Abstract
The present study was an investigation of the neuroprotective effects of probiotic bacteria in SH-SY5Y neuroblastoma cells experiencing oxidative stress. The bacterial strains were: commercial Lactobacillus rhamnosus GG; two isolated bacterial strains (Lactobacillus delbrueckii KU200170 and Lactobacillus plantarum KU200661); and probiotic Lactococcus lactis KC24. To evaluate the neuroprotective effects of the bacteria, a conditioned medium (CM) was prepared using HT-29 cells cultured with the heat-killed probiotic strains. Of the bacterial strains tested, the oxidatively stressed SH-SY5Y cells were most viable when cultured with L. lactis KC24-CM. L. lactis KC24-CM promoted the expression of brain-derived neurotropic factor (BDNF) in the HT-29 cells. It also significantly increased BDNF expression and reduced the apoptosis-related Bax/Bcl-2 ratio in the oxidatively stressed SH-SY5Y cells. Therefore, L. lactis KC24 is a potential psychobiotic for use in the functional food industry.
Keywords: Probiotics, Lactococcus lactis, Gut-brain-axis, Neuroprotective effect, Conditioned medium
Introduction
Neurobiological investigations into gut–brain crosstalk have revealed two-way communication that ensures gastrointestinal homeostasis, and have multiple effects on the neuronal activities of the brain that are associated with motivation and higher cognitive functions (Mayer, 2011; Foster and Neufeld, 2013). Two-way communication can be explained the autonomic nervous system, the enteric nervous system, the neurodendorcine system, and the immune system. These networks were affected by the gastrointestinal tract. The microbiota of the gut comprises over 5000 bacterial species (Carabotti et al., 2015). The modulatory effect of probiotics on the intestinal microbiota improves the intestinal barrier and mucosal immune response, thereby facilitating the treatment of microbe-related diseases (Holmes et al., 2011).
Reactive oxygen species (ROS) originate from both exogenous and endogenous sources, and can damage proteins, lipids, and other molecules. However, ROS are also essential for cellular function, and can be safely metabolized by an antioxidant mechanism (Phaniendra et al., 2015). The oxidative stress induced by ROS can contribute to pathologies including cancer (Valko et al., 2006), cardiovascular disease (Dhalla et al., 2000), diabetes (Giugliano et al., 1996), and aging (Sohal and Weindruch, 1996). The brain is particularly vulnerable to the effects of ROS because it has a high oxygen demand and an abundance of lipid cells (Leutner et al., 2001).
Several probiotics have been reported to have neuroprotective effects. Bifidobacterium longum 1714 had a positive effect on cognition in an anxious mouse (Savignac et al., 2015). Both Lactobacillus rhamnosus JB-1 and Bacteroides fragilis were able to activate intestinal afferent neurons ex vivo, and the polysaccharide A produced by B. fragilis completely represented the neuronal effects (Mao et al., 2013). Lactobacillus reuteri enhanced the emotionality of colonic neurons in naive rats by inhibiting Ca2+−dependent potassium channels (Kunze et al., 2009). Probiotic mixtures containing L. rhamnosus and Lactobacillus helveticus ameliorated maternal separation-induced depression by normalizing corticosterone levels (Gareau et al., 2007).
Psychobiotics are of interest to the functional food industry because the gut microbiota affects mental health. Therefore, the aim of this study was to investigate the neuroprotective effects with regard to oxidative stress of lactic acid bacteria isolated from traditional Korean fermented foods.
Materials and methods
Bacterial strains and culture conditions
Lactobacillus delbrueckii KU200170 (Lim et al., 2020), Lactobacillus plantarum KU200661 (Lim et al., 2020), Lactococcus lactis KC24 (Lee et al., 2015), and Lactobacillus rhamnosus GG (Cell Biotech., Ltd., Gimpo, Korea) were used in the present study. The lactic acid bacteria were propagated and maintained in lactobacilli MRS medium (MRS; BD Biosciences, Franklin Lakes, NJ, USA) at 37 °C, and were then cultured in MRS broth for 24 h. The bacterial cells were centrifuged (12,000×g at 4 °C for 10 min), and washed in phosphate-buffered saline (PBS; HyClone Laboratories Inc., Logan, UT, USA). The harvested bacteria were killed by heating at 121 °C for 15 min, and stored at − 80 °C until required.
