Abstract
Background.
Photoimmunotherapy (PIT) uses a target-specific photosensitizer based on a near-infrared (NIR) phthalocyanine dye, IR700, to induce tumor necrosis after irradiation with NIR light to kill cancer cells, such as those that remain after surgery. The purpose of the present study was to sterilize the surgical bed after pancreatic cancer resection with PIT in carcinoembryonic antigen (CEA)-expressing, patient-derived, orthotopic xenograft (PDOX) nude mouse models.
Methods.
After confirmation of tumor engraftment, mice were randomized to two groups: bright light surgery (BLS)-only and BLS + PIT. Each treatment arm consisted of seven tumor-bearing mice. BLS was performed under standard bright-field with an MVX10 long-working distance, high-magnification microscope on all mice. For BLS + PIT, anti-CEA antibody conjugated with IR700 (anti-CEA-IR700) (50 μg) was injected intravenously in all mice 24 h before surgery. After the surgery, the resection bed was then irradiated with a red-light-emitting diode at 690 ± 5 nm with a power density of 150 mW/cm2.
Results.
Anti-CEA-IR700 labelled and illuminated the pancreatic cancer PDOX. Minimal residual cancer of the PDOX was detected by fluorescence after BLS. The local recurrence rate was 85.7 % for BLS-only and 28.6 % for BLS + PIT-treated mice (p = 0.05). The average recurrent tumor weight was 1149.0 ± 794.6 mg for BLS-only and 210.8 ± 336.9 mg for BLS + PIT-treated mice (p = 0.015).
Conclusion.
Anti-CEA-IR700 was able to label and illuminate a pancreatic cancer PDOX nude mouse model sufficiently for PIT. PIT reduced recurrence by eliminating remaining residual cancer cells after BLS.
Complete tumor resection can improve overall survival of pancreatic cancer patients. However, the 5-year survival rate is only 5 %, indicating that a true R0 complete resection is rarely achieved.1 Thus, local and metastatic relapses often occur following attempted curative resection of the primary tumor as a result of invisible microscopic tumor deposits left behind. Therefore, adjuvant therapies are needed after surgical resection of pancreatic adenocarcinoma.
Mitsunaga et al. have developed a new type of molecular targeted cancer therapy, photoimmunotherapy (PIT), that uses a target-specific photosensitizer based on a near-infrared (NIR) phthalocyanine dye, IR700, conjugated to monoclonal antibodies (mAbs) targeting epidermal growth factor receptors.2 Cell death was induced immediately after irradiating mAb-IR700-bound target cancer cells with NIR light. Mitsunaga et al. also demonstrated in vivo tumor shrinkage after irradiation with NIR light in target cells expressing the epidermal growth factor receptor without any observed side effects.2
In a previous study, we used a monoclonal chimeric antibody specific for carcinoembryonic antigen (CEA) that was conjugated to IR700 (anti-CEA-IR700) to determine efficacy of PIT alone without surgical resection in an orthotopic model of human BxPC-3 pancreatic cancer.3 There was a significant difference in overall tumor burden between the PIT and control groups in favor of the treated groups when assessing for both tumor size and tumor weight 5 weeks after initial therapy. However, all of the PIT-treated mice had tumor recurrence. Given these results, we postulated that PIT might be more effective when used in an adjuvant setting after surgical resection of pancreatic cancer. Therefore, in the present study, the efficacy of using intraoperative PIT with anti-CEA-IR700 on the postsurgical resection bed was determined in a CEA-positive pancreatic cancer patient-derived orthotopic xenograft (PDOX) nude-mouse model.
MATERIALS AND METHODS
Animals
Athymic nu/nu nude mice (AntiCancer, Inc., San Diego, CA), 4–6 weeks old, were used in this study. Mice were kept in a barrier facility under HEPA filtration. Mice were fed with autoclaved laboratory rodent diet. All mouse surgical procedures and imaging were performed with the animals anesthetized by intramuscular injection of a solution of 50 % ketamine, 38 % xylazine, and 12 % acepromazine maleate (0.02 ml). All animal studies were conducted in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1.
