Abstract
Background:
Deficits in inhibitory control on a Stop Signal Task (SST) were previously observed to be of similar magnitude across schizophrenia, schizoaffective, and bipolar disorder with psychosis, despite variation in general cognitive ability. Understanding different patterns of performance on the SST may elucidate different pathways to the impaired inhibitory control each group displayed. Comparing nonpsychotic bipolar disorder to the psychosis groups on SST may also expand our understanding of the shared neurobiology of this illness spectrum.
Methods:
We tested schizophrenia (n=220), schizoaffective (n=216), bipolar disorder with (n=192) and without psychosis (n=67), and 280 healthy comparison participants with a SST and the Brief Assessment of Cognition in Schizophrenia (BACS), a measure of general cognitive ability.
Results:
All patient groups had a similar degree of impaired inhibitory control over prepotent responses. However, bipolar groups differed from schizophrenia and schizoaffective groups in showing speeded responses and inhibition errors that were not accounted for by general cognitive ability. Schizophrenia and schizoaffective groups had a broader set of deficits on inhibition and greater general cognitive deficit, which fully accounted for the inhibition deficits. No differences were found between the clinically well-matched bipolar with and without psychosis groups, including for inhibitory control or general cognitive ability.
Conclusions:
We conclude that 1) while impaired inhibitory control on a SST is of similar magnitude across the schizo-bipolar spectrum, including nonpsychotic bipolar, different mechanisms may underlie the impairments, and 2) history of psychosis in bipolar disorder does not differentially impact inhibitory behavioral control or general cognitive abilities.
Keywords: stop signal task, response inhibition, schizophrenia, schizoaffective disorder, bipolar disorder, psychosis
1. Introduction
Deficient inhibitory behavioral control in bipolar disorder has been related to impulsivity and risk-taking behaviors such as aggression, substance use, and suicide (Moeller et al., 2001; Swann et al., 2005; Swann et al., 2010). Inhibitory control deficits also characterize psychotic disorders including schizophrenia (SZ), schizoaffective disorder (SAD), and bipolar disorder with psychosis (PBD) (Ethridge et al., 2014). The shared feature of poor inhibitory control fits with current conceptualization of the relatedness of these disorders, including overlapping familiality (Lee et al., 2013; Smoller et al., 2013) and similar patterns of biomarker alterations (Clementz et al., 2016; Du et al., 2017; Ivleva et al., 2017).
Typically all psychosis groups show deficiencies across neurocognitive modalities compared to healthy participants, with PBD showing less severe impairment, SAD showing intermediate, and SZ showing the greatest magnitude of impairment (Hill et al., 2013; Tamminga et al., 2014). However, some studies of inhibitory control have yielded scores that diverge from this pattern. Specifically, a prior report from our group (Ethridge et al., 2014) indicated deficits on a Stop Signal Task (SST), a commonly used measure of inhibition of prepotent responses, were of a similar magnitude across SZ, SAD, and PBD. This was observed in the context of the usual more severe general cognitive impairment in SZ/SAD relative to PBD. Hence, a common magnitude of impaired inhibitory control may be present along the schizo-bipolar spectrum in the context of varying degrees of general cognitive impairment. This observation raises important questions about whether there is variation underlying the inhibition deficits and about the extent of deficits along the schizo-bipolar spectrum.
First, do inhibition errors occur for different reasons in PBD relative to schizophrenia-spectrum illness given the variation in general cognitive ability? Consideration of this question is facilitated by the use of SSTs as they fit the “race model” of inhibitory control to guide interpretation. This cognitive model describes inhibition invoked in a SST as a race between two processes: a “Go” process initiates after a go cue and, when an external stop signal occurs, a “Stop” process begins. Response inhibition will succeed if the Stop process completes prior to the Go process completing (Logan & Cowan, 1984). Each process, and their relation, may be influenced by different factors, which can therefore influence the outcome of successful inhibition. One set of factors are characterized as reactive stopping and proactive stopping processes, each of which can be operationalized in SSTs. SSTs include a range of intervals, or Stop Signal Delays (SSDs), between the Go and the Stop signals. This range allows estimation of the time it takes for the stop process to be completed successfully, an estimate of the internal speed of stopping known as the stop signal reaction time (SSRT). In neural systems models, SSRT is often associated with reactive stopping processes, initiated in response to an external signal (Aron, 2011). The prior study (Ethridge et al., 2014) did not find differences between psychosis groups on SSRT, but did report proactive stopping process alterations. These were identified as failure to show adequately slowed reaction times in the context of interleaved Stop and Go signals (in comparison with reaction times to stimuli occurring without the possibility of Stop signals). This adaptive slowing is thought to reflect more internally motivated preparations to stop, and as such, has relevance for understanding the impulsivity disturbances in psychotic disorders where symptoms or other characteristics (inattentiveness, preoccupation, emotional distress, etc.) may impact successful generation of readiness to stop, though reactive and proactive stopping processes cannot be entirely disentangled (Aron, 2011).
Other SST outcomes, such as errors on different trial types and reaction times of specific trial types were not examined in the prior study, but may help further elucidate the nature of the mechanisms contributing to inhibition dysfunction and group difference. For example, speeded basic reaction time may render Go processes more difficult for Stop processes to get ahead of. Or, elevated error rates on not only inhibition trials, but also other kinds of trials during a SST may qualify inhibition errors as part of a more generalized response deficiency, which may be consistent with more impaired general cognition.
Another important question is whether SST deficits seen for PBD are also present in nonpsychotic bipolar disorder (NPBD). Clarifying this may improve understanding of the common and divergent neurobiology along the schizo-bipolar spectrum. The literature on cognitive differences between NPBD and PBD is mixed. Several studies report no differences between NPBD and PBD across cognitive domains assessed (Burton et al., 2018; Demmo et al., 2016; Lahera et al., 2008; Savitz et al., 2009; Selva et al., 2007). Others report greater deficits in PBD compared to NPBD for verbal memory, executive function, working memory, semantic fluency, or social cognition (Allen et al., 2010; Aminoff et al., 2013; Bora et al., 2010; Glahn et al., 2006, 2007; Jiménez-López et al., 2017; Levy et al., 2013; Martinez-Aran et al., 2008; Simonsen et al., 2011; Thaler et al., 2013). Hence, the pattern, and particularly the inclusion of executive function among the distinguishing measures, suggests that higher order cognition may be more impaired in the psychotic variant of bipolar disorder relative to NPBD. As inhibitory control is also a higher order function due to its association with frontal lobe development (Dempster, 1992), SSTs may distinguish PBD from NPBD. Limited prior work using SSTs in NPBD and a mixed sample of PBD and NPBD indicates deficits exist relative to healthy controls (Farahmand et al., 2015; Fortgang et al., 2016; Hidiroğlu et al., 2015), but whether deficits are of differing magnitude for the two bipolar phenotypes has not been directly tested.
In the current study, we sought to address these questions. First, we predicted deficient inhibitory control across the schizo-bipolar spectrum, including NPBD. We further predicted there would be differences distinguishing SZ/SAD from both bipolar disorder (BD) groups on additional SST measures not examined previously (error rates and reaction times for specific trial types), supporting interpretation of inhibition deficits as reflecting problems adapting to task demands vs. problems related to speeded responding. Second, we predicted that SST performance would be more impaired in PBD patients with a history of psychosis compared to NPBD patients without lifetime history of psychosis.
2. Methods and Materials
2.1. Participants
As part of the Bipolar-Schizophrenia Network for Intermediate Phenotypes 2 study (B-SNIP2) and the Psychosis and Affective Research Domains and Intermediate Phenotypes (PARDIP) projects, participants were recruited at 5 sites (Tamminga et al., 2014). Groups were 259 BD, 216 SAD, 220 SZ, and 280 healthy controls (HC). All provided written informed consent and the study was approved by Institutional Review Boards at each site. This PARDIP/BSNIP-2 sample was fully different from that used in our prior study, which was the B-SNIP1 sample (Ethridge et al., 2014).
DSM-IV diagnoses were established via the Structured Clinical Interview for DSM-IV (First et al., 2002). HC had no personal history of lifetime psychotic or bipolar disorders or recurrent major depressive disorder, and no known history of psychotic or bipolar disorders in first-degree relatives. All participants met the following criteria: no known neurological disorders or decompensated medical disorders that could affect central nervous system function, no history of head trauma with >10 minutes unconsciousness, no positive urine drug screen on testing day, and no substance abuse within 1 month or dependence within 3 months. Patient participants were clinically stable and there were no changes to psychopharmacological treatment for at least one month. Chlorpromazine equivalent antipsychotic daily dose (CPZ) was calculated using the Andreasen (2010) method.
2.2. Procedure
2.2.1. Clinical and general cognitive assessments.
All subjects completed the self-report Barratt Impulsiveness Scale (Patton et al., 1995), which yields an overall impulsivity rating as well as three subscale scores – attentional, motor, and non-planning impulsiveness. Clinical symptoms were rated using the Positive and Negative Syndrome Scale (Lançon et al., 2000), Young Mania Rating Scale (Young et al., 1978), Montgomery-Asberg Depression Rating Scale (Montgomery & Asberg, 1979), and Schizo-Bipolar Scale (SBS; Keshavan et al., 2011). Suicide was also rated in terms of lifetime presence/absence of a suicide attempt, highest medical lethality of suicide attempts (0–7 scale), and lifetime number of attempts. For general cognition, the Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al., 2004) was administered, as in Ethridge et al. (2014). The BACS consists of six subtests which together comprise a composite score, corrected for age and gender using stratified norms and expressed as a z-score (Keefe et al., 2008).
