Abstract
Objective
To investigate differences in sleep quality by race in participants with and without a prior myocardial infarction (MI).
Design
Case-control study
Setting
Emory-affiliated hospitals in Atlanta, Georgia
Participants
273 individuals (190 Black) ≤ 60 years of age with a verified MI in the previous 8 months, and 100 community controls (44 Black) without a history of MI.
Measurements
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). Psychological factors were assessed using standardized questionnaires and clinical risk factors through medical history and chart review.
Results
A significant interaction existed between race and MI status on sleep quality (p=0.01), such that Black individuals with a history of MI, but not controls, reported worse sleep quality than their non-Black counterparts. Among MI cases, being Black was independently associated with higher PSQI scores after adjusting for baseline demographics (B =2.17, 95% CI 1.17, 3.17, p=0.006). Clinical risk factors, psychological factors and socioeconomic status (household income and years of education) all contributed equally to explain race-related disparities in sleep among MI cases. After further adjustment for these factors, the association was attenuated and no longer significant (B=0.70, 95% CI= −0.10, 1.21, p=0.26).
Conclusion
Black post-MI patients, but not healthy controls, have significantly poorer sleep quality than non-Blacks. This difference is driven by a combination of factors, including clinical risk factors, psychological factors as well as adverse socioeconomic conditions among Black individuals with MI.
Keywords: sleep quality, Pittsburgh Sleep Quality Index, coronary artery disease, racial differences
Introduction
Well-established racial disparities between Black adults and other demographic groups exist in cardiovascular health outcomes across the United States.1,2 National statistics indicate that Black adults have a higher burden of prevalent coronary artery disease (CAD), heart failure, and stroke.3 Equally as concerning is the fact that Black adults have higher mortality rates from cardiovascular disease, and deaths occur at a younger age compared to other groups.3,4 Potential mechanisms for these observed health disparities are likely multifactorial and multi-level, including societal, community and individual factors, and remain overall not well understood.5
Short sleep durations, poor sleep quality and sleep related disorders such as insomnia and obstructive sleep apnea are all recognized as independent risk factors for the development and progression of cardiovascular disease.6–9 These factors also appear to differentially affect Black individuals compared to other groups, and race differences have been reported across multiple sleep domains.10–15 Most prior literature has focused on racial differences in sleep duration and prevalence of sleep disorders, and data on racial disparities in broadly defined sleep quality are limited.14–16 These questions have never been addressed among patients with CAD, a high-risk group among whom such disparities could be exacerbated. Understanding the magnitude of racial differences in sleep quality among patients with CAD could help mitigate racial disparities in the outcome of CAD.
One of the hypothesized explanations for racial differences in sleep quality is disparities in the socioeconomic status (SES) between racial groups.17 However, few studies have directly examined the role of SES in explaining disparities in sleep quality by race, and these studies have produced conflicting results.18–23 It is important to understand the factors explaining the disparities in sleep quality by race in order to develop successful interventions to improve sleep and reducing health inequalities.
In the present study, we investigated differences in sleep quality by race in participants with and without a prior MI, and further explored the role of SES in any differences found. We hypothesized that, among adults with a history of MI, poor sleep quality is more common among Blacks than among other racial groups, and that these differences in sleep quality are partially mediated by SES.
Methods
Study population
The Myocardial Infarction and Mental Stress 2 study included patients with myocardial infarction (MI) and community controls without a history of CAD.24 Cases and controls were recruited between June 2011 and March 2016. Cases with MI were recruited from a pool of patients admitted at Emory-affiliated hospitals in Atlanta, Georgia with a documented MI in the previous 8 months and who were 18–60 years of age at the time of screening. We first recruited the MI cases, and then recruited the population controls sampling them according to the cases’ distribution of age and sex, as commonly done in case-control studies. The diagnosis of MI was verified by medical record review based on standard criteria of increases in troponin level together with symptoms of ischemia and ECG changes or other evidence of myocardial necrosis.25 Control subjects were recruited from a community-based sample from the Atlanta area without established CAD, congestive heart failure, or stroke.26 This community sample was selected using a two stage approach. The first stage included a random sample of Black and White residents of metropolitan Atlanta; the second stage included a subset who participated in an in-person study visit. Only community individuals whose age was in the same range as the MI cases were considered. The availability of this existing community sample for whom we already had information on demographic factors and past medical history allowed us to select controls without a previous history of CAD who could be matched to the cases. Cases and controls were frequency matched by age and sex, with a goal of achieving approximately 50% women and a similar mean age in both samples.27 Data on sociodemographic and psychosocial variables were collected for all participants. All participants provided written informed consent and the protocol was approved by the Emory University Institutional Review Board.
Patient assessments
Both cases and controls were evaluated by study nurses and physicians during in-person clinical examination, with medical record review for cases. Baseline demographics (age, sex, race, years of education, and household income) were obtained using standardized self-reported questionnaires. Given that only 18 individuals (4.8%) were neither Black nor White, race was examined in 2 categories: Black and non-Black. Household income was classified into 2 groups (≤ $50,000, or > $50,000). Previous history of co-morbid conditions and risk factors (diabetes, hypertension, smoking, congestive heart failure, and obesity) was obtained by study nurses or physicians through medical history, clinical examination and by reviewing medical records. Angina symptoms were assessed with the Seattle Angina Questionnaire’s angina-frequency subscale, which measures frequency of angina and use of nitroglycerin for chest pain over the previous 4 weeks, and with higher scores indicating less chest pain.28
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), which is a self-rated questionnaire. The scale includes 19 items that generate seven component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. Each individual item is scored from 0 to 3, and a composite score is calculated as the sum of the seven components, ranging from 0 to 21, with a higher score denoting worse sleep quality. Poor sleep quality was defined as a PSQI > 5, as previously established.29 In addition, the time to fall asleep in minutes, and total hours of sleep were measured using the raw numbers provided by the individual.