Establishment of Patient-Derived Orthotopic Xenograft of Pancreatic Cancer
Pancreatic cancer patient tumor tissues were obtained at surgery and cut into fragments (3 mm3) and originally transplanted subcutaneously in nude mice.4,5 The subcutaneous tumors were then passaged in nude mice both orthotopically and subcutaneously. All patients provided written, informed consent under the approval of the Institutional Review Board of the University of California San Diego.
Orthotopic Tumor Implantation
A small 6- to 10-mm transverse incision was made on the left flank of the mouse through the skin and peritoneum. The tail of the pancreas was exposed through this incision, and a single tumor fragment (3 mm3) from subcutaneous tumors was sutured to the tail of the pancreas using 8–0 nylon surgical sutures (Ethilon; Ethicon Inc., NJ). On completion, the tail of the pancreas was returned to the abdomen, and the incision was closed in one layer using 6–0 nylon surgical sutures (Ethilon).4,6
Antibody Dye Conjugation
A water-soluble, silicon-phthalocyanine derivative, IRDye 700DX NHS ester, was obtained from LI-COR Bioscience (Lincoln, NE). Chimeric anti-CEA antibody (Genara Biosciences LLC, Morgan Hill, CA) [2 mg (~14 nmol)] at a concentration of 2 mg/ml in 0.1 M Na2HPO4 (pH 8.6) was incubated for 2 h at room temperature with IR700dye NHS ester (135 μg, 70 nmol) prepared in anhydrous DMSO at 5 mmol/L. After the incubation period, the IR700-conjugate was buffer exchanged and purified with phosphate buffer saline (PBS, pH 7.1) using Amicon Ultra Centrifugal Filter Units (EMD Millipore Corporation, Billerica, MA). The IR700-mAb conjugate was repeatedly diluted with 10-ml volumes of PBS and then concentrated using the filter units until less than 2 % of the unconjugated IR700 dye species remained, as determined by size exclusion HPLC (SE-HPLC). Analysis of the conjugates by SE-HPLC was performed using an Agilent 1100 HPLC system fitted with a TSKgel G2000SW × 1 column (Tosoh Biosciences, Tokyo, Japan). The SE-HPLC elution buffer was 1× PBS (pH 7.1) with a flow rate of 1 ml/min. UV/Vis detection at 280 and 690 nm was used to determine the average dye-to-antibody ratio (DAR) for each conjugates. With this sample, a purity of 97.6 % with 0.5 % free dye and a dye to antibody ratio of 4.1 was achieved.
Fluorescence Imaging
The Olympus OV100 Small Animal Imaging System (Olympus Corp.), containing an MT-20 light source (Olympus Biosystems, Planegg, Germany) and DP70 CCD camera (Olympus Corp., Tokyo, Japan) was used for imaging tumors in live mice.7
Tumor Resection and PIT Treatment
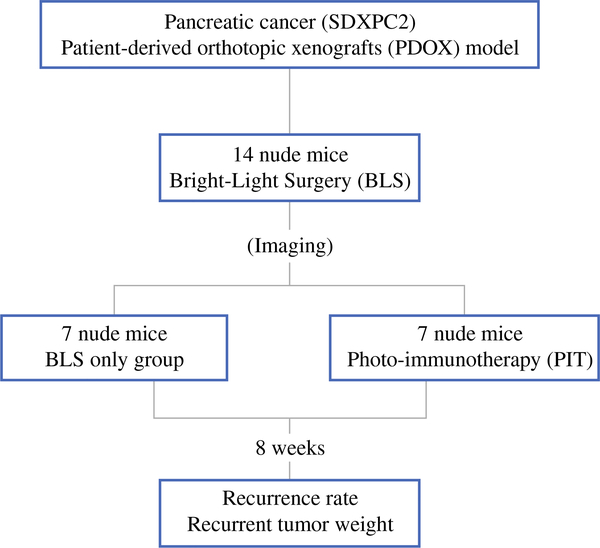
After confirmation of the tumor engraftment, mice were randomized to two groups: BLS only and BLS + PIT. Each treatment arm involved seven tumor-bearing mice (Fig. 1). For surgery, a 15-mm transverse incision was made on the left flank of the mouse through the skin and peritoneum and kept widely open with a retractor. The tail of the pancreas was exposed through this incision. BLS was performed under standard bright-field using the MVX10 microscope (Olympus, Tokyo, Japan) on all mice. For BLS + PIT group, anti-CEA antibody conjugated with IR700 (anti-CEA-IR700) (50 μg) was injected intravenously via the tail vein of mice 24 h before surgery. The resection bed was then irradiated with light from a red-light-emitting diode at wavelengths of 690 ± 5 nm (MRL-III-690–800mW, Ultralasers, Inc., Toronto, Canada) and a power density of 150 mW/cm2, as measured with an optical power meter (PM 100, Thorlabs, Inc., Newton, NJ) for 30 min (Supplemental Fig. 1). The surrounding normal tissues were protected with aluminum foil during PIT. After completion of treatment, the incision was closed in one layer using 6–0 nylon surgical sutures, and the mice were allowed to recover in their cages.
