Highlights
-
•
Double-blind, randomized, sham-controlled clinical trial of alpha-rhythm NFB.
-
•
PTSD severity decreased in the experimental group only, comparison to sham ns.
-
•
Evidence of normalized of DMN and SN connectivity post-treatment.
-
•
Decreased PTSD severity and NFB performance correlated to SN and DMN changes.
-
•
After treatment, 61.1% of experimental group no longer met criteria for PTSD.
Keywords: PTSD, Neurofeedback, fMRI, Connectivity, Default mode network, Salience network
Objective
The default-mode network (DMN) and salience network (SN) have been shown to display altered connectivity in posttraumatic stress disorder (PTSD). Restoring aberrant connectivity within these networks with electroencephalogram neurofeedback (EEG-NFB) has been shown previously to be associated with acute decreases in symptoms. Here, we conducted a double-blind, sham-controlled randomized trial of alpha-rhythm EEG-NFB in participants with PTSD (n = 36) over 20-weeks. Our aim was to provide mechanistic evidence underlying clinical improvements by examining changes in network connectivity via fMRI. Methods: We randomly assigned participants with a primary diagnosis of PTSD to either the experimental group (n = 18) or sham-control group (n = 18). We collected resting-state fMRI scans pre- and post-NFB intervention, for both the experimental and sham-control PTSD groups. We further compared baseline brain connectivity measures pre-NFB to age-matched healthy controls (n = 36). Results: With regard to the primary outcome measure of PTSD severity, we found a significant main effect of time in the absence of a group × time interaction. Nevertheless, we found significantly decreased PTSD severity scores in the experimental NFB group only, when comparing post-NFB (dz = 0.71) and 3-month follow-up scores (dz = 0.77) to baseline measures. Interestingly, we found evidence to suggest a shift towards normalization of DMN and SN connectivity post-NFB in the experimental group only. Both decreases in PTSD severity and NFB performance were correlated to DMN and SN connectivity post-NFB in the experimental group. Critically, remission rates of PTSD were significant higher in the experimental group (61.1%) as compared to the sham-control group (33.3%). Conclusion: The current study shows mechanistic evidence for therapeutic changes in DMN and SN connectivity that are known to be associated with PTSD psychopathology with no patient dropouts. This preliminary investigation merits further research to demonstrate fully the clinical efficacy of EEG-NFB as an adjunctive therapy for PTSD.
1. Introduction
1.1. PTSD and current treatment approaches
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric condition that can develop after exposure to trauma (APA, 2013) and involves symptoms of persistent intrusive recollections (vivid unwanted memories, flashbacks, and nightmares), avoidance of trauma-related stimuli (thoughts, feelings, and external reminders), alterations in cognition and mood (negative self-beliefs and expectations, difficulty concentrating, and an inability to experience positive emotions), and alterations in arousal and reactivity (aggression, destructive behaviour, hypervigilance, and problems sleeping). It is critical that recent advancements in our understanding of the neurobiological mechanisms associated with PTSD be integrated into current therapeutic approaches in order to improve treatment outcomes and provide optimal functional recovery (Lanius et al., 2015, Krystal et al., 2017, Etkin et al., 2019, Szeszko and Yehuda, 2019, Nicholson et al., 2020a). Currently, mixed response rates of psychotherapy and suboptimal response to pharmacological treatments have been reported in PTSD (Haagen et al., 2015, Krystal et al., 2017), where some research suggests that approximately 40% of patients with PTSD can fail to respond to these types of interventions (Bradley et al., 2005, Stein et al., 2006, Ravindran and Stein, 2009). Furthermore, dropout rates from psychological therapies remain a critical barrier to recovery (Bisson et al., 2013, Goetter et al., 2015) and are significantly higher for trauma-focused therapies (Lewis et al., 2020). As such, it is clear that not every therapeutic approach will benefit every patient in the same way and that novel adjunctive treatments are in high demand for the treatment of PTSD (Etkin et al., 2019, Nicholson et al., 2020b).
1.2. Restoring intrinsic connectivity networks in PTSD
Emerging research has begun to elucidate the role of intrinsic connectivity networks (ICNs) in the manifestation and maintenance of PTSD symptoms (e.g., Akiki et al., 2018; Szeszko and Yehuda, 2019; Nicholson et al., 2020a). The default mode network (DMN) possesses core nodes within the posterior cingulate cortex (PCC), precuneus, ventromedial prefrontal cortex (vmPFC), dorsomedial PFC (dmPFC), and hippocampus (Greicius et al., 2003, Buckner et al., 2008, Spreng et al., 2008, Qin and Northoff, 2011). DMN functional disruptions in PTSD patients are hypothesized to be related to modified and often negative self-referential thoughts, as well as to altered social cognition and autobiographical memory in the aftermath of trauma (Bluhm et al., 2009; Daniels et al., 2010; Van der Kolk, 2014; Tursich et al., 2015b, Akiki et al., 2017, Fenster et al., 2018, Frewen et al., 2020; Lanius et al., 2020). Studies investigating DMN functional connectivity at rest in PTSD report disrupted connectivity among multiple DMN structures (Bluhm et al., 2009, Sripada et al., 2012, Chen and Etkin, 2013, Lanius et al., 2015, Tursich et al., 2015b, Yehuda et al., 2015, Koch et al., 2016, Akiki et al., 2017, Barredo et al., 2018, Nicholson et al., 2020a). More recent work suggests that connectivity within the posterior community of the DMN (PCC, precuneus) may be intact or exacerbated relative to decreased connectivity within the anterior community of the DMN (mPFC) (Shang et al., 2014, Kennis et al., 2016, Akiki et al., 2018, Holmes et al., 2018). Indeed, it has been reported that decreased mPFC connectivity with the DMN may be a major risk factor predisposing individuals to the development of PTSD (Qin et al., 2012).
The salience network (SN), with core nodes consisting of the insula, dorsal anterior cingulate cortex (dACC), and the amygdala has been reported to be involved in environmental salience monitoring, interoceptive processing, autonomic regulation, and approach/avoidance behaviours (Dosenbach et al., 2007, Seeley et al., 2007, Sridharan et al., 2008, Modinos et al., 2009, Gogolla, 2017, Namkung et al., 2017). Moreover, the SN decodes innate alarm system signals in the context of threatening stimuli, detecting and integrating both emotion and sensory information (Lanius et al., 2017, Szeszko and Yehuda, 2019). Critically, alterations within the SN have been linked to PTSD symptoms of hyperarousal, hypervigilance, avoidance, and altered interoception (Sripada et al., 2012, Tursich et al., 2015, Yehuda et al., 2015, Akiki et al., 2017, Harricharan et al., 2019, Allen, 2020, McCurry et al., 2020, Nicholson et al., 2020a). Individuals with PTSD often display elevated SN connectivity during rest, particularly to anterior subregions of the insular cortex, with less connectivity to emotion regulation areas in the dlPFC (Sripada et al., 2012, Lanius et al., 2015, Koch et al., 2016, Harricharan et al., 2019, Jeong et al., 2019, Nicholson et al., 2020a). Critically, a recent review suggests that treatment response in PTSD is associated with lower functional activity within the anterior insula and better communication between the regulatory CEN and the DMN (Szeszko and Yehuda, 2019). Taken together, it has been hypothesized that normalizing the neural circuitry within large scale ICNs is an essential treatment avenue for reducing PTSD symptoms (Lanius et al., 2015, Koek et al., 2019; Szeszko and Yehuda, 2019; Nicholson et al., 2020a).
Indeed, this notion is supported directly by research in the field of neurofeedback (NFB) aimed at modulating such ICN dynamics in PTSD, where results of these studies suggest that NFB may indeed be a fruitful treatment approach (Peniston and Kulkosky, 1991, Ros et al., 2013, Zotev et al., 2018, Kluetsch et al., 2014, van der Kolk et al., 2016, Nicholson et al., 2018, Misaki et al., 2019, Rogel et al., 2020). Electroencephalogram (EEG)-NFB is a brain-computer interface that allows individuals to directly self-regulate neural states. Notably, whereas most evidence-based therapies for PTSD focus on the processing of trauma memories, the specific target of NFB is self-regulation of brain regions or networks (Ros et al., 2014, Sitaram et al., 2017). Recent systematic reviews of neurofeedback suggest that this intervention is associated with symptom improvements in patients with PTSD (Schoenberg and David, 2014, Panisch and Hai, 2018) and may be particularly beneficial among individuals who have been resistant to standard treatments (van der Kolk et al., 2016, Nicholson et al., 2020c, Rogel et al., 2020). Given the diversity of brain circuits associated with PTSD, modern NFB technology may facilitate a more personalized approach to medicine by targeting specific neural dynamics that are associated with unique symptoms among patients.
1.3. Modulating the default mode and salience networks with neurofeedback
Several studies suggest covariation between EEG alpha-rhythms and changes in the aforementioned ICNs (Laufs et al., 2003, Sadaghiani et al., 2010) that are particularly implicated in PTSD and its treatment (Lanius et al., 2015, Nicholson et al., 2020c). Specifically, alpha oscillations (8–12 Hz) correspond to a state of resting wakefulness positively correlated with DMN activity among healthy individuals and patients with PTSD (Mantini et al., 2007, Jann et al., 2009, Clancy et al., 2020). Among those with PTSD, alpha-rhythm reductions are commonly observed, particularly over the main hubs of the in DMN (PCC and mPFC) (Clancy et al., 2020), which is hypothesized to be a global index of chronic hyperarousal associated with SN connectivity (Ros et al., 2014, Liberzon and Abelson, 2016, Abdallah et al., 2017, Clancy et al., 2017, Clancy et al., 2020, Sitaram et al., 2017, Nicholson et al., 2020c). Several studies have provided preliminary evidence to suggest that alpha-based NFB may be a viable treatment avenue for PTSD. Notably, a single session of alpha-rhythm NFB has been shown to plastically alter connectivity within both the DMN and SN in PTSD (Kluetsch et al., 2014), resulting in acute symptom decreases in arousal (Kluetsch et al., 2014, Ros et al., 2016). Interestingly, alpha-rhythm desynchronization NFB has also been shown to restore PTSD alpha-rhythms towards levels found in the normal population (Ros et al., 2016), corresponding to a “homeostatic rebound” of alpha-rhythms (Kluetsch et al., 2014, Sitaram et al., 2017). In tandem, alpha-rhythm EEG-NFB has further been associated with a shift in amygdala complex functional connectivity (an area involved in the SN) away from the hippocampus and defense processing areas in the midbrain (periaqueductal grey), towards ventromedial PFC areas involved in executive functioning and emotion regulation (Nicholson et al., 2016).
In support of this work, a randomized controlled trial (RCT) in patients with chronic PTSD found that 24-sessions of EEG-NFB led to significant improvements in both PTSD symptoms and patients’ capacity for emotion regulation (van der Kolk et al., 2016). Speaking to the use of NFB as a valuable adjunctive treatment for PTSD, participants in this study consisted of numerously traumatized individuals who had not responded to at least six months of trauma-focused psychotherapy (van der Kolk et al., 2016). Similarly, a recent pilot EEG-NFB RCT in children with developmental trauma also demonstrates reductions on PTSD symptoms and improved executive functioning among patients with severe histories of abuse and neglect (Rogel et al., 2020). In the current trial, we sought to extend the knowledge-base surrounding alpha-based EEG-NFB by providing crucial mechanistic evidence underlying its long-term therapeutic effect in PTSD. Indeed, there remains a critical gap in the current PTSD literature, where a randomized controlled trial of alpha-based EEG-NFB examining plastic changes in large-scale network connectivity has yet to be conducted.
1.4. Study objective and hypotheses
We conducted a double-blind randomized controlled trial to investigate changes in clinical symptoms and DMN/SN connectivity following alpha-rhythm EEG-NFB over a 20-week period, relative to a sham-control group. Here, we first compared DMN and SN connectivity of all PTSD patients at baseline to a healthy control group in order to better characterize underlying aberrant neural network connectivity related to psychopathology before NFB. Here, based on the current literature reviewed above, we hypothesized that at baseline individuals with PTSD would demonstrate DMN hyper-connectivity with posterior communities of the DMN (precuneus and PCC), in addition to hypo-connectivity with anterior communities of the DMN (mPFC), as compared to healthy individuals. Furthermore, we predicted that PTSD patients would show SN hyper-connectivity to the insula at baseline, as compared to healthy controls.
Following the NFB intervention, we predicted that the PTSD experimental group would demonstrate significant reductions on the primary outcome measure of PTSD severity following the intervention as compared to the sham-control group. We further hypothesized that symptom decreases in the NFB experimental group would be associated with a normalization of DMN and SN connectivity patterns. Specifically, we predicted that NFB would result in decreased DMN connectivity with posterior communities of the network (precuneus and PCC) and increased connectivity with anterior communities (mPFC). In addition, we hypothesized that the SN would display less connectivity with the insula post-NFB as compared to the sham-control group.
