Abstract
Background:
A 2017 meta-analysis of data from 25 randomised controlled trials of vitamin D supplementation for the prevention of acute respiratory infections revealed a protective effect of the intervention. Since then, 20 new RCTs have been completed.
Methods:
Systematic review and meta-analysis of data from randomised controlled trials (RCTs) of vitamin D for ARI prevention using a random effects model. Pre-specified sub-group analyses were done to determine whether effects of vitamin D on risk of ARI varied according to baseline 25-hydroxyvitamin D (25[OH]D) concentration or dosing regimen. We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and the ClinicalTrials.gov registry from inception to 1st May 2020. Double-blind RCTs of supplementation with vitamin D or calcidiol, of any duration, were eligible if they were approved by a Research Ethics Committee and if ARI incidence was collected prospectively and pre-specified as an efficacy outcome. Aggregate data, stratified by baseline 25(OH)D concentration, were obtained from study authors. The study was registered with PROSPERO (no. CRD42020190633).
Findings:
We identified 45 eligible RCTs (total 73,384 participants). Data were obtained for 46,331 (98.0%) of 47,262 participants in 42 studies, aged 0 to 95 years. For the primary comparison of vitamin D supplementation vs. placebo, the intervention reduced risk of ARI overall (Odds Ratio [OR] 0.91, 95% CI 0.84 to 0.99; P for heterogeneity 0.01). No statistically significant effect of vitamin D was seen for any of the sub-groups defined by baseline 25(OH)D concentration. However, protective effects were seen for trials in which vitamin D was given using a daily dosing regimen (OR 0.75, 95% CI 0.61 to 0.93); at daily dose equivalents of 400–1000 IU (OR 0.70, 95% CI 0.55 to 0.89); and for a duration of ≤12 months (OR 0.82, 95% CI 0.72 to 0.93). No significant interaction was seen between allocation to vitamin D vs. placebo and dose frequency, dose size, or study duration. Vitamin D did not influence the proportion of participants experiencing at least one serious adverse event (OR 0.97, 95% CI 0.86 to 1.09). Risk of bias within individual studies was assessed as being low for all but three trials. A funnel plot showed left-sided asymmetry (P=0.008, Egger’s test).
Interpretation:
Vitamin D supplementation was safe and reduced risk of ARI, despite evidence of significant heterogeneity across trials. Protection was associated with administration of daily doses of 400–1000 IU vitamin D for up to 12 months. The relevance of these findings to COVID-19 is not known and requires investigation.
Introduction
Interest in the potential for vitamin D supplementation to reduce risk of acute respiratory infections (ARI) has increased since the emergence of the COVID-19 pandemic.1 This stems from findings of laboratory studies, showing that vitamin D metabolites support innate immune responses to respiratory viruses,2 together with observational studies reporting independent associations between low circulating levels of 25-hydroxyvitamin D (25[OH]D, the widely accepted biomarker of vitamin D status) and increased risk of ARI caused by other pathogens.3,4 Randomised controlled trials (RCTs) of vitamin D for the prevention of ARI have produced heterogeneous results, with some showing protection, and others reporting null findings. We previously meta-analysed individual participant data from 10,933 participants in 25 RCTs5–29 and showed a protective overall effect that was stronger in those with lower baseline 25(OH)D levels, and in trials where vitamin D was administered daily or weekly rather than in more widely spaced bolus doses.30 Since the date of the final literature search performed for that study (December 2015), 20 RCTs with 62,063 participants fulfilling the same eligibility criteria have been completed.31–50 We therefore sought data from these more recent studies for inclusion in an updated meta-analysis of stratified aggregate (trial-level) data to determine whether vitamin D reduced ARI risk overall, and to evaluate whether effects of vitamin D on ARI risk varied according to baseline 25(OH)D concentration and/or dosing regimen (frequency, dose size, and trial duration).
Methods
Protocol, Registration and Ethical Approvals
Methods were pre-specified in a protocol that was registered with the PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=190633). Research Ethics Committee approval to conduct this meta-analysis was not required in the UK; local ethical permission to contribute data from primary trials was required and obtained for studies by Camargo et al13 (The Ethics Review Committee of the Mongolian Ministry of Health), Murdoch et al14 (Southern Health and Disability Ethics Committee, ref. URB/09/10/050/AM02), Rees et al17 (Committee for the Protection of Human Subjects, Dartmouth College, USA; Protocol # 24381), Tachimoto et al28 (Ethics committee of the Jikei University School of Medicine, ref 26–333: 7839), Tran et al18 (QIMR Berghofer Medical Research Institute Human Research Ethics Committee, P1570) and Urashima et al6,20 (Ethics committee of the Jikei University School of Medicine, ref 26–333: 7839).
Eligibility Criteria
Randomised, double-blind, trials of supplementation with vitamin D3, vitamin D2 or 25(OH)D of any duration, with a placebo or low-dose vitamin D control, were eligible for inclusion if they had been approved by a Research Ethics Committee and if data on incidence of ARI were collected prospectively and pre-specified as an efficacy outcome. The latter requirement was imposed in order to minimise misclassification bias (prospectively designed instruments to capture ARI events were deemed more likely to be sensitive and specific for this outcome). Studies reporting results of long-term follow-up of primary RCTs were excluded.
Study Identification and Selection
Two investigators (ARM and DAJ) searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science and the ClinicalTrials.gov registry using the electronic search strategies described in the Methods Section of Supplementary Material. Searches were regularly updated up to and including 1st May 2020. No language restrictions were imposed. These searches were supplemented by searching review articles and reference lists of trial publications. Collaborators were asked if they knew of any additional trials. Three investigators (DAJ, CAC and ARM) determined which trials met the eligibility criteria.
Data Collection Processes
Summary data from trials which contributed to our previous meta-analysis of individual participant data30 were extracted from our central database, with permission from the Principal Investigators. Summary data relating to the primary outcome (overall and by sub-group) and secondary outcomes (overall only) from newly identified trials were requested from Principal Investigators. On receipt, they were assessed for consistency with associated publications. Study authors were contacted to provide missing data and to resolve any queries arising from these consistency checks. Once queries had been resolved, clean summary data were uploaded to the study database, which was held in STATA IC v14.2 (StataCorp, College Station, TX).
Data relating to study characteristics were extracted for the following variables: study setting, eligibility criteria, 25(OH)D assay and levels, details of intervention and control regimens, trial duration, case definitions for ARI and number entering primary analysis (after randomisation). Follow-up summary data were requested for the proportions of participants experiencing one or more ARI during the trial, both overall and stratified by baseline serum 25(OH)D concentration, where this was available. We also requested summary data on the proportions of participants who experienced one or more of the following events during the trial: upper respiratory infection (URI); lower respiratory infection (LRI); Emergency Department attendance and/or hospital admission for ARI; death due to ARI or respiratory failure; use of antibiotics to treat an ARI; absence from work or school due to ARI; a serious adverse event; death due to any cause; and potential adverse reactions to vitamin D (hypercalcaemia and renal stones).
Risk of Bias Assessment for Individual Studies
We used the Cochrane Collaboration Risk of Bias tool51 to assess the following variables: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, completeness of outcome data, evidence of selective outcome reporting and other potential threats to validity. Study quality was assessed independently by two investigators (ARM and DAJ), except for the five trials for which DAJ and/or ARM were investigators, which were assessed by CAC. Discrepancies were resolved by consensus.
Definition of outcomes
The primary outcome of the meta-analysis was the proportion of participants experiencing one or more ARIs, with the definition of ARI encompassing events classified as URI, LRI and ARI of unclassified location (i.e. infection of the upper and/or lower respiratory tract). Secondary outcomes were incidence of URI and LRI, analysed separately; incidence of Emergency Department attendance and/or hospital admission for ARI; death due to ARI or respiratory failure; use of antibiotics to treat an ARI; absence from work or school due to ARI; incidence of serious adverse events; death due to any cause; and incidence of potential adverse reactions to vitamin D (hypercalcaemia and renal stones).
Synthesis Methods
Data were analysed by DAJ; results were checked and verified by JDS. Our meta-analysis approach followed published guidelines.52 The primary comparison was of participants randomised to vitamin D vs. placebo: this was performed for all of the outcomes listed above. For trials that included higher-dose, lower-dose and placebo arms, data from higher-dose and lower-dose arms were pooled for analysis of the primary comparison. A secondary comparison of participants randomised to higher vs. lower doses of vitamin D was performed for the primary outcome only. A log odds ratio and its standard error was calculated for each outcome within each trial from the proportion of participants experiencing one or more events in the intervention vs. control arm. These were meta-analysed in a random effects model using the Metan package53 within STATA IC v14.2 to obtain a pooled odds ratio with a 95% confidence interval and a measure of heterogeneity summarized by the I2 statistic and its corresponding P value.
