Abstract
Background:
Ketamine appears to have a therapeutic role in certain mental disorders, most notably depression. However, the comparative performance of different formulations of ketamine is less clear.
Objectives:
This study aimed to assess the comparative efficacy and tolerability of racemic and esketamine for the treatment of unipolar and bipolar major depression.
Design:
Systematic review and meta-analysis.
Data sources:
We searched PubMed, MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Clinical Trials, and the Cochrane Database of Systematic Reviews for relevant studies published since database inception and December 17, 2019.
Study eligibility criteria:
We considered randomized controlled trials examining racemic or esketamine for the treatment of unipolar or bipolar major depression.
Outcomes:
Primary outcomes were response and remission from depression, change in depression severity, suicidality, retention in treatment, drop-outs, and drop-outs due to adverse events.
Analysis:
Evidence from randomized controlled trials was synthesized as rate ratios (RRs) for treatment response, disorder remission, adverse events, and withdrawals and as standardized mean differences (SMDs) for change in symptoms, via random-effects meta-analyses.
Findings:
24 trials representing 1877 participants were pooled. Racemic ketamine relative to esketamine demonstrated greater overall response (RR = 3.01 vs. RR = 1.38) and remission rates (RR = 3.70 vs. RR = 1.47), as well as lower dropouts (RR = 0.76 vs. RR = 1.37).
Conclusions:
Intravenous ketamine appears to be more efficacious than intranasal esketamine for the treatment of depression.
Keywords: Esketamine, Ketamine, Depressive disorder, Major, Bipolar disorder, Depression, Randomized controlled trials, Meta-analysis
INTRODUCTION
Depression is the leading cause of disability in the world, affecting nearly 300 million individuals globally (Charlson et al., 2019; Herrman et al., 2019). Although depressive symptoms may be reduced within several weeks following the initiation of conventional antidepressants, approximately one-third of patients fail to achieve meaningful recovery (Corriger and Pickering, 2019). Consequently, there is an ongoing search for effective treatments for treatment-resistant depression (TRD) (Shah, 2016).
To that end, there is an emerging role for different formulations of ketamine in the management of TRD (Li and Vlisides, 2016). Racemic ketamine was first introduced into clinical practice in the 1960s as an invaluable anesthetic, however, its use in the management of TRD is a much more recent addition to the therapeutic armamentarium in depression (Li and Vlisides, 2016). Early ketamine studies demonstrated rapid, potent reductions in depressive symptoms following the administration of a single sub-anesthetic dose of intravenous racemic ketamine (Berman et al., 2000; Hu et al., 2016; Ionescu et al., 2015; Wilkinson et al., 2018). While these early results were promising, effective means of maintaining the acute effects were actively sought (Phillips et al., 2019). To date, the use of other glutamatergic agents to prolong the acute antidepressant effects of ketamine have been largely inconsistent, with some successful case reports and small open-label studies (Caddy et al., 2015; Ibrahim et al., 2012; Mathew et al., 2010; McCloud et al., 2015; Zarate et al., 2005). However, repeat doses of intravenous racemic ketamine have been shown to help sustain the short-term antidepressant effects (Ghasemi et al., 2014; Ionescu et al., 2019; López-Díaz et al., 2017; Murrough et al., 2013b).
In addition to antidepressant properties, racemic ketamine can rapidly reduce suicidal thoughts within one day and for up to one week in depressed patients with suicidal ideation—partially independent of its effects on mood (Grunebaum et al., 2018; López-Díaz et al., 2017; Reinstatler and Youssef, 2015; Wilkinson et al., 2018; Williams et al., 2019; Witt et al., 2020). Given the current limitations of most existing treatments for reducing suicide ideations and plans in patients suffering from moderate to severe major depression, this additional property of ketamine may be particularly helpful in the emergent management of patients in acute crisis.
Racemic ketamine has led to a lot of preclinical and biomarker findings (Zanos et al., 2016; Zanos and Gould, 2018), which are leading to new possibilities in terms of safer alternatives to mitigate dissociation and reduce the propensity for misuse or diversion of ketamine (Burger et al., 2016; Lener et al., 2017; Newport et al., 2015). To that end, the rapid antidepressant effects of ketamine seen in individuals with TRD appears to be predictive of a sustained effect (Atigari and Healy, 2013; Ionescu et al., 2014; Murrough et al., 2011, 2013b).
Fortunately, ketamine appears to ameliorate the symptoms of depression at subanesthetic doses among individuals with major depressive disorder as well as bipolar depression (Lener et al., 2017). Despite the efficacy of racemic ketamine at low doses, its dissociative effects and abuse potential persist (Zanos et al., 2018). Alongside the impracticality and high costs of intravenous ketamine administration (Cohen et al., 2018; Smith-Apeldoorn et al., 2019), clinicians and researchers have sought alternative formulations and delivery systems for ketamine (Jelen et al., 2018). Subsequently, oral (Arabzadeh et al., 2018; Domany et al., 2019; Jafarinia et al., 2016), subcutaneous (George et al., 2017; Hardy et al., 2012; Loo et al., 2016), intranasal (Canuso et al., 2018; Daly et al., 2018; Galvez et al., 2018; Lapidus et al., 2014), and intramuscular (Chilukuri et al., 2014; Loo et al., 2016) ketamine delivery routes have all been explored across the literature with promising findings in several studies. With the isolation of the enantiomeric S-ketamine (esketamine)—which is four-fold more potent for the NMDA receptor—there was also an option of providing much lower doses of ketamine and the opportunity to reduce the dose-dependent dissociative properties of ketamine (Correia-Melo et al., 2018). As esketamine was also available through an intranasal delivery system, it presented a substantially more practical option than intravenous racemic ketamine (Schatzberg, 2019; Tibensky et al., 2016). Ultimately, intranasal esketamine was approved by the US Food and Drug Administration on March 5th, 2019 for use in TRD (Kim et al., 2019); on December 19th, 2019, Europe followed suit with approval for esketamine for the same indication (Wei et al., 2020).
Despite its potential for benefit, there are several concerns about the efficacy and tolerability of esketamine nasal spray for TRD (Fedgchin et al., 2019; Ochs-Ross et al., 2019; Wei et al., 2020). For example, dissociative symptoms are still observed in studies using 86 mg of intranasal esketamine, which are of comparable severity to racemic intravenous ketamine (Lapidus et al., 2014; Vlerick et al., 2020). To that end, there has been an in-depth exploration into the potential mechanisms behind ketamine’s antidepressant effects and adverse effects (Li and Vlisides, 2016; Sleigh et al., 2014; Zorumski et al., 2016). While the mechanisms behind ketamine’s antidepressant effects have not been fully elucidated, ketamine is known to antagonize glutamatergic N-methyl-D-aspartate receptors (NMDAR) in the central nervous system (Corriger and Pickering, 2019; Newport et al., 2015). Emerging evidence suggests ketamine’s mechanisms extend beyond the glutamatergic system, involving opioids, GABA, and complex second messenger pathways culminating in varied neuroplastic and neurogenic responses (Kadriu et al., 2019; Lener et al., 2017; Zanos and Gould, 2018). To date, proposed mechanisms include activation of the NMDAR and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) systems, traditional monoamines like serotonin and dopamine, brain-derived neurotrophic factor (BDNF), the mammalian target of rapamycin (mTOR), low-voltage-sensitive T-type calcium channels, endogenous options, transforming growth factor β1, as well as the gut microbiome (Newport et al., 2015; Sleigh et al., 2014; Wei et al., 2020). In addition, accumulating evidence from preclinical studies indicate that (R)-ketamine (arketamine) has greater potency and longer lasting antidepressant effects than (S)-ketamine in animal models of depression, and that arketamine has fewer detrimental side effects than both (R,S)-ketamine or (S)-ketamine (Hashimoto, 2019; Hashimoto and Yang, 2019; Zhang and Hashimoto, 2019a).
Although clinical studies of (R)-ketamine in humans are now underway, the level of proof of efficacy remains low and more RCTs are needed to explore efficacy and safety issues of ketamine in depression (Corriger and Pickering, 2019). To date, esketamine and racemic R,S-ketamine have not been robustly compared in clinical contexts, and no extant or ongoing studies have yet investigated the comparative efficacy of racemic ketamine versus esketamine.
OBJECTIVE
We aimed to examine the available evidence for racemic ketamine and esketamine to ascertain the comparative efficacy of these two formulations ketamine on remission from and symptoms of depression—both unipolar and bipolar. We also examined the safety of ketamine for the treatment of depression, including all-cause, serious, and treatment-related adverse events and study withdrawals.
METHODS
Protocol and registration
We registered this study with the Open Science Framework (https://osf.io/ksvnb/). We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) (Liberati et al., 2009).
Eligibility criteria
We included randomized controlled trials examining the use of ketamine in adults (aged 18 years or older) to treat primary unipolar or bipolar depression. We considered studies examining any intravenous ketamine or intranasal esketamine as a standalone treatment or in combination with psychotropic medications or psychotherapies. As per existing systematic reviews examining the efficacy of ketamine for depressive disorders, we limited eligibility to randomized controlled trials (Abdallah et al., 2015; Fond et al., 2014; Han et al., 2016; Kennedy et al., 2016; Lee et al., 2015; McGirr et al., 2015; Reinstatler and Youssef, 2015; Wilkinson et al., 2018; Witt et al., 2020). We excluded observational designs (i.e., cross-sectional studies, cohort studies, case-control studies), reviews of mechanisms of ketamine, commentary articles, and clinical overviews that did not assess and synthesize individual studies. We also excluded studies pairing ketamine with a neurostimulation-based treatment. We only included studies reporting at least one primary outcome—either remission or change in depression symptomology.
Information sources and search
We searched MEDLINE, Embase, PsycINFO, the Cochrane Central Register of Controlled Clinical Trials (CENTRAL), and the Cochrane Database of Systematic Reviews via Ovid for studies published from inception to December 13, 2019 (Appendix 1). To identify ongoing or unpublished studies, we also searched ClinicalTrials.gov, the EU Clinical Trials Register, and the Australian and New Zealand Clinical Trials Registry using the keywords “ketamine” and “depression.” We also hand-searched reference lists of included studies and topical reviews for potentially relevant articles.
Study selection
Two reviewers (AB, GV) independently examined titles and abstracts by use of the web-based systematic review programme Covidence (Veritas Health Innovation, 2019). Relevant articles were obtained in full and assessed for inclusion independently by the two reviewers. The disagreement between reviewers was resolved via discussion to reach consensus.
Data collection process and data items
Two reviewers extracted data via a pre-piloted, standardized data extraction tool in Microsoft Excel 2016. We extracted data on details of the populations, interventions, comparisons, outcomes of significance to the mental disorder, study methods, ketamine dose and route of administration, study withdrawals, and study withdrawals due to adverse events. Where there was missing data, we contacted the authors for additional information. When authors reported multiple analyses (e.g., intention-to-treat or per-protocol), we extracted the more conservative analysis with a preference for intention-to-treat analyses. We used Review Manager (RevMan), version 5.3, for generating the risk of bias plots (The Cochrane Collaboration, 2014).
Outcomes
We used the following seven outcome measures:
Improvement in depression score, defined as the change in depression severity from baseline to study endpoint using a validated depression rating scale.
Response to treatment, defined as the proportion of participants who achieved a minimum reduction of 50% in their baseline depression score.
Remission from depression, defined as the proportion of participants who had a depression rating of less than or equal to 12 on the Montgomery-Åsberg Depression Rating Scale or seven on the Hamilton Depression Rating Scale.
Improvement in suicidality, defined as the change in suicidal ideation severity from baseline to study endpoint using a validated suicidality rating scale.
Completion of treatment, defined as the proportion of participants who remained in the study until its primary endpoint.
Drop-outs, defined as the proportion of participants who prematurely discontinued their participation in the study for any cause.
Drop-outs due to adverse events, defined as the proportion who dropped out of the study prematurely due to adverse events.
