Abstract
Background
Chronic spontaneous urticaria (CSU) is a common pruritic skin condition, the pathogenesis of which remains unclear. Interleukin-31 (IL-31) is a major pruritogenic cytokine that plays a role in inducing pruritus in various skin diseases.
Aim.
To 1) compare serum IL-31 levels among CSU patients, psoriasis patients with pruritic symptoms, and healthy subjects, 2) examine the correlations between serum IL-31 levels and disease severity, and 3) compare IL-31 levels in patients with and without CSU-associated auto-antibodies.
Methods
Patients with CSU, psoriasis with pruritic symptoms, and healthy volunteers were recruited in the study. Serum IL-31 levels were measured with commercial kits. Baseline characteristics, urticaria activity score, psoriasis area severity index, pruritic intensity score, and related laboratory results were collected.
Results
Sixty-five CSU patients, 30 psoriasis patients who had pruritus, and 31 healthy subjects participated in our study. The CSU patients had significantly higher mean serum IL-31 levels than the psoriasis patients (252.4 ± 115.5 vs 121.4 ± 16.6 pg/mL, P < 0.001). Both CSU and psoriasis patients also had significantly higher mean serum IL-31 when compared with the healthy subjects. Serum IL-31 levels of CSU and psoriasis patients did not differ significantly according to disease or itching severity. Thyroid antibodies and antinuclear antibodies were positive in 22 (33.8%) and 28 (43.1%) CSU patients, respectively. The CSU patients with ANA titers ≥1:160 had significantly higher mean serum IL-31 levels than in those who were negative for ANA and those with titers of 1:80 (P < 0.003 and P < 0.008, respectively).
Conclusion
Higher serum IL-31 levels were found in patients with CSU and psoriasis with pruritic symptoms. This suggests that IL-31 has a possible role in the pathogenesis of CSU and psoriasis with pruritic symptoms.
Keywords: Pathology, Pathophysiology, Immunology, Immune system, Internal medicine, Dermatology, Antibody, Interleukin, Pruritus, Psoriasis, Urticaria
Pathology; Pathophysiology; Immunology; Immune system; Internal medicine; Dermatology; Antibody, Interleukin, Pruritus, Psoriasis, Urticaria.
1. Introduction
Chronic spontaneous urticaria (CSU) is a common skin disease characterized by itchy wheals, angioedema, or both occurring at least twice a week for longer than 6 weeks without inducible causes [1]. Mast cells and basophils play key roles in the disease and can be activated through the binding of allergen-specific IgE and its high-affinity receptor (FcεRI). Circulating IgG autoantibodies against IgE or FcεRI are also capable of stimulating mast cells and basophils and are detected in about one-third of CSU patients [2, 3]. Degranulation of these cells releases pre-formed vasoactive mediators, mainly histamine, pro-inflammatory mediators, and newly synthesized mediators including tumor necrosis factor, cytokines, leukotrienes, prostaglandins, and platelet-activating factor [4]. However, only approximately 60% of CSU patients respond to a fourfold increase in antihistamine dosage [5], with others requiring other treatment options such as cyclosporine, omalizumab, or a short course of systemic corticosteroids. This is likely due to the fact that the disease is autoimmune in nature or because of other immunologic mechanisms. Omalizumab, an anti-IgE monoclonal antibody, has been shown to be around 80% effective in treating CSU [6]. It binds to free serum IgE, leading to reductions in serum IgE levels and thus downregulation of FcεRI receptors [7, 8, 9]. Some patients do not respond to anti-IgE monoclonal antibody, which means there are likely mechanisms involved in CSU pathogenesis other than autoimmunity and mast cell or basophil activation.
In addition to mast cells, mononuclear cell infiltration is also found in chronic urticaria lesions, especially in CD4+ T-helper (Th) cells [10], which can produce various cytokines. Interleukin-31 (IL-31), a major pruritogenic cytokine and a member of the gp130/IL-6 family, is mainly produced by activated Th2 cells. Mast cells, macrophages, dendritic cells, eosinophils, and basophils can also express IL-31 [11]. IL-31 initially signals through a heterodimeric receptor complex composed of IL-31 receptor alpha (IL-31RA) and oncostatin M receptor beta (OSMRβ) and subsequently activates signaling pathways, including the JAK/STAT (Janus-activated kinase/signal transducer and activator of transcription), PI3K/AKT (phosphatidylinositol 3-kinase/protein kinase), and MAPK (mitogen-activated protein kinase) pathways, resulting in the release of chemokines and proinflammatory cytokines [12, 13]. Several studies have shown that IL-31 plays a key role in inducing pruritus in chronic inflammatory skin diseases such as atopic dermatitis [14, 15, 16], prurigo nodularis [17], contact dermatitis [18], psoriasis [19, 20], and CSU [21, 22, 23, 24], but no study has yet been conducted to compare CSU and inflammatory dermatitis (such as psoriasis). We, therefore, investigated differences in serum IL-31 levels among CSU patients, psoriasis vulgaris patients who had pruritus, and healthy subjects as well as possible correlations between IL-31 levels and disease severity or itch intensity. We also investigated the possible association of autoimmune diseases with IL-31 levels in CSU patients.
