Abstract
Until recently, it was thought that there were no lymphatic vessels in the central nervous system (CNS). Therefore, all metabolic processes were assumed to take place only in the circulation of the cerebrospinal fluid (CSF) and through the blood-brain barrier’s (BBB), which regulate ion transport and ensure the functioning of the CNS. However, recent findings yield a new perspective: There is an exchange of CSF with interstitial fluid (ISF), which is drained to the paravenous space and reaches lymphatic nodes at the end. This circulation is known as the glymphatic system. The glymphatic system is an extensive network of meningeal lymphatic vessels (MLV) in the basal area of the skull that provides another path for waste products from CNS to reach the bloodstream. MLV develop postnatally, initially appearing around the foramina in the basal part of the skull and the spinal cord, thereafter sprouting along the skull’s blood vessels and spinal nerves in various areas of the meninges. VEGF-C protein (vascular endothelial growth factor), expressed mainly by vascular smooth cells, plays an important role in the development of the MLV. The regenerative potential and plasticity of MLV and the novel discoveries related to CNS drainage offer potential for the treatment of neurodegenerative diseases such as dementia, hydrocephalus, stroke, multiple sclerosis, and Alzheimer disease (AD). Herein, we present an overview of the structure and function of the glymphatic system and MLV, and their potential involvement in the pathology and progression of neurodegenerative diseases.
Keywords: Glymphatic system, meningeal lymphatic vessels (MLV), cerebrospinal fluid (CSF), blood-brain barrier (BBB), functional anatomy, interstitial fluid of the brain, aquaporin-4, clinical perspective, neurodegenerative disorders
1. Introduction
Almost all mammalian tissues have blood and lymphatic vessels (LV) [1]. In peripheral organs, lymphatic drainage promotes homeostasis of interstitial fluid (ISF). Immune surveillance is provided by absorbing waste products, macromolecules and immune cells, filtering them in lymph nodes (LN), and collecting them into the systemic circulation [1-4]. The brain has high metabolic activity; therefore, it requires a robust system for moving metabolites. The system provides drainage from ISF into the cerebrospinal fluid (CSF) and thence into the venous sinuses and lymphatic vessels of the dura mater [1, 5-8]. The recent discovery of the meningeal lymphatic system in the dura mater provides an additional way for the central nervous system (CNS) to connect to the systemic circulation [9].
Isolated lymphatic vessels pass through skull channels from the brain and CSF circulates into cervical lymphatic vessels. Some groups of perivascular and perineural spaces are means to transport fluid to extracranial LV [10]. The presence of a functioning lymphatic system in the CNS means that current postulates about the stability and immune privileges of the brain need to be reconsidered. The potential importance of meningeal lymphatic vessels (MLV) in the etiology and pathogenesis of neurovascular, neuroinflammatory and neurodegenerative diseases, such as Alzheimer’s disease (AD), dementia, hydrocephalus, stroke, and multiple sclerosis (MS), is significant.
Systematic, rigorous analysis and integration of currently available information are first few important steps [1, 6, 11 - 26]. In the present review, we offer a critical analysis of the literature complemented by our own experiences regarding the structure and function of the glymphatic system and meningeal lymphatic vessels, as well as their association with brain pathologies.
2. Glymphatic system, blood-brain barrier (BBB) and their role in the metabolism of the CNS
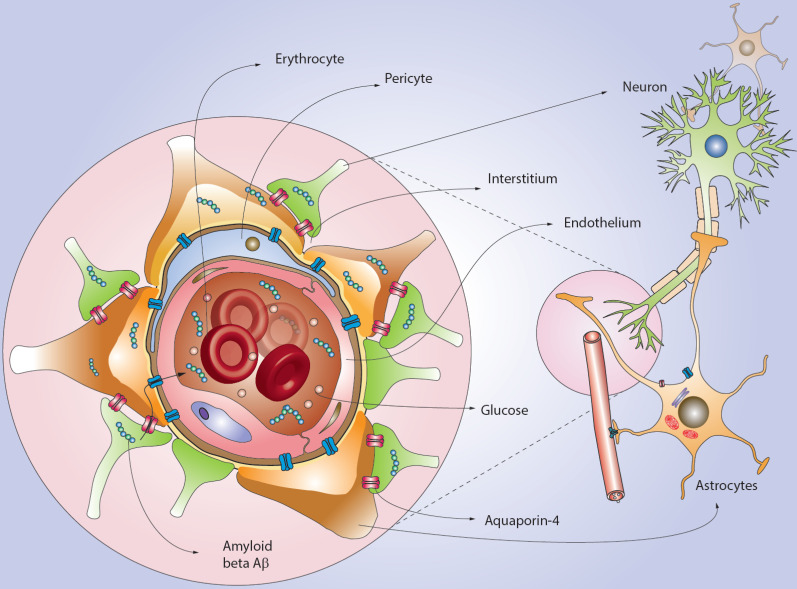
The BBB protects the brain from circulating blood and potential contaminants [6]. The BBB consists of endothelial cells that are supported by astrocytes and pericytes, and by blood vessels [6, 27]. Only lipophilic substances and small molecules like water and some gases are able to pass through the BBB via passive diffusion [15-17]. In addition, there is active transport using carrier proteins for glucose and amino acids among others [18]. The BBB protects the brain from ionic changes and maintains an optimal state of the intercellular fluid. Blood-borne substances that cross the BBB can initiate inflammatory reactions, disrupting the activity of the CNS [17].