Exploration of variation in effects
To explore reasons for heterogeneity of effect of the intervention between trials we performed a stratified analysis according to baseline vitamin D status (serum 25[OH]D <25 vs. 25–49.9 vs. 50–74.9 vs. ≥75 nmol/L) and according to age at baseline (<1 vs. 1–15.99 vs. 16–64.99 vs. ≥65 yrs). We additionally conducted sub-group analyses according to vitamin D dosing regimen (administration of daily vs. weekly vs. monthly or less frequent doses), dose size (daily equivalent <400 IU vs. 400–1000 IU vs. 1001–2000 IU vs. >2,000 IU), trial duration (≤12 months vs. >12 months) and presence of airway disease (trial restricted to participants with asthma vs. those restricted to participants with COPD vs. those in which participants without airway disease were eligible). The thresholds for baseline 25(OH)D concentration used in sub-group analyses were selected a priori on the basis that they represent cut-offs that are commonly used to distinguish profound vitamin D deficiency (<25 nmol/L), moderate vitamin D deficiency (25–49.9 nmol/L) and sub-optimal vitamin D status (50–74.9 nmol/L).54 An exploratory analysis restricted to studies with optimal dosing frequency, dose size and duration was also performed.
To investigate factors associated with heterogeneity of effect between subgroups of trials, we performed multivariable meta-regression analysis on trial-level characteristics, namely, dose frequency, dose size and trial duration, to produce an adjusted odds ratio, a 95% confidence interval and a P value for interaction for each factor. Independent variables were dichotomised to create a more parsimonious model (serum 25(OH)D of <25 vs. ≥25 nmol/L; administration of daily vs. non-daily doses; daily equivalent of ≤1000 IU vs. >1000 IU; and trial duration of ≤12 vs. >12 months). The meta-regression analysis excluded data from two trials that included higher-dose, lower-dose and placebo arms,18,47 since the higher-dose and lower-dose arms spanned the 1,000 IU/day cut-off, rendering them unclassifiable for the purposes of this analysis.
Quality Assessment Across Studies
For the primary analysis, the likelihood of publication bias was investigated through the construction of a contour-enhanced funnel plot.55 We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias)56 to assess the quality of the body of evidence contributing to analyses of the primary efficacy outcome and major secondary outcomes of our meta-analysis.
Sensitivity analyses
We conducted three exploratory sensitivity analyses for the primary comparison of the primary outcome: one excluded RCTs where risk of bias was assessed as being unclear; one excluded RCTs in which incidence of ARI was not the primary or co-primary outcome; and one substituted diary-defined ARI events (available for 2598 participants) for survey-defined ARI events (available for n=16,000 participants) from the trial by Pham et al.44
Role of the funding source
This study was conducted without external funding.
Results
Study selection and data obtained
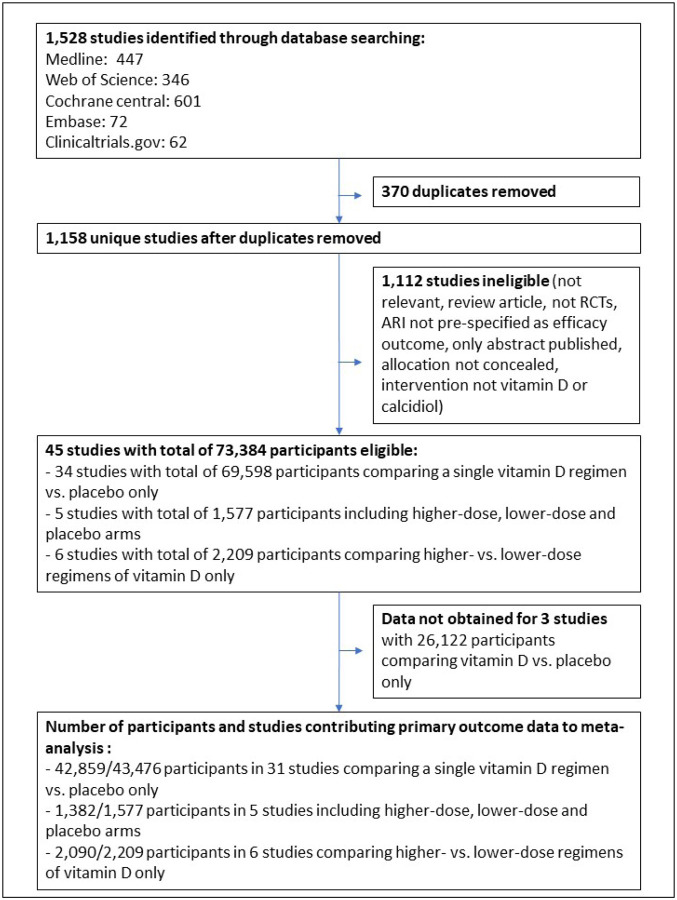
The study selection process is illustrated in Figure 1. Our search identified a total of 1,528 unique studies that were assessed for eligibility, of which 45 studies with a total of 73,384 randomised participants fulfilled eligibility criteria. Studies for which full text was reviewed prior to exclusion due to ineligibility are listed in Table S1. Of the 45 eligible studies identified, 34 compared effects of a single vitamin D regimen vs. placebo only,5–17,19,20,22,23,25–28,31,33,36,38,39,41–45,48–50,5 compared effects of higher-dose, lower-dose and placebo arms,18,21,24,40,47 and 6 compared effects of higher-vs. lower-dose regimens of vitamin D only.29,32,34,35,37,46 Stratified aggregate data were sought and obtained for all but 3 eligible studies.48–50 Data for the primary outcome (proportion of participants with one or more ARI) were obtained for 46,331 (98.0%) of 47,262 participants in 42 studies.5–29,31–47
Figure 1:
Flow chart of study selection
Study and participant characteristics
Characteristics of the 42 studies contributing data to this meta-analysis and their participants are presented in Table 1. Trials were conducted in 18 different countries on 5 continents, and enrolled participants of both sexes from birth to 95 years of age. Baseline serum 25(OH)D concentrations were determined in 34 of 42 trials: mean baseline 25(OH)D concentration ranged from 18.9 to 90.9 nmol/L (to convert to ng/ml, divide by 2.496). Forty-one studies administered oral vitamin D3 to participants in the intervention arm, while 1 study administered oral 25(OH)D. Vitamin D was given as monthly to 3-monthly bolus doses in 13 studies; as weekly doses in 6 studies; as daily doses in 21 studies; and as a combination of bolus and daily doses in 2 studies. Trial duration ranged from 8 weeks to 5 years. Incidence of ARI was primary or co-primary outcome for 22 studies, and a secondary outcome for 20 studies.