Assessment of heterogeneity
We assessed between-study heterogeneity using the I2 statistic: values of 0–39% were low, 40–74% as moderate, and 75–100% as high (Cochrane Collaboration, 2014).
Risk of bias in individual studies
We assessed the risk of bias within individual trials using the Cochrane risk of bias tool for randomized controlled trials. Specifically, the risk of bias tool assesses indicators of selection bias, performance bias, detection bias, attrition bias, and reporting bias (Higgins et al., 2011). The risk of bias assessments were completed independently by two reviewers (AB or GV). Inter-reviewer disagreements were resolved via discussion to reach consensus.
Summary measures
Continuous outcomes (outcomes 1 and 4) were pooled as standardized mean differences (SMDs) and dichotomous outcomes (outcomes 2, 3, 5, 6, and 7) as rate ratios (RRs), with random-effects, generic inverse variance meta-analyses.
Analytic methods
As we anticipated high heterogeneity, we undertook a random effects meta-analytic strategy, rather than using a fixed-effects model. For pairwise meta-analyses, we applied a Mantel-Haenszel approach and a DerSimonian-Laird estimator for heterogeneity using the meta package within R studio version 3.5.3 (Schwarzer, 2007). A continuity correction of 0.5 was applied to studies with zero events. We also considered the comparative performance of intravenous ketamine and intranasal esketamine across several time points: overall, at 24 hours, 48 hours, 72 hours, one week, two weeks, three weeks, four weeks, six weeks, and eight weeks post-treatment. Where raw depression scores were provided without corresponding response rates, a validated method of imputation was applied as per previous meta-analyses (Samara et al., 2013). For crossover studies, the reported results refer to the first period before crossover.
Risk of bias across studies
To assess publication bias, we applied a weighted linear regression of the treatment effect on the inverse of the total sample size using the variance of the average event rate as weights (Peters et al., 2006). The test statistic follows a t distribution with the number of studies minus two degrees of freedom (df = k-2); this test is available for meta-analyses comparing two binary outcomes or combining single proportions.
Role of the funding source
This study was not funded; thus, funders had no role in study design, data collection, data analysis, data interpretation, the writing of the report, or the decision to submit the paper for publication. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Study selection
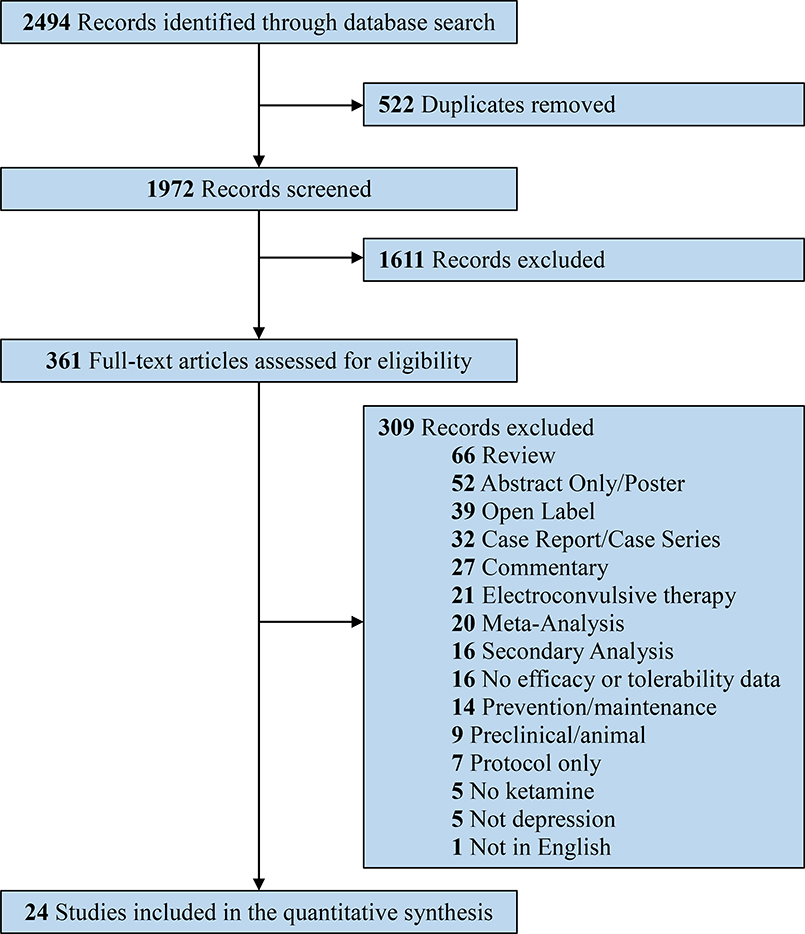
The search strategy identified a total of 2494 records (Figure 1). After duplicates were removed, a total of 1972 unique records were screened by title and abstract for potential relevance in the systematic review and meta-analysis. After title and abstract screening, 1611 irrelevant records were excluded, leaving 361 documents for full-text review. After full-text review, 24 randomized controlled trials met the final inclusion criteria for the systematic review and meta-analysis (Berman et al., 2000; Canuso et al., 2018; Correia-Melo et al., 2020; Daly et al., 2018; Diazgranados et al., 2010; Fava et al., 2018; Fedgchin et al., 2019; Grunebaum et al., 2017, 2018; Hu et al., 2016; Ionescu et al., 2019; Kudoh et al., 2002; Lapidus et al., 2014; Li et al., 2016; Murrough et al., 2013b; Ochs-Ross et al., 2019; Phillips et al., 2019; Popova et al., 2019; Singh et al., 2016a, 2016b; Sos et al., 2013; Su et al., 2017; Zarate et al., 2006, 2012).
Figure 1.
PRISMA flow diagram outlining the systematic review process.
Characteristics of studies, participants, and interventions
Table 1 provides an overview of study characteristics. Seven trials (Berman et al., 2000; Diazgranados et al., 2010; Lapidus et al., 2014; Phillips et al., 2019; Sos et al., 2013; Zarate et al., 2006, 2012) were crossover, while the remainder were parallel arm trials. By country, the majority of studies were from the United States (71%) or Taiwan (13%). Across trials, the total number of participants with depression was 1877. The majority (n=1836; 97.8%) were diagnosed with unipolar major depression; the remaining 41 were diagnosed with a bipolar spectrum depression (n=41; 2.2%). Diagnoses were confirmed by standardized means of assessments, with the most frequently used instruments being the Structured Clinical Interview for the DSM (First, 2015; Spitzer et al., 1994) or the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). There was considerable variation in sample sizes between the studies, as the total number of participants with depression ranged from nine participants (Berman et al., 2000) to 342 participants (Fedgchin et al., 2019). Three studies had a sample size of more than 100 participants (Fedgchin et al., 2019; Ochs-Ross et al., 2019; Popova et al., 2019). The mean age ranged from 35.9 to 70.0 years. All studies included both male and female participants, with an overall proportion of females of 60.7% (n=1139/1877). Three trials (Berman et al., 2000; Murrough et al., 2013a; Zarate et al., 2006) tested ketamine as a monotherapy (i.e., participants were required to discontinue any concomitant psychotropic medications before ketamine initiation). In contrast, the remainder tested ketamine as an adjunctive treatment (i.e., in augmentation of concomitant psychotropic medications). The majority of trials involved participants with TRD, defined as having an inadequate response to a minimum of one (21%), two (62%), or three (15%) previous antidepressant trials; only six trials involved non-TRD (Berman et al., 2000; Canuso et al., 2018; Grunebaum et al., 2018, 2017; Kudoh et al., 2002; Sos et al., 2013).
Table 1.
Study characteristics
| Study | Design | Population | N | Female (%) | Mean age | Formulation | Dose | Scale | Comparator |
|---|---|---|---|---|---|---|---|---|---|
| Berman 2000 (Berman et al., 2000) | Crossover | Non-TRD MDD | 9 | 55.6 | 37.0 | Racemic, monotherapy | 0.5 mg/kg IV, multiple | HDRS | Placebo |
| Kudoh 2002 (Kudoh et al., 2002) | Parallel | Non-TRD | 70 | N/R | 47.6 | Racemic, adjunctive | 1 mg/kg IV, single | HDRS | Placebo |
| Zarate 2006 (Zarate et al., 2006) | Crossover | TRD MDD | 18 | 66.7 | 46.7 | Racemic, monotherapy | 0.5 mg/kg IV, multiple | HDRS | Placebo |
| Diazgranados 2010 (Diazgranados et al., 2010) | Crossover | TRD BD | 9 | 66.7 | 47.9 | Racemic, adjunctive | 0.5 mg/kg IV, multiple | MADRS | Placebo |
| Zarate 2012 (Zarate et al., 2012) | Crossover | TRD BD | 15 | 53.3 | 46.7 | Racemic, adjunctive | 0.5 mg/kg IV, multiple | MADRS | Placebo |
| Sos 2013 (Sos et al., 2013) | Crossover | Non-TRD | 30 | 50.0 | 43.4 | Racemic, adjunctive | 0.5 mg/kg IV, single | MADRS | Placebo |
| Murrough 2013 (Murrough et al., 2013a) | Parallel | TRD MDD | 72 | 51.4 | 44.8 | Racemic, monotherapy | 0.5 mg/kg IV, single | MADRS | Midazolam |
| Lapidus 2014 (Lapidus et al., 2014) | Crossover | TRD MDD | 20 | 25.0 | 48.0 | Esketamine, adjunctive | 50 mg/day IN | MADRS | Placebo |
| Hu 2016 (Hu et al., 2016) | Parallel | TRD MDD | 27 | 63.0 | 38.9 | Racemic, adjunctive | 0.5 mg/kg IV, multiple | MADRS | Placebo |
| Singh 2016a (Singh et al., 2016b) | Parallel | TRD MDD | 67 | 70.6 | 43.0 | Racemic, adjunctive | 0.5 mg/kg IV, multiple | MADRS | Placebo |
| Singh 2016b (Singh et al., 2016a) | Parallel | TRD MDD | 40 | 57.9 | 43.7 | Esketamine, adjunctive | 0.2–0.4 mg/kg IV, single | MADRS | Placebo |
| Li 2016 (Li et al., 2016) | Parallel | TRD MDD | 64 | 75.0 | 46.6 | Racemic, adjunctive | 0.2–0.5 mg/kg IV | HDRS | Placebo |
| Grunebaum 2017 (Grunebaum et al., 2017) | Parallel | Non-TRD BD | 16 | 62.5 | 41.0 | Racemic, adjunctive | 0.5 mg/kg IV, single | HDRS | Midazolam |
| Su 2017 (Su et al., 2017) | Parallel | TRD MDD | 95 | 71.0 | 47.3 | Racemic, adjunctive | 0.2–0.5 mg/kg IV, single | HDRS | Placebo |
| Canuso 2018 (Canuso et al., 2018) | Parallel | Non-TRD | 66 | 65.2 | 35.9 | Esketamine, adjunctive | 84 mg twice/week IN | MADRS | Placebo |
| Grunebaum 2018 (Grunebaum et al., 2018) | Parallel | TRD MDD | 80 | 60.0 | 39.6 | Racemic, adjunctive | 0.5 mg/kg IV, single | HDRS | Midazolam |
| Daly 2018 (Daly et al., 2018) | Parallel | Non-TRD | 133 | 57.0 | 45.4 | Esketamine, adjunctive | 28–84 mg twice/week IN | MADRS | Placebo |
| Fava 2018 (Fava et al., 2018) | Parallel | TRD MDD | 99 | 57.6 | 46.5 | Racemic, adjunctive | 0.1–1 mg/kg IV, single | HDRS | Midazolam |
| Phillips 2019 (Phillips et al., 2019) | Crossover | TRD MDD | 43 | 55.8 | 41.7 | Racemic, adjunctive | 0.5 mg/kg IV, single | MADRS | Midazolam |
| Ionescu 2019 (Ionescu et al., 2019) | Parallel | TRD MDD | 26 | 38.5 | 45.4 | Racemic, adjunctive | 0.5 mg/kg IV, multiple | HDRS | Placebo |
| Popova 2019 (Popova et al., 2019) | Parallel | TRD MDD | 223 | 61.9 | 45.7 | Esketamine, adjunctive | 56–84 mg twice/week IN | MADRS | Placebo |
| Fedgchin 2019 (Fedgchin et al., 2019) | Parallel | TRD MDD | 455 | 71.1 | 46.6 | Esketamine, adjunctive | 56–84 mg twice/week IN | MADRS | Placebo |
| Correia-Melo 2019 (Correia-Melo et al., 2020) | Parallel | TRD MDD | 63 | 60.3 | 47.1 | Esketamine, adjunctive | 0.25 mg/kg IN, single | MADRS | Racemic ketamine (0.5 mg/kg IV) |
| Ochs-Ross 2020 (Ochs-Ross et al., 2020) | Parallel | TRD MDD | 137 | 62.0 | 70.0 | Esketamine, adjunctive | 28– 84 mg twice/week IN | MADRS | Placebo |
IV = intravenous; IN = intranasal; TRD = Treatment-Resistant Depression; MADRS = Montgomery-Åsberg Depression Rating Scale; HDRS = Hamilton Depression Rating Scale
Exclusion criteria across studies
In most studies, individuals with other significant medical or psychiatric conditions were not eligible for participation. Psychotic disorders (such as schizophrenia or schizoaffective disorder), acute medical complications, and severe substance use disorders (involving ketamine or other substances) were exclusion criteria for the majority of trials. Participants with acute suicidality were excluded from most studies unless the trial was explicitly intended for the treatment of acute suicidality with ketamine. Finally, pregnant and breastfeeding women were not permitted to participate in any of the trials.
