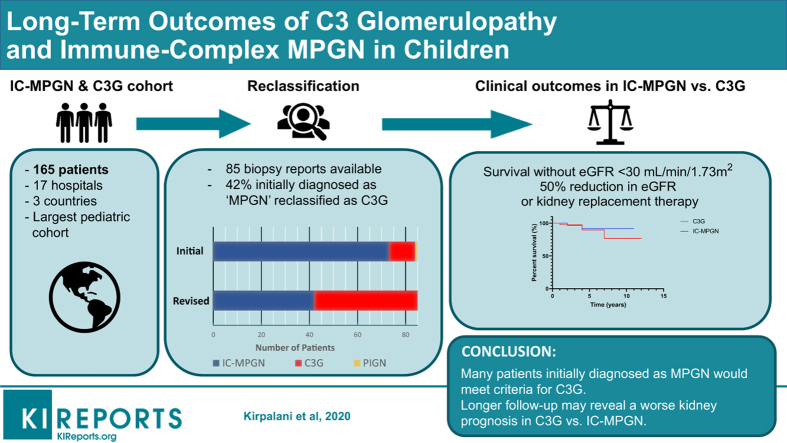
Abstract
Introduction
The reclassification of membranoproliferative glomerulonephritis (MPGN) into immune-complex MPGN (IC-MPGN) and C3 glomerulopathy (C3G) has provided insights into 2 distinct diseases. Although outcomes in adults are poor in both diseases, the pediatric literature is scarce and limited to small, single-center cohorts.
Methods
We conducted a retrospective analysis of 165 pediatric patients across 17 hospitals to compare outcomes between children with IC-MPGN and C3G.
Results
Forty-two percent of patients initially diagnosed with MPGN were reclassified as C3G after a review of renal biopsy reports. There was a trend toward higher serum creatinine levels in patients with C3G compared with IC-MPGN both at diagnosis (mean 168.9 [range 45.4–292.4] vs. 93.7 [range 70.7–116.6] μmol/l, P = 0.25) and after a mean follow-up time of 4 years (mean 145.0 (range −8.1 to 298.1) vs 99.1 (range 46.3–151.9) μmol/l, P = 0.47), although the estimated glomerular filtration rate (eGFR) was not significantly different. Steroid treatment was associated with a significant improvement in eGFR versus no steroids in C3G (mean +43.0 (range 12.9–73.0) vs. −3.0 (range −23.1 to 17.2) ml/min per 1.73 m2, P = 0.02) but not in IC-MPGN. Overall kidney function was preserved in both groups although hypertension remained prevalent in 42.5% of the cohort at the last follow-up, and the urine protein/creatinine ratio remained elevated (mean 253.8 [range 91.9–415.7] mg/mmol).
Conclusion
This large pediatric IC-MPGN/C3G cohort revealed nearly half of the patients were misclassified, and there may be a trend toward worse renal prognosis in C3G although they may have greater steroid responsiveness. The overall prognosis appears to be more favorable than in adults; however, persistent hypertension and proteinuria suggest suboptimal disease control.
Keywords: C3 glomerulonephritis, C3 glomerulopathy, dense deposit disease, hypertension, immune-complex membranoproliferative glomerulonephritis, proteinuria
Graphical abstract
MPGN has previously been used as an umbrella term to categorize a spectrum of hypocomplementemic glomerular diseases. More recently, the disease entity has been reclassified into 2 diseases: IC-MPGN and C3G.1 Despite the recent awareness of 2 distinct disease processes, these updated classification criteria left much to be understood about how best to differentiate these 2 diseases clinically in terms of diagnosis, optimal treatment, and prognosis. MPGN suggests a pattern of injury with characteristic mesangial cellularity and thickening of glomerular capillary walls due to subendothelial deposition of immune complexes or complement factors.2 Recent criteria have been suggested based on the predominance of C3, rather than Igs, on immunofluorescence in tissue with an otherwise MPGN-like pattern of glomerular disease to differentiate C3G from IC-MPGN.1
Beyond these histologic differences, different molecular mechanisms underlie the 2 disease processes, which may have diagnostic, therapeutic, and prognostic implications.3, 4, 5 Central to the pathophysiology of both diseases is activation of the body’s complement system.6,7
IC-MPGN has been thought to result from antigen-antibody immune complexes that activate the classical complement pathway in the setting of persistent antigenemia.8,9 In adults with IC-MPGN, the most common antigens identified result from infection, autoimmune disease, or monoclonal gammopathies.8 However, in children, antigenemia in IC-MPGN is most commonly idiopathic.6
In contrast, C3G is a disorder of primary alternative complement pathway dysregulation. The constitutive activation of the alternative complement cascade is due to impaired regulatory mechanisms and ultimately triggers the downstream activation of the terminal complement cascade and membrane attack complex.7 In children, the etiology is most commonly attributable to the formation of autoantibodies that protect C3 convertase from degradation (C3, C4, or C5 nephritic factors). Other causes include genetic mutations resulting in impaired function of alternative complement pathway regulators, with the remaining cases labeled as idiopathic (which may reflect genetic mutations not yet known).7,10,11 There are limited data on the epidemiology of these rare disorders, with estimates of 1 to 2 per 106 children 5 to 15 years of age.12
The management of IC-MPGN is still largely untargeted and centers on treatment of the underlying cause, if applicable, along with blockade of the renin-angiotensin-aldosterone system (RAS) in proteinuric patients and the use of corticosteroids and either oral cyclophosphamide or mycophenolate mofetil for the treatment of severe progressive IC-MPGN.13 Most data concerning outcomes in IC-MPGN come from adult literature and show that the disease invariably results in renal deterioration with most patients reaching end-stage kidney disease (ESKD) within a decade of diagnosis. Moreover, this poor prognosis has persisted without notable improvement in patient outcomes over the last several decades.14,15
Although the literature from adults has shown a poor renal prognosis in both disorders, the overall disease trajectory in children remains unclear. Because these diseases are even more rare in children, it is difficult to amass enough patients to directly compare the 2 entities, and the paucity of available literature to date has been only from case series or small cohorts with predominantly single-center experiences.3 As more targeted therapeutic options may be on the horizon, particularly with regard to modulation of the complement system, it is imperative to further elucidate the clinical course of these newly differentiated diseases in children using multicenter registries to better understand the natural histories of these 2 diseases and to inform potential future therapies. To help address this unmet need, this study aimed to expand our understanding of the demographic, clinical, and laboratory differences between IC-MPGN and C3G in children using data from the largest pediatric IC-MPGN/C3G registry, which includes patient data from multiple geographically diverse medical centers in 3 countries.
Materials and Methods
Setting and Participants
We built the largest known pediatric international registry of patients with IC-MPGN and C3G to date, with the support of investigators at 17 pediatric centers across 3 countries (Canada, the United States, and Australia) through the Pediatric Nephrology Research Consortium (formerly the Midwest Pediatric Nephrology Consortium). Patients < 18 years of age with a diagnosis of MPGN based on a renal biopsy performed between 2003 and 2012 were included in the original KidCOM registry. Patient data were collected at the time of enrollment into KidCOM and retrospectively from disease onset. The original goal of the KidCOM project was to monitor disease progression and biochemical markers in patients with MPGN. After publication of C3G consensus criteria,1 requests were sent to all participating centers to collect biopsy reports to allow for the reclassification of enrolled patients for comparison between both groups. Patients for whom renal biopsy reports were made available were reclassified as either C3G or IC-MPGN based on the recently published consensus criteria.1,16
A second round of enrollment was conducted at The Hospital for Sick Children, Toronto, Canada, to include all prevalent patients with a histologic diagnosis of C3G or IC-MPGN as part of the C3 Glomerulopathy and Membranoproliferative Glomerulonephritis: Pediatric Outcomes (C3PO) study. Patients < 18 years of age with a diagnosis of IC-MPGN or C3G based on renal biopsy performed between 2012 and 2019 were included in the C3PO registry. Patient data were again collected at disease onset (baseline) and at the time of the last available follow-up.
