Abstract
Background
The only conclusive way to diagnose Alzheimer’s is to carry out brain autopsy of the patient’s brain tissue and ascertain whether the subject had Alzheimer’s or any other form of dementia. However, due to the non-feasibility of such methods, to diagnose and conclude the conditions, medical practitioners use tests that examine a patient’s mental ability.
Objective
Accurate diagnosis at an early stage is the need of the hour for initiation of therapy. The cause for most Alzheimer’s cases still remains unknown except where genetic distinctions have been observed. Thus, a standard drug regimen ensues in every Alzheimer’s patient, irrespective of the cause, which may not always be beneficial in halting or reversing the disease progression. To provide a better life to such patients by suppressing existing symptoms, early diagnosis, curative therapy, site-specific delivery of drugs, and application of hyphenated methods like artificial intelligence need to be brought into the main field of Alzheimer’s therapeutics.
Methods
In this review, we have compiled existing hypotheses to explain the cause of the disease, and highlighted gene therapy, immunotherapy, peptidomimetics, metal chelators, probiotics and quantum dots as advancements in the existing strategies to manage Alzheimer’s.
Conclusion
Biomarkers, brain-imaging, and theranostics, along with artificial intelligence, are understood to be the future of the management of Alzheimer’s.
Keywords: Artificial intelligence, biomarkers, brain imaging, mild cognitive impairment, theranostics, gene therapy
1. Introduction
Alzheimer's disease (AD), a major cause of dementia, is a progressive neurodegenerative disorder. Dementia includes memory loss and difficulties with thinking, language and problem-solving skills. As per the WHO update, on epidemiology of AD in 2013, the number of people suffering from dementia worldwide is likely to triple by 2050 which was approximately 35.6 million in 2010. The incidence of dementia increases with age, approximately 5-8% are affected over age of 65, the number increases to 25-50% as the age rises over 85. The prevalence of AD for men was lower than that for women by 19-29%. China, USA, India, Japan, Germany, Russia, France and Brazil were the nine countries in descending order of incidence of people suffering from dementia in 2010, and the numbers more than 1 million (USFDA 2013) [1]. There is a protein formed in the brain to form structures called 'plaques' and 'tangles. Microscopically, the neurotic plaques forming amyloid beta peptide (Aβ42) and neurofibrillary tangles (NFTs) composed of hyper phosphorylated tau are the indicators of AD. These proteins are the precursors for the loss of connections between nerve cells, and eventually to the death of nerve cells and loss of brain tissue. We have summarized the pathophysiology, current strategies and future approaches for improvement of the treatment and diagnosis of AD in our review. We aim to give an insight into the current status of diagnosis and future directions to overcome the associated limitations with artificial intelligence. Several proposed theories explain the pathophysiology of neurodegeneration and cause of dementia and are explained in brief in the next section with current advances in understanding.
1.1. Mechanisms of Alzheimer’s Disease
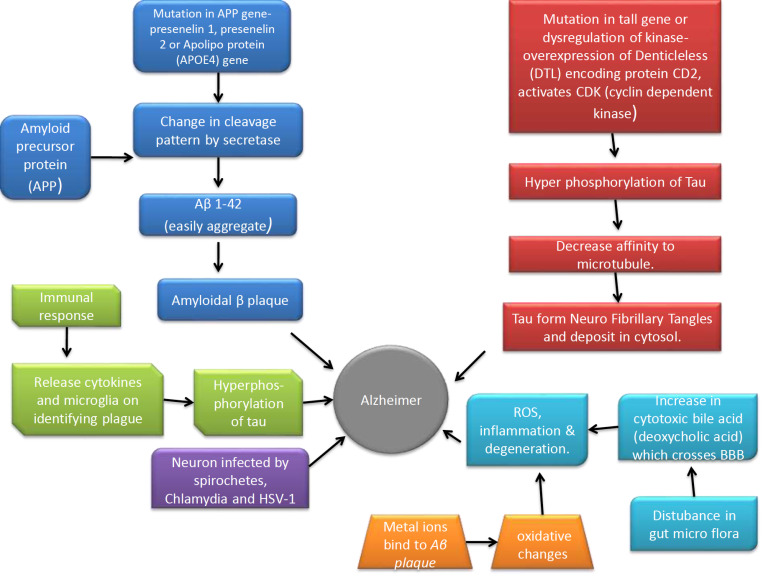
1.1.1. Aβ Plaque Associated Neurodegeneration
According to this hypothesis, Aβ plaques are formed and get deposited in different regions of the brain. These plaques are recognised as foreign material by the brain initiating an inflammatory and immune response by activating the microglia and release of cytokines, which eventually lead to cell death and neurodegeneration. The Aβ plaque comprises of Aβ peptides obtained from amyloid precursor protein (APP) by the enzymatic cleavage via secretases (α, β and γ) [2]. The primary step in the generation of Aβ plaque is the cleavage of amyloid precursor protein (APP) by β-secretase to produce a C-terminal membrane attached with fragments of 89 or 99 amino acids. This β-secretase includes BACE 1 (β-site APP cleaving enzyme), which is also called Asp2 or memapsin2. APP is cleaved at β-sites Asp1 and Glu11 by BACE 1. The C-terminal membrane-bound fragment of 99 amino acid residues is further cleaved by γ-secretase to produce Aβ1-40 and Aβ1-42 isoforms. γ-secretase mainly include presenilin 1 (PS1) or presenilin 2 (PS2). Aβ1-40 is the normal soluble isoform, but if the cleavage pattern changes it may give rise to Aβ1-42, which aggregates easily and forms the plaque due to two additional amino acids isoleucine and alanine [3]. This change in the cleavage pattern happens due to mutations in the APP gene, presenelin 1, presenelin 2 genes or apolipoprotein E (APOE4) gene. Apart from the genetic mutations, many neuropeptides are likely to be involved in the formation of the plaque for e.g. low levels of corticotrophin-releasing hormone (CRH), somatostatin and neuropeptide Y levels whereas higher levels of Angiotensin II are likely to be involved in either irregular cleavage of the APP or impaired removal of Aβ1-42 fragment [2]. Both Aβ and tau aggregates and cause impaired synaptic plasticity and neuronal cell death [4]. However, there has been a lot of controversy over this hypothesis and a recent report indicates that drugs that act to inhibit amyloid plaque formation show no effect in reversing or halting the cognitive decline. This suggests that either the hypothesis is incorrect or the brain becomes refractory to treatment after a certain time. Thus, one should focus on finding therapeutic interventions that act on non-amyloid targets like tau proteins, inflammation, oxidative stress, etc. [5].
1.1.2. Neurofibrillary Degeneration
Tau proteins are micro tubular neuronal proteins. The tau proteins have a microtubule binding domain, which is involved in polymerization and stabilization of the microtubule assembly to maintain the integrity of the cytoskeleton. This binding is regulated by phosphorylation of the serine/ threonine residues by a variety of kinases like Fyn Kinase, glycogen synthase kinase-3β (GSK3β) and cyclin-dependent kinase-5 (CDK5). CDK5 plays a potential role in the formation of neurofibrillary tangles. Aβ activates calpain and deregulates p35 which is an activator of CDK5. Due to cytosolic calcium overload, p35 splits into p25, which hyperactivates cyclin-dependent kinase-5 (CDK5) and leads to hyper phosphorylation of tau [6]. Hyper phosphorylation results in decreased affinity of the tau proteins to microtubules. The hyper phosphorylated tau forms NFTs and gets deposited in the cytosol and can no longer perform the function of maintaining the structure of the cell. Moreover, this deposition affects normal cellular function like synaptic transmission, axonal transport, signal transduction and the cell undergo degeneration gradually. The reason stated for the hyper phosphorylation is a mutation in the tau genes or dysregulation of kinases as mentioned below and phosphatases which catalyze the phosphorylation process [7].
1.1.3. Synaptic Dysfunction and Neurotransmitter Imbalance
The cholinergic system is involved in the process of cognition. The dysfunction of this system is responsible for various dementias, one of which is AD. Cholinergic neurons in the nucleus basalis of Meynert selectively display the deposition of amyloid plaque and NFTs, eventually undergo degeneration due to the initiation of pro-inflammatory events as described earlier, which further deteriorate cognition. Cholinergic deficit also alters the permeability of the blood brain barrier causing erroneous transportation of metabolites and hampering the removal of amyloid plaque, worsening the disease condition [8]. Alteration in Ca2+ permeable n-acetylcholine-receptor (nAChR) can cause impairment in synaptic integrity. In hippocampal and cortical synaptic region Aβ exhibit maximum binding to α7- and α4β2-nAChRs [3]. The expression of choline acetyl transferase is reduced and that of acetylcholinesterase (AChE) is increased, contributing to the depletion of acetylcholine and worsening of dementia. AchE also interacts with Aβ peptide and promotes plaque formation [9]. It has been observed that degeneration of noradrenergic neurons in the locus coerulus is also related to cognitive impairment and neurodegeneration. Further it is reported that noradrenergic receptors are densely present on the astrocytes, the activation of which improves synaptic plasticity and hence learning and memory [10]. Recently, it has been reported that serotonin is equally involved in AD pathogenesis. There is a loss of serotonergic neurons from the brainstem of many AD patients and the levels of this neurotransmitter are also found to be less. The serotonergic cortical input from the midbrain raphe nuclei, is responsible for modulation of cortical plasticity and formation of memory. A dysfunction of this pathway causes memory loss [11]. Glutamate by acting on the N-Methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor is a key neurotransmitter in maintaining synaptic plasticity. An imbalance in the glutamate/glutamine metabolism causes persistent depolarization of the neurons resulting in excitotoxicity leading to synaptic injury. Apart from this, Aβ induces hypersensitivity of NMDA receptors and also disrupts the regulatory control of NMDA activity causing excitotoxicity [12]. There is also a close association of GABA and serotonin in the dorsal raphae nuclei, a region of the brain stem that has many clusters of serotonergic neurons. A study reports that 5HT6R antagonist improves cognitive decline by enhancing serotonin levels via GABAergic neurons. The same molecule also reduces the formation of amyloid plaque since it reduces the gamma-secretase activity without having any effect on β secretase [13]. Loss of inhibitory control of GABAergic neurons on the cholinergic and glutamatergic neurons is linked to synaptic injury in AD patients. Thus, a complex interplay of several neurotransmitters is essential to keep the cognition intact. An imbalance in any one of the above neurotransmitters may contribute to further deterioration of AD symptoms.
Aβ, tau, and AD genetic risk factors affect dendritic integrity and disease progression [14]. The amyloid plaque formation starts at the post synapse. Tau phosphorylation is a protective mechanism against toxic amyloid deposition. Phosphorylated tau dissociates from the post synaptic site and becomes a substrate for other kinases resulting in hyperphosphorylation at various sites. This hyperphosphorylated tau spreads gradually from the post synaptic site to the dendrites and the cell body, finally from the axon to other neurons by intra-axonal connections. This process results in synaptic dysfunction contributing to dementia and neurodegeneration [15].
1.1.4. Neuroinflammation
Neuro-inflammation plays a central role in the pathogenesis of AD. Acute inflammation has a protective role in defending against brain injury such as the presence of Aβ plaque. However, persistent activation of microglia makes them incapable of removing the plaque, but their ability to release pro inflammatory cytokines is retained, resulting in an imbalance between the pro and anti-inflammatory cytokines. Aβ deposits activate various Toll like receptors (TLR2, TLR4 and TLR6), as well as their co-receptors, including CD36, CD14 and CD47 expressed by microglia. On the detection of microbes, proinflammatory cytokines of IL-1β family are produced by the immune system, including cytokine IL-1β and IL-18. These cytokines are expressed by caspase-1 or caspase-8 on activation. Inflammasome like NLR (‘Nod-like receptor’) family or PYHIN (‘pyrin and HIN domain–containing’) assist activation of caspase-1. NLRP3 is the primary inflammasome which can sense Ab aggregates. These proinflammatory cytokines can impair dendritic spines and also disrupt microglial clearance of Aβ. Neuron and glial cell on an encounter with proinflammatory cytokines express inducible isoforms of NO synthase, which enhances the synthesis of nitric oxide (NO). This increases the peptide’s ability to aggregate and makes it more potent in suppressing synaptic plasticity [16]. Under the influence of these cytokines, CDKs get activated causing hyperphosphorylation of tau and increase the formation of Aβ plaque as discussed earlier. Due to the dysregulation of GABAergic mechanism in AD, the inhibitory role of GABA on activated microglia is also lost which further contributes to the release of pro-inflammatory cytokines [17]. Other cells like endothelial cells, oligodendrocytes and neurons can also cause neuroinflammation. Several inflammation protective molecules are present in neuron, like fractalkine, the complement defence protein CD59 and CD200. Immune molecules like IL-1β, IL-6 and CCL2 are produced by brain endothelial cells against Aβ plaque [18].
1.1.5. Infectious Disease Hypothesis
Aβ has been attributed to have antimicrobial properties. It has been suggested that neurons infected by Spirochetes and other pathogens like Chlamydia and HSV-1 have more of Aβ deposition and NFTs. Thus, the persistent untreated infection could be one of the causes of AD [19]. In the presence of infection, the immune system gets activated. A component of the innate immune system referred to as “Toll like proteins” as mentioned above, is activated due to it [20].
1.1.6. Gut Microbiome Disruption
Brain and gut have a bidirectional communication via the brain-gut metabolic axis. Several studies have established a positive correlation between AD and microbial disruption in the gut. Disturbance in the gut microflora results in increased production of secondary cytotoxic bile acid, mainly deoxycholic acid, which can cross the blood brain barrier and get deposited in the brain leading to apoptosis, generation of reactive oxygen species, inflammation and neurodegeneration [21]. Alternatively, dysbiosis of the gut microbiome causes systemic inflammation, neuroinflammation, insulin resistance, which are all related to the pathogenesis of AD [22]. Gut microbes influence the formation, absorption and transport of serotonin and GABA in the brain, moreover some bacterial species influence the formation of amyloid plaque which initiates the inflammatory cascade and hence susceptibility to AD [23]. Thus, a faulty gut microbiome along with other factors contributes to the progression of AD.