Overview of results of pairwise meta-analyses
All trials reported depression rating scores and rates of response, the proportion of participants who completed the trial, the proportion who experienced adverse events, and the proportion who dropped out due to adverse events. Rates of remission were available for 19 trials (Arabzadeh et al., 2018; Berman et al., 2000; Canuso et al., 2018; Correia-Melo et al., 2020; Daly et al., 2018; Diazgranados et al., 2010; Domany et al., 2019; Fedgchin et al., 2019; George et al., 2017; Grunebaum et al., 2018, 2017; Hu et al., 2016; Ionescu et al., 2019; Jafarinia et al., 2016; Loo et al., 2016; Murrough et al., 2013a; Ochs-Ross et al., 2019; Phillips et al., 2019; Popova et al., 2019; Singh et al., 2016a, 2016b; Sos et al., 2013; Su et al., 2017; Zarate et al., 2006, 2012), while suicidality was reported by 11 trials (Canuso et al., 2018; Grunebaum et al., 2018, 2017; Hu et al., 2016; Ionescu et al., 2019; Kudoh et al., 2002; Murrough et al., 2013a; Phillips et al., 2019; Sos et al., 2013; Su et al., 2017; Zarate et al., 2012).
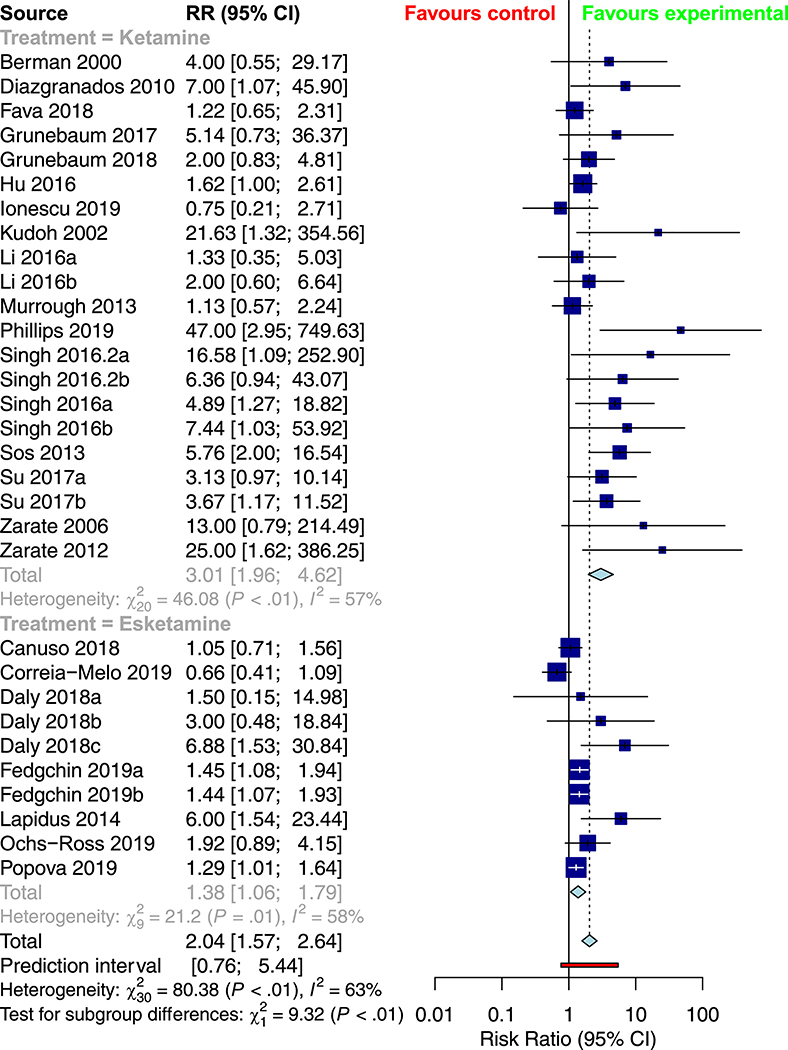
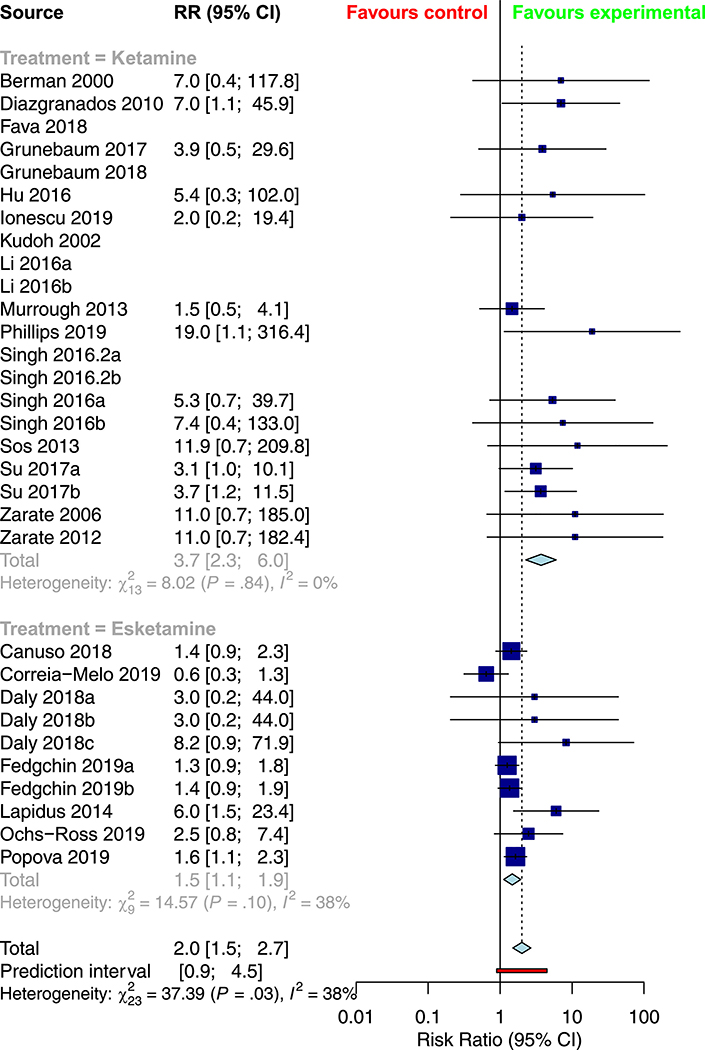
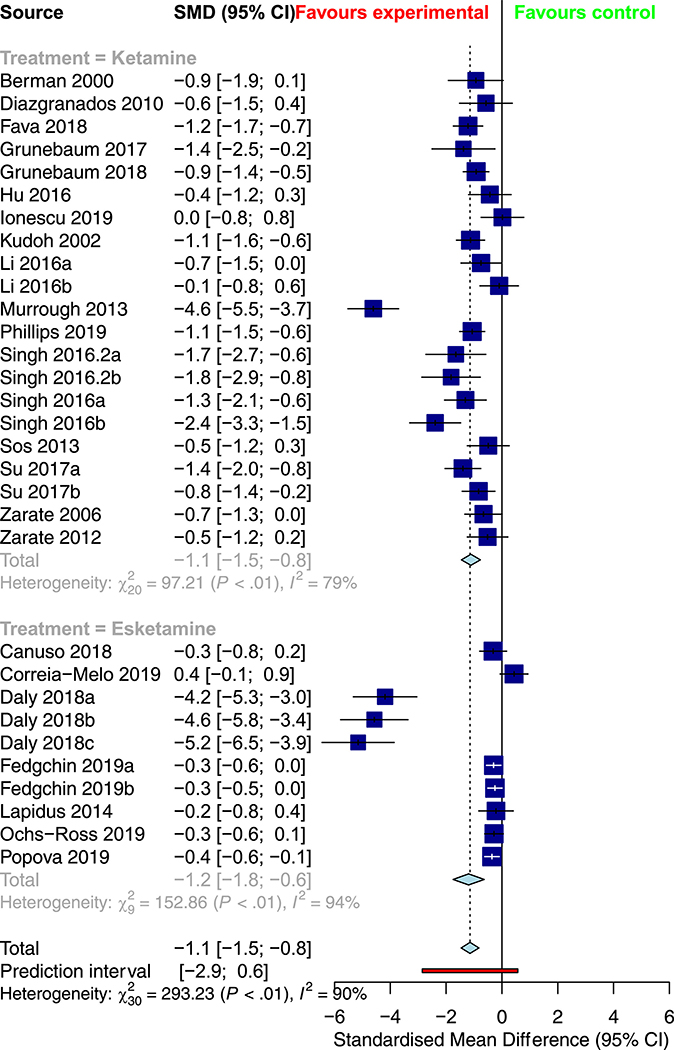
Table 2 provides a summary of the pooled meta-analysis outcomes—both crude and corrected for publication bias. Overall, ketamine demonstrated a significant improvement in response (RR = 2.0382, 95% CI: 1.5748; 2.6380, Figure 2) and remission rates (RR = 2.0029 [1.5005; 2.6735], Figure 3) relative to control conditions, alongside a significant reduction in depression severity (SMD = −1.1430 [−1.4613; −0.8247], Figure 4) and suicidality scores (SMD = −0.3867 [−0.7082; −0.0653]).
Table 2.
Summary of meta-analysis results (overall).
| Outcome | Random effects model | Corrected for publication bias | z | p-value | I2 | k |
|---|---|---|---|---|---|---|
| Response | RR = 2.0382 [1.5748; 2.6380] | RR = 1.4209 [1.0950; 1.8438] | 5.41 | < 0.0001 | 62.7% | 31 |
| Remission | RR = 2.0029 [1.5005; 2.6735] | RR = 1.5521 [1.1472; 2.1000] | 4.71 | < 0.0001 | 38.5% | 24 |
| Score | SMD = −1.1430 [−1.4613; −0.8247] | SMD = −0.4832 [−0.8453; −0.1212] | −7.04 | < 0.0001 | 89.8% | 31 |
| Suicidality | SMD = −0.3867 [−0.7082; −0.0653] | SMD = −0.5034 [−0.8180; −0.1888] | −2.36 | 0.0184 | 71.3 | 12 |
| Completion | RR = 0.9929 [0.9681; 1.0182] | RR = 0.9876 [0.9576; 1.0185] | −0.56 | 0.5773 | 14.0 | 31 |
| Dropouts | RR = 0.9664 [0.7234; 1.2911] | RR = 0.9229 [0.6864; 1.2410] | −0.23 | 0.8173 | 40.5 | 24 |
| Adverse events | RR = 1.8703 [1.0271; 3.4076] | RR = 2.0087 [1.1150; 3.6188] | 2.05 | 0.0406 | 0.0 | 14 |
RR = rate ratio; SMD = standardized mean difference; z = z-score (on normal distribution); I2 = measure of heterogeneity (closer to 100.0 indicates higher heterogeneity); k = number of trials involved in the sub analysis.
