Highlights
-
•
Frontal functional connectivity (FC) alterations in ALS emerge over six months.
-
•
The frontal FC alterations over time are related to executive dysfunction in ALS.
-
•
The middle frontal gyrus is a key area for the frontoparietal breakdown in ALS.
-
•
This study offers new potential markers for monitoring altered FC in ALS over time.
Abbreviations: rs-FC, resting-state functional connectivity; rs-fMRI, rs functional MRI; TAP, Test of Attentional Performance; MFG, middle frontal gyrus; IC, independent component
Keywords: Amyotrophic lateral sclerosis, Functional connectivity, Resting-state fMRI, Fronto-connected networks
Abstract
Objective
To investigate the progression of resting-state functional connectivity (rs-FC) changes in patients with amyotrophic lateral sclerosis (ALS) and their relationship with frontal cognitive alterations.
Methods
This is a multicentre, observational and longitudinal study. At baseline and after six months, 25 ALS patients underwent 3D T1-weighted MRI, resting-state functional MRI (rs-fMRI), and the computerized Test of Attentional Performance (TAP). Using independent component analysis, rs-FC changes of brain networks involving connections to frontal lobes and their relationship with baseline cognitive scores and cognitive changes over time were assessed. With a seed-based approach, rs-FC longitudinal changes of the middle frontal gyrus (MFG) were also explored.
Results
After six months, ALS patients showed an increased rs-FC of the left anterior cingulate, left middle frontal gyrus (MFG) and left superior frontal gyrus within the frontostriatal network, and of the left MFG, left supramarginal gyrus and right angular gyrus within the left frontoparietal network. Within the frontostriatal network, a worse baseline performance at TAP divided attention task was associated with an increased rs-FC over time in the left MFG and a worse baseline performance at the category fluency index was related with increased rs-FC over time in the left frontal superior gyrus. After six months, the seed-based rs-FC analysis of the MFG with the whole brain showed decreased rs-FC of the right MFG with frontoparietal regions in patients compared to controls.
Conclusions
Rs-FC changes in ALS patients progressed over time within the frontostriatal and the frontoparietal networks and are related to frontal-executive dysfunction. The MFG seems a potential core region in the framework of a frontoparietal functional breakdown, which is typical of frontotemporal lobar degeneration. These findings offer new potential markers for monitoring extra-motor progression in ALS.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is the most common type of motor neuron disease, a fatal and heterogeneous neurodegenerative disorder characterized by progressive damage to upper and lower motor neurons. (Swinnen and Robberecht, 2014) At present, its multisystem nature and clinical, pathological, and genetic overlap with frontotemporal lobar degeneration (FTLD) are firmly established. (Proudfoot et al., 2018) Cognitive and behavioural disturbances in ALS have been observed in about 50% of patients, (van Es et al., 2017) with executive functions, verbal fluency, and apathy as the most frequently affected domains. (Goldstein and Abrahams, 2013) Being increasingly recognized, these non-motor features in ALS have been shown to hold significant prognostic implications. (Agosta et al., 2019).
In recent years, several neuroimaging techniques, such as magnetic resonance imaging (MRI), have proved to be useful in the search of biomarkers of ALS in vivo. Resting-state functional connectivity (rs-FC) is offering a unique contribution for understanding the integrity of brain networks related to motion, cognition and behavior in ALS. (Smith et al., 2009) Cross-sectional rs-functional MRI (rs-fMRI) studies reported rs-FC alterations within motor and extra-motor networks in ALS patients relative to healthy controls. (Agosta et al., 2013, Mohammadi et al., 2009)
Longitudinal studies are critical for understanding, monitoring and timing the progression of neurodegenerative diseases. In ALS, these studies are particularly relevant in order to identify novel biomarkers of motor and cognitive decline and to better define the patient’s prognosis. Up to date, only few studies have investigated rs-FC changes over time in ALS. (Menke et al., 2018, Schulthess et al., 2016, Shen et al., 2018, Trojsi et al., 2020) Most of them have used network-based approaches and have commonly showed increased rs-FC within the sensorimotor network after six months of follow-up. On the other hand, although findings in extra-motor networks are heterogeneous, all studies evidenced altered rs-FC of the frontal regions. (Menke et al., 2018, Schulthess et al., 2016, Shen et al., 2018, Trojsi et al., 2020) At present, no studies have yet investigated the relationship between the progressive rs-FC changes involving the connections to the frontal lobes and ALS cognitive alterations over time. Specifically, it is not clear whether and how the progression of brain frontal alterations are related to the cognitive dysfunction in ALS, and therefore whether they reflect a sign of disease progression or a mechanism of compensation.
It is common knowledge that cognitive assessment in ALS patients requires a correction for motor speed. This adjustment has been excellently achieved by the Edinburgh Cognitive and Behavioural ALS Screen (ECAS), (Abrahams et al., 2014) a brief neuropsychological battery for detecting cognitive and behavioural disturbances in ALS patients. However, as a screening tool, ECAS may have the limitation of not identifying subtle cognitive deficits in ALS patients. On this purpose, other approaches, such as computer-based batteries, might allow for a deeper investigation of ALS-typical altered cognitive functions and, at the same time, accommodate for motor disability.
In this study, we collected and analyzed clinical, cognitive computer-based, and fronto-connected network (those networks which hold functional connections that reach the frontal lobes, such as frontostriatal and frontoparietal networks) rs-fMRI data in ALS patients at baseline and after six months. We also assessed the relationship of longitudinal rs-FC alterations with baseline and longitudinal performance of patients at a neuropsychological computer-based battery (the Test of Attentional Performance-TAP), (Zimmermann and Fimm, 1992) which investigates the whole spectrum of frontal involvement in ALS accounting for motor impairment. We hypothesized that rs-FC changes could provide an extra-motor brain marker for the study of ALS disease progression.
2. Material and methods
2.1. Participants
From a large sample of 255 MND cases, twenty-five patients with a clinical diagnosis of probable or definite ALS according to the revised El Escorial criteria (Brooks et al., 2000) with no significant respiratory failure were selected from those attending two centres in Milan, Italy (IRCCS San Raffaele Scientific Institute and IRCCS Istituto Auxologico Italiano). We selected only patients with two visits (at baseline and six months), each including a clinical evaluation, an MRI scan and a computer-based battery assessing attention and executive functions. A standard neuropsychological assessment covering all cognitive domains was also obtained at study entry. Thirty-nine healthy controls, age-, sex-, and education-matched with patients, were recruited among non-consanguineous relatives and by word of mouth based on the following criteria: normal neurological exam, mini mental state examination (MMSE) score ≥ 28, and no family history of neurodegenerative diseases. All healthy controls underwent the baseline visit, which included the standard neuropsychological assessment and an MRI scan. Ten healthy subjects performed also the same MRI protocol at six months. All participants were excluded if they had significant medical illnesses or substance abuse that could interfere with cognitive functioning; any (other) major systemic, psychiatric, or neurological illnesses; and (other) causes of focal or diffuse brain damage, including cerebrovascular disease at conventional MRI scans. No participants were excluded for motion-related artifacts in the MR images. Local ethical standards committee on human experimentation approved the study protocol and all participants provided written informed consent.