2.2.2. Stop Signal Task.
We used the same task and analysis approach as in Ethridge et al. (2014). Trials began with presentation of a white central-fixation crosshair (1.5 degrees) for a random interval between 750–1500 ms (Figure 1). On Go trials, a green circle (Go cue; 1.75 degrees) appeared 12 degrees right or left of the center for 650 ms. On Stop trials, a Stop signal (red stop sign; 1.75 degrees) was presented at central fixation at variable SSDs (50–282 ms) after the Go stimulus appeared. Participants were instructed to respond as quickly and accurately as possible via button press. Practice trials were completed to verify comprehension of task instructions. Then, a Baseline task of 50 consecutive Go trials was administered to help establish a prepotent response, followed by ~300 “SST trials” with pseudorandomly interleaved Go and Stop trials (40% Stop). To maintain a prepotent Go response tendency, lack of response within 650 ms on Go trials resulted in trial termination with a red ‘X’ and the word “faster.” For every third Go trial without a timely response, a Go trial was added later. A red ‘X’ over the Stop signal gave performance feedback for incorrect Stop trials. Rater administration notes were screened prior to sample selection and poor quality data for 19 participants (3 HC, 4 BP, 7 SAD, 5 SZ) was excluded.
Figure 1.
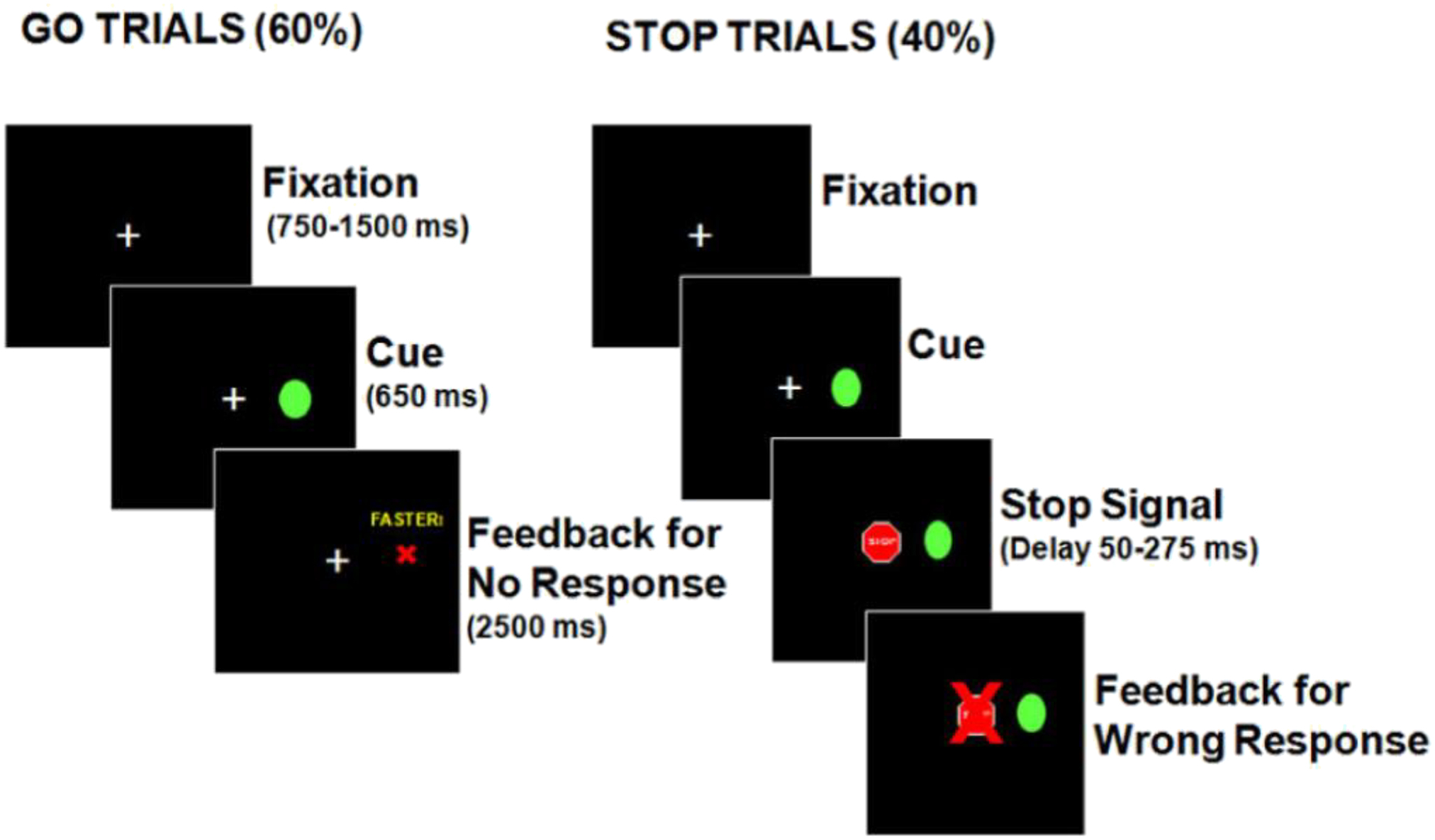
Stop Signal Task (4). In Go trials, participants pressed the corresponding left or right button when a green circle appeared to the right or left of the center. During Stop trials, they were to refrain from pushing the buttons. When participants did not respond within 650 ms on Go trials, a red ‘X’ and the word ‘faster’ were presented for 2500 ms indicating that the participant failed a trial. On Stop trials in which the participants pressed a button, a red ‘X’ appeared over the stop sign to provide performance feedback indicating the failure to inhibit a response.
Scores were computed for errors on SST Stop trials (failure to inhibit a response to a Go signal combined with a Stop signal), Baseline Go trial errors (omissions of response when there are no Stop signals), SST Go trial errors (omissions/overly-slow responding), and speed of correct response for Baseline and SST Go trials. Additionally, SSRT (estimated latency of successful Stop process) and adaptive slowing (mean response latency for SST Go minus mean for Baseline Go trials, with greater slowing indicating better adaptation to task demands) were examined using procedures consistent with prior work (Ethridge et al., 2014; Schmitt et al., 2018). SSRT was calculated using the integration method (Verbruggen et al., 2013; see Supplement section A), with shorter SSRT reflecting faster reactive stopping (Logan, 1994).
2.3. Statistical Analyses
Analysis of variance (ANOVA) and Chi-Square statistics were used to compare demographic and clinical variables across groups, and analysis of covariance (ANCOVA) was used to compare groups on the BACS composite z-score (global cognitive function), with race as covariate (BACS normative scores were already stratified by age and gender). For the SST, separate ANCOVAs with age, sex, and race as covariates were used to compare groups on errors for SST Stop trials, SST Go trials, Baseline Go trials, SSRT, and adaptive slowing. Adaptive slowing analyses were followed up with repeated measures ANCOVA on the components of the adaptive slowing measure – mean response latencies from Baseline Go trials and SST Go trials as the repeated measures with age, sex, and race as covariates, followed by univariate analyses as needed. Next, to examine the extent that global cognitive function accounted for SST outcomes, ANCOVA analyses were repeated with the BACS composite z-score as a covariate. Correlations among BACS and SST performance and clinical variables were explored using Spearman’s rho and were evaluated as significant with a False Discovery Rate (FDR) correction for multiple comparisons (Benjamini & Hochberg, 1995). For descriptive purposes, we conducted correlations among the SST variables within each group (see Supplement section G). All variables were assessed for study site differences, and none were identified. Comparison of two SST variables (errors for Stop trials and adaptive slowing) were conducted between the current B-SNIP2 SZ, SAD, and PBD groups and the B-SNIP1 sample (Ethridge et al., 2014) to verify comparability of the task (see Supplement section B).
3. Results
3.1. Patient and Healthy Control Comparisons
3.1.1. Characteristics.
Groups were matched for age, which ranged from 18 to 62 years old, but had other demographic differences (Table 1). Medication and symptom differences among the patient groups were as expected, including fewer BD patients prescribed antipsychotic medications and a larger proportion prescribed lithium compared to SAD and SZ groups. Clinically, the BD group endorsed less severe positive and negative symptoms than other disease groups. For the BIS, HC had lower impulsivity ratings than all disease groups. SZ had lower attentional and non-planning impulsivity than BD and SAD and lower motor and total impulsivity than BD.
Table 1.
Demographic and clinical data.
