Abstract
Current guidelines recommend angiotensin receptor blocker neprilysin inhibitors (ARNI) (sacubitril/valsartan) as a replacement for angiotensin-converting-enzymeinhibitor (ACE-I) in heart failure with reduced ejection fraction (HFrEF) who remain symptomatic despite optimal medical therapy. The effects of ARNIs have not previously been assessed in a systematic review. We searched for relevant trials until October 2019 in CENTRAL, MEDLINE, Embase, LILACS, BIOSIS, CNKI, VIP, WanFang and CBM. Our primary outcomes were all-cause mortality and serious adverse events. We systematically assessed the risks of random errors and systematic errors. PROSPERO registration: CRD42019129336. 48 trials randomising 19 086 participants were included. The ARNI assessed in all trials was sacubitril/valsartan. ACE-I or ARB were used as control interventions. Trials randomising HFrEF participants (27 trials) and heart failure with preserved ejection fraction (HFpEF) participants (four trials) were analysed separately. In HFrEF participants, meta-analyses and Trial Sequential Analyses showed evidence of a beneficial effect of sacubitril/valsartan when assessing all-cause mortality (risk ratio (RR), 0.86; 95% CI, 0.79 to 0.94) and serious adverse events (RR, 0.89; 95% CI, 0.86 to 0.93); and the results did not differ between the guideline recommended target population and HFrEF participants in general. We found no evidence of an effect of sacubitril/valsartan in HFpEF participants. Sacubitril/valsartan compared with either ACE-I or ARB seems to have a beneficial effect in patients with HFrEF. Our results indicate that sacubitril/valsartan might be beneficial in a wider population of patients with heart failure than the guideline recommended target population. Sacubitril/valsartan does not seem to show evidence of a difference compared with valsartan in patients with HFpEF.
Keywords: heart failure, heart failure treatment, renin-angiotensin system
Key questions.
What is already known about this subject?
Sacubitril/valsartan is recommended as an alternative to angiotensin-converting-enzyme inhibitor in patients with heart failure with reduced ejection fraction who remain symptomatic despite optimal medical therapy.
No former systematic review has been conducted.
What does this study add?
Meta-analysis and Trial Sequential Analyses shows that sacubitril/valsartan reduces the risk of all-cause mortality, serious adverse events, hospitalisations and NT-proBNP as well as increases quality of life and ejection fraction.
How might this impact on clinical practice?
Our results indicate that sacubitril/valsartan might be beneficial in a wider population of patients with heart failure with reduced ejection fraction.
Introduction
Worldwide, an estimated 37 million people have a diagnosis of heart failure.1 2 The lifetime risk for developing heart failure is approximately 20%.3 The prevalence of heart failure is increasing, presumably caused by an increase in both life expectancy and risk factors leading to heart failure as well as improved treatment of acute cardiovascular events.1 2 4 5
Guidelines recommend treatment of heart failure with reduced ejection fraction with a beta-blocker and an inhibitor of the renin-angiotensin-aldosterone system (angiotensin-converting-enzyme inhibitor (ACE-I) or angiotensin II receptor blocker (ARB)). It is recommended to add a mineralocorticoid-receptor antagonist in patients who remain symptomatic after this initial treatment.3 6
New drugs for heart failure have been developed and approved that combine inhibition of the renin-angiotensin-aldosterone system pathway (with an ARB) with inhibition of the neprilysin enzyme. These new types of drugs are classified as angiotensin receptor blocker neprilysin inhibitors (ARNIs).7
The European Society of Cardiology recommends ARNIs as a replacement for ACE-I in patients with heart failure with reduced ejection fraction (HFrEF) (EF <35%) who remain symptomatic (New York Heart Association (NYHA) II to IV) despite optimal medical therapy with an ACE-I, a beta-blocker and a mineralocorticoid-receptor antagonist (unless there are contraindications).6 The American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America, make similar recommendations.8
To our knowledge, the effects of ARNIs have not been assessed previously in a systematic review.9
Methods
This systematic review has been developed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidelines for reporting systematic reviews evaluating interventions in healthcare (online supplemental S1 text).9 10 Our methodology was predefined and described in detail in our pre-published protocol.11
openhrt-2020-001294supp001.pdf (789.4KB, pdf)
In short, we included all trials assessing the beneficial and harmful effects of ARNIs in participants with any type of heart failure.11 We included randomised clinical trials irrespective of trial design, setting, publication status, publication year and language. We searched from their inception to October 2019 for relevant trials in the Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica database (Embase), Latin American and Caribbean Health Sciences Literature (LILACS), Science Citation Index Expanded on Web of Science, Chinese Biomedical Literature Database (CBM), China National Knowledge Infrastructure (CNKI), Chinese Science Journal Database (VIP), WanFang, SINOMED and BIOSIS. The search strategy can be found in (online supplemental S2 text). Additionally, we hand searched reference lists, major pharmaceutical companies and several databases for relevant publications.
Outcomes and subgroup analyses
Our primary outcomes were all-cause mortality, serious adverse events; secondary outcomes were myocardial infarction, quality of life, non-serious adverse events and hospitalisations; and exploratory outcomes were cardiovascular mortality, ejection fraction, 6-min walking distance, and NT-proBNP. We used the trial results reported at maximum follow-up.11 We planned several subgroup analyses (test of interaction)12 and sensitivity analyses11 (see ‘Results’). In addition we added three subgroup analyses: (1) trials comparing different co-interventions, (2) trials published in English compared with Chinese and (3) trials using guideline criteria for inclusion compared with trials with broader inclusion criteria.
Data collection and risk of bias
Three authors (EEN, JF and F-LB) extracted data and assessed risks of bias of the included trials using standardised extraction sheets. Disagreements were resolved by discussion with a third author (JCJ). Our bias risk assessment was based on the results of meta-epidemiological studies (online supplemental S2 ref). Hence, risks of bias were assessed using the domains random sequence generation, allocation concealment, blinding of participants and treatment providers, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, for profit bias and other risks of bias.13 14 We contacted all authors by email in order to retrieve missing information.
openhrt-2020-001294supp002.pdf (216.3KB, pdf)
Data synthesis and assessment of significance
We used the statistical software Review Manager 5.3 provided by Cochrane to analyse data.12 We assessed our intervention effects with both random-effects meta-analyses15 and fixed-effect meta-analyses.16 We primarily used the most conservative point estimate of the two.17 We assessed two primary outcomes, and therefore, we considered a p value of 0.033 as the threshold for statistical significance.17 We investigated possible heterogeneity through subgroup analyses (test of interaction).12 In order to control the risks of type I errors and type II errors, we performed Trial Sequential Analysis.17
Results
Study characteristics
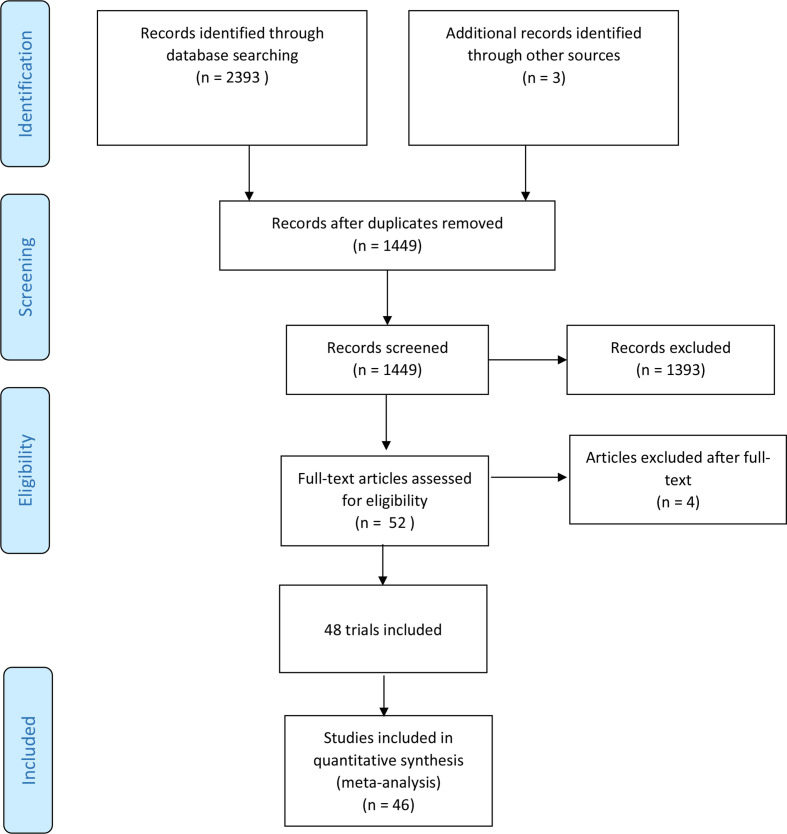
We identified 2393 potentially relevant studies through our literature search conducted in October 2019. In addition, three potential studies were identified through Novartis clinical registry. We included a total of 48 trials randomising 19 086 participants (figure 1). In all trials, the experimental intervention was 97 mg sacubitril/ 103 mg valsartan two times per day. The trials were conducted between 2012 and 2019 in 48 different countries. Nine of the included trials were written in English and published in Western databases and these trials accounted for 83% of the included participants. Two of these trials randomised 71% of all participants.18 19 Thirty-nine trials were conducted and published in China. These trials were generally small (34 to 180 participants) and reported mostly on surrogate outcomes (left ventricular ejection fraction, 6-min walking distance and NT-proBNP). Characteristics of included studies are summarised in (table 1).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram.
Table 1.