The KidCOM and C3PO registries were subsequently combined into a single cohort, which created an unprecedented number of children with IC-MPGN and C3G, along with nearly 2 decades of cases in a multinational collaborative study.
Renal Biopsy
Renal biopsy reports were reassessed for all patients with available renal biopsy reports (n = 85). Blinded investigators (n = 2) classified the revised diagnosis as either IC-MPGN or C3G based on light and electron microscopy as well as immunofluorescence as per consensus guidelines (Table 1). In cases in which patients underwent multiple renal biopsies, the most recent biopsy was used for determination of the revised diagnosis. The reported diagnosis from investigators at the time of the initial patient enrollment served as the initial diagnosis for each patient.
Table 1.
Summary of histologic findings in glomeruli of patients with C3 glomerulopathya
| Light microscopy | Electron microscopy | Immunofluorescence |
|---|---|---|
|
|
|
Variables of Interest
The demographic data collected included patient age, sex, race, and home institution. Clinical parameters included height, weight, presence or absence of hematuria on urinalysis, hypertension (defined as blood pressure > 95th percentile for age, sex, and height), presence or absence of nephrotic syndrome (defined as edema, protein-to-creatinine ratio of 200 mg/mmol or greater, and serum albumin < 25 g/l), presence or absence of antihypertensives, number of antihypertensives, number of each class of antihypertensive (including angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), and presence or absence of steroid use.
Laboratory data included complete blood counts, serum electrolytes (sodium, potassium, chloride, bicarbonate, phosphate, magnesium, and calcium), albumin, urea, creatinine, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, C3, and C4. Urine studies included a urine dipstick test, protein, and creatinine.
Data were converted wherever necessary to SI units. eGFR was calculated using the Schwartz formula. Proteinuria was quantified using urine protein–to-creatinine ratios expressed in mg/mmol wherein a value >200 was defined as nephrotic-range proteinuria. For a secondary outcome of kidney survival, a composite outcome of eGFR < 30 ml/min per 1.73 m2, 50% reduction in eGFR, or need for kidney replacement therapy was used.
Statistical Analyses
All continuous variables are presented as means with 95% confidence intervals and were analyzed using Student t tests. Categorical data were analyzed using the Fisher exact test for significance. P < 0.05 was used for statistical significance. For kidney survival, data are displayed in Kaplan-Meier curves.
Results
Study Design
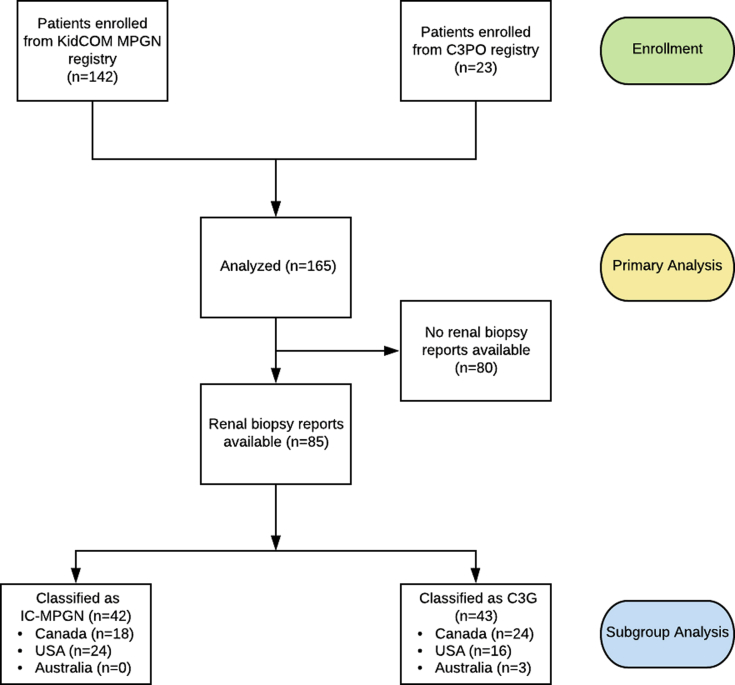
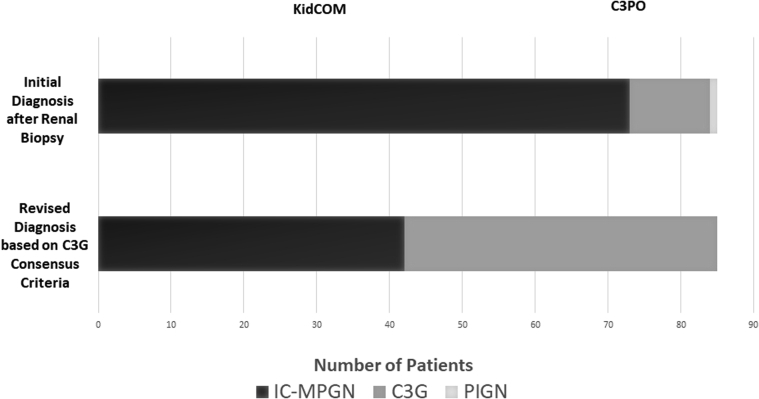
Renal biopsy reports were available for 85 patients in the cohort, and 42 patients were classified as IC-MPGN, with the other 43 classified as C3G (Figure 1). All patients enrolled in the KidCOM registry and many of the patients in the C3PO registry carried an initial diagnosis of MPGN, and upon reevaluation of the available biopsy reports, we found that approximately half of these would be classified as C3G today, with the remainder falling into the IC-MPGN class (Figure 2). Overall, the new consensus criteria resulted in the need for reclassification of 42.4% of patients who had previously been diagnosed with the historic “MPGN” label.
Figure 1.
The STROBE chart of the KidCOM study design. C3G, C3 glomerulopathy; C3PO, C3 Glomerulopathy and Membranoproliferative Glomerulonephritis: Pediatric Outcomes study of patients under 18 years of age at The Hospital for Sick Children, Toronto, Ontario, Canada, with a diagnosis of immune-complex membranoproliferative glomerulonephritis (IC-MPGN) or C3G based on renal biopsy performed between 2012 and 2019; KidCOM, registry of patients under 18 years of age with a diagnosis of membranoproliferative glomerulonephritis based on renal biopsy performed between 2003 and 2012.
Figure 2.
Renal biopsy reclassification based on the new consensus criteria. The initial diagnosis was the reported diagnosis based on renal histology when first reported by the local pathologist. Patients from the KidCOM and C3PO registries were subsequently combined into the study cohort. Revised diagnosis is based on revisions of renal biopsy reports by the 2 study pathologists based on the new C3G consensus criteria.1,17 C3G, C3 glomerulopathy; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; PIGN, postinfectious glomerulonephritis.
Patient Demographics
Patients in this study had a mean age of 9.3 years at diagnosis and 13.4 years at the last follow-up (the mean follow-up time was 4 years). The cohort was 47.3% male and predominantly white. There were no significant differences in age, sex, race, or anthropometrics between patients with IC-MPGN and those with C3G (Table 2).