Figure 2.
Subgroup meta-analysis of response rates in the treatment of depression with racemic ketamine versus esketamine
Figure 3.
Subgroup meta-analysis of remission rates in the treatment of depression with ketamine versus esketamine
Figure 4.
Subgroup meta-analysis of depression rating scores in the treatment of depression with ketamine versus esketamine
Study completion and drop-out rates were proxies for ketamine tolerability. Of the 1011 participants who were to receive ketamine, 147 (14.5%) dropped out, compared to 141/980 (14.4%) who were to receive control interventions (RR 0.97, 95% CI 0.72—1.29, z = −0.23, p = 0.82). Across studies, adverse events resulting in study discontinuation were only observed in 11 of the 31 trials (Canuso et al., 2018; Daly et al., 2018; Diazgranados et al., 2010; Fedgchin et al., 2019; Ionescu et al., 2016; Li and Vlisides, 2016; Murrough et al., 2013b; Ochs-Ross et al., 2019; Popova et al., 2019; Singh et al., 2016a, 2016b). Across studies, 52 such adverse events resulting in study discontinuation were observed, with 37 in experimental arms and 15 in control arms. One study reported cardiovascular side-effects in 2 of 47 patients (n = 1 refractory hypertension, n = 1 hypotension and bradycardia) who received ketamine and no such side-effects among control patients (Murrough et al., 2013a). The only recorded induction of mania/hypomania occurred in a patient with BD who was receiving saline placebo infusion (Diazgranados et al., 2010). No severe psychotic symptoms occurred in any patient.
Performance of ketamine over time
Table 3 provides an overview of the efficacy and tolerability of ketamine and esketamine over time points ranging from 24 hours to four weeks following the receipt of treatment. The pooled response and remission rates, as well as the change in depression rating scores, were statistically significant across all timepoints. However, reductions in suicidality were not statistically significant at the two- or four-week timepoints. While there was no clear pattern in the effect sizes observed for the response or remission rates, the effect on suicidality appeared to decrease over time.
Table 3.
Time-course analysis of outcomes
| Outcome | Random effects model | z | p-value | I2 | k |
|---|---|---|---|---|---|
| 4–12 hours | |||||
| Suicidality | SMD = −0.7045 [−1.2148; −0.1942] | −2.71 | 0.0068 | 82.9 | 9 |
| 24 hours | |||||
| Response | RR = 2.6011 [1.8599; 3.6378] | 5.59 | < 0.0001 | 61.0 | 28 |
| Remission | RR = 3.2823 [2.0966; 5.1385] | 5.20 | < 0.0001 | 8.7 | 14 |
| Score | SMD = −1.0636 [−1.3926; −0.7346] | −6.34 | < 0.0001 | 89.3 | 28 |
| Suicidality | SMD = −0.6876 [−1.1461; −0.2291] | −2.94 | 0.0033 | 81.2 | 9 |
| 48 hours | |||||
| Response | RR = 1.4124 [1.0217; 1.9524] | 2.09 | 0.0366 | 57.2 | 12 |
| Score | SMD = −1.0474 [−1.5189; −0.5759] | −4.36 | < 0.0001 | 79.5 | 12 |
| 72 hours | |||||
| Response | RR = 2.1836 [1.4397; 3.3120] | 3.67 | 0.0002 | 68.5 | 18 |
| Remission | RR = 2.3576 [1.1980; 4.6396] | 2.48 | 0.0130 | 51.0 | 8 |
| Score | SMD = −0.8763 [−1.2076; −0.5450] | −5.18 | < 0.0001 | 75.4 | 18 |
| Suicidality | SMD = −0.9243 [−1.5804; −0.2683] | −2.76 | 0.0058 | 79.9 | 5 |
| One week | |||||
| Response | RR = 1.8660 [1.3805; 2.5220] | 4.06 | < 0.0001 | 56.5 | 25 |
| Remission | RR = 2.5868 [1.2728; 5.2574] | 2.63 | 0.0086 | 50.2 | 11 |
| Score | SMD = −1.0179 [−1.3615; −0.6743] | −5.81 | < 0.0001 | 89.6 | 24 |
| Suicidality | SMD = −0.4287 [−0.8202; −0.0373] | −2.15 | 0.0318 | 67.6 | 8 |
| Two weeks | |||||
| Response | RR = 1.5796 [1.1926; 2.0921] | 3.19 | < 0.0001 | 50.2 | 15 |
| Remission | RR = 7.5979 [2.8489; 20.2632] | 4.05 | < 0.0001 | 0.0 | 5 |
| Score | SMD = −0.6418 [−0.9020; −0.3817] | −4.84 | < 0.0001 | 75.8 | 15 |
| Suicidality | SMD = −0.2506 [−0.5182; 0.0170] | −1.84 | 0.0665 | 0.0 | 5 |
| Three weeks | |||||
| Response | RR = 5.4566 [2.7713; 10.7437] | 4.91 | < 0.0001 | 70.2 | 7 |
| Remission | RR = 4.9525 [1.0471; 23.4241] | 2.02 | 0.0436 | 10.2 | 2 |
| Score | SMD = −0.2618 [−0.3908; −0.1328] | −3.96 | < 0.0001 | 0.0 | 7 |
| Four weeks | |||||
| Response | RR = 1.3891 [1.1655; 1.6557] | 3.67 | 0.0002 | 27.8 | 7 |
| Remission | RR = 1.5309 [1.2056; 1.9438] | 3.49 | 0.0005 | 25.2 | 7 |
| Score | SMD = −0.3037 [−0.4346; −0.1728] | −4.55 | < 0.0001 | 0.0 | 6 |
| Suicidality | SMD = −0.1602 [−0.4472; 0.1268] | −1.09 | 0.2741 | 0.0 | 4 |
RR = rate ratio; SMD = standardized mean difference; z = z-score (on normal distribution); I2 = measure of heterogeneity (closer to 100.0 indicates higher heterogeneity); k = number of trials involved in the sub analysis
Moderator analyses
Table 4 provides an overview of the results of the subgroup analyses for racemic ketamine vs. esketamine; TRD vs. non-TRD; unipolar vs. bipolar depression; crossover vs. parallel trial; monotherapy vs. adjunctive ketamine; and placebo vs. active control. Relative to intranasal esketamine, intravenous ketamine demonstrated more significant overall response and remission rates, as well as lower drop-outs due to adverse events. As well, more substantial response and remission rates were observed in crossover trials, while more significant improvements in depression rating scores were observed in parallel trials. There was no significant association between treatment resistance, depression type, treatment strategy, or comparator type on any of the seven outcome measures. There was no significant association between mean age (in years) or the study-level proportion of female participants (%) on any of the seven outcomes.
Table 4.
Summary of subgroup meta-analyses.
| Outcome Treatment | Ketamine | Esketamine | Subgroup test (p-value) |
|---|---|---|---|
| Response | RR = 3.0096 [1.9599; 4.6220] | RR = 1.3779 [1.0623; 1.7874] | 0.0023 |
| Remission | RR = 3.6999 [2.2772; 6.0112] | RR = 1.4724 [1.1197; 1.9361] | 0.0012 |
| Score | SMD = −1.1140 [−1.4551; −0.7729] | SMD = −1.1932 [−1.7539; −0.6326] | 0.8129 |
| Suicidality | SMD = −0.4323 [−0.7729; −0.0917] | SMD = 0.0450 [−0.4385; 0.5284] | 0.1137 |
| Completion | RR = 1.0088 [0.9553; 1.0652] | RR = 0.9759 [0.9313; 1.0227] | 0.3662 |
| Dropouts | RR = 0.7557 [0.5245; 1.0889] | RR = 1.3616 [0.9129; 2.0307] | 0.0331 |
| Adverse events | RR = 1.0601 [0.4307; 25601] | RR = 3.0168 [1.3412; 6.7856] | 0.0860 |
| Treatment-resistance | Non-TRD | TRD | Subgroup test (p-value) |
| Response | RR = 3.0967 [1.2143; 7.8973] | RR = 1.9265 [1.4637; 2.5358] | 0.3404 |
| Remission | RR = 2.5747 [0.9236; 7.1776] | RR = 2.0454 [1.4754; 2.8356] | 0.6751 |
| Score | SMD = −0.8008 [−1.1184; −0.4831] | SMD = −1.2343 [−1.6159; −0.8526] | 0.0871 |
| Suicidality | SMD = −0.3173 [−0.8435; 0.2090] | SMD = −0.4347 [−0.8777; 0.0083] | 0.7379 |
| Completion | RR = 1.0048 [0.9555; 1.0566] | RR = 0.9887 [0.9578; 1.0205] | 0.5950 |
| Dropouts | RR = 0.9245 [0.5309; 1.6099] | RR = 0.9754 [0.6922; 1.3744] | 0.8722 |
| Adverse events | RR = 4.4286 [0.5467; 35.8730] | RR = 1.7319 [0.9262; 3.2386] | 0.3994 |
| Depression type | MDD only | BD only | Subgroup test (p-value) |
| Response | RR = 1.8658 [1.4505; 2.4000] | RR = 7.9859 [2.3698; 26.9114] | 0.0564 |
| Remission | RR = 1.8233 [1.3733; 2.4208] | RR = 6.1295 [1.7744; 21.1733] | 0.1176 |
| Score | SMD = −1.1906 [−1.5378; −0.8434] | SMD = −0.7111 [−1.2257; −0.1964] | 0.3116 |
| Suicidality | SMD = −0.2988 [−0.6110; 0.0134] | SMD = −1.0438 [−2.5431; 0.4556] | 0.3404 |
| Completion | RR = 0.9921 [0.9652; 1.0197] | RR = 0.9908 [0.7574; 1.2960] | 0.9988 |
| Dropouts | RR = 0.9507 [0.6843; 1.3207] | RR = 1.2615 [0.7435; 2.1402] | 0.6725 |
| Adverse events | RR = 2.2547 [1.1751; 4.3260] | RR = 0.6667 [0.1440; 3.0855] | 0.1515 |
| Trial type | Crossover trial | Parallel trial | Subgroup test (p-value) |
| Response | RR = 7.2920 [3.8053; 13.9737] | RR = 1.5838 [1.2761; 1.9657] | < 0.0001 |
| Remission | RR = 8.1568 [3.5519; 18.7320] | RR = 1.5500 [1.2431; 1.9327] | 0.0002 |
| Score | SMD = −0.6863 [−0.9428; −0.4339] | SMD = −1.3112 [−1.7015; −0.9208] | 0.0088 |
| Suicidality | SMD = −0.7928 [−1.8251; 0.2395] | SMD = −0.2752 [−0.5706; 0.0203] | 0.3447 |
| Completion | RR = 1.0521 [0.8948; 1.2371] | RR = 0.9906 [0.9637; 1.0183] | 0.4723 |
| Dropouts | RR = 1.0468 [0.8013; 1.3675] | RR = 0.9766 [0.6191; 1.5404] | 0.7966 |
| Adverse events | RR = 0.5848 [0.1472; 2.3238] | RR = 2.4527 [1.2603; 4.7731] | 0.0666 |
| Treatment strategy | Monotherapy | Adjunctive | Subgroup test (p-value) |
| Response | RR = 2.5714 [0.5883; 11.2394] | RR = 2.0586 [1.5727; 2.6946] | 0.7712 |
| Remission | RR = 2.8075 [0.7458; 10.5678] | RR = 1.9845 [1.4670; 2.6845] | 0.6170 |
| Score | SMD = −2.0618 [−4.5233; 0.3997] | SMD = −1.0288 [−1.3281; −0.7294] | 0.4142 |
| Suicidality | SMD = −0.1999 [−0.6863; 0.2864] | SMD = −0.4051 [−0.7604; −0.0499] | 0.5043 |
| Completion | RR = 1.1001 [0.9705; 1.2470] | RR = 0.9891 [0.9643; 1.0145] | 0.1031 |
| Dropouts | RR = 0.3923 [0.1199; 1.2830] | RR = 1.0158 [0.7516; 1.3730] | 0.1272 |
| Adverse events | RR = 2.6842 [0.1339; 53.8059] | RR = 1.8429 [0.9993; 3.3985] | 0.8096 |
| Comparator | Placebo | Active | Subgroup test (p-value) |
| Response | RR = 2.2107 [1.6780; 2.9125] | RR = 1.6145 [0.7625; 3.4184] | 0.4408 |
| Remission | RR = 2.0228 [1.5364; 2.6633] | RR = 1.8103 [0.5393; 6.0762] | 0.8609 |
| Score | SMD = −1.0634 [−1.3862; −0.7406] | SMD = −1.4133 [−2.4375; −0.3892] | 0.5230 |
| Suicidality | SMD = −0.3431 [−0.8222; 0.1360] | SMD = −0.4828 [−0.8570; −0.1087] | 0.6524 |
| Completion | RR = 0.9901 [0.9603; 1.0209] | RR = 0.9998 [0.9510; 1.0510] | 0.7457 |
| Dropouts | RR = 0.9989 [0.6997; 1.4259] | RR = 0.9178 [0.6388; 1.3185] | 0.7439 |
| Adverse events | RR = 1.9688 [1.0552; 3.6735] | RR = 1.0035 [0.1135; 8.8691] | 0.5600 |
RR = rate ratio; SMD = standardized mean difference: TRD = treatment-resistant depression; MDD = major depressive disorder (i.e., unipolar depression); BD = bipolar depression.