2. Methods
2.1. Participants
Our neuroimaging sample consisted of 76 participants [PTSD (n = 40); healthy controls (n = 36)]. A total of 4 participants were excluded from the PTSD group due to incomplete fMRI resting-states scans; hence, our final sample consisted of 72 participants [PTSD (n = 36); healthy controls (n = 36); see Table 1]. The sample size of this preliminary investigation was based on trial feasibility during the time of recruitment. Thirty-six adults between 21 and 59 years-of-age who met criteria for a primary diagnosis of PTSD were randomized to either the experimental EEG-NFB group (n = 18) or the sham-control EEG-NFB group (n = 18). These participants completed resting-state fMRI scans pre- and post-NFB. The healthy control group was recruited to compare baseline network connectivity before EEG-NFB with resting-state fMRI, and this group did not receive the NFB intervention. This allowed us to evaluate aberrant neural network dynamics in the PTSD group before NFB treatment. There were no statistically significant differences between groups for age or sex, and the majority of the sample consisted of female participants (see Table 1). Prevalence of current major depressive disorder and other Axis I disorder diagnoses did not differ significantly between the PTSD experimental and sham-control groups [experimental group: MDD n = 5 (28%), somatization disorder n = 1 (6%); sham-control group: MDD n = 7 (39%), somatization disorder n = 3 (17%), specific phobia n = 1 (6%)]. With respect to trauma type, in the experimental NFB group, PTSD diagnoses were associated with military occupational trauma (n = 3), first responder occupational trauma (n = 2), and civilian physical/sexual abuse or neglect (n = 13). In the sham-control group, PTSD diagnoses were similarly associated military occupational trauma (n = 3), first responder occupational trauma (n = 1), and civilian physical/sexual abuse or neglect (n = 14). Critically, trauma type did not differ significantly between groups. Participants were recruited from 2014 to 2018 through referrals from family physicians, mental health professionals, psychology/psychiatric clinics, community programs for traumatic stress, and posters/advertisements within the London, Ontario community.
Table 1.
Baseline participant demographic and clinical information.
| PTSD Experimental Group | PTSD Sham-Control Group | Healthy Control Group | |
|---|---|---|---|
| N | 18 | 18 | 36 |
| Sex | 12 females | 14 females | 23 females |
| Age | 40.28 (12.21) | 46.28 (12.37) | 40 (10.33) |
| CAPS-Total | 36.86 (10.36) | 39.94 (7.83) | 0.7 (3.17)* |
| CTQ-Total | 54.61 (19.88) | 63.88 (19.94) | 33.30 (10.33)* |
| MDI- Total | 52.89 (14.87) | 67.88 (20.79) | 33.20 (3.34)* |
| MDD | current = 5, past = 8 | current = 7, past = 5 | current = 0, past = 0 |
| Somatization Disorder | current = 1, past = 0 | current = 3, past = 0 | current = 0, past = 0 |
| Specific Phobia | current = 0, past = 0 | current = 1, past = 0 | current = 0, past = 0 |
| Medication | 12 | 12 | 0 |
Brackets indicate standard deviation. Asterisks indicate significantly lower values in the healthy control group. PTSD groups did not differ with regard to CAPS, CTQ and MDI scores, as well as MDD and other Axis I disorder diagnoses. Abbreviations: PTSD = Posttraumatic Stress Disorder, CAPS = Clinician Administered PTSD Scale (Normalized to CAPS-5), CTQ = Childhood Trauma Questionnaire (none or minimal childhood trauma = 25–36, moderate = 56–68, extreme trauma > 72), MDD = Major Depressive Disorder.
The inclusion criteria for PTSD participants included a primary diagnosis of PTSD as determined using the Clinician-Administered PTSD Scale [CAPS; versions IV (n = 4) and 5 (n = 32)] and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (Blake et al., 1995, First et al., 2002, Weathers et al., 2013). Exclusion criteria for PTSD patients included alcohol or substance abuse/dependence not in sustained full remission within the last 3 months prior to the onset of the study and a lifetime diagnosis of bipolar or psychotic disorders. PTSD patients were also excluded from the study if they engaged in another primary trauma-focused psychotherapy treatment, if they received past or current biofeedback treatment, if they had had prominent current suicidal ideation within the past 3 months, if they exhibited self-injurious behaviours in the last 3 months requiring medical attention, if they had unstable living conditions (e.g., homeless, living in a shelter), or if they were currently involved in a violent relationship. Exclusion criteria for the healthy control group included a lifetime Axis-I psychiatric disorders, evaluated using the SCID and CAPS, current or past biofeedback, and current use of any psychotropic medications. Exclusion criteria for all participants included noncompliance with 3 Tesla fMRI safety standards, significant untreated medical illness, pregnancy, a history of neurological or pervasive developmental disorders, and previous head injury with loss of consciousness.
Patients with PTSD currently receiving psychotropic medication were on a stable dose prior to the start of the NFB trial and were asked not to change medication regime if at all possible. Individuals currently receiving psychotropic medications (n = 24) did not differ significantly between the experimental group (n = 12) and the sham-control groups (n = 12), nor did the class of medications, which included antidepressants (total n = 19: SSRIs, n = 15; SNRIs, n = 3; tricyclics, n = 1), atypical antipsychotics (total n = 6), sedatives (total n = 8: benzodiazepines, n = 6; cyclopyrrolone, n = 3), and stimulants (methylphenidate, n = 2). During the NFB trial, n = 3 participants in the PTSD experimental group increased their medication dosage (SSRIs, n = 1; benzodiazepines, n = 1; atypical antipsychotics, n = 1), and n = 2 participants in the sham-control group increased their dosage of medication once during the trial (SSRIs, n = 2). Average dose within a particular class of psychotropic medication did not differ significantly between groups at baseline nor during the clinical trial. When including both psychotropic medication as well as change in medication over the NFB trial as separate covariates in our subsequent analyses, results did not change beyond slight variations in cluster size.
The study was approved by the Research Ethics Board (REB) at Western University, Canada. Participants gave written and informed consent and received financial compensation for participating in the study. Neither the letter of information nor the informed consent gave any indication of our hypotheses. Participants with PTSD were informed that they would be randomly assigned to either the experimental or sham-control neurofeedback groups and that our research goal was to examine whether they could learn to control their brain activity and how they would go about achieving it. Participants in the sham-control neurofeedback group were offered active EEG-NFB following study completion. Our preliminary investigation was a pilot study and was therefore not pre-registered as a clinical trial. As such, we were highly restrictive with the outcome measures that we examined, with the primary outcome measure being PTSD severity scores (CAPS). Secondary outcome measures were additionally restricted to neuroimaging metrics of SN/DMN connectivity, as these networks have been shown to display plastic normalization after 1-session of the same alpha desynchronizing NFB protocol in our previous publications (Kluetsch et al., 2014, Nicholson et al., 2016).
2.2. Experimental procedures
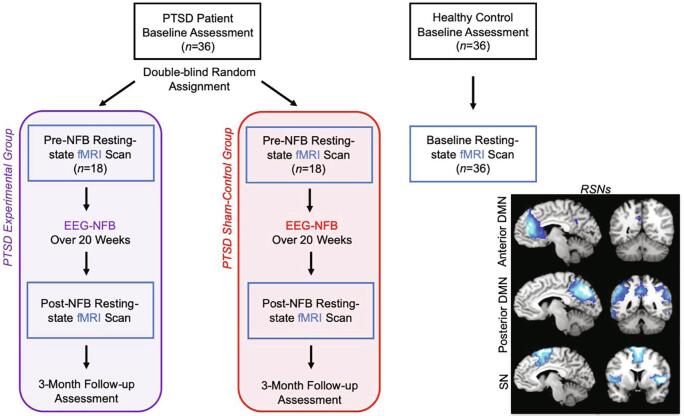
We first conducted baseline assessments on the CAPS and the SCID, where participants also completed the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 2003) and the Multiscale Dissociation Inventory (MDI) (Briere, 2002). Subsequently, all participants underwent a 6-minute eyes closed resting-state fMRI scan (Fig. 1).
Fig. 1.
Neurofeedback experimental design with pre- and post-intervention resting-state fMRI scans. All PTSD patients were compared to healthy controls at baseline with regard to resting-state fMRI network connectivity. PTSD patients were randomly allocated in a double-blind manner to either the active experimental group or sham-control group. Pre- versus post-intervention changes in resting-state fMRI network connectivity was compared for the experimental and sham-control groups. Here, all resting-state fMRI connectivity analyses consisted of group comparisons with regard to the data driven DMN and SN networks generated from the independent component analysis (spatial depiction of networks shown in the lower right panel). Clinical information was collected at baseline, post-intervention and at 3-month follow-up. Acronyms: EEG-NFB = electroencephalogram neurofeedback intervention, PTSD = posttraumatic stress disorder, RSNs = resting-state networks, DMN = default mode network, SN = salience network.
One week later, participants with PTSD then began and scheduled weekly sessions dedicated to EEG-NFB over a 20-week period. During the first NFB session, participants established goals for treatment, received an introduction to NFB technology and equipment, and baseline EEG recordings were collected. It was during participants’ second NFB session that they then started alpha-rhythm training, which consisted of alpha desynchronizing feedback for 20 min. Hence, our NFB analyses examining training effects focused on alpha-dynamics from session 2 onwards. One week after each participant’s last EEG-NFB session, we conducted a full clinical assessment using the CAPS and SCID. On this visit we also collected a second fMRI resting-state scan. Participants were then invited back to the laboratory 3-months later to receive a third clinical assessment.
2.3. EEG neurofeedback paradigm
Upon meeting inclusion criteria for the PTSD groups, participants were randomly assigned to either the experimental or sham-control NFB group under double-blind conditions (Fig. 1). For data to be included in the current study, participants had to schedule and complete a minimum of 15 weekly EEG-NFB training sessions, with a maximum of 20 weekly sessions being available [mean number of total completed sessions for experimental NFB group: 19.6 (SD 0.98); sham-control NFB group: 19.9 (SD 0.24)]. No participant completed <17 sessions in total during the trial, and groups did not differ in the duration of treatment [average duration for the experimental group: 161.1 days (SD = 36.3); sham-control NFB group: 182.2 (SD = 39.7)]. We implemented the same EEG-NFB training protocol as described previously (Ros et al., 2013, Kluetsch et al., 2014). During the first NFB session, participants established goals for treatment, received psychoeducation and an orientation to NFB technology, and baseline EEG recordings were collected. Subsequently, during participants’ second NFB session, alpha-rhythm EEG-NFB training commenced. During each of these NFB sessions, initial 3-minute baseline EEG recordings without feedback were also collected, where participants were asked to relax with their eyes open, refrain from excessive eye movements, and gaze at a blank wall. Subsequently, participants in the experimental NFB group down-regulated alpha amplitude (8–12 Hz) using real-time EEG feedback signal from the midline parietal cortex (Pz- electrode). PTSD patients in the sham-control NFB group were given the same instructions, but received yoked sham NFB signal, corresponding to a replayed feedback signal from a successful participant in the experimental group in order to ensure similar motivational states (Sorger et al., 2019). Here, EEGer sham-training was implemented in order to give the impression of real feedback, where the NFB signal was still sensitive to real-time artifacts such as eye blinks and muscular activity. Participants did not receive explicit strategies on how to down-regulate the alpha signal and were told to explore individual strategies that allow them to do this.
We selected the Pz electrode for the NFB signal as alpha rhythm is commonly maximal in this location (Ergenoglu et al., 2004). Participants completed EEG-NFB through interactive gaming. Consistent with a trauma-informed model of treatment and in order to be responsive to personal preference and to keep attention high over the 20-week trial, two visual NFB interfaces (i.e., visual presentation of feedback) were provided to participants. Furthermore, we offered two forms of feedback in case one of the interfaces was emotionally triggering for the participant. For each session, participants could select continuous visual feedback in the form of either i) a photo that had been divided into a grid, with individual grid pieces appearing as alpha amplitude was supressed; or ii) a cartoon character that moved across the screen as alpha amplitude was supressed. As such, the aim of the NFB training was to use the feedback signal to i) learn how to complete the image piece by piece; or ii) learn how to keep the cartoon character moving across the screen. Participants also received auditory feedback in the form of a series of single beeps, which occurred when they were suppressing alpha amplitude and corresponded to their visual feedback. Participants received the same auditory feedback regardless of which visual gaming interface they chose.
The EEG-NFB signal was infinite impulse response band-pass filtered in order to extract alpha oscillations with an epoch size of 0.5 s. The reward threshold was initially set such that participants would receive positive feedback about 65% of the time and receive negative feedback about 35% of the time. In order to ensure that all participants received comparable frequencies of reward, we readjusted reward thresholds to meet the desired ratio when they achieved disproportionately higher (>90%) or lower (<50%) rates of reward during feedback. Each 20-minute neurofeedback session was divided into 7 training periods (6 × 3-minute time periods, and 1 × 2-minute time period). Readjustments were made at the beginning of the next training period based on the EEG of the preceding 30 s (Kluetsch et al., 2014). After each training period, participant scores were displayed.