NO is secreted on activation of N-Methyl-D-aspartate (NMDA) receptors by glutamate and acts as a primary neurotransmitter of noradrenergic, noncholinergic and enteric nervous system. Gut microbes like Bifidobacteria and Lactobacilli convert nitrite and nitrate to NO. Gut bacilli and Streptomyces can also synthesize NO by their NO synthetase (NOS). Alteration in the activity of any of these gut microbes along with increase nitrate intake, can lead to overproduction of NO, which can cause axonal degeneration, neuro inflammation and neurodegenerative disorders [24].
Alteration in microbiome can lead to the secretion of immunogenic lipopolysaccharides (LPSs) and amyloids which can diffuse across brain and aggravate AD symptoms. Receptor for advanced glycosylation products (RAGE) mediate amyloid brain influx across blood brain barrier (BBB) and is controlled by chaperones and apolipoprotein E and J. Low-density lipoprotein receptor related protein 1 is known to control amyloid clearance. Impairment in all these transportation mechanisms is associated with AD. Moreover, prion like proteins can cause penetration of monocyte trans endothelial which can enhance the inflammation. Due to the alteration in the gut microbiome and transportation, the bacteria derived amyloids may leak from gastro intestinal track and escalate the level of proinflammatory cytokines (TNFα, IL10, IL6, IL17A, IL12p40, IL23p19, and IL22). This may further increase the levels of reactive oxygen species (ROS) and activate signalling of nuclear factor- kB (NF-kB). As a result, proinflammatory miRNA-34a is up regulated, causing downregulation of TREM-2 (trigger receptor expressed in microglial/myeloid cell-2), which can cause deposition of Aβ-42 peptide [25]. Certain viral and bacterial pathogens can also cause AD. It was identified that patients infected with bacteria (like Chlamydia pneumonia, Borrelia burgdorferi and Helicobacter pylori) showed increased levels of Aβ-40 and Aβ-42 as well as an increase in levels of inflammatory mediators associated with AD [26].
1.1.7. Genetic Mutations
APOE gene has been closely related to the incidence of AD in most cases [27]. Other genes involved are presenilin 1, presenelin 2 and mutations in β amyloid precursor protein [28]. Due to mutations in the genes mentioned above, the person becomes susceptible to AD.
1.1.8. Oxidative Stress
Mitochondrial dysfunction and oxidative stress have long been implicated in the pathogenesis of early AD. Decreased level of cytochrome c oxidase can cause dysfunction of mitochondria. Additionally, hyper excitation of glycogen synthase kinase (GSK-3) due to oxidative stress (OS) can alter the permeability of mitochondria. This could lead to overproduction of ROS [24]. Metal ions specifically zinc and copper may bind to the Aβ plaque and produce ROS. ROS thus produced brings about oxidative change in the Aβ peptide itself making its removal difficult and also cause lipid and protein oxidation of the cell membrane making it permeable and hence susceptible to degeneration [24, 29, 30]. Calcium ions storage in the endoplasmic reticulum can be impaired by Aβ plaque, which increases calcium levels in the cytosol. This increase in calcium levels depletes glutathione and over-accumulation of ROS inside the cells. Hyperactivation of N-Methyl-D-aspartate-type glutamate receptors (NMDARs) can also lead to increase calcium influx by promoting cell permeability, which leads to the formation of reactive nitrogen species (RNS) and ROS. Aβ proteins can directly activate nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase to initiate the synthesis of free radicals [31].
1.1.9. Autophagy
It is known as a housekeeping system or the waste management system of the cell. It involves engulfing of the damaged proteins or cell constituents. Nucleation is the first step in this process by which a phagophore is formed. Then the phagophore extends to enclose all the damaged proteins or the dysfunctional organelles. This leads to formation of closed vacuoles known as Autophagosomes. Finally, the autophagosomes fuse with lysosomes to form autolysosomes which degrade all its contents. Various growing factors for AD like presenelin 1 proteins, oxidative stress, tau neurofibrillary tangles can cause dysfunction of autophagic vacuoles and hence can contribute in the pathogenesis of AD [32, 33].
Fig. 1 compiles all the important mechanisms explored till now, which contributes towards having an understanding of the cause of the disease.
Fig. (1).
Mechanisms involved in pathogenesis of Alzheimer’s disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
1.2. Diagnostics of Alzheimer’s Disease
Currently, the diagnosis of AD is primarily dependent on positron emission tomography (PET) of tracer molecules and analysis of cerebrospinal fluid (CSF) protein [34]. Phosphorylated tau 181 (P-tau181) can be used as a confirmatory and prognostic biomarker for the diagnosis of AD. It gets deposited in the brain and secreted in CSF which crosses blood brain barrier to enter the blood where it can be used as a biomarker for AD [35, 36]. Recently, the diagnostic accuracy has increased with a specialized PET scan, which exhibits 100% specificity and 96% sensitivity in AD as well as in patients with the milder condition. Florbetapir, florbetaben and flutemetamol are used as PET ligands for diagnosis, but not widely used due to its high cost. Examination of CSF for p-tau, Aβ42 and total tau protein content is less costly. This method has an accuracy of 85-90% in diagnosing AD, but needs long duration for obtaining results due to invasive method (lumbar puncture) and the dearth of laboratory facilities involved in the analysis of fluid. However, both PET imaging and CSF analysis exhibit similar accuracy, and suggest that the optimum test for the diagnosis will depend upon patient/provider preference, cost and availability of facilities [37]. PET scanning and CSF analysis revealed that the pathological changes are initiated two decades before the appearance of symptoms [38]. Due to this reason, the diagnosis of AD on the onset of symptoms would not be very helpful as pathological changes and cognitive impairment would have already accelerated to higher stages. As a result, for better diagnosis and treatment, we suggest the use of biomarkers, which can help in early detection of AD as mentioned below.
2. Newer strategies in early detection of Alzheimer’s disease
2.1. Biomarkers
A biomarker is an indicator considered for evaluation of any normal biological as well as pathogenic processes and pharmacological effects of any therapy. In the case of AD, a biomarker can be used to assess the overall health and diseased condition of aged patients [39, 40]. An extracellular deposition of amyloid-β (Aβ) protein and aggregated form of hyper phosphorylated tau protein in the brain are two main pathological characteristics of AD [41]. Recently all the important molecular biomarkers of Alzheimer’s disease were critically discussed for their status and prospects by Lashley et al. (2018) [42].
They detailed the use of cerebrospinal fluid and blood biomarkers with positron emission tomography (PET) imaging techniques for Aβ42 and phosphorylated tau concentration, axonal and synaptic degeneration, glial activation, trans active response DNA-binding protein 43 and α-synuclein pathology determination. Fillit (2018) of the Alzheimer's Drug Discovery Foundation recently emphasized the need for new biomarkers for AD in Scientific American [43]. With increased emphasis on drugs targeting beta-amyloid proteins all these years, they have not yet yielded many positive results. Although prognostic and diagnostic biomarkers are available for Alzheimer’s disease, only a limited number of patients have been tested with these clinically available biomarkers due to the high cost and restricted access. Thus, studies are suggesting to come up with an affordable and feasible blood test to be performed in any clinical setup creating a big impact on AD patients.
2.2. Cerebrospinal Fluid (CSF) Proteins
More specific to AD, CSF measures of Aβ1-42, t-tau, and p-tau and molecular imaging using PET have become widely adopted with improving assays and ligands. Some of the previous studies carried out in vitro and human trials have indicated the role of non-essential heavy metals cadmium (Cd), mercury (Hg), lead (Pb), and arsenic (As) in causing Aβ protein aggregation along with worrisome levels of tau hyper phosphorylation [44]. An ELISA assay called as INNOTEST has been used for two decades for quantification of t-tau, p-tau and Aβ42 in CSF which gives a unique ‘Alzheimer’s CSF profile’ where an increased level of t-tau and p-tau is observed with decreased Aβ42 level [45, 46]. C- reactive protein (CRP) levels are majorly associated with ApoE genotype and the CSF amyloid levels [47]. Mean CRP levels decreased significantly in AD in study conducted by O'Bryant et al. (2009) [48]. They also evaluated the link between CRP and AD among Mexican Americans. In the mentioned study they observed decreased levels of CRP among Mexican American AD patients [49].
The CRP has a role in amyloid pathology as studied and shown in APP/PS1 (amyloid precursor protein/presenilin 1) mice; pentameric CRP dissociation takes place into monomeric forms prompted by amyloid plaque [50, 51]. Monomeric CRP acts as a linker between vascular trauma and inflammation and also is associated with other events like plaque generation, neuronal injury, and dementia [52]. There are many studies proving the statistical influence of the ApoE genotype on the peripheral CRP levels. Hubacek et al. (2010) showed that ApoE4 genotype carriers have lower levels of plasma CRP than in ApoE3 carriers [53]. Elevated CRP is found to be associated with decreased cognition in “The Project in Sado for Total Health” (PROST) study in the Japanese population. The present study revealed a clear association between serum CRP concentration and cognitive impairment in a community-dwelling elderly population in Japan [54].
A range of other markers namely vascular endothelial growth factor (VEGF), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), interferon gamma-induced protein 10 (IP-10), soluble vascular cell adhesion molecule-1 (sVCAM-1), macrophage migration inhibitory factor (MIF), and complement component 1q (C1q) have been strongly associated independently of patient’s age with the pathological tau levels. Thus, the effect of aging on CSF marker levels vary for each of these molecules. The CSF tau level increases in the subsequent stages of AD accompanied by events such as neuronal death owing to inflammatory signalling in response to tissue damage. It is concluded that CSF levels of these markers correspond to the pathological activity in the brain only in the later stages with cognitive demand tasks in AD [55].
For biomarkers estimation from CSF sample, it has limitations with respect to invasive process and high cost and limited access for PET studies. CSF fluid collection by lumbar puncture causes nausea, backache and weakness in aged people. It is essential to identify new biomarkers in other less invasive and easily collectible body fluids like serum, urine etc. With blood samples being used for diagnosis, it can help in analysing different types of blood cells (mononuclear cells, lymphocytes, monocytes or platelets), which can be categorically associated with AD pathologies [56]. Recently there has been a surge in finding blood biomarkers appropriate for repeated evaluations throughout the disease progression over a time-frame for an interventional/treatment study [57]. Other technical advancements in mass spectrometry-based methods and highly sensitive immunoassays hold great promises for blood biomarkers, which can be implemented for screening neurodegeneration and amyloid deposition in the brain [58].
2.3. Blood and Urine Biomarkers of AD
Plasma Aβ42 and Aβ40 levels are now established biomarkers for AD. In AD transgenic mouse models, plasma and CSF levels of Aβ42 and Aβ40 increase with age but later decrease with Aβ accumulation in the brain leading to onset of cognitive impairment. Schupf et al. (2008) determined the predictive value of Aβ levels in elderly people, and investigated the change in these biomarkers over time with the onset of cognitive impairment or AD. Monitoring the levels, it was seen that those with increased Aβ42 levels over the follow-up period, compared with decreasing levels were three times more likely to develop AD [59]. Yang et al. (2018) showed increased risks of AD in individuals with high arsenic or low dimethylarsinic acid percentage with a study based on propensity-score match [44]. In another study, an immuno‐infrared‐sensor was used for the extraction of the total Aβ fraction from plasma samples, wherein the biomarker amide I band were significantly lower for AD individuals. Amide I biomarker could detect AD before its clinical diagnosis and without dementia symptoms in individuals. The test had a positive likelihood ratio of 7.9 (ESTHER: Alzheimer’s society in Toronto), which indicated good evidence of this biomarker to identify AD in the general population [60].
2.4. Lipid Biomarkers of AD Present in the Blood
Brain lipids are important molecules as they play various biological and physiological roles in impulse conduction and cell signalling in the central nervous system (CNS). Out of all the brain lipids, the most abundant are cholesterol, ceramides, glucosyl ceramides, phosphatidylcholine, sphingomyelin, and sulfatides [61, 62]. Besides them, cholesterol oxide derivatives and other long chain derivatives of fatty acids including prostaglandins, leukotrienes and neuroprotectins are present [63, 64]. The levels of sphingolipids are also affected (decreased) in AD [65]. These brain lipids are found in the glial cells (astrocytes, oligodendrocytes), microglial cells, and neurons of the CNS. They are also present in myelin and helps in the nervous influx by favouring good conduction. The identification of above-mentioned lipid biomarkers may be explored as a less invasive diagnostic method and as predictors of AD for future disease progression and treatment response. Biochemical and physical methods are frequently used; however histological and cytological methods are also possible for lipid characterization and quantification since lot of dyes are being used in tissues and cells to identify and trace various types of lipids [66].
2.5. Genetic Markers
AD is complex and heterogeneous and is inherited according to Mendelian genetics. There are more than 160 mutations reported in three important genes which are responsible for coding amyloid precursor, presenilin 1, and presenilin 2 [67]. ε4 allele of the APOE gene which occurs with a frequency of 14% is the other major risk factor for developing AD; and its frequency increases to ~40% in AD patients and is also related to the onset of earlier age AD dementia and increased Aβ pathology. Amyloid plaques are superabundant in ε4 carriers, with lower Aβ1-42 concentration in CSF, with increased Pittsburgh compound B (PiB) shown bound to Aβ aggregates on PET imaging [4]. Familial Alzheimer's disease is inherited from parents and it currently accounts for < 1% of the AD burden [45]. Late-onset AD is genetically and etiologically heterogeneous in nature, with innumerable genes and environmental factors involved in disease progression rate and risk. The strongest and most reliable genetic association involves the epsilon 4 (ε4) allele at the ApoE locus for increased risk of late-onset AD [68, 69]. The ApoE gene encodes for a protein responsible for lipid transport; ε4 allele carriers have increased deposition of amyloid and also show adverse effects on memory and executive function [70]. However, the genotyping of the ApoE locus is complicated as the allelic denomination is determined by a pair of polymorphisms thus a small sample of blood or a cheek swab, can be used for deoxyribonucleic acid (DNA) isolation and thus the determination of ApoE genotype. From now onwards, the association between the ApoE ε4 allele carriers and the occurrence of AD is well-accepted as it also lowers the overall age for onset for AD [66, 67], but does not hold any specific inference for the individual carrier. Therefore, it is to add more accuracy to a complete biomarker panel for AD [71].