Table 2.
Patient demographics for the combined KidCOM and C3 Glomerulopathy and Membranoproliferative Glomerulonephritis: Pediatric Outcomes cohorts
| Patient demographics | Total cohort (N = 165) | IC-MPGN (n = 42) | C3G (n = 43) | P value (IC-MPGN vs. C3G) |
|---|---|---|---|---|
| Age at diagnosis (yr) | 9.3 (8.7–10.0) | 9.6 (8.7 – 10.5) | 10.1 (9.0–11.3) | NS (0.5647) |
| Age at last follow-up (yr) | 13.4 (12.6–14.0) | 13.6 (12.5–14.7) | 14.1 (13.0–15.2) | NS (0.5104) |
| Follow-up time (yr) | 4.0 (3.4–4.6) | 4.0 (3.1–5.0) | 4.0 (3.1–4.8) | NS (0.9072) |
| Sex | ||||
| Male | 78 | 21 | 15 | NS (0.1910) |
| Female | 87 | 21 | 28 | |
| Race | ||||
| White | 109 | 27 | 22 | NS (0.2744) |
| Asian | 13 | 5 | 4 | NS (0.7334) |
| American Indian | 2 | 1 | 0 | NS (0.4941) |
| Black | 13 | 3 | 2 | NS (0.6761) |
| Mixed race | 7 | 4 | 2 | NS (0.4331) |
| Native Hawaiian/Pacific Islander | 2 | 0 | 0 | NS (>0.9999) |
| Unknown | 19 | 2 | 13 | 0.0034a |
| Weight at diagnosis (kg) | 38.6 (34.6–42.6) | 36.4 (32.3–40.7) | 42.6 (34.4–50.8) | NS (0.1883) |
| Weight at last follow-up (kg) | 49.5 (45.6–53.4) | 51.4 (45.2–57.6) | 52.2 (46.8–57.7) | NS (0.8610) |
| Height at diagnosis (cm) | 133.0 (127.9–138.0) | 138.1 (132.3–144.0) | 135.9 (127.1–144.7) | NS (0.6691) |
| Height at last follow-up (cm) | 149.0 (145.0–153.0) | 154.0 (148.6–159.4) | 155.5 (150.2–160.8) | NS (0.6337) |
C3G, C3 glomerulopathy; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; NS, not significant.
Numeric data are shown as the mean with 95% confidence interval.
Statistically significant.
Clinical Parameters at Presentation and Last Follow-Up
The majority of patients had hematuria at the time of the initial presentation, and at the time of the last follow-up, there was a significant reduction in the prevalence. Hypertension was present in 57.5% of patients at diagnosis, and there was no significant change in the prevalence at the last follow-up (42.5%) (Table 3). The mean number of antihypertensives per patient was 2.0 at diagnosis and 1.5 at the last follow-up, and the most common class was angiotensin-converting enzyme inhibitors. Approximately half of the total cohort was treated with RAS blockade at diagnosis, and at the last follow-up, most patients were on an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker treatment (75.0%). Only 11.8% of all patients presented with nephrotic syndrome at the initial diagnosis. Steroids were used at diagnosis in less than half (43.7%) of the total cohort, and there was a significant reduction in the number of patients remaining on corticosteroids at the last follow-up (31.7%). There were no significant differences in any clinical parameters at any time points between children with IC-MPGN or those with C3G.
Table 3.
Clinical parameters for the combined KidCOM and C3 Glomerulopathy and Membranoproliferative Glomerulonephritis: Pediatric Outcomes cohorts
| Clinical parameters | Total cohort (N = 165) | IC-MPGN (n = 42) | C3G (n = 43) | P value (IC-MPGN vs. C3G) |
|---|---|---|---|---|
| Nephrotic syndrome at diagnosis | 11.8% | 22.0% | 11.9% | NS (0.25) |
| Hypertension at diagnosis | 57.5% | 57.1% | 57.6% | NS (0.38) |
| Hypertension at last follow-up | 42.5% | 70.4% | 42.4% | NS (0.40) |
| P value | NS (0.05) | NS (0.31) | NS (0.44) | |
| Hematuria at diagnosis | 80.9% | 81.0% | 61.9% | NS (0.19) |
| Hematuria at last follow-up | 48.5% | 57.1% | 38.1% | NS (0.82) |
| P value | <0.0001a | 0.0273a | 0.0005a | |
| On antihypertensives at diagnosis | 55.6% | 70.7% | 46.3% | 0.04a |
| On antihypertensives at last follow-up | 65.0% | 71.4% | 74.4% | NS (0.81) |
| P value | NS (0.09) | NS (>0.99) | 0.01a | |
| Antihypertensives per patient at diagnosis | 2.0 (1.6–2.3) | 2.2 (1.4–3.0) | 1.3 (0.73–1.9) | NS (0.16) |
| Antihypertensives per patient at last follow-up | 1.5 (1.3–1.7) | 1.7 (1.3–2.1) | 0.86 (0.56–1.2) | NS (0.06) |
| P value | 0.01a | NS (0.19) | NS (0.14) | |
| Corticosteroids at diagnosis | 43.7% | 68.3% | 57.1% | NS (0.37) |
| Corticosteroids at last follow-up | 31.7% | 31.7% | 40.5% | NS (0.49) |
| P value | 0.049a | 0.0018a | NS (0.19) | |
| ACEi/ARB at diagnosis | 49.5% | 53.3% | 40% | NS (0.42) |
| ACEi/ARB at last follow-up | 75.0% | 76.7% | 77.8% | NS (>0.99) |
| P value | 0.0002a | NS (0.10) | 0.0036a |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; C3G, C3 glomerulopathy; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; NS, not significant.
Corticosteroids include oral prednisone, oral prednisolone, or i.v. methylprednisolone. Numeric data shown as the mean with 95% confidence interval.
Statistically significant.
Biochemical Parameters at Presentation and Last Follow-Up
Patients in the cohort had a moderately depressed mean eGFR at presentation, with a significant increase noted in the total cohort and within each disease group at the last follow-up (Table 4). There was a trend toward higher serum creatinine levels at the initial presentation and at the last follow-up in patients with C3G compared with those with IC-MPGN, although this did not reach statistical significance, and eGFR was similar in both groups at both time points.
Table 4.