2.4. EEG recording, preprocessing and analysis
Scalp voltages were recorded using the Phoenix A202 2-channel EEG amplifier. The ground electrode was placed on the right earlobe and the reference electrode on the left earlobe. The EEG was recorded continuously, digitized at a sampling rate of 250 Hz, and stored on a hard drive for offline analysis. EEG data were then filtered with a 0.5–40 Hz bandpass filter offline.
The raw EEG signal from electrode Pz was imported into the MATLAB toolbox EEGLAB and statistically defined artifacting was then carried out with the FASTER plug-in (Nolan et al., 2010), removing segments based on extremal deviations of amplitude and variance from the mean (−2 < z-score > 2). Absolute alpha amplitude (8–12 Hz) was then estimated with a standard FFT approach using Welch's method (Matlab “pwelch” function) and a Hanning windowing function (2 s epoch, 50% overlap). The NFB-training alpha power dynamic for each participant was estimated by the average correlation coefficient between alpha power and the seven training periods within each EEG-NFB session.
2.5. fMRI paradigm, image acquisition and preprocessing
Participants with PTSD completed a 6-minute resting-state scan before and after the EEG-NFB intervention, which occurred on separate designated visits to the laboratory. Participants in the age-matched healthy control group only completed a baseline resting-state fMRI scan, as they did not receive the NFB intervention. We utilized a 3 Tesla MRI Scanner (Trio, Siemens Medical Solutions, Erlangen, Germany) with a 32-channel phased array head coil for brain imaging. We collected 120 volumes of whole brain BOLD (blood oxygen level dependent) images during a resting-state scan, acquired with the manufacturer’s standard T2* gradient-echo planar imaging (EPI) pulse sequence (single-shot, blipped-EPI, interleaved slice acquisition order and tridimensional prospective acquisition correction) with the following parameters: TR = 3000 ms, TE = 20 ms, isotropic resolution 2 mm, FOV = 192 × 192 × 128 mm3 (94 × 94 matrix, 64 slices), flip angle = 90°. High-resolution T1-weighted anatomical images were acquired with a Magnetization-Prepared Rapid Acquisition Gradient Echo sequence (192 slices, 1 mm isotropic resolution). For the resting-state procedure, participants were instructed to close their eyes and let their minds wander while trying not to focus on anything in particular for 6-minutes (Fransson, 2005, Bluhm et al., 2009).
Preprocessing of the functional images was performed with SPM12 (Wellcome Department of Cognitive Neurology, London, UK) within MATLAB R2017. Our standard preprocessing routine included discarding 4 initial volumes, re-orientation to the AC-PC axis, spatial alignment to the mean image using a rigid body transformation, reslicing, and coregistration of the functional mean image to the subject’s anatomical image. The co-registered images were segmented using the “New Segment” method implemented in SPM12. The functional images were normalized to MNI space (Montréal Neurological Institute) and were smoothed with a FWHM Gaussian kernel of 6 mm. Additional correction for motion was implemented using the ART software package (Gabrieli Lab, McGovern Institute for Brain Research, Cambridge, MA), which computes regressors that account for outlier volumes. The smoothed functional images were then bandpass filtered (high-pass 0.012 Hz, low-pass 0.1 Hz) (software by co-author Jean Théberge).
2.6. fMRI network connectivity analysis
Group spatial independent component analysis (ICA) was performed on the resting-state fMRI data with all subjects in order to identify spatially independent networks using the Group ICA of fMRI Toolbox (GIFT v4.0b) (Calhoun et al., 2001, Calhoun et al., 2009; Allen et al., 2011). A dimensionality of 20 ICA components was utilized, as this has been shown to provide behaviorally specific and easily interpretable ICNs (Calhoun et al., 2008, Smith et al., 2009, Laird et al., 2011, Vanasse et al., 2019). Our procedure consisted of the following steps: i) data reduction at the individual level through PCA; ii) concatenation into a group dataset; iii) further data reduction with PCA; iv) decomposition into group-independent components by using the Infomax Algorithm; and v) group ICA back- reconstruction of individual maps and calculation of Z-scores (Calhoun et al., 2001, Calhoun et al., 2008). In order to ensure reliability of the components, the ICA estimation was repeated 20 times through ICASSO (Himberg et al., 2004). This procedure resulted in a set of group aggregate spatial maps (which included brain regions that represent a network/component) and corresponding time courses of the BOLD signal change across time. For each component, the calculated z-scores denote the strength of each voxel’s connectivity with the aggregate component’s time course.
We first visually inspected the obtained components for the presence of artifacts (ensuring peak activations in gray matter, low spatial overlap with known vascular, ventricular, motion, and susceptibility artifacts, and investigated signal time course frequency fluctuations) (Allen et al., 2011). Subsequently, the spatial sorting function within the GIFT toolbox was used to identify components that shared features with reference network templates in the literature. Here, we utilized reference ICN masks derived from the GIFT toolbox (GIFT v4.0b) and from https://findlab.stanford.edu/functional_ROIs.html (Shirer et al., 2012, Rabellino et al., 2015, Nicholson et al., 2018).
2.6.1. Spatial comparison of intrinsic networks
The resulting component spatial maps denoting networks-of-interest were then entered into second-level analyses within SPM12. Here, we strictly followed the recent publication “Minimum statistical standards for submissions to Neuroimage: Clinical” (Roiser et al., 2016) to guide our analyses. Network maps were created by entering the z-scores from the subject-specific ICNs from all groups into a voxel-wise one-sample t-test that was orthogonal (independent) to the subsequent contrasts used to draw inferences (Calhoun et al., 2008, Vanasse et al., 2019), thresholded at p-FWE p < .05 k = 10. Each significant voxel provided an ICN-specific network mask. This ensured that all connectivity results would be restricted to brain regions actually contributing to a respective component generated by the ICA (Ros et al., 2013, Kluetsch et al., 2014, Vanasse et al., 2019) and effectively avoided problems of non-independence errors (Roiser et al., 2016, Vanasse et al., 2019).
Our aim was to examine the strength of network regional functional connectivity i) between the PTSD and healthy control groups pre-neurofeedback in order to characterize aberrant brain connectivity at baseline characteristic of PTSD; and ii) to evaluate changes in brain connectivity in response to NFB treatment in the PTSD experimental and sham-control groups. Using subject-level spatial-maps denoting network functional connectivity, we conducted separate two-sample t-tests for each ICN between all PTSD patients and healthy controls pre-NFB intervention. This allowed us to characterize aberrant ICN functional connectivity in the PTSD group as compared to the healthy control group. Following this step, for each ICN separately, we conducted a full factorial 2 (group) by 2 (timepoint) split plot ANOVA, where we inputted subject-level spatial-maps denoting ICN functional connectivity for the PTSD experimental and sham-control groups, for both pre- and post-NFB timepoints. We then conducted follow-up within-group comparisons examining pre- and post-NFB scans for the experimental and sham-control PTSD groups separately. We also compared ICN functional connectivity post-NFB between the PTSD experimental and sham-control groups. As a critical control, we compared ICN functional connectivity between the PTSD experimental and sham groups pre-NFB, where we found non-significant differences for all ICNs. All statistical tests were evaluated at the same conservative family-wise error protection rate for multiple comparisons (voxel-wise p-FWE < 0.05 k = 10) (Eklund et al., 2016, Roiser et al., 2016), where we set the initial uncorrected threshold in SPM at p < .001, k = 10. For all t-tests, we also conducted a region-of-interest (ROI) analysis for DMN and SN hubs showing significant changes in network connectivity after 1-session of alpha-rhythm EEG-NFB from a separate data set (Kluetsch et al., 2014). These regions included the bilateral insula, dorsal ACC and PCC [right middle insula: 40–2 10 (6 mm); left posterior insula: −36–12 14 (6 mm); left dACC: −10 26 40 (12 mm); PCC: 12–54 24 (12 mm)] (Kluetsch et al., 2014) and were strictly evaluated at the same conservative threshold (voxel-wise p-FWE < 0.05, k = 10).
2.7. Baseline clinical comparisons
We first evaluated baseline values with regard to clinical measures on the CAPS, CTQ, and MDI as well as participant age using independent sample t-tests between the PTSD experimental and sham-control groups. Using Pearson’s chi-squared and Fisher’s exact tests, we also compared sex, current major depressive disorder diagnoses and other Axis I disorder diagnoses between the PTSD experimental and sham control groups. Similarly, comparisons between the healthy control group and the collective PTSD group were made with regard to baseline clinical measures on the CAPS, CTQ, and MDI, as well as age and sex.
2.8. Behavioural data analysis
Change in CAPS score (representing PTSD severity) was evaluated as the primary outcome measure in our preliminary EEG-NFB trial. In order to include both CAPS-5 and earlier CAPS-IV measures, we normalised all scores to the CAPS-5 scale. Here, we divided participants CAPS-IV scores (4 participants in total, utilized prior to the release of CAPS-5) with the maximum available for CAPS-IV; we then multiplied this with the maximum score available for the CAPS-5. SPSS version 26 was used for behavioural statistical analyses. In order to evaluate changes on the CAPS, we conducted a split plot repeated measures ANOVA with the between-subjects factor group (experimental and sham groups) and within-subjects factor of time (pre-NFB, post-NFB, 3-month follow-up). Paired-sample t-tests were used to examine within group changes on the CAPS. Independent sample t-tests were also used to compare CAPS scores between PTSD groups at post-NFB and 3-month follow-up. Post-hoc tests evaluating changes in our primary outcome measure were Bonferroni corrected for multiple comparisons (p = 0.05/6).
2.9. PTSD severity and NFB performance correlations with resting-state fMRI network connectivity
The ImCalc function in SPM12 was used to calculate post-minus-pre NFB z-score fMRI connectivity change maps for each participant and network-of-interest, which served as the dependent variables in linear regression analyses. Here, the index that quantified a single subject’s performance during NFB was the training alpha power dynamic. This was defined as the correlation coefficient between alpha power and the seven training periods within each EEG-NFB session, which was then averaged across all sessions to obtain a single average correlation coefficient per subject. More negative correlation coefficients indicated temporally decreasing alpha amplitudes during NFB training sessions, i.e., better NFB performance. Specifically, we regressed the EEG-training alpha dynamic against individual z-score connectivity change maps of ICNs. Regarding the primary clinical outcome measure (CAPS), we conducted linear regression analyses between individual clinical symptom change scores (pre-NFB minus post-NFB scores on CAPS) with i) individual z-score connectivity change maps for each network; and with ii) individual z-score connectivity maps for each network post-NFB intervention. The results of all multiple regression analyses were evaluated at the same conservative threshold corrected for multiple comparisons (voxel-wise correction p-FWE < 0.05, k = 10), where we conducted separate regressions for the experimental and sham-control groups. We also conducted the same aforementioned ROI-analysis using coordinates from our previous alpha-rhythm EEG-NFB study (Kluetsch et al., 2014).
3. Results
3.1. Demographic and clinical characteristics
3.1.1. Baseline measures
At baseline pre-NFB, we found that PTSD patients in the experimental and sham-control groups did not differ significantly with regard to global PTSD severity scores (CAPS total scores normalized to CAPS-5 version), exposure to childhood trauma as measured by the CTQ, and dissociation scores as measured by the MDI (Table 1). Moreover, these groups did not differ significantly with regard to age, sex, current major depressive disorder or other Axis I disorder diagnoses. The healthy control group was age and sex matched to the PTSD group and as expected had significantly lower scores on the CAPS, CTQ and MDI.
3.1.2. Neurofeedback improvements on PTSD symptoms
With regard to our primary outcome measure of PTSD severity (CAPS score), we found a significant main effect of time (F(1.42, 48.37) = 14.20, η2 = 0.295, p < .0001), where the group × time interaction did not reach statistical significance (F(1.42, 48.37) = 0.911, η2 = 0.026, ns) (Greenhouse-Geisser corrected). Post-hoc t-tests nevertheless showed that only the PTSD experimental group demonstrated significant reductions on CAPS-totals scores from pre- to post-NFB (t(17) = 3.00, p < .008, dz = 0.71) and from pre-NFB to the 3-month follow-up (t(17) = 3.24, p < .005, dz = 0.77) (see Table 2 & Fig. 2). Comparisons to baseline scores were found to be non-significant for the sham-control PTSD group from pre- to post-NFB (t(17) = 2.38, ns, dz = 0.56) and from pre-NFB to the 3-month follow-up (t(17) = 2.68, ns, dz = 0.63). Homogeneity of variance and normality assumptions were not violated for paired-sample and independent samples t-tests. Independent-samples t-tests comparing the experimental and sham-control groups at post-NFB and 3-month follow-up did not reach statistical significance.