Table 1:
Characteristics of the 42 eligible trials and their participants
| Study first author, year | Setting | Participants | Mean age, years (s.d.) [range] | Male: Female | 25(OH)D assay, EQA scheme | Mean baseline 25(OH)D, nmol/L (s.d.) | Baseline 25(OH)D <25 nmol/L (%) | Mean attained 25(OH)D, intervention arm, nmo/L (s.d.) | Intervention: Control (total) | Oral dose of vitamin D3, intervention arm | Control | Trial duration | ARI definition | ARI primary or secondary outcome? | N contributing data / N randomised (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Li-Ng 20095 | USA | Healthy adults | 57.9 (13.6) [21.4 −80.6] | 34:128 | RIA (DiaSorin), DEQAS | 63.7 (25.5) | 3/150 (2.0) | 88.5 (23.2) | 84:78 (162) | 50 μg daily | Placebo | 3 mo | URI: ≥2 URI symptoms in absence of allergy symptoms | Primary | 157/162 (96.9) |
| Urashima 20106 | Japan | Schoolchildren | 10.2 (2.3) [6.0 – 15.0] | 242:188 | Not determined | Not determined | Not determined | Not determined | 217:213 (430) | 30 μg daily | Placebo | 4 mo | URI: influenza A/B diagnosed by RIDT or RIDT-negative ILI | Primary | 334/430 (77.7) |
| Manaseki-Holland 20107 | Afghanistan | Pre-school children with pneumonia | 1.1 (0.8) [0.1 – 3.3] | 257:196 | Not determined | Not determined | Not determined | Not determined | 224:229 (453) | 2.5 mg bolus once | Placebo | 3 mo | LRI: repeat episode of pneumonia – age-specific tachypnoea without wheeze | Secondary | 453/453 (100.0) |
| Laaksi 20108 | Finland | Military conscripts | 19.1 (0.6) [18.0 – 21.0] | 164:0 | EIA (IDS OCTEIA) | 75.9 (18.7) | 0/73 (0.0) | 71.6 (22.9) | 80:84 (164) | 10 μg daily | Placebo | 6 mo | ARI: medical record diagnosis | Primary | 164/164 (100.0) |
| Majak 20119 | Poland | Children with asthma | 10.9 (3.3) [6.0 – 17.0] | 32:16 | RIA (BioSource Europe), RIQAS | 88.9 (38.2) | 0/48 (0.0) | 37.6 (13.1) | 24:24 (48) | 12.5 μg daily | Placebo | 6 mo | ARI: self-report | Secondary | 48/48 (100.0) |
| Trilok-Kumar 201110 | India | Low birthweight infants | 0.1 (0.0) [0.0 – 0.3] | 970:1109 | -- | Not determined | Not determined | 55.0 (22.5) | 1039:1040 (2079) | 35 μg weekly | Placebo | 6 mo | ARI: medical record diagnosis of events causing hospitalisation | Secondary | 2064/2079 (99.3) |
| Lehouck 201211 | Belgium | Adults with COPD | 67.9 (8.3) [48.0 – 86.0] | 145:37 | RIA (Diasorin), DEQAS | 49.8 (29.2) | 31/182 (17.0) | 130.0 (44.7) | 91:91 (182) | 2.5 mg bolus monthly | Placebo | 1 yr | URI: self-report | Secondary | 175/182 (96.2) |
| Manaseki-Holland 201212 | Afghanistan | Infants | 0.5 (0.3) [0.0 – 1.0] | 1591:1455 | -- | Not determined | Not determined | 32.7 (17.1) | 1524:1522 (3046) | 2.5 mg bolus 3-monthly | Placebo | 1.5 yr | LRI: pneumonia confirmed by chest radiograph | Primary | 3011/3046 (98.9) |
| Camargo 201213 | Mongolia | 3rd/4th grade schoolchildren | 10.0 (0.9) [7.0 – 12.7] | 129:118 | LC-MS/MS, DEQAS | 18.9 (9.7) | 192/245 (78.4) | 49.1 (15.1) | 143:104 (247) | 7.5 μg daily | Placebo | 7 wk | ARI: parent-reported ‘chest infections or colds’ | Secondary | 244/247 (98.8) |
| Murdoch 201214 | New Zealand | Healthy adults | 48.1 (9.7) [18.0 – 67.6] | 81:241 | LC-MS/MS, DEQAS | 72.1 (22.1) | 5/322 (1.6) | 123.6 (27.5) | 161:161 (322) | 2 × 5 mg bolus monthly then 2.5 mg bolus monthly | Placebo | 1.5 yr | URI: assessed with symptom score | Primary | 322/322 (100.0) |
| Bergman 201215 | Sweden | Adults with increased susceptibility to ARI | 53.1 (13.1) [20.0 – 77.0] | 38:102 | CLA (DiaSorin), DEQAS | 49.3 (23.2) | 15/131 (11.45) | 94.9 (38.1) | 70:70 (140) | 100 μg daily | Placebo | 1 yr | URI: assessed with symptom score | Secondary | 124/140 (88.6) |
| Marchisio 201316 | Italy | Children with recurrent acute otitis media | 2.8 (1.0) [1.3 – 4.8] | 64:52 | CLA (DiaSorin), ISO9001 | 65.3 (17.3) | 2/116 (1.7) | 90.3 (21.1)] | 58:58 (116) | 25 μg daily | Placebo | 6 mo | URI: doctor-diagnosed acute otitis media | Primary | 116/116 (100.0) |
| Rees 201317 | USA | Adults with previous colorectal adenoma | 61.2 (6.6) [47.1 – 77.9] | 438:321[a] | RIA (IDS), DEQAS | 62.5 (21.3) | 0/759 (0.0) | 186.9 (455.1) | 399:360 (759) | 25 μg daily | Placebo | 13 mo (average) | URI: assessed from daily symptom diary | Secondary | 759/759 (100.0) |
| Tran 201418 | Australia | Healthy older adults | 71.7 (6.9) [60.3 – 85.2] | 343:301 | CLA (DiaSorin), DEQAS | 41.7 (13.5) | 66/643 (10.3) | 71.0 (19.6) | 430:214 (644) | 0.75 mg bolus vs. 1.5 mg bolus monthly | Placebo | 1 yr | URI: self-reported cold | Secondary | 594/644 (92.2) |
| Goodall 201419 | Canada | Healthy university students | 19.6 (2.2) [17.0 – 33.0] | 218:382 | Not determined | Not determined | Not determined | Not determined | 300:300 (600) | 0.25 mg weekly (factorial with gargling) | Placebo | 8 wk | URI: self-reported cold | Primary | 492/600 (82.0) |
| Urashima 201420 | Japan | High school students | 16.5 (1.0) [15.0 – 18.0] | 162:85 | Not determined | Not determined | Not determined | Not determined | 148:99 (247) | 50 μg daily | Placebo | 2 mo | URI: influenza A diagnosed by RIDT or RIDT-negative ILI | Primary | 247/247 (100.0) |
| Grant 201421 | New Zealand | Pregnant women and offspring | Offspring unborn at baseline | 0:260 (pregnant women) 121:128 (offspring) | LC-MS/MS, DEQAS | 54.8 (25.8) | 30/200 (15.0) | 92.9 (41.6) | 173:87 (pregnant women, 260) 164:85 (offspring, 249) | Pregnant women: 25 μg vs. 50 μg daily. Offspring: 10 μg vs. 20 μg daily | Placebo | 9 mo (3 mo in pregnancy + 6 mo in infancy) | ARI: doctor-diagnosed ARI precipitating primary care consult | Secondary | 236/260 (90.8) |
| Martineau 2015a22 [ViDiCO] | UK | Adults with COPD | 64.7 (8.5) [40.0 – 85.0] | 144:96 | LC-MS/MS, DEQAS | 46.1 (25.7) | 50/240 (20.8) | 67.3 (27.5) | 122:118 (240) | 3 mg bolus 2-monthly | Placebo | 1 yr | URI: assessed from daily symptom diary | Co-primary | 240/240 (100.0) |
| Martineau 2015b23 [ViDiAs] | UK | Adults with asthma | 47.9 (14.4) [16.0 – 78.0] | 109:141 | LC-MS/MS, DEQAS | 49.6 (24.7) | 36/250 (14.4) | 69.4 (21.0) | 125:125 (250) | 3 mg bolus 2-monthly | Placebo | 1 yr | URI: assessed from daily symptom diary | Co-primary | 250/250 (100.0) |
| Martineau 2015c24 [ViDiFlu] | UK | Older adults and their carers | 67.1 (13.0) [21.4 – 94.0] | 82:158 | LC-MS/MS, DEQAS | 42.9 (23.0) | 60/240 (25.0) | 84.8 (24.1) | 137:103 (240) | Older adults: 2.4 mg bolus 2-monthly + 10 μg daily Carers: 3 mg 2-monthly | Older adults: placebo + 10 μg daily Carers: placebo | 1 yr | URI & LRI, both assessed from daily symptom diary | Co-primary | 240/240 (100.0) |
| Simpson 201525 | Australia | Healthy adults | 32.2 (12.2) [18.0 – 52.0] | 14:20 | LC-MS/MS, DEQAS | 67.9 (23.0) | 0/33 (0.0) | Not determined | 18:16 (34) | 0.5 mg weekly | Placebo | 17 wk | ARI assessed with symptom score | Primary | 34/34 (100.0) |
| Dubnov-Raz 201526 | Israel | Adolescent swimmers with vitamin D insufficiency | 15.2 (1.6) [12.9 – 18.6] | 34:20 | RIA (DiaSorin), DEQAS | 60.4 (11.9) | 0/54 (0.0) | 73.7 (16.6) | 27:27 (54) | 50 μg daily | Placebo | 12 wk | URI assessed with symptom score | Primary | 25/54 (46.3) |
| Denlinger 201627 | USA | Adults with asthma | 39.2 (12.9) [18.0 – 85.0] | 130:278 | CLA (DiaSorin), VDSP | 47.0 (16.9) | 55/408 (13.5) | 104.3 (32.4) | 201:207 (408) | 2.5 mg bolus then 100 μg daily | Placebo | 28 wk | URI assessed with symptom score | Secondary | 408/408 (100.0) |