Several psychological factors were assessed using standardized questionnaires. Depressive symptoms were measured using the Beck Depression Inventory, a 21-item self-administered scale.30 Posttraumatic Stress Disorder (PTSD) symptoms were assessed with the 17-item scale PTSD Symptom Checklist.31 To avoid overlap with the PSQI, the sleep item in both the Beck Depression Inventory and PTSD Symptom Checklist were excluded. Trait anxiety was measured using with the State-Trait Anxiety Inventory scale.32,33 For measurement of anger trait, we used the Spielberger’s State-Trait Anger Expression Inventory was used,33 and to measure general stress, the Perceived Stress Scale.34 In order to assess overall psychological burden, a composite score of the above psychosocial scales was constructed as previously described.35,36 First, for each psychological scale, a Z-score was constructed and the Z-scores were then summed to derive a composite psychological distress index. Since sleep disturbance is part of the Beck Depression Inventory and the PTSD Symptom Checklist, the composite scores were calculated after exclusion of the questions related to sleep disturbances from these two scales.
Statistical analysis
We compared non-Blacks and Blacks within each group (controls and MI cases) for demographic, behavioral, and clinical characteristics, and sleep quality indices using t tests for continuous variables and χ-squared tests for categorical variables. Linear regression analysis was used to determine the association between race and the PSQI score as the outcome variable. We constructed a series of sequential models. Model 1 adjusted for age and sex. Model 2 adjusted for all variables in Model 1 plus history of hypertension and obesity, and, among MI cases only, smoking, and congestive heart failure. Model 3 adjusted for all variables in Model 2 plus the composite psychological distress index. Model 4 adjusted for all variables in Model 3 plus angina frequency. Model 5 adjusted for all variables in Model 4 plus SES variables (years of education and household income). The interaction of race with case-control status was formally tested by entering the interaction term in the regression models. In order to characterize the contribution of each variable in the models predicting sleep quality, we performed relative importance analysis,37 to represent the percentage of variance explained in the criterion that can be attributed to PSQI.37 The STATA command DOMIN was used to generate general dominance weights and produce additive decompositions of R2 indexes ascribing what can be interpreted as the “relative importance” of each variable in the prediction of PSQI as the outcome.
We also performed formal parallel mediation analysis with bootstrapping (1,000 bootstrap samples and a 95% confidence interval) to test the hypothesis that SES (household income and years of education) mediates the relationship between race and sleep disturbances using the method by Preacher and Hayes.38 All analyses were conducted using Stata 14 (StataCorp, College Station, Texas). A P-value of < 0.05 was considered statistically significant.
Results
Table 1 describes demographic, behavioral, and clinical characteristics, and sleep quality measures between non-Blacks and Black adults in each group (controls and MI cases). Among both controls and MI cases, Blacks had lower education and a lower household income (Table 1). Blacks with previous MI had a more adverse psychosocial profile (more depression, more PTSD symptoms, and more perceived stress) compared with non-Blacks with previous MI. Differences in psychosocial factors were less pronounced among controls. The rates of cardiovascular risk factors (diabetes, hypertension, and congestive heart failure), and angina frequency were also higher in Blacks with MI in comparison to non-Blacks; several risk factors were also disproportionately present in Blacks among controls (Table 1).
Table 1.
Baseline characteristics by case-control status and race.
| Controls (N=100) | p-value | MI Cases (N=273) | p-value | |||
|---|---|---|---|---|---|---|
| Non-Black (N=56) |
Black (N=44) |
Non-Black (N=83) |
Black (N=190) |
|||
| Sociodemographics | ||||||
| Age, Mean (SD) | 48 (10) | 50 (6) | 0.33 | 52 (5) | 50 (7) | 0.07 |
| Female, N (%) | 27 (48.2) | 25 (56.8) | 0.39 | 32 (38.6) | 106 (55.8) | 0.009 |
| Education, Years (SD) | 17 (3) | 15 (2) | 0.005 | 14 (3) | 13 (2) | <0.001 |
| Household income, N (%) | 0.62 | <0.001 | ||||
| ≤ $50,000 | 15 (24.6) | 13 (28.9) | 37 (36.3) | 144 (81.8) | ||
| > $50,000 | 46 (75.4) | 32 (71.1) | 65 (63.7) | 32 (18.2) | ||