2. Materials and methods
2.1. Participants
This was a cross-sectional, analytical study conducted at Khon Kaen University's Srinagarind Hospital in Thailand. Patients diagnosed with CSU and psoriasis vulgaris with pruritus between October 2017 and December 2019 were included. CSU was defined as itchy wheals, angioedema, or both occurring at least twice a week for longer than 6 weeks. The diagnosis of psoriasis vulgaris was based on clinical examination and histological confirmation. Exclusion criteria included urticarial vasculitis, physical urticaria, and administration of any systemic steroids, immunosuppressive drugs, or phototherapy within 3 months before participating in the study. The healthy group consisted of healthy adults undergoing plastic reconstructive or excisional surgical procedures at Srinagarind Hospital with no pruritic symptoms or co-morbid diseases including malignancy. This study was approved by Khon Kaen University's ethics committee [HE601361]. Informed consent was obtained from all participants.
2.2. Disease activity assessment
Chronic spontaneous urticaria severity was assessed based on patients' urticaria activity scores (which were the sum of their hives and itch severity scores) over 7 consecutive days (UAS7; range, 0–42). The hives score ranges from 0 to 3: 0 = no hives, 1 = mild (<20 hives/24 h), 2 = moderate (20–50 hives/24 h), 3 = severe (>50 hives/24 h or large confluent area of hives), and the itch severity score ranges from 0 to 3: 0 = no itch, 1 = mild (present, but not annoying), 2 = moderate (troublesome, but does not disturb normal daily activity or sleep), and 3 = severe (disturbs normal daily activity or sleep). Patients’ UAS7 scores were categorized into 3 disease activity states, as follows: 0–15 = mild, 16–27 = moderate, and 28–42 = severe. Weekly itch severity was classified into 3 levels as follows: 0–7 = mild, 8–14 = moderate, and 15–21 = severe.
Psoriasis severity was evaluated based on patients’ PASI scores (Psoriasis Activity and Severity Index), which range from 0 to 72 points and were categorized into 3 levels: 0–10 = mild, 11–20 = moderate, and >20 = severe. The average intensity of pruritus was assessed using an 11-point numeric rating scale (NRS-11) ranging from 0 (no itch) to 10 (worst imaginable itch). These scores were classified into 3 levels as follows: 0–3 = mild, 4–7 = moderate, and 8–10 = severe.
2.3. Baseline characteristics and laboratory parameters
Demographic data and baseline characteristics, including age, sex, duration and severity of the disease, laboratory results, and IL-31 levels, of all participants were recorded. Complete blood count and serum creatinine levels were obtained for all participants. Serum antinuclear antibodies (ANA) and thyroid antibodies, including anti-thyroid peroxidase (anti-TPO) antibodies and anti-thyroglobulin (anti-TG) antibodies, were tested only in CSU patients. All laboratory tests were performed at the hospital's central laboratory. Serum ANA was detected by indirect immunofluorescence assay and results in which titer ≥1:80 were considered positive. Anti-TPO and anti-TG were performed using electrochemiluminescence immunoassay, and results were considered positive if anti-TPO titer was >34 IU/mL or anti-TG was >115 IU/mL.
2.4. Analysis of IL-31 serum levels
Serum specimens were collected and stored at -80 °C. Concentrations of IL-31 in serum were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) with standard kits according to the manufacturer's instructions (Human IL-31 ELISA Kit, Abcam, Cambridge, MA, USA).
2.5. Statistical analysis
Statistical analysis was performed using STATA version 10.1 (College Station, Texas, USA). Parametric data were presented as mean ± standard deviation (SD), and a one-way ANOVA and Bonferroni post hoc test were used to compare between the groups. Non-parametric data were presented as median with inter-quartile range (IQR) and a Kruskal-Wallis test was used to assess the significant differences between the groups. P-values below 0.05 were considered statistically significant.
3. Results
3.1. Patient characteristics
There were 65 CSU patients, 30 psoriasis patients, and 31 healthy subjects who participated in this study. The median duration of disease in CSU and psoriasis patients was 8 and 24 months, respectively. Fifty-eight (89.2%) of the 65 CSU patients were taking an antihistamine at the time of recruitment. The CSU group differed from the psoriasis and healthy groups significantly in terms of sex, total lymphocyte count, and total basophil count (Table 1).
Table 1.