2.2. Clinical assessment
Disease severity in patients was assessed at baseline and after six months using the ALS Functional Rating Scale-revised (ALSFRS-R, with a maximum score of 48). (Cedarbaum et al., 1999) The rate of disease progression was defined according to the following formula: (48–ALSFRS-R score)/time between symptom onset and first visit.
2.3. Cognitive and behavioral assessment
At the two centers, neuropsychological assessments were performed by experienced neuropsychologists, who were trained by the same senior neuropsychologist and were unaware of the MRI results. Cognitive evaluation consisted in the administration of: a comprehensive standard neuropsychological battery (to patients and controls at study entry) in order to define the potential presence of cognitive and/or behavioural impairment according to Strong’s criteria, (Strong et al., 2017) and a computer-based battery (to patients at baseline and after six months) as cognitive outcome to monitor the cognitive progression over time.
In the standard battery, the following cognitive functions were evaluated: global cognitive functioning with the mini mental state examination (MMSE); (Folstein et al., 1975) long and short term verbal memory with the Rey Auditory Verbal Learning Test (Carlesimo et al., 1996) and the digit span forward, (Orsini et al., 1987) respectively; executive functions with the digit span backward, (Monaco et al., 2013) the Cognitive Estimation Task (CET), (Della Sala et al., 2003) the Weigl’s Sorting test, (Spinnler and Tognoni, 1987) the Wisconsin Card Sorting Test (Laiacona et al., 2000) and the Raven’s colored progressive matrices (CPM); (Basso et al., 1987) fluency with the phonemic and semantic fluency tests (Novelli et al., 1986) and the relative fluency indices (controlling for individual motor disabilities); (Abrahams et al., 2000) language with the Italian battery for the assessment of aphasic disorders; (Miceli et al., 1994) mood with the Beck Depression Inventory (BDI); (Beck et al., 1961) and the presence of behavioral disturbances with the Amyotrophic Lateral Sclerosis-Frontotemporal Dementia-Questionnaire (ALS-FTD-Q) (Raaphorst et al., 2012) administered to patients’ caregivers. Healthy controls underwent the entire assessment except for the CET and the Weigl’s Sorting test, which were used in patients to deeply investigate executive functions.
The computer-based assessment consisted in the administration of six subtests of the TAP (alertness, divided attention, crossmodal integration, go/nogo, incompatibility and sustained attention). (Zimmermann and Fimm, 1992) The TAP battery accounts for patients’ verbal and/or physical impairment and allows the investigation of cognitive abilities, such as attention and executive functions, requiring the involvement of the frontal regions typically affected in ALS. The TAP administration followed strictly standardized procedures. In order to account for patients’ verbal and/or physical impairment, patients performed each task with a response-box consisting in a single facilitator press-button for all sub-tests (with the only exception of the Incompatibility sub-test which required two press-buttons), not either requiring patient fine movements nor strength.
2.4. MRI acquisition
Using a 3.0 T scanner (Intera, Philips Medical Systems, Best, the Netherlands), the following brain MRI sequences were obtained from all participants at the same center (IRCCS San Raffaele Scientific Institute): T2-weighted spin echo (repetition time [TR] = 3500 ms; echo time [TE] = 85 ms; echo train length = 15; flip angle = 90 [degrees]; 22 contiguous, 5-mm-thick, axial slices; matrix size = 512 × 512; field of view [FOV] = 230 × 184 mm2); fluid-attenuated inversion recovery (TR = 11 s; TE = 120 ms; flip angle = 90 [degrees]; 22 contiguous, 5-mm-thick, axial slices; matrix size = 512 × 512; FOV = 230 mm2); 3D T1-weighted fast field echo (FFE) (TR = 25 ms; TE = 4.6 ms; flip angle = 30 [degrees]; 220 contiguous axial slices with voxel size = 0.89 × 0.89 × 0.8 mm, matrix size = 256 × 256, FOV = 230 × 182 mm2); and T2*-weighted single-shot echo planar imaging sequence for rs-fMRI (TR = 3000 ms; TE = 35 ms; flip angle = 90; FOV = 240 × 240 mm (Proudfoot et al., 2018); matrix size = 128 × 128; slice thickness = 4 mm; 200 sets of 30 contiguous axial slices; acquisition time = 10 min). Before starting the rs-fMRI scanning, the technician talked with the participants through their earphones instructing them to remain motionless, to keep their eyes closed, not to fall asleep, and not to think about anything in particular. At the end of the rs-fMRI acquisition, the technician talked again with the participants asking whether they remained awake during the sequence.
2.5. MRI analysis
MRI analysis was performed at the Neuroimaging Research Unit, IRCCS Scientific Institute San Raffaele, Milan, Italy.
2.5.1. Resting-state fMRI preprocessing
Rs-fMRI data processing was carried out using the FMRIB software library (FSLv5.0) as described previously. (Canu et al., 2020) The first four volumes of the rs-fMRI data were removed to reach complete magnet signal stabilization. The following FSL-standard preprocessing pipeline was applied: (1) motion correction using MCFLIRT; (2) high-pass temporal filtering (lower frequency: 0.01 Hz); (3) spatial smoothing (Gaussian Kernel of FWHM 6 mm); (4) single-session independent component analysis-based automatic removal of motion artifacts (ICA_AROMA) (Pruim et al., 2015) in order to identify those independent components (ICs) representing motion-related artifacts.
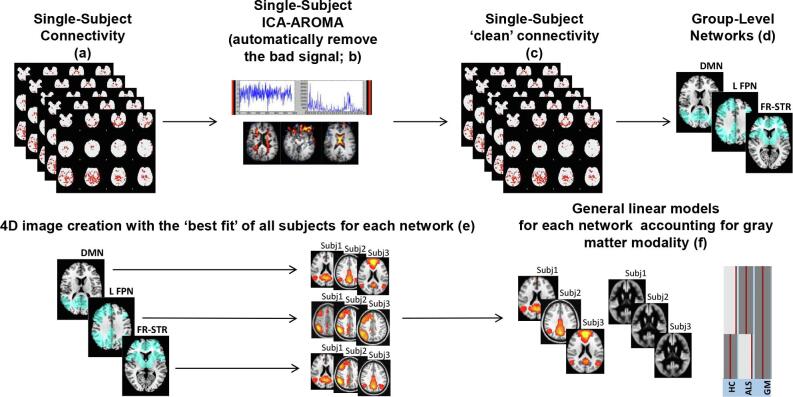
Network-based functional connectivity: Independent component analysis (for a brief overview of the method see Fig. 1).
Fig. 1.
Schematic representation of the procedure for the network-based independent component (IC) analysis. In each single-subject connectivity map (a), independent component (IC) analysis-based automatic removal of motion artifacts (ICA_AROMA) was applied (b). Pre-processed ‘clean’ resting state functional MRI data (c) were temporally concatenated across participants to create 4D group-level IC networks (d). A dual-regression procedure was performed and spatial maps of all participants were collected into single 4D files for each original IC (e). Finally, functional connectivity was investigated within each IC according to a specific general linear model. Here we provided the illustrative example of analysis at baseline (f): functional connectivity was compared between ALS patients and controls within each IC using a general linear model which includes the group as independent factor and accounts for voxel-based grey matter density. Abbreviations: ALS = amyotrophic lateral sclerosis; HC = healthy controls; GM = grey matter.