2.6. Brain Derived Neurotrophic Factor (BDNF)
The decline in memory with the reduction in hippocampal volume (HV) corresponding with high Aβ levels has been already reported in healthy individuals; Lim et al. (2013) showed that BDNF Val66Met worsens these conditions more in the preclinical stage of AD [72]. In the study conducted in healthy adults having a high Aβ level and carrying the Met allele, the rate of HV reduction was significantly more. A greater reduction rate in HV was observed in Val/Val homozygotes who were having high Aβ levels than with low Aβ, irrespective of their BDNF Val66Met polymorphism over 36 months. In another study, BDNFMet carriers exhibited greater memory decline and hippocampal atrophy in a span of 36 months compared to BDNF Val homozygotes. While increased memory decline and hippocampal atrophy have been reported previously in adults with an MCI and high Aβ, Lim et al., (2014) provided a preliminary report and linked high Aβ and BDNF Val66Met polymorphism in prodromal AD suggesting them as important prognostic markers of increased memory decline and hippocampal atrophy [73].
2.7. Kidney/Brain (KIBRA) Protein, a Memory-Associated Protein
KIBRA protein is expressed in various parts of the brain, mostly enriched in the postsynaptic density in the hippocampus [74]. It interacts with several postsynaptic proteins including dendrin and synaptopodin and function in memory-related processes through these interactions [75]. Tracy et al. (2016) identified an abnormal acetylation of K274 and K281 on tau in AD brains, which increased memory loss and disrupted synaptic plasticity by reducing the postsynaptic KIBRA protein [76]. The transgenic mice capable of acetylating K274 and K281 exhibited memory deficits and impairment of hippocampal long-term potentiation (LTP). The LTP deficit could be saved by increasing the polymerization of actin or by expression of the KIBRA protein. An increase in tau acetylation was linked to the loss of KIBRA in AD patients with dementia. This study led to a novel mechanism of pathogenic tau caused a role in causing synaptic dysfunction and cognitive decline in AD.
Genes- APOE, BDNF, KIBRA, klotho (KL), Spondin-1 (SPON1), CUB and Sushi multiple domains 1 (CSMD1) are important measures of cognitive change and the influences of these individual genes have been investigated and recorded thoroughly. Porter et al. (2018) emphasized on determining the gene risk profiling for preclinical trials in AD with cognition to assess the therapeutic efficacy [77].
2.8. Brain Imaging
There are profound changes that occur in the brain’s structure and its function due to normal aging and AD. AD follows an extensive cortical neuronal loss, with loss of connections in brain systems. Recent advancements in brain imaging have supported unique interruptions in functional neural networks. Concomitantly the potential of brain imaging has expanded rapidly with innovations in tools to acquire images and its analysis. Now they address structural, functional, and molecular aspects with imaging in AD. Magnetic resonance imaging (MRI) is being used for both structural and functional and PET for evaluation of both amyloid and cerebral metabolisms. Structural and functional MRI, fluoro-deoxy glucose (FDG) and amyloid PET are the most commonly used imaging techniques. Other MRI techniques in development that are also adding to the knowledge of AD are diffusion tensor imaging (DTI) and associated tractography technologies, arterial spin labeling measures of cerebral blood flow and PET tracers targeted at the cholinergic system, microglial activation and other tracers [78]. The PET imaging has utility of diagnostic decision making, confidence in diagnosis and management planning for patients with cognitive impairment [79]. Recent developments in neuroimaging studies of AD provide useful information to clinicians, including a new in vivo amyloid imaging. MRI, single photon emission computed tomography and 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG PET) are currently available for clinical use [80]. The first-in-human study by Lohith et al. (2019) reported the utility of 18F-MK-6240 as an NFT imaging PET tracer in a small cohort of 4 cognitively normal HE (Healthy Elderly) subjects, 4 subjects with clinically probable AD, and 2 subjects with amnestic MCI due to AD. They found that 18F-MK-6240 exhibits regional retention in a plausible pattern for NFT pathology in AD. Importantly, in NFT depositing regions, signal magnitude in terms of semi quantitative SUVR or fully quantitative VT values were higher in AD/MCI subjects compared with HE subjects, with no evidence of off-target binding in HE subjects [81].
2.9. Markers of Mild Cognitive Impairment (MCI)
According to the American Psychiatric Association 1987, dementia is an acquired cognitive impairment that interferes with social and occupational abilities. Cognitive functioning is the hallmark of MCI, which is bad for age but not as bad enough to be called dementia. A related concept, known as Cognitive Impairment, No Dementia (CIND) has the presence of cognitive impairment with the absence of dementia [82]. Thus, a fine distinction between both (or CIND) is the level of the cognitive impairment itself, and thus as reflected in its functional responses. MCI is always referred at an early stage of AD on the global deterioration scale [83] and to another factor comprising DSM-III-R (Diagnostic and Statistical Manual of Mental Disorder, third edition, Revised) and ICD-10 (International Classification of Diseases, Tenth Revision)diagnostic criteria [84], its most popular and cited version is the Mayo Criteria or Petersen Criteria [85] which is now called Amnestic MCI. IWG or Winblad Criteria also included non-amnestic impairments in the definition of MCI [86]. This criterion set is the prevailing standard for the MCI syndrome in current times, and is reflected in the NIAAA work group guidelines for the diagnosis of MCI due to AD [87]. It includes that the person is neither normal nor with dementia; there is a sign of decline in cognitive functions, and daily living activities are preserved with complex and intricate functions either being intact or minimally impaired [88].
Among various volumetric MRI-based techniques, medial temporal volume loss is considered as an important biomarker for assessing the progression of AD in patients with pre-existing MCI of regional brain atrophy. “Brain atrophy score” was derived using the linear discrimination analysis from healthy controls and AD patient’s data, found to be better for predicting one-year cognitive decline than atrophy in medial temporal structures alone. This atrophy score considered the volume of the various parts of the brain using a linear discrimination analysis which best-differentiated AD patients from healthy controls. Later this brain atrophy score was ameliorated by Mini-Mental State Exam (MMSE) scores and APOE ε4 alleles number to the model [89]. Kauppi et al. (2018) recently improved the prediction of AD among MCI patients by uniting age-sensitive polygenic hazard score (PHS) with structural neuroimaging relating to cognitive ability. An improved assessment of AD risk among old patients with subjective memory complaint is said to be helpful in clinical practice to determine treatment plans, and for testing newer interventions in the disease [90].
When CSF beta amyloid (CSF Aβ1-42) and tau protein levels were tested in MCI subjects, CSF tau was increased whereas levels of CSF Aβ1–42 were decreased compared to healthy controls with good comparable accuracy. Aβ1–42 and CSF tau showed a sensitivity of 59% and 83% with a specificity of 100% and 90% respectively [91].
The MCI diagnosis is usually based on the clinical judgment made by experts in agreement with a group, by using psychometric algorithm or rating scale. For differentiation purposes, dementia is diagnosed by standard measures such as ICD-10 [92], clinical dementia rating scale and criteria such as DSM-III-R [88, 93]. Diagnostic measures and markers of MCI mostly focus on etiological subtypes and stages of the disease. Few neuropsychological tests [94] and other measures including related autosomal dominant genes, medial temporal atrophy, β amyloid and tau proteins levels in CSF, temporoparietal glucose metabolism, amyloid imaging, and their validation for clinical use are being considered as markers of MCI. Positive serology in HIV infection and positive testing for CAG repeat units on chromosome 4 in Huntington’s disease [95] would be a diagnostic marker for MCI in future [88].
Begcevic et al. (2018) evaluated 30 candidate brain-related protein as prospective biomarkers for diagnosis of AD dementia and MCI due to AD [4]. Amyloid beta precursor like protein 1 (APLP1), secreted phosphoprotein 1 (SPP1) and contactin 2 (CNTN2) showed discriminating potential for diagnosing cognitively impaired patients and MCI due to AD from controls. APLP1 was elevated in MCI patients. However, these findings are yet to be validated in a bigger independent cohort of MCI and AD dementia patients. According to the revised guidelines for AD diagnosis in 2011, MCI symptoms and increased beta-amyloid levels in MCI suggest an early stage of the AD. Recently a postsynaptic protein named neurogranin (Ng) has been identified as a biomarker for MCI. It has significant association with memory and execution in MCI subjects with an increased level in the CSF of AD and MCI patients compared to control with normal cognitive ability. CSF Ng level can be used to determine and assess the effect of treatment as well as during the progression of AD [96].
3. Current strategies in the treatment of Alzheimer’s disease
Till date, efforts are made to target and counterbalance the neurotransmitter disturbances aimed to relieve symptoms of the disease. A major drawback for the unavailability of a specific treatment for the underlying pathology is that the emergence of AD-related pathologic changes begins quite early, almost a decade before the person shows the symptoms.
3.1. FDA Approved Medicines
Drugs that target cholinergic or glutamatergic neurotransmission are currently available treatments for AD. These drugs only relieve the symptoms. No drug is available that has a curative effect, though there are many ongoing clinical trials for such drugs. These newly developed molecules target the amyloid and tau proteins [97]. Currently approved drugs that affect cholinergic transmission are three acetylcholinesterase inhibitors: donepezil, rivastigmine, and galantamine. These drugs improve cognition in the patient and ease the social and economic burden. These drugs are effective in mild to moderate AD. To treat moderate to severe form of AD, FDA approved Memantine in 2003. It is a NMDA receptor antagonist and reduces the excitotoxicity observed in AD, caused due to excess of glutamatergic transmission. Table 1 shows the latest drugs developed for the treatment of AD, however failed to enter the market due to clinical trial failure. Each drug has a different mechanism of action such as BACE I inhibitors which inhibit the enzyme responsible for the processing of APP to Aβ, RAGE inhibitors which inhibit the influx of Aβ, PPAR-γ agonist which bring back insulin sensitivity and hence reduce Aβ formation or 5HT6 antagonist which modulate neurotransmission and hence improve cognition.
Table 1.
Drugs developed for the treatment of Alzheimer’s disease [98].
| Name of the Drug | Company | Mechanism of Action | Clinical Trial | Reason for Failure |
|---|---|---|---|---|
| Verubecestat | Merck | β-site amyloid precursor protein-cleaving enzyme 1 (BACE 1) inhibitors | EPOCH trial APECS trial |
No effect on slowing the progression of AD |
| Lanabecestat | Astra Zeneca & Eli Lilly |
BACE 1 inhibitors | AMARANTH and DAYBREAK-ALZ | Failure of interim futility analysis |
| Atabecestat | Janssen | BACE 1 inhibitors | EARLY | Liver toxicity |
| Solanezumab | Eli Lilly | Monoclonal antibody for AB | EXPEDITION III (Phase III) | No positive effect on cognitive decline |
| Azeliragon | vTv Therapeutics | Receptor for Advanced Glycation End products (RAGE) inhibitor | STEADFAST (Phase III) | Lack of efficacy |
| Pioglitazone | Takeda and Zinfandel Pharmaceuticals | Peroxisome Proliferator-Activated Receptor γ (PPAR-γ) |
- | - |
| Idalopirdine | Lundbeck & Otsuka | 5HT6 antagonist | STARSHINE, STARBEAM, STARBRIGHT (Phase III) | Did not improve cognition |
Looking into the failure rate of AD drugs, researchers are simultaneously developing agents that provide symptomatic relief in AD, the list of such drugs that are in clinical trials is long, and some of them are mentioned in Table 2.
Table 2.
Some important drugs currently in clinical trials.
| Name of the Drug | Company | Mechanism of Action | Clinical Trial | Symptoms to be Relieved |
|---|---|---|---|---|
| Brexpiprazole | Otsuka &Lundbeck | D2 partial agonist | Phase III | agitation |
| Tetrahydrocannabinol | Johns Hopkins University | CB1/CB2 partial agonist | Phase II trials | |
| Nabilone | Sunnybrook Health Sciences Centre |
semisynthetic cannabinoid derivative | small-scale phase III | |
| Pimavanserin | Acadia | selective 5-HT2A serotonin inverse agonist | phase II/III | psychotic |
| Suvorexant | Merck | dual antagonists of the orexin receptors | Phase III | insomnia |
| Lemborexant | Eisai | dual antagonists of the orexin receptors | Phase II |
Apart from the drugs mentioned above, immunotherapy is being made use of to develop anti-amyloid and anti-tau agents, also alternative strategies targeting the misfolded tau proteins and neuronal regeneration are under investigation [98]. It has been reported that there is a lag phase of many years between the pathological changes in the brain and the onset of symptoms. If the therapy is initiated in this lag phase or when the first symptom of AD is observed, it would be of great benefit to the patient. Hence, the 2013 FDA draft guidance encourages the inclusion of AD patients who are in the initial stages of the disease for clinical trials. Another hurdle is diagnosis/identification of such patients for inclusion in clinical trials [99, 100].
