Abstract
Objectives:
American Indian and Alaska Native (AI/AN) populations have higher gastric cancer rates than the general U.S. population. This study provides a comprehensive overview of incidence rates among AI/AN persons during 2005–2016 compared with non-Hispanic whites (whites).
Methods:
Population-based cancer registry data for 2005–2016 were linked with the Indian Health Service (IHS) patient registration databases to address racial misclassification. Age-adjusted gastric cancer incidence rates were expressed per 100,000 per year. Incidence and trend analyses were restricted to purchased/referred care delivery area (PRCDA) counties in 6 geographic regions, comparing gastric cancer incidence rates for AI/AN versus white populations in the U.S.
Results:
Gastric cancer rates were higher in the AI/AN compared to white populations in nearly every U.S. region. Incidence rates for central/distal portions of the stomach were higher in AI/AN individuals compared to whites. Rates of later stage gastric cancer were higher in AI/AN populations overall and in every region except the Pacific Coast and East. Incidence rates decreased significantly over time in both populations. Declining rates in the AI/AN populations were driven by changes in the Pacific Coast and Northern Plains regions.
Conclusions:
AI/AN populations have a disproportionately high incidence of gastric cancer, especially in Alaska. High incidence in the central/distal portions of the stomach among AI/AN populations likely reflects a high prevalence of Helicobacter pylori infection in these populations. These data can be used to develop interventions to reduce risk factors and improve access to health services among AI/AN peoples at high risk for gastric cancer.
Keywords: gastric cancer, disparity, American Indian, Alaska Native, cancer incidence
INTRODUCTION
Despite the decline in incidence rates of gastric cancer in the general U.S. population over the past 10 years [1, 2], survival remains relatively low [3]. Gastric cancer patients are generally diagnosed with late stage disease, which is difficult to treat and often results in poorer prognosis [4–6]. In the United States, the American Indian and Alaska Native (AI/AN) populations have some of the highest rates of gastric cancer incidence and death [2, 7–9]. The prevalence of Helicobactor pylori (H. pylori), which is a known risk factor for gastric cancer, is particularly high among Alaska Native people [10–13]. Because of these high rates, much of the current gastric cancer research in AI/AN people focuses specifically on the Alaska Native population [14–17]. The prevalence and effect of other gastric cancer risk factors for AI/AN populations have remained largely uncharacterized.
The study of H. pylori as a risk factor for gastric cancer has enhanced researchers’ understanding of gastric cancer etiology [18]. Changes and variations in gastric cancer incidence can also be associated with variations in other factors linked to gastric cancer incidence, including consumption of fruits and vegetables, intake of foods preserved with salt or by smoking, availability of refrigeration, and reduced or varied prevalence of H. pylori infection [1, 5, 19, 20]. The prevalence of these factors varies by racial and ethnic subgroup, which suggests possible reasons for the disparities in gastric cancer incidence by race and ethnicity in the United States.
The purpose of this study is to provide an updated, comprehensive overview of the burden of gastric cancer, by region, in AI/AN populations in the United States using the non-Hispanic white (white) population for comparison. Racial misclassification is addressed using previously described methodologies for linking cancer registry data with Indian Health Service (IHS) patient registration databases [21]. Understanding regional variations in gastric cancer incidence in AI/AN populations may provide additional opportunities to prevent gastric cancer in this population.
MATERIALS AND METHODS
Gastric cancer cases diagnosed during 2005–2016 were identified from population-based registries that participate in the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) or the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute or both programs [22, 23]. During the time covered by this study, tumor histology, tumor behavior, and primary cancer site (topography) were classified according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) [24].
For this study, eligible cases included all malignant neoplasms of the stomach (ICD-O-3 topography cases C16.0–C16.9 and ICD-O-3 behavior code 3) except lymphomas and Kaposi sarcomas. Benign and in situ tumors (ICD-O-3 behavior codes 0 and 2, respectively) and tumors of uncertain or unknown behaviors (ICD-O-3 behavior code 1) were excluded.
Results from previous studies have shown that AI/AN patients are often misclassified as non-Native in central cancer registries that rely solely on medical records for identification [25, 26]. For the current analysis, cancer registry records were linked with IHS patient services files to identify AI/AN individuals with proof of membership in a federally recognized tribe receiving health care from the IHS. These linkages were conducted using LinkPlus, a probabilistic software program developed by CDC that uses key patient identifiers (e.g., social security number, first name, last name, middle initial, date of birth).
These methods for addressing racial misclassification for AI/AN cases are most effective in geographic areas that are well-served by IHS [27]. Therefore, we restricted our analyses to purchased/referred care delivery area (PRCDA) counties, as defined by IHS. These counties (previously called contract health service delivery area counties) contain or are located adjacent to federally recognized tribal lands and have higher proportions of AI/AN residents than non-PRCDA counties. This use of PRCDA counties, as well as a description of the regions, was described in a previous publication and are shown in Supplemental Figure 1 [2].
Our analysis also examined the anatomic site of primary tumor growth for gastric cancer cases [4]. Generally, cancers that arise in the central/distal regions of the stomach are associated more closely with H. pylori infection than cancers in the proximal stomach. To characterize the distribution of primary site, cases were grouped as follows: proximal (cardia and fundus) distal (gastric body, lesser curvature, greater curvature, antrum, and pylorus), and overlapping/unknown (overlapping sites or unknown primary site). Consistent with other studies of gastric cancer etiology, our analysis of cases by anatomic subsite was restricted to patients with adenocarcinoma [28].
Cancer cases diagnosed during 2005–2016 were staged using the following Seer Summary Stage 2000 [29] categories: localized for disease restricted to the stomach, regional for disease extended directly into organs and adjacent areas, distant for disease metastasized to parts of the body not directly adjacent to the stomach, and unstaged for instances where stage was undocumented in the medical record or when there was insufficient documentation in the medical record to determine the stage at diagnosis.
Previous analyses have found that updated bridged intercensal population estimates significantly overestimate AI/AN populations of Hispanic origin [27, 30]. Therefore, we limited all analyses to non-Hispanic populations. Non-Hispanic white was chosen as the referent group. Because all analyses reported here were limited to non-Hispanic populations, we omitted the term “non-Hispanic” from the present study when discussing both groups.
Statistical analysis
Average annual age-adjusted incidence rate were calculated using the direct method [31]. Rates were expressed per 100,000 and adjusted by 19 age groups to the 2000 U.S. standard population [32]. Rate ratios (RR) with 95% confidence intervals (CI) were calculated for comparison of incidence rates between AI/AN and white populations, overall and by sex, region, age group (<40, 40–59, 60–74 and ≥75), stage, and primary site according to methods described by Tiwari et al using SEER*Stat software 8.3.2 [33]. Denominators for rate calculations were derived from population estimates from the U.S. Bureau of the Census.