| Psychological factors | ||||||
| BDI score, Mean (SD) | 6.1 (1.2) | 6.3 (1.1) | 0.86 | 9.6 (0.9) | 13.4 (0.7) | 0.006 |
| PCL score, Mean (SD) | 24.6 (111) | 24.5 (10.2) | 0.97 | 27.2 (1.2) | 33.9 (1.1) | <0.001 |
| STAI score, Mean (SD) | 30.2 (10.1) | 29.0 (12.2) | 0.52 | 35.1 (13.1) | 36.6 (13.0) | 0.35 |
| STAXI score, Mean (SD) | 16.7 (5.6) | 17.5 (6.7 | 0.45 | 17.8 (5.8) | 18.7 (7.6) | 0.28 |
| PSS score, Mean (SD) | 10.7 (6.7) | 9.8 (5.82) | 0.45 | 14.5 (6.2) | 17.1 (8.4) | 0.02 |
| Summary score*, Mean (SD) | 54.8 (25.3) | 55.2 (23.7) | 0.92 | 134.1 (71.0) | 158.2 (71.1) | 0.005 |
| Comorbidities | ||||||
| Diabetes, N (%) | 2 (3.6) | 5 (11.4) | 0.13 | 16 (19.3) | 69 (36.3) | 0.005 |
| Hypertension, N (%) | 14 (25.0) | 16 (36.4) | 0.21 | 56 (67.5) | 169 (88.9) | <0.001 |
| Congestive heart failure, N (%) | 0 | 0 | 3 (2.8) | 29 (14.1) | 0.002 | |
| Ejection Fraction, Mean (SD) | - | - | 51.2 (12.0) | 50.0 (11.4) | 0.67 | |
| Obesity, N (%) | 10 (17.9) | 26 (59.1) | <0.001 | 39 (47) | 107 (56.3) | 0.23 |
| Current Smoking, N (%) | 3 (5.4) | 2 (4.5) | 0.85 | 16 (19.3) | 50 (26.3) | 0.21 |
| Angina Frequency score, Mean (SD) | 1.5 (6.6) | 3.4 (8.3) | 0.25 | 12.0 (17.3) | 20.1 (21.6) | 0.002 |
BDI: Beck Depression Inventory, PCL: Posttraumatic Stress Disorder Symptom Checklist, STAI: State-Trait Anxiety Inventory, STAXI: State-Trait Anger Expression Inventory, PSS: perceived stress scale, PSQI: Pittsburgh Sleep Quality Index
Summary score aggregate of BDI, PCL, STAI, STAXI, and PSS scores
As shown in Table 2, among those with prior MI, Blacks exhibited higher PSQI scores as well as higher scores in multiple dimensions of the PSQI, denoting worse sleep compared to non-Black participants. Differences by race in the hours of sleep were modest among controls. Among both controls and MI cases, however, Blacks reported fewer hours of sleep (P for all < 0.01). There was a significant interaction between race and case-control status on PSQI scores (p=0.01), such that the association between PSQI and race was larger among those with previous MI (Table 2). Similarly, racial differences in poor sleep quality and time to fall asleep were larger among those with MI (Table 2).
Table 2.
Sleep quality measures by case-control status and race.
| Controls (N=100) | Magnitude of difference (95% CI) | p-value | MI Cases (N=273) | Magnitude of difference (95% CI) | p-value | P for Interaction | |||
|---|---|---|---|---|---|---|---|---|---|
| Non-Black (N=56) |
Black (N=44) |
Non-Black (N=83) |
Black (N=190) |
||||||
| PSQI Scale Scores | |||||||||
| PSQI total score, Mean (SD) | 5.8 (0.5) | 6.5 (0.6) | 0.6 (−0.2, 0.1) | 0.20 | 6.7 (0.3) | 8.8 (0.3) | 2.2 (1.2, 3.2) | <0.001 | 0.01 |
| Poor quality sleep (PSQI > 5), N (%) | 23 (41.1) | 22 (50.0) | - | 0.37 | 52 (61.9) | 147 (75.0) | - | 0.02 | 0.31 |
| Subjective sleep quality | 0.8 (0.6) | 1.1 (0.8) | 0.2 (−0.01, 0.5) | 0.07 | 1.1 (0.7) | 1.4 (0.9) | 0.38 (0.1, 0.6) | 0.001 | 0.12 |
| PSQI Subscales | |||||||||
| Sleep latency | 1.1 (1.0) | 1.0 (1.0) | 0.03 (−0.4, 0.3) | 0.88 | 1.1 (0.8) | 1.5 (1.0) | 0.43 (0.1, 0.7) | <0.001 | 0.009 |
| Sleep duration | 0.3 (0.6) | 0.9 (0.9) | 0.58 (0.2, 0.9) | <0.001 | 0.6 (0.9) | 1.2 (1.1) | 0.52 (0.2, 0.8) | <0.001 | 0.74 |
| Habitual sleep efficiency | 0.3 (0.6) | 0.5 (0.9) | 0.25 (−0.06, 0.5) | 0.11 | 0.5 (0.9) | 1.0 (1.1) | 0.42 (0.1, 0.7) | 0.002 | 0.02 |
| Sleep disturbance | 1.31 (0.6) | 1.35 (0.5) | 0.04 (−0.2, 0.2) | 0.72 | 1.5 (0.6) | 1.8 (0.7) | 0.25 (0.08, 0.4) | 0.005 | 0.01 |
| Use of sleeping medication | 0.6 (1.1) | 0.7 (1.2) | 0.14 (−0.3, 0.6) | 0.53 | 0.7 (1.1) | 0.8 (1.2) | 0.08 (−0.2, 0.4) | 0.54 | 0.83 |
| Day time sleep dysfunction | 0.6 (0.6) | 0.6 (0.8) | 0.02 (−0.2, 0.3) | 0.89 | 0.9 (0.7) | 1.0 (0.8) | −0.09 (−0.1, 0.3) | 0.34 | 0.61 |
| Time to fall asleep (Minutes) | 22.8 (23.4) | 25.2 (22.8) | 2.6 (−6.5, 11.8) | 0.57 | 23.5 (21.6) | 41.4 (43.8) | 18.6 (9.3, 27.8) | <0.001 | 0.03 |
| Hours of sleep | 7.0 (1.0) | 6.1 (1.1) | −0.9 (−1.3, −0.5) | <0.001 | 6.5 (1.5) | 5.8 (1.8) | −0.6 (−1.0, −0.2) | 0.006 | 0.41 |
A higher score in the PSQI subscales indicates worse sleep quality.
Multiple regression analysis
In a multiple regression model adjusting for demographics, clinical and psychological factors, both race (B= 1.2, 95% CI= 0.4–1.9, for Blacks vs. non-Blacks) and case-control status (B= 1.1, 95% CI= 0.1–2.1, for MI vs. controls) were independently associated with higher PSQI scores. In addition, both lower income (B= −0.85, 95% CI= −1.57, −0.12) and lower levels of education (B= −0.18, 95% CI= −0.06, −0.007) were independently associated with higher PSQI scores.