FIG. 1.
Schema of the experimental design. After confirmation of the tumor growth, the PDOXs (SDXPC2) were randomized in the two groups: BLS only and BLS + PIT. Each treatment arm involved seven tumor-bearing mice. BLS was performed under standard bright-field using the MVX10 microscope on both treatment groups. The PDOXs were imaged before and after BLS with the OV100, and the excised tumors also were imaged and weighed. Anti-CEA-IR700 (50 μg) was injected in the tail vain of the mice in the BLS + PIT group 24 h before surgery. An infrared laser (690 ± 5 nm, 150 mW/cm2) was used to irradiate the resection bed of the mice treated with BLS + PIT for 30 min. Eight weeks after surgery, animals underwent laparotomy, and tumors were imaged, weighed, and harvested for analysis
Tissue Histology
Tumor samples from two different PDOX models of pancreatic cancer (SDXPC1 and SDXPC2) were removed with surrounding normal tissues. Fresh tissue samples were fixed in 10 % formalin and embedded in paraffin before sectioning and staining. Tissue sections (3 μm) were deparaffinized in xylene and rehydrated in an ethanol series. Hematoxylin and eosin (H & E) staining was performed according to standard protocols. For immunohistochemistry, the sections were then treated for 30 min with 0.3 % hydrogen peroxide to block endogenous peroxidase activity. The sections were subsequently washed with PBS and unmasked in citrate antigen unmasking solution (Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan) in a water bath for 40 min at 98 °C. After incubation with 10 % normal goat serum, the sections were incubated with anti-CEA antibody (1:100) at 4 °C overnight. The bound primary antibodies were detected by binding with an antimouse secondary antibodies and an avidin/biotin/horseradish peroxidase complex (DAKO Cytomation, Kyoto, Japan) for 30 min at room temperature. The labeled antigens were visualized with the DAB kit (DAKO Cytomation). Sections were counterstained with hematoxylin and examined using an Olympus BH-2 microscope equipped with an INFINITY1 2.0 megapixel CMOS digital camera (Lumenera Corporation, Ottawa, Canada). All images were acquired using INFINITY ANALYZE software (Lumenera Corporation) without post-acquisition processing.
Evaluation of Tumor Recurrence and Progression
To assess for recurrence postoperatively, animals underwent laparotomy 8 weeks after surgery, and the tumors were imaged with an EOS 60D digital camera with an EF–S18–55 IS lens (Canon), excised, weighed, and harvested for analysis.
Statistical Analysis
PASWStatistics 18.0 (SPSS, Inc.) was used for statistical analyses. Tumor weight is expressed as mean ± SD. The two-tailed Student’s t test was used to compare continuous variables between two groups. Comparisons between categorical variables were analyzed using Fisher’s exact test. A p value <0.05 was considered statistically significant for all comparisons.
RESULTS
Differential CEA-Expression Levels of Pancreatic Cancer PDOXs
Both SDXPC1 and SDXPC2 were diagnosed as moderately differentiated adenocarcinoma with H & E staining (Supplemental Figs. 2A and C). SDXPC2 strongly stained with anti-CEA antibody (Supplemental Fig. 2D), whereas only a weak signal was detected in SDXPC1 (Supplemental Fig. 2B). Based on the result of immunohistochemistry, SDXPC1 was defined as CEA-negative and SDXPC2 was defined as CEA-positive.