Various studies have shown that aquaporin-4 contained at the ends of astrocytes helps in the normal redistribution of CSF during glucose metabolism in neurons [19, 28]. Beta-amyloid is a polypeptide of 39-43 amino acids that are formed in normal cells during metabolism and are present in the blood plasma, cerebral intercellular fluid, ISF, and CSF [20]. Its concentration in the brain is regulated by the mechanisms of the inflow and outflow of the fluids [16]. Unbound beta-amyloid can pass through the BBB using receptors and transporters such as the apical-side endothelial receptor for advanced glycation end-products (RAGE), efflux transporters P-glycoprotein/ABCB1 and BCRP/ABCG2 [8, 20] (Fig. 1). With respect to neuropathology, several studies have shown that, for example, the accumulation of amyloid-beta (Aβ), contributes to the development of AD [5, 11, 13-17, 22, 29, 30].
Fig. (1).
Amyloid beta (Aβ) accumulation and glymphatic tract. By several pathological mechanisms Aß accumulates in the central nervous system. However, Aß may leak through via the glymphatic tract from interstitium to lymphatic vessels. It is reported that the role of Aquaporin-4, located in astrocytes, is critical for regulating the Aß equilibrium in the brain. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The glymphatic (a portmanteau of glial and lymphatic) system was first identified in the rodent brain in 2012 [23]. It supports CSF currents into the brain along with the perivascular arterial spaces and then into the interstitium of the brain through aquaporin-4 [31] (Fig. 2). The dysfunction of the glymphatic system may be associated with the influx of CSF, which depends on arterial pulsation [12, 32]. With an increased inflow, more solutes are transferred to the perivascular space, but they cannot leave the space due to the depolarization of aquaporin-4 and dysfunction of the glymphatic system [15]. When this happens, beta-amyloid accumulates inside the brain and in blood vessels’ walls. This leads to a narrowing of the perivascular space and, ultimately, complete blockage of the glymphatic system’s cleansing pathway [8, 21, 23-39].
Fig. (2).
Role of the astrocytes and endothelial cells (EC) in the regulation of Aß levels. Astrocytes’ feet surround EC controlling the flux of metabolites as glucose and avoiding the passage of toxins and detrimental factors through the brain. Interestingly, the Aß molecules produced by neurons may be leak through the astrocytes feet and moved to CSF and blood. The glymphatic system serves as a transport media; however, the influx of Aß to CSF depends on arterial dynamics and Aquaporin-4 depolarization. Dysfunction of the glymphatic system may lead to unnecessary Aß accumulation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.1. Lymphogenesis
During embryogenesis, the lymphatic system arises from cells budding off the anterior and posterior cardinal veins, forming the initial lymphatic network [22]. Lymphatic capillaries are thin-walled vessels with blind ends. They are responsible for the drainage of fluids and consist of a single layer of lymphatic endotheliocytes, which are not covered by pericytes, and smooth muscle cells (SMC) [2-4, 39-40]. Unlike the continuous connections between endothelial cells (EC) of blood vessels, lymphatic capillaries have specific intercellular, intermittent connections that are characterized by parallel linear segments of the vascular endothelial protein cadherin [29, 39]. The gaps between the endothelium compounds permit fluids and macromolecules to passively enter the lumen of the vessel [40-47]. Recently, it was reported that angiopoietin 2 is one of the essential proteins involved in lymphatic capillary formation [39].
A unique feature of the lymphatic capillary is the possible absence of a basement membrane. Lymphatic capillaries are connected to the surrounding tissue by anchor filaments that are attached to the interstitial collagen fibers. They consist of emilin-1 and fibrillin [1, 41]. These filaments are involved actively in draining excess extracellular fluids: increased interstitial pressure can stretch connective tissue fibers, fixing filaments and endothelial cells by increasing the diameter of the lymphatic vessels [41]. Furthermore, impairment of these features can lead to defects in the lymphatic system, such as the inhibition of lymphatic drainage, lymphatic leakage, and the development of lymphedema [41].
At the cellular level, blood and lymphatic endotheliocytes have significant importance in the formation of the new vessels [41]. They both need external stimulation (through growth factors or cytokines) to form the initial section of the vessel, where the cells acquire two different profiles: the cells of the endings of the pre-lymphatic capillary produce numerous filopodia in order to “sense” the growth factor gradient and direct the growth process to it, and the cells of the main division, which proliferates to form new vessels [33-35, 39, 44].
2.2. Anatomy of the Meningeal Lymphatic Vessels
The overall organization of the meningeal lymphatic vascular (MLV) network has several distinctive features. The anatomical structure of the network is complex and is defined by the periarterial pathways of CSF infusion and perivenous clearance pathways that are functionally related to the interstitial currents supported by astrocytic aquaporin-4 molecules [1, 5-10, 28, 31, 48] (Fig. 3). An MRI study using a contrast agent demonstrated that humans, monkeys, and rodents present similar glymphatic pathways and CSF efflux [4, 10, 24].
Fig. (3).
Meningeal system and lymphatic vessels location in brain. The meningeal system is composed of several layers including Dura mater, Pia mater, and the Arachnoid layer. Below the Arachnoids (subarachnoid) space, is the cerebrospinal fluid CSF. CSF extends by several cisterns and continues to spinal medulla serving as a transport media for metabolites, ions, and waste product removal. Lymphatic vessels participate in the CSF dynamic and serve as drainage media (lymphatic vessel is highlighted by dashed lines). The most important brain tissue components of the brain are depicted in the figure. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
In this respect, the glymphatic pathway originates from the orbital region and passes over the olfactory bulbs, before reaching the venous sinuses of the hard shell. It covers a small amount of tissue, forming a network consisting of thin vessels, which are larger in the transverse sinuses than in the upper sagittal sinus [1, 5, 6, 9, 10, 23, 24]. Differences in their structure may reflect the environment in which the vessels are located. For example, the higher pressure of CSF in the CNS as compared with the pressure of the intercellular fluid in the peripheral tissues may affect branching of the vessels and limit their growth [11].
