mosquitoes. Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant difference.
We next examined the responses of our Gr mutants to a panel of ten CO2 mimics all of which evoked strong responses in the cpA neuron of wild-type mosquitoes. As expected, the largely silent cpA neuron of Gr23−/− and Gr24−/− mutants was indifferent to these compounds, (Supplemental Figure S4), as well as to stimulation with compounds that evoke non-CO2-like excitatory responses in wild-type cpA neurons (Supplemental Figure S5). However, all ten of our CO2 mimics stimulated obvious, albeit significantly reduced cpA responses in Gr22−/− mutants compared to wild-type and Gr22+/− heterozygotic mosquitoes which both showed robust and indistinguishable responses (Figure 5D). Of these, one heterocyclic compound, pyridine, displayed the strongest response in Gr22−/− mutants which was nevertheless still weaker when compared to wild-type and Gr22+/− firing rates (Figure 5D). Two other pyridine-derived compounds, 2-acetypyridine and 5-ethyl-2-methylpyridine, also evoke moderate cpA spike trains in both wild-type and Gr22+/− heterozygotes, but at significant lower frequencies in Gr22−/− mutants (Figure 5E). These data do not seem to align with recent studies indicating that pyridine triggers stronger responses in the Drosophila ab1C neurons expressing Ae. aegypti Gr2 and Gr3 but lacking Gr1 as compared to similar preparations expressing Ae. aegypti Gr1, Gr2 and Gr3 (Kumar et al., 2020).
Discussion
We have used in vivo gene-targeting in An. coluzzii to examine the requirements of the CO2 receptor triad encoded by Grs22, 23 and 24. These genes and the putative proteins they produce are highly conserved, but interestingly not universal components of insect CO2 receptor complexes that underlie electrophysiological responses on the maxillary palp and other chemosensory appendages. Importantly, our data is broadly consistent with previous studies that directly place these Grs at the heart of CO2 responses in fruit flies and mosquitoes (Lu et al., 2007; Kwon et al., 2007; McMeniman et al., 2014). While the results from our work are, for the most part, consistent with the recent findings in Aedes and Culex (Kumar et al., 2020; Xu et al., 2020), there are several inconsistencies. To begin with the aligned data, it can now be said with confidence that in mosquitoes: (1) Gr23 (Gr2 in Aedes and Culex) is not a redundant triad component but rather is necessary for comprehensive responses to diverse CO2 stimuli and, in all likelihood, for successful reproduction; (2) Gr23 and Gr24 (Gr2 and Gr3 in Aedes and Culex, respectively) together form a partially functional complex that is able to broadly detect CO2; (3) the presence of Gr22 (Gr1 in Aedes and Culex) is required for the complete functionality of the Gr23/24 CO2-sensing complex (Figure 6).