Table 2.
Primary outcome measure PTSD severity.
| PTSD Experimental Group |
PTSD Sham-Control Group |
|||||
|---|---|---|---|---|---|---|
| Pre-NFB | Post-NFB | 3- Month Follow-up | Pre-NFB | Post-NFB | 3-Month Follow-up | |
| CAPS-Total | 36.86 (10.36) | 24.39 (15.61)* | 23.58 (14.09) * | 39.94 (7.83) | 32.78 (12.27) | 31.78 (12.86) |
| % CAPS Reduction | – | 33.8% (Clinically meaningful reduction) | 36.0% (Clinically meaningful reduction) | – | 17.9% | 20.4% |
| Result Summary | – | Reduced compared to Pre-NFB (p < .008, dz = 0.71) | Reduced compared to Pre-NFB (p < .005, dz = 0.77) | – | ns (dz = 0.56) | ns (dz = 0.63). |
PTSD = Posttraumatic Stress Disorder, CAPS = Clinician Administered PTSD Scale (Normalized to CAPS-5), NFB = Neurofeedback. *Indicates significantly reduced clinical measures within a PTSD group as compared to pre-NFB baseline.
Fig. 2.
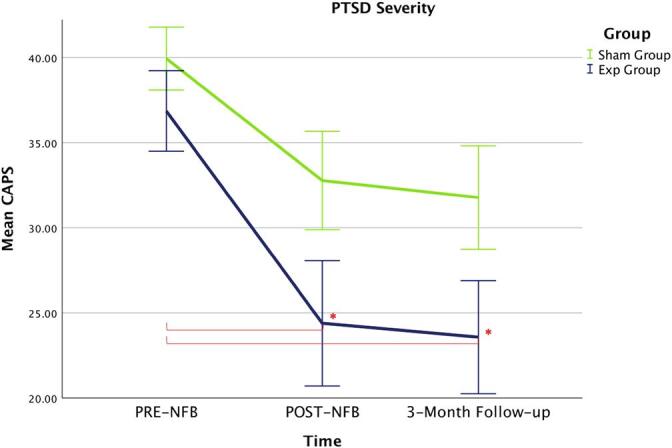
The primary outcome measure of PTSD severity (CAPS) changed significantly over the NFB-intervention for the experimental NFB group only as compared to baseline measures. No significant changes were detected for the sham-control group over the NFB-intervention as compared to baseline measures. Acronyms: Exp = experimental neurofeedback PTSD group, Sham = sham-control PTSD group, PTSD = posttraumatic stress disorder, CAPS = clinician administered PTSD scale.
Notably, at the 3-month follow-up assessment, 61.1% of participants in the experimental NFB group no longer met criteria for PTSD as compared to 33.3% of participants in the sham-control group. Here, groups differed significantly in the rate of PTSD remission (p = 0.040, bootstrapped 95%CI, number of repetitions = 100000). All PTSD patients meeting remission had clinically meaningful changes in CAPS (>30% reduction; Halvorsen, 2016). Of importance, average reductions on CAPS scores in the experimental NFB group were clinically meaningful when comparing baseline to post-NFB CAPS scores (33.8%) and when comparing baseline to 3-month follow-up CAPS scores (36.0%). By contrast, the average change in CAPS scores in the sham-control group were below threshold when comparing baseline to post-NFB (17.9%) and when comparing baseline to 3-month follow-up (20.4%) (see Table 2). Notably, double-blinding was maintained throughout the entire study, where participants reported that they could not identify accurately which group they were in.
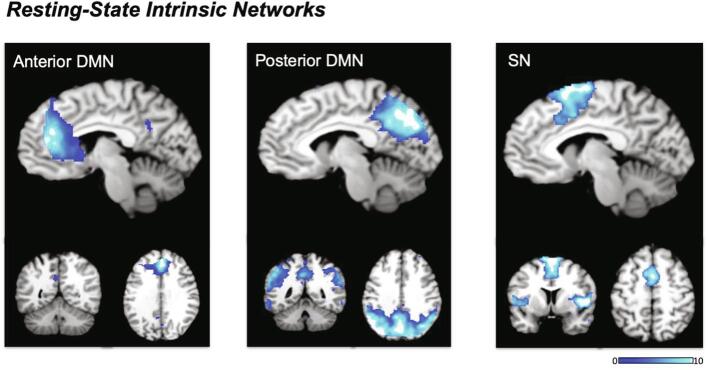
3.2. Intrinsic network identification
We identified three artifact-free components corresponding to the anterior DMN, posterior DMN, and the salience network (Fig. 3). The anterior (a)DMN component was comprised predominantly of the ACC and the mPFC, in addition to the PCC. The posterior (p)DMN component primarily covered a large posterior area of the brain, which included the bilateral precuneus, the PCC, the SPL, the temporal gyrus and the angular gyrus. The SN component was comprised of the anterior and posterior dorsal ACC, the supplementary motor area, the bilateral insulae, the rolandic operculum, and the temporal gyrus.
Fig. 3.
Resting-state intrinsic connectivity networks used for group comparisons generated by the independent component analysis. Acronyms: DMN = default mode network, SN = salience network.
3.3. Spatial comparison of intrinsic networks
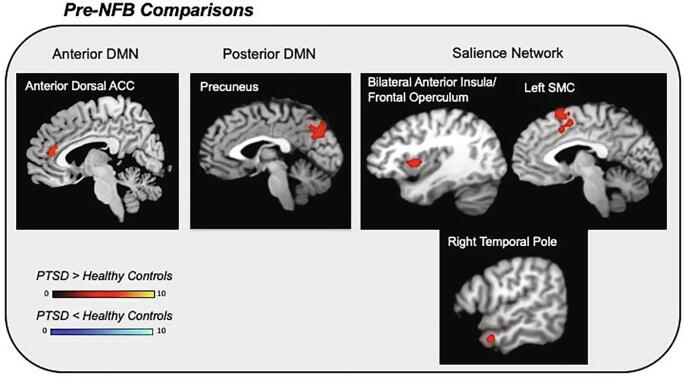
In summary, when comparing the functional connectivity of resting-state intrinsic networks between PTSD and healthy control groups at baseline, we discovered aberrant connectivity patterns among PTSD patients before the NFB intervention (see Fig. 4 & Table 3). Critically, this pattern of abnormal connectivity observed in the PTSD group was found to move towards normalization after treatment intervention in the experimental NFB group only (see Fig. 5 & Table 4). Importantly, PTSD experimental and sham-control groups did not differ significantly at baseline with regard to network functional connectivity of the DMN nor the SN. For all ICNs, group by time interactions, as well as post-NFB group comparisons were found to be non-significant at this conservative statistical threshold.
Fig. 4.
Baseline comparisons between PTSD (n = 36) and healthy controls (n = 36) pre-NFB intervention (voxel-wise pFWE < 0.05, k = 10). All PTSD patients were grouped together at baseline and compared to age-matched healthy controls in terms of network connectivity. Red clusters correspond to increased network connectivity among PTSD patients as compared to healthy controls. Blue clusters would indicate increased network connectivity among healthy controls as compared to PTSD patients. Importantly, the PTSD experimental group and PTSD sham-control groups did not different significantly at baseline with regard to network connectivity. Acronyms: DMN = default mode network, ACC = anterior cingulate cortex, SMC = supplementary motor cortex, PTSD = posttraumatic stress disorder, NFB = neurofeedback. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Baseline comparisons: intrinsic network spatial comparison between PTSD patients and healthy controls.
| Intrinsic Network | Contrast | Brain Region | Cluster Size | MNI Coordinate |
t Stat. | Z score | p FWE Peak | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Anterior DMN | All PTSD > Healthy | Anterior Dorsal ACC | 1447 | 8 | 40 | 10 | 7.29 | 6.25 | <0.001 |
| All PTSD < Healthy | ns | ||||||||
| PTSD Exp > PTSD Sham | ns | ||||||||
| PTSD Exp < PTSD Sham | ns | ||||||||
| Posterior DMN | All PTSD > Healthy | Left Precuneus | 384 | −6 | −68 | 32 | 4.96 | 4.57 | 0.037 |
| All PTSD < Healthy | ns | ||||||||
| PTSD Exp > PTSD Sham | ns | ||||||||
| PTSD Exp < PTSD Sham | ns | ||||||||
| SN | All PTSD > Healthy | Right Anterior Insula/Frontal Operculum | 315 | 36 | 16 | 4 | 6.90 | 6.00 | <0.001 |
| Right Temporal Pole | 10 | 54 | 4 | −25 | 5.20 | 4.76 | 0.006 | ||
| Left Supplementary Motor Cortex | 388 | −2 | 16 | 60 | 4.91 | 4.53 | 0.016 | ||
| Left Frontal Operculum/Anterior Insula | 31 | −50 | 10 | 0 | 4.70 | 4.36 | 0.032 | ||
| All PTSD < Healthy | ns | ||||||||
| PTSD Exp > PTSD Sham | ns | ||||||||
| PTSD Exp < PTSD Sham | ns | ||||||||
Direct group comparisons of intrinsic network functional connectivity at baseline pre-NFB among patients with PTSD (n = 36) and healthy controls (n = 36) (voxel-wise pFWE < 0.05, k = 10). All PTSD patients were grouped together at baseline and compared to age-matched healthy controls in terms of network connectivity. Additionally, we report here that the PTSD experimental group and PTSD sham-control groups did not different significantly at baseline with regard to network connectivity. Acronyms: DMN = default mode network, SN = salience network, ACC = anterior cingulate cortex, PTSD = posttraumatic stress disorder, PTSD Exp = posttraumatic stress disorder experimental neurofeedback group, PTSD Sham = posttraumatic stress disorder sham-control neurofeedback group.
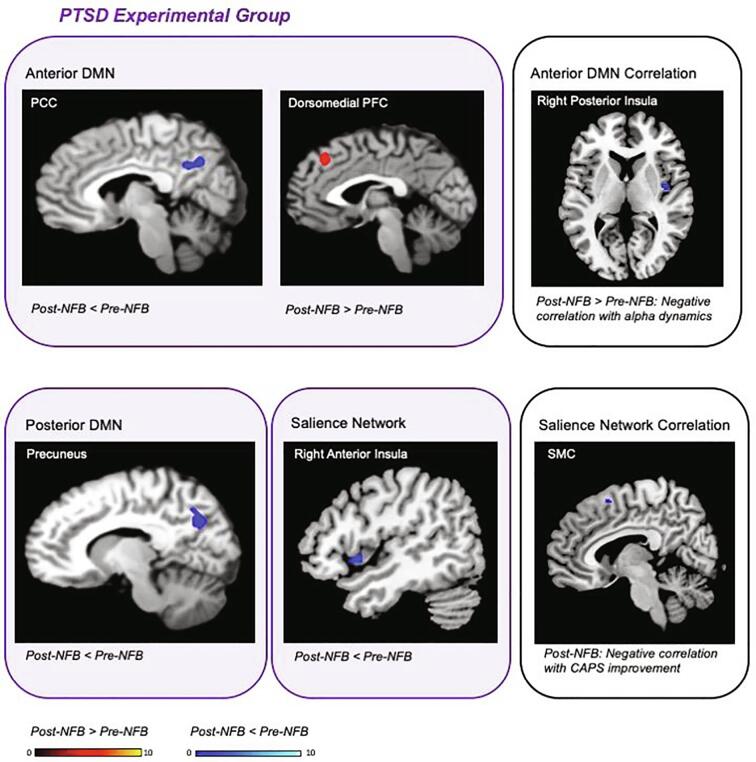
Fig. 5.
Alterations in network connectivity unique to the PTSD experimental group, with correlations to PTSD symptom reductions and NFB performance post NFB treatment (voxel-wise pFWE < 0.05, k = 10). Red clusters correspond to increased connectivity post as compared to pre-NFB intervention. Blue clusters correspond to decreased connectivity post as compared to pre-NFB. The right panels outlined in black correspond to the linear regression analysis between network connectivity and both NFB-training alpha power and PTSD severity improvement on the CAPS within the experimental group. Acronyms: PCC = posterior cingulate cortex, PFC = prefrontal cortex, SMC = supplementary motor cortex, DMN = default mode network, NFB = neurofeedback, CAPS = clinician administered PTSD scale (primary outcome measure), PTSD = posttraumatic stress disorder. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Within-group pre- versus post-neurofeedback intrinsic network spatial comparisons for the PTSD experimental group and PTSD sham-control group.
| Intrinsic Network | Group | Contrast | Brain Region | Cluster Size | MNI Coordinate |
t Stat. | Z score | p FWE Peak | ||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||
| Anterior DMN | Experimental | Post < Pre NFB | Posterior Cingulate Cortex* | 39 | 6 | −50 | 30 | 4.40 | 4.11 | 0.013 |
| Post > Pre NFB | Dorsomedial PFC* | 10 | 0 | 34 | 40 | 3.83 | 3.76 | <0.05 | ||
| Sham | Post < Pre NFB | ns | ||||||||
| Post > Pre NFB | ns | |||||||||
| Posterior DMN | Experimental | Post < Pre NFB | Right Precuneus | 233 | 12 | −64 | 30 | 5.73 | 5.16 | 0.002 |
| Post > Pre NFB | ns | |||||||||
| Sham | Post < Pre NFB | ns | ||||||||
| Post > Pre NFB | ns | |||||||||
| SN | Experimental | Post < Pre NFB | Right Anterior Insula | 31 | 43 | 16 | −6 | 4.50 | 4.19 | <0.05 |
| Post > Pre NFB | ns | |||||||||
| Sham | Post < Pre NFB | ns | ||||||||
| Post > Pre NFB | ns | |||||||||
Pre- and post-neurofeedback intervention comparisons for the PTSD experimental group and sham-control group (voxel-wise pFWE < 0.05, k = 10). * Denotes region-of-interest analysis also evaluated at the same error protection rate. Acronyms: DMN = default mode network, SN = salience network, PFC = prefrontal cortex, NFB = neurofeedback.