Rs-fMRI data set (‘clean’ from motion-related ICs) were co-registered to the participant’s 3D T1-weighted TFE image using affine boundary-based registration as implemented in FLIRT (Greve and Fischl, 2009) and subsequently transformed to the Montreal Neurological Institute (MNI) 152 standard space with 4 mm isotropic resolution using non-linear registration through FNIRT. (Andersson et al., 2007) Pre-processed rs-fMRI data for each subject were temporally concatenated across participants to create a single 4D data set. This rs-fMRI data set was then decomposed into ICs with a free estimate of the number of components using MELODIC (Multivariate Exploratory Linear Optimized Decomposition into Independent Components). (Beckmann et al., 2005) Among group-IC spatial maps (Fig. A.1), ICs of interest (frontostriatal, left and right frontoparietal networks) were selected by visual inspection of neuroimaging experts based on previous literature. (Smith et al., 2009) In order to identify the subject-specific temporal dynamics and spatial maps associated with each group IC, a dual regression analysis was applied. (Filippini et al., 2009) Finally, spatial maps of all participants were collected into single 4D files for each original IC.
To assess rs-FC changes in ALS patients, delta rs-FC maps for each IC (network) were obtained by subtracting baseline subject-specific spatial maps (in MNI standard space) from follow-up maps.
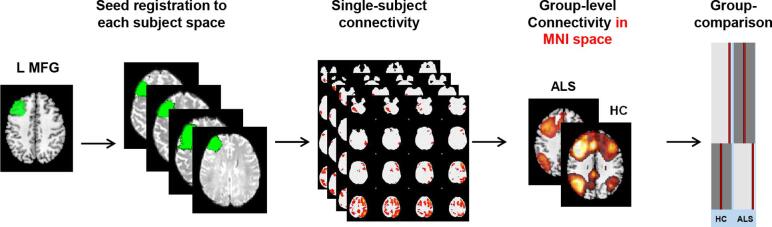
Post-hoc seed-based resting-state functional connectivity (for a brief overview of the method see Fig. 2).
Fig. 2.
Schematic representation of the procedure for the seed-based resting state functional connectivity. Each seed of interest (in the figure an example is provided for the left middle frontal gyrus) was defined in MNI space and moved to each subject native space. From each seed, mean time-series were extracted and subject-level maps of all positively and negatively predicted voxels for each regressor were obtained. Subject-level maps were finally registered to the MNI standard template and were ready for the statistical analysis. Here we provided the illustrative example of analysis at baseline: seed-based functional connectivity was investigated in each patient group versus the matched group of controls using a general linear model which includes the group as independent factor. Abbreviations: ALS = amyotrophic lateral sclerosis; HC = healthy controls; L = left; MFG = middle frontal gyrus.
Based on findings observed from the independent component analyses (i.e., an increased rs-FC of the MFG was present in ALS patients after six months within the frontostriatal and the left frontoparietal networks, and was related with the patients cognitive dysfunction at baseline; see Results for details), we performed a seed-based analysis, as previously described. (Canu et al., 2020) Two regions of interest were selected: left and right MFG. These regions were defined in the MNI space using the automated anatomical labelling atlas (AAL) in WFU PickAtlas (toolbox of SPM12), moved to each subject native T1-weighted space trough non-linear and affine registrations, and visually inspected in the individual brains by neuroimaging expert researchers. Seed-based rs-FC was then performed using a 2-step regression analysis as implemented in the FMRIB software library (FSLv5). First, time series of white matter (WM), cerebrospinal fluid, and whole brain volumes in rs-fMRI native space were extracted from the preprocessed and denoised data and their effects were regressed out using the FMRI Expert Analysis Tool. ROI mean time-series were then calculated. The output of this step is represented by subject-level maps of all positively and negatively predicted voxels for each regressor. Subject-level maps were registered to the MNI standard template to enter the statistical analysis.
For the longitudinal analysis in ALS patients, delta rs-FC maps for each seed of interest were obtained by subtracting baseline subject-level maps from follow-up maps.
2.6. Statistical analysis
2.6.1. Demographic, clinical and cognitive data.
To compare the baseline demographic characteristics and cognitive performance between groups at baseline, T-test models and Pearson’s χ2 test were performed for continuous and categorical variables, respectively. The statistical analyses were performed with SPSS software (version 24.0; IBM Corp., Armonk, NY, USA).
For the longitudinal analysis of cognitive (TAP) scores in ALS patients, we used general linear models (GLM) for repeated-measures in SPSS accounting for changes in ALSFRS-R and time between scans. Analyses were thresholded at p < 0.05 adjusted for multiple comparisons using Bonferroni’s correction. We finally investigated a potential relationship between significant TAP changes over time and patients’ mood at baseline assessed with the Beck Depression Inventory (BDI) using partial correlations accounted for ALSFRS-R changes and time between scans.
2.6.2. Network-based functional connectivity: Independent component analysis.
We performed: a) between-group (patients vs controls) rs-FC comparisons within each rs-network of interest at baseline; b) in ALS patients (within-group), analysis of each rs-network changes between baseline and follow-up; and c) in patients, correlations between rs-FC changes within each network of interest and measures of TAP, letter and category verbal fluency indices, and CPM (baseline and changes over time). All analyses were carried out using GLMs and nonparametric permutation tests (5000 permutations), and were restricted within the spatial rs-network of interest using binary masks obtained by thresholding the corresponding Z map image (Z > 2.3). A family-wise error (FWE) correction for multiple comparisons was performed, implementing the threshold-free cluster enhancement using a significance threshold of p < 0.05. Between-group comparisons at baseline a) were performed with GLMs including 4D spatial maps of all participants as dependent variable accounting for voxel-based gray matter (GM) density. Within-group analyses b) in ALS patients were performed with GLMs including patients’ delta rs-FC maps as dependent variables and delta GM density maps, ALSFRS-R changes, and time between scans as nuisance variables. Finally, for correlations c), GLMs included delta rs-FC maps of patients masked for significant (in b) findings as dependent variable, TAP scores and standard neuropsychological scores (fluency indices and CPM) at baseline and TAP and standard score changes over time as covariates of interest, and delta GM density maps, ALSFRS-R changes, and time between scans as nuisance variables. In order to exclude the possibility that our results could be driven by those patients with behavioral impairment (N = 3) and with C9orf72 mutation (N = 2), all analyses were performed also by excluding those patients.
2.6.3. Post-hoc seed-based resting-state functional connectivity.
Seed-based rs-FC was compared between patients (at baseline and at follow-up) and healthy controls using GLM, which included rs-FC maps as dependent variables. In ALS patients, changes over time were assessed applying GLM using delta rs-FC maps as dependent variables accounting for ALSFRS-R changes and time between scans. Corrections for multiple comparisons were carried out at a cluster level using Gaussian random field theory, z > 2.3; cluster significance: p < 0.05, corrected for multiple comparisons.