| Healthy Controls (HC) | Bipolar with and without Psychosis (BD) | Schizoaffective (SAD) | Schizophrenia (SZ) | |||||
|---|---|---|---|---|---|---|---|---|
| N | 280 | 259 | 216 | 220 | ||||
| Variable | N | % | N | % | N | % | N | % |
| Sex*, a | ||||||||
| Male | 138 | 49.3 | 100 | 38.6 | 99 | 45.8 | 123 | 55.9 |
| Female | 142 | 50.7 | 159 | 61.4 | 117 | 54.2 | 97 | 44.1 |
| Race**, b | ||||||||
| Caucasian | 130 | 46.4 | 175 | 67.6 | 93 | 43.1 | 77 | 35.0 |
| African American | 113 | 40.4 | 60 | 23.2 | 86 | 39.8 | 102 | 46.4 |
| Other | 37 | 13.2 | 24 | 9.3 | 37 | 17.1 | 41 | 18.6 |
| On antipsychotic medication**, c | - | - | 141 | 54.4 | 158 | 73.1 | 148 | 67.3 |
| On lithium**, d | - | - | 51 | 19.7 | 25 | 11.6 | 7 | 3.2 |
| On valproic acid*, e | - | - | 33 | 12.7 | 22 | 10.2 | 13 | 5.9 |
| On sedative medication**, f | - | - | 73 | 28.2 | 39 | 18.1 | 28 | 12.7 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Chlorpromazine equivalent (mg/day)*, g,^ | - | - | 274.82 | 222.86 | 375.71 | 281.53 | 398.81 | 312.50 |
| Lithium dose (mg/day)^ | - | - | 909.80 | 397.75 | 858.70 | 307.35 | 885.71 | 705.76 |
| Valproic acid dose (mg/day)^ | - | - | 1061.72 | 482.06 | 1052.63 | 602.88 | 1076.92 | 449.36 |
| Age (years) | 38.43 | 12.05 | 37.69 | 12.06 | 39.62 | 11.56 | 39.13 | 12.00 |
| Years of Education**, h | 15.33 | 2.46 | 14.59 | 2.50 | 13.41 | 2.26 | 13.05 | 2.38 |
| WRAT4 Reading**, i | 99.88 | 13.50 | 99.36 | 14.85 | 94.00 | 14.57 | 93.50 | 14.31 |
| BACS Composite**, h | -.299 | .758 | −.709 | .789 | −.971 | .798 | −1.146 | .826 |
| PANSS Total*, g | - | - | 59.14 | 18.99 | 67.28 | 20.78 | 63.32 | 17.31 |
| PANSS Positive**, j | - | - | 13.52 | 5.05 | 18.23 | 7.06 | 16.61 | 5.87 |
| PANSS Negative*, g | - | - | 14.30 | 6.45 | 16.08 | 6.79 | 16.05 | 5.84 |
| MADRS Total**, k | - | - | 14.44 | 11.15 | 14.19 | 10.55 | 9.31 | 8.53 |
| YMRS Total*, g | - | - | 8.90 | 7.72 | 11.00 | 7.68 | 9.42 | 6.10 |
| SBS Total**, j | - | - | 1.89 | 1.21 | 5.10 | 1.41 | 7.64 | 1.11 |
| BIS Total**,l | 52.61 | 9.04 | 71.32 | 14.54 | 69.89 | 12.14 | 65.39 | 12.50 |
| BIS Attention**, l | 12.91 | 3.19 | 18.88 | 5.10 | 18.69 | 4.57 | 16.88 | 4.27 |
| BIS Motor**, m | 19.42 | 3.65 | 25.27 | 5.53 | 24.02 | 4.51 | 22.83 | 5.67 |
| BIS Non-Planning**, l | 20.29 | 4.56 | 27.18 | 6.07 | 27.18 | 5.84 | 25.68 | 5.73 |
WRAT = Wide Range Achievement Test; BACS = Brief Assessment of Cognition in Schizophrenia; PANSS = Positive and Negative Syndrome Scale; MADRS = Montgomery-Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; SBS = Schizo-Bipolar Scale; BIS = Barratt Impulsiveness Scale.
Bonferroni corrected for multiple comparisons;
p < .05;
p < .001;
Means calculated with only those prescribed each medication
Greater proportion of females in the BD group compared to HC and other disease groups.
Greater proportion of Caucasians and fewer African Americans in BD compared to HC and other disease groups.
Fewer BD on antipsychotic medication than SAD and SZ groups.
Greater proportion of BD on lithium than SAD and SZ groups.
Greater proportion of BD and SAD on valproic acid than SZ.
Greater proportion of BD on sedative medication than SAD and SZ, and SAD greater than SZ.
BD < SAD
HC > BD > SZ; SAD = SZ
HC, BD > SAD, SZ
BD < SAD, SZ
BD, SAD > SZ
HC < SZ < BD, SAD
HC < SZ, SAD < BD
3.1.2. Global cognitive function.
All patient groups had significantly lower BACS composite scores than HC (Figure 2; F(3, 961) = 54.63, p <.001, ηp2 = .15). Consistent with prior reports in the B-SNIP1 sample (Hill et al., 2013), SZ had greater impairment than BD (Mdiff = −.29, SE = .07, p <.001), while SAD did not differ from either BD (Mdiff = −.15, SE = .07, p = .22) or SZ (Mdiff = .14, SE = .07, p = .36).
Figure 2.
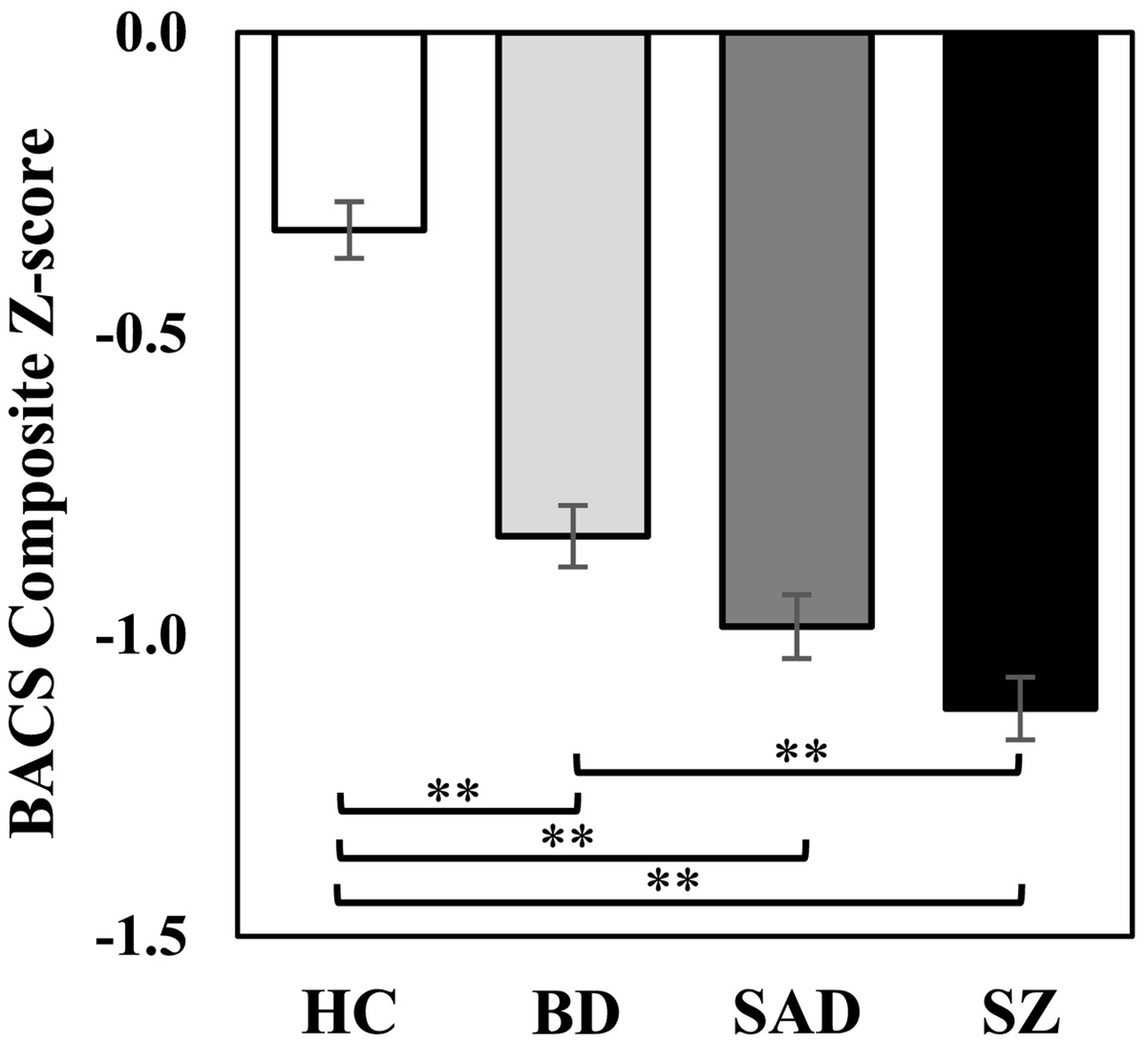
Brief Assessment of Cognition in Schizophrenia (BACS) z-scores across groups. All disease groups showed significant general cognitive deficit compared to healthy controls, with the schizophrenia group showing the greatest magnitude of deficit. ** p <.001.
3.1.3. Inhibitory control error rate.
There was a significant main effect of group for error rates on Stop trials, the key inhibition measure (Figure 3; F(3, 967) = 10.97, p <.001, ηp2 = .03), with higher error rates for each disease group compared to HC and no differences between disease groups (see also Supplement section D for a comparison of errors rates across SSD intervals). For SST Go trials, the SZ group committed more errors than HC (Mdiff = .05, SE = .01, p < .001) and BD (Mdiff = .03, SE = .01, p = .002), while SAD committed more errors than HC (Mdiff = .03, SE = .01, p = .002). For Baseline Go trials, SZ committed more errors compared to HC (Mdiff = .03, SE = .01, p < .001) and BD (Mdiff = .02, SE = .01, p = .02). Covarying for BACS composite z-score in all of these analyses eliminated all group differences except that the error rate for Stop trials remained significantly higher for BD compared to HC (Mdiff = .04, SE = .01, p = .01).
Figure 3.