| Tachimoto 201628 | Japan | Children with asthma | 9.9 (2.3) [6.0 – 15.0] | 50:39 | RIA (DiaSorin), CAP | 74.9 (24.6) | 1/89 (1.1) | 85.7 (24.5) | 54:35 (89) | 20 μg daily, first 2 mo. | Placebo | 6 mo | URI: assessed with symptom score | Secondary | 89/89 (100.0) |
| Ginde, 201629 | USA | Institutionalised older adults | 80.7 (9.9) [60.0 – 95.0] | 45:62 | LC-MS/MS, VDSP | 57.3 (22.7) | 12/107 (11.2) | Not determined | 55:52 (107) | 2.5 mg bolus monthly + ≤25 μg per day equivalent | Placebo + 10–25 μg per day equivalent | 1 yr | ARI: medical record diagnosis | Primary | 107/107 (100.0) |
| Gupta 201631 | India | Children with pneumonia | 1.4 (1.1) [0.5 – 5.0] | 226:98 | RIA (Immunotech SAS/ DiaSorin) | 43.9 (33.4) | 104/312 (33.3) | 64.1 (43.9) | 162:162 (324) | 2.5 mg bolus, single dose | Placebo | 6 mo | Physician confirmed recurrent pneumonia | Co-primary | 314/324 (96.9) |
| Aglipay 201732 | Canada | Healthy children | 2.7 (1.5) [1.0 – 5.0] | 404:296 | CLA (Roche ELECSYS) | 90.9 (20.9) | 1/703 (0.1) | High dose: 121.6 (2.2); Low dose: 91.9 (1.7) | 349:354 | 50 μg daily | 10 μg daily | 4–8 mo (mean 6.3 mo) | URI: lab confirmed | Primary | 699/703 (99.4) |
| Arihiro 201833 | Japan | Adults with diagnosis of inflammatory bowel disease | 44.7 (1.3) [18.0 – 82.0] | 136:87 | RIA (Diasorin) | 58.6 (22.0) | 5/223 (2.2) | 80.4 (21.5) | 119:118 (237) | 12.5 μg daily | Placebo | 6 mo | Lab confirmed influenza | Primary | 223/237 (94.1) |
| Hibbs 201834 | USA | African American preterm infants | Offspring unborn at baseline | 166:133[b] | RIA | 55.4 (22.2) | 0/300 (0.0) | 95.0 (21.2) | 153:147 (300) | 10 μg daily, regardless of dietary intake | 10 μg daily, only if dietary intake was <5 μg daily | 1 yr | ARI: self-reported URI/LRI | Secondary | 300/300 (100.0) |
| Lee 201835 | USA | Children and young adults with sickle cell disease | 9.9 (3.9) [3.0 – 20.0] | 30:32 | LC-MS/MS, DEQAS | 35.7 (16.5) | 18/62 (29.0) | 92.4 (23.7) | 31:31 (62) | 2.5 mg bolus monthly | 0.3 mg monthly | 2 yrs | Self-reported respiratory events, including ARI | Primary | 62/62 (100.0) |
| Loeb 201836 | Vietnam | Healthy children and adolescents | 8.5 (4.0) [3.0 – 17.0] | 621:679 | CLA (DiaSorin), DEQAS | 65.5 (16.8) | 5/1153 (43.4) | 91.8 (23.6) | 650:650 (1300) | 0.35 mg weekly | Placebo | 8 mo | RT-PCR confirmed influenza A or B | Primary | 1153/1300 (88.7) |
| Rosendahl 201837 | Finland | Healthy infants | Offspring unborn at baseline | 495:492 | CLA (IDS-iSYS) VDSP | 81.5 (25.9) | 0/879 (0.0) | 117.7 (26.1) | 492:495 (987) | 30 μg daily | 10 μg daily | 2 yrs | Parent reported infections, including ARI | Co-primary | 897/987 (90.9) |
| Shimizu 201838 | Japan | Healthy adults | 52.7 (6.5) [45.0 – 74.0] | 66:149 | RIA (DiaSorin) | 48.9 (13.5) | 1/214 (0.5) | 114.6 (32.7) | 126:126 (252) | 10 μg daily (25[OH] D)[c] | Placebo | 4 mo | URI: self-reported | Primary | 215/252 (85.3) |
| Aloia 201939 | USA | Healthy African American women aged over 60 years | 69.0 (5.3) [65.4 – 72.5] | 0:260 | LC-MS/MS, NIST | 54.4 (16.7) | 9/258 (3.5) | 117.0 (28.0) | 130:130 (260) | 50 μg daily | Placebo | 3 mo | ARI: self-reported cold/flu | Secondary | 260/260 (100.0) |
| Hauger 201940 | Denmark | Healthy children | 6.6 (1.5) [4.0 – 8.0] | 61:69 | LC-MS/MS, DEQAS | 56.8 (12.5) | 0/118 (0.0) | 20 μg arm: 75.8 (11.5) 10 ug arm: 61.8 (10.6) | 43:44:43 (130) | 20 μg / 10 μg daily | Placebo | 5 mo | ARI: self-reported | Secondary | 118/130 (90.8) |
| Camargo 202041 | New Zealand | Older adults | 66.4 (8.3) [50.0 –84.0] | 2,935:2,121 | LC-MS/MS, DEQAS | 63.4 (23.6) | 89/5056 (1.8) | 135.0 (39.9) | 2558:2552 (5110) | 5 mg bolus loading dose; then 2.5 mg bolus monthly | Placebo | 3 yrs | ARI: self-reported cold/flu | Secondary | 5056/5110 (98.9) |
| Ganmaa, 202042 | Mongolia | Healthy school children | 9.4 (1.6) [6.0 – 13.0] | 4485:4366 | EIA (Biomerieux), DEQAS | 29.7 (10.5) | 2813/8851 (31.8) | 77.4 (22.7) | 4418:4433 (8851) | 0.35 mg weekly | Placebo | 3 yrs | ARI: self-reported | Secondary | 8851/8851 (100.0) |
| Mandlik 202043 | India | Healthy children | 8.1 (1.2) [6.0 – 12.0] | 158:127 | EIA (DLD diagnostics) | 58.9 (10.9) | 0/237 (0.0) | 80 (23.3) | 135:150 (285) | 25 μg daily + 500 mg calcium | Placebo | 6 mo | URI: self-reported | Secondary | 244/285 (85.6) |
| Pham 202044 | Australia | Older adults | 69.3 (5.5) [60.0 – 86.0] | 8678:7322 | LC-MS/MS, VDSP | Not determined | Not determined | 114.8 (30.3)[d] | 8000:8000 (16000) | 1.5 mg bolus monthly | Placebo | 5 yrs | ARI: self-reported | Secondary | 16,000/16,000 (100.0) |
| Rake 202045 | England | Healthy older adults | 72.2 (4.9) [65.0 – 84.0] | 408:379 | CLA (Cobas 6000 Roche) | 50.2 (27.1) | 127/787 (16.1) | 109.2 (33.9) | 395:392 (787) | 2.5 mg bolus monthly | Placebo | 2 yrs | URI/LRI: GP recorded | Secondary | 787/787 (100.0) |
| Golan-Tripto, unpublished46 | Israel | Prematurely born infants | 0 (0) | 21:29 | CLA (DiaSorin) | 33.6 (29.7) | 19/46 (41.3) | 20 μg arm: 78.0 (75.0) 10 ug arm: 81.0 (73.0) | 25:25 (50) | 20 μg daily | 10 μg daily | 1 yr | ARI: GP recorded | Secondary | 25/50 (50.0) |
| Reyes, unpublished47 | Chile | Healthy pre-school children | 2.2 (0.5) [1.3 – 3.3] | 168:135 | LC-MS/MS | 62.2 (15.5) | 1/194 (0.5) | 0.14 mg arm: 82.4 (24.5) 0.28 mg arm: 104.6 (52.9) | 99:103:101 (303) | 0.14 mg / 0.28 mg weekly | Placebo | 6 mo | ARI: self-reported | Primary | 194/303 (64.0) |
Sex missing for two participants randomised to intervention arm and subsequently excluded from analysis due to lack of outcome data.
Sex missing for one participant.
equivalent to 30 ug vitamin D3.60 1 μg vitamin D3 = 40 international units (IU); 25(OH)D concentrations reported in ng/ml were converted to nmol/L by multiplying by 2.496.
from subset of participants randomised to intervention; for comparison, mean 25(OH)D at follow-up in subset of participants randomised to placebo was 77.5 nmol/L (sd 25.2 nmol/L); 25(OH)D, 25-hydroxyvitamin D; RIDT, rapid influenza diagnostic test; COPD, chronic obstructive pulmonary disease; D3, vitamin D3 (cholecalciferol); p.o., per os (orally); mo, month; yr, year; wk, week. ARI, acute respiratory infection; CAP, College of American Pathologists, CLA, chemiluminescent assay; DEQAS, Vitamin D External Quality Assessment Scheme; EIA, enzyme immunoassay; EQA, external quality assessment; GP, general practitioner; LC-MS/MS, liquid chromatography tandem-mass spectrometry, RIA, radio-immunoassay; URI, upper respiratory infection; LRI, lower respiratory infection; ILI, influenza-like illness; RIQAS, Randox International Quality Assessment Scheme; VDSP, Vitamin D Standardisation Program of the Office of Dietary Supplements, National Institutes of Health, USA
Risk of Bias Within Studies
Details of the risk of bias assessment are provided in supplementary Table S2. Four trials were assessed as being at unclear risk of bias due to high loss to follow-up. In the trial by Laaksi and colleagues,8 37% of randomised participants were lost to follow-up. In the trial by Dubnov-Raz and colleagues,26 52% of participants did not complete all symptom questionnaires. In the unpublished trial by Reyes and colleagues,47 loss to follow-up ranged from 33% to 37% across the three study arms, and in the unpublished trial by Golan-Tripto and colleagues,46 50% of participants were lost to follow-up. All other trials were assessed as being at low risk of bias for all seven aspects assessed.