Publication bias
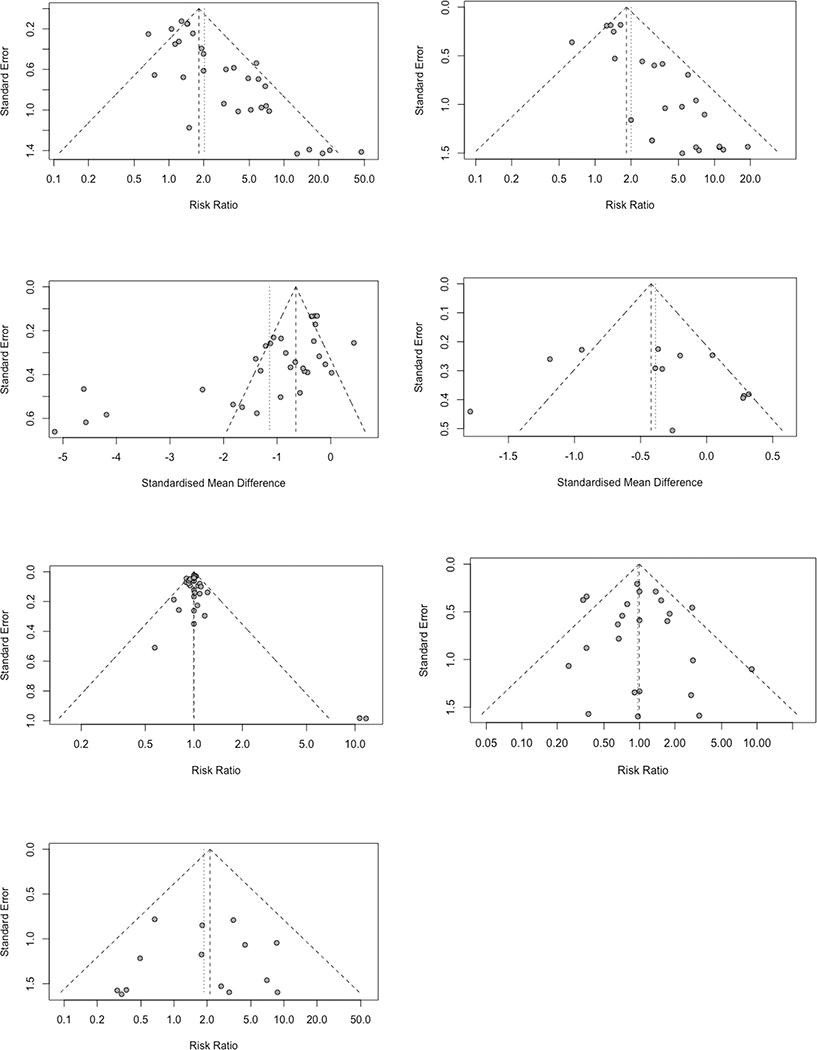
The results of publication bias assessments are illustrated in Figure 5. In summary, there were significant publication bias in response, remission, and depression rating scores. However, there was lower evidence for publication bias in the other four outcomes. Given this finding, the overall results in Table 2 were corrected for publication bias using the trim and fill method. Consequently, there were substantial reductions in the effect sizes for response rates (RR = 1.4209 [1.0950; 1.8438]), remission rates (RR = 1.5521 [1.1472; 2.1000]), and depression rating scores (SMD = −0.4832 [−0.8453; −0.1212]). There was a small increase in the effect size for suicidality reduction following correction for publication bias (SMD = −0.5034 [−0.8180; −0.1888]); however, the remaining three outcomes were not significantly changed following correction for publication bias.
Figure 5.
Funnel plots and publication bias assessment for response rates (top left), remission rates (top right), depression rating scores (upper middle left), suicidality (upper middle right), completion (lower middle left), drop-outs (lower middle right), and drop-outs due to adverse events (bottom left)
Study quality and risk of bias
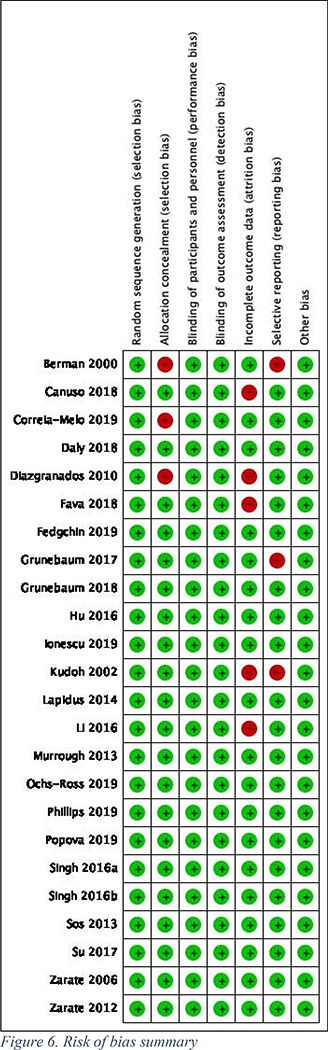
The overall quality of the 24 trials included in the meta-analysis was very high, with only a handful of studies having any “high risk” domains (Figure 6).
Figure 6.
Risk of bias summary
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis that has compared the performance of intravenous ketamine to intranasal esketamine for the treatment of unipolar and bipolar depression. Relative to intranasal esketamine, intravenous racemic ketamine demonstrated more significant overall response and remission rates, as well as lower drop-outs due to adverse events. In contrast, we did not find any significant differences between the effect of racemic ketamine or esketamine in TRD vs. non-TRD or between MDD vs. BD populations. Thus, while intravenous racemic ketamine tended to outperform intranasal ketamine, the specific differences at the subgroup level were nonsignificant. Furthermore, this points to a need for additional head-to-head studies in order to determine the specific reasons for this finding.
Several previous reviews have demonstrated the merits of intravenous racemic ketamine for the treatment of depression, either as a standalone treatment or in combination with electroconvulsive therapy (Caddy et al., 2015; Corriger and Pickering, 2019; Fond et al., 2014; Lee et al., 2015; McCloud et al., 2015; McGirr et al., 2015; Xu et al., 2016). While the present data suggest that intravenous racemic ketamine is superior to intranasal esketamine, the latter is FDA-approved and has more long-term data and larger sample sizes. The evidence base to date would suggest the recommendation of intravenous ketamine over intranasal esketamine for treatment-resistant major depressive disorders, as there are no published studies on the efficacy of the latter for the treatment of bipolar depression. In contrast, several prior studies indicate that there is a role for intravenous ketamine in the treatment of bipolar depression (Alberich et al., 2017; Bobo et al., 2016; Gałuszko-Węgielnik et al., 2019; Ionescu et al., 2015; Kraus et al., 2017; López-Díaz et al., 2017). In the present meta-analysis, there was no significant difference in clinical response between patients with unipolar major depression and bipolar depression to intravenous ketamine. Thus, it remains somewhat unclear if the clinical responsiveness to ketamine differs between patients with major depression or bipolar depression. For very short-term use, the available data demonstrates a clear and consistent antidepressive effect of ketamine vs esketamine treatment, relative to a variety of control conditions, beginning within hours of administration, and lasting up to 7 days after a single dose.
There is a real necessity in our therapeutic armamentarium for discovering and adding more effective and safer treatments for patients who have an unsatisfactory response or intolerable side effects to the current conventional antidepressive treatments (Gao et al., 2016). All studies of ketamine and esketamine for major depression enrolled patients that were resistant to one or more conventional antidepressants, second-generation antipsychotics or mood-stabilizing medications. However, the specific definitions of TRD varied, with the minimum number of unsuccessful trials required for trial participation ranging from one to three, indicating ketamine’s role as a ‘last resort’ treatment. Thus, it remains unclear how ketamine may perform in individuals with non-TRD depression (Aan Het Rot et al., 2012).
Part of the challenge in elucidating the comparative performance of different formulations of ketamine may lie in the lack of a clear consensus on the mechanisms underlying ketamine’s therapeutic effects (Strasburger et al., 2017; Zanos and Gould, 2018). While intravenous racemic ketamine has more side effects than intranasal esketamine, a recent open-label trial with the former seemed to have lower dissociative side effects. Ketamine blockade of glutamatergic neurotransmission via antagonism of the NMDA pathway promotes AMPA receptor activation (Aleksandrova et al., 2017; Zorumski et al., 2016). AMPA activation triggers second messenger pathways required for several neuroplastic changes, ultimately conferring the rapid and sustained antidepressant effects of ketamine (Evans et al., 2018; Maeng and Zarate, 2007).
While the antagonism of the NMDA pathway represents the primary antidepressant mechanism of ketamine, some studies have implied a role for opioid neurotransmission, as ketamine also appears to activate the mu, kappa, and delta-opioid receptors (Finck and Ngai, 1982; Freye et al., 1994, p.; Jonkman et al., 2018; Sarton et al., 2001). While the precise implications of these properties are currently under investigation, available studies indicate that the endogenous opioid system plays a role in mediating the antidepressant properties of ketamine (Mathew and Rivas-Grajales, 2019; Williams et al., 2019, 2018). To that end, the antidepressant effects of ketamine appear to require the activation of the opioid system, as the administration of the opioid antagonist naloxone abolishes the antidepressant properties of ketamine (Williams et al., 2018); however, another study contested these findings, claiming a lack of opioid system involvement in the antidepressant effects of ketamine (Zhang and Hashimoto, 2019b). Still, the role of the opioid system to ketamine’s antidepressant effects remains unclear and must consider the risk of abuse.
Outside of depressive contexts, ketamine is an adjuvant to opioid-based pharmacotherapy of pain (Bell et al., 2003). Ketamine appears to counter opioid-induced respiratory depression (Jonkman et al., 2018), which suggests that there may be a farther-reaching interplay between the ketamine and opioid neurotransmitter systems outside of only depression. Furthermore, ketamine and esketamine have shown great potential as potent and rapid anti-suicidal agents (Grunebaum et al., 2018; López-Díaz et al., 2017; Reinstatler and Youssef, 2015; Wilkinson et al., 2018; Williams et al., 2019; Witt et al., 2020). Given the current limitations of most existing treatments for reducing suicide ideations and plans in patients suffering from moderate to severe major depression, this additional property of ketamine may be helpful in the emergent management of patients in acute crisis.