Biochemical parameters for the combined KidCOM and C3 Glomerulopathy and Membranoproliferative Glomerulonephritis: Pediatric Outcomes cohorts
| Clinical parameters | Total cohort (N = 165) | IC-MPGN (n = 42) | C3G (n = 43) | P value (IC-MPGN vs. C3G) |
|---|---|---|---|---|
| Cr at diagnosis (μmol/l) | 148.6 (107.5–189.8) | 93.7 (70.7–116.6) | 168.9 (45.4–292.4) | NS (0.2528) |
| Cr at last follow-up (umol/l) | 132.2 (77.4–186.9) | 99.1 (46.3–151.9) | 145.0 (−8.1 to 298.1) | NS (0.4669) |
| P value | NS (0.6382) | NS (0.8389) | NS (0.8097) | |
| eGFR at diagnosis (ml/min per 1.73 m2) | 71.6 (63.0–80.3) | 76.0 (62.6–89.5) | 82.8 (67.5–98.2) | NS (0.4112) |
| eGFR at last follow-up (ml/min per 1.73 m2) | 94.6 (86.1–103.0) | 93.4 (80.8–106.2) | 108.7 (93.3–124.0) | NS (0.3237) |
| P value | <0.0001a | 0.0315a | 0.0134a | |
| Delta eGFR (ml/min per 1.73 m2) | 22.9 (13.1–32.7) | 17.4 (1.7–33.2) | 25.8 (5.7–45.9) | NS (0.5200) |
| Albumin at diagnosis (g/l) | 28 (26–30) | 27 (23–31) | 27 (24–30) | NS (0.4510) |
| Albumin at last follow-up (g/l) | 38 (36–39) | 35 (31–38) | 39 (37–41) | 0.0226a |
| P value | <0.0001a | 0.0033a | <0.0001a | |
| C3 at diagnosis (g/l) | 0.38 (0.30–0.46) | 0.26 (0.16–0.36) | 0.39 (0.19–0.59) | NS (0.71) |
| C3 at last follow-up (g/l) | 0.71 (0.61–0.81) | 0.50 (0.30–0.70) | 0.82 (0.63–1.0) | 0.0141a |
| P value | <0.0001a | 0.0066a | 0.0003a | |
| Delta C3 (g/l) | 0.33 (0.24–0.42) | 0.24 (0.07–0.40) | 0.43 (0.22–0.64) | NS (0.1485) |
| C4 at diagnosis (g/l) | 0.22 (0.17–0.27) | 0.21 (0.14–0.27) | 0.25 (0.10–0.40) | NS (0.6937) |
| C4 at last follow-up (g/l) | 0.22 (0.19–0.25) | 0.22 (0.18–0.32) | 0.21 (0.16–0.25) | NS (0.5284) |
| P value | NS (0.9733) | NS (0.8054) | NS (0.5105) | |
| Urine protein/creatinine ratio at diagnosis (mg/mmol) | 338.4 (222.2–454.6) | 460.3 (204.6–716.0) | 395.6 (160.7–630.5) | NS (0.5297) |
| Urine protein/creatinine ratio at last follow-up (mg/mmol) | 253.8 (91.9–415.7) | 207.9 (49.7–366.0) | 117.9 (52.1–183.7) | NS (0.2067) |
| P value | NS (0.4289) | NS (0.1170) | 0.0267a |
C3, serum C3 level (reference range 0.83–1.52 g/l); C4, serum C4 level (reference range 0.13–0.37 g/l); C3G, C3 glomerulopathy; Cr, serum creatinine; eGFR, estimated glomerular filtration rate; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; NS, not significant.
Numeric data shown as the mean with 95% confidence interval.
Statistically significant.
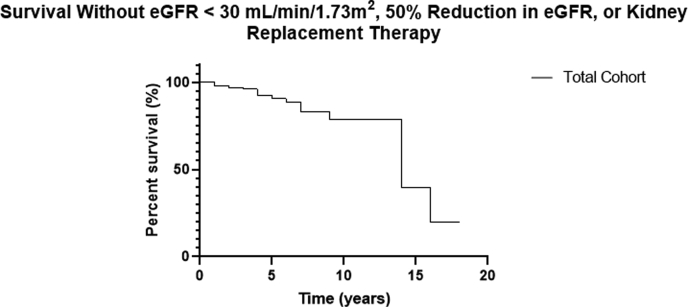
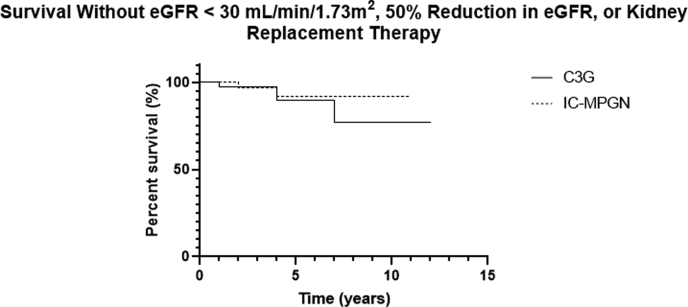
Kidney survival decreased over time in the total cohort (Figure 3), and there was a trend toward lower kidney survival in patients with C3G compared with IC-MPGN (Figure 4). Hypoalbuminemia and nephrotic-range proteinuria were noted at diagnosis in both groups, and although hypoalbuminemia improved significantly, the reduction in urine protein did not reach statistical significance in the total cohort, although it did in the subgroup with C3G. The mean serum C3 was low in patients in both groups at diagnosis and increased significantly in both groups by the last follow-up. The mean serum albumin and serum C3 were significantly lower in patients with IC-MPGN compared with those with C3G at the last follow-up.
Figure 3.
The Kaplan-Meier curve of kidney survival in the total cohort. The composite outcome of eGFR under 30 ml/min per 1.73 m2, 50% reduction in eGFR, or need for kidney replacement therapy (KRT) was used to define the primary end point. The graph shows survival free of this end point.
Figure 4.
The Kaplan-Meier curve of kidney survival in the IC-MPGN and C3G subgroups. The composite outcome of eGFR under 30 ml/min per 1.73 m2, 50% reduction in eGFR, or need for kidney replacement therapy (KRT) was used to define the primary end point. The graph shows survival free of this end point.
Clinical and Laboratory Findings Associated With Hypertension at Presentation
Patients who were hypertensive at the time of diagnosis had a lower mean eGFR initially and at the last follow-up in the total cohort, although this effect was not seen at the last follow-up within each subgroup (Table 5). Hypertension at the initial diagnosis was also associated with the need for a higher mean number of antihypertensives per patient at the last follow-up.
Table 5.