3.2. Gene Therapy in Alzheimer Disease
Gene therapy interventions are aimed to tackle a disease at its source, mostly a faulty DNA/gene/protein, to repair it and allow the cells to fix the problem. After revealing various genes involved in Alzheimer’s pathology, it opens up vast avenues for gene therapy, which involves inserting new genetic material into living cells using viruses. Due to the recent developments in gene therapy associated approaches in recombinant adeno-associated viruses (rAAVs), the possibility for treating these diseases in human beings is foreseen. In an effort to test the ability to degenerate neurons in AD towards a nervous system growth factor (NGF), Tuszynski et al. (2015) subjected ten patients with early AD with NGF gene ex vivo or in vivo therapy [101]. The findings indicated positive response of neurons showing cell hypertrophy, axonal sprouting, and activation of functional markers. Growth factor therapy induced sprouting by NGF, which persisted for 10 years after gene transfer and appeared safe. In another study, scientists delivered a gene PGC1-alpha (Peroxisome proliferator-activated receptor gamma coactivator 1-alpha) using a modified virus to the brain cells of mice which reduced the development of Alzheimer's. The treated mice showed better memory, no loss of brain cells in the hippocampus and had very few amyloid plaques after four months of injection. Those that had not been treated had multiple plaques in their brain [102]. This study was planned on the basis of earlier results of PGC-1α, where it exhibited a decrease in the generation of amyloid-β (Aβ) in cell culture by the same group. Recently, Rafii et al. (2018) reported the results of a multicentre randomized clinical trial of intracerebral gene delivery in Alzheimer’s disease (AD) patients [103]. This trial demonstrated the feasibility of sham-surgery-controlled stereotactic gene delivery studies in patients with AD. Adeno-associated viral vector (serotype 2)-nerve growth factor (AAV2-NGF) delivery was feasible and well-tolerated but without any clinical outcomes. Thus, the study needs confirmation of precise gene targeting. In June 2018, scientists at the Massachusetts Institute of Technology in a recent breakthrough found a cure for Alzheimer’s disease. A genetic snipping technique can be used to turn APOE4 gene responsible to cause amyloid beta proteins in the brain, into APOE3. Those with one copy and two copies of the APOE4 gene have double and twelve times more chances of Alzheimer’s respectively. APOE2 might protect and lower risk of Alzheimer's. To overcome this issue, gene editing could be the answer [104].
3.3. Theranostics in Alzheimer’s Disease
Theranostics is the latest technology, which combines therapeutics and diagnostics rather than using them in isolation. Theranostics may completely change the available therapy as well as diagnostics for AD. Gold nanorods incorporated with two known Aβ inhibitors (Aβ15-20 and polyoxometalates) are capable of detecting, inhibiting and destroying the preformed Aβ aggregates by NIR (near infra-red radiation) irradiation. Depending on the process of Aβ aggregation, the absorbance of gold nanorods changes and hence the progression of the disease can be monitored [105]. Dao et al. (2017) have synthesized phenothiazine derivatives which work as near infra-red fluorescent probes, having a high affinity for the amyloid plaque and can detect it in the brain and retinal tissue of transgenic mice [106]. These derivatives also have the ability to prevent the aggregation of Aβ plaque and break already formed Aβ fibrils. Charged molecules specifically (E)-4-(4-(dibutylamino) styryl)-1-(2-hydroxyethyl) quinolin-1-ium chloride (DBA-SLOH) show high affinity for Aβ aggregates and display enhanced fluorescence upon binding. It effectively prevents the aggregation of Aβ1-40 and Aβ1-42 fragments providing therapy for AD [107]. A recombinant design has been used to develop a blood brain barrier shuttle which uses an antibody-mAb158 that binds to Aβ fibrils. This antibody is fused with two single chain variable fragments of the transferrin receptor antibody-8D3. 8D3 is known to enhance uptake in the brain. Due to this fusion, the shuttle selectively permeates the BBB and delivers mAb158 in the brain which works as Aβ immunotherapy and also as a radio-ligand for PET diagnosis, aiding in the evaluation of the therapy simultaneously [108]. Metal ions are known to act as a catalyst for plaque formation and invariably, there is always an excess of these ions. Based on this observation, scientists have developed a nanoprobe containing a chelator-8-hydroxyquinoline-2-carboxylic acid, which can detect copper concentration upon exposure to 980 nm. Chelators capture excess copper ions in the brain and inhibit amyloid plaque induced apoptosis and also causes the structural transformation of Aβ [109]. Iron oxide magnetic nanoparticles incorporated with Congo red/Rutin have been developed which can not only detect amyloid plaque by magnetic resonance imaging but also achieve controlled release of drugs and prevent oxidative damage [110]. Thus, the field of theranostics which uses a single chemical entity for diagnosis and treatment of AD may soon have applications in personalized medicine.
Theranostics also involves Quantum dots (QDs), which are nanoparticles with bright fluorescence. This is due to their unique optical and electronic properties. QDs have been widely used in imaging and diagnostics. This is because emission from different QDs can be estimated simultaneously in a single assay using one wavelength. Recently there has been a lot of interest in developing theranostics application of QDs where simultaneous imaging, diagnosis and therapy is possible [111].
Xiao et al. (2016) used graphene quantum dots (GQDs) coated with glycine-proline-glutamate in APP/PS1 transgenic mice. The aggregation of amyloid plaque along with the levels of pro-inflammatory cytokines was reduced whereas the levels of anti-inflammatory cytokines increased, thus demonstrating strong potential as a therapy in AD [112]. Wavelengths ranging from 632.8 to 400 nm like low level laser, can reduce inflammation and oxidative stress in various disease conditions. However, this therapy is not useful since the amount of light actually penetrating the brain is very minute. With the use of technology named “Bioluminescence Resonance Energy Transfer to Quantum Dots” (BRET-Qdots), this problem can be overcome. These CdSe or CdTe quantum dots act as a source of near infra-red radiations and reduce the amyloid plaque induced inflammation and oxidative stress in animal models. This low-level laser therapy (LLLT) can be further exploited to treat other diseases that involve inflammation and oxidative stress pathologies [113].
Mars et al. (2018) described a sensitive method based on dual electrochemical and fluorescence detection of APOE4 DNA, responsible of Alzheimer and coronary artery diseases [114]. Curcumin was used for its dual-sensing transducer response due to its highly sensitive fluorescence properties. Graphene quantum dots were employed to enhance the electrochemical response. The analytical response showed a sensitive decrease in the presence of different APO e4 DNA target, which was due to the blocked photo-electron transfer activity due to formation of DNA complex. The curcumin-GQDs system exhibited a high selectivity and efficacy for which it was also investigated in the clinical fluid. The results showed relative standard deviation c.a. 4.7% toward DNA target in human blood plasma. Incorporation of QDs into microarrays is a relatively new venture, where current scanners have limitations in obtaining fluorescent signals under appropriate excitation levels for red and green lasers. Morales-Narvaez et al. (2012) studied the biosensing ability of QDs (QD655) and a fluorescent dye (A647) recruited in the sandwich ELISA as reporters in microarray format and compared it with a conventional ELISA by using ApoE as model analyte [115]. A minimal volume of human serum was sufficient for assay with high sensitivity and good precision. This idea is proposed for the detection of ApoE multiple isoforms and other AD biomarkers. In another study Medina-Sanchez et al. (2013) used cadmium-selenide/zinc-sulfide QDs as labelling agents for assaying and detecting ApoE. They used them within a magnetic zone with magnetic beads for sample purification and concentration [116]. With such requirements, the development of an integrated, miniaturized portable and affordable systems is possible.
3.4. Immunotherapy for Alzheimer
Various mechanisms have been hypothesized for Aβ immunotherapy. The soluble equilibrium mechanism includes antibodies neutralising and solubilising the Aβ plaque both centrally and peripherally. Phagocytosis mechanism is based on opsonisation of Aβ plaque which stimulates microglia associated phagocytosis. Antibodies also bind to Amyloid seed in the initial stages and can prevent its propagation [117]. There are several other mechanisms like direct method (in which Aβ plaque are unbundled) and peripheral sink mechanism (removal of Aβ from the brain to plasma) [118].
According to the pathophysiology of Alzheimer's, Amyloid β plaques are the primary target for immunotherapy. Various immunotherapy strategies are under investigation for Alzheimer's and which primarily include active immunisation and passive immunisation. Active immunization with Aβ-42 can stimulate B-cells, T-cells and microglia for immune response. Active immunization with Aβ fragments which are attached to carrier protein resembling helper T-cell epitopes is another approach that stimulates T-cells which releases signaling cytokines to activate antibodies releasing B-cells. Other immunotherapy includes passive administration of monoclonal antibodies (mAb) [119]. There are various monoclonal antibodies that are tested which includes bapineuzumab, solanezumab, gantenerumab, crenezumab and ponezumab out of which some are in phase 3 clinical trials. But majority of these drugs have severe side effects like ARIA (Amyloid related imaging abnormalities), which are associated with vasogenic edema (ARIA-E) or indicate micro haemorrhage and hemosiderosis (ARIA-H) [120].
Various engineered antibodies or second-generation antibodies are in the development phase. It includes BAN2401 (that selectively binds to large soluble Aβ protofibrils), SAR255952 (which primarily targets soluble protofibrillar and fibrillar species of Aβ), Aducanumab (which binds to the N-terminus of Aβ3-6), etc. These antibodies are mainly engineered and have low binding activity at Fcγ regions which are mainly involved in side effects of first line monoclonal antibodies [121]. Therapeutics based on active and passive mechanisms are compiled in Table 3.
Table 3.
Active and passive immunotherapeutic [122].
| Name | Mechanism | Phase of Clinical Trial |
|---|---|---|
| ACI-24 | Cause production of antibodies against Aβ without activating inflammatory cells. | Phase 1 |
| ACI-35 | Liposome based vaccine which generates antibodies against phosphorylated tau. | Phase 1 |
| ABvac40 | Targets C-terminus of Aβ40 | Phase 2 |
| AADvac-1 | Consist of Peptide (KDNIKHVPGGGS) which generate antibodies against tau. | Phase 3 |
| CAD106 | Virus based active vaccine which target Aβ without activating T cells. | Phase 2 |
| LuAF20513 (engineered mixed peptide antigen) |
Generate anti Aβ antibodies without microglial activation. | Phase 1 |
| DNA based vaccine | Translation of Aβ based DNA leads to generation of antibodies. | Early stage of development |
| Passive Immunotherapeutic | ||
| Drug | Mechanism | Phase of Clinical Trials |
| Aducanumab | Monoclonal antibody against Aβ | Phase 3 |
| Crenezumab | Humanised monoclonal antibody which mainly identifies polymorphic form of Aβ | Phase 3 |
| Gantenerumab | Binds to Aβ and induce phagocytosis by activating microglia. | Phase 3 |
| BAN2401 | Preferentially binds to soluble photofibrils of Aβ | Phase 2 |
| Bapineuzumab (Humanised form of murine monoclonal antibody) |
Target N- terminal region of Aβ | Failed in clinical trials |
| Solanczumab | Targets monomeric and non-fibrillary form of Aβ peptides | Phase 3 |
| BIIB092 | Targets N terminal fragment of tau | Phase 2 |
| C2N 8E12 | Targets extracellular tau aggregates. | Phase 2 |
3.5. Peptidomimetics
Peptidomimetics are analogues of natural proteins that interact with biological targets to exhibit equivalent or superior biological effect [122]. These peptide inhibitors can be derived from Aβ sequence. They could also target phosphorylated tau protein as well as Human β-Secretase [123]. The majority of these peptides can further be divided into peptide containing natural or modified amino acid.
In case of tau and Aβ aggregates the major recognition, sites are of the central hydrophobic core. For tau it is VQIVYK or VQIINK and for Aβ it is KLVFF. The peptidomimetics reacts or bind to these regions and prevents further progression of their deposition. Some of them also dissolve the pre formed aggregates. As proven inhibiting only aggregation cannot prevent AD therefore, multifunctional peptidomimetics were developed, which targeted other factors involved in AD. For example, Thy-KSrVSrFSr(P5) multifunctional peptidomimetic (based on KLVFF recognition site) is a potent inhibitor of Aβ. It stimulates the degradation of Aβ by activating autophagy mechanism in a yeast model. Another example includes multifunctional peptidomimetic GHKSrVSrFSr(P6), which chelates Cu2 released from Aβ42-Cu2 and protects plasmid DNA and other cells from ROS and metal induced toxicity [122].
There are various peptidomimetics designed against Aβ aggregates. They could further be subdivided to peptide targeting central hydrophobic core (KLVFFA) or c-terminus. Peptidomimetics based on central hydrophobic core containing natural amino acid included Aβ (15-22), Aβ (16-23), Aβ (17-24), iAβ11 (RDLPFFPVRID), iAβ5 (LPFFD), iAβ5p (Ac-LPFFD-amide), etc. and those containing modified amino acid included PPI-368 [cholyl-(LVFFA) -OH], PPI-433 [cholyl-(lvffa) -OH], PPI457 [cholyl-(lvffa)-NH2], NH2-K(Me-L) V (Me-F) F(Me-A) ECONH2, pgklvya, kklvffarrrra, kklvffa etc. Peptide inhibitor that target C terminal and contained natural amino acid included Gly-Val-Val-Ile-Ala-NH2, Arg-Val-Val-Ile-Ala-NH2, Aβ (31-42), Aβ(39-42), Aβ(20-29), tachykinins, etc. and those with modified amino acid included Pr-IIGLa (propionyl-Ile-Ile-Gly-Leu amide), RIIGLa, Aβ(25-35) with Gly33 N-methylated and Leu34 N-methylated, N-methylated hexapeptides (28-31) etc. [124].
3.6. Metal Chelators
Interaction of metals with Aβ leads to the generation of ROS as well as abnormal metal ion homeostasis which is linked to the pathogenesis of AD. Metal chelators are agents that break the Aβ-metal complex and restore metal ion homeostasis and hence reduce neuro toxicity. Chelators usually have low molecular weight, small molecular size and neutral or low charge to penetrate blood brain barrier. Chelators do not exhibit strong specific activity towards metal ions as it can deplete its level and lead to abnormal metal ion concentration [125].
In AD the level of metal ions in the brain mainly copper, iron, zinc increases almost 3 times the normal. As a result, these were the main metals targeted for chelation. Deferoxamine (DFO) and Deferiprone are main metal chelators for iron. They treat memory impairment due to iron and also prevent ROS production as well as metal induced toxicity. There are various analogues which target copper ions. They include pyrrolidine dithiocarbonate (PDTC), pyridine derivatives, reduced Schiff base derivatives, Bis(hydrazide), bis(thiosemicarbazones) (BTSC), peptides and multifunctional ligands [126].
Clioquinol from class 8-hydroxyquinoline has shown better results in treating neurodegenerative disease. It is a bi-dentate ligand which has 2:1 activity against copper and zinc ions. It is known to transfer metal ions from extracellular environment to intra cellular environment of the cell to maintain metal ion balance and also dissolves Ab-Cu2+/Zn2+aggregates [125, 126].