Characteristics of included studies
| Study (year) | Region | Type of heart failure | Chronic/ acute | Control intervention | Dates | Number of participants | Maximum follow-up (months) |
| AWAKE-HF et al (2019)30 |
English | HFrEF | Chronic | Enalapril | 2016–2018 | 70 | 4 |
| CLCZ696B2223 et al (2013)31 |
English | HFrEF | Chronic | Valsartan | 2011–2012 | 8 | 0.23 |
| EVALUATE-HF et al (2019)32 |
English | HFrEF | Chronic | Enalapril | 2016–2018 | 232 | 2.75 |
| OUTSTEP-HF et al (2019)33 |
English | HFrEF | Chronic | Enalapril | 2016–2018 | 310 | 4 |
| PARADIGM-HF et al (201419 | English | HFrEF | Chronic | Enalapril | 2009–2012 | 4209 | 27 |
| PARAGON-HF et al (2019)18 |
English | HFpEF | Chronic | Valsartan | 2014–2016 | 2419 | 35 |
| PARAMOUNT et al (2012)22 |
English | HFpEF | Chronic | Valsartan | 2009–2011 | 149 | 21 |
| PIONEER-HF et al (2018)34 |
English | HFrEF | Acute | Enalapril | 2016–2018 | 443 | 2 |
| PRIME-HF et al (2019)35 |
English | HFrEF | Chronic | Valsartan | 2016–2017 | 60 | 12 |
| Chai DJ et al (2019)36 |
Chinese | HFrEF | Chronic | Milinon | 2017–2018 | 48 | 0.01 (72 hours) |
| Chen CW et al (2019)37 |
Chinese | Unclear | Chronic | Enalapril | 2018–2019 | 40 | 1 |
| Chen L et al (2019)38 |
Chinese | HFrEF | Unclear | Enalapril | 2017–2018 | 32 | 6 |
| Dai WL et al (2019)39 |
Chinese | HFrEF | Chronic | Ramipril | 2017–2019 | 98 | 6 |
| Dong L et al (2019)40 |
Chinese | HFrEF | Chronic | Valsartan | 2017–2018 | 30 | 2.75 |
| Fan JF et al (2019)41 |
Chinese | HFrEF | Chronic | Benazepril | 2017–2018 | 27 | 2.75 |
| Fan TT et al (2019)42 |
Chinese | Mixed | Chronic | Valsartan | 2018–2018 | 35 | 1 |
| Fan ZL et al (2019)43 |
Chinese | Unclear | Chronic | Enalapril | 2017–2018 | 26 | 4 |
| Gao Y et al (2019)44 |
Chinese | HFrEF | Chronic | Valsartan | 2017–2018 | 17 | 1.75 |
| Han ZQ et al (2019)23 |
Chinese | HFpEF | Unclear | Valsartan | 2016–2018 | 39 | 2.25 |
| Hao QM et al (2019)45 |
Chinese | HFrEF | Chronic | Valsartan | 2017–2018 | 30 | 1.75 |
| Huang SB et al (2019)46 |
Chinese | HFpEF | Chronic | Usual care | 2017–2018 | 39 | 6 |
| Ke ZF et al (2019)47 |
Chinese | HFrEF | Chronic | Usual care | 2017–2018 | 35 | 3 |
| Li GX et al (2019)48 |
Chinese | Unclear | Chronic | Benazepril | 2017–2018 | 27 | 3 |
| Li J (1) et al (2019)49 |
Chinese | HFrEF | Chronic | Enalapril | 2017–2018 | 47 | 6 |
| Li J (2) et al (2019)50 |
Chinese | Unclear | Chronic | Benazepril | 2017–2017 | 62 | 12 |
| Liang HB et al (2019)51 |
Chinese | HFrEF | Unclear | Perindopril | 2018–2019 | 50 | 3 |
| Liu DN et al (2019)52 |
Chinese | HFrEF | Chronic | Usual care | 2017–2018 | 48 | NR |
| Liu YH et al (2019)53 |
Chinese | HFrEF | Chronic | Benazepril/candesartan | 2017–2018 | 26 | 6 |
| Pu SH et al (2019)54 |
Chinese | HFrEF | Chronic | ACE-I/ARB | 2017–2018 | 90 | 6 |
| Shen JH et al (2019)55 |
Chinese | Unclear | Chronic | Valsartan | 2017–2018 | 51 | 3 |
| Song Z et al (2019)56 |
Chinese | Unclear | Chronic | Usual care | 2016–2018 | 48 | 1 |
| Sun X et al (2019)57 |
Chinese | Unclear | Chronic | Usual care | 2017–2018 | 36 | 1 |
| Sun XN et al (2018)58 |
Chinese | Unclear | Chronic | Enalapril | 2017–2018 | 58 | 3 |
| Tang J et al (2018)59 |
Chinese | Unclear | Chronic | Bisoprolol | 2017–2018 | 50 | 1 |
| Wang QD et al (2019)60 |
Chinese | Unclear | Acute | Usual care | 2017–2018 | 30 | 1 |
| Wei ZX et al (2019)61 |
Chinese | HFrEF | Unclear | Valsartan | 2017–2018 | 30 | 3 |
| Wu MM et al (2018)62 |
Chinese | HFrEF | Chronic | Benazepril | 2017–2018 | 20 | 1 |
| Yang J et al (2019)63 |
Chinese | Unclear | Chronic | Benazepril | 2016–2018 | 46 | 3 |
| Yang RC et al (2018)64 |
Chinese | Unclear | Chronic | Valsartan | 2017–2018 | 57 | 2 |
| Yao LN et al (2019)65 |
Chinese | HFrEF | Chronic | ACE-I/ARB | 2018–2019 | 27 | NR |
| Yu H et al (2019)66 |
Chinese | HFrEF and HFmrEF | Chronic | Valsartan | 2018–2019 | 40 | 2.75 |
| Yu ZL et al (2018)67 |
Chinese | Unclear | Chronic | Usual care | 2017–2018 | 42 | 0,3 |
| Zhang H et al (2018)68 |
Chinese | HFrEF | Unclear | Enalapril | 2017–2017 | 36 | 1 |
| Zhang JW et al (2019)69 |
Chinese | HFrEF and HFmrEF | Chronic | Enalapril | 2017–2018 | 41 | 6 |
| Zhang XJ et al (2019)70 |
Chinese | Unclear | Chronic | Benazepril | 2017–2017 | 40 | 2.25 |
| Zhang Y et al (2019)71 |
Chinese | HFrEF | Chronic | Valsartan | 2017–2018 | 28 | 3 |
| Zhang YZ et al (2019)72 |
Chinese | HFrEF | Chronic | Benazepril | 2017–2018 | 40 | 3 |
| Zhao YQ et al (2019)73 |
Chinese | Unclear | Unclear | Usual care | 2016–2017 | 85 | 12 |
ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; HFmrEF, heart failure with midrange ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
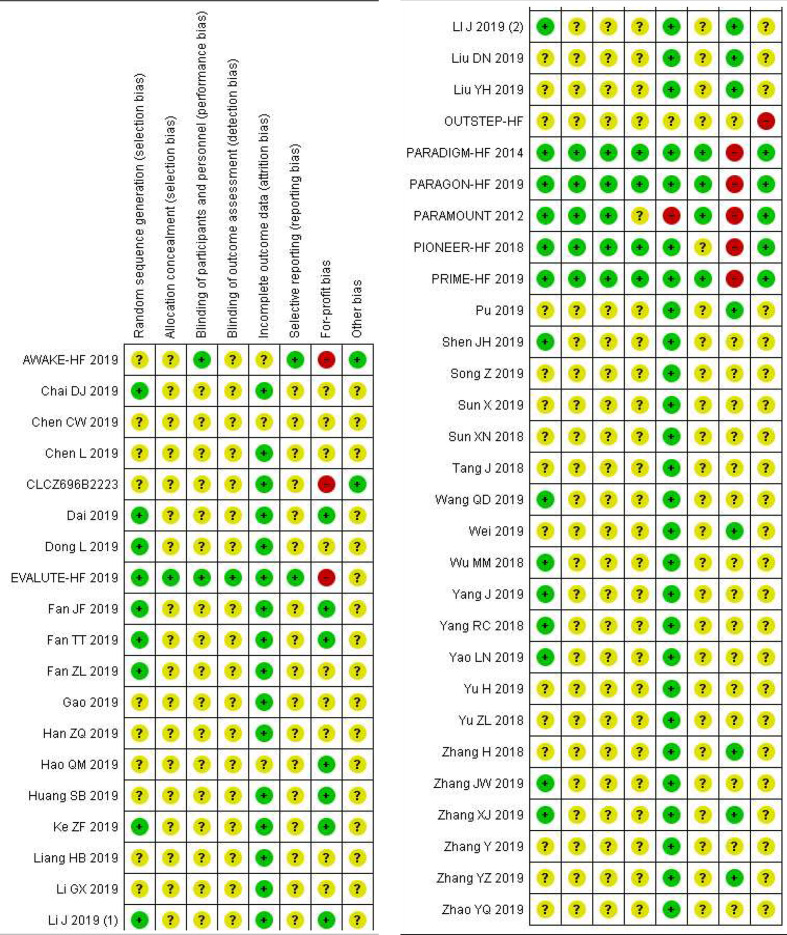
All nine trials published in English-language journals were judged to be high risk of bias mainly due to industry funding; Novartis Pharmaceuticals funded all nine trials. All trials published in Chinese were judged to be of high risk of bias and of low methodological quality; for example, none of the trials registered/ published protocols, used blinding and only 18/39 reported how the randomisation process was conducted (figure 2).
Figure 2.
Risk of bias summary.
Twenty-seven trials randomised a total of 12 311 HFrEF participants and four trials randomised 5278 heart failure with preserved ejection fraction (HFpEF) participants. The remaining 17 trials randomising a total of 1497 participants either did not specify which type of heart failure they assessed, or they included participants with different types of heart failure. The majority (93.0%) of the total number of randomised participants had NYHA II or III. The mean age of the trial participants was 65.9 years and the mean proportion of women was 34.2%. Baseline characteristics are summarised in (table 2).
