Abstract
Accurate translation of genetic information into proteins is vital for cell sustainability. ProXp-ala prevents proteome-wide Pro-to-Ala mutations by hydrolyzing misacylated Ala-tRNAPro, which is synthesized by prolyl-tRNA synthetase. Bacterial ProXp-ala was previously shown to combine a size-based exclusion mechanism with conformational and chemical selection for the recognition of the alanyl moiety, whereas tRNAPro is selected via recognition of tRNA acceptor-stem elements G72 and A73. The identity of these critical bases changed during evolution with eukaryotic cytosolic tRNAPro possessing a cytosine at the corresponding positions. The mechanism by which eukaryotic ProXp-ala adapted to these changes remains unknown. In this work, recognition of the aminoacyl moiety and tRNA acceptor stem by human (Homo sapiens, or Hs) ProXp-ala was examined. Enzymatic assays revealed that Hs ProXp-ala requires C72 and C73 in the context of Hs cytosolic tRNAPro for efficient deacylation of mischarged Ala-tRNAPro. The strong dependence on these bases prevents cross-species deacylation of bacterial Ala-tRNAPro or of Hs mitochondrial Ala-tRNAPro by the human enzyme. Similar to the bacterial enzyme, Hs ProXp-ala showed strong tRNA acceptor-stem recognition but differed in its amino acid specificity profile relative to bacterial ProXp-ala. Changes at conserved residues in both the Hs and bacterial ProXp-ala substrate-binding pockets modulated this specificity. These results illustrate how the mechanism of substrate selection diverged during the evolution of the ProXp-ala family, providing the first example of a trans-editing domain whose specificity evolved to adapt to changes in its tRNA substrate.
Keywords: transfer RNA (tRNA), aminoacyl-tRNA synthetase, evolution, translation, protein synthesis, protein–nucleic acid interaction, deacylation, editing, translational quality control, identity elements
Correct pairing of amino acids with their corresponding tRNAs by aminoacyl-tRNA synthetases (ARSs) is fundamental for faithful translation of genetic information into proteins. These enzymes are divided into two classes based on structural differences within their aminoacylation active sites. The chemical and structural similarities of certain classes of amino acids challenge the ability of the synthetic active site, wherein aminoacyl-adenylate formation occurs, to properly select only cognate amino acids at this first step. Consequently, ARSs frequently ligate tRNAs with the wrong amino acid during the second step of aminoacylation (1). If left uncorrected, misacylation may lead to the incorporation of amino acids in response to the wrong codon (i.e. mistranslation); accumulation of these errors has been linked to cell defects in diverse organisms (1–5). To prevent mistranslation, many ARSs have acquired quality control mechanisms that enable deacylation of tRNAs immediately after their synthesis in a reaction known as post-transfer editing. This reaction may occur in cis in a domain distinct from the aminoacylation active site, and seven members of both classes of ARSs possess such an editing domain (1). Class II synthetases have additionally been shown to edit aminoacyl-tRNAs in trans, that is, following substrate dissociation and rebinding (6–8). In addition, free-standing editing domains that are evolutionally related to the editing domains of three class II ARSs have been identified in all domains of life; these enzymes lack aminoacylation activity and catalyze deacylation exclusively in trans (8).
The critical role of aminoacyl-tRNA editing is highlighted by the different phenotypes associated with editing deficiencies (9–11). For example, murine cells harboring an editing-deficient transgene for valyl-tRNA synthetase transition to an apoptotic-like state because of the incorporation of Thr at Val codons (2), whereas a mild defect in the editing activity of AlaRS provokes ataxia in mice (3). In Escherichia coli, severe oxidative conditions inactivate the editing activity of threonyl-tRNA synthetase, which impairs cell growth (12), whereas heritable genome mutations have been identified in aging E. coli cells resulting from accumulation of translational errors from an editing-defective isoleucyl-tRNA synthetase (13).
Prolyl-tRNA synthetases (ProRSs) commonly misactivate Ala and Cys, which are smaller or similar in size to cognate Pro (14–17). In most bacteria, mistranslation of Pro codons with Ala is prevented by the ProRS editing domain, which is known as the insertion (INS) domain because of its location between the class II consensus motifs 2 and 3 (18). This domain specifically hydrolyzes Ala-tRNAPro but rejects Pro- and Cys-tRNAPro (14). The later error is corrected by a single-domain trans-editing enzyme known as YbaK, which is homologous to the INS domain but possesses unique specificity for Cys-tRNAs (19). Although tRNAPro misacylation with Ala is an inherent characteristic of ProRSs, the INS domain is not present in some bacteria, nor is it found in most eukaryotes (15). Instead, many organisms that lack INS encode ProXp-ala, another homolog of the INS domain that hydrolyzes Ala-tRNAPro in trans (20–22). Structural studies revealed that the INS domain from Enterococcus faecium and ProXp-ala from Caulobacter crescentus (Cc) share a conserved tertiary structure and key catalytic residues (23). Biochemical studies revealed that Cc ProXp-Ala and E. coli (Ec) ProRS share similar size-exclusion and chemical-based mechanisms for recognition of the aminoacyl moiety, limiting their ability to hydrolyze tRNAPro charged with cognate Pro, as well as other amino acids that are larger than Ala (24, 25). In contrast to their shared mechanism of amino acid recognition, INS and bacterial ProXp-ala developed distinct strategies for tRNAPro selection. The INS domain lacks intrinsic tRNA specificity and relies on the recognition of the tRNA anticodon by the anticodon binding domain of ProRS, whereas Cc ProXp-ala displays strong recognition of the acceptor-stem elements of bacterial tRNAPro (26). The tRNA specificity of both enzymes is crucial for preventing deacylation of cognate Ala-tRNAAla.
Bacterial tRNAPro sequences show high conservation of the A73 discriminator base and the C1:G72 base pair, which is unique to the tRNAPro acceptor stem (27). Cc ProXp-ala recognizes tRNAPro through specific interactions with G72 and A73 (26). These bases are also critical for aminoacylation of tRNAPro by bacterial ProRS (28); thus, they play a dual role in ensuring the correct aminoacylation of tRNAPro with Pro and in editing of misacylated Ala-tRNAPro. The identity of these bases at the top of the tRNAPro acceptor stem changed during evolution; eukaryotic cytosolic (cyto) tRNAPro encodes C73 and G1:C72 (Fig. 1A) (27). These differences in the acceptor stem prevent aminoacylation of human (Homo sapiens, or Hs) cyto tRNAPro by bacterial ProRS (29) as well as deacylation of Hs cyto Ala-tRNAPro (hereafter referred to as Hs Ala-tRNAPro) by bacterial ProXp-ala (26). Even though bacterial tRNAPro is a poor substrate for Hs ProRS, the human enzyme only weakly relies on recognition of the tRNAPro acceptor-stem bases, while strongly relying on interactions with the anticodon, as well as other structural features of Hs tRNAPro (30). A previous study using a variant of tRNAPro that could readily be misacylated with Ala by alanyl-tRNA synthetase (AlaRS) concluded that Hs ProXp-ala has amino acid specificity but lacks tRNA specificity (31). Here, we prepared native aminoacyl-tRNAPro substrates to investigate the amino acid and tRNA specificity of Hs ProXp-ala. Our findings suggest that the substrate-binding pocket of Hs ProXp-ala displays different amino acid selectivity relative to that of the bacterial enzyme, but the human enzyme maintains strong acceptor-stem recognition. We also identified residues in the substrate-binding pocket of both bacterial and Hs ProXp-ala that modulate their amino acid specificity. The results reported here provide insights into the evolutionary relationship between class II aminoacyl-tRNA synthetases and editing domains.
Figure 1.