Another approach included designing of hybrid multifunctional modulators (HMMs). HMMs inhibit aggregation of Aβ associated with metal ions and prevent mitochondrial damage. Among them, TGR86 gave best results for inhibiting Aβ by sequestering it from copper ions followed by TGR88, TGR87 and Clq. They mainly bind to 20FAEDVGSNKG29 of Aβ-42 isoforms. TGR86forms additional hydrogen bonds with Asn27, Lys28 and Ser26 region of Aβ due to which it showed maximum activity. TGR86 also increases cell viability [122]. As ROS is one of the major factor associated with AD which can cause neurotoxicity, metal chelators are the best line of therapy which can be used to prevent it in the future.
3.7. Probiotics, Prebiotics and Exercise
Alteration in gut microbiota can lead to AD as gut microbes are associated with the modulation of neurotransmitter and immune response by its interaction with the brain through brain-gut metabolic axis. Various studies are conducted on animals as well as humans to identify the effects of prebiotics and probiotics on AD. Probiotics facilitate the growth of gut microbes with the help of prebiotics which provide a suitable environment and nutrients for its growth. Lactobacillus and Bifidobacterium are the two main classes of gut microbes which are widely studied. Probiotics like VSL#3 containing 8-gram positive bacteria showed an increase in Actinobacteria and Bacteroidetes in animal models whereas supplementation with Bifidobacterium breve strain A1 reduces memory impairment, neuronal inflammation and immune responses in animal models [127]. Another study for animal model involved treatment with FRAMELIN which consist of probiotics like Lactobacillus acidophilus lysates, Bifidobacterium longum, omega 3 fatty acids, vitamins (A, D, B1, B3, B6, B9, B12) and exercise which included running on treadmill for a particular period of time. The results were evaluated with the help of Morris Water Maze Test, open field test, microbiome investigation and spontaneous alteration tests. It was concluded that regular physical exercise and probiotic supplementation prevent further progression of AD and alleviates its symptoms [128].
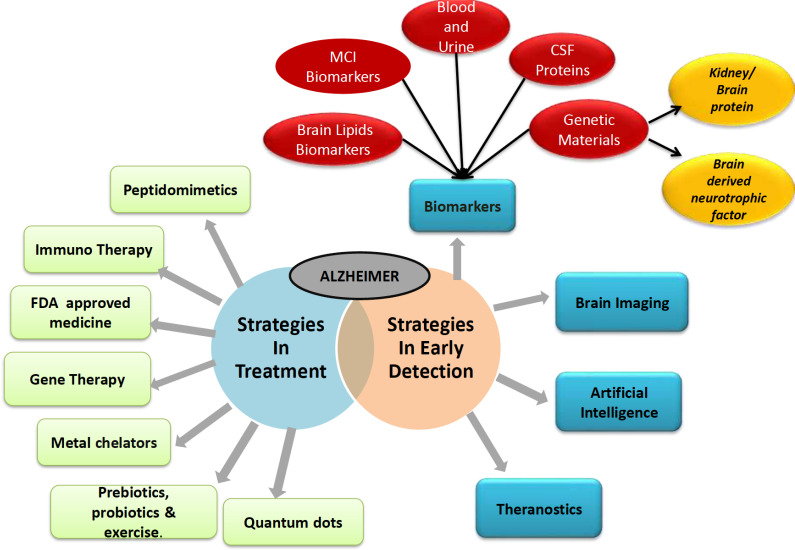
Study on human included intake of probiotics which belonged to genera Lactobacillus (L. casei, L. acidophilus, L. plantarum, L. salivarius, L. paracasei) and Bifidobacterium (Bifidobacterium lactis and Bifidobacterium bifidum) along with Lactococcus lacti. Vitamin D was given additionally. Results were produced which showed activation of immune system. The probiotic supplements should be mild and not very intensive as it can lead to disruption of gut microbiota activating the immune system and leading to neuroinflammation [129]. Further studies are still required in this field as they can provide guaranteed results in inhibiting the pathogenesis of AD. Fig. 2 gives a combined overview of current and future strategies of early detection and treatment of the disease.
Fig. (2).
Current strategies in treatment and newer strategies in early detection and treatment of Alzheimer’s disease. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3.8. Voice as a Biomarker Using Artificial Intelligence
In addition to dementia Alzheimer's is also known to cause loss of language skills which leads to difficulty in speaking and impair communication with the environment. The clinical manifestations mainly include anomia and aphasia.
At different stages of AD, patients experience different communication problems:
Early stage (ES) - The patient is unable to detect the right word in the speech or in the sentence frame.
Intermediate stage (IS) - They impoverish their vocabulary and language of daily use.
Advanced stage (AS) - They speak very less and their response is very limited.
Therefore, speech or voice analysis along with analysis of emotional temperature of patients suffering from AD is very important and can be used as a diagnostic tool for detecting Alzheimer's in its preliminary stage [130].
Various approaches have been made to demonstrate the potential of speech in detecting Alzheimer's or dementia.
Jarrold et al. (2014) differentiated between different types of dementia. Speech data of 9 controls, 9 patients suffering from AD and 30 patients suffering from fronto-temporal lobar degeneration (13 with semantic dementia, 9 with fronto-temporal dementia and 8 with progressive non-fluent aphasia) from NIH funded studies performed at UCSF Memory and Aging Centre were collected. Speech samples were collected from semi-structured interviews in which the patients were asked to describe a picture. The recording of patient’s speech was then used as input to a machine learning algorithm. Using multi-layer perceptron algorithm score of 88% was achieved [131].
Orimaye et al. (2014) also performed a diagnostic method to identify patients suffering from Alzheimer. Dementia Bank dataset was used to extract the audio data. The dataset consisted of audio recordings of interviews of people suffering from Alzheimer and dementia. The interview was taken in the English language on the basis of description of cookie theft picture. The study included binary diagnosis of Dementia and control groups. The dementia group included 314 patients (249 diagnosed with probable AD, 21 suffering from possible AD, 5 with VD (Vascular dementia), 3 with problems related to memory, 43 with Mild cognitive impairment and 4 with undiscovered form of dementia) and 242 healthy people were included in the control group. Nine syntactic features (coordinated sentences, subordinated sentences, reduced sentences, number of predicates, the average number of predicates, dependency distance, number of dependencies, average dependencies per sentence and production rules) and eleven lexical features (utterances, Mean Length of Utterances, function words, unique words, word count, character length, total sentences, repetitions, revisions, lexical bigrams and morphemes) were thoroughly analysed. Four different machine learning classification algorithms were explored. 74% accuracy was achieved by using SVM (Support vector machine) classifier with 10% cross validation [132]. Lopez-de-Ipiña et al. (2015) performed a diagnosis AD on the basis of emotional temperature and spontaneous speech analysis. Multicultural and multilingual (Chinese, English, French, Catalan, Spanish, Basque, Portuguese and Arabian) database of video recording was created. The study included 20 healthy and 20 patients suffering from AD. Then the data was segmented in 600 segments which were analysed thoroughly. It was done to identify the importance of emotions encapsulated in spontaneous speech. The test showed positive as well as promising results in differentiating different stages of AD. It concluded that there is a decrease in fluency in speech of people suffering from AD [130]. Konig et al. (2015) using four short vocal tasks performed an experiment. The participants were categorized into 3 different groups i.e. healthy control (HC), people suffering from Alzheimer (AD) and people suffering from mild cognitive impairment (MCI). The method involved pre-processing of the recordings, analysis and extraction of four main features - countdown and picture description, sentence repeating, semantic fluency and classification procedure. The method showed an accuracy of 80% in differentiating people suffering from MCI and AD, 87% between AD and HC and 79% between MCI and HC [133]. Fraser et al. (2016) studied the importance of linguistic features in detecting Alzheimer disease. The data was extracted from the dementia bank data set. The study consisted of two groups i.e. Dementia group and the control group. The dementia group included participants diagnosed with probable AD or possible AD which resulted in 240 speech samples from 167 participants. The Control group included 97 participants with 233 speech samples. The method extracted and analysed 370 features which included part-of-speech, syntactic complexity, grammatical constituents, psycholinguistics, vocabulary richness, information content and repetitiveness, acoustics. Two machine learning classification algorithms were applied to analyse the data. Accuracy of 92% was obtained in distinguishing people suffering from AD and healthy control group using top 25 ranked features [134]. Al-Hameed et al. (2016) studied the potential of audio-based biomarker in detecting Alzheimer disease. Dementia bank data set was utilised. Description Boston cookie theft picture was used as a speech sample from the participants. The speech samples were transcribed using Codes for the Human Analysis of Transcripts transcription format Mac Whinney. The sample size used by Fraser et al. was considered. It included 97 healthy participants (HC) and 167 AD diagnosed patients with a total of 473 recordings. Total 263 features were extracted and were divided into 3 groups. Four different classifiers which included Trees-Random Forest (RF), Bayesian Networks (BN), Meta- Bagging (MB) and AdaboostM1 (AB) were used. Weka software was used to run the experiment, which classified the most important features which can provide the best accuracy into 4 configurations. The highest classification accuracy was achieved by BN classifier (94.71%) in fourth configuration. It was then again followed by BN classifier in configuration three with 93.66% accuracy, while (MB) and (RF) classifiers showed an accuracy of 92.38% and 90.90% in configuration two and one [135].
Based on all the approaches, it can be concluded that voice can be used as a biomarker for Alzheimer. It is cheap, non-invasive method which can identify AD even in its preliminary stages. More detailed studies need to be done to identify the potential role of voice along with other factors affecting it in a disease like Alzheimer.
4. Future Directions in Prediction of Alzheimer’s disease with Artificial Intelligence
The field of bioscience has undergone an exponential expansion with recent advances in the field of genomics, proteomics, transcriptomics, epigenomics, metagenomics and metabolomics etc. These developments in the field have offered huge amount of unprocessed data, which called for the unprecented rise of Artificial intelligence (AI) to use and process abundant imaging data with computer-controlled robot and information technology. AI is able to integrate the accumulated data and to generate valuable predictions for therapeutic applications. As it’s impossible to conclude one particular gene for a disease, the recent failure of a BACE1 inhibitor for Alzheimer’s has drawn attention towards the distinctive role of multiple genes in pertinent biological pathways and the importance of data reproducibility to address serious issues in this field. AI has capacity to process a massive amount of whole-genome data to recognize the most relevant pathways, and increase the probability to find the best target for the therapy. These studies have identified non-coding regions like THAP9-AS1 as the topmost targets for AD. These targets could potentially answer more fundamental questions about memories in the future [136]. The advantage of AI is the algorithm can be applied to many disparate and unassociated data sets. An early detection has become a prerequisite for the betterment of AD. By the time the clinical symptoms become evident, the neurons are already dead and the condition is impossible to reverse.
McGill University’s department of psychiatry is using public data from about 800 individuals from normal control, Alzheimer’s patients or the ones suffering from mild cognitive impairment. AI interface is using their MRI scans, a highly associated genetic marker in AD, and a simple cognition activity profile to identify patients with decline signs despite the diagnosis. Efforts are being made to test if the tool can be used advanced for clinical set-up, as data can remain useful even after many years since it is first collected for prediction of neurodegeneration over time in the individuals. With the advent of AI, doctors are hopeful to get certainty to judge the risk for decline in elderly or middle-aged subjects [137].
In a recent advancement with the tool, Ding et al. (2018) used deep learning algorithm with fluorine, 18F-FDG PET scan of the brain for early prediction of AD. They attained 82% specificity with 100% sensitivity, at an average of 75.8 months ahead of the final diagnosis. They used data from 1,002 patients from the Alzheimer’s disease Neuroimaging Initiative (ADNI) and 40 patients for the retrospective independent test set between 2005 and 2017. The deep learning algorithm developed itself learning from 90% of the dataset and then tested it on the remaining 10% of the dataset. Through deep learning, the algorithm trained itself to read metabolic patterns that correlated to Alzheimer’s and was able to predict every single case of AD. As the variations in the glucose uptake pattern in the brain are delicate and scattered, it’s important to trap the metabolic changes to represent a broader yet subtle process [138]. Such studies are suggested to be validated in a larger multi-institutional group. Future should focus on training the deep learning algorithm to search for specific patterns might be analogous to the marker’s accumulation in the brain specific to AD [139]. But looking into this vast information without AI, is a cumbersome process; AI can help us rely on machine learning tools, where a computer software is made smart enough to read and process as fine as glucose metabolism patterns and relate it to complex AD symptoms.
Other approaches included building a Neuroimaging identification system with the help of Git Bash programming aid software, Inception V3 image sorter and Unix commands. MRI of 2542 patients diagnosed with Alzheimer and 500 MRI of healthy patients from ADNI (Alzheimer disease Neuroimaging Initiative) were used. Hippocampal atrophy and volumetric reduction of entorhinal cortex were mainly analysed by the system to differentiate AD and healthy patients. To perform this analysis, the photos of the brain scan were uploaded and then segregated into pixels. The colours of the pixel were examined and bottlenecks, text files were created which were reviewed 3 million times. Then the results were generated in descending order of possibilities differentiating between AD and healthy with good accuracy [140].
Another approach included Novel Machine Learning (MC) which identify subject with pre-MCI (Mild cognitive impairment) and MCI and their conversion to AD following 3 years. Support Vector Machine was used as its algorithm which assigned 16 features to a category to multiply the differences between the MCI and AD diagnosed patients. First the program was trained to differentiate between AD-control and it gave excellent results (AUC of 0.996). After that the algorithm was used to predict MCI conversion to AD which showed slight drop in its performance (AUC of 0.821) [141].
There are several AI systems that help the AD patients to improve their life quality and also assist them in life Daily Activities (LDA). AICS (Alzheimer’s Intelligence care system) is a highly personalized system which creates treatment regimen for the patient on the basis of MMSE (Mini-Mental State Examination) stages as enlisted in Table 4. MMSE is a widely used test to measure and screen cognitive ability. The treatment regimen is created on the basis of MMSE results and the approval of doctors and care givers. The system mainly includes reminder system, schema therapy to review specific life events and alarm system for health, drug and daily living. After all this, the system also receives feedback and, on its basis, modifies the regimen [142].