Table 2.
Baseline characteristics
| Trials providing information | ARNI | No. analysed (ARNI) | Control | No. analysed (Control) | |
| Age - years (SD) | 46 | 66.1 (9.8) | 9433 | 66.0 (9.7) | 9408 |
| Male sex - n (%) | 46 | 6214 (65.9) | 9433 | 6178 (66.0)) | 9358 |
| Female sex - n (%) | 46 | 3220 (34.1) | 9433 | 3194 (34.0) | 9358 |
| Mean body mass index (SD) | 10 | 29.0 (5.3) | 7600 | 29.0 (5.3) | 7611 |
| History of atrial fibrillation - n (%) | 6 | 2530 (34.7) | 7290 | 2610 (35.7) | 7301 |
| Diabetes - n (%) | 11 | 2757 (36.3) | 7578 | 2748 (36.2) | 7591 |
| Hypertension - n (%) | 15 | 6024 (77.8) | 7744 | 5995 (77.3) | 7751 |
| Previous heart failure - hospitalisation – n (%) | 5 | 3960 (56.3) | 7034 | 4050 (57.7) | 7044 |
| Previous myocardial infarction - n (%) | 6 | 2454 (33.7) | 7278 | 2417 (33.1) | 7287 |
| NYHA-class – n (%) | 26 | 8735 | 8335 | ||
| NYHA 1 | 291 (0.35) | 307 (0.37) | |||
| NYHA 2 | 5558 (66.7) | 5478 (65.7) | |||
| NYHA 3 | 2205 (26.5) | 2289 (27.4) | |||
| NYHA 4 | 257 (3.1) | 256 (3.1) | |||
| Heart failure classification – n (%) | 32 | 8694 | 8722 | ||
| Heart failure with reduced ejection fraction | 6025 | 6069 | |||
| Heart failure with midrange ejection fraction | 0 | 0 | |||
| Heart failure with preserved ejection fraction | 2669 | 2653 | |||
| Baseline medications – n (%) | |||||
| Beta-blockers | 10 | 6634 (85.8) | 7731 | 6608 (85.6) | 7716 |
| Diuretics | 8 | 6284 (82.9) | 7583 | 6291 (82.5) | 7618 |
| MRA | 9 | 3164 (41.3) | 7654 | 3353 (43.7) | 7667 |
| Pretrial ACE-I | 3 | 3363 (76.5) | 4396 | 3361 (76.0) | 4422 |
| Pretrial ARB | 3 | 1032 (23.5) | 4396 | 1068 (24.2) | 4422 |
| Pretrial ARB/ACE-I | 8 | 6936 (91.5) | 7583 | 6988 (92.0) | 7598 |
ACE-I, angiotensin-converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ARNI, angiotensin receptor blocker neprilysin inhibitor; MRA, mineralocorticoid-receptor antagonists; NYHA, New York Heart Association.
The trials used different control interventions: 14 trials used valsartan, 20 trials used an ACE-I (enalapril=12, benazepril=7, perindopril=1 and ramipril=1); 8 trials did not administer any comparator to the control group besides usual care, which was planned to be administered in both groups (as co-intervention); and 4 trials used either an unspecified ARB or an unspecified ACE-I. One trial used intravenous milrinone as control intervention. All trials used guideline recommended co-interventions (usual care) planned to be delivered similarly in both intervention groups, that is, beta-blockers, mineralocorticoid-receptor antagonists, diuretics and digitalis, if indicated.
Visual inspection of the forest plots and statistical tests (I2 statistics) showed signs of heterogeneity (figure 3 and online supplemental S1 figure). When trials randomising HFrEF participants and trials randomising HFpEF participants were analysed separately, then the heterogeneity was mostly resolved. Hence, we chose to report results separately for each type of heart failure.11
Figure 3.
Forest plot of subgroup based on type of heart failure on all-cause mortality.
openhrt-2020-001294supp003.pdf (226.3KB, pdf)
Participants with heart failure with reduced ejection fraction
Primary outcomes
All-cause mortality
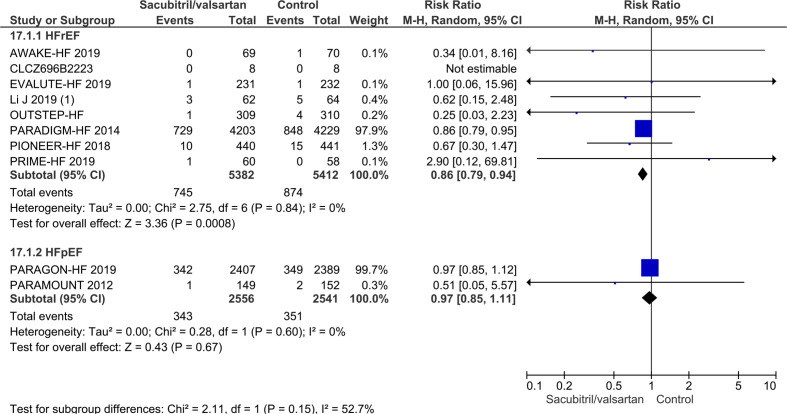
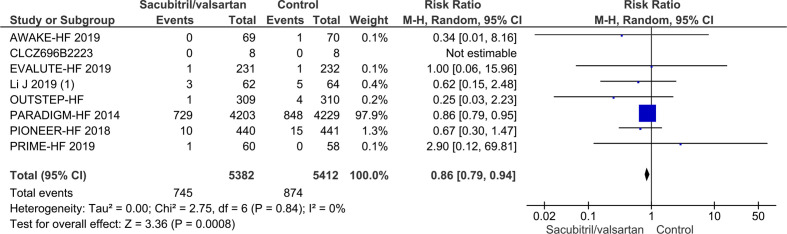
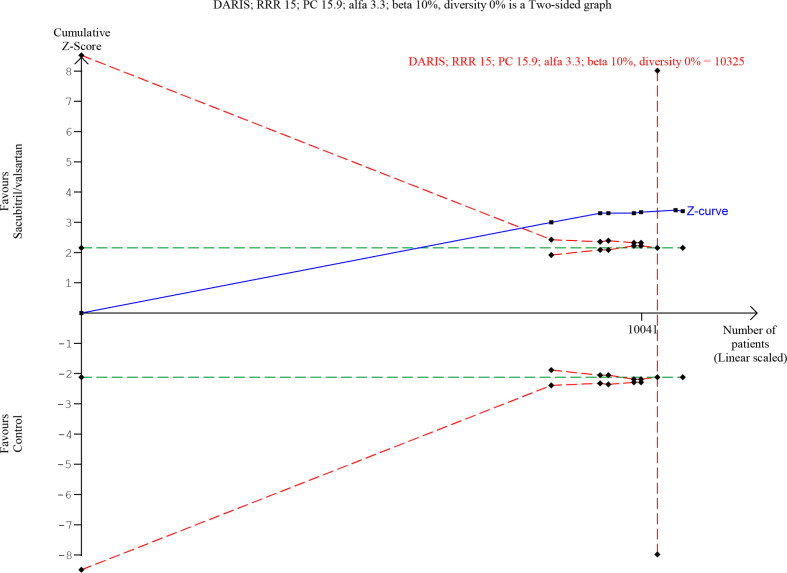
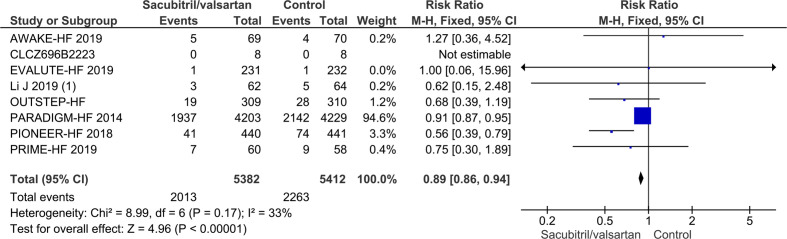
Seven trials randomising a total of 10 794 HFrEF participants reported on all-cause mortality. A total of 745/5382 (13.8%) sacubitril/valsartan participants died compared with 874/5412 (16.1%) control participants (mean follow-up of 22.8 months). Meta-analysis (risk ratio (RR), 0.86; 95% CI, 0.79 to 0.94; p=0.0008) showed evidence of a beneficial effect of sacubitril/valsartan compared with control (figure 4). Neither visual inspection of the forest plot nor tests for statistical heterogeneity (I2=0%; p=0.84) indicated significant heterogeneity. Trial Sequential Analysis showed that there was enough information to confirm that sacubitril/valsartan compared with control reduced the risk of death by 15% (figure 5). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S2 and S3 figures). The following tests of interaction showed no evidence of a difference: (1) acute decompensated heart failure participants compared with chronic heart failure participants (p=0.53); (2) different types of control intervention (valsartan, enalapril and benazepril) (p=0.68); (3) trials published in English compared with trials published in Chinese (p=0.64); and (4) trials using guideline criteria for inclusion compared with trials with broader inclusion criteria (p=0.77) (online supplemental S4–S7 figures). None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
Figure 4.
Forest plot of participants with heart failure with reduced ejection fraction on all-cause mortality.
Figure 5.