Time trends (1999–2016) were estimated by joinpoint regression using Joinpoint Regression Program 4.3.10 (National Cancer Institute, Bethesda, MD) [34, 35]. Average annual percent change (AAPC) was used to describe fixed interval trends during 1999–2016 [33].
RESULTS
Gastric cancer incidence rates varied by geographic region, sex and race (Table 1). During 2005–2016, a total of 1,505 gastric cancer cases were diagnosed in AI/AN populations residing in PRCDA counties (compared to 41,319 in the U.S. white population, not shown). For all regions combined, rates for AI/AN populations were about double those of the white population for both sexes. Rates varied substantially by region, ranging from 9.0 in the Pacific Coast to 27.0 in Alaska among AI/AN males and from 6.3 in the Pacific Coast to 15.7 in Alaska among AI/AN females. Rate ratios were the highest in Alaska, with gastric cancer rates nearly 4 times higher in AI/AN males than white males and over 5 times higher in AI/AN females compared to white females.
Table 1.
Gastric cancer incidence by region and sex for American Indian/Alaska Nativea and white populations in PRCDA counties, 2005–2016.
| Region | Sex | AI/AN Count | AI/AN Rateb | 95% CI for AI/AN Rate | White Rateb | RRc (AI/AN:W) | 95% CI for RR |
|---|---|---|---|---|---|---|---|
| Northern Plains | Both sexes | 225 | 10.4 | 9.0–12.0 | 5.1 | 2.04d | 1.75–2.35 |
| Males | 137 | 14.6 | 11.9–17.6 | 7.5 | 1.94d | 1.59–2.35 | |
| Females | 88 | 7.2 | 5.7–−9.0 | 3.1 | 2.33d | 1.84–2.91 | |
| Alaska | Both sexes | 211 | 20.9 | 18.1–−24.1 | 4.9 | 4.23d | 3.47–5.17 |
| Males | 130 | 27.0 | 22.2–32.6 | 6.9 | 3.93d | 3.04–5.07 | |
| Females | 81 | 15.7 | 12.3–19.6 | 2.9 | 5.39d | 3.83–7.59 | |
| Southern Plains | Both sexes | 282 | 8.8 | 7.7–9.9 | 4.7 | 1.85d | 1.62–2.10 |
| Males | 166 | 12.1 | 10.2–14.2 | 7.0 | 1.73d | 1.44–2.06 | |
| Females | 116 | 6.4 | 5.2–7.7 | 2.9 | 2.20d | 1.78–2.69 | |
| Pacific Coast | Both sexes | 212 | 7.4 | 6.4–8.6 | 5.1 | 1.46d | 1.25–1.68 |
| Males | 117 | 9.0 | 7.2–11.0 | 7.4 | 1.20 | 0.97–1.47 | |
| Females | 95 | 6.3 | 5.0–7.7 | 3.1 | 2.02d | 1.61–2.50 | |
| East | Both sexes | 92 | 8.1 | 6.4–10.0 | 6.0 | 1.34d | 1.07–1.66 |
| Males | 53 | 10.0 | 7.3–13.4 | 8.6 | 1.17 | 0.85–1.56 | |
| Females | 39 | 6.4 | 4.5–−8.9 | 4.0 | 1.63d | 1.14–2.24 | |
| Southwest | Both sexes | 483 | 11.9 | 10.8–−13.1 | 4.3 | 2.78d | 2.51–3.06 |
| Males | 295 | 17.4 | 15.3–19.6 | 6.0 | 2.89d | 2.53–3.27 | |
| Females | 188 | 8.1 | 6.9–−9.3 | 2.7 | 2.93d | 2.50–3.42 | |
| Total | Both sexes | 1,505 | 10.4 | 9.9–11.0 | 5.3 | 1.98d | 1.87–2.09 |
| Males | 898 | 14.2 | 13.2–15.2 | 7.6 | 1.87d | 1.74–2.01 | |
| Females | 607 | 7.6 | 7.0–−8.2 | 3.3 | 2.27d | 2.09–2.47 |
Source: Cancer Registries in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute
PRCDA purchased/referred care delivery areas; IHS Indian Health Service; AI/AN American Indian/Alaska Native; CI confidence interval; W non-Hispanic white
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Updated bridged intercensal population estimates significantly overestimate AI/AN populations of Hispanic origin. All analyses are limited to non-Hispanic AI/AN populations. Non-Hispanic white was chosen as the reference population. The term “non-Hispanic” is omitted when discussing both groups
Rates are per 100,000 and age-adjusted to the 2000 US Standard Population (19 age groups-Census P25–1130) Confidence Intervals (Tiwari model) are 95% for rates and ratios
Rate ratios are AI/AN versus white and calculated in SEER*Stat software before rounding of rates and may not equal the RR calculated from rates presented in table
Indicates that the AI/AN rate is significantly different than the rate for whites (p < 0.05).
Regions defined as follows: AK*, Northern Plains (IL, IN,* IA,* MO,* MN,* MT,* NE,* ND,* SD,* WI,* WY*), Southern Plains (OK,* KS,* TX*), Southwest (AZ,* CO,* NV,* NM,* UT* ), Pacific Coast (CA,* ID,* OR,* WA,* HI), and East (AL,* AR, CT,* DE, FL,* GA, KY, LA,* ME,* MD, MA,* MS,* NH, NJ, NY,* NC,* OH, PA,* RI,* SC,* TN, VT, VA, WV, DC). The * identifies states with at least 1 country designated as PRCDA
Gastric cancer incidence rates increased with age for both populations across all regions and for the total U.S. population for males and females combined (Table 2). In AI/AN males, incidence rates were higher in all age groups in Alaska and the Southwest, with the highest rates occurring in the oldest age group in Alaska (111.5). In AI/AN males aged 60–74 and ≥75 years, rates were higher compared to the white population for all regions, except in the East and Pacific Coast. The largest rate ratio (comparing AI/AN to white incidence rates) was observed in the youngest age group for AI/AN males in Alaska (RR = 14.39). In females, rates in Alaska and the Southwest were significantly higher in the AI/AN compared to white populations for all age groups. In the Northern Plains, Southern Plains, Pacific Coast, and Southwest, rates were higher in AI/ANs than in whites for females aged ≥40 years. In females, the highest rates were in AI/ANs aged >75 in Alaska (75.3) and the Southwest (57.6). Similar to AI/AN males in Alaska, AI/AN women in Alaska had the largest rate ratio in the youngest age group (21.7).
Table 2.