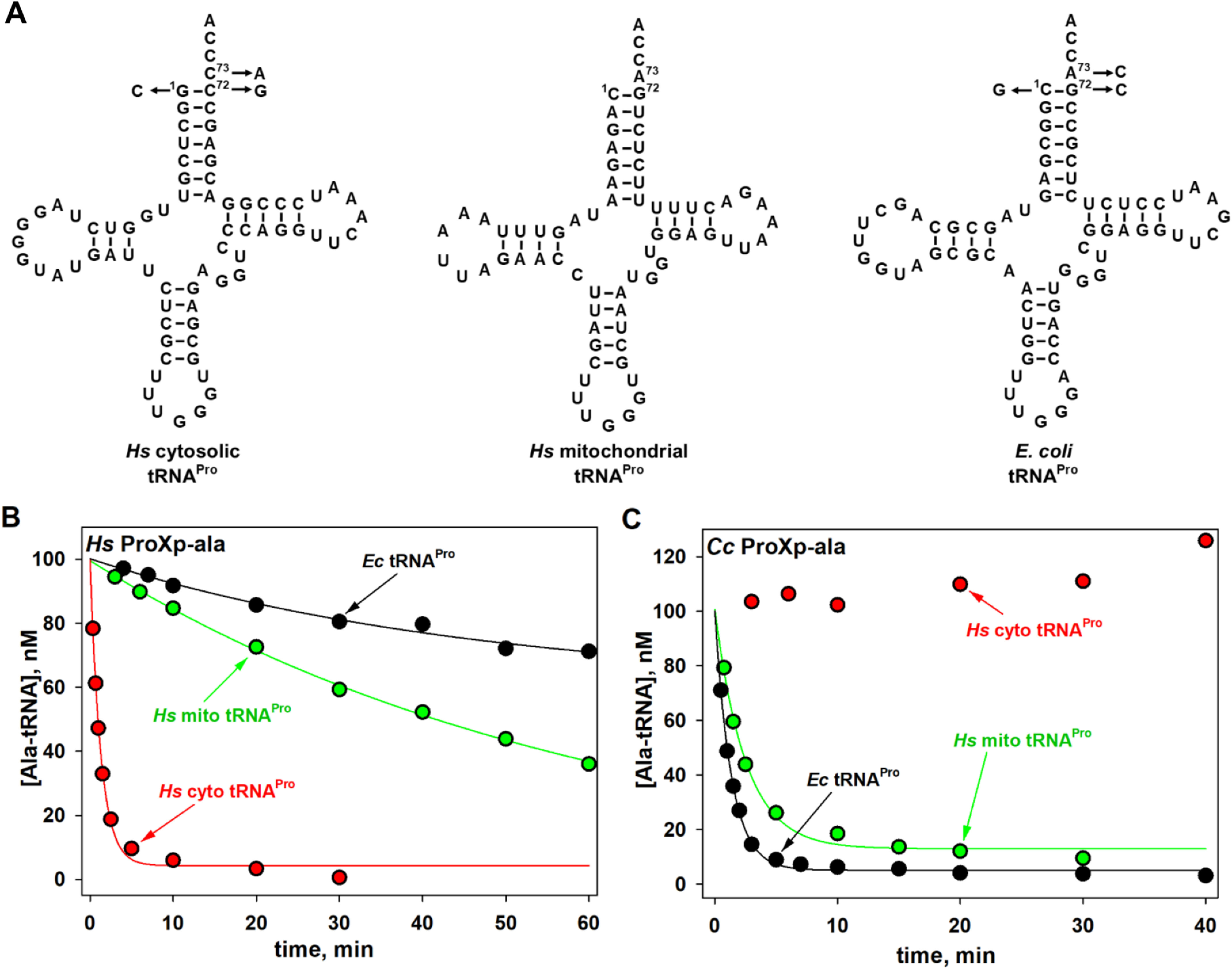
Cross-species deacylation of Hs and Cc ProXp-ala. A, predicted secondary structures of Hs cyto, mito, and Ec tRNAPro. Arrows indicate the acceptor-stem positions mutated in this study. B, deacylation of Hs cyto, mito, and Ec Ala-tRNAPro by Hs ProXp-ala. C, deacylation of Hs cyto, mito, and Ec Ala-tRNAPro by Cc ProXp-ala. Representative time courses of three independent experiments are shown. The reactions were performed with 1.5 μm Hs ProXp-ala and 100 nm Ala-tRNAPro at 30 °C (B) or with 0.75 μm Cc ProXp-ala and 100 nm Ala-tRNAPro at 18 °C (C), as described under “Experimental procedures.”
Results
Hs ProXp-ala robustly deacylates Ala-tRNAPro
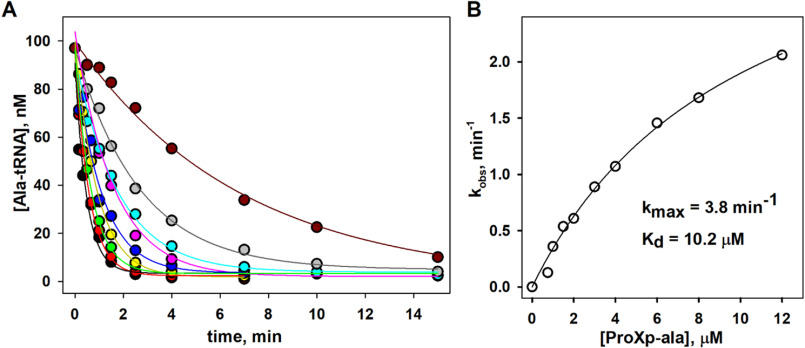
In this work, we used in vitro transcribed Hs cyto tRNAPro (Fig. 1A) and full-length recombinant Hs ProXp-ala expressed and purified in E. coli. To obtain sufficient yield of misacylated Hs Ala-tRNAPro substrates without the need for tRNA acceptor-stem mutation, we used flexizyme technology, which allows tRNAs to be charged with virtually any amino acid (proteinogenic or nonproteinogenic) (Fig. S1) (32). Our initial experiments showed that Hs ProXp-ala robustly deacylated Ala-tRNAPro in vitro. Based on an analysis of Hs Ala-tRNAPro deacylation by varying concentrations of Hs ProXp-ala under single-turnover conditions, we determined an apparent dissociation constant (Kd) of 10.2 μm and a maximum rate constant (kmax) of 3.8 min−1 (Fig. 2). Relative to the cyto tRNA, much weaker deacylation of Hs mitochondrial (mito) Ala-tRNAPro by Hs ProXp-ala was observed (Fig. 1B). Conversely, Cc ProXp-Ala deacylates mito Ala-tRNAPro with similar efficiency as bacterial Ala-tRNAPro (Fig. 1C). We hypothesize that differences in the acceptor-stem sequences of these two tRNAs (Fig. 1A) may account for these differences in deacylation activity.
Figure 2.
Deacylation of mischarged Hs cytosolic Ala-tRNAPro by Hs ProXp-ala under single-turnover conditions. A, time course of Ala-tRNAPro deacylation with varying concentrations (0.75, 1.0, 1.5, 2.0, 3.0, 4.0, 6.0, 8.0, and 12 μm) of Hs ProXp-ala. The reactions were performed with 100 nm Hs Ala-tRNAPro at 30 °C. Deacylation curves were fit to a single exponential equation. B, hyperbolic fit of the observed rate constant versus Hs ProXp-ala concentration yielded a kmax of 3.8 min−1 and a Kd, app of 10.2 μm. The results are based on a single experiment at each enzyme concentration.
Human ProXp-ala specificity is determined by tRNA acceptor-stem elements
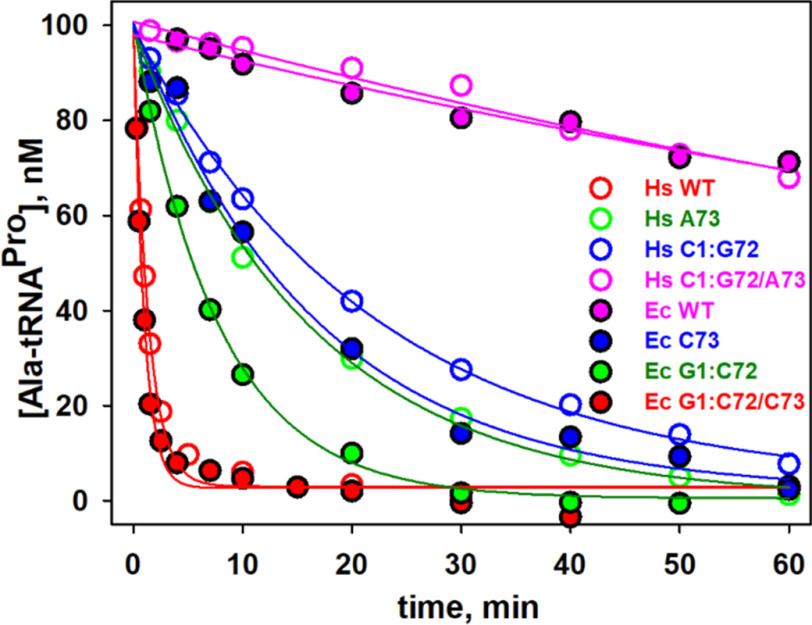
We previously showed that bacterial ProXp-ala cannot efficiently deacylate Hs cyto Ala-tRNAPro because of the differences in the identities of the bases at positions 72 and 73 in this eukaryotic tRNAPro (Fig. 1C) (26). To test whether Hs ProXp-ala exhibits cross-species deacylation capability, assays with Ec Ala-tRNAPro were performed under single-turnover conditions. We found that Hs ProXp-ala only weakly deacylated Ec Ala-tRNAPro with a 66-fold decrease in kobs (Fig. 3 and Table 1). This is consistent with the observation that Hs mito Ala-tRNAPro (which shares the C1:G72 and A73 acceptor-stem elements of Ec tRNAPro) is a poor substrate for Hs ProXp-ala (Fig. 1B). To identify the tRNA features that determine the specificity of Hs ProXp-ala, we prepared three Hs cyto tRNAPro acceptor-stem mutants (C73A, double mutant G1C/C72G, and triple mutant G1C/C72G/C73A) that mimic the acceptor stem of Ec tRNAPro (Fig. 1A). We found that the C73A and G1C/C72G tRNAPro mutants were hydrolyzed 13- and 16-fold slower than WT Ala-tRNAPro, respectively, whereas the triple mutant G1C/C72G/C73A tRNAPro was deacylated at an ∼100-fold reduced rate, similar to the decrease observed with Ec WT tRNAPro (Fig. 3 and Table 1). These data indicate that the first base pair and discriminator base of tRNAPro are important elements for Hs ProXp-ala recognition and deacylation activity. To further validate the role of these bases, we prepared a series of Ec tRNAPro acceptor-stem variants containing Hs tRNAPro elements (Fig. 1A). We found that Ec C73 and G1:C72 tRNAPro mutants were significantly better substrates for the human enzyme than Ec WT tRNAPro, whereas Ec G1:C72/A73 tRNAPro was deacylated slightly more efficiently than Hs WT tRNAPro (Fig. 3 and Table 1). Together, these results show that the deacylation activity of Hs ProXp-ala depends strongly on the identity of the nucleotides at the top of the acceptor stem of Hs cyto tRNAPro.