Table 4.
MMSE score card for measuring cognitive ability [136]
| Score | Description | Stage |
|---|---|---|
| 26-30 | Normal range | Can be normal |
| 20-25 | Mild cognitive impairment | Elementary |
| 10-19 | Clear moderate cognitive impairment | Intermediate |
| 0-9 | Severe cognitive impairment | Advanced |
Various mobile-based applications like RxMind me, Mymeds, Alzheimer’s caregiver buddy, Alzheimer’s daily companion are designed as a virtual caregiver to remind the patient regarding LDA.
Albright (2019) predicted the progression of Alzheimers using “All-Pairs” technique with data of 1737 patients, comparing possible pairs of temporal data points. When a separate data set (110 patients) was used for machine learning models, a neural network model showed good result (mAUC = 0.866) at predicting the progression of Alzheimer in normal and mild cognitive impairment diagnosed patients [143]. AD and other causes of cognitive impairment were also differentiated with machine learning and neuropsychological testing. With samples of 158 patients divided on the basis of Mini Mental State Examination (MMSE) into early and a late stage group (labelled as non-AD and AD), around 82% patients were clearly differentiated as AD and non-AD in total group and the accuracy increased to 89% [144]. Farooq et al. (2017) used deep learning-based framework to analyse MRI scans and classified them into normal cognitive (NC), initial stage of mild cognitive impairment (MCI), later stage of mild cognitive impairment and Alzheimer’s disease (AD). Around 98.88% accuracy was obtained by this method in diagnosing AD [145]. Most of the above studies used artificial neural-networks (also known as deep learning), highly successful due to its virtual structure resembling the biological neural network. Reproducibility of results and ease of rewriting them are some of the advantages associated with artificial intelligence. Further artificial intelligence and artificial neural network can be used to analyse petabyte of data from each neuron and model other biological factors like glial cells, hormones and even microbiome which affect the functioning of neuron [146].
Conclusion
Dementia is a syndrome characterized by functional and cognitive decline. Various hypotheses, however unable to explain the complete picture, have been put forth for the pathogenesis of AD. The cause in many cases remains untraceable, hence no treatment options can halt or reverse disease progression, although some may temporarily ameliorate symptoms. Gene therapy and Quantum dots are the recent additions as therapeutic agents for AD, however they are yet to be clinically approved. Early diagnosis is of utmost importance since it not only benefits the patient by providing prompt treatment but also aids in the inclusion of patients in the initial stages of the disease for the conduct of clinical trials of promising drug candidates. Detection of AD with cerebrospinal fluid markers, blood test for inflammatory biomarkers, amyloid imaging with concurrent markers, volumetric MRI, are promising diagnostic indicators. As early diagnosis allows an access to treatment options and cost-effective caregiving, we have emphasized on use of immunotherapy, theranostics, and artificial intelligence in diagnosis with biomarkers in AD patients. Most of the immunotherapeutics is in Phase 3 clinical trials. Approaches such as peptidomimetics, speech as an indicator, metal ion chelators, etc. are still under investigation and research thus not widely used.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Duthey B. https://www.who.int/medicines/areas/priority_medicines/BP6_11Alzheimer.pdf(Accessed October 18, 2019)
- 2.Barage S.H., Sonawane K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Grimm M.O.W., Hartmann T. Recent understanding of the molecular mechanisms of Alzheimer’s disease. J. Addict. Res. Ther. 2012;004(S5) [Google Scholar]
- 4.Begcevic I., Brinc D., Brown M., Martinez-Morillo E., Goldhardt O., Grimmer T., Magdolen V., Batruch I., Diamandis E.P. Brain-related proteins as potential CSF biomarkers of Alzheimer’s disease: A targeted mass spectrometry approach. J. Proteomics. 2018;182:12–20. doi: 10.1016/j.jprot.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 5.Mullane K., Williams M. Alzheimer’s disease (AD) therapeutics - 1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochem. Pharmacol. 2018;158:359–375. doi: 10.1016/j.bcp.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Crews L., Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010;19(R1):R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang F., Wang M., Liu R., Wang J.Z., Schadt E., Haroutunian V., Katsel P., Zhang B., Wang X. CDT2-controlled cell cycle reentry regulates the pathogenesis of Alzheimer’s disease. Alzheimers Dement. 2019;15(2):217–231. doi: 10.1016/j.jalz.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H., Mesulam M.M., Cuello A.C., Farlow M.R., Giacobini E., Grossberg G.T., Khachaturian A.S., Vergallo A., Cavedo E., Snyder P.J., Khachaturian Z.S. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018;141(7):1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira-Vieira T.H., Guimaraes I.M., Silva F.R., Ribeiro F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leanza G., Gulino R., Zorec R. Noradrenergic hypothesis linking neurodegeneration-based cognitive decline and astroglia. Front. Mol. Neurosci. 2018;11:254. doi: 10.3389/fnmol.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vakalopoulos C. Alzheimer’s Disease: The alternative serotonergic hypothesis of cognitive decline. J. Alzheimers Dis. 2017;60(3):859–866. doi: 10.3233/JAD-170364. [DOI] [PubMed] [Google Scholar]
- 12.Guo L., Tian J., Du H. Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s disease. J. Alzheimers Dis. 2017;57(4):1071–1086. doi: 10.3233/JAD-160702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun H.M., Park K.R., Kim E.C., Kim S., Hong J.T. Serotonin 6 receptor controls Alzheimer’s disease and depression. Oncotarget. 2015;6(29):26716–26728. doi: 10.18632/oncotarget.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cochran J.N., Hall A.M., Roberson E.D. The dendritic hypothesis for Alzheimer’s disease pathophysiology. Brain Res. Bull. 2014;103:18–28. doi: 10.1016/j.brainresbull.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ittner A., Ittner L.M. Dendritic tau in Alzheimer’s Disease. Neuron. 2018;99(1):13–27. doi: 10.1016/j.neuron.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015;16(3):229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 17.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heppner F.L., Ransohoff R.M., Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015;16(6):358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- 19.Ashraf G.M., Tarasov V.V., Makhmutova A., Chubarev V.N., Avila-Rodriguez M., Bachurin S.O., Aliev G. The possibility of an infectious etiology of Alzheimer Disease. Mol. Neurobiol. 2019;56(6):4479–4491. doi: 10.1007/s12035-018-1388-y. [DOI] [PubMed] [Google Scholar]
- 20.Bir S.C., Chernyshev O.Y., Minagar A. Roles of toll-like receptors in pathophysiology of Alzheimer’s Disease and multiple sclerosis. Neuroinflammation; 2018. pp. 541–562. [Google Scholar]
- 21.Mahmoudian D.S., Arnold M., Nho K., Ahmad S., Jia W., Xie G., Louie G., Kueider-Paisley A., Moseley M.A., Thompson J.W., St. John W.L., Tenenbaum J.D., Blach C., Baillie R., Han X., Bhattacharyya S., Toledo J.B., Schafferer S., Klein S., Koal T., Risacher S.L., Kling M.A., Motsinger-Reif A., Rotroff D.M., Jack J., Hankemeier T., Bennett D.A., De Jager P.L., Trojanowski J.Q., Shaw L.M., Weiner M.W., Doraiswamy P.M., van Duijn C.M., Saykin A.J., Kastenmüller G., Kaddurah-Daouk R. Alzheimer’s Disease neuroimaging initiative and the Alzheimer disease metabolomics consortium. altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement. 2019;15(1):76–92. doi: 10.1016/j.jalz.2018.07.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., Han Y., Wang J., Hou D., Deng H., Deng Y.L., Song Z. Comparative epidemiological investigation of Alzheimer’s disease and colorectal cancer: the possible role of gastrointestinal conditions in the pathogenesis of AD. Front. Aging Neurosci. 2018;10:176. doi: 10.3389/fnagi.2018.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de J R De-Paula V.; Forlenza, A.S.; Forlenza, O.V.; Forlenza, O.V. Relevance of gutmicrobiota in cognition, behaviour and Alzheimer’s disease. Pharmacol. Res. 2018;136:29–34. doi: 10.1016/j.phrs.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Luca M., Di Mauro M., Di Mauro M., Luca A. Gut microbiota in alzheimer’s disease, depression, and type 2 diabetes mellitus: the role of oxidative stress. Oxid. Med. Cell. Longev. 2019;2019:4730539. doi: 10.1155/2019/4730539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pistollato F., Sumalla Cano S., Elio I., Masias Vergara M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74(10):624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 26.Angelucci F., Cechova K., Amlerova J., Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflammation. 2019;16(1):108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanatsu K., Tomita T. Molecular mechanisms of the genetic risk factors in pathogenesis of Alzheimer disease. Front. Biosci. 2017;22:180–192. doi: 10.2741/4480. [DOI] [PubMed] [Google Scholar]
- 28.Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 2016;18(5):421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta. 2010;1802(1):2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Zhou T., Ziegler A.C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017;2017:2525967. doi: 10.1155/2017/2525967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasekhar K., Govindaraju T. Current progress, challenges and future prospects of diagnostic and therapeutic interventions in Alzheimer’s disease. RSC Advances. 2018;8:23780–23804. doi: 10.1039/C8RA03620A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funderburk S.F., Marcellino B.K., Yue Z. Cell “self-eating” (autophagy) mechanism in Alzheimer’s disease. Mt. Sinai J. Med. 2010;77(1):59–68. doi: 10.1002/msj.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bateman R.J., Barthélemy N.R., Horie K. Another step forward in blood-based diagnostics for Alzheimer’s disease. Nat. Med. 2020;26(3):314–316. doi: 10.1038/s41591-020-0797-4. [DOI] [PubMed] [Google Scholar]
- 35.Janelidze S., Mattsson N., Palmqvist S., Smith R., Beach T.G., Serrano G.E., Chai X., Proctor N.K., Eichenlaub U., Zetterberg H., Blennow K., Reiman E.M., Stomrud E., Dage J.L., Hansson O. Plasma P-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020;26(3):379–386. doi: 10.1038/s41591-020-0755-1. [DOI] [PubMed] [Google Scholar]
- 36.Thijssen E.H., La Joie R., Wolf A., Strom A., Wang P., Iaccarino L., Bourakova V., Cobigo Y., Heuer H., Spina S., VandeVrede L., Chai X., Proctor N.K., Airey D.C., Shcherbinin S., Duggan Evans C., Sims J.R., Zetterberg H., Blennow K., Karydas A.M., Teunissen C.E., Kramer J.H., Grinberg L.T., Seeley W.W., Rosen H., Boeve B.F., Miller B.L., Rabinovici G.D., Dage J.L., Rojas J.C., Boxer A.L., Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020;26(3):387–397. doi: 10.1038/s41591-020-0762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weller J., Budson A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Res. 2018;7:1–9. doi: 10.12688/f1000research.14506.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bateman R.J., Xiong C., Benzinger T.L.S., Fagan A.M., Goate A., Fox N.C., Marcus D.S., Cairns N.J., Xie X., Blazey T.M., Holtzman D.M., Santacruz A., Buckles V., Oliver A., Moulder K., Aisen P.S., Ghetti B., Klunk W.E., McDade E., Martins R.N., Masters C.L., Mayeux R., Ringman J.M., Rossor M.N., Schofield P.R., Sperling R.A., Salloway S., Morris J.C. Dominantly Inherited Alzheimer Network. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strimbu K., Tavel J.A. What are biomarkers? Curr. Opin. HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Humpel C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011;29(1):26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansson O., Seibyl J., Stomrud E., Zetterberg H., Trojanowski J.Q., Bittner T., Lifke V., Corradini V., Eichenlaub U., Batrla R., Buck K., Zink K., Rabe C., Blennow K., Shaw L.M. Swedish BioFINDER study group; Alzheimer’s Disease Neuroimaging Initiative. CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470–1481. doi: 10.1016/j.jalz.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lashley T., Schott J.M., Weston P., Murray C.E., Wellington H., Keshavan A., Foti S.C., Foiani M., Toombs J., Rohrer J.D., Heslegrave A., Zetterberg H. Molecular biomarkers of Alzheimer’s disease: progress and prospects. 2018. [DOI] [PMC free article] [PubMed]
- 43.Fillit H.M. We need new biomarkers for Alzheimer’s Disease. https://blogs.scientificamerican.com/observations/we-need-new-biomarkers-for-alzheimers-disease
- 44.Yang Y.W., Liou S.H., Hsueh Y.M., Lyu W.S., Liu C.S., Liu H.J., Chung M.C., Hung P.H., Chung C.J. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case-control study using propensity score matching. Toxicol. Appl. Pharmacol. 2018;356:8–14. doi: 10.1016/j.taap.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 45.Blennow K., Zetterberg H. Biomarkers for Alzheimer’s disease: current status and prospects for the future. J. Intern. Med. 2018;284(6):643–663. doi: 10.1111/joim.12816. [DOI] [PubMed] [Google Scholar]