According to experiments on mice, the MLV network is developed postnatally [10, 33-35]. Its development begins when the groups of venous endothelial cells in the common cardinal veins become associated with lymphatic cells [10]. After the formation of the first lymphatic plexus, the subsequent expansion of the vascular lymphatic tree occurs mainly by sprouting branches of previously formed LV, through a steadily increasing number of lymphatic vessels. Their concentration in the plexus contributes to meningeal lymphogenesis [10, 39-44].
Analyses of the effects of drugs on the meninges at different periods after birth, have shown that the development of MLV takes place during the first postnatal week. This occurs concurrently with veins and arteries, and cranial and spinal nerves. Developing MLV first appears around the holes for entry and exit of blood vessels and nerves; thus forming an extensive network with many branches. Formation ends about 3-4 weeks postnatally [14].
However, MLV have been found in mice prenatally. They were localized in the skull around the foramen magnum and sphenopalatine artery (branches of the internal carotid artery) [10, 39]. After birth, MLV from the area of the foramen magnum expand along blood vessels and then connect on the ventral side of the midline with further growth to the sagittal suture of the skull [11].
Para-sinusoidal MLV develop along the jugular veins and cranial nerves, which enter the skull through the jugular foramen. These are associated with deep cervical lymph nodes [1, 5, 6, 9, 10]. In addition, valves were found in these vessels [1]. Basically, the basal distribution of valves in MLV shows that they are exposed to various fluid flows and have certain functional properties in different areas around the brain and the spinal cord [24]. Furthermore, these findings suggest that the growth of intracranial LV along nerves and blood vessels takes place against the direction of lymph flows in subsequent functional LV. Intracranial and para-sinusoidal LV are directed upwards dorsolaterally along the sigmoid sinus, and then converge along the transverse sinus to the superior sagittal sinus, extending towards the front of the skull [11]. LV were discovered in the dura mater covering the cerebellum in association with the veins branching from the transverse sinus [10]. In the dura mater, LV are attached to the frontal bone, covering the olfactory bulb before the merging of two transverse sinuses into the superior sagittal. These vessels form the lymphatic network around the perforated plate, piercing and connecting with the nasal LV that merge into the deep cervical LV and reaching the lymph nodes [25, 26, 33-35]. Additionally, extracranial LV have been found on the ocular orbit after the enucleation of the eye [24]. In the base of the skull, MLV begin to develop soon after birth, concentrating around the cranial openings along the cranial nerves and the associated blood vessels [10]. By the end of the first month of life, MLV have developed in all parts of the skull.
3. MLV in the spinal cord
Cerebrospinal fluid enters the brain cavity through the paravascular spaces along the arteries, mixes with the interstitial fluid, and then leaves them through the paravascular spaces along the veins. The development of MLV in the spinal membranes occurs in the early postnatal period. A similar pattern of the development is present in the brain. The cervical, thoracic, and lumbar regions develop in parallel along the spinal nerves and connect with blood vessels [1]. LV surround a large hole in the skull, connecting with MLV in the cervical spine [1]. In contrast, MLV do not connect in the middle with the ventral side of the vertebral canal but instead are on the dorsal side, forming a connected uniform pattern throughout the subarachnoid space. Closer to the caudal region, spinal MLV become elongated, adjusting to the growing vertebrae [10, 11]. The completed MLV are connected along the large number of openings on both dorsal and ventral sides, covering the area of the intervertebral disc ventrally and intervertebral ligamentous apparatus dorsally [14]. Finally, MLV depart from the spinal canal laterally with the spinal nerves and blood vessels [10]. The architectonics of spinal MLV change along the ventral-dorsal and cranio-caudal axes of the spine, which reflect the functional properties of the MLV that are typical for this departure from the CNS [10, 11, 14, 24].
4. Biomechanical factors
VEGF-C (vascular endothelial growth factor C), but not VEGF-D, is essential for the development of MLV [39, 41-46]. VEGF-C is expressed by smooth muscle cells (SMC) of blood vessels located along the direction of the growth of LV [41]. Numerous co-receptors for VEGFR (vascular endothelial growth factor receptor) have been described, such as neuropilins, heparan sulfate, integrins, or cadherins [41]. This adds another level of regulation for VEGFR expression and inhibition through its modulation in time and space [22]. For example, the function group neuropilin (receptors of semaphorin-1 and 2) has been identified as pivotal factors in the promotion of VEGFR [26, 33]. Moreover, obstacles in the interaction of VEGF-C with VEGFR3 (vascular endothelial growth factor receptor-3) worsen the development of LV, and the removal of VEGFR3 altogether blocks LV development. Inhibition of its signaling has been shown to cause regression of MLV in adult mice [19, 20].
Other findings reveal that matrix metalloproteinases (MMPs) regulate angiogenesis through the extracellular matrix (ECM) remodelling [26]. Additionally, matrix metalloproteinases-2 (MMP-2) is involved in lymphogenesis in both mice and aquarium fish, controlling the migration of lymphatic endothelial cells in the Type 1 collagen matrix by acting similar to interstitial collagenase [33]. Membrane 1-matrix metalloproteinase (MT1-MMP), on the contrary, is capable of splitting the endothelial receptor-1 of hyaluronan in the lymphatic vessel (LYVE-1) into lymphatic EC and inhibiting lymphangiogenic reactions mediated by it [26, 33].