Clinical and laboratory results of patients by hypertension at diagnosis
| Clinical parameters | Total cohort HTN (n = 84) | Total cohort Nn HTN (n = 78) | P value | IC-MPGN HTN (n = 24) | IC-MPGN no HTN (n = 18) | P value | C3G HTN (n = 19) | C3G no HTN (n = 23) | P value |
|---|---|---|---|---|---|---|---|---|---|
| eGFR at diagnosis (ml/min per 1.73 m2) | 63.9 (53.5–74.2) | 84.0 (69.8–98.1) | 0.0204a | 75.5 (60.2–90.8) | 80.0 (52.8–107.3) | NS (0.7377) | 67.3 (43.8–90.8) | 99.6 (79.0–120.3) | 0.036a |
| eGFR at last follow-up (ml/min per 1.73 m2) | 82.4 (71.2– 93.7) | 103.9 (92.6–115.2) | 0.0088a | 87.6 (71.5–103.6) | 107.2 (95.0–119.4) | NS (0.0972) | 110.4 (86.4–134.3) | 100.4 (78.4–122.3) | NS (0.5231) |
| Delta eGFR (ml/min per 1.73 m2) | 29.8 (9.9–49.7) | 20.3 (5.5–35.1) | NS (0.4452) | 10.0 (−10.4to −30.4) | 27.0 (−7.5 to 61.4) | NS (0.1876) | 44.3 (1.0–87.6) | 14.4 (−13.2 to 42.1) | NS (0.2275) |
| C3 at diagnosis (g/l) | 0.40 (0.32–0.29) | 0.46 (0.34–0.57) | NS (0.4521) | 0.31 (0.19–0.42) | 0.49 (0.15–0.83) | NS (0.1595) | 0.24 (0.12–0.36) | 0.51 (0.27–0.75) | NS (0.0742) |
| C3 at last follow-up (g/l) | 0.66 (0.54–0.79) | 0.71 (0.56–0.85) | NS (0.6278) | 0.52 (0.27–0.76) | 0.35 (0.001–0.70) | NS (0.4119) | 0.73 (0.50–0.96) | 0.78 (0.50–1.1) | NS (0.7956) |
| Urine protein/creatinine ratio at diagnosis (mg/mmol) | 437.8 (225.2–650.4) | 360.4 (132.4–588.3) | NS (0.6192) | 433.8 (220.7–646.8) | 499.5 (−277.9 to 1277) | NS (0.7973) | 504.0 (98.6–909.3) | 229.3 (40.0–418.6) | NS (0.1797) |
| Urine protein/creatinine ratio at last follow-up (mg/mmol) | 210.4 (15.8–405.0) | 232.4 (82.9–381.8) | NS (0.8597) | 171.2 (51.0–291.4) | 244.7 (−84.3 to 573.7) | NS (0.5848) | 82.9 (36.4–129.3) | 143.6 (59.1–228.2) | NS (0.2173) |
| On antihypertensives at diagnosis | 71.6% | 23.1% | 0.0012a | 75.0% | 33.3% | NS (0.2010) | 75.0% | 66.7% | NS (>0.9999) |
| On antihypertensives at last follow-up | 71.1% | 59.0% | NS (0.1363) | 87.5% | 50.0% | 0.0144a | 68.4% | 78.3% | NS (0.5038) |
| Antihypertensives per patient at diagnosis | 2.4 (2.1–2.7) | 1.3 (0.8–1.8) | 0.0004a | 2.5 (1.7–3.2) | 1.4 (0.25–2.6) | NS (0.1455) | 2.3 (1.7–3.0) | 0.6 (−0.2 to 1.4) | 0.0011a |
| Antihypertensives per patient at last follow-up | 1.9 (1.6–2.3) | 1.4 (1.1–1.7) | 0.0166a | 1.9 (1.5–2.4) | 1.5 (0.2–2.7) | NS (0.3516) | 1.4 (0.7–2.2) | 1.2 (0.7–1.6) | NS (0.4902) |
C3, serum C3 level (reference range 0.83–1.52 g/l); Cr, serum creatinine; eGFR, estimated glomerular filtration rate based on the bedside Schwartz formula; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; NS, not significant.
Numeric data shown as the mean with 95% confidence interval.
Statistically significant.
Clinical and Laboratory Findings Associated With Steroid Use at Presentation
In the total cohort, patients treated with steroids at the initial diagnosis did not have any significant difference in eGFR at diagnosis or at the last-follow-up compared with those who did not receive steroids (Table 6). There was a higher prevalence of hypertension at diagnosis and the last follow-up in patients treated with steroids.
Table 6.
Clinical and laboratory results of patients by steroid use at presentation
| All steroids (n = 62) | All no steroids (n = 80) | P value | IC-MPGN steroids (n = 28) | IC-MPGN no steroids (n = 13) | P value | C3G steroids (n = 24) | C3G no steroids (n = 19) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| eGFR at diagnosis (ml/min per 1.73 m2) | 71.4 (61.1–81.6) | 69.1 (53.4–84.8) | NS (0.8054) | 76.8 (62.2–91.4) | 71.7 (31.3–112.0) | NS (0.7580) | 62.6 (43.8–81.4) | 115.6 (97.4–134.0) | 0.0002a |
| eGFR at last follow-up (ml/min per 1.73 m2) | 87.2 (76.9–97.5) | 88.0 (74.7–101.4) | NS (0.9212) | 92.4 (77.9–106.9) | 102.6 (81.8–123.5) | NS (0.4611) | 101.6 (78.7–124.5) | 106.2 (84.6–127.8) | NS (0.7699) |
| Delta eGFR (ml/min per 1.73 m2) | 18.4 (6.1–30.8) | 21.5 (5.5–37.5) | NS (0.7616) | 15.6 (−1.8 to 32.9) | 34.1 (−16.8 to 85.1) | NS (0.3469) | 43.0 (12.9–73.0) | −3.0 (−23.1 to 17.2) | 0.0197a |
| C3 at diagnosis (g/l) | 0.34 (0.25– 0.42) | 0.49 (0.39–0.58) | 0.0224a | 0.34 (0.210–0.46) | 0.37 (−0.011 to 0.82) | NS (0.8443) | 0.33 (0.13–0.54) | 0.45 (0.22–0.69) | NS (0.4072) |
| C3 at last follow-up (g/l) | 0.57 (0.42–0.72) | 0.74 (0.59–0.88) | NS (0.1038) | 0.34 (0.14–0.55) | 0.82 (0.45–1.2) | 0.0165a | 0.78 (0.57–0.98) | 0.74 (0.44–1.0) | NS (0.8093) |
| Urine protein/creatinine ratio at diagnosis (mg/mmol) | 494.1 (261.8–726.5) | 373.1 (84.7–661.4) | NS (0.5149) | 534.9 (220.1–849.6) | 254.0 (−43.4 to 551.5) | NS (0.2665) | 518.4 (162.5–874.4) | 161.0 (27.0–295.0) | NS (0.0785) |
| Urine protein/creatinine ratio at last follow-up (mg/mmol) | 135.4 (78.0–192.8) | 314.4 (25.8–603.0) | NS (0.2049) | 239.9 (65.6–414.2) | 80.0 (0.59–159.4) | NS (0.2768) | 104.8 (43.5–166.2 | 131.9 (39.4–224.4) | NS (0.5898) |
| Hypertension at diagnosis | 66.1% | 42.5% | 0.0067a | 67.9% | 30.8% | 0.0425a | 58.3% | 27.8% | NS (0.0653) |
| Hypertension at last follow-up | 81.0% | 59.5% | 0.0479a | 85.0% | 16.7% | 0.0045a | 66.7% | 50% | NS (0.6570) |
| On antihypertensives at diagnosis | 82.3% | 36.2% | <0.0001a | 82.1% | 85.7% | NS (>0.9999) | 69.6% | 70.8% | NS (>0.9999) |
| On antihypertensives at last follow-up | 77.4% | 55.0% | 0.0076a | 46.2% | 38.5% | NS (>0.9999) | 16.7% | 77.8% | 0.0006a |
| Antihypertensives per patient at diagnosis | 2.4 (2.0–2.8) | 2.4 (1.9–2.8) | NS (0.9980) | 2.3 (1.6–3.1) | 1.6 (0.81–2.5) | NS (0.3661) | 2.1 (1.4–2.8) | 0.5 (−0.13 to 1.1) | 0.0048a |
| Antihypertensives per patient at last follow-up | 1.9 (1.5–2.3) | 1.8 (1.5–2.2) | NS (0.8485) | 1.9 (1.4–2.4) | 1.0 (1.0–1.0) | NS (0.1284) | 1.7 (0.9–2.5) | 1.0 (0.6–1.4) | NS (0.0769) |
C3, serum C3 level (reference range 0.83–1.52 g/l); C3G, C3 glomerulopathy; eGFR, estimated glomerular filtration rate based on bedside Schwartz formula; Cr, serum creatinine; IC-MPGN, immune-complex membranoproliferative glomerulonephritis; NS, not significant.
Numeric data shown the mean with 95% confidence interval.
Statistically significant.
In patients with C3G, those who received steroids at diagnosis had a significantly lower eGFR initially but a similar eGFR at the last-follow-up with a significant improvement in renal function. This same effect on eGFR was not seen in IC-MPGN patients treated with steroids.