Figure 3.
Deacylation of Ala-tRNAPro acceptor-stem mutants by Hs ProXp-ala. Hs tRNAPro mutants (open circles) and Ec tRNAPro variants (filled circles). The same color was chosen for tRNAPro mutants with identical nucleotides at positions 1:72 and 73: Hs WT and Ec G1:C72/C73 (red), Hs A73 and Ec G1:C72 (green), Hs C1:G72 and Ec C73 (blue), and Hs C1:G72/A73 and Ec WT (magenta). The reactions were performed with 1.5 μm Hs ProXp-ala and 100 nm Ala-tRNAPro at 30 °C. Representative time courses of three independent experiments are shown.
Table 1.
Rate constants (kobs) for deacylation of Ala-tRNA variants by Hs ProXp-ala
Deacylation assays were performed under single-turnover conditions at 30 °C using 1.5 μm Hs ProXp-ala and 100 nm Ala-tRNA. All fold changes are relative to WT Hs tRNAPro. The results are the averages of three independent trials with the standard deviation indicated.
| tRNA | Acceptor stem | kobs | Fold change |
|---|---|---|---|
| min−1 | |||
| Hs tRNAPro WT | G1:C72/C73 | 0.79 ± 0.12 | |
| Hs tRNAPro A73 | G1:C72/A73 | 0.059 ± 0.007 | 13 |
| Hs tRNAPro C1:G72 | C1:G72/C73 | 0.049 ± 0.002 | 16 |
| Hs tRNAPro C1:G72/A73 | C1:G72/A73 | 0.007 ± 0.001 | 113 |
| Hs mito tRNAPro | C1:G72/A73 | 0.019 ± 0.005 | 42 |
| Ec tRNAPro WT | C1:G72/A73 | 0.012 ± 0.001 | 66 |
| Ec tRNAPro C73 | C1:G72/C73 | 0.071 ± 0.019 | 11 |
| Ec tRNAPro G1:C72 | G1:C72/A73 | 0.13 ± 0.009 | 6 |
| Ec tRNAPro G1:C72/C73 | G1:C72/C73 | 1.2 ± 0.16 | 0.66 |
| Ec tRNASer | G1:C72/G73 | 0.018 ± 0.001 | 44 |
| Ec tRNAAla | G1:C72/A73 | 0.055 ± 0.002 | 14 |
| Ec tRNACys | G1:C72/U73 | 0.098 ± 0.009 | 8 |
To further study the effect of different substitutions at the 73 position, we charged or mischarged three Ec tRNAs with Ala: tRNAAla, tRNASer, and tRNACys, which contain A73, G73, and U73, respectively (all have G1:C72). We found that the efficiency of Ala-tRNAAla deacylation is very similar to other G1:C73 tRNAPro variants with A73 (Table 1). Ala-tRNACys (U73) was a slightly better substrate, and Ala-tRNASer (G73) displayed the lowest kobs value of the three. These results emphasize a key role of the discriminator base for tRNA deacylation by Hs ProXp-ala.
Aminoacyl substrate specificity of Hs ProXp-ala
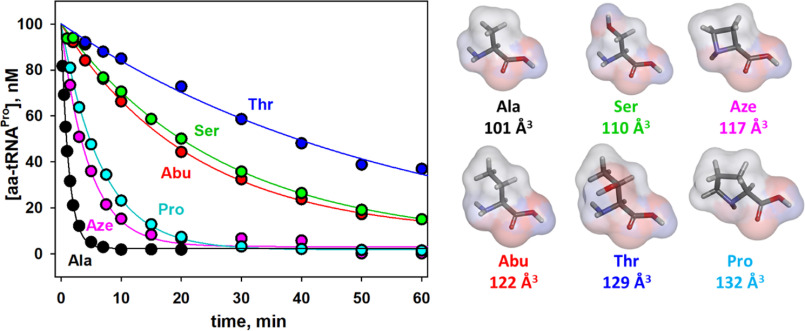
We next investigated the aminoacyl specificity of Hs ProXp-ala using Hs tRNAPro charged with amino acids of different classes including 2-aminobutyric acid (Abu), Ser, Thr, Pro, and azetidine-2-carboxylic acid (Aze) (Fig. 4). The deacylation rate of tRNAPro charged with the larger nonpolar Abu amino acid is 20-fold slower than Ala-tRNAPro (Table 2). Likewise, for tRNAPro charged with polar amino acids, the larger Thr-tRNAPro is a poorer substrate than Ser-tRNAPro. Thus, for these amino acids, the deacylation efficiency correlates with size of the aminoacyl moiety, with a preference for the smaller substrate. In contrast, Aze, a four-membered ring analog of Pro, with a molecular volume slightly larger than Ser, was deacylated 6-fold more efficiently than Ser and only 3.4-fold less efficiently than Ala. Aze-tRNAPro deacylation is also slightly greater (∼2-fold) than the rate of Pro-tRNAPro deacylation by the human enzyme, which surprisingly is only ∼5-fold lower than that of Ala-tRNAPro (Table 2).
Figure 4.
Hs ProXp-ala deacylation of Hs tRNAPro charged with various amino acids. Deacylation of Ala-tRNAPro (black), Aze-tRNAPro (magenta), Pro-tRNAPro (cyan), Abu-tRNAPro (red), Ser-tRNAPro (green), and Thr-tRNAPro (blue). The reactions were performed with 1.5 μm Hs ProXp-ala and 100 nm Hs aa-tRNAPro at 30 °C. Representative time courses of three independent experiments are shown. The structure and size of each amino acid, calculated as described under “Experimental procedures,” are shown on the right.
Table 2.
Rate constants (kobs) for deacylation of Hs aa-tRNAPro by Hs ProXp-ala
Deacylation assays were performed at 30 °C using 1.5 μm Hs ProXp-ala and 100 nm aa-tRNAPro. Fold change is reported relative to Hs Ala-tRNAPro. The results are the averages of three independent trials with the standard deviation indicated.
| Aminoacyl-tRNAPro | Amino acid volume | kobs | Fold change |
|---|---|---|---|
| Å3 | min−1 | ||
| Ala-tRNAPro | 101 | 0.79 ± 0.12 | |
| Abu-tRNAPro | 122 | 0.039 ± 0.007 | 20 |
| Ser-tRNAPro | 110 | 0.039 ± 0.003 | 20 |
| Thr-tRNAPro | 129 | 0.015 ± 0.002 | 53 |
| Aze-tRNAPro | 117 | 0.23 ± 0.026 | 3.4 |
| Pro-tRNAPro | 132 | 0.16 ± 0.012 | 5.0 |
The amino acid specificity of Hs ProXp-ala is tunable
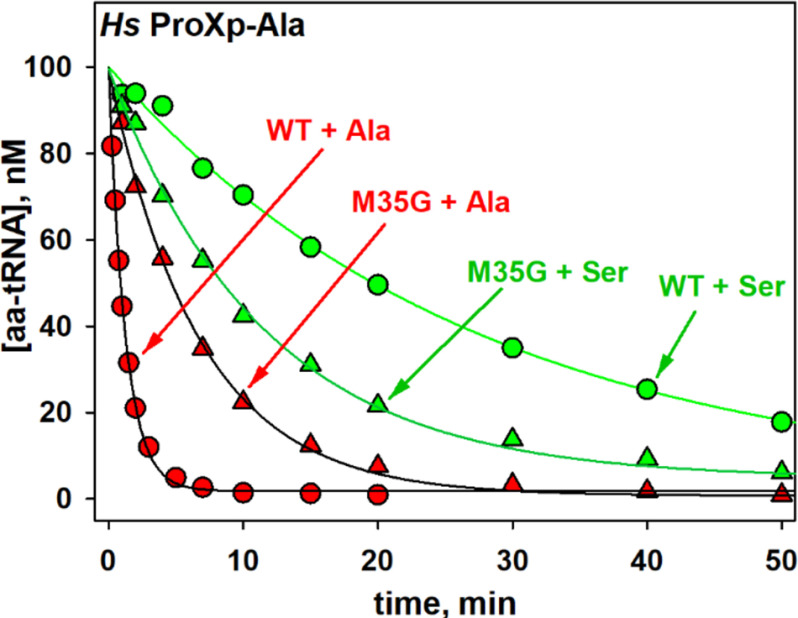
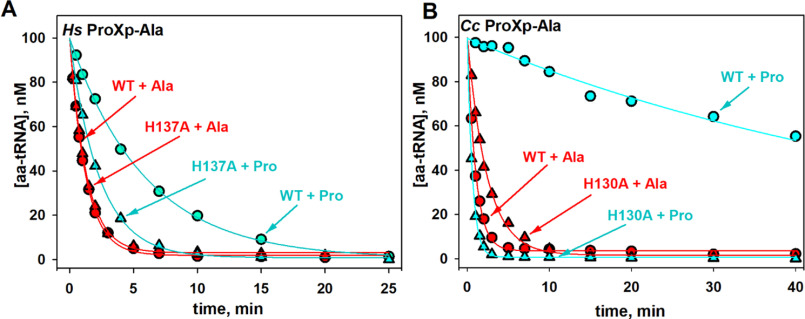
The aminoacyl specificity of the Ec INS editing domain is modulated by conserved residues in the substrate-binding pocket that include Leu266 and His369 (18, 25). Whether the corresponding residues play a role in determining the overall specificity of Hs ProXp-ala is unknown. Leu266 and His369 of Ec INS align with Met35 and His137 of Hs ProXp-ala, respectively (Fig. 5). We previously showed that Leu266 plays a role in the specificity of the INS toward Ser-tRNA; when this residue in Ec INS is mutated to Ala, Ala-tRNAPro is deacylated less efficiently, whereas the mutant enzyme hydrolyzes Ser-tRNAPro more robustly than WT INS (25). We now show that replacing Met35 of Hs ProXp-ala with smaller Gly increases the rate of Ser-tRNAPro deacylation by 2.3-fold while decreasing the rate of Ala-tRNAPro deacylation by 4.7-fold (Fig. 6 and Table 3). The highly conserved His residue in the substrate-binding pocket of Ec INS has previously been shown to function to prevent cognate substrate deacylation; mutation of this residue to Ala significantly increased Pro-tRNAPro deacylation (18). We now show that a homologous mutation (H137A) in Hs ProXp-ala also improves its activity toward Pro-tRNAPro, which is now deacylated only 2-fold weaker than Ala-tRNAPro by the same mutant (Fig. 7A and Table 3). Similarly, a Cc H130A ProXp-ala variant showed strong deacylation of Pro-tRNAPro (Fig. 7B). Interestingly, the Ala-tRNAPro deacylation activities of Cc H130A and Hs H137A ProXp-ala were not significantly different from those of the WT enzymes.