Limitations
Although this review has several strengths, a few fundamental limitations deserve some expansion here.
While the risk of bias assessments indicated that there was a low level of bias in individual studies, there was significant publication bias at the review-level. Thus, negative studies—particularly regarding response and remission rates—may not have been identified by our search protocol, which may inflate the effect sizes.
Our review attempted to cover as much follow-up time as possible following the administration of ketamine treatment, there remains minimal information regarding longer-term follow-up. The longest trials considered by this review only offered a follow-up to the four to the eight-week mark. Hence, the results of our study are also limited to this treatment window; extrapolation beyond this point is beyond the scope of the presented analyses.
Participants in the trials were mostly unrepresentative of the real-world population with depression. While some of the trials captured individuals with treatment-resistant depression, most trials excluded participants who had significant psychiatric or medical comorbidity, which is an unlikely scenario in most clinical settings. Thus, the results of the trials may not represent the real-world efficacy of ketamine.
One of the paper’s main aims was to evaluate the acceptability of racemic ketamine and esketamine. However, we only reported on dropout rates and general adverse event rates). Unfortunately, we could not report on specific side effects given inconsistent reporting across studies for dissociation, headaches, nausea, or other adverse effects.
In our review, we observed greater efficacy ratings for intravenous racemic ketamine in terms of response and remission. However, this superiority in performance appeared to drop after the fourth week after administration, when only the reduction of depression scale scores was observed. Thus, when appraising the relative efficacy of racemic ketamine to intranasal esketamine, one must also consider the timepoint.
The high heterogeneity within the selected studies could have impacted our results. Specifically, there were differences between unipolar and bipolar depressive patients, and patients with TRD vs. non-TRD. As well, some studies explored single doses while others involved repeated administration of ketamine (for example, Singh et al. 2016 and Ionescu et al. 2019 used repeated ketamine administration). Finally, some RCTs administered ketamine as a monotherapy, while others used it in augmentation with other psychotropics. We accounted for these sources of heterogeneity using subgroup analyses and meta-regression, however, statistical strategies can only account for measurable contributions. Hence, it is likely that there is unmeasurable, residual heterogeneity in our review.
Conclusions
This review finds that relative to intranasal esketamine, intravenous ketamine demonstrated more significant overall response and remission rates, as well as lower drop-outs due to adverse events. It is essential to underscore that, in contrast to esketamine, there is no current FDA approval of racemic ketamine for the treatment of major bipolar or unipolar depression (Commissioner, 2019; Kim et al., 2019). Therefore, the prescription of racemic ketamine for the treatment of depression remains an off-label intervention. While racemic ketamine has demonstrated significant short-term benefits in several clinical studies, the long-term benefits remain insufficiently explored, and this may be a contributor to the current lack of FDA approval for racemic ketamine. At present, the level of proof of efficacy remains low and more randomized controlled trials are needed to explore efficacy and safety issues for the administration of all forms of ketamine in the treatment of depression. Moreover, although ketamine represents an innovative, rapidly acting, experimental treatment for bipolar and unipolar depression, the route of administration presents a practical limitation that has been solved to some extent with the intranasal formulation of esketamine.
Supplementary Material
Highlights.
We reviewed the peer-reviewed academic literature to synthesize evidence for the comparative efficacy and acceptability of racemic ketamine and esketamine.
24 randomized controlled trials were identified and data across studies were pooled by way of systematic review and meta-analysis.
24 trials representing 1877 participants were pooled. Racemic ketamine relative to esketamine demonstrated greater overall response (RR = 3.01 vs. RR = 1.38) and remission rates (RR = 3.70 vs. RR = 1.47), as well as lower dropouts (RR = 0.76 vs. RR = 1.37).
Racemic ketamine appears to be more efficacious than esketamine for the treatment of depression. Head to head comparisons are needed to confirm the present findings.
Appendix 1. Search Strategy
MEDLINE: inception to December 19, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | ketamine.mp. or exp Ketamine/ | 20289 |
| 2. | depression.mp. or exp Depression/ | 407001 |
| 3. | 1 and 2 | 2444 |
| 4. | limit 3 to humans | 1126 |
| 5. | limit 4 to randomized controlled trial | 189 |
PsycINFO: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | exp Ketamine/ or ketamine.mp. | 3745 |
| 2. | exp Major Depression/ or exp Treatment Resistant Depression/ or depression.mp. | 332697 |
| 3. | 1 and 2 | 1256 |
| 4. | limit 3 to (human and “0300 clinical trial”) | 99 |
EMBASE: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | ketamine.mp. or exp ketamine/ | 42871 |
| 2. | exp depression/ or depression.mp. | 720295 |
| 3. | 1 and 2 | 6555 |
| 4. | limit 3 to human | 5033 |
| 5. | limit 4 to (clinical trial or randomized controlled trial or controlled clinical trial or multicenter study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial) | 1040 |
Cochrane Library: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | Ketamine | 5025 |
| 2. | Depression | 76586 |
| 3. | 1 and 2 | 896 |
ClinicalTrials.gov: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | “Ketamine” and “depression” | 190 |
The EU Clinical Trials Register: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | “Ketamine” and “depression” | 37 |
The Australian and New Zealand Clinical Trials Registry: inception to December 13, 2019
| Step | Search Criteria | Citations |
|---|---|---|
| 1. | “Ketamine” and “depression” | 43 |
Footnotes
Financial Disclosures: nil
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aan Het Rot M, Zarate CAJ, Charney DS, Mathew SJ, 2012. Ketamine for depression: where do we go from here? Biol. Psychiatry 72, 537–547. 10.1016/j.biopsych.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, Krystal JH, 2015. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu. Rev. Med. 66, 509–523. 10.1146/annurev-med-053013-062946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberich S, Martínez-Cengotitabengoa M, López P, Zorrilla I, Núñez N, Vieta E, González-Pinto A, 2017. Efficacy and safety of ketamine in bipolar depression: A systematic review. Rev. Psiquiatr. Salud Ment. 10, 104–112. 10.1016/j.rpsm.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Aleksandrova LR, Phillips AG, Wang YT, 2017. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J. Psychiatry Neurosci. JPN 42, 222–229. 10.1503/jpn.160175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabzadeh S, Hakkikazazi E, Shahmansouri N, Tafakhori A, Ghajar A, Jafarinia M, Akhondzadeh S, 2018. Does oral administration of ketamine accelerate response to treatment in major depressive disorder? Results of a double-blind controlled trial. J. Affect. Disord. 235, 236–241. 10.1016/j.jad.2018.02.056 [DOI] [PubMed] [Google Scholar]
- Atigari OV, Healy D, 2013. Sustained antidepressant response to ketamine. BMJ Case Rep. 2013. 10.1136/bcr-2013-200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Eccleston C, Kalso E, 2003. Ketamine as an adjuvant to opioids for cancer pain. Cochrane Database Syst. Rev CD003351 10.1002/14651858.CD003351 [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH, 2000. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 47, 351–354. 10.1016/S0006-3223(99)00230-9 [DOI] [PubMed] [Google Scholar]
- Bobo WV, Vande Voort JL, Croarkin PE, Leung JG, Tye SJ, Frye MA, 2016. Ketamine for Treatment-Resistant Unipolar and Bipolar Major Depression: Critical Review and Implications for Clinical Practice. Depress. Anxiety 33, 698–710. 10.1002/da.22505 [DOI] [PubMed] [Google Scholar]
- Burger J, Capobianco M, Lovern R, Boche B, Ross E, Darracq MA, McLay R, 2016. A Double-Blinded, Randomized, Placebo-Controlled Sub-Dissociative Dose Ketamine Pilot Study in the Treatment of Acute Depression and Suicidality in a Military Emergency Department Setting . Mil. Med. 181, 1195–1199. 10.7205/MILMED-D-15-00431 [DOI] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A, 2015. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst. Rev 10.1002/14651858.CD011612.pub2 [DOI] [PubMed] [Google Scholar]
- Canuso CM, Singh JB, Fedgchin M, Alphs L, Lane R, Lim P, Pinter C, Hough D, Sanacora G, Manji H, Drevets WC, 2018. Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of Symptoms of Depression and Suicidality in Patients at Imminent Risk for Suicide: Results of a Double-Blind, Randomized, Placebo-Controlled Study. Am. J. Psychiatry 175, 620–630. 10.1176/appi.ajp.2018.17060720 [DOI] [PubMed] [Google Scholar]
- Charlson F, Ommeren M. van, Flaxman A, Cornett J, Whiteford H, Saxena S, 2019. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. The Lancet 394, 240–248. 10.1016/S0140-6736(19)30934-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilukuri H, Reddy NP, Pathapati RM, Manu AN, Jollu S, Shaik AB, 2014. Acute antidepressant effects of intramuscular versus intravenous ketamine. Indian J. Psychol. Med. 36, 71 10.4103/0253-7176.127258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane Collaboration, 2014. Cochrane Handbook: General Methods for Cochrane Reviews. [WWW Document]. Heterogeneity; URL https://handbook-5-1.cochrane.org/chapter_9/9_5_heterogeneity.htm (accessed 7.17.19). [Google Scholar]
- Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, Viscusi ER, Narouze S, Davis FN, Ritchie EC, Lubenow TR, Hooten WM, 2018. Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med 43, 521–546. 10.1097/AAP.0000000000000808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commissioner, O. of the, 2019. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic [WWW Document]. FDA; URL http://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified (accessed 2.5.20). [Google Scholar]
- Correia-Melo FS, Leal GC, Carvalho MS, Jesus-Nunes AP, Ferreira CBN, Vieira F, Magnavita G, Vale LAS, Mello RP, Nakahira C, Argolo FC, Cardoso T, Souza CDS, Fontes ATC, Ferreira MB, Araújo-de-Freitas L, Tuena MA, Echegaray MVF, Cavalcanti DE, Lucchese AC, Bandeira ID, Telles M, Lima CS, Sampaio AS, Silva SS, Marback RF, Del-Porto JA, Abreu JN, Sarin LM, Paixão CS, Carvalho LP, Machado PRL, Turecki G, Lacerda ALT, Quarantini LC, 2018. Comparative study of esketamine and racemic ketamine in treatment-resistant depression. Medicine (Baltimore) 97 10.1097/MD.0000000000012414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia-Melo FS, Leal GC, Vieira F, Jesus-Nunes AP, Mello RP, Magnavita G, Caliman-Fontes AT, Echegaray MVF, Bandeira ID, Silva SS, Cavalcanti DE, Araújo-de-Freitas L, Sarin LM, Tuena MA, Nakahira C, Sampaio AS, Del-Porto JA, Turecki G, Loo C, Lacerda ALT, Quarantini LC, 2020. Efficacy and safety of adjunctive therapy using esketamine or racemic ketamine for adult treatment-resistant depression: A randomized, double-blind, non-inferiority study. J. Affect. Disord. 264, 527–534. 10.1016/j.jad.2019.11.086 [DOI] [PubMed] [Google Scholar]