Interestingly, in adult mice, lymphatic vessels regressed after VEGF-C removal. Its excess, to the contrary, induced meningeal lymphogenesis [22]. Growth of MLV may occur in response to VEGF-C gene transfer without affecting blood vessels [9, 10, 42-44]. In addition, VEGF-C, which is necessary for the development of LV, was most prevalent among the SMC of blood vessels of the pituitary and pineal gland. These glands are the most vascularized in the brain [34]. Moreover, VEGF-C’s ability to induce the growth of vessels is known to stimulate the contractile activity of smooth myocytes around the gathering LV [10].
Finally, regarding the expression of genes responsible for the development of BBB, it is known that non-coding single-nucleotide polymorphism in aquaporin-4 affects the cognitive and functional progression of AD [36, 49]. These combined results suggest the importance of VEGF-C and aquaporin-4 in the development of MLV and BBB.
5. Diseases associated with brain drainage systems
People with dementia have increased BBB permeability. Disturbances caused by the weakening of dense contacts between endothelial cells may appear not only in old age but also at other stages of life [27, 49-63].
Pathology, like AD, is a result of the accumulation of Aβ, leading to interruption and deregulation of the BBB. Under normal circumstances, improperly folded proteins are purified by proteasome degradation, autophagy, and the glymphatic system [49-53, 64]. However, when their concentration increases, they can form aggregates, resulting in the accumulation of neurotoxic beta-amyloid plaques and Tau oligomers. These are evident several years before the onset of AD [29, 30, 49].
Disturbances may occur due to the transport of proteins that change with constant production. With age, the flow of beta-amyloid into the brain increases and the effectiveness of BBB and CNS purification are reduced by the expression of protein-transporter genes [59]. Some studies have shown that the destruction of the BBB is correlated with the accumulation of Aβ and increases in neurodegeneration [29, 51].
The most common cause of dementia is small vessel disease, including capillaries, which affect microcirculation [52]. Given a large number of pathologies involving an imbalance of angiogenesis or lymphangiogenesis, it is important to pay close attention to the characteristics of each vascular network in order to develop more effective treatments for such diseases [41].
It is assumed that the features of small vessels diseases, including the increased formation of perivascular spaces and abluminal protein deposits, can be explained by factors regulating glymphatic function. Emphasis has been placed on the role of aquaporin-4, cerebrovascular pulsation, and clearance of metabolites in CNS [10, 31, 32, 48, 64]. With age, there is a dysfunction of the glymphatic system, for example, due to damages in the regulation of water transport in astrocytes [29, 57].
It has been shown experimentally that mice with depleted aquaporin-4 evidence a reduced CSF current and a deteriorated CSF-ISF cycle [53-55]. The purification of Aβ in the brain was 55 percent less when compared with normal mice [50]. Other mice had a mutation in which superexpression occurred, so the deletion of aquaporins-4 led to a significant increase in its concentration, although the level of proteins included in the synthesis and degradation of Aβ remained the same. Clearly, the mechanism involves a complex interaction, including oxidative stress and energy failure, that likely play central roles [59-63].
Furthermore, it has been shown that glymphatic system activity is higher during sleep and lower during wakefulness [50]. Therefore, poor quality sleep can disrupt the circadian rhythms of cortisol secretion, which also is associated with the development of dementia [49-58]. During wakefulness, the extracellular level of metabolites in the brain increases, including the production of Tau oligomers that, in turn, contribute to the pathogenesis of AD [29, 30, 49-63]. During sleep, the accumulation of metabolites in the brain decreases, as removal occurs from the extracellular space by the convective flow of interstitial and para-arterial fluid in the paravenous space [41, 45-61]. However, when the “sleep-wakefulness” cycle is disrupted (which is characterized by an increased level of the neuropeptide orexin, responsible for awakening), the degree of purification of the CNS from extracellular metabolites is reduced [51]. “Sleep-wakefulness” disorders are associated with increased oxidative stress on neurons and impaired hematoencephalic barrier functioning, which presumably play a role in the development and progression of AD [30, 49]. It is noteworthy that recent studies in humans and transgenic animal models have shown that pathophysiological processes commence long before the clinical onset of neurodegenerative disease, such as the deposition of Aβ in the brain and circadian rhythm disruption [53]. Taken together, these results suggest a mechanistic relationship between pathogenesis, sleep disruptions, and waking cycles that can accelerate the progression of the AD [50].
Moreover, it has been shown that a diet high in refined sugars, salt, animal proteins and fats, as well as low in fruits and vegetables, is associated with a higher risk of developing the disease [52]. In contrast, it has been shown that a diet that restores adequate cortisol concentrations can alleviate sleep disorders and brain clearance, consequently reducing the risk of cognitive impairment and dementia [49-52]. In this aspect, the recently discovered glymphatic system provides a new means to bridge the gap [64].
6. Perspectives and conclusion
Recent discoveries regarding the anatomy of the drainage of the CNS allow us to evaluate it in a fundamentally new way [2-4, 65]. The glymphatic system and the MLV have leading roles in the transport of waste products and other substances [9, 29]. The removal of waste products from CNS with the help of ISF via the glymphatic system was first described by researchers from the medical center of the University of Rochester, when they analyzed the intake and elimination of alcohol and interstitial flow in the brain using 2-photon microscopy in vivo [38, 53-64, 66-74]. MLV was discovered through several experiments conducted on mice. These experiments also showed that during a stroke the marrow of the skull bones supplies neutrophils to the damaged nerve tissue actively and in greater concentration than heretofore thought [49-55].