Figure 5.
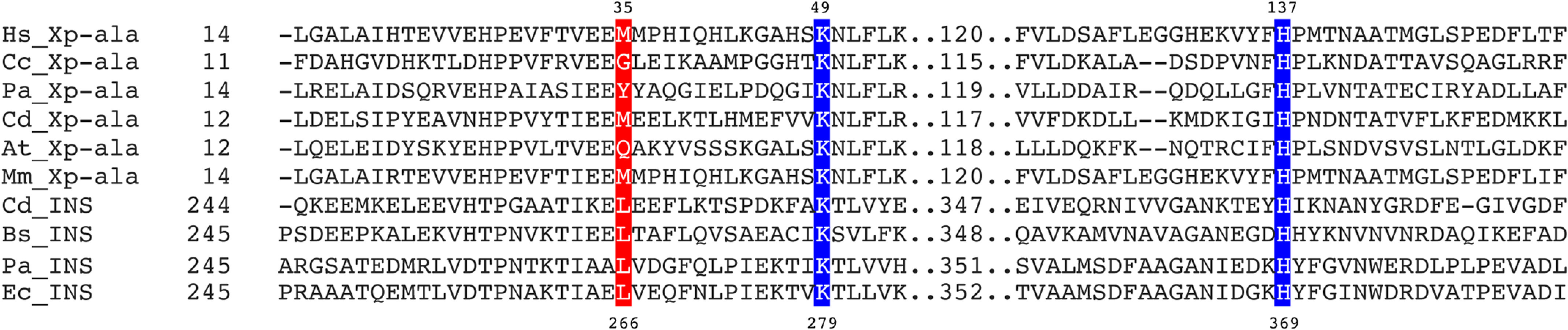
Multiple sequence alignment of ProRS INS and ProXp-ala. Sequences of Ec, Clostridioides difficile (Cd), Bacillus subtilis (Bs), and Pseudomonas aeruginosa (Pa) INS domains were aligned with Hs, M. musculus (Mm), A. thaliana (At), Cc, P. aeruginosa (Pa), and Cd ProXp-ala sequences using Clustal Omega (47). Corrections to the resulting alignment were made based on the structural alignment of Cc ProXp-ala (Protein Data Bank entry 5VXB) and Pa ProRS (Protein Data Bank entry 5UCM). Strictly and partially conserved residues within the ProXp-ala and INS families investigated here are highlighted in blue and red, respectively. Numbered residues correspond to the sequences of Ec INS and Hs ProXp-ala, respectively.
Figure 6.
Deacylation of Hs Ser- and Ala-tRNAPro by WT and mutant ProXp-ala. Deacylation of tRNAPro mischarged with Ala (red) and Ser (green) by WT (circles) and M35G (triangles) Hs ProXp-ala. The reactions were performed with 1.5 μm Hs ProXp-ala and 100 nm Hs aminoacyl-tRNAPro at 30 °C. Representative time courses of three independent experiments are shown.
Table 3.
Rate constants (kobs) for deacylation of Hs aa-tRNAPro by WT and mutant Hs ProXp-ala
Deacylation assays were performed at 30 °C using 1.5 μm Hs ProXp-ala and 100 nm Hs aa-tRNAPro. With the exception of Pro-tRNAPro deacylation by Hs ProXp-ala M35G (one trial), all results are the averages of three independent trials with the standard deviation indicated.
| Ala-tRNAPro |
Ser-tRNAPro |
Pro-tRNAPro |
|||
|---|---|---|---|---|---|
| kobs | kobs | Fold change (Ala/Ser) | kobs | Fold change (Ala/Pro) | |
| min−1 | min−1 | min−1 | |||
| WT | 0.79 ± 0.12 | 0.039 ± 0.003 | 20 | 0.16 ± 0.012 | 5.0 |
| M35G | 0.17 ± 0.008 | 0.089 ± 0.011 | 1.9 | 0.029 | 5.9 |
| H137A | 0.80 ± 0.019 | 0.045 ± 0.004 | 18 | 0.38 ± 0.056 | 2.1 |
Figure 7.
Deacylation of Ala- and Pro-tRNAPro by WT and mutant ProXp-ala. A, deacylation of Hs tRNAPro charged with Ala (red) and Pro (cyan) by WT (circles) and H137A (triangles) Hs ProXp-ala. B, deacylation of Ec tRNAPro charged with Ala (red) and Pro (cyan) by WT (circles) and H130A (triangles) Cc ProXp-ala. The reactions were performed with 1.5 μm Hs ProXp-ala and 100 nm Hs aminoacyl-tRNAPro at 30 °C or with 0.75 μm Cc ProXp-ala and 100 nm Ec aminoacyl-tRNAPro at 18 °C. Representative time courses of three independent experiments are shown.
Phylogeny and distribution of the ProXp-ala family
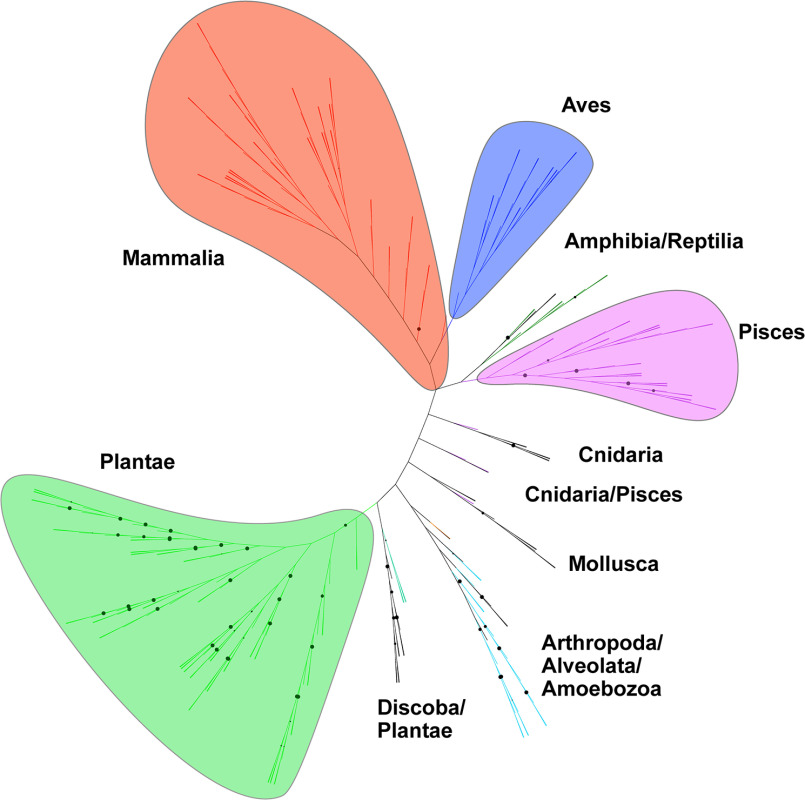
Previous bioinformatic analyses showed that bacterial ProXp-ala is primarily encoded in organisms belonging to the Alphaproteobacteria, Lactobacillales, Clostridia, and Negativicutes classes (20, 22), whereas eukaryotic ProXp-ala have only been reported for Hs, Mus musculus, Arabidopsis thaliana, Trypanosoma brucei, and Plasmodium falciparum (20). To update our understanding of the overall phylogenetic distribution of ProXp-ala in Eukarya, we searched all available 540 eukaryotic sequenced genomes and identified ProXp-ala genes in ∼54% of the organisms analyzed (Table S1 and Fig. 8). ProXp-ala is widely distributed in mammals, birds, fish, reptiles, and amphibians, whereas it is absent in other analyzed Animalia groups such as insects, arthropods, and worms. ProXp-ala is also encoded by almost all sequenced plants. The predicted plant ProXp-ala gene sequence is almost twice the size (∼320 amino acids) of that found in bacteria and the Animalia kingdom. The novel C-terminal extension of plant ProXp-ala shares no primary sequence homology with any known protein, and its function is unknown. In contrast to plants and animals, ProXp-ala is fused to the N terminus of ProRS in a group of animal parasites belonging to the Stramenopila, Aveolates, and Rhizaria supergroup, which includes P. falciparum and Toxoplasma gondii. This ProXp-ala-ProRS fusion is also observed in T. brucei ProRS and some other species from Kinetoplasida (e.g. Leishmania). We also identified putative ProXp-ala genes in several archaeal species including members of the Asgard archaea superphylum, Candidatus Prometheoarchaeum syntrophicum MK-D1 and Lokiarchaeum sp. GC14_75. Both organisms have been associated with eukaryogenesis and may be provide a link to the evolution of ProXp-ala (33, 34).