- Corriger A, Pickering G, 2019. Ketamine and depression: a narrative review. Drug Des. Devel. Ther. 13, 3051–3067. 10.2147/DDDT.S221437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, Thase ME, Winokur A, Van Nueten L, Manji H, Drevets WC, 2018. Efficacy and Safety of Intranasal Esketamine Adjunctive to Oral Antidepressant Therapy in Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry 75, 139–148. 10.1001/jamapsychiatry.2017.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CAJ, 2010. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry 67, 793–802. 10.1001/archgenpsychiatry.2010.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domany Y, Bleich-Cohen M, Tarrasch R, Meidan R, Litvak-Lazar O, Stoppleman N, Schreiber S, Bloch M, Hendler T, Sharon H, 2019. Repeated oral ketamine for out-patient treatment of resistant depression: randomised, double-blind, placebo-controlled, proof-of-concept study. Br. J. Psychiatry 214, 20–26. 10.1192/bjp.2018.196 [DOI] [PubMed] [Google Scholar]
- Evans JW, Szczepanik J, Brutsche N, Park LT, Nugent AC, Zarate CAJ, 2018. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol. Psychiatry 84, 582–590. 10.1016/j.biopsych.2018.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fava M, Freeman MP, Flynn M, Judge H, Hoeppner BB, Cusin C, Ionescu DF, Mathew SJ, Chang LC, Iosifescu DV, Murrough J, Debattista C, Schatzberg AF, Trivedi MH, Jha MK, Sanacora G, Wilkinson ST, Papakostas GI, 2018. Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol. Psychiatry 1–12. 10.1038/s41380-018-0256-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, Vitagliano D, Blier P, Fava M, Liebowitz M, Ravindran A, Gaillard R, Ameele HVD, Preskorn S, Manji H, Hough D, Drevets WC, Singh JB, 2019. Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). Int. J. Neuropsychopharmacol. 22, 616–630. 10.1093/ijnp/pyz039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck AD, Ngai SH, 1982. Opiate receptor mediation of ketamine analgesia. Anesthesiology 56, 291–297. 10.1097/00000542-198204000-00011 [DOI] [PubMed] [Google Scholar]
- First MB, 2015. Structured Clinical Interview for the DSM (SCID), in: The Encyclopedia of Clinical Psychology. American Cancer Society, pp. 1–6. 10.1002/9781118625392.wbecp351 [DOI] [Google Scholar]
- Fond G, Loundou A, Rabu C, Macgregor A, Lancon C, Brittner M, Micoulaud-Franchi J-A, Richieri R, Courtet P, Abbar M, Roger M, Leboyer M, Boyer L, 2014. Ketamine administration in depressive disorders: a systematic review and meta-analysis. [Review]. Psychopharmacology (Berl.) 231, 3663–3676. 10.1007/s00213-014-3664-5 [DOI] [PubMed] [Google Scholar]
- Freye E, Latasch L, Schmidhammer H, Portoghese P, 1994. [Interaction of S-(+)-ketamine with opiate receptors. Effects on EEG, evoked potentials and respiration in awake dogs]. Anaesthesist 43 Suppl 2, S52–58. [PubMed] [Google Scholar]
- Gałuszko-Węgielnik M, Wiglusz MS, Słupski J, Szałach Ł, Włodarczk A, Górska N, Szarmach J, Jakuszkowiak-Wojten K, Wilkowska A, Cubała WJ, 2019. Efficacy of Ketamine in bipolar depression: focus on anhedonia. Psychiatr. Danub. 31, 554–560. [PubMed] [Google Scholar]
- Galvez V, Li A, Huggins C, Glue P, Martin D, Somogyi AA, Alonzo A, Rodgers A, Mitchell PB, Loo CK, 2018. Repeated intranasal ketamine for treatment-resistant depression - the way to go? Results from a pilot randomised controlled trial. J. Psychopharmacol. Oxf. Engl. 32, 397–407. 10.1177/0269881118760660 [DOI] [PubMed] [Google Scholar]
- Gao M, Rejaei D, Liu H, 2016. Ketamine use in current clinical practice. Acta Pharmacol. Sin. 37, 865–872. 10.1038/aps.2016.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D, Gálvez V, Martin D, Kumar D, Leyden J, Hadzi-Pavlovic D, Harper S, Brodaty H, Glue P, Taylor R, Mitchell PB, Loo CK, 2017. Pilot Randomized Controlled Trial of Titrated Subcutaneous Ketamine in Older Patients with Treatment-Resistant Depression . Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 25, 1199–1209. 10.1016/j.jagp.2017.06.007 [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Kazemi MH, Yoosefi A, Ghasemi A, Paragomi P, Amini H, Afzali MH, 2014. Rapid antidepressant effects of repeated doses of ketamine compared with electroconvulsive therapy in hospitalized patients with major depressive disorder. Psychiatry Res. 215, 355–361. 10.1016/j.psychres.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Ellis SP, Keilp JG, Moitra VK, Cooper TB, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2017. Ketamine versus midazolam in bipolar depression with suicidal thoughts: A pilot midazolam-controlled randomized clinical trial. Bipolar Disord. 19, 176–183. 10.1111/bdi.12487 [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS, Marver JE, Burke AK, Milak MS, Sublette ME, Oquendo MA, Mann JJ, 2018. Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression: A Midazolam-Controlled Randomized Clinical Trial. Am. J. Psychiatry 175, 327–335. 10.1176/appi.ajp.2017.17060647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Chen J, Zou D, Zheng P, Li Q, Wang H, Li P, Zhou X, Zhang Y, Liu Y, Xie P, 2016. Efficacy of ketamine in the rapid treatment of major depressive disorder: a meta-analysis of randomized, double-blind, placebo-controlled studies. Neuropsychiatr. Dis. Treat. 12, 2859–2867. 10.2147/NDT.S117146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Quinn S, Fazekas B, Plummer J, Eckermann S, Agar M, Spruyt O, Rowett D, Currow DC, 2012. Randomized, double-blind, placebo-controlled study to assess the efficacy and toxicity of subcutaneous ketamine in the management of cancer pain. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30, 3611–3617. 10.1200/JCO.2012.42.1081 [DOI] [PubMed] [Google Scholar]
- Hashimoto K, 2019. Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective. Psychiatry Clin. Neurosci. 73, 613–627. 10.1111/pcn.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yang C, 2019. Is (S)-norketamine an alternative antidepressant for esketamine? Eur. Arch. Psychiatry Clin. Neurosci. 269, 867–868. 10.1007/s00406-018-0922-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrman H, Kieling C, McGorry P, Horton R, Sargent J, Patel V, 2019. Reducing the global burden of depression: a Lancet–World Psychiatric Association Commission. The Lancet 393, e42–e43. 10.1016/S0140-6736(18)32408-5 [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JAC, 2011. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y-D, Xiang Y-T, Fang J-X, Zu S, Sha S, Shi H, Ungvari GS, Correll CU, Chiu HFK, Xue Y, Tian T-F, Wu A-S, Ma X, Wang G, 2016. Single i.v. ketamine augmentation of newly initiated escitalopram for major depression: results from a randomized, placebo-controlled 4-week study. Psychol. Med. 46, 623–635. 10.1017/S0033291715002159 [DOI] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CAJ, 2012. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 37, 1526–1533. 10.1038/npp.2011.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Bentley KH, Eikermann M, Taylor N, Akeju O, Swee MB, Pavone KJ, Petrie SR, Dording C, Mischoulon D, Alpert JE, Brown EN, Baer L, Nock MK, Fava M, Cusin C, 2019. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: A randomized, double blind, placebo controlled trial. J. Affect. Disord. 243, 516–524. 10.1016/j.jad.2018.09.037 [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Slonena EE, Voort JLV, Brutsche NE, Jr CAZ, 2014. Effect of Baseline Anxious Depression on Initial and Sustained Antidepressant Response to Ketamine. J. Clin. Psychiatry 75, 932–938. 10.4088/JCP.14m09049 [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CAJ, 2015. A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord. 17, 438–443. 10.1111/bdi.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionescu DF, Swee MB, Pavone KJ, Taylor N, Akeju O, Baer L, Nyer M, Cassano P, Mischoulon D, Alpert JE, Brown EN, Nock MK, Fava M, Cusin C, 2016. Rapid and Sustained Reductions in Current Suicidal Ideation Following Repeated Doses of Intravenous Ketamine: Secondary Analysis of an Open-Label Study . J. Clin. Psychiatry 77, e719–725. 10.4088/JCP.15m10056 [DOI] [PubMed] [Google Scholar]
- Jafarinia M, Afarideh M, Tafakhori A, Arbabi M, Ghajar A, Noorbala AA, Saravi MA, Agah E, Akhondzadeh S, 2016. Efficacy and safety of oral ketamine versus diclofenac to alleviate mild to moderate depression in chronic pain patients: A double-blind, randomized, controlled trial. J. Affect. Disord. 204, 1–8. 10.1016/j.jad.2016.05.076 [DOI] [PubMed] [Google Scholar]
- Jelen LA, King S, Stone JM, 2018. Alternatives to ketamine in depression: state-of-the-art and future perspectives. Ther. Adv. Psychopharmacol. 8, 95–98. 10.1177/2045125317749456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman K, van Rijnsoever E, Olofsen E, Aarts L, Sarton E, van Velzen M, Niesters M, Dahan A, 2018. Esketamine counters opioid-induced respiratory depression. Br. J. Anaesth. 120, 1117–1127. 10.1016/j.bja.2018.02.021 [DOI] [PubMed] [Google Scholar]
- Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA, 2019. Glutamatergic Neurotransmission: Pathway to Developing Novel Rapid-Acting Antidepressant Treatments . Int. J. Neuropsychopharmacol. 22, 119–135. 10.1093/ijnp/pyy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, Hasnain M, Jollant F, Levitt AJ, MacQueen GM, McInerney SJ, McIntosh D, Milev RV, Müller DJ, Parikh SV, Pearson NL, Ravindran AV, Uher R, 2016. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder. Can. J. Psychiatry Rev. Can. Psychiatr. 61, 540–560. 10.1177/0706743716659417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Farchione T, Potter A, Chen Q, Temple R, 2019. Esketamine for Treatment-Resistant Depression - First FDA-Approved Antidepressant in a New Class. N. Engl. J. Med. 381, 1–4. 10.1056/NEJMp1903305 [DOI] [PubMed] [Google Scholar]
- Kraus C, Rabl U, Vanicek T, Carlberg L, Popovic A, Spies M, Bartova L, Gryglewski G, Papageorgiou K, Lanzenberger R, Willeit M, Winkler D, Rybakowski JK, Kasper S, 2017. Administration of ketamine for unipolar and bipolar depression. Int. J. Psychiatry Clin. Pract. 21, 2–12. 10.1080/13651501.2016.1254802 [DOI] [PubMed] [Google Scholar]
- Kudoh A, Takahira Y, Katagai H, Takazawa T, 2002. Small-Dose Ketamine Improves the Postoperative State of Depressed Patients. Anesth. Analg. 95, 114 10.1097/00000539-200207000-00020 [DOI] [PubMed] [Google Scholar]
- Lapidus KAB, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, Feder A, Iosifescu DV, Charney DS, Murrough JW, 2014. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry 76, 970–976. 10.1016/j.biopsych.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EE, Della Selva MP, Liu A, Himelhoch S, 2015. Ketamine as a novel treatment for major depressive disorder and bipolar depression: a systematic review and quantitative meta-analysis. Gen. Hosp. Psychiatry 37, 178–184. 10.1016/j.genhosppsych.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Lener MS, Kadriu B, Zarate CA, 2017. Ketamine and Beyond: Investigations into the Potential of Glutamatergic Agents to Treat Depression. Drugs 77, 381–401. 10.1007/s40265-017-0702-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-T, Chen M-H, Lin W-C, Hong C-J, Yang B-H, Liu R-S, Tu P-C, Su T-P, 2016. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Hum. Brain Mapp. 37, 1080–1090. 10.1002/hbm.23085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Vlisides PE, 2016. Ketamine: 50 Years of Modulating the Mind. Front. Hum. Neurosci. 10 10.3389/fnhum.2016.00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLOS Med 6, e1000100 10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo CK, Galvez V, O’Keefe E, Mitchell PB, Hadzi-Pavlovic D, Leyden J, Harper S, Somogyi AA, Lai R, Weickert CS, Glue P, 2016. Placebo-controlled pilot trial testing dose titration and intravenous, intramuscular and subcutaneous routes for ketamine in depression. Acta Psychiatr. Scand. 134, 48–56. 10.1111/acps.12572 [DOI] [PubMed] [Google Scholar]