- 46.Pawlowski M., Meuth S.G., Duning T. Cerebrospinal fluid biomarkers in Alzheimer’s disease-from brain starch to bench and bedside. Diagnostics (Basel) 2017;7(3):42. doi: 10.3390/diagnostics7030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kok E.H., Alanne-Kinnunen M., Isotalo K., Luoto T., Haikonen S., Goebeler S., Perola M., Hurme M.A., Haapasalo H., Karhunen P.J. CRP gene variation affects early development of Alzheimer’s disease-related plaques. J. Neuroinflammation. 2011;8:96. doi: 10.1186/1742-2094-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Bryant S.E., Waring S.C., Hobson V., Hall J.R., Moore C.B., Bottiglieri T., Massman P., Diaz-Arrastia R. Decreased C-reactive protein levels in Alzheimer disease. J. Geriatr. Psychiatry Neurol. 2010;23(1):49–53. doi: 10.1177/0891988709351832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Bryant S.E., Johnson L., Edwards M., Soares H., Devous M.D., Ross S., Rohlfing G., Hall J. Texas Alzheimer’s Research & Care Consortium. The link between C-reactive protein and Alzheimer’s disease among Mexican Americans. J. Alzheimers Dis. 2013;34(3):701–706. doi: 10.3233/JAD-122071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strang F., Scheichl A., Chen Y.C., Wang X., Htun N.M., Bassler N., Eisenhardt S.U., Habersberger J., Peter K. Amyloid plaques dissociate pentameric to monomeric C-reactive protein: a novel pathomechanism driving cortical inflammation in Alzheimer’s disease? Brain Pathol. 2012;22(3):337–346. doi: 10.1111/j.1750-3639.2011.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi B.T., Lin H.B., Cheng Y.F., Zhou H., Lin T., Zhang M.Z., Li T.J., Xu J.P. Promotion of β-amyloid production by C-reactive protein and its implications in the early pathogenesis of Alzheimer’s disease. Neurochem. Int. 2012;60(3):257–266. doi: 10.1016/j.neuint.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Slevin M., Matou S., Zeinolabediny Y., Corpas R., Weston R., Liu D., Boras E., Di Napoli M., Petcu E., Sarroca S., Popa-Wagner A., Love S., Font M.A., Potempa L.A., Al-Baradie R., Sanfeliu C., Revilla S., Badimon L., Krupinski J. Monomeric C-reactive protein--a key molecule driving development of Alzheimer’s disease associated with brain ischaemia? Sci. Rep. 2015;5:13281. doi: 10.1038/srep13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hubacek J.A., Peasey A., Pikhart H., Stavek P., Kubinova R., Marmot M., Bobak M. APOE polymorphism and its effect on plasma C-reactive protein levels in a large general population sample. Hum. Immunol. 2010;71(3):304–308. doi: 10.1016/j.humimm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe Y., Kitamura K., Nakamura K., Sanpei K., Wakasugi M., Yokoseki A., Onodera O., Ikeuchi T., Kuwano R., Momotsu T., Narita I., Endo N. Elevated C-reactive protein is associated with cognitive decline in outpatients of a general hospital: the Project in Sado for Total Health (PROST). Dement. Geriatr. Cogn. Disord. Extra. 2016;6(1):10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brosseron F., Traschütz A., Widmann C.N., Kummer M.P., Tacik P., Santarelli F., Jessen F., Heneka M.T. Characterization and clinical use of inflammatory cerebrospinal fluid protein markers in Alzheimer’s disease. Alzheimers Res. Ther. 2018;10(1):25. doi: 10.1186/s13195-018-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma S., Verma S., Kapoor M., Saini A., Nehru B. Alzheimer’s disease like pathology induced six weeks after aggregated amyloid-beta injection in rats: increased oxidative stress and impaired long-term memory with anxiety-like behavior. Neurol. Res. 2016;38(9):838–850. doi: 10.1080/01616412.2016.1209337. [DOI] [PubMed] [Google Scholar]
- 57.Shi L., Baird A.L., Westwood S., Hye A., Dobson R., Thambisetty M., Lovestone S. A decade of blood biomarkers for Alzheimer’s disease research: an evolving field, improving study designs, and the challenge of replication. J. Alzheimers Dis. 2018;62(3):1181–1198. doi: 10.3233/JAD-170531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 59.Schupf N., Tang M.X., Fukuyama H., Manly J., Andrews H., Mehta P., Ravetch J., Mayeux R. Peripheral Abeta subspecies as risk biomarkers of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2008;105(37):14052–14057. doi: 10.1073/pnas.0805902105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nabers A., Perna L., Lange J., Mons U., Schartner J., Güldenhaupt J., Saum K.U., Janelidze S., Holleczek B., Rujescu D., Hansson O., Gerwert K., Brenner H. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med. 2018;10(5):e8763. doi: 10.15252/emmm.201708763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Björkhem I., Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004;24(5):806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 62.Cermenati G., Mitro N., Audano M., Melcangi R.C., Crestani M., De Fabiani E., Caruso D. Lipids in the nervous system: from biochemistry and molecular biology to patho-physiology. Biochim. Biophys. Acta. 2015;1851(1):51–60. doi: 10.1016/j.bbalip.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 63.Otaegui-Arrazola A., Menéndez-Carreño M., Ansorena D., Astiasarán I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010;48(12):3289–3303. doi: 10.1016/j.fct.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Savary S., Trompier D., Andréoletti P., Le Borgne F., Demarquoy J., Lizard G. Fatty acids - induced lipotoxicity and inflammation. Curr. Drug Metab. 2012;13(10):1358–1370. doi: 10.2174/138920012803762729. [DOI] [PubMed] [Google Scholar]
- 65.Beal E. Lipid biomarkers for early-stage Alzheimer disease. Nat. Rev. Neurol. 2011;7(9):474. doi: 10.1038/nrneurol.2011.125. [DOI] [PubMed] [Google Scholar]
- 66.Zarrouk A., Debbabi M., Bezine M., Karym E.M., Badreddine A., Rouaud O., Moreau T., Cherkaoui-Malki M., El Ayeb M., Nasser B., Hammami M., Lizard G. Lipid biomarkers in Alzheimer’s Disease. Curr. Alzheimer Res. 2018;15(4):303–312. doi: 10.2174/1567205014666170505101426. [DOI] [PubMed] [Google Scholar]
- 67.Tanzi R.E., Kovacs D.M., Kim T.W., Moir R.D., Guenette S.Y., Wasco W. The gene defects responsible for familial Alzheimer’s disease. Neurobiol. Dis. 1996;3(3):159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 68.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., Jones N., Thomas C., Stretton A., Morgan A.R., Lovestone S., Powell J., Proitsi P., Lupton M.K., Brayne C., Rubinsztein D.C., Gill M., Lawlor B., Lynch A., Morgan K., Brown K.S., Passmore P.A., Craig D., McGuinness B., Todd S., Holmes C., Mann D., Smith A.D., Love S., Kehoe P.G., Hardy J., Mead S., Fox N., Rossor M., Collinge J., Maier W., Jessen F., Schürmann B., Heun R., van den Bussche H., Heuser I., Kornhuber J., Wiltfang J., Dichgans M., Frölich L., Hampel H., Hüll M., Rujescu D., Goate A.M., Kauwe J.S., Cruchaga C., Nowotny P., Morris J.C., Mayo K., Sleegers K., Bettens K., Engelborghs S., De Deyn P.P., Van Broeckhoven C., Livingston G., Bass N.J., Gurling H., McQuillin A., Gwilliam R., Deloukas P., Al-Chalabi A., Shaw C.E., Tsolaki M., Singleton A.B., Guerreiro R., Mühleisen T.W., Nöthen M.M., Moebus S., Jöckel K.H., Klopp N., Wichmann H.E., Carrasquillo M.M., Pankratz V.S., Younkin S.G., Holmans P.A., O’Donovan M., Owen M.J., Williams J. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41(10):1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poirier J., Davignon J., Bouthillier D., Kogan S., Bertrand P., Gauthier S. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342(8873):697–699. doi: 10.1016/0140-6736(93)91705-Q. [DOI] [PubMed] [Google Scholar]
- 70.Wolk D.A., Dickerson B.C., Apolipoprotein E. Alzheimer’s Disease neuroimaging initiative. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2010;107(22):10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barber R.C. Biomarkers for early detection of Alzheimer disease. J. Am. Osteopath. Assoc. 2010;110(9) Suppl. 8:S10–S15. [PubMed] [Google Scholar]
- 72.Lim Y.Y., Villemagne V.L., Laws S.M., Ames D., Pietrzak R.H., Ellis K.A., Harrington K.D., Bourgeat P., Salvado O., Darby D., Snyder P.J., Bush A.I., Martins R.N., Masters C.L., Rowe C.C., Nathan P.J., Maruff P. Australian Imaging, Biomarkers and Lifestyle (AIBL) Research Group. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol. Aging. 2013;34(11):2457–2464. doi: 10.1016/j.neurobiolaging.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 73.Lim Y.Y., Villemagne V.L., Laws S.M., Ames D., Pietrzak R.H., Ellis K.A., Harrington K., Bourgeat P., Bush A.I., Martins R.N., Masters C.L., Rowe C.C., Maruff P., AIBL Research Group Effect of BDNF Val66Met on memory decline and hippocampal atrophy in prodromal Alzheimer’s disease: a preliminary study. PLoS One. 2014;9(1):e86498. doi: 10.1371/journal.pone.0086498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johannsen S., Duning K., Pavenstädt H., Kremerskothen J., Boeckers T.M. Temporal-spatial expression and novel biochemical properties of the memory-related protein KIBRA. Neuroscience. 2008;155(4):1165–1173. doi: 10.1016/j.neuroscience.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 75.Kauppi K., Nilsson L.G., Adolfsson R., Eriksson E., Nyberg L. KIBRA polymorphism is related to enhanced memory and elevated hippocampal processing. J. Neurosci. 2011;31(40):14218–14222. doi: 10.1523/JNEUROSCI.3292-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tracy T.E., Sohn P.D., Minami S.S., Wang C., Min S.W., Li Y., Zhou Y., Le D., Lo I., Ponnusamy R., Cong X., Schilling B., Ellerby L.M., Huganir R.L., Gan L. Acetylated tau obstructs KIBRA-mediated signaling in synaptic plasticity and promotes tauopathy related memory loss. Neuron. 2016;90(2):245–260. doi: 10.1016/j.neuron.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Porter T., Villemagne V.L., Savage G., Milicic L., Lim Y.Y., Maruff P., Masters C.L., Ames D., Bush A.I., Martins R.N., Rainey-Smith S., Rowe C.C., Taddei K., Groth D., Verdile G., Burnham S.C., Laws S.M. Cognitive gene risk profile for the prediction of cognitive decline in presymptomatic Alzheimer’s disease. Pers. Med. Psychiatry. 2018;7–8:14–20. doi: 10.1016/j.pmip.2018.03.001. [DOI] [Google Scholar]
- 78.Johnson K.A., Fox N.C., Sperling R.A., Klunk W.E. Brain imaging in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(4):a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fantoni E.R., Chalkidou A., O’ Brien J.T., Farrar G., Hammers A. A systematic review and aggregated analysis on the impact of amyloid pet brain imaging on the diagnosis, diagnostic confidence, and management of patients being evaluated for Alzheimer’s Disease. J. Alzheimers Dis. 2018;63(2):783–796. doi: 10.3233/JAD-171093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferreira L.K., Busatto G.F. Neuroimaging in Alzheimer’s disease: current role in clinical practice and potential future applications. Clinics (São Paulo) 2011;66(Suppl. 1):19–24. doi: 10.1590/S1807-59322011001300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lohith T.G., Bennacef I., Vandenberghe R., Vandenbulcke M., Salinas C.A., Declercq R., Reynders T., Telan-Choing N.F., Riffel K., Celen S., Serdons K., Bormans G., Tsai K., Walji A., Hostetler E.D., Evelhoch J.L., Van Laere K., Forman M., Stoch A., Sur C., Struyk A. First-in-human brain imaging of Alzheimer dementia patients and elderly controls with 18F-MK-6240, a PET tracer targeting neurofibrillary tangle pathology. J. Nucl. Med. 2019;60(1):107–114. doi: 10.2967/jnumed.118.208215. [DOI] [PubMed] [Google Scholar]
- 82.Graham J.E., Rockwood K., Beattie B.L., Eastwood R., Gauthier S., Tuokko H., McDowell I. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349(9068):1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 83.Flicker C., Ferris S.H., Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/WNL.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 84.Zaudig M. A new systematic method of measurement and diagnosis of “mild cognitive impairment” and dementia according to ICD-10 and DSM-III-R criteria. Int. Psychogeriatr. 1992;4(4) Suppl. 2:203–219. doi: 10.1017/S1041610292001273. [DOI] [PubMed] [Google Scholar]
- 85.Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 86.Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O., Nordberg A., Bäckman L., Albert M., Almkvist O., Arai H., Basun H., Blennow K., de Leon M., DeCarli C., Erkinjuntti T., Giacobini E., Graff C., Hardy J., Jack C., Jorm A., Ritchie K., van Duijn C., Visser P., Petersen R.C. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 87.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C., Gamst A., Holtzman D.M., Jagust W.J., Petersen R.C., Snyder P.J., Carrillo M.C., Thies B., Phelps C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ganguli M. The unbearable lightness of MCI. Int. Psychogeriatr. 2014;26(3):353–359. doi: 10.1017/S1041610213002275. [DOI] [PubMed] [Google Scholar]
- 89.McEvoy L.K., Fennema-Notestine C., Roddey J.C., Hagler D.J., Jr, Holland D., Karow D.S., Pung C.J., Brewer J.B., Dale A.M. Alzheimer’s Disease Neuroimaging Initiative. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251(1):195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kauppi K., Fan C.C., McEvoy L.K., Holland D., Tan C.H., Chen C.H., Andreassen O.A., Desikan R.S., Dale A.M. Alzheimer’s Disease neuroimaging initiative. combining polygenic hazard score with volumetric mri and cognitive measures improves prediction of progression from mild cognitive impairment to Alzheimer’s Disease. Front. Neurosci. 2018;12:260. doi: 10.3389/fnins.2018.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hampel H., Teipel S.J., Fuchsberger T., Andreasen N., Wiltfang J., Otto M., Shen Y., Dodel R., Du Y., Farlow M., Möller H.J., Blennow K., Buerger K. Value of CSF β-amyloid1-42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol. Psychiatry. 2004;9(7):705–710. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 92.World Health Organization The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. https://www.who.int/classifications/icd/en/bluebook.pdf