In order to confirm our results at follow-up, seed-based analyses were repeated comparing ALS patients at month six with a sub-sample of 10 age-matched healthy controls who performed the same MRI protocol both at baseline and follow-up (see Table B.1 for sociodemographic features of ALS patients and healthy controls at baseline).
3. Results
At baseline, ALS patients and controls did not significantly differ in age, sex and education (Table 1). Two patients were C9orf72 mutation carriers. According to Strong’s criteria, (Strong et al., 2017) three patients were classified as behaviourally impaired and none held cognitive abnormalities. The standard neuropsychological battery did not reveal differences between groups, except for subtle mood alterations (evident at the BDI) in ALS patients compared to controls (Table 1). However, no patient showed clinical depression according to BDI neither to clinical interview. After six months, patients showed a clinical progression of the disease (ALSFRS-R at six months: 37.79 ± 6.63; p < 0.01), and the computer-based battery identified significant cognitive changes in the alertness (with and without cue) TAP subtests (Table 2). No significant associations were observed between patients’ mood at baseline and TAP significant changes over time (p > 0.05).
Table 1.
Sociodemographic, clinical and neuropsychological features of ALS patients and healthy controls at baseline.
| ALS | HC | P-value | |
|---|---|---|---|
| N | 25 | 39 | |
| Sex, women | 6 (25%) | 19 (47.5%) | 0.07 |
| Age at MRI [years] | 61.56 ± 10.79 | 64.17 ± 7.44 | 0.29 |
| Education [years] | 10.96 ± 3.92 | 12.28 ± 4.15 | 0.21 |
| Disease duration [months] | 17.56 ± 14.06 | – | – |
| ALSFRS-R baseline, 0–48 | 42.36 ± 4.02 | – | – |
| Disease Progression Rate | 0.47 ± 0.40 | – | – |
| Site of onset (limb/bulbar/limb + bulbar) | 21/3/1 | – | – |
| Visit time interval [months] | 5.1 ± 2.1 | – | – |
| Global cognition | |||
| MMSE | 28.94 ± 1.14 | 29.33 ± 0.92 | 0.21 |
| Memory | |||
| Digit span, forward | 6.20 ± 1.48 | 6.10 ± 0.98 | 0.85 |
| RAVLT, immediate | 47.00 ± 11.13 | 45.55 ± 10.05 | 0.75 |
| RAVLT, delayed | 8.50 ± 2.43 | 9.03 ± 3.32 | 0.71 |
| Spatial span, forward | – | 4.83 ± 1.01 | – |
| Executive function | |||
| CPM | 32.50 ± 2.88 | 31.11 ± 3.19 | 0.34 |
| Digit span, backward | 5.00 ± 1.23 | 4.40 ± 1.16 | 0.30 |
| CET | 13.00 ± 4.42 | – | – |
| Weigl’s test | 10.83 ± 2.71 | – | – |
| WCST, perseverative responses | 5.50 ± 1.73 | 11.75 ± 7.27 | 0.13 |
| Language | |||
| BADA (nouns) | 30.00 ± 0.00 | 29.31 ± 1.45 | 0.08 |
| BADA (actions) | 27.50 ± 0.83 | 27.25 ± 1.77 | 0.75 |
| Fluency | |||
| Phonemic fluency | 33.05 ± 12.63 | 38.21 ± 8.54 | 0.11 |
| Phonemic fluency, Index | 6.28 ± 3.54 | 4.82 ± 2.27 | 0.14 |
| Semantic fluency | 42.48 ± 10.39 | 44.03 ± 8.66 | 0.57 |
| Semantic fluency, Index | 3.89 ± 1.09 | 3.93 ± 1.08 | 0.85 |
| Mood & Behavior | |||
| BDI | 10.32 ± 6.78 | 4.35 ± 3.64 | 0.002* |
| ALS-FTD-Q | 22.00 ± 10.74 | – | – |
Values denote mean ± standard deviations or numbers (percentages). Neuropsychological values are reported as raw scores. P-values refer to T-test models and Pearson’s χ2 test. *=significant differences between groups at p < 0.05. Abbreviations: ALS = amyotrophic lateral sclerosis; ALS-FTD-Q = amyotrophic lateral sclerosis-frontotemporal dementia-questionnaire; ALSFRS-R = ALS Functional Rating Scale Revised; BADA = battery for the analysis of aphasic deficits; BDI = Beck Depression Inventory; CET = cognitive estimation test; CPM = coloured progressive matrices; HC = healthy controls; MMSE = mini-mental state examination; MRI = Magnetic Resonance Imaging. RAVLT = Rey auditory verbal learning test; WCST = Wisconsin card sorting test. Disease Progression Rate has been obtained as following: (48–ALSFRS-R score)/time between symptom onset and first visit. Fluency indices have been obtained as following: time for generation condition - time for control condition (reading or writing generated words)/total number of items generated.
Table 2.
Cognitive performance of ALS patients at the Test of Attentional Performance (TAP), fluency tests and coloured progressive matrices at baseline and at six months of follow-up.
| Outcome | Value | Baseline | Month 6 | P-value |
|---|---|---|---|---|
| Alertness, no cue | RT | 344.00 ± 131.86 | 383.96 ± 210.29 | 0.01* |
| Alertness, no cue | Omissions | 0.00 ± 0.00 | 0.20 ± 1.00 | 0.13 |
| Alertness, no cue | T-MDN | 40.17 ± 11.54 | 37.13 ± 10.76 | 0.02* |
| Alertness with cue | RT | 312.32 ± 98.98 | 349.48 ± 166.42 | 0.01* |
| Alertness, with cue | Omissions | 0.00 ± 0.00 | 0.04 ± 0.20 | 0.35 |
| Alertness with cue | T-MDN | 41.71 ± 11.99 | 39.13 ± 11.53 | 0.06 |
| Sustained attention | RT | 715.64 ± 120.89 | 718.55 ± 141.13 | 0.84 |
| Sustained attention | Errors | 11.27 ± 9.89 | 12.32 ± 16.98 | 0.64 |
| Sustained attention | T-MDN | 41.92 ± 7.84 | 41.50 ± 8.70 | 0.79 |
| Go/NoGo | RT | 643.35 ± 103.20 | 665.00 ± 115.74 | 0.15 |
| Go/NoGo | Errors | 2.22 ± 3.94 | 1.39 ± 2.46 | 0.17 |
| Go/NoGo | T-MDN | 45.09 ± 13.14 | 42.68 ± 15.19 | 0.16 |
| Incompatibility | RT | 633.72 ± 157.42 | 634.94 ± 181.54 | 0.96 |
| Incompatibility | Errors | 5.00 ± 7.66 | 3.55 ± 5.38 | 0.19 |
| Incompatibility | T-MDN | 42.44 ± 11.80 | 41.83 ± 12.93 | 0.76 |
| Divided attention | RT | 562.40 ± 87.18 | 568.52 ± 127.67 | 0.74 |
| Divided attention | Omissions | 0.56 ± 1.12 | 0.32 ± 0.85 | 0.46 |
| Divided attention | T-MDN | 47.24 ± 14.47 | 48.24 ± 13.50 | 0.67 |
| Crossmodal | RT | 518.73 ± 119.30 | 514.59 ± 135.36 | 0.87 |
| Crossmodal | Errors | 2.14 ± 4.04 | 1.18 ± 2.06 | 0.21 |
| Crossmodal | T-MDN | 44.50 ± 7.07 | 46.50 ± 10.41 | 0.52 |
| Letter fluency, Index | Adjusted score | 6.28 ± 3.54 | 8.43 ± 10.69 | 0.40 |
| Category fluency, Index | Adjusted score | 3.89 ± 1.09 | 4.37 ± 2.01 | 0.32 |
| CPM | Correct items | 32.50 ± 2.88 | 31.29 ± 4.15 | 0.05 |
Values denote means (reaction times or accuracy) and T-value of the median (T-MDN) ± standard deviations. RT (reaction times) have been reported in terms of seconds. Comparisons among the two time points were assessed using general linear models for repeated-measures, corrected for changes at the ALS Functional Rating Scale Revised (ALSFRS-R) and time between scans, adjusting for multiple comparisons using Bonferroni’s correction. *=significant differences between groups at p < 0.05. T is a standardized value with a mean of 50 and a standard deviation of 10. Higher T values mean better performance. Abbreviations: CPM = coloured progressive matrices. Fluency indices have been obtained as follows: time for generation condition - time for control condition (reading or writing generated words)/total number of items generated.