- López-Díaz Á, Fernández-González JL, Luján-Jiménez JE, Galiano-Rus S, Gutiérrez-Rojas L, 2017. Use of repeated intravenous ketamine therapy in treatment-resistant bipolar depression with suicidal behaviour: a case report from Spain. Ther. Adv. Psychopharmacol. 7, 137–140. 10.1177/2045125316675578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, 2007. The role of glutamate in mood disorders: Results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr. Psychiatry Rep. 9, 467–474. 10.1007/s11920-007-0063-1 [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Murrough JW, aan het Rot M, Collins KA, Reich DL, Charney DS, 2010. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int. J. Neuropsychopharmacol. 13, 71–82. 10.1017/S1461145709000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Rivas-Grajales AM, 2019. “Does the opioid system block or enhance the antidepressant effects of ketamine?” Chronic Stress Thousand Oaks Calif 3 10.1177/2470547019852073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C, Brett D, Amit BH, McShane R, Hamadi L, Hawton K, Cipriani A, 2015. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst. Rev CD011611 10.1002/14651858.CD011611.pub2 [DOI] [PubMed] [Google Scholar]
- McGirr A, Berlim MT, Bond DJ, Neufeld NH, Chan PY, Yatham LN, Lam RW, 2015. A systematic review and meta-analysis of randomized controlled trials of adjunctive ketamine in electroconvulsive therapy: efficacy and tolerability. J. Psychiatr. Res. 62, 23–30. 10.1016/j.jpsychires.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ, 2013a. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am. J. Psychiatry 170, 1134–1142. 10.1176/appi.ajp.2013.13030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Mathew SJ, Charney DS, 2011. A case of sustained remission following an acute course of ketamine in treatment-resistant depression. J. Clin. Psychiatry 72, 414–415. 10.4088/JCP.10l06447blu [DOI] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV, 2013b. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol. Psychiatry 74, 250–256. 10.1016/j.biopsych.2012.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, APA Council of Research Task Force on Novel Biomarkers and Treatments, 2015. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am. J. Psychiatry 172, 950–966. 10.1176/appi.ajp.2015.15040465 [DOI] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, Adler C, McShane R, Gaillard R, Wilkinson ST, Singh JB, 2020. Efficacy and Safety of Esketamine Nasal Spray Plus an Oral Antidepressant in Elderly Patients With Treatment-Resistant Depression-TRANSFORM-3. Am. J. Geriatr. Psychiatry Off. J. Am. Assoc. Geriatr. Psychiatry 28, 121–141. 10.1016/j.jagp.2019.10.008 [DOI] [PubMed] [Google Scholar]
- Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, Hough D, Manji H, Drevets WC, Sanacora G, Steffens DC, Adler C, McShane R, Gaillard R, Wilkinson ST, Singh JB, 2019. Efficacy and Safety of Esketamine Nasal Spray Plus an Oral Antidepressant in Elderly Patients With Treatment-Resistant Depression—TRANSFORM-3. Am. J. Geriatr. Psychiatry 10.1016/j.jagp.2019.10.008 [DOI] [PubMed] [Google Scholar]
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L, 2006. Comparison of two methods to detect publication bias in meta-analysis. JAMA 295, 676–680. 10.1001/jama.295.6.676 [DOI] [PubMed] [Google Scholar]
- Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, Owoeye O, Batten LA, Blier P, 2019. Single, Repeated, and Maintenance Ketamine Infusions for Treatment-Resistant Depression: A Randomized Controlled Trial . Am. J. Psychiatry 176, 401–409. 10.1176/appi.ajp.2018.18070834 [DOI] [PubMed] [Google Scholar]
- Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, Mazzucco C, Hough D, Thase ME, Shelton RC, Molero P, Vieta E, Bajbouj M, Manji H, Drevets WC, Singh JB, 2019. Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. Am. J. Psychiatry 176, 428–438. 10.1176/appi.ajp.2019.19020172 [DOI] [PubMed] [Google Scholar]
- Reinstatler L, Youssef NA, 2015. Ketamine as a Potential Treatment for Suicidal Ideation: A Systematic Review of the Literature. Drugs RD 15, 37–43. 10.1007/s40268-015-0081-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara MT, Spineli LM, Furukawa TA, Engel RR, Davis JM, Salanti G, Leucht S, 2013. Imputation of response rates from means and standard deviations in schizophrenia. Schizophr. Res. 151, 209–214. 10.1016/j.schres.2013.10.029 [DOI] [PubMed] [Google Scholar]
- Sarton E, Teppema LJ, Olievier C, Nieuwenhuijs D, Matthes HWD, Kieffer BL, Dahan A, 2001. The Involvement of the μ-Opioid Receptor in Ketamine-Induced Respiratory Depression and Antinociception . Anesth. Analg. 93, 1495–1500. 10.1097/00000539-200112000-00031 [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, 2019. A Word to the Wise About Intranasal Esketamine . Am. J. Psychiatry 176, 422–424. 10.1176/appi.ajp.2019.19040423 [DOI] [PubMed] [Google Scholar]
- Schwarzer G, 2007. meta: An R package for meta-analysis, R News; R, USA. [Google Scholar]
- Shah AA, 2016. Novel Approaches for Managing Treatment-Resistant Depression . Psychiatr. Ann. 46, 220–222. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–33;quiz 34–57. [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, Tadic A, Sienaert P, Wiegand F, Manji H, Drevets WC, Van Nueten L, 2016a. Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study . Biol. Psychiatry 80, 424–431. 10.1016/j.biopsych.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly EJ, De Boer P, Cooper K, Lim P, Pinter C, Murrough JW, Sanacora G, Shelton RC, Kurian B, Winokur A, Fava M, Manji H, Drevets WC, Van Nueten L, 2016b. A Double-Blind, Randomized, Placebo-Controlled, Dose-Frequency Study of Intravenous Ketamine in Patients With Treatment-Resistant Depression . Am. J. Psychiatry 173, 816–826. 10.1176/appi.ajp.2016.16010037 [DOI] [PubMed] [Google Scholar]
- Sleigh J, Harvey M, Voss L, Denny B, 2014. Ketamine – More mechanisms of action than just NMDA blockade. Trends Anaesth. Crit. Care 4, 76–81. 10.1016/j.tacc.2014.03.002 [DOI] [Google Scholar]
- Smith-Apeldoorn SY, Veraart JKE, Kamphuis J, van Asselt ADI, Touw DJ, aan het Rot M, Schoevers RA, 2019. Oral esketamine for treatment-resistant depression: rationale and design of a randomized controlled trial. BMC Psychiatry 19, 375 10.1186/s12888-019-2359-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sos P, Klirova M, Novak T, Kohutova B, Horacek J, Palenicek T, 2013. Relationship of ketamine’s antidepressant and psychotomimetic effects in unipolar depression. Neuro Endocrinol. Lett. 34, 287–293. [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Kroenke K, Linzer M, deGruy FV, Hahn SR, Brody D, Johnson JG, 1994. Utility of a New Procedure for Diagnosing Mental Disorders in Primary Care: The PRIME-MD 1000 Study. JAMA 272, 1749–1756. 10.1001/jama.1994.03520220043029 [DOI] [PubMed] [Google Scholar]
- Strasburger SE, Bhimani PM, Kaabe JH, Krysiak JT, Nanchanatt DL, Nguyen TN, Pough KA, Prince TA, Ramsey NS, Savsani KH, Scandlen L, Cavaretta MJ, Raffa RB, 2017. What is the mechanism of Ketamine’s rapid-onset antidepressant effect? A concise overview of the surprisingly large number of possibilities. J. Clin. Pharm. Ther. 42, 147–154. 10.1111/jcpt.12497 [DOI] [PubMed] [Google Scholar]
- Su T-P, Chen M-H, Li C-T, Lin W-C, Hong C-J, Gueorguieva R, Tu P-C, Bai Y-M, Cheng C-M, Krystal JH, 2017. Dose-Related Effects of Adjunctive Ketamine in Taiwanese Patients with Treatment-Resistant Depression. Neuropsychopharmacol. Off.Publ. Am. Coll. Neuropsychopharmacol. 42, 2482–2492. 10.1038/npp.2017.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration, 2014. Review Manager (RevMan), The Nordic Cochrane Centre; Copenhagen. [Google Scholar]
- Tibensky BN, de Léséleuc L, Perras C, Picheca L, 2016. Esketamine for Treatment-Resistant Depression, in: CADTH Issues in Emerging Health Technologies. Canadian Agency for Drugs and Technologies in Health, Ottawa (ON). [PubMed] [Google Scholar]
- Veritas Health Innovation, 2019. Covidence systematic review software. Melbourne, Australia. [Google Scholar]
- Vlerick L, Devreese M, Peremans K, Dockx R, Croubels S, Duchateau L, Polis I, 2020. Pharmacokinetics, absolute bioavailability and tolerability of ketamine after intranasal administration to dexmedetomidine sedated dogs. PLOS ONE 15, e0227762 10.1371/journal.pone.0227762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Chang L, Hashimoto K, 2020. A historical review of antidepressant effects of ketamine and its enantiomers. Pharmacol. Biochem. Behav. 190, 172870 10.1016/j.pbb.2020.172870 [DOI] [PubMed] [Google Scholar]
- Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, Sos P, Wang G, Zarate CA, Sanacora G, 2018. The Effect of a Single Dose of Intravenous Ketamine on Suicidal Ideation: A Systematic Review and Individual Participant Data Meta-Analysis. Am. J. Psychiatry 175, 150–158. 10.1176/appi.ajp.2017.17040472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Bentzley BS, Blasey C, Sudheimer KD, Hawkins J, Lyons DM, Schatzberg AF, 2019. Attenuation of antidepressant and antisuicidal effects of ketamine by opioid receptor antagonism. Mol. Psychiatry 24, 1779–1786. 10.1038/s41380-019-0503-4 [DOI] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, Schatzberg AF, 2018. Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. Am. J. Psychiatry 175, 1205–1215. 10.1176/appi.ajp.2018.18020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt K, Potts J, Hubers A, Grunebaum MF, Murrough JW, Loo C, Cipriani A, Hawton K, 2020. Ketamine for suicidal ideation in adults with psychiatric disorders: A systematic review and meta-analysis of treatment trials. Aust. N. Z. J. Psychiatry 54, 29–45. 10.1177/0004867419883341 [DOI] [PubMed] [Google Scholar]
- Xu Y, Hackett M, Carter G, Loo C, Gálvez V, Glozier N, Glue P, Lapidus K, McGirr A, Somogyi AA, Mitchell PB, Rodgers A, 2016. Effects of Low-Dose and Very Low-Dose Ketamine among Patients with Major Depression: a Systematic Review and Meta-Analysis . Int. J. Neuropsychopharmacol 19 10.1093/ijnp/pyv124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Gould TD, 2018. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 23, 801–811. 10.1038/mp.2017.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KSS, Fang Y, Huang X-P, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA, Gould TD, 2016. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, Pereira EFR, Albuquerque EX, Thomas CJ, Zarate CA, Gould TD, 2018. Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacol. Rev. 70, 621–660. 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK, 2006. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63, 856–864. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- Zarate CAJ, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA, 2012. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry 71, 939–946. 10.1016/j.biopsych.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr., Quiroz JA, Singh JB, Denicoff KD, De Jesus G, Luckenbaugh DA, Charney DS, Manji HK, 2005. An Open-Label Trial of the Glutamate-Modulating Agent Riluzole in Combination with Lithium for the Treatment of Bipolar Depression. Biol. Psychiatry 57, 430–432. 10.1016/j.biopsych.2004.11.023 [DOI] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K, 2019a. An update on ketamine and its two enantiomers as rapid-acting antidepressants. Expert Rev. Neurother. 19, 83–92. 10.1080/14737175.2019.1554434 [DOI] [PubMed] [Google Scholar]
- Zhang K, Hashimoto K, 2019b. Lack of Opioid System in the Antidepressant Actions of Ketamine. Biol. Psychiatry 85, e25–e27. 10.1016/j.biopsych.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Zorumski CF, Izumi Y, Mennerick S, 2016. Ketamine: NMDA Receptors and Beyond. J. Neurosci. 36, 11158–11164. 10.1523/JNEUROSCI.1547-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.