Figure 8.
Unrooted phylogenetic tree of eukaryotic ProXp-ala. ProXp-ala genes are primarily encoded in organisms belonging to the Animalia (Mammalia, Aves, Amphibia, Reptilia, Pisces, Cnidaria, Mollusca, and Arthropoda) and Plantae kingdoms as well as in the Alveolata, Discoba, and Amoebozoa clades. Branches with bootstrap values higher than 90 are indicated by black circles.
A phylogenetic tree of the ProXp-ala family revealed the formation of distinct clades corresponding to eukaryotic and bacterial ProXp-ala with only two instances of possible horizontal gene transfer events in the eukaryotes Entamoeba dispar and Ostreococcus tauri (Fig. 9). The phylogenic relationship of the ProXp-ala family underlines their divergence during evolution, which is reflected in distinct substrate features.
Figure 9.
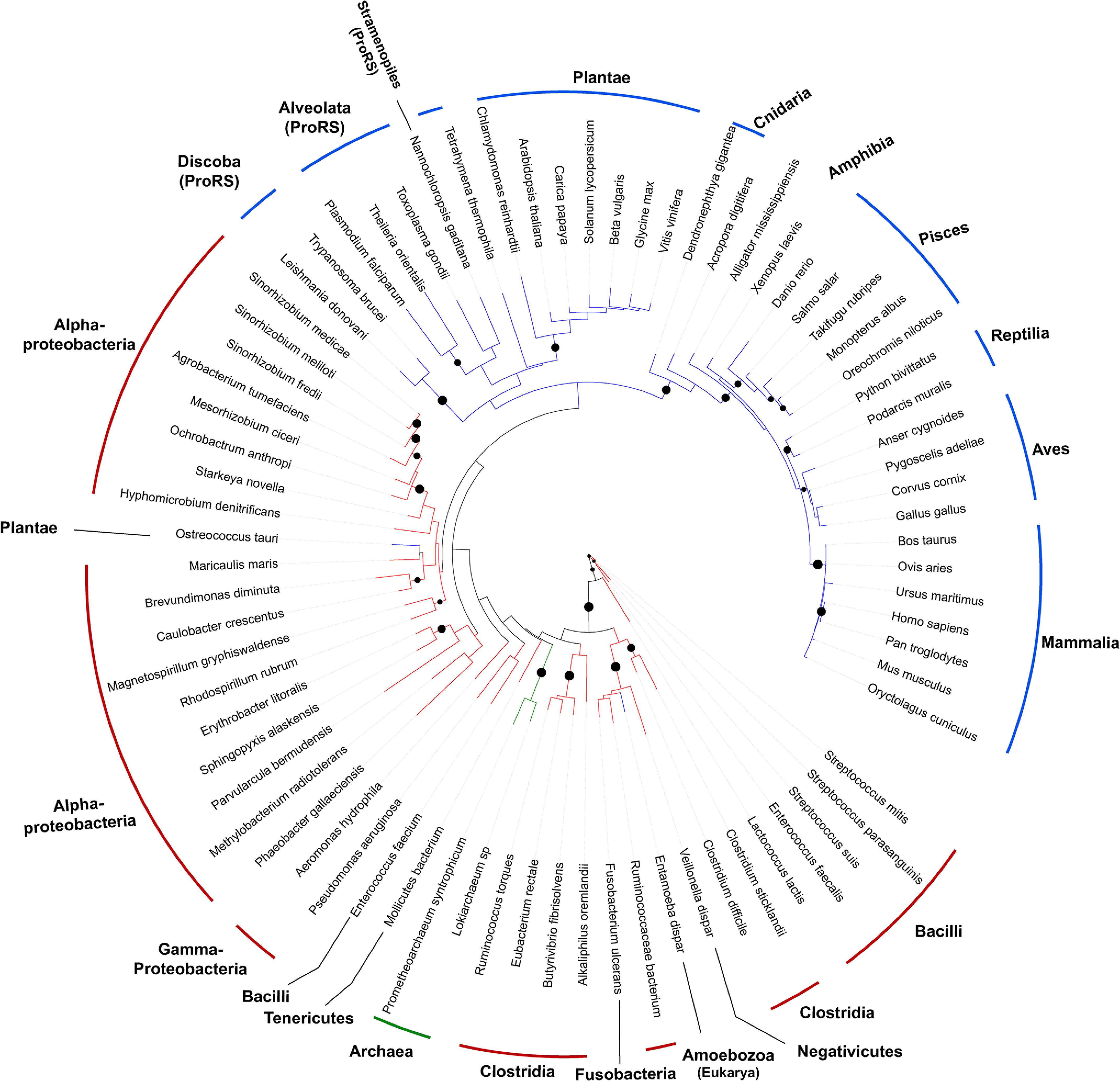
Phylogeny of ProXp-ala. The branches corresponding to bacterial, eukaryotic, and archaeal ProXp-ala are colored in red, blue, and green, respectively. The phylum, clade, or kingdom to which each organism belongs are indicated. ProXp-ala is fused to ProRS in organisms from the Discoba, Alveolata, and Stramenopiles clades. Branches with bootstrap values higher than 70 are indicated by black dots.
Discussion
In contrast to a previous report, which concluded that Hs cyto ProXp-ala lacks tRNA specificity (31), we found a strong dependence on elements at the top of the acceptor stem for efficient deacylation by the Hs enzyme (Table 1). In the earlier study, the reported activity was weak even at an elevated enzyme concentration of 5 μm (31). It is possible that the relatively weak deacylation activity was due to the use of a tRNAPro mutant with three substitutions (C3G, G70U, and C73A) relative to WT tRNAPro. These mutations were introduced to facilitate the preparation of Ala-tRNAPro using AlaRS (31). Although we did not test the third base pair in our work directly, we found that C73 is a critical recognition element for Hs ProXp-ala, and G3:U70-containing Ec Ala-tRNAAla is a relatively poor substrate despite the fact that it contains G1:C72 (Table 1). In addition, differences in the N terminus of the recombinant Hs ProXp-ala used previously relative to the genome-encoded sequence available in UniProt (accession number A6NEY8) used in our study (35) may have impacted enzymatic function (Fig. S2) (31).
The changes in the sequence of the acceptor stem of tRNAPro during evolution led to the development of distinct modes of tRNA recognition by ProRSs across the three domains of life (28, 30, 36). Here we found that these same changes also exerted pressure during the evolution of the trans-editing enzyme ProXp-ala from Bacteria to Eukarya, enabling its adaptation to cytosolic tRNAPro. However, Hs ProRS and ProXp-ala altered their substrate selection mechanisms in response to the tRNAPro acceptor stem changes differently. Hs ProRS relaxed its requirement for specific acceptor-stem nucleotides and instead gained a stronger affinity for the anticodon, as well as a specific D-stem loop requirement (30). In contrast, Hs ProXp-ala specificity relies on the G1:C72/C73 nucleotides of tRNAPro (Fig. 3 and Table 1), which are the same acceptor-stem positions recognized by bacterial ProXp-ala, albeit with different base identities (i.e. C1:G72/A73) (26). Consequently, Hs ProXp-ala only weakly deacylated bacterial and mitochondrial Ala-tRNAPro (Fig. 1B), and its strong tRNA specificity likely prevents efficient deacylation of Ala-tRNAAla (Table 1). These results, as well as the fact that C73, which is rarely found at this position and is a universal feature of all eukaryotic cyto tRNAPro, indicate that ProXp-ala is likely to act exclusively in the cytosol of higher eukaryotes. Studies to determine the cellular localization of Hs ProXp-ala are required to confirm this hypothesis. Thus, whereas acceptor-stem elements of bacterial tRNAPro play a dual role in aminoacylation and editing, the acceptor-stem elements that only weakly mark cytosolic tRNAPro for aminoacylation with Pro by Hs ProRS play a more important role in the proofreading of aminoacyl-tRNAPro by Hs ProXp-ala.