Figure 6.
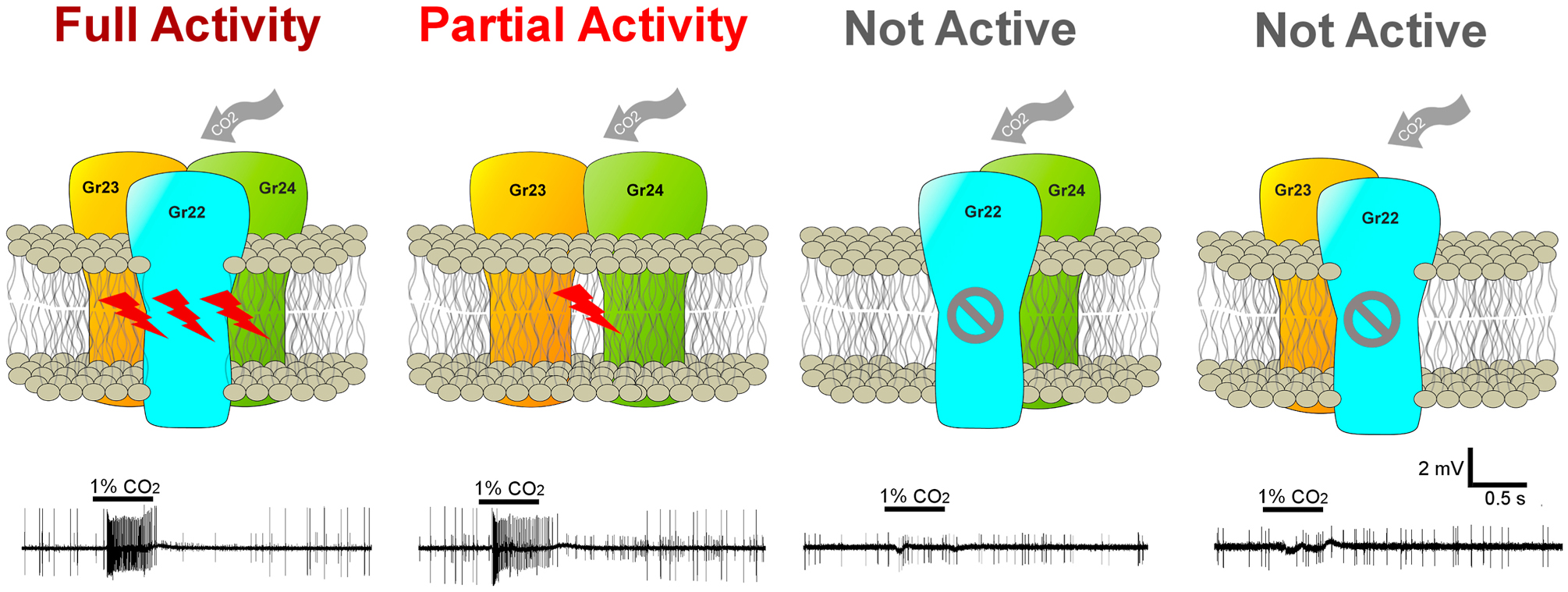
Model for organization of Grs in CO2 reception. While Gr23 and Gr24 are obligatory components in detecting CO2, Gr22 acts as an enhancer for the Gr23/24 complex in response to CO2, especially at the lower concentration.
Regarding the inconsistencies, we report that in vivo An. coluzzii Gr22−/− mutant maxillary palp cpA neurons display uniformly decreased sensitivity to CO2, CO2 mimics as well as to odorants that evoke non-CO2-like excitatory responses. In genetically engineered heterologous expression complexes utilizing the “empty” Drosophila ab1c neuron, lack of Ae. aegypti Gr1 also decreases the sensitivity to CO2 while increasing sensitivity for mimics such as pyridine (Kumar et al., 2020). The most parsimonious explanation for this minor discrepancy is that while it is clearly faithful in many regards, Drosophila empty neuron systems may simply not express, traffic or otherwise utilize mosquito Gr genes with the same competence as observed in vivo. Furthermore, studies of Culex Gr1 (Xu et al., 2020) suggest that it most likely acts as a negative modulator of the Gr2/3 complex, which directly contradicts the Drosophila-based studies of Aedes Gr1 as well as our in vivo findings in Anopheles that demonstrate that Gr22 enhances the sensitivity of the Gr23/24 complex to CO2. Once again, this is most likely due to the limitations of the in vitro Xenopus expression system and underscores the relevance of in vivo gene-targeting studies.
The first mosquito with a CO2-sensitive Gr gene knockout was generated in Ae. aegypti Gr3, the ortholog of Anopheles Gr24 (McMeniman et al., 2014). As we now report for Gr24−/− mutants in An. coluzzii, the cpA neuron in Gr3-deficient Ae. aegypti was shown to be completely silenced exhibiting neither background activity nor any response to CO2 or other compounds that typically evoke excitatory responses. This same phenomenon was observed in two studies using Drosophila expression platforms that lacked the fly CO2 receptor gene Gr63a, which is orthologous to Gr24 in Anopheles (Jones et al., 2007; Kwon et al., 2007). Taken together, these studies across broadly diverged dipteran insects confirm the absolute functional requirement of the Gr63a/Gr3/Gr24 component of the CO2-sensing apparatus.