3.3.1. Anterior default mode network
Before NFB, we found increased anterior dACC connectivity with the aDMN among PTSD patients as compared to healthy individuals (see Fig. 4 & Table 3). Experimental and sham-control PTSD NFB groups did not differ significantly before NFB with regard to aDMN connectivity.
Supporting our initial hypotheses, we found decreased PCC connectivity with the aDMN post-NFB as compared to pre-NFB in the PTSD experimental group only (see Fig. 5 & Table 4). Moreover, our results showed increased dorsomedial PFC connectivity with the aDMN post-NFB as compared to pre-NFB in the experimental group. Non-significant differences were found when comparing pre and post-NFB connectivity maps for the sham-control group, as well as when comparing the PTSD experimental and sham-control groups post-NFB.
3.3.2. Posterior default mode network
Before NFB, we found increased left precuneus connectivity with the pDMN among PTSD patients as compared to healthy individuals (see Fig. 4 & Table 3). Experimental and sham-control PTSD NFB groups did not differ significantly before NFB with regard to pDMN connectivity.
In line with our hypotheses, we demonstrated decreased right precuneus connectivity with the pDMN post-NFB as compared to pre-NFB in the PTSD experimental group only (see Fig. 5 & Table 4), suggesting network shifts towards normalization in the experimental group. Non-significant differences were found when comparing pre and post-NFB connectivity maps for the sham-control group, as well as when comparing the PTSD experimental and sham-control groups post-NFB.
3.3.3. Salience network
Before NFB, our results showed increased SN connectivity among PTSD patients to the bilateral anterior insula/frontal operculum, the right temporal pole, and the left SMC, as compared to healthy individuals (see Fig. 4 & Table 3). Patients with PTSD in the experimental and sham-control NFB groups did not differ significantly at pre-NFB with regard to SN connectivity.
As predicted, we found decreased right anterior insula connectivity with the SN post-NFB as compared to pre-NFB in the experimental NFB group only (see Fig. 5 & Table 4), suggesting a shift of salience network connectivity towards normalization in the experimental group. Non-significant differences were found when comparing pre and post-NFB connectivity maps for the sham-control group, as well as when comparing the PTSD experimental and sham-control groups post-NFB.
3.4. PTSD severity and NFB performance correlations with resting-state fMRI network connectivity
With regard to our primary outcome measure, we found a significant negative correlation between decreases on the CAPS (pre-NFB minus post-NFB scores) and SN connectivity post-NFB with the midline SMC in the experimental NFB group only (see Table 5 & Fig. 5). In other words, stronger decreases in PTSD severity were associated with less SN connectivity with the SMC post-NFB.
Table 5.
Intrinsic network functional connectivity multiple regression analysis.
| Network | Group | Contrast | Measure | Correlation | Brain Region | Cluster Size | MNI Coordinate |
t Stat. | Z score | p FWE Peak | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||||||
| SN | Experimental | Post NFB | CAPS Decrease | Negative | Supplementary Motor Cortex | 13 | 4 | 12 | 58 | 6.09 | 4.32 | 0.040 |
| Anterior DMN | Experimental | Post > Pre NFB | EEG-Training Alpha Dynamic | Negative | Right Posterior Insula* | 12 | 40 | −6 | 6 | 4.68 | 3.66 | 0.015 |
Pre- and post-neurofeedback intervention correlations for the PTSD experimental group and sham-control group (voxel-wise pFWE < 0.05, k = 10). * Denotes region-of-interest analysis also evaluated at the same error protection rate. Acronyms: DMN = default mode network, SN = salience network, CAPS = clinician administered PTSD scale (PTSD severity), NFB = neurofeedback.
Likewise, our results revealed that alpha power dynamics during NFB training correlated negatively to aDMN connectivity (post-NFB as compared to pre-NFB) with the right posterior insula in the experimental group only (see Table 5). Since the goal was to reduce alpha power, better NFB performance correlated with increased aDMN connectivity to the right posterior insula post-NFB. Taken together, both primary outcome clinical change scores and NFB training performance measures correlated significantly with SN and DMN connectivity patterns. These correlations were found only for the PTSD experimental group, where we did not find any significant correlations with network connectivity in the sham-control group.
3.5. Neurofeedback performance and EEG spectral analysis
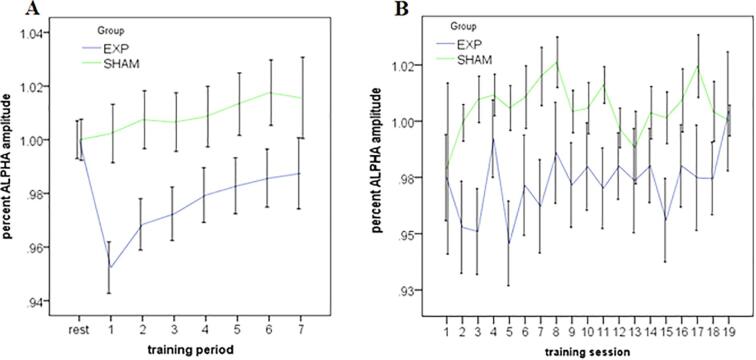
To confirm that NFB resulted in differential changes of the controlled parameter (alpha amplitude), we report the 20-min NFB session dynamics (6 × 3-minute training periods, and 1 × 2-minute training period) for the experimental and sham-control NFB groups. Here, we expressed training alpha power (within periods 1–7) as average percent change from baseline alpha power within each respective session (i.e., the initial rest period of that session). As depicted in Fig. 6A, and consistent with the NFB protocol, the experimental NFB group exhibited a more sustained reduction of alpha amplitudes as compared to the sham-control NFB group. For the feedback channel Pz, the alpha amplitude time course significantly differed between the experimental and sham-control NFB groups [group × time period interaction: F(7, 3458) = 8.16, p < 0.01]. Longitudinal changes in alpha percent change across all training sessions is depicted in Fig. 6B. Here, an ANOVA revealed a significant omnibus effect between experimental and sham-control NFB groups [group, F(1, 19) = 65.3, p < 0.01] in the absence of both a significant main effect of session and a group by session interaction. This indicates the experimental NFB group demonstrated lower percent alpha power values compared to the sham-control group, on average, over the whole course of the treatment.
Fig. 6.
. A. Within-session alpha amplitude for the experimental NFB group and the sham-control NFB group averaged over all NFB-training sessions (1–19). Rest represents the initial 3-min rest recording directly before training, where the subsequent feedback training was subdivided into 7 periods (over 20 min total). Alpha amplitude at the feedback site (channel Pz) was expressed as % change relative to the rest baseline of the respective session. B. Across-session alpha amplitude for the experimental NFB group and the sham-control NFB groups averaged over all training periods (1–7). Alpha amplitude was expressed as % change relative to the rest baseline of the respective session.
With regard to individual strategies used by participants to regulate their alpha-rhythms in the current NFB-intervention, most participants reported that they tried to “quiet their mind”. Additionally, some participants reported focusing on colours within the NFB gaming interface or imagining themselves in the photo presented. Lastly, some participants also reported mentally focusing on the feedback sound (reward beeps) that occurred when alpha was being suppressed.
4. Discussion
In this preliminary investigation, we report that alpha-rhythm EEG-NFB led to reductions on PTSD severity scores as well as increased rates of PTSD remission for the experimental NFB group. Our data qualitatively replicate previous clinical trials of EEG-NFB in patients with PTSD (van der Kolk et al., 2016, Rogel et al., 2020) and extend the mechanistic insights from our previous neuroimaging studies investigating a single session of alpha desynchronizing EEG-NFB (Ros et al., 2013, Kluetsch et al., 2014). Critically, aberrant DMN and SN connectivity patterns detected among PTSD patients at baseline (compared to healthy controls) in the current trial were found to shift towards normalization after active NFB treatment. Here, greater decreases on PTSD severity scores were associated with less SN connectivity with the SMC in the experimental group post-NFB. Similarly, our results revealed that better NFB performance correlated with an increase of aDMN connectivity with the right posterior insula after NFB as compared to baseline in the experimental group. Hence, both the primary outcome clinical measure and NFB training performance correlated significantly with SN and DMN connectivity dynamics. Importantly, our PTSD experimental and sham-control groups did not differ significantly at baseline.
Notably, at the 3-month follow-up assessment, 61.1% of participants in the experimental NFB group no longer met criteria for PTSD, a remission rate which was significantly higher than the sham control-group (33.3%). In support of this, only average reductions of CAPS scores in the experimental NFB group were clinically meaningful (>30% reduction; Halvorsen, 2016) when comparing baseline to post-NFB CAPS scores (33.8%) and when comparing baseline to 3-month follow-up CAPS scores (36.0%). By contrast, the average change in CAPS scores in the sham-control group were below threshold when comparing baseline to post-NFB (17.9%) and when comparing baseline to 3-month follow-up (20.4%). Another highly relevant clinical finding was that we had no patient dropouts from the NFB groups, which speaks to the tolerability of alpha NFB among individuals with PTSD. Indeed, dropout rates from psychological therapies in PTSD remain a critical barrier to recovery (Bisson et al., 2013, Goetter et al., 2015, Lewis et al., 2020). Finally, our EEG-NFB trial remission rates (61.1%) are comparable to those of current, gold standard treatments for PTSD (Bradley et al., 2005, Ehring et al., 2014, van der Kolk et al., 2016, Rogel et al., 2020). Taken together, NFB may represent a valuable adjunctive treatment for PTSD, where it has been suggested previously that NFB may be especially beneficial for PTSD patients experiencing high levels of anxiety, dissociation, and emotion dysregulation related to less optimal response to other forms of trauma-focused therapy (van der Kolk et al., 2016, Rogel et al., 2020).
4.1. Default mode network connectivity at baseline and after NFB
In line with our hypotheses, we found increased precuneus connectivity with the pDMN and increased anterior dACC connectivity with the aDMN among PTSD patients at baseline as compared to healthy individuals. Indeed, such DMN functional disruptions in PTSD patients are hypothesized to mediate negative self-referential thoughts as well as altered social cognition, bodily self-consciousness, and autobiographical memory related to trauma (Cavanna and Trimble, 2006; Bluhm et al., 2009; Daniels et al., 2010; Van der Kolk, 2014; Tursich et al., 2015b, Akiki et al., 2017, Fenster et al., 2018, Frewen et al., 2020; Lanius et al., 2020). After the NFB intervention, the PTSD experimental group displayed a decrease in precuneus connectivity with the pDMN as compared to baseline. Additionally, the PTSD experimental group was found to display decreased PCC connectivity and increased dorsomedial PFC connectivity with the aDMN after NFB as compared to baseline.
DMN functional connectivity at rest has been shown previously to be negatively correlated to PTSD symptoms (Patel et al., 2012, Lanius et al., 2015; Yehuda et al., 2015; Koch et al., 2016, Akiki et al., 2017, Akiki et al., 2018). A core hub of the DMN is the precuneus, an area essential to self-referential processing, episodic memory retrieval, bodily self-consciousness, and first-person perspective taking (Greicius et al., 2003, Cavanna and Trimble, 2006, Cabanis et al., 2013). In support of our findings of increased precuneus connectivity at baseline among PTSD patients, recent studies suggest that connectivity within the posterior community of the DMN (PCC and precuneus) may be intact or exacerbated relative to decreased connectivity within the anterior community of the DMN (vmPFC and dmPFC) (Shang et al., 2014, Kennis et al., 2016, Akiki et al., 2018, Holmes et al., 2018). It has also been shown via graph theoretical analyses that the DMN is characterized by less overall efficiency of communication across the network with increased segregation in PTSD patients (Akiki et al., 2018). This may represent deviations from an optimal cost-effective connectivity pattern within the DMN, reflecting a tendency of increased regional specialization with attenuated long-range functional connections in this network (Akiki et al., 2018). In relation to the increased anterior dACC connectivity with the aDMN among PTSD patients at baseline, the dACC has been shown to be hyperactive in PTSD patients and related to emotional expression, PTSD fear and anxiety symptoms, and to a vulnerability that predisposes individuals to the development of PTSD (Patel et al., 2012, Pitman et al., 2012, Fenster et al., 2018). Furthermore, the dACC is a core hub within the SN (Patel et al., 2012). Increased dACC connectivity with the aDMN at baseline in PTSD may therefore suggest greater resting-state SN interference with the DMN (Koch et al., 2016, Akiki et al., 2017), reflective of a chronic flight-or-flight response in PTSD.