3.1. Network-based functional connectivity: Independent component analysis.
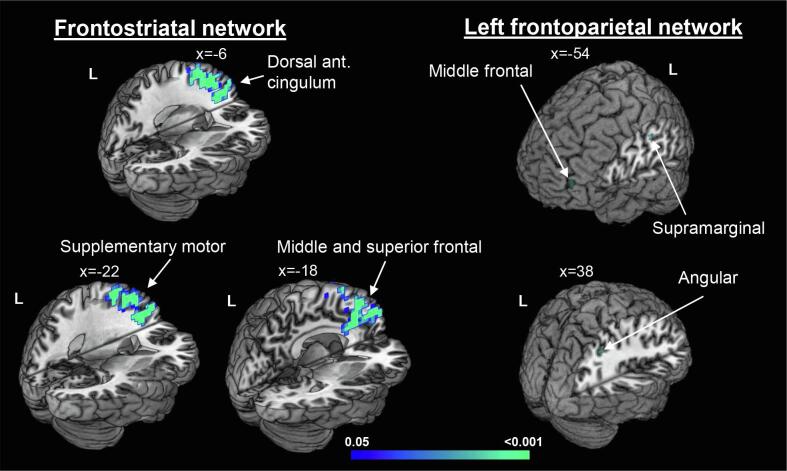
At baseline, we did not observe differences between groups. Over six months, ALS patients showed increased rs-FC in the bilateral supplementary motor area, and left dorsal anterior cingulate cortex (ACC), MFG, superior frontal and superior medial frontal gyri within the frontostriatal network; and in the left MFG and supramarginal gyrus and right angular gyrus within the left frontoparietal network (Fig. 3; Table B.2).
Fig. 3.
Independent component analysis. Increased resting-state functional connectivity in ALS patients after six months accounting for changes at the ALS Functional Rating Scale Revised (ALSFRS-R), time between scans and voxel-based grey matter density. Results are overlaid on the Montreal Neurological Institute (MNI) standard brain in neurological convention and displayed at p < 0.05 Family-wise error corrected for multiple comparisons. X values denote x-MNI coordinates. Colour bar represents p values.
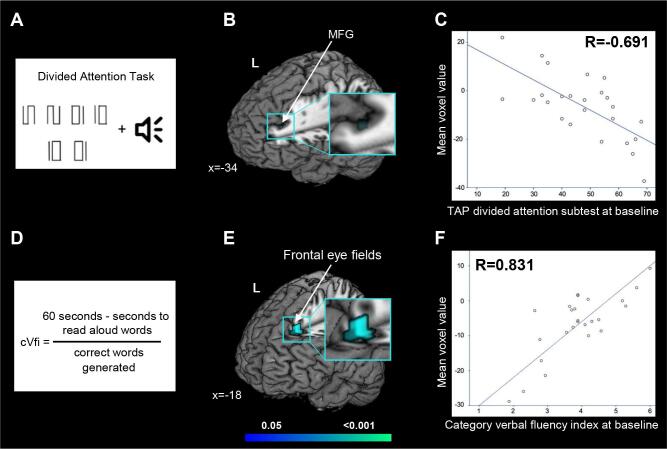
In ALS patients, within the frontostriatal network, we observed that: a worse performance at the TAP divided attention subtest (the T normative value of the median) was related with increased rs-FC over time in the left MFG (MNI coordinates, x = -34, y = 38; z = 32; Z = 0.959; p < 0.001; Pearson correlation coefficient, R = -0.691), and a worse performance at the category fluency (index scores) at baseline was related with increased rs-FC over time in the left frontal superior gyrus (MNI coordinates, x = -18, y = 30; z = 44; Z = 0.999; p < 0.001; R = 0.822) (Fig. 4). No other significant correlations were observed.
Fig. 4.
Cognitive-fMRI correlations. In ALS patients, worse performance at baseline TAP divided attention subtest (A) related with increased functional connectivity over time in the left middle frontal gyrus (MFG) within the frontostriatal network (B) (p < 0.001; R = -0.691 [C]), and worse performance at baseline category verbal fluency index, cVfi (D) related with increased functional connectivity over time in the frontal superior gyrus within the frontostriatal network (E) (p < 0.001; R = 0.831 [F]). Results are overlaid on the Montreal Neurological Institute (MNI) standard brain and displayed at p < 0.05 Family-wise error corrected for multiple comparisons. Delta gray matter density maps, changes at the ALS Functional Rating Scale Revised (ALSFRS-R) and time between scans were included as nuisance variables. X values denotes x-MNI coordinates. Colour bar represents p values.
The analysis performed excluding 5 ALS patients with behavioral imapirment and C9orf72 mutation confirmed the findings of the entire sample (Figs. A.2 and A.3).
3.2. Post-hoc seed-based resting-state functional connectivity
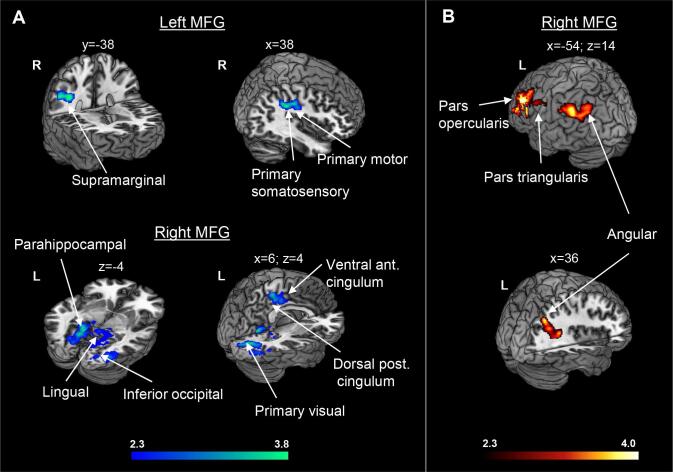
At baseline, compared to controls, ALS patients showed increased rs-FC between the left MFG and the right supramarginal gyrus, primary somatosensory and primary motor cortices; and between the right MFG and the bilateral primary visual and primary somatosensory cortices, bilateral dorsal posterior cingulate cortex and lingual gyrus, left parahippocampal gyrus, left precunes, right supramarginal gyrus, right primary motor cortex, right ventral ACC, right fusiform and inferior occipital gyri (Fig. 5A, Table B.3). At follow-up, we observed that compared to controls, ALS patients presented reduced rs-FC between the right MFG and the bilateral angular gyrus, left supramarginal gyrus, and left inferior frontal gyrus (pars opercularis and triangularis) (Fig. 5B, Table B.3). No rs-FC changes over time within ALS patients survived the correction for multiple comparisons. The results of the analysis in which we compared the 25 ALS patients with a sub-sample of 10 controls at the six month of follow-up confirmed a pattern of significant, although less extended, decreased rs-FC between the right and left MFG and anterior (mainly frontal) and posterior brain regions (Fig. A.4).