As was the case for Gr24−/−, maxillary palp cpA neurons are completely silent in An. coluzzii mutants lacking Gr23 demonstrating that loss of either gene is sufficient to render these neurons inactive across a wide spectrum of normally excitatory stimuli. Taken together, these data demonstrate that in Anopheles, and by extension many other insects, CO2-sensitive receptor complexes require both Gr23 and Gr24 as complexes lacking either receptor are inactive. This suggests that Gr23 and its homologs which are notably absent in the fully functional D. melanogaster CO2 receptor complex nevertheless provides an essential functionality to these divergent receptors. In light of the narrow CO2 specialization of the Drosophila Gr21a/63a complex (Kwon et al., 2007) it is reasonable to suggest that Gr2/23’s role in the mosquito receptor is related to its broader sensitivity to a wide range of non-CO2 stimuli (Lu et al., 2007; Coutinho-Abreu et al., 2019). While we have been unable to uncover any cpA neuron electrophysiological distinctions between the equally inactive Gr24−/− and Gr23−/− mutants, the unique inability of Gr23−/− mutants to self-reproduce in laboratory mating studies (data not shown) suggests there are nevertheless important functional distinctions between these complexes that may be developmental or otherwise salient for mosquito reproduction. Current efforts seek to resolve the mechanism of the Gr23−/− mutant’s reproductive deficiency which despite several backcrosses to establish a wild-type background, may also be the result of a closely-linked off-target sterile recessive mutation.
Our data suggests that in An. coluzzii, loss of Gr22 alters both the firing intensity and temporal dynamics of the cpA neuron’s response to CO2 stimulation characteristics that have been implicated in the physiological coding of compounds in the peripheral neuronal system of insects (De Bruyne et al., 2001; Hallem and Carlson, 2004). Indeed, changes in either firing frequency or temporal dynamics have been shown to influence the secondary processing in the antennal lobe as well as decision-making in the mushroom body of insect brains, which ultimately impair normal odorant perception in insects (Owald et al., 2015; Silbering et al., 2008; Turner et al., 2008). Therefore, while An. coluzzii Gr22−/− mutants still respond to CO2, their diminished firing frequency and altered temporal dynamics are likely to have dramatic impacts on their behavioral responses to CO2 and their host-seeking ability. Further behavioral assays will be conducted to more fully characterize Gr mutant mosquito’s response to CO2.
Although other CO2-mimic compounds, such as cycloheptanone, activate the cpA neuron in the maxillary palp and is behaviorally attractive for Culex quinquefasiatus in semi-field experiments (Tauxe et al., 2013), a recent field study combining both heat and pyridine/cycloheptanone mimics failed to trap Culex or Anopheles mosquitoes (Zhou et al., 2018). These conflicting studies suggest the comprehensive behavioral functionality of this class of semiochemicals remains largely undetermined leaving open the question of the intriguing effect of CO2 mimics on the behavioral responses of mosquitoes. In our studies, it is noteworthy that most of the maxillary palp CO2 mimics also activate antennal neurons (data not shown), suggesting additional complexity of neuronal coding by these compounds.
Conclusions
The malaria mosquito An. coluzzii uses three putative gustatory receptors, encoded by Gr22, 23 and 24 to generate a triad complex to detect CO2 from blood meal hosts as well as environmental sources (Figure 6). Gene knockout studies now demonstrate that in the maxillary palp CO2-sensitive cpA neuron of Anopheles and other mosquitoes, Gr22 functions as a modulatory component that enhances the sensitivity, discrimination and functionality of the obligatory Gr23/Gr24 complex.
Supplementary Material
Supplemental Figure S1. Comparison of responses of cpB/C neuron to 1-Octen-3-ol among wild-type and Gr23 mutant mosquitoes. (A) Spontaneous activity of cpB/C neuron in wild-type, Gr23+/− and Gr23−/− mosquito lines. (B) Firing frequency of cpB/C neuron in wild-type, Gr23+/− and Gr23−/− mosquito lines in response to 1-Octen-3-ol (10−7 v/v). Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 as significant difference, NS indicating non-significance.
Supplemental Figure S2. (A) Comparison of cpA neuron’s response to different concentrations of CO2 among wild-type, heterozygous Gr22+/−, and homozygous Gr22−/− mutant mosquitoes (n=6–10). BA=background activity. Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant difference. (B) Temporal analysis of cpA neuron’s response to 1% CO2 over 2 sec after stimulation. The Kurtosis value is calculated to indicate the response pattern tendency (tonic or phasic).