SDXPC2 was Brightly Labeled with Anti-CEA Antibody
To examine whether anti-CEA-IR700 could label the pancreatic tumors, anti-CEA-IR700 (50 μg) was injected into the tail veins of the mice with SDXPC1 and SDXPC2 tumors. Twenty-four hours later, whole-body images were taken with the OV100. SDXPC2 was brightly labeled with anti-CEA-IR700 (Supplemental Fig. 1H), whereas the fluorescence signal from SDXPC1 labeled with anti-CEA-IR700 was very weak (Supplemental Fig. 1F). These results were consistent with the immunohistochemical results, and based on them, we decided to use the SDXPC2 PDOX model for further PIT experiments.
Anti-CEA-IR700 Allowed for Detection of Residual Tumor Undetectable with BLS in the PDOX Model
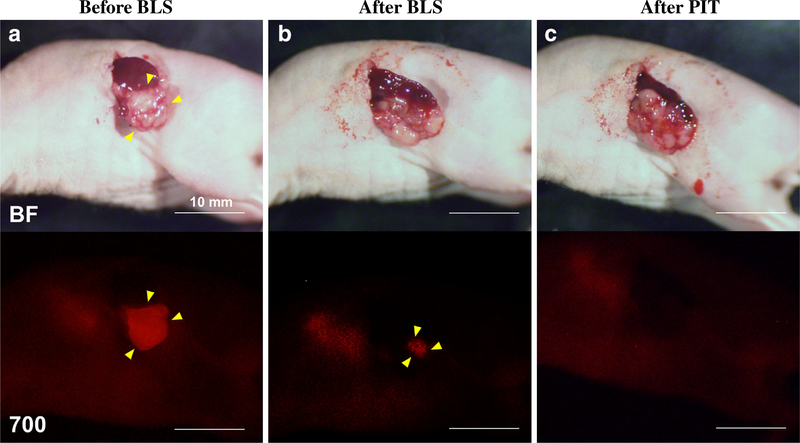
BLS was performed under standard bright-field using the MVX10 microscope on both treatment groups. The PDOXs were imaged before and after BLS with the OV100, and the excised tumors also were imaged and weighed. The average excised tumor weight of the BLS-only and the BLS + PIT-treated mice was 217.1 ± 122.0 and 262.4 ± 134.3 mg, respectively. There was no difference between two groups (p = 0.522). Anti-CEA-IR700 (50 μg) was injected in the tail vain of the mice in the BLS + PIT group 24 h before surgery, which brightly illuminated the PDOXs (Fig. 2a). Fluorescence signals from the residual tumors after BLS were still clearly detected (Fig. 2b), and those signals became undetectable after PIT treatment (Fig. 2c). This result suggests that anti-CEA-IR700 could illuminate the residual tumor sufficiently for PIT treatment.
FIG. 2.
Representative images of treated mice. Upper panels are bright field (BF) images and lower panels are fluorescence images with IR 700 (700). Anti-CEA-IR700 brightly illuminated the PDOXs (a). Fluorescence signals from the residual tumors after BLS were still bright (b), and those signals disappeared after PIT treatment (c). Yellow arrowheads indicate tumors. Scale bars 10 mm
PIT in Combination with BLS Reduced Recurrence
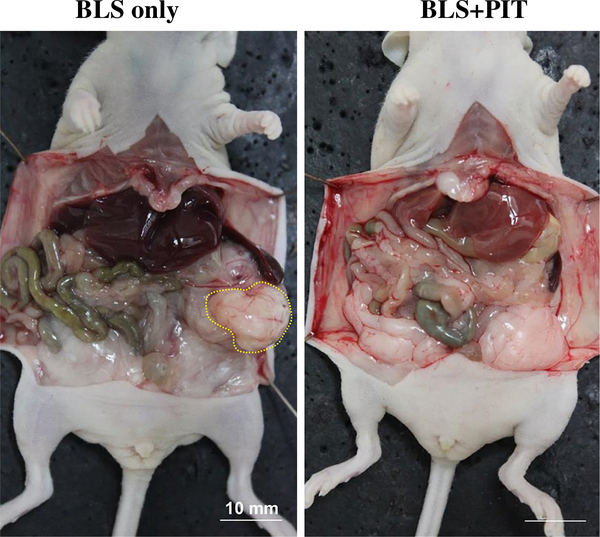
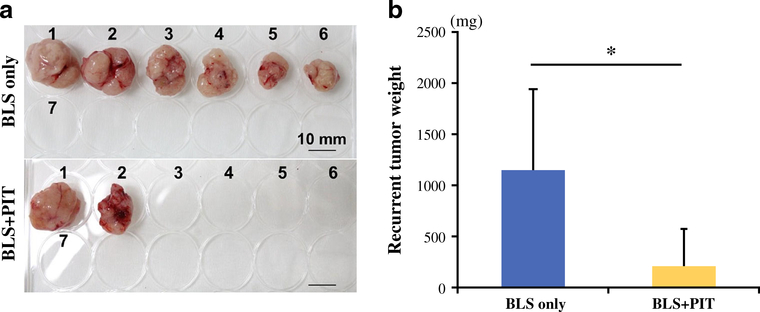
The local recurrence rate of BLS-only and BLS + PIT-treated mice was 85.7 and 28.6 %, respectively (p = 0.051). Figure 3 shows representative whole-body images in each treatment group. No metastatic recurrence was detected in either group. The average recurrent tumor weight of BLS-only and BLS + PIT-treated mice was 1149.0 ± 794.6 and 210.8 ± 336.9 mg, respectively (p = 0.015; Fig. 4). No body weight loss was found in either treatment group. These results suggest that PIT treatment reduces the residual tumors after BLS, resulting in less local recurrence and better outcome.
FIG. 3.
Recurrence in each treatment group. Representative whole-body images in each treatment group. A large local recurrent tumor (yellow dashed line) was detected in BLS-only treated mice. In contrast, the recurrent tumors were fewer in the BLS + PIT-treated mice. Scale bars 10 mm