Overall Results, Primary Outcome
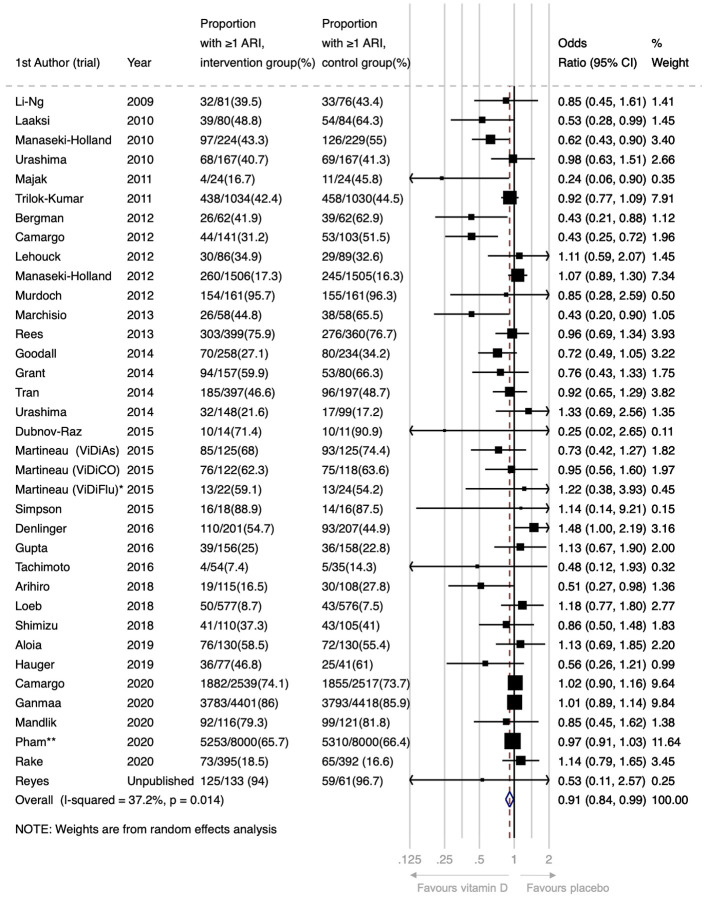
For the primary comparison of vitamin D vs. placebo control, supplementation resulted in a statistically significant reduction in the proportion of participants experiencing at least one ARI (Odds Ratio [OR] 0.91, 95% Confidence Interval [CI] 0.84 to 0.99; 44,009 participants in 36 studies; Figure 2, Table 2; Cates Plot, Figure S1). Heterogeneity of effect was moderate (I2 37.2%, P for heterogeneity 0.01).
Figure 2:
Forest plot of placebo-controlled RCTs reporting proportion of participants experiencing 1 or more acute respiratory infection.
*This analysis includes data from the subset of ViDiFlu trial participants who were randomised to vitamin D vs. placebo control. **For this trial, participants were asked to report the occurrence of ARI during the one month prior to completing each annual urvey (max surveys=5). The numerator is the number of participants who reported an ARI on at least one survey. The ARI outcomes for participants who completed fewer than 5 surveys and who did not report an ARI (N=2239; 14%) were estimated based on the % affected among those who completed all 5 surveys (N=12152; 76%).
Table 2: Placebo controlled RCTs:
Proportion of participants experiencing at least one acute respiratory infection, overall and stratified by potential effect-modifiers
| Potential effect-modifier | No of trials | Proportion with ≥1 ARI, intervention group (%) | Proportion with ≥1 ARI, control group (%) | Odds ratio (95% CI) | I2 % | P for heterogeneity |
|---|---|---|---|---|---|---|
| Overall | 36 | 13685/22288 (61.4) | 13565/21721 (62.5) | 0.91 (0.84 to 0.99) | 37.2 | 0.01 |
| Baseline 25(OH)D, nmol/L[a] | ||||||
| <25 | 19 | 1348/1798 (75.0) | 1388/1819 (76.3) | 0.78 (0.53 to 1.16) | 47.2 | 0.01 |
| 25 – 49.9 | 28 | 3439/4666 (73.7) | 3347/4501 (74.4) | 1.03 (0.92 to 1.15) | 0.4 | 0.46 |
| 50 – 74.9 | 29 | 1679/2839 (59.1) | 1565/2578 (60.7) | 0.90 (0.76 to 1.06) | 11.2 | 0.29 |
| ≥75 | 25 | 945/1543 (61.2) | 908/1471 (61.7) | 0.97 (0.81 to 1.16) | 0.0 | 0.78 |
| Dosing frequency | ||||||
| Daily | 18 | 1056/2134 (49.5) | 1020/1871 (54.5) | 0.75 (0.61 to 0.93) | 52.5 | 0.005 |
| Weekly | 6 | 4482/6421 (69.8) | 4447/6335 (70.2) | 0.97 (0.88 to 1.06) | 0.0 | 0.48 |
| Monthly or less frequently | 12 | 8147/13733 (59.3) | 8098/13515 (59.9) | 0.98 (0.93 to 1.03) | 0.0 | 0.57 |
| Daily dose equivalent, IU[b] | ||||||
| <400 | 2 | 482/1175 (41.0) | 511/1133 (45.1) | 0.65 (0.31 to 1.37) | 86.3 | 0.007 |
| 400–1000 | 10 | 656/1236 (53.1) | 627/1069 (58.7) | 0.70 (0.55 to 0.89) | 31.2 | 0.16 |
| 1001–2000 | 15 | 9946/15885 (62.6) | 10022/15817 (63.4) | 0.97 (0.92 to 1.03) | 1.0 | 0.44 |
| >2000 | 7 | 2291/3462 (66.2) | 2250/3444 (65.3) | 1.05 (0.84 to 1.31) | 37.1 | 0.15 |
| Trial duration, months | ||||||
| ≤12 | 29 | 1977/4887 (40.5) | 1866/4368 (42.7) | 0.82 (0.72 to 0.93) | 38.1 | 0.02 |
| >12 | 7 | 11708/17401 (67.3) | 11699/17353 (67.4) | 0.99 (0.95 to 1.04) | 0.0 | 0.91 |
| Age, yrs[a] | ||||||
| <1 | 5 | 875/2901 (30.2) | 839/2796 (30.0) | 0.95 (0.82 to 1.10) | 18.7 | 0.30 |
| 1–15.9 | 15 | 4297/5994 (71.7) | 4303/5877 (73.2) | 0.71 (0.57 to 0.90) | 46.0 | 0.03 |
| 16–64.9 | 21 | 3137/4876 (64.3) | 3087/4727 (65.3) | 0.97 (0.93 to 1.09) | 11.5 | 0.31 |
| ≥65 | 16 | 5376/8589 (62.6) | 5352/8394 (63.4) | 0.96 (0.90 to 1.02) | 0.0 | 0.67 |
| Airway disease | ||||||
| Asthma only | 4 | 203/404 (50.2) | 202/391 (51.7) | 0.73 (0.36 to 1.49) | 71.7 | 0.01 |
| COPD only | 2 | 106/208 (51.0) | 104/207 (50.2) | 1.01 (0.68 to 1.51) | 0.0 | 0.71 |
| Unrestricted | 30 | 13376/21676 (61.7) | 13259/21123 (62.8) | 0.91 (0.84 to 0.99) | 35.0 | 0.03 |
The number of trials in each category for this variable adds up to more than 36, since this is a participant-level variable, i.e. some trials contributed data from participants who fell into more than one category
For the secondary comparison of higher- vs. lower-dose vitamin D, we observed no statistically significant difference in the proportion of participants with at least one ARI (OR 0.87, 95% CI 0.73 to 1.04; 3,047 participants in 11 studies; I2 0.0%, P for heterogeneity 0.50; Figure S2).
Sub-group analyses, Primary Outcome
To investigate reasons for the observed heterogeneity of effect for the primary comparison of vitamin D vs. placebo control, we stratified this analysis by two participant-level factors (baseline vitamin D status and age) and by four trial-level factors (dose frequency, dose size, trial duration, and airway disease comorbidity). Results are presented in Table 2 and Figures S3–S8. No statistically significant effect of vitamin D was seen for participants with baseline 25(OH)D <25 nmol/L (OR 0.78, 95% CI 0.53 to 1.16; 3,617 participants in 19 studies), 25–49.9 nmol/L (OR 1.03, 95% CI 0.92 to 1.15; 9,167 participants in 28 studies), 50–74.9 nmol (OR 0.90, 95% CI 0.76 to 1.06; 5,417 participants in 29 studies), or ≥75 nmol/L (OR 0.97, 95% CI 0.81 to 1.16; 3,014 participants in 25 studies; Figure S3). A statistically significant protective effect of vitamin D was seen for participants aged 1–15.9 years (OR 0.71, 95% CI 0.57 to 0.90; 11,871 participants in 15 studies), but not in participants aged <1 year (OR 0.95, 95% CI 0.82 to 1.10; 5,697 participants in 5 studies), 16–64.99 years (OR 0.97, 95% CI 0.93 to 1.09; 9,603 participants in 21 studies), or ≥65 years (OR 0.96, 95% CI 0.90 to 1.02; 16,983 participants in 16 studies; Figure S7).