Fig. 5.
Seed-based functional connectivity analysis. Regions where ALS patients showed increased (cold colors) or reduced (warm colors) functional connectivity with the left and right middle frontal gyrus (MFG) compared to healthy controls at baseline (A) and at follow-up (6 months) (B), accounting for changes at the ALS Functional Rating Scale Revised (ALSFRS-R) and time between scans. Results are overlaid on the Montreal Neurological Institute (MNI) standard brain and displayed at p < 0.05 Family-wise error corrected for multiple comparisons at a cluster level. X, y and z values denotes MNI coordinates. Colour bar represents Z values.
The analysis performed excluding 5 ALS patients with behavioral imapirment and C9orf72 mutation confirmed the findings of the entire sample (Figs. A.2 and A.3).
4. Discussion
In this study, three major findings emerge: 1) rs-FC alterations of the frontostriatal and frontoparietal networks progressed in ALS patients over six months; 2) such alterations are related to patients’ frontal-executive low performance at baseline; 3) pivotal ‘frontal-executive’ regions, such as the MFG, are subject to a progressive frontoparietal breakdown.
Over time, in ALS patients, we observed increased rs-FC in middle and superior frontal gyri and ACC within the frontostriatal and left frontoparietal networks, and a progressive reduced rs-FC between the MFG and other frontal and parietal regions. Previous studies assessing longitudinal rs-FC changes in ALS demonstrated a mixed picture of decreased and increased rs-FC within motor and non-motor cortical networks, (Menke et al., 2018, Schulthess et al., 2016, Shen et al., 2018, Trojsi et al., 2020) together with a loss of integrity of WM connections (Menke et al., 2018) and progressive disability. (Menke et al., 2018, Schulthess et al., 2016) The ‘direction’ of rs-FC alterations seems to be dependent on the period of observation, with longer follow-up having higher chance of detecting decreased connectivity. (Menke et al., 2018, Trojsi et al., 2020)
Additionally, the hypothesis of a cerebral disconnection in ALS when increasing FC is present comes from several imaging studies which combined structural and functional connectivity using standard and connectome approaches. (Agosta et al., 2018) In line with neuropathological studies, these works demonstrated that local FC increased even when structural connectivity with distant brain regions of a network is disrupted. (Basaia et al., 2020) Although the observations of increased FC in established ALS might reflect a compensatory plasticity, (Mohammadi et al., 2009) a loss of local inhibitory neuronal circuits, which then overlaps with the established concept of cortical excitability, is becoming the most consistent hypothesis. (Douaud et al., 2011) In our cohort of ALS, the correlations that we observed between increased FC over time and low cognitive performances at baseline corroborate the disconnection hypothesis.
The expanding involvement of frontal and parietal networks in the progression of the disease is in keeping with pathological studies (Braak et al., 2013, Brettschneider et al., 2013) and supports the notion of ALS as part of the FTLD spectrum. (Burrell et al., 2016) Specifically, the finding of altered rs-FC in the ACC during the disease course well reflects a recent neuropathological study on TDP-43 which reported that agranular/disgranular regions of the ACC became involved in advanced pathological stages of sporadic ALS. (Braak and Del Tredici, 2018) Pathological changes in this region have been identified also in cases of frontotemporal dementia. (Seeley, 2008, Santillo et al., 2013) Beside neuropathology, our data are also in line with a recent connectome study, (Meier et al., 2020) which described a similar spatiotemporal progression of WM damage in ALS by simulating the disease propagation from the motor cortex using a network-based analysis.
In this study, we observed that the MFG plays a central role in the complex pattern of rs-FC changes in ALS patients. Specifically, we observed a progressive increased rs-FC of this region within both the frontostriatal and the left frontoparietal network, its association with a low performance at the TAP divided attention (dual task) subtest at baseline, and a continuous de-coupling between the MFG and a set of frontoparietal regions over time. MFG is known to have functional connections with the lateral and medial parietal structures (i.e., supramarginal gyrus, angular gyrus and posterior cingulate cortex), medial frontal cortices (i.e., ventral ACC), (Japee et al., 2015) and striatum. (Di Martino et al., 2008) In healthy individuals, all these regions subtend attentional and executive functions, and in particular working memory, with MFG being one of the regions with high network centrality (in terms of WM connectivity) and frontostriatal network having the unique role of not only maintaining but also updating working memory information (as represented by our dual task). (Ekman et al., 2016) The MFG has been shown to be involved in ALS in previous studies using functional imaging (Abrahams et al., 1996) (Abrahams et al., 2004) (Trojsi et al., 2017) and other imaging modalities. (Alruwaili et al., 2018, Verstraete et al., 2012) A study used a word-fluency paradigm (which requires high-order executive functions including working memory) during positron emission tomography and task-based fMRI. (Abrahams et al., 1996) Authors found reduced metabolism and activation, respectively, in both cognitively impaired and unimpaired ALS patients compared to controls in a cluster including the MFG and dorsolateral prefrontal cortices. (Abrahams et al., 1996) Furthermore, MFG rs-FC was found altered within the salience and the left frontoparietal networks in ALS patients who exhibited affective and cognitive theory of mind (a complex frontal function) deficits after six months and was observed to be related with patients’ disease duration. (Trojsi et al., 2017) A previous study (Pettit et al., 2013) also observed that abnormal dual-task performance in ALS patients correlated with low fractional anisotropy in the WM underneath the MFG, confirming the important role of this region in executive dysfunctions typical of ALS patients.
The computer-test battery (TAP) (Zimmermann and Fimm, 1992) revealed the progression of patients’ frontal dysfunctions, despite most of our patients had normal cognition according to Strong’s criteria (Strong et al., 2017) at study entry, suggesting that this is a valid tool to monitor even subtle frontal changes in cognitively unimpaired patients. It would be important to compare the performance of TAP in measuring cognitive changes over time with that of alternative versions of the ECAS, whenever available in Italian. We observed increased reaction times in patients’ alertness after six months. Alertness alterations in ALS patients may be attributed to several factors other than a frontal pathological involvement, such as hypoxia due to respiratory failure, progressive physical impairment and abnormal mood. However, we paid particular attention in reducing the impact of all these factors. Specifically, we excluded patients with significant respiratory failure; the TAP accounts for motor impairment and cognitive analyses were adjusted for ALSFRS-R changes; and we verified and excluded the potential relationship between patients’ mood and alertness.