Gastric cancer incidence rates and rate ratios by age, sex, and region for American Indian/Alaska Nativea and white populations in PRCDA counties, 2005–2016
| Both Sexes | Males | Females | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | Stage | AI/AN Rateb | W Rateb | RRc (AI/AN:W) | 95% CI | AI/AN Rateb | W Rateb | RRc (AI/AN:W) | 95% CI | AI/AN Rateb | W Rateb | RRc (AI/AN:W) | 95% CI | ||
| Northern Plains | <40 Years | 0.6d | 0.3 | 2.49 | (1.16–4.63) | 0.8 | 0.3 | 2.76 | (0.97–6.10) | 0.5 | 0.2 | 2.19 | (0.58–5.65) | ||
| 40–59 Years | 11.1d | 4.1 | 2.72 | (2.17–3.38) | 13.9d | 5.4 | 2.59 | (1.93–3.42) | 8.5d | 2.8 | 3.1 | (2.10–4.37) | |||
| 60–74 Years | 29.5d | 17.7 | 1.67 | (1.30–2.11) | 38.4d | 26.7 | 1.44 | (1.03–1.95) | 22.1d | 9.2 | 2.41 | (1.61–3.48) | |||
| ≥75 Years | 66.2d | 33.5 | 1.98 | (1.46–2.62) | 106.2d | 51.5 | 2.06 | (1.39–2.95) | 39.5d | 21.2 | 1.86 | (1.10–2.96) | |||
| Alaska | <40 Years | 3.1d | 0.2 | 17.59 | (6.37–59.74) | 2.7d | 0.2 | 14.39 | (3.54–81.20) | 3.4d | 0.2 | 21.7 | (4.78–197.70) | ||
| 40–59 Years | 27.4d | 4.3 | 6.38 | (4.67–8.72) | 36.5d | 5.1 | 7.14 | (4.84–10.56) | 18.4d | 3.3 | 5.49 | (3.18–9.48) | |||
| 60–74 Years | 62.5d | 17.6 | 3.56 | (2.56–4.90) | 86.4d | 26 | 3.32 | (2.23–4.88) | 40.9d | 7.9 | 5.21 | (2.78–9.79) | |||
| ≥75 Years | 89.1d | 30.9 | 2.88 | (1.80–4.54) | 111.5d | 44.3 | 2.51 | (1.28–4.68) | 75.3d | 18.3 | 4.11 | (1.98–8.48) | |||
| Southern Plains | <40 Years | 0.5 | 0.3 | 1.97 | (0.91–3.81) | 0.6 | 0.3 | 1.79 | (0.60–4.26) | 0.5 | 0.2 | 2.29 | (0.65–6.31) | ||
| 40–59 Years | 8.3d | 4.3 | 1.94 | (1.52–2.44) | 9.0d | 6.1 | 1.48 | (1.06–2.03) | 7.7d | 2.5 | 3.01 | (2.08–4.30) | |||
| 60–74 Years | 30.4d | 17 | 1.78 | (1.45–2.17) | 40.6d | 25.2 | 1.61 | (1.24–2.07) | 21.5d | 9.7 | 2.21 | (1.56–3.06) | |||
| ≥75 Years | 51.0d | 27.6 | 1.85 | (1.41–2.38) | 84.3d | 41.8 | 2.01 | (1.42–2.78) | 30.4d | 18.2 | 1.67 | (1.05–2.54) | |||
| Pacific Coast | <40 Years | 0.6d | 0.3 | 2.19 | (1.07–3.97) | 0.7 | 0.3 | 2.23 | (0.86–4.71) | 0.5 | 0.2 | 2.12 | (0.56–5.44) | ||
| 40–59 Years | 6.3d | 4.2 | 1.51 | (1.15–1.94) | 6.4 | 5.6 | 1.13 | (0.77–1.61) | 6.2d | 2.7 | 2.29 | (1.54–3.28) | |||
| 60–74 Years | 22.1 | 17.7 | 1.25 | (0.98–1.56) | 29.3 | 26.6 | 1.1 | (0.81–1.46) | 15.7d | 9.5 | 1.65 | (1.10–2.38) | |||
| ≥75 Years | 51.2d | 32.8 | 1.56 | (1.18–2.04) | 62.7 | 49.4 | 1.27 | (0.83–1.86) | 45.1d | 20.9 | 2.16 | (1.45–3.11) | |||
| East | <40 Years | 0.4 | 0.3 | 1.37 | (0.27–3.90) | 0.3 | 0.3 | 1.04 | (0.03–5.18) | 0.6 | 0.3 | 1.68 | (0.20–6.02) | ||
| 40–59 Years | 8.4d | 4.9 | 1.71 | (1.17–2.42) | 11.6d | 6.7 | 1.73 | (1.06–2.66) | 5.5 | 3.2 | 1.73 | (0.88–3.07) | |||
| 60–74 Years | 25.4 | 20.5 | 1.24 | (0.85–1.74) | 34 | 30.1 | 1.13 | (0.70–1.72) | 17.6 | 11.9 | 1.47 | (0.75–2.59) | |||
| ≥75 Years | 48.9 | 39.7 | 1.23 | (0.77–1.87) | 53 | 57.6 | 0.92 | (0.42–1.75) | 46.9 | 27.9 | 1.68 | (0.89–2.88) | |||
| Southwest | <40 Years | 1.0d | 0.2 | 4.34 | (2.75–6.62) | 0.9d | 0.2 | 3.76 | (1.85–7.02) | 1.1d | 0.2 | 4.97 | (2.63–8.89) | ||
| 40–59 Years | 7.8d | 3.6 | 2.16 | (1.76–2.63) | 9.2d | 4.8 | 1.92 | (1.45–2.49) | 6.6d | 2.4 | 2.73 | (1.98–3.69) | |||
| 60–74 Years | 36.8d | 14.1 | 2.6 | (2.21–3.04) | 56.8d | 20.9 | 2.72 | (2.23–3.29) | 21.3d | 7.8 | 2.73 | (2.03–3.59) | |||
| ≥75+ Years | 90.1d | 28.6 | 3.15 | (2.67–3.71) | 140.8d | 40.3 | 3.5 | (2.81–4.31) | 57.6d | 19.3 | 2.9 | (2.26–3.86) | |||
| Total | <40 Years | 0.9d | 0.3 | 3.06 | (2.40–3.84) | 0.9d | 0.3 | 2.83 | (2.00–3.89) | 0.8d | 0.3 | 3.34 | (2.36–4.60) | ||
| 40–59 Years | 9.5d | 4.3 | 2.18 | (1.99–2.40) | 11.4d | 5.9 | 1.95 | (1.72–2.20) | 7.7d | 2.8 | 2.72 | (2.35–3.15) | |||
| 60–74 Years | 31.9d | 18 | 1.77 | (1.62–1.93) | 44.6d | 26.8 | 1.66 | (1.49–1.86) | 21.2d | 10 | 2.12 | (1.83–2.45) | |||
| ≥75+ Years | 67.5d | 34.3 | 1.96 | (1.77–2.17) | 99.3d | 50.6 | 1.96 | (1.71–2.24) | 47.0d | 23.1 | 2.04 | (1.73–2.38) | |||
Source: Cancer Registries in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute
PRCDA purchased/referred care delivery areas; IHS Indian Health Service; AI/AN American Indian/Alaska Native; CI confidence interval; W non-Hispanic white
AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. Updated bridged intercensal population estimates significantly overestimate AI/AN populations of Hispanic origin. All analyses are limited to non-Hispanic AI/AN populations. Non-Hispanic white was chosen as the reference population. The term “non-Hispanic” is omitted when discussing both groups
Rates are per 100,000 and age-adjusted to the 2000 US standard population (19 age groups-Census P25–1130). Confidence intervals (Tiwari model) are 95% for rates and ratios
Rate ratios are AI/AN versus white and calculated in SEER*Stat software before rounding of rates and may not equal the RR calculated from rates presented in table
The rate ratio indicates that the AI/AN rate is significantly different than the rate for whites (P<0.05).