It has been described that almost all intracranial and MLV are developed in mice during the first month after birth. The progression is from the base of the skull, expanding main branches and merging clusters of lymphatic endothelial cells located next to the previously existing blood vessels and cranial nerves (synchronous joint development) [10]. Since these vessels are located between the membranes of the brain, they are difficult to detect. Furthermore, it was found that the drainage of half of the extracranial CSF occurred through the lymphatic pathways with the subsequent establishment of functional connections of the spinal subarachnoid space with both the lymphatic and glymphatic systems [5].
There is a clear need for a deeper analysis regarding the effects of lymphatic transport interruption on the antigen presentation when they pass through the lymph nodes [38, 49, 54-63, 66-72]. It has been shown that some components of CSF are able to stimulate the immune response in the deep lymph nodes of the neck [38, 63].
We theorize that the most likely pathway for this process is through the cribriform plate into the lymph of the nasal mucosa [53-63]. To determine the direct connection of meningeal lymph nodes with deep cervical lymph nodes, mice were injected with Evans’s blue dye in the ventricles of the brain. Thirty (30) minutes after administration, the dye was detected in the MLV and sinus, as well as in deep cervical lymph nodes [38, 75]. It is noteworthy that there was an initial absence of the dye in the superficial LV, but it was evident after a longer period of time [38]. At the same time, there were no traces of dye in the surrounding tissue. Interestingly, when the dye was injected directly into the nasal mucosa, it was not found in the deep cervical lymph nodes [38]. These findings suggest that the MLV are the primary pathway to drain the CSF into the deep cervical lymph nodes, not the lymph vessels of the nasal mucosa [38]. The drainage of CSF in MLV may be used in addition to the previously described filtration of cerebrospinal fluid through the arachnoid granulations [53]. This result suggests that there are several ways of CSF to be released from the CNS [5]. Recently discovered MLV are a new way for CSF drainage, and they represent a plausible manner for immune cells to exit from the CNS [2-4, 10, 29] (Fig. 4). Additional work should be undertaken in order to fully understand the drainage pathways of glymphatic systems, connecting the brain to peripheral vessels [2-5, 29, 64].
Fig. (4).
Potential mechanism of Aß transport in the brain. It is suggested that Aß may leak through interstitium and blood capillary via Aquaporin-4. Then Aß pass to perivascular space and meningeal lymphatic vessels. It is possible that Aß dissolved in CSF may be released from brain by arachnoid granulations. The meningeal lymphatic system may be associated with the CSF drainage. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Finally, the modulation of MLV and channels of the glymphatic system can provide opportunities to control the outflow of CSF, macromolecules, and cells during harmful conditions such as spinal cord damage, multiple sclerosis, and syringomyelia [53-64, 66-73, 76-78]. The selective removal of MLV by various means could provide a new understanding of its role in normal and pathological conditions [10]. The development of safe ways to promote therapeutic lymphatic filtration and improve the outflow of fluid, cells, and macromolecules from CNS is an important subject for future research. One possible method is to control spatial and temporal delivery and proteolytic activation of VEGF-C [9, 10, 20]. Future studies should be aimed at determining whether the modulation of the functions of MLV can be used safely and reliably in the prevention or treatment of various neuropathological conditions [78].
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This work was supported by the Russian Academic Excellence Project “5-100” for the Sechenov University, Moscow, Russia. This research was also supported within the framework of the grant provided by the CSP Ministry of the Health Russian Federation, and by the RFBR under scientific project No. 18-33-20209, Russian Federation.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Louveau A., Smirnov I., Keyes T.J., Eccles J.D., Rouhani S.J., Peske J.D., Derecki N.C., Castle D., Mandell J.W., Lee K.S., Harris T.H., Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradbury M.W.B., Cole D.F. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J. Physiol. 1980;299:353–365. doi: 10.1113/jphysiol.1980.sp013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspelund A., Antila S., Proulx S.T., Karlsen T.V., Karaman S., Detmar M., Wiig H., Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015;212(7):991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raper D., Louveau A., Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016;39(9):581–586. doi: 10.1016/j.tins.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plog B.A., Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu. Rev. Pathol. 2018;13:379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louveau A., Plog B.A., Antila S., Alitalo K., Nedergaard M., Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J. Clin. Invest. 2017;127(9):3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennels M.L., Blaumanis O.R., Grady P.A. Rapid solute transport throughout the brain via paravascular fluid pathways. Adv. Neurol. 1990;52:431–439. [PubMed] [Google Scholar]
- 8.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakka L., Chazal J. The meninges, an anatomical point of view. Morphologie. 2005;89(284):35–42. doi: 10.1016/S1286-0115(05)83236-9. [DOI] [PubMed] [Google Scholar]