With regard to dosing frequency, a statistically significant protective effect was seen for trials where vitamin D was given daily (OR 0.75, 95% CI 0.61 to 0.93; 4,005 participants in 18 studies), but not for trials in which it was given weekly (OR 0.97, 95% CI 0.88 to 1.06; 12,756 participants in 6 studies), or monthly to 3-monthly (OR 0.98, 95% CI 0.93 to 1.03; 27,248 participants in 12 studies; Figure S4). Statistically significant protective effects of the intervention were also seen in trials where vitamin D was administered at daily equivalent doses of 400–1000 IU (OR 0.70, 95% CI 0.55 to 0.89; 2,305 participants in 10 studies), but not where the daily dose equivalent was <400 IU (OR 0.65, 95% CI 0.31 to 1.37; 2,308 participants in 2 studies), 1001–2000 IU (OR 0.97, 95% CI 0.92 to 1.03; 31,702 participants in 15 studies), or >2000 IU (OR 1.05, 95% CI 0.84 to 1.31; 6,906 participants in 7 studies; Figure S5). Statistically significant protective effects were also seen for trials with a duration of ≤12 months (OR 0.82, 95% CI 0.72 to 0.93; 9,255 participants in 29 studies) but not in those lasting >12 months (OR 0.99, 95% CI 0.95 to 1.04; 34,754 participants in 7 studies; Figure S6).
Finally, statistically significant protective effects were also seen for trials that were not restricted to participants with asthma or COPD (OR 0.91, 95% CI 0.84 to 0.99; 42,799 participants in 30 studies), but not in trials that exclusively enrolled participants with asthma (OR 0.73, 95% CI 0.36 to 1.49; 795 participants in 4 studies), or COPD (OR 1.01, 95% 0.68 to 1.51; 415 participants in 2 studies; Figure S8).
An exploratory analysis restricted to eight placebo-controlled trials investigating effects of daily dosing at doses of 400–1000 IU/day with duration ≤12 months (for which mean baseline 25(OH)D level ranged from 54.8 nmol/L to 88.9 nmol/L) showed a statistically significant reduction in the proportion of participants experiencing at least one ARI (OR 0.58, 95% CI 0.45 to 0.75; 1,232 participants in 8 studies; Figure S9; Cates Plot, Figure S1). Heterogeneity of effect for this exploratory analysis was low (I2 0.0%, P for heterogeneity 0.67).
Multivariable Meta-Regression Analysis
Multivariable meta-regression analysis of trial-level sub-groups did not identify a statistically significant interaction between allocation to vitamin D vs. placebo and dose frequency, size or trial duration (Table S3).
Secondary outcomes
Meta-analysis of secondary outcomes was performed for results of placebo-controlled trials only; results are presented in Table 3. Overall, without consideration of participant- or trial-level factors, vitamin D supplementation did not have a statistically significant effect on the proportion of participants with one or more URI, LRI, courses of antimicrobials for ARI, work/school absences due to ARI, hospitalisations or emergency department attendances for ARI, serious adverse events of any cause, death due to ARI or respiratory failure, death due to any cause, or episodes of hypercalcaemia or renal stones.
Table 3: Placebo-controlled studies:
Secondary outcomes
| Variables | No of trials | Proportion with ≥1 event, intervention group (%) | Proportion with ≥1 event, control group (%) | Odds ratio (95% CI) | I2 % | P for heterogeneity |
|---|---|---|---|---|---|---|
| Efficacy outcomes | ||||||
| Upper respiratory infection* | 28 | 7931/13493 (58.8) | 7823/13034 (60.0) | 0.96 (0.90 to 1.02) | 4.4 | 0.40 |
| Lower respiratory infection* | 14 | 3583/12167 (29.4) | 3601/12027 (29.9) | 0.98 (0.93 to 1.04) | 0.0 | 0.56 |
| Emergency department attendance and/or hospital admission due to ARI | 18 | 117/9887 (1.2) | 125/9769 (1.3) | 0.90 (0.70 to 1.16) | 0.0 | 1.00 |
| Death due to ARI or respiratory failure | 33 | 14/13612 (0.1) | 11/13058 (0.1) | 1.04 (0.61 to 1.78) | 0.0 | 1.00 |
| Use of antibiotics to treat an ARI* | 13 | 2035/7562 (26.9) | 2082/7423 (28.0) | 0.91 (0.82 to 1.02) | 13.4 | 0.31 |
| Absence from work or school due to ARI | 10 | 378/1527 (24.7) | 364/1044 (34.9) | 0.91 (0.69 to 1.20) | 35.3 | 0.13 |
| Safety outcomes | ||||||
| Serious adverse event of any cause* | 35 | 545/13861 (3.9) | 554/13326 (4.2) | 0.97 (0.86 to 1.09) | 0.0 | 1.00 |
| Death due to any cause | 34 | 117/13854 (0.8) | 97/13293 (0.7) | 1.15 (0.90 to 1.49) | 0.0 | 1.00 |
| Hypercalcaemia | 21 | 50/9294 (0.5) | 39/8919 (0.4) | 1.20 (0.81 to 1.79) | 0.0 | 1.00 |
| Renal stones | 20 | 110/11540 (1.0) | 128/11138 (1.1) | 0.85 (0.66 to 1.10) | 0.0 | 1.00 |
This analysis includes a subset of participants in the trial by Pham et al, who completed symptom diaries.
Risk of bias across studies
A funnel plot for the proportion of participants experiencing at least one ARI (Figure S10) showed left-sided asymmetry, confirmed with an Egger’s regression test57 (P=0.008). This might reflect heterogeneity of effect across trials, or publication bias arising from omission of small trials showing non-protective effects of vitamin D from the meta-analysis.58 Given the latter possibility, the quality of the body of evidence contributing to analyses of the primary efficacy outcome and major secondary outcomes was downgraded to moderate (Table S4).
Sensitivity Analyses
Results of exploratory sensitivity analyses are presented in Table S5. Meta-analysis of the proportion of participants in placebo-controlled trials experiencing at least one ARI, excluding 4 studies assessed as being at unclear risk of bias,8,26,46,47 revealed protective effects of vitamin D supplementation consistent with the main analysis (OR 0.93, 95% CI 0.86 to 1.00; 43,626 participants in 33 studies). Sensitivity analysis for the same outcome, excluding 18 placebo-controlled trials that investigated ARI as a secondary outcome, did not show a statistically significant protective effect (OR 0.89, 95% CI 0.77 to 1.03; 7,537 participants in 18 studies). A sensitivity analysis for the same outcome, substituting diary-defined ARI events (available for 2598 participants) for survey-defined ARI events (available for n=16,000 participants) in the trial by Pham et al44 revealed protective effects of vitamin D supplementation consistent with the main analysis (OR 0.90, 95% CI 0.83 to 0.99; 30,607 participants in 36 studies).
Discussion
This updated meta-analysis of RCTs of vitamin D supplementation for the prevention of ARI includes data from an additional 17 studies completed since December 2015, when we performed the final literature search for our prior individual participant data meta-analysis.30 For expediency during the COVID-19 pandemic, we used a trial-level approach for this update, which includes data from a total of 46,331 participants in 42 trials. Overall, we report a modest statistically significant protective effect of vitamin D supplementation, as compared with placebo (OR 0.91, 95% CI 0.84 to 0.99). As expected, there was significant heterogeneity (P=0.01) across trials, which might have led to an under-estimate of the protective effect, and contributed to the asymmetry observed in the funnel plot.58 Alternatively, left-sided asymmetry in the funnel plot may reflect publication bias, which might have led to an over-estimate of the protective effect. In contrast to findings of our previous meta-analysis,30 we did not observed enhanced protection in those with the lowest 25(OH)D levels at baseline. However, there was evidence that efficacy of vitamin D supplementation varied according to dosing regimen and trial duration, with protective effects associated with daily administration of doses of 400–1000 IU vitamin D given for ≤12 months. An exploratory analysis restricted to data from 8 trials fulfilling these design criteria revealed a larger protective effect (OR 0.58, 95% CI 0.45 to 0.75) without significant heterogeneity across trials (P for heterogeneity 0.67).