Supplemental Figure S3. Compounds that elicited inhibitory responses on the cpA neuron with a firing frequency <=10 spikes/s (n=6–8).
Supplemental Figure S4. The cpA neuron of Gr23−/− (n=4) and Gr24−/− (n=6) mosquito presented no response to a panel of 10 CO2 mimics, which evoked obvious excitatory responses in wild-type mosquitoes (n=6).
Supplemental Figure S5. The responses of cpA neuron in Gr23−/− (n=4) and Gr24−/− (n=6) mosquitoes to two non-CO2 like compounds, which evoked obvious excitatory cpA responses in wild-type mosquitoes (n=6)
Highlights.
An. Coluzzii uses three putative gustatory receptors, encoded by Gr22, 23 and 24 to generate a triad complex to detect CO2 on its maxillary palp.
Both Gr23 and 24 are obligatory components of a functional CO2 detector
The Gr22 protein is not obligatory but instead functions as a modulatory component that enhances the sensitivity, discrimination and functionality of the obligatory Gr23/Gr24 CO2 receptor complex.
Acknowledgments
We thank the laboratory of Dr. Andrea Crisanti (Imperial College, UK) for their generous advice for Anopheles gene-targeting, Dr. H. Willi Honegger, Dr. Ann Carr, Stephen Ferguson and other members of the Zwiebel lab for critical suggestions during the course of this work and acknowledge the initial studies of Dr. Pingxi Xu (University of California, Davis) in framing these experiments. We also thank Zhen Li and Samuel Ochieng for mosquito rearing and technical help. This work was conducted with the support of Vanderbilt University and a grant from the National Institutes of Health (NIAID, AI137157) to LJZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests
The authors declare no competing financial interests.
Literature Cited
- Aly ASI, Vaughan AM, Kappe SHI, 2009. Malaria parasite development in the mosquito and infection of the mammalian host. Annu. Rev. Microbiol 63, 195–221. 10.1146/annurev.micro.091208.073403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier JC, 1998. Malaria parasite development in mosquitoes. Annu. Rev. Entomol 43, 519–543. 10.1146/annurev.ento.43.1.519 [DOI] [PubMed] [Google Scholar]
- Benoit JB, Adelman ZN, Reinhardt K, Dolan A, Poelchau M, Jennings EC, Szuter EM, Hagan RW, Gujar H, Shukla JN, Zhu F, Mohan M, Nelson DR, Rosendale AJ, Derst C, Resnik V, Wernig S, Menegazzi P, Wegener C, Peschel N, Hendershot JM, Blenau W, Predel R, Johnston PR, Ioannidis P, Waterhouse RM, Nauen R, Schorn C, Ott MC, Maiwald F, Johnston JS, Gondhalekar AD, Scharf ME, Peterson BF, Raje KR, Hottel BA, Armisén D, Crumière AJJ, Refki PN, Santos ME, Sghaier E, Viala S, Khila A, Ahn SJ, Childers C, Lee CY, Lin H, Hughes DST, Duncan EJ, Murali SC, Qu J, Dugan S, Lee SL, Chao H, Dinh H, Han Y, Doddapaneni H, Worley KC, Muzny DM, Wheeler D, Panfilio KA, Vargas Jentzsch IM, Vargo EL, Booth W, Friedrich M, Weirauch MT, Anderson MAE, Jones JW, Mittapalli O, Zhao C, Zhou JJ, Evans JD, Attardo GM, Robertson HM, Zdobnov EM, Ribeiro JMC, Gibbs RA, Werren JH, Palli SR, Schal C, Richards S, 2016. Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat. Commun 7, 1–10. 10.1038/ncomms10165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee M, Hunt RH, Wilkerson R, Torre A. Della, Coulibaly MB, Besansky NJ, 2013. Anopheles coluzzii and Anopheles amharicus, new members of the anopheles gambiae complex. Zootaxa 3619, 246–274. 10.11646/zootaxa.3619.3.2 [DOI] [PubMed] [Google Scholar]
- Cooperband MF, Carde RT, 2006. Orientation of Culex mosquitoes to carbon dioxide-baited traps: flight manoeuvres and trapping efficiency. Med. Vet. Entomol 20, 11–26. 10.1111/j.1365-2915.2006.00613.x [DOI] [PubMed] [Google Scholar]