Baseline characteristics and laboratory results of chronic spontaneous urticaria (CSU) patients, psoriasis patients, and healthy subjects.
| Factors | CSU patients n = 65 | Psoriasis patients n = 30 | Healthy subjects n = 31 | P-values∗ |
|---|---|---|---|---|
| Age (years): mean ± SD | 43 ± 15 | 44 ± 18 | 44 ± 18 | 0.92 |
| Female sex, n (%) | 51 (76.1) | 15 (45.5) | 19 (57.6) | 0.008 |
| Hemoglobin (g/dL): median (IQR) | 13.2 (12.6–14.1) | 13.5 (12.9–14.8) | 13.5 (12.6–14.2) | 0.35 |
| White blood cell (cells/mm3): median (IQR) | 7710 (6390–9040) | 6900 (5800–9670) | 6800 (5860–8250) | 0.30 |
| Total neutrophil count (cells/mm3): median (IQR) | 4418 (3177–5756) | 4109 (3334–4827) | 4210 (3075–5363) | 0.72 |
| Total lymphocyte count (cells/mm3): median (IQR) | 2477 (1977–2893) | 1841 (1579–2769) | 1978 (1780–2297) | 0.002, 0.004, 0.48 |
| Total monocyte count (cells/mm3): median (IQR) | 533 (400–673) | 512 (390–842) | 475 (402–560) | 0.23 |
| Total eosinophil count (cells/mm3): median (IQR) | 132 (83–218) | 197 (92–463) | 143 (74–284) | 0.15 |
| Total basophil count (cells/mm3): median (IQR) | 26 (16–38) | 48 (26–88) | 41 (30–59) | 0.0002, 0.0002, 0.24 |
| Serum creatinine, mg/dL (mean ± SD) | 0.85 ± 0.2 | 0.89 ± 0.2 | 0.82 ± 0.2 | 0.99, 0.99, 0.41 |
Abbreviations: g/dL, gram/deciliter; IQR, Interquartile range; mg/dL, milligram/deciliter; mm3, cubic millimeter; SD, Standard deviation.
P-values between CSU and healthy subjects, CSU and psoriasis, and psoriasis and healthy subjects, respectively.
3.2. Serum IL-31 levels in CSU, psoriasis, and healthy groups
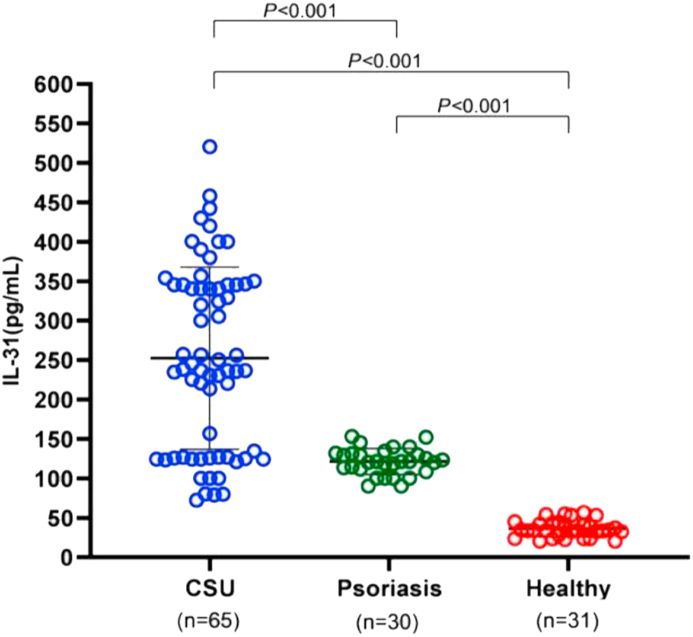
Serum Interleukin-31 levels in the CSU group were significantly higher than in the psoriasis and healthy groups (Figure 1). Mean IL-31 in the CSU group was about seven times higher than in the healthy group (252.4 ± 115.5 vs 36.3 ± 10.7 pg/mL, P < 0.001) and was about twice as high as in the psoriasis group (252.4 ± 115.5 vs 121.4 ± 16.6 pg/mL, P < 0.001). Additionally, mean IL-31 in the psoriasis group was also significantly higher than in the healthy group (P < 0.001; Figure 1).
Figure 1.
Comparison of serum IL-31 levels among CSU patients, psoriasis patients, and healthy subjects. Serum IL-31 levels were significantly higher in CSU patients than in those with psoriasis or healthy subjects.