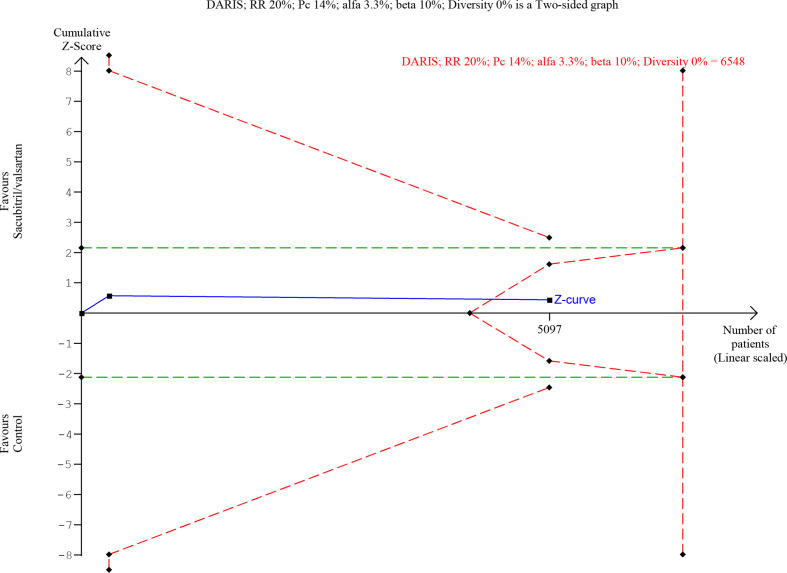
Trial sequential analysis of participants with heart failure with reduced ejection fraction on all-cause mortality.
openhrt-2020-001294supp004.pdf (145.9KB, pdf)
openhrt-2020-001294supp005.pdf (145.3KB, pdf)
openhrt-2020-001294supp006.pdf (230.7KB, pdf)
openhrt-2020-001294supp007.pdf (258.9KB, pdf)
openhrt-2020-001294supp008.pdf (226.9KB, pdf)
openhrt-2020-001294supp009.pdf (227.4KB, pdf)
Serious adverse events
Seven trials randomising a total of 10 794 HFrEF participants reported on serious adverse events. A total of 2013/5382 (37.4%) sacubitril/valsartan participants had a serious adverse event compared with 2263/5412 (41.8%) control participants (mean follow-up of 22.8 months). Meta-analysis (RR, 0.89; 95% CI, 0.86 to 0.94; p=<0.00001) showed evidence of a beneficial effect of sacubitril/valsartan compared with control (figure 6). Neither visual inspection of the forest plot nor test for statistical heterogeneity (I2=33%; p=0.17) indicated significant heterogeneity. Trial Sequential Analysis showed that there was enough information to confirm that sacubitril/valsartan compared with control reduced the risk of serious adverse events by 15% (figure 7). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S8 and S9 figures). The following tests of interaction showed no evidence of a difference: (1) different types of control intervention (valsartan, enalapril and and benazepril) (p=0.81); (2) trials published in English compared with trials published in Chinese (p=0.77); (3) trials using guideline criteria for inclusion compared with trials with broader inclusion criteria (p=0.62) (online supplemental S10–S12 figures). Test of interaction showed evidence of a difference when comparing acute decompensated heart failure participants to chronic heart failure participants (p=0.004) (online supplemental S13 figure). One trial randomised 881 participants with acute decompensated heart failure. However, this trial did not publish a full list of serious adverse events but only reported a predefined composite of serious clinical events. The remaining six trials randomised participants with chronic heart failure. None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
Figure 6.
Forest plot of participants with heart failure with reduced ejection fraction on serious adverse events.
Figure 7.
Trial sequential analysis of participants with heart failure with reduced ejection fraction on serious adverse events.
openhrt-2020-001294supp010.pdf (141.3KB, pdf)
openhrt-2020-001294supp011.pdf (140.8KB, pdf)
openhrt-2020-001294supp012.pdf (257.6KB, pdf)
openhrt-2020-001294supp013.pdf (226.6KB, pdf)
openhrt-2020-001294supp014.pdf (221.3KB, pdf)
openhrt-2020-001294supp015.pdf (229.1KB, pdf)
All serious adverse events were analysed individually and can be found in supplemental appendix (online supplemental S1 table). As a hypothesis generating analyses, we performed meta-analyses on all serious adverse events. Meta-analysis showed evidence of a beneficial effect of sacubitril/valsartan compared with control, when assessing the risk of hyperkalaemia (RR 0.44; 95% CI, 0.26 to 0.76; p=0.003), fatigue (RR 0.10; 95% CI, 0.10 to 0.79; p=0.03) and syncope (RR 0.62; 95% CI, 0.43 to 0.91; P 0.01). Prior neprilysin in combination with ACE-I have shown an elevated risk of angioedema. Meta-analysis showed no evidence of a difference between sacubitril/valsartan compared with control on angioedema (RR 1.01; 95% CI, 0.27 to 3.72; p=0.99).
Secondary outcomes
Myocardial infarction
Two trials randomising a total of 9051 HFrEF participants reported on myocardial infarction. A total of 70/4513 (1.6%) sacubitril/valsartan participants had a myocardial infarction compared with 72/4538 (1.6%) control participants. Meta-analysis (RR, 0.98; 95% CI, 0.71 to 1.35; p=0.89) showed no evidence of a difference between sacubitril/valsartan and control (online supplemental S14 figure). Neither visual inspection of the forest plot nor tests for statistical heterogeneity (I2=0%; p=0.62) indicated significant heterogeneity. Trial Sequential Analysis showed that there was not enough information to confirm or reject that sacubitril/valsartan compared with control reduced the risk of myocardial infarction by 15% (online supplemental S15 figure). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S16 and S17 figures). None of the planned subgroup analyses could be conducted due to lack of relevant data.11
openhrt-2020-001294supp016.pdf (94KB, pdf)
openhrt-2020-001294supp017.pdf (303.5KB, pdf)
openhrt-2020-001294supp018.pdf (93.3KB, pdf)
openhrt-2020-001294supp019.pdf (85.8KB, pdf)
Quality of life
Two Chinese trials randomising 232 HFrEF participants reported on quality of life using the Minnesota Living with Heart Failure Questionnaire (MLHFQ). Meta-analysis (mean difference (MD), −5.19; 95% CI, −8.37 to −2.01; p=0.001) showed evidence of a beneficial effect of sacubitril/valsartan compared with control (online supplemental S18 figure). Both visual inspection of the forest plot and tests for statistical heterogeneity (I2=85%; p=0.01) indicated substantial signs of heterogeneity which could not be resolved. Trial Sequential Analysis showed that there was enough information to confirm a MD of 5 (online supplemental S19 figure). Incomplete outcome data alone did not seem to have the potential to influence the results. Only one trial randomising 8442 participants reported on quality of life using the Kansas City Cardiomyopathy Questionnaire (KCCQ).20 The trial had a follow-up of 36 months and found a least squared mean of 2.28 (0.73), with a p value of 0.002. None of the planned subgroup analyses could be conducted due to lack of relevant data.
openhrt-2020-001294supp020.pdf (96.7KB, pdf)
openhrt-2020-001294supp021.pdf (429.3KB, pdf)
Non-serious adverse events
Nine trials randomising a total of 10 401 HFrEF participants reported on non-serious adverse events. A total of 2738/5186 (52.8%) sacubitril/valsartan participants had a non-serious adverse event compared with 2855/5215 (54.7%) control participants. Meta-analysis (RR, 0.91; 95% CI, 0.74 to 1.11; p=0.35) showed no evidence of a difference between sacubitril/valsartan and control (online supplemental S20 figure). Both visual inspection of the forest plot and tests for statistical heterogeneity (I2=60%; p=0.009) indicated moderate signs of heterogeneity which could not be resolved. Trial Sequential Analysis showed that there was not enough information to confirm or reject that sacubitril/valsartan compared with control reduced the risk of non-serious adverse events by 15% (online supplemental S21 figure). Incomplete outcome data alone did not have the potential to influence the meta-analysis results (online supplemental S22 and S23 figures). Test of interaction showed evidence of a difference when comparing trials only including patients according to current guidelines compared with trials including all HFrEF participants (online supplemental S24 figure). Three subgroup analyses showed no evidence of a difference (online supplemental figures 25–27). None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
openhrt-2020-001294supp022.pdf (155.4KB, pdf)
openhrt-2020-001294supp023.pdf (436.9KB, pdf)
openhrt-2020-001294supp024.pdf (156.3KB, pdf)
openhrt-2020-001294supp025.pdf (155KB, pdf)
openhrt-2020-001294supp026.pdf (240.1KB, pdf)
openhrt-2020-001294supp027.pdf (289.2KB, pdf)
openhrt-2020-001294supp029.pdf (241KB, pdf)
openhrt-2020-001294supp028.pdf (231.9KB, pdf)
Hospitalisations
Four trials randomising a total of 9476 HFrEF participants reported on hospitalisations during follow-up. A total of 594/4724 (12.6%) sacubitril/valsartan participants were hospitalised during follow-up compared with 756/4752 (15.9%) control participants. Meta-analysis (RR, 0.79; 95% CI, 0.72 to 0.87; p=<0.00001) showed evidence of a beneficial effect of sacubitril/valsartan compared with control (online supplemental S28 figure). Neither visual inspection of the forest plot nor tests for statistical heterogeneity (I2=31%; p=0.09) indicated significant heterogeneity. Trial Sequential Analysis showed that there was enough information to confirm that sacubitril/valsartan compared with control reduced the risk of hospitalisations by 15% (online supplemental S29 figure). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S30 and S31 figures). Three subgroup analyses showed no evidence of a difference (online supplemental S32–S34 figures). None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