FIG. 4.
a Gross images of recurrent tumors in each treatment group. Local recurrent tumors were detected in six mice in the BLS-only group (85.7 %) and two mice in the BLS + PIT group (28.6 %). No metastatic recurrence was detected in either group. The tumor volume in the BLS + PIT-treated mice was less than in BLS-only treated mice. Scale bars 10 mm. b Recurrent tumor weight of each treatment group. The average recurrent tumor weight was 1149.0 ± 794.6 mg for BLS-only treated mice and 210.8 ± 336.9 mg for BLS + PIT treated mice (p = 0.015)
DISCUSSION
In the present study, we used a clinically similar model of pancreatic cancer, PDOX, to determine the efficacy of PIT to eradicate residual cancer after BLS. We found that anti-CEA-IR700 could label and illuminate the pancreatic tumor strongly enough for PIT in both subcutaneous and PDOX models. The use of sequential BLS and PIT resulted in no tumor recurrence and apparent cures in more than 70 % of the animals. In addition, the current study was on a patient-derived orthotopic xenograft (PDOX) model.4,8–14 This is a major advance in the case of adjuvant PIT for curative pancreatic cancer therapy. In our previous study with the use of PIT only, all of the mice with pancreatic cancer recurred after treatment.3 Also, the previous study was performed with a pancreatic cancer cell line and not a PDOX model.3
PIT treatment avoids the side effects of conventional PDT with a targeted-photosensitizer, which involves two components: a monoclonal antibody (mAb), which recognizes specific proteins on the surface of cancer cells, and a photosensitizer, which can be activated by light for targeted PIT only when bound to specific antigens.2
Previous studies by Kobayashi et al. showed that panitumumab-IR700 killed tumor cells seconds after near-infrared light irradiation.15 There also was a positive correlation between the intensity of the excitation light and percentage of cell death. Infrared light alone or panitumumab-IR700 conjugate alone did not cause any damage to normal cells. When mice with tumors were treated with panitumumab-IR700 and near-infrared light, significant tumor shrinkage was observed. With fractionated administration of mAB–IR700 conjugate followed by systematic repeated NIR light irradiation of the tumor, 80 % of the cancer cells were eradicated and mouse survival was significantly prolonged.15
In conclusion, we used a chimeric antibody to CEA for conjugation to IR700 for PIT. CEA is not expressed in normal pancreatic tissue, but it is frequently expressed in pancreatic cancer tissues.16,17 We showed that anti-CEA-IR700 is able to label and illuminate a pancreatic cancer PDOX nude mouse model sufficiently for PIT, which reduced recurrence by targeting residual cancer cells after BLS.