3.3. Serum IL-31 levels and disease severity in the CSU and psoriasis groups
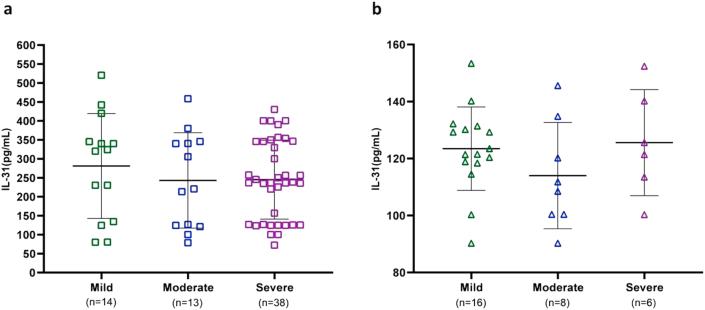
We assessed the mean IL-31 levels in the CSU group according to disease severity. Based on patients’ UAS7 scores, 14 had mild (281 ± 138.4 pg/mL), 13 had moderate (242.8 ± 125.7 pg/mL), and 38 had severe (245.1 ± 104.0 pg/mL) CSU. There were no significant differences in IL-31 levels among these groups (P = 0.58; Figure 2). Psoriasis severity was evaluated based on PASI scores and was mild in 16 patients (123.5 ± 14.6 pg/mL), moderate in 8 (114 ± 18.7 pg/mL), and severe in 6 (125.6 ± 18.6 pg/mL). There were no significant differences in mean IL-31 among these groups (P = 0.34; Figure 2).
Figure 2.
Comparison of serum IL-31 levels and disease severity in CSU according to UAS7 (a) and in psoriasis based on PASI (b). There were no significant differences in IL-31 levels among groups.
3.4. Serum IL-31 levels and pruritus intensity in the CSU and psoriasis groups
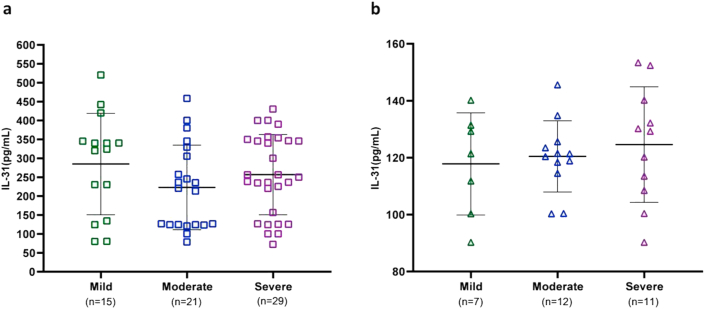
Pruritus intensity in CSU patients was measured based on weekly itch severity and was mild in 15 patients (285 ± 134.2 pg/mL), moderate in 21 (222.9 ± 111.9 pg/mL), and severe in 29 (256.9 ± 106.2 pg/mL). No significant differences in mean IL-31 were detected among these groups (P = 0.28; Figure 3). In the psoriasis group, itch intensity was evaluated according to the NRS-11. Seven patients had mild (117.8 ± 17.9 pg/mL), 12 had moderate (120.4 ± 12.5 pg/mL), and 11 had severe itch (124.6 ± 20.3 pg/mL). There were no significant differences in mean IL-31 among these groups (P = 0.69; Figure 3).
Figure 3.
Comparison of serum IL-31 levels and pruritus intensity in CSU according to weekly itch intensity (a) and in psoriasis based on NRS-11 (b). There were no significant differences in IL-31 levels among groups.
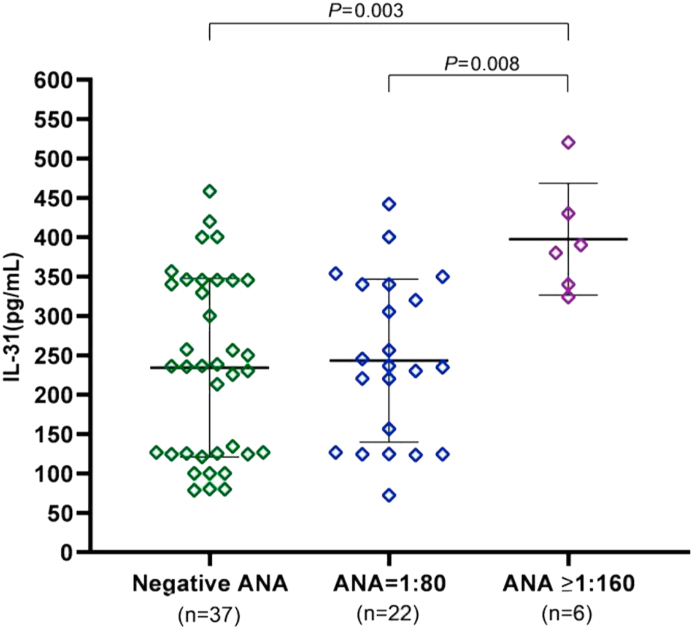
3.5. Serum IL-31 levels and thyroid autoimmunity and ANA in the CSU group
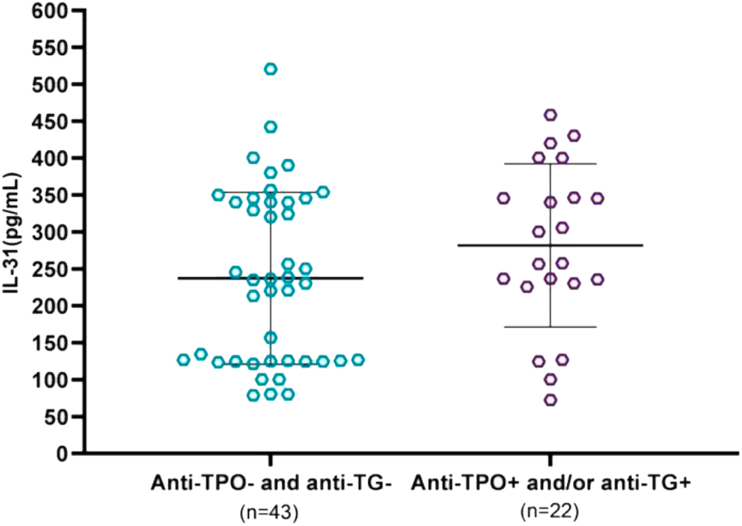
Patients were categorized as having thyroid autoimmunity if they tested positive for anti-TPO or anti-TG. Thyroid autoantibody was detected in 22 (33.8%) of 65 patients (16 [24.6%] anti-TPO, 15 [23.1%] anti-TG, and 9 [13.8%] both anti-TPO and anti-TG). Mean IL-31 was 281.7 ± 110.4 pg/mL in patients with thyroid autoimmunity and 237.4 ± 116.4 pg/mL in those without, with no significant difference between groups (P = 0.15; Figure 4). Twenty-eight (43.1%) of the 65 patients were positive for ANA: 22 (33.8%) with titers of 1:80 and 6 (9.2%) with titers ≥1:160. The mean IL-31 levels in the patients with ANA titers ≥1:160 were significantly higher than in those who were negative for ANA (397.6 ± 71 vs 234.3 ± 113.4 pg/mL, P < 0.003) and those with titers of 1:80 (397.6 ± 71 vs 243.3 ± 103.5 pg/mL, P < 0.008; Figure 5).
Figure 4.
Comparison of serum IL-31 levels and thyroid autoimmunity in CSU patients. There was no significant difference in IL-31 levels between groups.
Figure 5.
Comparison of serum IL-31 levels and ANA in CSU patients. Serum IL-31 levels were significantly higher in CSU patients with ANA titers ≥1:160 than in those who were negative for ANA and those with titers of 1:80.