openhrt-2020-001294supp030.pdf (107.8KB, pdf)
openhrt-2020-001294supp031.pdf (420KB, pdf)
openhrt-2020-001294supp032.pdf (109KB, pdf)
openhrt-2020-001294supp033.pdf (109.2KB, pdf)
openhrt-2020-001294supp034.pdf (187.6KB, pdf)
openhrt-2020-001294supp035.pdf (184.5KB, pdf)
openhrt-2020-001294supp036.pdf (190.2KB, pdf)
Exploratory outcomes
Cardiovascular mortality, ejection fraction, 6-min walking distance and NT-proBNP were analysed as exploratory outcomes. Meta-analyses showed evidence of a beneficial effect of sacubitril/valsartan compared with control when assessing ejection fraction (online supplemental S35 figure), 6-min walking distance (online supplemental S36 figure) and NT-proBNP (online supplemental S37 figure). Only one trial assessed cardiovascular mortality so meta-analysis could not be performed.21
openhrt-2020-001294supp037.pdf (260.3KB, pdf)
openhrt-2020-001294supp038.pdf (238.8KB, pdf)
openhrt-2020-001294supp039.pdf (31.8KB, pdf)
Participants with heart failure with preserved ejection fraction
Primary outcome
All-cause mortality
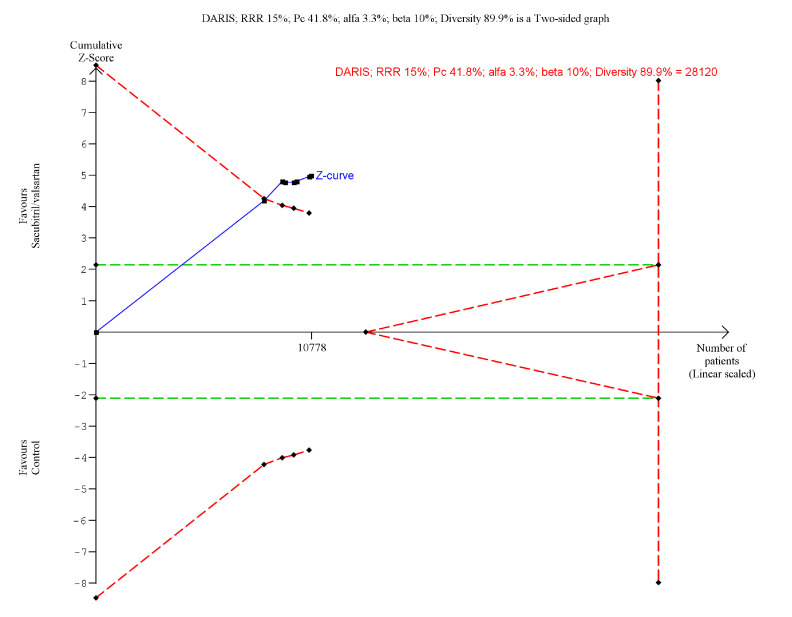
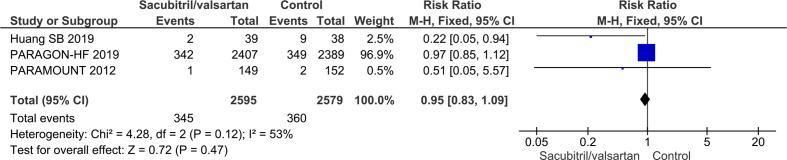
Three trials randomising a total of 5174 HFpEF participants reported on all-cause mortality. A total of 345/2595 (13.3%) sacubitril/valsartan participants died compared with 360/2579 (14.0%) control participants (mean follow-up of 34.1 months). Meta-analysis (RR, 0.95; 95% CI, 0.83 to 1.09; p=0.47) showed no evidence of a beneficial effect of adding sacubitril/valsartan compared with control (figure 8). Both visual inspection of the forest plot and test for statistical heterogeneity (I2=53%; p=0.12) indicated moderate heterogeneity. When removing the trial published in China assessing sacubitril/valsartan compared with usual care, with an extreme result, no heterogeneity was observed. Trial Sequential Analysis showed that there was not enough information to confirm or reject that sacubitril/valsartan compared with control reduced the risk of death by 15% (online supplemental S38 figure). A post-hoc Trial Sequential Analysis showed that there was enough information to confirm that sacubitril/valsartan compared with control did not reduce the risk of death by 20% (figure 9). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S39 and S40 figures). Test of interaction showed evidence of a difference between (1) trials published in English compared with Chinese (p=0.05) and (2) different control interventions (valsartan and usual care) (p=0.05) (online supplemental S41 and S42 figures). None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
Figure 8.
Forest plot of participants with heart failure with preserved ejection fraction on all-cause mortality.
Figure 9.
Trial sequential analysis of participants with heart failure with preserved ejection fraction on all-cause mortality.
openhrt-2020-001294supp040.pdf (413.8KB, pdf)
openhrt-2020-001294supp041.pdf (100.4KB, pdf)
openhrt-2020-001294supp042.pdf (102.3KB, pdf)
openhrt-2020-001294supp043.pdf (180.1KB, pdf)
openhrt-2020-001294supp044.pdf (182.6KB, pdf)
Serious adverse events
Three trials randomising a total of 5174 HFpEF participants reported on serious adverse events. A total of 1448/2607 (55.5%) sacubitril/valsartan participants had a serious adverse event compared with 1455/2592 (56.1%) control participants (mean follow-up of 34.1 months). Meta-analysis (RR, 0.99; 95% CI, 0.94 to 1.04; p=0.63) showed no evidence of a difference between sacubitril/valsartan and control (online supplemental S43 figure). Both visual inspection of the forest plot and test for statistical heterogeneity (I2=64%; p=0.06) indicated moderate heterogeneity. When removing the trial published in China assessing sacubitril/valsartan compared with usual care, with an extreme result, no heterogeneity was observed. Trial Sequential Analysis showed that there was enough information to reject that sacubitril/valsartan compared with control reduced the risk of serious adverse events by 15% (online supplemental S44 figure). Incomplete outcome data alone did not seem to have the potential to influence the meta-analysis results (online supplemental S45 and S46 figures). Test of interaction showed evidence of a difference between (1) trials published in English compared with Chinese (p=0.04) and (2) different control interventions (valsartan and usual care) (p=0.04) (online supplemental S47 and S48 figures). None of the remaining planned subgroup analyses could be conducted due to lack of relevant data.11
openhrt-2020-001294supp045.pdf (96.2KB, pdf)
openhrt-2020-001294supp046.pdf (423.9KB, pdf)
openhrt-2020-001294supp047.pdf (100.5KB, pdf)
openhrt-2020-001294supp048.pdf (178.1KB, pdf)
openhrt-2020-001294supp049.pdf (179.9KB, pdf)
openhrt-2020-001294supp050.pdf (100.4KB, pdf)
Secondary outcomes
Two trials randomising a total of 5122 HFpEF participants reported on myocardial infarction. Meta-analysis (RR, 0.94; 95% CI, 0.59 to 1.50; p=0.79, I2=0%) showed no evidence of a difference between sacubitril/valsartan and control (online supplemental S49 figure). The same trials reported on non-serious adverse events. Meta-analysis (RR, 0.96; 95% CI, 0.85 to 1.09; p=0.56, I2=50%) showed no evidence of a difference between sacubitril/valsartan and control (online supplemental S50 figure). Only one trial assessed quality of life using the KCCQ – OSS score and found no evidence of a difference.22
openhrt-2020-001294supp051.pdf (92.7KB, pdf)
openhrt-2020-001294supp052.pdf (98.4KB, pdf)
Exploratory outcomes
Cardiovascular mortality, ejection fraction, 6-min walking distance and NT-proBNP were analysed as exploratory outcomes. Meta-analysis showed no evidence of a difference when assessing ejection fraction (online supplemental S51 figure). Six-minute walking distance23 and NT-proBNP22 were only assessed in one trial each so meta-analysis could not be performed. No trials reported cardiovascular mortality.
openhrt-2020-001294supp053.pdf (114.1KB, pdf)
Summary of findings
Our main results are presented in the summary of findings tables (tables 3 and 4).
Table 3.
Summary of findings table – heart failure with reduced ejection fraction
| Sacubitril/valsartan compared with control for heart failure with reduced ejection fraction | |||||
| Patient or population: heart failure with reduced ejection fraction Intervention: sacubitril/valsartan Comparison: control | |||||
| Outcomes | No of participants (studies) follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with control | Risk difference with ARNI | ||||
| All-cause mortality follow-up: mean 23 months | 10 794 (seven RCTs) |
⨁⨁⨁◯ MODERATE* |
RR 0.86 (0.79 to 0.94) |
161 per 1.000 |
23 fewer per 1.000 (34 fewer to 10 fewer) |
| Serious adverse events follow-up: mean 23 months | 10 794 (seven RCTs) |
⨁⨁⨁◯ MODERATE* |
RR 0.89 (0.86 to 0.94) |
418 per 1.000 |
46 fewer per 1.000 (59 fewer to 25 fewer) |
| Myocardial infarction follow-up: mean 25 months | 9051 (two RCTs) |
⨁◯◯◯ VERY LOW*†‡ |
RR 0.98 (0.71 to 1.35) |
16 per 1.000 |
0 fewer per 1.000 (5 fewer to six more) |
| Quality of life assessed with: MLHFQ follow-up: mean 2 months | 232 (two RCTs) |
⨁◯◯◯ VERY LOW§¶ |
– | MD 5.19 score lower (8.37 lower to 2.01 lower) |
|
| Non-serious adverse events follow-up: mean 23 | 10 401 (nine RCTs) |
⨁⨁◯◯ LOW*† |
RR 0.95 (0.84 to 1.08) |
547 per 1.000 |
27 fewer per 1.000 (88 fewer to 44 more) |
| Rehospitalisations follow-up: mean 25 months | 9476 (four RCTs) |
⨁⨁◯◯ LOW*‡ |
RR 0.77 (0.69 to 0.87) |
159 per 1.000 |
37 fewer per 1.000 (49 fewer to 21 fewer) |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE, Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
*Downgraded 1 for risk of bias, due to industry funding.
†Downgraded 1 for risk of inconsistency due to moderate heterogeneity.
‡Downgraded 1 for imprecision due to Trial Sequential Analysis showing that there was not enough information to confirm or reject a RRR of 15%. Moreover, the meta-analysis showed wide CI.
§Downgraded 2 for risk of bias.
¶Downgraded 2 for risk of inconsistency due to substantial heterogeneity.
RR, risk ratio; MD, mean difference; ARNI, angiotensin receptor blocker neprilysin inhibitor; RCTs, randomised controlled trials; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MD, mean difference.