After stratification by MI status (Table 3), Black race remained significantly associated with higher PSQI scores among MI cases after adjusting for baseline demographics (Model 1). Adjustment for clinical risk factors (Model 2) attenuated the association by 30.8%. This association was further attenuated after adjusting for the composite psychological score (Model 3), and angina (Model 4) by 26.0% and 9.0%, respectively, but it remained statistically significant. After further adjusting for SES (income and education), the association between higher PSQI and Black race was further attenuated by 30.7% and no longer significant (Model 5). Exclusion of those who did not identify themselves as White/Black did not change the associations (Supplemental Table 1).
Table 3.
Multivariable regression investigating the association between PSQI (outcome) and race stratified by case-control status
| Controls (N=100) B (95% CI) |
MI Cases (N=273) B (95% CI) |
P for interaction |
Percent of Attenuation among MI cases |
|||
|---|---|---|---|---|---|---|
| Non-Black (N=56) |
Black (N=44) |
Non-Black (N=83) |
Black (N=190) |
|||
| Model 1 | 0 (Reference) | 0.91 (−0.59, 2.41) | 0 (Reference) | 2.17 (1.17, 3.17) | 0.01 | |
| Model 2 | 0 (Reference) | 0.54 (−1.04, 2.13) | 0 (Reference) | 1.51 (0.47, 2.56) | 0.02 | 30.8% |
| Model 3 | 0 (Reference) | 0.43 (−1.01, 1.88) | 0 (Reference) | 1.11 (0.23, 1.99) | 0.02 | 26.0% |
| Model 4 | 0 (Reference) | 0.78 (−0.87, 2.47) | 0 (Reference) | 1.01 (0.10, 1.92) | 0.02 | 9.0% |
| Model 5 | 0 (Reference) | 0.70 (−0.68, 2.08) | 0 (Reference) | 0.70 (−0.10, 1.21) | 0.03 | 30.7% |
The B coefficient expresses the increment in PSQI total score points (reflecting worse sleep quality) in Blacks compared with non-Blacks.
Model 1. Adjusted for age, and sex
Model 2. Adjusted for all variables in Model 2 plus history of hypertension and obesity, and, among MI cases only, also smoking and congestive heart failure.
Model 3. Adjusted for all variables in Model 3 plus the summary score aggregate of psychological factors
Model 4, Adjusted for all variables in Model 4 plus the angina frequency
Model 5. Adjusted for all variables in Model 5 plus years of education, and household income
Further analysis of the individual sleep quality component scores revealed that among those with previous MI, Black race was associated with worse scores in most of the components, including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, and sleep disturbances, even after adjusting for clinical and psychological factors (Table 4). When further adjusting for SES, these associations were attenuated and only remained significant for sleep duration. Exclusion of those who did not identify themselves as White/Black did not change the associations (Supplemental Table 2).
Table 4.
Multivariable regression investigating the association of PSQI total score and individual PSQI component scores with race among patients with MI
| MI Cases (N=273) B (95% CI) |
|||
|---|---|---|---|
| Non-Black(N=83) | Black (N=190) | p- Value | |
| Model 1 | |||
| PSQI total | 0 (Reference) | 2.17 (1.17, 3.17) | 0.005 |
| Subjective sleep quality | 0 (Reference) | 0.027 (0.04, 0.50) | 0.01 |
| Sleep latency | 0 (Reference) | 0.25 (0.02, 0.48) | 0.027 |
| Sleep duration | 0 (Reference) | 0.38 (0.11, 0.64) | 0.004 |
| Habitual sleep efficiency | 0 (Reference) | 0.50 (0.22, 0.78) | <0.001 |
| Sleep disturbance | 0 (Reference) | 0.36 (0.08, 0.65) | 0.010 |
| Use of sleeping medication | 0 (Reference) | 0.04 (−0.25, 0.34) | 0.76 |
| Day time sleep dysfunction | 0 (Reference) | 0.10 (−0.10, 0.31) | 0.31 |
| Time to fall asleep | 0 (Reference) | 17.5 (7.7, 27.2) | <0.001 |
| Hours of sleep | 0 (Reference) | −0.61 (−1.0, −0.17) | 0.007 |
| Model 2 | |||
| PSQI total | 0 (Reference) | 1.51 (0.47, 2.56) | 0.028 |
| Subjective sleep quality | 0 (Reference) | 0.19 (0.03, 0.41) | 0.01 |
| Sleep latency | 0 (Reference) | 0.21 (0.05, 0.49) | 0.009 |