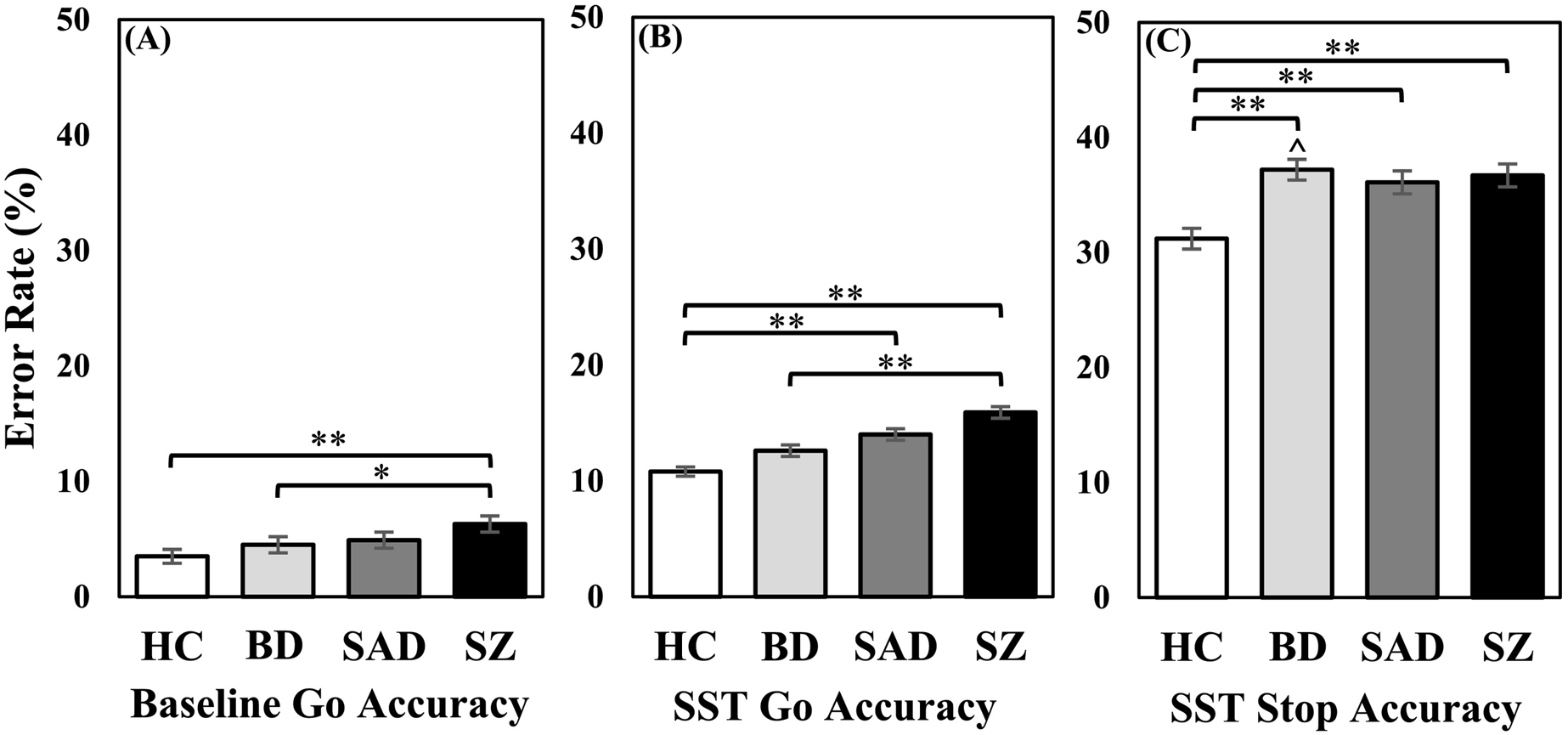
(A) Error rate for Baseline Go trials. (B) Error rate for SST Go trials. (C) Error rate for SST Stop trials. While the schizophrenia group had the highest error rate across Baseline and SST Go trials, the bipolar group (with and without psychosis) had higher Stop trial error rates than all other groups, before and after controlling for BACS. ** p <.001. Ŝignificant differences after controlling for BACS.
3.1.4. Inhibitory control reaction time.
Estimated SSRTs differed across groups (Figure 4; F(3, 967) = 7.73, p <.001, ηp2 = .03), with SZ taking longer to complete the Stop process than BD (Mdiff = 14.85, SE = 3.93, p = .001) and HC (Mdiff = 15.82, SE = 3.72, p <.001). After covarying for the BACS composite, group differences were no longer significant (F(3, 958) = 2.31, p = .08, ηp2 = .01). For adaptive slowing (difference in Baseline and SST Go reaction time), HC slowed their reaction times on SST Go trials more than SAD and SZ, while BD slowed more than SZ (Figure 5; F(3, 967) = 8.82, p <.001, ηp2 = .03) (see also Supplement section E for a comparison of percent increase in reaction time). Reaction time for Baseline Go trials and SST Go trials were then examined to further explore this effect. As expected, reaction time was slower overall during SST Go trials compared to Baseline Go trials (F(1, 950) = 616.16, p <.001, ηp2 = .39). However, there was also a significant condition by group interaction (F(3, 950) = 6.43, p <.001, ηp2 = .02) in which SZ showed slower reaction times for Baseline Go trials compared to all other groups (F(3, 967) = 13.02, p <.001, ηp2 = .04), and SAD had slower reaction times than BD (Mdiff = 14.52, SE = 4.48, p = .01). During SST Go trials, BD demonstrated faster reaction times than all other groups (F(3, 967) = 8.70, p <.001, ηp2 = .03). After covarying for the BACS composite, group differences for adaptive slowing and reaction time for SAD/SZ were no longer significant. However, BD still had faster reaction times than HC and all other disease groups for SST Go trials (F(3, 958) = 8.34, p <.001, ηp2 = .03), and a new effect emerged such that BD was also faster than all groups for Baseline Go trials (F(3, 958) = 9.40, p <.001, ηp2 = .03). Supplemental analyses of intraindividual variability of these reaction time scores indicated greater variability in BD for SST Go trials compared to all other groups, with or without BACS as a covariate (Supplement section F).
Figure 4.
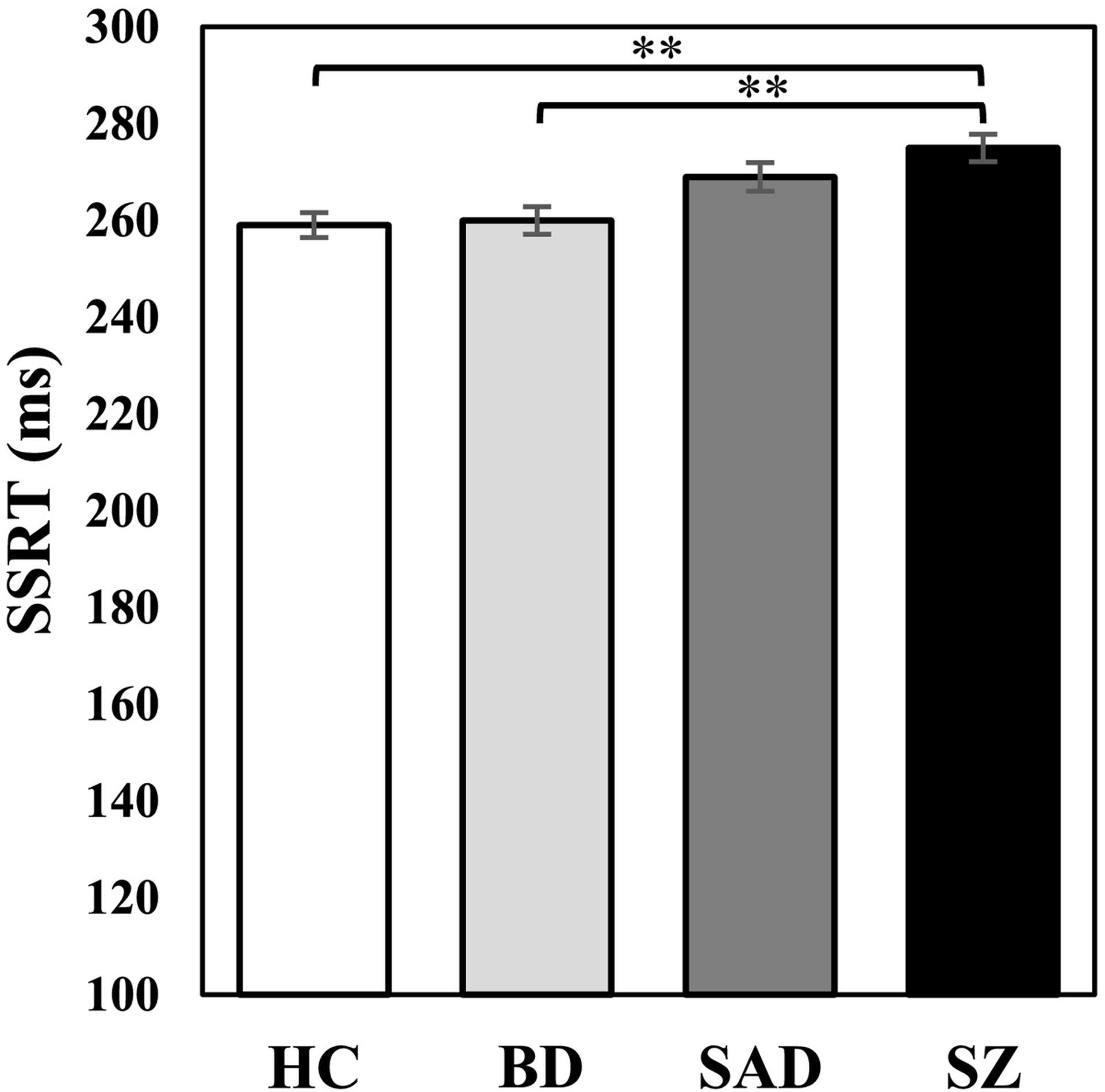
Stop Signal Reaction time (SSRT). While the schizophrenia group demonstrated prolonged SSRT compared to HC and BD, this difference was attenuated after controlling for BACS composite. **p < .001.
Figure 5.