This cohort was predominantly white, with approximately equal proportions of male and female patients. Neither race nor sex appeared to portend a difference in renal prognosis or the last follow-up (Supplementary Tables S1 and S2).
Discussion
This study presents the clinical and laboratory presentations and outcomes of the largest pediatric IC-MPGN/C3G cohort to date in a multicenter, multinational collaborative study. We found that nearly half of the patients originally diagnosed as MPGN would be reclassified as C3G today. Children with C3G had a trend toward higher serum creatinine at both time points, although this did not reach statistical significance. Although clinical and biochemical parameters were similar between both groups, we noted a significant improvement in renal function after steroid treatment in C3G, which was not noted in IC-MPGN. The overall renal outcomes were favorable in both groups with normal eGFR after a mean follow-up time of 4 years; however, despite treatment, the burden of hypertension and proteinuria remained significant.
Histologic Reclassification
We found that approximately half of the patients previously diagnosed as MPGN would today be considered C3G under the most recent histologic classification guidelines. In the present study, we found that patients divided by histology into 1 of the 2 diseases did not appear to have any significant differences in the available medium-term outcomes. Comparison with historic studies is challenging because of the heterogeneity of the definition of MPGN in studies published before and shortly after the reclassification of the disease entities of IC-MPGN and C3G and because studies performed after reclassification have been limited in pediatrics with small sample sizes.
In a retrospective study of 20 adult patients with IC-MPGN and 15 with C3G followed for a median duration of 68 months, no significant differences were noted in renal survival or treatment response between patients in either disease group.17 Within the multiple small cohort studies of children with IC-MPGN/C3G conducted postreclassification, some have demonstrated worse renal prognoses in C3G,3,18 whereas others showed no significant differences between the groups.19 These studies have all been limited by small sample sizes.
In our cohort, we noticed a trend toward higher creatinine in children with C3G compared with those with IC-MPGN. Although this was not statistically significant, there was a trend toward worse kidney survival in C3G, which raises the questions as to whether there may be a worse renal prognosis in C3G that may become evident with a longer follow-up. This may be attributed to ongoing alternative complement pathway activity inadequately controlled with the current therapy. Although the initial and final eGFR measurements were not statistically different in C3G and IC-MPGN, we acknowledge that there are many limitations to eGFR equations used in pediatrics.20 Although our data show no significant differences between clinical presentation and prognosis in pediatric patients with either disease, there may be increased steroid responsiveness in C3G. Of note, hypocomplementemia remained significantly more pronounced in IC-MPGN at the last follow-up, although serum C3 levels had normalized in patients with C3G. Patients with C3G also had a statistically significant reduction in proteinuria, which was not achieved in patients with IC-MPGN, although between-group differences in urinary protein-to-creatinine ratios were not significant.
The reclassification of MPGN into the 2 disease entities has led to a paradigm shift, identifying a primary complement-driven disease in approximately 50% of the patient population. Histology provides evidence of a distinct pathophysiology between these disease groups, driven by underlying complement activity. However, our results suggest that histologic classification alone may be insufficient to adequately risk stratify patients. In addition, it has previously been shown that pathologic criteria alone may not distinguish between disease activity driven by the complement alternative pathway and that genetics in combination with susceptibility factors (such as complement activity or pathologic antibodies) may be better indicators of prognosis.21
As previously discussed, it is postulated that in IC-MPGN classical pathway activation may be secondarily triggered by immune-complex deposition, whereas C3G may result from a primary dysfunction of complement proteins or regulators. It was also suggested in the 2013 C3 glomerulopathy consensus report that the morphologic appearance can indicate the likelihood of the C3 glomerulopathy disease process.1 However, our results and previous genetic analyses now appear to highlight the limitations of renal biopsy alone to differentiate the 2 disease processes.21
Comparison to Adult Patients
Data have been limited in pediatric patients with IC-MPGN and C3G, with most data coming from adult studies or mixed cohorts with relatively few children. The literature from adults suggests a poor renal prognosis in both diseases,4,22 although previously it has been unclear as to whether this held true in children. Patients in our cohort had several key differences from the data published in prior adult IC-MPGN and C3G cohorts. Of note the most common initial presentation of C3G reported in adult studies is nephrotic syndrome,22 whereas in the present study only 11.8% of pediatric patients presented with nephrotic syndrome, with no significant difference noted between patients with IC-MPGN and C3G.
Bomback et al.22 previously found a 40% rate of progression to advanced chronic kidney disease (CKD), ESKD, or death in a large American cohort study of 111 patients (approximately one-third pediatric) with C3G. Similar results have been found in MPGN; Nakagawa et al.5 found worse renal outcomes in adults compared with children with MPGN (types I and III) in a cross-sectional survey of patients in a Japanese renal biopsy registry. These results would suggest a more favorable disease course in children versus adults; however, as mixed cohorts, the generalizability of these studies to pediatrics has been unclear because the number of children in each study was relatively small compared with adults.
In our study of exclusively pediatric patients, advanced CKD and ESKD were rare findings, with only 7.3% of C3G patients, 5.7% of IC-MPGN patients, and 12.5% of the total cohort reaching an eGFR ≤ 15 ml/min per 1.73 m2 at the last follow-up (mean time of 4 years) with no documented deaths. We also demonstrate a longer time to loss of kidney survival in this cohort, which is in keeping with the univariate analysis by Bomback et al.22 that noted a significantly longer time to ESKD or death in pediatric patients compared with adults.
The finding of preserved eGFR in the present study may be related to fewer comorbidities in this pediatric population compared with prior adult studies. Furthermore, the etiology of IC-MPGN in children is most often idiopathic or “primary IC-MPGN,” as opposed to secondary causes such as infection, autoimmune disease, or monoclonal gammopathy in adults, which may portend a worse prognosis in this subset of patients.
Therapeutic Challenges
The treatment of IC-MPGN or C3G is centered on ameliorating the underlying immune dysregulation as well as minimizing the complications of hypertension and proteinuria. The present management largely relies on RAS blockade and immunosuppression (typically with steroids and/or mycophenolate mofetil) because much of the evidence for therapy again comes from adult data.
In a mixed adult-pediatric cohort study of patients with C3G, Meena et al.23 showed a significant increase in time to ESKD in patients treated with steroids compared with those who did not receive steroid therapy at diagnosis, and the protective effect of steroid therapy remained significant in the multivariate analysis. In our cohort, with a similar follow-up period, no difference in eGFR was noted between patients treated with steroids compared with patients who did not receive initial steroid therapy. However, we did find that steroid-treated patients with C3G had a significant improvement in eGFR over the following 4 years, whereas steroid-treated patients with IC-MPGN did not.
These findings suggest that the pathophysiology of primary complement dysregulation in C3G may be more amenable to treatment. Terminal complement blockade has been attempted in C3G with mixed success,12,24, 25, 26, 27, 28 and targeted therapy remains an area of interest in this disease. Although indications and guidelines for complement blockade have not yet been firmly established, our results suggest a potential future role for such disease-modifying therapy.