Supplementary Material
ACKNOWLEDGMENT
This study was supported in part by National Cancer Institute grants CA132971 and 142669 (to Michael Bouvet and AntiCancer, Inc.) and JSPS KAKENHI Grant Numbers 26830081 to Yukihiko Hiroshima, 26462070 to Itaru Endo and 24592009 to Kuniya Tanaka.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4553-9) contains supplementary material, which is available to authorized users.
REFERENCES
- 1.Kato K, Yamada S, Sugimoto H, et al. Prognostic factors for survival after extended pancreatectomy for pancreatic head cancer: influence of resection margin status on survival. Pancreas. 2009;38(6):605–12. [DOI] [PubMed] [Google Scholar]
- 2.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat Med 2011;17(12):1685–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maawy A, Hiroshima Y, Zhang Y, et al. Near infra-red photoimmunotherapy with anti-CEA-IR700 results in extensive tumor lysis and a significant decrease in tumor burden in orthotopic mouse models of pancreatic cancer. PLoS One. 2015;10(3): e0121989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu X, Guadagni F, Hoffman RM. A metastatic nude-mouse model of human pancreatic cancer constructed orthotopically with histologically intact patient specimens. Proc Natl Acad Sci U S A. 1992;89(12):5645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furukawa T, Kubota T, Watanabe M, Kitajima M, Hoffman RM. A novel “patient-like” treatment model of human pancreatic cancer constructed using orthotopic transplantation of histologically intact human tumor tissue in nude mice. Cancer Res. 1993;53(13):3070–2. [PubMed] [Google Scholar]
- 6.Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17(4):343–59. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi K, Yang M, Jiang P, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 2006;66(8):4208–14. [DOI] [PubMed] [Google Scholar]
- 8.Astoul P, Colt HG, Wang X, Hoffman RM. Metastatic human pleural ovarian cancer model constructed by orthotopic implantation of fresh histologically-intact patient carcinoma in nude mice. Anticancer Res. 1993;13(6A):1999–2002. [PubMed] [Google Scholar]
- 9.Fu X, Hoffman RM. Human ovarian carcinoma metastatic models constructed in nude mice by orthotopic transplantation of histologically-intact patient specimens. Anticancer Res. 1993; 13(2):283–286. [PubMed] [Google Scholar]
- 10.Fu X, Le P, Hoffman RM. A metastatic orthotopic-transplant nude-mouse model of human patient breast cancer. Anticancer Res. 1993;13(4):901–904. [PubMed] [Google Scholar]
- 11.Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci U S A. 1991;88(20):9345–9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiroshima Y, Zhang Y, Zhang N, et al. Establishment of a patient-derived orthotopic xenograft (PDOX) model of HER-2-positive cervical cancer expressing the clinical metastatic pattern. PLoS One. 2015;10(2):e0117417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Togo S, Shimada H, Kubota T, Moossa AR, Hoffman RM. Host organ specifically determines cancer progression. Cancer Res. 1995;55(3):681–684. [PubMed] [Google Scholar]
- 14.Wang X, Fu X, Hoffman RM. A new patient-like metastatic model of human lung cancer constructed orthotopically with intact tissue via thoracotomy in immunodeficient mice. Int J Cancer. 1992;51(6):992–995. [DOI] [PubMed] [Google Scholar]
- 15.Mitsunaga M, Nakajima T, Sano K, Choyke PL, Kobayashi H. Near-infrared theranostic photoimmunotherapy (PIT): repeated exposure of light enhances the effect of immunoconjugate. Bioconjug Chem. 2012;23(3):604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaguchi K, Enjoji M, Tsuneyoshi M. Pancreatoduodenal carcinoma: a clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19–9. J Surg Oncol. 1991;47(3):148–54. [DOI] [PubMed] [Google Scholar]
- 17.Xu C, Sun Y, Wang Y, et al. CEA promoter-regulated oncolytic adenovirus-mediated Hsp70 expression in immune gene therapy for pancreatic cancer. Cancer Lett 2012;319(2):154–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.