- 93.American Psychiatric Association Diagnostic and statistical manual of mental disorders. https://www.who.int/substance_abuse/ terminology/diagnostic/en/
- 94.Jedynak B.M., Lang A., Liu B., Katz E., Zhang Y., Wyman B.T., Raunig D., Jedynak C.P., Caffo B., Prince J.L. Alzheimer’s Disease Neuroimaging Initiative. A computational neurodegenerative disease progression score: method and results with the Alzheimer’s disease Neuroimaging Initiative cohort. Neuroimage. 2012;63(3):1478–1486. doi: 10.1016/j.neuroimage.2012.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.American Psychiatric Association https://www.psychiatry.org/ psychiatrists/practice/dsm
- 96.Headley A., De Leon-Benedetti A., Dong C., Levin B., Loewenstein D., Camargo C., Rundek T., Zetterberg H., Blennow K., Wright C.B., Sun X. Alzheimer’s Disease Neuroimaging Initiative. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology. 2018;90(10):e887–e895. doi: 10.1212/WNL.0000000000005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y., Fu A.K.Y., Ip N.Y. Synaptic dysfunction in Alzheimer’s disease: Mechanisms and therapeutic strategies. Pharmacol. Ther. 2019;195:186–198. doi: 10.1016/j.pharmthera.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 98.Fish P.V., Steadman D., Bayle E.D., Whiting P. New approaches for the treatment of Alzheimer’s disease. Bioorg. Med. Chem. Lett. 2019;29(2):125–133. doi: 10.1016/j.bmcl.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 99. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm519875.html
- 100. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM596728.pdf
- 101.Tuszynski M.H., Yang J.H., Barba D. U, H.S.; Bakay, R.A.; Pay, M.M.; Masliah, E.; Conner, J.M.; Kobalka, P.; Roy, S.; Nagahara, A.H. Nerve growth factor gene therapy: activation of neuronal responses in Alzheimer Disease. JAMA Neurol. 2015;72(10):1139–1147. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Katsouri L., Lim Y.M., Blondrath K., Eleftheriadou I., Lombardero L., Birch A.M., Mirzaei N., Irvine E.E., Mazarakis N.D., Sastre M. PPARγ-coactivator-1α gene transfer reduces neuronal loss and amyloid-β generation by reducing β-secretase in an Alzheimer’s disease model. Proc. Natl. Acad. Sci. USA. 2016;113(43):12292–12297. doi: 10.1073/pnas.1606171113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rafii M.S., Tuszynski M.H., Thomas R.G., Barba D., Brewer J.B., Rissman R.A., Siffert J., Aisen P.S. AAV2-NGF Study Team. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with Alzheimer Disease: A randomized clinical trial. JAMA Neurol. 2018;75(7):834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. https://www.barchester.com/news/new-gene-therapy-technique-could-reverse-alzheimer%EAlzheimers
- 105.Li M., Guan Y., Zhao A., Ren J., Qu X. Using multifunctional peptide conjugated Au nanorods for monitoring β-amyloid aggregation and chemo-photothermal treatment of Alzheimer’s Disease. Theranostics. 2017;7(12):2996–3006. doi: 10.7150/thno.18459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dao P., Ye F., Liu Y., Du Z.Y., Zhang K., Dong C.Z., Meunier B., Chen H. Development of Phenothiazine-based theranostic compounds that act both as inhibitors of β-amyloid aggregation and as imaging probes for amyloid plaques in Alzheimer’s Disease. ACS Chem. Neurosci. 2017;8(4):798–806. doi: 10.1021/acschemneuro.6b00380. [DOI] [PubMed] [Google Scholar]
- 107.Li Y., Xu D., Ho S.L., Li H.W., Yang R., Wong M.S. A theranostic agent for in vivo near-infrared imaging of β-amyloid species and inhibition of β-amyloid aggregation. Biomaterials. 2016;94:84–92. doi: 10.1016/j.biomaterials.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 108.Hultqvist G., Syvänen S., Fang X.T., Lannfelt L., Sehlin D. Bivalent brain shuttle increases antibody uptake by monovalent binding to the transferrin receptor. Theranostics. 2017;7(2):308–318. doi: 10.7150/thno.17155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cui Z., Bu W., Fan W., Zhang J., Ni D., Liu Y., Wang J., Liu J., Yao Z., Shi J. Sensitive imaging and effective capture of Cu(2+): Towards highly efficient theranostics of Alzheimer’s disease. Biomaterials. 2016;104:158–167. doi: 10.1016/j.biomaterials.2016.06.056. [DOI] [PubMed] [Google Scholar]
- 110.Hu B., Dai F., Fan Z., Ma G., Tang Q., Zhang X. Nanotheranostics: Congo Red/Rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of Alzheimer’s Disease in APPswe/PS1dE9 transgenic mice. Adv. Mater. 2015;27(37):5499–5505. doi: 10.1002/adma.201502227. [DOI] [PubMed] [Google Scholar]
- 111.Matea C.T., Mocan T., Tabaran F., Pop T., Mosteanu O., Puia C., Iancu C., Mocan L. Quantum dots in imaging, drug delivery and sensor applications. Int. J. Nanomedicine. 2017;12:5421–5431. doi: 10.2147/IJN.S138624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao S., Zhou D., Luan P., Gu B., Feng L., Fan S., Liao W., Fang W., Yang L., Tao E., Guo R., Liu J. Graphene quantum dots conjugated neuroprotective peptide improve learning and memory capability. Biomaterials. 2016;106:98–110. doi: 10.1016/j.biomaterials.2016.08.021. [DOI] [PubMed] [Google Scholar]
- 113.Bungart B.L., Dong L., Sobek D., Sun G.Y., Yao G., Lee J.C. Nanoparticle-emitted light attenuates amyloid-β-induced superoxide and inflammation in astrocytes. Nanomedicine (Lond.) 2014;10(1):15–17. doi: 10.1016/j.nano.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mars A., Hamami M., Bechnak L., Patra D., Raouafi N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta. 2018;1036:141–146. doi: 10.1016/j.aca.2018.06.075. [DOI] [PubMed] [Google Scholar]
- 115.Morales-Narváez E., Montón H., Fomicheva A., Merkoçi A. Signal enhancement in antibody microarrays using quantum dots nanocrystals: application to potential Alzheimer’s disease biomarker screening. Anal. Chem. 2012;84(15):6821–6827. doi: 10.1021/ac301369e. [DOI] [PubMed] [Google Scholar]
- 116.Medina-Sánchez M., Miserere S., Morales-Narváez E., Merkoçi A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014;54:279–284. doi: 10.1016/j.bios.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 117.Demattos R.B., Lu J., Tang Y., Racke M.M., Delong C.A., Tzaferis J.A., Hole J.T., Forster B.M., McDonnell P.C., Liu F., Kinley R.D., Jordan W.H., Hutton M.L. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76(5):908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 118.Panza F., Lozupone M., Seripa D., Imbimbo B.P. Amyloid-β immunotherapy for Alzheimer disease: Is it now a long shot? Ann. Neurol. 2019;85(3):303–315. doi: 10.1002/ana.25410. [DOI] [PubMed] [Google Scholar]
- 119.Schenk D. Amyloid-β immunotherapy for Alzheimer’s disease: the end of the beginning. Nat. Rev. Neurosci. 2002;3(10):824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- 120.van Dyck C.H. Anti-Amyloid-β monoclonal antibodies for Alzheimer’s Disease: Pitfalls and promise. Biol. Psychiatry. 2018;83(4):311–319. doi: 10.1016/j.biopsych.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sumner I.L., Edwards R.A., Asuni A.A., Teeling J.L. Antibody engineering for optimized immunotherapy in Alzheimer’s Disease. Front. Neurosci. 2018;12:254. doi: 10.3389/fnins.2018.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajasekhar K., Mehta K., Govindaraju T. Hybrid multifunctional modulators inhibit multifaceted aβ toxicity and prevent mitochondrial damage. ACS Chem. Neurosci. 2018;9(6):1432–1440. doi: 10.1021/acschemneuro.8b00033. [DOI] [PubMed] [Google Scholar]
- 123.Hom R.K., Fang L.Y., Mamo S., Tung J.S., Guinn A.C., Walker D.E., Davis D.L., Gailunas A.F., Thorsett E.D., Sinha S., Knops J.E., Jewett N.E., Anderson J.P., John V. Design and synthesis of statine-based inhibitors of human –secretase. J. Med. Chem. 2003;46:1799–1802. doi: 10.1021/jm025619l. [DOI] [PubMed] [Google Scholar]
- 124.Goyal D., Shuaib S., Mann S., Goyal B. Rationally designed peptides and peptidomimetics as inhibitors of amyloid-β (Aβ) Aggregation: Potential therapeutics of Alzheimer’s Disease. ACS Comb. Sci. 2017;19(2):55–80. doi: 10.1021/acscombsci.6b00116. [DOI] [PubMed] [Google Scholar]
- 125.Budimir A. Metal ions, Alzheimer’s disease and chelation therapy. Acta Pharm. 2011;61(1):1–14. doi: 10.2478/v10007-011-0006-6. [DOI] [PubMed] [Google Scholar]
- 126.Robert A., Liu Y., Nguyen M., Meunier B. Regulation of copper and iron homeostasis by metal chelators: a possible chemotherapy for Alzheimer’s disease. Acc. Chem. Res. 2015;48(5):1332–1339. doi: 10.1021/acs.accounts.5b00119. [DOI] [PubMed] [Google Scholar]
- 127.Wong C.B., Kobayashi Y., Xiao J-Z. Probiotics for preventing cognitive impairment in Alzheimer’s Disease. Gut Microbiota - Brain Axis; Evrensel, A.; Ünsalver, B.O. Neuroscience. 2018;•••:85–104. [Google Scholar]
- 128.Abraham D., Feher J., Scuderi G.L., Szabo D., Dobolyi A., Cservenak M., Juhasz J., Ligeti B., Pongor S., Gomez-Cabrera M.C., Vina J., Higuchi M., Suzuki K., Boldogh I., Radak Z. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019;115:122–131. doi: 10.1016/j.exger.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 129.Leblhuber F., Steiner K., Schuetz B., Fuchs D., Gostner J.M. Probiotic supplementation in patients with Alzheimer’s Dementia - An explorative intervention study. Curr. Alzheimer Res. 2018;15(12):1106–1113. doi: 10.2174/1389200219666180813144834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopez-de-Ipiña K., Alonso J.B., Solé-Casals J., Barroso N., Henriquez P., Faundez-Zanuy M., Travieso C., Ecay-Torres M., Martinez-Lage P., Egiraun H. On Automatic diagnosis of alzheimer’s disease based on spontaneous speech analysis and emotional temperature. Cognit. Comput. 2015;7:44–55. doi: 10.1007/s12559-013-9229-9. [DOI] [Google Scholar]
- 131.Jarrold W., Peintner B., Wilkins D., Vergryi D., Richey C., Gorno-Tempini M.L., Ogar J. Aided diagnosis of dementia type through computer-based analysis of spontaneous speech. 2014.
- 132.Orimaye S.O., Wong J.S-M., Golden K.J. Learning predictive linguistic features for Alzheimer’s disease and related dementia using verbal utterances. Association for Computational Linguistics; 2014. pp. 78–87. [Google Scholar]
- 133.König A., Satt A., Sorin A., Hoory R., Toledo-Ronen O., Derreumaux A., Manera V., Verhey F., Aalten P., Robert P.H., David R. Automatic speech analysis for the assessment of patients with predementia and Alzheimer’s disease. 2015. [DOI] [PMC free article] [PubMed]
- 134.Fraser K.C., Meltzer J.A., Rudzicz F. Linguistic features identify Alzheimer’s Disease in narrative speech. J. Alzheimers Dis. 2016;49(2):407–422. doi: 10.3233/JAD-150520. [DOI] [PubMed] [Google Scholar]
- 135.Al-Hameed S., Benaissa M., Christensen H. Simple and robust audio-based detection of biomarkers for Alzheimer’s disease; [Google Scholar]
- 136. https://labiotech.eu/features/artificial-intelligence-alzheimer
- 137. https://globalnews.ca/news/4594878/alzheimers-disease-artificial-intelligence-ai/
- 138.Ding Y., Sohn J.H., Kawczynski M.G., Trivedi H., Harnish R., Jenkins N.W., Lituiev D., Copeland T.P., Aboian M.S., Mari Aparici C., Behr S.C., Flavell R.R., Huang S.Y., Zalocusky K.A., Nardo L., Seo Y., Hawkins R.A., Hernandez Pampaloni M., Hadley D., Franc B.L. A Deep learning model to predict a diagnosis of Alzheimer Disease by using 18F-FDG PET of the brain. Radiology. 2019;290(2):456–464. doi: 10.1148/radiol.2018180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Radiological Society of North America Artificial intelligence predicts Alzheimer’s years before diagnosis. htm.sciencedaily.com/
- 140.De Brito Sanchez R., de Barros L., Rodrigues S.C.M., Fernandes J.C.L., Bondioli A.C.V., de Campos Mundin H.A., de Sousa V.D.S., da Silva L.H.B.O. 2019. [Google Scholar]
- 141.Graham S.A., Depp C.A. Artificial intelligence and risk prediction in geriatric mental health: what happens next? Int. Psychogeriatr. 2019;31(7):921–923. doi: 10.1017/S1041610219000954. [DOI] [Google Scholar]
- 142.Rahmanian M., Mosalanejad L., Abdolallahifard S. An Alzheimer’s Intelligence Care System (AICS) to assist Alzheimer’s Patients : Design and development of application. J. Res. Med. Dental. Sci. 2018;6(6):9–15. [Google Scholar]
- 143.Albright J. Forecasting the progression of Alzheimer’s disease using neural networks and a novel preprocessing algorithm. Alzheimers Dement. (N. Y.) 2019;5:483–491. doi: 10.1016/j.trci.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gurevich P., Stuke H., Kastrup A., Stuke H., Hildebrandt H. Neuropsychological testing and machine learning distinguish Alzheimer’s disease from other causes for cognitive impairment. Front. Aging Neurosci. 2017;9:114. doi: 10.3389/fnagi.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Farooq A., Anwar S.M., Awais M., Alnowami M. Proceedings of the IEEE International Smart Cities Conference; Wuxi, China. 2017. pp. 1–4. [Google Scholar]
- 146.Maoz U., Linstead E. Brain imaging and artificial intelligence. Casting Light on the Dark Side of Brain Imaging; Raz, A.; Thibault, R.T. Elsevier; 2019. pp. 99–103. [Google Scholar]