Table 4.
Summary of findings table – heart failure with preserved ejection fraction
| Sacubitril/valsartan compared with control for heart failure with preserved ejection fraction | |||||
| Patient or population: heart failure with preserved ejection fraction Intervention: sacubitril/valsartan Comparison: control | |||||
| Outcomes | No of participants (studies) follow-up |
Certainty of the evidence (GRADE) |
Relative effect (95% CI) |
Anticipated absolute effects | |
| Risk with control | Risk difference with ARNI | ||||
| All-cause mortality follow-up: mean 34 months | 5174 (three RCTs) |
⨁◯◯◯ VERY LOW*†‡ |
RR 0.95 (0.83 to 1.09) |
140 per 1.000 |
7 fewer per 1.000 (24 fewer to 13 more) |
| Serious adverse events follow-up: mean 34 months | 5174 (three RCTs) |
⨁⨁◯◯ LOW*† |
RR 0.99 (0.94 to 1.04) |
564 per 1.000 |
6 fewer per 1.000 (34 fewer to 23 more) |
| Myocardial infarction follow-up: mean 34 months | 5122 (two RCTs) |
⨁⨁◯◯ LOW*‡ |
RR 0.94 (0.59 to 1.50) |
14 per 1.000 |
1 fewer per 1.000 (6 fewer to 7 more) |
| Non-serious adverse events follow-up: mean 34 months | 5122 (two RCTs) |
⨁⨁◯◯ LOW*† |
RR 0.96 (0.85 to 1.09) |
934 per 1.000 |
37 fewer per 1.000 (140 fewer to 84 more) |
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
GRADE, Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect.
*Downgraded 1 for serious risk of bias.
†Downgraded 1 for inconsistency due to moderate heterogeneity.
‡Downgraded 1 for imprecision due to Trial Sequential Analysis showing that there was not enough information to confirm or reject a RRR of 15%. Moreover, the meta-analysis showed wide CI.
RR, risk ratio; ARNI, angiotensin receptor blocker neprilysin inhibitor; RCTs, randomised controlled trials.
Discussion
We included a total of 48 trials. HFrEF participants and HFpEF participants were analysed separately due to heterogeneity showing difference in effect between these two types of participants. Twenty-seven trials randomised a total of 12 391 HFrEF participants and four trials randomised a total of 5278 HFpEF participants. The remaining 17 trials randomised a total of 1497 participants, did either not report the participants’ type of heart failure or included a combination of both HFpEF and HFrEF participants. Nevertheless, these 17 trials did not report any data on our primary outcomes, and very limited data for our secondary outcomes. All trials and outcome results were at high risk of bias. The certainty of the evidence according to GRADE was judged to be moderate to very low (tables 3 and 4).
Meta-analyses and Trial Sequential Analyses showed that sacubitril/valsartan compared with control decreases the risk of death, risk of serious adverse events, risk of hospitalisations and NT-proBNP; and seems to increase quality of life using the MLHFQ, ejection fraction and 6-min walking distance; and have no effect on myocardial infarction and non-serious adverse events. Current guidelines recommend sacubitril/valsartan as a replacement for ACE-I in HFrEF patients (EF <35%) who remain symptomatic (NYHA II to IV) despite optimal medical therapy with ACE-I, beta-blocker and mineralocorticoid-receptor antagonist.6 8 Our meta-analyses showed no signs of heterogeneity when including trials regardless of prior treatment with ACE-I, NYHA class and NT-proBNP, that is, our results indicate that sacubitril/valsartan seems to be beneficial in HFrEF patients in general. In addition, we performed a post-hoc subgroup analyses on our primary outcomes, we assessed the difference between trials using guideline recommended inclusion criteria with trials randomising patients with HFrEF irrespective of prior treatment with ACE-I, NYHA class and NT-proBNP. We found no significant subgroup difference (see Primary outcomes). Our results suggest that sacubitril/valsartan might be beneficial for patients with HFrEF in general and not only in the guideline recommended target population. Furthermore, current guidelines highlight that sacubitril/valsartan is associated with an increased risk of hypotension and angioedema. Our results showed no evidence of a difference between sacubitril/valsartan and control when assessing risk of hypotension and angioedema.
In HFpEF participants, meta-analysis and Trial Sequential Analysis showed no evidence of a difference between sacubitril/valsartan and control when assessing all-cause mortality, serious adverse events, myocardial infarction, non-serious adverse events, quality of life and ejection fraction. Not enough data was available for the remaining outcomes.
Our review has several strengths. We followed our protocol, which was registered and published prior to the systematic literature search.11 Data were extracted by two authors in order to minimise the risk of inaccurate data extraction. This systematic review considered both risks of random errors and risks of systematic errors. Bias was assessed according to Cochrane13 and Lundh.14 We used GRADE to assess the certainty of the evidence,24 25 Trial Sequential Analysis to assess the risks of random errors, (online supplemental S3 ref) the eight-step assessment suggested by Jakobsen et al to assess if the thresholds for significance were crossed,17 subgroup analyses to assess possibly heterogeneity and sensitivity analyses to test the potential impact of incomplete outcome data bias.17 We included data from both unpublished and published trials.
Our review also has several limitations. The majority of our participants came from two trials,18 19 which held the largest weight in our meta-analysis. However, the heterogeneity of the trials in our meta-analysis was judged very low, both by test of heterogeneity (p=0.84), and by visual inspection. In addition, the point estimates of the smaller trials is mostly centred around the RR of the meta-analysis results, indicating that the results of the larger trials are reproducible. All included trials had high risk of bias, which might bias our review results.14 All nine English published trials were sponsored by Novartis, which currently produces the only licensed sacubitril/valsartan. This might introduce high risk of bias as study-sponsored studies tend to show more favourable efficacy results and conclusions than trials receiving sponsorship by other sources.14 These limitations should be considered when interpreting the results. There were differences in the choice of control intervention for trials including HFrEF participants. The majority of trials with HFrEF participants used enalapril as control intervention with a target dose of 10 mg twice daily, below the guideline recommended dose of 20 mg. However, the dose is higher than in the trials that lay the basis for recommending enalapril in the first place. In our meta-analysis of trials with HFpEF participants, all trials used valsartan as control except one small trial. However, valsartan is not recommended in guidelines for HFpEF patients, due to their failure to show benefit in large randomised trials.26 27 The choice of control interventions has been an issue of debate and our present results should be interpreted accordingly.28 29 Subgroup analyses assessing the potential difference between control interventions, showed no significant subgroup difference.
We identified 12 ongoing trials. Characteristics of the ongoing trials are summarised in (online supplemental S2 table).
Conclusions
Sacubitril/valsartan compared with either ACE-I or ARB seems to have a beneficial effect in patients with heart failure with reduced ejection fraction. Our results indicate that sacubitril/valsartan might be beneficial in a wider population of patients with heart failure than the guideline recommended target population. Sacubitril/valsartan does not seem to show evidence of a difference compared with valsartan in patients with heart failure with preserved ejection fraction.
Acknowledgments
Department of Internal Medicine – Cardiology Section, Holbæk Hospital, Denmark, and the Institute of Regional Health Research, University of Southern Denmark, Odense, Denmark, paid the salary for all authors during the writing of the review.
Footnotes
Contributors: EEN conceived the systematic review, conducted the literature search, data extraction, data analysis, data interpretation and wrote the article. JF conducted the literature search, data extraction, aided in data interpretation and amended the article. F-LB conducted the literature search, data extraction and amended the article. MHO, IR, and FS-H helped conceive the systematic review, provided invaluable comments and amended the article. JCJ conceived the systematic review, aided in data interpretation and amended the article.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Disclaimer: No ethics approval was obtained, as all data is anonymised and all data used is obtained from clinical trials in which informed consent has already been obtained by trial investigators.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol 2011;8:30–41. 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol 2016;13:368–78. 10.1038/nrcardio.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WRITING COMMITTEE MEMBERS, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of cardiology Foundation/American heart association Task force on practice guidelines. Circulation 2013;128:e240–327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 4.Maggioni AP. Epidemiology of heart failure in Europe. Heart Fail Clin 2015;11:625–35. 10.1016/j.hfc.2015.07.015 [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart 2000;83:596–602. 10.1136/heart.83.5.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of cardiology (ESC). developed with the special contribution of the heart failure association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 7.Jhund PS, McMurray JJV. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart 2016;102:1342–7. 10.1136/heartjnl-2014-306775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 2017;23:628–51. 10.1016/j.cardfail.2017.04.014 [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Shamseer L, Clarke M, Ghersi D, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. 10.1136/bmj.g7647 [DOI] [PubMed] [Google Scholar]
- 11.Nielsen EE, Feinberg J, Raymond I, et al. The effects of adding angiotensin receptor neprilysin inhibitors to usual care in patients with heart failure: a protocol for a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Syst Rev 2019;8:251. 10.1186/s13643-019-1173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Review Manager [program]. 5.3 version 2014.