Cell line culture
HT-29 human intestinal cells (KCLB 30038) and SH-SY5Y human neuroblastoma cells (KCLB 22266) were obtained from the Korean Cell Line Bank (Seoul, Korea). The HT-29 and SH-SY5Y cells were grown in Roswell Park Memorial Institute medium 1640 (RPMI 1640; Gibco, Grand Island, NY, USA) and Dulbecco’s modified Eagle’s medium (DMEM; HyClone Laboratories Inc.), respectively. The cells were kept at 37 °C in 5% CO2. All media were supplemented with 5% fetal bovine serum (HyClone) with 1% (v/v) penicillin–streptomycin (HyClone).
Preparation of conditioned medium (CM) from HT-29 cells
The conditioned medium (CM) was prepared using HT-29 cells cultured with heat-killed probiotics (Park et al., 2017). The probiotics culture was centrifuged at 14,000×g, 4 °C for 10 min, and the collected cell was washed twice with PBS. The harvested bacteria were then heated at 121 °C for 15 min to produce heat-killed lactic acid bacteria (LAB), which were stored at − 80 °C until required. The monolayered HT-29 cells were treated with two concentrations (1.0 × 107 and 1.0 × 108 CFU/well) of the heat-killed LAB for 24 h. After incubation, the supernatant was collected by centrifugation (12,000×g for 10 min) and filtered by syringe filtration (0.45-μm membrane filter). This CM was stored at -80 °C until required.
Protective effect of LAB-CM on SH-SY5Y neuroblastoma cells experiencing hydrogen peroxide-induced oxidative stress
The neuroprotective effect on the oxidatively stressed SH-SY5Y cells was determined by the following methods (Park et al., 2017). The SH-SY5Y cells (1.0 × 105 cells/well) were incubated to form a confluent monolayer on a 96-well plate. After pretreatment with 80 μL of LAB-CM for 4 h, the SH-SY5Y cells in each well were treated with 20 μL of 50 μM hydrogen peroxide (H2O2; Junsei Chemical Co. Ltd., Tokyo, Japan) for 20 h. After incubation, the media were removed, and the cells were incubated with 100 μL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL) for 1 h. The absorbance was measured using a microplate reader at 540 nm. The cell viability (%) was calculated using the Eq. (1):
| 1 |
BDNF gene expression in the HT-29 cells
BDNF gene expression was determined using the following modified methods (Park et al., 2017). The HT-29 cells were inoculated into 6-well plates at a density of 1.0 × 106 cells/well, and incubated to form a confluent monolayer. After incubation, the cells were treated with heat-killed LAB for 24 h. The total RNA of the HT-29 cells was isolated using a total RNA isolation kit (Qiagen, Hilden, Germany), and converted to complementary DNA (cDNA) using a cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA, USA). Brain-derived neurotrophic factor (BDNF) gene expression was confirmed by real-time polymerase chain reaction (PCR) analysis.
Confirmation of BDNF, Bax, and Bcl-2 gene expression in the SH-SY5Y cells experiencing H2O2-induced oxidative stress by reverse transcription polymerase chain reaction (RT-PCR) analysis
The SH-SY5Y cells (1.0 × 106 cells/well) were seeded into a 6-well plate and incubated to form a confluent monolayer. After incubation, the cells were treated with 800 μL of CM for 4 h. Subsequently, 200 μL of H2O2 (50 μM) was added to induce oxidative stress for 20 h (Cheon et al., 2020; Park et al., 2017). The total RNA was isolated using an RNeasy Mini total RNA isolation kit and primers. The cDNA was manufactured using a cDNA synthesis kit (Thermo Fisher Scientific, Waltham, MA). The primers were those described by Cheon et al. (2020). The PCR data were evaluated using the 2−ΔΔCT method, and are expressed as fold changes.
Statistical analysis
All data are represented as the mean and standard deviation by three replicates. Analysis of variance (ANOVA) was used to determine significant differences. The mean values were utilized for the Duncan’s multiple range test for post hoc verification (p < 0.05).