Biochemical characterization also showed that Hs ProXp-ala displays an aminoacyl specificity profile that does not follow a simple relationship between the rate of hydrolysis and the size (molecular volume) of the amino acid. For example, Hs ProXp-ala deacylated Abu-tRNAPro with similar efficiency as Ser-tRNAPro, despite Ser being significantly smaller than Abu. In contrast, tRNAPro charged with Aze (which resembles the volume of Ser) was deacylated severalfold better than Ser-tRNAPro and with similar efficiency as Pro-tRNAPro (Fig. 4 and Table 2). Thus, compared with the bacterial system (24), the Hs ProXp-ala active site better accommodates the pyrrolidine and azetidine side chains of Pro and Aze and shows greater discrimination against polar OH-containing amino acids. Consequently, Cc and Hs ProXp-ala appear to have evolved not only different tRNA acceptor-stem recognition specificities but also different preferences in amino acid selection.
Our study uncovered key residues that define the amino acid specificity of the ProXp-ala family. In Hs ProXp-ala, mutations at Met35 and His137 modulated amino acid recognition, similar to the corresponding Leu and His residues in the substrate-binding pocket of the Ec INS domain (25). Substitution of Met35 with Gly enlarges the Hs enzyme's binding pocket and allowed better accommodation of Ser-tRNAPro. The corresponding position in Cc ProXp-ala corresponds to G32, and WT Cc ProXp-ala has a binding pocket that enables it to efficiently accommodate and robustly hydrolyze Ser-tRNAPro (24).
Our results also confirmed that a highly conserved His residue in the aminoacyl-binding pocket of INS/ProXp-ala family members functions as a universal gatekeeper to prevent or reduce cognate Pro-tRNAPro deacylation. In the case of INS, the H369A mutation led to a switch of specificity from Ala- to Pro-tRNAPro (18), whereas the analogous mutation in Cc and Hs ProXp-ala resulted in dual activity for Ala- and Pro-tRNAPro. Notably, His137 of Hs ProXp-ala is less effective at preventing Pro-tRNAPro deacylation relative to the bacterial system (Fig. 7). The rather efficient deacylation of cognate Pro-tRNAPro observed for the Hs trans-editing enzyme in vitro is surprising and suggests that other factors in the cell likely prevent this undesired reaction. Based on the ability of bacterial elongation factor Tu to protect correctly charged tRNAs from free-standing editing domains, including bacterial ProXp-ala (22, 37), we propose that Hs EF-1A may outcompete Hs ProXp-ala for binding to Pro-tRNAPro, allowing only deacylation of the mischarged Ala-tRNAPro species in cells. If editing occurred in cis (i.e. by a ProRS editing domain prior to substrate dissociation) rather than in trans, this competition would be less likely to be successful and may explain why eukaryotic ProRSs rely on a free-standing trans-editing enzyme for proofreading. Whether Aze-tRNAPro is protected by elongation factors is unknown. Aze is a proline analog synthesized in many plants; it is also misactivated by Hs ProRS (15). High levels of Aze administered to zebrafish embryos lead to Aze-tRNAPro accumulation and cell toxicity (38). Thus, it is possible that the dual activity toward Ala and Aze, although weaker for the latter, displayed by Hs ProXp-ala serves to prevent mistranslation of Pro codons with either amino acid when present at low levels.
The results reported here underscore the complex evolutionary relationship between class II aminoacyl-tRNA synthetases and editing domains. The tRNAPro-specific INS/ProXp-ala family of editing domains likely arose from the biological pressure to prevent mistranslation of Pro codons caused by the critical nature of this amino acid for protein structure and function in all domains of life. The ProRS INS domain, found only in bacteria (14, 39), and the ProXp-ala family, found primarily in bacteria and eukarya (Fig. 9), have the same specificity toward editing of Ala-tRNAPro; yet ProXp-ala is usually encoded as a free-standing domain (with a few exceptions, see Fig. 9), and INS is always found appended to ProRS (14, 20, 22, 39). The latter observation may be due to the lack of strong tRNA acceptor-stem specificity of this domain (26), which would result in promiscuous trans-editing of Ala-tRNAAla. Each organism has likely evolved to use the type of Ala-tRNAPro editing domain (INS versus ProXp-ala) and domain structure (free-standing or synthetase-appended) to ensure optimal levels of fidelity required for cell survival.
Experimental procedures
Materials
All amino acids, nucleotides, enzymes, and other materials were purchased from Millipore–Sigma unless otherwise noted.
Protein preparation
All WT and mutant proteins used in this study were prepared as previously described: Hs ProXp-Ala (38), Cc ProXp-Ala (22), Hs ProRS (40), Ec ProRS K279A (25), and Ec tRNA nucleotidyltransferase (5). Briefly, BL-21-CodonPlus(DE3)-RIL cells (Agilent) were used for protein expression. Overexpression was carried out upon induction with 0.1 mm isopropyl β-d-1-thiogalactopyranoside (Gold Biotechnology) for 18–20 h at 18 °C. His6-tagged proteins were purified on His-Select nickel affinity gel using a 5–250 mm imidazole gradient. Purified proteins were buffer-exchanged into the storage buffer (50 mm sodium phosphate, pH 7.5, 150 mm NaCl, 1 mm DTT), mixed with 40% glycerol, and stored at −20 °C. Enzyme concentrations were determined using a Bio-Rad protein assay kit (Bio-Rad). Mutations in Hs ProXp-ala and Cc ProXp-Ala were introduced using either QuikChange site-directed mutagenesis (Agilent) or site-directed, ligase-independent mutagenesis (41). The successful mutagenesis was confirmed by DNA sequencing (The Genomics Shared Resource at the Ohio State University Comprehensive Cancer Center).
Preparation of tRNAs and aminoacyl-tRNA substrates
All tRNA variants used in these studies were prepared by in vitro transcription as previously described (14). Briefly, for each tRNA, a linear DNA template for T7 RNA polymerase was obtained by BstN1 (NEB) digestion of a plasmid carrying the corresponding gene. Transcribed tRNA was purified using denaturing 10% PAGE. To ensure high yields of tRNA variants with cytidine at position 1, a hammerhead ribozyme was inserted at 5′ of the tRNA gene (42). A plasmid encoding Hs tRNAPro C1:72G variant was ordered from Genewiz; all other acceptor-stem tRNAPro mutants were made by site-directed, ligase-independent mutagenesis (41) and confirmed by DNA sequencing (The Genomics Shared Resource at the Ohio State University Comprehensive Cancer Center). All tRNAs used in deacylation assays were labeled with 32P at the 3´-A76 using tRNA nucleotidyltransferase (43). Preparation of aminoacylated Hs tRNAPro was carried out in 50 mm HEPES, pH 7.5, 20 mm KCl, 4 mm ATP, 10 mm MgCl2, 0.1 mg/ml BSA, and 1 mm DTT by incubating 10 μm Hs ProRS, 10 μm tRNAPro, and 0.03 mg/ml pyrophosphatase (Roche) with the following amino acids: 900 mm Ala, 30 mm Pro, or 300 mm Aze for 5 min at 37 °C. Ec Pro- and Ala-tRNAPro were prepared under similar conditions with editing-deficient Ec K279A ProRS (25). Aminoacyl-tRNAs were phenol chloroform-extracted followed by ethanol precipitation. Acceptor-stem tRNAPro variants, as well as Ec tRNASer, Ec tRNAAla, and Ec tRNACys, were charged with Ala using dinitro-flexizyme (dFx) and Ala–3,5-dinitrobenzyl ester (Ala-DBE) as described (44). Briefly, 32P-labeled tRNA and dFx were refolded by heating at 90 °C for 1 min followed by addition of MgCl2 and cooling to room temperature for 3 min. The aminoacylation reaction was carried in 100 mm HEPES-KOH (pH 7.5), 20 mm MgCl2, 25 μm tRNA, 25 μm dFx, 5 mm Ala-DBE, Ser-DBE, or Abu-DBE for 20 h on ice. Thr-tRNAPro was prepared using enhanced flexizyme and Thr–4-chlorobenzyl thioester (44). Substrates for deacylation assays were ethanol-precipitated; dissolved in 3 mm sodium acetate, pH 5.2; and stored at −80 °C prior to use.