Regions defined as follows: AK*; Northern Plains (IL, IN,* IA,* MO,* MN,* MT,* NE,* ND,* SD,* WI,* WY*); Southern Plains (OK,* KS,* TX*); Southwest (AZ,* CO,* NV,* NM,* UT*); Pacific Coast (CA,* ID,* OR,* WA,* HI); East (AL,* AR, CT,* DE, FL,* GA, KY, LA,* ME,* MD, MA,* MS,* NH, NJ, NY,* NC,* OH, PA,* RI,* SC,* TN, VT, VA, WV, DC)
Identifies states with at least 1 country designated as PRCDA
Distribution and incidence rates of gastric cancer cases by stage of diagnosis are shown in Figure 1 and Supplemental Table 1. For both sexes, between 25.4% (Southwest) to 42.2% (Alaska) of gastric cancers in AI/AN people (compared to 23%–35.5% in the white population) were diagnosed at distant disease (Supplemental Table 1). In AI/AN males, rates of distant disease were significantly higher in the AI/AN compared to white population in the Northern Plains (RR=2.67), Alaska (RR=4.22), Southern Plains (RR=1.77) and Southwest (RR=2.50). In AI/AN females, rates of distant diseases were higher in the AI/AN versus white population in the Northern Plains (RR = 1.93), Alaska (RR = 2.58), Southern Plains (RR = 2.61), and Southwest (RR = 2.02), with the highest rate occurring in Alaska (3.0/100,000).
Figure 1:

Gastric cancer incidence ratesa and rate ratios by stage of diagnosis, sex, and Indian Health Service region for American Indian/Alaska Nativeb and white populations in PRCDA counties, 2005–2016.
Figure 1 contains two bar separate bar graphs, one for male and one for females. Each show gastric cancer incidence rates for AI/AN compared to white populations by stage of disease and Indian Health service region for those regions that are Purchased/Referred Care Delivery Area (PRCDA) counties. The figures also show the AI/AN versus white rate ratio.
aRates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups-Census P25–1130) standard; Confidence Intervals (Tiwari model) are 95% for rates and ratios
b AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. The updated bridged intercensal population estimates significantly overestimate AI/AN populations of Hispanic origin. All analyses are limited to non-Hispanic AI/AN populations. Non-Hispanic White was chosen as the reference population. The term “non-Hispanic” is omitted when discussing both groups
c The rate ratio indicates that the AI/AN rate is significantly different than the rate for whites (P<0.05). Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups-Census P25–1130) standard; Confidence Intervals (Tiwari model) are 95% for rates and ratios
Localized: Disease restricted to the stomach
Regional: Disease extended into organ and areas adjacent to the stomach.
Distant: Disease metastasized to portions of the body not adjacent to the stomach
Unstaged: Diagnosis could not be determined
Incidence rates of gastric adenocarcinoma by anatomic subsite are shown in Figure 2 and Supplemental Table 2. For all regions combined, incidence rates of adenocarcinoma of the proximal stomach were similar in the AI/AN and white populations for both sexes combined, as well as for males (Supplemental Table 2). Rates in AI/AN females were higher than rates in white females. Rates of adenocarcinoma of the central/distal stomach were significantly higher in AI/AN males than in whites for each region individually, with rate ratios ranging from 2 in the East to over 11 in Alaska, and for all regions combined (overall RR = 3.74, 95% CI 3.30–4.22). Similarly, rates of central/distal cancer were higher in AI/AN females compared to white females, for each region individually, except in the East, and for all regions combined (overall RR = 3.30, 95% CI 2.86–3.79). The highest rate ratio was in Alaska (RR = 8.58).