- 10.Antila S., Karaman S., Nurmi H., Airavaara M., Voutilainen M.H., Mathivet T., Chilov D., Li Z., Koppinen T., Park J-H., Fang S., Aspelund A., Saarma M., Eichmann A., Thomas J-L., Alitalo K. Development and plasticity of meningeal lymphatic vessels. J. Exp. Med. 2017;214(12):3645–3667. doi: 10.1084/jem.20170391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H., Xie L., Yu M., Kang H., Feng T., Deane R., Logan J., Nedergaard M., Benveniste H. The effect of body posture on brain glymphatic transport. J. Neurosci. 2015;35(31):11034–11044. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limanaqi F., Biagioni F., Busceti C.L., Ryskalin L., Soldani P., Frati A., Fornai F. Cell clearing systems bridging neuro-immunity and synaptic plasticity. Int. J. Mol. Sci. 2019;20(9):2197. doi: 10.3390/ijms20092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bower N.I., Hogan B.M. Brain drains: new insights into brain clearance pathways from lymphatic biology. J. Mol. Med. (Berl.) 2018;96(5):383–390. doi: 10.1007/s00109-018-1634-9. [DOI] [PubMed] [Google Scholar]
- 14.Da Mesquita S., Louveau A., Vaccari A., Smirnov I., Cornelison R.C., Kingsmore K.M., Contarino C., Onengut-Gumuscu S., Farber E., Raper D., Viar K.E., Powell R.D., Baker W., Dabhi N., Bai R., Cao R., Hu S., Rich S.S., Munson J.M., Lopes M.B., Overall C.C., Acton S.T., Kipnis J. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560(7717):185–191. doi: 10.1038/s41586-018-0368-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verheggen I.C.M., Van Boxtel M.P.J., Verhey F.R.J., Jansen J.F.A., Backes W.H. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev. 2018;90:26–33. doi: 10.1016/j.neubiorev.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 16.Ramanan V.K., Risacher S.L., Nho K., Kim S., Shen L., McDonald B.C., Yoder K.K., Hutchins G.D., West J.D., Tallman E.F., Gao S., Foroud T.M., Farlow M.R., De Jager P.L., Bennett D.A., Aisen P.S., Petersen R.C., Jack C.R., Jr, Toga A.W., Green R.C., Jagust W.J., Weiner M.W., Saykin A.J. Alzheimer’s disease neuroimaging initiative (adni). gwas of longitudinal amyloid accumulation on 18f-florbetapir pet in alzheimer’s disease implicates microglial activation gene IL1RAP. Brain. 2015;138(Pt 10):3076–3088. doi: 10.1093/brain/awv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehsan S-K. Gene-Jack, W.; Corinde, E. W., Sukru, B, Demiral.; Min, G.; Sung, W. K.; Elsa, L.; Veronica, R.; Amna, Zehra.; Clara, F.; Gregg, M.; Peter, M.; Tansha, S.; Susan, De Santi.; Dardo, T.; Helene, B.; and Nora, D. V. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA. 2018;115:4483–4488. doi: 10.1073/pnas.1721694115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rennels M.L., Gregory T.F., Blaumanis O.R., Fujimoto K., Grady P.A. Evidence for a ‘Paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. J. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- 19.Benveniste H., Lee H., Volkow N.D. The glymphatic pathway: waste removal from the cns via cerebrospinal fluid transport. Neuroscientist. 2017;23(5):454–465. doi: 10.1177/1073858417691030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucchieri F., Farina F., Zummo G., Cappello F. Lymphatic vessels of the dura mater: a new discovery? J. Anat. 2015;227(5):702–703. doi: 10.1111/joa.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou W., Pu T., Feng W., Lu M., Zheng Y., Du R., Xiao M., Hu G. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegener. 2019;8:7. doi: 10.1186/s40035-019-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao X., Xu H., Feng W., Su D., Xiao M. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res. Bull. 2018;143:83–96. doi: 10.1016/j.brainresbull.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Kerjaschki D. The lymphatic vasculature revisited. J. Clin. Invest. 2014;124(3):874–877. doi: 10.1172/JCI74854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mader S., Brimberg L. Aquaporin-4 Water channel in the brain and its implication for health and disease. Cells. 2019;8(2):90. doi: 10.3390/cells8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hui F.K. Clearing your mind: a glymphatic system? World Neurosurg. 2015;83(5):715–717. doi: 10.1016/j.wneu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Roth C., Stitz H., Roth C., Ferbert A., Deinsberger W., Pahl R., Engel H., Kleffmann J. Craniocervical manual lymphatic drainage and its impact on intracranial pressure - a pilot study. Eur. J. Neurol. 2016;23(9):1441–1446. doi: 10.1111/ene.13055. [DOI] [PubMed] [Google Scholar]
- 27.Brightman M. Ultrastructure of brain endothelium. In: Bradbury M.W.B., editor. Physiology and Pharmacology of the Blood-Brain Barrier. Berlin, Heidelberg: Springer-Verlag; 1992. pp. 1–22. [Google Scholar]
- 28.Nagelhus E.A., Ottersen O.P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 2013;93(4):1543–1562. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iliff J.J., Chen M.J., Plog B.A., Zeppenfeld D.M., Soltero M., Yang L., Singh I., Deane R., Nedergaard M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014;34(49):16180–16193. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louveau A., Da Mesquita S., Kipnis J. Lymphatics in neurological disorders: A neuro-lymphovascular component of multiple sclerosis and Alzheimer’s disease? Neuron. 2016;91(5):957–973. doi: 10.1016/j.neuron.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papadopoulos M.C., Verkman A.S. Aquaporin water channels in the nervous system. Nat. Rev. Neurosci. 2013;14(4):265–277. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iliff J.J., Wang M., Zeppenfeld D.M., Venkataraman A., Plog B.A., Liao Y., Deane R., Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J. Neurosci. 2013;33(46):18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas J-L., Jacob L., Boisserand L. Med. Sci. (Paris) 2019;35(1):55–61. doi: 10.1051/medsci/2018309. [Lymphatic system in central nervous system]. [DOI] [PubMed] [Google Scholar]