- 13.Higgins JPT GS. Cochrane Handbook for systematic reviews of interventions version 5.1.0, 2011. www.cochrane-handbook.org [Google Scholar]
- 14.Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017;2:Mr000033. 10.1002/14651858.MR000033.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials 2015;45:139–45. 10.1016/j.cct.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341–8. 10.1002/sim.4780060325 [DOI] [PubMed] [Google Scholar]
- 17.Jakobsen JC, Wetterslev J, Winkel P, et al. Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 2014;14:120. 10.1186/1471-2288-14-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–20. 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 19.McMurray JJV, Packer M, Desai AS, et al. Angiotensin–Neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 20.Lewis EF, Claggett BL, McMurray JJV, et al. Health-Related quality of life outcomes in PARADIGM-HF. Circulation 2017;10 10.1161/CIRCHEARTFAILURE.116.003430 [DOI] [PubMed] [Google Scholar]
- 21.McMurray JJV, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Zile M, Pieske B, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012;380:1387–95. 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- 23.Han Z. nuoxintuozhiliao zuo xinshi she xie fenshu baoliu de xinshuai linchuang liaoxiao guancha [Clinical observation of noxin injection in the treatment of heart failure with left ventricular ejection fraction]. yangsheng baojian zhinan [Health Guide] 2019;29:140. [Google Scholar]
- 24.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Schünemann HJ, et al. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol 2011;64:380–2. 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 26.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359:2456–67. 10.1056/NEJMoa0805450 [DOI] [PubMed] [Google Scholar]
- 27.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved trial. Lancet 2003;362:777–81. 10.1016/S0140-6736(03)14285-7 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro MA. Paradigms and PARAGON-HF. JACC Heart Fail 2018;6:86. 10.1016/j.jchf.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 29.Bernardez-Pereira S, Ramires FJA, de Melo RFT, et al. Was the enalapril dose too low in the PARADIGM-HF trial? Cardiol Rev 2018;26:196–200. 10.1097/CRD.0000000000000193 [DOI] [PubMed] [Google Scholar]
- 30.Owens RL, Birkeland K, Heywood JT, et al. Sleep outcomes from AWAKE-HF, a randomized clinical trial with open-label extension of sacubitril/valsartan versus enalapril in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;73:849 10.1016/S0735-1097(19)31456-1 [DOI] [Google Scholar]
- 31.pharmaceuticals N A randomized, double-blind, controlled, crossover study to evaluate the sodium excretion of LCZ696 in patients with stable heart failure, in patients with hypertension, and in healthy volunteers. Identifier CLCZ696B2223. Novartis Pharmaceuticals Clinical registry, 2013. [Google Scholar]
- 32.Desai AS, Solomon SD, Shah AM, et al. Effect of Sacubitril-Valsartan vs enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2019:1077–10. 10.1001/jama.2019.12843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pharmaceuticals N. randOmized stUdy using acceleromeTry to compare Sacubitril/valsarTan and enalapril in patients with heart failure 2016. Available: https://clinicaltrials.gov/ct2/show/nct02900378
- 34.Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-Neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539–48. 10.1056/NEJMoa1812851 [DOI] [PubMed] [Google Scholar]
- 35.Kang D-H, Park S-J, Shin S-H, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation 2019;139:1354–65. 10.1161/CIRCULATIONAHA.118.037077 [DOI] [PubMed] [Google Scholar]
- 36.Chai DJ, Cheng K, Tu X, et al. shakubaquxieshatan zhiliao wanguxingxinlishuaijie de liaoxiao yu anquanxingguancha [The efficacy and safety of entresto in treatment of patients with refractory heart failure ]. xindian yu xunhuan 2019;38:301–3. [Google Scholar]
- 37.Chen CW. shakubaquxieshatan zhiliao manxing xinlishuaijie de xiaoguo tantao [Effect of Sacubitril Valsartan on the Treatment of Chronic Heart Failure]. zhongxiyijiehe dianzi zazhi 2019;22:40–1. [Google Scholar]
- 38.Chen L, Lu W, Wu Y, et al. shakubaquxieshatan duishexie fenshu jiangdi de xinlishuaijie ji huanzhe shenghua zhibiao he zuo xinshi jiegou de ganyu zuoyong [ Effects of Saeubitril/Valsartan on biochemical indicators and on left ventricular structure in NYHA l Vheart failure with reduced ejection fraction patients]. zhonghua laonianyixue zazhi 2019;38. [Google Scholar]
- 39.Dai WL, Wu X, Wang S, et al. shakubaquxieshatan zhiliao manxing chongxue xing xinlishuaijie huanzhe de linchuang yanjiu [Clinical research on sacubitril/valsartan treating patients with chronic congestive heart failure]. zhongguo yiyao 2019;9:1297–301. [Google Scholar]
- 40.Dong L, Tian Y, Liu S, et al. shakubaquxieshatan napiandui manxing xinlishuaijie huanzhe de liaoxiao ji dui shenjing neifenmi jisu yingxiang [Efficacy of sacubitril/valsartan sodium tablets in treatment of chronic heart failure and the effect on neuroendocrine hormone activity ]. zhongguo yiyao 2019;14:655–8. [Google Scholar]
- 41.Fan JF, Fang Y, Zheng C. shakubaquxieshatanna zhiliao manxing xinlishuaijie huanzhe de linchuang liaoxiao fenxi [Clinical efficacy of sacubitril valsartan in the treatment of chronic heart failure ]. zhongguo linchuang yaolixue yu zhiliaoxue 2019;24:810–4. [Google Scholar]
- 42.Fan TT, He F, Wang X. shakubaquxieshatanyuxieshatan zhiliao manxing xinlishuaijie de zaoqi linchuang liaoxiao bijiao [Comparison of early clinical efficacy of sacubitril/ valsartan(LCZ696) and valsartan in treatment of chronic heart failure ]. anhui yixue 2019;40:618–21. [Google Scholar]
- 43.Fan ZL, Liu S, Xie G, et al. shakubaquxieshatan dui chongxue xing xinlishuaijie huanzhe xueguan neipi sunshang yu xinshi chongsu zhuangtai de yingxiangyanjiu [Study on the Influence of Sacubitril Valsartan for the Vascular Endothelial Injury and Ventricular Remodeling State of Patients with Congestive Heart-failure]. zhongguo yixue chuangxin 2019;16:51–4. [Google Scholar]
- 44.Gao Y, Luan B, Gao Y, et al. shakubaquxieshatan zhiliao manxing xinlishuaijie de linchuang guancha [Clinical observation of Sacrubitril /Valsartan in the treatment of chronic heart failure]. zhongguo xun zheng xinxueguan yixue zazhi 2019;11:595–7. [Google Scholar]
- 45.Hao QM, Cheng J, Xue Y, et al. bijiao shakubaquxieshatan yuxieshatandui manxing xinshuai huanzhe xinsheng de yingxiang [Comparison of the Effects of Sacubitril/valsartan and Valsartan on Heart and Kidney Function in Patients with Chronic Heart Failure ]. xiandai shengwuyixue jinzhan 2019;4:2691–4. [Google Scholar]
- 46.Huang SB, Chen H, Zhou H. shakubaquxieshatan zhiliao xinzangshexiefenshuxiajiang de xinlishuaijiehuanzhe liaoxiaoguancha [Curative Effect Observation of Sacubitril Valsartan in Patients with Heart Failure with Decreased Cardiac Ejection Fraction ]. Neike 2019;14:337–8. [Google Scholar]
- 47.ZF K, Liang Y, Zhang Z, et al. shakubaquxieshatan zhiliao manxing she xie fenshu jiangdi xinlishuaijie li linchuang guancha [Clinical observation of sacubitril-valsartan in chronic heart failure with reduced ejection fraction]. guangdong yikedaxue xuebao 2019;37:335–8. [Google Scholar]
- 48.GX L. shakubaquxieshatan nayubeinapuli zhiliao manxing xinshuai de linchuang xiaoguo pingjia [Clinical evaluation of the treatment of chronic heart failure with Sacubitril Valsartan sodium and benazepril]. ren jiankang 2019;19:125. [Google Scholar]
- 49.Li J, Cao J, Liu W, et al. shakubaquxieshatan zhiliao laonianren kuozhang xing xinji bing zhi manxing xinlishuaijie de liaoxiao guancha [Efficacy of Sacubitril/Valsartan in the treatment of chronic heart failure in elderly patients with dilated cardiomyopathy ]. zhonghua laonianyixue zazhi 2019;38. [Google Scholar]
- 50.Li J, Cao J, Danzeng L, et al. shakubaquxieshatan napian zhiliao gaoyuan diqu gaoxueya manxing xinlishuaijie huanzhe de linchuang xiaoguo [Clinical effect of sacubitril valsartan sodium tablets on hypertensive patients with chronic heart failure in plateau area]. zhongguo yiyao 2019;14:321–4. [Google Scholar]
- 51.Liang HB. shakubaquxieshatan yu pei duo pu li zhiliao she xie fenshu jiangdi de xinlishuaijie de linchuang liaoxiao bijiao [Comparison of the clinical efficacy in the treatment of heart failure with reduced ejection fraction by of sacubitril/valsartan and perindopril ]. guangxi yikedaxue 2019. [Google Scholar]