Figure 2:
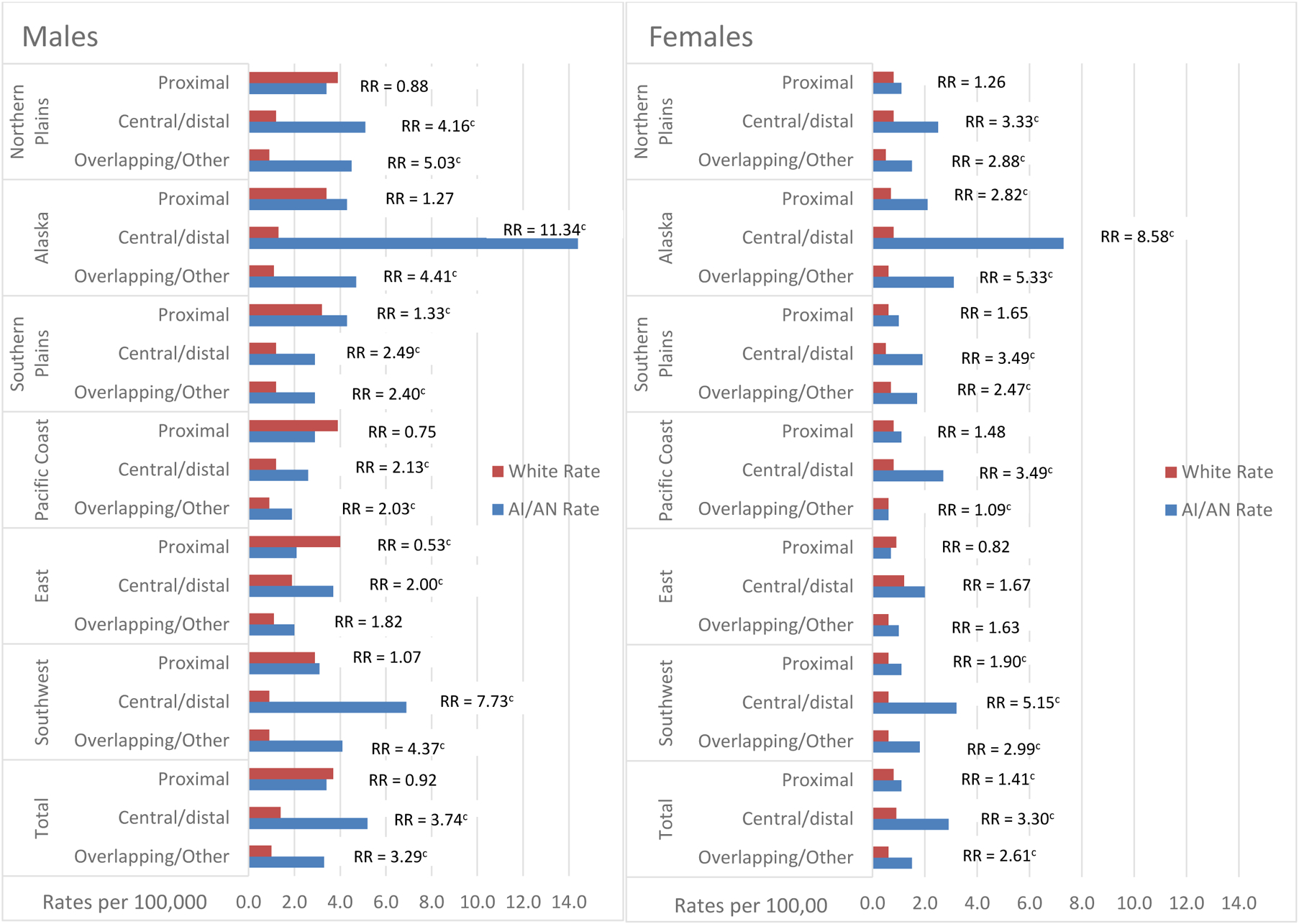
Gastric adenocarcinoma incidence ratesa and rate ratios by anatomic subsite, sex, and Indian Health Service region for American Indian/Alaska Nativeb and white populations in PRCDA counties, 2005–2016.
Figure 2 contains two bar separate bar graphs, one for male and one for females. Each show gastric cancer incidence rates for AI/AN compared to white populations by anatomic subsite and Indian Health service region for those regions that are Purchased/Referred Care Delivery Area (PRCDA) counties. The figures also show the AI/AN versus white rate ratio.
aRates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups-Census P25–1130) standard; Confidence Intervals (Tiwari model) are 95% for rates and ratios
b AI/AN race is reported by NPCR and SEER registries or through linkage with the IHS patient registration database. The updated bridged intercensal population estimates significantly overestimate AI/AN populations of Hispanic origin. All analyses are limited to non-Hispanic AI/AN populations. Non-Hispanic White was chosen as the reference population. The term “non-Hispanic” is omitted when discussing both groups
c The rate ratio indicates that the AI/AN rate is significantly different than the rate for whites (P<0.05). Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups-Census P25–1130) standard; Confidence Intervals (Tiwari model) are 95% for rates and ratios
d Includes primary Sites C16.0-C16.1 (topography codes from the International Classification of Diseases for Oncology)
e Includes primary sites C16.2-C16.6
f Includes primary sites C16.8-C16.9
Overall gastric cancer incidence trends during 1995–2016 for males and females separately are shown in Figure 3; average annual percent change (AAPC) by region and sex is shown in Supplemental Table 3. Overall in the United States, rates for both sexes combined have decreased significantly in the AI/AN (AAPC −1.4) and white (AAPC −1.5) populations. Overall, decreases in rates in AI/AN males (AAPC −1.7) were similar, largely driven by the significant decrease in incidence rates in the Pacific Coast (AAPC −4.0) (Supplemental Table 3). Decreases in rates in AI/AN males in other regions, including Alaska, were not significant, while rates in white males decreased significantly in the East, Southwest, and Pacific Coast. In AI/AN females, significant decreases in rates were observed only in the Northern Plains (AAPC −4.3, Supplemental Table 3), while a significant increase was observed in the East (AAPC 4.4). No significant decreases in rates were observed for the remaining regions or overall (Figure 3, Supplemental Table 3) in AI/AN females. In white females, significant decreases in gastric cancer incidence rates were observed overall (AAPC −1.2) in the Pacific Coast (AAPC −1.2) and Southwest (AAPC −0.8). Gastric cancer incidence rates in Alaska did not decrease by either race or sex.
Figure 3.
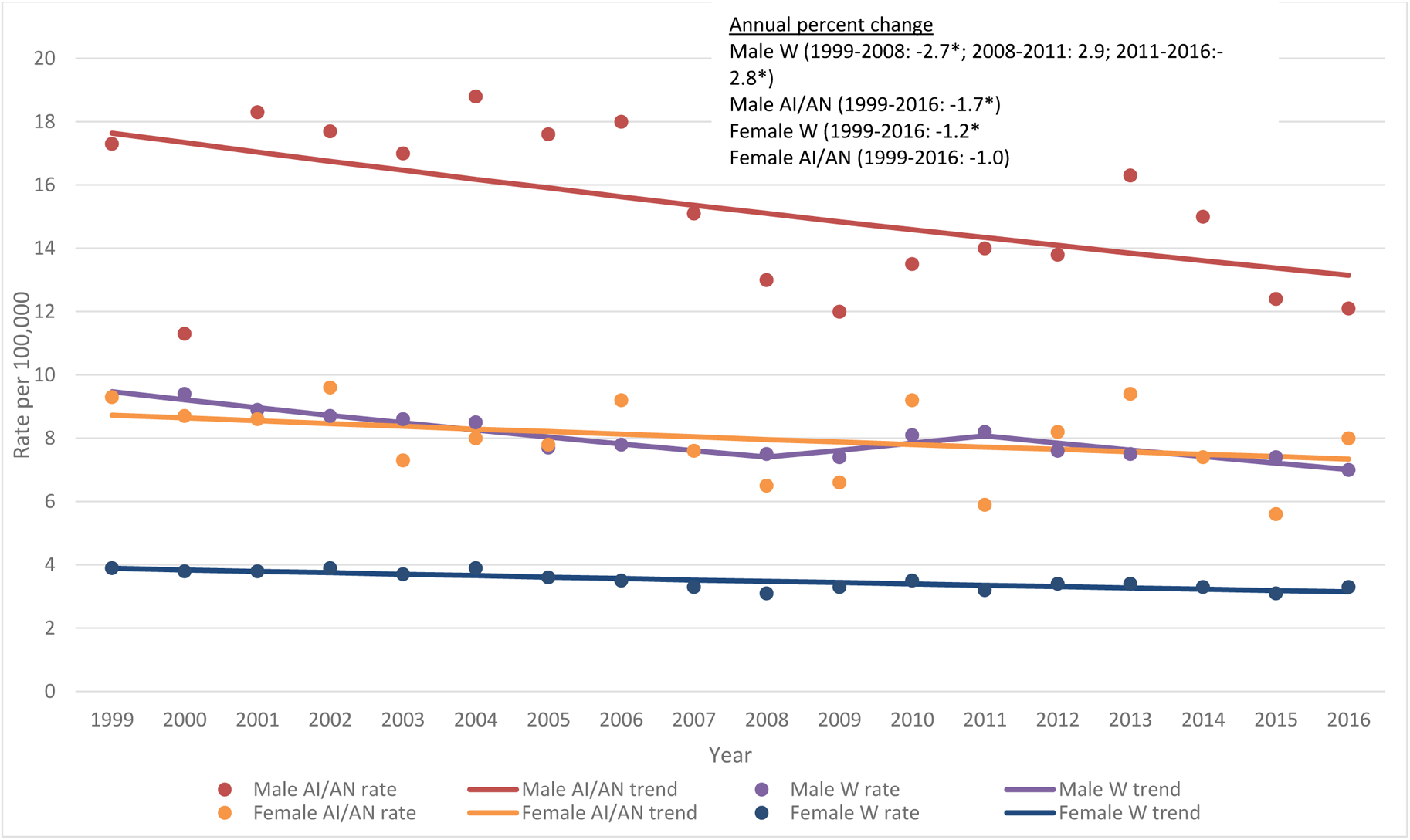
Trends in gastric cancer incidence for American Indian/Alaska Native and white males and females, all regions, PRCDA counties, 1999–2016
Figure 3 contains four lines describing the gastric cancer incidence rates and trends between 1999—2016 for AI/AN males, AI/AN females, white males and white females. It also contains the average annual percent change in incidence rates for these groups.