Deacylation assays
Single-turnover aminoacyl-tRNAPro deacylation reactions were performed as previously described (24). Hs ProXp-Ala (WT and mutants) reactions typically contained 0.1 μm 32P-labeled aminoacyl-tRNAPro and 1.5 μm ProXp-ala in deacylation buffer A (50 mm HEPES, pH 7.5, 20 mm KCl, 5 mm MgCl2, 0.1 mg/ml BSA, 1 mm DTT) and were performed at 30 °C. Cc ProXp-Ala reactions contained 0.1 μm 32P-labeled aminoacyl-tRNAPro and 0.75 μm ProXp-ala in deacylation buffer B (150 mm potassium phosphate, pH 7.0, 5 mm MgCl2, 0.1 mg/ml BSA) and were performed at 18 °C. The reactions were initiated by mixing equal volumes of aminoacyl-tRNAPro and enzyme. At the chosen time points, 2-μl aliquots were quenched into a solution containing 20 units of S1 nuclease, 1 mm zinc acetate, and 200 mm NaOAc mm sodium acetate, pH 5.0. Product formation was monitored by separating aminoacyl-A76 from A76 on polyethyleneimine-cellulose plates (EMD Millipore) using a mobile phase of 0.05% ammonium chloride, 5% acetic acid. Radioactive products were detected by autoradiography using a Typhoon FLA 9500 (GE Healthcare) and quantified using ImageQuant TL 8 software (GE Healthcare). Comparative analysis of numerous tRNA and enzyme variants was performed using single-turnover rate conditions with the concentration of Hs ProXp-ala severalfold smaller than the apparent Kd. Under these conditions, the observed rate constant (kobs) reflects kcat/Km and allows comparison of the enzyme efficiency for various substrates. For Kd determination, deacylation reactions were performed with varying Hs ProXp-Ala concentrations from 0.75 to 12 μm. The Kd and kmax were obtained by fitting the kobs versus [Hs ProXp-Ala] plot with the Michaelis–Menten equation: kmax = kobs [Hs ProXp-Ala]/(Kd + [Hs ProXp-Ala]). Observed rate constants were obtained by fitting the time course for aminoacyl-tRNA deacylation with a single-exponential equation using SigmaPlot (Systat Software). Each reported rate constant is an average of three independent assays. The graphs show representative time courses. A control reaction in the absence of enzyme was performed for each aa-tRNA substrate. All data points were corrected for nonenzymatic buffer hydrolysis (Figs. S3 and S4).
Amino acid volume calculations
The structure of each free amino acid (Ala, Abu, Aze, Pro, Ser, and Thr) was obtained from the Protein Data Bank. Molecular volumes for each amino acid were calculated by loading each .pdb file into the Volume Assessor module of the 3V webserver (http://3vee.molmovdb.org/volumeCalc.php) using a 0.1 Å probe radius and high grid resolution (0.5 Å voxel size) (45).
Phylogenetic analysis of ProXp-ala
A total of 542 eukaryotic sequenced genomes (retrieved from the KEGG database, RRID:SCR_004165, and the Joint Genome Institute Genomes Online Database (46)) were individually searched for ProXp-ala genes using the National Center for Biotechnology Information BLASTp service (Table S1). ProXp-ala reference sequences from Hs, Cc, Clostridium sticklandii, Agrobacterium tumefaciens, and A. thaliana were used as queries. A global search of ProXp-ala genes in Archaea was also carried out. The resulting putative genes were designated as ProXp-ala only if they had the signature residues of ProXp-ala (i.e. Lys45, His130, GXXX(P/A), numbering based on C. crescentus ProXp-ala). The eukaryotic ProXp-ala phylogenetic tree was built via multiple sequence alignment using Clustal Omega (47) followed by a maximum likelihood analysis with 100 bootstraps using MEGA X with default settings (48). The ProXp-ala family tree was built using the NGPhylogeny.fr platform (49). A workflow with Clustal Omega sequence alignment followed by tree inference using PhyML with 100 bootstraps was used. The bacterial, eukaryotic, and archaeal ProXp-ala sequences from the organisms listed in Table S1 were used for the tree. The iTOL online tool was used to display both trees (50).
Data availability
All data are contained within the article.
Supplementary Material
Acknowledgments
We thank Irina Shulgina for protein and tRNA preparation, Dr. Marie Sissler (Université de Strasbourg) for providing the plasmid encoding the human mitochondrial tRNAPro, Dr. Ana Crnkovic for critical reading of the manuscript, and Drs. Mom Das and Sandeep Kumar for enlightening discussions and suggestions.
This article contains supporting information.
Author contributions—O. V.-R., M. B., and K. M.-F. conceptualization; O. V.-R. and M. B. data curation; O. V.-R., M. B., and D. M. formal analysis; O. V.-R. and M. B. visualization; O. V.-R., M. B., D. M., J. A., Y. G., H. S., and K. M.-F. writing-original draft; O. V.-R., M. B., and K. M.-F. writing-review and editing; M. B., D. M., and J. A. investigation; M. B., D. M., J. A., Y. G., H. S., and K. M.-F. methodology; Y. G., H. S., and K. M.-F. funding acquisition; H. S. and K. M.-F. supervision; K. M.-F. project administration.
Funding and additional information—This work was supported by National Institutes of Health Grant RO1 GM113656 (to K. M.-F.) and by KAKENHI Grants JP20H05618 (to H. S.) and JP20H02866 (to Y. G.) from the Japan Society for the Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- Cc
- C. crescentus
- Ec
- E. coli
- Hs
- H. sapiens
- ARS
- aminoacyl-tRNA synthetase
- ProRS
- prolyl-tRNA synthetase
- AlaRS
- alanyl-tRNA synthetase
- tRNA
- transfer RNA
- aa-tRNA
- aminoacyl-tRNA
- Abu
- 2-aminobutyric acid
- Aze
- azetidine-2-carboxylic acid
- cyto
- cytosolic
- INS
- insertion domain
- mito
- mitochondrial
- dFx
- dinitro-flexizyme
- DBE
- 3,5-dinitrobenzyl ester.
References
- 1. Mohler K., and Ibba M. (2017) Translational fidelity and mistranslation in the cellular response to stress. Nat. Microbiol. 2, 17117 10.1038/nmicrobiol.2017.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nangle L. A., Motta C. M., and Schimmel P. (2006) Global effects of mistranslation from an editing defect in mammalian cells. Chem. Biol. 13, 1091–1100 10.1016/j.chembiol.2006.08.011 [DOI] [PubMed] [Google Scholar]
- 3. Lee J. W., Beebe K., Nangle L. A., Jang J., Longo-Guess C. M., Cook S. A., Davisson M. T., Sundberg J. P., Schimmel P., and Ackerman S. L. (2006) Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 10.1038/nature05096 [DOI] [PubMed] [Google Scholar]
- 4. Hilander T., Zhou X. L., Konovalova S., Zhang F. P., Euro L., Chilov D., Poutanen M., Chihade J., Wang E. D., and Tyynismaa H. (2018) Editing activity for eliminating mischarged tRNAs is essential in mammalian mitochondria. Nucleic Acids Res. 46, 849–860 10.1093/nar/gkx1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Y., Satz J. S., Vo M. N., Nangle L. A., Schimmel P., and Ackerman S. L. (2014) Deficiencies in tRNA synthetase editing activity cause cardioproteinopathy. Proc. Natl. Acad. Sci. U.S.A. 111, 17570–17575 10.1073/pnas.1420196111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ling J., So B. R., Yadavalli S. S., Roy H., Shoji S., Fredrick K., Musier-Forsyth K., and Ibba M. (2009) Resampling and editing of mischarged tRNA prior to translation elongation. Mol. Cell 33, 654–660 10.1016/j.molcel.2009.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen M., Kuhle B., Diedrich J., Liu Z., Moresco J. J., Yates Iii J. R., Pan T., and Yang X. L. (2020) Cross-editing by a tRNA synthetase allows vertebrates to abundantly express mischargeable tRNA without causing mistranslation. Nucleic Acids Res. 48, 6445–6457 10.1093/nar/gkaa469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuzmishin Nagy A. B., Bakhtina M., and Musier-Forsyth K. (2020) trans-Editing by aminoacyl-tRNA synthetase-like editing domains. In The Enzymes: Biology of Aminoacyl-tRNA Synthetases (Ribas de Pouplana L., and Kaguni L., eds) Academic Press, Orlando, FL: [DOI] [PubMed] [Google Scholar]
- 9. Drummond D. A., and Wilke C. O. (2009) The evolutionary consequences of erroneous protein synthesis. Nat. Rev. Genet. 10, 715–724 10.1038/nrg2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ling J., Reynolds N., and Ibba M. (2009) Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 10.1146/annurev.micro.091208.073210 [DOI] [PubMed] [Google Scholar]
- 11. Yadavalli S. S., and Ibba M. (2012) Quality control in aminoacyl-tRNA synthesis: its role in translational fidelity. Adv. Protein Chem. Struct. Biol. 86, 1–43 10.1016/B978-0-12-386497-0.00001-3 [DOI] [PubMed] [Google Scholar]
- 12. Ling J., and Söll D. (2010) Severe oxidative stress induces protein mistranslation through impairment of an aminoacyl-tRNA synthetase editing site. Proc. Natl. Acad. Sci. U.S.A. 107, 4028–4033 10.1073/pnas.1000315107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bacher J. M., and Schimmel P. (2007) An editing-defective aminoacyl-tRNA synthetase is mutagenic in aging bacteria via the SOS response. Proc. Natl. Acad. Sci. U.S.A. 104, 1907–1912 10.1073/pnas.0610835104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beuning P. J., and Musier-Forsyth K. (2000) Hydrolytic editing by a class II aminoacyl-tRNA synthetase. Proc. Natl. Acad. Sci. U.S.A. 97, 8916–8920 10.1073/pnas.97.16.8916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beuning P. J., and Musier-Forsyth K. (2001) Species-specific differences in amino acid editing by class II prolyl-tRNA synthetase. J. Biol. Chem. 276, 30779–30785 10.1074/jbc.M104761200 [DOI] [PubMed] [Google Scholar]