4. Discussion
The pathogenesis of chronic spontaneous urticaria (CSU) is complex and not yet completely understood. IL-31 is a T cell-derived cytokine mainly produced by activated Th2 cells, but mast cells, macrophages, dendritic cells, eosinophils, and basophils are also a major source [11]. Recent studies have shown that IL-31 plays a role in chronic pruritic inflammatory skin diseases and autoimmune skin conditions including psoriasis [19, 20] and CSU [21, 22, 23, 24]. This study showed that patients with CSU had significantly higher serum IL-31 levels than those with psoriasis and healthy subjects. Moreover, serum IL-31 levels in psoriasis patients who had pruritus were also significantly higher than those of healthy subjects. This means that itching symptoms in CSU and psoriasis with pruritus might have a similar mechanism related to IL-31. Previous studies have found serum IL-31 levels to be significantly higher in patients with uremic pruritus and intrahepatic cholestasis of pregnancy, both of which are chronic pruritic skin conditions with subtle or no skin inflammation [25, 26]. In a recent study, we also found that serum IL-31 levels were significantly higher in patients who had chronic pruritus of unknown origin without skin lesions than in healthy subjects [27]. These findings imply that there is an association between IL-31 and itching. One study found that IL-31-deficient (Il31−/−) mice had reduced scratching frequency and duration but not reduced inflammation during contact hypersensitivity induced by FITC and DNFB [28]. Other recent studies found that anti-IL-31 receptor A antibody significantly reduced pruritus in moderate to severe atopic patients [29, 30]. Our study might indicate that IL-31 is responsible for chronic pruritic conditions regardless of inflammation. The difference in serum IL-31 levels in patients with CSU and those with psoriasis with pruritic symptoms might be due to the itching in each condition being caused by a separate mechanism. The exact mechanism of IL-31 in chronic pruritic skin conditions needs to be further investigated.
Several reports have demonstrated that serum IL-31 levels are correlated with disease severity in atopic dermatitis patients [15, 31, 32] and with itching severity in uremic pruritus [25] and CSU patients [22]. One study found that serum IL-31 levels decreased significantly in psoriatic patients after 20 sessions of narrow-band ultraviolet radiation exposure [19]. Moreover, a study in CSU patients showed significant reductions in serum IL-31 in patients successfully treated with omalizumab [23]. Another recent study also showed a significant correlation between improvement of clinical symptoms and decreases in IL-31-secreting T cells in CSU patients who responded to omalizumab [21]. In this study, IL-31 levels in CSU and psoriasis patients were not significantly correlated with disease severity (as assessed using UAS7 and PASI scores, respectively) nor with itching severity (based on weekly itch and NRS-11 scores, respectively). This might be due to the subjective assessment of pruritus severity, individual variations in pruritic threshold, and/or the small number of subjects examined.