Source: Cancer Registries in the National Program of Cancer Registries (NPCR) of the Centers for Disease Control and Prevention and the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute
PRCDA Purchased/Referred Care Delivery Areas; IHS Indian Health Service; AI/AN; American Indian/Alaska Native; W; non-Hispanic white
a APC (Annual Percent Change) is based on rates that were age-adjusted to the 2000 US standard population (11 age groups, Census P25–1130).
* 2-sided P < 0.05
DISCUSSION
The results of this study indicate significant differences between gastric cancer incidence rates in AI/AN populations compared to the white population in the United States. Rates also varied by region in AI/AN populations, with the highest rates occurring in Alaska for both males and females. Rates of central/distal gastric cancer were 3.5 times higher in AI/AN people compared to whites overall and significantly higher in Alaska Native populations compared with all other IHS sites. The high rates of gastric cancer in AI/AN people, particularly of central/distal gastric cancer, could indicate a disproportionate prevalence of important gastric cancer risk factors, including prevalence of H. pylori infection [1, 5]. These data also showed that a higher percentage of gastric cancers in AI/AN populations were diagnosed at a later stage, with rates 5 times higher than the general U.S. population.
Previous evidence has established an association between H. pylori infection and gastric cancer that has provided an important understanding of variation in gastric cancer incidence rates worldwide [12, 18]. H. pylori is a common gram-negative bacteria that is typically acquired early in life through person-to-person contact and known to cause chronic gastritis and peptic ulcer disease [12]. Chronic infection with H. pylori has been characterized by the International Agency for Research on Cancer as a Class 1 carcinogen in humans [36]. H. pylori infection results in a chronic, active immune response that, when left untreated, persists for the life of the host [37].
In the United States, the prevalence and incidence of H. pylori infection varies by age, geographic location, and race [38]. Most research on H. pylori prevalence in AI/AN populations focuses on Alaska Native populations, where the burden of H. pylori infection is particularly high (ranging from 64% to 81%) [15, 39]. Similar data regarding H. pylori prevalence is not currently available for the other regions examined in the present study. Although socioeconomic factors are strongly linked with H. pylori prevalence in studies of Alaska Native people and gastric cancers in other populations [15, 40–42], little data are available on H. pylori prevalence and associated risk factors in American Indian populations in other regions. Further research in this area is needed to understand the variation in gastric cancer incidence in AI/AN populations.
H. pylori infection has been associated with living in a more crowded or multifamily household because H. pylori transmission is mainly intrafamilial [17]. Water source and lack of in-home water have also been identified as risk factors [17]. In addition to the high rates of seroprevalence of H. pylori infection, a recent study conducted in three regions of Alaska found a cumulative reinfection rate of 16.1% within the first 2 years of H. pylori treatment [16], which is much higher than the rate in the general U.S. population. Disparities in rates of H. pylori infection, as well as factors associated with transmission and reinfection, could be driving the higher rates of gastric cancers (specifically central/distal gastric cancer) in the Alaska Native population [11, 13]. Further research is needed to characterize the prevalence of H. pylori infection and reinfection in American Indian populations outside of Alaska.
Although H. pylori is a known risk factor, gastric cancer is a multifactorial disease [1, 43], and some of the observed variation in incidence rates could be due to differences in the prevalence of risk factors other than H. pylori that may also contribute to gastric cancer incidence. To date, little research has been done to assess these risk factors specifically in AI/AN populations. Previous research suggests that environmental and behavioral factors play an important role in the development of gastric cancer. Dietary factors, such as the high consumption of fresh fruits and vegetables, have been associated with lowered risk of gastric cancer [20, 44]. Conversely, high intake of salt, nitrites, and nitrates have been associated with higher risk [35, 45, 46]. Refrigeration may have played a role in reducing gastric cancer rates over the last 60 years by decreasing reliance on food preservation methods, such as salt curing, pickling, and meat smoking, which have been shown to be sources of carcinogenic compounds associated with cancer [19, 47, 48]. Although these dietary factors have been associated with gastric cancer in studies of other populations, none of these studies have specifically assessed the effect of dietary patterns in AI/AN populations on gastric cancer incidence.
Although many studies are unable to take into account the role of potential confounders (such as H. pylori infection and dietary intake), substantial evidence exists that smoking is associated with gastric cancer [4, 5, 48–50]. Recent studies have shown that this association persists across gender and race [49]. Further work is needed to understand how this association might be affected by other gastric cancer risk factors.
Family history may also play a role in gastric cancer risk [51]. Case control studies of gastric cancer have reported that the odds ratios associated with family history varied from 2 to 10, depending on the country [52], and 10%–30% of gastric cancer patients had a family history of the disease [51, 53, 54]. Since early detection and diagnosis of gastric cancer play an important role in cancer-related outcomes [1], future studies of family history of gastric cancer in AI/AN populations could help focus screening and intervention programs.