Another area of interest to improve outcomes in these patients is the management of hypertension. The present study highlights the prevalence of hypertension in children with IC-MPGN and C3G, which was not explored in the study by Bomback et al.22 and is in contrast to findings from the Japan renal biopsy registry.5 In our cohort, 57.5% of patients were hypertensive at the time of diagnosis, many requiring multiple antihypertensives. Although the incidence of hypertension was similar in both IC-MPGN and C3G at diagnosis, fewer patients in the C3G group were treated with antihypertensives initially. It is unclear why this was the case, although this may be related to the use of more RAS blockade primarily for proteinuria in the IC-MPGN group because these patients had more proteinuria and a lower serum albumin. The difference in antihypertension use at diagnosis may also be related to absolute blood pressure differences or a difference in the etiology of hypertension (e.g., suspicion of transient cause in the C3G group) not captured in the data. At the last follow-up, the use of antihypertensives was similar in both groups, and there was a significant increase in the number of patients with C3G started on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Despite RAS blockade in many patients, there was still a significant proportion of children with uncontrolled hypertension after a mean treatment time of 4 years (42.5%), and among those children who were hypertensive at presentation, 71.6% remained hypertensive at the last follow-up.
Patients who were hypertensive at the time of diagnosis also had a significantly lower eGFR than normotensive counterparts both at diagnosis and at the last follow-up with worse overall kidney survival (Supplementary Figure S1), and they required more antihypertensive medications both at diagnosis and at the last follow-up. These results may reflect the impact of CKD on blood pressure, with a lower eGFR potentially making blood pressure control more difficult. These results are consistent with data in adults that hypertension at the initial presentation is an independent risk factor for poor renal outcomes.5
Proteinuria was another suboptimally controlled target parameter in the patients in our study. Although proteinuria trended toward improvement in our patients in both groups, the mean proteinuria was still significantly elevated 4 years later at the last follow-up in this cohort in both groups. As seen in this cohort, RAS blockade was widely used (75%) in patients at the last follow-up. Although medication doses were not available in the present study, future studies may benefit from directly assessing the potential value of optimization of antiproteinuric therapy to slow or prevent progressive CKD in this patient population.
Strengths and Limitations
Our study represents the largest pediatric IC-MPGN/C3G cohort since reclassification and the only multinational and multicenter study regarding these diseases. We were also able to use renal biopsy, the gold standard for disease classification, for many patients to compare between both disease groups. The diversity of the participating centers may have increased the reliability of these findings; however, we note that as a predominantly white cohort, the genetic profile of the patients may differ from other ethnic groups, and this may limit the generalizability of the findings to all patient populations.29
A comparison between the subgroups of patients with C3G and IC-MPGN was limited to 85 of the 165 patients because detailed reports (i.e., electron microscopy) could not be obtained for all enrolled patients for reclassification. However, we do not anticipate that this sample differs significantly from the rest of the registry and plan to collect the remaining biopsy reports for all patients in subsequent studies. Confounding factors such as socioeconomic status, lifestyle interventions, and medication adherence were unable to be assessed in this study but would be important to consider in future prospective studies. This registry of patients did not have information regarding detailed complement assays (i.e., soluble C5b9, C3d, and factors H and I), genetics, and immunosuppression (e.g., mycophenolate mofetil and eculizumab), all of which will be collected in future studies. However, we do note that studies suggest only approximately 20% of patients with C3G are expected to have an identified genetic mutation21,30; therefore, it is unlikely that these results will significantly impact this cohort. We used eGFR based on the Schwartz formula to assess renal function, and there are many limitations to the use of eGFR equations in children across different ages, ethnicities, and sizes.20 Finally, because of the indolent nature of these disease processes, the long-term clinical outcomes of these complement-mediated diseases may not be known for several more years because of the limited follow-up available to date in the current study. However, trends toward clinically important differences between these newly distinguished disease entities are apparent even after only 4 years of mean follow-up, which suggests that longer follow-ups in future studies may reveal statistically significant differences in outcomes between IC-MPGN and C3G.
Conclusion and Future Perspectives
In summary, we have found that pediatric patients with IC-MPGN and C3G appear to have a much more favorable clinical course compared with adult patients. Renal function may be preserved, and progression to advanced CKD appears to be rare in children. Despite this, using current treatment approaches, hypertension and proteinuria remain suboptimally controlled in these diseases as modifiable disease risk factors, even after 4 years of active management. Our results further suggest there may be a trend toward worse renal prognosis in C3G compared with IC-MPGN and that early steroids may be beneficial in C3G. Current histologic criteria alone may be insufficient to distinguish disease prognosis in children with IC-MPGN and C3G. Given the exciting potential for new highly targeted complement therapy on the horizon, it is even more important now to better understand the clinical and molecular similarities and differences between these 2 diseases in both children and adults. Future studies in children and adults with IC-MPGN and C3G should use sequential measurements of serum complement activity and autoantibody titres as well as genetic analysis both as prognostic biomarkers and to further delineate the pathophysiology and likelihood for response to future targeted molecular therapies.
Acknowledgments
We thank the Midwest Nephrology Consortium, its participating centers, physicians, study, and nurse coordinators for their contributions toward the collection of the plasma samples used in this study. We wish to express our deep gratitude to Ms. Michelle Mayne (nee Frieling), who, especially during its early phase, was the face of KidCOM. She made major contributions to establishing the KidCOM database and biorepository, built the relationships within the Midwest Pediatric Nephrology Consortium that became the foundation of KidCOM’s success, and from the beginning shared the vision that KidCOM had and has the potential of making a crucial contribution to changing the lives of children with severe renal disease. Without her contribution, KidCOM would not have been successful, and this paper would not have been published.
Author Contributions
CL and WES established the KidCOM registry and biorepository. CL, AK, and NJ established the C3PO registry. All authors analyzed and interpreted the data and drafted, edited, and approved the final manuscript.
Footnotes
Table S1. Clinical and laboratory results of patients by sex. C3G, C3 glomerulopathy; Cr, serum creatinine; eGFR, estimated glomerular filtration rate based on bedside Schwartz formula; IC-MPGN, immune-complex membranoproliferative glomerulonephritis. Numeric data shown as the mean with 95% confidence interval.
Table S2. Clinical and laboratory results of patients by race. C3G, C3 glomerulopathy; Cr, serum creatinine; eGFR, estimated glomerular filtration rate based on bedside Schwartz formula; IC-MPGN, immune-complex membranoproliferative glomerulonephritis. Numeric data shown as the mean with 95% confidence interval.
Figure S1. Kaplan-Meier curve of kidney survival in patients with and without hypertension. The composite outcome of estimated glomerular filtration rate (eGFR) under 30 ml/min per 1.73 m2, 50% reduction in eGFR, or need for kidney replacement therapy (KRT) was used to define the primary end point. The graph shows survival free of this end point. HTN, hypertension.
STROBE Statement.
Contributor Information
Christoph Licht, Email: christoph.licht@sickkids.ca.