The magnitude of the overall protective effect seen in the current analysis (OR 0.91, 95% CI 0.84 to 0.99) is modest, and similar to the value reported in our previous meta-analysis of individual participant data (adjusted OR 0.88, 95% CI 0.81 to 0.96).30 In keeping with our previous study, the point estimate for this effect was lower among those with baseline 25(OH)D <25 nmol/L than in those with higher baseline vitamin D status. However, in contrast to our previous finding, a statistically significant protective effect of vitamin D was not seen in those with the lowest 25(OH)D concentrations. This difference reflects the inclusion of null data from four new RCTs in which vitamin D was given in relatively high doses at weekly or monthly intervals over 2–5 years.41,42,44,45 Null results of these studies contrast with protective effects reported from earlier trials in which smaller daily doses of vitamin D were given over shorter periods.8,9,13,16 These differing findings suggest that the frequency, amount and duration of vitamin D supplementation may be key determinants of its protective efficacy. In keeping with this hypothesis, statistically significant protective effects of vitamin D were seen for meta-analysis of trials where vitamin D was given daily; where it was given at doses of 400–1000 IU/day; and where it was given for 12 months or less. When results of trials that investigated daily administration of 400–1000 IU over ≤12 months were pooled in an exploratory meta-analysis, a protective effect was seen (OR 0.58, 95% CI 0.45 to 0.75) with low heterogeneity (I2 0.0%, P for heterogeneity 0.67). Greater protective efficacy of lower vs higher doses of vitamin D might reflect deleterious effects of higher-dose vitamin D on its own metabolism, or on host responses to respiratory pathogens: head-to-head mechanistic studies in individuals randomised to different regimens of vitamin D supplementation are needed to investigate this issue.
The current study has several strengths: it contains the very latest RCT data available in this fast-moving field, including findings from three large phase 3 trials published in 202041,42,44 as well as some as-yet unpublished studies.46,47 The inclusion of additional studies allowed us to analyse results of placebo-controlled studies vs. high-dose / low-dose studies separately, and gave us the power to investigate reasons for heterogeneity of effect observed across trials. For example, we could distinguish the effects of daily vs. weekly dosing, which were previously pooled.30
Our work also has limitations. Given the need to generate a rapid update of our previous work in the context of the COVID-19 pandemic, we meta-analysed aggregate (trial-level) data, rather than individual participant data; this allowed us to proceed rapidly, without the delays introduced by the need to establish multiple data sharing agreements. However, we did contact authors to get unpublished estimates of effect that were stratified by pre-defined baseline 25(OH)D levels, harmonised across studies: thus, we were able to provide accurate data for the major participant-level effect-modifier of interest. Despite the large number of trials overall, relatively few compared effects of lower- vs. higher-dose vitamin D: our power for this secondary comparison was therefore limited. We lacked the individual participant data to investigate race/ethnicity and obesity as potential effect-modifiers. We also could not account for other factors that might influence the efficacy of vitamin D supplements for ARI prevention (e.g., taking the supplement with or without food) or secular trends that would influence trials, such as the increased societal use of vitamin D supplements;59 concurrent use of standard dose vitamin D supplements or multivitamins in the “placebo” group would effectively render these as high- vs. low-dose trials and potentially drive results toward the null. A final limitation relates to the funnel plot, which suggests that the overall effect size may have been over-estimated due to publication bias; we have mitigated this by inclusion of data from unpublished studies identified by searching clinicaltrials.gov where this was obtainable.
In summary, this updated meta-analysis of data from RCTs of vitamin D for the prevention of ARI showed a statistically significant overall protective effect of the intervention. The protective effect was heterogenous across trials; it also may have been over-estimated due to publication bias. In contrast to findings of our previous meta-analysis of individual participant data, we did not see a protective effect of vitamin D supplementation among those with the lowest baseline vitamin D status. The vitamin D dosing regimen of most benefit was daily and used standard doses (e.g., 400 to 1000 IU) for up to 12 months. The relevance of these findings to COVID-19 is not known and requires investigation.
Supplementary Material
Research in context.
Evidence before this study
The active vitamin D metabolite, 1,25-dihydroxyvitamin D, induces innate immune responses to respiratory viruses and bacteria. A previous meta-analysis of individual participant data from 10,933 participants in 25 randomised controlled trials of vitamin D supplementation for the prevention of acute respiratory infection demonstrated an overall protective effect (adjusted Odds Ratio [aOR] 0.88, 95% confidence interval 0.81 to 0.96). Sub-group analysis revealed most benefit in those with the lowest vitamin D status at baseline who received daily or weekly supplementation (aOR 0.30, 0.17 to 0.53).
Added value of this study
Our meta-analysis of aggregate data from 46,331 participants in 42 randomised controlled trials, stratified by baseline 25(OH)D concentration, provides an updated estimate of the protective effects of vitamin D against acute respiratory infection overall, and in sub-groups defined by baseline vitamin D status and dosing frequency, amount and duration.
Implications of all the available evidence
Overall, vitamin D reduced the risk of having one or more acute respiratory infections (OR 0.91, 0.84 to 0.99), but there was evidence of significant heterogeneity across trials (P for heterogeneity 0.01). A funnel plot showed left-sided asymmetry, which may reflect publication bias and/or heterogeneity of effect across trials. No statistically significant effect of vitamin D was seen for any of the sub-groups defined by baseline 25(OH)D concentration. However, protective effects were seen in trials where vitamin D was given using a daily dosing regimen (OR 0.75, 0.61 to 0.93); at daily dose equivalents of 400–1000 IU (OR 0.70, 0.55 to 0.89); and for a duration of ≤12 months (OR 0.82, 0.72 to 0.93). The relevance of these findings to COVID-19 is not known and requires investigation.
Acknowledgements
This study was conducted without external funding. DAJ is supported by a Barts Charity Lectureship (ref MGU045). ARM is supported by the United Kingdom Office for Students. The views expressed are those of the authors and not necessarily those of Barts Charity or the Office for Students. Sources of support for individual trials are detailed in Supplementary Material. We thank all the people who participated in primary randomised controlled trials, and the teams who conducted them.
Funding: None
Transparency Declaration
DAJ and ARM are the manuscript’s guarantors and they affirm that this is an honest, accurate, and transparent account of the study being reported and that no important aspects of the study have been omitted. All analyses were pre-specified in the study protocol, other than the exploratory analyses whose results are presented in Table 2 (sub-group analyses by age and presence of asthma/COPD, requested by reviewers), Table S5 and Figure S7.
Footnotes
Competing Interests
All authors have completed the ICMJE uniform disclosure form. No author has had any financial relationship with any organisations that might have an interest in the submitted work in the previous three years. No author has had any other relationship, or undertaken any activity, that could appear to have influenced the submitted work.
Data Sharing: the study dataset is available from d.a.jolliffe@qmul.ac.uk.