Some limitations of our study should be noted. The sample of patients is relatively small and this could have led to negative findings, mainly in the correlation analysis. Second, we did not report longitudinal data for healthy controls, for both imaging and cognitive features. However, the sub-analysis that we performed with 10 healthy controls who underwent the MRI scan also after six months, confirmed our findings. Finally, the cognitive data of the present study have been collected before the publication of the Italian version of the ECAS. Thus, we cannot compare data obtained by using this ALS specific battery with those observed with the TAP. Despite these shortcomings, this manuscript has several strengths. The ALS sample has been cognitively well defined using standard and computer-based batteries. Finally, we used both network- and seed-based rs-FC, thus revealing comprehensive ALS extra-motor brain alterations.
5. Conclusions
In conclusion, this study investigated the relationship between cognitive and rs-fMRI longitudinal changes in ALS. In ALS, the increased connectivity in frontal regions in relation with lower frontal-executive performance at study entry suggests that it is likely not a mechanism of compensation but rather a sign of disease progression, as previously observed in patients with FTLD. (Farb et al., 2013) This is also revealed by the reduced rs-FC between MFG and antero-posterior regions at follow-up that could reflect the typical FTLD functional disconnection. (Reyes et al., 2018) The combined information of cognitive and fMRI alterations (and their relationship) can be useful in ALS to predict the disease progression beyond the motor network, even in patients with not frank cognitive impairment. We believe that these findings offer new potential markers for monitoring the ALS evolution. Further studies are needed to verify whether the information of cognitive and fMRI alterations may be able to classify new ALS patients with a FTLD-like progression at the single subject level.
CRediT authorship contribution statement
Veronica Castelnovo: Conceptualization, Methodology, Resources, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization. Elisa Canu: Conceptualization, Methodology, Resources, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Davide Calderaro: Formal analysis, Writing - review & editing. Nilo Riva: Resources, Writing - review & editing. Barbara Poletti: Resources, Writing - review & editing. Silvia Basaia: Resources, Formal analysis, Writing - review & editing. Federica Solca: Resources, Writing - review & editing. Vincenzo Silani: Resources, Writing - review & editing, Funding acquisition. Massimo Filippi: Conceptualization, Resources, Methodology, Writing - review & editing, Funding acquisition, Supervision. Federica Agosta: Conceptualization, Methodology, Supervision, Writing - original draft, Writing - review & editing, Funding acquisition, Project administration.
Acknowledgments
Acknowledgements
This work has been supported by the Italian Ministry of Health, Italy [RF-2010-2313220; RF-2011-02351193].
Declaration of competing interest
V. Castelnovo, D. Calderaro, N. Riva, B. Poletti, S. Basaia, F. Solca report no disclosures.
E. Canu has received research supports from the Italian Ministry of Health.
V. Silani received compensation for consulting services and/or speaking activities from AveXis, Cytokinetics, Italfarmaco, and Zambon; and receives research supports from the Italian Ministry of Health (Grant RF-2013-02355764), Fondazione Regione per la Ricerca Biomedica Regione Lombardia (Project nr.2015-0023), and E-RARE JTC 2018 (Project Repetomics).
M. Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
F. Agosta is Section Editor of NeuroImage: Clinical; has received speaker honoraria from Biogen Idec, Novartis and Philips; and receives or has received research supports from the Italian Ministry of Health, AriSLA (Fondazione Italiana di Ricerca per la SLA), and the European Research Council.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102509.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Swinnen B., Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2014;10:661–670. doi: 10.1038/nrneurol.2014.184. [DOI] [PubMed] [Google Scholar]
- Proudfoot M., Bede P., Turner M.R. Imaging Cerebral Activity in Amyotrophic Lateral Sclerosis. Front. Neurol. 2018;9:1148. doi: 10.3389/fneur.2018.01148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es M.A., Hardiman O., Chio A. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Goldstein L.H., Abrahams S. Changes in cognition and behaviour in amyotrophic lateral sclerosis: nature of impairment and implications for assessment. Lancet Neurol. 2013;12:368–380. doi: 10.1016/S1474-4422(13)70026-7. [DOI] [PubMed] [Google Scholar]
- Agosta F., Spinelli E.G., Riva N. Survival prediction models in motor neuron disease. Eur. J. Neurol. 2019;26:1143–1152. doi: 10.1111/ene.13957. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. U.S.A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Canu E., Valsasina P. Divergent brain network connectivity in amyotrophic lateral sclerosis. Neurobiol. Aging. 2013;34:419–427. doi: 10.1016/j.neurobiolaging.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Mohammadi B., Kollewe K., Samii A., Krampfl K., Dengler R., Munte T.F. Changes of resting state brain networks in amyotrophic lateral sclerosis. Exp. Neurol. 2009;217:147–153. doi: 10.1016/j.expneurol.2009.01.025. [DOI] [PubMed] [Google Scholar]
- Menke R.A.L., Proudfoot M., Talbot K., Turner M.R. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. Neuroimage Clin. 2018;17:953–961. doi: 10.1016/j.nicl.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulthess I., Gorges M., Muller H.P. Functional connectivity changes resemble patterns of pTDP-43 pathology in amyotrophic lateral sclerosis. Sci. Rep. 2016;6:38391. doi: 10.1038/srep38391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D.C., Xu Y.Y., Hou B. Monitoring Value of Multimodal Magnetic Resonance Imaging in Disease Progression of Amyotrophic Lateral Sclerosis: A Prospective Observational Study. Chin. Med. J. (Engl) 2018;131:2904–2909. doi: 10.4103/0366-6999.247214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojsi F., Di Nardo F., Siciliano M. Frontotemporal degeneration in amyotrophic lateral sclerosis (ALS): a longitudinal MRI one-year study. CNS Spectr. 2020:1–10. doi: 10.1017/S109285292000005X. [DOI] [PubMed] [Google Scholar]
- Abrahams S., Newton J., Niven E., Foley J., Bak T.H. Screening for cognition and behaviour changes in ALS. Amyotrophic lateral sclerosis & frontotemporal degeneration. 2014;15:9–14. doi: 10.3109/21678421.2013.805784. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP) Psytest. 1992 [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on Motor Neuron D. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N., Malta E. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Abrahams S., Goldstein L.H. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): Revised diagnostic criteria. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:153–174. doi: 10.1080/21678421.2016.1267768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Carlesimo G.A., Caltagirone C., Gainotti G. The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur. Neurol. 1996;36:378–384. doi: 10.1159/000117297. [DOI] [PubMed] [Google Scholar]