Interestingly, the experimental NFB group displayed decreased precuneus connectivity with the pDMN as well as decreased PCC connectivity with the aDMN post-NFB as compared to baseline. This may reflect normalized connectivity within over utilized posterior DMN communities consisting of the precuneus and PCC (Akiki et al., 2018, Holmes et al., 2018) after NFB treatment. Our NFB protocol also resulted in an increase of dorsomedial PFC connectivity to the aDMN within the experimental group post-NFB. Importantly, this plastic change in DMN connectivity may reflect an increased recruitment of anterior communities in the DMN that are typically shut-down (Shang et al., 2014, Akiki et al., 2018, Holmes et al., 2018). Indeed, it has been reported that decreased mPFC connectivity with the DMN may be a major risk factor predisposing patients to the development of PTSD (Qin et al., 2012). These findings are also supported by our previous experiment investigating a single session of alpha-desynchronizing NFB in PTSD, where post-training we found that increased DMN connectivity with the dorsomedial PFC was correlated with increased calmness (Kluetsch et al., 2014). Furthermore, we demonstrated that this same NFB protocol was associated with an increase in ventromedial PFC connectivity to the amygdala (Nicholson et al., 2016), a limbic area known to be associated with the SN and highly implicated in PTSD (Nicholson et al., 2015, Fenster et al., 2018). Taken together, these results suggest that medial PFC connectivity may play a key role in restoring anterior portions of the DMN and thus enabling downregulation of limbic interference on DMN structures (Nicholson et al., 2016). Interestingly, although our previous investigations showed that one-session of EEG-NFB leads to acute decreases in arousal symptoms (Kluetsch et al., 2014, Nicholson et al., 2016), not surprisingly, participants neither displayed a significant reduction in PTSD severity nor did they demonstrate remission from PTSD. By contrast, the current NFB trial over 20-weeks shows that remission rates/decreases in PTSD symptoms are comparable to that of current gold-standard treatments for PTSD (Bradley et al., 2005, Ehring et al., 2014, van der Kolk et al., 2016). Future investigations are required to delineate the most optimal dosage of EEG-NFB necessary to produce best possible effects.
4.2. Salience network connectivity at baseline and after NFB
Confirming our initial hypotheses, patients with PTSD displayed increased SN connectivity with the bilateral anterior insula/frontal operculum, the right temporal pole, and the left SMC at baseline as compared to healthy individuals. This pattern of “pathological connectivity” in the PTSD group is strongly supported by the current knowledge base (Lanius et al., 2011, Wang et al., 2016, Akiki et al., 2017, Fenster et al., 2018, Vanasse et al., 2019), where such disruptions in SN connectivity have been shown to be associated with PTSD symptoms of hyperarousal, hypervigilance, avoidance, and altered interoception (Sripada et al., 2012, Tursich et al., 2015, Yehuda et al., 2015, Akiki et al., 2017, Harricharan et al., 2019, Allen, 2020, McCurry et al., 2020, Nicholson et al., 2020a). The temporal pole is a paralimbic region that is highly interconnected with the amygdala and orbitofrontal cortex and is involved in both sensory and limbic processing of emotional states (Olson et al., 2007). Furthermore, the SMC is a region engaged in planning action sequences (Nachev et al., 2008) and has been shown to be hyperconnected to sensorimotor networks at rest in PTSD (Vanasse et al., 2019). In direct support of a therapeutic shift in SN connectivity after the NFB intervention, our regression analysis revealed that greater decreases on PTSD severity scores were associated with less SN connectivity with the SMC post-NFB. Hence, although speculative, the current NFB intervention may normalize aberrant resting-state SN connectivity that is known to underlie PTSD symptoms related to hyperarousal, hypervigilance, and fight-or-flight defensive posturing involving the SMC (Yehuda et al., 2015, Shalev et al., 2017, Fenster et al., 2018, Vanasse et al., 2019).
Interestingly, we additionally found decreased SN connectivity with the right anterior insula post-NFB as compared to pre-NFB in the experimental NFB group only. Decreased SN connectivity with the anterior insula post treatment suggests a normalization of the aberrant SN neural circuitry reported at baseline in the current study. Indeed, PTSD patients have been found to display elevated resting-state SN connectivity to the anterior insula while exhibiting less connectivity to emotion regulation regions in the dlPFC (Sripada et al., 2012, Lanius et al., 2015, Koch et al., 2016, Harricharan et al., 2019, Jeong et al., 2019, Nicholson et al., 2020a). Importantly, the anterior insula has connections to the posterior insula (Craig, 2011, Namkung et al., 2017). Here, information relating to constantly changing physiological states arrives at the level of the posterior insula via ascending brainstem and thalamic inputs (Namkung et al., 2017). Viscerosensory information in the posterior insula is then projected rostrally through the mid insula to the anterior insula, where it is integrated with emotion and cognitive signals, supporting unique subjective feeling states (Namkung et al., 2017). In relation to the current results of decreased anterior insula connectivity with the SN post-NFB, a recent review also suggests that treatment response in PTSD is associated with lower functional activity and connectivity within the anterior insula, resulting in more controlled SN processing (Szeszko and Yehuda, 2019).
Although increased anterior insula activation has been widely reported in PTSD at rest, the posterior insula appears to be hypoactive (Patel et al., 2012, Koch et al., 2016, Wang et al., 2016, Akiki et al., 2017, Fenster et al., 2018). Specifically, it has been suggested that increased anterior insula activity may coincide with enhanced salience processing of environmental cues and PTSD symptoms of hypervigilance, hyperarousal, and re-experiencing, whereas decreased posterior insula activity has been suggested to reflect diminished internally focused thoughts, numbing and somatosensory awareness in PTSD (Hopper et al., 2007, Patel et al., 2012, Koch et al., 2016, Akiki et al., 2017). In further support of normalized SN dynamics in PTSD after our NFB intervention, we found that better NFB performance correlated with increased right posterior insula connectivity with the aDMN post-NFB as compared to pre-NFB in the experimental group only. Indeed, the posterior insula is heavily involved in somatosensory processing as well as integrating bodily physiological states, which together support DMN-related functions of self-awareness and self-referential processing (Frewen et al., 2020). Similarly, we have previously shown that a single session of alpha-desynchronizing EEG-NFB leads to increased posterior insula connectivity post-NFB, which was associated with increased calmness among PTSD patients (Kluetsch et al., 2014). An interesting parallel here is that both the NFB brain target (alpha waves) and the DMN are thought to be associated with mindfulness meditation (for review see Frewen et al., 2020). Interestingly, mindfulness training has been shown to increase posterior insula connectivity to DMN areas and facilitate interoceptive processing (Farb et al., 2013, Kirk et al., 2014, Jang et al., 2018, Frewen et al., 2020). Indeed, it has been hypothesized that mindfulness meditation may share overlapping mechanisms with both neurofeedback and biofeedback (Frewen et al., 2020), where future investigations are warranted. Taken together, it appears that plasticity of the insula and the SN may be central mechanisms underlying therapeutic effects among PTSD patients in the current RCT of alpha-based EEG-NFB. This interpretation is based on the currently observed results implicating the insula and SN in both the normalization of aberrant connectivity post-NFB, and associations with improvements on PTSD severity scores and NFB performance.
4.3. Limitations and future directions
The current preliminary study was not pre-registered as a clinical trial as ethical (REB) approval occurred when this was not yet a standard practice in the field. As such, we were highly restrictive with the outcome measures we examined as described above. Furthermore, it has been suggested recently that diverging evidence with regard to previous DMN connectivity findings in PTSD may be related to the use of ICA and graph theoretical data driven methodology as compared to specific a-priori seed selection, which may not provide optimal representation of connectivity within networks as a whole (Kennis et al., 2016, Akiki et al., 2018). Although we implemented an ICA in the current study, further replication using graph theory will be essential. Recent cytoarchitectonic and insula tracing studies have shown that the insula can be divided into as many as 16 subregions (Namkung et al., 2017, Allen, 2020). As the insula has been shown to be a key brain area related to currently observed PTSD symptom decreases, it will be critical for future studies to investigate these insula subregions further.
Additionally, significant group differences on CAPS scores were not found when comparing PTSD experimental and sham-control groups post-NFB and at the 3-month follow-up. This may be related to trending reductions on CAPS scores observed in the sham-control group. We hypothesize that this may be due to participants in both groups building supportive relationships with highly trained trauma-informed clinicians during regular weekly visits to the clinic. In addition, all participants were encouraged to be present and grounded for 20 min once a week during the NFB trial. Hence, future studies designed to compare mental strategies (for example mindfulness) and no-training control groups (for example waitlist and treatment as usual control groups) (Sorger et al., 2019) with ideally powered larger sample sizes are warranted.
Future studies will also need to compare directly NFB protocols that target both the up- and down-regulation of different neural oscillations (i.e., delta, theta, or beta), different electrode placements, and examine optimal reinforcement schedules and treatment dose. Moreover, the effect of psychotropic medication on NFB induced changes in resting-state connectivity should be investigated in follow-up studies (McCabe and Mishor, 2011, Rzepa et al., 2017). Although we matched frequencies of feedback reward between NFB groups and group blinding was maintained throughout the trial, a potential disadvantage of yoked signal feedback may be a lack of signal controllability experienced by participants (Sorger et al., 2019). Additionally, although auditory feedback was the same for all participants, choice of visual feedback interface (i.e., NFB game choice) was not recorded for all participants. We could therefore not calculate quantitative statistics with regard to the choice of visual stimuli in the NFB versus sham conditions. Finally, future studies should examine the mediation/moderation effects between NFB, ICN changes, and PTSD severity scores (Misaki et al., 2019).
5. Conclusions
In this preliminary double-blind randomized controlled trial, we demonstrate for the first time that alpha-rhythm EEG-NFB may restore abnormal network mechanisms associated with PTSD psychopathology. Critically, we found significantly decreased PTSD severity scores in the experimental NFB group only when comparing post-NFB and 3-month follow-up scores to baseline. Interestingly, we also found evidence to suggest a shift towards normalization with regard to DMN and SN connectivity post-NFB in the experimental group. Both decreases in PTSD severity and NFB performance were correlated to DMN and SN connectivity changes in the experimental group only. Overall, the current NFB intervention was well tolerated with no dropouts and led to significant symptom improvements, where 61.1% of patients in the experimental group no longer met criteria for PTSD following completion of the trial. The current study suggests mechanistic evidence for therapeutic changes in DMN and SN connectivity resulting from EEG-NFB, where this preliminary investigation merits further research to demonstrate clinical efficacy of NFB as an adjunctive therapy for PTSD.
CRediT authorship contribution statement
Andrew A. Nicholson: Conceptualization, Investigation, Data curation, Funding acquisition, Formal analysis, Software, Writing - original draft. Tomas Ros: Conceptualization, Investigation, Formal analysis, Methodology. Maria Densmore: Conceptualization, Methodology, Writing - review & editing. Paul A. Frewen: Conceptualization, Methodology, Writing - review & editing. Richard W.J. Neufeld: Conceptualization, Methodology, Writing - review & editing. Jean Théberge: Conceptualization, Methodology, Writing - review & editing. Rakesh Jetly: Conceptualization, Methodology, Writing - review & editing. Ruth A. Lanius: Conceptualization, Supervision, Data curation, Funding acquisition, Writing - review & editing.
Acknowledgements
This NFB trial was funded by the Canadian Institute for Veteran Health Research (CIMVHR; grant agreement No. W7716-125624/001/SV), and ANS Research, London, United Kingdom. Andrew A. Nicholson has received funding support from the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Individual Fellowship (grant agreement No. 897709). We also thank Suzy Southwell, Barbara Whelan, and Stephanie Nevill for their contributions with data collection and curation.