Results and discussion
Protective effects of LAB-CM in SH-SY5Y cells experiencing H2O2-induced oxidative stress
L. delbrueckii KU200170, L. plantarum KU200661, and L. lactis KC24 was reported on their probiotic properties included antimicrobial effect (Lee et al., 2015; Lim et al., 2020). Therefore, this study was dealt on neuroprotective effects using heat-killed probiotics. Cell viability was determined to evaluate the neuroprotective effects of LAB-CM in oxidatively stressed SH-SY5Y cells (Fig. 1). The positive control treated with H2O2 exhibited 47.46 ± 1.38% cell viability. The LAB-CM (1.0 × 108 CFU/mL) using L. rhamnosus GG, L. delbrueckii KU200170, L. plantarum KU200661, and L. lactis KC24 exhibited 55.93 ± 5.53%, 63.28 ± 5.08%, 65.00 ± 2.73%, and 67.23 ± 5.59% cell viability, respectively. LAB-CM concentrations of 1.0 × 107 CFU/mL and 1.0 × 108 CFU/mL resulted in similar or higher cell viability values to that in the positive control. These results confirm that in Ruminococcus albus-CM, the viability of oxidatively stressed SH-SY5Y cells depends on the concentration of colony-forming units (106–108 CFU/mL) (Park et al., 2017). The antioxidant effects of L. rhamnosus GG, and of L. lactis KC24 and LGG have been evaluated by determining ferric reducing ability of plasma (FRAP) values, and the inhibition of β-carotene and linoleic acid (Lee et al., 2015; Mishra et al., 2015). Therefore, the neuroprotective effects of L. rhamnosus GG and L. lactis KC24 may be influenced by their antioxidant effects.
Fig. 1.
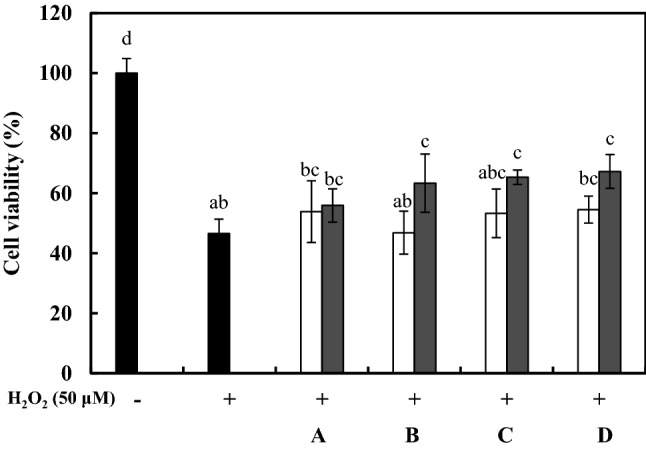
Protective effects of heat-killed lactic acid bacteria–conditioned medium (LAB-CM) on cell viability in SH-SY5Y cells experiencing oxidative stress induced by H2O2 (50 μM). Cell viability was evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. A, Lactobacillus rhamnosus GG; B, Lactobacillus delbrueckii KU200170; C, Lactobacillus plantarum KU200661; D, Lactobacillus lactis KC24. The error bars indicate standard deviations from three independent experiments. The letters on each bar exemplify significant differences (p < 0.05). open square, 1.0 × 107 CFU/mL; filled square, 1.0 × 108 CFU/mL
BDNF gene expression in HT-29 cells following treatment with heat-killed LAB
Microbial dysbiosis can reduce BDNF expression in the brain and gut (Bistoletti et al., 2019). BDNF expression is also involved in the homeostatic regulation of intestinal barrier integrity (Li et al., 2018; Yu et al., 2017). Therefore, probiotic bacterial strains may increase the expression of BDNF by modulating intestinal cells. The HT-29 cells were treated with 1.0 × 108 CFU/mL of heat-killed LAB, and RT-PCR analysis was used to determine the level of BDNF gene expression from the extracted RNA (Fig. 2). The levels of BDNF gene expression in the HT-29 cells treated with heat-killed L. delbrueckii KU200170 and L. plantarum KU200661 were not significantly different to those in the PBS-treated controls. The levels of BDNF gene expression in the HT-29 cells treated with heat-killed L. rhamnosus GG and with L. lactis KC24 were 2.23 ± 0.13- and 1.84 ± 0.07-fold, respectively. Therefore, heat-killed L. rhamnosus GG and L. lactis KC24 may be useful for the treatment of intestinal conditions.