| Sleep duration | 0 (Reference) | 0.42 (0.12, 0.72) | 0.005 |
| Habitual sleep efficiency | 0 (Reference) | 0.34 (0.04, 0.64) | 0.02 |
| Sleep disturbance | 0 (Reference) | 0.20 (0.07, 0.29) | 0.02 |
| Use of sleeping medication | 0 (Reference) | −0.11 (−0.43, 0.19) | 0.45 |
| Day time sleep dysfunction | 0 (Reference) | −0.03 (−0.24, 0.17) | 0.72 |
| Time to fall asleep | 0 (Reference) | 13.4 (3.1, 23.7) | 0.01 |
| Hours of sleep | 0 (Reference) | −0.55 (−1.03, −0.07) | 0.02 |
| Model 3 | |||
| PSQI total | 0 (Reference) | 1.11 (0.23, 1.99) | 0.015 |
| Subjective sleep quality | 0 (Reference) | 0.18 (0.02, 0.39) | 0.02 |
| Sleep latency | 0 (Reference) | 0.20 (0.05, 0.46) | 0.008 |
| Sleep duration | 0 (Reference) | 0.41 (0.11, 0.70) | 0.006 |
| Habitual sleep efficiency | 0 (Reference) | 0.33 (0.03, 0.63) | 0.02 |
| Sleep disturbance | 0 (Reference) | 0.19 (0.04, 0.26) | 0.02 |
| Use of sleeping medication | 0 (Reference) | −0.12 (−0.43, 0.18) | 0.43 |
| Day time sleep dysfunction | 0 (Reference) | −0.05 (−0.24, 0.13) | 0.55 |
| Time to fall asleep | 0 (Reference) | 13.4 (3.1, 23.7) | 0.01 |
| Hours of sleep | 0 (Reference) | −0.54 (−1.02, −0.06) | 0.02 |
| Model 4 | |||
| PSQI total | 0 (Reference) | 1.01 (0.10, 1.92) | 0.023 |
| Subjective sleep quality | 0 (Reference) | 0.17 (0.01, 0.29) | 0.021 |
| Sleep latency | 0 (Reference) | 0.17 (0.03, 0.35) | 0.01 |
| Sleep duration | 0 (Reference) | 0.40 (0.09, 0.66) | 0.01 |
| Habitual sleep efficiency | 0 (Reference) | 0.32 (0.02, 0.59) | 0.03 |
| Sleep disturbance | 0 (Reference) | 0.11 (0.02, 0.20) | 0.03 |
| Use of sleeping medication | 0 (Reference) | −0.10 (−0.40, 0.14) | 0.53 |
| Day time sleep dysfunction | 0 (Reference) | −0.06 (−0.14, 0.09) | 0.34 |
| Time to fall asleep | 0 (Reference) | 13.0 (2.8, 21.5) | 0.02 |
| Hours of sleep | 0 (Reference) | −0.55 (−1.00, −0.03) | 0.031 |
| Model 5 | |||
| PSQI total | 0 (Reference) | 0.70 (−0.10, 1.21) | 0.191 |
| Subjective sleep quality | 0 (Reference) | 0.15 (−0.06, 0.38) | 0.16 |
| Sleep latency | 0 (Reference) | 0.12 (−0.15, 0.41) | 0.37 |
| Sleep duration | 0 (Reference) | 0.39 (0.07, 0.72) | 0.016 |
| Habitual sleep efficiency | 0 (Reference) | 0.30 (−0.02, 0.62) | 0.09 |
| Sleep disturbance | 0 (Reference) | 0.05 (−0.12, 0.24) | 0.53 |
| Use of sleeping medication | 0 (Reference) | −0.09 (−0.44, 0.24) | 0.57 |
| Day time sleep dysfunction | 0 (Reference) | −0.11 (−0.32, 0.08) | 0.25 |
| Time to fall asleep | 0 (Reference) | 12.2 (1.1, 24.3) | 0.03 |
| Hours of sleep | 0 (Reference) | −0.61 (−1.1, −0.11) | 0.01 |
The B coefficient expresses the increment in PSQI total score or subscore points (reflecting worse sleep quality) in Blacks compared with non-Blacks.
Model 1. Adjusted for age and sex
Model 2. Adjusted for all variables in Model 2 plus history of diabetes, hypertension, obesity, smoking and congestive heart failure
Model 3. Adjusted for all variables in Model 3 plus the summary score aggregate of psychological factors
Model 4. Adjusted for all variables in Model 4 plus angina frequency
Model 5. Adjusted for all variables in Model 5 plus years of education, and household income
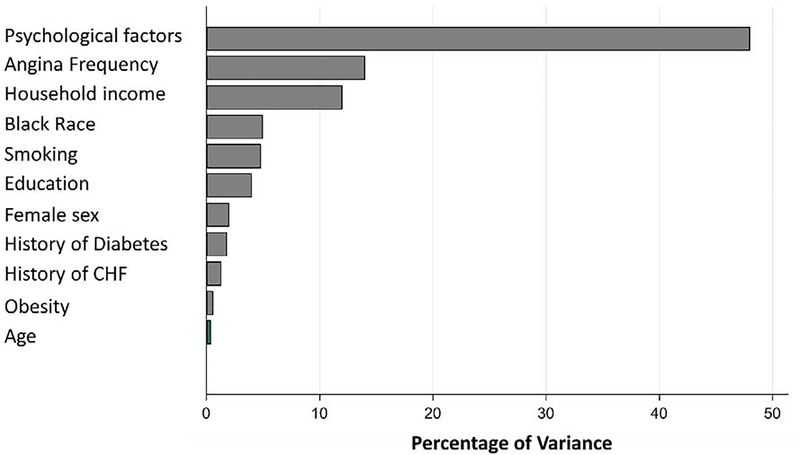
Relative importance analysis was performed to characterize the contribution of each variable to sleep quality in the models with PSQI as outcome among individuals with prior MI (Supplemental Table 3). As shown in Figure 1 and Supplemental Table 3, the composite psychological distress index was the most important contributor to sleep quality (47.4%), followed by the frequency of angina symptoms (14.1%), and household income (12.2%), respectively. With these factors in the model, race was only a minor contributor to differences in sleep quality.
Figure 1.
Significant (p<0.05) predictors of PSQI among subjects with prior myocardial infarction. Variables displayed are the statistically significant predictors of PSQI subscales on univariate analysis.