Midwest Pediatric Nephrology Consortium:
Gina-Marie Barletta, Sharon Bartosh, Neal Blatt, Tom Blydt-Hansen, Patrick Brophy, Lawrence Copelovitch, Brad Dixon, Anne Durkan, Matthew Eison, Larry Greenbaum, Guillermo Hidalgo, Deborah Jones, Mini Michael, John Sanders, Donald Weaver, and Amy Wilson
Appendix
List of the Midwest Pediatric Nephrology Consortium
Drs. Gina-Marie Barletta (Helen Devos Children’s Hospital, Grand Rapids, Michigan, USA), Sharon Bartosh (University of Wisconsin, Madison, Wisconsin, USA), Neal Blatt (University of Michigan, Ann Arbor, Michigan, USA), Tom Blydt-Hansen (British Columbia Children’s Hospital, Vancouver, British Columbia, Canada), Patrick Brophy (Children’s Hospital, University of Iowa, Iowa City, Iowa, USA), Lawrence Copelovitch (Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania, USA), Brad Dixon (Cincinnati Children’s Hospital, Cincinnati, Ohio, USA), Anne Durkan (The Children’s Hospital at Westmead, Westmead, Australia), Matthew Eison (University of Texas Health Science Center, Greenville, South Carolina, USA), Larry Greenbaum (Emory University School of Medicine, Atlanta, Georgia, USA), Guillermo Hidalgo (East Carolina University, Greenville, North Carolina, USA), Deborah Jones (Monroe Carell Jr. Children’s Hospital at Vanderbilt, Nashville, Tennessee, USA), Mini Michael (Texas Children’s Hospital, Houston, Texas, USA), John Sanders (Sanford Children’s Hospital, Sioux Falls, South Dakota, USA), Donald Weaver (Carolinas Medical Center, Charlotte, North Carolina, USA), and Amy Wilson (Riley Hospital for Children, Indianapolis, Indiana, USA).
Disclosure
All the authors declared no competing interests.
Supplementary Material
Supplementary File (PDF)
References
- 1.Pickering M.C., D’Agati V.D., Nester C.M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S., Nester C.M., Smith R.J.H. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81:434–441. doi: 10.1038/ki.2011.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuda Y., Ishikura K., Hamada R. Membranoproliferative glomerulonephritis and C3 glomerulonephritis: frequency, clinical features, and outcome in children. Nephrology (Carlton) 2015;20:286–292. doi: 10.1111/nep.12382. [DOI] [PubMed] [Google Scholar]
- 4.Ravindran A., Fervenza F.C., Smith R.J.H., De Vriese A.S., Sethi S. C3 glomerulopathy: ten years’ experience at Mayo Clinic. Mayo Clin Proc. 2018;93:991–1008. doi: 10.1016/j.mayocp.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa N., Hasebe N., Hattori M. Clinical features and pathogenesis of membranoproliferative glomerulonephritis: a nationwide analysis of the Japan renal biopsy registry from 2007 to 2015. Clin Exp Nephrol. 2018;22:797–807. doi: 10.1007/s10157-017-1513-7. [DOI] [PubMed] [Google Scholar]
- 6.Licht C., Schlötzer-Schrehardt U., Kirschfink M., Zipfel P.F., Hoppe B. MPGN II – genetically determined by defective complement regulation? Pediatr Nephrol. 2007;22:2–9. doi: 10.1007/s00467-006-0299-8. [DOI] [PubMed] [Google Scholar]
- 7.Riedl M., Thorner P., Licht C. C3 glomerulopathy. Pediatr Nephrol. 2017;32:43–57. doi: 10.1007/s00467-015-3310-4. [DOI] [PubMed] [Google Scholar]
- 8.Noris M., Remuzzi G. Glomerular diseases dependent on complement activation, including atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, and C3 glomerulopathy: core curriculum 2015. Am J Kidney Dis. 2015;66:359–375. doi: 10.1053/j.ajkd.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S., Fervenza F.C. Membranoproliferative glomerulonephritis — a new look at an old entity. N Engl J Med. 2012;366:1119–1131. doi: 10.1056/NEJMra1108178. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Meyer N.C., Fervenza F.C. C4 nephritic factors in C3 glomerulopathy: a case series. Am J Kidney Dis. 2017;70:834–843. doi: 10.1053/j.ajkd.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marinozzi M.-C., Chauvet S., Le Quintrec M. C5 nephritic factors drive the biological phenotype of C3 glomerulopathies. Kidney Int. 2017;92:1232–1241. doi: 10.1016/j.kint.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Lebreton C., Bacchetta J., Dijoud F., Bessenay L., Fremeaux-Bacchi V., Sellier-Leclerc A.L. C3 glomerulopathy and eculizumab: a report on four paediatric cases. Pediatr Nephrol. 2017;32:1023–1028. doi: 10.1007/s00467-017-3619-2. [DOI] [PubMed] [Google Scholar]
- 13.Cattran D.C., Feehally J., Cook H.T. Kidney disease: improving global outcomes (KDIGO) glomerulonephritis work group. KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2:139–274. [Google Scholar]
- 14.Little M.A., Dupont P., Campbell E., Dorman A., Walshe J.J. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 2006;69:504–511. doi: 10.1038/sj.ki.5000084. [DOI] [PubMed] [Google Scholar]
- 15.Wilson G.J., Cho Y., Teixiera-Pinto A. Long-term outcomes of patients with end-stage kidney disease due to membranoproliferative glomerulonephritis: an ANZDATA registry study. BMC Nephrol. 2019;20:417. doi: 10.1186/s12882-019-1605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou J., Markowitz G.S., Bomback A.S. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85:450–456. doi: 10.1038/ki.2013.340. [DOI] [PubMed] [Google Scholar]
- 17.Aksoy E., Mirioglu S., Uludag O. SP181 comparison of various features and outcomes in adult patients with immune complex membranoproliferative glomerulonephritis and C3 glomerulopathy. Nephrol Dial Transplant. 2019;34(suppl 1) [Google Scholar]
- 18.Kawasaki Y., Kanno S., Ono A. Differences in clinical findings, pathology, and outcomes between C3 glomerulonephritis and membranoproliferative glomerulonephritis. Pediatr Nephrol. 2016;31:1091–1099. doi: 10.1007/s00467-015-3307-z. [DOI] [PubMed] [Google Scholar]
- 19.Holle J., Berenberg-Goßler L., Wu K. Outcome of membranoproliferative glomerulonephritis and C3-glomerulopathy in children and adolescents. Pediatr Nephrol. 2018;33:2289–2298. doi: 10.1007/s00467-018-4034-z. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz G.J., Muñoz A., Schneider M.F. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iatropoulos P., Noris M., Mele C. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol. 2016;71:131–142. doi: 10.1016/j.molimm.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Bomback A.S., Santoriello D., Avasare R.S. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney Int. 2018;93:977–985. doi: 10.1016/j.kint.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Meena P., Bhargava V., Gupta P. Clinico-pathological features and predictors of end-stage renal disease (ESRD) in C3 glomerulopathy: a retrospective study. Clin Nephrol Res. 2019;3:37–42. [Google Scholar]
- 24.Gurkan S., Fyfe B., Weiss L., Xiao X., Zhang Y., Smith R.J. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975–1981. doi: 10.1007/s00467-013-2503-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inman M., Prater G., Fatima H., Wallace E. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J. 2015;8:445–448. doi: 10.1093/ckj/sfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Quintrec M., Lionet A., Kandel C. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484–489. doi: 10.1053/j.ajkd.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 27.Welte T., Arnold F., Kappes J. Treating C3 glomerulopathy with eculizumab. BMC Nephrol. 2018;19:7. doi: 10.1186/s12882-017-0802-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Le Quintrec M., Lapeyraque A.-L., Lionet A. Patterns of clinical response to eculizumab in patients with C3 glomerulopathy. Am J Kidney Dis. 2018;72:84–92. doi: 10.1053/j.ajkd.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Kumar A., Nada R., Ramachandran R. Outcome of C3 glomerulopathy patients: largest single-centre experience from South Asia. J Nephrol. 2020;33:539–550. doi: 10.1007/s40620-019-00672-5. [DOI] [PubMed] [Google Scholar]
- 30.Servais A., Noël L.-H., Roumenina L.T. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454–464. doi: 10.1038/ki.2012.63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File (PDF)