Obesity has also been associated with gastric cancer incidence, particularly gastric cancers of the stomach cardia or proximal site [55–58]. Previous data from the Behavioral Risk Factor Surveillance System suggest that prevalence of obesity is higher in AI/AN populations compared to whites [14] and that prevalence varies by region [59]. Risk factors associated with higher rates of certain subtypes of gastric cancer may be the same risk factors associated with the increasing rates of esophageal adenocarcinoma in the United States, including obesity and gastroesophageal reflux disease [51, 53, 54].
Although this study used the most accurate and current data for cancer incidence in AI/AN populations, it has limitations. Because racial misclassification was addressed by linking with the IHS patient registration database, this correction applied only to people who had ever accessed services through IHS and were members of federally recognized tribes. People who lived in urban, non-PRCDA areas, who were members of a non-federally recognized tribe, or who had not accessed services through IHS were underrepresented. Therefore, our results may not be generalizable to all AI/AN people in the United States or in individual geographic regions. Additionally, information regarding prevalence of important gastric cancer risk factors, including H. pylori infection, was not available. Finally, the exclusion of non-Hispanic AI/AN people may affect rates for certain regions.
This study highlights higher incidence of gastric cancer in AI/AN populations, with particularly high rates in Alaska and the Southwest. These disparities call for new, comprehensive prevention and treatment strategies to reduce disease and death related to gastric cancer, including strategies to identify and address risk factors associated with gastric cancer. Screening and early detection are critical to improving outcomes because later stage disease is difficult to treat and often results in poorer prognosis. While screening high-risk populations for gastric cancer is appropriate in countries with a relatively high incidence rate, screening is generally thought to be costly and unwarranted in countries with low rates of gastric cancer, such as the United States[1]. However, recent studies have shown that gastric cancer screening in high-risk populations can be both cost effective and effective at reducing mortality[60, 61]. Future research is needed to identify people at high risk by considering all potential risk factors and to support, for example, development of a risk prediction or risk stratification model for targeted interventions for this population [62]. Monitoring regional variations in gastric cancer incidence may help identify important differences in risk factors. The resulting data could be used to focus future resources on reducing persistent disparities in gastric cancer incidence in AI/AN populations.
Supplementary Material
Acknowledgements
CDC coauthors participated as a part of their official duties. Additional support was provided by Contract HHSN261201800014I, Task Order HHSN26100001 from the National Cancer Institute (C.Wiggins) and grant number T32CA09168 National Cancer Institute (NCI) (D.Pete).
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
No conflicts of interest to disclose.
REFERENCES
- 1.Karimi P, Islami F, Anandasabapathy S, et al. Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev 2014;23:700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melkonian SC, Jim MA, Haverkamp D, et al. Disparities in Cancer Incidence and Trends among American Indians and Alaska Natives in the United States, 2010–2015. Cancer Epidemiol Biomarkers Prev 2019;28:1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jim MA, Pinheiro PS, Carreira H, et al. Stomach cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:4994–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley JR, Duggan JM. Gastric cancer epidemiology and risk factors. J Clin Epidemiol 2003;56:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Layke JC, Lopez PP. Gastric cancer: diagnosis and treatment options. Am Fam Physician 2004;69:1133–40. [PubMed] [Google Scholar]
- 7.Wiggins CL, Perdue DG, Henderson JA, et al. Gastric cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer 2008;113:1225–33. [DOI] [PubMed] [Google Scholar]
- 8.White MC, Espey DK, Swan J, et al. Disparities in cancer mortality and incidence among American Indians and Alaska Natives in the United States. Am J Public Health 2014;104 Suppl 3:S377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinson HA, Shelby NJ, Alberts SR, et al. Gastric cancer in Alaska Native people: A cancer health disparity. World J Gastroenterol 2018;24:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IARC Helicobacter pylori Working Group (2014). Helicobacter pylori Eradication as a Strategy for Preventing Gastric Cancer. Lyon, France: International Agency for Research on Cancer (IARC Working Group Reports, No. 8) Available from: http://www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php. [Google Scholar]
- 11.McMahon BJ, Bruce MG, Koch A, et al. The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: Expert Commentary. Epidemiol Infect 2016;144:225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stolte M, Meining A. Helicobacter pylori and Gastric Cancer. Oncologist 1998;3:124–128. [PubMed] [Google Scholar]
- 13.Wroblewski LE, Peek RM Jr. Helicobacter pylori, Cancer, and the Gastric Microbiota. Adv Exp Med Biol 2016;908:393–408. [DOI] [PubMed] [Google Scholar]
- 14.Cobb N, Espey D, King J. Health behaviors and risk factors among American Indians and Alaska Natives, 2000–2010. Am J Public Health 2014;104 Suppl 3:S481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keck JW, Miernyk KM, Bulkow LR, et al. Helicobacter pylori infection and markers of gastric cancer risk in Alaska Native persons: a retrospective case-control study. Can J Gastroenterol Hepatol 2014;28:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMahon BJ, Bruce MG, Hennessy TW, et al. Reinfection after successful eradication of Helicobacter pylori: a 2-year prospective study in Alaska Natives. Aliment Pharmacol Ther 2006;23:1215–23. [DOI] [PubMed] [Google Scholar]
- 17.Miernyk KM, Bulkow LR, Gold BD, et al. Prevalence of Helicobacter pylori among Alaskans: Factors associated with infection and comparison of urea breath test and anti-Helicobacter pylori IgG antibodies. Helicobacter 2018;23:e12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wroblewski LE, Peek RM Jr., Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 2010;23:713–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang X, Wei J, He X, et al. Landscape of dietary factors associated with risk of gastric cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Cancer 2015;51:2820–32. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, et al. Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Int J Cancer 2012;131:2910–9. [DOI] [PubMed] [Google Scholar]
- 21.Espey DK, Wiggins CL, Jim MA, et al. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer 2008;113:1120–30. [DOI] [PubMed] [Google Scholar]
- 22.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev 1999;8:1117–21. [PubMed] [Google Scholar]