This study also found that CSU patients differed from those with psoriasis and healthy subjects in terms of sex, total lymphocyte count, and total basophil count (Table 1). There were more female CSU patients than males, which corresponds with the findings of other studies [33, 34]. This might be due to autoimmune processes, as thyroid autoantibodies and ANA were more commonly detected in female patients than in their male counterparts (32 [84.2%] of 38 patients with thyroid autoantibodies or ANA were female; data not shown), confirming the findings of a previous report [34]. In terms of basopenia in CSU, previous studies have found decreases in peripheral blood basophils in patients with chronic urticaria as compared to healthy subjects [35, 36, 37], which one study found to be related to the presence of autoantibodies and serum histamine releasing activity [36]. Peripheral blood basopenia in CSU may be due to basophil recruitment from the blood into urticarial lesions (which was detected by skin biopsy) [38, 39] or to increased lymphocyte production (which was found in our study), resulting in relatively low peripheral blood basophil counts. The association of lymphocytes and basophils in CSU should thus be investigated further. However, there was no correlation between total basophil count and urticarial activity in CSU patients in this study (P = 0.49).
Approximately 30%–40% of CSU patients have circulating IgG autoantibodies against IgE or the high-affinity IgE receptor (FcεRI) [2, 3], which is often classified as an autoimmune disease. Chronic spontaneous urticaria is also associated with other autoimmune diseases such as autoimmune thyroid diseases, type 1 diabetes mellitus, rheumatoid arthritis, celiac disease, vitiligo, pernicious anemia, autoimmune polyglandular syndromes, and systemic lupus erythematosus [40]. A previous study found autoimmune thyroid disease in from 3.7-37.1% CSU patients [41], and another found that 0–31.9% were positive for ANA [40]. In this study, thyroid autoimmunity was detected in a similar percentage of CSU patients (33.8%), but the percentage of patients with ANA (43.1%) was higher than in previous studies. There was no significant correlation between IL-31 levels and thyroid autoimmunity. However, we found that CSU patients with ANA titers ≥1:160 had significantly higher serum IL-31 levels than in those who were negative for ANA and those with titers of 1:80. Various studies have shown that IL-31 plays a role in autoimmune skin diseases including CSU and autoimmune connective tissue diseases (systemic lupus erythematosus and dermatomyositis) [11]. These findings imply that high serum IL-31 levels in CSU patients might be a marker for seeking autoimmune connective tissue disease comorbidities.
There were some limitations to this study. First, only IL-31 was measured. Second, there was a relatively small total number of participants and a different number with each level of disease severity due to the non-randomized study design. Third, no ASST was performed in this study; however, a previous report found no difference in serum IL-31 levels in patients with positive versus negative ASST [24]. Lastly, ours was a cross-sectional study, necessitating follow-up research on complete remission after treatment to demonstrate the role of IL-31 in the pathogenesis of the disease.
5. Conclusion
Serum IL-31 levels were significantly higher in CSU and psoriasis patients than in healthy subjects. Moreover, serum IL-31 levels in psoriasis patients were also significantly higher than in healthy subjects. There was no significant difference between serum IL-31 levels and disease severity or itch intensity in CSU and psoriasis. However, serum IL-31 levels were significantly higher in CSU patients with ANA titers ≥1:160 than in those who were negative for ANA and those with titers of 1:80.
Declarations
Author contribution statement
S. Chaowattanapanit: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
C. Choonhakarn: Conceived and designed the experiments; Performed the experiments.
K. Salao: Performed the experiments; Contributed reagents, materials, analysis tools or data.
K. Winaikosol: Performed the experiments.
N. Julanon and R. Wongjirattikarn: Conceived and designed the experiments.
C. Foocharoen: Analyzed and interpreted the data.
M. Sompornrattanaphan: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Khon Kaen University, Thailand (IN61211).
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to thank Dr. Dylan Southard (USA) for editing this manuscript.