References
- Abdallah C.G., Southwick S.M., Krystal J.H. Neurobiology of posttraumatic stress disorder (PTSD): a path from novel pathophysiology to innovative therapeutics. Neurosci. Lett. 2017;649:130–132. doi: 10.1016/j.neulet.2017.04.046. [DOI] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Abdallah C.G. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr. Psychiatr. Rep. 2017 doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiki T.J., Averill C.L., Wrocklage K.M., Scott J.C., Averill L.A., Schweinsburg B., Alexander-Bloch A., Martini B., Southwick S.M., Krystal J.H., Abdallah C.G. Default mode network abnormalities in posttraumatic stress disorder: a novel network-restricted topology approach. Elsevier Ltd NeuroImage. 2018;176:489–498. doi: 10.1016/j.neuroimage.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E.A., Erhardt E.B., Damaraju E., Gruner W., Segall J.M., Silva R.F., Havlicek M., Rachakonda S., Fries J., Kalyanam R., Michael A.M., Caprihan A., Turner J.A., Eichele T., Adelsheim S., Bryan A.D., Bustillo J., Clark V.P., Feldstein Ewing S.W., Filbey F., Ford C.C., Hutchison K., Jung R.E., Kiehl K.A., Kodituwakku P., Komesu Y.M., Mayer A.R., Pearlson G.D., Phillips J.P., Sadek J.R., Stevens M., Teuscher U., Thoma R.J., Calhoun V.D. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5:1–23. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen M. Unravelling the neurobiology of interoceptive inference. Elsevier Ltd Trends Cogn. Sci. 2020;24:265–266. doi: 10.1016/j.tics.2020.02.002. [DOI] [PubMed] [Google Scholar]
- APA, 2013. Diagnostic and statistical manual of mental disorders. American Journal of Psychiatry 5th edn (ed. V. A. P. Publishing) Washington DC: Arlington, VA: American Psychiatric Publishing.
- Barredo J., Aiken E., Van ’T Wout-Frank M., Greenberg B.D., Carpenter L.L., Philip N.S. Network functional architecture and aberrant functional connectivity in post-traumatic stress disorder: a clinical application of network convergence. Brain Connect. 2018;8:549–557. doi: 10.1089/brain.2018.0634. [DOI] [PubMed] [Google Scholar]
- Bernstein D.P., Stein J.A., Newcomb M.D., Walker E., Pogge D., Ahluvalia T., Stokes J., Handelsman L., Medrano M., Desmond D., Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bisson J., Roberts N., Andrew M., Cooper R., Lewis C. Psychological therapies for chronic post-traumatic stress disorder (PTSD) in adults. Cochrane Database Syst. Rev. 2013;12:CD00338. doi: 10.1002/14651858.CD003388.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake D.D., Weathers F.W., Nagy L.M., Kaloupek D.G., Gusman F.D., Charney D.S., Keane T.M. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm R.L., Williamson P.C., Osuch E., Frewen P., Stevens T.K., Boksman K., Neufeld R.W.J., Théberge J., Lanius R. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J. Psychiatr. Neurosci. 2009;34:187–194. [PMC free article] [PubMed] [Google Scholar]
- Bradley, R., Greene, J., Russ, E., Dutra, L., Westen, D., Al, E.T., 2005. Reviews and overviews a multidimensional meta-analysis of psychotherapy for PTSD. 214–227. [DOI] [PubMed]
- Briere J. Psychological Assessment Resources; Odessa, Florida: 2002. Multiscale Dissociation Inventory Professional Manual. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain’s default network anatomy. Function Relevance Disease. 2008;38:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cabanis M., Pyka M., Mehl S., Müller B.W., Loos-Jankowiak S., Winterer G., Wölwer W., Musso F., Klingberg S., Rapp A.M., Langohr K., Wiedemann G., Herrlich J., Walter H., Wagner M., Schnell K., Vogeley K., Kockler H., Shah N.J., Stöcker T., Thienel R., Pauly K., Krug A., Kircher T. The precuneus and the insula in self-attributional processes. Cogn. Affect. Behav. Neurosci. 2013;13:330–345. doi: 10.3758/s13415-012-0143-5. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. A method for making group inferences from functional MRI data using independent component analysis. 2001;151:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Kiehl K.A., Pearlson G.D. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adal T. NeuroImage A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:163–172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chen A.C., Etkin A. Hippocampal network connectivity and activation differentiates post-traumatic stress disorder from generalized anxiety disorder. Nature Publishing Group Neuropsychopharmacology. 2013;38:1889–1898. doi: 10.1038/npp.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy, K., Ding, M., Bernat, E., Schmidt, N.B., Li, W., 2017. Restless ‘rest’: intrinsic sensory hyperactivity and disinhibition in post-traumatic stress disorder. [DOI] [PMC free article] [PubMed]
- Clancy, K.J., Andrzejewski, J.A., Simon, J., Ding, M., Schmidt, N.B., Li, W., 2020. Posttraumatic stress disorder is associated with α dysrhythmia across the visual cortex and the default mode network. eNeuro 7. [DOI] [PMC free article] [PubMed]
- Craig A.D.B. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Daniels J.K., Mcfarlane A.C., Bluhm R.L., Moores K., Richard Clark C., Shaw M.E., Williamson P.C., Densmore M., Lanius R. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J. Psychiatr. Neurosci. 2010;35:258–266. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U.F., Fair D., Miezin F.M., Cohen A.L., Wenger K.K., Dosenbach R.a.T., Fox M.D., Snyder A.Z., Vincent J.L., Raichle M.E., Schlaggar B.L., Petersen S.E. Distinct brain networks for adaptive and stable task control in humans. PNAS. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T., Welboren R., Morina N., Wicherts J.M., Freitag J., Emmelkamp P.M.G. Meta-analysis of psychological treatments for posttraumatic stress disorder in adult survivors of childhood abuse. Elsevier B.V. Clin. Psychol. Rev. 2014;34:645–657. doi: 10.1016/j.cpr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. 2016;113:201602413. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergenoglu T., Demiralp T., Bayraktaroglu Z., Ergen M., Beydagi H., Uresin Y. Alpha rhythm of the EEG modulates visual detection performance in humans. Cogn. Brain Res. 2004;20:376–383. doi: 10.1016/j.cogbrainres.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Etkin A., Maron-Katz A., Wu W., Fonzo G.A., Huemer J., Vértes P.E., Patenaude B., Richiardi J., Goodkind M.S., Keller C.J., Ramos-Cejudo J., Zaiko Y.V., Peng K.K., Shpigel E., Longwell P., Toll R.T., Thompson A., Zack S., Gonzalez B., Edelstein R., Chen J., Akingbade I., Weiss E., Hart R., Mann S., Durkin K., Baete S.H., Boada F.E., Genfi A., Autea J., Newman J., Oathes D.J., Lindley S.E., Abu-Amara D., Arnow B.A., Crossley N., Hallmayer J., Fossati S., Rothbaum B.O., Marmar C.R., Bullmore E.T., O’Hara R. Using fMRI connectivity to define a treatment-resistant form of post-traumatic stress disorder. Sci. Transl. Med. 2019;11:eaal3236. doi: 10.1126/scitranslmed.aal3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A.S., Segal Z.V., Anderson A.K. Mindfulness meditation training alters cortical representations of interoceptive attention. Social Cogn. Affect. Neurosci. 2013;8:15–26. doi: 10.1093/scan/nss066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster R.J., Lebois L.A.M., Ressler K.J., Suh J. Springer US Nature Reviews Neuroscience; 2018. Brain Circuit Dysfunction in Post-Traumatic Stress Disorder: From Mouse to Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Biometrics Research, New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P, 11/2002 revision). for DSMIV. [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P., Schroeter M.L., Riva G., Cipresso P., Fairfield B., Padulo C., Kemp A.H., Palaniyappan L., Owolabi M., Kusi-Mensah K., Polyakova M., Fehertoi N., D’Andrea W., Lowe L., Northoff G. Neuroimaging the consciousness of self: review, and conceptual-methodological framework. Neurosci. Biobehav. Rev. 2020 doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- Goetter, E.M., Bui, E., Ojserkis, R.A., Zakarian, R.J., Brendel, R.W., Simon, N.M., 2015. A systematic review of dropout from psychotherapy for posttraumatic stress disorder among Iraq and Afghanistan Combat Veterans. 401–409. [DOI] [PubMed]
- Gogolla N. The insular cortex. Elsevier Curr. Biol. 2017;27:R580–R586. doi: 10.1016/j.cub.2017.05.010. [DOI] [PubMed] [Google Scholar]
- Greicius, M.D., Krasnow, B., Reiss, A.L., Menon, V., 2003. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. 100. [DOI] [PMC free article] [PubMed]
- Haagen J.F.G., Smid G.E., Knipscheer J.W., Kleber R.J. The efficacy of recommended treatments for veterans with PTSD: a metaregression analysis. Elsevier Ltd Clin. Psychol. Rev. 2015;40:184–194. doi: 10.1016/j.cpr.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Halvorsen J.Ø. Defining clinically significant change. British Journal of Psychiatry. 2016;209(1):85. doi: 10.1192/bjp.209.1.85. [DOI] [PubMed] [Google Scholar]
- Harricharan S., Nicholson A.A., Thome J., Densmore M., McKinnon M.C., Théberge J., Frewen P.A., Neufeld R.W.J., Lanius R.A. PTSD and its dissociative subtype through the lens of the insula: anterior and posterior insula resting-state functional connectivity and its predictive validity using machine learning. Psychophysiology. 2019:1–23. doi: 10.1111/psyp.13472. [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyva A., Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Holmes S.E., Scheinost D., DellaGioia N., Davis M.T., Matuskey D., Pietrzak R.H., Hampson M., Krystal J.H., Esterlis I. Cerebellar and prefrontal cortical alterations in PTSD: structural and functional evidence. Chronic Stress. 2018;2 doi: 10.1177/2470547018786390. 247054701878639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J.W., Frewen P.A., van der Kolk B.A., Lanius R.A. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J. Trauma. Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Jang J.H., Kim J.H., Yun J.Y., Choi S.H., An S.C., Kang D.H. Differences in functional connectivity of the insula between brain wave vibration in meditators and non-meditators. Mindfulness. 2018;9:1857–1866. doi: 10.1007/s12671-018-0928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Dierks T., Boesch C., Kottlow M., Strik W., Koenig T. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Elsevier Inc. NeuroImage. 2009;45:903–916. doi: 10.1016/j.neuroimage.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Jeong H., Park S., Dager S.R., Lim S.M., Lee S.L., Hong H., Ma J., Ha E., Hong Y.S., Kang I., Lee E.H., Yoon S., Kim J.E., Kim J., Lyoo I.K. Altered functional connectivity in the fear network of firefighters with repeated traumatic stress. Br. J. Psychiatry. 2019;214:347–353. doi: 10.1192/bjp.2018.260. [DOI] [PubMed] [Google Scholar]
- Kennis M., Van Rooij S.J.H., Van Den Heuvel M.P., Kahn R.S., Geuze E. Functional network topology associated with posttraumatic stress disorder in veterans. The Authors NeuroImage: Clin. 2016;10:302–309. doi: 10.1016/j.nicl.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk U., Gu X., Harvey A.H., Fonagy P., Montague P.R. Mindfulness training modulates value signals in ventromedial prefrontal cortex through input from insular cortex. Elsevier B.V. NeuroImage. 2014;100:254–262. doi: 10.1016/j.neuroimage.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluetsch R.C., Ros T., Théberge J., Frewen P., Calhoun V.D., Schmahl C., Jetly R., Lanius R. Plastic modulation of PTSD resting-state networks and subjective wellbeing by EEG neurofeedback. Acta Psychiatr. Scand. 2014;130:123–136. doi: 10.1111/acps.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depression Anxiety. 2016;14:n/a-n/a. doi: 10.1002/da.22478. [DOI] [PubMed] [Google Scholar]
- Koek R.J., Roach J., Athanasiou N., van ‘t Wout-Frank M., Philip N.S. Neuromodulatory treatments for post-traumatic stress disorder (PTSD) Elsevier Progr. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;92:148–160. doi: 10.1016/j.pnpbp.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Krystal J.H., Davis L.L., Neylan T.C., Raskind A.M., Schnurr P.P., Stein M.B., Vessicchio J., Shiner B., Gleason T.D., Huang G.D. It Is Time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD psychopharmacology working group. Biol. Psychiatry. 2017;82:e51–e59. doi: 10.1016/j.biopsych.2017.03.007. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, Mckay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT (2011) Behavioral Interpretations of Intrinsic Connectivity Networks. 4022–4037. [DOI] [PMC free article] [PubMed]
- Lanius R., Bluhm R.L., Frewen P. How understanding the neurobiology of complex post-traumatic stress disorder can inform clinical practice: a social cognitive and affective neuroscience approach. Acta Psychiatr. Scand. 2011;124:331–348. doi: 10.1111/j.1600-0447.2011.01755.x. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Frewen P.A., Tursich M., Jetly R., Mckinnon M.C. Restoring large-scale brain networks in PTSD and related disorders: a proposal for neuroscientifically-informed treatment interventions. 2015;1:1–12. doi: 10.3402/ejpt.v6.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanius R.A., Rabellino D., Boyd J.E., Harricharan S., Frewen P.A., McKinnon M.C. The innate alarm system in PTSD: conscious and subconscious processing of threat. Elsevier Ltd Curr. Opin. Psychol. 2017;14:109–115. doi: 10.1016/j.copsyc.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Lanius R.A., Terpou B.A., Mckinnon M.C., Lanius R.A., Terpou B.A., Mckinnon M.C., Lanius R.A. The sense of self in the aftermath of trauma : lessons from the default mode network in posttraumatic stress disorder network in posttraumatic stress disorder. European Journal of Psychotraumatology. 2020;11(1) doi: 10.1080/20008198.2020.1807703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H., Kleinschmidt a., Beyerle a., Eger E., Salek-Haddadi a., Preibisch C., Krakow K. EEG-correlated fMRI of human alpha activity. NeuroImage. 2003;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Lewis C., Roberts N.P., Gibson S., Bisson J.I. Dropout from psychological therapies for post-traumatic stress disorder (PTSD) in adults: systematic review and meta-analysis. Taylor & Francis Eur. J. Psychotraumatol. 2020;11 doi: 10.1080/20008198.2019.1709709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I., Abelson J.L. Context Processing and the Neurobiology of Post-Traumatic Stress Disorder. Elsevier Inc. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. Electrophysiological signatures of resting state networks in the human brain. PNAS. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C., Mishor Z. Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. NeuroImage. 2011;57(4):1317–1323. doi: 10.1016/j.neuroimage.2011.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurry K.L., Frueh B.C., Chiu P.H., King-Casas B. Opponent effects of hyperarousal and re-experiencing on affective habituation in posttraumatic stress disorder. Elsevier Inc Biol. Psychiatr. Cogn. Neurosci. Neuroimaging. 2020;5:203–212. doi: 10.1016/j.bpsc.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misaki M., Phillips R., Zotev V., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Brain activity mediators of PTSD symptom reduction during real-time fMRI amygdala neurofeedback emotional training. Elsevier NeuroImage: Clin. 2019;24:102047. doi: 10.1016/j.nicl.2019.102047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modinos G., Ormel J., Aleman A. Activation of anterior insula during self-reflection. PLoS ONE. 2009;4:e4618. doi: 10.1371/journal.pone.0004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachev P., Kennard C., Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- Namkung H., Kim S.H., Sawa A. The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Elsevier Ltd Trends Neurosci. 2017;40:200–207. doi: 10.1016/j.tins.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A., Densmore M., Frewen P.A., Théberge J., Neufeld R.W.J., McKinnon M.C., Lanius The dissociative subtype of posttraumatic stress disorder : unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Harricharan S., Densmore M., Neufeld R.W.J., Ros T., McKinnon M.C., Frewen P.A., Théberge J., Jetly R., Pedlar D., Lanius R.A. Classifying heterogeneous presentations of PTSD via the default mode, central executive, and salience networks with machine learning. Elsevier NeuroImage: Clin. 2020;27:102262. doi: 10.1016/j.nicl.2020.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson, A.A., Mckinnon, M.C., Jetly, R., Lanius, R.A., 2020b. Uncovering the heterogeneity of posttraumatic stress disorder: towards a personalized medicine approach for military members. 6.