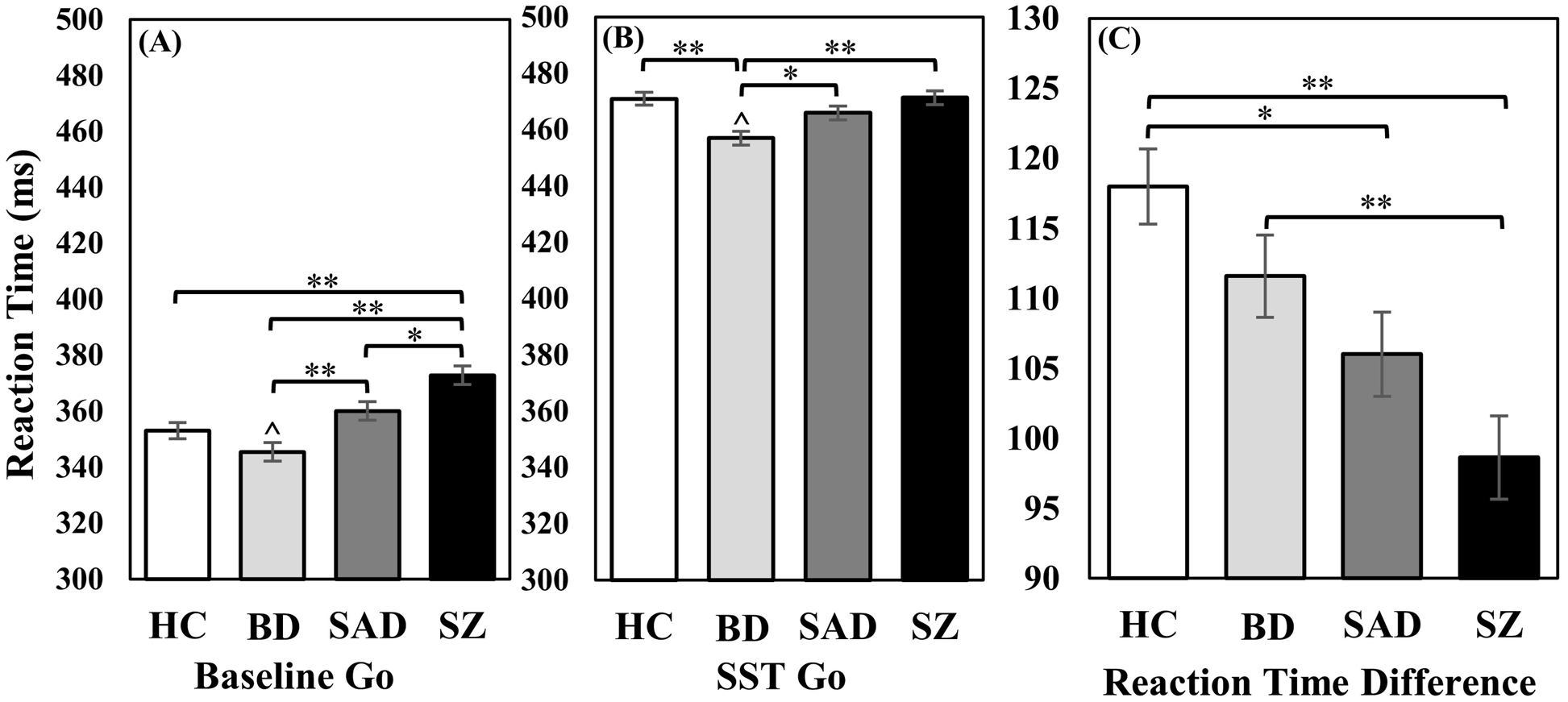
(A) Reaction time for Baseline Go trials. As expected, the schizophrenia group demonstrated slower reaction times than all other groups for Baseline Go trials. (B) Reaction time for SST Go trials. For SST Go trials, the bipolar group (with and without psychosis) had faster reaction times than all other groups, which was apparent during all Go trials and remained significant after controlling for BACS. (C) Difference in Baseline Go and SST Go reaction time. Adaptive slowing was reduced for schizoaffective and schizophrenia groups compared to healthy controls. The schizophrenia group also had reduced adaptive slowing compared to the bipolar group (with and without psychosis). However, these group differences were not significant when the BACS composite was included as a covariate. * p <.05; ** p <.001. Ŝignificant differences after controlling for BACS.
3.2. Comparison of Bipolar with and without Psychosis
NPBD and PBD did not differ on any SST variable, including errors, SSRT, adaptive slowing, or reaction time, nor on the BACS composite score (Table 2). There were no differences between PBD and NPBD on clinical characteristics, including all symptom measures. While more subjects in the PBD group were taking antipsychotic medications, PBD and NPBD did not differ in CPZ equivalent daily dose (F(1,98) = .01, p = .91). NPBD and PBD also did not differ in frequency of use or daily dose of lithium (χ2(1) = .42, p = .52; F(1,49) = 1.06, p = .31), valproic acid (χ2(1) = .05, p = .82; F(1,31) = .71, p = .41), or use of sedative medication (χ2(1) = 3.72, p = .054).
Table 2.
Demographic and clinical differences between bipolar with and without psychosis.
| Bipolar (NPBD) | Bipolar with Psychosis (PBD) | ||||
|---|---|---|---|---|---|
| N | 67 | 192 | |||
| Variable | N | % | N | % | |
| Sex* | |||||
| Male | 19 | 28.4 | 81 | 42.2 | |
| Female | 48 | 71.6 | 111 | 57.8 | |
| Race | |||||
| Caucasian | 46 | 68.7 | 129 | 67.2 | |
| African American | 15 | 22.4 | 45 | 23.4 | |
| Other | 6 | 9.0 | 18 | 9.4 | |
| On antipsychotic medication* | 26 | 38.8 | 115 | 59.9 | |
| On lithium | 15 | 22.4 | 36 | 18.8 | |
| On valproic acid | 8 | 11.9 | 25 | 13.0 | |
| On sedative medication | 25 | 37.3 | 48 | 25.0 | |
| Mean | SD | Mean | SD | ||
| Chlorpromazine equivalent dose (mg/day)^ | 269.43 | 218.37 | 275.93 | 225.06 | |
| Lithium dose (mg/day)^ | 825 | 354.97 | 948.57 | 414.89 | |
| Valproic acid dose (mg/day)^ | 925 | 600.17 | 1100 | 450.69 | |
| Age (years)* | 41.39 | 12.72 | 36.41 | 11.58 | |
| Years of Education | 14.49 | 2.98 | 14.62 | 2.32 | |
| WRAT4 Reading | 97.19 | 15.09 | 100.11 | 14.72 | |
| BACS Composite | −.673 | .830 | −.722 | .776 | |
| PANSS Total | 60.59 | 16.59 | 58.41 | 20.12 | |
| PANSS Positive | 13.08 | 3.75 | 13.74 | 5.59 | |
| PANSS Negative | 15.37 | 6.52 | 13.77 | 6.37 | |
| MADRS Total | 15.02 | 9.82 | 14.15 | 11.79 | |
| YMRS Total | 9.59 | 7.20 | 8.54 | 7.97 | |
| SBS Total | 1.80 | .96 | 1.93 | 1.31 | |
| BIS Total | 71.32 | 12.57 | 71.32 | 14.54 | |
| BIS Attention | 19.31 | 4.43 | 18.72 | 5.32 | |
| BIS Motor | 24.94 | 5.09 | 25.39 | 5.69 | |
| BIS Non-planning | 27.08 | 5.57 | 27.22 | 6.25 | |
| SST Proportion Errors Baseline Go Trials | .039 | .048 | .036 | .048 | |
| SST Proportion Errors SST Go Trials | .141 | .092 | .113 | .082 | |
| SST Proportion Errors SST Stop Trials | .379 | .124 | .376 | .137 | |
| SST Baseline Go RT | 347.94 | 44.70 | 340.76 | 49.05 | |
| SST Proper Go RT | 459.50 | 36.26 | 454.03 | 37.28 | |
| SST Baseline-Proper Go RT Difference | 111.56 | 38.31 | 113.27 | 40.71 | |
| SSRT | 268.10 | 6.37 | 260.62 | 3.95 | |
WRAT = Wide Range Achievement Test; BACS = Brief Assessment of Cognition in Schizophrenia; PANSS = Positive and Negative Symptom Scale; MADRS = Montgomery-Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; SBS = Schizo-Bipolar Scale; BIS = Barratt Impulsiveness Scale.
p <.05;
Includes only those prescribed each medication.
3.3. Clinical Correlations
Among symptom severity ratings, only negative symptoms showed significant correlations with test performance (Table 3). For both the BD and SAD group, more severe negative symptoms were associated with lower BACS composite score. For the BD group, negative symptoms were also associated with higher Stop trial errors and reduced adaptive slowing. For SZ, negative symptoms were associated with reduced adaptive slowing, higher SST Go trial errors, and slower reaction time for Baseline Go trials. There were no significant associations between BACS or SST with rated aspects of suicidal behavior or self-rated impulsivity. There were also no significant correlations between medication dose and task performance. As an alternative to categorical diagnostic grouping, correlations with the SBS scale and SST performance were conducted, with results detailed in Supplement section C.
Table 3.