- Coutinho-Abreu IV, Sharma K, Cui L, Yan G, Ray A, 2019. Odorant ligands for the CO2 receptor in two Anopheles vectors of malaria. Sci. Rep 9, 1–7. 10.1038/s41598-019-39099-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyne M, Foster K, Carlson JR, 2001. Odor coding in the Drosophila antenna. Neuron 30, 537–552. 10.1016/S0896-6273(01)00289-6 [DOI] [PubMed] [Google Scholar]
- Den Otter CJ, Behan M, Maes FW, 1980. Single cell responses in female Pieris brassicae (Lepidoptera: Pieridae) to plant volatiles and conspecific egg odours. J. Insect Physiol 26, 465–472. 10.1016/0022-1910(80)90117-1 [DOI] [Google Scholar]
- Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ, 2001. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl. Acad. Sci. U. S. A 98, 14693–14697. 10.1073/pnas.261432998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier M, Boeckh J, 1999. A new Y-tube olfactometer for mosquitoes to measure the attractiveness of host odours. Entomol. Exp. Appl 92, 9–19. 10.1046/j.1570-7458.1999.00519.x [DOI] [Google Scholar]
- Ghaninia M, Ignell R, Hansson BS, 2007. Functional classification and central nervous projections of olfactory receptor neurons housed in antennal trichoid sensilla of female yellow fever mosquitoes, Aedes aegypti. Eur. J. Neurosci 26, 1611–1623. 10.1111/j.1460-9568.2007.05786.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, Aghajanian JG, O’Connell RJ, Wigton BE, 1995. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J. Comp. Physiol. A 177, 389–396. 10.1007/BF00187475 [DOI] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR, 2004. The odor coding system of Drosophila. Trends Genet 20, 453–459. 10.1016/j.tig.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, Burt A, Windbichler N, Crisanti A, Nolan T, 2016. A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat. Biotechnol 34, 78–83. 10.1038/nbt.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy TP, Copland MJW, 1995. Activation of Anopheles gambiae mosquitoes by carbon dioxide and human breath. Med. Vet. Entomol 9, 331–336. 10.1111/j.1365-2915.1995.tb00143.x [DOI] [PubMed] [Google Scholar]
- Hoel DF, Kline DL, Allan SA, Grant A, 2007. Evaluation of carbon dioxide, 1-octen-3-ol, and lactic acid as baits in mosquito Magnettm Pro traps for Aedes albopictus in North Central Florida. 10.2987/8756-971X(2007)23[11:EOCDOA]2.0.CO;2 23, 11–17. [DOI] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Grunwald Kadow I, Vosshall LB, 2007. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 445, 86–90. 10.1038/nature05466 [DOI] [PubMed] [Google Scholar]
- Kline DL, Bernier UR, Hogsette JA, 2012. Efficacy of three attractant blends tested in combination with carbon dioxide against natural populations of mosquitoes and biting flies at the lower Suwannee wildlife refuge. J. Am. Mosq. Control Assoc 28, 123–127. 10.2987/11-6200R.1 [DOI] [PubMed] [Google Scholar]
- Kline DL, Takken W, Wood JR, Carlson DA, 1990. Field studies on the potential of butanone, carbon dioxide, honey extract, l-octen-3-ol, L-lactic acid and phenols as attractants for mosquitoes. Med. Vet. Entomol 4, 383–391. 10.1111/j.1365-2915.1990.tb00455.x [DOI] [PubMed] [Google Scholar]
- Kumar A, Tauxe GM, Perry S, Scott CA, Dahanukar A, Ray A, 2020. Contributions of the conserved insect carbon dioxide receptor subunits to odor detection. Cell Rep 31, 107510 10.1016/j.celrep.2020.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JY, Dahanukar A, Weiss LA, Carlson JR, 2007. The molecular basis of CO2 reception in Drosophila. Proc. Natl. Acad. Sci. U. S. A 104, 3574–3578. 10.1073/pnas.0700079104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, Valen E, 2019. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res 47, W171–W174. 10.1093/nar/gkz365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chen L, Appel AG, Liu N, 2013. Olfactory responses of the antennal trichoid sensilla to chemical repellents in the mosquito, Culex quinquefasciatus. J. Insect Physiol 59, 1169–1177. 10.1016/j.jinsphys.2013.08.016 [DOI] [PubMed] [Google Scholar]