- 16. Ahel I., Stathopoulos C., Ambrogelly A., Sauerwald A., Toogood H., Hartsch T., and Söll D. (2002) Cysteine activation is an inherent in vitro property of prolyl-tRNA synthetases. J. Biol. Chem. 277, 34743–34748 10.1074/jbc.M206928200 [DOI] [PubMed] [Google Scholar]
- 17. Kamtekar S., Kennedy W. D., Wang J., Stathopoulos C., Söll D., and Steitz T. A. (2003) The structural basis of cysteine aminoacylation of tRNAPro by prolyl-tRNA synthetases. Proc. Natl. Acad. Sci. U.S.A. 100, 1673–1678 10.1073/pnas.0437911100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong F. C., Beuning P. J., Nagan M., Shiba K., and Musier-Forsyth K. (2002) Functional role of the prokaryotic proline-tRNA synthetase insertion domain in amino acid editing. Biochemistry 41, 7108–7115 10.1021/bi012178j [DOI] [PubMed] [Google Scholar]
- 19. An S., and Musier-Forsyth K. (2004) trans-Editing of Cys-tRNAPro by Haemophilus influenzae YbaK protein. J. Biol. Chem. 279, 42359–42362 10.1074/jbc.C400304200 [DOI] [PubMed] [Google Scholar]
- 20. Ahel I., Korencic D., Ibba M., and Söll D. (2003) Trans-editing of mischarged tRNAs. Proc. Natl. Acad. Sci. U. S. A. 100, 15422–15427 10.1073/pnas.2136934100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruan B., and Söll D. (2005) The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNACys deacylase. J. Biol. Chem. 280, 25887–25891 10.1074/jbc.M502174200 [DOI] [PubMed] [Google Scholar]
- 22. Vargas-Rodriguez O., and Musier-Forsyth K. (2013) Exclusive use of trans-editing domains prevents proline mistranslation. J. Biol. Chem. 288, 14391–14399 10.1074/jbc.M113.467795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crepin T., Yaremchuk A., Tukalo M., and Cusack S. (2006) Structures of two bacterial prolyl-tRNA synthetases with and without a cis-editing domain. Structure 14, 1511–1525 10.1016/j.str.2006.08.007 [DOI] [PubMed] [Google Scholar]
- 24. Danhart E. M., Bakhtina M., Cantara W. A., Kuzmishin A. B., Ma X., Sanford B. L., Vargas-Rodriguez O., Košutić M., Goto Y., Suga H., Nakanishi K., Micura R., Foster M. P., and Musier-Forsyth K. (2017) Conformational and chemical selection by a trans-acting editing domain. Proc. Natl. Acad. Sci. U.S.A. 114, E6774–E6783 10.1073/pnas.1703925114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar S., Das M., Hadad C. M., and Musier-Forsyth K. (2012) Substrate specificity of bacterial prolyl-tRNA synthetase editing domain is controlled by a tunable hydrophobic pocket. J. Biol. Chem. 287, 3175–3184 10.1074/jbc.M111.313619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Das M., Vargas-Rodriguez O., Goto Y., Suga H., and Musier-Forsyth K. (2014) Distinct tRNA recognition strategies used by a homologous family of editing domains prevent mistranslation. Nucleic Acids Res. 42, 3943–3953 10.1093/nar/gkt1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin B. Y., Chan P. P., and Lowe T. M. (2019) tRNAviz: explore and visualize tRNA sequence features. Nucleic Acids Res. 47, W542–W547 10.1093/nar/gkz438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu H., Peterson R., Kessler J., and Musier-Forsyth K. (1995) Molecular recognition of tRNAPro by Escherichia coli proline-tRNA synthetase in vitro. Nucleic Acids Res. 23, 165–169 10.1093/nar/23.1.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burke B., Yang F., Chen F., Stehlin C., Chan B., and Musier-Forsyth K. (2000) Evolutionary coadaptation of the motif 2–acceptor stem interaction in the class II prolyl-tRNA synthetase system. Biochemistry 39, 15540–15547 10.1021/bi001835p [DOI] [PubMed] [Google Scholar]
- 30. Stehlin C., Burke B., Yang F., Liu H., Shiba K., and Musier-Forsyth K. (1998) Species-specific differences in the operational RNA code for aminoacylation of tRNAPro. Biochemistry 37, 8605–8613 10.1021/bi980364s [DOI] [PubMed] [Google Scholar]
- 31. Ruan L.-L., Zhou X.-L., Tan M., and Wang E.-D. (2013) Human cytoplasmic ProX edits mischarged tRNAPro with amino acid but not tRNA specificity. Biochem. J. 450, 243–252 10.1042/BJ20121493 [DOI] [PubMed] [Google Scholar]
- 32. Murakami H., Ohta A., Ashigai H., and Suga H. (2006) A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 3, 357–359 10.1038/nmeth877 [DOI] [PubMed] [Google Scholar]
- 33. Imachi H., Nobu M. K., Nakahara N., Morono Y., Ogawara M., Takaki Y., Takano Y., Uematsu K., Ikuta T., Ito M., Matsui Y., Miyazaki M., Murata K., Saito Y., Sakai S., et al. (2020) Isolation of an archaeon at the prokaryote-eukaryote interface. Nature 577, 519–525 10.1038/s41586-019-1916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spang A., Saw J. H., Jørgensen S. L., Zaremba-Niedzwiedzka K., Martijn J., Lind A. E., van Eijk R., Schleper C., Guy L., and Ettema T. J. G. (2015) Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521, 173–179 10.1038/nature14447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. UniProt C. (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 10.1093/nar/gky1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burke B., Lipman R. S., Shiba K., Musier-Forsyth K., and Hou Y. M. (2001) Divergent adaptation of tRNA recognition by Methanococcus jannaschii prolyl-tRNA synthetase. J. Biol. Chem. 276, 20286–20291 10.1074/jbc.m100456200 [DOI] [PubMed] [Google Scholar]
- 37. Kuncha S. K., Mazeed M., Singh R., Kattula B., Routh S. B., and Sankaranarayanan R. (2018) A chiral selectivity relaxed paralog of DTD for proofreading tRNA mischarging in Animalia. Nat. Commun. 9, 511 10.1038/s41467-017-02204-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Song Y., Zhou H., Vo M. N., Shi Y., Nawaz M. H., Vargas-Rodriguez O., Diedrich J. K., Yates J. R., Kishi S., Musier-Forsyth K., and Schimmel P. (2017) Double mimicry evades tRNA synthetase editing by toxic vegetable-sourced non-proteinogenic amino acid. Nat. Commun. 8, 2281 10.1038/s41467-017-02201-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yaremchuk A., Cusack S., and Tukalo M. (2000) Crystal structure of a eukaryote/archaeon-like protyl-tRNA synthetase and its complex with tRNAPro(CGG). EMBO J. 19, 4745–4758 10.1093/emboj/19.17.4745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heacock D., Forsyth C. J., Shiba K., and Musier-Forsyth K. (1996) Synthesis and aminoacyl-tRNA synthetase inhibitory activity of prolyl adenylate analogs. Bioorg. Chem. 24, 273–289 10.1006/bioo.1996.0025 [DOI] [Google Scholar]
- 41. Chiu J., March P. E., Lee R., and Tillett D. (2004) Site-directed, ligase-Independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res. 32, e174 10.1093/nar/gnh172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fechter P., Rudinger J., Giegé R., and Théobald-Dietrich A. (1998) Ribozyme processed tRNA transcripts with unfriendly internal promoter for T7 RNA polymerase: production and activity. FEBS Lett. 436, 99–103 10.1016/S0014-5793(98)01096-5 [DOI] [PubMed] [Google Scholar]
- 43. Ledoux S., and Uhlenbeck O. C. (2008) [3'-32P]-Labeling tRNA with nucleotidyltransferase for assaying aminoacylation and peptide bond formation. Methods 44, 74–80 10.1016/j.ymeth.2007.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Goto Y., Katoh T., and Suga H. (2011) Flexizymes for genetic code reprogramming. Nat. Protoc. 6, 779–790 10.1038/nprot.2011.331 [DOI] [PubMed] [Google Scholar]
- 45. Voss N. R., and Gerstein M. (2010) 3V: cavity, channel and cleft volume calculator and extractor. Nucleic Acids Res. 38, W555–W562 10.1093/nar/gkq395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mukherjee S., Stamatis D., Bertsch J., Ovchinnikova G., Katta H. Y., Mojica A., Chen I. A., Kyrpides N. C., and Reddy T. (2019) Genomes OnLine database (GOLD) v.7: updates and new features. Nucleic Acids Res. 47, D649–D659 10.1093/nar/gky977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sievers F., Wilm A., Dineen D., Gibson T. J., Karplus K., Li W., Lopez R., McWilliam H., Remmert M., Söding J., Thompson J. D., and Higgins D. G. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stecher G., Tamura K., and Kumar S. (2020) Molecular evolutionary genetics analysis (MEGA) for macOS. Mol. Biol. Evol. 37, 1237–1239 10.1093/molbev/msz312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lemoine F., Correia D., Lefort V., Doppelt-Azeroual O., Mareuil F., Cohen-Boulakia S., and Gascuel O. (2019) NGPhylogeny.fr: new generation phylogenetic services for non-specialists. Nucleic Acids Res. 47, W260–W265 10.1093/nar/gkz303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Letunic I., and Bork P. (2019) Interactive tree of life (iTOL) v4: recent updates and new developments. Nucleic Acids Res. 47, W256–W259 10.1093/nar/gkz239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the article.