Fig. 2.
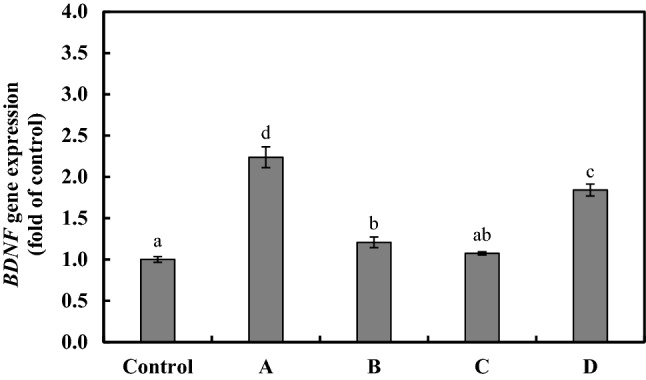
mRNA expression of the BDNF gene in HT-29 cells treated with heat-killed lactic acid bacteria (LAB). A, Lactobacillus rhamnosus GG; B, Lactobacillus delbrueckii KU200170; C, Lactobacillus plantarum KU200661; D, Lactobacillus lactis KC24. The control was treated with phosphate-buffered saline (PBS). The error bars indicate standard deviations from three independent experiments. The letters on each bar exemplify significant differences (p < 0.05)
BDNF gene expression and anti-apoptosis in heat-killed LAB-CM-treated SH-SY5Y neuroblastoma cells experiencing H2O2-induced oxidative stress
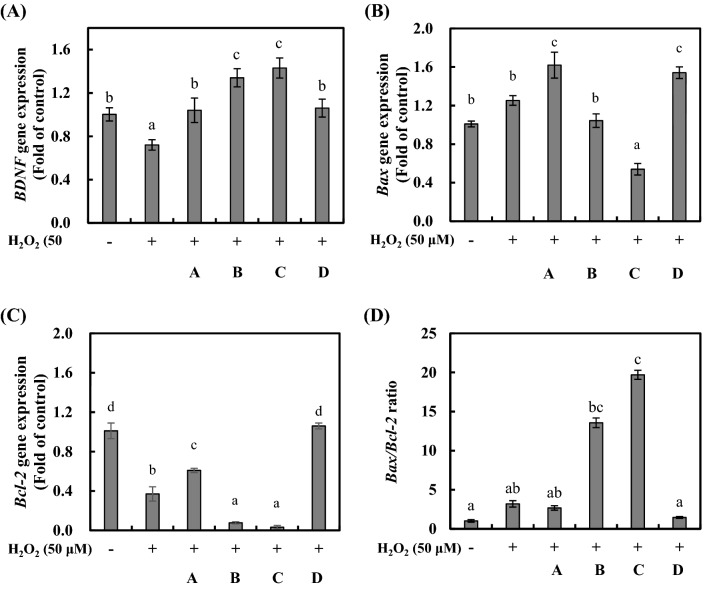
Oxidative stress was induced in the SH-SY5Y cells by treatment with H2O2, and BDNF production was determined using heat-killed LAB-CM (Fig. 3A). BDNF gene expression in the H2O2-treated positive control was reduced by 0.72 ± 0.05-fold compared to that in the negative control. The BDNF gene expression rates were higher in the CMs than in the negative control group. The BDNF gene expression rates in the SH-SY5Y cells treated with the LAB-CM (1.0 × 108 CFU/mL) using heat-killed L. rhamnosus GG, L. delbrueckii KU200170, L. plantarum KU200661, and L. lactis KC24 were 1.04 ± 0.11-, 1.34 ± 0.08-, 1.43 ± 0.09-, and 1.05 ± 0.08-fold, respectively. There was no difference in the level of BDNF expression between the intestinal cells and the neuroblastoma cells. The administration of L. rhamnosus modulates the gut microbiota of zebrafish, and the expression of BDNF in the gut and brain (Borrelli et al., 2016).