- 34.Herz J., Louveau A., Da Mesquita S., Kipnis J. Morphological and functional analysis of cns-associated lymphatics. Methods Mol. Biol. 2018;1846:141–151. doi: 10.1007/978-1-4939-8712-2_9. [DOI] [PubMed] [Google Scholar]
- 35.Hägerling R., Pollmann C., Andreas M., Schmidt C., Nurmi H., Adams R.H., Alitalo K., Andresen V., Schulte-Merker S., Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32(5):629–644. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimana O.M., Joseph M., Rutkowski J., Brandon D., Witold K., Jacqueline D.S., Melody A.S. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circ. Res. 2010;106:920–931. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan N.N., Qingguang Z., David C., Amanda C., Timothy M.R., Patrick J.D. Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. eLife. 2019;8:e44278. doi: 10.1101/485995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maloveska M., Danko J., Petrovova E., Kresakova L., Vdoviakova K., Michalicova A., Kovac A., Cubinkova V., Cizkova D. Dynamics of Evans blue clearance from cerebrospinal fluid into meningeal lymphatic vessels and deep cervical lymph nodes. Neurol. Res. 2018;40(5):372–380. doi: 10.1080/01616412.2018.1446282. [DOI] [PubMed] [Google Scholar]
- 39.Morfoisse F., Noel A. Lymphatic and blood systems: Identical or fraternal twins? Int. J. Biochem. Cell Biol. 2019;114:105562. doi: 10.1016/j.biocel.2019.105562. [DOI] [PubMed] [Google Scholar]
- 40.Oliver G. Lymphatic vasculature development. Nat. Rev. Immunol. 2004;4(1):35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 41.Detry B., Erpicum C., Paupert J., Blacher S., Maillard C., Bruyère F., Pendeville H., Remacle T., Lambert V., Balsat C., Ormenese S., Lamaye F., Janssens E., Moons L., Cataldo D., Kridelka F., Carmeliet P., Thiry M., Foidart J-M., Struman I., Noël A. Matrix metalloproteinase-2 governs lymphatic vessel formation as an interstitial collagenase. Blood. 2012;119(21):5048–5056. doi: 10.1182/blood-2011-12-400267. [DOI] [PubMed] [Google Scholar]
- 42.Rezaei M., Hashemi M., Sanaei S., Mashhadi M.A., Taheri M. Association between vascular endothelial growth factor gene polymorphisms with breast cancer risk in an iranian population. Breast Cancer (Auckl.) 2016;10:85–91. doi: 10.4137/BCBCR.S39649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castro Dias M., Mapunda J.A., Vladymyrov M., Engelhardt B. Structure and junctional complexes of endothelial, epithelial and glial brain barriers. Int. J. Mol. Sci. 2019;20(21):E5372. doi: 10.3390/ijms20215372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikeshima-Kataoka H. Neuroimmunological implications of AQP4 in astrocytes. Int. J. Mol. Sci. 2016;17(8):1306. doi: 10.3390/ijms17081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong H.L., Jin G., Cao R., Zhang S., Cao Y., Zhou Z. MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat. Commun. 2016;7:10824. doi: 10.1038/ncomms10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levet S., Ciais D., Merdzhanova G., Mallet C., Zimmers T.A., Lee S.J., Navarro F.P., Texier I., Feige J.J., Bailly S., Vittet D. Bone morphogenetic protein 9 (BMP9) controls lymphatic vessel maturation and valve formation. Blood. 2013;122(4):598–607. doi: 10.1182/blood-2012-12-472142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baluk P., Fuxe J., Hashizume H., Romano T., Lashnits E., Butz S., Vestweber D., Corada M., Molendini C., Dejana E., McDonald D.M. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johanson C., Flaherty S., Duncan J. Aging rat brain: A model for analyzing interactions among CSF dynamics, ventriculomegaly and the beta-amyloid retention of Alzheimer’s disease. Cerebrospinal Fluid Res. 2005;2:S6. doi: 10.1186/1743-8454-2-S1-S6. [DOI] [Google Scholar]
- 49.Demiral Ş.B., Tomasi D., Sarlls J., Lee H., Wiers C.E., Zehra A., Srivastava T., Ke K., Shokri-Kojori E., Freeman C.R., Lindgren E., Ramirez V., Miller G., Bandettini P., Horovitz S., Wang G-J., Benveniste H., Volkow N.D. Apparent diffusion coefficient changes in human brain during sleep - Does it inform on the existence of a glymphatic system? Neuroimage. 2019;185:263–273. doi: 10.1016/j.neuroimage.2018.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elvsåshagen T., Mutsaerts H.J., Zak N., Norbom L.B., Quraishi S.H., Pedersen P.Ø., Malt U.F., Westlye L.T., van Someren E.J., Bjørnerud A., Groote I.R. Cerebral blood flow changes after a day of wake, sleep, and sleep deprivation. Neuroimage. 2019;186:497–509. doi: 10.1016/j.neuroimage.2018.11.032. [DOI] [PubMed] [Google Scholar]
- 51.Bernardi G., Cecchetti L., Siclari F., Buchmann A., Yu X., Handjaras G., Bellesi M., Ricciardi E., Kecskemeti S.R., Riedner B.A., Alexander A.L., Benca R.M., Ghilardi M.F., Pietrini P., Cirelli C., Tononi G. Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage. 2016;129:367–377. doi: 10.1016/j.neuroimage.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim Y-K., Nam K.I., Song J. The Glymphatic System in Diabetes-Induced Dementia. Front. Neurol. 2018;9:867. doi: 10.3389/fneur.2018.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veening J.G., Barendregt H.P. The regulation of brain states by neuroactive substances distributed via the cerebrospinal fluid; a review. Cerebrospinal Fluid Res. 2010;7:1. doi: 10.1186/1743-8454-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldmann J., Kwidzinski E., Brandt C., Mahlo J., Richter D., Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J. Leukoc. Biol. 2006;80(4):797–801. doi: 10.1189/jlb.0306176. [DOI] [PubMed] [Google Scholar]