Mediation Analysis
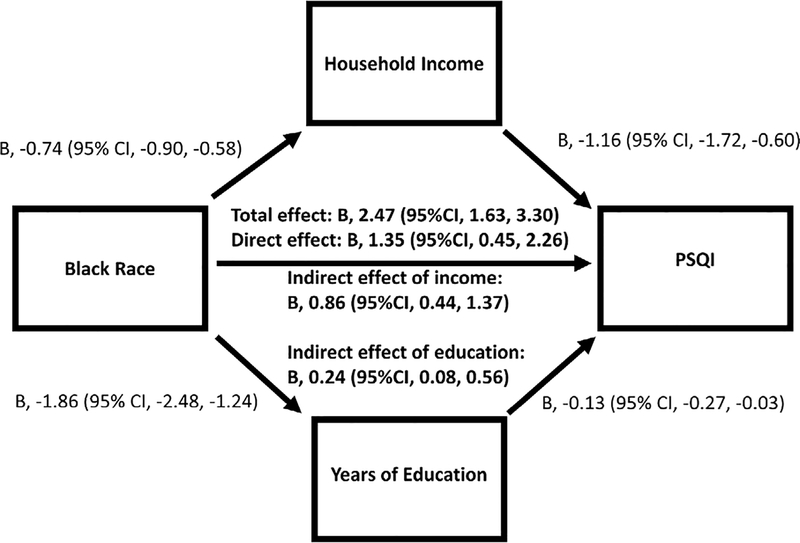
We performed mediation analysis to examine the hypothesized pathway that among those with a prior MI, markers of lower SES mediate the relationship between black race and worse sleep quality. As shown in Figure 2, among cases with prior MI, Black race had direct relationships with both household income and education, as well as PSQI levels (P<0.01), after adjusting for other risk factors. Household incomes and years of education, when added to the model, mediated the relationship between Black race and PSQI by 34%, and 10% respectively.
Figure 2.
Mediation analysis for socioeconomic status as mediator of the relationship between Black race and sleep disturbances among the MI cases (N=273)
Discussion
In the present study, we found that the racial differences in PSQI was more than 2 times higher among cases with prior MI compared to controls. Among those with prior MI, worse sleep quality was explained by the more adverse cardiovascular risk profile, worse psychosocial profile and fewer years of education and lower household income in Black patients compared to their non-Black counterparts. However, among controls, these factors were overall similar by race, which could help explain why the differences in sleep quality were not as pronounced among Blacks compared to non-Blacks. Consistent with our initial hypothesis, we found that SES was a major explanatory factor for race differences in sleep. However, we also found that, in addition to SES, differences in traditional risk factors and psychological factors contributed to the race-related differences in sleep quality among MI patients.
Poor sleep quality was shown to increase the incidence of cardiovascular risk factors, such as obesity,39 hypertension,40 and diabetes,41 and is also linked to a higher risk of cardiovascular events including MI42 and total mortality. Following a first-time acute MI, sleep impairment and insomnia have shown to increase the risk of future MI, stroke, heart failure and death.43,44 Both the quantity and quality of sleep have been shown to influence the pathophysiology of cardiovascular disease via inflammatory and autonomic pathways, among others.45 The higher degree of systemic inflammation and autonomic dysregulation associated with poor sleep quality could make the post-MI population especially vulnerable to the long-term adverse effects of poor sleep.
In our sample, Black adults had more risk factors and comorbidities than non-Blacks in both MI and control groups, but, as expected, the prevalence of these conditions was much higher in the MI cases. Black adults in the MI group had higher rates of hypertension, diabetes and congestive heart disease compared to non-Blacks. All these factors have shown to be associated with poor sleep quality.46–48 Therefore, the fact that in our sample there were mostly no significant differences in the PSQI scores between Black and non-Black participants without CAD could be related to the lower risk profile of the control group. In addition, the disparity in SES indicators between Black and non-Black adults was less pronounced among controls compared to individuals with MI, which could also explain why these individuals had a more similar sleep quality. However, Blacks in the control group did sleep fewer hours than their non-Black counterparts. This difference could be partly attributed to higher prevalence of obesity in Blacks compared to non-Blacks in the control group, which could increase the rate of obstructive sleep apnea in the former group.
Among MI cases, clinical risk factors including diabetes, hypertension and congestive heart failure explained a considerable portion of the sleep disparity by race. These data suggest that an improvement in the clinical risk factors of Blacks with MI could potentially improve their quality of sleep. Nonetheless, even after accounting for clinical and psychosocial differences, Black patients with previous MI still had worse sleep quality than non-Blacks. It was only after accounting for differences in socioeconomic status that most of the differences in sleep were explained.
Consistent with prior literature, we found that Black participants reported fewer hours of sleep compared to non-Blacks. These differences were seen among both those with and without MI, and were independent of medical and psychological factors. Our findings are in line with a previous meta-analysis of subjective and objective measures of sleep that showed that, on average, Black people sleep fewer hours than non-Blacks, and that these differences are at least partly moderated by biopsychosocial factors.15 Prior observational studies have linked a shorter sleep duration to the development of obesity, diabetes, and their downstream effects on cardiovascular health.45,49 In another meta-analysis of over 400,000 participants, the relative risk for developing or dying from CAD or stroke was increased by almost 50% among those who slept less than 6 hours per night compared to those who slept between 7 – 8 hours per night.50 These findings highlight the importance of understanding the reasons for short sleep durations in Black individuals as it may directly influence their future risk of cardiovascular disease incidence and mortality. Our results suggest that social factors related to low socioeconomic resources are important determinants of observed disparities in sleep quality by race.
In our study, we demonstrated that markers of SES including low income and fewer years of education were among the most important predictors of worse sleep quality among participants with prior MI, and more important than clinical risk factors. A lower SES (household income and education) among Black individuals with MI explained approximately one third of the relationship between racial disparities and sleep disturbances after adjustment of other factors. Previous studies have documented a direct relationship between SES and sleep health.51–54 Furthermore, income level is associated with behavioral factors known to influence sleep, such as smoking, excessive alcohol, overweight and lack of exercise,55 as well as with higher rates of anxiety and depressive symptoms.56,57 Individuals with lower income are also more likely to live in crowded households, to work later shifts, to hold multiple jobs, and to work long hours, all of which can have profound implications for sleep quality and timing.58,59 These findings suggest that income and education as markers of SES may play an important role in sleep health both directly and also through modulation of physical and mental health. Psychological disturbances were by far the most important predictor of poor sleep, and were more prevalent among Black study participants with MI than those of other race/ethnicity groups. Thus, it is not surprising that they did contribute to explain a substantial portion of the race differences in sleep quality among MI cases.