- Lorenz LM, Keane A, Moore JD, Munk CJ, Seeholzer L, Mseka A, Simfukwe E, Ligamba J, Turner EL, Biswaro LR, Okumu FO, Killeen GF, Mukabana WR, Moore SJ, 2013. Taxis assays measure directional movement of mosquitoes to olfactory cues. Parasites and Vectors 6 10.1186/1756-3305-6-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJA, Takken W, Carlson JR, Zwiebel LJ, 2007. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr. Biol 17, 1533–1544. 10.1016/j.cub.2007.07.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB, 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. 10.1016/j.cell.2013.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misof B, Liu S, Meusemann K, Peters RS, Donath A, Mayer C, Frandsen PB, Ware J, Flouri T, Beutel RG, Niehuis O, Petersen M, Izquierdo-Carrasco F, Wappler T, Rust J, Aberer AJ, Aspöck U, Aspöck H, Bartel D, Blanke A, Berger S, Böhm A, Buckley TR, Calcott B, Chen J, Friedrich F, Fukui M, Fujita M, Greve C, Grobe P, Gu S, Huang Y, Jermiin LS, Kawahara AY, Krogmann L, Kubiak M, Lanfear R, Letsch H, Li Yiyuan, Li Z, Li J, Lu H, Machida R, Mashimo Y, Kapli P, McKenna DD, Meng G, Nakagaki Y, Navarrete-Heredia JL, Ott M, Ou Y, Pass G, Podsiadlowski L, Pohl H, Von Reumont BM, Schütte K, Sekiya K, Shimizu S, Slipinski A, Stamatakis A, Song W, Su X, Szucsich NU, Tan M, Tan X, Tang M, Tang J, Timelthaler G, Tomizuka S, Trautwein M, Tong X, Uchifune T, Walzl MG, Wiegmann BM, Wilbrandt J, Wipfler B, Wong TKF, Wu Q, Wu G, Xie Y, Yang S, Yang Q, Yeates DK, Yoshizawa K, Zhang Q, Zhang R, Zhang W, Zhang Yunhui, Zhao J, Zhou C, Zhou L, Ziesmann T, Zou S, Li Yingrui, Xu X, Zhang Yong, Yang H, Wang Jian, Wang Jun, Kjer KM, Zhou X, 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science (80-. ) 346, 763–767. 10.1126/science.1257570 [DOI] [PubMed] [Google Scholar]
- Owald D, Felsenberg J, Talbot CB, Das G, Perisse E, Huetteroth W, Waddell S, 2015. Activity of defined mushroom body output neurons underlies learned olfactory behavior in Drosophila. Neuron 86, 417–427. 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondeville E, Puchot N, Meredith JM, Lynd A, Vernick KD, Lycett GJ, Eggleston P, Bourgouin C, 2014. Efficient φc31 integrase-mediated site-specific germline transformation of Anopheles gambiae. Nat. Protoc 9, 1698–1712. 10.1038/nprot.2014.117 [DOI] [PubMed] [Google Scholar]
- Qiu YT, Smallegange RC, Hoppe S, van Loon JJA, Bakker E-J, Takken W, 2004. Behavioural and electrophysiological responses of the malaria mosquito Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) to human skin emanations. Med. Vet. Entomol 18, 429–438. 10.1111/j.0269-283X.2004.00534.x [DOI] [PubMed] [Google Scholar]
- Robertson HM, Kent LB, 2009. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J. Insect Sci 9, 1–14. 10.1673/031.009.1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roiz D, Roussel M, Munõz J, Ruiz S, Soriguer R, Figuerola J, 2012. Efficacy of mosquito traps for collecting potential west nile mosquito vectors in a natural mediterranean wetland. Am. J. Trop. Med. Hyg 86, 642–648. 10.4269/ajtmh.2012.11-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbering AF, Okada R, Ito K, Galizia CG, 2008. Olfactory information processing in the Drosophila antennal lobe: Anything goes? J. Neurosci 28, 13075–13087. 10.1523/JNEUROSCI.2973-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzen J, Smallegange RC, Takken W, 2008. Effect of human odours and positioning of CO2 release point on trap catches of the malaria mosquito Anopheles gambiae sensu stricto in an olfactometer. Physiol. Entomol 33, 116–122. 10.1111/j.1365-3032.2008.00612.x [DOI] [Google Scholar]