- 55.Fitzner D., Schnaars M., van Rossum D., Krishnamoorthy G., Dibaj P., Bakhti M., Regen T., Hanisch U-K., Simons M. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J. Cell Sci. 2011;124(Pt 3):447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 56.Struckhoff G. Coculture of meningeal and astrocytic cells – a model for the formation of the glial limiting membrane. Int. J. Dev. Neurosci. 1995;13:595–606. doi: 10.1016/0736-5748(95)00040-n. [DOI] [PubMed] [Google Scholar]
- 57.Karman J., Ling C., Sandor M., Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J. Immunol. 2004;173(4):2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 58.Noé F.M., Marchi N. Central nervous system lymphatic unit, immunity, and epilepsy: Is there a link? Epilepsia Open. 2019;4(1):30–39. doi: 10.1002/epi4.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng W., Achariyar T.M., Li B., Liao Y., Mestre H., Hitomi E., Regan S., Kasper T., Peng S., Ding F., Benveniste H., Nedergaard M., Deane R. Suppression of glymphatic fluid transport in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2016;93:215–225. doi: 10.1016/j.nbd.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ludewig P., Winneberger J., Magnus T. The cerebral endothelial cell as a key regulator of inflammatory processes in sterile inflammation. J. Neuroimmunol. 2019;326:38–44. doi: 10.1016/j.jneuroim.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Kim J.H., Kang D.S., Kim J.H., Kong M.H., Song K.Y. Chronic subdural hematoma treated by small or large craniotomy with membranectomy as the initial treatment. J. Korean Neurosurg. Soc. 2011;50(2):103–108. doi: 10.3340/jkns.2011.50.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharon L.Y., Wai Hoe N.G. ‘Subarachnoid cyst’ after evacuation of chronic subdural hematoma: Case report of an unusual postoperative morbidity. Asian J. Neurosurg. 2016;11(3):316. doi: 10.4103/1793-5482.144210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Modesti L.M., Hodge C.J., Barnwell M.L. Intracerebral hematoma after evacuation of chronic extracerebral fluid collections. Neurosurgery. 1982;10(6 Pt 1):689–693. doi: 10.1227/00006123-198206010-00002. [DOI] [PubMed] [Google Scholar]
- 64.Iliff J.J., Goldman S.A., Nedergaard M. Implications of the discovery of brain lymphatic pathways. Lancet Neurol. 2015;14(10):977–979. doi: 10.1016/S1474-4422(15)00221-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jessen N.A., Munk A.S.F., Lundgaard I., Nedergaard M. The glymphatic system: A beginner’s guide. Neurochem. Res. 2015;40(12):2583–2599. doi: 10.1007/s11064-015-1581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molly J. Sullan, Breton M. Asken, Michael S. Jaffee, Steven T. De Kosky, Russell M. Bauer. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 2018;84:316–324. doi: 10.1016/j.neubiorev.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 67.Chomicki A., Sakka L., Avan P., Khalil T., Lemaire J.J., Chazal J. Derivation of cerebrospinal fluid: consequences on inner ear biomechanics in adult patients with chronic hydrocephalus. Neurochirurgie. 2007;53(4):265–271. doi: 10.1016/j.neuchi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 68.Mestre H., Kostrikov S., Mehta R.I., Nedergaard M. Perivascular spaces, glymphatic dysfunction, and small vessel disease. Clin. Sci. (Lond.) 2017;131(17):2257–2274. doi: 10.1042/CS20160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giammattei L., Messerer M., Daniel R.T. Contribution of glymphatic system in pathogenesis of secondary brain injury and its modulation. World Neurosurg. 2018;117:473–474. doi: 10.1016/j.wneu.2018.06.173. [DOI] [PubMed] [Google Scholar]
- 70.Catala M. Embryonic and fetal development of structures associated with the cerebro-spinal fluid in man and other species. Part I: The ventricular system, meninges and choroid plexuses. Arch. Anat. Cytol. Pathol. 1998;46(3):153–169. [PubMed] [Google Scholar]
- 71.Silver I., Li B., Szalai J., Johnston M. Relationship between intracranial pressure and cervical lymphatic pressure and flow rates in sheep. Am. J. Physiol. 1999;277(6):R1712–R1717. doi: 10.1152/ajpregu.1999.277.6.R1712. [DOI] [PubMed] [Google Scholar]
- 72.Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsu M., Rayasam A., Kijak J.A., Choi Y.H., Harding J.S., Marcus S.A., Karpus W.J., Sandor M., Fabry Z. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat. Commun. 2019;10(1):229. doi: 10.1038/s41467-018-08163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bordon Y. Neuroimmunology: A brain drain. Nat. Rev. Immunol. 2015;15(7):404. doi: 10.1038/nri3878. [DOI] [PubMed] [Google Scholar]
- 75.Cserr H.F. Physiology of the choroid plexus. Physiol. Rev. 1971;51(2):273–311. doi: 10.1152/physrev.1971.51.2.273. [DOI] [PubMed] [Google Scholar]
- 76.Hladky S.B., Barrand M.A. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS. 2014;11(1):26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kress B.T., Iliff J.J., Xia M., Wang M., Wei H.S., Zeppenfeld D., Xie L., Kang H., Xu Q., Liew J.A., Plog B.A., Ding F., Deane R., Nedergaard M. Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol. 2014;76(6):845–861. doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Benjamin A. Plo. The glymphatic system in CNS health and disease: past, present and future. Annu. Rev. Pathol. 2018;13:379–394. doi: 10.1146/annurev-pathol-051217-111018. [DOI] [PMC free article] [PubMed] [Google Scholar]