- 52.Liu DN, Li X, Ma J, et al. shakubaquxieshatanna zhiliao she xie fenshu jianshao manxing xinlishuaijie de yanjiu [Therapeutic Effects of Sacubitril/ Valsrtan in Heart Failure Patients and Reduced Ejection Fraction ]. ningxia yixue zazhi 2019;4:1–4. [Google Scholar]
- 53.Liu YH, Li F, Wang J, et al. shakubaquxieshatan zhiliao manxing xinlishuaijie [Observation on the Therapeutic Effect of Sacubitril Valsartan on Chronic Heart Failure]. changchun zhongyi yao daxue xuebao 2019;35:457–60. [Google Scholar]
- 54.SH P, Guan Y, Yang Z, et al. shakubaquduanshatan zhiliao zuo xinshi she xie fenshu jiangdi xinlishuaijie huanzhe de linchuang liaoxiao guancha [The efficiency of Sacubitril/Valsartan therapy in patients with heart failure with reduced ejection fraction]. zhongguo yanjiu 2019;17. [Google Scholar]
- 55.Shen JH. shakubaquxieshatan zhiliao manxing xinlishuaijie de liaoxiao ji anquanxing yanjiu [Efficacy and safety of saponin and valsartan in the treatment of chronic heart failure]. zhongguo baojian yingyang 2019;29:266. [Google Scholar]
- 56.Song Z, Peng J, Liu Z, et al. shakubaquxieshatan lianhe qumeitaqin zhiliao manxing xinlishuaijie de linchuang guancha [Clinical efficacy of combined sacubitril-valsartan and trimetazidine in chronic heart failure ]. guangdong yikedaxue xuebao 2019;37:331–4. [Google Scholar]
- 57.Sun X, Jia Y. shakubaquxieshatanna zhiliao manxing xinlishuaijie de linchuang liaoxiao guancha [Clinical observation of the efficacy of sirubatra and valsartan in the treatment of chronic heart failure]. shijie zuixin yixue xinxi wenzhai 2019;19:125. [Google Scholar]
- 58.Sun XN, Qu F. nuoxintuodui manxing xin gongneng bu quan huanzhe shenghuozhiliang de yingxiang [Effect of Entresto on quality of life in patients with chronic heart failure ]. jiankang bi dou 2018;17:20–1. [Google Scholar]
- 59.Tang J. shakubaquxieshatan lianhe bisuo luo er zhiliao manxing xinlishuaijie de linchuang xiaoguo guancha [Clinical observation of the treatment of chronic heart failure with saponin and bisoprolol ]. linchuang heli yongyao zazhi 2018;11:39–40. [Google Scholar]
- 60.Wang QD, Lai M. shakubaquxiesh tan duijixingduan taigao xing qian bi xinjigengsi bing xinlishuaijie huanzhe xin yingxiang [Effect of Sacubitril Valsartan on cardiac function in patients with acute ST- segment elevation myocardial infarction of the anterior wall complicated with heart failure ]. guangzhou yikedaxue xuebao 2019;47:92–5. [Google Scholar]
- 61.Wei ZX, Dongo L, Yuan Z, et al. shakubaquxieshatan zhiliao zuoxinjibing xiangguanxing feidongmaigaoya de linchuangyanjiu [Clinical trial of sacubitril/valsartan in the treatment of Pulmonary hypertension (PH) due to left heart disease (LHD)]. zhongguo linchuang yaolixue yu zhiliaoxue 2019;24:57–62. [Google Scholar]
- 62.MM W. shakubaquxieshatan duishexie fenshu jiangdi de xinlishuaijie huanzhe xin yingxiang [Effect of Sacubitril/valsartan on heart function in patients with heart failure reducedd ejection fraction]. hebei yikedaxue 2018. [Google Scholar]
- 63.Yang J. shakubaquxieshatan nayubeinapuli zhiliao manxing xinshuai de liaoxiao guancha [Therapeutic effect of saponin and valsartan on chronic heart failure]. mu ying shijie 2019;13:134–6. [Google Scholar]
- 64.Yang RC, Wang S. shakubaquxieshatan zhiliao tangniaobing xinji bing xin gongneng bu quan de linchuang liaoxiao guancha[Observation on Clinical Therapeutic Effect of Sacubitril uba and Valsartan on Cardiac Dysfunction in Patients with Diabetic Cardiomyopathy ]. zhongguo heli yongyao tansuo 2019;16:121–3. [Google Scholar]
- 65.Yao LN. shakubaquxieshatan zhiliao gaoling she xie fenshu jiangdi xinlishuaijie [Sacubitril Valsartan in the treatment of advanced ejection fraction reduces heart failure]. zhongxiyijiehe dianzi zazhi 2019;7:5–6. [Google Scholar]
- 66.Yu H, Zhao T, Liu K. shakubaquxieshatan napian zhiliao laonian manxing xinlishuaijie de xiaoguo [Effect of Sacubitril Valsartan Tablets on Elderly Patients with Chronic Heart Failure]. zhongguo laonianxue zazhi 2019;39:3620–2. [Google Scholar]
- 67.ZL Y. shakubaquxieshatan zhiliao wanguxingxinlishuaijie de xiaoguoguancha [Effect of Shakuba Qusarsar on the Treatment of Refractory Heart Failure]. haixia yaoxue 2018;30:194–5. [Google Scholar]
- 68.Zhang H. shakubaquxieshatan yuyinapuli dui quexue xing xinji binghuan zhe xueliu jie dao de xieguankuozhang gongneng ji jingdongmai neizhong mo houdu de yingxiang [The effects of sacubitril/valsartan versus enalapril on flow-mediated dilation and carotid intima-media thickness in patients with heart failure and obstructive coronary artery disease] 2018.
- 69.Zhang JW, Xie Z, Wu F, et al. sha ku ba qu xie sha tan na pian nuo xin tuo dui manxing xinlishuaijie huanzhe liaoxiao ji shuiping de [The effect of Sacubitril Valsartan Sodium Tablets (Entresto) on the curative effect and the influence of BNP of in patients with chronic heart failure]. jilin yixue 2019;40:1430–3. [Google Scholar]
- 70.Zhang XJ, Huang W, Xu Y. shakubaquxieshatanhe yansuan beinapuli pian duixin li shuaijie huanzhe xinshi chongsu yanzheng yinzi de yingxiang [Clinical study of the effects of entresto and lotensin on ventricular remodeling and inflammatory factors in patients with heart failure ]. shiyong yixue zazhi 2019;35:795–9. [Google Scholar]
- 71.Zhang Y, Yang G, Li D, et al. shakubaquxieshatan zhiliao manxing xinlishuaijie fei dongmai gaoya de liaoxiao fenxi [Efficacy of sacubitril/valsartan on pulmonary hypertension associated with left heart failure with reduced ejection fraction]. yiyao luntan zazhi 2019;40:5–8. [Google Scholar]
- 72.Zhang YZ, Zhao C, Cao Q, et al. shakubaquxieshatan nayubeinapuli zhiliao manxing xinshuai de liaoxiao guancha [Therapeutic effect of saponin and valsartan on chronic heart failure]. shijie zuixin yixue xinxi wenzhai 2019;19:128–9. [Google Scholar]
- 73.Zhao YQ, Liu H, Yang Y, et al. shakubaquxieshatan lianhe andiantong zhiliao hebing zhen fa xing fang chan de laonian xing xinlishuaijie de liaoxiao guancha [Therapeutic effects of sacubitril valsartan combined with amiodarone on senile heart failure complicated by paroxysmal atrial fibrillation ]. hebei yiyao 2019;41:404–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2020-001294supp001.pdf (789.4KB, pdf)
openhrt-2020-001294supp002.pdf (216.3KB, pdf)
openhrt-2020-001294supp003.pdf (226.3KB, pdf)
openhrt-2020-001294supp004.pdf (145.9KB, pdf)
openhrt-2020-001294supp005.pdf (145.3KB, pdf)
openhrt-2020-001294supp006.pdf (230.7KB, pdf)
openhrt-2020-001294supp007.pdf (258.9KB, pdf)
openhrt-2020-001294supp008.pdf (226.9KB, pdf)
openhrt-2020-001294supp009.pdf (227.4KB, pdf)
openhrt-2020-001294supp010.pdf (141.3KB, pdf)
openhrt-2020-001294supp011.pdf (140.8KB, pdf)
openhrt-2020-001294supp012.pdf (257.6KB, pdf)
openhrt-2020-001294supp013.pdf (226.6KB, pdf)
openhrt-2020-001294supp014.pdf (221.3KB, pdf)
openhrt-2020-001294supp015.pdf (229.1KB, pdf)
openhrt-2020-001294supp016.pdf (94KB, pdf)
openhrt-2020-001294supp017.pdf (303.5KB, pdf)
openhrt-2020-001294supp018.pdf (93.3KB, pdf)
openhrt-2020-001294supp019.pdf (85.8KB, pdf)
openhrt-2020-001294supp020.pdf (96.7KB, pdf)
openhrt-2020-001294supp021.pdf (429.3KB, pdf)
openhrt-2020-001294supp022.pdf (155.4KB, pdf)
openhrt-2020-001294supp023.pdf (436.9KB, pdf)
openhrt-2020-001294supp024.pdf (156.3KB, pdf)
openhrt-2020-001294supp025.pdf (155KB, pdf)
openhrt-2020-001294supp026.pdf (240.1KB, pdf)
openhrt-2020-001294supp027.pdf (289.2KB, pdf)
openhrt-2020-001294supp029.pdf (241KB, pdf)
openhrt-2020-001294supp028.pdf (231.9KB, pdf)
openhrt-2020-001294supp030.pdf (107.8KB, pdf)
openhrt-2020-001294supp031.pdf (420KB, pdf)
openhrt-2020-001294supp032.pdf (109KB, pdf)
openhrt-2020-001294supp033.pdf (109.2KB, pdf)
openhrt-2020-001294supp034.pdf (187.6KB, pdf)
openhrt-2020-001294supp035.pdf (184.5KB, pdf)
openhrt-2020-001294supp036.pdf (190.2KB, pdf)
openhrt-2020-001294supp037.pdf (260.3KB, pdf)
openhrt-2020-001294supp038.pdf (238.8KB, pdf)
openhrt-2020-001294supp039.pdf (31.8KB, pdf)
openhrt-2020-001294supp040.pdf (413.8KB, pdf)
openhrt-2020-001294supp041.pdf (100.4KB, pdf)
openhrt-2020-001294supp042.pdf (102.3KB, pdf)
openhrt-2020-001294supp043.pdf (180.1KB, pdf)
openhrt-2020-001294supp044.pdf (182.6KB, pdf)
openhrt-2020-001294supp045.pdf (96.2KB, pdf)
openhrt-2020-001294supp046.pdf (423.9KB, pdf)
openhrt-2020-001294supp047.pdf (100.5KB, pdf)
openhrt-2020-001294supp048.pdf (178.1KB, pdf)
openhrt-2020-001294supp049.pdf (179.9KB, pdf)
openhrt-2020-001294supp050.pdf (100.4KB, pdf)
openhrt-2020-001294supp051.pdf (92.7KB, pdf)
openhrt-2020-001294supp052.pdf (98.4KB, pdf)
openhrt-2020-001294supp053.pdf (114.1KB, pdf)