Fig. 3.
mRNA expression of the BDNF gene (A), Bax gene (B), Bcl-2 gene (C), and the Bax/Bcl-2 ratio (D) in oxidatively stressed SH-SY5Y cells treated with heat-killed lactic acid bacteria–conditioned medium (LAB-CM). Oxidative stress was induced by H2O2 (50 μM). A, Lactobacillus rhamnosus GG; B, Lactobacillus delbrueckii KU200170; C, Lactobacillus plantarum KU200661; D, Lactobacillus lactis KC24. The error bars indicate standard deviations from three independent experiments. The letters on each bar exemplify significant differences (p < 0.05)
Bax is a pro-apoptotic Bcl-2-family protein in cytosol and translocated to mitochondria upon induction of apoptosis (Hsu et al., 1997). The Bax/Bcl-2 ratio is more important than the expression for each gene in determining susceptibility to apoptosis (Raisova et al., 2001).
The mRNA levels of the pro-apoptotic factor Bax was increased 1.25-fold under H2O2 treatment compared with the negative control (Fig. 3B). CM using L. rhamnosus GG and L. lactis KC24 increased by 1.62- and 1.54-fold. While L. delbrueckii KU200170 and L. plantarum KU200661 was 1.04- and 0.53-fold. The mRNA levels of anti-apoptotic factor, Bcl-2 was decreased 0.37-fold under H2O2 treatment compared with the negative control (Fig. 3C). The LAB-CM using L. rhamnosus GG, L. delbrueckii KU200170, and L. plantarum KU200661 reduced 0.37-, 0.08-, and 0.04-fold, respectively. However, L. lactis KC24 maintained by 1.06-fold.
A decrease in apoptosis can be explained by a reduced Bax/Bcl-2 ratio. The Bax/Bcl-2 ratio improved significantly in the SH-SY5Y cells that had been co-cultured with H2O2, resulting in apoptosis. The Bax/Bcl-2 ratios in the negative control and in the positive control that had received H2O2 treatment were 1.01 ± 0.17 and 3.19 ± 0.41, respectively (Fig. 3D). The Bax/Bcl-2 ratios in the SH-SY5Y cells that had been treated with the LAB-CM (1.0 × 108 CFU/mL) using L. rhamnosus GG, L. delbrueckii KU200170, L. plantarum KU200661, and L. lactis KC24 were 2.67 ± 0.28, 13.56 ± 0.62, 19.69 ± 0.58, and 1.45 ± 0.58, respectively. Therefore, the L. lactis KC24 ratio was similar to that in the negative control. L. lactis KC24-CM exhibited the highest neuroprotective effect of all the samples in the oxidatively stressed SH-SY5Y cells. A probiotic cocktail comprising L. rhamnosus GG, Bifidobacterium animalis subsp. lactis, and Lactobacillus acidophilus prevented neurodegenerative diseases in mice (Srivastav et al., 2019). R. albus-CM significantly affected the Bax/Bcl-2 ratio (by 1.3–1.5) in oxidatively stressed SH-SY5Y cells (Park et al., 2017). Therefore, L. lactis KC24 has the potential to prevent neurodegenerative diseases.
In conclusion, the antimicrobial, antioxidant, and anticancer effects of the probiotic L. lactis KC24 have been reported; the present study evaluated its neuroprotective effects in oxidatively stressed SH-SY5Y neuroblastoma cells. Treatment with L. lactis KC24 increased cell viability, increased BDNF gene expression, and reduced the Bax/Bcl-2 ratio in oxidatively stressed SH-SY5Y cells. These results suggest it has potential as a prophylactic therapy for neuroprotection.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sung-Min Lim, Email: bluenoah37@nate.com.
Na-Kyoung Lee, Email: lnk11@konkuk.ac.kr.
Hyun-Dong Paik, Email: hdpaik@konkuk.ac.kr.