References
- 1.Zuberbier T., Aberer W., Asero R., Abdul Latiff A.H., Baker D., Ballmer-Weber B. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–1414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 2.Hide M., Francis D.M., Grattan C.E., Hakimi J., Kochan J.P., Greaves M.W. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N. Engl. J. Med. 1993;328(22):1599–1604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 3.Fiebiger E., Maurer D., Holub H., Reininger B., Hartmann G., Woisetschlager M. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J. Clin. Invest. 1995;96(6):2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Theoharides T.C., Valent P., Akin C. Mast cells, mastocytosis, and related disorders. N. Engl. J. Med. 2015;373(2):163–172. doi: 10.1056/NEJMra1409760. [DOI] [PubMed] [Google Scholar]
- 5.Guillen-Aguinaga S., Jauregui Presa I., Aguinaga-Ontoso E., Guillen-Grima F., Ferrer M. Updosing nonsedating antihistamines in patients with chronic spontaneous urticaria: a systematic review and meta-analysis. Br. J. Dermatol. 2016;175(6):1153–1165. doi: 10.1111/bjd.14768. [DOI] [PubMed] [Google Scholar]
- 6.Metz M., Ohanyan T., Church M.K., Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J. Dermatol. Sci. 2014;73(1):57–62. doi: 10.1016/j.jdermsci.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Steiss J.O., Strohner P., Zimmer K.P., Lindemann H. Reduction of the total IgE level by omalizumab in children and adolescents. J. Asthma. 2008;45(3):233–236. doi: 10.1080/02770900701883782. [DOI] [PubMed] [Google Scholar]
- 8.Metz M., Staubach P., Bauer A., Brehler R., Gericke J., Kangas M. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcepsilonRI-positive cells in the skin. Theranostics. 2017;7(5):1266–1276. doi: 10.7150/thno.18304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deza G., Bertolin-Colilla M., Pujol R.M., Curto-Barredo L., Soto D., Garcia M. Basophil FcepsilonRI expression in chronic spontaneous urticaria: a potential immunological predictor of response to omalizumab therapy. Acta Derm. Venereol. 2017;97(6):698–704. doi: 10.2340/00015555-2654. [DOI] [PubMed] [Google Scholar]
- 10.Mekori Y.A., Giorno R.C., Anderson P., Kohler P.F. Lymphocyte subpopulations in the skin of patients with chronic urticaria. J. Allergy Clin. Immunol. 1983;72(6):681–684. doi: 10.1016/0091-6749(83)90629-2. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs B.F., Patsinakidis N., Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front. Immunol. 2019;10:1383. doi: 10.3389/fimmu.2019.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Salvo E., Ventura-Spagnolo E., Casciaro M., Navarra M., Gangemi S. IL-33/IL-31 Axis: a potential inflammatory pathway. Mediat. Inflamm. 2018;2018:3858032. doi: 10.1155/2018/3858032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakashima C., Otsuka A., Kabashima K. Interleukin-31 and interleukin-31 receptor: new therapeutic targets for atopic dermatitis. Exp. Dermatol. 2018;27(4):327–331. doi: 10.1111/exd.13533. [DOI] [PubMed] [Google Scholar]
- 14.Bilsborough J., Leung D.Y., Maurer M., Howell M., Boguniewicz M., Yao L. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2006;117(2):418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 15.Raap U., Wichmann K., Bruder M., Stander S., Wedi B., Kapp A. Correlation of IL-31 serum levels with severity of atopic dermatitis. J. Allergy Clin. Immunol. 2008;122(2):421–423. doi: 10.1016/j.jaci.2008.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Raap U., Weissmantel S., Gehring M., Eisenberg A.M., Kapp A., Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr. Allergy Immunol. 2012;23(3):285–288. doi: 10.1111/j.1399-3038.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- 17.Sonkoly E., Muller A., Lauerma A.I., Pivarcsi A., Soto H., Kemeny L. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006;117(2):411–417. doi: 10.1016/j.jaci.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 18.Neis M.M., Peters B., Dreuw A., Wenzel J., Bieber T., Mauch C. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J. Allergy Clin. Immunol. 2006;118(4):930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Narbutt J., Olejniczak I., Sobolewska-Sztychny D., Sysa-Jedrzejowska A., Slowik-Kwiatkowska I., Hawro T. Narrow band ultraviolet B irradiations cause alteration in interleukin-31 serum level in psoriatic patients. Arch. Dermatol. Res. 2013;305(3):191–195. doi: 10.1007/s00403-012-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czarnecka-Operacz M., Polanska A., Klimanska M., Teresiak-Mikolajczak E., Molinska-Glura M., Adamski Z. Itching sensation in psoriatic patients and its relation to body mass index and IL-17 and IL-31 concentrations. Postepy. Dermatol. Alergol. 2015;32(6):426–430. doi: 10.5114/pdia.2015.56097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rauber M.M., Pickert J., Holiangu L., Mobs C., Pfutzner W. Omalizumab response correlates with reduced IFN-gamma-, IL-10- and IL-31-secreting cells in chronic spontaneous urticaria. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16216. [DOI] [PubMed] [Google Scholar]