Clinical correlations by group.
| BD | SAD | SZ | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BACS | BGErr | SGErr | SErr | BACS | BGErr | SGErr | SErr | BACS | BGErr | SGErr | SErr | |
| Current Symptoms | ||||||||||||
| PANSS Positive | −.10 | .16 | .12 | .12 | −.11 | .07 | .14 | .20 | −.13 | .12 | <.01 | .11 |
| PANSS Negative | −.38* | .23 | .18 | .25* | −.24* | .17 | .18 | .17 | −.20 | .13 | .26* | .13 |
| MADRS Total | −.13 | .09 | .04 | .08 | −.15 | .18 | .01 | .09 | .03 | <.01 | −.01 | .01 |
| YMRS Total | .01 | .02 | .05 | −.03 | .03 | −.07 | −.04 | .03 | −.12 | .03 | −.13 | .01 |
| Medication | ||||||||||||
| CPZ Dose | −.16 | .15 | .12 | .07 | −.05 | .04 | .15 | <.01 | −.06 | −.03 | .05 | −.06 |
| Lithium Dose | −.03 | −.01 | .15 | .02 | .01 | −.09 | .04 | <.01 | .02 | .09 | .03 | .02 |
| Valproic Acid Dose | <.01 | .03 | .04 | −.07 | .06 | −.02 | .05 | .05 | −.05 | −.01 | .01 | −.02 |
| Sedative Use | <.01 | −.05 | −.03 | .12 | −.13 | .09 | .12 | .08 | <.01 | −.02 | .06 | −.03 |
| Impulsivity | ||||||||||||
| BIS | −.05 | <.01 | −.06 | −.05 | −.16 | .10 | .02 | .08 | −.14 | .05 | −.03 | .14 |
| Total | ||||||||||||
| BIS Attentional | .07 | −.01 | −.06 | −.05 | −.07 | .08 | −.02 | .02 | −.09 | .04 | −.02 | .13 |
| BIS Motor | −.02 | −.03 | −.10 | −.05 | −.06 | .01 | .03 | .08 | −.08 | −.01 | <.01 | .08 |
| BIS Non-planning | −.14 | .05 | .01 | −.04 | −.19 | .13 | .02 | .08 | −.10 | .07 | −.03 | .14 |
| Suicide | ||||||||||||
| History of suicide | .03 | −.02 | −.05 | −.01 | .08 | .03 | −.06 | .08 | .09 | −.08 | .04 | <.01 |
| Suicide severity | .03 | −.01 | −.05 | .01 | .06 | .08 | <.01 | .11 | .07 | −.06 | .02 | .08 |
| Suicide count | .02 | −.03 | −.06 | −.06 | −.02 | .06 | −.10 | .08 | .14 | −.10 | −.04 | −.08 |
| BD | SAD | SZ | ||||||||||
| BGRT | SGRT | AS | SSRT | BGRT | SGRT | AS | SSRT | BGRT | SGRT | AS | SSRT | |
| Current Symptoms | ||||||||||||
| PANSS Positive | .03 | <.01 | −.04 | .10 | −.15 | −.02 | −.18 | .16 | .06 | −.06 | −.12 | −.02 |
| PANSS Negative | .19 | −.03 | −.25* | .22 | .14 | .06 | −.12 | .20 | .28* | .08 | −.28* | .14 |
| MADRS Total | .01 | −.09 | −.10 | −.01 | .05 | −.07 | −.11 | −.02 | .07 | −.05 | −.12 | −.09 |
| YMRS Total | .04 | .03 | −.02 | −.02 | −.06 | −.11 | −.03 | −.03 | −.05 | −.17 | −.09 | −.15 |
| Medication | ||||||||||||
| CPZ Dose | .11 | .02 | −.11 | .12 | .13 | .08 | −.09 | .03 | .05 | .10 | .02 | −.02 |
| Lithium Dose | .06 | .10 | .02 | .13 | −.06 | −.03 | .06 | −.04 | <.01 | <.01 | <.01 | .05 |
| Valproic Acid Dose | −.01 | .09 | .10 | −.01 | <.01 | .01 | .01 | .07 | .03 | .06 | .01 | .08 |
| Sedative Use | −.10 | −.10 | −.02 | −.08 | .07 | −.04 | −.14 | .02 | .03 | .05 | <.01 | .03 |
| Impulsivity | ||||||||||||
| BIS Total | −.13 | −.08 | .08 | −.13 | .04 | −.05 | −.08 | .10 | <.01 | −.14 | −.11 | −.02 |
| BIS Attentional | −.14 | −.10 | .07 | −.16 | .02 | .01 | −.02 | .13 | .02 | −.09 | −.10 | .04 |
| BIS Motor | −.17 | −.09 | .11 | −.15 | −.01 | −.08 | −.05 | −.02 | .07 | −.08 | −.15 | <.01 |
| BIS Non-planning | −.04 | −.02 | .02 | −.05 | .07 | −.04 | −.11 | .12 | −.10 | −.15 | −.02 | −.04 |
| Suicide | ||||||||||||
| History of suicide | .01 | −.06 | −.07 | <.01 | .04 | −.05 | −.09 | .01 | .03 | .05 | <.01 | .06 |
| Suicide severity | −.03 | −.08 | −.04 | .01 | −.01 | −.12 | −.09 | .01 | .02 | .06 | .03 | .04 |
| Suicide count | <.01 | .01 | <.01 | −.05 | .06 | −.05 | −.12 | −.01 | −.01 | −.03 | −.01 | −.03 |
BACS = Brief Assessment of Cognition in Schizophrenia; PANSS = Positive and Negative Symptom Scale; MADRS = Montgomery-Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale; CPZ = Chlorpromazine equivalent antipsychotic dose; BIS = Barratt Impulsiveness Scale; BGErr = Baseline Go errors; SGErr = SST Go errors; SErr = Stop errors; BGRT = Baseline Go reaction time; SGRT = SST Go reaction time; AS = adaptive slowing; SSRT = Stop Signal Reaction Time.
FDR corrected p-value.
4. Discussion
This study was designed to investigate a variety of metrics associated with deficient inhibitory control during a SST across the schizo-bipolar spectrum, seeking characteristics that distinguish psychosis groups. We also wanted to determine if prior findings of a specific deficit in inhibitory control in PBD extends to NPBD. We found that the magnitude of impairment for inhibitory control across disorders was consistent with prior findings (Ethridge et al., 2014), and that different processes may underlie inhibition errors across groups. We extended the findings of impaired inhibitory control to include NPBD, as the BD groups performed similarly in terms of inhibition as well as general cognitive function.
4.1. Impaired Response Inhibition across Patient Groups
A key finding was the comparable magnitude of inhibitory control deficits (Stop trial errors) across all patient groups. This measure of inhibitory control could be an important biomarker defining a common neurocognitive deficit present across the schizo-bipolar spectrum. There were, however, unique features among disease groups that were captured by other SST variables suggesting that distinct alterations may underlie inhibitory control deficits within each group. For example, the combined BD group had shorter latencies during both types of Go trials, and intact Go trial accuracy and adaptive slowing. Hence, the BD group was able to slow their reaction times from Baseline to SST Go trials, demonstrating some capacity for proactive inhibition. However, the adaptation did not appear to be successful as there was also a high error rate for Stop trials. This was consistent with the faster Baseline and SST Go trial latencies seen in only the BD group. Shorter Go trial reaction times have been reported elsewhere in bipolar disorder (Hidiroğlu et al., 2015; Houshmand et al., 2010) and support the notion that inhibitory control deficits in BD may be best conceptualized as errors associated with speeded responding (see also Supplement section G for discussion of possibly stronger correlation of Baseline Go RT and SSRT in BD). Further, the BD group showed high variability in their reaction times (Supplement section F), particularly SST Go trial reaction times, and this group distinction was not accounted for by general cognitive ability. This suggests performance was characterized by inconsistently failed attempts at slowing or monitoring.
Broadly, inhibitory control is thought to be supported by a fronto-basal-ganglia network involving the right inferior frontal gyrus (IFG), subthalamic nucleus (STN), and presupplementary motor area (preSMA) (Aron, 2011). Using this model, Go processes are initiated through the direct pathway of the basal ganglia, while the IFG and preSMA send a Stop command to intercept the Go process through connections with the basal ganglia, specifically the STN. This network is responsible for initiating both reactive stopping to inhibit behavior in response to an external cue and proactive stopping to slow down and prepare for an inhibitory behavioral response. Striatum and frontal brain regions involved in working memory are also thought to play a role in proactive stopping processes by modulating the reactive stopping circuitry. Hence, the capacity for adaptive slowing found in the BD group may be consistent with less severe working memory impairments compared to SZ/SAD (Park & Gooding, 2014). We further speculate that the BD deficits observed in our study could reflect overactivity of the Go process due to reduced initiation of reactive inhibition from the IFG and preSMA. Moreover, this is consistent with meta-analytic findings of reduced right IFG volume (Selvaraj et al., 2012) and attenuated activity in the IFG and associated striatal/limbic regions found in bipolar disorder (Chen et al., 2011).
In contrast, SAD/SZ showed reduced adaptive slowing and slower SSRT, consistent with dysfunction that involves both reactive and proactive stopping processes. Further, SAD/SZ also had more errors on Baseline and interleaved Stop and Go trials, suggestive of both Go and Stop process deficiencies. This was consistent with some differential correlations among SST variables for schizophrenia in particular (Supplement section G). Despite slowed Go reaction times, SAD/SZ were still unable to initiate stopping processes and took longer to internally complete the Stop process. This pattern may reflect a variety of altered processes – reduced proactive anticipation and poor preparation for Stop trials, altered capacity to execute reactive stop processes, and/or inattentiveness. Overall, this is consistent with an interpretation of inhibitory control deficits in SZ/SAD that entails multiple alterations, including dysfunction in higher-order self-control and planning, and more poorly controlled motor impulsivity. This was also consistent with the more impaired general cognition we observed for SZ/SAD, as reported previously (Hill et al., 2013), and may reflect greater widespread alterations rather than specific fronto-striatal dysfunction.
Further, the relationship between general cognition and response inhibition replicated prior findings (Ethridge et al., 2014) and was informative in terms of characterizing the magnitude of inhibitory control deficits relative to other cognitive alterations. Specifically, the BACS composite accounted for impairments on all SST indices for SZ and SAD, suggesting that these measures are part of the generalized cognitive deficit across cognitive domains in SZ/SAD (Hill et al., 2008; Hochberger et al., 2016; Reilly & Sweeney, 2014). On the other hand, controlling for general cognitive ability did not attenuate the high error rate on Stop trials in BD. Hence, for BD, impairment captured by SST appears largely related to more circumscribed difficulty with speeded reaction times and impulsive behavior rather than the generalized cognitive deficit associated with nonaffective psychotic disorders. This suggests that in BD, impaired inhibitory control may represent a deficit not entirely overlapping the group’s general cognitive impairment.