References
- 1.Lanham-New SA, Webb AR, Cashman KD, et al. Vitamin D and SARS-CoV-2 virus/COVID-19 disease. BMJ Nutrition, Prevention & Health 2020. [DOI] [PMC free article] [PubMed]
- 2.Greiller CL, Martineau AR. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015; 7(6): 4240–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolliffe DA, Griffiths CJ, Martineau AR. Vitamin D in the prevention of acute respiratory infection: systematic review of clinical studies. J Steroid Biochem Mol Biol 2013; 136: 321–9. [DOI] [PubMed] [Google Scholar]
- 4.Pham H, Rahman A, Majidi A, Waterhouse M, Neale RE. Acute Respiratory Tract Infection and 25-Hydroxyvitamin D Concentration: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health 2019; 16(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li-Ng M, Aloia JF, Pollack S, et al. A randomized controlled trial of vitamin D3 supplementation for the prevention of symptomatic upper respiratory tract infections. Epidemiol Infect 2009; 137(10): 1396–404. [DOI] [PubMed] [Google Scholar]
- 6.Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in schoolchildren. Am J Clin Nutr 2010; 91(5): 1255–60. [DOI] [PubMed] [Google Scholar]
- 7.Manaseki-Holland S, Qader G, Isaq Masher M, et al. Effects of vitamin D supplementation to children diagnosed with pneumonia in Kabul: a randomised controlled trial. Trop Med Int Health 2010; 15(10): 1148–55. [DOI] [PubMed] [Google Scholar]
- 8.Laaksi I, Ruohola JP, Mattila V, Auvinen A, Ylikomi T, Pihlajamaki H. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. J Infect Dis 2010; 202(5): 809–14. [DOI] [PubMed] [Google Scholar]
- 9.Majak P, Olszowiec-Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. J Allergy Clin Immunol 2011; 127(5): 1294–6. [DOI] [PubMed] [Google Scholar]
- 10.Trilok Kumar G, Sachdev HS, Chellani H, et al. Effect of weekly vitamin D supplements on mortality, morbidity, and growth of low birthweight term infants in India up to age 6 months: randomised controlled trial. BMJ 2011; 342: d2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehouck A, Mathieu C, Carremans C, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med 2012; 156(2): 105–14. [DOI] [PubMed] [Google Scholar]
- 12.Manaseki-Holland S, Maroof Z, Bruce J, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet 2012; 379(9824): 1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camargo CA Jr., Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012; 130(3): e561–7. [DOI] [PubMed] [Google Scholar]
- 14.Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA 2012; 308(13): 1333–9. [DOI] [PubMed] [Google Scholar]
- 15.Bergman P, Norlin AC, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open 2012; 2(6): e001663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marchisio P, Consonni D, Baggi E, et al. Vitamin D supplementation reduces the risk of acute otitis media in otitis-prone children. Pediatr Infect Dis J 2013; 32(10): 1055–60. [DOI] [PubMed] [Google Scholar]
- 17.Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 Supplementation and Upper Respiratory Tract Infections in a Randomized, Controlled Trial. Clin Infect Dis 2013. [DOI] [PMC free article] [PubMed]
- 18.Tran B, Armstrong BK, Ebeling PR, et al. Effect of vitamin D supplementation on antibiotic use: a randomized controlled trial. Am J Clin Nutr 2014; 99(1): 156–61. [DOI] [PubMed] [Google Scholar]
- 19.Goodall EC, Granados AC, Luinstra K, et al. Vitamin D3 and gargling for the prevention of upper respiratory tract infections: a randomized controlled trial. BMC Infectious Diseases 2014; 14: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urashima M, Mezawa H, Noya M, Camargo CA Jr. Effects of vitamin D supplements on influenza A illness during the 2009 H1N1 pandemic: a randomized controlled trial. Food & Function 2014; 5(9): 2365–70. [DOI] [PubMed] [Google Scholar]
- 21.Grant CC, Kaur S, Waymouth E, et al. Reduced primary care respiratory infection visits following pregnancy and infancy vitamin D supplementation: a randomised controlled trial. Acta Paediatr 2014. [DOI] [PubMed]
- 22.Martineau AR, James WY, Hooper RL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. The Lancet Respiratory Medicine 2015; 3(2): 120–30. [DOI] [PubMed] [Google Scholar]
- 23.Martineau AR, MacLaughlin BD, Hooper RL, et al. Double-blind randomised placebo-controlled trial of bolus-dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015; 70(5): 451–7. [DOI] [PubMed] [Google Scholar]
- 24.Martineau AR, Hanifa Y, Witt KD, et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015; 70(10): 953–60. [DOI] [PubMed] [Google Scholar]
- 25.Simpson SJ, van der Mei I, Stewart N, Blizzard L, Tettey P, Taylor B. Weekly cholecalciferol supplementation results in significant reductions in infection risk among the vitamin D deficient: results from the CIPRIS pilot RCT. BMC Nutrition 2015; 1(7). [Google Scholar]
- 26.Dubnov-Raz G, Rinat B, Hemila H, Choleva L, Cohen AH, Constantini NW. Vitamin D supplementation and upper respiratory tract infections in adolescent swimmers: a randomized controlled trial. Pediatric Exercise Science 2015; 27(1): 113–9. [DOI] [PubMed] [Google Scholar]
- 27.Denlinger LC, King TS, Cardet JC, et al. Vitamin D Supplementation and the Risk of Colds in Patients with Asthma. Am J Respir Crit Care Med 2016; 193(6): 634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved Control of Childhood Asthma with Low-Dose, Short-Term Vitamin D Supplementation: A Randomized, Double-Blind, Placebo-Controlled Trial. Allergy 2016. [DOI] [PubMed]
- 29.Ginde AA, Blatchford P, Breese K, et al. High-Dose Monthly Vitamin D for Prevention of Acute Respiratory Infection in Older Long-Term Care Residents: A Randomized Clinical Trial. J Am Geriatr Soc 2017; 65(3): 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 2017; 356: i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta P, Dewan P, Shah D, et al. Vitamin D Supplementation for Treatment and Prevention of Pneumonia in Under-five Children: A Randomized Double-blind Placebo Controlled Trial. Indian Pediatr 2016; 53(11): 967–76. [DOI] [PubMed] [Google Scholar]
- 32.Aglipay M, Birken CS, Parkin PC, et al. Effect of High-Dose vs Standard-Dose Wintertime Vitamin D Supplementation on Viral Upper Respiratory Tract Infections in Young Healthy Children. JAMA 2017; 318(3): 245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arihiro S, Nakashima A, Matsuoka M, et al. Randomized Trial of Vitamin D Supplementation to Prevent Seasonal Influenza and Upper Respiratory Infection in Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis 2019; 25(6): 1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hibbs AM, Ross K, Kerns LA, et al. Effect of Vitamin D Supplementation on Recurrent Wheezing in Black Infants Who Were Born Preterm: The D-Wheeze Randomized Clinical Trial. JAMA 2018; 319(20): 2086–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MT, Kattan M, Fennoy I, et al. Randomized phase 2 trial of monthly vitamin D to prevent respiratory complications in children with sickle cell disease. Blood Adv 2018; 2(9): 969–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loeb M, Dang AD, Thiem VD, et al. Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza Other Respir Viruses 2019; 13(2): 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosendahl J, Valkama S, Holmlund-Suila E, et al. Effect of Higher vs Standard Dosage of Vitamin D3 Supplementation on Bone Strength and Infection in Healthy Infants: A Randomized Clinical Trial. JAMA Pediatrics 2018; 172(7): 646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu Y, Ito Y, Yui K, Egawa K, Orimo H. Intake of 25-Hydroxyvitamin D3 Reduces Duration and Severity of Upper Respiratory Tract Infection: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Comparison Study. J Nutr Health Aging 2018; 22(4): 491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aloia JF, Islam S, Mikhail M. Vitamin D and Acute Respiratory Infections-The PODA Trial. Open Forum Infectious Diseases 2019; 6(9): ofz228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hauger H, Ritz C, Mortensen C, et al. Winter cholecalciferol supplementation at 55 degrees N has little effect on markers of innate immune defense in healthy children aged 4–8 years: a secondary analysis from a randomized controlled trial. European Journal of Nutrition 2019; 58(4): 1453–62. [DOI] [PubMed] [Google Scholar]
- 41.Camargo CA, Sluyter J, Stewart AW, et al. Effect of monthly high-dose vitamin D supplementation on acute respiratory infections in older adults: A randomized controlled trial. Clin Infect Dis 2020; 71: 311–7. [DOI] [PubMed] [Google Scholar]
- 42.Ganmaa D, Uyanga B, Zhou X, et al. Vitamin D Supplements and Prevention of Tuberculosis Infection and Disease. N Engl J Med 2020; 383: 359–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mandlik R, Mughal Z, Khadilkar A, et al. Occurrence of infections in schoolchildren subsequent to supplementation with vitamin D-calcium or zinc: a randomized, double-blind, placebo-controlled trial. Nutr Res Pract 2020; 14(2): 117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pham H, Waterhouse M, Baxter C, et al. The effect of vitamin D supplementation on acute respiratory tract infection in older Australian adults: an analysis of data from the D-Health Trial: a randomised controlled trial. The Lancet Diabetes & Endocrinology 2020; in press. [DOI] [PubMed]
- 45.Rake C, Gilham C, Bukasa L, et al. High-dose oral vitamin D supplementation and mortality in people aged 65–84 years: the VIDAL cluster feasibility RCT of open versus double-blind individual randomisation. Health Technol Assess 2020; 24(10): 1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Golan-Tripto I. The Effect of Vitamin D Administration to Premature Infants on Vitamin D Status and Respiratory Morbidity (NCT02404623). https://clinicaltrials.gov/ct2/show/NCT02404623. [DOI] [PubMed]
- 47.Reyes ML, Vizcaya C, Borzutzky A, et al. Study of Vitamin D for the Prevention of Acute Respiratory Infections in Children (NCT02046577). https://clinicaltrials.gov/ct2/show/NCT02046577.
- 48.Ducharme FM, Jensen M, Mailhot G, et al. Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial. Trials 2019; 20(1): 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreno A. Effect of Supplementation With Vitamin D on the Acute Bronchitis Prevention During the First Year of Life (NCT01875757). https://clinicaltrials.gov/ct2/show/NCT01875757.
- 50.Camargo CA. Effects of Vitamin D and Omega-3 Fatty Acids on Infectious Diseases and hCAP18 (VITAL Infection; NCT01758081).
- 51.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane 2019. [DOI] [PMC free article] [PubMed]
- 53.Harris R, Bradburn M, Deeks J, et al. METAN: Stata module for fixed and random effects meta-analysis, revised 23 Sep 2010. Statistical Software Components S456798. Boston College Department of Economics; 2006.
- 54.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. BMJ 2010; 340: b5664. [DOI] [PubMed] [Google Scholar]
- 55.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008; 61(10): 991–6. [DOI] [PubMed] [Google Scholar]
- 56.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336(7650): 924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315(7109): 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011; 343: d4002. [DOI] [PubMed] [Google Scholar]
- 59.Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. Trends in Dietary Supplement Use Among US Adults From 1999–2012. JAMA 2016; 316(14): 1464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jetter A, Egli A, Dawson-Hughes B, et al. Pharmacokinetics of oral vitamin D(3) and calcifediol. Bone 2014; 59: 14–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.