Our study has a number of limitations. First, the cross-sectional design precludes any conclusions on the causality of the associations found. Second, sleep quality was evaluated subjectively, and objective measures of sleep were not available. Third, the lack of significant associations between race and sleep disturbances among the controls could be attributed to the relatively small sample size of this group. However, except for sleep duration, the effect size for race was unequivocally smaller among controls compared to the MI cases, with significant interactions for many sleep domains. Fourth, while our relative importance analysis showed race to be a minor contributor to differences in sleep quality, this could also be attributed to the fact that proximal factors demonstrate a higher level of relative importance in this method of analysis. Fifth, data on sleep disordered-breathing or other sleep disorders were not collected in our sample, which could also have a role in the variance seen in sleep quality especially among MI patients. Sixth, the control group could be affected by self-selection of participants who agreed to a study visit. However, the MI sample was also invited for a study visit; thus, if there is a selection bias, it should be non-differential. Finally, data regarding clinical outcomes were not available. Our group has recently shown that both short and long sleep duration is associated with higher all-cause mortality in both Blacks and non-Blacks with CAD.60 Future studies are needed to investigate whether higher rates of sleep disturbances among Black adults with MI translate into worse future outcomes. Strengths of this study include the comprehensive phenotyping of both cases and controls across multiple levels, including sociodemographic, medical and psychosocial data, which allowed us to investigate the role of all these factors on racial disparities in sleep. The design including both MI cases and controls without CAD represents another important strength of this study.
In conclusion, we showed that Black individuals who have suffered an MI are especially vulnerable to sleep disturbance. Clinical risk factors, psychological factors and adverse SES all play important roles in race-related disparities in sleep. This group may potentially benefit for targeted interventions to ameliorate sleep. Future prospective studies should address the impact of sleep quality and duration on racial disparities in MI recurrence and mortality, and test the usefulness of interventions aimed at improving sleep in this population.
Supplementary Material
Acknowledgments
Funding:
This work was supported by grants P01 HL101398, R01 HL109413, R01HL109413-02S1, R01HL125246, K24HL077506, K24 MH076955, UL1TR000454, KL2TR000455, K23HL127251, and T32HL130025 from the National Institutes of Health.
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson AR. Unequal treatment: report of the Institute of Medicine on racial and ethnic disparities in healthcare. Ann Thorac Surg. 2003;76(4):S1377–1381. [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. [DOI] [PubMed] [Google Scholar]
- 3.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111(10):1233–1241. [DOI] [PubMed] [Google Scholar]
- 4.Writing Group M, Mozaffarian D, Benjamin EJ, et al. Executive Summary: Heart Disease and Stroke Statistics−−2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):447–454. [DOI] [PubMed] [Google Scholar]
- 5.Williams DR, Sternthal M. Understanding racial-ethnic disparities in health: sociological contributions. J Health Soc Behav. 2010;51 Suppl:S15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med 2010;71(5):1027–1036. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Jin X, Shan Z, et al. Relationship of Sleep Duration With All-Cause Mortality and Cardiovascular Events: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. J Am Heart Assoc. 2017;6(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lao XQ, Liu X, Deng HB, et al. Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. J Clin Sleep Med 2018;14(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008;118(10):1080–1111. [DOI] [PubMed] [Google Scholar]
- 10.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health. 2015;36:417–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutson KL. Sociodemographic and cultural determinants of sleep deficiency: implications for cardiometabolic disease risk. Soc Sci Med. 2013;79:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingsbury JH, Buxton OM, Emmons KM. Sleep and its Relationship to Racial and Ethnic Disparities in Cardiovascular Disease. Curr Cardiovasc Risk Rep 2013;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adenekan B, Pandey A, McKenzie S, Zizi F, Casimir GJ, Jean-Louis G. Sleep in America: role of racial/ethnic differences. Sleep Med Rev 2013;17(4):255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrov ME, Lichstein KL. Differences in sleep between black and white adults: an update and future directions. Sleep Med. 2016;18:74–81. [DOI] [PubMed] [Google Scholar]
- 15.Ruiter ME, Decoster J, Jacobs L, Lichstein KL. Normal sleep in African-Americans and CaucasianAmericans: A meta-analysis. Sleep Med 2011;12(3):209–214. [DOI] [PubMed] [Google Scholar]
- 16.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med 2010;8(4):246–259. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DA, Jackson CL, Williams NJ, Alcantara C . Are sleep patterns influenced by race/ethnicity - a marker of relative advantage or disadvantage? Evidence to date. Nat Sci Sleep. 2019;11:79–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ertel KA, Berkman LF, Buxton OM. Socioeconomic status, occupational characteristics, and sleep duration in African/Caribbean immigrants and US White health care workers. Sleep. 2011;34(4):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164(1):5–16. [DOI] [PubMed] [Google Scholar]
- 20.Hall MH, Matthews KA, Kravitz HM, et al. Race and financial strain are independent correlates of sleep in midlife women: the SWAN sleep study. Sleep. 2009;32(1):73–82. [PMC free article] [PubMed] [Google Scholar]