4.2. Differences Between Bipolar with and without Psychosis
This is the first study to directly compare a sample of NPBD and PBD using a SST. We note the two groups were very well matched on a variety of clinical characteristics. In contrast to our predictions, findings indicated a similar magnitude of impairment across SST metrics in BD groups. The similar performance, including errors and reaction time, suggests similar mechanisms underlying impaired inhibition in NPBD and PBD. Although some work has indicated higher-order cognitive impairment in PBD compared to NPBD, our study is consistent with the literature that shows no cognitive differences. Indeed, our PBD and NPBD groups also did not differ on general cognitive ability, consistent with prior reports of estimates of premorbid and general intellectual ability not differing between PBD and NPBD (Allen et al., 2010; Glahn et al., 2007; Jiménez-López et al., 2017; Levy et al., 2013; Martinez-Aran et al., 2008; Simonsen et al., 2011; Thaler et al., 2013). This is consistent with evidence of minimal to no differences in gray or white matter assessed in MRI studies (Ji et al., 2017; Godwin et al., 2018; Hibar et al., 2018). Thus, alternative characteristics are needed for distinguishing psychosis risk in bipolar disorder, such as genetic factors (Benedetti et al., 2016; Ivleva et al., 2010) or reward learning (Abohamza et al., 2020; Barch et al., 2017). Another possibility, however, is that any lifetime psychosis experience vs. none, which defines PBD vs. NPBD membership, is of limited value. Alternative grouping approaches, such as higher frequency of psychosis defining PBD, or neurobiological rather than symptom-based nosologies (Clementz et al., 2016; Drysdale et al., 2017; Ivleva et al., 2017; Williams, 2017) may be more useful.
4.3. Clinical Correlations
Symptom correlates with test performance indicated a relationship between greater negative symptom severity and worse cognitive performance. This included the BACS composite, adaptive slowing, and error scores variously across disease groups. Prior analysis from an independent sample failed to find correlations between SST performance and clinical symptoms (Ethridge et al., 2014). This could be due to the modest magnitude of the correlations, and they could be spurious findings despite FDR significance correction. Prior findings are mixed in terms of symptoms correlating with other measures of inhibitory control, such as anti-saccade task performance (Donohoe et al., 2006; Harris et al., 2009; Reilly et al., 2014; Winograd-Gurvich et al., 2008). More consistent associations have been found with the Stroop test and other measures of executive function in schizophrenia (Donohoe & Robertson, 2003; Westerhausen et al., 2011), but these correlations also have been modest.
4.4. Limitations
Although the present study included a large sample of individuals with schizo-bipolar spectrum disorders and healthy controls, it was relatively limited in its NPBD sample, rendering analyses comparing PBD to NPBD somewhat insensitive. Along the lines of sensitivity concerns, we note that the BACS is a brief cognitive battery that may not be as sensitive as prior work using a large battery to comprehensively compare performance between NPBD and PBD on other neuropsychological domains that were outside the focus of this study (Levy et al., 2013; Martinez-Aran et al., 2008).
4.5. Conclusion
Inhibitory control appears similarly impaired across the schizo-bipolar spectrum. The impairment is accounted for by generalized cognitive deficits in SZ/SAD, while for BD, inhibitory control appears more related to speeded responding, regardless of psychosis history. One important implication of these observations is that there is heterogeneity in how inhibition deficiencies may arise, which must be accounted for in future approaches designed to target inhibition problems in these serious psychiatric disorders. This also informs how interventions in BD may be developed that seek to utilize relative strengths of some capacity for proactive inhibitory control (e.g., monitoring behavior, keeping goal-relevant information in working memory) to compensate. For SZ/SAD treatment development, the findings suggest any improvement upon general cognitive function may also have impact on inhibitory control deficits, or possibly vice versa. In sum, our evolving understanding of inhibition in psychotic disorders may contribute to a better understanding of approaches to improve cognitive deficits across the schizo-bipolar spectrum.
Supplementary Material
Acknowledgment
The authors would like to thank the participants who contributed their time and effort to this study.
Role of the Funding Source
This work was supported by National Institute of Mental Health Grant Nos. MH103368 (to ESJ), MH096913 and MH077851 (to CAT), MH096957 and MH077945 (to GDP), MH096942 and MH078113 (to MSK), and MH096900 and MH103366 (to BAC). The NIMH had no role in study design; in the collection, analysis and interpretation of the data; in the writing of the report; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
M.Y. Gotra, S.K. Hill, E.S. Gershon, C.A. Tamminga, E.I. Ivleva, G.D. Pearlson, B.A. Clementz, J.E. McDowell, P.F. Buckley, J.A. Sweeney, and S.K. Keedy report no financial interests or potential conflicts. M.S. Keshavan has served as an adviser for Alkermes.
References
- Abohamza E, Weickert T, Ali M, Moustafa AA, 2020. Reward and punishment learning in schizophrenia and bipolar disorder. Behav. Brain Res 10.1016/j.bbr.2019.112298 [DOI] [PubMed] [Google Scholar]
- Allen DN, Randall C, Bello D, Armstrong C, Frantom L, Cross C, Kinney J, 2010. Are working memory deficits in bipolar disorder markers for psychosis? Neuropsychology. 10.1037/a0018159 [DOI] [PubMed] [Google Scholar]
- Aminoff SR, Hellvin T, Lagerberg TV, Berg AO, Andreassen OA, Melle I, 2013. Neurocognitive features in subgroups of bipolar disorder. Bipolar Disord. 10.1111/bdi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC, 2010. Antipsychotic dose equivalents and dose-years: A standardized method for comparing exposure to different drugs. Biol. Psychiatry 10.1016/j.biopsych.2009.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, 2011. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 10.1016/j.biopsych.2010.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Carter CS, Gold JM, Johnson SL, Kring AM, MacDonald AW, … Strauss ME, 2017. Explicit and implicit reinforcement learning across the psychosis spectrum. J. Abnorm. Psychol 10.1037/abn0000259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Colombo C, Lorenzi C, Pirovano A, Smeraldi E, 2010. Association between catechol-O-methyltransferase Val(108/158)Met polymorphism and psychotic features of bipolar disorder. J. Affect. Disord 10.1016/j.jad.2010.01.005 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y, 1995. Controlling the False Discovery Rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Bora E, Yücel M, Pantelis C, 2010. Neurocognitive markers of psychosis in bipolar disorder: A meta-analytic study. J. Affect. Disord 10.1016/j.jad.2010.02.117 [DOI] [PubMed] [Google Scholar]
- Burton CZ, Ryan KA, Kamali M, Marshall DF, Harrington G, McInnis MG, Tso IF, 2018. Psychosis in bipolar disorder: Does it represent a more “severe” illness? Bipolar Disord. 10.1111/bdi.12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET, 2011. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of distinct psychosis biotypes using brain-based biomarkers. Am. J. Psychiatry 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmo C, Lagerberg TV, Aminoff SR, Hellvin T, Kvitland LR, Simonsen C, … Ueland T, 2016. History of psychosis and previous episodes as potential explanatory factors for neurocognitive impairment in first-treatment bipolar I disorder. Bipolar Disord. 10.1111/bdi.12377 [DOI] [PubMed] [Google Scholar]
- Dempster FN, 1992. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Dev. Rev 10.1016/0273-2297(92)90003-K [DOI] [Google Scholar]
- Donohoe G, Robertson IH, 2003. Can specific deficits in executive functioning explain the negative symptoms of schizophrenia? A review. Neurocase 10.1076/neur.9.2.97.15075 [DOI] [PubMed] [Google Scholar]
- Donohoe G, Reilly R, Clarke S, Meredith S, Green B, Morris D, … Robertson IH, 2006. Do antisaccade deficits in schizophrenia provide evidence of a specific inhibitory function? J. Int. Neuropsychol. Soc 10.1017/S135561770606108X [DOI] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, … Liston C, 2017. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat. Med 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Pearlson GD, Lin D, Sui J, Chen J, Salman M, … Calhoun VD, 2017. Identifying dynamic functional connectivity biomarkers using GIG-ICA: Application to schizophrenia, schizoaffective disorder, and psychotic bipolar disorder. Hum. Brain Mapp 10.1002/hbm.23553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Soilleux M, Nakonezny PA, Reilly JL, Kristian Hill S, Keefe RSE, … Sweeney JA, 2014. Behavioral response inhibition in psychotic disorders: Diagnostic specificity, familiality and relation to generalized cognitive deficit. Schizophr. Res 10.1016/j.schres.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahmand Z, Tehrani-Doost M, Amini H, Mohammadi A, Mirzaei M, Mohamadzadeh A, 2015. Working memory and response inhibition in patients with bipolar I disorder during euthymic period. Iran. J. Psychiatry Behav. Sci 10.5812/ijpbs.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J, 2002. Structured clinical interview for DSMIV-TR axis I disorders, research version, patient edition (SCID-I/P). American Psychiatric Press, Inc.; New York, NY. [Google Scholar]