- 23.Thoburn KK, German RR, Lewis M, et al. Case completeness and data accuracy in the Centers for Disease Control and Prevention’s National Program of Cancer Registries. Cancer 2007;109:1607–16. [DOI] [PubMed] [Google Scholar]
- 24.Fritz A, Percy C, Jack A, Shanmugaratnum K, Sobin L, Parkin DM, Whelan S (Editors). International Classification of Diseases for Oncology. Third Edition World Health Organization; Geneva, 2000. [Google Scholar]
- 25.Frost F, Taylor V, Fries E. Racial misclassification of Native Americans in a surveillance, epidemiology, and end results cancer registry. J Natl Cancer Inst 1992;84:957–62. [DOI] [PubMed] [Google Scholar]
- 26.Partin MR, Rith-Najarian SJ, Slater JS, et al. Improving cancer incidence estimates for American Indians in Minnesota. Am J Public Health 1999;89:1673–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jim MA, Arias E, Seneca DS, et al. Racial misclassification of American Indians and Alaska Natives by Indian Health Service Contract Health Service Delivery Area. Am J Public Health 2014;104 Suppl 3:S295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng XJ, Lin JC, Tu SP. Etiology and Prevention of Gastric Cancer. Gastrointest Tumors 2016;3:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA (eds). SEER Summary Staging Manual-2000: Codes and Coding Instructions, National Cancer Institute, NIH Pub. No. 01–4969, Bethesda, MD, 2001. [Google Scholar]
- 30.Suryaprasad A, Byrd KK, Redd JT, et al. Mortality caused by chronic liver disease among American Indians and Alaska Natives in the United States, 1999–2009. Am J Public Health 2014;104 Suppl 3:S350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothman KJ, Greenland S. Modern Epidemiology, Second Edition (pp. 260–261). Lippincott, Williams and Wilkins, Philadelphia; 1998. [Google Scholar]
- 32.Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population Healthy People 2010 Statistical Notes, No. 20 Hyattsville, Maryland: National Center for Health Statisics; January 2001. [PubMed] [Google Scholar]
- 33.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 35.Ge S, Feng X, Shen L, et al. Association between Habitual Dietary Salt Intake and Risk of Gastric Cancer: A Systematic Review of Observational Studies. Gastroenterol Res Pract 2012;2012:808120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou CK, Pfeiffer RM, Cleary SD, et al. Relationship between male pattern baldness and the risk of aggressive prostate cancer: an analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Clin Oncol 2015;33:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Logan RP, Walker MM. ABC of the upper gastrointestinal tract: Epidemiology and diagnosis of Helicobacter pylori infection. BMJ 2001;323:920–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everhart JE, Kruszon-Moran D, Perez-Perez GI, et al. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis 2000;181:1359–63. [DOI] [PubMed] [Google Scholar]
- 39.Parkinson AJ, Gold BD, Bulkow L, et al. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol 2000;7:885–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol 2010;25:479–86. [DOI] [PubMed] [Google Scholar]
- 41.Jarosz M, Rychlik E, Siuba M, et al. Dietary and socio-economic factors in relation to Helicobacter pylori re-infection. World J Gastroenterol 2009;15:1119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malaty HM, Paykov V, Bykova O, et al. Helicobacter pylori and socioeconomic factors in Russia. Helicobacter 1996;1:82–7. [DOI] [PubMed] [Google Scholar]
- 43.Talley NJ, Fock KM, Moayyedi P. Gastric Cancer Consensus conference recommends Helicobacter pylori screening and treatment in asymptomatic persons from high-risk populations to prevent gastric cancer. Am J Gastroenterol 2008;103:510–4. [DOI] [PubMed] [Google Scholar]
- 44.Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev 2006;15:1998–2001. [DOI] [PubMed] [Google Scholar]
- 45.D’Elia L, Galletti F, Strazzullo P. Dietary salt intake and risk of gastric cancer. Cancer Treat Res 2014;159:83–95. [DOI] [PubMed] [Google Scholar]
- 46.Zhang FX, Miao Y, Ruan JG, et al. Association Between Nitrite and Nitrate Intake and Risk of Gastric Cancer: A Systematic Review and Meta-Analysis. Med Sci Monit 2019;25:1788–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer 2007;10:75–83. [DOI] [PubMed] [Google Scholar]
- 48.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol 2009;472:467–77. [DOI] [PubMed] [Google Scholar]
- 49.Nomura AM, Wilkens LR, Henderson BE, et al. The association of cigarette smoking with gastric cancer: the multiethnic cohort study. Cancer Causes Control 2012;23:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henley SJ, Thomas CC, Sharapova SR, et al. Vital Signs: Disparities in Tobacco-Related Cancer Incidence and Mortality - United States, 2004–2013. MMWR Morb Mortal Wkly Rep 2016;65:1212–1218. [DOI] [PubMed] [Google Scholar]
- 51.Bernini M, Barbi S, Roviello F, et al. Family history of gastric cancer: a correlation between epidemiologic findings and clinical data. Gastric Cancer 2006;9:9–13. [DOI] [PubMed] [Google Scholar]
- 52.Yaghoobi M, Bijarchi R, Narod SA. Family history and the risk of gastric cancer. Br J Cancer 2010;102:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000;343:78–85. [DOI] [PubMed] [Google Scholar]
- 54.Zanghieri G, Di Gregorio C, Sacchetti C, et al. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer 1990;66:2047–51. [DOI] [PubMed] [Google Scholar]
- 55.Yang P, Zhou Y, Chen B, et al. Overweight, obesity and gastric cancer risk: results from a meta-analysis of cohort studies. Eur J Cancer 2009;45:2867–73. [DOI] [PubMed] [Google Scholar]
- 56.Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer 2008;44:465–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindblad M, Rodriguez LA, Lagergren J. Body mass, tobacco and alcohol and risk of esophageal, gastric cardia, and gastric non-cardia adenocarcinoma among men and women in a nested case-control study. Cancer Causes Control 2005;16:285–94. [DOI] [PubMed] [Google Scholar]
- 58.Jee SH, Yun JE, Park EJ, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer 2008;123:1892–6. [DOI] [PubMed] [Google Scholar]
- 59.Melkonian SC, Jim MA, Reilley B, et al. Incidence of primary liver cancer in American Indians and Alaska Natives, US, 1999–2009. Cancer Causes Control 2018;29:833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saumoy M, Schneider Y, Shen N, et al. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018;155:648–660. [DOI] [PubMed] [Google Scholar]
- 61.Zhang X, Li M, Chen S, et al. Endoscopic Screening in Asian Countries Is Associated With Reduced Gastric Cancer Mortality: A Meta-analysis and Systematic Review. Gastroenterology 2018;155:347–354 e9. [DOI] [PubMed] [Google Scholar]
- 62.Nolen LD, Vindigni SM, Parsonnet J; Symposium Leaders, Bruce MG, Martinson HA,Thomas TK, Sacco F, Nash S, Olnes MJ, Miernyk K, Bruden D, Ramaswamy M, McMahon B, Goodman KJ, Bass AJ, Hur C, Inoue M, Camargo MC, Cho SJ, Parnell K, Allen E, Woods T, Melkonian S. Combating gastric cancer in Alaska Native people: An expert and community symposium: Alaska Native Gastric Cancer Symposium. Gastroenterology. 2019. December 10 pii: S0016–5085(19)41901-X. doi: 10.1053/j.gastro.2019.11.299. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