- 21.Mezick EJ, Matthews KA, Hall M, et al. Influence of race and socioeconomic status on sleep: Pittsburgh SleepSCORE project. Psychosom Med 2008;70(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troxel WM, Buysse DJ, Matthews KA, et al. Marital/cohabitation status and history in relation to sleep in midlife women. Sleep. 2010;33(7):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaccarino V, Sullivan S, Hammadah M, et al. Mental Stress-Induced-Myocardial Ischemia in Young Patients With Recent Myocardial Infarction: Sex Differences and Mechanisms. Circulation. 2018;137(8):794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020–2035. [DOI] [PubMed] [Google Scholar]
- 26.Morris AA, Zhao L, Ahmed Y, et al. Association between depression and inflammation--differences by race and sex: the META-Health study. Psychosom Med. 2011;73(6):462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturmer T, Brenner H. Degree of matching and gain in power and efficiency in case-control studies. Epidemiology. 2001;12(1):101–108. [DOI] [PubMed] [Google Scholar]
- 28.Spertus JA, Winder JA, Dewhurst TA, et al. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–341. [DOI] [PubMed] [Google Scholar]
- 29.Bruno RM, Palagini L, Gemignani A, et al. Poor sleep quality and resistant hypertension. Sleep Med. 2013;14(11):1157–1163. [DOI] [PubMed] [Google Scholar]
- 30.Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. 2013;35(4):416–431. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669–673. [DOI] [PubMed] [Google Scholar]
- 32.Wiglusz MS, Landowski J, Cubala WJ. Psychometric properties and diagnostic utility of the State-Trait Anxiety Inventory in epilepsy with and without comorbid anxiety disorder. Epilepsy Behav. 2019;92:221–225. [DOI] [PubMed] [Google Scholar]
- 33.Lievaart M, Franken IH, Hovens JE. Anger Assessment in Clinical and Nonclinical Populations: Further Validation of the State-Trait Anger Expression Inventory-2. J Clin Psychol 2016;72(3):263–278. [DOI] [PubMed] [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 35.Pimple P, Lima BB, Hammadah M, et al. Psychological Distress and Subsequent Cardiovascular Events in Individuals With Coronary Artery Disease. Journal of the American Heart Association. 2019;8(9):e011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pimple P, Hammadah M, Wilmot K, et al. The Relation of Psychosocial Distress With Myocardial Perfusion and Stress-Induced Myocardial Ischemia. Psychosom Med 2019;81(4):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LeBreton JM, Tonidandel S. Multivariate relative importance: extending relative weight analysis to multivariate criterion spaces. J Appl Psychol. 2008;93(2):329–345. [DOI] [PubMed] [Google Scholar]
- 38.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. [DOI] [PubMed] [Google Scholar]
- 39.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring). 2008;16(3):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calhoun DA. Sleep disorders and hypertension risk. J Hum Hypertens. 2017;31(6):371–372. [DOI] [PubMed] [Google Scholar]
- 41.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33(2):414–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daghlas I, Dashti HS, Lane J, et al. Sleep Duration and Myocardial Infarction. Journal of the American College of Cardiology. 2019;74 (10):1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark A, Lange T, Hallqvist J, Jennum P, Rod NH. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37(5):851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conden E, Rosenblad A. Insomnia predicts long-term all-cause mortality after acute myocardial infarction: A prospective cohort study. Int J Cardiol. 2016;215:217–222. [DOI] [PubMed] [Google Scholar]
- 45.Hall MH, Brindle RC, Buysse DJ. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am Psychol 2018;73(8):994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larcher S, Benhamou PY, Pepin JL, Borel AL. Sleep habits and diabetes. Diabetes Metab. 2015;41(4):263–271. [DOI] [PubMed] [Google Scholar]
- 47.Lo K, Woo B, Wong M, Tam W. Subjective sleep quality, blood pressure, and hypertension: a meta-analysis. J Clin Hypertens (Greenwich). 2018;20(3):592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St-Onge MP, Grandner MA, Brown D, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation. 2016;134(18):e367–e386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9 Suppl 1:S23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011;32(12):1484–1492. [DOI] [PubMed] [Google Scholar]
- 51.Grandner MA, Petrov ME, Rattanaumpawan P, Jackson N, Platt A, Patel NP. Sleep symptoms, race/ethnicity, and socioeconomic position. J Clin Sleep Med. 2013;9(9):897–905; 905A-905D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lallukka T, Arber S, Rahkonen O, Lahelma E. Complaints of insomnia among midlife employed people: the contribution of childhood and present socioeconomic circumstances. Sleep Med 2010;11(9):828–836. [DOI] [PubMed] [Google Scholar]
- 53.Stamatakis KA, Kaplan GA, Roberts RE. Short sleep duration across income, education, and race/ethnic groups: population prevalence and growing disparities during 34 years of follow-up. Ann Epidemiol. 2007;17(12):948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: do negative emotions play a role? Psychol Bull 2003;129(1):10–51. [DOI] [PubMed] [Google Scholar]
- 56.Alvaro PK, Roberts RM, Harris JK. A Systematic Review Assessing Bidirectionality between Sleep Disturbances, Anxiety, and Depression. Sleep. 2013;36(7):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lovato N, Gradisar M. A meta-analysis and model of the relationship between sleep and depression in adolescents: recommendations for future research and clinical practice. Sleep Med Rev. 2014;18(6):521–529. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Y, Du CL, Hwang JJ, Chen IS, Chen MF, Su TC. Working hours, sleep duration and the risk of acute coronary heart disease: a case-control study of middle-aged men in Taiwan. Int J Cardiol. 2014;171(3):419–422. [DOI] [PubMed] [Google Scholar]
- 59.Bannai A, Tamakoshi A. The association between long working hours and health: a systematic review of epidemiological evidence. Scand J Work Environ Health. 2014;40(1):5–18. [DOI] [PubMed] [Google Scholar]
- 60.Kim JH, Hayek SS, Ko YA, et al. Sleep Duration and Mortality in Patients With Coronary Artery Disease. Am J Cardiol. 2019;123(6):874–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.