- Orsini A., Grossi D., Capitani E., Laiacona M., Papagno C., Vallar G. Verbal and spatial immediate memory span: normative data from 1355 adults and 1112 children. Ital. J. Neurol. Sci. 1987;8:539–548. doi: 10.1007/BF02333660. [DOI] [PubMed] [Google Scholar]
- Monaco M., Costa A., Caltagirone C., Carlesimo G.A. Forward and backward span for verbal and visuo-spatial data: standardization and normative data from an Italian adult population. Neurol Sci. 2013;34:749–754. doi: 10.1007/s10072-012-1130-x. [DOI] [PubMed] [Google Scholar]
- Della Sala S., MacPherson S.E., Phillips L.H., Sacco L., Spinnler H. How many camels are there in Italy? Cognitive estimates standardised on the Italian population. Neurol Sci. 2003;24:10–15. doi: 10.1007/s100720300015. [DOI] [PubMed] [Google Scholar]
- Spinnler H., Tognoni G. Standardizzazione e taratura italiana di test neuropsicologici. Ital. J. Neurol. Sci. 1987;6(suppl 8):44–46. [PubMed] [Google Scholar]
- Laiacona M., Inzaghi M.G., De Tanti A., Capitani E. Wisconsin card sorting test: a new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurol. Sci. 2000;21:279–291. doi: 10.1007/s100720070065. [DOI] [PubMed] [Google Scholar]
- Basso A., Capitani E., Laiacona M. Raven's coloured progressive matrices: normative values on 305 adult normal controls. Funct. Neurol. 1987;2:189–194. [PubMed] [Google Scholar]
- Novelli G., Laiacona M., Papagno C., Vallar G., Capitani E., Cappa S.F. Three clinical tests to research and rate the lexical performance of normal subjects. Arch. Psicol. Neurol. Psichiatr. 1986;47:477–506. [Google Scholar]
- Abrahams S., Leigh P.N., Harvey A., Vythelingum G.N., Grise D., Goldstein L.H. Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS) Neuropsychologia. 2000;38:734–747. doi: 10.1016/s0028-3932(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Miceli G., Laudanna A., Burani C., Capasso R. A Battery for the Assessment of Aphasic Disorders] CEPSAG; Roma: 1994. Batteria per l'Analisi del Deficit Afasico. B.A.D.A. [B.A.D.A. [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Raaphorst J., Beeldman E., Schmand B. The ALS-FTD-Q: a new screening tool for behavioral disturbances in ALS. Neurology. 2012;79:1377–1383. doi: 10.1212/WNL.0b013e31826c1aa1. [DOI] [PubMed] [Google Scholar]
- Canu E., Agosta F., Tomic A. Breakdown of the affective-cognitive network in functional dystonia. Hum. Brain Mapp. 2020;41:3059–3076. doi: 10.1002/hbm.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H.R., Mennes M., van Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48:63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L., Jenkinson M., Smith S. Technical Report; FMRIB Centre, Oxford, United Kingdom: 2007. Non-linear registration, aka spatial normalisation. [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005;360:1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N., MacIntosh B.J., Hough M.G. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosta F., Spinelli E.G., Filippi M. Neuroimaging in amyotrophic lateral sclerosis: current and emerging uses. Expert Rev. Neurother. 2018;18:395–406. doi: 10.1080/14737175.2018.1463160. [DOI] [PubMed] [Google Scholar]
- Basaia S., Agosta F., Cividini C. Structural and functional brain connectome in motor neuron diseases: A multicenter MRI study. Neurology. 2020 doi: 10.1212/WNL.0000000000010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Filippini N., Knight S., Talbot K., Turner M.R. Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain. 2011;134:3470–3479. doi: 10.1093/brain/awr279. [DOI] [PubMed] [Google Scholar]
- Braak H., Brettschneider J., Ludolph A.C., Lee V.M., Trojanowski J.Q., Del Tredici K. Amyotrophic lateral sclerosis–a model of corticofugal axonal spread. Nat. Rev. Neurol. 2013;9:708–714. doi: 10.1038/nrneurol.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brettschneider J., Del Tredici K., Toledo J.B. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013;74:20–38. doi: 10.1002/ana.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell J.R., Halliday G.M., Kril J.J. The frontotemporal dementia-motor neuron disease continuum. Lancet. 2016;388:919–931. doi: 10.1016/S0140-6736(16)00737-6. [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K. Anterior Cingulate Cortex TDP-43 Pathology in Sporadic Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2018;77:74–83. doi: 10.1093/jnen/nlx104. [DOI] [PubMed] [Google Scholar]
- Seeley W.W. Selective functional, regional, and neuronal vulnerability in frontotemporal dementia. Curr. Opin. Neurol. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santillo A.F., Nilsson C., Englund E. von Economo neurones are selectively targeted in frontotemporal dementia. Neuropathol. Appl. Neurobiol. 2013;39:572–579. doi: 10.1111/nan.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J.M., van der Burgh H.K., Nitert A.D. Connectome-Based Propagation Model in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2020;87:725–738. doi: 10.1002/ana.25706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japee S., Holiday K., Satyshur M.D., Mukai I., Ungerleider L.G. A role of right middle frontal gyrus in reorienting of attention: a case study. Front. Syst. Neurosci. 2015;9:23. doi: 10.3389/fnsys.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Scheres A., Margulies D.S. Functional connectivity of human striatum: a resting state FMRI study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Ekman M., Fiebach C.J., Melzer C., Tittgemeyer M., Derrfuss J. Different Roles of Direct and Indirect Frontoparietal Pathways for Individual Working Memory Capacity. J. Neurosci. 2016;36:2894–2903. doi: 10.1523/JNEUROSCI.1376-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahams S., Goldstein L.H., Kew J.J. Frontal lobe dysfunction in amyotrophic lateral sclerosis. A PET study. Brain. 1996;119(Pt 6):2105–2120. doi: 10.1093/brain/119.6.2105. [DOI] [PubMed] [Google Scholar]
- Abrahams S., Goldstein L.H., Simmons A. Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain. 2004;127:1507–1517. doi: 10.1093/brain/awh170. [DOI] [PubMed] [Google Scholar]
- Trojsi F., Di Nardo F., Santangelo G. Resting state fMRI correlates of Theory of Mind impairment in amyotrophic lateral sclerosis. Cortex. 2017;97:1–16. doi: 10.1016/j.cortex.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Alruwaili A.R., Pannek K., Coulthard A., Henderson R., Kurniawan N.D., McCombe P. A combined tract-based spatial statistics and voxel-based morphometry study of the first MRI scan after diagnosis of amyotrophic lateral sclerosis with subgroup analysis. J. Neuroradiol. 2018;45:41–48. doi: 10.1016/j.neurad.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Verstraete E., Veldink J.H., Hendrikse J., Schelhaas H.J., van den Heuvel M.P., van den Berg L.H. Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2012;83:383–388. doi: 10.1136/jnnp-2011-300909. [DOI] [PubMed] [Google Scholar]
- Pettit L.D., Bastin M.E., Smith C., Bak T.H., Gillingwater T.H., Abrahams S. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis. Brain. 2013;136:3290–3304. doi: 10.1093/brain/awt243. [DOI] [PubMed] [Google Scholar]
- Farb N.A., Grady C.L., Strother S. Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex. 2013;49:1856–1873. doi: 10.1016/j.cortex.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Reyes P., Ortega-Merchan M.P., Rueda A. Functional Connectivity Changes in Behavioral, Semantic, and Nonfluent Variants of Frontotemporal Dementia. Behav. Neurol. 2018;2018:9684129. doi: 10.1155/2018/9684129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.