- Syed Z, Leal WS, 2007. Maxillary palps are broad spectrum odorant detectors in culex quinquefasciatus. Chem. Senses 32, 727–738. 10.1093/chemse/bjm040 [DOI] [PubMed] [Google Scholar]
- Terrapon N, Li C, Robertson HM, Ji L, Meng X, Booth W, Chen Z, Childers CP, Glastad KM, Gokhale K, Gowin J, Gronenberg W, Hermansen RA, Hu H, Hunt BG, Huylmans AK, Khalil SMS, Mitchell RD, Munoz-Torres MC, Mustard JA, Pan H, Reese JT, Scharf ME, Sun F, Vogel H, Xiao J, Yang W, Yang Zhikai, Yang Zuoquan, Zhou J, Zhu J, Brent CS, Elsik CG, Goodisman MAD, Liberles DA, Roe RM, Vargo EL, Vilcinskas A, Wang J, Bornberg-Bauer E, Korb J, Zhang G, Liebig J, 2014. Molecular traces of alternative social organization in a termite genome. Nat. Commun 5, 1–12. 10.1038/ncomms4636 [DOI] [PubMed] [Google Scholar]
- Turner GC, Bazhenov M, Laurent G, 2008. Olfactory representations by Drosophila mushroom body neurons. J. Neurophysiol 99, 734–746. 10.1152/jn.01283.2007 [DOI] [PubMed] [Google Scholar]
- Whitten MMA, Shiao SH, Levashiva EA, 2006. Mosquito midguts and malaria: cell biology, compartmentalization and immunology. Parasite Immunol 28, 121–130. 10.1111/j.1365-3024.2006.00804.x [DOI] [PubMed] [Google Scholar]
- Xu P, Wen X, Leal WS, 2020. CO2 per se activates carbon dioxide receptors. Insect Biochem. Mol. Biol 117, 103284 10.1016/j.ibmb.2019.103284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Liu F, Sun H, Barker M, Pitts RJ, Zwiebel LJ, 2020. Heterogeneous expression of the ammonium transporter AgAmt in chemosensory appendages of the malaria vector, Anopheles gambiae. Insect Biochem. Mol. Biol 120 10.1016/j.ibmb.2020.103360 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Comparison of responses of cpB/C neuron to 1-Octen-3-ol among wild-type and Gr23 mutant mosquitoes. (A) Spontaneous activity of cpB/C neuron in wild-type, Gr23+/− and Gr23−/− mosquito lines. (B) Firing frequency of cpB/C neuron in wild-type, Gr23+/− and Gr23−/− mosquito lines in response to 1-Octen-3-ol (10−7 v/v). Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 as significant difference, NS indicating non-significance.
Supplemental Figure S2. (A) Comparison of cpA neuron’s response to different concentrations of CO2 among wild-type, heterozygous Gr22+/−, and homozygous Gr22−/− mutant mosquitoes (n=6–10). BA=background activity. Nonparametric Mann-Whitney test is applied in the statistical analysis with P<0.05 (*), P<0.01 (**) and P<0.001 (***) as significant difference. (B) Temporal analysis of cpA neuron’s response to 1% CO2 over 2 sec after stimulation. The Kurtosis value is calculated to indicate the response pattern tendency (tonic or phasic).
Supplemental Figure S3. Compounds that elicited inhibitory responses on the cpA neuron with a firing frequency <=10 spikes/s (n=6–8).
Supplemental Figure S4. The cpA neuron of Gr23−/− (n=4) and Gr24−/− (n=6) mosquito presented no response to a panel of 10 CO2 mimics, which evoked obvious excitatory responses in wild-type mosquitoes (n=6).
Supplemental Figure S5. The responses of cpA neuron in Gr23−/− (n=4) and Gr24−/− (n=6) mosquitoes to two non-CO2 like compounds, which evoked obvious excitatory cpA responses in wild-type mosquitoes (n=6)