- 22.Lin W., Zhou Q., Liu C., Ying M., Xu S. Increased plasma IL-17, IL-31, and IL-33 levels in chronic spontaneous urticaria. Sci. Rep. 2017;7(1):17797. doi: 10.1038/s41598-017-18187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altrichter S., Hawro T., Hanel K., Czaja K., Luscher B., Maurer M. Successful omalizumab treatment in chronic spontaneous urticaria is associated with lowering of serum IL-31 levels. J. Eur. Acad. Dermatol. Venereol. 2016;30(3):454–455. doi: 10.1111/jdv.12831. [DOI] [PubMed] [Google Scholar]
- 24.Raap U., Wieczorek D., Gehring M., Pauls I., Stander S., Kapp A. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp. Dermatol. 2010;19(5):464–466. doi: 10.1111/j.1600-0625.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 25.Ko M.J., Peng Y.S., Chen H.Y., Hsu S.P., Pai M.F., Yang J.Y. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J. Am. Acad. Dermatol. 2014;71(6):1151–9 e1. doi: 10.1016/j.jaad.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Basile F., Santamaria A., Mannucci C., Rizzo L., Gangemi S., D'Anna R. Interleukin 31 is involved in intrahepatic cholestasis of pregnancy. J. Matern. Fetal Neonatal Med. 2017;30(9):1124–1127. doi: 10.1080/14767058.2016.1205025. [DOI] [PubMed] [Google Scholar]
- 27.Salao K., Sawanyawisuth K., Winaikosol K., Choonhakarn C., Chaowattanapanit S. Interleukin-31 and chronic pruritus of unknown origin. Biomark. Insights. 2020;15 doi: 10.1177/1177271920940712. 1177271920940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takamori A., Nambu A., Sato K., Yamaguchi S., Matsuda K., Numata T. IL-31 is crucial for induction of pruritus, but not inflammation, in contact hypersensitivity. Sci. Rep. 2018;8(1):6639. doi: 10.1038/s41598-018-25094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruzicka T., Hanifin J.M., Furue M., Pulka G., Mlynarczyk I., Wollenberg A. Anti-Interleukin-31 receptor A antibody for atopic dermatitis. N. Engl. J. Med. 2017;376(9):826–835. doi: 10.1056/NEJMoa1606490. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg J.I., Pinter A., Pulka G., Poulin Y., Bouaziz J.D., Wollenberg A. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J. Allergy Clin. Immunol. 2020;145(1):173–182. doi: 10.1016/j.jaci.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Ezzat M.H., Hasan Z.E., Shaheen K.Y. Serum measurement of interleukin-31 (IL-31) in paediatric atopic dermatitis: elevated levels correlate with severity scoring. J. Eur. Acad. Dermatol. Venereol. 2011;25(3):334–339. doi: 10.1111/j.1468-3083.2010.03794.x. [DOI] [PubMed] [Google Scholar]
- 32.Kim S., Kim H.J., Yang H.S., Kim E., Huh I.S., Yang J.M. IL-31 serum protein and tissue mRNA levels in patients with atopic dermatitis. Ann. Dermatol. 2011;23(4):468–473. doi: 10.5021/ad.2011.23.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sachdeva S., Gupta V., Amin S.S., Tahseen M. Chronic urticaria. Indian J. Dermatol. 2011;56(6):622–628. doi: 10.4103/0019-5154.91817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asero R. Sex differences in the pathogenesis of chronic urticaria. J. Allergy Clin. Immunol. 2003;111(2):425–426. doi: 10.1067/mai.2003.15. [DOI] [PubMed] [Google Scholar]
- 35.Rorsman H. Basopenia in urticaria. Acta Allergol. 1961;16:185–215. doi: 10.1111/j.1398-9995.1961.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 36.Grattan C.E., Walpole D., Francis D.M., Niimi N., Dootson G., Edler S. Flow cytometric analysis of basophil numbers in chronic urticaria: basopenia is related to serum histamine releasing activity. Clin. Exp. Allergy. 1997;27(12):1417–1424. doi: 10.1046/j.1365-2222.1997.1630972.x. [DOI] [PubMed] [Google Scholar]
- 37.Grattan C.E., Dawn G., Gibbs S., Francis D.M. Blood basophil numbers in chronic ordinary urticaria and healthy controls: diurnal variation, influence of loratadine and prednisolone and relationship to disease activity. Clin. Exp. Allergy. 2003;33(3):337–341. doi: 10.1046/j.1365-2222.2003.01589.x. [DOI] [PubMed] [Google Scholar]
- 38.Ying S., Kikuchi Y., Meng Q., Kay A.B., Kaplan A.P. TH1/TH2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J. Allergy Clin. Immunol. 2002;109(4):694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 39.Ito Y., Satoh T., Takayama K., Miyagishi C., Walls A.F., Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66(8):1107–1113. doi: 10.1111/j.1398-9995.2011.02570.x. [DOI] [PubMed] [Google Scholar]
- 40.Kolkhir P., Borzova E., Grattan C., Asero R., Pogorelov D., Maurer M. Autoimmune comorbidity in chronic spontaneous urticaria: a systematic review. Autoimmun. Rev. 2017;16(12):1196–1208. doi: 10.1016/j.autrev.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Kolkhir P., Metz M., Altrichter S., Maurer M. Comorbidity of chronic spontaneous urticaria and autoimmune thyroid diseases: a systematic review. Allergy. 2017;72(10):1440–1460. doi: 10.1111/all.13182. [DOI] [PubMed] [Google Scholar]