- Nicholson A.A., Rabellino D., Densmore M., Frewen P.A., Paret C., Kluetsch R., Schmahl C., Théberge J., Ros T., Neufeld R.W.J., Mckinnon M.C., Reiss J.P., Jetly R., Lanius R.A. Intrinsic connectivity network dynamics in PTSD during amygdala downregulation. Hum. Brain Mapp. 2018:1–18. doi: 10.1002/hbm.24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Frewen P.A., Densmore M., Théberge J., Kluetsch R.C., Lanius R.A. Alpha oscillation neurofeedback modulates amygdala complex connectivity and arousal in posttraumatic stress disorder. Authors NeuroImage: Clin. 2016;12:506–516. doi: 10.1016/j.nicl.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A.A., Ros T., Jetly R., Lanius R.A. Regulating posttraumatic stress disorder symptoms with neurofeedback : Regaining control of the mind. J. Military, Veteran Family Health. 2020;6 [Google Scholar]
- Nolan H., Whelan R., Reilly R.B. FASTER: fully automated statistical thresholding for EEG artifact rejection. Elsevier B.V. J. Neurosci. Methods. 2010;192:152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Olson I.R., Plotzker A., Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Panisch, L.S., Hai, A.H., 2018. The effectiveness of using neurofeedback in the treatment of post-traumatic stress disorder: a systematic review. [DOI] [PubMed]
- Patel R., Spreng R.N., Shin L.M., Girard T. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Elsevier Ltd Neurosci. Biobehav. Rev. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Peniston E.G., Kulkosky P.J. Alpha-theta brainwave neuro-feedback for vietnam veterans with combat- related post-traumatic stress disorder. Medical Psychother. 1991;4:47–60. [Google Scholar]
- Pitman R.K., Rasmusson A.M., Koenen K.C., Shin L.M., Orr S.P., Gilbertson M.W., Milad M.R., Liberzon I. Biological studies of post-traumatic stress disorder. Nature Publishing Group Nat. Rev. Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L., Wang Z., Sun Y., Wan J., Su S., Zhou Y., Xu J. A preliminary study of alterations in default network connectivity in post-traumatic stress disorder patients following recent trauma. Elsevier Brain Res. 2012;1484:50–56. doi: 10.1016/j.brainres.2012.09.029. [DOI] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? Elsevier Inc NeuroImage. 2011;57:1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Rabellino D., Tursich M., Frewen P.A., Daniels J.K., Densmore M., Théberge J., Lanius R.A. Intrinsic Connectivity Networks in post-traumatic stress disorder during sub- and supraliminal processing of threat-related stimuli. Acta Psychiatr. Scand. 2015 doi: 10.1111/acps.12418. [DOI] [PubMed] [Google Scholar]
- Ravindran L.N., Stein M.B. Pharmacotherapy of PTSD: premises, principles, and priorities. Elsevier B.V. Brain Res. 2009;1293:24–39. doi: 10.1016/j.brainres.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Loomis A.M., Hamlin E., Hodgdon H., Spinazzola J., van der Kolk B. The impact of neurofeedback training on children with developmental trauma: a randomized controlled study. Psychol. Trauma: Theory Res. Pract. Policy. 2020 doi: 10.1037/tra0000648. [DOI] [PubMed] [Google Scholar]
- Roiser J.P., Linden D.E., Gorno-Tempinin M.L., Moran R.J., Dickerson B.C., Grafton S.T. Minimum statistical standards for submissions to Neuroimage. Clinical. NeuroImage: Clin. 2016;12:1045–1047. doi: 10.1016/j.nicl.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Frewen P., Théberge J., Michela A., Kluetsch R., Mueller A., Candrian G., Jetly R., Vuilleumier P., Lanius R.A. Neurofeedback tunes scale-free dynamics in spontaneous brain activity. Cereb. Cortex. 2016:1–12. doi: 10.1093/cercor/bhw285. [DOI] [PubMed] [Google Scholar]
- Ros T., Baars J.B., Lanius R., Vuilleumier P. Tuning pathological brain oscillations with neurofeedback: a systems neuroscience framework. Front. Hum. Neurosci. 2014;8:1–22. doi: 10.3389/fnhum.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros T., Théberge J., Frewen P., Kluetsch R., Densmore M., Calhoun V.D., Lanius R. Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Elsevier Inc. NeuroImage. 2013;65:324–335. doi: 10.1016/j.neuroimage.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepa E., Dean Z., McCabe C. Bupropion Administration Increases Resting-State Functional Connectivity in Dorso-Medial Prefrontal Cortex. International Journal of Neuropsychopharmacology. 2017;20(6):455–462. doi: 10.1093/ijnp/pyx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A.-L., Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: a simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci.: Off. J. Soc. Neurosci. 2010;30:10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenberg P.L.A., David A.S. Biofeedback for psychiatric disorders: a systematic review. Appl. Psychophysiol. Biofeedback. 2014;39:109–135. doi: 10.1007/s10484-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. Off. J. Soc. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev A., Liberzon I., Marmar C. Post-traumatic stress disorder. N. Engl. J. Med. 2017;376:2459–2469. doi: 10.1056/NEJMra1612499. [DOI] [PubMed] [Google Scholar]
- Shang J., Lui S., Meng Y., Zhu H., Qiu C., Gong Q., Liao W., Zhang W. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS ONE. 2014;9:e96834. doi: 10.1371/journal.pone.0096834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirer W.R., Ryali S., Rykhlevskaia E., Menon V., Greicius M.D. Decoding subject-driven cognitive states with whole-brain connectivity patterns. 2012;3:158–165. doi: 10.1093/cercor/bhr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R., Ros T., Stoeckel L., Haller S., Scharnowski F., Lewis-Peacock J., Weiskopf N., Blefari M.L., Rana M., Oblak E., Birbaumer N., Sulzer J. Closed-loop brain training: the science of neurofeedback. Nat. Rev. Neurosci. 2017;18:86–100. doi: 10.1038/nrn.2016.164. [DOI] [PubMed] [Google Scholar]
- Smith, S.M., Fox, P.T., Miller, K.L., Glahn, D.C., Fox, P.M., Mackay, C.E., Filippini, N., Watkins, K.E., Toro, R., Laird, A.R., Beckmann, C.F., 2009. Correspondence of the brain’s functional architecture during activation and rest. [DOI] [PMC free article] [PubMed]
- Sorger B., Scharnowski F., Linden D.E.J., Hampson M., Young K.D. Control freaks: towards optimal selection of control conditions for fMRI neurofeedback studies. Elsevier Ltd NeuroImage. 2019;186:256–265. doi: 10.1016/j.neuroimage.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng, R.N., Mar, R.A., Kim, A.S.N., 2008. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. 489–510. [DOI] [PubMed]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;911 doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D., Ipser J., Seedat S., Sager C., Amos T. Pharmacotherapy for post traumatic stress disorder (PTSD) Cochrane Database Syst. Rev. CD002795. 2006 doi: 10.1002/14651858.CD002795.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko P.R., Yehuda R. Magnetic resonance imaging predictors of psychotherapy treatment response in post-traumatic stress disorder: a role for the salience network. Elsevier Ireland Ltd Psychiatr. Res. 2019;277:52–57. doi: 10.1016/j.psychres.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Tursich M., Ros T., Frewen P.A., Kluetsch R.C., Calhoun V.D., Lanius R.A. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. Acta Psychiatr. Scand. 2015;132:29–38. doi: 10.1111/acps.12387. [DOI] [PubMed] [Google Scholar]
- Tursich, M., Ros, T., Pa, F., Rc, K., Vd, C., Ra, L., 2015b. Distinct intrinsic network connectivity patterns of post-traumatic stress disorder symptom clusters. 29–38. [DOI] [PubMed]
- Vanasse T.J., Franklin C., Salinas F.S., Ramage A.E., Calhoun V.D., Robinson P.C., Kok M., Peterson A.L., Mintz J., Litz B.T., Young-Mccaughan S., Resick P.A., Fox P.T. A resting-state network comparison of combat-related PTSD with combat-exposed and civilian controls. Social Cogn. Affect. Neurosci. 2019;14:933–945. doi: 10.1093/scan/nsz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kolk B.A. Viking; New York: 2014. The body keeps the score: Brain, mind, and body in the healing of trauma. [Google Scholar]
- van der Kolk B.A., Hodgdon H., Gapen M., Musicaro R., Suvak K., Hamlin E., Spinazzola J. A randomized controlled study of neurofeedback for chronic PTSD. PLOS ONE. 2016:1–18. doi: 10.1371/journal.pone.0166752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu J., Zhang J., Zhan W., Li L., Wu M., Huang H., Zhu H., Kemp G.J., Gong Q. Altered resting-state functional activity in posttraumatic stress disorder: a quantitative meta-analysis. Nature Publishing Group Sci. Rep. 2016;6:1–14. doi: 10.1038/srep27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers, F.W., Blake, D.D., Schnurr, P.P., Kaloupek, D.G., Marx, B.P., Keane, T.M., 2013. The clinician‐administered PTSD scale for DSM‐5 (CAPS‐5). Interview available from the National Center for PTSD at www.ptsd.va.gov.
- Yehuda R., Hoge C.W., McFarlane A.C., Vermetten E., Lanius R.A., Nievergelt C.M., Hobfoll S.E., Koenen K.C., Neylan T.C., Hyman S.E. Post-traumatic stress disorder. Macmillan Publishers Limited Nat. Rev. Disease Primers. 2015;15057 doi: 10.1038/nrdp.2015.57. [DOI] [PubMed] [Google Scholar]
- Zotev V., Phillips R., Misaki M., Wong C.K., Wurfel B.E., Krueger F., Feldner M., Bodurka J. Real-time fMRI neurofeedback training of the amygdala activity with simultaneous EEG in veterans with combat-related PTSD. NeuroImage: Clinical. 2018;19:106–121. doi: 10.1016/j.nicl.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]