- Fortgang RG, Hultman CM, Van Erp TGM, Cannon TD, 2016. Multidimensional assessment of impulsivity in schizophrenia, bipolar disorder, and major depressive disorder: Testing for shared endophenotypes. Psychol. Med 10.1017/S0033291716000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI, Soares JC, 2006. Differential working memory impairment in bipolar disorder and schizophrenia: Effects of lifetime history of psychosis. Bipolar Disord. 10.1111/j.1399-5618.2006.00296.x [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC, Velligan DI, 2007. The neurocognitive signature of psychotic bipolar disorder. Biol. Psychiatry 10.1016/j.biopsych.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Godwin D, Alpert KI, Wang L, Mamah D, 2018. Regional cortical thinning in young adults with schizophrenia but not psychotic or non-psychotic bipolar I disorder. Int. J. Bipolar Disord 10.1186/s40345-018-0124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MSH, Reilly JL, Thase ME, Keshavan MS, Sweeney JA, 2009. Response suppression deficits in treatment-naïve first-episode patients with schizophrenia, psychotic bipolar disorder and psychotic major depression. Psychiatry Res. 10.1016/j.psychres.2008.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, … Andreassen OA, 2018. Cortical abnormalities in bipolar disorder: An MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry 10.1038/mp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidiroğlu C, Torres IJ, Er A, Işik G, Yalin N, Yatham LN, Ceylan D, Özerdem A, 2015. Response inhibition and interference control in patients with bipolar I disorder and first-degree relatives. Bipolar Disord. 10.1111/bdi.12335 [DOI] [PubMed] [Google Scholar]
- Hill SK, Sweeney JA, Hamer RM, Keefe RSE, Perkins DO, Gu H, McEvoy JP, Lieberman JA, 2008. Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J. Int. Neuropsychol. Soc 10.1017/S1355617708080570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RSE, Gold JM, Bishop JR, Gershon ES, … Sweeney JA, 2013. Neuropsychological impairments in schizophrenia and psychotic Bipolar disorder: Findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am. J. Psychiatry 10.1176/appi.ajp.2013.12101298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberger WC, Hill SK, Nelson CLM, Reilly JL, Keefe RSE, Pearlson GD, Keshavan MS, Tamminga CA, Clementz BA, Sweeney JA, 2016. Unitary construct of generalized cognitive ability underlying BACS performance across psychotic disorders and in their first-degree relatives. Schizophr. Res 10.1016/j.schres.2015.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houshmand K, Bräunig P, Gauggel S, Kliesow K, Sarkar R, Krüger S, 2010. Emotional vulnerability and cognitive control in patients with bipolar disorder and their healthy siblings: A pilot study. Acta Neuropsychiatr. 10.1111/j.1601-5215.2010.00451.x [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Clementz BA, Dutcher AM, Arnold SJM, Jeon-Slaughter H, Aslan S, … Tamminga CA, 2017. Brain structure biomarkers in the psychosis biotypes: Findings from the Bipolar-Schizophrenia Network for Intermediate Phenotypes. Biol. Psychiatry 10.1016/j.biopsych.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA, 2010. Genetics and intermediate phenotypes of the schizophrenia-bipolar disorder boundary. Neurosci. Biobehav. Rev 10.1016/j.neubiorev.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji A, Godwin D, Rutlin J, Kandala S, Shimony JS, Mamah D, 2017. Tract-based analysis of white matter integrity in psychotic and nonpsychotic bipolar disorder. J. Affect. Disord 10.1016/j.jad.2016.11.038 [DOI] [PubMed] [Google Scholar]
- Jiménez-López E, Aparicio AI, Sánchez-Morla EM, Rodriguez-Jimenez R, Vieta E, Santos JL, 2017. Neurocognition in patients with psychotic and non-psychotic bipolar I disorder. A comparative study with individuals with schizophrenia. J. Affect. Disord 10.1016/j.jad.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L, 2004. The Brief Assessment of Cognition in Schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr. Res 10.1016/j.schres.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Harvey PD, Goldberg TE, Gold JM, Walker TM, Kennel C, Hawkins K, 2008. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS). Schizophr. Res 10.1016/j.schres.2008.03.024 [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Morris DW, Sweeney JA, Pearlson G, Thaker G, Seidman LJ, Eack SM, Tamminga C, 2011. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: The Schizo-Bipolar Scale. Schizophr. Res 10.1016/j.schres.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahera G, Montes JM, Benito A, Valdivia M, Medina E, Mirapeix I, Sáiz-Ruiz J, 2008. Theory of mind deficit in bipolar disorder: Is it related to aprevious history of psychotic symptoms? Psychiatry Res. 10.1016/j.psychres.2007.08.009 [DOI] [PubMed] [Google Scholar]
- Lançon C, Auquier P, Nayt G, Reine G, 2000. Stability of the five-factor structure of the Positive and Negative Syndrome Scale (PANSS). Schizophr. Res 10.1016/S0920-9964(99)00129-2 [DOI] [PubMed] [Google Scholar]
- Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, … Wray NR, 2013. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet 10.1038/ng.2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B, Medina AM, Weiss RD, 2013. Cognitive and psychosocial functioning in bipolar disorder with and without psychosis during early remission from an acute mood episode: A comparative longitudinal study. Compr. Psychiatry 10.1016/j.comppsych.2012.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, 1984. On the ability to inhibit thought and action: A theory of an act of control. Psychol. Rev 10.1037/0033-295X.91.3.295 [DOI] [PubMed] [Google Scholar]
- Logan GD, 1994. On the ability to inhibit thought and action: A users guide to the stop-signal paradigm In Dagenbach D & Carr TH (Eds.), Inhibitory Processes in Attention, Memory, and Language. 189–239. San Diego, CA: Academic Press. [Google Scholar]
- Martinez-Aran A, Torrent C, Tabares-Seisdedos R, Salamero M, Daban C, Balanza-Martinez V, … Vieta E, 2008. Neurocognitive impairment in bipolar patients with and without history of psychosis. J. Clin. Psychiatry 10.4088/JCP.v69n0209 [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC, 2001. Psychiatric aspects of impulsivity. Am. J. Psychiatry 10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M, 1979. A new depression scale designed to be sensitive to change. Br. J. Psychiatry 10.1192/bjp.134.4.382 [DOI] [PubMed] [Google Scholar]
- Park S, Gooding DC., 2014. Working memory impairmentas an endophenotypic marker of a schizophrenia diathesis. Schizophr Res Cogn https://doi:10.1016/j.scog.2014.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol [DOI] [PubMed] [Google Scholar]
- Reilly JL, Frankovich K, Hill S, Gershon ES, Keefe RSE, Keshavan MS, Pearlson GD, Tamminga CA, Sweeney JA, 2014. Elevated antisaccade error rate as an intermediate phenotype for psychosis across diagnostic categories. Schizophr. Bull 10.1093/schbul/sbt132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JL, Sweeney JA, 2014. Generalized and specific neurocognitive deficits in psychotic disorders: Utility for evaluating pharmacological treatment effects and as intermediate phenotypes for gene discovery. Schizophr. Bull 10.1093/schbul/sbu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Van Der Merwe L, Stein DJ, Solms M, Ramesar R, 2009. Neuropsychological status of bipolar I disorder: Impact of psychosis. Br. J. Psychiatry 10.1192/bjp.bp.108.052001 [DOI] [PubMed] [Google Scholar]
- Schmitt LM, White SP, Cook EH, Sweeney JA, Mosconi MW, 2018. Cognitive mechanisms of inhibitory control deficits in autism spectrum disorder. J. Child Psychol. Psychiatry Allied Discip 10.1111/jcpp.12837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selva G, Salazar J, Balanzá-Martínez V, Martínez-Arán A, Rubio C, Daban C, … Tabarés-Seisdedos R, 2007. Bipolar I patients with and without a history of psychotic symptoms: Do they differ in their cognitive functioning? J. Psychiatr. Res 10.1016/j.jpsychires.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Selvaraj S, Arnone D, Job D, Stanfield A, Farrow TFD, Nugent AC, … McIntosh AM, 2012. Grey matter differences in bipolar disorder: A meta-analysis of voxel-based morphometry studies. Bipolar Disord 10.1111/j.1399-5618.2012.01000.x [DOI] [PubMed] [Google Scholar]
- Simonsen C, Sundet K, Vaskinn A, Birkenaes AB, Engh JA, Færden A, … Andreassen OA, 2011. Neurocognitive dysfunction in bipolar and schizophrenia spectrum disorders depends on history of psychosis rather than diagnostic group. Schizophr. Bull 10.1093/schbul/sbp034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Kendler K, Craddock N, Lee PH, Neale BM, Nurnberger JN, … O’Donovan M, 2013. Identification of risk loci with shared effects on five major psychiatric disorders: A genome-wide analysis. Lancet 10.1016/S0140-6736(12)62129-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Steinberg JL, Moeller FG, 2005. Increased impulsivity associated with severity of suicide attempt history in patients with bipolar disorder. Am. J. Psychiatry 10.1176/appi.ajp.162.9.1680 [DOI] [PubMed] [Google Scholar]
- Swann AC, Lijffijt M, Lane SD, Steinberg JL, Moeller FG, 2010. Interactions between bipolar disorder and antisocial personality disorder in trait impulsivity and severity of illness. Acta Psychiatr. Scand 10.1111/j.1600-0447.2009.01528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G, 2014. Bipolar and schizophrenia network for intermediate phenotypes: Outcomes across the psychosis continuum. Schizophr. Bull 10.1093/schbul/sbt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler NS, Allen DN, Sutton GP, Vertinski M, Ringdahl EN, 2013. Differential impairment of social cognition factors in bipolar disorder with and without psychotic features and schizophrenia. J. Psychiatr. Res 10.1016/j.jpsychires.2013.09.010 [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, Logan GD, 2013. Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychol. Sci 10.1177/0956797612457390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Kompus K, Hugdahl K, 2011. Impaired cognitive inhibition in schizophrenia: A meta-analysis of the Stroop interference effect. Schizophr. Res 10.1016/j.schres.2011.08.025 [DOI] [PubMed] [Google Scholar]
- Williams LM, 2017. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress. Anxiety 10.1002/da.22556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Gurvich C, Fitzgerald PB, Georgiou-Karistianis N, Millist L, White O, 2008. Inhibitory control and spatial working memory: A saccadic eye movement study of negative symptoms in schizophrenia. Psychiatry Res. 10.1016/j.psychres.2007.02.004 [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 10.1192/bjp.133.5.429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