References
- Bistoletti M, Caputi V, Maranzini N, Marchesi N, Filpa V, Marsilio I, Cerantola S, Terova G, Baj A, Grimaldi A, Pascale A, Frigo G, Crema F, Giron MC, Giaroni C. Antibiotic treatment-induced dysbiosis differently affects BDNF and TrkB expression in the brain and in the gut of juvenile mice. PLoS One. 2019;14:e0212856. doi: 10.1371/journal.pone.0212856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli L, Aceto S, Agnisola C, De Paolo S, Dipineto L, Stilling RM, Dinan TG, Cryan JF, Menna LF, Fioretti A. Probiotic modulation of the microbiota-gut-brain axis and behavior in zebrafish. Sci. Rep. 2016;6:30046. doi: 10.1038/srep30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Cheon MJ, Lim SM, Lee NK, Paik HD. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int. J. Mol. Sci. 2020;21:1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Foster JA, Neufeld KAM. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Gareau MG, Jury J, MacQueen G, Sherman PM, Perdue MH. Probiotic treatment of rat pups normalises corticosterone release and ameliorates colonic dysfunction induced by maternal separation. Gut. 2007;56:1522–1528. doi: 10.1136/gut.2006.117176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diab. Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Holmes E, Li JV, Athanasiou T, Ashrafian H, Nicholson JK. Understanding the role of gut microbiome–host metabolic signal disruption in health and disease. Trends Microbiol. 2011;19:349–359. doi: 10.1016/j.tim.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Natl. Acad. Sci. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze WA, Mao YK, Wang B, Huizinga JD, Ma X, Forsythe P, Bienenstock J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009;13:2261–2270. doi: 10.1111/j.1582-4934.2009.00686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT Food Sci. Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- Leutner S, Eckert A, Müller WE. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001;108:955–967. doi: 10.1007/s007020170015. [DOI] [PubMed] [Google Scholar]
- Li C, Cai YY, Yan ZX. Brain-derived neurotrophic factor preserves intestinal mucosal barrier function and alters gut microbiota in mice. Kaohsiung J. Med. Sci. 2018;34:134–141. doi: 10.1016/j.kjms.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Lee NK, Paik HD. Antibacterial and anticavity activity of probiotic Lactobacillus plantarum 200661 isolated from fermented foods against Streptococcus mutans. LWT Food Sci. Technol. 2020;118:108840. doi: 10.1016/j.lwt.2019.108840. [DOI] [Google Scholar]
- Mao YK, Kasper DL, Wang B, Forsythe P, Bienenstock J, Kunze WA. Bacteroides fragilis polysaccharide A is necessary and sufficient for acute activation of intestinal sensory neurons. Nat. Commun. 2013;4:1465. doi: 10.1038/ncomms2478. [DOI] [PubMed] [Google Scholar]
- Mayer EA. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J. Probiotics as potential antioxidants: A systematic review. J. Agric. Food Chem. 2015;63:3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- Park J, Lee J, Yeom Z, Heo D, Lim YH. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci. Rep. 2017;7:14520. doi: 10.1038/s41598-017-15163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisova M, Hossini AM, Eberle J, Riebeling C, Orfanos CE, Geilen CC, Weider T, Sturm I, Daniel PT. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Invest. Dermatol. 2001;117:333–340. doi: 10.1046/j.0022-202x.2001.01409.x. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, Cryan JF. Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behav. Brain Res. 2015;287:59–72. doi: 10.1016/j.bbr.2015.02.044. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastav S, Neupane S, Bhurtel S, Katila N, Maharjan S, Choi HJ, Hong JT, Choi DY. Probiotics mixture increases butyrate, and subsequently rescues the nigral dopaminergic neurons from MPTP and rotenone-induced neurotoxicity. J. Nutr. Biochem. 2019;69:73–86. doi: 10.1016/j.jnutbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes C, Moncol J, Izakovic MM, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Yu YB, Zhao DY, Qi QQ, Long X, Li X, Chen FX, Luo SL. BDNF modulates intestinal barrier integrity through regulating the expression of tight junction proteins. Neurogastroent. Motil. 2017;29:12967. doi: 10.1111/nmo.12967. [DOI] [PubMed] [Google Scholar]